Abstract
Ring-opening reaction via selective cleavage of C–C bond is known as a powerful strategy for construction of complex molecules. Complementary to the ionic process focusing on mostly small ring systems, radical-mediated C–C bond cleavage offers a solution for further diverse enantioselective functionalization benefited from its mild conditions, whereas such asymmetric transformations are still limited to three-membered rings so far. Herein, we describe radical-mediated ring-opening and enantioselective cyanation of four- and five-membered cycloketone oxime esters to access chiral 1,5- and 1,6-dinitriles. Employment of dual photoredox/copper catalysis is essential for the asymmetric ring-opening cyanation of cyclopentanone oxime esters. Both reactions proceed under mild conditions giving chiral dinitriles in high yields and enantioselectivity with low catalyst loading and broad substrate scope. The products dinitriles can be converted to valuable optically active diamides and diamines. Mechanistic studies indicate that the benzylic radical generated via C–C single bond cleavage is involved in the catalytic cycle.
Subject terms: Stereochemistry, Synthetic chemistry methodology
Ring-opening reaction via selective cleavage of C–C bond is a powerful strategy to increase molecular complexity. Here the authors present the facile synthesis of chiral 1,5- and 1,6-dinitriles via the radical-mediated ring-opening and enantioselective cyanation of cycloketone oxime esters.
Introduction
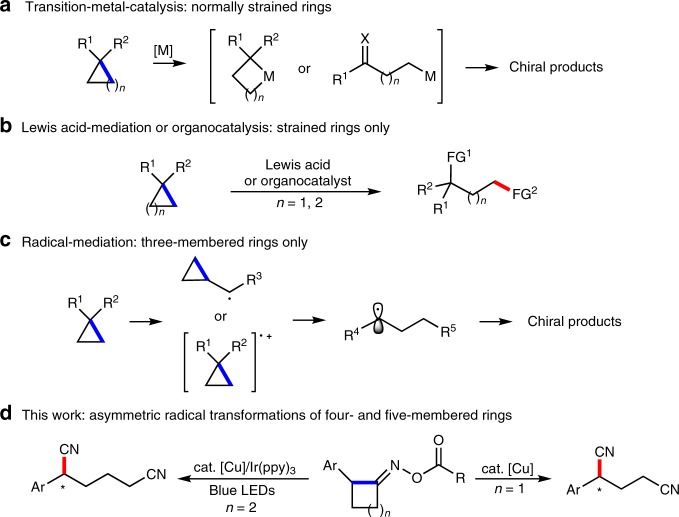
Synthesis of complex natural products or functional molecules in an efficient and quick manner always represents a major challenge in organic chemistry. C–C single bond is basic element and linkage in organic molecules, selective cleavage and functionalization of which is of great significance and provides a straightforward and atom-economical method to access target molecule1–6. Indeed, ring-opening reaction via selective cleavage of C–C bond has long been used as a powerful strategy in retrosynthetic analysis to build up complexity of a molecule in a single step7–10. Due to the thermodynamic stability of C–C single bond, high temperature is usually required to enhance the reactivity, which is normally considered unfavorable to achieve high enantioselectivity. Thus, asymmetric reactions induced by ring-opening C–C bond cleavage still remain challenging since there is requirement of a balanced relationship between selectivity and reactivity in such transformations11. To date, the known asymmetric reactions via ring-opening C–C bond cleavage are mainly studied in the field of transition-metal catalysis12–19 (Fig. 1a), Lewis acid catalysis20–23, and organocatalysis24–27 (Fig. 1b). However, most of these reactions via ionic pathway focused on C–C bond cleavage of small ring systems (3 or 4-membered rings), in which intrinsic strain release is exploited as a major driving force. Complementary to the ionic processes, radical-mediated C–C bond cleavage offers an efficient pathway for further asymmetric functionalization due to the mild conditions and reaction diversity28–31. However, such asymmetric reactions involving radical-promoted ring-opening C–C bond cleavage are still limited to three-membered rings so far (Fig. 1c)32–38.
Fig. 1.
Enantioselective functionalization via ring-opening C−C bond cleavage. a Transition-metal-catalysis: normally strained rings. b Lewis acid-mediation or organocatalysis: strained rings only. c Radical-mediation: three-membered rings only. d This work: asymmetric radical transformations of four- and five-membered rings.
Dinitriles, known as synthetically useful motifs in many pharmaceuticals or biologically active compounds39–44, are also widely used in polymer materials, enzyme catalysis and organic synthesis45–50. For instance, the most widely used dinitrile, adiponitrile, serves as a key precursor to produce the polymer nylon-66, which is used as fibers for clothing, tire cords, carpets, conveyor belts, and brushes due to its excellent dyeability and durability51. Despite the widespread application, the efficient synthesis of chiral dinitriles is still underdeveloped and remains as a big challenge. To our best knowledge, the only catalytic method to make chiral dinitriles is the asymmetric addition of cyano-containing building blocks to α,β-unsaturated nitriles52–55. According to the pioneering discoveries of Zard56,57 and recent works from other groups58,59, iminyl radical-mediated ring-opening of cyclic oxime derivatives is an efficient pathway to cleave C–C single bond via β-scission. As such transformation generates cyanoalkyl radical in situ, we envision this radical could be further trapped by chiral transition-metal catalyst, followed by reductive elimination from the metal cyanide complex to furnish corresponding optically active dinitrile60–66.
Herein, we report enantioselective ring-opening cyanation for facile synthesis of chiral 1,5- and 1,6-dinitriles (Fig. 1d). It is worthy of note that C–C single bond cleavage and asymmetric radical cyanation of less strained 5-membered cycloketone oxime ester can be expediently achieved by merging photoredox catalysis with copper catalysis. Under mild conditions, both asymmetric transformations demonstrate high catalytic reactivity, excellent enantioselectivity, low catalyst loading and broad substrate scope. The key to success is the capture of the cyanoalkyl radical in situ generated via ring-opening C–C cleavage with chiral copper catalyst resulting in an enantioselective cyanation through stereoselective reductive elimination.
Results
Optimization of the asymmetric ring-opening cyanation
Our studies commenced with cyclopentanone oxime ester 1a used as the pilot substrate, and trimethylsilyl cyanide (TMSCN) as the coupling partner in the presence of a catalytic amount of Cu(MeCN)4PF6 (10 mol%) at room temperature. To our delight, the desired ring-opening cyanation product 2a was afforded with 91% ee when chiral bis(oxazoline) L1 was used as the ligand, albeit in a rather low yield (23%, entry 1, Table 1). Considering high energy requirement of ring-opening due to the thermodynamic stability of C–C single bond in less strained five-membered ring, we elevated the temperature to 50 °C to facilitate the process, and the yield was improved markedly to 63%, however expectedly, with a relatively lower ee (88%, entry 2). While decreasing catalyst loading resulted in significant recovery of 1a, the increase of copper loading could not further improve the yield because of the side reactions involved at this temperature (entries 3–4). To further enhance the reactivity, photoredox catalysis was merged with copper catalytic system to provide extra energy. We reckoned that such system could react at lower temperature to inhibit side reactions and simultaneously improve the enantioselectivity. As expected, addition of Ir(ppy)3 as the photocatalyst under the irradiation of 5 W blue LEDs at room temperature could raise the yield to 71% with 91% ee, and the copper catalyst loading could be reduced to only 2 mol% (entry 5). Moreover, a careful solvent screening (entries 5–11) indicated DMAc afforded a slighter higher yield (73%) and ee (92%, entry 11). Additionally, further screening of bis(oxazoline) ligands revealed that L1 was the optimal ligand for this asymmetric transformation (entries 11–16). To our satisfaction, the yield could be further improved to 78% when the amount of TMSCN was reduced to 1.5 equiv (entry 17). Finally, the control experiments showed only trace amount of the desired dinitrile 2a was obtained in the absence of light, Ir(ppy)3 or Cu(MeCN)4PF6 (entries 18–20), which clearly indicated that photoredox catalytic system and copper catalysis were both essential for this highly efficient transformation.
Table 1.
Optimization of reaction conditionsa.
 | |||||
|---|---|---|---|---|---|
| entry | [Cu]/mol% | ligand | Solvent | T/oC | Yield (ee)/% |
| 1b,c | 10 | L1 | DCM | RT | 23 (91) |
| 2b,c | 10 | L1 | DCM | 50 | 63 (88) |
| 3c,d | 5 | L1 | DCM | 50 | 55 (90) |
| 4c,e | 15 | L1 | DCM | 50 | 58 (88) |
| 5 | 2 | L1 | DCM | RT | 71 (91) |
| 6 | 2 | L1 | CH3CN | RT | 70 (91) |
| 7 | 2 | L1 | Acetone | RT | 24 (93) |
| 8 | 2 | L1 | Benzene | RT | 81 (87) |
| 9 | 2 | L1 | PhCl | RT | 80 (87) |
| 10 | 2 | L1 | DMF | RT | 73 (91) |
| 11 | 2 | L1 | DMAc | RT | 73 (92) |
| 12 | 2 | L2 | DMAc | RT | 68 (88) |
| 13 | 2 | L3 | DMAc | RT | 70 (86) |
| 14 | 2 | L4 | DMAc | RT | 58 (75) |
| 15 | 2 | L5 | DMAc | RT | 40 (73) |
| 16 | 2 | L6 | DMAc | RT | 56 (-43) |
| 17f | 2 | L1 | DMAc | RT | 78 (92) |
| 18g | 2 | L1 | DMAc | RT | 12 (90) |
| 19h | 2 | L1 | DMAc | RT | 9 (91) |
| 20i | 0 | L1 | DMAc | RT | 14 (0) |
Ir(ppy)3 Tris(2-phenylpyridine)iridium, DCM Dichloromethane, DMF N,N-Dimethylformamide, DMAc N,N-Dimethylacetamide, RT room temperature
aReaction conditions: 1a (0.10 mmol), TMSCN (3 equiv), Cu(MeCN)4PF6 (2 mol%), ligand (3 mol%), Ir(ppy)3 (0.5 mol%) in solvent (1.0 mL) at RT for 36 h under the irradiation of 5 W blue LEDs
bCu(MeCN)4PF6 (10 mol%), L1 (12 mol%)
c24 h
dCu(MeCN)4PF6 (5 mol%), L1 (6 mol%)
eCu(MeCN)4PF6 (15 mol%), L1 (18 mol%)
fTMSCN (1.5 equiv)
gIn dark
hNo Ir(ppy)3
iNo Cu(MeCN)4PF6
Substrate scope of the cyclopentanone oxime esters 1
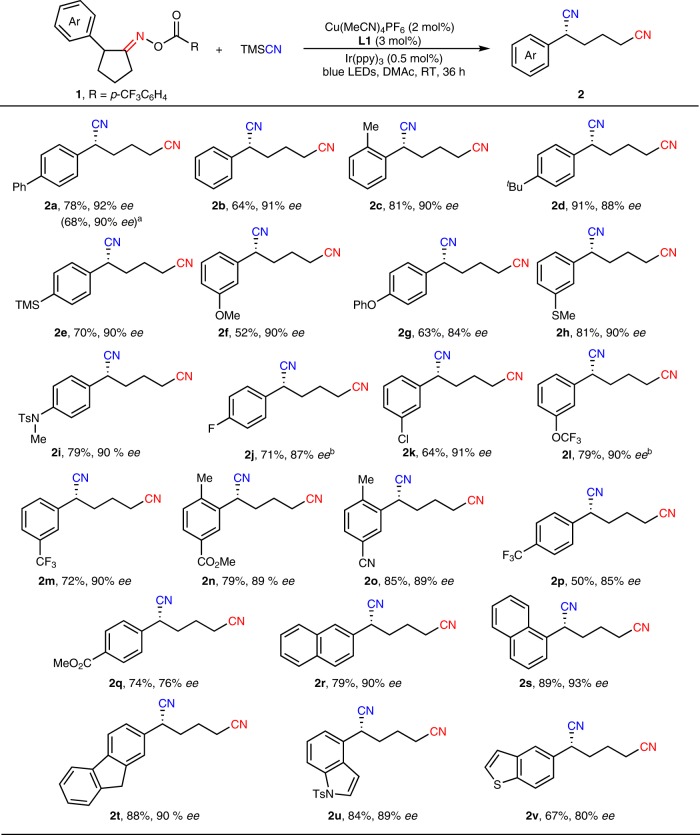
With the optimized conditions in hand, we next started to expand the substrate scope of this asymmetric ring-opening cyanation. As shown in Fig. 2, a variety of cyclopentanone oxime esters 1 installed with various substituted aromatic rings were compatible with this protocol, affording chiral 2-arylhexanedinitriles 2 in good to excellent yields and enantioselectivity. The substituent effect of the aryl rings on oxime esters 1 was first investigated. A number of all carbon five-membered rings bearing a scope of para-, meta- as well as ortho-substituted aryl rings were smoothly opened for asymmetric cyanation in high enantioselectivity (84–92% ee). Meanwhile, both electron-donating groups such as phenyl (1a), methyl (1c), tert-butyl (1d), trimethylsilyl (1e), methoxy (1f), phenoxy (1g), methylthio (1h), amino (1i), and electron-withdrawing groups such as fluoro (1j), chloro (1k), trifluoromethoxy (1l), trifluoromethyl (1m, 1p), ester (1n, 1q), cyano (1o) were all tolerated under this mild catalytic system. Of note is that the tolerance of halogens (2j, 2k) enabled further synthetic elaboration of such chiral dinitriles via known transition-metal-catalyzed cross-coupling reactions. To our satisfaction, such asymmetric ring-opening cyanation reactions of 2-naphthyl, 1-naphthyl, fluorenyl-derived substrates also proceeded smoothly to give the desired products 2r, 2s, 2t with good yields and high ee values. Remarkably, the cyclopentanone oxime esters bearing heteroarenes, including indolyl (1u) and benzothiophenyl (1v), were also compatible with this catalytic system, albeit in slightly lower yields.
Fig. 2.
Substrate scope of the cyclopentanone oxime esters 1. Reaction conditions: 1 (0.10 mmol), TMSCN (1.5 equiv), Cu(MeCN)4PF6 (2 mol%), L1 (3 mol%), Ir(ppy)3 (0.5 mol%) in DMAc (1.0 mL) at RT for 36 h under the irradiation of 5 W blue LEDs. a1.0 mmol scale. bCu(MeCN)4PF6 (1.5 mol%), L1 (2.25 mol%). TMS Trimethylsilyl. Ts p-Methyl benzenesulfonyl.
Substrate scope of the cyclobutanone oxime esters 3
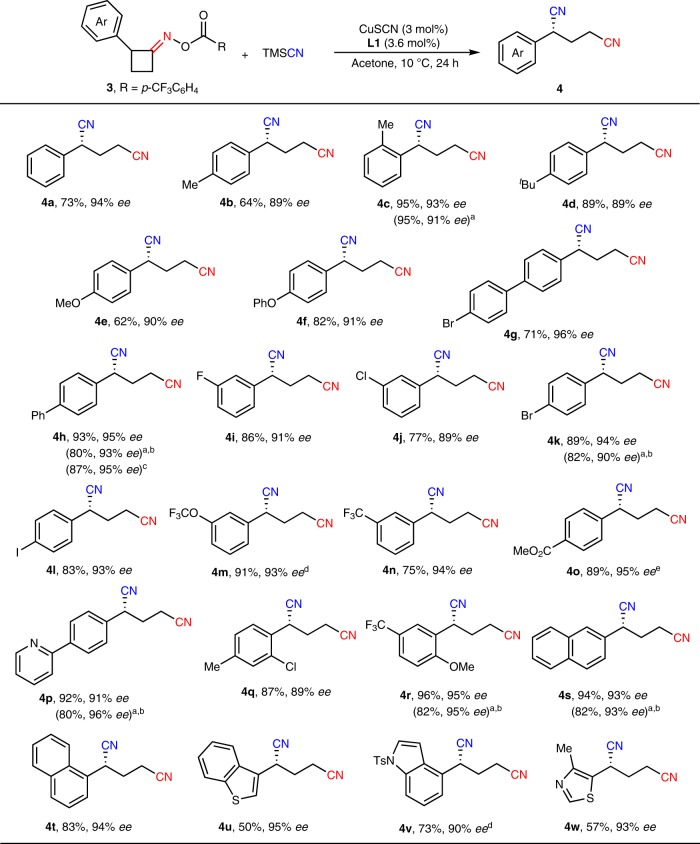
After the photoredox and copper-catalyzed ring-opening cyanation of cyclopentanone oxime esters has been established, we next set out to explore the transformations of cyclobutanone derivatives for enantioselective synthesis of 2-arylpentanedinitriles. After careful screening of reaction conditions (Supplementary Tables 4–6), as expected, the asymmetric ring-opening cyanation of oxime ester 3 could be accessed by copper catalysis alone, presumably because higher inclination of strain release of four-membered ring provided major driving force for C–C single bond cleavage. Since the reaction could be performed under even lower temperature (10 °C), the enantioselectivity was further improved (up to 96% ee), and more active substituents could be well tolerated in this transformation. As shown in Fig. 3, not only relatively inactive halides including F and Cl, but also more active halides including Br and I were compatible with this catalytic reaction, which clearly broaden synthetic potential of such chiral dinitriles for further transformations (3i–3l). Additionally, both electron-rich and electron-deficient substituents were well compatible with this reaction system (3a–3h, 3m–3r). Notably, cyclobutanone oxime esters installed with fused rings including 1-naphthyl and 2-naphthyl, as well as heteroarenes including benzothiophenyl, indolyl and thiazolyl were also suitable in this asymmetric reaction to give corresponding products with good results (3s-3w). To our delight, the reactions proceeded smoothly with good efficiency and excellent enantioselectivity even if copper catalyst loading was decreased to 1 mol% (4c, 4h, 4k, 4p, 4r, and 4s). The absolute configuration of 4g was determined to be R by X-ray crystallography.
Fig. 3.
Substrate scope of the cyclobutanone oxime esters 3. Reaction conditions: 3 (0.10 mmol), TMSCN (1.5 equiv), CuSCN (3 mol%), L1 (3.6 mol%) in acetone (1.0 mL) at 10 °C for 24 h. aCuSCN (1 mol%), L1 (1.2 mol%). b48 h. c1.0 mmol scale. dCuSCN (5 mol%), L1 (6 mol%). eee of the crude product.
Synthetic applications
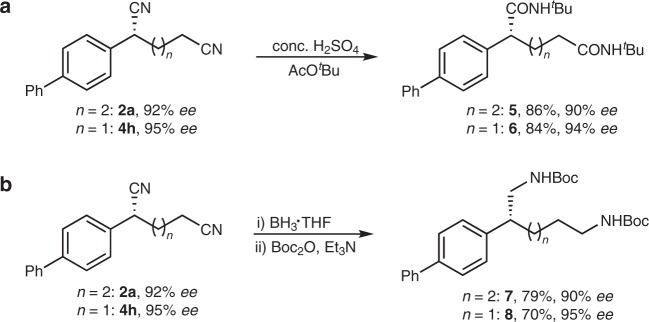
To demonstrate the utility of this methodology, further derivatizations of the chiral dinitriles were next carried out (Fig. 4). Firstly, treatment of the chiral dinitriles 2a and 4h with concentrated sulphuric acid in AcOtBu, known as a modified Ritter reaction, afforded the corresponding N-tert-butyl diamides 5 and 6, respectively, in good yields (86%, 84%) with excellent enantioselectivity (90% ee, 94% ee)67. Moreover, reduction of chiral 2a and 4h by BH3-THF complex followed by treatment with (Boc)2O furnished Boc-protected diamines 7 and 8, respectively, in good yields (79%, 70%) without obvious loss of enantioselectivity65.
Fig. 4.
Synthetic applications. a 2a or 4 h was converted to chiral diamides. b 2a or 4 h was converted to chiral diamines. Boc2O Di-tert-butyl decarbonate.
Mechanistic studies
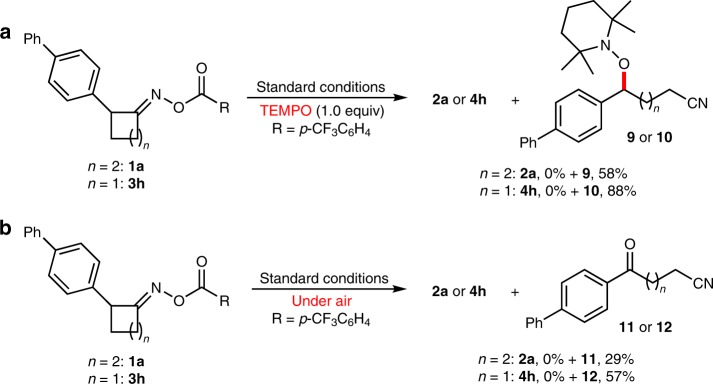
To gain some insights into mechanism of the reactions, several control experiments were conducted (Fig. 5). Firstly, the subjection of 1.0 equiv of TEMPO to the standard conditions for 1a and 3h could completely inhibited the desired reactions, and the corresponding TEMPO-trapped products 9 and 10 were isolated in 58 and 88% yields, respectively. Meanwhile, when the reactions of 1a and 3h were carried out under air atmosphere, both gave only cyano-containing ketones 11 and 12, respectively, (29 and 57% yields) and no desired dinitriles was obtained. All these results clearly indicated that benzylic radicals were involved in both catalytic cycles.
Fig. 5.
Preliminary mechanistic studies. a Radical trapping experiment with TEMPO. b Radical trapping experiment with O2. TEMPO 2,2,6,6-Tetramethylpiperidinooxy.
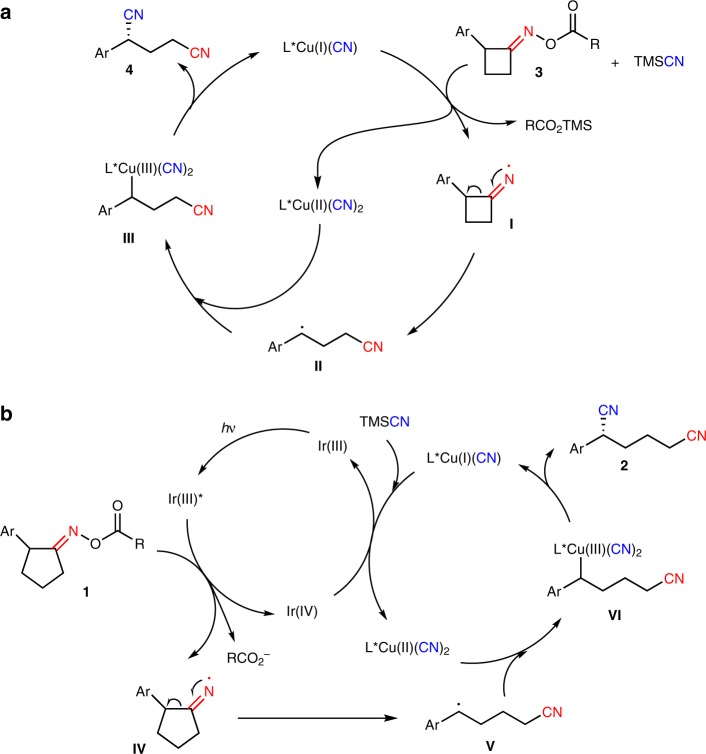
Based on the above observations and previous reports60–66,68,69, the proposed mechanisms of two catalytic processes were depicted in Fig. 6. As for cyclobutanone oxime esters 3, initially, substrates 3 would undergo SET (single electron transfer) with L*Cu(I)(CN) in the presence of TMSCN affording L*Cu(II)(CN)2 and iminyl radical I, which generated benzylic radical II via a radical-mediated C–C bond cleavage. The capture of radical II by L*Cu(II)(CN)2 to give Cu(III) species III, followed by reductive elimination furnished dinitriles 4 and regenerated the catalyst L*Cu(I)(CN) (Cycle a, Fig. 6). Meanwhile, as for cyclopentanone oxime esters 1, the catalytic cycle regarding a dual photoredox/copper catalysis was described as cycle b. Firstly, the SET between oxime esters 1 and the excited state of photocatalyst Ir(III)* provided iminyl radical IV under irradiation of blue LEDs, and then the resulting Ir(IV) species oxidized L*Cu(I)(CN) to L*Cu(II)(CN)2 with participation of TMSCN. The benzylic radical V, which was generated by the C–C bond cleavage of iminyl radical IV, could be trapped by L*Cu(II)(CN)2 to deliver Cu(III) species VI. Final reductive elimination of intermediate VI would give desired products 2 and regenerate the Cu(I) catalyst. Definitely, the catalytic process enabled by single copper catalysis could not be excluded in the ring-opening reaction of cyclopentanone oxime esters (entries 18–19, Table 1). Of note is that the Cu(I)/Cu(II) catalytic cycle involving direct cyano group transfer from chiral copper cyanide could not be excluded for both catalytic processes at this stage.
Fig. 6.
Proposed mechanisms. a copper catalysis. b a dual photoredox/copper catalysis.
Discussion
In conclusion, we have developed an enantioselective ring-opening cyanation of cycloketone oxime esters for facile access to chiral 1,5- and 1,6-dinitriles. Specifically, C–C single bonds in less strained 5-membered cycloketone oxime esters can be cleaved for asymmetric cyanation via a radical pathway by combining photoredox and copper catalysis. And, as for strained cyclobutanone oxime esters, asymmetric ring-opening cyanation can be realized by single copper catalysis. Both reactions feature mild conditions, high catalytic reactivity, excellent enantioselectivity, low catalyst loading and broad substrate scope. This radical relay strategy offers a solution for remote functionalization by ring-opening C–C single bonds cleavage, and provides an efficient approach for rapid formation of chiral dinitriles. The synthetic utility of the protocols has been demonstrated by further transformations of the chiral dinitriles to diamides and diamines without obvious loss of enantioselectivity. Further exploration on enantioselective remote difunctionalization via C–C single bonds cleavage are currently ongoing in our laboratory.
Methods
Procedure for the asymmetric ring-opening cyanation of oxime esters 1
To a 25 mL Schlenk tube, Cu(CH3CN)4PF6 (3.7 mg, 0.01 mmol), chiral bisoxazoline ligand L1 (5.3 mg, 0.015 mmol), Ir(ppy)3 (1.6 mg, 0.0025 mmol) were added in super dry DMAc (5 mL) under N2 atmosphere. The tube was sealed with a Teflon-lined cap, then the mixture was stirred at room temperature for 0.5 h. The catalyst solution I was prepared firstly and used in the next step.
To a 25 mL Schlenk tube containing substrate 1 (0.1 mmol, 1.0 equiv), catalyst solution I (1 mL) and TMSCN (0.15 mmol, 1.5 equiv) were sequentially added under N2 atmosphere. The tube was sealed with a Teflon-lined cap, and the mixture was stirred at a distance of ~5 cm from a 5 W blue LEDs at room temperature for 36 h.The reaction mixture was diluted with ethyl acetate (10 mL). The organic layer was washed with brine (3 × 5 mL) and dried over anhydrous Na2SO4. After filtration and concentration, the residue was purified by silica gel chromatography with petroleum ether and ethyl acetate (PE/EA = 9:1~3:1) to afford the product 2.
Procedure for the asymmetric ring-opening cyanation of oxime esters 3
To a 5 mL Schlenk tube, CuSCN (1.8 mg, 0.015 mmol), chiral bisoxazoline ligand L1 (6.4 mg, 0.018 mmol) were added in degassed acetone (1 mL) under N2 atmosphere. The tube was sealed with a Teflon-lined cap, then the mixture was stirred at room temperature for 0.5 h. The catalyst solution II was prepared firstly and used in the next step.
To a 25 mL Schlenk tube containing substrate 3 (0.1 mmol, 1.0 equiv), 0.8 mL acetone, catalyst solution II (200 uL) and TMSCN (0.15 mmol, 1.5 equiv) were sequentially added under N2 atmosphere. The tube was sealed with a Teflon-lined cap, and the mixture was stirred at 10 °C for 24 h.Then solvent was removed under vacuum and the residue was purified by silica gel chromatography with petroleum ether and ethyl acetate (PE/EA = 10:1~5:1) to afford the product 4.
Supplementary information
Acknowledgements
We gratefully acknowledge the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB20000000), the National Basic Research Program of China (973 Program 2015CB856600), the National Science Foundation of China (21971228, 21772187, 21522208) for financial support.
Author contributions
T.W. designed and performed the experiments. Y.-N.W., R.W., B.-C.Z., C.Y., and Y.-L.L. helped to complete the experiments. X.-S.W. directed the project and wrote the manuscript. All authors interpreted the results on the manuscript.
Data availability
The authors declare that all the data supporting the findings of this research are available within the article and its supplementary information. The characterization data (Supplementary Figs. 1–268), the optimization of reaction conditions (Supplementary Tables 1–6) and the crystallography data (Supplementary Tables 7–12, Supplementary Fig. 269) are available within Supplementary Information. The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 1911616 (4g) and can be obtained free of charge from www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Junkai Fu, Hua-Jian Hua-Jian and Yiyuan Peng for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-13369-x.
References
- 1.Jun C-H. Transition metal-catalyzed carbon–carbon bond activation. Chem. Soc. Rev. 2004;33:610–618. doi: 10.1039/B308864M. [DOI] [PubMed] [Google Scholar]
- 2.Dong, G. C–C bond activation 346 (Springer-Verlag, Berlin, 2014).
- 3.Chen F, Wang T, Jiao N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon−carbon bonds. Chem. Rev. 2014;114:8613–8661. doi: 10.1021/cr400628s. [DOI] [PubMed] [Google Scholar]
- 4.Souillart L, Cramer N. catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 2015;115:9410–9464. doi: 10.1021/acs.chemrev.5b00138. [DOI] [PubMed] [Google Scholar]
- 5.Murakami M, Ishida N. Potential of metal-catalyzed C–C single bond cleavage for organic synthesis. J. Am. Chem. Soc. 2016;138:13759–13769. doi: 10.1021/jacs.6b01656. [DOI] [PubMed] [Google Scholar]
- 6.Kim D-S, Park W-J, Jun C-H. Metal−organic cooperative catalysis in C–H and C–C bond activation. Chem. Rev. 2017;117:8977–9015. doi: 10.1021/acs.chemrev.6b00554. [DOI] [PubMed] [Google Scholar]
- 7.Seiser T, Saget T, Tran DN, Cramer N. Cyclobutanes in catalysis. Angew. Chem. Int. Ed. 2011;50:7740–7752. doi: 10.1002/anie.201101053. [DOI] [PubMed] [Google Scholar]
- 8.Mack DJ, Njardarson JT. Recent advances in the metal-catalyzed ring expansions of three- and four-membered rings. ACS Catal. 2013;3:272–286. doi: 10.1021/cs300771d. [DOI] [Google Scholar]
- 9.Rosa D, Nikolaev A, Nithiy N, Orellana A. Palladium-catalyzed cross-coupling reactions of cyclopropanols. Synlett. 2015;26:441–448. doi: 10.1055/s-0034-1380156. [DOI] [Google Scholar]
- 10.Fumagalli G, Stanton S, Bower JF. Recent methodologies that exploit C–C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 2017;117:9404–9432. doi: 10.1021/acs.chemrev.6b00599. [DOI] [PubMed] [Google Scholar]
- 11.Souillart, L., Parker, E., Cramer, N. In C–C Bond Activation (ed. Dong, G.) 163–194 (Springer, Berlin Heidelberg, 2014).
- 12.Matsuda T, Shigeno M, Makino M, Murakami M. Enantioselective C–C bond cleavage creating chiral quaternary carbon centers. Org. Lett. 2006;8:3379–3381. doi: 10.1021/ol061359g. [DOI] [PubMed] [Google Scholar]
- 13.Seiser T, Roth OA, Cramer N. Enantioselective synthesis of indanols from tert-cyclobutanols using a rhodium-catalyzed C–C/C–H activation sequence. Angew. Chem. Int. Ed. 2009;48:6320–6323. doi: 10.1002/anie.200903189. [DOI] [PubMed] [Google Scholar]
- 14.Seiser T, Cramer N. Rhodium(I)-catalyzed 1,4-silicon shift of unactivated silanes from aryl to alkyl: enantioselective synthesis of indanol derivatives. Angew. Chem. Int. Ed. 2010;49:10163–10167. doi: 10.1002/anie.201005399. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Ishida N, Murakami M. Atom- and step-economical pathway to chiral benzobicyclo[2.2.2]octenones through carbon−carbon bond cleavage. Angew. Chem. Int. Ed. 2012;51:2485–2488. doi: 10.1002/anie.201108446. [DOI] [PubMed] [Google Scholar]
- 16.Xu T, Ko HM, Savage NA, Dong G. Highly enantioselective Rh-catalyzed carboacylation of olefins: efficient syntheses of chiral poly-fused rings. J. Am. Chem. Soc. 2012;134:20005–20008. doi: 10.1021/ja309978c. [DOI] [PubMed] [Google Scholar]
- 17.Souillart L, Parker E, Cramer N. Highly enantioselective rhodium(I)-catalyzed activation of enantiotopic cyclobutanone C–C bonds. Angew. Chem. Int. Ed. 2014;53:3001–3005. doi: 10.1002/anie.201311009. [DOI] [PubMed] [Google Scholar]
- 18.Deng L, Xu T, Li H, Dong G. Enantioselective Rh-catalyzed carboacylation of C=N bonds via C–C activation of benzocyclobutenones. J. Am. Chem. Soc. 2016;138:369–376. doi: 10.1021/jacs.5b11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y-L, et al. Enantioselective cross-exchange between C−I and C–C σ bonds. Angew. Chem. Int. Ed. 2019;58:6747–6751. doi: 10.1002/anie.201902029. [DOI] [PubMed] [Google Scholar]
- 20.Parsons AT, Smith AG, Neel AJ, Johnson JS. Dynamic kinetic asymmetric synthesis of substituted pyrrolidines from racemic cyclopropanes and aldimines: reaction development and mechanistic insights. J. Am. Chem. Soc. 2010;132:9688–9692. doi: 10.1021/ja1032277. [DOI] [PubMed] [Google Scholar]
- 21.Xia Y, Liu X, Zheng H, Lin L, Feng X. Asymmetric synthesis of 2,3-dihydropyrroles by ring-opening/cyclization of cyclopropyl ketones using primary amines. Angew. Chem. Int. Ed. 2015;54:227–230. doi: 10.1002/anie.201407880. [DOI] [PubMed] [Google Scholar]
- 22.Wang D-C, et al. Enantioselective dearomative [3 + 2] cycloaddition reactions of benzothiazoles. Angew. Chem. Int. Ed. 2016;55:14111–14115. doi: 10.1002/anie.201607852. [DOI] [PubMed] [Google Scholar]
- 23.Perrotta D, Wang M-M, Waser J. Lewis acid catalyzed enantioselective desymmetrization of donor−acceptor meso-diaminocyclopropanes. Angew. Chem. Int. Ed. 2018;57:5120–5123. doi: 10.1002/anie.201800494. [DOI] [PubMed] [Google Scholar]
- 24.Lv H, Mo J, Fang X, Chi YR. Formal Diels−Alder reactions of chalcones and formylcyclopropanes catalyzed by chiral N-heterocyclic carbenes. Org. Lett. 2011;13:5366–5369. doi: 10.1021/ol202250s. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Diez E, et al. Organocatalytically generated donor−acceptor cyclopropanes in domino reactions. one-step enantioselective synthesis of pyrrolo[1,2-a]quinolines. Org. Lett. 2016;18:1270–1273. doi: 10.1021/acs.orglett.6b00173. [DOI] [PubMed] [Google Scholar]
- 26.Blom J, et al. Directing the activation of donor−acceptor cyclopropanes towards stereoselective 1,3-dipolar cycloaddition reactions by Brønsted base catalysis. Angew. Chem. Int. Ed. 2017;56:11831–11835. doi: 10.1002/anie.201706150. [DOI] [PubMed] [Google Scholar]
- 27.Ortega A, et al. Catalytic enantioselective Cloke−Wilson rearrangement. Angew. Chem. Int. Ed. 2018;57:8225–8229. doi: 10.1002/anie.201804614. [DOI] [PubMed] [Google Scholar]
- 28.Dowd P, Zhang W. Free radical-mediated ring expansion and related annulations. Chem. Rev. 1993;93:2091–2115. doi: 10.1021/cr00022a007. [DOI] [Google Scholar]
- 29.Wu X, Zhu C. Recent advances in ring-opening functionalization of cycloalkanols by C–C σ-bond cleavage. Chem. Rec. 2018;18:587–598. doi: 10.1002/tcr.201700090. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Xu W, Xie J, Yu S, Zhu C. Distal radical migration strategy: an emerging synthetic means. Chem. Soc. Rev. 2018;47:654–667. doi: 10.1039/C7CS00507E. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Zhu C. Recent advances in radical-mediated C–C bond fragmentation of non-strained molecules. Chin. J. Chem. 2019;37:171–182. [Google Scholar]
- 32.Hashimoto T, Kawamata Y, Maruoka K. An organic thiyl radical catalyst for enantioselective cyclization. Nat. Chem. 2014;6:702–705. doi: 10.1038/nchem.1998. [DOI] [PubMed] [Google Scholar]
- 33.Amador AG, Sherbrook EM, Yoon TP. Enantioselective photocatalytic [3 + 2] cycloadditions of aryl cyclopropyl ketones. J. Am. Chem. Soc. 2016;138:4722–4725. doi: 10.1021/jacs.6b01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryss JM, Turek AK, Miller SJ. Disulfide-bridged peptides that mediate enantioselective cycloadditions through thiyl radical catalysis. Org. Lett. 2018;20:1621–1625. doi: 10.1021/acs.orglett.8b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woźniak Ł, Magagnano G, Melchiorre P. Enantioselective photochemical organocascade catalysis. Angew. Chem. Int. Ed. 2018;57:1068–1072. doi: 10.1002/anie.201711397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X, et al. Asymmetric [3 + 2] photocycloadditions of cyclopropanes with alkenes or alkynes through visible-light excitation of catalyst-bound substrates. Angew. Chem. Int. Ed. 2018;57:5454–5458. doi: 10.1002/anie.201802316. [DOI] [PubMed] [Google Scholar]
- 37.Hao W, Harenberg JH, Wu X, MacMillan SN, Lin S. Diastereo- and enantioselective formal [3 + 2] cycloaddition of cyclopropyl ketones and alkenes via Ti-catalyzed radical redox relay. J. Am. Chem. Soc. 2018;140:3514–3517. doi: 10.1021/jacs.7b13710. [DOI] [PubMed] [Google Scholar]
- 38.Yang S, et al. Copper-catalyzed asymmetric aminocyanation of arylcyclopropanes for synthesis of γ-amino nitriles. ACS Catal. 2019;9:716–721. doi: 10.1021/acscatal.8b03768. [DOI] [Google Scholar]
- 39.Caron S, Vazquez E. The synthesis of a selective PDE4/TNFα inhibitor. Org. Process Res. Dev. 2001;5:587–592. doi: 10.1021/op010223p. [DOI] [Google Scholar]
- 40.Marfat, A. Therapeutically active compounds based on indazole bioisostere replacement of catechol in PDE4 inhibitors. US2002058687A1 (2002).
- 41.Christensen, S. B. IV & Forster, C. J. 3-Cyano-3-(3,4-disubstituted) phenylcyclohexyl-1-carboxylates US6300372B1 (2001).
- 42.Imogai, H. J. et al. 1,4-Disubstituted 3-cyano-pyridone derivatives and their use as positive allosteric modulators of mGluR2-receptors. WO2007104783A2 (2007).
- 43.Vialard, J. E. et al Quinolinone derivatives as PARP and TANK inhibitors. WO2008107478A1 (2008).
- 44.Fadda AA, Afsah EM, Awad RS. Synthesis and antimicrobial activity of some new benzo and naphthonitrile derivatives. Eur. J. Med. Chem. 2013;60:421–430. doi: 10.1016/j.ejmech.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 45.Hartmann RW, Batzl C, Pongratz TM, Mannschreck A. Synthesis and aromatase inhibition of 3-cycloalkyl-substituted 3-(4-aminophenyl)piperidine-2,6-diones. J. Med. Chem. 1992;35:2210–2214. doi: 10.1021/jm00090a010. [DOI] [PubMed] [Google Scholar]
- 46.Gavagan JE, et al. Chemoenzymic production of lactams from aliphatic α,ω-dinitriles. J. Org. Chem. 1998;63:4792–4801. doi: 10.1021/jo9804386. [DOI] [Google Scholar]
- 47.Effenberger F, Oßwald S. Selective hydrolysis of aliphatic dinitriles to monocarboxylic acids by a nitrilase from arabidopsis thaliana. Synthesis. 2001;12:1866–1872. doi: 10.1055/s-2001-17523. [DOI] [Google Scholar]
- 48.Therkelsen FD, Hansen A-L, Pedersen EB, Nielsen C. Multiple pathways in the synthesis of new annelated analogues of 6-benzyl-1-(ethoxymethyl)-5-isopropyluracil (emivirine) Org. Biomol. Chem. 2003;1:2908–2918. doi: 10.1039/b303658h. [DOI] [PubMed] [Google Scholar]
- 49.Mukherjee C, Zhu D, Biehl ER, Hua L. Exploring the synthetic applicability of a cyanobacterium nitrilase as catalyst for nitrile hydrolysis. Eur. J. Org. Chem. 2006;2006:5238–5242. doi: 10.1002/ejoc.200600699. [DOI] [Google Scholar]
- 50.Kholkhoev BCh, Burdukovskii VF, Mognonov DM. Preparation of aromatic polyamidines and their transformation in polybenzimidazoles. Express Polym. Lett. 2014;8:635–646. doi: 10.3144/expresspolymlett.2014.67. [DOI] [Google Scholar]
- 51.Yu L, Wang J, Cao H, Ding K, Xu Q. A review on catalyzed dimerization of acrylonitrile. Chin. J. Org. Chem. 2014;34:1986–1991. doi: 10.6023/cjoc201405004. [DOI] [Google Scholar]
- 52.Wang Y, Liu X, Deng L. Dual-function cinchona alkaloid catalysis: catalytic asymmetric tandem conjugate addition−protonation for the direct creation of nonadjacent stereocenters. J. Am. Chem. Soc. 2006;128:3928–3930. doi: 10.1021/ja060312n. [DOI] [PubMed] [Google Scholar]
- 53.Xie J, Xing W-L, Sha F, Wu X-Y. Enantioselective cascade reaction of α-cyano ketones and isatylidene malononitriles: asymmetric construction of spiro[4H-pyran-oxindoles] Eur. J. Org. Chem. 2016;2016:3983–3992. doi: 10.1002/ejoc.201600432. [DOI] [Google Scholar]
- 54.Liu W, Hu Z-P, Yan Y, Liao W-W. Highly diastereo- and enantioselective construction of phthalide-oxindole hybrids bearing vicinal quaternary chiral centers via an organocatalytic allylic alkylation. Tetrahedron Lett. 2018;59:3132–3135. doi: 10.1016/j.tetlet.2018.07.002. [DOI] [Google Scholar]
- 55.Yu K-M, Zhu H-K, Zhao X-L, Shi M, Zhao M-X. Organocatalyzed asymmetric tandem conjugate addition−protonation of isocyanoacetates to 2-chloroacrylonitrile. Org. Biomol. Chem. 2019;17:639–645. doi: 10.1039/C8OB02833H. [DOI] [PubMed] [Google Scholar]
- 56.Boivin J, Fouquet E, Zard SZ. Ring opening induced by iminyl radicals derived from cyclobutanones: new aspects of tin hydride cleavage of S-phenyl sulfenylimines. J. Am. Chem. Soc. 1991;113:1055–1057. doi: 10.1021/ja00003a057. [DOI] [Google Scholar]
- 57.Boivin J, Fouquet E, Zard SZ. Iminyl radicals: Part II. Ring opening of cyclobutyl- and cyclopentyliminyl radicals. Tetrahedron. 1994;50:1757–1768. doi: 10.1016/S0040-4020(01)80850-4. [DOI] [Google Scholar]
- 58.Sivaguru P, Wang Z, Zanoni G, Bi X. Cleavage of carbon–carbon bonds by radical reactions. Chem. Soc. Rev. 2019;48:2615–2656. doi: 10.1039/C8CS00386F. [DOI] [PubMed] [Google Scholar]
- 59.Yin W, Wang X. Recent advances in iminyl radical-mediated catalytic cyclizations and ring-opening reactions. N. J. Chem. 2019;43:3254–3264. doi: 10.1039/C8NJ06165C. [DOI] [Google Scholar]
- 60.Wang F, Chen P-H, Liu G. Copper-catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 2018;51:2036–2046. doi: 10.1021/acs.accounts.8b00265. [DOI] [PubMed] [Google Scholar]
- 61.Zhang W, et al. Enantioselective cyanation of benzylic C–H bonds via copper-catalyzed radical relay. Science. 2016;353:1014–1018. doi: 10.1126/science.aaf7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang D-H, Zhu N, Chen P-H, Lin Z-Y, Liu G. Enantioselective decarboxylative cyanation employing cooperative photoredox catalysis and copper catalysis. J. Am. Chem. Soc. 2017;139:15632–15635. doi: 10.1021/jacs.7b09802. [DOI] [PubMed] [Google Scholar]
- 63.Sha W, et al. Merging photoredox and copper catalysis: enantioselective radical cyanoalkylation of styrenes. ACS Catal. 2018;8:7489–7494. doi: 10.1021/acscatal.8b01863. [DOI] [Google Scholar]
- 64.Lu F-D, et al. Asymmetric propargylic radical cyanation enabled by dual organophotoredox and copper catalysis. J. Am. Chem. Soc. 2019;141:6167–6172. doi: 10.1021/jacs.9b02338. [DOI] [PubMed] [Google Scholar]
- 65.Guo Q, et al. Dual-functional chiral Cu-catalyst-induced photoredox asymmetric cyanofluoroalkylation of alkenes. ACS Catal. 2019;9:4470–4476. doi: 10.1021/acscatal.9b00209. [DOI] [Google Scholar]
- 66.Bao X, Wang Q, Zhu J. Dual photoredox/copper catalysis for the remote C(sp3)−H functionalization of alcohols and alkyl halides by N-alkoxypyridinium salts. Angew. Chem. Int. Ed. 2019;58:2139–2143. doi: 10.1002/anie.201813356. [DOI] [PubMed] [Google Scholar]
- 67.Reddy KL. An efficient method for the conversion of aromatic and aliphatic nitriles to the corresponding N-tert-butyl amides: a modified Ritter reaction. Tetrahedron Lett. 2003;44:1453–1455. doi: 10.1016/S0040-4039(03)00006-6. [DOI] [Google Scholar]
- 68.Yu X-Y, Zhao Q-Q, Chen J, Chen J-R, Xiao W-J. Copper-catalyzed radical cross-coupling of redox-active oxime esters, styrenes, and boronic acids. Angew. Chem. Int. Ed. 2018;57:15505–15509. doi: 10.1002/anie.201809820. [DOI] [PubMed] [Google Scholar]
- 69.Tian L, et al. Copper-catalyzed ring-opening C(sp3)–N coupling of cycloketone oxime esters: access to 1°, 2° and 3° alkyl amines. Chem. Commun. 2019;55:5347–5350. doi: 10.1039/C9CC02030F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this research are available within the article and its supplementary information. The characterization data (Supplementary Figs. 1–268), the optimization of reaction conditions (Supplementary Tables 1–6) and the crystallography data (Supplementary Tables 7–12, Supplementary Fig. 269) are available within Supplementary Information. The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 1911616 (4g) and can be obtained free of charge from www.ccdc.cam.ac.uk/data_request/cif.