Abstract
Previous molecular phylogenetic studies have shown that families in Zoopagales are not monophyletic. To test the monophyly of genera and species in the order, we used a single-cell approach to generate nuclear 18S rRNA (18S) sequences for 10 isolates representing nine taxa. We provide the first sequences for the genus Zoopage and additional sequences for taxa in Cocholonema, Acaulopage, and Zoophagus. Our results reveal that Zoophagus, Zoopage, and Acaulopage tetraceros are not monophyletic. We conclude that morphology alone is not sufficient to delineate genera and species in the order and encourage studies that increase genetic sampling of taxa including type species.
Keywords: Acaulopage, Cochlonema, single-cell amplification, Stylopage, Zoopage, Zoopagomycota, Zoophagus
INTRODUCTION
Predation has arisen multiple times in Fungi. A fungus is considered a predator if it physically or chemically immobilizes multiple prey individuals from a population for use as a nutrient and/or energy source. It is estimated that there are over 300 predacious species belonging to Ascomycota, Basidiomycota, Chytridiomycota, and the former Zygomycota (Barron 2004). Predacious fungi trap a range of motile organisms, such as nematodes, rotifers, and amoebae. The predacious zygomycetes, along with many parasitic taxa, are placed in Zoopagales (Benny et al. 2016b) and, unlike predacious taxa in Dikarya, cannot be cultured in the absence of a prey population. The Zoopagales were recently placed in the newly described Zoopagomycota, a lineage formerly belonging to Zygomycota that primarily associates with other microbes and animals (Spatafora et al. 2016). The Zoopagales contains five families delineated using ecology and morphology and an uncertain number of genera (Benny et al. 2016b). Previous molecular phylogenetic studies have shown that the families Helicocephalidaceace, Piptocephalidaceae, Sigmoideomycetaceae, and Zoopagaceae are not monophyletic (e.g., Tanabe et al. 2000; Michel et al. 2015). Genera and species of Zoopagales are similarly delineated based on ecology and morphology. In Syncephalis, morphology has been shown to be phylogenetically inconsistent (Benny et al. 2016a; Lazarus et al. 2017). However, because zoopagalean taxa are largely uncultured and molecular data scarce, the monophyly of most genera and species has not been tested using molecular phylogenetics.
Genera in Zoopagales are delineated based on conidium and conidiophore morphology, and species are delineated based on ecology, morphology of conidia/conidiophores, and/or method of penetrating the host/prey (see Kirk et al. 2010, Benny et al. 2016b, and zygomycetes.org). However, the inability of morphological and ecological characters to delineate monophyletic families and the inconsistency of morphology in Syncephalis (Lazarus et al. 2017) brings the monophyly of the genera and species into question. Further, it can be inferred from Corsaro et al. (2018) that the position of conidia is insufficient to distinguish genera such as Acaulopage and Stylopage.
ur objective was to increase the number of nuc 18S rRNA (18S) sequences of Zoopagales available for phylogenetic analysis by obtaining sequences from uncultured predaceous taxa. To do so, we screened samples from across the United States for additional taxa of Zoopagales. In order to obtain sequence data from predatory fungi, we used a whole-genome amplification approach from single or few cells through multiple displacement amplification (MDA; Gawad et al. 2016). The error rate of MDA is considerably lower than that of Taq polymerase (Binga et al. 2008), but it can result in chimeras in the same manner that polymerase chain reaction (PCR) does. The additional step of MDA before PCR may introduce some errors into the resulting DNA sequence; however, many studies have successfully used MDA templates for analysis of single cells by PCR or genome sequencing (Gonzalez et al. 2005; Hosokawa et al. 2017; Ahrendt et al. 2018). Using this method, we demonstrate its feasibility for analysis of unculturable predatory fungi by generating 18S sequences for 10 isolates representing nine taxa. Phylogenetic analysis of these new sequence data demonstrates that none of the predacious genera of Zoopagales examined are monophyletic.
MATERIALS AND METHODS
Collection of isolates.—
Soil, water, vegetation, dung, and basidiocarp samples were collected in Alabama, Alaska, Arizona, California, Georgia, Michigan, and Ohio (SUPPLEMENTARY TABLE 1). Methods of finding predacious fungi include plating a portion of the sample on a weak nutrient agar plate, which allows for the prey population to expand and the fungi to sporulate (Barron 1977). Thus, we plated soil samples on water agar amended with sea salt (Michel et al. 2014) and quarter-strength cornmeal agar (Becton, Dickinson, and Co., Sparks, Maryland). At least four replicates were made of each soil sample: water agar amended with sea salt, quarter-strength cornmeal, water agar amended with sea salt plus a suspension of Rhabditis sp. or C. elegans (Barron 1977), and quarter-strength cornmeal agar plus a suspension of Rhabditis sp. or C. elegans (Barron 1977). The Rhabditis culture was obtained from Carolina Biological (Burlington, North Carolina) and maintained on potato plugs according to their care manual. The C. elegans culture was wild-type N2 and was maintained on nematode growth medium (NGM) (Stiernagle et al. 2006). We also plated pieces of basidiocarps on water agar amended with sea salt and quarter-strength cornmeal agar. Plates were sealed with parafilm and incubated at room temperature (RT) for up to 3 mo. Water samples were incubated with sesame seeds in a sterile Petri dish for 1 mo. Dung samples were incubated in sterile Petri dishes with moist filter paper at RT for 1 mo. We screened the samples for fungi periodically using a Zeiss Discovery V8 stereomicroscope (Carl Zeiss, Jena, Germany), at times inverted on a Zeiss Imager A2 compound microscope. Fungi were imaged directly on the plate or as a wet mount using a Zeiss AxioCam MRc camera. Isolates were identified using morphology based on the original species descriptions (Dreschler 1935a, 1935b, 1935c, 1936; Prowse 1954; Duddington 1955; Saikawa and Morikawa 1985).
We made microscope slides as vouchers of some isolates (TABLE 1). Other samples could not be vouchered, as the entire sample was utilized for DNA sequencing. Material was placed in a drop of lactolphenol (Remel, Lenexa, Kansas) with a coverslip then baked at 60 C for 1–3 d. The edges of the coverslips were coated with neutral balsam (Fisher Scientific, Fair Lawn, New Jersey) and baked again at 60 C for 1–3 d. Microscope slides were deposited in the University of Michigan Herbarium (MICH).
Table 1.
Metadata associated with the species and isolates used in the phylogenetic analysis.
| Species-isolate | Microhabitat | Geographic location | Latitude, longitude | Date of sample collection | 18S GenBank accession | MICH accession | iNaturalist observation |
|---|---|---|---|---|---|---|---|
| Acaulopage acanthospora Ac | Soil beneath Sphaeralcea sp. | Nina Mason Pulliam Rio Salado Audubon Center 3131 S Central Ave, Phoenix, Arizona | 33.419109, −112.07213 | Feb 2017 | MG920176 | 231388 | https://www.inaturalist.org/observations/5305874 |
| Acaulopage sp. Asp1 | Soil beneath a walnut tree | Glenmount Ave, Akron, Ohio | 41.022588, −81.515119 | Aug 2016 | MG953913 | N/A | N/A |
| Acaulopage tetraceros AT | Soybean field | Fosters Loop Rd, Fosters, Alabama | 33.087807, −87.632033 | Sep 2015 | MG920179 | 231390 | https://www.inaturalist.org/observations/2573149 |
| Cochlonema odontosperma E | Auricularia auricular collected from a stump | Manchester Road Akron, Ohio | 40.941076, −81.568438 | Mar 2017 | MG920177 | 231389 | https://www.inaturalist.org/observations/6037406 |
| Piptocephalis sp. | Moose dung | Fairbanks, Alaska | Oct 2016 | MG920184 | N/A | https://www.inaturalist.org/observations/4512283 | |
| Stylopage hadra SOG | Soil and leaf litter in mixed hardwood forest | South side of Cassandra Bog, Edwin Smith George Reserve, Michigan | 42.4600, −84.0231 | Oct 2015 | MG920178 | N/A | N/A |
| Zoopage sp. C | Soil beneath leaf litter in poplar stand | Chenoweth Drive, Akron, Ohio | 41.001887, −81.497566 | Aug 2016 | MG920182 | N/A | N/A |
| Zoopage sp. Zo2 | Soil beneath Sphaeralcea sp. | Nina Mason Pulliam Rio Salado Audubon Center 3131 S Central Ave, Phoenix, Arizona | 33.419109, −112.07213 | Feb 2017 | MG920181 | N/A | https://www.inaturalist.org/observations/5306362 |
| Zoophagus insidians Zi | Bog water | Bryant’s Bog, Pellston, Michigan | 45.566851, −84.711864 | Aug 2016 | MG920180 | N/A | https://www.inaturalist.org/observations/3900383 |
| Zoophagus pectospora AZ | Soil beneath brush | Hilltop Lane, Augusta, Michigan | 42.368664, −85.29438 | Sep 2017 | MG920183 | N/A | https://www.inaturalist.org/observations/8579416 |
Note. When possible, a preserved slide was made and was deposited in the University of Michigan Herbarium (MICH). When possible, observation of the isolates was reported to iNaturalist.org. Species-isolate column represents species name followed by isolate codes, e.g., Ac, Asp1.
Multiple displacement amplification and PCR.—
Because most fungi in the Zoopagales have never been successfully cultured, we used methods taken from single-cell genomic approaches involving multiple displacement amplification (MDA; Gawad et al. 2016) to obtain sufficient amounts of material for genetic analysis. We used half reactions of the Qiagen REPLI-g Single Cell Kit (Qiagen, Gaithersburg, Maryland) to extract and amplify genomic DNA. We plucked 10–30 spores using a dental file (Henry Schein, Melville, New York) and placed them in 2 μL of ultraviolet (UV) light–sterilized phosphate-buffered saline. To lyse the spores, we added 1.5 µL of lysis buffer, incubated them on the thermocycler for 10 min at 65 C, and added 1.5 μL of the stop buffer. After adding the MDA master mix, the lysed spores were incubated at 30 C for 6 h. One to three reactions were performed per isolate.
MDA products were diluted 1:100. The 18S was amplified using the primer pairs SR1R/NS4 (Vilgalys and Hester 1990; White et al. 1990) and SSU0817F/SSU1536–3R (Borneman and Hartin 2000). Amplicons were sequenced via Sanger sequencing at the University of Michigan Sequencing Core (https://seqcore.brcf.med.umich.edu/) and assembled into a contiguous sequence in Sequencher 5.3. Sequences were deposited at GenBank (MG920176–MG920184; MG953913).
Phylogenetic analysis.—
Sequences belonging to the Zoopagales, Kickxellales, and Entomophthorales were downloaded from GenBank, including the environmental sequences identified in Corsaro et al. (2018) and additional, phylogenetically closely related environmental sequences identified by BLASTn of the National Center for Biotechnology Information (NCBI) database. Sequences were aligned in MAFFT 7 (Katoh et al. 2002, 2017). The alignment was filtered using Gblocks 0.91b (Castresana 2000) with minimum number of sequences for conserved and flanking positions set to 31, the maximum number of contiguous nonconserved positions set to 8, the minimum block length set to 5, and gaps allowed. A maximum likelihood (ML) tree was inferred with RAxML 8.2.8 (Stamatakis 2006) under the GTR+gamma model with rapid bootstrapping (Stamatakis et al. 2008) and rooted with the Kickxellales and Entomophthorales. The tree and alignment were deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S22606). A Bayesian inference (BI) tree was inferred using MrBayes 3.2.6 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003) with a GTR+gamma model. Appropriate values for rate, nucleotide frequencies, and gamma distribution parameters in the BI model were determined with maximum likelihood in RAxML. The BI analysis was run using four chains for 5 million generations sampled every 500 generations. The consensus tree and posterior probabilities were calculated after discarding the first 2500 generations. Scripts and raw output files are available on GitHub (https://github.com/wjdavis90/ZyGoLife/tree/master/Zoopagales_18s_paper).
RESULTS
We screened 31 soil samples, 7 water samples, 5 dung samples, and 3 basidiocarps (SUPPLEMENTARY TABLE 1). We observed members of Zoopagales in 20 soil samples (65%), 4 water samples (57%), 1 dung sample (20%), and 1 fungal basidiocarp (Auricularia auricula) (33%) (SUPPLEMENTARY TABLE 1). The most commonly observed morphospecies were Acaulopage tetraceros (5 soil samples) and Stylopage hadra (5 soil samples).
We generated 18S sequences for Acaulopage acanthospora, Acaulopage tetraceros, Cochlonema odontosperma, Piptocephalis sp., Stylopage hadra, Zoopage spp., Zoophagus insidians, and Zoophagus pectospora (TABLE 1). Our sequence of Acaulopage tetraceros (isolate AT) was 93% similar to the sequence under the same name in GenBank (JQ288098). Our sequence for Zoophagus insidians (isolate Zi) was 99% similar to the sequence under the same name in GenBank (AB016009); our sequence for Stylopage hadra (SOG) was 99% similar to the sequence in GenBank (EF546661).
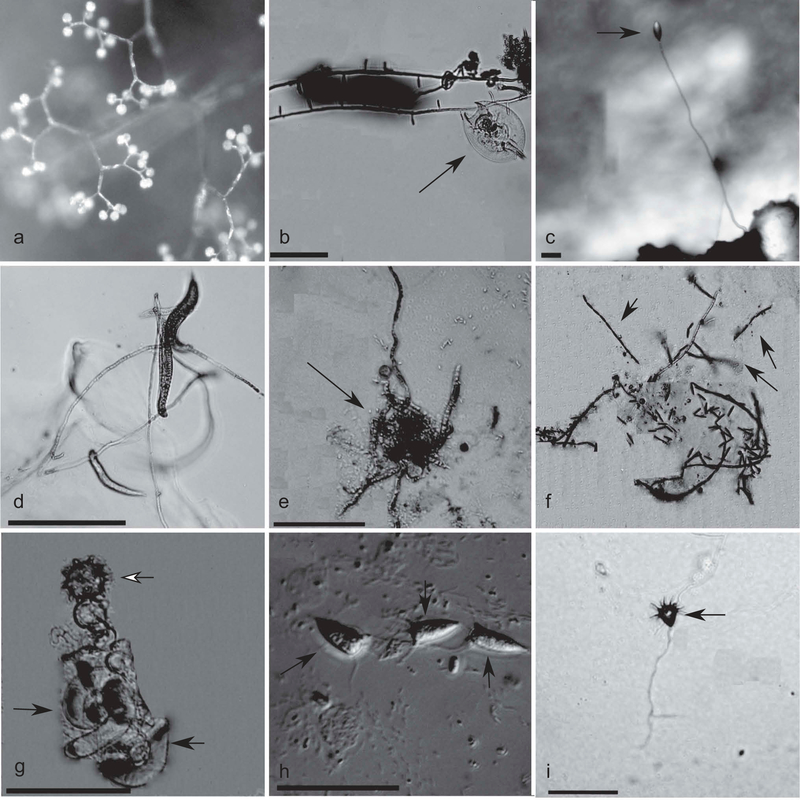
Our 18S alignment contained 61 sequences with 1327 sites retained following Gblocks masking. After phylogenetic analyses, we recovered a strain morphologically identified as Piptocephalis sp. (FIGS. 1, 2A) from moose dung collected in Alaska that grouped with Piptocephalis lepidula with 93% bootstrap support. Sister to the Piptocephalis clade (FIG. 1), which includes one sequence labeled Kuzuhaea moniliformis, but with 46% bootstrap support was a clade containing our two isolates of Zoophagus spp., the only zoopagalean genus that forms peg-like traps, and Stylopage hadra, recovered from Michigan soils and waters. The Zoophagus insidians isolate was identified based on its peg-like traps and presence of trapped rotifers (FIG. 2B) and placed with sequence from a culture (Tanabe et al. 2000) with 100% bootstrap support. The Zoophagus insidians clade was sister to a clade containing our isolates of Stylopage hadra and Zoophagus pectospora with 94% bootstrap support. The Stylopage hadra isolate was characterized with conidia borne on erect conidiophores (FIG. 2C) and a swollen haustorium at the point of penetration into the nematode. It was sister to a clone sequence from GenBank putatively identified as Stylopage hadra with 100% bootstrap support. Zoophagus pectospora was identified based on the presence of peg-like traps with captured Bunonema nematodes (FIG. 2D) and was sister to S. hadra with 100% bootstrap support.
Figure 1.
ML tree of Zoopagales inferred from 18S data. Nodes with >90% bootstrap support and/or Bayesian posterior probability values >0.95 are in bold. Tip labels of new sequences are in bold. For ease of reading, support values are not displayed for internal branches of the Acaulopage clade. Clades without a Bayesian posterior probability did not appear in the Bayesian consensus tree. Major clades within Zoopagales have been labeled, and a silhouette of their prey/host placed beside their label. All silhouettes were downloaded from phylopic.org. The silhouette of the rotifer was created by Ferando Carezzano. The silhouettes of the nematode and amoeba are copyrighted by Gareth Monger under a creative commons attributation 3.0 unported license (http://creativecommons.org/licenses/by/3.0/).
Figure 2.
A. Sporangiophore of Piptocephalis sp. Photograph taken at 80× with a stereomicroscope. B. Rotifer (black arrow) trapped on hyphae of Zoophagus insidians. Photograph taken at 100×. C. Conidiophore and conidium (black arrow) of Stylopage hadra. Photograph taken at 80× with a stereomicroscope. D. Hyphae of Zoophagus pectospora with trapped Bunoema nematodes. Photograph taken at 100×. E. Amoeba (black arrow) invaded and partially digested by the Ohio isolate of Zoopage sp. Photograph taken at 400×. F. Catenulated conidia chains (black arrows) of the Arizona Zoopage sp. isolate. Photograph taken at 100× through the agar plate. G. Thallus (black arrows) and zygospore (white arrow) of Cochlonema odontosperma. Photograph taken at 200×. H. Conidia (black arrows) of Acaulopage tetraceros. Photograph taken at 400× with DIC. I. Conidium (black arrow) of Acaulopage acanthospora. Photograph taken at 100× through the agar plate. Bars = 50 μm.
The Ohio isolate of Zoopage sp. was identified based on external hyphae invading an amoeba (FIG. 2E) with conidia produced in erect aerial chains; it formed a clade with two environmental sequences with 98% bootstrap support. The Ohio Zoopage sp. + environmental sequences clade was sister to a clade containing the Arizona Zoopage sp. isolate and two isolates of Cochlonema with 99% bootstrap support. The Arizona strain of Zoopage sp. featured an external mycelium with erect chains of aerial conidia (FIG. 2F) and was in a clade with Cochlonema euryblastum with 100% bootstrap support. The isolate of Cochlonema odontosperma was identified based on the coiled thallus, the zygospore covered with tooth-like projections (FIG. 2G), and warty conidia produced in erect aerial chains; it formed a clade with the Arizona Zoopage sp. isolate and the Cochlonema euryblastum isolate with 58% bootstrap support.
The Zoopage-Cochlonema clade formed a sister group to a lineage containing all of the Acaulopage isolates and an isolate of Stylopage araea with 63% bootstrap support (FIG. 1). The Acaulopage clade had 100% bootstrap support, but the internal branches were short and most of the nodes had <50% bootstrap support. From the Alabama soils, we recovered an isolate that was morphologically identified as Acaulopage tetraceros (FIG. 2H); the observed conidia of A. tetraceros were 20–25 μm long and 5 μm wide, with an average of four appendages on the apex. It is sister to a clade containing environmental sequences, our Acaulopage acanthospora isolate, and a nonsporulating Acaulopage sp. isolate with 50% bootstrap support. The A. acanthospora isolate had turbinate conidia approximately 10 μm wide by 10 μm long, with many conspicuous apical appendages on the apex (FIG. 2I). The Acaulopage isolate was identified as a member of the Zoopagales based on the presence of trapped amoeba and identified as a member of Acaulopage based on the molecular phylogeny. The Acaulopage clade also included an isolate of A. tetraceros from Germany, which was sister to the rest of the clade with 100% bootstrap support and to a German isolate of Stylopage araea.
DISCUSSION
We successfully implemented procedures to perform phylogenetic analyses on uncultured predacious taxa in Zoopagales. Using observation of substrates placed on water agar or weak corn meal agar, we were able to observe and photograph taxa that could generally be attributed to genera and often species of Zoopagales and obtain DNA sequences of the 18S rRNA gene using single-cell approaches. Our results demonstrate that morphology alone is not sufficient to delineate genera and species within the Zoopagales. None of the predatory genera held up as monophyletic. Isolates morphologically identified as Acaulopage tetraceros fell into distinct clades, and Acaulopage, Cochlonema, Stylopage, Zoopage, and Zoophagus were shown to be nonmonophyletic. The placement of Zoophagus pectospora sister to Stylopage hadra and not Zoophagus insidians further indicates lack of consistency in morphological characters, as does the placement of Stylopage aerea within Acaulopage (Corsaro et al. 2018). These findings are consistent with those of Benny et al. (2016a) and Lazarus et al. (2017), who found morphological characters inconsistent with molecular phylogenetics in Syncephalis.
Although all of the sequences belonging to Acaulopage group together, sequences belonging to the morphospecies Acaulopage tetraceros are in separate clades, which suggests convergence onto a similar distinct conidium morphology with four or more apical appendages. An alternative explanation to the disjunct phylogenetic distribution of the Acaulopage tetraceros isolates is that the MDA and subsequent PCR introduced errors into our sequence, causing false divergence. We do not think this is the case, as our sequences of Zoophagus insidians and Stylopage hadra, which were also derived from MDA and PCR, are 99% similar to sequences derived from PCR alone. Another example of polyphyly is the placement of the two Zoopage sp. isolates. The isolate from Ohio clusters with environmental sequences recovered from a sludge digester (Matsubayashi et al. 2017) and from a sulfidic karst spring, whereas the Arizona isolate clusters with an environmental sequence from the compost of mushrooms in a clade sister to Cochlonema euryblastum. Drescher (1935) noted that the catenulated conidia of Zoopage and Cochlonema are very similar in terms of development and morphology; thus, it is not surprising that the two genera are in the same clade. However, our results indicate that Zoopage is not monophyletic and that the predacious strategy and associated morphology are either ancestral or convergent. It will take additional isolates of Cochlonema and Zoopage to revise the taxonomy of these genera and map transitions between predation and endoparasitism and associated transitions in morphology.
Morphology in Zoophagus was also found to be phylogenetically inconsistent. Whereas our isolate of Zoopagus insidians groups with a sequence derived from a culture (Tanabe et al. 2000), our isolate of Zoophagus pectospora is sister to a clade containing Stylopage hadra. Zoopagus insidians was originally placed in the Oomycota (Prowse 1954) until an accumulation of biochemical and ultrastructural data necessitated its transfer to the Zoopagales (Dick 1990). Zoophagus pectospora was originally described as a species of Acaulopage (Dreschler 1962) but was transferred to Zoophagus (Dick 1990) based on ultrastructural data (Saikawa and Morikawa 1985) and the ability to trap both nematodes and rotifers (Saikawa et al. 1988). Our results support the close relationship of Zoophagus pectospora and Z. insidians but indicate that the genus is not monophyletic.
Our results show that all sampled predatory genera in Zoopagales are not monophyletic and at least one species is not monophyletic. However, only 9 of the 22 genera and 18 of the 193 species are represented in our phylogeny. Further sampling of additional taxa is clearly needed. Additionally, we show that single-cell amplification methods are a viable way of obtaining molecular data for fungi that cannot be grown in axenic culture. These methods should be broadly applicable to unculturable host-associated fungi where hosts are microscopic, e.g., Cryptomycota, Entomophthorales, Chytridiomycota, as well as biotrophic taxa of larger hosts, e.g., Pucciniales and Peronosporaceae. Moreover, the application of single-cell methods could allow for the sampling of additional molecular markers linked to the same taxon, which may result in a better-resolved phylogeny.
Supplementary Material
ACKNOWLEDGMENTS
We thank Robert Harold Davis, Lovina Davis, Jim Davis, Vickie Davis, and Elizabeth Rhodes for the samples yielding the Ohio Acaulopage sp., Zoopage sp., and Cochlonema odontosperma isolates; Jason Martin for the sample yielding the Alabama isolate of Acaulopage tetroceros; and Brandon Hassett for the moose dung. We thank Robby Robinson and the ZyGoLife project for the opportunity to sample in Tempe, Arizona.
FUNDING
This project was funded by National Science Foundation (NSF) grant DEB 1441677.
Footnotes
Supplemental data for this article can be accessed on the publisher’s Web site.
LITERATURE CITED
- Ahrendt SR, Quandt CA, Ciobanu D, Clum A, Salamov A, Andreopoulos B, Cheng J-F, Woyke T, Pelin A, Henrissat B, Benny GL, Smith ME, James TY, Grigoriev IV. 2018. Leveraging single-cell genomics to expand the Fungal Tree of Life. Nature Microbiology, doi: 10.1038/s41564-018-0261-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron GL. 1977. The nematode-destroying fungi. Topics in Mycobiology No. 1. Guelph, Ontario, Canada: Canadian Biological Publications; 140 p. [Google Scholar]
- Barron GL. 1980. The biological role of Rhopalomyces magnus. Mycologia 72:427–430. [Google Scholar]
- Barron GL. 2004. Fungal parasites and predators of rotifers, nematodes, and other invertebrates In: Mueller GM, Bills GF, Foster MS, eds. Biodiversity of Fungi: inventory and monitoring methods. Amsterdam, The Netherlands: Elsevier Academic Press; p. 435–450. [Google Scholar]
- Benny GL, Smith ME, Kirk PM, Tretter ED, White MM. 2016b. Challenges and future perspectives in the systematics of Kickxellomycotina, Mortierellamycotina, Mucoromycotina, and Zoopagomycotina In: Li D-W, ed. Biology of microfungi. Cham, Switzerland: Springer International Publishing; p. 65–126. [Google Scholar]
- Benny GL, Ho H-M, Lazarus KL, Smith ME. 2016a. Five new species of the obligate mycoparasite Syncephalis (Zoopagales, Zoopagomycotina) from soil. Mycologia 108:1114–1129. [DOI] [PubMed] [Google Scholar]
- Binga EK, Lasken RS, Neufeld JD. 2008. Something from (almost) nothing: the impact of multiple displacement amplification on microbial ecology. The ISME Journal 2:233–241. [DOI] [PubMed] [Google Scholar]
- Borneman J, Hartin RJ. 2000. PCR primers that amplify fungal rRNA genes from environmental samples. Applied and Environmental Microbiology 66:4356–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17:540–552. [DOI] [PubMed] [Google Scholar]
- Corsaro D, Köhsler M, Wylezich C, Venditti D, Walochnik J, Michel R. 2018. New insights from molecular phylogenetics of amoebophagous fungi (Zoopagomycota, Zoopagales). Parasitology Research 117:157–167. [DOI] [PubMed] [Google Scholar]
- Dick MW. 1990. The systematic position of Zoophagus insidians. Mycological Research 94:347–354. [Google Scholar]
- Dreschler C 1935a. Some conidial phycomycetes destructive to terricolous amoebae. Mycologia 27:6–40. [Google Scholar]
- Dreschler C 1935b. Some non-catenulate conidial phycomycetes preying on terricolous amoebae. Mycologia 27:176–205. [Google Scholar]
- Dreschler C 1936. A new species of Stylopage preying on nematodes. Mycologia 28:241–246. [Google Scholar]
- Dreschler C 1962. A nematode-capturing phycomycete with distally adhesive branches and proximally imbedded fusiform conidia. Canadian Journal of Botany 49:1089–1095. [Google Scholar]
- Dreshcler C 1935c. A new species of conidial phycomyete preying on nematodes. Mycologia 27:206–215. [Google Scholar]
- Duddington CL. 1955. A new species of Stylopage capturing nematodes. Mycologia 47:245–248. [Google Scholar]
- Gawad C, Koh W, Quake SR. 2016. Single-cell genome sequencing: current state of the science. Nature Reviews Genetics 17:175–188. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Portillo MC, Saiz-Jimenez C. 2005. Multiple displacement amplification as a pre-polymerase chain reaction (pre-PCR) to process difficult to amplify samples and low copy number sequences from natural environments. Environmental Microbiology 7:1024–1028. [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Nishikawa Y, Kogawa M, Takeyama H. 2017. Massively parallel whole genome amplification for single-cell sequencing using droplet microfluidics. Scientific Reports 7:5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755. [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30:3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. 2017. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics bbx108. [DOI] [PMC free article] [PubMed]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. 2010. Dictionary of the Fungi. 10th ed. Wallingford, UK: CABI; 771 p. [Google Scholar]
- Lazarus KL, Benny GL, Ho H-M, Smith ME. 2017. Phylogenetic systematics of Syncephalis (Zoopagales, Zoopagomycotina), a genus of ubiquitous mycoparasites. Mycologia 109:333–349. [DOI] [PubMed] [Google Scholar]
- Matsubayashi M, Shimada Y, Li Y-Y, Harada H, Kubota K. 2017. Phylogenetic diversity and in situ detection of eukaryotes in anaerobic sludge digesters. PLoS ONE 12:e0172888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel R, Steid P, Köhsler M, Walochnik J. 2015. Acaulopage tetraceros Dreschler 1935 (Zoopagales): cultivation, prey pattern, and molecular characterization. Journal of Endocytobiosis and Cell Research 26:76–82. [Google Scholar]
- Michel R, Walochnik J, Steid P. 2014. Article for the “Free-living amoeba special issue”: isolation and characterisation of various amoebophagous fungi and evaluation of their prey spectrum. Experimental Parasitology 145:5131–5136. [DOI] [PubMed] [Google Scholar]
- Prowse GA. 1954. Sommerstorffia spinose and Zoophagus insidians predaceous on rotifers, and Rozellaopsis inflata, the endoparasite of Zoophagus. Transactions of the British Mycological Society 37:134–150. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19:1572–1574. [DOI] [PubMed] [Google Scholar]
- Saikawa M, Morikawa C. 1985. Electron microscopy on a nematode-trapping fungus, Acaulopage pectospora. Canadian Journal of Botany 63:1386–1390. [Google Scholar]
- Saikawa M, Yamaguchi K, Morikawa C. 1988. Capture of rotifers by Acaulopage pectospora, and further evidence of its similarity to Zoophagus insidians. Mycologia 80:880–884. [Google Scholar]
- Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A, James TY, O’Donnell K, Taylor TN, Uehling J, Vilgalys R, White MM, Stajich JE. 2016. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108:1028–1046.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analysis with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemount J. 2008. A rapid algorithm for the RAxML web servers. SystBiol 57:758–771. [DOI] [PubMed] [Google Scholar]
- Stiernagle T 2006. Maintenance of C. elegans. In: Wormbook ed. The C. elegans Research Community, doi: 10.1895/wormbook.1.101.1; http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Tanabe Y, O’Donnell K, Saikawa M, Sugiyama J. 2000. Molecular phylogeny of parasitic Zygomycota (Dimargaritales, Zoopagales) based on nuclear small sub-unit ribosomal DNA sequences. Molecular Phylogenetics and Evolution 16:253–262. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds. PCR protocols: a guide to the methods and applications. New York: Academic Press; p. 315–322. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.