Abstract
Women are twice as likely than men to suffer from posttraumatic stress disorder (PTSD). While women have increased exposure to traumatic events of many types and have greater prevalence of comorbid psychiatric disorders compared to men, these differences do not account for the overall sex difference in the prevalence of PTSD. The current review summarizes significant findings that implicate the role of estradiol, progesterone, and allopregnanolone in female risk for PTSD symptoms and dysregulation of fear psychophysiology that is cardinal to PTSD. We also discuss how these steroid hormones influence the stress axis and neural substrates critical for the regulation of fear responses. Understanding the role of ovarian steroid hormones in risk and resilience for trauma-related adverse mental health outcomes across the lifespan in women has important translational, clinical, and intergenerational implications for mitigating the consequences of trauma exposure.
Keywords: Women, estrogen, progesterone, PTSD, fear psychophysiology, neuroimaging
1. Introduction
Post-traumatic stress disorder (PTSD) is a debilitating psychiatric condition that occurs after exposure to a psychological traumatic event [1]. Epidemiological studies indicate that approximately 70% of the general population will experience a traumatic event in their lifetime, but only 7.8% of the general population in the United States will go on to develop PTSD [2]. Prospective studies indicate that while the majority of trauma victims experience the cardinal symptoms of PTSD, including re-experiencing, avoidance, and hyperarousal, immediately following trauma [3], for most trauma survivors these symptoms subside and eventually disappear [3]. For a significant minority, however, the symptoms persist and develop into syndromal PTSD that is associated with significant comorbidities including major depression [4], substance and alcohol abuse [5], suicide [6], and cardiometabolic disease [7; 8].
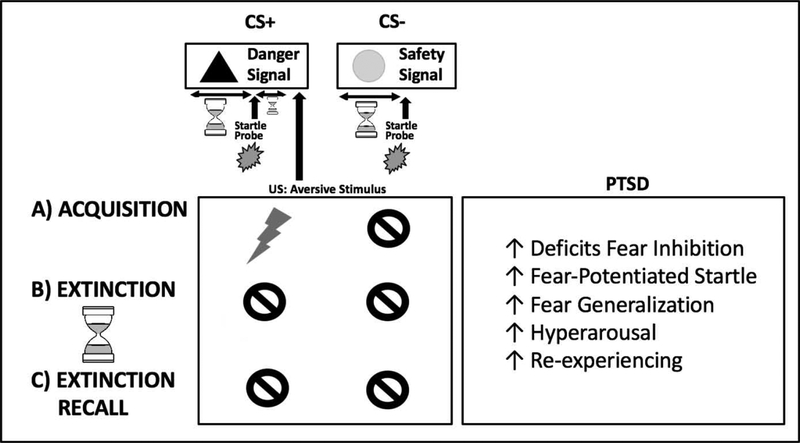
PTSD is a heterogeneous psychiatric syndrome that is characterized by re-experiencing, avoidance, hyperarousal symptoms, and negative cognitions [9]. The etiology and maintenance of PTSD is linked to deficits in fear regulation, including increased fear response and deficits in fear extinction [10; 11; 12], that are assessed in humans and in rodents by objectively measuring psychophysiological changes, such as skin conductance or startle responses, in response to fear conditioning paradigms [13; 14; 15; 16]. Classical fear conditioning paradigms typically consist of a habituation phase, a fear conditioning (acquisition) phase, and a fear extinction phase (Figure 1). In the habituation phase, subjects become familiar with the study environment. In the fear-conditioning phase (acquisition), a neutral stimulus (conditioned stimulus; CS) is paired with an aversive unconditioned stimulus (such as a mild shock or aversive air blast; US). In the fear extinction phase, the CS is no longer presented with the aversive US, and participants should be able to dissociate the CS from danger. An extinction recall phase is sometimes used to assess the presence of fear extinction learning acquired during the extinction phase of the paradigm. Some paradigms have multiple neutral stimuli, where only one (CS+) is paired with the US (danger cue) while the other (CS−) is not (safety cue). These fear-conditioning paradigms can assess stimulus (CS+ vs. CS−) and context discrimination (environment or cue-based), as well as fear generalization, the expression of fear in inappropriate contexts. Deficits in fear inhibition during fear conditioning are analogous to the fear generalization symptoms seen in PTSD, while deficits in fear extinction and extinction recall point to the re-experiencing and hyperarousal symptoms characteristic of PTSD in traumatized individuals [10].
Figure 1. Illustration of fear conditioning paradigms and fear responses related to PTSD.
Classical fear conditioning paradigms typically consist of a (A) conditioning (acquisition) phase, an (B) extinction phase, and sometimes an (C) extinction recall phase. In the conditioning phase (acquisition), a neutral stimulus (conditioned stimulus; CS) is paired with an aversive unconditioned stimulus (US; such as a mild shock or aversive air blast). Some paradigms have multiple neutral stimuli, where only one (CS+; danger cue) is paired with the aversive US while the other (CS−; safety cue) is not. In the extinction phase, the CS+ is no longer presented with the aversive US, and participants should learn to dissociate the CS from danger. In some paradigms, recall of the extinction memory (dissociation of the CS+ from danger) is assessed at a later time (extinction retention phase). Fear potentiated startle is another paradigm used, in which the startle response is measured in response to a loud but harmless tone (startle probe) in the presence of the CS+ and CS−. Deficits in fear inhibition during fear conditioning are analogous to the increased fear and fear generalization symptoms seen in PTSD, while deficits in extinction and extinction recall point to the re-experiencing and hyperarousal symptoms characteristic of PTSD.
Many studies over the last few decades have identified environmental risk factors for individual vulnerability to the development of PTSD in trauma survivors, including severity and duration of trauma, childhood abuse and lack of family and social support [17; 18]. Recent work has also identified genetic factors that influence individual susceptibility to PTSD, as PTSD is 30–40% heritable [19]. Candidate gene studies suggest that genetic polymorphisms at loci that influence the activity of biological mechanisms, including the stress axis and fear circuitry, increase individual vulnerability to the development of PTSD [20].
One factor known to affect risk for and severity of PTSD is biological sex, as women are twice as likely than men to suffer from PTSD [17; 21; 22; 23]. While women have increased exposure to traumatic events that confer greater risk for PTSD and have greater prevalence of comorbid psychiatric disorders compared to men, these differences do not account for the overall sex difference in the prevalence of PTSD [21; 22; 24]. In the current non-systematic review, we will summarize significant findings that implicate the role of ovarian steroid hormones in female risk for PTSD symptoms and dysregulation of fear psychophysiology that is cardinal to PTSD. More specifically, we will summarize how natural fluctuations in estradiol, progesterone and its metabolite allopregnanolone, the primary endogenous female steroid hormones, over the ovarian cycle of women are associated with changes in PTSD symptoms and how these hormones influence neural substrates critical for the regulation of fear responses. In parallel, we will highlight translational and preclinical animal model studies that manipulate ovarian steroid hormones to assess their mechanistic role in PTSD pathophysiology. Understanding the role of ovarian steroid hormones in risk and resilience for trauma-related adverse mental health outcomes has important translational, clinical, and intergenerational implications for mitigating the consequences of trauma exposure.
2. Physiology of ovarian hormone fluctuations over the menstrual cycle
Estradiol (E2) is the primary endogenous estrogen that is produced by the aromatase enzyme, which converts testosterone into E2. E2 acts primarily via estrogen receptors alpha (ERα) and beta (ERβ), both nuclear steroid hormone receptor transcription factors that are localized within the cell nucleus where once the ligand binds, dimerize to bind estrogen response elements within promoter regions of DNA to influence gene transcription [25]. While ERα and ERβ mediate most central E2 effects, it is important to note that these two receptors can also interact with other transcription factors when they are not bound to E2 [26]. These non-classical effects of E2 receptors have been labeled as ligand-independent [27].
Progesterone (P4) is the primary endogenous progestin that is synthesized from cholesterol and pregnanolone by cytochrome P45017 and 3β-hydroxysteroid-dehydrogenase (3β-HSD), respectively [28]. P4 influences gene transcription via its own steroid hormone receptor transcription factor, the progesterone receptor (PR) [29], whose expression is increased by E2 [30]. Progesterone can have fast-acting, neuroactive effects via its conversion to its metabolite, 3α-5α-tetrahydroprogesterone, more commonly known as allopregnanolone. Allopregnanolone is a positive allosteric modulator of γ-aminobutyric acid (GABAA) receptors that is ten times more potent than benzodiazepines [31]. This conversion of progesterone to allopregnanolone is a two-step process, requiring 5α-reductase to convert progesterone into 5α-dihydroprogesterone (5α-DHP), and 3α-hydroxysteroid-dehydrogenase (3α-HSD) to convert 5α-DHP into allopregnanolone [31].
Importantly, concentrations E2 and P4 vary over the course of the menstrual cycle in women and non-human primates, and the estrus cycle in rodents. The fluctuations of E2 and P4 are regulated by the fine-tuned control of the hypothalamic-pituitary-gonadal (HPG) axis. More specifically, gonadotropin-releasing hormone (GnRH) is secreted from neurons in the medialbasal hypothalamus in a pulsatile manner [32; 33], that in turns binds GnRH receptors in the anterior pituitary to induce the secretion of luteinizing hormone (LH) and follicle stimulating hormone (FSH) into the vasculature. LH acts on receptors in the theca cells of the ovaries to stimulate androgen synthesis, follicle rupture, and ovulation, while FSH acts to stimulate follicle maturation and increase aromatase activity for E2 biosynthesis from the granulosa cells [34].
At the beginning of the ovarian cycle in the early follicular (women) or estrus (rodent) phase, concentrations of E2 are at their with lowest levels due to negative feedback inhibition of the HPG axis [35]. However, over the course of the follicular phase, E2 levels increase steadily due to continued GnRH, and subsequent LH, and FSH release. A switch from negative to positive inhibition of the HPG axis occurs after E2 levels reach a threshold wherein they excite rather than inhibit GnRH neurons (via kisspeptin, reviewed in [36]), inducing the LH surge that is critical for the induction of ovulation at mid-cycle [37]. In the luteal (women) or diestrus (rodent) phase of the ovarian cycle, the ruptured follicle degenerates into the corpus luteum, a body that secretes progesterone in preparation for embryo implantation in the uterus [34]. Thus, the luteal phase is characterized by an increase in P4 concentrations that wanes slowly over time [38]. E2 concentrations decline following ovulation but rise again following the mid-luteal increase in P4 [38]. In parallel with P4 concentrations, plasma concentrations of allopregnanolone increase in the luteal compared to the follicular phase in women [28; 39]. Peripheral concentrations of E2, P4, and allopregnanolone all decrease if pregnancy does not occur, triggering menses and the beginning of the next cycle [38].
Fluctuations in steroid hormones concentrations over the menstrual cycle have been associated with changes in affective symptoms in women, as approximately 20% of reproductive-aged women in the United States experience significant increases in mood and anxiety symptoms in the premenstrual (luteal) phase of the cycle [40]. Decreasing levels of E2 [41; 42; 43] and P4/allopregnanolone [44; 45; 46] in the luteal phase have all been implicated in the etiology of mood dysregulation and premenstrual dysphoric disorder (PMDD), a mood disorder described in the DSM-5 whose onset is defined by menstrual cycle phase [9]. Although PMDD is characterized primarily by increases in depressed mood and anhedonia, it is also associated with increased anxiety symptoms [9; 47]. While low levels of E2 and diminishing concentrations of P4/allopregnanolone have been associated with increased risk for anxiety disorders in the premenstruum [48], the role of ovarian steroids in the etiology and maintenance of PTSD in women is less clear.
3. Ovarian hormones influence fear responses in women and rodents
3.1. Menstrual cycle phase impacts memory and fear responses in healthy women.
Studies probing memory for emotionally-arousing stimuli demonstrate that menstrual cycle phase is associated with changes in memory across both declarative and nondeclarative forms of memory (Table 1). Work across a number of studies leveraging classical fear-conditioning and list-learning tasks links phases of the menstrual cycle with high E2 levels with facilitated learning in humans, particularly for emotionally-arousing stimuli. For example, healthy women in the luteal phase of the menstrual cycle are more likely to experience spontaneous intrusive memories after watching an emotional film and to remember details of an emotional story compared to hearing a neutral story, relative to the early follicular phase [49; 50; 51]. Women in the early luteal phase are also more likely to experience intrusive memories for several days after watching a stressful film compared to women in the late luteal phase (low E2) or mid-follicular phase (high E2) of the cycle [52]. Such effects of menstrual cycle phase on emotional declarative memory may not be specific to negative or threatening stimuli, as women in the late-follicular phase of the menstrual cycle are more likely to remember positive images compared to women in the early-follicular phase, but found no effect of cycle phase on memory for negative images [53]. However, the findings are not entirely consistent across the literature, as data from at least one study showed that women in the early-follicular phase (low E2 levels) were more likely to remember negative picture stimuli compared to women in the mid-luteal phase [54].
Table 1.
Summary of clinical data assessing the effects of menstrual cycle phase and E2 levels on fear acquisition, extinction, extinction recall and inhibition in women.
| Clinical Status | Reference | Sample Size | Phenotype Assessed | Menstrual Cycle Phase or Hormone Assessment | Effect of E2 or Higher- E2 Cycle Phase | |
|---|---|---|---|---|---|---|
| Healthy | [49, 50] | Feree et. al. | 40 (19 follicular phase, 21 luteal phase); 48 (16 follicular phase, 16 luteal phase, 16 men) | Emotional declarative memory | Menstrual cycle phase confirmed by salivary E2/P4 | ↑ intrusive recollection of negative stimuli |
| Healthy | [51] | Nielsen et. al. | 98 (28 follicular phase, 31 luteal phase, 39 men) | Emotional declarative memory | Menstrual cycle phase confirmed by salivary E2/P4 | ↑ arousal-related enhancement of recall |
| Healthy | [52] | Soni et. al | 42 (11 late luteal phase, 15 early luteal phase, 15 mid-follicular phase) | Emotional declarative memory | Menstrual cycle phase confirmed by salivary E2/P4 | ↑ intrusive recollection of negative stimuli |
| Healthy | [54] | Bayer et.. al. | 22 (early follicular, and mid-luteal) | Emotional declarative memory | Menstrual cycle phase confirmed by salivary E2/P4 | ↓ declarative memory for negative emotional stimuli |
| Healthy | [53] | Pompili et al. | 38 (20 early follicular phase, 18 peri-ovulatory phase) | Emotional declarative memory | Menstrual cycle phase confirmed by salivary E2/P4 | No effect on declarative memory for negative stimuli; ↑ declarative memory for positive stimuli |
| Healthy | [58] | Glover et. al. | 28 (14 follicular phase, 14 luteal phase) | Classical conditioning | Menstrual cycle phase (self-report based on first day of menses) | ↑ fear acquisition to danger signal; ↑ fear discrimination |
| Healthy | [55] | Milad et. al. | 28 (14 early follicular phase, 14 late follicular phase) | Classical conditioning | Menstrual cycle phase (self-report based on first day of menses) | No cycle effect on fear acquisition; ↓ extinction recall |
| Healthy | [57] | Lonsdorf et. al. | 337 (18 follicular, 22 luteal, 172 hormonal contraceptives, 116 men) | Classical conditioning | Menstrual cycle phase, self-report; Hormonal contraceptive use | No cycle effect on fear inhibition; No cycle effect on extinction; Contraceptives (low E2/P4) ↓ fear inhibition |
| Healthy* | [56] | Pineles et. al. | 16 (repeated- measures in early follicular and mid- luteal phases) | Classical conditioning | Menstrual cycle phase, assessed by ovulation kit, confirmed by plasma E2/P4 | No effect on acquisition; ↑ extinction recall |
| Healthy | [66] | Wegerer et. al. | 37 (16 early follicular phase, 21 luteal phase) | Classical conditioning (violent film clips as US) | Salivary E2 | No effect on acquisition; ↑ fear extinction; ↓ memory for negative stimuli |
| Healthy* | [67] | Glover et. al. | 49 (24 low E2, 25 high E2) | Classical conditioning | Serum E2 | No effect on acquisition; no effect on fear inhibition; no effect on extinction |
| Healthy | [60] | Milad et. al. | 54 (18 low E2, 18 high E2, 18 men) | Classical conditioning | Serum E2 | No effect on acquisition; no effect on extinction training; ↑ extinction recall |
| Healthy | [61] | White & Graham | 73 (20 high E2, 19 low E2, 16 hormonal contraceptives, 18 males) | Classical conditioning | Serum E2 | No effect on fear inhibition; ↑ extinction recall |
| Healthy & Spider Phobic | [62] | Li & Graham | 60 (30 high E2, 30 low E2; 26 healthy, 34 spider phobic) | Classical conditioning (pictures of spiders as CS) | Serum E2 | No effect on extinction training; ↑ fear inhibition to CS-; ↑ extinction recall |
| PTSD* | [58, 67] | Glover et al. | 44 (22 high E2, 22 low E2); 32 (17 low E2, 15 high E2) | Classical conditioning | Serum E2 | No effect on acquisition; ↑ fear inhibition to CS-; ↑ extinction |
| PTSD* | [56] | Pineles et al. | 16 (repeated- measures in early follicular and mid- luteal phases) | Classical conditioning | Menstrual cycle phase, assessed by ovulation kit, confirmed by plasma E2/P4 | No effect on acquisition; ↓ extinction recall |
Studies that found differences between a healthy group and a patient group are listed twice to distinguish the separate results of E2 in each group.
The above-mentioned results from studies assessing the effects of menstrual cycle phase on emotional declarative memory are also not in full concordance with studies leveraging fear-conditioning paradigms to assess menstrual cycle effects on fear memory. For the majority of studies, there was no effect of menstrual cycle phase on the initial learning or acquisition of the fear memory [55; 56; 57]. Only one study found an effect on fear acquisition, such that healthy women in the luteal phase of the menstrual cycle displayed stronger acquisition of fear to a threat versus non-threat-linked stimulus (fear discrimination between danger and safety signals), while women in the follicular phase did not [58].
Instead, in classical conditioning paradigms, menstrual cycle appears to have stronger effects on fear extinction and particularly the long-term retention of extinction memory (“extinction recall”), which likely rely upon different neural substrates than the initial acquisition of fear. As in the case of emotional declarative memory, fear extinction and extinction recall require the participation of the hippocampus, medial prefrontal cortex, and amygdala, whereas the associations formed during fear acquisition primarily reside within the amygdala [59]. Perhaps more importantly, the most consistent effects of cycle phase are observed for extinction recall, such that high-E2 phases of the menstrual cycle are associated with better recall [56; 60; 61; 62]. Such recall paradigms are conducted 24 hours after the initial formation of the extinction memory, entailing consolidation processes that depend upon genetic effects for the formation of new stable synapses via transcriptional regulation by ERα, ERβ, and/or PR receptors. Together, such findings suggest that menstrual cycle phase has its strongest effects on transcription-dependent memory consolidation processes.
Discrepancies between studies may also arise from possible confounding of when memory learning, consolidation and recall where assessed relative to menstrual cycle phase at the time of each assessment, as well as methodological differences between studies, such as the specific phenotypes assessed (emotional memory vs. conditioning), and methods of assessment of menstrual cycle phase (Table 1). For example, some studies use self-report measures of menstrual cycle phase based on recall of first day of menses [55; 58], while others measure salivary E2 and P4 concentrations to verify menstrual cycle phase and categorize into follicular vs. luteal [49; 50; 51; 52; 53; 54]. Also contributing to the noise in menstrual cycle influences on fear responses and memory in women is the inherent variation in concentrations of E2 and P4 within menstrual cycle phases themselves (e.g. low E2 during early follicular vs. moderate E2 during mid-follicular vs. high E2 during ovulation/late follicular).
3.2. Menstrual cycle phase influences on PTSD and fear responses in traumatized women.
A handful of studies have linked PTSD symptoms with menstrual cycle-related changes in ovarian hormones. Among recently traumatized women, flashback memories—a hallmark symptom of PTSD—were significantly higher during the luteal phase compared to other cycle phases [63]. This study also showed that women who reported having experienced the initial trauma during the luteal phase additionally reported greater difficulties with flashbacks [63]. However, the evidence must be considered preliminary as the measurement of cycle timing relied on self-report of last menstrual period, a notoriously unreliable timing method [64], in a relatively small cohort of women (N=138). The only other study assessing the effects of menstrual cycle phase (confirmed by E2/P4 hormone assays) on PTSD symptoms (N=49) found that some PTSD symptoms are exacerbated in the early follicular phase of the menstrual cycle as opposed to the mid-luteal phase [65]. More specifically, women with PTSD show higher depression and phobic anxiety during the early follicular phase as compared to the mid-luteal phase confirmed by E2 and P4 concentrations [65]. While these limited available data on menstrual cycle phase and PTSD symptoms suggest that different aspects of PTSD symptomology change over the varying phases of the menstrual cycle (with memory-related symptoms such as intrusive recall increasing during the early/mid luteal phase, and anxiety symptoms peaking during the early follicular phase), a more comprehensive, large cohort study is necessary to better describe menstrual cycle effects on PTSD symptomology in traumatized women.
Laboratory-based studies of fear conditioning and extinction show that extinction recall in women with PTSD is impaired during the early luteal phase of the menstrual cycle as confirmed by tracking of the ovulatory LH surge [56]. Such findings suggest that the hormonal changes shortly after ovulation (decrease in E2, increase in P4) negatively impact the consolidation and/or post-encoding stabilization of the extinction memory in women with PTSD, with relatively little effect on the initial formation and extinction of fear memory. These results contrast somewhat with the findings from healthy participants, who show greater fear acquisition to threat [58], and greater extinction memory [55], and worse extinction recall during the follicular phase [56]. Together these limited data suggest that menstrual cycle stage influences PTSD symptoms and fear psychophysiology in traumatized women. However, given that there has only been one fear conditioning study in women to assess menstrual cycles effects on PTSD [56], additional work is needed to better understand the effects of ovarian hormone fluctuations on PTSD and fear responses in traumatized women over the menstrual cycle, as well as other periods of significant hormonal change (i.e. menopause, postpartum). Furthermore, the above studies linking menstrual cycle phase to alterations in PTSD symptoms and fear learning do not examine the direct association between steroid hormones concentrations themselves, and PTSD symptoms and fear responses (Table 1). However, many studies in women suggest that concentrations of E2 and P4/allogpregnanolone (independent of menstrual cycle phase) are associated with risk for PTSD and altered fear-learning outcomes.
3.3. Ovarian hormone concentrations are associated with PTSD and fear responses in women
In healthy women, low E2 concentrations are associated with greater intrusive memories after fear conditioning with short and violent film clips as the unconditioned stimuli [66]. Similarly, low E2 concentrations in women are associated with higher PTSD symptoms compared to women with high E2 levels [67]. Furthermore, low E2 levels are associated with poorer extinction recall, even though women with low E2 levels report a conscious expectation that the CS+ was no longer associated with threat [60; 61; 62]. In fact, women with high E2 levels do not differ from men in extinction recall, suggesting that E2 is at least partly responsible for the increased vulnerability for PTSD in women [60]. Use of hormonal contraceptives, which inhibit endogenous E2 production and reduce overall E2 concentrations, is also associated with poorer extinction recall [61; 68]. Although little experimental work has been conducted, initial evidence suggests that this deficit in extinction recall is rescued by a single oral dose of E2 [68].
Estradiol levels influence fear extinction training in women as well (Table 1). Healthy women with high endogenous E2 concentrations demonstrate fear stimulus discrimination, lower fear potentiated startle to a safety cue, and lower skin conductance responses during extinction recall, while women with low endogenous E2 levels do not [58; 62]. Healthy women with lower E2 concentrations also display higher skin conductance responses during fear extinction than women with higher E2 concentrations [66]. In women with PTSD, low E2 levels are associated with greater fear potentiated startle during extinction [67]. Together, these data suggest that lower E2 concentrations may increase vulnerability for PTSD symptoms and poorer fear learning outcomes in traumatized women (Table 1). This notion is supported by a systematic review of the literature on E2 and menstrual cycle phase on fear learning and PTSD [69], as well as data showing that the suppression of endogenous E2 concentration via hormonal contraceptives in healthy women leads to lower stimulus discrimination during extinction recall [57].
While a substantial literature on the effects of E2 on fear-related outcomes exists as described above in women, P4 concentrations have more reliably been used as a way to confirm menstrual cycle phase as opposed to assessing their relationships to PTSD symptoms and fear psychophysiology [69]. Thus, only a handful of studies assess the association between P4 concentrations on similar outcomes in healthy women [69], and the results have been equivocal. P4 concentrations are not associated with emotion regulation of psychophysiological fear responses [70] or intrusive memories after fear conditioning [66] in healthy women. P4 levels are also not associated with changes in conditioned fear extinction and recall in healthy women over the menstrual cycle [60; 61; 71]. However, salivary P4 concentrations in the luteal phase of the cycle predict spontaneous intrusive memories after exposure to emotional films in healthy women [49], and high P4 concentrations in healthy women during the early-luteal phase predict intrusive memories for several days after watching a stressful film [52].
Very few studies assess the relationship between P4/allopregnanlone and PTSD-related outcomes in women with PTSD. Data from a singular study focused on fear extinction showed that extinction recall during the midluteal phase is associated with high P4 concentrations in women with PTSD [56]. Plasma and cerebral spinal fluid (CSF) concentrations of allopregnanolone are lower in women with PTSD [28; 72]. This decrease in allopregnanolone occurs in parallel with an increase of its precursor, 5α-DHP (5α-dihydroprogesterone), consistent with the notion of a deficiency in the activity of the allopregnanolone synthetic enzyme 3α-hydroxysteroid dehydrogenase (3α-HSD) [28]. PTSD symptom severity is associated with lower allopregnanolone to 5α-DHP ratio in women during the early luteal phase [28]. Although these studies indicate that P4 and allopregnanolone impact PTSD-related symptoms and fear extinction, the ability to assess the unique effects of high P4/allopregnanolone on outcomes on interest is difficult due to the fact that P4/allopregnanolone concentrations are high when E2 concentrations are as well [73]. Significant work in rodent models has addressed this issue by not only assessing the effects of estrus cycle phase on fear-related outcomes, but also by directly manipulating hormones levels.
3.4. Ovarian hormones concentrations are associated with altered fear responses in rodents.
Preclinical studies in rodent models highlight the associations between estrus cycle phase and fear psychophysiology. For example, naturally cycling rats in the proestrus phase (high E2 and P4) show better extinction recall compared to rats in the metestrus phase (low E2 and P4) [68]. Rats in the proestrus phase of the estrus cycle also display less freezing behavior (indicative of less fear) during extinction recall compared to rats in the metestrus phase [74]. This enhanced recall during the proestrus phase in female rats is extinguished when E2 and/or P4 receptor antagonists are administered before extinction training [74]. Likewise, exogenous administration of E2 and/or P4 prior to extinction training during the metestrus phase results in less freezing during extinction recall, suggesting that differences in extinction recall due to differences in estrus cycle phase are directly influenced by ovarian hormones [74].
The above notion is highlighted by data showing that E2 administration 24-hours prior to fear conditioning improves extinction [75]. Furthermore, direct manipulation of endogenous ovarian hormones in female rats via hormonal contraceptive treatment results in more freezing during extinction training and extinction recall compared to naturally cycling rats [68]. This effect is dose-dependent, as higher doses of hormonal contraceptives exacerbate this effect, increasing freezing during extinction and augmenting poorer extinction recall [68]. While these data indicate that high E2 levels may confer resilience by facilitating fear extinction learning, E2 administration during proestrus phase when endogenous E2 concentrations are high results in an impairment of extinction [76], suggesting that the effects of E2 on extinction processes may work as an inverted U function wherein low and high E2 levels impair extinction and moderate E2 levels facilitate extinction in females [76]. It is important to note however, that the findings of the above studies are limited due to presence of hormonal fluctuations of both E2 and P4. To assess the direct effects of E2 and P4 on fear psychophysiology, studies in rodent models have used hormone replacement following ovariectomy (to remove endogenous hormones) to assess the direct effects of these steroid hormones on fear processes.
Table 2 summarizes findings on direct E2 manipulation and fear learning processes while highlighting methodological differences between studies. Chronic E2 administration over 16 days also facilitates fear extinction of conditioned taste aversion in both gonadectomized male and female rats [77]. Replacement of E2 in ovariectomized (OVX) female rats results in fast fear generalization [78], in facilitation of extinction recall [79], and in decreased freezing in a novel context after contextual fear conditioning [80; 81]. While this result suggests that E2 may increase the ability to discriminate between novel and threatening contexts for female rats [80], other studies provide evidence that high E2 concentrations are associated with poorer fear learning outcomes. For example, OVX mice supplemented with estradiol benzoate for 10 days display greater anxiety-like behavior and more freezing after fear conditioning compared to OVX mice without E2 replacement [82]. This increased freezing after fear conditioning appears when OVX mice are treated with E2 both acutely (two days) and chronically (two weeks) at high doses [83]. The equivocal nature of these results may be due to alterations in estrogen dosage administered, type of estrogen administered, or type of fear conditioning assessed (contextual versus cue-based). For example, low doses of 17β-estradiol and 17α-estradiol enhance contextual fear conditioning, while high doses impair contextual fear conditioning [84]. High and low doses of estrone do not have an effect on contextual fear conditioning in old female rats, but a middle dose impairs contextual fear conditioning [84].
Table 2.
Summary of preclinical studies assessing the effects of direct E2 manipulation on fear acquisition, extinction, extinction recall and inhibition in ovariectomized (OVX) and orchidectomized (ODX) rodents.
| Reference | Species | Sex | Sample Size | Type of Fear Conditioning | Hormone Replacement Used | Hormone Dosage | Effects of E2 | |
|---|---|---|---|---|---|---|---|---|
| [77] | Yuan & Chambers | Rat | Male & Female | ~10 animals per group | Conditioned taste aversion | OVX/ODX animals implanted with capsule containing crystalline E2 for 8 days, 16 days, or 20 days before conditioned taste aversion occurred. | 0 mm, 10 mm, or 30 mm capsule filled with crystalline E2 | No effect on acquisition; ↑ extinction |
| [79] | Graham & Daher | Rat | Female | 11–16 per group | Auditory fear conditioning | OVX females administered E2 benzoate 24 hours after conditioning and again 24 hours before extinction training | 10 μg in 150 μg sesame oil | No effect on extinction training; ↑ extinction recall |
| [82] | Morgan & Pfaff | Mouse | Female | 15–17 animals per group | Auditory fear conditioning | OVX females implanted with capsule containing E2 benzoate 2 weeks before fear conditioning | 25, 50, or 75 μg in 0.03 mL sesame oil | ↑ acquisition |
| [76] | Graham & Scott | Rat | Female | 7–17 animals per group | Auditory fear conditioning | Estrous cycle phase confirmed by daily vaginal smears, low or high dose of β-E2 administered 30 minutes before extinction training | 15 μg/kg (low dose) or 100 μg/kg (high dose) of β-E2 | No effect on extinction training in proestrus; ↑ extinction training in metestrus; Low dose ↑ extinction recall when endogenous E2 is low; Low dose ↓ extinction recall or has no effect when endogenous E2 is high; High dose ↓ extinction recall when endogenous E2 is high |
| [80] | Barker & Galea | Rat | Male & Female | 8 per group | Contextual/ auditory fear conditioning | OVX/ODX animals received E2 benzoate for 15 days, ending the day before the conditioning procedure began | 33 μg/kg in 0.1 mL sesame oil | No effect on acquisition; ↑ fear inhibition |
| [78] | Lynch et al. | Rat | Male & Female | 9–12 per group | Contextu al fear conditioning | OVX females implanted with capsule containing 17β-E2 9 days before conditioning | 5 mm capsule filled with 17β-E2 | ↓ fear inhibition |
| [81] | Gupta et al. | Rat | Male & Female | 25 or 29 females, 12 males | Contextu al fear conditioning | OVX animals administered E2 benzoate 48 hours before conditioning and again 4 hours before conditioning | 10 μg in 0.1 mL peanut oil | ↓ consolidation of acquisition |
| [83] | Matsumoto et al. | Mouse | Female | 4–9 animals per group | Contextual fear conditioning | OVX females administered different doses of E2 benzoate either 2 days or 2 weeks before conditioning | 0.5 μg, 5 μg, or 50 μg injection in .1 mL oil, or 0.03 μg, 0.3 μg, 3 μg, or 30 μg in implanted capsule | ↑ acquisition |
| [84] | Barha et al. | Rat | Female | 7–9 animals per group | Contextual fear conditioning | OVX females administered 17β- E2, 17α-E2, or estrone 30 minutes before conditioning | 0.3 μg/0.1 mL, 1 μg/0.1 mL, or 10 μg/0.1 mL 17β- E2, 17α -E2, or estrone in sesame oil | No effect on fear inhibition; No effect on cued fear acquisition;, Low levels (P and a) ↑ contextual fear acquisition; High levels ↓ contextual fear acquisition |
| [90] | Lynch et al. | Rat | Female | 5–10 animals per group | Contextual fear conditioning | OVX females acutely administered E2 benzoate 24 hours, 6 hours, or 1 hour before training and were tested 24 hours or 6 days post training. Other OVX females acutely administered E2 benzoate 24 hours after training and 6 days, 2 days, 24 hours, 6 hours, or 1 hour before testing. Other OVX females were given ERα agonist or ERβ agonist 24 hours following training (24 hours before testing) | 15 μg E2 benzoate in 0.1 mL sesame oil, 1 mg/kg or 2.5 mg/kg of ERα agonist or ERβ agonist | ↓ fear inhibition |
| [91] | Lynch et al. | Rat | Female | 5–16 animals per group | Contextual fear conditioning | OVX females acutely administered cytosolic ER antagonist, E2 benzoate (subcutaneous injection or intracranial infusion), or ER agonists 24 hours post-training and 24 hours before testing | 15 μg/0.1 mL E2 benzoate (subcutaneous injection), 2 μL of 10 mM solution of E2 benzoate and dimethylsulfoxide (DMSO) (intracranial infusion), 100 μg in 2 μL DMSO of cytosolic ER antagonist, 0.2 pg/μL ERα agonist in DMSO, 40 pg/μL ERβ agonist in DMSO | ↓ fear inhibition |
| [125] | Chang et al. | Rat | Male & Female | 6–18 animals per group | Contextual fear conditioning | OVX females administered E2 benzoate (subcutaneous injection) 24 hours before conditioning. Other OVX females administered E2 benzoate (hippocampal infusion), ERα agonist, or ERβ agonist 1 hour before conditioning | 10 μg or 20 μg E2 benzoate dissolved in sesame oil (subcutaneous injection), 0.5 μM E2 benzoate in DMSO (hippocampal infusion), 5 μM ERα agonist, 5 μM ERβ agonist | No effect on acquisition, ↑ extinction recall |
Studies in female rats indicate that P4 facilitates the recall of fear extinction [74], corroborating data showing that replacement of P4 to OVX female mice enhances more general cognitive performance [85]. However, another study showed that fear extinction recall is potentiated by P4 replacement six hours following extinction training in E2-replaced OVX rats, whereas P4’s potentiating effects on extinction recall is abolished if extinction training occurs 24 hours after P4 is administered [79]. These studies indicate that P4’s ability to modulate fear extinction recall in E2-treated OVX rodents is biphasic and dependent on the time interval between P4 replacement and fear extinction training [79]. This notion that timing from maximal P4 concentrations is important for its influence on affective symptoms is further highlighted by data in women showing that withdrawal from high levels of P4/allopregnanolone during the menstrual cycle and in the postpartum period are associated with increased depressive symptoms [86].
Although concentrations of allopregnanolone also fluctuate with those of P4 across the menstrual and estrus cycles, much less research has directly manipulated allopregnanolone levels in preclinical models to assess the neuroactive steroid’s influence on fear responses. However, data from translational rodent models of PTSD show that decreases in allopregnanolone are induced by exposure to either prolonged social isolation or a single-prolonged stressor and associated with risk for PTSD-like phenotypes, including enhanced contextual fear condition and deficits in contextual fear extinction and recall [87; 88; 89]. Importantly, these fear deficits in rodents are normalized upon exogenous treatment with allopregnanolone itself [87], a synthetic analog (ganaxolone) [88], and other pharmaceutical agents that increase endogenous allopregnanolone levels in the brain [89], suggesting that allopregnanolone may be an effective treatment for PTSD [90].
3.5. Summary, synthesis and caveats
Overall, available preclinical, translational, and clinical data suggest that low levels of E2 in pre-menopausal, reproductive aged women confer risk for increased PTSD symptoms and dysregulation of fear responses, whereas high concentrations of E2 bestow resilience to PTSD and maladaptive alterations in fear psychophysiology in traumatized women by facilitating fear extinction learning and recall (Table 1). This notion that high E2 levels confer resilience to dysregulation of fear responses is corroborated by translational rodent studies (Table 2). Available cross-sectional data also suggest that the effects of E2 and P4 on fear regulation are impacted by the presence of PTSD in women. This is exemplified by findings showing that low E2 is associated with fear extinction deficits only in women with PTSD [67], and data showing that fear extinction recall is enhanced in healthy women, but impaired in women with PTSD during the early luteal phase of the menstrual cycle [56].
Although findings from many studies indicate that high E2 status confers resilience from PTSD symptoms and deficits in fear psychophysiology in women (Table 1), there are some inconsistencies in results, including null findings, that may be attributable to methodological differences between studies. For example, Milad and colleagues found that healthy women in the middle of the menstrual cycle (when E2 levels are high) have impaired extinction recall [55], while several other studies have found that high E2 is associated with enhanced extinction recall in women [60; 61; 62] or that E2 has no effect on extinction [67]. Subtle differences in the conditioning paradigms used, such as the number of conditioning trials assessed in extinction and how early and late extinction were defined between studies, may contribute to inconsistent results between studies and null findings. Furthermore, the study by Milad and colleagues compared women in different phases of the menstrual cycle and relied solely on self-reporting of menstrual cycle phase by participants, while the other studies assessed absolute E2 concentrations and compared participants based on E2 level [60; 61; 62]. Finally, it is also important to note that differences in results may arise due to difference in whether ovarian hormone concentrations were assessed in saliva or blood, as salivary levels are a better reflection of free and bioactive levels, whereas blood levels also include bound and inactive steroid hormone levels [91]. Another factor that may contribute to the equivocal nature of existing data are the nuisances of different fear conditioning paradigms used by investigators. For instance, Barker and Galea found that E2 reduces fear generalization [80], while Lynch and colleagues reported that E2 increases fear generalization [78; 92; 93]. Barker and Galea assessed fear by measuring freezing in a novel context [80], while Lynch and colleagues measured latency to cross to another part of the conditioning chamber [78; 92; 93].
Robust studies implicating P4 role in modulating risk and resilience for PTSD in women are generally lacking due to being used more as confirmatory maker of cycle phase and high co-linearity with E2 concentrations in the luteal phase [73]. However, fluctuations, withdrawal, and reductions of P4/allopregnanolone have been associated with increased risk for deficits in contextual fear conditioning and fear extinction and recall in rodents [87; 88; 89] and in women with PTSD [28; 56]. In the next section, we discuss two mechanisms by which E2 and P4/allopregnanolone influence fear psychophysiology and PTSD-related symptoms, via the modulation of fear neurocircuitry and the activity of the hypothalamic-pituitary-adrenal (HPA) axis.
4. Mechanisms of ovarian hormone action on fear and PTSD symptoms
4.1. Ovarian hormone actions on fear neurocircuitry.
Neurocircuitry models of the regulation of fear involve the amygdala, insula, hippocampus, and sub-regions of the prefrontal cortex, including the medial prefrontal cortex (mPFC) and anterior cingulate cortex (for reviews see [94; 95]). Dysfunction in various aspects of these circuits has been implicated in the pathophysiology of PTSD. For example, PTSD has been consistently linked with amygdala hyper-reactivity accompanied by reduced functional [96; 97] and structural [98; 99] connectivity with ventromedical PFC (vmPFC) subregions containing putative inhibitory projections to the amygdala, such as Brodmann Area 25. Individuals with PTSD also show increased functional connectivity between the amygdala and components of the salience network including dorsal ACC/dmPFC and the insula [100; 101; 102].
Fluctuations in steroid hormones are very likely to influence the neurocircuitry supporting acute threat reactivity and extinction learning. The human amygdala, hippocampus, and prefrontal cortex are densely populated with E2 receptors (ERα, ERβ) [103; 104]. E2 activation of ERβ has broad influences on synaptic plasticity (facilitating dendritic growth) [105; 106] as well as amygdala-mediated anxiolytic effects [107]. The amygdala, hippocampus, and vmPFC show relatively low progesterone receptor (PR) expression, but do show high levels of membrane-expressed progesterone receptors (mPR) [108]. Although steroid binding and biological functions of mPRs are only beginning to be explored in humans, these membrane-expressed receptors may be a critical component of how P4 influences cellular function. For example, these receptors have been implicated the precipitous drop in P4 that is responsible for stimulating labor in humans [109]. Interestingly, post-mortem concentrations of P4 are highest in the amygdala, hypothalamus, and cerebellum relative to other brain regions [110]. The post-mortem concentration of allopregnanolone also appears to be relatively low within the amygdala, hippocampus, and vmPFC [110], with greater expression in the substantia nigra and basal hypothalamus. However, even very low concentrations of allopregnanolone are known to potentiate the GABAa receptor [111], producing a number of neuroprotective effects such as stimulating the proliferation of human neural progenitor cells in the hippocampus [112].
Furthermore, anatomical MRI studies in women indicate that effects of female hormonal fluctuations during a single menstrual cycle have surprisingly gross global effects on the brain that can be observed at the coarse spatial resolution (~1mm3) observable with MRI neuroimaging. For example, a study mapping brain-wide gray matter, white matter, and CSF at 4 longitudinal timepoints within a cycle showed a 1.8% increase in gray matter volume concurrent with the peak in blood serum E2 (at ovulation), relative to other cycle timepoints [113]. The hippocampus, in particular, shows structural changes over the menstrual cycle in healthy women. During the mid-follicular phase of the cycle, hippocampal volume increases relative to the late luteal phase when E2 is low, with corresponding increases in declarative memory performance [114; 115]. A systematic review of structural neuroimaging studies shows that effects of both E2 and P4 are consistently observed within the fear neurocircuitry, particularly in the amygdala, hippocampus, nearby medial temporal lobe regions, and the anterior cingulate cortex [116].
The menstrual cycle and steroid hormone levels have similarly been linked with changes in human brain function. E2 appears to be linked with greater connectivity and activation in the vmpFC and hippocampus, and facilitates their modulatory influence on amygdala reactivity. For example, healthy women in the mid-follicular phase of the menstrual cycle show greater resting state activity (measured via glucose metabolism) in the prefrontal cortex and medial temporal lobe (including the hippocampus) compared to when they were in the early follicular phase [117]. Women with higher E2 levels concentrations show greater resting state amygdala-prefrontal cortex connectivity compared to women with low E2 levels [118]. E2 concentrations positively correlate with vmPFC activity in healthy women during the presentation of highly-arousing unpleasant film clips [119], and these findings have been interpreted as reflecting a beneficial prefrontal modulation of arousal responses to a potentially traumatizing stimulus.
In contrast, P4 appears to have opposing effects on the amygdala and its connections with the vmPFC and hippocampus. One particularly powerful neuroimaging study actively manipulated P4 levels [120], giving a single oral dose of micronized P4 during the early follicular phase when endogenous P4 and E2 are typically low. This was contrasted with a within-subjects placebo condition at the same phase of the next menstrual cycle. Blood assays confirmed that P4 and allopregnanalone increased to a level biologically expected during the mid-luteal phase or during early pregnancy. P4 produced an increase in the reactivity of the right basolateral amygdala to negative emotional face stimuli, and had no effect on any other brain region. Furthermore, P4 administration decreased the functional connectivity between the amygdala and the fusiform gyrus (involved in face perception) and increased connectivity with dACC. Similar findings have been observed in naturally-cycling women in the mid-luteal phase when endogenous P4 is high, who show increased amygdala and hippocampal responses to negative emotional stimuli relative to the early follicular phase [121], potentially indicating increased arousal or vigilance. Such P4-related alterations in human brain function closely parallel many of the neuroimaging correlates of PTSD.
A growing body of literature has addressed the effects of E2 on neural circuit function during fear conditioning and extinction. Women display greater activation of the right amygdala during fear acquisition compared to men [122]. A more detailed look at the contribution of E2 to this sex difference shows that only those women with high E2 concentrations display this heightened pattern of amygdala reactivity, whereas women with low E2, and women taking oral contraceptives did not differ from men [122], and a similar pattern has been observed for hypothalamic activation [123]. E2 concentrations in women appear to be linearly associated with activity in the amygdala and hypothalamus [123].
During fear extinction, successful extinction is expected to produce a reduction in amygdala reactivity to the CS+ (given that it is no longer associated with threat), accompanied by activation in the vmPFC and hippocampus which both facilitate the formation and expression of extinction memories. In one study of cycling women, those with low levels of E2 showed lower activation of vmPFC during extinction learning, and of vmPFC and hippocampus during extinction recall, compared to those with high levels of E2 [71; 123]. Similarly, women taking oral contraceptives with resulting suppression of endogenous hormones show heightened amygdala reactivity to the CS+ during extinction (relative to men and naturally cycling women), reflecting a potential failure or slowing of extinction [124]. Together, the findings suggest that when E2 is lower, amygdala reactivity during fear acquisition is also lower, but there is also a (potentially maladaptive) decrease in vmPFC and hippocampal activation during fear extinction and recall and a corresponding increase in the amygdala response to the extinguished CS+. It may thus be hypothesized that, during times of low or fluctuating E2, trauma-exposed women may fail to engage the vmPFC and hippocampus in extinguishing the fear associated with the trauma memory in the weeks and months following the trauma. While no neuroimaging study has yet investigated the direct effects of steroid hormone manipulation on fear neurocircuitry in women with PTSD, preclinical studies in rodent models have informed our understanding of how ovarian hormones affect the neurocircuitry regulating fear responses.
Estrogens influence the structure and function of the neurocircuitry regulating learning and cognition (for review please see [125]). In the regards to contextual fear learning known to be impaired in individuals with PTSD and linked to function of the hippocampus [94; 95], E2 replacement in OVX rats in reduces hippocampal long-term potentiation (LTP) while concurrently attenuating freezing behavior, suggesting that E2 reduces consolidation of the contextual fear memories [81]. This effect of E2 on hippocampal consolidation of the contextual fear is mediated via E2 actions on ERβ, as male and female mice lacking ERβ (BERKO) display less freezing in the context in which fear conditioning occurred [126] and OVX rats injected with an ERβ agonist in the dorsal hippocampus show enhanced retrieval of a contextual fear extinction memory [127]. ERβ in the dorsal CA1 region of the hippocampus also mediates the fear generalization enhancing effects of E2 in OVX rats by affecting retrieval of the fear memory [92; 93].
Estradiol also affects fear conditioning in a rodent model of PTSD wherein rats undergo a single prolonged stress that induces deficits in fear extinction and other PTSD-like phenotypes [128]. Supplementation of E2 in females who underwent a single prolonged stress show less freezing after contextual freezing compared to animals not given E2 [129]. This beneficial effect of E2 supplementation occurs concomitantly with E2-induced increases in levels of brain derived neurotrophic factor (BDNF; a neurotrophin that promotes cell generation and survival) and increased cell density in the hippocampus compared to animals supplemented with vehicle alone [129]. These data suggest that E2 promotes neurogenesis and cell survival in the hippocampus to reduce fear behaviors.
Estrogens can also impact the function of the amygdala to modulate fear psychophysiology in a sub-region specific manner. For instance, the activity in the basolateral amygdala (BLA) is higher in female than male rats, and varies over the course of the estrus cycle in female rats [130]. Similar to effects seen in the hippocampus, E2 affects synaptic plasticity in the amygdala. Inhibition of aromatase, the enzyme that converts testosterone into E2, in the BLA reduces spine density and blocks LTP in female rats [131]. The ability of E2 to affect the activity and plasticity of the amygdala has direct ramifications for the amydala’s capacity to regulate fear learning and extinction. For example, naturally cycling rats administered an ERβ agonist systemically 30 minutes prior to fear extinction training show less freezing during fear extinction recall compared to animals who received an ERα agonist or vehicle, suggesting ERβ facilitates extinction consolidation [71]. Additionally, systemic E2 administration immediately following extinction training (during the metestrus phase) show better fear extinction recall (less freezing) the next day compared to animals administered E2 four hours after extinction training. This systemic E2 administration following extinction training induces lower c-fos expression (an immediate early gene that marks neuronal activation) in the amygdala, suggesting that ERβ activation immediately following extinction training may modify synaptic plasticity in the amygdala to improve extinction memory consolidation [71].
The actions of E2 on modulating synaptic plasticity within the amygdala to improve the consolidation of fear extinction training are also sub-region specific. E2 treatment reduces c-fos expression in the lateral amygdala and increases c-fos expression in the central nucleus of the amygdala (CeA) during extinction learning [132]. However, during extinction recall, E2 treatment reduces c-fos expression in the central amygdala [132]. These results suggest that the E2-induced reduction of lateral amygdala activity during extinction learning results in the dissociation between the CS and the US [132], and that the E2-incuded reduction of CeA activity during extinction recall reduces the expression of fear behaviors [71; 132].
One mechanism by which E2 may be impacting the structure and function of the amygdala is by modulating the expression, and thus actions, of HDAC4, a histone deacetylase implicated in learning and memory [133; 134; 135; 136]. Female mice in metestrus when E2 concentrations are low show increased HDAC4 expression in the amygdala after fear conditioning compared to females that remained in their home cage and did not undergo fear conditioning [137]. A similar increase in HDAC4 expression in the amygdala is also seen in fear-conditioned OVX females. However, fear-conditioning in intact female mice during proestrus when E2 concentrations are high and in OVX females treated with E2 does not result in increased HDAC4 expression, suggesting that beneficial effects of E2 on fear learning and extinction may be mediated by its effects on HDAC4 [137]. This notion is supported by evidence that alterations in methylation within the HDAC4 gene are association with PTSD in women [137]. Furthermore, a single nucleotide polymorphism (SNP) within the HDAC4 gene is associated with greater expression of fear, deficits in fear extinction, increased resting state functional connectivity of the amygdala in traumatized women [137]. Overall, these data suggest that E2’s ability to modulate HDAC4 may contribute to PTSD in women [137].
Another area critical for fear learning whose structure and function shows sex differences [138; 139] and whose activity is modulated by E2 in females is the PFC, including the mPFC and the vmPFC (known as the infralimbic cortex in rodents) [132]. E2 administration immediately following fear extinction training in female rats results in greater activation of infralimbic cortex as indicated by increased c-fos expression in the infralimbic cortex [71]. Because the infralimbic cortex typically acts to inhibit the amygdala, these results suggest that E2’s ability to reduce amygdala activity and decrease fear behaviors is mediated by increasing the activity of the infralimbic cortex [71; 132]. Overall, these data recapitulate findings from women showing that natural variation in E2 concentrations over the menstrual cycle influence fear extinction recall and vmPFC/amygdala activation [71].
Although P4 replacement in aged, OVX mice increases freezing in a contextual fear conditioning task mediated by the hippocampus and the mPFC, reports of the effects of P4 on the neurocircuitry of fear are more rare than findings focused on E2, mostly due to the inability to disentangle the effects of E2 from P4 in gonadally-intact systems in reproductive aged females. However, because allopregnanolone acts as a positive allosteric modulator of GABAA receptors independent of the progesterone receptor, its ability to modulate the activity of fear neurocircuitry is better defined. Indeed, fluctuations in allopregnanolone over the estrus cycle in rodents can modulate both the phasic and tonic inhibitory tone of neurons within fear neurocircuitry in a manner that is dependent on the reorganization GABAA receptor subunits [140; 141]. Allopregnanolone’s ability to facilitate fear extinction in mice with stress-induced decreases in central allopregnanolone concentrations is thought to be mediated by inhibition of LTP or enhancement of long-term depression (LTD) within the hippocampus [90].
4.2. Ovarian hormone actions on stress axis regulation and function in females.
Another mechanism by which E2 and P4/allopregnanolone confer risk and resilience to PTSD is their modulation of activity and regulation of the hypothalamic-pituitary-adrenal (HPA) axis, which has also been implicated in the pathophysiology of PTSD [142]. Individuals with PTSD show lower levels of basal cortisol [142], enhanced glucocorticoid negative feedback inhibition of the HPA axis as assessed by a dexamethasone suppression test (DST) [143], increased glucocorticoid sensitivity [144], heightened levels of central and peripheral concentrations of corticotropin-releasing factor (CRF) [145; 146], and decreased levels of FKBP5, a co-chaperone of the glucocorticoid receptor (GR) that prevents cortisol from binding and thus inhibits the translocation of GRs into the nucleus [147]. Importantly, preclinical and translational work has shown that E2 and P4/allopregnanolone can modulate the function of the HPA axis.
Basal (unprovoked, resting) cortisol and ACTH concentrations can be augmented by E2 in rats [148; 149; 150], monkeys [151; 152; 153; 154], and replacement of E2 in postmenopausal women increases cortisol [155; 156]. Estradiol increases the expression of CRF mRNA in the CeA [157; 158] and paraventricular nucleus (PVN) of the hypothalamus in OVX female rats and mice, respectively [159]. Replacement of E2 in OVX rhesus monkeys results in augmented CRH mRNA levels in the PVN [160] as well increased CRF peptide levels in the hypothalamus [161]. These effects of E2 on CRH, ACTH and cortisol are linked to E2’s enhancement of glucocorticoid negative feedback inhibition of the HPA axis via modulation of GR and mineralocorticoid receptors [150; 162]. Indeed, replacement of E2 in OVX monkeys increases the responsiveness of the HPA axis by diminishing glucocorticoid negative feedback in response to a DST [163]. Taken together, these data suggest that E2 facilitates HPA responsivity under basal, unprovoked conditions.
Replacement of E2 in OVX female rodents also increases ACTH and corticosterone release following exposure to a novel environment [150] and restraint stress [164]. This increase in stress-induced ACTH and corticosterone by E2 occurs concomitantly with stress-induced increases in CRF within the PVN [165]. Although these preclinical studies also suggest that E2 facilitates HPA responsiveness during stress exposure, a handful of studies also support the notion that E2 attenuates stress-induced activity of the HPA axis. For instance, E2 replacement in OVX rats attenuates stress-induced release of ACTH but not corticosterone [166]. Similarly, E2 supplementation in perimenopausal women reduces increases in cortisol and ACTH induced by a cognitive, arithmetic challenge [167]. The equivocal nature of these results suggests that other factors may influence E2’s ability to modulate HPA responsiveness.
One such factor is a background of chronic stress exposure, as chronic psychosocial stress in female macaques exacerbates the effects of E2 on glucocorticoid negative feedback in response to a DST [163]. Furthermore, chronic stress exposure itself can result in low E2 concentrations due to hypogonadism induced by suppression of the HPG axis due to cortisol and CRF actions (CITES) [168]. Another factor that may influence E2’s ability to influence HPA function is genetic variability in genes critical for regulation. For example, a single nucleotide polymorphism (SNP) in the ADCYAP1R1 gene is associated with dysregulation of fear responses, greater PTSD symptoms, increased odds for a PTSD diagnosis in women [169; 170], as well as increases reactivity of the hippocampus and amygdala to threat stimuli and decreased functional connectivity between hippocampus and amygdala in traumatized women [171]. This SNP lays within an estrogen response element within the ADCYAP1R1 gene, thus influencing E2’s ability to modulate the downstream actions of PACAP (pituitary adenylate cyclase-activating polypeptide) [172], an important regulator of the HPA axis [173].
Another factor that influences E2’s effects on the HPA axis is whether P4 is on board. For instance, the facilitating effects of E2 on activity within the PVN of OVX female rodents is reversed if P4 is replacement concurrently with E2 [174]. Similarly, replacement of E2 in combination with P4 in OVX monkeys abrogates the increase in CRH mRNA levels in the PVN induced by E2 replacement alone [160], as well as the facilitation of glucocorticoid negative feedback by E2 replacement alone [163]. These effects of P4 on HPA axis function are mediated via allopregnanlone, as GABAergic inhibition is critical for modulating the activity of CRH neurons within the PVN [175] and microinfusion of neuroactive steroids similar to allopregnanolone decrease circulating levels of corticosterone in mice [176]. These data, and findings showing that allopregnanolone concentrations increase following acute stress and act to restore homeostasis by inhibiting the release of CRH and cortisol [177; 178; 179] indicate that decreases in allopregnanolone over the menstrual cycle in women with PTSD who also show lower levels of central allopregnanolone [72] may facilitate the activity of the HPA axis and exacerbate PTSD symptoms and deficits in fear responses by heightening amygdala activity.
5. Conclusions and future directions
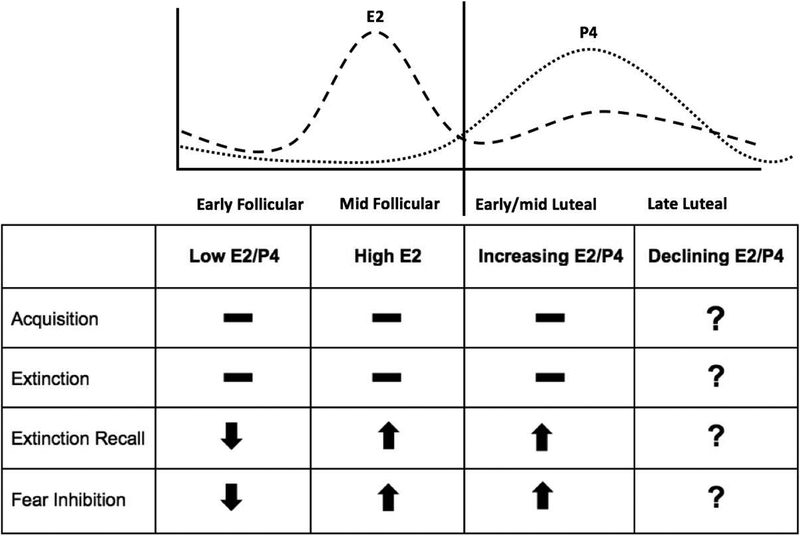
To date, a significant amount of data indicates that low concentrations of E2 and P4/allopregnanolone (early follicular phase) are associated with increased PTSD-related symptoms and deficits in fear regulation in healthy and traumatized women, and that high or increasing levels of E2 and P4/allopregnanolone (mid follicular and early luteal phase) may be protective for traumatized women (Figure 2). However, it is important to note that not all studies assessing the relationships between these steroid hormones and PTSD symptoms and fear responses are congruent. The equivocal nature of some of the findings highlight the importance of accounting for factors that may influence the association and our interpretation of the data (Table 1 and 2), including (but not limited to) the sample assessed (clinical versus community based), the context of fear conditioning paradigms used, species studied, whether studies were done in gonadally-intact or OVX condition, and type, timing, dosing, and absolute concentrations of hormone replacement regimes administered.
Figure 2. Relationship between cycle phase, ovarian hormones, and fear conditioning.
Existing studies in women indicate no relationship between menstrual cycle phase/ovarian hormone levels on acquisition or extinction processes. Low E2/P4 concentrations (as seen in the early follicular phase) are associated with impaired extinction recall and fear inhibition. High E2 concentrations (seen in the late follicular phase) and high E2/P4 concentrations (seen in the early to mid luteal phase) are associated with enhanced extinction recall and fear inhibition. The effects of declining E2/P4 concentrations during the late luteal phase on fear conditioning processes remain to be assessed.
In human studies, race is another critical factor to consider, as concentrations of E2 and P4 during the menstrual cycle are greater in AA compared to Caucasian women [180; 181]. Furthermore, race may influence skin conductance that is assessed as an outcome measure for fear learning paradigms. This notion is highlighted by the study by Pineles and colleauges that found that only 28% of skin conductance responders (participants who had an adequate skin conductance response to the unconditioned stimulus) were African-American, while 71% of skin conductance non-responders were African-American [56]. It therefore may be problematic to rely on skin conductance as a measure of fear if the measure does not adequately report fear responses in African-Americans. Given that African-Americans are at the highest risk for PTSD [182], it is critical that studies modeling processes that go awry in PTSD use measures that are effective in the African-American population. To date, the majority of studies examining the role of ovarian hormones on fear learning and/or PTSD symptoms in humans feature overwhelmingly white populations, or fail to report racial demographics of the sample.
Some critical gaps in knowledge that currently exist regarding the influences of E2 and P4/allopregnanolone on risk and resilience to PTSD stem from the lack of studies assessing these relationships throughout the lifespan, when dramatic changes in concentrations of these steroid hormones occur concomitantly with increased risk for anxiety symptoms (reviewed in [48]). The sex difference in stress- and trauma-related disorders favoring females to males by two to one emerges during puberty [183], highlighting the need to better understand how menarche interacts with stress and trauma exposure to impact the neurobehavioral development of neurocircuitry implicated in fear in girls and young women.
Another period in the female reproductive lifespan whose effects on risk and resilience to PTSD has been largely understudied is pregnancy, a period of nine months characterized by a profound increase in ovarian steroid hormones, as concentrations of E2 and P4 increase by tenfold [184; 185]. Results from the few existing studies assessing the effects of pregnancy on PTSD symptoms are equivocal in nature [186; 187; 188], as some studies show that lower levels of PTSD symptoms in pregnant compared to non-pregnant women [187; 189] and another shows greater hyperarousal and deficits in fear inhibition [190]. Because epidemiological studies show that rates of PTSD are 4–5% higher in pregnant compared to non-pregnant women [188] and that stress exposure during gestation impacts risk for adverse birth outcomes [191; 192] and intergenerational risk for adverse health outcomes in offspring [193; 194; 195], it is important to determine the mechanisms by which changes in E2 and P4/allopregnanolone during pregnancy may impact PTSD and dysregulation of fear responses.
Significant changes in circulating E2 and P4/allopregnanolone also occur during reproductive senescence, as the menopausal transition has also been associated with increased risk for affective disruption in women, including augmented symptoms of depression and anxiety [48]. While some studies assessing the effects of E2 on PTSD symptoms and fear extinction in traumatized women have included postmenopausal women due to the upper limit of their inclusion criteria [67], to our knowledge there are no studies that specifically assess the effects of menopause on PTSD symptoms and fear psychophysiology in traumatized women. Similarly, there are a lack of studies systematically assessing the effects of different hormone replacement regimens on PTSD symptoms and fear psychophysiology in traumatized post-menopausal women. Because chronic PTSD is linked to accelerated aging [196] and heightened risk for physical health condition linked to increased morbidity and mortality (such as cardiometabolic disorders) [197], this should be a focus of future research in women’s health.
Similarly, future studies are necessary to better understand the long-term consequences of hormonal oral contraceptive use on PTSD symptoms in reproductive aged women. The data from preclinical and clinical studies showing that the inhibition of endogenous E2 and P4/allopregnanolone with hormonal contraceptive use is associated with poorer fear response regulation [57; 61; 68] and increased amygdala reactivity [124] suggest that taking such contraceptives may exacerbate PTSD in traumatized women. Largely unexplored is also the effect of hormonal contraceptive use, cycle phase, and E2 and P4/allopregnanolone concentrations at the time of trauma exposure on prospective risk for developing PTSD. A singular study assessing the effects of emergency contraceptive on PTSD symptom development following sexual assault supports the notion that manipulation of ovarian hormones in the immediate aftermath of trauma alters risk for PTSD development [198].
In summary, ovarian steroid hormones and their fluctuations over the course of the menstrual cycle confer risk and resilience to PTSD-related symptoms and deficits in fear psychophysiology in females. These data underscore the notion that supplementation of E2 and/or P4/allopregnanolone during times when these levels of these hormones are endogenously low and in concert with evidence-based treatment for PTSD, such as prolonged exposure therapy [199; 200], may serve as novel therapeutic interventions for traumatized women with PTSD. The promise of the therapeutic potential of such interventions is highlighted by the recent demonstration in a phase III clinical trial that a positive allosteric modulator of the GABAA receptor is efficacious in the treatment postpartum depression [201]. Future studies are clearly warranted to assess such hormonal intervention as therapies, and to better understand the impact of ovarian steroid hormones on risk for PTSD across the lifespan in women. Results from these studies will also have important implications for transgender individuals with PTSD [202]. The recent mandate by the National Institutes of Health that requires investigators to address sex as a biological variable in their proposals and studies will help expedite our understanding of female risk and resilience to PTSD and other adverse trauma-related sequelea [203].
Research Highlights:
Women are twice as likely than men to suffer from posttraumatic stress disorder (PTSD).
Ovarian hormones are associated with PTSD symptoms and fear psychophysiology.
Ovarian hormones impact the stress axis and neural substrates critical for fear response regulation.
Gaps in knowledge exist regarding these relationships across the lifespan in women.
Funding and disclosure
This review was supported in part by the National Institute of Health: MH115174 (VM), MH117009 (JSS) and HD085850. Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378. The authors declare no conflict of interest.
References:
- [1].Dedert EA, Calhoun PS, Watkins LL, Sherwood A, and Beckham JC, Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med 39 (2010) 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Keane TM, Marx BP, and Sloan DM, Post-Traumatic Stress Disorder: Definition, Prevalence, and Risk Factors in: Shiromani PJ, Keane TM, and LeDoux JE, (Eds.), Post-Traumatic Stress Disorder: Basic Science and Clinical Practice, Humana Press, New York, 2009, pp. 1–19. [Google Scholar]
- [3].Rothbaum B, Foa EB, Riggs DS, Murdock T, and Walsh W, A Prospective Examination of Posttraumatic Stress Disorder in Rape Victims. Journal of Traumatic Stress (1992) 455–475. [Google Scholar]
- [4].Rytwinski NK, Scur MD, Feeny NC, and Youngstrom EA, The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J Trauma Stress 26 (2013) 299–309. [DOI] [PubMed] [Google Scholar]
- [5].Pietrzak RH, Goldstein RB, Southwick SM, and Grant BF, Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord 25 (2011) 456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Thibodeau MA, Welch PG, Sareen J, and Asmundson GJ, Anxiety disorders are independently associated with suicide ideation and attempts: propensity score matching in two epidemiological samples. Depress Anxiety 30 (2013) 947–54. [DOI] [PubMed] [Google Scholar]
- [7].Boscarino JA, A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med 70 (2008) 668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weiss T, Skelton K, Phifer J, Jovanovic T, Gillespie CF, Smith A, Umpierrez G, Bradley B, and Ressler KJ, Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. Gen Hosp Psychiatry 33 (2011) 135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].APA APA, Diagnostic and Statistical Manual of Mental Disorders. (2013).
- [10].Jovanovic T, Kazama A, Bachevalier J, and Davis M, Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62 (2012) 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Norrholm SD, Glover EM, Stevens JS, Fani N, Galatzer-Levy IR, Bradley B, Ressler KJ, and Jovanovic T, Fear load: The psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int J Psychophysiol 98 (2015) 270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, and Ressler KJ, Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological psychiatry 69 (2011) 556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Davis M, The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15 (1992) 353–75. [DOI] [PubMed] [Google Scholar]
- [14].Fendt M, and Fanselow MS, The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience and biobehavioral reviews 23 (1999) 743–60. [DOI] [PubMed] [Google Scholar]
- [15].Maren S, Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24 (2001) 897–931. [DOI] [PubMed] [Google Scholar]
- [16].Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, and Duncan EJ, Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biological psychiatry 57 (2005) 1559–64. [DOI] [PubMed] [Google Scholar]
- [17].Breslau N, The epidemiology of posttraumatic stress disorder: what is the extent of the problem? The Journal of clinical psychiatry 62 Suppl 17 (2001) 16–22. [PubMed] [Google Scholar]
- [18].Roberts AL, Rosario M, Corliss HL, Koenen KC, and Austin SB, Childhood gender nonconformity: a risk indicator for childhood abuse and posttraumatic stress in youth. Pediatrics 129 (2012) 410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stein MB, Jang KL, Taylor S, Vernon PA, and Livesley WJ, Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. The American journal of psychiatry 159 (2002) 1675–81. [DOI] [PubMed] [Google Scholar]
- [20].Michopoulos V, Norrholm SD, and Jovanovic T, Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biological psychiatry 78 (2015) 344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kessler RC, Sonnega A, Bromet E, Hughes M, and Nelson CB, Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry 52 (1995) 1048–60. [DOI] [PubMed] [Google Scholar]
- [22].Olff M, de Vries GJ, Guzelcan Y, Assies J, and Gersons BP, Changes in cortisol and DHEA plasma levels after psychotherapy for PTSD. Psychoneuroendocrinology 32 (2007) 619–26. [DOI] [PubMed] [Google Scholar]
- [23].Tolin DF, and Foa EB, Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull 132 (2006) 959–92. [DOI] [PubMed] [Google Scholar]
- [24].Breslau N, Chilcoat HD, Kessler RC, and Davis GC, Previous exposure to trauma and PTSD effects of subsequent trauma: results from the Detroit Area Survey of Trauma. The American journal of psychiatry 156 (1999) 902–7. [DOI] [PubMed] [Google Scholar]
- [25].Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, and Gustafsson JA, Mechanisms of estrogen action. Physiol Rev 81 (2001) 1535–65. [DOI] [PubMed] [Google Scholar]
- [26].Kushner PJ, Agard D, Feng WJ, Lopez G, Schiau A, Uht R, Webb P, and Greene G, Oestrogen receptor function at classical and alternative response elements. Novartis Found Symp 230 (2000) 20–6; discussion 27–40. [DOI] [PubMed] [Google Scholar]
- [27].Blaustein JD, Minireview: Neuronal steroid hormone receptors: they’re not just for hormones anymore. Endocrinology 145 (2004) 1075–81. [DOI] [PubMed] [Google Scholar]
- [28].Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Arditte Hall KA, Hauger R, Miller MW, Resick PA, Orr SP, and Rasmusson AM, PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology 93 (2018) 133–141. [DOI] [PubMed] [Google Scholar]
- [29].Schumacher M, Mattern C, Ghoumari A, Oudinet JP, Liere P, Labombarda F, Sitruk-Ware R, De Nicola AF, and Guennoun R, Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Progress in neurobiology 113 (2014) 6–39. [DOI] [PubMed] [Google Scholar]
- [30].Leavitt WW, Chen TJ, and Allen TC, Regulation of progesterone receptor formation by estrogen action. Annals of the New York Academy of Sciences 286 (1977) 210–25. [DOI] [PubMed] [Google Scholar]
- [31].Majewska MD, Harrison NL, Schwartz RD, Barker JL, and Paul SM, Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor Science (New York, N.Y: 232 (1986) 1004–7. [DOI] [PubMed] [Google Scholar]
- [32].Ordog T, Chen MD, Nishihara M, Connaughton MA, Goldsmith JR, and Knobil E, On the role of gonadotropin-releasing hormone (GnRH) in the operation of the GnRH pulse generator in the rhesus monkey. Neuroendocrinology 65 (1997) 307–13. [DOI] [PubMed] [Google Scholar]
- [33].Clarke IJ, and Cummins JT, The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111 (1982) 1737–9. [DOI] [PubMed] [Google Scholar]
- [34].Channing CP, Schaerf FW, Anderson LD, and Tsafriri A, Ovarian follicular and luteal physiology. Int Rev Physiol 22 (1980) 117–201. [PubMed] [Google Scholar]
- [35].Yamaji T, Dierschke DJ, Bhattacharya AN, and Knobil E, The negative feedback control by estradiol and progesterone of LH secretion in the ovariectomized rhesus monkey. Endocrinology 90 (1972) 771–7. [DOI] [PubMed] [Google Scholar]
- [36].Kauffman AS, Clifton DK, and Steiner RA, Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends in neurosciences 30 (2007) 504–11. [DOI] [PubMed] [Google Scholar]
- [37].Karsch FJ, Weick RF, Butler WR, Dierschke DJ, Krey LC, Weiss G, Hotchkiss J, Yamaji T, and Knobil E, Induced LH surges in the rhesus monkey: strength-duration characteristics of the estrogen stimulus. Endocrinology 92 (1973) 1740–7. [DOI] [PubMed] [Google Scholar]
- [38].Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, and Merriam GR, Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. American journal of obstetrics and gynecology 158 (1988) 5–11. [DOI] [PubMed] [Google Scholar]
- [39].Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, Cintron D, Thompson KD, Khine KK, and Schmidt PJ, 5alpha-Reductase Inhibition Prevents the Luteal Phase Increase in Plasma Allopregnanolone Levels and Mitigates Symptoms in Women with Premenstrual Dysphoric Disorder. Neuropsychopharmacology 41 (2016) 1093–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Strine TW, Chapman DP, and Ahluwalia IB, Menstrual-related problems and psychological distress among women in the United States. J Womens Health (Larchmt) 14 (2005) 316–23. [DOI] [PubMed] [Google Scholar]
- [41].Douma SL, Husband C, O’Donnell ME, Barwin BN, and Woodend AK, Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv Nurs Sci 28 (2005) 364–75. [DOI] [PubMed] [Google Scholar]
- [42].Pigott TA, Anxiety disorders in women. Psychiatr Clin North Am 26 (2003) 621–72, vi-vii. [DOI] [PubMed] [Google Scholar]
- [43].Rapkin AJ, Mikacich JA, Moatakef-Imani B, and Rasgon N, The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Rep 4 (2002) 419–28. [DOI] [PubMed] [Google Scholar]
- [44].Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, and Genazzani AR, Serum allopregnanolone in women with postpartum “blues”. Obstetrics and gynecology 97 (2001) 77–80. [DOI] [PubMed] [Google Scholar]
- [45].Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, Petraglia F, and Genazzani AR, Allopregnanolone concentrations and premenstrual syndrome. European journal of endocrinology / European Federation of Endocrine Societies 142 (2000) 269–73. [DOI] [PubMed] [Google Scholar]
- [46].Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, and Mahesh VB, Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstetrics and gynecology 90 (1997) 709–14. [DOI] [PubMed] [Google Scholar]
- [47].Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, and Yonkers KA, Premenstrual dysphoric disorder: evidence for a new category for DSM-5. The American journal of psychiatry 169 (2012) 465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hantsoo L, and Epperson CN, Anxiety Disorders Among Women: A Female Lifespan Approach. Focus (Am Psychiatr Publ) 15 (2017) 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ferree NK, Kamat R, and Cahill L, Influences of menstrual cycle position and sex hormone levels on spontaneous intrusive recollections following emotional stimuli. Conscious Cogn 20 (2011) 1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ferree NK, and Cahill L, Post-event spontaneous intrusive recollections and strength of memory for emotional events in men and women. Conscious Cogn 18 (2009) 126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nielsen SE, Ahmed I, and Cahill L, Sex and menstrual cycle phase at encoding influence emotional memory for gist and detail. Neurobiology of learning and memory 106 (2013) 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Soni M, Curran VH, and Kamboj SK, Identification of a narrow post-ovulatory window of vulnerability to distressing involuntary memories in healthy women. Neurobiology of learning and memory 104 (2013) 32–8. [DOI] [PubMed] [Google Scholar]
- [53].Pompili A, Arnone B, D’Amico M, Federico P, and Gasbarri A, Evidence of estrogen modulation on memory processes for emotional content in healthy young women. Psychoneuroendocrinology 65 (2016) 94–101. [DOI] [PubMed] [Google Scholar]
- [54].Bayer J, Schultz H, Gamer M, and Sommer T, Menstrual-cycle dependent fluctuations in ovarian hormones affect emotional memory. Neurobiology of learning and memory 110 (2014) 55–63. [DOI] [PubMed] [Google Scholar]
- [55].Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, and Rauch SL, Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behavioral neuroscience 120 (2006) 1196–203. [DOI] [PubMed] [Google Scholar]
- [56].Pineles SL, Nillni YI, King MW, Patton SC, Bauer MR, Mostoufi SM, Gerber MR, Hauger R, Resick PA, Rasmusson AM, and Orr SP, Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. J Abnorm Psychol 125 (2016) 349–55. [DOI] [PubMed] [Google Scholar]
- [57].Lonsdorf TB, Haaker J, Schumann D, Sommer T, Bayer J, Brassen S, Bunzeck N, Gamer M, and Kalisch R, Sex differences in conditioned stimulus discrimination during context-dependent fear learning and its retrieval in humans: the role of biological sex, contraceptives and menstrual cycle phases. J Psychiatry Neurosci 40 (2015) 368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, Ressler KJ, and Jovanovic T, Inhibition of fear is differentially associated with cycling estrogen levels in women. J Psychiatry Neurosci 38 (2013) 341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Dunsmoor JE, and Kroes MCW, Episodic memory and Pavlovian conditioning: ships passing in the night. Curr Opin Behav Sci 26 (2019) 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, and Goldstein JM, The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience 168 (2010) 652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].White EC, and Graham BM, Estradiol levels in women predict skin conductance response but not valence and expectancy ratings in conditioned fear extinction. Neurobiology of learning and memory 134 Pt B (2016) 339–48. [DOI] [PubMed] [Google Scholar]
- [62].Li S, and Graham BM, Estradiol is associated with altered cognitive and physiological responses during fear conditioning and extinction in healthy and spider phobic women. Behavioral neuroscience 130 (2016) 614–23. [DOI] [PubMed] [Google Scholar]
- [63].Bryant RA, Felmingham KL, Silove D, Creamer M, O’Donnell M, and McFarlane AC, The association between menstrual cycle and traumatic memories. Journal of affective disorders 131 (2011) 398–401. [DOI] [PubMed] [Google Scholar]
- [64].Wilcox AJ, Dunson D, and Baird DD, The timing of the “fertile window” in the menstrual cycle: day specific estimates from a prospective study. BMJ 321 (2000) 1259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nillni YI, Pineles SL, Patton SC, Rouse MH, Sawyer AT, and Rasmusson AM, Menstrual cycle effects on psychological symptoms in women with PTSD. J Trauma Stress 28 (2015) 1–7. [DOI] [PubMed] [Google Scholar]
- [66].Wegerer M, Kerschbaum H, Blechert J, and Wilhelm FH, Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiology of learning and memory 116 (2014) 145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, and Norrholm SD, Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological psychiatry 72 (2012) 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Graham BM, and Milad MR, Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biological psychiatry 73 (2013) 371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Garcia NM, Walker RS, and Zoellner LA, Estrogen, progesterone, and the menstrual cycle: A systematic review of fear learning, intrusive memories, and PTSD. Clin Psychol Rev 66 (2018) 80–96. [DOI] [PubMed] [Google Scholar]
- [70].Graham BM, Ash C, and Den ML, High endogenous estradiol is associated with enhanced cognitive emotion regulation of physiological conditioned fear responses in women. Psychoneuroendocrinology 80 (2017) 7–14. [DOI] [PubMed] [Google Scholar]
- [71].Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, and Milad MR, Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological psychiatry 70 (2011) 920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, and Guidotti A, Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biological psychiatry 60 (2006) 704–13. [DOI] [PubMed] [Google Scholar]
- [73].Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, and Young E, Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146 (2005) 1650–73. [DOI] [PubMed] [Google Scholar]
- [74].Milad MR, Igoe SA, Lebron-Milad K, and Novales JE, Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164 (2009) 887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Rivas-Arancibia S, and Vazquez-Pereyra F, Hormonal modulation of extinction responses induced by sexual steroid hormones in rats. Life sciences 54 (1994) PL363–7. [DOI] [PubMed] [Google Scholar]
- [76].Graham BM, and Scott E, Effects of systemic estradiol on fear extinction in female rats are dependent on interactions between dose, estrous phase, and endogenous estradiol levels. Hormones and behavior 97 (2018) 67–74. [DOI] [PubMed] [Google Scholar]
- [77].Yuan DL, and Chambers KC, Estradiol accelerates extinction of a conditioned taste aversion in female and male rats. Hormones and behavior 36 (1999) 1–16. [DOI] [PubMed] [Google Scholar]
- [78].Lynch J 3rd, Cullen PK, Jasnow AM, and Riccio DC, Sex differences in the generalization of fear as a function of retention intervals Learning & memory (Cold Spring Harbor, N.Y: 20 (2013) 628–32. [DOI] [PubMed] [Google Scholar]
- [79].Graham BM, and Daher M, Estradiol and Progesterone have Opposing Roles in the Regulation of Fear Extinction in Female Rats. Neuropsychopharmacology 41 (2016) 774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Barker JM, and Galea LA, Males show stronger contextual fear conditioning than females after context pre-exposure. Physiology & behavior 99 (2010) 82–90. [DOI] [PubMed] [Google Scholar]
- [81].Gupta RR, Sen S, Diepenhorst LL, Rudick CN, and Maren S, Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1). Brain research 888 (2001) 356–365. [DOI] [PubMed] [Google Scholar]
- [82].Morgan MA, and Pfaff DW, Effects of estrogen on activity and fear-related behaviors in mice. Hormones and behavior 40 (2001) 472–82. [DOI] [PubMed] [Google Scholar]
- [83].Matsumoto YK, Kasai M, and Tomihara K, The enhancement effect of estradiol on contextual fear conditioning in female mice. PloS one 13 (2018) e0197441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Barha CK, Dalton GL, and Galea LA, Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology 35 (2010) 547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Frye CA, and Walf AA, Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiology of learning and memory 90 (2008) 171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Smith SS, Withdrawal properties of a neuroactive steroid: implications for GABA(A) receptor gene regulation in the brain and anxiety behavior. Steroids 67 (2002) 519–28. [DOI] [PubMed] [Google Scholar]
- [87].Pibiri F, Nelson M, Guidotti A, Costa E, and Pinna G, Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America 105 (2008) 5567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Pinna G, and Rasmusson AM, Ganaxolone improves behavioral deficits in a mouse model of posttraumatic stress disorder. Front Cell Neurosci 8 (2014) 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhang LM, Qiu ZK, Zhao N, Chen HX, Liu YQ, Xu JP, Zhang YZ, Yang RF, and Li YF, Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in animal models of post-traumatic stress disorder. Int J Neuropsychopharmacol 17 (2014) 1659–69. [DOI] [PubMed] [Google Scholar]
- [90].Rasmusson AM, Marx CE, Pineles SL, Locci A, Scioli-Salter ER, Nillni YI, Liang JJ, and Pinna G, Neuroactive steroids and PTSD treatment. Neuroscience letters 649 (2017) 156–163. [DOI] [PubMed] [Google Scholar]
- [91].Bellem A, Meiyappan S, Romans S, and Einstein G, Measuring estrogens and progestagens in humans: an overview of methods. Gend Med 8 (2011) 283–99. [DOI] [PubMed] [Google Scholar]
- [92].Lynch JF 3rd, Dejanovic D, Winiecki P, Mulvany J, Ortiz S, Riccio DC, and Jasnow AM, Activation of ERbeta modulates fear generalization through an effect on memory retrieval. Hormones and behavior 66 (2014) 421–9. [DOI] [PubMed] [Google Scholar]
- [93].Lynch JF 3rd, Winiecki P, Vanderhoof T, Riccio DC, and Jasnow AM, Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiology of learning and memory 130 (2016) 83–92. [DOI] [PubMed] [Google Scholar]
- [94].Hartley CA, and Phelps EA, Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology 35 (2010) 136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, and Konrad C, Human fear conditioning and extinction in neuroimaging: a systematic review. PloS one 4 (2009) e5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, and Ressler KJ, Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of psychiatric research 47 (2013) 1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jin C, Qi R, Yin Y, Hu X, Duan L, Xu Q, Zhang Z, Zhong Y, Feng B, Xiang H, Gong Q, Liu Y, Lu G, and Li L, Abnormalities in whole-brain functional connectivity observed in treatment-naive posttraumatic stress disorder patients following an earthquake. Psychol Med 44 (2014) 1927–36. [DOI] [PubMed] [Google Scholar]
- [98].Costanzo ME, Jovanovic T, Pham D, Leaman S, Highland KB, Norrholm SD, and Roy MJ, White matter microstructure of the uncinate fasciculus is associated with subthreshold posttraumatic stress disorder symptoms and fear potentiated startle during early extinction in recently deployed Service Members. Neuroscience letters 618 (2016) 66–71. [DOI] [PubMed] [Google Scholar]
- [99].Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, and Olff M, Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: a diffusion tensor imaging study. J Psychiatry Neurosci 42 (2017) 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Brown VM, LaBar KS, Haswell CC, Gold AL, Mid-Atlantic MW, McCarthy G, and Morey RA, Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 39 (2014) 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, and Stein MB, Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological psychiatry 68 (2010) 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, and Liberzon I, Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci 37 (2012) 241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ostlund H, Keller E, and Hurd YL, Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences 1007 (2003) 54–63. [DOI] [PubMed] [Google Scholar]
- [104].Pilgrim C, and Hutchison JB, Developmental regulation of sex differences in the brain: can the role of gonadal steroids be redefined? Neuroscience 60 (1994) 843–55. [DOI] [PubMed] [Google Scholar]
- [105].Woolley CS, Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Hormones and behavior 34 (1998) 140–8. [DOI] [PubMed] [Google Scholar]
- [106].Bi R, Foy MR, Vouimba RM, Thompson RF, and Baudry M, Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proceedings of the National Academy of Sciences of the United States of America 98 (2001) 13391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Oyola MG, Portillo W, Reyna A, Foradori CD, Kudwa A, Hinds L, Handa RJ, and Mani SK, Anxiolytic effects and neuroanatomical targets of estrogen receptor-beta (ERbeta) activation by a selective ERbeta agonist in female mice. Endocrinology 153 (2012) 837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Pang Y, Dong J, and Thomas P, Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors delta and {epsilon} (mPRdelta and mPR{epsilon}) and mPRdelta involvement in neurosteroid inhibition of apoptosis. Endocrinology 154 (2013) 283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, and Thomas P, Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term Molecular endocrinology (Baltimore, Md: 20 (2006) 1519–34. [DOI] [PubMed] [Google Scholar]
- [110].Bixo M, Andersson A, Winblad B, Purdy RH, and Backstrom T, Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain research 764 (1997) 173–8. [DOI] [PubMed] [Google Scholar]
- [111].Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, and Costa E, Neurosteroids act on recombinant human GABAA receptors. Neuron 4 (1990) 759–65. [DOI] [PubMed] [Google Scholar]
- [112].Wang JM, Johnston PB, Ball BG, and Brinton RD, The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci 25 (2005) 4706–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW, and Gaser C, Changes in brain size during the menstrual cycle. PloS one 6 (2011) e14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, and Stern E, Hippocampal structural changes across the menstrual cycle. Hippocampus 18 (2008) 985–8. [DOI] [PubMed] [Google Scholar]
- [115].Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, and Kerschbaum HH, Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain research 1348 (2010) 55–62. [DOI] [PubMed] [Google Scholar]
- [116].Catenaccio E, Mu W, and Lipton ML, Estrogen- and progesterone-mediated structural neuroplasticity in women: evidence from neuroimaging. Brain Struct Funct 221 (2016) 3845–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Reiman EM, Armstrong SM, Matt KS, and Mattox JH, The application of positron emission tomography to the study of the normal menstrual cycle. Human reproduction (Oxford, England) 11 (1996) 2799–805. [DOI] [PubMed] [Google Scholar]
- [118].Engman J, Linnman C, Van Dijk KR, and Milad MR, Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology 63 (2016) 34–42. [DOI] [PubMed] [Google Scholar]
- [119].Miedl SF, Wegerer M, Kerschbaum H, Blechert J, and Wilhelm FH, Neural activity during traumatic film viewing is linked to endogenous estradiol and hormonal contraception. Psychoneuroendocrinology 87 (2018) 20–26. [DOI] [PubMed] [Google Scholar]
- [120].van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, and Fernandez G, Progesterone selectively increases amygdala reactivity in women. Molecular psychiatry 13 (2008) 325–33. [DOI] [PubMed] [Google Scholar]
- [121].Andreano JM, and Cahill L, Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage 53 (2010) 1286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lebron-Milad K, Graham BM, and Milad MR, Low estradiol levels: a vulnerability factor for the development of posttraumatic stress disorder. Biological psychiatry 72 (2012) 6–7. [DOI] [PubMed] [Google Scholar]
- [123].Hwang MJ, Zsido RG, Song H, Pace-Schott EF, Miller KK, Lebron-Milad K, Marin MF, and Milad MR, Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry 15 (2015) 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, and Wolf OT, Neuronal correlates of extinction learning are modulated by sex hormones. Soc Cogn Affect Neurosci 7 (2012) 819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, and McEwen BS, Uncovering the mechanisms of estrogen effects on hippocampal function. Frontiers in neuroendocrinology 29 (2008) 219–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Day M, Sung A, Logue S, Bowlby M, and Arias R, Beta estrogen receptor knockout (BERKO) mice present attenuated hippocampal CA1 long-term potentiation and related memory deficits in contextual fear conditioning. Behavioural brain research 164 (2005) 128–31. [DOI] [PubMed] [Google Scholar]
- [127].Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, and Hsu KS, Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus 19 (2009) 1142–50. [DOI] [PubMed] [Google Scholar]
- [128].Armario A, Escorihuela RM, and Nadal R, Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neuroscience and biobehavioral reviews 32 (2008) 1121–35. [DOI] [PubMed] [Google Scholar]
- [129].Saffari S, Abrari K, Arezu R, Rashidy-Pour A, Goudarzi I, and Elahdadi Salmani M, Correlation of fear memory in a PTSD animal model and hippocampal BDNF in response to β-estradiol treatment. Journal of Paramedical Sciences 6 (2015) 22–34. [Google Scholar]
- [130].Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ, DeJoseph MR, Urban JH, and Rosenkranz JA, Sex- and Estrus-Dependent Differences in Rat Basolateral Amygdala. J Neurosci 37 (2017) 10567–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Bender RA, Zhou L, Vierk R, Brandt N, Keller A, Gee CE, Schafer MK, and Rune GM, Sex-Dependent Regulation of Aromatase-Mediated Synaptic Plasticity in the Basolateral Amygdala. J Neurosci 37 (2017) 1532–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Maeng LY, Cover KK, Taha MB, Landau AJ, Milad MR, and Lebron-Milad K, Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. J Neurosci Res 95 (2017) 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Fitzsimons HL, Schwartz S, Given FM, and Scott MJ, The histone deacetylase HDAC4 regulates long-term memory in Drosophila. PloS one 8 (2013) e83903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kim MS, Akhtar MW, Adachi M, Mahgoub M, Bassel-Duby R, Kavalali ET, Olson EN, and Monteggia LM, An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J Neurosci 32 (2012) 10879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sando R 3rd, Gounko N, Pieraut S, Liao L, Yates J 3rd, and Maximov A, HDAC4 governs a transcriptional program essential for synaptic plasticity and memory. Cell 151 (2012) 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wang WH, Cheng LC, Pan FY, Xue B, Wang DY, Chen Z, and Li CJ, Intracellular trafficking of histone deacetylase 4 regulates long-term memory formation. Anat Rec (Hoboken) 294 (2011) 1025–34. [DOI] [PubMed] [Google Scholar]
- [137].Maddox SA, Kilaru V, Shin J, Jovanovic T, Almli LM, Dias BG, Norrholm SD, Fani N, Michopoulos V, Ding Z, Conneely KN, Binder EB, Ressler KJ, and Smith AK, Estrogen-dependent association of HDAC4 with fear in female mice and women with PTSD. Molecular psychiatry (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Garrett JE, and Wellman CL, Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience 162 (2009) 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kolb B, and Stewart J, Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. Journal of neuroendocrinology 3 (1991) 95–9. [DOI] [PubMed] [Google Scholar]
- [140].Maguire J, and Mody I, Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci 27 (2007) 2155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Maguire J, and Mody I, Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology 34 Suppl 1 (2009) S84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Yehuda R, Neuroendocrine aspects of PTSD. Handbook of experimental pharmacology (2005) 371–403. [DOI] [PubMed] [Google Scholar]
- [143].Yehuda R, Boisoneau D, Lowy MT, and Giller EL Jr., Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Archives of general psychiatry 52 (1995) 583–93. [DOI] [PubMed] [Google Scholar]
- [144].Yehuda R, Golier JA, Yang RK, and Tischler L, Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biological psychiatry 55 (2004) 1110–6. [DOI] [PubMed] [Google Scholar]
- [145].Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, and Geracioti TD Jr., Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. The American journal of psychiatry 162 (2005) 992–4. [DOI] [PubMed] [Google Scholar]
- [146].de Kloet CS, Vermetten E, Geuze E, Lentjes EG, Heijnen CJ, Stalla GK, and Westenberg HG, Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Progress in brain research 167 (2008) 287–91. [DOI] [PubMed] [Google Scholar]
- [147].Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Muller-Myhsok B, Holsboer F, and Buxbaum JD, Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biological psychiatry 66 (2009) 708–11. [DOI] [PubMed] [Google Scholar]
- [148].Viau V, and Meaney MJ, Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology 129 (1991) 2503–11. [DOI] [PubMed] [Google Scholar]
- [149].Burgess LH, and Handa RJ, Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology 131 (1992) 1261–9. [DOI] [PubMed] [Google Scholar]
- [150].Carey MP, Deterd CH, de Koning J, Helmerhorst F, and de Kloet ER, The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. The Journal of endocrinology 144 (1995) 311–21. [DOI] [PubMed] [Google Scholar]
- [151].Stavisky RC, Watson SL, Anthony MS, Manuck SB, Adams MR, and Kaplan JR, Influence of estradiol on cortisol secretion in ovariectomized cynomolgus macaques (Macaca fascicularis). American journal of primatology 60 (2003) 17–22. [DOI] [PubMed] [Google Scholar]
- [152].Wood CE, Cline JM, Anthony MS, Register TC, and Kaplan JR, Adrenocortical effects of oral estrogens and soy isoflavones in female monkeys. The Journal of clinical endocrinology and metabolism 89 (2004) 2319–25. [DOI] [PubMed] [Google Scholar]
- [153].Giussani DA, Farber DM, Jenkins SL, Yen A, Winter JA, Tame JD, and Nathanielsz PW, Opposing effects of androgen and estrogen on pituitary-adrenal function in nonpregnant primates. Biology of reproduction 62 (2000) 1445–51. [DOI] [PubMed] [Google Scholar]
- [154].Smith CJ, and Norman RL, Influence of the gonads on cortisol secretion in female rhesus macaques. Endocrinology 121 (1987) 2192–8. [DOI] [PubMed] [Google Scholar]
- [155].Gudmundsson A, Goodman B, Lent S, Barczi S, Grace A, Boyle L, Ershler WB, and Carnes M, Effects of estrogen replacement therapy on the circadian rhythms of serum cortisol and body temperature in postmenopausal women. Exp Gerontol 34 (1999) 809–18. [DOI] [PubMed] [Google Scholar]
- [156].Burleson MH, Malarkey WB, Cacioppo JT, Poehlmann KM, Kiecolt-Glaser JK, Berntson GG, and Glaser R, Postmenopausal hormone replacement: effects on autonomic, neuroendocrine, and immune reactivity to brief psychological stressors. Psychosom Med 60 (1998) 17–25. [DOI] [PubMed] [Google Scholar]
- [157].Jasnow AM, Schulkin J, and Pfaff DW, Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Hormones and behavior 49 (2006) 197–205. [DOI] [PubMed] [Google Scholar]
- [158].Li XF, Mitchell JC, Wood S, Coen CW, Lightman SL, and O’Byrne KT, The effect of oestradiol and progesterone on hypoglycaemic stress-induced suppression of pulsatile luteinizing hormone release and on corticotropin-releasing hormone mRNA expression in the rat. Journal of neuroendocrinology 15 (2003) 468–76. [DOI] [PubMed] [Google Scholar]
- [159].Paulmyer-Lacroix O, Hery M, Pugeat M, and Grino M, The modulatory role of estrogens on corticotropin-releasing factor gene expression in the hypothalamic paraventricular nucleus of ovariectomized rats: role of the adrenal gland. Journal of neuroendocrinology 8 (1996) 515–9. [DOI] [PubMed] [Google Scholar]
- [160].Roy BN, Reid RL, and Van Vugt DA, The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology 140 (1999) 2191–8. [DOI] [PubMed] [Google Scholar]
- [161].Kerdelhue B, Jones GS, Gordon K, Seltman H, Lenoir V, Melik Parsadaniantz S, Williams RF, and Hodgen GD, Activation of the hypothalamo-anterior pituitary corticotropin-releasing hormone, adrenocorticotropin hormone and beta-endorphin systems during the estradiol 17 beta-induced plasma LH surge in the ovariectomized monkey. J Neurosci Res 42 (1995) 228–35. [DOI] [PubMed] [Google Scholar]
- [162].Patchev VK, and Almeida OF, Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci 16 (1996) 7077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wilson ME, Legendre A, Pazol K, Fisher J, and Chikazawa K, Gonadal steroid modulation of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine 26 (2005) 89–97. [DOI] [PubMed] [Google Scholar]
- [164].Handa RJ, Burgess LH, Kerr JE, and O’Keefe JA, Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones & Behavior 28 (1994) 464–76. [DOI] [PubMed] [Google Scholar]
- [165].Lund TD, Munson DJ, Haldy ME, and Handa RJ, Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. Journal of neuroendocrinology 16 (2004) 272–8. [DOI] [PubMed] [Google Scholar]
- [166].Young EA, Altemus M, Parkison V, and Shastry S, Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology 25 (2001) 881–91. [DOI] [PubMed] [Google Scholar]
- [167].Komesaroff PA, Esler MD, and Sudhir K, Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. The Journal of clinical endocrinology and metabolism 84 (1999) 606–10. [DOI] [PubMed] [Google Scholar]
- [168].Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, and Dhabhar FS, Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81 (1997) 689–97. [DOI] [PubMed] [Google Scholar]
- [169].Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, and May V, Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470 (2011) 492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Conneely KN, and Ressler KJ, ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. Am J Med Genet B Neuropsychiatr Genet 162B (2013) 262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, Reiser E, Ely TD, Dhanani R, Glover EM, Jovanovic T, and Ressler KJ, PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proceedings of the National Academy of Sciences of the United States of America (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Mercer KB, Dias B, Shafer D, Maddox SA, Mulle JG, Hu P, Walton J, and Ressler KJ, Functional evaluation of a PTSD-associated genetic variant: estradiol regulation and ADCYAP1R1. Transl Psychiatry 6 (2016) e978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Legradi G, Hannibal J, and Lechan RM, Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neuroscience letters 246 (1998) 145–8. [DOI] [PubMed] [Google Scholar]
- [174].Figueiredo HF, Dolgas CM, Bruestle A, and Herman JP, Estrogen and progesterone differentially modulate the activation of HPA axis-related brain regions following stress., Society for Neuroscience, New Orleans, 2003. [Google Scholar]
- [175].Cullinan WE, Ziegler DR, and Herman JP, Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct 213 (2008) 63–72. [DOI] [PubMed] [Google Scholar]
- [176].Sarkar J, Wakefield S, MacKenzie G, Moss SJ, and Maguire J, Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci 31 (2011) 18198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Purdy RH, Morrow AL, Moore PH Jr., and Paul SM, Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proceedings of the National Academy of Sciences of the United States of America 88 (1991) 4553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Patchev VK, Hassan AH, Holsboer DF, and Almeida OF, The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 15 (1996) 533–40. [DOI] [PubMed] [Google Scholar]
- [179].Patchev VK, Shoaib M, Holsboer F, and Almeida OF, The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 62 (1994) 265–71. [DOI] [PubMed] [Google Scholar]
- [180].Setiawan VW, Haiman CA, Stanczyk FZ, Le Marchand L, and Henderson BE, Racial/ethnic differences in postmenopausal endogenous hormones: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 15 (2006) 1849–55. [DOI] [PubMed] [Google Scholar]
- [181].Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, and Le Marchand L, Ethnic and racial differences in the smoking-related risk of lung cancer. The New England journal of medicine 354 (2006) 333–42. [DOI] [PubMed] [Google Scholar]
- [182].Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, and Ressler KJ, Trauma exposure and stress-related disorders in inner city primary care patients. General hospital psychiatry 31 (2009) 505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Garza K, and Jovanovic T, Impact of Gender on Child and Adolescent PTSD. Curr Psychiatry Rep 19 (2017) 87. [DOI] [PubMed] [Google Scholar]
- [184].Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, and Soldin SJ, Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertility and sterility 84 (2005) 701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Jung C, Ho JT, Torpy DJ, Rogers A, Doogue M, Lewis JG, Czajko RJ, and Inder WJ, A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. The Journal of clinical endocrinology and metabolism 96 (2011) 1533–40. [DOI] [PubMed] [Google Scholar]
- [186].Seng JS, Low LK, Sperlich M, Ronis DL, and Liberzon I, Prevalence, trauma history, and risk for posttraumatic stress disorder among nulliparous women in maternity care. Obstetrics and gynecology 114 (2009) 839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Smith MV, Poschman K, Cavaleri MA, Howell HB, and Yonkers KA, Symptoms of posttraumatic stress disorder in a community sample of low-income pregnant women. The American journal of psychiatry 163 (2006) 881–4. [DOI] [PubMed] [Google Scholar]
- [188].Seng JS, Rauch SA, Resnick H, Reed CD, King A, Low LK, McPherson M, Muzik M, Abelson J, and Liberzon I, Exploring posttraumatic stress disorder symptom profile among pregnant women. J Psychosom Obstet Gynaecol 31 (2010) 176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Onoye JM, Shafer LA, Goebert DA, Morland LA, Matsu CR, and Hamagami F, Changes in PTSD symptomatology and mental health during pregnancy and postpartum. Archives of women’s mental health 16 (2013) 453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Michopoulos V, Rothbaum AO, Corwin E, Bradley B, Ressler KJ, and Jovanovic T, Psychophysiology and posttraumatic stress disorder symptom profile in pregnant African-American women with trauma exposure. Archives of women’s mental health 18 (2015) 639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Koen N, Brittain K, Donald KA, Barnett W, Koopowitz S, Mare K, Zar HJ, and Stein DJ, Psychological trauma and posttraumatic stress disorder: risk factors and associations with birth outcomes in the Drakenstein Child Health Study. Eur J Psychotraumatol 7 (2016) 28720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Kertes DA, Kamin HS, Hughes DA, Rodney NC, Bhatt S, and Mulligan CJ, Prenatal Maternal Stress Predicts Methylation of Genes Regulating the Hypothalamic-Pituitary-Adrenocortical System in Mothers and Newborns in the Democratic Republic of Congo. Child Dev 87 (2016) 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Sack WH, Clarke GN, and Seeley J, Posttraumatic stress disorder across two generations of Cambodian refugees. J Am Acad Child Adolesc Psychiatry 34 (1995) 1160–6. [DOI] [PubMed] [Google Scholar]
- [194].Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, and Berkowitz GS, Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. The Journal of clinical endocrinology and metabolism 90 (2005) 4115–8. [DOI] [PubMed] [Google Scholar]
- [195].Yehuda R, Bell A, Bierer LM, and Schmeidler J, Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. Journal of psychiatric research 42 (2008) 1104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Wolf EJ, Maniates H, Nugent N, Maihofer AX, Armstrong D, Ratanatharathorn A, Ashley-Koch AE, Garrett M, Kimbrel NA, Lori A, Va Mid-Atlantic Mirecc W, Aiello AE, Baker DG, Beckham JC, Boks MP, Galea S, Geuze E, Hauser MA, Kessler RC, Koenen KC, Miller MW, Ressler KJ, Risbrough V, Rutten BPF, Stein MB, Ursano RJ, Vermetten E, Vinkers CH, Uddin M, Smith AK, Nievergelt CM, and Logue MW, Traumatic stress and accelerated DNA methylation age: A meta-analysis. Psychoneuroendocrinology 92 (2018) 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Rosenbaum S, Stubbs B, Ward PB, Steel Z, Lederman O, and Vancampfort D, The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism 64 (2015) 926–33. [DOI] [PubMed] [Google Scholar]
- [198].Ferree NK, Wheeler M, and Cahill L, The influence of emergency contraception on posttraumatic stress symptoms following sexual assault. Journal of forensic nursing 8 (2012) 122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Maples-Keller JL, Price M, Rauch S, Gerardi M, and Rothbaum BO, Investigating Relationships Between PTSD Symptom Clusters Within Virtual Reality Exposure Therapy for OEF/OIF Veterans. Behav Ther 48 (2017) 147–155. [DOI] [PubMed] [Google Scholar]
- [200].Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, and Foa EB, A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev 30 (2010) 635–41. [DOI] [PubMed] [Google Scholar]
- [201].Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, Li H, Sankoh AJ, Clemson C, Schacterle A, Jonas J, and Kanes S, Brexanolone injection in postpartum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 392 (2018) 1058–1070. [DOI] [PubMed] [Google Scholar]
- [202].Nguyen HB, Loughead J, Lipner E, Hantsoo L, Kornfield SL, and Epperson CN, What has sex got to do with it? The role of hormones in the transgender brain. Neuropsychopharmacology 44 (2019) 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Clayton JA, Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiology & behavior 187 (2018) 2–5. [DOI] [PubMed] [Google Scholar]