Abstract
Acute kidney injury (AKI) is a strong independent predictor of mortality and often results in incomplete recovery of renal function, leading to progressive chronic kidney disease (CKD). Many clinical trials have been conducted on the basis of promising preclinical data, but no therapeutic interventions have been shown to improve long-term outcomes after AKI. This is partly due to the failure of preclinical studies to accurately model clinically relevant injury and long-term outcomes on CKD progression. Here, we evaluated the long-term effects of AKI on CKD progression in three animal models reflecting diverse etiologies of AKI: repeat-dose cisplatin, rhabdomyolysis, and ischemia-reperfusion injury. Using transdermal measurement of glomerular filtration rate as a clinically relevant measure of kidney function and quantification of peritubular capillary density to measure capillary rarefaction, we showed that repeat-dose cisplatin caused capillary rarefaction and decreased renal function in mice without a significant increase in interstitial fibrosis, whereas rhabdomyolysis-induced AKI led to severe interstitial fibrosis, but renal function and peritubular capillary density were preserved. Furthermore, long-term experiments in mice with unilateral ischemia-reperfusion injury showed that restoration of renal function 12 wk after a contralateral nephrectomy was associated with increasing fibrosis, but a reversal of capillary rarefaction was seen at 4 wk. These data demonstrate that clear dissociation between kidney function and fibrosis in these models of AKI to CKD progression and suggest that peritubular capillary rarefaction is more strongly associated with CKD progression than renal fibrosis.
Keywords: acute kidney injury, capillary rarefaction, chronic kidney disease, glomerular filtration rate, renal fibrosis
INTRODUCTION
Acute kidney injury (AKI), defined as a rapid decline in kidney function that occurs over a 7-day period or less (11, 27, 50), is a heterogeneous clinical syndrome that is precipitated by a variety of different injurious events including dehydration, heart failure, blood loss, major trauma (including surgery), muscle injury (rhabdomyolysis), sepsis, and drug toxicities (5, 27, 50). Despite its heterogeneity, diagnosis of AKI is a strong independent predictor of mortality and often results in incomplete recovery, resulting in progressive chronic kidney disease (CKD) (5, 27, 50). However, despite the significance of the problem and the large number of clinical trials that have been conducted on the basis of results of promising preclinical data (60), no therapeutic interventions have been shown to definitively prevent, or to improve, long-term outcomes after AKI (6, 39, 42, 71). In part, this failure to translate preclinical data into effective clinical interventions results from the limited use of models and study end points that reflect the diversity of injurious events and important clinical and pathological outcomes in patients with AKI (16). In addition, the relatively short-term observation periods used in many preclinical studies, often only 24–72 h after the initiating injurious event, do not reflect the long-term effects of interventions on CKD progression and patient survival after an episode of AKI (60). To address these concerns, we have evaluated the long-term effects of two toxin models of AKI and of ischemia-reperfusion injury (IRI), simulating the effects of cisplatin (CP) chemotherapy, rhabdomyolysis, and renal hypoperfusion, on CKD progression in patients with AKI.
CP was one of the first and is still one of the most widely used chemotherapeutics for the treatment of a wide range of solid organ malignancies (22). By far the most common and clinically significant side effect of CP is AKI, which limits its use in many patients (10, 43, 69). Typical chemotherapy treatment regimens in patients involve repeat cycles of CP at 3- to 4-wk intervals. Even with hydration and avoidance of other nephrotoxic agents, about one-third of these patients develop AKI 7–10 days after CP dosing (10). In addition, the risk of AKI increases with each subsequent dose, and survivors of cancer who have undergone repeat cycles of CP are at increased risk of CKD progression (45). Despite this, until recently, the majority of preclinical studies have only evaluated short-term outcomes, 24–72 h, after injection of rodents with a single high (lethal) dose of CP (43). There are more limited data on the long-term effects of CP on CKD progression after AKI. More recent attempts to model the human condition have evaluated the effects of repeat dose CP (RDCP) in mice either with (48, 49) or without an associated tumor burden (7, 31, 55, 56, 68). Although dosing regimens vary between studies and strains of mice used, RDCP studies have shown that mice develop a progressive, stepwise increase in blood urea nitrogen (BUN) and serum creatinine values after the initial injury and that this is associated with increased tubulointerstitial fibrosis (TIF) in some (31, 48, 55) but not all studies (7, 68).
Rhabdomyolysis is a condition in which skeletal muscle rapidly breaks down, releasing myoglobin, electrolytes, and enzymes such as creatine kinase, lactate dehydrogenase, and aldolase into the bloodstream (9, 67). Common causes of rhabdomyolysis include direct trauma to the muscle (crush injury), strenuous exercise, drugs and toxins, muscle ischemia, infections, and both acquired and inherited myopathies (9, 67). AKI is the most significant complication of rhabdomyolysis, and the severity of rhabdomyolysis is an independent risk factor for AKI (25). In rodents, a single intramuscular dose of 50% glycerol in water causes disruption of myofiber plasma membranes (32), release of free myoglobin into the circulation, and rhabdomyolysis-induced AKI (Rb-AKI) (57). Like CP-induced AKI, the majority of preclinical Rb-AKI studies in rodents have evaluated short-term outcomes, 24–72 h, after injection of glycerol (44), precluding evaluation of long-term effects of interventions on renal functional and histological recovery. More limited long-term outcome studies have indicated that there is extensive TIF, but renal function is only mildly impaired 30–60 days after injury in mice and rats (4, 62).
The choice of primary end points that are used to evaluate AKI to CKD progression in experimental models of AKI should be chosen to reflect important clinical and pathological outcomes in patients (16). Commonly used end points include the evaluation of serum BUN and creatinine and measures of renal fibrosis, including histochemical staining for tubulointerstitial atrophy, fibrosis, and collagen deposition. However, serum creatinine and BUN are insensitive measures of kidney function, particularly in mice (19, 70), and renal fibrosis does not always correlate with renal function in long-term outcome studies that have been performed in rodents models of RDCP and Rb-AKI (4, 7, 62, 68). Direct measurement of glomerular filtration rate (GFR) avoids the limitations of serum creatinine overestimating GFR in mice with mild CKD (19); however, it is impractical to perform the repeated timed urine collections and/or blood draws that are required for inulin clearance studies, the gold standard for measuring GFR, on large cohorts of mice that are required to evaluate long-term outcomes after experimental AKI (41, 47). More recently, direct analysis of GFR in larger cohorts of mice has become feasible with the use of transdermal GFR monitoring devices (51, 53). These determine GFR by transdermal measurement of fluorescence decay after a single intravenous injection FITC-conjugated sinistrin, a fluorescently conjugated inulin analog (53). This assay can be performed relatively easily on cohorts of 20–30 conscious mice over a 3- to 4-h period (51). Peritubular capillary (PTC) density has also been used to evaluate the long-term impact of AKI on CKD progression in experimental models of AKI (3). PTC loss occurs in patients with progressive CKD from different causes (1, 63) and is associated with CKD progression in rodent models of aging, nephron reduction [5/6 nephrectomy (Nx)], ureteric obstruction, and Alport syndrome (1, 28–30). PTC loss has also been described in rodents after IRI and folic acid-induced AKI (1, 2, 34, 75). Since PTC loss perpetuates tissue hypoxia and TIF, which are characteristics of the kidney in CKD (14, 72, 75), this has led to the belief that PTC loss is a major driver of CKD progression after AKI (3, 36, 46, 66). Despite this, the relationship between GFR, fibrosis, and PTC density has not been evaluated in other models of AKI to CKD progression, including RDCP and Rb-AKI.
In the present study, therefore, we evaluated the long-term effects of RDCP and rhabdomyolysis models of kidney injury in mice by direct measurement of GFR, as determined by transdermal FITC-sinistrin clearance, as well as the assessment of renal fibrosis and PTC density. Mice that received weekly doses of CP have markedly reduced GFR 4 wk after the initial injury without a significant increase in TIF, whereas mice with severe Rb-AKI have preserved GFR 5 wk after the initial injury despite developing marked TIF. Decreased GFR in RDCP mice is, however, associated with reduced PTC density, whereas PTC density is preserved 5 wk after Rb-AKI. Consistent with these findings, we showed that there was a reversal of impaired renal function in mice after unilateral IRI with a delayed contralateral Nx [delayed Nx (DN)-IRI model] at 12 versus 4 wk after the initiating injury and that this was associated with restoration of PTC density, despite increasing renal fibrosis. These findings demonstrate a clear dissociation between fibrosis and CKD progression (GFR) after AKI in different models of experimental AKI and suggest that reduced PTC density is more closely associated with CKD progression after RDCP, Rb-AKI, and IR-AKI than renal fibrosis. These data also underscore the importance of evaluating GFR and PTC density as primary end points in models of AKI to CKD progression in mice.
MATERIALS AND METHODS
Animals were purchased from Charles River (Wilmington, MA). Mice were maintained on a 12:12-h light-dark cycle with free access to standard food and water, except where specified. For all injury models, body weight was monitored throughout the study, and blood and kidneys were collected at the time of euthanization. Blood was collected for the determination of serum creatinine and BUN, and kidneys were harvested for histopathology and quantitative RT-PCR for fibrosis markers. All animal experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Repeat-dose CP.
Male FVB/N mice aged 14 wk (24–32 g) were given 7 mg/kg ip CP (Fresenius Kabi, Lake Zurich, IL) in 0.9% saline (normal saline) once a week for 4 wk, as previously described by Sharp et al. (55). Mice were given 0.5 ml of 0.9% saline subcutaneously immediately and 1 and 2 days after each CP dose to treat salt wasting (24). BUN was assessed at baseline, before each CP injection (days 7, 14, and 21), and 1 and 3 days after each CP injection (days 8, 10, 15, 17, 22, and 24). GFR was assessed at day 26 by transdermal measurement of FITC-sinistrin clearance, as previously described (51). Mice were euthanized on day 28 by cervical dislocation. We used uninjured age- and sex-matched littermate controls for these experiments.
Rhabdomyolysis-induced AKI.
Male BALB/c mice aged 18 wk (22–25 g) were given 6.0 ml/kg im of 50% glycerol (Invitrogen, Carlsbad, CA) in sterile water after 18 h of water deprivation. Glycerol (50%) was injected into anterior thigh muscles while mice were anesthetized with a ketamine-xylazine mixture (120–150 mg/kg ketamine and 12–15 mg/kg xylazine) intraperitoneally, with the glycerol dose split equally between the two hindlimbs. Pain relief (0.015 mg buprenorphine sq) was provided every 12 h for 24 h starting immediately after intramuscular injections. Mice were also given 0.5 ml sc of 0.9% saline immediately after and 24 h after intramuscular injections. BUN was assessed at baseline and 1, 3, 7, 14, 21, 28, and 36 days after glycerol injection. GFR was assessed at day 35 by transdermal FITC-sinistrin clearance, as previously described (51). Mice were euthanized on day 36 by cervical dislocation. Age- and sex-matched littermates were injected with 6.0 ml/kg of sterile water intramuscularly as controls for these experiments. A separate cohort of Rb-AKI mice was euthanized at week 9, with GFR measurements at weeks 5 and 9.
DN-IRI model.
Male BALB/c mice age 10 wk (22–29 g) underwent 30-min unilateral left renal pedicle clamping followed by a right Nx on day 8, as previously described (58). Briefly, mice were anaesthetized with a ketamine-xylazine mixture (120–150 mg/kg ip ketamine and 12–15 mg/kg ip xylazine) and placed prone on a heating pad at 38°C. The kidney was exteriorized via a dorsal incision, and a clamp was placed on the left renal pedicle for 30 min, or the right kidney was tied off with silk suture and removed; 0.5 ml sc of 0.9% saline was given immediately after and the morning after each surgery. Pain relief was provided with buprenorphine subcutaneously for 48 h after surgery. All surgeries were started in the morning and completed by noon.
Assessment of renal function.
Blood was collected by submandibular vein or cardiac puncture (terminal) into lithium-heparin-coated microcuvette tubes. Four microliters of plasma were collected for BUN and measured in duplicate, according to the manufacturer’s instructions (Infinity Urea, Thermo Scientific, Waltham, MA). Plasma creatinine was measured in 5 μl plasma by LC-MS/MS at the O’Brien Core Center for AKI Research, University of Alabama (64, 74). Transdermal FITC-sinistrin clearance was performed in conscious mice, as previously described (51). The FITC-sinistrin half-life was calculated using a three-compartment model with linear fit using MPD Studio software (MediBeacon, Mannheim, Germany). The FITC-sinistrin half-life (in min) was converted to GFR (in μl/min) as previously described (53) with correction for mouse body weight.
Picrosirius red staining.
A transverse section of the kidney was processed and stained with picrosirius red (PSR) solution as previously described (52). Quantification of PSR staining was performed by an observer blinded to the injury conditions (R. Delgada and A. Menshikh), as previously described (52).
Periodic acid-Schiff staining.
Five-micrometer paraffin sections were generated as described above and stained using a periodic acid-Schiff (PAS) staining kit (Sigma-Aldrich, St Louis, MO), as described by the manufacturer. More than 20 images of the cortex and outer stripe of the outer medulla (OSOM) were captured with light microscopy at ×400 magnification. Tubular injury and glomerular pathology were scored by a pathologist (H. Yang) blinded to the injury conditions. Tubular injury was based on the following scoring system on a scale of 0−4: 0 = no injury, 1 = 1–25% of area injured, 2 = 26–50% of area injured, 3 = 51–75% of area injured, and 4 = 76–100% of area injured. Acute tubulointerstitial injury was defined as interstitial edema with loss of the brush border, shedding of both necrotic and viable epithelial cells into the tubular lumen, intratubular cast formation, tubular dilation, or naked tubular basement membrane. Chronic tubulointerstitial injury was defined as a matrix-rich expansion of the interstitium with sloughing of tubular epithelial cells, tubular cast formation, tubular dilation, tubular atrophy, or thickening of the tubular basement membrane. For glomerular scoring, individual glomeruli were scored on a scale of 0–4, according to the percent area of mesangial expansion or sclerosis: 0 = no injury, 1 = 1–25% of area injured, 2 = 26–50% of area injured, 3 = 51–75% of area injured, and 4 = 76–100% of area injured. The final glomerular damage score was calculated as follows: final glomerular damage score = 0 × (% grade 0 glomeruli) + 1 × (% grade 1 glomeruli) + 2 × (% grade 2 glomeruli) + 3 × (% grade 3 glomeruli) + 4 × (% grade 4 glomeruli). More than 50 glomeruli were scored per mouse.
CD31 staining and quantification.
A transverse section of the middle of the kidney was fixed in 10% buffered formalin at 4°C for 4 h, extensively washed in PBS, and embedded in paraffin. Five-micrometer sections were deparaffinized and hydrated, and the heat-induced antigen retrieval method was used by incubating sections with BOND Epitope Retrieval Solution 2 (Leica Biosystems, Buffalo Grove, IL) for 20 min at 100°C. Sections were blocked with an avidin-biotin blocking kit (Vector Laboratories, Burlingame, CA) for 30 min and rinsed; additional blocking was performed by universal blocking reagent 1X (BioGenex, Freemont, CA) for 30 min at room temperature. Sections were incubated with rat anti-mouse CD31 antibody (Clone SZ31, Dianova, Hamburg, Germany) diluted 1:100 at 4°C overnight. Slides were then incubated with biotin SP-conjugated AffiniPure donkey anti-rat IgG secondary antibody (Jackson Immuno Research Laboratories, Bar Harbor, ME) diluted 1:1,000 for 1 h at room temperature. After sections were washed in PBS, they were incubated with Neutravidin Dylight 650 (ThermoFisher Scientific, Waltham, MA) diluted in 1:250 for 30 min at room temperature and then with Hoechst 33342 (ThermoFisher Scientific) diluted 1:1,000 for 5 min. Slides were mounted with SlowFade Gold antifade reagent (Invitrogen, ThermoFisher Scientific). Quantification of CD31 staining was performed by an observer blinded to the injury conditions (A. Menshikh). For this, at least eight images of the cortex and OSOM were captured using a Ti-Eclipse inverted microscope (Nikon). Images were captured with a Plan-Apochromat lambda ×20/0.50 DLL Ph1 objective (Nikon) through a Cy5 common filter set cube. Excluding glomeruli and muscularized vessels, the percent CD31-positive area within each image was calculated using ImageJ, and the mean percent CD31-positive area was calculated for each animal. Results are expressed as fold changes versus controls. Glomeruli and vessels were manually identified and excluded from the analysis.
Quantitative RT-PCR.
A transverse section of the middle of the kidney was snap frozen in liquid nitrogen, and RNA was extracted, cDNA was synthesized, and quantitative RT-PCR was performed as previously described (52). The following primer sequences were used: GAPDH, forward 5′-TGGAGAAACCTGCCAAGTATGA-3′ and reverse 5′-GAAGAGTGGGAGTTGCTGTTGA-3′; α-smooth muscle actin (α-SMA), forward 5′-CGCTGTCAGGAACCCTGAGA-3′ and reverse 5′-CGAAGCCGGCCTTACAGA-3′; lysyl oxidase-like 2 (LoxL2), forward 5′-GATCTTCAGCCCCGATGGA-3′ and reverse 5′-CAAGGGTTGCTCTGGCTTGT-3′; collagen type I-α1 (Col1-α1), forward 5′-CCCGCCGATGTCGCTAT-3′ and reverse 5′-GCTACGCTGTTCTTGCAGTGAT-3′; and collagen type III-α1 (Col3-α1), forward 5′-AGGCAACAGTGGTTCTCCTG-3′ and reverse 5′-GACCTCGTGCTCCAGTTAGC-3′.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism 7 software (San Diego, CA). An unpaired, two-tailed t-test was used to compare control and injured groups, one-way ANOVA was used to compare multiple groups, and two-way ANOVA was used to compare changes between groups over time. Post hoc Dunnett’s or Sidak’s tests were used for multiple comparisons, as indicated in the figures. P values of <0.05 were considered statistically significant.
RESULTS
Repeat-dose CP.
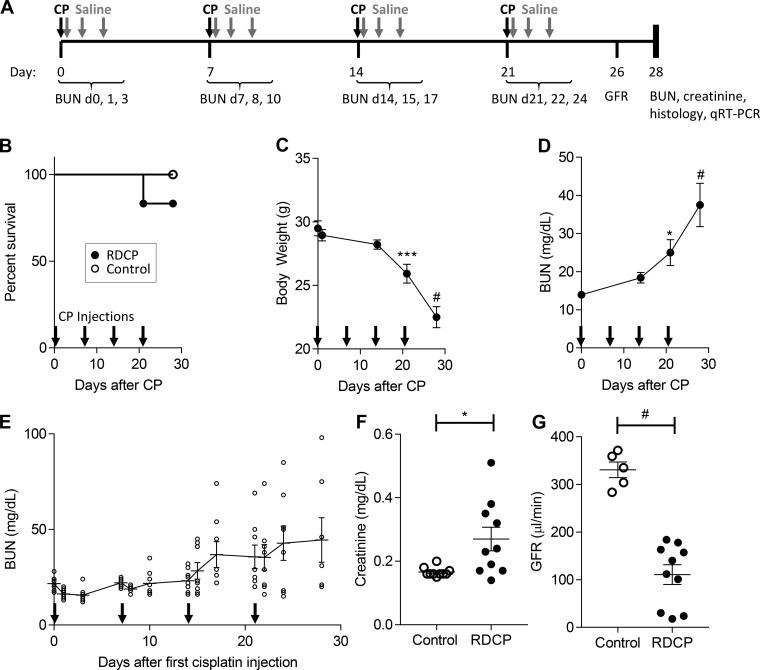
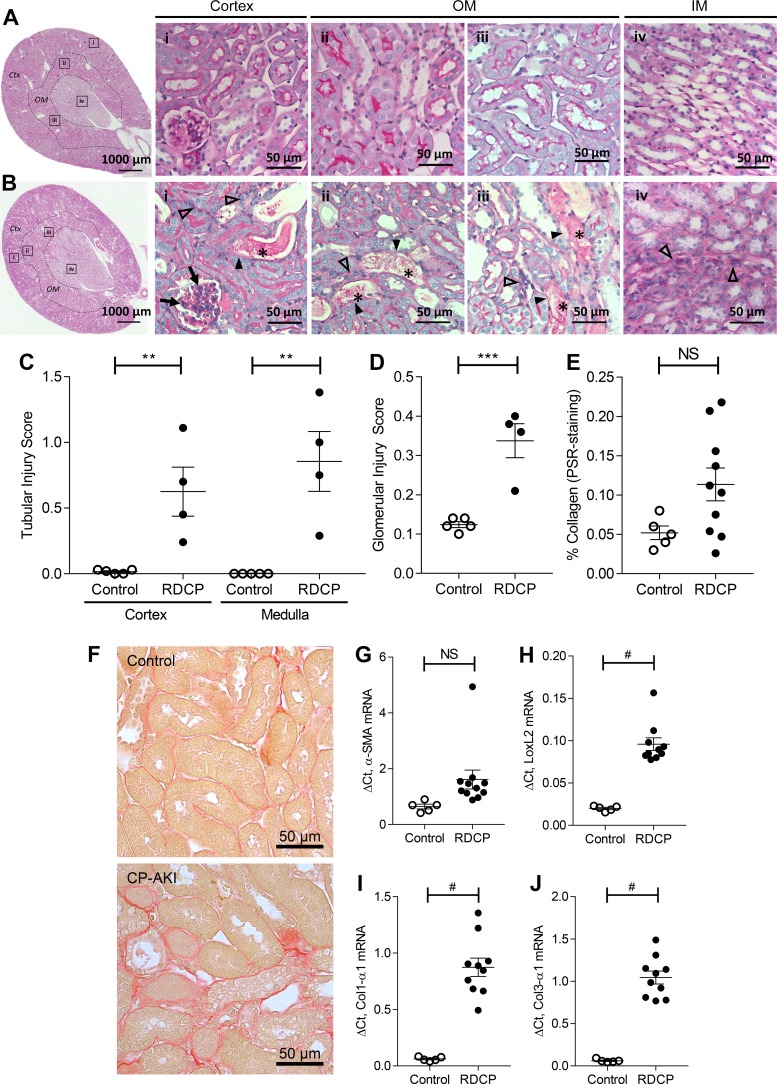
We established the RDCP model in male FVB/N mice in our laboratory, as previously described by Sharp et al. (55). Fig. 1A shown an outline of the RDCP study design. Mortality was 16.7% in the RDCP group (2 of 12 mice died by day 21) and 0% in the control group (0 of 5 mice died; Fig. 1B). RDCP mice progressively lost weight during the study and weighed significantly less than at baseline from day 21 onward (Fig. 1C). BUN levels in RDCP mice also progressively increased throughout the study, becoming significantly elevated above baseline levels from day 21 onward, reaching highest levels at the study end point (Fig. 1D). BUN levels steadily increased in a stepwise fashion after repeated CP dosing over time, with peak value achieved 3 days after each CP treatment (Fig. 1E). Plasma creatinine was significantly elevated at the study end point, but there was considerable overlap with age-matched healthy control littermates (Fig. 1F). There was, however, a marked and nonoverlapping reduction in GFR in RDCP mice compared with healthy controls at the study end point (Fig. 1G). In addition, there was evidence of chronic tubulointerstitial injury in PAS-stained kidney sections, including tubular dilation, protein casts, and expansion of interstitial matrix in the cortex and OSOM compared with healthy controls (Fig. 2, A and B). This was confirmed by semiquantitative analysis of chronic tubular injury scores in RDCP mice compared with healthy controls in both the cortex and OSOM (Fig. 2C). There was also mild glomerulosclerosis, with focal mesangial expansion and proliferation, in RDCP mice (Fig. 2, B and D). Renal fibrosis, as assessed by quantifying the percent PSR staining in the OSOM, was increased in RDCP mice compared with control mice, but showed considerable overlap and was not significantly increased in RDCP mice compared with healthy control mice (Fig. 2, E and F). We also evaluated mRNA expression of the fibrosis markers α-SMA, LoxL2, Col1-α1, and Col3-α1 in renal tissue. α-SMA was not significantly increased in RDCP mice compared with control mice, but LoxL2, Col1-α1, and Col3-α1 mRNAs were markedly increased (Fig. 2, G–J).
Fig. 1.
Repeated-dose cisplatin (RDCP) model in male FVB/N mice. A: schematic of the RDCP study design. Fourteen-week-old male mice were administered a low dose (7 mg/kg) of CP (RDCP; n = 12) or vehicle (control; n = 5) once weekly for 4 wk (black arrows). Saline (0.5 ml of 0.9% saline) was administered immediately and 1 and 2 days after each CP injection to treat salt wasting (gray arrows). B: survival of mice over time. C: changes in body weight over time. D: changes in blood urea nitrogen (BUN) levels over time. E: BUN levels measured before and 1 and 3 days after each CP injection in a separate cohort of mice (n = 10). F: serum creatinine at the study end point (day 28). G: glomerular filtration rate (GFR) calculated from transdermal measurement of FITC-sinistrin clearance on day 26. Data points in B represent percent survival. Data points in C and D represent means ± SE. Data points in E–G represent individual animals, with means ± SE of the groups indicated, and the solid line in E connects the mean values over time. One-way ANOVA was used to determine statistical significance, and post hoc Dunnett’s multiple-comparison test was used to compare with baseline day 0 (C and D). A two-sided t-test was used to compare RDCP to controls in F and G. *P ≤ 0.05; ***P ≤ 0.001; #P ≤ 0.0001.
Fig. 2.
Tubular injury and fibrosis after repeated-dose cisplatin (RDCP). A–D: data from a separate cohort of mice given 7 mg/kg CP (n = 4) or vehicle (n = 5) weekly for 4 wk. A and B: representative images of a control kidney (A) and a CP-induced acute kidney injury (CP-AKI) kideny (B) at day 28. The highlighted boxes (i–iv) in the low-power images correspond to the magnified images to the right. In injured kidneys (B), dilated tubules with casts (*) show deepithelialization (solid arrowheads). Cortical glomeruli have mesangial expansion (arrows). Intratubular fibrosis and hypercellularity (open arrowheads) are found in the cortex (Ctx; i), outer medulla (OM; ii and iii), and inner medulla (IM; iv). C: tubular injury scores in the Ctx and OM from periodic acid-Schiff-stained kidney sections at day 28. D: glomerular injury score from periodic acid-Schiff-stained kidney sections at day 28. E–I: data from the same cohort of mice shown in Fig. 1. E: quantification of renal fibrosis (picrosirius red staining) in the OM at day 28. F: representative images of the OM of picrosirius red-stained kidney sections. G−J: mRNA expression of the following fibrosis markers: α-smooth muscle actin (α-SMA; G), lysyl oxidase-like 2 (LoxL2; H), collagen type I-α1 (Col1-α1; I), and collagen type III-α3 (Col3-α1; J) relative to GAPDH on day 28. Data points in C–E and G–J represent individual animals, with means ± SE of the groups indicated. A two-sided t-test was used to compare RDCP to control mice in C–E and G–J. NS, not significant (P > 0.05). **P ≤ 0.01; ***P ≤ 0.001; #P ≤ 0.0001.
Rhabdomyolysis.
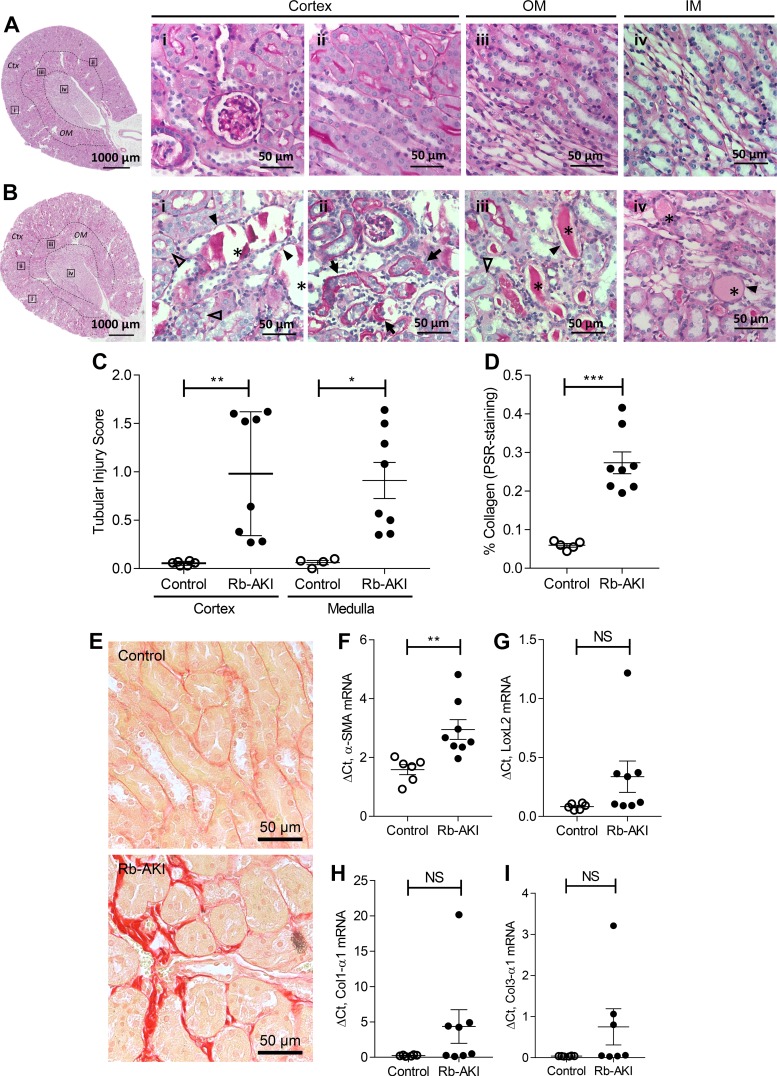
Figure 3A shows an outline of the Rb-AKI study design in male BALB/c mice. Mortality was 20% in Rb-AKI mice (2 of 10 mice died by day 5) and 0% in water-injected control mice (Fig. 3B). Body weight in Rb-AKI mice initially decreased after glycerol injections, reaching the lowest point at day 3, but then progressively increased for the remainder of the study (Fig. 3C). BUN levels were markedly elevated 1 and 3 days after glycerol injection, rapidly decreasing by day 7. BUN was elevated but not statistically increased compared with controls after day 3 and progressively decreased for the remainder of the study (Fig. 3D). Interestingly, while plasma creatinine levels were elevated in Rb-AKI mice (Fig. 3E), GFR was unchanged at the study end point (Fig. 3F). There was extensive tubulointerstitial injury in the cortex and OSOM, including features of acute injury with necrotic tubules, granular casts, vacuoles in proximal tubule cells, and interstitial inflammation (Fig. 4, A and B). Unlike the RDCP model, we did not observe any evidence of glomerular injury in Rb-AKI mice. Semiquantitative analysis of acute tubular injury scores demonstrated a marked increase in tubular injury in the both cortex and OSOM of Rb-AKI mice at day 36 (Fig. 4C). This was associated with a marked increase in fibrosis, as assessed by quantifying the percent PSR-positive area in the OSOM (Fig. 4, D and E). In contrast, while α-SMA mRNA was elevated in Rb-AKI mice, LoxL2, Col1-α1, and Col3-α1 mRNAs were not significantly increased (Fig. 4, F–H).
Fig. 3.
Rhabdomyolysis-induced acute kidney injury (Rb-AKI) model in male BALB/c mice. A: schematic of the Rb-AKI study design. Eighteen-week-old male BALB/c mice were water restricted for 18 h before intramuscular administration of 6 ml/kg of 50% glycerol or vehicle. B: survival of mice over time. C: changes in body weight over time. D: changes in blood urea nitrogen (BUN) levels over time. E: creatinine measured at the study end point (day 36). F: glomerular filtration rate (GFR) calculated from transdermal measurement of FITC-sinistrin clearance on day 35. Data points in B represent percent survival. Data points in C and D represent means ± SE. Data points in E and F represent individual animals, with means ± SE of the groups indicated. One-way ANOVA (C) and two-way ANOVA (D) were used to determine statistical significance, and a post hoc Dunnett’s multiple-comparisons test was used to compare with baseline day 0 (C and D). A two-sided t test was used to compare Rb-AKI with control mice in E and F. NS, not significant (P > 0.05). *P ≤ 0.05; #P ≤ 0.0001.
Fig. 4.
Tubular injury and fibrosis after rhabdomyolysis-induced acute kidney injury (Rb-AKI). A and B: representative images of a control kidney (A) and a Rb-AKI kidney (B) at day 36. The highlighted boxes (i–iv) in the low-power images correspond to the magnified images to the right. In injured kidneys (B), cortical tubules were dilated with friable casts (i, *), while the outer (OM; iii) and inner medulla (IM; iv) had solid casts (*). There was also peritubular fibrosis with interstitial proliferation in the cortex (Ctx; ii, arrows) and OM (not shown). Tubules also showed deepithelialization (closed arrowheads) and epithelial vacuoles (open arrowheads). C: tubular injury scores in the Ctx and OM from periodic acid-Schiff-stained kidney sections at day 36. D: quantification of renal fibrosis (picrosirius red staining) in the OM at day 36. E: representative images of the OM of picrosirius red-stained kidney sections. F−I: mRNA expression of the following fibrosis markers: α-smooth muscle actin (α-SMA; F), lysyl oxidase-like 2 (LoxL2; G), collagen type I-α1 (Col1-α1; H), and collagen type III-α1 (Col3-α1; I) relative to GAPDH on day 36. Data points in C, D, and F–I represent individual animals, with means ± SE of the groups indicated. A two-sided t-test was used to compare Rb-AKI with control mice in C, D, and F–I. NS, not significant (P > 0.05). **P ≤ 0.01; ***P ≤ 0.001.
Long-term evaluation of kidney function and fibrosis after Rb-AKI.
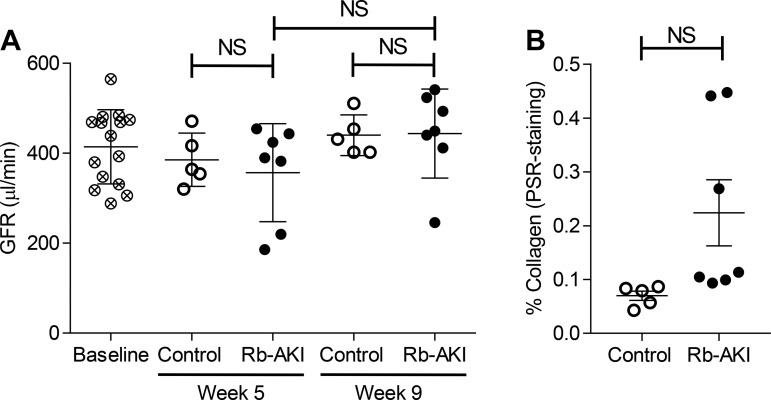
To determine whether the failure to see a decrease in GFR in Rb-AKI mice was because the kidney injury had not progressed sufficiently after 5 wk to impact function, we repeated the experiments in a separate cohort of mice and evaluated GFR at week 5 (day 35) and week 9 after injury (day 65). GFR was unchanged in Rb-AKI mice at weeks 5 and 9 (Fig. 5A), while PSR staining in the OSOM was not statistically increased compared with controls after 9 wk (Fig. 5B). These findings indicate that Rb-AKI does not give rise to long-term CKD progression.
Fig. 5.
Renal function and fibrosis 9 wk after rhabdomyolysis-induced acute kidney injury (Rb-AKI). A separate cohort of Rb-AKI (n = 7) and control (n = 5) mice underwent the same experimental protocol shown in Fig. 3A except that mice were monitored for 9 wk. A: glomerular filtration rate (GFR) calculated from transdermal measurement of FITC-sinistrin clearance at baseline, week 5, and week 9. B: quantification of renal fibrosis (picrosirius red staining) in the outer medulla at week 9. Data points represent individual animals, with means ± SE of the groups indicated. One-way ANOVA was used to determine statistical significance, and a post hoc Dunnett’s multiple-comparison test was used to determine pairwise significance. A two-sided t-test was used to compare Rb-AKI with control mice in B. NS, not significant (P > 0.05).
Comparative analysis of fibrosis and PTC density in RDCP and Rb-AKI.
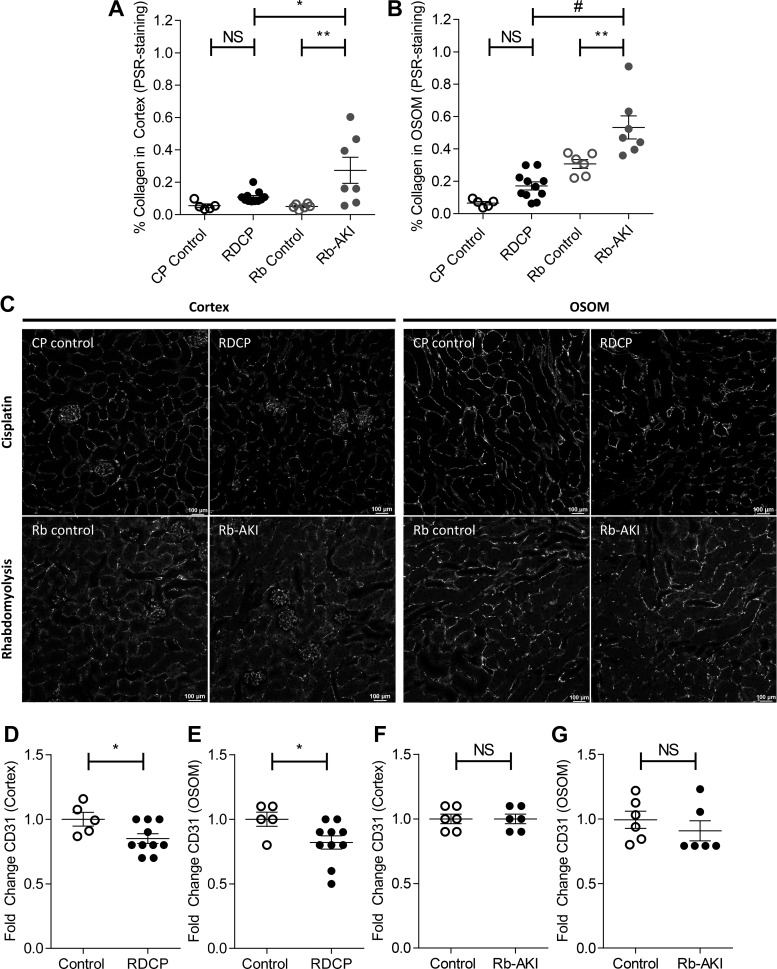
To determine whether reduced GFR was associated with increased fibrosis in RDCP mice compared with Rb-AKI mice, we compared fibrosis experiments at the study end points in the two models. Because there are changes in baseline levels of PSR staining between batches of assays, PSR staining was repeated on both RDCP and Rb-AKI samples at the same time to allow for direct comparison between models. We also evaluated fibrosis in the cortex and OSOM to determine whether there were differences in the distribution of PSR staining (which reflects collagen deposition) between models. RDCP mice had slightly increased PSR staining in both the cortex and OSOM, but this was not significantly different from uninjured controls (Fig. 6, A and B). In contrast, Rb-AKI mice had markedly increased fibrosis in both the cortex and OSOM compared with their controls. Moreover, direct comparison of PSR staining between the two models showed that Rb-AKI mice had more fibrosis than RDCP mice, despite preservation of GFR after Rb-AKI.
Fig. 6.
Renal fibrosis and capillary rarefaction in the repeated-dose cisplatin (RDCP) and rhabdomyolysis (Rb)-induced acute kidney injury (Rb-AKI) models. Quantification of fibrosis [picrosirius red (PSR) staining] was repeated to compare both models side by side. A and B: renal fibrosis (PSR staining) in the cortex (A) and outer stripe of the outer medulla (OSOM; B) at day 28 in RDCP and CP control mice and at day 36 in Rb-AKI and Rb control mice. C–G: capillary rarefaction was quantified as the fold change in CD31 staining in the cortex and OSOM at day 28 in CP-AKI and CP control mice (D and E) and at day 36 in Rb-AKI and Rb control mice (F and G). Data points represent individual animals, with means ± SE of the groups indicated. One-way ANOVA was used to determine statistical significance, and a post hoc Dunnett’s multiple-comparisons test was used to determine pairwise significance to compare RDCP vs. CP control, Rb-AKI vs. Rb control, and RDCP vs. Rb-AKI mice. P values were adjusted for multiple comparisons using Sidak’s multiple-comparison test. A two-sided t-test was used to compare RDCP and Rb-AKI groups with their respective control groups in D–G. NS, not significant (P > 0.05). *P ≤ 0.05; **P ≤ 0.01; #P ≤ 0.0001.
PTC rarefaction.
Dissociation between fibrosis and GFR was unexpected and suggests that differences in the effects of RDCP and Rb-AKI on CKD progression do not solely result from differences in fibrosis. Since PTC loss is also associated with CKD progression in diverse rodent models (1, 2, 28–30, 34, 75), we quantified PTC density by calculating the surface area of CD31 staining in the cortex and OSOM (excluding glomeruli and muscularized vessels) (Fig. 6C), as previously described (1, 35). Because there were differences in background staining in controls between the two mouse strains, for comparative purposes, data were expressed as fold changes versus controls for each study. The fold change in CD31 surface area was significantly decreased in both the cortex and OSOM of mice after RDCP (Fig. 6, C–E) but not in Rb-AKI mice at the study end point (Fig. 6, C, F, G). These data indicate that PTC density correlates with GFR changes and suggest that PTC rarefaction is a better marker of CKD progression than fibrosis in the RDCP and Rb-AKI models.
Reversible PTC rarefaction after ischemia-reperfusion-induced AKI with a delayed Nx.
To determine whether changes in PTC density correlate with changes in CKD progression in other models of AKI, we evaluated long-term outcomes in a model we developed in which unilateral IRI is followed by a contralateral Nx 8 days after the initial injury [delayed Nx (DN-IRI) model] (58). We have used this approach to evaluate effects of therapeutic and genetic interventions on functional and histological recovery after IR-AKI 4 wk after the initiating injury (12, 13, 58, 59, 61). However, a delayed contralateral Nx has also been shown to improve long-term functional and histological recovery in a rat model of unilateral IRI (21). Therefore, we evaluated changes in GFR and renal fibrosis over a 12-wk time course, to determine whether there was longer-term progression or resolution of CKD in the DN-IRI model. As shown in Fig. 7A, we evaluated two separate cohorts of mice, both with littermate controls that underwent a Nx alone. One cohort was evaluated at 4 wk, and the other cohort underwent GFR measurements at multiple time points up to 12 wk after unilateral IRI. DN-IRI mice had reduced GFR up to 4 wk after the initial injury, but by 8 wk, GFR was indistinguishable from Nx controls (Fig. 7B). To understand why decreased GFR 4 wk after IRI normalizes over time, we evaluated renal fibrosis by PSR staining for collagen in the OSOM of kidneys from mice euthanized 4 and 12 wk after the initiating injury. To our surprise, there was increased PSR staining of the OSOM in mice 12 wk after IRI compared with both Nx controls and PSR staining of the OSOM 4 wk after IRI (Fig. 7, C and D). Interestingly, while this was associated with increased mRNA expression of the fibrosis markers LoxL2, Col1α1, and Col3α1 (but not α-SMA) in the kidneys 12 wk after IRI compared with Nx controls, levels were lower than those at 4 wk after IRI (Fig. 7, E–H), suggesting that by 12 wk, active new transcription of fibrosis genes is reduced despite persistence of actual fibrosis, indicating that mRNA expression for markers is not a good marker for the progression of fibrosis in long-term studies. Next, we evaluated PTC density in the OSOM of kidneys of mice 4 and 12 wk after DN-IRI. These experiments demonstrated that the anticipated reduction in PTC density in the OSOM at 4 wk was no longer seen 12 wk after DN-IRI (Fig. 8, A and B). These findings are consistent with our findings in the RDCP and Rb-AKI mouse models, and they further indicate that changes in PTC density that correlate with changes in GFR in the two models are dynamically regulated in the DN-IRI model in which there is a spontaneous improvement in GFR over time.
Fig. 7.
Ischemia-reperfusion injury (IRI) with delayed contralateral nephrectomy (Nx) (DN-IRI model) in male BALB/c mice. A: schematic of the DN-IRI study design. Eleven-week-old male BALB/c mice underwent 30 min of unilateral renal ischemia followed by a contralateral Nx 8 days later (n = 5) or Nx only (n = 5). B: glomerular filtration rate (GFR) calculated from transdermal measurement of FITC-sinistrin clearance at baseline, day 9 (1 day after Nx), and weeks 2, 3, 4, 6, 8, and 12. C: quantification of renal fibrosis [picrosirius red (PSR) staining] in the outer medulla at weeks 4 and 12. D: representative images of the outer medulla of PSR-stained kidney sections. E−G: fold changes of mRNA expression of the following fibrosis markers: α-smooth muscle actin (α-SMA; E), lysyl oxidase-like 2 (LoxL2; F), collagen type I-α1 (Col1-α1; G), and collagen type III-α1 (Col3-α1; H) relative to GAPDH at weeks 4 and 12. Bars in B represent means ± SE. Data points in C and E–H represent individual animals, with means ± SE of the groups indicated. Two-way ANOVA (B) or one-way ANOVA (C, E–H) was used to determine statistical significance, and a post hoc Dunnett’s multiple-comparison test was used to determine pairwise significance compared with Nx control mice within each time point. NS, not significant (P > 0.05). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Fig. 8.
Capillary rarefaction after ishemia-repefusion injury with a delayed contralateral nephrectomy (Nx) (DN-IRI model). A and B: capillary rarefaction was quantified as the fold change in CD31 staining in the cortex and outer medulla at weeks 4 and 12 in DN-IRI and Nx control mice. Data points represent individual animals, with means ± SE of the groups indicated. One-way ANOVA was used to determine statistical significance, and a post hoc Dunnett’s multiple-comparison test was used to determine pairwise significance. NS, not significant (P > 0.05). *P ≤ 0.05; **P ≤ 0.01.
DISCUSSION
In the present study, we evaluated the long-term effects of three different AKI models, which simulate the effects of RDCP, acute rhabdomyolysis, and renal hypoperfusion, on CKD progression in patients with AKI. One of the most striking findings is that despite the severity of renal fibrosis and tubular injury, rhabdomyolysis-induced AKI does not result in progressive CKD after 9 wk, whereas RDCP gives rise to progressive CKD after 4 wk, despite having only a slight increase in renal fibrosis. These findings were reinforced by our long-term experiments of unilateral IRI with a delayed contralateral Nx, in which increasing renal fibrosis was associated with improvement in renal function between 4 and 12 wk after the initial injury. Although it has been proposed that fibrosis contributes to tissue ischemia by impairing postglomerular capillary blood flow and by impairing oxygen delivery from PTCs to the tubules (36, 40), our findings suggest that fibrosis alone is unlikely to account for progressive CKD after AKI. On the surface, these findings conflict with the widely held belief that renal fibrosis is the main determinant of CKD progression after AKI. This is based largely on analyses of patients and models of CKD in which fibrosis correlates closely with a progressive decline in renal function (40, 54). However, our study evaluated the process of CKD progression that occurs after an episode of AKI in which the dynamics and mechanisms of CKD may be quite different.
Our results indicate that the level of PTC rarefaction is more closely associated with CKD progression or resolution than the level of renal fibrosis on long-term followup in three different models of AKI. While there are extensive experimental data showing a direct relationship between fibrosis and PTC rarefaction after bilateral or unilateral ischemia-repefusion-induced AKI (1, 2, 14, 26, 34, 35), we are only aware of one study that evaluated the effect of another AKI model on fibrosis and PTC rarefaction, in this case, acute crystal nephropathy induced by high-dose folic acid (75). Our CP experiments identified an additional model of AKI, in which PTC rarefaction is associated with a long-term reduction in GFR but, in this case, with only a minor increase in fibrosis. However, our study also provides the first clear evidence that fibrosis and PTC rarefaction are dissociated in other models of AKI. In particular, we have shown that there is a marked increase in renal fibrosis despite preservation of PTC density and GFR on long-term followup of mice after Rb-AKI and DN-IRI. Moreover, by evaluating different time points in mice after DN-IRI, we have shown that restoration of renal function 12 wk after injury was associated with reversal of PTC rarefaction seen at 4 wk. These are the first data that we are aware of in which reversal of capillary rarefaction is associated with an improvement in renal function despite progressively increasing fibrosis. These findings support a direct causal relationship between PTC rarefaction, rather than fibrosis, on CKD progression after CP, rhabdomyolysis, and ischemic AKI.
As it has been shown that capillary rarefaction persists in the outer medulla of mice 8 wk after severe bilateral IRI (34) and 56 days after unilateral IRI (18), it is likely that restoration of capillary density 12 wk after DN-IRI results from the delayed Nx that is performed 8 days after the initial injury. The mechanism by which this occurs remains to be determined. However, delayed contralateral Nx has also been shown to improve long-term functional and histological recovery in a rat model of unilateral IRI (21). This was associated with increased GFR and an increase in renal plasma flow but not in calculated single nephron GFRs (21). These findings suggest that the Nx promotes the recruitment of functioning nephrons that would otherwise have become atrophic in the injured kidney. It is unclear from our results whether restoration of PTC density is the cause or consequence of increased nephron recruitment. However, these data do suggest that therapeutic interventions that increase renal blood flow and nephron recruitment after injury might also reduce CKD progression after AKI. They also underscore the importance not only of evaluating renal fibrosis but also of evaluating PTC density in models of AKI to CKD transition in rodents.
Because of the ethical and clinical challenges obtaining renal biopsies for research in patients with AKI (17), aside from a study of long-term allograft dysfunction in patients after renal transplantation (63), there are no published data on the association between PTC rarefaction, fibrosis, and CKD progression in long-term studies of patients with AKI. However, recent biomarker studies from the TRIBE-AKI consortium showing that higher levels of the circulating antiangiogenic factor soluble VEGF receptor 1 and reduced levels of the proangiogenic factors VEGF and placental growth factor after cardiac surgery are independently associated with worse outcomes and longer duration of AKI suggest that the regulation of renal angiogenesis, and by inference PTC density, may play a wider role in determining long-term recovery after AKI (37). On this basis, while there are limited patient data, there is evidence that the level of PTC rarefaction may also play an important role in determining functional recovery in patients with AKI.
The Siskind laboratory (55, 56) modified the standard single high-dose CP protocol to a RDCP protocol to create a clinically relevant AKI to CKD progression model of CP nephropathy. Using this model, we have shown that there was only a minor increase in fibrosis despite a profound reduction in GFR in RDCP mice. These findings are consistent with two other studies that also showed only a minor increase in fibrosis despite marked reductions in GFR 4 and 25 wk after RDCP treatment (7, 68). Furthermore, while the Siskind laboratory initially reported significantly increased fibrosis in the RDCP model at 4 wk (55), these results were not replicated in a later publication (56), which only reported significantly increased fibrosis compared with controls at 6 mo, but not 4 wk. This suggests that the extent of renal fibrosis at the 4-wk time point is mild, at worst, and is unlikely to explain the effects of RDCP on GFR. Histological analysis of RDCP kidneys revealed not only that there was chronic tubulointerstitial atrophy but also mild glomerulosclerosis with focal mesangial expansion and proliferation. Sharp and colleagues noted periglomerular fibrosis at 6 mo (56) but did not report any glomerular changes at week 4 (55). This may, therefore, contribute to the reduction in GFR seen in the RDCP model. In this respect, it is notable that we saw no evidence of glomerular injury in the Rb-AKI model in which GFR was preserved at the study end points. However, the extent of glomerular injury that we saw in the RDCP model was mild and unlikely to account for the severe reduction in GFR seen after 4 wk in this model. Furthermore, the mechanisms of glomerular injury in this model are unclear. CP has been shown to be cleared by glomerular filtration (73). It is toxic to the glomerulus in guinea pigs, affecting all glomerular components, including podocytes (33), although we could not find any published data on CP and glomerulosclerosis in humans. However, albuminuria is often seen in patients with CP-induced kidney injury (15, 38), suggesting that glomerular damage may occur in CP nephrotoxicity. Glomerular pathology has also been reported in two different, repeat-dose, genetic models of diphtheria toxin-induced renal tubular cell depletion (23, 65). Thus, it is also possible that glomerular injury occurs after RDCP because tubular cell death has indirect effects on glomerular pathophysiology, potentially caused by impaired tubuloglomerular cross-talk, effects of peritubular inflammation, or PTC rarefaction.
Despite the severity of the initial injury after glycerol injection in our model of Rb-AKI (based on marked elevation in BUN 1–3 days after the glycerol injection), there was complete recovery of GFR 5 wk after the initiating injury. Furthermore, longer-term followup, 9 wk after the initiating injury, also failed to demonstrate a reduction in GFR in injured mice. Thus, while we cannot be certain that even longer-term studies may not demonstrate a decline in renal function after injury, in practical terms, our study indicates that there is unlikely to be progressive CKD after Rb-AKI in BALB/c mice. These data are consistent with limited long-term outcome studies in mice and rats with Rb-AKI, showing that renal function is only mildly impaired 30−60 days after injury (4, 62). In addition, limited outcome data in combat casualties who developed dialysis-dependent AKI as a result of traumatic rhabdomyolysis indicate that creatinine-based GFR estimates return to baseline on long-term followup (8, 20). However, proteinuria develops in ~25% of patients on long-term followup (8), suggesting that there may be chronic tubular and/or glomerular injury in patients after severe traumatic Rb-AKI. Although we saw no evidence of glomerular pathology after Rb-AKI, these data are consistent with our observation that there was extensive tubular injury after 5 wk. In addition, the fact that serum creatinine levels were increased after 5 wk despite normal GFRs are consistent with the hypothesis that Rb-AKI induces chronic tubular injury. Increased serum creatinine in the absence of other indicators of renal dysfunction (decreased GFR) could be due to ongoing muscle damage from the initial intramuscular glycerol injection. However, muscle injury is only present 3−7 days after glycerol injection in this model (32). On this basis, increased serum creatinine levels are more likely to result from chronic tubular dysfunction, resulting in reduced tubular creatinine secretion, since these mice have normal GFRs and tubular secretion normally accounts for up to 50% of creatinine clearance in mice (19).
In conclusion, by evaluating and quantifying changes in fibrosis, GFR, and PTC rarefaction across three different long-term models of AKI, our study provides the first clear evidence that fibrosis, GFR, and PTC rarefaction are dissociated in different models of AKI to CKD transition. Our data show that repeat doses of CP cause capillary rarefaction and decreased renal function in mice without a significant increase in interstitial fibrosis, whereas Rb-AKI leads to severe interstitial fibrosis but renal function and peritubular capillary density are preserved. Furthermore, long-term outcome experiments in mice with unilateral ischemia-reperfusion-induced AKI showed that restoration of renal function 12 wk after a contralateral Nx is associated with increasing fibrosis but reversal of PTC rarefaction seen after 4 wk. Taken together, these findings support the hypothesis that there is a direct causal relationship between PTC rarefaction rather than fibrosis on long-term functional recovery after CP, rhabdomyolysis, and ischemia-repefusion-induced AKI and underscore the importance not only of evaluating renal fibrosis but also of evaluating PTC density in models of AKI to CKD transition in rodents.
GRANTS
This work was supported by the Vanderbilt Center for Kidney Disease and was funded by the following grants: Department of Defense PR-161028 and National Institutes of Health (NIH) Grant R01-DK-112688. We acknowledge the Translational Pathology Shared Resource for tissue processing for histology (Vanderbilt University Medical Center), supported by NIH Grants 5-P30-CA-68485-19 and 2-U24-DK-059637-16 (Vanderbilt Mouse Metabolic Phenotyping Center). We acknowledge support from the University of Alabama at Birmingham-University of California-San Diego O'Brien Core Center for Acute Kidney Injury Research for performing serum creatinine assays (NIH Grant P30-DK-079337).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M., L.S., and M.P.d.C. conceived and designed research; A.M., L.S., R.D., and C.F. performed experiments; A.M., L.S., R.D., Y.Z., and H.Y. analyzed data; A.M., L.S., and M.P.d.C. interpreted results of experiments; A.M., L.S., and Y.Z. prepared figures; A.M., L.S., and M.P.d.C. drafted manuscript; A.M., L.S., and M.P.d.C. edited and revised manuscript; A.M., L.S., R.D., C.F., Y.Z., H.Y., and M.P.d.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Leah Siskind (University of Louisville, Louisville, KY) for help setting up the RDCP model.
REFERENCES
- 1.Bábíčková J, Klinkhammer BM, Buhl EM, Djudjaj S, Hoss M, Heymann F, Tacke F, Floege J, Becker JU, Boor P. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int 91: 70–85, 2017. doi: 10.1016/j.kint.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 2.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001. doi: 10.1152/ajprenal.00050.2001. [DOI] [PubMed] [Google Scholar]
- 3.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Belliere J, Casemayou A, Ducasse L, Zakaroff-Girard A, Martins F, Iacovoni JS, Guilbeau-Frugier C, Buffin-Meyer B, Pipy B, Chauveau D, Schanstra JP, Bascands JL. Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. J Am Soc Nephrol 26: 1363–1377, 2015. doi: 10.1681/ASN.2014040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 380: 756–766, 2012. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 6.Billings FT IV, Shaw AD. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract 127: 89–93, 2014. doi: 10.1159/000363725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black LM, Lever JM, Traylor AM, Chen B, Yang Z, Esman SK, Jiang Y, Cutter GR, Boddu R, George JF, Agarwal A. Divergent effects of AKI to CKD models on inflammation and fibrosis. Am J Physiol Renal Physiol 315: F1107–F1118, 2018. doi: 10.1152/ajprenal.00179.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolanos JA, Yuan CM, Little DJ, Oliver DK, Howard SR, Abbott KC, Olson SW. Outcomes after post-traumatic AKI requiring RRT in United States military service Members. Clin J Am Soc Nephrol 10: 1732–1739, 2015. doi: 10.2215/CJN.00890115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med 361: 62–72, 2009. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 10.Campbell GA, Hu D, Okusa MD. Acute kidney injury in the cancer patient. Adv Chronic Kidney Dis 21: 64–71, 2014. doi: 10.1053/j.ackd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA; Acute Disease Quality Initiative Workgroup 16.. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13: 241–257, 2017. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 12.Chiba T, Skrypnyk NI, Skvarca LB, Penchev R, Zhang KX, Rochon ER, Fall JL, Paueksakon P, Yang H, Alford CE, Roman BL, Zhang MZ, Harris R, Hukriede NA, de Caestecker MP. Retinoic acid signaling coordinates macrophage-dependent injury and repair after AKI. J Am Soc Nephrol 27: 495–508, 2016. doi: 10.1681/ASN.2014111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, Chiba T, Novitskaya T, Woods C, West J, Korotchenko VN, McDermott L, Day BW, Davidson AJ, Harris RC, de Caestecker MP, Hukriede NA. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol 24: 943–953, 2013. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements ME, Chaber CJ, Ledbetter SR, Zuk A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One 8: e70464, 2013. doi: 10.1371/journal.pone.0070464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugaard G, Abildgaard U, Holstein-Rathlou NH, Bruunshuus I, Bucher D, Leyssac PP. Renal tubular function in patients treated with high-dose cisplatin. Clin Pharmacol Ther 44: 164–172, 1988. doi: 10.1038/clpt.1988.132. [DOI] [PubMed] [Google Scholar]
- 16.de Caestecker M, Humphreys BD, Liu KD, Fissell WH, Cerda J, Nolin TD, Askenazi D, Mour G, Harrell FE Jr, Pullen N, Okusa MD, Faubel S; ASN AKI Advisory Group . Bridging translation by improving preclinical study design in AKI. J Am Soc Nephrol 26: 2905–2916, 2015. doi: 10.1681/ASN.2015070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Caestecker M, Harris R. Translating knowledge into therapy for acute kidney injury. Semin Nephrol 38: 88–97, 2018. doi: 10.1016/j.semnephrol.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehling J, Bábíčková J, Gremse F, Klinkhammer BM, Baetke S, Knuechel R, Kiessling F, Floege J, Lammers T, Boor P. Quantitative micro-computed tomography imaging of vascular dysfunction in progressive kidney diseases. J Am Soc Nephrol 27: 520–532, 2016. doi: 10.1681/ASN.2015020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisner C, Faulhaber-Walter R, Wang Y, Leelahavanichkul A, Yuen PS, Mizel D, Star RA, Briggs JP, Levine M, Schnermann J. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int 77: 519–526, 2010. doi: 10.1038/ki.2009.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faulk T, Walker LE, Howard JT, Janak JC, Sosnov JA, Stewart IJ. Rhabdomyolysis among critically ill combat casualties: long-term outcomes. Am J Nephrol 48: 399–405, 2018. doi: 10.1159/000494337. [DOI] [PubMed] [Google Scholar]
- 21.Finn WF. Enhanced recovery from postischemic acute renal failure. micropuncture studies in the rat. Circ Res 46: 440–448, 1980. doi: 10.1161/01.RES.46.3.440. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem 88: 102925, 2019. doi: 10.1016/j.bioorg.2019.102925. [DOI] [PubMed] [Google Scholar]
- 23.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamdi T, Latta S, Jallad B, Kheir F, Alhosaini MN, Patel A. Cisplatin-induced renal salt wasting syndrome. South Med J 103: 793–799, 2010. doi: 10.1097/SMJ.0b013e3181e63682. [DOI] [PubMed] [Google Scholar]
- 25.Harrois A, Soyer B, Gauss T, Hamada S, Raux M, Duranteau J; Traumabase® Group . Prevalence and risk factors for acute kidney injury among trauma patients: a multicenter cohort study. Crit Care 22: 344, 2018. doi: 10.1186/s13054-018-2265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hörbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, Sutton TA. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 293: F688–F695, 2007. doi: 10.1152/ajprenal.00452.2006. [DOI] [PubMed] [Google Scholar]
- 27.Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 14: 607–625, 2018. doi: 10.1038/s41581-018-0052-0. [DOI] [PubMed] [Google Scholar]
- 28.Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis 37: 601–611, 2001. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- 29.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12: 1448–1457, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol 12: 1434–1447, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Katagiri D, Hamasaki Y, Doi K, Negishi K, Sugaya T, Nangaku M, Noiri E. Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide-sensitive amine oxidase inhibition. Kidney Int 89: 374–385, 2016. doi: 10.1038/ki.2015.327. [DOI] [PubMed] [Google Scholar]
- 32.Kawai H, Nishino H, Kusaka K, Naruo T, Tamaki Y, Iwasa M. Experimental glycerol myopathy: a histological study. Acta Neuropathol 80: 192–197, 1990. doi: 10.1007/BF00308923. [DOI] [PubMed] [Google Scholar]
- 33.Kohn S, Fradis M, Ben-David J, Zidan J, Robinson E. Nephrotoxicity of combined treatment with cisplatin and gentamicin in the guinea pig: glomerular injury findings. Ultrastruct Pathol 26: 371–382, 2002. doi: 10.1080/01913120290104683. [DOI] [PubMed] [Google Scholar]
- 34.Kramann R, Tanaka M, Humphreys BD. Fluorescence microangiography for quantitative assessment of peritubular capillary changes after AKI in mice. J Am Soc Nephrol 25: 1924–1931, 2014. doi: 10.1681/ASN.2013101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee D, Shenoy S, Nigatu Y, Plotkin M. Id proteins regulate capillary repair and perivascular cell proliferation following ischemia-reperfusion injury. PLoS One 9: e88417, 2014. doi: 10.1371/journal.pone.0088417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu ZZ, Bullen A, Li Y, Singh P. Renal oxygenation in the pathophysiology of chronic kidney disease. Front Physiol 8: 385, 2017. doi: 10.3389/fphys.2017.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansour SG, Zhang WR, Moledina DG, Coca SG, Jia Y, Thiessen-Philbrook H, McArthur E, Inoue K, Koyner JL, Shlipak MG, Wilson FP, Garg AX, Ishibe S, Parikh CR; TRIBE-AKI Consortium . The association of angiogenesis markers with acute kidney injury and mortality after cardiac surgery. Am J Kidney Dis 74: 36–46, 2019. doi: 10.1053/j.ajkd.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon KR, Harel-Sterling M, Pizzi M, Huynh L, Hessey E, Zappitelli M. Long-term renal follow-up of children treated with cisplatin, carboplatin, or ifosfamide: a pilot study. Pediatr Nephrol 33: 2311–2320, 2018. doi: 10.1007/s00467-018-3976-5. [DOI] [PubMed] [Google Scholar]
- 39.Molitoris BA, Okusa MD, Palevsky PM, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene TH, Faubel SG, Kellum JA, Wald R, Chertow GM, Levin A, Waikar SS, Murray PT, Parikh CR, Shaw AD, Go AS, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA; Trials of Patients with Sepsis and in Selected Hospital Settings . Design of clinical trials in AKI: a report from an NIDDK workshop. Trials of patients with sepsis and in selected hospital settings. Clin J Am Soc Nephrol 7: 856–860, 2012. doi: 10.2215/CJN.12821211. [DOI] [PubMed] [Google Scholar]
- 40.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992. doi: 10.1016/S0272-6386(12)80312-X. [DOI] [PubMed] [Google Scholar]
- 41.Noonan WT, Lorenz JN. Clearance studies in genetically altered mice. Methods Mol Med 86: 315–327, 2003. doi: 10.1385/1-59259-392-5:315. [DOI] [PubMed] [Google Scholar]
- 42.Okusa MD, Molitoris BA, Palevsky PM, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Faubel S, Kellum JA, Wald R, Chertow GM, Levin A, Waikar SS, Murray PT, Parikh CR, Shaw AD, Go AS, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene TH, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA. Design of clinical trials in acute kidney injury: a report from an NIDDK workshop−prevention trials. Clin J Am Soc Nephrol 7: 851–855, 2012. doi: 10.2215/CJN.12811211. [DOI] [PubMed] [Google Scholar]
- 43.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 44.Panizo N, Rubio-Navarro A, Amaro-Villalobos JM, Egido J, Moreno JA. Molecular mechanisms and novel therapeutic approaches to rhabdomyolysis-induced acute kidney injury. Kidney Blood Press Res 40: 520–532, 2015. doi: 10.1159/000368528. [DOI] [PubMed] [Google Scholar]
- 45.Perazella MA, Moeckel GW. Nephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology, and prevention/therapy. Semin Nephrol 30: 570–581, 2010. doi: 10.1016/j.semnephrol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Polichnowski AJ. Microvascular rarefaction and hypertension in the impaired recovery and progression of kidney disease following AKI in preexisting CKD states. Am J Physiol Renal Physiol 315: F1513–F1518, 2018. doi: 10.1152/ajprenal.00419.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- 48.Ravichandran K, Wang Q, Ozkok A, Jani A, Li H, He Z, Ljubanovic D, Weiser-Evans MC, Nemenoff RA, Edelstein CL. CD4 T-cell knockout does not protect against kidney injury and worsens cancer. J Mol Med (Berl) 94: 443–455, 2016. doi: 10.1007/s00109-015-1366-z. [DOI] [PubMed] [Google Scholar]
- 49.Ravichandran K, Holditch S, Brown CN, Wang Q, Ozkok A, Weiser-Evans MC, Nemenoff R, Miyazaki M, Thiessen-Philbrook H, Parikh CR, Ljubanovic D, Edelstein CL. IL-33 deficiency slows cancer growth but does not protect against cisplatin-induced AKI in mice with cancer. Am J Physiol Renal Physiol 314: F356–F366, 2018. doi: 10.1152/ajprenal.00040.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 51.Scarfe L, Schock-Kusch D, Ressel L, Friedemann J, Shulhevich Y, Murray P, Wilm B, de Caestecker M. Transdermal measurement of glomerular filtration rate in mice. J Vis Exp 140: 58520, 2018. doi: 10.3791/58520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scarfe L, Menshikh A, Newton E, Zhu Y, Delgado R, Finney C, de Caestecker MP. Long-term outcomes in mouse models of ischemia-reperfusion induced acute kidney injury. Am J Physiol Renal Physiol 317: 1068–1080, 2019. doi: 10.1152/ajprenal.00305.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S, Koenig S, Heinrich R, Hoecklin F, Pill J, Friedemann J, Schweda F, Gretz N, Schock-Kusch D. Transcutaneous measurement of renal function in conscious mice. Am J Physiol Renal Physiol 303: F783–F788, 2012. doi: 10.1152/ajprenal.00279.2012. [DOI] [PubMed] [Google Scholar]
- 54.Sethi S, D’Agati VD, Nast CC, Fogo AB, De Vriese AS, Markowitz GS, Glassock RJ, Fervenza FC, Seshan SV, Rule A, Racusen LC, Radhakrishnan J, Winearls CG, Appel GB, Bajema IM, Chang A, Colvin RB, Cook HT, Hariharan S, Herrera Hernandez LP, Kambham N, Mengel M, Nath KA, Rennke HG, Ronco P, Rovin BH, Haas M. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int 91: 787–789, 2017. doi: 10.1016/j.kint.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Sharp CN, Doll MA, Dupre TV, Shah PP, Subathra M, Siow D, Arteel GE, Megyesi J, Beverly LJ, Siskind LJ. Repeated administration of low-dose cisplatin in mice induces fibrosis. Am J Physiol Renal Physiol 310: F560–F568, 2016. doi: 10.1152/ajprenal.00512.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharp CN, Doll MA, Megyesi J, Oropilla GB, Beverly LJ, Siskind LJ. Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease. Am J Physiol Renal Physiol 315: F161–F172, 2018. doi: 10.1152/ajprenal.00636.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh AP, Junemann A, Muthuraman A, Jaggi AS, Singh N, Grover K, Dhawan R. Animal models of acute renal failure. Pharmacol Rep 64: 31–44, 2012. doi: 10.1016/S1734-1140(12)70728-4. [DOI] [PubMed] [Google Scholar]
- 58.Skrypnyk NI, Harris RC, de Caestecker MP. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J Vis Exp 78: 50495, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skrypnyk NI, Sanker S, Skvarca LB, Novitskaya T, Woods C, Chiba T, Patel K, Goldberg ND, McDermott L, Vinson PN, Calcutt MW, Huryn DM, Vernetti LA, Vogt A, Hukriede NA, de Caestecker MP. Delayed treatment with PTBA analogs reduces postinjury renal fibrosis after kidney injury. Am J Physiol Renal Physiol 310: F705–F716, 2016. doi: 10.1152/ajprenal.00503.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skrypnyk NI, Siskind LJ, Faubel S, de Caestecker MP. Bridging translation for acute kidney injury with better preclinical modeling of human disease. Am J Physiol Renal Physiol 310: F972–F984, 2016. doi: 10.1152/ajprenal.00552.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skrypnyk NI, Voziyan P, Yang H, de Caestecker CR, Theberge MC, Drouin M, Hudson B, Harris RC, de Caestecker MP. Pyridoxamine reduces postinjury fibrosis and improves functional recovery after acute kidney injury. Am J Physiol Renal Physiol 311: F268–F277, 2016. doi: 10.1152/ajprenal.00056.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soares TJ, Costa RS, Volpini RA, Da Silva CG, Coimbra TM. Long-term evolution of the acute tubular necrosis (ATN) induced by glycerol: role of myofibroblasts and macrophages. Int J Exp Pathol 83: 165–172, 2002. doi: 10.1046/j.1365-2613.2002.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steegh FM, Gelens MA, Nieman FH, van Hooff JP, Cleutjens JP, van Suylen RJ, Daemen MJ, van Heurn EL, Christiaans MH, Peutz-Kootstra CJ. Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol 22: 1024–1029, 2011. doi: 10.1681/ASN.2010050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int 71: 266–271, 2007. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 65.Takaori K, Nakamura J, Yamamoto S, Nakata H, Sato Y, Takase M, Nameta M, Yamamoto T, Economides AN, Kohno K, Haga H, Sharma K, Yanagita M. Severity and frequency of proximal tubule injury determines renal prognosis. J Am Soc Nephrol 27: 2393–2406, 2016. doi: 10.1681/ASN.2015060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 307: F1187–F1195, 2014. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 67.Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J 15: 58–69, 2015. [PMC free article] [PubMed] [Google Scholar]
- 68.Torres R, Velazquez H, Chang JJ, Levene MJ, Moeckel G, Desir GV, Safirstein R. Three-dimensional morphology by multiphoton microscopy with clearing in a model of cisplatin-induced CKD. J Am Soc Nephrol 27: 1102–1112, 2016. doi: 10.1681/ASN.2015010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsang RY, Al-Fayea T, Au HJ. Cisplatin overdose: toxicities and management. Drug Saf 32: 1109–1122, 2009. doi: 10.2165/11316640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 70.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol 23: 13–21, 2012. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weisbord SD, Palevsky PM. Design of clinical trials in acute kidney injury: lessons from the past and future directions. Semin Nephrol 36: 42–52, 2016. doi: 10.1016/j.semnephrol.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Yang B, Lan S, Dieudé M, Sabo-Vatasescu JP, Karakeussian-Rimbaud A, Turgeon J, Qi S, Gunaratnam L, Patey N, Hébert MJ. Caspase-3 is a pivotal regulator of microvascular rarefaction and renal fibrosis after ischemia-reperfusion injury. J Am Soc Nephrol 29: 1900–1916, 2018. doi: 10.1681/ASN.2017050581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci 334: 115–124, 2007. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 74.Young S., Struys E., Wood T.. Quantification of creatine and guanidinoacetate using GC-MS and LC-MS/MS for the detection of cerebral creatine deficiency syndromes. Curr Protoc Hum Genet Chap. 17: Unit 17.3, 2007. doi: 10.1002/0471142905.hg1703s54. [DOI] [PubMed] [Google Scholar]
- 75.Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol 163: 2289–2301, 2003. doi: 10.1016/S0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]