Abstract
Cyanobacteria played an important role in the evolution of Early Earth and the biosphere. They are responsible for the oxygenation of the atmosphere and oceans since the Great Oxidation Event around 2.4 Ga, debatably earlier. They are also major primary producers in past and present oceans, and the ancestors of the chloroplast. Nevertheless, the identification of cyanobacteria in the early fossil record remains ambiguous because the morphological criteria commonly used are not always reliable for microfossil interpretation. Recently, new biosignatures specific to cyanobacteria were proposed. Here, we review the classic and new cyanobacterial biosignatures. We also assess the reliability of the previously described cyanobacteria fossil record and the challenges of molecular approaches on modern cyanobacteria. Finally, we suggest possible new calibration points for molecular clocks, and strategies to improve our understanding of the timing and pattern of the evolution of cyanobacteria and oxygenic photosynthesis.
Keywords: Biosignatures, Cyanobacteria, Evolution, Microfossils, Molecular clocks, Precambrian
Graphical abstract
Highlights
-
•
Origin and evolution of cyanobacteria, oxygenic photosynthesis and plasts are debated.
-
•
Cyanobacterial fossil record starts unambiguously at 1.89–1.84 Ga.
-
•
Classic and new cyanobacterial signatures, and their fossil record are reassessed.
-
•
Challenges of molecular phylogenies and clocks are reviewed.
-
•
New possible calibration points for molecular clocks are suggested.
1. Introduction
Modern cyanobacteria constitute an ancient and well-diversified bacterial phylum, with unique complex morphologies and cellular differentiation. They play a key role in food webs as primary producers performing oxygenic photosynthesis. Cyanobacteria also played a major role in early biogeochemical fluxes and in Life and Earth evolution. They are the only prokaryotic organisms that perform oxygenic photosynthesis, and are thus generally held responsible for the rise of oxygen in the atmosphere and oceans around 2.4 Ga, during the so-called Great Oxidation Event (GOE [1,2]), facilitated by geological processes [3]. Oxygenic photosynthesis has enabled the oxygenation of oceanic and terrestrial niches, and the diversification of complex life [4]. Indeed, most modern eukaryotes need a minimal concentration of molecular oxygen to synthesize their sterol membranes [5]. They diversified from a last eukaryotic common ancestor (LECA), an aerobe protist with a mitochondrion [6]. Further increased oxygen concentration was required for the metabolic activity of mobile macroscopic metazoans [7]. At least 1.05 Ga ago, oxygenic photosynthesis spread among some eukaryotic clades, giving rise to diverse types of algae and later to plants. This important evolutionary step was due to the primary endosymbiosis of a cyanobacterium within a unicellular eukaryote [8,9], and subsequent higher-order endosymbiotic events [10]. The endosymbiotic theory is well supported by biochemical, ultrastructural, ecological and molecular data [11], although the identity and habitat of the cyanobacterial ancestor of the chloroplast are still debated [[12], [13], [14], [15], [16], [17]].
Despite the importance of cyanobacteria in the early evolution of Earth and life, fundamental questions remain about their origin, the timing and pattern of their diversification, and the origin of oxygenic photosynthesis, ranging from the Archean to the GOE [18]. One crucial problem to solve is the discrepancy between the unambiguous cyanobacterial fossil record, starting at 1.9 Ga, the GOE at 2.4 Ga, and the report of several older geochemical data suggestive of oxygenic photosynthesis ([19]; but see Ref. [20]) [[21], [22], [23], [24], [25]]; and [26,27].
Several types of evidence are used to reconstruct the fossil record of cyanobacteria, but all have their limitations and challenges. Stromatolites are usually associated to cyanobacterial activity. However, although conical stromatolites seem to plead for oxygenic photosynthesis [28], others types of stromatolites and microbially induced sedimentary structures (MISS) [[29], [30], [31]] may have been produced by non-cyanobacterial lineages, such as anoxygenic phototrophs [28,32], or by/in association with methanotrophs [33]. This suggests that stromatolites and MISS are not necessarily indicative of cyanobacteria activity, and perhaps not even of photosynthesis [34]. Biomarkers (fossil molecules) can indicate the presence of metabolisms such as oxygenic photosynthesis, but they are preserved only in well-preserved unmetamorphosed rocks, and contamination is a challenge [35]. Among those, lipids such as 2-methyl-hopanes are produced by cyanobacteria [36] but not only [37], and pigments such as porphyrins with N isotope composition [38]. Other geochemical (redox and isotopic) proxies can also inform on the presence of molecular oxygen in the water column or biologically-induced isotopic fractionation due to oxygenic photosynthesis, but their interpretation is often debated. Finally, microfossils may provide direct evidence for cyanobacteria, but their identification is often ambiguous. At the present time, the cyanobacterial identity of only three fossil taxa is not debated: Eoentophysalis, Eohyella and Polybessurus. Eoentophysalis belcherensis is the oldest microfossil interpreted with certainty as a cyanobacterium [39]. This microfossil has been described from 1.89–1.84 Ga silicified stromatolites of the Belcher Supergroup, Hudson Bay, Canada [39].
Microbiology of modern cyanobacteria pairs the geological and paleobiological approaches. The accumulation of modern cyanobacterial genetic data in public databases increasingly allows phylogenetic reconstructions and molecular clock analyses aimed at estimating the origin of the phylum and the origin of oxygenic photosynthesis. However, due to the lack of tree calibrations from the fossil record, contamination of genetic sequences, chosen dataset, and limitations or differences in models, these estimates are quite variable [40]. Thus, discrepancies between the geological and fossil records and molecular phylogenies remain, and the origin and evolution of cyanobacteria, oxygenic photosynthesis, and the chloroplast are still debated.
In this paper, we review classic and new biosignatures of cyanobacteria, critically assess their fossil record, and suggest possible new calibration points for molecular clocks. We also briefly discuss molecular phylogenies, molecular clocks and their discrepancies. We finally make some suggestions for future research, to improve our understanding about the evolution of cyanobacteria and its consequences on Earth and biosphere evolution.
| Fundamental and unresolved questions regarding the early evolution of cyanobacteria |
|---|
| What are the timing, pattern, and environment of cyanobacteria origin and evolution? How to interpret the discrepancies between the fossil record and molecular phylogenies, and how to reconcile these records? What are the origin and timing of oxygenic photosynthesis? Which among geochemical redox proxies and stromatolites are reliable indicators of oxygenic photosynthesis? What is the origin, timing, and environment of chloroplast acquisition by endosymbiosis and evolution of eukaryotic photosynthesis? |
2. Identification of cyanobacteria in the fossil record
Paleontologists have to rely on information other than the genomic data and internal cellular organization to identify the biological affinities of early microfossils. In some cases, conventional biosignatures such as morphology, division mode, presence of ornamentation, ultrastructure, and chemistry of carbonaceous cell walls, combined with their distribution pattern within the hosting rocks and the characteristics of their preservational environments may help deciphering their biological affinities [[41], [42], [43]].
Recently, new cyanobacterial signatures, such as intracellular biominerals, molecular fossils of lipids and pigments, and isotopic signatures of carbon and nitrogen, measured on single molecules or whole microfossils, were proposed as tools to better constrain the early evolution of cyanobacteria and their role in early ecosystems. These conventional and new biosignatures of cyanobacteria are discussed below.
2.1. Morphology and division pattern
Cyanobacteria were traditionally described as algae and referred to as ‘Cyanophyta’ or ‘Blue green algae’. Until the end of the 20th century, the nomenclatural system of cyanobacteria followed the International Code of Botanical Nomenclature. In the late seventies, Stanier and colleagues [44] recognized the prokaryotic nature of the cyanobacteria and proposed to follow the International Code of Nomenclature for Bacteria. According to the Bergey's Manual of Systematic Bacteriology, and following the Stanier approach and Rippka et al. (1979) [45] concepts, cyanobacteria were divided into two groups: unicellular and multicellular filamentous cyanobacteria; and five subsections based on morphological criteria, and corresponding to five former cyanobacterial orders: Chroococcales (section I) and Pleurocapsales (section II) harbor unicellular cells, which divide by binary fission in one or multiple plans and are solitary or arranged in colonies. Pleurocapsales can also produce small easily dispersed cells (baeocytes) after division by multiple fissions. Within the multicellular filamentous cyanobacteria, Oscillatoriales (section III) have only vegetative cells arranged in filaments, whereas Nostocales (section IV), and Stigonematales (section V) are capable of producing specialized cells, the heterocytes, which allow N-fixation in an anoxic compartment; or can exhibit environmental stress resistant cells called akinetes. Besides, cyanobacteria from section V are also characterized by their ability to divide in more than one plane and form true branched trichomes [45].
Since that time, the taxonomic classification of cyanobacteria has been continually reevaluated with the development of electron microscopy and genetic characterization methods [46]. The classification into subsections is practical but does not reflect phylogeny because they are not all monophyletic except for the Nostocales and Stigonematales.
Interpreting microfossils as cyanobacteria is generally based on morphological criteria, and their mode of division. However, the simple shape of many microfossils makes an unambiguous identification very difficult [40]. The size of cyanobacteria cells or filaments may be used as a taxonomic criterion for microfossil biological affinity. However, microfossil size does not correspond exactly to the size of living microorganisms due to modification by taphonomic processes, including diagenesis, collapsing and flattening [47]. Moreover, different fossil and modern groups of bacteria, cyanobacteria and eukaryotic algae have overlapping size ranges. Therefore, microfossil size alone does not constitute a reliable criterion for the interpretation of a microfossil as a cyanobacterium [48] or even as a prokaryote or a eukaryote [49].
Cyanobacteria form spheroidal or rod-shaped cells, filaments or tubes. Some of them occur as spiral filaments (e.g. Spirulina, modern counterpart of Obruchevella [50]) but this morphology also occurs in other bacteria (Leptospira [51]). Cyanobacteria from the Nostocales and Stigonematales orders may present some of the most complex morphologies among prokaryotes, including specialized cells such as larger elongated cells with thicker walls (akinetes) and round cells in or at the end of the filament (heterocytes, fixing nitrogen). The complex multicellular filamentous forms of nostocalean and (uniseriate or multiseriate) stigonematalean cyanobacteria may also display false or true branching. These more complex cyanobacteria may present unique characters allowing to not only identify their fossils as cyanobacteria, but also as specific cyanobacterial clades, more useful as calibrating points.
However, most cyanobacteria have simple morphologies, widespread in the three domains of life. Simple prokaryotic shapes may lead to erroneous interpretations since abiotic processes, such as mineral growth, fluid inclusions, organics migration, or interstitial spaces between grains, can mimic biogenic forms [40,47,[52], [53], [54], [55], [56], [57]]. Once the biogenicity of a microfossil is established, morphological observations need to be combined with other criteria, including the wall ultrastructure and molecular composition, in order to confirm unambiguously its identity [58,59].
The division pattern of fossil cells, when preserved, indicates their reproduction, and in some cases, may be indicative of particular taxonomic groups [60,61]. Cyanobacteria reproduce asexually by binary or multiple fissions. Multiple fissions may lead to the formation of baeocytes, which are small cells formed within the parental cell [62]. They can also divide in two or three planes of division, such as Entophysalis spp., the modern counterpart of Eoentophysalis. Moreover, some cyanobacteria occur as colonies either within (e.g. Gloeocapsa spp., modern counterpart of Gloeodiniopsis) or without (e.g. Cyanobium spp., Synechococcus spp., and Synechocystis spp.) a thick polysaccharide envelope. They can also present more or less organized and dense aggregates (e.g. Microcystis spp., modern counterpart of Eomicrocystis) and even cells organized as tablets (e.g. Merismopedia spp.). Some cyanobacteria can also reproduce with the aid of hormogonia, which are short filaments resulting from break up of longer filaments [63]. Trichomes are ensheathed individual filaments [63].
2.2. Ultrastructure
The wall ultrastructure of modern cyanobacteria consists of a peptidoglycan layer of varying thickness in the periplasmic space between a cytoplasmic and an outer membrane, with generally an external S-layer [64]. In some cases, a transparent or pigmented exopolysaccharidic (EPS) envelope, the so called sheath, may surround cells, filaments or colonies. Cyanobacterial EPS includes two forms, one attached to the cell wall and one released in the environments. They may be composed by up to twelve different monosaccharides, including pentoses, hexoses, and acid hexoses as well as methyl sugars and/or amino sugars (e.g. N-acetyl glucosamine, 2,3-O-methyl rhamnose, 3-O-methyl rhamnose, 4-O-methyl rhamnose, 3-O-methyl glucose, see Ref. [65] for a review). In the fossil record, sheaths of cyanobacteria are very common given that they are more readily fossilized, or less easily degraded, than the unsheathed ones [66]. The association of sheaths with clay minerals is more frequent than with unsheathed cyanobacteria, which helps the preservation of these structures [67,68]. This association would be due to differences in the chemical composition between the EPS and the sheath, and the rapid coating of sheaths [67]. Precipitation of other minerals such as nano-aragonite [68] or silica [69] may also enhance preservation.
As phototrophs, all cyanobacteria but Gloeobacter spp [70]. possess internal membranes called thylakoids hosting the photosynthetic apparatus, the two photosystems and their pigments. The arrangement of the thylakoids in cells is well organized and coincide with cyanobacterial lineages taxonomy [71]. For example, in tested strains from Nostocales/Stigonematales thylakoids are coiled and concentrated at the periphery [71]. Since cyanobacteria are the only oxygenic photosynthetic organisms among prokaryotes, the presence of preserved thylakoids in microfossils would be a reliable criterion to confirm that they were able to make this type of photosynthesis [72]. So far, ultralaminae interpreted as thylakoids based on their stacking and thickness, were preserved in microfossils as old as 155 Ma [72]. In acid extraction of 600 Ky microbial mats also revealed the resilience of thylakoids displaying their concentric structure over the hosting cell walls [68]. In both cases, the preservation of thylakoids made of lipids, pigments and proteins was favored by clay minerals ([68,72] and discussion therein). Moreover, although eukaryotes also have thylakoids, they are compartmentalized in chloroplasts and often are arranged differently than in cyanobacteria. Therefore, the distinction between cyanobacterial and eukaryotic thylakoids would be possible in fossils if they are preserved [72].
2.3. Paleoecology and behavior
Geochemical proxies may provide indirect evidence for oxygenation, at the planetary scale, such as the GOE, or at the scale of basins [[73], [74], [75], [76], [77]], and permit the reconstruction of the evolution of paleoredox conditions. However, as mentioned above, their interpretation can be challenging and do not necessarily imply the existence of oxygenic photosynthesis. In the best cases, they represent average values of local conditions over a relatively large time scale compared to the life span and sedimentation of individual taxa within a given microfossil assemblage (which itself also represents a time average, biased by taphonomy). Thus, establishing the paleoecology of fossil assemblages is difficult, although combining micropaleontology and geochemistry at high-resolution along a paleoenvironmental gradient may be informative [54,75], review in Ref. [78]. Hence, examining the consistent trends in distribution of some microfossil taxa in photic zones, in silicified stromatolites or other carbonates, or in microbial mats preserved in siliciclastic rocks, might give a hint to their metabolism and point to (anoxygenic or oxygenic) photosynthesis. Nevertheless, paleontologists and biologists have to keep in mind that the paleoecology of fossil organisms might differ from their modern relatives.
At the microscale, the distribution and the orientation of microfossils within rocks may also provide insights about their ecology [41]. Moreover, their orientation might help to infer the behavior of microfossils, such as phototropism of filaments erected towards the light or chemotropism, rock-boring by endolithic microbes, mat-building benthic microbes, or plankton settling down with no preferential orientation [79,80]. For example, Green et al. [81] and Golubic and Seong-Joo [61] concluded that Eohyella was a euendolithic cyanobacterium owing to its orientation in oolithes, by analogy with its modern counterpart, Hyella [41]. Euendoliths are rock-inhabiting microorganisms, which dissolve mineralized substrates to penetrate the rock [82], in contrast to other endoliths, e.g. chasmoendoliths, which are endoliths that colonize existing rock fissures [82].
2.4. Molecular fossils
Molecular fossils include complex organic molecules produced only by biology and, in some cases, are indicative of particular metabolisms or lineages [83]. Cyanobacteria produce lipids (2-methyl-hopanes – [83,84] and pigments that can potentially be preserved in the unmetamorphosed geological record [35,36]. So far, the lipids 2-methyl-hopanes were extracted from bitumen in black shales as old as 1.6 Ga (McArthur basin, Australia) [83,84]. Their oldest record at 2.7 Ga [85] was reassessed as younger contaminants [86,87]. These fossilized lipids were first attributed to cyanobacteria [84], but it is now acknowledged that they might be signatures of other bacterial lineages [37].
Pigments are also used as signatures for (anoxygenic and oxygenic) photosynthesis. More precisely, the presence of ancient chlorophylls can be detected by the preservation of their nitrogen-containing tetrapyrrole (porphyrin) core. Moreover, some fossilized forms of carotenoids, such as okenane and isorenieratane evidence the presence of purple S-bacteria, green S-bacteria and other bacterial lineages (incl. cyanobacteria) [83]. Even if they are not unique to cyanobacteria, their report shows pigment can be preserved in relatively old unmetamorphosed rocks.
The oldest porphyrins reported so far are preserved in shales of the 1.1 Ga Taoudeni basin, Mauritania [38], which also preserves exquisite microfossils, including eukaryotes and cyanobacteria [88]. These fossilized pigments exhibit a specific N isotope fractionation indicating a cyanobacterial source and permit to suggest that cyanobacteria were the dominant primary producers in mid-proterozoic oceans [38].
Other UV-protective (sunscreen) pigments may be used as signature for bacterial life, such as the mycosporin-like amino acids (MAAs), and two colored molecules specific of cyanobacteria: scytonemin and gloeocapsin. For instance, the combined analyses of modern cultures and fossil (4500 years BP) microbial mats of cyanobacteria from Antarctica revealed that cells and pigmented filamentous sheaths can withstand acetolysis (used to isolate them from the mineral matrix) and retain their molecular signature identified by FTIR microspectroscopy [68]. They are also preserved in siliciclastic sediments by precipitation of nano-aragonite and clay minerals [68]. FTIR microspectroscopy of microfossils enables the non-destructive analysis of the biopolymer composition of cell walls or sheaths. Comparison with taxonomically informative polymers unique to particular modern clades permit to identify the microfossils, in combination with the morphology and wall ultrastructure [58]. However, the scarce knowledge of the composition of pigments, preservable cells, cysts and other structures produced by modern microorganisms, and of their transformation and alteration through fossilization, limits this approach. Moreover, high temperature and pressure (metamorphism) after burial can alter even more or erase original biological properties [89]. Raman microspectroscopy enables estimating the temperature at which the organic material has been submitted (e.g. Ref. [90]), which is necessary to interpret properly the spectra obtained by FTIR microspectroscopy [89]. Raman spectroscopy also permits to characterize molecules in modern organisms, including cyanobacteria [68,[91], [92], [93], [94]]. Scytonemin is a molecule consisting of phenolic and indolic subunits [95]. Today it is notably biosynthesized in benthic filaments of Calothrix sp. [68], and in the endolithic cyanobacteria Hyella sp. and Solentia sp., from coastal carbonates [93]. It may be a promising signature of cyanobacteria given that it can be fossilized [68,96]. In older deposits from Antarctica, derivatives of scytonemin and carotenoids can be extracted from 125000 years BP sediments [97]. However, the preservation potential of scytonemin in older rocks is not known. Artificial taphonomic experiments of decaying cyanobacterial cultures showed the recalcitrance of filamentous polysaccharide sheaths, possibly helped by the presence of pigments [66]. However, in lake sediments from Antarctica, both brown (scytonemin-rich) and transparent (scytonemin-poor) filamentous sheaths were well preserved; hence, scytonemin probably was not the factor driving their preservation [68].
Gloeocapsin, an enigmatic pigment detected in the thick sheath enveloping colonies of the cyanobacterial genus Gloeocapsa growing on carbonate surfaces [93] and in lichens (with cyanobacterial symbionts), might also become a useful indicator, but its molecular composition remains to be characterized and its preservation potential is currently unknown [93].
2.5. Isotopic fractionation
Carbon isotopes fractionation do not permit to discriminate oxygenic photosynthesis from other metabolisms that have overlapping range of fractionation, except for methanogenesis [98,99]. At the microscale, C and N isotopic composition can be measured on single microfossils with undisputed biogenicity [[100], [101], [102]] and might reveal some information on inferred paleobiology and metabolism, but only when combined with their morphology, ultrastructure, molecular composition, paleoecology and behavior.
Analyses of N isotopic fractionation measured on molecular fossils can also indicate metabolism and cyanobacterial affinity. Phototrophic organisms have a specific nitrogen isotopic offset between total biomass and chloropigments [38]. Based on laboratory experiments, this offset is independent of the nitrogen source (NH4+, N2, or NO3−) and its isotopic composition and from redox conditions during cell growth. The N isotopic offset remains relatively constant within different phototrophic organisms such as cyanobacteria, bacteria, red or green algae or plants, and thus may help identify the source organisms. This offset was notably measured on in 1.1 Ga porphyrins, permitting to relate them to cyanobacteria [38].
2.6. Intracellular biomineralization
Passive biomineralization leads to precipitation of minerals on filaments, sheaths, or cells and enhance their preservation potential but is not specific of particular microorganisms [103,104]. Active biomineralization is controlled by the cell or the organism and might be indicative of its metabolism and taxonomic identity. In modern oceans and alkaline lakes, some cyanobacteria have the capacity to form beads of intracellular Ca-carbonates [57,105], but their preservation potential is unknown. Some extant cyanobacteria also have the capacity to produce intracellular ferric phosphates [106]. In the 1.88 Ga Gunflint Formation, Canada, specific microfossil taxa preserved in silicified stromatolites contain internal Fe-silicate and Fe-carbonate nanocrystals, absent from the external wall surfaces. This feature and its distribution pattern are consistent with intracellular biomineralization, with subsequent recrystallization, and not with known patterns of diagenesis. Thus, the combination of large size, morphology and intracellular Fe biominerals is consistent with a cyanobacterial affinity, and not with other known Fe-mineralizing microorganisms [107]. High-resolution observations, as illustrated in Ref. [107], might possibly reveal new cyanobacteria signatures at the nano-scale, in the rock record.
3. The fossil record
Microfossils interpreted as cyanobacteria have been reported in rocks as old as the early Archean, but their biogenicity and interpretation is highly debated. The necessity of reliable criteria for the study of microfossils is well illustrated with the famous controversy surrounding the “microfossils” from the 3.45 Ga Apex Chert, Australia [108]. These traces interpreted as fossil cyanobacteria based on their morphology and the geological context [108] were subsequently reassessed either as pseudofossils [[109], [110], [111]], contaminants [48], mineral artefacts [53], or microfossils [[112], [113], [114]] and the geological context was revised [110].
In this section, we discuss a selection of (1) unambiguous cyanobacteria microfossils for which morphological features and habitats coincide strikingly with modern lineages, (2) probable and possible cyanobacteria microfossils that share morphological similarities both with a taxon belonging to the cyanobacterial phylum and with other lineages belonging to another phylum or domain of life. The limited number of preservable characters, along with their taphonomic alteration, and possible morphological convergence, limits the interpretation of the fossil record. Therefore, additional signatures would strengthen the confidence in the identification of fossil cyanobacteria. Table 1 summarizes the morphology, dimensions, habitats, and geological occurrences of the fossil taxa discussed below and their possible modern counterparts. Supplementary Table 1 summarizes the geochronological information dating these fossil occurrences.
Table 1.
Summary of microfossil morphological features, habitat, occurrences and their modern analogues.
| Microfossil | Morphology | Dimensions (μm) (length x width) |
Habitat | Occurrences Formation/Group, (age in Ga), country |
Modern analogue |
|---|---|---|---|---|---|
| Anhuithrix magna | Uniserial, straight, curved, bent or twisted filament. Presence of globose cells (akinetes) in or at the end of the filament. In aggregates, filaments are in a common mucilaginous matrix. If isolated, trichomes may be enveloped by an extracellular sheath (thin and non-lamillated). | Filament: up to 2 cm x 141-615 Cells: 56–425 × 113-614 Globose cells: 364-800 |
Transitional to offshore zones. Benthic organism. | Liulaobei Fm (0.84), North China [128] | Nostocales and Stigonematales |
| Archaeoellipsoides | Ellispoidal vesicle with a smooth or ribbed wall. With rounded, flat or slightly depressed ends, Solitary or in groups | 50–100 × 15-25 | Peritidal platform | Francevillian Group (2.1–2.04), Gabon [135]; McArthur Group (1.653–1.647), Australia [136]; Salkhan Limestone (1.6), India [173]; Gaoyuzhuang Fm Jixian section (∼1.58), China [144]; Kotuikan Fm & Yusmastakh Fm (1.5), Siberia [133]; Wumishan Fm (1.425), China [174]; Dismal Lake Group (1.4), Canada [175]; Debengda Fm (1.265–1.04), Siberia [176]; Shorikha Fm (1), Siberia [177]; Kirgitey & Lopatinskaya Fm (0.8), Siberia [178]; Chichkan Fm (0.65), South Kazakhstan [179] |
Anabaena (akinetes) Possible Nostocales or Stigonematales |
| Eoentophysalis belcherensis | Spheroidal to ellipsoidal cells arranged in dyads, tetrads, octets or in loose or palmelloid colonies (spherical, hemispherical or mushroom-like shape), Division by binary fission in three perpendicular planes. The outer layers of colonies are pigmented. | 2,5-9 | Intertidal, mudflats and shallow sub- and supratidal zone | Belcher Supergroup (1.89–1.84), Canada [39]; Amelia Dolomite (McArthur Group – 1.653–1.647), Australia [180]; Kotuikan Fm & Yumastakh Fm & Avzyan Fm (1.5), Ural Mountains [133]; Kheinjua Fm (1.5–1), India [181]; Balbirini Fm (1.483), Australia [182]; Gaoyuzhuang Fm & Wumishan Fm (Changcheng Group – 1.425), China [139]; Sukhaya Tunguska Fm (1), Siberia [154]; Bitter Springs Fm (0.81–0.79), Australia [140]; Deoban Limestone & Jammu Limestone Fm (1–0.967), India [183]; Jiudingshan Fm (0.8), China [184]; Min’Yar Fm (0.79–0.68), Southern Ural Mountains [185]; Svanbergfjellet Fm (0.75–0.7), Spitsbergen [171]; Narssârssuk Fm (0.688), Greenland [186]; Ediacaran Shuurgat Fm (0.635), Mongolia [187] |
Entophysalis Chrooccocales |
| Eohyella | Uni-, bi- or multiseriate pseudofilament which penetrates the substrate. Branched or unbranched. | 4–21 × 8,5-<50 | Euendolithic in silicified ooids, intertidal and subtidal environments | Dahongyu Fm (Changcheng Group – 1.63), China [126]; Koldaha Shale Fm (1.5–1), India [188]; Backlundtoppen Fm (0.8–0.7), Spitsbergen [189]; Eleonore Bay Group (0.95–0.68), Greenland [81]; Red Pine Shale unit (Uinta Mountains Group – ∼0.75), USA [190]; Nagod Limestone Fm (0.625 or 0.75–0.65), Central India [191] |
Hyella Pleurocapsales |
| Eomicrocystis | Subspheroidal colonies, or packets, of spheroidal to ellipsoidal vesicles with a single layered wall. | 0,8–6,5 or 15-17 | Subtidal to intertidal environment | Kotuikan Fm (1.48–1.47), Siberia [138]; Widely distributed in Meso-Neoproterozoic |
Microcystis Chroococcales |
| Gloeodiniopsis | Spheroidal vesicles, sometimes solitary but commonly in colonies. Vesicles are usually with multilayered envelope. | 0,8-8 | Intertidal environment | Bitter Springs Fm (0.81–0.79), Australia [140]; widely distributed in Meso-Neoproterozoic |
Gloeocapsa or Chroococcus Chroococcales |
| Obruchevella | Tightly or loosely coiled empty tube with loose or regular cylindrical spirals. | Width: 0,8-8; 11–25; 27-55 |
Intertidal to supratidal; in open shelf; in tidal flats | Gaoyuzhuang Fm (∼1.58), China [144]; Kamo Group (1.5–1.05), Russia [192]; Thule Supergroup (1.3–1.2), Greenland [193]; Avadh Fm (1.25–1.15), India [194]; Atar/El Mreïti Group (1.1), Mauritania [75]; Mbuji-Mayi Supergroup (1.03–0.95), DRC [165]; Bylot Supergroup (1.092 ± 59 Ma), Canada [163]; Burgess Shale (0.5), Canada [147]; | Spirulina for narrower diameter, and Arthrospira for larger forms, Oscillatoriales, or eukaryotes |
| Oscillatoriopsis | Uniseriate and unbranched trichome, without sheath, formed by discoidal to cylindrical cells whose length is less or equal their diameter. Apices may sometimes be tapered. Solitary or in mat-like mass. | Cell length: 1,8-12 Trichome width: 1-11; 25; 63 |
Shallow water marine environments, subtidal shelf environments, peritidal flat and pluvial lakes | Bitter Springs Fm (0.81–0.79), Australia [140]; widely distributed in Proterozoic |
Oscillatoria, Oscillatoriales or other bacteria |
| Palaeolyngbya | Uniseriate unbranched trichome formed by discoidal to cylindrical cells. Trichome surrounded by an uni- or multilayered smooth sheath. | 2–8 x 8-85 | Peritidal flat, restricted tidal flat or open shelf | Bitter Springs Fm (0.81–0.79), Australia [140]; Widely distributed in Proterozoic |
Lyngbya Oscillatoriales, or other bacteria |
| Polybessurus | Multilamellated cylindrical stalk with concave and regularly spaced layers and with funnel-like shape. The top of the stalk is open or is ended by preserved cells. | Cell: 25–85 × 20-60 Stalk: 20–600 × 15-150 |
Intertidal to subtidal environments | Avzyan Fm (1.35–1.01), Russia [123]; Society Cliffs Fm (1.2), North America [169]; Hunting Fm (1.2), North America [195]; Sukhaya Tunguska Fm (1), Siberia [196]; Seryi Klyuch Fm (1.1–0.85), Siberia [197]; Skillogalee Dolomite (0.77), Australia [198]; Svanbergfjellet Fm (0.75–0.7), Spitsbergen [171]; Eleonore Bay Group (0.95–0.68), Greenland [121] | Cyanostylon -like, Pleurocapsales |
| Polysphaeroides filiformis | Spheroidal vesicles arranged in a filamentous aggregate surrounded by a common sheath closed at both ends. Cells are dispersed or arranged in pairs, tetrads, octets or in colonies. Colonies sometimes arranged in pairs forming pseudobranched filament. | Sheath: 300–500 x 7–13,5 Cell width: 3,5-8 |
Shallow subtidal shelf | Ust’-Il'ya Fm & Kotuikan Fm (1.48–1.46), Siberia [199]; Mbuji-Mayi Supergroup (1.03–0.95), DRC [165]; Miroedikha Fm (0.85), Siberia [162]; Vychegda Fm (0.635–0.55), East European Platform [200] |
Stigonema robustum, Stigonematales, or green and red algae |
| Siphonophycus | Cylindrical empty tube, unbranched with non-septate. Solitary or generally arranged in mass. | ≤120 x 1–3,7 | Subtidal environments | Bitter Springs Fm (0.81–0.79), Australia [140]; Widely distributed in Proterozoic |
Oscillatoria –like, Oscillatoriales, Or other bacteria |
3.1. Unambiguous microfossils of cyanobacteria
Although microfossils attributed to cyanobacteria are abundant during the Proterozoic, many of them are identified with some ambiguities. Knoll and Golubic [41] determined a confidence range for these microfossils, since most of them are identified based on morphology, sometimes coupled with their occurrence in the photic zone, despite the possibility of morphological convergence. So far, only three taxa are unambiguously identified as cyanobacteria: Eoentophysalis, Polybessurus and Eohyella, because they also present distinctive modes of division.
3.1.1. Eoentophysalis
The cyanobacteria fossil record starts around 1.9 billion years ago with the most emblematic Proterozoic microfossil identified so far with certainty as a cyanobacterium, Eoentophysalis belcherensis (Fig. 1A). E. belcherensis was first described in the 1.89–1.84 Ga Belcher Supergroup, Hudson Bay, Canada, where this colonial microorganism formed mats in silicified stromatolites [39,115].
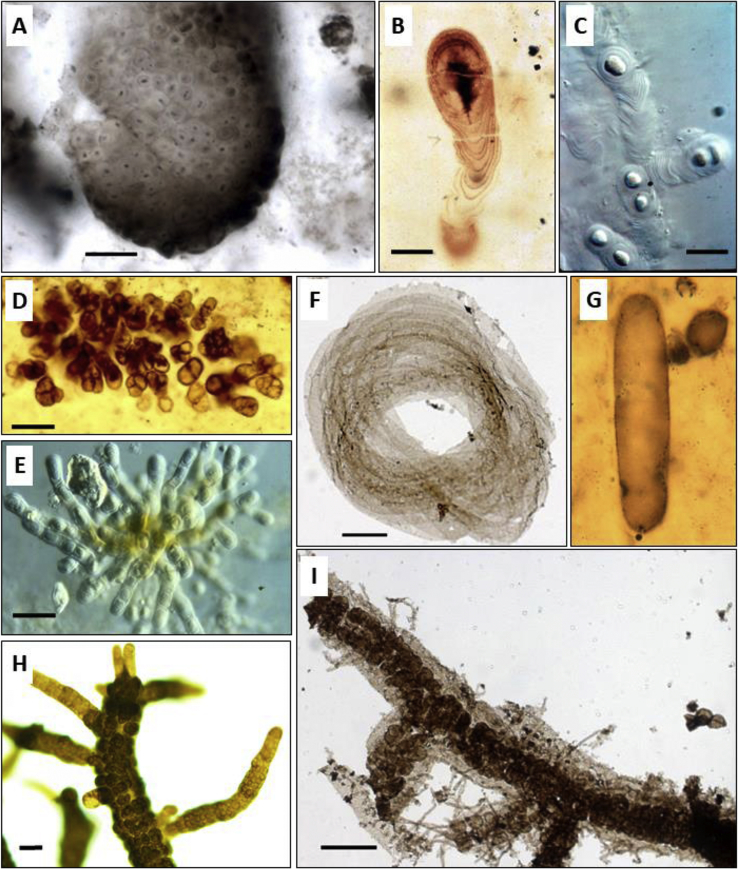
Fig. 1.
Microphotographs of fossils with some of their modern analogues. A) Eoentophysalis belcherensis from the 1.89–1.84 Ga Kasegalik Formation, Belcher Supergroup, Canada; B) Polybessurus from the 800-750 Ma Draken Formation, Svalbard, photo courtesy of A. H. Knoll; C) Cyanostylon, the modern analogue of Polybessurus, photo courtesy of A. H. Knoll; D) Eohyella, the euendolithic cyanobacterium from the 950-680 Ma Eleonore Bay Group, central East Greenland, photo courtesy of A. H. Knoll; E) Hyella, the modern analogue of Eohyella, photo courtesy of A. H. Knoll; F) Obruchevella from the 1.03–0.95 Ga Mbuji-Mayi Supergroup, Democratic Republic of the Congo, photo courtesy of B. K. Baludikay; G) Archaeoellipsoides from the 1.48–1.3 Ga Billyakh Group, Siberia, photo courtesy of A. H. Knoll; H) Stigonema robustum, the modern analogue of Polysphaeroides filiformis, photo courtesy of T. Hauer; I) Polysphaeroides filiformis of the 1.03–0.95 Ga Mbuji-Mayi Supergroup, Democratic Republic of the Congo, photo courtesy of B. K. Baludikay. Scale bars = 20 μm in A, B, E, F, G and H; = 10 μm in C; = 100 μm in D; = 50 μm in I.
The identification of E. belcherensis is based on comparison with the modern cyanobacterium genus, Entophysalis [115]. Based on morphology, Entophysalis belongs to the order Chroococcales [116] and consists of coccoidal unicells forming characteristic pustular palmelloid colonies. The morphology of Entophysalis colonies is due to its mode of cell division by binary fission in three perpendicular planes. Modern Entophysalis produce highly hydrated exopolymer envelopes that expand during cell division. With this expansion, the older exopolymer envelopes are moved outward. Another particularity of Entophysalis colonies is the presence in the outer layers of the colonies of a yellow-brown UV-protecting pigment, scytonemin, that is produced by the most external cells for protection against intense solar radiation [117]. Entophysalis cells grow in the form of mats in the intertidal range of shallow marine basins [115,117]. This cyanobacterial genus may also precipitate micrite in stromatolites such as those in the shallow hypersaline Hamelin Pool, Shark Bay (Western Australia), in association with other cyanobacteria but also other Bacteria, and halophilic and methanogenic Archaea [34,[117], [118], [119]]. Entophysalis is dominant in pustular mats, but also in smooth and colloform coccoidal mats and precipitate micrite, playing a key role in lithifying the stromatolites compared to filamentous forms [34]. They are also reported to enable the stabilization of loose sandy substrate and contribute to the formation of stromatolites by passively trapping sediment particles on the mat's surface between its irregularities [115,117].
The microfossils Eoentophysalis belcherensis were discovered in silicified stromatolites from the Kasegalik and McLeary formations of the Paleoproterozoic Belcher Supergroup (1.89–1.84 Ga). The geological context of these two formations corresponds to intertidal mudflats as well as shallow subtidal and supratidal zones [39,120]. These paleoenvironments are thus comparable to the modern ecological niches occupied by Entophysalis. In addition, E. belcherensis and Entophysalis have similar morphological attributes and, both consist of coccoidal cells showing the same size range (Table 1). They both reproduce by binary fission in three perpendicular planes with a high production of exopolymeric envelopes. Finally, both fossil and modern colonies present the same characteristic warty (pustular) mamillate shape and darkened external layers [115]. However, the nature of this dark outer layer in the fossil colonies remains to be elucidated, as it could be simply due to desiccation instead of specific pigments [59,115]. The morphology of those cells and colonies occurs exclusively in Chroococcales [41]. Therefore, the combination of those criteria (morphology, development, environment and colony pigmentation) enabled the unambiguous identification of this microfossil.
3.1.2. Polybessurus
Polybessurus is another Proterozoic microfossil unambiguously identified as a cyanobacterium [121]. Polybessurus was formally described from silicified carbonates of the ca. 950-680 Ma Eleonore Bay Group (East Greenland) [121], following Fairchild, who was the first who described Polybessurus in his unpublished PhD thesis [122]. Its oldest occurrence is in the ca. 1.35–1.01 Ga Avzyan Formation, Russia [123].
Polybessurus is a fossil of a coccoidal unicellular microorganism with a very particular morphology unique to cyanobacteria (Fig. 1B). This microfossil is composed of a cylindrical stalk produced by means of asymmetric and unidirectional secretion of extracellular mucopolysaccharide envelopes, growing from within the sediments upward. Ellipsoidal cells, surrounded by multiple envelopes, are present at the higher end of the stalk. The primordial role of this stalk is to maintain the cells at the sediment-water interface [124]. The morphology of Polybessurus resembles a stack of cup shaped envelopes.
This particular morphology with cells jetted upward by the stalk is similar to the modern cyanobacterium Cyanostylon belonging to the order Chroococcales (Fig. 1C). However, Polybessurus reproduced by the formation of baeocytes (smaller cells) which is characteristic of the order Pleurocapsales, but is unlike the modern Cyanostylon [121]. Indeed, the reproduction mode of Polybessurus was inferred from preserved clusters of narrow tubes radiating from the same point. This particularity allows to suggest that those narrow tubes were produced by small cells [121]. Thus, the modern counterpart of Polybessurus is a not yet described Pleurocapsales Cyanostylon-like cyanobacterium discovered in peritidal environments of the Bahama Banks, environments similar to the paleoenvironment of Polybessurus [79]. Actually, it is the discovery of the fossil Polybessurus that permitted to predict the environment where to look for its modern counterpart [121,124]. Taken together, the particular morphology of Polybessurus, its mode of reproduction and ecology enable its affiliation to cyanobacteria, probably within the Pleurocapsales.
3.1.3. Eohyella
The Limestone-Dolomite series of the ca. 950-680 Ma Eleonore Bay Group (central East Greenland) also preserve a group of particular microfossils showing a distinct endolithic behavior [81]. Among them, Eohyella is a coccoidal microfossil forming pseudofilaments (where juxtaposed cells do not share a common wall), sometimes branching depending on the microfossil species, by the juxtaposition of several cells surrounded by extracellular envelopes (Fig. 1D). Eohyella was qualified as an euendolith cyanobacterium because of its orientation within the substrate: they crosscut the substrate laminae [81,125]. Eohyella microfossils occur in oolites and pisolites of shallow peritidal environment. Within the euendolithic cyanobacteria group of the Limestone-Dolomite series, Eohyella was the most abundant genus. So far, its oldest occurrence was found in the 1.63 Ga Dahongyu Formation, China [126].
The morphology, behavior and paleoenvironment of Eohyella are similar to those of the modern genus Hyella (Fig. 1E), which enabled the identification of this microfossil as a cyanobacterium, of the order of Pleurocapsales. In the present day, Hyella is an euendolithic cyanobacterial taxon present in ooids of the shallow subtidal environment in the Bahamas Banks [81].
3.2. Probable and possible cyanobacteria microfossils
Other microfossils are identified with less confidence but still considered as probable or possible cyanobacteria, depending on the authors. They are discussed below in alphabetical order. While analyses of fossil porphyrins suggest that cyanobacteria were the dominant primary producers in mid-proterozoic oceans [38], it is still unknown whether they were planktonic or benthic, and mostly small and coccoidal or filamentous, or both. The geological record seems to preserve mostly benthic cyanobacteria in the form of microbial mats or endoliths, although some microfossils, such as Eomicrocystis, are possible planktonic cyanobacteria. Modern Microcystis colonies overwinter on lake sediment after summer blooms and reinvade the water column in the spring [127]. This alternance of benthic and planktonic stage of life may have evolved early in cyanobacteria.
This review does not illustrate all the Proterozoic microfossils interpreted as cyanobacteria, often displaying simple colonial morphologies also encountered in other bacterial clades. Sergeev et al. [50] list additional taxa such as Eosynechococcus, Leiosphaeridia, Myxococcoides in their extensive review about all the Proterozoic microfossils currently interpreted as cyanobacteria. Here, we discuss those we consider the most relevant, common or distinctive taxa that could be used directly, or after further characterization, as new possible calibration points.
3.2.1. Anhuithrix
Pang et al. [128] described a new mat-forming filamentous microfossil, Anhuithrix magna, from the Tonian Liulaobei Formation (0.84 Ga), North China. They interpreted this fossil as a heterocytous N-fixing cyanobacterium of subsections IV or V (Nostocales or Stigonematales), based on the occurrence of large globose cells, observed between smaller vegetative cells within a filament, or at filament ends. These large cells were interpreted as probable akinetes according to their dimensions (364–800 μm in diameter) and their location in filaments. This microfossil reproduced by the production of hormogonia and grew by binary fission. However, the preservation of those microfossils as carbonaceous compressions might lead to cell deformation, making difficult the interpretation based on size and simple morphology.
This new fossil genus is, as Archaeoellipsoides (discussed below), a promising calibration point for molecular clocks to provide a minimum age of the Nostocales or Stigonematales. Therefore, this probable interpretation should be strengthened by microanalyses (ultrastructure, chemistry) of extracted microfossils to confirm its identity.
3.2.2. Archaeoellipsoides
Nostocales and Stigonematales are modern cyanobacterial orders that, as mentioned above, have evolved specialized cells, the heterocytes [129], and in some cases akinetes [130]. Akinetes, formed from vegetative cells, differ from those by their larger size, a thicker cell wall and absence of cell division. Modern akinetes, in all known species of akinete-bearing cyanobacteria, have an ellipsoidal to cylindrical morphology and range from 2 to 450 μm in length and to 1.8–30 μm in width [128,131]. The microfossils Archaeoellipsoides are cylindrical cells that include several species differing by their size [50] (Fig. 1G). Golubic et al. [132] hypothesized that Archaeoellipsoides from the 1.48–1.3 Ga Billyakh Group (Siberia) were fossil akinetes, based on morphological characteristics (size, elongated shape, and absence of cell division), by comparison with the akinetes of the extant analogue Anabaena. Anabaena, a nostocacean cyanobacterium, produces akinetes ranging from 7 to 90 μm in length and to 1.8–25 μm in width [128,131]. Moreover, Archaeoellipsoides are associated, in the Billyakh assemblage, with short trichomes. Those trichomes were interpreted as possible products of akinete germination [133], but the relationship between the akinetes and the co-occurring trichomes is discussed [134]. Older occurrences include poorly preserved microfossils in the 2.1–2.04 Ga Francevillian Supergroup, Gabon [135] and better preserved specimens in the 1.65 Ga McArthur Supergroup, Australia [136].
However, the simple morphology of Archaeoellipsoides might also occur among other microorganisms, such as some giant Firmicutes, e.g. the parasitic Epulopiscium [137] or green algae, such as Spirotaenia or Stichococcus [134]. Therefore, the identity of Archaeoellipsoides remains to be confirmed by other evidence than morphology alone.
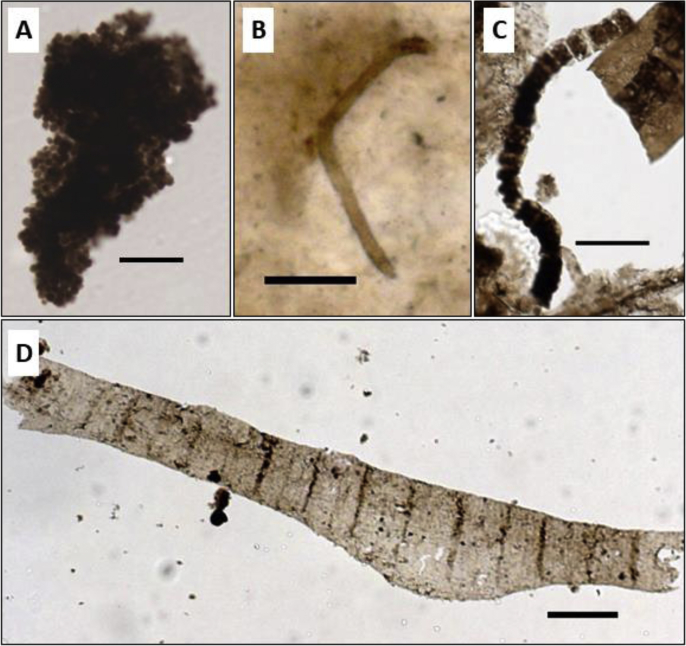
3.2.3. Eomicrocystis
Eomicrocystis is a microfossil genus described in 1984 by Golovenok and Belova [138], and interpreted as a cyanobacteria. It was named according to its possible modern analogue, Microcystis, a planktonic coccoid cyanobacterium that forms colonies in freshwater lakes and ponds [133,138]. Eomicrocystis also formed colonies composed of small spheroidal to ellipsoidal cells (Fig. 2A), but preserved in marine environments. It may dominate assemblages and occur as blooms in specific levels of the 1.1 Ga El Mreiti Group, Mauritania [88]. Sergeev et al. [133] suggested that Eomicrocystis was a junior synonym of the genus Coniunctiophycus that Zhang [139] had also described and interpreted as the fossil analogue of the extant Microcystis. Eomicrocystis’ oldest occurrence is in the 1.48–1.46 Ga Kotuikan Formation, Siberia [133]. However, the simple morphology of this microfossil does not enable a confident interpretation as a cyanobacterium. Indeed, this morphology is also encountered among eukaryotic algae (e.g. Nannochloropsis) [63] and other bacteria.
Fig. 2.
Microphotographs of fossils considered as probable or possible cyanobacteria. A) Eomicrocystis from the 1.1 Ga Atar/El Mreïti Group, Taoudeni Basin, Mauritania. B) Siphonophycus from the 1.48–1.3 Ga Billyakh Group, Siberia; C) Palaeolyngbya from the 1.03–0.95 Ga Mbuji-Mayi Supergroup, Democratic Republic of the Congo, photo courtesy of B. K. Baludikay; D) Tortunema from the 1.03–0.95 Ga Mbuji-Mayi Supergroup, Democratic Republic of the Congo, photo courtesy of B. K. Baludikay. Scale bars = 20 μm.
3.2.4. Gloeodiniopsis
Gloeodiniopsis is also another possible fossil of a benthic chroococcacean cyanobacterium [140]. Its stratigraphic range starts with the ∼1.58 Ga Gaoyuzhuang Formation, China, and the 1.55 Ga Satka Formation, the Southern Ural Mountains [139,141].
Gloeodiniopsis consists of several spheroidal to ellipsoidal vesicles surrounded by a multilayered envelope. They are generally grouped in colonies but they may also occur occasionally as isolated cells. This morphology resembles that of modern Gloeocapsa or Chroococcus. These two possible modern analogues show both a similar morphology but differ slightly by the presence (Gloeocapsa) or the absence (Chroococcus) of a colored sheath and the thickness of this sheath. The distinction between these two modern cyanobacteria is still debated because some consider this difference between Gloeocapsa and Chroococcus as minor [50], and moreover, molecular analyzes show that they both are polyphyletic groups [45,142].
Although the morphology of Gloeodiniopsis is very similar to Gloeocapsa, some green algae may also present a similar morphology, e.g. Volvox [143], Sphaerocystis or Eudorina [63]. Again here, new analyzes of ultrastructure and chemistry, including the presence of unique pigments [93] in microfossils and modern specimens might help the discrimination.
3.2.5. Obruchevella
Obruchevella is a microfossil that consists of an empty helically coiled tube (Fig. 1F). This fossil genus includes several species differing by their tube and spiral diameters. The stratigraphic range of Obruchevella starts in the third member of the ∼1.58 Ga Gaoyuzhuang Formation, China [144]. When preserved as carbonaceous compressions in shale, the helicoidal filaments are compressed into more or less tight spirals. When preserved in 3D in chert, they occur as screw-like coiled filaments [145].
Reitlinger [146] first described Obruchevella specimens as foraminifera. Its biological affinity was however reassessed as cyanobacteria with Spirulina as modern counterpart, a cyanobacterium belonging to the Oscillatoriales [147,148]. The interpretation of Obruchevella was essentially based on its helically coiled morphology and its ecology, both similar to Spirulina, a planktonic helically coiled cyanobacterium. However, this morphology is also known in other cyanobacteria (Arthrospira) [149], and other bacteria, e.g. the parasitic but also free-living helicoidal species of Leptospira [51], Pararhodospirillum [150,151] and in some eukaryotic algae (e.g. Ophiocytium, a Tribophycean alga [63]). Some species of Leptospirales are associated with marine stromatolites [34]. Leptospira has a much thinner diameter (0.1 μm) and does not overlap with the thinnest Obruchevella (0.8 μm). Some Obruchevella microfossils present dimensions similar to Spirulina (tube diameter 0.5–3 μm, see in Ref. [149]), while most other species have sizes close to Arthrospira dimensions (tube diameter 2.5–16 μm, see in Ref. [149]. A few other Obruchevella species have a tube diameter wider than 20 μm, broader than Arthrospira and Spirulina, and may perhaps be closer to eukaryotic organisms. Other organisms in the fossil record also have spiral morphology with a larger size and a eukaryotic interpretation. The Mesoproterozoic specimens of Grypania spiralis, a coiled filamentous fossil, reach macroscopic size and have been interpreted as a eukaryotic organism based on its size, preserved septae and external sheath, and cell length and size suggesting a coenocytic organization. The older 1.9 Ga Grypania are smaller, thinner and do not preserve internal structure, and resemble more ripped-up microbial mat fragments (see review in Ref. [59]).
Thus, although Obruchevella is a probable cyanobacteria, these hypotheses are only based on morphology and size, and would be strengthened by ultrastructure and chemical analyzes.
3.2.6. Oscillatoriopsis
Oscillatoriacean cyanobacteria are reported as the most represented group of cyanobacteria in the fossil record [152]. One of those is Oscillatoriopsis, an unsheathed cellular filament with more or less isodiametric cells (Length:Width ≤ 1) [140,153,154] (Fig. 2D). Oscillatoriopsis microfossils are slightly constricted at intercellular septa [153].
Oscillatoriopsis microfossils are commonly found in shallow water marine environments but they also may be found in lacustrine deposits or pluvial lakes [50,140,155]. The stratigraphic record of this genus starts with the ca. 2.2–1.8 Ga silicified carbonates from the Duck Creek Dolomite Formation, Australia [155].
The interpretation is only based on morphology, similar to modern Oscillatoria. However, this type of simple morphology is also found among other prokaryotes such as Beggiatoa, a sulfide-oxidizing proteobacterium [41,50,155,156] or among eukaryotes such as Ulothrix, a green alga [157]. Oscillatoriacean cyanobacteria often reproduce by the formation of hormogonia. The fossil occurrence of such short filamentous microfossils interpreted as Oscillatoriopsis could support its identificationas hormogonia of oscillatoriacean cyanobacteria [141]. However, other bacteria, again including Beggiatoa, may also produce hormogonia. Therefore, the interpretation of Oscillatoriopsis as an oscillatoriacean cyanobacterium, albeit plausible, is still debated [41].
3.2.7. Palaeolyngbya
Palaeolyngbya is interpreted as a hormogonian oscillatoriocean cyanobacterium microfossil found first in the 0.81–0.79 Ga Bitter Springs Formation, Central Australia [140,158], but its oldest occurrence is in the 1.60 Ga Gaoyuzhuang Formation, China [159], and in 1.48–1.46 Ga Kotuikan Formation, Siberia [160]. It is a sheathed filament with a smooth wall (Fig. 2C). Regular and uniseriate discoidal cells are arranged inside the single sheath [153].
As several other possible cyanobacteria microfossils, Palaeolyngbya has been interpreted as such based only on its morphology [140,161] and therefore is debatable.
3.2.8. Polysphaeroides filiformis
Polysphaeroides is a fossil genus described by Hermann [162], which included several fossil species, until 1994, when Hofmann and Jackson [163] moved nearly all of the species of Polysphaeroides to the genus Chlorogloeaopsis, because of their similar morphology. Only one species remained, Polysphaeroides filiformis [164]. Polysphaeroides filiformis consists of spheroidal cells arranged in a loose multiseriate filamentous aggregate and surrounded by a common envelope with closed ends (Fig. 1I). The colonies formed by the spheroidal cells may branch. The 1.48–1.46 Ga Kotuikan Formation, Siberia, is the oldest formation in which Polysphaeroides filiformis was recorded so far [164].
Polysphaeroides is compared to modern stigonemataleans [164,165], although some authors suggested a possible affinity to eukaryotic algae, either green or red [166], for example the red algae Polysiphonia (Figs. 16–42 in Ref. [63]). However, the morphology of Polysphaeroides filiformis, characterized by a thick sheath surrounding multiseriate filament arrangement and occasional branching, fits the description of the recently re-evaluated modern genus Stigonema [167]. For instance, Polysphaeroides filiformis from the 1.03–0.95 Ga Mbuji-Mayi Supergroup, DRC [165], displays cell shape, arrangement, and diameters, as well as the presence of the thick sheath and the occurrence of branching (Fig. 1I) that are strikingly similar to the modern multiseriate species, Stigonema robustum (Fig. 1H). We consider that this fossil cyanobacterium may represent a good alternative calibration for future molecular clock analyses as modern taxa belonging to this genus form a monophyletic clade. Modern multiseriate Stigonema species including the recently described S. informe, and S. robustum, are generally epilithic [167] while Polysphaeroides filiformis from Mbuji-Mayi Supergroup was associated with intertidal or subtidal environments [165].
3.2.9. Siphonophycus
Siphonophycus is one of the most common filamentous microfossils in the Proterozoic. It is commonly found in shallow water deposits in Proterozoic mat assemblages [41,168], preserved in situ in chert [41,80,124,140,169] or as bundles ripped off mats in shales [54], or as the main stromatolite builders [50]. Siphonophycus is an unbranched, non-septate and empty smooth-walled filamentous sheath [140] (Fig. 2B). Several species are distinguished based on the diameter range of the filamentous sheath [153]. Broad (15–25 μm) filaments of Siphonophycus transvaalensis reported in the latest Archean 2.52 Ga Gamohaan Formation of South Africa were interpreted as non-heterocytous cyanobacteria similar to modern Oscillatoriales [170]. Similar Oscillatoriales-like microfossils occur through all the Proterozoic.
Siphonophycus specimens are generally interpreted as sheaths of oscillatoriacean cyanobacteria. Schopf [140] occasionally observed transverse thickenings that were placed along Siphonophycus filamentous sheaths. Therefore, he suggested that modern counterparts of Siphonophycus microfossils would be LPP-like cyanobacteria (Lyngbya, Phormidium and Plectonema) [140,141,171]. Nevertheless, this simple morphology is also encountered in other bacteria. For example, minute Siphonophycus sheaths may be comparable to Chloroflexi-like photosynthetic bacteria [41,168]. Large Siphonophycus microfossils might also be the remains of filamentous eukaryotic algae [50]. Some Siphonophycus may present a sheath with a thickness of around 2 μm. Thick sheaths are generally common among cyanobacteria and not among other bacterial phyla [172]. They may thus be a criterion of a cyanobacterial affinity for those Siphonophycus specimens, in addition to alternating vertical and horizontal disposition in mats, which may indicate phototropism or chemotropism, a behavior not unique to cyanobacteria [41,134].
4. Molecular dating
The understanding of cyanobacterial phylum evolution has progressed significantly with the emergence of molecular biology techniques and new sequencing technologies. Since the late 90's a myriad of phylogenetic studies based on single loci (i.e., 16S rRNA or some protein) have been published (e.g. Refs. [9,201]). Even if the five major sections of Cyanobacteria were not yet represented in genomic databases, the first studies to use a phylogenomic (i.e. multilocus) dataset were the works of Rodriguez-Ezpeleta et al. (2005) [202] and then of Criscuolo and Gribaldo (2011) [203]. In 2013, the large CyanoGEBA (Genomic Encyclopedia of Bacteria and Archaea) sequencing project led to an improvement in terms of genomic coverage of cyanobacterial taxa, notably by sequencing genomes belonging to sections II and V [201]. Since then, the number of publicly available cyanobacterial genomes has dramatically increased. Yet, their quality, especially contamination of cyanobacterial assemblies by non-cyanobacterial DNA, has gone worse in parallel, which is a problem for phylogenetic analyses [204]. Moreover, the real biodiversity of cyanobacteria is still under-represented in genomic databases, mainly because of a biased sampling in the sequencing effort [205,206]. Nevertheless, since Shih et al. (2013) [201], many authors have taken advantage of these new genomes to carry out phylogenomic analyses [13,15,[207], [208], [209], [210], [211], [212], [213]]. Most of these studies focussed on integrating new genomic data to the same set of 100–200 hundreds loci (but see Ref. [15]). By doing so, few tried to handle the methodological difficulties associated with the use of large-scale data to resolve the phylogeny of old groups such as Cyanobacteria, whether during dataset assembly (e.g., contamination, horizontal gene transfer, hidden paralogy) or phylogenetic inference (e.g., substitutional saturation, compositional bias, heterogeneous evolutionary rate) [[214], [215], [216], [217]], except [203]. Consequently, among the ten phylogenomic studies cited above, only three are in agreement on the cyanobacterial backbone [13,201,210].
4.1. Calibration, models, and datasets
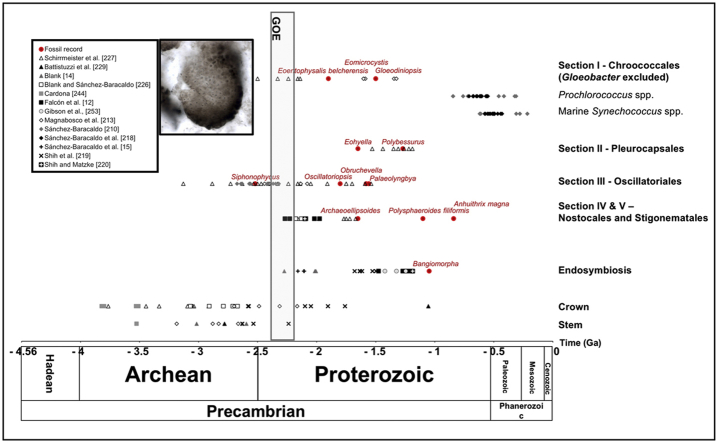
For molecular clock reconstructions, microfossils of cyanobacteria are needed as a source of calibration of the molecular phylogenies. However, only few calibration points are available to date oxygenic photosynthesis, the endosymbiotic event having given rise to the chloroplast, as well as the origin of cyanobacteria. For the latter, estimates range from the early Archean to the GOE, in the Paleoproterozoic (Fig. 3). Beyond the lack of congruence of the phylogenomic studies and the polyphyletic nature of many cyanobacterial groups (including well-known genera such as Synechoccocus and Leptolyngbya), the issue is complicated by the absence in genomic databases of modern counterparts (Entophysalis, Hyella or Cyanostylon-like) of the unambiguous cyanobacteria microfossils (Eoentophysalis, Eohyella and Polybessurus). As we have no reliable indication of the phylogenetic position of these important modern taxa, researchers often use flimsy affiliations as calibration points. Usually, only few cyanobacterial fossils are used as constraints for molecular clock analyses. They include Archaeoellipsoides for monophyletic Nostocales and Stigonematales lineages, and Eohyella for Pleurocapsales lineages, despite the absence of a genetic characterization of the modern counterpart Hyella (e.g. Refs. [210,218]). The occurrence of fossil diatoms is often used for the advent of the endosymbiont Rivularia intracellularis, and the fossil red algae Bangiomorpha for the minimum age of the primary endosymbiosis. Moreover, some authors have chosen not to use cyanobacteria microfossils in their analysis, but instead fossils from eukaryotic lineages such as plants [219], or the occurrence of horizontal gene transfer [213], or the GOE to set a lower bound on Cyanobacteria as a phylum [220]. In a non-exhaustive survey, we observed more than 89 different approaches used to estimate the evolution of cyanobacterial phylum. This diversity of phylogenies, and calibration points, but also of clock models and dating software packages, has led to a large variety of age estimates.
Fig. 3.
Microfossils record of unambiguous, probable and possible cyanobacteria (see text for discussion, Table 1, and Supplementary Table 1), and of Bangiomorpha as an unambiguous red alga, and minimum median age estimates for the divergence of sections I, II, III, IV and V as described by Rippka et al. [45]; for phylogenetic nodes supporting stem and crown group cyanobacteria according to the literature; and for the primary endosymbiosis. Note that the age of Polysphaeroides filiformis considered here corresponds to its record in Baludikay et al. [165].
So far, all the attempts to date the evolutionary events of the cyanobacterial phylum used a fixed-node approach, where researchers manually select nodes to place calibration points. This strategy has the disadvantage of inducing an unmeasurable uncertainty on the inferred node ages, due to errors in taxonomic affiliation and/or specified geological ages. The affiliation error is often due to misleading morphological similarities between unrelated extant organisms and (ambiguous) microfossils. Moreover, polyphyletic groups make impossible to specify node calibrations, except by reporting their origin to a (much) older common ancestor far back in the tree. This problem could be even worse if some scarcely sampled extant organisms used for calibration are actually polyphyletic. Regarding ages, they are specified as prior distributions partly based on the minimum age (lower bound or oldest occurrence) of a given microfossil in the paleontological record. However, owing to issues due to taphonomy and extinction of stem groups, this may introduce an unmeasurable divergence between the ages specified for the fossil and the real geological span of the organism. Ultimately, inferred node ages are thus highly dependent on the completeness of the paleontological record [221].
Because of these limitations, an alternative strategy, termed tip-dating, would be more suitable for dating the evolution of Cyanobacteria. In such an approach, the placement of the microfossils within the tree is guided by a morphological matrix and supported by statistical values, the posterior probabilities [222]. The consequence of this “automatic” placement is that tip-dating enables the use of a wider range of microfossils, not only the unambiguous ones, but also the numerous ambiguous microfossils. Further, by explicitly modeling stem groups within the tree [221], tip-dating is able to test (and thus either confirm or reject) the affiliation of microfossils to extant organisms, which is usually taken for granted in the paleontological literature and many molecular dating studies building upon it.
4.2. Origin of cyanobacteria and oxygenic photosynthesis
In most cyanobacterial phylogenetic analyses that are using a non-cyanobacterial outgroup to root the tree, the reference strain Gloeobacter violaceus PCC7421 has a basal position [9,[223], [224], [225]]. Consequently, the phylogenetic node bearing Gloeobacter and the rest of modern cyanobacterial lineages serves as calibration for the origin of cyanobacteria in several studies [[226], [227], [228]]. In these studies, the authors have set different root limit ages, so that the maximum root age may vary between the earliest estimate of abiogenesis around 4.2 Ga [226], the end of the Late Heavy Bombardment at 3.85 Ga [228], or the GOE at 2.4 Ga [229].
Recently, two newly discovered lineages were proposed as sister groups of the cyanobacterial phylum, the Melainabacteria [230] and the Sericytochromatia [231]. Of note, these lineages (mostly known as metagenomics assemblies) do not contain genes required for photosynthesis nor carbon fixation [231]. The integration of genetic data from Melainabacteria and Sericytochromatia as outgroups for molecular clock analyses suggested that cyanobacteria evolved just before the GOE [213,219]. Taken together, this suggests that oxygenic photosynthesis has evolved after the separation of cyanobacteria from Melainabacteria [213,219]. However, the loss of photosynthetic capability in the ancestor of the three lineages before or at the time of GOE has been suggested as an alternative hypothesis that cannot be ruled out [232].
Among bacterial phototrophs (cyanobacteria, green S-bacteria, green non sulfur bacteria, purple bacteria, heliobacteria, some acidobacteria and gemmatimonadetes), Cyanobacteria is the only lineage that possesses two photosynthetic reaction centers of the Fe–S type (RCI) and Quinone type (RCII), whereas anoxygenic bacteria possess either the Fe–S or Quinone type. So far, three hypotheses were proposed to explain the presence of both types of RC in modern cyanobacteria. Two of these hypotheses suggest that both RCs would have been present in an anoxygenic phototrophic ancestor. In a first hypothesis, RCs evolved within the common ancestor of (all) bacterial phototrophs. Both of them were kept in the cyanobacterial lineage whereas there was a selective loss of one type of each in the modern anoxygenic lineage ancestors [233,234]. In the second hypothesis, the RCI and RCII would have emerged in the protocyanobacterial ancestor by duplication of a unique ancestral RC. This was followed by lateral transfer of a different RC type to the ancestors of the modern anoxygenic phototrophs [235]. The existence of an anoxygenic cyanobacterial ancestor may be supported by the occurrence of several genes involved in anoxygenic photosynthesis in modern cyanobacterial genomes [236], and the co-occurrence of both anoxygenic and oxygenic photosynthesis in several lineages of modern cyanobacteria clades [237,238]. A third hypothesis rather suggests the independent evolution of the two RCs in separate lineages of anoxygenic phototrophs and their lateral transfer into a protocyanobacterial ancestor, the so-called fusion hypothesis [239,240]. At least in purple bacteria, the genes for RCII are clustered in an ensemble of operons, the photosynthesis gene cluster (PGC), some organisms even harboring the PGC on large plasmids. This observation makes the transfer of full photosystems highly plausible, and recent events of such kind have been convincingly inferred in Rhodobacteraceae [241].
In order to test the likelihood of the ancient transfer of photosystems between the bacterial phototrophic lineages, Magnabosco and colleagues [213] added horizontal gene transfer events information of two genes (encoding for Mg-chelatase and S-adenosyl-l-homocysteine hydrolase) as additional constraints to their models to estimate the stem age of the bacterial phototrophs (Cyanobacteria, green S-bacteria and green non-sulfur bacteria). These authors assumed that such estimates would allow them to investigate the feasibility and timing of the RC transfer events between phototrophic lineages. Their results excluded the possibility of a RC transfer from the green sulfur bacteria to cyanobacteria, and thus, invalidated the fusion hypothesis. However, they were not able to choose between the two hypotheses suggesting that both RCs emerged from a common ancestor [213].
First, the reaction centers RCI and RCII would operate separately and asynchronously in the same ancestral anoxygenic phototroph organism. The RCI would catalyze H2S oxidation as in green S-bacteria, while the RCII would act as a light-dependent electron transporter as in purple bacteria [242]. A water-splitting RCII could also have evolved from an ancestral RCII type already capable of photosynthesis and manganese oxidation [243]. However, the evolution of these processes, and the early or late evolution of oxygenic photosynthesis, are still debated e.g. Refs. [99,244].
4.3. Diversification
Several studies suggested that ancestral cyanobacteria first inhabited freshwater ecosystems [13,15,17,210,218,226], but see e.g. Ref. [16] for a marine origin. Nevertheless, these estimates are based on comparison with the modern ecology of basal clades of cyanobacteria, which are likely to have changed through time. Moreover, the fossil record of cyanobacteria is almost exclusively estuarine and shallow marine, often from the intertidal zone, or hypersaline lacustrine. However, terrestrial deposits are less commonly preserved in the geological record, and this might bias our view of the fossil ecological ranges.
A couple time calibrated phylogenies based on low-resolution alignments of 16S rRNA gene sequences or on a large multilocus dataset [227,228] suggest that multicellular forms of cyanobacteria were potentially present when the GOE started, implying a pre-GOE origin of the cyanobacterial phylum. Furthermore, their results hint at an acceleration of the diversification rate after the substantial increase of atmospheric oxygen concentration [227]. The acquisition of the multicellularity would be an advantage for UV resistance and substrate adhesion [40]. However, multicellularity is polyphyletic and convergent several times across extant species – especially in cyanobacteria.
Other studies hypothesize that the origin of marine planktonic cyanobacteria would have happened after the evolution of crown groups in freshwater, terrestrial and benthic coastal modern environments [210]. Benthic (terrestrial and coastal) cyanobacteria may have dominated the oxygenic photosynthesis from the late Archean and possibly until the mid-Neoproterozoic [245].
However, these hypotheses remain to be confirmed since the record of Archean cyanobacteria is controversial as explained above, and the fossil record is biased towards benthic forms. Benthic filamentous cyanobacteria forming mats or preserved in silicified stromatolites are preserved preferentially to small planktonic cells sedimenting in the water column. Moreover shallow-water deposits are more common in the Precambrian then deeper sediments.
Non-heterocytous N-fixing unicellular and filamentous Trichodesmium spp. would have appeared later, during the late Neoproterozoic [210,218]. This observation possibly coincides with an increase of bioavailable Mo (an essential co-factor of nitrogenase) in the open ocean [40,218,246,247]. However, cyanobacteria likely had to invent a new N2-fixation machinery that could operate in the presence of the rising O2, leading to the evolution of heterocytous cyanobacterial taxa, probably as early as the GOE [227], and possibly supported by Paleoproterozoic [[134], [135], [136]] and Neoproterozoic [128] microfossils. However, this hypothesis might be questioned as heterocytous cyanobacteria age estimates resulted from models that used poorly preserved putative fossil akinetes from 2.1 Ga Franceville Gabon. In the Paleoproterozoic redox stratified oceans, cyanobacteria may have performed anoxygenic (rather than oxygenic) photosynthesis using H2S above euxinic layers, or may have been outcompeted by anoxygenic photosynthetic bacteria metabolizing H2S or Fe2+ above ferruginous water [248]. However, they became important primary producers in the still stratified mid-Proterozoic oceans [38].
4.4. Origin of chloroplast
Chloroplasts form a monophyletic cluster within the Cyanobacteria phylum [12]. This observation is elegantly interpreted as the result of a primary endosymbiosis at the origin of the chloroplast [249]. Although this theory is well accepted in the scientific community (but see Ref. [250] for a more complex model involving Chlamydiales), the precise position of the plastid within the extant diversity of Cyanobacteria has been a matter of discussion. Two major scenarios are opposed, one postulating an ancient origin (early-branching) [13,14,105,202,203,209,211,220] and the other one postulating a (relatively) recent (late-branching) origin [12,208,251]. The hypothesis of an early origin is more frequent in the literature, and it has recently been strengthened by the study of Ponce-Toledo et al. (2017) [13], who identified the early-branching Gloeomargarita lithophora as the closest extant relative of plastids. In an attempt to date the primary endosymbiosis, Falcón and colleagues [12] assumed that the closest relative of chloroplasts was a unicellular N-fixing cyanobacterium (late-branching hypothesis) and that it occurred during the middle of the Proterozoic [12] (Fig. 3). However the calibration used included Archean ages for the highly controversial Apex chert microstructures and sterane that were subsequently reassessed as contaminants (see above). Later studies of ATP synthases subunits and elongation factors permitted to estimate the first endosymbiosis event at approximately 0.9 Ga [220]. Taking advantage of the recent discovery of Gloeomargarita [13,252], Sanchez-Baraccaldo et al. (2017) estimated the age of the origin of the chloroplast at 1.9 Ga (2.12–1.75 Ga) [15]. This result is similar to the one reported in Ref. [14], although the topologies used in the two studies were slightly different with respect to plastid position. In contrast, Shih et al., 2017 [219] recovered a quite different age for plastids (1.1 Ga). Interestingly, this latter estimate is more similar to the result of [12], even if assuming an early-branching hypothesis for plastid emergence. This suggests that discrepancies in estimated chloroplast ages rather stem from differences in calibration points and/or dating models than from topologies (early vs late-branching hypotheses). Indeed, if differences do exist in tree topologies concerning chloroplast emergence, the wide age intervals obtained in the various analyses often exceed the branch length variation implied by topological changes. This is not surprising given the very short length of the corresponding internodes in the cyanobacterial backbone.
As exploitable cyanobacterial microfossils are not numerous, a logical strategy is to use the morphologically more complex and more recent eukaryotic algae as calibration points. However, the oldest unambiguous fossil record of eukaryotic algae are silicified multicellular bangiophyte red algae preserved in hypersaline shallow-water environment [195] and recently well dated at 1.047 Ga [253]. These fossils were interpreted as benthic multicellular red algae based on their morphology, longitudinal division pattern, attachment structures, and ecology [195]. Other 1.6 Ga microfossils are interpreted as more divergent Florideophyte red algae based on morphology [254], but their age is debated because of the complex geology of the area [253]. Microfossils interpreted as green algae may also provide an estimation of the minimum age for chloroplast acquisition. Their fossil record ranges from unambiguous 0.6 Ga prasinophytes based on wall ultrastructure [255], 0.8 Ga probable siphonocladalean chlorophytes and probable hydrodictyacean chlorophyte based on distinctive morphology and ecology [134], the latter also found in 1.1–0.9 Ga lower Shaler Group of arctic Canada [256], to 1.65 Ga acritarchs whose putative algal interpretation needs confirmation [257]. Using the new age of 1.047 Ga calibration for Bangiomorpha, Gibson et al. [253] estimated the primary chloroplast endosymbiosis at 1.25 Ga, consistent with most of the unambiguous algal fossil record.
5. Conclusions
Cyanobacterial fossil record starts unambiguously at 1.89–1.84 Ga and the minimum age for the oxygenic photosynthesis starts with the GOE around 2.4 Ga. Eoentophysalis, Polybessurus and Eohyella microfossils present a combination of distinctive morphologies, modes of division and ecology that are diagnostic of the cyanobacteria phylum [41]. Therefore, their placement into this phylum is strongly supported, unlike other Proterozoic microfossils that display a simpler morphology widespread among other prokaryotes. Older possible geochemical traces of oxygenation and the metabolisms involved in stromatolites and MISS builders in the Archean are discussed. Moreover, the origin and timing of oxygenic photosynthesis is also still debated although some studies corroborate that the evolution of oxygenic photosynthesis happened right before the GOE which would then be a consequence of this evolution. The origin, timing and environment of the primary endosymbiotic event giving rise to eukaryotic algae are also still debated. Therefore, it is essential to define new biosignatures indicative of cyanobacteria in order to reassess their fossil record and provide new calibration points for molecular clocks. Those biosignatures will combine analyses of the morphology, ultrastructure and ecology of promising microfossils identified in this review, with their molecular (lipids and pigments), metal and isotopic composition. Identifying these fossils, not only as cyanobacteria, but of specific clades within the cyanobacteria, will improve our understanding of their diversification record and provide new calibration points. Coupling these new microfossil calibration points with improved molecular phylogenies and alternative molecular clocks (such as tip-dating) will then enable to date the minimum ages of important biological events such as the origin of oxygenic photosynthesis and the acquisition of chloroplasts among photosynthetic eukaryotic lineages.
Acknowledgments
We thank the editors W. Fischer & J. Valentine for their invitation to write this review for a special issue “How did life come to tolerate and thrive in an oxygenated world?”. Research funding came from the European Research Council StG ELiTE [grant number: FP7/308074], the BELSPO IAP PLANET TOPERS, the FRS-FNRS-FWO EOS ET-HoME project, FRS–FNRS, and the ULiege mini-ARC PUMA project. A. Wilmotte is research associate of the FRS-FNRS. We also thank Prof. A.H. Knoll, Dr T. Hauer, Dr B.K. Baludikay and J. Beghin for providing pictures to illustrate this review. Finally, we also thank anonymous reviewers for their insights and comments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.freeradbiomed.2019.05.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bekker A., Holland H.D., Wang P., Iii D.R., Stein H.J., Hannah J.L., Coetzee L.L., Beukes N.J. Dating the rise of atmospheric oxygen. Nature. 2004;427:117–120. doi: 10.1038/nature02260. [DOI] [PubMed] [Google Scholar]
- 2.Hannah J.L., Bekker A., Stein H.J., Markey R.J., Holland H.D. Primitive Os and 2316 Ma age for marine shale: implications for Paleoproterozoic glacial events and the rise of atmospheric oxygen. Earth Planet. Sci. Lett. 2004;225:43–52. [Google Scholar]
- 3.Kasting J.F. What caused the rise of atmospheric O2? Chem. Geol. 2013;362:13–25. [Google Scholar]
- 4.Lewis L.A. Hold the salt: freshwater origin of primary plastids. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114:9759–9760. doi: 10.1073/pnas.1712956114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold D.A., Caron A., Fournier G.P., Summons R.E. Paleoproterozoic sterol biosynthesis and the rise of oxygen. Nature. 2017;543:420–423. doi: 10.1038/nature21412. [DOI] [PubMed] [Google Scholar]
- 6.Roger A.J., Muñoz-Gómez S.A., Kamikawa R. The origin and diversification of mitochondria. Curr. Biol. 2017;27:R1177–R1192. doi: 10.1016/j.cub.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Knoll A.H., Hewitt D. Major Transitions Evol. Revisited. MIT Press; Cambridge: 2011. Phylogenetic, functional and geological perspectives on complex multicellularity; pp. 251–270. [Google Scholar]
- 8.Sagan L. On the origin of mitosing cells. J. Theor. Biol. 1967;14:225–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 9.Schirrmeister B.E., Anisimova M., Antonelli A., Bagheri H.C. Evolution of cyanobacterial morphotypes. Taxa required for improved phylogenomic approaches. Commun. Integr. Biol. 2011;11:45. doi: 10.4161/cib.4.4.16183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delwiche C.F. Tracing the thread of plastid diversity through the tapestry of life. Am. Nat. 1999;154:S164–S177. doi: 10.1086/303291. [DOI] [PubMed] [Google Scholar]
- 11.Giovannoni S.J., Turner S., Olsen G.J., Barns S., Lane D.J., Pace N.R. Evolutionary relationships among cyanobacteria and green chloroplasts. J. Bacteriol. 1988;170:3584–3592. doi: 10.1128/jb.170.8.3584-3592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falcón L.I., Magallón S., Castillo A. Dating the cyanobacterial ancestor of the chloroplast. ISME J. 2010;4:777–783. doi: 10.1038/ismej.2010.2. [DOI] [PubMed] [Google Scholar]
- 13.Ponce-Toledo R.I., Deschamps P., López-García P., Zivanovic Y., Benzerara K., Moreira D. An early-branching freshwater cyanobacterium at the origin of plastids. Curr. Biol. 2017;27:386–391. doi: 10.1016/j.cub.2016.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blank C.E. Origin and early evolution of photosynthetic eukaryotes in freshwater environments: reinterpreting proterozoic paleobiology and biogeochemical processes in light of trait evolution. J. Phycol. 2013;49:1040–1055. doi: 10.1111/jpy.12111. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Baracaldo P., Raven J.A., Pisani D., Knoll A.H. Early photosynthetic eukaryotes inhabited low-salinity habitats. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114:E7737–E7745. doi: 10.1073/pnas.1620089114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakov T., Boyko J.D., Alverson A.J., Beaulieu J.M. Models with unequal transition rates favor marine origins of Cyanobacteria and photosynthetic eukaryotes. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114:E10606–E10607. doi: 10.1073/pnas.1716692114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sánchez-Baracaldo P., Bianchini G., Huelsenbeck J.P., Raven J.A., Pisani D., Knoll A.H. Reply to Nakov et al.: model choice requires biological insight when studying the ancestral habitat of photosynthetic eukaryotes. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114:E10608–E10609. doi: 10.1073/pnas.1717417114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer W.W., Hemp J., Johnson J.E. Evolution of oxygenic photosynthesis. Annu. Rev. Earth Planet Sci. 2016;44:647–683. [Google Scholar]
- 19.Rosing M.T., Frei R. U-rich Archaean sea-floor sediments from Greenland - indications of >3700 Ma oxygenic photosynthesis. Earth Planet. Sci. Lett. 2004;217:237–244. [Google Scholar]
- 20.Shen Y., Buick R. The antiquity of microbial sulfate reduction. Earth Sci. Rev. 2004;64:243–272. [Google Scholar]
- 21.Buick R. When did oxygenic photosynthesis evolve? Philos. Trans. R. Soc. B Biol. Sci. 2008;363:2731–2743. doi: 10.1098/rstb.2008.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono S., Beukes N.J., Rumble D., Fogel M.L. Early evolution of atmospheric oxygen from multiple-sulfur and carbon isotope records of the 2.9 Ga Mozaan Group of the Pongola Supergroup, Southern Africa. S. Afr. J. Geol. 2006;109:97–108. [Google Scholar]
- 23.Crowe S.A., Døssing L.N., Beukes N.J., Bau M., Kruger S.J., Frei R., Canfield D.E. Atmospheric oxygenation three billion years ago. Nature. 2013;501:535–538. doi: 10.1038/nature12426. [DOI] [PubMed] [Google Scholar]
- 24.Frei R., Gaucher C., Poulton S.W., Canfield D.E. Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes. Nature. 2009;461:250. doi: 10.1038/nature08266. [DOI] [PubMed] [Google Scholar]
- 25.Planavsky N.J., Asael D., Hofmann A., Reinhard C.T., Lalonde S.V., Knudsen A., Wang X., Ossa Ossa F., Pecoits E., Smith A.J.B., Beukes N.J., Bekker A., Johnson T.M., Konhauser K.O., Lyons T.W., Rouxel O.J. Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nat. Geosci. 2014;7:283–286. [Google Scholar]
- 26.Anbar A.D., Duan Y., Lyons T.W., Arnold G.L., Kendall B., Creaser R.A., Kaufman A.J., Gordon G.W., Scott C., Garvin J., Buick R. A whiff of oxygen before the Great oxidation event? Science. 2007;317:1903–1906. doi: 10.1126/science.1140325. [DOI] [PubMed] [Google Scholar]
- 27.Lyons T.W., Reinhard C.T., Planavsky N.J. The rise of oxygen in Earth's early ocean and atmosphere. Nature. 2014:307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 28.Bosak T., Liang B., Sim M.S., Petroff A.P. Morphological record of oxygenic photosynthesis in conical stromatolites. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:10939–10943. doi: 10.1073/pnas.0900885106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noffke N., Gerdes G., Klenke T., Krumbein W.E. Microbially induced sedimentary structures - a new category within the classification of primary sedimentary structures - discussion. J. Sediment. Res. 2001;72:587–588. [Google Scholar]
- 30.Heubeck C. An early ecosystem of Archean tidal microbial mats (Moodies Group, South Africa, ca. 3.2 Ga) Geology. 2009;37:931–934. [Google Scholar]
- 31.Homann M., Sansjofre P., Van Zuilen M., Heubeck C., Gong J., Killingsworth B., Foster I.S., Airo A., Van Kranendonk M.J., Ader M., Lalonde S.V. Microbial life and biogeochemical cycling on land 3,220 million years ago. Nat. Geosci. 2018;11:665–671. [Google Scholar]
- 32.Bosak T., Knoll A.H., Petroff A.P. The meaning of stromatolites. Annu. Rev. Earth Planet Sci. 2013;41:21–44. [Google Scholar]
- 33.Slotznick S.P., Fischer W.W. Examining archean methanotrophy. Earth Planet. Sci. Lett. 2016;441:52–59. [Google Scholar]
- 34.Suosaari E.P., Reid R.P., Playford P.E., Foster J.S., Stolz J.F., Casaburi G., Hagan P.D., Chirayath V., Macintyre I.G., Planavsky N.J., Eberli G.P. New multi-scale perspectives on the stromatolites of Shark Bay, western Australia. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alleon J., Summons R.E. Organic geochemical approaches to understanding early life. Free Radic. Biol. Med. 2019 doi: 10.1016/j.freeradbiomed.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Schinteie R., Brocks J.J. Paleoecology of Neoproterozoic hypersaline environments: biomarker evidence for haloarchaea, methanogens, and cyanobacteria. Geobiology. 2017;15:641–663. doi: 10.1111/gbi.12245. [DOI] [PubMed] [Google Scholar]
- 37.Rashby S.E., Sessions A.L., Summons R.E., Newman D.K. Biosynthesis of 2-methylbacteriohopanepolyols by an anoxygenic phototroph. Proc. Natl. Acad. Sci. Unit. States Am. 2007;104:15099–15104. doi: 10.1073/pnas.0704912104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gueneli N., Mckenna A.M., Ohkouchi N., Boreham C.J., Beghin J., Javaux E.J., Brocks J.J. 1.1-billion-year-old Porphyrins establish a marine ecosystem dominated by bacterial primary producers. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115:E6978–E6986. doi: 10.1073/pnas.1803866115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofmann H.J. Precambrian microflora, belcher islands, Canada: significance and systematics. J. Paleontol. 1976;50:1040–1073. [Google Scholar]
- 40.Schirrmeister B.E., Sanchez-Baracaldo P., Wacey D. Cyanobacterial evolution during the precambrian. Int. J. Astrobiol. 2016;15:187–204. [Google Scholar]
- 41.Knoll A.H., Golubic S. Proterozoic and living cyanobacteria. In: Schidlowski P., Golubic S., Kimberley M., McKirdy D., Trudinger, editors. Early Org. Evol. Implic. Miner. Energy Resour. Springer; 1992. pp. 450–462. [Google Scholar]
- 42.Javaux E.J., Knoll A.H., Walter M.R. TEM evidence for eukaryotic diversity in mid-Proterozoic oceans. Geobiology. 2004;2:121–132. [Google Scholar]
- 43.Willman S., Cohen P.A. Quantifying Evol. Early Life; 2011. Ultrastructural approaches to the microfossil record: assessing biological affinities by use of transmission electron microscopy; pp. 301–320. [Google Scholar]
- 44.Stanier R.Y., Sistrom W.R., Hansen T.A., Whitton B.A., Castenholz R.W., Pfennig N., Gorlenko V.N., Kondratieva E.N., Eimhjellen K.E., Whittenbury R., Gherna R.L., Trüper H.G. Proposal to place the nomenclature of the cyanobacteria (Blue-Green algae) under the rules of the international Code of nomenclature of bacteria. Int. J. Syst. Evol. Microbiol. 1978;28:335–336. [Google Scholar]
- 45.Rippka R., Deruelles J., Waterbury J.B., Herdman M., Stanier R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology. 1979;111:1–61. [Google Scholar]
- 46.Komárek J., Kaštovský J., Mareš J., Johansen J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia. 2014;86:295–335. [Google Scholar]
- 47.Javaux E.J., Benzerara K., Microfossils Comptes. Rendus - Palevol. 2009;8:605–615. [Google Scholar]
- 48.Altermann W., Kazmierczak J. Archean microfossils : a reappraisal of early life on Earth. Res. Microbiol. 2003;154:611–617. doi: 10.1016/j.resmic.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Javaux E.J., Knoll A.H., Walter M. Recognizing and interpreting the fossils of early eukaryotes. Orig. Life Evol. Biosph. 2003;33:75–94. doi: 10.1023/a:1023992712071. [DOI] [PubMed] [Google Scholar]
- 50.Sergeev V.N., Sharma M., Shukla Y. Proterozoic fossil cyanobacteria. Palaeobotanist. 2012;61:189–358. [Google Scholar]
- 51.Adler B., de la Pena Moctezuma A., Leptospira, Leptospirosis Vet. Microbiol. 2010;140:287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 52.García-Ruiz J.M., Hyde S.T., Carnerup A.M., Christy A.G., Van Kranendonk M.J., Welham N.J. Self-assembled silica-carbonate structures and detection of ancient microfossils. Science. 2003;302:1194–1197. doi: 10.1126/science.1090163. [DOI] [PubMed] [Google Scholar]
- 53.Wacey D., Saunders M., Kong C., Brasier A., Brasier M. 3.46 Ga Apex chert ‘microfossils’ reinterpreted as mineral artefacts produced during phyllosilicate exfoliation. Gondwana Res. 2016;36:296–313. [Google Scholar]
- 54.Javaux E.J., Knoll A.H. Micropaleontology of the lower Mesoproterozoic Roper Group , Australia , and implications for early eukaryotic evolution. J. Paleontol. 2017;91:199–229. [Google Scholar]
- 55.Knoll A.H., Bergmann K.D., Strauss J.V. Life: the first two billion years. Philos. Trans. R. Soc. B Biol. Sci. 2016;371 doi: 10.1098/rstb.2015.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rouillard J., García-Ruiz J.M., Gong J., van Zuilen M.A. A morphogram for silica-witherite biomorphs and its application to microfossil identification in the early earth rock record. Geobiology. 2018;16:279–296. doi: 10.1111/gbi.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benzerara K., Skouri-Panet F., Li J., Ferard C., Gugger M., Laurent T., Couradeau E., Ragon M., Cosmidis J., Menguy N., Margaret-Oliver I., Tavera R., Lopez-Garcia P., Moreira D. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111:10933–10938. doi: 10.1073/pnas.1403510111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Javaux E.J., Marshal C.P. A new approach in deciphering early protist paleobiology and evolution: combined microscopy and microchemistry of single Proterozoic acritarchs. Rev. Palaeobot. Palynol. 2006;139:1–15. [Google Scholar]
- 59.Javaux E.J., Lepot K. The Paleoproterozoic fossil record: implications for the evolution of the biosphere during Earth's middle-age. Earth Sci. Rev. 2018:68–86. [Google Scholar]
- 60.Knoll A.H., Golubic S. Anatomy and taphonomy of a precambrian algal stromatolite. Precambrian Res. 1979;10:115–151. [Google Scholar]
- 61.Golubic S., Seong-Joo L. Early cyanobacterial fossil record: preservation, palaeoenvironments and identification. Eur. J. Phycol. 1999;34:339–348. [Google Scholar]
- 62.Castenholz R.W., Wilmotte A., Herdman M., Rippka R., Waterbury J.B., Iteman I., Hoffmann L., Phylum B.X. Cyanobacteria. Bergey’s Manual® Syst. Bacteriol. 2001;437:473–599. [Google Scholar]
- 63.Graham L.E., Wilcox L.W. Prentice Hall; Upper Saddle River: 2000. Algae. [Google Scholar]
- 64.Šmarda J., Šmajs D., Komrska J., Krzyžánek V. S-layers on cell walls of cyanobacteria. Micron. 2002;33:257–277. doi: 10.1016/s0968-4328(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 65.Pereira S., Zille A., Micheletti E., Moradas-ferreira P., De Philippis R., Tamagnini P. biosynthesis and assembly. 2009:917–941. doi: 10.1111/j.1574-6976.2009.00183.x. [DOI] [PubMed] [Google Scholar]
- 66.Bartley J.K. Actualistic taphonomy of cyanobacteria; implications for the Precambrian fossil record. Palaios. 1996;11:571–586. [Google Scholar]
- 67.Newman S.A., Klepac-Ceraj V., Mariotti G., Pruss S.B., Watson N., Bosak T. Experimental fossilization of mat-forming cyanobacteria in coarse-grained siliciclastic sediments. Geobiology. 2017;15:484–498. doi: 10.1111/gbi.12229. [DOI] [PubMed] [Google Scholar]
- 68.Lepot K., Compère P., Gérard E., Namsaraev Z., Verleyen E., Tavernier I., Hodgson D.A., Vyverman W., Gilbert B., Wilmotte A., Javaux E.J. Organic and mineral imprints in fossil photosynthetic mats of an East Antarctic lake. Geobiology. 2014;12:424–450. doi: 10.1111/gbi.12096. [DOI] [PubMed] [Google Scholar]
- 69.Konhauser K.O., Jones B., Phoenix V.R., Ferris G., Renaut R.W. The microbial role in hot spring silicification. AMBIO A J. Hum. Environ. 2009;33:552–558. doi: 10.1579/0044-7447-33.8.552. [DOI] [PubMed] [Google Scholar]
- 70.Rippka R., Waterbury J., Cohen-Bazire G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 1974;100:419–436. [Google Scholar]
- 71.Komárek J., Kaštovský J. Coincidences of structural and molecular characters in evolutionary lines of cyanobacteria. Arch. Hydrobiol. Suppl. Algol. Stud. 2003;109:305–325. [Google Scholar]
- 72.Pacton M., Gorin G.E., Fiet N. Unravelling the origin of ultralaminae in sedimentary organic matter: the contribution of bacteria and photosynthetic organisms. J. Sediment. Res. 2008;78:654–667. [Google Scholar]
- 73.Anbar A.D. Oceans: elements and evolution. Science. 2008;322:1481–1483. doi: 10.1126/science.1163100. [DOI] [PubMed] [Google Scholar]
- 74.Diamond C.W., Planavsky N.J., Wang C., Lyons T.W. What the ∼1.4 Ga Xiamaling Formation can and cannot tell us about the mid-Proterozoic ocean. Geobiology. 2018;16:219–236. doi: 10.1111/gbi.12282. [DOI] [PubMed] [Google Scholar]
- 75.Beghin J., Guilbaud R., Poulton S.W., Gueneli N., Brocks J.J., Storme J.Y., Blanpied C., Javaux E.J. A palaeoecological model for the late mesoproterozoic – early neoproterozoic atar/el Mreïti group, Taoudeni basin, Mauritania, northwestern Africa. Precambrian Res. 2017;299:1–14. [Google Scholar]
- 76.Guilbaud R., Slater B.J., Poulton S.W., Harvey T.H.P., Brocks J.J., Nettersheim B.J., Butterfield N.J. Oxygen minimum zones in the early Cambrian ocean. Geochemical Perspect. Lett. 2018:33–38. [Google Scholar]
- 77.Raiswell R., Hardisty D.S., Lyons T.W., Canfield D.E., Owens J.D., Planavsky N.J., Poulton S.W., Reinhard C.T. The iron paleoredox proxies: a guide to the pitfalls, problems and proper practice. Am. J. Sci. 2018;318:491–526. [Google Scholar]
- 78.Porter S.M., Agiæ H., Riedman L.A. Anoxic ecosystems and early eukaryotes. Emerg. Top. Life Sci. 2018;2:299–309. doi: 10.1042/ETLS20170162. [DOI] [PubMed] [Google Scholar]
- 79.Knoll A.H. Univ. Press; Princeton, NJ: 2003. Life on a Young Planet; p. 277. [Google Scholar]
- 80.Knoll A.H., Worndle S., Kah L.C. Covariance of microfossil assemblages and microbialite textures across an upper mesoproterozoic carbonate platform. Palaios. 2013;28:453–470. [Google Scholar]
- 81.Green J.W., Knoll A.H., Swett K. Microfossils from oolites and pisolites of the upper proterozoic Eleonore Bay group, central East Greenland. J. Paleontol. 1988;62:835–852. doi: 10.1017/s0022336000030109. [DOI] [PubMed] [Google Scholar]
- 82.Golubic S., Friedman I., Schneider J. The lithobiontic ecological niche, with special reference to microorganisms. SEPM J. Sediment. Res. 1981;51:475–478. [Google Scholar]
- 83.Brocks J.J., Love G.D., Summons R.E., Knoll A.H., Logan G.A., Bowden S.A. Biomarker evidence for green and purple sulphur bacteria in a stratified Palaeoproterozoic sea. Nature. 2005;437:866–870. doi: 10.1038/nature04068. [DOI] [PubMed] [Google Scholar]
- 84.Summons R.E., Jahnke L.L., Hope J.M., Logan G.A. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature. 1999;400:554–557. doi: 10.1038/23005. [DOI] [PubMed] [Google Scholar]
- 85.Brocks J.J., Logan G.A., Buick R., Summons R.E. Archean molecular fossils and the early rise of eukaryotes. Science. 1999;285:1033–1036. doi: 10.1126/science.285.5430.1033. [DOI] [PubMed] [Google Scholar]
- 86.Rasmussen B., Fletcher I.R., Brocks J.J., Kilburn M.R. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature. 2008;455:1101–1104. doi: 10.1038/nature07381. [DOI] [PubMed] [Google Scholar]
- 87.French K.L., Hallmann C., Hope J.M., Schoon P.L., Zumberge J.A., Hoshino Y., Peters C.A., George S.C., Love G.D., Brocks J.J., Buick R., Summons R.E. Reappraisal of hydrocarbon biomarkers in Archean rocks. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112:5915–5920. doi: 10.1073/pnas.1419563112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beghin J., Storme J.-Y., Blanpied C., Gueneli N., Brocks J.J., Poulton S.W., Javaux E.J. Microfossils from the late mesoproterozoic – early neoproterozoic atar/el Mreïti group, Taoudeni basin, Mauritania, northwestern Africa. Precambrian Res. 2017;291:63–82. [Google Scholar]
- 89.Marshall C.P., Javaux E.J., Knoll A.H., Walter M.R. Combined micro-Fourier transform infrared (FTIR) spectroscopy and micro-Raman spectroscopy of Proterozoic acritarchs: a new approach to Palaeobiology. Precambrian Res. 2005;138:208–224. [Google Scholar]
- 90.Baludikay B.K., François C., Sforna M.C., Beghin J., Cornet Y., Storme J.-Y., Fagel N., Fontaine F., Littke R., Baudet D., Delvaux D., Javaux E.J. Raman microspectroscopy, bitumen reflectance and illite crystallinity scale: comparison of different geothermometry methods on fossiliferous Proterozoic sedimentary basins (DR Congo, Mauritania and Australia) Int. J. Coal Geol. 2018;191:80–94. [Google Scholar]
- 91.Edwards H.G.M., Garcia-Pichel F., Newton E.M., Wynn-Williams D.D. Vibrational Raman spectroscopic study of scytonmenin, the UV-protective cyanobacterial pigment. Spectrochim. Acta. 1999:193–200. doi: 10.1016/s1386-1425(99)00218-8. [DOI] [PubMed] [Google Scholar]
- 92.Jehlièka J., Oren A. Raman spectroscopy in halophile research. Front. Microbiol. 2013;4:1–7. doi: 10.3389/fmicb.2013.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Storme J.-Y., Golubic S., Wilmotte A., Kleinteich J., Velázquez D., Javaux E.J. Raman characterization of the UV-protective pigment gloeocapsin and its role in the survival of cyanobacteria. Astrobiology. 2015;15:843–857. doi: 10.1089/ast.2015.1292. [DOI] [PubMed] [Google Scholar]
- 94.De Oliveira V.E., Miranda M.A.C.N., Soares M.C.S., Edwards H.G.M., De Oliveira L.F.C. Study of carotenoids in cyanobacteria by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;150:373–380. doi: 10.1016/j.saa.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 95.Proteau P.J., Gerwick W.H., Garcia-Pichel F., Castenholz R. The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia. 1993;49:825–829. doi: 10.1007/BF01923559. [DOI] [PubMed] [Google Scholar]
- 96.Fulton J.M., Arthur M.A., Freeman K.H. Subboreal aridity and scytonemin in the holocene black sea. Org. Geochem. 2012;49:47–55. [Google Scholar]
- 97.Hodgson D.A., Wright S.W., Davies N. Mass Spectrometry and reverse phase HPLC techniques for the identification of degraded fossil pigments in lake sediments and their application in palaeolimnology. J. Paleolimnol. 1997;18:335–350. [Google Scholar]
- 98.Knoll A.H., Canfield D.E., Konhauser K.O. John Wiley; 2012. Fundamentals of Geobiology. [Google Scholar]
- 99.Ward L.M., Shih P.M. The evolution and productivity of carbon fixation pathways in response to changes in oxygen concentration over geological time. Free Radic. Biol. Med. 2019 doi: 10.1016/j.freeradbiomed.2019.01.049. S0891584918315132. [DOI] [PubMed] [Google Scholar]
- 100.House C.H., Schopf J.W., McKeegan K.D., Coath C.D., Harrison T.M., Stetter K.O. Carbon isotopic composition of individual Precambrian microfossils. Geology. 2000;28:707–710. doi: 10.1130/0091-7613(2000)28<707:cicoip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 101.Delarue F., Robert F., Sugitani K., Tartèse R., Duhamel R., Derenne S. Nitrogen isotope signatures of microfossils suggest aerobic metabolism 3.0 Gyr ago. Geochemical Perspect. Lett. 2018;7:32–36. [Google Scholar]
- 102.Williford K.H., Ushikubo T., Schopf J.W., Lepot K., Kitajima K., Valley J.W. Preservation and detection of microstructural and taxonomic correlations in the carbon isotopic compositions of individual Precambrian microfossils. Geochem. Cosmochim. Acta. 2013;104:165–182. [Google Scholar]
- 103.Konhauser K.O. Bacterial iron biomineralisation in nature. FEMS Microbiol. Rev. 1997;20:315–326. [Google Scholar]
- 104.Li J., Benzerara K., Bernard S., Beyssac O. The link between biomineralization and fossilization of bacteria : insights from fi eld and experimental studies. Chem. Geol. 2013;359:49–69. [Google Scholar]
- 105.Couradeau E., Benzerara K., Gérard E., Moreira D., Bernard S., Brown G.E., López-García P. An early-branching microbialite cyanobacterium forms intracellular carbonates. Science. 2012;336:459–462. doi: 10.1126/science.1216171. [DOI] [PubMed] [Google Scholar]
- 106.Brown I.I., Bryant D.A., Casamatta D., Thomas-Keprta K.L., Sarkisova S.A., Shen G., Graham J.E., Boyd E.S., Peters J.W., Garrison D.H., McKay D.S. Polyphasic characterization of a thermotolerant siderophilic filamentous cyanobacterium that produces intracellular iron deposits. Appl. Environ. Microbiol. 2010;76:6664–6672. doi: 10.1128/AEM.00662-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lepot K., Addad A., Knoll A.H., Wang J., Troadec D., Béché A., Javaux E.J. Iron minerals within specific microfossil morphospecies of the 1.88 Ga Gunflint Formation. Nat. Commun. 2017;8:14890. doi: 10.1038/ncomms14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schopf J.W., Packer B.M. Early archean (3.3-billion to 3.5-billion-year-old) microfossils from warrawoona group, Australia. Science. 1987;237:70–73. doi: 10.1126/science.11539686. [DOI] [PubMed] [Google Scholar]
- 109.Bower D.M., Steele A., Fries M.D., Green O.R., Lindsay J.F. Raman imaging spectroscopy of a putative microfossil from the ∼3.46 Ga Apex chert: insights from quartz grain orientation. Astrobiology. 2016;16:169–180. doi: 10.1089/ast.2014.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brasier M.D., Green O.R., Lindsay J.F., McLoughlin N., Steele A., Stoakes C. Critical testing of Earth's oldest putative fossil assemblage from the ∼3.5 Ga Apex chert, Chinaman Creek, Western Australia. Precambrian Res. 2005;140:55–102. [Google Scholar]
- 111.Pinti D.L., Mineau R., Clement V. Hydrothermal alteration and microfossil artefacts of the 3,465-million-year-old Apex chert. Nat. Geosci. 2009;2:640–643. [Google Scholar]
- 112.De Gregorio B.T., Sharp T.G., Flynn G.J., Wirick S., Hervig R.L. Biogenic origin for Earth's oldest putative microfossils. Geology. 2009;37:631–634. [Google Scholar]
- 113.Schopf J.W. Microfossils of the early archean Apex chert: new evidence of the antiquity of life. Science. 1993;260:640–646. doi: 10.1126/science.260.5108.640. [DOI] [PubMed] [Google Scholar]
- 114.Schopf J.W., Kitajima K., Spicuzza M.J., Kudryavtsev A.B., Valley J.W. SIMS analyses of the oldest known assemblage of microfossils document their taxon-correlated carbon isotope compositions. Proc. Natl. Acad. Sci. Unit. States Am. 2017;115:53–58. doi: 10.1073/pnas.1718063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Golubic S., Hofmann H.J. Comparison of holocene and mid-precambrian entophysalidaceae (cyanophyta) in stromatolitic algal mats: cell division and degradation. J. Paleontol. 1976;50:1074–1082. [Google Scholar]
- 116.Komárek J. Spektrum Akademischer Verlag; Heidelberg: 1999. Cyanoprokaryota 1. Teil: Chroococcales, Subwasserflora von Mitteleuropa. 19 1–548. [Google Scholar]
- 117.Golubic S., Abed R.M.M. Entophysalis mats as environmental regulators. Natl. Acad. Sci. Planet. Biol. 2010:237–251. [Google Scholar]
- 118.Burns B.P., Anitori R., Butterworth P., Henneberger R., Goh F., Allen M.A., Ibañez-Peral R., Bergquist P.L., Walter M.R., Neilan B.A. Modern analogues and the early history of microbial life. Precambrian Res. 2009;173:10–18. [Google Scholar]
- 119.Papineau D., Walker J.J., Mojzsis S.J., Pace N.R. Composition and structure of microbial communities from stromatolites of Hamelin Pool in Shark Bay, western Australia, appl. Environ. Microbiol. 2005;71:4822–4832. doi: 10.1128/AEM.71.8.4822-4832.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hofmann H.J., Jackson G.D. Precambrian (aphebian) microfossils from belcher islands, Hudson Bay. Can. J. Earth Sci. 1969;6:1137–1144. [Google Scholar]
- 121.Green J.W., Knoll A.H., Golubic S., Swett K. Paleobiology of distinctive benthic microfossils from the upper Proterozoic Limestone-Dolomite “Series,” central East Greenland. Am. J. Bot. 1987;74:928–940. [PubMed] [Google Scholar]
- 122.Fairchild T. Doctoral dissertation University of California; 1975. The Geologic Setting and Paleobiology of a Late Precambrian Stromatolitic Microflora from South Australia. [Google Scholar]
- 123.Sergeev V.N. Microfossils in cherts from the middle riphean (mesoproterozoic) Avzyan Formation, southern ural Mountains, Russian federation. Precambrian Res. 1994;65:231–254. doi: 10.1016/0301-9268(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 124.Knoll A.H. updated edition. Princeton University Press; 2015. Life on a Young Planet: the First Three Billion Years of Evolution on Earth. [Google Scholar]
- 125.Tribollet A., Golubic S. Coral Reefs an Ecosyst. Transit.; 2011. Reef bioerosion: agents and processes; pp. 435–449. [Google Scholar]
- 126.Zhang Y. Proterozoic stromatolitic micro-organisms from Hebei, North China: cell preservation and cell division. Precambrian Res. 1988;38:165–175. [Google Scholar]
- 127.Visser P.M., Ibelings B.W., Mur L.R., Walsby A.E. The ecophysiology of the harmful cyanobacterium Microcystis. In: Huisman J., Matthijs H., Visser P.M., editors. Harmful Cyanobacteria. Springer; Dordrecht: 2005. pp. 109–142. [Google Scholar]
- 128.Pang K., Tang Q., Chen L., Wan B., Niu C., Yuan X., Xiao S. Nitrogen-fixing heterocystous cyanobacteria in the tonian period. Curr. Biol. 2018;28:616–622. doi: 10.1016/j.cub.2018.01.008. e1. [DOI] [PubMed] [Google Scholar]
- 129.Wolk C.P., Ernst A., Elhai J. Heterocyst metabolism and development. In: Bryant D.A., editor. Mol. Biol. Cyanobacteria. Adv. Photosynth. Springer; Dordrecht: 1994. [Google Scholar]
- 130.Kaplan-Levy R.N., Hadas O., Summers M.L., Rücker J., Sukenik A. Akinetes: Dormant cells of Cyanobacteria. In: Lubzens E., editor. Dormancy and Resistance in Harsh Environments. Springer; Berlin: 2010. pp. 5–27. [Google Scholar]
- 131.Komárek J. Springer Spektrum; 2013. Cyanoprokaryota. 3. Teil: Heterocytous genera, Süßwasserflora von Mitteleuropa. [Google Scholar]
- 132.Golubic S., Sergeev V.N., Knoll A.H. Mesoproterozoic Archaeoellipsoides: akinetes of heterocystous cyanobacteria. Lethaia. 1995;28:285–298. doi: 10.1111/j.1502-3931.1995.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 133.Sergeev V.N., Knoll A.H., Grotzinger J.P. Paleobiology of the mesoproterozoic Billyakh group, anabar uplift, northern Siberia. J. Paleontol. 1995;69:1–37. [PubMed] [Google Scholar]
- 134.Butterfield N.J. Proterozoic photosynthesis - a critical review. Palaeontology. 2015;58:953–972. [Google Scholar]
- 135.Amard B., Bertrand-Sarfati J. Microfossils in 2000 Ma old cherty stromatolites of the Franceville group. Gabon, Precambrian Res. 1997;81:197–221. [Google Scholar]
- 136.Tomitani A., Knoll A.H., Cavanaugh C.M., Ohno T. The evolutionary diversification of cyanobacteria: molecular-phylogenetic and paleontological perspectives. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103:5442–5447. doi: 10.1073/pnas.0600999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schulz-Vogt H.N., Angert E.R., Garcia-Pichel F. Giant bacteria. Encycl. Life Sci. 2007:1–7. [Google Scholar]
- 138.Golovenok V.K., Belova M.Y. Riphean microbiotas in cherts of the Billyakh group on the anabar uplift. Paleontol. Zh. 1984;4:20–30. [Google Scholar]
- 139.Zhang Y. Proterozoic stromatolite microfloras of the Gaoyuzhuang Formation (early sinian: riphean), hebei, China. J. Paleontol. 1981;55:485–506. [Google Scholar]
- 140.Schopf J.W. Microflora of the bitter Springs Formation, late precambrian, central Australia. J. Paleontol. 1968;42:651–688. [Google Scholar]
- 141.Sergeev V.N., Sharma M., Shukla Y. Mesoproterozoic silicified microbiotas of Russia and India-Characteristics and Contrasts. Palaeobotanist. 2008;57:323–358. [Google Scholar]
- 142.Komárková J., Jezberová J., Komárek O., Zapomìlová E. Variability of Chroococcus (cyanobacteria) morphospecies with regard to phylogenetic relationships. Hydrobiologia. 2010;639:69–83. [Google Scholar]
- 143.Nozaki H. Morphology, sexual reproduction and taxonomy of Volvox carteri f. kawasakiensis f. nov. (Chlorophyta) from Japan. Phycologia. 1988;27:209–220. [Google Scholar]
- 144.Shi M., Feng Q., Khan M.Z., Zhu S. An eukaryote-bearing microbiota from the early mesoproterozoic Gaoyuzhuang Formation, Tianjin, China and its significance. Precambrian Res. 2017;303:709–726. [Google Scholar]
- 145.Song X.L. Obruchevella from the early cambrian meishaucun stage of the meishucun section, jinning, yunnan, China. Geol. Mag. 1984;121:179–183. [Google Scholar]
- 146.Reitlinger E. Kimbrijskie foraminifery yakutii (cambrian foraminifers of yakutsk), byullelin ‘moskovskogo obs. Ispytateki Prir. Geol. 1948;23:77–81. [Google Scholar]
- 147.Mankiewicz C. Obruchevella and other microfossils in the Burgess Shale: preservation and affinity. J. Paleontol. 1992;66:717–729. [Google Scholar]
- 148.Luchinina V.A. Nauka; Novosibirsk: 1975. Paleoal’gologicheskaya Kharakteristika Rannego Kembriya Sibirskoi Platformy (Paleoalgological Characteristics of the Lower Cambrian of the Siberian Plat Form) p. 100. [Google Scholar]
- 149.Sili C., Torzillo G., Vonshak A. Arthrospira (Spirulina) In: Whitton B.A., editor. Ecol. Cyanobacteria II Their Divers. Sp. Time. Springer; 2012. pp. 677–706. [Google Scholar]
- 150.Giesberger G. Some observations on the culture, physiology and morphology of some brown-red Rhodospirillum-species. Antonie Leeuwenhoek. 1947;13:137–148. [Google Scholar]
- 151.Lakshmi K.V.N.S., Divyasree B., Ramprasad E.V.V., Sasikala C., Ramana C.V. Reclassification of Rhodospirillum photometricum Molisch 1907, Rhodospirillum sulfurexigens Anil Kumar et al. 2008 and Rhodospirillum oryzae Lakshmi et al. 2013 in a new genus, Pararhodospirillum gen. nov., as Pararhodospirillum photometricum comb. nov. Int. J. Syst. Evol. Microbiol. 2013;64:1154–1159. doi: 10.1099/ijs.0.059147-0. [DOI] [PubMed] [Google Scholar]
- 152.Schopf J.W. The paleobiological record of photosynthesis. Photosynth. Res. 2011;107:87–101. doi: 10.1007/s11120-010-9577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Butterfield N.J., Knoll A.H., Swett K. Paleobiology of the neoproterozoic svanbergfjellet formation. Spitsbergen, Foss. Strat. 1994;34:1–84. [Google Scholar]
- 154.V Mendelson C., Schopf J.W. Proterozoic microfossils from the sukhaya tunguska, shorikha, and yudoma formations of the siberian platform, USSR. J. Paleontol. 1982;56:42–83. [Google Scholar]
- 155.Knoll A.H., Strother P.K., Rossi S. Distribution and diagenesis of microfossils from the lower proterozoic duck creek dolomite, Western Australia. Precambrian Res. 1988;38:257–279. doi: 10.1016/0301-9268(88)90005-8. [DOI] [PubMed] [Google Scholar]
- 156.Schulz-Vogt H.N. Beggiatoa. In: Springer D., editor. Encycl. Geobiol. 2011. pp. 111–112. [Google Scholar]
- 157.Permyakov A., Osipova S., Bondarenko N., Obolkina L., Timoshkin O., Boedeker C., Geist B., Schäffner A.R. Proteins homologous to aquaporins of higher plants in the freshwater alga Ulothrix zonata (Ulotrichales, Chlorophyta) Eur. J. Phycol. 2016;51:99–106. [Google Scholar]
- 158.Macdonald F.A., Schmitz M.D., Crowley J.L., Roots C.F., Jones D.S., Maloof A.C., Strauss J.V., Cohen P.A., Johnston D.T., Schrag D.P. Calibrating the cryogenian. Science. 2010;327:1241–1243. doi: 10.1126/science.1183325. [DOI] [PubMed] [Google Scholar]
- 159.Zhang P.Y., Yan X.L. Microfossils from the Gaoyuzhuang Formation in laishui county, hebei, China. Acta Geol. Sin. 1984;58:196–203. [Google Scholar]
- 160.Yakschin M.S. Algal microbiota from the lower riphean deposits of the anabar uplift. Nauk. Novosib. 1991:61. [Google Scholar]
- 161.Oehler J.H. Experimental studies in Precambrian paleontology: structural and chemical changes in blue-green algae during simulated fossilization in synthetic chert. Bull. Geol. Soc. Am. 1976;87:117–129. [Google Scholar]
- 162.Timofeev B.V., Hermann T.N., Mikhailova N.S. Nauk. Leningr.; 1976. Microphytofossils of the Precambrian, Cambrian, and Ordovician. [Google Scholar]
- 163.Hofmann H.J., Jackson G.D. Baffin Island; Canada: 1994. Shale-facies Microfossils from the Proterozoic Bylot Supergroup; pp. 1–34. [Google Scholar]
- 164.Vorob’eva N.G., Sergeev V.N., Petrov P.Y. Kotuikan Formation assemblage: a diverse organic-walled microbiota in the Mesoproterozoic Anabar succession, northern Siberia. Precambrian Res. 2015;256:201–222. [Google Scholar]
- 165.Baludikay B.K., Storme J., François C., Baudet D., Javaux E.J. A diverse and exquisitely preserved organic-walled microfossil assemblage from the Meso – neoproterozoic Mbuji-Mayi Supergroup (Democratic Republic of Congo) and implications for Proterozoic biostratigraphy. Precambrian Res. 2016;281:166–184. [Google Scholar]
- 166.Vorob’eva N.G., Sergeev V.N., Knoll A.H. Neoproterozoic microfossils from the northeastern margin of the East european platform. J. Paleontol. 2009;83:161–196. [Google Scholar]
- 167.Mareš J., Lara Y., Dadáková I., Hauer T., Uher B., Wilmotte A., Kaštovský J. Phylogenetic analysis of cultivation-resistant terrestrial cyanobacteria with massive sheaths (Stigonema spp. and Petalonema alatum, Nostocales, Cyanobacteria) using single-cell and filament sequencing of environmental samples. J. Phycol. 2015;51:288–297. doi: 10.1111/jpy.12273. [DOI] [PubMed] [Google Scholar]
- 168.Javaux E.J., Lepot K., Van Zuilen M.A., Melezhik V.A., V Medvedev P. Palaeoproterozoic microfossils. In: Melezhik V.A., editor. Read. Arch. Earth's Oxyg. Springer; 2013. pp. 1352–1370. [Google Scholar]
- 169.Kah L.C., Knoll A.H. Microbenthic distribution of Proterozoic tidal flats: environmental and taphonomic considerations. Geology. 1996;24:79–82. doi: 10.1130/0091-7613(1996)024<0079:mdoptf>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 170.Klein C., Beukes N.J., Schopf J.W. Filamentous microfossils in the early proterozoic transvaal Supergroup: their morphology, significance, and paleoenvironmental setting. Precambrian. 1987;36:81–94. [Google Scholar]
- 171.Knoll A.H., Swett K., Mark J. Paleobiology of a neoproterozoic tidal flat/lagoonal complex: the draken conglomerate formation. Spitsbergen, J. Paleontol. 1991;65:531–570. doi: 10.1017/s0022336000030663. [DOI] [PubMed] [Google Scholar]
- 172.Lekele Baghekema S.G., Lepot K., Riboulleau A., Fadel A., Trentesaux A., El Albani A. Nanoscale analysis of preservation of ca. 2.1 Ga old Francevillian microfossils. Gabon, Precambrian Res. 2017:1–18. [Google Scholar]
- 173.Sharma M. Small-sized akinetes from the mesoproterozoic salkhan Limestone, semri group, Bihar, India. J. Palaeontol. Soc. India. 2006;51:109–118. [Google Scholar]
- 174.Yun Z. Stromatolitic microbiota from the middle proterozoic wumishan formation (jixian group) of the ming tombs, beijing, China. Precambrian Res. 1985;30:277–302. [Google Scholar]
- 175.Horodyski R.J., Donaldson J.A. Microfossils from the middle proterozoic dismal lakes General geology the Dismal Lakes Group is exposed in a sinuous belt that extends for a distance of more than 300 km from the north shore of Great Bear Lake al- most to the coast of Coronation Gulf. Precambrian Res. 1980;11 [Google Scholar]
- 176.Sergeev V.N., Knoll A.H., Kolosova S.P., Kolosov P.N. Microfossils in cherts from the mesoproterozoic (middle riphean) debengda formation, the olenek uplift, northeastern Siberia. Stratigr. Geol. Correl. 1994;2:19–33. http://europepmc.org/abstract/MED/11539429 [PubMed] [Google Scholar]
- 177.Sergeev V.N. Paleobiology of the neoproterozoic (upper riphean) shorikha and burovaya silicified microbiotas, turukhansk uplift, Siberia. J. Paleontol. 2001;75:427–448. [Google Scholar]
- 178.Golovenok V.K., Belova M.Y. Riphean microbiotas in cherts of the yeniseyskiy kryazh (ridge) Paleontol. Zh. 1985;2:94–103. [Google Scholar]
- 179.Sergeev V.N., Ogurtsova R.N. Microbiota from the lower cambrian phosphatic deposits of the maly karatau (south Kazakhstan) Izv. Akad. Nauk. SSSR - Seriya Geol. 1989;3:58–66. [Google Scholar]
- 180.Muir M.D. Microfossils from the middle precambrian McArthur group, northern territory, Australia. Orig. Life. 1974;5:105–118. [PubMed] [Google Scholar]
- 181.McMenamin D.S., Kumar S., Awramik S.M. Microbial fossils from the kheinjua formation, middle proterozoic semri group (lower vindhyan) son valley area, central India. Precambrian Res. 1983;21:247–271. [Google Scholar]
- 182.Oehler D.Z. Microflora of the middle proterozoic balbirini dolomite (McArthur group) of Australia. Alcheringa. 1978;2:269–309. [Google Scholar]
- 183.Kumar S., Srivastava P. Middle to late proterozoic microbiota from the deoban Limestone, garhwal himalaya, India. Precambrian Res. 1992;56:291–318. [Google Scholar]
- 184.Liu X., Lin Z., Zhang Z., Xu X. A study of late Precambrian microfossil algal community from Jinning County, Jiangshu Province. Acta Micropalaeontol. Sin. 1984;1:171–182. [Google Scholar]
- 185.Sergeev V.N. Geos; Moscow: 2006. Silicified Microfossils from the Precambrian: Nature, Classification, and Biostratigraphic Significance. [Google Scholar]
- 186.Strother P.K., Knoll A.H., Barghoorn E.S. Micro-organisms from the late precambrian narssarssuk formation, north-western Greenland. Palaeontology. 1983;26:1–32. [Google Scholar]
- 187.Anderson R.P., McMahon S., Bold U., Macdonald F.A., Briggs D.E.G. Palaeobiology of the early ediacaran shuurgat formation, zavkhan terrane, south-western Mongolia. J. Syst. Palaeontol. 2017;15:947–968. [Google Scholar]
- 188.Prasad R., Uniyal S.N., Asher Organic-walled microfossils from the proterozoic vindhyan Supergroup of son valley, Madhya Pradesh , India. Palaeobotanist. 2005;54:13–60. [Google Scholar]
- 189.Knoll A.H., Swett K., Burkhardt E. Paleoenvironmental distribution of microfossils and stromatolites in the upper proterozoic backlundtoppen formation, spitsbergen. J. Paleontol. 1989;63:129–145. [PubMed] [Google Scholar]
- 190.Sprinkel D.A., Waanders G. Stratigraphy, organic microfossils, and thermal maturity of the neoproterozoic uinta Mountains group in the eastern uinta Mountains, northeastern Utah. In: C.M D., Pederson J.L., Sprinkel D.A., Kowallis B.J., editors. Uinta Mt. Geol. 2005. pp. 63–73. [Google Scholar]
- 191.Singh V.K., Babu R., Shukla M. Heterolithic prokeryotes from the coated grains bearing carbonate facies of Bhander Group, Madhya Pradesh, India. J. Appl. Biosci. 2011;37:80–90. [Google Scholar]
- 192.Nagovitsin K. Tappania-bearing association of the Siberian platform: biodiversity, stratigraphic position and geochronological constraints. Precambrian Res. 2009;173:137–145. [Google Scholar]
- 193.Samuelsson J., Dawes P.R., Vidal G. Organic-walled microfossils from the proterozoic thule Supergroup, northwest Greenland. Precambrian Res. 1999;96:1–23. [Google Scholar]
- 194.Prasad B., Asher R. Acritarch biostratigraphy and lithostratigraphic classification of proterozoic and lower paleozoic sediments (Pre-unconformity sequence) of ganga basin, India, paleontogr. Indica. 2001;5:151. [Google Scholar]
- 195.Butterfield N.J., Knoll A.H., Swett K. A bangiophyte red alga from the proterozoic of arctic Canada. Science. 1990;250:104–107. doi: 10.1126/science.11538072. [DOI] [PubMed] [Google Scholar]
- 196.Golovenok V.K., Belova M.Y. Microfossils in cherts from the sukhaya tunguska formation, riphean, turukhansk uplift, dokl. Earth Sci. 1992;323:114–118. [Google Scholar]
- 197.Nagovitsin K.E. Silicified microbiotas of the upper riphean of the yenisei ridge: news in paleontology and stratigraphy. Geol .Geof. 2000;41:7–31. [Google Scholar]
- 198.Schopf J.W. Biostratigraphic usefulness of stromatolitic precambrian microbiotas: a preliminary analysis. Precambrian Res. 1977;5:143–173. [Google Scholar]
- 199.Sergeev V.N., Vorob’eva N.G., Petrov P. Yu. The biostratigraphic conundrum of Siberia: do true Tonian–Cryogenian microfossils occur in Mesoproterozoic rocks? Precambrian Res. 2017;299:282–302. [Google Scholar]
- 200.Vorob’eva N.G., Sergeev V.N., Knoll A.H. Neoproterozoic microfossils from the margin of the East European Platform and the search for a biostratigraphic model of lower Ediacaran rocks. Precambrian Res. 2009;173:163–169. [Google Scholar]
- 201.Shih P.M., Wu D., Latifi A., Axen S.D., Fewer D.P., Talla E., Calteau A., Cai F., Tandeau de Marsac N., Rippka R., Herdman M., Sivonen K., Coursin T., Laurent T., Goodwin L., Nolan M., Davenport K.W., Han C.S., Rubin E.M., Eisen J.A., Woyke T., Gugger M., Kerfeld C.A. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110:1053–1058. doi: 10.1073/pnas.1217107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rodríguez-Ezpeleta N., Brinkmann H., Burey S.C., Roure B., Burger G., Löffelhardt W., Bohnert H.J., Philippe H., Lang B.F. Monophyly of primary photosynthetic eukaryotes: green plants, red algae, and glaucophytes. Curr. Biol. 2005;15:1325–1330. doi: 10.1016/j.cub.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 203.Criscuolo A., Gribaldo S. Large-scale phylogenomic analyses indicate a deep origin of primary plastids within cyanobacteria. Mol. Biol. Evol. 2011;28:3019–3032. doi: 10.1093/molbev/msr108. [DOI] [PubMed] [Google Scholar]
- 204.Cornet L., Meunier L., Van Vlierberghe M., Léonard R.R., Durieu B., Lara Y., Misztak A., Sirjacobs D., Javaux E.J., Philippe H., Wilmotte A., Baurain D. Consensus assessment of the contamination level of publicly available cyanobacterial genomes. PLoS One. 2018;13:1–26. doi: 10.1371/journal.pone.0200323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dvořák P., Poulíčková A., Hašler P., Belli M., Casamatta D.A., Papini A. Species concepts and speciation factors in cyanobacteria, with connection to the problems of diversity and classification. Biodivers. Conserv. 2015;24:739–757. [Google Scholar]
- 206.Cornet L., Wilmotte A., Javaux E.J., Baurain D. A constrained SSU-rRNA phylogeny reveals the unsequenced diversity of photosynthetic Cyanobacteria (Oxyphotobacteria) BMC Res. Notes. 2018;11:435. doi: 10.1186/s13104-018-3543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dagan T., Roettger M., Stucken K., Landan G., Koch R., Major P., Gould S.B., Goremykin V.V., Rippka R., De Marsac N.T., Gugger M., Lockhart P.J., Allen J.F., Brune I., Maus I., Pühler A., Martin W.F. Genomes of stigonematalean cyanobacteria (subsection V) and the evolution of oxygenic photosynthesis from prokaryotes to plastids. Genome Biol. Evol. 2013;5:31–44. doi: 10.1093/gbe/evs117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ochoa de Alda J.A.G., Esteban R., Diago M.L., Houmard J. The plastid ancestor originated among one of the major cyanobacterial lineages. Nat. Commun. 2014;5:4937. doi: 10.1038/ncomms5937. [DOI] [PubMed] [Google Scholar]
- 209.Li B., Cox C.J., Lopes J.S., Foster P.G., Embley T.M. Compositional biases among synonymous substitutions cause conflict between gene and protein trees for plastid origins. Mol. Biol. Evol. 2014;31:1697–1709. doi: 10.1093/molbev/msu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sánchez-Baracaldo P. Origin of marine planktonic cyanobacteria. Sci. Rep. 2015;5:14–17. doi: 10.1038/srep17418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Uyeda J.C., Harmon L.J., Blank C.E. A comprehensive study of cyanobacterial morphological and ecological evolutionary dynamics through deep geologic time. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Walter J.M., Coutinho F.H., Dutilh B.E., Swings J., Thompson F.L., Thompson C.C. Ecogenomics and taxonomy of Cyanobacteria phylum. Front. Microbiol. 2017;8:1–18. doi: 10.3389/fmicb.2017.02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Magnabosco C., Moore K.R., Wolfe J.M., Fournier G.P. Dating phototrophic microbial lineages with reticulate gene histories. Geobiology. 2018;16:179–189. doi: 10.1111/gbi.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Philippe H., Brinkmann H., Lavrov D.V., Littlewood D.T.J., Manuel M., Wörheide G., Baurain D. Resolving difficult phylogenetic questions: why more sequences are not enough. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Philippe H., de Vienne D.M., Ranwez V., Roure B., Baurain D., Delsuc F. Pitfalls in supermatrix phylogenomics. Eur. J. Taxon. No 283 Pitfalls Supermatrix Phylogenomics. 2017;283:1–25. [Google Scholar]
- 216.Simion P., Philippe H., Baurain D., Jager M., Richter D.J., Di Franco A., Roure B., Satoh N., Queinnec E., Ereskovsky A. A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr. Biol. 2017;27:958–967. doi: 10.1016/j.cub.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 217.Irisarri I., Baurain D., Brinkmann H., Delsuc F., Sire J.-Y., Kupfer A., Petersen J., Jarek M., Meyer A., Vences M. Phylotranscriptomic consolidation of the jawed vertebrate timetree. Nat. Ecol. Evol. 2017;1:1370–1378. doi: 10.1038/s41559-017-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sánchez-Baracaldo P., Ridgwell A., Raven J.A. A neoproterozoic transition in the marine nitrogen cycle. Curr. Biol. 2014;24:652–657. doi: 10.1016/j.cub.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 219.Shih P.M., Hemp J., Ward L.M., Matzke N.J., Fischer W.W. Crown group Oxyphotobacteria postdate the rise of oxygen. Geobiology. 2017;15:19–29. doi: 10.1111/gbi.12200. [DOI] [PubMed] [Google Scholar]
- 220.Shih P.M., Matzke N.J. Primary endosymbiosis events date to the later Proterozoic with cross-calibrated phylogenetic dating of duplicated ATPase proteins. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110:12355–12360. doi: 10.1073/pnas.1305813110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gavryushkina A., Heath T.A., Ksepka D.T., Stadler T., Welch D., Drummond A.J. Bayesian total-evidence dating reveals the recent crown radiation of penguins. Syst. Biol. 2017;66:57–73. doi: 10.1093/sysbio/syw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ronquist F., Lartillot N., Phillips M.J. Closing the gap between rocks and clocks using total-evidence dating. Phil. Trans. R. Soc. B. 2016;371:20150136. doi: 10.1098/rstb.2015.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Seo P.-S., Yokota A. The phylogenetic relationships of cyanobacteria inferred from 16S rRNA, gyrB, rpoC1 and rpoD1 gene sequences. J. Gen. Appl. Microbiol. 2003;49:191–203. doi: 10.2323/jgam.49.191. [DOI] [PubMed] [Google Scholar]
- 224.Hoffmann L., Komárek J., Kaštovský J. System of cyanoprokaryotes (cyanobacteria) – state in 2004. Arch. Hydrobiol. Suppl. Algol. Stud. 2005;117:95–115. [Google Scholar]
- 225.Gupta R.S., Mathews D.W. Signature proteins for the major clades of Cyanobacteria. BMC Evol. Biol. 2010;10:1–20. doi: 10.1186/1471-2148-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Blank C.E., Sanchez-Baracaldo P. Timing of morphological and ecological innovations in the cyanobacteria – a key to understanding the rise in atmospheric oxygen. Geobiology. 2010;8:1–23. doi: 10.1111/j.1472-4669.2009.00220.x. [DOI] [PubMed] [Google Scholar]
- 227.Schirrmeister B.E., de Vos J.M., Antonelli A., Bagheri H.C. Evolution of multicellularity coincided with increased diversification of cyanobacteria and the Great Oxidation Event. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110:1791–1796. doi: 10.1073/pnas.1209927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schirrmeister B.E., Gugger M., Donoghue P.C.J. Cyanobacteria and the Great oxidation event: evidence from genes and fossils. Palaeontology. 2015;58:769–785. doi: 10.1111/pala.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Battistuzzi F.U., Feijao A., Hedges S.B. A genomic timescale of prokaryote evolution: insights into the origin of methanogenesis, phototrophy, and the colonization of land. BMC Evol. Biol. 2004;4:1–14. doi: 10.1186/1471-2148-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Di Rienzi S.C., Sharon I., Wrighton K.C., Koren O., Hug L.A., Thomas B.C., Goodrich J.K., Bell J.T., Spector T.D., Banfield J.F., Ley R.E. The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to Cyanobacteria. Elife. 2013;2013:1–25. doi: 10.7554/eLife.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Soo R.M., Hemp J., Parks D.H., Fischer W.W., Hugenholtz P. On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria. Science. 2017;355(80):1436–1440. doi: 10.1126/science.aal3794. [DOI] [PubMed] [Google Scholar]
- 232.Martin W.F., Bryant D.A., Beatty J.T. A physiological perspective on the origin and evolution of photosynthesis. FEMS Microbiol. Rev. 2018;42:205–231. doi: 10.1093/femsre/fux056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Olson J.M. The evolution of photosynthesis. Science. 1970;168:438. doi: 10.1126/science.168.3930.438. http://science.sciencemag.org/content/168/3930/438.abstract LP-446. [DOI] [PubMed] [Google Scholar]
- 234.Olson J.M., Pierson B.K. Origin and evolution of photosynthetic reaction centers. Orig. Life Evol. Biosph. 1987;17:419–430. [Google Scholar]
- 235.Allen J.F., Martin W. Out of thin air. Nature. 2007;445:610–612. doi: 10.1038/445610a. [DOI] [PubMed] [Google Scholar]
- 236.Mulkidjanian A.Y., Koonin E.V., Makarova K.S., Mekhedov S.L., Sorokin A., Wolf Y.I., Dufresne A., Partensky F., Burd H., Kaznadzey D., Haselkorn R., Galperin M.Y. The cyanobacterial genome core and the origin of photosynthesis. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103:13126–13131. doi: 10.1073/pnas.0605709103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cohen Y., Jorgensen B.B., Revsbech N.P., Poplawski R. Adaptation to hydrogen sulfide of oxygenic and anoxygenic photosynthesis among cyanobacteria. Appl. Environ. Microbiol. 1986;51:398–407. doi: 10.1128/aem.51.2.398-407.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Klatt J.M., de Beer D., Häusler S., Polerecky L. Cyanobacteria in sulfidic spring microbial mats can perform oxygenic and anoxygenic photosynthesis simultaneously during an entire diurnal period. Front. Microbiol. 2016;7:1–10. doi: 10.3389/fmicb.2016.01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Blankenship R.E. Origin and early evolution of photosynthesis. Photosynth. Res. 1992;33:91–111. [PubMed] [Google Scholar]
- 240.Mathis P. Compared structure of plant and bacterial photosynthetic reaction centers. Evolutionary implications. Biochim. Biophys. Acta Bioenerg. 1990;1018:163–167. [Google Scholar]
- 241.Brinkmann H., Göker M., Koblížek M., Wagner-Döbler I., Petersen J. Horizontal operon transfer, plasmids, and the evolution of photosynthesis in Rhodobacteraceae. ISME J. 2018;12:1994–2010. doi: 10.1038/s41396-018-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shestakov S.V., Karbysheva E.A. The origin and evolution of cyanobacteria. Biol. Bull. Rev. 2017;7:259–272. [Google Scholar]
- 243.Fischer W.W., Hemp J., Johnson J.E. Manganese and the evolution of photosynthesis. Orig. Life Evol. Biosph. 2015;45:351–357. doi: 10.1007/s11084-015-9442-5. [DOI] [PubMed] [Google Scholar]
- 244.Cardona T. Early archean origin of heterodimeric photosystem I. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.V Lalonde S., Konhauser K.O. Benthic perspective on Earth's oldest evidence for oxygenic photosynthesis. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112:995–1000. doi: 10.1073/pnas.1415718112. http://www.pnas.org/content/112/4/995.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Arnold G.L., Anbar A.D., Barling J., Lyons T.W. Molybdenum isotope evidence for widespread anoxia in mid-proterozoic oceans. Science. 2004;304:87–90. doi: 10.1126/science.1091785. [DOI] [PubMed] [Google Scholar]
- 247.Anbar A.D., Knoll A.H. proterozoic ocean chemistry and evolution: a bioinorganic bridge? Science. 2002;297:1137–1142. doi: 10.1126/science.1069651. [DOI] [PubMed] [Google Scholar]
- 248.Johnston D.T., Wolfe-Simon F., Pearson A., Knoll A.H. Anoxygenic photosynthesis modulated Proterozoic oxygen and sustained Earth's middle age. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:16925–16929. doi: 10.1073/pnas.0909248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Archibald J.M. The puzzle of plastid evolution. Curr. Biol. 2009;19:R81–R88. doi: 10.1016/j.cub.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 250.Cenci U., Bhattacharya D., Weber A.P.M., Colleoni C., Subtil A., Ball S.G. Biotic host–pathogen interactions as major drivers of plastid endosymbiosis. Trends Plant Sci. 2017;22:316–328. doi: 10.1016/j.tplants.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 251.Deschamps P., Plancke C., Colleoni C., D'Hulst C., Dauvillée D., Raj J.N., Ball S., Suzuki E., Nakamura Y., Putaux J.-L., Buléon A., Haebel S., Ritte G., Steup M., Falcón L.I., Moreira D., Löffelhardt W. Metabolic symbiosis and the birth of the plant kingdom. Mol. Biol. Evol. 2007;25:536–548. doi: 10.1093/molbev/msm280. [DOI] [PubMed] [Google Scholar]
- 252.Ragon M., Benzerara K., Moreira D., Tavera R., López-García P. 16S rDNA-based analysis reveals cosmopolitan occurrence but limited diversity of two cyanobacterial lineages with contrasted patterns of intracellular carbonate mineralization. Front. Microbiol. 2014;5:1–11. doi: 10.3389/fmicb.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gibson T.M., Shih P.M., Cumming V.M., Fischer W.W., Crockford P.W., Hodgskiss M.S.W., Wörndle S., Creaser R.A., Rainbird R.H., Skulski T.M., Halverson G.P. Precise age of Bangiomorpha pubescens dates the origin of eukaryotic photosynthesis. Geology. 2018;46:135–138. [Google Scholar]
- 254.Bengtson S., Sallstedt T., Belivanova V., Whitehouse M. Three-dimensional preservation of cellular and subcellular structures suggests 1.6 billion-year-old crown-group red algae. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Arouri K.R., Greenwood P.F., Walter M.R. Biological affinities of Neoproterozoic acritarchs from Australia: microscopic and chemical characterisation. Org. Geochem. 2000;31:75–89. [Google Scholar]
- 256.Loron C.C., Rainbird R.H., Turner E.C., Greenman J.W., Javaux E.J. Organic-walled microfossils from the late mesoproterozoic to early neoproterozoic lower shaler Supergroup (arctic Canada): diversity and biostratigraphic significance. Precambrian Res. 2019;321:349–374. [Google Scholar]
- 257.Agiæ H., Moczyd³owska M., Yin L.M. Affinity, life cycle, and intracellular complexity of organic-walled microfossils from the Mesoproterozoic of Shanxi, China. J. Paleontol. 2015;89:28–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.