Abstract
The number of older patients admitted to acute hospitals has increased; however, their needs are heterogeneous and there is no gold‐standard method of triaging them towards practicing comprehensive geriatric assessment (CGA). In our hospital, the SAFE (Specialist Advice for the Frail Elderly) team provide an initial geriatric assessment of all emergency admissions of patients aged ≥75 years (with some assessments also occurring in those aged 65 to 74 years) and recommend as to whether CGA in a dedicated Department of Medicine for the Elderly (DME) ward may be required. SAFE assessments include routine screening for geriatric syndromes using validated tools. Our aim was to compare the characteristics (age, gender, acute illness severity on admission as per modified early warning score (MEWS), Charlson Comorbidity Index, Clinical Frailty Scale (CFS), presence of dementia and delirium) and outcomes (length of stay, delayed discharge, inpatient mortality, discharge to usual place of residence, and new institutionalization) of patients listed to a DME ward, to those not listed. We analyzed all SAFE team assessments of patients admitted nonelectively between February 2015 and November 2016. Of 6192 admissions, 16% were listed for a DME ward. Those were older, had higher MEWS and CFS score, were more often affected by cognitive impairment, had longer hospital stay, higher inpatient mortality, and more often required new institutionalization. Higher CFS and presence of dementia and delirium were the strongest predictors of DME ward recommendation. Routine measurement of markers of geriatric complexity may help maximize access to finite inpatient CGA resources.
Keywords: clinical frailty scale, frail older adults, geriatrics, hospital medicine
1. INTRODUCTION
The number of older patients admitted nonelectively to acute hospitals in England continues to rise;1 however, their clinical presentations are heterogeneous, from those who are fitter and present with simpler, single‐organ pathologies, to those who present with more complex geriatric syndromes.2 The latter have also continued to increase in English hospitals,3 and their hospital care is complex because of multimorbidity, multicausality, high risk of adverse effects, lack of an evidence base for guideline‐based treatment options, and need to personalize care plans.4 In the acute hospital setting, the best evidence‐based approach for the clinical care of these complex patients is the provision of comprehensive geriatric assessment (CGA) in dedicated inpatient multidisciplinary wards. CGA is a multidimensional, multidisciplinary diagnostic and therapeutic process conducted to determine the medical, mental, and functional problems of older people with frailty so that a coordinated and integrated plan for treatment and follow‐up can be developed. Older patients are more likely to be alive and in their own homes at follow‐up if they received CGA on admission to hospital.5
Owing to the growing number of older people presenting to acute hospitals with complex care needs and the finite number of geriatric beds, strategies are needed to maximize the accessibility of acute geriatric care for appropriate patients, including timely transfer from nongeriatric to geriatric wards.6 In our tertiary university English hospital, a team called SAFE (Specialist Advice for the Frail Elderly) composed of senior nurses and therapists (occupational therapist and physiotherapists) provide an initial geriatric assessment of all emergency admissions of patients aged ≥75 years (with some assessments also occurring in those aged 65 to 74 years) and recommend as to whether CGA in a dedicated Department of Medicine for the Elderly (DME) ward may be required ( https://www.cuh.nhs.uk/specialist-advice-for-frail-elderly-safe). Whilst most SAFE assessments take place in the Emergency Department (ED), those who are not seen in ED are reviewed on the ward unless directly admitted to a DME ward. SAFE assessments include routine screening for frailty, delirium, and dementia using validated tools.
There is no gold?standard method of triaging frail complex older patients to geriatric wards; however, the service provided by our SAFE team could help maximize access to geriatric medicine beds to those who need them the most. The aim of our study was to retrospectively evaluate our SAFE team activity and compare the characteristics and outcomes of patients listed to a DME ward, to those who were not listed.
2. METHODS
2.1. Setting
This retrospective service evaluation was conducted in a large tertiary university hospital (Addenbrooke's hospital) in Cambridge, England ( https://www.cuh.nhs.uk/about-us/our-profile/facts-and-figures). Of around 1000 beds in the hospital, 150 are dedicated Department of Medicine for the Elderly (DME) CGA beds.
2.2. Sample
We analyzed all SAFE team assessments of patients admitted nonelectively between February 1, 2015, and November 30, 2016. Anonymized data were obtained electronically using the hospital's electronic medical records system (EPIC).
2.3. Measures
Routinely collected information included:
Demographics age, sex.
Acute illness severity in the Emergency Department using the Modified Early Warning Score (ED‐MEWS).7
Clinical Frailty Scale (CFS, http://geriatricresearch.medicine.dal.ca/clinical_frailty_scale.htm). Since 2013, all patients aged 75 years or older admitted nonelectively to our hospital are routinely screened for frailty using the CFS within 72 hours,8 resulting in a score ranging between 1 (very fit) to 9 (terminally ill, life expectancy <6 months).
Charlson Comorbidity Index (CCI, without age adjustment).9 The CCI is based on the discharge diagnoses, as coded by the 10th version of the WHO International Classification of Diseases (ICD‐10).
Known history of dementia (yes or no), based on the clinical history and known previous medical records.
Delirium In the absence of known dementia, it was defined as an abnormal (ie, <4) 4‐item Abbreviated Mental Test (4‐ATM) score.10 In the English National Health Service (NHS) acute hospitals, cognitive screening in older adults is nationally mandated.11
Patient outcomes: mean length of stay (LOS, days), inpatient mortality (%), discharge specialty, discharge to usual place of residence, and new institutionalization (admission to care home).
2.4. Statistical analyses
Statistical analyses were performed using SPSS. Descriptives were given as count with percentage (%) and mean with standard deviation (SD). Bivariate comparisons were performed with chi‐square (dichotomous variables) or Mann‐Whitney U test (continuous variables). Multivariate predictors of being listed to DME were obtained by binary logistic regression. Odds ratios were reported with 95% confidence intervals (CI). P‐values <0.05 were considered statistically significant. The probability level for the regression model was saved on the dataset to calculate the area under the receiver operating characteristic curve (AUC).
2.5. Ethical approval
This study received service evaluation approval by Addenbrooke's Hospital's Patient Safety Department (Reference number PRN 7147).
3. RESULTS
A total of 6191 patients were included. Table 1 describes their characteristics and outcomes.
Table 1.
Descriptives of patients included in the study
| Sample descriptives | % (n) or mean (range; SD) | Missing data % (n) |
|---|---|---|
| Age, y | 84.6 (65‐105; 6.3) | 0.0% |
| Age 65‐74 y | 4.0% (249) | |
| Age 75 or more years | 96.0% (5942) | |
| Female | 58.3% (3610) | 0.0% |
| Male | 41.7% (2581) | |
| Not listed for a DME ward | 83.8% (5191) | 0.0% |
| Listed for DME ward | 16.2% (1000) | |
| Mean ED‐MEWS | 2.6 (0‐9; 1.4) | 0.9% (54) |
| Mean CCI | 2.0 (0‐11; 1.8) | 0.0% |
| Mean CFS | 5.0 (1‐9; 1.5) | 6.7% (417) |
| Dementia | 11.6% (716) | 0.0% |
| Delirium | 11.8% (733) | 0.0% |
| Discharged by General Medicine | 29.2% (1805) | 0.0% |
| Discharged by Geriatric Medicine | 35.4% (2191) | 0.0% |
| Mean LOS (d) | 8.7 (0‐155; 12.2) | 0.0% |
| Inpatient death | 4.4% (274) | 0.0% |
| Discharge to usual place of residence | 80.2% (4964) | 0.0% |
| New institutionalization | 9.2% (570) | 0.0% |
CCI, Charlson Comorbidity Index; CFS: Clinical Frailty Scale score; DME, Department of Medicine for the Elderly; ED‐MEWS, Emergency Department Modified Early Warning Score; LOS, length of stay.
About 16% were listed by SAFE for a DME ward. As Table 2 shows, those listed were significantly older, more acute, frailer, more often affected by dementia or delirium, and had longer hospital stay, higher inpatient mortality, and more often required new institutionalization.
Table 2.
Comparison of the characteristics and outcomes of those listed vs not listed by SAFE to DME ward
| Not listed for DME ward (5191) | Listed for DME ward (1000) | P‐value | |
|---|---|---|---|
| Mean age, y (SD) | 84.1 (6.3) | 87.3 (5.7) | <0.001*** |
| Female sex (%) | 58.0 | 60.1 | 0.210 |
| Mean ED‐MEWS (SD) | 2.6 (1.4) | 2.9 (1.5) | <0.001*** |
| Mean CCI (SD) | 2.0 (1.8) | 2.0 (1.7) | 0.05* |
| Mean CFS (SD) | 4.8 (1.5) | 6.1 (1.0) | <0.001*** |
| Dementia (%) | 8.5 | 27.6 | <0.001*** |
| Delirium (%) | 8.9 | 27.1 | <0.001*** |
| Discharged by General Medicine (%) | 30.5 | 22.2 | <0.001*** |
| Discharged by Geriatric Medicine (%) | 30.1 | 62.7 | <0.001*** |
| Mean LOS, days (SD) | 7.7 (11.2) | 14.0 (15.2) | <0.001*** |
| Inpatient death (%) | 3.9 | 7.3 | <0.001*** |
| Discharge to usual place of residence (%) | 83.3 | 63.9 | <0.001*** |
| New institutionalization (%) | 6.5 | 23.2 | <0.001*** |
CCI, Charlson Comorbidity Index; CFS, Clinical Frailty Scale score; DME, Department of Medicine for the Elderly; ED‐MEWS, Emergency Department Modified Early Warning Score; LOS, length of stay; SAFE, Specialist Advice for the Frail Elderly team.
Statistical significance is marked as P‐value (*<0.05, **<0.01, ***<0.001).
Table 3 shows the multivariate logistic regression model to predict being listed to DME by SAFE. The number included in the model was 5729 (92.5% of total sample). The strongest predictors of being listed were the presence of dementia, delirium, and clinical frailty. The AUC for the model was 0.80 (95% CI: 0.79‐0.82, P < 0.001), indicating a fair to good discrimination for the clinical decision to list (Figure 1).
Table 3.
Multivariate predictors of being listed to DME ward by SAFE
| Odds Ratio | 95% CI for Odds Ratio | P‐value | |
|---|---|---|---|
| Age | 1.04 | 1.03‐1.06 | <0.001*** |
| Female sex | 0.91 | 0.77‐1.06 | 0.232 |
| ED‐MEWS | 1.01 | 0.96‐1.07 | 0.650 |
| CCI | 0.94 | 0.89‐0.98 | <0.01** |
| CFS | 2.02 | 1.89‐2.20 | <0.001*** |
| Dementia | 2.15 | 1.77‐2.61 | <0.001*** |
| Delirium | 2.13 | 1.76‐2.58 | <0.001*** |
CCI: Charlson Comorbidity Index; CFS: Clinical Frailty Scale Score; DME: Department of Medicine for the elderly; ED‐MEWS: Emergency Department Modified Early Warning Score; SAFE: Specialist Advice for the Frail Elderly team.
Statistical significance is marked by stars, where P < 0.05 is represented by *, P < 0.01 by **, and P < 0.001 by *** with odds ratio (OR) and 95% confidence interval (95% CI).
Figure 1.
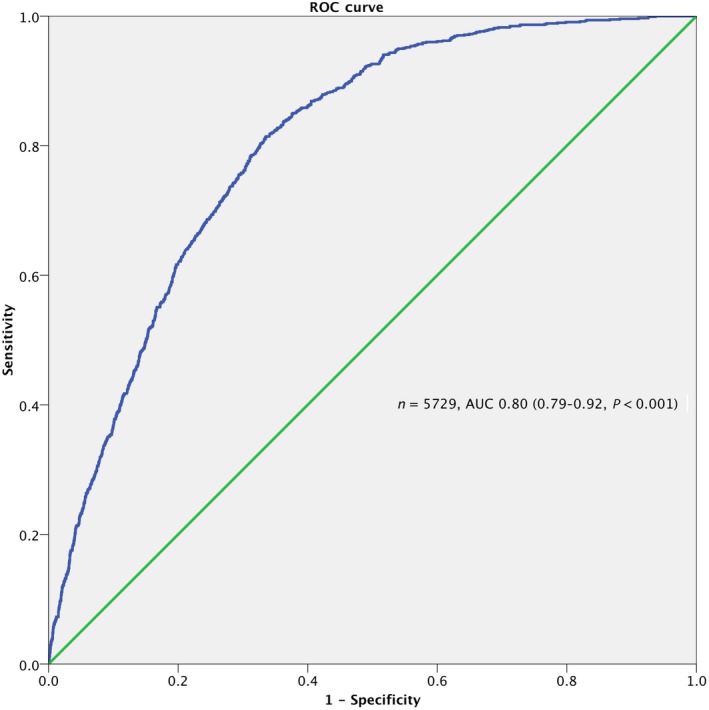
Area Under the Receiver Operating Characteristic (ROC) Curve of the Multivariable Model to Predict SAFE Decision to List for DME Ward
4. DISCUSSION
Results suggest that those listed for DME were more complex and had longer LOS and higher risk of mortality and institutionalization. Frailty and cognition seemed to strongly influence the decision to list.
Cognitive impairment may be a focus for CGA in specialist geriatric wards, as these may have more streamlined discharge planning processes for patients with dementia.12 Acute geriatric ward hospitalization may be associated with reduced incident delirium in older medical inpatients.13
Frailty may be another focus for CGA because it results in poor restoration of homeostasis after a stressor event.14, 15 There is no gold‐standard frailty tool in acute care,16 but measures of frailty based on brief geriatric assessment (eg, CFS) may help identify ED patients at higher risk.17, 18, 19, 20
SAFE listed a small proportion (16%); this highlights their awareness of the need to balance risks and benefits of ward moves21 and may reflect the support they provide outside DME through general ward education and coordinating consultations with other specialties (Pharmacy, Liaison Psychiatry, Geriatric Medicine).
Our evaluation is limited by its retrospective observational nature. Findings are not externally valid or generalizable. Data could not tell us how many patients were listed in ED as opposed to ward. About 37% of listed patients were not discharged by DME; data did not provide reasons, but this may reflect bed capacity issues as well as the fact that SAFE continues to review all listed patients, and a proportion are “de‐listed” prior to transfer when patients are sufficiently supported on‐site. About 30% of the nonlisted were discharged by DME; many in this group could have presented to ED out‐of‐hours and admitted directly to DME.
In conclusion, SAFE was able to identify a group of more vulnerable older patients, and their clinical impression can be evidence‐based. Frailty and cognitive impairment are two related syndromes22 where multicomponent interventions may be effective.23 Quality improvement initiatives such as SAFE aim to support hospitals in delivering evidence‐based care for older people with frailty and urgent care needs.24 Research is necessary to establish if these interventions have a causal effect on patient outcomes.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS’ CONTRIBUTIONS
SAS drafted the manuscript, reviewed it for important intellectual content, and approved the final version. DDS, PS, VLK, and SJW reviewed the manuscript for important intellectual content and approved the final version. RRO obtained service evaluation approval, collected the data, performed statistical analyses, reviewed the manuscript for important intellectual content, and approved the final version.
Alabaf Sabbaghi S, De Souza D, Sarikonda P, Keevil VL, Wallis SJ, Romero‐Ortuno R. Allocating patients to geriatric medicine wards in a tertiary university hospital in England: A service evaluation of the Specialist Advice for the Frail Elderly (SAFE) team. Aging Med. 2018;1:120–124. 10.1002/agm2.12029
REFERENCES
- 1. National Audit Office . Reducing emergency admissions 27 February, 2018, 2018, National Audit Office https://www.nao.org.uk/wpcontent/uploads/2018/02/Reducing-emergency-admissions.pdf. Accessed June 29, 2018.
- 2. Romero‐Ortuno R, O'Shea D. Fitness and frailty: opposite ends of a challenging continuum! Will the end of age discrimination make frailty assessments an imperative? Age Ageing. 2013;42(3):279‐280. [DOI] [PubMed] [Google Scholar]
- 3. Soong J, Poots AJ, Scott S, et al. Quantifying the prevalence of frailty in English hospitals. BMJ Open. 2015;5(10):e008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olde Rikkert MG, Gussekloo J. The complexity of medical care of frail older patients. Ned Tijdschr Geneeskd. 2014;159:A8710. [PubMed] [Google Scholar]
- 5. Ellis G, Gardner M, Tsiachristas A, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev, 2017;9:CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kafetz K. How effective are acute geriatric wards at admitting geriatric patients? Clin Med (Lond). 2010;10(4):420‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romero‐Ortuno R, Wallis S, Biram R, Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: an observational study. Eur J Intern Med. 2016;35:24‐34. [DOI] [PubMed] [Google Scholar]
- 8. Wallis SJ, Wall J, Biram RW, Romero‐Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108(12):943‐949. [DOI] [PubMed] [Google Scholar]
- 9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 10. Romero‐Ortuno R, Forsyth DR, Wilson KJ, et al. The Association of geriatric syndromes with hospital outcomes. J Hosp Med. 2017;12(2):83‐89. [DOI] [PubMed] [Google Scholar]
- 11. Harrison JR. Improving inpatient care for older adults: implementing Dementia Commissioning for Quality and Innovation (CQUIN). BMJ Qual Improv Rep, 2017;6(1) 10.1136/bmjquality.u212202.w4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Briggs R, O'Shea E, de Siún A, et al. Does admission to a specialist geriatric medicine ward lead to improvements in aspects of acute medical care for older patients with dementia? Int J Geriatr Psychiatry 2017;32(6):624‐632. [DOI] [PubMed] [Google Scholar]
- 13. Bo M, Martini B, Ruatta C, et al. Geriatric ward hospitalization reduced incidence delirium among older medical inpatients. Am J Geriatr Psychiatry. 2009;17(9):760‐768. [DOI] [PubMed] [Google Scholar]
- 14. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hatheway OL, Mitnitski A, Rockwood K. Frailty affects the initial treatment response and time to recovery of mobility in acutely ill older adults admitted to hospital. Age Ageing. 2017;46(6):920‐925. [DOI] [PubMed] [Google Scholar]
- 16. Elliott A, Hull L, Conroy SP. Frailty identification in the emergency department‐a systematic review focussing on feasibility. Age Ageing. 2017;46(3):509‐513. [DOI] [PubMed] [Google Scholar]
- 17. Brousseau AA, Dent E, Hubbard R, et al. Identification of older adults with frailty in the Emergency Department using a frailty index: results from a multinational study. Age Ageing. 2018;47(2):242‐248. [DOI] [PubMed] [Google Scholar]
- 18. Conroy SP, Turpin S. New horizons: urgent care for older people with frailty. Age Ageing. 2016;45(5):577‐584. [DOI] [PubMed] [Google Scholar]
- 19. Wall J, Wallis S. Frailty in the emergency department: are bed allocation pressures prioritised over patient frailty in the allocation of geriatric beds? Age Ageing. 2014;43:i30. [Google Scholar]
- 20. Wall J, Wallis S. Can a frailty scale be used to triage elderly patients from emergency department to geriatric wards? Age Ageing, 2014. 43:i30. [Google Scholar]
- 21. McMurdo ME, Witham MD. Unnecessary ward moves. Age Ageing. 2013;42(5):555‐556. [DOI] [PubMed] [Google Scholar]
- 22. Malmstrom TK, Morley JE. Frailty and cognition: linking two common syndromes in older persons. J Nutr Health Aging. 2013;17(9):723‐725. [DOI] [PubMed] [Google Scholar]
- 23. Quinlan N, Marcantonio ER, Inouye SK, Gill TM, Kamholz B, Rudolph JL. Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc. 2011;59(Suppl 2):S262‐S268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conroy S, Thompson D, Griffiths S, et al. Improving acute care for older people at scale ‐ the Acute Frailty Network. Acute Med. 2016;15(4):185‐192. [PubMed] [Google Scholar]


