Abstract
Immature B cells in the bone marrow (BM) emigrate into the spleen during adult lymphopoiesis. Here, we report that emigration is shifted to earlier B-cell stages in mice with orthotopic breast cancer, spontaneous ovarian cancer, and possibly in human breast carcinoma. Using mouse and human BM aspirates and mouse models challenged with highly metastatic 4T1 breast cancer cells, we demonstrated that this was the result of secretion of thymic stromal lymphopoietin (TSLP) by cancer cells. First, TSLP downregulated surface expression of BM retention receptors CXCR4 and VLA4 in B-cell precursors, increasing their motility and, presumably, emigration. Then, TSLP supported peripheral survival and proliferation of BM B-cell precursors such as pre-B-like cells. 4T1 cancer cells used the increased pool of circulating pre-B-like cells to generate metastasis-supporting regulatory B-cells. As such, the loss of TSLP expression in cancer cells alone or TSLPR deficiency in B-cells, blocked both accumulation of pre-B-like cells in circulation and cancer metastasis, implying that the pre-B cell-TSLP axis can be an attractive therapeutic target.
INTRODUCTION
The role of B cells in cancer remains poorly understood, although their infiltration in tumors can be associated with an unfavorable disease outcome (1). They can promote androgen-independent growth of prostate cancer cells by expressing lymphotoxin α/β (2) or HPV16-induced tumor progression (3) and breast cancer lymph node (LN) metastasis (4) by producing immunoglobulin. IgA, IL-10, and PD-L1 from liver resident plasma cells inhibit CD8+ T cells that prevent progression of hepatocellular carcinoma in humans and mice with non-alcoholic fatty liver disease (5). B cells also differentiate into regulatory B cells (Bregs), a phenotypically and functionally heterogeneous group of cells that include CD25+ tBregs (6), IL-10+ B10 cells (7), T2-MZP Bregs (8), IL-33+ Bregs (9), IL-35+ Bregs (10), and GrB+ Bregs (11). At least for some, their generation is controlled by cancer-secreted factors. We reported that 4T1 breast cancer cells, a BALB/c mouse model of human triple-negative breast carcinoma (12), uses 5-lipooxygenase metabolites to convert circulating B cells into CD25+ Bregs (tBregs) (13), which then promote lung metastasis by inducing the generation of FoxP3+ Tregs and activating myeloid-derived suppressive cells (MDSC) (6,14,15). Murine and human tumors, such as breast cancer cells, also express thymic stromal lymphopoietin (TSLP) (16,17). Although TSLP is expressed by epithelial cells, keratinocytes, mast cells, and basophils in response to a range of stimuli, including cancer cell-derived IL1α, IL-1β, TNFα, IL-13, and TGFβ (18), progression and metastasis of some cancers also require TSLP production from cancer cells themselves. TSLP’s purpose is to induce production of survival factors (19) or to activate invasive and angiogenic properties of alveolar macrophages (20). We reported that 4T1 cancer cell-produced TSLP induces Th2-skewed and CCL17-elevated lung environment (16) to enable co-infiltration of CCR4+ cancer cells and their protector CCR4+ Tregs in the lungs (6,16,21). This presumably explains why cancer cell-specific downregulation of TSLP is sufficient to abrogate lung metastasis (16,20).
TSLP acts through heterodimeric TSLPR and IL-7Rα receptor (22). It participates in adult B-cell lymphopoiesis in the bone marrow (BM) (23), where newly generated B cells undergo consecutive differentiation stages termed pro-B, pre-BI, large and small pre-BII, and immature B cells or fraction A-D cells, according to the Basel (24) and the Philadelphia nomenclatures (25), respectively. Although most B-cell precursors express both chains of TSLP receptor, TSLP primarily promotes proliferation of large pre-BII cells (23). The generation of pre-BI cells requires expression of the surrogate light chain (SLC) genes (VpreB and λ5) and the lymphoid-specific recombination-activating enzymes RAG1 and RAG2 to rearrange D to JH chains of the H chain locus. After completion of VHDJH rearrangement, pre-BI cells differentiate into large pre-BII cells, where the μH chain associates with SLC and signaling molecules Igα and Igβ as pre-BCR to support their proliferation in concert with IL7Rα. The pre-BII cells turn off expression of the SLC gene, downregulate pre-BCR and IL-7 signaling, and upregulate transcription factors Foxo1 and Pax5 to express RAG1, RAG2, and BLNK. This leads to generation of non-proliferating small pre-BII cells which, upon completion of the Ig L chain loci recombination, differentiate into immature IgM+ B cells and then emigrate from BM into spleen to further differentiate (26). However, circulating RAG+ BM B-cell precursors are noted in mice and humans after some inflammatory perturbations (27,28) and in transgenic Notch-deficient mice overexpressing TSLP in keratinocytes (29), suggesting that inflammation can cause premature emigration of BM B-cell precursors into an inhospitable for them splenic/peripheral environment (30). The functional relevance of this “unnatural” peripheral presence of B-cell precursors, and whether it is also increased in cancers producing TSLP, remains unknown.
Here, we report that some murine and human cancers can indeed induce accumulation of B-cell precursors in circulation. Using TSLP-sufficient and TSLP-deficient cancer cells, and mice with TSLPR deficiency, we linked this process to TSLP secreted from cancer cells. It presumably caused premature emigration of primary B-cell precursors in mice and humans by downregulating their surface expression of CXCR4 and α4β1 integrin, because loss of these two receptors would detach and thus cause the exit of pro-B cells from BM under sheer stress generated from intrasinusoidal turbulent blood flow and plasma transudation (30–32). The circulating B-cell precursors are then used by 4T1 cancer cells to generate tBregs, which then downregulate antitumor immune responses and promote lung metastasis.
MATERIALS AND METHODS
Mice and cells:
The animal protocols were approved by the IACUC institutional review board and performed under the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, 1985). Mice (5–20 weeks of age, females) were housed in a pathogen-free environment at the National Institute on Aging (NIA). BALB/cj and C57BL/6j mice were purchased from Jackson Laboratory (Bar Harbor, ME). We also used BALB/c-backcrossed μMT mice (C.129S2-Ighmth1Cgn, a gift from Professor Dr. Blankenstein, Max-Delbrück-Center for Molecular Medicine, Berlin, Germany) and Tslpr −/− mice (TSLPR KO, a gift from Dr. W.J. Leonard, NHLBI, Bethesda, MD), which were described previously (16), and mogp-Tag mice (in C57BL/6 background, a gift from Professor Dr. I. Miyoshi, Tohoku University Graduate School of Medicine, Japan). Murine breast cancer 4T1 and EMT6 cells, melanoma B16-F10 cells, 70Z/3 pre-B cells (TIB-158™), and human MCF7 cells were purchased from the American Type Culture Collection (ATCC). Murine AT3 cells were a gift from Dr. S. Abrams (Roswell Park Cancer Institute, Buffalo, NY). The 4T1.2 cells, a subset of 4T1 cells, selected for higher lung metastasis, were a gift from Dr. R.L. Anderson (Peter McCallum Cancer Center, Australia). The TSLP-deficient 4T1.2 cells (generated by shRNA knock down, cloneA6, TslpKD) and TSLP-sufficient 4T1.2 cells (control shRNA, clone3, 4T1.2ctrl) were previously described (6). Cells were free of mycoplasma, as shown by a mycoplasma detection kit (Lonza, Walkersville, MD) or by IDEXX BioAnalytics (Glen Burnie, MD). They were cultured in cRPMI: RPMI 1640 supplemented with 10% Fetal bovine serum, 1X of HEPES, sodium pyruvate, non-essential amino acids solution, Penicillin-Streptomycin-Glutamine (Gibco), and 55 μM 2-mercaptethanol. Cancer cell conditioned medium (CM) was prepared as described previously (6).
Tissues and blood processing:
All primary human samples were collected with written informed consent, including human bone marrow aspirates, by the Health Apheresis Unit and the Clinical Core Laboratory, NIA, under Human Subject Protocol # 2003054 and Tissue Procurement Protocol # 2003-071; PBMC and tonsils were obtained from healthy female adults, and human tumor and non-tumor breast tissues from patients with breast cancer (BC) the day before the surgery diagnosed and treated in the adjuvant setting at the Institute Jules Bordet under the institute’s approved ethics committee protocol (EC1981). Bone marrow, tumor, and lung tissues were dissociated with dissociation kit (MiltenyiBiotec, Auburn, CA) using GentleMACS™ Dissociator (MiltenyiBiotec) following the manufactureŕs instruction. Blood was collected in tubes with 2 mg/ml of Na-heparin (Sigma). Red blood cells were lysed with ACK buffer and immune cells were enriched by density gradient centrifugation in Ficoll-Paque (GE Healthcare, Pittsburgh, PA). Human tumor tissues were manually minced before two rapid rounds of mechanical dissociation with the GentleMACS™ Dissociator as described elsewhere (33). Murine single cell suspensions were prepared using a 70 μm cell strainer (BD Falcon, Bedford, MA).
Flow cytometry (FACS):
Antibodies (Ab) were purchased from Biolegend, eBioscience, BD Bioscience, and R&D Systems (STable 1). For cytokine intracellular (IC) staining, cells were stimulated with 50 ng/ml phorbol-12-myristate13-acetate (PMA, Tocris Bioscience, Minneapolis, MN) and 500 ng/ml Ionomycin (Tocris Bioscience) for 1–2 h, followed by Golgi stop for 3–4 h using 10 μmol/L of Monensin or Brefeldin A (eBioscience, ThermoFisher Scientific, Waltham, MA); and then stained following manufacturer’s instruction for IC fixation & permeabilization (eBioscience). Data were analyzed on FACS Canto II (BD Biosciences, San Jose, CA) or CytoFLEX (Beckman Coulter, Inc., Indianapolis, INC) using FlowJo software (Tree Star, Inc., Ashland, OR) or CytExpert software (Beckman Coulter, Inc.). Human samples were collected on a Navios (Beckman Coulter, Inc.) and analyzed using Kaluza Flow Cytometry Analysis v1.2 software. For FACS or magnetic sorting, murine B cells were pre-depleted of lineage positive (Lin+) cells (Gr1/CD11b/CD3e/CD8a/TER119/CD49b) with biotin-labelled Ab and EasySep Mouse Streptavidin RapidSpheres Isolation Kit (STEMCELL Technologies, Cambridge, MA) or PE-labelled Ab and anti-PE microbeads (Miltenyi Biotec, Auburn, MA) following the manufacturer’s instructions. B-cells were positively sorted with anti-CD19-PE using autoMACS Pro (Miltenyi Biotec) and then further enriched using relevant Ab in MoFlo XDP (Beckman Coulter, Inc.). Patient CD45+CD19+ B cells were sorted using Astrios sorter (Beckman Coulter, Inc.) and human BM B cells were purified using human CD19 MircoBeads (Miltenyi Biotech). Cell purity was 95–98%.
In vitro and in vivo assays:
Mouse and human B cells (106 cells/ml) were cultured in cRPMI supplemented with or without 50–100 ng/ml murine BAFF/Blys (R&D Systems, Minneapolis, MN), or with murine or human TSLP (R&D Systems), or 16–50% of cancer CM for 2–4 days. The generation of tBregs was described elsewhere (6). In brief, sorted Lin (GR-1, CD3, CD11b, CD49b, TER119)− CD19+ IgM− or IgM+ BM B cells (106 cells/ml) were cultured with 50% conditioned medium (CM) from 4T1.2 cells in cRPMI for 7 days. Then, after staining with viability dye Efluor780, live cells were resorted to perform the T cell suppression assay, as previously described (21). In brief, splenic CD3+ T cells (purified using mouse CD3+ T-cell enrichment column, R&D Systems) were labeled with eFluor450 Cell Proliferation Dye (eBioscience) and stimulated with anti-mouse CD3e Ab (1.5–2 μg/ml, BD Biosciences) or Dynabeads anti-CD3/28 beads (1 bead for 3 cells, Thermo Fisher Scientific, Waltham, MA) in the presence of an equal number of B cells for 4–5 days.
For immune profiling, 4T1, 4T1.2, 4T1.2ctrl, TslpKD, or EMT6 cells (0.5–2×106) were injected subcutaneously (s.c.) in the 4th mammary gland of BALB/c and TSLPR KO mice; AT3 breast cancer or B16-F10 (B16) melanoma cells (0.5×106) were s.c. injected in C57BL/6 mice. To enrich tBregs, BALB/c mice with 4T1.2 cancer were injected intraperitoneally (i.p.) with anti-CD20 Ab (clone 5D2, Genentech, 250 μg/mouse, 2 times), or isotype-control IgG as previously described (14), or with AMD3100 (200 μg/mouse, Sigma) every 2–3 days after tumor challenge. For B-cell trafficking experiments, μMT mice with 4T1 or 4T1.2 cancer were injected intravenously (i.v.) with an equal number of eFluor450-labelled CD23− BM B cells and eFluor670-labelled splenic B cells or with CellVue Burgundy-labelled CD20− BM B cells to analyze them after 1–7 days. For lung metastasis, mice were s.c. challenged with 0.5×105 4T1.2 cancer cells in the 4th mammary gland and the lungs were analyzed as we previously described (21). To evaluate the role of B cells, µMT and TSLPR KO mice were adoptively i.v. transferred with sort-purified B cell subsets of interest (1–3×106 cells) 2–4 days post tumor challenge. Alternatively, 4T1 tumor-bearing BALB/c mice were i.p. treated with 20 μg of MK-886 (sodium salt, Cayman Chemical, Ann Arbor, MI) at days 3, 5, 8, 11, and 13 to eliminate tBregs (13), and then at day 15 mice were i.v. transferred with sort-purified cells (5×106) from spleen of tumor bearing BALB/c mice.
RNA analysis:
RNA was isolated with RNeasy Plus Micro Kit (QIAGEN, Germantown, MD) or TRIzol Reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s instruction. After reverse transcription with Maxima First Strand cDNA Synthesis Kit, gene expression was quantified with qRT-PCR (Thermo Fisher Scientific) after normalization against Gapdh (see the list of primers, STable 2). Microarray analysis was performed using the Illumina and Agilent platforms. The sample raw intensity values were quantile-normalized and log2-transformed; a gene expression heatmap was generated with ComplexHeatmap (34). The data were compared with the microarray expression profiles from the Immunological Genome Project (35,36) (NCBI GEO accession GSE15907). To reconcile the datasets from different microarray instruments, a cross-platform normalization was performed using XPN to allow comparative analysis (37). Principal component analysis was performed with prcomp (R Core Team 2018, https://www.R-project.org/) and visualized with the ggfortify package. For the human Cancer Genome Atlas database (TCGA, https://portal.gdc.cancer.gov) survival analysis, we used gene expression values recomputed by Toil (38). The upper 25% (high gene expresser) and the lower 25% (low gene expresser) were compared for each gene and type of cancer of interest. A log rank test was applied to detect significant differences in overall survival between low and high expressers with a significance threshold set at 0.05. A false discovery rate was controlled with Benjamini & Hochberg procedure. Wilcoxon Signed Rank Test was used to detect changes in expression of genes with a significance threshold set at 0.05. We adjusted P-values for multiple testing using Holm’s correction. To evaluate VPREB3 in single cell expression data (39), the values for Z-score-normalized imputed expression in B cells were extracted and used.
Statistical Analysis:
The results are presented as the mean with each individual data point or in bar graph ± SD. GraphPad Prism (Prism 6; Graph Pad Software, Inc., San Diego, CA) was used to perform statistical analysis. Data were analyzed using non-parametric Mann-Whitney U or Welchś t-test, or one-way ANOVA. A P-value less than 0.05 was considered significant (*P < 0.05, **P < 0.01, and ***P < 0.001).
RESULTS
Circulating Pre-B-like cells accumulate in mice and humans with cancer
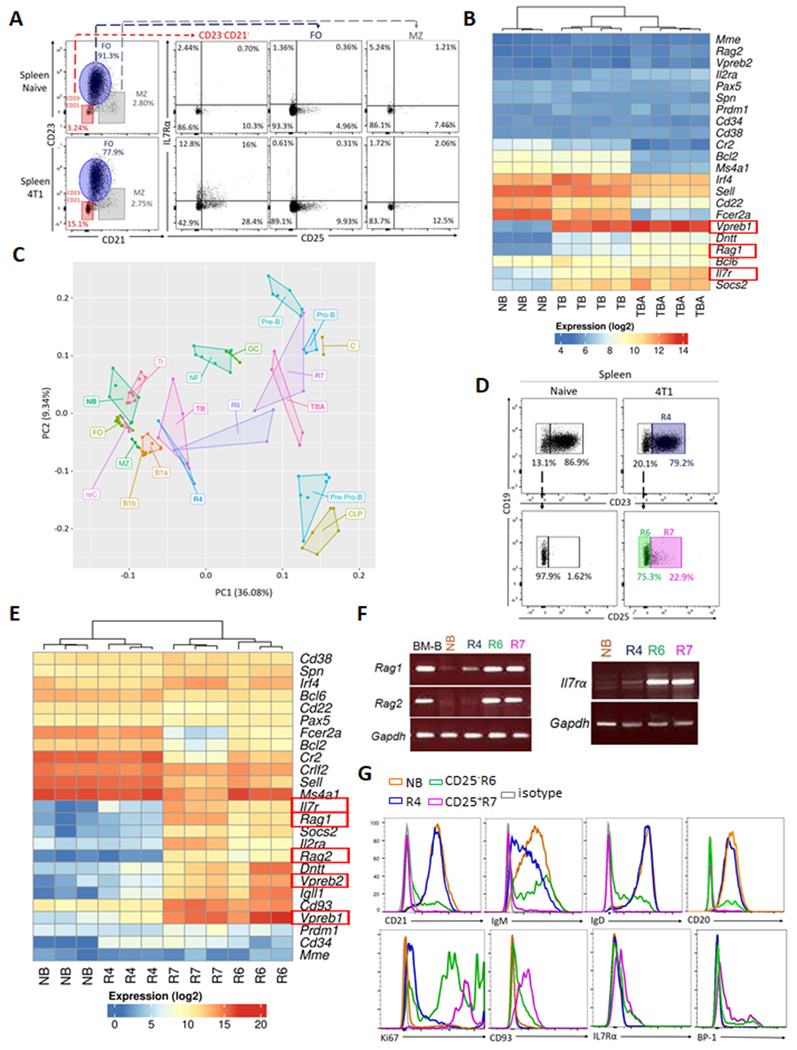
We previously reported that the frequency of cancer-induced tBregs can be increased by depletion of B cells with anti-CD20 antibody (Ab) (14). Therefore, this strategy was used to characterize these cells in spleens of mice with 4T1 cancer using a flow cytometric analysis. Compared with cancer-free mice (NB), cancer-bearing mice (TB) markedly increased cells resembling CD25+ and CD25− BM B-cell precursors (CD23−CD21−IL7Rα+, in terms of numbers and frequency, Fig. 1A), which was further increased after treatment with anti-CD20 Ab of TB mice (TBA, Supplementary Fig. S1A). To confirm this result, we performed an RNA microarray analysis of FACS-sorted B cells from spleens of TBA, TB and NB mice. The BM B-cell precursor-specific transcripts, such as Rag1, Vpreb1, and IL7rα (40), were significantly enriched in TB, and particularly TBA (Fig. 1B and Supplementary Table S3). Principal component analysis (PCA) of the Immunological Genome Project database (35,36), linked B cells from TBA closer to pro- and pre-B cells than to other B-cell subsets (Fig. 1C). In contrast, transcription profile of control splenic B cells from anti-CD20 Ab-treated cancer-free mice differed from that of BM B cells (aCD20_NB, Supplementary Fig. S1B,C). We repeated transcription analysis after FACS-sort purification of the CD25+ (R7) and CD25− (R6) subsets of the B-cell precursor-like cells from spleen of TB mice (Fig. 1D). The R7, and at a lesser extent R6, were again markedly enriched in expression of BM B-cell precursor genes (Fig. 1E,F and Supplementary Table S4) and closely associated them to TBA and BM B-cell precursors (Fig. 1C). While R6 appeared to be pro-B cells, R7 cells resembled proliferating (Ki67+) large Fr.C’ pre-B cells from BM (IgM−IgD− CD20−/LoCD21− CD23− CD24HiCD25+CD43+CD93+BP1+IL7Rα+ B220Int, Fig. 1A,G and Supplementary Fig. S2A–C). These cells (termed pre-B-like cells; from here on we mostly discuss R7 cells, CD25+CD23−CD21− CD20−/LoB220IntBCR− CD19+, unless specified) were also significantly increased in the circulation and primary tumors of 4T1 cancer-bearing mice (p<0.001, n=14–32, both in terms of numbers and frequency, Fig. 2A,B and Supplementary Fig. S3A). They were also increased in the circulation of C57BL/6 mice with spontaneous ovarian cancer (mogp), but were absent in C57BL/6 and BALB/c mice with orthotopic B16-F10 melanoma and EMT6 breast cancer cells, respectively (Fig. 2C–E and Supplementary Fig. S3B,C). Analysis of the Cancer Genome Atlas database (TCGA, https://portal.gdc.cancer.gov) also found significant upregulation of BM B-cell precursor-specific/associated genes (IGLL1, IGLL5, VPREB1, VPREB3, RAG1, RAG2, IL2RA, and IL7Ra) in various human tumors (p<0.05, Supplementary Table S5). In single cell RNAseq data (39), VPREB3 expression was also increased in B cells infiltrated human breast tumors as compared to healthy tissues (Supplementary Fig. S3D). To confirm these results, we evaluated cryopreserved PBMC from humans with breast cancer (BC, n=85) and age-and sex-matched healthy donors (HD, n=18). The frequency of CD10+CD19+ B cells (which include pre-B and immature B cells (41)) was significantly increased in BC as compared to HD (Fig. 2F). RT/PCR assay confirmed expression of VPREB and RAG½ in tumor-infiltrating B cells (Fig. 2G and Supplementary Fig. S3E). Hence, circulating pre-B-like cells can also accumulate in humans with cancer.
Figure 1. Pre-B-like cells accumulate in the spleens of mice with 4T1 cancer.
Compared with follicular (FO) B cells (CD23+CD21+CD19+, R4) and MZ B cells (CD23−/LoCD21+CD19+), BM B-cell precursor-like CD25+ (R7) and CD25− (R6) IL7Rα+ CD21−CD23−CD19+ cells were increased in spleens of mice with and without 4T1 cancer, as shown in FACS dot plot (A and D), gene expression heat map (B and E, n=3–4) and transcription PCA analysis (C, n=3–6) of splenic B cells (B and C) and their FACS-sorted R7, R6, and R4 subsets (C and E) from BALB/c mice with 4T1.2 cancer, which were treated with control IgG (Ab, TB) or anti-CD20 Ab (TBA cells), or without cancer (NB). The PCA analysis (C) links TBA and R7 closer to BM B-cell precursors (pro-B and pre-B) than to other B cells (common lymphoid precursors, CLP; pre-pro-B; germinal center, GC; FOB; marginal zone, MZ; B1a; B1b; Transitional, Tr, newly formed, NF; and recirculating, reC) in the Immunological Genome Project. Expression of the BM B-cell precursor-specific genes (highlighted in Red, B and E) were confirmed with RT/PCR (F) and FACS analysis (G). The results were independently reproduced at least twice.
Figure 2.
Murine and human cancers increase circulating pre-B-like cells, as revealed by FACS phenotyping in the indicated tissues and primary tumors of BALB/c mice with 4T1 cancer (n=14–32; shown frequency, A, and absolute numbers, B, of CD25+ Pre-B-like cells ) and C57BL/6 mice with spontaneous ovarian cancer (n=6–13, mogp, C). Mice with EMT6 cells, despite having a larger tumor than 4T1 cells (D), inefficiently increased CD25+pre-B-like cells (E, n=5, the result was reproduced twice). Compared with healthy people (HD, n=18), peripheral blood of humans with breast cancer markedly increased the frequency of CD10+CD19+ B cells (BC, n=85, F). RT/PCR assay confirmed expression of RAG1, RAG2, and VPREB in tumor infiltrating B cells in humans with BC (TIL-B, n=5, relative to GAPDH, G). Controls were tonsils (n=2, G). A–F, each symbol is for a single mouse or human and G shows mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant in Mann-Whitney Wilcoxon t-test (A–F).
Cancer-produced TSLP impairs BM retention of B-cell precursors
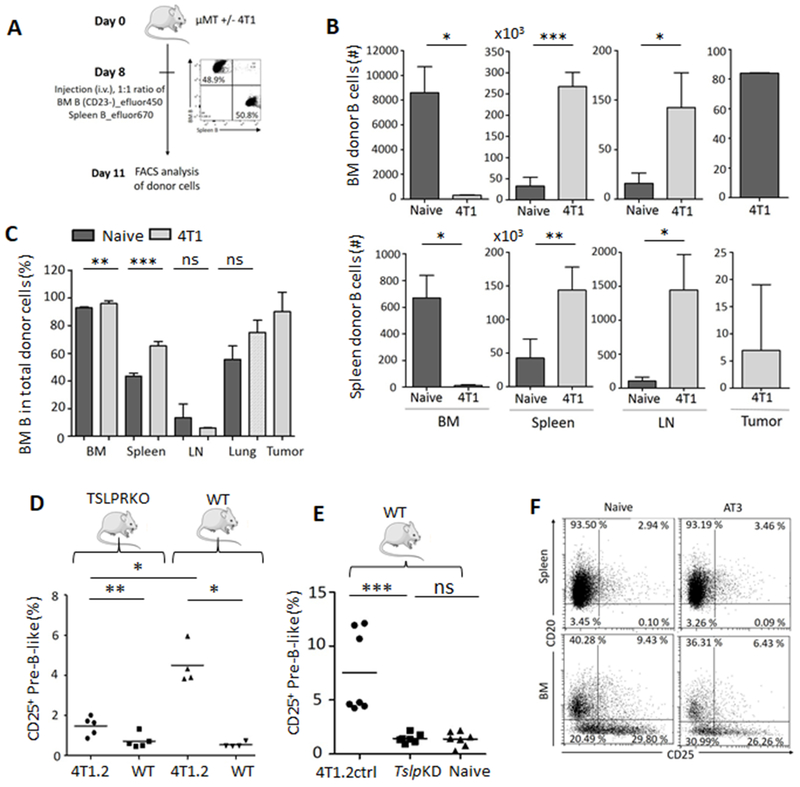
Unlike circulating pre-B-like cells, their CD25+ counterparts were decreased in BM of mice with 4T1 cancer (Supplementary Fig. S4A–C). Given that overexpression of TSLP in keratinocytes can similarly increase BM Rag+ B-cells in circulation (29) and that 4T1 cancer cells abundantly express TSLP (16), we sought whether cancer TSLP caused emigration of B-cell precursors from BM. To test this, we i.v. transferred a 1:1 mixture of BM B-cell precursors (stained with eFluor450) and total splenic B cells (eFluor670+) from naïve mice into B-cell-deficient μMT mice with and without 4T1 cancer (Fig. 3A). At day 3, the majority of donor cells in BM (>80%) and about 40% in the spleen and lungs of cancer-free mice were of BM origin (Fig. 3B,C). In contrast, cancer caused significant decrease of BM cell migration into BM and instead increased their homing into spleen and lungs (Fig. 3B). Interestingly, almost 90% of transferred cells in primary tumor were BM B cells (Fig. 3C). In a repeat of this experiment with BM CD20−/Lo B-cell precursors (included CD25+ and CD25− subsets), the cells remained increased in circulation but further decreased in BM of mice with 4T1 cancer even after 26 days (Supplementary Fig. S4D–F), implying the pre-B-like cell accumulation in circulation could be due to dysregulation of BM microenvironment and cell migration. To link this process to TSLP, we quantified pre-B-like cells in TSLPR deficient (TSLPR KO) and WT BALB/c mice with or without 4T1 cancer. Despite comparable growth of primary tumors and total B-cell counts (Supplementary Fig. S4G,H), the increase of circulating pre-B-like cell was markedly abrogated in TSLPR KO mice as compared to WT mice (both frequency and numbers, Fig. 3D and Supplementary Fig. S4I). We also quantified pre-B-like cells in WT BALB/c mice bearing s.c. 4T1.2 cells whose TSLP expression was either blocked with shRNA knockdown (TslpKD) or unaffected with an irrelevant shRNA (4T1.2ctrl) (16). While 4T1.2ctrl cells readily increased pre-B-like cells in circulation, their TslpKD subset failed to do so (Fig. 3E and Supplementary Fig. S4J). Moreover, BALB/c and C57BL/6 mice, respectively, challenged with EMT6 and AT3 breast cancer cells, which do not express TSLP (19,20), did not increase pre-B-like cells (Fig. 2E and Fig. 3F). Together, these results suggest that the B-cell precursor emigration from BM and accumulation in circulation require the TSLP/TSLPR axis.
Figure 3. TSLP/RSLPR axis controls emigration of BM B-cell precursors.
A, Shown is schema of in vivo tracking experiment with a 1:1 mixture of BM B-cell precursors (eFluor450 labeled) and splenic B-cells (eFluor670) after their adoptive transfer into μMT mice with (light grey) and without 4T1 cancer (dark, n=3). Donor B-cells were quantified 3 days after transfer in the indicated tissues (numbers, B, and frequency, C). Shown is mean ± SD of a representative experiment reproduced twice. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant in Welch’s t-test. Compared with WT, TSLRP KO mice poorly accumulated CD25+ pre-B-like cells in spleen (D, n=5–4). Similarly, unlike 4T1.2ctrl cells, the TSLP-deficient 4T1.2 cells (TslpKD, (E, n=7) or AT3 cells (F, n=5) do not increase CD25+ pre-B-like cells in spleen of WT mice. In D and E, each symbol is for an individual mouse, P-values were calculated with Mann-Whitney Wilcoxon; data were reproduced at least twice. In F, shown are BM and splenic B cells expressing CD25 and CD20 (%).
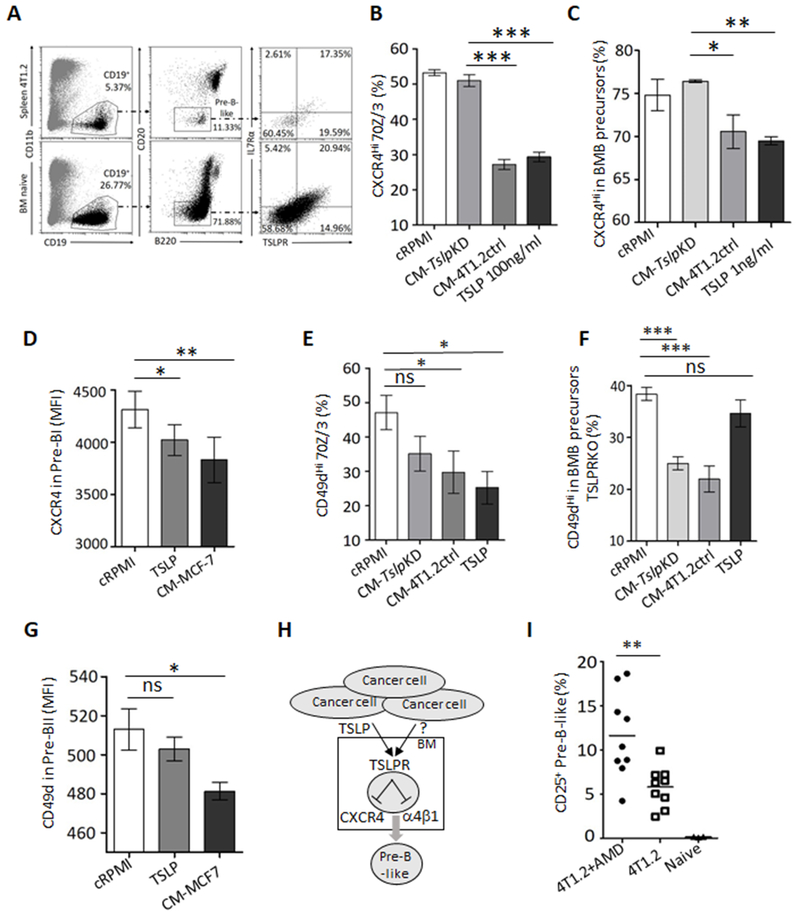
Because loss of CXCR4 signaling and consequent reduction of α4β1 integrin in pro-B cells causes their premature emigration from BM under sheer stress generated from intrasinusoidal turbulent blood flow and plasma transudation in BM (30–32), we sought whether it was mediated by TSLP. First, we evaluated expression of TSLPR in murine BM primary B-cell precursors and their immortalized 70Z/3 cells and found that they expressed both chains of TSLPR (Fig. 1B,E–G, Fig. 4A, and Supplementary Fig. S5A,B). We therefore treated 70Z/3 cells with recombinant TSLP or conditioned medium (CM) from TSLP-sufficient and -deficient 4T1.2 cells (CM-4T1.2ctrl and CM-TslpKD, respectively). Only TSLP and TSLP-containing CM significantly downregulated CXCR4 (Fig. 4B and Supplementary Fig. S5C). We repeated this experiment using purified BM B-cell precursors from naïve WT or TSLPR KO mice. Only TSLP and CM-4T1.2ctrl, but not CM-TslpKD, significantly inhibited expression of CXCR4 on the surface of B-cell precursors from WT, but not TSLPR KO, mice (Fig. 4C and Supplementary Fig. S5D). We also evaluated response to TSLP in primary B-cell precursors purified from BM aspirates of healthy human donors. Their pre-BI cells markedly downregulated CXCR4 after treatment with human recombinant TSLP or CM from human MCF7 breast cancer cells (Fig. 4D), which naturally secrete TSLP (16,17). Although TSLP downregulated α4β1 in 70Z/3 cells (Fig. 4E and Supplementary Fig. S5E), the expression of this integrin on the surface of primary B-cell precursors of mice and humans was primarily inhibited with cancer CM independent of TSLP (Fig. 4F,G and Supplementary Fig. S5F). Thus, we concluded that by impairing the CXCR4 and α4β1 in B-cell precursors (Fig. 4H), cancer-produced TSLP and other factors presumably caused their premature emigration from BM. In concordance, 4T1 cancer-bearing mice further increased circulating pre-B-like cells after treatment with a CXCR4-specific inhibitor AMD3100 (Fig. 4I) (42) without affecting tumor growth and metastasis (Supplementary Fig. S5G,H).
Figure 4. Cancer-produced TSLP targets receptors of BM retention in B-cell precursors.
Representative FACS dot plots show that BM and splenic B-cell precursors express both chains of TLSPR, TSLPR, and IL7Rα in mice with and without 4T1.2 cancer (n=5, A). Compared with cRPMI or CM-TslpKD, treatment with TSLP or TSLP-sufficient cancer medium (CM-4T1.2ctrl) downregulates expression of CXCR4 on the surface of 70Z/3 pre-B cells (%, B) and early B-cell precursors from BM of mice (%, C) and pre-BI cells from BM of humans (D, MFI in CD34+CD10+ IgM−CD19+). TSLP also downregulates α4β1 integrin (CD49d) on the surface of 70Z/3 cells (CD49dHi, E). In contrast, only cancer CM inhibits CD49d on the surface of early BM B-cell precursors from BM of WT (E) and TSLPR KO mice (%, F) and human BM pre-BII cells (G, MFI) independently of TSLP. Shown are results after overnight treatment with murine TSLP (100 ng/ml, B, E, and F; and 1 ng/ml, C) and human recombinant TSLP (500 ng/ml, D and G) at 100 ng/ml (B, D–F) and 1 ng/ml (C); or CM-TslpKD and CM-4T1.2ctrl (16% in cRPMI, B and C) and CM from MCF7 cells (50% in cRPMI, D and G). The accumulation of CD25+ pre-B-like cells in BALB/c mice with 4T1.2 cancer was further increased by inhibition of CXCR4 signaling (AMD3100, 200 μg/mouse, n=9, I). Shown are representative results from experiments reproduced at least twice . P-values were calculated with Welch’s t-test (B–G) and with Mann-Whitney Wilcoxon (I). The summary figure to show that by acting via TSLPR, cancer-produced TSLP downregulates CXCR4 and α4β1 integrin in BM B cell precursors, presumably causing their detachment and emigration from BM (H).
TSLP supports pre-B-like cells in circulation
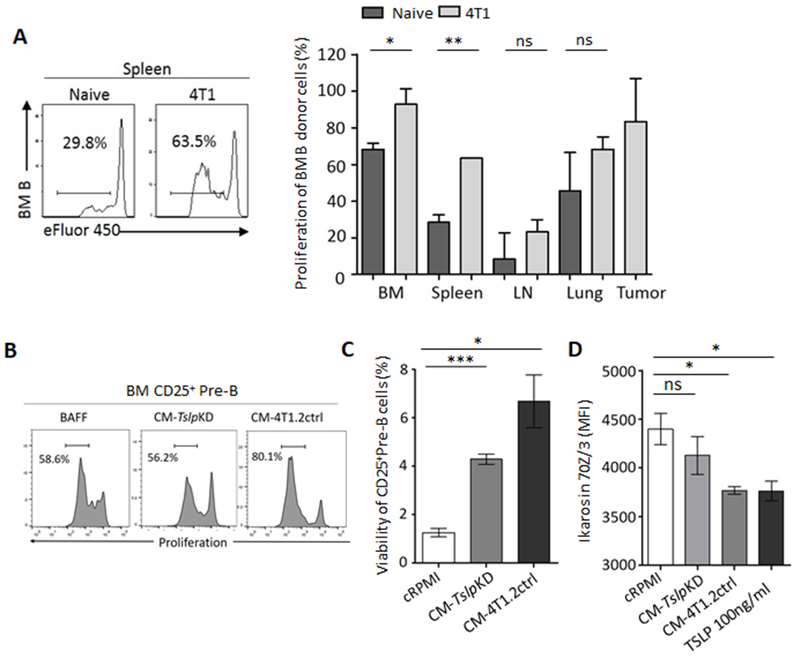
B-cell progenitors do not survive in an inhospitable splenic/peripheral microenvironment (30). However, our circulating pre-B-like cells exhibited signs of increased proliferation (expressed Ki67, Fig. 1G), suggesting that cancer also promoted their peripheral survival and expansion. To test this possibility, we adoptively transferred eFluor450-labelled BM B-cell precursors from naive mice into μMT mice, with or without 4T1 cancer, to quantify B-cell proliferation after 4–5 days. Compared with cancer-free mice, mice with 4T1 cancer significantly increased their eFluor450 dilution in circulation (Fig. 5A), suggesting an enhanced proliferation/survival. To link this result to cancer-produced TSLP, we in vitro cultured eFluor450+ BM B-cell precursors from naïve mice with CM-4T1.2ctrl, CM-TslpKD, or with BAFF/BlyS (a B-cell-supporting factor that does not induce tBregs (6)) for 4 days. Compared to BAFF or CM-TslpKD, the TSLP-sufficient CM-4T1.2ctrl indeed significantly increased proliferation (Fig. 5B) and viability (Fig. 5C) of CD25+ B-cell precursors. Because transcription factor Ikaros regulates survival and proliferation of BM B-cell precursors by suppressing c-Myc (43), we tested whether TSLP expanded pre-B-like cells by targeting this pathway in 70Z/3 cells. As early as 6 h after treatment with recombinant TSLP or TSLP-sufficient, but not deficient, CM, the cells markedly downregulated expression of Ikaros (Fig. 5D).
Figure 5. TSLP supports pre-B-like cells in circulation.
Compared with cancer-free mice, proliferation of i.v. transferred BM B-cell precursors is increased in the circulation of μMT mice with 4T1 cancer. Shown are eFluor450 dilution in donor cells (a representative histogram in spleen, left panel) and summary quantification in various tissues, right panel, n=3, A). In vitro experiments (B–D) suggest that cancer-produced TSLP increases proliferation (a representative histogram of eFluor450 dilution, B) and survival (C) of BM CD25+ pre-B cells by downregulating their Ikaros expression (in 70Z/3 pre-B cells, D). TSLP downregulated Ikaros expression in murine (D, MFI). Data are from a 4-day (B and C) or overnight (D) culture with BAFF-supplemented cRPMI (BAFF), CM-4T1.2ctrl, CM-TslpKD, or 100 ng/ml TSLP. Shown are mean % or MFI ± SD of triplicate experiments reproduced at least twice. P-values were calculated with Welch’s t-test.
Cancer uses pre-B-like cells to generate metastasis-promoting tBregs
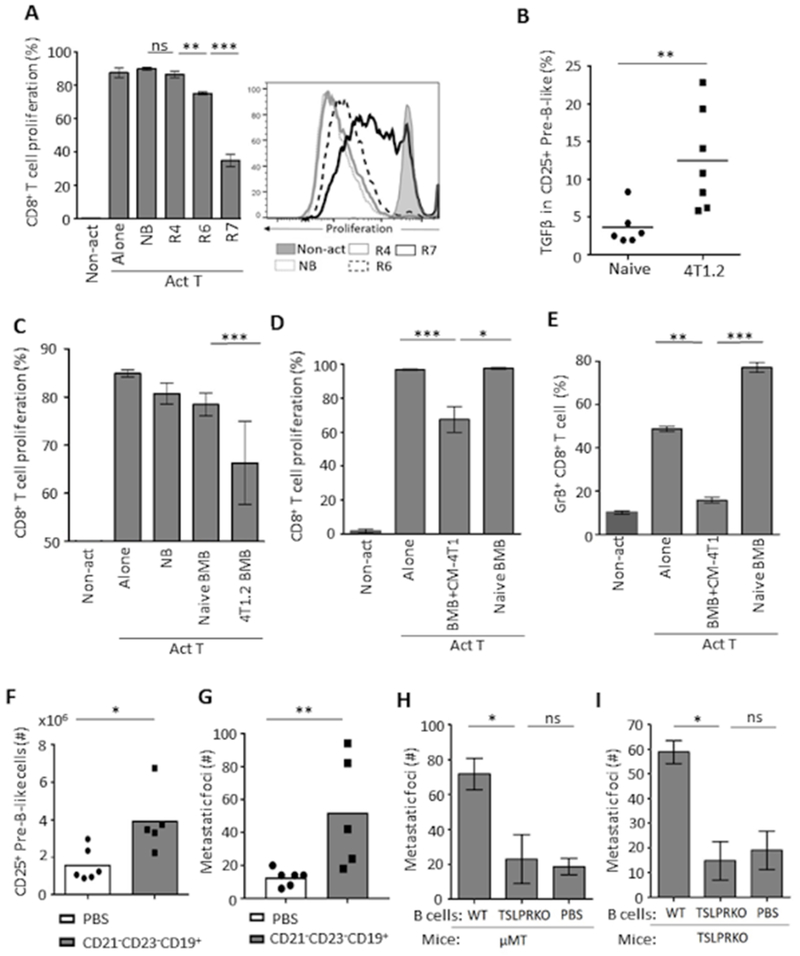
Circulating pre-B-like cells and tBregs share some phenotypic features, and their accumulation appears to mirror each other, such as being present in mice with TSLP-producing breast cancer and absent in mice with TSLPR or B-cell deficiency (6,14,15). Given that the tBreg generation requires 4T1 cancer cell-produced factors such as 5-LO metabolites (13), we hypothesized that the accumulation of B-cell precursors in circulation was to provide a source for the generation of tBregs. First, we tested the immunoregulatory function of FACS-sorted splenic R7 (CD25+) and R6 (CD25−) pre-B-like cells and FOB (R4) cells from 4T1 cancer-bearing mice by co-culturing them with eFluor450+ T cells from congenic naïve mice in the presence of anti-mouse CD3e Ab for 4–5 days. Compared to control FOB cells from mice with or without cancer, the R7, and at lesser extent R6, cells significantly inhibited eFluor450 dye dilution of CD8+ and CD4+ T cells (Fig. 6A and Supplementary Fig. S6A). The R7 cells also suppressed IFN-γ and IL-2 production from activated CD4+ T cells (Supplementary Fig. S6B). Moreover, only circulating R7 cells from cancer-bearing, but not cancer-free, mice markedly upregulated expression of TGFβ (Fig. 6B), a key factor that mediates immunoregulation of tBregs (6,13), implying that their regulatory function was not intrinsic but rather induced by cancer. To test this possibility, we performed T cell suppression assay with BM B-cell precursors from 4T1 cancer-bearing and cancer-free mice. Only B cells from mice with cancer inhibited proliferation of CD8+ and CD4+ T cells (Fig. 6C and Supplementary Fig. S6C). Conversely, sort-purified BM B-cell precursors from cancer-free mice were treated with 4T1-CM for 7 days. Unlike mock-treated or freshly isolated BM B-cell precursors, only cancer CM-treated B-cell precursors inhibited proliferation and granzyme-B expression of CD8+ and CD4+ T cells stimulated with anti-CD3/CD28 beads (Fig. 6D,E and Supplementary Fig. S6D,E). Next, we tested whether this conversion also enhances lung metastasis of 4T1 cells implanted in mammary glands of BALB/c mice, the feature we previously linked to tBregs (6,15). First, 4T1 cancer-bearing mice were treated with MK886 (at days 3, 5, 8, 11, and 13), a 5-LO/FLAP inhibitor which abolishes tBregs (13). Then, two days after the last treatment, mice were i.v. transferred with sort-purified pre-B-like cells from spleen of mice with 4T1.2 cancer. The transfer did not affect the growth of primary tumors (Supplementary Fig. S6F) but instead significantly increased the number of circulating pre-B-like cells (Fig. 6F) and, importantly, metastatic foci in the lungs (Fig. 6G). To confirm this result, we performed a series of independent experiments by transferring B cells into either μMT or TSLPR KO mice with 4T1.2 cancer, which cannot support metastasis due to the inability to generate tBregs (6,14). Only the transfer of B cells from WT, but not TSLPR KO, mice with 4T1 cancer significantly increased lung metastasis (Fig. 6H,I), but not tumor weight (Supplementary Fig. S6G,H). The transfer of FOB failed to do so (Supplementary Fig. S6I).
Figure 6. 4T1 cancer uses pre-B-like cells to generate tBregs (A–F) which promote metastasis (G–I).
Shown are representative data from in vitro CD8+ T cell suppression assay with pre-B-like cells from spleen (R6 and R7, A) or with BM B cells (both CD25+ and CD25− IgM− B-precursors, BMB, C) from mice with 4T1 cancer (4T1.2 BMB) or without cancer (NB). Control cells were FOB (R4) and BMB from naïve mice. Non-act is for unstimulated T cells and Act T is for T cells stimulated with anti-CD3e Ab (A) or with anti-CD3e/anti-CD28 Ab beads (C-E). Unlike circulating pre-B-like cells from cancer-free mice, pre-B-like cells from mice with 4T1 cancer express high levels of TGFβ (intracellular FACS staining result, B). To demonstrate that 4T1 cancer confers B-cell precursors with a regulatory activity, naïve mouse BM B-cell precursors (BMB) are cultured in CM-4T1 or in cRPMI for 7 days (D and E). Only CM-4T1-treated BMB suppressed proliferation (D) and granzyme B expression (E) in CD8+ T cells in in vitro suppression assay. In A, C, and D, proliferation is measured by the dilution of eFluor450 (a representative histogram, right panel in A) and depicted as mean ± SD of triplicate experiments reproduced at least twice (n=3-5). In F–G, BALB/c mice bearing 4T1.2 cancer (pretreated with 20 μg MK886 to eliminate endogenous tBregs) are i.v. injected with PBS or purified pre-B-like cells from spleen of syngeneic mice with 4T1.2 cancer (n=5). Shown are numbers of CD25+ pre-B-like cells in spleen (F) and metastatic foci in the lungs (G). Similarly, splenic B cells from WT or TSLPR KO mice with 4T1.2 cancer were i.v. transferred into μMT (H, n=4) and TSLPR KO mice with 4T1.2 cancer (I, n=4). Each symbol is for a single mouse (B, F, G) and Y-axis in G, H, I is for mean of metastatic foci ± SD in the lungs 34 days post tumor challenge. Results are reproduced at least twice. P-values were calculated with one-way ANOVA followed by Tukey test (A), Welch’s t-test (D, E), and Mann-Whitney Wilcoxon t-test (B, C, F–I).
DISCUSSION
Cancer growing at distant sites can remotely affect BM hematopoiesis by inhibiting B-cell lymphopoiesis and skewing the differentiation of precursor cells towards myelopoiesis (44). Here we report that cancer can also cause premature emigration of newly generated B cells ensuing “unnatural” accumulation of pro-B and pre-B cells in spleen and primary tumor. Although the molecular mechanism of this process is a topic of a different study, we link it to cancer-produced TSLP which, by acting through its cognate receptor, targeted Ikaros to abrogate attachment of B-cell precursors in BM. Consistent with the importance of Ikaros in signaling of CXCR4 and the focal adhesion and expression of integrins (45), TSLP markedly downregulated the key receptors, CXCR4 and α4β1 integrin, which keep B-cell precursors in BM. The loss of CXCR4 signaling or α4β1 integrin binding to VCAM1-expressing stromal cells alone is sufficient to cause exit of B-cell precursors from the BM under sheer stress generated from intrasinusoidal turbulent blood flow and plasma transudation (30–32). Interestingly and consistent with their enrichment in spleen of mice treated with CXCR4 inhibitor AMD3100 (42,46), emigration of advanced B-cell precursors and myeloid cells from BM appeared to also depend on downregulation of CXCR4 but via a TSLP-independent process. This also explains why the circulating pre-B-like cells were significantly decreased, but not completely lost, in TSLPR KO mice with 4T1 cancer or in WT mice with TSLP-deficient cancer cells.
The splenic/peripheral microenvironment is inhospitable for BM B-cell precursors (30), as they quickly disappear when induced to prematurely emigrate after some immune perturbations (27,28). To circumvent this, cancer also utilized TSLP. It appeared to enhance proliferation and survival of our pre-B-like cells in circulation by, at least in part, downregulating Ikaros because its loss promotes proliferation of pre-B cells (43). Thus, the inability of pre-B-like cells to prematurely emigrate from BM and survive in circulation presumably explains their absence in circulation of mice with TSLP-deficient cancer cells (TslpKD 4T1.2, EMT6 and AT3 breast cancer cells, and B16-F10 melanoma cells) (19,20). The requirement for TSLP produced from cancer cells is puzzling as its expression can be induced in the host by various inflammatory and cancer-induced stimuli (TNFα, TGFβ, IL1β, and IL1α) (16,19,21). Although we cannot rule out the role of its local production from metastasized cancer cells in the BM, several lines of evidence suggest that TSLP remotely targeted BM cells. First, we reported that 4T1 cancer in mammary gland can activate distant tissues — the lungs — by increasing the concentration of TSLP in blood (16). Second, overexpression of TSLP alone in keratinocytes can increase Rag+ B-cells (possibly pre-B-like cells) in circulation of Notch-deficient mice (29). This phenomenon appears to also occur in humans with cancer, as their tumor cells express TSLP (16,17) and VPREB and RAG -expressing B cells were detectable in circulation and in primary tumors of patients with breast and ovarian carcinomas. In support, analysis of single-cell RNA-seq (39) and the Cancer Genome Atlas databases revealed significant transcription activation of VPREB, IGLL, and RAG, alone or paired with CD79A (BCR alpha chain), in human breast cancer TIB.
The functional and clinical relevance of circulating pre-B-like cells remains unknown. Unlike its role in promoting cancer-supporting CD4+ T cells (16), TSLP primarily caused the pre-B-like cell accumulation without affecting their function, as the pre-B cells per se did not express TGFβ, an important factor utilized by tBregs in the regulation of T cell activity, the generation of FoxP3+ Tregs, and education of MDSCs to successfully support cancer metastasis (6,15). We think that their subsequent fate is determined by the inflammatory milieu, causing extranodal differentiation, cryoglobulinemia, and self-reactive antibody (47,48). In mice with 4T1 cancer, the circulating pre-B-like cells differentiated into CD25+ tBregs presumably in response to cancer-produced factors such as 5-LO metabolites, which we reported to cause the CD25+ tBreg conversion from peripheral B cells (13). In support, upon treatment with CM of 4T1 cancer cells, pre-B cells upregulated expression of TGFβ and suppressed T cell activity. Although 4T1 cancer does not generate T2-MZP Bregs and, conversely, our pre-B-like cells were absent in mice with B16 melanoma that accumulate T2-MZP Bregs (8), we cannot rule out differentiation of the pre-B-like cells into transitional 2-marginal zone precursor (T2-MZP) B cells under different inflammatory conditions. Unlike IL-10-expressing Bregs or suppressive FasHigh B cells differentiated from CpG-ODN treated pro-B cells (49), the CpG-ODN stimulation converts tBregs into potent inducers of cytolytic antitumor CD8+ T cells (14). Similarly, the inflammatory milieu can similarly determine the fate of human pre-B-like cells, explaining why expression of B-cell precursor-specific genes (VPREB, IGLL, and RAG genes) in the human RNA-seq tumor database can be associated with both overall shorter survival in breast, ovarian, and cervical cancers and improved disease outcome in kidney renal clear cell and liver carcinomas (p<0.05, Supplementary Fig. S7A–E).
Overall, expanding our previous report that TSLP from cancer cells induce metastasis-supporting Th2-skewed CD4+ T cell response (16), we demonstrate that this pluripotent cytokine causes premature emigration of early BM B-cell precursors from BM and their subsequent survival and expansion in circulation. In the case of some cancers, it is exploited to generate immunoregulatory B cells and, thereby, to escape from immune surveillance. We propose that this axis can be targeted to improve the outcome in cancer, because when the source of tBregs (i.e., circulating pre-B-like cells) was lost in μMT mice, which cannot support B-cell differentiation beyond the pro-B-cell stage (50), or in TSLPR KO mice, cancer failed to generate tBregs and efficiently metastasize (14,16).
Supplementary Material
SIGNIFICANCE.
Cancer cells induce premature emigration of B-cell precursors from the bone marrow to generate regulatory B cells
ACKNOWLEDGMENTS
We are grateful to Dr. A.C. Chan (Genentech, Inc., San Fracisco, CA) for providing anti-CD20 Ab; S. Abrams (Roswell Park Cancer Institute, Buffalo, NY), and R.L. Anderson (Peter McCallum Cancer Center, Australia) for the gift of valuable cells; Drs. E. Lehrmann and Y. Zhang (NIA/NIH) for microarray assay and bioinformatics analysis; and Ana Lustig (NIA/NIH) for proofreading. The authors are also grateful to Cindy Clark (NIH Library) for copyediting of the manuscript. This research was supported by the Intramural Research Program of the National Institute on Aging, NIH and in part by a CRADA with Janssen Research & Development, LLC (E.R, K.M, M.B, CC, X.W, T.B, K.G.B, R.W.M, and A.B.) and by the Russian Scientific Foundation grant 14-44-00077 (F.G and E.R).
Financial support: This research was supported by the Intramural Research Program of the National Institute on Aging, NIH, and in part by the Russian Scientific Foundation grant 14-44-00077; and a CRADA with Janssen Research & Development, LLC.
Footnotes
SUPPLEMENTARY MATERIALS contain Supplementary Fig. S1–7 and Supplementary Tables S1–5.
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.
Reference
- 1.Biragyn A, Lee-Chang C. A new paradigm for an old story: the role of regulatory B cells in cancer. Frontiers in immunology 2012;3:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 2010;464:302–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005;7:411–23 [DOI] [PubMed] [Google Scholar]
- 4.Gu Y, Liu Y, Fu L, Zhai L, Zhu J, Han Y, et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat Med 2019;25:312–22 [DOI] [PubMed] [Google Scholar]
- 5.Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2017;551:340–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4⁺ T cells to T-regulatory cells. Cancer Res 2011;71:3505–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. The Journal of clinical investigation 2011;121:4268–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America 2011;108:10662–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sattler S, Ling GS, Xu D, Hussaarts L, Romaine A, Zhao H, et al. IL-10-producing regulatory B cells induced by IL-33 (Breg(IL-33)) effectively attenuate mucosal inflammatory responses in the gut. Journal of autoimmunity 2014;50:107–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nature Medicine 2014;20:633–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TFE, et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res 2013;73:2468–79 [DOI] [PubMed] [Google Scholar]
- 12.Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P, et al. A novel orthotopic model of breast cancer metastasis to bone. ClinExpMetastasis 1999;17:163–70 [DOI] [PubMed] [Google Scholar]
- 13.Wejksza K, Lee-Chang C, Bodogai M, Bonzo J, Gonzalez FJ, Lehrmann E, et al. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor alpha. Journal of immunology 2013;190:2575–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP, et al. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer research 2013;73:2127–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodogai M, Moritoh K, Lee-Chang C, Hollander CM, Sherman-Baust CA, Wersto RP, et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res 2015;75:3456–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. Journal of immunology 2011;186:5656–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. The Journal of experimental medicine 2011;208:479–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebastian K, Borowski A, Kuepper M, Friedrich K. Signal transduction around thymic stromal lymphopoietin (TSLP) in atopic asthma. Cell Commun Signal 2008;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuan EL, Ziegler SF. A tumor-myeloid cell axis, mediated via the cytokines IL-1alpha and TSLP, promotes the progression of breast cancer. Nat Immunol 2018;19:366–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burkard-Mandel L, O’Neill R, Colligan S, Seshadri M, Abrams SI. Tumor-derived thymic stromal lymphopoietin enhances lung metastasis through an alveolar macrophage-dependent mechanism. Oncoimmunology 2018;7:e1419115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res 2009;69:5996–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Pre-B cell receptor expression is necessary for thymic stromal lymphopoietin responsiveness in the bone marrow but not in the liver environment. Proc Natl Acad Sci U S A 2004;101:11070–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osmond DG, Rolink A, Melchers F. Murine B lymphopoiesis: towards a unified model. Immunol Today 1998;19:65–8 [DOI] [PubMed] [Google Scholar]
- 25.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol 2001;19:595–621 [DOI] [PubMed] [Google Scholar]
- 26.Uckun FM. Regulation of human B-cell ontogeny. Blood 1990;76:1908–23 [PubMed] [Google Scholar]
- 27.Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature 1999;400:682–7 [DOI] [PubMed] [Google Scholar]
- 28.Gartner F, Alt FW, Monroe RJ, Seidl KJ. Antigen-independent appearance of recombination activating gene (RAG)-positive bone marrow B cells in the spleens of immunized mice. J Exp Med 2000;192:1745–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demehri S, Liu Z, Lee J, Lin MH, Crosby SD, Roberts CJ, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol 2008;6:e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med 2004;200:1145–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck TC, Gomes AC, Cyster JG, Pereira JP. CXCR4 and a cell-extrinsic mechanism control immature B lymphocyte egress from bone marrow. J Exp Med 2014;211:2567–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fistonich C, Zehentmeier S, Bednarski JJ, Miao R, Schjerven H, Sleckman BP, et al. Cell circuits between B cell progenitors and IL-7(+) mesenchymal progenitor cells control B cell development. J Exp Med 2018;215:2586–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garaud S, Gu-Trantien C, Lodewyckx JN, Boisson A, De Silva P, Buisseret L, et al. A simple and rapid protocol to non-enzymatically dissociate fresh human tissues for the analysis of infiltrating lymphocytes. J Vis Exp 2014; 94 doi: 10.3791/52392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016;32:2847–9 [DOI] [PubMed] [Google Scholar]
- 35.Painter MW, Davis S, Hardy RR, Mathis D, Benoist C, Immunological Genome Project C. Transcriptomes of the B and T lineages compared by multiplatform microarray profiling. J Immunol 2011;186:3047–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mabbott NA, Gray D. Identification of co-expressed gene signatures in mouse B1, marginal zone and B2 B-cell populations. Immunology 2014;141:79–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudy J, Valafar F. Empirical comparison of cross-platform normalization methods for gene expression data. BMC Bioinformatics 2011;12:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vivian J, Rao AA, Nothaft FA, Ketchum C, Armstrong J, Novak A, et al. Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol 2017;35:314–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018;174:1293–308 e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsubata T, Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med 1990;172:973–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaminski DA, Wei C, Qian Y, Rosenberg AF, Sanz I. Advances in human B cell phenotypic profiling. Front Immunol 2012;3:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 2003;102:2728–30 [DOI] [PubMed] [Google Scholar]
- 43.Ma S, Pathak S, Mandal M, Trinh L, Clark MR, Lu R. Ikaros and Aiolos inhibit pre-B-cell proliferation by directly suppressing c-Myc expression. Mol Cell Biol 2010;30:4149–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreau JM, Mielnik M, Berger A, Furlonger C, Paige CJ. Tumor-secreted products repress B-cell lymphopoiesis in a murine model of breast cancer. Eur J Immunol 2016;46:2835–41 [DOI] [PubMed] [Google Scholar]
- 45.Joshi I, Yoshida T, Jena N, Qi X, Zhang J, Van Etten RA, et al. Loss of Ikaros DNA-binding function confers integrin-dependent survival on pre-B cells and progression to acute lymphoblastic leukemia. Nat Immunol 2014;15:294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Q, Li Z, Gao JL, Wan W, Ganesan S, McDermott DH, et al. CXCR4 antagonist AMD3100 redistributes leukocytes from primary immune organs to secondary immune organs, lung, and blood in mice. Eur J Immunol 2015;45:1855–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meffre E, Chiorazzi M, Nussenzweig MC. Circulating human B cells that express surrogate light chains display a unique antibody repertoire. J Immunol 2001;167:2151–6 [DOI] [PubMed] [Google Scholar]
- 48.Astrakhan A, Omori M, Nguyen T, Becker-Herman S, Iseki M, Aye T, et al. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat Immunol 2007;8:522–31 [DOI] [PubMed] [Google Scholar]
- 49.Montandon R, Korniotis S, Layseca-Espinosa E, Gras C, Megret J, Ezine S, et al. Innate pro-B-cell progenitors protect against type 1 diabetes by regulating autoimmune effector T cells. Proceedings of the National Academy of Sciences of the United States of America 2013;110:E2199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 1991;350:423–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.