Abstract
Introduction
Agitation in individuals with Alzheimer's disease (AD) may predict institutionalization. This study assessed the incremental risk and costs associated with agitation in individuals with AD.
Methods
A retrospective analysis of the National Alzheimer's Coordinating Center Uniform Data Set (June 2005–February 2018) was conducted. Incremental risk of institutionalization associated with agitation was estimated and used with the number of institutionalized individuals with AD and agitation and costs of living by residential setting in the United States (literature-based), to estimate incremental institutionalization costs.
Results
The analysis included 11,348 individuals with AD: 6603 (58.2%) with and 4745 (41.8%) without agitation. Compared with individuals without agitation, those with agitation were 20% more likely to be institutionalized (odds ratio = 1.20; 95% CI = 1.08–1.33). Total incremental cost of institutionalization associated with agitation was $4.3 billion ($50,588/individual).
Discussion
Agitation is associated with a higher risk of institutionalization among patients with AD, which translates into a substantial economic burden.
Keywords: Alzheimer's disease, Agitation, Institutionalization, Long-term care, Nursing home, Costs
Highlights
-
•
Agitation in Alzheimer's disease portends higher risk of institutionalization.
-
•
Higher institutionalization risk translates into a substantial economic burden.
-
•
Agitation-related institutionalization cost was ∼$4.3 billion in the United States.
1. Background
Alzheimer's disease (AD), the most common form of dementia, is a complex neurodegenerative brain disease that affects over 5 million Americans [1]. The defining clinical features of AD include progressive decline in cognition and functional abilities, and a range of behavioral symptoms—including agitation, mood disorders, psychotic symptoms, and sleep disorders—that manifest throughout the disease process [2,3].
Agitation is among the most persistent and distressing behavioral symptoms of AD [4,5]. However, until 2015, there was no established definition of agitation, leading to variability in reports of disease burden and epidemiological data. Recently, a working group led by the International Psychogeriatric Association developed a consensus definition of agitation in patients with cognitive disorders. Agitation is defined as excessive motor activity, verbal aggression, or physical aggression that is associated with observed or inferred evidence of emotional distress, severe enough to produce excess disability, which in the physician's opinion is beyond that due to the cognitive impairment and not attributable solely to another comorbid psychiatric or medical condition [6].
Agitation is a frequent behavioral symptom in individuals with AD, but, owing to the lack of an established definition until recently, prevalence estimates vary greatly in the literature [4,7,8]. This is perhaps best illustrated by a recent large care home survey, in which 40% of patients with dementia were reported to have clinically significant agitation based on the Cohen-Mansfield Agitation Inventory (score ≥45), whereas 32% were reported to have clinically significant agitation based on the Neuropsychiatric Inventory (NPI; score ≥4 on the agitation domain) and 57% were reported to have any agitation (score ≥1 on the NPI agitation domain) [9]. Other studies have also reported prevalence of agitation for individuals with dementia in residential facilities to be between 28% and 60% [5,8,10,11]. Among community-dwelling individuals with dementia, the prevalence of agitation was reported to be between 24% and 67% [7,12].
In patients with dementia, agitation is associated with more rapid decline and deterioration in cognitive function and quality of life, which impede daily activities and relationships including those with family and caregivers [8,9,13]. In addition, agitation may be an important predictor of institutionalization [12,14,15]. Among patients with dementia, more severe behavioral symptoms and disruptive behaviors are important predictors of earlier institutionalization [[16], [17], [18]]. However, prior studies have been limited to very small sample sizes and largely focused on behavioral symptoms in general.
The main components of the costs attributable to dementia are the costs associated with institutional and home-based long-term care rather than medical care. Together, residential and formal and informal home care represent 75% to 84% of costs attributable to dementia in the United States [19]. Across disabilities, costs associated with long-term care residential facilities has been estimated at over $200 billion in the United States in 2012—representing almost 10% of all the US personal health care spending [20]. Given that agitation may increase the risk of institutionalization, it is likely associated with a substantial economic burden, yet there is a paucity of published data on costs of institutionalization associated with agitation in individuals with AD.
The primary objective of this study was to assess the incremental risk of institutionalization associated with agitation, as defined by the agitation domain of the NPI Questionnaire (NPI-Q), in individuals with AD. The secondary objective was to estimate the incremental costs of institutionalization associated with agitation in individuals with AD.
2. Methods
2.1. Data sources
This study utilized two data sources; the primary analyses were conducted using data from the National Alzheimer's Coordinating Center Uniform Data Set (NACC-UDS, September 2005–February 2018), and sensitivity analyses were conducted using the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (September 2005–September 2017).
The NACC-UDS reflects the enrollment of individuals from approximately 39 past and present Alzheimer's Disease Centers across the United States, which are supported by the US National Institute on Aging/National Institute of Health. The NACC-UDS includes information on over 39,000 individuals with a range of cognitive status—normal cognition, mild cognitive impairment, and different types of dementia. More specifically, the NACC-UDS comprises longitudinal data on individual demographics, family history, medical history (including medication use), cognitive status (based on validated instruments such as the Mini–Mental State Examination and Clinical Dementia Rating scale [CDR® Dementia Staging Instrument]), functional status (evaluated using the Functional Assessment Questionnaire), and behavioral symptoms (evaluated using the NPI-Q). The NACC-UDS protocol requires annual follow-up visits as long as the individual is able to participate.
The ADNI (adni.loni.usc.edu), a public–private partnership led by the Principal Investigator Michael W. Weiner, MD, is a longitudinal multicenter study of individuals over 55 years of age, designed to identify clinical, imaging, genetic, and biochemical markers for the early detection and tracking of AD. The study includes over 1000 individuals in the United States; 25% with dementia, 50% with mild cognitive impairment of the amnestic type, and 25% without cognitive impairment. For up-to-date information, see www.adni-info.org. Individuals in the ADNI database have been observed for up to 12 years after study enrollment, with visits scheduled every 6 months.
Enrollment in both the NACC-UDS and the ADNI databases is protocol specific and is not intended to be representative of the broader US population. Written informed consent was obtained from all participants and informants to the NACC-UDS and ADNI. Both databases comply with the Health Insurance Portability and Accountability Act. This study received an exemption from institutional review from the New England Independent Review Board on the basis that the data do not include any identifiable individual information.
In addition, for the estimation of the incremental institutionalization/living costs associated with agitation in AD, a targeted literature review was conducted to identify aggregated unit costs by type of living place in the US, including costs of living in a regular home without assistance, costs of assisted living, and costs of living in a skilled nursing home facility.
2.2. Study design, sample selection, and cohort definition
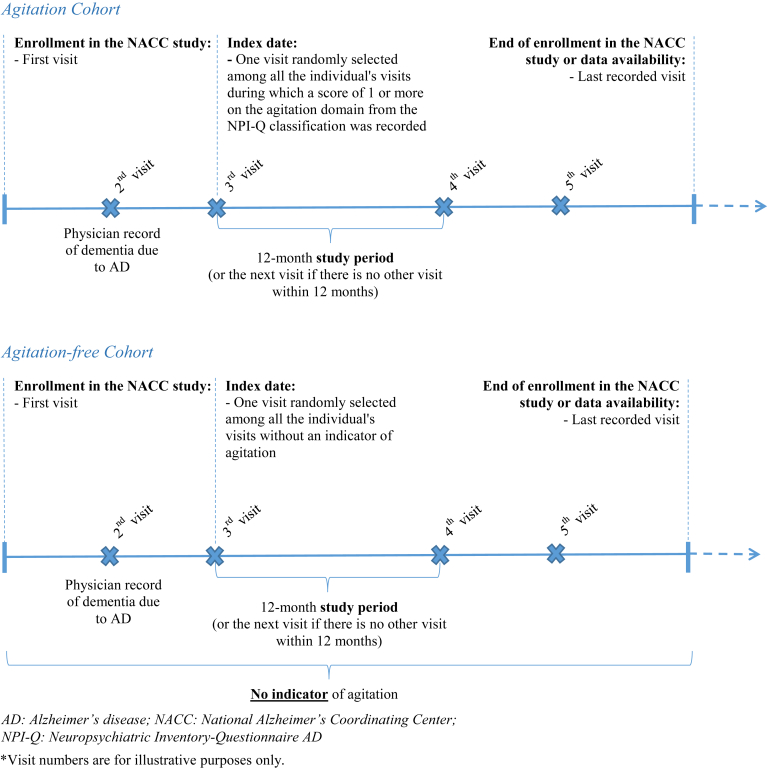
A retrospective cohort design was employed. Individuals were selected for this study if they (1) were enrolled in the NACC-UDS study and had mild cognitive impairment or dementia; (2) had known information on key variables (e.g., agitation domain of the NPI-Q, institutionalization) at any time; and (3) had dementia with presumptive etiologic diagnosis of AD at any time before the index date or during the study period (defined below). Individuals meeting the selection criteria were classified into two mutually exclusive cohorts. Individuals were classified in the Agitation cohort if they had at least one visit with a score of 1 or more on the agitation domain from the NPI-Q classification (i.e., at least a noticeable change in the patient being resistive to help from others at times, or hard to handle since the patient first began to experience memory problems) [21] recorded at any time. Individuals were classified in the Agitation-free cohort if they had no visit with an indicator of agitation recorded at any time. An indicator of agitation was defined as a score of 1 or more on the agitation domain from the NPI-Q classification, a meaningful change in behavior related to agitation based on clinician assessment as reported in the NACC-UDS, or a mention of any type of agitation in other psychiatric disorders. Individuals with an indicator of agitation, but without a score of 1 or more on the agitation domain of the NPI-Q at any time, were excluded from the study.
For each individual in the Agitation cohort, one visit was randomly selected (using equal probability sampling) among all of the individual's visits where a score of 1 or more on the agitation domain from the NPI-Q classification was recorded, and the date of the selected visit was defined as the index date. For each individual in the Agitation-free cohort, one visit was randomly selected (using equal probability sampling) among all of the individual's visits without an indicator of agitation, and the date of the selected visit was defined as the index date. For both cohorts, the study period was defined as the 12-month period after the index date (or the next visit if there was no other visit within 12 months) (Fig. 1).
Fig. 1.
Study design.
Entropy balancing was applied to reweight the demographic and clinical characteristics of individuals included in the two cohorts [22]. Individuals in the Agitation-free cohort were reweighted so that the distribution of specified covariates had the exact same moments (mean and standard deviation) as the distribution of covariates for individuals in the Agitation cohort. Weights were normalized so that the sum of weights was equal to the number of individuals in each cohort. The following characteristics were balanced between cohorts as of the index date: age, gender, race, ethnicity, education level, primary language, marital status, systolic and diastolic blood pressure, resting heart rate, active depression, score on the Clinical Dementia Rating, score on the Geriatric Depression Scale, score on the Modified Hachinski Ischemia Scale, and score on the Functional Activities Questionnaire (high [score >9] vs. low [score ≤9]).
For the sensitivity analysis using the ADNI database, similar study design and selection criteria were used, but agitation was identified based on the score on the agitation domain of either the NPI or NPI-Q (based on availability).
2.3. Measures, outcomes, and statistical analyses
Descriptive statistics were used to summarize individual characteristics including demographics and AD-related clinical characteristics, separately for the Agitation cohort and Agitation-free cohort, before and after reweighting. Means, standard deviations, and medians were presented for continuous variables, and frequencies and percentages were presented for categorical variables. Standardized differences between the two cohorts were calculated both before and after reweighting.
2.3.1. Institutionalization risk
Institutionalization risk was assessed from a prevalence-based perspective and was measured at any visit before or during the study period. Individuals were considered institutionalized if they reported living in a residential care facility where individuals are expected to require assistance with activities related to daily living (e.g., personal care, meal preparation, administration of medication). More specifically, the following two categories were considered: (1) an assisted living facility, adult family home, boarding home, or (2) skilled nursing facility, nursing home, hospital, or hospice. Institutionalization rates were calculated for each cohort as the weighted number of individuals institutionalized divided by the total number of individuals in the cohort. Incremental institutionalization risk associated with agitation was estimated using a weighted logistic regression model, where the dependent variable was institutionalization and the independent variable was a dummy variable for the Agitation cohort. Results were reported as odds ratios (ORs) with their 95% confidence intervals (CIs) and P values.
2.3.2. Institutionalization costs
The total institutionalization costs incurred by individuals with AD and agitation in the United States were estimated based on the following components: the number of individuals with AD and agitation institutionalized in the United States (obtained from the literature); the incremental risk of institutionalization associated with agitation in individuals with AD (estimated in this study); and the unit costs of institutionalization and the costs of living in a regular home without assistance in the United States (obtained from the literature) [1,10,[23], [24], [25], [26], [27]]. The incremental institutionalization costs associated with agitation in individuals with AD were estimated based on the difference between the institutionalization costs and the costs of living in a regular home without assistance, multiplied by the incremental number of institutionalized individuals with AD and agitation in the United States. Unit costs obtained from the literature were adjusted for inflation and expressed in 2018 US dollars using the Consumer Price Index, Urban All Items Component.
3. Results
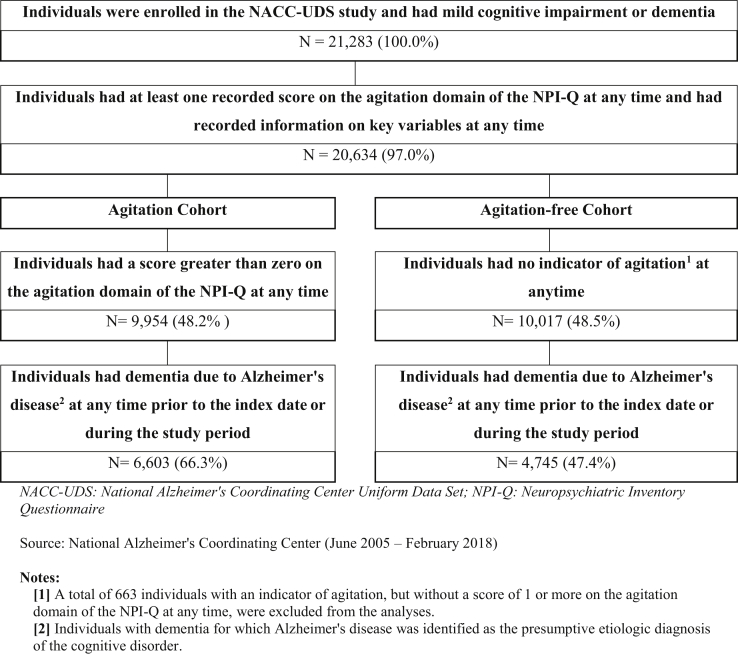
A total of 11,348 individuals from the NACC-UDS met the sample selection criteria for the study: 6603 individuals (58.2%) were included in the Agitation cohort and 4745 individuals (41.8%) were included in the Agitation-free cohort (Fig. 2). Before entropy balancing, the Agitation cohort showed greater cognitive impairment in terms of AD severity and clinical and cognitive assessment than the Agitation-free cohort. The mean score on the Clinical Dementia Rating Scale was 8.4 in the Agitation cohort and 6.0 in the Agitation-free cohort; similarly, the mean score on the Functional Activities Questionnaire was 19.2 versus 14.5, respectively, with standardized differences between cohorts >0.50 for both scores (Table 1). After applying entropy balancing, the Agitation and Agitation-free cohorts had similar demographics and AD severity, as well as similar clinical and cognitive assessment scores (i.e., all standardized differences <0.20).
Fig. 2.
Sample selection—NACC-UDS database.
Table 1.
Individual characteristics (original and balanced cohort)—NACC-UDS database
| Characteristics∗ | Agitation cohort N = 6603 |
Original cohort |
Balanced cohort |
||
|---|---|---|---|---|---|
| Agitation-free cohort |
Standardized difference | Agitation-free cohort |
Standardized difference | ||
| N = 4745 | N = 4745 | ||||
| Age, mean ± SD [median] | 75.9 ± 9.9 [77.0] | 76.1 ± 10.0 [77.0] | 0.020 | 75.9 ± 9.9 [77.0] | 0.000 |
| Female, N (%) | 3515 (53.2%) | 2704 (57.0%) | 0.080 | 2526 (53.2%) | 0.000 |
| Race, N (%) | |||||
| Asian | 132 (2.0%) | 84 (1.8%) | 0.020 | 95 (2.0%) | 0.000 |
| Black | 732 (11.1%) | 412 (8.7%) | 0.080 | 526 (11.1%) | 0.000 |
| White | 5304 (80.3%) | 4019 (84.7%) | 0.120 | 3812 (80.3%) | 0.000 |
| Other† | 257 (3.9%) | 130 (2.7%) | 0.060 | 185 (3.9%) | 0.000 |
| Unknown | 178 (2.7%) | 100 (2.1%) | 0.040 | 128 (2.7%) | 0.000 |
| Ethnicity, N (%) | |||||
| Hispanic | 700 (10.6%) | 388 (8.2%) | 0.080 | 503 (10.6%) | 0.000 |
| Non-Hispanic | 5891 (89.2%) | 4341 (91.5%) | 0.080 | 4233 (89.2%) | 0.000 |
| Unknown | 12 (0.2%) | 16 (0.3%) | 0.030 | 9 (0.2%) | 0.000 |
| Education level‡, N (%) | |||||
| Less than high school | 863 (13.1%) | 523 (11.0%) | 0.060 | 620 (13.1%) | 0.000 |
| High school degree | 1546 (23.4%) | 1105 (23.3%) | 0.000 | 1111 (23.4%) | 0.000 |
| Some college | 1108 (16.8%) | 818 (17.2%) | 0.010 | 796 (16.8%) | 0.000 |
| University degree | 3036 (46.0%) | 2274 (47.9%) | 0.040 | 2182 (46.0%) | 0.000 |
| Unknown | 50 (0.8%) | 25 (0.5%) | 0.030 | 36 (0.8%) | 0.000 |
| Primary language, N (%) | |||||
| English | 5914 (89.6%) | 4294 (90.5%) | 0.030 | 4250 (89.6%) | 0.000 |
| Spanish | 536 (8.1%) | 315 (6.6%) | 0.060 | 385 (8.1%) | 0.000 |
| Other§ | 149 (2.3%) | 131 (2.8%) | 0.030 | 107 (2.3%) | 0.000 |
| Unknown | 4 (0.1%) | 5 (0.1%) | 0.020 | 3 (0.1%) | 0.000 |
| Marital status, N (%) | |||||
| Married | 4258 (64.5%) | 3017 (63.6%) | 0.020 | 3060 (64.5%) | 0.000 |
| Widowed | 1461 (22.1%) | 1089 (23.0%) | 0.020 | 1050 (22.1%) | 0.000 |
| Divorced | 524 (7.9%) | 381 (8.0%) | 0.000 | 377 (7.9%) | 0.000 |
| Separated | 59 (0.9%) | 42 (0.9%) | 0.000 | 42 (0.9%) | 0.000 |
| Never married | 213 (3.2%) | 130 (2.7%) | 0.030 | 153 (3.2%) | 0.000 |
| Living as married/domestic partner | 72 (1.1%) | 63 (1.3%) | 0.020 | 52 (1.1%) | 0.000 |
| Unknown | 16 (0.2%) | 23 (0.5%) | 0.040 | 11 (0.2%) | 0.000 |
| Blood pressure (sitting) | |||||
| Systolic, mean ± SD [median] | 127.9 ± 34.1 [131.0] | 125.9 ± 38.3 [131.0] | 0.250 | 127.9 ± 34.1 [131.0] | 0.000 |
| Diastolic, mean ± SD [median] | 70.8 ± 18.9 [73.0] | 69.5 ± 21.2 [72.0] | 0.190 | 70.8 ± 18.9 [73.0] | 0.000 |
| Resting heart rate (pulse), mean ± SD [median] | 65.1 ± 18.4 [67.0] | 63.1 ± 20.4 [66.0] | 0.230 | 65.1 ± 18.4 [66.0] | 0.000 |
| Comorbidities, N (%) | |||||
| Hypertension | 3821 (57.9%) | 2607 (54.9%) | 0.060 | 2675 (56.4%) | 0.030 |
| Hypercholesterolemia | 3760 (56.9%) | 2546 (53.7%) | 0.070 | 2576 (54.3%) | 0.050 |
| Depression in the last 2 years | 3175 (48.1%) | 1671 (35.2%) | 0.260 | 2282 (48.1%) | 0.000 |
| Incontinence—urinary | 2148 (32.5%) | 1093 (23.0%) | 0.210 | 1428 (30.1%) | 0.050 |
| Cardiovascular disease | 2019 (30.6%) | 1417 (29.9%) | 0.020 | 1433 (30.2%) | 0.010 |
| Clinical and cognitive assessment | |||||
| Mini–Mental State Examination, Mean ± SD [Median] | 18.4 ± 7.7 [20.0] | 20.5 ± 6.5 [22.0] | 0.300 | 18.0 ± 7.4 [19.0] | 0.050 |
| Individuals with missing values, N (%) | 570 (8.6%) | 744 (15.7%) | 0.220 | 486 (10.2%) | 0.050 |
| Clinical Dementia Rating scale, Mean ± SD [Median] | 8.4 ± 5.2 [7.0] | 6.0 ± 4.3 [5.0] | 0.520 | 8.4 ± 5.2 [7.0] | 0.000 |
| Individuals with missing values, N (%) | 0 (0.0%) | 0 (0.0%) | 0.000 | 0 (0.0%) | 0.000 |
| Functional Activities Questionnaire, Mean ± SD [median] | 19.2 ± 9.2 [21.0] | 14.5 ± 9.4 [14.0] | 0.510 | 18.7 ± 9.3 [20.0] | 0.060 |
| Individuals with missing values, N (%) | 41 (0.6%) | 57 (1.2%) | 0.060 | 29 (0.6%) | 0.000 |
| Geriatric Depression Scale, mean ± SD [median] | 2.6 ± 2.8 [2.0] | 2.4 ± 2.6 [2.0] | 0.080 | 2.6 ± 2.8 [2.0] | 0.000 |
| Individuals with missing values, N (%) | 628 (9.5%) | 333 (7.0%) | 0.090 | 451 (9.5%) | 0.000 |
| Modified Hachinski Ischemia Scale, mean ± SD [median] | 1.1 ± 1.5 [1.0] | 1.1 ± 1.5 [1.0] | 0.030 | 1.1 ± 1.5 [1.0] | 0.000 |
| Individuals with missing values, N (%) | 422 (6.4%) | 651 (13.7%) | 0.250 | 303 (6.4%) | 0.000 |
| Any medication use, N (%) | 6341 (96.0%) | 4539 (95.7%) | 0.020 | 4528 (95.4%) | 0.030 |
| FDA-approved medication for AD symptoms | 4542 (68.8%) | 3007 (63.4%) | 0.110 | 3196 (67.4%) | 0.030 |
| Antihypertensive or blood pressure medication | 3591 (54.4%) | 2573 (54.2%) | 0.000 | 2554 (53.8%) | 0.010 |
| Lipid-lowering medication | 2761 (41.8%) | 2045 (43.1%) | 0.030 | 1998 (42.1%) | 0.010 |
| Antidepressant | 2919 (44.2%) | 1646 (34.7%) | 0.200 | 1940 (40.9%) | 0.070 |
| Anticoagulant or antiplatelet agent | 2590 (39.2%) | 1824 (38.4%) | 0.020 | 1782 (37.6%) | 0.030 |
| Nonsteroidal anti-inflammatory medication | 2480 (37.6%) | 1679 (35.4%) | 0.050 | 1639 (34.6%) | 0.060 |
| Beta-adrenergic blocking agent (beta-blocker) | 1362 (20.6%) | 977 (20.6%) | 0.000 | 943 (19.9%) | 0.020 |
| Angiotensin converting enzyme inhibitor | 1241 (18.8%) | 874 (18.4%) | 0.010 | 880 (18.5%) | 0.010 |
| Diuretic | 954 (14.4%) | 718 (15.1%) | 0.020 | 711 (15.0%) | 0.010 |
| Calcium channel blocking agent | 985 (14.9%) | 710 (15.0%) | 0.000 | 701 (14.8%) | 0.000 |
| Anxiolytic, sedative, or hypnotic agent | 856 (13.0%) | 443 (9.3%) | 0.120 | 453 (9.5%) | 0.110 |
| Antipsychotic agent | 870 (13.2%) | 195 (4.1%) | 0.330 | 316 (6.7%) | 0.220 |
| Diabetes medication | 729 (11.0%) | 439 (9.3%) | 0.060 | 472 (9.9%) | 0.040 |
NOTE. Bold face indicate that the standardized difference is greater than 0.20.
Abbreviations: AD, Alzheimer's disease; FDA, Food and Drug Administration; NACC-UDS, National Alzheimer's Coordinating Center Uniform Data Set; SD, standard deviation.
Individual characteristics were measured as of the index date. In the situation where specific information was not available as of the index date, the most recent visit with complete information prior to the index date was used.
Other race was defined as American Indian/Alaskan, Hawaiian/other place of origin, more than one race.
Less than high school was defined as less than 12 years of education, high school degree was defined as 12 years of education, some college was defined as 13 to 15 years of education, and university degree was defined as 16 or more years of education.
Other primary language was defined as Mandarin, Cantonese, Russian, Japanese, and other specified primary language.
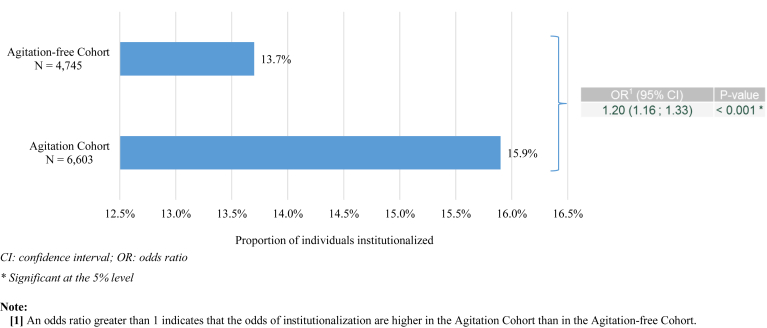
Compared with individuals in the Agitation-free cohort, those in the Agitation cohort were 20% more likely to be institutionalized (OR: 1.20; 95% CI: 1.08–1.33) (Fig. 3). In the sensitivity analysis using the ADNI database (N = 567), agitation was also found to be associated with a higher risk of institutionalization (OR: 3.48; 95% CI: 1.16–10.44) (Supplementary Table 1).
Fig. 3.
Odds of institutionalization associated with agitation in individuals with Alzheimer's disease (balanced cohorts)—NACC-UDS database.
Based on a prevalence of agitation in institutionalized individuals with AD of 50% [10] and an incremental risk of institutionalization associated with agitation in individuals with AD of 1.20, an estimated excess of 85,089 individuals with AD were institutionalized because of agitation, and the estimated total incremental costs of institutionalization associated with agitation in individuals with AD was $4.3 billion in 2018 ($50,588 for each additional institutionalized individual with AD) (Table 2). Owing to the lack of consensus in the literature on the prevalence of agitation in institutionalized individuals with AD, a sensitivity analysis was conducted using prevalence estimates of 28% and 60% [5,8,10,11]. With a prevalence of 28%, the total annual incremental cost of institutionalization associated with agitation in individuals with AD was estimated at $2.4 billion. With a prevalence of 60%, the total annual incremental cost of institutionalization associated with agitation in individuals with AD was estimated at $5.1 billion (results not presented). Thus, it is estimated that the incremental costs of institutionalization associated with agitation represent 2.9% to 6.1% of the total absolute costs of institutionalization in individuals with AD, which were estimated at $84.1 billion in 2018.
Table 2.
Incremental costs of institutionalization associated with agitation in individuals with Alzheimer's disease—NACC-UDS database
| Components | Label | Estimate∗ |
|---|---|---|
| Incremental costs of institutionalization associated with agitation in individuals with AD | ||
| Excess number of individuals with AD in institutionalized settings associated with agitation† | [A] | 85,089 |
| Average annual incremental costs of institutionalization per individual with AD‡ | [B] | $50,588 |
| Total excess costs of institutionalization associated with agitation in individuals with AD | [C] | $4,304,468,479 |
| Total costs of institutionalization in individuals with AD | ||
| Total number of individuals with AD in institutionalized settings§ | [D] | 1,021,068 |
| Average annual costs per individual with AD in institutionalized settings¶ | [E] | $82,331 |
| Total absolute costs of institutionalization in individuals with AD | [F] | $84,065,869,488 |
| Total excess costs of institutionalization/total absolute costs of institutionalization | [G] | 5.1% |
Calculations:
[C] = [A] x [B]
[F] = [D] x [E]
[G] = [C]/[F]
Abbreviations: AD, Alzheimer's disease; NACC-UDS, National Alzheimer's Coordinating Center Uniform Data Set.
Combined results from the literature, public databases, and results from the present study. Costs are presented in 2018 United States dollars.
Excess number of individuals with AD in institutionalized settings associated with agitation is based on a 50% prevalence of agitation in institutionalized individuals with AD [10], total number of individuals with AD in institutionalized and noninstitutionalized settings [23].
Average annual incremental costs of institutionalization per individual with AD is based on the average annual costs per individual with AD in institutionalized settings [27] and the average annual costs per individual with AD in noninstitutionalized settings [1,[24], [25], [26]].
Based on Long-Term Care Providers and Services Users in the United States: Data From the National Study of Long-Term Care Providers, there were 1,021,068 individuals with AD in institutionalized settings (nursing home n = 690,329; assisted living facility n = 330,739) [23].
The average annual costs per individual with AD in institutionalized settings is based on a an average annual costs of $94,637 per individual with AD in nursing homes and $56,646 per individual with AD in assisted living facilities. These costs were derived from the Market Survey of Long-Term Care Costs [27].
4. Discussion
This study, drawing on data from two large-scale longitudinal studies of AD, found that among individuals with AD, those with agitation were more likely to be institutionalized compared with individuals without agitation. In addition, the annual total incremental costs of institutionalization associated with agitation in individuals with AD were estimated at $4.3 billion in 2018.
Findings based on the NACC-UDS data were consistent with those from the sensitivity analysis based on the ADNI data. However, some variations in the estimated risk of institutionalization based on NACC-UDS and ADNI data were observed, which may be explained by the smaller sample size from the ADNI data resulting in wider CIs for the estimates. In addition, differences in the underlying populations of the two databases may also contribute to observed variations in the estimates. In particular, individuals in the ADNI database had less severe AD, higher educational attainment, and were more likely to be white. Given that the extent of cognitive impairment has consistently been shown to be associated with higher risk of institutionalization, it is possible that the marginal impact of agitation on the risk of institutionalization is higher among patients with less severe AD [14,15,28]. There is also some evidence to suggest racial and ethnic disparities in the risk of institutionalization among patients with dementia; in the United States, African Americans and Hispanics have been associated with a lower rate of placement in residential care facilities compared with non-Hispanic whites [28].
Results from the present study are in line with prior findings suggesting an association between behavioral symptoms and institutionalization [14,15,28]. However, this study benefits from a larger and more generalizable sample than prior research samples. Moreover, agitation has been proposed as one of the most important factors influencing the decision to transfer an individual with dementia to a residential care facility [28,29]. The increased risk of institutionalization among agitated individuals may be attributed to several factors. Agitated behavior adversely influences the patient's environment, and raises concerns about risk of self-inflicted harm and deterioration in quality of life and cognitive function, leading to a high burden on patients' family and caregivers [8,13,30]. In addition, the therapeutic management of agitation in patients with AD remains challenging, such that a large proportion of patients remain untreated [10]. Although nonpharmacological approaches are generally appropriate, in many instances, pharmacological treatment is necessary for the optimal management of patients with severe agitation symptoms [31,32]. Yet, there is currently no treatment approved by the US Food and Drug Administration (FDA) for the full spectrum of agitation symptoms in dementia [32]. The absence of FDA-approved treatments combined with concerns about polypharmacy in this patient population may lead to a suboptimal management of agitation symptoms [29].
The present study focuses on the incremental costs of institutionalization, but agitation may be associated with a much higher overall burden. A study in the United Kingdom estimated that the additional costs of managing agitation accounted for approximately 12% of the costs of dementia or £4091 ($7236) per individual with AD when including additional components such as health care costs [33]. In the US setting, a recent claims-based retrospective study found that patients with dementia and behavioral disturbances including agitation had a higher prevalence of comorbidities, greater use of comedications, and greater health care utilization, resulting in higher health care costs ($9644) compared with patients with dementia without behavioral disturbances. Future initiatives including multisite collaborations to collect data on agitation representative of the US population of patients with dementia are warranted to validate and extend results of registry-based studies.
The evidence base for treatment of agitation in individuals with dementia—including both nonpharmacological and pharmacological interventions—is growing [31,32]. Several ongoing trials are evaluating the efficacy of psychosocial and environmental behavior management strategies centered around patients and caregivers (e.g., social engagement and sensory interventions), as well as pharmacological interventions (e.g., antipsychotics, selective serotonin reuptake inhibitors, and cannabinoids) for the treatment of agitation in individuals with dementia [3,[34], [35], [36]]. Addressing agitation represents a great opportunity for therapeutic intervention and the alleviation of individual suffering, family burden, and societal costs. Reducing agitation could also reduce caregiver burden and prevent or delay the institutionalization of individuals with dementia. In the absence of FDA-approved treatments for dementia-related agitation, effective, safe, and well-tolerated pharmacological treatments are needed to manage the full spectrum of agitation symptoms in individuals with AD. Efforts to promote uptake of clinical practice guidelines and a systematic approach to the treatment of agitation in individuals with AD in real-world practice may also help reduce risk of institutionalization through effective management of agitation symptoms. Management of agitation symptoms in individuals with AD remains largely inadequate. For example, prior studies have noted that a considerable proportion of patients do not receive nonpharmacological treatment as recommended by practice guidelines [10].
Findings from this study should be interpreted in the light of some limitations. First, the population of the NACC-UDS and ADNI databases may not be fully representative of the general population of patients with AD as it includes individuals who are predominantly white and have a higher socioeconomic status with better access to care than the general AD patient population. Second, in the context of this study, agitation was defined based on the agitation domain of the NPI-Q, which evaluates whether the patient is resistive to help from others at times or hard to handle. Although the validity of the NPI-Q as a measure of agitation has been shown [37], it may not inform on the impact of specific manifestations that could be considered aggressive or disruptive for a given stakeholder. Moreover, the NPI-Q score was assessed at irregular intervals, on average every 12 months in the NACC-UDS database, and may not have been assessed at every visit or may have been incorrectly entered. To the extent that data entry errors did not occur in a systematic way or differently across cohorts, it is unlikely that these measurement errors significantly influenced the findings of the present study. Third, because there is no single data source to measure costs of institutionalization associated with agitation in individuals with AD, several estimates from the literature and governmental publications were combined, which may result in inconsistencies. Fourth, many factors may be involved in the institutionalization decision process. Although important predictors of institutionalization such as age, gender, race, education level, and dementia severity were balanced between cohorts, there may be remaining unobserved differences between cohorts that could potentially have an impact on institutionalization risk. Finally, the present study considered the impact of agitation on the risk of institutionalization based on any level of changes in the patient behavior. Further studies are warranted to understand how the risk of institutionalization is impacted by agitation severity.
To our knowledge, this is the first study to put forth estimates of the incremental risk of institutionalization and associated costs in a US setting among patients with symptoms of agitation associated with AD. Findings from two separate databases show that agitation is associated with a higher risk of institutionalization among patients with AD, which translates into a substantial economic burden. Findings from this study highlight the need to better address symptoms of agitation in individuals with AD.
Research in Context.
-
1.
Systematic review: Agitation is one of the most common and distressing behavioral symptoms of Alzheimer's disease (AD). More severe behavioral symptoms are important predictors of institutionalization. However, most studies to date lack a specific focus on agitation symptoms and are limited to small sample sizes.
-
2.
Interpretation: Utilizing data from two large-scale longitudinal studies of AD, this study found that among individuals with AD, those with agitation were more likely to be institutionalized compared with individuals without agitation. The annual incremental cost of institutionalization associated with agitation in individuals with AD was estimated at $4.3 billion, equivalent to $50,588 for each additional institutionalized individual with AD.
-
3.
Future directions: Approved safe and effective treatments for the full spectrum of agitation symptoms in AD are needed to address the suboptimal management of the condition. Future large-scale studies representative of the United States population are also warranted.
Acknowledgments
Medical writing assistance was provided by Sara Kaffashian, PhD, an employee of Analysis Group, Inc.
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Data collection and sharing was funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). The ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. The ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
This study was funded by Otsuka Pharmaceutical Development & Commercialization, Inc.
Footnotes
Conflicts of Interest: M.C., M.G.L., P.G.S., and A.G. are employees of Analysis Group, Inc. that has received consultancy fees from Otsuka America Pharmaceutical. A.H. and K.G. are employees of Lundbeck Pharmaceuticals. R.A.B., R.D., and M.S.A. are employees of Otsuka America Pharmaceutical.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.10.004.
Supplementary data
References
- 1.Alzheimer's Association. AD Facts and Figures 2017. https://www.alz.org/alzheimers-dementia/facts-figures. Accessed October 5, 2019.
- 2.Desai A.K., Schwartz L., Grossberg G.T. Behavioral disturbance in dementia. Curr Psychiatry Rep. 2012;14:298–309. doi: 10.1007/s11920-012-0288-5. [DOI] [PubMed] [Google Scholar]
- 3.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 4.Ballard C., Corbett A. Agitation and aggression in people with Alzheimer's disease. Curr Opin Psychiatry. 2013;26:252–259. doi: 10.1097/YCO.0b013e32835f414b. [DOI] [PubMed] [Google Scholar]
- 5.Gauthier S., Cummings J., Ballard C., Brodaty H., Grossberg G., Robert P. Management of behavioral problems in Alzheimer's disease. Int Psychogeriatrics. 2010;22:346–372. doi: 10.1017/S1041610209991505. [DOI] [PubMed] [Google Scholar]
- 6.Cummings J., Mintzer J., Brodaty H., Sano M., Banerjee S., Devanand D.P. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr. 2015;27:7–17. doi: 10.1017/S1041610214001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tractenberg R.E., Weiner M.F., Thal L.J. Estimating the prevalence of agitation in community-dwelling persons with Alzheimer's disease. The J Neuropsychiatry Clin Neurosci. 2002;14:11–18. doi: 10.1176/jnp.14.1.11. [DOI] [PubMed] [Google Scholar]
- 8.Zuidema S., Koopmans R., Verhey F. Prevalence and predictors of neuropsychiatric symptoms in cognitively impaired nursing home patients. J Geriatr Psychiatry Neurol. 2007;20:41–49. doi: 10.1177/0891988706292762. [DOI] [PubMed] [Google Scholar]
- 9.Livingston G., Barber J., Marston L., Rapaport P., Livingston D., Cousins S. Prevalence of and associations with agitation in residents with dementia living in care homes: MARQUE cross-sectional study. BJPsych Open. 2017;3:171–178. doi: 10.1192/bjpo.bp.117.005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kverno K.S., Rabins P.V., Blass D.M., Hicks K.L., Black B.S. Prevalence and treatment of neuropsychiatric symptoms in advanced dementia. J Gerontol Nurs. 2008;34:8–15. doi: 10.3928/00989134-20081201-03. quiz 6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margallo-Lana M., Swann A., O'Brien J., Fairbairn A., Reichelt K., Potkins D. Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. Int J Geriatr Psychiatry. 2001;16:39–44. doi: 10.1002/1099-1166(200101)16:1<39::aid-gps269>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Lyketsos C.G., Steinberg M., Tschanz J.T., Norton M.C., Steffens D.C., Breitner J.C. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Colaco Harmand M., Meillon C., Rullier L., Avila-Funes J.A., Bergua V., Dartigues J.F. Cognitive decline after entering a nursing home: a 22-year follow-up study of institutionalized and noninstitutionalized elderly people. J Am Med Dir Assoc. 2014;15:504–508. doi: 10.1016/j.jamda.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Gaugler J.E., Yu F., Krichbaum K., Wyman J.F. Predictors of nursing home admission for persons with dementia. Med Care. 2009;47:191–198. doi: 10.1097/MLR.0b013e31818457ce. [DOI] [PubMed] [Google Scholar]
- 15.Okura T., Plassman B.L., Steffens D.C., Llewellyn D.J., Potter G.G., Langa K.M. Neuropsychiatric symptoms and the risk of institutionalization and death: the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59:473–481. doi: 10.1111/j.1532-5415.2011.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez O.L., Wisniewski S.R., Becker J.T., Boller F., DeKosky S.T. Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer disease. Arch Neurol. 1999;56:1266–1272. doi: 10.1001/archneur.56.10.1266. [DOI] [PubMed] [Google Scholar]
- 17.Park D.G., Lee S., Moon Y.M., Na D.L., Jeong J.H., Park K.W. Predictors of institutionalization in patients with Alzheimer's disease in South Korea. J Clin Neurol. 2018;14:191–199. doi: 10.3988/jcn.2018.14.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scarmeas N., Brandt J., Blacker D., Albert M., Hadjigeorgiou G., Dubois B. Disruptive behavior as a predictor in Alzheimer disease. Arch Neurol. 2007;64:1755–1761. doi: 10.1001/archneur.64.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurd M.D., Martorell P., Delavande A., Mullen K.J., Langa K.M. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Health Policy Forum 2014 National spending for long-term services and supports (LTSS) 2012. http://www.nhpf.org/library/details.cfm/2783 Accessed October 5, 2019.
- 21.Cummings J. The Neuropsychiatric Inventory Questionnaire: Background and Administration. 1994. https://www.alz.org/media/Documents/npiq-questionnaire.pdf Accessed October 5, 2019.
- 22.Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal, 20:21.
- 23.Harris-Kojetin L., Sengupta M., Park-Lee E., Valverde R., Caffrey C., Rome V. Long-Term Care Providers and services users in the United States: data from the National Study of Long-Term Care Providers, 2013-2014. Vital Health Stat. 2016;3 x-xii, 1-105. [PubMed] [Google Scholar]
- 24.Genworth Genworth 2015 Cost of Care Survey: Home Care Providers, Adult Day Health Care Facilities, Assisted Living Facilities and Nursing Homes. 2015. https://www.genworth.com/dam/Americas/US/PDFs/Consumer/corporate/130568_040115_gnw.pdf Accessed October 5, 2019.
- 25.United States Department of Labor: Bureau of Labor Statistics Consumer expenditure surveys. https://www.bls.gov/cex/ Accessed October 5, 2019.
- 26.McCann J.J., Hebert L.E., Li Y., Wolinsky F.D., Gilley D.W., Aggarwal N.T. The effect of adult day care services on time to nursing home placement in older adults with Alzheimer's disease. Gerontologist. 2005;45:754–763. doi: 10.1093/geront/45.6.754. [DOI] [PubMed] [Google Scholar]
- 27.Market Survey of Long-Term Care Costs: The 2012 MetLife Market Survey of Nursing Home, Assisted Living, Adult Day Services, and Home Care Costs.
- 28.Yaffe K., Fox P., Newcomer R., Sands L., Lindquist K., Dane K. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 29.Livingston G., Kelly L., Lewis-Holmes E., Baio G., Morris S., Patel N. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18:1–226. doi: 10.3310/hta18390. v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed S., Rosenheck R., Lyketsos C.G., Schneider L.S. Caregiver burden in Alzheimer disease: cross-sectional and longitudinal patient correlates. Am J Geriatr Psychiatry. 2010;18:917–927. doi: 10.1097/JGP.0b013e3181d5745d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kales H.C., Lyketsos C.G., Miller E.M., Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer's disease: an international Delphi consensus. Int Psychogeriatr. 2019;31:83–90. doi: 10.1017/S1041610218000534. [DOI] [PubMed] [Google Scholar]
- 32.Reus V.I., Fochtmann L.J., Eyler A.E., Hilty D.M., Horvitz-Lennon M., Jibson M.D. The American psychiatric association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173:543–546. doi: 10.1176/appi.ajp.2015.173501. [DOI] [PubMed] [Google Scholar]
- 33.Morris S., Patel N., Baio G., Kelly L., Lewis-Holmes E., Omar R.Z. Monetary costs of agitation in older adults with Alzheimer's disease in the UK: prospective cohort study. BMJ Open. 2015;5:e007382. doi: 10.1136/bmjopen-2014-007382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cummings J.L., Lyketsos C.G., Peskind E.R., Porsteinsson A.P., Mintzer J.E., Scharre D.W. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314:1242–1254. doi: 10.1001/jama.2015.10214. [DOI] [PubMed] [Google Scholar]
- 35.Garay R.P., Citrome L., Grossberg G.T., Cavero I., Llorca P.M. Investigational drugs for treating agitation in persons with dementia. Expert Opin Investig Drugs. 2016;25:973–983. doi: 10.1080/13543784.2016.1193155. [DOI] [PubMed] [Google Scholar]
- 36.Porsteinsson A.P., Antonsdottir I.M. An update on the advancements in the treatment of agitation in Alzheimer's disease. Expert Opin Pharmacother. 2017;18:611–620. doi: 10.1080/14656566.2017.1307340. [DOI] [PubMed] [Google Scholar]
- 37.Kaufer D.I., Cummings J.L., Ketchel P., Smith V., MacMillan A., Shelley T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.