Colistin is a drug of last resort for the treatment of many multidrug-resistant Gram-negative bacteria, including carbapenem-resistant Klebsiella pneumoniae (CRKP). However, bacteria readily acquire resistance to this antibiotic via lipopolysaccharide modifications caused by spontaneous mutations or from enzymes acquired by lateral gene transfer. The fitness cost associated with these modifications remains poorly understood.
KEYWORDS: lytic, colistin resistance, Klebsiella pneumoniae, fitness cost, virulence, colonization, carbapenem-resistant Klebsiella pneumoniae (CRKP), bacteriophage
ABSTRACT
Colistin is a drug of last resort for the treatment of many multidrug-resistant Gram-negative bacteria, including carbapenem-resistant Klebsiella pneumoniae (CRKP). However, bacteria readily acquire resistance to this antibiotic via lipopolysaccharide modifications caused by spontaneous mutations or from enzymes acquired by lateral gene transfer. The fitness cost associated with these modifications remains poorly understood. In this report, we show that colistin-resistant CRKP is more susceptible to killing by a newly isolated lytic phage than the colistin-sensitive parent strain. We observed this behavior for colistin resistance conferred by a horizontally transferred mcr-1-containing plasmid and also from the inactivation of the chromosomal gene mgrB. By measuring zeta potentials, we found that the phage particles were negatively charged at neutral pH and that colistin-resistant bacteria had less negative zeta potentials than did the wild type. These results suggest that the decreased negative surface charge of colistin-resistant cells lowers the level of electrostatic repulsion between the phage and bacteria, thereby promoting phage adherence and subsequent infection. To further explore this, we tested the effect of phage treatment on CRKP growing in several different environments. We found that colistin-resistant cells were more susceptible to phage than were the wild-type cells when growing in biofilms or infected moth larvae and when colonizing the mammalian gut. A better understanding of these fitness costs may lead to new treatment approaches that minimize the emergence and spread of colistin-resistant pathogens in human and environmental reservoirs.
INTRODUCTION
The rise of multidrug-resistant Gram-negative bacteria represents an increasing threat to public health (1). For example, the increased prevalence of carbapenem-resistant Klebsiella pneumoniae (CRKP), which belongs to the family Enterobacteriaceae and is an opportunistic pathogen commonly associated with nosocomial infections, has greatly compromised the efficacy of carbapenem antibiotics (2). Colistin is a cyclic cationic antimicrobial peptide (CAMP) that was discovered in 1949, but it subsequently fell into disuse for systemic treatment due to its toxic side effects. However, in the last 2 decades, colistin has been resurrected as the drug of last resort for the treatment of multidrug-resistant Enterobacteriaceae (3). Unfortunately, bacteria readily acquire colistin resistance (colistinr), greatly reducing the utility of this antibiotic and of similar CAMPs and peptide mimetics. The primary mode of resistance occurs through envelope modifications that change the charge and possibly other physicochemical properties of the outer membrane. These modifications include the addition of aminoarabinose and phosphoethanolamine to lipid A and the polysaccharide core of lipopolysaccharides (LPS). Most Enterobacteriaceae spp. encode in their genomes the enzymes to make these modifications and regulate their expression through two-component signaling systems (4). In contrast to some antimicrobial peptides, colistin has little effect on the stimulation of these systems, but spontaneous resistance can emerge through mutations that render these regulatory systems constitutively active. Among colistinr clinical isolates of Klebsiella spp., the majority have mutations, such as insertions by IS elements, in mgrB (5, 6), which encodes a small membrane protein whose function was first identified in Escherichia coli (7). MgrB inhibits the protein PhoQ, a sensor kinase that is critical for antimicrobial peptide resistance in Enterobacteriaceae. PhoQ is strongly activated in MgrB-null strains, leading to resistance to colistin and other CAMPs in such strains. In the last few years, another mode of resistance has emerged through the horizontal transfer of mobile elements containing the mcr-1 gene, which encodes a phosphoethanolamine transferase (8, 9). Reports of transmissible plasmids containing mcr-1 and related genes (mcr homologs, including mcr-2 to mcr-9, all of which encode phosphoethanolamine transferase enzymes) in clinical isolates and in agricultural settings have risen at a remarkable pace (9–12), raising concerns that the emergence of colistin resistance will only continue to accelerate in the near future.
Clearly, there is an increasing urgency to address the following question: what are the fitness costs of the membrane modifications associated with colistin resistance? The fact that the enzymes responsible for these modifications are tightly regulated in most Enterobacteriaceae suggests that their expression comes with a fitness cost. However, to date, growth defects associated with MgrB-null strains of CRKP have not been observed (13). Moreover, only small fitness defects, if any, have been reported for plasmid carriage of mcr-1 (14). In this study, we isolated lytic bacteriophages of CRKP and compared their lytic efficiencies between colistin-sensitive (colistins) and colistinr CRKP strains. We found that colistinr CRKP was significantly more sensitive to phage infection. The use of bacteriophage is reemerging as a tool for combatting bacterial infections and antibiotic resistance (15, 16). Our results suggest that phage may also be useful in combination therapy with colistin treatment to prevent the emergence of resistance. In addition, phage treatment may provide a means to purge reservoirs of colistin-resistant strains in the environment.
RESULTS AND DISCUSSION
CRKP lytic phage isolation and characterization.
To isolate lytic phages for multidrug-resistant (MDR) CRKP, we screened sewage water samples near hospitals in different regions of China. We obtained five phages that could efficiently lyse a clinical CRKP isolate, A2312NM (17) (see Table S1 in the supplemental material), producing clear plaques (1 to 3 mm in diameter) after a 12-h incubation. In addition, all of these phages were able to infect a panel of MDR CRKP clinical isolates (Table S1). We sequenced the phage genomes and determined that all five were approximately 50 kb and belonged to the Tunavirinae subfamily (Table S2). We selected one of the phages, ФNJS1, for further characterization. Transmission electron microscopy (Fig. 1A) revealed that the phage possessed a capsid with a long noncontractile tail, suggesting that this phage is a member of the family Siphoviridae. ФNJS1 has a 49,292-bp genome (G+C content of 50.6%). The phage is highly similar to phage T1 of enterobacteria (Fig. S1A in the supplemental material) and closely related to a number of other previously isolated phages (Fig. S1B). The phage has 71 predicted open reading frames (ORFs), 25 of which have annotated gene functions predicted by BLASTp, including T1-like core genes encoding proteins involved in DNA replication, DNA packaging, morphogenesis, recombination, and lysis (Fig. S1C). ФNJS1 was able to infect CRKP at a low multiplicity of infection (MOI) (Fig. S2A) and, based on a one-step growth curve analysis, showed a short latency period of approximately 10 min with a burst size of about 75 PFU/cell (Fig. S2B). The phage was also stable over a wide pH range (Fig. S2C). Finally, ФNJS1 appears to show high specificity for CRKP, as it could not infect other members of Enterobacteriaceae that we tested (Fig. S2D).
FIG 1.
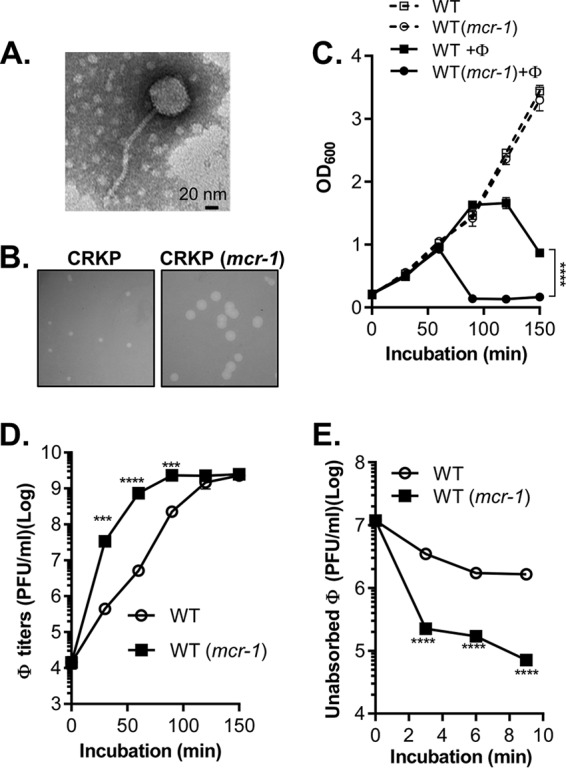
Effects of a CRKP phage on CRKP with and without mcr-1-containing plasmid pHNSHP45. (A) Transmission electron micrograph of ФNJS1. Scale bar, 20 nm. Representative image is shown. (B) Plaque morphology. ФNJS1 (150 PFU) was mixed with 108 CRKP and CRKP (mcr-1) in top agar and incubated at 37°C for 12 h. Representative images are shown. (C and D) ФNJS1 infection dynamics (C) and phage titers (D). Approximately 108 CRKP cells alone, or CRKP cells mixed with phages (MOI = 10−4), were incubated aerobically at 37°C. Samples were withdrawn at the time points indicated, and OD600 was measured. The number of free phages was determined by serial dilution and plating on the seeded top agar plates (D). (E) Phage adsorption. Bacterial cells (108 CFU/ml) were incubated with 107 PFU/ml phages in LB in a 37°C water bath for 10 min. Samples were withdrawn at the time points indicated and filtered through 0.22-μm-pore-size filters. The PFU of free phages in filtrates was then determined. Means of results from three independent assays are shown, and error bars represent the standard deviations. ***, P < 0.0005 (2-way analysis of variance [ANOVA]); ****, P < 0.0001.
CRKP phage is more infectious toward colistin-resistant CRKP.
To test whether colistin resistance in CRKP affects phage lysis, we introduced mcr-1-containing plasmid pHNSHP45 (the plasmid in which mcr-1 was first discovered [8]) into a colistins CRKP clinical isolate, A2312NM (colistin MIC < 0.5 μg/ml), which rendered the resulting strain highly resistant to colistin (MIC > 4 μg/ml). We found that ФNJS1 formed significantly larger plaques on colistinr CRKP (mcr-1) than on wild-type (WT) CRKP (Fig. 1B). In liquid cultures, the phage lysed CRKP (mcr-1) considerably faster than it lysed WT CRKP (Fig. 1C; see also Fig. S3). In addition, the phage titer from the infection of CRKP (mcr-1) was drastically higher than the corresponding titer from the infection of WT (Fig. 1D). Moreover, the number of unadsorbed phage particles from CRKP (mcr-1) culture supernatants dropped rapidly over time compared to those from WT (Fig. 1E). These data suggest that mcr-1-containing CRKP is more sensitive than WT to phage lysis and also adsorbs NJS1 phage more efficiently.
To test whether it is specifically the mcr-1 gene in the pHNSHP45 plasmid that contributes to phage sensitivity of CRKP, we replaced the mcr-1 gene with a kanamycin resistance cassette in pHNSHP45 (Δmcr-1::Kmr). We also engineered a second plasmid in which mcr-1 was restored at another position in the mcr-1::Kmr plasmid (Δmcr-1+mcr-1) to test for complementation. We then compared the levels of phage lysis of the CRKP strains containing these constructs. We found that the rapid lysis of ФNJS1 on CRKP was dependent on the presence of the mcr-1 gene, as it was evident only in CRKP (mcr-1) and the complemented strain, CRKP (Δmcr-1+mcr-1) (Fig. 2A). To further confirm that alteration of phage sensitivity resulted from acquired colistin resistance, we compared the levels of phage lysis efficiency of a CRKP strain containing an mcr-1 variant harbored in a different plasmid (mcr-1.6 [18]) and also of CRKP ΔmgrB. Panel B of Fig. 2 shows that all three colistinr strains were more susceptible to phage infection than the corresponding colistins strains from which they were derived. These results suggest that colistin resistance in CRKP, due to either the expression of mcr-1 or mgrB deletion, leads to increased phage infectivity.
FIG 2.
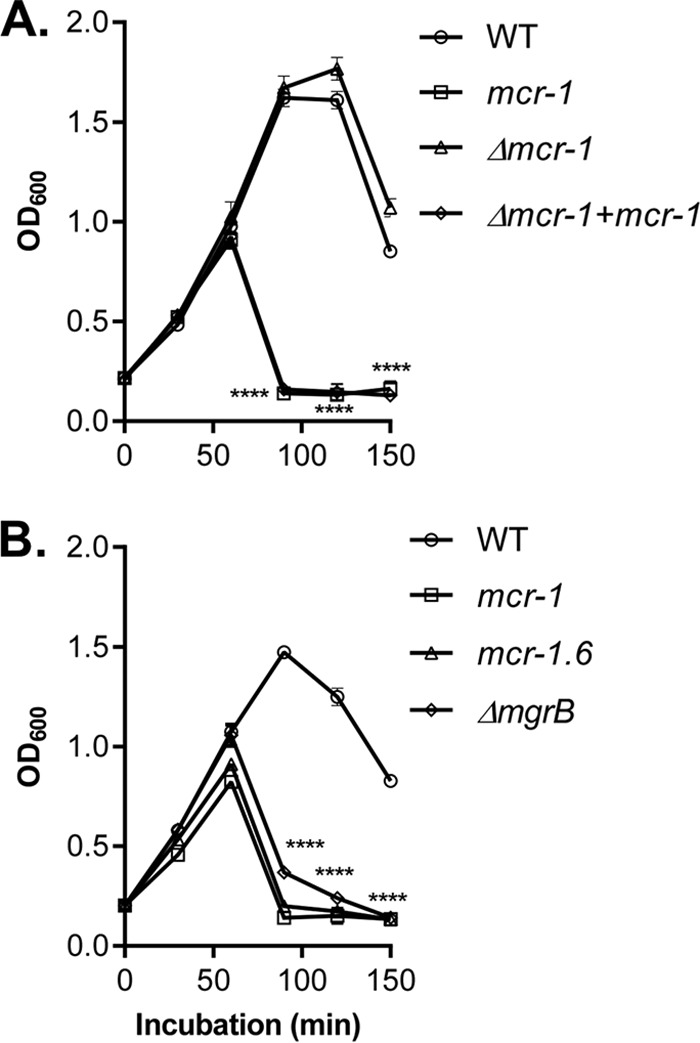
Colistin resistance and phage sensitivity. (A) The role of the mcr-1 gene in phage sensitivity. Freshly grown WT, CRKP containing the mcr-1 plasmid, mcr-1::Kmr, and Δmcr-1+mcr-1 bacteria were mixed with ФNJS1 at MOI = 10−4 and incubated at 37°C with aeriation. OD600 was measured at the time points indicated. (B) The colistinr effects on phage sensitivity. Freshly grown WT, ΔmgrB, or WT containing the mcr-1 plasmid or the mcr-1.6 plasmid was mixed with ФNJS1 at MOI = 10−4 and incubated at 37°C with aeriation. OD600 was measured at the time points indicated. Means of results from three independent assays are shown, and error bars represent the standard deviations. ****, P < 0.0001 (2-way ANOVA).
Colistin resistance-enhanced phage sensitivity is spread across CRKP strains and their phages.
To examine whether the enhanced phage sensitivity associated with colistin resistance described above is common among CRKP-phage interactions, we introduced the mcr-1 plasmid into five distinct CRKP clinical isolates and compared their levels of phage sensitivity to those of their parental strains. We found that they all displayed colistin resistance-dependent hypersensitivity to the NJS1 phage (Fig. 3A). In addition, we tested four other lytic CRKP phages that we isolated (Table S2) for their infectivity of colistins and colistinr CRKP. Panel B of Fig. 3 shows that these phages were also more virulent against colistinr strains than against colistins strains. Taken together, these data suggest that the acquisition of colistin resistance in CRKP does indeed come with a fitness cost: susceptibility to lytic phage infection.
FIG 3.
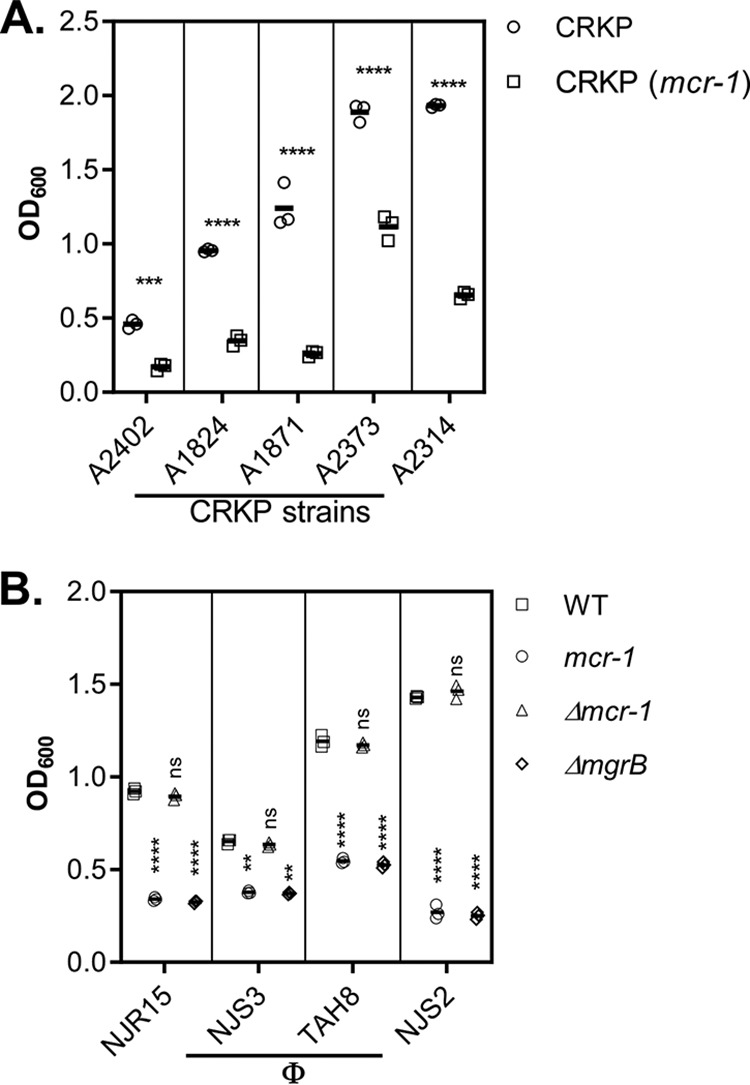
Colistinr effects on phage sensitivity in different CRKP strains and toward different phage isolates. (A) CRKP clinical isolates. The mcr-1 plasmid was introduced into different CRKP isolates, and the levels of phage sensitivity of the resulting strains were compared with the levels measured for the parental strains using the methods described in the Fig. 2 legend. Means of results from three independent assays are shown, and error bars represent the standard deviations. ***, P < 0.0005 (one-way ANOVA); ****, P < 0.0001. (B) Different CRKP phages. Freshly grown cultures of WT, ΔmgrB, or WT containing the mcr-1 plasmid or mcr-1::Kmr were mixed with ФNJR15 (at MOI = 10−2), ФNJS3 (MOI = 10−1), ФTAH8 (MOI = 10−2), and ФNJS2 (MOI = 10−3). At the time points indicated, samples were withdrawn and OD600 was measured. Means of results from three independent assays are shown, and error bars represent the standard deviations. ****, P < 0.0001 (2-way ANOVA). ns, no significance.
Alteration of surface charges promotes phage sensitivity.
Since many phage virions are negatively charged at neutral pH (19), we hypothesized that these macromolecular assemblies would interact more strongly with the modified outer membranes that characterize ΔmgrB or mcr-1+ colistinr cells. Thus, we assayed the surface charges of CRKP and phage particles by taking zeta potential measurements, a technique that has been applied in bacteriological studies to address the hypothesis that the susceptibility of bacteria to CAMPs is related to the charge on the bacterial cell surface (20, 21). Panel A of Fig. 4 shows that the zeta potential of ФNJS1 has a value of +4.7 mV at pH 2 and decreases to −37.1 mV at pH 7, indicating a net negative charge at neutral pH. In addition, CRKP cells with colistin resistance acquired from either mgrB deletion or the mcr-1 plasmid have less negative zeta potentials than the wild-type (colistin-susceptible) cells or those with the mcr-1-deleted plasmid (Fig. 4B), which is consistent with previous observations (20). These data suggest that a decrease in the net negative surface charge of colistinr cells lowers the level of electrostatic repulsion between the phage and bacteria, thereby promoting phage adherence and infection.
FIG 4.
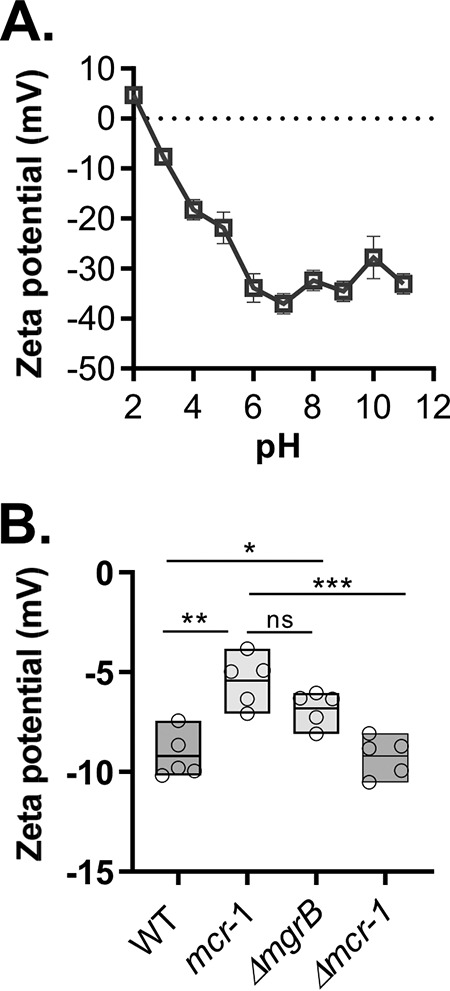
Zeta potential measurements of surface charge of the phage and of CRKP. (A) Isoelectric point values of ФNJS1. Phage particles (108 PFU/ml) were resuspended in 2 μM KCl with different pH, and the zeta potential was measured. Means of results from three independent assays are shown, and error bars represent the standard deviations. (B) Zeta potential of colistins and colistinr CRKP. Mid-log-phase cells of WT, ΔmgrB, or WT containing the mcr-1 plasmid or mcr-1::Kmr were rinsed with ddH2O, and approximately 5 × 104 CFU/ml cells resuspended in ddH2O were used to measure zeta potential. *, P < 0.05 (2-way ANOVA); **, P < 0.005; ***, P < 0.0005; ns, no significance.
Colistinr-mediated enhanced phage efficacy reduces CRKP virulence.
Due to the surge of antibiotic resistance, there has been renewed interest in phage therapy to treat infections by antibiotic-resistant bacteria (22, 23). Since we found that colistinr MDR K. pneumoniae is more susceptible to phage lysis, we tested if we could exploit this fitness cost as a means to control colistin resistance. We first examined whether colistinr CRKP is more sensitive to phage in biofilms. Biofilm formation has been shown previously to promote resistance to antibiotics and to be important for bacterial pathogenesis in many contexts (24). We grew wild-type and CRKP (mcr-1) in glass tubes for 24 h and, after biofilms formed, replaced the culture fluid with fresh Luria-Bertani (LB) medium with or without phages. Panel A of Fig. 5 shows that phages had little effect on the biofilm of wild-type but significantly reduced the biofilm mass of CRKP (mcr-1). These data suggest that the enhanced phage sensitivity associated with colistin resistance also occurs in CRKP biofilms. Alternatively, it is possible that after lysing colistinr CRKP, phages disperse from the colistinr CRKP biofilm structure more effectively than from the colistins one. In either case, colistin resistance renders the CRKP biofilms more sensitive to dispersal by phages.
FIG 5.
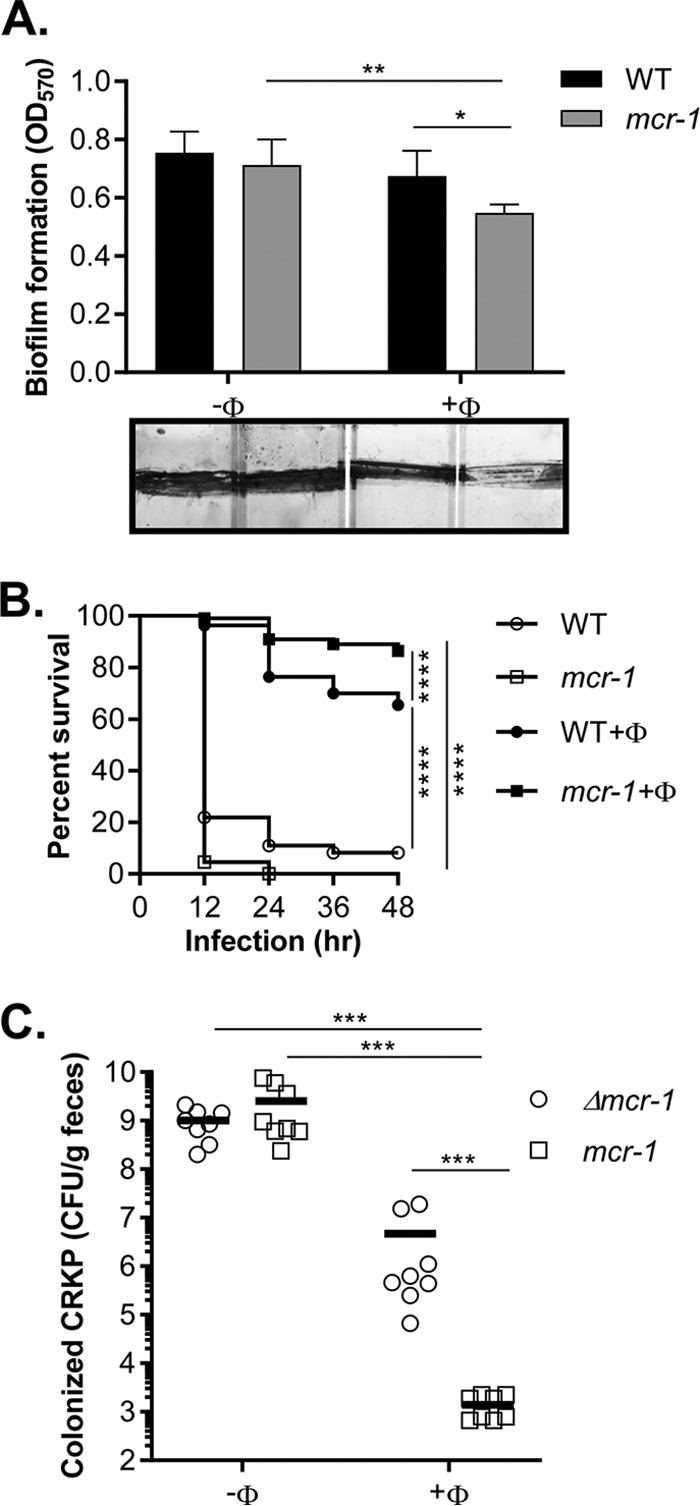
Fitness cost of colistin resistance. (A) Biofilm dispersal. CRKP or CRKP containing the mcr-1 plasmid was grown in LB at 37°C for 24 h. After planktonic cells were removed and biofilms were rinsed, 1 ml fresh LB with or without 104 PFU of cells was added and incubated at 37°C for 4 h. Biofilm mass was then quantified by using crystal violet staining as described previously (37). Means of 5 independent assays are shown, and error bars represent the standard deviations. *, P < 0.05 (2-way ANOVA); **, P < 0.01. Representative photos of crystal violet-stained biofilms are shown at the bottom of the graph. (B) Wax moth infection model. The larvae of the wax moth Galleria mellonella were infected with 10 μl (106 CFU) of WT or WT containing the mcr-1 plasmid and incubated at 37°C in the dark. After 2 h, larvae were injected with 10 μl SM buffer or SM buffer containing approximately 102 PFU NJS1 and incubated at 37°C in the dark. The survival rate was scored every 12 h. n = 110. *, P < 0.05 (Gehan-Breslow-Wilcoxon test); ***, P < 0.0002; ****, P < 0.0001. (C) Intestinal colonization. A total of 108 cells of CRKP containing the mcr-1 plasmid and Δmcr-1 plasmid were intragastrically administered at a 1:1 ratio to 5-week-old CD-1 mice treated with streptomycin. SM buffer or SM buffer containing 105 PFU of phages was then administered at 12 h. Fecal pellets were collected at day 2 postinfection, and CRKP numbers were determined. Horizontal lines represent the average CFU level of CRKP colonized from 8 mice. ***, P < 0.0005 (Mann-Whitney test).
We then used a wax moth (Galleria mellonella) larva model, which is a simple system for evaluating CRKP pathogenesis and gene expression (25). We found that CRKP with or without the mcr-1 plasmid effectively killed G. mellonella larvae in 24 h (Fig. S4; see also Fig. 5B, open symbols). However, when ФNJS1 was injected 2 h postinfection, significantly more larvae survived (Fig. 5B, closed symbols). Strikingly, the phage protected CRKP (mcr-1)-infected larvae more effectively than those infected with CRKP lacking mcr-1 (Fig. 5B), indicating that, similarly to what we found in vitro, ФNJS1 can kill colistinr CRKP more effectively than colistins CRKP in the wax moth larva infection model. Next, we tested the effect of phage treatment in a mouse colonization model. Panel C of Fig. 5 shows that without phage, CRKP (mcr-1::Kmr) and CRKP (mcr-1) colonized mouse intestines equally well. However, when ФNJS1 was fed to the mice, the number of colonized CRKP (mcr-1::Kmr) was reduced, whereas the number of CRKP (mcr-1) was reduced more dramatically than that of CRKP without mcr-1. These data strongly suggest that we can take advantage of phage sensitivity to eliminate colistinr bacteria.
Concluding remarks.
In this study, we found that the sensitivity to phage infection of colistinr CRKP was greater than that of colistins CRKP. Acquisition of colistin resistance in Gram-negative bacteria through mutations or horizontal gene transfer results in chemical modifications of the lipopolysaccharide molecules of the outer membrane. These substantial cellular changes presumably come with a fitness cost, at least for those species that do not make these modifications constitutively. Surprisingly, no major fitness cost for CRKP associated with mcr-1 in naturally occurring plasmids or with inactivation of mgrB had been reported prior to this work. However, our studies indicated that colistin resistance conferred by either mgrB deletion or mcr-1 renders CRKP more sensitive to phage infection. Although numerous groups are now exploring the utility of phage therapy to combat various pathogens, to our knowledge there have been no previous studies using phage to preferentially target the antibiotic-resistant forms of any pathogen. In addition, phage cocktails (without colistin) may be a valuable tool to combat reservoirs of colistin-resistant bacteria in agricultural settings.
MATERIALS AND METHODS
Bacterial strains and growth condition.
Carbapenem-resistant K. pneumoniae A2312NM, a clinical isolate that belongs to the serotype 11 (ST-11) serogroup K47 capsule type (wzi gene sequenced by using primers described in reference 26), was used as the wild-type strain in this study. Detailed information on this strain and other CRKP clinical isolates used in this study is provided in Table S1 in the supplemental material. The ΔmgrB mutant was obtained by spreading an A2312NM overnight culture on LB plates containing 5 μg/ml colistin. Colistin-resistant colonies were then purified, and the mgrB locus was amplified and sequenced. An isolate with a 65-bp deletion in the mgrB sequence was selected and is annotated as strain ΔmgrB. The colistin resistance plasmids containing mcr-1 or mcr-1.6 were introduced into different strains by conjugation. Briefly, cell pellets of 1-ml overnight cultures of E. coli K-12 containing either mcr-1 or mcr-1.6 plasmids were mixed with that of A2312NM and incubated on LB plates for 4 h. The transconjugants were selected by streaking the conjugation mixes on LB plates containing streptomycin and colistin. Susceptibility tests for colistin were performed by using the broth microdilution method described in reference 27. All strains were cultured in Luria-Bertani (LB) medium or on LB agar plates at 37°C, unless otherwise noted.
Plasmid construction.
Primers used in this study are listed in Table S3. The mcr-1 gene on plasmid pHNSHP45 was deleted and replaced with FRT-cat-FRT (where FRT is “flippase recognition target”) by recombineering (to produce plasmid pAC21) (28). The FRT-cat-FRT cassette was amplified from pKD3 by PCR (28) using primers mcr1_LRed_u1 and mcr1_LRed_l1. To complement the resulting deletion, the FRT-cat-FRT cassette was first replaced with FRT-Kan-FRT using pCP20 (29), producing pAC22. The FRT-Kan-FRT cassette was obtained from pKD4 by PCR (28) using primers psyn-u1 and oriR6kseqprim1. The mcr-1 gene, with its native promoter, was then amplified by PCR from pHNSHP45 using primers mcr1_IS_LRed_u1 and mcr1_IS_LRed_l1 and integrated by recombineering at a site that was different from the original location of mcr-1, giving rise to pAC23. Engineered plasmids were moved into CRKP strains by conjugation.
Phage isolation, propagation, and characterization.
Phages capable of lysing wild-type CRKP were isolated from raw sewage near different hospital sites by standard enrichment techniques and double-agar overlay plaque assays (30). To preserve the phages, plaques were picked and dispersed into SM buffer (10 mM MgSO4, 100 mM NaCl, 0.01% gelatin, 50 mM Tris-HCl, pH 7.5) and stored at 4°C. For measuring lysis activity of the phages against CRKP strains, mid-log cultures were mixed with different MOIs (multiplicities of infection) and incubated at 37°C. Samples were collected at the indicated time points, and the optical density at 600 nm (OD600) was measured. The one-step growth curves and phage adsorption rates were measured as described previously (31–33).
Transmission electron microscopy.
High-titer phage lysate was prepared accordingly to a described previously method (34) and centrifuged at 25,000 × g for 60 min. The phage pellet was washed with 0.1 M ammonium acetate (pH 7.0) and resuspended in SM buffer. The samples were then deposited on carbon-coated Formvar films and stained with 2% uranyl acetate. Microscopy was performed with a model H7650 transmission electron microscope (Hitachi, Tokyo, Japan).
Phage genome sequencing and analysis.
Extraction and purification of genomic DNA from CRKP phage suspensions were carried out using a Norgen phage DNA isolation kit (Norgen Biotek Corp., Ontario, Canada), according to the manufacturer’s protocol. DNA library preparation and whole-genome sequencing were performed at the next-generation-sequencing (NGS) core of the Department of Medicine at the University of Pennsylvania. The DNA library was prepared using an Illumina Nextera DNA library preparation kit. Whole-phage DNA was sequenced by the use of an Illumina MiSeq next-generation sequencing platform, which generated 250-bp paired-end reads. De novo assembly of short raw reads was accomplished using SPAdes 3.10.1 genome assembler software. Open reading frames (ORFs) were predicted using Glimmers, FGENES V (Softberry, Inc., USA) and GeneMarkS, and ribosomal binding sites (RBSs) were predicted using RBSfinder for confirmation of ORF predictions (J. Craig Venter Institute, San Diego, CA, USA). The predicted ORFs were annotated for specific functions using GATU software and the RAST and PHASTER programs. Comparisons of genome sequences with those of other phages were performed using the NCBI database (https://www.ncbi.nlm.nih.gov/), MAUVE software (version 2.3.1), the MUMmer3 program, and Easyfig software (version 2.1). BLASTP and PSI-BLAST searches (http://www.ebi.ac.uk/Tools/sss/fasta/) were used to determine the similarity of all putative proteins in the genome sequence.
Zeta potential measurement.
Zeta potentials were measured as described previously (35, 36) but with the following modifications. Mid-log-phase bacterial cells were collected by centrifugation, and the pellets were rinsed twice with double-distilled water (ddH2O) and resuspended in ddH2O. Zeta potential measurements were made on approximately 5 × 104 CFU/ml cells using a Zetasizer Nano ZS90 analyzer (Malvern Panalytical Ltd., United Kingdom). To determine the phage isoelectric point, phage suspensions were centrifuged (27,000 × g; 60 min; 4°C), and precipitated particles were washed twice and resuspended with ddH2O. The phage suspensions were then filtered using a 0.22-μm-pore-size membrane and stored at 4°C. Phage samples (108 PFU/ml) in the range of pH 2 to 12 with an ionic strength value of 2 μM KCl were used to examine zeta potential.
Biofilm assays.
Overnight cultures of CRKP and CRKP (mcr-1) were inoculated at 1% into 1 ml LB medium containing 0.1% porcine mucin (Sigma-Aldrich, St. Louis, MO) in glass tubes and grown at 37°C with slow shaking for 24 h. The tubes were washed four times with sterile water to remove planktonic cells. 1 ml fresh LB with or without 104 PFU of ФNJS1 was then added, and the tubes were incubated at 37°C for 4 h. Biofilms were then rinsed and stained with 0.1% crystal violet and imaged (37). (All four tubes were photographed at the same time, and the image was subsequently cropped.) To quantify the biofilms, crystal violet was dissolved with 1 ml dimethyl sulfoxide (DMSO), and OD570 was measured.
Infection of G. mellonella larvae.
The larvae of the Galleria mellonella wax moth were used to assess pathogenesis of CRKP as described previously (25) with modifications. After surface disinfection was performed using ethanol (70% [vol/vol]), the last right proleg of each larva was injected with 10 μl (106 CFU) of wild-type or CRKP (mcr-1) resuspended in phosphate-buffered saline (PBS) by use of a Hamilton syringe with a 50-gauge needle. After 2 h, larvae were injected with 10 μl SM buffer or SM buffer containing approximately 102 PFU ФNJS1 and incubated at 37°C in the dark. Larvae were scored as dead (not responding to physical stimuli) or alive at 12-h intervals. For each group, 20 or 30 larvae were used and five independent experiments were performed.
CRKP colonization of adult mice.
All animal experiments were carried out in strict accordance with animal protocols that were approved by the Ethical Committee of Animal Experiments of Nanjing Agricultural University [permit number SYXK (Su) 2017-0007]. All efforts were made to minimize animal suffering. Euthanasia was performed by CO2 inhalation.
CD-1 mice (5 weeks of age) were provided drinking water with 0.5% (wt/vol) streptomycin and 0.5% aspartame throughout the experiment. One day after the streptomycin treatment, mixtures of CRKP (Δmcr-1) and CRKP (mcr-1) (1:1) (approximately 108 CFU) were intragastrically administered to mice. After 12 h of incubation, 50 μl SM buffer or SM buffer containing 105 PFU of ФNJS1 was inoculated into mice intragastrically. Fecal pellets were collected on day 2 postinfection, and the colonized CRKP bacteria were enumerated by serial dilution and plating on selective LB agar plates containing either 5 μg/ml colistin or 50 μg/ml kanamycin. Phage titers in fecal samples were determined by double-agar-overlay plaque assays.
Data availability.
The complete genome sequences of phage NJS1, NJS2, NJS3, TAH8, and NJR15 were deposited in the NCBI database under the GenBank accession numbers MH445453, MH633485, MH633486, MH633484, and MH633487, respectively.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ge Liu, Yuanqi Jia, and Xia Chen for technical support.
This study was supported by an NSFJ grant (SBK2019022717 to H.W.), the Priority Project on Infectious Disease Control and Prevention (2018ZX10714-002 to B.K.), and NIH grants AI125814 (to M.G. and J.Z.) and AI120489 (to J.Z.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01609-19.
REFERENCES
- 1.Rojas LJ, Salim M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Marshall S, Rudin SD, Domitrovic TN, Hujer AM, Hujer KM, Doi Y, Kaye KS, Evans S, Fowler VG, Bonomo RA, van Duin D, Antibacterial Resistance Leadership Group. 2017. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: laboratory detection and impact on mortality. Clin Infect Dis 64:711–718. doi: 10.1093/cid/ciw805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin RM, Bachman MA. 2018. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol 8:4. doi: 10.3389/fcimb.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zavascki AP, Goldani LZ, Li J, Nation RL. 2007. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother 60:1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- 4.Olaitan AO, Morand S, Rolain JM. 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannatelli A, D'Andrea MM, Giani T, Di Pilato V, Arena F, Ambretti S, Gaibani P, Rossolini GM. 2013. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob Agents Chemother 57:5521–5526. doi: 10.1128/AAC.01480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron S, Hadjadj L, Rolain JM, Olaitan AO. 2016. Molecular mechanisms of polymyxin resistance: knowns and unknowns. Int J Antimicrob Agents 48:583–591. doi: 10.1016/j.ijantimicag.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Lippa AM, Goulian M. 2009. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet 5:e1000788. doi: 10.1371/journal.pgen.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L-F, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J-H, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Zhang H, Liu YH, Feng Y. 7 March 2018, posting date Towards understanding MCR-like colistin resistance. Trends Microbiol doi: 10.1016/j.tim.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Yang YQ, Li YX, Lei CW, Zhang AY, Wang HN. 2018. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother 73:1791–1795. doi: 10.1093/jac/dky111. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Wang Y, Zhou Y, Li J, Yin W, Wang S, Zhang S, Shen J, Shen Z, Wang Y. 2018. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect 7:122. doi: 10.1038/s41426-018-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO, Wiedmann M. 2019. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype Typhimurium Isolate. mBio 10:e00853-19. doi: 10.1128/mBio.00853-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannatelli A, Santos-Lopez A, Giani T, Gonzalez-Zorn B, Rossolini GM. 2015. Polymyxin resistance caused by mgrB inactivation is not associated with significant biological cost in Klebsiella pneumoniae. Antimicrob Agents Chemother 59:2898–2900. doi: 10.1128/AAC.04998-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Q, Li M, Spiller OB, Andrey DO, Hinchliffe P, Li H, MacLean C, Niumsup P, Powell L, Pritchard M, Papkou A, Shen Y, Portal E, Sands K, Spencer J, Tansawai U, Thomas D, Wang S, Wang Y, Shen J, Walsh T. 2017. Balancing mcr-1 expression and bacterial survival is a delicate equilibrium between essential cellular defence mechanisms. Nat Commun 8:2054. doi: 10.1038/s41467-017-02149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viertel TM, Ritter K, Horz HP. 2014. Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J Antimicrob Chemother 69:2326–2336. doi: 10.1093/jac/dku173. [DOI] [PubMed] [Google Scholar]
- 16.Kortright KE, Chan BK, Koff JL, Turner PE. 2019. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. doi: 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Chen D, Zhou H. 2018. Study of the population structure and drug resistance mechanism of carbapenem-resistant Klebsiella pneumoniae. Chin J Antibiot 43:507–512. doi: 10.13461/j.cnki.cja.006245. [DOI] [Google Scholar]
- 18.Lu X, Hu Y, Luo M, Zhou H, Wang X, Du Y, Li Z, Xu J, Zhu B, Xu X, Kan B. 2017. MCR-1.6, a new MCR variant carried by an IncP plasmid in a colistin-resistant Salmonella enterica serovar Typhimurium isolate from a healthy individual. Antimicrob Agents Chemother 61:e02632-16. doi: 10.1128/AAC.02632-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michen B, Graule T. 2010. Isoelectric points of viruses. J Appl Microbiol 109:388–397. doi: 10.1111/j.1365-2672.2010.04663.x. [DOI] [PubMed] [Google Scholar]
- 20.Soon RL, Nation RL, Cockram S, Moffatt JH, Harper M, Adler B, Boyce JD, Larson I, Li J. 2011. Different surface charge of colistin-susceptible and -resistant Acinetobacter baumannii cells measured with zeta potential as a function of growth phase and colistin treatment. J Antimicrob Chemother 66:126–133. doi: 10.1093/jac/dkq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouhara K, Komatsuzawa H, Yamada S, Shiba H, Fujiwara T, Ohara M, Sayama K, Hashimoto K, Kurihara H, Sugai M. 2005. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, {beta}-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother 55:888–896. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- 22.Cisek AA, Dąbrowska I, Gregorczyk KP, Wyżewski Z. 2017. Phage therapy in bacterial infections treatment: one hundred years after the discovery of bacteriophages. Curr Microbiol 74:277–283. doi: 10.1007/s00284-016-1166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills S, Ross RP, Hill C. 2017. Bacteriocins and bacteriophage; a narrow-minded approach to food and gut microbiology. FEMS Microbiol Rev 41:S129–S153. doi: 10.1093/femsre/fux022. [DOI] [PubMed] [Google Scholar]
- 24.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 25.Insua JL, Llobet E, Moranta D, Perez-Gutierrez C, Tomas A, Garmendia J, Bengoechea JA. 2013. Modeling Klebsiella pneumoniae pathogenesis by infection of the wax moth Galleria mellonella. Infect Immun 81:3552–3565. doi: 10.1128/IAI.00391-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brisse S, Passet V, Haugaard AB, Babosan A, Kassis-Chikhani N, Struve C, Decré D. 2013. wzi gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol 51:4073–4078. doi: 10.1128/JCM.01924-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chew KL, La MV, Lin RTP, Teo J. 2017. Colistin and polymyxin B susceptibility testing for carbapenem-resistant and mcr-positive Enterobacteriaceae: comparison of Sensititre, MicroScan, Vitek 2, and Etest with broth microdilution. J Clin Microbiol 55:2609–2616. doi: 10.1128/JCM.00268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. doi: 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 30.Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP. 2009. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol Biol 501:69–76. doi: 10.1007/978-1-60327-164-6_7. [DOI] [PubMed] [Google Scholar]
- 31.Pajunen M, Kiljunen S, Skurnik M. 2000. Bacteriophage phiYeO3-12, specific for Yersinia enterocolitica serotype O:3, is related to coliphages T3 and T7. J Bacteriol 182:5114–5120. doi: 10.1128/jb.182.18.5114-5120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sillankorva S, Neubauer P, Azeredo J. 2008. Isolation and characterization of a T7-like lytic phage for Pseudomonas fluorescens. BMC Biotechnol 8:80. doi: 10.1186/1472-6750-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drulis-Kawa Z, Mackiewicz P, Kęsik-Szeloch A, Maciaszczyk-Dziubinska E, Weber-Dąbrowska B, Dorotkiewicz-Jach A, Augustyniak D, Majkowska-Skrobek G, Bocer T, Empel J, Kropinski AM. 2011. Isolation and characterisation of KP34–a novel phiKMV-like bacteriophage for Klebsiella pneumoniae. Appl Microbiol Biotechnol 90:1333–1345. doi: 10.1007/s00253-011-3149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drulis-Kawa Z, Olszak T, Danis K, Majkowska-Skrobek G, Ackermann HW. 2014. A giant Pseudomonas phage from Poland. Arch Virol 159:567–572. doi: 10.1007/s00705-013-1844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esposito F, Fernandes MR, Lopes R, Munoz M, Sabino CP, Cunha MP, Silva KC, Cayo R, Martins W, Moreno AM, Knobl T, Gales AC, Lincopan N. 2017. Detection of colistin-resistant MCR-1-positive Escherichia coli by use of assays based on inhibition by EDTA and zeta potential. J Clin Microbiol 55:3454–3465. doi: 10.1128/JCM.00835-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dika C, Duval JF, Francius G, Perrin A, Gantzer C. 2015. Isoelectric point is an inadequate descriptor of MS2, Phi X 174 and PRD1 phages adhesion on abiotic surfaces. J Colloid Interface Sci 446:327–334. doi: 10.1016/j.jcis.2014.08.055. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Mekalanos JJ. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell 5:647–656. doi: 10.1016/S1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete genome sequences of phage NJS1, NJS2, NJS3, TAH8, and NJR15 were deposited in the NCBI database under the GenBank accession numbers MH445453, MH633485, MH633486, MH633484, and MH633487, respectively.


