Abstract
Background
Medical cannabis use is an emerging topic of interest in orthopedics. Although there is a large amount of literature on medical cannabis use for managing various types of pain, few studies have focused on orthopedic conditions. There is little high-quality evidence in core orthopedic areas. The objective of this study was to summarize the literature on the efficacy of cannabis use for pain related to orthopedic conditions.
Methods
We conducted a systematic review of the literature on the use of cannabinoids for pain management in core orthopedic conditions. Two independent reviewers extracted information on reporting quality, risk of bias, drugs, population, control, duration of study, pain outcomes and the authors’ conclusions regarding efficacy for pain outcomes.
Results
We identified 33 orthopedic studies, including 21 primary studies and 12 reviews. Study quality was generally low to moderate. Six of the included studies had a control group and 15 were noncontrolled studies. Methodologies, drugs and protocols of administration varied greatly across studies. Study conclusions were generally positive in noncontrolled studies and mixed in controlled studies. Studies using higher doses tended to conclude that cannabis use was effective, but the potential for harmful effects may also be increased with higher doses.
Conclusion
Variability in the methodologies used in cannabis research makes it challenging to draw conclusions about dosing, routes and frequency of administration. Most of the existing evidence suggests that medical cannabis use is effective, but this efficacy has been demonstrated only when either there is no comparator or cannabis is compared with placebo. Studies using an active comparator have not demonstrated efficacy. Future research should focus on improving study reporting and methodologic quality so that protocols that optimize pain control while minimizing harmful effects can be determined.
Abstract
Contexte
La consommation de cannabis à des fins médicales est un sujet d’intérêt émergent en orthopédie. Malgré l’existence d’un important corpus de littérature médicale sur l’utilisation du cannabis pour traiter divers types de douleurs, peu d’études ont porté sur les problèmes orthopédiques. On dispose de peu de données probantes de grande qualité relatives aux principaux domaines de l’orthopédie. L’objectif de cette étude était de résumer la littérature sur l’efficacité du cannabis à soulager les douleurs orthopédiques.
Méthodes
Nous avons réalisé une revue systématique de la littérature sur l’utilisation des cannabinoïdes pour la prise en charge de la douleur associée aux principaux problèmes orthopédiques. Deux examinateurs indépendants ont extrait l’information sur la qualité des rapports, le risque de biais, les médicaments, les populations et groupes témoins, la durée des études, les scores de douleur et les conclusions des auteurs quant à l’efficacité au plan des scores de douleur.
Résultats
Nous avons recensé 33 études orthopédiques, dont 21 études primaires et 12 revues. La qualité des études était généralement de faible à moyenne. Six des études incluses étaient contrôlées et 15 ne l’étaient pas. Les méthodologies, les médicaments et les protocoles d’administration variaient grandement d’une étude à l’autre. Les conclusions étaient généralement positives dans les études non contrôlées, et mixtes dans les études contrôlées. Les études qui utilisaient des doses plus fortes avaient tendance à conclure que le cannabis était efficace, mais le risque d’effets négatifs pouvait également être proportionnel à la dose.
Conclusion
En raison de la variabilité des méthodologies utilisées dans la recherche sur le cannabis, il est difficile de tirer des conclusions sur la posologie, les voies et la fréquence d’administration. La plupart des preuves disponibles donnent à penser que le cannabis médical est efficace, mais cette efficacité n’a été démontrée que s’il n’y avait pas de comparateur ou si le cannabis était comparé à un placebo. Les études ayant utilisé un comparateur actif n’ont pas fait état d’efficacité. La recherche future devrait veiller à améliorer les rapports et la qualité méthodologique des études afin de déterminer quels protocoles améliorent la maîtrise de la douleur tout en réduisant les effets négatifs.
Medical cannabis is an emerging topic of interest in the field of orthopedics. Given the recent focus on the dangers of opioids1,2 and the trend toward legalizing medical and recreational cannabis use in Canada and some American states,3 there is a need for safe strategies to manage pain in orthopedic conditions. Osteoarthritis pain affects 27 million people in the United States,4 back pain affects one-quarter of Americans5 and up to 40% of the pain of patients with chronic pain originated from trauma or surgery.6 The pain of patients with orthopedic conditions can be complicated and difficult to treat because it can be chronic and/or acute, nociceptive, inflammatory and/or neuropathic. As populations age internationally, there is concern that the burden of pain related to orthopedic conditions will increase7 and therefore there will be an increased need for alternative and adjuvant strategies to manage pain associated with orthopedic conditions. Medical cannabis use has been hypothesized as a possible solution both to help control pain and to reduce opioid use.
There has been a considerable amount of research on medical cannabis use, particularly for the management of pain. A systematic review of cannabis use for any indication concluded that cannabis is a promising medication for pain as well as several other conditions/symptoms.8 For example, a controlled clinical trial found that vapourized cannabis improves neuropathic pain,9 and several studies have investigated the role of cannabis in fibromyalgia10 and spasticity caused by multiple sclerosis.11 Additionally, cannabis use may play a role in reducing opioid, alcohol and illicit drug use among patients with pain.12 There is also some preliminary evidence that medical cannabis use can help patients with pain related to orthopedic conditions. Blake and colleagues13 found that the pain of patients with rheumatoid arthritis was significantly improved when they used cannabis, and the authors identified the need for further investigation in this area.
Despite a large body of evidence, previous studies have not been able to identify the optimal type of cannabis, dosage, route and frequency of administration. Previous systematic reviews on medical cannabis use have not focused on orthopedic conditions. A recent scoping review on medical cannabis use for the management of musculoskeletal pain identified a need for further high-quality studies in 4 key orthopedic areas: arthritis, back pain, postsurgical pain and posttrauma pain.14
The objective of this study was to summarize the literature on the efficacy of cannabis use for pain related to orthopedic conditions. We focused on the efficacy of cannabis use in the context of the methodologies used in the published cannabis literature. We further identified which protocols of cannabis administration (e.g., dose, route, frequency, type), comparators and outcomes are commonly used.
Methods
We conducted a systematic review of the available literature on the use of cannabinoids for pain management in core orthopedic conditions (posttrauma pain, postsurgery pain, back pain and arthritis) as part of a large scoping review.14
Literature search
On the basis of previous systematic reviews in the field, we developed a systematic search strategy of the MEDLINE, Embase, PsycINFO and Cochrane databases using keywords related to cannabis, marijuana or related cannabinoid terms AND pain search terms on May 1, 2017. We used medical subject heading (MeSH) terms wherever possible. We did not use any language or date limits. Full search strategies for each database were previously reported.14 We used the Ovid search interface and RefWorks software to manage the references.
Study eligibility
Using DistillerSR systematic review management software (evidencepartners.com), 2 of 4 reviewers (K.M., A.G., N.J.V., F.M.B.) independently reviewed each title and abstract for eligibility. Disagreements resulted in inclusion in the next stage. At the full-text stage, 2 reviewers independently reviewed full-text papers and disagreements were resolved by discussion and consultation with a senior author (M.B.) if necessary. Inclusion criteria included the following: (a) primary clinical research in humans (i.e., not animal or basic science studies or nonsystematic reviews), (b) treatment with a cannabis-based medication (i.e., a medication containing or derived from tetrahydrocannabinol [THC] and/or cannabidiol [CBD]), (c) conducted in a therapeutic context (i.e., not recreational) regarding pain management and (d) not exclusively about cancer pain. We did not have any restrictions on study design other than the fact that we excluded nonsystematic reviews. At the final stage, we excluded all studies that were not related to 1 of 4 core orthopedic topics: (a) posttrauma pain, (b) post-surgical pain, (c) back pain and (d) arthritis.
Data extraction
We used DistillerSR software to design a study-specific data extraction form. We extracted reporting quality; risk of bias/methodologic quality; drug name, dose, route and frequency; the population; control; duration of study; pain outcomes; and the authors’ conclusions on efficacy relating to pain.
Reporting quality
Wherever possible, we selected well-used reporting quality tools for each study type included in this review, which are endorsed by the Enhancing the Quality and Transparency of Research (EQUATOR) network (equator-network.org). The EQUATOR network is an international organization dedicated to improving the reporting quality of health research by developing and promoting the use of reporting guidelines. For randomized controlled trials (RCTs), we used the Consolidated Standards of Reporting Trials (CONSORT) checklist;15 for cohort studies, we used Strengthening the Reporting of Observational Studies in Epidemiology (STROBE);16 for systematic reviews, we used Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA);17 for qualitative studies, we used Standards of Reporting for Qualitative Research (SRQR);18 and for case series and case reports, we used the Case Report (CARE) checklist.19 Surveys do not have a well-established reporting guideline, so we used the “Good practice in the conduct and reporting of survey research” list developed by Kelley and colleagues.20 We scored each item on the checklists as adequately reported, not reported or not applicable. For example, some trials did not have binary outcomes, so they were exempt from reporting both absolute and relative effects and were scored as not applicable. We did not count items that were not applicable in the denominator when calculating the percentage of adequately reported items.
Risk of bias and methodologic quality
We used the Cochrane Risk of Bias tool21 and the Cochrane Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool22 to assess the methodologic quality of randomized trials and observational studies, respectively. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines to assess the credibility of systematic reviews and meta-analyses.23
Statistical analysis
All data are presented descriptively as means and standard deviations where possible for continuous data and as frequencies and percentages for categorical data. We could not perform a meta-analysis because of differences in the drugs, doses, routes, frequencies, populations, comparison drugs and outcomes used across studies. Instead, for studies that had a comparsion group, we summarized study conclusions for pain outcomes as (a) cannabis performed better than comparator for pain outcomes, (b) cannabis performed worse than comparator for pain outcomes or (c) no difference on pain outcomes. For noncontrolled studies such as case series and surveys, we categorized studies on the basis of the authors’ conclusions as (a) cannabis performed well in a noncontrolled study, (b) cannabis did not perform well in a noncontrolled study or (c) inconclusive or mixed results in a noncontrolled study.
Results
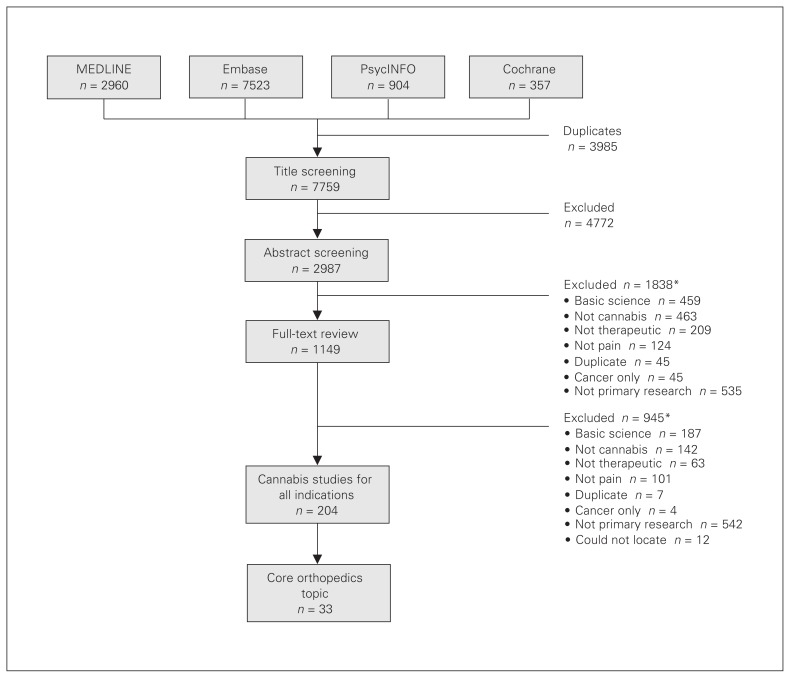
After removal of duplicates we reviewed 7759 potentially eligible studies, of which 118 studies assessed cannabis use for treatment of general musculoskeletal pain. We included 33 core orthopedic studies in the current review, but we focused on 21 primary studies because the 12 systematic reviews included mostly overlapping studies. The full study flow diagram is shown in Figure 1.
Fig. 1.
Study flow diagram. *Studies can be excluded for multiple reasons.
Reporting quality
Reporting quality was relatively low to moderate for all study designs (Table 1). The mean percentage of correctly reported items was 69% for RCTs, 65% for observational studies and 65% for systematic reviews (means exclude studies that were abstracts only). Some individual studies performed well on the reporting tools; 5 studies scored 80% or higher,24–28 including 1 study that scored 100%.26 However, 6 studies scored 50% or lower (excluding abstracts).13,29–33
Table 1.
Reporting quality of included studies
| Study type; study | Adequately reported items, no. * | Adequately reported items, % |
|---|---|---|
| Randomized controlled trials | ||
| Beaulieu et al. 200634 | 24/35 | 65.6 |
| Blake et al. 200613 | 15/34 | 44.1 |
| Frank et al. 200835 | 27/34 | 79.4 |
| Kantor and Hopper 198150† | 2/33 | 6.0 |
| Levin et al. 201724 | 31/35 | 88.6 |
| Nonrandomized studies | ||
| Cohort study | ||
| Holdcroft et al. 200629 | 14/33 | 42.4 |
| Case series and case reports | ||
| Aggarwal et al. 200930 | 13/26 | 50.0 |
| Barbosa-Hernandez et al. 201331† | 10/26 | 38.5 |
| Gofeld et al. 200536 | 14/26 | 53.8 |
| Haroutiunian et al. 201152† | 8/26 | 30.8 |
| Haroutiunian et al. 200831 | 11/26 | 42.3 |
| Haroutiunian et al. 201637 | 19/26 | 73.0 |
| Hornby et al. 200938 | 17/26 | 65.4 |
| Ware et al. 200239 | 18/26 | 69.2 |
| Surveys | ||
| Harris et al. 200053 | 6/10 | 60.0 |
| Hazekamp et al. 201325 | 8/10 | 80.0 |
| Piper et al. 201754 | 6/10 | 60.0 |
| Ste-Marie et al. 201626 | 10/10 | 100 |
| Swift et al. 200527 | 8/10 | 80.0 |
| Walsh et al. 201328 | 8/10 | 80.0 |
| Qualitative study | ||
| Peters 201355 | 11/21 | 52.4 |
| Systematic reviews | ||
| Campbell et al. 200145 | 18/25 | 72.0 |
| Covarrubias-Gomez 200848† | 5/25 | 20.0 |
| Deshpande et al. 201541 | 17/25 | 68.0 |
| Fitzcharles et al. 201642 | 18/25 | 72.0 |
| Hwang et al. 201632 | 11/25 | 44.0 |
| Khaiser et al. 201633 | 12/25 | 48.0 |
| Kung et al. 201149† | 6/27 | 22.2 |
| Lynch and Ware 201546 | 16/25 | 64.0 |
| MacFarlane et al. 201147 | 16/25 | 64.0 |
| Martín-Sánchez et al. 200943 | 21/27 | 77.8 |
| Stevens and Higgins 201744 | 20/26 | 76.9 |
| Wang et al. 200840 | 17/25 | 68.0 |
Denominators may differ across studies because we judged some items to be not applicable and did not count them in the denominator when we calculated the percentage of adequately reported items.
Abstract only.
Methodologic quality
The methodologic quality of the included RCTs was mostly unclear, particularly for sequence generation, allocation concealment and blinding outcome assessors. We rated 3 studies as being at high risk of bias for “other bias” owing to potential conflicts of interest.13,34,35 One RCT achieved a low risk of bias rating on all domains.24 Most observational studies were rated as being at high risk of bias for confounding, selection and measurement bias. We also identified selective outcome reporting bias in 5 studies.30,36–39 No observational studies achieved a low risk of bias rating in each domain. Most systematic reviews were rated as very low (2 studies32,40) or low quality (5 studies32,41–44). Three systematic reviews achieved a GRADE rating of moderate quality,45–47 but none were considered high quality. We were unable to assess the GRADE quality of 2 systematic reviews48,49 because only abstracts were available. Most reasons for downgrading the quality were because of risk of bias, indirectness and inconsistency (Table 2, Table 3, Table 4).
Table 2.
Bias and methodologic quality of the randomized controlled trials included in this review
| Study | Quality indicator | ||||||
|---|---|---|---|---|---|---|---|
| Sequence generation | Allocation concealment | Blinding participants | Blinding assessors | Incomplete outcomes | Selective reporting | Other bias | |
| Beaulieu et al. 200634 | ? | ? | + | ? | ? | ? | − |
| Blake et al. 200613 | ? | ? | ? | ? | + | + | − |
| Frank et al. 200835 | ? | ? | + | ? | − | + | − |
| Kantor and Hopper 198150* | ? | ? | ? | ? | ? | ? | ? |
| Levin et al. 201724 | + | + | + | + | + | + | + |
+ = low risk of bias; ? = unclear risk of bias; − = high risk of bias.
Abstract only.
Table 3.
Bias and methodologic quality of nonrandomized studies included in this review
| Study type; study | Quality indicator | |||||||
|---|---|---|---|---|---|---|---|---|
| Confounding | Selection | Classification | Intervention deviation | Missing data | Measurement | Selective reporting | Other | |
| Cohort study | ||||||||
| Holdcroft et al. 200629 | + | ? | + | + | + | + | ? | − |
| Case series/case reports | ||||||||
| Aggarwal et al. 200930 | ? | − | ? | ? | + | − | − | ? |
| Barbosa-Hernandez et al. 201331* | ? | ? | ? | ? | ? | ? | ? | ? |
| Gofeld et al. 200536 | ? | − | + | + | + | − | − | ? |
| Haroutiunian et al. 201152* | ? | ? | ? | ? | ? | ? | ? | ? |
| Haroutiunian et al. 200831 | − | ? | + | ? | − | ? | ? | ? |
| Haroutiunian et al. 201637 | − | − | + | − | − | + | + | + |
| Hornby et al. 200938 | − | − | + | + | + | + | − | + |
| Ware et al. 200239 | − | − | + | + | + | − | − | + |
| Survey | ||||||||
| Harris et al. 200053 | − | − | + | − | + | − | ? | + |
| Hazekamp et al. 201325 | − | − | + | + | + | − | ? | − |
| Piper et al. 201754 | − | − | + | + | ? | − | ? | − |
| Ste-Marie et al. 201626 | − | − | + | + | + | − | ? | − |
| Swift et al. 200527 | − | − | + | + | + | − | ? | + |
| Walsh et al. 201328 | − | − | + | + | + | − | ? | + |
| Qualitative study | ||||||||
| Peters 201355 | − | − | + | + | + | − | − | + |
+ = low risk of bias; ? = unclear risk of bias; − = high risk of bias.
Abstract only.
Table 4.
Bias and methodologic quality of the systematic reviews included in this review
| Study | Design of the included studies | Quality assessment | Reason(s) for downgrading (if applicable) |
|---|---|---|---|
| Campbell et al. 200145 | RCT | +++○ Moderate |
Indirectness |
| Covarrubias-Gomez 200848* | Unclear | Not enough information | |
| Deshpande et al. 201541 | RCT | ++○○ Low |
Risk of bias, indirectness |
| Fitzcharles et al. 201642 | RCT | ++○○ Low |
Risk of bias, indirectness |
| Hwang et al. 201632 | RCT | ++○○ Low |
Risk of bias, inconsistency |
| Khaiser et al. 201633 | Observational | +○○○ Very low |
Risk of bias, indirectness |
| Kung et al. 201149* | RCT | Not enough information | |
| Lynch and Ware 201546 | RCT | +++○ Moderate |
Risk of bias |
| MacFarlane et al. 201147 | RCT | +++○ Moderate |
Publication bias |
| Martín-Sánchez et al. 200943 | RCT | ++○○ Low |
Risk of bias, indirectness |
| Stevens and Higgins 201744 | RCT | ++○○ Low |
Inconsistency, indirectness |
| Wang et al. 200840 | Observational | +○○○ Very low |
Risk of bias, inconsistency, indirectness |
RCT = randomized controlled trial.
Abstract only.
Study design
Of the 33 included studies, 5 were RCTs,13,24,34,35,50 1 was a nonrandomized intervention study,29 8 were case reports/ case series,30,31,36–39,51,52 6 were surveys,25–28,53,54 1 was a qualitative study54 and 12 were systematic reviews.32,33,40,41–49 Of the 12 systematic reviews, 9 included RCTs,32,41–47,49 2 included nonrandomized studies33,40 and 1 review48 was unclear on the design of the included studies.
Population
The 6 controlled intervention studies13,24,29,34,35,50 (5 RCTs and 1 nonrandomized intervention study) included a total of 681 patients. Of these patients, 171 (25.1%) underwent directly orthopedic-related procedures, although in 1 study50 it was unclear what type of surgery 81 patients underwent. Four of the 6 studies evaluated cannabis use for acute postsurgical pain,24,29,34,50 1 evaluated patients with rheumatoid arthritis13 and 1 evaluated chronic neuropathic pain,35 including 25 orthopedic patients (Table 5).
Table 5.
Characteristics, outcomes and conclusions of controlled studies
| Study type; study | Characteristic | Pain outcomes and conclusions* | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Drug | Dose | Route of administration | Frequency of administration | Population | Control | Duration | ||
| Randomized controlled trials | ||||||||
|
| ||||||||
| Beaulieu 200634 | Nablione | 1 mg, 2 mg | Oral capsule | Every 8 h | 41 major surgery patients (18 orthopedic) using a PCA device | Ketoprofen, placebo | 24 h | NRS (−) |
|
| ||||||||
| Blake et al. 200613 | Nabiximols | Mean 14.6 mg THC and 13.5 mg CBD | Oral spray | Daily | 58 patients with rheumatoid arthritis with pain not adequately controlled by medication | Placebo | 5 wk | NRS, McGill pain (+) |
|
| ||||||||
| Frank et al. 200835 | Nabilone | 250 μg escalating to 2 mg | Oral capsule | Daily | 96 patients with chronic neuropathic pain (25 orthopedic) | Dihydrocodeine crossover | 14 wk | VAS (−) |
|
| ||||||||
| Kantor and Hopper 198150† | Levonantradol | 1.5–3.0 mg | Oral capsule | Once | 81 postsurgical patients | Placebo | Unclear | SPID (+) |
| 0.25 mg, 0.5 mg, 1.0 mg | Intramuscular | |||||||
|
| ||||||||
| Levin et al. 201724 | Nabilone | 0.5 mg | Oral capsule | Once | 340 postsurgical patients (47 orthopedic) at risk for nausea and vomiting | Placebo | 300 min | NRS (=) |
|
| ||||||||
| Nonrandomized interventional study | ||||||||
|
| ||||||||
| Holdcroft et al. 200629 | Cannabis extract | 5 mg, 10 mg, 15 mg | Oral capsule | Once | 65 postsurgical patients (23 orthopedic) | Low compared with medium and high doses | 6 h | Rescue analgesia, VRS (+) (higher doses better than lower doses) |
CBD = cannabidiol; NR = not reported; NRS = numeric rating scale; PCA = patient-controlled analgesia; SPID = sum of pain intensity difference; THC= tetrahydrocannabinol; VAS = visual analogue scale; VRS = verbal rating scale.
(+) = cannabis performed significantly better than comparator for pain outcomes; (=) = no difference for pain outcomes; (−) = cannabis performed worse than comparator for pain outcomes.
Abstract only.
The 15 noncontrolled studies included 4629 patients. Of these patients, at least 2552 (55.1%) underwent directly orthopedic-related procedures (the numbers were unclear for 4 studies26,28,37,53). (Table 6).
Table 6.
Characteristics, outcomes and conclusions of noncontrolled studies
| Study type; study | Characteristic | Pain outcomes and conclusions* | ||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Dose | Route of administration | Frequency of administration | Population | Control | Duration | ||
| Case series/case reports | ||||||||
| Aggarwal et al. 200930 | Unspecified | Unspecified | Various | Unspecified | 139 pain clinic patients (72 back pain, 43 arthritis pain) | None | Retrospective 11 d – 8.3 yr | McGill pain (+) |
| Barbosa-Hernandez et al. 201331† | Dronabinol | 2.5 mg | Oral | Twice per d | 1 25-yr-old male, posttrauma pain, opioid-tolerant | None | 6 d | VAS (+) |
| Gofeld et al. 200536 | Nabilone | 1 mg, 2 mg | Oral | Twice per d | 1 29-yr-old male, postsurgical pain resistant to standard analgesia | None | 4 d | PCA morphine consumption (+) |
| Haroutiunian et al. 201152† | Unspecified | NR | NR | NR | 42 pain clinic patients (19% back pain) | None | 3–6 mo | BPI, pain symptoms (+) |
| Haroutiunian et al. 200831 | Cannabis extract | 5 mg | Sublingual | 2–3 times/d | 13 pain clinic patients (5 back pain, 1 joint pain, 1 unspecified bone pain) | None | 5 d – 36 mo | TOPS (=) |
| Haroutiunian et al. 201637 | Herbal cannabis, cannabis extract | 1 puff or drop | Oral drops, edibles or smoked | 1–3 times/d | 206 pain clinic patients | None | 6 mo | TOPS (+) |
| Hornby et al. 200958 | Herbal cannabis | Various | Smoked, oral capsules, and oral tincture | Various | 1 33--yr-old male, uncontrolled posttrauma pain | None | 15 mo | Unspecified pain score (+) |
| Ware et al. 200239 | Herbal cannabis | 2–8 puffs | Smoked | Various, median 4 times daily | 15 pain clinic patients (3 back pain, 2 arthritis pain, 1 unspecified MSK) | None | Cross-sectional | Perceived effectiveness (+) |
| Surveys | ||||||||
| Harris et al. 200053 | Unspecified | NR | NR | At least once/wk | 100 adults legally using medical cannabis | None | Cross-sectional | Perceived effectiveness (+) |
| Hazekamp et al. 201325 | Dronabinol, nabilone, nabiximols, vapourized THC, herbal cannabis | Various | Smoked, vapourized, sublingual or oral tincture | Various | 953 adults using cannabis as medicine (135 back pain, 59 trauma pain, 19 arthritis pain) | None | Cross-sectional | None |
| Piper et al. 201754 | Unspecified | Various | Various including smoked, vapourized, edibles and tinctures | Various | 1513 medical dispensary members (176 trauma pain, 798 back/neck pain, 200 postsurgical pain) | None | Cross-sectional | Perceived effectiveness (+) |
| Ste-Marie et al. 201626 | Herbal cannabis | Mean 1.4 g, max 5 g | Smoked, vapourized, oral and topical | Various | 1000 rheumatology patients (most arthritis pain) | 28 cannabis users v. 972 nonusers‡ | Cross-sectional | None (only baseline pain measured; VAS) |
| Swift et al. 200527 | Unspecified | NR | Edibles, tea, smoked vapourized | Various | 128 (14 back pain) | None | Cross-sectional | Perceived effectiveness (+) |
| Walsh et al. 201328 | Unspecified | Various | Smoked, vapourized oral | Various | 628 medical cannabis users (unclear number of patients with back pain and arthritis pain) | None | Cross-sectional | None |
| Qualitative study | ||||||||
| Peters 201355 | Unspecified | Various | Various, mostly smoked and oral | Various | 28 pain patients (6 postsurgical pain, 2 back pain, 6 arthritis pain, 6 hip or knee pain) | None | Cross-sectional | Patient- reported (qualitative) (+) |
BPI = brief pain inventory; MSK = musculoskeletal; NR = not reported; PCA = patient-controlled analgesia; THC = tetrahydrocannabinol; TOPS = treatment outcomes of pain survey; VAS = visual analogue scale.
(+) = cannabis performed significantly better than comparator for pain outcomes; (=) = no difference for pain outcomes.
Abstract only.
This study technically had a control group; however, we included it with the noncontrolled studies because it assessed only the demographic characteristics of cannabis users versus nonusers; there was no comparison of pain outcomes across groups.
Outcomes measured
The 6 controlled intervention studies used 6 methods to measure pain outcomes: a numeric rating scale (NRS), a verbal rating scale (VRS), a visual analogue scale (VAS), the sum of pain intensity differences (SPID), the McGill pain questionnaire and the amount of rescue analgesia required (Table 5).
The noncontrolled study used 7 methods to measure pain outcomes: the McGill pain questionnaire, patient-controlled analgesia (PCA) morphine required, perceived effectiveness, the brief pain inventory (BPI), the treatment outcomes of pain survey (TOPS), a VAS and a qualitative interview asking about pain. In 1 study38 the method used to measure the pain outcome was unspecified and in 3 studies25,26,28 there were no pain outcomes (Table 6).
Efficacy — controlled studies
There were only 6 controlled studies, including 5 RCTs and a nonrandomized intervention study (Table 5). Three studies evaluated the efficacy of nabilone,24,34,35 an oral synthetic cannabinoid. One study evaluated levonantradol, 50 another synthetic cannabinoid that can be administered orally or intramuscularly. One study evaluated nabiximols13 (trade name Sativex), which is an oral cannabis extract spray that delivers a set dosage of THC and CBD per spray. The nonrandomized study29 evaluated an oral capsule containing cannabis extract (trade name Cannador).
Oral nabilone
Beaulieu,34 Frank and colleagues35 and Levin and colleagues24 conducted RCTs to evaluate nabilone. These 3 studies included 477 patients who underwent surgery or who had chronic neuropathic pain, only 90 of whom were orthopedic patients. One study was a dose-escalation study evaluating doses of 250 μg to 2 mg and the other 2 studies evaluated 0.5 mg, 1 mg and 2 mg doses. All studies used oral capsules. The frequency of administration also varied greatly between the 3 studies, with 1 study only administering nabilone once, 1 study administering the drug once daily and 1 study administering the drug 3 times daily. One study used a placebo comparator, 1 study used an active comparator (dihydrocodeine) and 1 study had a placebo arm and an active comparator arm (ketoprofen). The studies were generally short in duration, ranging from 300 minutes to 14 weeks. All 3 studies had a pain outcome, using 2 different pain rating scales (VAS pain and NRS pain). None of the 3 studies showed a significant improvement in pain symptoms. In the 2 studies with active comparators, cannabis performed worse than the active comparator in terms of pain relief. Additionally, nabilone had more side effects than dihydrocodeine and a similar number of side effects compared with ketoprofen.
Nabiximols oral spray
One study13 evaluated nabiximols as an oral spray. Participants started with 1 spray per day before bed and were allowed to increase this to 6 sprays as tolerated. Sprays delivered 2.7 mg THC and 2.5 mg CBD each. Participants used a mean of 14.6 mg THC and 13.5 mg CBD per day by the end of the study period. This study comprised 58 patients with rheumatoid arthritis whose regular medication was not adquately controlling their pain. The study used a placebo as a comparator. The study duration was 5 weeks. Nabiximols use showed an improvement in pain control on the McGill pain score and NRS pain compared with placebo, and there were fewer dropouts from the nabiximols group because of adverse events than from the placebo group.
Oral and intramuscular levonantradol
One study50 evaluated the use of levonantradol as intramuscular injections. Patients who had undergone surgery (n = 61) were randomly assigned to receive 1-time doses of 1 of 3 strengths of intramuscular levonantradol (0.25 mg, 0.5 mg or 1.0 mg) or placebo. It is unclear how many of these patients underwent orthopedic surgery. The 0.25 mg dose of levonantradol performed similarly to placebo, but the higher doses had analgesic effects. The authors warned that there may have been adverse effects on the central nervous system, although they were mild. The authors also stated that 20 patients were given 1.5 to 3.0 mg of oral levonantradol but only preliminary results of this investigation were reported.
Oral cannabis extract
One study29 evaluated an oral cannabis extract in capsule form. This was a nonrandomized dose-escalation study evaluating 1-time administration of 5 mg, 10 mg or 15 mg of cannabis. This study included 65 patients who had undergone surgery, 23 of whom had undergone orthopedic surgical procedures. Although the study evaluated only the effect of cannabis over 6 hours, higher doses reduced pain significantly better than low doses on the basis of the amount of rescue analgesia needed and scores on VRS pain scales.
Efficacy — noncontrolled studies
Fifteen studies were noncontrolled. These studies did not specify a predefined dose, route frequency or study drug (Table 6). As such, the characteristics of the cannabis medications varied greatly. Seven studies did not specify the type of cannabis used.27,28,30,52–55 Five studies investigated herbal cannabis or extracts,26,31,37–39 1 studied dronabinol,51 1 studied nabilone36 and 1 study25 had a mix of dronabinol, nabilone, nabiximols, vapourized THC and herbal cannabis. Most of the studies had various or unspecified doses and frequencies of administration. Routes of administration also varied greatly and included oral capsules, edibles, tinctures, oral spray, inhaled (both smoked and vapourized) and topical. Eight studies were cross-sectional and the remaining 7 studies had durations of 4 days to 8.3 years.
Of the 8 case series/case reports, 7 concluded that cannabis use reduced patients’ pain from baseline.30,36–39,51,52 The remaining study31 found that sublingual cannabis use did not significantly reduce patients’ pain from baseline. Results of 3 of the 6 included surveys indicated that patients were satisfied with cannabis use as a means of reducing their pain.27,53,54 Pain outcomes were not reported for the remaining 3 surveys.25,26,28 The qualitative study found that patients were satisfied with using cannabis to treat their pain.54
Results from previous systematic reviews can be found in Appendix 1 (available at canjsurg.ca/001018-a1).
Route of administration and dose
Table 7 shows a summary of study conclusions by route of administration and dose, for all studies where a specific route and dose could be identified. This table shows a lack of comparisons with an active comparator. Only nabilone oral capsules were compared with an active comparator, and all studies showed that cannabis performed worse than the active comparator. Conclusions were generally positive (5/7 positive conclusions for oral capsules). Generally, higher doses performed better than lower doses. Three doses of intramuscular levonantradol were identified, with higher doses performing better than lower doses. Smoked cannabis was evaluated only in noncomparative studies, but the conclusions of these studies were positive. Although oral spray was evaluated in only 1 study, the conclusions were also positive. Sublingual oil was also evaluated in only 1 noncomparative study, with mixed results.
Table 7.
Summary of study conclusions by route of administration and dose
| Route of administration | Comparison; dose and conclusion* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cannabis v. placebo (or no comparison) | Cannabis v. active comparator | ||||||||
| Oral capsule | 0.5 mg nabilone (=) | 1–2 mg nabilone (+) | 2.5 mg dronabinol (+) | 1.5–3 mg levonantradol (++) | 5 mg extract (−) | 10 mg extract (++) | 15 mg extract (++) | 1 mg nabilone (−) | 2 mg nabilone (− −) |
| Intramuscular | 0.25 mg levonantradol (=) | 0.5 mg levonantradol (++) | 1 mg levonantradol (++) | — | — | — | — | — | — |
| Smoked 1 puff (+) | 2–8 puffs (+) | — | — | — | — | — | — | — | |
| Oral spray | 14.6 mg THC/13.5 mg CBD nabiximols (++) | — | — | — | — | — | — | — | — |
| Sublingual oil | 5 mg extract (==) | — | — | — | — | — | — | — | — |
(++) = cannabis performed significantly better than comparator for pain outcomes; (+) = cannabis performed well in a noncontrolled study; (=) = inconclusive or no difference on pain outcomes; (==) inconclusive or mixed results in a noncontrolled study; (−) = cannabis performed worse than comparator for pain outcomes; (− −) = cannabis did not perform well in a noncontrolled study; CBD = cannabidiol; THC = tetrahydrocannabinol.
This table includes studies where the dosage and route are clear.
Discussion
This systematic review of medical cannabis use for the management of pain in orthopedic conditions assessed the efficacy of medical cannabis use with a particular focus on study methodology. Although it appears on the surface that there is a large body of medical cannabis literature, we found that there is a paucity of literature focusing on orthopedic conditions like arthritis pain, postsurgical pain, posttrauma pain and back pain. One concern is that although most of the existing evidence suggests that medical cannabis use is effective, this efficacy has been demonstrated only when either there is no comparator or cannabis is compared with placebo. Studies using an active comparator do not demonstrate efficacy. We have identified a need for improved reporting of study methodology and methodologic quality. Many of the studies included in our review were noncomparative and were therefore limited in terms of the evidence that they could provide for the efficacy of medical cannabis. Most comparative studies included a small number of patients, an even smaller number of whom were orthopedic patients (25% of patients in comparative studies and 55% of patients in noncomparative studies had conditions that were directly orthopedic related). Despite these limitations in the current body of evidence, the overall results provide preliminary evidence that cannabinoids are effective as an intervention for pain management and justify the need for future larger studies in the area.
A large degree of heterogeneity is present in the literature because of differences in the drugs, doses, routes, frequencies, populations, comparison drugs and outcomes across studies. As a result, we were unable conduct a metaanalysis and provide a single estimate of the pooled results. However, we are able to provide some qualitative evidence that higher doses of cannabis, in general, had better analgesic properties than lower doses. Oral capsules are the most well-studied route of administration. They typically performed well when compared with placebo, but they performed worse than active comparators. More information is needed from comparisions of cannabis with standard medications as well as from studies using cannabis as an adjunct to standard pain medications.
In this systematic review we did not focus on the harmful effects of cannabis use, although we acknowledge that benefits must be studied alongside harms in clinical trials. A previous systematic review focusing on the harmful effects of cannabis use found that 96.6% of the harmful effects of cannabis use are not serious, and there is no evidence that serious adverse events are more common among patients given cannabis than among patients in control groups (rate ratio 1.04, 95% confidence interval 0.79–1.39).40 However, most studies followed patients for only a short time.40 The most common nonserious harmful effects included neurologic disorders, gastrointestinal disorders and administration-site conditions.40 Any future studies should attempt to find an administration protocol that maximizes benefit and minimizes harm. Wang and colleagues40 found that many studies did not fully report harms, so this should be a priority for future research.
The strengths of this scoping review include the fact that we conducted an exhaustive literature search in duplicate, using several medical databases. Additionally, we were able to include a broad range of study designs. We also included all published abstracts where a full-text article was not available. These strengths also lead to a key limitation: we were unable to conduct a meta-analysis because of heterogeneity, so we present a qualitative summary of study conclusions with a particular focus on methodology (e.g., study design, comparator, dosage, route of administration). This summary gives readers a comprehensive overview of the available literature on the topic.
More studies focusing on orthopedic patients are required to assess the efficacy of cannabinoids in pain management. Further large, high-quality studies are needed as there have been few controlled studies in this population. International collaboration on large, high-quality studies will contribute to the generalizability of study results and will improve researchers’ ability to recruit large numbers of patients. Most of the studies included in our study were of short duration; more long-duration studies are needed to assess efficacy in chronic conditions like arthritis. Most studies used a placebo or no comparator rather than an active comparator. Future studies could include an active comparator arm if the aim is to demonstrate the efficacy of cannabis compared with standard medications. The next steps for research in this field should include identifying the optimal dosing and methods of administration of cannabis, evaluating the cost-effectiveness of cannabis relative to other pain treatments, evaluating the efficacy of cannabis compared with and in conjunction with standard pain medications and assessing patients’ preferences regarding medical cannabis use.
Conclusion
There is minimal high-quality evidence for the efficacy of medical cannabis in pain management within the core orthopedic areas of arthritis pain, postsurgical pain, back pain and posttrauma pain. Although most of the existing evidence suggests that medical cannabis use is effective, this efficacy has been demonstrated only when either there is no comparator or cannabis is compared with placebo. Studies using an active comparator have not demonstrated the efficacy of cannabis use. Additionally, more studies are required to determine factors such as optimal dosing and method of administration. Variability in the methodologies of cannabis research makes it difficult to gain insights about dosing, routes and frequency of administration. Future research should improve reporting and methodologic quality so that protocols that optimize pain control while minimizing harmful effects can be determined.
Acknowledgements
The authors thank Ms. Tristiana Dalchand, Ms. Simrun Chona, Mr. Joshua George and Dr. Hassan Baldawi for their assistance with article screening.
Footnotes
Funding: K. Madden is funded by a Canadian Institutes of Health Research (CIHR) doctoral award (GSD-134929). M. Bhandari is funded in part by a Canada Research Chair. Funding for this program of research was provided by Beleave Inc. (a licensed cannabis producer). The funders played no role in the design or execution of the study or in the analysis or interpretation of the findings.
Competing interests: A. George and N. van der Hoek declare no competing interests. K. Madden received an honorarium to prepare this review from OrthoEvidence Inc., an orthopedics knowledge translation company. At the time of this study, G. Mammen was employed as a clinical research and collaboration liaison for Beleave Inc. (a licensed cannabis producer) for 6 months to develop consumer-focused educational content; his compensation was not tied in any way to the findings of this study. M. Bhandari received a grant from Beleave Inc. during the conduct of this study.
Contributors: K. Madden and M. Bhandari designed the study. K. Madden, A. George, N. van der Hoek and F. Borim acquired the data, which K. Madden, A. George, G. Mammen and M. Bhandari analyzed. K. Madden, A. George, N. van der Hoek and M. Bhandari wrote the article, which K. Madden, A. George, F. Borim, G. Mammen and M. Bhandari reviewed. All authors approved the article for publication and agreed to be accountable for all aspects of the work.
References
- 1.Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189:E659–66. doi: 10.1503/cmaj.170363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy M. Opioids should be last resort to treat chronic pain, says draft CDC guideline. BMJ. 2015;351:h6905. doi: 10.1136/bmj.h6905. [DOI] [PubMed] [Google Scholar]
- 3.Ko GD, Bober SL, Mindra S, et al. Medical cannabis — the Canadian perspective. J Pain Res. 2016;9:735–44. doi: 10.2147/JPR.S98182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plotnikoff R, Karunamuni N, Lytvyak E, et al. Osteoarthritis prevalence and modifiable factors: a population study. BMC Public Health. 2015;15:1195. doi: 10.1186/s12889-015-2529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manchikanti L, Singh V, Falco FJ, et al. Epidemiology of low back pain in adults. Neuromodulation. 2014;17(Suppl 2):3–10. doi: 10.1111/ner.12018. [DOI] [PubMed] [Google Scholar]
- 6.Crombie IK, Davies HT, Macrae WA. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain. 1998;76:167–71. [PubMed] [Google Scholar]
- 7.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21:1145–53. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowal MA, Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2010–2014. Cannabinoids. 2016;11:1–18. [Google Scholar]
- 9.Wilsey B, Marcotte TD, Deutsch R, et al. Low dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136–48. doi: 10.1016/j.jpain.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walitt B, Klose P, Fitzcharles MA, et al. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7:CD011694. doi: 10.1002/14651858.CD011694.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184:1143–50. doi: 10.1503/cmaj.110837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas P, Walsh Z, Crosby K, et al. Substituting cannabis for prescription drugs, alcohol and other substances among medical cannabis patients: The impact of contextual factors. Drug Alcohol Rev. 2016;35:326–33. doi: 10.1111/dar.12323. [DOI] [PubMed] [Google Scholar]
- 13.Blake DR, Robson P, Ho M, et al. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45:50–2. doi: 10.1093/rheumatology/kei183. [DOI] [PubMed] [Google Scholar]
- 14.Madden K, van der Hoek NJ, Baldawi H, et al. Cannabinoids in the management of musculoskeletal pain: a scoping review. JBJS Rev. 2018;6(5):e7. doi: 10.2106/JBJS.RVW.17.00153. [DOI] [PubMed] [Google Scholar]
- 15.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245–51. doi: 10.1097/ACM.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 19.Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013;2:38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley K, Clark B, Brown V, et al. Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003;15:261–6. doi: 10.1093/intqhc/mzg031. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin DN, Dulberg Z, Chan AW, et al. A randomized-controlled trial of nabilone for the prevention of acute postoperative nausea and vomiting in elective surgery. Can J Anaesth. 2017;64:385–95. doi: 10.1007/s12630-017-0814-3. [DOI] [PubMed] [Google Scholar]
- 25.Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids — an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199–210. doi: 10.1080/02791072.2013.805976. [DOI] [PubMed] [Google Scholar]
- 26.Ste-Marie PA, Shir Y, Rampakakis E, et al. Survey of herbal cannabis (marijuana) use in rheumatology clinic attenders with a rheumatologist confirmed diagnosis. Pain. 2016;157:2792–7. doi: 10.1097/j.pain.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 27.Swift W, Gates P, Dillon P. Survey of Australians using cannabis for medical purposes. Harm Reduct J. 2005;2:18. doi: 10.1186/1477-7517-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh Z, Callaway R, Belle-Isle L, et al. Cannabis for therapeutic purposes: patient characteristics, access, and reasons for use. Int J Drug Policy. 2013;24:511–6. doi: 10.1016/j.drugpo.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Holdcroft A, Maze M, Doré C, et al. A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology. 2006;104:1040–6. doi: 10.1097/00000542-200605000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal SK, Carter GT, Sullivan MD, et al. Characteristics of patients with chronic pain accessing treatment with medical cannabis in Washington State. J Opioid Manag. 2009;5:257–86. doi: 10.5055/jom.2009.0028. [DOI] [PubMed] [Google Scholar]
- 31.Haroutiunian S, Rosen G, Shouval R, et al. Open-label, add-on study of tetrahydrocannabinol for chronic nonmalignant pain. J Pain Palliat Care Pharmacother. 2008;22:213–7. doi: 10.1080/15360280802251215. [DOI] [PubMed] [Google Scholar]
- 32.Hwang JK, Clarke H. Cannabis and pain: a review. J Pain Manag. 2016;9:395–413. [Google Scholar]
- 33.Khaiser M, Peng M, Ahrari S, et al. Medical cannabis dosing strategies in pain-related conditions: a scoping review of current literature. J Pain Manag. 2016;9:449–63. [Google Scholar]
- 34.Beaulieu P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can J Anaesth. 2006;53:769–75. doi: 10.1007/BF03022793. [DOI] [PubMed] [Google Scholar]
- 35.Frank B, Serpell MG, Hughes J, et al. Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ. 2008;336:199–201. doi: 10.1136/bmj.39429.619653.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gofeld M, Robinson S, Faclier G. Administration of nabilone for postoperative pain control in the marijuana-addicted: case study. Acute Pain. 2006;8:29–32. [Google Scholar]
- 37.Haroutounian S, Ratz Y, Ginosar Y, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain. 2016;32:1036–43. doi: 10.1097/AJP.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 38.Hornby AP, Sharma M, Stegman B. Standardized natural product cannabis in pain management and observations at a Canadian compassion society: a case report. Cases J. 2009;2:7487. doi: 10.1186/1757-1626-2-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ware MA, Gamsa A, Persson J, et al. Cannabis for chronic pain: case series and implications for clinicians. Pain Res Manag. 2002;7:95–9. doi: 10.1155/2002/380509. [DOI] [PubMed] [Google Scholar]
- 40.Wang T, Collet J-P, Shapiro S, et al. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008;178:1669–78. doi: 10.1503/cmaj.071178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deshpande A, Mailis-Gagnon A, Zoheiry N, et al. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: systematic review of randomized controlled trials. Can Fam Physician. 2015;61:e372–81. [PMC free article] [PubMed] [Google Scholar]
- 42.Fitzcharles MA, Ste-Marie PA, Häuser W, et al. Efficacy, tolerability, and safety of cannabinoid treatments in the rheumatic diseases: a systematic review of randomized controlled trials. Arthritis Care Res (Hoboken) 2016;68:681–8. doi: 10.1002/acr.22727. [DOI] [PubMed] [Google Scholar]
- 43.Martín-Sánchez E, Furukawa TA, Taylor J, et al. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009;10:1353–68. doi: 10.1111/j.1526-4637.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 44.Stevens AJ, Higgins MD. A systematic review of the analgesic efficacy of cannabinoid medications in the management of acute pain. Acta Anaesthesiol Scand. 2017;61:268–80. doi: 10.1111/aas.12851. [DOI] [PubMed] [Google Scholar]
- 45.Campbell FA, Tramèr MR, Carroll D, et al. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2001;323:13–6. doi: 10.1136/bmj.323.7303.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: an updated systematic review of randomized controlled trials. J Neuroimmune Pharmacol. 2015;10:293–301. doi: 10.1007/s11481-015-9600-6. [DOI] [PubMed] [Google Scholar]
- 47.Macfarlane GJ, El-Metwally A, De Silva V, et al. Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 2011;50:1672–83. doi: 10.1093/rheumatology/ker119. [DOI] [PubMed] [Google Scholar]
- 48.Covarrubias-Gómez A. The role of cannabinoids to manage pain. Revista Mexicana de Anestesiologia. 2008;31:191–200. [Google Scholar]
- 49.Kung T, Hochman J, Sun Y, et al. Efficacy and safety of cannabinoids for pain in musculoskeletal diseases: a systematic review and meta-analysis. J Rheumatol. 2011;38:1171. [Google Scholar]
- 50.Kantor TG, Hopper M. A study of levonantradol, a cannabinol derivative, for analgesia in post operative pain. Pain. 1981;11:S37. [Google Scholar]
- 51.Barbosa-Hernandez GF, Masri V, Eismon J, et al. Multimodal approach for managing acute post-traumatic pain in a heroin addict. Reg Anesth Pain Med. 2013;38:353–69. [Google Scholar]
- 52.Haroutiunian S, Ratz Y, Rosen G, et al. Evaluation of pain and health-related quality of life (HRQOL) outcomes in chronic pain patients treated with cannabis. Eur J Pain Suppl. 2011;5(S1):277. [Google Scholar]
- 53.Harris D, Jones RT, Shank R, et al. Self-reported marijuana effects and characteristics of 100 San Francisco medical marijuana club members. J Addict Dis. 2000;19:89–103. doi: 10.1300/J069v19n03_07. [DOI] [PubMed] [Google Scholar]
- 54.Piper BJ, Beals ML, Abess AT, et al. Chronic pain patients’ perspectives of medical cannabis. Pain. 2017;158:1373–9. doi: 10.1097/j.pain.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peters DC., II Patients and caregivers report using medical marijuana to decrease prescription narcotics use. Humboldt J Soc Relat. 2013;35:24–40. [Google Scholar]