Abstract
Polycystic ovary syndrome (PCOS) is a common, complex condition that affects up to 18% of reproductive- aged women, causing reproductive, metabolic and psychological dysfunctions. We performed an overview and appraisal of methodological quality of systematic reviews that assessed medical and surgical treatments for reproductive outcomes in women with PCOS. Databases (MEDLINE, EMBASE, CINAHL PLUS and PROSPERO) were searched on the 15th of September 2017. We included any systematic review that assessed the effect of medical or surgical management of PCOS on reproductive, pregnancy and neonatal outcomes. Eligibility assessment, data extraction and quality assessment by the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) tool were performed in duplicate. We identified 53 reviews comprising 44 reviews included in this overview; the majority were moderate to high quality. In unselected women with PCOS, letrozole was associated with a higher live birth rate than clomiphene citrate (CC), while CC was better than metformin or placebo. In women with CC-resistant PCOS, gonadotrophins were associated with a higher live birth rate than CC plus metformin, which was better than laparoscopic ovarian drilling (LOD). LOD was associated with lower multiple pregnancy rates than other medical treatments. In women with PCOS undergo- ing in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI), the addition of metformin to gonadotro- phins resulted in less ovarian hyperstimulation syndrome (OHSS), and higher pregnancy and live birth rates than gonadotrophins alone. Gonadotrophin releasing hormone (GnRH) antagonist was associated with less OHSS, gonadotrophin units and shorter stimulation length than GnRH agonist. Letrozole appears to be a good first line treatment and gonadotrophins, as a second line treatment, for anovulatory women with PCOS. LOD results in lower multiple pregnancy rates. However, due to the heterogeneous nature of the included popula- tions of women with PCOS, further larger scale trials are needed with more precise assessment of treatments according to heterogeneous variants of PCOS.
Keywords: Infertility, Polycystic Ovary Syndrome, Review, Therapeutics, Treatment Outcome
Introduction
Polycystic ovary syndrome (PCOS) is one of the most important dilemmas in reproductive medicine. PCOS is a member of the World Health Organization group II ovulation disorders, and has a 9-18% prevalence among reproductive-aged women (1) and nearly 80% among infertile anovulatory women (1, 2). There is an ongoing debate related to its definition, aetiology, diagnosis and treatment for its clinical phenotypes (3). Since first described by Stein and Leventhal (4), a number of reports and meetings have suggested diagnostic criteria for this condition (3, 5, 6). However, the criteria reported by ESHRE/ASRM in Rotterdam in 2003 are most commonly used both in research and clinical care. These criteria propose that two out of three domains should be present to establish a diagnosis of PCOS. These domains are: an-/oligo-ovulation, hyperandrogenism (clinical ± biochemical) and polycystic ovary morphology on ultrasound examination, with exclusion of other causes of hyperandrogenism (6). In 2012, the National Institute of Health reinforced the need for identification of four phenotypes within the Rotterdam criteria in women with PCOS, which refer to the combination of diagnostic criteria (7). By using the possible combinations of these criteria, four different phenotypes of PCOS are now identified: i. Hyperandrogenism (clinical or biochemical) and chronic anovulation (H-CA), ii. Hyperandrogenism and polycystic ovaries on ultrasound (PCOm), but with ovulatory cycles (H-PCOm), iii. Chronic anovulation and polycystic ovaries without hyperandrogenism (CA-PCOm), and iv. Hyperandrogenism, chronic anovulation and polycystic ovaries (H-CA-PCOm). The identification of specific phenotypes in women with PCOS seems to be justified from the metabolic point (3).
This heterogeneous condition manifests with many clinical presentations, including infertility, pregnancy complications, and psychological and metabolic features. The reproductive problems associated with PCOS consist mainly of menstrual dysfunction, infertility and pregnancy complications. Many treatments are proposed by different guidelines for infertility with PCOS, and include clomiphene citrate (CC), letrozole and gonadotrophins. However, there is a lack of clarity around the relative efficacy of these different treatments. Despite the agreement between most guidelines of the importance and priority of lifestyle modification in PCOS and weight loss, where women are overweight or obese, there are still limited studies that compare lifestyle modification and pharmacological drugs for reproductive outcomes (8). With regards to pharmacological treatment in isolation, CC is recommended as first-line treatment for ovulation induction (OI) in infertile women with POCS with the alternative treatment, letrozole, which has encouraging results in many recent trials (1, 2, 8-10). Although the insulin sensitizer metformin has been recently recommended as a firstline treatment (11), its role and specific indication are controversial (1-3). The second-line treatment is usually recommended as gonadotrophins or laparoscopic ovarian drilling (LOD) (2). Additional issues relating to treatment of reproductive outcomes which are still somewhat controversial include the best time to use in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) in women who failed to become pregnant after pharmacological treatment, and the potential benefit of modern techniques like in vitro maturation (IVM) (2, 3).
The aim of this review was to perform an overview to summarize and appraise the content, results and quality of systematic reviews that assess medical or surgical treatments for reproductive outcomes in women with PCOS.
Materials and Methods
Inclusion criteria
The Participant, Intervention, Comparison, Outcomes and Studies (PICOS) framework was used for this review. This overview is part of a larger overview of systematic reviews. For this broader overview, we included any systematic review or meta-analysis where the assessment or management of PCOS was the primary focus of the review, either as interventions in PCOS or a comparison of women with and without PCOS for a specific outcome. Exclusion criteria were studies where PCOS was a secondary condition assessed as part of a broader topic. For this specific overview, we included any systematic review that assessed the effect of medical or surgical management of PCOS on reproductive outcomes. The specific inclusion criteria were: i. Published from 2009 onwards, as this was the date of publication of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement as a guideline for conducting systematic reviews (12), ii. Must have included a search strategy that contained at least key words or terms, iii. Must include the number of identified and included articles, and iv. The review needed to conduct some form of quality appraisal of the articles. The comparisons term was not applicable in this review context. The outcomes assessed were reproductive outcomes, specifically live birth, clinical pregnancy, miscarriage, ovulation, multiple pregnancy, menstrual cycle frequency, follicular size, pregnancy related outcomes (gestational diabetes, pregnancy-induced hypertension and pre-eclampsia), neonatal outcomes, costs and side effects. Only articles published in English were included. The protocol is registered in the International Prospective Register of Systematic Reviews PROSPERO (CRD42016052649).
Article selection
A comprehensive database search was conducted on the 17th of October 2016, which was last updated on 15th September 2017. The following electronic databases were used to identify relevant published literature: Medline in-process and other non-indexed citations [Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present]; EMBASE (EBM Reviews- Cochrane Database of Systematic Reviews 2005 to September 15, 2017, EBM Reviews- ACP Journal Club 1991 to September 2017, EBM Reviews- Database of Abstracts of Reviews of Effects 1st Quarter 2016, EBM Reviews- Cochrane Central Register of Controlled Trials September 2017, EBM Reviews- Cochrane Methodology Register 3rd Quarter 2012, EBM Reviews- Health Technology Assessment 4th Quarter 2016, EBM Reviews- NHS Economic Evaluation Database 1st Quarter 2016); and CINAHL PLUS. The search strategy is documented in Supplementary Appendix 1 (See Supplementary On line Information at www.celljournal.org). This search was modified for EMBASE and CINAHL using their subject headings instead of the MeSH subject headings. The International Prospective Register of Systematic Reviews PROSPERO (http://www.crd.york.ac.uk/PROSPERO/) was additionally searched on the 15th September 2017 using the key words “PCOS” or “polycystic ovary syndrome”. In addition, experts in the field were asked to provide any potentially relevant studies for consideration. Two independent reviewers, who were not blinded to the names of investigators or sources of publication, identified and selected the articles that met the inclusion criteria (L.J.M, D.H or C.T.T). Disagreements between reviewers were discussed and resolved by consensus or arbitration with a third reviewer.
Data extraction
All eligible systematic reviews included were examined and extracted independently by two reviewers (L.J.M, M.G or C.T.T). Disagreements were discussed and resolved by consensus or arbitration with a third reviewer. The data extracted included information on author(s), year, country of author, inclusion criteria, study methodology, study outcomes, number of studies identified, number of participants in the review, whether a meta-analysis was conducted, and quality of identified articles in each review (as reported by the systematic review authors as overall quality of the entire study or evidence or reported as unclear if not summarized by the systematic review authors).
Data synthesis
A narrative description of the included reviews was performed. We presented results per reproductive outcome.
Quality assessment of systematic reviews
All included reviews were evaluated by two independent reviewers (L.J.M, M.G or C.T.T) using the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) tool (13, 14). Disagreements were discussed and resolved by consensus or arbitration with a third reviewer. The AMSTAR tool contains 11 items to appraise the methodological aspects of the systematic reviews. Each item was scored 1 for “yes” and 0 for “no” or “not applicable” with a total score range from 0 to 11. The methodological quality for each review was classified as low [≤ 3], moderate [4-7] and high [8-11] (15) .
Results
Characteristics of included reviews
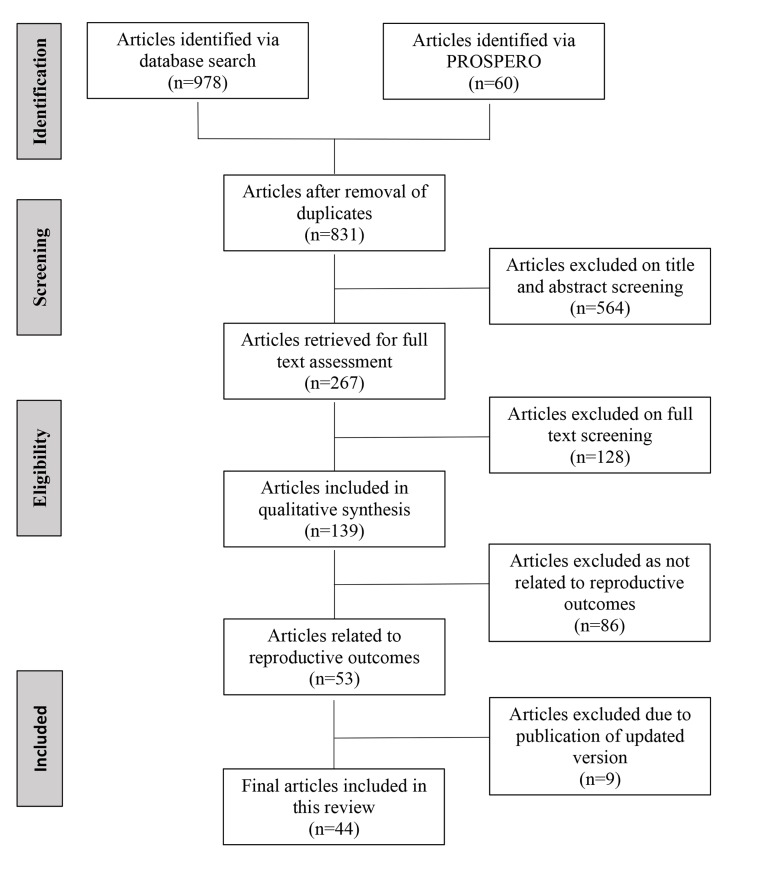
The search yielded 978 citations, with 60 citations identified from PROSPERO and one citation identified from expert assessors, for a total of 1039 citations. There were 831 citations that remained after removal of duplicates. Based on a priori selection criteria, screening for title or abstract identified 276 articles for assessment of the full text. Of these, 128 articles were excluded for the following: not conducting quality assessment, not in English, no search terms detailed or no identified search strategy (Supplementary Appendix 2) (See Supplementary Online Information at www.celljournal.org). We included 139 full-text articles for our final analysis, of which 53 articles were related to the theme of medical or surgical treatment on reproductive outcomes in PCOS, with the remaining eligible articles assessed in separate overviews of systematic reviews and excluded from this specific review. These 53 articles comprised 44 reviews (Fig .1).
Fig 1.
Study selection.
The characteristics of these reviews are summarized in Supplementary Appendix 3 (See Supplementary Online Information at www.celljournal.org). The number of included studies in each review ranged between none (16, 17) and 66 (18). The type of included studies in each review was only randomised controlled trials (RCTs) in 22 reviews (16, 18-38), RCTs and crossover trials until first inclusion in 11 reviews (17, 33, 39-47), RCTs and systematic reviews of RCTs in two reviews (48, 49), any study design in two reviews (50, 51), any study with control group in three reviews (52-54), RCTs and prospective studies in one review (55) and not stated in three reviews (56-58). Participants in the included reviews were treatment-naive women in two reviews (27, 28), women resistant to CC in six reviews (19, 23, 29, 32, 33, 56), women whose treatment status was undefined in 32 reviews (16-18, 20-22, 24-26, 30, 31, 35-42, 44-51, 55, 57, 59), pregnant women with PCOS in four reviews (52-54, 58), adolescents with PCOS (11-19 years old) in one review (34), and women with PCOS not trying to conceive in one review (43). Twenty-two reviews were conducted according to prior guidelines for conducting systematic reviews such as PRISMA, Meta-analyses Of Observational Studies in Epidemiology (MOOSE), Quality of Reporting of Meta-analyses (QUORUMS) or Cochrane (16, 17, 19, 23, 25, 27, 29, 32, 34, 36, 39-46, 50, 51, 58, 59). Meta-analyses were performed in 39 reviews (18-32, 34- 43, 45-50, 52-59). The systematic reviews did not apply language restrictions in 28 reviews (16, 17, 19, 20, 24-26, 28, 29, 34, 36, 38-47, 50, 53-56, 58, 59), restricted the search to articles in English in 12 reviews (18, 21, 22, 27, 32, 33, 35, 37, 48, 49, 52, 57), restricted the search to articles in English and Chinese in two reviews (30, 31) and did not state if language restrictions were applied in two reviews (23, 51). The quality of included studies in each review was not reported by authors or was not able to be easily interpreted in 31 reviews (16, 17, 20-29, 32, 36, 38, 39, 42, 43, 45-47, 50-59), low or insufficient in eight reviews (18, 31, 34, 35, 37, 40, 41, 44), low to moderate in two reviews (19, 48) and low to high in three reviews (30, 33, 49)
Quality of included reviews
The quality of the included reviews are presented in Supplementary Appendix 4 (See Supplementary Online Information at www.celljournal.org). Seven reviews were of low quality (28, 30, 33, 36, 51, 52, 58), 22 reviews were of moderate quality (16, 20-27, 31, 32, 35, 37, 38, 40, 50, 53-57, 59) and 15 reviews were of high quality (17-19, 29, 34, 39, 41-49). Twenty reviews had pre-specified their clinical question and inclusion criteria (16-19, 29, 33, 34, 39-49, 55, 59). Nineteen reviews conducted study selection and data extraction in duplicate (17-19, 21, 23, 26, 27, 29, 32, 34, 37, 39, 42-45, 50, 55, 57). Twenty-eight reviews conducted a comprehensive literature search (16-19, 21, 24-26, 28- 31, 34, 38-49, 53, 54, 59). Twenty reviews included grey literature searches (16, 17, 19, 25, 26, 29, 34, 38- 47, 53, 54, 59). Twenty-four reviews listed included and excluded studies (16, 17, 19, 23-27, 29, 32, 34, 38, 39, 41-46, 48-50, 57, 59). Forty reviews described the characteristics of the included studies (18-29, 32-59). Thirty-eight reviews assessed study quality (16-27, 29- 35, 37-50, 54, 56, 57, 59, 60). Nineteen reviews used the scientific quality of their included studies in formulating results (18, 20-22, 24, 25, 29, 31, 32, 34, 35, 39, 40, 45-49, 57). Thirty-seven reviews combined the studies using appropriate methods (18-32, 35-43, 45, 46, 48-50, 52-59). Twenty-two reviews addressed the risk of reporting bias, and used a statistical test where appropriate (16-19, 32, 34, 35, 37-39, 41-44, 46, 47, 50, 52, 53, 55, 56, 58). Seven reviews addressed the potential for conflict of interest (16, 17, 29, 43, 47-49).
Types of interventions
Letrozole
Six reviews (three high quality (19, 39, 49) and three moderate quality (20, 27, 32) assessed interventions that contained letrozole, comprising a total of 89 trials and 14 008 participants. Of these, five assessed letrozole ± other OI drugs versus OI drugs, including letrozole alone (20, 27, 32, 39, 49) and one assessed letrozole versus LOD (19). The populations studied were women with PCOS who were treatment-naïve (27), CC resistant (32), or treatment-naïve ± CC resistant or unknown treatment status (20, 39, 49).
The meta-analyses reported statistically significant results for higher live birth, pregnancy and ovulation after letrozole compared to CC followed by timed intercourse in overall women with PCOS, and higher live birth and pregnancy after letrozole in women with PCOS and body mass index (BMI) >25 kg/m2 (20, 27, 39, 49). In women with CC resistance, letrozole with or without metformin resulted in higher live births compared to CC with metformin (32, 39), letrozole resulted in higher pregnancy and ovulation than anastrozole and higher ovulation than LOD (49). Long-term letrozole (10 days) resulted in higher pregnancy than short-term letrozole (5 days) (Tables1, 2) (49).
Table 1.
Results of main medical interventions
| Review | Population | Outcomes assessed | Comparison | Outcomes with significant results | |
|---|---|---|---|---|---|
| Letrozole | |||||
| Abu Hashim et al. (32), 2015 | CC resistant PCOS | Live birth | CC+metformin vs. Letrozole | Live birth/woman | OR: 0.21, 95% CI: 0.05 to 0.87 |
| Pregnancy | |||||
| Ovulation | |||||
| Miscarriage | |||||
| Multiple pregnancy | |||||
| OHSS | |||||
| Franik et al. (39), 2014 | PCOS, reproductive age | Live birth | Letrozole vs. CC (BMI >25 kg/m2) | Live birth/woman | OR: 1.67, 95% CI: 1.31 to 2.11 |
| Pregnancy | Letrozole vs. CC (with or without adjuncts followed by timed intercourse) | Live birth/woman | OR: 1.64, 95% CI: 1.32 to 2.04 | ||
| Miscarriage | Letrozole vs. CC (with or without adjuncts followed by IUI) | Pregnancy/woman | OR: 1.71, 95% CI: 1.30 to 2.25 | ||
| Multiple pregnancy | Letrozole vs. CC (overall with or without adjuncts followed by timed intercourse) | Pregnancy/woman | OR: 1.40, 95% CI: 1.18 to 1.65 | ||
| OHSS | Letrozole vs. CC+rFSH and rFSH only | Pregnancy/woman | OR: 1.66, 95% CI: 1.23 to 2.22 | ||
| Letrozole+metformin vs. CC+metformin | Live birth/woman | OR: 4.5, 95% CI: 1.09 to 18.50 | |||
| He and Jiang (20), 2011 | PCOS | Pregnancy | Letrozole vs. CC | Mature follicles/cycle | SMD: 1.41, 95% CI: 1.54 to 1.28 |
| Ovulation | |||||
| Miscarriage | |||||
| Multiple pregnancy | |||||
| OHSS | Letrozole vs. CC | Ovulation/cycle | RR: 1.29, 95% CI: 1.12 to 1.49 | ||
| Mature follicles | |||||
| Misso et al. (49), 2012 | PCOS | Live birth | Letrozole long-term (10 days) vs. Letrozole short-term (5 days) | Pregnancy/cycle | Higher in long-term (10 days) |
| Pregnancy | Letrozole vs. Anastrozole | Ovulation/cycle | Higher in letrozole | ||
| Ovulation | Letrozole vs. Anastrozole | Pregnancy/woman | Higher in letrozole | ||
| Miscarriage | Letrozole vs. CC | Ovulation/woman | OR: 2.90, 95% CI: 1.72 to 4.88 | ||
| Multiple pregnancies | Letrozole vs. LOD | Ovulation/cycle | Higher in letrozole | ||
| Adverse events | |||||
| Cost effectiveness | |||||
| Roque et al. (27), 2015 | PCOS (therapy naïve) | Live birth | Letrozole vs. CC | Live birth/woman | RR: 1.55, 95% CI: 1.26 to 1.90 |
| Clinical pregnancy | |||||
| Ovulation | |||||
| Miscarriage | |||||
| Multiple pregnancy | Letrozole vs. CC | Pregnancy/woman | RR: 1.38, 95% CI: 1.05 to 1.83 | ||
| CC | |||||
| Abu Hashim et al. (32), 2015 | CC resistant PCOS | Live birth | CC+metformin vs. Gonadotrophins | Live birth/woman | OR: 0.33, 95% CI: 0.13 to 0.85 |
| Pregnancy | CC+metformin vs. Gonadotrophins | Ovulation/woman | OR: 0.25, 95% CI: 0.15 to 0.41 | ||
| Ovulation | CC+metformin vs. CC + NAC | Ovulation/woman | OR: 8.93, 95% CI: 4.61 to 17.32 | ||
| Miscarriage | CC+metformin vs. Gonadotrophins | Pregnancy/woman | OR: 0.45, 95% CI: 0.27 to 0.75 | ||
| Multiple pregnancy | CC+metformin vs. CC + NAC | Pregnancy/woman | OR: 5.28, 95% CI: 1.91 to 14.62 | ||
| OHSS | |||||
| Brown and Farquhar (46), 2017 | WHO group 2 anovulation | Live birth | CC vs. Placebo | Pregnancy/woman | OR: 5.91, 95% CI: 1.77 to 19.68 |
| Pregnancy | CC vs. Gonadotrophins | Live birth/woman | OR: 0.64, 95% CI: 0.41 to 0.98 | ||
| Ovulation | CC vs. Gonadotrophins | Pregnancy/woman | OR: 0.61, 95% CI: 0.40 to 0.93 | ||
| Miscarriage | CC 5 day vs. CC 10 day | Live birth/woman | OR: 0.10, 95% CI: 0.02 to 0.45 | ||
| Multiple pregnancy | CC 5 day vs. CC 10 day | Pregnancy/woman | OR: 0.18, 95% CI: 0.06 to 0.55 | ||
| OHSS | CC+DEX vs. CC | Pregnancy/woman | OR: 6.2, 95% CI: 2.20 to 17.48 | ||
| Adverse effects | Early CC vs. late CC | Pregnancy/woman | OR: 2.81, 95% CI: 1.02 to 7.75 | ||
| CC+OCP vs. CC | Pregnancy/woman | OR: 27.18, 95% CI: 3.14 to 235.02 | |||
| Ding et al. (36), 2016 | PCOS | Pregnancy | Late CC vs. Early CC | Mature follicles/cycle | MD: 1.82, 95% CI: 0.86 to 2.78 |
| Ovulation | |||||
| Miscarriage | |||||
| Number of follicles | |||||
| Farquhar et al. (19), 2012 | CC resistant PCOS | Live birth | LOD vs. CC+metformin | Live birth/woman | OR: 0.44, 95% CI: 0.24 to 0.82 |
| Pregnancy | LOD vs. CC+metformin | Costs | MD: 3711.3, 95% CI: 3585.17 to 3837.43 | ||
| Ovulation | |||||
| Miscarriage | |||||
| Multiple pregnancy | |||||
| OHSS | |||||
| Costs | |||||
| Gill et al. (33), 2014 | CC resistant PCOS, reproductive age | Pregnancy | CC+metformin vs. CC | Ovulation/woman | Higher in CC+metformin |
| Ovulation | CC+metformin vs. CC | Pregnancy/woman | Higher in CC+metformin | ||
| Palomba et al. (24), 2009 | PCOS | Live birth | Metformin vs. CC+metformin | Live birth/woman | OR: 0.23, 95% CI: 0.13 to 0.40 |
| Pregnancy | Metformin vs. CC+metformin | Ovulation/woman | OR: 0.23, 95% CI: 0.15 to 0.34 | ||
| Ovulation | Metformin vs. CC+metformin | Pregnancy/woman | OR: 0.23, 95% CI: 0.14 to 0.37 | ||
| Miscarriage | |||||
| Adverse events | |||||
| Siebert et al. (28), 2012 | PCOS (therapy naïve) | Live birth | Metformin vs. CC | Live birth/woman | OR: 0.48, 95% CI: 0.31 to 0.73 |
| Pregnancy | Metformin vs. CC | Ovulation/woman | OR: 0.48, 95% CI: 0.41 to 0.57 | ||
| Ovulation | CC+metformin vs. CC | Ovulation/woman | OR: 1.6, 95% CI: 1.2 to 2.1 | ||
| CC+metformin vs. CC | Pregnancy/woman | OR: 1.3, 95% CI: 1.0 to 1.6 | |||
| Tang et al. (41), 2012 | PCOS | Live birth | Metformin vs. CC (BMI≥ 30) | Live birth/woman | OR: 0.3, 95% CI: 0.17 to 0.52 |
| Metformin vs. CC (BMI ≥ 30) | Ovulation/woman | OR: 0.43, 95% CI: 0.36 to 0.51 | |||
| Pregnancy | CC+metformin vs. CC (CC resistant PCOS) | Ovulation/woman | OR: 4.86, 95% CI: 2.43 to 9.74 | ||
| Ovulation | CC+metformin vs. CC (BMI<30) | Ovulation/woman | OR: 1.75, 95% CI: 1.27 to 2.39 | ||
| Miscarriage | CC+metformin vs. CC (BMI≥30) | Ovulation/woman | OR: 1.78, 95% CI: 1.51 to 2.1 | ||
| Multiple pregnancy | Metformin vs. CC (BMI ≥ 30) | Pregnancy/woman | OR: 0.34, 95% CI: 0.21 to 0.55 | ||
| Menstrual frequency | Metformin vs. CC (BMI <30) | Pregnancy/woman | OR: 1.94, 95% CI: 1.19 to 3.16 | ||
| CC+metformin vs. CC | Pregnancy/woman | OR: 1.51, 95% CI: 1.17 to 1.96 | |||
| CC+metformin vs. CC (BMI ≥30) | Pregnancy/woman | OR: 1.76, 95% CI: 1.26 to 2.47 | |||
| CC+metformin vs. CC | Side effects | OR: 3.31, 95% CI: 2.11 to 5.20 | |||
| CC+metformin vs. CC | Side effects (GIT) | OR: 3.4, 95% CI: 2.08 to 5.54 | |||
| Thakker et al. (47), 2015 | PCOS | Live birth | NAC vs. Placebo (CC resistant PCOS) | Live birth/woman | OR: 3.0, 95% CI: 1.05 to 8.6 |
| Ovulation | NAC vs. Placebo (CC resistant PCOS) | Ovulation/woman | OR: 8.4, 95% CI: 4.5 to 15.67 | ||
| Miscarriage, Multiple pregnancy OHSS | NAC vs. Placebo (CC resistant PCOS) | Pregnancy/woman | OR: 4.83, 95% CI: 2.30 to 10.13 | ||
| Menstrual regularity | |||||
| Xiao et al. (3), 2012 | PCOS, <35 years | Pregnancy | Metformin vs. CC | Ovulation/woman | OR: 0.48, 95% CI: 0.26 to 0.87 |
| Ovulation | Metformin+CC vs. CC | Pregnancy/woman | OR: 1.56, 95% CI: 1.16 to 2.08 | ||
| Miscarriage | |||||
| Insulin sensitizers | |||||
| Tang et al. (41), 2012 | PCOS | Live birth | Metformin vs. Placebo | Side effects (GIT) | OR: 4.27, 95% CI: 2.4 to 7.59 |
| Clinical pregnancy | Metformin vs. Placebo (BMI < 30) | Menstrual frequency | OR: 21.15, 95% CI: 1.01 to 445.0 | ||
| Ovulation | Metformin vs. Placebo (BMI ≥30) | Menstrual frequency | OR: 1.57, 95% CI: 1.03 to 2.41 | ||
| Miscarriage | Metformin vs. Placebo (BMI<30) | Pregnancy/woman | OR: 2.35, 95% CI: 1.44 to 3.82 | ||
| Multiple pregnancy | Metformin vs. Placebo | Menstrual frequency | OR: 1.72, 95% CI: 1.14 to 2.61 | ||
| Menstrual frequency | Metformin vs. Placebo | Ovulation/woman | OR: 1.81, 95% CI: 1.13 to 2.93 | ||
| Metformin vs. Placebo | Pregnancy/woman | OR: 2.31, 95% CI: 1.52 to 3.51 | |||
| Feng et al. (52), 2015 | PCOS, pregnant and took metformin to get conception | GDM, PE, Miscarriage, Premature delivery | Metformin during pregnancy vs. Placebo | Miscarriage | RR: 0.32, 95% CI: 0.19 to 0.56 |
| Metformin during pregnancy vs. Placebo | Preterm birth | RR: 0.4, 95% CI: 0.18 to 0.91 | |||
| Tan et al. (58), 2016 | PCOS and pregnant | GDM, PIH/PE, Miscarriage Preterm delivery | Metformin during pregnancy vs. Placebo | GDM | OR: 0.28, 95% CI: 0.10 to 0.75 |
| Metformin during pregnancy vs. Placebo | Miscarriage | OR: 0.20, 95% CI: 0.12 to 0.31 | |||
| Fetal abnormality, Fetal birth weight | Metformin during pregnancy vs. Placebo | Preterm birth | OR: 0.33, 95% CI: 0.18 to 0.60 | ||
| Metformin during pregnancy vs. Placebo (Non RCTs) | GDM | OR: 0.14, 95% CI: 0.09 to 0.24 | |||
| Metformin during pregnancy vs. Placebo (Non RCTs) | PIH/PE | OR: 0.28, 95% CI: 0.16 to 0.48 | |||
| Zhuo et al. (54), 2014 | PCOS and pregnant | GDM | Metformin during pregnancy vs. Placebo | GDM | OR: 0.19, 95% CI: 0.13 to 0.27 |
| Zeng et al. (53), 2016 | PCOS and pregnant | Live birth | Metformin during pregnancy vs. Placebo | GDM | OR: 0.02, 95% CI: 0.14 to 0.87 |
| Miscarriage | Metformin during pregnancy vs. Placebo | IUGR | OR: 0.17, 95% CI: 0.08 to 0.33 | ||
| Preterm delivery GDM | Metformin during pregnancy vs. Placebo | Live birth/woman | OR: 5.23, 95% CI: 3.12 to 8.75 | ||
| PIH/PE | Metformin during pregnancy vs. Placebo | Miscarriage | OR: 0.19, 95% CI: 0.12 to 0.28 | ||
| IUGR | Metformin during pregnancy vs. Placebo | PIH/PE | OR: 0.22, 95% CI: 0.13 to 0.38 | ||
| Fetal malformation | Metformin during pregnancy vs. Placebo | Preterm birth | OR: 0.37, 95% CI: 0.20 to 0.68 | ||
| Neonatal death Macrosomia | |||||
| Li et al. (22), 2011 | PCOS | Pregnancy | Metformin vs. Thiazolidinediones (3 months duration) | Side effects | OR: 8.88, 95% CI: 3.54 to 22.27 |
| Ovulation | |||||
| Menstrual regularity | Metformin vs. Thiazolidinediones (6 months duration) | Side effects | OR: 12.22, 95% CI: 3.53 to 42.31 | ||
| Thakker et al.(47), 2015 | PCOS | Live birth | NAC vs. Metformin | Ovulation/woman | OR: 0.13, 95% CI: 0.08 to 0.22 |
| Ovulation | NAC vs. Metformin | Pregnancy/woman | OR: 0.4, 95% CI: 0.23 to 0.71 | ||
| Miscarriage, Multiple pregnancy OHSS | |||||
| Menstrual regularity | |||||
| Al Khalifah et al. (34), 2016 | Adolescents with PCOS (11-19 year old) | Menstrual regulation | OCP vs. Metformin | Menstrual frequency | MD; 0.27, 95% CI: -0.33 to -0.21 |
| Fang et al. (37), 2017 | PCOS | Dominant follicles Menstrual regularity | Vitamin D + metformin vs. Metformin | Menstrual frequency | OR: 1.85, 95% CI: 1.01 to 3.39 |
| Pundir et al. (38), 2017 | PCOS | Live birth Clinical pregnancy | Inositol vs. Placebo | Ovulation/woman | RR: 2.3, 95% CI: 1.1 to 4.7 |
| Ovulation Miscarriage | Inositol vs. Placebo | Menstrual frequency | RR: 6.8, 95% CI: 2.8 to 16.6 | ||
| Menstrual regulation | Pioglitazone vs. Placebo | Menstrual frequency | OR: 8.88, 95% CI: 2.35 to 33.61 | ||
| Roziglitazone vs. Placebo | Menstrual frequency | OR: 5.59, 95% CI: 2.20 to 14.19 | |||
BMI; Body mass index, CC; Clomiphene citrate, DEX; Dexamethasone, GDM; Gestational diabetes mellitus, GIT; Gastrointestinal tract, IUGR; Intra-uterine growth restriction, IUI; Intra uterine insemination, LOD; Laparoscopic ovarian drilling, MD; Mean difference, NAC; N-acetyl cysteine, OCP; Oral contraceptive pills, OHSS; Ovarian hyper-stimulation syndrome, OR: Odds ratio, PCOS; Polycystic ovary syndrome, PIH/PE; Pregnancy induced hypertension/Preeclampsia, RCT; Randomized controlled trial, rFSH: Recombinant follicle stimulating hormone, RR; Risk ratio, SMD; Standardized mean difference, and WHO; World Health Organization.
Table 2.
Results of their interventions
| Gonadotrophins | |||||
|---|---|---|---|---|---|
| Bordewijk et al. (45), 2017 | PCOS and anovulatory women | Live birth | FSH+metformin vs. FSH in PCOS resistant | Live birth/woman | OR: 2.31, 95% CI: 1.23 to 4.34 |
| Clinical pregnancy | FSH+metformin vs. FSH in PCOS resistant | Pregnancy/woman | OR: 2.46, 95% CI: 1.36 to 4.46 | ||
| Ovulation | |||||
| Multiple pregnancy Miscarriage | |||||
| OHSS | |||||
| Adverse effects | |||||
| Farquhar et al. (19), 2012 | CC resistant PCOS | Live birth | LOD vs. Gonadotrophins long-term | Costs | MD: -2235.0, 95% CI: -4433.16 to -36.84 |
| Pregnancy | LOD vs. Gonadotrophins short-term | Costs | MD: -1115.75, 95% CI: -1309.72 to -921.77 | ||
| Ovulation | |||||
| Miscarriage | |||||
| Multiple pregnancy | |||||
| OHSS | LOD vs. Gonadotrophins | Multiple pregnancy | OR: 0.13, 95% CI: 0.03 to 0.52 | ||
| Costs | |||||
| Moazami et al. (23), 2014 | CC-resistant PCOS | Live birth Pregnancy Miscarriage | LOD vs. Gonadotropins | Live birth/woman | OR: 0.446, 95% CI: 0.269 to 0.74 |
| Multiple pregnancies | LOD vs. Gonadotropins | Multiple pregnancy | OR: 0.127, 95% CI: 0.028 to 0.579 | ||
| Multiple pregnancy OHSS | Gonadotrophins+metformin vs. Gonadotrophins in OI | Pregnancy/woman | OR: 2.25, 95% CI: 1.50 to 3.38 | ||
| Gonadotrophins+metformin vs. Gonadotrophins in OI | Cancellation/cycle | OR: 0.41, 95% CI: 0.24 to 0.72 | |||
| Gonadotrophins+metformin vs. Gonadotrophins in OI | Gonadotrophins units | MD: 306.62, 95% CI: -500.02 to -113.22 | |||
| Gonadotrophins+metformin vs. Gonadotrophins in OI | Stimulation length | MD: -3.28, 95% CI: -6.23 to -0.32 | |||
| Palomba et al. (40), 2014 | PCOS | Live birth Pregnancy Miscarriage | Gonadotrophins+metformin vs. Gonadotrophins in OI | Live birth/woman | |
| Multiple pregnancy OHSS | Gonadotrophins+metformin vs. Gonadotrophins in OI | Pregnancy/woman | |||
| Gonadotrophins+metformin vs. Gonadotrophins in OI | Cancellation/cycle | ||||
| Gonadotrophins+metformin vs. Gonadotrophins in OI | Gonadotrophins units | ||||
| Gonadotrophins+metformin vs. Gonadotrophins in OI | Stimulation length | ||||
| Weiss et al. (29), 2015 | CC-resistant ± failure PCOS Women treated with prior metformin use +/- CCWomen with prior electro cautery of ovaries. | Live birth | rFSH vs. All urinary gonadotrophins | Gonadotrophins units | MD: -105.44, 95% CI: -154.21, -56.68 |
| rFSH vs. HMG | Gonadotrophins units | MD: -283.94, 95% CI: -449.10 to -118.78 | |||
| Clinical pregnancy Miscarriage | rFSH vs. uFSH | Gonadotrophins units | MD: -88.4, 95% CI: -139.44 to -37.36 | ||
| rFSH vs. All urinary gonadotrophins | Stimulation length | MD: -0.66, 95% CI: -1.04 to -0.28 | |||
| Multiple pregnancy OHSS | rFSH vs. HMG | Stimulation length | MD: -2.28, 95% CI: -3.49 to -1.07 | ||
| rFSH vs. uFSH | Stimulation length | MD: -0.49, 95% CI: -0.88 to -0.09 | |||
| Laparoscopic ovarian drilling (LOD) | |||||
| Farquhar et al. (19), 2012 | CC resistant PCOS | Live birth | LOD vs. Metformin | Pregnancy/woman | OR: 2.47, 95% CI: 1.05 to 5.81 |
| Pregnancy | LOD vs. Other medical treatments | Multiple pregnancy | OR: 0.21, 95% CI: 0.08 to 0.58 | ||
| Ovulation | |||||
| Miscarriage | |||||
| Multiple pregnancy | |||||
| OHSS | |||||
| Costs | |||||
| Baghdadi et a. (56), 2012 | CC resistant PCOS | Pregnancy | Lean vs. Obese PCOS | Ovulation/cycle | RR: 1.90, 95% CI: 1.46 to 2.48 |
| Lean vs. Obese PCOS | Ovulation/woman | RR: 1.43, 95% CI: 1.22 to 1.66 | |||
| Ovulation | Lean vs. Obese PCOS | Pregnancy/cycle | RR: 4.14, 95% CI: 2.08 to 8.23 | ||
| Lean vs. Obese PCOS | Pregnancy/woman | RR: 1.73, 95% CI: 1.39 to 2.17 | |||
| IUI/IVF/ICSI related interventions | |||||
| Luo et al. (57), 2014 | PCOS undergoing COS/IUI | Live birth | GnRH antagonist +IUI vs. Control IUI | LH | MD: 4.6, 95% CI: 0.9 to 8.31 |
| Clinical pregnancy | GnRH antagonist +IUI vs. Control IUI | Premature lutenization rate | OR: 4.36, 95% CI: 2.15 to 8.84 | ||
| Miscarriage | GnRH antagonist +IUI vs. Control IUI | Progesterone | MD: 0.31, 95% CI: 0.24 to 0.37 | ||
| Kollman et al. (18), 2016 | PCOS | Inositol vs. Placebo IVF | Pregnancy/woman | RR: 1.41, 95% CI: 1.05 to 1.89 | |
| Live birth/ ongoing pregnancy | Myo-inositol vs. D-chiro-inositol | Pregnancy/woman | RR: 2.86, 95% CI: 1.14 to 7.16 | ||
| Clinical pregnancy Miscarriage | Antagonist vs. Agonist | OHSS | RR: 0.63, 95% CI: 0.49 to 0.80 | ||
| OHSS | Mannitol vs. Placebo | OHSS | RR: 0.54, 95% CI: 0.39 to 0.77 | ||
| Palomba et al. (59), 2013 | PCOS undergoing IVF cycles | Live birth Pregnancy Miscarriage | Gonadotrophins+metformin vs. Gonadotrophins (Metformin stopping time until 12 weeks of gestation) | Live birth/woman | OR: 75.6, 95% CI: 8.03 to 711.5 |
| Gonadotrophins+metformin vs. Gonadotrophins | Miscarriage | OR: 0.50, 95% CI: 0.30 to 0.83 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (Metformin stopping time until 12 weeks of gestation) | Miscarriage | OR: 0.08, 95% CI: 0.02 to 0.39 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (Pretreatment length effect for long-term >3 weeks) | Miscarriage | OR: 0.41, 95% CI: 0.21 to 0.78 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (Pretreatment length effect for short-term ≤ 3 weeks) | OHSS | OR: 0.20, 95% CI: 0.07 to 0.54 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (Metformin stopping time until oocyte retrieval, ET and HCG injection) | OHSS | OR: 0.22, 95% CI: 0.11 to 0.42 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (no pretreatment period) | OHSS | OR: 0.14, 95% CI: 0.05 to 0.38 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (higher dose >1000 mg daily) | OHSS | OR: 0.40, 95% CI: 0.20 to 0.80 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (lower dose <=1000 mg/daily) | OHSS | OR: 0.15, 95% CI: 0.06 to 0.38 | |||
| OHSS | Gonadotrophins+metformin vs. Gonadotrophins | OHSS | OR: 0.27, 95% CI: 0.16 to 0.46 | ||
| Gonadotrophins+metformin vs. Gonadotrophins | Oocyte number retrieved | WMD: -1.11, 95% CI: -1.86 to -0.36 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (higher dose >1000 mg daily) | Oocyte number retrieved | WMD: -1.16, 95% CI: -1.96 to -0.37 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (Pretreatment length effect for long-term >3 weeks) | Oocyte number retrieved | WMD: -1.45, 95% CI: -2.37 to -0.53 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (Metformin stopping time until pregnancy test) | Oocyte number retrieved | WMD: -1.32, 95% CI: -2.40 to -0.23 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (Pretreatment length effect for long-term >3 weeks) | Implantation/embryo | OR: 0.28, 95% CI: 0.12 to 0.62 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (higher dose > 1000 mg daily) | Implantation/embryo | OR: 1.42, 95% CI: 1.24 to 2.75 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (Metformin stopping time until pregnancy test) | Stimulation length | WMD: 0.85, 95% CI: 0.02 to 1.68 | |||
| Gonadotrophins+metformin vs. Gonadotrophins (lower dose <=1000 mg/daily) | Gonadotrophins units | WMD: -326.84, 95% CI: -505.99 to -147.69 | |||
| Huang et al. (21), 2015 | PCOS undergoing IVF/ICSI in non-donor cycles | Live birth Clinical pregnancy | Metformin vs. Placebo | OHSS | RR: 0.44; 95%CI 0.26 to 0.77 |
| Miscarriage Multiple pregnancy | |||||
| OHSS | |||||
| Tso et al. (42), 2014 | PCOS and of reproductive age undergoing IVF or ICSI | Live birth | Metformin vs. Placebo | Pregnancy/woman | OR: 1.52, 95% CI: 1.07 to 2.15 |
| Clinical pregnancy | Metformin vs. Placebo | Side effects | OR: 4.49, 95% CI: 1.88 to 10.72 | ||
| Miscarriage OHSS | Metformin vs. Placebo | OHSS | OR: 0.29, 95% CI: 0.18 to 0.49 | ||
| Side effects | Metformin vs. Placebo (long protocol GnRH agonist) | OHSS | OR: 0.29, 95% CI: 0.16 to 0.51 | ||
| Pundir et al. (26), 2012 | PCOS undergoing IVF with or without ICSI | Live birth | GnRH antagonist vs. Agonist | Gonadotrophins units | WMD: -0.28, 95% CI: -0.43 to -0.13) |
| Clinical pregnancy | GnRH antagonist vs. Agonist | Moderate and severe OHSS | RR: 0.59, 95% CI: 0.45 to 0.76 | ||
| Ongoing pregnancy | GnRH antagonist vs. Agonist | OHSS (moderate & severe) | RR: 0.60, 95% CI: 0.48 to 0.76 | ||
| Miscarriage | GnRH antagonist vs. Agonist | Stimulation length | WMD: -0.74, 95% CI: -1.12 to -0.36 | ||
| OHSS | |||||
| Siristatidis et al. (50), 2015 | PCOS, PCO and control undergoing IVM | Live birth | IVM in (PCOS vs. Control) | Cancellation/cycle | OR: 0.15, 95% CI: 0.05 to 0.44 |
| Clinical pregnancy | IVM in (PCOS vs. Non PCOS) | Cancellation/cycle | OR: 0.18, 95% CI: 0.06 to 0.47 | ||
| Miscarriage | IVM in (PCOS vs. PCO) | Cancellation/cycle | OR: 0.25, 95% CI: 0.07 to 0.92 | ||
| Oocyte maturation | IVM in (PCOS vs. Non PCOS) | Implantation/embryo | OR: 1.73, 95% CI: 1.06 to 2.81 | ||
| IVM in (PCOS vs. Control) | Maturation/oocyte | OR: 0.74, 95% CI: 0.59 to 0.93 | |||
| IVM in (PCOS vs. Control) | Pregnancy/cycle | OR: 3.09, 95% CI: 1.46 to 6.53 | |||
| IVM in (PCOS vs. Non-PCOS) | Pregnancy/cycle | OR: 2.23, 95% CI: 1.45 to 3.43 | |||
| IVM in (PCOS vs. Control) | Pregnancy/woman | OR: 3.29, 95% CI: 1.42 to 7.62 | |||
| IVM in (PCOS vs. Non PCOS) | Pregnancy/woman | OR: 2.37, 95% CI: 1.53 to 3.68 | |||
| Xiao et al. (31), 2013 | PCOS | Clinical pregnancy | GnRH antagonist vs. GnRH agonist | Moderate-severe OHSS | OR: 0.36, 95% CI: 0.25 to 0.52 |
CC; Clomiphene citrate, COS; Controlled ovarian stimulation, ET; Embryo transfer, FSH: Follicle stimulating hormone, GnRH; Gonadotrophins releasing hormone, HCG; Human chorionic gonadotrophin, HMG; Human menopausal gonadotrophin, ICSI; Intra cytoplasmic sperm injection, IUI; Intra uterine insemination, IVF; In vitro fertilization, IVM; In vitro maturation, LH; Luteinizing hormone, LOD; Laparoscopic ovarian drilling, MD; Mean difference, OHSS; Ovarian hyper-stimulation syndrome, OI; Ovulation induction, OR; Odds ratio, PCOS; Polycystic ovary syndrome, rFSH; Recombinant follicle stimulating hormone, RR; Risk ratio, uFSH; Urinary follicle stimulating hormone, and WMD; Weighted mean difference
Clomiphene citrate
Seventeen reviews, [seven high quality (19, 39, 41, 46- 49), six moderate quality (16, 20, 24, 27, 32, 38) and four low quality (28, 30, 33, 36)] assessed interventions that contained CC, comprising a total of 203 trials with 26 731 participants. One review assessed CC versus LOD (19). One review assessed early follicular versus late luteal CC administration (36). The remaining 14 reviews assessed CC ± other OI drugs such as metformin, inositol, N-acetyl cysteine (NAC) and others versus other OI drugs, including CC. The populations studied were women with PCOS who were treatment-naïve (27), CC resistant (19, 32, 33) and women with PCOS who were treatment-naïve ± CC resistant PCOS or unknown treatment status.
The meta-analyses reported in overall women with PCOS that CC compared to placebo had statistically higher pregnancy and ovulation (46). Early follicular CC had higher pregnancy than late luteal CC (46) but with less mature follicles (36). Higher live birth, pregnancy, and ovulation resulted after CC compared to metformin mainly in women with BMI ≥30 kg/m2 (28, 30, 41) while metformin resulted in higher pregnancy than CC in women with BMI <30 kg/m2 (41). CC plus metformin was of more benefit than CC or metformin alone with regards to live birth (24), pregnancy and ovulation, but had higher gastrointestinal side effects (24, 28, 30, 33, 41). Higher live birth and pregnancy resulted after gonadotrophins compared to CC and 10 days of CC compared to 5 days of CC, respectively (46).
In women with CC resistant PCOS, gonadotrophins resulted in statistically higher live birth, pregnancy and ovulation than CC plus metformin (32, 46) which, in turn, resulted in higher live birth than LOD (19). In the same population of women, the addition of dexamethasone, NAC or contraceptive pills to CC resulted in higher live births, pregnancy and ovulation than CC alone (46, 47). Furthermore, the addition of metformin to CC resulted in more favourable outcomes compared with the addition of NAC with regards to pregnancy and ovulation. However, the cost of treatment was greater for gonadotrophins followed by LOD then CC plus metformin (19).
Gonadotrophins
Ten reviews [six high quality (19, 29, 39, 45, 46, 49) and four moderate quality (23, 32, 40, 59)] assessed interventions containing gonadotrophins, which comprised 146 trials with 18 379 participants. Two reviews assessed gonadotrophins versus LOD (19, 23). Three reviews assessed the effectiveness of adding metformin to gonadotrophins during OI (40, 45) and IVF (59). Two reviews assessed gonadotrophins versus anti-oestrogens ± adjunctive drugs (32, 46). Two reviews assessed gonadotrophins versus aromatase inhibitors (39, 49). One review assessed the effectiveness of different types of gonadotrophins (29). The populations studied were women with CC resistant PCOS (19, 23, 29, 32) and women who were treatment-naïve ± CC resistant PCOS women or unknown treatment status.
The meta-analyses reported that in women with CC resistant PCOS, gonadotrophins resulted in statistically higher live births, multiple pregnancies, and costs of short- and long-term treatment in comparison to LOD (19, 23) and higher live births, pregnancy and ovulation in comparison to CC ± metformin (32, 46), but lower pregnancy in comparison to letrozole (39). Adding metformin to gonadotrophins, compared to gonadotrophins alone, resulted in higher live birth and pregnancy in OI (40, 45) and higher live birth, implantation rate, lower miscarriage, ovarian hyperstimulation syndrome (OHSS) and number of oocyte retrieved in IVF (59). Recombinant follicle stimulating hormone (FT.) resulted in lower dose and stimulation duration than other urinary gonadotrophins in OI (29).
Insulin sensitizers
Thirty reviews (12 reviews of high quality (18, 19, 34, 39, 41, 42, 44-49), 13 reviews of moderate quality (16, 21, 22, 24, 25, 32, 35, 37, 38, 40, 53, 54, 59) and five reviews of low quality (28, 30, 33, 52, 58) assessed interventions that contained insulin sensitizers comprising 398 trials with 45 031 participants. Four reviews assessed metformin versus placebo (18, 21, 41, 42). Four reviews assessed metformin during pregnancy (52-54, 58). One review assessed the effect of pre-gestational metformin on risk of miscarriage (25). One review assessed roziglitazone, plioglitazone, and D-chiro-inositol versus placebo (41). One review assessed metformin versus thiazolidinediones (22). One review assessed LOD versus metformin (19). One review assessed NAC versus placebo or metformin (47). One review assessed oral contraceptive pills versus metformin (34). One review assessed the benefit of adding vitamin D to metformin (37).Three reviews had CC resistant PCOS women as participants (19, 32, 33) while the others did not clarify the treatment status.
The meta-analyses reported that, overall in women with PCOS, metformin resulted in higher live births, pregnancy, and gastrointestinal side effects with lower OHSS than placebo when used in addition to IVF (18, 21, 42) and higher pregnancy, ovulation, side effects and menstrual frequency in OI (41). Metformin had higher gastrointestinal side effects than thiazolidinediones (22). In women with CC resistant PCOS, NAC resulted in higher live births, pregnancy and ovulation than placebo, but lower pregnancy and ovulation than metformin (47). Oral contraceptive pills were better than metformin in improving menstrual frequency (34). Adding vitamin D to metformin improved menstrual frequency than metformin alone (37). Inositol resulted in higher pregnancy than placebo with more benefit of myoinositol over D-chiro inositol in IVF (18), while inositol resulted in higher ovulation than placebo in OI. Roziglitaone, pioglitazone and inositol improved menstrual frequency in OI (38). In women with PCOS who became pregnant, metformin intake during pregnancy resulted in higher live birth and lower miscarriage, preterm labour, gestational hypertension, preeclampsia, gestational diabetes and intrauterine growth retardation (52-54, 58).
Laparoscopic ovarian drilling
Six reviews [four high quality (19, 32, 39, 49) and two moderate quality (23, 56)] assessed ovarian ablation therapy and LOD as an intervention in PCOS comprising 97 trials with 13 617 participants. Three reviews had participants as CC resistant PCOS (19, 23, 56).
The meta-analyses reported that LOD resulted in lower live births than CC plus metformin and gonadotrophins, respectively (19, 23), higher pregnancy than metformin alone (19), lower ovulation than letrozole (49), higher costs than CC plus metformin but lower than gonadotrophins (19) and lower multiple pregnancy rate than other medical treatments (19). Pregnancy and ovulation were higher in lean women (BMI <25 kg/m2) with CC resistant PCOS than in overweight and obese women (BMI ≥25 kg/m2) undergoing LOD (56).
Intrauterine insemination, in vitro fertilization, intracytoplasmic sperm injection related interventions
Nine reviews [three high quality (17, 18, 42)] and six moderate quality (21, 26, 31, 50, 57, 59) assessed different interventions in women with PCOS undergoing assisted reproductive techniques [intrauterine insemination (IUI), IVF/ICSI] comprising 126 trials with 12 298 participants in eight reviews and 333 cycles in the ninth review which did not report on the number of participants (57). Three reviews assessed gonadotrophin releasing hormone (GnRH) antagonist as an adjuvant intervention in controlled ovarian stimulation plus IUI (57) and in comparison with GnRH agonist during IVF/ICSI (26, 31). Three reviews assessed the effect of metformin during IVF/ICSI (21, 42, 59). Two reviews assessed the use of IVM (17, 50).
The meta-analyses reported statistically significant results for lower progesterone, luteinizing hormone (LH) and premature luteinisation rate during IUI after GnRH antagonist (57) and lesser dose, duration of gonadotrophins and OHSS rate after GnRH antagonist during lVF/ ICSI . Metformin compared to placebo in IVF resulted in higher live births (18, 59), pregnancy (18, 42), lower miscarriage (59), lower OHSS (18, 21, 42, 59), and lower oestradiol (E2), gonadotrophin dose and higher implantation rate (59); however, disadvantages included more, yet mild, gastrointestinal side effects (42). Compared to placebo, inositol resulted in higher pregnancy with better results after myoinositol than D-Chiro inositol, while mannitol resulted in lower OHSS (18). IVM used in women with PCOS had higher pregnancy, lower cancelled cycles, higher implantation but lower mature oocytes than IVM in non-PCOS patients (50).
Other interventions
A low quality review reported that bariatric surgery improved menstrual frequency in women with PCOS in six trials and 264 participants (51). A high quality review reported that statins did not improve menstrual frequency or ovulation in women with PCOS not trying to conceive in four trials and 244 participants (43). A high quality review (44) assessed the use of antidepressants in women with PCOS, and identified no studies reporting on any of the primary reproductive outcomes with the exception of one RCT that reported on endocrine and metabolic outcomes between fluoxetine with sibutramine found no significant difference between both drugs (61). A moderate quality review assessed orlistat versus other anti-obesity drugs and found no difference in reproductive outcomes (55).
Discussion
We reported the first overview of systematic reviews on treatment for reproductive outcomes in women with PCOS. This review follows a process of systematic reviews proposed by the Cochrane collaboration that summarizes evidence from more than one systematic review of different interventions for the same condition (62, 63). This type of review can be utilized as a rich source of data synthesis for developing and updating guidelines, and for health care policy makers. Our overview included 53 systematic reviews (9 older versions and 44 currently updated articles), 498 studies, and 56 057 participants. The quality of most included reviews was moderate to high, although the quality of included studies was variable.
Our results align with most current guidelines on PCOS. According to many guidelines, treatment of anovulation in PCOS should start with lifestyle modification before commencing pharmacological agents, especially in obese women with BMI >30 kg/m2 (1, 3, 8, 10, 11), The firstline pharmacological agent is usually CC (2, 3, 11, 64, 65) and some guidelines propose letrozole as an alternative (1, 8, 10). Our results suggest that, overall, in women with PCOS (with or without CC resistance), letrozole resulted in higher live birth and clinical pregnancy rates than other OI drugs, especially CC. This is consistent with many reviews and RCTs (9, 20, 27, 32, 39, 49, 66-68), despite the fact that letrozole is an off-label drug in OI. Nevertheless, the issue of safety in pregnancy for both CC and letrozole has not been completely resolved. Most large retrospective studies found no evidence of any difference between these drugs (69). Metformin is recommended in many guidelines as an adjunctive treatment with CC in women with glucose intolerance and in obese women (1-3, 8, 10), while the National Institute for Health and Clinical Excellence Guidance (NICE) recommended metformin alone or with CC as a first-line treatment (11). Our results suggest that, overall, in women with PCOS, CC plus metformin also resulted in in better reproductive outcomes than CC or metformin alone. The Australian National Health and Medical Research Council (NHMRC) evidence-based guidelines suggested that it is acceptable to use gonadotrophins as a first-line treatment (8). Our results suggest that the use of gonadotrophins resulted in higher live birth and clinical pregnancy rates than CC, overall, in women with PCOS.
CC is usually used for six months, which is recommended by many guidelines (1, 8, 11). After that, women are considered to be CC resistant, which necessitates a second-line treatment. Most fertility guidelines recommend low dose gonadotrophins or LOD as a second-line treatment (1-3, 8, 10, 11). CC plus metformin was also recommended by some guidelines, if not already used as a first-line treatment (8, 11). Gonadotrophins have the disadvantage of cost and increased rates of multiple pregnancies, while LOD has a risk with anaesthesia, decreased ovarian reserve, and the need to use adjuvant drugs for OI after surgery (3). Our results suggest that, in women with CC resistant PCOS, gonadotrophins resulted in better reproductive outcomes than many OI drugs with the disadvantages of increased multiple pregnancies and increased cost (19, 23, 32, 46). We found that women who used gonadotrophins had higher live birth than those who were prescribed CC plus metformin or LOD respectively, and higher clinical pregnancy and ovulation rates than CC plus metformin. CC plus metformin resulted in higher live birth rate and lower cost than LOD. Gonadotrophins are more expensive than LOD. LOD has the advantage of lower rates of multiple pregnancies compared to other interventions, such as gonadotrophins, in CC resistant PCOS (19). LOD in lean women seem to have better reproductive outcomes than in overweight and obese women.
Current recommendations state that IVF should be used in case of CC failure, which is defined by failure of conception after 6-9 months (1, 11). Our results support the current evidence for use of GnRH antagonists and addition of metformin to GnRH agonist to decrease OHSS (1). There is lack of data on use of IVM in PCOS (1), which is reported by one of included reviews (17). Another review by the same author reported higher pregnancy and implantation rates with lower cancellation rate in women with PCOS undergoing IVM compared to IVM in nonPCOS women (50).
Despite the large number of reviews and RCTs that have been conducted assessing different treatments for management of reproductive outcomes in women with PCOS, there are still a considerable number of research gaps. Recently, the international evidence-based guideline for the assessment and management of PCOS has issued new recommendations for the diagnosis and management of PCOS(70). These guidelines state that letrozole should be considered first-line pharmacological treatment for OI in women with PCOS with anovulatory infertility and no other infertility factors to improve ovulation, pregnancy and live birth rates. This is consistent with our results in this overview. They also stated that inositol (in any form) should currently be considered an experimental therapy in PCOS, with emerging evidence on efficacy highlighting the need for further research (70). Furthermore, research on the possible reasons for CC resistance and failure utilizing unified definitions is needed. This is particularly relevant given that some recent reviews revealed that the antiestrogenic effect of CC, specifically on endometrial tissue, is not enough rationale for resistance and failure (66). Furthermore, a recent crossover RCT found that there is no difference in clinical pregnancy and live birth rates between CC and letrozole when used as a second line treatment in women who failed to ovulate or conceive with CC or letrozole as first line of treatment (9). It is also important to note that a thorough study of the cost effectiveness of any of these treatments has not been performed, particularly in low income countries. Further investigation of metformin with regards to its cost effectiveness, safety, and effectiveness in non-obese women is also needed (1, 8). There is also a lack of data relating to the comparison between the use of LOD and medical treatment as a first line treatment, and the minimum efficient dose of LOD to induce ovulation without affecting ovarian reserve (1, 3, 11).
Limitations include our search strategy with reviews published from 2009 onwards, coinciding with the PRISMA statement publication for conducting systematic reviews. While this would miss earlier reviews, later included reviews would be likely to be of higher quality and aligned with the PRISMA statement. We applied language restrictions including only articles in English, which might lead to bias in exclusion of other languages. We found insufficient data on the quality of included studies in each review. We did not perform a quality assessment of each of the individual trials within each systematic review and relied instead on the judgement of the authors which varied from cursory to comprehensive; although we note that performing a quality assessment of 498 total studies would have been an extensive task. We note that the actual effect of different treatments in each treatment status and PCOS phenotypes is still unclear. We also note wide variability in the definition of outcomes across reviews and included studies. For instance, although pregnancy was reported as clinical pregnancy in most included reviews, ongoing pregnancy was reported in some reviews (26, 45) and pregnancy was not predefined in others (22, 24, 36, 40, 51, 56). The definition of clinical pregnancy varied across the included studies within each review.
Conclusion
We report here a significant contribution to the literature in the overview and synthesis of systematic reviews that assessed medical and surgical treatments for reproductive outcomes in women with PCOS. In agreement with most recent international guidelines on management of PCOS, letrozole was superior to other OI agents as a first-line pharmacological treatment with gonadotrophins a second-line pharmacological treatment for anovulatory women with PCOS.
Supplementary PDF
Acknowledgments
There is no financial support and Conflict of interest in this study.
Author’s Contributions
L.J.M., D.S.H., B.W.J.M., A.M., H.J.T., S.T., J.P., R.J.N., M.A.G., C.T.T.; Participated in conception and design. L.J.M., D.S.H., C.T.T.; Participated in identification and selection of included articles. L.J.M., M.A.G., C.T.T.; Participated in data extraction and quality assessment of included articles. M.A.G.; Drafted the manuscript, revising by all authors. All authors read and approved the final manuscript.
References
- 1.Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687–708. doi: 10.1093/humupd/dmw025. [DOI] [PubMed] [Google Scholar]
- 2.Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23(3):462–477. doi: 10.1093/humrep/dem426. [DOI] [PubMed] [Google Scholar]
- 3.Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4):P1–P29. doi: 10.1530/EJE-14-0253. [DOI] [PubMed] [Google Scholar]
- 4.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29(2):181–191. [Google Scholar]
- 5.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar- Morreale HF, Futterweit W, et al. The androgen excess and PCOS society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 7.7. Final Report National Institute of Health. Evidence-based Methodology Workshop on Polycystic Ovary Syndrome. Executive summary at https://prevention.nih.gov/sites/default/files/2018-06/ FinalReport.pdf. (3-5 Dec 2012)
- 8.Evidence-based guidelines for the assessment and management of polycystic ovary syndrome. Jean Hailes for Women’s Health on behalf of the PCOS Australian Alliance. Melbourne: 2015. [Google Scholar]
- 9.Amer SA, Smith J, Mahran A, Fox P, Fakis A. Double-blind randomized controlled trial of letrozole versus clomiphene citrate in subfertile women with polycystic ovarian syndrome. Hum Reprod. 2017;32(8):1631–1638. doi: 10.1093/humrep/dex227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, et al. american association of clinical endocrinologists, american college of endocrinology, and androgen excess and pcos society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome--part 2. Endocr Pract. 2015;21(12):1415–1426. doi: 10.4158/EP15748.DSCPT2. [DOI] [PubMed] [Google Scholar]
- 11.National Collaborating Centre for Women’s and Children’s Health (UK) London: Royal College of Obstetricians & Gynaecologists; 2013. Feb, Fertility: assessment and treatment for people with fertility problems. (NICE Clinical Guidelines, No156) Available from: https://wwwncbinlmnihgov/books/NBK247932/ (Feb 2013) [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10–10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shea BJ, Bouter LM, Peterson J, Boers M, Andersson N, Ortiz Z, et al. External Validation of a Measurement Tool to Assess Systematic Reviews (AMSTAR) PLoS One. 2007;2(12):e135–e135. doi: 10.1371/journal.pone.0001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva V, Grande AJ, Carvalho AP, Martimbianco AL, Riera R. Overview of systematic reviews - a new type of study.Part II. Sao Paulo Med J. 2015;133(3):206–217. doi: 10.1590/1516-3180.2013.8150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinawat S, Buppasiri P, Lumbiganon P, Pattanittum P. Long versus short course treatment with metformin and clomiphene citrate for ovulation induction in women with PCOS. Cochrane Database Syst Rev. 2012;10:CD006226–CD006226. doi: 10.1002/14651858.CD006226.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Siristatidis CS, Vrachnis N, Creatsa M, Maheshwari A, Bhattacharya S. In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database Syst Rev. 2013;(10):CD006606–CD006606. doi: 10.1002/14651858.CD006606.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Kollmann M, Martins WP, Lima ML, Craciunas L, Nastri CO, Richardson A, et al. Strategies for improving outcome of assisted reproduction in women with polycystic ovary syndrome: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2016;48(6):709–718. doi: 10.1002/uog.15898. [DOI] [PubMed] [Google Scholar]
- 19.Farquhar C, Brown J, Marjoribanks J. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2012;(6):CD001122–CD001122. doi: 10.1002/14651858.CD001122.pub4. [DOI] [PubMed] [Google Scholar]
- 20.He D, Jiang F. Meta-analysis of letrozole versus clomiphene citrate in polycystic ovary syndrome. Reprod Biomed Online. 2011;23(1):91–96. doi: 10.1016/j.rbmo.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Wang P, Tal R, Lv F, Li Y, Zhang X. A systematic review and meta-analysis of metformin among patients with polycystic ovary syndrome undergoing assisted reproductive technology procedures. Int J Gynaecol Obstet. 2015;131(2):111–116. doi: 10.1016/j.ijgo.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 22.Li XJ, Yu YX, Liu CQ, Zhang W, Zhang HJ, Yan B, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf) 2011;74(3):332–339. doi: 10.1111/j.1365-2265.2010.03917.x. [DOI] [PubMed] [Google Scholar]
- 23.Moazami Goudarzi Z, Fallahzadeh H, Aflatoonian A, Mirzaei M. Laparoscopic ovarian electrocautery versus gonadotropin therapy in infertile women with clomiphene citrate-resistant polycystic ovary syndrome: A systematic review and meta-analysis. Iran J Reprod Med. 2014;12(8):531–538. [PMC free article] [PubMed] [Google Scholar]
- 24.Palomba S, Pasquali R, Orio F Jr, Nestler JE. Clomiphene citrate, metformin or both as first-step approach in treating anovulatory infertility in patients with polycystic ovary syndrome (PCOS): a systematic review of head-to-head randomized controlled studies and meta-analysis. Clin Endocrinol (Oxf) 2009;70(2):311–321. doi: 10.1111/j.1365-2265.2008.03369.x. [DOI] [PubMed] [Google Scholar]
- 25.Palomba S, Falbo A, Orio F Jr, Zullo F. Effect of preconceptional metformin on abortion risk in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2009;92(5):1646–1658. doi: 10.1016/j.fertnstert.2008.08.087. [DOI] [PubMed] [Google Scholar]
- 26.Pundir J, Sunkara SK, El-Toukhy T, Khalaf Y. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod Biomed Online. 2012;24(1):6–22. doi: 10.1016/j.rbmo.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Roque M, Tostes AC, Valle M, Sampaio M, Geber S. Letrozole versus clomiphene citrate in polycystic ovary syndrome: systematic review and meta-analysis. Gynecol Endocrinol. 2015;31(12):917–921. doi: 10.3109/09513590.2015.1096337. [DOI] [PubMed] [Google Scholar]
- 28.Siebert TI, Viola MI, Steyn DW, Kruger TF. Is metformin indicated as primary ovulation induction agent in women with PCOS?. A systematic review and meta-analysis. Gynecol Obstet Invest. 2012;73(4):304–313. doi: 10.1159/000335253. [DOI] [PubMed] [Google Scholar]
- 29.Weiss NS, Nahuis M, Bayram N, Mol WB, Van der Veen F, van Wely M. Gonadotrophins for ovulation induction in women with polycystic ovarian syndrome. Cochrane Database Syst Rev. 2015;(9):CD010290–CD010290. doi: 10.1002/14651858.CD010290.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Xiao J, Chen S, Zhang C, Chang S. The effectiveness of metformin ovulation induction treatment in patients with PCOS: a systematic review and meta-analysis. Gynecol Endocrinol. 2012;28(12):956–960. doi: 10.3109/09513590.2012.705368. [DOI] [PubMed] [Google Scholar]
- 31.Xiao J, Chen S, Zhang C, Chang S. Effectiveness of GnRH antagonist in the treatment of patients with polycystic ovary syndrome undergoing IVF: a systematic review and meta analysis. Gynecol Endocrinol. 2013;29(3):187–191. doi: 10.3109/09513590.2012.736561. [DOI] [PubMed] [Google Scholar]
- 32.Abu Hashim H, Foda O, Ghayaty E. Combined metformin-clomiphene in clomiphene-resistant polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2015;94(9):921–930. doi: 10.1111/aogs.12673. [DOI] [PubMed] [Google Scholar]
- 33.Gill S, Gemmell A, Colleran R, Bt Zanuri N, O'Brien H, Poobalan A. Does metformin combined with clomiphene citrate improve fertility related outcomes in clomiphene resistant women with PCOS: a systematic review. Middle East Fertility Society Journal. 2014;19(2):81–88. Available from: http://wwwsciencedirectcom/science/article/pii/S1110569014000326 . [Google Scholar]
- 34.Al Khalifah RA, Florez ID, Dennis B, Thabane L, Bassilious E. Metformin or oral contraceptives for adolescents with polycystic ovarian syndrome: a meta-analysis. Pediatrics. 2016;137(5) doi: 10.1542/peds.2015-4089. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YY, Hou LQ, Zhao TY. Effects of acarbose on polycystic ovary syndrome: a meta-analysis. Exp Clin Endocrinol Diabetes. 2014;122(6):373–378. doi: 10.1055/s-0034-1375676. [DOI] [PubMed] [Google Scholar]
- 36.Ding N, Chang J, Jian Q, Liang X, Liang Z, Wang F. Luteal phase clomiphene citrate for ovulation induction in women with polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol Endocrinol. 2016;32(11):866–871. doi: 10.1080/09513590.2016.1197196. [DOI] [PubMed] [Google Scholar]
- 37.Fang F, Ni K, Cai Y, Shang J, Zhang X, Xiong C. Effect of vitamin D supplementation on polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2017;26:53–60. doi: 10.1016/j.ctcp.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Pundir J, Psaroudakis D, Savnur P, Bhide P, Sabatini L, Teede H, et al. Inositol treatment of anovulation in women with polycystic ovary syndrome: a meta-analysis of randomised trials. BJOG. 2018;125(3):299–308. doi: 10.1111/1471-0528.14754. [DOI] [PubMed] [Google Scholar]
- 39.Franik S, Kremer JA, Nelen WL, Farquhar C. Aromatase inhibitors for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2014;(2):CD010287–CD010287. doi: 10.1002/14651858.CD010287.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Palomba S, Falbo A, La Sala GB. Metformin and gonadotropins for ovulation induction in patients with polycystic ovary syndrome: a systematic review with meta-analysis of randomized controlled trials. Reprod Biol Endocrinol. 2014;12:3–3. doi: 10.1186/1477-7827-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2012;(5):CD003053–CD003053. doi: 10.1002/14651858.CD003053.pub5. [DOI] [PubMed] [Google Scholar]
- 42.Tso LO, Costello MF, Albuquerque LE, Andriolo RB, Macedo CR. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD006105.pub3. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raval AD, Hunter T, Stuckey B, Hart RJ. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565–CD008565. doi: 10.1002/14651858.CD008565.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Zhuang J, Wang X, Xu L, Wu T, Kang D. Antidepressants for polycystic ovary syndrome. Cochrane Database Syst Rev. 2013;(5):CD008575–CD008575. doi: 10.1002/14651858.CD008575.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bordewijk EM, Nahuis M, Costello MF, Van der Veen F, Tso LO, Mol BW, et al. Metformin during ovulation induction with gonadotrophins followed by timed intercourse or intrauterine insemination for subfertility associated with polycystic ovary syndrome. Cochrane Database Syst Rev. 2017;1:CD009090–CD009090. doi: 10.1002/14651858.CD009090.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown J, Farquhar C. Clomiphene and other antioestrogens for ovulation induction in polycystic ovarian syndrome. Cochrane Database Syst Rev. 2016;12:CD002249–CD002249. doi: 10.1002/14651858.CD002249.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thakker D, Raval A, Patel I, Walia R. N-Acetylcysteine for polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled clinical trials. Obstet Gynecol Int. 2015;2015:817849–817849. doi: 10.1155/2015/817849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misso ML, Costello MF, Garrubba M, Wong J, Hart R, Rombauts L, et al. Metformin versus clomiphene citrate for infertility in non-obese women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2013;19(1):2–11. doi: 10.1093/humupd/dms036. [DOI] [PubMed] [Google Scholar]
- 49.Misso ML, Wong JL, Teede HJ, Hart R, Rombauts L, Melder AM, et al. Aromatase inhibitors for PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(3):301–312. doi: 10.1093/humupd/dms003. [DOI] [PubMed] [Google Scholar]
- 50.Siristatidis C, Sergentanis TN, Vogiatzi P, Kanavidis P, Chrelias C, Papantoniou N, et al. In Vitro Maturation in women with vs.without polycystic ovarian syndrome: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0134696–e0134696. doi: 10.1371/journal.pone.0134696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butterworth J, Deguara J, Borg CM. Bariatric Surgery, polycystic ovary syndrome, and infertility. J Obes. 2016;2016:1871594–1871594. doi: 10.1155/2016/1871594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng L, Lin XF, Wan ZH, Hu D, Du YK. Efficacy of metformin on pregnancy complications in women with polycystic ovary syndrome: a meta-analysis. Gynecol Endocrinol. 2015;31(11):833–839. doi: 10.3109/09513590.2015.1041906. [DOI] [PubMed] [Google Scholar]
- 53.Zeng XL, Zhang YF, Tian Q, Xue Y, An RF. Effects of metformin on pregnancy outcomes in women with polycystic ovary syndrome: a meta-analysis. Medicine (Baltimore) 2016;95(36):e4526–e4526. doi: 10.1097/MD.0000000000004526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhuo Z, Wang A, Yu H. Effect of metformin intervention during pregnancy on the gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and meta-analysis. J Diabetes Res. 2014;2014:381231–381231. doi: 10.1155/2014/381231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graff SK, Mario FM, Ziegelmann P, Spritzer PM. Effects of orlistat vs.metformin on weight loss-related clinical variables in women with PCOS: systematic review and meta-analysis. Int J Clin Pract. 2016;70(6):450–461. doi: 10.1111/ijcp.12787. [DOI] [PubMed] [Google Scholar]
- 56.Baghdadi LR, Abu Hashim H, Amer SA, Palomba S, Falbo A, Al-Ojaimi E, et al. Impact of obesity on reproductive outcomes after ovarian ablative therapy in PCOS: a collaborative meta-analysis. Reprod Biomed Online. 2012;25(3):227–241. doi: 10.1016/j.rbmo.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 57.Luo S, Li S, Li X, Bai Y, Jin S. Effect of gonadotropin-releasing hormone antagonists on intrauterine insemination cycles in women with polycystic ovary syndrome: a meta-analysis. Gynecol Endocrinol. 2014;30(4):255–259. doi: 10.3109/09513590.2013.863862. [DOI] [PubMed] [Google Scholar]
- 58.Tan X, Li S, Chang Y, Fang C, Liu H, Zhang X, et al. Effect of metformin treatment during pregnancy on women with PCOS: a systematic review and meta-analysis. Clin Invest Med. 2016;39(4):E120–E131. doi: 10.25011/cim.v39i4.27091. [DOI] [PubMed] [Google Scholar]
- 59.Palomba S, Falbo A, La Sala GB. Effects of metformin in women with polycystic ovary syndrome treated with gonadotrophins for in vitro fertilisation and intracytoplasmic sperm injection cycles: a systematic review and meta-analysis of randomised controlled trials. BJOG. 2013;120(3):267–276. doi: 10.1111/1471-0528.12070. [DOI] [PubMed] [Google Scholar]
- 60.Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Syst Rev. 2009;(4):CD002249–CD002249. doi: 10.1002/14651858.CD002249.pub4. [DOI] [PubMed] [Google Scholar]
- 61.Karabacak IY, Karabacak O, Toruner FB, Akdemir O, Arslan M. Treatment effect of sibutramine compared to fluoxetine on leptin levels in polycystic ovary disease. Gynecol Endocrinol. 2004;19(4):196–201. doi: 10.1080/09513590400012077. [DOI] [PubMed] [Google Scholar]
- 62.Thomson D, Russell K, Becker L, Klassen T, Hartling L. The evolution of a new publication type: Steps and challenges of producing overviews of reviews. Res Synth Methods. 2010;1(3-4):198–211. doi: 10.1002/jrsm.30. [DOI] [PubMed] [Google Scholar]
- 63.Becker LA OA. Overviews of reviews. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. 2011. Available from: https://communitycochraneorg/book_pdf/764. (02 Nov 2018) [Google Scholar]
- 64.Moghetti P, Carmina E, De Leo V, Lanzone A, Orio F, Pasquali R, et al. How to manage the reproductive issues of PCOS: a 2015 integrated endocrinological and gynecological consensus statement of the Italian Society of Endocrinology. J Endocrinol Invest. 2015;38(9):1025–1037. doi: 10.1007/s40618-015-0274-y. [DOI] [PubMed] [Google Scholar]
- 65.Vause TDR, Cheung AP. Reproductive Endocrinology and Infertility Committee.Ovulation induction in polycystic ovary syndrome. J Obstet Gynaecol Can. 2010;32(5):495–502. doi: 10.1016/S1701-2163(16)34504-2. [DOI] [PubMed] [Google Scholar]
- 66.Gadalla MA, Huang S, Wang R, Norman RJ, Abdullah SA, El Saman AM, et al. Effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(1):64–76. doi: 10.1002/uog.18933. [DOI] [PubMed] [Google Scholar]
- 67.Wang R, Kim BV, van Wely M, Johnson NP, Costello MF, Zhang H, et al. Treatment strategies for women with WHO group II anovulation: systematic review and network meta-analysis. BMJ. 2017;356:j138–j138. doi: 10.1136/bmj.j138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma S, Ghosh S, Singh S, Chakravarty A, Ganesh A, Rajani S, et al. Congenital malformations among babies born following letrozole or clomiphene for infertility treatment. PLoS One. 2014;9(10):e108219–e108219. doi: 10.1371/journal.pone.0108219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. doi: 10.1093/humrep/dey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.