Abstract
Vachellia and Senegalia are the most important genera in the subfamily Mimosoideae (Fabaceae). Recently, species from both genera were separated from the long-characterized Acacia due to their macro-morphological characteristics. However, this morpho-taxonomic differentiation struggles to discriminate some species, for example, Vachellia nilotica and Senegalia senegal. Therefore, sequencing the chloroplast (cp) genomes of these species and determining their phylogenetic placement via conserved genes may help to validate the taxonomy. Hence, we sequenced the cp genomes of V. nilotica and S. senegal, and the results showed that the sizes of the genomes are 165.3 and 162.7 kb, respectively. The cp genomes of both species comprised large single-copy regions (93,849~91,791 bp) and pairs of inverted repeats (IR; 26,093~26,008 bp). The total numbers of genes found in the V. nilotica and S. senegal cp genomes were 135 and 132, respectively. Approximately 123:130 repeats and 290:281 simple sequence repeats were found in the S. senegal and V. nilotica cp genomes, respectively. Genomic characterization was undertaken by comparing these genomes with those of 17 species belonging to related genera in Fabaceae. A phylogenetic analysis of the whole genome dataset and 56 shared genes was undertaken by generating cladograms with the same topologies and placing both species in a new generic system. These results support the likelihood of identifying segregate genera from Acacia with phylogenomic disposition of both V. nilotica and S. senegal in the subfamily Mimosoideae. The current study is the first to obtain complete genomic information on both species and may help to elucidate the genome architecture of these species and evaluate the genetic diversity among species.
Introduction
Senegalia senegal (L.) Britton and Vachellia nilotica (L.) P.J.H. Hurter & Mabb are the most important species of the genera Senegalia and Vachellia, which belong to the family Fabaceae [1]. S. senegal was formerly known as Acacia senegal (L.) Wild, and V. nilotica was known as Acacia nilotica [2]. Both species were placed in different genera due to their morphological and taxonomical differences. S. senegal is a deciduous tree native to arid and semi-desert regions of sub-Saharan Africa but can also be found in other parts of the world, such as the Indian sub-continent and the Arabian peninsula [3]. The genera are well-known for their exudate gum arabic, a non-timber forest product in international trade possessing medicinal, ecological and commercial importance [3]. The gum derived from the tree is used in such industries as food, pharmaceutical and cosmetics [4]. Moreover, this gum is also used in lithographic ink due to its unique emulsification, encapsulation and film-forming properties, adding to the commercial importance of these species [5, 6]. Furthermore, S. senegal has been noted to increase soil fertility through efficient nutrient fixation, whereas the tree provides shade, fodder, wood fuel [7]. In terms of medicinal uses, gum and tree parts have been known to play bioactive roles in cancer, inflammation, oxidative stress and abdominal complications [7, 8].
In a similar vein, V. nilotica, a multipurpose legume tree and drought-resistant species, has been well-regarded as a means of rehabilitating dry land ecosystems [9]. This tree increases soil organic carbon, total and available forms of nitrogen and phosphorus under its canopy and can thus be used in soil amelioration [10]. Nitrogenous fertilizers are highly expensive for large-scale afforestation [11]. Utilizing alternative species, such as V. nilotica, can assist in fixing atmospheric nitrogen to increase soil fertility [5]. The nutrients generated by V. nilotica trees through biological nitrogen fixation can be exploited within the production system, either simultaneously as an intercropping plant or sequentially, as in rotational fallow systems [9]. V. nilotica has also been well-documented to possess essential chemical constituents that have been suggested to play roles in fighting cancer, microbial pathogenesis, inflammation, sexually transmitted diseases, oxidative stress, diabetes and mutagenesis[12, 13]. Despite the strong medicinal and local uses of both V. nilotica and S. senegal, the taxonomy of these species has not been elucidated. These two species were formerly placed in the genus Acacia, despite their major variation from the other species of Acacia [14]. The genus Acacia comprised 1350 species distributed in most of the continents, except Antarctica [13]. The 2011 IBC (International Botanical Congress) meeting in Melbourne finally ratified the previous decision, despite the long-standing controversy, paving the way for name changes to Vachellia for a smaller and pan-tropical group [15]. This meeting suggested the use of the genera Senegalia and Vachellia in the classification of S. senegal and V. nilotica, respectively. Morphological, biochemical, and palynological data are highly important for the classification of plants into their respective genera [16]. However, emphasis has been placed exegetically to further understand and create more genomic datasets to elucidate these difficult-to-classify and important species [17].
In this regard, chloroplast, the most important organelle in plant cells, plays an important role in photosynthesis, carbon fixation, fatty and amino acid synthesis [18, 19] and has been a focus of attention in recent decades to understand taxonomy, evolution and biological processes. Ideally, a chloroplast (cp) genome of angiosperms exhibits a quadripartite structure size ranging from 110 kb -160 kb. The quadripartite structure is usually composed of a large single copy (LSC) region, a small single copy region (SSC) region and a pair of inverted repeats (IR), which are mirror images of each other [19]. Angiosperm cp genomes generally contain 80 protein-coding genes, 4 ribosomal RNA (rRNA) genes, and 30 transfer RNA (tRNA) genes [20]. The majority of cp genomes exhibit highly conserved structures, some reveal structural variations, IR loss, and gene loss as a result of adaptation to their environments [21, 22]. Next-generation technologies have allowed the rapid sequencing of many cp genomes in recent years [23]. These abundant cp genomes have facilitated the verification of evolutionary relationships and allowed detailed phylogenetic classifications to be conducted at the group, family, and even generic level in plants [24, 25]. Furthermore, cp genomes can be used for species identification through DNA barcoding and molecular markers that enable morphologically similar species to be distinguished [26]. Despite the highly economic, biological, ecological and social importance of these genera, very little information is available on the comparative chloroplast genomes of Senegalia and Vachellia. It is difficult to demarcate monophyletic lineages within these genera, despite morphological differences, and they face classification issues [27, 28]. In this study, we sequenced the chloroplast genomes of V. nilotica and S. senegal, and complete phylogenomic analysis was performed to validate their placement in the genera Vachellia and Senegalia, respectively. Our study provides sequence resources for future studies of population diversity and taxonomy.
Materials and methods
Chloroplast DNA extraction and sequencing
Young and immature green fresh photosynthetic leaves of V. nilotica and S. senegal were ground to fine powder in liquid nitrogen, and the contamination-free chloroplast DNA was isolated according to the modified protocol of Shi et al., [29]. The Ion Torrent sequencing platform was used for sequencing intact chloroplast DNA using the Ion torrent S5 sequencer with the Ion Torrent server (Life Technologies, USA). Genomic libraries were prepared according to the manufacturer’s instructions (Life Technologies, USA). The total chloroplast DNA of each sample was sheared enzymatically into approximately 400-bp fragments using the Ion Shear Plus Reagents kit, and libraries were prepared using the Ion Xpress Plus gDNA Fragment Library kit. Prepared libraries were quantified and qualified on a Qubit 3.0 fluorimeter and an Agilent 2100 Bioanalyzer system. Library preparations were followed by template amplification (Ion one touch 2 instrument, Life Technologies, USA), and enrichment of the amplified template was performed (Ion OneTouch™ ES enrichment system, Life Technologies, USA) using Ion 520 and 530 OT2 reagents. The prepared libraries were loaded onto the Ion S5 sequencing chip, and sequencing was performed according to the Ion torrent S5 protocol (Life Technologies USA).
Genome assembly
The sequencing of V. nilotica and S. senegal resulted in 185,114 and 137,673 reads, respectively. The obtained reads of both Vachellia and Senegelia species were mapped to the selected reference genome of Vachellia flava and Senegalia laeta using Bowtie ((v.2.2.3) [30] in Geneious Pro (v.10.2.3) [31] software. The mean coverage of the reads for V. nilotica and S. senegal were 134X and 168X, respectively. The IR (inverted repeat) junction regions were selected from the reference genomes to adjust the sequence length, and the iteration method was used with MITObim (v.1.8) [32].
Genome annotation
Chloroplast genomes of the sequenced species were annotated by using Dual Organellar Genome Annotator (DOGMA)[33], and the number and position of ribosomal RNAs, transfer RNAs and coding genes present in chloroplast genomes were identified and analyzed using BLASTX and BLASTN, and tRNAscan-SE version 1.21 [34] software was used to annotate tRNA genes. Additionally, for manual adjustment, Geneious (v11.0) and tRNAscan-SE [34] were used to compare the genome with previously reported reference genomes. Correspondingly, the start and stop codons and intron boundaries were also manually adjusted compared with the pre-published reference cp genome. In addition, the structural features of the cp genomes of both V. nilotica and S. senegal species were illustrated using OGDRAW [35]. Correspondingly, the MEGA6 software [36] was used to determine the relative synonymous codon usage and deviations in synonymous codon usage by avoiding the influence of amino acid composition. The divergence of V. nilotica and S. senegal species taxa genomes from those of other related species (Fig 5) was determined using mVISTA [37] in Shuffle-LAGAN mode and using V. nilotica and S. senegal as reference genomes.
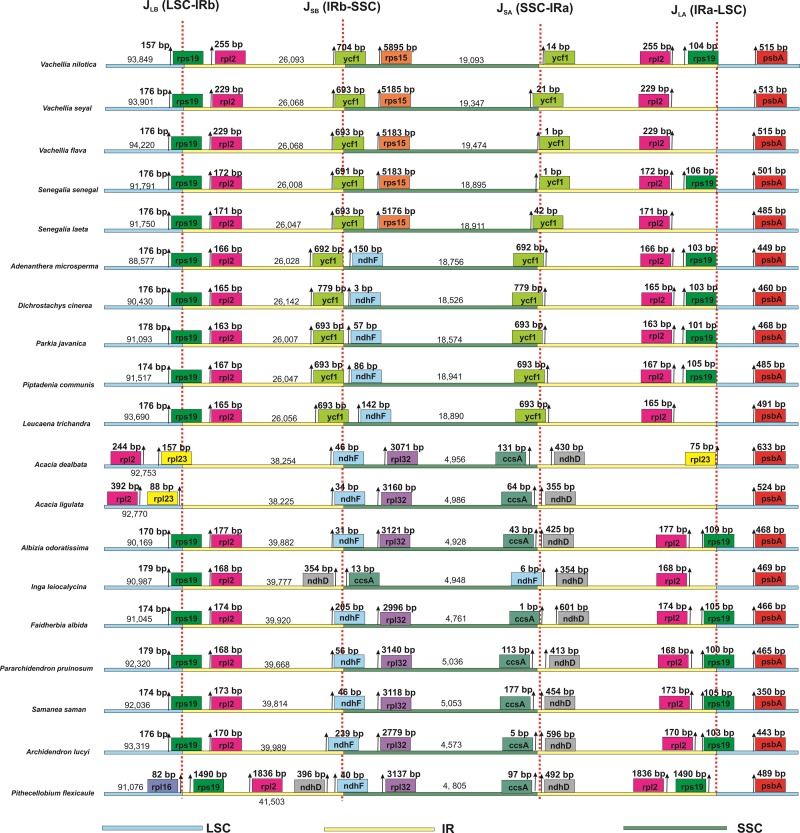
Fig 5. Comparison of border distance between adjacent genes and junctions of LSC, SSC and two IR regions among the chloroplast genomes of V. nilotica and S. senegal with related species.
Boxes above or below the main line indicate the adjacent border genes. The figure is not to scale with respect to sequence length and only shows relative changes at or near the IR/SC borders.
Repeat identification
REPuter software [38] was used for the identification of palindromic, forward and tandem repeats present in the genome. The criterion was a minimum >15 base pairs with a sequence identity of 90%. Furthermore, SSRs were determined using Phobos version 3.3.12 [39] with the search parameters set for mononucleotide repeats ≥ 10 repeat units, dinucleotide repeats ≥ 8 repeat units, tri- and tetranucleotide repeats ≥ 4 repeat units, and pentanucleotide and hexanucleotide repeats ≥ 3 repeat units. Tandem Repeats Finder version 4.07 b [40] with default settings was used to determine tandem repeats.
Sequence divergence and phylogenetic analysis
The average pairwise sequence divergence of the complete cp genomes of Vachellia and Senegalia species with related species was determined. Comparative sequence analysis after comparing gene order and performing multiple sequence alignment was used to identify missing and ambiguous gene annotations. MAFFT version 7.222 [41], with default parameters was used for the alignment of complete genomes, and pairwise sequence divergence was calculated by selected Kimura’s two-parameter (K2P) model [42]. To resolve the phylogenetic position of V. nilotica and S. senegal within the family Fabaceae, cp genomes were downloaded from the NCBI database. Alignment of the complete cp genomes was constructed on the basis of conserved gene order and structure of the cp genome. Four methods were used to infer the phylogenetic trees, including maximum parsimony (MP) implemented with PAUP 4.0100, neighbour-joining (NJ) and maximum likelihood (ML) with MEGA 6[36] and Bayesian inference (BI) with MrBayes 3.1.299 [43] using setting derived from Asaf et al [44] and Wu et al [45]. ML analysis parameters were adjusted with a BIONJ tree with 1000 bootstrap replicates using the Kimura 2-parameter model with gamma-distributed rate heterogeneity and invariant sites. A heuristic search for MP analysis was run with 1000 random addition sequence replicates with the tree-bisection-reconnection (TBR) branch-swapping tree search criterion. The best substitution model GTR + G model was used according to the Akaike information criterion (AIC) by jModelTest version 2102 for Bayesian posterior probabilities (PP) in the BI analyses. The Markov Chain Monto Carlo (MCMC) was run with 4 incrementally heated chains for 1,000,000 generations, starting from random trees and sampling 1 out of every 100 generations. The first 25% of trees were discarded as burn-in to estimate the value of posterior probabilities. In another phylogenetic study, 65 shared genes from the cp genomes of the 102 Fabaceae members downloaded from NCBI were aligned in MAFFT version 7.222 [38]. The above four phylogenetic-inference methods were used to infer trees from these 65 concatenated genes using the same settings described above. The assembled and annotated complete chloroplast genome was submitted to NCBI under the accession numbers MK645904 (V. nilotica) and MK645903 (S. senegal).
Results
General features of V. nilotica and S. senegal chloroplast genomes
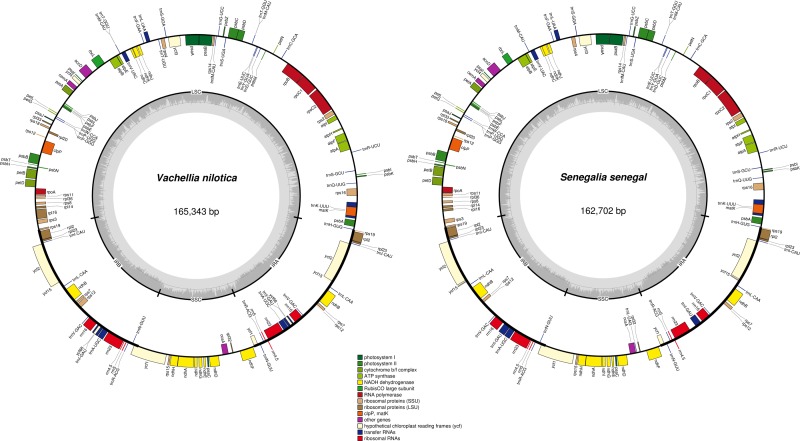
The complete chloroplast genomes of V. nilotica and S. Senegal exhibited typical sizes of 165,343 bp and 162,702 bp, respectively. These genomes showed a typical quadripartite structure with a large single copy region (LSC) and a small single copy region (SSC) and a pair of inverted repeats (Fig 1). The completely sequenced genomes of V. nilotica and S. Senegal were compared with seventeen other chloroplast genomes, where the results showed that the sizes of compared genomes ranged from 178,887 bp (Pithecellobium flexicaula) to 159,389 bp (Adenanthera micrsperma). The overall GC content in V. nilotica was found (35.4%) to be less than that in S. senegal (35.7%). The LSC regions were 39,849 bp and 91,791 bp, while the SSC regions were 19,308 bp and 18,895 bp, respectively, in V. nilotica and S. senegal. The IR region in the two cp genomes was found to be similar in V. nilotica (26,093) and S. senegal (26,008). The number of rRNAs (04) in all the sequenced and compared genomes was the same, while the numbers of tRNAs in the genomes were 37 and 38 in S. senegal and V. nilotica, respectively (Table 1).
Fig 1. Genome map of the Vachellia nilotica and Senegalia senegal chloroplast genome.
Thick lines indicate the extent of the inverted repeat regions (IRa and IRb), which separate the genome into small (SSC) and large (LSC) single copy regions. Genes drawn inside the circle are transcribed clockwise, and those outside are transcribed counterclockwise. Genes belonging to different functional groups are color-coded. The dark grey in the inner circle corresponds to the GC content, and the light grey corresponds to the AT content.
Table 1. Composition of Vachellia nilotica and Senegalia senegal cp genomes with related species.
| Size (bp) | Overall GC contents | LSC size in bp | SSC size in bp | IR size in bp | Protein coding regions size in bp | tRNA size in bp | rRNA size in bp | Number of genes | Number of protein coding genes | Number of rRNA | Number of tRNA | Genes with introns | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V. nilotica | 165,343 | 35.4 | 93849 | 19308 | 26093 | 79520 | 2847 | 9052 | 135 | 89 | 8 | 38 | 22 |
| V. seyal | 165,383 | 35.3 | 93901 | 19347 | 26068 | 78009 | 2828 | 9052 | 127 | 82 | 8 | 37 | 21 |
| V. flava | 165,829 | 35.3 | 94220 | 19474 | 26068 | 78033 | 2828 | 9052 | 127 | 82 | 8 | 37 | 21 |
| S. Senegal | 162702 | 35.7 | 91791 | 18895 | 26008 | 75571 | 2794 | 9052 | 132 | 87 | 8 | 37 | 22 |
| S. Laeta | 162754 | 35.8 | 91750 | 18911 | 26047 | 77997 | 2829 | 9052 | 127 | 82 | 8 | 37 | 20 |
| S. saman | 176717 | 35.3 | 92036 | 5053 | 39814 | 89202 | 2793 | 9052 | 138 | 92 | 8 | 37 | 21 |
| p. flexicaula | 178887 | 35.1 | 91076 | 4805 | 41503 | 89784 | 2793 | 9078 | 139 | 94 | 8 | 37 | 23 |
| P. communis | 162,552 | 35.9 | 91517 | 18941 | 26047 | 77949 | 2793 | 9052 | 130 | 83 | 8 | 37 | 23 |
| P. javanica | 161,681 | 35.9 | 91093 | 18574 | 26007 | 78075 | 2794 | 9052 | 130 | 83 | 8 | 37 | 23 |
| P. pruinosum | 176,692 | 35.3 | 92320 | 5036 | 39668 | 89271 | 2793 | 9052 | 138 | 92 | 8 | 37 | 24 |
| L. trichandra | 164692 | 35.6 | 93690 | 18890 | 26056 | 78759 | 2815 | 9049 | 129 | 84 | 8 | 37 | 22 |
| I. leiocalycina | 175489 | 35.5 | 90987 | 4948 | 39777 | 89244 | 2820 | 9056 | 137 | 92 | 8 | 37 | 24 |
| F. albida | 175646 | 35.3 | 91045 | 4761 | 39920 | 88638 | 2793 | 9068 | 138 | 90 | 8 | 37 | 23 |
| D. cinerea | 161240 | 35.9 | 90430 | 18526 | 26142 | 77958 | 2793 | 9068 | 130 | 83 | 8 | 37 | 23 |
| A. lucyi | 176870 | 35.2 | 92319 | 4573 | 39989 | 89133 | 2793 | 9052 | 138 | 92 | 8 | 37 | 24 |
| A. odoratissima | 174861 | 35.6 | 90169 | 4928 | 39882 | 89130 | 2793 | 9066 | 138 | 92 | 8 | 37 | 24 |
| A. microsperma | 159389 | 36.5 | 88577 | 18756 | 26028 | 78030 | 2793 | 9052 | 130 | 83 | 8 | 37 | 23 |
| A. ligulata | 174233 | 35.4 | 92770 | 4986 | 38225 | 88107 | 2802 | 9062 | 134 | 89 | 8 | 37 | 22 |
| A. daelbata | 174217 | 35.4 | 92753 | 4956 | 38254 | 88551 | 2793 | 9060 | 137 | 91 | 8 | 37 | 22 |
Important genes and base composition in sequenced cp genome
Furthermore, the gene content, gene size and gene order of V. nilotica and S. senegal were largely similar, comprising 135 and 132 genes, respectively. Among all the compared genomes, P. flexicaula possessed the highest number of genes (139), and S. laeta showed the lowest number of genes (127). The numbers of protein coding genes (PCGs) were 89 and 87 in V. nilotica and S. senegalia, respectively. However, these numbers were found to be highest (94) in the P. flexicaula cp genome. The PCGs in the chloroplast genome include some important genes responsible for photosynthesis, i.e., Photosystem I (psaA, B, C, I, J) and Photosystem II (psbA, B, C, D, E, F, H, I, J, K, L, M, N, T, Z). The genes responsible for tRNA, rRNA, large subunit and small subunit of ribosomal proteins were also present in the chloroplast genome. Other important genes annotated in the chloroplast genome were matK, clpP, cemA, accD, ccsA, ycf1, 3, 4, 15, which were also present in the chloroplast genome (Table 2). Approximately 22 intron-containing genes were observed in both sequenced genomes (Table 1).
Table 2. Genes in the sequenced V. nilotica and S. senegal species chloroplast genomes.
| Category | Group of genes | Name of genes |
|---|---|---|
| Self-replication | Large subunit of ribosomal proteins | rpl2, 14, 16, 20, 22, 23, 32, 33, 36 |
| Small subunit of ribosomal proteins | rps2, 3, 4, 7, 8, 11, 12, 14,15, 16, 18, 19 | |
| DNA dependent RNA polymerase | rpoA, B, C1, C2 | |
| rRNA genes | rrn 4.5, rrn 5, rrn 16, rrn23 | |
| tRNA genes | trnC-GCA, trnD-GUC, trnfM-CAU, trnG-UCC, trnH-GUG, trnI-CAU, trnI-GAU, trnK-UUU, trnL-CAA, trnL-UAA, trnL-UAG, trnM-CAU, trnN-GUU, trnP-GGG, trnP-UGG, trnQ-UUG, trnR-ACG, trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-UAC, trnW-CCA, trnY-GUA | |
| Photosynthesis | Photosystem I | psaA, B, C, I, J, |
| Photosystem II | psbA, B, C, D, E, F, H, I, J, K, L, M, N, T, Z | |
| Cytochrome b6/f complex | petA, B, D, G, L, N | |
| ATP synthase | atpA, B, E, F, H, I | |
| Rubisco | rbcL | |
| Other genes | Maturase | matK |
| Protease | clpP | |
| Envelop membrane protein | cemA | |
| Subunit Acetyl- CoA-Carboxylate | accD | |
| c-type cytochrome synthesis gene | ccsA | |
| Unknown | Conserved open reading frames | ycf1, 3,4, 15 |
In the complete genome, the composition of (T) is higher than other base nucleotides present in the genome, which is 32.9% and 32.7% in V. nilotica and S. senegal, respectively. Adenine (A), which comprises the first position in both V. nilotica and S. senegal, accounts for 34.47 and 30.8, respectively. The (T/U) base at the 2nd position was found to be higher than the other genomes, accounting for 33.59 and 32.2 in V. nilotica and S. senegal, respectively. Similarly, the (T/U) base was also found to be abundant at the 3rd position (Table 3).
Table 3. Base composition of the Vachellia nilotica and Senegalia senegal chloroplast genome.
| T/U | C | A | G | Length (bp) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V.N | S.S | V.N | S.S | V.N | S.S | V.N | S.S | V.N | S.S | |
| Genome | 32.9 | 32.7 | 17.9 | 18 | 31.7 | 31.6 | 17.5 | 17.7 | 165343 | 162702 |
| LSC | 34.6 | 34.3 | 16.7 | 16.9 | 32.9 | 32.7 | 15.8 | 16 | 93849 | 91791 |
| SSC | 35.8 | 35.8 | 14.2 | 14.4 | 34.5 | 34.2 | 15.5 | 15.5 | 193.8 | 18895 |
| IR | 28.4 | 28.3 | 22.1 | 22.1 | 28.9 | 29.1 | 20.6 | 20.6 | 26093 | 26008 |
| tRNA | 25.3 | 24.9 | 23.3 | 23.4 | 22.1 | 22.2 | 29.3 | 29.5 | 2847 | 2794 |
| rRNA | 19 | 18.9 | 23.7 | 23.7 | 25.6 | 25.7 | 31.7 | 31.7 | 9052 | 9052 |
| Protein Coding genes | 31.9 | 32.1 | 17.4 | 17.3 | 30.6 | 30.7 | 20.1 | 19.9 | 79520 | 75571 |
| 1st position | 23.36 | 25.2 | 17.43 | 18.20 | 34.47 | 30.8 | 25.57 | 25.16 | 26506 | 25189 |
| 2nd position | 33.59 | 32.2 | 19.09 | 19.78 | 29.26 | 29.62 | 19.44 | 17.49 | 26506 | 25189 |
| 3rd position | 40.12 | 38.7 | 15.24 | 13.8 | 27.82 | 31.6 | 15.15 | 14.4 | 26506 | 25189 |
V.N = Vachellia nilotica, S. S = Senegalia Senegal.
Comparison of sequenced genomes with other genomes
Comparison of the currently two sequenced and seventeen other genomes from the database (NCBI) revealed that the P. flexicaula (178,887 bp) cp genome was the largest, and that of A. microsperma (159,389) was the smallest (Table 1). V. nilotica contains the highest number of tRNAs (38) among all the compared genomes. The highest number of genes was found in the P. flexicaula (139), and the lowest number was found in 127 genes and was similar in the V. seyal, V. flava and S. laeta chloroplast genomes. The highest number of PCGs (protein coding genes) was observed in P. flexicaula (94 genes), and the lowest number (82) was found to be similar in V. seyal, V. flava and S. laeta. The number of rRNAs was similar in all of the compared and sequenced chloroplast genomes, while the number of tRNA- and intron-containing genes varied in all of the chloroplast genomes (Table 1). The largest LSC region was found in V. flava (94,220 bp), and the smallest LSC was observed in A. microsperma (88,577 bp), which is also the smallest genome. The largest SSC region was found in V. flava (19,474 bp), while the smallest was found in A. lucyi (4,573 bp).
Comparative sequence divergent regions in genome
The complete chloroplast genomes of V. nilotica and S. senegal were compared with seventeen species for sequence divergent regions from the NCBI database using mVISTA [37]. The comparative analyses of the chloroplast genome showed a high level of similarity. Overall, the comparison of these chloroplast genomes observed similarity in coding regions, while non-coding regions had more variation, which is almost two times that of coding regions (S1 Fig). The V. nilotica chloroplast genome was used as a reference genome. The comparative analyses of V. nilotica with related species revealed high sequence similarity with no obvious difference from V. flava. The most variable coding regions found in these genomes are tran K, rps16, rpoC1, petB, petD, ycf2, rrn23, and ndhA. In particular, the ycf1 gene displayed more variation among all variable regions (S1 Fig).
Analysis of repetitive sequences in genomes
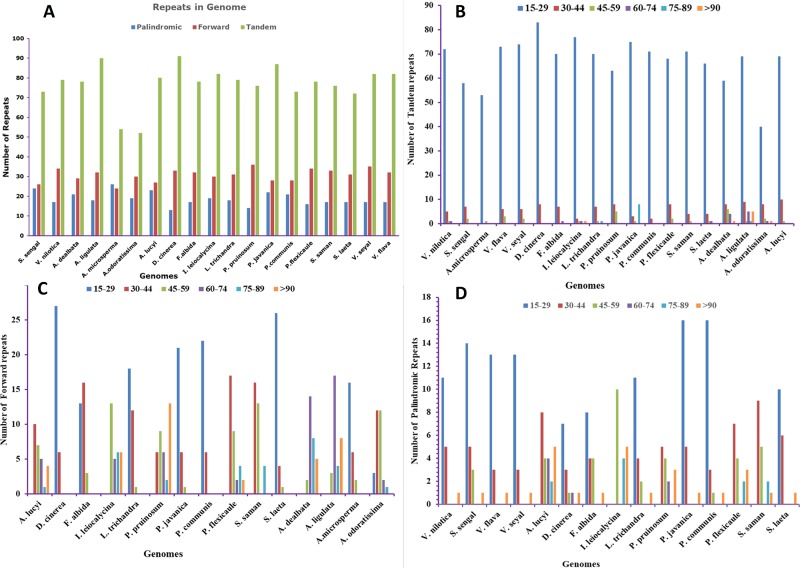
Repeat analysis of the sequenced cp genomes showed that there were 123 repeats in the S. senegal cp genome, which comprised 24 palindromic repeats, 26 forward repeats and 73 tandem repeats. Similarly, in V. nilotica, 130 repeats were present, containing 17 palindromic, 34 forward and 79 tandem repeats (Fig 2). In V. nilotica, the highest number of repeats was observed, and the sizes ranged from 15–29 in all palindromic, forward and tandem repeats containing 11, 20 and 72 repeats, respectively. A similar trend was observed in S. senegal containing 15–29 repeat sizes with 18, 23 and 70 repeats, respectively. Analysis of total repeats showed that V. nilotica had similarity with Archidendron lucyi in repeat number, with each containing 130 repeats. Similarly, V. flava and V. seyal also had 131 and 134 repeats, which showed similarity in repeat number to V. nilotica. The other species that was similar to S. senegal regarding repeats was S. laeta, containing 120 repeats, suggesting that S. senegal shows similarity in terms of repeats. Overall, in the compared genomes, Acacia ligulata comprised the highest number of repeats (140), and Albizia odoratissima contained the lowest number of repeats (101) (Fig 2).
Fig 2. Analysis of repeated sequences in V. nilotica and S. senegal.
(A) Totals of three repeat types, (B) Frequency of palindromic repeats by length, (C) Frequency of forward repeats by length and (D) Frequency of tandem repeats by length.
SSRs in the genomes
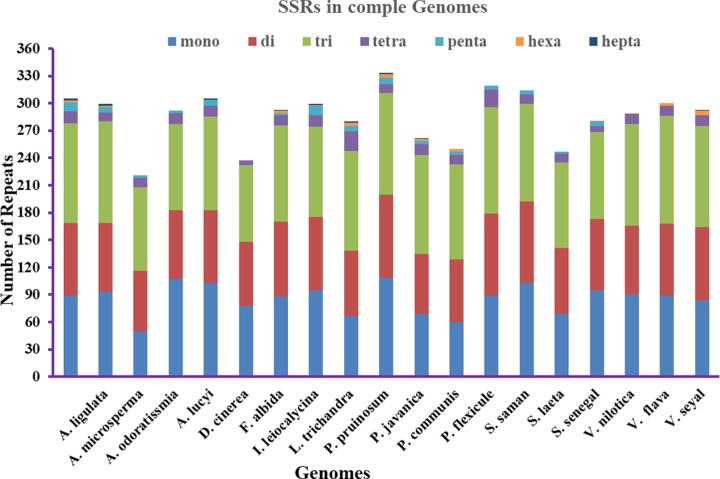
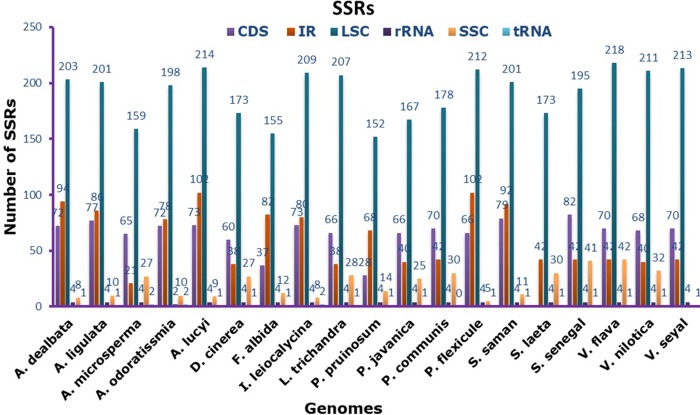
The SSRs (1–7) present in the V. nilotica genome were analyzed, and a total of 290 and 281 SSRs were present in V. nilotica and S. senegal, respectively. In V. nilotica, the most numerous SSRs were trinucleotide repeats (111) followed by mononucleotide (90) and dinucleotide (76) SSRs (Fig 3). The highest number of SSR nucleotides present in the V. nilotica genome was an octanucleotide (1). Similarly, in S. senegal, the total number of SSRs found was 281, where the highest number of nucleotides were trinucleotide (95) followed by mononucleotide (94) and dinucleotide repeats (78). Furthermore, V. nilotica contains the least number of SSRs when compared to other Vachellia species, i.e., V. flava and V. seyal with 302 and 295 SSR repeats, respectively. S. senegal had the highest number of SSRs compared to S. laeta. The number of SSRs was abundant in coding regions of all the sequenced and compared cp genomes (Fig 4). V. nilotica and S. senegal had 211 and 195 SSRs in the coding regions, respectively. Furthermore, V. flava contained the highest number (218) of SSRs in the coding region among all the compared genomes (Fig 4).
Fig 3. Analysis of simple sequence repeat (SSR) in V. nilotica and S. senegal genomes with related species cp genomes.
Number of different SSR types detected in these genomes.
Fig 4. Analysis of simple sequence repeat (SSR) in the V. nilotica and S. senegal genomes.
Frequency of identified SSRs in the Small Single-Copy (SSC), Large Simple-Copy (LSC), Inverted Repeat (IR), transfer RNA (tRNA), ribosomal RNA (rRNA), and coding sequence (CDS) regions.
Contraction and expansion of IR regions
Comprehensive comparative analysis of the junction region was performed among the 19 species for the contraction and expansion in JLb (LSC-IRb), JSB (IRb-SSC), JSA (SSC-IRa), and JLA (IRa-LSC) and also for the position of genes present on these junctions. The largest inverted repeat region was found in the largest chloroplast genome of P. flexicaula, which was 41,503 bp in size, and the smallest IR region was found in the P. javanica (26,007 bp) chloroplast genome.
Although genomic structure and gene composition are highly conserved among these genomes, there are some differences in the IR region. Comparison of the JSB junction of Vachellia species (V. nilotica, V. seyal, V. flava) and Senegalia species (S. senegal, S. laeta) revealed small differences, and the genes at the junction regions are also conserved. In the junction regions of all the compared genomes, the ycf1 gene is conserved and present at the same position (Fig 5). In the JSB junction in Vachellia and Senegalia species, the ycf 1 gene is present, while in the remaining species, it is located in the IRb region. Furthermore, at the JLB junction in all the genomes, the rpl2 gene is located in the IRb region, except for A. ligulata and A. dealbata, in which the rpl2 gene is present in the LSC region. Moreover, at the JSB junction, the rps15 gene was found in the SSC region of all Vachellia and Senegalia species, while other compared genomes were absent. In addition, rpl23 was only at the JLB junction in Accacia dealbata. Similarly, in S. senegal, the ycf1 gene is present at the JSA junction, while in V. nilotica, it was found 14 bp away from JSA in the IRa region (Fig 5).
Phylogenetic analyses: Confirmation of recent classification based on complete CP
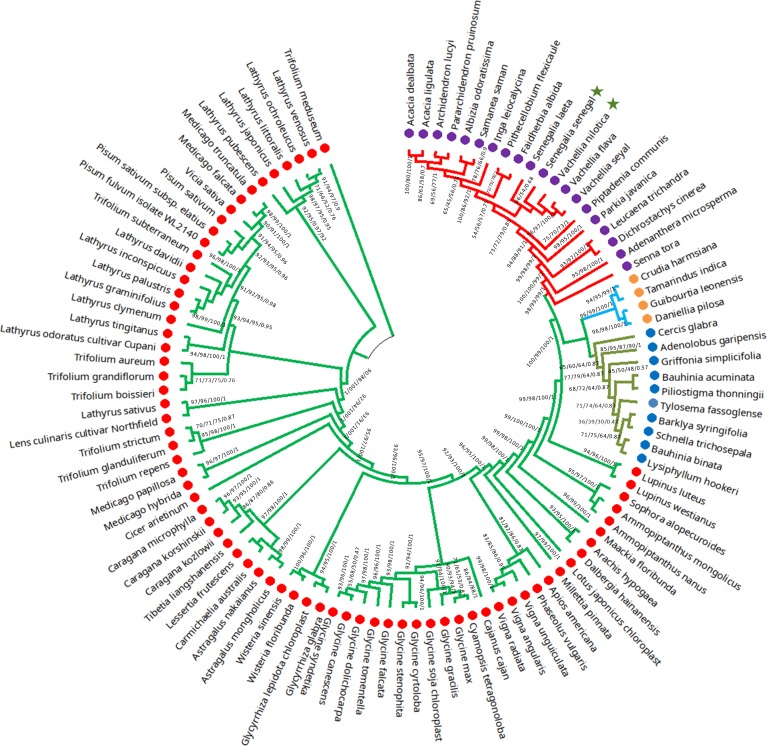
Previously, numerous studies were conducted to resolve the phylogenetic position of Mimosoideae [46], but no study to date has investigated the basis of the complete chloroplast genome of Vachellia and Senegalia species. In this study, the phylogenetic position of V. nilotica and S. senegal within the family Fabaceae was established by analyzing multiple sequence alignments of complete cp genomes and 56 shared genes of 104 Fabaceae members (Fig 6 and S2). The 56 shared genes (from all species) and the complete cp genome sequence generated phylogenetic trees with identical topologies (Fig 6 and S2). In these phylogenetic trees, S. senegal formed a sister clade with S. laeta, while V. nilotica shared a sister clade with V. flava and V. seyal with high posterior probability and bootstrap support values using four different methods (Fig 6). Our results supported the recent classification of V. nilotica and S. senegal in the genera Vachellia and Senegal, respectively, and did not support the former placement of these species in the genus Acacia.
Fig 6. Phylogenetic tree constructed on the basis of whole genome dataset using four different methods: Bayesian inference (BI), maximum likelihood (ML), maximum parsimony (MP), and neighbor-joining (NJ).
Numbers above the branches are the posterior probabilities of BI and bootstrap values for ML, MP and NJ. The star represents the position of V. nilotica and S. senegal.
Discussion
This study reports the complete chloroplast genomes of S. Senegal and V. nilotica, ranging from 162.7~165.3 kb in length. Both cp genomes exhibit a typical quadripartite conserved structure, as reported for other angiosperm genomes [44, 47]. Both V. nilotica and S. Senegal encode 135 and 132 genes, including 89 and 87 protein-coding genes, respectively. Similar differences in the protein coding genes were also observed, as in previously reported genomes [46]. The important genes present in these genomes were also similar to those of previously reported angiosperm cp genomes [44, 48]. The main reason for size variation among the chloroplast genomes is the contraction and expansion in the IR regions of the genome [49]. The size variation was observed (161,681 bp ~178,887 bp) to be in keeping with the previously reported angiosperm genomes [46]. Genome conservation was observed in both genera with some minor changes in IR/SSC regions, which reveal evidence of variation in the chloroplast genomes and also provides some information in the evolutionary context of chloroplast genomes [50]. Divergence hotspots among the species facilitate comparative genomics, species identification [51] and phylogenetic studies at different levels [52]. Comparative analysis of these genomes through mVISTA revealed that coding regions, such as rps16, rpoC2, atpF, rpoC1, accD, clpP, petD, rpl16, ycf1, ycf2 and ndhA, were more divergent than the non-coding regions, which is similar to the findings obtained with previously reported cp genomes [46, 53]. The significance of these divergent regions can be further used as potential DNA markers for phylogenetic studies, population genetics studies and species identification studies [54]. Some of the protein coding genes present in the plastid genomes were found to have versatile roles in the resolution of phylogenetic relationships of complex plant taxa, such as rpoA, psal, petB and rps19 in Notopterygium species [55] and ycf1 in Anemopaegma species [56]. Moreover, in some other species, such as Veroniceae, the petD-rpoA, ycf4-cemA, and rpl32-trnL genes were used for the identification of the species. In our study, the PCG regions were more conserved and showed less sequence divergence than the intergenic spacer region, which had a higher degree of divergence among the compared species. Surprisingly, the IR regions in these compared cp genomes were less divergent compared to the LSC and SSC regions, which were also previously reported [53].
Repetitive sequences within the chloroplast genome play a crucial role in evolution, divergence studies and cp genome rearrangement. Moreover, microsatellite-like SSRs play an important role in molecular-level identification and in population genetics [57, 58]. The identification of repetitive sequences in the IGS provides useful information in various angiosperm species [58]. Among all the compared genomes, Albizia odoratissima had the lowest number of total repeats (104), and Acacia ligulata had the highest number of total repeats in cp genomes of subfamily Mimosoideae [46] and among other angiosperms [59]. The Adenanthera microsperma genome was found to contain the highest number of palindromic repeats (26), and the lowest number (16) was reported in Pithecellobium flexicaule. The highest number of forward repeats (36) was found in Pararchidendron pruinosum, while the lowest (24) was found in A. microsperma. The tandem repeats were highest (91) in Dichrostachys cinerea, and the lowest (52) was in Albizia odoratissima. Plastome size variation leads to the variation in tandem repeats [60] and dispersed repeats as previously reported by [57]. Earlier studies also showed that these repeats play an important role in structural variation [61]. The highest number of SSRs among these genomes was 333 in Parachidendron pruinosum, while the lowest number of SSRs was observed in A. microsperma. This result was consistent with the previously reported chloroplast genome of wild roses [62].
The phylogenetic relationship of the genus Vachellia and Senegalia belonging to the sub-family Mimosoideae (Fabaceae) was poorly resolved previously using only a few plastid markers [63–65]. Phylogenomic analysis based on the complete chloroplast genome can be widely used to resolve the complex relationship at the family level, as previously reported in orchiaceae [66], and Bambusoideae [67]. A detailed comprehensive study of the subfamily Mimosoideae was reported by Wang et al.[46], but there was no mention of the phylogenomic placement of V. nilotica and S. senegal into the genus Vachellia and Senegalia. The results of our study indicate that phylogenetic trees based on the complete genome dataset and 56 shared genes of V. nilotica and S. senegal contain the same phylogenetic signals and support the recent classification of V. nilotica and S. senegal in the genera Vachellia and Senegal, respectively (Fig 6). A complete phylogeny of Mimosoideae was constructed to resolve the evolutionary relationship of Mimosoideae with Fabaceae. Structural rearrangement in the chloroplast genome is an important phylogenetic signal and is used to define monophyletic lineages in plant groups [68].
Conclusion
We sequenced the chloroplast genome of V. nilotica and S. senegal. Both genomes shared the same gene organization and overall genome structure, which were also found in related species. The quadripartite structure (LSC/SSC/IRA/IRB) of the genomes was compared for Mimosoideae species, and no significant variation was noted in these genomes, instead showing the closest similarity to these species. The phylogenetic relationships of these species, which were formerly classified in the genus Acacia and later placed in the genera Vachellia and Senegalia, were validated on the basis of the complete chloroplast genome. Furthermore, the phylogenetic analyses revealed that both V. nilotica and S. senegal formed monophyletic clades, while V. nilotica further shared sub-monophyletic clades with V. flava and V. seyal, while the S. senegal shared the same clade with S. laeta. These findings may help to elucidate the complex taxonomy of these genera and the studied species V. nilotica and S. senegal.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Supporting information
VISTA-based identity plot showing sequence identity among nineteen species, using V. nilotica as a reference genome. The vertical scale indicates the percentage of identity, ranging from 50% to 100%. The horizontal axis indicates the coordinates within the chloroplast genome. Arrows indicate the annotated genes and their transcriptional direction.
(PDF)
A phylogenetic tree was constructed for 104 species from the family Fabaceae based on 56 shared protein coding genes. The following four different methods were used for the 56 shared gene data sets: Bayesian inference (BI), maximum likelihood (ML), maximum parsimony (MP), and neighbor-joining (NJ). Numbers above the branches are the posterior probabilities of BI and bootstrap values for ML, MP and NJ.
(PDF)
Data Availability
The assembled and annotated complete chloroplast genome were submitted to NCBI under the accession number MK645904 (V. nilotica) and MK645903 (S. senegal).
Funding Statement
The authors are thankful to The Research Council, Oman for their financial support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Haque A. Investigation of the fungi associated with dieback of prickly acacia (Vachellia nilotica subsp. indica) in Northern Australia. PhD, The University of Queensland. 2015.
- 2.Kyalangalilwa B, Boatwright JS, Daru BH, Maurin O, van der Bank M. Phylogenetic position and revised classification of A cacia sl (F abaceae: M imosoideae) in A frica, including new combinations in V achellia and S enegalia. Botanical Journal of the Linnean Society. 2013;172(4):500–23. [Google Scholar]
- 3.Beshai AA. The economics of a primary commodity: Gum Arabic. Oxford bulletin of economics and statistics. 1984;46(4):371–81. [Google Scholar]
- 4.Khan IA, Abourashed EA. Leung's encyclopedia of common natural ingredients: used in food, drugs and cosmetics: John Wiley & Sons; 2011. [Google Scholar]
- 5.Al-Assaf S, Phillips GO, Aoki H, Sasaki Y. Characterization and properties of Acacia senegal (L.) Willd. var. senegal with enhanced properties (Acacia (sen) SUPER GUM™): Part 1—Controlled maturation of Acacia senegal var. senegal to increase viscoelasticity, produce a hydrogel form and convert a poor into a good emulsifier. Food Hydrocolloids. 2007;21(3):319–28. [Google Scholar]
- 6.Motlagh S, Ravines P, Karamallah K, Ma Q. The analysis of Acacia gums using electrophoresis. Food hydrocolloids. 2006;20(6):848–54. [Google Scholar]
- 7.Arce LR, Banks H. A preliminary survey of pollen and other morphological characters in neotropical Acacia subgenus Aculeiferum (Leguminosae: Mimosoideae). Botanical Journal of the Linnean Society. 2001;135(3):263–70. [Google Scholar]
- 8.Salih AA, El Fadl MA, Kaarakka V, Luukkanen O. Symbiotic nitrogen fixation in eight Acacia senegal provenances in dryland clays of the Blue Nile Sudan estimated by the 15 N natural abundance method. Plant and Soil. 2005;275(1–2):261–9. [Google Scholar]
- 9.Bargali K, Bargali S. Acacia nilotica: a multipurpose leguminous plant. Nature and Science. 2009;7(4):11–9. [Google Scholar]
- 10.Raj A, Chandrawanshi S. Acacia nilotica: a multipurpose tree and source of Indian gum Arabic. South Indian Journal of Biological Sciences. 2015;1(2):66–9. [Google Scholar]
- 11.Good A. Toward nitrogen-fixing plants. Science. 2018;359(6378):869–70. 10.1126/science.aas8737 [DOI] [PubMed] [Google Scholar]
- 12.Donalisio M, Cagno V, Civra A, Gibellini D, Musumeci G, Rittà M, et al. The traditional use of Vachellia nilotica for sexually transmitted diseases is substantiated by the antiviral activity of its bark extract against sexually transmitted viruses. Journal of ethnopharmacology. 2018;213:403–8. 10.1016/j.jep.2017.11.039 [DOI] [PubMed] [Google Scholar]
- 13.Rather LJ, Mohammad F. Acacia nilotica (L.): a review of its traditional uses, phytochemistry, and pharmacology. Sustainable Chemistry and Pharmacy. 2015;2:12–30. [Google Scholar]
- 14.Daru BH, Kyalangalilwa B, Maurin O, van der Bank M, Boatwright JS. Phylogenetic position and revised classification of Acacia s.l. (Fabaceae: Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia. Botanical Journal of the Linnean Society. 2013;172(4):500–23. 10.1111/boj.12047 [DOI] [Google Scholar]
- 15.Orchard AE, Maslin BR. The case for conserving Acacia with a new type. Taxon. 2005;54(2):509–12. [Google Scholar]
- 16.Gardner FP, Pearce RB, Mitchell RL. Physiology of crop plants: Scientific Publishers; 2017. [Google Scholar]
- 17.Taylor DB, Dhileepan K. Implications of the changing phylogenetic relationships of Acacia sl on the biological control of Vachellia nilotica ssp. indica in Australia. Annals of Applied Biology. 2019;174(2):238–47. [Google Scholar]
- 18.Jansen RK, Ruhlman TA. Plastid genomes of seed plants. Genomics of chloroplasts and mitochondria: Springer; 2012. p. 103–26. [Google Scholar]
- 19.Daniell H, Lin C-S, Yu M, Chang W-J. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome biology. 2016;17(1):134 10.1186/s13059-016-1004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wicke S, Schneeweiss GM, Müller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant molecular biology. 2011;76(3–5):273–97. 10.1007/s11103-011-9762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delannoy E, Fujii S, Colas des Francs-Small C, Brundrett M, Small I. Rampant gene loss in the underground orchid Rhizanthella gardneri highlights evolutionary constraints on plastid genomes. Molecular Biology and Evolution. 2011;28(7):2077–86. 10.1093/molbev/msr028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wicke S, Müller KF, de Pamphilis CW, Quandt D, Wickett NJ, Zhang Y, et al. Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the broomrape family. The Plant Cell. 2013;25(10):3711–25. 10.1105/tpc.113.113373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Land M, Hauser L, Jun S-R, Nookaew I, Leuze MR, Ahn T-H, et al. Insights from 20 years of bacterial genome sequencing. Functional & integrative genomics. 2015;15(2):141–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks M, Cronn R, Liston A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC biology. 2009;7(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen RK, Cai Z, Raubeson LA, Daniell H, Leebens-Mack J, Müller KF, et al. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proceedings of the National Academy of Sciences. 2007;104(49):19369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim K, Lee S-C, Lee J, Lee HO, Joh HJ, Kim N-H, et al. Comprehensive survey of genetic diversity in chloroplast genomes and 45S nrDNAs within Panax ginseng species. PloS one. 2015;10(6):e0117159 10.1371/journal.pone.0117159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross JH. A conspectus of African acacia species1979.
- 28.Miller JT, Bayer RJ. Molecular phylogenetics of Acacia subgenera Acacia and Aculeiferum (Fabaceae: Mimosoideae), based on the chloroplast matK coding sequence and flanking trnK intron spacer regions. Australian Systematic Botany. 2003;16(1):27–33. [PubMed] [Google Scholar]
- 29.Shi C, Hu N, Huang H, Gao J, Zhao Y-J, Gao L-Z. An improved chloroplast DNA extraction procedure for whole plastid genome sequencing. Plos one. 2012;7(2):e31468 10.1371/journal.pone.0031468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature methods. 2012;9(4):357 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–9. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn C, Bachmann L, Chevreux B. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads—a baiting and iterative mapping approach. Nucleic acids research. 2013;41(13):e129–e. 10.1093/nar/gkt371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20(17):3252–5. 10.1093/bioinformatics/bth352 [DOI] [PubMed] [Google Scholar]
- 34.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic acids research. 2005;33(suppl_2):W686–W9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lohse M, Drechsel O, Bock R. OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Current genetics. 2007;52(5–6):267–74. 10.1007/s00294-007-0161-y [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Nei M, Dudley J, Tamura K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings in Bioinformatics. 2008;9(4):299–306. 10.1093/bib/bbn017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic acids research. 2004;32(suppl_2):W273–W9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic acids research. 2001;29(22):4633–42. 10.1093/nar/29.22.4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraemer L, Beszteri B, Gäbler-Schwarz S, Held C, Leese F, Mayer C, et al. S TAMP: Extensions to the S TADEN sequence analysis package for high throughput interactive microsatellite marker design. BMC bioinformatics. 2009;10(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic acids research. 1999;27(2):573 10.1093/nar/27.2.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular biology and evolution. 2013;30(4):772–80. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of molecular evolution. 1980;16(2):111–20. 10.1007/bf01731581 [DOI] [PubMed] [Google Scholar]
- 43.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–4. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- 44.Asaf S, Khan AL, Khan MA, Waqas M, Kang S-M, Yun B-W, et al. Chloroplast genomes of Arabidopsis halleri ssp. gemmifera and Arabidopsis lyrata ssp. petraea: Structures and comparative analysis. Scientific reports. 2017;7(1):7556 10.1038/s41598-017-07891-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Z, Tembrock LR, Ge S. Are differences in genomic data sets due to true biological variants or errors in genome assembly: an example from two chloroplast genomes. PLoS One. 2015;10(2):e0118019 10.1371/journal.pone.0118019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y-H, Qu X-J, Chen S-Y, Li D-Z, Yi T-S. Plastomes of Mimosoideae: structural and size variation, sequence divergence, and phylogenetic implication. Tree genetics & genomes. 2017;13(2):41. [Google Scholar]
- 47.Yang JB, Li DZ, Li HT. Highly effective sequencing whole chloroplast genomes of angiosperms by nine novel universal primer pairs. Molecular Ecology Resources. 2014;14(5):1024–31. 10.1111/1755-0998.12251 [DOI] [PubMed] [Google Scholar]
- 48.Asaf S, Waqas M, Khan AL, Khan MA, Kang S-M, Imran QM, et al. The complete chloroplast genome of wild rice (Oryza minuta) and its comparison to related species. Frontiers in plant science. 2017;8:304 10.3389/fpls.2017.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raubeson LA, Peery R, Chumley TW, Dziubek C, Fourcade HM, Boore JL, et al. Comparative chloroplast genomics: analyses including new sequences from the angiosperms Nuphar advena and Ranunculus macranthus. BMC Genomics. 2007;8(1):174 10.1186/1471-2164-8-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S-D, Jin J-J, Chen S-Y, Chase MW, Soltis DE, Li H-T, et al. Diversification of Rosaceae since the Late Cretaceous based on plastid phylogenomics. New Phytologist. 2017;214(3):1355–67. 10.1111/nph.14461 [DOI] [PubMed] [Google Scholar]
- 51.Ahmed I, Matthews PJ, Biggs PJ, Naeem M, McLenachan PA, Lockhart PJ. Identification of chloroplast genome loci suitable for high-resolution phylogeographic studies of Colocasia esculenta (L.) Schott (Araceae) and closely related taxa. Molecular Ecology Resources. 2013;13(5):929–37. 10.1111/1755-0998.12128 [DOI] [PubMed] [Google Scholar]
- 52.Downie SR, Jansen RK. A comparative analysis of whole plastid genomes from the Apiales: expansion and contraction of the inverted repeat, mitochondrial to plastid transfer of DNA, and identification of highly divergent noncoding regions. Systematic Botany. 2015;40(1):336–51. [Google Scholar]
- 53.Dong W-L, Wang R-N, Zhang N-Y, Fan W-B, Fang M-F, Li Z-H. Molecular Evolution of Chloroplast Genomes of Orchid Species: Insights into Phylogenetic Relationship and Adaptive Evolution. International Journal of Molecular Sciences. 2018;19(3):716 10.3390/ijms19030716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye X, Hu D, Guo Y, Sun R. Complete Chloroplast Genome of Castanopsis sclerophylla (Lindl.) Schott: Genome Structures, Comparative and Phylogenetic Analysis. BioRxiv. 2019:540617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Vázquez L, Chen X, Li H, Zhang H, Liu Z, et al. Development of Chloroplast and Nuclear DNA Markers for Chinese Oaks (Quercus Subgenus Quercus) and Assessment of Their Utility as DNA Barcodes. Frontiers in Plant Science. 2017;8(816). 10.3389/fpls.2017.00816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Firetti F, Zuntini AR, Gaiarsa JW, Oliveira RS, Lohmann LG, Van Sluys M-A. Complete chloroplast genome sequences contribute to plant species delimitation: A case study of the Anemopaegma species complex. American Journal of Botany. 2017;104(10):1493–509. 10.3732/ajb.1700302 [DOI] [PubMed] [Google Scholar]
- 57.Weng M-L, Blazier JC, Govindu M, Jansen RK. Reconstruction of the ancestral plastid genome in Geraniaceae reveals a correlation between genome rearrangements, repeats, and nucleotide substitution rates. Molecular biology and evolution. 2013;31(3):645–59. 10.1093/molbev/mst257 [DOI] [PubMed] [Google Scholar]
- 58.Xue J, Wang S, Zhou S-L. Polymorphic chloroplast microsatellite loci in Nelumbo (Nelumbonaceae). American Journal of Botany. 2012;99(6):e240–e4. 10.3732/ajb.1100547 [DOI] [PubMed] [Google Scholar]
- 59.Khan AL, Al-Harrasi A, Asaf S, Park CE, Park G-S, Khan AR, et al. The first chloroplast genome sequence of Boswellia sacra, a resin-producing plant in Oman. PloS one. 2017;12(1):e0169794 10.1371/journal.pone.0169794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dugas DV, Hernandez D, Koenen EJ, Schwarz E, Straub S, Hughes CE, et al. Mimosoid legume plastome evolution: IR expansion, tandem repeat expansions, and accelerated rate of evolution in clpP. Scientific reports. 2015;5:16958 10.1038/srep16958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greiner S, Wang X, Rauwolf U, Silber MV, Mayer K, Meurer J, et al. The complete nucleotide sequences of the five genetically distinct plastid genomes of Oenothera, subsection Oenothera: I. Sequence evaluation and plastome evolution. Nucleic acids research. 2008;36(7):2366–78. 10.1093/nar/gkn081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeon J-H, Kim S-C. Comparative Analysis of the Complete Chloroplast Genome Sequences of Three Closely Related East-Asian Wild Roses (Rosa sect. Synstylae; Rosaceae). Genes. 2019;10(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouchenak-Khelladi Y, Maurin O, Hurter J, Van der Bank M. The evolutionary history and biogeography of Mimosoideae (Leguminosae): an emphasis on African acacias. Molecular Phylogenetics and Evolution. 2010;57(2):495–508. 10.1016/j.ympev.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 64.Luckow M, Miller JT, Murphy DJ, Livshultz T. A phylogenetic analysis of the Mimosoideae (Leguminosae) based on chloroplast DNA sequence data. Advances in legume systematics, part. 2003;10:197–220. [Google Scholar]
- 65.Miller JT, Seigler D. Evolutionary and taxonomic relationships of Acacia sl (Leguminosae: Mimosoideae). Australian Systematic Botany. 2012;25(3):217–24. [Google Scholar]
- 66.Givnish TJ, Spalink D, Ames M, Lyon SP, Hunter SJ, Zuluaga A, et al. Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1814):20151553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wysocki WP, Clark LG, Attigala L, Ruiz-Sanchez E, Duvall MR. Evolution of the bamboos (Bambusoideae; Poaceae): a full plastome phylogenomic analysis. BMC evolutionary biology. 2015;15(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henry RJ. Plant diversity and evolution: genotypic and phenotypic variation in higher plants: Cabi Publishing; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VISTA-based identity plot showing sequence identity among nineteen species, using V. nilotica as a reference genome. The vertical scale indicates the percentage of identity, ranging from 50% to 100%. The horizontal axis indicates the coordinates within the chloroplast genome. Arrows indicate the annotated genes and their transcriptional direction.
(PDF)
A phylogenetic tree was constructed for 104 species from the family Fabaceae based on 56 shared protein coding genes. The following four different methods were used for the 56 shared gene data sets: Bayesian inference (BI), maximum likelihood (ML), maximum parsimony (MP), and neighbor-joining (NJ). Numbers above the branches are the posterior probabilities of BI and bootstrap values for ML, MP and NJ.
(PDF)
Data Availability Statement
The assembled and annotated complete chloroplast genome were submitted to NCBI under the accession number MK645904 (V. nilotica) and MK645903 (S. senegal).
All data generated or analyzed during this study are included in this published article.