Abstract
Liver cirrhosis (LC) is the final usual outcome of liver damage induced by various chronic liver diseases. Because of asymptomatic nature of LC, it is usually diagnosed at late and advanced stages, and patients are easy to miss the best timing for treatment. Thus, the early detection of LC is needed. In the prospective Korean Cancer Prevention Study-II (K-II), we aimed to identify valuable biomarkers for LC using metabolomics to distinguish subjects with incident LC (LC group) from subjects free from LC (control group) during a mean 7-year follow-up period. Metabolic alterations were investigated using baseline serum specimens acquired from 94 subjects with incident LC and 180 age- and sex-matched LC-free subjects via ultra-performance liquid chromatography (UPLC)-linear-trap quardrupole (LTQ)-Orbitrap mass spectrometry (MS). As a result of the metabolic analysis, 46 metabolites were identified. Among them, 11 and 18 metabolite level showed a significant increase and decrease, respectively, in the LC group compared to the control group. Nine metabolic pathways, including glyoxylate and dicarboxylate metabolism, amino acid metabolism, fatty acid metabolism, linoleic acid metabolism, α-linolenic acid metabolism, and arachidonic acid metabolism, were significantly different between the two groups. Logistic regression demonstrated that the LC emergence was independently affected by serum levels of myristic acid, palmitic acid, linoleic acid, eicosapentaenoic acid (EPA), lysophosphatidic acid (LPA) (18:1), glycolic acid, lysophosphatidylcholine (lysoPC) (22:6), and succinylacetone (R2 = 0.837, P < 0.001). This prospective study revealed that dysregulation of various metabolism had the clinical relevance on the LC development. Moreover, myristic acid, palmitic acid, linoleic acid, EPA, LPA (18:1), glycolic acid, lysoPC (22:6), and succinylacetone were emerged as independent variables influencing the incidence of LC. The results support that the early biomarkers found in this study may useful for predicting and remedying the risk of LC.
Keywords: liver cirrhosis, metabolic dysregulation, early biomarkers, Korean cohort, prospective study
Introduction
Various chronic liver diseases induce liver damage, finally leading to liver cirrhosis (LC) as the final common pathological outcome (Zhou et al., 2014). The cause of LC includes alcoholism, chronic hepatitis B (Asia-Pacific region) or C (western countries) virus infection, and non-alcoholic fatty liver disease (Liaw et al., 2008; Innes et al., 2013). Over early cirrhosis, the liver can compensate the changes leaded by bridging fibrosis, and most patients do not have any special symptoms unless entering the decompensated cirrhosis stage (Qi et al., 2012). Decompensated cirrhosis, also called end-stage liver disease, is a general factor of global mortality; liver disease is the 7th and 12th cause of death in Europe and United States, respectively (Peng et al., 2019). In Korean population (aged 20–65), liver disease is the 8th leading cause of overall death in 2017 (Korean Statistical Information Service [KOSIS], 2018). Because of asymptomatic nature of LC, LC is usually diagnosed at late and advanced stages and patients often miss the best opportunity for therapy. Therefore, for betterment of the diagnosis and prognosis, identifying additional and reliable markers possibly used for early and accurate detection of LC is necessary.
Metabolomics scientifically provides new aspects of disease biomarker by analyzing biological samples and by offering all detectable metabolites (metabolic profiling) (Jasbi et al., 2019). Indeed, for observing metabolic alterations caused by various disease, mass spectrometry-based metabolic profiling is a good analytical platform (Jasbi et al., 2019); and this technology is widely used to discover new biomarkers (Chen et al., 2013; Lu et al., 2015). Researchers have attempted to investigate metabolic profiling of LC, but the results regarding pre-diagnostic biomarkers of LC are contradictory. Furthermore, few studies have focused on prospective settings. Therefore, in the prospective Korean Cancer Prevention Study-II (KCPS-II), which enrolled a cancer-free cohort at baseline, we applied metabolomics to distinguish individuals with LC (LC group) from age- and sex-matched controls who were free of LC (healthy controls) over a mean 7-year follow-up period. Our objective was to identify early biomarkers with value in differentiating these two groups using their baseline serum metabolic profiles, as measured using ultra-performance liquid chromatography (UPLC)-linear-trap quadrupole (LTQ)-Orbitrap mass spectrometry (MS).
Materials and Methods
Study Population
Study participants were recruited from the KCPS-II Biobank during routine health check-up at 18 health promotion centers in Seoul and Gyeonggi-do, South Korea (from 2004 to 2013). Detailed information of the KCPS-II is described in a previous publication (Jee et al., 2018). Inclusion criterion was normal levels of liver enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), which reflect normal liver function and hepatocyte damage, respectively; upper limits of normal ALT were 34 and 24 U/L in men and women, respectively, and upper limits of normal AST were 32 and 26 U/L in men and women, respectively. These values were set according to a previous report for Koreans (Sohn et al., 2013). Exclusion criteria were underweight or overweight/obesity [18 kg/m2 ≤ body mass index (BMI) < 25 kg/m2]; hypertension (systolic/diastolic blood pressure ≤ 140/90 mmHg); diabetes (fasting glucose ≤ 126 mg/dL); alcohol abuse/alcoholic; or any medication.
Among the 156,701 KCPS-II subjects, who were fully given study explanation and provided written consent, 94 individuals developed LC during the 7-year follow-up period (LC group); at baseline, these individuals had had normal liver function and no diagnostic evidences of LC during the health check-up. At an approximately 1:2 ratio, 180 age- and sex-matched healthy subjects (normal liver function at baseline and did not develop LC during the 7-year follow-up period) were included as a control group in the final analysis. The study, which complied with the principle in the Declaration of Helsinki, was reviewed and approved by the Institutional Review Board of Yonsei University.
Blood Collection and Biochemical and Anthropometric Assessments
For all assessment, we used baseline serum samples to determine any clinical and biochemical differences between the control and LC groups prior to LC occurrence. For acquiring the serum samples, peripheral venous blood was obtained after a fasting period (minimum 12 h) from each study participants and the serum was separated from the specimens. The serum aliquots were then stored at –70°C prior to further examination.
For biochemical assessments, fasting serum glucose, total cholesterol, triglyceride, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, AST, ALT, and high-sensitivity C-reactive protein (hs-CRP) levels were assessed via automatic analyzers. Each laboratory measurement was carried out according to the internal and external quality control (QC) procedures specified by the Korean Association of Laboratory QC. The agreement for each measurement across respective hospitals was high (correlation coefficients ranging from 0.96 to 0.99) (Jee et al., 2014).
Regarding the anthropometric assessments, height (cm) and body weight (kg) were measured with light clothing. To calculate BMI, body weight (kg) was divided into height in meter squared (m2). Waist circumference (cm) was measured midway between the lower rib and the iliac crest. Systolic and diastolic blood pressures (mmHg) were measured after a rest period of at least 15 min. To gather smoking and alcohol consumption histories, each study participants were interviewed with a structured questionnaire.
Diagnosis of LC
From the National Health Insurance Service (NHIS), health insurance claim information about LC incident were acquired. Among the data obtained from the NHIS, patients who were hospitalized and outpatients more than once were defined as having LC. LC was coded as K74 according to the International Classification of Diseases, Tenth Revision (ICD-10) (World Health Organization [WHO], 2011).
Non-targeted (Global) Metabolic Profiling Using Serum
Sample Preparation and UPLC-LTQ-Orbitrap MS
Detailed description regarding a preparation of serum samples was reported elsewhere (Jee et al., 2016). Briefly, 100-μL aliquots of the baseline serum samples, obtained during the health check-up, were mixed with acetonitrile (800 μL) by vortexing. The mixtures were centrifuged (10,000 rpm, 5 min, 4°C), and then supernatant was dried with N2 gas. After drying process, remaining pallet was dissolved in 10% methanol, vortexed, and centrifuged (10,000 rpm, 5 min, 4°C). The supernatant was then transferred to a vial.
Full information of UPLC-LTQ-Orbitrap MS analysis was also described in our previous research as a Supplementary Material (Yoo et al., 2018). In brief, an Acquity UPLC-BEH-C18 column (2.1 × 50 mm, 1.7 μm; Waters, Milford, MA, United States) was equipped in a Thermo UPLC system (Ultimate 3000 BioRS; Dionex-Thermo Fisher Scientific, Bremen, Germany). The prepared samples were extracted and injected (5 μL) into the column, and liquid chromatographic separation was carried out in both electrospray ionization (ESI)-positive and -negative modes. The metabolites, separated by the column under each ESI-positive and -negative mode, were then applied to an LTQ-Orbitrap MS (Thermo Fisher Scientific, Waltham, MA, United States) operated in full scan mode for Fourier transform MS. The spectra were gathered from m/z 50 to m/z 1,000. For QC, a pooled QC sample was prepared and injected into every 5th sample. The metabolites’ MS/MS spectra were acquired by applying a collision energy ramping from 20 to 55%.
Data Processing and Putative Identification of Metabolites
A SIEVE 2.2 (Thermo Fisher Scientific, Waltham, MA, United States) software was used to process relevant spectral data. Analysis parameters were set as following: retention time width 2.5 min, m/z width 5 ppm and m/z range 50–1,000. Metabolites were putatively identified by searching based on the databases: ChemSpider1, Human Metabolome2, KEGG3, Lipid MAPS4, and MassBank5. Selected metabolites were confirmed according to the retention times and mass spectra of standard samples.
Statistical Analysis
As a statistic analysis tool, a SPSS version 23.0 (IBM/SPSS, Chicago, IL, United States) was utilized. To compare nominal and continuous variables between the two groups, a chi-squared test and an independent t-test were conducted. To determine independent effects of each variable on the LC incident, a logistic regression analysis was performed. A two-tailed P-values less than 0.05 (P < 0.05) was thought to have statistical significance. To avoid errors of multiple comparison with regard to metabolites, P-values were adjusted by false discovery rate (FDR); FDR-corrected q-values were calculated by a R package “fdrtool” and q-values less than 0.05 (q < 0.05) were regarded as having a statistical significance.
For a multivariate analysis, a SIMCA-P+ 14.0 (Umetrics Inc., Umeå, Sweden) was used to export spectrometric data. Pareto scaling was used to all data prior to an in-depth analysis. To analyze our models, as a supervised classification tool, orthogonal projection to latent structures-discriminant analysis (OPLS-DA) was used. The validity of the models was evaluated using Q2Y and R2Y parameters. A metabolic pathway analysis was conducted by MetaboAnalyst 3.06.
Results
Baseline Clinical Characteristics
During the 7 years (mean follow-up period), 94 subjects emerged LC among the 156,701 KCPS-II subjects. The 94 subjects with incident LC were used as the LC group and 180 age- and sex-matched healthy subjects (remained free from LC after the follow-up) were set as the control group. After adjusting for confounding factors (age, sex, BMI, and smoking and drinking status), weight, waist circumference, systolic/diastolic blood pressure, glucose, triglyceride, total cholesterol, HDL-cholesterol, LDL-cholesterol, and hs-CRP did not show any statistical significance between the two groups (Table 1). However, the LC group showed slightly but significantly higher levels of AST (P = 0.018), ALT (P = 0.030), and γ-glutamyltransferase (GGT) (P = 0.022) than the control group. Moreover, the LC group showed a trend toward a decrease in leukocyte number (white blood cell; P = 0.057). Additionally, hepatitis B virus surface antigen (HBsAg) and hepatitis C virus antibody (HCV Ab) as one of the causes of LC were assessed at baseline and shown in the Supplementary Table S1.
TABLE 1.
Clinical characteristics according to onset of liver cirrhosis (LC) in the subjects with normal liver function.
| Control group | LC group | Pa | Pb | |
| (n = 180) | (n = 94) | |||
| Age (years) | 45.2 ± 0.70 | 43.8 ± 0.99 | 0.270 | – |
| Male/female n, (%) | 110 (61.1)/69 (38.3) | 57 (60.6)/37 (39.4) | 0.763 | – |
| Current smoker n, (%) | 40 (24.1) | 23 (24.5) | 0.904 | – |
| Alcohol drinker n, (%) | 139 (82.7) | 67 (71.3) | 0.030 | – |
| BMI (kg/m2) | 23.2 ± 0.20 | 22.0 ± 0.18 | <0.001 | – |
| Weight (kg) | 64.6 ± 0.77 | 60.7 ± 0.86 | 0.001 | 0.177 |
| Waist circumference (cm) | 79.7 ± 0.67 | 78.7 ± 0.51 | 0.007 | 0.673 |
| Systolic blood pressure (mmHg) | 116.2 ± 1.17 | 113.9 ± 1.08 | 0.143 | 0.537 |
| Diastolic blood pressure (mmHg) | 72.9 ± 0.78 | 70.7 ± 0.87 | 0.073 | 0.894 |
| Glucose (mg/dL)∮ | 91.7 ± 1.48 | 87.5 ± 1.15 | 0.060 | 0.375 |
| Triglyceride (mg/dL)∮ | 128.8 ± 7.33 | 110.4 ± 7.26 | 0.184 | 0.764 |
| Total cholesterol (mg/dL)∮ | 186.0 ± 2.25 | 188.6 ± 3.04 | 0.456 | 0.260 |
| HDL-cholesterol (mg/dL)∮ | 52.3 ± 0.75 | 55.4 ± 1.30 | 0.039 | 0.241 |
| LDL-cholesterol (mg/dL)∮ | 111.4 ± 2.08 | 113.8 ± 2.72 | 0.398 | 0.205 |
| AST (IU/L)∮ | 19.5 ± 0.27 | 20.6 ± 0.41 | 0.022 | 0.018 |
| ALT (IU/L)∮ | 17.9 ± 0.45 | 19.0 ± 0.64 | 0.193 | 0.030 |
| GGT (IU/L)∮ | 25.1 ± 1.14 | 27.5 ± 1.86 | 0.280 | 0.022 |
| White blood cell (×103/UL)∮ | 5.74 ± 0.12 | 5.35 ± 0.13 | 0.035 | 0.057 |
| hs-CRP (mg/dL)∮ | 0.12 ± 0.02 | 0.20 ± 0.05 | 0.315 | 0.518 |
Mean ± SE. ∮tested by logarithmic transformation. Pa-values derived from a chi-squared test and an independent t-test for nominal variables and continuous variables, respectively. Pb-values derived from an ANCOVA to adjust age, sex, BMI, and smoking and drinking status.
Metabolic Profiling in the Serum Samples Using UPLC-LTQ-Orbitrap MS
Non-targeted (Global) Metabolic Pattern Analysis
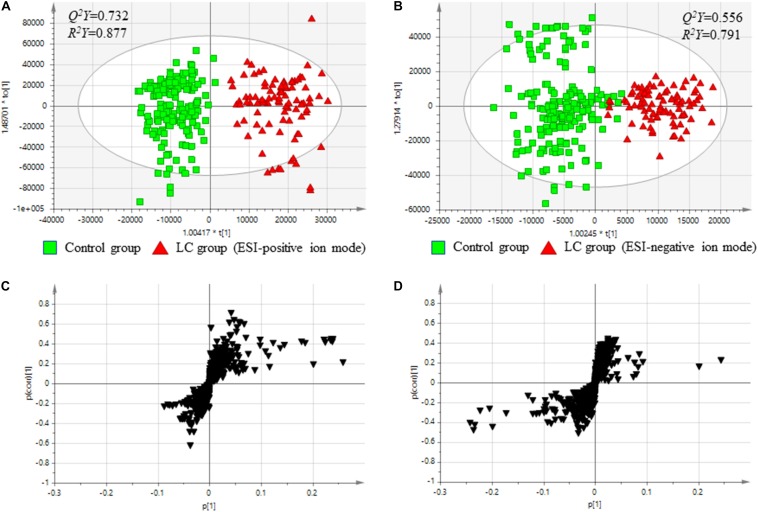
Using an OPLS-DA score plots, both ESI-positive and -negative ion modes chromatography data on serum metabolites were analyzed. The quality of OPLS-DA was assessed by the values of Q2Y and R2Y to evaluate the predictive ability of each model and to confirm that the models were not over-fitted, respectively. Figures 1A,B showed the results of comparisons of baseline metabolite levels between the control and LC groups in ESI-positive and -negative modes, respectively. The OPLS-DA models displayed acceptable predictive ability and well fitted in ESI-positive ion mode (Figure 1A; Q2Y = 0.732, R2Y = 0.877) and ESI-negative ion mode (Figure 1B; Q2Y = 0.556, R2Y = 0.791).
FIGURE 1.
Comparison between serum metabolites in the control and Cirrhosis groups. (A) Score plots from OPLS-DA models classifying two groups in positive mode. (B) Score plots from OPLS-DA models classifying two groups in negative mode. (C) S-plots for covariance [p] and reliability correlation [p(corr)] from OPLS-DA models in positive mode. (D) S-plots for covariance [p(1)] and reliability correlation [p(corr)(1)] from OPLS-DA models in negative mode. (A,B) Comparison between the baseline levels of the control group (free from cirrhosis at baseline and follow-up; n = 180) and the baseline levels of the cirrhosis group (free from cirrhosis at baseline and incident cirrhosis at follow-up; n = 94).
The results indicated that the two groups could be discriminated by discrepancies in amount of the metabolites. To confirm potential metabolites providing the differences, S-plots of p(1) and p(corr)(1) were created for the OPLS-DA models using Pareto scaling in ESI-positive and -negative ion modes (Figures 1C,D). The S-plots showed that metabolites having higher or lower values of p(corr) were more related to separate between the control and the LC groups.
Baseline Serum Metabolites
Peaks (serum metabolites) that importantly discriminate between the control and LC groups were selected in accordance with the variable importance in the projection (VIP) values (VIP ≥ 1.5). As comparing the metabolites, detected from the baseline serum samples, between the two groups, a total of 156 metabolites (both ESI-positive and -negative ion modes) had VIP values more than 1.5; of these, 46 metabolites were putatively identified based on the metabolites’ database, whereas the remaining were novel (Supplementary Figure S1). Table 2 presents the serum metabolites in the groups. In the LC group, 18 metabolites had significantly lower peak intensities than in the control group; glycolic acid (q = 1.112 × 10–11), myristic acid (q = 0.019), 3-hydroxytetradecanedioic acid (q = 0.025), α-linolenic acid (q = 1.877 × 10–5), linoleic acid (q = 3.805 × 10–5), oleic acid (q = 0.025), 9-HOTE (q = 0.001), 13S-hydroxyoctadecadienoic acid (q = 3.326 × 10–5), 9-cis-retinoic acid (q = 7.158 × 10–6), eicosapentaenoic acid (q = 5.311 × 10–5), 5-HEPE (q = 3.021 × 10–6), 5-HETE (q = 4.986 × 10–7), leukotriene B4 (q = 3.412 × 10–4), 17-HdoHE (q = 4.587 × 10–7), dehydroepiandrosterone sulfate (q = 9.100 × 10–4), LPA (16:0) (q = 0.005), LPA (18:1) (q = 2.097 × 10–5), and PE (P-16:0e) (q = 4.878 × 10–5). On the other hand, compared to the control group, the LC group showed 11 metabolites having significantly higher peak intensities; succinylacetone (q = 3.242 × 10–10), phenylalanine (q = 0.023), tyrosine (q = 0.004), indoleacrylic acid (q = 2.931 × 10–6), tryptophan (q = 5.017 × 10–6), palmitic acid (q = 0.043), palmitoylcarnitine (q = 8.624 × 10–4), oleoylcarnitine (q = 7.053 × 10–4), lysoPC (16:0) (q = 0.006), lysoPC (18:2) (q = 0.017), and lysoPC (22:6) (q = 0.026) (Table 2).
TABLE 2.
Putative identification of serum metabolites according to onset of liver cirrhosis (LC) in the subjects with normal liver function.
| m/z [M+H]† [M−H]‡ | Molecular formula | Putatively identified metabolites | VIP | t-test | Cohen’s d | Change trend | |
| P | q | ||||||
| 75.009 | C2H4O3 | Glycolic acid‡ | 7.885 | 3.109E-13 | 1.112E-11 | –0.821 | ↓ |
| 132.101 | C6H13NO2 | L-Leucine† | 3.513 | 0.876 | 0.267 | 0.018 | ↑ |
| 159.065 | C7H10O4 | Succinylacetone† | 2.072 | 1.352E-11 | 3.242E-10 | 2.097 | ↑ |
| 166.086 | C9H11NO2 | L-Phenylalanine† | 3.803 | 0.033 | 0.023 | 0.257 | ↑ |
| 182.081 | C9H11NO3 | L-Tyrosine† | 2.486 | 0.004 | 0.004 | 0.340 | ↑ |
| 188.070 | C11H9NO2 | Indoleacrylic acid† | 1.861 | 6.461E-07 | 2.931E-06 | 0.601 | ↑ |
| 205.096 | C11H12N2O2 | L-Tryptophan† | 4.394 | 1.210E-06 | 5.017E-06 | 0.615 | ↑ |
| 227.202 | C14H28O2 | Myristic acid‡ | 2.385 | 0.026 | 0.019 | –0.259 | ↓ |
| 247.153 | C12H22O5 | 3-Hydroxydodecanedioic acid† | 1.961 | 0.123 | 0.067 | –0.172 | ↓ |
| 253.216 | C16H30O2 | Palmitoleic acid‡ | 2.869 | 0.096 | 0.056 | –0.202 | ↓ |
| 255.233 | C16H32O2 | Palmitic acid‡ | 9.512 | 0.070 | 0.043 | 0.236 | ↑ |
| 275.185 | C14H26O5 | 3-Hydroxytetradecanedioic acid† | 2.186 | 0.037 | 0.025 | –0.222 | ↓ |
| 279.231 | C18H30O2 | α-Linolenic acid† | 3.423 | 5.897E-06 | 1.877E-05 | –0.493 | ↓ |
| 279.233 | C18H32O2 | Linoleic acid‡ | 8.057 | 1.383E-05 | 3.805E-05 | –0.511 | ↓ |
| 281.249 | C18H34O2 | Oleic acid‡ | 7.365 | 0.037 | 0.025 | –0.240 | ↓ |
| 283.264 | C18H36O2 | Stearic acid‡ | 3.644 | 0.803 | 0.250 | 0.030 | ↑ |
| 295.226 | C18H30O3 | 9-HOTE† | 2.010 | 0.001 | 0.001 | –0.355 | ↓ |
| 295.227 | C18H32O3 | 13S-Hydroxyoctadecadienoic acid‡ | 2.400 | 1.167E-05 | 3.326E-05 | –0.477 | ↓ |
| 301.215 | C20H28O2 | 9-cis-Retinoic acid† | 3.109 | 1.842E-06 | 7.158E-06 | –0.521 | ↓ |
| 303.231 | C20H30O2 | Eicosapentaenoic acid† | 3.694 | 2.079E-05 | 5.311E-05 | –0.466 | ↓ |
| 303.232 | C20H32O2 | Arachidonic acid‡ | 4.486 | 0.497 | 0.172 | –0.078 | ↓ |
| 305.247 | C20H34O2 | 11,14,17-Eicosatrienoic acid‡ | 1.945 | 0.980 | 0.290 | 0.003 | ↑ |
| 317.211 | C20H30O3 | 5-HEPE‡ | 1.809 | 6.711E-07 | 3.021E-06 | –0.542 | ↓ |
| 319.227 | C20H32O3 | 5-HETE‡ | 3.039 | 7.857E-08 | 4.986E-07 | –0.589 | ↓ |
| 327.232 | C22H32O2 | Docosahexaenoic acid‡ | 4.894 | 0.143 | 0.075 | –0.173 | ↓ |
| 329.248 | C22H34O2 | Docosapentaenoic acid‡ | 1.906 | 0.186 | 0.090 | –0.158 | ↓ |
| 335.222 | C20H32O4 | Leukotriene B4‡ | 1.745 | 1.943E-04 | 3.412E-04 | –0.404 | ↓ |
| 343.227 | C22H32O3 | 17-HdoHE‡ | 3.320 | 7.074E-08 | 4.587E-07 | –0.594 | ↓ |
| 367.157 | C19H28O5S | Dehydroepiandrosterone sulfate‡ | 1.847 | 6.909E-04 | 9.100E-04 | –0.394 | ↓ |
| 400.341 | C23H45NO4 | L-Palmitoylcarnitine† | 1.618 | 6.438E-04 | 8.624E-04 | 0.518 | ↑ |
| 409.236 | C19H39O7P | LPA (16:0)‡ | 6.033 | 0.006 | 0.005 | –0.325 | ↓ |
| 426.356 | C25H47NO4 | Oleoylcarnitine† | 2.161 | 4.898E-04 | 7.053E-04 | 0.498 | ↑ |
| 433.236 | C21H39O7P | LPA (18:2)‡ | 2.276 | 0.997 | 0.293 | −4.360E-4 | ↓ |
| 435.251 | C21H41O7P | LPA (18:1)‡ | 3.459 | 6.726E-06 | 2.097E-05 | –0.550 | ↓ |
| 436.283 | C21H44NO6P | PE (P-16:0e)‡ | 1.814 | 1.873E-05 | 4.878E-05 | –0.467 | ↓ |
| 468.307 | C22H46NO7P | LysoPC (14:0)† | 1.765 | 0.127 | 0.069 | 0.195 | ↑ |
| 480.343 | C24H50NO6P | LysoPC (P-16:0)† | 2.471 | 0.404 | 0.146 | 0.116 | ↑ |
| 494.322 | C24H48NO7P | LysoPC (16:1)† | 2.040 | 0.760 | 0.240 | 0.037 | ↑ |
| 496.338 | C24H50NO7P | LysoPC (16:0)† | 6.143 | 0.007 | 0.006 | 0.351 | ↑ |
| 510.353 | C25H52NO7P | LysoPC (17:0)† | 1.781 | 0.429 | 0.153 | 0.106 | ↑ |
| 518.322 | C26H48NO7P | LysoPC (18:3)† | 1.620 | 0.361 | 0.134 | –0.102 | ↓ |
| 520.338 | C26H50NO7P | LysoPC (18:2)† | 3.481 | 0.023 | 0.017 | 0.298 | ↑ |
| 522.353 | C26H52NO7P | LysoPC (18:1)† | 2.949 | 0.281 | 0.116 | 0.137 | ↑ |
| 524.369 | C26H54NO7P | LysoPC (18:0)† | 3.716 | 0.838 | 0.259 | 0.027 | ↑ |
| 544.338 | C28H50NO7P | LysoPC (20:4)† | 1.512 | 0.170 | 0.085 | 0.175 | ↑ |
| 568.337 | C30H50NO7P | LysoPC (22:6)† | 1.831 | 0.039 | 0.026 | 0.303 | ↑ |
†Metabolites obtained by ESI-positive ion mode (M/Z [M+H]). †Metabolites obtained by ESI-negative ion mode (M/Z [M-H]). VIP is variable important in the projection. P-values derived from an independent t-test between groups; control group (n = 180) and LC group (n = 94). q-Value is adjusted p-value that controls the false discovery rate (FDR). Cohen’s d is an effect size for the comparison between two means; difference between two means divided by a pooled standard deviation; it is defined as “small, d = 0.20,” “medium, d = 0.50,” and “large, d = 0.80,” Change trend is determined by a comparison of the metabolites’ peak intensities in the LC group with those in the control group.
Metabolic Pathways Regarding the LC Pathogenesis in Healthy Subjects
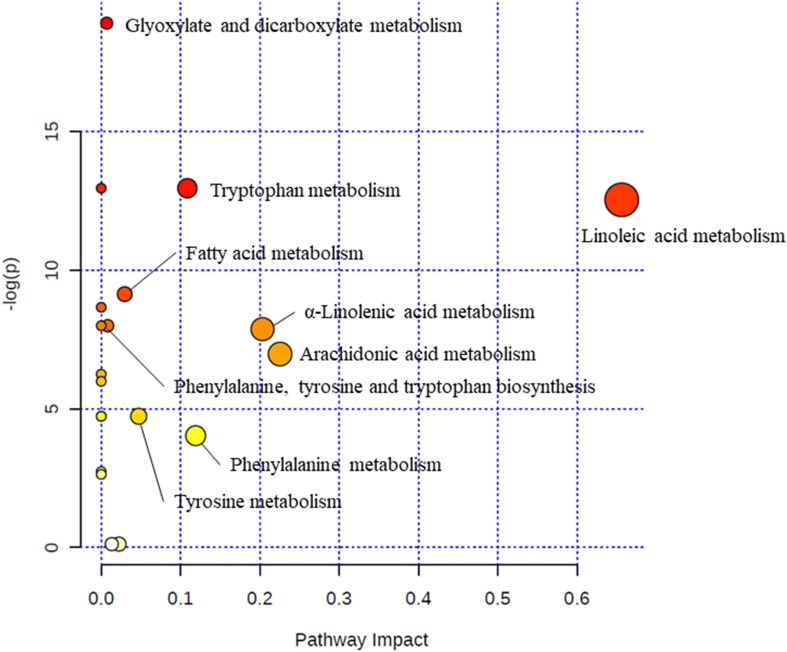
Via MetaboAnalyst 3.0, a web-based analysis module (see text footnote 6), a metabolic pathway analysis was carried out to verify the proper pathways associated with the selected metabolites (Figure 2). Glyoxylate and dicarboxylate metabolism (glycolic acid; impact = 0.007), tryptophan metabolism (tryptophan; impact = 0.109), linoleic acid metabolism (linoleic acid and 13S-hydroxyoctadecadienoic acid; impact = 0.656), fatty acid metabolism (palmitoylcarnitine and palmitic acid; impact = 0.030), phenylalanine, tyrosine and tryptophan biosynthesis (tryptophan, tyrosine, and phenylalanine; impact = 0.008), α-linolenic acid metabolism (α-linolenic acid; impact = 0.203), arachidonic acid metabolism (5-HETE, leukotriene B4, and arachidonic acid; impact = 0.226), tyrosine metabolism (tyrosine; impact = 0.047), phenylalanine metabolism (tyrosine and phenylalanine; impact = 0.119) were relevant to incidence of LC (Table 3).
FIGURE 2.
Metabolic pathway analysis. The “metabolome view” presents pathways arranged according to the scores based on enrichment analysis (y axis) and topology analysis (x axis). The color and size of each circle is based on P-values and pathway impact values, respectively.
TABLE 3.
Metabolic pathway analysis.
| Metabolic pathways | Hits | P | FDR | Impact |
| Glyoxylate and dicarboxylate metabolism | Glycolic acid | 6.11.E-09 | 1.22.E-07 | 0.007 |
| Tryptophan metabolism | L-Tryptophan | 2.36.E-06 | 1.57.E-05 | 0.109 |
| Linoleic acid metabolism | Linoleic acid | 3.58.E-06 | 1.79.E-05 | 0.656 |
| 13S-Hydroxyoctadecadienoic acid | ||||
| Fatty acid metabolism | L-Palmitoylcarnitine | 1.08.E-04 | 4.31.E-04 | 0.030 |
| Palmitic acid | ||||
| Phenylalanine, tyrosine and tryptophan biosynthesis | L-Tryptophan | 3.37.E-04 | 8.43.E-04 | 0.008 |
| L-Tyrosine | ||||
| L-Phenylalanine | ||||
| α-Linolenic acid metabolism | α-Linolenic acid | 3.82.E-04 | 8.49.E-04 | 0.203 |
| Arachidonic acid metabolism | 5-HETE | 0.001 | 0.002 | 0.226 |
| Leukotriene B4 | ||||
| Arachidonic acid | ||||
| Tyrosine metabolism | L-Tyrosine | 0.009 | 0.012 | 0.047 |
| Phenylalanine metabolism | L-Tyrosine | 0.018 | 0.022 | 0.119 |
| L-Phenylalanine |
Hits: the actually matched metabolites uploaded in MetaboAnalyst 3.0. FDR: the P-values adjusted using false discovery rate (FDR). Impact: the pathway impact value calculated from pathway topology analysis.
Logistic Regression Analysis
For evaluating independent influence of following variables on LC incident, logistic regression analysis was performed: AST, ALT, GGT, and 29 metabolites with q-values less than 0.05. In all study participants, the incidence of LC was affected by myristic acid, palmitic acid, linoleic acid, eicosapentaenoic acid, LPA (18:1), glycolic acid, lysoPC (22:6), and succinylacetone (R2 = 0.837, P < 0.001).
Discussion
In present-day society, discovering biomarkers of LC is one of the valuable goal to decrease mortality related to liver disease. Although researchers have attempted to find LC-related biomarker, there are still lack of prospective studies to identify early biomarker of LC before its incident. In this study, total 29 metabolites showed the significant differences between the control and LC groups at baseline (before LC incident); 11 and 18 metabolites had significantly increased and decreased peak intensities in the LC group, respectively, compared to the control group. In addition, through the metabolomics technology and metabolic pathway analysis, the present prospective study suggested that deregulation of nine metabolic pathways showed the clinical relevance on LC occurrence in subjects with normal liver function and free from LC at baseline. Thus, these metabolic processes’ alterations are possibly a potential underlying mechanism on LC development. To discuss the metabolites involved in the metabolic alteration regarding incident of LC, we classified them by their type.
Amino Acids
In the present study, higher levels of phenylalanine, tyrosine, and tryptophan in the LC group in comparison with the control group definitely showed the alteration of amino acid metabolism. These three amino acids are a member of aromatic amino acids (AAAs) and they have been reported to increase in the serum of hepatocellular carcinoma and LC patients (Gao et al., 2009; Fages et al., 2015). While, branched chain amino acids (BCAAs), including leucine, isoleucine, and valine have been informed to decrease in the same condition, thus, an imbalance of the amino acids has been revealed under the hepatocellular carcinoma and LC condition (Gao et al., 2009; Fages et al., 2015). However, the present metabolomics study showed that serum levels of BCAAs were not significantly different between the control and the LC groups. These findings may support the following pathologic progression of LC: initially, BCAAs and AAAs are increased via altered amino acid metabolism; and then, eventual degradation of BCAAs may be occurred by carnitines, which oxidizes BCAAs (Hoppel, 2003).
Acylcarnitines
Acyl group arising from the metabolism of BCAAs has been suggested to be contributing proportionately to the acyl-carnitine fraction in cirrhosis (Fuller and Hoppel, 1983). During fatty acid oxidation, acylcarnitines are generated as intermediates; and their accumulation is caused by metabolic dysfunctions as a consequence of the insufficient integration between β-oxidation and Krebs cycle (Al-Bakheit et al., 2016). Long-chain acylcarnitines accumulate during certain conditions including hepatic cirrhosis (Fuller and Hoppel, 1983). Additionally, a new pilot study revealed that in patients with hepatocellular carcinoma, serum acylcarnitines had high concentration compared to normal subjects (Yaligar et al., 2016). In this study, even before LC development, the LC group showed higher levels of palmitoylcarnitine and oleoylcarnitine than the control group. Both palmitoylcarnitine and oleoylcarnitine are long-chain acylcarnitines formed from long-chain fatty acid [palmitic acid (C16:0) and oleic acid (C18:1), respectively,] (Kuhajda et al., 1994), therefore, our results are in agree with the studies previously mentioned.
Saturated Fatty Acids
In this study, in comparison with the control group, the levels of palmitic acid (C16:0) and myristic acid (C14:0) were higher and lower in the LC group, respectively. Palmitic acid (C16:0) is reported to induce lipoapoptosis in hepatocytes via mechanisms, including mitochondrial dysfunction and stress of endoplasmic reticulum (Martínez et al., 2015). Myristic acid (C14:0), which is more rapidly metabolized (both β-oxidation and elongation) in hepatocyte than palmitic acid (C16:0) (Rioux et al., 2000), has been known not to be lipotoxic unlike palmitic acid (C16:0); however, a recent study reported a synergistic effect of myristic acid (C14:0) in palmitic acid (C16:0)-induced lipotoxicity (Martínez et al., 2015). In the present study, lower levels of myristic acid (C14:0) observed in the LC group might be due to its rapid metabolism in hepatocyte; and the rapid metabolism might contribute to accumulation of palmitic acid (C16:0) in the LC group. Furthermore, the logistic regression analysis revealed the independent effect of myristic acid (C14:0) and palmitic acid (C16:0) on LC progression and metabolic pathway analysis showed that palmitic acid (C16:0) involved in fatty acid metabolism which altered in the LC group. These findings, therefore, suggest that early changes in these fatty acids need to be observed to prevent LC development.
Polyunsaturated Fatty Acids
α-Linolenic acid (C18:3, ω-3 PUFA) is a major precursor of ω-3 PUFA metabolites. In this study, the levels of α-linolenic acid (C18:3) and its downstream ω-3 PUFA metabolites, including eicosapentaenoic acid (C20:5), docosapentaenoic acid (C22:5), and docosahexaenoic acid (C22:6), were lower in the LC group than the control group; however, docosapentaenoic acid (C22:5) and docosahexaenoic acid (C22:6) did not show significant differences. Studies have shown that reduction of circulating levels of ω-3 PUFAs is observed in cirrhotic patients (Watanabe et al., 1999; Ristić-Medić et al., 2013; Enguita et al., 2019). In addition, especially, depletion of docosahexaenoic acid (C22:6) may influence on homeostasis of liver tissue, likely fibrosis progression (Enguita et al., 2019). In the present study, the reason why the LC group had lower levels of ω-3 PUFAs than the control group was hard to know because of limited information. Factors of ω-3 PUFAs deficient could be diverse. First of all, ω-3 PUFAs are essential fatty acids which cannot be synthesized in the body and therefore have to be consumed from diet; thus, a lack of ingestion of ω-3 PUFAs may be a potential reason. Other factors, including malabsorption and changes in microbiota, also can be a reason related to altered circulation levels of ω-3 PUFAs (Enguita et al., 2019). Conclusionally, since the LC group showed a decrease of ω-3 PUFA levels even before LC incident, our results suggest that low levels of ω-3 PUFAs should be carefully observed for preventing future risk of LC development.
In case of ω-6 PUFA, the LC group also showed a significant decrease in linoleic acid (C18:2) compared to the control group. The levels of 13S-hydroxyoctadecadienoic acid, which is a lipoxygenation product derived from linoleic acid (C18:2) (The Human Metabolome Database [HMDB], 2019), was also significantly lower in the LC group. Meanwhile, the levels of arachidonic acid (C20:4), converted from linoleic acid (C18:2), did not show any statistical difference between the groups. There are conflicting reports on the levels of ω-6 PUFAs. A study revealed that arachidonic acid (C20:4) levels showed marked decrease in patients with hepatitis, cirrhosis, and liver cancer compared to healthy controls (Safaei et al., 2016). On the other hand, high ω-6 PUFA to ω-3 PUFA ratio induced by large amount of consumption of ω-6 PUFAs is observed in liver diseases (Wree et al., 2013); and a study demonstrated that high intake of ω-6 PUFAs should be avoid for mitigating progression of liver disease due to their pro-inflammatory characteristics (Ullah et al., 2019). The LC group of this study had low levels of ω-6 PUFAs rather than the control group before LC development; in addition, the inflammatory marker, hs-CRP, was not statistically different between the groups. Thus, inflammation caused by ω-6 PUFAs may not be a principal reason of future LC progression in this study.
Arachidonic Acid (C20:4) and Eicosapentaenoic Acid (C20:5)-Related Metabolites
5-HETE and leukotriene B4 are metabolites associated with arachidonic acid (C20:4) metabolism. Arachidonic acid (C20:4) released by phospholipases from cell membrane is metabolized by lipoxygenases (LOX); especially, 5-LOX generates 5-HETE and leukotriene B4 from arachidonic acid (C20:4) (Harizi et al., 2008). These two metabolites induce chemotactic response and chemokinesis of leukocytes and show increased levels in inflammatory lesions for immune function, therefore, 5-HETE and leukotriene B4 may play a key role on leukocyte function (Goetzl et al., 1982). In the present study, leukotriene B4 and 5-HETE levels were significantly lower in the LC group than the control group and the leukocyte number in the LC group had a trend toward decrease compared to the control group. This result corresponds to a previous research that LC subjects showed significant downregulated levels of eicosanoids including leukotriene in comparison with healthy controls (Fitian et al., 2014). Thus, relatively low leukocyte number, which may be associated with low levels of 5-HETE and leukotriene B4, may lead to an improper inflammatory/immune function against future LC progression.
Meanwhile, 5-HEPE is also a metabolite processed by 5-LOX from eicosapentaenoic acid (C20:5) (Onodera et al., 2017). A study demonstrated that eicosapentaenoic acid (C20:5) and 5-HEPE have anti-inflammatory effects on chronic inflammation disease including hepatic steatosis by enhancing anti-inflammatory immune cells, such as T regulatory cells (Onodera et al., 2017). In addition, 5-HEPE lessens inflammatory reaction of macrophage through the JNK pathway and has a protection effect against hepatic steatosis (Wang et al., 2017). Therefore, these metabolites can be considered as an effective factor for preventing early stage of liver disease (before progression of LC). In the present study, both eicosapentaenoic acid (C20:5) and 5-HEPE were significantly lower in the LC group than the control group; moreover, the former was revealed as an independent factor for incidence of LC. Thus, eicosapentaenoic acid (C20:5) and 5-HEPE may become a potent biomarker for prediction LC development.
LysoPCs
LysoPC is a metabolite released from phospholipids by phospholipase A2, an enzyme that involves in hydrolysis the ester bond of sn-2 position in phospholipids (Kokotou et al., 2017). This metabolite has been reported to be associated with oxidative stress and inflammation (Zhang et al., 2019), which are related to a progression of liver diseases; oxidative stress has been well known to play important role in liver diseases and chronic inflammation also has been revealed to result in liver fibrosis, cirrhosis, and hepatocellular carcinoma by causing persistent liver injury (Yuan et al., 2019). Indeed, many studies proved that lysoPCs are connected with liver damage. Kakisaka et al. (2012) demonstrated that lysoPCs lead to hepatocyte lipoapoptosis that is germane to liver diseases via various mechanism. Other studies showed that greater concentration of lysoPC in hepatic tissue are observed in non-alcohol steatohepatitis patients compared to healthy controls (Puri et al., 2007; Han et al., 2008). Finally, Yang et al. (2014) showed that lysoPCs were identified as a potential biomarker for early diagnosis of liver fibrosis and cirrhosis. In the current study, the levels of lysoPCs (16:0, 18:2, and 22:6) were detected to be significantly higher in the LC group compared to the control group at baseline. Therefore, our results can support the role of lysoPC as an early biomarker for LC.
From lysoPC species, lysophosphatidic acids (LPAs) are generated by enzymes, autotaxin (ATX) which has lysophospholipase D activity (Kremer et al., 2010). Several studies have been demonstrated that ATX-LPA axis is an important pathological pathway regarding liver diseases (Nakagawa et al., 2011; Erstad et al., 2017; Valdés-Rives and González-Arenas, 2017). In addition, a study showed that elevated serum levels of ATX are positively associated with the severity of LC; and the ATX has a positive correlation with LPA levels in liver cirrhotic patients (Pleli et al., 2014). Unlikely these studies, however, our data showed decreased levels of LPA (16:0 and 18:1) in the LC group. This discordance may come from disease status of individuals. In this study, the time point at which biological samples obtained of the LC group was before LC occurrence, whereas the studies mentioned previously measured the markers under disease existence. Perhaps, the increase of lysoPC levels may precede the LPA elevation in a pathologic progression of LC. Moreover, lysoPC (22:6) and LPA (18:1) were revealed as independent factors for future LC incident via logistic regression analysis. Thus, lysoPC increase and LPA decrease may need to be carefully observed before LC incident.
Other Metabolites
Glycolic acid and succinylacetone were also revealed as independent factors associated with the incidence of LC in the present study. In the liver, glycolic acid is generated from ethylene glycol by enzymes, alcohol dehydrogenase and aldehyde dehydrogenase; and then is metabolized to oxalic acid by enzymes, glycolate oxidase and glycolate dehydrogenase. Eventually, oxalic acid is excreted in urine via kidney (Corley et al., 2005). Glycolic acid and oxalic acid have been reported to cause metabolic acidosis and acute renal failure (Yamamoto et al., 2011; Tuero et al., 2018). Although our study detected glycolic acid in the LC group, the levels were lower than the control group and their clinical relevance on the liver has not been focused so far. Thus, an attempt to elucidate relationships between glycolic acid and future LC development is needed.
Succinylacetone is a metabolite of tyrosine and has liver toxicity. Fumarylacetoacetate hydrolase (FAH) plays a key role in tyrosine metabolism to produce succinylacetone; and FAH deficiency mainly induced by a heredity problem of FAH encoded gene leads to type 1 tyrosinemia, in which increased levels of succinylacetone are observed along with liver diseases including hepatic failure, LC, and liver cancer (Scott, 2006; Yang et al., 2017). In addition to succinylacetone, levels of tyrosine, methionine, and phenylalanine also have been reported to increase in genetic deficiency of FAH-induced type 1 tyrosinemia (Scorza et al., 2014). Although, in the present study, there were no participants with congenital type 1 tyrosinemia caused by FAH gene abnormality, not only succinylacetone but also tyrosine and phenylalanine levels showed significant elevated levels in the LC group compared to the control group. There are limits to know an exact mechanism with our information, however, we suggest a possibility based on our findings: activities of tyrosine metabolism-related enzymes may be altered before LC development.
The present study has limitations. First, even though we obtained the information of LC occurrence from the NHIS record (K74 according to the ICD-10), an exact cause of LC in each subject is still ambiguous; because we did not directly review the patients’ charts and carry out standardized test for LC diagnosis. In addition, depending on which cause or underlying disease leaded to the onset of LC, the metabolic profile at baseline might be possible to vary slightly. Nonetheless, in this study, LC was a final phenotype after all; accordingly, our data can warn about a future risk of LC development. Second, notably, in this study, the exposure assessment was performed at a single time point (baseline; before LC incident) and changes which might be occurred during the follow-up were not measured although the follow-up period was long (7 years); therefore, exact mechanisms on future LC development cannot be fully elucidate with our metabolomics data interpretation. Third, an external validation of our result with an independently different cohort should have performed; however, due to the limitation of available resources (funding, samples, time, etc.), it could not be possible. Instead, we have reviewed carefully our metabolomics data to support their pathological relevance on LC development. Lastly, diurnal variations might exist among the study subjects because sample collection time was varied (a.m. or p.m.). For remedying the shortcomings, further study is needed.
Conclusion
Despite these limitations, still, many studies can support our results and vice versa. Similar to previous metabolomics studies, the clinical relevance of deregulations of amino acid metabolism, linoleic acid metabolism, fatty acid metabolism, α-linolenic acid metabolism, and arachidonic acid metabolism were observed. In addition, myristic acid, palmitic acid, linoleic acid, eicosapentaenoic acid, LPA (18:1), glycolic acid, lysoPC (22:6), and succinylacetone were revealed as the independent variables related to the future LC development. Metabolic patterns found in this study before LC progression provide meaningful and potential biomarkers for future LC development. The results can provide pathological insight of LC incident and the biomarkers may be useful in early diagnosis of LC.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The study participants were fully given study explanation and provided written consent. The Institutional Review Board of Yonsei University reviewed and approved the study, which complied with the principles in the Declaration of Helsinki.
Author Contributions
HY analyzed and interpreted the data, prepared the manuscript, and contributed to critical revision of the manuscript. KJ acquired the data, provided the blood samples, and prepared the manuscript. MkK, MjK, and MsK analyzed and interpreted the data. SJ provided the blood samples and research funding. YC provided the blood samples. JL interpreted the data, and prepared the manuscript. All authors contributed to the conception and design of the study and minor revision of the manuscript, and have approved the final version of the manuscript for the publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This study was funded by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, South Korea (HI14C2686).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.01421/full#supplementary-material
References
- Al-Bakheit A., Traka M., Saha S., Mithen R., Melchini A. (2016). Accumulation of palmitoylcarnitine and its effect on pro-inflammatory pathways and calcium influx in prostate cancer. Prostate 76 1326–1337. 10.1002/pros.23222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Kong H., Lu X., Li Y., Yin P., Zeng Z., et al. (2013). Pseudotargeted metabolomics method and its application in serum biomarker discovery for hepatocellular carcinoma based on ultra high-performance liquid chromatography/triple quadrupole mass spectrometry. Anal. Chem. 85 8326–8333. 10.1021/ac4016787 [DOI] [PubMed] [Google Scholar]
- Corley R. A., Bartels M. J., Carney E. W., Weitz K. K., Soelberg J. J., Gies R. A., et al. (2005). Development of a physiologically based pharmacokinetic model for ethylene glycol and its metabolite, glycolic Acid, in rats and humans. Toxicol. Sci. 85 476–490. 10.1093/toxsci/kfi119 [DOI] [PubMed] [Google Scholar]
- Enguita M., Razquin N., Pamplona R., Quiroga J., Prieto J., Fortes P. (2019). The cirrhotic liver is depleted of docosahexaenoic acid (DHA), a key modulator of NF-κB and TGFβ pathways in hepatic stellate cells. Cell Death Dis. 10:14. 10.1038/s41419-018-1243-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erstad D. J., Tager A. M., Hoshida Y., Fuchs B. C. (2017). The autotaxin-lysophosphatidic acid pathway emerges as a therapeutic target to prevent liver cancer. Mol. Cell. Oncol. 4:e1311827. 10.1080/23723556.2017.1311827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fages A., Duarte-Salles T., Stepien M., Ferrari P., Fedirko V., Pontoizeau C., et al. (2015). Metabolomic profiles of hepatocellular carcinoma in a European prospective cohort. BMC Med. 13:242. 10.1186/s12916-015-0462-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitian A. I., Nelson D. R., Liu C., Xu Y., Ararat M., Cabrera R. (2014). Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/MS-MS. Liver Int. 34 1428–1444. 10.1111/liv.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. K., Hoppel C. L. (1983). Elevated plasma carnitine in hepatic cirrhosis. Hepatology 3 554–558. 10.1002/hep.1840030413 [DOI] [PubMed] [Google Scholar]
- Gao H., Lu Q., Liu X., Cong H., Zhao L., Wang H., et al. (2009). Application of 1H NMR-based metabonomics in the study of metabolic profiling of human hepatocellular carcinoma and liver cirrhosis. Cancer Sci. 100 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Goldman D. W., Naccache P. H., Sha’afi R. I., Pickett W. C. (1982). Mediation of leukocyte components of inflammatory reactions by lipoxygenase products of arachidonic acid. Adv. Prostaglandin Thromboxane Leukot Res. 9 273–282. [PubMed] [Google Scholar]
- Han M. S., Park S. Y., Shinzawa K., Kim S., Chung K. W., Lee J. H., et al. (2008). Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 49 84–97. 10.1194/jlr.m700184-jlr200 [DOI] [PubMed] [Google Scholar]
- Harizi H., Corcuff J. B., Gualde N. (2008). Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol. Med. 14 461–469. 10.1016/j.molmed.2008.08.005 [DOI] [PubMed] [Google Scholar]
- Hoppel C. (2003). The role of carnitine in normal and altered fatty acid metabolism. Am. J. Kidney Dis. 41 S4–S12. [DOI] [PubMed] [Google Scholar]
- Innes H. A., Hutchinson S. J., Barclay S., Cadzow E., Dillon J. F., Fraser A., et al. (2013). Quantifying the fraction of cirrhosis attributable to alcohol among chronic hepatitis C virus patients: implications for treatment cost-effectiveness. Hepatology 57 451–460. 10.1002/hep.26051 [DOI] [PubMed] [Google Scholar]
- Jasbi P., Wang D., Cheng S. L., Fei Q., Cui J. Y., Liu L., et al. (2019). Breast cancer detection using targeted plasma metabolomics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1105 26–37. 10.1016/j.jchromb.2018.11.029 [DOI] [PubMed] [Google Scholar]
- Jee S. H., Batty G. D., Jang Y., Oh D. J., Oh B. H., Lee S. H., et al. (2014). The Korean Heart Study: rationale, objectives, protocol, and preliminary results for a new prospective cohort study of 430,920 men and women. Eur. J. Prev. Cardiol. 21 1484–1492. 10.1177/2047487313497602 [DOI] [PubMed] [Google Scholar]
- Jee S. H., Kim M., Kim M., Kang M., Seo Y. W., Jung K. J., et al. (2016). Clinical relevance of glycerophospholipid, sphingomyelin and glutathione metabolism in the pathogenesis of pharyngolaryngeal cancer in smokers: the Korean Cancer Prevention Study-II. Metabolomics 12:164. 10.1067/mlc.2001.117161 [DOI] [PubMed] [Google Scholar]
- Jee Y. H., Emberson J., Jung K. J., Lee S. J., Lee S., Back J. H., et al. (2018). Cohort profile: the korean cancer prevention study-II (KCPS-II) Biobank. Int. J. Epidemiol. 47 385f–386f. [DOI] [PubMed] [Google Scholar]
- Kakisaka K., Cazanave S. C., Fingas C. D., Guicciardi M. E., Bronk S. F., Werneburg N. W., et al. (2012). Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 302 G77–G84. 10.1152/ajpgi.00301.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotou M. G., Limnios D., Nikolaou A., Psarra A., Kokotos G. (2017). Inhibitors of phospholipase A2 and their therapeutic potential: an update on patents (2012-2016). Expert. Opin. Ther. Pat. 27 217–225. 10.1080/13543776.2017.1246540 [DOI] [PubMed] [Google Scholar]
- Korean Statistical Information Service [KOSIS], (2018). Cause of Death Statistics. Available at: http://kosis.kr/ (accessed September 19, 2018). [Google Scholar]
- Kremer A. E., Martens J. J., Kulik W., Ruëff F., Kuiper E. M., van Buuren H. R., et al. (2010). Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology 139 1008–1018. 10.1053/j.gastro.2010.05.009 [DOI] [PubMed] [Google Scholar]
- Kuhajda F. P., Jenner K., Wood F. D., Hennigar R. A., Jacobs L. B., Dick J. D., et al. (1994). Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc. Natl. Acad. Sci. U.S.A. 91 6379–6383. 10.1073/pnas.91.14.6379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw Y. F., Leung N., Kao J. H., Piratvisuth T., Gane E., Han K. H., et al. (2008). Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol. Int. 2 263–283. 10.1007/s12072-008-9080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Nie H., Li Y., Zhan C., Liu X., Shi X., et al. (2015). Comprehensive characterization and evaluation of hepatocellular carcinoma by LC–MS based serum metabolomics. Metabolomics 11 1381–1393. 10.1007/s11306-015-0797-4 [DOI] [Google Scholar]
- Martínez L., Torres S., Baulies A., Alarcón-Vila C., Elena M., Fabriàs G., et al. (2015). Myristic acid potentiates palmitic acid-induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaning de novo ceramide synthesis. Oncotarget 6 41479–41496. 10.18632/oncotarget.6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H., Ikeda H., Nakamura K., Ohkawa R., Masuzaki R., Tateishi R., et al. (2011). Autotaxin as a novel serum marker of liver fibrosis. Clin. Chim. Acta 412 1201–1206. 10.1016/j.cca.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Onodera T., Fukuhara A., Shin J., Hayakawa T., Otsuki M., Shimomura I. (2017). Eicosapentaenoic acid and 5-HEPE enhance macrophage-mediated Treg induction in mice. Sci. Rep. 7:4560. 10.1038/s41598-017-04474-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J. K., Hepgul N., Higginson I. J., Gao W. (2019). Symptom prevalence and quality of life of patients with end-stage liver disease: a systematic review and meta-analysis. Palliat. Med. 33 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleli T., Martin D., Kronenberger B., Brunner F., Köberle V., Grammatikos G., et al. (2014). Serum autotaxin is a parameter for the severity of liver cirrhosis and overall survival in patients with liver cirrhosis–a prospective cohort study. PLoS One 9:e103532. 10.1371/journal.pone.0103532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P., Baillie R. A., Wiest M. M., Mirshahi F., Choudhury J., Cheung O., et al. (2007). A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46 1081–1090. [DOI] [PubMed] [Google Scholar]
- Qi S. W., Tu Z. G., Peng W. J., Wang L. X., Ou-Yang X., Cai A. J., et al. (2012). 1H NMR-based serum metabolic profiling in compensated and decompensated cirrhosis. World J. Gastroenterol. 18 285–290. 10.3748/wjg.v18.i3.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux V., Lemarchal P., Legrand P. (2000). Myristic acid, unlike palmitic acid, is rapidly metabolized in cultured rat hepatocytes. J. Nutr. Biochem. 11 198–207. 10.1016/s0955-2863(00)00065-6 [DOI] [PubMed] [Google Scholar]
- Ristić-Medić D., Takić M., Vučić V., Kandić D., Kostić N., Glibetić M. (2013). Abnormalities in the serum phospholipids fatty acid profile in patients with alcoholic liver cirrhosis - a pilot study. J Clin. Biochem. Nutr. 53 49–54. 10.3164/jcbn.12-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaei A., Arefi Oskouie A., Mohebbi S. R., Rezaei-Tavirani M., Mahboubi M., Peyvandi M., et al. (2016). Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol. Hepatol. Bed Bench 9 158–173. [PMC free article] [PubMed] [Google Scholar]
- Scorza M., Elce A., Zarrilli F., Liguori R., Amato F., Castaldo G. (2014). Genetic diseases that predispose to early liver cirrhosis. Int. J. Hepatol. 2014:713754. 10.1155/2014/713754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. R. (2006). The genetic tyrosinemias. Am. J. Med. Genet. Part C Semin. Med. Genet. 142C 121–126. 10.1002/ajmg.c.30092 [DOI] [PubMed] [Google Scholar]
- Sohn W., Jun D. W., Kwak M. J., Park Q., Lee K. N., Lee H. L., et al. (2013). Upper limit of normal serum alanine and aspartate aminotransferase levels in Korea. J. Gastroenterol. Hepatol. 28 522–529. 10.1111/j.1440-1746.2012.07143.x [DOI] [PubMed] [Google Scholar]
- The Human Metabolome Database [HMDB], (2019). 13S-Hydroxyoctadecadienoic Acid. Available at: http://www.hmdb.ca/ (accessed January 11, 2019). [Google Scholar]
- Tuero G., González J., Sahuquillo L., Freixa A., Gomila I., Elorza M. Á., et al. (2018). Value of glycolic acid analysis in ethylene glycol poisoning: a clinical case report and systematic review of the literature. Forensic Sci. Int. 290 e9–e14. 10.1016/j.forsciint.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Ullah R., Rauf N., Nabi G., Ullah H., Shen Y., Zhou Y.-D., et al. (2019). Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: recent updates. Int. J. Biol. Sci. 15 265–276. 10.7150/ijbs.30121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés-Rives S. A., González-Arenas A. (2017). Autotaxin-lysophosphatidic acid: from inflammation to cancer development. Mediators Inflamm. 2017:9173090. 10.1155/2017/9173090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liu W., Yao L., Zhang X., Zhang X., Ye C., et al. (2017). Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br. J. Pharmacol. 174 2358–2372. 10.1111/bph.13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Saito S., Tsuchida T., Higuchi K., Okita M. (1999). Low plasma levels of docosahexaenoic acid in patients with liver cirrhosis and its correction with a polyunsaturated fatty acid-enriched soft oil capsule. Nutrition 15 284–288. 10.1016/s0899-9007(99)00004-0 [DOI] [PubMed] [Google Scholar]
- World Health Organization [WHO], (2011). International Statistical Classification of Diseases and Related Health Problems, 3rd Edn Geneva: WHO Press. [Google Scholar]
- Wree A., Broderick L., Canbay A., Hoffman H. M., Feldstein A. E. (2013). From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 10 627–636. 10.1038/nrgastro.2013.149 [DOI] [PubMed] [Google Scholar]
- Yaligar J., Teoh W. W., Othman R., Verma S. K., Phang B. H., Lee S. S., et al. (2016). Longitudinal metabolic imaging of hepatocellular carcinoma in transgenic mouse models identifies acylcarnitine as a potential biomarker for early detection. Sci. Rep. 6:20299. 10.1038/srep20299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R., Morita S., Aoki H., Nakagawa Y., Yamamoto I., Inokuchi S. (2011). Acute renal failure and metabolic acidosis due to oxalic acid intoxication: a case report. Tokai J. Exp. Clin. Med. 36 116–119. [PubMed] [Google Scholar]
- Yang H., Rossignol F., Cyr D., Laframboise R., Wang S. P., Soucy J. F., et al. (2017). Mildly elevated succinylacetone and normal liver function in compound heterozygotes with pathogenic and pseudodeficient FAH alleles. Mol. Genet. Metab. Rep. 14 55–58. 10.1016/j.ymgmr.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Zheng X., Xing F., Zhuo H., Liu C. (2014). Serum metabolomic characteristics of patients with liver cirrhotic ascites. Integr. Med. Int. 1 136–143. [Google Scholar]
- Yoo H. J., Kim M., Kim M., Kang M., Jung K. J., Hwang S., et al. (2018). Analysis of metabolites and metabolic pathways in breast cancer in a Korean prospective cohort: the Korean Cancer Prevention Study-II. Metabolomics 14:85. 10.1007/s11306-018-1382-4 [DOI] [PubMed] [Google Scholar]
- Yuan J. M., Grouls M., Carmella S. G., Wang R., Heskin A., Jiang Y., et al. (2019). Prediagnostic levels of urinary 8-epi-prostaglandin F2α and prostaglandin E2 metabolite, biomarkers of oxidative damage and inflammation, and risk of hepatocellular carcinoma. Carcinogenesis 40 989–997. 10.1093/carcin/bgy180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Liu Y., Liu M., Liu B., Li N., Dong X., et al. (2019). UHPLC-QTOF/MS-based metabolomics investigation for the protective mechanism of Danshen in Alzheimer’s disease cell model induced by Aβ1–42. Metabolomics 15:13. [DOI] [PubMed] [Google Scholar]
- Zhou W. C., Zhang Q. B., Qiao L. (2014). Pathogenesis of liver cirrhosis. World J. Gastroenterol. 20 7312–7324. 10.3748/wjg.v20.i23.7312 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.