Significance
Thermogenesis is a fundamental aspect of energy homeostasis. Here, we present evidence that adipose tissue NAD+ metabolism is essential for thermogenesis. We found cold exposure activates NAD+ biosynthesis mediated by a rate-limiting enzyme, NAMPT, in mouse and human brown adipose tissue (BAT). Loss of NAMPT impairs the gene programs involved in thermogenesis and mitochondrial function in BAT. Mice lacking NAMPT in both BAT and white adipose tissue (WAT) but not in BAT alone have impaired thermogenic responses to cold exposure, fasting, and β-adrenergic stimulation. In WAT, NAMPT deletion decreases adrenergic-mediated lipolysis through inactivation of caveolin-1, which likely impairs whole-body thermogenesis. Nicotinamide mononucleotide administration normalized these metabolic derangements. These findings demonstrate the importance of adipose tissue NAD+ biology in energy metabolism.
Keywords: NAD, adipose tissue, thermogenesis, lipolysis, energy metabolism
Abstract
Nicotinamide adenine dinucleotide (NAD+) is a critical coenzyme for cellular energy metabolism. The aim of the present study was to determine the importance of brown and white adipose tissue (BAT and WAT) NAD+ metabolism in regulating whole-body thermogenesis and energy metabolism. Accordingly, we generated and analyzed adipocyte-specific nicotinamide phosphoribosyltransferase (Nampt) knockout (ANKO) and brown adipocyte-specific Nampt knockout (BANKO) mice because NAMPT is the rate-limiting NAD+ biosynthetic enzyme. We found ANKO mice, which lack NAMPT in both BAT and WAT, had impaired gene programs involved in thermogenesis and mitochondrial function in BAT and a blunted thermogenic (rectal temperature, BAT temperature, and whole-body oxygen consumption) response to acute cold exposure, prolonged fasting, and administration of β-adrenergic agonists (norepinephrine and CL-316243). In addition, the absence of NAMPT in WAT markedly reduced adrenergic-mediated lipolytic activity, likely through inactivation of the NAD+–SIRT1–caveolin-1 axis, which limits an important fuel source fatty acid for BAT thermogenesis. These metabolic abnormalities were rescued by treatment with nicotinamide mononucleotide (NMN), which bypasses the block in NAD+ synthesis induced by NAMPT deficiency. Although BANKO mice, which lack NAMPT in BAT only, had BAT cellular alterations similar to the ANKO mice, BANKO mice had normal thermogenic and lipolytic responses. We also found NAMPT expression in supraclavicular adipose tissue (where human BAT is localized) obtained from human subjects increased during cold exposure, suggesting our finding in rodents could apply to people. These results demonstrate that adipose NAMPT-mediated NAD+ biosynthesis is essential for regulating adaptive thermogenesis, lipolysis, and whole-body energy metabolism.
Nonshivering thermogenesis is fundamental to whole-body energy expenditure in rodents, and it accounts for ∼20% of total energy expenditure in people (1). The complex mechanisms responsible for regulating nonshivering and whole-body thermogenesis involve both brown and white adipose tissues (BAT and WAT). BAT dissipates the energy of the mitochondrial protein gradient as heat by uncoupling protein 1 (UCP1)-dependent and UCP1-independent mechanisms and has a central role in thermogenesis after cold exposure (1–3). Free fatty acids (FFAs) are a major energy substrate for BAT heat production and directly activate UCP1 and mitochondrial function (1, 3). Data obtained from recent studies conducted in several adipocyte-specific knockout mouse models demonstrate that lipolytic activity in WAT and release of FFAs into the circulation are critical in regulating thermogenesis by providing fuel for BAT and other tissues after cold exposure, prolonged fasting, and administration of a β3-adrenergic receptor agonist (4–8). In addition, WAT-secreted adipokines, such as adiponectin and leptin, affect BAT thermogenesis by modulating thermogenic genes and sympathetic nerve activity (9). Consistent with these data from rodent studies, cold- or catecholamine-induced BAT activation is accompanied by increased WAT lipolysis and an altered adipokine profile in people (1–3). These findings underscore the importance of both BAT and WAT in regulating whole-body thermogenesis.
Nicotinamide adenine dinucleotide (NAD+) is a classical redox coenzyme that acts as a key cellular energy sensor in many species. NAD+ is regulated by various NAD+ biosynthetic and degradative enzymes, such as nicotinamide phosphoribosyltransferase (NAMPT), a rate-limiting NAD+ biosynthetic enzyme, and poly(ADP-ribose) polymerases (PARPs). Manipulating NAD+ metabolism has a profound impact on key energy metabolic pathways in a tissue-specific fashion (10–14). In skeletal muscle, for example, loss of NAMPT triggers a dystrophic phenotype by impairing mitochondrial oxidative metabolism and ATP production (15), whereas PARP-2 inhibition enhances mitochondrial function and mitigates diet- or age-induced metabolic complications (16). In liver, NAD+ level is positively associated with energy status, Nampt ablation impairs FFA metabolism, and CD38 inhibition improves mitochondrial respiratory function and glucose metabolism (12–14). Recently, we and others found that WAT NAMPT is essential for regulating whole-body glucose metabolism, adiponectin production, and adipose tissue expansion in response to high-fat diet feeding (17–19). In addition, recent work shows Nampt knockout impairs mitochondrial respiratory function in BAT (18). However, the role of adipose tissue NAD+ metabolism in whole-body thermogenesis and energy metabolism is still unclear.
The major aim of the present study was to test the hypothesis that NAMPT-mediated NAD+ biosynthesis in BAT and WAT is indispensable for regulating thermogenesis and energy metabolism. To this end, we first evaluated the relationship between the cold-induced BAT thermogenesis and NAD+ metabolism. To determine the roles of NAMPT-mediated NAD+ biosynthesis in thermogenesis, we generated a mouse model, which we have named brown adipocyte-specific Nampt knockout (BANKO) mice, and studied both BANKO mice and pan adipocyte-specific Nampt knockout (ANKO) mice. We also evaluated the potential clinical relevance of the studies by assessing NAMPT expression in supraclavicular adipose tissue [where human BAT is localized (1, 2)] biopsy samples obtained during thermoneutral and cold conditions in human subjects.
Results
NAMPT-Mediated NAD+ Biosynthesis Is Associated with Brown Adipose Tissue Activity in Mice and Humans.
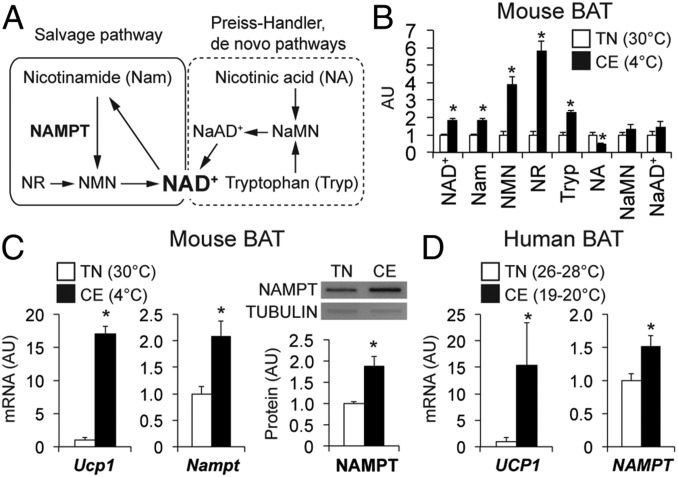
To determine the role of NAD+ in thermogenesis, we evaluated key NAD+ metabolites (Fig. 1A) in BAT obtained from mice kept in thermoneutral (30 °C) and cold (4 °C) environments. NAD+ levels were nearly double in BAT during cold than during thermoneutral conditions (Fig. 1B). NAD+ metabolites involved in the salvage pathway (Fig. 1A), nicotinamide, nicotinamide mononucleotide (NMN), and nicotinamide riboside (NR), were also markedly increased by cold exposure (Fig. 1B). In addition, BAT gene expression of Ucp1 and Nampt and NAMPT protein levels were increased by cold exposure (Fig. 1C). Consistent with these animal data, UCP1 and NAMPT gene expression were significantly increased in supraclavicular BAT obtained from human subjects (SI Appendix, Table S1) during cold conditions (∼22 °C) compared with thermoneutral conditions (26–28 °C) (Fig. 1D).
Fig. 1.
NAMPT-mediated NAD+ biosynthesis is associated with BAT activity in mice and humans. (A) In mammals, NAMPT functions as a key enzyme in the salvage biosynthetic pathway and converts nicotinamide into NMN. NaMN, nicotinic acid mononucleotide; NaAD+, nicotinic acid adenine dinucleotide. The levels of NAD+ and key intermediates (B), gene expression of Ucp1 and Nampt, and NAMPT protein expression (C) were measured in BAT obtained from mice kept under thermoneutrality (TN, 30 °C) and cold exposure (CE, 4 °C) (n = 4 to 5 per group). (D) Gene expression of UCP1 and NAMPT was determined in BAT obtained from human subjects under the thermoneutral (TN, 26–28 °C) and cold (CE, ∼22 °C) conditions. *Value significantly different from the TN value (P < 0.05). Values are means ± SEM.
ANKO Mice Have Severe Cold Intolerance.
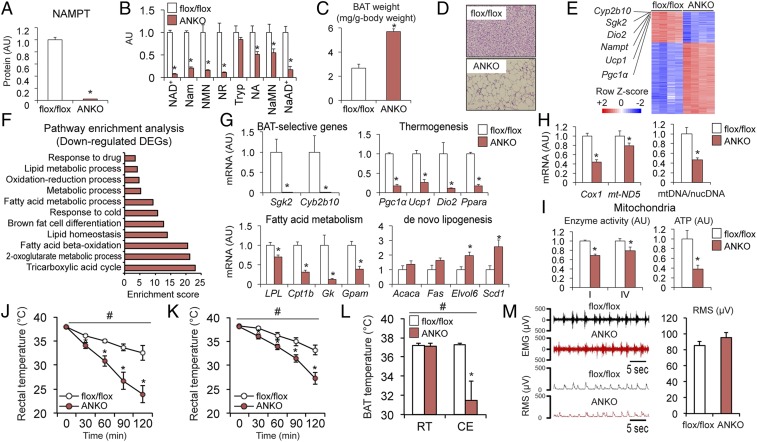
The close relationship between NAMPT-mediated NAD+ biosynthesis and cold-induced BAT activity in mice and people led us to hypothesize that NAD+ regulates BAT function and thermogenesis. To test this hypothesis, we analyzed ANKO mice lacking NAMPT in both BAT and WAT (17, 20). The expression of NAMPT protein in BAT was nearly completely (∼98%) abolished in ANKO mice compared with flox/flox mice (Fig. 2A and SI Appendix, Fig. S1). ANKO mice also had marked decreases in levels of NAD+ and salvage pathway intermediates (Fig. 2B). Loss of NAMPT caused BAT hypertrophy (Fig. 2C) and induced whitening of BAT, manifested by the appearance of large, unilocular lipid droplets (Fig. 2D). RNA-sequencing (RNA-seq) revealed BAT-selective genes (e.g., Cyp2b10, Sgk2) and thermogenic genes (e.g., Pgc1α, Ucp1, Dio2) were among the most down-regulated differentially expressed genes (DEGs) in ANKO mice compared with flox/flox mice (Fig. 2E). Down-regulated DEGs were enriched in pathways involved in FFA metabolism, thermogenesis, and mitochondrial function, whereas up-regulated DEGs were enriched in pathways involved in immune function (Fig. 2F and SI Appendix, Fig. S2). Real-time PCR confirmed the loss of NAMPT caused a decrease in gene expression of BAT-selective genes and key regulators of thermogenesis and FFA metabolism but increased the gene expression of lipogenic enzymes (Fig. 2G). In addition, ANKO mice had decreases in gene expression of mitochondrially encoded proteins, mitochondrial DNA content (Fig. 2H), the protein content of the subunits of the electron transport chain (ETC) enzyme complex (SI Appendix, Fig. S3), enzyme activities of ETC complex-I and -IV, and ATP contents in isolated mitochondria (Fig. 2I). Taken together, these findings suggest NAD+ is an important regulator of the metabolic programs involved in thermogenesis and mitochondrial function in BAT. Consistent with the BAT cellular phenotype, ANKO mice had a much greater decline in rectal temperature during cold exposure than control mice, after acclimation to thermoneutrality (30 °C) (Fig. 2J) and room temperature (22 °C) (Fig. 2K). In addition, BAT temperature during cold exposure was lower in ANKO mice than in flox/flox mice (Fig. 2L). However, data obtained from electromyography (EMG) studies found no difference in muscle shivering during cold exposure between genotypes (Fig. 2M), suggesting impaired muscle shivering is unlikely responsible for hypothermia in ANKO mice. No difference in the cold tolerance was detected between flox/flox and Adiponectin-Cre (Cre) mice (SI Appendix, Fig. S4). Feeding rescued the hypothermia of ANKO and control mice during cold exposure but resulted in a greater increase in body temperature in ANKO mice than in control mice (SI Appendix, Fig. S5).
Fig. 2.
ANKO mice have severe cold intolerance. The levels of NAMPT protein (A) and NAD+ metabolites (B) were determined in BAT (n = 3 to 5 per group). (C) BAT weight normalized by body weight (n = 5 per group). (D) Images of hematoxylin and eosin (H&E) staining of BAT. (E) Heat map of DEGs induced by Nampt knockout in BAT (n = 4 per group). Z-scores were calculated from the log-transformed fragments per kilobase of transcript per million fragments mapped values in RNA-seq data. (F) Enriched pathways of the down-regulated DEGs. (G) Gene expression of BAT-selective markers and proteins involved in thermogenesis and FFA metabolism and lipogenic enzymes (n = 4 per group). (H) BAT gene expression of mitochondrially encoded proteins and mitochondrial DNA (mtDNA) contents normalized to nuclear DNA (nucDNA) contents (n = 4 to 5 per group). (I) ETC complex-I and -IV activities (n = 3 to 6 per group) and ATP contents (n = 2 to 3 per group) in isolated mitochondria. Rectal temperature during cold exposure after acclimation to thermoneutrality (J) or room temperature (K) (n = 6 to 7 per group). Mice were fasted during cold exposure. (L) BAT temperature before and after 5 h cold exposure (n = 5 per group). (M) Representative EMG and the rms traces and quantification of the rms during 1 h cold exposure (n = 5 per group). #Repeated measures ANOVA revealed a significant group × time interaction (P < 0.05). *Value significantly different from the control value (P < 0.05). Values are means ± SEM.
BANKO Mice Have Normal Cold Tolerance.
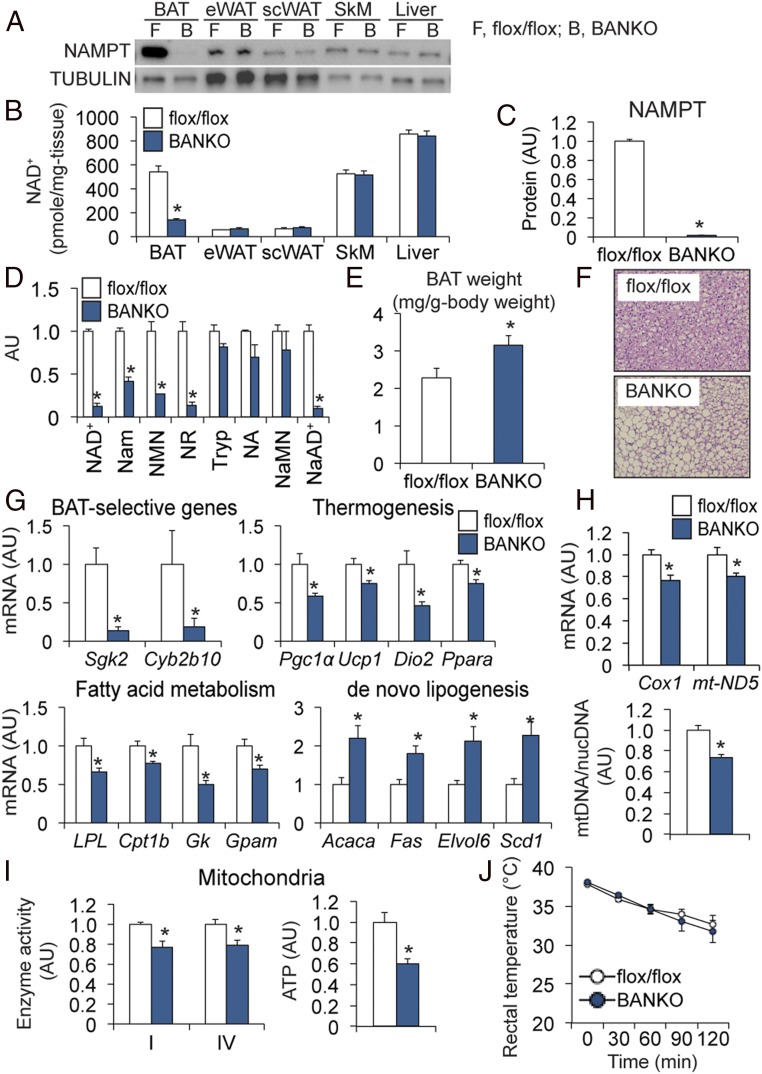
Several lines of recent evidence have pointed to an important role of WAT in regulating BAT thermogenesis (4–7). Therefore, we generated BANKO mice by using Ucp1-Cre mice and determined the WAT- and BAT-specific roles of NAD+ biosynthesis in whole-body thermogenesis by comparing phenotypes in ANKO and BANKO mice. BANKO mice had marked decreases in NAMPT protein and NAD+ concentrations in BAT but not in WAT depots or other organs (Fig. 3 A and B). In contrast to ANKO mice, which exhibit insulin resistance, hyperinsulinemia, and hypoadiponectinemia (17, 20), key metabolic parameters were not affected in BANKO mice (SI Appendix, Fig. S6). In addition, plasma extracellular NAMPT (eNAMPT) level; WAT gene expression of markers of brown, white, and beige fat; and WAT structure were not altered in BANKO mice (SI Appendix, Fig. S6 J–L). The knockout efficiency on BAT NAMPT in BANKO mice (∼98%) was identical to that in ANKO mice (Fig. 3C and SI Appendix, Fig. S1). Loss of NAMPT in BANKO mice markedly decreased key NAD+ metabolites and increased BAT mass and number of unilocular lipid droplets (Fig. 3 D–F). Gene expression of BAT-selective genes and genes that regulate thermogenesis and FFA metabolism were decreased, and lipogenic enzymes were up-regulated in BANKO mice (Fig. 3G). In addition, Nampt deletion impaired markers of mitochondrial function (Fig. 3 H and I and SI Appendix, Fig. S3). These findings confirm the importance of NAD+ biosynthesis in regulating BAT thermogenic genes and mitochondrial function that was also observed in ANKO mice. However, despite such alterations in BAT metabolic programs, BANKO mice had the normal cold tolerance (Fig. 3J). Loss of NAMPT in BAT did not cause increased WAT expression of thermogenic genes during cold exposure (SI Appendix, Fig. S6M), suggesting no compensatory browning of WAT in BANKO mice.
Fig. 3.
BANKO mice have normal cold tolerance. NAMPT protein expression (A) and NAD+ concentrations (B) in key metabolic organs (n = 5 to 8 per group). eWAT, epididymal WAT; scWAT, subcutaneous WAT; SkM, skeletal muscle. The levels of NAMPT protein (C) and NAD+ intermediates (D) were determined in BAT (n = 4 to 5 per group). (E) BAT weight normalized by body weight (n = 7 to 11 per group). (F) Images of H&E staining of BAT. (G) Gene expression of BAT-selective markers, proteins involved in thermogenesis and FFA metabolism, and lipogenic enzymes (n = 7 to 9 per group). (H) BAT gene expression of mitochondrially encoded proteins and mtDNA (n = 5 to 9 per group). (I) ETC complex activities (n = 3 to 6 per group) and ATP contents (n = 3 per group) in BAT mitochondria. (J) Rectal temperature during cold exposure (n = 6 to 7 per group). Mice were fasted during cold exposure. *Value significantly different from the control value (P < 0.05). Values are means ± SEM.
ANKO Mice, but Not BANKO Mice, Have Impaired Thermogenic and Energy Responses to Fasting and β-Adrenergic Stimulation.
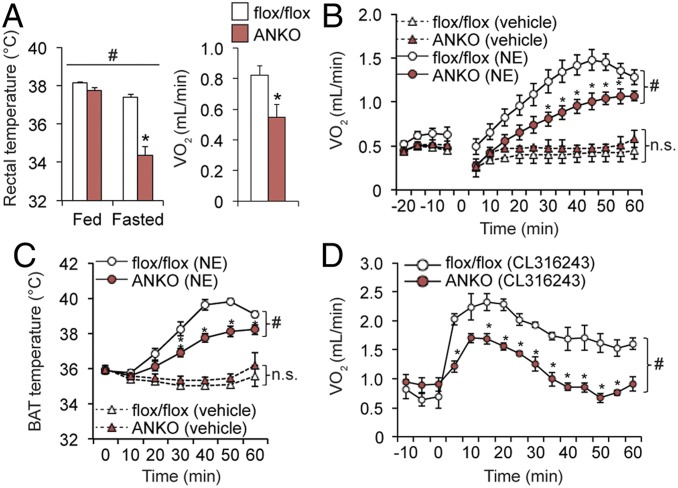
In addition to a cold intolerance phenotype, ANKO mice, but not BANKO mice, had marked decreases in rectal temperature and oxygen consumption (VO2) after prolonged fasting compared to flox/flox mice (Fig. 4A and SI Appendix, Fig. S7 A and B). We next evaluated the energetic responses to administration of a β-adrenergic ligand norepinephrine (NE) in mice kept at thermoneutrality. We confirmed vehicle administration had no effect on VO2 and BAT temperature (Fig. 4 C and D). ANKO mice had blunted VO2 and BAT temperature responses to NE administration (Fig. 4 C and D). In contrast, NE administration increased VO2 and BAT temperature similarly in BANKO and control mice (SI Appendix, Fig. S7 C and D). In addition, ANKO mice, but not BANKO mice, exhibited blunted VO2 responses to administration of the β3-adrenergic agonist CL-316243 (Fig. 4E and SI Appendix, Fig. S7E). The marked differences in the thermogenic responses between ANKO and BANKO mice prompted us to hypothesize that loss of NAMPT impairs adrenergic-induced lipolysis in WAT, which could result in insufficient fuel supply for BAT thermogenesis.
Fig. 4.
ANKO mice have impaired thermogenic responses to fasting and adrenergic stimulation. (A) Rectal temperature before and after 48 h fasting (n = 5 to 8 per group) and VO2 during the 12 h dark period under fasting conditions (n = 4 per group). VO2 (B) and BAT temperature (C) before and after a subcutaneous injection of NE (1 mg/kg) (n = 5 per group) or vehicle (n = 3 per group). n.s., not significant. (D) VO2 values before and after an intraperitoneal injection of CL-316243 (0.6 mg/kg) (n = 3 to 4 per group). #Repeated measures ANOVA revealed a significant group × time interaction (P < 0.05). *Value significantly different from the control value (P < 0.05). Values are means ± SEM.
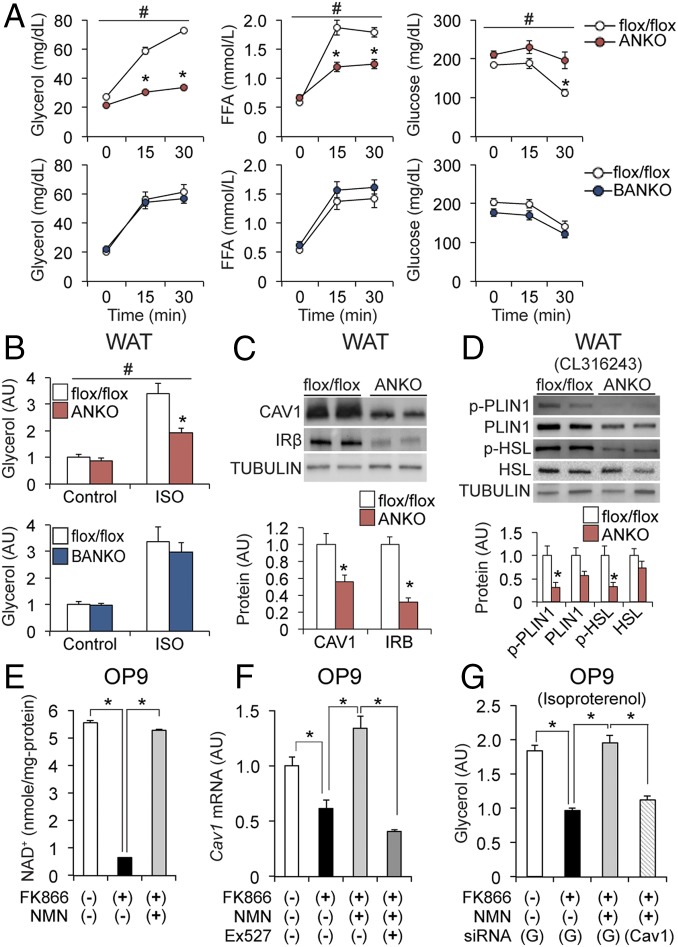
Loss of NAMPT Impairs Adrenergic-Mediated Lipolytic Activity through Inactivation of Caveolin-1 in WAT.
To test the hypothesis, we first evaluated the effects of adrenergic stimulation on WAT lipolytic activity. Administration of CL-316243 markedly increased plasma concentrations of glycerol and FFAs but decreased blood glucose concentrations in BANKO, flox/flox, and Cre mice (Fig. 5A and SI Appendix, Fig. S4B). However, these metabolic responses were severely blunted in ANKO mice (Fig. 5A). In addition, data obtained from ex vivo studies found isoproterenol (ISO)-mediated glycerol release was suppressed in WAT explants from ANKO mice compared with those from BANKO and flox/flox mice (Fig. 5B). These findings demonstrate NAMPT-mediated NAD+ biosynthesis is essential for catecholamine-induced lipolysis in WAT.
Fig. 5.
Loss of NAMPT impairs adrenergic-mediated lipolytic activity through inactivation of caveolin-1 in WAT. (A) Plasma concentrations of glycerol and FFA and blood glucose concentrations before and after an injection of CL-316243 (1 mg/kg) (n = 4 to 8 per group). (B) WAT depots were incubated in the presence or absence of ISO for 2 h (n = 5 to 8 per group). (C) Western blot analysis of CAV1 and IRβ in WAT (n = 4 to 5 per group). (D) Western blot analysis of phospho-PLIN1 (Ser522), PLIN1, phospho-HSL (Ser563), and HSL in WAT after CL-316243 injection (n = 4 per group). NAD+ concentrations (E) and Cav1 gene expression (F) in OP9 adipocytes in the presence or absence of FK866, NMN, and Ex527 (n = 4 per group). (G) ISO-stimulated glycerol release from GFP (G) or Cav1 siRNA-treated OP9 adipocytes (n = 4 per group). #Repeated measures ANOVA revealed a significant group × time interaction (P < 0.05). Data were analyzed by Student’s unpaired t test (A–D) and one-way ANOVA with Tukey’s post hoc test (E–G). *P < 0.05. Values are means ± SEM.
Recent studies show that loss of caveolin-1 (CAV1), a key component of the caveolae plasma membranes, causes WAT dysfunction that is similar to that of ANKO mice, including insulin resistance, hypoadiponectinemia, and impaired adrenergic lipolysis and thermogenesis (21–25). We therefore hypothesized that CAV1 is a mediator that links NAD+ biology and lipolysis in WAT. Supporting the hypothesis, loss of NAMPT decreased WAT expression levels of CAV1 and insulin receptor β (IRβ), an important downstream target of CAV1 (22) (Fig. 5C and SI Appendix, Fig. S8A). Compared to flox/flox mice, ANKO mice had marked decreases in protein levels of phosphorylated perilipin-1 (PLIN1) and HSL in WAT after CL-316243 administration (Fig. 5D). In addition, loss of NAMPT reduced gene expression of β3-adrenergic receptor (ADRB3) in WAT (SI Appendix, Fig. S8B). Given that CAV1 regulates PLIN1, HSL, and ADRB3 in adipose tissue (23–26), diminished CAV1 expression could be responsible for impaired adrenergic-mediated lipolysis induced by NAMPT inhibition. To test this possibility, we next used mouse OP9 preadipocytes to further evaluate the relationships among NAD+, CAV1, and lipolysis. Cav1 mRNA expression in OP9 adipocytes was significantly reduced after treatment with FK866, a potent NAMPT inhibitor, which markedly decreased NAD+ concentration (Fig. 5 E and F). Both NAD+ and Cav1 mRNA levels were fully restored in the presence of NMN, a NAMPT product that can be converted to NAD+ (Fig. 5 E and F). However, Ex527, a specific inhibitor of the NAD+-dependent deacetylase SIRT1, abolished the effects of NMN on Cav1 expression in FK866-treated adipocytes. Consistent with the changes in NAD+ and Cav1 levels, ISO-stimulated glycerol release was markedly reduced by FK866 treatment and fully restored by coincubation with NMN. However, small interfering RNA (siRNA)-mediated knockdown of Cav1 (∼80%) abolished the effects of NMN treatment on ISO-mediated glycerol release in FK866-treated cells (Fig. 5G and SI Appendix, Fig. S8C). Taken together, these findings suggest that CAV1 regulates adrenergic lipolysis as a key downstream mediator of NAMPT-mediated NAD+ biosynthesis and SIRT1.
NMN Administration Restores Thermogenesis in ANKO Mice.
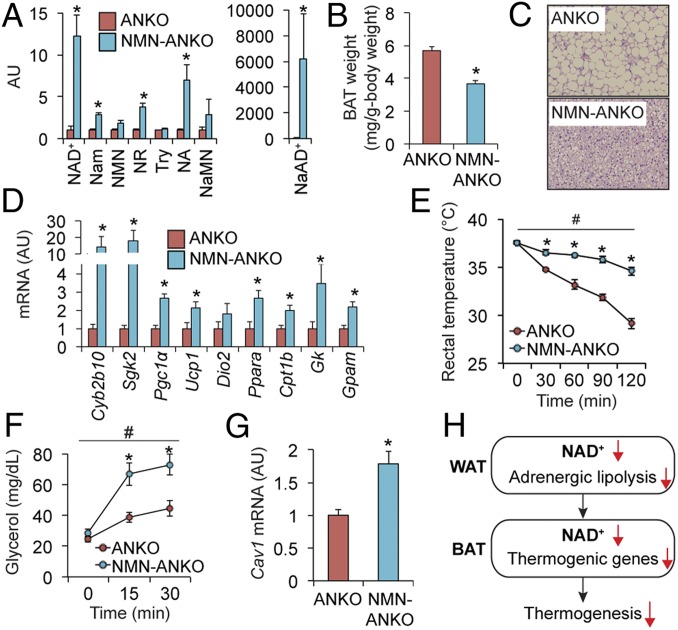
Given the pleotropic role of NAMPT (12), loss of NAMPT function, besides disturbing intracellular NAD+ homeostasis, might also contribute to impaired thermogenesis in ANKO mice. To test the possibility of the off-target effects induced by Nampt knockout, we provided NMN in drinking water to ANKO mice and found NMN administration increased BAT NAD+ metabolites (Fig. 6A), decreased BAT weight (Fig. 6B), diminished BAT whitening (Fig. 6C), and restored gene expression of BAT-selective genes and key proteins involved in thermogenesis, FFA metabolism, and mitochondrial function (Fig. 6D). NMN-treated ANKO mice had greater cold tolerance than ANKO mice (Fig. 6E). In addition, NMN administration increased plasma glycerol responses to an injection of CL-316243 (Fig. 6F) and restored Cav1 expression in WAT (Fig. 6G). In contrast, NMN administration did not affect cold tolerance or adrenergic responses in flox/flox mice (SI Appendix, Fig. S9). Although we cannot exclude the possibility that NMN administration affects energy metabolism in other metabolic organs (12, 13), these findings support the conclusion that adipose tissue NAD+ biosynthesis is an important regulator of thermogenesis and whole-body energy metabolism (Fig. 6H).
Fig. 6.
NMN administration restores thermogenesis in ANKO mice. NMN was administered in drinking water to ANKO mice (500 to 1,000 mg/kg). BAT NAD+ metabolites (A), tissue weight (B), H&E staining (C), gene expression of BAT-selective genes and key proteins involved in thermogenesis and FFA metabolism (D), cold tolerance (E), plasma glycerol concentrations before and after administration of CL-316243 (1 mg/kg) (F), and WAT gene expression of Cav1 (G) were determined in ANKO and NMN-treated ANKO mice (n = 3 to 5 per group). (H) A schematic overview of the proposed thermoregulation mediated by adipose tissue NAD+ biosynthesis. #Repeated measures ANOVA revealed a significant group × time interaction (P < 0.05). *Value significantly different from the control value (P < 0.05). Values are means ± SEM.
Discussion
The results from the present study provide insights into the importance of adipose tissue NAD+ biology in the regulation of thermogenesis and whole-body energy metabolism. Our study demonstrates defects in NAMPT-mediated NAD+ biosynthesis dysregulate the gene programs involved in thermogenesis, mitochondrial biogenesis, and FFA metabolism, leading to “whitening” of BAT. Despite marked cellular alterations in BAT, NAMPT deletion in BAT alone did not affect whole-body thermogenesis, possibly due to compensatory BAT hypertrophy observed in knockout mice. Nonetheless, these results demonstrate NAD+ is a key physiological regulator of thermogenic and mitochondrial genes, such as UCP1 and PGC1α, in BAT. This notion is supported by data from previous studies that found boosting NAD+ levels inhibiting PARP1 activity or administering NR increases UCP1 and PGC1α expression and enhances mitochondrial biogenesis in BAT (27, 28). The potential translation of these results to humans is supported by our observation that cold-induced BAT activation and UCP1 expression are accompanied by increased NAMPT expression in people. The precise molecular mechanism responsible for the effect of NAD+ on BAT metabolism is not clear but likely involves NAD+-dependent protein deacetylases sirtuins. Recent studies show cold exposure increases SIRT6 expression in BAT and Sirt6 deletion decreases the expression of thermogenic genes and genes involved in FFA metabolism and mitochondrial biogenesis, resulting in whitening of BAT (29). Similarly, Sirt1 deficiency induces whitening of BAT by reducing thermogenic genes and ETC complexes (30). Data obtained from in vitro studies showed mitochondrial SIRT3 regulates UCP1 and PGC1α expression and oxygen consumption in brown adipocytes (31). However, whole-body Sirt3-deficient mice and adipocyte-specific mitochondrial Sirt3 knockout (AMiSKO) mice have normal BAT function and cold tolerance (32, 33), suggesting SIRT3 alone is not a major downstream mediator in vivo. Nonetheless, we cannot exclude the possibility of the functional redundancy of mitochondrial sirtuins (SIRT3-5), which was recently found in retinal cells (34). Additional studies that involve mice lacking multiple sirtuins are needed to determine the precise molecular mechanism that links NAD+ and the thermogenic gene program.
The major factor responsible for the differences in whole-body metabolic function between ANKO and BANKO mice likely involves alterations in WAT function. Several possible mechanisms could link WAT with the BAT thermogenesis. First, alterations in the production of adiponectin and possibly other adipokine(s) from WAT could contribute to BAT dysfunction. Adiponectin administration increases BAT expression of UCP1 and rectal temperature (9), so it is possible that hypoadiponectinemia observed in ANKO mice contributes to BAT thermogenic dysfunction (17). Second, a decrease in eNAMPT could affect BAT function and thermogenesis in ANKO mice. Plasma eNAMPT increases after prolonged fasting and regulates NAD+ and SIRT1 activity in the hypothalamus (12, 20). Given the sympathetic nerve–mediated neuronal communication between BAT and the hypothalamus (1), eNAMPT could regulate BAT thermogenic proteins by modulating the sympathetic nerve activity. Third, an inadequate fuel supply from plasma FFA due to impaired WAT lipolytic sensitivity to adrenergic stimulation could directly deactivate UCP1 expression in BAT (1). We also found NAMPT deletion decreases the expression of proteins involved in FFA uptake and β-oxidation (e.g., LPL, CPT1) in BAT, suggesting both inadequate supply of FFAs and impaired intracellular FFA metabolism can act in synergy to impair thermogenic function. Last, systemic metabolic abnormalities in ANKO mice, such as hyperinsulinemia and insulin resistance, could negatively impact BAT thermogenesis. Additional studies are needed to further investigate the mechanisms involved in the complex communication between WAT and BAT in NAD+-mediated regulation of whole-body thermogenesis.
Our results underscore the role of WAT lipolysis in BAT thermogenesis, which is consistent with recent findings demonstrating genetic ablation of a key WAT lipolytic activator, such as ATGL or CGI-58, disrupts thermogenic responses to cold exposure, fasting, and β3-adrenergic stimulation (4–7). Although we cannot exclude the possibility of the involvement of other organs, such as liver, muscle, and heart (4), impaired WAT lipolytic responses to β-adrenergic stimulation are likely responsible for an insufficient supply of energy substrates for BAT thermogenesis. Our results suggest that CAV1 acts as a key downstream mediator of NAMPT-mediated NAD+ biosynthesis and SIRT1 to regulate β-adrenergic-stimulated lipolysis in WAT. It is possible this defective NAD+–SIRT1–CAV1 axis contributes to the blunted lipolytic response to β-adrenergic stimulation observed in obesity and older age in people (35, 36) because NAMPT-mediated NAD+ biosynthesis and SIRT1 activity are impaired in WAT by obesity and aging (12, 19). Diminished CAV1 expression could contribute to other metabolic abnormalities in ANKO mice. For example, we previously found ANKO mice have a marked decrease in plasma concentration of adiponectin (17, 19), which is a key downstream target of CAV1 (21). More recently, Nielsen et al. (18) found loss of NAMPT increases adipose tissue fibrosis under high-fat diet conditions, which is also consistent with previous findings that CAV1 is involved in the pathogenesis of adipose tissue fibrosis (26). In addition, both Cav1-deficient and adipocyte-specific Nampt knockout mice exhibit impaired glucose metabolism and multiorgan insulin sensitivity (17, 18, 21, 22). Taken together, these findings support the notion that NAD+ deficiency in WAT has important effects on whole-body metabolic function, particularly in people with obesity and older adults.
In conclusion, our data demonstrate that NAD+ is an essential regulator of adipose tissue metabolism and thermogenesis and illustrate the importance of adipocytes in regulating whole-body energy homeostasis. The current commercial availability and increasing popularity of NAD+ boosters, such as NR and NMN (10–14, 19), make it particularly important to further evaluate the translational potential of these experimental findings in rodents and cell systems to people and to determine whether NAD+ is a promising molecular target for enhancing adipose tissue function and whole-body energy homeostasis.
Materials and Methods
All animal studies were approved by the Washington University Institutional Animal Care and Use Committee. Written informed consent was obtained from all subjects before their participation in this study, which was approved by the Institutional Review Board of Washington University School of Medicine in St. Louis, MO. Details of all other experimental procedures are described in SI Appendix. All RNA-seq data used in this study have been deposited into the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) database under accession number GSE137149.
Supplementary Material
Acknowledgments
The authors thank Dr. Takeshi Egawa, Dr. Sangeeta Adak, Terri Pietka (Washington University School of Medicine), Melanie Schmitt, and Jiane Feng (University of Michigan) for technical assistance. We also thank Dr. Raja Ramaswamy and Dr. J. Daniel Giardina (Washington University School of Medicine) for performing BAT biopsies, the nursing staff of the Clinical Translational Research Unit for help in conducting the studies, and the study subjects for their participation. This study was funded by National Institutes of Health Grants DK104995 (J.Y.) and DK098659 (D.W.P.), a Pilot & Feasibility grant from the Diabetes Research Center (DRC) (DK020579 to S.C.G.), and grants from the Diabetes Research Connection (S.C.G.) and the Pershing Square Foundation (S.K.). This study was also supported by the Washington University Nutrition Obesity Research Center (NORC) (DK056341), DRC (DK020579), Digestive Diseases Research Core Center (DK052574), the Intellectual and Developmental Disability Research Center (HD087011), the Genome Technology Access Center (CA91842, UL1TR002345), the University of Michigan DRC (DK020572), NORC (DK089503), and Mouse Metabolic Phenotyping Center (1U2CDK110678-01). S.Y. was supported by the Sumitomo Life Welfare and Culture Foundation. M.C. was supported by a postdoctoral fellowship from the American Heart Association (17POST33060003).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. W.L.K. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE137149).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909917116/-/DCSupplemental.
References
- 1.Cannon B., Nedergaard J., Brown adipose tissue: Function and physiological significance. Physiol. Rev. 84, 277–359 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Sidossis L., Kajimura S., Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Invest. 125, 478–486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chouchani E. T., Kazak L., Spiegelman B. M., New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 29, 27–37 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Schreiber R., et al. , Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab 26, 753–763.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin H., et al. , Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab. 26, 764–777.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heine M., et al. , Lipolysis triggers a systemic insulin response essential for efficient energy replenishment of activated brown adipose tissue in mice. Cell Metab. 28, 644–655.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Ahmadian M., et al. , Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13, 739–748 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chondronikola M., et al. , Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab. 23, 1200–1206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamauchi T., et al. , The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Katsyuba E., Auwerx J., Modulating NAD+ metabolism, from bench to bedside. EMBO J. 36, 2670–2683 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang E. F., et al. , NAD+ in aging: Molecular mechanisms and translational implications. Trends Mol. Med. 23, 899–916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshino J., Baur J. A., Imai S. I., NAD+ intermediates: The biology and therapeutic potential of NMN and NR. Cell Metab. 27, 513–528 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajman L., Chwalek K., Sinclair D. A., Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metab. 27, 529–547 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chini E. N., Chini C. C. S., Espindola Netto J. M., de Oliveira G. C., van Schooten W., The pharmacology of CD38/NADase: An emerging target in cancer and diseases of aging. Trends Pharmacol. Sci. 39, 424–436 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frederick D. W., et al. , Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 24, 269–282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai P., et al. , PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 13, 450–460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stromsdorfer K. L., et al. , NAMPT-mediated NAD(+) biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 16, 1851–1860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen K. N., et al. , NAMPT-mediated NAD+ biosynthesis is indispensable for adipose tissue plasticity and development of obesity. Mol. Metab. 11, 178–188 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi S., Yoshino J., Adipose tissue NAD+ biology in obesity and insulin resistance: From mechanism to therapy. Bioessays 39, 1600227 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon M. J., et al. , SIRT1-mediated eNAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell Metab. 21, 706–717 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asterholm I. W., Mundy D. I., Weng J., Anderson R. G., Scherer P. E., Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab. 15, 171–185 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen A. W., et al. , Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am. J. Physiol. Cell Physiol. 285, C222–C235 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Cohen A. W., et al. , Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes 53, 1261–1270 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Cohen A. W., Schubert W., Brasaemle D. L., Scherer P. E., Lisanti M. P., Caveolin-1 expression is essential for proper nonshivering thermogenesis in brown adipose tissue. Diabetes 54, 679–686 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Mattsson C. L., Csikasz R. I., Shabalina I. G., Nedergaard J., Cannon B., Caveolin-1-ablated mice survive in cold by nonshivering thermogenesis despite desensitized adrenergic responsiveness. Am. J. Physiol. Endocrinol. Metab. 299, E374–E383 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Martin S., et al. , Caveolin-1 deficiency leads to increased susceptibility to cell death and fibrosis in white adipose tissue: Characterization of a lipodystrophic model. PLoS One 7, e46242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai P., et al. , PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13, 461–468 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crisol B. M., et al. , Nicotinamide riboside induces a thermogenic response in lean mice. Life Sci. 211, 1–7 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Yao L., et al. , Cold-inducible SIRT6 regulates thermogenesis of brown and beige fat. Cell Rep. 20, 641–654 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Xu F., et al. , Diet-induced obesity and insulin resistance are associated with brown fat degeneration in SIRT1-deficient mice. Obesity (Silver Spring) 24, 634–642 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Shi T., Wang F., Stieren E., Tong Q., SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J. Biol. Chem. 280, 13560–13567 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Porter L. C., et al. , NAD+-dependent deacetylase SIRT3 in adipocytes is dispensable for maintaining normal adipose tissue mitochondrial function and whole body metabolism. Am. J. Physiol. Endocrinol. Metab. 315, E520–E530 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lombard D. B., et al. , Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 27, 8807–8814 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J. B., et al. , NAMPT-mediated NAD(+) biosynthesis is essential for vision in mice. Cell Rep. 17, 69–85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horowitz J. F., Klein S., Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am. J. Physiol. Endocrinol. Metab. 278, E1144–E1152 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Lönnqvist F., Nyberg B., Wahrenberg H., Arner P., Catecholamine-induced lipolysis in adipose tissue of the elderly. J. Clin. Invest. 85, 1614–1621 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.