Abstract
In this review, we describe the sex differences in prevalence, onset, symptom profiles, and disease outcome that are evident in schizophrenia, bipolar disorder, and post‐traumatic stress disorder. Women with schizophrenia tend to exhibit less disease impairment than men. By contrast, women with post‐traumatic stress disorder are more affected than men. The most likely candidates to explain these sex differences are gonadal hormones. This review details the clinical evidence that oestradiol and progesterone are dysregulated in these psychiatric disorders. Notably, existing data on oestradiol, and to a lesser extent, progesterone, suggest that low levels of these hormones may increase the risk of disease development and worsen symptom severity. We argue that future studies require a more inclusive, considered analysis of gonadal steroid hormones and the intricacies of the interactions between them, with methodological rigour applied, to enhance our understanding of the roles of steroid hormones in psychiatric disorders.
LINKED ARTICLES
This article is part of a themed section on The Importance of Sex Differences in Pharmacology Research. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.21/issuetoc
Abbreviations
- APA
American Psychiatric Association
- BD
bipolar disorder
- DHEA
dehydroepiandrosterone
- PTSD
post‐traumatic stress disorder
1. INTRODUCTION
Sex differences are prevalent in disorders affecting the CNS. Schizophrenia, bipolar disorder (BD), post‐traumatic stress disorder (PTSD), major depressive disorder, autism spectrum disorder, Alzheimer's disease, multiple sclerosis, motor neuron disease, and Parkinson's disease are examples of such conditions that report sexually dimorphic incidence rates, symptomology, or prognosis (Christiansen & Hansen, 2015; Diflorio & Jones, 2010; Gogos et al., 2015; Pinares‐Garcia, Stratikopoulos, Zagato, Loke, & Lee, 2018; Sanchez, Bourque, Morissette, & Di Paolo, 2010). While there are a number of biological, cultural, and environmental factors that may underlie these sex differences, the important role of gonadal steroid hormones has become increasingly evident in recent decades. Steroids have potent and widespread effects in the brain, and the role of gonadal hormones has been of particular interest (Gogos et al., 2015; Pinares‐Garcia et al., 2018). Accumulating evidence demonstrates the influence of gonadal steroid hormones on cognition and various pathophysiological pathways, in addition to their therapeutic potential. Throughout the lifespan, mammalian females experience various endogenous fluctuations in ovarian hormone levels, which can influence the symptom profile of CNS disorders. Such fluctuations include the recurring oscillations of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1013 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2377 across the reproductive cycle (i.e., the menstrual cycle), the dramatic changes throughout pregnancy and post‐partum that orchestrate reproduction, and the overall decline in circulating hormone levels associated with reproductive senescence (i.e., menopause; Sbisa, van den Buuse, & Gogos, 2017; Sun, Walker, Dean, van den Buuse, & Gogos, 2016). Researchers have utilised these natural variations in oestradiol and progesterone to investigate their effects on the expression of psychiatric disorders and subsequently glean insights into the mechanisms of endogenous gonadal hormones and how they may act in concert with each other. More recently, the effects of exogenous hormone administration have been investigated, highlighting their therapeutic potential (Kulkarni et al., 2014; Kulkarni, Gavrilidis, Worsley, & Hayes, 2012). In this review, therefore, we summarise the literature describing sex differences in certain psychiatric disorders and then probe whether these differences are due to the influence of selected steroid hormones. We outline the evidence of steroid hormone dysregulation in each disorder, emphasising the similarities across disorders. The integral role of oestradiol and progesterone in psychiatry is highlighted, and a number of unanswered questions posed as improvements for further studies are suggested.
This review will focus on schizophrenia, BD, and PTSD, three sexually dimorphic psychiatric disorders that show similarities in clinical/cognitive symptomatology, aetiology, and neurobiology. In the next section, we detail evidence of sex differences in these three disorders, focussing on the differences between men and women in disease incidence, onset, and symptom expression (summarised in Table 1).
Table 1.
Converged sex and hormone‐related effects across disorders
| Schizophrenia | Bipolar disorder | Post‐traumatic stress disorder | |
|---|---|---|---|
| Differences at the level of sex | |||
| Incidence | M > F |
M > F Type I M < F Type II |
M < F |
| Age of onset | M < F | M < F # | Unknown |
| Symptom severity | M > F | F = M, depending on BD type | M < F |
| Symptom presentation | M > F for comorbid issues |
M > F for mania M < F for depression |
M < F for dissociation and threat sense |
| Treatment response | M < F for response to treatment, remission, recovery | Unknown | M < F for response to treatment |
| Interactions and effects of gonadal steroid hormones | |||
| Blood oestradiol levels | ↓ in M and F | Mixed/inconclusive | Unknown |
| Blood progesterone levels | ↓ in M and F | Mixed/inconclusive | ↓ in F |
| Menstrual cycle effects | ↑ symptoms during low‐hormone phases | ↑ symptoms during low‐hormone phases | ↑ symptoms during low‐hormone phases# |
| Effects of menopause | ↑ symptoms, prevalence | ↑ depression and ↓ mania | Unknown |
| Effects of exogenous oestradiol | Oestradiol and raloxifene improved symptoms in M and F | Oestradiol improved manic and psychotic symptoms | Oestradiol improved performance in analogue trauma tasks in non‐PTSD subjects |
M: Male; F: Female; <: less or worse; >: greater or better; ↑: increased; ↓: decreased; #: preliminary or weaker evidence.
2. SEX DIFFERENCES IN PSYCHIATRIC DISORDERS
2.1. Schizophrenia
Schizophrenia is a chronic disorder characterised by multiple symptom categories, including positive psychotic symptoms, negative symptoms, disordered thoughts or speech, and cognitive deficits (American Psychiatric Association [APA], 2013). Sex differences in schizophrenia are widely documented, reportedly affecting several domains including disease onset, course of illness, and symptom profiles (Gogos et al., 2015; Ochoa, Usall, Cobo, Labad, & Kulkarni, 2012; Sun et al., 2016). The incidence of schizophrenia is greater in men (1.4:1 male : female ratio; McGrath et al., 2004), who typically experience peak onset between the ages of 18 and 25, approximately 4 years earlier than women (Galderisi, Bucci, Ucok, & Peuskens, 2012; Hafner, 2003). Additionally, women uniquely exhibit a second surge of disease onset around 45–49 years, suggested to be attributed to the decline of ovarian hormones due to the menopause (Hafner, 2003; Sun et al., 2016). Sex differences in disease progression and prognosis have also been observed, with men showing less responsiveness to antipsychotic medication (Szymanski et al., 1995) and longer, more frequent hospitalisations (Szymanski et al., 1995; Usall et al., 2003). Men also exhibit greater substance abuse (Koster, Lajer, Lindhardt, & Rosenbaum, 2008; Ochoa et al., 2012), social isolation, and withdrawal than women (Koster et al., 2008; Zhang et al., 2012), who have better social prognoses, including retention of marriages, interpersonal relationships (Hafner, 2003; Vila‐Rodriguez, Ochoa, Autonell, Usall, & Haro, 2011), and employment (Cotton et al., 2009; Vila‐Rodriguez et al., 2011). Furthermore, women experience higher rates of remission and recovery than men (Carpiniello, Pinna, Tusconi, Zaccheddu, & Fatteri, 2012). While the sex differences in schizophrenia are typically observed postdiagnosis, a recent review suggests their presence prior to the onset of clinically detectable symptoms, with men displaying poorer premorbid functioning than women, including greater social withdrawal, isolation, and poor self‐care (Mendrek & Mancini‐Marie, 2016).
In addition to the sex differences in social and behavioural domains, men with schizophrenia experience more brain morphological abnormalities than women. For example, gross anatomical studies show greater ventricular enlargement (Narr et al., 2001), and more severe frontal and temporal lobe atrophy in men (Bryant, Buchanan, Vladar, Breier, & Rothman, 1999; Narr et al., 2001). Furthermore, men also show greater abnormalities in white matter microstructure (Kanaan et al., 2012; Kelly et al., 2018).
Research regarding sex differences in symptomology in schizophrenia remains less clear. Some studies report that women suffer more positive (Thorup et al., 2007) and affective symptoms than men (Ochoa et al., 2012; Zhang et al., 2012), and several studies suggest milder symptoms overall in women, including fewer cognitive deficits (Han et al., 2012) and less severe negative symptoms (Bakhshi & Chance, 2015; Galderisi et al., 2012; Grossman, Harrow, Rosen, Faull, & Strauss, 2008; Koster et al., 2008). By contrast, others failed to identify any sex differences in positive or negative symptoms (Han et al., 2012; Ochoa et al., 2012; Szymanski et al., 1995). Furthermore, both men (Morgan, Castle, & Jablensky, 2008) and women (Szymanski et al., 1995) have been reported as suffering greater depressive symptoms, and women experience worse negative symptoms than men (Galderisi et al., 2012). There are several possible explanations for these discrepancies, including inconsistent methodology, sampling variation and size, and the use of different symptom evaluation tools, particularly when comparing earlier versus recent studies. Sex‐specific differences in comorbid and psychosocial factors including substance abuse (Riecher‐Rössler, Butler, & Kulkarni, 2018), and access to, or engagement with social supports may also explain some of the sex differences observed (Grossman et al., 2008). These issues highlight the need for further investigation into sex differences using a systematic approach to control for confounding variables.
2.2. Bipolar disorder
BD is a complex disorder characterised by extreme fluctuations in mood, from manic highs to depressive lows (APA, 2013). Two diagnostic subtypes are recognised: BD I—characterised by at least one episode of full blown mania that affects functioning or BD II—characterised by a more short‐lived and less severe form of mania, called hypomania, that occurs alongside episodes of depression. The lifetime incidence of BD is approximately 1:1 in men and women, although the incidence of manic episodes and unipolar mania is higher in men with the disease (Diflorio & Jones, 2010). Research suggests an increased prevalence of BD II and hypomania in women, with general functioning being significantly better for men with this BD subtype (Diflorio & Jones, 2010). Women with BD also report increased rates of rapid cycling in some but not all studies (Baldassano et al., 2005; Diflorio & Jones, 2010; Robb, Young, Cooke, & Joffe, 1998). Reports of sex differences in psychosis symptoms are inconsistent, with some studies finding an increased prevalence in men versus women (Morgan, Mitchell, & Jablensky, 2005) or vice versa (Bräunig, Sarkar, Effenberger, Schoofs, & Krüger, 2009) and others finding no differences at all (Kessing, 2004). Comorbid phobia, panic disorder, PTSD, eating disorders, and borderline personality disorder are more frequently reported in women than men with BD, while higher rates of comorbid conduct and substance use disorders are reported in men (Diflorio & Jones, 2010; Suominen et al., 2009).
Most extant studies indicate an equal age of onset across the sexes, although some have reported that women may be slightly older than men when the disease is manifested (Diflorio & Jones, 2010; Kawa et al., 2005; Robb et al., 1998; Suppes et al., 2001). Recurrent depressive polarity and a depressive or mixed onset has been shown to predominate in women with BD (Kessing, 2004; Viguera, Baldessarini, & Tondo, 2001), while mania may be more prevalent in men at first onset (Kawa et al., 2005; Suppes et al., 2001). Owing to inconsistent literature, it is not clear if there are sex differences in the number of depressive or manic episodes (Baldassano et al., 2005; Diflorio & Jones, 2010; Robb et al., 1998). However, some studies do show an increased use of antidepressant treatment in women with BD, as well as of benzodiazepines, ECT, and psychotherapy (Baldassano et al., 2005; Karanti et al., 2015). On the other hand, men appear to be treated with lithium more often (Karanti et al., 2015), but sex differences in its clinical response are not evident (Viguera et al., 2001). Women with BD have much increased rates of hypothyroidism (when lithium‐treated) and are at increased risk of migraine, compared to men; while rates of metabolic syndrome appear to be equal across the sexes (Diflorio & Jones, 2010; Saunders et al., 2014).
Sex‐specific effects on cognitive profiles have been largely unexplored in the BD literature. However, there is some evidence to show that men with BD perform worse on visuospatial construction (Gogos, Joshua, & Rossell, 2010) and better on spatial memory and sustained attention tasks (Bücker et al., 2014) compared to women with BD but not controls. Men with BD also have worse verbal memory performance (Carrus et al., 2010), worse recognition of happy prosody (Van Rheenen & Rossell, 2013), and reduced sensorimotor gating (Gogos, van den Buuse, & Rossell, 2009) compared to male controls; while worse recognition of surprise and fear prosody (Bozikas et al., 2007) and increased sensorimotor gating (Gogos et al., 2009) are seen in women with BD, compared with female controls. One study showed that women with BD experience greater cognitive benefit from vigorous physical activity than men with BD (Fellendorf et al., 2017).
In terms of brain morphology, there is no evidence of sex differences in global grey or white matter, limbic, or ventricular and sulcal volumes (Jogia, Dima, & Frangou, 2012). Reduced grey matter volume of the medial prefrontal cortex, however, has been reported in men with BD compared to male controls (Almeida et al., 2009; Jogia et al., 2012). Further, right hippocampal volume loss is evident in women versus men with BD (Shi et al., 2018) but not controls. Women with BD also exhibit abnormal neural engagement of the caudate and prefrontal cortex during social–cognitive tasks compared to female controls (Jogia et al., 2012). Overall, compared to schizophrenia, there is very little research investigating sex differences in BD. However, the available data show that there are sex differences in a number of domains of BD, supporting the idea that sex is an important consideration in BD.
2.3. Post‐traumatic stress disorder
PTSD is a common disorder that occurs after experiencing a traumatic event involving threat to life or physical integrity (APA, 2013). It is characterised by re‐experiencing symptoms including intrusive memories and nightmares of the trauma, avoidance of trauma reminders, hyper‐arousal, and dysregulation in mood, cognition, and in the hypothalamic–pituitary axis (Pitman et al., 2012). There are several known risk factors for PTSD, including previous psychiatric or trauma history, family psychiatric history, and age at trauma onset. One of the most robust known risk factors of PTSD is being female. Large scale epidemiological and prospective studies consistently report that women develop PTSD at approximately twice the rate of men following trauma (Christiansen & Hansen, 2015; Kessler et al., 2017; Olff, 2017; Tolin & Foa, 2006). Longitudinally, the PTSD sex difference is highest between the ages of 21 and 25, with men and women reporting their highest rates of PTSD in their 40s and 50s respectively (Ditlevsen & Elklit, 2010).
Sex differences in the risk of PTSD are not attributable to measurement errors, reporting bias or methodological issues (Christiansen & Hansen, 2015; Tolin & Foa, 2006). A multiplicity of factors (social, cognitive, biological, and genetic) may contribute to this sex difference. For example, women are exposed to greater interpersonal and sexual violence, which lead to greater rates of PTSD (Christiansen & Hansen, 2015; Forbes et al., 2014; Kessler et al., 2017; Olff, 2017). In contrast, men are typically exposed to more industrial accidents, war, and combat trauma and physical assaults (Kessler et al., 2017). This raises the question, if women are associated with more toxic forms of trauma exposure such as sexual violence, might this explain their heightened risk of developing PTSD? The most definitive test of whether sex differences in PTSD can be attributed to sex differences in prevalence of exposure to different types of trauma was in the largest study in a U.S. sample of >34,000 participants (Blanco et al., 2018). Lifetime prevalence for PTSD was 9.48% in women and 5.97% in men, and women experienced childhood maltreatment and assaultive violence more often than men. However, differences in the prevalence of exposure to varying trauma types did not explain the differential risk of PTSD in women and men. In fact, most types of trauma (17 out of 19) were associated with greater risk of PTSD in women than in men. A key finding of this study was that a difference in reactivity to trauma, regardless of the type of trauma, explained more than 60% of the differential PTSD risk in women and men (Blanco et al., 2018).
In epidemiological studies, there are sex differences in initial reactivity to trauma with increased peri‐traumatic dissociation and perception of life threat reported as more prevalent immediate responses in women than men (Bryant & Harvey, 2003; Irish et al., 2011; Solomon, Gelkopf, & Bleich, 2005). Differences in symptom presentation appear to dissipate somewhat over time, with studies reporting similar symptomology between men and women, albeit women reporting stronger intensity across all symptoms (Carragher et al., 2016; Murphy, Elklit, Chen, Ghazali, & Shevlin, 2018). There are few studies reporting sex differences in standard experimental tasks that assess PTSD mechanisms and risk factors. In one imaging study, however, increased blood‐oxygen‐level dependent activation was observed in the right amygdala and anterior cingulate cortex of healthy women compared to men during a fear conditioning task, a task where reduction in arousal to analogue trauma (e.g., electric shock) is measured physiologically (Lebron‐Milad et al., 2012). Further, we showed that women have greater negative memory consolidation than men in response to stress‐induction, suggesting a potential mechanism for the greater female susceptibility to develop PTSD (Felmingham, Tran, Fong, & Bryant, 2012). In this study, participants viewed negative, neutral, and positive images and were then exposed to a stress‐induction test (i.e., cold‐pressor stress task) immediately following encoding and stress hormones (cortisol and salivary alpha amylase) were recorded. Although women and men showed similar stress‐induced increases in cortisol, women showed significantly greater recall of negative images that suggests greater negative memory consolidation following stress in women (Felmingham, Tran, et al., 2012).
Women appear to be more responsive to PTSD treatment than men, with studies reporting better treatment gains and maintenance to exposure and behavioural therapies in female patients (Felmingham & Bryant, 2012; Galovski, Blain, Chappuis, & Fletcher, 2013; Stenmark, Guzey, Elbert, & Holen, 2014). Most recently, a meta‐analysis of 48 of these studies confirmed that women indeed have better treatment responses than men, though this primarily examined studies employing behavioural interventions, and differences in responses to pharmacological treatment were not reported (Wade et al., 2016). Interestingly, it is also reported that women with, but not without, PTSD express higher fear responses to a conditioned stimulus than men (Inslicht et al., 2013), which may explain the success during exposure therapy, due to the reliance of the technique on effective reactivation of trauma memories. However, there has not been sufficient research to identify why treatment responses are better in women, with tentative explanations involving increased learning capacity in females during both trauma experience and extinction training (Felmingham & Bryant, 2012).
3. ARE GONADAL STEROID HORMONES THE MEDIATING FACTOR?
The neurobiological complexity of the psychiatric disorders described above contributes to the fact that the exact mechanisms underlying the aetiology of these disorders remain elusive. However, the most commonly proposed candidate to explain the sex differences described above is the dysfunction of neuroactive steroid systems (Gogos et al., 2015; Sun et al., 2016). For example, the above observations underpin the oestrogen hypothesis of schizophrenia, which postulates a protective role of oestrogens against the development and severity of the disorder (Gogos et al., 2015; Seeman & Lang, 1990). Similarly, in relation to PTSD, evidence of sex differences has led to the hypothesis that low oestradiol results in poor extinction recall. The reproductive life cycle in women makes them particularly sensitive to rapid and/or cyclical variation in levels of circulating ovarian hormones at different time periods. In this section, we briefly describe the two primary ovarian hormones and then detail evidence of gonadal steroid dysfunction in each of the psychiatric disorders of interest (summarised in Table 1).
3.1. The main female steroid hormones: Oestrogens and progesterone
The ovarian hormones, oestrogens and progesterone, are also neuroactive steroids that play a crucial role in glial and neuronal development, structure, and function (Barth, Villringer, & Sacher, 2015). Of these, progestogens are a class of neuroactive steroids that bind to and activate progesterone receptors. In humans, endogenous progesterone (P4) is the most potent and abundant progestogen subtype. It is synthesised both centrally and peripherally from http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2376, a cholesterol‐derived steroid precursor (Sun et al., 2016; Tsutsui, 2008; Figure 1). As a precursor to oestrogens, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2858 (the main male gonadal hormone), and various glucocorticoids and mineralocorticoids, progesterone plays a crucial role in both male and female health and development. In adult males, serum progesterone levels reportedly range between 1 and 3 nM with little change relative to age (Oettel & Mukhopadhyay, 2004; Sun et al., 2016). In contrast, non‐pregnant premenopausal women experience variable serum progesterone levels relative to their menstrual cycle (Figure 2), with lowest concentrations reported during the early follicular phase (0.3–2 nM) and maximum concentrations during the midluteal phase (20–55 nM; Mumford et al., 2010; Sun et al., 2016). Progesterone levels are highest during pregnancy, with serum progesterone steadily increasing throughout the gestational period and reaching peak concentrations of up to fourfold non‐pregnant levels at term (Byrns, 2014; Schock et al., 2016). By contrast, serum progesterone levels dramatically decline following menopause (Santoro, 2005).
Figure 1.
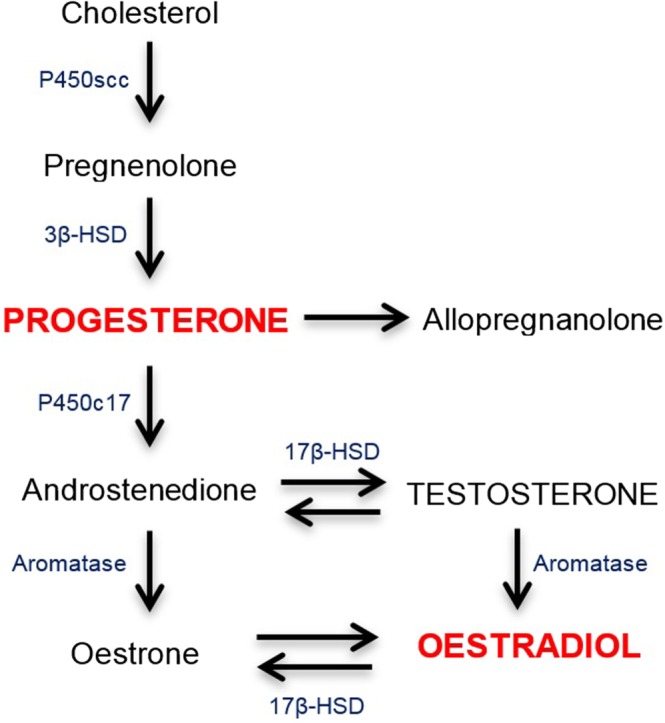
Simplified biosynthesis pathway for progesterone and oestradiol. HSD: hydroxysteroid dehydrogenase; P450: cytochrome P450; P450scc: cholesterol side‐chain cleavage enzyme (adapted from Sun et al., 2016)
Figure 2.
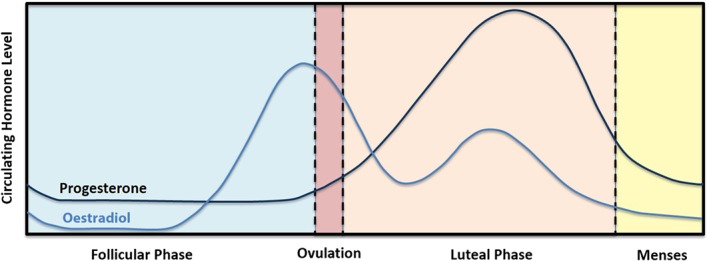
Fluctuating circulating levels of 17β‐oestradiol and progesterone in women across the menstrual cycle (adapted from Sun et al., 2016; Widmaier, Raff, & Strang, 2014)
The term oestrogen describes a class of structurally and functionally similar compounds, the most common of which are http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2818 (E1); http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1013” (E2); and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2821 (E3; Thomas & Potter, 2013). Of these, oestradiol is the most potent endogenous oestrogen in humans (Gogos et al., 2015; Jeyakumar, Carlson, Gunther, & Katzenellenbogen, 2011) and the focal oestrogen of this review. The synthesis of oestrogens occurs both centrally and peripherally and is mediated via aromatisation of testosterone (Figure 1). Oestradiol is present in both males and females, although adult males show considerably lower total serum oestradiol than females (140 pM; Sun et al., 2016; Yamamoto, Hibi, Katsuno, & Miyake, 1995), with minimal fluctuations or age‐related decline (Greenblatt, Oettinger, & Bohler Clorinda, 1976; Orwoll et al., 2006). Conversely, non‐pregnant premenopausal women exhibit biphasic cyclic variation in serum oestradiol during their 28‐day menstrual cycle (Figure 2), with the lowest oestradiol levels during the early follicular phase (median ~150 pM), the highest levels during ovulation (median ~670 pM), and a second, smaller peak during the midluteal phase (median ~500 pM; Stricker, Eberhart, Chevailler, Quinn, & Bischof, 2006; Sun et al., 2016). During pregnancy, oestradiol levels increase up to ninefold but drop dramatically post‐partum (Schock et al., 2016). Similarly, oestradiol levels decline dramatically following menopause, reaching levels ≤100 pM (Burger et al., 1998).
Steroid hormones may act via either organisational or activational effects. Organisational mechanisms refer to the effects of steroid hormones during prenatal and early postnatal development that result in permanent effects on the brain, while activational mechanisms are the receptor‐mediated transient actions of steroid hormones that occur predominantly in adulthood. Throughout the brain, progesterone and oestradiol function by directly binding to receptors that act via genomic and nongenomic mechanisms (Gogos et al., 2015; Newton‐Mann, Finney, Purves‐Tyson, & Gogos, 2017; Petersen et al., 2013). Of these, the most common of the genomic receptors are ligand‐modulated transcription factors known as http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=627‐A and ‐B, and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=96‐α and ‐β. Following the diffusion of the steroid hormone across the cellular membrane, it then activates its cytoplasmic receptor, triggering conformational changes that result in receptor homo‐ or hetero‐dimerisation. The dimerised receptors then translocate to the nucleus where they bind to promoter regions of target genes and regulate transcription (Ellmann et al., 2009). Dimerised oestrogen receptors specifically bind to activator protein 1 sites or oestrogen response elements, enabling transcription of oestrogen‐responsive genes (Newton‐Mann et al., 2017). The non‐genomic effects of progesterone are primarily mediated via several membrane‐bound receptors whereas oestrogenic signalling also occurs via membrane‐bound receptors and the G protein‐coupled oestrogen receptor 1. The non‐genomic effects of these steroids are rapid and involve activation of a range of downstream effects (Newton‐Mann et al., 2017; Petersen et al., 2013).
To summarise, in women, oestradiol and progesterone levels fluctuate throughout the reproductive lifespan. Further, these levels are much higher in women than in men. While progesterone primarily exerts its effects via activating its receptors, it also has significant indirect effects via its conversion into a number of neuroactive molecules, including testosterone and oestrogens. This constant, complex interaction between steroid hormones needs to be recognised before one can consider the effects of hormones across the reproductive life cycle.
3.2. Evidence of gonadal steroid hormone dysregulation in schizophrenia
Women with schizophrenia often experience symptom fluctuation relative to their menstrual cycle phase and also experience high rates of irregular menses—a common symptom of gonadal hormone dysfunction (Gleeson et al., 2016). Further, the cyclical exacerbation of symptoms corresponds to periods of low levels of oestradiol and progesterone in the menstrual cycle (Bergemann, Parzer, Runnebaum, Resch, & Mundt, 2007; Hoff et al., 2001; Kumari et al., 2010), during which times women also experience increased rates of hospitalisation (Bergemann et al., 2002). Similarly, symptom improvements during pregnancy and worsening of symptoms post‐partum correspond to increased and decreased gonadal hormone levels respectively (Riecher‐Rössler, 2017). This is also true for the midlife surge of female diagnoses, which correspond with the menopause (Ochoa et al., 2012), with women over the age of 40 receiving new diagnoses at approximately twice the rate of men (Riecher‐Rössler, 2017).
The above findings may be explained, at least in part, to chronic hypooestrogenism in schizophrenic patients. Low serum oestradiol has been reported in both male (Belvederi Murri et al., 2016; Doğan Bulut, Bulut, & Güriz, 2016) and female (Bergemann et al., 2002; Bergemann et al., 2007) patients with schizophrenia and first‐episode psychosis with serum oestradiol varying relative to menstrual phase in females. Women showed reduced oestradiol levels throughout the entire menstrual cycle, with hypooestrogenism identified during the follicular phase (defined as serum oestradiol below 110 pM) and the peri‐ovulatory phase (defined as serum oestradiol below 370 pM; Bergemann et al., 2005). It is important to note that these effects are thought to be unrelated to antipsychotic drug treatment‐induced hyperprolactinemia, which can itself induce a hypooestrogenic state (McGregor, Riordan, & Thornton, 2017). Similarly, compared to healthy controls, lower serum progesterone has been reported in both male and female patients with schizophrenia (Bicikova, Hill, Ripova, Mohr, & Hampl, 2013; Doğan Bulut et al., 2016; Ritsner, Maayan, Gibel, & Weizman, 2007). Antipsychotic medications were reported to significantly increase pregnenolone levels in the brain, suggesting that correction of aberrant progesterone signalling may be partially involved in their efficacy (Ritsner, 2011). A recent meta‐analysis found elevated levels of circulating http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2370 sulphate (DHEA‐S) and testosterone levels in both male and female patients with schizophrenia or first‐episode psychosis (Misiak et al., 2018). Numerous studies report a negative correlation between serum testosterone and the severity of negative symptoms and cognitive deficits in male patients with schizophrenia (Akhondzadeh et al., 2006; Ko et al., 2007; Shirayama, Hashimoto, Suzuki, & Higuchi, 2002; Sisek‐Sprem et al., 2015). While further research is needed, these findings suggest dysfunction at multiple levels of the neurosteroid biosynthetic pathway.
At present, research into the use of hormonal therapies in schizophrenia is providing promising results (reviewed in Gogos et al., 2015; Sbisa et al., 2017). Studies by Kulkarni and colleagues demonstrated that combined antipsychotic and oestradiol therapy significantly improved symptoms in premenopausal female patients, particularly the positive symptoms, and improved general psychopathology in male patients, without causing feminising side effects (reviewed in Kulkarni et al., 2012). Similarly, a double‐blind placebo‐controlled trial found that combined administration of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=86 and a synthetic oestradiol significantly improved both positive and negative symptoms in premenopausal women with chronic schizophrenia (Akhondzadeh et al., 2003). Unfortunately, long‐term use of oestradiol may be associated with side effects including increased cardiovascular and cancer risk (Sbisa et al., 2017). Additionally, the ligand‐binding domains for oestrogen receptor‐α and ‐β are highly homologous (Newton‐Mann et al., 2017), making it difficult to selectively target these receptors using orthosteric ligands. In an attempt to subvert these issues, researchers are exploring the therapeutic potential of selective oestrogen receptor modulators, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2820, that elicit tissue‐dependent and/or receptor‐dependent agonist or antagonist effects (Sbisa et al., 2017). While the exact mechanism of action of raloxifene in the CNS is unclear, it has high affinity for both oestrogen receptor‐α and ‐β (Newton‐Mann et al., 2017). However, depending on sex, reproductive status, and hormone levels, the clinical efficacy of raloxifene is likely to vary (Sbisa et al., 2017). Nevertheless, reviews have concluded that combined antipsychotic and raloxifene therapy improves both positive and negative symptoms in women with schizophrenia and improves negative symptoms in men with schizophrenia (Bratek, Krysta, Drzyzga, Baranska, & Kucia, 2016; McGregor et al., 2017). In perimenopausal and postmenopausal women (aged 40–70 years) with treatment‐resistant schizophrenia, raloxifene reduced symptom severity and improved likelihood of clinical response (Kulkarni et al., 2016). By contrast, another study found that severely ill, postmenopausal women with schizophrenia receiving adjunct raloxifene did not show symptom improvement and in fact experienced worsened positive and negative symptoms (Weiser et al., 2017). The authors concluded that while raloxifene may be beneficial for some patients, further research is needed to identify suitable subgroups for safe administration.
Interestingly, one study found that adjunctive low‐dose pregnenolone improved positive symptoms, extrapyramidal side effects, and several measures of executive function in chronically ill male and female patients (Ritsner, 2010). Furthermore, progesterone administration has been shown to improve cognition in healthy women (Berent‐Spillson et al., 2015) suggesting it may be of benefit for the cognitive deficits in schizophrenia. However, progesterone and its receptors have received considerably less attention than oestradiol in the neuropsychiatric community. Thus, further research is needed to better understand the role of progesterone and other neuroactive compounds in schizophrenia (Sun et al., 2016). Overall, there is strong evidence for a role of steroid hormones in schizophrenia and, in particular, that reduced oestradiol levels may be a predisposing factor to disease development and symptom severity in at‐risk populations.
3.3. Evidence of gonadal steroid hormone dysregulation in BD
A large proportion of women with BD have menstrual abnormalities that precede diagnosis (Rasgon et al., 2005). There are a number of studies providing evidence that reproductive life cycle events associated with hormonal fluctuations, specifically a reduction in hormone levels, can influence the course of BD. For example, perimenstrual mood changes are reported in 40–65% of BD women (Blehar et al., 1998; Teatero, Mazmanian, & Sharma, 2014), and those women who experience perimenstrual mood changes are at higher risk for post‐partum relapse (Perich et al., 2017), a worse course of illness and increased symptom severity (Dias et al., 2011). Rapid cycling, comorbid anxiety, and mixed mood episodes, as well as an earlier age of mania or depression onset are also more frequently reported in BD women who have reported reproductive life cycle associated mood changes (Perich et al., 2017). The luteal phase of the menstrual cycle (both early and late luteal, i.e., low hormone levels) has been associated with greater manic/depressive symptoms compared to other phases (Rasgon, Bauer, Glenn, Elman, & Whybrow Peter, 2003; Shivakumar et al., 2008). An elevation of mood and psychosis symptoms post‐partum is common (Blehar et al., 1998; Jones, Chandra, Dazzan, & Howard, 2014), with one study reporting an eightfold increase in the prevalence of mania symptoms in BD women in the week following childbirth compared with the prevalence during pregnancy (Heron, Haque, Oyebode, Craddock, & Jones, 2009). Some clinical studies also show high rates of emotional disturbance with an onset in pregnancy, although epidemiological studies indicate that the prevalence rate for first time hospitalisations for a mood episode during pregnancy is low (Jones et al., 2014). The risk of BD relapse during the post‐partum period is estimated at 35%, with higher relapse rates in women who are medication‐naïve during pregnancy compared to those that are not (Wesseloo et al., 2015). Notably, conversion from major depressive disorder to BD also appears to be several fold higher in the first 6 months post‐partum than typically seen annually (Sharma et al., 2014). Transition to menopause is also commonly characterised by an increase in mood disturbance in women with BD (Blehar et al., 1998; Marsh, Gershenson, & Rothschild, 2015). Longitudinal evidence indicates that progression from premenopausal to postmenopausal stages is associated with a decrease in mood stability associated with increasing depressive and decreasing mania symptoms (Marsh et al., 2012).
While this clinical evidence implicates fluctuations in gonadal hormones in the phenomenology of BD in women, research on gonadal hormone and neurosteroid profiles in the disorder is sparse. One study reported significantly decreased CSF levels of pregnenolone in BD patients irrespective of sex, which was driven by patients who were depressed rather than euthymic at the time of testing (George et al., 1994). Another study reported that relative to controls, euthymic BD women had increased circulating progesterone and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4108 (active metabolite of progesterone) but not cortisol concentrations during the luteal phase of the menstrual cycle (Hardoy et al., 2006). In contrast, two other studies found that the progesterone and oestradiol levels of women with BD (either rapid cycling or with post‐partum BD relapse) did not differ from control women during either the midluteal or follicular phases of the menstrual cycle (Karadag et al., 2004; Wieck et al., 2003). Finally, testosterone levels were lower in depressed men with BD, but higher in depressed women with BD, compared to their respective controls (Wooderson, Gallagher, Watson, & Young, 2015). Higher testosterone levels during a depressive or mixed episode have been associated with an increased incidence of mania and suicide attempts in BD patients, irrespective of sex (Sher et al., 2012), with baseline testosterone predictive of future suicidal behaviour in depressed or mixed episode BD women (Sher et al., 2014).
Further evidence for the involvement of gonadal hormones in BD comes from studies where women were receiving exogenous oestrogenic treatments. A small study reported that there were no significant mood changes in women with BD who were taking oral contraceptives (containing combined synthetic oestradiol and progesterone) on the active versus inactive (placebo) days, but there was a slight but significant worsening of mood in BD women not taking oral contraceptives (first 7 days of cycle versus last seven days; Rasgon et al., 2003). Several small clinical trials also highlight the efficacy of the selective oestrogen receptor modulator, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1016 and one study showed benefits using the synthetic progesterone, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2879, for the treatment of acute mania (Kulkarni et al., 2014; Meinhard, Kessing, & Vinberg, 2014). In a study of women with post‐partum psychosis and oestrogen deficiency, psychosis symptoms were ameliorated with daily oestradiol treatment (Ahokas, Aito, & Rimón, 2000). Pregnenolone has also been shown to be efficacious for the treatment of BD depression, although the mechanism of action is unclear given that changes in depressive symptoms did not correlate with changes in serum pregnenolone or other neurosteroids (Brown et al., 2014). The evidence for steroid hormone dysregulation in women with BD is supported by studies showing a worsening of symptoms during times of low levels of gonadal hormones and by clinical trials suggesting that certain steroid hormones can improve specific symptoms of BD.
3.4. Evidence of gonadal steroid hormone dysregulation in PTSD
Gonadal hormones are thought to be a critical factor in the contrasting rates of PTSD diagnoses between men and women (Ney, Matthews, Bruno, & Felmingam, 2018; Rasmusson et al., 2017). Human studies have identified oestradiol as an important regulator of the recall of extinction memories (Glover, Jovanovic, & Norrholm, 2015), which is the quintessential process underlying recovery from a traumatic experience. This may be expected, given the known importance of oestradiol on memory, learning, and cognitive processes more generally (Frick, Kim, Tuscher, & Fortress, 2015; Gogos, Wu, Williams, & Byrne, 2014; Sbisa et al., 2017). In studies of fear extinction in healthy women, it has been found that higher oestradiol levels are associated with improved task performance, where participants learn that a previously aversive stimulus is no longer associated with an aversive experience (e.g., an electric shock) with success gauged by change in physiological arousal upon presentation of the stimulus (Graham & Milad, 2013; Milad et al., 2010; Wegerer, Kerschbaum, Blechert, & Wilhelm, 2014; Zeidan et al., 2011). Similar studies have found that women with higher levels of endogenous oestradiol more successfully extinguish fear memories compared to women using hormonal contraceptives (Graham & Milad, 2013; Li & Graham, 2016; White & Graham, 2016). The effect of menstrual cycle phase on fear extinction in healthy women is less consistent, with a large‐scale study finding no significant differences between phases (Lonsdorf et al., 2015).
Although no study has examined long‐term oestradiol levels in PTSD patients, there has been some research measuring gonadal hormones in PTSD. One study found that women with PTSD and low oestradiol levels had intensified physiological responses to an aversive laboratory stimulus, compared to non‐PTSD women with low oestradiol levels. This effect was absent in women with high oestradiol levels, where PTSD and non‐PTSD participants displayed similar responding to the aversive stimulus, strongly suggesting a protective effect of oestradiol on fear learning (Glover et al., 2012). Women with PTSD were also shown to exhibit increased phobic anxiety and depression during the early follicular, compared to midluteal, phase of the menstrual cycle (Nillni et al., 2015). In another study, women with PTSD in the midluteal phase of the menstrual cycle exhibited impaired fear extinction compared to women in the early follicular phase (Pineles et al., 2016), which is the reverse finding of healthy participants. While it is unclear what the mechanistic implications of these findings might be, recent genetic studies have identified oestradiol‐related risk factors for PTSD, with oestrogen‐response elements on the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=370&familyId=67&familyType=GPCR gene, PAC1R, associated with PTSD severity in women but not men (Lind et al., 2017). This is a significant finding as deficiency of the endogenous ligand, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2258, is associated with impaired production of transcriptional factors underlying both the hypothalamic–pituitary–adrenal and sympathetic stress responses in mice (Stroth & Eiden, 2010). The recent meta‐analysis by Lind et al. (2017) therefore provides evidence of hormone influences on stress reactivity at the genetic level and a possible diverging factor in prevalence and severity of PTSD between the sexes. Further, oestrogen‐dependent effects on DNA methylation on the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2659 gene may be associated with fear learning and memory in PTSD (Maddox et al., 2018).
Whereas oestrogenic signalling is implicated in effective recovery following a traumatic experience, consolidation of emotional memories may instead be associated with progesterone levels at the time of trauma. Studies of real‐world trauma have shown that increased reports of flashback memories and increased PTSD symptomology are associated with hospitalisation during the midluteal phase (Bryant et al., 2011), which is characterised by peak progesterone levels and elevated oestradiol. Similarly, survivors of sexual assault who neglected to take emergency contraception (which contains high dose progesterone) were significantly more likely to report higher PTSD symptomology compared to those who did take emergency contraception (Ferree, Wheeler, & Cahill, 2012). In the laboratory, healthy women who are in the midluteal phase report more intrusive memories of negative stimuli (Ertman, Andreano, & Cahill, 2011; Wassell, Rogers, Felmingham, Pearson, & Bryant, 2015), and this effect is correlated with levels of progesterone but not oestradiol at encoding (Ertman et al., 2011; Felmingham, Fong, & Bryant, 2012; Wassell et al., 2015). Only one study has found that oestradiol was associated with increased reports of intrusive memories (Cheung, Chervonsky, Felmingham, & Bryant, 2013); conversely, another study found that oestradiol was associated with decreased reports of intrusions (Wegerer et al., 2014). Overall, a number of studies support a facilitative effect of progesterone levels on intrusive memories of emotionally negative stimuli during encoding, with inconsistent or negligible effects of oestradiol.
4. FUTURE DIRECTIONS AND IMPLICATIONS
The exact mechanisms underlying the sex differences in the severe psychiatric disorders described above are unclear but are likely to involve genetic factors, biological, cognitive, and social influences. In this review, we have focussed on the role of gonadal and neuroactive hormones, particularly oestradiol and progesterone, to explain the sex differences. While it is common for researchers to investigate the role of gonadal hormones in women, what about men? Oestradiol and progesterone may also play an important role on symptom severity in men (Kulkarni, Gavrilidis, Worsley, Van Rheenen, & Hayes, 2013) and, conversely, it may be that testosterone is the key hormone influencing disease development and severity in men (Ko et al., 2007). Further, there is a strong link between testosterone and oestrogens, whereby the enzyme http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1362 regulates the conversion of testosterone to oestradiol (Figure 1). The role of hormones in men is an important issue and yet an understudied area, again highlighting that future research should always consider both males and females.
Based on the evidence presented here, it is clear that female hormone variation informs similar symptom profiles across schizophrenia, BD, and PTSD. In both schizophrenia and BD, low levels of oestradiol are associated with increased manic and psychotic‐like symptoms, as demonstrated in both experimental and observational studies (Gogos et al., 2015; Meinhard et al., 2014). In PTSD, low oestradiol levels are associated with poorer recovery following a traumatic experience and continuity of PTSD symptoms (Glover et al., 2015), while progesterone levels may influence the consolidation of emotional memories at the time of trauma. In all of these disorders, higher oestradiol is generally associated with improved symptomology; this is most relevant to the cognitive domain and represents a significant novel treatment avenue (Glover et al., 2015; Gogos et al., 2015; Meinhard et al., 2014).
An intriguing contradiction between symptom profiles of these three disorders is that, despite high oestradiol being recognised as a key protective factor explaining lower rates of schizophrenia and BD II in women, low oestradiol is conversely believed to drive higher prevalence of PTSD in women. Given that women have higher oestradiol levels than men, if oestradiol was a primary aetiological protective factor in all three disorders, it might have been expected that women would have lower, rather than higher, rates of PTSD, as is observed in schizophrenia. This phenomenon is likely to be explained by the differential influence of gonadal hormone fluctuation during different stages of PTSD. In PTSD, trauma causes an extreme biological stress response resulting in down‐regulation of the hypothalamic–pituitary–gonadal axis and lower production of oestradiol and progesterone that results in impaired extinction learning long term (Ney et al., 2018; Toufexis, Rivarola, Lara, & Viau, 2014). Restoration of these hormones in PTSD, and similarly up‐regulation in schizophrenia and BD, therefore appears to be a promising treatment option for similar reasons between the disorders, including through augmentation of cognition. However, high levels of gonadal hormones may similarly be a risk factor for PTSD through facilitation of learning, which acts to enhance consolidation of trauma memories at the time of trauma. The differential effect of hormones in this case reflects PTSD as a delayed disorder, characterised by an initial stressor and a later onset of symptomology (APA, 2013).
It is important to recognise that steroid hormones show dynamic interactions, meaning that treatment target options may include more than just oestradiol. For example, in PTSD, down‐regulation of the hypothalamic–pituitary–gonadal axis coincides with interruption of cortisol production in the hypothalamic–pituitary–adrenal axis following extreme stress created during trauma (Ney et al., 2018; Pitman et al., 2012; Toufexis et al., 2014). Restoration of either of these pathways can improve PTSD symptomology through the cognitive aspects of fear extinction learning (Glover et al., 2015; Ney et al., 2018; Pitman et al., 2012). It is necessary to consider that these axes, as with all steroid hormone production, are inseparable and are dual effectors in disorders that are stress‐related; not only in PTSD, but cortisol production is chronically dysregulated in both schizophrenia and BD (Girshkin, Matheson, Shepherd, & Green, 2014). Similarly, the effects attributed to oestradiol and progesterone in each of these disorders might be better explained by the increasingly recognised influence of other neuroactive steroids (which include precursors and metabolites of oestradiol and progesterone; Figure 1) in psychiatric disorders, such as pregnenolone, allopregnanolone, testosterone, and DHEA (Melcangi, Panzica, & Garcia‐Segura, 2011; Rasmusson et al., 2017; Ritsner, 2011; Sun et al., 2016; Zheng, 2009). For instance, research has highlighted the importance of allopregnanolone and DHEA for fear extinction learning and PTSD symptomology (Rasmusson et al., 2017). Unlike progesterone, allopregnanolone is a potent allosteric modulator of the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=72 a receptor implicated in psychiatric disorders (Melcangi et al., 2011; Sun et al., 2016). A recent study showed that men with psychotic features of schizophrenia, BD, or major depression had elevated DHEA levels compared to those without a history of psychosis (Buoli, Caldiroli, Serati, Grassi, & Altamura, 2016). Given the known interactions between neuroactive steroids, it is important to identify the underlying imbalances in steroid levels for each disorder. For example, in women, PTSD was associated with lower levels of 5α‐dihydroprogesterone, the primary metabolite of progesterone and precursor of allopregnanolone (Pineles et al., 2018), suggesting that pharmacological targeting of 5α‐dihydroprogesterone, rather than allopregnanolone or progesterone, may be beneficial. Findings of an imbalance of various neuroactive steroids in these psychiatric disorders underscores the importance of further research into steroid interactions instead of isolated targets such as oestradiol.
Overall, this review provides compelling evidence for cross‐disorder oestradiol‐related protective mechanisms, which may be expected given the known importance of both genomic and non‐genomic effects of oestrogens on cognitive processes more generally (Frick et al., 2015). However, oestradiol and progesterone have broad functional diversity in the brain, with both types of receptor implicated in many processes such as neurogenesis, microglia expression, inflammation, and bioenergetics (Gogos et al., 2015; Rettberg, Yao, & Brinton, 2014; Sun et al., 2016). Given this diversity, it is unlikely that steroid hormones play a single role in the pathogenesis and pathophysiology of PTSD, schizophrenia, or BD—rather, their role is likely to regulate various signalling, neurodevelopmental, neuroplastic, and epigenetic processes. As an example, all three disorders are characterised by reductions in hippocampal volume and functioning (Lieberman et al., 2018; Pitman et al., 2012; Shi et al., 2018), which is attributable to various neurobiological mechanisms associated with learning and memory. Oestradiol has been shown to modulate phosphorylation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2109, and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=284 pathways (Fortress, Fan, Orr, Zhao, & Frick, 2013; Hasegawa et al., 2015; Kim, Szinte, Boulware, & Frick, 2016) and can increase dendritic spine density of hippocampal neurons (Luine & Frankfurt, 2013). Phosphorylation of these pathways in turn mediate oestrogenic genomic actions including DNA methylation and histone acetylation in the hippocampus, subsequently affecting levels of proteins such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4872 (Zhao, Fan, & Frick, 2010), which is a potent effector of learning and memory and recognised as a critical factor in psychiatric disorders (Luine & Frankfurt, 2013; Pitman et al., 2012; Wu, Hill, Gogos, & van den Buuse, 2013). Given the well‐established role of neurotransmitter dysfunction in psychiatric disorders, it is important to note that both oestradiol and progesterone modulate the activity of dopaminergic, serotonergic, glutamatergic, and GABAergic systems (e.g., Kokras et al., 2018; reviewed in Barth et al., 2015; Gogos et al., 2015; Sun et al., 2016). Neurobiological pathways involved in the pathophysiology of these disorders, specifically the prefrontal and limbic networks and their connectivity, have also been shown to have sex specificity (Felmingham et al., 2010). Oestradiol receptors are expressed throughout the hippocampus (Österlund, Gustafsson, Keller, & Hurd, 2000), as well as the dorsolateral prefrontal cortex (Montague et al., 2008), for which crosstalk is involved in increasing psychotic symptomology (Lieberman et al., 2018) and being critical to fear circuitry (Pitman et al., 2012). Based on this converging evidence, it is likely that the similarities in symptomology between these three disorders is attributed to dysfunction across similar oestrogenic‐mediated mechanisms. In support, the most recent clinical studies have detected large genome‐wide association overlaps in heritability of vulnerability towards PTSD and schizophrenia, with a moderate shared effect between these disorders and BD (Duncan et al., 2018). Future research will need to assess the extent to which similar mechanisms mediate symptomology across disorders, and lessons may be learnt by combining methodologies and theoretical models between fields.
Finally, there are some common limitations across studies. Examining the specific effects of gonadal hormones in a naturalistic human setting is particularly difficult. Inconsistencies in the literature are likely, because of varied methodologies in the population selection (e.g., women in different reproductive life stages) and the symptom/task/hormone measures (e.g., estimated cycle phase compared to blood levels or saliva assays). To compound this, the lack of methodological detail makes it very difficult to compare across studies. For example, when one discusses differences across the menstrual cycle, it is often unclear what the effects observed are actually due to—is it really a change in oestradiol level? Or is it the accompanying increase in progesterone (Sun et al., 2016) or testosterone levels that are the mediating factors? To complicate matters further, it is not known if the differences observed in blood hormone levels correspond to CNS hormone levels. Therefore, using changes in blood levels as evidence for these hormones mediating the sex differences in psychiatric disorders should be treated with caution. Another confounding issue is the increasing prevalence of hormonal contraceptive use by women, which means that studies need to report on contraceptive use, including type of contraception and duration of use. This is particularly important given the known influence of contraceptives, containing synthetic oestrogen and progestin, on cognition (Gogos et al., 2014). Using animal models of psychiatric disorders is one way we can control for many of the confounding variables (Gogos et al., 2015; Sbisa et al., 2017). Future studies require a more inclusive, considered effort of steroid hormones and the intricacies of the interactions between them, with methodological rigour applied, to enhance our understanding of the roles of steroid hormones in psychiatric disorders.
5. CONCLUSIONS
Psychiatric disorders such as schizophrenia, BD and PTSD are chronic and debilitating diseases that have no easy treatment and in some cases, they are incurable. Current medications are inadequate, they do not treat all patients of all symptoms, nor are they side‐effect free. Thus, the need for the development of better treatments, or perhaps adjunctive treatments, is clearly important. Could gonadal hormones, such as oestradiol, provide the framework for the development of future therapeutics? In this review, three different psychiatric disorders were examined. However, there are a number of similarities between them, including cognitive impairment, genetic predisposition, neurotransmitter dysfunction, and inflammation. It has been proposed that a mechanism uniting the genetic and neurobiological factors implicated in psychiatric disorders might be endocrine responses, specifically oestradiol, which has been described as the ‘master regulator’ of these systems (Rettberg et al., 2014). Given the fact that the steroid receptors are widespread throughout the CNS (Newton‐Mann et al., 2017), and that steroid hormones have wide‐ranging effects on the CNS (Gogos et al., 2015), it is not surprising that oestradiol and progesterone can modulate the expression of psychiatric disorders. Although unclear, and not reviewed here, it is likely that the effects of steroid hormones on psychiatric disorders involves a combination of organisational and activational effects as well as genomic and non‐genomic mechanisms. One thing is obvious, there are marked sex differences across multiple disorders; women with schizophrenia tend to exhibit less disease impairment than men (Gogos et al., 2015), but women with PTSD are more affected than men (Blanco et al., 2018). We implore researchers and clinicians to, at the very least, acknowledge that sex differences exist. Thus, future studies should be balanced for sex, and in the clinic, women may need to be classified according to their hormone status, before starting treatment. Illuminating the extent to which steroid hormones facilitate these purported sex differences, and the associated mechanisms, may lead to avenues for novel therapeutic approaches and allow for precision medicine for men and women. This has important potential therapeutic implications for the preponderance of neuropsychiatric diseases with sexually dimorphic incidence, symptomology, and treatment responses.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Cidlowski et al., 2017; Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Peters et al., 2017).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
We gratefully acknowledge the financial support of the National Health and Medical Research Council of Australia (AG CDF ID1108098; TVR ECF ID1088785; KLF program grant APP1073041). The Florey Institute of Neuroscience and Mental Health acknowledges the support from the Victorian Government's Operational Infrastructure Support Grant. TVR has received funding from the Helen McPherson Smith Trust, Swinburne University of Technology, University of Melbourne, Society of Mental Health Research, Rebecca L. Cooper Foundation, Jack Brockhoff Foundation, CASS Foundation, Ian Potter Foundation, and the Henry Freeman Trust.
Gogos A, Ney LJ, Seymour N, Van Rheenen TE, Felmingham KL. Sex differences in schizophrenia, bipolar disorder, and post‐traumatic stress disorder: Are gonadal hormones the link?. Br J Pharmacol. 2019;176:4119–4135. 10.1111/bph.14584
REFERENCES
- Ahokas, A. , Aito, M. , & Rimón, R. (2000). Positive treatment effect of estradiol in postpartum psychosis: A pilot study. The Journal of Clinical Psychiatry, 61(3), 166–169. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh, S. , Nejatisafa, A. A. , Amini, H. , Mohammadi, M. R. , Larijani, B. , Kashani, L. , … Kamalipour, A. (2003). Adjunctive estrogen treatment in women with chronic schizophrenia: A double‐blind, randomized, and placebo‐controlled trial. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 27(6), 1007–1012. 10.1016/S0278-5846(03)00161-1 [DOI] [PubMed] [Google Scholar]
- Akhondzadeh, S. , Rezaei, F. , Larijani, B. , Nejatisafa, A. A. , Kashani, L. , & Abbasi, S. H. (2006). Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophrenia Research, 84(2–3), 405–410. 10.1016/j.schres.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174(Suppl 1), S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174, S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, J. R. , Akkal, D. , Hassel, S. , Travis, M. J. , Banihashemi, L. , Kerr, N. , … Phillips, M. L. (2009). Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: Significant effects of gender and trait anxiety. Psychiatry Research: Neuroimaging, 171(1), 54–68. 10.1016/j.pscychresns.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association: Washington D.C., USA. [Google Scholar]
- Bakhshi, K. , & Chance, S. A. (2015). The neuropathology of schizophrenia: A selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neuroscience, 303, 82–102. [DOI] [PubMed] [Google Scholar]
- Baldassano, C. F. , Marangell, L. B. , Gyulai, L. , Nassir Ghaemi, S. , Joffe, H. , Kim, D. R. , … Cohen, L. S. (2005). Gender differences in bipolar disorder: Retrospective data from the first 500 STEP‐BD participants. Bipolar Disorders, 7(5), 465–470. 10.1111/j.1399-5618.2005.00237.x [DOI] [PubMed] [Google Scholar]
- Barth, C. , Villringer, A. , & Sacher, J. (2015). Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience, 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvederi Murri, M. , Fanelli, F. , Pagotto, U. , Bonora, E. , Triolo, F. , Chiri, L. , … Tarricone, I. (2016). Neuroactive steroids in first‐episode psychosis: A role for progesterone? Schizophrenia Research and Treatment, 2016, 1942828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berent‐Spillson, A. , Briceno, E. , Pinsky, A. , Simmen, A. , Persad, C. C. , Zubieta, J. K. , & Smith, Y. R. (2015). Distinct cognitive effects of estrogen and progesterone in menopausal women. Psychoneuroendocrinology, 59, 25–36. 10.1016/j.psyneuen.2015.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergemann, N. , Mundt, C. , Parzer, P. , Jannakos, I. , Nagl, I. , Salbach, B. , … Resch, F. (2005). Plasma concentrations of estradiol in women suffering from schizophrenia treated with conventional versus atypical antipsychotics. Schizophrenia Research, 73(2–3), 357–366. 10.1016/j.schres.2004.06.013 [DOI] [PubMed] [Google Scholar]
- Bergemann, N. , Parzer, P. , Nagl, I. , Salbach, B. , Runnebaum, B. , Mundt, C. , & Resch, F. (2002). Acute psychiatric admission and menstrual cycle phase in women with schizophrenia. Archives of Women's Mental Health, 5(3), 119–126. 10.1007/s00737-002-0004-2 [DOI] [PubMed] [Google Scholar]
- Bergemann, N. , Parzer, P. , Runnebaum, B. , Resch, F. , & Mundt, C. (2007). Estrogen, menstrual cycle phases, and psychopathology in women suffering from schizophrenia. Psychological Medicine, 37(10), 1427–1436. [DOI] [PubMed] [Google Scholar]
- Bicikova, M. , Hill, M. , Ripova, D. , Mohr, P. , & Hampl, R. (2013). Determination of steroid metabolome as a possible tool for laboratory diagnosis of schizophrenia. The Journal of Steroid Biochemistry and Molecular Biology, 133, 77–83. [DOI] [PubMed] [Google Scholar]
- Blanco, C. , Hoertel, N. , Wall, M. M. , Franco, S. , Peyre, H. , Neria, Y. , … Limosin, F. (2018). Toward understanding sex differences in the prevalence of posttraumatic stress disorder: Results from the national epidemiologic survey on alcohol and related conditions. The Journal of Clinical Psychiatry, 79(2), 16m11364 10.4088/JCP.16m11364 [DOI] [PubMed] [Google Scholar]
- Blehar, M. C. , DePaulo, J. R. Jr. , Gershon, E. S. , Reich, T. , Simpson, S. G. , & Nurnberger, J. I. Jr. (1998). Women with bipolar disorder: Findings from the NIMH Genetics Initiative sample. Psychopharmacology Bulletin, 34(3), 239–243. [PubMed] [Google Scholar]
- Bozikas, V. P. , Kosmidis, M. H. , Tonia, T. , Andreou, C. , Focas, K. , & Karavatos, A. (2007). Impaired perception of affective prosody in remitted patients with bipolar disorder. Journal of Neuropsychiatry and Clinical Neurosciences, 19(4), 436–440. [DOI] [PubMed] [Google Scholar]
- Bratek, A. , Krysta, K. , Drzyzga, K. , Baranska, J. , & Kucia, K. (2016). The role of selective estrogen receptor modulators in the treatment of schizophrenia. Psychiatria Danubina, 28(Suppl‐1), 45–48. [PubMed] [Google Scholar]
- Bräunig, P. , Sarkar, R. , Effenberger, S. , Schoofs, N. , & Krüger, S. (2009). Gender differences in psychotic bipolar mania. Gender Medicine, 6(2), 356–361. [DOI] [PubMed] [Google Scholar]
- Brown, E. S. , Park, J. , Marx, C. E. , Hynan, L. S. , Gardner, C. , Davila, D. , … Holmes, T. (2014). A randomized, double‐blind, placebo‐controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology, 39(12), 2867–2873. 10.1038/npp.2014.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, N. L. , Buchanan, R. W. , Vladar, K. , Breier, A. , & Rothman, M. (1999). Gender differences in temporal lobe structures of patients with schizophrenia: A volumetric MRI study. The American Journal of Psychiatry, 156(4), 603–609. 10.1176/ajp.156.4.603 [DOI] [PubMed] [Google Scholar]
- Bryant, R. A. , Felmingham, K. L. , Silove, D. , Creamer, M. , O'Donnell, M. , & McFarlane, A. C. (2011). The association between menstrual cycle and traumatic memories. Journal of Affective Disorders, 131(1–3), 398–401. 10.1016/j.jad.2010.10.049 [DOI] [PubMed] [Google Scholar]
- Bryant, R. A. , & Harvey, A. G. (2003). Gender differences in the relationship between acute stress disorder and posttraumatic stress disorder following motor vehicle accidents. The Australian and New Zealand Journal of Psychiatry, 37(2), 226–229. [DOI] [PubMed] [Google Scholar]
- Bücker, J. , Popuri, S. , Muralidharan, K. , Kozicky, J.‐M. , Baitz, H. A. , Honer, W. G. , … Yatham, L. N. (2014). Sex differences in cognitive functioning in patients with bipolar disorder who recently recovered from a first episode of mania: Data from the Systematic Treatment Optimization Program for Early Mania (STOP‐EM). Journal of Affective Disorders, 155, 162–168. 10.1016/j.jad.2013.10.044 [DOI] [PubMed] [Google Scholar]
- Buoli, M. , Caldiroli, A. , Serati, M. , Grassi, S. , & Altamura, A. C. (2016). Sex steroids and major psychoses: Which role for DHEA‐S and progesterone. Neuropsychobiology, 73(3), 178–183. 10.1159/000444922 [DOI] [PubMed] [Google Scholar]
- Burger, H. G. , Cahir, N. , Robertson, D. M. , Groome, N. P. , Dudley, E. , Green, A. , & Dennerstein, L. (1998). Serum inhibins A and B fall differentially as FSH rises in perimenopausal women. Clinical Endocrinology, 48(6), 809–813. 10.1046/j.1365-2265.1998.00482.x [DOI] [PubMed] [Google Scholar]
- Byrns, M. C. (2014). Regulation of progesterone signaling during pregnancy: Implications for the use of progestins for the prevention of preterm birth. The Journal of Steroid Biochemistry and Molecular Biology, 139, 173–181. [DOI] [PubMed] [Google Scholar]
- Carpiniello, B. , Pinna, F. , Tusconi, M. , Zaccheddu, E. , & Fatteri, F. (2012). Gender differences in remission and recovery of schizophrenic and schizoaffective patients: Preliminary results of a prospective cohort study. Schizophrenia Research and Treatment, 2012, 576369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher, N. , Sunderland, M. , Batterham, P. J. , Calear, A. L. , Elhai, J. D. , Chapman, C. , & Mills, K. (2016). Discriminant validity and gender differences in DSM‐5 posttraumatic stress disorder symptoms. Journal of Affective Disorders, 190, 56–67. 10.1016/j.jad.2015.09.071 [DOI] [PubMed] [Google Scholar]
- Carrus, D. , Christodoulou, T. , Hadjulis, M. , Haldane, M. , Galea, A. , Koukopoulos, A. , … Frangou, S. (2010). Gender differences in immediate memory in bipolar disorder. Psychological Medicine, 40(8), 1349–1355. 10.1017/S0033291709991644 [DOI] [PubMed] [Google Scholar]
- Cheung, J. , Chervonsky, L. , Felmingham, K. L. , & Bryant, R. A. (2013). The role of estrogen in intrusive memories. Neurobiology of Learning and Memory, 106, 87–94. 10.1016/j.nlm.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Christiansen, D. M. , & Hansen, M. (2015). Accounting for sex differences in PTSD: A multi‐variable mediation model. European Journal of Psychotraumatology, 6, 26068 10.3402/ejpt.v6.26068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton, S. M. , Lambert, M. , Schimmelmann, B. G. , Foley, D. L. , Morley, K. I. , McGorry, P. D. , & Conus, P. (2009). Gender differences in premorbid, entry, treatment, and outcome characteristics in a treated epidemiological sample of 661 patients with first episode psychosis. Schizophrenia Research, 114(1–3), 17–24. 10.1016/j.schres.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Dias, R. S. , Lafer, B. , Russo, C. , Del Debbio, A. , Nierenberg, A. A. , Sachs, G. S. , & Joffe, H. (2011). Longitudinal follow‐up of bipolar disorder in women with premenstrual exacerbation: Findings from STEP‐BD. American Journal of Psychiatry, 168(4), 386–394. 10.1176/appi.ajp.2010.09121816 [DOI] [PubMed] [Google Scholar]
- Diflorio, A. , & Jones, I. (2010). Is sex important? Gender differences in bipolar disorder. International Review of Psychiatry, 22(5), 437–452. [DOI] [PubMed] [Google Scholar]
- Ditlevsen, D. N. , & Elklit, A. (2010). The combined effect of gender and age on post traumatic stress disorder: Do men and women show differences in the lifespan distribution of the disorder? Annals of General Psychiatry, 9, 32–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doğan Bulut, S. , Bulut, S. , & Güriz, O. (2016). The relationship between sex hormone profiles and symptoms of schizophrenia in men. Comprehensive Psychiatry, 69, 186–192. [DOI] [PubMed] [Google Scholar]
- Duncan, L. E. , Ratanatharathorn, A. , Aiello, A. E. , Almli, L. M. , Amstadter, A. B. , Ashley‐Koch, A. E. , … Koenen, K. C. (2018). Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Molecular Psychiatry, 23(3), 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellmann, S. , Sticht, H. , Thiel, F. , Beckmann, M. W. , Strick, R. , & Strissel, P. L. (2009). Estrogen and progesterone receptors: From molecular structures to clinical targets. Cellular and Molecular Life Sciences, 66(15), 2405–2426. 10.1007/s00018-009-0017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertman, N. , Andreano, J. M. , & Cahill, L. (2011). Progesterone at encoding predicts subsequent emotional memory. Learning & Memory, 18(12), 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellendorf, F. T. , Kainzbauer, N. , Platzer, M. , Dalkner, N. , Bengesser, S. A. , Birner, A. , … Reininghaus, E. Z. (2017). Gender differences in the association between physical activity and cognitive function in individuals with bipolar disorder. Journal of Affective Disorders, 221, 232–237. 10.1016/j.jad.2017.06.048 [DOI] [PubMed] [Google Scholar]
- Felmingham, K. , Williams, L. M. , Kemp, A. H. , Liddell, B. , Falconer, E. , Peduto, A. , & Bryant, R. (2010). Neural responses to masked fear faces: Sex differences and trauma exposure in posttraumatic stress disorder. Journal of Abnormal Psychology, 119(1), 241–247. 10.1037/a0017551 [DOI] [PubMed] [Google Scholar]
- Felmingham, K. L. , & Bryant, R. A. (2012). Gender differences in the maintenance of response to cognitive behavior therapy for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 80(2), 196–200. [DOI] [PubMed] [Google Scholar]
- Felmingham, K. L. , Fong, W. C. , & Bryant, R. A. (2012). The impact of progesterone on memory consolidation of threatening images in women. Psychoneuroendocrinology, 37(11), 1896–1900. [DOI] [PubMed] [Google Scholar]
- Felmingham, K. L. , Tran, T. P. , Fong, W. C. , & Bryant, R. A. (2012). Sex differences in emotional memory consolidation: The effect of stress‐induced salivary alpha‐amylase and cortisol. Biological Psychology, 89(3), 539–544. [DOI] [PubMed] [Google Scholar]
- Ferree, N. K. , Wheeler, M. , & Cahill, L. (2012). The influence of emergency contraception on post‐traumatic stress symptoms following sexual assault. Journal of Forensic Nursing, 8(3), 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, D. , Lockwood, E. , Phelps, A. , Wade, D. , Creamer, M. , Bryant, R. A. , … O'Donnell, M. (2014). Trauma at the hands of another: Distinguishing PTSD patterns following intimate and nonintimate interpersonal and noninterpersonal trauma in a nationally representative sample. Journal of Clinical Psychiatry, 75(2), 147–153. 10.4088/JCP.13m08374 [DOI] [PubMed] [Google Scholar]
- Fortress, A. M. , Fan, L. , Orr, P. T. , Zhao, Z. , & Frick, K. M. (2013). Estradiol‐induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learning & Memory, 20(3), 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick, K. M. , Kim, J. , Tuscher, J. J. , & Fortress, A. M. (2015). Sex steroid hormones matter for learning and memory: Estrogenic regulation of hippocampal function in male and female rodents. Learning & Memory, 22(9), 472–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi, S. , Bucci, P. , Ucok, A. , & Peuskens, J. (2012). No gender differences in social outcome in patients suffering from schizophrenia. European Psychiatry, 27(6), 406–408. [DOI] [PubMed] [Google Scholar]
- Galovski, T. E. , Blain, L. M. , Chappuis, C. , & Fletcher, T. (2013). Sex differences in recovery from PTSD in male and female interpersonal assault survivors. Behaviour Research and Therapy, 51(6), 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, M. S. , Guidotti, A. , Rubinow, D. , Pan, B. , Mikalauskas, K. , & Post, R. M. (1994). CSF neuroactive steroids in affective disorders: Pregnenolone, progesterone, and DBI. Biological Psychiatry, 35(10), 775–780. [DOI] [PubMed] [Google Scholar]
- Girshkin, L. , Matheson, S. L. , Shepherd, A. M. , & Green, M. J. (2014). Morning cortisol levels in schizophrenia and bipolar disorder: A meta‐analysis. Psychoneuroendocrinology, 49, 187–206. [DOI] [PubMed] [Google Scholar]
- Gleeson, P. C. , Worsley, R. , Gavrilidis, E. , Nathoo, S. , Ng, E. , Lee, S. , & Kulkarni, J. (2016). Menstrual cycle characteristics in women with persistent schizophrenia. The Australian and New Zealand Journal of Psychiatry, 50(5), 481–487. 10.1177/0004867415590459 [DOI] [PubMed] [Google Scholar]
- Glover, E. M. , Jovanovic, T. , Mercer, K. B. , Kerley, K. , Bradley, B. , Ressler, K. J. , & Norrholm, S. D. (2012). Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological Psychiatry, 72(1), 19–24. 10.1016/j.biopsych.2012.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, E. M. , Jovanovic, T. , & Norrholm, S. D. (2015). Estrogen and extinction of fear memories: Implications for posttraumatic stress disorder treatment. Biological Psychiatry, 78(3), 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos, A. , Joshua, N. , & Rossell, S. L. (2010). Use of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) to investigate group and gender differences in schizophrenia and bipolar disorder. Australian and New Zealand Journal of Psychiatry, 44, 220–229. [DOI] [PubMed] [Google Scholar]
- Gogos, A. , Sbisa, A. M. , Sun, J. , Gibbons, A. , Udawela, M. , & Dean, B. (2015). A role for estrogen in schizophrenia: Clinical and preclinical findings. International Journal of Endocrinology, 2015, 615356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos, A. , van den Buuse, M. , & Rossell, S. (2009). Gender differences in prepulse inhibition (PPI) in bipolar disorder: Men have reduced PPI, women have increased PPI. International Journal of Neuropsychopharmacology, 12(9), 1249–1259. [DOI] [PubMed] [Google Scholar]
- Gogos, A. , Wu, Y. C. , Williams, A. S. , & Byrne, L. K. (2014). The effects of ethinylestradiol and progestins (“the pill”) on cognitive function in pre‐menopausal women. Neurochemical Research, 39(12), 2288–2300. [DOI] [PubMed] [Google Scholar]
- Graham, B. M. , & Milad, M. R. (2013). Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biological Psychiatry, 73(4), 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt, R. B. , Oettinger, M. , & Bohler Clorinda, S. S. (1976). Estrogen‐androgen levels in aging men and women: Therapeutic considerations*. Journal of the American Geriatrics Society, 24(4), 173–178. [DOI] [PubMed] [Google Scholar]
- Grossman, L. S. , Harrow, M. , Rosen, C. , Faull, R. , & Strauss, G. P. (2008). Sex differences in schizophrenia and other psychotic disorders: A 20‐year longitudinal study of psychosis and recovery. Comprehensive Psychiatry, 49(6), 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner, H. (2003). Gender differences in schizophrenia. Psychoneuroendocrinology, 28(Suppl 2), 17–54. [DOI] [PubMed] [Google Scholar]
- Han, M. , Huang, X. F. , Chen, D. C. , Xiu, M. H. , Hui, L. , Liu, H. , … Zhang, X. Y. (2012). Gender differences in cognitive function of patients with chronic schizophrenia. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 39(2), 358–363. 10.1016/j.pnpbp.2012.07.010 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardoy, M. C. , Serra, M. , Carta, M. G. , Contu, P. , Pisu, M. G. , & Biggio, G. (2006). Increased neuroactive steroid concentrations in women with bipolar disorder or major depressive disorder. Journal of Clinical Psychopharmacology, 26(4), 379–384. [DOI] [PubMed] [Google Scholar]
- Hasegawa, Y. , Hojo, Y. , Kojima, H. , Ikeda, M. , Hotta, K. , Sato, R. , … Kawato, S. (2015). Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Research, 1621, 147–161. 10.1016/j.brainres.2014.12.056 [DOI] [PubMed] [Google Scholar]
- Heron, J. , Haque, S. , Oyebode, F. , Craddock, N. , & Jones, I. (2009). A longitudinal study of hypomania and depression symptoms in pregnancy and the postpartum period. Bipolar Disorders, 11(4), 410–417. [DOI] [PubMed] [Google Scholar]
- Hoff, A. L. , Kremen, W. S. , Wieneke, M. H. , Lauriello, J. , Blankfeld, H. M. , Faustman, W. O. , … Nordahl, T. E. (2001). Association of estrogen levels with neuropsychological performance in women with schizophrenia. American Journal of Psychiatry, 158(7), 1134–1139. 10.1176/appi.ajp.158.7.1134 [DOI] [PubMed] [Google Scholar]
- Inslicht, S. S. , Metzler, T. J. , Garcia, N. M. , Pineles, S. L. , Milad, M. R. , Orr, S. P. , … Neylan, T. C. (2013). Sex differences in fear conditioning in posttraumatic stress disorder. Journal of Psychiatric Research, 47(1), 64–71. 10.1016/j.jpsychires.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish, L. A. , Fischer, B. , Fallon, W. , Spoonster, E. , Sledjeski, E. M. , & Delahanty, D. L. (2011). Gender differences in PTSD symptoms: An exploration of peritraumatic mechanisms. Journal of Anxiety Disorders, 25(2), 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakumar, M. , Carlson, K. E. , Gunther, J. R. , & Katzenellenbogen, J. A. (2011). Exploration of dimensions of estrogen potency: Parsing ligand binding and coactivator binding affinities. The Journal of Biological Chemistry, 286(15), 12971–12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogia, J. , Dima, D. , & Frangou, S. (2012). Sex differences in bipolar disorder: A review of neuroimaging findings and new evidence. Bipolar Disorders, 14(4), 461–471. 10.1111/j.1399-5618.2012.01014.x [DOI] [PubMed] [Google Scholar]
- Jones, I. , Chandra, P. S. , Dazzan, P. , & Howard, L. M. (2014). Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post‐partum period. The Lancet, 384(9956), 1789–1799. [DOI] [PubMed] [Google Scholar]
- Kanaan, R. A. , Allin, M. , Picchioni, M. , Barker, G. J. , Daly, E. , Shergill, S. S. , … McGuire, P. K. (2012). Gender differences in white matter microstructure. PLoS One, 7(6), e38272 10.1371/journal.pone.0038272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadag, F. , Akdeniz, F. , Erten, E. , Pirildar, S. , Yucel, B. , Polat, A. , & Atmaca, M. (2004). Menstrually related symptom changes in women with treatment‐responsive bipolar disorder. Bipolar Disorders, 6(3), 253–259. 10.1111/j.1399-5618.2004.00112.x [DOI] [PubMed] [Google Scholar]
- Karanti, A. , Bobeck, C. , Osterman, M. , Kardell, M. , Tidemalm, D. , Runeson, B. , … Landén, M. (2015). Gender differences in the treatment of patients with bipolar disorder: A study of 7354 patients. Journal of Affective Disorders, 174, 303–309. 10.1016/j.jad.2014.11.058 [DOI] [PubMed] [Google Scholar]
- Kawa, I. , Carter, J. D. , Joyce, P. R. , Doughty, C. J. , Frampton, C. M. , Wells, J. E. , … Olds, R. J. (2005). Gender differences in bipolar disorder: Age of onset, course, comorbidity, and symptom presentation. Bipolar Disorders, 7(2), 119–125. 10.1111/j.1399-5618.2004.00180.x [DOI] [PubMed] [Google Scholar]
- Kelly, S. , Jahanshad, N. , Zalesky, A. , Kochunov, P. , Agartz, I. , Alloza, C. , … Donohoe, G. (2018). Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA schizophrenia DTI working group. Molecular Psychiatry, 23(5), 1261–1269. 10.1038/mp.2017.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing, L. V. (2004). Gender differences in the phenomenology of bipolar disorder. Bipolar Disorders, 6(5), 421–425. [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , Aguilar‐Gaxiola, S. , Alonso, J. , Benjet, C. , Bromet, E. J. , Cardoso, G. , … Koenen, K. C. (2017). Trauma and PTSD in the WHO World Mental Health Surveys. European Journal of Psychotraumatology, 8, 1353383 10.1080/20008198.2017.1353383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Szinte, J. S. , Boulware, M. I. , & Frick, K. M. (2016). 17β‐Estradiol and agonism of G‐protein‐coupled estrogen receptor enhance hippocampal memory via different cell‐signaling mechanisms. Journal of Neuroscience, 36(11), 3309–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, Y. H. , Jung, S. W. , Joe, S. H. , Lee, C. H. , Jung, H. G. , Jung, I. K. , … Lee, M. S. (2007). Association between serum testosterone levels and the severity of negative symptoms in male patients with chronic schizophrenia. Psychoneuroendocrinology, 32(4), 385–391. 10.1016/j.psyneuen.2007.02.002 [DOI] [PubMed] [Google Scholar]
- Kokras, N. , Pastromas, N. , Papasava, D. , de Bournonville, C. , Cornil, C. A. , & Dalla, C. (2018). Sex differences in behavioral and neurochemical effects of gonadectomy and aromatase inhibition in rats. Psychoneuroendocrinology, 87, 93–107. 10.1016/j.psyneuen.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Koster, A. , Lajer, M. , Lindhardt, A. , & Rosenbaum, B. (2008). Gender differences in first episode psychosis. Social Psychiatry and Psychiatric Epidemiology, 43(12), 940–946. [DOI] [PubMed] [Google Scholar]
- Kulkarni, J. , Berk, M. , Wang, W. , Mu, L. , Scarr, E. , Van Rheenen, T. E. , … Davis, S. R. (2014). A four week randomised control trial of adjunctive medroxyprogesterone and tamoxifen in women with mania. Psychoneuroendocrinology, 43, 52–61. 10.1016/j.psyneuen.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Kulkarni, J. , Gavrilidis, E. , Gwini, S. M. , Worsley, R. , Grigg, J. , Warren, A. , … Davis, S. R. (2016). Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: A randomized clinical trial. JAMA Psychiatry, 73(9), 947–954. 10.1001/jamapsychiatry.2016.1383 [DOI] [PubMed] [Google Scholar]
- Kulkarni, J. , Gavrilidis, E. , Worsley, R. , & Hayes, E. (2012). Role of estrogen treatment in the management of schizophrenia. CNS Drugs, 26(7), 549–557. [DOI] [PubMed] [Google Scholar]
- Kulkarni, J. , Gavrilidis, E. , Worsley, R. , Van Rheenen, T. , & Hayes, E. (2013). The role of estrogen in the treatment of men with schizophrenia. International Journal of Endocrinology and Metabolism, 11(3), 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari, V. , Konstantinou, J. , Papadopoulos, A. , Aasen, I. , Poon, L. , Halari, R. , & Cleare, A. J. (2010). Evidence for a role of progesterone in menstrual cycle‐related variability in prepulse inhibition in healthy young women. Neuropsychopharmacology, 35(4), 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron‐Milad, K. , Abbs, B. , Milad, M. R. , Linnman, C. , Rougemount‐Bucking, A. , Zeidan, M. A. , … Goldstein, J. M. (2012). Sex differences in the neurobiology of fear conditioning and extinction: A preliminary fMRI study of shared sex differences with stress‐arousal circuitry. Biology of Mood & Anxiety Disorders, 2, 7 10.1186/2045-5380-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , & Graham, B. M. (2016). Estradiol is associated with altered cognitive and physiological responses during fear conditioning and extinction in healthy and spider phobic women. Behavioral Neuroscience, 130(6), 614–623. [DOI] [PubMed] [Google Scholar]
- Lieberman, J. A. , Girgis, R. R. , Brucato, G. , Moore, H. , Provenzano, F. , Kegeles, L. , … Small, S. A. (2018). Hippocampal dysfunction in the pathophysiology of schizophrenia: A selective review and hypothesis for early detection and intervention. Molecular Psychiatry, 23(8), 1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind, M. J. , Marraccini, M. E. , Sheerin, C. M. , Bountress, K. , Bacanu, S. A. , Amstadter, A. B. , & Nugent, N. R. (2017). Association of posttraumatic stress disorder with rs2267735 in the ADCYAP1R1 gene: A meta‐analysis. Journal of Traumatic Stress, 30(4), 389–398. 10.1002/jts.22211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf, T. B. , Haaker, J. , Schumann, D. , Sommer, T. , Bayer, J. , Brassen, S. , … Kalisch, R. (2015). Sex differences in conditioned stimulus discrimination during context‐dependent fear learning and its retrieval in humans: The role of biological sex, contraceptives and menstrual cycle phases. Journal of Psychiatry and Neuroscience, 40(6), 368–375. 10.1503/jpn.140336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine, V. , & Frankfurt, M. (2013). Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience, 239, 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox, S. A. , Kilaru, V. , Shin, J. , Jovanovic, T. , Almli, L. M. , Dias, B. G. , … Smith, A. K. (2018). Estrogen‐dependent association of HDAC4 with fear in female mice and women with PTSD. Molecular Psychiatry, 23(3), 658–665. 10.1038/mp.2016.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, W. K. , Gershenson, B. , & Rothschild, A. J. (2015). Symptom severity of bipolar disorder during the menopausal transition. International Journal of Bipolar Disorders, 3(1), 17 10.1186/s40345-015-0035-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, W. K. , Ketter, T. A. , Crawford, S. L. , Johnson, J. V. , Kroll‐Desrosiers, A. R. , & Rothschild, A. J. (2012). Progression of female reproductive stages associated with bipolar illness exacerbation. Bipolar Disorders, 14(5), 515–526. 10.1111/j.1399-5618.2012.01026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, J. , Saha, S. , Welham, J. , El Saadi, O. , MacCauley, C. , & Chant, D. (2004). A systematic review of the incidence of schizophrenia: The distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Medicine, 2, 13 10.1186/1741-7015-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor, C. , Riordan, A. , & Thornton, J. (2017). Estrogens and the cognitive symptoms of schizophrenia: Possible neuroprotective mechanisms. Frontiers in Neuroendocrinology, 47, 19–33. [DOI] [PubMed] [Google Scholar]
- Meinhard, N. , Kessing, L. V. , & Vinberg, M. (2014). The role of estrogen in bipolar disorder, a review. Nordic Journal of Psychiatry, 68(2), 81–87. [DOI] [PubMed] [Google Scholar]
- Melcangi, R. C. , Panzica, G. , & Garcia‐Segura, L. M. (2011). Neuroactive steroids: Focus on human brain. Neuroscience, 191, 1–5. [DOI] [PubMed] [Google Scholar]
- Mendrek, A. , & Mancini‐Marie, A. (2016). Sex/gender differences in the brain and cognition in schizophrenia. Neuroscience & Biobehavioral Reviews, 67, 57–78. [DOI] [PubMed] [Google Scholar]
- Milad, M. R. , Zeidan, M. A. , Contero, A. , Pitman, R. K. , Klibanski, A. , Rauch, S. L. , & Goldstein, J. M. (2010). The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience, 168(3), 652–658. 10.1016/j.neuroscience.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak, B. , Frydecka, D. , Loska, O. , Moustafa, A. A. , Samochowiec, J. , Kasznia, J. , & Stańczykiewicz, B. (2018). Testosterone, DHEA and DHEA‐S in patients with schizophrenia: A systematic review and meta‐analysis. Psychoneuroendocrinology, 89, 92–102. 10.1016/j.psyneuen.2018.01.007 [DOI] [PubMed] [Google Scholar]
- Montague, D. , Weickert, C. S. , Tomaskovic‐Crook, E. , Rothmond, D. A. , Kleinman, J. E. , & Rubinow, D. R. (2008). Oestrogen receptor alpha localisation in the prefrontal cortex of three mammalian species. Journal of Neuroendocrinology, 20(7), 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, V. A. , Castle, D. J. , & Jablensky, A. V. (2008). Do women express and experience psychosis differently from men? Epidemiological evidence from the Australian National Study of Low Prevalence (Psychotic) Disorders. The Australian and New Zealand Journal of Psychiatry, 42(1), 74–82. [DOI] [PubMed] [Google Scholar]
- Morgan, V. A. , Mitchell, P. B. , & Jablensky, A. V. (2005). The epidemiology of bipolar disorder: Sociodemographic, disability and service utilization data from the Australian National Study of Low Prevalence (Psychotic) Disorders. Bipolar Disorders, 7(4), 326–337. [DOI] [PubMed] [Google Scholar]
- Mumford, S. L. , Schisterman, E. F. , Siega‐Riz, A. M. , Browne, R. W. , Gaskins, A. J. , Trevisan, M. , … Wactawski‐Wende, J. (2010). A longitudinal study of serum lipoproteins in relation to endogenous reproductive hormones during the menstrual cycle: Findings from the BioCycle study. The Journal of Clinical Endocrinology & Metabolism, 95(9), E80–E85. 10.1210/jc.2010-0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S. , Elklit, A. , Chen, Y. Y. , Ghazali, S. R. , & Shevlin, M. (2018). Sex differences in PTSD symptoms: A differential item functioning approach. Psychological Trauma: Theory, Research, Practice and Policy, 11(3), 319–327. 10.1037/tra0000355 [DOI] [PubMed] [Google Scholar]
- Narr, K. L. , Thompson, P. M. , Sharma, T. , Moussai, J. , Blanton, R. , Anvar, B. , … Toga, A. W. (2001). Three‐dimensional mapping of temporo‐limbic regions and the lateral ventricles in schizophrenia: Gender effects. Biological Psychiatry, 50(2), 84–97. 10.1016/S0006-3223(00)01120-3 [DOI] [PubMed] [Google Scholar]
- Newton‐Mann, E. , Finney, C. , Purves‐Tyson, T. , & Gogos, A. (2017). Estrogen receptors: Mechanism of action and relevance to schizophrenia. Current Psychiatry Reviews, 13(1), 55–64. [Google Scholar]
- Ney, L. J. , Matthews, A. , Bruno, R. , & Felmingam, K. L. (2018). Modulation of the endocannabinoid system by sex hormones: Implications for posttraumatic stress disorder. Neuroscience & Biobehavioral Reviews, 94, 302–320. [DOI] [PubMed] [Google Scholar]
- Nillni, Y. I. , Pineles, S. L. , Patton, S. C. , Rouse, M. H. , Sawyer, A. T. , & Rasmusson, A. M. (2015). Menstrual cycle effects on psychological symptoms in women with PTSD. Journal of Traumatic Stress, 28(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Ochoa, S. , Usall, J. , Cobo, J. , Labad, X. , & Kulkarni, J. (2012). Gender differences in schizophrenia and first‐episode psychosis: A comprehensive literature review. Schizophrenia Research and Treatment, 2012, 916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettel, M. , & Mukhopadhyay, A. K. (2004). Progesterone: The forgotten hormone in men? The aging male: the official journal of the International Society for the Study of the Aging Male, 7(3), 236–257. [DOI] [PubMed] [Google Scholar]
- Olff, M. (2017). Sex and gender differences in post‐traumatic stress disorder: An update. European Journal of Psychotraumatology, 8, 1351204 10.1080/20008198.2017.1351204 [DOI] [Google Scholar]
- Orwoll, E. , Lambert, L. C. , Marshall, L. M. , Phipps, K. , Blank, J. , Barrett‐Connor, E. , … Cummings, S. (2006). Testosterone and estradiol among older men. The Journal of Clinical Endocrinology & Metabolism, 91(4), 1336–1344. 10.1210/jc.2005-1830 [DOI] [PubMed] [Google Scholar]
- Österlund, M. K. , Gustafsson, J.‐A. K. , Keller, E. , & Hurd, Y. L. (2000). Estrogen receptor β (ERβ) messenger ribonucleic acid (mRNA) expression within the human forebrain: Distinct distribution pattern to ERα mRNA1. The Journal of Clinical Endocrinology & Metabolism, 85(10), 3840–3846. [DOI] [PubMed] [Google Scholar]
- Perich, T. A. , Roberts, G. , Frankland, A. , Sinbandhit, C. , Meade, T. , Austin, M.‐P. , & Mitchell, P. B. (2017). Clinical characteristics of women with reproductive cycle–associated bipolar disorder symptoms. Australian and New Zealand Journal of Psychiatry, 51(2), 161–167. 10.1177/0004867416670015 [DOI] [PubMed] [Google Scholar]
- Petersen, S. L. , Intlekofer, K. A. , Moura‐Conlon, P. J. , Brewer, D. N. , Del Pino Sans, J. , & Lopez, J. A. (2013). Novel progesterone receptors: Neural localization and possible functions. Frontiers in Neuroscience, 7, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinares‐Garcia, P. , Stratikopoulos, M. , Zagato, A. , Loke, H. , & Lee, J. (2018). Sex: A significant risk factor for neurodevelopmental and neurodegenerative disorders. Brain Sciences, 8(8). 10.3390/brainsci8080154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles, S. L. , Nillni, Y. I. , King, M. W. , Patton, S. C. , Bauer, M. R. , Mostoufi, S. M. , … Orr, S. P. (2016). Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. Journal of Abnormal Psychology, 125(3), 349–355. 10.1037/abn0000138 [DOI] [PubMed] [Google Scholar]
- Pineles, S. L. , Nillni, Y. I. , Pinna, G. , Irvine, J. , Webb, A. , Arditte Hall, K. A. , … Rasmusson, A. M. (2018). PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology, 93, 133–141. 10.1016/j.psyneuen.2018.04.024 [DOI] [PubMed] [Google Scholar]
- Pitman, R. K. , Rasmusson, A. M. , Koenen, K. C. , Shin, L. M. , Orr, S. P. , Gilbertson, M. W. , … Liberzon, I. (2012). Biological studies of post‐traumatic stress disorder. Nature Reviews Neuroscience, 13(11), 769–787. 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon, N. , Bauer, M. , Glenn, T. , Elman, S. , & Whybrow Peter, C. (2003). Menstrual cycle related mood changes in women with bipolar disorder. Bipolar Disorders, 5(1), 48–52. 10.1034/j.1399-5618.2003.00010.x [DOI] [PubMed] [Google Scholar]
- Rasgon, N. L. , Altshuler, L. L. , Fairbanks, L. , Elman, S. , Bitran, J. , Labarca, R. , … Mintz, J. (2005). Reproductive function and risk for PCOS in women treated for bipolar disorder. Bipolar Disorders, 7(3), 246–259. 10.1111/j.1399-5618.2005.00201.x [DOI] [PubMed] [Google Scholar]
- Rasmusson, A. M. , Marx, C. E. , Pineles, S. L. , Locci, A. , Scioli‐Salter, E. R. , Nillni, Y. I. , … Pinna, G. (2017). Neuroactive steroids and PTSD treatment. Neuroscience Letters, 649, 156–163. 10.1016/j.neulet.2017.01.054 [DOI] [PubMed] [Google Scholar]
- Rettberg, J. R. , Yao, J. , & Brinton, R. D. (2014). Estrogen: A master regulator of bioenergetic systems in the brain and body. Frontiers in Neuroendocrinology, 35(1), 8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecher‐Rössler, A. (2017). Oestrogens, prolactin, hypothalamic‐pituitary‐gonadal axis, and schizophrenic psychoses. The Lancet Psychiatry, 4(1), 63–72. [DOI] [PubMed] [Google Scholar]
- Riecher‐Rössler, A. , Butler, S. , & Kulkarni, J. (2018). Sex and gender differences in schizophrenic psychoses—A critical review. Archives of Women's Mental Health, 21(6), 627–648. 10.1007/s00737-018-0847-9 [DOI] [PubMed] [Google Scholar]
- Ritsner, M. , Maayan, R. , Gibel, A. , & Weizman, A. (2007). Differences in blood pregnenolone and dehydroepiandrosterone levels between schizophrenia patients and healthy subjects. European Neuropsychopharmacology, 17(5), 358–365. [DOI] [PubMed] [Google Scholar]
- Ritsner, M. S. (2010). Pregnenolone, dehydroepiandrosterone, and schizophrenia: Alterations and clinical trials. CNS Neuroscience & Therapeutics, 16(1), 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner, M. S. (2011). The clinical and therapeutic potentials of dehydroepiandrosterone and pregnenolone in schizophrenia. Neuroscience, 191, 91–100. [DOI] [PubMed] [Google Scholar]
- Robb, J. C. , Young, L. T. , Cooke, R. G. , & Joffe, R. T. (1998). Gender differences in patients with bipolar disorder influence outcome in the medical outcomes survey (SF‐20) subscale scores. Journal of Affective Disorders, 49, 189–193. [DOI] [PubMed] [Google Scholar]
- Sanchez, M. G. , Bourque, M. , Morissette, M. , & Di Paolo, T. (2010). Steroids‐dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neuroscience & Therapeutics, 16(3), e43–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro, N. (2005). The menopausal transition. The American Journal of Medicine, 118(12), 1405 10.1016/j.amjmed.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Saunders, E. F. , Nazir, R. , Kamali, M. , Ryan, K. A. , Evans, S. , Langenecker, S. , … McInnis, M. G. (2014). Gender differences, clinical correlates, and longitudinal outcome of bipolar disorder with comorbid migraine. The Journal of Clinical Psychiatry, 75(5), 512–519. 10.4088/JCP.13m08623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbisa, A. M. , van den Buuse, M. , & Gogos, A. (2017). The effect of estradiol and its analogues on cognition in preclinical and clinical research: Relevance to schizophrenia In Gargiulo P. A., & Mesones‐Arroyo H. L. (Eds.), Psychiatry and neuroscience update: Bridging the divide (Vol. 2) (pp. 355–374). Cham, Switzerland: Springer. [Google Scholar]
- Schock, H. , Zeleniuch‐Jacquotte, A. , Lundin, E. , Grankvist, K. , Lakso, H. A. , Idahl, A. , … Fortner, R. T. (2016). Hormone concentrations throughout uncomplicated pregnancies: A longitudinal study. BMC Pregnancy and Childbirth, 16(1), 146 10.1186/s12884-016-0937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman, M. V. , & Lang, M. (1990). The role of estrogens in schizophrenia gender differences. Schizophrenia Bulletin, 16(2), 185–194. [DOI] [PubMed] [Google Scholar]
- Sharma, V. , Xie, B. , Campbell, M. K. , Penava, D. , Hampson, E. , Mazmanian, D. , & Pope, C. J. (2014). A prospective study of diagnostic conversion of major depressive disorder to bipolar disorder in pregnancy and postpartum. Bipolar Disorders, 16(1), 16–21. [DOI] [PubMed] [Google Scholar]
- Sher, L. , Grunebaum, M. F. , Sullivan, G. M. , Burke, A. K. , Cooper, T. B. , Mann, J. J. , & Oquendo, M. A. (2012). Testosterone levels in suicide attempters with bipolar disorder. Journal of Psychiatric Research, 46(10), 1267–1271. 10.1016/j.jpsychires.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher, L. , Grunebaum, M. F. , Sullivan, G. M. , Burke, A. K. , Cooper, T. B. , Mann, J. J. , & Oquendo, M. A. (2014). Association of testosterone levels and future suicide attempts in females with bipolar disorder. Journal of Affective Disorders, 166, 98–102. 10.1016/j.jad.2014.04.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Guo, H. , Fan, F. , Fan, H. , An, H. , Wang, Z. , … Tan, Y. (2018). Sex differences of hippocampal structure in bipolar disorder. Psychiatry Research: Neuroimaging, 273, 35–41. 10.1016/j.pscychresns.2017.11.011 [DOI] [PubMed] [Google Scholar]
- Shirayama, Y. , Hashimoto, K. , Suzuki, Y. , & Higuchi, T. (2002). Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophrenia Research, 58(1), 69–74. 10.1016/S0920-9964(01)00367-X [DOI] [PubMed] [Google Scholar]
- Shivakumar, G. , Bernstein, I. H. , Suppes, T. , & Network aTSFB (2008). Are bipolar mood symptoms affected by the phase of the menstrual cycle? Journal of Women's Health, 17(3), 473–478. [DOI] [PubMed] [Google Scholar]
- Sisek‐Sprem, M. , Krizaj, A. , Jukic, V. , Milosevic, M. , Petrovic, Z. , & Herceg, M. (2015). Testosterone levels and clinical features of schizophrenia with emphasis on negative symptoms and aggression. Nordic Journal of Psychiatry, 69(2), 102–109. [DOI] [PubMed] [Google Scholar]
- Solomon, Z. , Gelkopf, M. , & Bleich, A. (2005). Is terror gender‐blind? Gender differences in reaction to terror events. Social Psychiatry and Psychiatric Epidemiology, 40(12), 947–954. [DOI] [PubMed] [Google Scholar]
- Stenmark, H. , Guzey, I. C. , Elbert, T. , & Holen, A. (2014). Gender and offender status predicting treatment success in refugees and asylum seekers with PTSD. European Journal of Psychotraumatology, 5 10.3402/ejpt.v3405.20803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker, R. , Eberhart, R. , Chevailler, M. C. , Quinn, F. A. , & Bischof, P. (2006). Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clinical Chemistry and Laboratory Medicine, 44(7), 883–887. [DOI] [PubMed] [Google Scholar]
- Stroth, N. , & Eiden, L. E. (2010). Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase‐activating polypeptide signaling. Neuroscience, 165(4), 1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Walker, A. J. , Dean, B. , van den Buuse, M. , & Gogos, A. (2016). Progesterone: The neglected hormone in schizophrenia? A focus on progesterone‐dopamine interactions. Psychoneuroendocrinology, 74, 126–140. [DOI] [PubMed] [Google Scholar]
- Suominen, K. , Mantere, O. , Valtonen, H. , Arvilommi, P. , Leppämäki, S. , & Isometsä, E. (2009). Gender differences in bipolar disorder type I and II. Acta Psychiatrica Scandinavica, 120(6), 464–473. [DOI] [PubMed] [Google Scholar]
- Suppes, T. , Leverich, G. S. , Keck, P. E. , Nolen, W. A. , Denicoff, K. D. , Altshuler, L. L. , … Post, R. M. (2001). The Stanley Foundation Bipolar Treatment Outcome Network: II. Demographics and illness characteristics of the first 261 patients. Journal of Affective Disorders, 67(1), 45–59. [DOI] [PubMed] [Google Scholar]
- Szymanski, S. , Lieberman, J. A. , Alvir, J. M. , Mayerhoff, D. , Loebel, A. , Geisler, S. , … Kane, J. (1995). Gender differences in onset of illness, treatment response, course, and biologic indexes in first‐episode schizophrenic patients. The American Journal of Psychiatry, 152(5), 698–703. 10.1176/ajp.152.5.698 [DOI] [PubMed] [Google Scholar]
- Teatero, M. L. , Mazmanian, D. , & Sharma, V. (2014). Effects of the menstrual cycle on bipolar disorder. Bipolar Disorders, 16(1), 22–36. [DOI] [PubMed] [Google Scholar]
- Thomas, M. P. , & Potter, B. V. (2013). The structural biology of oestrogen metabolism. The Journal of Steroid Biochemistry and Molecular Biology, 137, 27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup, A. , Petersen, L. , Jeppesen, P. , Ohlenschlaeger, J. , Christensen, T. , Krarup, G. , … Nordentoft, M. (2007). Gender differences in young adults with first‐episode schizophrenia spectrum disorders at baseline in the Danish OPUS study. The Journal of Nervous and Mental Disease, 195(5), 396–405. 10.1097/01.nmd.0000253784.59708.dd [DOI] [PubMed] [Google Scholar]
- Tolin, D. F. , & Foa, E. B. (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132(6), 959–992. [DOI] [PubMed] [Google Scholar]
- Toufexis, D. , Rivarola, M. A. , Lara, H. , & Viau, V. (2014). Stress and the reproductive axis. Journal of Neuroendocrinology, 26(9), 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui, K. (2008). Progesterone biosynthesis and action in the developing neuron. Endocrinology, 149(6), 2757–2761. [DOI] [PubMed] [Google Scholar]
- Usall, J. , Ochoa, S. , Araya, S. , Marquez, M. , & NEDES Group (Assessment Research Group in Schizophrenia) (2003). Gender differences and outcome in schizophrenia: A 2‐year follow‐up study in a large community sample. European Psychiatry, 18(6), 282–284. [DOI] [PubMed] [Google Scholar]
- Van Rheenen, T. E. , & Rossell, S. L. (2013). Auditory‐prosodic processing in bipolar disorder; from sensory perception to emotion. Journal of Affective Disorders, 151(3), 1102–1107. [DOI] [PubMed] [Google Scholar]
- Viguera, A. C. , Baldessarini, R. J. , & Tondo, L. (2001). Response to lithium maintenance treatment in bipolar disorders: Comparison of women and men. Bipolar Disorders, 3(5), 245–252. [DOI] [PubMed] [Google Scholar]
- Vila‐Rodriguez, F. , Ochoa, S. , Autonell, J. , Usall, J. , & Haro, J. M. (2011). Complex interaction between symptoms, social factors, and gender in social functioning in a community‐dwelling sample of schizophrenia. The Psychiatric Quarterly, 82(4), 261–274. [DOI] [PubMed] [Google Scholar]
- Wade D, Varker T, Kartal D, Hetrick S, O'Donnell M, Forbes D (2016). Gender difference in outcomes following trauma‐focused interventions for posttraumatic stress disorder: Systematic review and meta‐analysis. Psychological Trauma: Theory, Research, Practice and Policy 8: 356–364. [DOI] [PubMed] [Google Scholar]
- Wassell, J. , Rogers, S. , Felmingham, K. L. , Pearson, J. , & Bryant, R. A. (2015). Progesterone and mental imagery interactively predict emotional memories. Psychoneuroendocrinology, 51, 1–10. [DOI] [PubMed] [Google Scholar]
- Wegerer, M. , Kerschbaum, H. , Blechert, J. , & Wilhelm, F. H. (2014). Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiology of Learning and Memory, 116, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser, M. , Levi, L. , Burshtein, S. , Hagin, M. , Matei, V. P. , Podea, D. , … Davis, J. M. (2017). Raloxifene plus antipsychotics versus placebo plus antipsychotics in severely Ill decompensated postmenopausal women with schizophrenia or schizoaffective disorder: A randomized controlled trial. Journal of Clinical Psychiatry, 78(7), e758–e765. 10.4088/JCP.15m10498 [DOI] [PubMed] [Google Scholar]
- Wesseloo, R. , Kamperman, A. M. , Munk‐Olsen, T. , Pop, V. J. , Kushner, S. A. , & Bergink, V. (2015). Risk of postpartum relapse in bipolar disorder and postpartum psychosis: A systematic review and meta‐analysis. American Journal of Psychiatry, 173(2), 117–127. [DOI] [PubMed] [Google Scholar]
- White, E. C. , & Graham, B. M. (2016). Estradiol levels in women predict skin conductance response but not valence and expectancy ratings in conditioned fear extinction. Neurobiology of Learning and Memory, 134(Pt B), 339–348. [DOI] [PubMed] [Google Scholar]
- Widmaier, E. P. , Raff, H. , & Strang, K. T. (2014). Vander's human physiology: The mechanisms of body function (13th ed.). McGraw Hill: New York. [Google Scholar]
- Wieck, A. , Davies, R. , Hirst, A. , Brown, N. , Papadopoulos, A. , Marks, M. , … Campbell, I. C. (2003). Menstrual cycle effects on hypothalamic dopamine receptor function in women with a history of puerperal bipolar disorder. Journal of Psychopharmacology, 17(2), 204–209. 10.1177/0269881103017002009 [DOI] [PubMed] [Google Scholar]
- Wooderson, S. C. , Gallagher, P. , Watson, S. , & Young, A. H. (2015). An exploration of testosterone levels in patients with bipolar disorder. BJPsych Open, 1(2), 136–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. C. , Hill, R. A. , Gogos, A. , & van den Buuse, M. (2013). Sex differences and the role of estrogen in animal models of schizophrenia: Interaction with BDNF. Neuroscience, 239, 67–83. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M. , Hibi, H. , Katsuno, S. , & Miyake, K. (1995). Serum estradoiol levels in normal men and men with idiopathic infertility. International Journal of Urology, 2(1), 44–46. [PubMed] [Google Scholar]
- Zeidan, M. A. , Igoe, S. A. , Linnman, C. , Vitalo, A. , Levine, J. B. , Klibanski, A. , … Milad, M. R. (2011). Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry, 70(10), 920–927. 10.1016/j.biopsych.2011.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. Y. , Chen, D. C. , Xiu, M. H. , Yang, F. D. , Haile, C. N. , Kosten, T. A. , & Kosten, T. R. (2012). Gender differences in never‐medicated first‐episode schizophrenia and medicated chronic schizophrenia patients. The Journal of Clinical Psychiatry, 73(7), 1025–1033. [DOI] [PubMed] [Google Scholar]
- Zhao, Z. , Fan, L. , & Frick, K. M. (2010). Epigenetic alterations regulate estradiol‐induced enhancement of memory consolidation. Proceedings of the National Academy of Sciences of the United States of America, 107(12), 5605–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, P. (2009). Neuroactive steroid regulation of neurotransmitter release in the CNS: Action, mechanism and possible significance. Progress in Neurobiology, 89, 134–152. [DOI] [PubMed] [Google Scholar]


