Abstract
Objectives:
To determine the safety, tolerability, pharmacokinetics, and immunomodulatory effects of a six-week course of atorvastatin in acute KD patients with coronary artery aneurysms (CAA).
Study design:
We performed a Phase I/IIa, 2-center, dose-escalation study of atorvastatin (0.125-0.75 mg/kg/day) in 34 acute patients with Kawasaki disease (age 2-17 yrs.) with echocardiographic evidence of CAA. We measured the brain metabolite 24(S)-hydroxycholesterol (24-OHC), serum lipids, acute phase reactants, liver enzymes, creatine phosphokinase, peripheral blood mononuclear cell populations, and coronary artery (CA) internal diameter normalized for body surface area pre-treatment and two and six weeks post-initiation of therapy.
Results:
A 6-week course of up to 0.75 mg/kg/day of atorvastatin was well-tolerated by the 34 subjects (median age 5.3 yrs, IQR 2.6-6.4) with no serious adverse events attributable to the study drug. The area under the curve for atorvastatin and its metabolite was larger than in adults suggesting a slower rate of metabolism in young children. The levels of 24-OHC were similar between the atorvastatin-treated subjects and matched controls.
Conclusions:
Atorvastatin was safe and well-tolerated in children with acute KD and CAA. A Phase III efficacy trial is warranted in this patient population that may benefit from the known anti-inflammatory and immunomodulatory effects of this drug.
Trial registration:
Keywords: Kawasaki disease, atorvastatin, statin, coronary artery abnormalities
Despite the fact that over 40 million adults in the United States use statin medications on a daily basis for their lipid-lowering and anti-inflammatory effects, this class of drugs has not been systematically studied in young children.1 Statin therapy is FDA approved for use in children at least 8 years of age for familial hypercholesterolemia.2 Statins are also commonly used following heart transplantation in infants and young children and are recommended in the 2010 International Society of Heart and Lung Transplantation Guidelines for pediatric heart transplant patients with coronary allograft vasculopathy.3-5 However, no data are available to guide dosing in the pediatric age group.
Statins have pleotropic anti-oxidant and anti-inflammatory effects that help promote endothelial cell homeostasis and block myofibroblast transformation, thus making this class of drugs potentially useful in the treatment of vascular inflammation.6-8 Studies have also shown that the statins induce autophagy and mitophagy and thus inhibit NRLP3 inflammasome activation and inflammatory cytokines such IL-1β, which is linked to the pathogenesis of KD.9-11 Kawasaki disease), the leading cause of acquired heart disease in children, is a pediatric vasculitis that can lead to coronary artery aneurysms (CAA) resulting from oxidative stress, immune activation, and vessel wall infiltration by cells of the innate and adaptive immune system associated with secretion of pro-inflammatory cytokines, elastases, and matrix metalloproteinases (MMPs).10, 12-16 Based on the new American Heart Association (AHA) definition of aneurysms in KD, in a single center study, 6.4% of patients with Kawasaki disease developed new CAA despite standard treatment with intravenous immunoglobulin (IVIG, 2 g/kg) and aspirin (ASA) within the first 10 days after fever onset.17, 18 The prognosis is even worse for infants younger than 6 months of age in whom more than half have persistent CAA despite timely IVIG therapy.19 The rationale for statins as adjunctive therapy for acute KD was based on their inhibition of MMP secretion and myofibroblast transformation, both of which have been implicated in the formation of CAA in KD10, 15, 20, 21.
In the United States, the number of young adults with a history of KD and CAA is projected to increase by 1400 individuals annually without a new therapeutic approach to intervene in this process.22 Atorvastatin was chosen for this study because it is FDA-approved for children at least 8 years of age with FH in the United States and data support its anti-inflammatory role in pathways critical to CAA formation in KD.23-29 To study the safety and tolerability, to generate PK data, and to understand the immunomodulatory effects of atorvastatin in young children, we performed a Phase I/IIa, dose-escalation study of atorvastatin for acute KD patients with CAA.
METHODS:
This study recruited patients from Rady Children’s Hospital San Diego (RCHSD), California and Children’s Hospital Colorado in Aurora, Colorado between October 1, 2012, and December 31, 2017. Children ages 2 to 17 years who had at least 3 days of fever with at least two clinical signs of KD per the AHA guidelines and a coronary artery (CA) internal diameter normalized for body surface area (Z score) of the left anterior descending coronary artery (LAD) or right coronary artery (RCA) of at least 2.5 within the first 20 days after fever onset were eligible for enrollment. Children with a chronic disease, screening creatine phosphokinase greater than three times the upper limit of normal, or who had taken a CYP3A4 inhibitor (ie, cyclosporine, clarithromycin or doxycycline) in the previous seven days were excluded. The IND issued by the FDA for this study limited enrollment to KD patients at least 2 years of age due to the lack of juvenile toxicity data in infant rats submitted by Pfizer at the time of the initial atorvastatin licensing.
All patients received IVIG (2 g/kg), ASA (30-50 or 80-100 mg/kg/day while hospitalized; lowered to 3-5 mg/kg/day, max 81 mg, at time of discharge), and infliximab (5 or 10 mg/kg IV) prior to study entry. The primary outcome measure was the safety and tolerability of atorvastatin. The secondary outcome measures included the PK, change in markers of inflammation including levels of C-reactive protein (CRP), white blood cell count (WBC), and erythrocyte sedimentation rate (ESR), change in levels of protein carbonyls, and the change in coronary artery Z-score. Peripheral blood mononuclear cell (PBMC) populations were enumerated at the two-week visit and compared with a cohort of age- and Z score-similar, historical control KD patients treated with IVIG, ASA, and infliximab at RCHSD.
Five of the patients treated with atorvastatin (0.5-0.75mg/kg/day) were age-matched with five patients treated with only IVIG and infliximab and their PBMC separated by Ficoll-Hypaque at the subacute stage (Day 15-30). Phenotyping of innate and adaptive immune cells in these ten subjects was performed using the antibodies described below.
As measures of oxidative stress, plasma carbonyl levels were measured in nine patients treated with atorvastatin (0.125-0.75 mg/kg/day) and age- and Illness day- matched 1:2 with 18 KD control subjects treated only with IVIG and infliximab. Details of KD and controls subjects are given in Table I (available at www.jpeds.com).
Table 1; online:
Comparison of demographic and clinical laboratory characteristics of atorvastatin-treated KD subjects and controls evaluated for protein carbonyl levels
| Atorvastatin KD subjects (N=9) |
KD controls† (N=18) |
P value | |
|---|---|---|---|
| Statin concentration 0.125 mg/kg, N | 1 | NA | NA |
| 0.25 mg/kg, N | 1 | NA | NA |
| 0.75 mg/kg, N | 7 | NA | NA |
| Age, y* | 2.9 (2.8-5.1) | 3.5 (2.5-5.4) | 0.8 |
| Males, N (%) | 8 (89) | 13 (72) | 0.6 |
| Days of illness at sample collection* | |||
| Pre-IVIG | 5 (4-6) | 5 (4-6) | 0.7 |
| 2 weeks | 20 (20-22) | 20 (17-23) | 0.6 |
| White blood cell count, ×109/L* | 11.7 (10.7-13.2) | 10.4 (7.8-14.5) | 0.4 |
| CRP, mg/dL* | 8.1 (6.0-10.1) | 5.5 (4.1-17.7) | 1.0 |
| ESR, mm/hr* | 60 (33-80) | 42 (27-60) | 0.6 |
| ALT, IU/L* | 63 (35-107) | 87 (46-108) | 0.4 |
| Zmax* | 3.2 (2.8-3.7) | 2.0 (1.3-2.8) | 0.1 |
Median (IQR)
Controls are KD subjects treated only with IVIG and infliximab
WBC: white blood cell count, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, ALT: alanine aminotransferase, NA: Not applicable
The study protocol was reviewed and approved by the University of California San Diego’s Institutional Review Board (IRB) and the Colorado Multiple IRB. Written informed consent was obtained from the parents or legal guardians and assent, when appropriate, was obtained from the patient. This study was registered with ClinicalTrials.gov (). The FDA-issued IND # was 113304.
Administration of study drug
Atorvastatin was provided for this study by Pfizer, Inc. Study drug was easily and rapidly dissolved by combining one 10 mg atorvastatin tablet with either 2.5 or 5.0 mL water and equal volumes of OraSweet™ syrup to produce a final concentration of 1-2 mg/mL. Immediately after mixing, the appropriate volume (weight-based dosing) was drawn up in a syringe and administered orally prior to breakfast. An additional 2.5- 5 mL of water was then drawn up in the syringe and administered to the subject to deliver any residual drug.
Dose escalation protocol
Details of the study protocol and planned analysis were previously published.30 Briefly, the dose-escalation protocol was designed to enroll a minimum of three subjects per dose level (Dose Level, dose (mg/kg/day); 1, 0.125 mg/kg/day; 2, 0.25 mg/kg/day; 3, 0.5 mg/kg/day; 4, 0.75 mg/kg/day). Based on the adult maximum dose, no subject received a dose greater than 80 mg/day. The “3+3 dose escalation design” used the number of dose limiting toxicities (DLTs) to determine the maximum tolerated dose (MTD).31 A DLT was defined as any of the following at the two- or six-week time point: (1) creatine kinase (CK) elevation > 10 times the upper limit of normal or symptoms of muscle pain due to myositis; (2) a decrease in total cholesterol level that was at least 10% lower than entry level AND below 100 mg/dl (~2.5th percentile for children age 2 yrs.); or (3) alanine aminotransferase (ALT) or (aspartate aminotransferase) AST more than three times the upper limit of the age and sex-adjusted normal range. A subject who experienced a DLT discontinued atorvastatin immediately and was monitored for resolution of the toxicity as medically appropriate.
Dose escalation depended on the number of subjects with a DLT at a given dose level: (1) if 0 of 3 subjects had a DLT, then 3 subjects were enrolled at the next dose level; (2) if 1 of 3 subjects had a DLT, then an additional 3 subjects were enrolled at that dose level and further dose escalation was dependent on the number of DLTs in those additional three subjects; (3) if 2 of 3 subjects had a DLT, 3 additional subjects were enrolled at the next lowest dose. The MTD was defined as the highest dose of atorvastatin studied at which no more than one in six patients experienced a DLT during the 6 weeks of treatment. Once the MTD was determined, the remaining subjects were enrolled in the ‘dose expansion’ phase of the study at the MTD to further assess the safety and activity of atorvastatin at the MTD.
Monitoring drug toxicity
The main side effects of statins are elevated serum aminotransferase concentrations and myopathy. The following laboratory studies were obtained at baseline, 2 weeks (+/− 4 days), and 6 weeks (+/− 4 days) after enrollment: complete blood count, fasting lipid panel, CRP, ALT, AST, and CK. Given the importance of cholesterol in brain development and to ensure that brain cholesterol levels were not significantly affected by atorvastastin therapy, the plasma concentrations of the brain-specific cholesterol metabolite, 24(S)-hydroxycholesterol (24-OHC), were measured at 2 and 6 weeks in a subset of the subjects (9 KD patients on atorvastatin and 7 KD controls matched for age, illness day, and infliximab treatment who were not treated with atorvastatin). The cartridges to isolate lipids from the serum were equilibrated by passing 3 mL of 100% methanol followed by 3 mL of distilled water. Serum samples (100μl) were mixed with 200μl of 100% methanol and 150μl of isopropanol. The solution was then vortexed for 1 minute and centrifuged for 10 minutes at 12,000 × g. The supernatant was then loaded onto the cartridges (Supelco Cat.57012). After washing, the analyte was eluted using 3 mL of 85% methanol. The eluate was then dried by passing nitrogen gas over the collection tube. Dried samples were kept at 4°C unt il analyzed by ELISA. 24-OHC was measured using a commercially available kit (Enzo 24-OHC ELISA kit, Cat. ADI-900-210, Lot. 03171704), following the manufacturer’s instruction.
All subjects were monitored for both adverse events (AEs) and serious adverse events (SAEs) during the 6 weeks of the study. These were classified according to severity and relationship to study drug. A Data Safety Monitoring Board reviewed the clinical data at the end of each dose level, granted permission for escalation to the next dose level, and determined the MTD at the end of enrollment in the Phase I study.
Pharmacokinetic analyses
Samples were collected before the first dose of oral atorvastatin and then 1, 2, 6, 12, and 24 hours after the dose. Trough levels were drawn just prior to giving an observed oral dose in clinic at 2 and 6 weeks after enrollment. The samples were analyzed for levels of atorvastatin, the parent drug, as well as the o-hydroxyatorvastatin metabolite (Q2 Solutions, Morrisville, North Carolina). For concentrations that were below the limit of detection, the value was set to 0.125 ng/mL or 1.25 ng/mL, depending on the detection limit of the assay. The maximum plasma concentration (Cmax), corresponding time (Tmax), half-life (t1/2), and area under the curve (AUC)0–last for atorvastatin and the o-hydroxyatorvastatin metabolite were calculated as previously described.32 Noncompartmental oral clearance rate (Cl/FNC) was calculated as the ratio of dose to AUC0–∞. Apparent volume of distribution (Vd/FNC) was calculated as Cl/FNC over λz. was calculated as 0.693/λz. Plasma atorvastatin trough concentrations collected in weeks 2 and 6 were compared with the 24 hour post-dose concentration collected during the acute phase (study day 1).
Genotyping
Samples from 10 KD subjects enrolled in this study were available for both pharmacokinetic and genetic analysis. DNA was collected from either whole blood or mouthwash samples as previously described and genotyping for rs4149056 in SLCO1B1 (solute carrier organic anion transporter family, member 1B1) was performed using TaqMan SNP genotype assays following manufacturer’s instruction (Life Technologies, USA).33 This locus was chosen because this polymorphism in SLCO1B1 has been associated with reduced activity of the OATP1B1 pathway and increased levels of atorvastatin and have been associated with statin myopathy.34
Protein Carbonyls
As measures of oxidative stress, plasma carbonyl levels were measured in nine patients treated with atorvastatin (0.125-0.75 mg/kg/day) and age- and Illness day- matched 1:2 with 18 KD control subjects treated only with IVIG and infliximab. We measured the concentration of plasma protein carbonyl groups using an ELISA (Cell Biolabs, Inc, San Diego, CA.) as described by the vendor. The results for all samples fell within the standard curve of 0.375 – 7.5 nmol protein carbonyl/mg protein using 4-parameter curve fitting.
Echocardiographic evaluation
2-D transthoracic echocardiograms (2-D Echo) were obtained on all KD subjects according to a strict pre-determined protocol. All echocardiographic images from the two-sites were evaluated by a single cardiologist (BP) at the Core Echo Lab at RCHSD, who was blinded to patient clinical status. The internal diameter of the coronary arteries was adjusted for body surface area (Z-score) from 2-D echocardiograms at baseline, 2 and 6 weeks. Analyses included comparison across dose levels for Z-max, defined as the maximum Z-score of either the LAD or RCA measured on any echocardiogram during the 6 weeks of the study, and the change in Z scores between baseline and 2 and 6 weeks after study entry.
Immune phenotyping
Given the role of T cells and myeloid dendritic cells (DC) in the pro- and anti-inflammatory pathways in KD, we focused on immune phenotyping these cell populations. Myeloid DC populations were characterized and enumerated by flow cytometry using the following monoclonal antibodies (mAb) : anti-human CD11c-APC, mouse IgG1κ, clone B-ly6, anti-human CD11b-APC-Cyanin 7 (Cy7), mouse IgG1κ, clone ICRF44, anti-human CD14-phycoerythrin (PE) Cy7, mouse IgG2aκ, clone M5E2, anti-human CD86-fluorescein isothiocyanate (FITC), mouse IgG1κ, clone 2331 (FuN-1) (eBioscience).
T cell populations were characterized and enumerated by flow cytometry using the following mAb: CD25 BV421, mouse IgG1 k, clone M-A251 (BD Bioscience), anti-human CD4-percp-Cy5.5, mouse IgG1k, clone RPA-T4, anti-human CD8, Alexa Fluor 700, clone RPA-T8, mouse IgG1k, anti-human CD45RA APC, mouse IgG2b k, clone HI100, anti-human CD127 FITC, mouse IgG1 k, clone eBioRDR5, anti-human HLA-DR APC-H7 clone G46-6, mouse IgG2a k (eBioscience). Data were acquired with FACS ARIA II and analyzed using FACSDiva (BS Biosciences, San Jose, CA).
Statistical analyses
Incidence rates of adverse events and the proportion of subjects prematurely withdrawn from the study due to adverse events was compiled. For continuous variables, the Kruskal Wallis Test was performed to compare the 4 dose levels. For categorical variables, the Fisher exact test was performed. Statistical analyses were performed using the statistical software R (version 3.4.4) (http://www.r-project.org). Analyses were performed following the intent-to-treat principle. No adjustments for multiple comparisons were made for secondary analyses, and a P value of .05 was considered statistically significant.
RESULTS:
Clinical and Laboratory Profiles of Study Patients
The participant flow as well as the baseline demographic and clinical characteristics for the study population overall (n=34) and by dose level are summarized in Figure 1 (available at www.jpeds.com)and Table 2. Except for race, for which there were more white subjects in dose levels 2 and 4 (p= 0.01), there were no significant differences in baseline characteristics across the four dose levels. There were no significant differences in the change in laboratory values by dose level between baseline and week 2 and 6 across dosage levels (Table 3; available at www.jpeds.com).
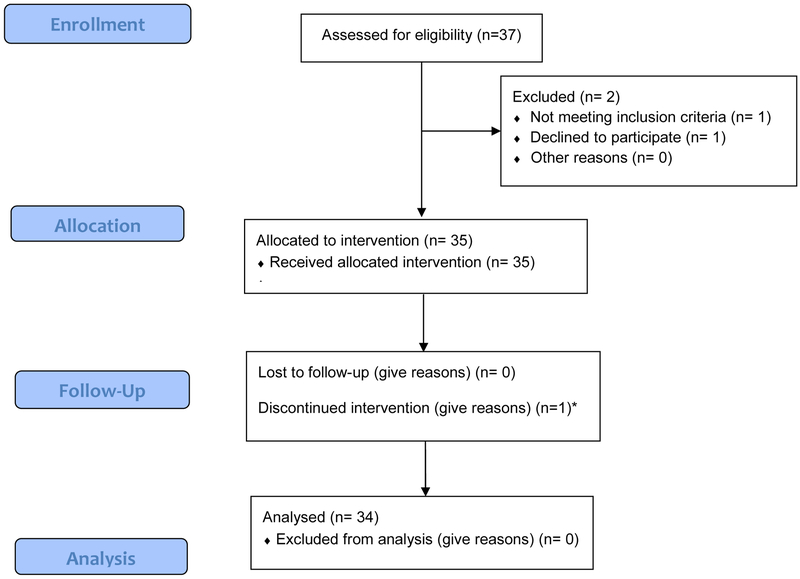
Figure 1 online.
Participant CONSORT flow diagram. Of the 37 subjects assessed for eligibility, one did not meet inclusion criteria (had a chronic underlying disorder that excluded participation), one declined (concerned about risks of atorvastatin) and one discontinued atorvastatin upon discharge home.
Table 2.
Baseline demographic and clinical characteristics of study population
| All dose levels N=34 |
Dose 1 (0.125 mg/kg/day) N=6 |
Dose 2 (0.25 mg/kg/day) N=7 |
Dose 3 (0.5 mg/kg/day) N=3 |
Dose 4 (0.75 mg/kg/day) N=18 |
P value (across doses) |
|
|---|---|---|---|---|---|---|
| Age, y* | 4 (2.1–16.5) | 7.3 (2.5-12.8) | 3.3 (2.2-16.5) | 5.3 (3.9-11.9) | 3 (2.1-14.5) | 0.19 |
| Males, N (%) | 24 (70.6) | 2 (33.3) | 5 (71.4) | 3 (100) | 14 (77.8) | 0.18 |
| Race, N (%) | ||||||
| White | 21 (61.8) | 1 (16.7) | 5 (71.4) | 1 (33.3) | 14 (77.8) | 0.01 |
| Asian | 3 (8.8) | 0 (0) | 0 (0) | 2 (66.7) | 1 (5.6) | |
| African American | 1 (2.9) | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | |
| Other† | 1 (2.9) | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | |
| More than one | 8 (23.5) | 3 (50) | 2 (28.6) | 0 (0) | 3 (16.7) | |
| Hispanic ethnicity, N (%) | 13 (38.2) | 1 (16.7) | 4 (57.1) | 1 (33.3) | 7 (38.9) | 0.62 |
| Days of illness at study enrollment*^ | 8 (4-21) | 8 (4-17) | 10 (4-21) | 7 (5-9) | 7.5 (5-15) | 0.62 |
| White blood | ||||||
| cell count, ×109/L* | 12.9 (4.7-36.7) | 11.9 (6-26.7) | 14.2 (8.7-36.7) | 12 (6.2-16.8) | 13.6 (4.7-23.5) | 0.72 |
| zHgb, *‡ | −1.6 (−3.8 to 2.67) | −1.3 (−3.5 to 1.2) | −2.6 (−3.8 to −0.8) | −2.7 (−2.8 to −1.7) | −1.3 (−3.7 to 2.7) | 0.14 |
| Platelet count, ×109/L* | 356 (66-624) | 385 (184-624) | 389 (308-550) | 283 (201-429) | 346 (66-554) | 0.56 |
| CRP, mg/dL* | 8.8 (0.5-36.3) | 6.5 (0.5-21.7) | 17.2 (4-36.3) | 17.7 (6.9-25.9) | 8.6 (3.5-31.7) | 0.38 |
| ESR, mm/hr* | 68 (10->140) | 60 (38-72) | 80 (37->140) | 99 (45->140) | 69 (10->140) | 0.40 |
| ALT, U/L* | 62 (15-473) | 55 (19-191) | 49 (19-268) | 62 (25-166) | 68 (15-473ss) | 0.81 |
| GGT, U/L* | 62 (11-270) | 128 (16-211) | 84 (13-270) | 95 (25-136) | 31 (11-249) | 0.58 |
| Albumin, g/dL* | 3.5 (2.4-5.9) | 3.5 (3.3-5.9) | 3.6 (2.9-4.2) | 3.5 (3.3-3.8) | 3.6 (2.4-4.4) | 0.97 |
| Z scores*§ Proximal LAD | 2.7 (0.4-9.1) | 2.6 (0.6-4.2) | 5.2 (0.4-9.1) | 3.3 (3.2-3.5) | 2.5 (1.4-6.2) | 0.36 |
| Proximal RCA | 2.3 (−0.7-8.9) | 2.4 (0.8-3.7) | 2.7 (0.8-8.9) | 3.3 (2.4-4.1) | 1.9 (−0.7-5.3) | 0.30 |
Median (Range)
Other: Afghani
One subject was enrolled on day 21 of illness rather than within the first 20 days of illness
zHgb, standard deviation units from the mean for age-adjusted hemoglobin values1
LAD, left anterior descending coronary artery; RCA, right coronary artery; Z scores were calculated as previously published2
Gunn L, Nechyba C. The Harriet Lane Handbook:16th edition. Philadelphia: Mosby; 2002.
de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133:254-8.
Table 3; online:
Change in laboratory characteristics of study population by dose level between baseline and week 2 and 6 of the study*
| Overall | Dose 1 (0.125 mg/kg) N=6 |
Dose 2 (0.25 mg/kg) N=7 |
Dose 3 (0.5 mg/kg) N=3 |
Dose 4 (0.75 mg/kg) N=18 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta 2 weeks minus baseline |
Delta 6 weeks minus baseline |
Delta 2 weeks minus baseline |
Delta 6 weeks minus baseline |
Delta 2 weeks minus baseline |
Delta 6 weeks minus baseline |
Delta 2 weeks minus baseline |
Delta 6 weeks minus baseline |
Delta 2 weeks minus baseline |
Delta 6 weeks minus baseline |
|
| WBC, ×109/L | −6.3 (−8.4 to −2.4) | −6.2 (−9.4 to −3.9) | −7.5 (−7.7 to −3.3) | −6.1 (−8.3 to −3.4) | −6.4 (−12.3 to −4.3) | −7 (−13 to −5.1) | −4.4 (−6.3 to −1.8) | −4 (−5 to −2.5) | −5.3 (−8.9 to −1.5) | −6.2 (−9.4 to −4.8) |
| Polys, % | −30 (−37 to −17) | −22.4 (−32.3 to −14.2) | −30 (−35.5 to −1.1) | −18.3 (−33.3 to −11.1) | −30 (−38 to −25) | −25 (−29.5 to −22.5) | −35 (−36 to −13.5) | −7 (−20 to 0) | −28 (−35.7 to −17.6) | −22.4 (−30.7 to −14.2) |
| Bands, % | −4 (−12 to −1) | −4 (−11 to 0) | −4 (−15 to −2.5) | −5 (−16 to −2.5) | −1 (−8 to 1) | −1 (−8 to −1) | −12 (−16.5 to −8) | −12 (−17.5 to −8) | −3 (−11 to −0.5) | −1 (−10.5 to 0.25) |
| Lymphs, % | 29.3 (22 to 35) | 27.5 (22 to 38.3) | 26 (21 to 32) | 35 (26.1 to 39) | 29.4 (26 to 37.5) | 36 (25.6 to 38.5) | 34 (24.5 to 36) | 38 (19.5 to 38.5) | 29.1 (22.2 to 35) | 25.2 (21.1 to 29.4) |
| ZHgb‡ | 0.7 (−0.7 to 1.8) | 1.4 (0.7 to 3) | 0.7 (0.7 to 1.3) | 1.8 (1.4 to 2.9) | 1.8 (0.6 to 2.1) | 1.4 (0.3 to 2.5) | 0.2 (−0.1 to 1.1) | 1.5 (1 to 2.1) | 0.1 (−1.2 to 1) | 1.3 (0.7 to 3.2) |
| Platelets ×109/L | 16.5 (−74 to 175) | −34 (−122 to 37.8) | −7 (−157 to 28) | −71.5 (−144.3 to 8.8) | −24 (−78.5 to 125) | −40 (−102 to 43.5) | 52 (20 to 175.5) | 17 (5.5 to 69) | 20 (−57 to 200) | −23 (−127 to 36.5) |
| CRP, mg/dL | −13.4 (−21 to −5.8) | −15.1 (−21 to −4.5) | −12 (−19.9 to −3.6) | −7.4 (−19.7 to −4.5) | −18.2 (−21.6 to −8.3) | −15.1 (−18.7 to −10.6) | −17.2 (−17.2 to −17.2) | −17.2 (−17.2 to −17.2) | −10.2 (−15.5 to −5.8) | −13.9 (−23.5 to −4.4) |
| ESR, mm/hr | −12 (−35 to 2) | −46 (−67 to −34) | −12 (−15 to 30) | −51 (−54 to −38) | −13 (−19 to −5) | −46 (−99 to −38) | −36 (−71 to 30) | −80 (−99 to −56) | −7 (−42 to 4) | −46 (−60 to −29) |
| GGT, IU/L | −43 (−90 to −3) | −43 (−104 to −9) | −115 (−132 to −72.5) | −133 (−163 to −88) | −55 (−75 to −6.5) | −62 (−84 to −11) | −43 (−67 to −21) | −68 (−94 to −39) | −13 (−77 to −1) | −19 (−85 to −7) |
| ALT, IU/L | −23 (−75 to −3) | −23 (−90 to 2) | −34 (−42 to 0) | −42 (−52 to 6) | −15 (−53 to −2) | −22 (−61 to 3) | −16 (−66 to −8) | −26 (−76 to −12) | −27 (−85 to −6) | −18 (−98 to −8) |
| AST, IU/L† | 9 (−11 to 17) | 2 (−20 to 11) | 18 (−2 to 33) | −9 (−18 to 2) | −7 (−32 to 12.5) | −5 (−52 to 11) | 1 (−3 to 5) | 1 (−6 to 8) | 12 (3 to 17) | 3 (−7 to 6) |
| CK, IU/L† | 18 (6-29) | 57 (34-83) | 23 (9-35) | 44 (28-59) | 11 (6-18) | 75 (50-75) | 24 (2-28) | 63 (56 to 81) | 19 (8-27) | 55 (26-92) |
| hsCRP, mg/L† | −62.9 (−105.9 to −31.1) | −63.4 (−120.6 to −31.9) | −78.4 (−83.1 to −64) | −78.5 (−83.5 to −64.2) | −30.7 (−62.8 to −8.2) | −32.3 (−63.7 to −8.3) | −53.4 (−68.8 to −43.4) | −56 (−70.9 to −44.2) | −66.3 (−130.9 to −35.1) | −67.5 (−142.7 to −32) |
| Cholesterol, mmol/L† | 15 (−5 to 37) | −7 (−25 to 3) | 28 (10 to 57) | 1 (−14 to 22) | 22 (−27 to 33) | −32 (−42 to −13) | −1 (−10 to 18) | −7 (−8 to 5) | 15 (−8 to 35) | −7 (−22 to 2.3) |
| LDL, mmol/L | −7 (−24 to 13) | −24 (−38 to −12) | −11 (−30 to 27) | −14 (−37 to −1) | 7 (−48 to 14) | −34 (−63 to −19) | −11 (−16 to −9) | −13 (−26 to −8) | −1 (−24 to 15) | −24 (−33 to −12) |
| HDL, mmol/L | 29 (21 to 36) | 35 (26 to 40) | 33 (26 to 36) | 40 (34 to 43) | 26 (23 to 30) | 29 (26 to 37) | 32 (22 to 35 | 34 (30 to 36) | 28 (21 to 41) | 36 (26 – |
| Triglycerides, mmol/L | −58 (−97 to −30) | −87 (−122 to −55) | −53 (−174 to −45) | −91 (−208 to −81) | −91 (−104 to −20) | −90 (−98 to −48) | −61 (−89 to −8) | −90 (−98 to −48) | −53 (−83 to −35) | 41) −67 (−127 to −62) |
Median (IQR)
As these laboratory values were followed once subjects had enrolled in the atorvastatin trial, the baseline values are post-IVIG.
For the following laboratory values, the number of subjects differed from 34: Bands (N=24), Platelets (N=32), CRP (N=19), ESR (N=32), ALT (N=32), AST (N=24), CK (N=27), hsCRP (N=30), cholesterol (N=28)
zHgb, standard deviation units from the mean for age-adjusted hemoglobin values1
Gunn L, Nechyba C. The Harriet Lane Handbook:16th edition. Philadelphia: Mosby; 2002.
Safety of Atorvastatin
As per the 3+3 protocol, the number of DLTs dictated the minimum number of subjects enrolled in each dose level. As there were no DLTs in dose levels 1 and 3, the minimum of three subjects was enrolled in each of these dose levels. One DLT occurred in dose level 2 thus requiring a minimum of six subjects at that dose level. Overall, we enrolled 6 subjects in dose level 1 (3 more than needed as we awaited DSMB approval to open next dose level), 7 in dose level 2 (1 more than needed as we awaited DSMB approval to open next dose level), 3 in dose level 3, and 18 in dose level 4, as the latter also included subjects in the dose expansion cohort. Two subjects had laboratory abnormalities during the dose escalation phase of the study that met criteria for a DLT. The first subject was receiving 0.25 mg/kg/day of atorvastatin and had a total cholesterol of 95 mg/dL after 6 weeks of study drug, and thus qualified as a DLT as the cholesterol was less than 100 mg/dL and more than 10% below the initial cholesterol level of 140 mg/dL. As this was the end of the study, atorvastatin was discontinued as per the study protocol and the cholesterol increased to 148 mg/dL by 4 weeks later. The second subject was receiving 0.75 mg/kg/day of atorvastatin and had an ALT of 82 IU/L that had decreased from 114 IU/L at the time of KD diagnosis. However, as this ALT level after 2 weeks of atorvastatin was still 3 times above the upper limit of normal for age, the study drug was discontinued as per study protocol. The ALT decreased to within the normal range (26 IU/L) four weeks later. During the expansion phase, one additional subject receiving 0.75 mg/kg/day of atorvastatin had a total cholesterol of 95 mg/dL after 2 weeks of study drug. The study drug was discontinued as the cholesterol was less than 100 mg/dL and more than 10% below the initial cholesterol level of 139 mg/dL. The cholesterol increased to 113 mg/dL four weeks after discontinuation of study drug. Thus, atorvastatin was discontinued because of low total cholesterol in 1 of 7 (14.3%) subjects at the second dose level (0.25 mg/kg/day) and because of either low total cholesterol or elevated ALT in 2 of 18 (11.1%) subjects at the highest dose level (0.75mg/kg/day).
Adverse events
The AEs are summarized in Table 4. No patient experienced an elevation in plasma CK. Of the 34 subjects, 24 (70.6%) experienced an AE. There was no difference in the proportion of subjects with at least one AE across dose levels. The most common adverse events included fever, either due to treatment resistance or a concomitant viral illness, and bruising due to antiplatelet or anticoagulant therapy for CAA (Table 5; available at www.jpeds.com). No SAE was related to the study drug. Four SAEs requiring either readmission or prolonged hospitalization occurred, including recurrence of fever in two subjects (one receiving 0.5 mg/kg/day and one receiving 0.75 mg/kg/day) and two worsening CAA at the highest dose level of 0.75 mg/kg/day (Table 4). There was no difference in the proportion of subjects with at least one SAE across dose levels.
Table 4.
Adverse events during the six-week study period stratified by dose level.
| All dose levels N=34 |
Dose 1 (0.125 mg/kg/day) N=6 |
Dose 2 (0.25 mg/kg/day) N=7 |
Dose 3 (0.5 mg/kg/day) N=3 |
Dose 4 (0.75 mg/kg/day) N=18 |
P value | |
|---|---|---|---|---|---|---|
| Total adverse events, N | 87 | 11 | 14 | 6 | 56 | |
| Subjects who experienced | ||||||
| ≥1 adverse event, N (%) | 24(70.6) | 4 (66.7) | 4 (57.1) | 2 (66.7) | 14 (77.8) | 0.754 |
| ≥2 adverse events, N (%) | 20(58.8) | 2(33.3) | 3 (42.9) | 2 (66.7) | 13 (72.2) | 0.263 |
| AE study drug relationship | ||||||
| Probably related to study | 3 (3.5) | 0 (0) | 1 (7.1) | 0 (0) | 2 (3.6) | >0.99 |
| drug, N (%) | ||||||
| Possibly related to study drug, N (%) | 6 (6.9) | 0 (0) | 0 (0) | 0 (0) | 6 (10.7) | 0.16 |
| Serious adverse events, N | 4 | 0 | 0 | 1 | 3 | |
| Number (%) of subjects who | 4 (11.8) | 0 (0) | 0 (0) | 1 (33.3) | 3 (16.7) | 0.369 |
| experienced SAE | ||||||
| Recrudescent fever† | 2 | 0 | 0 | 1 | 1 | |
| Worsening CAA† | 2 | 0 | 0 | 0 | 2 |
These events led to prolonged hospitalization and thus qualified as an SAE
Table 5; online:
Adverse events by body system
| All dose levels N=87* |
Dose 1 (0.125 mg/kg/day) N=11* |
Dose 2 (0.25 mg/kg/day) N=14* |
Dose 3 (0.5 mg/kg/day) N=6* |
Dose 4 (0.75 mg/kg/day) N=56* |
|
|---|---|---|---|---|---|
| Cardiac, (n, %) | 2 (2.3) | - | - | - | Worsening coronary |
| - | - | artery aneurysm | |||
| Dermatologic | 12 (13.8) | Hives/Viral exanthema | Rash, Hair loss, Insect bites | ||
| Gastrointestinal | 5 (5.8) | Constipation | Diarrhea | Emesis | Emesis, elevated AST or ALT |
| Hematologic | 15 (17.2) | Bruising/Epistaxis | Bruising/Epistaxis | - | Bruising/Epistaxis |
| Immunologic | 19 (21.8) | Fever | Fever | Fever | Fever |
| Metabolic | 3 (3.5) | - | Decreased cholesterol | - | Decreased cholesterol |
| Musculoskeletal | 12 (13.4) | Myalgia with transient thigh or foot pain | Myalgia with bilateral leg pain | Arthritis, arthralgia | |
| Neurologic | 7 (8.1) | - | Headache | Headache | Irritability, Headache |
| Ophthalmic | 1 (1.2) | - | - | - | Viral conjunctivitis |
| Oropharyngeal | 2 (2.3) | - | - | - | Otitis media, otalgia |
| Respiratory | 8 (9.2) | Viral illness | Viral illness | Congestion | Viral illness |
| Urologic | 1 (1.2) | - | - | - | Urinary tract infection |
N reflects number of adverse events for the dose level
24(S)-hydroxycholesterol (24-OHC)
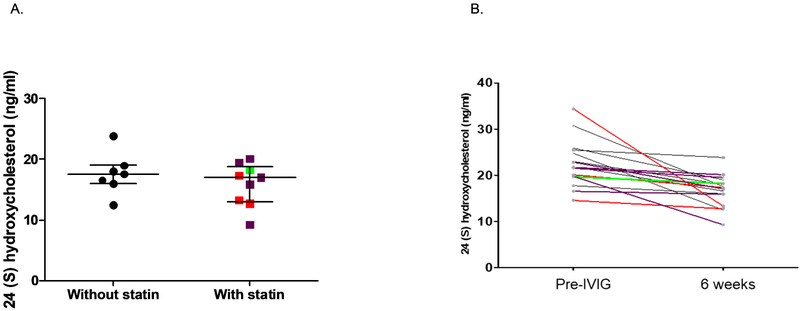
Detection of 24-OHC using extracted serum samples was tested in linearity assays (82.3% recovery) and the intra-assay coefficient of variation for the ELISA was 0-11.7%. As compared with control KD patients who had received standard therapy with IVIG but no atorvastatin, there was no difference in 24-OHC levels after 6 weeks of treatment with atorvastatin regardless of dose (Figure 2, A). Across all subjects, the serum levels of 24-OHC were elevated pre-IVIG treatment and decreased by 6 weeks later, regardless of whether atorvastatin was administered (Figure 2, B).
Figure 2.
Levels of 24(S)-hydroxycholesterol with and without atorvastatin therapy and across dose levels. (A) 24(S)-hydroxycholesterol levels after 6 weeks of KD diagnosis is shown in KD subjects who received IVIG and infliximab versus IVIG, infliximab and atorvastatin. (B) The individual 24(S) hydroxycholesterol level for each KD subject treated with atorvastatin is shown by dose level. Red: 0.25 mg/kg, Green: 0.5 mg/kg, Purple: 0.75 mg/kg, Black: without atorvastatin treatment.
Pharmacokinetics of atorvastatin
Noncompartmental PK analysis
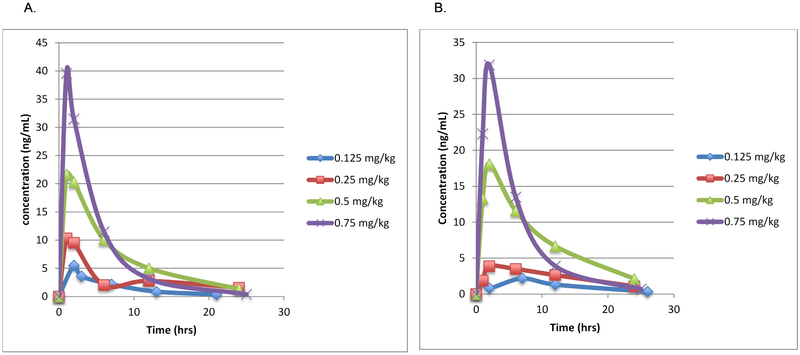
Of the 21 subjects in whom PK samples were collected during the dose escalation phase of the study, 15 had sufficient data for PK evaluation. Increasing the weight-based dose of atorvastatin led to an increase in the median Cmax and AUC of atorvastatin as well as of the ortho-hydroxyatorvastatin metabolite (Figure 3 and Table 6 [available at www.jpeds.com]). The median Tmax was reached at one hour for atorvastatin (range 1 to 2 hours) and two hours for the O-hydroxyatorvastatin metabolite (range 1 to 6 hours) (Table 6).
Figure 3.
Median concentration of (A) atorvastatin and (B) ortho-hydroxyatorvastatin metabolite versus time by dose in mg/kg. Increasing doses of atorvastatin and its active metabolite, ortho-hydroxyatorvastatin, are shown. Blue, 0.125 mg/kg/day; red, 0.25 mg/kg/day; green 0.5 mg/kg/day; purple 0.75 mg/kg/day.
Table 6; online.
Atorvastatin and O-hydroxyatorvastatin pharmacokinetic parameters by dose level
| Atorvastatin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) |
Cmax (ng/mL) |
C24 (ng/mL) | Trough 2 weeks (ng/mL) |
Trough 6 weeks (ng/mL) |
Tmax (hours) |
Half Life (hours) |
AUC0-∞ (ng*hr/mL) |
CL/F (L/hr/kg) |
Vd/F (L/kg) |
| 0.125 (n=6) | 5.80 (2.32-8.53) | BQL (BQL-0.38) | BQL (BQL-0.91) | BQL (BQL-0.26) | 1.5 (1-2) | 5.5 (4.4-7.9) | 41.9 (31.5-42.9) | 29.8 (16.6-39.8) | 222 (190-287) |
| 0.25 (n=5) | 9.49 (4.02-22.5) | BQL (BQL-1.54) | BQL (BQL-1.11) | BQL (BQL-2.19) | 1 (1-6) | 6.0 (5.6-6.2) | 122.8 (38.4-171.7) | 20.2 (14.6-60.00) | 163 (130-519) |
| 0.5 (n=3) | 21.70, 82.6 | BQL, 1.33 | 4.30 (BQL-12.8) | BQL, 6.0 | 1, 2 | 5.3 (4.2-6.3) | 330.9 (188.1-473.6) | 18.7 (10.6-26.80) | 153 (65-242) |
| 0.75 (n=6) | 41.4 (29.3-469) | BQL (BQL-2.89) | BQL (BQL-10.7) | BQL (BQL-5.56) | 1 (1-2) | 4.3 (3.1-5.1) | 356.5 (210.6-2890.9) | 21.7 (14.7-34.20) | 158 (65-212) |
| O-hydroxyatorvastatin metabolite | |||||||||
| Dose (mg/kg) |
Cmax (ng/mL) |
C24 (ng/mL) | Trough 2 weeks (ng/mL) | Trough 6 weeks (ng/mL) | Tmax (hours) |
Half Life (hours) | AUC0-∞ (ng*hr/mL) |
CL/F (L/hr/kg) |
Vd/F (L/kg) |
| 0.125 (n=6) | 1.87 (0.92-3.42) | BQL (BQL-0.67) | BQL (BQL-1.97) | BQL (BQL-0.87) | 6 (2-6) | 6.2 (4.1-9.1) | 40.2 (18.1-42.7) | N/A | N/A |
| 0.25 (n=5) | 4.91 (3.86-5.35) | BQL (BQL-1.07) | BQL (BQL-1.55) | BQL (BQL-2.59) | 2 (1-6) | 5.6 (2.7-8.5) | 64.6 (45.6-81.0) | N/A | N/A |
| 0.5 (n=3) | 11.10, 18.10 | BQL, 2.13 | 7.01 (BQL-17.7) | BQL, 12.4 | 1 , 2 | 6.8 (6.3-7.3) | 178.6 (145.5-211.8) | N/A | N/A |
| 0.75 (n=6) | 24.6 (9.27-49.9) | BQL (BQL-5.64) | BQL (BQL-9.57) | BQL (BQL-13.1) | 2 (1-6) | 5.3 (4.3-6.9) | 238.4 (154.9-1062.1) | N/A | N/A |
BQL: Below quantitative level (<0.25 or <2.5 ng/mL)
Values are reported as medians with a range. If only two values are available, both are reported.
Genotyping of SLCO1B1
In the 10 subjects for whom both DNA and PK data were available, two were heterozygous for the C risk allele for SLCO1B1 at rs4149056. One of these subjects had the highest Cmax and AUC of all subjects (Figure 3).
Protein Carbonyls
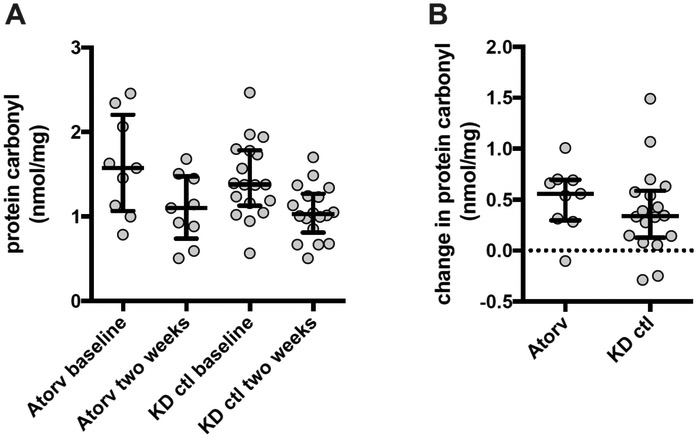
There was no difference in plasma protein carbonyl concentration between the atorvastatin-treated and matched control KD subjects at either baseline (pre-IVIG) or at 2 weeks (Figure 4; available at www.jpeds.com). However, there was a significant decrease in plasma protein carbonyl concentration from baseline to 2 weeks in both the atorvastatin–treated and the matched control KD subjects (p = 0.0078 and p = 0.0005, respectively), although there was no difference in the change between groups.
Figure 4 online:
Plasma protein carbonyl concentration. A) The plasma protein carbonyl concentration (median and IQR) is are shown for samples acquired prior to IVIG administration (baseline) and after two weeks of atorvastatin compared with samples from matched KD control (ctl) patients B) The difference in plasma protein carbonyl concentration (baseline minus 2 week) for study subjects and matched control KD patients (median and IQR).
Echocardiographic evaluation
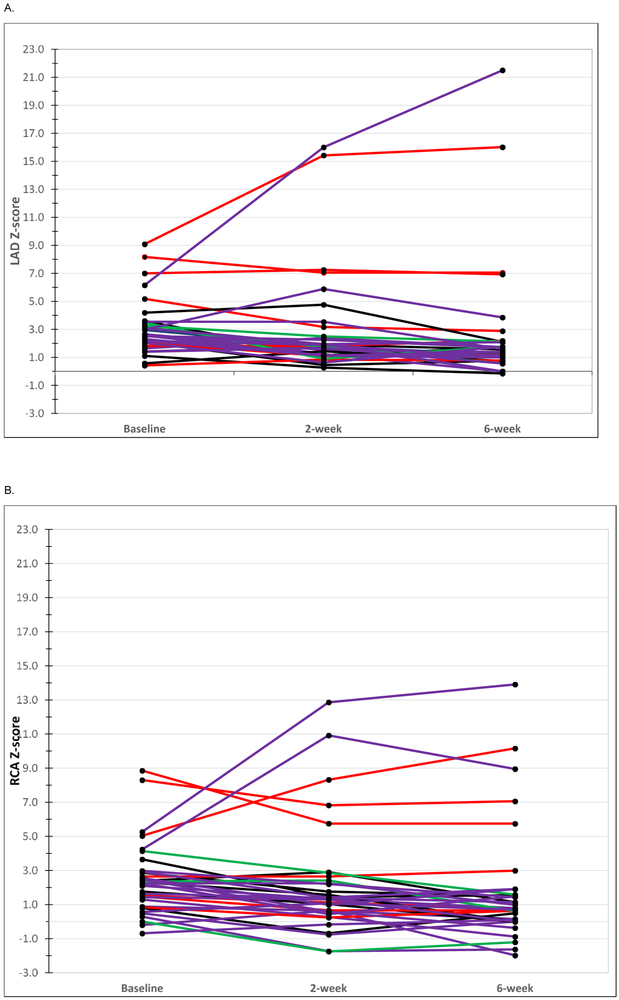
The baseline Z score of the LAD or RCA did not differ significantly by dose level (Table 2). The maximum Z scores for each coronary artery segment at any time point (Z max) and the difference in Z-scores from baseline to 2 and 6 weeks were similar across all four dose levels based on the Echo Core Lab readings (Table 7; available at www.jpeds.com). The evolution of Z scores is outlined for subjects who had a Zmax ≥ 5 (Table 8; available at www.jpeds.com). Two subjects treated with 0.75mg/kg/day had progression of their CAA while on the study drug (Figure 5; available at www.jpeds.com).
Table 7; online.
Comparison of z max and change in Z score for the study population over time by dose level*
| Overall | Dose 1 (0.125 mg/kg) N= 6 |
Dose 2 (0.25 mg/kg) N= 7 |
Dose 3 (0.5 mg/kg) N= 3 |
Dose 4 (0.75 mg/kg) N= 18 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zmax† | 2.9 (2.5 to 3.6) | 3 (2.9 to 3.5) | 5.2 (2.5 to 8.6) | 3.5 (3.3 to 3.8) | 2.6 (2.5 to 3.1) | |||||
| 2 week Z score minus baseline |
6 week Z score minus baseline |
2 week Z score minus baseline |
6 week Z score minus baseline |
2 week Z score minus baseline |
6 week Z score minus baseline |
2 week Z score minus baseline |
6 week Z score minus baseline |
2 week Z score minus baseline |
6 week Z score minus baseline |
|
| LAD‡ | −0.9 (−1.4 to 0.3) | −1.2 (−1.5 to −0.2) | −1 (−1.5 to 0.2) | −1.5 (−1.8 to −1.3) | 0 (−1 to 0.3) | −0.1 (−1 to 0.4) | −1.4 (−2 to −1.1) | −1.7 (−2.2 to −1.4) | −0.8 (−1.2 to 0.2) | −1.2 (−1.2 to −0.6) |
| RCA‡ | −0.7 (−1.5 to 0) | −1.1 (−1.8 to −0.4) | −0.9 (−1.4 to −0.7) | −1.4 (−1.8 to −1.3) | −0.6 (−1.2 to −0.2) | −0.8 (−1.0 to 0.1) | −0.6 (−0.9 to −0.3) | −2.2 (−2.4 to −1.9) | −0.5 (−1.4 to 0.4) | −0.9 (−1.6 to −0.3) |
Median (IQR)
LAD, left anterior descending coronary artery; RCA, right coronary artery. z scores were calculated as previously published 1
Zmax is the largest Z score of either the LAD or RCA over the 6 week course of therapy
Table 8 online.
Evolution of Z scores for subjects with Zmax† ≥ 5 over the course of atorvastatin therapy
| Age, y | Days of illness at study enrollment |
Dose of atorvastatin (mg/kg) |
Baseline LAD Z score‡ |
Baseline RCA Z score‡ |
2 week LAD Z score‡ |
2 week RCA Z score‡ |
6 week LAD Z score‡ |
6 week RCA Z score‡ |
|---|---|---|---|---|---|---|---|---|
| 2.75 | 15 | 0.25 | 5.18 | 1.51 | 3.17 | 1.12 | 2.88 | 0.73 |
| 16.50 | 21 | 0.25 | 7.00 | 8.30 | 7.25 | 6.82 | 6.93 | 7.06 |
| 4.73 | 15 | 0.25 | 8.17 | 8.85 | 7.05 | 5.75 | 7.05 | 5.75 |
| 5.44 | 10 | 0.25 | 9.08 | 5.03 | 15.42 | 8.32 | 16.01 | 10.16 |
| 2.94 | 5 | 0.75 | 2.98 | 4.23 | 5.88 | 10.92 | 3.85 | 8.95 |
| 2.80 | 7 | 0.75 | 6.15 | 5.26 | 16.00 | 12.86 | 21.50 | 13.91 |
LAD, left anterior descending coronary artery; RCA, right coronary artery.
Zmax is the largest Z score of either the LAD or RCA over the 6 week course of therapy
Figure 5 online:
Z-score over course of treatment with atorvastatin. (A) Left anterior descending (LAD) coronary artery Z score at baseline (pre-IVIG) and 2 and 6 weeks after treatment with atorvastatin. (B) Right coronary artery (RCA) Z score pre-IVIG and 2 and 6 weeks after treatment with atorvastatin. Blue, 0.125 mg/kg/day; red, 0.25 mg/kg/day; green 0.5 mg/kg/day; purple 0.75 mg/kg/day.
Immune phenotyping
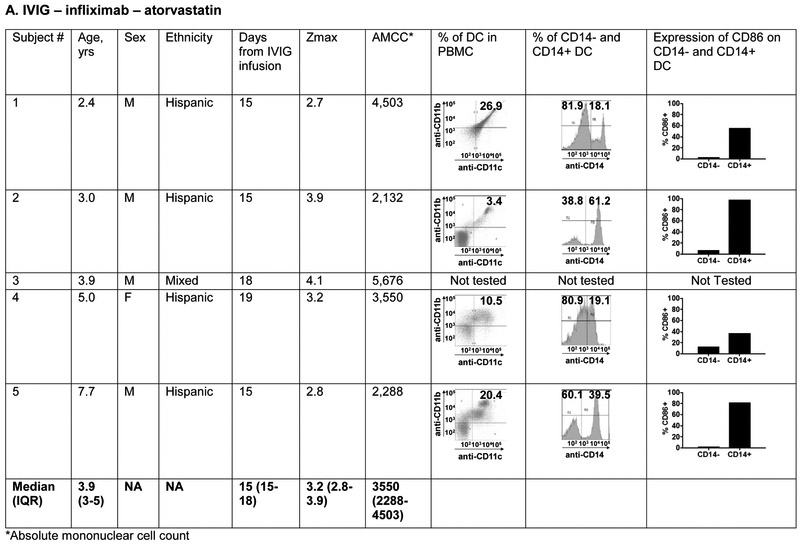
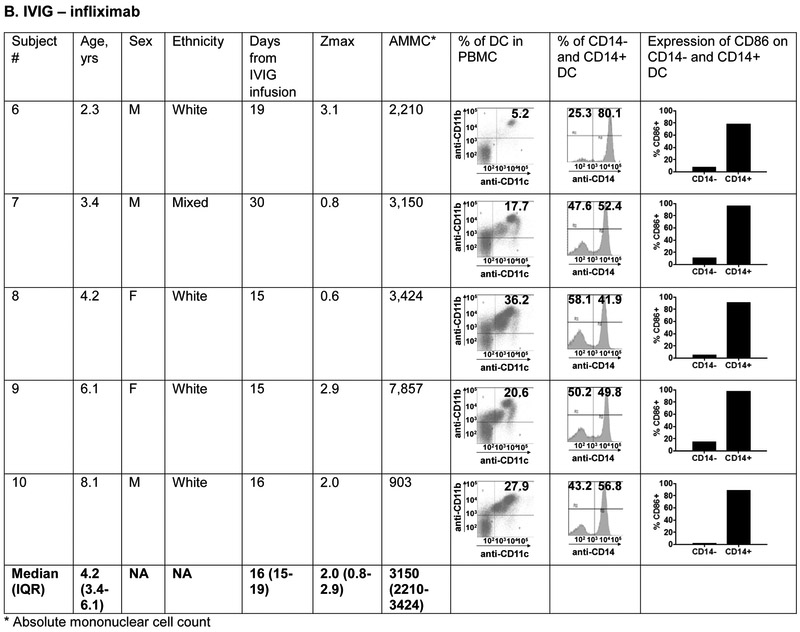
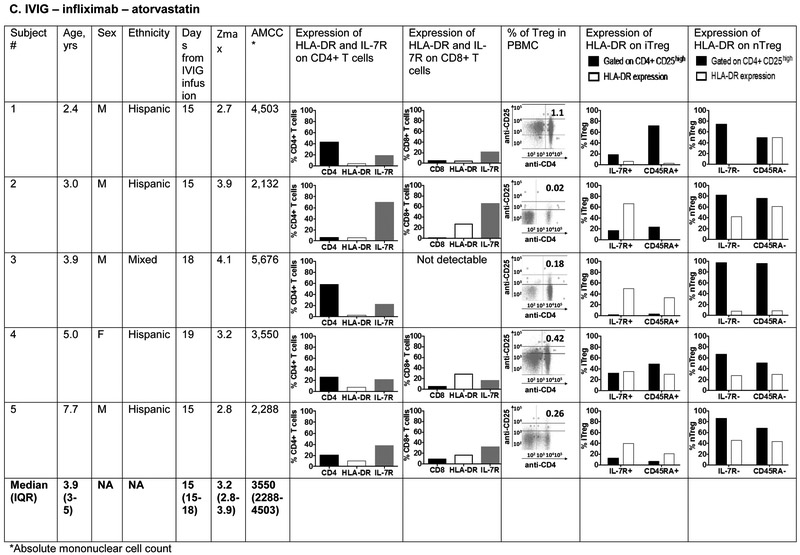
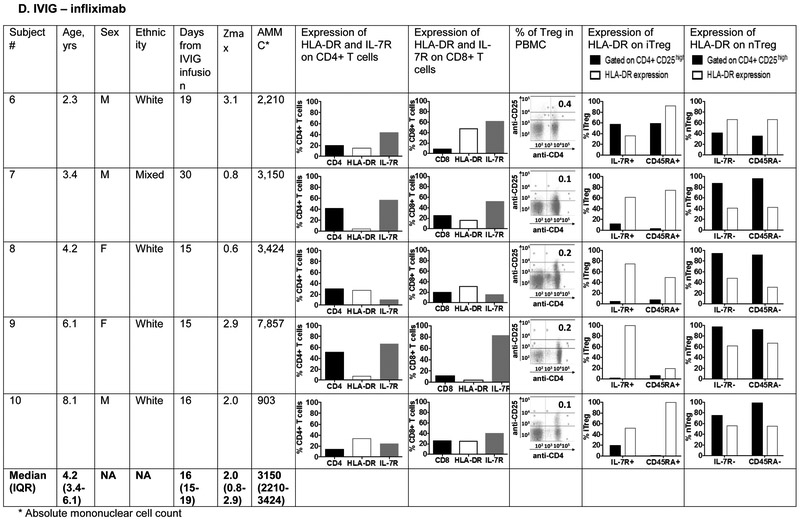
The phenotype of innate and adaptive PBMC at the subacute phase (Illness Day 15-30) was compared between 5 patients treated with IVIG, ASA, infliximab, and atorvastatin (0.5-0.75mg/kg/day) and five patients treated with IVIG, ASA, and infliximab alone (Figure 6; available at www.jpeds.com). The groups did not differ with respect to median age. The predominant innate PBMC were myeloid dendritic cells (mDC) defined by the expression of CD11c and CD11b. Within the mDC population, a large percentage expressed CD14 as a marker of tolerogenic mDC that have been previously described in KD 35. These CD14+ cells expressed CD86 as a maturation and activation marker. There was no significant difference in the distribution of mDC populations between the two treatment groups.
Figure 6 online:
Immunophenotyping of peripheral blood mononuclear cells in patients with Kawasaki disease treated with IVIG, infliximab, and atorvastatin or IVIG and infliximab only. Subjects 1, 2, 4 and 5 received 0.75 mg/kg/day of atorvastatin and subject 3 received 0.5 mg/kg/day.
Panels A and B: Characterization of myeloid dendritic cells (mDC) by flow cytometry: CD11c+ CD11b+ mDC (dot plots) were gated on CD14− and CD14+ populations (histograms) and evaluated for CD86 expression to determine their maturation/activation stage.
Panels C and D: Characterization of T cell lineages by flow cytometry: CD4+ and CD8+ T cells were enumerated and evaluated for their activation/expansion by measuring DR and IL-7R expression. Regulatory T cells (Treg) were enumerated by gating on CD4+ CD25high cells (dot plots) and evaluated for IL-7R and CD45RA expression that define peripherally induced Treg (iTreg). Natural Treg that mature in the thymus (nTreg) are IL-7R – and CD45RA−. DR expression on iTreg and nTreg defines their activation stage.
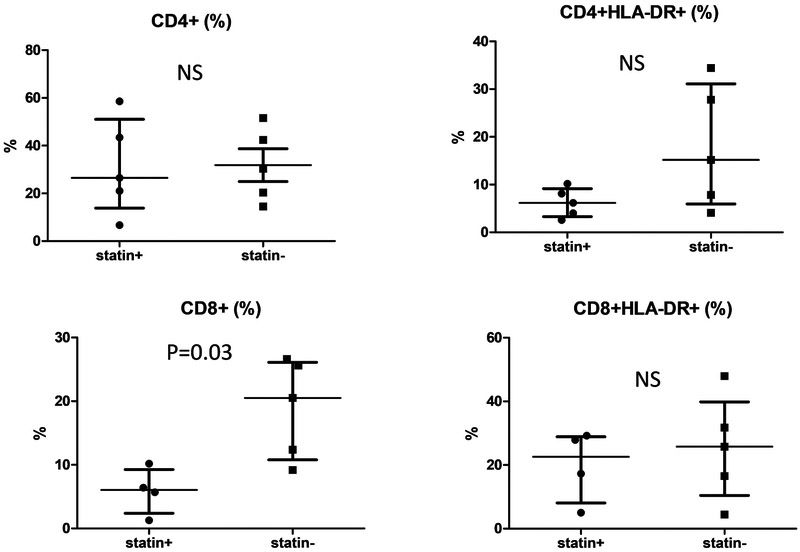
T cell lineages were compared between the 2 groups using markers for activation (DR), expansion (IL7R), and regulation (CD25high). CD4+ CD25high T cells were further characterized as peripherally induced (iTreg: IL-7R+ CD45RA+) and natural Treg (nTreg: IL-7R− CD45RA−). The only consistent difference between the patients treated with or without atorvastatin was a lower percentage of circulating CD8+ T cells in patients who received atorvastatin (p=0.03) (Figure 7; available at www.jpeds.com). However, the percent activated (DR+) CD8+ was similar between groups. Although iTreg have been described as induced by atorvastatin treatment in adults, these cells are not abundant in the circulation in children in general, and were a minor cell population in both groups of KD patients regardless of treatment 36. nTreg that were previously described to downregulate inflammation in acute KD were equally represented in the two treatment groups 37.
Figure 7 online.
Percentage of circulating CD4+ and CD8+ T cells, including activated (HLA-DR+) T cells for KD patients treated with (statin +) and without (statin −) atorvastatin.
DISCUSSION:
This study assessed the safety and tolerability of a six-week course of atorvastatin in children with acute KD and CAA and to determine the PK around the first dose. Atorvastatin at doses ranging from 0.125-0.75 mg/kg/day was safe and well-tolerated in this young patient population. Based on this study in a multiethnic population, 0.75 mg/kg/day of atorvastatin for a 6 week period was well-tolerated in acute KD patients with CAA.
As cholesterol is important in brain development in the pediatric age group, a conservative lower threshold of 100 mg/dl was set for plasma cholesterol as a DLT. Although cholesterol did drop to 95 mg/dL in two subjects, the cholesterol level improved after the atorvastatin was discontinued. To assess whether a six-week course of atorvastatin decreased the brain cholesterol metabolite (24-OHC) levels in children, we compared 24-OHC levels of KD patients treated with and without atorvastatin. The lack of significant change in 24-OHC levels compared with KD patients not treated with atorvastatin reaffirms the safety of a six-week course of atorvastatin during acute KD. We were also reassured by the lack of increase in CK in our subjects after a six-week course of atorvastatin. There were no signs of myopathy by physical examination or parental report. Although it is sometimes difficult to assess muscle tenderness in young children, data from the adult clinical trials found that true myopathy is rare and is consistently associated with CK elevation.38
The PK data from this study may help to guide dosing in other pediatric populations, although the acute inflammatory state of our KD patients may have altered both drug absorption and metabolism. The Cmax and AUC0-∞ of atorvastatin and O-hydroxyatorvastatin were larger in our study population as compared with similar weight based dosing using an average adult weight of 70 kg.39, 40 In contrast, the Tmax was similar between our subjects and published data from adults.41 This would suggest that the rate of absorption of atorvastatin is similar but the extent of absorption is greater and/or the overall metabolism is slower in children. Absorption and metabolism of oral atorvastatin may also be affected by changes in gastrointestinal permeability which appear to occur in acute KD. In the Lactobacillus caseii cell wall extract (LCWE) mouse model of KD, the mice exhibit intestinal leakage and this dysfunction is associated with increased serum levels of zonulin, a protein marker of intestinal permeability.42 Although the enzymes required to metabolize atorvastatin are nearing adult activity by one year of age, pediatric liver weight lags behind total body weight making the mass of the liver in proportion to the body smaller in children.43, 44 This developmental detail could contribute to these findings. Host genetics also influence statin metabolism and the subject with the highest Cmax was heterozygous for the C risk allele for SLCO1B1 at rs4149056.45 This polymorphism can potentially reduce the metabolism and first pass loss of atorvastatin via the OATP1B1 pathway and lead to higher circulating concentrations. Another subject who was heterozygous for the risk allele had the third highest Cmax among the genotyped subjects. Neither subject experienced any toxicity from these high levels of atorvastatin.
The transmural inflammation in the coronary arteries during CAA formation in acute KD destroys the normal architecture and the arterial wall is most likely never functionally normal again, even in remodeled vessels with a normal lumen.46, 47 Pro-inflammatory cytokines including interleukin (IL)-1b and tumor necrosis factor (TNF) a and MMPs circulate at high levels in acute KD patients and these aspects of the systemic vasculitis have been recapitulated in a murine model using intraperitoneal injection of LCWE.48-51 Genetic studies suggest that allelic variants in MMP genes are associated with both KD susceptibility and CAA14. Using mouse splenocytes incubated with LCWE, Blankier et al demonstrated that T cell proliferation, MMP-9 secretion, and TNFa production were reduced in a dose-dependent manner in response to ex vivo exposure to atorvastatin.52 These observations lend support to the potential beneficial role of statins in protecting the arterial wall during the acute vasculitis of KD.
The importance of cytotoxic T cells in mediating the destruction of the arterial wall is well-documented in KD. Immunohistochemistry studies have demonstrated infiltration of CD8+ T cells into the media and gene expression studies from the arterial wall of KD autopsies have shown increased transcripts associated with activated CD8+ T cells.53, 54 In a mouse model of myocarditis, statin treatment of CD8+ T cells ameliorated disease induction.55 The exploratory immunophentoyping in our cohort suggests that circulating CD8+ T cells may be reduced in children on atorvastatin therapy, but these preliminary findings will need to be validated in a larger cohort. This type of immune monitoring, available as a routine laboratory test in many hospital clinical laboratories, could be incorporated into a Phase III trial in the future.
In previously published work, blood samples from several subjects in this study were evaluated in an in vitro model of endothelial-to-mesenchymal transition (Endo-MT) using human umbilical vein endothelial cells (HUVEC).56 Pre-treatment sera when incubated with HUVEC initiated a molecular cascade involving KLF4, mir-483, and CTGF that resulted in transition of the HUVEC to a mesenchymal phenotype. In contrast, KD patient sera after six weeks of atorvastatin therapy induced higher levels of mir-483, an inhibitor of CTGF and Endo-MT, in the HUVEC cultures as compared with sera from age-, illness day- and Z-score- matched KD patients who had been treated only with IVIG and infliximab. These in vitro data support the potential benefit of atorvastatin therapy in acute KD patients with early signs of CA damage. Additional support comes from analysis of KD autopsies from the acute phase of the illness. Immunohistochemistry of the arterial wall demonstrated α-smooth muscle actin (SMA)-positive, smoothelin-negative myofibroblast-like cells in the thickened intima that co-expressed IL-17, lending more support to the injurious role of myofibroblasts in acute KD.15
Autopsy studies from the late convalescent phase have demonstrated luminal myofibroblastic proliferation in the arterial wall resulting in significant stenosis.57 For this reason, the convalescent use of statins in older KD patients with CAA has been widely adopted. A report from Japan described a significant reduction in high sensitivity (hs) CRP in 11 KD patients with CAA treated with fluvastatin for 12 months an average of 12.4 years after the acute phase.58 In a Canadian study of 20 KD patients with giant CAA, atorvastatin at a dose of either 5 or 10 mg/day administered for a median of 2.5 years beginning at least two years after the acute phase was determined to be safe but no PK studies were performed.59 Another study of 13 KD patients with CAA at least one year after the acute phase demonstrated improved flow-mediated dilation of the brachial artery and lower hsCRP after 6 months of pravastatin therapy.60 In two case reports of adult KD patients with giant CAA and inflammation in the arterial wall demonstrated by FDG-PET, the inflammation signal was greatly attenuated after the initiation of statin therapy but returned in the one KD patient in whom the statin was discontinued.61,62
We recognize both strengths and weaknesses in the current study. This is the first dose-escalation PK study of a statin for children with acute cardiovascular inflammation and the first study to demonstrate the safety of atorvastatin in children with acute KD. However, rare adverse events related to atorvastatin could have been missed in this study due to small sample size. As a Phase I/IIa study, this clinical trial was neither placebo-controlled nor powered to determine the effectiveness of atorvastatin in reducing laboratory measures of inflammation or change in Zmax. In this study, only children ≥ 2 years of age were eligible for this trial given that there was no juvenile toxicity data in infant rats submitted to the FDA at the time of initial drug approval. Thus the safety of atorvastatin in children <2 years who have the greatest risk of coronary artery aneurysms remains unanswered. The limited number of patients studied for immune phenotyping limited the strength of the conclusions that could be drawn. The standard clinical laboratory values that were compared between atorvastatin-treated subjects and controls were likely insensitive measures of statin effect. With respect to the protein carbonyl studies, treatment with IVIG and infliximab alone was likely sufficient to reduce reactive oxygen species. Measurement of specific MMP and pro-inflammatory cytokines within a shorter time frame (e.g. 24-72 hours after initiation of statin therapy) might have been more informative and should be considered in future trial designs.
With this study, we have established a range of safe doses of atorvastatin in children with acute KD and CAA. The potential benefit of the anti-inflammatory and immunomodulatory actions of atorvastatin warrant a Phase III efficacy trial to test the hypothesis that children with acute KD and early signs of CA damage will benefit from the addition of atorvastatin to standard therapy.
ACKNOWLEDGEMENTS:
We thank the members of the Data and Safety Monitoring Board: Lori Daniels, MD, MAS; Edmund Capparelli, PharmD; Antonio Arrieta, MD; and Rema Raman, PhD. We are grateful to our study support staff, pharmacists, physician colleagues, and, most importantly, patients and their families for their contribution to this study.
Supported in part by NIH (R01-HD081296 and R01-HL140898 [to A.T.]), an Investigator-Initiated Research Award from Pfizer Inc (to A.T.), a grant from the Gordon and Marilyn Macklin Foundation (to J.B.), the American Heart Association (15GRNT22760008 [to A.F.]), an NIH Research Supplement to Promote Diversity (to A.T. for R.P.), the NIH/NCATS Colorado CTSI (UL1 TR000154 [to P.J.]), and support from the Kawasaki Kids Foundation (to P.J.). Pfizer Inc, the manufacturer of atorvastatin, provided commercial-grade drug for this study. The authors declare no conflicts of interest.
List of abbreviations:
- AE
Adverse event
- AHA
American Heart Association
- ALT
Alanine aminotransferase
- ASA
Aspirin
- AST
Aspartate aminotransferase
- CA
Coronary artery
- CAA
Coronary artery aneurysms
- CK
Creatine kinase
- Cmax
Maximum plasma concentration
- CRP
C-reactive protein
- DLT
Dose limiting toxicity
- ESR
Erythrocyte sedimentation rate
- IL
Interleukin
- IVIG
Intravenous immunoglobulin
- LAD
Left anterior descending coronary artery
- LCWE
Lactobacillus caseii cell wall extract
- mDC
Myeloid dendritic cell
- MMP
Matrix metalloproteinases
- MTD
Maximum tolerated dose
- OHC
Hydroxycholesterol
- PBMC
Peripheral blood mononuclear cells
- PK
Pharmacokinetic
- RCA
Right coronary artery
- RCHSD
Rady Children’s Hospital San Diego
- SAE
Serious adverse event
- Treg
T regulatory cells
- n
natural
- i
peripherally induced
- Tmax
Time to maximum plasma concentration
- TNF
Tumor necrosis factor
- WBC
White blood cell count
- Zmax
Maximal Z score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented, in part, as an oral presentation at the International Kawasaki Disease Symposium on February 4, 2015, << >>.
References
- [1].Mercado C, DeSimone AK, Odom E, Gillespie C, Ayala C, Loustalot F. Prevalence of Cholesterol Treatment Eligibility and Medication Use Among Adults--United States, 2005-2012. MMWR Morb Mortal Wkly Rep. 2015;64:1305–11. [DOI] [PubMed] [Google Scholar]
- [2].McCrindle BW, Urbina EM, Dennison BA, Jacobson MS, Steinberger J, Rocchini AP, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115:1948–67. [DOI] [PubMed] [Google Scholar]
- [3].Mahle WT, Vincent RN, Berg AM, Kanter KR. Pravastatin therapy is associated with reduction in coronary allograft vasculopathy in pediatric heart transplantation. J Heart Lung Transplant. 2005;24:63–6. [DOI] [PubMed] [Google Scholar]
- [4].Chin C, Lukito SS, Shek J, Bernstein D, Perry SB. Prevention of pediatric graft coronary artery disease: atorvastatin. Pediatr Transplant. 2008;12:442–6. [DOI] [PubMed] [Google Scholar]
- [5].Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–56. [DOI] [PubMed] [Google Scholar]
- [6].Mihos CG, Salas MJ, Santana O. The pleiotropic effects of the hydroxy-methyl-glutaryl-CoA reductase inhibitors in cardiovascular disease: a comprehensive review. Cardiology in review. 2010;18:298–304. [DOI] [PubMed] [Google Scholar]
- [7].Benton JA, Kern HB, Leinwand LA, Mariner PD, Anseth KS. Statins block calcific nodule formation of valvular interstitial cells by inhibiting alpha-smooth muscle actin expression. Arterioscler Thromb Vasc Biol. 2009;29:1950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tremoulet AH. The role of statins in inflammatory vasculitides. Autoimmunity. 2015;48:177–80. [DOI] [PubMed] [Google Scholar]
- [9].Peng S, Xu LW, Che XY, Xiao QQ, Pu J, Shao Q, et al. Atorvastatin Inhibits Inflammatory Response, Attenuates Lipid Deposition, and Improves the Stability of Vulnerable Atherosclerotic Plaques by Modulating Autophagy. Front Pharmacol 2018;9:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fury W, Tremoulet AH, Watson VE, Best BM, Shimizu C, Hamilton J, et al. Transcript abundance patterns in Kawasaki disease patients with intravenous immunoglobulin resistance. Hum Immunol. 2010;71:865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Andres AM, Hernandez G, Lee P, Huang C, Ratliff EP, Sin J, et al. Mitophagy is required for acute cardioprotection by simvastatin. Antioxid Redox Signal. 2014;21:1960–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Franco A, Shimizu C, Tremoulet AH, Burns JC. Memory T-cells and characterization of peripheral T-cell clones in acute Kawasaki disease. Autoimmunity. 2010;43:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shimizu C, Jain S, Davila S, Hibberd ML, Lin KO, Molkara D, et al. Transforming growth factor-beta signaling pathway in patients with Kawasaki disease. Circulation. 2011;4:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shimizu C, Matsubara T, Onouchi Y, Jain S, Sun S, Nievergelt CM, et al. Matrix metalloproteinase haplotypes associated with coronary artery aneurysm formation in patients with Kawasaki disease. J Hum Genet. 2010;55:779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shimizu C, Oharaseki T, Takahashi K, Kottek A, Franco A, Burns JC. The role of TGF-beta and myofibroblasts in the arteritis of Kawasaki disease. Human pathology. 2013;44:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yahata T, Hamaoka K. Oxidative stress and Kawasaki disease: how is oxidative stress involved from the acute stage to the chronic stage? Rheumatology (Oxford). 2017;56:6–13. [DOI] [PubMed] [Google Scholar]
- [17].McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017;135:e927–e99. [DOI] [PubMed] [Google Scholar]
- [18].Skochko SM, Jain S, Sun X, Sivilay N, Kanegaye JT, Pancheri J, et al. Kawasaki Disease Outcomes and Response to Therapy in a Multiethnic Community: A 10-Year Experience. J Pediatr. 2018;203:408–15 e3. [DOI] [PubMed] [Google Scholar]
- [19].Salgado AP, Ashouri N, Berry EK, Sun X, Jain S, Burns JC, et al. High Risk of Coronary Artery Aneurysms in Infants Younger than 6 Months of Age with Kawasaki Disease. J Pediatr. 2017;185:112–6 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].de Jongh S, Lilien MR, op't Roodt J, Stroes ES, Bakker HD, Kastelein JJ. Early statin therapy restores endothelial function in children with familial hypercholesterolemia. J Am Coll Cardiol. 2002;40:2117–21. [DOI] [PubMed] [Google Scholar]
- [21].Porter KE, Turner NA, O'Regan DJ, Ball SG. Tumor necrosis factor alpha induces human atrial myofibroblast proliferation, invasion and MMP-9 secretion: inhibition by simvastatin. Cardiovasc Res. 2004;64:507–15. [DOI] [PubMed] [Google Scholar]
- [22].Gordon JB, Kahn AM, Burns JC. When children with Kawasaki disease grow up: Myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54:1911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Izidoro-Toledo TC, Guimaraes DA, Belo VA, Gerlach RF, Tanus-Santos JE. Effects of statins on matrix metalloproteinases and their endogenous inhibitors in human endothelial cells. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:547–54. [DOI] [PubMed] [Google Scholar]
- [24].Mahajan N, Dhawan V. Inhibition of C-reactive protein induced expression of matrix metalloproteinases by atorvastatin in THP-1 cells. Mol Cell Biochem. 2010;338:77–86. [DOI] [PubMed] [Google Scholar]
- [25].Schweitzer M, Mitmaker B, Obrand D, Sheiner N, Abraham C, Dostanic S, et al. Atorvastatin modulates matrix metalloproteinase expression, activity, and signaling in abdominal aortic aneurysms. Vascular and endovascular surgery. 2010;44:116–22. [DOI] [PubMed] [Google Scholar]
- [26].Tang TT, Song Y, Ding YJ, Liao YH, Yu X, Du R, et al. Atorvastatin upregulates regulatory T cells and reduces clinical disease activity in patients with rheumatoid arthritis. J Lipid Res. 2011;52:1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Avis HJ, Vissers MN, Stein EA, Wijburg FA, Trip MD, Kastelein JJ, et al. A systematic review and meta-analysis of statin therapy in children with familial hypercholesterolemia. Arterioscler Thromb Va c Biol. 2007;27:1803–10. [DOI] [PubMed] [Google Scholar]
- [28].Krmar RT, Ferraris JR, Ramirez JA, Sorroche P, Legal S, Cayssials A. Use of atorvastatin in hyperlipidemic hypertensive renal transplant recipients. Pediatr Nephrol. 2002;17:540–3. [DOI] [PubMed] [Google Scholar]
- [29].Gandelman K, Glue P, Laskey R, Jones J, LaBadie R, Ose L. An eight-week trial investigating the efficacy and tolerability of atorvastatin for children and adolescents with heterozygous familial hypercholesterolemia. Pediatr Cardiol. 2011;32:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tremoulet AH, Jain S, Burns JC. Evaluating a novel treatment for coronary artery inflammation in acute Kawasaki disease: A Phase I/IIa trial of atorvastatin. Expert Opin Orphan Drugs. 2015;3:967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–37. [PubMed] [Google Scholar]
- [32].Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, Jafri HS, et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008;153:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Khor CC, Davila S, Breunis WB, Lee YC, Shimizu C, Wright VJ, et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet. 2011;43:1241–6. [DOI] [PubMed] [Google Scholar]
- [34].Group SC, Link E, Parish S, Armitage J, Bowman L, Heath S, et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008;359:789–99. [DOI] [PubMed] [Google Scholar]
- [35].Franco A, Kumar J, Lin G, Behnamfar N, Hsieh LE, Shimizu C, et al. Pediatric tolerogenic DCs expressing CD4 and immunoglobulin-like transcript receptor (ILT)-4 secrete IL-10 in response to Fc and adenosine. Eur J Immunol. 2018;48:482–91. [DOI] [PubMed] [Google Scholar]
- [36].Zhang D, Wang S, Guan Y, Wang L, Xie W, Li N, et al. Effect of oral atorvastatin on CD4+CD25+ regulatory T cells, FoxP3 expression, and prognosis in patients with ST-segment elevated myocardial infarction before primary percutaneous coronary intervention. J Cardiovasc Pharmacol. 2011;57:536–41. [DOI] [PubMed] [Google Scholar]
- [37].Burns JC, Song Y, Bujold M, Shimizu C, Kanegaye JT, Tremoulet AH, et al. Immune-monitoring in Kawasaki disease patients treated with infliximab and intravenous immunoglobulin. Clin Exp Immunol. 2013;174:337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61. [DOI] [PubMed] [Google Scholar]
- [39].Kantola T, Kivisto KT, Neuvonen PJ. Effect of itraconazole on the pharmacokinetics of atorvastatin. Clin Pharmacol Ther. 1998;64:58–65. [DOI] [PubMed] [Google Scholar]
- [40].Lilja JJ, Kivisto KT, Neuvonen PJ. Grapefruit juice increases serum concentrations of atorvastatin and has no effect on pravastatin. Clin Pharmacol Ther. 1999;66:118–27. [DOI] [PubMed] [Google Scholar]
- [41].Woo HI, Kim SR, Huh W, Ko JW, Lee SY. Association of genetic variations with pharmacokinetics and lipid-lowering response to atorvastatin in healthy Korean subjects. Drug Des Devel Ther. 2017;11:1135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Noval Rivas M, Wakita D, Abe M, Franklin MK, Chen S, Shimada K, et al. Role of Intestinal Permeability and Secretory Iga in the Development of Cardiovascular Pathology in a Murine Model of Kawasaki Disease. Circulation. 2017;136. [Google Scholar]
- [43].Kanamori M, Takahashi H, Echizen H. Developmental changes in the liver weight- and body weight-normalized clearance of theophylline, phenytoin and cyclosporine in children. Int J Clin Pharmacol Ther. 2002;40:485–92. [DOI] [PubMed] [Google Scholar]
- [44].Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther. 2014;19:262–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82:726–33. [DOI] [PubMed] [Google Scholar]
- [46].Takahashi K, Oharaseki T, Yokouchi Y. Histopathological aspects of cardiovascular lesions in Kawasaki disease. Int J Rheum Dis. 2018;21:31–5. [DOI] [PubMed] [Google Scholar]
- [47].Sugimura T, Kato H, Inoue O, Takagi J, Fukuda T, Sato N. Vasodilatory response of the coronary arteries after Kawasaki disease: evaluation by intracoronary injection of isosorbide dinitrate. J Pediatr. 1992;121:684–8. [DOI] [PubMed] [Google Scholar]
- [48].Furukawa S, Matsubara T, Jujoh K, Yone K, Sugawara T, Sasai K, et al. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clin Immunol Immunopathol. 1988;48:247–51. [DOI] [PubMed] [Google Scholar]
- [49].Chua PK, Melish ME, Yu Q, Yanagihara R, Yamamoto KS, Nerurkar VR. Elevated levels of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 during the acute phase of Kawasaki disease. Clin Diagn Lab Immunol. 2003;10:308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Maury CP, Salo E, Pelkonen P. Circulating interleukin-1 beta in patients with Kawasaki disease. N Engl J Med. 1988;319:1670–1. [DOI] [PubMed] [Google Scholar]
- [51].Lee Y, Schulte DJ, Shimada K, Chen S, Crother TR, Chiba N, et al. Interleukin-1beta is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation. 2012;125:1542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Blankier S, McCrindle BW, Ito S, Yeung RS. The role of atorvastatin in regulating the immune response leading to vascular damage in a model of Kawasaki disease. Clin Exp Immunol. 2011;164:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184:940–3. [DOI] [PubMed] [Google Scholar]
- [54].Rowley AH, Wylie KM, Kim KY, Pink AJ, Yang A, Reindel R, et al. The transcriptional profile of coronary arteritis in Kawasaki disease. BMC Genomics. 2015;16:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bu DX, Tarrio M, Grabie N, Zhang Y, Yamazaki H, Stavrakis G, et al. Statin-induced Kruppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. The Journal of clinical investigation. 2010;120:1961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].He M, Chen Z, Martin M, Zhang J, Sangwung P, Woo B, et al. miR-483 Targeting of CTGF Suppresses Endothelial-to-Mesenchymal Transition: Therapeutic Implications in Kawasaki Disease. Circ Res. 2017;120:354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Orenstein JM, Shulman ST, Fox LM, Baker SC, Takahashi M, Bhatti TR, et al. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS ONE. 2012;7:e38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hamaoka A, Hamaoka K, Yahata T, Fujii M, Ozawa S, Toiyama K, et al. Effects of HMG-CoA reductase inhibitors on continuous post-inflammatory vascular remodeling late after Kawasaki disease. J Cardiol 2010;56:245–53. [DOI] [PubMed] [Google Scholar]
- [59].Niedra E, Chahal N, Manlhiot C, Yeung RS, McCrindle BW. Atorvastatin safety in Kawasaki disease patients with coronary artery aneurysms. Pediatr Cardiol. 2014;35:89–92. [DOI] [PubMed] [Google Scholar]
- [60].Duan C, Du ZD, Wang Y, Jia LQ. Effect of pravastatin on endothelial dysfunction in children with medium to giant coronary aneurysms due to Kawasaki disease. World J Pediatr. 2014;10:232–7. [DOI] [PubMed] [Google Scholar]
- [61].Suda K, Tahara N, Honda A, Yoshimoto H, Kishimoto S, Kudo Y, et al. Statin reduces persistent coronary arterial inflammation evaluated by serial (1)(8)fluorodeoxyglucose positron emission tomography imaging long after Kawasaki disease. Int J Cardiol 2015;179:61–2. [DOI] [PubMed] [Google Scholar]
- [62].Bekki M, Tahara N, Tahara A, Honda A, Igata S, Sugiyama Y, et al. Anti-inflammatory effect of statin in coronary aneurysms late after Kawasaki disease. J Nucl Cardiol. 2019;26:671–3. [DOI] [PubMed] [Google Scholar]