Abstract
Purpose of review:
Neuropathic pain may arise from multiple mechanisms and locations. Efficacy of current treatments for painful diabetic neuropathy is limited to an unpredictable sub-set of patients, possibly reflecting diversity of pain generator mechanisms, and there is a lack of targeted treatments for individual patients. This review summarizes preclinical evidence supporting a role for spinal disinhibition in painful diabetic neuropathy, the physiology and pharmacology of rate dependent depression (RDD) of the spinal H-reflex and the translational potential of using RDD as a biomarker of spinally-mediated pain.
Recent Findings:
Impaired RDD occurs in animal models of diabetes and was also detected in diabetic patients with painful versus painless neuropathy.
Summary:
RDD status can be determined using standard neurophysiological equipment. Loss of RDD may provide a clinical biomarker of spinal disinhibition, thereby enabling a personalized medicine approach to selection of current treatment options and enrichment of future clinical trial populations.
Keywords: Rate dependent depression, spinal disinhibition, neuropathic pain, diabetic neuropathy, H-reflex, KCC2
Introduction
Pain is a debilitating consequence of both type 1 and type 2 diabetes [1] that afflicts some 15–30% of people with diabetic neuropathy [2–5]. The diversity of the manifestations of painful diabetic neuropathy, including spontaneous tingling, lancinating or burning pain that frequently presents alongside touch-evoked pain and numbness [2, 6], suggests involvement of complex mechanisms downstream of initiating events such as impaired insulin signaling or hyperglycemia. However, the pathogenesis of painful diabetic neuropathy remains poorly understood and pain is frequently unresponsive to treatment with frontline analgesics or requires doses that also produce intolerable side effects [7].
The transduction of sensory signals originates with activation of receptors present in primary afferent terminals embedded in specialized end organs or in peripheral tissues. Signals are transmitted along primary afferent axons to sensory terminals in the superficial dorsal horn. Sensory information is subsequently integrated by projection neurons in the superficial and deep laminae of the spinal cord which transmit information via ascending pathways to the periaqueductal grey (PAG), rostroventral medulla (RVM) and thalamus of the brain [8]. The PAG and RVM in turn send projections back down to the spinal cord that can additionally facilitate or inhibit spinal excitability. Disruption of the sensory neuraxis leading to neuropathic pain can result from signal amplification or loss of inhibition (disinhibition) at any point along this system [9]. Indeed, changes in peripheral drive, central amplification and brain processing of nociceptive information have all been described in rodent models of diabetes [9].
For the purposes of this review, we will confine our discussion to the role of altered spinal excitability, arising from spinal disinhibition, in the development of painful diabetic neuropathy and the limited efficacy of current therapeutics.
Spinal Disinhibition
Melzack and Wall first proposed that peripheral sensory inputs could be modulated by spinal inhibitory circuits to modify the message ultimately received and interpreted by the brain [10]. The intimate association between inhibitory interneurons and spinal nociceptive circuits [11] implies that local tonic spinal inhibition, mediated by the inhibitory neurotransmitters GABA and glycine, plays a critical role in regulating spinal sensory processing. Pharmacological studies support this inference. Intrathecal administration of GABA or glycine receptor antagonists decreases nociceptive thresholds and produces behavioral indices of hypersensitivity in response to both painful and non-painful stimuli [12–15], indicating that spinal sensory systems are under tonic inhibition. Conversely, enhancing inhibitory neurotransmission by administering inhibitory receptor agonists or by genetic manipulation to enhance release of inhibitory neurotransmitters, increases nociceptive thresholds and reverses indices of pain in multiple models of neuropathy [16–19]. Spinal disinhibition due to loss of inhibitory interneurons, impaired storage and/or release of inhibitory neurotransmitters or impaired post-synaptic receptor activity, has been proposed as an important pathogenic mechanism underlying neuropathic pain [20, 21].
A mechanism of spinal disinhibition in diabetes
Reduced spinal GABAergic tone occurs in a number of models of neuropathic pain [22, 23]. However, there is no evidence that inhibitory GABAergic interneurons are compromised by diabetes. Indeed, both basal and stimulus-evoked spinal GABA levels are elevated in the spinal cord of diabetic rats [24] in association with unchanged spinal GABAA receptor protein levels [25]. Reports of decreased GABAB receptor protein and activity in the spinal cord of diabetic rodents could compromise tonic presynaptic suppression of excitatory neurotransmitter release [26, 27] but stimulus-evoked release of glutamate is paradoxically reduced in diabetic rats with painful neuropathy [24]. The answer to the conundrum of how elevated spinal GABA co-exists with increased nociceptive behavior in diabetic rats appears to lie with a change in GABAA receptor function. Diabetes causes a reduction in protein levels of the spinal potassium chloride co-transporter 2 (KCC2) [25, 28] that is restricted to the dorsal horn [29, 30]. KCC2 is specifically localized to post-synaptic neuronal membranes in the spinal cord [31] and maintains the chloride reversal potential that is critical for the inhibitory function of ionotropic GABAA receptors [32]. Reduced spinal KCC2 activity would be expected to cause GABA, acting at ionotropic GABAA receptors, to have impaired inhibitory, or even overtly excitatory, influence on post-synaptic membranes and depletion of this pump has been implicated in the genesis of a number of pain states involving spinal disinhibition [33]. While the diabetes-induced reductions in spinal KCC2 protein are modest (~20%), such changes in pump activity are considered sufficient to disrupt neurotransmission [34]. The involvement of excess GABA, acting via GABAA receptors, in pain behaviors of diabetic rats is further supported by the observation that spinal administration of the GABAA receptor antagonist bicuculline alleviated tactile allodynia and formalin-evoked hyerperalgesia [25] rather than having the pro-nociceptive effect expected if GABAA receptors were operating in a tonic inhibitory manner.
KCC2 gene expression in the brain is regulated by a variety of factors including insulin-like growth factor-1 (IGF-1) and brain derived neurotrophic factor (BDNF), with the former promoting KCC2 expression and the latter down regulating it [35]. BDNF is produced by activated microglia in the injured spinal cord and microglial-derived BDNF contributes to the down regulation of KCC2 and spinal disinhibition in a variety of nerve injury models of neuropathic pain [36, 37]. There are also reports that markers of spinal microglia activation are elevated in diabetic rodents and that disruption of microglial activation restores spinal KCC2 expression and ameliorates behavioral indices of neuropathic pain [38–41]. Further, spinal delivery of BDNF to normal rats promotes a diabetes-like pain state, with reduced dorsal horn KCC2 expression accompanied by GABAA receptor-driven allodynia and hyperalgesia, whereas spinal delivery of a BDNF sequestering agent to diabetic rats reverses allodynia [29]. However, we have been unable to identify a diabetes-induced increase in activated spinal microglia in our diabetic rats (Lee-Kubli and Calcutt, unpublished data) and have therefore explored the possibility that excess spinal BDNF in diabetic rats could be derived from sources outside the spinal cord. BDNF is expressed by primary afferent neurons and transported along central projections of the axon to the spinal cord, where its release modifies synaptic functions and sensory processing [42]. Interestingly, BDNF mRNA [43] and protein (Figure 1) expression are elevated in the dorsal root ganglia of diabetic rats. It is therefore plausible that diabetes-induced increased primary afferent BDNF expression, transport and central release contributes to spinal disinhibition and subsequent allodynia.
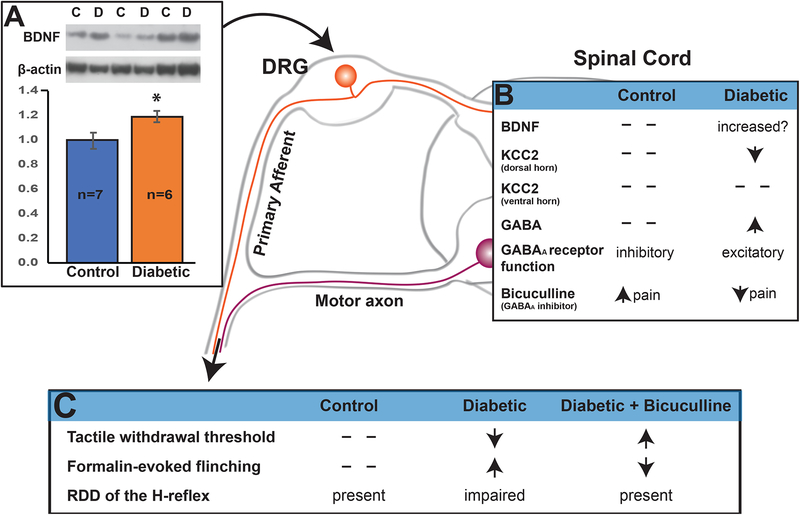
FIGURE 1: The impact of diabetes on spinal modulation of sensory function in rodents.
Diabetes significantly (* = P<0.05 vs control by unpaired t test) increases BDNF protein in dorsal root ganglia (Panel A: Lee-Kubli and Calcutt, unpublished data showing group mean±SEM), while excess spinal GABA and BDNF promote decreased KCC2 protein in the dorsal horn, loss of the inhibitory function of GABAA receptors and anti-nocicpetive effects of spinal delivery of the GABAA receptor antagonist bicuculline (Panel B). These biochemical disorders drive behavioral indices of neuropathic pain and loss of rate dependent depression (RDD) of the H-reflex, all of which are restored by spinal bicuculline (Panel C). See references [25, 29, 24, 30].
Rate-dependent depression of the H-reflex as a biomarker of spinal disinhibition
Our working hypothesis, that spinal disinhibition contributes to pain in diabetic neuropathy, is not easy to test directly in patients, as this would require measuring spinal BDNF, KCC2 and GABAA receptor function. The prediction from our animal studies that a spinal GABAA receptor antagonist would alleviate neuropathic pain in diabetic subjects could plausibly be assessed, but runs counter to the currently accepted wisdom that GABAA agonists are analgesic [44, 45]. We therefore sought a more clinically acceptable method of assessing spinal GABAergic tone based on the phenomenon of diminished rate dependent depression (RDD) of the Hoffman (H)-reflex, a long-established electrophysiological phenomenon that has more recently been linked with spinal KCC2 levels[46, 25].
The H-reflex has been described as the electrophysiological analog of the tendon reflex [47] and H-reflex latency is used to measure sensory nerve conduction velocities in humans [48] and rodents [49, 50]. The H-reflex also has several properties that can be used to evaluate spinal function. For example, the ratio of the amplitude of the maximum H-reflex (Hmax) to the maximum M-wave (Mmax) can provide information about excitability of motor neurons because Mmax relies on the direct activation of motor fibers, while Hmax relies upon both the excitability of the motor neuron soma and the strength of the synaptic connections between Ia afferents and motor neurons [51, 47]. Further, while the H-reflex is only detected in a subset of muscle groups under normal conditions, patients with CNS lesions show both increased Hmax/Mmax ratio and the presence of H-reflexes in previously silent muscles, indicating that tonic descending inhibition of spinal sensory circuits is impaired [52–56]. Changes to the Hmax/Mmax ratio have also been detected in astronauts, likely reflecting reduced motor neuron excitability in zero gravity conditions [57]. The H-reflex can be inhibited by parallel external input such as vibration or muscle stretch and this inhibition is enhanced after cerebral lesions [58] but impaired in patients with spinal cord injury [59–61]. The properties of the H-reflex therefore offer insight into the function of spinal and supraspinal modulatory systems during disease or other homeostatic disruptions when viewed with appropriate caution [62].
The spinal H-reflex is modified when exposed to a second simulation within 0.2–1.0s of the initial stimulation. This results in a decrease in H wave amplitude relative to the amplitude of the original H wave (Figure 2) and is variably referred to as RDD, frequency-dependent depression or paired-pulse depression [63–66]. As RDD of the H-reflex may be measured by a simple electrophysiological procedure that is commonly employed for nerve conduction studies, it can be adapted to evaluate function of spinal inhibitory systems in humans. Indeed, there is an extensive literature demonstrating that RDD is lost after spinal cord injury [52, 64, 67]. Diminution of RDD after spinal cord injury has been associated with spasticity and rigidity of the lower limbs [64, 65] arising from reduced spinal inhibition [68] due to damage to descending inhibitory systems and/or selective loss of inhibitory interneurons [65, 69, 70]. Attenuated RDD of the H-reflex has also been reported in hyperkinetic children and patients with psychosis or Parkinson’s disease and can be modulated with treatment [71–73]. These data suggest that RDD impairment is not exclusive to conditions that involve overt physical damage to local spinal inhibitory circuitry, and implicate the higher CNS in H-reflex modulation. However, few studies have examined the pharmacological underpinnings of RDD of the H-reflex and little detail is known about the neurotransmitters and circuitry involved.
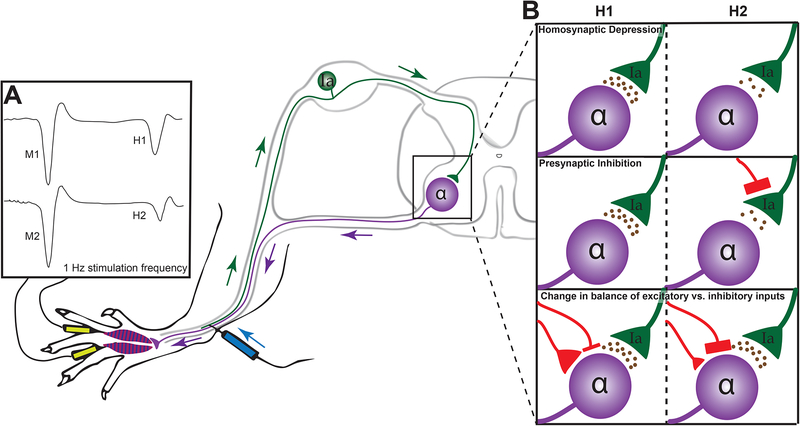
FIGURE 2: Rate dependent depression of the H-reflex.
Initial stimulation of the tibial nerve produces an electromyogram at the paw (Panel A) consisiting of an early M wave (M1) arising from orthodromic stimulation of motor neurons and a later H wave resulting from stimulation of adjacent primary afferents that synapse onto motor neurons in the ventral horn of the spinal cord (H1). Repetitive stimulation at 1Hz results in diminution of the amplitude of subsequent H waves (H2<H1) while the M wave retains its former amplitude (M2=M1). Rate dependent depression of the H-reflex (H1–H2) may be modified by a variety of mechanisms (Panel B) including homoynaptic depression, presynaptic inhibition or modified excitability of the post-synapse. Recent appreciation of the role of the KCC2 in post-synaptic membranes provides another means of altering RDD, while the selective loss of KCC2 protein in the dorsal horn of diabetic rats and its association with loss of RDD (see Figure 1) contributes to a growing appreciation that RDD is more than a simple monosynaptic reflex [62].
The H-reflex, like the tendon reflex, has been considered to be the result of activation of a monosynaptic reflex pathway [74], in which Ia afferents synapse directly onto ventral horn motor neurons (Figure 2). As the duration of post-synaptic inhibition of the motor neuron does not last long enough to contribute to RDD, diminution of H-reflex amplitude is frequently attributed to reduced excitatory neurotransmitter release onto motor neurons due to presynaptic inhibition or homosynaptic depression [75–78, 64, 66]. However, more recent studies implicate direct inhibition of the motor neuron itself in some forms of RDD. For example, reduced KCC2 expressed by ventral motor neurons is associated with impaired RDD following spinal injury [79, 46]. It should also be noted that small polysynaptic group II afferents also contribute to the H-reflex [80] and that the excitatory post-synaptic potential (EPSP) of the H-reflex in humans and rats has a sufficiently long rise time to allow for the contribution of oligosynaptic inputs [81, 82]. This potential complexity of the H-reflex is supported by evidence from cats [83, 84] and humans [85, 86] indicating that group Ia afferents make both monosynaptic and oligosynaptic excitatory connections onto homonymous muscles, with only the most excitable afferent fibers causing a monosynaptic discharge of motor neurons without also activating interneurons [87]. Further evidence in support of an oligosynaptic origin of inhibition during RDD comes from the loss of RDD in hind limbs of dogs that underwent temporary ischemia and selectively damaged spinal interneurons [88]. It is therefore plausible that inhibition of any neuron that contributes to the final excitation of ventral motor neurons could affect the H-reflex amplitude by shifting the balance between excitatory and inhibitory input.
Insight into cellular mechanisms underlying RDD impairment can be extrapolated from interventions that alleviate RDD deficits associated with physical spinal cord injuries or disease. For example, impaired RDD of the H-reflex following spinal cord injury is restored by treadmill training in patients [70] and passive exercise in rats [89–93], presumably via reinforcement of ventral inhibition. Exercise regimens following spinal injury restore both RDD and spinal KCC2 expression [94]. These results suggest that RDD depends upon maintenance of the post-synaptic motor neuron chloride gradient by KCC2 that facilitates post-synaptic hyperpolarization via the GABAA receptor chloride ion channel. There are relatively few reports of pharmacological interventions that restore RDD after loss through injury or disease. The inhibitory neurotransmitter GABA has been implicated in RDD because both GABAA and GABAB receptors are involved in presynaptic inhibition of H-reflex circuitry in the cat [95–97]. RDD is also impaired in response to anesthetic agents, including pentobarbital, Nembutal, saffan and etomidate, all of which interact with GABA receptors [98, 63]. The importance of spinal GABA to RDD was confirmed by a study showing that the combination of lentiviral-induced overexpression of GAD65 with a GABA reuptake inhibitor tiagabine, restored depression of the spinal H-reflex in a rat model of spinal cord injury induced spasticity [99]. The metobotropic GABAB receptor agonist, baclofen, which alleviates spasticity in patients [100, 101], has also been reported to restore RDD impairment in an animal model of cortical infarction resulting in forelimb spasticity [102]. However, interpretation of studies with baclofen is complex as it can also reduce the absolute amplitude of the H-reflex or block the H-reflex completely by directly inhibiting post-synaptic motor neurons [103–105, 65, 101]. L-Dopa has been shown to restore RDD in spinal cord injured rats [106], as has modafinil, an eugeroic drug with a multiplicity of potential targets, including monoamines such as 5-HT [107] used to treat narcolepsy. 5-HT restored the H-reflex recovery curve in patients with psychiatric disorders [108] while duloxetine, a serotonin-norepinephrine re-uptake inhibitor (SNRI) or DOI, a selective 5-HT2A/C receptor agonist, restored impaired RDD in diabetic rats [30], supporting a contribution of supraspinal inputs to RDD. Such a contribution may itself be via spinal GABA-mediated inhibition because a specific 5-HT2A receptor agonist was found to upregulate the function of spinal KCC2 in motor neurons and restore RDD in spinal cord injured rats [79]. Conversely, a recent report linked severity of spasticity after spinal cord injury with impaired RDD of the H-reflex and increased expression of 5-HT2A receptors on motor neurons [109]. However, serotonergic fibers were also significantly reduced due to the spinal cord injury, so it is unclear which, if either, of these features had an effect on RDD. While the intricacies of RDD remain to be untangled, it is clear that the circuitry and pharmacology of RDD exhibits much greater complexity than the common characterization of RDD as a monosynaptic spinal reflex [62].
Rate-dependent depression of the H-reflex in experimental diabetes
Our interest in using RDD impairment as a biomarker for loss of spinal GABAergic inhibition and subsequent pain arising from spinal disinhibition developed from apparent mechanistic parallels between diabetic rats and rats treated with the KCC2 antagonist DIOA. Diabetic rats had reduced spinal KCC2 and both diabetic and DIOA-treated rats exhibited hyperalgesia to paw formalin and allodynia to paw mechanical stimulation [25]. Both models also showed loss of RDD. Moreover, whereas the GABAA antagonist bicuculline diminished RDD in control rats, establishing the role of GABAA receptor function in normal RDD, the antagonist restored RDD in both diabetic and DIOA-treated rats. These data indicate that GABA, acting via spinal GABAA receptors contributes to loss of RDD in these models, and suggests that KCC2 depletion and subsequent loss of the chloride gradient in post-synaptic neurons attenuates the hyperpolarizing actions of GABAA receptors and potentially induced inversion of GABA function to excitatory. These data led us to consider RDD as an index of GABAergic inhibitory tone and loss of RDD as a biomarker for GABAA-mediated disinhibition that contributes to the pain state of these animals.
The viability of RDD as a biomarker for spinal inhibitory tone was further tested in studies that manipulated spinal BDNF as a means of altering local KCC2 expression. BDNF delivery to the spinal cord of control rats reduced KCC2 expression, diminished RDD and induced allodynia [29]. The impact of BDNF on RDD and tactile sensation is mediated via GABAA receptors, as spinal delivery of bicuculline restored RDD and reduced allodynia and hyperalgesia in BDNF-treated rats, as also occurs in both diabetic and DIOA-treated rats [25]. We then showed that allodynia, hyperalgesia and loss of RDD in diabetic rats were all functions of spinal BDNF, as all disorders were reversed by sequestering this neurotrophic factor. Sequestration of spinal BDNF also allowed GABA to resume its normal role of contributing to RDD. A further test of the capacity of the loss of RDD to discriminate pain associated with spinal disinhibition was provided by examining rats treated with paclitaxel, a model of chemotherapy-induced neuropathic pain that has been attributed to increased primary afferent drive [110, 111]. In this model, the same behavioral indices of neuropathic pain seen in diabetic, BDNF-treated or DIOA-treated rats were present but, unlike the other models, RDD was normal and GABA agonists (not antagonists) alleviated indices of pain [29]. One somewhat unexpected finding was that while KCC2 protein expression was selectively reduced in the dorsal horn of diabetic and BDNF-treated rats, paclitaxel-treated rats had selectively reduced KCC2 in the ventral horn. These data suggest that paclitaxel-induced neuropathic pain may include a spinal component related to disinhibition of ventral motor neurons to accompany increased primary afferent drive, have focused our interest on BDNF-regulated KCC2 expression in post-synaptic neurons of the dorsal horn and support a polysynaptic contribution to RDD.
Our most recent studies have extended observations of impaired RDD in rodent models of type 1 diabetes from rats to mice and also to a rat model of type 2 diabetes. We also demonstrated that the initiating pathogenic event was not acute ambient hyperglycemia and that impaired RDD could be corrected by treatment with insulin or C-peptide, as is tactile allodynia [112, 113, 30]. The SNRI duloxetine, which is used to treat painful diabetic neuropathy [114] and alleviates tactile allodynia of diabetic rats in a spinal 5HT2a receptor dependent manner [115], also restored RDD in diabetic rats via a similar mechanism [30]. Thus, neuropathic pain and loss of RDD not only appear to share a common pathogenic mechanism involving spinal KCC2 depletion and disinhibition caused by inversion of GABAA receptor function but also exhibit common responses to therapeutic intervention outside the root pathogenic mechanism. This supports the concept that RDD status may be a viable biomarker for both identifying the dominant generator site in individual patients with neuropathic pain and for predicting efficacy of therapeutic strategies that target alleviation of spinal disinhibition.
Rate dependent depression of the H-reflex in patients with diabetic neuropathy
RDD has been studied in humans with spinal injury and assorted diseases for over half a century. Both humans and rats show similar magnitudes of RDD and loss of RDD after spinal cord injury, suggesting that RDD findings in animal models of disease may be relevant to the human condition [116, 82, 117, 93]. Accumulating evidence that loss of RDD and indices of neuropathic pain share a common pathogenic mechanism in rodents and that RDD can serve as a biomarker for predicting efficacy of a spinally acting analgesic prompted our exploratory study to determine whether RDD was impaired by diabetes and associated with neuropathic pain.
We measured the magnitude of RDD of the H-reflex in patients with type I diabetes segregated into painful and painless neuropathy and in age-matched healthy controls [30]. Importantly, indices of metabolic control did not differ significantly between patients with and without painful neuropathy, suggesting that immediate severity of diabetes was not the cause of pain. Conventional measures of large and small fiber neuropathy were also similar in the two diabetic groups, so that there was no association between standard measures of neuropathy and pain. RDD was detected in all groups and, consistent with previous human studies, there was a progressive increase in the magnitude of RDD with increasing stimulation frequency. However, mean RDD was significantly reduced and notably more variable in the painful neuropathy group compared to both healthy controls and patients with painless diabetic neuropathy. Detailed analysis revealed that approximately half of the painful neuropathy group exhibited RDD that was more that 2 standard deviations from the mean of the control group such that the painful neuropathy group could be sub-divided into those with relatively normal RDD or notably impaired RDD. Within the painful neuropathy group, no significant correlation was observed between severity of RDD impairment and the severity of small and large fiber neuropathy, indicating that loss of RDD was independent of severity of neuropathy. Subjects also underwent in-vivo corneal confocal microscopy, which has been shown to be an objective and sensitive test to detect abnormalities in small nerve fiber morphology in patients with diabetes [118–122]. Nerve density in the corneal sub-basal nerve plexus was reduced in both groups of patients diagnosed with diabetic neuropathy. This loss was more dramatic in those with painful neuropathy [30] but within the painful neuropathy group did not correlate with impaired RDD. Thus, while pain in patients with diabetic neuropathy is likely to be complex in origin [8, 9], our finding of RDD deficits in a sub-set of diabetic patients with pain suggest that abnormalities in spinal excitability and disinhibition contribute to the development of painful symptoms in a proportion of diabetic patients who may be identified by impaired RDD.
The frequency dependence curves for RDD in normal rodents and humans are remarkably similar [29, 30], despite the procedural difference that rodents are anesthetized during the procedure whereas human are not. However, it is notable that diabetic rodents exhibit a much more homogeneous neuropathy, neuropathic pain and impaired RDD phenotypes than diabetic humans, in whom only approximately 60% will ever develop neuropathy, 20% (one third of those with neuropathy) will develop pain and, from our admittedly small scale exploratory study, only 10% (half of those with pain) will show RDD deficits. This is presumably due to the genetic homogeneity of experimental rat and mouse strains. It is also important to acknowledge that a number of patients in our study were taking anti-neuropathic pain medication and that their therapeutic response was not studied in a systematic manner. To validate the use of RDD as a biomarker of spinal disinhibition in patients with diabetic neuropathy, further larger scale studies that control for the use of anti-neuropathic pain medication and/or systematically assess therapeutic response are required. One potential drawback to using RDD as a biomarker in diabetic subjects is that the H-reflex can be difficult to elicit in a proportion of patients with diabetic neuropathy [123] and RDD may therefore be of limited use in screening patients with severe neuropathy. H-reflex amplitude can also be modulated by factors such as muscle stretch or contraction and postural variables [124] and should therefore be performed using strictly defined procedural criteria.
Similarities in RDD frequency-dependence and the impact of diabetes suggest, but do not demonstrate, similarities in the spinal circuitry between species. While understanding of the spinal physiology and pharmacology of RDD in rodents is emerging, further mechanistic studies comparing RDD with the nociceptive flexion reflex [125], which also undergoes inter-stimulus interval dependent habituation [126], and measures of diffuse noxious inhibitory control such as conditioned pain modulation, which is impaired in patients with painful diabetic neuropathy who show a greater therapeutic response to SNRIs [127], may help further elucidate the underlying spinal circuitry in humans.
Conclusions and Future Directions
RDD, used in combination with a measure of primary afferent activity such as microneurography, may allow assessment of the balance between peripheral vs spinal drive in patients with painful diabetic neuropathy and guide treatment strategies. For example, patients with diabetic neuropathy whose pain is driven by spinal disinhibition will demonstrate impaired RDD and may respond preferentially to anti-neuropathic pain medications that overcome disinhibition such as SNRI’s [115, 114]. Conversely patients with pain driven predominantly by peripheral mechanisms such as nociceptor hyperexcitability might show greater therapeutic response to drugs acting on ion channels of primary afferents [128]. Using RDD as a simple to employ biomarker may also be useful to select patients likely to be most responsive to a given therapy undergoing clinical trial based on the primary mechanism of pain generation. Thus, trials of peripheral acting therapies could enroll patients with minimal impairment of spinal inhibitory pathways whereas therapies that modulate spinal inhibition could select patients with evidence of impaired spinal disinhibition. This approach may allow number needed to treat (NNT) and number needed to harm (NNH) values to be optimized and pave the way towards personalized medicine.
Acknowledgments
Supported by National Institutes for Health awards DK057629 (NAC), DP3DK108245 (NAC) and DK081082 (NAC and RAM) and American Diabetes Association Award 1-17-ICTS-062 (AGM, NAC). This is a post-peer-review, pre-copyedit version of an article published in Current Diabetes Reports. The final authenticated version is available online at: http://dx.doi.org/10.1007/s11892-018-0969-5
Footnotes
Conflict of Interest
Corinne Lee-Kubli, Andrew G. Marshall, Rayaz A. Malik, and Nigel A. Calcutt declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
Previously unpublished data (Figure 1) was collected by Corinne Lee-Kubli and Nigel Calcutt using the UCSD IACUC-approved protocol S-02059R.
REFERENCES
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–54. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220–4. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518–22. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 4.Pruitt J 3rd, Moracho-Vilrriales C, Threatt T, Wagner S, Wu J, Romero-Sandoval EA. Identification, prevalence, and treatment of painful diabetic neuropathy in patients from a rural area in South Carolina. J Pain Res. 2017;10:833–43. doi: 10.2147/JPR.S129139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, Group KS. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. 2009;10(2):393–400. doi: 10.1111/j.1526-4637.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- 6.Baron R, Tolle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. Pain. 2009;146(1–2):34–40. doi: 10.1016/j.pain.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Finnerup NB, Sindrup SH, Jensen TS. Management of painful neuropathies. Handbook of Clinical Neurology. 2013;115:279–90. [DOI] [PubMed] [Google Scholar]
- 8.D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101(1):8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 9.Lee-Kubli CA, Calcutt NA. Painful neuropathy: Mechanisms. Handb Clin Neurol. 2014;126:533–57. doi: 10.1016/B978-0-444-53480-4.00034-5. [DOI] [PubMed] [Google Scholar]
- 10.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–9. [DOI] [PubMed] [Google Scholar]
- 11.Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 1996;16(1):283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts LA, Beyer C, Komisaruk BR. Nociceptive responses to altered GABAergic activity at the spinal cord. Life Sci. 1986;39(18):1667–74. [DOI] [PubMed] [Google Scholar]
- 13.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72(1):169–79. [DOI] [PubMed] [Google Scholar]
- 14.Sorkin LS, Puig S, Jones DL. Spinal bicuculline produces hypersensitivity of dorsal horn neurons: effects of excitatory amino acid antagonists. Pain. 1998;77(2):181–90. [DOI] [PubMed] [Google Scholar]
- 15.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37(1):111–23. [DOI] [PubMed] [Google Scholar]
- 16.Gwak YS, Tan HY, Nam TS, Paik KS, Hulsebosch CE, Leem JW. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J Neurotrauma. 2006;23(7):1111–24. doi: 10.1089/neu.2006.23.1111. [DOI] [PubMed] [Google Scholar]
- 17.Hammond DL, Drower EJ. Effects of intrathecally administered THIP, baclofen and muscimol on nociceptive threshold. Eur J Pharmacol. 1984;103(1–2):121–5. [DOI] [PubMed] [Google Scholar]
- 18.Miletic G, Draganic P, Pankratz MT, Miletic V. Muscimol prevents long-lasting potentiation of dorsal horn field potentials in rats with chronic constriction injury exhibiting decreased levels of the GABA transporter GAT-1. Pain. 2003;105(1–2):347–53. [DOI] [PubMed] [Google Scholar]
- 19.Wilson PR, Yaksh TL. Baclofen is antinociceptive in the spinal intrathecal space of animals. Eur J Pharmacol. 1978;51(4):323–30. [DOI] [PubMed] [Google Scholar]
- 20.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.•.Prescott SA. Synaptic inhibition and disinhibition in the spinal dorsal horn. Prog Mol Biol Transl Sci. 2015;131:359–83. doi: 10.1016/bs.pmbts.2014.11.008. [DOI] [PubMed] [Google Scholar]; A clear and conscise recent review of the role of spinal disinhibition in neuropathic pain.
- 22.Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993;620(2):287–91. [DOI] [PubMed] [Google Scholar]
- 23.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22(15):6724–31. doi:20026611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malmberg AB, O’Connor WT, Glennon JC, Cesena R, Calcutt NA. Impaired formalin-evoked changes of spinal amino acid levels in diabetic rats. Brain Res. 2006;1115(1):48–53. doi: 10.1016/j.brainres.2006.07.077. [DOI] [PubMed] [Google Scholar]
- 25.Jolivalt CG, Lee CA, Ramos KM, Calcutt NA. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain. 2008;140(1):48–57. doi: 10.1016/j.pain.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang XL, Zhang HM, Chen SR, Pan HL. Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol. 2007;579(Pt 3):849–61. doi: 10.1113/jphysiol.2006.126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang XL, Zhang Q, Zhang YZ, Liu YT, Dong R, Wang QJ et al. Downregulation of GABAB receptors in the spinal cord dorsal horn in diabetic neuropathy. Neurosci Lett. 2011;490(2):112–5. doi: 10.1016/j.neulet.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 28.Morgado C, Pinto-Ribeiro F, Tavares I. Diabetes affects the expression of GABA and potassium chloride cotransporter in the spinal cord: a study in streptozotocin diabetic rats. Neurosci Lett. 2008;438(1):102–6. doi: 10.1016/j.neulet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 29.Lee-Kubli CA, Calcutt NA. Altered rate-dependent depression of the spinal H-reflex as an indicator of spinal disinhibition in models of neuropathic pain. Pain. 2014;155(2):250–60. doi: 10.1016/j.pain.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.••.Marshall AG, Lee-Kubli C, Azmi S, Zhang M, Ferdousi M, Mixcoatl-Zecuatl T et al. Spinal Disinhibition in Experimental and Clinical Painful Diabetic Neuropathy. Diabetes. 2017;66(5):1380–90. doi: 10.2337/db16-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first report of impaired RDD in type 1 diabetic patients with neuropathic pain. Diabetic patients with similar neuropathy but no pain had normal RDD. Preclinical studies extended measures of impaired RDD to rat model of type 2 diabetes.
- 31.•.Javdani F, Hollo K, Hegedus K, Kis G, Hegyi Z, Docs K et al. Differential expression patterns of K(+) /Cl(−) cotransporter 2 in neurons within the superficial spinal dorsal horn of rats. J Comp Neurol. 2015;523(13):1967–83. doi: 10.1002/cne.23774. [DOI] [PubMed] [Google Scholar]; A thorough demonstration of the exclusive localization of KCC2 to dendrites and neuronal perikarya in the spinal dorsal horn, variability of KCC2 distribution between neurons and its absence from presynaptic membranes.
- 32.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 2014;15(10):637–54. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424(6951):938–42. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 34.•.Doyon N, Prescott SA, De Koninck Y. Mild KCC2 Hypofunction Causes Inconspicuous Chloride Dysregulation that Degrades Neural Coding. Front Cell Neurosci. 2015;9:516. doi: 10.3389/fncel.2015.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper uses computer simulations to demonstrate that modest deceases in KCC2 function can impede normal neurotransmission. This finding helps address concerns that the detected reduction of spinal KCC2 protein during injury or disease was too small to produce the associated pain phenotype.
- 35.Watanabe M, Fukuda A. Development and regulation of chloride homeostasis in the central nervous system. Front Cell Neurosci. 2015;9:371. doi: 10.3389/fncel.2015.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 37.Ferrini F, De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013;2013:429815. doi: 10.1155/2013/429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM et al. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70(4):1246–54. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- 39.Morgado C, Pereira-Terra P, Cruz CD, Tavares I. Minocycline completely reverses mechanical hyperalgesia in diabetic rats through microglia-induced changes in the expression of the potassium chloride co-transporter 2 (KCC2) at the spinal cord. Diabetes Obes Metab. 2011;13(2):150–9. doi: 10.1111/j.1463-1326.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 40.Tsuda M, Ueno H, Kataoka A, Tozaki-Saitoh H, Inoue K. Activation of dorsal horn microglia contributes to diabetes-induced tactile allodynia via extracellular signal-regulated protein kinase signaling. Glia. 2008;56(4):378–86. doi: 10.1002/glia.20623. [DOI] [PubMed] [Google Scholar]
- 41.Wodarski R, Clark AK, Grist J, Marchand F, Malcangio M. Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats. Eur J Pain. 2009;13(8):807–11. doi: 10.1016/j.ejpain.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55(1):1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Fernyhough P, Diemel LT, Brewster WJ, Tomlinson DR. Altered neurotrophin mRNA levels in peripheral nerve and skeletal muscle of experimentally diabetic rats. J Neurochem. 1995;64(3):1231–7. [DOI] [PubMed] [Google Scholar]
- 44.McCarson KE, Enna SJ. GABA pharmacology: the search for analgesics. Neurochem Res. 2014;39(10):1948–63. doi: 10.1007/s11064-014-1254-x. [DOI] [PubMed] [Google Scholar]
- 45.Zeilhofer HU, Ralvenius WT, Acuna MA. Restoring the spinal pain gate: GABA(A) receptors as targets for novel analgesics. Adv Pharmacol. 2015;73:71–96. doi: 10.1016/bs.apha.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16(3):302–7. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 47.Palmieri RM, Ingersoll CD, Hoffman MA. The hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004;39(3):268–77. [PMC free article] [PubMed] [Google Scholar]
- 48.Wager EW Jr., Buerger AA. A linear relationship between H-reflex latency and sensory conduction velocity in diabetic neuropathy. Neurology. 1974;24(8):711–4. [DOI] [PubMed] [Google Scholar]
- 49.Cliffer KD, Tonra JR, Carson SR, Radley HE, Cavnor C, Lindsay RM et al. Consistent repeated M- and H-Wave recording in the hind limb of rats. Muscle Nerve. 1998;21(11):1405–13. [DOI] [PubMed] [Google Scholar]
- 50.Stanley EF. Sensory and motor nerve conduction velocities and the latency of the H reflex during growth of the rat. Exp Neurol. 1981;71(3):497–506. [DOI] [PubMed] [Google Scholar]
- 51.Bandaru SP, Liu S, Waxman SG, Tan AM. Dendritic spine dysgenesis contributes to hyperreflexia after spinal cord injury. J Neurophysiol. 2015;113(5):1598–615. doi: 10.1152/jn.00566.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angel RW, Hofmann WW. The H Reflex in Normal, Spastic, and Rigid Subjects. Arch Neurol. 1963;9:591–6. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Mullin R, Mayer RF. H reflexes in acute and chronic hemiplegia. Brain. 1972;95(3):559–72. [DOI] [PubMed] [Google Scholar]
- 54.Little JW, Halar EM. H-reflex changes following spinal cord injury. Arch Phys Med Rehabil. 1985;66(1):19–22. [PubMed] [Google Scholar]
- 55.Taylor S, Ashby P, Verrier M. Neurophysiological changes following traumatic spinal lesions in man. J Neurol Neurosurg Psychiatry. 1984;47(10):1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teasdall RD, Park AM, Languth HW, Magladery JW. Electrophysiological studies of reflex activity in patients with lesions of the nervous system. II. Disclosure of normally suppressed monosynaptic reflex discharge of spinal motoneurones by lesions of lower brain-stem and spinal cord. Bull Johns Hopkins Hosp. 1952;91(4):245–56. [PubMed] [Google Scholar]
- 57.Reschke MF, Anderson DJ, Homick JL. Vestibulospinal reflexes as a function of microgravity. Science. 1984;225(4658):212–4. [DOI] [PubMed] [Google Scholar]
- 58.Ashby P, Verrier M, Warsh JJ, Price KS. Spinal reflexes and the concentrations of 5-HIAA, MHPG, and HVA in lumbar cereborspinal fluid after spinal lesions in man. J Neurol Neurosurg Psychiatry. 1976;39(12):1191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Field-Fote EC, Brown KM, Lindley SD. Influence of posture and stimulus parameters on post-activation depression of the soleus H-reflex in individuals with chronic spinal cord injury. Neurosci Lett. 2006;410(1):37–41. doi: 10.1016/j.neulet.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nielsen J, Petersen N, Ballegaard M, Biering-Sorensen F, Kiehn O. H-reflexes are less depressed following muscle stretch in spastic spinal cord injured patients than in healthy subjects. Exp Brain Res. 1993;97(1):173–6. [DOI] [PubMed] [Google Scholar]
- 61.Phadke CP, Thompson FJ, Kukulka CG, Nair PM, Bowden MG, Madhavan S et al. Soleus H-reflex modulation after motor incomplete spinal cord injury: effects of body position and walking speed. J Spinal Cord Med. 2010;33(4):371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve. 2003;28(2):144–60. doi: 10.1002/mus.10372. [DOI] [PubMed] [Google Scholar]
- 63.Ho SM, Waite PM. Effects of different anesthetics on the paired-pulse depression of the h reflex in adult rat. Exp Neurol. 2002;177(2):494–502. [DOI] [PubMed] [Google Scholar]
- 64.Ishikawa K, Ott K, Porter RW, Stuart D. Low frequency depression of the H wave in normal and spinal man. Exp Neurol. 1966;15(1):140–56. [DOI] [PubMed] [Google Scholar]
- 65.Kakinohana O, Hefferan MP, Nakamura S, Kakinohana M, Galik J, Tomori Z et al. Development of GABA-sensitive spasticity and rigidity in rats after transient spinal cord ischemia: a qualitative and quantitative electrophysiological and histopathological study. Neuroscience. 2006;141(3):1569–83. doi: 10.1016/j.neuroscience.2006.04.083. [DOI] [PubMed] [Google Scholar]
- 66.Lloyd DP, Wilson VJ. Reflex depression in rhythmically active monosynaptic reflex pathways. J Gen Physiol. 1957;40(3):409–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olsen PZ, Diamantopoulos E. Excitability of spinal motor neurones in normal subjects and patients with spasticity, Parkinsonian rigidity, and cerebellar hypotonia. J Neurol Neurosurg Psychiatry. 1967;30(4):325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mukherjee A, Chakravarty A. Spasticity mechanisms - for the clinician. Front Neurol. 2010;1:149. doi: 10.3389/fneur.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsushita A, Smith CM. Spinal cord function in postischemic rigidity in the rat. Brain Res. 1970;19(3):395–410. [DOI] [PubMed] [Google Scholar]
- 70.Trimble MH, Kukulka CG, Behrman AL. The effect of treadmill gait training on low-frequency depression of the soleus H-reflex: comparison of a spinal cord injured man to normal subjects. Neurosci Lett. 1998;246(3):186–8. [DOI] [PubMed] [Google Scholar]
- 71.Metz J, Goode DJ, Meltzer HY. Descriptive studies of H-reflex recovery curves in psychiatric patients. Psychol Med. 1980;10(3):541–8. [DOI] [PubMed] [Google Scholar]
- 72.Pivik RT, Mercier L. Spinal motoneuronal excitability in hyperkinesis: H-reflex recovery function and homosynaptic depression during wakefulness. J Clin Neuropsychol. 1981;3(3):215–36. [DOI] [PubMed] [Google Scholar]
- 73.Sabbahi M, Etnyre B, Al-Jawayed I, Jankovic J. H-reflex recovery curves differentiate essential tremor, Parkinson’s disease, and the combination of essential tremor and Parkinson’s disease. J Clin Neurophysiol. 2002;19(3):245–51. [DOI] [PubMed] [Google Scholar]
- 74.Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin. 2000;30(2):67–80. [DOI] [PubMed] [Google Scholar]
- 75.Curtis DR, Eccles JC. Synaptic action during and after repetitive stimulation. J Physiol. 1960;150:374–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Decandia M, Provini L, Taborikova H. Mechanisms of the reflex discharge depression in the spinal motoneurone during repetitive orthodromic stimulation. Brain Res. 1967;4(2):284–91. [DOI] [PubMed] [Google Scholar]
- 77.Fuortes MG, Frank K, Becker MC. Steps in the production of motoneuron spikes. J Gen Physiol. 1957;40(5):735–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108(3):450–62. [DOI] [PubMed] [Google Scholar]
- 79.Bos R, Sadlaoud K, Boulenguez P, Buttigieg D, Liabeuf S, Brocard C et al. Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci U S A. 2013;110(1):348–53. doi: 10.1073/pnas.1213680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayer RF, Mawdsley C. Studies in Man and Cat of the Significance of the H Wave. J Neurol Neurosurg Psychiatry. 1965;28:201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burke D, Gandevia SC, McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol. 1983;339:535–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meinck HM. Occurrence of the H reflex and the F wave in the rat. Electroencephalogr Clin Neurophysiol. 1976;41(5):530–3. [DOI] [PubMed] [Google Scholar]
- 83.Jankowska E, Johannisson T, Lipski J. Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. J Physiol. 1981;310:381–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jankowska E, McCrea D, Mackel R. Oligosynaptic excitation of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol. 1981;316:411–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol. 1984;52(3):435–48. [DOI] [PubMed] [Google Scholar]
- 86.Fukushima Y, Yamashita N, Shimada Y. Facilitation of H-reflex by homonymous Ia-afferent fibers in man. J Neurophysiol. 1982;48(5):1079–88. [DOI] [PubMed] [Google Scholar]
- 87.Magladery JW. Some observations on spinal reflexes in man. Pflugers Arch Gesamte Physiol Menschen Tiere. 1955;261(4):302–21. [DOI] [PubMed] [Google Scholar]
- 88.Gelfan S. Altered spinal motoneurons in dogs with experimental hind-lamb rigidity. J Neurophysiol. 1966;29(4):583–611. [DOI] [PubMed] [Google Scholar]
- 89.Cote MP, Azzam GA, Lemay MA, Zhukareva V, Houle JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28(2):299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garrison MK, Yates CC, Reese NB, Skinner RD, Garcia-Rill E. Wind-up of stretch reflexes as a measure of spasticity in chronic spinalized rats: The effects of passive exercise and modafinil. Exp Neurol. 2011;227(1):104–9. doi: 10.1016/j.expneurol.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ollivier-Lanvin K, Keeler BE, Siegfried R, Houle JD, Lemay MA. Proprioceptive neuropathy affects normalization of the H-reflex by exercise after spinal cord injury. Exp Neurol. 2010;221(1):198–205. doi: 10.1016/j.expneurol.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reese NB, Skinner RD, Mitchell D, Yates C, Barnes CN, Kiser TS et al. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord. 2006;44(1):28–34. doi: 10.1038/sj.sc.3101810. [DOI] [PubMed] [Google Scholar]
- 93.Yates CC, Charlesworth A, Reese NB, Skinner RD, Garcia-Rill E. The effects of passive exercise therapy initiated prior to or after the development of hyperreflexia following spinal transection. Exp Neurol. 2008;213(2):405–9. doi: 10.1016/j.expneurol.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cote MP, Gandhi S, Zambrotta M, Houle JD. Exercise modulates chloride homeostasis after spinal cord injury. J Neurosci. 2014;34(27):8976–87. doi: 10.1523/JNEUROSCI.0678-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eccles JC, Schmidt RF, Willis WD. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J Physiol. 1962;161:282–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stuart GJ, Redman SJ. The role of GABAA and GABAB receptors in presynaptic inhibition of Ia EPSPs in cat spinal motoneurones. J Physiol. 1992;447:675–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Willis WD. John Eccles’ studies of spinal cord presynaptic inhibition. Prog Neurobiol. 2006;78(3–5):189–214. doi: 10.1016/j.pneurobio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 98.Duke M, Advokat C. Pentobarbital-induced modulation of flexor and H-reflexes in spinal rats. Brain Res. 2000;881(2):217–21. [DOI] [PubMed] [Google Scholar]
- 99.Kakinohana O, Hefferan MP, Miyanohara A, Nejime T, Marsala S, Juhas S et al. Combinational spinal GAD65 gene delivery and systemic GABA-mimetic treatment for modulation of spasticity. PLoS One. 2012;7(1):e30561. doi: 10.1371/journal.pone.0030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abbruzzese G. The medical management of spasticity. Eur J Neurol. 2002;9 Suppl 1:30–4; discussion 53–61. [DOI] [PubMed] [Google Scholar]
- 101.Macdonell RA, Talalla A, Swash M, Grundy D. Intrathecal baclofen and the H-reflex. J Neurol Neurosurg Psychiatry. 1989;52(9):1110–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee S, Toda T, Kiyama H, Yamashita T. Weakened rate-dependent depression of Hoffmann’s reflex and increased motoneuron hyperactivity after motor cortical infarction in mice. Cell Death Dis. 2014;5:e1007. doi: 10.1038/cddis.2013.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Azouvi P, Roby-Brami A, Biraben A, Thiebaut JB, Thurel C, Bussel B. Effect of intrathecal baclofen on the monosynaptic reflex in humans: evidence for a postsynaptic action. J Neurol Neurosurg Psychiatry. 1993;56(5):515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Corleto JA, Bravo-Hernandez M, Kamizato K, Kakinohana O, Santucci C, Navarro MR et al. Thoracic 9 Spinal Transection-Induced Model of Muscle Spasticity in the Rat: A Systematic Electrophysiological and Histopathological Characterization. PLoS One. 2015;10(12):e0144642. doi: 10.1371/journal.pone.0144642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dachy B, Dan B. Electrophysiological assessment of the effect of intrathecal baclofen in dystonic children. Clin Neurophysiol. 2004;115(4):774–8. doi: 10.1016/j.clinph.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 106.Liu H, Skinner RD, Arfaj A, Yates C, Reese NB, Williams K et al. L-Dopa effect on frequency-dependent depression of the H-reflex in adult rats with complete spinal cord transection. Brain Res Bull. 2010;83(5):262–5. doi: 10.1016/j.brainresbull.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yates CC, Charlesworth A, Reese NB, Ishida K, Skinner RD, Garcia-Rill E. Modafinil normalized hyperreflexia after spinal transection in adult rats. Spinal Cord. 2009;47(6):481–5. doi: 10.1038/sc.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Metz JT, Holcomb HH, Meltzer HY. Effect of 5-hydroxytryptophan on H-reflex recovery curves in normal subjects and patients with affective disorders. Biol Psychiatry. 1988;23(6):602–11. [DOI] [PubMed] [Google Scholar]
- 109.Ryu Y, Ogata T, Nagao M, Kitamura T, Morioka K, Ichihara Y et al. The swimming test is effective for evaluating spasticity after contusive spinal cord injury. PLoS One. 2017;12(2):e0171937. doi: 10.1371/journal.pone.0171937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C. Terminal arbor degeneration--a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci. 2011;33(9):1667–76. doi: 10.1111/j.1460-9568.2011.07652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain. 2008;135(3):262–70. doi: 10.1016/j.pain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain. 1996;68(2–3):293–9. [DOI] [PubMed] [Google Scholar]
- 113.•.Jolivalt CG, Rodriguez M, Wahren J, Calcutt NA. Efficacy of a long-acting C-peptide analogue against peripheral neuropathy in streptozotocin-diabetic mice. Diabetes Obes Metab. 2015;17(8):781–8. doi: 10.1111/dom.12477. [DOI] [PubMed] [Google Scholar]; C-peptide, a product of pro-insulin processing, has neuroprotective properties in diabetic mice including preventing loss of RDD. A first demonstration that a systemically delivered therapy can protect RDD, as opposed to acute pharmacological manipulation of RDD with spinally delivered drugs.
- 114.Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurol. 2008;8:29. doi: 10.1186/1471-2377-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mixcoatl-Zecuatl T, Jolivalt CG. A spinal mechanism of action for duloxetine in a rat model of painful diabetic neuropathy. Br J Pharmacol. 2011;164(1):159–69. doi: 10.1111/j.1476-5381.2011.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gozariu M, Roth V, Keime F, Le Bars D, Willer JC. An electrophysiological investigation into the monosynaptic H-reflex in the rat. Brain Res. 1998;782(1–2):343–7. [DOI] [PubMed] [Google Scholar]
- 117.Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J Neurophysiol. 1992;68(5):1473–86. [DOI] [PubMed] [Google Scholar]
- 118.Chen X, Graham J, Dabbah MA, Petropoulos IN, Ponirakis G, Asghar O et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. 2015;38(6):1138–44. doi: 10.2337/dc14-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Clerck EE, Schouten JS, Berendschot TT, Kessels AG, Nuijts RM, Beckers HJ et al. New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3(8):653–63. doi: 10.1016/S2213-8587(15)00136-9. [DOI] [PubMed] [Google Scholar]
- 120.Jiang MS, Yuan Y, Gu ZX, Zhuang SL. Corneal confocal microscopy for assessment of diabetic peripheral neuropathy: a meta-analysis. Br J Ophthalmol. 2016;100(1):9–14. doi: 10.1136/bjophthalmol-2014-306038. [DOI] [PubMed] [Google Scholar]
- 121.Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148–54. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- 122.Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33(8):1792–7. doi: 10.2337/dc10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Millan-Guerrero R, Trujillo-Hernandez B, Isais-Millan S, Prieto-Diaz-Chavez E, Vasquez C, Caballero-Hoyos JR et al. H-reflex and clinical examination in the diagnosis of diabetic polyneuropathy. J Int Med Res. 2012;40(2):694–700. doi: 10.1177/147323001204000233. [DOI] [PubMed] [Google Scholar]
- 124.Burke D, Adams RW, Skuse NF. The effects of voluntary contraction on the H reflex of human limb muscles. Brain. 1989;112 (Pt 2):417–33. [DOI] [PubMed] [Google Scholar]
- 125.Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans -- review article. Pain. 2002;96(1–2):3–8. [DOI] [PubMed] [Google Scholar]
- 126.von Dincklage F, Olbrich H, Baars JH, Rehberg B. Habituation of the nociceptive flexion reflex is dependent on inter-stimulus interval and stimulus intensity. J Clin Neurosci. 2013;20(6):848–50. doi: 10.1016/j.jocn.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 127.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153(6):1193–8. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 128.Todorovic SM. Painful Diabetic Neuropathy: Prevention or Suppression? Int Rev Neurobiol. 2016;127:211–25. doi: 10.1016/bs.irn.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]