Abstract
Pregnancy-associated plasma protein-A (PAPP-A) is a metalloproteinase with a well-established role in releasing bioactive insulin-like growth factor-1 (IGF-1) from IGF-binding protein-2, -4, and -5 by proteolytic processing of these. The IGF system has repeatedly been suggested to be involved in the pathology of atherosclerosis, and both PAPP-A and IGF-1 are proposed biomarkers and therapeutic targets for this disease. Several experimental approaches based on atherosclerosis mouse models have been undertaken to obtain causative and mechanistic insight to the role of these molecules in atherogenesis. However, reports seem conflicting. The literature suggests that PAPP-A is detrimental, while IGF-1 is beneficial. This raises important questions that need to be addressed. Here we summarize the various studies and discuss potential underlying explanations for this seemingly inconsistency with the objective of better understanding complexities and limitations when manipulating the IGF system in mouse models of atherosclerosis. A debate clarifying what’s up and what’s down is highly warranted going forward with the ultimate goal of improving atherosclerosis therapy by targeting the IGF system.
Keywords: atherosclerosis, IGF system, mouse models, PAPP-A
INTRODUCTION
The intention of this review is to account for the seemingly inconsistent studies reporting an effect of the insulin-like growth factor-1 (IGF-1) system on atherosclerosis. Even among studies based on widely used and reproducible atherosclerosis mouse models reports are incongruent. In this review, we focus on all reported studies accessible in the PubMed database to date in which the IGF system is directly manipulated in mouse models of atherosclerosis. We discuss potential reasons for the inconsistent conclusions emphasizing problematic issues resulting from manipulating the IGF system in a whole organism setting, which could complicate interpretation of results.
It should be noted that the majority of reported in vivo studies involve genetic manipulation of vascular smooth muscle cells (VSMCs). The reason for this apparent bias is presumably that VSMCs are known to be the principal producers of IGF components in the artery wall (see expression of igf-1, igf-1r, and papp-a in atherosclerosis) and could therefore be assumed to be most relevant. Indeed, endothelial cell- and macrophage-dependent processes of atherosclerosis have been proposed, and future genetic in vivo models addressing these cell types will most likely deepen our understanding of the involvement of the IGF system in atherosclerosis. A summary of the numerous cellular mechanisms proposed to be affected by the IGF system in atherosclerosis is not within the scope of this review. Instead we refer to recent reviews (44, 137).
PREGNANCY-ASSOCIATED PLASMA PROTEIN-A AND THE IGF SYSTEM
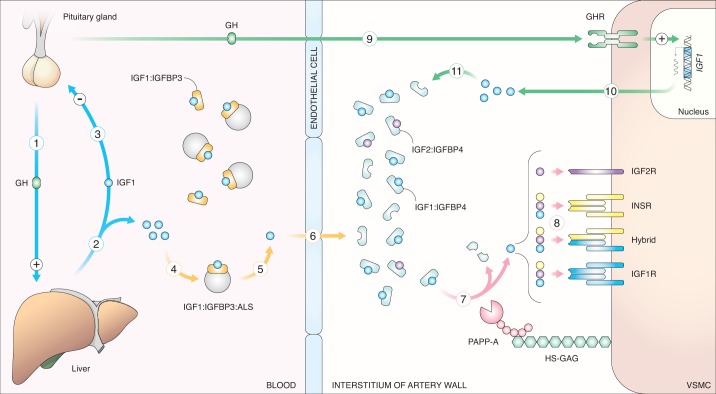
In the classic view of the somatotroph axis, growth hormone (GH) secreted from the pituitary gland stimulates hepatocytes to secrete IGF-1, which in turn acts on tissues expressing IGF-1 receptors (IGF-1R) and/or insulin receptors (INSRs) (72). As its name implies, IGF-1 is structurally and functionally related to insulin (90, 91, 95); however, while insulin secretion is restricted to β-cells of the endocrine pancreas (10), IGF-1 is expressed in most tissues and its actions are exerted by endo-, auto-, and paracrine modes (30, 105, 135). IGF-1 is not a hormone like insulin as it is present at a relatively steady level in the blood (106). Consequently, several levels of extracellular regulation are required between IGF-1 secretion and receptor activation. First, the majority of IGF-1 is bound by one of six IGF-binding proteins (IGFBPs), which typically sequester IGF-1 and prevent receptor activation (34). Second, IGFBP proteinases can liberate IGF-1 by proteolytic cleavage of IGFBPs (64), and third, IGFBP proteinases are regulated by proteinase inhibitors (52, 60, 80, 81). IGFBP-bound IGF-1 can therefore be considered a reservoir of IGF-1 that can be liberated in a spatiotemporal manner by IGFBP proteolysis depending on the presence and activity of various proteinases in different tissues (82) (Fig. 1).
Fig. 1.
Insulin-like growth factor-1 (IGF-1): from secretion to receptor activation. Blue arrows: growth hormone (GH) secreted from the pituitary gland (1) stimulates hepatic IGF-1 secretion into the circulation (2). In turn, IGF-1 reduces GH secretion by negative feedback (3). Yellow arrows: IGF-1 is bound by IGF-binding protein 3 (IGFBP-3) alone or in a ternary complex with IGFBP-3 and acid labile subunit (ALS) (4). Liberated IGF-1 (5) is able to cross the endothelium. Upon entry into the artery interstitium, IGF-1 is met by an IGFBP-4-dominated environment and is bound by IGFBP4 (6). Red arrows: IGF-1 is liberated from IGFBP4 by proteolytic activity of pregnancy-associated plasma protein-A (PAPP-A) (7). PAPP-A is associated with cell surfaces by electrostatic interaction with heparan sulfate glycosaminoglycans (HS-GAGs). IGF-1 is thereby liberated in close proximity to its receptors. Free IGF-1 and IGF-2 and insulin can bind and activate IGF receptor-1 (IGF-1R), insulin receptors (INSR; depending on isotype), and hybrids hereof, while IGF-2 can bind IGF-2R (8). Green arrows: GH can enter the artery wall and stimulate GH receptor (GHR)-expressing cells (e.g., vascular smooth muscle cells (VSMCs) to synthesize IGF-1 (9). IGF-1 produced by local vascular cells (10) is bound by IGFBP-4 and thereby enters the reservoir of PAPP-A-dependent releasable IGF-1 (11).
Pregnancy-associated plasma protein-A (PAPP-A) is a well-established IGFBP proteinase capable of leaving IGFBP-2, -4, and -5 (65, 67, 75). To date no other proteinases are known to target IGFBP-4 under physiological conditions (in contrast to IGFBP-2 and -5), thus PAPP-A is believed to be critical for liberation of IGFBP-4-bound IGF-1 (82). By its elongated zinc-binding motif, PAPP-A is classified as a metzincin metalloproteinase (15). However, unlike other members of this superfamily, e.g., several of the matrix metalloproteinases, which often cleave promiscuously, substrates other than IGFBPs have not been identified for PAPP-A (82). PAPP-A consists of multiple protein modules, some of which are well characterized while the function of others remains unknown. Besides the proteolytic domain, PAPP-A has three Lin12-Notch repeats (LNRs) critical for IGFBP-4 substrate specificity (14, 130) and five complement control protein (CCP) modules, also termed short consensus repeats, of which CCP3 and CCP4 are important for the ability of PAPP-A to bind to cell surfaces via heparan sulfate glycosaminoglycans (HS-GAGs) (66). The ability of PAPP-A to interact with HS-GAGs on cell surfaces enables efficient regulation of IGF-1 signaling (66). By liberating IGF-1 from IGFBP-4 in close proximity to IGF-1Rs, rebinding of IGF-1 to other high-affinity IGFBPs is minimized. In fact, although counterintuitive, Igfbp4−/− mice are smaller because IGFBP-4 cooperates closely with PAPP-A to ensure efficient delivery of IGF-1 to its receptor (64, 77, 78).
By tight association with cellular surfaces, PAPP-A thus functions in tissues, close to the receptors of target cells. This is in contrast to PAPP-A2, the only paralog of PAPP-A, which has proteolytic activity toward IGFBP-3 and IGFBP-5 and is unable to associate with cellular surfaces (66). PAPP-A2 is therefore believed to function mainly in the circulation to ensure a steady level of free IGF-1 for tissues (3). Two families with PAPP-A2 deficiencies have recently been identified, directly supporting this interpretation (29). In humans, no families with inactivating PAPP-A mutations have yet been identified.
Little is known about the regulation of PAPP-A gene expression, but three endogenous PAPP-A inhibitors have been identified: The proform of eosinophil major basic protein, which inactivates the vast majority of circulating placenta-derived PAPP-A in pregnancy (80, 81), and stanniocalcin-1 and -2 (STC1 and -2), which appear to regulate PAPP-A within different tissues, including arteries (51, 52, 60, 112). STC1 inhibits by high-affinity (pM) binding to PAPP-A or PAPP-A2, while proform of eosinophil major basic protein or STC2 inhibition requires the formation of a covalent bond to PAPP-A or PAPP-A2.
Several lines of evidence underline the crucial role of PAPP-A in growth regulation and in the IGF system. First, genetic variants of PAPP-A strongly associate with height in genome-wide association studies (GWAS) such as the GIANT GWAS (62, 132). Second, Pappa−/− mice are proportional dwarfs with a 40% reduction in birth weight (26). Interestingly, this effect is abolished in Pappa−/−/Igfbp4−/− mice (78) underlining that IGFBP-4 is the principal substrate of PAPP-A. Third, STC2 is one of seven genes including other principal components of the IGF system to have major impact on dog breed weight (89). Moreover, in a GWAS including >700,000 individuals, coding variants of STC2 resulting in compromised proteolytic inhibition of PAPP-A were reported to increase human height by up to 2.1 centimeters per allele (71). Collectively, these findings strongly indicate that PAPP-A and other key factors in the regulation IGF bioavailability are crucial in human physiology.
EXPRESSION OF IGF-1, IGF-1R, AND PAPP-A IN ATHEROSCLEROSIS
IGF-1 and IGF-1R are expressed by endothelial cells, VSMCs, and macrophages (32, 47, 76, 92). In early human atherosclerotic lesion, IGF-1 and IGF-1R are associated primarily with medial VSMCs (79), and expression of IGF-1 and IGF-1R is reduced in advanced lesions (11, 79, 84, 128), possibly as a consequence of VSMC apoptosis (32).
PAPP-A is expressed by medial and intimal VSMCs (73) in the healthy artery wall and throughout lesion development (112). In advanced plaques, PAPP-A colocalizes with endothelial cells, VSMCs, and macrophages, and plaques classified as vulnerable have elevated levels of PAPP-A (6). Indeed, factors present in the atherosclerotic milieu such as various proinflammatory cytokines (TNFα and IL-1β) stimulate VSMC PAPP-A expression (24). Cultivated VSMCs from various species produce abundant PAPP-A (7, 24, 112) and express approximately ten times more PAPP-A than endothelial cells (24). Interestingly, although macrophages stain positive for PAPP-A in atherosclerosis specimens, macrophages do not express PAPP-A, even upon cytokine stimulation (23).
In contrast to human atherosclerosis, little is known about expression patterns in mouse plaques.
CONFLICTING REPORTS?
Circulating PAPP-A was first identified as a biomarker for acute coronary syndrome in 2001 (6). Since then, a multitude of studies have reported diagnostic and prognostic values of PAPP-A in cardiovascular cohorts (53). The late realization that administration of the anticoagulant heparin artificially elevates circulating PAPP-A by detachment from tissue HS-GAGs has, however, seeded doubt in studies not reporting whether or not heparin was used as well of the timing of heparin administration relative to blood sampling (118). Nonetheless, these positive findings prompted hypotheses as to the involvement of PAPP-A in atherosclerosis. Papp-a-deficient Apoe−/− mice have a marked reduction in lesion development compared with Apoe−/− littermates in both constitutive and inducible knockout model systems (4, 43) (Table 1). Vice versa, SM22α promoter (altered to prevent decreased promoter activity during plaque formation (125)-driven transgenic overexpression of human PAPP-A by VSMCs but also bladder and uterus smooth muscle cells aggravated atherosclerosis (25). To assess which domains of PAPP-A the proatherogenic effect is attributed to, mice overexpressing mutants of human PAPP-A (driven by the altered SM22α-promoter) were generated (13). Disruption of either the proteolytic domain, CCP3 (responsible for HS-GAG binding) or LNR3 (responsible for IGFBP-4 specificity) reduced the atheropromoting effect, highlighting the critical involvement of these domains and hence a crucial function of the interplay between PAPP-A and IGFBP-4 in atherosclerosis. Finally, in more therapeutic-oriented studies, treating Apoe−/− mice with either human STC2 (adeno-associated virus-mediated hepatic overexpression) or an antibody inhibiting IGFBP-4-specific proteolytic activity of PAPP-A reduced atherogenesis (21, 112). PAPP-A expression is increased in inflammatory environments (24) and atherosclerotic plaques (6, 112), perhaps through downregulation of miR-430–3p, which in turn inhibits PAPP-A translation (115).
Table 1.
Summary of seemingly conflicting reports
| Model System (Apoe−/− Background Unless Stated Otherwise) | Effect on Atherosclerosis Burden | Plasma Cholesterol | Endogenous Igf-1 | Other Reported Effects | Reference |
|---|---|---|---|---|---|
| Papp-a−/− (global knockout, constitutive) | Reduction | Unaffected | Similar in circulation in newborns (26) | Reduced body weight by ~40% (26) | 43 |
| Papp-a−/− (global knockout, induced 5 wk postdiet initiation) | Reduction | Unaffected | ND | None | 4 |
| Papp-a inhibition (AAV-mediated hepatic secretion of human STC2 into the circulation) | Reduction | Unaffected | ND | None | 112 |
| Papp-a inhibition (antibody targeting IGFBP-4 proteolysis) | Reduction | Unaffected | ND | None | 21 |
| Human PAPP-A overexpression from VSMCs (and other tissues, especially bladder and uterus) (altered SM22α promoter) | Increase | Unaffected | Unaffected | None | 25 |
| Mutated human PAPP-A overexpression from VSMCs (altered SM22α promoter) | Increase | ND | ND | None | 13 |
| Human IGF-1 treatment | Reduction | Unaffected | Reduced in circulation by ~40% | None | 114 |
| Long R3 IGF-1 treatment | Reduction | Unaffected | Unaffected | None | 120 |
| Rat Igf-1 overexpression from VSMCs (altered α-SMA promoter) | None (stabilizing) | Unaffected | Unaffected | Body weight unaffected Reduced aorta size |
99 |
| Hepatic Igf1−/− (induced at age 3 mo), Paigen diet (not Apoe−/−) | Increase (only females) | Increase (females) | Reduced in circulation by ~80% Artery mRNA: increased by ~200% |
Reduced body weight by ~20% | 117 |
| Hepatic Jak2−/− (both Apoe−/− and Ldlr −/−) As above + Long R3 IGF-1 treatment or hepatic overexpression of rat Igf-1 |
Increase Reduction |
Unaffected ND |
Major reduction in circulation Reversing reducing effect |
Hepatic steatosis: increased GH in circulation by ~400% Reversing effects above |
104 |
| Myeloid Igf1r−/− (lyz-cre) | Increase | Unaffected | Unaffected | None | 45 |
| VSMC and fibroblast Igf1r−/− (SM22α-cre) | Increase | ND | Reduced in circulation by ~25% Vascular mRNA unaffected |
Reduced body weight by ~25% Reduced aorta size Increased circulating GH |
113 |
| C3H.6T+/+ (with Igf-1 genomic region transferred from CH3 background) | Increase | Unaffected | Reduced in circulation by ~20% | None | 100 |
| Igf2−/− (global knockout) | Reduction | Reduced by ~45% | ND | Reduced body weight by ~60% Reduced aorta size Increased insulin level by ~200% |
138 |
IGF-1, insulin-like growth factor 1; AAV, adeno-associated virus; VSMC: vascular smooth muscle cell; α-SMA, α-smooth muscle actin; PAPP-A, pregnancy-associated plasma protein-A; STC2, stanniocalcin-2; GH, growth hormone.
The uniform conclusion of these studies is that by increasing IGF-1 bioavailability in the artery tissue, PAPP-A is an atheropromoting molecule.
However, this conclusion is in opposition to a number of studies focusing on IGF-1 itself (Table 1) (44). Treating Apoe−/− mice with human recombinant IGF-1 (114) or the LR3 IGF-1 analog (120) had atheroprotective effects. Vice versa, Apoe−/− mice harboring a genomic region from the C3H strain have a 20% reduction in circulating IGF-1 and increased atherosclerosis; however, it was stated that other genetic components of the C3H strain could contribute to this phenotype (100). Likewise, adult-onset hepatocyte-specific Igf-1 knockout mice with an 80% reduction in circulating Igf-1 showed more pronounced fatty streak formation in Paigen diet (a high-fat diet supplemented with cholate)-fed female mice (but not in males), although this effect may in part be attributed to increased plasma cholesterol and IL-6 levels, as stated by the authors (117). In a more complex setting, hepatocyte-specific Jak2 knockout mice on Apoe−/− background, which develop profound hepatic steatosis and reduced circulating Igf-1, also showed aggravated atherosclerosis, which could be counteracted by either infusion of LR3 IGF-1 or by hepatic overexpression of rat Igf-1 (104). As emphasized by the authors of this study, a direct effect of IGF-1 on the vascular wall could not be concluded as hepatic steatosis was also attenuated by IGF-1 treatment. Transgenic rat Igf-1 expression driven by a α-smooth muscle actin (α-SMA) promoter fragment (SMP8) in Apoe−/− mice was used to determine the effect of local VSMC and fibroblast produced IGF-1 in the artery wall (ex-vascular production was also detected) (99). Although there was no effect on plaque burden (in contrast to studies based on elevating Igf-1 in the circulation), features of plaque stability (content of SM22α- and α-SMA-positive cells and reduced necrotic core size) were enhanced upon Igf-1 overexpression. On the other hand, Igf-1r deletion in VSMCs and fibroblasts (SM22α-CRE driven) resulted in atherosclerosis aggravation (113), as did Igf-1r depletion (lyz2-CRE driven) in macrophages (45).
Taken together, the current literature appears to be conflicting. However, as discussed in the following sections, there are several potential explanations for this apparent dispute.
NOT JUST A MATTER OF UP AND DOWN
It has previously been emphasized that the effect of manipulating IGF-1 signaling depends on several parameters. Timing (40) and duration (121, 140) of IGF-1 signaling are essential for resulting biological responses and phenotypes in various physiological and pathophysiological settings (27). This notion includes atherosclerosis. While gene expression is regulated dynamically by various disease stimuli, transgene expression is constant (indeed, as mentioned above, efforts are made to alter promoters to ensure chronic transgene expression) and the level not readily controllable. Therefore, chronic overexpression of a transgene is not simply a spatiotemporal parallel increase in the expression of its endogenous counterpart.
This possibly explains paradoxical observations such as the absence of an effect on lesion burden when overexpressing rat Igf-1 by VSMCs (99) as opposed to the increase in atherosclerosis resulting from knocking out endogenous VSMC/fibroblast Igf-1r (113) or why both constitutive and inducible Pappa−/− mice as well as transgenic VSMC PAPP-A overexpression all reduce neointima formation during mechanical vascular injury (5, 22, 88). On the other hand, in healthy aortas where endogenous gene expression is constant, observations are consistent: overexpression of IGF-1 leads to VSMC hyperplasia (127) and altered dimensions of the aorta including increased thickness of the medial layer (99), while Igf-1r deficiency of VSMCs reduces aorta dimensions (113). Accordingly, overexpression of IGFBP-4 by VSMCs results in VSMC hypoplasia (129), and when a PAPP-A-resistant variant of IGFBP-4 is overexpressed, hypoplasia is accentuated (139). Moreover, overexpression of PAPP-A increases arterial Igfbp-5 expression (25) [an Igf-1-responsive gene (33)] indicating enhanced Igf-1 activity. It is of note that the fact that healthy arteries display marked dimensional differences in these models is a problem, as they likely bias subsequent atherogenesis. Taken together, manipulating the IGF system is not simply a matter of “up and down.”
SOMATOTROPH AXIS FEEDBACK
An additional set of problems arise upon systemic manipulation of the IGF system. Manipulation of any component of any biological system is bound to set in motion counterregulatory effects. The IGF system is not an exception. The problem is evident from many of the studies addressed here. Artificial lowering or raising of circulating IGF-1 feeds back on GH release from the pituitary gland either directly or indirectly via reduced GH-releasing hormone from the hypothalamus. The result is a reciprocal production of endogenous Igf-1. For instance, hepatocyte-specific Igf1−/− mice having an 80% reduction in circulating Igf-1 display an increase in circulating GH (138) and a twofold increase in arterial Igf-1 mRNA in females (117). Likewise, circulating GH is elevated in hepatocyte-specific Jak2 knockout mice on Apoe−/− background and reduced upon LR3 IGF-1 treatment (104). Vice versa, administration of recombinant human IGF-1 resulted in a 40% reduction in circulating endogenous Igf-1 (114). These counterregulatory effects give rise to a number of questions. How does the experimental approach affect net IGF-1 signaling in the artery tissue examined? What is the direct effect of GH on cells involved in atherosclerosis [indeed, the relevant cells of atherosclerosis are GH responsive (70, 110, 119)]. Since GH not only regulates expression of IGF-1 but also other factors of the IGF system, including IGFBPs (74, 122), interpretation is further complicated. That said, cell-type specific gene manipulation would be expected to circumvent these issues; however, that does not appear to be the case, as VSMC-specific Igf-1r deficiency resulted in concomitant elevation of circulating GH and an ~25% reduction in circulating Igf-1 as well as reduced body and aorta size (113).
Although circulating Igf-1 was unaffected in Papp-a−/− mice (26) or upon vascular overexpression of human PAPP-A (25) it is likely that unidentified counterregulatory effects occur in these model systems as well.
IGF-2/IGF-2R NEGLECT
The presumed reason for the scarcity of experimental data on IGF-2 in rodents and the neglect of IGF-2 in most of the studies summarized above is that rodent Igf-2 expression decreases dramatically after birth (in contrast to humans) (68) and that Igf-2 deficiency does not affect postnatal growth (31) and therefore may be considered insignificant in postnatal life. Although the direct effect on atherosclerosis by Igf-2 cannot be determined from the Igf2−/−/Apoe−/− mouse phenotype due to concomitant effects on plasma cholesterol (reduction in both atherosclerosis and total cholesterol) (138), Igf-2 mRNA is not detectable in normal aortas, but elevated in atherosclerotic aortas (138), and evidently the vasculature is highly reactive to Igf-2 as Igf-2 overexpression by VSMCs (driven by an altered α-SMA promoter) results in spontaneous focal neointima formation (138). Moreover, translating to the human situation, IGF-2 seems to be at play in postnatal life. IGF-2 prevails in the circulation throughout life (30), and recently an IGF-2 mutation was shown to cause both pre- and postnatal growth restriction (9). PAPP-A liberates both IGF-1 and IGF-2 from its binding protein substrates, and depending on the relative abundance of Igf-1, Igf-2, and Papp-a substrates in healthy and diseased mouse arteries, the resulting phenotype of manipulating Papp-a would be a combined effect of increased bioavailability of both Igfs. Likewise, manipulating the level of Igf-1 or Igf-1r cannot be done without affecting Igf-2 signaling through the Igf-1 receptor. Although IGF-1 and IGF-2 are similar and both bind to IGF-1R and INSR (depending on INSRA/INSRB isoform) (36, 108), IGF-2 is the only one binding the IGF-2 receptor (IGF-2R), which is structurally and functionally distinct from IGF-1R (61). Whether or not IGF-2R is relevant in the context of vascular biology and atherosclerosis is unknown; however, in vitro studies do point to important roles of Igf-2r in VSMC modulation and migration (41, 42).
In summary, the observed effects on atherosclerosis in the studies summarized above are net effects of altered Igf-1 and Igf-2 signaling, which complicates interpretation of these studies.
INSULIN SIGNALING
The IGFs and insulin are ligands of the homologous IGF-1R and INSR, and hybrid receptors hereof, and bind these with varying affinity (107, 108). Similar to disrupting the balance between IGF-1 and IGF-2, manipulating the bioavailability of IGFs (e.g., by overexpression or deletion of Igf-1, Igf-1r, or Papp-a) would affect the constellation and/or usage of receptors. For instance, reducing Igf-1r expression was shown to increase insulin sensitivity by increasing the relative number of insulin holoreceptors in the mouse aorta (1). Indeed, INSRs are present on all vascular as well as inflammatory cells relevant for atherosclerosis (54). Immunoprecipitation of Igf-1r and Insr indicated a 0:2:1 ratio of Igf-1r holoreceptors, hybrid receptors, and Insr holoreceptors, respectively, on macrophages (45). Deletion of Igf-1r would thereby result in exclusive expression of Insr holoreceptors on macrophages. Although insulin-dependent Akt phosphorylation was unaffected in Igf-1r-deficient macrophages, it is difficult to exclude an effect on insulin signaling, since other known (e.g., the MAPK/Erk pathway) and potentially unknown pathways exist (58). Taken together, manipulation of the Igf-1 system cannot be done in an isolated manner without affecting insulin signaling. The consequence of insulin signaling on atherosclerosis is controversial and may depend on dose, context, and pathway use (87), yet there is no doubt that manipulation of insulin signaling affects atherosclerosis (58). Furthermore, other cell surface proteins (e.g., αVβ3-integrin and integrin-associated protein) interacting with IGF-1R can modulate not only which intracellular pathways that are initiated but also the duration of signaling (20). In this way, the exact cellular response to a given dose of IGF depends on the protein composition of the individual cell. These mechanisms of IGF signaling modulation probably account for the various reported cellular responses to IGF, including proliferation, survival, differentiation, migration, and insulin-like effects on metabolism (57). Depriving cells of Igf-1r could thus affect systems other than the IGF system and insulin signaling. These effects will also contribute to the net phenotype observed in the studies summarized here. In extension, unforeseen tampering with insulin signaling is likely to affect blood glucose levels, especially when manipulating the IGF system on a whole organism level. Since hyperglycemia is a recognized driver of atherosclerosis, blood glucose should be reported in future studies.
IGF SYSTEM-INDEPENDENT FUNCTIONS OF PAPP-A
One immediate conclusion of the above-listed conflicting studies is that PAPP-A simply has atheropromoting effects independent of its established role in increasing IGF-1 bioavailability. Since the role of several PAPP-A domains has yet to be identified, this is indeed a possibility, and some studies may propound this notion. The most direct evidence for an IGF system-independent function of PAPP-A was demonstrated in zebrafish, as pappa knockdown impairing early zebrafish development could be rescued by overexpression of a proteolytically inactive pappa mutant (59). However, it seems that most of the atheropromoting effect of PAPP-A is dependent on IGFBP-4 proteolysis. As mentioned above, genetic disruption of the LNR3 domain, required for cleavage of IGFBP-4, resulted in a marked reduction of the atheropromoting effect of PAPP-A (13), and targeting the LNR3 domain with an antibody reduced atherogenesis (21). Indeed, since a common strategy of these two approaches is disruption/targeting of the LNR3 domain to selectively inhibit IGFBP-4 proteolysis, an alternative role of LNR3 could theoretically confound the conclusion. Alternatively, cleavage of IGFBP-4 could have other effects than liberating IGF-1. Indeed, IGF system-independent roles have been described for all three PAPP-A substrates (8, 48, 49, 94, 109, 116, 136). In the case of IGFBP-4, one example of this is the reported effect on cardiomyocyte growth and differentiation, which appear to be mediated via the Wnt/β-catenin pathway (131, 134, 142), but other IGF-independent effects have also been suggested (85, 102, 133).
Taken together, PAPP-A may have atheropromoting effects by means of functions that are not related to the IGF system; however, if this is indeed the case, they would have to overpower the apparent atheroprotective effect of increasing IGF-1 bioavailability (45, 99, 100, 104, 113, 114, 117, 120). Moreover, they would have to be specific for atherosclerosis.
GENETIC EVIDENCE
A few hypothesis-driven studies have indicated atheroprotecting effects of IGF-1. In hypertensive but not normotensive individuals, a 192-basepair deletion within the IGF-1 promoter is associated with a 20% decrease in circulating IGF-1 and a 4% increase in carotid intima-media thickness (97), and in a different study, the C-allele of rs35767 was associated with a 7% increase in circulating IGF-1 and a 6.5% decrease in carotid intima-media thickness (98).
Two single nucleotide polymorphisms of PAPPA have also been reported to be associated with atherosclerosis, although the direction of effect is less clear. The intron-located rs13290387 was associated with acute myocardial (83), while the C allele rs7020782 was reported to be associated with ischemic cerebrovascular (126) and carotid plaque development (141). rs7020782 is located in exon 14 and the A to C substitution causes a missense mutation (tyrosine to serine) in the CCP1 module of PAPP-A. CCP3 and CCP4 have been demonstrated to be important in HS-GAG interaction and cell-surface binding (66); however, any specific function of CCP1 remains to be demonstrated. Of note, rs7020782 was previously associated with recurrent miscarriage during pregnancy (118) further highlighting a potential functional importance of the CCP1 module.
Curiously, however, despite that most genes encoding components of the IGF system contain effectful variants evident from strong associations with, e.g., height (17), there is no evidence for associations between these genes and measurable proxies for atherosclerotic disease such as coronary artery disease or myocardial infarction in current leading GWAS (17, 18, 28). The reason for this could be that the observed mouse phenotypes suffer from what has previously been referred to as “the knock out conundrum of experimental atherosclerosis” (69); namely, that the quick pace of experimental atherosclerosis (weeks/months) limits time for compensatory mechanisms to take over as they would in the human decades-long disease development and in this way not be as crucial as suggested by a mouse phenotype. Alternatively, genes encoding components of the IGF system may eventually be added to the ever-growing list of susceptibility loci with increasing power of GWAS. The above-mentioned hypothesis-driven genetic studies indeed propound this possibility. Finally, current large-scale GWAS are based on single nucleotide polymorphisms masking other types of variation such as the 192-basepair deletion within the IGF-1 promoter (97).
ASSOCIATING CIRCULATING PAPP-A AND IGF-1 WITH MEASURES OF ATHEROSCLEROSIS
Human studies associating either circulating IGF-1 or PAPP-A with measures of atherosclerosis suffer from methodological issues in part due to biological complexity. Recognizing that heparin administration causes artificial elevations in circulating PAPP-A (118) (presumably by outcompeting its binding to tissue heparan sulfate) complicates interpretation of studies not reporting on the use of this anticoagulant. Moreover, since a yet to be assessed proportion of circulating PAPP-A may be in complex with STC1 or STC2 in nonpregnant individuals, it may be that a variable fraction of quantified circulating PAPP-A is inactive. Also, total circulating PAPP-A could be underestimated if epitopes are masked by these inhibitors.
IGF-1 has for long been known not to circulate freely. Around 99% of IGF-1 in the circulation is in a complex with IGFBP-3 and acid labile subunit (ALS) (12, 101). Efforts are made to measure the free or “bioavailable” portion of IGF-1 as this is considered more physiologically relevant than the reservoir of latent IGF-1 present in complex with an IGFBP. It remains elusive what mechanism governs the transfer of circulating IGF-1 (free or bound) to its receptor on the cell surface of target cell in the vascular wall; however, it likely involves transfer of IGF-1 from the IGFBP-3/ALS complex to IGFBP types dominating the vascular tissue, e.g., IGFBP-4 (2, 39). In support of this idea, proteins regulating IGF signaling locally (i.e., PAPP-A and its inhibitors) appear to be more critical than the concentration of circulating IGF-1, since knockout or transgenic overexpression of genes encoding these has profound effects on physiology without affecting circulating Igf-1 (19, 26, 38, 124). Taken together, although highly convenient in terms of obtaining a blood sample, the biological and methodological complexities complicate data interpretation. Consequently, reports linking IGF-1, circulating binding proteins, or PAPP-A to measures of atherosclerosis point in all directions (16, 35, 37, 50, 53, 55, 56, 63, 93, 96, 103, 111, 123).
The next problem is the interpretation of an elevated level of circulating IGF-1 or PAPP-A. A positive correlation between a circulating molecule and a disease measure is usually interpreted as the molecule being a disease-promoting role. However, it may also be that the tissue expresses the molecule as a way to counteract disease progression.
Circulating PAPP-A is low in healthy individuals, and circulating elevations in disease states are probably an artifact with no physiological purpose. This notion is substantiated as PAPP-A must be bound to the cell surface in close proximity to IGF receptors to enable local liberation of IGF to avoid that IGFs are rebound to new IGFBPs (66). Circulating levels of IGFBP-4 fragments may circumvent the above-mentioned complications and be a better measure of tissue PAPP-A activity (46, 86).
CONCLUSION AND FUTURE DIRECTIONS
Therefore, what’s up and what’s down? As discussed in this review, there are many potential ways to explain the seemingly conflicting reports all of which point to the complexity of the IGF system and the problems of manipulating it. Experimental approaches addressing whether or not the proatherosclerotic effects of PAPP-A are in fact mediated by IGF-1 or whether IGF system-independent effects are involved should be a high priority. This would require, e.g., complex model systems enabling assessment of consequences of PAPP-A overexpression in an Igf-1r-deficient system. Moreover, future studies should strive to use inducible cell type-specific genetic manipulation (e.g., tamoxifen- or doxycycline-inducible cre-recombinase activation systems) to circumvent many of the problems highlighted above.
At this point, the literature does not provide a clear message, but we hope that the issues discussed here will be considered when navigating in the literature of this field and when designing future experiments aiming at dissecting the role of PAPP-A and the IGF system in atherosclerosis with the overall aim of identifying novel treatment regimens for this disease.
GRANTS
This work was supported by the Centre for Individualized Medicine in Arterial Diseases, Odense University Hospital (to L. B. Steffensen); National Heart, Lung, and Blood Institute Grant R01-HL-074871 (to C. A. Conover); and Independent Research Fund Denmark (to C. Oxvig).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.B.S. prepared figures; L.B.S. drafted manuscript; L.B.S., C.A.C., and C.O. edited and revised manuscript; L.B.S., C.A.C., and C.O. approved final version of manuscript.
REFERENCES
- 1.Abbas A, Imrie H, Viswambharan H, Sukumar P, Rajwani A, Cubbon RM, Gage M, Smith J, Galloway S, Yuldeshava N, Kahn M, Xuan S, Grant PJ, Channon KM, Beech DJ, Wheatcroft SB, Kearney MT. The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes 60: 2169–2178, 2011. doi: 10.2337/db11-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson P, Gustafsson T, Arnqvist HJ. Insulin-like growth factor binding proteins-2 to -6 are expressed by human vascular smooth muscle cells. J Endocrinol 163: 281–288, 1999. doi: 10.1677/joe.0.1630281. [DOI] [PubMed] [Google Scholar]
- 3.Argente J, Chowen JA, Pérez-Jurado LA, Frystyk J, Oxvig C. One level up: abnormal proteolytic regulation of IGF activity plays a role in human pathophysiology. EMBO Mol Med 9: 1338–1345, 2017. doi: 10.15252/emmm.201707950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bale LK, Chakraborty S, Conover CA. Inducible reduction in pregnancy-associated plasma protein-A gene expression inhibits established atherosclerotic plaque progression in mice. Endocrinology 155: 1184–1187, 2014. doi: 10.1210/en.2013-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bale LK, Resch ZT, Harstad SL, Overgaard MT, Conover CA. Constitutive expression of pregnancy-associated plasma protein-A in arterial smooth muscle reduces the vascular response to injury in vivo. Am J Physiol Endocrinol Metab 304: E139–E144, 2013. doi: 10.1152/ajpendo.00376.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR Jr, Virmani R, Oxvig C, Schwartz RS. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med 345: 1022–1029, 2001. doi: 10.1056/NEJMoa003147. [DOI] [PubMed] [Google Scholar]
- 7.Bayes-Genis A, Schwartz RS, Lewis DA, Overgaard MT, Christiansen M, Oxvig C, Ashai K, Holmes DR Jr, Conover CA. Insulin-like growth factor binding protein-4 protease produced by smooth muscle cells increases in the coronary artery after angioplasty. Arterioscler Thromb Vasc Biol 21: 335–341, 2001. doi: 10.1161/01.ATV.21.3.335. [DOI] [PubMed] [Google Scholar]
- 8.Beattie J, Allan GJ, Lochrie JD, Flint DJ. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochem J 395: 1–19, 2006. doi: 10.1042/BJ20060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begemann M, Zirn B, Santen G, Wirthgen E, Soellner L, Büttel HM, Schweizer R, van Workum W, Binder G, Eggermann T. Paternally inherited IGF2 mutation and growth restriction. N Engl J Med 373: 349–356, 2015. doi: 10.1056/NEJMoa1415227. [DOI] [PubMed] [Google Scholar]
- 10.Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in β-cell function. Nature 414: 788–791, 2001. doi: 10.1038/414788a. [DOI] [PubMed] [Google Scholar]
- 11.Beneit N, Fernández-García CE, Martín-Ventura JL, Perdomo L, Escribano Ó, Michel JB, García-Gómez G, Fernández S, Díaz-Castroverde S, Egido J, Gómez-Hernández A, Benito M. Expression of insulin receptor (IR) A and B isoforms, IGF-IR, and IR/IGF-IR hybrid receptors in vascular smooth muscle cells and their role in cell migration in atherosclerosis. Cardiovasc Diabetol 15: 161, 2016. doi: 10.1186/s12933-016-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol 170: 63–70, 2001. doi: 10.1677/joe.0.1700063. [DOI] [PubMed] [Google Scholar]
- 13.Boldt HB, Bale LK, Resch ZT, Oxvig C, Overgaard MT, Conover CA. Effects of mutated pregnancy-associated plasma protein-a on atherosclerotic lesion development in mice. Endocrinology 154: 246–252, 2013. doi: 10.1210/en.2012-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldt HB, Kjaer-Sorensen K, Overgaard MT, Weyer K, Poulsen CB, Sottrup-Jensen L, Conover CA, Giudice LC, Oxvig C. The Lin12-notch repeats of pregnancy-associated plasma protein-A bind calcium and determine its proteolytic specificity. J Biol Chem 279: 38525–38531, 2004. doi: 10.1074/jbc.M405222200. [DOI] [PubMed] [Google Scholar]
- 15.Boldt HB, Overgaard MT, Laursen LS, Weyer K, Sottrup-Jensen L, Oxvig C. Mutational analysis of the proteolytic domain of pregnancy-associated plasma protein-A (PAPP-A): classification as a metzincin. Biochem J 358: 359–367, 2001. doi: 10.1042/bj3580359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boquist S, Ruotolo G, Skoglund-Andersson C, Tang R, Björkegren J, Bond MG, de Faire U, Brismar K, Hamsten A. Correlation of serum IGF-I and IGFBP-1 and -3 to cardiovascular risk indicators and early carotid atherosclerosis in healthy middle-aged men. Clin Endocrinol (Oxf) 68: 51–58, 2008. doi: 10.1111/j.1365-2265.2007.02998.x. [DOI] [PubMed] [Google Scholar]
- 17.Canela-Xandri O, Rawlik K, Tenesa A. An atlas of genetic associations in UK Biobank. Nat Genet 50: 1593–1599, 2018. doi: 10.1038/s41588-018-0248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardiovascular Disease Knowledge Portal Team.. Cardiovascular Disease Knowledge Portal (Online). http://broadcvdi.org/home/portalHome [1 April 2019].
- 19.Chang AC, Hook J, Lemckert FA, McDonald MM, Nguyen MA, Hardeman EC, Little DG, Gunning PW, Reddel RR. The murine stanniocalcin 2 gene is a negative regulator of postnatal growth. Endocrinology 149: 2403–2410, 2008. doi: 10.1210/en.2007-1219. [DOI] [PubMed] [Google Scholar]
- 20.Clemmons DR, Maile LA. Minireview: Integral membrane proteins that function coordinately with the insulin-like growth factor I receptor to regulate intracellular signaling. Endocrinology 144: 1664–1670, 2003. doi: 10.1210/en.2002-221102. [DOI] [PubMed] [Google Scholar]
- 21.Conover CA, Bale LK, Oxvig C. Targeted inhibition of pregnancy-associated plasma protein-a activity reduces atherosclerotic plaque burden in mice. J Cardiovasc Transl Res 9: 77–79, 2016. doi: 10.1007/s12265-015-9666-9. [DOI] [PubMed] [Google Scholar]
- 22.Conover CA, Bale LK, Powell DR. Inducible knock out of pregnancy-associated plasma protein-a gene expression in the adult mouse: effect on vascular injury response. Endocrinology 154: 2734–2738, 2013. doi: 10.1210/en.2013-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conover CA, Harrington SC, Bale LK, Oxvig C. Surface association of pregnancy-associated plasma protein-A accounts for its colocalization with activated macrophages. Am J Physiol Heart Circ Physiol 292: H994–H1000, 2007. doi: 10.1152/ajpheart.00798.2006. [DOI] [PubMed] [Google Scholar]
- 24.Conover CA, Harrington SC, Bale LK. Differential regulation of pregnancy associated plasma protein-A in human coronary artery endothelial cells and smooth muscle cells. Growth Horm IGF Res 18: 213–220, 2008. doi: 10.1016/j.ghir.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conover CA, Mason MA, Bale LK, Harrington SC, Nyegaard M, Oxvig C, Overgaard MT. Transgenic overexpression of pregnancy-associated plasma protein-A in murine arterial smooth muscle accelerates atherosclerotic lesion development. Am J Physiol Heart Circ Physiol 299: H284–H291, 2010. doi: 10.1152/ajpheart.00904.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Füchtbauer EM, Oxvig C, van Deursen J. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development 131: 1187–1194, 2004. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- 27.Conover CA. Discrepancies in insulin-like growth factor signaling? No, not really. Growth Horm IGF Res 30-31: 42–44, 2016. doi: 10.1016/j.ghir.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Crawford KM, Gallego-Fabrega C, Kourkoulis C, Miyares L, Marini S, Flannick J, Burtt NP, von Grotthuss M, Alexander B, Costanzo MC, Vaishnav NH, Malik R, Hall JL, Chong M, Rosand J, Falcone GJ; International Stroke Genetics Consortium . Cerebrovascular disease knowledge portal: an open-access data resource to accelerate genomic discoveries in stroke. Stroke 49: 470–475, 2018. doi: 10.1161/STROKEAHA.117.018922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dauber A, Muñoz-Calvo MT, Barrios V, Domené HM, Kloverpris S, Serra-Juhé C, Desikan V, Pozo J, Muzumdar R, Martos-Moreno GÁ, Hawkins F, Jasper HG, Conover CA, Frystyk J, Yakar S, Hwa V, Chowen JA, Oxvig C, Rosenfeld RG, Pérez-Jurado LA, Argente J. Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability. EMBO Mol Med 8: 363–374, 2016. doi: 10.15252/emmm.201506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev 10: 68–91, 1989. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- 31.DeChiara TM, Efstratiadis A, Robertsen EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 345: 78–80, 1990. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 32.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 24: 435–444, 2004. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 33.Duan C, Hawes SB, Prevette T, Clemmons DR. Insulin-like growth factor-I (IGF-I) regulates IGF-binding protein-5 synthesis through transcriptional activation of the gene in aortic smooth muscle cells. J Biol Chem 271: 4280–4288, 1996. doi: 10.1074/jbc.271.8.4280. [DOI] [PubMed] [Google Scholar]
- 34.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 23: 824–854, 2002. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 35.Fischer F, Schulte H, Mohan S, Tataru MC, Köhler E, Assmann G, von Eckardstein A. Associations of insulin-like growth factors, insulin-like growth factor binding proteins and acid-labile subunit with coronary heart disease. Clin Endocrinol (Oxf) 61: 595–602, 2004. doi: 10.1111/j.1365-2265.2004.02136.x. [DOI] [PubMed] [Google Scholar]
- 36.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 19: 3278–3288, 1999. doi: 10.1128/MCB.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedrich N, Haring R, Nauck M, Lüdemann J, Rosskopf D, Spilcke-Liss E, Felix SB, Dörr M, Brabant G, Völzke H, Wallaschofski H. Mortality and serum insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J Clin Endocrinol Metab 94: 1732–1739, 2009. doi: 10.1210/jc.2008-2138. [DOI] [PubMed] [Google Scholar]
- 38.Gagliardi AD, Kuo EYW, Raulic S, Wagner GF, DiMattia GE. Human stanniocalcin-2 exhibits potent growth-suppressive properties in transgenic mice independently of growth hormone and IGFs. Am J Physiol Endocrinol Metab 288: E92–E105, 2005. doi: 10.1152/ajpendo.00268.2004. [DOI] [PubMed] [Google Scholar]
- 39.Grant MB, Wargovich TJ, Ellis EA, Tarnuzzer R, Caballero S, Estes K, Rossing M, Spoerri PE, Pepine C. Expression of IGF-I, IGF-I receptor and IGF binding proteins-1, -2, -3, -4 and -5 in human atherectomy specimens. Regul Pept 67: 137–144, 1996. doi: 10.1016/S0167-0115(96)00124-3. [DOI] [PubMed] [Google Scholar]
- 40.Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Prog Neurobiol 70: 443–462, 2003. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Han Y, Cui J, Tao J, Guo L, Guo P, Sun M, Kang J, Zhang X, Yan C, Li S. CREG inhibits migration of human vascular smooth muscle cells by mediating IGF-II endocytosis. Exp Cell Res 315: 3301–3311, 2009. doi: 10.1016/j.yexcr.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Han Y, Luan B, Sun M, Guo L, Guo P, Tao J, Deng J, Wu G, Liu S, Yan C, Li S. Glycosylation-independent binding to extracellular domains 11-13 of mannose-6-phosphate/insulin-like growth factor-2 receptor mediates the effects of soluble CREG on the phenotypic modulation of vascular smooth muscle cells. J Mol Cell Cardiol 50: 723–730, 2011. doi: 10.1016/j.yjmcc.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res 100: 1696–1702, 2007. doi: 10.1161/CIRCRESAHA.106.146183. [DOI] [PubMed] [Google Scholar]
- 44.Higashi Y, Gautam S, Delafontaine P, Sukhanov S. IGF-1 and cardiovascular disease. Growth Horm IGF Res 45: 6–16, 2019. doi: 10.1016/j.ghir.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higashi Y, Sukhanov S, Shai SY, Danchuk S, Tang R, Snarski P, Li Z, Lobelle-Rich P, Wang M, Wang D, Yu H, Korthuis R, Delafontaine P. Insulin-like growth factor-1 receptor deficiency in macrophages accelerates atherosclerosis and induces an unstable plaque phenotype in apolipoprotein E-deficient mice. Circulation 133: 2263–2278, 2016. doi: 10.1161/CIRCULATIONAHA.116.021805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hjortebjerg R, Lindberg S, Pedersen S, Mogelvang R, Jensen JS, Oxvig C, Frystyk J, Bjerre M. Insulin-like growth factor binding protein 4 fragments provide incremental prognostic information on cardiovascular events in patients with ST-segment elevation myocardial infarction. J Am Heart Assoc 6: 83, 2017. doi: 10.1161/JAHA.116.005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hochberg Z, Hertz P, Maor G, Oiknine J, Aviram M. Growth hormone and insulin-like growth factor-I increase macrophage uptake and degradation of low density lipoprotein. Endocrinology 131: 430–435, 1992. doi: 10.1210/endo.131.1.1612024. [DOI] [PubMed] [Google Scholar]
- 48.Huynh H, Zheng J, Umikawa M, Zhang C, Silvany R, Iizuka S, Holzenberger M, Zhang W, Zhang CC. IGF binding protein 2 supports the survival and cycling of hematopoietic stem cells. Blood 118: 3236–3243, 2011. doi: 10.1182/blood-2011-01-331876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang JR, Huh JH, Lee Y, Lee SI, Rho SB, Lee JH. Insulin-like growth factor-binding protein-5 (IGFBP-5) inhibits TNF-α-induced NF-κB activity by binding to TNFR1. Biochem Biophys Res Commun 405: 545–551, 2011. doi: 10.1016/j.bbrc.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 50.Janssen JA, Stolk RP, Pols HA, Grobbee DE, Lamberts SW. Serum total IGF-I, free IGF-I, and IGFBP-1 levels in an elderly population: relation to cardiovascular risk factors and disease. Arterioscler Thromb Vasc Biol 18: 277–282, 1998. doi: 10.1161/01.ATV.18.2.277. [DOI] [PubMed] [Google Scholar]
- 51.Jepsen MR, Kløverpris S, Bøtkjær JA, Wissing ML, Andersen CY, Oxvig C. The proteolytic activity of pregnancy-associated plasma protein-A is potentially regulated by stanniocalcin-1 and -2 during human ovarian follicle development. Hum Reprod 31: 866–874, 2016. doi: 10.1093/humrep/dew013. [DOI] [PubMed] [Google Scholar]
- 52.Jepsen MR, Kløverpris S, Mikkelsen JH, Pedersen JH, Füchtbauer EM, Laursen LS, Oxvig C. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J Biol Chem 290: 3430–3439, 2015. doi: 10.1074/jbc.M114.611665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jespersen CH, Vestergaard KR, Schou M, Teisner B, Goetze JP, Iversen K. Pregnancy-associated plasma protein-A and the vulnerable plaque. Biomarkers Med 8: 1033–1047, 2014. doi: 10.2217/bmm.14.53. [DOI] [PubMed] [Google Scholar]
- 54.Jialal I, Crettaz M, Hachiya HL, Kahn CR, Moses AC, Buzney SM, King GL. Characterization of the receptors for insulin and the insulin-like growth factors on micro- and macrovascular tissues. Endocrinology 117: 1222–1229, 1985. doi: 10.1210/endo-117-3-1222. [DOI] [PubMed] [Google Scholar]
- 55.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jørgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation 106: 939–944, 2002. doi: 10.1161/01.CIR.0000027563.44593.CC. [DOI] [PubMed] [Google Scholar]
- 56.Kawachi S, Takeda N, Sasaki A, Kokubo Y, Takami K, Sarui H, Hayashi M, Yamakita N, Yasuda K. Circulating insulin-like growth factor-1 and insulin-like growth factor binding protein-3 are associated with early carotid atherosclerosis. Arterioscler Thromb Vasc Biol 25: 617–621, 2005. doi: 10.1161/01.ATV.0000154486.03017.35. [DOI] [PubMed] [Google Scholar]
- 57.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev 21: 215–244, 2000. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 58.King GL, Park K, Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: The 2015 Edwin Bierman Award Lecture. Diabetes 65: 1462–1471, 2016. doi: 10.2337/db16-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kjaer-Sorensen K, Engholm DH, Kamei H, Morch MG, Kristensen AO, Zhou J, Conover CA, Duan C, Oxvig C. Pregnancy-associated plasma protein A (PAPP-A) modulates the early developmental rate in zebrafish independently of its proteolytic activity. J Biol Chem 288: 9982–9992, 2013. doi: 10.1074/jbc.M112.426304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kløverpris S, Mikkelsen JH, Pedersen JH, Jepsen MR, Laursen LS, Petersen SV, Oxvig C. Stanniocalcin-1 potently inhibits the proteolytic activity of the metalloproteinase pregnancy-associated plasma protein-A. J Biol Chem 290: 21915–21924, 2015. doi: 10.1074/jbc.M115.650143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem 61: 307–330, 1992. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 62.Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, Willer CJ, Jackson AU, Vedantam S, Raychaudhuri S, Ferreira T, Wood AR, Weyant RJ, Segrè AV, Speliotes EK, Wheeler E, Soranzo N, Park JH, Yang J, Gudbjartsson D, Heard-Costa NL, Randall JC, Qi L, Vernon Smith A, Mägi R, Pastinen T, Liang L, Heid IM, Luan J, Thorleifsson G, Winkler TW, Goddard ME, Sin Lo K, Palmer C, Workalemahu T, , et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467: 832–838, 2010. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab 89: 114–120, 2004. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 64.Laursen LS, Kjaer-Sorensen K, Andersen MH, Oxvig C. Regulation of insulin-like growth factor (IGF) bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and -5. Mol Endocrinol 21: 1246–1257, 2007. doi: 10.1210/me.2006-0522. [DOI] [PubMed] [Google Scholar]
- 65.Laursen LS, Overgaard MT, Søe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett 504: 36–40, 2001. doi: 10.1016/S0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- 66.Laursen LS, Overgaard MT, Weyer K, Boldt HB, Ebbesen P, Christiansen M, Sottrup-Jensen L, Giudice LC, Oxvig C. Cell surface targeting of pregnancy-associated plasma protein A proteolytic activity. Reversible adhesion is mediated by two neighboring short consensus repeats. J Biol Chem 277: 47225–47234, 2002. doi: 10.1074/jbc.M209155200. [DOI] [PubMed] [Google Scholar]
- 67.Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR 3rd, Conover CA. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci USA 96: 3149–3153, 1999. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LeRoith D, Roberts CT Jr. The insulin-like growth factor system and cancer. Cancer Lett 195: 127–137, 2003. doi: 10.1016/S0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 69.Libby P. ALX-chemy: adding spice to the inflammatory soup of atherosclerosis. Cardiovasc Res 105: 3–5, 2015. doi: 10.1093/cvr/cvu245. [DOI] [PubMed] [Google Scholar]
- 70.Lu C, Kumar PA, Sun J, Aggarwal A, Fan Y, Sperling MA, Lumeng CN, Menon RK. Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. J Biol Chem 288: 15725–15735, 2013. doi: 10.1074/jbc.M113.460212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marouli E, Graff M, Medina-Gomez C, Lo KS, Wood AR, Kjaer TR, Fine RS, Lu Y, Schurmann C, Highland HM, Rüeger S, Thorleifsson G, Justice AE, Lamparter D, Stirrups KE, Turcot V, Young KL, Winkler TW, Esko T, Karaderi T, Locke AE, Masca NG, Ng MC, Mudgal P, Rivas MA, Vedantam S, Mahajan A, Guo X, Abecasis G, Aben KK, Adair LS, Alam DS, Albrecht E, Allin KH, Allison M, , et al.; EPIC-InterAct Consortium; CHD Exome+ Consortium; ExomeBP Consortium; T2D-Genes Consortium; GoT2D Genes Consortium; Global Lipids Genetics Consortium; ReproGen Consortium; MAGIC Investigators . Rare and low-frequency coding variants alter human adult height. Nature 542: 186–190, 2017. doi: 10.1038/nature21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathews LS, Norstedt G, Palmiter RD. Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci USA 83: 9343–9347, 1986. doi: 10.1073/pnas.83.24.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mikkelsen JH, Steffensen LB, Oxvig C. Development of a recombinant antibody towards PAPP-A for immunohistochemical use in multiple animal species. J Immunol Methods 404: 33–40, 2014. doi: 10.1016/j.jim.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Mohan S, Strong DD, Lempert UG, Tremollieres F, Wergedal JE, Baylink DJ. Studies on regulation of insulin-like growth factor binding protein (IGFBP)-3 and IGFBP-4 production in human bone cells. Acta Endocrinol (Copenh) 127: 555–564, 1992. doi: 10.1530/acta.0.1270555. [DOI] [PubMed] [Google Scholar]
- 75.Monget P, Mazerbourg S, Delpuech T, Maurel MC, Manière S, Zapf J, Lalmanach G, Oxvig C, Overgaard MT. Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: identification of cleavage site and characterization of IGFBP-2 degradation. Biol Reprod 68: 77–86, 2003. doi: 10.1095/biolreprod.102.007609. [DOI] [PubMed] [Google Scholar]
- 76.Nagaoka I, Trapnell BC, Crystal RG. Regulation of insulin-like growth factor I gene expression in the human macrophage-like cell line U937. J Clin Invest 85: 448–455, 1990. doi: 10.1172/JCI114458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ning Y, Schuller AG, Bradshaw S, Rotwein P, Ludwig T, Frystyk J, Pintar JE. Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein-3, -4, and -5. Mol Endocrinol 20: 2173–2186, 2006. doi: 10.1210/me.2005-0196. [DOI] [PubMed] [Google Scholar]
- 78.Ning Y, Schuller AG, Conover CA, Pintar JE. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol Endocrinol 22: 1213–1225, 2008. doi: 10.1210/me.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okura Y, Brink M, Zahid AA, Anwar A, Delafontaine P. Decreased expression of insulin-like growth factor-1 and apoptosis of vascular smooth muscle cells in human atherosclerotic plaque. J Mol Cell Cardiol 33: 1777–1789, 2001. doi: 10.1006/jmcc.2001.1441. [DOI] [PubMed] [Google Scholar]
- 80.Oxvig C, Haaning J, Kristensen L, Wagner JM, Rubin I, Stigbrand T, Gleich GJ, Sottrup-Jensen L. Identification of angiotensinogen and complement C3dg as novel proteins binding the proform of eosinophil major basic protein in human pregnancy serum and plasma. J Biol Chem 270: 13645–13651, 1995. doi: 10.1074/jbc.270.23.13645. [DOI] [PubMed] [Google Scholar]
- 81.Oxvig C, Sand O, Kristensen T, Gleich GJ, Sottrup-Jensen L. Circulating human pregnancy-associated plasma protein-A is disulfide-bridged to the proform of eosinophil major basic protein. J Biol Chem 268: 12243–12246, 1993. [PubMed] [Google Scholar]
- 82.Oxvig C. The role of PAPP-A in the IGF system: location, location, location. J Cell Commun Signal 9: 177–187, 2015. doi: 10.1007/s12079-015-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park S, Youn JC, Shin DJ, Park CM, Kim JS, Ko YG, Choi D, Ha JW, Jang Y, Chung N. Genetic polymorphism in the pregnancy-associated plasma protein-A associated with acute myocardial infarction. Coron Artery Dis 18: 417–422, 2007. doi: 10.1097/MCA.0b013e328241d967. [DOI] [PubMed] [Google Scholar]
- 84.Patel VA, Zhang QJ, Siddle K, Soos MA, Goddard M, Weissberg PL, Bennett MR. Defect in insulin-like growth factor-1 survival mechanism in atherosclerotic plaque-derived vascular smooth muscle cells is mediated by reduced surface binding and signaling. Circ Res 88: 895–902, 2001. doi: 10.1161/hh0901.090305. [DOI] [PubMed] [Google Scholar]
- 85.Perks CM, Bowen S, Gill ZP, Newcomb PV, Holly JM. Differential IGF-independent effects of insulin-like growth factor binding proteins (1-6) on apoptosis of breast epithelial cells. J Cell Biochem 75: 652–664, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 86.Postnikov AB, Smolyanova TI, Kharitonov AV, Serebryanaya DV, Kozlovsky SV, Tryshina YA, Malanicev RV, Arutyunov AG, Murakami MM, Apple FS, Katrukha AG. N-terminal and C-terminal fragments of IGFBP-4 as novel biomarkers for short-term risk assessment of major adverse cardiac events in patients presenting with ischemia. Clin Biochem 45: 519–524, 2012. doi: 10.1016/j.clinbiochem.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 87.Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab 3: 46–56, 2007. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 88.Resch ZT, Simari RD, Conover CA. Targeted disruption of the pregnancy-associated plasma protein-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia after vascular injury. Endocrinology 147: 5634–5640, 2006. doi: 10.1210/en.2006-0493. [DOI] [PubMed] [Google Scholar]
- 89.Rimbault M, Beale HC, Schoenebeck JJ, Hoopes BC, Allen JJ, Kilroy-Glynn P, Wayne RK, Sutter NB, Ostrander EA. Derived variants at six genes explain nearly half of size reduction in dog breeds. Genome Res 23: 1985–1995, 2013. doi: 10.1101/gr.157339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem 253: 2769–2776, 1978. [PubMed] [Google Scholar]
- 91.Rinderknecht E, Humbel RE. Primary structure of human insulin-like growth factor II. FEBS Lett 89: 283–286, 1978. doi: 10.1016/0014-5793(78)80237-3. [DOI] [PubMed] [Google Scholar]
- 92.Rom WN, Basset P, Fells GA, Nukiwa T, Trapnell BC, Crysal RG. Alveolar macrophages release an insulin-like growth factor I-type molecule. J Clin Invest 82: 1685–1693, 1988. doi: 10.1172/JCI113781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ruotolo G, Båvenholm P, Brismar K, Eféndic S, Ericsson CG, de Faire U, Nilsson J, Hamsten A. Serum insulin-like growth factor-I level is independently associated with coronary artery disease progression in young male survivors of myocardial infarction: beneficial effects of bezafibrate treatment. J Am Coll Cardiol 35: 647–654, 2000. doi: 10.1016/S0735-1097(99)00591-4. [DOI] [PubMed] [Google Scholar]
- 94.Russo VC, Azar WJ, Yau SW, Sabin MA, Werther GA. IGFBP-2: The dark horse in metabolism and cancer. Cytokine Growth Factor Rev 26: 329–346, 2015. doi: 10.1016/j.cytogfr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Sato A, Nishimura S, Ohkubo T, Kyogoku Y, Koyama S, Kobayashi M, Yasuda T, Kobayashi Y. Three-dimensional structure of human insulin-like growth factor-I (IGF-I) determined by 1H-NMR and distance geometry. Int J Pept Protein Res 41: 433–440, 1993. doi: 10.1111/j.1399-3011.1993.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 96.Schuler-Lüttmann S, Monnig G, Enbergs A, Schulte H, Breithardt G, Assmann G, Kerber S, von Eckardstein A. Insulin-like growth factor-binding protein-3 is associated with the presence and extent of coronary arteriosclerosis. Arterioscler Thromb Vasc Biol 20: 1175, 2000. doi: 10.1161/01.ATV.20.4.e10. [DOI] [PubMed] [Google Scholar]
- 97.Schut AF, Janssen JA, Deinum J, Vergeer JM, Hofman A, Lamberts SW, Oostra BA, Pols HA, Witteman JC, van Duijn CM. Polymorphism in the promoter region of the insulin-like growth factor I gene is related to carotid intima-media thickness and aortic pulse wave velocity in subjects with hypertension. Stroke 34: 1623–1627, 2003. doi: 10.1161/01.STR.0000076013.00240.B0. [DOI] [PubMed] [Google Scholar]
- 98.Sesti G, Mannino GC, Andreozzi F, Greco A, Perticone M, Sciacqua A, Marini MA, Perticone F. A polymorphism at IGF1 locus is associated with carotid intima media thickness and endothelium-dependent vasodilatation. Atherosclerosis 232: 25–30, 2014. doi: 10.1016/j.atherosclerosis.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 99.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe-/- mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler Thromb Vasc Biol 30: 1916–1924, 2010. doi: 10.1161/ATVBAHA.110.210831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Rosen CJ, Delafontaine P. Low circulating insulin-like growth factor I increases atherosclerosis in ApoE-deficient mice. Am J Physiol Heart Circ Physiol 300: H1898–H1906, 2011. doi: 10.1152/ajpheart.01081.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Silha JV, Murphy LJ. Insights from insulin-like growth factor binding protein transgenic mice. Endocrinology 143: 3711–3714, 2002. doi: 10.1210/en.2002-220116. [DOI] [PubMed] [Google Scholar]
- 102.Singh P, Dai B, Dhruva B, Widen SG. Episomal expression of sense and antisense insulin-like growth factor (IGF)-binding protein-4 complementary DNA alters the mitogenic response of a human colon cancer cell line (HT-29) by mechanisms that are independent of and dependent upon IGF-I. Cancer Res 54: 6563–6570, 1994. [PubMed] [Google Scholar]
- 103.Sirbu A, Nicolae H, Martin S, Barbu C, Copaescu C, Florea S, Panea C, Fica S. IGF-1 and insulin resistance are major determinants of common carotid artery thickness in morbidly obese young patients. Angiology 67: 259–265, 2016. doi: 10.1177/0003319715586499. [DOI] [PubMed] [Google Scholar]
- 104.Sivasubramaniyam T, Schroer SA, Li A, Luk CT, Shi SY, Besla R, Dodington DW, Metherel AH, Kitson AP, Brunt JJ, Lopes J, Wagner KU, Bazinet RP, Bendeck MP, Robbins CS, Woo M. Hepatic JAK2 protects against atherosclerosis through circulating IGF-1. JCI Insight 2: e93735, 2017. doi: 10.1172/jci.insight.93735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sjögren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Törnell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA 96: 7088–7092, 1999. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skjaerbaek C, Frystyk J, Kaal A, Laursen T, Møller J, Weeke J, Jørgensen JO, Sandahl Christiansen J, Orskov H. Circadian variation in serum free and total insulin-like growth factor (IGF)-I and IGF-II in untreated and treated acromegaly and growth hormone deficiency. Clin Endocrinol (Oxf) 52: 25–33, 2000. doi: 10.1046/j.1365-2265.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 107.Slaaby R, Schäffer L, Lautrup-Larsen I, Andersen AS, Shaw AC, Mathiasen IS, Brandt J. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J Biol Chem 281: 25869–25874, 2006. doi: 10.1074/jbc.M605189200. [DOI] [PubMed] [Google Scholar]
- 108.Soos MA, Field CE, Siddle K. Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. Biochem J 290: 419–426, 1993. doi: 10.1042/bj2900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sorrell AM, Shand JH, Tonner E, Gamberoni M, Accorsi PA, Beattie J, Allan GJ, Flint DJ. Insulin-like growth factor-binding protein-5 activates plasminogen by interaction with tissue plasminogen activator, independently of its ability to bind to plasminogen activator inhibitor-1, insulin-like growth factor-I, or heparin. J Biol Chem 281: 10883–10889, 2006. doi: 10.1074/jbc.M508505200. [DOI] [PubMed] [Google Scholar]
- 110.Spadaro O, Goldberg EL, Camell CD, Youm YH, Kopchick JJ, Nguyen KY, Bartke A, Sun LY, Dixit VD. Growth hormone receptor deficiency protects against age-related NLRP3 inflammasome activation and immune senescence. Cell Rep 14: 1571–1580, 2016. doi: 10.1016/j.celrep.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spallarossa P, Brunelli C, Minuto F, Caruso D, Battistini M, Caponnetto S, Cordera R. Insulin-like growth factor-I and angiographically documented coronary artery disease. Am J Cardiol 77: 200–202, 1996. doi: 10.1016/S0002-9149(96)90600-1. [DOI] [PubMed] [Google Scholar]
- 112.Steffensen LB, Conover CA, Bjørklund MM, Ledet T, Bentzon JF, Oxvig C. Stanniocalcin-2 overexpression reduces atherosclerosis in hypercholesterolemic mice. Atherosclerosis 248: 36–43, 2016. doi: 10.1016/j.atherosclerosis.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 113.Sukhanov S, Higashi Y, Shai SY, Snarski P, Danchuk S, D’Ambra V, Tabony M, Woods TC, Hou X, Li Z, Ozoe A, Chandrasekar B, Takahashi SI, Delafontaine P. SM22α (smooth muscle protein 22-α) promoter-driven IGF1R (insulin-like growth factor 1 receptor) deficiency promotes atherosclerosis. Arterioscler Thromb Vasc Biol 38: 2306–2317, 2018. doi: 10.1161/ATVBAHA.118.311134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 27: 2684–2690, 2007. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 115.Sun Y, Chen D, Cao L, Zhang R, Zhou J, Chen H, Li Y, Li M, Cao J, Wang Z. MiR-490-3p modulates the proliferation of vascular smooth muscle cells induced by ox-LDL through targeting PAPP-A. Cardiovasc Res 100: 272–279, 2013. doi: 10.1093/cvr/cvt172. [DOI] [PubMed] [Google Scholar]
- 116.Sureshbabu A, Okajima H, Yamanaka D, Tonner E, Shastri S, Maycock J, Szymanowska M, Shand J, Takahashi S, Beattie J, Allan G, Flint D. IGFBP5 induces cell adhesion, increases cell survival and inhibits cell migration in MCF-7 human breast cancer cells. J Cell Sci 125: 1693–1705, 2012. doi: 10.1242/jcs.092882. [DOI] [PubMed] [Google Scholar]
- 117.Svensson J, Sjögren K, Levin M, Borén J, Tivesten Å, Ohlsson C. Increased diet-induced fatty streak formation in female mice with deficiency of liver-derived insulin-like growth factor-I. Endocrine 52: 550–560, 2016. doi: 10.1007/s12020-015-0809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Terkelsen CJ, Oxvig C, Nørgaard BL, Glerup S, Poulsen TS, Lassen JF, Møller HJ, Thuesen L, Falk E, Nielsen TT, Andersen HR. Temporal course of pregnancy-associated plasma protein-A in angioplasty-treated ST-elevation myocardial infarction patients and potential significance of concomitant heparin administration. Am J Cardiol 103: 29–35, 2009. doi: 10.1016/j.amjcard.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 119.Thøgersen VB, Heickendorff L, Ledet T. Effect of insulin and growth hormone on the synthesis of radiolabelled proteoglycans from cultured human arterial smooth-muscle cells. Eur J Endocrinol 134: 326–330, 1996. doi: 10.1530/eje.0.1340326. [DOI] [PubMed] [Google Scholar]
- 120.von der Thüsen JH, Borensztajn KS, Moimas S, van Heiningen S, Teeling P, van Berkel TJ, Biessen EA. IGF-1 has plaque-stabilizing effects in atherosclerosis by altering vascular smooth muscle cell phenotype. Am J Pathol 178: 924–934, 2011. doi: 10.1016/j.ajpath.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tran D, Bergholz J, Zhang H, He H, Wang Y, Zhang Y, Li Q, Kirkland JL, Xiao ZX. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell 13: 669–678, 2014. doi: 10.1111/acel.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Underwood LE, Thissen JP, Lemozy S, Ketelslegers JM, Clemmons DR. Hormonal and nutritional regulation of IGF-I and its binding proteins. Horm Res 42: 145–151, 1994. doi: 10.1159/000184187. [DOI] [PubMed] [Google Scholar]
- 123.van den Beld AW, Bots ML, Janssen JA, Pols HA, Lamberts SW, Grobbee DE. Endogenous hormones and carotid atherosclerosis in elderly men. Am J Epidemiol 157: 25–31, 2003. doi: 10.1093/aje/kwf160. [DOI] [PubMed] [Google Scholar]
- 124.Varghese R, Gagliardi AD, Bialek PE, Yee SP, Wagner GF, Dimattia GE. Overexpression of human stanniocalcin affects growth and reproduction in transgenic mice. Endocrinology 143: 868–876, 2002. doi: 10.1210/endo.143.3.8671. [DOI] [PubMed] [Google Scholar]
- 125.Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22α promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res 95: 981–988, 2004. doi: 10.1161/01.RES.0000147961.09840.fb. [DOI] [PubMed] [Google Scholar]
- 126.Wang H, Song Y, Zhang C, Zhan J, Zhang R, Wang H. Genetic relationship between serum pregnancy-associated plasma protein-A gene polymorphism and ischemic cerebrovascular disease in a Northern Han Chinese population. Neural Regen Res 7: 528–533, 2012. doi: 10.3969/j.issn.1673-5374.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang J, Niu W, Nikiforov Y, Naito S, Chernausek S, Witte D, LeRoith D, Strauch A, Fagin JA. Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest 100: 1425–1439, 1997. doi: 10.1172/JCI119663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J, Razuvaev A, Folkersen L, Hedin E, Roy J, Brismar K, Hedin U. The expression of IGFs and IGF binding proteins in human carotid atherosclerosis, and the possible role of IGF binding protein-1 in the regulation of smooth muscle cell proliferation. Atherosclerosis 220: 102–109, 2012. doi: 10.1016/j.atherosclerosis.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 129.Wang J, Niu W, Witte DP, Chernausek SD, Nikiforov YE, Clemens TL, Sharifi B, Strauch AR, Fagin JA. Overexpression of insulin-like growth factor-binding protein-4 (IGFBP-4) in smooth muscle cells of transgenic mice through a smooth muscle alpha-actin-IGFBP-4 fusion gene induces smooth muscle hypoplasia. Endocrinology 139: 2605–2614, 1998. doi: 10.1210/endo.139.5.5986. [DOI] [PubMed] [Google Scholar]
- 130.Weyer K, Boldt HB, Poulsen CB, Kjaer-Sorensen K, Gyrup C, Oxvig C. A substrate specificity-determining unit of three Lin12-Notch repeat modules is formed in trans within the pappalysin-1 dimer and requires a sequence stretch C-terminal to the third module. J Biol Chem 282: 10988–10999, 2007. doi: 10.1074/jbc.M607903200. [DOI] [PubMed] [Google Scholar]
- 131.Wo D, Peng J, Ren DN, Qiu L, Chen J, Zhu Y, Yan Y, Yan H, Wu J, Ma E, Zhong TP, Chen Y, Liu Z, Liu S, Ao L, Liu Z, Jiang C, Peng J, Zou Y, Qian Q, Zhu W. Opposing roles of Wnt inhibitors IGFBP-4 and Dkk1 in cardiac ischemia by differential targeting of LRP5/6 and β-catenin. Circulation 134: 1991–2007, 2016. doi: 10.1161/CIRCULATIONAHA.116.024441. [DOI] [PubMed] [Google Scholar]
- 132.Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, Chu AY, Estrada K, Luan J, Kutalik Z, Amin N, Buchkovich ML, Croteau-Chonka DC, Day FR, Duan Y, Fall T, Fehrmann R, Ferreira T, Jackson AU, Karjalainen J, Lo KS, Locke AE, Mägi R, Mihailov E, Porcu E, Randall JC, Scherag A, Vinkhuyzen AA, Westra HJ, Winkler TW, Workalemahu T, Zhao JH, Absher D, Albrecht E, Anderson D, , et al.; Electronic Medical Records and Genomics (eMEMERGEGE) ConsortiumMIGen ConsortiumPAGEGE Consortium; LifeLines Cohort Study . Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet 46: 1173–1186, 2014. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wright RJ, Holly JM, Galea R, Brincat M, Mason HD. Insulin-like growth factor (IGF)-independent effects of IGF binding protein-4 on human granulosa cell steroidogenesis. Biol Reprod 67: 776–781, 2002. doi: 10.1095/biolreprod.101.001511. [DOI] [PubMed] [Google Scholar]
- 134.Xue Y, Yan Y, Gong H, Fang B, Zhou Y, Ding Z, Yin P, Zhang G, Ye Y, Yang C, Ge J, Zou Y. Insulin-like growth factor binding protein 4 enhances cardiomyocytes induction in murine-induced pluripotent stem cells. J Cell Biochem 115: 1495–1504, 2014. doi: 10.1002/jcb.24804. [DOI] [PubMed] [Google Scholar]
- 135.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96: 7324–7329, 1999. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yasuoka H, Hsu E, Ruiz XD, Steinman RA, Choi AM, Feghali-Bostwick CA. The fibrotic phenotype induced by IGFBP-5 is regulated by MAPK activation and egr-1-dependent and -independent mechanisms. Am J Pathol 175: 605–615, 2009. doi: 10.2353/ajpath.2009.080991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu XH, He LH, Gao JH, Zhang DW, Zheng XL, Tang CK. Pregnancy-associated plasma protein-A in atherosclerosis: Molecular marker, mechanistic insight, and therapeutic target. Atherosclerosis 278: 250–258, 2018. doi: 10.1016/j.atherosclerosis.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 138.Zaina S, Pettersson L, Ahrén B, Brånén L, Hassan AB, Lindholm M, Mattsson R, Thyberg J, Nilsson J. Insulin-like growth factor II plays a central role in atherosclerosis in a mouse model. J Biol Chem 277: 4505–4511, 2002. doi: 10.1074/jbc.M108061200. [DOI] [PubMed] [Google Scholar]
- 139.Zhang M, Smith EP, Kuroda H, Banach W, Chernausek SD, Fagin JA. Targeted expression of a protease-resistant IGFBP-4 mutant in smooth muscle of transgenic mice results in IGFBP-4 stabilization and smooth muscle hypotrophy. J Biol Chem 277: 21285–21290, 2002. doi: 10.1074/jbc.M112082200. [DOI] [PubMed] [Google Scholar]
- 140.Zhao G, Sutliff RL, Weber CS, Wang J, Lorenz J, Paul RJ, Fagin JA. Smooth muscle-targeted overexpression of insulin-like growth factor I results in enhanced vascular contractility. Endocrinology 142: 623–632, 2001. doi: 10.1210/endo.142.2.7941. [DOI] [PubMed] [Google Scholar]
- 141.Zhou S, Cui M, Yin Z, Li R, Zhu J, Zhou H. Correlation of single nucleotide polymorphisms in the pregnancy-associated plasma protein-A gene with carotid plaques. BMC Cardiovasc Disord 15: 60, 2015. doi: 10.1186/s12872-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, Naito AT, Nishi J, Ueno H, Umezawa A, Minamino T, Nagai T, Kikuchi A, Asashima M, Komuro I. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature 454: 345–349, 2008. [Erratum in Nature 506: 254, 2014.] doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]