Abstract
Prothrombin time (PT) is a measure of coagulation status and was assessed in the majority of patients in the rivaroxaban phase II and III clinical trials as a pharmacodynamic marker. In the absence of sufficient phase III pharmacokinetic (PK) data to provide individual exposure measures for input into rivaroxaban exposure–response analyses, the aim of the present study was to investigate the use of PT‐adjustment approaches (i.e., the use of observed individual PT measurements) to enhance the prediction of individual rivaroxaban exposure metrics (derived using a previously developed integrated population PK model) based on the observed linear relationship between PT and rivaroxaban plasma concentrations. The PT‐adjustment approaches were established using time‐matched PK and PT measurements, which were available from 1,779 patients across four phase II trials and one phase III trial of rivaroxaban. PT‐adjusted exposure estimates improved the identification of statistically significant effects when compared with covariate‐only exposure estimates.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The pharmacokinetics (PK) of rivaroxaban were demonstrated to be predictable by an integrated population PK model across indications. Prothrombin time (PT), measured systematically across the rivaroxaban clinical development program, was found to be strongly associated with rivaroxaban concentration in plasma.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Can the use of PT‐adjustment approaches (i.e., observed individual PT measurements from the phase III studies) enhance the quality of model‐predicted individual rivaroxaban exposure metrics in the absence of PK measurements?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ A PT‐adjustment procedure has been established that, in the absence of PK data, considerably enhances the quality of model‐predicted rivaroxaban exposure metrics based on PT measurements.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
☑ These enhanced rivaroxaban exposure estimates will provide a basis for exposure–response analyses assessing clinical outcomes in large phase III studies to further examine the benefit–risk balance of rivaroxaban.
Rivaroxaban is a direct factor Xa inhibitor and non‐vitamin K antagonist oral anticoagulant that is approved for the prevention or treatment of several thromboembolic disorders.1, 2 In the United States, rivaroxaban is approved for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE) in patients undergoing knee or hip replacement surgery.2 It is also indicated for the treatment of DVT, for the treatment of PE, and in patients at continued risk of recurrent DVT and/or PE after initial treatment lasting ≥6 months, for the reduction in the risk of these events.2 In addition, rivaroxaban is indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF).2 Rivaroxaban is authorized for similar clinical use in adults in Europe, where it is also indicated for the prevention of atherothrombotic events in adults after acute coronary syndrome (ACS) with elevated biomarkers in combination with aspirin alone or with aspirin plus clopidogrel or ticlopidine.1 Recently, rivaroxaban coadministered with aspirin was also approved in Europe for the prevention of atherothrombotic events in adult patients with coronary artery disease or symptomatic peripheral artery disease at high risk of ischemic events1 and in the United States to reduce the risk of major cardiovascular events (cardiovascular death, myocardial infarction, and stroke) in patients with chronic coronary artery disease or peripheral artery disease.2
In 2015, the European Medicines Agency's (EMA's) Committee for Medicinal Products for Human Use requested additional analyses of the relationships between rivaroxaban dose and exposure, pharmacodynamic markers, and efficacy and safety outcomes for each of the licensed indications. It was agreed with the EMA that the requested exposure–response (ER) analyses would be based on the large phase III trials for the approved indications. Accurate rivaroxaban exposure estimates are essential to enable large‐scale ER analyses to be performed across indications. However, pharmacokinetic (PK) data were not collected for the majority of patients in phase III studies of rivaroxaban, except for a small subset of patients enrolled in Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) (N = 161).3
Previously, an integrated population PK (popPK) model including a covariate analysis was developed to predict rivaroxaban exposure across indications based on dose and patient characteristics.4 The model used pooled data from 4,918 patients in seven phase II and III clinical trials and was found to provide reliable rivaroxaban exposure estimates across all approved indications.4 Patient characteristics examined in the popPK model, including creatinine clearance, age, and body weight, were identified as sources of interindividual variability (IIV) in rivaroxaban exposure.4 However, as is usual, a considerable degree of variability remained unexplained after adjustment for these characteristics in the popPK model (IIV for the absorption rate constant, clearance, and volume of distribution were 93.5%, 42.6%, and 20.0% coefficient of variation (CV), respectively).4 Therefore, using patient characteristics alone to predict individual PK might not fully reflect the variability expected. The resulting underestimation of the variability may then reduce the probability of successfully establishing ER relationships.
Prothrombin time (PT) is a measure of coagulation status and was assessed in the majority of patients in the rivaroxaban phase II and III clinical trials as a pharmacodynamic marker for rivaroxaban. There is a linear correlation between rivaroxaban plasma concentrations and the PT when the PT is measured with a thromboplastin reagent that is sensitive to the anticoagulant effects of rivaroxaban,5 both in healthy volunteers6 and in patients receiving rivaroxaban for different indications.7, 8, 9, 10 A simple linear relationship between rivaroxaban plasma concentrations and PT has been reported at therapeutic rivaroxaban doses of 5–20 mg, which begins to plateau at supratherapeutic doses. This effect may be because of the high International Sensitivity Index value of the PT reagent used in some rivaroxaban studies (e.g., the phase II Oral DIrect FactorXa inhibitor BAY 59‐7939 in the prevention of VTE in patients undergoing total KNEE replacement (ODIXa‐KNEE) study), but is not thought to be relevant to clinical practice.7 In the absence of sufficient phase III PK data to provide individual exposure measures for input into ER analyses, the aim of the present study was to investigate the use of observed individual PT measurements to enhance the prediction of individual covariate‐only exposure metrics in patients in the phase III rivaroxaban trials.
Methods
Studies included in the current analysis
For each study included in the current analysis, the protocol received ethics committee or institutional review board approval. Written informed consent was obtained from the study participants.
Studies included in the development of the popPK model have been described previously.4 The PT‐adjustment functions described here were established using PK and PT measurements from four phase II trials Oral Direct Factor Xa Inhibitor BAY 59‐7939 in the Prevention of Venous Thromboembolism in Patients Undergoing Total Hip Replacement 2 (ODIXa‐HIP2) (NCT00398905),11 ODIXa‐KNEE (NCT00402467),12 Oral Direct Factor Xa Inhibitor BAY 59‐7939 in Patients With Acute Symptomatic Deep‐Vein Thrombosis (ODIXa‐DVT) (NCT00839163),13 and Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Deep Vein Thrombosis (EINSTEIN‐DVT) Dose‐Ranging Study (NCT00395772)),14 plus one phase III clinical trial of rivaroxaban (ROCKET AF (NCT00403767))15 (Table 1).
Table 1.
Description of studies used in the derivation of the PT‐adjustment functions and description of studies for which the PT‐adjustment function was applied
| Description of studies used in the derivation of the PT‐adjustment function | |||||
|---|---|---|---|---|---|
| Indication | Prevention of VTE in patients undergoing hip or knee replacement surgery | Treatment of VTE | AF | ||
| Study | ODIXa‐HIP2 | ODIXa‐KNEE | ODIXa‐DVT | EINSTEIN‐DVT | ROCKET AF |
| Study number | 10944 | 10945 | 11223 | 11528 | AFL3001 |
| ClinicalTrials.gov identifier | NCT00398905 | NCT00402467 | NCT00839163 | NCT00395772 | NCT00403767 |
| Phase | II | II | II | II | III |
| N (n with valid PK and PT samples) | 553 (508) | 501 (322) | 470 (437) | 400 (351) | 161 (161) |
| Number of PK samples per patient, median (range) | 4 (1–10) | 4 (1–10) | 8 (1–9) | 3 (1–5) | 5 (2–15) |
| Number of PT samples per patient, median (range) | 7 (2–12) | 6 (1–10) | 9 (3–9) | 4 (1–5) | 7 (3–18) |
| Dose (mg) and regimen | 2.5, 5, 10, 20, 30 b.d. Dose‐ranging | 2.5, 5, 10, 20, 30 b.d. | 10, 20, 30 b.d. 40 o.d. | 20, 30, 40 o.d. Dose‐ranging | 20 (15 in patients with CrCl 30–49 mL/min) o.d. |
| Treatment duration | 9 ± 2 days | 8 ± 2 days | 12 weeks | 12 weeks | Median 590 days |
| Description of studies for which the PT adjustment was applied | ||||||
|---|---|---|---|---|---|---|
| Indication | Prevention of VTE in patients undergoing hip or knee replacement surgery | Treatment of VTE | AF | |||
| Study | RECORD1 | RECORD2 | RECORD3 | RECORD4 | EINSTEIN‐DVT/PE | ROCKET AF |
| Study number | 11354 | 11357 | 11356 | 11355 | 11702 | AFL3001 |
| ClinicalTrials.gov identifier(s) | NCT00329628 | NCT00332020 | NCT00361894 | NCT00362232 | NCT00440193/NCT00439777 | NCT00403767 |
| Phase | III | III | III | III | III | III |
| Number of patients for ER analysis | 2,183 | 1,197 | 1,191 | 1,526 | 4,130 | 7,111 |
| Number of patients with PT adjustment | 2,059a | 1,051a | 1,081a | 1,102a | 3,582b | 5,681c |
| Number of PT samples per patient without baseline samples, median (range) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–5) | 2 (1–3)d |
| Dose (mg) and regimen, and treatment duration | 10 o.d. 35 ± 4 days | 10 o.d. 35 ± 4 days | 10 o.d. 12 ± 2 days | 10 o.d. 12 ± 2 days | 15 b.d. for 3 weeks followed by 20 o.d. for 3, 6, or 12 months | 20 o.d. (15 o.d. for patients with moderate renal impairment); median 590 days |
AF, atrial fibrillation; b.d., twice daily; CrCl, creatinine clearance; DVT, deep vein thrombosis; ER, exposure–response; o.d., once a day; PE, pulmonary embolism; PK, pharmacokinetic; PT, prothrombin time; VKA, vitamin K antagonist; VTE, venous thromboembolism.
aAcross studies of patients undergoing hip or knee replacement surgery, data from 5,293 patients were used for PT adjustment. In 24 cases, a missing covariate value was imputed. PT measurements under the influence of VKA were excluded, as were PT measurements <72 hours postsurgery. In this early postsurgery phase, neither PK nor PT data reflect a steady‐state PK/PT relationship. A median of two samples per participant were available. bIn 30 cases, a missing covariate value was imputed. For eight patients, no information on comedication intake was available for the 20 mg o.d. phase; these patients were treated as nonusers in this phase. PT measurements under the influence of VKA were excluded, as were PT measurements in the initial 3‐week 15 mg b.d. treatment period. PT measurements in the first 3 days, after the switch from 15 mg b.d. to 20 mg o.d. treatment, were also excluded because they cannot be assumed to reflect a 20 mg o.d. steady‐state PK model. A median of two samples per participant were available. cIncludes the subset of patients with PT measurements (including the 161 patients from the PK subgroup). In 13 cases, a missing covariate value was imputed. A median of two samples per participant were available. dFor patients not in the PK subset.
We define PT‐adjustment functions as equations that relate a steady‐state exposure metric (area under the plasma concentration–time curve from 0 to 24 hours (AUC0–24), maximum plasma concentration (Cmax), or trough plasma concentration at the end of the dosing interval before the next dose (Ctrough) to observed PT to enhance individual PK predictions.
The developed PT‐adjustment functions were applied to a series of phase III studies of rivaroxaban in which PT data were available, but PK measurements were either not available at all or available for only a small subset of patients Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism (RECORD1) (NCT00329628),16 RECORD2 (NCT00332020),17 RECORD3 (NCT00361894),18 RECORD4 (NCT00362232),19 EINSTEIN‐DVT (NCT00440193),20 Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Pulmonary Embolism (EINSTEIN‐PE) (NCT00439777),21 and ROCKET AF (NCT00403767))15 (Table 1). PT was not measured in the global phase III study in patients with ACS Anti‐Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction (ATLAS ACS 2‐TIMI) 51 (ACS 3001, NCT00809965)),22 which compared the use of antiplatelet therapies (aspirin with or without a thienopyridine) with rivaroxaban or placebo.
PT data
All PT measures used in the derivation of the PT‐adjustment functions were determined in central laboratories with a rivaroxaban‐sensitive thromboplastin assay (STA Neoplastine or STA Neoplastine CI Plus (Diagnostica Stago, Asnières‐sur‐Seine, France)) as described previously.3, 5, 7, 8 Using these assays, PT values of approximately 10–40 seconds were reported in healthy volunteers receiving rivaroxaban and in patients receiving rivaroxaban for the prevention or treatment of venous thromboembolism (VTE).6, 7, 8
All PT measurements were used in the assessment of the PK/PT relationship for time‐matched PK and PT samples. Subsequently, strict rules were applied to ensure the reliability of PT values included in the development and application of the PT‐adjustment functions. Specifically, PT measurements >50 seconds were considered clinically unrealistic and were excluded for all indications. In addition, PT measurements observed >30 hours after the last rivaroxaban dose were considered uninformative and unlikely to be related to rivaroxaban exposure given the half‐life of rivaroxaban (5–13 hours).1 For studies conducted in patients undergoing hip or knee replacement surgeries, PT measurements <72 hours after surgery were excluded owing to a potential influence of the surgical procedure. For studies conducted in patients receiving rivaroxaban for the treatment of VTE (including the prevention of recurrent VTE), PT measurements in the first 3 weeks were excluded owing to the state of disease (thrombus burden, clot formation) and potential related effects.
Derivation of the PT‐adjustment functions
Two approaches, denoted the “η” and “PT covariate” approaches, have been established based on data from patients with PK and PT observations (PK–PT data set) and tested for the ability to enhance the covariate‐only exposure predictions using PT data (Figure 1). Individual exposure estimates were derived using the integrated popPK model based on all available covariate information and individual PK samples (denoted as “observed exposure” estimates) for the PK–PT data set. Expected exposure metric estimates were then derived based only on dose and covariates, without consideration of the available individual PK data (covariate‐only exposure estimates). The prediction‐corrected exposure was defined as the ratio of observed/covariate‐only exposure.
Figure 1.
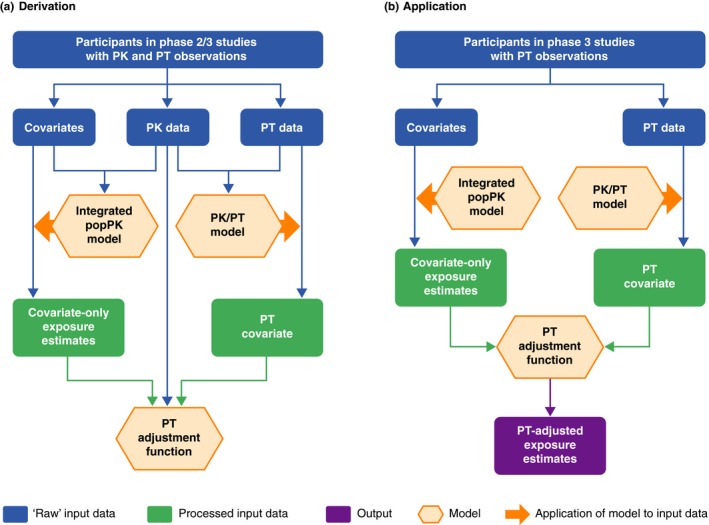
Overview of the (a) derivation and (b) application of the PT‐adjustment functions. PK, pharmacokinetic; popPK, population pharmacokinetic; PT, prothrombin time.
The η approach was similar to the popPK approach used in the presence of PK sampling.4 Using the previously developed popPK model4 and the linear relationship between measured PT and PK samples (Figures S1 and S2 ),6, 9 PK estimates were derived based on measured PT values only. These post hoc PK estimates were used to derive the steady‐state exposure metrics of interest.
The alternative PT covariate approach (Figure 1 a) aggregated all measured PT values from each patient into one value. This covariate was tested for its correlation with the observed exposure.
To this aim, in a first step, the PT data were prediction corrected by using patient covariate information combined with the popPK model and the linear PK/PT model (expected PT values). A PT covariate value was subsequently derived for each patient by calculating the geometric mean of the prediction‐corrected PT values. The PT covariate can be interpreted as a covariate containing the aggregated prediction‐corrected (in terms of influence of PK covariates and dose) PT information for an individual patient. The relationship between the prediction‐corrected PT and prediction‐corrected exposure metrics (AUC0–24, Cmax, and Ctrough) was investigated, and adjustment functions were established using regression analyses to translate the PT covariate into an exposure adjustment factor (dependent variable = prediction‐corrected exposure; independent variable = PT covariate).
The prediction‐corrected exposure was used for the qualification of both the η and PT covariate approaches because it was already corrected for the influence of different doses and treatment regimens (e.g., depending on indication) and the identified PK covariates. Thus, the extent to which each approach could account for the remaining unexplained IIV in exposure could be assessed. PT adjustment was considered useful if there was a positive correlation between the predicted and observed prediction‐corrected exposure metrics. In addition, both approaches were compared graphically and quantitatively by considering bias (observed minus predicted) for prediction‐corrected AUC0–24, Cmax, and Ctrough. The distribution of bias in the analysis population was described using mean, SD, 5th to 95th percentiles, and boxplots. The approach showing the strongest correlation between predicted and observed exposures (indicated by R 2) and least bias was considered superior.
Linear functions for the PK/PT relationship and PT adjustment were derived using NONMEM (version 7.3; ICON Development Solutions, San Antonio, TX) on Windows Server 2012 R2 (Microsoft, Redmond, WA).
Application of the PT‐adjustment functions
An overview of the application of the PT‐adjustment functions is shown in Figure 1 b. The PT‐adjustment functions were applied to adjust the covariate‐only exposure estimates for patients who received rivaroxaban in phase III studies and had valid PT, but not PK, assessments (Table 1).
Covariate information, including demographic characteristics and use of relevant comedications, was considered for the included patients, as reported previously for the integrated popPK model.4 Age and sex were known for each of these patients. In the case of a missing covariate, this value was imputed using the median value of the respective age and sex group of the relevant study. For patients taking comedications, constant comedication factors for the study period were assumed and derived as described previously.4 No time‐changing covariates were considered in the popPK model because a constant dose was assumed and exposure metrics were time invariant.4
PT adjustment was performed under a number of assumptions. First, the observed PT values in all phase III studies were assumed to be measured at steady state. Second, the steady‐state PK/PT relationship was assumed to be identical in all studies. Third, the PT‐adjustment functions were assumed to be identical for all studies.
Assessment of the use of PT‐adjusted exposure estimates in clinical trial simulations based on phase III data for the treatment of VTE
Clinical trial simulations were conducted to assess whether the use of PT‐adjusted exposure estimates improved the likelihood of identifying ER relationships when compared with the use of covariate‐only exposure estimates. The observed exposure from the phase II study populations as well as the respective PT‐adjusted (using the most adequate PT‐adjustment approach) and covariate‐only exposure estimates were used to generate a virtual population identical to that in the EINSTEIN‐DVT/PE phase III trials (N = 4,130). A relationship between Ctrough and response was assumed. The multiplicative factor of the effect size of the Ctrough–efficacy relationship varied from 0–3, with 0 representing no exposure/Ctrough effect and 3 representing a very strong relationship between Ctrough and response. For each multiplicative factor change in the effect size of the Ctrough–efficacy relationship (0, 1, 2, and 3), 1,000 studies were simulated. Based on these studies, the rate at which the effect was correctly identified as being statistically significant (the true positive rate) was determined using the observed exposure estimate for Ctrough (derived using the popPK model), the PT‐adjusted Ctrough, or the covariate‐only Ctrough.
Results
Derivation and comparison of the PT‐adjustment approaches
Data from 1,779 patients were used in the derivation of the PT‐adjustment function, including 831 patients undergoing hip or knee replacement surgery (median of four PT samples per patient), 788 patients receiving treatment for VTE (median of three PT samples per patient), and 161 patients with AF (median of seven PT samples per patient).
A linear model that relates observed rivaroxaban plasma concentration (CP) to the corresponding PT value was used in the initial development of the η and PT covariate approaches as follows: PT = BASE + SLOPE·CP, where BASE is the estimated baseline PT value for a patient before rivaroxaban was given, and SLOPE denotes the change in PT (in seconds) with increasing rivaroxaban concentration (in micrograms per liter). The estimated BASE PT value was 13.1 seconds (with an estimated IIV of 0.006 (SD = 0.0757, CV = 7.76%)), and the SLOPE value was 0.031 seconds/μg/L (with an estimated IIV of 0.136 (SD = 0.369, CV = 38.2%)).
Using the η approach, individual PK estimates (e.g., for parameters such as absorption rate constant, clearance, and volume) could be determined post hoc for all patients.
For AUC0–24, Cmax, and Ctrough, the PT covariate (geometric mean of prediction‐corrected PT) was a statistically significant (P < 0.001) predictor of individual prediction‐corrected exposure (Table 2; Figure 2 a–c). The linear functions fitted to the data resembled the general trends in the data, as demonstrated by the local regression fits, and were therefore used as the adjustment functions for all exposure measures. A comparison of the linear PT‐adjustment functions for AUC0–24, Cmax, and Ctrough is shown in Figure 2 d. The numerically strongest association was seen between Ctrough and PT, indicating that the PT adjustment had the greatest influence on Ctrough predictions. The adjustment functions for AUC0–24, Cmax, and Ctrough all pass close to the point at which both the prediction‐corrected exposure and geometric mean of the prediction‐corrected PT are equal to 1. As would be expected, this means that little to no adjustment of the exposure values estimated by the covariate‐only model is required in the case that the expected PT value matches the observed PT value.
Table 2.
Regression of prediction‐corrected exposure metric and PT covariate exposure (intercept and slope)
| Exposure measure | Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|---|
| AUC0–24, μg*L/hour | Intercept | –0.0147 | –0.117 | 0.088 | 0.778 |
| Slope | 1.02 | 0.924 | 1.120 | <0.001 | |
| Cmax, μg/L | Intercept | 0.34 | 0.272 | 0.408 | <0.001 |
| Slope | 0.659 | 0.594 | 0.725 | <0.001 | |
| Ctrough, μg/L | Intercept | –1.14 | –1.41 | –0.868 | <0.001 |
| Slope | 2.27 | 2.01 | 2.530 | <0.001 | |
AUC0–24, area under the plasma concentration–time curve from 0 to 24 hours; CI, 95% confidence interval; Cmax, maximum concentration; Ctrough, trough concentration at the end of the dosing interval before the next dose; PT, prothrombin time.
Figure 2.
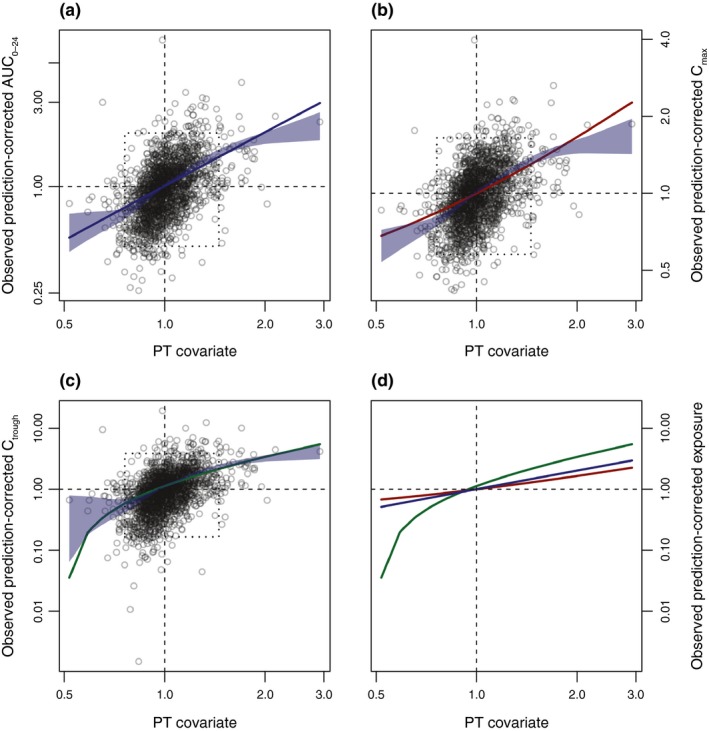
Derivation of the PT‐adjustment function. Relationship between the PT covariate and observed prediction‐corrected exposure estimates for (a) AUC 0–24, (b) Cmax, and (c) Ctrough. (d) Comparison of the linear PT‐adjustment functions for AUC 0–24, Cmax, and Ctrough. Blue shading shows the 95% confidence bound for the locally estimated scatterplot smoothing regression. Dotted boxes highlight areas where 95% of the data are located. Solid colored lines show the respective estimated linear relationship. AUC 0–24, area under the plasma concentration–time curve over 24 hours; Cmax, maximum concentration; Ctrough, trough concentration at the end of the dosing interval before the next dose; PT, prothrombin time.
A comparison of the η and PT covariate approaches was conducted based on the AUC0–24 because this was considered the most relevant summary exposure parameter. The absolute bias in predicting the prediction‐corrected AUC0–24 was slightly lower for the PT covariate than the η approach in terms of mean (−2e–15 vs. 0.111), SD (0.385 vs. 0.44), and 5th to 95th percentile (−0.462 vs. −0.56 and 0.608 vs. 0.777, respectively) (Figure S3 ). As shown in Figure S4, both approaches used PT information in a similar way and, in principle, potential bias introduced by the PT information was independent of the approach used. Figure S4 also provides further confirmation that the performance of the PT covariate approach was slightly better than that of the η approach.
In general, both the η and PT covariate approaches enhanced the information to predict the prediction‐corrected AUC0–24 because the locally estimated scatterplot smoothing regression and the R 2 value indicated a clear correlation (Figure 3). As shown in Figure 3, the width of the histograms representing the frequency distribution of the AUC0–24 differs between the observed and predicted prediction‐corrected AUC0–24, indicating that the observed, unexplained IIV in AUC0–24 could not be fully reproduced by either of the two methods tested. This finding was consistent with expectations because it was not anticipated that the biomarker PT could completely replace measured PK concentrations. Notably, for the η approach, the frequency distribution was bimodal in shape, indicating that, for a subset of patients of one indication (VTE prevention), the prediction‐corrected AUC0–24 was considerably overpredicted.
Figure 3.
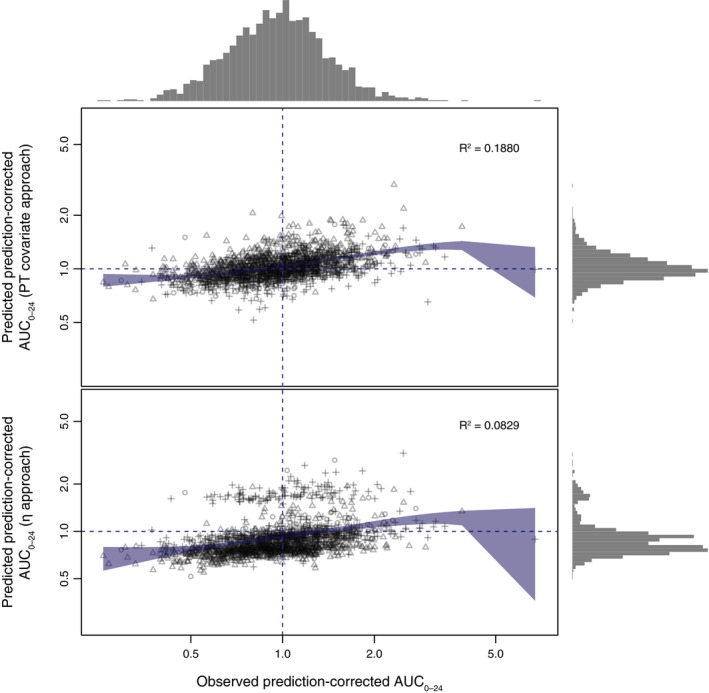
Comparison of the PT covariate and η approaches for predicted vs. observed prediction‐corrected AUC 0–24. Blue shaded area indicates the 95% confidence interval of the locally estimated scatterplot smoothing regression fit. The gray histograms on top and on the right indicate the frequency distribution of the observed and predicted AUC 0–24. Different symbols indicate different indications (atrial fibrillation = circles, venous thromboembolism prevention = triangles, venous thromboembolism treatment = crosses). AUC 0–24, area under the plasma concentration–time curve from 0 to 24 hours; PT, prothrombin time.
PT‐adjusted exposure estimates for patients in phase III studies
PT‐adjusted exposure estimates were generated for patients who received rivaroxaban in phase III studies and had valid PT assessments (Table 3). The observed and expected PT values (based on covariate‐only exposure estimates and the linear PK/PT model) and the covariate‐only and PT‐adjusted exposure estimates (AUC0–24, Cmax, and Ctrough) for all patients in the phase III EINSTEIN‐DVT trial are illustrated in Figure S5 . Results for two individual patients, as examples of PT adjustment increasing and decreasing predicted exposure, are also highlighted in this figure. The difference in exposure estimates between these two individual patients was ≤10% for all covariate‐only exposure estimates but, following PT adjustment, increased to 45%, 35%, and 65% for AUC0–24, Cmax, and Ctrough, respectively. For all indications assessed, the median adjustment ratios were >1 for all three exposure estimates (Table 3), indicating an overall upward trend when covariate‐only exposure estimates were adjusted by PT data.
Table 3.
Range of PT covariate and exposure adjustment factors in phase III trials
| Indication (N) | Minimum | 5th percentile | Median | 95th percentile | Maximum | |
|---|---|---|---|---|---|---|
| AF (5,681) | PT covariate | 0.55 | 0.77 | 1.02 | 1.56 | 3.19 |
| AUC0–24 adjustment factor | 0.55 | 0.77 | 1.03 | 1.58 | 3.24 | |
| Cmax adjustment factor | 0.70 | 0.85 | 1.01 | 1.37 | 2.44 | |
| Ctrough adjustment factor | 0.10 | 0.60 | 1.18 | 2.41 | 6.10 | |
| Treatment of VTE (3,582) | PT covariate | 0.58 | 0.83 | 1.06 | 1.39 | 2.95 |
| AUC0–24 adjustment factor | 0.58 | 0.84 | 1.07 | 1.41 | 3.00 | |
| Cmax adjustment factor | 0.72 | 0.89 | 1.04 | 1.26 | 2.28 | |
| Ctrough adjustment factor | 0.18 | 0.75 | 1.27 | 2.02 | 5.55 | |
| Prevention of VTE in patients undergoing hip or knee replacement surgery (5,293) | PT covariate | 0.71 | 0.88 | 1.05 | 1.41 | 2.81 |
| AUC0–24 adjustment factor | 0.71 | 0.89 | 1.06 | 1.42 | 2.86 | |
| Cmax adjustment factor | 0.81 | 0.92 | 1.03 | 1.27 | 2.19 | |
| Ctrough adjustment factor | 0.47 | 0.86 | 1.25 | 2.05 | 5.25 | |
AF, atrial fibrillation; AUC0–24, area under the plasma concentration–time curve from 0 to 24 hours; Cmax, maximum concentration; Ctrough, trough concentration at the end of the dosing interval before the next dose; PT, prothrombin time; VTE, venous thromboembolism.
Summaries of PT‐adjusted exposure estimates, grouped by indication, are shown in Tables S1 , S2 , and S3 ; these values will be used in future ER analyses. The CV for each exposure parameter was increased from that seen in the covariate‐only model, with limited impact on median or mean values, indicating that the PT adjustment resulted in a greater spread of data.
Assessment of the use of PT‐adjusted exposure estimates in clinical trial simulations based on phase III data for the treatment of VTE
When the impact of Ctrough on efficacy in the phase III EINSTEIN‐DVT/PE trials was examined in simulated clinical trials, the true positive rate was estimated to be 98.2% using observed Ctrough data (assumed reference ER effect size), 59.7% using covariate‐only exposure estimates, and 75.0% using PT‐adjusted exposure estimates. A higher true positive rate was consistently obtained with PT‐adjusted exposure estimates than with covariate‐only exposure estimates across a wide range of effect sizes of the Ctrough–efficacy relationship (when the multiplicative factor of the effect size was ~0.2–2.2; Figure 4).
Figure 4.
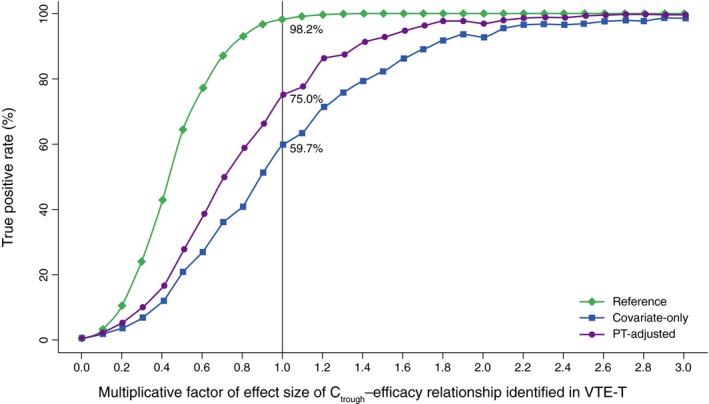
Estimated true positive rates (%) based on clinical trial simulations. Ctrough, trough concentration at the end of the dosing interval before the next dose; PT, prothrombin time; VTE‐T, venous thromboembolism treatment.
Discussion
As requested by the EMA, ER analyses will be conducted to examine how exposure to rivaroxaban in phase III studies impacted efficacy and safety outcomes. Individual rivaroxaban exposure estimates are essential to enable large‐scale ER analyses to be performed in several indications. An integrated popPK model based on phase II studies and a small subset of patients from a phase III trial was initially developed to estimate rivaroxaban exposure for the phase III population based on dose and patient characteristics.4 However, because this covariate‐only popPK model cannot incorporate any additional IIV (in the absence of PK data), the distribution of the exposure estimates is restricted, and this negatively impacts the ability to establish ER relationships in subsequent analyses. To address this limitation, the present analyses took a novel approach to enhance rivaroxaban covariate‐only exposure estimates by using available PT data from phase III trials. This procedure is based on the known linear relationship between rivaroxaban concentrations and PT values that was established during the early phases of rivaroxaban development.6, 9 It combines the information obtained from both PK and PT measurements to increase the likelihood of identifying any significant relationship between rivaroxaban exposure and clinical outcomes, which may not be identifiable using a covariate‐only approach.
The current study demonstrates that using the observed PT data to adjust the PK predictions considerably enhances the spread in the predicted exposure and the quality of PK predictions. Simulated clinical trials based on data from the phase III studies of rivaroxaban in the treatment of VTE assessed the impact of Ctrough on efficacy and demonstrated that the use of PT‐adjusted exposure estimates improved the true positive rate for detection of a statistically significant ER relationship relative to covariate‐only exposure estimates. Thus, these simulations support the use of PT‐adjusted rivaroxaban exposure estimates in future ER analyses.
Including PT significantly improved the prediction of exposure when compared with a covariate‐only model. However, using PT also added some uncertainty to the estimation of exposure when compared with the observed exposure estimates. Figure 2 a,b show the relationship between the prediction‐corrected exposure and the PT covariate geometric mean of prediction‐corrected PT values. The respective regression models show a clear correlation in the expected direction, but with discernable deviations around the regression line. If PT and concentration were fully correlated, no scatter around the regression line would be expected. This confirms the expectation that PT samples are not a completely equivalent replacement for PK samples.
The enhanced precision of rivaroxaban exposure estimates following PT adjustment is expected to enhance the quality of future ER analyses, yet several factors should be considered when using PT‐adjusted exposure estimates. First, the approach was relatively conservative, with small PT‐adjustment factors used. A disadvantage of this was that the magnitude of IIV, as for observed exposures, could not be fully reproduced. However, the potential for PT adjustments to introduce misspecifications is likely to be limited. Second, using PT as a quantitative measurement of rivaroxaban exposure relies on all samples being tested at a central laboratory and the use of a rivaroxaban‐sensitive thromboplastin.9 PT samples tested under these conditions were available for the current analyses, but these requirements may limit the applicability of this PT‐adjustment approach to other data set. Finally, although simulated clinical trials indicated that the use of PT‐adjusted exposure estimates could increase the true positive rate relative to covariate‐only exposure estimates, the rate remained lower than that obtained using observed exposure data derived using the popPK model from observed PK measurements.
To our knowledge, the current study is the first in which a method has been developed to use PT measurements to enhance the quality of individual exposure estimates in cases in which there is a lack of individual PK sampling. This approach was adopted for EMA‐requested ER analyses of rivaroxaban in the indications of prophylaxis and treatment of VTE, AF, and ACS. The outcome of these analyses, including the PT‐adjustment approach that was described in this study, will be reported in separate papers in the future. The novel approach described herein could potentially be applied to other anticoagulants with well‐characterized PK/PT relationships. A similar approach may also be useful for the study of other compounds with limited or missing PK information but with available PK‐related biomarker data.
In conclusion, a PT‐adjustment procedure has been established that, in the absence of PK data, considerably enhances the quality of rivaroxaban exposure estimates from phase III studies based on PT measurements. These enhanced rivaroxaban exposure estimates will provide a basis for future ER analyses assessing clinical outcomes in large phase III studies.
Funding
This analysis was supported by Bayer AG, Leverkusen, Germany. Tamzin Gristwood, PhD, and Emma Bolton, DPhil, of Oxford PharmaGenesis, Oxford, UK, provided medical writing support, which was funded by Bayer AG, Berlin, Germany.
Conflict of Interest
A.S., M.F., D.K., W.M., S.W., and D.G. are employees of Bayer AG. S.D.B. and T.E.S. are employees of Bayer U.S. LLC. A.H.‐V. and L.Z. are employees of Janssen Research & Development, LLC. X.Y. was an employee of Janssen Research & Development at the time that this work was carried out. He is currently employed by The Chinese University of Hong Kong.
Author Contributions
All authors wrote the manuscript, all authors designed the research, all authors performed the research, and all authors analyzed the data. The authors meet the authorship criteria recommended by the International Committee of Medical Journal Editors and received no direct compensation related to the development of the article.
Supporting information
Figure S1. Overview of the derivation (a) and application (b) of the η approach.
Figure S2. Time‐matched PK, PT samples from the PK–PT data set.
Figure S3. Absolute bias of the η and PT covariate approaches in predicting prediction‐corrected AUC0–24.
Figure S4. Absolute bias of the η approach vs. the PT covariate approach in predicting prediction‐corrected AUC0–24.
Figure S5. The observed and expected PT values (a), and the covariate‐only and PT‐adjusted estimates for AUC0–24 (b), Cmax (c), and Ctrough (d) in the phase III EINSTEIN‐DVT study.
Table S1. Range of exposure estimates based on the covariate‐only model, including PT adjustment, and used in the exposure–response analysis in the AF indication.
Table S2. Range of exposure estimates based on the covariate‐only model, including PT adjustment, and used in the exposure–response analysis in patients undergoing hip or knee replacement surgery.
Table S3. Range of exposure estimates based on the covariate‐only model, including PT adjustment, and used in the exposure–response analysis in patients receiving treatment for VTE.
Model Code.
Acknowledgments
The authors would like to thank Gary Peters, MD, for his contributions related to the work described in this article.
References
- 1. European Medicines Agency . XARELTO (rivaroxaban) summary of product characteristics <https://www.ema.europa.eu/documents/product-information/xarelto-epar-product-information_en.pdf> (2018). Accessed March 15, 2019.
- 2. Janssen Pharmaceuticals Inc . XARELTO (rivaroxaban) tablets, for oral use <http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf> (2019). Accessed March 15, 2019.
- 3. Girgis, I.G. et al Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non‐valvular atrial fibrillation: results from ROCKET AF. J. Clin. Pharmacol. 54, 917–927 (2014). [DOI] [PubMed] [Google Scholar]
- 4. Willmann, S. et al Integrated population pharmacokinetic analysis of rivaroxaban across multiple patient populations. CPT Pharmacometrics Syst. Pharmacol. 7, 309–320 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ten Cate, H. et al The prothrombin time does not predict the risk of recurrent venous thromboembolism or major bleeding in rivaroxaban‐treated patients. Thromb. Res. 170, 75–83 (2018). [DOI] [PubMed] [Google Scholar]
- 6. Mueck, W. , Becka, M. , Kubitza, D. , Voith, B. & Zuehlsdorf, M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban‐an oral, direct factor xa inhibitor‐in healthy subjects. Int. J. Clin. Pharmacol. Ther. 45, 335–344 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Mueck, W. et al Population pharmacokinetics and pharmacodynamics of rivaroxaban‐an oral, direct factor Xa inhibitor‐in patients undergoing major orthopaedic surgery. Clin. Pharmacokinet. 47, 203–216 (2008). [DOI] [PubMed] [Google Scholar]
- 8. Mueck, W. , Lensing, A.W. , Agnelli, G. , Decousus, H. , Prandoni, P. & Misselwitz, F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep‐vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin. Pharmacokinet. 50, 675–686 (2011). [DOI] [PubMed] [Google Scholar]
- 9. Mueck, W. , Stampfuss, J. , Kubitza, D. & Becka, M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin. Pharmacokinet. 53, 1–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu, X.S. et al Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with acute coronary syndromes. Br. J. Clin. Pharmacol. 74, 86–97 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eriksson, B.I. et al Oral, direct Factor Xa inhibition with BAY 59‐7939 for the prevention of venous thromboembolism after total hip replacement. J. Thromb. Haemost. 4, 121–128 (2006). [DOI] [PubMed] [Google Scholar]
- 12. Turpie, A.G. et al BAY 59‐7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose‐ranging study. J. Thromb. Haemost. 3, 2479–2486 (2005). [DOI] [PubMed] [Google Scholar]
- 13. Agnelli, G. et al Treatment of proximal deep‐vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59‐7939): the ODIXa‐DVT (Oral Direct Factor Xa Inhibitor BAY 59‐7939 in Patients with Acute Symptomatic Deep‐Vein Thrombosis) study. Circulation 116, 180–187 (2007). [DOI] [PubMed] [Google Scholar]
- 14. Buller, H.R. et al A dose‐ranging study evaluating once‐daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein‐DVT Dose‐Ranging Study. Blood 112, 2242–2247 (2008). [DOI] [PubMed] [Google Scholar]
- 15. Patel, M.R. et al Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 365, 883–891 (2011). [DOI] [PubMed] [Google Scholar]
- 16. Eriksson, B.I. et al Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N. Engl. J. Med. 358, 2765–2775 (2008). [DOI] [PubMed] [Google Scholar]
- 17. Kakkar, A.K. et al Extended duration rivaroxaban versus short‐term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double‐blind, randomised controlled trial. Lancet 372, 31–39 (2008). [DOI] [PubMed] [Google Scholar]
- 18. Lassen, M.R. et al Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N. Engl. J. Med. 358, 2776–2786 (2008). [DOI] [PubMed] [Google Scholar]
- 19. Turpie, A.G. et al Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 373, 1673–1680 (2009). [DOI] [PubMed] [Google Scholar]
- 20. Bauersachs, R. et al Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 363, 2499–2510 (2010). [DOI] [PubMed] [Google Scholar]
- 21. Buller, H.R. et al Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 366, 1287–1297 (2012). [DOI] [PubMed] [Google Scholar]
- 22. Mega, J.L. et al Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 366, 9–19 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Overview of the derivation (a) and application (b) of the η approach.
Figure S2. Time‐matched PK, PT samples from the PK–PT data set.
Figure S3. Absolute bias of the η and PT covariate approaches in predicting prediction‐corrected AUC0–24.
Figure S4. Absolute bias of the η approach vs. the PT covariate approach in predicting prediction‐corrected AUC0–24.
Figure S5. The observed and expected PT values (a), and the covariate‐only and PT‐adjusted estimates for AUC0–24 (b), Cmax (c), and Ctrough (d) in the phase III EINSTEIN‐DVT study.
Table S1. Range of exposure estimates based on the covariate‐only model, including PT adjustment, and used in the exposure–response analysis in the AF indication.
Table S2. Range of exposure estimates based on the covariate‐only model, including PT adjustment, and used in the exposure–response analysis in patients undergoing hip or knee replacement surgery.
Table S3. Range of exposure estimates based on the covariate‐only model, including PT adjustment, and used in the exposure–response analysis in patients receiving treatment for VTE.
Model Code.


