Abstract
Background and Purpose:
Albuminuria is associated with stroke risk among individuals with diabetes. However, the association of albuminuria with incident stroke among non-diabetic patients is less clear.
Methods:
We performed a post-hoc analysis of the SPRINT trial, which examined the effect of higher versus lower intensity blood pressure management on mortality in 8,913 participants without diabetes. We fit unadjusted and adjusted Cox proportional-hazards models to estimate the association of baseline albuminuria (urinary albumin-to-creatinine ratio ≥30mg/g versus<30mg/g) with stroke risk. We also assessed effect modification according to treatment arms.
Results:
Mean age was 68±9 years, 35% were female, and 30% were black. Median follow-up was 3.2 years and 19% patients had baseline albuminuria. Incident stroke occurred in 129 individuals during follow-up. Albuminuria was associated with increased stroke risk (unadjusted hazard ratio [HR] 2.24; 95% CI 1.55-3.23; adjusted HR 1.73; 95% CI 1.17-2.56). The association of albuminuria with incident stroke differed according to the randomized treatment arm (P-interaction=0.03). In the intensive treatment arm, the association of albuminuria and stroke was non-significant (unadjusted HR 1.25; 95% CI 0.69-2.28), whereas, in the standard treatment arm, it was significant (unadjusted HR 3.44; 95% CI 2.11-5.61).
Conclusions:
In a post-hoc analysis of SPRINT, baseline albuminuria (versus not) was associated with a higher risk of incident stroke, but this relationship appeared to be restricted to those in the standard treatment arm. Further studies are required to conclusively determine if reduction of albuminuria in itself is beneficial in reducing stroke risk
Clinical Trial Registration Information:
The original SPRINT trial () is registered at https://clinicaltrials.gov/ct2/show/NCT01206062.
Keywords: Albuminuria, Stroke, Hypertension
Subject Terms: stroke, hypertension, risk factors
Introduction
Vascular disease, including stroke, has been associated with impaired endothelial function. Albuminuria is an independent marker of systemic endothelial dysfunction and is associated with adverse cardiovascular events.1 A meta-analysis of 48,596 patients found an association of microalbuminuria with higher risk of incident stroke across various populations after cardiovascular risk adjustment.1 However, there are limited data regarding this association in patients without diabetes and history of prior stroke.
In the SPRINT trial, intensive blood pressure (BP) treatment of participants at high risk for cardiovascular events, but without diabetes or prior stroke, resulted in lower rates of the primary composite cardiovascular outcome.2 Although there were fewer strokes in the intensive treatment group, the difference was not statistically significant.
Our aim is to analyze the association between stroke and albuminuria in a population without diabetes or prior stroke.
Materials and Methods
Study design and population
We performed a post-hoc analysis of SPRINT (), which randomized 9,361 hypertensive patients with increased cardiovascular event risk to a systolic BP target <140mmHg (standard) or <120mmHg (intensive). Patients with diabetes, prior stroke, urinary protein excretion ≥1g/day, or urinary albumin excretion ≥600g/day were excluded. Detailed methods of SPRINT have been published.2 The institutional review board at Hospital Garcia de Orta deemed this secondary analysis to be exempt from further review. The data used in this study are subject to a data use agreement and cannot be shared directly by the authors.
Exposure and outcomes
We defined baseline albuminuria as a urinary albumin-to-creatinine ratio (ACR) ≥30 mg/g at randomization. We excluded patients with missing ACR (n=448; supplementary Table I). The primary outcome was incident stroke (all types), which was adjudicated by a blinded endpoints committee. As secondary outcomes, we evaluated myocardial infarction (MI), other acute coronary syndromes (ACS), heart failure (HF), death from CVD and a composite CVD outcome (MI, other ACS, stroke, HF, or death from CVD).
Statistical Analysis
We evaluated time to incident outcomes using ACR as continuous (log-transformed) or categorical variables (ACR≥30mg/g vs <30mg/g). We used Cox proportional-hazards regression models, with site stratification. We performed restricted cubic spline analyses with three-knots to flexibly display the ACR and incident stroke relationship. We adjusted multivariable models for age, sex, race, smoking status, systolic BP, antihypertensive agents, prior CVD, body mass index, estimated glomerular filtration rate (eGFR), triglycerides, total cholesterol, HDL cholesterol, plasma glucose, statin use, aspirin use, and randomized treatment arm. These variables were selected a priori based on biological plausibility. We used likelihood ratio tests to assess for effect modification of the association of albuminuria with stroke, according to randomized treatment arm and race (black vs non-black). We considered p-values<0.05 statistically significant. We performed analyses with Stata® 14.2.
Results
Baseline characteristics
We analyzed 8,913 participants with a 3.2-year median follow-up, mean age of 68±9 years; 35% were female and 30% were black. At baseline, 1,730(19.4%) had albuminuria. Participants with albuminuria were more likely to be black, smokers, use more antihypertensive agents and statins, have prior CVD and CKD, higher systolic BP, total cholesterol and triglycerides (Table I).
Table I –
Baseline Characteristics of study participants by albuminuria status
| No albuminuria (n=7183) |
Albuminuria (n=1730) |
P-value | |
|---|---|---|---|
| Age (years) | 67.4 (9.2) | 70.3 (10.1) | <0.001 |
| Female (n,%) | 2568 (35.8) | 579 (33.5) | 0.07 |
| Race/ethnic group (n,%) | |||
| Non-Hispanic white | 4121 (57.4) | 995 (57.5) | 0.04 |
| Non-Hispanic black | 2138 (29.8) | 552 (31.9) | |
| Hispanic | 789 (11.0) | 154 (8.9) | |
| Other | 135 (1.9) | 29 (1.7) | |
| Baseline blood pressure (mmHg) | |||
| Systolic | 139±15 | 144±17 | <0.001 |
| Diastolic | 78±12 | 78±13 | 0.16 |
| Antihypertensive agents (n) | 2 (1-2) | 2 (1-3) | <0.001 |
| Cardiovascular disease (n,%) | 1381 (19.2) | 423 (24.5) | <0.001 |
| Body mass index (kg/m2) | 29.9±5.7 | 29.7±6.1 | 0.27 |
| Fasting plasma glucose (mg/dL) | 99±13 | 99±15 | 0.25 |
| Fasting total cholesterol (mg/dL) | 191±41 | 185±42 | <0.001 |
| Fasting high-density lipoprotein cholesterol (mg/dL) | 53±14 | 52±16 | 0.23 |
| Fasting total triglycerides (mg/dL) | 106 (77-149) | 109 (78-157) | 0.02 |
| Statin use (n,%) | 3056 (42.8) | 837 (48.7) | <0.001 |
| Aspirin use (n,%) | 3632 (50.7) | 908 (52.5) | 0.17 |
| Current smoker (n,%) | 3011 (41.9) | 786 (45.4) | 0.01 |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 74±19 | 63±24 | <0.001 |
| Chronic kidney disease (n,%) | 1736 (24.2) | 818 (47.3) | <0.001 |
| Urinary albumin-to-creatinine ratio (mg/g) | 7.7 (5.1-12.6) | 69.7 (42.1-162.1) | <0.001 |
Albuminuria and stroke association
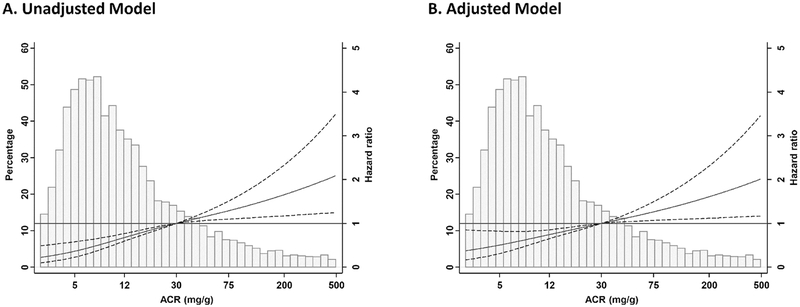
During follow-up, stroke occurred in 129 participants. Higher logACR was associated with higher stroke risk [hazard ratio (HR) 1.46(1.30–1.64); adjusted HR (aHR) 1.35(1.18–1.54)]. The unadjusted (Figure 1A) and adjusted (Figure 1B) restricted cubic spline analysis was consistent with these results and supports that the association of logACR with stroke risk is linear.
Figure 1.
Incident stroke risk according to the logarithm of baseline urinary albumin-to-creatinine ratio (ACR) as continuous variable. ACR=30 mg/g was used as reference value. Unadjusted(A) and adjusted(B) model.
Stroke occurred in 46(2.7%) participants with albuminuria and in 83(1.2%) without albuminuria. Albuminuria was associated with an increased stroke risk [HR 2.24(1.55–3.23); aHR 1.73(1.17–2.56)]. There was no evidence for effect modification according to race (p=0.13). Similar patterns of associations were noted for secondary outcomes (supplementary table II)
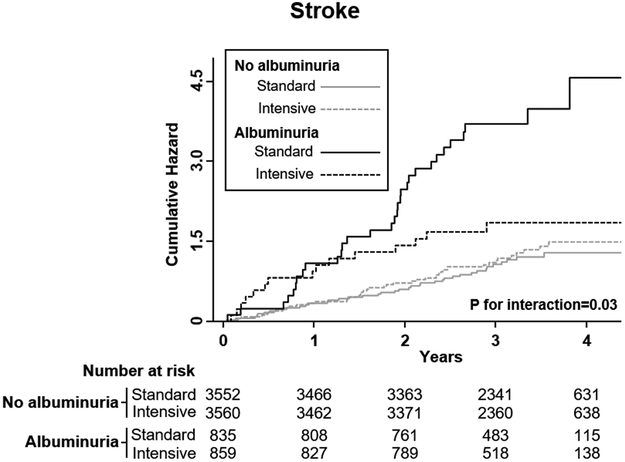
Albuminuria and stroke according to treatment arm
There was evidence for effect modification between baseline albuminuria and BP treatment group regarding stroke risk (Fig. 2; P-interaction=0.03). In the intensive treatment arm, the association of albuminuria and stroke was non-significant [HR 1.25(0.69–2.28); aHR 0.93(0.48–1.78)], whereas in the standard treatment arm this association was significant [HR 3.44(2.11–5.61); aHR 2.71(1.61–4.55)]. Similar patterns of interaction were noted for HF (p-interaction=0.04) and MI (p-interaction=0.06) (supplementary table III), but not for the other outcomes (p-interaction >0.1).
Figure 2 –
Stroke incidence by treatment arm stratified by baseline albuminuria.
Discussion
This post-hoc analysis of SPRINT shows an association between baseline albuminuria and increased incident stroke risk in non-diabetic hypertensive patients. Furthermore, we report evidence for effect modification according to treatment arms, such that the association of albuminuria with incident stroke appeared to be restricted to those in the standard BP arm.
Our findings replicate previous reports of an association of albuminuria with stroke and other CVD. In a pooled-analysis of community-based cohorts, who had prior CVD (except stroke) and diabetes, albuminuria was associated with increased stroke risk.3 Nevertheless, there are few studies addressing the association of albuminuria with stroke in patients without prior stroke or diabetes.
Higher stroke risk in patients with albuminuria may reflect damage to small arterioles/capillaries. Both kidney and brain have low-resistance arterial beds, where blood flow is kept constant by autoregulatory mechanisms.4 Their failure may result in microvascular damage: renal glomerular sclerosis and cerebral lacunar infarcts/white matter lesions.4 In fact, UACR positively correlated with white matter lesion burden in SPRINT-MIND.2,5 Furthermore, blood pressure variability has also been associated with increased stroke risk6.
We found evidence for effect modification according to treatment arm, such that the association of albuminuria with stroke only remained significant for those in the standard treatment arm. This pattern of association was also found for MI and HF. Interestingly, in the ACCORD trial, which only included diabetic patients, with higher albuminuria burden, intensive BP treatment was associated with decreased stroke risk.7 In the PREVEND-IT trial, fosinopril-treatment significantly reduced albuminuria and stroke risk, while the primary outcome of CVD mortality/hospitalization was reduced only in the albuminuria subgroup (>50mg/24h).8 Thus, in patients with albuminuria, BP-lowering interventions may decrease stroke risk. In fact, a recent systematic review concluded that the magnitude of BP reduction is linearly related to risk reduction of CV events.9 However, it remains unclear if albuminuria is a modifiable risk factor, or simply a surrogate marker of favorable response to BP treatment. Indeed, it is unknown whether albuminuria reduction, without BP lowering, provides any stroke risk benefit. Nevertheless, our data argue that more intensive BP targets in individuals with albuminuria may be warranted, pending studies specifically designed to address this question.
Our study has limitations. We were unable to stratify by stroke subtype/etiology. Furthermore, patients were categorized based on one UACR measurement, and, despite risk factor adjustment, we cannot exclude residual confounding. The modest number of stroke events is a further limitation. This analysis must be regarded as exploratory as it was not pre-specified.
In a post-hoc analysis of SPRINT, baseline albuminuria was associated with higher stroke risk, with evidence of effect modification according to the randomized treatment arm. Albuminuria should be further researched as a risk factor for first stroke among non-diabetic individuals.
Supplementary Material
Acknowledgements
SPRINT participants; NEJM-SPRINT Data Analysis Challenge.
Funding sources
Dr Mc Causland is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK102511.
Footnotes
Disclosures
None.
References
- 1.Lee M, Saver JL, Chang K-H, Liao H-W, Chang S-C, Ovbiagele B. Impact of microalbuminuria on incident stroke: a meta-analysis. Stroke. 2010;41:2625–2631. [DOI] [PubMed] [Google Scholar]
- 2.SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmoodi BK, Yatsuya H, Matsushita K, Sang Y, Gottesman RF, Astor BC, et al. Association of kidney disease measures with ischemic versus hemorrhagic strokes: pooled analyses of 4 prospective community-based cohorts. Stroke. 2014;45:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro P, Azevedo E, Rocha I, Sorond F, Serrador JM. Chronic kidney disease and poor outcomes in ischemic stroke: is impaired cerebral autoregulation the missing link? BMC Neurol. 2018;18:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner DE, Gaussoin SA, Nord J, Auchus AP, Chelune GJ, Chonchol M, et al. Cognitive Function and Kidney Disease: Baseline Data From the Systolic Blood Pressure Intervention Trial (SPRINT). Am. J. Kidney Dis 2017;70:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsivgoulis G, Pikilidou M, Katsanos AH, Stamatelopoulos K, Michas F, Lykka A, et al. Association of Ambulatory Blood Pressure Monitoring parameters with the Framingham Stroke Risk Profile. J Neurol Sci. 2017;15;380:106–111. [DOI] [PubMed] [Google Scholar]
- 7.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouwers FP, Asselbergs FW, Hillege HL, de Boer RA, Gansevoort RT, van Veldhuisen DJ, et al. Long-term effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria:Ten years of follow-up of Prevention of Renal and Vascular End-stage Disease Intervention Trial (PREVEND IT). Am. Heart J 2011;161:1171–1178. [DOI] [PubMed] [Google Scholar]
- 9.Katsanos AH, Filippatou A, Manios E, Deftereos S, Parissis J, Frogoudaki A, et al. Blood pressure reduction and secondary stroke prevention: A systematic review and metaregression analysis of randomized clinical trials. Hypertension. 2017;69:171–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.