Abstract
Guanylyl cyclase C (GUCY2C) is the index cancer mucosa antigen, an emerging class of immunotherapeutic targets for the prevention of recurrent metastases originating in visceral epithelia. GUCY2C is an autoantigen principally expressed by intestinal epithelium, and universally by primary and metastatic colorectal tumors. Immunization with adenovirus expressing the structurally unique GUCY2C extracellular domain (GUCY2CECD; Ad5-GUCY2C) produces prophylactic and therapeutic protection against GUCY2C-expressing colon cancer metastases in mice, without collateral autoimmunity. GUCY2C antitumor efficacy is mediated by a unique immunological mechanism involving lineage-specific induction of antigen-targeted CD8+ T cells, without CD4+ T cells or B cells. Here, the unusual lineage specificity of this response was explored by integrating high-throughput peptide screening and bioinformatics, revealing the role for GUCY2C-directed CD8+ T cells targeting specific epitopes in antitumor efficacy. In BALB/c mice vaccinated with Ad5-GUCY2C, CD8+ T cells recognize the dominant GUCY2C254–262 epitope in the context of H-2Kd, driving critical effector functions including interferon gamma secretion, cytolysis ex vivo and in vivo, and antitumor efficacy. The ability of GUCY2C to induce lineage-specific responses targeted to cytotoxic CD8+ T cells recognizing a single epitope mediating antitumor efficacy without autoimmunity highlights the immediate translational potential of cancer mucosa antigen–based vaccines for preventing metastases of mucosa-derived cancers.
Keywords: Cancer immunotherapy, Guanylyl cyclase C, Cancer mucosa antigens, Cytotoxic T lymphocyte
Introduction
Despite the recent FDA approval of sipuleucel-T [1], the first therapeutic cancer vaccine marketed in the United States, there remains a significant mechanistic and clinical gap in cancer immunotherapeutics. One principal hurdle in developing efficacious cancer immunotherapeutics is the inadequacy of target self-antigens, reflecting poor immunogenicity, inter-patient heterogeneity in expression, and autoimmunity. In that context, cancer mucosa antigens (CMAs) are an emerging class of vaccine targets for colorectal and other mucosa-derived tumors [2]. CMAs are expressed by immunoprivileged mucosae and uniformly by derivative metastatic cancers. They provide enhanced immunoefficacy as therapeutic targets to prevent secondary metastases, reflecting their mucosal immunoprivilege associated with attenuated systemic tolerance [2]. Moreover, CMAs offer therapeutic efficacy without autoimmunity, leveraging the paucity of immunological cross-talk between systemic and mucosal compartments [2].
Guanylyl cyclase C (GUCY2C), the index CMA, is one of a family of receptors that synthesize the second messenger cyclic GMP, activating downstream protein kinase G (PKG)-dependent signaling pathways [3]. Under physiological conditions, GUCY2C is principally expressed by intestinal epithelial cells from the duodenum to the rectum [4]. Unlike many other tumor-associated antigens, GUCY2C is universally over-expressed by metastatic colorectal cancer cells [4–6]. Indeed, GUCY2C serves as a sensitive and specific biomarker for enhancing disease staging and monitoring in colorectal cancer patients, reflecting compartmentalization of this antigen normally in the mucosa, but systemically following tumor metastasis [7, 8].
These same characteristics of differential compartmentalization in health and disease suggest GUCY2C as an ideal tumor-associated antigen in vaccines for the secondary prevention of metastatic colorectal cancer. Guanylyl cyclase isoforms share significant homology within the cytosolic catalytic domain, but diverge in the extracellular receptor binding domain, reflecting identical enzymatic function but unique ligand specificities, respectively [3, 9]. Thus, recombinant viral vectors expressing the unique GUCY2C extracellular domain (GUCY2CECD) were explored as targeted immunotherapy for colorectal cancer. Immunization with recombinant adenovirus expressing mouse GUCY2CECD (Ad5-GUCY2C) produced GUCY2C-specific immune responses associated with prophylactic and therapeutic antitumor efficacy in mice in the absence of autoimmunity [9, 10]. Surprisingly, antitumor efficacy was associated with the induction of GUCY2C-specific CD8+ T cells in the absence of antigen-targeted CD4+ T cells and antibodies [9]. While this unusual lineage-specific response was effective against metastatic colon cancer cells, the precise antigenic targets and effector mechanisms mediating selective CD8+ T cell antitumor immunity remain to be defined. Here, using a combination of high-throughput peptide screening and bioinformatics, we explored the induction of systemic lineage-specific GUCY2C-targeted cytotoxic T cell responses, identified the dominant GUCY2C epitope directing those responses, and defined the antitumor effector functions of those antigen-targeted cytotoxic T cells ex vivo and in vivo.
Results
The mucosal autoantigen GUCY2C is a target for colorectal cancer immunotherapy. In humans, GUCY2C expression is normally confined to intestinal epithelium from duodenum to rectum, without expression in systemic tissues, for example lung, liver, and skin [4, 7, 11, 12]. Similarly, GUCY2C also is principally expressed by intestinal enterocytes in mice (Fig. 1a), confirming the utility of rodent models to examine GUCY2C-specific immunotherapy for colorectal cancer. While previous studies targeted a signaling-deficient truncation mutant of GUCY2C in colorectal cancer metastases [9, 10], mouse GUCY2CECD-expressing adenovirus (Ad5-GUCY2C) administered as a single intramuscular immunization induced systemic responses that protected against metastatic colorectal cancer cells expressing full-length mouse GUCY2C as well (Fig. 1a–c). The use of engineered mouse cell lines is necessary for these experiments, because most mouse (CT26, MC38, CMT93) and human (SW480, HCT116, etc.) colorectal cancer cell lines do not express GUCY2C in vitro, despite the nearly universal expression of GUCY2C in cancers in vivo [4–6]. Importantly, we previously demonstrated that CT26 cells engineered to express GUCY2C, do so at levels comparable to normal mouse intestine and human colorectal cancer cell lines [9]. Despite robust expression of GUCY2C in epithelium throughout the intestines, no immune infiltrate was observed in the gut, and mice were free of intestinal pathology [9]. To confirm that antitumor responses were mediated by antigen-specific CD8+ T cell effectors, GUCY2C epitopes were identified and reactive T cells were characterized.
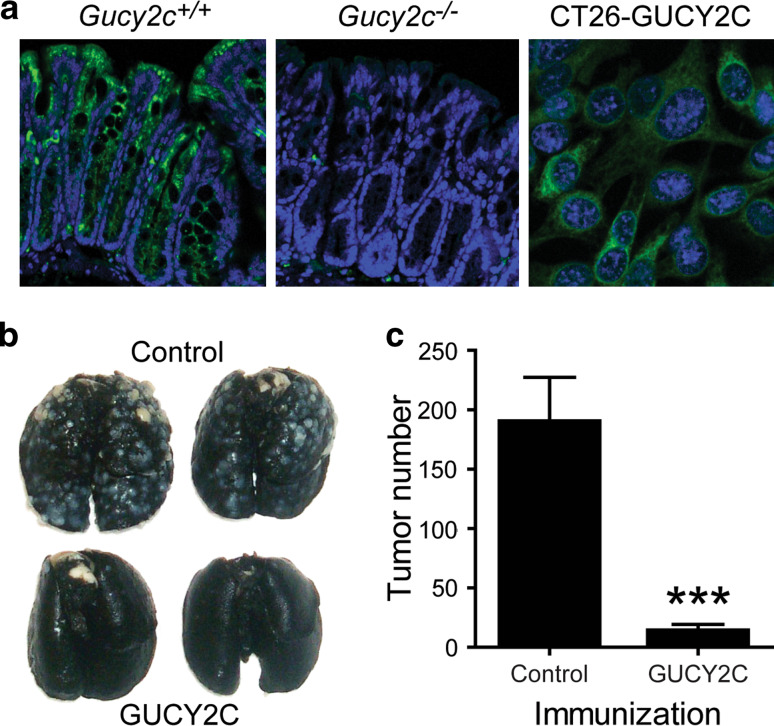
Fig. 1.
The mucosal autoantigen GUCY2C is a target for colorectal cancer immunotherapy. a GUCY2C is an intestinal differentiation antigen. Wild-type (Gucy2c +/+) or GUCY2C-deficient (Gucy2c −/−) mouse colons were stained with a GUCY2C-specific monoclonal antibody (green), revealing GUCY2C expression throughout the crypt–villus axis in Gucy2c +/+, but not Gucy2c −/−, mice. CT26 cells stably expressing full-length mouse GUCY2C (CT26-GUCY2C) exhibit levels of GUCY2C that are comparable to intestine. b, c Ad5-GUCY2C produces antitumor immunity in Gucy2c +/+ BALB/c mice. BALB/c mice (n = 7 per group) were immunized with control Ad5 or Ad5-GUCY2C and challenged 7 days later with CT26-GUCY2C cells by tail vein to produce lung metastases. Following necropsy and staining of lungs 17 days later (b), tumor metastases were enumerated (c)
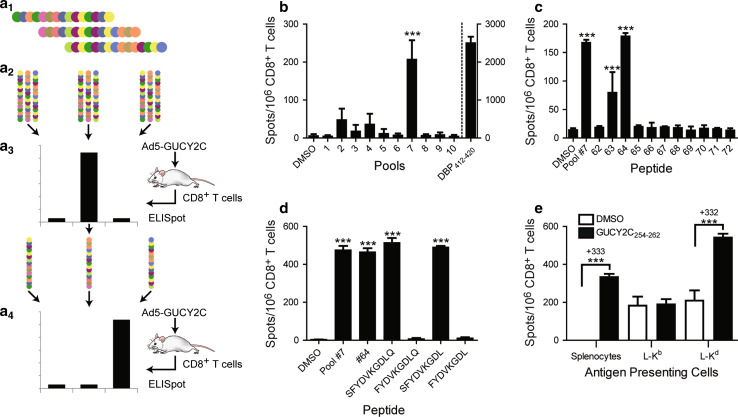
Epitope identification overview. The extracellular domain of GUCY2C (GUCY2CECD) is comprised of 429 amino acids and possesses <20% homology with any known guanylyl cyclase in mice or humans [9]. To identify CD8+ T cell epitopes within the extracellular domain, a combination of high-throughput peptide screening and bioinformatics was employed (Fig. 2a). A library of peptides 15 amino acids in length spanning the mouse GUCY2C extracellular domain and overlapping with adjacent peptides by 11 amino acids was produced (Fig. 2a1). The library possesses all possible epitopes up to 11 amino acids in length, as well as many epitopes 12–15 amino acids in length, ensuring the presence of any CD8+ T cell epitope, which are typically 8–10 amino acids in length. To increase screening efficiency, pools of 10–11 peptides were created for initial screening (Fig. 2a2). Pools were tested in ELISpot assays with purified CD8+ T cells from Ad5-GUCY2C-immunized BALB/c mice (Fig. 2a3). Peptides from pools producing positive responses were then analyzed individually by ELISpot (Fig. 2a4).
Fig. 2.
GUCY2C-specific CD8+ T cells recognize a dominant Kd-presented epitope. a 1 a library of 105 GUCY2CECD-derived peptides 15 amino acids in length was synthesized. These peptides overlapped adjacent peptides by 11 amino acids and covered the GUCY2C extracellular domain (residues 1–429) construct employed in the Ad5-GUCY2C vaccine. Peptides were pooled into groups of 10 or 11 peptides (a 2) that were used to stimulate CD8+ T cells obtained from Ad5-GUCY2C-immunized mice (a 3). a 4 peptides from positive pools were tested individually to identify epitope-containing peptides. b GUCY2C-specific CD8+ T cells recognize a single pool of peptides. Purified CD8+ T cells from Ad5-GUCY2C-immunized BALB/c mice were stimulated with naïve splenocytes (APCs) and peptide pools at 10 μg/ml each peptide and responses analyzed by IFNγ-ELISpot. DMSO served as the vehicle control while the dominant Ad5-derived DBP412–420 epitope served as a positive control (right Y axis). Data are combined from 4 experiments using pooled T cells from 3–8 immunized mice. c Two overlapping peptides represent all of pool #7 reactivity. Individual peptides from pool #7 were tested as in b, identifying peptides #63 and #64 as those recognized by GUCY2C-specific CD8+ T cells (data are representative of 3 experiments using pooled T cells from 3 to 8 immunized mice). d GUCY2C254–262 is the dominant H-2d CD8+ T cell epitope. Algorithms were used to predict the minimum epitope within the overlapping region of peptides #63 and #64 (Table 1), and identified peptides were synthesized and tested to reveal SFYDVKGDL (GUCY2C254–262) but not FYDVKGDL (GUCY2C255–262) as the CD8+ T cell epitope (data obtained using pooled T cells from 6 mice). e GUCY2C254–262 is H-2Kd-presented. GUCY2C254–262 was predicted to bind Kd, but not Dd or Ld (Table 1). Therefore, L929 cells stably expressing H-2 Kb (negative control) or H-2Kd were pulsed with GUCY2C254–262 and used to present epitope to GUCY2C-specific CD8+ T cells. Peptide-pulsed L-Kd cells produced specific responses that were equivalent to peptide-pulsed splenocytes (332 vs. 333 spots/106 cells, respectively). Data are representative of 2 experiments using pooled T cells from 4–6 immunized mice
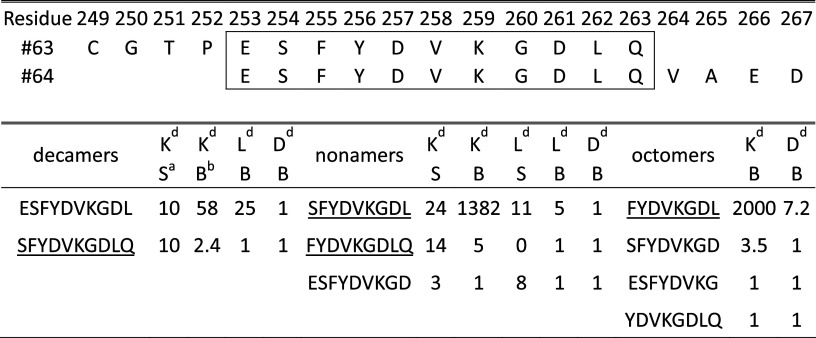
GUCY2C 254–262 is the dominant H-2K d-presented epitope. Ad5-GUCY2C immunization reproducibly induced robust CD8+ T cell responses recognizing peptides in pool #7, suggesting the presence of an immunodominant epitope. In contrast, pools #2 to #4 elicited attenuated responses with substantial inter-experimental variability, suggesting the presence of weaker subdominant epitopes (Fig. 2b). Examination of individual peptides in pool #7 revealed responses to adjacent peptides #63 and #64 (Fig. 2c). The 11-amino acid overlap region spanning peptides #63 and #64 was analyzed with SYFPEITHI [13] and BIMAS [14] algorithms to produce a score for each 8-, 9- and 10-amino acid epitope (Table 1). Based on MHC-binding motifs, these algorithms predicted that GUCY2C255–262 (FYDVKGDL) and GUCY2C254–262 (SFYDVKGDL) were likely candidates for the minimum epitope. Four candidate peptides containing the predicted sequence were synthesized (Table 1) and ELISpot revealed GUCY2C254–262 (SFYDVKGDL) as the minimum epitope (Fig. 2d). To confirm H-2Kd-restriction of this predicted epitope, L929 cells stably expressing H-2Kd (L-Kd) were pulsed with GUCY2C254–262 peptide and used as antigen-presenting cells with GUCY2C-specific CD8+ T cells (Fig. 2e). L-Kd cells pulsed with GUCY2C254–262 peptide restimulated IFNγ-secreting GUCY2C-specific CD8+ T cells equally to splenocytes employed as APCs (~330 net spots over unpulsed cells; Fig. 2e). In contrast, L929 cells expressing the H-2b haplotype molecule (L-Kb) and pulsed with GUCY2C254–262 did not restimulate GUCY2C-specific CD8+ T cells (Fig. 2e).
Table 1.
GUCY2C253–263 fine-mapping
Boxed region indicates overlap between peptides #63 and #64. Scores from 0–1 were rounded up to 1. ELISpot-tested peptides are underlined
aSYFPETHI
bBIMAS
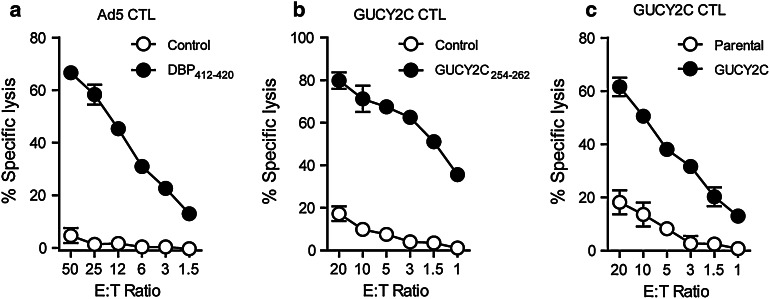
GUCY2C 254–262-specific CD8 + T cells exhibit cytolytic effector function ex vivo. Because cytolysis can be uncoupled from IFNγ secretion [15], which was the principal endpoint for epitope mapping, the cytolytic capacity of GUCY2C254–262-specific CD8+ T cells was examined. BALB/c mice were immunized with Ad5-GUCY2C, and splenocytes collected 2 weeks later were restimulated with the adenovirus DNA-binding protein [16] DBP412–420 (positive control; Fig. 3a) or GUCY2C254–262 (Fig. 3b, c). After 7 days, cytolysis by effector CD8+ T cells was assessed using CT26 mouse colon cancer cells expressing β-galactosidase and pulsed with DBP412–420 (Fig. 3a), GUCY2C254–262 (Fig. 3b), or control peptide (Control), or CT26 cells expressing GUCY2C (Fig. 3c). Indeed, CD8+ T cells from mice immunized with Ad5-GUCY2C specifically lysed CT26 cells pulsed with DBP412–420 (Fig. 3a) or GUCY2C254–262 (Fig. 3b) peptides, or CT26 cells expressing GUCY2C (Fig. 3c), confirming that GUCY2C254–262-specific T cells possess both IFNγ and cytolytic effector functions ex vivo.
Fig. 3.
GUCY2C-specific CD8+ T cells exhibited cytolytic effector function ex vivo. Ad5-GUCY2C immunization produces Ad5 and GUCY2C-specific cytotoxic T lymphocytes (CTLs). BALB/c mice were immunized with Ad5-GUCY2C, and 2 weeks later, splenocytes were collected and restimulated for 7–14 days with the dominant Ad5 epitope, DBP412–420 (a) or GUCY2C254–262 (b, c) and IL-2. a Ad5 CTL cultures were then tested for their ability to lyse target cells stably expressing β-galactosidase and pulsed with control peptide or DBP412–420 peptide. GUCY2C CTL cultures were tested for their ability to lyse target cells pulsed with control peptide or GUCY2C254–262 peptide (b) as well as parental and full-length GUCY2C-expressing target cells (c). Data are representative of two experiments using pooled splenocytes from 5 immunized mice
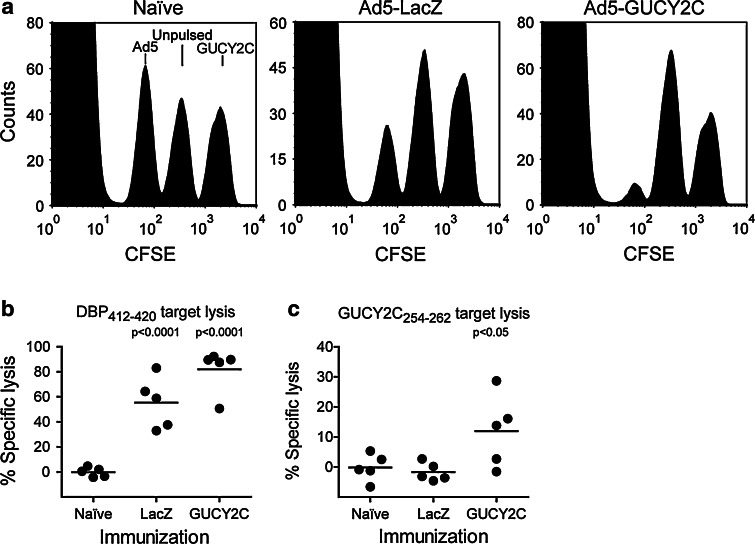
GUCY2C 254–262-specific CD8 + T cells exhibit cytolytic effector function in vivo. To quantify in vivo cytolytic function, we utilized an in vivo CTL assay [17–19]. Naïve peptide-pulsed splenocytes are labeled by incubation with different concentrations of CFSE to produce different levels of green fluorescence. These targets can then be administered intravenously to immune mice and will be targeted for cytolysis. The 16-h incubation period used here allows for cytolysis, but is too short to allow CFSE dilution by target cell division [18, 19]. Moreover, inclusion of naïve mice reveals the baseline for each target population, and inclusion of control-pulsed target populations provides an internal control for normalization within each mouse. Here, naïve BALB/c mice or those immunized with Ad5-LacZ or Ad5-GUCY2C were challenged 2 weeks later with a 1:1:1 mixture of three CFSE-labeled target cell populations: unpulsed, DBP412–420-pulsed, or GUCY2C254–262-pulsed. These populations were produced by labeling three naïve splenocyte samples with CFSE concentrations producing different fluorescent intensities. Following peptide-pulsing of each population, they were mixed to produce 3 distinct peaks by FACS analysis. Administration of the mixture to naïve mice revealed all 3 populations in a ratio of ~1:1:1 (Fig. 4a, left). Mice immunized with Ad5-LacZ eliminated DBP412–420-pulsed cells (Fig. 4a, middle and b), but not GUCY2C254–262-pulsed cells (Fig. 4a, middle and c). In contrast, Ad5-GUCY2C immunization eliminated both DBP412–420 and GUCY2C254–262-pulsed target cells (Fig. 4a, right and b–c). Lysis of DBP412–420-pulsed targets was more efficient than that of GUCY2C254-262-pulsed targets, reflecting the ~10-fold greater responses to the immunodominant Ad5 peptide than to GUCY2C observed by ELISpot (Fig. 2b).
Fig. 4.
GUCY2C-specific CD8+ T cells exhibit cytolytic effector function in vivo. a–c Ad5-GUCY2C immunization produces Ad5 and GUCY2C-specific CTLs in vivo. Naïve BALB/c mice or those immunized with Ad5-LacZ or Ad5-GUCY2C were challenged 2 weeks later with a mixture of three CFSE-labeled splenocyte populations: unpulsed, DBP412–420 or GUCY2C254–262 peptide-pulsed. a Lysis of CFSE-labeled splenocytes was assessed by FACS. b Ad5-LacZ and Ad5-GUCY2C-immunized, but not naïve, mice specifically lysed DBP412–420-pulsed targets (P < 0.001 LacZ and GUCY2C vs. naïve). c Ad5-GUCY2C-immunized, but not naïve or Ad5-LacZ-immunized, mice specifically lysed GUCY2C254–262-pulsed targets (P < 0.05 GUCY2C vs. naïve). N = 5 mice per group (individual mice are shown)
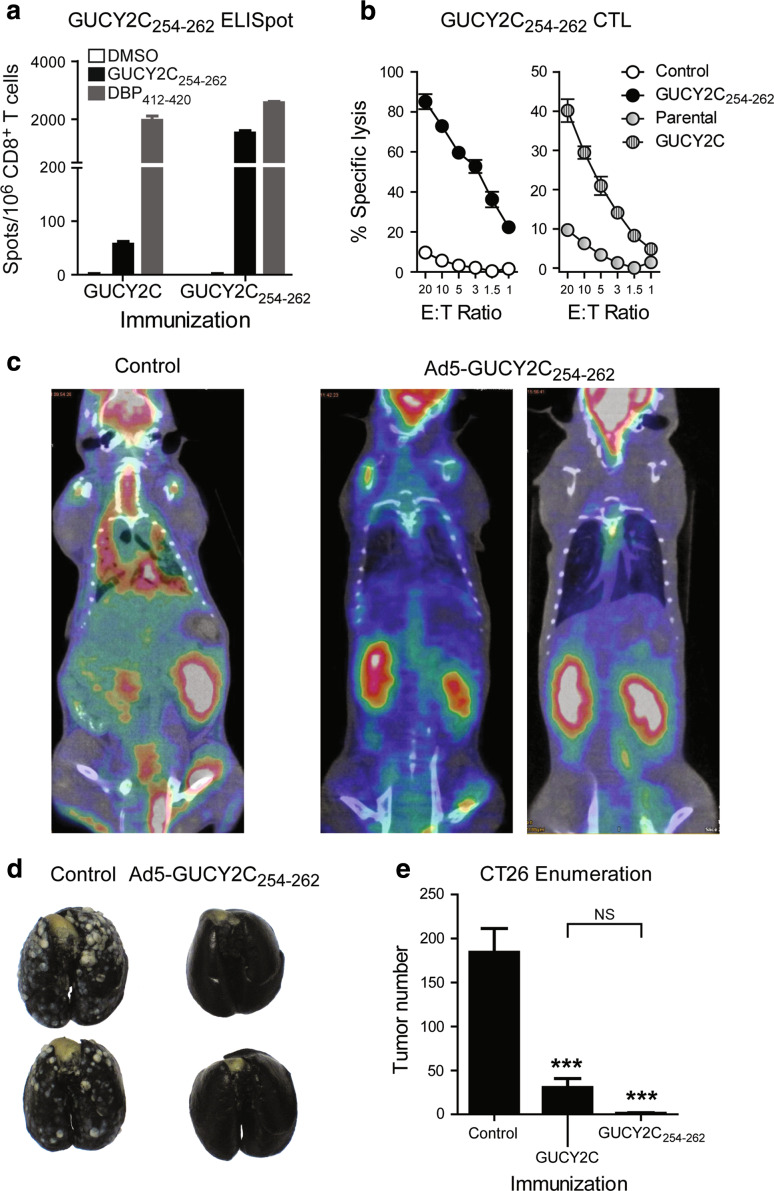
GUCY2C 254–262-specific CD8 + T cells exhibit antitumor efficacy in vivo. A recombinant adenovirus expressing minigene GUCY2C254–262 (Ad5-GUCY2C254–262) was produced possessing an initiating methionine, the GUCY2C254–262 epitope, and a stop codon. Ad5-GUCY2C254–262 immunization produced epitope-specific CD8+ T cell responses quantified by IFNγ ELISpot that exceeded responses to Ad5-GUCY2C by an order of magnitude (Fig. 5a). Similarly, Ad5-GUCY2C254–262 immunization produced CD8+ T cells that exhibit antigen-specific cytolytic effector function targeting cells expressing full-length GUCY2C or GUCY2C254–262-pulsed (Fig. 5b). Moreover, BALB/c mice were immunized with control or Ad5-GUCY2C254–262 and challenged 7 days later with GUCY2C-expressing CT26 cells to establish lung metastases. Mice immunized with control Ad5 exhibited substantial tumor burden on day 17, visualized by PET/CT (Fig. 5c) and quantified by tumor enumeration (Fig. 5d, e). In contrast, tumor growth was substantially inhibited by a single Ad5-GUCY2C254–262 immunization (Fig. 5c–e). This antitumor effect was greater, though the average tumor number was not statistically different, than that using Ad5-GUCY2C (Fig. 5e).
Fig. 5.
GUCY2C254–262-specific CD8+ T cells exhibit antitumor efficacy in vivo. a Ad5-GUCY2C254–262 immunization produces GUCY2C-specific CD8+ T cell responses. BALB/c mice were immunized with Ad5-GUCY2C or Ad5-GUCY2C254–262, and GUCY2C254–262-specific responses were measured by ELISpot. GUCY2C-specific responses were ~25× higher following Ad5-GUCY2C254–262 than Ad5-GUCY2C (P < 0.001). Data are representative of 2 experiments using 3–4 mice/group. b Ad5-GUCY2C254–262 immunization produces GUCY2C-specific CTLs. CTL cultures produced from BALB/c mice immunized with Ad5-GUCY2C254–262 were tested for their ability to lyse GUCY2C254–262 peptide-pulsed targets (left) or those expressing full-length GUCY2C (right) by β-galactosidase release. Negative controls were control peptide–pulsed targets or parental (non-GUCY2C-expressing) targets, respectively. Data are representative of two experiments using pooled splenocytes from 5 immunized mice. c–e Ad5-GUCY2C254–262 immunization produces GUCY2C-specific antitumor CTLs. BALB/c mice were immunized with control Ad5 or Ad5-GUCY2C254–262 and challenged 7 days later with GUCY2C-expressing CT26 cells by tail vein to establish lung metastases. Tumor burden was reduced in Ad5-GUCY2C254–262-immunized mice by PET/CT (c) and tumor enumeration (d, e). For comparison, Ad5-GUCY2C-primed mice were included in the tumor enumeration study (e). ***P < 0.0001, one-way ANOVA, Tukey’s comparison to control immunization; NS not statistically different between GUCY2C and GUCY2C254–262 immunized. The difference in tumor frequency of Ad5-GUCY2C-primed mice (100%) and Ad5-GUCY2C254–262-primed mice (40%) was statistically significant (P < 0.05, Fisher’s exact test). N = 10 mice per group for tumor enumeration
Discussion
One gap in evolving effective immunological approaches for primary therapy and secondary prevention in cancer is the identification of self-antigens that serve as ideal antitumor targets [20]. These antigens should induce potent antitumor responses to maximize efficacy; universally associate with tumors to provide broad disease coverage; and discriminate tumor from normal cells to minimize off target adverse effects. In that context, cancer mucosa antigens may particularly qualify as immunotherapeutic targets. Their physiological expression confined to mucosa should support the generation of robust immune responses due to limited systemic tolerance [2]. Moreover, the paucity of cross-talk between immunological compartments should produce systemic antitumor responses in the absence of mucosal autoimmunity [2]. GUCY2C, the index cancer mucosa antigen, is principally expressed by intestinal epithelial cells from the duodenum to the rectum, and universally over-expressed by primary and metastatic colorectal tumors [4–6, 21]. Immunization with viral vectors expressing the extracellular domain of GUCY2C induces robust antigen-specific systemic immune responses associated with prophylactic and therapeutic antitumor efficacy against parenchymal colon cancer metastases [9]. These antitumor responses are achieved without autoimmunity, colitis, or other inflammatory sequelae that could limit translation of this paradigm [10].
Unexpectedly, GUCY2C induced antigen-specific CD8+ T, but not CD4+ T or B cell responses [9]. While this unusual lineage-specific immunity provided effective protection against parenchymal metastases, the antigenic targets and effector mechanisms mediating antitumor responses have remained undefined. The present study reveals that GUCY2C immunization produces CD8+ T cell responses targeting a single dominant epitope. Induction of epitope-specific CD8+ T cells reflected by IFNγ production was coupled with antigen-dependent tumor cell lysis ex vivo and in vivo and efficacy against parenchymal metastases. Moreover, immunization with an Ad5 vector expressing only the GUCY2C254–262 epitope qualitatively recapitulated, and quantitatively exceeded, immune responses produced by an Ad5 vector expressing the entire extracellular domain of GUCY2C (Fig. 5a). The immunological and antitumor efficacy of the minigene construct, harboring only a single epitope that binds class I MHC, confirms that CD8+ T cells alone oppose GUCY2C-expressing tumor cells in vivo [9]. The strict lineage specificity of these immunological responses coupled with their clinical efficacy underscores the importance of defining mechanisms by which GUCY2C-targeted CD8+ T cells are engaged in the absence of antigen-specific CD4+ T cell help canonically required for CTL induction.
Conversely, these considerations highlight the unique evolution of lineage-restricted tolerance to GUCY2C, in which antigen-specific CD4+ T helper and B cell, but not CD8+ T cell, responses are eliminated [9, 10]. This concept of lineage-restricted tolerance is reinforced by the failure of GUCY2C to induce antigen-targeted CD4+ T cell and antibody responses following repeated immunizations with a heterologous viral prime-boost regimen [9, 10]. Lineage-restricted tolerance does not reflect a unique structural characteristic of the antigen since Ad5-GUCY2C produces CD8+ T, CD4+ T and B cell responses in mice in which GUCY2C expression was eliminated [9, 10]. Rather, cell-specific tolerance likely reflects selective expression of GUCY2C in the anatomically, functionally, and immunologically compartmentalized intestinal mucosa. Cellular and molecular mechanisms underlying differential susceptibility of GUCY2C-specific B and CD4+ T cells, compared to CD8+ T cells, to tolerance reflecting mucosal restriction of antigen remain undefined. Tolerant cells may encounter GUCY2C protein and peptide–MHC complexes centrally in thymus or bone marrow, or peripherally. Although GUCY2C was not detected in thymus or bone marrow [4, 22], expression in rare tolerance-inducing cells cannot be excluded. In that context, autoimmune regulator (AIRE)-dependent promiscuous peripheral antigen expression in medullary thymic epithelial cells is critical to induce tolerance to peripheral antigens and prevent autoimmunity [23]. The contribution of these and other mechanisms to lineage-restricted tolerance to GUCY2C and their generalizability to other cancer mucosal antigens are being explored.
Importantly, antigen-presenting cells can present MHC class I antigen complexes and activate CD8+ T cells in the absence of CD4+ T cell help. However, the mechanism and precise role of CD4+ T cell help in CD8+ T cell responses are not yet fully defined. Early studies highlighted the importance of dendritic cells as an often critical mediator of CD4+ T cell help to CD8+ T cell responses [24–26]. Like B cell help, the molecular mechanism was shown to be CD40–CD40 ligand interaction, as CD40 agonist could replace CD4+ T cell help. However, in some experiments utilizing MHC-II or CD4-deficient mice or antibody-mediated CD4+ T cell depletion, CD8+ T cell responses to several pathogens were CD4+ T cell independent [27–29]. Thus, like B cells, there is a help-independent portion of CD8+ T cell responses, and while the underlying molecular mechanisms remain to be defined, this allows the generation of GUCY2C-specific CD8+ T cell responses following Ad5-GUCY2C immunization.
Although mechanisms shaping tolerance to endogenous mucosa self-antigens have not yet been explored, transgenic mouse models provide limited insights. A chicken ovalbumin (OVA) transgene controlled by the intestinal fatty acid binding protein (IFABP) promoter produces OVA expression restricted to small intestine [30–32]. In transgenic mice, OVA epitopes are presented in mesenteric lymph nodes and activate adoptively transferred OVA-specific CD8+ T cells [31]. However, in contrast to GUCY2C, transgenic mice were completely tolerant, without CD8+ T cell responses, to OVA-specific immunization using bacterial or viral vectors [30, 32]. These observations highlight the limitations of model antigen systems to predict tolerance mechanisms to endogenous products. Interestingly, transgenic mouse models express supra-physiological levels of OVA [30], potentially confounding mechanistic interpretations since antigen levels drive tolerance [33]. Ultimately, dissection of tolerance mechanisms to bona fide cancer mucosa antigens, including GUCY2C, will define molecular and cellular pathways mediating lineage-restricted tolerance and their generalizability to compartmentalized antigens.
Results here support established principles developed with conventional tumor immunotherapeutics, in which CD8+ T cells are generally accepted as principal mediators of antitumor efficacy [34]. Indeed, there is a well-established prognostic relationship between tumor-infiltrating cytotoxic CD8+ T cells and disease-free survival in colorectal cancer patients [35–38]. These observations suggest that immunotherapeutics that elicit new, or amplify endogenous, cytotoxic CD8+ T cell responses should be the most efficacious in colorectal cancer. Unfortunately, many colorectal cancer vaccines to date have proven suboptimal in inducing CD8+ T cell responses [39]. Clinical efficacy against the human oncofetal antigen 5T4 was mediated exclusively by antibodies, without induction of CD8+ T cell responses [40]. Moreover, single immunization with recombinant vaccinia virus expressing carcinoembryonic antigen (CEA) failed to induce CD8+ T cell responses [41]. In contrast, single administration of Ad5-GUCY2C produced GUCY2C-specific cytotoxic CD8+ T cells with antitumor efficacy.
Although immunotherapy has been largely unsuccessful in clinical trials [42], a substantial need exists for more than 500,000 patients who die annually from colorectal cancer [43]. Here, we demonstrate that immunization with Ad5-GUCY2C induces cytotoxic CD8+ T cells targeting an MHC class I–restricted epitope that mediate potent antitumor responses. In the context of its demonstrated safety [10], Ad5-GUCY2C may serve as an effective vaccine strategy for colorectal cancer patients for the secondary prevention of metastatic disease. Lineage-specific immune cell responses revealed here offer for the first time the possibility of antitumor immunotherapy using vectors expressing only MHC-compatible GUCY2C epitopes. Moreover, this study reinforces the importance of defining mechanisms underlying lineage-specific immune responses, to enable strategies that abrogate lineage-restricted tolerance and engage CD4+ and CD8+ T and B cells to maximize the impact of GUCY2C-targeted immunotherapy. Clinical translation of these unique lineage-specific mechanisms to effective immunotherapy will be tested in a planned trial of an Ad5-GUCY2C vector in stage I and II colon cancer patients.
Materials and methods
Immunofluorescence
Formalin-fixed, paraffin-embedded tissues or methanol-fixed cells were stained with Alexa-488-conjugated MS20 mouse monoclonal antibody specific for mouse GUCY2C.
Mice and immunizations
BALB/c mice were obtained from the NCI Animal Production Program (Frederick, MD). Animal protocols were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee. Adenovirus expressing the extracellular domain of mouse GUCY2C (Ad5-GUCY2C) and Ad5-LacZ (control Ad5) were described previously [9]. Adenovirus expressing minigene GUCY2C254–262 (Ad5-GUCY2C254–262) was produced as previously described [9]. For immunizations, mice received 1 × 108 IFU of adenovirus by IM injection of the anterior tibialis.
Peptide library
A library of 105 GUCY2CECD-derived peptides 15 amino acids in length, with 11-amino acid overlap with adjacent peptides, was synthesized (JPT Peptide Technologies, Berlin, Germany). Aliquots of individual peptides or pools of 10–11 peptides were dissolved in DMSO and used in ELISpot assays at a final concentration of 5–10 μg/mL each peptide with ≤1% DMSO.
ELISpot
ELISpot assays were described previously [9]. Briefly, multiscreen filtration plates (Millipore) were coated with anti-mouse IFNγ-capture antibody (BD Biosciences). CD8+ T cells were MACS-purified (Miltenyi Biotec, Bergisch Gladbach, Germany) from immunized mice, and ~250,000 were plated with ~50,000 naïve splenocytes per well serving as antigen-presenting cells and 5–10 μg/mL peptide. After ~24 h of peptide stimulation, spots were developed with biotinylated anti-IFNγ detection antibody (BD Biosciences, San Jose, California) and alkaline phosphatase-conjugated streptavidin (Pierce, Rockford, Illinois), followed by NBT/BCIP substrate (Pierce). Spot-forming cells were enumerated using computer-assisted video imaging analysis (ImmunoSpot v5, Cellular Technology, Shaker Heights, Ohio). In some assays, L-Kb or L-Kd (L929 cells stably expressing H-2Kb or H-2Kd, respectively) were pulsed for 1 h at 37°C with 10 μg/mL peptide and washed and 50,000 were used per well as antigen-presenting cells in lieu of splenocytes and soluble peptide.
In silico epitope prediction
For fine-mapping of the dominant GUCY2C epitope, the 11-amino acid region spanning recognized peptides #63 and #64 was analyzed using the SYFPEITHI [13] and BIMAS [14] algorithms. The score of each epitope 8, 9 and 10 amino acids in length was obtained for each H-2d MHC class I molecule available. The four short peptides indicated in Table 1 were synthesized and tested by ELISpot.
Ex vivo β-gal-release cytotoxic T cell (CTL) assay
Splenocytes were collected from mice 2 weeks after immunization with Ad5-GUCY2C254–262 or an Ad5-GUCY2C construct containing the C-terminal amino acids SVSSFERFEIFPK. Cells were restimulated in upright T25 flasks with 10 u/mL recombinant human IL-2 (NCI-Frederick Cancer Research and Development Center, Biological Resources Branch) and 10 μg/mL peptide—GUCY2C254–262 or the dominant adenovirus epitope DBP412–420 [16]. Ad5 CTLs were cultured for 7 days, while GUCY2C CTLs were cultured for 14 days, reflecting the lower CTL frequency of GUCY2C CTLs than Ad5 CTLs. Target cells, CT26 cells stably expressing β-galactosidase (CT26-CL25, ATCC, Manassas, Virginia), were pulsed with 10 μg/mL GUCY2C254–262, adenovirus DBP412–420, or control peptide (mouse Her263–71) for 1 h at 37°C and washed. CT26-CL25 cells expressing full-length GUCY2C (CT26-GUCY2C) were produced by retroviral transduction and selection using pMSCV-Puro (Clontech, Mountain View, CA). Effector CTLs (E) were incubated at 37°C with target cells (T) for 4 h. Released β-galactosidase was measured in the media using the Galacto-Light Plus System (Applied Biosystems, Carlsbad, California) [44]. Maximum release was determined from supernatants of cells that were lysed by the addition of supplied lysis buffer. Spontaneous release was determined from target cells incubated without effector cells. The following equation was used to calculate % specific lysis for Ad5-specific and GUCY2C-specific CTLs:
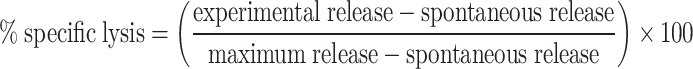 |
In vivo CTL assay [17, 18]
Splenocytes were collected from naïve BALB/c mice and labeled with 0.1, 0.8, or 6.4 μM CFSE (Invitrogen, Carlsbad, California) to produce cell populations of CFSElo, CFSEmed, and CFSEhi fluorescence intensities. Subsequently, each population was not further treated or was pulsed for 1 h at 37°C with 10 μg/mL GUCY2C254–262 or adenovirus DBP412–420 peptide. After washing, the three populations were mixed at equal ratios, and 1.5 × 107 total cells were administered by tail vein to naïve mice or mice immunized 2 weeks earlier with Ad5-LacZ or Ad5-GUCY2C. The next day, splenocytes were collected and analyzed by FACS, quantifying the number of CFSElo, CFSEmed, and CFSEhi cells. The following equation was used to calculate % specific lysis for Ad5-specific and GUCY2C-specific CTLs in each mouse:
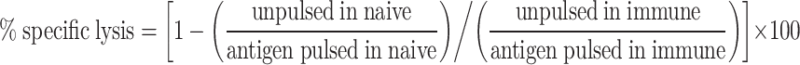 |
Metastatic tumor model
BALB/c-derived CT26 colorectal cancer cells were from ATCC. The GUCY2C1–461-expressing CT26 (CT26-GUCY2CTM) cell line was described previously [9]. CT26 cells expressing full-length GUCY2C (CT26-GUCY2C) were similarly produced by retroviral transduction and selection. BALB/c mice were immunized 7 days prior to the administration of 5 × 105 CT26 cells via tail vein injection to establish lung metastases. For PET/CT, mice received 0.45 mCi18F-fluorodeoxyglucose 17 days after tumor challenge, and PET images were collected 2 h later on a Mosaic scanner (Philips Medical Systems, Andover, Massachusetts). CT images were acquired on a microCAT II (Imtek, Inc, Knoxville, Tennessee). Other mice were euthanized and metastases enumerated 17 days after challenge [45].
Statistical analysis
Differences between peptides in ELISpot assays were analyzed by one-way ANOVA using Dunnett’s multiple comparison test in which DMSO served as control. For ELISpot assays employing L929 cells or splenocytes as APCs, one-way ANOVA with Bonferroni’s multiple comparison test was used, comparing peptide-pulsed to DMSO-pulsed for each APC group. Differences between immunizations in in vivo CTL assays were analyzed by one-way ANOVA using Dunnett’s multiple comparison test in which naive mice served as control. Tumor enumeration employed Student’s t test (Fig. 1) or one-way ANOVA using Tukey’s multiple comparison test (Fig. 5). Statistical analyses were carried out using GraphPad Prism Software v5 (La Jolla, California).
Acknowledgments
We would like to thank Dr. Mathew Thakur and the members of the Small Animal Imaging Core Facility, Thomas Jefferson University, for their assistance in PET/CT imaging. Financial support was provided by the National Institutes of Health (CA75123, CA95026) and Targeted Diagnostic and Therapeutics Inc. (to S.A.W.); Measey Foundation Fellowship (to A.E.S.); S.A.W is the Samuel M.V. Hamilton Endowed Professor. This project is funded, in part, by a grant from the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions. S.A.W is the chair of the Data Safety Monitoring Board for the C-Cure Trial™ sponsored by Cardio Biosciences, and the chair (uncompensated) of the Scientific Advisory Board to Targeted Diagnostics and Therapeutics, Inc., which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
Abbreviations
- CMA
Cancer mucosa antigen
- GMP
Guanosine monophosphate
- GUCY2C
Guanylyl cyclase C
- PKG
Protein kinase G
References
- 1.DeFrancesco L. Landmark approval for Dendreon’s cancer vaccine. Nat Biotechnol. 2010;28(6):531–532. doi: 10.1038/nbt0610-531. [DOI] [PubMed] [Google Scholar]
- 2.Snook AE, Eisenlohr LC, Rothstein JL, Waldman SA. Cancer Mucosa antigens as a novel immunotherapeutic class of tumor-associated antigen. Clin Pharmacol Ther. 2007;82(6):734–739. doi: 10.1038/sj.clpt.6100369. [DOI] [PubMed] [Google Scholar]
- 3.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52(3):375–414. [PubMed] [Google Scholar]
- 4.Carrithers SL, Barber MT, Biswas S, Parkinson SJ, Park PK, Goldstein SD, Waldman SA. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc Natl Acad Sci USA. 1996;93(25):14827–14832. doi: 10.1073/pnas.93.25.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birbe R, Palazzo JP, Walters R, Weinberg D, Schulz S, Waldman SA. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum Pathol. 2005;36(2):170–179. doi: 10.1016/j.humpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Schulz S, Hyslop T, Haaf J, Bonaccorso C, Nielsen K, Witek ME, Birbe R, Palazzo J, Weinberg D, Waldman SA. A validated quantitative assay to detect occult micrometastases by reverse transcriptase-polymerase chain reaction of guanylyl cyclase C in patients with colorectal cancer. Clin Cancer Res. 2006;12(15):4545–4552. doi: 10.1158/1078-0432.CCR-06-0865. [DOI] [PubMed] [Google Scholar]
- 7.Waldman SA, Hyslop T, Schulz S, Barkun A, Nielsen K, Haaf J, Bonaccorso C, Li Y, Weinberg DS. Association of GUCY2C expression in lymph nodes with time to recurrence and disease-free survival in pN0 colorectal cancer. Jama. 2009;301(7):745–752. doi: 10.1001/jama.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson MR. Previstage GCC colorectal cancer staging test: a new molecular test to identify lymph node metastases and provide more accurate information about the stage of patients with colorectal cancer. Mol Diagn Ther. 2009;13(1):11–14. doi: 10.2165/01250444-200913010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Snook AE, Stafford BJ, Li P, Tan G, Huang L, Birbe R, Schulz S, Schnell MJ, Thakur M, Rothstein JL, Eisenlohr LC, Waldman SA. Guanylyl cyclase C-induced immunotherapeutic responses opposing tumor metastases without autoimmunity. J Natl Cancer Inst. 2008;100(13):950–961. doi: 10.1093/jnci/djn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snook AE, Li P, Stafford BJ, Faul EJ, Huang L, Birbe RC, Bombonati A, Schulz S, Schnell MJ, Eisenlohr LC, Waldman SA. Lineage-specific T-cell responses to cancer mucosa antigen oppose systemic metastases without mucosal inflammatory disease. Cancer Res. 2009;69(8):3537–3544. doi: 10.1158/0008-5472.CAN-08-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cagir B, Gelmann A, Park J, Fava T, Tankelevitch A, Bittner EW, Weaver EJ, Palazzo JP, Weinberg D, Fry RD, Waldman SA. Guanylyl cyclase C messenger RNA is a biomarker for recurrent stage II colorectal cancer. Ann Intern Med. 1999;131(11):805–812. doi: 10.7326/0003-4819-131-11-199912070-00002. [DOI] [PubMed] [Google Scholar]
- 12.Waldman SA, Cagir B, Rakinic J, Fry RD, Goldstein SD, Isenberg G, Barber M, Biswas S, Minimo C, Palazzo J, Park PK, Weinberg D. Use of guanylyl cyclase C for detecting micrometastases in lymph nodes of patients with colon cancer. Dis Colon Rectum. 1998;41(3):310–315. doi: 10.1007/BF02237484. [DOI] [PubMed] [Google Scholar]
- 13.Rammensee HG, Friede T, Stevanoviic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41(4):178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 14.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152(1):163–175. [PubMed] [Google Scholar]
- 15.Einarsdottir T, Lockhart E, Flynn JL. Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection. Infect Immun. 2009;77(10):4621–4630. doi: 10.1128/IAI.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKelvey T, Tang A, Bett AJ, Casimiro DR, Chastain M. T-cell response to adenovirus hexon and DNA-binding protein in mice. Gene Ther. 2004;11(9):791–796. doi: 10.1038/sj.gt.3302232. [DOI] [PubMed] [Google Scholar]
- 17.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161(10):5338–5346. [PubMed] [Google Scholar]
- 18.Aichele P, Brduscha-Riem K, Oehen S, Odermatt B, Zinkernagel RM, Hengartner H, Pircher H. Peptide antigen treatment of naive and virus-immune mice: antigen-specific tolerance versus immunopathology. Immunity. 1997;6(5):519–529. doi: 10.1016/S1074-7613(00)80340-4. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Zhang M, Zhou C, Zhao X, Iijima N, Frankel FR. Novel vaccination protocol with two live mucosal vectors elicits strong cell-mediated immunity in the vagina and protects against vaginal virus challenge. J Immunol. 2008;180(4):2504–2513. doi: 10.4049/jimmunol.180.4.2504. [DOI] [PubMed] [Google Scholar]
- 20.Buckwalter MR, Srivastava PK. “It is the antigen(s), stupid” and other lessons from over a decade of vaccitherapy of human cancer. Semin Immunol. 2008;20(5):296–300. doi: 10.1016/j.smim.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrithers SL, Parkinson SJ, Goldstein S, Park P, Robertson DC, Waldman SA. Escherichia coli heat-stable toxin receptors in human colonic tumors. Gastroenterology. 1994;107(6):1653–1661. doi: 10.1016/0016-5085(94)90804-4. [DOI] [PubMed] [Google Scholar]
- 22.Conzelmann M, Dieterle CP, Linnemann U, Berger MR. Cytokeratin 20 and guanylyl cyclase C mRNA is largely present in lymph node and liver specimens of colorectal cancer patients. Int J Cancer. 2003;107(4):617–628. doi: 10.1002/ijc.11425. [DOI] [PubMed] [Google Scholar]
- 23.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 24.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393(6684):480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 25.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 26.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393(6684):474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 27.Buller RM, Holmes KL, Hugin A, Frederickson TN, Morse HC., III Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987;328(6125):77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- 28.Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353(6340):180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y, Liu Y. Viral induction of co-stimulatory activity on antigen-presenting cells bypasses the need for CD4+ T-cell help in CD8+ T-cell responses. Current biology: CB. 1994;4(6):499–505. doi: 10.1016/S0960-9822(00)00110-X. [DOI] [PubMed] [Google Scholar]
- 30.Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12(5):505–514. doi: 10.1016/S1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 31.Vezys V, Lefrancois L. Cutting edge: inflammatory signals drive organ-specific autoimmunity to normally cross-tolerizing endogenous antigen. J Immunol. 2002;169(12):6677–6680. doi: 10.4049/jimmunol.169.12.6677. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Lefrancois L. Intestinal epithelial antigen induces mucosal CD8 T cell tolerance, activation, and inflammatory response. J Immunol. 2004;173(7):4324–4330. doi: 10.4049/jimmunol.173.7.4324. [DOI] [PubMed] [Google Scholar]
- 33.Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188(2):409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titu LV, Monson JR, Greenman J. The role of CD8(+) T cells in immune responses to colorectal cancer. Cancer Immunol Immunother. 2002;51(5):235–247. doi: 10.1007/s00262-002-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 36.Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67(5):1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 37.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 38.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 39.Mocellin S, Rossi CR, Lise M, Nitti D. Colorectal cancer vaccines: principles, results, and perspectives. Gastroenterology. 2004;127(6):1821–1837. doi: 10.1053/j.gastro.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, Myers KA, Drury N, Kingsman SM, Hawkins RE, Carroll MW. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12(11):3416–3424. doi: 10.1158/1078-0432.CCR-05-2732. [DOI] [PubMed] [Google Scholar]
- 41.Conry RM, Khazaeli MB, Saleh MN, Allen KO, Barlow DL, Moore SE, Craig D, Arani RB, Schlom J, LoBuglio AF. Phase I trial of a recombinant vaccinia virus encoding carcinoembryonic antigen in metastatic adenocarcinoma: comparison of intradermal versus subcutaneous administration. Clin Cancer Res. 1999;5(9):2330–2337. [PubMed] [Google Scholar]
- 42.Nagorsen D, Thiel E. Clinical and immunologic responses to active specific cancer vaccines in human colorectal cancer. Clin Cancer Res. 2006;12(10):3064–3069. doi: 10.1158/1078-0432.CCR-05-2788. [DOI] [PubMed] [Google Scholar]
- 43.Colorectal Cancer (2003) In: Stewart BW, Kleihues P (eds) World cancer report. International Agency for Research on Cancer, Lyon, pp 198–202
- 44.Schafer H, Schafer A, Kiderlen AF, Masihi KN, Burger R. A highly sensitive cytotoxicity assay based on the release of reporter enzymes, from stably transfected cell lines. J Immunol Methods. 1997;204(1):89–98. doi: 10.1016/s0022-1759(97)00040-9. [DOI] [PubMed] [Google Scholar]
- 45.Mule JJ, Shu S, Schwarz SL, Rosenberg SA. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984;225(4669):1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]