Abstract
The ability to observe, interpret, and learn behaviors and emotions from conspecifics is crucial for survival, as it bypasses direct experience to avoid potential dangers and maximize rewards and benefits. The anterior cingulate cortex (ACC) and its extended neural connections are emerging as important networks for the detection, encoding, and interpretation of social signals during observational learning. Evidence from rodents and primates (including humans) suggests that the social interactions that occur while individuals are exposed to important information in their environment lead to transfer of information across individuals that promotes adaptive behaviors in the form of either social affiliation, alertness, or avoidance. In this review, we first showcase anatomical and functional connections of the ACC in primates and rodents that contribute to the perception of social signals. We then discuss species-specific cognitive and social functions of the ACC and differentiate between neural activity related to ‘self’ and ‘other’, extending into the difference between social signals received and processed by the self, versus observing social interactions among others. We next describe behavioral and neural events that contribute to social learning via observation. Finally, we discuss some of the neural mechanisms underlying observational learning within the ACC and its extended network.
Keywords: Social learning, Vicarious learning, Empathy, Social cues, Social transmission, Social dominance, Fear learning, Fear conditioning by proxy, Amygdala, Anterior cingulate cortex, Prefrontal cortex, Rodents, Primates, Facial expression
1. Introduction
The ability to monitor behaviors in others and to learn by observation to maximize rewards and avoid dangers is a fundamental higher-level cognitive function shared by multiple species (Bastiaansen et al., 2009; Panksepp and Lahvis, 2011; Preston and de Waal, 2002). Findings from rodents, non-human primates, and humans provide evidence that exposure to behavioral cues during natural social interactions (e.g., witnessing aggression towards a social partner and responding with protective behaviors), initiates a learning process that connects the observed behaviors to outcomes (e.g., social bonding). During this process, the perceived social signals are integrated to produce adaptive behaviors. This phenomenon has been referred in the literature as social or observational learning. For example, during observational fear learning, exposure of a mouse or rat to cues that signal threat to others, including the smell, sight, and sound of a distressed conspecific, induces fear responses (Allsop et al., 2018; Carrillo et al., 2015; Jeon and Shin, 2011). Likewise, fear to snakes in demonstrator monkeys induces fear of snakes in laboratory-reared monkeys (Cook and Mineka, 1989; Mineka et al., 1984), and fear of a “visual cliff’ (a graphical illusion painted on a flat floor) in mothers inhibits human babies from crawling over it (Gibson and Walk, 1960). These findings highlight that the ability of learning through observation is highly conserved across species.
Social cues can also modulate behavior via a non-associative process. Fear learning, for example, can be either attenuated or enhanced by the presence of social cues. The presence of a social partner or a familiar conspecific signals safety and can attenuate the acquisition, consolidation, and expression of fear memories, a phenomenon known as social buffering of aversive responses (Beauchamp, 2008; Davitz and Mason, 1955; Guzmán et al., 2009; Harb and Taylor, 2015; Hennessy et al., 2009; Howell et al., 2017; Kikusui et al., 2006; Kiyokawa, 2017; Morrison and Hill, 1967). Social cues can also sensitize the brain and enhance fear learning reinforced by non-social unconditional stimuli at a later moment (Ito et al., 2015; Knapska et al., 2010). Likewise, vicarious social defeat can cause chronic anhedonia by altering the affective state of the observer (Iñiguez et al., 2018; Warren et al., 2013). The mechanisms by which the brain perceives, processes, and learns social cues to translate the learned information into adaptive behaviors are not well understood, although multiple processes have been implicated. These processes include the recognition of emotional facial cues in others (reviewed by Niedenthal and Brauer, 2012), mimicry or imitation (reviewed by Kavanagh and Winkielman, 2016), heightened attention to others (reviewed by Miklósi, 1999; Oláh et al., 2016), theory of mind (reviewed by Emery and Clayton, 2009), and the vicarious motivational state when observing the actions of others (reviewed by Apps et al., 2016).
Recent work in observational learning has started to home in on its neurobiological underpinning. For instance, studies in non-human primates (Apps et al., 2013; Chang et al., 2013), rodents (Allsop et al., 2018; Jeon et al., 2010; Kim et al., 2012), and humans (Lockwood et al., 2015; Olsson and Phelps, 2007) ascribe the anterior cingulate cortex (ACC) an important role in observational learning. Such attribution to the ACC comes by virtue of distinct neural specialization for processing social information that is supported by a vast network of connections to other structures with known social-cognitive functions.
Although many findings implicate the ACC-centered circuits in an organism’s ability to learn socially transmitted information, the mechanisms by which neurons in these circuits compute information about others to guide learning and produce adaptive behavior are not completely understood. Likewise, little is known about the behavioral factors that promote social transmission of information, and the cellular and synaptic mechanisms involved. By bringing together insights from anatomical, neurophysiological, and behavioral studies across species, in this review we aim to examine the critical components of a larger ACC network to help formulate new mechanistic hypotheses regarding behavioral factors and synaptic processes by which evaluating and computing socially salient information about others can be tied to ACC circuits.
2. Brain regions constituting the ACC
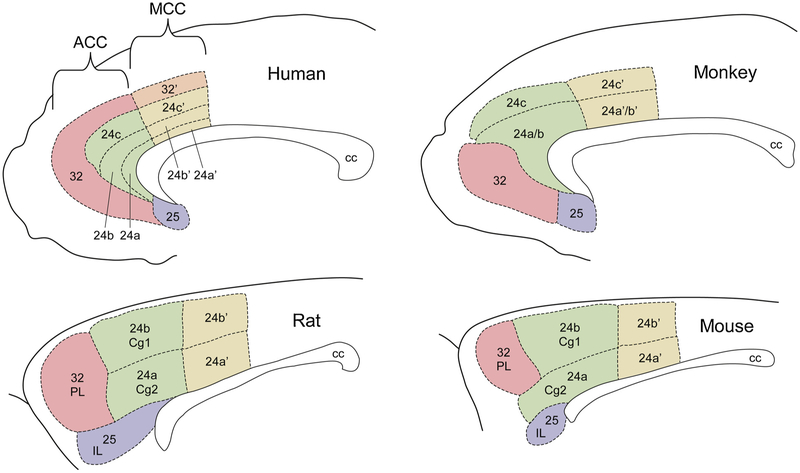
The cortical areas of the ACC are divided in phylogenetically older agranular or dysgranular areas, that lack or contain a rather faint internal granular cortical layer 4 and granular areas that are present only in humans and non-human primates (Barbas and García-Cabezas, 2016; Carlén, 2017; Fuster, 2015). Despite the lack of full homology between the ACC of primates and other mammalian species including rodents, this area performs, in multiple species, overlapping cognitive functions, such as emotional regulation, motivational drives, and social cognition (Apps et al., 2016; Bicks et al., 2015; Fuster, 2001; Paus, 2001; Watanabe, 2017; Laubach et al., 2018). ACC regions form an arc around the genu of the corpus callosum and face towards the medial wall of the frontal lobes. In primates, ACC regions generally include Brodmann area 32 (often referred to as the dorsal anterior cingulate cortex, dACC), area 24 (often referred to as the ventral anterior cingulate cortex, vACC), and area 25 (often referred to as the subgenual anterior cingulate cortex, sACC). In addition, these ACC areas have been further subdivided into 32′, 24a, 24b, and 24c, based on relative location and cytoarchitectonic features (i.e., cell composition, size, spacing, density, and lamination). Fig. 1 illustrates the relative location of these ACC subdivisions.
Fig. 1. Brain regions constituting the ACC in primates and rodents.
Diagrams represent midsagittal sections of the human, monkey, rat, and mouse brains, and illustrate distinct brain regions that form part of the anterior cingulate cortex. While the construction of these diagrams considered various previous studies and brain atlases (Bush et al., 2000; Paxinos and Franklin, 2019; Paxinos and Watson, 2014; Rushworth et al., 2004; Vogt and Paxinos, 2014), the sizes and boundaries illustrated for each brain region are not precise. Numerical labels represent Brodmann nomenclature, whereas alphabetical labels represent other definitions given to some of the regions in rodents. ACC, anterior cingulate cortex; MCC, mid anterior cingulate cortex; Cg1, cingulate area 1; Cg2, cingulate area 2; PL, prelimbic cortex; IL, infralimbic cortex; CC, corpus callosum.
Frontal cortices in rodents are evolutionarily less developed than in primates. However, despite much debate (Carlén, 2017; Laubach et al., 2018), distinct regions of the medial prefrontal cortex in rodents exhibit anatomical and functional features that resemble those observed in the primate ACC, and have been therefore recently redefined using the Brodmann nomenclature scheme (i.e., areas 32, 24, and 25; Vogt and Paxinos, 2014). Such rodent ACC areas substitute the areas previously known as the cingulate area 1 (Cg1, redefined to area 24b), cingulate area 2 (Cg2, redefined to area 24a), prelimbic cortex (PL, redefined to area 32), and infralimbic cortex (IL, redefined to area 25) (Paxinos and Franklin, 2019, 2004; Paxinos and Watson, 2014, 1998). This alternative Brodmann-based naming scheme provides great opportunities for neuroscientists to become more consistent with nomenclature, as well as with comparisons of ACC function across species.
While areas 24/Cg and 32/PL share many similarities in their pattern of anatomical connectivity with other brain regions, area 25/IL exhibits input and output patterns that are particularly distinct when compared to areas 24/Cg and 32/PL (Gabbott et al., 2005; Heilbronner et al., 2016; Hoover and Vertes, 2007; Morecraft et al., 2012; Vertes, 2004). Furthermore, area 25/IL exhibits functional roles that are quite distinct from the functional roles of areas 24/Cg and 32/PL (e.g., Balleine and O’Doherty, 2010; Burgos-Robles et al., 2013, 2009, Burgos-Robles et al., 2007; Peters et al., 2009; Wallis et al., 2017). For these reasons, in this review we do not intend to highlight the differences across these regions, but rather we intend to discuss a set of studies that have highlighted social functions in ACC areas, and intend to establish some ideas pertaining to how the ACC in general perceives and integrates social signals to facilitate observational learning. Yet, we recognize that the larger majority of the studies we discuss mostly examined areas 24/Cg and 32/PL, unless otherwise indicated.
Fig. 2 reviews some of the anatomical connections of areas 24/Cg and 32/PL in rodents, with layer specificity, and categorizes them into broad functional groups. Their specialized function and contribution to the processing of complex social signals are discussed elsewhere in this review.
Fig. 2. ACC connectivity in rodents is organized to integrate complex sensory, cognitive, and emotional information.
Left, Schematic of cortical layers in the rodent ACC. Cortical layers are color-coded. CC stands for corpus callosum. Right, Summary of connections of individual ACC layers with other brain areas involved in sensation, cognition, affect/arousal, and neuromodulation, based on anatomical tracing and electrophysiology studies (Aston-Jones and Waterhouse, 2016; Bissière et al., 2008; Cruikshank et al., 2012; Gabbott et al., 2005; Hoover and Vertes, 2007; Kamigaki, 2018; Lee et al., 2005; Little and Carter, 2012; Sara and Hervé-Minvielle, 1995; Vertes, 2004). Thicker and thinner arrows represent stronger and weaker connections, respectively. While stronger sensory inputs to the ACC tend to arrive through the supragranular layers 2/3, and stronger outputs originate in the infragranular layer 5, cells in both supragranular and infragranular layers also form extensive reciprocal connections with many brain areas involved in sensation, cognition, affect/arousal, and neuromodulation. The strong reciprocal connectivity of the ACC with this extended network may be optimal for integrating different types of information, including social and environmental cues, to facilitate observational learning.
3. The anatomical and functional connections of the rodent and primate ACC; homologies and unique features
Different subdivisions of the ACC are bidirectionally connected to distinct nuclear groups of the amygdala (Amaral and Insausti, 1992; Morecraft et al., 2012), whose function in mediating defensive behaviors is highly conserved among species including rodents and humans (Terburg et al., 2018). In primates, projections originating from basolateral nuclei of the amygdala target both the superficial layers and the deep layers of areas 24a and 24b (Morecraft et al., 2007; Morecraft and Van Hoesen, 1998). To clarify the amygdala nomenclature, the basolateral nuclei refer to the cortical nuclei of the amygdala that include the lateral, basal, and accessory basal nuclei in primates, or the lateral, basolateral, and basomedial counterparts in rodents, respectively (LeDoux, 2007). The pattern of connectivity from the amygdala to the cingulate cortex is truly exceptional as cortical projections originating in the amygdala typically terminate in the superficial layers of the cortex (Freese and Amaral, 2006). The amygdalo-cingulate projections that terminate in the deep layers of cingulate cortex where the motor neurons reside, create a direct pathway for limbic inputs from the amygdala to be translated into motor behaviors (Morecraft et al., 2007). In rodents, the amygdalo-cingulate projections also originate from the basal (basolateral) and accessory basal (basomedial) nuclei and to a small extent in the lateral nucleus (Reppucci and Petrovich, 2016). The rich, and relatively punctate connectivity of the ACC with the basolateral nuclei of the amygdala allows, in principle, the presence of multiple parallel loops that may carry out different functions.
The ACC receives other inputs from numerous areas that respond to social signals. Social signals including faces and bodies, certain types of touch, vocalization, certain odors, gestures and postures, biological motion, etc., arrive to the ACC from multisensory association areas, such as the superior temporal sulcus and the temporo-parietal area (Carmichael and Price, 1995; Heimer and Van Hoesen, 2006; Li et al., 2013; Vogt, 2016). The ACC is mostly agranular or dysgranular limbic cortex, therefore it is unlikely to participate in serial reprocessing of sensory thalamic inputs (Shipp, 2005), i.e., this area does not function as a quintessential sensory processor, but as a receiver of highly processed multi-sensory information that contributes to the sensory, decision-making, and motor dimensions of the ongoing behavior. Likewise, early sensory areas in primates are not known to project to the ACC. The ACC, like the amygdala, and the limbic areas of the insula, receives highly processed sensory signals, i.e., signals of high stimulus dimensions, such as faces. It may be therefore, that a highly salient social feature such the eyes, are not sufficient by themselves as a high-contrast visual feature, to activate neurons in these areas, but the eyes that are socially engaged (e.g., in eye contact) may activate strongly the ACC. Indeed, neurons in the amygdala that project to the ACC signal that the subject is seeking and is engaged in eye contact (Mosher et al., 2014).
The ACC of rodents follows the same connectivity scheme, with few exceptions (reviewed by Laubach et al., 2018). Based on these inputs, the ACC of rodents and primates alike integrates socially salient information about others into a coherent social percept, and generates, based on this percept, outcome estimates for the actions of others and self. Support for these functions of the cingulate come from its activation during tasks that require the subjects to predict the (social) behaviors of others (Apps et al., 2013; Apps and Ramnani, 2014; Chang et al., 2013; de Araujo et al., 2012; Haroush and Williams, 2015). The ACC is also activated when animals witness a conspecific receiving rewards or aversive stimuli (Hillman and Bilkey, 2012; Jeon et al., 2010; Jones and Monfils, 2016; Kavaliers et al., 2005), or when humans observe the facial expressions (e.g., Simon et al., 2006; Vrticka et al., 2009) and vocalizations (e.g., Johnstone et al., 2006) of others. The same types of stimuli also activate the primate amygdala (Chang et al., 2015; Gothard et al., 2007; Livneh et al., 2012; Sliwa and Freiwald, 2017). Coactivation of the ACC and the amygdala supports processing vicarious rewards and emotional resonance in rats (Amemiya et al., 2016), monkeys (Livneh and Paz, 2012), and humans (Seara-Cardoso et al., 2016). Indeed, the neural activity recorded simultaneously from these two brain areas show overlap and complementarity (Klavir et al., 2013) but also remarkable differences. A recent report showed that information coding in the human ACC is more efficient, while coding in the non-human primates is more robust (Pryluk et al., 2019). Furthermore, neural activity in the ACC and the amygdala increases with the acute administration of the pro-social peptide oxytocin (Pisansky et al., 2017), further supporting a notion that signaling mechanisms within the ACC-amygdala circuit are important for emphatic behaviors.
3.1. ACC networks for cognitive functions during social learning
The ACC is one of the most interconnected areas of the mammalian brain. An excellent recent review by Apps et al. (2016) regarding the role of the ACC in social behavior organized the connectivity of the cingulate cortex into three main networks. The delineation of these networks is based on (1) the known anatomical connections of each subdivision of the cingulate cortex, (2) dissociable activation networks revealed by neuroimaging in humans, and (3) lesion studies and single unit recordings from cingulate area in multiple species. The three networks support (a) mentalizing, (b) action observation, and (c) value-based, affective-cognitive processing.
The mentalizing network processes abstract features of other-oriented information such as shame and guilt, attributing agency to perceived actions (Yoshida and Burling, 2011), or adjusting subjective rewards to social norms (Rizzolatti and Craighero, 2004). This network connects portions of the anterior cingulate (areas 24a and 24b) to the temporo-parietal junction and to the dorsomedial prefrontal cortex. In light of a recent review of the homologies between the rodent and the primate prefrontal cortex (Laubach et al., 2018), this network may be less prominent in rodents because the granular dorsomedial prefrontal cortex of primates may not have a rodent homologue (all prefrontal areas in rodents are agranular cingulate cortex).
The action observation network connects the anterior cingulate to ventral premotor and parietal sensory-motor areas. These areas become active when organisms observe the actions of others. The connections of the cingulate with premotor and sensory-motor areas may mediate imitation and mirroring the actions of others (Rizzolatti and Craighero, 2004). Anatomical evidence suggests that hand movements in humans and monkeys are coordinated by cortical action execution/recognition circuit that includes the insula (Di Cesare et al., 2018). Interestingly, neurons in this network, specifically in the ventral premotor area, respond not only when the self and others perform an action but also when a forbidden action is not performed, either by the self or by others (Bonini et al., 2014).
The third, value processing network, connects the anterior cingulate to the amygdala, the insula, and ventromedial, ventrolateral and orbitofrontal prefrontal areas (in primates, Broadman areas, 10, 11, 12, 13, 14, 25 and 32). This network incorporates an embodied, emotion-mirroring system (complementary but different from action mirroring included in the action observation network), which includes the insula, the amygdala, and the anterior cingulate. These areas meet the criteria for mirror mechanisms of emotion, namely they produce a specific emotion when artificially stimulated, contain neurons that respond to displays of that emotion, and lesions cause behavioral impairments in experience that emotion (Rizzolatti and Caruana, 2017). Given that little is known about the mirroring system in rodents, the conserved core of this network, namely the amygdala - orbitofrontal cortex -cingulate loop, may be the common denominator of observational learning across species. The amygdalo-cingulate limbic-motor loop (Gothard, 2014; Morecraft et al., 2012, 2007) coordinates reward and affect-motivated movements. Limbic-motor circuits set in register the motivation state, or value representation (of the self and of other) with actions (of self or of others). Indeed, neurons in the cingulate respond to the subjective value of rewards (ventral bank in primates) and to the actions required to obtain rewards (dorsal bank) (Cai and Padoa-Schioppa, 2012). Likewise, neurons in the primate amygdala respond to the facial expressions of others (Gothard et al., 2007) and to the facial expressions of self (Mosher et al., 2016). The value signals that motivate actions elaborated in the ACC appear to come from the amygdala via the OFC, as lesions of the amygdala alter more profoundly value encoding in OFC than in the ACC (Rudebeck et al., 2013).
3.2. ACC networks for autonomic regulation during social learning
The rich connectivity of the cingulate cortex to subcortical areas involved in autonomic regulation also contributes to socio-emotional behavior, and implicitly to observational learning. These pathways ensure that the energetic state of the organism (e.g., arousal or depressed mood) meets its behavioral agenda (e.g., exploratory drive, hide for safety). Specifically, area 25, or the subgenual cingulate in primates and the homologous region in rodents, is tightly connected to monoaminergic brainstem nuclei such as the ventral tegmental area, raphe nuclei, and locus coeruleus (Chiba et al., 2001; Freedman et al., 2000; Gabbott et al., 2005; Lee et al., 2005; Sara and Hervé-Minvielle, 1995; Vertes, 2004), and to visceral afferents such as nucleus of the solitary tract and the parabrachial nuclei (Showers and Crosby, 1958). Although the connections between the parabrachial nuclei, solitary tract and the amygdala have been analyzed in more detail in the context of pain (Neugebauer, 2015; Palmiter, 2018), the same pathways appear to carry non-painful visceral stimuli that contribute to socio-cognitive states (Fulwiler and Saper, 1984). These connections may set the “tone” and the reactivity level in the cingulate. It has been proposed that these inputs to the subgenual cingulate sustain arousal during anticipation of positive outcomes thereby contributing to positive emotional states (Rudebeck et al., 2014). Failure to maintain positive anticipatory states reduces motivation to act and causes depression (Drevets et al., 1997; Keedwell et al., 2005). This might explain why in select patients stimulation of this area relieves treatment-resistant depression (Mayberg et al., 2005).
4. Other cognitive and social functions of the ACC shared by rodents and primates
Despite species-specific differences in cytoarchitectonics, arealization, and connectivity of the medial frontal/prefrontal cortex (Heilbronner et al., 2016; Laubach et al., 2018; Morecraft and Van Hoesen, 1993; Neubert et al., 2015; Vogt and Paxinos, 2014), the anterior cingulate of rodents and primates (human and non-human alike) carries out an overlapping list of computations, relying on somewhat different algorithms and neural implementation. These functions include but are not restricted to processing rewards and punishment (Chudasama et al., 2013; Rudebeck et al., 2013, 2008), cost-benefit estimations (Kennerley et al., 2006; Kennerley and Wallis, 2009; Kolling and Rushworth, 2015) including the estimation of effort (Cowen et al., 2012; Hillman and Bilkey, 2012), error monitoring and error prediction (Behrens et al., 2007; Fu et al., 2019; Kerns et al., 2004; Kolling et al., 2016; Shenhav et al., 2013; Sheth et al., 2012), and the elaboration of motor behaviors (e.g., Procyk et al., 2016). Some of these functions are useful in non-social contexts, yet the role of the anterior cingulate in social behavior does not emerge from adopting these general functions to social behavior. A recent review by Apps et al. (2016) argues that the cingulate cortex contains specialization for social behavior. A key specialization for observational learning is the presence in the ACC of neurons that respond selectively to information that pertains to the subjective experience and to the actions of others. These neurons are intermingled, and most likely interconnected with other neurons involved in cognitive control and error monitoring (Fu et al., 2019).
Through error monitoring an organism can compare, at both short and long-time scales, the outcome resulting from an action to the expected outcome. When errors are detected, new strategies are elaborated to avoid in the future similar errors (cognitive control). Although the neural events causal to and correlated with cognitive control have been studied primarily in behavioral paradigms motivated by primary reinforcers (e.g., food or juice), it is highly likely that the cognitive control of social behaviors exploits the same error monitoring and corrective system (Platt et al., 2016). Indeed, observational learning maximizes rewards or desired outcomes based on monitoring the action-outcomes experienced by others. By observing the actions of others and the subsequent errors, the observer is able to estimate the outcome of new strategies or behaviors. This is persuasively illustrated by neurons in the anterior cingulate of non-human primates that encode plans to switch to alternative actions based on outcome estimation (Kennerley et al., 2006; Sheth et al., 2012; Shima and Tanji, 1998). A recent report on neural correlates of error-monitoring and post-error adjustments in the human cingulate and the adjacent pre-supplementary motor area, indicate that cognitive control is instantiated by the coactivity of subpopulations of neurons that signal error, error history, conflict, and cognitive control state (Fu et al., 2019).
The most prominent physiological marker of error monitoring in the ACC is the Error-Related-Negativity (ERN), an evoked potential coinciding with errors that is large enough to be measurable with scalp electrodes in humans. The typical behavioral consequence of an error is the Post Error Slowing (PES), a delay in motor behavior in trials immediately following an error. This might be due to the inhibition of the motor loops originating in the ACC and other motor areas. Indeed, stimulation of these motor areas in the context of error-prone tasks, delays behaviors in order to avoid errors (Stuphorn and Schall, 2006). Although in rodents the ERN is less obvious, inactivation of the rodent cingulate cortex eliminates post-error slowing (Narayanan et al., 2013). In non-human primates, disruption of the cingulate cortex and of adjacent areas of the medial prefrontal cortex prevents suboptimal reward history to promote the elaboration of new behavioral strategies (Kennerley et al., 2006; Shima and Tanji, 1998).
In most species studied, observational learning requires the direct adjustment of actions of self to the observed actions of others. Reviewing the similarities between the rodent and primate cingulate Laubach and colleagues concluded that the rat and monkey ACC performs similar computations when organisms sequence (and plan) actions and outcomes (monkey: Procyk et al., 2000) (rat: Lapish et al., 2008), and when they monitor and adjust their performance (monkey: Ito et al., 2003) (rat: Narayanan and Laubach, 2008). The motor functions of cingulate cortex emerge from the anterior part of the midcingulate or dorsal cingulate in primates that is homologous to the Cg1 and Cg2 in the rodent ACC (Laubach et al., 2018; Vogt, 2016). These areas of the cingulate coordinate motor behaviors incentivized by rewards (e.g., obtaining juice reward) and by social imperatives (e.g., reassurance through facial expressions) that are strongly driven by limbic inputs (Gothard, 2014). The limbic-motor function is illustrated by the observation that motor actions coordinated by the cingulate cortex are selected based on the expected reward (Shima and Tanji, 1998). In the same vein, the activation of the orofacial motor representation in anterior midcingulate in humans depends on the amount of juice reward delivered as feedback during an exploration task (Amiez et al., 2013).
5. Social signals versus interactive social signals in the ACC
Many areas of the social brain respond to social cues, but few respond to social interactions. Sliwa and Freiwald (2017) used whole brain functional magnetic resonance imaging (fMRI) in monkeys to compare brain activity elicited by (1) interacting objects, (2) inactive monkeys, (3) active but not interacting monkeys, and (4) interactive monkeys. The videos that depicted active but not interactive monkeys enhanced neural responses in the action observation/mirror neuron system localized to ventral premotor and parietal areas. Watching videos of social interactions, one can observe the activation of multiple subdivision of cingulate cortex (areas 32, 24) along with dorsomedial prefrontal areas, premotor and polysensory areas of the frontal and temporal lobe, and areas that process reward and valence, including the amygdala. The strongest indication that the cingulate cortex and other medial prefrontal areas such as pre-supplementary motor area (Broadman area 6) are specialized to support social interactions comes from the differential and simultaneous representations of self and other (e.g., Decety and Sommerville, 2003; Rizzolatti and Sinigaglia, 2010; Tomlin et al., 2006; Yoshida et al., 2011). For example, when the monkeys have to use the actions of their social partner to inform their own behavior, neurons in medial prefrontal cortex respond differentially to the same action when perform by self and by the social partner (Yoshida et al., 2011). These neurons signal agency, an obligatory ingredient for separating the perceived outcomes into those caused by the self and by others (Frith and Frith, 2006). This differentiates the ACC and the dorsomedial prefrontal cortex from other components of the social brain (e.g., the face patches) that respond selectively to social signals but not social interactions. While selective responses to social signals serve social perception, selective, and simultaneously active responses to self and other serve social interactions. Social interactions recruit the motor system, even synchronizing motor cortical activity in social partners (Tseng et al., 2018).
As rank in a hierarchically structured primate society is defined relative to others and relative to the size of the social group, the covariation of neural activity in the ACC with social status also supports the role of the ACC in processing interactive social signals (Noonan et al., 2011; Sallet et al., 2011). Dominance and social ranking within mouse colonies also implicate the ACC. Rodent models allow meaningful manipulations to further explore this role, such as altering social status by altering plasticity in ACC neurons. Specifically, increasing synaptic strength by viral expression of the GluA4 subunit of AMPA receptor or Ras in the ACC neurons increased dominance rank measured by the tube test. In the tube test two mice are placed at the two ends of the tube and the subordinate mouse will retreat to allow the dominant mouse advance through the tube. Conversely, artificial decreases in synaptic strength by overexpression of the GluA4 C-tail or Rap1, decreased the dominance rank (Wang et al., 2011). Furthermore, sociability is decreased by shifting the excitation/inhibition balance in the medial prefrontal cortex towards inhibition (Yizhar et al., 2011). Decisions to conform to social rules and norms also activate the ACC, so that the subjective value of rewards is defined in relations to valuation of the same reward by others (Apps and Ramnani, 2017; Izuma, 2013). From these studies it is clear that beyond processing social cues and rewards (whether to self and other), the ACC is processing social interactive cues, where rewards (or status) and actions are defined and evaluated in relation to the rewards and actions of others. Neurons in the ACC seem to encode separately but keep track simultaneously of self-and other, which indicates a higher-level, multidimensional representation of social information.
6. Interaction and communication in observational learning
How do individuals select information they should attend to and retain from their environment? To date, the majority of non-human animal models of learning focus on creating an association between a conditioned stimulus and an unconditioned stimulus through direct experience (e.g., classical fear conditioning). Direct exposure to an event, however, is not the only way that individuals acquire memories or form associations. Information can also be acquired indirectly, via social transmission between conspecifics. This form of learning may, in some contexts, prove to be a more effective and safer means of acquiring information, since it enables the learner to acquire knowledge while minimizing risks. Much research has been devoted to understanding how human and non-human primates acquire information through observation (Cook et al., 1985; Cook and Mineka, 1987; Hygge and Ohman, 1978; Mineka et al., 1984; Mineka and Cook, 1988; Olsson and Phelps, 2004); yet, research on the social transmission of fear in non-primate mammals has only more recently emerged (Allsop et al., 2018; Atsak et al., 2011; Ben-Ami Bartal et al., 2011; Bredy and Barad, 2009; Bruchey et al., 2010; Carrillo et al., 2015; Chen et al., 2009; Guzmán et al., 2009; Jeon et al., 2010; Jones et al., 2014; Jones and Monfils, 2016; Kavaliers et al., 2005; Kim et al., 2010; Knapska et al., 2010; Langford et al., 2006; Masuda and Aou, 2009; Pereira et al., 2012).
6.1. Observational fear learning paradigms
Observational learning can be broadly defined as the acquisition of new information through observation of a conspecific’s response to stimuli. Through this form of learning, a naïve animal may identify an associative relationship between two stimuli (observational associative learning) or learn to associate a behavioral response with the presentation of a stimulus (observational operant learning). In this section, we focus on the former. In a typical observational fear learning paradigm, rats (or mice) are placed on either side of a divider. For a brief overview of observational operant learning, please refer to a recently published review by Monfils and Agee (2019). The “demonstrator” is directly exposed to an aversive US (usually a footshock), and the “observer” witnesses the event. The divider can be transparent or opaque, to allow (or not) visual access. As such, rather than being an “eye-witness”, the observer may have access to visual and/or non-visual cues to process the information. Jeon et al. (2010; 2011) performed an elegant series of experiments using this paradigm, and found that a mouse that witnessed another mouse receiving shocks in a context showed fear to that same context when tested at a later time, suggesting that observational fear learning may be used to study long-lasting acquisition of socially-transferred emotional information. It is important to note that in a typical observational learning paradigm, the number, intensity, duration and frequency of shocks administered are typically quite high (e.g., in Jeon et al. [2010]: 24 shocks, 2 s in duration, 1 mA intensity, and an inter-trial interval of 10 s), and the observer and demonstrator do not have the opportunity to physically engage with one another during the demonstrator’s experience with the US (and the associated behavioral display).
Bruchey et al. (Bruchey et al., 2010) developed a different paradigm in which rats can acquire fear responding to a neutral stimulus in the absence of an unconditioned stimulus. In this paradigm, termed fear conditioning by proxy, a subset of rats acquire fear to a stimulus simply by being allowed to freely interact with a previously conditioned conspecific while the latter re-experiences the conditioned stimulus and displays a conditioned response. This experimental design was developed to determine whether fear responding could be socially transmitted between conspecifics during retrieval of a discrete memory. Bruchey et al. (2010) found that during the fear conditioning by proxy session, in which the observer and demonstrator rats can freely interact, the fear conditioned rats shows significant freezing, while the fear conditioned by proxy rat does not. When tested at a long-term memory test the next day, the observer rats (those conditioned by proxy) show a broad range of variability, with some rats not freezing at all, and others freezing for as much as 80% of the conditioned stimulus (Jones and Monfils, 2016). The degree of pro-social interactions (e.g. grooming, paw to body contact, nose-to-nose contact) between the fear conditioned and fear conditioned by proxy rats during training is a significant predictor of freezing during the long-term memory test.
There are important differences between fear conditioning by-proxy and other social fear learning paradigms. Unlike other models of social transmission of fear, fear conditioning by proxy does not involve an unconditioned stimulus-demonstrator unconditioned response association. Rather, during the fear conditioning by proxy interaction, the response displayed by the demonstrator is a conditioned response. It is also important to emphasize that whereas most observers acquire fear in a typical “observer-demonstrator” paradigm, only a subset acquire fear in the fear conditioning by proxy paradigm. The precise reasons underlying why only a subset of individuals acquire fear conditioning by proxy remain to be determined, though we have some clues. It appears that one of the best predictors for freezing at the long-term memory test following fear conditioning by proxy training is the amount of social contact the fear conditioned by proxy rat displays toward the fear conditioned rat during the training session. This, in turn, appears to be, at least in part, by dominance status (Jones and Monfils, 2016), and familiarity/kinship (Jones et al., 2014).
How is fear acquired via social transmission in rodents? The answer to this question is, in part, paradigm-dependent, though there are elements that appear to be common across social transmission of fear experimental designs. Importantly, as this is an emerging and dynamically evolving field of research, it is likely that a number of factors contributing to this form of learning have yet to be uncovered. Here, we at least describe the role of ultrasonic vocalizations (USVs) and chemosensation as modes of communication that may enable the transfer of information between conspecific rats or mice. Next, we briefly discuss the phenomenon of emotional contagion that might play a role in social transmission of information in rodents.
6.2. Ultrasonic vocalizations in observational learning
Vocalizations in rats in the lower spectrum of the ultrasonic range around 22 kHz are associated with negative affect elicited in situations where the rat is experiencing a fear-inducing state, such as the presence of a predator (Blanchard et al., 1991), painful (Calvino et al., 1996; Han et al., 2005) or startling stimuli (Kaltwasser, 1991), or in aggressive encounters with other rats (Thomas et al., 1983). Higher frequency vocalizations (typically referred to as 50 kHz calls) generally occur with activities with a more positive affect such as play (Knutson et al., 1998), social exploration (Wöhr and Schwarting, 2007), and anticipation of reward (Burgdorf et al., 2000).
USVs in mice have been found to emit a wide range of frequency-modulated ultrasonic vocalizations (typically within the 30–110 kHz range) that can be divided into predictable syllables (Ferhat et al., 2016; Gourbal et al., 2004; Holy and Guo, 2005; Portfors, 2007). These vocalizations have traditionally thought to be primarily linked to male courtship behavior (Chabout et al., 2015; Holy and Guo, 2005), though more recent research indicates that male and female mice alike may also emit these calls as a territorial response to intruders (Hammerschmidt et al., 2012; Scattoni et al., 2011) (for review, see Portfors and Perkel, 2014). Research into how USVs in mice might communicate affective states is more limited, though USV emission has been observed to change following restraint stress, suggestive of production during an aversive experience (Chabout et al., 2012; Gaub et al., 2016; Grimsley et al., 2016) (for review, see Ehret, 2018). It is also important to note that Chen et al. (2009) have reported that squeaks produced by demonstrator mice are sufficient to enable social fear transmission. Outside of Chen et al.’s experiment, explicit research into whether USVs in mice influence social learning does not, to our knowledge, exist, though the aforementioned results do provide evidence that they could indeed possibly serve this function.
Auditory information appears to be an important component of social transmission of fear paradigms, with the presence of negative affect vocalizations (22 kHz range) (Kim et al., 2010), or the sudden onset of silence (Pereira et al., 2012) being important indicators of danger in a social setting. Jones and Monfils (2016) found that in fear conditioning by proxy, the majority of rats do not vocalize at all during the social transmission of fear, but this does not preclude a conspecific from learning about associative fear. In line with this findings, other groups have found evidence suggesting that 22 kHz vocalizations have little to no effect in naïve rats (Kisko et al., 2017; Wöhr et al., 2015). Still, for the rats that do vocalize in the 22 kHz range during fear conditioning by proxy, the duration of the vocalizations is positively correlated with the amount of fear acquired socially, as measured by freezing displayed by the observer the following day (Jones and Monfils, 2016).
6.3. Chemosensation in observational learning
Primates are considered microsmatic and most of them are lacking the vomeronasal organ and the accessory olfactory bulb (Smith et al., 2014) present in rodents. Nevertheless, olfaction plays a role in their social behaviors (McGann, 2017; Shepherd, 2004), as illustrated by chimpanzees recognizing group members and kin based on olfactory cues (Henkel and Setchell, 2018) and humans choosing mates based on olfactory signals (Jaworska et al., 2017).
In rodents, unlike in primates, chemosensory pathways transmit the largest proportion of social information (Arakawa et al., 2008; Camats Perna and Engelmann, 2017; Ferretti et al., 2019; Ishii and Touhara, 2019; Stowers and Kuo, 2015; Wesson, 2013). Secreted chemicals activate receptors in the main olfactory epithelium and Gruenberg ganglion, which transmit the information to the main olfactory bulb and then to the piriform cortex, medial amygdala and hypothalamus (Stowers and Kuo, 2015). They also activate receptors in the vomeronasal epithelium, which transmit the signal to the accessory olfactory bulb, and then to the medial amygdala, hypothalamus, and bed nucleus of the stria terminalis (Anderson, 2016; Matsuo et al., 2015; Stowers and Kuo, 2015). While the main and accessory olfactory bulbs do not project to ACC, their downstream targets send axons to ACC, although these are relatively sparse (Hoover and Vertes, 2007; Price et al., 1991). Loss of main olfactory system has been shown to produce deficits in a wide range of social behaviors (Matsuo et al., 2015). These findings suggest an important role of chemosensory pathways in social learning in rodents.
6.4. Emotional contagion in social learning
Emotional contagion refers to the process in which an observed behavioral change in one individual leads to the reflexive production of the same behavior by other individuals in close proximity, with the likely outcome of converging emotionally (Panksepp and Lahvis, 2011). One of the classic displays of contagion in primates is that of yawning—yawning on one individual’s part appears to strongly correlate with yawning from others in the vicinity (Anderson et al., 2004). Dogs, as well as rats and mice, also appear to display yawning contagion (Joly-Mascheroni et al., 2008; Li et al., 2010; Moyaho et al., 2015). Blinking, in the context of highly engaged social interactions (e.g., eye contact during prosocial or angry encounters) also seems to be contagious: both humans and non-human primates synchronize their blinks (with a delay of approximately ½ s) to the blinks of their social partner (Ballesta et al., 2016; Nakano and Kitazawa, 2010). Observational fear learning can, in part, be explained by contagion. Effectively, in the typical “observer-demonstrator” paradigm, an immediate response (e.g., freezing, heart rate changes) to witnessing the event is generally observed in rats and mice (Atsak et al., 2011; Chen et al., 2009; Gonzalez-Liencres et al., 2014; Jeon et al., 2010).
7. Neural mechanisms for the learning of emotionally salient social cues
Studies in rodents have begun to elucidate the neural mechanisms associated to the encoding and integration of emotionally salient social signals during observational learning. Particularly, studies using the “observer-demonstrator” fear learning paradigm (Allsop et al., 2018; Jeon et al., 2010; Jeon and Shin, 2011) have become substantially successful to establish novel mechanistic explanations on how the ACC and its extended network promote observational learning. For instance, Allsop et al. (2018) recently validated a variation of the observational task used by Jeon and colleagues (detailed in Section 6.1; 2010; 2011) in which electrical shocks to demonstrators were signaled by an auditory cue (Fig. 3A). In contrast to the contextual-based observational paradigm used by Jeon and colleagues, the cue-based paradigm by Allsop et al. (2018) time-locked distress social signals to the cue, thereby transferring aversive properties to the auditory cue itself. This led to the formation of a cued fear memory in observer mice that elicited more prominent fear-related reactions during cue epochs than baseline epochs, even in the absence of demonstrator mice, and regardless of the contextual environment in which observer mice were tested (Allsop et al., 2018). Another advantage of the cue-based observational fear paradigm was that it allowed a multi-trial structure that was ideal to track the evolution of changes in neural firing across multiple stages of learning (i.e., habituation, conditioning, and test), while still maintaining high statistical power. Thus, the simplicity and high controllability of experimental conditions in this cue-based observational fear paradigm offers great opportunities to examine neural mechanisms and dynamics during multiple aspects of social learning, such as the perception and processing of socially transmitted distress signals, as well as their association with the environmental cues that predict them. Although Jeon and Allsop’s observational learning paradigms have been focused on aversive forms of learning, similar principles could easily be implemented to examine neural computations and circuit dynamics during reward-related learning (Behrens et al., 2009, 2008), such as social transmission of food preference (Galef, 2003, 1992, Galef, 1990).
Fig. 3. Activity in the ACC-BLA pathway during observational fear conditioning. Illustrations summarize findings by Allsop et al. (2018).
A, Schematic of the observational fear paradigm. Demonstrator mice received tone-shock pairings, while observer mice witnessed these events through a transparent wall from the adjacent compartment. B, Schematic of in vivo neural recordings combined with optogenetic strategies to monitor the activity of either ACC-to-BLA projectors or BLA neurons during the task. C, ACC-to-BLA projectors acquired prominent responses to the shock-predicting cue. D, BLA neurons also acquired prominent responses to the cue, but such responses were abolished by photoinhibition of the ACC input.
7.1. Integration of social and environmental signals in the ACC during observational fear learning
The ACC shows prominent neural correlates during observational fear learning. For instance, the ACC in mice develops potentiated neural oscillations in the theta frequency range during this type of learning (Jeon et al., 2010). Furthermore, large proportions of ACC neurons exhibit potentiated responses during observational fear learning (Allsop et al., 2018). Such ACC responses are mostly excitatory (i.e., increased activity compared to baseline) and occur while mice perceive the cues that predict electric shocks to demonstrators (Fig. 3B-C), as well as during the shock periods themselves when the demonstrators display the most prominent aversive reactions (Allsop et al., 2018). During similar observational tasks in rats, a recent study demonstrated that the ACC contains neurons that exhibit pronounced elevated activity when demonstrators receive electric shocks (Carrillo et al., 2019). Such elevation of neural activity in the ACC was attributed to a “mirror-like” emotional contagion effect (i.e., similar patterns of activity were observed when rats experienced pain themselves and when they witnessed pain in other rats) (Carrillo et al., 2019). These electrophysiological findings highlight strong ACC participation during observational fear learning in rodents and suggest that the ACC integrates social and environmental signals to promote this type of learning.
While electrophysiological findings could just have highlighted ACC participation, additional examination has revealed that ACC activity is vital for observational fear learning. It has been shown in mice that the acquisition of fear memory through observation is impaired by temporal inactivation of the ACC (Jeon et al., 2010; Kim et al., 2012). Similarly, fear in rats while witnessing others receiving shocks is impaired by ACC inactivation (Carrillo et al., 2019). Observational fear learning can also be disrupted by ACC-restricted deletion of Cav1.2L-type calcium channels (Jeon et al., 2010), which have been shown to be highly expressed in the ACC (Day et al., 2002), mediate aversive experience-dependent gene expression (Bavley et al., 2017), and contribute to the excitability and plasticity of ACC neurons (Liauw et al., 2005). In addition, observational fear learning can be disrupted by local administration of either dopaminergic antagonists or serotonergic agonists into the ACC (Kim et al., 2014). Finally, to highlight bidirectional modulation, it has been reported that observational fear learning can be facilitated by electrical stimulation of the ACC (Kim et al., 2012).
Although ACC activity facilitates observational fear learning, ACC activity is trivial for the recall of this type of memory. It has been shown that pharmacological inactivation of the ACC during test sessions (e.g., 24 h after observational fear training) fails to disrupt the recall (Jeon et al., 2010). This suggests that the role of ACC activity during this type of learning is to promote memory formation in downstream regions, from which these memories can be later retrieve.
7.2. The amygdala as a crucial downstream region of the ACC during observational fear learning
The amygdala, especially the basolateral nuclei (including in rodents the lateral, basolateral, and basomedial regions; which from now on we will refer to as the “BLA”) is unequivocally fundamental for learning fear associations through classical conditioning methods in which direct experience of punishment is required (for review, see Aggleton, 2000; Duvarci and Pare, 2014; Janak and Tye, 2015; LeDoux, 2007, 2000; Maren and Quirk, 2004). Growing evidence indicates that BLA is also fundamental for learning fear associations through observational methods (for review, see Debiec and Olsson, 2017; Ferretti and Papaleo, 2019; Olsson and Phelps, 2007). In this section we focus on some studies in rodents demonstrating that neural activity in the BLA and its interactions with the ACC support observational fear learning.
The BLA exhibits potentiated theta oscillations during observational fear learning that are highly synchronized with the theta oscillations observed in the ACC (Jeon et al., 2010). Similarly to the ACC, many neurons in BLA develop potentiated responses to social and environmental cues during observational fear learning (Fig. 3D; Allsop et al., 2018). However, compared to ACC neurons, BLA neurons require a relatively larger amount of training to develop potentiated responses during observational fear learning. This suggests an ACC-to-BLA directionality for the encoding of these memories. Supporting this hypothesis, Allsop et al. (2018) combined electrophysiology and optogenetic strategies to demonstrate that ACC neurons that directly project to the BLA exhibit stronger responses during the observational fear task than other ACC subpopulations. They also found that selective inhibition of ACC inputs to the BLA profoundly disrupts both, BLA responses and behavioral responses, during this task. In addition, they found that selective inhibition in the opposite direction (i.e., BLA-to-ACC) did not affect observational fear learning. These findings provide important evidence for the hypothesis that ACC-to-BLA communication promotes memory formation during observational fear learning.
Finally, BLA activity is necessary during the long-term recall of fear memories learned through observation. This was shown by Jeon et al. (2010) using pharmacological inactivation of the BLA, and contrasts the finding that ACC activity is trivial during memory recall in this task. Consistent with these findings, Allsop et al. (2018) demonstrated that selective optogenetic-mediated inhibition of ACC inputs to the BLA fails to disrupt fear recall after observational fear learning. Therefore, it seems that once fear memories learned through observation have been formed within the BLA, recalling these memories later on only requires BLA activity but not ACC involvement.
7.3. Working model for ACC-BLA interactions during observational fear learning
To summarize the findings on this section, network interactions between the ACC and BLA play a crucial role during observational fear learning. While neural activation in the ACC supports observational fear learning by signaling emotionally salient social cues, the ACC transmits this information to the BLA to further promote memory encoding. Observationally-learned fear memories can be subsequently accessed from the BLA to modulate behavior, even in the absence of ACC involvement (Allsop et al., 2018). This model is consistent with previously suggested models that also take into account findings from human studies (Debiec and Olsson, 2017; Olsson and Phelps, 2007).
8. Observational fear learning produces enduring reorganization of the ACC-BLA network
While the ACC-BLA network exhibits fast computations that promote memory formation during observational fear learning, this network also exhibits enduring synaptic adaptations that may affect other behaviors. Two types of neuronal adaptations identified so far include synaptic changes in ACC inputs to BLA principal neurons and in the local GABAergic inputs to the principal neurons in the ACC layer 5.
The synaptic transmission from ACC to BLA was tested ex vivo by recording postsynaptic responses in BLA neurons during blue light stimulation of the ACC axons expressing channelrhodopsin (Ito et al., 2015). It was reported that observational fear training decreased the ratio between the AMPA and NMDA receptor-mediated responses. This effect was specific to the ACC-BLA pathway, as no changes were found in inputs from the TeA (temporal cortex, association area), which integrates multimodal sensory information (McDonald, 1998; Sheinberg and Logothetis, 1997). The decrease in AMPA/NMDA ratio was also social learning-specific, as no changes were found after non-social stressors, including two-hour immobilization or classical auditory fear conditioning reinforced by mild electrical footshock. The reduction in the AMPAR/NMDAR ratio resulted from increases in the NMDAR currents and coincided with the emergence of silent synapses, which contain NMDA receptors but lack AMPA receptors. There was no reduction in the AMPAR currents, which suggests that silent synapses were generated de novo and not by withdrawal of AMPAR from functional synapses. Because silent synapses can become functional after insertion of AMPAR, they provide a substrate for synaptic facilitation. The acquired propensity for increased facilitation in the ACC-BLA pathway has been confirmed both ex vivo and in vivo during inhibitory avoidance training (Ito and Morozov, 2019). It remains unknown how these synaptic changes contribute to observational fear memories per se, but they provide a potential mechanism for the enhanced inhibitory avoidance learning in mice that experienced observational fear training in the past (Ito et al., 2015).
9. Observational fear learning reorganizes GABAergic circuits in the rodent ACC
Following observational fear learning, GABA circuits in ACC may also undergo reorganization in the form of potentially switching the patterns of interactions between the ACC and other brain areas (Liu et al., 2017). In ACC, observational fear altered the short-term plasticity of GABAergic inputs to the layer 5 principal neurons (PNs). The presynaptic GABAb autoreceptors normally suppress these inputs, which causes the decline in the inhibitory postsynaptic currents in the principal cells during repeated stimulation of GABAergic neurons. Observational fear had opposing effects on GABAergic inputs from two classes of GABAergic neurons. It attenuated the GABAbR suppression of inputs from the somatostatin-expressing interneurons (Sst-INs), but enhanced the suppression of inputs from the parvalbumin-expressing cells (PV-INs) (Liu et al., 2017). Given that PV-INs target mostly the somas and proximal dendrites of PNs, whereas the Sst-INs target distal dendrites (Markram et al., 2004), observational fear likely shifts inhibitory drive along the somato-dendritic axis of PNs from the soma and proximal dendrites to the distal dendrites. The shift is expected to enhance connectivity among the layer 5 neurons, because they project mainly to the proximal basal dendrites of one another (Markram et al., 1997), and to attenuate connectivity from the layer 2/3 neurons to the layer 5 neurons, because the inputs target apical dendrites (Thomson and Bannister, 1998). At the same time, it is expected to weaken the remote connectivity from the thalamus, whose axons travel via the cortical layer 1 and target apical dendrites, and to enhance the connectivity with the BLA, whose axons are abundant in the layer 5 and target the basal proximal dendrites (Hoover and Vertes, 2007; Oh et al., 2014). Thus, the GABAergic adaptations to observational fear are likely to rearrange both local and remote connectivity of neurons in ACC and change its interactions with other areas of the brain. Based on these findings, it is possible that the formation of silent synapses in the ACC-BLA pathway and changes in GABAergic transmission are not cell-specific. Nevertheless, by altering remote connectivity or synchronization, they can facilitate plasticity in specific neuronal ensembles that store socially-reinforced memories.
10. Concluding remarks
We compiled evidence for well-conserved neural networks centered on the ACC that detect, interpret, and encode socially-derived signals during social learning across multiple species. As social learning is a highly dynamic and rapidly evolving field of research, our interpretation of the existing literature may soon require further revisions. Nevertheless, the ideas emerging from the evaluation of the current literature provide a framework for compartmentalizing the ACC networks in subdivisions that may support distinct yet related forms of social learning.
10.1. Future avenues
Studies in humans and non-human primates suggest that the cingulate cortex (including areas of the anterior cingulate, mid-cingulate, and posterior cingulate cortex) is activated by a multitude of functions, many of which serve social behaviors and observational learning. The diversity of functions attributed to the cingulate cortex, might be explained, by its rich connectivity with other cortical and subcortical areas, specifically with the components of the “social brain” (Dunbar, 1998). The amygdala is a prominent node of the social brain that is bidirectionally connected to multiple subregions of the cingulate cortex. It appears that neural ensembles in the ACC respond to particular social situations (Livneh et al., 2012; Mosher et al., 2014; Rutishauser et al., 2015; Wang et al., 2014) that are linked to neural events in the amygdala (Klavir et al., 2013). Recent studies in rodents showed that social learning elicits broad synaptic changes that influence functional connectivity between the ACC and the amygdala (Allsop et al., 2018; Ito et al., 2015; Liu et al., 2017). One challenge for future studies is to keep identifying highly selective social learning neural engrams to better understand how they map onto diverse functions of the ACC-amygdala circuits and how they enable behavioral adaptation in response to social cues. Future studies may determine whether learning through observation recruits the same or different neural ensembles that are typically recruited when learning through direct experience.
An emerging question in the field is how social factors such as dominance status affects the neural mechanisms controlling social transmission and learning. In case of fear learning by social transmission, dominance status plays an important role (Jones and Monfils, 2016). Whereas subordinate rats readily acquire fear by proxy when they interact with a previously fear-conditioned dominant conspecific, dominant rats do not reliably acquire fear via social transmission from subordinates. In a similar vein, the decision making and anxiety level of human subjects (correlated with increased activity levels in the ACC) was negatively impacted by collaborating with a social partner of low reputation (Qi et al., 2018). Future studies examining differences in circuit mechanisms between dominant and subordinate animals should shed light on the neurobiological players by which dominance status influences social fear learning.
Gender specific mechanisms of social learning have been scarcely addressed. A few early studies suggest some differences in the neural mechanisms of social learning between males and females. For example, it has been reported that male but not female rats exhibit increased c-fos activation in the BLA and ACC areas during some forms of social transmission (Knapska et al., 2006; Mikosz et al., 2015). In humans, women, but not men, show correlations of right hemisphere activation and empathy (Rueckert and Naybar, 2008).
Finally, social learning is particularly important during development because it may form the bases for the development of emotion regulation and social-cognitive function. Recent studies in rodents have shown that a caregiver can impart fear learning in the offspring via a process called social referencing (Chang and Debiec, 2016; Debiec and Sullivan, 2017, 2014), while human studies suggest that infants are more sensitive to caregiver fear and anxiety (Aktar et al., 2018; Murray et al., 2008). In addition, developmental differences in social learning appeared to be linked to a developmental shift in the processing of experienced and observed feedback during learning (Rodriguez Buritica et al., 2018). Altogether, these findings indicate the importance of future studies to examine the neurobiological mechanisms underlying social learning during development, as well as other factors that influence developmental differences in the ability to use observed versus experienced feedback.
Acknowledgements
The authors assembled this review article based on a recent scientific symposium held at the International Behavioral Neuroscience Society meeting (IBNS 2018) in which they presented work related to the topic. The symposium was titled “Social transmission of information in mammals: Key insights from rodents and non-human primates”. The authors thank Dr. Gregory J. Quirk for his helpful comments and suggestions on the manuscript. A.B.-R. acknowledges support by the Brain and Behavior Research Foundation (NARSAD Young Investigator Award) and 3 R01 MH102441-01 (Research Supplement to Promote Diversity in Health-Related Sciences). K.M.G. acknowledges support by the NIH/NIMH grant P50- MH100023. M.H.M. acknowledges support by the NSF grant NSF-1748911. A.M. acknowledges support by the NIH grants R01-MH 112093-01 and 5-R21 MH112093-02.
Footnotes
Competing interests
The authors declare no conflicts of interest.
References
- Aggleton JP, 2000. The Amygdala: A Functional Analysis. Oxford University Press. [Google Scholar]
- Aktar E, Van Bockstaele B, Pérez-Edgar K, Wiers RW, Bögels SM, 2018. Intergenerational transmission of attentional bias and anxiety. Dev. Sci e12772 10.1111/desc.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang C-J, Felix-Ortiz AC, Vienne A, Beyeler A, Izadmehr EM, Glober G, Cum MI, Stergiadou J, Anandalingam KK, Farris K, Namburi P, Leppla CA, Weddington JC, Nieh EH, Smith AC, Ba D, Brown EN, Tye KM, 2018. Corticoamygdala transfer of socially derived information gates observational learning. Cell 173, 1329–1342. 10.1016/j.cell.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, 1992. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp. Brain Res 88, 375–388. 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- Amemiya S, Kubota N, Umeyama N, Nishijima T, Kita I, 2016. Noradrenergic signaling in the medial prefrontal cortex and amygdala differentially regulates vicarious trial-and-error in a spatial decision-making task. Behav. Brain Res 297, 104–111. 10.1016/j.bbr.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Amiez C, Neveu R, Warrot D, Petrides M, Knoblauch K, Procyk E, 2013. The location of feedback-related activity in the midcingulate cortex is predicted by local morphology. J. Neurosci. Off. J. Soc. Neurosci 33, 2217–2228. 10.1523/JNEUROSCI.2779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, 2016. Circuit modules linking internal states and social behaviour in flies and mice. Nat. Rev. Neurosci 17, 692–704. 10.1038/nrn.2016.125. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Myowa-Yamakoshi M, Matsuzawa T, 2004. Contagious yawning in chimpanzees. Proc. Biol. Sci 271 (Suppl 6), S468–470. 10.1098/rsbl.2004.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps Ma.J., Green R, Ramnani N, 2013. Reinforcement learning signals in the anterior cingulate cortex code for others’ false beliefs. NeuroImage 64, 1–9. 10.1016/j.neuroimage.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Apps M, Ramnani N, 2017. Contributions of the medial prefrontal cortex to social influence in economic decision-making. Cereb. Cortex 1991 (27), 4635–4648. 10.1093/cercor/bhx183. [DOI] [PubMed] [Google Scholar]
- Apps MAJ, Ramnani N, 2014. The anterior cingulate gyrus signals the net value of others’ rewards. J. Neurosci. Off. J. Soc. Neurosci 34, 6190–6200. 10.1523/JNEUROSCI.2701-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MAJ, Rushworth MFS, Chang SWC, 2016. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron 90, 692–707. 10.1016/j.neuron.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ, 2008. Scent marking behavior as an odorant communication in mice. Neurosci. Biobehav. Rev 32, 1236–1248. 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Waterhouse B, 2016. Locus coeruleus: from global projection system to adaptive regulation of behavior. Brain Res. 1645, 75–78. 10.1016/j.brainres.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, Moita M, Keysers C, 2011. Experience modulates vicarious freezing in rats: a model for empathy. PLoS One 6, e21855 10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP, 2010. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 35, 48–69. 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta S, Mosher CP, Szep J, Fischl KD, Gothard KM, 2016. Social determinants of eyeblinks in adult male macaques. Sci. Rep 6, 38686 10.1038/srep38686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, García-Cabezas MÁ, 2016. How the prefrontal executive got its stripes. Curr. Opin. Neurobiol 40, 125–134. 10.1016/j.conb.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen JJ, Thioux M, Keysers C, 2009. Evidence for mirror systems in emotions. Philos. Trans. R. Soc. Lond., B, Biol. Sci 364, 2391–2404. 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavley CC, Fischer DK, Rizzo BK, Rajadhyaksha AM, 2017. Cav1.2 channels mediate persistent chronic stress-induced behavioral deficits that are associated with prefrontal cortex activation of the p25/Cdk5-glucocorticoid receptor pathway. Neurobiol. Stress 7, 27–37. 10.1016/j.ynstr.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp G, 2008. What is the magnitude of the group-size effect on vigilance? Behav. Ecol 19, 1361–1368. 10.1093/beheco/arn096. [DOI] [Google Scholar]
- Behrens TEJ, Hunt LT, Rushworth MFS, 2009. The computation of social behavior. Science 324, 1160–1164. 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MFS, 2008. Associative learning of social value. Nature 456, 245–249. 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Walton ME, Rushworth MFS, 2007. Learning the value of information in an uncertain world. Nat. Neurosci 10, 1214–1221. 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Decety J, Mason P, 2011. Empathy and pro-social behavior in rats. Science 334, 1427–1430. 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicks LK, Koike H, Akbarian S, Morishita H, 2015. Prefrontal cortex and social cognition in mouse and man. Front. Psychol 6, 1805 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissière S, Plachta N, Hoyer D, McAllister KH, Olpe H-R, Grace AA, Cryan JF, 2008. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biol. Psychiatry 63, 821–831. 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM, 1991. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol. Behav 50, 967–972. [DOI] [PubMed] [Google Scholar]
- Bonini L, Maranesi M, Livi A, Fogassi L, Rizzolatti G, 2014. Ventral premotor neurons encoding representations of action during self and others’ inaction. Curr. Biol. CB 24, 1611–1614. 10.1016/j.cub.2014.05.047. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Barad M, 2009. Social modulation of associative fear learning by pheromone communication. Learn. Mem. Cold Spring Harb. N 16, 12–18. 10.1101/lm.1226009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchey AK, Jones CE, Monfils M-H, 2010. Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behav. Brain Res 214, 80–84. 10.1016/j.bbr.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, 2000. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav. Neurosci 114, 320–327. [PubMed] [Google Scholar]
- Burgos-Robles A, Bravo-Rivera H, Quirk GJ, 2013. Prelimbic and infralimbic neurons signal distinct aspects of appetitive instrumental behavior. PLoS One 8, e57575 10.1371/journal.pone.0057575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ, 2009. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J. Neurosci. Off. J. Soc. Neurosci 29, 8474–8482. 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ, 2007. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53, 871–880. 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI, 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. (Regul. Ed.) 4, 215–222. [DOI] [PubMed] [Google Scholar]
- Cai X, Padoa-Schioppa C, 2012. Neuronal encoding of subjective value in dorsal and ventral anterior cingulate cortex. J. Neurosci. Off. J. Soc. Neurosci 32, 3791–3808. 10.1523/JNEUROSCI.3864-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvino B, Besson JM, Boehrer A, Depaulis A, 1996. Ultrasonic vocalization (22-28 kHz) in a model of chronic pain, the arthritic rat: effects of analgesic drugs. Neuroreport 7, 581–584. [DOI] [PubMed] [Google Scholar]
- Camats Perna J, Engelmann M, 2017. Recognizing others: rodent’s social memories. Curr. Top. Behav. Neurosci 30, 25–45. 10.1007/7854_2015_413. [DOI] [PubMed] [Google Scholar]
- Carlén M, 2017. What constitutes the prefrontal cortex? Science 358, 478–482. 10.1126/science.aan8868. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL, 1995. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol 363, 642–664. 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Han Y, Migliorati F, Liu M, Gazzola V, Keysers C, 2019. Emotional mirror neurons in the rat’s anterior cingulate cortex. Curr. Biol. CB 29, 1301–1312. 10.1016/j.cub.2019.03.024.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo M, Migliorati F, Bruls R, Han Y, Heinemans M, Pruis I, Gazzola V, Keysers C, 2015. Repeated witnessing of conspecifics in pain: effects on emotional contagion. PLoS One 10, e0136979 10.1371/journal.pone.0136979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabout J, Sarkar A, Dunson DB, Jarvis ED, 2015. Male mice song syntax depends on social contexts and influences female preferences. Front. Behav. Neurosci 9, 76 10.3389/fnbeh.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabout J, Serreau P, Ey E, Bellier L, Aubin T, Bourgeron T, Granon S, 2012. Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS One 7, e29401 10.1371/journal.pone.0029401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D-J, Debiec J, 2016. Neural correlates of the mother-to-infant social transmission of fear. J. Neurosci. Res 94, 526–534. 10.1002/jnr.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Fagan NA, Toda K, Utevsky AV, Pearson JM, Platt ML, 2015. Neural mechanisms of social decision-making in the primate amygdala. Proc. Natl. Acad. Sci. U. S. A 112, 16012–16017. 10.1073/pnas.1514761112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Gariépy J-F, Platt ML, 2013. Neuronal reference frames for social decisions in primate frontal cortex. Nat. Neurosci 16, 243–250. 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Panksepp JB, Lahvis GP, 2009. Empathy is moderated by genetic background in mice. PLoS One 4, e4387 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K, 2001. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 888, 83–101. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Daniels TE, Gorrin DP, Rhodes SEV, Rudebeck PH, Murray EA, 2013. The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cereb. Cortex 1991 (23), 2884–2898. 10.1093/cercor/bhs266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M, Mineka S, 1989. Observational conditioning of fear to fear-relevant versus fear-irrelevant stimuli in rhesus monkeys. J. Abnorm. Psychol 98, 448–459. [DOI] [PubMed] [Google Scholar]
- Cook M, Mineka S, 1987. Second-order conditioning and overshadowing in the observational conditioning of fear in monkeys. Behav. Res. Ther 25, 349–364. [DOI] [PubMed] [Google Scholar]
- Cook M, Mineka S, Wolkenstein B, Laitsch K, 1985. Observational conditioning of snake fear in unrelated rhesus monkeys. J. Abnorm. Psychol 94, 591–610. [DOI] [PubMed] [Google Scholar]
- Cowen SL, Davis GA, Nitz DA, 2012. Anterior cingulate neurons in the rat map anticipated effort and reward to their associated action sequences. J. Neurophysiol 107, 2393–2407. 10.1152/jn.01012.2011. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Ahmed OJ, Stevens TR, Patrick SL, Gonzalez AN, Elmaleh M, Connors BW, 2012. Thalamic control of layer 1 circuits in prefrontal cortex. J. Neurosci. Off. J. Soc. Neurosci 32, 17813–17823. 10.1523/JNEUROSCI.3231-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davitz JR, Mason DJ, 1955. Socially facilitated reduction of a fear response in rats. J. Comp. Physiol. Psychol 48, 149–151. [DOI] [PubMed] [Google Scholar]
- Day M, Olson PA, Platzer J, Striessnig J, Surmeier DJ, 2002. Stimulation of 5-HT(2) receptors in prefrontal pyramidal neurons inhibits Ca(v)1.2 L type Ca(2+) currents via a PLCbeta/IP3/calcineurin signaling cascade. J. Neurophysiol 87, 2490–2504. 10.1152/jn.00843.2001. [DOI] [PubMed] [Google Scholar]
- de Araujo MFP, Hori E, Maior RS, Tomaz C, Ono T, Nishijo H, 2012. Neuronal activity of the anterior cingulate cortex during an observation-based decision making task in monkeys. Behav. Brain Res 230, 48–61. 10.1016/j.bbr.2012.01.060. [DOI] [PubMed] [Google Scholar]
- Debiec J, Olsson A, 2017. Social fear learning: from animal models to human function. Trends Cogn. Sci. (Regul. Ed.) 21, 546–555. 10.1016/j.tics.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Sullivan RM, 2017. The neurobiology of safety and threat learning in infancy. Neurobiol. Learn. Mem 143, 49–58. 10.1016/j.nlm.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Sullivan RM, 2014. Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc. Natl. Acad. Sci. U. S. A 111, 12222–12227. 10.1073/pnas.1316740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Sommerville JA, 2003. Shared representations between self and other: a social cognitive neuroscience view. Trends Cogn. Sci. (Regul. Ed.) 7, 527–533. [DOI] [PubMed] [Google Scholar]
- Di Cesare G, Pinardi C, Carapelli C, Caruana F, Marchi M, Gerbella M, Rizzolatti G, 2018. Insula connections with the parieto-frontal circuit for generating arm actions in humans and macaque monkeys. Cereb. Cortex N. Y. N 1991 10.1093/cercor/bhy095. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME, 1997. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827. 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, 1998. The social brain hypothesis. Evol. Anthropol. Issues News Rev 6, 178–190. . [DOI] [Google Scholar]
- Duvarci S, Pare D, 2014. Amygdala microcircuits controlling learned fear. Neuron 82, 966–980. 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G, 2018. Chapter 18 - characteristics of vocalization in adult mice In: Brudzynski SM (Ed.), Handbook of Behavioral Neuroscience, Handbook of Ultrasonic Vocalization. Elsevier, pp. 187–195. 10.1016/B978-0-12-809600-0.00018-4. [DOI] [Google Scholar]
- Emery NJ, Clayton NS, 2009. Comparative social cognition. Annu. Rev. Psychol 60, 87–113. 10.1146/annurev.psych.60.110707.163526. [DOI] [PubMed] [Google Scholar]
- Ferhat A-T, Torquet N, Le Sourd A-M, de Chaumont F, Olivo-Marin J-C, Faure P, Bourgeron T, Ey E, 2016. Recording mouse ultrasonic vocalizations to evaluate social communication. J. Vis. Exp. JoVE 10.3791/53871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti V, Maltese F, Contarini G, Nigro M, Bonavia A, Huang H, Gigliucci V, Morelli G, Scheggia D, Managò F, Castellani G, Lefevre A, Cancedda L, Chini B, Grinevich V, Papaleo F, 2019. Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr. Biol. CB 29, 1938–1953. 10.1016/j.cub.2019.04.070.e6. [DOI] [PubMed] [Google Scholar]
- Ferretti V, Papaleo F, 2019. Understanding others: emotion recognition in humans and other animals. Genes Brain Behav. 18, e12544 10.1111/gbb.12544. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y, 2000. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J. Comp. Neurol 421, 172–188. [PubMed] [Google Scholar]
- Freese JL, Amaral DG, 2006. Synaptic organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. J. Comp. Neurol 496, 655–667. 10.1002/cne.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U, 2006. The neural basis of mentalizing. Neuron 50, 531–534. 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Fu Z, Wu D-AJ, Ross I, Chung JM, Mamelak AN, Adolphs R, Rutishauser U, 2019. Single-neuron correlates of error monitoring and post-error adjustments in human medial frontal cortex. Neuron 101, 165–177. 10.1016/j.neuron.2018.11.016.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB, 1984. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 319, 229–259. [DOI] [PubMed] [Google Scholar]
- Fuster J, 2015. The Prefrontal Cortex. Academic Press. [Google Scholar]
- Fuster JM, 2001. The prefrontal cortex–an update: time is of the essence. Neuron 30, 319–333. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ, 2005. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol 492, 145–177. 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Galef BG, 2003. Social learning of food preferences in rodents: rapid appetitive learning. Curr. Protoc. Neurosci 10.1002/0471142301.ns0805ds21. Chapter 8, Unit 8.5D. [DOI] [PubMed] [Google Scholar]
- Galef BG, 1992. Ontogeny and social transmission of food preferences in mammals: basic and applied research. Appetite 19, 309–311. [DOI] [PubMed] [Google Scholar]
- Galef BG, 1990. A historical perspective on recent studies of social learning about foods by Norway rats. Can. J. Psychol 44, 311–329. [DOI] [PubMed] [Google Scholar]
- Gaub S, Fisher SE, Ehret G, 2016. Ultrasonic vocalizations of adult male Foxp2-mutant mice: behavioral contexts of arousal and emotion. Genes Brain Behav. 15, 243–259. 10.1111/gbb.12274. [DOI] [PubMed] [Google Scholar]
- Gibson EJ, Walk RD, 1960. The “visual cliff.”. Sci. Am 202, 64–71. [PubMed] [Google Scholar]
- Gonzalez-Liencres C, Juckel G, Tas C, Friebe A, Brüne M, 2014. Emotional contagion in mice: the role of familiarity. Behav. Brain Res 263, 16–21. 10.1016/j.bbr.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Gothard KM, 2014. The amygdalo-motor pathways and the control of facial expressions. Front. Neurosci 8, 43 10.3389/fnins.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG, 2007. Neural responses to facial expression and face identity in the monkey amygdala. J. Neurophysiol 97, 1671–1683. 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Gourbal BEF, Barthelemy M, Petit G, Gabrion C, 2004. Spectrographic analysis of the ultrasonic vocalisations of adult male and female BALB/c mice. Naturwissenschaften 91, 381–385. 10.1007/s00114-004-0543-7. [DOI] [PubMed] [Google Scholar]
- Grimsley JMS, Sheth S, Vallabh N, Grimsley CA, Bhattal J, Latsko M, Jasnow A, Wenstrup JJ, 2016. Contextual modulation of vocal behavior in mouse: newly identified 12 kHz “Mid-Frequency” vocalization emitted during restraint. Front. Behav. Neurosci 10, 38 10.3389/fnbeh.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán YF, Tronson NC, Guedea A, Huh KH, Gao C, Radulovic J, 2009. Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav. Brain Res 201, 173–178. 10.1016/j.bbr.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J, 2012. The structure and usage of female and male mouse ultrasonic vocalizations reveal only minor differences. PLoS One 7, e41133 10.1371/journal.pone.0041133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Bird GC, Li W, Jones J, Neugebauer V, 2005. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J. Neurosci. Methods 141, 261–269. 10.1016/j.jneumeth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Harb R, Taylor JR, 2015. The fragrant power of collective fear. PLoS One 10, e0123908 10.1371/journal.pone.0123908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroush K, Williams ZM, 2015. Neuronal prediction of opponent’s behavior during cooperative social interchange in primates. Cell 160, 1233–1245. 10.1016/j.cell.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN, 2016. Circuit-based corticostriatal homologies between rat and primate. Biol. Psychiatry 80, 509–521. 10.1016/j.biopsych.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW, 2006. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci. Biobehav. Rev 30, 126–147. 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Henkel S, Setchell JM, 2018. Group and kin recognition via olfactory cues in chimpanzees (Pan troglodytes). Proc. Biol. Sci 285 10.1098/rspb.2018.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N, 2009. Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol 30, 470–482. 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hillman KL, Bilkey DK, 2012. Neural encoding of competitive effort in the anterior cingulate cortex. Nat. Neurosci 15, 1290–1297. 10.1038/nn.3187. [DOI] [PubMed] [Google Scholar]
- Holy TE, Guo Z, 2005. Ultrasonic songs of male mice. PLoS Biol. 3, e386 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP, 2007. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct 212, 149–179. 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Howell BR, McMurray MS, Guzman DB, Nair G, Shi Y, McCormack KM, Hu X, Styner MA, Sanchez MM, 2017. Maternal buffering beyond glucocorticoids: impact of early life stress on corticolimbic circuits that control infant responses to novelty. Soc. Neurosci 12, 50–64. 10.1080/17470919.2016.1200481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hygge S, Ohman A, 1978. Modeling processes in the acquisition of fears: vicarious electrodermal conditioning to fear-relevant stimuli. J. Pers. Soc. Psychol 36, 271–279. [DOI] [PubMed] [Google Scholar]
- Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, Sanchez DO, Lobo MK, Serrano PA, Braren SH, Castillo SA, 2018. Vicarious social defeat stress induces depression-related outcomes in female mice. Biol. Psychiatry 83, 9–17. 10.1016/j.biopsych.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii KK, Touhara K, 2019. Neural circuits regulating sexual behaviors via the olfactory system in mice. Neurosci. Res 140, 59–76. 10.1016/j.neures.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD, 2003. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science 302, 120–122. 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Ito W, Erisir A, Morozov A, 2015. Observation of distressed conspecific as a model of emotional trauma generates silent synapses in the prefrontal-amygdala pathway and enhances fear learning, but ketamine abolishes those effects. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 40, 2536–2545. 10.1038/npp.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito W, Morozov A, 2019. Prefrontal-amygdala plasticity enabled by observational fear. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 10.1038/s41386-019-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, 2013. The neural basis of social influence and attitude change. Curr. Opin. Neurobiol 23, 456–462. 10.1016/j.conb.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM, 2015. From circuits to behaviour in the amygdala. Nature 517, 284–292. 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworska J, Rapacz-Leonard A, Janowski TE, Jezierski T, 2017. Does the major histocompatibility complex influence choice of mate in humans and other mammals? - a review. Anim. Sci. Pap. Rep 35, 107–121. [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin S-Y, Rabah D, Kinet J-P, Shin H-S, 2010. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci 13, 482–488. 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Shin H-S, 2011. A mouse model for observational fear learning and the empathetic response. Curr. Protoc. Neurosci 10.1002/0471142301.ns0827s57. Chapter 8, Unit 8.27. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Oakes TR, Davidson RJ, 2006. The voice of emotion: an FMRI study of neural responses to angry and happy vocal expressions. Soc. Cogn. Affect. Neurosci 1, 242–249. 10.1093/scan/nsl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly-Mascheroni RM, Senju A, Shepherd AJ, 2008. Dogs catch human yawns. Biol. Lett 4, 446–448. 10.1098/rsbl.2008.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Monfils M-H, 2016. Dominance status predicts social fear transmission in laboratory rats. Anim. Cogn 19, 1051–1069. 10.1007/s10071-016-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Riha PD, Gore AC, Monfils M-H, 2014. Social transmission of Pavlovian fear: fear-conditioning by-proxy in related female rats. Anim. Cogn 17, 827–834. 10.1007/s10071-013-0711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltwasser MT, 1991. Acoustic startle induced ultrasonic vocalization in the rat: a novel animal model of anxiety? Behav. Brain Res 43, 133–137. [DOI] [PubMed] [Google Scholar]
- Kamigaki T, 2018. Prefrontal circuit organization for executive control. Neurosci. Res 10.1016/j.neures.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD, Choleris E, 2005. Kinship, familiarity and social status modulate social learning about “micropredators” (biting flies) in deer mice. Behav. Ecol. Sociobiol (Print) 58, 60–71. 10.1007/s00265-004-0896-0. [DOI] [Google Scholar]
- Kavanagh LC, Winkielman P, 2016. The functionality of spontaneous mimicry and its influences on affiliation: an implicit socialization account. Front. Psychol 7, 458 10.3389/fpsyg.2016.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML, 2005. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol. Psychiatry 58, 495–503. 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD, 2009. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur. J. Neurosci 29, 2061–2073. 10.1111/j.1460-9568.2009.06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TEJ, Buckley MJ, Rushworth MFS, 2006. Optimal decision making and the anterior cingulate cortex. Nat. Neurosci 9, 940–947. 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS, 2004. Anterior cingulate conflict monitoring and adjustments in control. Science 303, 1023–1026. 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y, 2006. Social buffering: relief from stress and anxiety. Philos. Trans. R. Soc. Lond., B, Biol. Sci 361, 2215–2228. 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Lee J, Bang M, Seo BA, Khalid A, Jung MW, Jeon D, 2014. Differential regulation of observational fear and neural oscillations by serotonin and dopamine in the mouse anterior cingulate cortex. Psychopharmacology (Berl.) 231, 4371–4381. 10.1007/s00213-014-3581-7. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kim ES, Covey E, Kim JJ, 2010. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One 5, e15077 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Mátyás F, Lee S, Acsády L, Shin H-S, 2012. Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proc. Natl. Acad. Sci. U. S. A 109, 15497–15501. 10.1073/pnas.1213903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisko TM, Wöhr M, Pellis VC, Pellis SM, 2017. From play to aggression: high-frequency 50-kHz ultrasonic vocalizations as play and appeasement signals in rats. Curr. Top. Behav. Neurosci 30, 91–108. 10.1007/7854_2015_432. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, 2017. Social odors: alarm pheromones and social buffering. Curr. Top. Behav. Neurosci 30, 47–65. 10.1007/7854_2015_406. [DOI] [PubMed] [Google Scholar]
- Klavir O, Genud-Gabai R, Paz R, 2013. Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron 80, 1290–1300. 10.1016/j.neuron.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Knapska E, Mikosz M, Werka T, Maren S, 2010. Social modulation of learning in rats. Learn. Mem. Cold Spring Harb. N 17, 35–42. 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Nikolaev E, Boguszewski P, Walasek G, Blaszczyk J, Kaczmarek L, Werka T, 2006. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc. Natl. Acad. Sci. U. S. A 103, 3858–3862. 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J, 1998. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J. Comp. Psychol 1983 (112), 65–73. [DOI] [PubMed] [Google Scholar]
- Kolling N, Rushworth MFS, 2015. What’s worth the risk? A neural circuit for trade-offs. Cell 161, 1243–1244. 10.1016/j.cell.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Kolling N, Wittmann MK, Behrens TEJ, Boorman ED, Mars RB, Rushworth MFS, 2016. Value, search, persistence and model updating in anterior cingulate cortex. Nat. Neurosci 19, 1280–1285. 10.1038/nn.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS, 2006. Social modulation of pain as evidence for empathy in mice. Science 312, 1967–1970. 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Lapish CC, Durstewitz D, Chandler LJ, Seamans JK, 2008. Successful choice behavior is associated with distinct and coherent network states in anterior cingulate cortex. Proc. Natl. Acad. Sci. U. S. A 105, 11963–11968. 10.1073/pnas.0804045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach M, Amarante LM, Swanson K, White SR, 2018. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 5 10.1523/ENEURO.0315-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, 2007. The amygdala. Curr. Biol. CB 17, R868–874. 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, 2000. Emotion circuits in the brain. Annu. Rev. Neurosci 23, 155–184. 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim M-A, Waterhouse BD, 2005. Retrograde double-labeling study of common afferent projections to the dorsal raphe and the nuclear core of the locus coeruleus in the rat. J. Comp. Neurol 481, 179–193. 10.1002/cne.20365. [DOI] [PubMed] [Google Scholar]
- Li L, Hu X, Preuss TM, Glasser MF, Damen FW, Qiu Y, Rilling J, 2013. Mapping putative hubs in human, chimpanzee and rhesus macaque connectomes via diffusion tractography. NeuroImage 80, 462–474. 10.1016/j.neuroimage.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-M, Collins GT, Paul NM, Grundt P, Newman AH, Xu M, Grandy DK, Woods JH, Katz JL, 2010. Yawning and locomotor behavior induced by dopamine receptor agonists in mice and rats. Behav. Pharmacol 21, 171–181. 10.1097/FBP.0b013e32833a5c68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liauw J, Wu L-J, Zhuo M, 2005. Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. J. Neurophysiol 94, 878–882. 10.1152/jn.01205.2004. [DOI] [PubMed] [Google Scholar]
- Little JP, Carter AG, 2012. Subcellular synaptic connectivity of layer 2 pyramidal neurons in the medial prefrontal cortex. J. Neurosci. Off. J. Soc. Neurosci 32, 12808–12819. 10.1523/JNEUROSCI.1616-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Ito W, Morozov A, 2017. GABAb receptor mediates opposing adaptations of GABA release from two types of prefrontal interneurons after observational fear. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 42, 1272–1283. 10.1038/npp.2016.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh U, Paz R, 2012. Amygdala-prefrontal synchronization underlies resistance to extinction of aversive memories. Neuron 75, 133–142. 10.1016/j.neuron.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Livneh U, Resnik J, Shohat Y, Paz R, 2012. Self-monitoring of social facial expressions in the primate amygdala and cingulate cortex. Proc. Natl. Acad. Sci. U. S. A 109, 18956–18961. 10.1073/pnas.1207662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Apps MAJ, Roiser JP, Viding E, 2015. Encoding of vicarious reward prediction in anterior cingulate cortex and relationship with trait empathy. J. Neurosci. Off. J. Soc. Neurosci 35, 13720–13727. 10.1523/JNEUROSCI.1703-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ, 2004. Neuronal signalling of fear memory. Nat. Rev. Neurosci 5, 844–852. 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Roth A, Sakmann B, 1997. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. (Paris) 500 (Pt 2), 409–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C, 2004. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci 5, 793–807. 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Masuda A, Aou S, 2009. Social transmission of avoidance behavior under situational change in learned and unlearned rats. PLoS One 4, e6794 10.1371/journal.pone.0006794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Hattori T, Asaba A, Inoue N, Kanomata N, Kikusui T, Kobayakawa R, Kobayakawa K, 2015. Genetic dissection of pheromone processing reveals main olfactory system-mediated social behaviors in mice. Proc. Natl. Acad. Sci. U. S. A 112, E311–320. 10.1073/pnas.1416723112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH, 2005. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660. 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, 1998. Cortical pathways to the mammalian amygdala. Prog. Neurobiol 55, 257–332. [DOI] [PubMed] [Google Scholar]
- McGann JP, 2017. Poor human olfaction is a 19th-century myth. Science 356 10.1126/science.aam7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklósi A, 1999. The ethological analysis of imitation. Biol. Rev. Camb. Philos. Soc 74, 347–374. [DOI] [PubMed] [Google Scholar]
- Mikosz M, Nowak A, Werka T, Knapska E, 2015. Sex differences in social modulation of learning in rats. Sci. Rep 5, 18114 10.1038/srep18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Cook M, 1988. Social learning and the acquisition of snake fear in monkeys. Social Learning: Psychological and Biological Perspectives. Lawrence Erlbaum Associates, Inc, Hillsdale, NJ, US, pp. 51–73. [Google Scholar]
- Mineka S, Davidson M, Cook M, Keir R, 1984. Observational conditioning of snake fear in rhesus monkeys. J. Abnorm. Psychol 93, 355–372. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Agee LA, 2019. Insights from social transmission of information in rodents. Genes Brain Behav. 18, e12534 10.1111/gbb.12534. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, van Hoesen GW, 2007. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J. Comp. Neurol 500, 134–165. 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN, 2012. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res. Bull 87, 457–497. 10.1016/j.brainresbull.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW, 1998. Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res. Bull 45, 209–232. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW, 1993. Frontal granular cortex input to the cingulate (M3), supplementary (M2) and primary (M1) motor cortices in the rhesus monkey. J. Comp. Neurol 337, 669–689. 10.1002/cne.903370411. [DOI] [PubMed] [Google Scholar]
- Morrison BJ, Hill WF, 1967. Socially facilitated reduction of the fear response in rats raised in groups or in isolation. J. Comp. Physiol. Psychol 63, 71–76. 10.1037/h0024175. [DOI] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, Fuglevand AJ, Gothard KM, 2016. Tactile stimulation of the face and the production of facial expressions activate neurons in the primate amygdala. eNeuro 3 10.1523/ENEURO.0182-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, Gothard KM, 2014. Neurons in the monkey amygdala detect eye contact during naturalistic social interactions. Curr. Biol. CB 24, 2459–2464. 10.1016/j.cub.2014.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyaho A, Rivas-Zamudio X, Ugarte A, Eguibar JR, Valencia J, 2015. Smell facilitates auditory contagious yawning in stranger rats. Anim. Cogn 18, 279–290. 10.1007/s10071-014-0798-0. [DOI] [PubMed] [Google Scholar]
- Murray L, de Rosnay M, Pearson J, Bergeron C, Schofield E, Royal-Lawson M, Cooper PJ, 2008. Intergenerational transmission of social anxiety: the role of social referencing processes in infancy. Child Dev. 79, 1049–1064. 10.1111/j.1467-8624.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- Nakano T, Kitazawa S, 2010. Eyeblink entrainment at breakpoints of speech. Exp. Brain Res 205, 577–581. 10.1007/s00221-010-2387-z. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Cavanagh JF, Frank MJ, Laubach M, 2013. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat. Neurosci 16, 1888–1895. 10.1038/nn.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M, 2008. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J. Neurophysiol 100, 520–525. 10.1152/jn.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert F-X, Mars RB, Sallet J, Rushworth MFS, 2015. Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc. Natl. Acad. Sci. U. S. A 112, E2695–2704. 10.1073/pnas.1410767112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, 2015. Amygdala pain mechanisms. Handb. Exp. Pharmacol 227, 261–284. 10.1007/978-3-662-46450-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal PM, Brauer M, 2012. Social functionality of human emotion. Annu. Rev. Psychol 63, 259–285. 10.1146/annurev.psych.121208.131605. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Mars RB, Rushworth MFS, 2011. Distinct roles of three frontal cortical areas in reward-guided behavior. J. Neurosci. Off. J. Soc. Neurosci 31, 14399–14412. 10.1523/JNEUROSCI.6456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, Mortrud MT, Ouellette B, Nguyen TN, Sorensen SA, Slaughterbeck CR, Wakeman W, Li Y, Feng D, Ho A, Nicholas E, Hirokawa KE, Bohn P, Joines KM, Peng H, Hawrylycz MJ, Phillips JW, Hohmann JG, Wohnoutka P, Gerfen CR, Koch C, Bernard A, Dang C, Jones AR, Zeng H, 2014. A mesoscale connectome of the mouse brain. Nature 508, 207–214. 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh K, Elekes F, Turcsán B, Kis O, Topál J, 2016. Social pre-treatment modulates attention allocation to transient and stable object properties. Front. Psychol 7, 1619 10.3389/fpsyg.2016.01619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Phelps EA, 2007. Social learning of fear. Nat. Neurosci 10, 1095–1102. 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA, 2004. Learned fear of “unseen” faces after Pavlovian, observational, and instructed fear. Psychol. Sci 15, 822–828. 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, 2018. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293. 10.1016/j.tins.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP, 2011. Rodent empathy and affective neuroscience. Neurosci. Biobehav. Rev 35, 1864–1875. 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, 2001. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci 2, 417–424. 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, 2019. The Mouse Brain in Stereotaxic Coordinates. Academic Press. [Google Scholar]
- Paxinos G, Franklin KBJ, 2004. The Mouse Brain in Stereotaxic Coordinates. Gulf Professional Publishing. [Google Scholar]
- Paxinos G, Watson C, 2014. The Rat Brain in Stereotaxic Coordinates, 7th ed. Academic Press. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1998. The Rat Brain in Stereotaxic Coordinates, 4th ed. Academic Press. [DOI] [PubMed] [Google Scholar]
- Pereira AG, Cruz A, Lima SQ, Moita MA, 2012. Silence resulting from the cessation of movement signals danger. Curr. Biol. CB 22, R627–628. 10.1016/j.cub.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ, 2009. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn. Mem. Cold Spring Harb. N 16, 279–288. 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisansky MT, Hanson LR, Gottesman II, Gewirtz JC, 2017. Oxytocin enhances observational fear in mice. Nat. Commun 8, 2102 10.1038/s41467-017-02279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Seyfarth RM, Cheney DL, 2016. Adaptations for social cognition in the primate brain. Philos. Trans. R. Soc. Lond., B, Biol. Sci 371, 20150096 10.1098/rstb.2015.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV, 2007. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab. Anim. Sci. JAALAS 46, 28–34. [PubMed] [Google Scholar]
- Portfors CV, Perkel DJ, 2014. The role of ultrasonic vocalizations in mouse communication. Curr. Opin. Neurobiol 28, 115–120. 10.1016/j.conb.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FBM, 2002. Empathy: its ultimate and proximate bases. Behav. Brain Sci 25, 1–20 discussion 20-71. [DOI] [PubMed] [Google Scholar]
- Price JL, Carmichael ST, Carnes KM, Clugnet M-C, Kuroda M, Ray JP, 1991. Olfactory input to the prefrontal cortex. Olfaction Model Syst. Comput. Neurosci 101–120. [Google Scholar]
- Procyk E, Tanaka YL, Joseph JP, 2000. Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat. Neurosci 3, 502–508. 10.1038/74880. [DOI] [PubMed] [Google Scholar]
- Procyk E, Wilson CRE, Stoll FM, Faraut MCM, Petrides M, Amiez C, 2016. Midcingulate motor map and feedback detection: converging data from humans and monkeys. Cereb. Cortex 1991 (26), 467–476. 10.1093/cercor/bhu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryluk R, Kfir Y, Gelbard-Sagiv H, Fried I, Paz R, 2019. A tradeoff in the neural code across regions and species. Cell 176, 597–609. 10.1016/j.cell.2018.12.032.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Footer O, Camerer CF, Mobbs D, 2018. A collaborator’s reputation can Bias decisions and anxiety under uncertainty. J. Neurosci. Off. J. Soc. Neurosci 38, 2262–2269. 10.1523/JNEUROSCI.2337-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD, 2016. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct. Funct 221, 2937–2962. 10.1007/s00429-015-1081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Caruana F, 2017. Some considerations on de Waal and Preston review. Nat. Rev. Neurosci 18, 769 10.1038/nrn.2017.139. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L, 2004. The mirror-neuron system. Annu. Rev. Neurosci 27, 169–192. 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C, 2010. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat. Rev. Neurosci 11, 264–274. 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Rodriguez Buritica JM, Heekeren HR, Li S-C, Eppinger B, 2018. Developmental differences in the neural dynamics of observational learning. Neuropsychologia 119, 12–23. 10.1016/j.neuropsychologia.2018.07.022. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Bannerman DM, Rushworth MFS, 2008. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn. Affect. Behav. Neurosci 8, 485–497. 10.3758/CABN.8.4.485. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Mitz AR, Chacko RV, Murray EA, 2013. Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron 80, 1519–1531. 10.1016/j.neuron.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, Rhodes SEV, Murray EA, 2014. A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc. Natl. Acad. Sci. U. S. A 111, 5391–5396. 10.1073/pnas.1317695111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert L, Naybar N, 2008. Gender differences in empathy: the role of the right hemisphere. Brain Cogn. 67, 162–167. 10.1016/j.bandc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM, 2004. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. (Regul. Ed.) 8, 410–417. 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Adolphs R, 2015. The primate amygdala in social perception - insights from electrophysiological recordings and stimulation. Trends Neurosci. 38, 295–306. 10.1016/j.tins.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Andersson JL, O’Reilly JX, Jbabdi S, Croxson PL, Jenkinson M, Miller KL, Rushworth MFS, 2011. Social network size affects neural circuits in macaques. Science 334, 697–700. 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Hervé-Minvielle A, 1995. Inhibitory influence of frontal cortex on locus coeruleus neurons. Proc. Natl. Acad. Sci. U. S. A 92, 6032–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN, 2011. Unusual repertoire of vocalizations in adult BTBR T + tf/J mice during three types of social encounters. Genes Brain Behav. 10, 44–56. 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seara-Cardoso A, Sebastian CL, Viding E, Roiser JP, 2016. Affective resonance in response to others’ emotional faces varies with affective ratings and psychopathic traits in amygdala and anterior insula. Soc. Neurosci 11, 140–152. 10.1080/17470919.2015.1044672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinberg DL, Logothetis NK, 1997. The role of temporal cortical areas in perceptual organization. Proc. Natl. Acad. Sci 94, 3408–3413. 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD, 2013. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79, 217–240. 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, 2004. The human sense of smell: are we better than we think? PLoS Biol. 2, E146 10.1371/journal.pbio.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN, 2012. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488, 218–221. 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Tanji J, 1998. Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282, 1335–1338. [DOI] [PubMed] [Google Scholar]
- Shipp S, 2005. The importance of being agranular: a comparative account of visual and motor cortex. Philos. Trans. R. Soc. Lond., B, Biol. Sci 360, 797–814. 10.1098/rstb.2005.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showers MJ, Crosby EC, 1958. Somatic and visceral responses from the cingulate gyrus. Neurology 8, 561–565. [DOI] [PubMed] [Google Scholar]
- Simon D, Craig KD, Miltner WHR, Rainville P, 2006. Brain responses to dynamic facial expressions of pain. Pain 126, 309–318. 10.1016/j.pain.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Sliwa J, Freiwald WA, 2017. A dedicated network for social interaction processing in the primate brain. Science 356, 745–749. 10.1126/science.aam6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TD, Laitman JT, Bhatnagar KP, 2014. The shrinking anthropoid nose, the human vomeronasal organ, and the language of anatomical reduction. Anat. Rec. Hoboken (Hoboken) 2007 (297), 2196–2204. 10.1002/ar.23035. [DOI] [PubMed] [Google Scholar]
- Stowers L, Kuo T-H, 2015. Mammalian pheromones: emerging properties and mechanisms of detection Curr. Opin. Neurobiol 34, 103–109. 10.1016/j.conb.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD, 2006. Executive control of countermanding saccades by the supplementary eye field. Nat. Neurosci 9, 925–931. 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- Terburg D, Scheggia D, Triana Del Rio R, Klumpers F, Ciobanu AC, Morgan B, Montoya ER, Bos PA, Giobellina G, van den Burg EH, de Gelder B, Stein DJ, Stoop R, van Honk J, 2018. The basolateral amygdala is essential for rapid escape: a human and rodent study. Cell 175, 723–735. 10.1016/j.cell.2018.09.028.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, Takahashi LK, Barfield RJ, 1983. Analysis of ultrasonic vocalizations emitted by intruders during aggressive encounters among rats (Rattus norvegicus). J. Comp. Psychol. Wash. DC 1983 97, 201–206. [PubMed] [Google Scholar]
- Thomson AM, Bannister AP, 1998. Postsynaptic pyramidal target selection by descending layer III pyramidal axons: dual intracellular recordings and biocytin filling in slices of rat neocortex. Neuroscience 84, 669–683. [DOI] [PubMed] [Google Scholar]
- Tomlin D, Kayali MA, King-Casas B, Anen C, Camerer CF, Quartz SR, Montague PR, 2006. Agent-specific responses in the cingulate cortex during economic exchanges. Science 312, 1047–1050. 10.1126/science.1125596. [DOI] [PubMed] [Google Scholar]
- Tseng P-H, Rajangam S, Lehew G, Lebedev MA, Nicolelis MAL, 2018. Interbrain cortical synchronization encodes multiple aspects of social interactions in monkey pairs. Sci. Rep 8, 4699 10.1038/s41598-018-22679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synap. N. Y. N 51, 32–58. 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vogt BA, 2016. Midcingulate cortex: structure, connections, homologies, functions and diseases. J. Chem. Neuroanat 74, 28–46. 10.1016/j.jchemneu.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Paxinos G, 2014. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct 219, 185–192. 10.1007/s00429-012-0493-3. [DOI] [PubMed] [Google Scholar]
- Vrticka P, Andersson F, Sander D, Vuilleumier P, 2009. Memory for friends or foes: the social context of past encounters with faces modulates their subsequent neural traces in the brain. Soc. Neurosci 4, 384–401. 10.1080/17470910902941793. [DOI] [PubMed] [Google Scholar]
- Wallis CU, Cardinal RN, Alexander L, Roberts AC, Clarke HF, 2017. Opposing roles of primate areas 25 and 32 and their putative rodent homologs in the regulation of negative emotion. Proc. Natl. Acad. Sci. U. S. A 114, E4075–E4084. 10.1073/pnas.1620115114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H, 2011. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334, 693–697. 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Wang S, Tudusciuc O, Mamelak AN, Ross IB, Adolphs R, Rutishauser U, 2014. Neurons in the human amygdala selective for perceived emotion. Proc. Natl. Acad. Sci. U. S. A 111, E3110–3119. 10.1073/pnas.1323342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iñiguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolaños-Guzmán CA, 2013. Neurobiological sequelae of witnessing stressful events in adult mice. Biol. Psychiatry 73, 7–14. 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M (Ed.), 2017. The Prefrontal Cortex as an Executive, Emotional, and Social Brain. Springer, Japan. [Google Scholar]
- Wesson DW, 2013. Sniffing behavior communicates social hierarchy. Curr. Biol. CB 23, 575–580. 10.1016/j.cub.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Schwarting RKW, 2007. Ultrasonic communication in rats: can playback of 50-kHz calls induce approach behavior? PLoS One 2, e1365 10.1371/journal.pone.0001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, van Gaalen MM, Schwarting RKW, 2015. Affective communication in rodents: serotonin and its modulating role in ultrasonic vocalizations. Behav. Pharmacol 26, 506–521. 10.1097/FBP.0000000000000172. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K, 2011. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178. 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Burling JM, 2011. A new perspective on embodied social attention. Cogn. Brain Behav. Interdiscip. J 15, 535–552. [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Saito N, Iriki A, Isoda M, 2011. Representation of others’ action by neurons in monkey medial frontal cortex. Curr. Biol. CB 21, 249–253. 10.1016/j.cub.2011.01.004. [DOI] [PubMed] [Google Scholar]