Abstract
Objective:
Neuroimaging studies show structural alterations of various brain regions in children and adults with ADHD, although non-replications are frequent. Our aim is to identify cortical characteristics related to ADHD using large-scale studies.
Methods:
Cortical thickness and surface area (based on the Desikan–Killiany atlas) were compared between cases (n=2246) and controls (n=1934) for children, adolescents, and adults separately in ENIGMA-ADHD, a consortium of 36 centers. To assess familial effects on cortical measures, cases, unaffected siblings, and controls in the NeuroIMAGE study (n=506) were compared. Associations of the attention scale from the Child Behavior Checklist with cortical measures were determined in a pediatric population sample (Generation-R, n=2707).
Results:
In ENIGMA-ADHD, lower surface area values were found in children with ADHD, mainly in frontal, cingulate, and temporal regions; the largest effect was for total surface area (Cohen’s d=−0.21; pFDR=<0.001). Fusiform gyrus and temporal pole cortical thickness was also lower in children with ADHD. Neither surface area nor thickness differences were found in the adolescents/adult groups. Familial effects were seen for surface area in several regions. In an overlapping set of regions, surface area, but not thickness, was associated with attention problems in Generation-R.
Conclusion:
Subtle differences in cortical surface area are widespread in children, but not in adolescents and adults with ADHD, confirming involvement of frontal cortex and highlighting regions deserving further attention. Importantly, the alterations behave like endophenotypes in families and are linked to ADHD symptoms in the population, extending evidence that ADHD behaves as a continuous trait in the population. Future longitudinal studies should clarify individual lifespan trajectories that lead to non-significant findings in adolescent/adult groups despite presence of an ADHD diagnosis.
Keywords: ADHD, cortical thickness, cortical surface area, lifespan, meta-analysis, imaging
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a common neuropsychiatric disorder characterized by age-inappropriate levels of inattention and/or hyperactivity and impulsivity. ADHD occurs in around 5-7% of children and 2.5% of adults (1, 2). ADHD can negatively affect multiple aspects of daily life of patients, and represents a major public health challenge (3). Neuroimaging studies in ADHD show differences between the brains of people with ADHD and those of healthy individuals in structure (4-9), function (8, 10, 11), and connectivity (12-14), albeit with small effect sizes (9). While informative, existing studies have several major limitations. First, most ADHD neuroimaging studies have been cross-sectional and performed during childhood; studies that either consider ADHD throughout the lifespan or have a longitudinal design are rare. In one such lifespan study, we recently showed that differences in intracranial volume (ICV) and subcortical volumes between patients and healthy individuals were largely restricted to childhood (9). Furthermore, an earlier longitudinal study showed slower, delayed development of cortical thickness and surface area in children with ADHD, especially in frontal-temporal regions (15). Nonetheless, large-scale studies of cerebral cortical architecture throughout the lifespan are lacking.
A second major limitation in the neuroimaging literature is that most studies on ADHD have small sample sizes and show limited reproducibility (16). Combining data from existing research by means of meta-/mega-analysis can produce more reliable results. For ADHD, meta-/mega-analyses of structural brain phenotypes are available for subcortical structures (9, 17), but the cortex has only been assessed in meta-analyses of brain-wide voxel-based morphometry (VBM) studies (5-8). The largest VBM study (931 patients and 822 controls) reported case-control differences for anterior cingulate, medial prefrontal cortex, ventromedial orbitofrontal cortex, and the insula (8). Here, we further the field by providing the first large-scale, mega-analytic examination of cortical measures across the lifespan in ADHD. We analyzed cortical surface area and thickness separately, as recent large-scale studies show that the biological mechanisms underlying such measures overlap only partially (18). Our large sample size also provides the power needed to examine clinical factors such as common comorbid disorders.
Neuroimaging analyses of ADHD have also largely not addressed a major question: are the observed brain differences a consequence of living with the disorder, or do the brain differences reflect underlying risk for the disorder? Different study designs can help us begin to address this question. Family-based studies can indicate if cortical changes are present in unaffected siblings of cases to indicate the involvement of shared genetic and/or environmental risk factors that underlie the cortical characteristics associated with the disorder. Several family studies (e.g. (19)) suggest that at least some of the brain alterations seen in patients are also present in their unaffected siblings and are associated with symptom severity in healthy individuals. Population-based studies can determine whether individuals with traits of ADHD show similar cortical changes to those associated with the full syndrome. The largest population study published to date (n=776 children) showed that higher levels of ADHD symptoms were associated with a thinner cortex in caudal middle frontal, temporal, and occipital regions (20). While this and similar studies (21) showed that brain alterations extend beyond the clinical disorder, no attempts have yet been made to directly assess the overlap between studies in clinical samples and the general population. Combined, family and population-based findings suggest that the brain differences seen in those with ADHD are not simply markers of the disorder, but larger studies, directly comparing brain phenotypes across different informative study designs, are needed to shed more light on this.
Here, we present a mega-analysis of cortical thickness and surface area in participants with ADHD and healthy controls across the lifespan from the ENIGMA-ADHD Working Group, a world-wide collaboration aiming to characterize the characteristics of the brain of people with ADHD. All partners used standardized methods (segmentation protocols and quality control procedures), limiting methodological heterogeneity more than in previous meta-analyses. In addition to assessing case-control differences in children, adolescents, and adults, we investigated cortical brain correlates of clinical features, assessed familiality of effects, and mapped the dimensionality of affected cortical regions in the large, independent pediatric Generation-R population study (22).
MATERIALS AND METHODS
Contributing studies
The ENIGMA-ADHD Working Group currently consists of 36 cohorts from around the world (http://enigma.ini.usc.edu/ongoing/enigma-adhd-working-group/). All cohorts have structural imaging data available for individuals with an ADHD diagnosis, and most sites also include data from healthy controls. An overview of the sites is given in ST1; details of image acquisition and study protocols are provided in ST2 and SA1. The dataset for the cortical analysis comprised 4,180 individuals: 2,246 people with ADHD with mean age of 19.22 years (SD= 11.31), age range of 4-62 years, 74.1% males; 1,934 healthy controls with mean age of 18.05 years (SD=11.26), age range of 4-63 years, 59.8% males.
For the analysis of dimensionally-assessed ADHD traits in the general population we used data from 2,707 individuals with mean age of 10.11 (SD=0.57) years, age range of 8.5-11.9 years, 49.4% males (ST3) from the Generation-R cohort (22).
For all participating cohorts, approval for the analysis was available from the responsible ethics committees.
Neuroimaging
Structural T1-weighted brain MRI data were acquired and processed at the individual sites. The images were analyzed using standardized protocols to harmonize analysis and quality control processes (http://enigma.ini.usc.edu/protocols/imaging-protocols/ and SA2) (23-25). Fully-automated and validated neuroimaging segmentation algorithms based on FreeSurfer versions 5.1 or 5.3 were used (ST2). Regions based on the Desikan–Killiany atlas were segmented, which resulted in cortical thickness and surface area values for 34 left and 34 right hemisphere regions. Two whole-hemisphere values for average thickness and average surface area were also computed. For further analysis, we used the mean of the bilateral values ((R+L)/2).
The Generation-R data were collected using a single, study-dedicated MRI scanner and processed using FreeSurfer version 6.0 on a high-performance computing system (Cartesius, surfsara.nl), for scanner sequence please see SA3. All imaging data were visually inspected for inaccuracies in the surface-based reconstruction. Data not suitable for analysis were excluded (for a flowchart see SF1), providing n=2707. For a non response analysis, please see SA4.
Case-control differences in cortical thickness and surface area in children, adolescents, and adults
Based on the age-specificity of earlier findings (9), three age groups were assessed: children: 4-14 years, 1081 cases, 1048 controls; adolescents: 15-21 years, 432 cases, 347 controls; adults: 22-63 years, 733 cases, 539 controls. As there are marked developmental changes across the 4 to 14 year age range, we also performed supplemental analyses on age tertiles of the childhood group. For each of the age groups we determined differences between participants with ADHD and healthy controls using mixed-effect models with ‘site’ as a random factor in the nlme package in R. Age and sex were included as additional covariates; for the surface area analysis, intracranial volume (ICV) was also added, as surface area scales with head size (24-26). We also included analyses without ICV as a covariate given the debate over whether it should be included or not (see SA5). To calculate Cohen’s d effect size estimates, adjusting for the appropriate covariates, we used the t-statistic from the Diagnosis (ADHD=1, control=0) predictor in the equation(27). To correct for multiple comparisons, we used a false discovery rate (FDR) at q=0.05.
Split-half validation of case-control findings
To ensure stability of effects, we performed a validation of our mega-analysis in age groups with significant results. Data were split into two halves, statistically matched for age, sex, and ICV within each site. Validation was defined as pFDR<0.05 in the first half and puncorrected<0.05 in the second half, with matching effect directions(28).
Exploration of the influence of sex, IQ and clinical factors on cortical regions affected in ADHD
For regions and age groups showing validated case-control differences, we examined potential effects of sex, IQ, comorbid disorders, medication use and ADHD symptoms (severity) (see details in SA6). Given the exploratory nature of these analyses, we report uncorrected p-values in the Results section.
Family study
Two subsets of the ENIGMA-ADHD sample (NeuroIMAGE Amsterdam and Nijmegen (29)) had collected brain data from patients (n=211), their unaffected siblings (n=175), and unrelated controls (n=120). To determine familial effects on ADHD-affected cortical regions, unaffected siblings were compared with healthy controls in those cortical regions. Levels of ADHD symptoms in the unaffected siblings had been shown to not differ from those of controls (19). Multiple comparisons correction was performed based on the effective number of independent tests (Meff) (30); differences between unaffected siblings and controls were considered significant at p<0.01 (Meff=5, for details please see SA7).
Association between ADHD symptoms and the cortex in the general population
ADHD symptoms were assessed in children from Generation-R using the Child Behavior Checklist (CBCL)(31). Both attention problems (Syndrome Scale) and ADHD problems (DSM-oriented scale) were examined for associations with surface/thickness in regions with validated case-control differences in ENIGMA-ADHD. R statistical software (version 3.3.3) was used to fit multiple linear regressions to model these associations. Primary analyses were adjusted for age at MRI scan, sex, ICV and ethnicity. In supplemental analyses, models were additionally adjusted for non-verbal IQ, ADHD medication status, MR-scanner software version, and motion during scanning (SA8).
RESULTS
Case-control differences in cortical surface area and thickness in children, adolescents, and adults
In children with ADHD versus control children, lower values of cortical surface area were widespread, with 24 out of 34 regions and total surface area being smaller in patients (Table 1, Figure 1, ST4). The largest effect was found for total surface area: d= −0.21, pFDR=<0.001. When the child group was further subdivided in post-hoc analyses, this effect size increased to d=−0.35, pFDR=<0.001 in the youngest tertile (4-9 years), which comprised 317 cases and 340 controls (ST5). More generally, the youngest group showed the largest case-control differences (ST5). No case-control differences were found in the adolescent and adult groups (ST6 and ST7; ST8 shows combined analysis of age groups). For results of the model without ICV, please see ST9.
Table 1.
Mega-analysis of case-control cortical surface area differences in children of 14 years of age and younger in ENIGMA-ADHD.
| Cortical region | Controls (N) | ADHD (N) | Cohen's d (standard error) |
95% confidence interval |
p-value | FDR p-value |
|---|---|---|---|---|---|---|
| total surface areaa | 1048 | 1081 | −0.21 (0.04) | −0.29 to −0.12 | <0.001 | <0.001 |
| superior frontal gyrusa | 1044 | 1074 | −0.19 (0.04) | −0.28 to −0.11 | <0.001 | <0.001 |
| lateral orbitofrontal cortexa | 1047 | 1081 | −0.17 (0.04) | −0.26 to −0.09 | <0.001 | <0.001 |
| medial orbitofrontal cortex | 1039 | 1070 | −0.16 (0.04) | −0.24 to −0.07 | <0.001 | 0.002 |
| posterior cingulate cortexa | 1042 | 1078 | −0.16 (0.04) | −0.25 to −0.08 | <0.001 | 0.002 |
| rostral anterior cingulate cortexa | 1041 | 1067 | −0.16 (0.04) | −0.25 to −0.08 | <0.001 | 0.002 |
| superior temporal gyrus | 987 | 993 | −0.15 (0.05) | −0.24 to −0.07 | <0.001 | 0.003 |
| caudal middle frontal gyrusa | 1046 | 1077 | −0.15 (0.04) | −0.23 to −0.06 | <0.001 | 0.003 |
| fusiform gyrus | 1043 | 1075 | −0.13 (0.04) | −0.21 to −0.04 | 0.004 | 0.01 |
| isthmus cingulate cortex | 1040 | 1079 | −0.13 (0.04) | −0.22 to −0.05 | 0.002 | 0.008 |
| middle temporal gyrusa | 1001 | 1024 | −0.13 (0.04) | −0.22 to −0.04 | 0.004 | 0.01 |
| rostral middle frontal gyrus | 1044 | 1079 | −0.13 (0.04) | −0.21 to −0.04 | 0.004 | 0.01 |
| supramarginal gyrus | 1036 | 1063 | −0.13 (0.04) | −0.22 to −0.05 | 0.002 | 0.008 |
| inferior parietal cortex | 1041 | 1078 | −0.12 (0.04) | −0.20 to −0.03 | 0.009 | 0.02 |
| inferior temporal gyrus | 1041 | 1064 | −0.12 (0.04) | −0.21 to −0.04 | 0.005 | 0.01 |
| lateral occipital cortex | 1047 | 1078 | −0.12 (0.04) | −0.21 to −0.04 | 0.005 | 0.01 |
| precuneus | 1044 | 1080 | −0.12 (0.04) | −0.20 to −0.03 | 0.008 | 0.02 |
| superior parietal cortex | 1045 | 1073 | −0.12 (0.04) | −0.21 to −0.04 | 0.004 | 0.01 |
| insula | 1042 | 1078 | −0.12 (0.04) | −0.21 to −0.04 | 0.006 | 0.01 |
| banks of superior temporal sulcus | 974 | 999 | −0.10 (0.05) | −0.19 to −0.01 | 0.02 | 0.04 |
| pars triangularis of inferior frontal gyrus | 1048 | 1074 | −0.10 (0.04) | −0.18 to −0.01 | 0.02 | 0.04 |
| postcentral gyrus | 1032 | 1060 | −0.10 (0.04) | −0.18 to −0.01 | 0.03 | 0.05 |
| precentral gyrus | 1041 | 1064 | −0.10 (0.04) | −0.19 to −0.02 | 0.02 | 0.03 |
| temporal pole | 1043 | 1075 | −0.10 (0.04) | −0.18 to −0.01 | 0.03 | 0.04 |
Note: Displayed are the significant regions surviving correction for multiple comparisons with FDR q-value<0.05. Regions are sorted based on the effect size of the difference between cases and controls (Cohen’s d), with the regions with the largest effects on top. Regions are the average of left and right hemisphere surface area. Model is adjusted for age, sex, intracranial volume (ICV), and site.
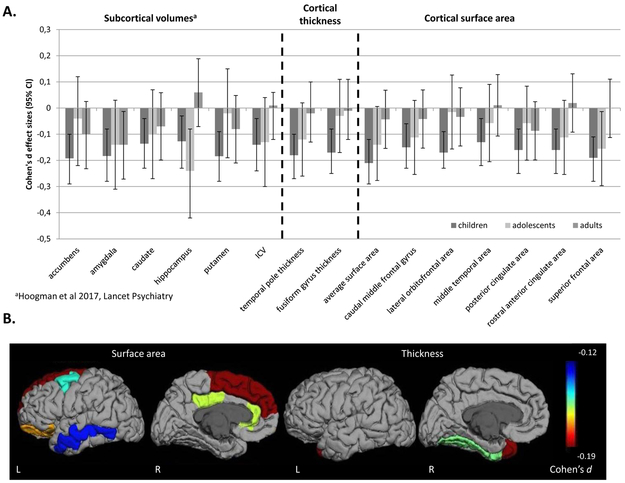
FIGURE 1. Subcortical and cortical brain differences across the lifespan.
A. Cohen’s d effect sizes with error bars showing the 95% confidence intervals for case-control differences in ENIGMA-ADHD cortical and subcortical structural features stratified by 3 age groups: children of 14 years of age and younger, adolescents from age 15 to 21 years, and adults older than 21 years. Structural features of all regions listed on the x-axis showed significant case-control differences in children; in analyses of cortical and subcortical features, no significant effects were seen in adolescents or adults. This is reflected in the effects sizes shown, all of which reached case-control statistical significance for children but not for adolescent and adult groups, except for the hippocampus, which shows a significant case-control difference in the adolescent group as well. B. Displayed are the heatmaps of validated case-control differences in the childhood subset for both surface area (left) and thickness (right) in each hemisphere.
Cortical thickness was affected in four regions (fusiform, parahippocampal, and precentral gyrus and temporal pole) in children, all being thinner in patients than controls (Table 2, Figure 1 and ST10). Further subdivision of the child group retained significant effects for fusiform gyrus (d= −0.31, pFDR=0.002) and temporal pole (d=−0.25, pFDR=0.02) in the group of children aged 10 and 11 (356 cases, 365 controls); in younger (4-9 years) and older (12-14 years) children, effects did not survive multiple comparisons correction (ST11). In adolescents and adults, no case-control differences were found (ST12 and ST13; ST14 shows combined analysis of age groups).
Table 2.
Mega-analysis of case-control cortical thickness differences in children of 14 years of age and younger in ENIGMA-ADHD.
| Controls (N) | ADHD (N) | Cohen’s d (standard error) |
95% confidence interval |
p-value | FDR p- value |
|
|---|---|---|---|---|---|---|
| temporal polea | 1042 | 1075 | −0.18 (0.04) | −0.27 to −0.10 | <0.001 | 0.001 |
| fusiform gyrusa | 1044 | 1077 | −0.17 (0.04) | −0.25 to −0.08 | <0.001 | 0.003 |
| precentral gyrus | 1040 | 1064 | −0.16 (0.04) | −0.25 to −0.07 | <0.001 | 0.003 |
| parahippocampal gyrus | 1041 | 1076 | −0.15 (0.04) | −0.23 to −0.06 | <0.001 | 0.008 |
Note: Displayed are the significant regions surviving correction for multiple comparisons with FDR q-value<0.05. Regions are sorted based on the effect size of the difference between cases and controls (Cohen’s d), with the regions with the largest effects on top. Regions are the average of left and right hemisphere thickness measures. Model is adjusted for age, sex and site.
Validation of case-control findings
The split-half validation analysis showed seven regions for surface area and two regions for thickness to be significant in both halves (Table 1 & 2, ST15 & ST16, Figure 1). For all other regions, the direction of effects was the same in both split-halves.
Effect sizes of the validated cortical differences across the age groups are plotted in Figure 1, together with the effect sizes of subcortical brain volumes from our earlier work (9). Post-hoc analysis by adding the term Agegroup*Diagnosis to the main model indicated differences in effect sizes across the lifespan for surface area of the superior frontal gyrus and thickness of the fusiform gyrus (ST17).
Exploration of effects of sex, IQ, comorbidity, psychostimulant medication, and ADHD severity
Extending the main findings, we investigated several factors linked to ADHD, which have shown to influence brain volume in their own right. No significant interaction effects of diagnosis-by-sex were found (ST18). Correcting for IQ in surface area analyses only led to minor changes in the level of significance in the case-control comparisons. In all thickness analyses, IQ was a non-significant contributor (ST19).
For comorbidity analyses, we had information on cases of the childhood subset (n=1081) available (comorbidity ever versus never, lifetime) for almost 50% of participants (ST20). In total, 194 children with ADHD (39%) were ever or currently diagnosed with a comorbid psychiatric disorder. The three most frequently co-occurring disorders were oppositional defiant disorder (ODD, present in n=79 cases (16.0%)), anxiety disorders (observed in n=39 (8.6%)), and mood disorders (seen in n=13 (3.0%)). Presence versus absence of comorbid disorders did not affect cortical surface area; a nominal effect of ever being diagnosed with a comorbid psychiatric disorder was found for fusiform gyrus thickness, with a thinner fusiform gyrus in cases with an additional disorder in the past or present (ST21).
Current stimulant use versus no current use had a nominally significant association with surface area of two regions in frontal cortex, with those taking medication having lower surface areas (ST21).
Hyperactivity/impulsivity severity ratings on Conners’ questionnaires, available for n=240 childhood patients, but not inattention, showed nominally significant correlation with surface area in rostral anterior cingulate cortex (r=−0.18, p=0.01), superior frontal gyrus (r=−0.19, p=0.01), and with total surface area (r=−0.15, p=0.03) (ST22).
Family study
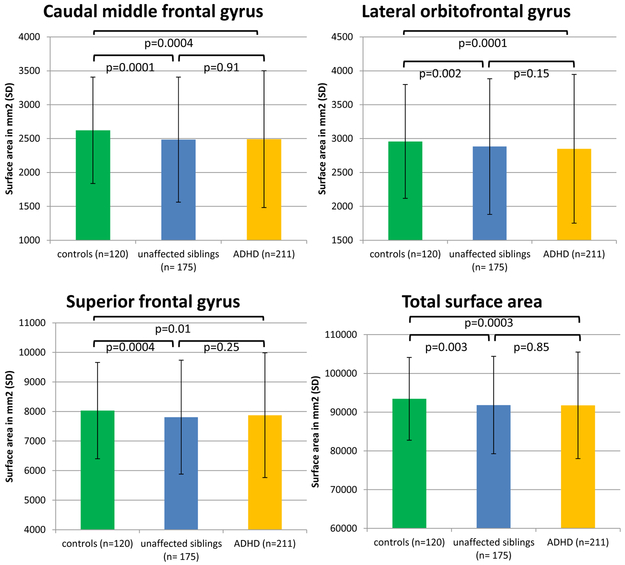
Among the validated ADHD-associated cortical features, surface area of caudal middle frontal, lateral orbital frontal, and superior frontal gyrus and the total surface area were significantly smaller in the unaffected siblings as compared with controls (Figure 2, ST23), indicating familial effects. A similar trend was seen for the majority of the other cortical measures (SF2).
FIGURE 2.
Bar graphs showing results of familiality analyses, comparing healthy controls, unaffaected siblings and cases, in the ADHD-affected cortical regions in the NeuroIMAGE datasets (n=506). Displayed are the cortical surface areas showing effects of familiality in the NeuroIMAGE datasets. For these regions, unaffected siblings differed from healthy controls (Meff-corrected results). Cortical values are adjusted for age, gender, ICV and site.
Effects of ADHD symptoms in the general population on the validated brain phenotypes
Population-based analysis showed caudal middle frontal gyrus, middle temporal gyrus, and total surface area to be associated with the attention problems scale of the CBCL (Table 3, SF3); higher levels of dimensional ADHD symptoms were associated with smaller surface areas. No associations were found with the two cortical thickness measures (Table 3). To ensure a linear fit was optimal and that the more severe end of the symptom continuum was not driving findings, models with quadratic and cubic symptom terms were also tested. AIC and BIC values were highly similar across models, suggesting little to no improvement over the simpler linear term (ST24).
Table 3.
Associations between validated cortical regions and CBCL syndrome scale attention problems in Generation-R.
| Cortical region | B | SE | CI lower | CI upper | β | p-value | FDR p- value |
|---|---|---|---|---|---|---|---|
| Surface area | |||||||
| caudal middle frontal gyrus | −14.10 | 5.49 | −24.87 | −3.33 | −0.04 | 0.01 | 0.03 |
| lateral orbitofrontal cortex | −8.28 | 5.01 | −18.10 | 1.54 | −0.02 | 0.10 | 0.11 |
| middle temporal gyrus | −13.63 | 5.86 | −25.12 | −2.14 | −0.03 | 0.02 | 0.04 |
| posterior cingulate cortex | −5.02 | 2.42 | −9.77 | −0.27 | −0.03 | 0.04 | 0.06 |
| rostral anterior cingulate cortex | −3.50 | 1.93 | −7.29 | 0.29 | −0.03 | 0.07 | 0.09 |
| superior frontal gyrus | −7.16 | 11.93 | −30.55 | 16.24 | −0.01 | 0.55 | 0.55 |
| total surface area | −323.79 | 77.50 | −475.75 | −171.82 | −0.04 | <0.001 | <0.001 |
| total surface area (residualized*) | −291.62 | 77.43 | −443.44 | −139.79 | −0.07 | <0.001 | <0.001 |
| Thickness | |||||||
| fusiform gyrus | 0.004 | 0.002 | 0.000 | 0.01 | 0.04 | 0.05 | 0.054 |
| temporal pole | 0.01 | 0.01 | −0.001 | 0.03 | 0.04 | 0.07 | 0.07 |
Note: Regions are the average of left and right hemisphere surface area, and are the regions showing significant group differences in split-half analyses (ST15 and ST16). Model is adjusted for age, sex, and ethnic background. ICV is also included as a covariate in the surface area analysis. B is the unstandardized regression coefficient for the square root transformed CBCL syndrome scale attention problems score, and CI is the 95% confidence interval of that regression coefficient. β is the standardized regression coefficient.
Given the high correlation between total surface area and ICV, we also tested a model where total surface area was first regressed on ICV, and the resulting residuals were used in the model described above, but without entering ICV. This shows that multicolinearity is not driving the effects. p-values in bold are considered significant, surviving correction for multiple comparisons with FDR q-value<0.05.
Adding non-verbal IQ or ADHD medication status to the analysis model of the attention problems, did not influence results (ST25). Results also remained stable when we tested the effect of MRI scanner software version and image quality (ST25). The quantitative amount of motion in the T1-weighted scan (32) did not seem to affect analyses (ST26).
DISCUSSION
Here, we report the largest study to date of ADHD and cortical surface area and thickness in clinical samples and a pediatric population sample. Compared with healthy controls, children with ADHD showed smaller surface area in frontal, temporal, and cingulate regions, with the effects being most prominent in the youngest children (4-9 years). Case control differences had small effect sizes, but survived validation. Differences in thickness were limited to the temporal pole and fusiform gyrus, which were thinner in children with ADHD. These differences were most prominent in the group aged 10 and 11 years. The influence of comorbidity and symptom ratings, available from subsamples, appeared limited. None of these covariates of interest showed effects surviving multiple testing correction. There were no significant associations between cortical alterations and either stimulant treatment or IQ. Family-based analyses revealed familial effects for four surface area regions, but not for any of the thickness measures. A set overlapping with family-based analyses (caudal middle frontal gyrus, total surface area) and/or severity rating analyses (total surface area) showed associations with CBCL-based ratings of attention problems, in the population-based sample; no such effects were found for thickness.
The regions affected in ADHD were widespread across the cortex. The frontal cortex differences in orbital, middle, and superior regions nicely confirmed earlier work (e.g. (8, 15)). These regions play a key role in cognitive processes related to reward and punishment, emotional processing, response inhibition, and attention - all known to be deficient in ADHD (33-35). Few studies yet have implicated structural differences in the cingulate cortex, an important structure linked to executive functioning and emotion (36), in ADHD (7, 37). Findings for the temporal cortex are particularly interesting, because both surface area and thickness were affected. The functions of this region are diverse, as it seems to be involved in semantic memory and processing of abstract concepts, attention, emotion processing and regulation (38). Integrating the current findings with our earlier subcortical results (9), the multitude of findings for brain regions involved in emotion processing is intriguing. In view of this, the network of orbito-frontal cortex, cingulate, and amygdala could be particularly interesting for future research (39, 40), as they may underlie the deficient emotional self-regulation often observed among ADHD patients (33).
Effect sizes of the observed brain differences were small, which is at a similar level as our earlier findings for subcortical volumes and ICV in ADHD (Figure 1) and comparable to effect sizes seen in other psychiatric disorders studied within the ENIGMA consortium (23, 24). Whether this reflects phenotypic heterogeneity, with only a subgroup of patients showing reduced brain structure of large(r) effect size, or homogeneously small effects existing in the majority of patients remains to be investigated. Effects were not driven by IQ. Findings in several areas seemed to scale with the severity of hyperactivity/impulsivity in patients, but the heterogeneity of assessment instruments limited the power of this analysis. As in our earlier analysis of subcortical volumes and ICV, we did not find any significant associations between psychostimulant medication and cortical dimensions, neither in case-control nor in population-based designs. However, given our observational design and reliance on legacy data, we would not want to draw any firm conclusions from those results.
Looking across the lifespan, all case-control differences were most pronounced in children and non-significant in adolescents and adults. The same phenomenon, albeit attenuated, was seen in our recent cross-sectional study of ICV and subcortical structures (9) (Figure 1). Post-hoc analysis of potential differences in effect sizes across the three age groups in the current study confirmed age-related attenuation of effects for several structures. Those findings are in line with an earlier longitudinal study, where case-control differences in cortical thickness observed in children attenuated with increasing age, suggesting a delayed cortical maturation (41). An alternative explanation for the age-related differences might be the existence of subgroups; the childhood patient group is likely to consist of a mix of individuals who will persist and remit in adulthood, while the adult group consists largely of persisters. We cannot yet rule out low power as a reason for not detecting significant effects in the older subgroups, which were half the size of the children’s group, and these initial findings concerning apparent differences across the lifespan should be confirmed in longitudinal studies.
The case-control differences observed in the childhood sample did not seem to be influenced by comorbidity. However, we noticed that the comorbidity rate in this subset was relatively low (39%). There could be several reasons for that. First, the sample we used in our analysis of comorbidity was very young (4-14 years), as we only focused on the subsample with significant case-control differences. The relatively young age could explain the lower than expected comorbidity rate, as children might simply not yet have developed some of the frequent comorbid psychiatric disorders (e.g. substance use disorders). In comparison, Taurines and coworkers (2010) (42) described in their review that 73% of 6-18 year olds with ADHD had one or more comorbid disorders. A second reason could lie in the fact that we are dealing with research diagnoses, in which comorbidity assessments were often limited to checking inclusion and exclusion criteria for a specific study aim. This is a clear limitation of dealing with legacy data from multiple different sites, where different protocols and different instruments of assessment of comorbidity and symptom severity were used. We adjusted our design accordingly and concentrated only on the three most frequent comorbidities, defining those as ever or never experienced.
Although our study was not designed to study causality, our results may shed some light on the issue of whether brain differences are a consequence of living with the disorder or a risk factor for the disorder. Our family analysis showed unaffected siblings of cases, i.e. those without a diagnosis and with levels of ADHD symptoms comparable to healthy controls, to have similar surface area differences from controls as their affected siblings. In addition, the relationship between ADHD symptoms and cortical phenotypes also held in the general population. Here, the dimensional assessment of attention problems was related to brain morphology in a linear fashion, suggesting the phenotype and underlying brain morphology to be independent of clinical diagnosis, operating along a continuum. The two different approaches show cortical alterations in ADHD-related regions to occur independent of diagnosis. The overlap between the findings from the different approaches was, however, not complete. Future studies could perform more direct comparisons between case-control and population samples using e.g. conjunction analysis (43). The two different approaches show cortical alterations in ADHD-related regions to occur independent of diagnosis, indicating that they are neither necessary nor sufficient to cause the disorder. The overlap between the findings from the different approaches was, however, not complete. Future studies could perform more direct comparisons between case-control and population samples using e.g. conjunction analysis (41). In such a design it would be interesting to test the liability-threshold model, to better understand which factors contribute to liability for the disorder. Also, whether the observed brain differences relative to controls are indeed risk factors for ADHD, remains to be investigated in prospective longitudinal designs. Future imaging genetics studies might further clarify the neurobiological pathways and mechanisms underlying cortical differences in ADHD. While genetic information is not available in sufficient numbers from ENIGMA-ADHD, the ENIGMA Genetics Working Group recently identified genetic factors determining cortical surface area and thickness in a largely healthy population (18). Those genetic factors might in turn constitute risk factors for ADHD given recent finding of genetic overlap between the genetic contribution to ADHD and to the total surface area of the cortex. As we have recently shown for subcortical volumes and intracranial volume, further work might delineate the individual genes or gene networks underlying such genetic overlap (Klein et al., Am. J. Psychiatry, in press; see also (44)).
The current study has several strengths and limitations. Our major strength lies in the large sample sizes in both the clinical (n=4180) and population-based (n=2707) samples, along with the use of harmonized segmentation protocols, which provided unprecedented power to detect effects. Another strength is the split-half validation combined with stringent multiple comparison correction, showing that our findings – despite small effect sizes – are stable. Also, results from the population study suggest little effect of motion during scanning on our cortical regions of interest. The combination of case-control with family- and population-based designs to identify mechanisms is an additional strength. A limitation is that we relied on legacy data in ENIGMA-ADHD, so the participating studies differ somewhat in their aims, methods, and assessments. Given this heterogeneity, our findings might underestimate the true effects, and we may have missed effects of comorbidity, medication, and symptom severity due to insufficient power. The limited sample size of the family study together with the small effect sizes for brain differences are probably the reason why the results of the family study found the expected staircase effect, at a trend level only.
In light of the findings from the current and the earlier (9) ENIGMA studies of ADHD, what should future neuroimaging studies in ADHD look like? Effect sizes observed are small (i.e. Cohen’s d=−0.21), with largest effects for measures of total brain volume and surface area in this and our previous study (9). Also, effects are restricted to childhood despite persistent ADHD diagnosis in adolescents and adults. Future studies should answer the question, whether (regional) effect sizes are comparable in everyone, or whether subgroups exist, in which certain regional effect sizes are more pronounced. This could be examined using clustering algorithms, such as community detection, and machine learning (45). An analysis of particular interest would be the comparison between children who remit in adulthood and those who persist. In-depth analysis of adult persisters versus adult remitters could add to our understanding of the null findings in adults, as it seems counterintuitive that the adult persisters, believed to be more severely affected, show no apparent signs of brain differences in adulthood, but the childhood group, which is likely to be a mix of remitters and persisters when they are adults, does. Subgroups may also provide information on comorbidity and links to symptom severity in the different behavioral domains of ADHD. Most importantly, longitudinal studies are needed to study the processes that lead to the apparent reductions of case-control effects from childhood to adolescence and adulthood; only very few longitudinal samples for ADHD are currently available (15, 29). We should also not forget that the segmentation used in the current study is based on classical neuroanatomical divisions rather than a partitioning based on biological functions (44, 46). Other cortical phenotypes such as gyrification (47), or more sophisticated methods to define regional gray matter structure, and analyses of other brain measures to be captured by neuroimaging in large sample sizes (e.g., white matter integrity (48); resting state functional MRI (49)) may help us find the presumed case-control differences in adults (50, 51).
In conclusion, we identify, for the first time, cortical phenotypes affected in ADHD that are robust, and show an association with ADHD beyond narrowly-defined clinical diagnoses. Our work suggests them to behave as endophenotypes and thus extends the evidence for ADHD as a continuous trait in the population, shown for behavioral measures and genetics (52), and now for to neuroimaging phenotypes. Future studies should clarify individual lifespan trajectories and identify the underlying genetic and environmental factors shaping these trajectories.
Supplementary Material
ACKNOWLEDGEMENTS
ZI-CAPS: We would like to acknowledge Isabella Wolf, Nathalie Holz and Regina Boecker-Schlier.
CAPS_UZH: We would like to acknowledge Tobias Hauser, Anthony Schläpfer and Reto Iannaccone.
MTA: The Multimodal Treatment Study of Children with ADHD (MTA) was a National Institute of Mental Health (NIMH) cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and finally under a National Institute on Drug Abuse (NIDA) contract. Collaborators from NIMH: Benedetto Vitiello, M.D. (Child & Adolescent Treatment and Preventive Interventions Research Branch), Joanne B. Severe, M.S. (Clinical Trials Operations and Biostatistics Unit, Division of Services and Intervention Research), Peter S. Jensen, M.D. (currently at REACH Institute and Mayo Clinic), L. Eugene Arnold, M.D., M.Ed. (currently at Ohio State University), Kimberly Hoagwood, Ph.D. (currently at New York University); previous contributors from NIMH to the early phases: John Richters, Ph.D. (currently at National Institute of Nursing Research); Donald Vereen, M.D. (currently at NIDA). Principal investigators and co-investigators from the sites are: University of California, Berkeley/San Francisco: Stephen P. Hinshaw, Ph.D. (Berkeley), Glen R. Elliott, Ph.D., M.D. (San Francisco); Duke University Medical Center: Karen C. Wells, Ph.D., Jeffery N. Epstein, Ph.D. (currently at Cincinnati Children's Hospital Medical Center), Desiree W. Murray, Ph.D.; previous Duke contributors to early phases: C. Keith Conners, Ph.D. (former PI); John March, M.D., M.P.H.; University of California, Irvine: James Swanson, Ph.D., Timothy Wigal, Ph.D.; previous contributor from UCLA to the early phases: Dennis P. Cantwell, M.D. (deceased); New York University: Howard B. Abikoff, Ph.D.; Montreal Children's Hospital/ McGill University: Lily Hechtman, M.D.; New York State Psychiatric Institute/Columbia University/Mount Sinai Medical Center: Laurence L. Greenhill, M.D. (Columbia), Jeffrey H. Newcorn, M.D. (Mount Sinai School of Medicine). University of Pittsburgh: Brooke Molina, Ph.D., Betsy Hoza, Ph.D. (currently at University of Vermont), William E. Pelham, Ph.D. (PI for early phases, currently at Florida International University). Follow-up phase statistical collaborators: Robert D. Gibbons, Ph.D. (University of Illinois, Chicago); Sue Marcus, Ph.D. (Mt. Sinai College of Medicine); Kwan Hur, Ph.D. (University of Illinois, Chicago). Original study statistical and design consultant: Helena C. Kraemer, Ph.D. (Stanford University). Collaborator from the Office of Special Education Programs/US Department of Education: Thomas Hanley, Ed.D. Collaborator from Office of Juvenile Justice and Delinquency Prevention/Department of Justice: Karen Stern, Ph.D. Additional investigators for Neuroimaging Substudy: Leanne Tamm, Ph.D., PI (Cincinnati Children's Hospital Medical Center), James Bjork, Ph.D. (Division of Clinical Neuroscience and Behavioral Research, NIDA; currently at Virginia Commonwealth University), Daniel Mathalon, M.D., Ph.D. (UC San Francisco), Allen Song, Ph.D. (Duke), Bradley Peterson, M.D. (currently USC), Steven Potkin, M.D. & Claudia Buss, Ph.D. (UC Irvine), Katerina Velanova, Ph.D. (Pittsburgh), Neuroimaging Consultants: Susan Tapert, Ph.D. & Joshua Kuperman, Ph.D. (UC San Diego), BJ Casey, Ph.D. & Leah Sommerville, Ph.D. (Sackler Institute, Cornell, currently at Yale and Harvard, respectively), Krista Lisdahl, Ph.D. (University of Wisconsin-Milwaukee). Neuroimaging Analysis and Interpretation: Terry Jernigan, Ph.D. & Anders Dale, Ph.D. (UC San Diego), F. Xavier Castellanos, M.D. & Clare Kelly, Ph.D. (New York University).
UCHZ: We would like to acknowledge Carmen Ghisleni, Steffen Bollmann, Lars Michels, Peter Klaver, Simon Shlomo Poil, Stefanie Kübel, Juliane Ball, Dominique Eich-Höchli, and Ernst Martin.
ADHD_Russia: We would like to acknowledge Vladimir Zelman, Boris Gutman, Anait Gevorkyan, Vladimir Smirnov
NICAP: We would like to acknowledge other investigators: Emma Sciberras, Daryl Efron, Vicki Anderson, Jan M. Nicholson, Philip Hazell, and all the staff and students of the Children’s Attention Project, as well as the RCH Medical Imaging staff for their assistance and expertise in the collection of the MRI data included in this study. We would also like to thank all of the many families and schools for their participation in this study.
Grant support
ENIGMA: received funding from the National Institutes of Health (NIH) Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centers of Excellence (BD2K). We also are supported by the European College for Neuropsychopharmacology (ECNP) by a grant for the ECNP Network ADHD across the lifespan.
ADHD-WUE: Data collection and analysis was supported by the Deutsche Forschungsgemeinschaft (KFO 125, TRR 58/A1 and A5, SFB-TRR 58/B01, B06 and Z02, RE1632/5-1) and the research leading to these results also received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no 602805 (Aggressotype) and the Horizon 2020 research and innovation programme under Grant no. 728018 (Eat2beNICE).
ADHD-DUB1 and DUB2: The ADHD-DUB1 and DUB2 studies received funding from the Health Research Board Ireland.
ADHD-Mattos: Ivanei Bramati, Paulo Mattos and Fernanda Tovar-Moll were supported by an IDOR intramural grant.
ADHD200-KKI: We would like to acknowledge Lindsey Koenig, Michelle Talley, Jessica Foster, Deana Crocetti, Lindsey MacNeil, Andrew Gaddis, Marin Ranta, Anita Barber, Mary Beth Nebel, John Muschelli, Suresh Joel, Brian Caffo, Jim Pekar, Stacy Suskauer. Research was made possible due to the following funding sources: The Autism Speaks Foundation and NIH (R01 NS048527, R01MH078160 and R01MH085328), Johns Hopkins General Clinical Research Center (M01 RR00052), National Center for Resource (P41 RR15241), Intellectual and Developmental Disabilities Research Center (HD-24061)
ADHD200-NYU: We would like to acknowledge Amy Roy, Andrea McLaughlin, Ariel Schvarcz, Camille Chabernaud, Chiara Fontani, Christine Cox, Daniel Margulies, David Anderson, David Gutman, Devika Jutagir, Douglas Slaughter, Dylan Gee, Emily Brady, Jessica Raithel, Jessica Sunshine, Jonathan Adelstein, Kristin Gotimer, Leila Sadeghi, Lucina Uddin, Maki Koyama, Natan Potler, Nicoletta Adamo, Rebecca Grzadzinski, Rebecca Lange, Samantha Adelsberg, Samuele Cortese, Saroja Bangaru, Xinian Zuo, Zarrar Shehzad and Zoe Hyde. Data collection was made possible thanks to funding from NIMH (R01MH083246), Autism Speaks, The Stavros Niarchos Foundation, The Leon Levy Foundation, and an endowment provided by Phyllis Green and Randolph Cowen.
ADHD200-Peking: we would like to acknowledge Jue-jing Ren, De-yi Wang, Su-fang Li, Zu-lai Peng, Peng Wang, Yun-yun Zhu, Zhao Qing. Research was made possible due to the following funding sources: The Commonwealth Sciences Foundation, Ministry of Health, China (200802073), The National Foundation, Ministry of Science and Technology, China (2007BAI17B03), The National Natural Sciences Foundation, China (30970802), The Funds for International Cooperation of the National Natural Science Foundation of China (81020108022), The National Natural Science Foundation of China (8100059), Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning
ADHD200-OHSU: We would like to acknowledge the Advanced Imaging Research Center, Bill Rooney, Kathryn L. Mills, Taciana G. Costa Dias, Michelle C. Fenesy, Bria L. Thurlow, Corrine A. Stevens, Samuel D. Carpenter, Michael S. Blythe, Colleen F. Schmitt. Research was made possible due to the following funding resources: K99/R00 MH091238 (Fair), R01 MH086654 (Nigg), Oregon Clinical and Translational Research Institute (Fair), Medical Research Foundation (Fair), UNCF/Merck (Fair), Ford Foundation (Fair)
ADHD-UKA: KFO-112 and IRTG1328 was supported by the German Research Foundation (DFG).
DAT-London: This work was supported in part by UK Medical Research, Council Grant G03001896 to J Kuntsi and NIH grants, R01MH62873 and R01MH081803 to SV Faraone.
IMpACT: The IMpACT study was supported by a grant from the Brain & Cognition Excellence Program and a personal Vici grant (to Barbara Franke) of the Netherlands Organization for Scientific Research (NWO, grant numbers 433-09-229 and 016-130-669) and in part by the Netherlands Brain Foundation (grant number, 15F07[2]27) and the BBMRI-NL (grant CP2010-33). Funding was also provided by a pilot grant of the Dutch National Research Agenda for the NeuroLabNL project (grant 400 17 602). The research leading to these results also received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 602805 (Aggressotype), no. 278948 (TACTICS), and no. 602450 (IMAGEMEND). In addition, the project received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 643051 (MiND), under grant agreement no. 667302 (CoCA) and the grant agreement no. 728018 (Eat2beNICE).
Niche: The structural neuroimaging studies of NICHE were supported by VIDI and VICI grants from the Netherlands Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek, NWO) to Sarah Durston (grant numbers Vidi-91.776.384 and Vici-453-10-005).
NYU ADHD: NYU data collection and sharing was supported by NIH grants T32MH67763, R01MH083246, K23MH087770, R01MH094639, and U01MH099059 and a grant from the Stavros S. Niarchos Foundation.
UAB-ADHD: The study and its contributors received funding from the Ministerio de Economía y Competitividad under research grant SAF2012-32362 and : PI12/01139 from the Department of Health of the Government of Catalonia. Additional funding was obtained from the Generalitat de Catalunya.
ZI-CAPS: The Neurofeedback study was partly funded by the project D8 of the Deutsche Forschungsgesellschaft collaborative research center 636.
ADHD-Rubia: The study was funded by the UK Department of Health via the National Institute of Health Research Centre (BRC) for Mental Health South London and the Maudsley NHS Foundation Trust and the Institute of Psychiatry, King's College London.
CAPS_UZH: The data contributed to this study were collected in two projects on ADHD and OCD in children and adolescents, supported by the Swiss National Science Foundation (projects No. 136249 Sinergia and No. 320030_130237) and the Hartmann Müller Foundation (No. 1460).
NeuroIMAGE: This work was supported by NIH Grant R01MH62873, NWO Large Investment Grant 1750102007010 and grants from Radboud University Medical Center, University Medical Center Groningen and Accare, and VU University Amsterdam. This work was also supported by grants from NWO Brain & Cognition (433-09-242 and 056-13-015) and from ZonMW (60-60600-97-193). Further support was received from the European Union FP7 programmes TACTICS (278948) and IMAGEMEND (602450).
MTA: Data collection and sharing for this project was funded by the NIDA MTA Neuroimaging Study (National Institute on Drug Abuse Grant Contract #: HHSN271200800009C).
NIH: studies were supported by intramural grants at the National Institute of Mental Health and National Human Genome Research Institute.
OHSU: The OHSU work was supported by NIMH grants R01MH86654, MH099064, and MH115357.
UCHZ: This work was supported by the University Research Priority Program “Integrative Human Physiology” at the University of Zurich.
ACPU: This research was conducted within the Academic Child Psychiatry Unit, University of Melbourne, Royal Children’s Hospital and the Developmental Imaging research group, Murdoch Children’s Research Institute, Melbourne, Victoria. National Health and Medical Research Council of Australia (NHMRC) project grants 384419 and 569533 provided funds for the data collection. It was also supported by the Murdoch Children’s Research Institute, the Royal Children’s Hospital and the Children’s MRI Centre, The Royal Children’s Hospital Foundation, and the RCH Mental Health Service, Department of Paediatrics The University of Melbourne and the Victorian Government's Operational Infrastructure Support Program. Tim Silk was supported by a NHMRC Career Development Award.
NICAP: The Neuroimaging of the Children’s Attention Project was funded by the National Medical Health and Research Council of Australia (NHMRC; project grant #1065895). Earlier funding of the Children’s Attention Project was as funded by an NHMRC project grant #1008522 and a grant from the Collier Foundation. This research was conducted within the Developmental Imaging research group, Murdoch Children’s Research Institute and the Children’s MRI Centre, The Royal Children's Hospital, Melbourne, Victoria. It was supported by the Murdoch Children’s Research Institute, The Royal Children’s Hospital, The Royal Children’s Hospital Foundation, Department of Paediatrics at The University of Melbourne and the Victorian Government's Operational Infrastructure Support Program.
Tübingen: The recruitment of the Tübingen sample was funded by the Deutsche Forschungsgemeinschaft (DFG grant: ET 112/5-1)
Dundee: This work was supported by a grant from TENOVUS SCOTLAND and was conducted in collaboration with the Dundee site of the ADHD Drugs Use Chronic Effects (ADDUCE) study (EU FP7 agreement No. 260576)
ePOD: The neuroimaging studies of the ePOD-MPH trial (NTR3103) were supported by faculty resources of the Academic Medical Center, University of Amsterdam, and by grant 11.32050.26 from the European Research Area Network Priority Medicines for Children (Sixth Framework Programme) to Liesbeth Reneman.
Sao Paulo: The present investigation was supported by a 2010 NARSAD Independent Investigator Award (NARSAD: The Brain and Behavior Research Fund) awarded to Geraldo F. Busatto. Geraldo F. Busatto is also partially funded by CNPq-Brazil. Marcus V. Zanetti is funded by FAPESP, Brazil (no. 2013/03905-4)
Sussex: This study was supported by funding from Brighton and Sussex Medical School and the Dr. Mortimer and Dame Theresa Sackler Foundation.
Clinic Barcelona: This work has received financial support from two grants, Fundació la Marató de TV3-2009 (project number: 091810) and Fondo de Investigaciones Sanitarias, of the Spanish Ministry of Health (project number: PI11/01419).
Generation R: Supercomputing resources were supported by the NWO Physical Sciences Division (Exacte Wetenschappen) and SURFsara (Cartesius compute cluster, www.surfsara.nl). The neuroimaging and neuroimaging infrastructure was supported by the Netherlands Organization for Health Research and Development (ZonMw) TOP project number 91211021 to TW. The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. We gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam. The general design of Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam, ZonMw, the Netherlands Organisation for Scientific Research (NWO), and the Ministry of Health, Welfare and Sport.
Martine Hoogman: supported by a personal Veni grant from of the Netherlands Organization for Scientific Research (NWO, grant number 91619115)
Maarten Mennes: supported by a Marie Curie International Incoming Fellowship within the 7th European Community Framework Programme, grant agreement n° 327340.
Jan Haavik: K.G. Jebsen Centre for Research on Neuropsychiatric Disorders, University of Bergen, Bergen, Norway
Steve Faraone: K.G. Jebsen Centre for Research on Neuropsychiatric Disorders, University of Bergen, Bergen, Norway
Footnotes
Potential conflict of Interest and financial support
These authors all declare no conflicts of Interest:
Hoogman, Muetzel, Guimaraes, Shumskaya, Mennes, Zwiers, Jahanshad, Sudre, Mostert, Wolfers, Earl, Soliva Vila, Vives-Gilabert, Khadka, Novotny, Hartman, Heslenfeld, Schweren, Ambrosino, Oranje, de Zeeuw, Chaim-Avancini, Rosa, Zanetti, Malpas, Kohls, von Polier, Seitz, Doyle, Epstein, Jernigan, Baur-Streubel, Ziegler, Zierhut, Schrantee, Høvik, Lundervold, Kelly, McCarthy, Skokauskas, O'Gorman Tuura, Calvo, Lera-Miguel, Nicolau, Chantiluke, Christakou, Vance, Cercignani, Gabel, Asherson, Baumeister, Hohmann, Bramati, Tovar-Moll, Fallgatter, Kardatzki, Schwarz, Anikin, Baranov, Gogberashvili, Kapilushniy, Solovieva, El Marroun, White, Namazova-Baranova, Ethofer, Plessen, Mehta, Paloyelis, Harrison, Bellgrove, Silk, Cubillo, Lazaro, Brem, Frodl, Zentis, Castellanos, Yoncheva, Reneman, Conzelmann, Pauli, Reif, Tamm, Oberwelland Weiss, Busatto, Louza, Durston, Oosterlaan, Stevens, Vilarroya, Fair, Nigg, Thompson, Shaw, Tiemeier, Bralten.
Potential conflicts of interest for the following authors are reported:
David Coghill served in an advisory or consultancy role for Lilly, Medice, Novartis, Oxford outcomes, Shire and Viforpharma. He received conference support or speaker’s fee by Janssen McNeil, Lilly, Medice, Novartis, Shire and Sunovian. He is/has been involved in clinical trials conducted by Lilly & Shire. The present work is unrelated to the above grants and relationships.
Jonna Kuntsi has given talks at educational events sponsored by Medice; all funds are received by King’s College London and used for studies of ADHD.
Theo Van Erp consulted for Roche Pharmaceuticals and has a contract with Otsuka Pharmaceutical, Ltd.
Anders Dale is a Founder of CorTechs Labs, Inc. He serves on the Scientific Advisory Boards of CorTechs Labs and Human Longevity, Inc., and receives research funding through a Research Agreement with General Electric Healhcare.
Paulo Mattos was on the speakers’ bureau and/or acted as consultant for Janssen-Cilag, Novartis, and Shire in the previous five years; he also received travel awards to participate in scientific meetings from those companies. The ADHD outpatient program (Grupo de Estudos do Déficit de Atenção/Institute of Psychiatry) chaired by Dr. Mattos has also received research support from Novartis and Shire.The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Tobias Banaschewski served in an advisory or consultancy role for Actelion, Hexal Pharma, Lilly, Lundbeck, Medice, Neurim Pharmaceuticals, Novartis and Shire. He received conference support or speaker’s fee by Lilly, Medice, Novartis and Shire. He is/has been involved in clinical trials conducted by Shire & Viforpharma. He received royalities from Hogrefe, Kohlhammer, CIP Medien, Oxford University Press. The present work is unrelated to the above grants and relationships.
Katya Rubia received speaker's fees form Shire, Medice and a grant from Lilly for another project.
Jan Haavik has received speaker fees from Lilly, Novartis and Janssen Cilag.
Stephen V. Faraone, in the past year, received income, potential income, travel expenses continuing education support and/or research support from Tris, Otsuka, Arbor, Ironshore, Shire, Akili Interactive Labs, VAYA, Ironshore, Sunovion, Supernus and Genomind. With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD.
Joseph Biederman is currently receiving research support from the following sources: The Department of Defense, Food & Drug Administration, Ironshore, Lundbeck, Magceutics Inc., Merck, PamLab, Pfizer, Shire Pharmaceuticals Inc., SPRITES, Sunovion, Vaya Pharma/Enzymotec, and NIH. In 2015, Dr. Joseph Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He has a US Patent Application pending (Provisional Number #61/233,686) through MGH corporate licensing, on a method to prevent stimulant abuse. In 2014, Dr. Joseph Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He received research support from AACAP, Alcobra, Forest Research Institute, and Shire Pharmaceuticals Inc. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH.
Kerstin Konrad received speaking fees from Medice, Lilly and Shire.
Josep-Antoni Ramos-Quiroga Josep-Antoni Ramos-Quiroga was on the speakers’ bureau and/or acted as consultant for Eli-Lilly, Janssen-Cilag, Novartis, Shire, Lundbeck, Almirall, Braingaze, Sincrolab, Medice and Rubió in the last 5 years. He also received travel awards (air tickets + hotel) for taking part in psychiatric meetings from Janssen-Cilag, Medice, Rubió, Shire, and Eli- Lilly. The Department of Psychiatry chaired by him received unrestricted educational and research support from the following companies in the last 5 years: Eli-Lilly, Lundbeck, Janssen- Cilag, Actelion, Shire, Ferrer, Oryzon, Roche, Psious, and Rubió.
Klaus-Peter Lesch served as a speaker for Eli Lilly and received research support from Medice, and travel support from Shire, all outside the submitted work.
Pieter Hoekstra received a research grant from Shire and was part of the advisory board of Shire.
Jan Buitelaar has been in the past 3 years a consultant to / member of advisory board of / and/or speaker for Janssen Cilag BV, Eli Lilly, Medice, Shire, Roche, and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties.
Barbara Franke has received educational speaking fees from Shire and Medice.
Susanne Walitza has received lecture honoraria from Eli-Lilly, Opopharma in the last five years and her outside professional activities and interests are declared under the link of the University of Zurich www.uzh.ch/prof/ssl-dir/interessenbindungen/client/web.
Daniel Brandeis serves as an unpaid scientific consultant for an EU-funded neurofeedback trial.
Georgii Karkashadze received payment for the authorship of the article and speaker fees from Sanofi and from Pikfarma.
REFERENCES
- 1.Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Rohde LA, Sonuga-Barke EJ, Tannock R, Franke B. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. [DOI] [PubMed] [Google Scholar]
- 2.Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135:e994–1001. [DOI] [PubMed] [Google Scholar]
- 3.Le HH, Hodgkins P, Postma MJ, Kahle J, Sikirica V, Setyawan J, Erder MH, Doshi JA. Economic impact of childhood/adolescent ADHD in a European setting: the Netherlands as a reference case. Eur Child Adolesc Psychiatry. 2014;23:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:1361–1369. [DOI] [PubMed] [Google Scholar]
- 5.Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125:114–126. [DOI] [PubMed] [Google Scholar]
- 7.Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based metaanalysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168:1154–1163. [DOI] [PubMed] [Google Scholar]
- 8.Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, Rubia K. Structural and Functional Brain Abnormalities in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder: A Comparative Meta-analysis. JAMA Psychiatry. 2016;73:815–825. [DOI] [PubMed] [Google Scholar]
- 9.Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, van Hulzen KJE, Medland SE, Shumskaya E, Jahanshad N, Zeeuw P, Szekely E, Sudre G, Wolfers T, Onnink AMH, Dammers JT, Mostert JC, Vives-Gilabert Y, Kohls G, Oberwelland E, Seitz J, Schulte-Rüther M, Ambrosino S, Doyle AE, Høvik MF, Dramsdahl M, Tamm L, van Erp TGM, Dale A, Schork A, Conzelmann A, Zierhut K, Baur R, McCarthy H, Yoncheva YN, Cubillo A, Chantiluke K, Mehta MA, Paloyelis Y, Hohmann S, Baumeister S, Bramati I, Mattos P, Tovar-Moll F, Douglas P, Banaschewski T, Brandeis D, Kuntsi J, Asherson P, Rubia K, Kelly C, Martino AD, Milham MP, Castellanos FX, Frodl T, Zentis M, Lesch KP, Reif A, Pauli P, Jernigan TL, Haavik J, Plessen KJ, Lundervold AJ, Hugdahl K, Seidman LJ, Biederman J, Rommelse N, Heslenfeld DJ, Hartman CA, Hoekstra PJ, Oosterlaan J, Polier GV, Konrad K, Vilarroya O, Ramos-Quiroga JA, Soliva JC, Durston S, Buitelaar JK, Faraone SV, Shaw P, Thompson PM, Franke B. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry. 2017;4:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. [DOI] [PubMed] [Google Scholar]
- 11.Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: A meta-analytic review of the fMRI literature. Neurosci Biobehav Rev. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki Y, Cortese S, Castellanos FX. Research Review: Diffusion tensor imaging studies of attention-deficit/hyperactivity disorder: meta-analyses and reflections on head motion. J Child Psychol Psychiatry. 2018;59:193–202. [DOI] [PubMed] [Google Scholar]
- 13.Cortese S, Castellanos FX, Eickhoff CR, D'Acunto G, Masi G, Fox PT, Laird AR, Eickhoff SB. Functional Decoding and Meta-analytic Connectivity Modeling in Adult Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2016;80:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Hu X, Ouyang L, He N, Liao Y, Liu Q, Zhou M, Wu M, Huang X, Gong Q. A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2016;68:838–847. [DOI] [PubMed] [Google Scholar]
- 15.Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson AD, Mathias JL, Banich MT. Corpus callosum morphology in children and adolescents with attention deficit hyperactivity disorder: a meta-analytic review. Neuropsychology. 2008;22:341–349. [DOI] [PubMed] [Google Scholar]
- 18.Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, Lind PA, Pizzagalli F, Ching CR, McMahon MA, Shatokhina N, Zsembik LCP, Agartz I, Alhusaini S, Almeida MA, Alnas D, Amlien IK, Andersson M, Ard T, Armstrong NJ, Ashley-Koch A, Bernard M, Brouwer RM, Buimer EE, Bülow R, Bürger C, Cannon DM, Chakravarty M, Chen Q, Cheung JW, Couvy-Duchesne B, Dale AM, Dalvie S, de Araujo TK, de Zubicaray GI, de Zwarte SM, den Braber A, Doan NT, Dohm K, Ehrlich S, Engelbrecht H-R, Erk S, Fan CC, Fedko IO, Foley SF, Ford JM, Fukunaga M, Garrett ME, Ge T, Giddaluru S, Goldman AL, Groenewold NA, Grotegerd D, Gurholt TP, Gutman BA, Hansell NK, Harris MA, Harrison MB, Haswell CC, Hauser M, Heslenfeld DJ, Hoehn D, Holleran L, Hoogman M, Hottenga J-J, Ikeda M, Janowitz D, Jansen IE, Jia T, Jockwitz C, Kanai R, Karama S, Kasperaviciute D, Kaufmann T, Kelly S, Kikuchi M, Klein M, Knapp M, Knodt AR, Krämer B, Lancaster TM, Lee PH, Lett TA, Lewis LB, Lopes-Cendes I, Luciano M, Macciardi F, Marquand AF, Mathias SR, Melzer TR, Milaneschi Y, Mirza-Schreiber N, Moreira JC, Mühleisen TW, Müller-Myhsok B, Najt P, Nakahara S, Nho K, Olde Loohuis LM, Papadopoulos Orfanos D, Pearson JF, Pitcher TL, Pütz B, Ragothaman A, Rashid FM, Redlich R, Reinbold CS, Repple J, Richard G, Riedel BC, Risacher SL, Rocha CS, Roth Mota N, Salminen L, Saremi A, Saykin AJ, Schlag F, Schmaal L, Schofield PR, Secolin R, Shapland CY, Shen L, Shin J, Shumskaya E, Sønderby IE, Sprooten E, Strike LT, Tansey KE, Teumer A, Thalamuthu A, Thomopoulos SI, Tordesillas-Gutiérrez D, Turner JA, Uhlmann A, Vallerga CL, van der Meer D, van Donkelaar MM, van Eijk L, van Erp TG, van Haren NE, Van Rooij D, van Tol M-J, Veldink JH, Verhoef E, Walton E, Wang Y, Wardlaw JM, Wen W, Westlye LT, Whelan CD, Witt SH, Wittfeld K, Wolf C, Wolfers T, Yasuda CL, Zaremba D, Zhang Z, Zhu AH, Zwiers MP, Artiges E, Assareh AA, Ayesa-Arriola R, Belger A, Brandt CL, Brown GG, Cichon S, Curran JE, Davies GE, Degenhardt F, Dietsche B, Djurovic S, Doherty CP, Espiritu R, Garijo D, Gil Y, Gowland PA, Green RC, Häusler AN, Heindel W, Ho B-C, Hoffmann WU, Holsboer F, Homuth G, Hosten N, Jack CR, Jang M, Jansen A, Kolskår K, Koops S, Krug A, Lim KO, Luykx JJ, Mathalon DH, Mather KA, Mattay VS, Matthews S, Mayoral Van Son J, McEwen SC, Melle I, Morris DW, Mueller BA, Nauck M, Nordvik JE, Nöthen MM, O'Leary DS, Opel N, Paillère Martinot M-L, Pike GB, Preda A, Quinlan EB, Ratnakar V, Reppermund S, Steen VM, Torres FR, Veltman DJ, Voyvodic JT, Whelan R, White T, Yamamori H, Adams HH, Bis JC, Debette S, Decarli C, Fornage M, Gudnason V, Hofer E, Ikram MA, Launer L, Longstreth WT, Lopez OL, Mazoyer B, Mosley TH, Roshchupkin GV, Satizabal CL, Schmidt R, Seshadri S, Yang Q, Alvim MK, Ames D, Anderson TJ, Andreassen OA, Arias-Vasquez A, Bastin ME, Baune BT, Blangero J, Boomsma DI, Brodaty H, Brunner HG, Buckner RL, Buitelaar JK, Bustillo JR, Cahn W, Calhoun V, Caseras X, Caspers S, Cavalleri GL, Cendes F, Corvin A, Crespo-Facorro B, Dalrymple-Alford JC, Dannlowski U, de Geus EJ, Deary IJ, Delanty N, Depondt C, Desrivières S, Donohoe G, Espeseth T, Fernández G, Fisher SE, Flor H, Forstner AJ, Francks C, Franke B, Glahn DC, Gollub RL, Grabe HJ, Gruber O, Håberg AK, Hariri AR, Hartman CA, Hashimoto R, Heinz A, Hillegers MH, Hoekstra PJ, Holmes AJ, Hong LE, Hopkins WD, Hulshoff Pol HE, Jernigan TL, Jönsson EG, Kahn RS, Kennedy MA, Kircher TT, Kochunov P, Kwok JB, Le Hellard S, Martin NG, Martinot J-L, McDonald C, McMahon KL, Meyer-Lindenberg A, Morey RA, Nyberg L, Oosterlaan J, Ophoff RA, Paus T, Pausova Z, Penninx BW, Polderman TJ, Posthuma D, Rietschel M, Roffman JL, Rowland LM, Sachdev PS, Sämann PG, Schumann G, Sim K, Sisodiya SM, Smoller JW, Sommer IE, St Pourcain B, Stein DJ, Toga AW, Trollor JN, Van der Wee NJ, van't Ent D, Völzke H, Walter H, Weber B, Weinberger DR, Wright MJ, Zhou J, Stein JL, Thompson PM, Medland SE. The genetic architecture of the human cerebral cortex. bioRxiv. 2018. [Google Scholar]
- 19.Bralten J, Greven CU, Franke B, Mennes M, Zwiers MP, Rommelse NN, Hartman C, van der Meer D, O'Dwyer L, Oosterlaan J, Hoekstra PJ, Heslenfeld D, Arias-Vasquez A, Buitelaar JK. Voxel-based morphometry analysis reveals frontal brain differences in participants with ADHD and their unaffected siblings. J Psychiatry Neurosci. 2016;41:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mous SE, Muetzel RL, El Marroun H, Polderman TJ, van der Lugt A, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H, Posthuma D, White T. Cortical thickness and inattention/hyperactivity symptoms in young children: a population-based study. Psychol Med. 2014;44:3203–3213. [DOI] [PubMed] [Google Scholar]
- 21.Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans A, Rapoport J, Giedd J. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White T, Muetzel RL, El Marroun H, Blanken LME, Jansen P, Bolhuis K, Kocevska D, Mous SE, Mulder R, Jaddoe VWV, van der Lugt A, Verhulst FC, Tiemeier H. Paediatric population neuroimaging and the Generation R Study: the second wave. Eur J Epidemiol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, Cheung JW, van Erp TGM, Bos D, Ikram MA, Vernooij MW, Niessen WJ, Tiemeier H, Hofman A, Wittfeld K, Grabe HJ, Janowitz D, Bülow R, Selonke M, Völzke H, Grotegerd D, Dannlowski U, Arolt V, Opel N, Heindel W, Kugel H, Hoehn D, Czisch M, Couvy-Duchesne B, Rentería ME, Strike LT, Wright MJ, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Goya-Maldonado R, Gruber O, Krämer B, Hatton SN, Lagopoulos J, Hickie IB, Frodl T, Carballedo A, Frey EM, van Velzen LS, Penninx BWJH, van Tol MJ, van der Wee NJ, Davey CG, Harrison BJ, Mwangi B, Cao B, Soares JC, Veer IM, Walter H, Schoepf D, Zurowski B, Konrad C, Schramm E, Normann C, Schnell K, Sacchet MD Gotlib IH, MacQueen GM, Godlewska BR, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Hall J, Sussmann JE, Li M, Walter M, Aftanas L, Brack I, Bokhan NA, Thompson PM, Veltman DJ. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boedhoe PSW, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, Arnold PD, Batistuzzo MC, Benedetti F, Beucke JC, Bollettini I, Bose A, Brem S, Calvo A, Calvo R, Cheng Y, Cho KIK, Ciullo V, Dallaspezia S, Denys D, Feusner JD, Fitzgerald KD, Fouche JP, Fridgeirsson EA, Gruner P, Hanna GL, Hibar DP, Hoexter MQ, Hu H, Huyser B, Jahanshad N, James A, Kathmann N, Kaufmann C, Koch K, Kwon JS, Lazaro L, Lochner C, Marsh R, Martínez-Zalacaín I, Mataix-Cols D, Menchón JM, Minuzzi L, Morer A, Nakamae T, Nakao T, Narayanaswamy JC, Nishida S, Nurmi E, O'Neill J, Piacentini J, Piras F, Reddy YCJ, Reess TJ, Sakai Y, Sato JR, Simpson HB, Soreni N, Soriano-Mas C, Spalletta G, Stevens MC, Szeszko PR, Tolin DF, van Wingen GA, Venkatasubramanian G, Walitza S, Wang Z, Yun JY, Thompson PM, Stein DJ, van den Heuvel OA, Group E-OW, Group EOW. Cortical Abnormalities Associated With Pediatric and Adult Obsessive-Compulsive Disorder: Findings From the ENIGMA Obsessive-Compulsive Disorder Working Group. Am J Psychiatry. 2018;175:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, Versace A, Bilderbeck AC, Uhlmann A, Mwangi B, Krämer B, Overs B, Hartberg CB, Abé C, Dima D, Grotegerd D, Sprooten E, Bøen E, Jimenez E, Howells FM, Delvecchio G, Temmingh H, Starke J, Almeida JRC, Goikolea JM, Houenou J, Beard LM, Rauer L, Abramovic L, Bonnin M, Ponteduro MF, Keil M, Rive MM, Yao N, Yalin N, Najt P, Rosa PG, Redlich R, Trost S, Hagenaars S, Fears SC, Alonso-Lana S, van Erp TGM, Nickson T, Chaim-Avancini TM, Meier TB, Elvsåshagen T, Haukvik UK, Lee WH, Schene AH, Lloyd AJ, Young AH, Nugent A, Dale AM, Pfennig A, McIntosh AM, Lafer B, Baune BT, Ekman CJ, Zarate CA, Bearden CE, Henry C, Simhandl C, McDonald C, Bourne C, Stein DJ, Wolf DH, Cannon DM, Glahn DC, Veltman DJ, Pomarol-Clotet E, Vieta E, Canales-Rodriguez EJ, Nery FG, Duran FLS, Busatto GF, Roberts G, Pearlson GD, Goodwin GM, Kugel H, Whalley HC, Ruhe HG, Soares JC, Fullerton JM, Rybakowski JK, Savitz J, Chaim KT, Fatjó-Vilas M, Soeiro-de-Souza MG, Boks MP, Zanetti MV, Otaduy MCG, Schaufelberger MS, Alda M, Ingvar M, Phillips ML, Kempton MJ, Bauer M, Landén M, Lawrence NS, van Haren NEM, Horn NR, Freimer NB, Gruber O, Schofield PR, Mitchell PB, Kahn RS, Lenroot R, Machado-Vieira R, Ophoff RA, Sarró S, Frangou S, Satterthwaite TD, Hajek T, Dannlowski U, Malt UF, Arolt V, Gattaz WF, Drevets WC, Caseras X, Agartz I, Thompson PM, Andreassen OA. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23:932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, Clarkson MJ, MacManus DG, Ourselin S, Fox NC. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–1255. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. [DOI] [PubMed] [Google Scholar]
- 28.Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, Allen NB, Alia-Klein N, Batalla A, Blaine S, Brooks S, Caparelli E, Chye YY, Cousijn J, Dagher A, Desrivieres S, Feldstein-Ewing S, Foxe JJ, Goldstein RZ, Goudriaan AE, Heitzeg MM, Hester R, Hutchison K, Korucuoglu O, Li CR, London E, Lorenzetti V, Luijten M, Martin-Santos R, May A, Momenan R, Morales A, Paulus MP, Pearlson G, Rousseau ME, Salmeron BJ, Schluter R, Schmaal L, Schumann G, Sjoerds Z, Stein DJ, Stein EA, Sinha R, Solowij N, Tapert S, Uhlmann A, Veltman D, van Holst R, Whittle S, Wright MJ, Yücel M, Zhang S, Yurgelun-Todd D, Hibar DP, Jahanshad N, Evans A, Thompson PM, Glahn DC, Conrod P, Garavan H, Group EAW. Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. Am J Psychiatry. 2018:appiajp201817040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Rhein D, Mennes M, van Ewijk H, Groenman AP, Zwiers MP, Oosterlaan J, Heslenfeld D, Franke B, Hoekstra PJ, Faraone SV, Hartman C, Buitelaar J. The NeuroIMAGE study: a prospective phenotypic, cognitive, genetic and MRI study in children with attention-deficit/hyperactivity disorder. Design and descriptives. Eur Child Adolesc Psychiatry. 2015;24:265–281. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb). 2005;95:221–227. [DOI] [PubMed] [Google Scholar]
- 31.Chen WJ, Faraone SV, Biederman J, Tsuang MT. Diagnostic accuracy of the Child Behavior Checklist scales for attention-deficit hyperactivity disorder: a receiver-operating characteristic analysis. J Consult Clin Psychol. 1994;62:1017–1025. [DOI] [PubMed] [Google Scholar]
- 32.White T, Jansen PR, Muetzel RL, Sudre G, El Marroun H, Tiemeier H, Qiu A, Shaw P, Michael AM, Verhulst FC. Automated quality assessment of structural magnetic resonance images in children: Comparison with visual inspection and surface-based reconstruction. Hum Brain Mapp. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry. 2014;171:276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luman M, Oosterlaan J, Sergeant J. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25:183–213. [DOI] [PubMed] [Google Scholar]
- 35.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. [DOI] [PubMed] [Google Scholar]
- 36.Stevens FL, Hurley RA, Taber KH. Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci. 2011;23:121–125. [DOI] [PubMed] [Google Scholar]
- 37.Puiu AA, Wudarczyk O, Goerlich KS, Votinov M, Herpertz-Dahlmann B, Turetsky B, Konrad K. Impulsive aggression and response inhibition in attention-deficit/hyperactivity disorder and disruptive behavioral disorders: Findings from a systematic review. Neurosci Biobehav Rev. 2018;90:231–246. [DOI] [PubMed] [Google Scholar]
- 38.Bonner MF, Price AR. Where is the anterior temporal lobe and what does it do? J Neurosci. 2013;33:4213–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffiths KR, Grieve SM, Kohn MR, Clarke S, Williams LM, Korgaonkar MS. Altered gray matter organization in children and adolescents with ADHD: a structural covariance connectome study. Transl Psychiatry. 2016;6:e947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X, Liu L, Chen W, Cao Q, Zepf FD, Ji G, Wu Z, An L, Wang P, Qian Q, Zang Y, Sun L, Wang Y. Integrity of Amygdala Subregion-Based Functional Networks and Emotional Lability in Drug-Naïve Boys With ADHD. J Atten Disord. 2016. [DOI] [PubMed] [Google Scholar]
- 41.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taurines R, Schmitt J, Renner T, Conner AC, Warnke A, Romanos M. Developmental comorbidity in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2010;2:267–289. [DOI] [PubMed] [Google Scholar]
- 43.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. [DOI] [PubMed] [Google Scholar]
- 44.Klein M, Walters RK, Demontis D, Stein JL, Hibar DP, Adams HH, Bralten J, Roth Mota N, Schachar R, Sonuga-Barke E, Mattheisen M, Neale BM, Thompson PM, Medland SE, Borglum AD, Faraone SV, Arias-Vasquez A, Franke B. Genetic markers of ADHD-related variations in intracranial volume. bioRxiv. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman ME. Finding community structure in networks using the eigenvectors of matrices. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;74:036104. [DOI] [PubMed] [Google Scholar]
- 46.Franke B, Stein JL, Ripke S, Anttila V, Hibar DP, van Hulzen KJE, Arias-Vasquez A, Smoller JW, Nichols TE, Neale MC, McIntosh AM, Lee P, McMahon FJ, Meyer-Lindenberg A, Mattheisen M, Andreassen OA, Gruber O, Sachdev PS, Roiz-Santiañez R, Saykin AJ, Ehrlich S, Mather KA, Turner JA, Schwarz E, Thalamuthu A, Shugart YY, Ho YY, Martin NG, Wright MJ, O'Donovan MC, Thompson PM, Neale BM, Medland SE, Sullivan PF, Consortium SWGotPG, Consortium E. Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat Neurosci. 2016;19:420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, Blangero J, Brouwer RM, Curran JE, de Zubicaray GI, Duggirala R, Fox PT, Hong LE, Landman BA, Martin NG, McMahon KL, Medland SE, Mitchell BD, Olvera RL, Peterson CP, Starr JM, Sussmann JE, Toga AW, Wardlaw JM, Wright MJ, Hulshoff Pol HE, Bastin ME, McIntosh AM, Deary IJ, Thompson PM, Glahn DC. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage. 2013;81:455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adhikari BM, Jahanshad N, Shukla D, Turner J, Grotegerd D, Dannlowski U, Kugel H, Engelen J, Dietsche B, Krug A, Kircher T, Fieremans E, Veraart J, Novikov DS, Boedhoe PSW, van der Werf YD, van den Heuvel OA, Ipser J, Uhlmann A, Stein DJ, Dickie E, Voineskos AN, Malhotra AK, Pizzagalli F, Calhoun VD, Waller L, Veer IM, Walter H, Buchanan RW, Glahn DC, Hong LE, Thompson PM, Kochunov P. A resting state fMRI analysis pipeline for pooling inference across diverse cohorts: an ENIGMA rs-fMRI protocol. Brain Imaging Behav. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Onnink AM, Zwiers MP, Hoogman M, Mostert JC, Kan CC, Buitelaar J, Franke B. Brain alterations in adult ADHD: Effects of gender, treatment and comorbid depression. Eur Neuropsychopharmacol. 2013. [DOI] [PubMed] [Google Scholar]
- 51.Mostert JC, Shumskaya E, Mennes M, Onnink AM, Hoogman M, Kan CC, Arias Vasquez A, Buitelaar J, Franke B, Norris DG. Characterising resting-state functional connectivity in a large sample of adults with ADHD. Prog Neuropsychopharmacol Biol Psychiatry. 2016;67:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Middeldorp CM, Hammerschlag AR, Ouwens KG, Groen-Blokhuis MM, Pourcain BS, Greven CU, Pappa I, Tiesler CMT, Ang W, Nolte IM, Vilor-Tejedor N, Bacelis J, Ebejer JL, Zhao H, Davies GE, Ehli EA, Evans DM, Fedko IO, Guxens M, Hottenga JJ, Hudziak JJ, Jugessur A, Kemp JP, Krapohl E, Martin NG, Murcia M, Myhre R, Ormel J, Ring SM, Standl M, Stergiakouli E, Stoltenberg C, Thiering E, Timpson NJ, Trzaskowski M, van der Most PJ, Wang C, Nyholt DR, Medland SE, Neale B, Jacobsson B, Sunyer J, Hartman CA, Whitehouse AJO, Pennell CE, Heinrich J, Plomin R, Smith GD, Tiemeier H, Posthuma D, Boomsma DI, Consortium EGaLEE, Group PGCAW. A Genome-Wide Association Meta-Analysis of Attention-Deficit/Hyperactivity Disorder Symptoms in Population-Based Pediatric Cohorts. J Am Acad Child Adolesc Psychiatry. 2016;55:896–905.e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.