Abstract
The term gut–liver axis is used to highlight the close anatomical and functional relationship between the intestine and the liver. The intestine has a highly specialized epithelial membrane which regulates transport across the mucosa. Due to dysbiosis, impairment of the intestinal barrier and altered immunity status, bacterial products can reach the liver through the portal vein, where they are recognized by specific receptors, activate the immune system and lead to a proinflammatory response. Gut microbiota and bacterial translocation play an important role in the pathogenesis of chronic liver diseases, including alcoholic and non-alcoholic fatty liver disease, cirrhosis, and its complications, such as portal hypertension, spontaneous bacterial peritonitis and hepatic encephalopaty. The gut microbiota also plays a critical role as a modulator of bile acid metabolism which can also influence intestinal permeability and portal hypertension through the farnesoid-X receptor. On the other hand, cirrhosis and portal hypertension affect the microbiota and increase translocation, leading to a “chicken and egg” situation, where translocation increases portal pressure, and vice versa. A myriad of therapies targeting gut microbiota have been evaluated specifically in patients with chronic liver disease. Further studies targeting intestinal microbiota and its possible hemodynamic and metabolic effects are needed. This review summarizes the current knowledge about the role of gut microbiota in the pathogenesis of chronic liver diseases and portal hypertension.
Keywords: Gut–liver axis, Cirrhosis, Portal hypertension, Microbiota, Translocation, LPS, Endotoxemia, Bile acids
Introduction
The gut microbiota comprises a wide spectrum of microorganism (mainly bacteria) that contributes to digestion, synthesis of vitamins, and resistance to colonization. The microbiota may also stimulate the immune system in the gastrointestinal tract and decrease pathogens by competing for nutrients and space [1]. Intestinal bacteria can be critically influenced by environmental factors, such as dietary modifications, alcohol consumption, or certain drugs (proton pump inhibitors or probiotics among others) [2, 3]. Various liver disorders such as alcoholic liver disease, non-alcoholic fatty liver disease and primary sclerosing cholangitis have been associated with qualitative and quantitative (overgrowth) changes in gut microbiota [4–6]. Dysbiosis, defined as the imbalance between protective and harmful bacteria, and the alteration of intestinal barrier may also influence the degree of hepatic steatosis, inflammation and fibrosis through multiple interactions with the host’s immune system and other cell types [7].
The term gut–liver axis has been coined to highlight the close anatomical and functional relationship between both organs. The liver is strategically placed in the proximity of the gut receiving 75% of its blood supply through the portal vein. The portal vein flow carries nutrients, but also translocated microbial products and bacteria which provide the liver with a broad spectrum of antigens. Most of them are harmless dietary and commensal products, but pathogens or bacterial-derived factors from the gastrointestinal tract can also reach the liver via the enterohepatic circulation [8]. Although a highly specialized epithelial barrier regulates transport across the intestinal mucosa, a wide variety of gut-derived antigens may reach the liver through the portal vein. The hepatic immune system must remain tolerant to harmless stimuli while, at the same time, must be activated against bacterial pathogens and prevent them from reaching the systemic circulation [8, 9]. The recognition of these bacteria-derived antigens by specific receptors and the subsequent proinflammatory response has been linked to the development and progression of chronic liver disease [10]. Therefore, the intestinal barrier, the microbiota, the liver and the immune system must establish complex interactions to maintain homeostasis. Disturbance of this balance can lead to increased intestinal permeability and result in disease.
This review intends to integrate and summarize the current knowledge about the role that the gut microbiota plays in the pathogenesis of chronic liver diseases and portal hypertension, and provides a glimpse of its clinical and therapeutics perspectives.
Intestinal barrier: the forefront of the defense
Only a small part of the intestinal luminal content reaches the portal circulation, since a highly specialized barrier limits direct interaction between this content, the underlying lamina propria and the immune system. This barrier promotes nutrient and water transport while also protecting against gut-derived pathogens [11]. The intestinal epithelial barrier is composed of a mucus lining, an epithelial monolayer of specialized cells, and the intercellular junctions that seal the space between them and control trafficking across the intestinal mucosa. The luminal mucus layer prevents large particles and intact bacteria from coming into contact with the epithelium [12]. The major components of the mucus are mucins, which are large glycoproteins secreted by intestinal globet cells [13]. Below this layer, the apical surface of the epithelium forms a single and continuous border that restricts the passage of most hydrophilic solutes. The apical junctional proteins (claudins, occludins, and zonula occludens), known as tight junctions and adherens junctions, regulate paracellular transport and seal the space between the epithelial cells.
Some hepatotoxic compounds, such as alcohol, can directly affect the intestinal epithelial tight junctions impairing the intestinal barrier and inducing translocation and endotoxemia [14–16]. Acetaldehyde, an oxidative metabolite that is generated by intestinal metabolism of alcohol, increases intestinal permeability by disrupting integrity and expression of epithelial tight junction molecules. Dunagan et al. showed that acetaldehyde mediates protein phosphatase 2A (PP2A) translocation to tight junctions and dephosphorylation of occludin on threonine residues [17]. Furthermore, alcohol may induce a change in the expression of zonula occludens-1 (ZO-1) and claudin-1, increasing intestinal epithelial barrier permeability [18]. Ethanol-induced miR-212 over-expression has been also linked to decreased ZO-1 protein levels [19]. Finally, tight junction-mediated permeability may be regulated by the local expression of pro- and anti-inflammatory interleukins, such as interferon gamma (IFN-γ), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) [20, 21].
Intestinal microbiota: the key regulator
Alteration of gut microbiota has been linked with liver disease development and progression, especially related to non-alcoholic liver disease (NAFLD) and alcoholic liver disease (ALD), although the exact mechanisms at play remain unknown [22–25].
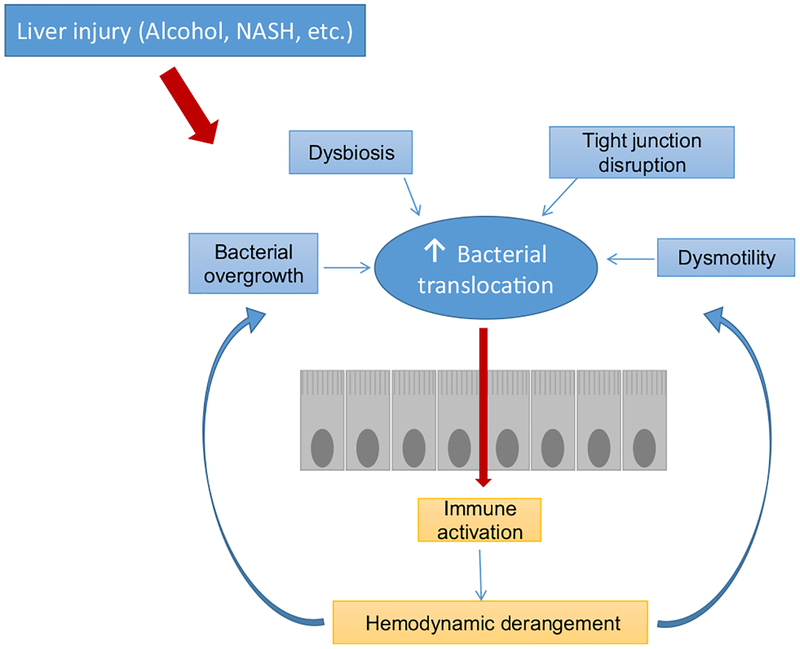
As cirrhosis progresses, portal hypertension leads to an increase in intestinal permeability due to the loosening of the tight junctions, higher transcytosis, and a decrease of mediators that limit contact between bacteria and intestinal microvilli [26]. In addition, intestinal bacterial overgrowth and changes in the composition of intestinal bacterial flora can promote bacterial translocation, defined as the migration of bacteria or bacterial products from the gut to the extra-intestinal space [27] (Fig. 1).
Fig. 1.
Mechanisms of bacterial translocation. Bacterial overgrowth, dysbiosis and altered intestinal permeability promote bacterial translocation from the intestinal lumen to the mesenteric lymph nodes and gut-associated lymphoid tissue. When the immune system capacity is overcome, bacteria may access the systemic circulation increasing the risk of infections and favoring hemodynamic derangement. This worsening in turn favors bacterial translocation, perpetuating the “chicken and egg” circle
Several studies have shown a decrease in beneficial autochthonous bacteria in cirrhotic patients, such as Lactobacillus, Bifidobacterium, and Bacteroides species. Decreased abundance of these species, in particular Bifidobacterium, can exacerbate hepatic injury and impede regeneration [28]. Furthermore, there is an increase in potentially pathogenic families such as Proteobacteria (particularly Enterobacteriaceae which is responsible of most of the spontaneous bacterial peritonitis episodes), Fusobacterium spp., Veillonellaceae, and Streptococcaceae [29–32] (Table 1). An interesting, and not yet addressed, question in liver diseases relates to the impact of metabolites produced by gut microbiota in the host immune response. There is some evidence about how microbiota-derived metabolites modulate the activation of the inflammasome (NLRP6 inflammasome signaling) to influence microbial community stability [33], but further studies are needed in patients with liver diseases.
Table 1.
Changes in microbiota composition in cirrhosis
Small intestine bacterial overgrowth (SIBO) in cirrhotic patients has been demonstrated by quantitative analyses of bacterial cultures from jejunal aspirates [34]. This fact is linked to a decrease in bile acid secretion and the impairment of intestinal motility, mainly characterized by prolonged transit time [35, 36]. Decreased intestinal motility contributes to SIBO, but may be observed in patients without bacterial overgrowth [37]. The pathogenesis of this dysmotility is multifactorial, including autonomic neuropathy, inflammatory mediators, dysbiosis and neuropeptides.
Disruption of intestinal barrier, translocation and liver immunology: the last stand
Dysbiosis, increased intestinal permeability induced by disruption of the intestinal barrier, and overwhelming of the defense mechanisms leads to bacterial translocation [10, 38]. Bacterial translocation occurs when bacteria adhere to the epithelium, pass through the intestinal wall and reach the mesenteric lymph nodes. The capacity of the lymph nodes to eliminate intestinal bacteria can be overcome in certain situations, favoring their access to other territories and increasing the risk of infections. In cirrhosis, this phenomenon is the consequence of a “leaky” intestinal barrier, and its occurrence is considered detrimental in the natural history of a patient with chronic liver disease [39]. This fact also contributes to the chronic activation of the immune system and inflammation in the liver [38].
Bacterial products are identified by the immune system through recognition of pathogen-associated molecular patterns (PAMPs), which are a limited and defined set of conserved molecular patterns carried by all microorganisms of a given class [40]. The most studied gut-derived PAMP is the bacterial endotoxin known as lipopolysaccharide (LPS), which is found in the outer membrane of Gram-negative bacteria. The administration of intraperitoneal LPS has been shown to increase portal pressure [41], which at the same time increases intestinal permeability [42, 43]. Furthermore, bacterial translocation, endotoxemia and proinflammatory cytokines impair the contractility of mesenteric vessels in patients with cirrhosis, increasing portal pressure [44, 45]. In a portal-vein ligation model, mice colonized with intestinal microbiota presented with significantly higher portal pressure as compared to germ-free mice [46]. On the other hand, Tabibian et al. [47] have recently shown that the absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis, and that this also increases cholangiocyte senescence.
Although LPS and other bacterial products can activate different signaling pathways promoting a proinflammatory cascade, anti-inflammatory and antifibrogenic mechanisms are present concurrently to balance these processes and maintain liver homeostasis and immunotolerance. Hepatic antigen-presenting cells (APC) are central to the tolerogenic nature of the liver due to their capacity to preferentially suppress adaptive immunity by killing or inactivating T cells or by inducing maturation of naïve T cells into T-regulatory cells that suppress CD4+ and CD8+ T cell responses [9].
Once bacterial products reach the liver, they can activate immune cells through pattern recognition receptors (PRRs) such as the membrane-bound toll-like receptors (TLRs) and the cytoplasmic nucleotide-binding oligomerization domain like receptors (NLRs). TLRs are transmembrane proteins that play a key role in the innate immune system, usually expressed in sentinel cells such as macrophages and dendritic cells; however, TLRs have been identified in response to LPS of hepatic non-immune cells, including hepatic stellate cells and endothelial cells [38, 48]. TLRs recognize structurally conserved PAMPs and activate an inflammatory response [49, 50]. There are 10 subtypes of TLRs [51], including TLR4 which activates the innate immune in response to LPS through the co-receptors CD14 or MD-2 [49, 52]. Downstream TLR4 signaling may be either MyD88-dependent or -independent [53]. The MyD88-dependent pathway involves nuclear translocation of NF-κB [54], which is a heterodimeric transcription factor constitutively expressed in all cell types. NF-κB has a central role in regulating the immune response to infection as well as in the pathogenesis of a wide variety of conditions affecting the liver, including viral hepatitis, steatohepatitis, cirrhosis and hepatocellular carcinoma [55]. NF-κB activation induces the release of pro-inflammatory cytokines such as TNFα, IL-6 and IL-1β [56]. On the other hand, the MyD88-independent pathway implies the phosphorylation of the interleukin regulatory factor 3, which leads to induction of type-I interferon [57, 58]. TLRs can also be activated by damage-associated molecular patterns (DAMPs) released from injured cells or tissues [59]. This may lead to sterile inflammation and plays a role in the pathogenesis of non-alcoholic and alcoholic liver disease [49, 60]. DAMPS may also activate NLRs which regulate inflammatory and apoptotic responses along with TLRs [61, 62]. Activation of NLRs results in the up-regulation of cytokines and chemokines, such as IL-1β and IL-18, which are then released to recruit and activate additional inflammatory cells [63, 64].
In normal conditions, the intestinal barrier prevents translocation of most of the bacteria, so only small amounts of microbial products can reach the liver. Once there, they are cleared by immune cells, particularly Kupffer cells [48]. In cirrhotic patients, due to a disrupted intestinal barrier, a greater influx of bacterial products to the liver occurs, leading to activation of liver immune cells and release of pro-inflammatory cytokines [65, 66]. This mechanism is especially relevant in alcoholic liver disease [67, 68], where studies in animal models have shown that the use of antibiotics or Kupffer cells elimination can alleviate alcohol-induced liver injury [69, 70]. Translocation of bacteria and their products from a leaky lumen has also been recognized as a major contributor to systemic inflammation in advanced cirrhosis and acute-on-chronic liver failure (ACLF) [71].
Role of bile acids in portal hypertension: the old-new players
The gut microbiota also plays a critical role as modulator of bile acids (BAs) pool size and composition, through defined enzymatic activities (dihydroxylation, oxidation and epimerization among others), and significantly modifying the signaling properties of BAs and their actions on BA receptors [22, 72, 73].
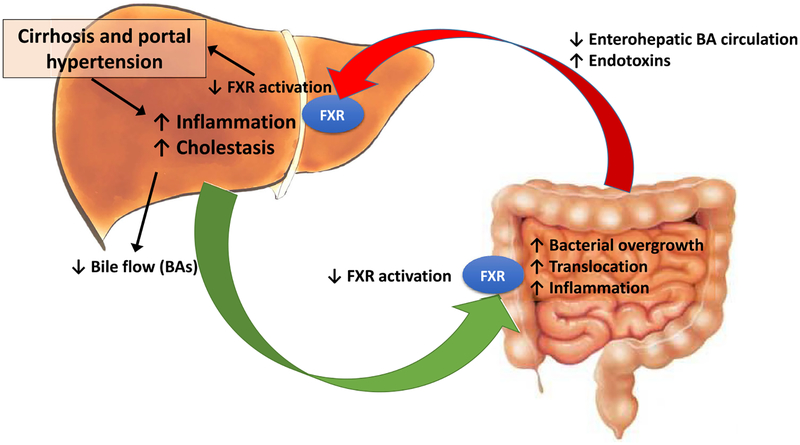
BAs mediate the absorption of dietary lipids but also play a pivotal role contributing to select intestinal microbiome [74] by means of their direct antimicrobial effects through the farnesoid-X receptor (FXR)-induced production of antimicrobial peptides such as angogenin1 and RNase family member 4 [75, 76]. These prevent bacterial overgrowth and promote epithelial cell integrity [77]. Bile acids therefore inhibit bacterial proliferation directly and indirectly by modulating host cell expression of antimicrobial genes. Consequently, decreased intraluminal concentration of bile acids on end-stage-chronic liver diseases may promote bacterial overgrowth [78]. Several studies on germ-free and FXR-deficient mice have shown that the gut microbiota also modulates BA profiles and influences FXR signaling [22, 79]. On the other hand, it has been described that FXR activation markedly inhibits inflammation and preserves the intestinal barrier function reducing bacterial translocation in inflammatory bowel disease [80] and in a rat model of cholestatic liver injury [81] (Fig. 2).
Fig. 2.
Schematic depicting the influence of cirrhosis and portal hypertension in bile acids and their circulation in relation to gut microbiota. BAs bile acids, FXR Farnesoid-X receptor
Modulation of the BAs nuclear receptor with obeticholic acid (OCA), a potent selective FXR agonist, has been shown to improve portal hypertension by two distinct pathways in a rat model of intoxication with thioacetamide and in the bile-duct-ligation (BDL) model of liver injury. In both models, treatment with OCA reactivated the FXR downstream signaling pathway and decreased portal pressure by lowering total intrahepatic vascular resistance without deleterious systemic hypotension [82]. Additionally, OCA has been shown to reduce bacterial translocation and reduce intestinal inflammation in ascitic cirrhotic rats [83]. Thus, modulation of BAs signaling could be a novel target for modulation of portal hypertension, in part through effects on the microbiome.
Clinical and therapeutic perspectives
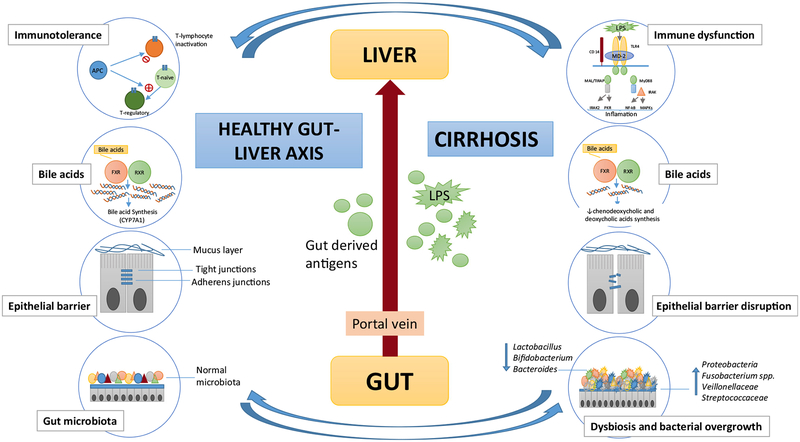
As previously described, the gut microbiota plays a pivotal role in liver disease natural history, both in the progression of the disease and in the development of its complications such as spontaneous bacterial peritonitis (SBP) and hepatic encephalopathy (HE) [84], but, at the same time, cirrhosis and portal hypertension affect the microbiota and increase translocation, leading to a “chicken and egg” situation. The initial process of translocation of bacterial products (“chicken”) from the gut to the bloodstream triggers a response in the liver increasing the portal pressure (“egg”), and the latter (“chicken”) in turn leads to intestinal edema, disruption of epithelial integrity and more translocation (“egg”) (Fig. 3).
Fig. 3.
Gut liver axis in health and disease. In immune response, hepatic antigen-presenting cells (APC) are central to tolerogenic nature of the liver due to their capacity to suppress adaptive immunity by killing or inactivating T cells or by inducing maturation of naïve cells into regulatory cells that suppress CD4+ and CD8+ cell responses. In cirrhosis, TLR4 activation by LPS leads to a proinflammatory response. Persistent activation of the immune system leads to cirrhosis immune dysfunction. The bile acids are natural ligands of farnesoid-X receptor (FXR). FXR is a nuclear receptor highly expressed in the liver and intestine. When activated, FXR translocates to the nucleus and forms a heterodimer with RXR. In the liver, FXR negatively regulates bile acid production by repressing CYP7A1. Bile acid secretion is decrease in cirrhosis, which contributes to bacterial translocation and inflammation. The epithelial barrier regulates transport across the mucosa and prevents gut-derived pathogens reach the portal system. In cirrhosis, intestinal permeability increase due to disruption and altered expression of tight junction proteins. The gut microbiota are beneficial autochthonous bacteria (Lactobacillus, Bifidobacterium, and Bacteroides species) which decrease in cirrhotic patients, meanwhile potentially pathogenic families such as Proteobacteria, Fusobacterium, Veillonellaceae, and Streptococcaceae are increased, leading to dysbiosis and bacterial overgrowth
Patients with liver disease from different etiologies (viral hepatitis, alcohol, NAFLD, primary biliary cholangitis, primary sclerosing cholangitis, etc.) have been shown to have elevated levels of LPS in plasma and endotoxemia [85–88]. For this reason, attempts to target gut microbiota (with probiotics, antibiotics or depletion of Kupffer cells) have been pursued with some success in alcoholic liver disease models [69, 70, 89]. The mutual interdependence between the pathogenesis of the cirrhotic process, intestinal bacterial translocation and portal pressure make the gut–liver axis an attractive target for specific therapeutic interventions. Non-selective beta-blockers (NSBB), broadly used in cirrhotic patients, have been shown to be beneficial by reducing bacterial translocation, while other therapies evaluated in patients with cirrhosis specifically aiming gut microbiota are antibiotics (rifaximin), prebiotic (lactulose), probiotics and proton-pump inhibitors (PPI), with different results. These drugs are discussed in more detail below.
Non-selective beta-blockers (NSBB)
NSBB reduce sympathetic tonus, decrease portal pressure, and protect against variceal hemorrhage in cirrhosis, by lowering cardiac output through blockade of β−1 adrenoreceptors, and increase splanchnic vasoconstriction through blockade of β−2 adrenoreceptors [90]. A meta-analysis also confirmed that NSBB protect against SBP independently of their hemodynamic effects [91]. In cirrhotic patients, treatment with NSBB has been shown to increase intestinal transit, reduce intestinal bacterial overgrowth, decrease intestinal permeability, and reduce bacterial translocation [92, 93].
Antibiotics
Despite pre-clinical models having shown that rifaximin treatment improves microbiota composition and function, clinical trials have not shown significant differences in those endpoints, probably due to these studies have been carried out in patients with advanced cirrhosis, and, therefore, the microbiota reflects the cirrhotic status rather than the effect of rifaximin [94, 95]. Regarding clinical endpoints, an elegant study by Bajaj et al. [96] showed that cirrhotic patients under rifaximin treatment, despite having a slight change in microbiota composition, have less endotoxemia and an improvement in cognition. In the same study, rifaximin treatment increased serum fatty acid metabolites, changed bile acid composition and promoted an anti-inflammatory status, changing the correlation networks between bacteria and metabolites. Recently, a randomized, double-blind, placebo-controlled trial in 54 patients with cirrhosis and ascites showed no effect on hemodynamics (hepatic venous pressure gradient or systemic hemodynamics) [97]. Currently, large studies are being carried out to evaluate the effect of rifaximin on the complications of decompensated cirrhosis and portal pressure (ClinicalTrials.gov ID: and ).
Prebiotics
Despite its widespread use, no studies have clearly shown that lactulose leads to significant changes in microbiota composition or function [98]. The proposed mechanisms of action of lactulose are as a laxative, increasing the volume of stools, and as a prebiotic, acidifying and modifying the colonic flora. This potential modification of the microbiota could lead to a displacement of urease-producing bacteria with non-urease-producing Lactobacillus, and, therefore, to a reduction in the formation of potentially toxic short-chain fatty acids (e.g., propionate, butyrate, and valerate) [99, 100]. Currently, a clinical trial is being carried out to elucidate the influence of lactulose intake on the gut microbiota composition ().
Probiotics
Probiotics, in particular lactose-fermenting Lactobacilli and Bifidobacteria, promote mucosal barrier function and modulate the gut microflora, suppressing pathogenic microbial growth [101–103]. Probiotics and synbiotics (a combination of probiotics and prebiotics in a form of synergism) have been used broadly in trials addressing HE. Among the different combinations Lactobacillus GG and VSL#3 have shown improvements in endotoxemia, endothelial dysfunction, and dysbiosis over placebo, along with modifications on bile acid pool composition [104–106]. VSL#3 has shown improvements in hepatic and systemic hemodynamics [107], but larger clinical trials with clinically significant outcomes are needed.
Fecal microbiota transplantation
The role of fecal microbiota transplantation is becoming increasingly recognized as an emergent treatment in gastrointestinal pathologies. Evidence regarding liver diseases includes potential benefits in primary sclerosing cholangitis, NAFLD, and, most recently, in preventing alcohol-induced liver injury in mice [108–110]. However, further studies are needed before proposing strong recommendations.
Proton-pump inhibitors (PPI)
Another widespread medication involves PPI, which have been reported to be used by half of cirrhotic patients on a regular basis [111]. PPI have been shown to change microbiota composition in cirrhotic and non-cirrhotic patients [112]. Omeprazole increases oral-origin Streptococcaceae in the stools, similar to the effect of the use of broad antibiotic therapy [112]. This could set the stage for SIBO or Clostridium difficile infection. In two recent studies, PPI were identified as a risk factor for HE and SBP in patients with cirrhosis with ascites, with the risk increasing with dose [111, 113].
Conclusions
The gut–liver axis plays an important role in the development and progression of cirrhosis and portal hypertension, interplaying a role of “chicken and egg”, where bacterial translocation from the intestine reaches the liver and increases portal pressure, while, on the other hand, portal hypertension leads to intestinal edema, disruption of epithelial integrity and more translocation. This disruption of the intestinal barrier leads to bacterial translocation recognized by TLRs and NLRs, which induces a proinflammatory response. Additionally, BAs receptors play a role in the gut barrier function and portal pressure. While no studies have shown a definitive beneficial effect of rifaximin, lactulose or probiotics in large clinical trials of portal hypertension, further studies to delineate the impact of therapies on bacterial composition and function, as well as therapies aimed to regulate intestinal permeability and bacterial translocation, are urgently needed.
Acknowledgements
This work was supported by grant(s) NIH DK59615 and AA021171 (VHS), the Clinical Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567). Arab JP was funded by an award from AASLD Foundation (AASLD/ LIFER Clinical and Translational Research Fellowship in Liver Diseases). The authors also thanks Mrs. Terri Johnson for her secretarial assistance.
Abbreviations
- ACLF
Acute-on-chronic liver failure
- APC
Antigen-presenting cells
- BAs
Bile acids
- BDL
Bile-duct ligation
- DAMPs
Damage-associated molecular patterns
- DDAH-2
Dimethylarginine dimethylaminohydrolase 2
- eNOS
Endothelial nitric oxide synthase
- FXR
Farnesoid-X receptor
- IFN-γ
Interferon gamma
- IL-6
Interleukin-6
- MLCK
Myosin-light chain kinase
- NAFLD
Non-alcoholic liver disease
- NLR
Nucleotide-binding oligomerization domain like receptors
- NSBB
Non-selective beta-blockers
- LPS
Lipopolysaccharide
- OCA
Obeticholic acid
- PAMPs
Pathogen-associated molecular patterns
- PP2A
Protein phosphatase 2A
- PPI
Proton-pump inhibitors
- PRRs
Pattern recognition receptors
- SIBO
Small intestine bacterial overgrowth
- TNF-α
Tumor necrosis factor-α
- TLRs
Toll-like receptors
- ZO-1
Zonula occludens-1
Footnotes
Conflict of interest Juan P. Arab, Rosa M. Martin-Mateos and Vijay H. Shah have nothing to disclose.
Human and animal rights This article does not contain any studies with human or animal subjects.
References
- 1.Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease. Eur J Pediatr 2015;174:151–167 [DOI] [PubMed] [Google Scholar]
- 2.Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016;65:749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vassallo G, Mirijello A, Ferrulli A, Antonelli M, Landolfi R, Gasbarrini A, et al. Review article: alcohol and gut microbiota—the possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Aliment Pharmacol Ther 2015;41:917–927 [DOI] [PubMed] [Google Scholar]
- 4.Bashiardes S, Shapiro H, Rozin S, Shibolet O, Elinav E. Nonalcoholic fatty liver and the gut microbiota. Mol Metab 2016;5:782–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabino J, Vieira-Silva S, Machiels K, Joossens M, Falony G, Ballet V, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016;65:1681–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bluemel S, Williams B, Knight R, Schnabl B. Precision medicine in alcoholic and nonalcoholic fatty liver disease via modulating the gut microbiota. Am J Physiol Gastrointest Liver Physiol 2016;311:G1018–G1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch M Gut microbiota and the liver: a tale of 2 cities: a narrative view in 2 acts. J Clin Gastroenterol 2016;50 Suppl 2,Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13–15, 2015:S183–S187 [DOI] [PubMed] [Google Scholar]
- 9.Crispe IN. Immune tolerance in liver disease. Hepatology 2014;60:2109–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis 2010;30:232–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol 2010;5:119–144 [DOI] [PubMed] [Google Scholar]
- 12.Johansson ME, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014;63:281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 2010;12:319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 1984;1:179–182 [DOI] [PubMed] [Google Scholar]
- 15.Draper LR, Gyure LA, Hall JG, Robertson D. Effect of alcohol on the integrity of the intestinal epithelium. Gut 1983;24:399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 2000;32:742–747 [DOI] [PubMed] [Google Scholar]
- 17.Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 2012;303:G1356–G1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Tong J, Chang B, Wang B, Zhang D, Wang B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol Med Rep 2014;9:2352–2356 [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res 2008;32:355–364 [DOI] [PubMed] [Google Scholar]
- 20.Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi M, Ereifej L, et al. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS ONE 2014;9:e85345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen P, Starkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 2015;61:883–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 2017;65:350–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boursier J, Diehl AM. Nonalcoholic fatty liver disease and the gut microbiome. Clin Liver Dis 2016;20:263–275 [DOI] [PubMed] [Google Scholar]
- 24.Quigley EM, Monsour HP. The gut microbiota and nonalcoholic fatty liver disease. Semin Liver Dis 2015;35:262–269 [DOI] [PubMed] [Google Scholar]
- 25.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016;63:764–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo YS, Shah VH. The role of gut–liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin Mol Hepatol 2012;18:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology 2005;41:422–433 [DOI] [PubMed] [Google Scholar]
- 28.Rayes N, Pilarski T, Stockmann M, Bengmark S, Neuhaus P, Seehofer D. Effect of pre- and probiotics on liver regeneration after resection: a randomised, double-blind pilot study. Benef Microbes 2012;3:237–244 [DOI] [PubMed] [Google Scholar]
- 29.Betrapally NS, Gillevet PM, Bajaj JS. Gut microbiome and liver disease. Transl Res 2017;179:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60:940–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011;54:562–572 [DOI] [PubMed] [Google Scholar]
- 32.Chen P, Schnabl B. Host-microbiome interactions in alcoholic liver disease. Gut Liver 2014;8:237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy M, Thaiss CA, Zeevi D, Dohnalova L, Zilberman-Schapira G, Mahdi JA, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 2015;163:1428–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer TM, Schwacha H, Steinbruckner B, Brinkmann FE, Ditzen AK, Aponte JJ, et al. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol 2002;97:2364–2370 [DOI] [PubMed] [Google Scholar]
- 35.Raedsch R, Stiehl A, Gundert-Remy U, Walker S, Sieg A, Czygan P, et al. Hepatic secretion of bilirubin and biliary lipids in patients with alcoholic cirrhosis of the liver. Digestion 1983;26:80–88 [DOI] [PubMed] [Google Scholar]
- 36.Gunnarsdottir SA, Sadik R, Shev S, Simren M, Sjovall H, Stotzer PO, et al. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol 2003;98:1362–1370 [DOI] [PubMed] [Google Scholar]
- 37.Chesta J, Defilippi C, Defilippi C. Abnormalities in proximal small bowel motility in patients with cirrhosis. Hepatology 1993;17:828–832 [PubMed] [Google Scholar]
- 38.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 2008;48:322–335 [DOI] [PubMed] [Google Scholar]
- 39.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 2014;60:1310–1324 [DOI] [PubMed] [Google Scholar]
- 40.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol 2011;30:16–34 [DOI] [PubMed] [Google Scholar]
- 41.Steib CJ, Hartmann AC, v Hesler C, Benesic A, Hennenberg M, Bilzer M, et al. Intraperitoneal LPS amplifies portal hypertension in rat liver fibrosis. Lab Invest 2010;90:1024–1032 [DOI] [PubMed] [Google Scholar]
- 42.Chiva M, Guarner C, Peralta C, Llovet T, Gomez G, Soriano G, et al. Intestinal mucosal oxidative damage and bacterial translocation in cirrhotic rats. Eur J Gastroenterol Hepatol 2003;15:145–150 [DOI] [PubMed] [Google Scholar]
- 43.Clements WD, Erwin P, McCaigue MD, Halliday I, Barclay GR, Rowlands BJ. Conclusive evidence of endotoxaemia in biliary obstruction. Gut 1998;42:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tazi KA, Moreau R, Herve P, Dauvergne A, Cazals-Hatem D, Bert F, et al. Norfloxacin reduces aortic NO synthases and proinflammatory cytokine up-regulation in cirrhotic rats: role of Akt signaling. Gastroenterology 2005;129:303–314 [DOI] [PubMed] [Google Scholar]
- 45.Wiest R, Das S, Cadelina G, Garcia-Tsao G, Milstien S, Groszmann RJ. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest 1999;104:1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moghadamrad S, McCoy KD, Geuking MB, Sagesser H, Kirundi J, Macpherson AJ, et al. Attenuated portal hypertension in germ-free mice: function of bacterial flora on the development of mesenteric lymphatic and blood vessels. Hepatology 2015;61:1685–1695 [DOI] [PubMed] [Google Scholar]
- 47.Tabibian JH, O’Hara SP, Trussoni CE, Tietz PS, Splinter PL, Mounajjed T, et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology 2016;63:185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009;27:147–163 [DOI] [PubMed] [Google Scholar]
- 49.Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair 2010;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract 2010;2010:710381. doi: 10.1155/2010/710381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology 2006;44:287–298 [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805–820 [DOI] [PubMed] [Google Scholar]
- 53.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol 2009;50:1258–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J Biol Chem 2009;284:24192–24203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakraborty JB, Mann DA. NF-kappaB signalling: embracing complexity to achieve translation. J Hepatol 2010;52:285–291 [DOI] [PubMed] [Google Scholar]
- 56.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783–801 [DOI] [PubMed] [Google Scholar]
- 57.Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol 2012;590:447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schafer SL, Lin R, Moore PA, Hiscott J, Pitha PM. Regulation of type I interferon gene expression by interferon regulatory factor-3. J Biol Chem 1998;273:2714–2720 [DOI] [PubMed] [Google Scholar]
- 59.Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci 2016;61:1294–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010;10:826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol 2007;82:259–264 [DOI] [PubMed] [Google Scholar]
- 62.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol 2009;27:229–265 [DOI] [PubMed] [Google Scholar]
- 63.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10:417–426 [DOI] [PubMed] [Google Scholar]
- 64.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004;20:319–325 [DOI] [PubMed] [Google Scholar]
- 65.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol 2009;183:1320–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis 2009;29:141–154 [DOI] [PubMed] [Google Scholar]
- 67.Szabo G, Mandrekar P, Petrasek J, Catalano D. The unfolding web of innate immune dysregulation in alcoholic liver injury. Alcohol Clin Exp Res 2011;35:782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szabo G gut–liver axis in alcoholic liver disease. Gastroenterology 2015;148:30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology 1994;20:453–460 [PubMed] [Google Scholar]
- 70.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 1995;108:218–224 [DOI] [PubMed] [Google Scholar]
- 71.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 2015;63:1272–1284 [DOI] [PubMed] [Google Scholar]
- 72.Ridlon JM, Bajaj JS. The human gut sterolbiome: bile acid-microbiome endocrine aspects and therapeutics. Acta Pharm Sin B 2015;5:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol 2014;30:332–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Best N, Jansen PL, Rensen SS. The gut microbiota of nonalcoholic fatty liver disease: current methods and their interpretation. Hepatol Int 2015;9:406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA 2006;103:3920–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parseus A, Sommer N, Sommer F, Caesar R, Molinaro A, Stahlman M, et al. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017;66(3):429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cariou B, Staels B. The expanding role of the bile acid receptor FXR in the small intestine. J Hepatol 2006;44:1213–1215 [DOI] [PubMed] [Google Scholar]
- 78.Lorenzo-Zuniga V, Bartoli R, Planas R, Hofmann AF, Vinado B, Hagey LR, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology 2003;37:551–557 [DOI] [PubMed] [Google Scholar]
- 79.Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol 2017;101:47–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011;60:463–472 [DOI] [PubMed] [Google Scholar]
- 81.Verbeke L, Farre R, Verbinnen B, Covens K, Vanuytsel T, Verhaegen J, et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol 2015;185:409–419 [DOI] [PubMed] [Google Scholar]
- 82.Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology 2014;59:2286–2298 [DOI] [PubMed] [Google Scholar]
- 83.Ubeda M, Lario M, Munoz L, Borrero MJ, Rodriguez-Serrano M, Sanchez-Diaz AM, et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol 2016;64:1049–1057 [DOI] [PubMed] [Google Scholar]
- 84.Cardenas A, Mendez-Bocanegra A. Report of the Baveno VI Consensus Workshop. Ann Hepatol 2016;15:289–290 [DOI] [PubMed] [Google Scholar]
- 85.Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, et al. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology 2007;133:1627–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol 1987;4:8–14 [DOI] [PubMed] [Google Scholar]
- 87.Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011;141:1220–1230, 1230 e1221–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med 1994;205:243–247 [DOI] [PubMed] [Google Scholar]
- 90.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823–832 [DOI] [PubMed] [Google Scholar]
- 91.Senzolo M, Cholongitas E, Burra P, Leandro G, Thalheimer U, Patch D, et al. beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int 2009;29:1189–1193 [DOI] [PubMed] [Google Scholar]
- 92.Perez-Paramo M, Munoz J, Albillos A, Freile I, Portero F, Santos M, et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology 2000;31:43–48 [DOI] [PubMed] [Google Scholar]
- 93.Senzolo M, Fries W, Buda A, Pizzuti D, Nadal E, Sturniolo GC, et al. Oral propranolol decreases intestinal permeability in patients with cirrhosis: another protective mechanism against bleeding? Am J Gastroenterol 2009;104:3115–3116 [DOI] [PubMed] [Google Scholar]
- 94.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 2012;302:G168–G175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol 2012;303:G675–G685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS ONE 2013;8:e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kimer N, Pedersen JS, Busk TM, Gluud LL, Hobolth L, Krag A, et al. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: A randomized, double-blind, placebo-controlled trial. Hepatology 2017;65:592–603 [DOI] [PubMed] [Google Scholar]
- 98.Bajaj JS, Gillevet PM, Patel NR, Ahluwalia V, Ridlon JM, Kettenmann B, et al. A longitudinal systems biology analysis of lactulose withdrawal in hepatic encephalopathy. Metab Brain Dis 2012;27:205–215 [DOI] [PubMed] [Google Scholar]
- 99.Riggio O, Varriale M, Testore GP, Di Rosa R, Di Rosa E, Merli M, et al. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. J Clin Gastroenterol 1990;12:433–436 [DOI] [PubMed] [Google Scholar]
- 100.Mortensen PB, Holtug K, Bonnen H, Clausen MR. The degradation of amino acids, proteins, and blood to short-chain fatty acids in colon is prevented by lactulose. Gastroenterology 1990;98:353–360 [DOI] [PubMed] [Google Scholar]
- 101.Pinzone MR, Celesia BM, Di Rosa M, Cacopardo B, Nunnari G. Microbial translocation in chronic liver diseases. Int J Microbiol 2012;2012:694629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gorbach SL. Probiotics and gastrointestinal health. Am J Gastroenterol 2000;95:S2–S4 [DOI] [PubMed] [Google Scholar]
- 103.Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 2005;39:540–543 [DOI] [PubMed] [Google Scholar]
- 104.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther 2014;39:1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wiest R, Chen F, Cadelina G, Groszmann RJ, Garcia-Tsao G. Effect of Lactobacillus-fermented diets on bacterial translocation and intestinal flora in experimental prehepatic portal hypertension. Dig Dis Sci 2003;48:1136–1141 [DOI] [PubMed] [Google Scholar]
- 106.Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 2014;147:1327–1337 e1323 [DOI] [PubMed] [Google Scholar]
- 107.Rincon D, Vaquero J, Hernando A, Galindo E, Ripoll C, Puerto M, et al. Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int 2014;34:1504–1512 [DOI] [PubMed] [Google Scholar]
- 108.Ferrere G, Wrzosek L, Cailleux F, Turpin W, Puchois V, Spatz M, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol 2017;66:806–815 [DOI] [PubMed] [Google Scholar]
- 109.Rodriguez EA, Carey EJ, Lindor KD. Emerging treatments for primary sclerosing cholangitis. Expert Rev Gastroenterol Hepatol 2017;11(5):451–459 [DOI] [PubMed] [Google Scholar]
- 110.Haque TR, Barritt ASt. Intestinal microbiota in liver disease. Best Pract Res Clin Gastroenterol 2016;30:133–142 [DOI] [PubMed] [Google Scholar]
- 111.Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology 2016;64:1265–1272 [DOI] [PubMed] [Google Scholar]
- 112.Bajaj JS, Cox IJ, Betrapally NS, Heuman DM, Schubert ML, Ratneswaran M, et al. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol 2014;307:G951–G957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, et al. Proton pump inhibitors increase risk for hepatic encephalopathy in patients with cirrhosis in a population study. Gastroenterology 2017;152:134–141 [DOI] [PubMed] [Google Scholar]
- 114.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004;39:1441–1449 [DOI] [PubMed] [Google Scholar]