Significance
Shift workers frequently experience circadian misalignment and are at increased risks of obesity. It is established that some characteristics of the circadian system and body weight regulation differ between males and females. However, whether there are sex differences in the effects of circadian misalignment on energy regulation remains unknown. Here we show that circadian misalignment disrupts energy balance in females and males through different pathways. Females had more disturbances in the energy homeostasis process, including a decrease in the satiety hormone and an increase in hunger hormone. Males had elevated cravings for energy-dense and savory foods. These data have potential implications for sex-specific weight gain prevention in shift work populations and other groups experiencing circadian rhythm disturbances.
Keywords: sex difference, circadian disruption, shift work, appetite, energy metabolism
Abstract
Shift work causes circadian misalignment and is a risk factor for obesity. While some characteristics of the human circadian system and energy metabolism differ between males and females, little is known about whether sex modulates circadian misalignment effects on energy homeostasis. Here we show—using a randomized cross-over design with two 8-d laboratory protocols in 14 young healthy adults (6 females)—that circadian misalignment has sex-specific influences on energy homeostasis independent of behavioral/environmental factors. First, circadian misalignment affected 24-h average levels of the satiety hormone leptin sex-dependently (P < 0.0001), with a ∼7% decrease in females (P < 0.05) and an ∼11% increase in males (P < 0.0001). Consistently, circadian misalignment also increased the hunger hormone ghrelin by ∼8% during wake periods in females (P < 0.05) without significant effect in males. Females reported reduced fullness, consistent with their appetite hormone changes. However, males reported a rise in cravings for energy-dense and savory foods not consistent with their homeostatic hormonal changes, suggesting involvement of hedonic appetite pathways in males. Moreover, there were significant sex-dependent effects of circadian misalignment on respiratory quotient (P < 0.01), with significantly reduced values (P < 0.01) in females when misaligned, and again no significant effects in males, without sex-dependent effects on energy expenditure. Changes in sleep, thermoregulation, behavioral activity, lipids, and catecholamine levels were also assessed. These findings demonstrate that sex modulates the effects of circadian misalignment on energy metabolism, indicating possible sex-specific mechanisms and countermeasures for obesity in male and female shift workers.
Approximately 20–30% of the workforce in the United States and Europe are engaged in shift work, including permanent night work, rotating shifts, and irregular schedules (1, 2). Shift workers frequently undergo circadian misalignment, a state where environmental/behavioral cycles are out of sync with the endogenous circadian system (3). Circadian misalignment may predispose individuals to weight gain by decreasing total energy expenditure and altering dietary choices (4, 5). Indeed, shift work is a well-recognized risk factor for excessive weight gain and obesity (6, 7). Understanding the physiological mechanisms underlying such risk can facilitate the development of evidence-based countermeasures. Importantly, many epidemiological studies also noticed sex differences in shift work–related risk of metabolic disorders (8). However, these studies appear contradictory, reporting shift work as having either stronger (9–11) or weaker (12, 13) association with obesity and/or metabolic syndrome in females as compared to males. The inconsistency between these studies may be due to systematic differences in the type of night work between males and females in different studies (e.g., differing shift schedules, physical activity, light exposure, food accessibility, eating patterns, etc). Thus, only studies in which these variables are kept constant can distinguish sex-specific effects of circadian misalignment on biology—that is, independent of type of shift work and environmental factors. To our knowledge, no such systematic studies have been performed.
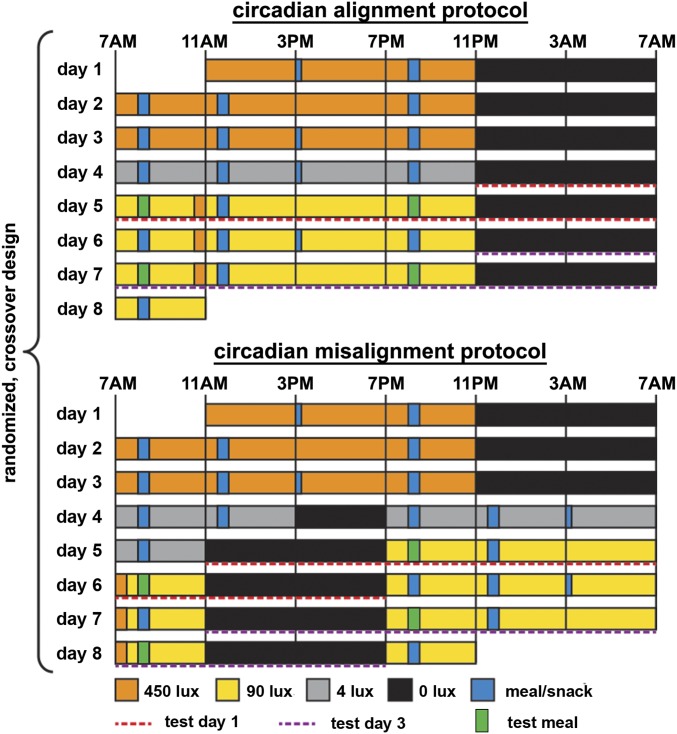
While controlled studies on the effects of circadian misalignment on energy control are critically lacking, sex differences have been well-characterized in the circadian system and energy metabolism separately. For example, females are more likely to be classified as morning types as compared to males (14). This is consistent with underlying biology, in which females on average also have a shorter intrinsic circadian period (cycle length) and earlier timing of core body temperature and melatonin rhythms than males (15, 16). Regarding the regulation of energy homeostasis, there are also profound sex differences, including more efficient fat storage, lower basal lipid oxidation, and more serum leptin per kilogram of body fat in females than in males (17). Females and males also show differences in the physiology of homeostatic and hedonic eating (18). Given these substantial sex differences and the deleterious metabolic consequences of shift work, it is critical to understand the different metabolic responses to circadian disruption in males and females. To address this knowledge gap, we performed a systematic assessment of energy balance–related measures between males and females using a randomized, cross-over study design with 2 highly controlled 8-d in-laboratory protocols (Fig. 1), including either circadian alignment or circadian misalignment conditions (a rapid 12-h shift of the behavioral/environmental cycle for 3 test days).
Fig. 1.
Circadian alignment protocol (Top) and circadian misalignment protocol (Bottom) as part of the randomized, cross-over design. Twenty-four-hour metabolic profiles were assessed on days 5 and 7 in the circadian alignment protocol and across days 5/6 and 7/8 in the circadian misalignment protocol (red and purple dashed lines as test day 1 and test day 3, respectively). Light levels indicated are in the horizontal angle of gaze. Green and blue bars represent test meals (green), other meals (wide blue), and snacks (narrow blue).
Results
Demographics and characteristics were similar between female and male participants, except that females had a higher body fat percentage, as expected (17) (Table 1; for details see SI Appendix, Table S1 and Fig. S1). We did not detect any significant interaction effects on the primary outcomes between “duration of exposure” (i.e., test day 1 vs. test day 3) and other main effects (i.e., misalignment [circadian alignment vs. circadian misalignment], sex, misalignment × sex). Thus, here we report all results with test days 1 and 3 combined.
Table 1.
Characteristics of study participants
| Female | Male | P values | |
| Number | 6 | 8 | NA |
| Age (y) | 27.8 ± 10 [20–45] | 27.4 ± 9.4 [21–49] | 0.93 |
| BMI (kg/m2) | 26.2 ± 2.6 [22.2–28.8] | 24.8 ± 2.7 [21–29.5] | 0.35 |
| Body fat (%) | 39 ± 7 [28.4–47.2] | 25.3 ± 4.8 [19.1–33.5] | <0.001 |
| BMI category | 2 normal | 5 normal | 0.14 |
| 4 overweight | 3 overweight | ||
| Chronotype | 2 moderate morning | 3 moderate morning | 0.85 |
| 4 intermediate | 4 intermediate | ||
| 1 moderate evening | |||
| Menstrual phase | 2 in follicular phase | NA | NA |
| 4 in luteal phase |
Data are presented as n or mean ± SD [range]. No female participants were on oral contraceptives during the study. NA, not applicable.
Sex Differences in Effects of Circadian Misalignment on Appetite-Regulating Hormones.
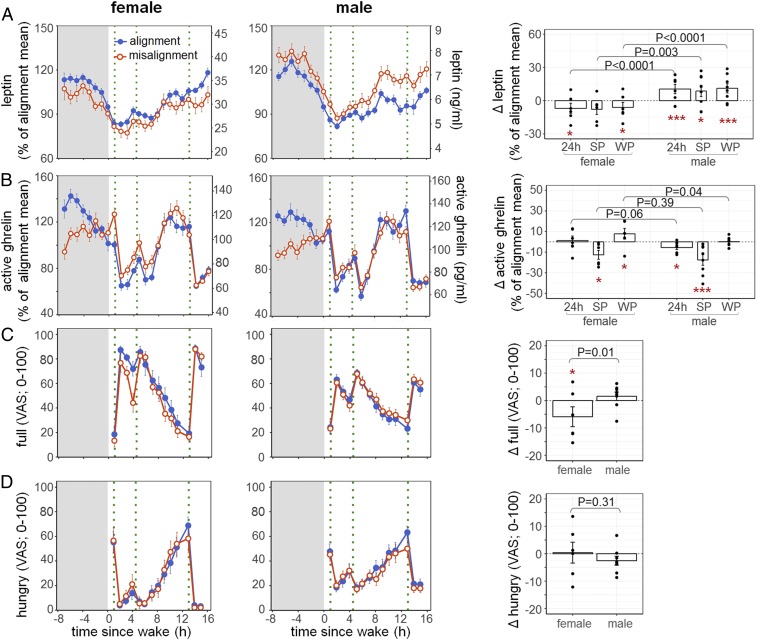
We found no significant overall effects of circadian misalignment on 24-h leptin levels (P = 0.23). This was attributed to the fact that females and males had opposite leptin responses to circadian misalignment (misalignment × sex: P < 0.0001; Fig. 2A). Indeed, circadian misalignment led to a decrease in 24-h leptin levels in females (−6.9 ± 4.6%, adjusted [adj.] P = 0.033) but an increase in males (+10.5 ± 4.2%, adj. P < 0.0001). We also tested the effects of circadian misalignment on appetite hormones during scheduled wake periods (WP; feeding) and sleep periods (SP; fasting) separately. This is because moderate changes in appetite hormones may have different influences on direct food intake during wakefulness versus sleep, given that the latter is less likely to result in changes in caloric intake unless the hunger drive is so strong that it crosses the threshold to arousal from sleep. Consistent with the 24-h leptin profiles, there were sex differences in the effects of circadian misalignment on WP and SP leptin profiles (P < 0.0001 and P = 0.0027, respectively), with females having significant decreases in WP leptin levels (−6.1 ± 4.6%, adj. P = 0.033) and males having significant increases in both WP and SP leptin levels when misaligned (+11.1 ± 4.2%, adj. P < 0.0001, and +8.7 ± 5.1%, adj. P = 0.034, respectively).
Fig. 2.
Effects of circadian misalignment on leptin (A) and active ghrelin (B) levels and hunger (C) and full (D) ratings in females (Left) and males (Middle). Gray bar represents sleep opportunity; green dotted line represents a meal. Percentage changes (A and B) or absolute changes (C and D) under circadian misalignment as compared to circadian alignment across 24 h or during SP and WP are shown in the bar graphs (Right). As aforementioned, since there were no significant interaction effects of duration of exposure and other main effects, values are reported as both test days combined. Each black dot represents an individual value. P values, statistical significance for interaction effect of misalignment and sex. Adj. P values for subgroup analysis by sex, *adj. P < 0.05; ***adj. P < 0.0001.
In addition, circadian misalignment increased the levels of the active form of the orexigenic hormone ghrelin during the wake period (P = 0.029). Such an increase was mostly attributed to females (misalignment × sex: P = 0.040; Fig. 2B), who had 7.8 ± 5.2% elevated WP active ghrelin levels under circadian misalignment as compared to alignment (adj. P = 0.020), while males did not show any changes (+0.2 ± 1.5%, adj. P = 0.99). There were no overall effects of circadian misalignment or its interaction effects with sex on 24-h active ghrelin levels (both P ≥ 0.062). Both females and males had similar reduction in active ghrelin levels during sleep under circadian misalignment vs. alignment (misalignment × sex: P = 0.39; females: −12.6 ± 4.3%, adj. P = 0.020, males: −17.6 ± 5.1%, adj. P < 0.0001).
Females Were Less Full under Circadian Misalignment, While Males Had Increased Cravings for Energy-Dense and Savory Foods.
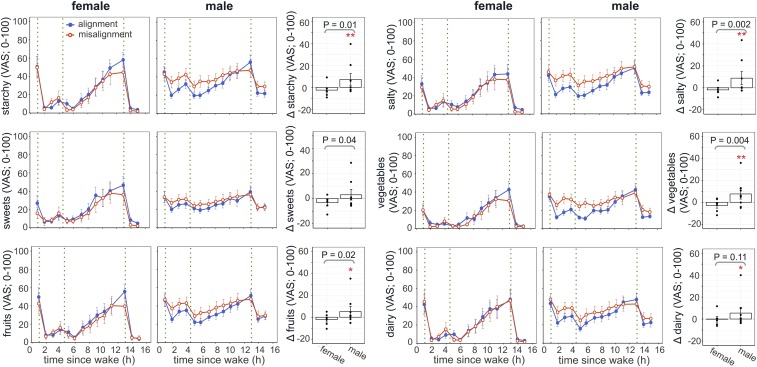
Consistent with the changes in appetite hormones, circadian misalignment induced different changes in self-reported fullness during wake periods in females and males (misalignment × sex: P = 0.008; Fig. 2C), with reduced fullness in females but not in males (−5.9 ± 3.6 [visual analog scale; 0–100], adj. P = 0.044 and +1.6 ± 1.5, adj. P = 0.62, respectively). There was no sex difference in the effect of circadian misalignment on self-reported hunger (P = 0.31; Fig. 2D). Surprisingly, under circadian misalignment, females and males also showed significantly different changes in cravings for starches (P = 0.011), sweets (P = 0.041), salt (P = 0.0015), fruits (P = 0.021), and vegetables (P = 0.0043). It seemed that while in males the homeostatic control of food intake remained undisturbed under circadian misalignment, their cravings for energy-dense and savory foods were significantly increased (starches: +7.2 ± 5.4, adj. P = 0.0052; salt: +8.8 ± 6, adj. P = 0.0008; fruits: +5.2 ± 4.7, adj. P = 0.014; vegetables: +7.7 ± 4.6, adj. P = 0.0004; dairy: +5.4 ± 5.2, adj. P = 0.002; Fig. 3). Such sex differences in misalignment-induced cravings for sweets and salt remained significant (P < 0.05), and for vegetables close to significant (P = 0.055), after removing the male individual with the largest change.
Fig. 3.
Effects of circadian misalignment on cravings for various foods in females (Left) and males (Middle). Green dotted line represents a meal. Changes under circadian misalignment as compared to circadian alignment are shown in the bar graphs (Right). Values are reported as both test days combined. Each black dot represents an individual value. P values, statistical significance for interaction effect of misalignment and sex. Adj. P values for subgroup analysis by sex, *adj. P < 0.05; **adj. P < 0.01.
Females Had Increased Energy Expenditure and Lipid Oxidation Rate under Circadian Misalignment, While Males Remained Unchanged.
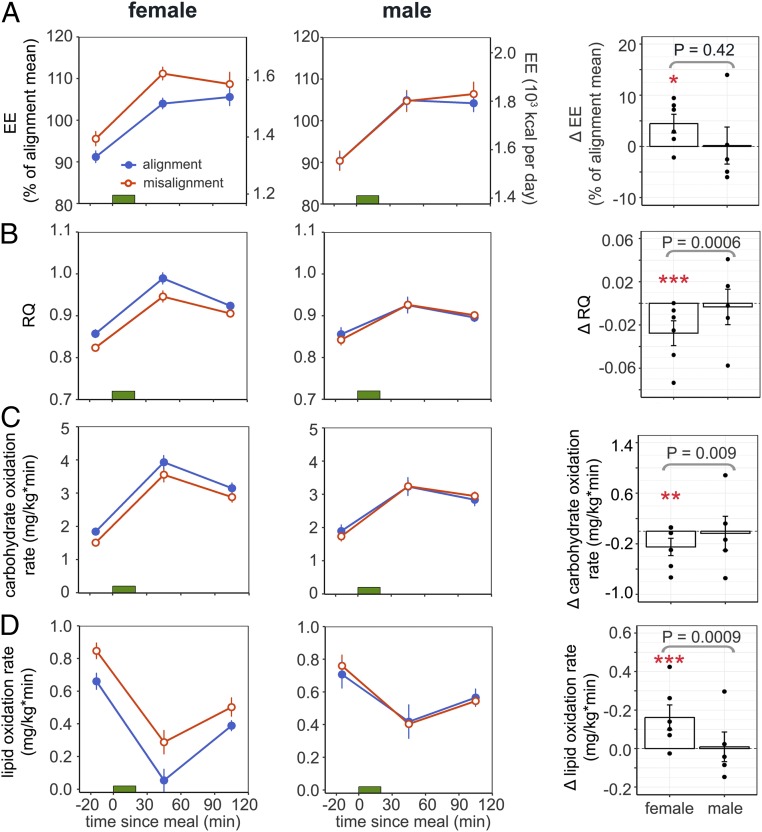
Either decreased energy intake or increased energy expenditure can result in negative energy balance and up-regulation of homeostatic appetite control (19). Because energy intake (per 24 h) in the 2 protocols was identical within each participant and body weight was stable throughout the study, we examined whether there were sex differences in energy expenditure under circadian misalignment as compared to alignment. Interestingly, females had a 4.5 ± 1.8% increase in fasting and postprandial energy expenditure under circadian misalignment (adj. P = 0.037) while males had no alterations (0.2 ± 3.6%, adj. P = 0.99), although no significant interaction effects were detected (misalignment × sex: P = 0.43; Fig. 4A).
Fig. 4.
Effects of circadian misalignment on energy expenditure (A), respiratory quotient (B), glucose oxidation rate (C), and lipid oxidation rate (D) in females (Left) and males (Middle). Green bar represents a meal. Percentage changes (A and B) or absolute changes (C and D) under circadian misalignment as compared to circadian alignment are shown in the bar graphs (Right). Values are reported as both test days combined. Each black dot represents an individual value. P values, statistical significance for interaction effect of misalignment and sex. Adj. P values for subgroup analysis by sex, *adj. P < 0.05; **adj. P < 0.01, ***adj. P < 0.0001.
Moreover, there were significant interaction effects of circadian misalignment and sex on fasting and postprandial substrate utilization as estimated by respiratory quotient (RQ; misalignment × sex: P = 0.006) and carbohydrate and lipid oxidation rates (P = 0.045 and P = 0.0093, respectively; Fig. 4). In females, circadian misalignment reduced RQ (−0.03 ± 0.01, adj. P < 0.0001) by decreasing the carbohydrate oxidation rate (−0.25 ± 0.14 mg/kg × min, adj. P = 0.0055) and increasing the lipid oxidation rate (+0.16 ± 0.06 mg/kg × min, adj. P < 0.0001), while these measurements remained unchanged in males (RQ: 0.0 ± 0.02, adj. P = 0.99; carbohydrate oxidation rate: −0.04 ± 0.27 mg/kg × min, adj. P = 0.92; lipid oxidation rate: 0.01 ± 0.08 mg/kg × min, adj. P = 0.99).
Because physical activity is another crucial contributor to daily energy expenditure (20), we next tested whether physical activity, as estimated by accelerometry, was differently influenced in females versus in males under circadian misalignment. Circadian misalignment significantly reduced 24-h activity counts (misalignment: P < 0.0001). And this effect was similar in females and males (misalignment × sex: P = 0.90; females: −4.7 ± 8.5%, adj. P = 0.02; males: −10.4 ± 4.9%, adj. P < 0.0001; SI Appendix, Fig. S2).
Rapid Shift in Behavioral Cycle by 12-hr Induced Circadian Misalignment and Decreased Total Sleep Time Similarly in Females and Males.
We found similar changes in the fitted peak time and magnitude of 24-h melatonin and cortisol profiles [determined by nonorthogonal spectral analysis (21)] in response to the 12-h shifted behavioral cycle between females and males (all P ≥ 0.21; SI Appendix, Fig. S3 and Table S2). There were also no significant differences between females and males in the changes in stages 1, 2, and 3, REM sleep, and total sleep time under circadian misalignment as compared to circadian alignment (all P ≥ 0.11; SI Appendix, Table S3). Thus, the above sex differences in the effects of circadian misalignment on metabolic measures were not likely attributed to the differences in degree of misalignment or sleep loss.
No Sex Differences in Effects of Circadian Misalignment on Markers of the Autonomic Nervous System.
The autonomic nervous system (ANS) plays an important role in the regulation of energy expenditure (22). Catecholamines are often used as measures of systemic sympathetic activity and are powerful stimulators of lipid oxidation (23). To examine whether the ANS may contribute to the sex differences in changes in energy metabolism under circadian misalignment, we examined estimates of 24-h systemic sympathetic activity from 24-h urinary norepinephrine and epinephrine excretion. We did not find significant sex differences in urinary catecholamines under circadian misalignment (misalignment × sex: both P ≥ 0.56; SI Appendix, Fig. S4A). Moreover, there were no interaction effects of circadian misalignment and sex on parasympathetic markers assessed by frequency and time indices of heart rate variability (all P ≥ 0.20; SI Appendix, Fig. S5B).
No Sex Differences in Effect of Circadian Misalignment on Circulating Free Fatty Acids or Triglyceride Levels.
Since increased lipid fuel availability can elevate lipid oxidation with a reciprocal decrease in glucose oxidation (24), we tested whether circulating free fatty acid (FFA) and triglyceride (TG) concentrations could explain the sex differences in substrate utilization under circadian misalignment. However, we did not detect significant interaction effects of misalignment and sex on either 24-h or WP circulating FFA and TG levels (all P ≥ 0.48; SI Appendix, Fig. S6).
Core Body Temperature.
Because a 1 °C increase in body temperature has been associated with a 10–13% increase in metabolic rate in humans (25), we tested whether changes in core body temperature (CBT) may help explain the increased energy expenditure in females under circadian misalignment. There was a significant sex difference in the effect of circadian misalignment on 24-h CBT (misalignment × sex: P = 0.043; SI Appendix, Fig. S7). However, we did not find significant changes in 24-h CBT under circadian misalignment as compared to alignment in either females or males (females: 0.26 ± 0.21% [equivalent to ∼0.1 °C increase in CBT], adj. P = 0.14; males: 0.14 ± 0.09% [∼0.05 °C increase in CBT], adj. P = 0.33).
Discussion
Here we have demonstrated sex differences in the effects of circadian misalignment on the regulation of energy homeostasis in healthy humans. Since we strictly controlled for food intake, activity, sleep timing, and light exposure, we could examine whether the influence of circadian misalignment per se differs between females and males. Here, we found that females had lower 24-h leptin levels and higher WP active ghrelin levels under circadian misalignment than under alignment, while males, opposite to females, had higher 24-h leptin levels when misaligned and no changes in WP active ghrelin levels. Consistent with the change in appetite hormones, females reported less fullness in circadian misalignment than in alignment. Interestingly, while circadian misalignment did not alter homeostatic hunger ratings (i.e., hungry and full) in males, males reported higher cravings for energy-dense and savory foods, indicating that males were more susceptible to hedonic eating than females when misaligned. On the other end of the energy balance equation, while physical activity estimates were reduced in both sexes under circadian misalignment, only females showed significant changes in energy expenditure (EE) and substrate utilization at rest, with ∼5% increased EE and decreased RQ (by decreased carbohydrate oxidation and increased lipid oxidation). Our results show that circadian misalignment alters energy balance factors in females and males through different pathways. While females showed more disturbances in the energy homeostasis process, males experienced increases in hedonic appetite accompanied by no increases in energy expenditure and no changes in homeostasis appetite, pointing to a direction of energy gain. These data have potential implications for sex-specific weight gain prevention in shift work populations.
Appetites and Appetite Hormones.
Findings from prior human in-laboratory studies suggest that appetite hormones are altered during circadian misalignment when food intake is controlled to meet energy balance in the aligned condition (26–28). Contrary to some of these studies (26, 28), we did not find that circadian misalignment decreased satiety hormone leptin levels in all participants combined, but found significant interaction effects with sex. According to the sex difference we uncovered here, the conflicting results from ours and prior studies could be partially influenced by the number of females in each study, with a high proportion of females contributing to a negative effect. Indeed, both studies with at least half female participants found that circadian misalignment lowered leptin (26, 28), while the study with fewer than half females, like the current study, saw no changes (27). The contrasting results could also be attributed to differences in study design. Our previous study utilized a 28-h-“day” forced desynchrony protocol under dim light conditions, which was less similar to the conditions experienced by real-life shift workers. Moreover, the 28-h day included an extra ∼4 h in addition to the ∼24-h circadian cycle. In the circadian-aligned day, the extra 4-h window was close to the peak of the endogenous circadian rhythm of leptin (circadian phase 0°–60°), while it was close to the trough (circadian phase 180°–240°) in the circadian-misaligned day (29). With a 16% peak-to-trough amplitude of mean leptin levels (29), such difference could have contributed to the observed lower leptin levels in the misaligned day than in the aligned day. As for the McHill et al. (26) study, the transient reduction in 24-h leptin levels on the first simulated night shift could have been partly due to the acute response to short-term negative energy balance (30, 31), which was brought about by the increased total daily EE (∼350 kJ more) and extended fasting duration (∼14.5 h for the first night shift vs. ∼11 h at baseline) during the preceding transition day. In the current study, we minimized some of the above limitations by measuring 24-h profiles of leptin (a full circadian cycle) and applying the same fasting durations in both alignment and misalignment protocols. In the aforementioned study, McHill et al. (26) also reported no changes in 24-h total ghrelin levels at 2 d of simulated night shift conditions (26), which is consistent with our finding of 24-h active ghrelin levels—the primary form for ghrelin’s appetite-stimulating action (32). We further found that circulating active ghrelin changed differently under circadian misalignment during wake (increase) versus sleep (decrease) periods. This can be attributed to the endogenous circadian rhythm of active ghrelin, for which the nadir (in the biological day) coincides with the sleep episodes of the circadian misalignment protocol and the peak (in the biological evening/night) coincides with the wake periods during circadian misalignment (33, 34). Moreover, we found that the change in active ghrelin during misaligned wake periods was modulated by sex, with females as the major contributors to the overall increase. Females also consistently reported reduced fullness. Considering that ghrelin infusion can acutely increase food intake (35), the elevated levels while awake may stimulate caloric intake. Future studies are needed to examine whether the decreased self-reported fullness in females leads to overeating when misaligned. Surprisingly, despite elevated leptin levels and unchanged hunger/fullness ratings, males reported greater cravings for energy-dense and savory foods when misaligned. This suggests that hedonic or reward-based appetite regulation can override the homeostatic pathway in males during circadian misalignment.
Energy Expenditure and Substrate Utilization.
Previous studies exploring effects of circadian misalignment on EE in humans have reported mixed results (26, 27, 36–38). The inconsistency may be attributed to variations in participants’ prior energy balance status (positive, negative, or neutral), degree of sleep debt, physiological state (e.g., resting vs. postabsorptive, 24 h vs. sleep or wake period), or the endogenous circadian phase at the time of assessment (resting and postprandial EE have circadian variations [37, 39]). Here we measured resting and postprandial EE, and ensured that the indirect calorimetry sessions between alignment and misalignment protocols had comparable fasting durations, circadian phases, and energy balance status. As we have previously published, we found no significant effects of circadian misalignment on resting and postprandial EE (39). This result is in line with 2 previous reports of no changes in resting EE after either 3 d or 3 wk of circadian misalignment induced by forced desynchrony protocols (27, 37). Surprisingly, when examining females and males separately, we found that females had a significant increase in EE (resting and postprandial combined) under circadian misalignment. In addition, sex differences were observed for substrate utilization in responses to circadian misalignment. Such sex differences could not be explained by variations in sleep efficiency or degree of circadian misalignment (40, 41), since females and males had similar sleep loss and change in timing or amplitude of circadian markers when misaligned. We also did not detect any sex-specific changes in autonomic markers and circulating FFA and TG, which suggested that pathways other than sympathetic activation or substrate availability may contribute to the increased EE and lipid oxidation in females. Interestingly, there was a significant interaction effect of sex and circadian misalignment on 24-h CBT, although we may have lacked the power to detect significant changes in females and males, separately, in responses to misalignment. Since body temperature has been positively associated with metabolic rate (42, 43), future studies are required to investigate whether sex differences in thermoregulation play a role in sex-specific changes in energy metabolism. We permitted participants to have some low-intensity free movement, which allowed us to observe the reduction in spontaneous physical activity in both females and males under circadian misalignment. This reduction may have contributed to a positive energy balance in males, as their energy intake and EE at rest remained unchanged and thus may have resulted in the aforementioned higher leptin levels under circadian misalignment. As for females, since we did not have 24-h EE measurement, it was not clear whether the reduced physical activity EE was offset by the increased EE at rest. Thus, further investigation is needed to see whether the decreased leptin levels in females are caused by potential transient negative energy balance under circadian misalignment. If not, this suggests that circadian misalignment may predispose females to weight gain by lowering their satiety signaling (leptin).
Strength and Limitations.
The strengths of this study include within-participant comparison of circadian alignment and circadian misalignment, comprehensive assessments of measures related to energy balance, and highly controlled in-laboratory protocols that were able to determine the impact of circadian misalignment and its interaction effect with sex independent of any behavioral and environmental factors. The limitations of the study include relatively small sample size, no measurements of sex hormones, and no power to test the effect of different menstrual phases. Nonetheless, this sample size is in keeping with similar prolonged, highly controlled within-participant studies.
Materials and Methods
Participants.
Fourteen healthy, drug- and medication-free young adults (mean age ± SD, 28 ± 9 y; body mass index [BMI], 25.4 ± 2.6 kg/m2, 6 females; see details in Table 1) completed the study in the Intensive Physiological Monitoring (IPM) Unit of the Brigham and Women’s Hospital Center for Clinical Investigation research facilities. No females were on oral contraceptives during the study (3 had never been on oral contraceptives, 2 had stopped >3 y before the study, and 1 had stopped 2 mo before the first in-laboratory visit). Health status was determined by extensive medical history and physical, psychological, and laboratory examination. For details on exclusion criteria, see previous publications (44–46). All participants provided written informed consent prior to enrollment, and ethical approval for all study procedures was granted by the Partners Healthcare Institutional Review Board.
Preinpatient Study Conditions.
To ensure a stable circadian rhythmicity, participants maintained a regular sleep–wake cycle of 8-h sleep per night for more than 11 d immediately before admission to the laboratory (verified by sleep/wake diaries, call-ins to a time-stamped voice recorder, and wrist actigraphy). Participants refrained from exercise and were provided with a standardized diet 3 d before each in-laboratory visit to ensure energy balance upon admission.
Study Design.
The study design is graphically depicted in Fig. 1. Each participant underwent one circadian alignment and one circadian misalignment protocol in a randomized, cross-over fashion, with a washout of 3–8 wk in between (detailed in SI Appendix). Females were admitted to the laboratory either on days 3–5 (n = 2, follicular phase) or on days 12–18 (n = 4, luteal phase) of their menstrual cycle. For their second visit, females were admitted at the menstrual phase similar to that of their first visit (mean ± SD for the difference between visits, −1.3 ± 1.2 menstrual cycle days). For both protocols, participants arrived at the IPM Unit at ∼10:30 AM and were admitted to a private, sound-attenuated, temperature-controlled suite. In the circadian alignment protocol, the sleep opportunity occurred between 11 PM and 7 AM for all 8 d. Days 1–3 of the circadian misalignment protocol had the same schedule as those of the circadian alignment protocol. Then on day 4 of the circadian misalignment protocol, a 12-h shifted behavioral cycle was achieved by including an 8-h wake episode and a 4-h sleep opportunity, thus maintaining the same scheduled sleep-to-wake ratio (1:2). Metabolic assessments were performed on days 5 and 7 in the circadian alignment protocol and across days 5/6 and 7/8 in the circadian misalignment protocol (1st and 3rd test days, respectively). 24-h blood samples (n = 14) were collected hourly starting shortly after bedtime (SI Appendix provides assay details).
Diet.
Participants consumed an isocaloric diet per 24 h after day 1 in each laboratory protocol, calculated according to the Harris-Benedict equation (activity factor of 1.4; 45–50% carbohydrate, 30–35% fat, 15–20% protein, and at least 2.5 L of water per 24 h). Participants were required to consume all their food. The diet was identical within each participant between the 2 in-laboratory visits (detailed in SI Appendix).
Hunger and Appetite Ratings.
Every hour during wake periods, participants used computerized visual analog scales (VASs; 0 as “not at all” and 100 as “very much”/“extremely”) to rate hunger, appetite, and food preferences (n = 14).
Indirect Calorimetry.
Indirect calorimetry measurements (n = 11; 5 females) for test meal sessions were obtained with a calibrated, open-circuit, ventilated hood system (Vmax Encore 29N; VIASYS Healthcare; detailed in SI Appendix). Technical difficulties precluded indirect calorimetry measurements in 3 of the 14 participants.
Statistical Analysis.
Participants’ characteristics (Table 1) and changes in melatonin and cortisol profiles were compared between groups with χ2 and t tests. The outcomes, including leptin, active ghrelin, and energy expenditure, were expressed as percentages of each participant’s average levels under control conditions (circadian alignment protocol) to focus on the percentage changes in response to circadian misalignment. Other outcomes, including hunger and appetite ratings, RQ, and carbohydrate/lipid oxidation rate, were expressed as raw values to focus on the absolute change in response to circadian misalignment. Linear mixed-effects model analyses were used with the participant as a random factor and 4 main fixed factors: 1) alignment condition (circadian alignment vs. circadian misalignment); 2) sex (female vs. male); 3) test day (test day 1 vs. test day 3); and 4) time (time since start of test meal for indirect calorimetry measurements; time since scheduled wake for all others). Mixed-model analyses included the interaction sex vs. alignment condition. Other 2/3-way interactions and “sequence” (the order of circadian alignment/misalignment conditions) were included if significant. Sequence did not alter any of the main fixed effects and their interactions. BMI was also tested as covariate, but was excluded from the final model since it was not statistically significant. To account for the autocorrelation of time-course measurements, we assumed a first-order autoregressive covariance structure. Two-sided P values of 0.05 were considered for statistical significance. Subgroup analyses were performed by females and males separately using a mixed-effect model to test the effects of alignment condition in each group. To reduce type I error for multiple comparisons, Bonferroni correction was applied for subgroup analyses. Analyses were performed with SAS software, version 9.4 (SAS Institute). Unless specified, data are presented as mean ± SEM.
Additional details of the study protocol, including polysomnography, physical activity measures, assays, etc., are provided in SI Appendix, Materials and Methods.
Data Availability.
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Supplementary Material
Acknowledgments
We thank the research volunteers, research staff, and recruiters, and the nursing and technical staff of Brigham and Women’s Hospital Center for Clinical Investigation. This study was supported by the National Institutes of Health (NIH) (R01HL094806) to F.A.J.L.S. and by the Clinical Translational Science Award (Award UL1RR025758) to Harvard University and Brigham and Women’s Hospital from the National Center for Research Resources. J.Q. was supported in part by the American Diabetes Association (Award 1-17-PDF-103) and by the NIH (Grant R01DK102696). R.C. was supported in part by the Minerva Scholarship and the Trustee Fund. W.W. was supported in part by the NIH (Grants R01DK099512, R01HL118601, and R01DK102696). M.G. was supported in part by The Spanish Government of Investigation, Development and Innovation (SAF2017-84135-R), including FEDER co-funding, and by the NIH (Grant R01DK105072). F.A.J.L.S. was supported in part by the NIH (Grants R01HL118601, R01DK099512, R01DK102696, R01DK105072, and R01HL140574).
Footnotes
Competing interest statement: C.J.M. reports receiving salary from Grünenthal Ltd., UK, and that this relationship is not related to the present article. F.A.J.L.S. received speaker fees from Bayer Healthcare, Sentara Healthcare, Philips, Kellogg Company, Vanda Pharmaceuticals, and Pfizer. J.Q., R.C., W.W., and M.G. declare no competing interests.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914003116/-/DCSupplemental.
References
- 1.European Foundation for the Improvement of Living and Working Conditions , Sixth European Working Conditions Survey – Overview Report (Luxembourg, Publications Office of the European Union, 2016).
- 2.Alterman T., Luckhaupt S. E., Dahlhamer J. M., Ward B. W., Calvert G. M., Prevalence rates of work organization characteristics among workers in the U.S.: Data from the 2010 National Health Interview Survey. Am. J. Ind. Med. 56, 647–659 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian J., Scheer F. A. J. L., Circadian system and glucose metabolism: Implications for physiology and disease. Trends Endocrinol. Metab. 27, 282–293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McHill A. W., Wright K. P. Jr, Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes. Rev. 18 (suppl. 1), 15–24 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Bonham M. P., Bonnell E. K., Huggins C. E., Energy intake of shift workers compared to fixed day workers: A systematic review and meta-analysis. Chronobiol. Int. 33, 1086–1100 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Pan A., Schernhammer E. S., Sun Q., Hu F. B., Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 8, e1001141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antunes L. C., Levandovski R., Dantas G., Caumo W., Hidalgo M. P., Obesity and shift work: Chronobiological aspects. Nutr. Res. Rev. 23, 155–168 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Kautzky-Willer A., Harreiter J., Pacini G., Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 37, 278–316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva-Costa A., et al. , Gender-specific association between night-work exposure and type-2 diabetes: Results from longitudinal study of adult health, ELSA-Brasil. Scand. J. Work Environ. Health 41, 569–578 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Karlsson B., Knutsson A., Lindahl B., Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup. Environ. Med. 58, 747–752 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y., et al. , Shift work and the relationship with metabolic syndrome in Chinese aged workers. PLoS One 10, e0120632 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan Y., et al. , Shift work and diabetes mellitus: A meta-analysis of observational studies. Occup. Environ. Med. 72, 72–78 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Son M., Ye B. J., Kim J. I., Kang S., Jung K. Y., Association between shift work and obesity according to body fat percentage in Korean wage workers: Data from the fourth and the fifth Korea National Health and Nutrition Examination Survey (KNHANES 2008–2011). Ann. Occup. Environ. Med. 27, 32 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey M., Silver R., Sex differences in circadian timing systems: Implications for disease. Front. Neuroendocrinol. 35, 111–139 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy J. F., et al. , Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. U.S.A. 108 (suppl. 3), 15602–15608 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cain S. W., et al. , Sex differences in phase angle of entrainment and melatonin amplitude in humans. J. Biol. Rhythms 25, 288–296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovejoy J. C., Sainsbury A.; Stock Conference 2008 Working Group , Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev. 10, 154–167 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Asarian L., Geary N., Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R1215–R1267 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods S. C., Seeley R. J., Porte D. Jr, Schwartz M. W., Signals that regulate food intake and energy homeostasis. Science 280, 1378–1383 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Ahima R. S., “Principles of energy homeostasis” in Metabolic Syndrome: A Comprehensive Textbook, Ahima R. S., Ed. (Springer International Publishing, Cham, 2016), pp. 311–326. [Google Scholar]
- 21.Czeisler C. A., et al. , Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284, 2177–2181 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Monroe M. B., et al. , Direct evidence for tonic sympathetic support of resting metabolic rate in healthy adult humans. Am. J. Physiol. Endocrinol. Metab. 280, E740–E744 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Axelrod J., Weinshilboum R., Catecholamines. N. Engl. J. Med. 287, 237–242 (1972). [DOI] [PubMed] [Google Scholar]
- 24.Groop L. C., Bonadonna R. C., Shank M., Petrides A. S., DeFronzo R. A., Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J. Clin. Invest. 87, 83–89 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Bois E. F., The basal metabolism in fever. JAMA 77, 352–357 (1921). [Google Scholar]
- 26.McHill A. W., et al. , Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. U.S.A. 111, 17302–17307 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonnissen H. K., et al. , Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am. J. Clin. Nutr. 96, 689–697 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Scheer F. A., Hilton M. F., Mantzoros C. S., Shea S. A., Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U.S.A. 106, 4453–4458 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shea S. A., Hilton M. F., Orlova C., Ayers R. T., Mantzoros C. S., Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J. Clin. Endocrinol. Metab. 90, 2537–2544 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolaczynski J. W., et al. , Responses of leptin to short-term fasting and refeeding in humans: A link with ketogenesis but not ketones themselves. Diabetes 45, 1511–1515 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Chin-Chance C., Polonsky K. S., Schoeller D. A., Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J. Clin. Endocrinol. Metab. 85, 2685–2691 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Broglio F., et al. , Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J. Clin. Endocrinol. Metab. 86, 5083–5086 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Qian J., Morris C. J., Caputo R., Garaulet M., Scheer F., Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int. J. Obes. (Lond) 43, 1644–1649 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McHill A. W., Hull J. T., McMullan C. J., Klerman E. B., Chronic insufficient sleep has a limited impact on circadian rhythmicity of subjective hunger and awakening fasted metabolic hormones. Front. Endocrinol. (Lausanne) 9, 319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wren A. M., et al. , Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 86, 5992 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Wefers J., et al. , Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 115, 7789–7794 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zitting K. M., et al. , Human resting energy expenditure varies with circadian phase. Curr. Biol. 28, 3685–3690.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buxton O. M., et al. , Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 4, 129ra43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris C. J., et al. , The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity (Silver Spring) 23, 2053–2058 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung C. M., et al. , Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J. Physiol. 589, 235–244 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markwald R. R., et al. , Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. U.S.A. 110, 5695–5700 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rising R., Keys A., Ravussin E., Bogardus C., Concomitant interindividual variation in body temperature and metabolic rate. Am. J. Physiol. 263, E730–E734 (1992). [DOI] [PubMed] [Google Scholar]
- 43.Landsberg L., Core temperature: A forgotten variable in energy expenditure and obesity? Obes. Rev. 13 (suppl. 2), 97–104 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Morris C. J., et al. , Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. U.S.A. 112, E2225–E2234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris C. J., Purvis T. E., Hu K., Scheer F. A., Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. U.S.A. 113, E1402–E1411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian J., Dalla Man C., Morris C. J., Cobelli C., Scheer F. A. J. L., Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes. Metab. 20, 2481–2485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.