Abstract
Background & Aims:
Quantitative fecal immunochemical tests (FITs) for hemoglobin are commonly used for colorectal cancer (CRC) screening. We aimed to quantify the change in CRC and advanced adenoma detection and number of positive test results at different positivity thresholds and by sex and age.
Methods:
We searched MEDLINE and EMBASE, selecting articles of FIT for CRC detection in asymptomatic adults undergoing screening. We calculated sensitivity and specificity, as well as detected number of cancers, advanced adenomas, and positive test results at positivity thresholds ≤10 µg hemoglobin/g feces, 10 to ≤20 µg/g, 20 to ≤30 µg/g, and >30 µg/g. We also analyzed results from stratified by patient sex, age, and reference standard.
Results:
Our meta-analysis comprised 46 studies with 2.4 million participants and 6478 detected cancers. Sensitivity for detection of CRC increased from 69% (95% CI, 63%–75%) at thresholds >10 µg/g and ≤20 µg/g to 80% (95% CI, 76%–83%) at thresholds ≤10 µg/g. At these threshold values, sensitivity for detection of advanced adenomas increased from 21% (95% CI, 18%–2%5) to 31% (95% CI, 27%–35%), whereas specificity decreased from 94% (95% CI, 93%–96%) to 91% (95% CI, 89%–93%). In 3 studies stratified by sex, sensitivity of CRC detection was 77% in men (95% CI, 75%–79%) and 81% in women (95% CI, 60%–100%) (P=.68). In 3 studies stratified by age groups, sensitivity of CRC detection was 85% for ages 50–59 years (95% CI, 71%–99%) and 73% for ages 60–69 years (95% CI, 71%–75%) (P=.10). All studies with colonoscopy follow up had similar sensitivity levels for detection of CRC to studies that analyzed 2-year registry follow-up data (74%; 95% CI, 68%–78% vs 75%; 95% CI, 73%–77%).
Conclusions:
In a meta-analysis of studies that analyzed detection of CRC and advanced adenomas at different FIT positivity thresholds, we found the sensitivity and specificity of detection to vary with positive cut-off value. It might be possible to decrease positive threshold values for centers with sufficient follow-up colonoscopy resources. More research is needed to precisely establish FIT thresholds for each sex and age subgroup.
Keywords: colon cancer, advanced neoplasia, fecal occult blood test, diagnostic performance
Graphical Abstract:
Lay summary:
Quantitative fecal immunochemical tests, or FITs, are commonly used for colorectal cancer screening. Screening programs could detect significantly more cancers and polyps by using lower thresholds to define a positive result, provided they have enough specialists to perform the necessary follow-up colonoscopies.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States.1 Randomized clinical trials have demonstrated that screening with guaiac fecal occult blood test (gFOBT) can reduce CRC mortality.2 Fecal immunochemical tests (FITs) are recommended for CRC screening3, 4 because they obtain better diagnostic performance and higher participation rates than gFOBT.5
The optimal positivity threshold of quantitative FIT for screening is unknown and may vary by sex and age; it can be adjusted to optimize CRC detection and be concordant with local colonoscopy resources.6 Some experts in the United States favor a uniform threshold of ≤20 µg hemoglobin/g feces, but evidence is limited because individual studies included small numbers of patients with CRC; data and consistent definitions for advanced adenoma detection were frequently not included;3, 7 variable comparison groups between studies;7 and variability between FIT brands8 and positivity thresholds.9, 10 Normal mean fecal hemoglobin concentrations varies significantly by sex and age,9, 11–13 as does cancer incidence; combined, these trends could have important impacts on FIT performance. Higher mean stool hemoglobin concentrations in men than in women might generate more positive results, potentially impacting both sensitivity and specificity (because more men would go to colonoscopy). Whether the quantitative abnormal cut-off should vary by sex and age, like peripheral complete blood cell counts, is largely unexplored, due to the difficulty of evaluating these subpopulations in individual studies.14–16
In this context, we substantially expanded prior systematic reviews7, 17 to provide more precise risks and benefits of varying FIT positivity thresholds and to explore the effects of patient (sex and age), test (FIT brand), and study characteristics (reference standard and geographic area) on optimal cut-offs for FIT performance.
Methods
We employed a protocol (PROSPERO CRD42017068760) based on standard guidelines for the systematic review of diagnostic tests. We followed the Standards for the Reporting of Diagnostic Accuracy Studies (STARD)18 and Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) of Diagnostic Test Accuracy Studies19 statements for reporting our systematic review. All authors had access to the study data and reviewed and approved the final manuscript.
Literature Search:
In addition to articles from a previous review with studies from 1996 to 2013,7 we searched for eligible articles published between January 1 2012 and May 30 2018, using MEDLINE (via Ovid), EMBASE, and Database of Abstracts of Reviews of Effects (Supplemental Table 1). We also manually searched bibliographies and reference lists of eligible papers and consulted experts in the field.
Study Selection:
Two investigators (KS, EL or CD) independently reviewed each pertinent title/abstract to determine eligibility. We included studies which: 1) evaluated asymptomatic screening participants with a mean age ≥40 years old; 2) evaluated the diagnostic accuracy of quantitative FIT for CRC (studies of qualitative FIT were excluded); 3) reported data for the calculation of the absolute numbers of true-positive, false-negative, true-negative, and false-positive observations at ≥1 FIT positivity thresholds; 4) included adequate follow-up, defined as colonoscopy for all participants or colonoscopy for patients with positive FIT result combined with ≥1-year follow-up with medical records or cancer registry of FIT-negative individuals as reference standard; and, 5) used a randomized trial or cohort study design. Data for advanced adenomas were extracted if available and a definition provided. Except where noted, advanced adenomas were defined as any adenoma ≥10 mm or containing villous histology or high-grade dysplasia (regardless of size). To avoid duplicate reporting of the same population, we manually reviewed papers and used data from the latest publication or studies with data from multiple positivity thresholds (Supplemental Table 2).
Data Extraction and Synthesis:
Two reviewers (KS, EL or CD) independently evaluated and extracted relevant information and assessed study quality using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) instrument.20 For studies with incomplete or unavailable information, we contacted the authors; additional data were provided that allowed us to include 5 additional studies.8, 21–24 Positivity thresholds were converted to micrograms of hemoglobin per gram of stool.25
Statistical Analysis:
For each study, we calculated the sensitivity and specificity for CRC detection, including 95% confidence intervals. For studies with colonoscopy follow-up of all participants, we also calculated the sensitivity for advanced adenoma detection and the specificity among those without advanced adenoma and CRC.
We first performed analyses of FIT accuracy for CRC and advanced adenomas stratified by positivity thresholds (≤10 μg hemoglobin/g of stool, >10 and ≤20 μg/g, >20 and ≤30 μg/g, and >30 μg/g) using a bivariate random-effects model.26 Studies could contribute sensitivity and specificity pairings at multiple positivity thresholds, if available. For this analysis, which included both CRCs and advanced adenomas, we restricted to studies with colonoscopy follow-up of all participants to minimize differential verification bias, as it can make lower positivity thresholds appear disadvantageous,27 and to provide consistent estimates of both CRC and advanced adenoma detection (which are typically asymptomatic). We then calculated the number of CRCs and advanced adenomas detected and number of positive tests generated at each positivity threshold per 100,000 individuals undergoing screening colonoscopy using the pooled prevalence from all prospective studies with colonoscopy follow-up of all participants (Supplementary Table 3).
For all other analyses, we included studies with both colonoscopy and registry follow-up using the primary positivity threshold from each study (not stratified by positivity threshold) (Table 1).7 We generated overall hierarchical summary receiver-operating characteristic (ROC) curves and calculated the area under the hierarchical summary ROC curve for CRC and advanced adenoma, respectively.28 We calculated FIT sensitivity and specificity for CRC stratified by sex and age including only studies that provided stratified results. Sex and age stratified bivariate random effects analyses could not be performed due to the small number of studies, and univariate random effects analyses were conducted instead. This approach does not account for the correlation between sensitivity and specificity across studies. However, in situations where the bivariate random effects model cannot be fit due to a small number of studies or sparse data, valid summary estimates of sensitivity and specificity can be obtained with univariate random effects models.29
Table 1:
Characteristics of included studies, listed by year. Some articles contributed more than one study. Additional information available in supplementary material.
| Author | Year | Country | FIT brand | Primary positivity threshold (µg/g) | Other thresholds | Reference standard | Total Cohort | Cancers detected | CRC Sensitivity (95%CI) | CRC Specificity (95%CI) | Advanced adenomas detecteda | AA sensitivity (95%CI) | AA specificity (95%CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Itoh62 | 1996 | Japan | OC-Hemodia | 10 | - | 2-year follow-up | 27860 | 89 | 87% (78–93) | 95% (95–95) | - | - | - |
| Nakama38 | 2001 | Japan | OC-Hemodia | 30 | 10, 60 | Colonoscopy | 4260 | 27 | 81% (62–94) | 96% (96–97) | 56b | 54% (40–67) | 97% (96–97) |
| Liu51 | 2003 | Taiwan | OC-Hemodia | Not specified | - | Colonoscopy | 1387 | 6 | 50% (12–88) | 98% (97–99) | 37b | 16% (6–32) | 98% (98–99) |
| Morikawa45 | 2005 | Japan | Magstream | 67 | - | Colonoscopy | 21805 | 79 | 66% (54–76) | 95% (94–95) | 648c | 22% (19–26) | 95% (95–95) |
| Launoy33 | 2005 | France | Magstream | 67 | 168, 251 | 2-year registry | 7421 | 28 | 86% (67–96) | 94% (94–95) | - | - | - |
| Sohn41 | 2005 | Korea | OC-Hemodia | 20 | - | Colonoscopy | 3794 | 12 | 25% (5–57) | 99% (98–99) | 67 | 6% (2–15) | 99% (98–99) |
| Nakazato34 | 2006 | Japan | OC-Hemodia | 16 | - | Colonoscopy | 3090 | 19 | 53% (29–76) | 87% (86–87) | 53b | 25% (14–38) | 87% (86–89) |
| Levi35 | 2007 | Israel | OC-Micro | 15 | - | Colonoscopy | 80 | 3 | 67% (9–99) | 83% (73–91) | 15 | 53% (27–79) | 92% (82–97) |
| Castiglione63 | 2007 | Italy | OC-Hemodia | 20 | - | 2-year registry | 27503 | 83 | 81% (71–89) | 96% (96–96) | - | - | - |
| Graser36 | 2009 | Germany | FOB-Gold | 2.38 | - | Colonoscopy | 285 | 1 | 100% (3–100) | 84% (79–88) | 24 | 29% (13–51) | 86% (81–90) |
| Park39 | 2010 | Korea | OC-Micro | 20 | 15 | Colonoscopy | 770 | 13 | 77% (46–95) | 94% (92–95) | 59 | 24% (14–37) | 94% (92–96) |
| Levi37 | 2011 | Israel | OC-Micro | 14 | - | 2-year registry | 1204 | 6 | 100% (54–100) | 88% (86–90) | - | - | - |
| Chen44 | 2011 | Taiwan | OC-Sensor | 20 | - | 1-year registry | 46355 | 115 | 61% (51–70) | 96% (96–96) | - | - | - |
| De Wijkers-looth64 | 2012 | Netherlands | OC-Sensor | 20 | 10, 15 | Colonoscopy | 1256 | 8 | 75% (35–97) | 95% (93–96) | 113 | 29% (21–39) | 97% (96–98) |
| Wong22 | 2012 | Canada | Magstream | 67 | - | Colonoscopy | 1075 | 2 | 100% (16–100) | 91% (90–93) | 67 | 36% (24–48) | 93% (92–95) |
| Brenner32 | 2013 | German | RIDASCREE | 24.5 | - | Colonoscopy | 2235 | 15 | 60% | 95% | 207 | 21% | 97% |
| y | N Hb | (32–84) | (94–96) | (15–27) | (96–98) | ||||||||
| Brenner32 | OC-Sensor | 6.1 | 73% (45–92) | 96% (95–96) | 207 | 22% (17–29) | 97% (97–98) | ||||||
| Shin53 | 2013 | Korea | n/a | n/a | - | 1-year registry | 354014 | 839 | 52% (48–55) | 97% (97–97) | - | - | - |
| Imperiale40 | 2014 | USA | OC-Sensor | 20 | - | Colonoscopy | 9989 | 65 | 74% (61–84) | 94% (93–94) | 757 | 24% (21–27) | 95% (94–95) |
| Hernandez45 | 2014 | Spain | OC-Sensor | 20 | 10, 15, 25, 30, 40 | Colonoscopy | 779 | 5 | 100% (48–100) | 94% (92–95) | 92 | 28% (19–39) | 96% (94–97) |
| Johnson21 | 2014 | USA | OC-Sensor | 20 | - | Colonoscopy | 193 | 2 | 100% (16–100) | 98% (95–99) | 25 | 4% (0–20) | 98% (95–100) |
| Symonds65 | 2015 | Australia | OC-Sensor | 10 | 10 | Colonoscopy | 1381 | 66 | 79% (67–88) | 80% (78–83) | 189 | 42% (35–50) | 84% (82–86) |
| Stegeman23 | 2015 | Netherlands | OC-Sensor | 10 | - | 2-year registry | 2871 | 20 | 75% (51–91) | 92% (91–93) | - | - | - |
| Lee52 | 2015 | Korea | HemoTecht | 19 | 6.3 | Colonoscopy | 1397 | 14 | 71% (42–92) | 96% (94–97) | 7 | 43% (10–82) | 96% (95–97) |
| Jensen54 | 2016 | USA | OC-Sensor | 20 | - | 1-year registry | 323349 | 645 | 84% (81–87) | 95% (95–95) | - | - | - |
| Chen55 | 2016 | Taiwan | OC-Sensor | 20 | - | 1-year registry | 141045 | 763 | 93% (91–95) | 94% (94–95) | - | - | - |
| Kim66 | 2016 | Korea | OC-Sensor | 20 | 10, 15 | Colonoscopy | 3990 | 79 | 73% (62–83) | 83% (81–84) | 376 | 38% (33–43) | 84% (82–86) |
| Chen43 | 2016 | Germany | FOB-Gold | 17 | 15, 28, 42, 82 | Colonoscopy | 3466 | 29 | 97% (82–100) | 90% (89–91) | 354 | 33% (28–38) | 93% (92–94) |
| Redwood50 | 2016 | USA | OC-Sensor | 20 | 20 | Colonoscopy | 424 | 4 | 75% (19–99) | 93% (90–95) | 56 | 29% (17–42) | 96% (93–98) |
| Kim46 | 2017 | Korea | OC-Sensor | 20 | - | Colonoscopy | 26316 | 16 | 69% (41–89) | 97% (97–97) | 154 | 19% (16–23) | 97% (97–97) |
| Aniwan47 | 2017 | Thailand | OC-Sensor | 20 | 5, 10, 30, 40 | Colonoscopy | 1479 | 14 | 79% (49–95) | 93% (92–95) | 123 | 16% (10–24) | 94% (93–96) |
| Van der Vlugt67 | 2017 | Netherlands | OC-Sensor | 10 | - | 2-year registry | 18716 | 116 | 77% (68–84) | 89% (89–89) | - | - | - |
| Haug68 | 2017 | Netherlands | OC-Sensor | 10 | - | 2-year registry | 4523 | 25 | 88% (69–97) | 92% (91–93) | - | - | - |
| Shapiro48 | 2017 | USA | OC-Sensor | 20 | - | Colonoscopy | 947 | 2 | 0% (0–84) | 97% (96–98) | 53 | 15% (7–28) | 98% (97–99) |
| Gies8 | 2018 | Germany | CAREprime | 6.3 | 7, 12, 15, 26 | Colonoscopy | 516 | 16 | 81% (54–96) | 88% (85–91) | 200 | 31% (25–38) | 91% (88–94) |
| Gies8 | Hb Elisa | 2 | 5, 15, 29 | 81% (54–96) | 82% (78–85) | 200 | 44% (37–51) | 86% (81–89) | |||||
| Gies8 | OC Sensor | 10 | 4, 7, 15, 18 | 69% (41–89) | 96% (93–97) | 200 | 18% (13–24) | 98% (95–99) | |||||
| Gies8 | RIDASCREE N Hb | 8 | 12, 15, 30 | 81% (54–96) | 87% (84–90) | 200 | 36% (29–43) | 91% (87–94) | |||||
| Gies8 | FOB-Gold | 17 | 2, 15, 18, 53 | 69% (41–89) | 95% (92–96) | 200 | 18% (13–24) | 96% (94–98) | |||||
| Gies8 | Eurolyser FOB test | 8.04 | 2, 6, 15, 21 | 63% (35–85) | 95% (93–97) | 200 | 19% (14–26) | 97% (94–99) | |||||
| Gies8 | ImmoCare C | 6.25 | 9, 15, 17, 37 | 81% (54–96) | 87% (84–90) | 200 | 35% (28–42) | 90% 86–93) | |||||
| Gies8 | QuantOn Hem | 3.7 | 10, 15, 18, 30 | 81% (54–96) | 82% (79–85) | 200 | 41% (35–49) | 86% (81–89) | |||||
| Gies8 | QuikRead go iFOBT | 15 | 23 | 63% 35–85) | 95% (92–97) | 200 | 19% (13–25) | 97% (94–98) | |||||
| Chen56 | 2018 | Taiwan | OC-Sensor | 20 | - | 2-year registry | 723113 | 2005 | 75% (73–77) | 96% (96–96) | - | - | - |
| Selby9 | 2018 | USA | OC-Sensor | 20 | 10, 15, 25, 30 | 2-year programmatic | 640859 | 1245 | 74% (72–77) | 93% (92–93) | - | - | - |
| Liles24 | 2018 | USA | OC-Micro | 20 | 10, 15, 25, 30 | Colonoscopy | 2771 | 2 | 100% (16–1) | 96% (95–97) | 209 | 13% (9–19) | 97% (96–97) |
FIT: fecal immunochemical test, CRC: colorectal cancer, AA: advanced adenomas (adenomas ≥ 1 cm,
All authors defined advanced adenomas as: ≥10mm, with villous histology, and/or with any high-grade dysplasia, unless specified
Launoy, Liu and Nakama defined advanced adenomas as ≥10 mm only
Morikawa et al defined advanced adenomas as ≥10 mm or with any high-grade dysplasia only
Sensitivity Analyses and Evaluation of Heterogeneity:
We performed sensitivity analyses for overall sensitivity and specificity for CRC by excluding studies which: used discontinued tests; had >1 FIT sample per patient; had a mean age <50 years; had >70% men; lacked a reported positivity threshold; or that included participants with a family history of CRC (Supplementary Table 4).
The inconsistency index (I2) test was used to estimate heterogeneity between studies using the sensitivity.30 We used Stata, version 14.2 (StataCorp, College Station, Texas) for all statistical analyses. All tests were 2-sided, and P-values less than 0.05 were considered statistically significant. We evaluated for causes of between-study heterogeneity using stratified analyses based on the reference standard (colonoscopy vs. clinical follow-up), geographic region of the study (North America, Europe, or Asia), and FIT brand (for brands with 3 or more included studies). OC-Sensor and OC-Micro were considered together.31 Sensitivity and specificity were compared between subgroups using bivariate, mixed-effects meta-regression.
Results
Study Selection:
The literature search in MEDLINE and EMBASE identified 1775 articles published between 2012 and 2018, of which 131 full-text articles were evaluated and 23 articles met the inclusion criteria (Supplemental Figure 1). These were supplemented by ten articles identified from our previous systematic review7 and 4 from manual searches, providing a total of 37 articles including 46 studies that met the inclusion criteria (Table 1, Supplement).
Characteristics of Included Studies:
Sample sizes ranged from 80 to 723,113 patients (Table 1), with a total of 2,412,518 participants and 6478 detected cancers. Thirty-four studies with 121,545 participants used colonoscopy as the reference standard (gold standard) in all participants, regardless of FIT result, and, among these, 32 reported sensitivity and specificity for advanced adenomas. The remaining 12 studies used longitudinal follow-up of patients with cancer registries and/or medical records during 1 to 2 years, with colonoscopy for those with positive FIT results. Twenty-two studies evaluated more than one positivity threshold. Only two articles8, 32 examined more than one FIT brand on the same study participants. The mean age ranged between 42 and 64 years and the proportion of men from 29% to 86%.
The sensitivities for CRC and advanced adenoma ranged from 0% to 100% and from 4% to 54%, respectively; specificities ranged from 80% to 99% and from 84% to 98% (Table 1). Thirteen quantitative FIT brands from 10 manufacturers were evaluated. OC-Sensor/OC-Micro was tested in 21 studies, OC-Hemodia (now discontinued) in 6, and FOB-Gold and Magstream in 3 studies; the remaining brands in 1 or 2 studies. Six studies analyzed the performance characteristics of 2 to 4 FIT samples, with 1 or more positive samples defined as a positive result.33–38 The positivity threshold values varied widely, ranging from 2 to 251 µg hemoglobin/g of stool; however, 30 included positivity thresholds between 10 and 20 µg/g, inclusive. Funding sources varied: 10 articles reported government funding only, 9 non-industry funding except for provision of the FIT kits by the manufacturer, 5 other forms of partial industry funding, 3 industry funding only,36, 39, 40 and 10 did not report a funding source.
Quality Assessment:
Overall results of the QUADAS-2 assessment from the 37 articles are shown in Supplemental Figure 2 and Supplemental Table 5. All 12 articles with registry follow-up were at high-risk of bias because of lack of blinding of endoscopists to FIT results and differential follow-up depending on FIT results. Six were at high risk because they used frozen stool samples.8, 32, 36, 41–43 Numerous articles had ‘patient selection’ applicability concerns, with 10 articles explicitly including patients with a family history of CRC22, 24, 35, 38, 42, 44–48 and 6 articles patients either younger than 40 years or older than 80 years38, 44, 46, 49–51. Three articles were rated as low risk in all risk of bias and applicability domains.39, 40, 52
Stratification of Studies with Colonoscopy Follow-Up by Positivity Threshold:
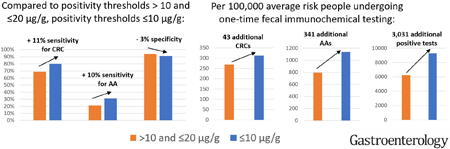
Sensitivity for CRC increased from 69% (95%CI 63–75) for studies with a threshold of >10 and ≤20 µg/g to 80% (95%CI 76–83) for studies with a threshold ≤10 µg/g, and specificity among those without CRC or an advanced adenoma decreased from 94% (95%CI 93–96) to 91% (95%CI 89–93) (Table 2). Statistical heterogeneity was moderate for these estimates, with I2 values between 30% and 52%.30 Sensitivity for advanced adenoma increased from 21% (95%CI 18–25) at >10 and ≤20 µg/g to 31% (95%CI 27–35) at ≤10 µg/g, and specificity decreased from 96% (95%CI 95–97) to 93% (95%CI 91–95). Differences of sensitivity and specificity between studies with thresholds >10 and ≤20 µg/g and higher were smaller.
Table 2:
Pooled sensitivity and specificity of quantitative fecal immunochemical tests for colorectal cancers and advanced adenomas, stratified by positivity threshold, limited to cohorts with colonoscopy follow-up. One study could contribute to more than one pooled analysis if additional positivity thresholds were available
| Positivity threshold (µg/g) | Number of studies | Number of CRC | Sensitivity for CRC (95% CI) | Specificity for CRC (95% CI) | I2 | Number of AA | Sensitivity for AA (95% CI) | Specificity for AA+CRC (95% CI) |
|---|---|---|---|---|---|---|---|---|
| ≤10 | 18 | 447 | 80% (76–83) | 91% (89–93) | 30% | 2,972 | 31% (27–35) | 93% (91–95) |
| >10 and ≤20 | 26 | 432 | 69% (63–75) | 94% (93–96) | 52% | 4,337 | 21% (18–25) | 96% (95–97) |
| >20 and ≤30 | 12 | 188 | 73% (62–81) | 96% (95–97) | 46% | 2,241 | 18% (13–23) | 98% (97–98) |
| >30 | 8 | 188 | 66% (55–75) | 96% (94–97) | 38% | 1,770 | 19% (14–25) | 97% (96–98) |
µg/g: micrograms of stool per gram of buffer; CRC: colorectal cancer; 95% CI: 95% confidence interval; I2: Inconsistency index; AA: advanced adenomas
Among the studies using the OC-Sensor/OC-Micro FIT, the sensitivity for CRC increased from 64% (95%CI 26–90) at >20 µg/g, to 71% (95%CI 64–78) at 10 to 20 µg/g, and 74% (95%CI 65–81) at ≤10 µg/g (Supplemental Figure 10). Specificity decreased from 96% (95%CI 95–97), to 94% (95%CI 92–96) and 90% (95%CI 85–93), respectively. Sensitivity for advanced adenomas increased from 23% (95%CI 18–29) at 10 to 20 µg/g to 33% (95%CI 28–39) at ≤10 µg/g (Supplementary Figure 11).
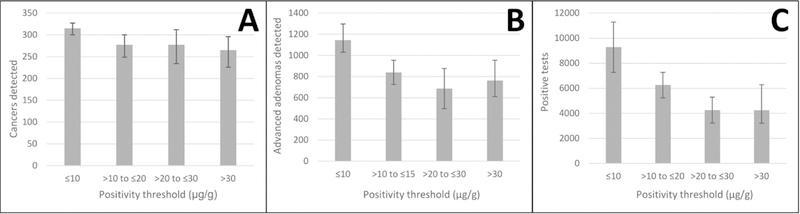
CRC and advanced adenomas detected at varying positivity thresholds in a theoretical screening population:
We calculated the effect of sensitivity and specificity values on a theoretical cohort of 100,000 participants (Figure 2 and Supplemental Table 3). The number of detected CRCs increased by 16%, from 269 (95%CI 245–292) at >10 and ≤20 µg/g to 312 (95% CI 296–323) at <10 µg/g (Figure 2). Advanced adenoma detection increased by 43%, from 794 (95% CI 681–946) at >10 and ≤20 µg/g to 1135 (95% CI 983–1286) at <10 µg/g. The number of positive tests increased by 49% from 6246 (95% CI 4230–7265) at >10 and ≤20 µg/g to 9277 (95% CI 7269–11,281) at <10 µg/g.
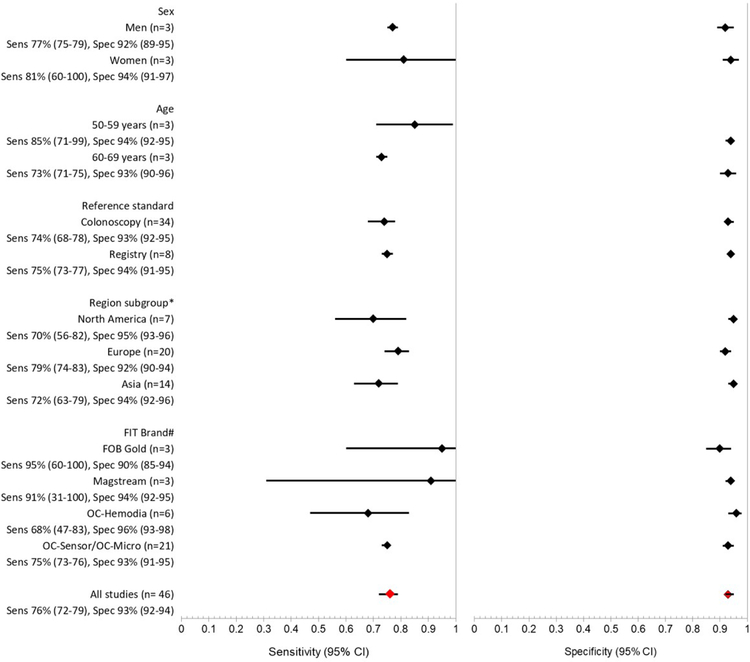
Figure 2: Pooled sensitivity and specificity for colorectal cancer, stratified by study characteristics.
*One study from Australia excluded
#Only includes brands with 3 or more available studies to allow pooled estimates
Overall Accuracy of FIT:
The sensitivity and specificity for CRC using the primary threshold of all included studies (i.e., colonoscopy and registry follow-up) were 76% (95% CI 72–80) and 94% (95% CI 92–95), respectively, with high heterogeneity (I2 = 91% [95%CI 89–93]). The exclusion of 4 registry studies with less than 2-year follow-up for all participants44, 53–55 resulted in similar estimates of sensitivity and specificity (76% [95%CI 72–79]) and 93% [95%CI 92–94] respectively) and decreased (moderate) heterogeneity (I2 = 53% [95%CI 36–69]). Registry studies with less than 2-years follow-up were therefore excluded from subsequent analyses. Summary receiver operator characteristic curves are in Supplemental Figures 6 and 9.
Stratified results by sex and age:
Three studies with 1,459,185 participants provided results stratified by sex.9, 15, 56 Pooled sensitivity by sex was 77% (95%CI 75–79) in men and 81% (95%CI 60–100) in women (Figure 2, P=0.68), with high heterogeneity (overall I2=99%). Specificity was 92% (95%CI 89–95) and 94% (95%CI 91–97), respectively (P=0.28). Four studies with 1,393,499 participants stratified by age;9, 15, 46, 56 pooled sensitivity for 3 studies was 85% for ages 50 to 59 (95%CI 71–99) and 73% for ages 60 to 69 (95%CI 71–75, P=0.10), with high heterogeneity (overall I2=80%).9, 15, 56 Specificity was 94% (95%CI 92–97) and 93% (95%CI 90–96) respectively (P=0.39). No studies reported FIT accuracy by race or ethnicity.
Sensitivity Analyses and Evaluation of Heterogeneity
Sensitivity analyses:
Excluding studies with discontinued FIT, unusually higher numbers of men or older participants, or atypical methods gave similar results (Supplemental Figure 7).
Stratification by reference standard:
Studies using colonoscopy to follow up all participants had a similar sensitivity (74% [95%CI 68–78]) as studies using 2-year registry follow-up (75% [95%CI 73–77]) (Figure 2). Specificity was also similar at 93% (95%CI 92–95) and 94% (95%CI 91–95), respectively.
Stratification by study region:
The pooled sensitivity of studies conducted in Asia (72% [95%CI 63–79]) and North America (sensitivity 70% [95%CI 56–82, P=0.06]) were similar, and lower than those in Europe (80% [95%CI 75–83], P=0.01 and >0.001 respectively). Pooled specificities for North America (95% [95%CI 93–96]) and Asia (94% [95%CI 92–96]) were similar, while for Europe they were lower (92% [95%CI 90–94], P<0.001 for both).
Stratification by FIT brand:
Four FIT brands (OC-Sensor/OC-Micro, OC-Hemodia, FOB Gold and Magstream) had 4 or more studies that could be pooled for subgroup analyses (Figure 2). OC-Sensor/OC-Micro was evaluated in 21 studies and had the most precise estimates for sensitivity and specificity, 75% (95%CI 73–76, I2 47% [95%CI 20–84]) and 93% (95%CI 91–95) respectively. When compared with OC-Sensor/OC-Micro, OC-Hemodia (discontinued) had lower sensitivity at 68% (95%CI 47–83, P=0.02) and a higher specificity (96% [95%CI 93–98, P<0.01]). The other two test (FOB Gold and Magstream) did not have statistically significantly higher sensitivities (P=0.86 and P=0.25, respectively).
Discussion
This meta-analysis found that the use of a positivity threshold ≤10 µg/g rather than between >10 and ≤20 µg/g increased sensitivity for CRCs from 69% to 80% and for advanced adenomas from 21% to 31%, with a corresponding decrease in specificity for CRC from 94% to 91%. Contrary to expectations, given lower mean fecal hemoglobin concentrations among women and younger participants,9, 11 we did not find statistically significant lower FIT sensitivity for CRC among women or younger patients.
Our results, with a favorable tradeoff of additional cancers and advanced adenomas detected to additional positive tests generated, should be interpreted in the context of three recent studies.9, 17, 57 First, our sensitivity of 80% from studies with a threshold ≤10 µg/g is consistent with an estimate from a meta-analysis by Imperiale et al that pooled studies with a threshold of <10 µg/g and equal to 10 µg/g separately.17 They found sensitivities of 78% and 91% respectively, suggesting a higher sensitivity at 10 µg/g than below, a surprising finding not supported by within study comparisons of varying thresholds.17 Overall, we had a larger number of studies because we included studies with registry follow-up. However, the choice of studies was similar for the comparison between positivity thresholds, because here we excluded studies with registry follow-up. Second, our finding that a threshold of ≤10 µg/g detects 16% more CRCs and 43% more advanced adenomas with 49% more positive tests is more favorable than a recent, community-based cohort with registry follow-up and multiple tests over 2 years.9 The registry follow-up study found that a decrease from 20 to 10 µg/g would result in 7% more cancers and 75% more positive tests.9 This is likely because registry follow-up cannot quantify advanced adenoma detection, we included studies with thresholds below 10 µg/g, and the current study primarily includes first-time screening participants undergoing colonoscopy, thus with a higher prevalence of cancers. Third, our findings are more favorable than a large meta-analysis of interval cancer incidence after FIT, which showed no decrease in interval cancers with lower quantitative thresholds.57 That study had large numbers of participants in later screening rounds who had fewer interval cancers, again suggesting that the advantages of lower thresholds may be lower during repeat screening. We applied our results to a theoretical cohort of 100,000 screening participants and the real-world trade-offs of various positivity thresholds are more complex.
The present study suggests that screening programs with adequate colonoscopy resources may wish to consider positivity thresholds at the lower end of the ≤20 µg/g range currently recommended by the U.S. Multi-Society Task Force on Colorectal Cancer Screening.3 OC-Sensor, the most commonly used FIT in the US, has been validated at thresholds as low as 4 µg/g and a small number of certified laboratories in the United States already use quantitative results to guide colonoscopy recommendations at thresholds below 20 µg/g (Helen Landicho, personal communication, November 19 2018).
This systematic review is the first to examine the effect of sex and age on CRC detection. Previous studies suggested important differences in FIT performance by sex and age9, 15, 58, 59 and possible benefits of stratifying FIT-positive patients by sex, age and quantitative result.60 Some did not have follow-up for all participants59, 60 or were performed on non-screening populations.58 Among the limited number of eligible studies with data by sex and age, we did not identify statistically significant differences in sensitivity or specificity for CRC. Studies of advanced adenomas suggest higher sensitivity in men than women (Supplemental Table 6).14–16, 61 We observed a trend towards decreasing sensitivity with age that did not reach statistical significance, though this was not seen in two studies of FIT accuracy for advanced neoplasia15, 61 (Supplemental Table 7). The trend in registry studies could be due to more rapid development of neoplasms in older age groups.
Contrary to a previous systematic review showing higher sensitivity in registry studies,7 studies with 2-year follow-up in this updated review had a similar pooled sensitivity as those with colonoscopy follow-up for all (Figure 2). Other reviews have excluded registry studies because of incomplete ascertainment of CRCs and advanced adenomas among those who do not undergo colonoscopy.10, 17 Nonetheless, they have larger sample sizes to allow subgroup analyses and represent real-world use of FIT that are less susceptible to overdiagnosis (i.e. detection of lesions that would never progress to symptomatic cancer). The finding of similar pooled sensitivity and specificity in studies with 2-year follow-up supports their utility, even if they are reporting on interval cancers rather than missed cancers at the time of a negative result.
Similar to previous reviews7, 10, 17 and a study directly comparing 9 different FITs,8 we did not find significant differences between currently available FIT brands in accuracy for CRC and AA detection (OC Hemodia is no longer sold). By far the largest number of studies examined the performance of the OC-Sensor FIT (Figure 2).
Despite the large number of studies conducted since our previous review, gaps remain for further research. Few studies have reported results stratified by sex and age and none have stratified by race/ethnicity in the same population. In addition, FIT has not been widely used at lower positivity thresholds (e.g., ≤10 µg/g) with annual screening or over multiple rounds of screening. Finally, methods used to define sensitivity and specificity varied widely in the 12 studies with registry follow-up.
Strengths of the current meta-analysis include the addition of several recent large studies, strict adherence to the PRISMA guidelines and comprehensive assessment of study quality. There are several potential limitations. First, there was moderate to high heterogeneity for several summary estimates. However, stratified estimates by quantitative threshold had lower heterogeneity (Table 2) and several subgroup analyses and sensitivity analyses gave similar results (Figures 2 and Supplemental Figure 7). Second, meta-analyses are subject to the detection, verification, and spectrum biases of the original studies. Third, results are dominated by one test (OC-Sensor/Micro) and may not be transferable to other FIT brands. Finally, greater than expected heterogeneity among studies with 1-y registry follow-up led to modification of the study protocol to evaluate more homogeneous strata.
The study provides important information on the diagnostic performance of FIT at varying positivity thresholds. Lower positivity thresholds (e.g. ≤10µg) may be preferable as the threshold value in settings with sufficient follow-up colonoscopy resources. Additional data are needed regarding the influence of sex and age on test performance. Future research should determine the impact of quantitative thresholds of ≤10 µg/g with multiple rounds of annual testing and provide better estimates of FIT performance in important subgroups.
Supplementary Material
Figure 1: Quantitative fecal immunochemical test performance at varying positivity thresholds in a theoretical cohort of 100,000 average risk adults.
Panel A: Number of colorectal cancers detected. Panel B: Number of advanced adenomasa detected. Panel C: Number of positive tests requiring colonoscopy follow-up. Cancer and advanced adenoma prevalence calculated based on pooled prevalence of included cohort studies.b Error bars represent 95% confidence intervals generated from pooled estimates of sensitivity and specificity in Table 3.
µg/g: micrograms of stool per gram of buffer
aAdvanced adenomas defined as adenomas ≥10 mm, containing villous histology, and/or with any high-grade dysplasia.
bCohort studies for follow-up limited to prospective cohorts with colonoscopy follow-up of all participants (Supplementary Table 5)
Table 3:
Studies reporting stratified results of fecal immunochemical test performance for colorectal cancer detection by sex and agea
| Author, Year | Reference standard | FIT brand, positivity threshold | Sensitivity for CRC among men (95% CI) | Sensitivity for CRC among women (95% CI) | P-value | Specificity among men (95% CI) | Specificity among women (95% CI) | P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Brenner,15 2018b | Colonoscopy | FOB Gold, 17 µg/g | 12/14 86% (57–98) |
11/11 100% (72–100) |
0.191 | 1,376/1,545 89% (87–91) |
1,518/1,641 93% (91–94) |
0.001 | ||
| Chen,56 2018 | 2-year registry | OC-Sensor, 20 µg/g | 813/1,065 76% (74–79) |
683/940 73% (70–75) |
0.059 | 262,840/275,977 95% (95–95) |
430,834/445,131 97% (97–97) |
<0.001 | ||
| Selby,9 2018 | 2-year programmatic | OC-Sensor, 20 µg/g | 552/717 77% (74–80) |
373/528 71% (67–75) |
0.011 | 277,174/302,554 92% (92–92) |
314,804/337,060 93% (93–93) |
<0.001 | ||
| Author, Year | Reference standard | FIT brand, positivity threshold | Sensitivity for CRC, age group 1 (95% CI) | Sensitivity for CRC, age group 2 (95% CI) | Sensitivity for CRC, age group 3 (95% CI) | P-value | Specificity, age group 1 (95% CI) | Specificity, age group 2 (95% CI) | Specificity, age group 3 (95% CI) | p-value |
| Kim,46 2017 | Colonoscopy | OC-Sensor, 20 µg/g | 30–39 years: 1/1 100% (3–100) |
40–49 years: 3/4 75% (19–99) |
≥50 years: 7/11 64% (31–89) |
0.719 | 30–39 years: 11,072/11,403 97% (97–98) |
40–49 years: 10,218/10,534 97% (97–97) |
≥50 years: 4,203/4,365 96% (96–97) |
0.075 |
| Brenner,15 2018b | Colonoscopy | FOB Gold, 17 µg/g | 50–59 years: 5/5 100% (48–100) |
60–69 years: 8/10 80% (44–98) |
70–79 years: 10/10 100% (69–100) |
0.196 | 50–59 years: 1,382/1,491 93% (91–94) |
60–69 years: 1,034/1,144 90% (89–92) |
70–79 years: 478/551 87% (84–90) |
<0.001 |
| Chen,56 2018 | 2-year registry | OC-Sensor, 20 µg/g | 50–59 years: 673/879 77% (74–79) |
60–69 years: 823/1,126 73% (70–76) |
0.076 | 50–59 years: 420,743/435,105 97% (97–97) |
60–69 years: 272,931/286,003 95% (95–96) |
<0.001 | ||
| Selby,9 2018 | 2-year programmatic | OC-Sensor, 20 µg/g | 50–59 years: 338/428 79% (75–83) |
60–69 years: 392/534 73% (69–77) |
70–75years: 195/283 69% (63–74) |
0.009 | 50–59 years: 302,444/323,427 94% (93–94) |
60–69 years: 215,168/ 234,131 92% (92–92) |
70–75 years: 74,366/82,056 91% (90–91) |
<0.001 |
FIT: fecal immunochemical test; CRC: colorectal cancer
Only showing results for those aged 50 years or above
Study not included in overall pooled analyses because of overlap with Chen 201643
BACKGROUND AND CONTEXT:
We performed a meta-analysis to determine whether quantitative fecal immunochemical test performance varies with test positivity threshold and among patient subgroups (by sex and age).
NEW FINDINGS:
Sensitivity and specificity for colorectal cancers and advanced adenomas is substantially improved at thresholds ≤10 µg/g. We did not find statistically significant differences in FIT accuracy by sex or age.
LIMITATIONS:
Estimates were based on 1-time FITs and not annual or biennial screening. Few studies compared subgroups, limiting comparisons by sex and age.
IMPACT:
Colorectal cancer screening programs with sufficient colonoscopy resources should consider using lower FIT positive thresholds.
Acknowledgments
Funding/Support: This study was conducted within the National Cancer Institute-funded (grant U54 CA163262) Population-based Research Optimizing Screening Through Personalized Regimens consortium, which conducts multisite, coordinated, transdisciplinary research to evaluate and improve cancer screening processes, and by grants K07 CA212057 from the National Cancer Institute and grant BIL KFS-3720-08-2015 of the Swiss Cancer Research Foundation.
Funding source: National Cancer Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors disclose no conflicts of interest.
Protocol: PROSPERO CRD42017068760
Bibliography
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol 2008;103:1541–9. [DOI] [PubMed] [Google Scholar]
- 3.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;152:1217–1237 e3. [DOI] [PubMed] [Google Scholar]
- 4.Force USPST, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 5.Vart G, Banzi R, Minozzi S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: a systematic review and meta-analysis. Prev Med 2012;55:87–92. [DOI] [PubMed] [Google Scholar]
- 6.Toes-Zoutendijk E, van Leerdam ME, Dekker E, et al. Real-Time Monitoring of Results During First Year of Dutch Colorectal Cancer Screening Program and Optimization by Altering Fecal Immunochemical Test Cut-Off Levels. Gastroenterology 2017;152:767–775. [DOI] [PubMed] [Google Scholar]
- 7.Lee JK, Liles EG, Bent S, et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014;160:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gies A, Cuk K, Schrotz-King P, et al. Direct Comparison of Diagnostic Performance of 9 Quantitative Fecal Immunochemical Tests for Colorectal Cancer Screening. Gastroenterology 2018;154:93–104. [DOI] [PubMed] [Google Scholar]
- 9.Selby K, Jensen CD, Lee JK, et al. Influence of Varying Quantitative Fecal Immunochemical Test Positivity Thresholds on Colorectal Cancer Detection: A Community-Based Cohort Study. Ann Intern Med 2018. [DOI] [PMC free article] [PubMed]
- 10.Gies A, Bhardwaj M, Stock C, et al. Quantitative fecal immunochemical tests for colorectal cancer screening. International Journal of Cancer 2018;143:234–244. [DOI] [PubMed] [Google Scholar]
- 11.McDonald Paula J, Strachan Judith A, Digby J, et al. Faecal haemoglobin concentrations by gender and age: implications for population-based screening for colorectal cancer. Clinical Chemistry and Laboratory Medicine. Volume 50, 2012:935. [DOI] [PubMed] [Google Scholar]
- 12.Ferlitsch M, Reinhart K, Pramhas S, et al. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA 2011;306:1352–1358. [DOI] [PubMed] [Google Scholar]
- 13.Brenner H, Niedermaier T, Chen H. Strong subsite-specific variation in detecting advanced adenomas by fecal immunochemical testing for hemoglobin. Int J Cancer 2017;140:2015–2022. [DOI] [PubMed] [Google Scholar]
- 14.Brenner H, Haug U, Hundt S. Sex differences in performance of fecal occult blood testing. The American journal of gastroenterology 2010;105:2457–64. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H, Qian J, Werner S. Variation of diagnostic performance of fecal immunochemical testing for hemoglobin by sex and age: results from a large screening cohort. Clin Epidemiol 2018;10:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grobbee EJ, Wieten E, Hansen BE, et al. Fecal immunochemical test-based colorectal cancer screening: The gender dilemma. United European Gastroenterology Journal 2017;5:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imperiale TF, Gruber RN, Stump TE, et al. Performance Characteristics of Fecal Immunochemical Tests for Colorectal Cancer and Advanced Adenomatous Polyps: A Systematic Review and Meta-analysisPerformance Characteristics of FITs for CRC and Advanced Adenomas. Annals of Internal Medicine 2019;170:319–329. [DOI] [PubMed] [Google Scholar]
- 18.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies. JAMA 2018;319:388–388. [DOI] [PubMed] [Google Scholar]
- 20.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- 21.Johnson DA, Barclay RL, Mergener K, et al. Plasma Septin9 versus Fecal Immunochemical Testing for Colorectal Cancer Screening: A Prospective Multicenter Study. PLoS One 2014;9:e98238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong CKW, Fedorak RN, Prosser CI, et al. The sensitivity and specificity of guaiac and immunochemical fecal occult blood tests for the detection of advanced colonic adenomas and cancer. International Journal of Colorectal Disease 2012;27:1657–1664. [DOI] [PubMed] [Google Scholar]
- 23.Stegeman I, Doorn SCV, Mundt MW, et al. Participation, yield, and interval carcinomas in three rounds of biennial FIT-based colorectal cancer screening. Cancer Epidemiol 2015;39:388–393. [DOI] [PubMed] [Google Scholar]
- 24.Liles EG, Perrin N, Rosales AG, et al. Performance of a quantitative fecal immunochemical test for detecting advanced colorectal neoplasia: A prospective cohort study. BMC Cancer 2018;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser CG, Allison JE, Halloran SP, et al. A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. J Natl Cancer Inst 2012;104:810–4. [DOI] [PubMed] [Google Scholar]
- 26.Reitsma JB, Glas AS, Rutjes AWS, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58:982–990. [DOI] [PubMed] [Google Scholar]
- 27.Rosman AS, Korsten MA. Effect of verification bias on the sensitivity of fecal occult blood testing: a meta-analysis. J Gen Intern Med 2010;25:1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutter CM, Gatsonis Ca. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Statistics in medicine 2001;20:2865–84. [DOI] [PubMed] [Google Scholar]
- 29.Takwoingi Y, Guo B, Riley RD, et al. Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Stat Methods Med Res 2017;26:1896–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ : British Medical Journal 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force. Rockville (MD), 2016. [PubMed] [Google Scholar]
- 32.Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 2013;49:3049–54. [DOI] [PubMed] [Google Scholar]
- 33.Launoy GD, Bertrand HJ, Berchi C, et al. Evaluation of an immunochemical fecal occult blood test with automated reading in screening for colorectal cancer in a general average-risk population. Int J Cancer 2005;115:493–6. [DOI] [PubMed] [Google Scholar]
- 34.Nakazato MY HO.; Matsushita HO.; Sato K.; Fujita K.; Yamanaka Y. Immunologic fecal occult blood test for colorectal cancer screening. Japan Med Assoc J 2006;49:203–207. [Google Scholar]
- 35.Levi Z, Rozen P, Hazazi R, et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med 2007;146:244–55. [DOI] [PubMed] [Google Scholar]
- 36.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut 2009;58:241–8. [DOI] [PubMed] [Google Scholar]
- 37.Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer 2011;128:2415–24. [DOI] [PubMed] [Google Scholar]
- 38.Nakama H, Zhang B, Zhang X. Evaluation of the optimum cut-off point in immunochemical occult blood testing in screening for colorectal cancer. Eur J Cancer 2001;37:398–401. [DOI] [PubMed] [Google Scholar]
- 39.Park DI, Ryu S, Kim YH, et al. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol 2010;105:2017–25. [DOI] [PubMed] [Google Scholar]
- 40.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;370:1287–97. [DOI] [PubMed] [Google Scholar]
- 41.Sohn DK, Jeong S-Y, Choi HS, et al. Single immunochemical fecal occult blood test for detection of colorectal neoplasia. Cancer research and treatment : official journal of Korean Cancer Association 2005;37:20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol 2012;107:1570–8. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Werner S, Brenner H. Fresh vs Frozen Samples and Ambient Temperature Have Little Effect on Detection of Colorectal Cancer or Adenomas by a Fecal Immunochemical Test in a Colorectal Cancer Screening Cohort in Germany. Clin Gastroenterol Hepatol 2016;15:1547–1556. [DOI] [PubMed] [Google Scholar]
- 44.Chen LS, Yen AMF, Chiu SYH, et al. Baseline faecal occult blood concentration as a predictor of incident colorectal neoplasia: Longitudinal follow-up of a Taiwanese population-based colorectal cancer screening cohort. The Lancet Oncology 2011;12:551–558. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez V, Cubiella J, Gonzalez-Mao MC, et al. Fecal immunochemical test accuracy in average-risk colorectal cancer screening. World J Gastroenterol 2014;20:1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim NH, Park JH, Park DI, et al. The fecal immunochemical test has high accuracy for detecting advanced colorectal neoplasia before age 50. Digestive and Liver Disease 2017;49:557–561. [DOI] [PubMed] [Google Scholar]
- 47.Aniwan S, Ratanachu Ek T, Pongprasobchai S, et al. The Optimal Cut-Off Level of The Fecal Immunochemical Test For Colorectal Cancer Screening in a Country with Limited Colonoscopy Resources: A Multi-Center Study from Thailand. Asian Pac J Cancer Prev 2017;18:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro JA, Bobo JK, Church TR, et al. A Comparison of Fecal Immunochemical and High-Sensitivity Guaiac Tests for Colorectal Cancer Screening. American Journal of Gastroenterology 2017;112:1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morikawa T, Kato J, Yamaji Y, et al. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 2005;129:422–428. [DOI] [PubMed] [Google Scholar]
- 50.Redwood DG, Asay ED, Blake ID, et al. Stool DNA Testing for Screening Detection of Colorectal Neoplasia in Alaska Native People. Mayo Clinic proceedings 2015. [DOI] [PubMed]
- 51.Liu HH, Huang TW, Chen HL, et al. Clinicopathologic significance of immunohistochemical fecal occult blood test in subjects receiving bidirectional endoscopy. Hepatogastroenterology 2003;50:1390–2. [PubMed] [Google Scholar]
- 52.Lee YH, Hur M, Kim H, et al. Optimal cut-off concentration for a faecal immunochemical test for haemoglobin by Hemo Techt NS-Plus C15 system for the colorectal cancer screening. Clin Chem Lab Med 2015;53:e69–e71. [DOI] [PubMed] [Google Scholar]
- 53.Shin A, Choi KS, Jun JK, et al. Validity of fecal occult blood test in the national cancer screening program, Korea. PLoS One 2013;8:e79292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jensen CD, Corley DA, Quinn VP, et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med 2016;164:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen CH, Wen CP, Tsai MK. Fecal immunochemical test for colorectal cancer from a prospective cohort with 513,283 individuals: Providing detailed number needed to scope (NNS) before colonoscopy. Medicine (Baltimore) 2016;95:e4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen SL-S, Hsu C-Y, Yen AM-F, et al. Demand for Colonoscopy in Colorectal Cancer Screening Using a Quantitative Fecal Immunochemical Test and Age-Sex-Specific Thresholds for Test Positivity. Cancer Epidemiology Biomarkers & Prevention 2018;27:704–710. [DOI] [PubMed] [Google Scholar]
- 57.Wieten E, Schreuders EH, Grobbee EJ, et al. Incidence of faecal occult blood test interval cancers in population-based colorectal cancer screening: a systematic review and meta-analysis. Gut 2018. [DOI] [PubMed]
- 58.van Turenhout ST, Oort FA, van der Hulst RW, et al. Prospective cross-sectional study on faecal immunochemical tests: sex specific cut-off values to obtain equal sensitivity for colorectal cancer? BMC Gastroenterol 2014;14:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arana-Arri E, Rubio I, Uranga B, et al. Population-based colorectal cancer screening programmes using a faecal immunochemical test: Should faecal haemoglobin cut-offs differ by age and sex?, 2017. [DOI] [PMC free article] [PubMed]
- 60.Auge JM, Pellise M, Escudero JM, et al. Risk Stratification for Advanced Colorectal Neoplasia According to Fecal Hemoglobin Concentration in a Colorectal Cancer Screening Program. Gastroenterology 2014;147:628–636.e1. [DOI] [PubMed] [Google Scholar]
- 61.Khalid-de Bakker CAJ, Jonkers DMAE, Sanduleanu S, et al. Test performance of immunologic fecal occult blood testing and sigmoidoscopy compared with primary colonoscopy screening for colorectal advanced adenomas. Cancer Prevention Research 2011;4:1563–1571. [DOI] [PubMed] [Google Scholar]
- 62.Itoh M, Takahashi K, Nishida H, et al. Estimation of the optimal cut off point in a new immunological faecal occult blood test in a corporate colorectal cancer screening programme. J Med Screen 1996;3:66–71. [DOI] [PubMed] [Google Scholar]
- 63.Castiglione G, Visioli CB, Ciatto S, et al. Sensitivity of latex agglutination faecal occult blood test in the Florence District population-based colorectal cancer screening programme. Br J Cancer 2007;96:1750–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Immunochemical Fecal Occult Blood Testing Is Equally Sensitive for Proximal and Distal Advanced Neoplasia. Am J Gastroenterol 2012;107:1570–1578. [DOI] [PubMed] [Google Scholar]
- 65.Symonds EP S.; Baker R.; Gopalsamy G.; Worthley D.; Murray D.; Bampton PA.; Fraser RJL.; Cole SR.; Lapoint L.; Young P. Increased Detection of Colorectal Cancer with a Combination Faecal and Blood Screening Test. United European Gastroenterol J 2015;3:A120. [Google Scholar]
- 66.Kim NH, Yang HJ, Park SK, et al. Does Low Threshold Value Use Improve Proximal Neoplasia Detection by Fecal Immunochemical Test? Dig Dis Sci 2016;61:2685–93. [DOI] [PubMed] [Google Scholar]
- 67.van der Vlugt M, Grobbee EJ, Bossuyt PMM, et al. Interval Colorectal Cancer Incidence Among Subjects Undergoing Multiple Rounds of Fecal Immunochemical Testing. Gastroenterology 2017;153:439–447 e2. [DOI] [PubMed] [Google Scholar]
- 68.Haug U, Grobbee EJ, Lansdorp-vogelaar I, et al. Immunochemical faecal occult blood testing to screen for colorectal cancer: can the screening interval be extended? Gut 2016;66:1262–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.