Abstract
Scattered tubular-like cells (STCs) contribute to repair neighboring injured renal tubular cells. Mitochondria mediate STC biology and function but might be injured by the ambient milieu. We hypothesized that the microenviroment induced by the ischemic and metabolic components of renovascular disease impairs STC mitochondrial structure and function in swine, which can be attenuated with mitoprotection. CD24+/CD133+ STCs were quantified in pig kidneys after 16 wk of metabolic syndrome (MetS) or lean diet (Lean) with or without concurrent renal artery stenosis (RAS) (n = 6 each). Pig STCs were isolated and characterized, and mitochondrial structure, membrane potential, and oxidative stress were assessed in cells untreated or incubated with the mitoprotective drug elamipretide (1 nM for 6 h). STC-protective effects were assessed in vitro by their capacity to proliferate and improve viability of injured pig tubular epithelial cells. The percentage of STCs was higher in MetS, Lean + RAS, and MetS + RAS kidneys compared with Lean kidneys. STCs isolated from Lean + RAS and MetS + RAS pigs showed mitochondrial swelling and decreased matrix density, which were both restored by mitoprotection. In addition, mitochondrial membrane potential and ATP production were reduced and production of reactive oxygen species elevated in MetS, Lean + RAS, and MetS + RAS STCs. Importantly, mitoprotection improved mitochondrial structure and function as well as the capacity of MetS + RAS STCs to repair injured tubular cells in vitro. Renovascular disease in swine is associated with a higher prevalence of STCs but induces structural and functional alterations in STC mitochondria, which impair their reparative potency. These observations suggest a key role for mitochondria in the renal reparative capacity of STCs.
Keywords: metabolic syndrome, mitochondria, renal artery stenosis, renovascular disease, scattered tubular cells
INTRODUCTION
Renovascular disease (RVD) remains an important cause of secondary hypertension and renal dysfunction that is frequently associated with several cardiovascular complications (47). In atherosclerotic RVD, the kidney is often affected by both ischemia and metabolic abnormalities. Progressive renal artery stenosis (RAS) produces a reduction in renal blood flow (RBF) and ischemia (38, 54), whereas metabolic abnormalities, including obesity, hyperlipidemia, and insulin resistance, can lead to irreversible inflammation and fibrosis, impeding renal recovery (7). However, whether the ischemic and metabolic components of RVD interfere with endogenous repair mechanisms in the poststenotic kidney remains unknown.
Scattered tubular-like cells (STCs) represent a dedifferentiated phenotype that can be adopted by surviving tubular epithelial cells. These cells are characterized by the coexpression of cell surface markers CD133 and CD24 (39, 44) but also express other genes characteristic of proximal tubule dedifferentiation, including the mesenchymal marker vimentin (29) and kidney injury marker (KIM)-1 (44). In preclinical models of acute kidney injury (AKI), delivery of STCs exerted important renoprotective effects comparable with those obtained with bone marrow-derived mesenchymal stem cells (17). Furthermore, the number of STCs has been shown to predict tissue recovery in patients with AKI (51), underscoring the potential of these cells to stimulate tissue regeneration after kidney injury.
As tubular cells, STCs are also equipped with a significant number of mitochondria, which produce cellular energy and regulate a variety of cellular metabolic functions, such as redox state and proliferation (31). We have previously shown that ischemia (11) and metabolic abnormalities (15) in swine RVD both induce structural and functional damage in tubular cell mitochondria. Furthermore, coexisting RAS and metabolic syndrome (MetS) in pigs synergistically aggravate tubular cell mitochondrial damage, contributing to structural injury and dysfunction in the poststenotic kidney (34a). However, whether RVD induces structural and functional abnormalities in swine STC mitochondria, impairing renal cellular reparative mechanisms, remains unknown. We hypothesized that kidney injury due to RVD in swine impairs STC mitochondrial structure and function, which can be attenuated with mitoprotection.
MATERIALS AND METHODS
All animal procedures were approved by the Institutional Animal Care and Use Committee. Twenty-four female domestic pigs (Manthei Hog Farm, Elk River, MN) were studied for 16 wk. At baseline, animals were fed a high-cholesterol/high-carbohydrate diet (to generate MetS) (35) or standard pig chow [lean diet (Lean)] for the entire course of the study (n = 12 each).
Six weeks later, all animals were anesthetized with intramuscular tiletamine hydrochloride/zolazepam hydrochloride (5 mg/kg, Telazol, Fort Dodge Animal Health, New York) and xylazine (2 mg/kg), and anesthesia was then maintained with intravenous ketamine (0.2 mg·kg−1·min−1) and xylazine (0.03 mg·kg−1·min−1). Unilateral RAS was induced in six MetS and six Lean pigs by placing an local irritant coil in the main renal artery using fluoroscopy (Siemens, Munich, Germany), as previously described (27). A sham procedure was performed in the remaining six Lean pigs and six MetS pigs.
Single kidney hemodynamics and function were determined 10 wk later using multidetector computed tomography (MDCT), and blood pressure was measured with an intra-arterial catheter (24). Before MDCT experiments, animals were similarly anesthetized, and the degree of stenosis in each animal was determined by angiography. Systemic blood samples were collected, and lipid panels (enzyme immunoassay kit, Roche Diagnostics, Indianapolis, IN) and fasting glucose and insulin levels were measured by standard procedures. Insulin resistance was assessed by the homeostasis model assessment of insulin resistance (35).
Animals were euthanized with pentobarbital sodium (100 mg/kg iv, Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MI) a few days after MDCT experiments. The kidneys were removed using a retroperitoneal incision and immediately dissected. Kidney cells were then dissociated, and the number of STCs was quantified by flow cytometry. STCs were isolated from fresh pig kidneys, cultured, characterized, and preserved in Trump’s fixative for electron microscopy or frozen in liquid nitrogen for in vitro experiments.
In vivo experiments.
MDCT experiments were performed in all animals to evaluate single kidney volume, RBF, and glomerular filtration rate (GFR; as previously described in Refs. 10 and 24). After a bolus of iopamidol (0.5 ml·kg−1·2 s−1), 70 multiscan exposures were acquired in a Flash 128 MDCT scanner (Somatom Definition Flash, Siemens Healthcare) at a cycle time of 0.67 s (flow study) followed by 70 scans at a cycle time of 2 s. Regions of interest were traced from cross-sectional images from the aorta, renal cortex, and medulla, and average tissue attenuation curves were fitted to calculate cortical and medullary hemodynamics. Single kidney renal volume was calculated by planimetry (Analyze, Biomedical Imaging Resource, Mayo Clinic, Rochester, MN), GFR from the cortical curve slope, and RBF by multiplying kidney volume (ml tissue) by renal perfusion (ml·min−1·ml tissue−1) (14, 24).
Ex vivo experiments: STCs in the swine kidney.
To detect STCs in frozen pig kidney sections, we performed double staining of CD133 (1:100, Novus Biologicals) and CD24 (1:100, Abcam, San Francisco, CA) and the tubular marker Phaseolus vulgaris erythroagglutinin (Invitrogen, Carlsbad, CA).
Flow cytometry (FlowSight, Amnis, Seattle, WA) was used to quantify the percentage of STCs in pig cortical and medullary sections. Fresh kidney sections from Lean, MetS, Lean + RAS, and MetS + RAS pigs were diced and digested with 0.05 mg/ml Liberase Thermolysin Low (Millipore Sigma, Burlington, MA) and 100 U/ml DNase (ThermoFisher Scientific). RPMI media (Sigma-Aldrich, St. Louis, MO) and 10% FBS were added, and the suspension was filtered through a cell strainer. The filtrate was then centrifuged at 300 g for 10 min. Cell pellets were then resuspended in 15 ml ice-cold autoMACs rinsing solution with 0.5% BSA and centrifuged at 1,000 reads per kilobase million (RPKM) for 5 min. Cellular pellets were washed twice in 2 ml autoMACs rinsing solution with 0.5% BSA, and cells were counted by mixing trypan blue dye (0.4%, Invitrogen) and cell suspension (1:1) using a disposable chamber slide and automated cell counter (Countess-II FL, Life Technologies, Carlsbad, CA). Cell suspensions were then centrifuged at 1,000 RPKM for 5 min at 4°C, resuspended in autoMACs rinsing solution with 0.5% BSA, aliquoted in low retention tubes, and centrifuged again at 1,000 RPKM for 5 min at 4°C. After the supernatant was removed, samples were incubated with blocking antibody for 20 min at 2–8°C (1:2) and subsequently stained with primary CD24 antibody (1:25, catalog no. ab134375, Abcam), CD133 (1:25, catalog no. NB120-16518G, Novus Biologics, Centennial, CO), and secondary anti-IgG antibody for CD24 conjugated to phycoerythrin (1:100, catalog no. P-852, Invitrogen). Cell suspensions were then centrifuged one last time at 1,000 RPKM for 5 min at 4°C, supernatants were removed, and cell pellets were washed twice in autoMACs rinsing solution with 0.5% BSA. Supernatants were discarded, and cells were suspended in 100 µl autoMACs rinsing solution with 0.5% BSA to be run for flow cytometry.
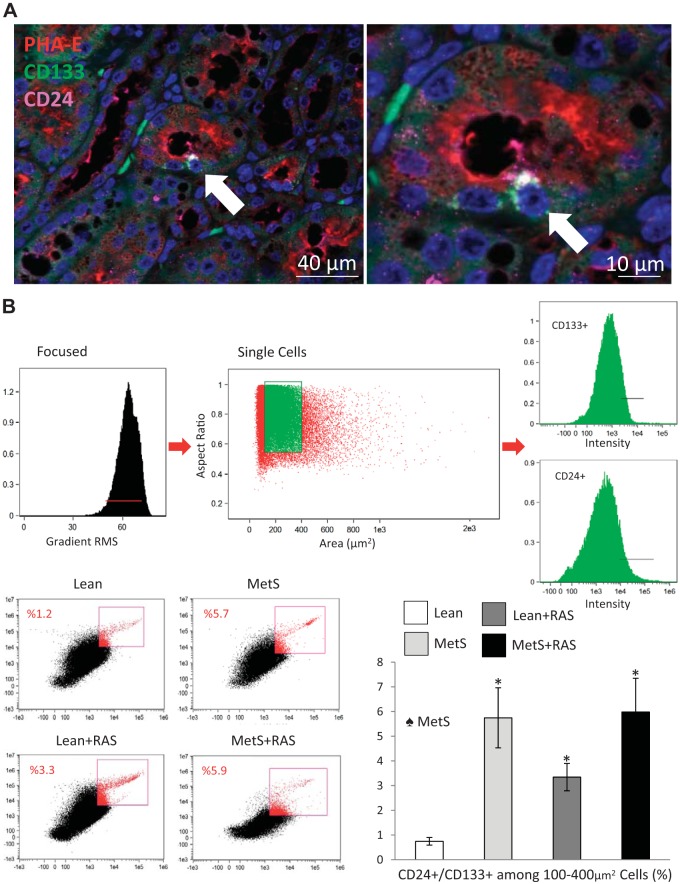
At least 100,000 events were acquired. STCs were quantified using a flow-gating strategy. After particle focusing, doublets, cell clusters, and debris were eliminated by gating on events with high aspect ratio (>0.6) and for cell size (10- to 20-µm diameter, 100- to 400-µm2 area) using bright-field area versus aspect ratio features. Single cells were further gated with the combination of CD133 and CD24 on a fluorescence intensity scatterplot, and STCs were identified by CD24/CD133 double-positive populations. Acquisition was performed using FlowSight Imaging Flow Cytometer (Amnis, Seattle, WA) equipped with INSPIRE software. Assessment of STC population was performed according to the cell surface expression of CD133 and CD24 as follows: number of double-positive events/number of singlet events between 10- and 20-µm diameter and expressed as a percentage.
In vitro experiments: STC isolation and characterization.
STCs were isolated from fresh pig kidneys, as our group has previously described (55). In brief, pig cortical and medullary sections were washed with PBS, diced, digested with 2 mg/ml collagenase for 1 h, and forced through a 60 mesh (250 μm) steel sieve to remove the fibrous component (50). The cellular fraction was then passed through a 100-μm cell strainer followed by the addition of medium 199 containing 3% FBS (GIBCO, Waltham, MA) (53) at 37°C in a humidified atmosphere with 5% CO2. The culture medium was replaced every 2 days to remove nonadherent cells. After about 2 wk, the adherent cells were harvested with TrypLE Express (GIBCO) treatment and subcultured. Cultured pig STCs were characterized using flow cytometry and immunofluorescence staining, which confirmed their positivity for CD24 (Abcam), CD133 (Novus Biologicals), vimentin (Abcam), and KIM-1 (R&D Systems).
STC mitochondrial morphology and function.
Mitochondrial morphology was assessed in STCs using digital electron microscopy (Phillips CM10 Transmission Electron Microscopy). Cells were preserved in Trump’s fixative solution (4% formaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer) overnight at room temperature, mounted on mesh grids, and stained with aqueous uranyl acetate and lead citrate at the Mayo Clinic’s electron microscopy core facility. Five representative STCs were randomly selected for examination, and mitochondrial spatial density (number per unit cellular area), area, matrix density, and number of fusion/fission events were determined using Image-J (version 1.5, National Institutes of Health), a software that converts electron microscopic images to binary images (42). Only mitochondria fully contained within the borders of the transmission electron microscopy images were manually traced using the “freehand tool” of ImageJ, which provides mitochondrial area in nanometers squared and mean gray values (brightness). Matrix density was calculated as 1/mean gray values and expressed as arbitrary units.
STC ATP production (ATP-to-ADP ratio) was assessed by colorimetric and fluorometric methods (catalog nos. ab83355 and ab83359, Abcam) (15). STC mitochondrial membrane potential was measured by tetramethylrhodamine ethyl ester (TMRE) staining (50 nM for 20 min at 37°C, catalog no. T669, ThermoFisher) (16), whereas mitochondrial reactive oxygen species (ROS) production was measured by Mito-SOX (2 μM for 30 min at 37°C, catalog no. M36008, ThermoFisher) (34). Mitochondrial structure and function were also assessed in Lean, MetS, Lean + RAS, and MetS + RAS STCs preincubated with the mitochondria-targeted peptide elamipretide (ELAM). This tetrapeptide targets and stabilizes mitochondrial inner membrane cardiolipin (45), inhibits the activity of cytochrome c peroxidase, prevents formation of the mitochondria permeability transition pore, and exerts protective effects on the kidney exposed to the ischemic (11, 13) and metabolic (12, 15) constituents of RVD. Cells were treated with 1 nM of ELAM for 6 h, a dose commonly used for in vitro studies (9, 36).
STC migration and proliferation.
STC migration was measured using a Boyden Chamber assay (catalog no. ECM506, Millipore 5 µm QCM Chemotaxis Cell Migration Assay, Millipore Sigma) (6). The Boyden Chamber system uses a hollow plastic chamber, sealed at one end with a porous membrane. STCs were placed inside the 24-well colorimetric chamber and allowed to migrate through the pores to the other side of the membrane. Migratory cells were then stained by crystal violet and quantified by spectrophotometry at an optical density of 56 nm (SynergyMx, BioTek Instruments, Winooski, VT).
STC proliferation was assessed in real time using IncuCyte Zoom (Essen Biosciences) (32, 33). Cells were harvested in medium 199 with 3% FBS, and 2,500 cells/well were seeded onto a 96-well tissue culture plate. Cell growth was monitored by capturing phase-contrast images every 2 h for 120 h and analyzed using the integrated confluence algorithm.
STC paracrine function.
STC-protective effects were evaluated by their capacity to improve viability (MTT assay, Roche Diagnostics) of pig proximal tubular epithelial cells (PK1 cells) coincubated with 10 ng/ml TNF-α and 10 µM antimycin-A (AMA), a model that mimics renal ischemic injury in vitro (28, 52). Injured PK1 cells were cocultured with Lean STCs or MetS + RAS STCs untreated or preincubated with ELAM (1 nM for 6 h).
Statistical analysis.
The Shapiro-Wilk test was used to test for deviation from normality. Normally distributed data are expressed as means ± SD and were compared using ANOVA and a Student’s t-test. Data that did not follow a Gaussian distribution are expressed as medians (interquartile ranges) and were compared using nonparametric methods (Wilcoxon or Kruskal-Wallis). Two-way ANOVA was performed to analyze the effects of RAS and MetS and their interactions, followed by a Tukey’s test as appropriate. For variables with nonnormal distributions, log-transformed values were used for two-way ANOVA. Statistical analysis was performed using JMP Pro 14.0 (SAS) software, and results were considered significant for P < 0.05.
RESULTS
Table 1 shows systemic characteristics and renal function of Lean, MetS, Lean + RAS, and MetS + RAS pigs at 16 wk. Body weight was equally elevated in MetS and MetS + RAS pigs, whereas blood pressure was similarly elevated in MetS, Lean + RAS, and MetS + RAS pigs compared with Lean pigs. RAS and MetS + RAS pigs developed moderate, but significant, stenosis of a comparable degree. Total cholesterol, LDL-cholesterol, and triglycerides were higher in MetS and MetS + RAS pigs compared with their respective controls. Fasting glucose levels did not differ among the groups, but fasting insulin levels and homeostasis model assessment of insulin resistance scores were higher in MetS and MetS + RAS groups compared with Lean and Lean + RAS groups, indicating prediabetic MetS.
Table 1.
Baseline characteristics and single kidney function in study groups
| Lean | MetS | Lean + RAS | MetS + RAS | |
|---|---|---|---|---|
| Body weight, kgd | 56.0 ± 6.2 | 90.5 ± 6.0a | 50.8 ± 6.4 | 91.2 ± 3.6a |
| Mean arterial pressure, mmHg | 98.4 ± 8.9 | 118.6 ± 15.1a | 119.6 ± 11.6a | 128.4 ± 8.4a |
| Degree of stenosis, %e | 0 | 0 | 72.3 ± 10.2a,b | 77.5 ± 11.3a,b |
| Total cholesterol, mg/dld | 82.8 ± 7.8 | 422.2 ± 40.1a | 85.5 ± 6.7b | 417.3 ± 57.8a,c |
| LDL cholesterol, mg/dld | 33.4 ± 6.5 | 361.1 ± 63.4a | 66.8 ± 32.7b | 296.7 ± 52.8a,c |
| Triglycerides, mg/dld | 7.5 ± 1.9 | 17.3 ± 7.9a | 6.2 ± 1.2b | 13.3 ± 5.2a,c |
| Fasting glucose, mg/dld | 129.5 ± 14.7 | 113.0 ± 8.9 | 114.7 ± 12.0 | 108.5 ± 12.8 |
| Fasting insulin, mU/mld | 0.4 [0.3–0.5] | 0.7 [0.7–0.8]a | 0.4 [0.3–0.4]b | 0.8 [0.7–0.8]a,c |
| HOMA-IR scored | 0.6 [0.6–0.7] | 1.9 [1.5–1.9]a | 0.6 [0.6–0.7]b | 1.8 [1.7–1.9]a,c |
| Renal volume, mld,e | 125.9 ± 12.3 | 217.9 ± 11.4a | 79.5 ± 10.3a,b | 169.1 ± 7.7a,b,c |
| Renal blood flow, ml/mind,e | 522.2 ± 18.2 | 872.0 ± 87.8a | 331.9 ± 22.3a,b | 651.5 ± 11.2a,b,c |
| Glomerular filtration rate, ml/mind,e | 74.7 ± 4.5 | 148.1 ± 6.1a | 39.2 ± 6.0a,b | 94.2 ± 4.8a,b,c |
Values are means ± SE; n = 7 each. Lean, lean diet fed; MetS, metabolic syndrome; RAS, renal artery stenosis; HOMA-IR, homeostasis model assessment of insulin resistance.
P < 0.05 vs. the Lean group;
P < 0.05 vs. the MetS group;
P < 0.05 vs. the Lean + RAS group.
MetS: significant effect of MetS (two-way ANOVA);
Ischemia: significant effect of ischemia (two-way ANOVA).
Single kidney volume, RBF, and GFR were higher in MetS versus Lean pigs, reflecting hyperfiltration, but decreased in Lean + RAS and MetS + RAS pigs compared with their respective controls. However, the coexistence of MetS and ischemia did not aggravate stenotic kidney hemodynamics and function (P > 0.05 for all, two-way ANOVA).
RVD is associated with a higher prevalence of STCs.
Immunohistological staining of frozen pig kidney sections confirmed the presence of CD133+/CD24+ cells in proximal tubules in situ (Fig. 1A). Flow cytometry analysis indicated that the percentage of CD24+/CD133+ STCs among dissociated kidney cells was higher in MetS, RAS, and MetS + RAS groups compared with the Lean group (Fig. 1B) and predominantly influenced by MetS (P = 0.01, two-way ANOVA).
Fig. 1.
Scattered tubular-like cells (STCs) in the pig kidney. A: representative immunofluorescence staining (original magnification: ×40 and ×100) for the STC surface markers CD133 (green) and CD24 (pink) and the tubular cell marker phytohemagglutinin [Phaseolus vulgaris erythroagglutinin (PHA-E); red] showing CD133+/CD24+ cells in renal proximal tubules. B: flow cytometry analysis indicated that the percentage of CD24+/CD133+ STCs from dissociated kidney cells was higher in the metabolic syndrome (MetS), renal artery stenosis (RAS), and MetS + RAS groups compared with the lean diet-fed (Lean) group. *P < 0.05 vs. the Lean group. ♠MetS: significant effect of MetS (two-way ANOVA).
STC characterization.
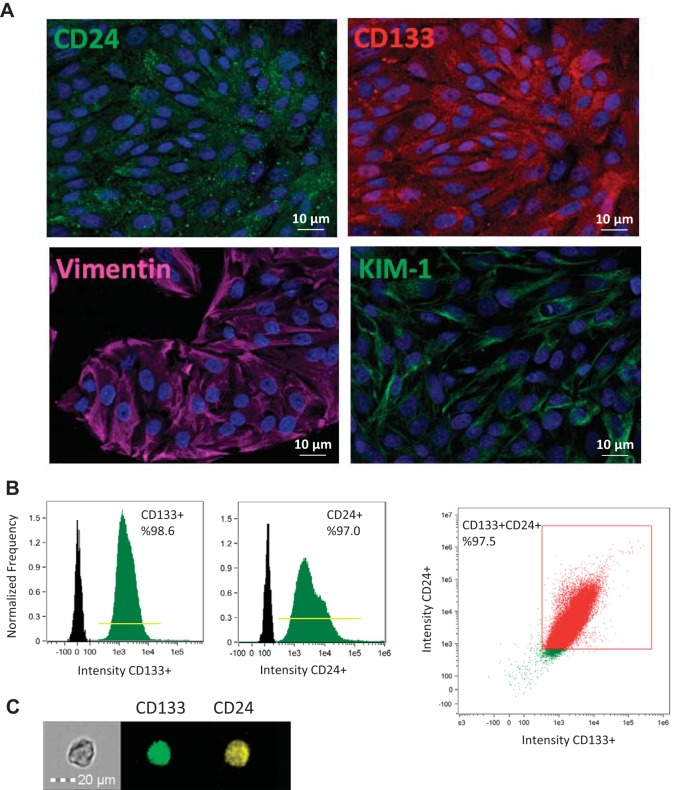
Cultured pig STCs were characterized using flow cytometry and immunofluorescence staining, which confirmed their positivity for CD24 (Abcam), CD133 (Novus Biologicals), vimentin (Abcam), and KIM-1 (R&D Systems) at a purity of 97.5% (Fig. 2).
Fig. 2.
Characterization of scattered tubular-like cells (STCs) isolated from pig kidneys. A: representative immunofluorescence staining (original magnification: ×40) for the STC surface markers CD24 (green), CD133 (red), vimentin (pink), and kidney injury molecule (KIM)-1 (green) in isolated swine STCs. B: flow cytometry analysis of isolated STCs showing that 98.6% of cells expressed CD133, 97.0% expressed CD24, and 97.5% were double positive for CD133 and CD24. C: representative image of a CD133+ (green)/CD24+ (yellow) cell.
RVD induces STC mitochondrial structural damage.
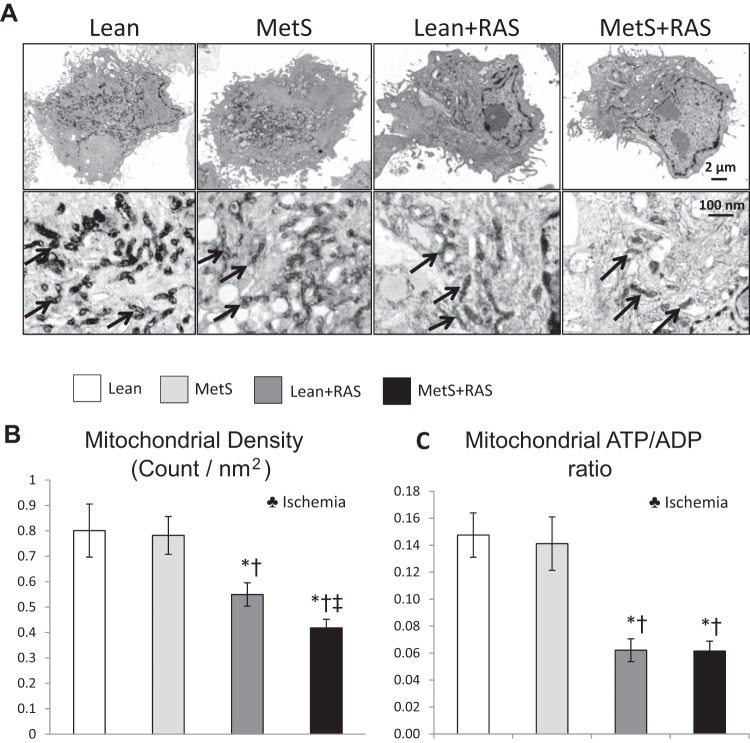
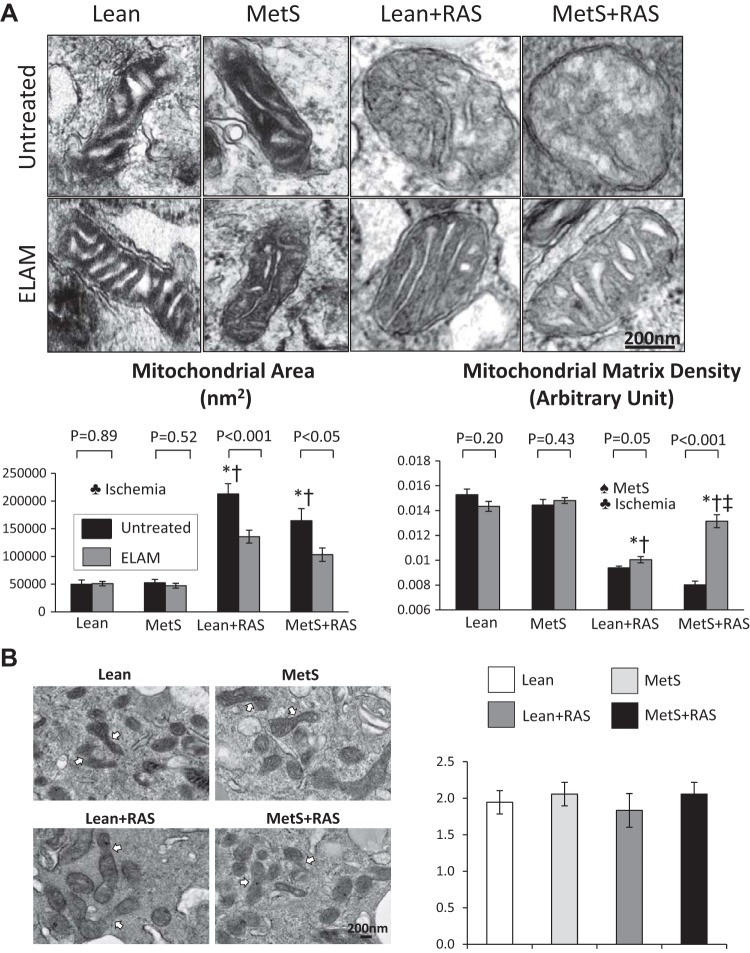
Mitochondrial density, which decreased in RAS groups compared with Lean and MetS groups, further decreased in the MetS + RAS group and was predominantly influenced by ischemia (Fig. 3, A and B). STC mitochondrial area increased in Lean + RAS and MetS + RAS groups compared with Lean and MetS groups, suggesting mitochondrial swelling, which was also influenced by the effect of ischemia (Fig. 4A). Contrarily, mitochondrial matrix density, which decreased in Lean + RAS and MetS + RAS groups versus their respective controls, further decreased in the MetS + RAS group, suggesting loss of cristae membranes. MetS and ischemia influenced matrix density (P < 0.05, two-way ANOVA). The number of fusion events on electron microscopy images did not differ among the groups (Fig. 4B).
Fig. 3.
Scattered tubular-like cell (STC) mitochondrial density and function. A: transmission electron microscopy (original magnification: ×5 k and ×100 k) of STC mitochondria. B: STC mitochondrial density decreased in the lean diet-fed (Lean) + renal artery stenosis (RAS) group compared with the Lean and metabolic syndrome (MetS) groups and further decreased in the MetS + RAS group. C: the ATP-to-ADP ratio was comparably lower in Lean + RAS and MetS + RAS STCs versus Lean and MetS STCs. *P < 0.05 vs. the Lean group; †P < 0.05 vs. the MetS group; ‡P < 0.05 vs. the Lean + RAS group. ♣Ischemia: significant effect of ischemia (two-way ANOVA).
Fig. 4.
Scattered tubular-like cell (STC) mitochondrial morphology. A: STC mitochondrial area increased in the lean diet-fed (Lean) + renal artery stenosis (RAS) and metabolic syndrome (MetS) + RAS groups compared with the Lean and MetS groups. Mitochondrial matrix density, which decreased in the Lean + RAS and MetS + RAS groups versus their respective controls, further decreased in the MetS + RAS group. Elamipretide (ELAM) decreased mitochondrial area and increased matrix density of Lean + RAS and MetS + RAS STCs. B: the number of fusion events was similar among the groups. *P < 0.05 vs. the Lean group; †P < 0.05 vs. the MetS group; ‡P < 0.05 vs. the Lean + RAS group. ♠MetS: significant effect of MetS (two-way ANOVA); ♣Ischemia: significant effect of ischemia (two-way ANOVA).
MetS and RAS contribute to STC mitochondrial dysfunction.
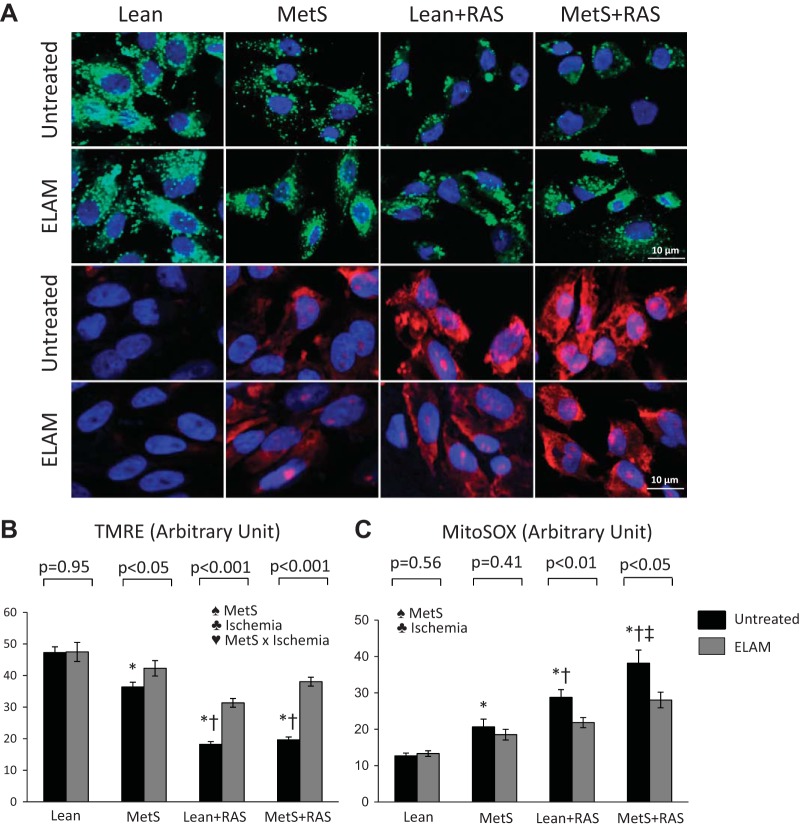
The ATP-to-ADP ratio was comparably lower in Lean + RAS and MetS + RAS STCs versus Lean and MetS STCs and was influenced by the effect of ischemia (Fig. 3C). Mitochondrial membrane potential decreased in MetS versus Lean groups, further decreased in RAS groups, and was influenced by MetS, ischemia, and their interaction (Fig. 5, A and B). However, production of mitochondrial ROS, which increased in the MetS group compared with the Lean group and further increased in the Lean + RAS group, increased even more in the MetS + RAS group (Fig. 5, A and C). MetS and ischemia had an additive effect to exacerbate mitochondrial ROS production (P < 0.05, two-way ANOVA).
Fig. 5.
Scattered tubular-like cell (STC) mitochondrial function. A: representative immunofluorescence staining (original magnification: ×40) for the mitochondrial membrane potential marker tetramethylrhodamine ethylester (TMRE; green) and the mitochondrial superoxide indicator (MitoSOX; red). B: mitochondrial membrane potential decreased in metabolic syndrome (MetS) STCs compared with lean diet-fed (Lean) STCs and further decreased in Lean + renal artery stenosis (RAS) and MetS + RAS STCs. Mitochondrial production of reactive oxygen species increased in MetS STCs versus Lean STCs, further increased in Lean + RAS STCs, and increased even more in MetS + RAS STCs. Elamipretide (ELAM) increased mitochondrial membrane potential and decreased oxidative stress in MetS, Lean + RAS, and MetS-RAS STCs. *P < 0.05 vs. the Lean group; †P < 0.05 vs. the MetS group; ‡P < 0.05 vs. the Lean + RAS group. ♠MetS: significant effect of MetS (two-way ANOVA); ♣Ischemia: significant effect of ischemia (two-way ANOVA); ♥MetS × Ischemia: significant effect of MetS × ischemia (two-way ANOVA).
MetS and RAS impair STC reparative capacity.
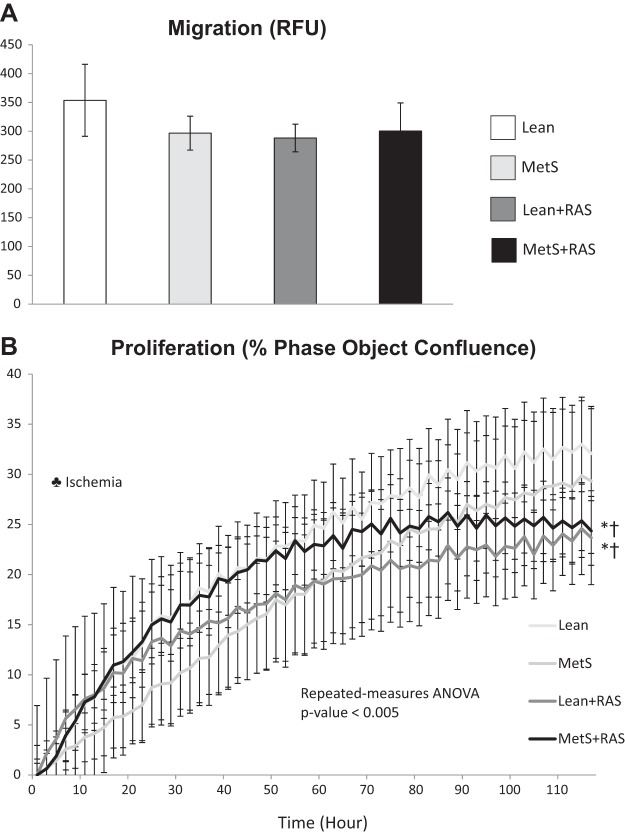
STC migration was similar among the groups (Fig. 6A). However, STC proliferation, which remained unchanged in the MetS group compared with the Lean group, equally decreased in Lean + RAS and MetS + RAS grous and was predominantly influenced by ischemia (P < 0.05, two-way ANOVA; Fig. 6B). TNF-α and AMA significantly impaired the viability of swine PK1 cells, which was improved by Lean STCs but not by MetS + RAS STCs (Fig. 7).
Fig. 6.
Scattered tubular-like cell (STC) migration and proliferation. A: migration of isolated STCs, measured by color density, was similar among study groups. B: STC proliferation, measured in percent phase object confluence unit, decreased in the lean diet-fed (Lean) + renal artery stenosis (RAS) and metabolic syndrome (MetS) + RAS groups compared with the Lean and MetS groups. *P < 0.05 vs. the Lean groups; †P < 0.05 vs. the MetS group; ‡P < 0.05 vs. the Lean + RAS group. ♣Ischemia: significant effect of ischemia (two-way ANOVA).
Fig. 7.
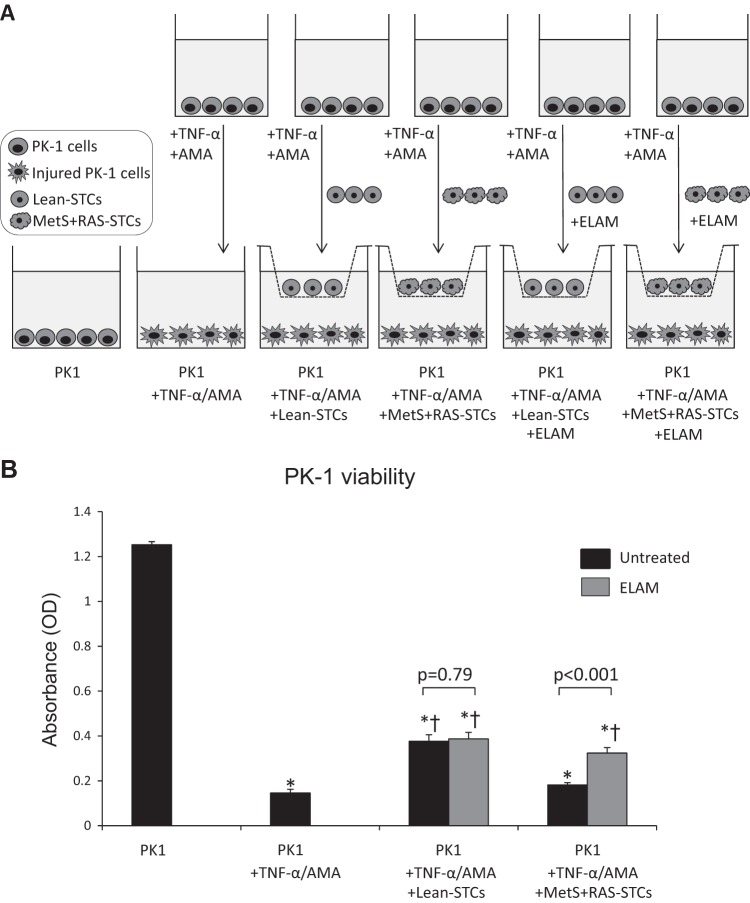
Scattered tubular-like cells (STCs) reparative capacity. A: STC-protective effects were assessed by their capacity to improve viability of proximal tubular epithelial cells (PK1 cells) coincubated with 10 ng/ml TNF-α and 10 µM antimycin-A (AMA). Injured PK1 cells were untreated or treated with lean diet-fed (Lean) STCs or metabolic syndrome (MetS) + renal artery stenosis (RAS) STCs untreated or preincubated with elamipretide (ELAM; 1 nM for 6 h). B: TNF-α and AMA impaired the viability of swine PK1 cells, which was improved by Lean STCs but not by MetS + RAS STCs. ELAM improved the capacity of MetS + RAS STCs to increase the viability of PK1 cells. *P < 0.05 vs. PK1; †P < 0.05 vs. PK1 + TNF-α/AMA.
Mitoprotection attenuates MetS + RAS-induced STC injury.
Treatment with mitochondria-targeted peptides decreased mitochondrial area and increased matrix density of Lean + RAS- and MetS + RAS STCs (Fig. 4). ELAM also increased mitochondrial membrane potential and decreased production of ROS of MetS, Lean + RAS, and MetS-RAS STCs (Fig. 5). Finally, mitoprotection improved the capacity of MetS + RAS STCs to increase the viability of PK1 cells (Fig. 7).
DISCUSSION
The present study shows that RVD in swine is associated with activation of renal repair mechanisms, such as STCs. However, the ischemic and metabolic components of RVD induce structural and functional alterations in STC mitochondria that impair the proliferative potential of these cells as well as their ability to repair tubular cells in vitro. Notably, mitoprotection restored mitochondrial structure and function and improved the viability of swine tubular cells in an in vitro model that mimics renal ischemic injury. These observations implicate mitochondrial damage in RVD-induced STC dysfunction in experimental RVD and position mitochondria as a therapeutic target.
The kidney has a limited ability to repair that primarily relies on surviving tubular cells that acquire a dedifferentiated phenotype with progenitor-like characteristics (19, 48). The key role of STCs in renal repair is supported by the observation that almost all proliferating cells in AKI biopsy samples are CD24+ and CD133+ (18, 44). Previous studies have shown that CD24+/CD133+ STCs have important proliferative potential and showed promising results in murine models of AKI. For example, delivery of STCs into the tail vein of mice with rhabdomyolysis-induced AKI conferred protective effects (3). Likewise, intra-arterial injection of STC-derived extracellular vesicles improved perfusion and oxygenation in the stenotic murine kidney, underscoring the regenerative potential of this endogenous repair system (55). However, it remains unclear if STCs immersed in a microenviroment of ischemia and metabolic abnormalities, such as the milieu of RVD, retain their protective properties.
In the present study, we used a swine model that recapitulates the ischemic and metabolic components of human RVD to assess the impact of a noxious cardiovascular milieu on this renal repair mechanism. We found that the percentage of STCs among dissociated kidney cells was higher in MetS, Lean + RAS, and MetS + RAS groups compared with the Lean group, suggesting that RVD activates STCs. These cells were characterized by coexpression of the glycosyl phosphatidylinositol-anchored protein CD24 and the pentaspanning transmembrane glycoprotein CD133 (18), which characterize immature or dedifferentiated cells (22, 43). Although the primary mechanisms of STC activation are largely unknown (23), studies in mouse models of AKI have suggested that transformation of adult renal tubular epithelial cells into STCs initiates with a dediferentiation process characterized by upregulation of genes involved in kidney development (e.g., SOX9) (26) and pathways that modulate cell proliferative and self-renewal signal (e.g., Wnt signaling) (37).
We then isolated these cells and confirmed their expression of the mesenchymal marker vimentin and the injury marker KIM-1, supporting previous observations in humans (18, 40, 44). Vimentin positivity is often associated with resiliency to injury and regeneration. After acute ischemic injury, tubular cells expressing vimentin do not undergo necrosis and remain anchored to the basal membrane (18). Surviving cells expressing vimentin also express markers indicating ongoing mitogenesis like proliferating cell nuclear antigen (49). Tubular cells that acquire the STC phenotype also express KIM-1, a transmembrane protein that is expressed on the surface of dedifferentiated tubular cells undergoing injury and has been implicated in tubular cell regeneration (20). KIM-1 is directly responsible for phagocytosis of apoptotic bodies, facilitating remodeling of injured tubular epithelia cells (21).
Although STCs have fewer mitochondria compared with differentiated tubular cells (18), these organelles modulate several critical aspects of their function. Electron microscopy analysis of STCs revealed that renal ischemia decreased mitochondrial density and induced structural mitochondrial injury. Lean + RAS and MetS + RAS were associated with dramatic changes in mitochondrial morphology, including swelling (increased area) and loss of cristae membranes (decreased matrix density). However, the number of fusion/fission events did not differ among the groups, arguing against changes in mitochondrial dynamics as a cause for mitochondrial enlargement.
Importantly, mitochondrial structural abnormalities were associated with functional impairment, disclosed by decreased mitochondrial membrane potential, the driving force for mitochondrial ATP synthesis. Indeed, ATP production was similarly blunted in Lean + RAS- and MetS + RAS STCs compared with Lean and MetS STCs. Mitochondrial membrane depolarization increases the activity of the respiratory complexes I and II, which subsequently contributes to excessive mitochondrial ROS production (46). In line with this, we found that production of mitochondrial superoxide anion, which slightly increased in MetS STCs versus Lean STCs, further increased in RAS groups. Overall, these observations are consistent with and extend our recent findings in differentiated tubular cells (34a), suggesting that the deleterious effects of cardiovascular risk factors on tubular cell mitochondria can also compromise renal repair mechanisms.
Importantly, mitochondrial structural damage and dysfunction impacted STC function. Although their migration remained unchanged, proliferation capacity was impaired in Lean + RAS and MetS + RAS STCs compared with Lean and MetS STCs. Mitochondria play an important role in cell proliferation (4). Inducible attenuation of mitochondrial membrane potential results in diminished cell proliferation (30), and excessive mitochondrial ROS generation negatively affects mitogenic cellular signaling (8). Therefore, RVD-induced changes in mitochondrial membrane potential and redox signaling could have contributed to impair STC proliferation.
Interestingly, we found that while the number of STCs in the pig kidney was predominantly affected by MetS, their ability to proliferate was influenced by ischemia. Remarkably, ischemia impacted on all aspects of mitochondrial damage. Therefore, the metabolic component of RVD may modulate STC activation, whereas ischemia-induced STC mitochondrial injury may limit STC proliferative capacity. However, the coexistence of MetS and RAS did not aggravate stenotic kidney dysfunction in the short time of our in vivo experiments. Possibly, RVD-induced STC mitochondrial damage and dysfunction may precede aggravated loss of renal function.
Importantly, coexisting MetS and ischemia did not aggravate mitochondrial injury in STCs, as opposed to differentiated tubular cells. We have previously shown in swine that mitochondrial matrix density and ATP generation in mature tubular cells were markedly influenced by the interaction of MetS and ischemia (34a). Contrarily, this study found that STC mitochondrial density was affected by MetS and ischemia but not by their interaction. Although MetS and ischemia decreased mitochondrial membrane potential, their coexistence, rather than aggravate it, preserved it at the Lean + RAS level. Possibly, being a reparative system, STCs are less susceptible to injury by the microenviroment induced by the ischemic and metabolic components of RVD than the surrounding tubular cells.
To test whether RVD-induced STC damage impairs their ability to repair tubular cells, we compared the capacity of Lean and MetS + RAS STCs to improve viability of proximal tubular epithelial cells in an in vitro model of renal ischemic injury (28, 52). We found that Lean STCs improved survival of injured PK1 cells, underscoring the potential of STCs to repair damaged tubular cells. The paracrine effects of STCs might have contributed to improve PK1 viability. Our group has previously shown that primary cultured swine STCs exert protective effects in injured tubular epithelial cells in vitro, partly by releasing extracellular vesicles that are uptaken by tubular cells (55). STC extracellular vesicles carry proteins, genes, and microRNAs capable of promoting in vitro survival and proliferation in target epithelial cells and release several growth factors that may control both cell proliferation and survival (1, 17). Contrarily, MetS + RAS STCs failed to improve the viability of PK1 cells, confirming that the ischemic and metabolic abnormalities of RVD mitigate the ability of STCs to repair injured tubular cells.
To further establish the contribution of mitochondria in RVD-induced STC dysfunction, we treated cells with the mitochondria-targeted peptide ELAM, which targets and stabilizes mitochondrial inner membrane cardiolipin (45). We have previously shown that this compound exerted protective effects on the kidney exposed to the ischemic (11, 13) and metabolic (12, 15) constituents of RVD. The present study demonstrates that mitoprotection also attenuates mitochondrial structural and functional damage in STCs, disclosed by improvements in mitochondrial area, matrix density, membrane potential, and mitochondrial production of ROS. Previous studies have shown that preservation of mitochondrial cardiolipin maintains the integrity of the inner mitochondrial membrane and restores cristae membranes. Cardiolipin plays a key structural role in cristae formation and organization of respiratory complexes into supercomplexes, allowing optimal oxidative phosphorylation (45). ELAM has a high affinity for cardiolipin, and the complex they form inhibits cytochrome c peroxidase activity, which catalyzes cardiolipin peroxidation and results in mitochondrial damage (5). Therefore, preservation of mitochondrial cardiolipin with ELAM could have improved mitochondrial morphology and function and reduced mitochondrial oxidative stress.
Furthermore, we found that coincubation with ELAM improved the capacity of MetS + RAS STCs to increase the viability of injured tubular cells. Although the exact mechanisms by which ELAM modulated STC paracrine activity warrants further investigation, our findings suggest that mitochondria are important determinants of the renal reparative capacity of STCs.
We acknowledge some limitations in our study. The RVD model uses relatively young pigs, and the duration of the disease is shorter than in humans. Nevertheless, our animals recapitulate the ischemic and metabolic components of human RVD. In addition, our study in vitro findings that RVD impairs the reparative capacity of STCs need to be validated by in vivo studies. High-dose TNF-α and AMA reduced the survival of PK1 cells by 80%, which could have limited the reparative potential of STCs. Yet, coculture with Lean STCs significantly increased the viability of PK1 cells. Despite these limitations, our observations demonstrate that 16 wk of RVD is sufficient to exert deleterious effects on this renal endogenous reparative system, which can be alleviated by mitoprotection. Nevertheless, additional long-term followup studies are needed to test whether RVD-induced changes in STC mitochondria perpetuate STC dysfunction and to determine the long-term effects of mitoprotection.
In summary, our study shows that experimental RVD leads to activation of STCs, which are characterized by mitochondrial structural abnormalities and dysfunction, reflected by changes in mitochondrial morphology, decreased membrane potential and ATP production, and increased production of ROS. Interestingly, we found that MetS modulates STC activation, whereas ischemia-induced STC mitochondrial injury limits their proliferative capacity. Treatment with mitoprotective drugs ameliorated mitochondrial injury and restored mitochondrial function. In addition, mitoprotection improved the capacity of MetS + RAS STCs to repair injured tubular cells in vitro. Therefore, these observations suggest a key role for mitochondria in the renal reparative capacity of STCs.
GRANTS
This work was supported by National Institutes of Health Grants DK-106427, DK-122137, DK-104273, DK-120292, DK-102325, and HL-123160.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.O.L. and A.E. conceived and designed research; A.A.N., X.Y.Z., S.M.C., I.M.S., and A.E. performed experiments; A.A.N., X.Y.Z., S.M.C., J.R.W., I.M.S., and A.E. analyzed data; A.A.N., X.Y.Z., S.M.C., J.R.W., I.M.S., L.O.L., and A.E. interpreted results of experiments; A.A.N. and A.E. prepared figures; A.A.N., L.O.L., and A.E. drafted manuscript; X.Y.Z., S.M.C., J.R.W., I.M.S., L.O.L., and A.E. edited and revised manuscript; A.A.N., X.Y.Z., S.M.C., J.R.W., I.M.S., L.O.L., and A.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank James D. Krier, Kyra L. Jordan, and Christopher M. Ferguson for support on this project. We also thank the Mayo Microscopy and Cell Analysis Core, Histology Core Facility, and Immunochemical Core laboratory.
REFERENCES
- 1.Aggarwal S, Grange C, Iampietro C, Camussi G, Bussolati B. Human CD133+ renal progenitor cells induce erythropoietin production and limit fibrosis after acute tubular injury. Sci Rep 6: 37270, 2016. doi: 10.1038/srep37270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 30: 1714–1725, 2012. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 4.Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC. Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal 16: 1150–1180, 2012. doi: 10.1089/ars.2011.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24: 1250–1261, 2013. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Herrmann SM, Zhu X, Jordan KL, Gloviczki ML, Lerman A, Textor SC, Lerman LO. Preserved function of late-outgrowth endothelial cells in medically treated hypertensive patients under well-controlled conditions. Hypertension 64: 808–814, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies MG, Saad WE, Bismuth J, Naoum JJ, Peden EK, Lumsden AB. Impact of metabolic syndrome on the outcomes of percutaneous renal angioplasty and stenting. J Vasc Surg 51: 926–932, 2010. doi: 10.1016/j.jvs.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Diebold L, Chandel NS. Mitochondrial ROS regulation of proliferating cells. Free Radic Biol Med 100: 86–93, 2016. doi: 10.1016/j.freeradbiomed.2016.04.198. [DOI] [PubMed] [Google Scholar]
- 9.Eirin A, Ebrahimi B, Kwon SH, Fiala JA, Williams BJ, Woollard JR, He Q, Gupta RC, Sabbah HN, Prakash YS, Textor SC, Lerman A, Lerman LO. Restoration of mitochondrial cardiolipin attenuates cardiac damage in swine renovascular hypertension. J Am Heart Assoc 5: e003118, 2016. doi: 10.1161/JAHA.115.003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Tang H, Crane JA, Lerman A, Textor SC, Lerman LO. Changes in glomerular filtration rate after renal revascularization correlate with microvascular hemodynamics and inflammation in Swine renal artery stenosis. Circ Cardiovasc Interv 5: 720–728, 2012. doi: 10.1161/CIRCINTERVENTIONS.112.972596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res 103: 461–472, 2014. doi: 10.1093/cvr/cvu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eirin A, Hedayat AF, Ferguson CM, Textor SC, Lerman A, Lerman LO. Mitoprotection preserves the renal vasculature in porcine metabolic syndrome. Exp Physiol 103: 1020–1029, 2018. doi: 10.1113/EP086988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eirin A, Li Z, Zhang X, Krier JD, Woollard JR, Zhu XY, Tang H, Herrmann SM, Lerman A, Textor SC, Lerman LO. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension 60: 1242–1249, 2012. doi: 10.1161/HYPERTENSIONAHA.112.199919. [DOI] [PubMed] [Google Scholar]
- 14.Eirin A, Saad A, Tang H, Herrmann SM, Woollard JR, Lerman A, Textor SC, Lerman LO. Urinary Mitochondrial DNA Copy Number Identifies Chronic Renal Injury in Hypertensive Patients. Hypertension 68: 401–410, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eirin A, Woollard JR, Ferguson CM, Jordan KL, Tang H, Textor SC, Lerman A, Lerman LO. The metabolic syndrome induces early changes in the swine renal medullary mitochondria. Transl Res 184: 45–56.e9, 2017. doi: 10.1016/j.trsl.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrelly E, Amaral MC, Marshall L, Huang SG. A high-throughput assay for mitochondrial membrane potential in permeabilized yeast cells. Anal Biochem 293: 269–276, 2001. doi: 10.1006/abio.2001.5139. [DOI] [PubMed] [Google Scholar]
- 17.Grange C, Moggio A, Tapparo M, Porta S, Camussi G, Bussolati B. Protective effect and localization by optical imaging of human renal CD133+ progenitor cells in an acute kidney injury model. Physiol Rep 2: e12009, 2014. doi: 10.14814/phy2.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansson J, Hultenby K, Cramnert C, Pontén F, Jansson H, Lindgren D, Axelson H, Johansson ME. Evidence for a morphologically distinct and functionally robust cell type in the proximal tubules of human kidney. Hum Pathol 45: 382–393, 2014. doi: 10.1016/j.humpath.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Huo W, Zhang K, Nie Z, Li Q, Jin F. Kidney injury molecule-1 (KIM-1): a novel kidney-specific injury molecule playing potential double-edged functions in kidney injury. Transplant Rev (Orlando) 24: 143–146, 2010. doi: 10.1016/j.trre.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 118: 1657–1668, 2008. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, Zeilstra J, Pals ST, Mehmet H, Stassi G, Medema JP. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res 70: 719–729, 2010. doi: 10.1158/0008-5472.CAN-09-1820. [DOI] [PubMed] [Google Scholar]
- 23.Kramann R, Kusaba T, Humphreys BD. Who regenerates the kidney tubule? Nephrol Dial Transplant 30: 903–910, 2015. doi: 10.1093/ndt/gfu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 281: F630–F638, 2001. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Liu J, Pang P, Krautzberger AM, Reginensi A, Akiyama H, Schedl A, Humphreys BD, McMahon AP. Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Reports 12: 1325–1338, 2015. doi: 10.1016/j.celrep.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 27.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10: 1455–1465, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Liang X, Chen Y, Zhang L, Jiang F, Wang W, Ye Z, Liu S, Yu C, Shi W. Necroptosis, a novel form of caspase-independent cell death, contributes to renal epithelial cell damage in an ATP-depleted renal ischemia model. Mol Med Rep 10: 719–724, 2014. doi: 10.3892/mmr.2014.2234. [DOI] [PubMed] [Google Scholar]
- 29.Lindgren D, Boström AK, Nilsson K, Hansson J, Sjölund J, Möller C, Jirström K, Nilsson E, Landberg G, Axelson H, Johansson ME. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol 178: 828–837, 2011. doi: 10.1016/j.ajpath.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martínez-Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, Mehta MM, Wang T, Santos JH, Woychik R, Dufour E, Spelbrink JN, Weinberg SE, Zhao Y, DeBerardinis RJ, Chandel NS. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol Cell 61: 199–209, 2016. doi: 10.1016/j.molcel.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFarland R, Taylor RW, Turnbull DM. Mitochondrial disease−its impact, etiology, and pathology. Curr Top Dev Biol 77: 113–155, 2007. doi: 10.1016/S0070-2153(06)77005-3. [DOI] [PubMed] [Google Scholar]
- 32.Miller JE, Monsanto SP, Ahn SH, Khalaj K, Fazleabas AT, Young SL, Lessey BA, Koti M, Tayade C. Interleukin-33 modulates inflammation in endometriosis. Sci Rep 7: 17903, 2017. doi: 10.1038/s41598-017-18224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran-Jones K, Brown LM, Samimi G. INC280, an orally available small molecule inhibitor of c-MET, reduces migration and adhesion in ovarian cancer cell models. Sci Rep 5: 11749, 2015. doi: 10.1038/srep11749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukhopadhyay P, Rajesh M, Yoshihiro K, Haskó G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358: 203–208, 2007. doi: 10.1016/j.bbrc.2007.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Nargesi AA, Zhang L, Tang H, Jordan KL, Saadiq IM, Textor SC, Lerman LO, Eirin A. Coexisting renal artery stenosis and metabolic syndrome magnifies mitochondrial damage, aggravating poststenotic kidney injury in pigs. J Hypertens 37: 2061–2073, 2019. doi: 10.1097/HJH.0000000000002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawar AS, Zhu XY, Eirin A, Tang H, Jordan KL, Woollard JR, Lerman A, Lerman LO. Adipose tissue remodeling in a novel domestic porcine model of diet-induced obesity. Obesity (Silver Spring) 23: 399–407, 2015. doi: 10.1002/oby.20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med 10: 291–315, 2008. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rinkevich Y, Montoro DT, Contreras-Trujillo H, Harari-Steinberg O, Newman AM, Tsai JM, Lim X, Van-Amerongen R, Bowman A, Januszyk M, Pleniceanu O, Nusse R, Longaker MT, Weissman IL, Dekel B. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Reports 7: 1270–1283, 2014. doi: 10.1016/j.celrep.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis 63: 186–197, 2014. doi: 10.1053/j.ajkd.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Romagnani P, Remuzzi G. CD133+ renal stem cells always co-express CD24 in adult human kidney tissue. Stem Cell Res (Amst) 12: 828–829, 2014. doi: 10.1016/j.scr.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 42.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirasawa T, Akashi T, Sakamoto K, Takahashi H, Maruyama N, Hirokawa K. Gene expression of CD24 core peptide molecule in developing brain and developing non-neural tissues. Dev Dyn 198: 1–13, 1993. doi: 10.1002/aja.1001980102. [DOI] [PubMed] [Google Scholar]
- 44.Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JF, Moeller MJ. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol 229: 645–659, 2013. doi: 10.1002/path.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 171: 2029–2050, 2014. doi: 10.1111/bph.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang X, Luo YX, Chen HZ, Liu DP. Mitochondria, endothelial cell function, and vascular diseases. Front Physiol 5: 175, 2014. doi: 10.3389/fphys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Textor SC, Lerman LO. Paradigm shifts in atherosclerotic renovascular disease: where are we now? J Am Soc Nephrol 26: 2074–2080, 2015. doi: 10.1681/ASN.2014121274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol 294: C22–C28, 2008. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]
- 49.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang G, Jia Y, Li C, Cheng Q, Yue W, Pei X. Hyperglycemic stress impairs the stemness capacity of kidney stem cells in rats. PLoS One 10: e0139607, 2015. doi: 10.1371/journal.pone.0139607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye Y, Wang B, Jiang X, Hu W, Feng J, Li H, Jin M, Ying Y, Wang W, Mao X, Jin K. Proliferative capacity of stem/progenitor-like cells in the kidney may associate with the outcome of patients with acute tubular necrosis. Hum Pathol 42: 1132–1141, 2011. doi: 10.1016/j.humpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Jiang F, Chen Y, Luo J, Liu S, Zhang B, Ye Z, Wang W, Liang X, Shi W. Necrostatin-1 attenuates ischemia injury induced cell death in rat tubular cell line NRK-52E through decreased Drp1 expression. Int J Mol Sci 14: 24742–24754, 2013. doi: 10.3390/ijms141224742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells 31: 117–125, 2013. doi: 10.1002/stem.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoccali C, Mallamaci F, Finocchiaro P. Atherosclerotic renal artery stenosis: epidemiology, cardiovascular outcomes, and clinical prediction rules. J Am Soc Nephrol 13, Suppl 3: S179–S183, 2002. doi: 10.1097/01.ASN.0000032548.18973.0F. [DOI] [PubMed] [Google Scholar]
- 55.Zou X, Kwon SH, Jiang K, Ferguson CM, Puranik AS, Zhu X, Lerman LO. Renal scattered tubular-like cells confer protective effects in the stenotic murine kidney mediated by release of extracellular vesicles. Sci Rep 8: 1263, 2018. doi: 10.1038/s41598-018-19750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]