Abstract
Mitochondria have emerged as a central factor in the pathogenesis and progression of heart failure (HF), as well as other cardiovascular diseases (CVD), but no therapies are available to treat mitochondrial dysfunction. The National Heart, Lung, and Blood Institute (NHLBI) convened a group of leading experts in HF, CVD, and mitochondria research in August 2018. These experts reviewed the current state of science and identified key gaps and opportunities in basic, translational and clinical research focusing on the potential of mitochondria-based therapeutic strategies in HF. The workshop provided short- and long-term recommendations for moving the field toward clinical strategies for the prevention and treatment of HF and CVD using mitochondria-based approaches.
Keywords: heart failure, mitochondria, cardiovascular diseases
Introduction
Cardiovascular disease (CVD) remains the number one killer in developed countries. Increases in life expectancy and improved treatment strategies for ischemic heart disease and myocardial infarction have led to a steady rise in HF prevalence. Despite the use of guideline-directed therapies, the morbidity and mortality of HF remain unacceptably high. There have been few new therapies for HF with reduced ejection fraction (HFrEF) in the last 20 years, and no compelling new therapies for HF with preserved ejection fraction (HFpEF). Novel approaches, orthogonal to traditional neurohormonal blockade, are thus greatly needed. Mitochondrial dysfunction and energy deficiency have been strongly implicated in the development of HF.1-3 Targeting mitochondrial dysfunction in HF may provide novel approaches that are both hemodynamically favorable and complementary to current, somewhat limited approaches. To date, however, mitochondrial-targeted therapies have not succeeded in impacting this disease process. Phase III trials targeting the mitochondrial permeability transition pore (mPTP) with cyclosporine, for example, were disappointingly negative despite encouraging phase I/II results, as were large trials with antioxidants like vitamin E.4, 5 A better understanding of mitochondria and their role in the pathobiology of HF, in conjunction with better tools for the delivery of mitochondria-targeted therapies and the monitoring of mitochondrial function in humans, are needed to translate this innovative treatment strategy.
Since the previous NHLBI mitochondria-focused workshop in 2007 entitled, “Modeling Mitochondrial Dysfunction in Cardiovascular Disease,”6 major advances have been made and substantial molecular information critical to our understanding has rapidly accumulated, bringing this classic discipline back to center stage. It is now recognized that mitochondria, traditionally viewed as the powerhouse of the cell, sense and respond to changes and stresses in the cellular environment and that they control critical cellular decision points. The recent workshop “Unlocking the Secrets of Mitochondria: Path to a Cure in Heart Failure,”7 held by the NHLBI on August 6-7, 2018 discussed major advances in mitochondrial science and identified key knowledge gaps in translating these advances to mitochondria-based therapies for HF. Here we report on the challenges and recommendations for five priority areas.
I. Multiplicity of Mitochondrial Functions in CVD and HF.
The heart demands a substantial amount of energy relative to other organs. Mitochondria occupy approximately one third of the volume of adult cardiomyocytes.8 Oxidative metabolism in mitochondria provides the majority of energy consumed by the heart, and inability to generate and transfer energy has long been considered a key mechanism of contractile failure.9, 10 It is increasingly recognized, however, that mitochondrial function extends far beyond that of a power plant and includes important biological and regulatory roles such as redox balance, biosynthesis, reactive oxygen species (ROS) signaling, cell growth and death, ion homeostasis, protein quality control and inflammation.3, 11-16 A picture has begun to emerge that the pathogenic role of mitochondria in HF and CVD not only involves decreased ATP production, but also a general maladaptation in the spectrum of its functions (Figure 1). These observations have opened the door for a large variety of new targets for mitochondria-based therapies.17, 18
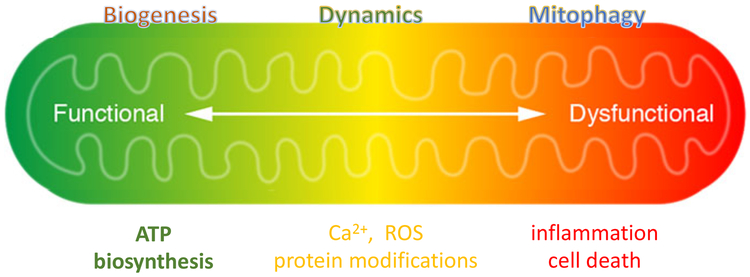
Figure 1. Multiplicity of mitochondrial function.
Mitochondria are known as the powerhouse of the cell. In addition to generating ATP, intermediate metabolism in the mitochondria produces metabolites for biosynthesis, protein modification, and signal transduction. Oxidative phosphorylation regulates NAD(H) redox state and is coupled with the generation of reactive oxygen species (ROS), both can modulate and/or trigger post-translational modifications. Mitochondrial metabolism is stimulated by Ca2+ - lower Ca2+ level impairs mitochondrial activity while calcium overload can trigger the opening of the mitochondrial permeability transition pore (mPTP). The release of mitochondrial contents, such as cytochrome C, induces apoptosis, or the loss of membrane potential (a consequence of prolonged mPTP opening) causes ATP deprivation and necrosis. Leakage of damage-associated molecular patterns (DAMPs), such as mitochondrial DNA and peptides, or excessive ROS generation can cause inflammation that results in further tissue damage. Mitochondrial function is also regulated by biogenesis, fission, and fusion dynamics, and protein quality control via mitophagy. The transition of mitochondria from a powerhouse to a death engine involves a shift of the entire spectrum of functions.
Research Gaps and Opportunities.
Despite striking observations in preclinical studies, in large part involving bioengineered mouse models, the relative contribution of each unique biological function of mitochondria to the development of HF remains unclear. Moreover, little is known about the integration of mitochondrial bioenergetics with each role. Identification of novel therapeutic targets relies on further elucidation of mechanisms that link processes involved in oxidative metabolism (e.g., fuel selection, energy production / transfer, and ROS generation / scavenging) with the numerous other functions of mitochondria. For example, decades of research have revealed that impaired myocardial energetics are accompanied by defects in substrate utilization, Krebs cycle flux and oxidative phosphorylation.1, 19-24 Effective therapies for improving energy supply of the failing heart are lacking.25-28 In addition, whether interventions targeting intermediary metabolism will be sufficient to overcome mitochondrial dysfunction and improve the outcome of HF is also an important unresolved question. Furthermore, little attention has been given to the non-energetic roles of mitochondria in the failing heart, which likely play a critical role in pathological remodeling through proteomic and epigenomic modifications.29, 30
Preclinical studies demonstrate that mitochondrial Ca2+ is a key regulator of energy metabolism and also a trigger of mitochondria-induced cell death via activating the mPTP.31 There is a paucity of information regarding the state of mitochondrial Ca2+ dynamics in human HF, highlighting an important knowledge gap. It remains controversial whether mitochondria in the failing heart are Ca2+ starved or overloaded.14, 32, 33 From the therapeutic perspective, any extreme increase or decrease in mitochondrial Ca2+ is likely to lead to negative outcomes, while more subtle interventions might be beneficial in the setting of HF or during ischemia-reperfusion injury. To address these gaps, we need to develop strategies that modulate mitochondrial Ca2+ levels within the physiological range. Similarly, it is also unclear whether altered rates of mitophagy in HF are sufficient to maintain the balance between biogenesis and degradation and what form of mitophagy may be dysfunctional. Subtle interventions to alter these processes may be necessary to fine-tune mitochondrial quality to optimize function in HF. Our incomplete understanding of why the metabolic gene program is altered in HF and how to safely activate cardiac mitochondrial biogenesis to reverse defects in oxidative capacity is a significant barrier to therapeutic targeting of mitochondria at the present time.
Although mitochondrial dysfunction appears integral to HFrEF, the role of mitochondrial function in HFpEF is poorly defined. The lack of effectiveness of proven HFrEF treatments to improve outcomes in HFpEF might likely indicate that the mechanisms involved in these distinct forms of HF differ significantly. Notably, the HFpEF population shares a number of characteristics with patient populations known to have impaired mitochondrial function, e.g. older age and obesity. Thus, deciphering mitochondrial mechanisms in HFpEF is highly warranted.
The knowledge gaps identified at the workshop provide an excellent road map for future work, especially translational research on mitochondria-focused HF therapy. Research in the past decade has identified not only multiple pathological mechanisms, but also a significant number of potential therapeutic targets. Moving these targets into therapy will require the collaborative efforts of biologists, engineers and clinicians that i) translate disease mechanisms to druggable targets; ii) devise effective strategies to engage the targets; and iii) develop monitoring systems to follow the biological outcome (see New Tools and Translation to Patients sections). We expect that the effort will yield promising leads for clinical testing. Recognizing the multitude of mitochondrial mechanisms in HF should drive the focus of future investigations towards a balance of critical regulators of mitochondrial function, such as Ca2+, ROS, and redox state. We will continue to build on the concept that mitochondrial dysfunction in HF represents a spectrum shift rather than the loss of a single function (Figure 1). Future work will strive to restore homeostasis rather than manipulate individual functions. New discovery in mitochondrial biology lies in the integration of mitochondrial bioenergetics with its role in regulating cell fate.
II. Intra- and Inter-cellular Communication
It has become increasingly clear that mitochondria do not work in isolation. Communication within a mitochondrion, between mitochondria, and between mitochondria and other cellular organelles, (e.g., the nucleus and sarco-endoplasmic reticulum), as well as crosstalk of mitochondria across different cells or tissues, are all increasingly recognized as important responses to environmental stressors. A number of studies have shown that mitochondrial metabolites, such as thioester (acyl-CoA) and ROS, can directly modify the mitochondrial proteome, thus rapidly modulating mitochondrial activity in response to environmental changes.34, 35 Such regulatory circuits present potential opportunities for intervention as they couple mitochondrial metabolism with its sensitivity to stress.11 Furthermore, communication between mitochondria and the nucleus via epigenetic modification is another powerful mode of determining cell fate. Defective oxidative phosphorylation significantly impacts both acetylation and methylation processes by altering acetyl CoA metabolism and the methionine cycle, respectively; changes that can be reversed by restoration of NADH redox state.36 Moreover, alpha-ketoglutarate, a Krebs cycle intermediate, plays a specific role in cytosine demethylation reactions as a cofactor for the Tet family of dioxygenases in the nucleus.37 In addition to the possibility that mitochondrial metabolism can alter the availability of epigenetic enzyme intermediates, emerging evidence indicates that mitochondrial genotype itself might influence nuclear DNA cytosine methylation patterns, as demonstrated in mitochondrial nuclear exchange (MNX) experiments.38 MNX mice display differential DNA methylation patterns and gene expression between strains having identical nDNA but different mtDNAs.39 Interestingly, this non-Mendelian control over gene expression was found to influence whole body metabolism and susceptibility to adiposity upon high fat feeding,40 as well as the susceptibility to cardiac volume overload.41 In humans, mtDNA mutations have been proposed to contribute to bioenergetic adaptation to dietary changes and are hypothesized to modulate disease susceptibility.42-44 This concept suggests that the natural variation of mtDNA background combined with co-evolution of its nucleus, significantly impacts the cellular response to stimuli and contributes to individual variability in predisposition to cardiometabolic disease. Different mtDNA-nDNA combinations can impact the susceptibility to HF, 41, 45 and it is possible that the underlying mitochondrial genomic response to meet environmental challenges may, in a retrograde fashion, regulate nuclear gene responses that further modulate disease susceptibility.
Research Gaps and Opportunities.
The nature of mitochondria-originating signals predicts a network of changes, which poses a challenge for identification of specific mechanisms and/or targets. While several emerging signaling mediators, such as mitochondria-derived metabolites, peptides, mtDNA, and ROS have been identified, little is known about their specific targets and functions in health and disease. There is no information on the mitochondrial metabolome in living cells. Direct evidence of how metabolites produced in mitochondria alter biochemical reactions in the nucleus remains elusive. In addition, we lack knowledge of site-specific quantitation of occupancy for each post-translational modification (PTM) of mitochondrial and/or nuclear proteins. The field is still perplexed by the association between a robust biological effect and modifications of a relatively small fraction of proteins. Since a variety of molecules can modify the same amino acid residue, the additive and even synergistic (or antagonistic) effects among protein populations bearing different modifications on the same site should be considered in its totality. Likewise, the role of PTMs of mitochondrial proteins and their effects on protein-protein interactions is poorly understood.
In order to comprehend more fully how mtDNA influence the susceptibility of patients to cardiovascular diseases and HF, we need to gain a thorough understanding of mtDNA polymorphisms and mutations in the general population. Tissue differences in mitochondrial variants and their impact on the pathogenesis and progression of HF have not been addressed. Animal studies focusing on the causal relationship between mitochondria-nuclear genome interactions are currently lacking. Evidence derived from large sample sizes is needed to determine whether mitochondrial genotype can be used for stratification and treatment of HF patients.
The workshop recognized that further understanding of how mitochondria control nuclear functions through epigenetics, damage-sensing pathways or simply by their maternal origin will substantially advance the field. The global impact of the vast number of changes that can occur through these mechanisms appears to be a problem to unravel by single pathway analysis, but can be tackled more readily through a systems approach. With emerging tools in hand or in development that integrate multi-omic analysis with deep mitochondrial phenotyping, we have an enormous opportunity for discovery and innovation. Future studies will resolve the mechanism by which proteomes and metabolomes translate biological signals into functional outputs (Figure 2). For example, we will define how specific metabolites are altered by various stresses, which metabolites are secreted from the mitochondria and to the extracellular space, how the rate of secretion is modulated by mitochondrial activity, and how these metabolites signal other cells. We will determine the mechanisms by which PTMs alter mitochondrial phenotype via networks and interactomes. Future therapeutics will be developed to target mechanisms for retrograde signaling to the nucleus or retrograde-anterograde crosstalk including novel pathways for modulating immunological responses, protein folding, endoplasmic reticular stress responses, and protein degradation processes.
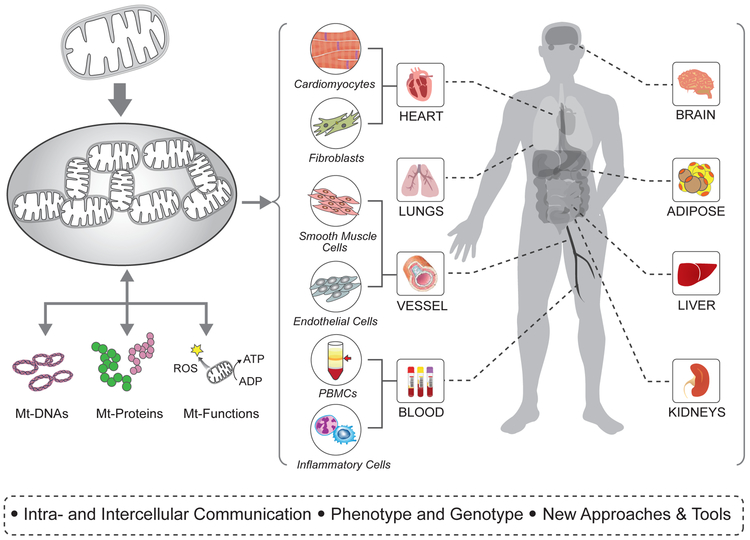
Figure 2: Targeting Mitochondrial Functions in Heart, Lung, and Blood (HLB) Health and Disease.
Mitochondria in multiple cell and organ types contribute to the pathogenesis of heart failure or its risk factors. A better understanding of basic mitochondrial biology including the multiplicity of functions played by mitochondria, their role in intra- and inter-cellular communication, and the relationship between mitochondrial genotype and phenotype is a high priority in developing mitochondria-based therapy. Other priority areas emphasized the need for improved tools and/or the development of new approaches for the study of mitochondrial function in humans and the development of strategies for the translation of basic observations to the clinic.
III. Phenotype and Genotype
The role of mitochondrial phenotype and genotype in determining the response to environmental stresses and the propensity for the development of HF is poorly understood. It is increasingly clear that mitochondrial function is heterogeneous across cell and tissue types, and from person-to-person. Mitochondrial genotype and phenotype are influenced by intrinsic factors such as genetics, race, age and sex. Such heterogeneity may influence the response to environmental factors such as diet, and contribute to the pathobiology of common risk factors for HF such as hypertension, diabetes, obesity, inflammation, and aging.
Pre-existent natural genetic variation is a likely basis for differential risk among individuals. In this respect, mtDNA mutations have been proposed as the basis for bioenergetic adaptation to changes in diet and climate in prehistoric times, and today are hypothesized to contribute to cardiometabolic disease susceptibility.42-44 Natural variation of mtDNA background combined with the co-evolution of the nDNA may impact the cellular response to environmental stimuli, and can account for the complexity of an individual’s predisposition to metabolic disease.41 Thus, different mtDNA – nDNA combinations can impact susceptibility to HF. The concept of mitochondrial - nuclear genetic interaction, or “mito-Mendelian” genetics may provide a means of central control for cellular function. Aging and sex are intrinsic determinants that may affect the mitochondrial phenotype. Aging, a major risk factor for HF, is associated with degradation of nuclear and mitochondrial genetic integrity due to telomere shortening, a process that is opposed by telomerase reverse transcriptase (TERT). Mitochondrial TERT is associated with beneficial and protective effects including improved metabolism, reduced ROS and increased mtDNA integrity.46 Sex differences have been noted with regard to mitochondria in the heart,47, 48 but the functional consequences are not clear. Estrogen and androgen receptors have been localized to mitochondria in various cell types, and a number of sex differences have been described with regard to mitochondrial efficiency, ROS production, antioxidant capacity, substrate preference, and Ca2+ handling in the heart. Studies have also noted key sex-dependent differences in the expression and PTM of key mitochondrial proteins.
Mitochondrial dysfunction may play a role in the pathobiology of important risk factors for HF including hypertension, diabetes, obesity, inflammation, and aging (Figure 2). Mitochondria are a major source of ROS, such as superoxide and hydrogen peroxide that lead to target organ damage, dysfunction, hypertrophy, and inflammation. Hypertension is associated with depletion and inactivation of the key mitochondrial deacetylase, sirtuin 3 (Sirt3), which is involved in the regulation of key metabolic steps. Depletion of Sirt3 promotes development of hypertension and cardiac fibrosis. Diabetes and obesity promote a shift in mitochondrial phenotype in favor of fatty acid (FA) oxidation and attenuation of glucose oxidation. Some of the earliest consequences of enhanced FA flux in the diabetic heart are increased mitochondrial uncoupling and generation of ROS, which result in decreased ATP synthesis despite the high energy demand. Initially adaptive mitochondrial responses may become maladaptive with depressed oxidative phosphorylation activity, reduced protein expression and/or function of electron transport chain (ETC) complexes, and accumulation of damaged mitochondria. Cardiac magnetic resonance spectroscopy has shown that stores of high energy creatine phosphate are decreased in association with oxidative modifications in ETC proteins.49, 50 The mitochondrial phenotype in type 2 diabetes / obesity appears to differ from that in models of HFrEF, suggesting that diabetes and obesity may interact with other risk factors for HF. Inflammation is a common feature associated with hypertension, diabetes, obesity, and other risk factors for HF. Although the levels of inflammatory cytokines are elevated in HF and correlate with its severity and prognosis, the source(s) of circulating cytokines have not been identified. As with other solid organs, however, the heart possesses a modest population of resident macrophages and dendritic cells that provide an immune surveillance function to maintain cellular homeostasis. The ancestral prokaryotic features of mitochondria retain bacterial signatures, which when released following mitochondrial stress, may function as damage-associated molecular patterns (DAMPS) to activate innate immunity that can amplify subsequent inflammation.51 Cardiomyocytes and skeletal muscle cells may thus function as non-professional immune cells which respond to perturbed mitochondrial quality control by initiating intracellular immune surveillance programs to cause inflammation, thereby promoting cardiac and skeletal muscle dysfunction.
Research Gaps and Opportunities
Mitochondrial phenotyping and genotyping in humans and animal models of HF, in combination with large nuclear gene-sequence databases, are needed to address several questions: How does mitochondrial phenotype differ across various etiologies of HF? How do underlying nuclear and mitochondrial genotype, sex, and age affect mitochondrial phenotype and contribute to increased or decreased susceptibility to HF and/or HF risk factors such as hypertension, diabetes, obesity, aging, and inflammation? How do changes in mitochondrial bioenergetics mediated by functional mutations in the mtDNA and/or nuclear DNA modulate cellular pathways and functions? How do the resulting changes in oxidant signaling and/or metabolite flux (e.g., citric acid cycle) shape the host cell response to changes in the cellular micro-environment? To address these and related questions, it will be important to develop a nuclear - mitochondrial genomic “fingerprint” in humans for comparing longitudinal data sets within cohorts. A long-term challenge will be identification of the mechanisms by which different mtDNA – nDNA genetic variations alter metabolic systems that affect disease susceptibility. Likewise, it will be important to determine how sex and age affect mitochondrial phenotype and genotype. What is the role of age-related telomere shortening in increasing the incidence of HF with age, and does TERT activation represent a potentially useful strategy for reducing the adverse consequences of cardiovascular aging? How do sex-related differences in mitochondrial biology and function contribute to HF? Is there a link between mitochondrial dysfunction and inflammation in the pathophysiology of HF? The answers to these and related questions will expand our knowledge of disease mechanisms in HF and identify opportunities for precision medicine in its prevention and treatment.
IV. New Tools
Like in other disciplines, advances in the cutting-edge technologies of genomics, proteomics, transcriptomics, metabolomics, and epigenomics have been driving ground-breaking discoveries in the field of mitochondrial biology. Our understanding of mitochondrial physiology is further enhanced by the availability of compartment specific sensors and the ability to assess mitochondrial respiration in intact cells. Despite increases in our knowledge, we lack the ability to discern and deeply phenotype mitochondria with respect to cardiac diseases. Importantly, enabling tools and technologies for translating basic discoveries to clinical practice are urgently needed.
Research Gaps and Opportunities.
First, we lack rigorous, quantitative information on genome, proteome, transcriptome, metabolome, interactome, and epigenome dynamics in different cells underlying cardiac phenotypes [both model systems and clinical cohorts, e.g., Trans-Omics for Precision Medicine (TOPMed)]. Specific examples of critically important gaps include: (i.) understanding mtDNA sequences in renewable cell lines (e.g., lymphoblastoid cell lines) as they relate to whole blood-derived DNA in the same individuals; how they are related to mitochondrial function and disease phenotypes; (ii.) elucidating a comprehensive map of proteome PTMs; (iii.) resolving the mechanism by which mitochondria communicate and signal to other compartments; and (iv.) obtaining quantitative structural information defining the interface/crosstalk between protein-protein interactomes. Second, we lack computational tools and platforms for deep phenotyping, which include extracting information from datasets and discerning causality versus correlation. We need new machine learning-based strategies for data analysis that are capable of embracing the inherent complexities and volume of mitochondrial datasets. Third, we lack well-organized datasets and we are missing a well-curated mitochondrial molecular atlas integrating various omics, functional and clinical datasets (e.g., mitochondrial knowledge graph). Existing datasets and tools are helpful but limited, and the relational organization of diverse datasets is obscure. As they currently stand, they are inadequate to support clinical translation and application of mitochondrial knowledge. Fourth, model systems are missing for integrated in vivo studies, such as large animal models, reporter mice, and models for investigating sex differences. These tools are critical to bridging the test tube and/or computational results to clinical testing. Fifth, we need tools to assess mitochondrial function in patients with CVD. As tissue sampling for mitochondrial analysis is at present impractical and largely impossible for HF, the current translational effort is seriously limited by the lack of surrogate readouts of tissue mitochondrial integrity or biomarkers of mitochondrial dysfunction. Lastly, only very limited tools/reagents are available to target mitochondria in the in vivo setting. Vehicles for delivering mitochondria-targeted cargo or methods to manipulate mtDNA remain a serious challenge in translational research.
These challenges set the stage for several exciting opportunities in mitochondrial research, illuminating the many ways that tools and technology may help to advance cardiovascular biology and medicine. Key action items were identified in the workshop in order to ultimately realize these opportunities. For the first time, we possess the tools to conduct deep clinical phenotyping by obtaining comprehensive, quantitative omics information for relevant phenotype via NHLBI’s TOPMed program. This will provide unparalleled characterization of genomic, proteomic, transcriptomic, metabolomic, and epigenetic information and will facilitate interactomic analysis. Further development of these tools and their capacities to resolve the different omic dimensions will enable the identification of statistically-significant and biologically-relevant changes in protein-protein, protein-metabolite and metabolite-metabolite interactions to reveal molecular-level details underpinning mitochondrial dysfunction.52-55 We can create model systems in which we systematically link mtDNA sequence information to functional phenotypes in order to understand the impact of genetic variations. Further investigations of mtDNA homoplasmic and heteroplasmic mutations56 in different cell types will provide an important foundation for understanding the contributions of the mitochondrial genome to disease. Using powerful artificial intelligence and machine learning-based computational platforms a large volume of information can be integrated into a relational molecular atlas through construction of a mitochondrial knowledge graph (MKG), providing a live demonstration of how omics/functional/clinical datasets can facilitate our understanding of HF pathogenesis.57, 58
The translation of mitochondria-based therapies will require new and improved methods to assess and modify mitochondrial function. The development and sharing of animal models that report mitochondrial function in an integrated setting will accelerate the translational effort. The development of biomarkers and imaging modalities that allow non-invasive and longitudinal assessment of mitochondrial function in patients is critical for moving discoveries to clinical testing and care. Biomarkers will also be used to identify patient subsets with different mitochondrial deficits that are most likely to respond to specific forms of therapy, and to track target engagement with such interventions. In accordance with the diverse examples provided above, these tools require integration of analytical, biochemical, molecular, genetic, bioinformatics, and machine learning approaches that measure and decode mitochondrial properties in vivo and in vitro. Innovations will advance research and therapeutic targeting of mitochondria.
V. Translation to Patients
Mitochondrial dysfunction has been implicated in the pathophysiology of HF of multiple etiologies and types (e.g., HFrEF vs. HFpEF). There has been significant progress in understanding the mechanisms of mitochondrial dysfunction and the identification of potential therapeutic targets. The successful translation of mitochondria-targeted therapy to patients with HF, however, remains elusive, with few clinical trials to date. Several promising approaches have been suggested by studies in animal models of HF including mitochondria-targeted antioxidants, interventions to restore NADH redox balance, elamipretide, coenzyme-Q, resveratrol, and a variety of small molecules that may act by preventing mitochondrial sodium overload. While each has had some level of success in preclinical models, very few have advanced to clinical trials in patients with HF. It is noteworthy that all of these potential therapies appear to act in ways that differ from currently used drugs for HF. The antioxidants decrease the effects of excessive ROS, elamipretide may stabilize cardiolipin, NAD+ precursors and resveratrol may augment sirtuin activity, while ranolazine, SGLT2 inhibitors and CGP-37157 may act by correcting sodium / calcium balance in mitochondria.59 Thus, the mechanism of action of mitochondria-targeted therapies, in general, is likely to be orthogonal to current therapies, and thereby has the potential to exert complementary beneficial effects.
One of the few mitochondria-targeted therapies to be tested in humans is coenzyme-Q, a lipid-soluble electron carrier that plays a central role in electron transport and ATP synthesis. Decreased levels of myocardial and plasma coenzyme-Q have been observed in some studies of patients with HF, and meta-analyses have suggested a possible clinical benefit.60, 61 Q-SYMBIO, a randomized trial of coenzyme-Q for 2 years in 420 patients with NYHA class III and IV HFrEF showed promising decreases in morbidity and mortality.62 Elamipretide is a small peptide that targets the mitochondrial inner membrane where association with cardiolipin occurs, and has been shown to lead to bioenergetic improvements in various models of mitochondrial dysfunction. In mice, elamipretide administered by osmotic pump for four weeks caused improvements in several aspects of adverse remodeling and cardiac function in models of HF caused by angiotensin infusion and pressure overload.63, 64 In dogs with embolization-induced HF, elamipretide administered by daily subcutaneous injection for three months led to improvements in myocardial hemodynamics and mitochondrial function.65 Clinical experience with elamipretide in HF has so far been limited to eight patients with HFrEF who received a single four-hour infusion.66 Elamipretide is also being tested in patients with diseases of mitochondrial dysfunction, including Barth syndrome, an ultra-rare genetic condition caused by defective remodeling of mitochondrial cardiolipin that is characterized by dilated cardiomyopathy and skeletal myopathy.
Alterations in NADH and NADPH redox state have been observed in HF. In addition to the consequences for cellular redox regulation, NAD+ is a co-substrate for multiple enzymes, including sirtuin deacetylases, and thereby plays critical roles in PTMs of proteins by lysine that are important to multiple cellular metabolic processes and energy transduction. In murine HF models the administration of NAD+ or the NAD+ precursors nicotinamide mononucleotide (NMN) or nicotinamide riboside (NR) slowed the development of HF.13, 67, 68 NR has entered clinical trials in systolic HF. Resveratrol is a naturally-occurring polyphenol with pleotropic effects including antioxidant properties and activation of sirtuins. Preclinical studies have suggested that resveratrol and synthetic, related flavonoid derivatives exert beneficial effects in a variety of HF models, and at least one randomized clinical trial of resveratrol is under way in patients with HF.
A major issue in the translation of mitochondria-targeted therapies to humans is the difficulty inherent in assessing cardiac mitochondrial function in vivo. Positron emission tomography (PET) and magnetic resonance spectroscopy (MRS) are the currently available noninvasive approaches for studying cardiac metabolism in vivo. The former has been primarily used to quantify substrate uptake and utilization and the latter for measuring cardiac high-energy phosphates (HEP) and turnover with 31P MRS. These techniques have been applied in both animal models of HF and in humans with HF, leading to several important findings including that: a) cardiac substrate utilization is altered in HF, b) cardiac HEP pools and the rate of ATP turnover through the creatine kinase reaction are significantly depressed, c) energetic changes often precede ventricular function and remodeling changes, and d) depressed cardiac energetics independently predict clinical HF outcomes and mortality. An alternative or complementary approach to assessing mitochondrial function in humans is the measurement of bioenergetics/mitochondrial function by respirometry or extracellular flux analysis in human circulating platelets and leukocytes.69-71 Observations in a variety of diseases including type 2 diabetes, pulmonary arterial hypertension and asthma have led to the suggestion that mitochondrial alterations in circulating cells could be utilized as a biomarker of disease. It remains to be determined how and to what extent systemic mitochondrial function relates to mitochondrial function in target organs including the heart, skeletal muscle and vasculature.
Research Gaps and Opportunities.
While preclinical work suggests the potential benefit of mitochondria-targeted therapies in HF, it remains to be established whether improving mitochondrial function will result in improved clinical outcomes in patients. As highlighted in the prior sections, success in translation to patients will be critically dependent on continued progress in understanding the pathophysiologic role of mitochondria in HF and the development of effective mitochondria-targeted therapeutic agents. Clinical translation presents obstacles not faced in preclinical studies. Patients are heterogeneous with regard to a myriad of factors that can affect mitochondrial genotype and phenotype, and thus, the susceptibility to both metabolic challenges and risk factors for HF such as hypertension, diabetes, obesity, aging, and inflammation. In addition to this inherent variability from patient-to-patient, HF is a syndrome with numerous etiologies that have distinct and overlapping pathophysiologic mechanisms. Consequently, there may be important patient- and etiology-specific differences in mitochondrial phenotype that have a bearing on the success of a particular mitochondria-targeted intervention. Accordingly, it will be important that the relationship between HF pathophysiology and mitochondrial phenotype be clarified and considered in the design of clinical trials of mitochondria-targeted agents. This pathophysiologic heterogeneity may be particularly important in regard to patients with HFpEF vs. HFrEF.
Another hurdle is the difficulty in measuring mitochondrial function in humans. PET and MRS are demanding technologies that are not widely available outside of teaching and research institutions. 31P and 13C MRS have limited sensitivity and relatively low spatial resolution, and their demonstrated ability to provide sophisticated measures of cardiac metabolism in isolated hearts has not been fully translated to in vivo studies in humans. Better, more robust, noninvasive means to measure in vivo mitochondrial function and energy metabolism are critically needed to probe the links between impaired mitochondrial metabolism and HF development in patients, to identify subsets of patients most likely to respond to a particular therapy, to assess the effect of therapy on mitochondrial function and to determine the relationship of changes in mitochondrial function to clinical parameters. Biomarkers and assays indicative of mitochondrial function in circulating cells could provide useful guidance in patient selection and assessment of therapeutic responses. Further studies are needed to determine the relations between such biomarkers and mitochondrial function in target organs such as heart and skeletal muscle. Finally, as opportunities for clinical trials increase, it will be important to develop a network-based approach that facilitates the use of standardized protocols, measurements and strategies, including stratification on key factors such as disease etiology, age, race, and sex.
Summary and Recommendations
The two-day workshop identified five priority areas for research to promote the development of mitochondria-directed therapies for HF. Several of the priorities addressed the need to better understand relevant aspects of basic mitochondrial biology including the multiplicity of functions played by mitochondria, their role in intra- and inter-cellular communication, and the relationship between mitochondrial genotype and phenotype (Fig. 2). Other priority areas emphasized the need for improved tools for the study of mitochondrial function in humans and the development of strategies for the translation of basic observations to the clinic. Recommendations fell into three main areas.
Recommendation 1:
Foster multi-disciplinary and integrated systems-based approaches to define the mechanistic role of mitochondria in cardiovascular health and disease, and to discover novel therapeutic opportunities.
Studies integrating the multiplicities of mitochondrial function extending beyond energy provision, such as mitochondria-nuclear communication, PTMs, ROS and Ca2+ homeostasis, and regulation of inflammatory responses, are critical to further development of the field and identification of new therapies.
The genotype-phenotype relationship warrants further investigation and should be included, such as assessment of the influence of age, sex, and race on the mitochondrial responses to stress.
It is highly recommended that future projects take a team science approach by bringing together investigators across disciplines, such as biologists, informaticians, engineers, epidemiologists, and clinicians.
Recommendation 2:
Develop and share with the community novel reagents, tools, and knowledge for mitochondrial research in humans and in translationally-relevant animal and cellular models, including imaging modalities, pharmacological agents, biomarkers, multi-omics, and computational approaches.
Develop novel reagents and tools to not only provide multi-omics information on mitochondria, but also generate knowledge maps that enable rapid translation of laboratory discoveries.
Technical innovations in modeling, intervening, and monitoring mitochondrial function in vitro and in vivo are particularly encouraged.
Biomarkers that identify specific patient populations who will benefit from mitochondria-based therapy are of high significance for clinical trials.
Recommendation 3:
Foster translational approaches to mitochondrial therapies for rapid translation of novel therapies and discoveries into clinical trials.
Develop techniques to assess cardiac mitochondrial function in HF patients, including identification of biomarkers, to identify mitochondrial phenotype, guide patient selection, and assess the effects of new therapeutic agents on mitochondrial function.
Perform proof-of-concept studies in relatively homogeneous patient populations selected with regard to key determinants of mitochondrial phenotype in order to assess the relationship between mitochondrial function and cardiac performance.
Use a network-based approach to clinical development that standardizes protocols, measurements, and strategies for patient selection.
ACKNOWLEDGEMENTS
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
SOURCES OF FUNDING
This workshop was supported by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health. Relevant grant support of authors are as listed below.
J.E.B.: NIH R01GM086688
M.M.B.: NIH R01DK103750, NIH 1UL1TR001430, and American Heart Association 16GRNT27660006
S.B.: NIH R01DK098646, NIH R01DK100826, and American Heart Association 16GRNT30990018
W.S.C.: NIH RO1HL064750 and NIH NO1HV-28178
G.W.D.: NIH R35HL135736, NIH R01HL128071, and the Harrington Discovery Institute
S.D.: NIH R01HL124116
R.A.G.: NIH P01HL112730, NIH R01HL132075, NIH R01HL144509
D.P.K.: NIH R01HL128349, NIH R01HL058493
M.J.K.: NIH R01HL136496
D.M.-R.: NIH R01HL52141
R.N.K.: NIH R01HL128071, NIH R01HL130861, NIH R01HL138475
E.D.L.: NIH R01HL049244, NIH R01HL132525, NIH R01HL113057
J.M.M: NIH R01HL125695
B.O.: NIH R01HL137259, NIH R01HL134821
K.D.O.: NIH R21HL126209, NIH P01HL092969
J.Y.P.: NIH R01HL126952
S.S.: NIH R01HL133003-01A1, NIH R01GM113816-01, and Hemophilia Center of Western Pennsylvania
S-S. S.: NIH R01HL122124, NIH R01HL093671, NIH R01HL137426, NIH R01HL142864, NIH R01HL137266
R.T.: NIH R01HL110349, NIH R01HL129510, NIH R01HL126209, NIH R01HL142628
D.C.W.: NIH R01NS021328, NIH R01MH108592, NIH R01OD010944, U.S. Department of Defense W81XWH-16-1-0401
R.W.: NIH R01HL61912, NIH R01HL63030
Appendix: Workshop Participant List
NHLBI Organizers:
Lisa Schwartz Longacre, PhD
Zorina S. Galis, PhD
Scarlet Shi, PhD
Renee Wong, PhD
Working Group Participants:
Co-Chairs:
Wilson S. Colucci, MD, Boston University
Rong Tian, MD, PhD, University of Washington
Members:
Zoltan Arany, MD, PhD, University of Pennsylvania
Markus M. Bachschmid, PhD, Boston University
Scott W. Ballinger, PhD, University of Alabama at Birmingham
Sihem Boudina, PhD, University of Utah
James E. Bruce, PhD, University of Washington
David W. Busija, PhD, Tulane University
Sergey Dikalov, PhD, Vanderbilt University Medical Center
Gerald W. Dorn II, MD, Washington University
Roberta A. Gottlieb, MD, Cedars-Sinai Medical Center
Daniel P. Kelly, MD, University of Pennsylvania
Richard N. Kitsis, MD, Albert Einstein College of Medicine
Mark J. Kohr, PhD, Johns Hopkins University
Daniel Levy, MD, National Institutes of Health
E. Douglas Lewandowski, PhD, Ohio State University
Joseph M. McClung, PhD, East Carolina University
Daria Mochly-Rosen, PhD, Stanford University
Kevin D. O’Brien, MD, University of Washington
Brian O’Rourke, PhD, Johns Hopkins University
Joon-Young Park, PhD, Temple University
Peipei Ping, PhD, University of California, Los Angeles
Michael N. Sack, MD, PhD, National Institutes of Health
Shey-Shing Sheu, PhD, Thomas Jefferson University
Sruti Shiva, PhD, University of Pittsburgh
Douglas C. Wallace, PhD, Children’s Hospital of Philadelphia Research Institute
Robert G. Weiss, MD, Johns Hopkins University
Hilary J. Vernon, MD, PhD, Johns Hopkins University
Footnotes
DISCLOSURES
G.W.D. is a founder of Mitochondria in Motion, Inc., a Saint Louis based biotech R&D company focused on enhancing mitochondrial trafficking and fitness in neurodegenerative diseases.
R.N.K. is co-founder of Aspida Therapeutics Inc. and consultant for Amaron Bio.
H.J.V. is the PI on a clinical trial sponsored by Stealth Biotherapeutics, Newton MA.
References
- 1.Ventura-Clapier R, Garnier A, Veksler V, Joubert F. Bioenergetics of the failing heart. Biochim Biophys Acta. 2011;1813:1360–1372. [DOI] [PubMed] [Google Scholar]
- 2.Neubauer S The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. [DOI] [PubMed] [Google Scholar]
- 3.Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guerin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De Poli F, Morice MC, Ider O, Dubois-Rande JL, Unterseeh T, Le Breton H, Beard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, Ovize M. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N Engl J Med. 2015; 373:1021–1031. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med. 2000;342:154–160. [DOI] [PubMed] [Google Scholar]
- 6.National Heart, Lung, and Blood Institute (NHLBI). Modeling Mitochondrial Dysfunction in Cardiovascular Disease. https://www.nhlbi.nih.gov/events/2007/modeling-mitochondrial-dysfunction-cardiovascular-disease. Date published March 3, 2008. Date accessed January 3, 2019.
- 7.National Heart, Lung, and Blood Institute (NHLBI). NHLBI Working Group Unlocking the Secrets of Mitochondria in the Cardiovascular System: Path to a Cure in Heart Failure - Executive Summary. https://www.nhlbi.nih.gov/events/2018/nhlbi-working-group-unlocking-secrets-mitochondria-cardiovascular-system-path-cure. Date published September 19, 2018. Date accessed January 3, 2019.
- 8.Jennings RB, Ganote CE. Mitochondrial structure and function in acute myocardial ischemic injury. Circ Res. 1976;38:I80–I91. [PubMed] [Google Scholar]
- 9.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. [DOI] [PubMed] [Google Scholar]
- 10.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey S, DeMazumder D, Sidor A, Foster DB, O’Rourke B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ Res. 2018;123:356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iglewski M, Hill JA, Lavandero S, Rothermel BA. Mitochondrial fission and autophagy in the normal and diseased heart. Curr Hypertens Rep. 2010;12:418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA, Edgar JS, Goo YA, Goodlett DR, Bruce JE, Tian R. Normalization of NAD+ Redox Balance as a Therapy for Heart Failure. Circulation. 2016;134:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, Carpenter AC, Kolmetzky D, Gao E, van Berlo JH, Tsai EJ, Molkentin JD, Chen X, Madesh M, Houser SR, Elrod JW. The mitochondrial Na(+)/Ca(2+) exchanger is essential for Ca(2+) homeostasis and viability. Nature. 2017;545:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol. 2013;61:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Karamanlidis G, Tian R. Novel targets for mitochondrial medicine. Sci Transl Med. 2016;8:326rv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. [DOI] [PubMed] [Google Scholar]
- 20.Kolwicz SC Jr., Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res. 2012; 111:728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahey R, Carley AN, Wang X, Glass CE, Accola KD, Silvestry S, O’Donnell JM, Lewandowski ED. Enhanced Redox State and Efficiency of Glucose Oxidation With miR Based Suppression of Maladaptive NADPH-Dependent Malic Enzyme 1 Expression in Hypertrophied Hearts. Circ Res. 2018;122:836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. [DOI] [PubMed] [Google Scholar]
- 23.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. [DOI] [PubMed] [Google Scholar]
- 24.Carley AN, Lewandowski ED. Triacylglycerol turnover in the failing heart. Biochim Biophys Acta. 2016;1861:1492–1499. [DOI] [PubMed] [Google Scholar]
- 25.Winter JL, Castro PF, Quintana JC, Altamirano R, Enriquez A, Verdejo HE, Jalil JE, Mellado R, Concepcion R, Sepulveda P, Rossel V, Sepulveda L, Chiong M, Garcia L, Lavandero S. Effects of trimetazidine in nonischemic heart failure: a randomized study. J Card Fail. 2014;20:149–154. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Lu Y, Jiang H, Zhang L, Sun A, Zou Y, Ge J. Additional use of trimetazidine in patients with chronic heart failure: a meta-analysis. J Am Coll Cardiol. 2012;59:913–922. [DOI] [PubMed] [Google Scholar]
- 27.Abozguia K, Elliott P, McKenna W, Phan TT, Nallur-Shivu G, Ahmed I, Maher AR, Kaur K, Taylor J, Henning A, Ashrafian H, Watkins H, Frenneaux M. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation. 2010;122:1562–1569. [DOI] [PubMed] [Google Scholar]
- 28.George CH, Mitchell AN, Preece R, Bannister ML, Yousef Z. Pleiotropic mechanisms of action of perhexiline in heart failure. Expert Opin Ther Pat. 2016;26:1049–1059. [DOI] [PubMed] [Google Scholar]
- 29.Keating ST, El-Osta A. Epigenetics and metabolism. Circ Res. 2015;116:715–736. [DOI] [PubMed] [Google Scholar]
- 30.Papanicolaou KN, O’Rourke B, Foster DB. Metabolism leaves its mark on the powerhouse: recent progress in post-translational modifications of lysine in mitochondria. Front Physiol. 2014;5:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denton RM, McCormack JG. The calcium sensitive dehydrogenases of vertebrate mitochondria. Cell Calcium. 1986;7:377–386. [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Takimoto E, Dimaano VL, DeMazumder D, Kettlewell S, Smith G, Sidor A, Abraham TP, O’Rourke B. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ Res. 2014;115:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu T, O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008;103:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. [DOI] [PubMed] [Google Scholar]
- 35.Stachowski MJ, Holewinski RJ, Grote E, Venkatraman V, Van Eyk JE, Kirk JA. Phospho-Proteomic Analysis of Cardiac Dyssynchrony and Resynchronization Therapy. Proteomics. 2018;18:e1800079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lozoya OA, Martinez-Reyes I, Wang T, Grenet D, Bushel P, Li J, Chandel N, Woychik RP, Santos JH. Mitochondrial nicotinamide adenine dinucleotide reduced (NADH) oxidation links the tricarboxylic acid (TCA) cycle with methionine metabolism and nuclear DNA methylation. PLoS Biol. 2018;16:e2005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Day JJ, Kennedy AJ, Sweatt JD. DNA methylation and its implications and accessibility for neuropsychiatric therapeutics. Annu Rev Pharmacol Toxicol. 2015;55:591–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kesterson RA, Johnson LW, Lambert LJ, Vivian JL, Welch DR, Ballinger SW. Generation of Mitochondrial-nuclear eXchange Mice via Pronuclear Transfer. Bio Protoc. 2016;6:e1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivian CJ, Brinker AE, Graw S, Koestler DC, Legendre C, Gooden GC, Salhia B, Welch DR. Mitochondrial Genomic Backgrounds Affect Nuclear DNA Methylation and Gene Expression. Cancer Res. 2017;77:6202–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunham-Snary KJ, Sandel MW, Sammy MJ, Westbrook DG, Xiao R, McMonigle RJ, Ratcliffe WF, Penn A, Young ME, Ballinger SW. Mitochondrial - nuclear genetic interaction modulates whole body metabolism, adiposity and gene expression in vivo. EBioMedicine. 2018;36:316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fetterman JL, Zelickson BR, Johnson LW, Moellering DR, Westbrook DG, Pompilius M, Sammy MJ, Johnson M, Dunham-Snary KJ, Cao X, Bradley WE, Zhang J, Wei CC, Chacko B, Schurr TG, Kesterson RA, Dell’italia LJ, Darley-Usmar VM, Welch DR, Ballinger SW. Mitochondrial genetic background modulates bioenergetics and susceptibility to acute cardiac volume overload. Biochem J. 2013;455:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunham-Snary KJ, Ballinger SW. Mitochondrial genetics and obesity: evolutionary adaptation and contemporary disease susceptibility. Free Radic Biol Med. 2013;65:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krzywanski DM, Moellering DR, Fetterman JL, Dunham-Snary KJ, Sammy MJ, Ballinger SW. The mitochondrial paradigm for cardiovascular disease susceptibility and cellular function: a complementary concept to Mendelian genetics. Lab Invest. 2011;91:1122–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004;303:223–226. [DOI] [PubMed] [Google Scholar]
- 45.McManus MJ, Picard M, Chen HW, De Haas HJ, Potluri P, Leipzig J, Towheed A, Angelin A, Sengupta P, Morrow RM, Kauffman BA, Vermulst M, Narula J, Wallace DC. Mitochondrial DNA Variation Dictates Expressivity and Progression of Nuclear DNA Mutations Causing Cardiomyopathy. Cell Metab. 2019;29:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ait-Aissa K, Kadlec AO, Hockenberry J, Gutterman DD, Beyer AM. Telomerase reverse transcriptase protects against angiotensin II-induced microvascular endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2018;314:H1053–H1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 2007;74:456–465. [DOI] [PubMed] [Google Scholar]
- 48.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106:1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luptak I, Sverdlov AL, Panagia M, Qin F, Pimentel DR, Croteau D, Siwik DA, Ingwall JS, Bachschmid MM, Balschi JA, Colucci WS. Decreased ATP production and myocardial contractile reserve in metabolic heart disease. J Mol Cell Cardiol. 2018;116:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sverdlov AL, Elezaby A, Behring JB, Bachschmid MM, Luptak I, Tu VH, Siwik DA, Miller EJ, Liesa M, Shirihai OS, Pimentel DR, Cohen RA, Colucci WS. High fat, high sucrose diet causes cardiac mitochondrial dysfunction due in part to oxidative post-translational modification of mitochondrial complex II. J Mol Cell Cardiol. 2015;78:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sack MN. Mitochondrial fidelity and metabolic agility control immune cell fate and function. J Clin Invest. 2018;128:3651–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chavez JD, Lee CF, Caudal A, Keller A, Tian R, Bruce JE. Chemical Crosslinking Mass Spectrometry Analysis of Protein Conformations and Supercomplexes in Heart Tissue. Cell Syst. 2018;6:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schweppe DK, Chavez JD, Lee CF, Caudal A, Kruse SE, Stuppard R, Marcinek DJ, Shadel GS, Tian R, Bruce JE. Mitochondrial protein interactome elucidated by chemical cross-linking mass spectrometry. Proc Natl Acad Sci U S A. 2017;114:1732–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, Baca LG, Kim E, Ghosh CC, Parikh SM, Jiang A, Chu Q, Forman DE, Lecker SH, Krishnaiah S, Rabinowitz JD, Weljie AM, Baur JA, Kasper DL, Arany Z. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim B, Jang C, Dharaneeswaran H, Li J, Bhide M, Yang S, Li K, Arany Z. Endothelial pyruvate kinase M2 maintains vascular integrity. J Clin Invest. 2018;128:4543–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wallace DC. Mitochondrial genetic medicine. Nat Genet. 2018;50:1642–1649. [DOI] [PubMed] [Google Scholar]
- 57.Garlid AO, Polson JS, Garlid KD, Hermjakob H, Ping P. Equipping Physiologists with an Informatics Tool Chest: Toward an Integerated Mitochondrial Phenome. Handb Exp Pharmacol. 2017;240:377–401. [DOI] [PubMed] [Google Scholar]
- 58.Ping P, Hermjakob H, Polson JS, Benos PV, Wang W. Biomedical Informatics on the Cloud: A Treasure Hunt for Advancing Cardiovascular Medicine. Circ Res. 2018;122:1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertero E, Maack C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ Res. 2018;122:1460–1478. [DOI] [PubMed] [Google Scholar]
- 60.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. [DOI] [PubMed] [Google Scholar]
- 61.Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension. 2018;71:1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, Steurer G, Littarru GP. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2:641–649. [DOI] [PubMed] [Google Scholar]
- 63.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24:1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown DA, Hale SL, Baines CP, del Rio CL, Hamlin RL, Yueyama Y, Kijtawornrat A, Yeh ST, Frasier CR, Stewart LM, Moukdar F, Shaikh SR, Fisher-Wellman KH, Neufer PD, Kloner RA. Reduction of early reperfusion injury with the mitochondria-targeting peptide bendavia. J Cardiovasc Pharmacol Ther. 2014;19:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S, Zhang K. Chronic Therapy With Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs With Advanced Heart Failure. Circ Heart Fail. 2016;9:e002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daubert MA, Yow E, Dunn G, Marchev S, Barnhart H, Douglas PS, O’Connor C, Goldstein S, Udelson JE, Sabbah HN. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ Heart Fail. 2017;10:e004389. [DOI] [PubMed] [Google Scholar]
- 67.Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, Gouge A, Gressette M, Manoury B, Blanc J, Breton M, Decaux JF, Lavery GG, Baczko I, Zoll J, Garnier A, Li Z, Brenner C, Mericskay M. Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy. Circulation. 2018;137:2256–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin AS, Abraham DM, Hershberger KA, Bhatt DP, Mao L, Cui H, Liu J, Liu X, Muehlbauer MJ, Grimsrud PA, Locasale JW, Payne RM, Hirschey MD. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight. 2017;2:e93885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardenes N, Corey C, Geary L, Jain S, Zharikov S, Barge S, Novelli EM, Shiva S. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood. 2014;123:2864–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chacko BK, Kramer PA, Ravi S, Benavides GA, Mitchell T, Dranka BP, Ferrick D, Singal AK, Ballinger SW, Bailey SM, Hardy RW, Zhang J, Zhi D, Darley-Usmar VM. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin Sci (Lond). 2014;127:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tyrrell DJ, Bharadwaj MS, Jorgensen MJ, Register TC, Molina AJ. Blood cell respirometry is associated with skeletal and cardiac muscle bioenergetics: Implications for a minimally invasive biomarker of mitochondrial health. Redox Biol. 2016; 10:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]