Abstract
What doesn’t kill you makes you stronger. Hormesis, the paradoxical beneficial effects of low-dose stressors, can be better defined as the biphasic dose-effect or time-effect relationship for any substance. Here we review hormesis-like phenomena in the context of chronic diseases for many substances, including lifestyle factors and endocrine factors. Intermittent or pulsatile exposure can generate opposite effects compared to continuous exposure. An initial exposure can elicit an adaptive stress response with long-lasting protection against subsequent exposures. Early-life stress can increase resilience in later life, and lack of stress can lead to vulnerability. Many stressors are naturally occurring and are required for healthy growth or homeostasis, which exemplifies how ‘illness is the doorway to health’.
Keywords: biphasic, dose-effect, time-effect, intermittent, resilience, stress response
Introduction
Hormesis was originally defined as a phenomenon in which exposure to a harmful substance gives beneficial effects to living organisms when the dose of the harmful substance is small. The radiation hormesis is among the first documented examples. Although high-dose radiation promotes mutagenesis and carcinogenesis, low-dose ionizing radiation such as X-ray has been shown to suppress tumor development [1]. Many chemical carcinogens such as DTT or α-benzene hexachloride, when given at a low dose, can protect against DNA damage and cytotoxic effects induced by subsequent exposures at a much higher dose [2]. As illustrated in the aphorism ‘what doesn’t kill you makes you stronger’, this original idea of hormesis involves an adaptive response upon the initial exposure and, therefore, can be referred to as ‘stress-response’ hormesis [3]. There are three components in this scenario: the initial stress exposure that ‘tries to kill you’, the subsequent stress exposure that you are more resilient against, and a time interval in between. If the initial low-dose exposure protects against subsequent exposure to the higher dose of the same substrate, it is a ‘single-mode’ stress response. If the initial low-dose exposure protects against a different substance, it is a ‘cross-mode’ stress response. If the time interval between the two exposures involves a developmental process, it is a ‘developmental’ stress-response hormesis.
The issue with this original definition is that the ‘harmfulness’ or ‘stress’ is not an intrinsic feature for any substance. It is the dose, and the time of the exposure, that determines toxicity. Many substances discussed in the context of hormesis are naturally occurring substances that the body is exposed to during evolution. Therefore, our body has likely evolved mechanisms to respond to, adapt to, or even rely on, the stressors for healthy growth and homeostasis. Similarly, what is ‘beneficial’ is also context-dependent. A beneficial effect of calorie restriction on glucose metabolism can come with a sacrifice on muscle mass or bone mineral density [4]. A thrifty phenotype can be beneficial when food is scarce but can be detrimental when food is in excess. Therefore, a more reasonable definition of hormesis seems to be the biphasic dose-effect or time-effect relationship for any substance [5,6], which defines a broad sense of hormesis. The biphasic time-effect can be mechanistically related to the stress response hormesis.
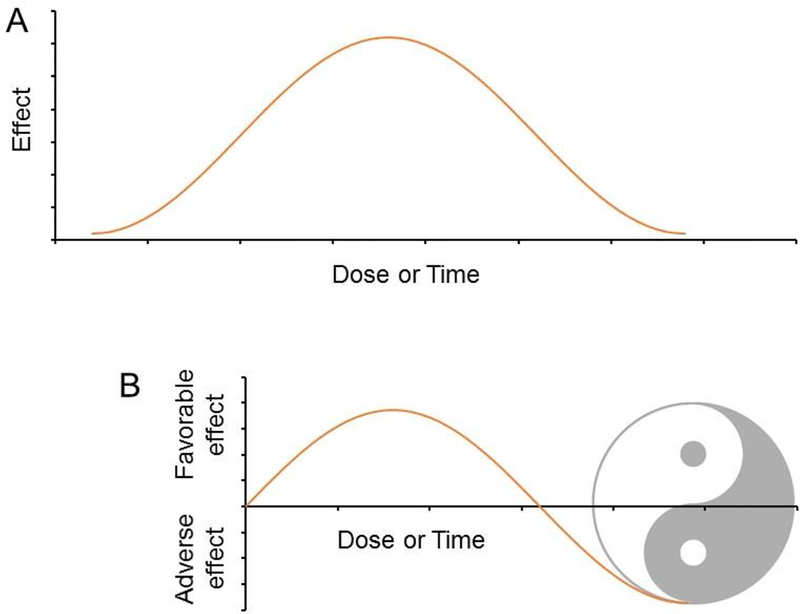
Hormesis can be counterintuitive but not entirely surprising. If ‘too much of a good thing is a bad thing’, a little bad thing can be good. The golden mean doctrine in philosophy suggests a Goldilocks zone for everything. A bell-like biphasic curve, therefore, is expected for the dose-effect or time-effect relationship for any substance (Fig 1A). A dose-effect relationship consistent with the original definition of hormesis appears only after we artificially define the baseline (Fig 1B). The transformation of an adverse effect into a favorable effect exemplifies how ‘illness is the doorway to health’ as articulated in the philosophy of Yin and Yang.
Figure 1.
(A) Bell-like curve depicting the optimal dose or time of an exposure. Time not only refers to the total duration but also includes the temporal pattern as defined by frequency and interval. (B) An artificial setting of the baseline leads to a biphasic curve aligned with the classic definition of hormesis - low-dose beneficial effects of harmful substances. The transformation of the adverse effect to the favorable effect exemplifies how ‘illness is the doorway to health’ as articulated in the ancient philosophy of Yin and Yang.
Hormesis induced by environmental chemicals and radiation has been nicely reviewed in the field of toxicology with the functional readout mainly on cell viability, cell proliferation, enzyme activity, or gene expression [6]. In this review, we focus on lifestyle factors (Box 1 and 2) and endocrine factors (Table 1) in the context of chronic diseases with the emphasis on in vivo studies in mammals. The underlying molecular mechanisms are unclear for most hormesis phenomena.
Box 1. Hormetic effects of lifestyle factors.
Cigarette smoke
Cigarette smoke can cause lung diseases. However, epidemiological studies also reveal a correlation between smoking and a lower incidence of Parkinson’s disease [108]. Specifically, the longer duration of smoking, not the higher intensity, is correlated with the lower risk of Parkinson’s disease [109]. Nicotine can be neuroprotective through nicotinic acetylcholine receptors that modulate neuroinflammation [110]. A major component of cannabis, delta-9-tetrahydrocannabinol (THC), can disrupt short-term memory. However, THC can improve neurocognitive functions in old animals when administered at low concentrations. THC promotes hippocampal neurogenesis and slows down the neurodegenerative processes in animal models of Alzheimer’s disease, which is associated with ameliorated inflammation and improved memory [111].
Alcohol consumption
Heavy alcohol consumption can cause alcoholic fatty liver and alcoholic cardiomyopathy. However, compared to abstainers, moderate alcohol consumption is associated with reduced mortality and lower risk of cardiovascular diseases, although there is controversy regarding methodologies [112,113]. Moderate alcohol consumption is associated with beneficial alterations in blood lipid profile, platelet function, fibrinolytic activity, insulin sensitivity, myocardial blood flow, cardiomyocytes survival signaling pathways, which can contribute to the lower risk of coronary events [113]. Moderate alcohol consumption can also be protective against cognitive dysfunction [114], although it is more controversial with the mechanism less well-defined [115].
Exercise
Moderate level of exercise reduces the level of pro-inflammatory cytokines and stimulates the production of anti-inflammatory cytokines in healthy individuals [116]. Exercise activates the sympatho-adrenomedullary and the hypothalamus-pituitary-adrenal axes, leading to release of catecholamines [117,118], which regulates inflammatory cytokines through the β2 adrenergic receptor [119]. Intense exercise, however, can induce the release of pro-inflammatory cytokines and inhibit the release of anti-inflammatory productions [116]. Sedentary diabetic rats subjected to an acute intense swimming exercise showed aggravated inflammatory profiles and oxidative stress [120]. Metabolic derangements in obese rats impaired the negative feedback mechanisms between IL-6 and noradrenaline, and excess high-intensity exercise can worsen the dysregulation, inducing pro-inflammatory effects [121]. Such a shifted balance between pro- and anti-inflammatory cytokine can cause excessive tissue damage and inflammatory diseases such as osteoarthritis [122].
Conversely, the role of inflammation in muscle tissue repair is also biphasic in the context of exercise. On one hand, a low degree of inflammation seems required for recovery of exercise-induced muscle microtrauma [123]. Post-exercise treatment of non-steroidal anti-inflammatory drugs (NSAID) can suppress satellite cell proliferation and reduce muscle protein synthesis [124]. On the other hand, intense exercise can cause muscle damage, as characterized by disruption of myofiber and release of myocellular proteins. This leads to an inflammatory response and cytokine discharge, which can, in turn, worsen the muscle performance [125]. Exercise also have biphasic effects on neurocognition. In a rat model of brain ischemia, low intensity exercise (LI), but not high intensity exercise (HI), performed better in spatial memory test compared to mice in the sedentary group. The HI group presented higher blood corticosterone levels, implicating a higher stress response. Expression levels of BDNF, Synapsin-I, and PSD-95 in the hippocampus were increased only in LI rats, indicating that low-intensity exercise led to better synaptic plasticity [126].
Box 2. Hormetic effects of fasting.
Prolonged fasting or starvation causes muscle wasting and impairs cardiac functions [127], while mild caloric restriction (CR) or intermittent fasting (IF) can have beneficial effects. Calorie restriction to 20% of ad libitum for 8 weeks in mice caused remarkable myocardial fibrosis with increased expression of caspase-1, IL-1β, and IL-18 compared to ad libitum control. In contrast, calorie restriction to 40% of ad libitum did not cause myocardial fibrosis and reduced expression of caspase-1, IL-1β, and IL-18 [128]. Mechanistically, one would assume that reduced calorie intake underlies fasting-induced metabolic benefits under overnutrition. However, it is not always the case. Time-restricted fasting, by only restricting the feeding time within 8–12 h per day, do not change total calorie intake in mice but can have many beneficial effects including improved glucose tolerance, suppressed insulin resistance, and ameliorated dyslipidemia [129]. IF can also promote adipose thermogenesis by upregulating vascular endothelial growth factor (VEGF) expression, and remodels adipose macrophages associated with adipose tissue browning [130].
Fasting can also facilitate cancer treatment. In preclinical mouse cancer models and patient xenograft models, fasting (6 cycles of 1-day feeding- 1-day fasting) can inhibit the development of both B cell and T cell acute lymphoblastic leukemia (ALL), but not acute myeloid leukemia. This is associated with upregulated expression of the leptin receptor (LepR) and its downstream signaling, potentially due to a compensatory expression elevation triggered by reduced circulating leptin level. The activated LepR signaling upregulated the PRDM1 gene, a known tumor suppressor, which contributed to the ALL inhibition effect [131]. In addition to the direct effect on tumor cells, fasting can affect immune cells and facilitate their recognition and clearance of tumor cells. When combined with chemotherapy, fasting-mimicking diet (FMD) increased the level of bone marrow lymphoid progenitor cells and promoted the accumulation of CD8+ tumor-infiltration lymphocytes in the tumor bed, and thus reduced the tumor progression of breast cancer and melanoma. FMD downregulated the expression of heme oxygenase-1 (HO-1) in breast tumor, which decreased regulatory T cells and facilitated the targeted attack of cancer cells [132]. Reduced circulating glucose, increased ketone bodies, and altered hormonal signals during fasting can also limit the glucose availability to cancer cells, which may contribute to the anti-cancer effects of fasting [133].
Table 1.
Biphasic dose-effect or time-effect of endocrine factors
| Hormone | Target | Effect | Reference |
|---|---|---|---|
| Parathyroid hormone | Bone | Constant high-level exposure causes bone loss, while intermittent exposure increases bone mass. | [65][66] |
| Insulin secretion | Low-dose stimulates glucose-induced insulin release, while high-dose inhibits it. | [67][68] | |
| Glucocorticoid | Muscle | Chronic treatment exacerbates muscle atrophy, while intermittent weekly treatment enhances muscle repair and improves muscle contractile function. | [69][70] |
| Cognitive function | Chronic high-level exposure is associated with increased risk of cognitive decline, while the acute exposure improves memory consolidation but inhibits memory retrieval. | [72][73] [74] |
|
| Thyroid hormone | Heart | Hyperthyroidism increases the risk of coronary heart diseases, pulmonary hypertension, and atrial fibrillation. Hypothyroidism causes left ventricular diastolic dysfunction and increases carotid intima-media thickness. | [76] |
| Metabolism | Thyroid hormone increases catabolism and energy expenditure. However, the T3 level is positively correlated with unfavorable metabolic parameters in some population. | [77,78] | |
| Adiponectin | Metabolism | Adiponectin improves glucose tolerance and endothelial functions while reduces inflammation and atherosclerosis. But the adiponectin level is positively associated with mortality in coronary heart disease. Adiponectin may exacerbate chronic inflammatory conditions. | [80] |
| Estrogen | Tumor cell proliferation | Estrogen stimulates tumor cell proliferation at low doses but promotes cell apoptosis at high doses. | [81][82] |
| Cardiovascular system | Low-dose activates, while high-dose inhibits, plasminogen activator in aortic endothelial cells. | [84] | |
| Bone | Estrogen stimulates endochondral bone formation at the start of puberty but induce epiphyseal closure at the end of puberty. In treating Turner syndrome, intermittent low-dose estrogen induces maximal ulnar growth, while high-dose estrogen fails to stimulate ulnar growth. | [86][87] | |
| Progesterone | Memory | Progesterone, when administrated shortly before test, potentiates estradiol-induced effects on extinction recall or spatial memory. However, when administrated a day before the test, progesterone abolishes the estradiol effects. | [89][90] |
| Immune response | Long exposure time is associated with poor immune responses to genital herpes. Short treatment is protective against HSV challenge. | [91] | |
| Growth hormone (GH) and IGF-1 | Lifespan | Both low and high IGF-I levels are associated with increased mortality. GH can increase muscle mass, reduce adiposity, and improve bone density. However, deficiencies in GH/IGf-1 signaling pathways increases lifespan and healthspan. | [92] [95,99] |
| Insulin | Blood glucose | Insulin lowers blood glucose levels in diabetes patients. However, suppressing hyperinsulinemia can improve insulin sensitivity and extend lifespan in diet-induced obesity. | [101] [102] |
| Irisin | Bone | Low-dose weekly treatment increases bone density. However, irisin deficiency also suppresses ovariectomy-induced bone resorption. | [106] |
Stress-response hormesis
Single-mode and cross-mode stress responses
Many xenobiotic chemicals induce the expression of detoxification enzymes, which is protective against subsequent higher-dose exposures [7]. Some xenobiotic chemicals can directly serve as ligands for nuclear receptors such as constitutive androstane receptor (CAR) or pregnane X receptor (PXR). Other xenobiotic chemicals can indirectly activate other transcription factors such as nuclear factor erythroid 2-related factor 2 (Nrf2) through altering the intracellular redox status or a variety of stress-response signaling pathways. Activation of these transcription factors upregulates the expression of genes that encode enzymes in xenobiotic metabolism. These enzymes are classified into three phases, including phase I enzymes such as cytochrome P450 family enzymes, phase II enzymes such as glutathione-based conjugation enzymes, and phase III enzymes such as ATP-binding cassette transporters, which are collectively responsible for detoxification and excretion of the xenobiotic chemicals from the body. Thus, priming the body with a low-dose xenobiotic chemical can confer protection against subsequent exposures of the same chemical [7]. This adaptive response in xenobiotic metabolism constitutes a classic example of single-mode stress-response hormesis.
If the initial exposure confers protection against a different substance, it is referred to as ‘cross-mode’ hormesis. In the abovementioned example of xenobiotic-mediated upregulation of detoxification enzymes, a combination of detoxification enzymes induced by one chemical can protect against many other chemicals. Such cross-model hormesis is the basis of chemoprevention, an approach to use low-toxicity chemicals to induce adaptive protective responses against many other carcinogenic chemicals [3]. Cross-mode hormesis exists in many situations. For example, exposure of cells to mild heat stress can make them more protected from oxidative stress or toxins such as cyanide [8]. Similarly, exposure to a low-dose mitochondrial uncoupling agent 2,4-dinitrophenol can make cells less vulnerable to being killed by ischemia [9]. This cross-mode aspect of hormesis may contribute to the broad benefits of exercise and dietary restriction [10] (Box 1 and 2). Below we examine a few other common stressors.
Reactive oxygen species (ROS)
Oxidative stress refers to the high level of ROS that causes damage to DNA, protein, or other cellular components. The free radical theory of aging suggests that oxidative stress causes aging [11]. As some DNA repair mediators are downregulated in aged organisms, DNA damage induced by oxidative stress can be left unrepaired, leading to genome instability. However, low-level ROS can function as signal molecules to initiate biological processes without the above detrimental effects and, therefore, can be beneficial [12]. For example, the increased oxidative stress, caused by depletion of the mitochondrial superoxide dismutase, extended lifespan in C. elegans [13]. Treatment with Paraquat, a superoxide generator, at a low dose can also increase the lifespan, while a high-dose Paraquat was deleterious [14]. Randomized clinical trials have shown that antioxidants, such as Vitamin C and Vitamin E, do not prolong lifespan in human [15,16] and can potentially increase the risk for cancer and diabetes [17].
In addition to the level, subcellular location also determines the role of ROS. While cytosolic ROS tends to shorten lifespan, mitochondrial ROS (mtROS) can extend the lifespan [18,19]. For example, skin wounding triggers local production of mtROS. Lowering mtROS levels by mitochondrial superoxide-specific antioxidants blocked actin-based wound closure. Paraquat, a pro-oxidant that induces mitochondrial superoxide, promoted actin-based wound closure. Mutations of superoxide dismutase in worms elevated mitochondrial superoxide, promoted wound closure, and enhanced survival. Mechanistically, mtROS was increased by wound-triggered calcium influx, which locally inhibited Rho GTPase activity via a redox-sensitive motif [20]. A similar role of mtROS was documented in mammalian skeletal muscle cells. Injury increased mitochondrial calcium uptake through the mitochondrial calcium uniporter, which transiently increased mtROS. Mitochondrial respiratory chain inhibitor rotenone and antimycin A increased injury-triggered mtROS production and showed beneficial effects on plasma membrane repair in a dose-dependent manner. Mechanistically, mtROS locally activated GTPase RhoA, triggered F-actin accumulation at the site of injury, which facilitated membrane repair. Quenching mtROS using mitoTEMPO in myofibers during eccentric exercise prevented injury-triggered RhoA activation and actin polymerization, leading to increased damage to myofibers and a greater loss of force [21]. Therefore, mtROS not only play a role in extending longevity but also is required for wound repair. This biphasic dose-effect of ROS and other substances in mitochondria is referred to as mitohormesis [22].
ROS not only serves as an intracellular signal but can also mediate inter-cellular communications. ROS generated by neutrophils plays a vital role in promoting liver repair [23]. Upon the tissue injury, neutrophils are recruited to the injured site, contributing to liver repair by causing the macrophage phenotypic conversion from a pro-inflammatory stage to a pro-regenerative stage. ROS from neutrophils acts as a mediator for the process because reducing neutrophil ROS by either depletion of neutrophils or genetically perturbation of neutrophil NADPH oxidase 2 (Nox2) blocked the macrophage phenotypic conversion. Conversely, transferring wild-type neutrophils, but not Nox2 knock-out neutrophils, can rescue the hepatic damage in neutrophil-depleted mice and promote the macrophage phenotypic conversion [23]. Thus, ROS is a critical inter-cellular signaling molecule that mediates the resolution of inflammation.
Hypoxia
Obstructive sleep apnea (OSA) is a risk factor for cardiovascular and liver diseases. Intermittent hypoxia (IH) is a major component of OSA and contributes to the hepatic, metabolic, and vascular effects of OSA. Nocturnal IH is independently associated with metabolic dyslipidemia and steatosis. IH (1 min cycle, FiO2 5–6% for 30 s, FiO2 20.9% for 30 s; for 9 h) can also cause insulin resistance in lean mice [24]. Chronic IH (1 min cycle, FiO2 6–7% for 30 s, FiO2 ~21% for 30 s) during the light phase (9 am- 9 pm) for 4 weeks exacerbates insulin resistance and glucose intolerance in diet-induced obesity [25].
Paradoxically, IH shows benefits in some conditions by facilitating the adaptation to reduced oxygen. Exposure to daily IH cycle for 4 days, each composed of 2-min at 6–8% O2 followed by 3-min re-oxygenation for 5 times, can protect the heart against ischemia-reperfusion injury [26]. IH also increases exercise tolerance in elderly men [27] and improves the myocardial perfusion in patients with severe coronary heart disease [28]. Mechanistically, IH improves nitric oxide (NO) bioavailability and storage [29]. Decreased O2 level can lead to less oxidization of NO to NO2− and NO3−, allowing more NO release from hemoglobin [30]. In addition, hypoxia can induce the expression of nitric oxide synthase through hypoxia-inducible factor HIF-1 [31]. IH (9.5–10% O2, 5–10 min, 5–8 times/day, for 20 days) suppressed hypertension in spontaneously hypertensive rats. The endothelial function was sustained in the IH group but decreased in the control group. This was associated with enhanced capacity of aortic rings to store NO and increased NO availability in vascular walls [32]. In a mouse model of metabolic syndromes, short-term IH (1 min cycle, FiO2 5% for 30 s, FiO2 21% for 30 s; 8 h/day) increased insulin and leptin levels, and prevented endothelial dysfunction caused by a high-fat diet. IH restored mitochondrial complex I activity and slightly increased complex II and IV activity, which may help boost mitochondrial oxidative-phosphorylation and reduce liver lipid accumulation [33].
Nitric oxide (NO)
NO can lead to mutagenesis and cell death at high concentrations through inhibiting DNA synthesis, disrupting cell membrane integrity, arresting the cell cycle, causing DNA strand break, and apoptosis [34]. Excess NO also impairs mitochondrial function and affects metabolic processes in neurons, contributing to neurodegenerative diseases [35]. However, NO at low concentrations modulates glutamatergic neurotransmission [36]. Depletion of neuronal NO synthase deletion impaired cognitive performance and synaptic plasticity [37]. In the cardiovascular system, NO promotes new vessel formation, limits vessel constriction, suppresses inflammation, and promotes blood flow [38].
Amyloid-β peptide (Aβ)
Excessive Aβ deposits causes synaptoxicity and memory dysfunction [39]. Beyond this amyloid hypothesis, emerging evidence suggests that Aβ and its precursor protein APP have physiological functions at low concentrations in the healthy brain [40,41]. APP is involved in cell proliferation, differentiation, neurite outgrowth, cell adhesion, and synaptogenesis [40]. In contrast to the detrimental effects at nanomolar concentrations, picomolar concentrations of Aβ42 enhances hippocampal long-term potential (LTP) formation by increasing acetylcholine and activation of nicotinic acetylcholine receptors [42,43], which suggests a positive role of Aβ in synaptic plasticity. Infusion of mice with low dose Aβ in hippocampus improved the fear memory [42]. Conversely, APP knockout mice showed impaired LTP and memory [44]. Aβ oligomers can also bind to the glycoproteins of the herpesvirus capsid, resulting in the entrapment of virus particles and protection of the brain from Herpesviridae infection [45]. The biphasic dose-response effects of Aβ might contribute to the failure of Aβ-targeting drugs in clinical trials in treating Alzheimer’s disease.
Developmental stress-response hormesis
During the stress-response hormesis, the initial exposure can occur at an early developmental stage. A classic example is the hygiene hypothesis, in which a lack of early childhood exposure to infectious agents and parasites can suppress the natural development of the immune system and increase susceptibility to allergic diseases [46]. The stress-inoculation hypothesis is a counterpart of the hygiene hypothesis in psychology. It suggests that mild or intermittent stress exposure early in life induces resilience to subsequent stress in adults [47].
The stress-inoculation hypothesis is supported by many animal studies. In one study, male mice were subjected to a variety of stresses manipulations such as maternal separation, early weaning, reduced nesting, isolation, handling, restraining, daily swim stress in early life, and were then tested for depression- or anxiety-like behaviors in adulthood following exposure to chronic social defeat stress. Some of the manipulations mitigated the deleterious consequences of adult stress [48]. Similarly, manipulations of female mice with early handling or limited nesting in early life can make them more resistant to similar aversive conditions in adult, as measured by the anxiety, depression, or sociability behaviors [49]. Rats exposed to physical stress during early-adolescence showed increased anxiety behaviors in adulthood, while mid-adolescence stress paradoxically reduced the anxiety-like behaviors in adulthood. These results suggest that the timing of adolescent adversity is essential to long-term outcomes [50]. In another study, early life stress of reduced bedding in mice led to resistance to social interaction deficits after chronic social defeat stress. It also mitigated acute restraint and tail-shock stress-induced impairments in hippocampal synaptic plasticity, an effect abolished by adrenalectomy [51]. Short-term separation stress in early life altered histone modifications and expression of genes involved in dopamine receptor signaling in the brain, a distinct effect from long-term separation stress, suggesting the potential involvement of the dopamine signaling and epigenetic changes in the underlying mechanisms [52]. Signaling pathways of other neurotransmitters or neuropeptides were also implicated in the process, including AVP and oxytocin [53].
The stress-inoculation theory also has support from human studies. Threat-related amygdala reactivity can be measured using task-based functional magnetic resonance imaging (fMRI) in human and is a biomarker of vulnerability to stress-related depression or anxiety [54]. In a study with adolescents, increased amygdala reactivity to an interpersonal threat was positively associated with better familial affective responsiveness, especially in adolescents reporting lower recent stressful life events [55]. The finding is in line with the studies examining the parenting style and the mental wellbeing of adolescents. Parental overprotection refers to a restrictive or indulgent parenting style when it comes to protecting the child from potential harm or risk. Parental overprotection is positively associated with later psychopathology, including dysfunctional attitudes, depression or anxiety disorders, and suicide intent [56,57]. Conversely, stress inoculation training (SIT) is a type of psychotherapy using intermittent exposure to mild stress to improve patients’ ability to deal with stress. SIT can reduce anxiety and depression for cancer patients and veterans with posttraumatic stress disorder or traumatic brain injury [58,59]. The paradoxical beneficial effect of early-life stress makes sense from an evolutionary perspective, considering that the brain needs stimulation from the environment for proper mental development during the young age. What we assume as a stressor can be actually perceived as a positive stimulation by the brain. Indeed, the brain responds to a variety of stressors in a similar way as to the enriched environment (EE), positive stimuli known to enhance synaptogenesis and intellectual development [60].
The resilience conferred by early-life stress likely represents an adaptation to the altered environment and is often a trade-off between different traits. Exposure of female zebra finches to time-restricted feeding and daily corticosterone injection from young ages reduced breeding performance during early adulthood but led to better breeding performance than the control when birds were bred in old adulthood [61]. Birds exposed to short episodes of mild heat stress in early life had ameliorated oxidative damage in adulthood when given heat stress, as compared to the control group without the early-life priming. Interestingly, birds with the early-life heat stress, but did not experience it again later in life, had a shorter lifespan than any other group [62]. Females blackbirds show decreased breeding success, but increased lifespan with increasing exposure to lead, but not cadmium [63]. These studies suggest that some stress can cause a rebalance of the trade-off between fecundity and longevity.
The trade-off can be either beneficial or harmful, depending on whether the late-life environment matches the early-life stress. If the outcome is harmful, it falls into the ‘Developmental Origins of Health and Disease’ (DOHaD) paradigm. A classic example of the later is the thrifty phenotype hypothesis, in which undernutrition during the fetal or infant stage rebalances the metabolism towards a more thrifty phenotype that favors energy storage, which increases the risk of developing obesity, diabetes, and other metabolic syndromes in later life when the food becomes plenty [64]. Therefore, the developmental stress-response hormesis and the DOHaD paradigm are two sides of the same coin.
Biphasic dose-effect or time-effect of endocrine factors
Parathyroid hormone (PTH)
Many endocrine factors show dose-dependent or time-dependent opposite effects, which falls within the broad definition of hormesis. Here, time not only refers to the total duration of the exposure but also include the temporal pattern. Intermittent or bipolar treatment can produce the opposite effects as chronic continuous treatment. Parathyroid hormone (PTH) causes bone loss at a constantly high level as in chronic hyperparathyroidism. However, intermittent exposure to PTH or its paralogue, at a rate of once per day, increases bone mass [65]. Such intermittent exposure strategy has been approved as a therapy for osteoporosis. The underlying mechanism is not completely understood. PTH promotes two opposite processes: bone formation by osteoblasts and bone reabsorption by osteoclasts, but with seemingly different kinetics [66]. Even within one cell type, PTH can have opposite effects. For example, in osteoblast cells, PTH inhibits apoptosis but also inhibits differentiation [66]. PTH also affects other cell types, including bone lining cells, osteoblast progenitor cells, osteoclast cells, lymphocytes, and macrophages. A variety of intracellular molecular signaling pathways, presumably downstream of the cell membrane receptors for PTH, are being characterized in different cell types upon PTH treatment [66]. However, what seems lacking is a detailed characterization of the kinetics of the cellular phenotypic changes in response to PTH with different temporal patterns. PTH also shows biphasic dose-dependent effects on insulin secretion from pancreatic islets cells. Low-dose PTH stimulates glucose-induced insulin release, while high-dose PTH inhibits it [67]. The PTH level for the maximal stimulatory effects is dependent on the extracellular calcium level. High-dose PTH reduces the intracellular ATP level and increases the resting intracellular calcium level, which contributes to impaired insulin release [68].
Glucocorticoid
Intermittent weekly glucocorticoids treatment can produce the opposite effect as chronic daily glucocorticoids treatment on muscle atrophy. In an acute focal muscle injury model in mice, pulsatile weekly treatment of glucocorticoids reduced injury area, macrophage infiltration, and injury associated fibrosis, which improved muscle performance recovery [69]. However, chronic daily glucocorticoids worsened muscle performance. In mouse models of muscular dystrophy, daily glucocorticoids exacerbated muscle atrophy, while weekly glucocorticoids ameliorated it [69][70]. The expression of several genes in muscle atrophy was upregulated in skeletal muscles upon daily glucocorticoids treatment but was downregulated upon weekly treatment. The effect of glucocorticoids on cognitive functions is also biphasic [71]. Chronic high levels of glucocorticoids are associated with increased risk of cognitive decline and neurodegeneration [72]. However, the acute rise of glucocorticoids improves memory consolidation in multiple models [73]. This positive effect of glucocorticoids on memory consolidation is accompanied by an adverse effect on memory retrieval [74]. Electrophysiological recording assessing long-term potentiation of rat CA1 population showed a positive correlation between low-level glucocorticoids with primed burst potentiation, but a negative correlation at high levels [75]. Thus, dosage, duration, and the temporal pattern collectively determine the outcome of glucocorticoids on cognitive functions by acting on multiple stages of memory processing.
Thyroid hormone
Thyroid hormone is a classic example of biphasic dose-effect, as both hyperthyroidism and hypothyroidism are medical conditions. Thyroid hormone has cardioprotective potentials because it inhibits apoptosis, activates mitochondrial metabolism, decreases fibrosis, and increases neoangiogenesis. Therefore, thyroid hormone replacement therapy could be used for myocardial infarction to induce positive cardiac remodeling. However, hyperthyroidism increases the risk of coronary heart diseases, atrial fibrillation, and pulmonary hypertension. Conversely, hypothyroidism causes left ventricular diastolic dysfunction and increases carotid intima-media thickness [76]. Thyroid hormone increases catabolism and energy expenditure, and therefore, is presumably negatively correlated with metabolic syndromes characterized by overnutrition. However, blood triiodothyronine (T3) and thyroid-stimulating hormone (TSH) levels in euthyroid human subjects are positively associated with metabolic syndromes such as higher body mass index and blood lipid or glucose levels [77,78]. It is speculated that the elevated T3 can be a failed compensatory mechanism aiming to maintain metabolic health under overnutrition [79]. It is also possible that the dose-effect curve of thyroid hormone on each component of the metabolic syndrome is biphasic, and certain unfavorable metabolic parameters can shift the dose-effect curve in a way that favors detection of only one side of the curve.
Adiponectin
Adiponectin has an elusive relationship with mortality, known as ‘adiponectin paradox’ [80]. Adiponectin improves glucose tolerance, reduces inflammation, improves endothelial functions, and inhibits atherosclerosis. However, blood levels of adiponectin, both total adiponectin and the high molecular-weight isoform, are positively associated with mortality across many clinical conditions such as coronary heart disease. One obvious explanation is that the elevated adiponectin is a compensatory response to potential adiponectin resistance in the situation of deteriorated health although this hypothesis has not been rigorously tested. It is also possible that adiponectin has biphasic effects on some of the physiological processes. This possibility is supported by observations that adiponectin may exacerbate inflammation in chronic inflammatory conditions such as colitis, rheumatoid arthritis, or Crohn disease [80]. It is unclear what determines such a switch from anti-inflammatory to pro-inflammatory effects for adiponectin.
Estrogen
Estrogen has biphasic effects on cell proliferation and tumor growth. It stimulates tumor cell proliferation at low doses but promotes cell apoptosis at high doses [81]. In mouse models of prostate cancer and breast cancer, exposure to low-dose 17β-estradiol (E2) led to larger tumors than placebo, while exposure to high-dose E2 led to smaller tumors [81][82]. Mechanistically, low-dose E2 decreases KLF5-dependent pro-apoptotic FOXO1 transcription through ERβ, which inhibits apoptosis and promotes tumor growth. High-dose E2 suppresses the expression of PDGFA and FOXO1, and thereby blocks angiogenesis and suppresses tumor growth [82]. High-dose E2 also activates extrinsic and intrinsic apoptosis pathways [83]. In the cardiovascular system, low-dose E2 activates plasminogen activator in aortic endothelial cells, while high-dose E2 inhibits plasminogen activator [84], which may contribute to the increased risk of myocardial infarction or ischemic stroke in young women who receive higher doses of estrogen as oral contraception [85]. Estrogen also has biphasic effects on bone remodeling. During bone development, both estrogen and androgen stimulates endochondral bone formation at the start of puberty but induce epiphyseal closure at the end of puberty [86]. In treating Turner syndrome, intermittent low-dose estrogen induces maximal ulnar growth, while high-dose estrogen fails to stimulate ulnar growth [87].
Progesterone
Progesterone has biphasic time-effects on estrogen-dependent regulation of memory. Intracranial infusion of progesterone in young ovariectomized mice increased dorsal hippocampal p42 ERK after 5 min but decreased its phosphorylation after 15 min, and intriguingly had no apparent effect after 30 min [88]. Estradiol facilitates extinction recall, an effect potentiated by progesterone if progesterone administration occurred 6 h before extinction training. However, when given 24 h before the extinction training, progesterone abolished the estradiol effect on extinction recall [89]. Similar time-dependent biphasic effects were found for spatial memory in estradiol-treated ovariectomy rats, progesterone augmented the beneficial effect of estradiol on spatial memory when administered 90 min before the test, but reversed estradiol’s effects when administered 24 h before the test [90]. The biphasic effect of progesterone on the immune system is also time-dependent. Longer exposure time with a long-acting progestin formulation was associated with poor innate and adaptive immune responses to genital herpes HSV. In contrast, mice immunized shortly after progesterone treatment were protected from HSV challenge [91].
Growth hormone (GH) and IGF-1
GH shares common ancestry with the insulin/insulin-like growth factor 1 (IGF-1). Epidemiological studies suggest that the relationship of IGF-1/GH levels with healthy aging is biphasic. Both low and high circulating IGF-I levels are associated with increased mortality in the general population [92] or increased cancer mortality in older men [93,94]. Interventional studies also revealed a perplexing relationship between GH/IGF-1 and aging. On one hand, GH administration in some elderly individuals can increase muscle mass, reduce adiposity, and improve bone mineral density, demonstrating anti-aging benefits [95]. On the other hand, mice with deficiency in GH, GH receptor, GH releasing hormone (GHRH), GHRH receptor, IGF-1, IGF-1 receptor, insulin receptor, insulin receptor substrate, or downstream molecules such as mTOR or p70 ribosomal protein S6 kinase 1 (S6K1) all have increased lifespan or healthspan [95–97]. The underlying mechanisms include improved antioxidant defenses, reduced inflammation, reduced insulin levels, reduced cell senescence, altered mitochondrial function and energy metabolism, and enhanced stress resistance [95]. Enhanced insulin sensitivity is particularly interesting, as mice with transgenic overexpression of GH receptor antagonist showed increased adiposity but improved glucose tolerance on a high-fat diet [98]. Humans with genetic deficiencies in the GH/IGF-1 signaling pathway are characterized by proportional short stature, central obesity, delayed puberty, but are generally healthy and protected from aging-related diseases such as cancer, diabetes, and atherosclerosis [95,99]. Thus, the general retardation of growth and reproduction seems to be a sacrifice for the longevity benefits [100]. It may be possible to get the best from both sides through careful timing of the intervention, i.e. reducing the GH/IGF-1 signaling pathway only after mid-life. The trade-off between survival and fecundity, or between two other distinct physiological processes, can potentially explain many paradoxical hormesis phenomena.
Insulin
Insulin is widely used to lower blood glucose levels in diabetes patients. However, suppressing hyperglycemia can improve glucose control and protect against obesity, which implicates a potential biphasic effect of insulin in metabolic disorders. Insulin is encoded by two genes, Ins1 and Ins2, in mice. Female Ins1−/−; Ins2+/− mice showed reduced hyperinsulinemia compared to the control Ins1−/−; Ins2+/+ mice after high-fat diet feeding, which was associated with attenuated weight gain, lower glucose levels, improved insulin sensitivity, and extended lifespan [101][102]. These results suggest that hyperglycemia contributes to insulin resistance in diet-induced obesity. Pharmacological reduction of insulin secretion also lowers body weight in obese people [103]. However, on the leptin-deficient Lepob/ob background, loss of 2 or 3 insulin alleles reduced body fat, but resulted in exacerbated glucose intolerance as compared to the control Ins1+/+; Ins2+/−; Lepob/ob mice [104]. These results suggest that reduced adiposity can be separated from improved glucose control. It also suggests that leptin is required for the effect on glucose control.
Irisin
Irisin is a myokine secreted as a cleaved product of fibronectin type III domain-containing protein 5 (FNDC5) from skeletal muscles in response to physical exercise [105]. The effect of irisin on bone is biphasic. Recombinant irisin, given at a low dose weekly in mice, increased cortical bone mineral density and positively modified bone geometry, upregulated pro-osteoblastic genes in bone marrow, increased the activity of osteoblasts, and reduced osteoclast numbers [106]. Consistently, irisin upregulated sclerostin in osteocyte-like cell line and in mice. However, FNDC5 null mice were resistant to ovariectomy (OVX)-induced trabecular bone loss and displayed a marked reduction in bone resorption [107]. Deficiency of FNDC5 suppressed bone resorption by reducing in osteoclast number and bone erosion, which ameliorated OVX-induced bone loss. Thus, although irisin could be a therapeutic target for osteoporosis, its other effects on bone remodeling should also be considered.
Conclusion
The golden mean principle in philosophy suggests an optimal dose, duration, temporal pattern, or spatial distribution for any exposure for a given effect. Any deviation from this Goldilocks condition, in either direction, results in suboptimal or harmful effects, which generates a biphasic or non-monotonic curve. In this broadest sense, everything can be hormesis. More often, we use ‘hormesis’ to refer to paradoxical low-dose beneficial effects of stressors. It might seem paradoxical because of our preconception regarding what constitutes ‘stress’ or ‘benefit’. It can also be counterintuitive because we are often cognitively biased towards the monotonic cause-effect relationships. For example, we are used to expecting opposite outcomes from gain-of-function and loss-of-function manipulations. We describe many molecular signaling events in a monotonic manner. Looking at biological processes through the lenses of hormesis could help explain or reconcile many paradoxical phenomena, particularly the opposite effects of the same substance, regardless whether it is a xenobiotic or endogenous substance, hormone or metabolite, genetic manipulation or epigenetic alteration, experimental intervention or natural event. Compared to the dose, the temporal pattern and duration of the exposure are underappreciated factors in determining the net outcome. Intermittent exposure often generates opposite effects as compared to continuous exposure.
Our body is highly adaptive. On one hand, exposure to a stressor can induce stress responses that are protective against subsequent exposures in a way that the body is anticipating more stress. On the other hand, constant high-level exposure can increase tolerance to avoid overstressing the system. Such adaptations are essential because the environmental changes can be unpredictable, and the body needs to adjust the trade-off among different functions such as fecundity versus longevity, or energy conservation versus expenditure. The endocrine, nervous, and immune systems are particularly amenable to these adaptations because these systems directly sense the environmental changes and communicate the perceived change to the rest of the body. These adaptations can be so common and successful during evolution that body becomes reliant on certain stress stimulations for ‘training’ purposes during healthy development and homeostasis. Removing the stressors, especially during early life, deprives this training opportunity and can reduce resilience, as exemplified by the hygiene hypothesis and stress-inoculating theory.
Outstanding questions:
When we measure an effect of an exposure, what exactly is the baseline?
What are the molecular and cellular changes, on a fine time-scale, upon an acute exposure to an environmental or endocrine factor?
What caused opposite effects of intermittent (pulsatile) versus continuous (sustained) exposures at the same dosage?
What are the mechanisms, epigenetic or non-epigenetic, underlying resilience induced by early-life stress?
Highlights.
Biphasic dose-effect or time-effect relationship are prevalent for many environmental and endocrine factors
Intermittent (pulsatile) exposure can have opposite effects as continuous (sustained) exposure
Early-life stress can increase resilience and lack of stress can lead to vulnerability
The nervous, endocrine, and immune systems are highly adaptive and responsive to stress
Acknowledgment
We owe apologies to colleagues whose work we failed to cite. We prefer to cite recent original research articles on mammals. We thank Dr. Mitchell Lazar for critical reading of the manuscript and helpful discussions. We thank Thomas Fair for assisting with the table. We thank NIH grants DK111436 and ES027544 for supporting the related work in our lab. We are also thankful for the Cardiovascular Research Institute, Dan L Duncan Comprehensive Cancer Center (P30CA125123), Texas Medical Center Digestive Diseases Center (P30DK056338), and the SPORE program in lymphoma (P50 CA126752) at Baylor College of Medicine, and Gulf Coast Center for Precision Environmental Health (P30ES030285).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaiserman A et al. (2018) Health Impacts of Low-Dose Ionizing Radiation: Current Scientific Debates and Regulatory Issues. Dose-Response 16, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukushima S et al. (2005) Hormesis and dose–response-mediated mechanisms in carcinogenesis: evidence for a threshold in carcinogenicity of non-genotoxic carcinogens. Carcinogenesis 26, 1835–1845 [DOI] [PubMed] [Google Scholar]
- 3.Gems D and Partridge L (2008) Stress-response hormesis and aging: “that which does not kill us makes us stronger.” Cell Metab 7, 200–203 [DOI] [PubMed] [Google Scholar]
- 4.Locher JL et al. (2016) Calorie restriction in overweight older adults: Do benefits exceed potential risks? Exp. Gerontol 86, 4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabrese EJ and Mattson MP (2017) How does hormesis impact biology, toxicology, and medicine? Npj Aging Mech. Dis 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calabrese EJ (2013) Hormetic mechanisms. Crit. Rev. Toxicol 43, 580–606 [DOI] [PubMed] [Google Scholar]
- 7.Omiecinski CJ et al. (2011) Xenobiotic Metabolism, Disposition, and Regulation by Receptors: From Biochemical Phenomenon to Predictors of Major Toxicities. Toxicol. Sci 120, S49–S75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L et al. (2002) Oxidative Stress and Cyclooxygenase-2 Induction Mediate Cyanide-Induced Apoptosis of Cortical Cells. Toxicol. Appl. Pharmacol 185, 55–63 [DOI] [PubMed] [Google Scholar]
- 9.Korde AS et al. (2005) The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J. Neurochem 94, 1676–1684 [DOI] [PubMed] [Google Scholar]
- 10.Mattson MP (2008) Dietary factors, hormesis and health. Ageing Res. Rev 7, 43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pomatto LCD and Davies KJA (2018) Adaptive homeostasis and the free radical theory of ageing. Free Radic. Biol. Med 124, 420–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schieber M and Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr. Biol. CB 24, R453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Raamsdonk JM and Hekimi S (2009) Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet 5, e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W and Hekimi S (2010) A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol 8, e1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virtamo J et al. (2003) Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA 290, 476–485 [DOI] [PubMed] [Google Scholar]
- 16.Hercberg S et al. (2010) Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int. J. Cancer 127, 1875–1881 [DOI] [PubMed] [Google Scholar]
- 17.Lippman SM et al. (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301, 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaar CE et al. (2015) Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS Genet 11, e1004972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scialò F et al. (2016) Mitochondrial ROS Produced via Reverse Electron Transport Extend Animal Lifespan. Cell Metab 23, 725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu S and Chisholm AD (2014) C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev. Cell 31, 48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horn A et al. (2017) Mitochondrial redox signaling enables repair of injured skeletal muscle cells. Sci. Signal 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ristow M and Schmeisser K (2014) Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose-Response Publ. Int. Hormesis Soc 12, 288–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang W et al. (2019) Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun 10, 1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iiyori N et al. (2007) Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am. J. Respir. Crit. Care Med 175, 851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drager LF et al. (2011) Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obes. Silver Spring Md 19, 2167–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milano G et al. (2013) Impact of the phosphatidylinositide 3-kinase signaling pathway on the cardioprotection induced by intermittent hypoxia. PloS One 8, e76659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burtscher M et al. (2004) Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int. J. Cardiol 96, 247–254 [DOI] [PubMed] [Google Scholar]
- 28.del Pilar Valle M et al. (2006) Improvement of myocardial perfusion in coronary patients after intermittent hypobaric hypoxia. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol 13, 69–74 [DOI] [PubMed] [Google Scholar]
- 29.Manukhina EB et al. (2006) Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp. Biol. Med. Maywood NJ 231, 343–365 [DOI] [PubMed] [Google Scholar]
- 30.Heyman SN et al. (1999) Tissue oxygenation modifies nitric oxide bioavailability. Microcirc. N. Y. N 1994 6, 199–203 [PubMed] [Google Scholar]
- 31.Melillo G et al. (1995) A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J. Exp. Med 182, 1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manukhina EB et al. (2011) Intermittent hypoxia conditioning prevents endothelial dysfunction and improves nitric oxide storage in spontaneously hypertensive rats. Exp. Biol. Med. Maywood NJ 236, 867–873 [DOI] [PubMed] [Google Scholar]
- 33.Trzepizur W et al. (2015) Vascular and hepatic impact of short-term intermittent hypoxia in a mouse model of metabolic syndrome. PloS One 10, e0124637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burney S et al. (1997) A mechanistic analysis of nitric oxide-induced cellular toxicity. Nitric Oxide Biol. Chem 1, 130–144 [DOI] [PubMed] [Google Scholar]
- 35.Ghasemi M et al. (2018) Nitric Oxide and Mitochondrial Function in Neurological Diseases. Neuroscience 376, 48–71 [DOI] [PubMed] [Google Scholar]
- 36.Raju K et al. (2015) Regulation of brain glutamate metabolism by nitric oxide and S-nitrosylation. Sci. Signal 8, ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardingham N et al. (2013) The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front. Cell. Neurosci 7, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghimire K et al. (2017) Nitric oxide: what’s new to NO? Am. J. Physiol. Cell Physiol 312, C254–C262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selkoe DJ and Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med 8, 595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawkins E and Small DH (2014) Insights into the physiological function of the β-amyloid precursor protein: beyond Alzheimer’s disease. J. Neurochem 129, 756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puzzo D et al. (2012) Hormetic effect of amyloid-β peptide in synaptic plasticity and memory. Neurobiol. Aging 33, 1484.e15–24. [DOI] [PubMed] [Google Scholar]
- 42.Puzzo D et al. (2008) Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. Off. J. Soc. Neurosci 28, 14537–14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morley JE et al. (2010) A physiological role for amyloid-beta protein:enhancement of learning and memory. J. Alzheimers Dis. JAD 19, 441–449 [DOI] [PubMed] [Google Scholar]
- 44.Palmeri A et al. (2017) Amyloid-β Peptide Is Needed for cGMP-Induced Long-Term Potentiation and Memory. J. Neurosci. Off. J. Soc. Neurosci 37, 6926–6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eimer WA et al. (2018) Alzheimer’s Disease-Associated β-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection. Neuron 99, 56–63.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Mutius E (2007) Allergies, infections and the hygiene hypothesis--the epidemiological evidence. Immunobiology 212, 433–439 [DOI] [PubMed] [Google Scholar]
- 47.Romeo RD (2015) Perspectives on stress resilience and adolescent neurobehavioral function. Neurobiol. Stress 1, 128–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peña CJ et al. (2019) Environmental Programming of Susceptibility and Resilience to Stress in Adulthood in Male Mice. Front. Behav. Neurosci 13, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santarelli S et al. (2014) Evidence supporting the match/mismatch hypothesis of psychiatric disorders. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol 24, 907–918 [DOI] [PubMed] [Google Scholar]
- 50.Wilkin MM et al. (2012) Intermittent physical stress during early- and mid-adolescence differentially alters rats’ anxiety- and depression-like behaviors in adulthood. Behav. Neurosci 126, 344–360 [DOI] [PubMed] [Google Scholar]
- 51.Hsiao Y-M et al. (2016) Early life stress dampens stress responsiveness in adolescence: Evaluation of neuroendocrine reactivity and coping behavior. Psychoneuroendocrinology 67, 86–99 [DOI] [PubMed] [Google Scholar]
- 52.Köhler JC et al. (2019) Early-Life Adversity Induces Epigenetically Regulated Changes in Hippocampal Dopaminergic Molecular Pathways. Mol. Neurobiol 56, 3616–3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry-Paldi A et al. (2019) Early Environments Shape Neuropeptide Function: The Case of Oxytocin and Vasopressin. Front. Psychol 10, 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swartz JR et al. (2015) A neural biomarker of psychological vulnerability to future life stress. Neuron 85, 505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farber MJ et al. (2019) Paradoxical associations between familial affective responsiveness, stress, and amygdala reactivity. Emot. Wash. DC 19, 645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim HH-S (2019) Parental overprotection and youth suicide behavior in low- and middle-income countries: a multilevel analysis of cross-national data. Int. J. Public Health 64, 173–184 [DOI] [PubMed] [Google Scholar]
- 57.Otani K et al. (2013) Parental overprotection engenders dysfunctional attitudes about achievement and dependency in a gender-specific manner. BMC Psychiatry 13, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kashani F et al. (2015) Effect of stress inoculation training on the levels of stress, anxiety, and depression in cancer patients. Iran. J. Nurs. Midwifery Res 20, 359–364 [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson S et al. (2019) Stress inoculation training outcomes among veterans with PTSD and TBI. Psychol. Trauma Theory Res. Pract. Policy DOI: 10.1037/tra0000432 [DOI] [PubMed] [Google Scholar]
- 60.Crofton EJ et al. (2015) Inoculation stress hypothesis of environmental enrichment. Neurosci. Biobehav. Rev 49, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marasco V et al. (2018) Environmental conditions shape the temporal pattern of investment in reproduction and survival. Proc. Biol. Sci 285, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monaghan P and Haussmann MF (2015) The positive and negative consequences of stressors during early life. Early Hum. Dev 91, 643–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fritsch C et al. (2019) Exposure to Pb impairs breeding success and is associated with longer lifespan in urban European blackbirds. Sci. Rep 9, 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wells JCK (2011) The thrifty phenotype: An adaptation in growth or metabolism? Am. J. Hum. Biol 23, 65–75 [DOI] [PubMed] [Google Scholar]
- 65.Miller PD et al. (2016) Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA 316, 722–733 [DOI] [PubMed] [Google Scholar]
- 66.Wein MN and Kronenberg HM (2018) Regulation of Bone Remodeling by Parathyroid Hormone. Cold Spring Harb. Perspect. Med 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fadda GZ et al. (1990) Direct effect of parathyroid hormone on insulin secretion from pancreatic islets. Am. J. Physiol 258, E975–984 [DOI] [PubMed] [Google Scholar]
- 68.Perna AF et al. (1990) Mechanisms of impaired insulin secretion after chronic excess of parathyroid hormone. Am. J. Physiol 259, F210–216 [DOI] [PubMed] [Google Scholar]
- 69.Quattrocelli M et al. (2017) Intermittent glucocorticoid steroid dosing enhances muscle repair without eliciting muscle atrophy. J. Clin. Invest 127, 2418–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quattrocelli M et al. (2017) Intermittent Glucocorticoid Dosing Improves Muscle Repair and Function in Mice with Limb-Girdle Muscular Dystrophy. Am. J. Pathol 187, 2520–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lupien SJ et al. (2007) The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn 65, 209–237 [DOI] [PubMed] [Google Scholar]
- 72.Ouanes S and Popp J (2019) High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci 11, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meir Drexler S and Wolf OT (2017) The role of glucocorticoids in emotional memory reconsolidation. Neurobiol. Learn. Mem 142, 126–134 [DOI] [PubMed] [Google Scholar]
- 74.de Quervain D et al. (2017) Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat. Rev. Neurosci 18, 7–19 [DOI] [PubMed] [Google Scholar]
- 75.Diamond DM et al. (1992) Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus 2, 421–430 [DOI] [PubMed] [Google Scholar]
- 76.Jabbar A et al. (2017) Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol 14, 39–55 [DOI] [PubMed] [Google Scholar]
- 77.Kim HJ et al. (2016) Triiodothyronine Levels Are Independently Associated with Metabolic Syndrome in Euthyroid Middle-Aged Subjects. Endocrinol. Metab. Seoul Korea 31, 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou Y-C et al. (2018) Exploring the association between thyroid- stimulating hormone and metabolic syndrome: A large population-based study. PloS One 13, e0199209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jun JE et al. (2017) Hormetic effect of triiodothyronine in metabolically healthy obese persons. Endocrine 57, 418–427 [DOI] [PubMed] [Google Scholar]
- 80.Menzaghi C and Trischitta V (2018) The Adiponectin Paradox for All-Cause and Cardiovascular Mortality. Diabetes 67, 12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakajima Y et al. (2016) Estrogen Exhibits a Biphasic Effect on Prostate Tumor Growth through the Estrogen Receptor β-KLF5 Pathway. Mol. Cell. Biol 36, 144–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shim WS et al. (2000) Estradiol hypersensitivity and mitogen-activated protein kinase expression in long-term estrogen deprived human breast cancer cells in vivo. Endocrinology 141, 396–405 [DOI] [PubMed] [Google Scholar]
- 83.Lewis-Wambi JS and Jordan VC (2009) Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. BCR 11, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strom JO et al. (2011) Hormesis and Female Sex Hormones. Pharm. Basel Switz 4, 726–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roach REJ et al. (2015) Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst. Rev DOI: 10.1002/14651858.CD011054.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vanderschueren D et al. (2004) Androgens and bone. Endocr. Rev 25, 389–425 [DOI] [PubMed] [Google Scholar]
- 87.Juul A (2001) The effects of oestrogens on linear bone growth. Hum. Reprod. Update 7, 303–313 [DOI] [PubMed] [Google Scholar]
- 88.Orr PT et al. (2012) The progesterone-induced enhancement of object recognition memory consolidation involves activation of the extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) pathways in the dorsal hippocampus. Horm. Behav 61, 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graham BM and Daher M (2016) Estradiol and Progesterone have Opposing Roles in the Regulation of Fear Extinction in Female Rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 41, 774–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sandstrom NJ and Williams CL (2001) Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci 115, 384–393 [PubMed] [Google Scholar]
- 91.Gillgrass AE et al. (2003) Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J. Virol 77, 9845–9851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burgers AMG et al. (2011) Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J. Clin. Endocrinol. Metab 96, 2912–2920 [DOI] [PubMed] [Google Scholar]
- 93.Svensson J et al. (2012) Both low and high serum IGF-I levels associate with cancer mortality in older men. J. Clin. Endocrinol. Metab 97, 4623–4630 [DOI] [PubMed] [Google Scholar]
- 94.Vitale G et al. (2017) GH/IGF-I/insulin system in centenarians. Mech. Ageing Dev 165, 107–114 [DOI] [PubMed] [Google Scholar]
- 95.Bartke A and Darcy J (2017) GH and ageing: Pitfalls and new insights. Best Pract. Res. Clin. Endocrinol. Metab 31, 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim S-S and Lee C-K (2019) Growth signaling and longevity in mouse models. BMB Rep 52, 70–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fontana L and Partridge L (2015) Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang T et al. (2015) Growth hormone receptor antagonist transgenic mice are protected from hyperinsulinemia and glucose intolerance despite obesity when placed on a HF diet. Endocrinology 156, 555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aguiar-Oliveira MH and Bartke A (2019) Growth Hormone Deficiency: Health and Longevity. Endocr. Rev 40, 575–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milman S et al. (2016) The Somatotropic Axis in Human Aging: Framework for the Current State of Knowledge and Future Research. Cell Metab 23, 980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Templeman NM et al. (2015) Suppression of hyperinsulinaemia in growing female mice provides long-term protection against obesity. Diabetologia 58, 2392–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Templeman NM et al. (2017) Reduced Circulating Insulin Enhances Insulin Sensitivity in Old Mice and Extends Lifespan. Cell Rep 20, 451–463 [DOI] [PubMed] [Google Scholar]
- 103.Kolb H et al. (2018) Insulin translates unfavourable lifestyle into obesity. BMC Med 16, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.D’souza AM et al. (2016) Suppressing hyperinsulinemia prevents obesity but causes rapid onset of diabetes in leptin-deficient Lepob/ob mice. Mol. Metab 5, 1103–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grygiel-Górniak B and Puszczewicz M (2017) A review on irisin, a new protagonist that mediates muscle-adipose-bone-neuron connectivity. Eur. Rev. Med. Pharmacol. Sci 21, 4687–4693 [PubMed] [Google Scholar]
- 106.Colaianni G et al. (2015) The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. U. S. A 112, 12157–12162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim H et al. (2018) Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 175, 1756–1768.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Quik M (2004) Smoking, nicotine and Parkinson’s disease. Trends Neurosci 27, 561–568 [DOI] [PubMed] [Google Scholar]
- 109.Chen H et al. (2010) Smoking duration, intensity, and risk of Parkinson disease. Neurology 74, 878–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Quik M et al. (2012) Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc 27, 947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Calabrese EJ and Rubio‐Casillas A (2018) Biphasic effects of THC in memory and cognition. Eur. J. Clin. Invest 48, e12920. [DOI] [PubMed] [Google Scholar]
- 112.Goel S et al. (2018) Effect of Alcohol Consumption on Cardiovascular Health. Curr. Cardiol. Rep 20, 19. [DOI] [PubMed] [Google Scholar]
- 113.Krenz M and Korthuis RJ (2012) Moderate Ethanol Ingestion and Cardiovascular Protection. J. Mol. Cell. Cardiol 52, 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gutwinski S et al. (2018) Drink and Think: Impact of Alcohol on Cognitive Functions and Dementia - Evidence of Dose-Related Effects. Pharmacopsychiatry 51, 136–143 [DOI] [PubMed] [Google Scholar]
- 115.Topiwala A and Ebmeier KP (2018) Effects of drinking on late-life brain and cognition. Evid. Based Ment. Health 21, 12–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Elenkov IJ and Chrousos GP (2002) Stress Hormones, Proinflammatory and Antiinflammatory Cytokines, and Autoimmunity. Ann. N. Y. Acad. Sci 966, 290–303 [DOI] [PubMed] [Google Scholar]
- 117.Neural regulation of endocrine and autonomic stress responses | Nature Reviews Neuroscience. . [Online] Available: https://www.nature.com/articles/nrn2647. [Accessed: 30-Jun-2019] [DOI] [PMC free article] [PubMed]
- 118.Clark A and Mach N (2016) Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J. Int. Soc. Sports Nutr 13, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dimitrov S et al. (2017) Inflammation and exercise: Inhibition of monocytic intracellular TNF production by acute exercise via β2-adrenergic activation. Brain. Behav. Immun 61, 60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Teixeira de Lemos E et al. (2011) Differential effects of acute (extenuating) and chronic (training) exercise on inflammation and oxidative stress status in an animal model of type 2 diabetes mellitus. Mediators Inflamm 2011, 253061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martín-Cordero L et al. (2011) The interleukin-6 and noradrenaline mediated inflammation-stress feedback mechanism is dysregulated in metabolic syndrome: effect of exercise. Cardiovasc. Diabetol 10, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wojdasiewicz P et al. (2014) The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014, 561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Michailidis Y et al. (2013) Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am. J. Clin. Nutr 98, 233–245 [DOI] [PubMed] [Google Scholar]
- 124.Peake JM et al. (2015) Modulating exercise-induced hormesis: Does less equal more? J. Appl. Physiol. Bethesda Md 1985 119, 172–189 [DOI] [PubMed] [Google Scholar]
- 125.Peake JM et al. (2017) Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. Bethesda Md 1985 122, 559–570 [DOI] [PubMed] [Google Scholar]
- 126.Shih P-C et al. (2013) Effects of exercise intensity on spatial memory performance and hippocampal synaptic plasticity in transient brain ischemic rats. PloS One 8, e78163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee SR et al. (2015) Influence of starvation on heart contractility and corticosterone level in rats. Pflugers Arch 467, 2351–2360 [DOI] [PubMed] [Google Scholar]
- 128.Wang Z et al. (2018) Role of pyroptosis in normal cardiac response to calorie restriction and starvation. Biochem. Biophys. Res. Commun 495, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 129.Chaix A et al. (2019) Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab 29, 303–319.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim K-H et al. (2017) Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res 27, 1309–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu Z et al. (2017) Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat. Med 23, 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Di Biase S et al. (2016) Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 30, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buono R and Longo VD (2018) Starvation, Stress Resistance, and Cancer. Trends Endocrinol. Metab. TEM 29, 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]