The R2R3 MYB activator WP1 associates with MtTT8 and MtWD40-1 and regulates floral carotenoid pigmentation by directly modulating carotenogenic gene expression in Medicago truncatula.
Abstract
Carotenoids are a group of natural tetraterpenoid pigments with indispensable roles in the plant life cycle and the human diet. Although the carotenoid biosynthetic pathway has been well characterized, the regulatory mechanisms that control carotenoid metabolism, especially in floral organs, remain poorly understood. In this study, we identified an anthocyanin-related R2R3-MYB protein, WHITE PETAL1 (WP1), that plays a critical role in regulating floral carotenoid pigmentation in Medicago truncatula. Carotenoid analyses showed that the yellow petals of the wild-type M. truncatula contained high concentrations of carotenoids that largely consisted of esterified lutein and that disruption of WP1 function via Tnt1 insertion led to substantially reduced lutein accumulation. WP1 mainly functions as a transcriptional activator and directly regulates the expression of carotenoid biosynthetic genes including MtLYCe and MtLYCb through its C-terminal acidic activation motif. Further molecular and genetic analyses revealed that WP1 physically interacts with MtTT8 and MtWD40-1 proteins and that this interaction facilitates WP1’s function in the transcriptional activation of both carotenoid and anthocyanin biosynthetic genes. Our findings demonstrate the molecular mechanism of WP1-mediated regulation of floral carotenoid pigmentation and suggest that the conserved MYB-basic-helix-loop-helix-WD40 regulatory module functions in carotenoid biosynthesis in M. truncatula, with specificity imposed by the MYB partner.
INTRODUCTION
Carotenoids are important secondary metabolites widely distributed in plants, algae, and certain types of bacteria and fungi (Grotewold, 2006; Nisar et al., 2015; Sun et al., 2018). Besides their critical functions in human nutrition and health (Fraser and Bramley, 2004), carotenoids play essential roles in the structure and function of the photosynthetic apparatus and provide precursors for the biosynthesis of phytohormones (Niyogi et al., 1997; Holt et al., 2005; Nambara and Marion-Poll, 2005; Al-Babili and Bouwmeester, 2015). Moreover, carotenoids impart vivid colors ranging from yellow to red in flowers and fruits, and these colors fulfill an important ecological function by attracting pollinator visitation and influencing reproductive success in flowering plants (Nisar et al., 2015; Sun et al., 2018).
Plant carotenoid biosynthesis has been studied extensively, and most key genes and enzymes in the carotenoid biosynthetic pathway have been well characterized (Fraser et al., 1994; Moise et al., 2014; Nisar et al., 2015; Sun et al., 2018). The first committed step for carotenoid biosynthesis involves the condensation of two geranylgeranyl diphosphate (GGPP) molecules into phytoene, catalyzed by phytoene synthase (PSY). The colorless phytoene is then subjected to a series of desaturation and isomerization reactions catalyzed by phytoene desaturase (PDS), ζ-carotene desaturase (ZDS), and carotenoid isomerase (CRTISO), resulting in the red pigment lycopene. Subsequent cyclization of lycopene by lycopene β-cyclase (LYCb) alone or with lycopene ε-cyclase (LYCe) produces orange β-carotene and α-carotene, respectively. These cyclized carotenes are further oxygenated by β-carotene hydroxylase (BCH), cytochromes P450 (CYP97A and CYP97C), zeaxanthin epoxidase, violaxanthin de-epoxidase, and neoxanthin synthase to yield yellow xanthophylls including lutein, zeaxanthin, antheraxanthin, violaxanthin, and neoxanthin. Further modification of xanthophylls and carotenes generates various species-specific carotenoids in diverse plants (Giuliano, 2014, 2017; Sun et al., 2018). Despite this well-established biosynthetic pathway, the regulatory control of carotenogenesis remains poorly understood.
Extensive studies have indicated that the diversity of carotenoid pigmentation in plants is largely associated with differential expression of carotenoid biosynthetic genes (Moehs et al., 2001; Ha et al., 2007; Chiou et al., 2010; Yamamizo et al., 2010). Recently, several families of transcription factors (TFs) have been demonstrated to directly regulate the carotenogenic genes and control carotenoid biosynthesis in diverse plant species. In Arabidopsis (Arabidopsis thaliana), the basic-helix-loop-helix (bHLH) TF PHYTOCHROME INTERACTING FACTOR1 (PIF1) and the basic leucine zipper TF LONG HYPOCOTYL5 (HY5) antagonistically regulate carotenoid accumulation by directly binding to the promoter of PSY in response to light signaling (Toledo-Ortiz et al., 2010, 2014). The APETALA2 (AP2)/ethylene-responsive element binding protein RAP2.2 has also been implicated in binding the cis-acting element ATCTA in the promoter of PSY (Welsch et al., 2007). Recently, CsMADS6 was found to directly activate LCYb1 expression in Citrus sinensis. Overexpression of CsMADS6 significantly increased carotenoid accumulation in Citrus calli and induced the expression of the carotenogenic genes LCYb1, PSY, and PDS (Lu et al., 2018). Although progress has been made in understanding the transcriptional control of carotenoid accumulation in diverse plants, little is known about the regulation of floral carotenoid pigmentation.
The R2R3-MYB proteins comprise one of the largest TF families and play essential roles in regulating primary and second metabolism in plants (Allan et al., 2008; Dubos et al., 2010). Previous studies have demonstrated that the R2R3-MYB TFs fulfill a key function in regulating the biosynthesis of proanthocyanidin (PA) and anthocyanin, the widely distributed flavonoid pigments that provide the red to blue colors in flowers and fruits, by interacting with bHLH and WD-repeat (WDR) proteins to form a conserved transcriptional activation complex (Baudry et al., 2004; Koes et al., 2005; Ramsay and Glover, 2005; Xu et al., 2015). R2R3-MYB TFs are emerging as modulators of carotenoid production. In Citrus reticulate, the R2R3-MYB protein CrMYB68 represses the biosynthesis of α- and β-branch carotenoids by downregulating CrBCH2 and CrNCED5 expression (Zhu et al., 2017). By contrast, disruption of the R2R3-MYB protein REDUCED CAROTENOID PIGMENTATION1 (RCP1) led to downregulation of all carotenoid biosynthetic genes and reduced carotenoid content in Mimulus lewisii flowers, indicating that RCP1 positively regulates carotenoid biosynthesis during flower development (Sagawa et al., 2016). Recent studies in kiwifruit (Actinidia deliciosa) revealed that AdMYB7 plays a role in modulating carotenoid accumulation by activating AdLCY-β expression (Ampomah-Dwamena et al., 2019), indicating that there may be a variety of MYBs from unrelated clades involved in carotenoid regulation in different plant species. Despite these enlightening reports, little is known about the regulatory mechanism of R2R3-MYB TFs in the regulation of carotenoid metabolism.
Medicago truncatula is a model legume species with vivid yellow flowers, generally assumed to be pigmented by carotenoids, with fine red veins of anthocyanin accumulation in the center of the vexillum petals (Xie et al., 2004; Jun et al., 2015). The availability of abundant floral pigment mutants in M. truncatula provides an ideal system for investigating the regulation of flower color and floral carotenoid pigmentation. Here, we report the identification and characterization of white petal1 (wp1) from an M. truncatula mutant that is defective in floral pigmentation. WP1 encodes a subgroup 6 R2R3-MYB TF that positively regulates lutein and anthocyanin accumulation in M. truncatula petals by directly activating carotenoid and anthocyanin biosynthetic genes. Our results demonstrate that WP1 physically interacts with the M. truncatula bHLH protein MtTT8 and WDR family member MtWD40-1 and that this interaction facilitates WP1 function both in floral carotenoid and anthocyanin production, suggesting that the MYB-bHLH-WDR (MBW) regulatory module plays a key role in carotenogenesis that is analogous to its role in the control of flavonoid biosynthesis.
RESULTS
Identification of the wp1 Mutants of M. truncatula
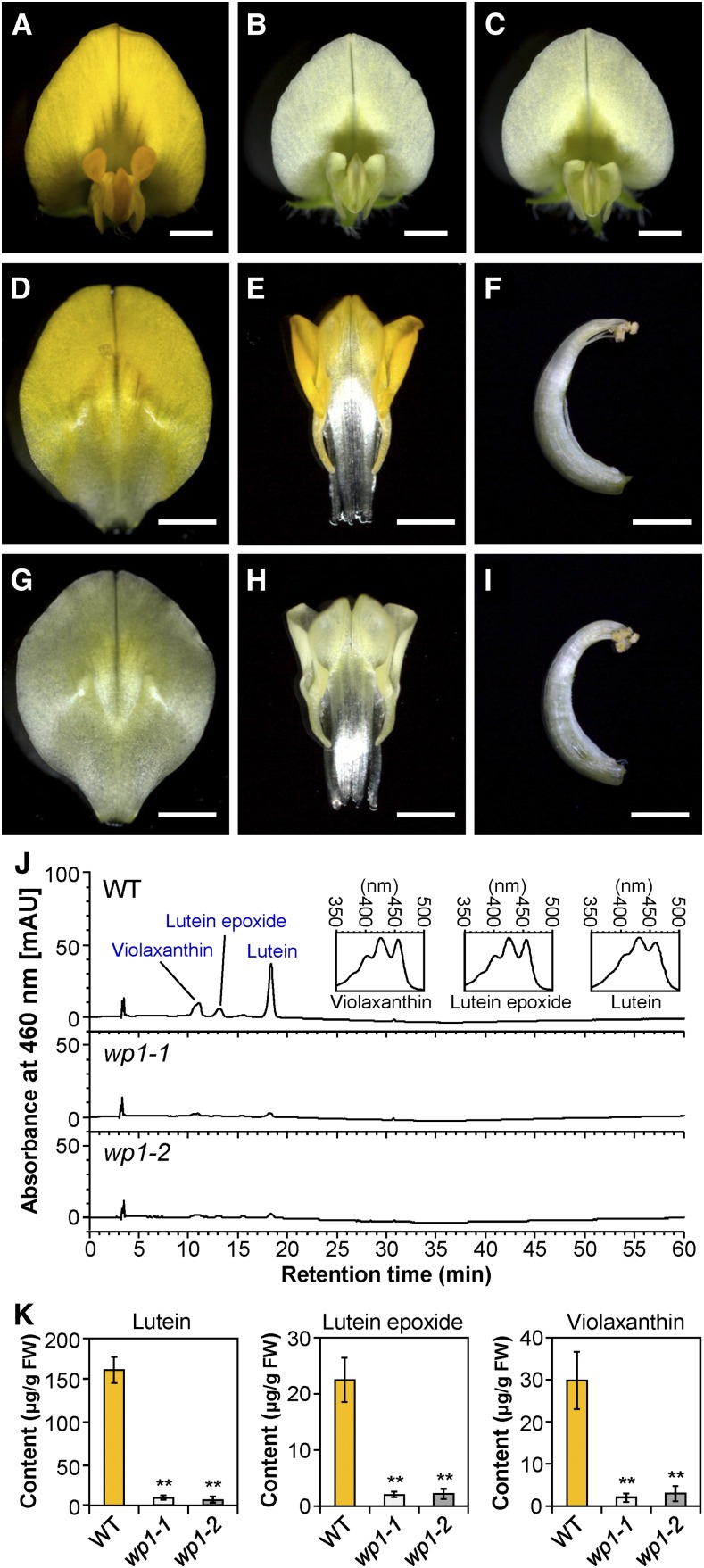
Two phenotypically similar loss-of-floral pigment mutants named wp1-1 and wp1-2 were identified by screening the M. truncatula Tnt1 insertion mutant population for visible white-flower phenotypes (Figures 1A to 1C). Genetic analysis revealed that wp1-1 and wp1-2 are allelic mutants (Supplemental Figure 1). In contrast to the wild-type R108, which has yellow petals, the wp1 mutants lack the typical yellow pigmentation in all vexillum, keel, and alae petals, which appear nearly white, but no obvious pigment loss was observed in anthers (Figures 1D to 1I). To examine whether the reduced petal coloration in the wp1 mutants was due to a reduction in carotenoid accumulation, we analyzed the carotenoid profiles of the wild-type, wp1-1, and wp1-2 petals. HPLC analysis with or without saponification revealed that the yellow petals of the wild-type M. truncatula contained high levels of carotenoids that largely consisted of esterified lutein but that the levels of lutein were drastically reduced in both wp1-1 and wp1-2 (Figures 1J and 1K; Supplemental Figures 2 and 3). In addition, the contents of lutein epoxide and violaxanthin were also decreased in the petals of wp1 mutants (Figures 1J and 1K). These results indicate that the wp1 mutants fail to accumulate lutein in petals and that WP1 function is highly relevant to carotenoid biosynthesis during M. truncatula flower development.
Figure 1.
Phenotype Analysis of wp1 Flowers.
(A) to (C) Flowers of the wild type (A), wp1-1 (B), and wp1-2 (C). Bars = 1.5 mm.
(D) to (F) Dissected flowers of wild type. The vexillum petal (D), the fused alae and keel petals (E), and the stamens (F) of the wild type. Bars = 1.5 mm.
(G) to (I) Dissected flowers of wp1-1. The vexillum petal (G), the fused alae and keel petals (H), and the stamens (I) of wp1-1. Bars = 1.5 mm.
(J) HPLC chromatograms and characteristic absorbance spectra of the extracted and saponified carotenoids from petals of the wild type and two wp1 mutants. The peaks for lutein, lutein epoxide, and violaxanthin are marked. Insets are spectra of indicated peaks. mAU, milli-absorbance unit; WT, wild type.
(K) Contents of lutein, lutein epoxide, and violaxanthin in petals of the wild type and two wp1 mutants. Bars represent means ± sd of three biological replicates. Asterisks indicate differences from the wild type (**P < 0.01, Student’s t test). FW, fresh weight; WT, wild type.
Molecular Cloning and Characterization of WP1
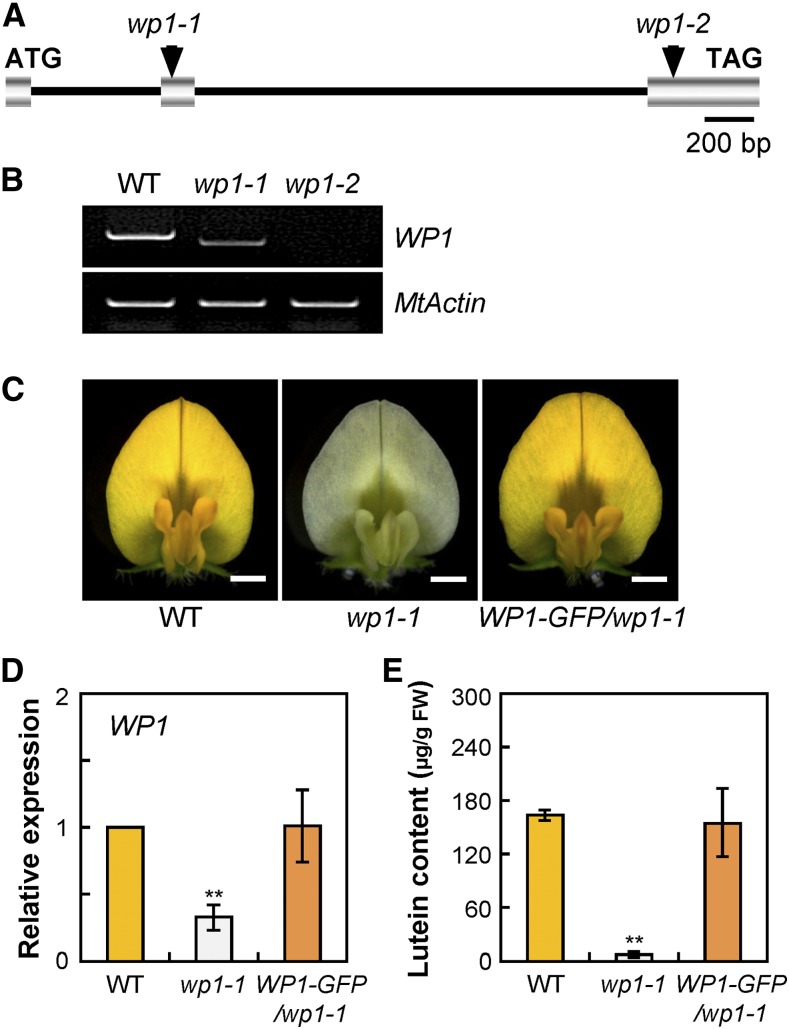
The WP1 gene was cloned by PCR-based genotyping of flanking sequence tags (FSTs) in segregating populations (Tadege et al., 2008). Flanking sequence analysis of the Tnt1 retrotransposon in the wp1-1 mutant by PCR-based genotyping revealed that one flanking sequence, FST 6, segregated with the mutant phenotype. The wp1-1 mutant phenotype segregated as a single Mendelian recessive mutation (i.e., heterozygous parents produced progeny that segregated 3:1 [71:25] for the wild-type-like and mutant plants), and all mutant plants were homozygous for FST 6. The full-length gene sequence corresponding to this particular FST was recovered and designated to represent the WP1 gene. The genomic sequence of WP1 contains three exons, and the Tnt1 retrotransposon in wp1-1 is inserted at the middle of exon 2 (Figure 2A). RT-PCR and sequencing analyses showed that this Tnt1 insertion led to an aberrantly spliced WP1 mRNA lacking exon 2 (130 bp), which resulted in a frameshift and generated a premature translation termination product (Figure 2B; Supplemental Figure 4). The transcript level of this exon-skipping isoform of WP1 was severely decreased in wp1-1 (Figure 2B). We further confirmed that in the wp1-2 mutant, the presence of a Tnt1 insertion in the third exon of WP1, 340 bp upstream of the translational stop, cosegregated with the phenotype (Figure 2A). RT-PCR analysis revealed that the full-length WP1 transcript was absent in the wp1-2 allele (Figure 2B). The identity of WP1 was further confirmed by genetic complementation. A construct (WP1-GFP) including a 3.0-kb WP1 promoter region, the entire WP1 genomic DNA sequence, a GFP gene fusing hemagglutinin tag, and the 1.5-kb downstream region of WP1 was introduced into wp1-1 plants by Agrobacterium tumefaciens-mediated transformation (Supplemental Figure 5). Phenotypic analysis showed that the floral pigmentation phenotypes of wp1-1 were complemented by the WP1-GFP transgene in all three transgenic lines and comparable to those of the wild type (Figure 2C). Molecular and HPLC analyses indicated that the WP1 transcript levels and lutein concentration were fully restored to the wild-type levels in WP1-GFP complementation plants (Figures 2D and 2E). Collectively, these data confirm that disruption of WP1 function results in the floral carotenoid pigmentation defects of wp1 mutants.
Figure 2.
Molecular Cloning and Confirmation of the WP1 Gene.
(A) Schematic representation of the gene structure of WP1 showing the Tnt1 insertion sites in wp1-1 and wp1-2. Introns are represented by a line, and exons are represented by a striped box.
(B) RT-PCR showing transcript abundance of WP1 in the flowers of the wild type and wp1 mutants. MtActin was used as the control. WT, wild type.
(C) Genetic complementation of wp1-1. Representative flowers of the wild type, wp1-1, and wp1-1 complemented with WP1-GFP (WP1-GFP/wp1-1). Bars = 1.5 mm. WT, wild type.
(D) Transcript levels of WP1 in petals of the wild type, wp1-1, and WP1-GFP/wp1-1. Bars represent means ± sd of three biological replicates. Asterisks indicate differences from the wild type (**P < 0.01, Student’s t test). WT, wild type.
(E) Lutein concentrations in petals of the wild type, wp1-1, and WP1-GFP/wp1-1. Bars represent means ± sd of three biological replicates. Asterisks indicate differences from the wild type (**P < 0.01, Student’s t test). WT, wild type.
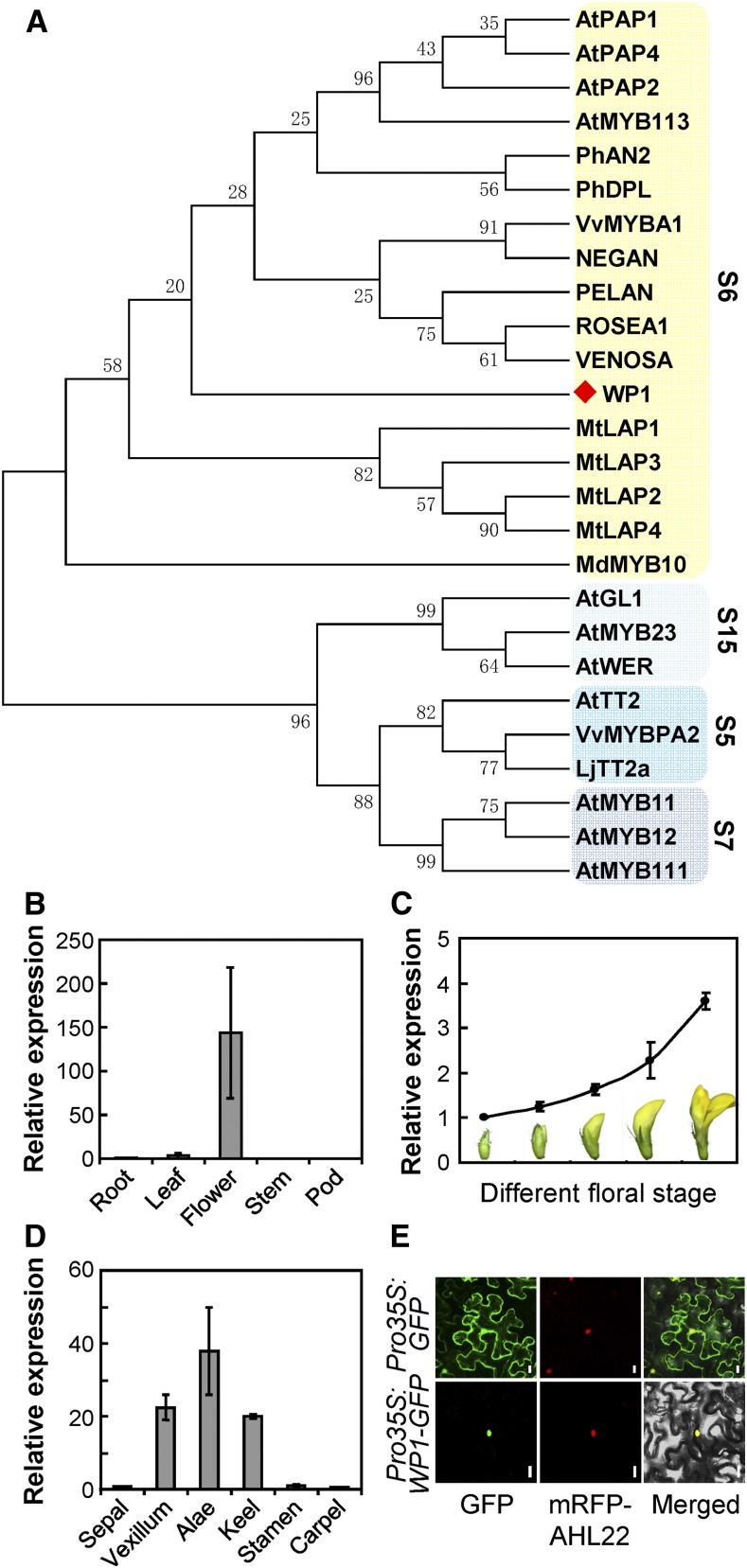
WP1 encodes a MYB TF belonging to the R2R3 class, based on the presence of conserved R2 and R3 MYB DNA binding domains (Stracke et al., 2001; Dubos et al., 2010). In a phylogenetic analysis using the R2R3 domains, WP1 was clustered with MYBs involved in the regulation of anthocyanin (subgroup 6; Figure 3A; Supplemental Data Set 1). A protein BLAST search against the National Center for Biotechnology Information (NCBI) database of Arabidopsis showed that the closest match for WP1 is AtMYB113, which controls anthocyanin regulation. WP1 was also clustered with subgroup 6 when analyzed using the web-based tool IT3F (An Interspecies Transcription Factor Function Finder for Plant). Accordingly, sequence analysis showed that WP1 contains the conserved bHLH-interacting motif and the ANDV motif within the R3 domain immediately following the R2 domain, and the [R/K]Px[P/A/R]xx[F/Y] motif downstream of the conserved R2 and R3 MYB DNA binding domains (Supplemental Figure 6), all of which are defining features of subgroup 6 MYBs (Stracke et al., 2001; Zimmermann et al., 2004; Dubos et al., 2010; Lin-Wang et al., 2010).
Figure 3.
Phylogenetic Analysis of the WP1 Protein and Expression Pattern of WP1.
(A) Phylogenetic analysis of WP1 (marked with a red diamond) and other MYBs using R2R3 MYB domains. Subgroup (S) names are indicated on the right, and the subgroups are shaded.
(B) to (D) RT-qPCR analysis of WP1 expression in various tissues (B), different floral stages (C), and dissected floral organs (D). Bars represent means ± sd of three biological replicates.
(E) Subcellular localization of WP1-GFP in N. benthamiana leaf epidermal cells. AHL22 was used as a nuclear marker. Bars = 20 μm.
Expression Patterns of WP1 and Subcellular Localization of the WP1 Protein
RT-qPCR analysis of different tissues revealed that WP1 expression is primarily detected in the flowers and that transcript abundance is highest at the mature floral stage (Figures 3B and 3C). Further RT-qPCR analysis of dissected floral organs showed that WP1 is predominantly expressed in the vexillum, keel, and alae petals (Figure 3D), which is consistent with WP1 function in the regulation of M. truncatula petal carotenoid pigmentation.
To determine the subcellular localization of WP1, we generated a Pro35S:WP1-GFP fusion construct and coexpressed it with the nuclear marker monomeric red fluorescent protein (mRFP)-AHL22 (Wang et al., 2013) in Nicotiana benthamiana leaf epidermal cells. Using fluorescence microscopy, we observed that the WP1-GFP fusion protein colocalized with the nuclear marker mRFP-AHL22 (Figure 3E), confirming that WP1 functions as a nuclear-localized transcription regulator.
WP1 Functions as a Transcriptional Activator
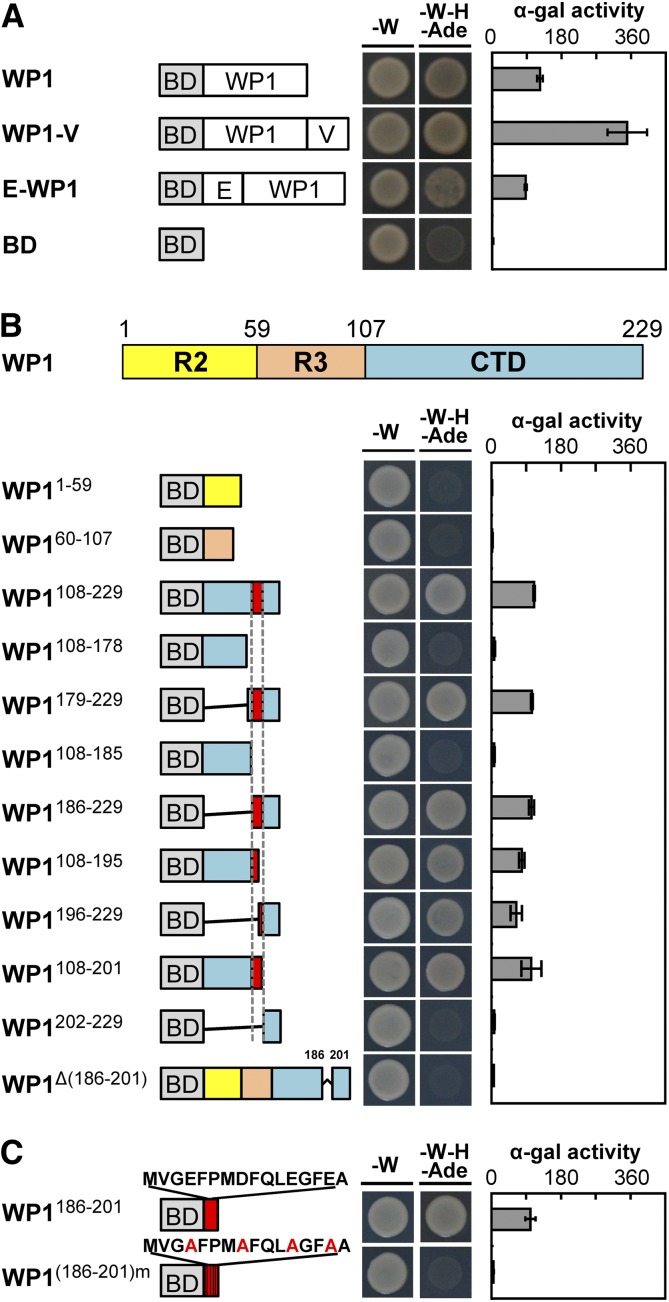
To gain insight into how WP1 regulates floral carotenoid pigmentation, we performed a transactivation activity assay in yeast (Saccharomyces cerevisiae) to test the transcriptional activation activity of WP1. The full-length WP1 was fused in-frame with the GAL4 DNA binding domain (BD) in the pGBKT7-GW vector, and the resultant construct was transformed into the Gold yeast strain. The growth of yeast carrying pGBKT7-WP1 on selective medium (SD/−Trp/−His/−Ade) along with an α-galactosidase assay indicated that WP1 protein has strong transcriptional activation activity compared with the empty pGBKT7-GW vector used as the negative control (Figure 4A). Furthermore, fusion of an exogenous activation domain, VP64, to WP1 enhanced its activation activity, whereas fusion of an exogenous repression module, EAR4 (tetrameric repeats of EAR), to WP1 diminished this activation (Figure 4A), confirming that WP1 mainly acts as a transcriptional activator.
Figure 4.
WP1 Acts as a Transcriptional Activator.
(A) Transactivation analysis of WP1, WP1-VP64, and EAR4-WP1 using a yeast assay. VP64 and EAR4 are an exogenous activation domain and an exogenous repression module, respectively. The GAL4 DNA BD alone was used as the negative control. Plate auxotroph and α-galactosidase assay showing transcriptional activation of each protein. Bars represent means ± sd of three independent experiments. α-gal, α-galactosidase; E, EAR4; V, VP64; -W, SD/−Trp; -W-H-Ade, SD/−Trp/−His/−Ade.
(B) Mapping of the transactivation motif of WP1 using a yeast assay. R2, amino acids 1 to 59; R3, amino acids 60 to 107; CTD, amino acids 108 to 229. Plate auxotroph and α-galactosidase assay showing transcriptional activation of each protein. Bars represent means ± sd of three independent experiments. α-gal, α-galactosidase; BD, GAL4 DNA binding domain; -W, SD/−Trp; -W-H-Ade, SD/−Trp/−His/−Ade.
(C) Transactivation analysis of the C-terminal motif MVGEFPMDFQLEGFEA using a yeast assay. BD stands for the GAL4 DNA binding domain. Mutations introduced into the C-terminal motif are indicated by red font. Plate auxotroph and α-galactosidase (α-gal) assay showing transcriptional activation by each protein. Bars represent means ±sd of three independent experiments. α-gal, α-galactosidase; -W, SD/−Trp; -W-H-Ade, SD/−Trp/−His/−Ade.
To explore how the structure of WP1 influences its transcriptional activation activity, we divided the WP1 protein into three major parts based on conserved domain analysis: the R2 repeat domain (R2; amino acids 1 to 59), R3 repeat domain (R3; amino acids 60 to 107), and C-terminal domain (CTD; amino acids 108 to 229; Figure 4B). Analyzing individual and combined domain deletions in the yeast assay indicated that the CTD, especially amino acids 186 to 229, is essential for the activation activity of WP1 (Figure 4B).
Further examination of the activation activity in different CTD deletion mutants revealed that a 16-amino acid activation motif, MVGEFPMDFQLEGFEA, located at the CTD and including amino acids 186 to 201, is important for the activation activity of WP1. Complete or partial deletion of this activation motif led to abolition or reduction, respectively, of WP1’s transactivation activity (Figure 4B). Notably, the MVGEFPMDFQLEGFEA motif on its own showed strong transcriptional activation activity, but mutations of acidic amino acid residues in the activation motif (Glu/Asp to Ala amino acid substitutions), similar to those reported by Ikeda et al. (2009), led to abolition of transactivation activity (Figure 4C), indicating that the acidic amino acids are critical for activation. Taken together, these data indicate that WP1 functions as a transcriptional activator and that the C-terminal motif MVGEFPMDFQLEGFEA is essential and sufficient for its activation activity in the yeast assay.
WP1 Directly Activates the Expression of Carotenogenic Genes
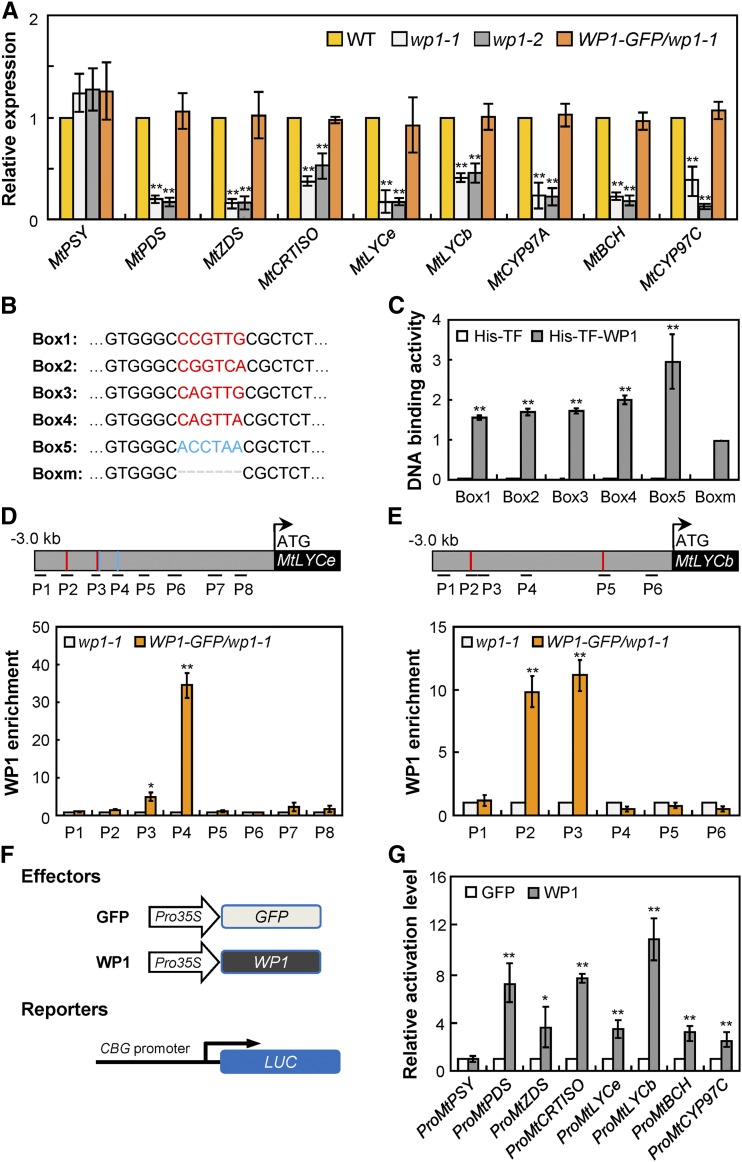
To determine the mechanism by which WP1 regulates floral carotenoid pigmentation in M. truncatula, we analyzed the transcript levels of carotenoid pathway genes in wp1 petals at the mature stage. RT-qPCR analysis showed that, with the exception of MtPSY, most carotenoid biosynthetic genes that are involved in the biosynthesis of lutein, including MtPDS, MtZDS, MtCRTISO, MtLYCe, MtLYCb, MtCYP97A, MtBCH, and MtCYP97C, were significantly downregulated in both wp1-1 and wp1-2 petals, while expression of these repressed genes was completely restored in WP1-complemented wp1-1 petals (Figure 5A). These data are consistent with the significant association of decreased expression of carotenoid biosynthetic genes with reduced floral lutein levels in wp1 mutants.
Figure 5.
WP1 Directly Activates the Expression of Carotenoid Biosynthesis Genes.
(A) Transcript levels of carotenoid biosynthesis genes in petals of the wild type, wp1-1, wp1-2, and WP1-GFP/wp1-1 revealed by RT-qPCR. Bars represent means ± sd of three biological replicates; asterisks indicate differences from the wild type (**P < 0.01, Student’s t test). WT, wild type.
(B) Putative MYB binding sites in the promoter regions of the carotenoid biosynthesis genes. The red and blue sequences correspond to the MYB-core and AC-rich element, respectively.
(C) DNA binding assay corresponding to the putative binding sites of WP1 shown in (B). The His-TFa protein was used as the negative control. Bars represent means ± sd of three biological replicates; asterisks indicate differences from Boxm (**P < 0.01, Student’s t test).
(D) and (E) ChIP assay showing the association of WP1 with several regions in the promoters of MtLYCe (D) and MtLYCb (E). The regions tested by ChIP assays are indicated in the schematic representation. The putative MYB-core and AC-rich elements are shown by red and blue lines, respectively. MtActin was used for normalization. Bars represent means ± sd of three biological replicates; asterisks indicate differences from wp1-1 (*P < 0.05, **P < 0.01, Student’s t test).
(F) Schematic representation of reporter and effector constructs used in transient expression assay. CBG, carotenoid biosynthesis gene.
(G) Transient expression assay in Arabidopsis protoplasts showing activation of carotenoid biosynthesis genes by the WP1 effector compared with the GFP control. Bars represent means ± sd of three biological replicates; asterisks indicate differences from the GFP control (*P < 0.05, **P < 0.01, Student’s t test).
Owing to the fact that WP1 acts as a transcriptional activator and noting the downregulated expression of carotenogenesis genes in wp1, we hypothesized that WP1 may directly regulate the expression of carotenogenesis genes and thus control floral carotenoid pigmentation. Plant R2R3-MYB family proteins have been reported to recognize and bind to the MYB-core element (C/T)NGTT(G/A) and AC-rich element ACC(A/T)(A/C/T)(A/C/T) (Gómez-Maldonado et al., 2004; Xu et al., 2015; Gao et al., 2016; Zhu et al., 2017). Sequence analysis using the PlantCARE program (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) predicted that one or multiple typical MYB binding sites (MYB-core and/or AC-rich elements) were present in the promoter regions of the downregulated carotenoid biosynthetic genes in wp1 (Figure 5B; Supplemental Figure 7). Using an in vitro DNA binding assay, we found that WP1 protein fused to a His and Trigger Factor tag (His-TFa-WP1) was able to bind to fragments containing these predicted MYB binding sequences, while binding was significantly diminished by deleting MYB-core and AC-rich elements (Figure 5C). These results indicated that WP1 activation of carotenoid biosynthetic genes may be mediated by binding to their specific promoter regions.
The direct in vivo binding of WP1 to the promoters of these carotenoid biosynthetic genes was further confirmed by chromatin immunoprecipitation (ChIP) assays. Using the WP1-GFP transgenic complementation plants and an anti-GFP antibody, we found that several of the promoter regions of the downregulated carotenogenesis genes MtLYCe and MtLYCb that contain MYB-core and AC-rich elements were significantly enriched in WP1-GFP chromatin (Figures 5D and 5E), indicating that WP1 directly binds to multiple regions on the promoters of these target genes, consistent with the in vitro DNA binding results.
To further examine whether WP1 could directly regulate the transcription of carotenogenesis genes, a transient expression assay was performed using the luciferase system in Arabidopsis leaf protoplasts (Figure 5F). We found that coexpression of the WP1 effector protein and luciferase reporter constructs driven by the endogenous promoters of MtPDS, MtZDS, MtCRTISO, MtLYCe, MtLYCb, MtBCH, and MtCYP97C, all of which are downregulated in wp1, resulted in significantly increased luminescence intensity compared to the GFP control (Figure 5G), while deletion of the C-terminal motif MVGEFPMDFQLEGFEA abolished WP1’s activation activity and consequently failed to activate downstream carotenogenesis genes (Supplemental Figure 8). On the other hand, no obvious activation was detected when the WP1 effector was coexpressed with the luciferase reporter driven by the promoter of MtPSY, whose expression was not downregulated in the wp1 mutants (Figures 5A and 5G). These results confirm that WP1 specifically recognizes the promoters of the downregulated carotenogenesis genes and can activate their expression in planta. These data together demonstrate that WP1 functions as a transcriptional activator that modulates floral carotenoid pigmentation by directly regulating the expression of multiple carotenoid biosynthetic genes in M. truncatula.
WP1 Physically Interacts with MtTT8 and MtWD40-1
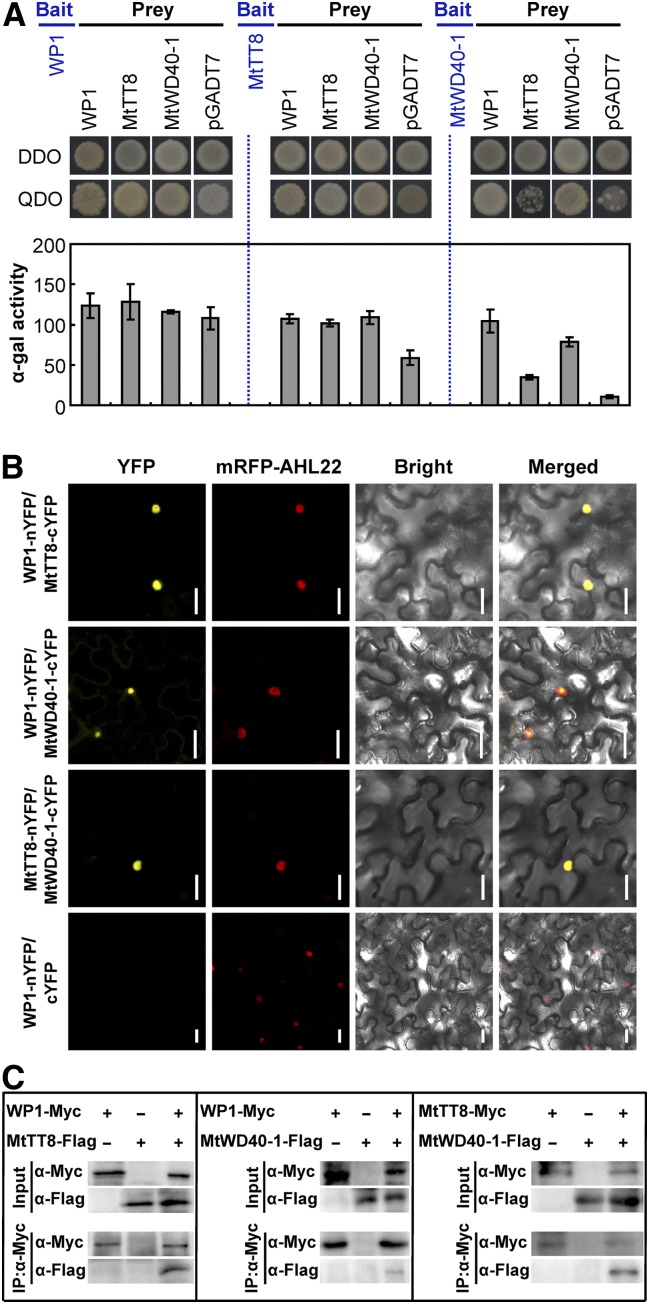
Previous studies have shown that the R2R3-MYB TFs regulate transcription by interacting with bHLH and WDR proteins to form a conserved MBW activator complex (Baudry et al., 2004; Koes et al., 2005; Ramsay and Glover, 2005; Xu et al., 2015). Given that WP1 protein contains the highly conserved bHLH-interacting motif, we examined whether WP1 functions within an MBW complex as well. In M. truncatula, the WDR protein MtWD40-1 and bHLH TF MtTT8 have been identified (Pang et al., 2009; Li et al., 2016) and shown to physically interact with several R2R3-MYB TFs to modulate flavonoid biosynthesis (Liu et al., 2014; Jun et al., 2015; Li et al., 2016). A yeast two-hybrid (Y2H) assay revealed that, despite WP1 and MtTT8 showing strong self-activation activities, different combinations of WP1, MtTT8, and MtWD40-1 exhibited interactions (Figure 6A). We also observed that WP1, MtTT8, and MtWD40-1 appear to form homodimers (Figure 6A).
Figure 6.
WP1 Interacts with MtTT8 and MtWD40-1 to Form an MBW Complex.
(A) Interaction between WP1, MtTT8, and MtWD40-1 in the Y2H assay. Plate auxotroph and α-galactosidase assay showing interaction of each protein. Bars represent means ± sd of three independent experiments. α-gal, α-galactosidase; DDO, double dropout; pGADT7, prey plasmid; QDO, quadruple dropout.
(B) Interaction between WP1, MtTT8, and MtWD40-1 in N. benthamiana leaf epidermal cells using a split YFP BiFC assay. AHL22 was used as a nuclear localization marker. Bars = 25 μm.
(C) Interaction between WP1, MtTT8, and MtWD40-1 in N. benthamiana using a Co-IP assay. Immunoblots of the total protein extracts (Input) and the IP product were performed using the anti-Myc antibody (α-Myc) or anti-Flag antibody (α-Flag), respectively.
These interactions and homodimerization among WP1, MtTT8, and MtWD40-1 were verified in N. benthamiana leaves by bimolecular fluorescence complementation (BiFC) assays using split yellow fluorescent protein (YFP; Figure 6B; Supplemental Figure 9). While sharp yellow fluorescence was clearly observed when WP1 was fused to the N-terminal half of YFP (nYFP) and MtTT8 or MtWD40-1 was fused to the C-terminal half (cYFP), or when MtTT8 and MtWD40-1 were fused to the two YFP halves (Figure 6B), no YFP fluorescence signal was detected in negative controls in which one-half of the split YFP (nYFP or cYFP) was fused to WP1, MtTT8, or MtWD40-1 and the other half was used alone (Figure 6B; Supplemental Figure 9).
The WP1-MtTT8-MtWD40-1 physical interaction was further confirmed in vivo by coimmunoprecipitation (Co-IP) assays. We transiently coexpressed Pro35S:WP1-Myc with Pro35S:MtTT8-Flag or Pro35S:MtWD40-1-Flag in N. benthamiana leaves. Total proteins were isolated and incubated with anti–C-MYC magnetic beads to immunoprecipitate WP1-Myc. Anti-Myc and anti-Flag antibodies were then used to detect immunoprecipitated proteins having the corresponding tag. MtTT8 or MtWD40-1 was detected in the immunoprecipitated WP1 complex, but not in the negative control without WP1-Myc input (Figure 6C), indicating that WP1 physically associates with MtTT8 and MtWD40-1 in planta. We also transiently coexpressed Pro35S:MtTT8-Myc with Pro35S:MtWD40-1-Flag in N. benthamiana leaves and detected the MtWD40-1-Flag protein in the immunoprecipitated MtTT8-Myc complex (Figure 6C), confirming the Y2H and BiFC results. Taken together, these data suggest that WP1 physically interacts with both MtTT8 and MtWD40-1 in vivo to potentially form an MBW complex.
MtTT8 and MtWD40-1 Are Involved in Floral Carotenoid Biosynthesis
MtTT8 and MtWD40-1 have been identified as essential regulators of anthocyanin and PA biosynthesis in seed coat and vegetative tissues (Pang et al., 2009; Li et al., 2016). Recent studies revealed that disruption of MtTT8 function also influences flower color (Li et al., 2016). Because MtTT8 and MtWD40-1 are strong interactors of WP1, we investigated their roles in floral carotenoid pigmentation by characterizing the mttt8 and mtwd40-1 mutants.
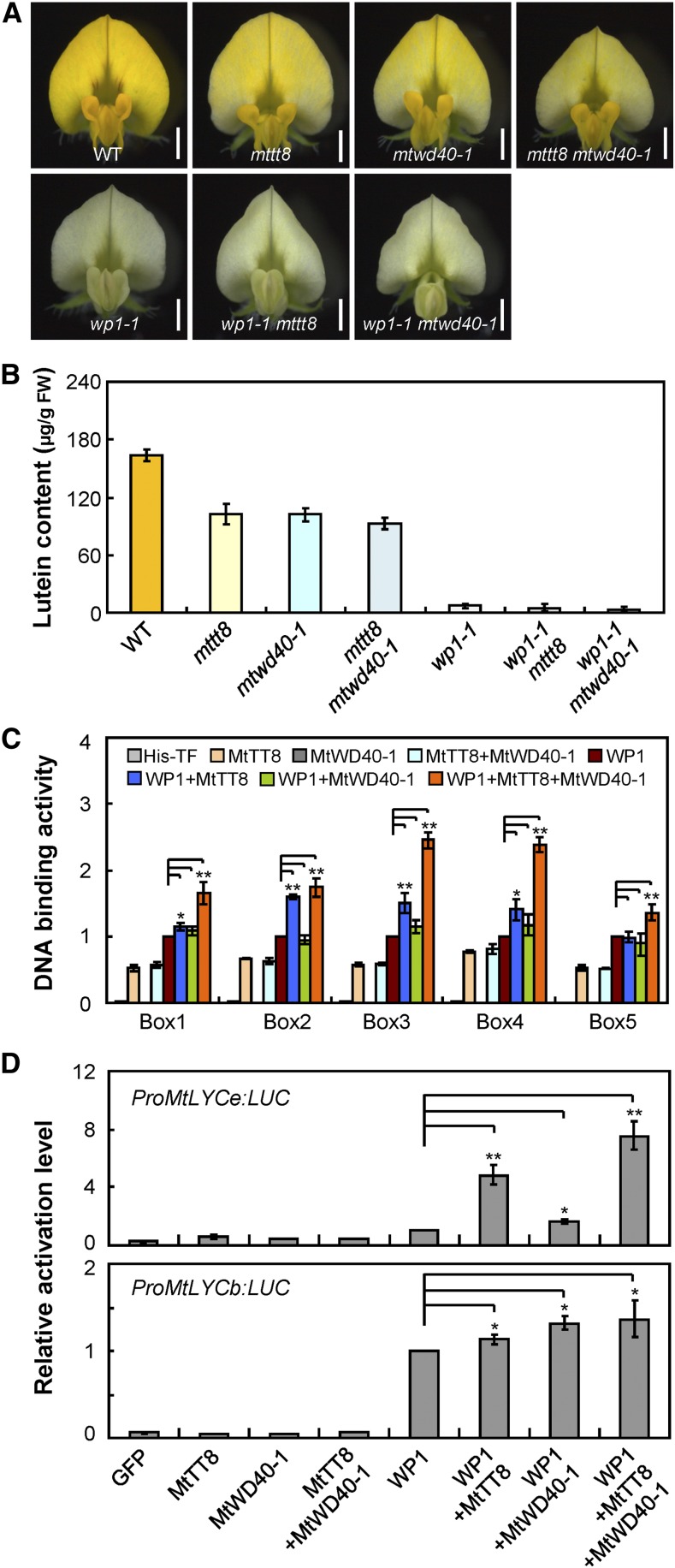
Consistent with previous findings (Pang et al., 2009; Li et al., 2016), the Tnt1 insertional loss-of-function mutants mttt8 and mtwd40-1 (Supplemental Figure 10) showed yellowish seed coat and lacked red pigmentation in leaflets and petioles as a result of reduced PA and anthocyanin accumulation, while seed coat and petiole pigmentations were normal in wp1 mutants and there were no obvious carotenoid profile changes in leaflets of wp1-1, mttt8, and mtwd40-1 relative to the wild type (Supplemental Figure 11). Interestingly, we found that both mttt8 and mtwd40-1 showed clearly pale-yellow petals with reduced lutein content compared with the vivid yellow petals of the wild type (Figures 7A and 7B). Consistent with the decreased lutein production, RT-qPCR analysis showed that transcripts of the carotenoid biosynthetic genes that were downregulated in wp1 (Figure 5A) were decreased in the petals of mttt8 and mtwd40-1 mutants as well (Supplemental Figure 12). Collectively, these results indicate that both MtTT8 and MtWD40-1 may be involved in floral carotenoid accumulation and affect carotenogenic gene expression in a manner similar to WP1.
Figure 7.
WP1 Associates with MtTT8 and MtWD40-1 to Modulate Floral Carotenoid Biosynthesis.
(A) Flowers of the wild type, mttt8, mtwd40-1, mttt8 mtwd40-1, wp1-1, wp1-1 mttt8, and wp1-1 mtwd40-1. Bars = 1.5 mm. WT, wild type.
(B) Lutein concentrations in the petals of the wild type, mttt8, mtwd40-1, mttt8 mtwd40-1, wp1-1, wp1-1 mttt8, and wp1-1 mtwd40-1. Bars represent means ± sd of three biological replicates. FW, fresh weight; WT, wild type.
(C) DNA binding assay showing the binding activity of WP1 in the presence or absence of MtTT8 and/or MtWD40-1. Box1 to Box5 indicate putative binding sites of WP1 shown in Figure 5B. The His-TFa protein was used as a negative control. Bars represent means ± sd of three biological replicates; asterisks indicate differences from WP1 alone (*P < 0.05, **P < 0.01, Student’s t test).
(D) Activation of MtLYCe and MtLYCb promoters in transient luciferase assay using Arabidopsis protoplasts. Various combinations of M. truncatula effectors (WP1, MtTT8, MtWD40-1, and GFP control) were used to transfect Arabidopsis protoplasts along with the MtLYCe or MtLYCb promoter fused to a luciferase reporter. Bars represent means ± sd of three biological replicates; asterisks indicate differences from WP1 alone (*P < 0.05, **P < 0.01, Student’s t test). LUC, luciferase.
The WP1-MtTT8-MtWD40-1 Complex Coordinately Regulates Carotenoid Pigmentation in M. truncatula Flowers
To clarify the roles of MtTT8 and MtWD40-1 with respect to WP1 function in regulating floral carotenoid pigmentation, we performed three complementary experiments. First, we generated double mutants involving combinations of wp1, mttt8, and mtwd40-1 and characterized the single and double mutants to investigate the genetic relationship among WP1, MtTT8, and MtWD40-1 in M. truncatula floral carotenoid pigmentation. Consistent with the scenario that the MYB protein acts as the chief regulator in the MBW complex, the wp1 flowers showed the most severe phenotype, with drastically reduced lutein relative to the mttt8 and mtwd40-1 single mutants, and the wp1 mttt8 and wp1 mtwd40-1 double mutants showed white petals with abolished lutein accumulation similar to the wp1 single mutant (Figures 7A and 7B). By contrast, both the mttt8 and mtwd40-1 single mutants and the mttt8 mtwd40-1 double mutant showed pale-yellow petals with partially reduced lutein contents (Figures 7A and 7B), indicating that the contributions of MtTT8 and MtWD40-1 to floral carotenoid pigmentation are secondary and redundant.
Second, using in vitro DNA binding assays, we tested the direct DNA binding activity of WP1 to its putative binding sites in the presence of MtTT8 and/or MtWD40-1. Coexpression of WP1 with MtTT8 usually resulted in a higher DNA binding capacity than WP1 alone, and addition of MtWD40-1 further enhanced activation (Figure 7C). In addition, MtTT8 was able to bind all of the putative WP1 binding sites even in the absence of WP1 and MtWD40-1, while no similar binding was detected for MtWD40-1 (Figure 7C). These results suggest that MtTT8 and MtWD40-1 facilitate the capacity of WP1 to recognize and bind to its target promoters.
Third, using a transient promoter activation assay in Arabidopsis protoplasts, we found that coexpression of WP1 with MtTT8 and/or MtWD40-1 and a luciferase reporter construct driven by the MtLYCe or MtLYCb promoter resulted in a significant increase in luminescence intensity compared with WP1 alone, while MtTT8 or MtWD40-1 alone produced luminescence from the reporter constructs comparable to that in the GFP control (Figure 7D). These data together suggest that WP1 associates with MtTT8 and MtWD40-1 to activate floral carotenoid production and that WP1 fulfills the major function within the activation complex.
WP1 Is a Positive Regulator of Floral Anthocyanin Biosynthesis
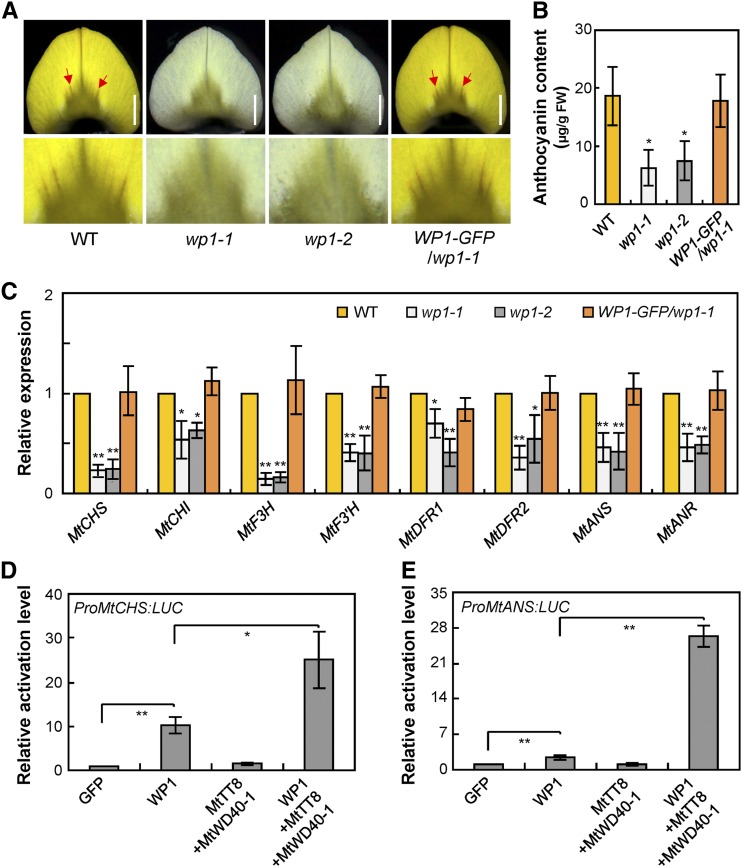
The M. truncatula vexillum petals are marked by a ray of darker veins that radiate from the middle of the base (Figure 8A) and are thought to be caused by anthocyanin accumulation (Xie et al., 2004; Jun et al., 2015). The color of these veins was visibly diminished in the vexillum petals of wp1 and the anthocyanin content was reduced in wp1 petals compared to the wild type, whereas introducing the WP1 genomic sequence restored the vein color and anthocyanin accumulation to the wild-type levels (Figures 8A and 8B). These data suggest that WP1 function may also be relevant to anthocyanin biosynthesis during flower development.
Figure 8.
WP1 Affects Anthocyanin Accumulation in M. truncatula Petals.
(A) Anthocyanin accumulation in the vexillum petals of the wild type, wp1-1, wp1-2, and WP1-GFP/wp1-1. Red arrows indicate the anthocyanin veins. Bars = 1.5 mm. WT, wild type.
(B) Anthocyanin contents in petals of the wild type, wp1-1, wp1-2, and WP1-GFP/wp1-1. Bars represent means ± sd of three biological replicates; asterisks indicate differences from the wild type (*P < 0.05, Student’s t test). FW, fresh weight; WT, wild type.
(C) Transcript levels of anthocyanin biosynthetic genes in petals of the wild type, wp1-1, wp1-2, and WP1-GFP/wp1-1 revealed by RT-qPCR. Bars represent means ± sd of three biological replicates; asterisks indicate differences from the wild type (*P < 0.05, **P < 0.01, Student’s t test).
(D) and (E) Activation of MtCHS (D) and MtANS (E) promoters in transient luciferase assay using Arabidopsis protoplasts. Various combinations of effectors (WP1, MtTT8, MtWD40-1, and GFP control) were used to transfect Arabidopsis protoplasts along with the MtCHS or MtANS promoter fused to a luciferase reporter. Bars represent means ± sd of three biological replicates; asterisks indicate significance differences (*P < 0.05, **P < 0.01, Student’s t test). LUC, luciferase.
To determine the molecular mechanism by which WP1 affects floral anthocyanin accumulation in M. truncatula, we analyzed the transcript levels of anthocyanin biosynthetic pathway genes in wp1 petals. RT-qPCR analysis showed that anthocyanin biosynthesis genes MtCHS, MtCHI, MtF3H, MtF3′H, MtDFR1, MtDFR2, MtANS, and MtANR were downregulated in wp1 petals (Figure 8C). Further transient expression assays showed that WP1 could activate luciferase reporter constructs driven by the promoters of MtCHS and MtANS (Figures 8D and 8E), which contain conserved MYB-core elements (Supplemental Figure 7), and that the presence of MtTT8 and MtWD40-1 could enhance these WP1-mediated activations (Figures 8D and 8E). Moreover, phenotypic observation revealed that both mttt8 and mtwd40-1 petals had veins with reduced anthocyanin content, similar to those of wp1 (Supplemental Figure 13). These results together suggest that WP1 associates with MtTT8 and MtWD40-1 and thereby regulates both carotenoid and anthocyanin production by activating their biosynthetic genes in M. truncatula petals.
DISCUSSION
WP1 Is Required for Both Carotenoid and Anthocyanin Production in M. truncatula Petals
The flowers of M. truncatula contain mainly yellow pigments, although a fine red vein of anthocyanin accumulates in the center of the vexillum petals (Xie et al., 2004; Jun et al., 2015). However, until now, neither the pigment components that impart M. truncatula flower color nor their regulatory controls have been clearly understood. In this study, we established that the yellow petals of the wild-type M. truncatula contained high levels of xanthophyll that largely consisted of esterified lutein (Figure 1J; Supplemental Figure 3). Functional disruption of WP1, an R2R3-MYB TF, by Tnt1 retrotransposon insertion resulted in loss of the vivid yellow floral pigment, which is associated with loss of lutein ester accumulation, as well as reduced of anthocyanin in the veins (Figures 1 and 8A and 8B; Supplemental Figure 3), suggesting that WP1 is required for both carotenoid and anthocyanin production in M. truncatula petals. We showed that WP1 is the central regulator of floral carotenoid pigmentation in M. truncatula by characterizing two independent but allelic mutants of ecotype R108 through forward genetics and genetic complementation (Figure 2; Supplemental Figure 1): introducing the WP1 genomic sequence into these mutants fully restored the yellow color and lutein accumulation to the wild-type levels.
These findings are intriguing from the perspectives of both lutein itself, which imparts the yellow color, and the R2R3-MYB regulator of the lutein biosynthetic pathway. Lutein accumulation has been reported as a major carotenoid in other yellow flowers, including the ray petals of chrysanthemums (Kishimoto et al., 2004). However, most flower colors, including blue, red, purple, and orange in popular ornamentals such as Rosa, Petunia, and carnation (Dianthus caryophyllus), are primarily determined by flavonoids (Forkmann, 1991; Grotewold, 2006). Even the bright yellow colors of snapdragon (Antirrhinum majus) and dahlia (Dahlia pinnata) flowers were reported to be conferred by flavonoids (Asen et al., 1972; Schwarz-Sommer et al., 2003). Plant flavonoids and carotenoids are produced in totally different pathways: flavonoids are derived from aromatic amino acids produced in the cytosol and on the endoplasmic reticulum through the shikimic acid pathway (Winkel-Shirley, 2001; Koes et al., 2005), while carotenoids are derived from GGPP produced in the plastids via the mevalonic acid pathway (Nisar et al., 2015; Sun et al., 2018). It is thus possible that flavonoids remain viable candidates for contributing to the yellow color to M. truncatula flowers.
To evaluate this possibility, we analyzed six of the commonly found major yellow flavonoids in plants including chalcone, apigenin, luteolin, quercetin, and two aurones (aureusidin and sulfuretin) in the petals of the wild type and wp1 mutants. We found that while the concentrations of chalcone and luteolin were unchanged, those of apigenin and quercetin were increased in the wp1 mutants compared with the wild type (Supplemental Table 1). Aureusidin and sulfuretin, on the other hand, were not detectable in either the wild type or the wp1 mutants (Supplemental Table 1). Thus, the yellow carotenoid lutein is most likely the major pigment responsible for the yellow petal of M. truncatula, although potential contributions from other flavonoids cannot be excluded.
Although anthocyanin levels were reduced (Figure 8B), those of chalcone and luteolin were almost unchanged and those of apigenin and quercetin were increased in wp1 mutant petals compared with the wild type (Supplemental Table 1), even though expression of several genes required for the biosynthesis of anthocyanin and these flavonoids was repressed in the mutant petals (Figure 8C). One possible explanation for these results might involve balancing feedback, that is, the decrease of anthocyanin in wp1 petals might be counterbalanced by an increase in other flavonoids. Consistent with this possibility, a previous study reported that loss of function of MYB regulator MtPAR led to substantially reduced PA in the M. truncatula seed coat, whereas levels of anthocyanin were indistinguishable and flavonoid glycoside content was higher (by 23.2%) in par mutants than in the wild-type controls despite the existence of a common pathway that generates precursors for both PA and anthocyanin biosynthesis (Verdier et al., 2012).
WP1 belongs to subgroup 6 of the R2R3-MYB proteins (Figure 3A), which is characterized by the signature bHLH-interacting motif, the ANDV motif, and the [R/K]Px[P/A/R]xx[F/Y] motif downstream of the conserved R2 and R3 MYB DNA binding domains (Supplemental Figure 6; Stracke et al., 2001; Zimmermann et al., 2004; Dubos et al., 2010; Lin-Wang et al., 2010). To date, all of the subgroup 6 MYBs characterized in Arabidopsis, M. truncatula, and other species appear to regulate anthocyanin biosynthesis (Liu et al., 2015; Allan and Espley, 2018). Our results showed that a member of this subgroup can also function in the regulation of carotenoids, directly connecting the two major pigment pathways. In light of this, recent studies in M. lewisii have reported that the subgroup 21 MYB protein RCP1, the first TF that positively regulates carotenoid biosynthesis during flower development, can simultaneously repress anthocyanin production in the petal lobe (Sagawa et al., 2016), indicating that there may be a variety of MYBs from different clades involved in both carotenoid and anthocyanin regulation in different plant species.
The presence of conserved MYB binding elements in the promoters of both anthocyanin and carotenoid biosynthesis genes suggests that plants may have evolved an efficient mechanism that produces and controls carotenoid and anthocyanin biosynthesis via conserved regulators (Supplemental Figure 7). Furthermore, tissue specificity appears to be remarkably precise. Although chlorophylls and important phytohormones such as gibberellin and abscisic acid are derived from the same intermediate (GGDP) as lutein, wp1-1 was indistinguishable from the wild type in other pigments and morphological features including lutein and anthocyanin in leaves (Supplemental Figure 11), suggesting an efficient mechanism for attaining specificity.
The C-Terminal Acidic Motif Is Essential for the Activation Function of WP1
Although transcriptional activation domains are essential for gene regulation, their intrinsic disorder and low primary sequence conservation have made it challenging to identify the amino acid composition features that underlie their activity. Molecular dissection of the transcriptional activator WP1 enabled us to gain insight into this activation mechanism. Our results showed that the C-terminal MVGEFPMDFQLEGFEA motif, which contains multiple acidic amino acid residues, is critical for the activation activity of WP1 (Figures 4B and 4C). Either deletion or mutation of acidic amino acid residues of the activation motif fully abolished WP1’s activation activity (Figures 4B and 4C; Supplemental Figure 8). Although BLASTP analysis revealed that WP1 had no similarity with any functionally identified activation motifs, such short acidic domains have been reported to be important components of MYB TFs involved in the activation of transcription. For example, the maize (Zea mays) C1 protein, the first TF identified in plants, was characterized as a MYB-related transcriptional activator defined by numerous acidic residues near the C terminus of the protein (Paz-Ares et al., 1987; Martin and Paz-Ares, 1997). These foregoing observations suggest that the acidic region provides a functional basis for the activation activity of MYB family TFs. In agreement with this, a recent study in yeast reported that acidic residues may underlie transcriptional activation domains by creating a permissive context for a hydrophobic short linear motif (Staller et al., 2018).
WP1 Associates with MtTT8 and MtWD40-1 to Modulate Floral Carotenoid and Anthocyanin Biosynthesis
In M. truncatula, several MYB TFs including MtPAR, MtMYB14, MtMYB5, and MtLAP1 have been reported to specifically regulate anthocyanin and PA biosynthesis in the presence of the bHLH protein MtTT8 and the WDR protein MtWD40-1 (Verdier et al., 2012; Liu et al., 2014; Li et al., 2016), establishing the so-called MBW complex similar to those in other species (Ramsay and Glover, 2005; Xu et al., 2015). The interactions between WP1, MtTT8, and MtWD40-1 indicate that the biosynthesis of carotenoids, anthocyanins, and PA may be regulated by different MBW complexes that share similar components and that R2R3 MYB might play a central role within the MBW complex in recognizing and activating targets at a specific time and in a specific tissue. Consistent with this hypothesis, both MtTT8 and MtWD40-1 are expressed in most tissues (Pang et al., 2009; Li et al., 2016), but their petal-specific function in carotenoid and anthocyanin regulation is brought about by the petal-specific expression of WP1 (Figures 3B to 3D). Nevertheless, it is worth noting that the presence of MtTT8 and MtWD40-1 enhances the WP1-mediated activation of both anthocyanin and carotenoid biosynthesis genes (Figures 7D and 8D and 8E), although the petals of M. truncatula contain mainly yellow xanthophyll and only a small amount of anthocyanin accumulated in the veins in the center of the vexillum petals.
These results might be explained by two nonexclusive possibilities. First, the anthocyanin biosynthesis/deposition in M. truncatula petals might be suppressed in areas outside of the veins by one or more unidentified repressors epistatic to WP1. Second, although the wp1, mttt8, and mtwd40-1 mutants showed reduced anthocyanin in veins, indicating that WP1, MtTT8, and MtWD40-1 are each indispensable for petal anthocyanin accumulation, it is possible that the function of WP1-dependent MBW complexes in the activation of vein-specific anthocyanin production is dependent on an additional partner that is present in the veins of vexillum petals. Further identification and characterization of the suppressors and potential partners of WP1 are needed to test these possibilities. Moreover, it is noteworthy that while lutein is severely reduced or absent in wp1 petals, the petals of mttt8 and mtwd40-1 showed only partial lutein reduction (Figure 7B), suggesting that the specific activation of carotenoid biosynthesis genes by WP1-dependent MBW complexes may also involve additional cofactors that remain to be characterized.
Although the MBW complex has been extensively studied in the context of the anthocyanin and flavonoid pathways, the complete complex, or members in the complex, have been co-opted in diverse plant taxa to regulate other unrelated pathways such as trichome initiation, root hair development, and seed coat differentiation (Stracke et al., 2001; Broun, 2005; Xu et al., 2015). Our results suggest that the carotenoid regulator WP1 may have been derived from the anthocyanin-regulating MYBs, retaining the ability to regulate anthocyanins and using a strikingly similar mechanism involving some of the same players. Likewise, recent studies in beets (Beta vulgaris) reported that an anthocyanin MYB-like protein, BvMYB1, activated the betalain red pigment pathway (Hatlestad et al., 2015). These findings suggest that plants may have evolved complex but efficient mechanisms that produce and control chemically unrelated pigments in distinct pathways. Taken together, our study demonstrates the molecular mechanism of WP1-mediated regulation of floral carotenoid and anthocyanin pigmentation in M. truncatula. Our findings provide a framework for comparative studies of flavonoid and carotenoid biosynthetic regulatory controls and offer mechanistic insights into the evolution of plant secondary metabolism.
METHODS
Plant Materials and Tnt1 Insertion Mutant Screening
Medicago truncatula ecotype R108 was used for all experiments described in this article. The wp1-1 (NF4496), wp1-2 (NF10625), mttt8 (NF15995), and mtwd40-1 (NF11228) mutants were identified from the Tnt1 retrotransposon-tagged mutant collection of M. truncatula (Tadege et al., 2008; Yarce et al., 2013). Primers used for genotyping are listed in Supplemental Table 2. Scarified M. truncatula seeds were germinated overnight in moist Petri dishes and placed at 4°C for 1 week. Plants were grown at 24°C day/22°C night temperature, 16-h day/8-h night photoperiod, 150 to 200 μE/m2/s light intensity (full-spectrum white fluorescent light bulbs), and 60 to 70% RH.
Molecular Cloning of WP1 and Identification of Insertion Sites in WP1
The Tnt1 FSTs of wp1 mutants were obtained from the M. truncatula mutant database (http://medicago-mutant.noble.org/mutant/) and genotyped by PCR using Tnt1-specific and gene-specific primers. FST 6, which segregated with the white-flower phenotype of wp1-1, was analyzed by BLAST searches against the M. truncatula genome at NCBI (http://www.ncbi.nlm.nih.gov/) and Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) to obtain the full-length sequence of WP1. PCR and RT-PCR were performed to amplify the WP1 genomic and coding sequences (CDS), respectively. The FST sequence corresponding to WP1 in the wp1-2 mutant was amplified using Tnt1- and WP1-specific primers. Tnt1 insertion fragments from PCR were further confirmed by sequencing. The primer sequences are listed in Supplemental Table 2.
Plasmid Construction and Plant Transformation
To make the complementation construct, a 1.5-kb fragment downstream of the termination site of WP1 was amplified from M. truncatula R108 DNA and inserted into the PstI site of the pCAMBIA2300 vector by the In-Fusion cloning system (no. 639648, Clontech) to generate the vector pCAMBIA2300-WP1 3′UTR. Subsequently, the 3-kb WP1 promoter region upstream of the translation start site and the 3097-bp genomic DNA fragment containing three exons and two introns were cloned into pCAMBIA2300-WP1 3′UTR, at the KpnI site, to generate the destination vector WP1-GFP. The Agrobacterium tumefaciens strain AGL1–mediated transformation was used to introduce the WP1-GFP construct into M. truncatula as described previously by Tadege et al., (2011). The primer sequences are listed in Supplemental Table 2.
Sequence Alignment and Phylogenetic Analysis
The amino acid sequences of WP1 and subgroup 6 MYB proteins were aligned using ClustalW (http://www.genome.jp/tools/clustalw/). Bootstrap values of 1000 permutations for the neighbor-joining phylogenetic tree were generated using MEGA 4.0.
mRNA Expression Analyses
Petals from fully opened flowers were collected and dissected for RNA isolation. Total RNA was isolated using TRIzol reagent (Invitrogen). cDNA was synthesized by RT with TransScript-Uni One-Step gDNA Removal and cDNA Synthesis SuperMix (no. AU311, TRAN). RT-PCR reactions were performed using 2×Taq PCR Master Mix (no. PM604-1, UPTECH) according to the manufacturers’ instructions, and the PCR amplicons were examined by electrophoresis on a 1% (w/v) agarose gel. RT-qPCR was performed as described previously by Wang et al., (2017), with three biological replicates. Gene expression was normalized using the expression of MtActin, a housekeeping gene. Relative gene expression for each gene in the mutant plants was compared with that obtained for the wild type, which was arbitrarily set to 1.0. All primers used are listed in Supplemental Table 2.
Carotenoid Analysis
Carotenoids were extracted as described previously, with minor modifications (Fraser et al., 2000). Dissected petals (15 mg) from fully opened flowers or leaves at the vegetative stage were ground into a powder in liquid nitrogen. For saponification, 200 μL of methanol containing 6% (w/v) KOH was added and mixed thoroughly and then incubated at 60°C for 1 h in the dark. After cooling to room temperature, 200 μL of 50 mM Tris-HCl buffer, pH 7.5 (containing 1 M NaCl), was added to the suspension and the mixture was incubated on ice for 10 min. Chloroform (800 µL) was then added to the mixture, which was incubated on ice for 10 min. After centrifugation at 3000g for 5 min at 4°C, a visible stratification formed. The lower phase was removed, and the aqueous phase was re-extracted with chloroform (800 µL). The pooled chloroform extracts were dried under a stream of nitrogen. For nonsaponification, 200 μL of methanol was added to the ground samples and the suspension was mixed by inversion for 5 min at 4°C. Two hundred μL of 50 mM Tris-HCl buffer, pH 7.5 (containing 1M NaCl) was added to the suspension and the extraction performed as described above. All dried extracts were stored at −80°C under an atmosphere of nitrogen prior to HPLC.
HPLC analysis for floral carotenoids was performed as described previously by Fraser et al., (2000), with minor modifications. Briefly, a reverse-phase C30 column (250 × 4.6 mm, 5 μm; YMC) coupled to a 20 × 4.6-mm C30 guard (YMC) with mobile phases consisting of methanol (A), water/methanol (20/80 by volume) (B), and tert-methyl butyl ether–based mobile phase (C) was also used with a 10Avp HPLC system (Shimadzu). The gradient elution used with this column was 95% A, 5% B isocratically for 12 min; a step to 80% A, 5% B, 15% C at 12 min; followed by a linear gradient to 30% A, 5% B, 65% C by 30 min. A reverse-phase C18 Hypersil ODS 5-μm (200 × 4.6-mm) column (Thermo Fisher Scientific) coupled to a 5-μm (10 × 4.6-mm) C18 guard column (Phenomenex) was used with same Shimadzu HPLC system for analyzing carotenoids in leaf tissues. Acetonitrile-based mobile phases were used as described in a previous report (Holloway et al., 2000). Throughout chromatography, the elution was monitored continuously from 200 to 600 nm by an online Shimadzu SPD-10Avp PDA detector. Column temperature was maintained at 25°C by a Shimadzu CTO-10ACVP column oven. In all cases, flow rates of 1 mL/min were used. Carotenoids were identified by their characteristic absorption spectra, typical retention time, and comparison with authentic standards. The content of each compound was calculated using the corresponding standard as an external standard. The carotenoid standards were neoxanthin (no. BCBZ3644, Sigma-Aldrich), violaxanthin (no. BCBZ4220, Sigma-Aldrich), lutein epoxide (no. 0232, CaroteNature), lutein (no. 0133, CaroteNature), antheraxanthin (no. ASB-00001885-00A, ChromaDex), and β-carotene (no. ASB-00003210-00A, ChromaDex).
Flavonoid Analysis
The extraction and analysis of the total content (free form and glycosides) of flavonoids were performed as described previously (Matsuda et al., 2009; Ng et al., 2015), with minor modifications. Dissected petals (10 mg) from fully opened flowers were ground into a powder in liquid nitrogen and extracted with 1 mL of 80% (v/v) methanol/water solution. After shaking overnight at 4°C in the dark, the supernatant was spun in a microcentrifuge at 16,000g for 10 min and then transferred to a fresh tube. After being evaporated in a SpeedVac centrifuge, the residue was dissolved in 400 μL of 2 M HCl, heated at 80°C for 90 min to deglycosylate flavonoids and subsequently extracted with 1 mL of ethyl acetate. Extracts (400 μL) were dried in a SpeedVac centrifuge and subsequently dissolved in 1 mL of 80% (v/v) methanol. The resulting solution was filtered with a 0.22-μm syringe filter, and the sample was analyzed with an ultraperformance liquid chromatography-tandem mass spectrometry system consisting of an Agilent 1290 Infinity LC pump and a 6495 triple-quadrupole mass spectrometer. Positive mode analysis was performed in multiple reaction monitoring mode. The mass spectrometry parameters were optimized using metabolite standards. The analytical conditions were as follows: the HPLC column was an Agilent ZORBAX Extend C18 (pore size, 1.8 mm; length, 2.1 × 100 mm); the solvent system was according to a previously described method (Matsuda et al., 2009) using acetonitrile (0.1% formic acid):water (0.1% formic acid); the gradient program was 1:99 (v/v) at 0 min, 1:99 (v/v) at 0.1 min, 99.5:0.5 at 15.5 min, 99.5:0.5 at 17.0 min; the flow rate was 0.4 mL/min; and the temperature was 35°C. The ion source parameters were set as follows: drying gas temperature, 325°C (nitrogen); drying gas flow, 7 liters/min; nebulizer, 40 pounds per square inch; sheath gas heater, 350°C; sheath gas flow, 12 liters/min; and capillary voltage, 3 kV (electrospray ionization). The content of each compound was calculated using the corresponding standard as an external standard. Flavonoid standards used were apigenin (no. 42251, Sigma-Aldrich), luteolin (no. 72511, Sigma-Aldrich), quercetin (no. PHR1488, Sigma-Aldrich), chalcone (no. A14734, Alfa Aesar), aureusidin (no. BBP05377, BioBioPha), and sulfuretin (no. B50928, Shyuanye).
Anthocyanin Analysis
Anthocyanin content was detected as described previously by Pang et al., (2009). Ten milligrams of ground petals was added to 300 μL of 0.1% (v/v) HCl/methanol, and the mixture was rotated overnight on a rotating wheel at 4°C in the dark. Following centrifugation at 2500g for 10 min at 4°C, the supernatant was transferred to a fresh tube and the absorption of the extraction was recorded at 530 nm. Total anthocyanin content was calculated based on the molar absorbance of a cyanidin 3-O-glucoside chloride standard (no. 52976, Sigma-Aldrich).
Subcellular Localization Analysis
The CDS of WP1 was cloned into pMDC83 by Gateway Cloning (Invitrogen) to generate Pro35S:WP1-GFP. A plasmid expressing mRFP-AHL22 protein was used as a nuclear localization marker (Wang et al., 2013). The plasmid pMDC32-GFP (Pro35S:GFP) expressing GFP protein alone, was used as the control. The A. tumefaciens strain GV2260 containing Pro35S:WP1-GFP or Pro35S:GFP, and the same strain containing mRFP-AHL22, were simultaneously infiltrated into the leaves of 4-week-old Nicotiana benthamiana plants. P19 was used to inhibit transgenic silencing. The fluorescence signal was observed 2 to 3 d after infiltration by confocal microscopy (TCS SP2 microscope, Leica). The primers used for plasmid construction are listed in Supplemental Table 2.
Transactivation Activity Assay in Yeast
The transactivation activity assay was performed as described previously by Zhang et al., (2016). WP1, VP64 (encoding an exogenous activation domain) or EAR4 (encoding an exogenous repression module) fused to WP1, different domains of WP1, and various truncated forms of WP1 were fused with the GAL4 DNA BD in the plasmid pGBKT7-GW using a Gateway recombination system. These constructs were then transformed into the yeast (Saccharomyces cerevisiae) strain Gold according to the instructions for the Frozen-EZ Yeast Transformation II kit (no. T2001, Zymo Research). Yeast colonies were patched onto SD/−Trp and SD/−Trp/−His/−Ade plates and grown at 28°C for 3 d. The α-galactosidase assay was performed according to the Yeast Protocols Handbook (no. PT3024-1, Clontech). The primer sequences are listed in Supplemental Table 2.
Y2H Assay
Y2H assays were performed as described previously (Niu et al., 2015). The CDSs of WP1, MtTT8, and MtWD40-1 were amplified and then inserted into the bait plasmid pGBKT7-GW or the prey plasmid pGADT7-GW. All clones were validated by sequencing. The bait and prey plasmids were cotransformed into yeast strain Gold using the Frozen-EZ Yeast Transformation II kit (no. T2001, Zymo Research). Yeast colonies were patched onto SD/−Leu/−Trp (double dropout) and SD/−Trp/−Leu/−His/−Ade (quadruple dropout) plates and grown at 28°C for 3 d. The α-galactosidase assay was performed according to the Yeast Protocols Handbook (no. PT3024-1, Clontech). The primer sequences are listed in Supplemental Table 2.
BiFC Assay
BiFC assays were performed as described previously (Lu et al., 2010; Niu et al., 2015). The CDSs of WP1, MtTT8, and MtWD40-1 were cloned into the pEarleyGate 201-YN or pEarleyGate 202-YC vectors (Lu et al., 2010) using a Gateway recombination system. The prepared vectors were introduced into A. tumefaciens strain GV2260. Combinations of YN and YC plasmids together with plasmids mRFP-AHL22 and P19 were agroinfiltrated into the leaves of 4-week-old N. benthamiana plants. Signals were observed 3 d after infiltration by confocal microscopy (TCS SP2 microscope, Leica). Primers used for plasmid construction are listed in Supplemental Table 2.
Co-IP Assay
The Co-IP assay was performed as described previously by Meng et al., (2013), with minor modifications. The CDSs of WP1, MtTT8, and MtWD40-1 were cloned into either the pGWB17-GW-Myc or pGWB11-GW-Flag vector, resulting in pGWB17-WP1-Myc, pGWB17-MtTT8-Myc, pGWB11-MtTT8-Flag, and pGWB11-MtWD40-1-Flag. For testing protein–protein interactions, A. tumefaciens strain GV2260 containing pairs of these constructs together with P19 was co-infiltrated into the leaves of 4-week-old N. benthamiana plants. Equal amount of samples (0.3 g) was collected 3 d after infiltration, ground in liquid nitrogen, and then homogenized in 1 mL of extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% Tween 20, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, and 1 tablet/50 mL of protease inhibitor cocktail). The lysates were incubated at 4°C for 15 min and centrifuged at 16,000g for 10 min at 4°C. The supernatant was precleared with 30 μL of Dynabeads Protein A (no. 10001D, Novex) at 4°C for 1 h. After a brief spin, the supernatants were incubated with 30 μL of suspensions of Pierce Anti-c-MYC magnetic beads (no. 88842, Thermo Fisher Scientific) at 4°C for 4 h and then washed four to five times with the extraction buffer. The proteins were eluted from the beads with 30 μL of 6× Protein Loading Buffer (no. L10215, TRAN), boiled for 5 min, and spun at 12,000 rpm for 10 min at room temperature. The supernatants were electrophoretically separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane (no. A10190852, GE Healthcare). Immunoblots were performed using an anti-Myc antibody (1:4000; no. M20002, Abmart) for probing WP1-Myc or MtTT8-Myc, and an anti-Flag antibody (1:4000; no. M20008, Abmart) for probing MtTT8-Flag or MtWD40-1-Flag, sequentially. The primer sequences are listed in Supplemental Table 2.
DNA Binding Assay
The CDSs of WP1, MtTT8, and MtWD40-1 were amplified and inserted into the EcoRI site of the pCOLD-TFa vector using the In-Fusion cloning strategy (Clontech). The recombinant constructs were transformed into Escherichia coli (BL21) and induced with 0.2 mM isopropyl-1-thio-d-galactopyranoside. Recombinant His-TFa-WP1, His-TFa-MtTT8, and His-TFa-MtWD40-1 proteins and His-TFa control protein were purified using Profinity IMAC Ni-charged resin (no. 1560131, Bio-Rad) according to the manufacturer’s protocol and quantified by the Bio-Rad protein assay reagent. The DNA binding assay was performed as described previously by Meng et al., (2013) and Wang et al., (2017). The putative MYB binding fragments (Box1 to Box5) or the mutant binding fragment (Boxm) were incubated with the His-TFa-WP1, His-TFa-MtTT8, and His-TFa-MtWD40-1 proteins, or combinations of these proteins, in the Ni-charged resin, and then the DNA binding activity (protein-bound DNA) was determined by real-time qPCR after washing and elution. Primers used are listed in Supplemental Table 2.
Transient Luciferase Assay
The transient dual-luciferase assay was performed as described previously (Wang et al., 2017), with minor modifications. The effector plasmids were constructed by cloning the WP1, WP1Δ(186-201), MtTT8, and MtWD40-1 CDSs into the pMDC32 or pEarleyGate203 vector using the Gateway Cloning system (Invitrogen), resulting in vectors pMDC32-WP1, pMDC32-WP1Δ(186-201), pEarleyGate203-MtTT8, and pEarleyGate203-MtWD40-1, respectively. The plasmid pMDC32-GFP was used as the negative control. The ∼3-kb promoter fragments upstream of the transcription start sites of MtPSY, MtPDS, MtZDS, MtCRTISO, MtLYCe, MtLYCb, MtBCH, MtCYP97C, MtCHS, and MtANS were cloned into pGreenII-0800-Luc using the In-Fusion cloning strategy (Clontech) to generate the corresponding reporter vectors. Arabidopsis (Arabidopsis thaliana) protoplasts were cotransformed with different combinations of plasmids, incubated for 12 to 14 h in darkness, and then collected and lysed for the detection of luciferase activity. The detection was performed according to the manufacturer’s recommendations for the Dual-Luciferase Reporter Assay System (E1910, Promega). Primers used are listed in Supplemental Table 2.
ChIP-PCR Assay
The WP1-GFP transgenic plants (T2 generation) were used for ChIP assays according to the previously described method (Cui et al., 2016), with minor modifications. Briefly, 1 g of fully opened flowers was ground into a fine powder with liquid nitrogen, cross-linked with 1% (v/v) formaldehyde, and then quenched with 0.125 M Gly for 5 min at 4°C. The chromatin complexes were isolated, sonicated, and then incubated with an anti-GFP antibody (no. ab290, Abcam). The washing, elution, reverse cross-linking, and DNA purification steps were performed as described previously (Bowler et al., 2004). The precipitated DNA was subjected to qPCR analysis. The ChIP-qPCR results were quantified by normalization of the immunoprecipitation signal with the corresponding input signal and are presented as the percentage of input (PCR signal of immunoprecipitation reaction/PCR signal of input). The primers used for the ChIP assays are listed in Supplemental Table 2.
Statistical Analysis
For statistical analysis, Student’s t test was used as specified in each figure and in Supplemental Data Set 2. Asterisks indicate statistical differences (*P < 0.05, **P < 0.01). Data represent mean values, and error bars are sd.
Accession Numbers
Sequence data from this article can be found in the NCBI (http://www.ncbi.nlm.nih.gov/) databases, M. truncatula Genome Database (http://www.medicagogenome.org/), or Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) under the following accession numbers: Antirrhinum majus: ROSEA1, ABB83826; VENOSA, ABB83828. A. thaliana: AtPAP1, AT1G56650; AtPAP4, AT1G66380; AtPAP2, AT1G66390; AtMYB113, AT1G66370; AtGL1, AT3G27920; AtMYB23, AT5G40330; AtWER, AT5G14750; AtTT2, AT5G35550; AtMYB11, AT3G62610; AtMYB12, AT2G47460; AtMYB111, AT5G49330. Lotus japonicus: LjTT2a, BAG12893. Malus × domestica: MdMYB10, ABB84753. Mimulus lewisii: NEGAN, AHJ80988; PELAN, AHJ80987. M. truncatula: WP1, Medtr0197s0010; MtLAP1, Medtr8g060940; MtLAP3, Medtr7g017260; MtLAP2, Medtr5g079290; MtLAP4, Medtr5g079220; MtTT8, Medtr1g072320; MtWD40-1, Medtr3g092840; MtPSY, Medtr5g076620; MtPDS, Medtr3g084830; MtZDS, Medtr1g081290; MtCRTISO, Medtr1g054965; MtLYCe, Medtr2g040060; MtLYCb, Medtr7g090150; MtCYP97A, Medtr7g079440; MtBCH, Medtr6g048440; MtCYP97C, Medtr1g062190; MtCHS, Medtr3g083910; MtCHI, Medtr1g115820; MtF3H, Medtr8g075890; MtF3′H, Medtr3g025230; MtDFR1, Medtr1g022445; MtDFR2, Medtr1g022440; MtANS, Medtr5g011250; MtANR, Medtr4g092080. Petunia × hybrida: PhAN2, AF146702; PhDPL, ADW94950. Vitis vinifera: VvMYBA1, BAD18977; VvMYBPA2, ACK56131.
Supplemental Data
Supplemental Figure 1. Genetic analysis of wp1-1 and wp1-2 mutants.
Supplemental Figure 2. HPLC analysis of carotenoids in wild-type R108 petals.
Supplemental Figure 3. Carotenoid profiles in wild-type, wp1-1, and wp1-2 petals.
Supplemental Figure 4. WP1 cDNA sequence and its deduced amino acid sequence in wp1-1.
Supplemental Figure 5. Molecular characterization of WP1-GFP complemented transgenic plants.
Supplemental Figure 6. Sequence alignment of WP1 and subgroup 6 R2R3-MYB proteins in M. truncatula and Arabidopsis.
Supplemental Figure 7. The predicted MYB binding sites in the promoters of downregulated carotenoid and anthocyanin biosynthesis genes in wp1.
Supplemental Figure 8. Transactivation analysis of the WP1Δ(186-201) protein using transient luciferase assay.
Supplemental Figure 9. Homodimerization of WP1, MtTT8, and MtWD40-1 proteins.
Supplemental Figure 10. Molecular characterization of the mttt8 and mtwd40-1 Tnt1 insertional mutants.
Supplemental Figure 11. Phenotype analysis of the wp1-1, mttt8, and mtwd40-1 mutants.
Supplemental Figure 12. Comparison of carotenoid biosynthesis gene expression in petals of wild type, wp1-1, mttt8, and mtwd40-1.
Supplemental Figure 13. Anthocyanin accumulation in the vexillum petals of wild type, mttt8, and mtwd40-1.
Supplemental Table 1. Quantification of flavonoids in petals of wild type (WT), wp1-1, wp1-2, and WP1-GFP/wp1-1 plants.
Supplemental Table 2. Primers used in this study.
Supplemental Data Set 1. Alignments used to generate the phylogeny presented in Figure 3A.
Supplemental Data Set 2. Summary of statistical tests.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank the Metabolomics Facility of the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences for assistance regarding flavonoid analysis. This work was supported by the National Transgenic Science and Technology Program (grants 2019ZX08010-003 and 2019ZX08010-002), the National Key R&D Program of China (grants 2018YFA0901000 and 2018YFA0901003), the National Natural Science Foundation of China (grant 31870284), the Beijing Municipal Science and Technology Commission (grant lj201812), and the Scientific Research Project for Major Achievements of the Agricultural Science and Technology Innovation Program (ASTIP; grant CAAS-ZDXT2019004).
AUTHOR CONTRIBUTIONS
Y.M., Z.W., L.N., and H. Lin designed the research. Y.M., Z.W., Y.W., C.W., B.Z., H. Liu, and W.J. performed the experiments. Y.M., Z.W., M.T., L.N., and H. Lin analyzed the data. J.W. and C.C. contributed analytical tools. Y.M., M.T., L.N., and H. Lin wrote the article with contributions by all authors.
Footnotes
Articles can be viewed without a subscription.
References
- Al-Babili S., Bouwmeester H.J. (2015). Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 66: 161–186. [DOI] [PubMed] [Google Scholar]
- Allan A.C., Espley R.V. (2018). MYBs drive novel consumer traits in fruits and vegetables. Trends Plant Sci. 23: 693–705. [DOI] [PubMed] [Google Scholar]
- Allan A.C., Hellens R.P., Laing W.A. (2008). MYB transcription factors that colour our fruit. Trends Plant Sci. 13: 99–102. [DOI] [PubMed] [Google Scholar]
- Ampomah-Dwamena C., Thrimawithana A.H., Dejnoprat S., Lewis D., Espley R.V., Allan A.C. (2019). A kiwifruit (Actinidia deliciosa) R2R3-MYB transcription factor modulates chlorophyll and carotenoid accumulation. New Phytol. 221: 309–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asen S., Norris K.H., Stewart R.N. (1972). Copigmentation of aurone and flavone from petals of Antirrhinum majus. Phytochemistry 11: 2739–2741. [Google Scholar]
- Baudry A., Heim M.A., Dubreucq B., Caboche M., Weisshaar B., Lepiniec L. (2004). TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 39: 366–380. [DOI] [PubMed] [Google Scholar]
- Bowler C., Benvenuto G., Laflamme P., Molino D., Probst A.V., Tariq M., Paszkowski J. (2004). Chromatin techniques for plant cells. Plant J. 39: 776–789. [DOI] [PubMed] [Google Scholar]
- Broun P. (2005). Transcriptional control of flavonoid biosynthesis: A complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr. Opin. Plant Biol. 8: 272–279. [DOI] [PubMed] [Google Scholar]
- Chiou C.Y., Pan H.A., Chuang Y.N., Yeh K.W. (2010). Differential expression of carotenoid-related genes determines diversified carotenoid coloration in floral tissues of Oncidium cultivars. Planta 232: 937–948. [DOI] [PubMed] [Google Scholar]
- Cui X., et al. (2016). REF6 recognizes a specific DNA sequence to demethylate H3K27me3 and regulate organ boundary formation in Arabidopsis. Nat. Genet. 48: 694–699. [DOI] [PubMed] [Google Scholar]
- Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L. (2010). MYB transcription factors in Arabidopsis. Trends Plant Sci. 15: 573–581. [DOI] [PubMed] [Google Scholar]
- Forkmann G. (1991). Flavonoids as flower pigments: The formation of the natural spectrum and its extension by genetic engineering. Plant Breed. 106: 1–26. [Google Scholar]
- Fraser P.D., Bramley P.M. (2004). The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 43: 228–265. [DOI] [PubMed] [Google Scholar]
- Fraser P.D., Pinto M.E., Holloway D.E., Bramley P.M. (2000). Technical advance: Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 24: 551–558. [DOI] [PubMed] [Google Scholar]
- Fraser P.D., Truesdale M.R., Bird C.R., Schuch W., Bramley P.M. (1994). Carotenoid biosynthesis during tomato fruit development (evidence for tissue-specific gene expression). Plant Physiol. 105: 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Jia S., Wang C., Wang F., Wang F., Zhao K. (2016). BjMYB1, a transcription factor implicated in plant defence through activating BjCHI1 chitinase expression by binding to a W-box-like element. J. Exp. Bot. 67: 4647–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G. (2014). Plant carotenoids: Genomics meets multi-gene engineering. Curr. Opin. Plant Biol. 19: 111–117. [DOI] [PubMed] [Google Scholar]
- Giuliano G. (2017). Provitamin A biofortification of crop plants: A gold rush with many miners. Curr. Opin. Biotechnol. 44: 169–180. [DOI] [PubMed] [Google Scholar]
- Gómez-Maldonado J., Avila C., Torre F., Cañas R., Cánovas F.M., Campbell M.M. (2004). Functional interactions between a glutamine synthetase promoter and MYB proteins. Plant J. 39: 513–526. [DOI] [PubMed] [Google Scholar]
- Grotewold E. (2006). The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 57: 761–780. [DOI] [PubMed] [Google Scholar]
- Ha S.H., Kim J.B., Park J.S., Lee S.W., Cho K.J. (2007). A comparison of the carotenoid accumulation in Capsicum varieties that show different ripening colours: Deletion of the capsanthin-capsorubin synthase gene is not a prerequisite for the formation of a yellow pepper. J. Exp. Bot. 58: 3135–3144. [DOI] [PubMed] [Google Scholar]
- Hatlestad G.J., Akhavan N.A., Sunnadeniya R.M., Elam L., Cargile S., Hembd A., Gonzalez A., McGrath J.M., Lloyd A.M. (2015). The beet Y locus encodes an anthocyanin MYB-like protein that activates the betalain red pigment pathway. Nat. Genet. 47: 92–96. [DOI] [PubMed] [Google Scholar]
- Holloway D.E., Yang M., Paganga G., Rice-Evans C.A., Bramley P.M. (2000). Isomerization of dietary lycopene during assimilation and transport in plasma. Free Radic. Res. 32: 93–102. [DOI] [PubMed] [Google Scholar]
- Holt N.E., Zigmantas D., Valkunas L., Li X.P., Niyogi K.K., Fleming G.R. (2005). Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307: 433–436. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Mitsuda N., Ohme-Takagi M. (2009). Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell 21: 3493–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J.H., Liu C., Xiao X., Dixon R.A. (2015). The transcriptional repressor MYB2 regulates both spatial and temporal patterns of proanthocyandin and anthocyanin pigmentation in Medicago truncatula. Plant Cell 27: 2860–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto S., Maoka T., Nakayama M., Ohmiya A. (2004). Carotenoid composition in petals of chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitamura). Phytochemistry 65: 2781–2787. [DOI] [PubMed] [Google Scholar]
- Koes R., Verweij W., Quattrocchio F. (2005). Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 10: 236–242. [DOI] [PubMed] [Google Scholar]
- Li P., Chen B., Zhang G., Chen L., Dong Q., Wen J., Mysore K.S., Zhao J. (2016). Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bHLH transcription factor MtTT8. New Phytol. 210: 905–921. [DOI] [PubMed] [Google Scholar]
- Lin-Wang K., Bolitho K., Grafton K., Kortstee A., Karunairetnam S., McGhie T.K., Espley R.V., Hellens R.P., Allan A.C. (2010). An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol. 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Jun J.H., Dixon R.A. (2014). MYB5 and MYB14 play pivotal roles in seed coat polymer biosynthesis in Medicago truncatula. Plant Physiol. 165: 1424–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Osbourn A., Ma P. (2015). MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant 8: 689–708. [DOI] [PubMed] [Google Scholar]
- Lu Q., Tang X., Tian G., Wang F., Liu K., Nguyen V., Kohalmi S.E., Keller W.A., Tsang E.W., Harada J.J., Rothstein S.J., Cui Y. (2010). Arabidopsis homolog of the yeast TREX-2 mRNA export complex: Components and anchoring nucleoporin. Plant J. 61: 259–270. [DOI] [PubMed] [Google Scholar]
- Lu S., Zhang Y., Zhu K., Yang W., Ye J., Chai L., Xu Q., Deng X. (2018). The citrus transcription factor CsMADS6 modulates carotenoid metabolism by directly regulating carotenogenic genes. Plant Physiol. 176: 2657–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Paz-Ares J. (1997). MYB transcription factors in plants. Trends Genet. 13: 67–73. [DOI] [PubMed] [Google Scholar]
- Matsuda F., Yonekura-Sakakibara K., Niida R., Kuromori T., Shinozaki K., Saito K. (2009). MS/MS spectral tag-based annotation of non-targeted profile of plant secondary metabolites. Plant J. 57: 555–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Li H., Wang Q., Liu B., Lin C. (2013). Blue light-dependent interaction between cryptochrome2 and CIB1 regulates transcription and leaf senescence in soybean. Plant Cell 25: 4405–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehs C.P., Tian L., Osteryoung K.W., Dellapenna D. (2001). Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Mol. Biol. 45: 281–293. [DOI] [PubMed] [Google Scholar]
- Moise A.R., Al-Babili S., Wurtzel E.T. (2014). Mechanistic aspects of carotenoid biosynthesis. Chem. Rev. 114: 164–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E., Marion-Poll A. (2005). Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 56: 165–185. [DOI] [PubMed] [Google Scholar]
- Ng J.L., Hassan S., Truong T.T., Hocart C.H., Laffont C., Frugier F., Mathesius U. (2015). Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell 27: 2210–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisar N., Li L., Lu S., Khin N.C., Pogson B.J. (2015). Carotenoid metabolism in plants. Mol. Plant 8: 68–82. [DOI] [PubMed] [Google Scholar]
- Niu L., Lin H., Zhang F., Watira T.W., Li G., Tang Y., Wen J., Ratet P., Mysore K.S., Tadege M. (2015). LOOSE FLOWER, a WUSCHEL-like Homeobox gene, is required for lateral fusion of floral organs in Medicago truncatula. Plant J. 81: 480–492. [DOI] [PubMed] [Google Scholar]
- Niyogi K.K., Björkman O., Grossman A.R. (1997). The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci. USA 94: 14162–14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y., et al. (2009). A WD40 repeat protein from Medicago truncatula is necessary for tissue-specific anthocyanin and proanthocyanidin biosynthesis but not for trichome development. Plant Physiol. 151: 1114–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J., Ghosal D., Wienand U., Peterson P.A., Saedler H. (1987). The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 6: 3553–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay N.A., Glover B.J. (2005). MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 10: 63–70. [DOI] [PubMed] [Google Scholar]
- Sagawa J.M., Stanley L.E., LaFountain A.M., Frank H.A., Liu C., Yuan Y.W. (2016). An R2R3-MYB transcription factor regulates carotenoid pigmentation in Mimulus lewisii flowers. New Phytol. 209: 1049–1057. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Davies B., Hudson A. (2003). An everlasting pioneer: The story of Antirrhinum research. Nat. Rev. Genet. 4: 657–666. [DOI] [PubMed] [Google Scholar]
- Staller M.V., Holehouse A.S., Swain-Lenz D., Das R.K., Pappu R.V., Cohen B.A. (2018). A High-throughput mutational scan of an intrinsically disordered acidic transcriptional activation domain. Cell Syst. 6: 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R., Werber M., Weisshaar B. (2001). The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 4: 447–456. [DOI] [PubMed] [Google Scholar]
- Sun T., Yuan H., Cao H., Yazdani M., Tadmor Y., Li L. (2018). Carotenoid metabolism in plants: The role of plastids. Mol. Plant 11: 58–74. [DOI] [PubMed] [Google Scholar]
- Tadege M., et al. (2011). STENOFOLIA regulates blade outgrowth and leaf vascular patterning in Medicago truncatula and Nicotiana sylvestris. Plant Cell 23: 2125–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadege M., Wen J., He J., Tu H., Kwak Y., Eschstruth A., Cayrel A., Endre G., Zhao P.X., Chabaud M., Ratet P., Mysore K.S. (2008). Large-scale insertional mutagenesis using the Tnt1 retrotransposon in the model legume Medicago truncatula. Plant J. 54: 335–347. [DOI] [PubMed] [Google Scholar]
- Toledo-Ortiz G., Huq E., Rodríguez-Concepción M. (2010). Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 107: 11626–11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Ortiz G., Johansson H., Lee K.P., Bou-Torrent J., Stewart K., Steel G., Rodríguez-Concepción M., Halliday K.J. (2014). The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 10: e1004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier J., Zhao J., Torres-Jerez I., Ge S., Liu C., He X., Mysore K.S., Dixon R.A., Udvardi M.K. (2012). MtPAR MYB transcription factor acts as an on switch for proanthocyanidin biosynthesis in Medicago truncatula. Proc. Natl. Acad. Sci. USA 109: 1766–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Niu L., Fu C., Meng Y., Sang D., Yin P., Wu J., Tang Y., Lu T., Wang Z.Y., Tadege M., Lin H. (2017). Overexpression of the WOX gene STENOFOLIA improves biomass yield and sugar release in transgenic grasses and display altered cytokinin homeostasis. PLoS Genet. 13: e1006649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Fan C., Zhang X., Zhu J., Fu Y.F. (2013). BioVector, a flexible system for gene specific-expression in plants. BMC Plant Biol. 13: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch R., Maass D., Voegel T., Dellapenna D., Beyer P. (2007). Transcription factor RAP2.2 and its interacting partner SINAT2: Stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiol. 145: 1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B. (2001). Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 126: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D.Y., Jackson L.A., Cooper J.D., Ferreira D., Paiva N.L. (2004). Molecular and biochemical analysis of two cDNA clones encoding dihydroflavonol-4-reductase from Medicago truncatula. Plant Physiol. 134: 979–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Dubos C., Lepiniec L. (2015). Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 20: 176–185. [DOI] [PubMed] [Google Scholar]
- Yamamizo C., Kishimoto S., Ohmiya A. (2010). Carotenoid composition and carotenogenic gene expression during Ipomoea petal development. J. Exp. Bot. 61: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarce J.C., Lee H.K., Tadege M., Ratet P., Mysore K.S. (2013). Forward genetics screening of Medicago truncatula Tnt1 insertion lines. Methods Mol. Biol. 1069: 93–100. [DOI] [PubMed] [Google Scholar]
- Zhang C., Liu J., Zhao T., Gomez A., Li C., Yu C., Li H., Lin J., Yang Y., Liu B., Lin C. (2016). A drought-inducible transcription factor delays reproductive timing in rice. Plant Physiol. 171: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., et al. (2017). An R2R3-MYB transcription factor represses the transformation of α- and β-branch carotenoids by negatively regulating expression of CrBCH2 and CrNCED5 in flavedo of Citrus reticulate. New Phytol. 216: 178–192. [DOI] [PubMed] [Google Scholar]
- Zimmermann I.M., Heim M.A., Weisshaar B., Uhrig J.F. (2004). Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J. 40: 22–34. [DOI] [PubMed] [Google Scholar]