Abstract
The importance of circadian biology has rarely been considered in pre-clinical studies, and even more when translating to the bedside. Circadian biology is becoming a critical factor for improving drug efficacy and diminishing drug toxicity. Indeed, there is emerging evidence showing that some drugs are more effective at nighttime than daytime, whereas for others it is the opposite. This suggests that the biology of the target cell will determine how an organ will respond to a drug at a specific time of the day, thus modulating pharmacodynamics. Thus, it is now time that circadian factors become an integral part of translational research.
Keywords: Circadian Biology, Drug metabolism, chronotherapy, clock genes, ADME, translation
Introduction
Differential day/night outcomes in humans
While the past two decades have witnessed a transformation in the understanding of the molecular underpinnings of biological clocks in model organisms, the bridge between circadian mechanisms and clinical studies remains incomplete. Reaching across the divide between molecular and clinical systems requires integration of circadian biology elements in medical practice (Bass and Lazar, 2016). For instance, a number of studies demonstrated that heart attacks occur more frequently during the morning hours, a finding which may be explained by vulnerabilities to day/night fluctuations in cardiovascular events such as circadian variation in blood pressure and heart rate (for review, see (Thosar et al., 2018), and day/night fluctuations in thromboembolic risk (Haus, 2007), arrhythmogenicity (Portaluppi and Hermida, 2007), and inflammation (Winter et al., 2018). Cardiovascular system challenges, such as cardiac surgery, provoke myocardial injury in a predictive way, and it turns out that injury severity is highly influenced by the time of day. Specifically, there is significantly lower risk when the surgery is performed in the afternoon compared to the morning (Montaigne et al., 2018). A similar effect was reported in rodents. Interestingly, the day/night fluctuation in rodent cardiac injury is not present in animals with a disrupted cardiomyocyte clock (Durgan et al., 2010). Furthermore, in the hospital environment, these processes are disrupted by enforced continuous activity throughout the day and night, including maintained lighting, wakening for medication, delivery of parenteral nutrition at night (in the opposite phase from the daily appetite and digestive cycle), and delivery of medication out of synchrony with drug-metabolizing enzyme expression. Each of these events represents conflict between internal and external timing. Similarly, staying up late at night and/or reading with blue-light emitting screens may desynchronize intrinsic circadian cycles with the natural light-dark cycle (Chang et al., 2015; Chinoy et al., 2018). The short list of circadian pathology signs includes emergencies that present at specific times of day, such as nocturnal asthma (Burioka et al., 2010) and glucose peak at dawn (Campbell et al., 1985). Evidence also suggests there is circadian control of cognition (Chellappa et al., 2018), memory performance (Kwapis et al., 2018), and mood (McClung, 2013), and a decline in the coherence of rhythmic processes may be a hallmark of age-related neurodegenerative disease (Musiek and Holtzman, 2016). Further, both clinical studies and cross-sectional evidence correlate shift work in human subjects with the heightened risk of disease, including metabolic, cardiovascular, inflammatory, and neoplastic disorders (Buxton et al., 2012; Ruger and Scheer, 2009; Wang et al., 2011). While shift work also results in confounding non-circadian effects like increased stress, eating, and smoking, these factors are also known consequences of fragmented sleep and sleep deprivation. These examples emphasize the exciting opportunity now available to apply an understanding of molecular timekeeping to human health.
Depression was one of the first diseases associated with circadian clock malfunction, observed as profound sleep disturbances and early morning awakenings (Gresham et al., 1965; Zung et al., 1964). This phenomenon is due to rhythm dysfunction: a disturbed sleep-wake cycle is the main symptom of major depression, and sleep disturbances are, in turn, a major risk factor for developing depression and other mood disorders. For instance, patients who suffer from seasonal affective disorder (SAD), a mood disorder that correlates with the extremely shortened daily light period during the winter season, have altered levels of the dark-phase hormone melatonin and exhibit an altered circadian cycle. SAD symptoms may be alleviated by chronobiological therapy, such as bright light and melatonin (Lewy et al., 2006). In fact, more or less all of the successful mood disorder treatments seem to affect circadian rhythms, and it appears as if rhythm stabilization, or even resetting the internal time, is helpful if not essential for therapeutic benefit. In modern societies, 80% of the world’s population is now exposed to light during the night (Falchi et al., 2016; Straif et al., 2007), and 20% of European and 29% of US employees engage in rotating shift work, actions that make them prone to rhythm disorders and disease. Thus, our circadian clocks appear crucial for health, and while many researchers studied circadian rhythms initially from a fundamental scientific perspective, we have been surprised—overwhelmed even—by the strong involvement of clock malfunction in a number of diseases. Consequently, circadian rhythm research has become more than a model area for biological research; it will also impact future clinical research, substantially and perhaps dramatically.
Oxaliplatin: how chronobiology cracked the timing riddle
Drug development includes defining a recommended dose for a potential new compound, based on the majority of subjects, irrespective of timing, sex, age, lifestyle, or comorbidities. In this regard, pharmaceutical companies have long tried to improve the translational path from animal work to humans by increasing the success rate of phase I, II, and III trials. However, numerous drugs continue to fail in these trials despite optimal pharmacokinetics/pharmacodynamics (PK/PD), target specificity, tissue availability, and careful patient group selection (Cook et al., 2014; Morgan et al., 2018). Indeed, unanticipated or overwhelming adverse effects represent severe limitations, and both result in drug attrition (Waring et al., 2015) and post-marketing withdrawal of otherwise effective medications (Zhang et al., 2012). For example, the toxicities of new agents sometimes outweigh the slight benefits in efficacy at a population level, thus making these new treatments too costly for the healthcare system. As a result, medication safety represents a crucial challenge that needs to be prioritized and addressed with new concepts and methods. While there are many factors that could contribute to the failures in translating drug treatments from rodent studies to humans (e.g., length of ovulatory cycles, low LDL cholesterol, or lifespan), circadian factors might also be involved in such failures. Do pre-clinical studies in nocturnal animals not test the drugs at the optimal time of day to simulate human biology? Conversely, does testing in humans not take timing into account, either insufficiently or not at all?
An illustration of how ignorance of timing effects could lead to abandoning a useful drug is provided by oxaliplatin. It has become one of the main drugs against colorectal cancer worldwide, despite its development being halted for excessive toxicities in a phase I clinical trial by a leading pharmaceutical company (Rhone-Poulenc-Rorer, France; (Extra et al., 1990). The drug was sold to a just-created pharmaceutical group (Debiopharm, Switzerland), which aimed at establishing oxaliplatin safety and efficacy through chronopharmacology. Circadian toxicity and efficacy studies in mice (Granda et al., 2002) guided the design of clinical chronotherapy trials in patients with metastatic colorectal cancer (reviewed in (Levi, 2001). The safety of the drug was established following its administration as a circadian chronomodulated infusion with peak delivery rate at 16:00 in phase I (Caussanel et al., 1990) and II (Levi et al., 1993) clinical trials. The first demonstration of its clinical efficacy in colorectal cancer was provided in a large phase II clinical trial using chronomodulated delivery (Levi et al., 1992), a finding that was later confirmed in randomized phase III trials (Levi et al., 1997). Consequently, the integration of chronopharmacology that accompanied the entire drug development process helped minimize adverse effects and maximize therapeutic efficacy through the identification of optimally timed drug delivery. Although to our knowledge this case is the only well-documented example of a misleading clinical trial linked to the lack of consideration of circadian aspects, the prior failure is an outstanding example that underscores the urgency for pre-clinical and clinical researchers to take circadian time into consideration along the clinical phases of the development of new drugs, as well as for clinical tests of novel treatments.
The clock system
Evolutionary conservation and relevance to physiology
Deciphering the molecular core clock mechanism in the fruit fly led to the 2017 Nobel Prize in Physiology or Medicine awarded to Michael Young, Michael Rosbash and Jeffrey Hall. This mechanism is fundamentally conserved within the animal kingdom (Allada et al., 2001; Rosbash, 2009; Takahashi, 2017; Young and Kay, 2001), although in mammals many clock components are present in multiple copies, a fact which increases the complexity and redundancy of the system (Clayton et al., 2001). The PERIOD protein (PER) appears to have the same structure and function in the core circadian clock of all animals, although its function in response to adaptions of the core clock to environmental signals varies among species (Albrecht, 2007; Partch et al., 2014; Sandrelli et al., 2008). In spite of small differences between fruit flies and mice in the cytoplasmic and gated nuclear entry of PER proteins (Curtin et al., 1995; Shafer et al., 2002), the basic molecular mechanism that generates rhythms is highly similar in all examined animals. Moreover, this timing mechanism is active in specialized brain areas (e.g., the accessory medulla (AME) in the fruit fly and the suprachiasmatic nucleus (SCN) of the mammalian hypothalamus) that have close anatomical and functional connections to the optic system (light input) and hormonal system (systemic output; (Helfrich-Forster, 2004, 2009). The master clocks in insects and mammals have a neuromodulatory function and employ a wide variety of neuropeptides as signaling molecules (Maywood et al., 2011; Yoshii et al., 2009). Both insect and mammalian master clocks influence behavioral rhythms and connect to peripheral clocks via a combination of humoral factors and the peripheral autonomic nervous system.
In mammals, the relationship between the SCN and peripheral organs is well established. In order to adjust its rhythm, the SCN receives information from the retina where intrinsically photoreceptive retinal ganglion cells (ipRGCs) relay photic signals to the SCN via retinohypothalamic fibers (Hannibal and Fahrenkrug, 2002; Hattar et al., 2002). The SCN communicates with the other clocks through autonomic innervation and, to a second degree, through the regulation of systemic cues such as body temperature, hormonal signaling, and feeding (Mohawk et al., 2012). The SCN directly influences behavioral and peripheral rhythms through neuropeptides (Cheng et al., 2002; Loh et al., 2011), but it also controls peripheral clocks indirectly via the autonomic innervation. For instance, the SCN influences glucose homeostasis in the liver or glucocorticoid secretion by the adrenal glands via the paraventricular nucleus (Ishida et al., 2005; Kalsbeek et al., 2004). Furthermore, clocks from the heart, kidney, pancreas, lung, and thyroid glands are also controlled by autonomic nervous connections. In contrast, feeding and body temperature rhythms, modulated by rest and activity cycles, are additional zeitgebers that entrain the liver, the pancreas, the heart, and the kidneys (Buhr et al., 2010; Dibner et al., 2010).
The identification of clock genes, and the cellular processes instructed by those genes, confidently explains how individual neurons can act as autonomous oscillators. While the ability of individual neurons to oscillate with circadian periodicities is now well established (Herzog et al., 1998; Welsh et al., 1995), in the recent past it was almost unimaginable that this phenomenon would be the case, given the long time constants involved. Moreover, according to a recent finding, even astrocyte clocks in the SCN can drive circadian rhythmicity of SCN neurons (Brancaccio et al., 2019). Although individual neurons possess this remarkable autonomous capability, the field has come to recognize that the timing of physiology and behavior is the outcome of autonomous oscillators that work together within and among tissues in synchronized multi-oscillator ensembles. Intriguingly, communication among clock cells leads to new properties at the network or tissue level. For example, synchronization among central clock neurons leads to the ability to encode and store day length, which provides information about the seasons, a major task of clocks in many organisms (Hastings et al., 2018; Helfrich-Forster, 2009; VanderLeest et al., 2007). This ability is not present at the single-neuron level. Further, the central clock in mammals is under the influence of both the external milieu -mostly light- and the internal milieu, and it integrates signals from other brain areas as well as from peripheral organs. These signals all change the properties of the central clocks in important ways, and the signals provide not only adaptability but robustness and precision.
The identification of the central molecular clock mechanism provided the foundational “building blocks” that now allow an understanding of how molecular feedback loops within individual neurons interact with similar mechanisms in other neurons. This information creates a neuronal network with both properties of the fundamental molecular oscillators and properties that emerge from interactions with other neurons (Hastings et al., 2018; Helfrich-Forster, 2009; VanderLeest et al., 2007). Clearly, understanding biological timing at multiple levels of molecular, cellular, and neural organization will be extremely salient for translation of circadian biology to humans.
Translational challenges
For practical reasons and workday organization, researchers typically perform experiments during the daytime, but this design creates unique difficulties when using laboratory rodents. The most critical difference is oft-cited and obvious: mice and rats are nocturnal. In fact, the National Association for Biomedical Research in the US reports that mice and rats represent 95% of all laboratory animals used for research. While mice and rats are nocturnal and have a high metabolic rate and increased behavioral activity and wakefulness during the night, humans are diurnal and have their active period during the day. The main problem is related to the fact that the majority of researchers who use rodents perform their experiments during the daytime, which corresponds to night time in humans, when drugs are not usually administered. These fundamental biological differences between nocturnal rodents and humans may pose a challenge when trying to translate pre-clinical research results to humans. As a result, the meaningful translation of pre-clinical data to humans may be partly missed.
Daytime experiments in rodents are different from human daytime
Given the strong time-dependency exhibited by most behavioral and metabolic tests, such as learning (Chaudhury and Colwell, 2002) or glucose tolerance tests (la Fleur et al., 2001), such variations are important to consider for the design of experimental protocols. However, the differences are in at least two respects subtler and more difficult to evaluate. First, rodents are polyphasic sleepers: although sleep is mostly consolidated into the daytime, a rodent will show frequent short bouts of sleep and waking during the night as well as the day in a way that has no human equivalent (Simasko and Mukherjee, 2009). The circadian system and the sleep homeostasis are tightly connected but are two separately driven systems in which the circadian drive for wakefulness is uniquely timed to the increasing homeostatic sleep drive in order to consolidate a 24 hr rhythm in sleep and wakefulness (Dijk and Czeisler, 1994). Thus, effects of “sleep pressure” are much harder to evaluate, in spite of the high conservation of both molecular and synaptic aspects of sleep. Moreover, perhaps due to the normal frequency of sleep, a four-hour period of enforced wakefulness represents a major perturbation equivalent to much longer periods of sleep deprivation in a human being. Second, rodent metabolism is tuned to frequent eating, which makes prolonged fasting an unnatural experience. Thus, typical conditions of food deprivation represent mild starvation, and a single day of fasting can result in up to 20% loss in body weight (Dohm et al., 1983). Similarly, small changes in temperature create large changes in food consumption behavior. For example, mice kept at 33°C ate 50% less than those kept at 20°C, and mice kept at 11°C ate 37% more (Bronson, 1987). Overall, performing experiments during the animal’s sleep phase can have major consequences on their physiology and the ability to translate the findings to humans.
Nocturnal versus diurnal circadian systems
Recently, the ancestral activity patterns of Mammalia were reconstructed. The data indicate that mammals went through a nocturnal bottleneck due to a temporal partitioning between early mammals (night activity) and dinosaurs (day activity) during the Mesozoic Era (Maor et al., 2017). Diurnality (day activity) appeared in mammals with the extinction of dinosaurs in the Cenozoic Era. With this change, several adaptations appeared in diurnal mammals to optimize their physiology to the inverted rest-activity cycle. Theoretically, three different types of adaptations were possible. First, the molecular clock could be changed so that it runs with the opposite phase in diurnal compared to nocturnal animals. Second, the input sensors could be changed such that they affect the molecular clock mechanism in opposite ways and, third, the interpretation of the clock signal could be opposite, which would lead to appropriate regulation of physiological pathways.
Deoxyglucose uptake experiments revealed that in the SCN, the rhythm of metabolic activity is similar in both nocturnal and diurnal animals (Schwartz et al., 1983). At the molecular level, the expression of clock genes shows the same phase in diurnal and nocturnal SCN, and their light-dependent synchronization is comparable (Mrosovsky et al., 2001). Interestingly, the behavioral response to light perceived at night is similar in nocturnal and diurnal animals as well. They respond with a delay of clock phase in response to light during the early part of the dark phase, whereas clock phase is advanced if light is perceived during the late portion of the dark phase (Mahoney et al., 2001). This finding indicates that the SCN clock ticks in a similar manner regardless of the activity preference of the species: there is a universal connection to solar time.
However, in SCN neurons, rhythmicity of putative clock target factors display opposite phases in diurnal and nocturnal animals, as exemplified by transforming growth factor alpha (TGFα; (Tournier et al., 2007). Interestingly, brain clock gene expression outside the SCN (Vosko et al., 2009) and in peripheral tissues (Mure et al., 2018) displays roughly opposite phases. Additionally, hormones (glucocorticoids, leptin, and ghrelin) and metabolites (glucose and free fatty acids) show opposite cycling phases in diurnal and nocturnal rodents (Kumar Jha et al., 2015), results that suggest the difference between nocturnal and diurnal animals may lie downstream of the SCN.
There is, however, evidence that diurnal and nocturnal mammals differ in the input pathways to the clock. For example, the diurnal species Tupaia (tree shrew) displays arrhythmic activity patterns under constant darkness conditions (Meijer et al., 1990), whereas nocturnal mice show rhythmic, free-running activity with a period slightly less than 24 hours under these same conditions. Under constant bright light conditions, however, the situation is inverted: nocturnal rodents can become arrhythmic, whereas the diurnal Tupaia displays robust, rhythmic, and free-running activity (Meijer et al., 1990). The acute light response is also qualitatively different (Meijer et al., 1990). Taken together with their similar core clock phases, the difference between diurnal and nocturnal species may be at the cellular and neuronal network level rather than the genetic level. Evidence for anatomical adaptation to diurnality comes from the observation that in diurnal animals, classical photoreceptors play a more important role in the light response than in nocturnal animals (van Diepen et al., 2013). Older studies revealed that the ratio between light-suppressed and light-activated cells differs between diurnal and nocturnal animals (Meijer et al., 1989), results that suggest the light-input mechanism is not identical. This observation is consistent with the finding described above that constant light and darkness do not have the same effect on activity patterns in animals that occupy opposing temporal niches. Taken together, it appears that adaptation to diurnality may also include pathways upstream of the SCN. It also needs to be mentioned that the light sensitivity for phase entrainment is much higher in nocturnal animals.
The observations described above are largely consistent with a recent study that systematically analyzed the diurnal transcriptome in neural and peripheral tissues of the baboon (Papio anubis; (Mure et al., 2018). Comparison between the diurnal baboon and the nocturnal mouse revealed that, with the exception of the SCN, the peak phase of most clock genes is opposite between comparable tissues. Since common cycling clock target genes in peripheral tissues of baboon and mouse did not consistently show an opposite expression pattern (Mure et al., 2018), it is unlikely that there is a single nocturnal-diurnal “switch” downstream of the SCN. Indeed, in all mammalian species, independent of nocturnal or diurnal activity, the pineal gland secretes melatonin exclusively at night (Pevet, 2003). This phenomenon suggests that in peripheral tissues, temporal organization is diverse and may be autonomous. The dominance of feeding-fasting cycles on the clock phase entrainment of peripheral organs (but not the SCN) in mice and rats (Damiola et al., 2000; Stokkan et al., 2001), and probably also in humans, suggests that time of food uptake is a main synchronizer in peripheral organs. Insulin signaling drives the synthesis of the clock protein PER to entrain circadian rhythms with feeding time (Crosby et al., 2019). This may explain the opposite cycling of peripheral oscillators observed between nocturnal and diurnal species, because feeding times are following activity patterns. Although clock rhythms in the SCN are comparable in both nocturnal and diurnal species, the peripheral oscillators are not. However, all species maintain a constant phase relationship between the SCN and peripheral organs in order to organize body physiology in a temporal manner. As mentioned above, the rodent SCN integrates the light entrainment into the autonomous molecular and electrical oscillations, which in turn are relayed to the body via neural and humoral pathways. Whereas glucocorticoids communicate SCN signals to peripheral clocks, feeding signals can entrain peripheral clocks without SCN influence (Damiola et al., 2000), which appears to use insulin and IGF-1 as a major signaling pathways (Crosby et al., 2019). Hence, model organisms for developing treatments of clock-related diseases in humans may be useful taking into consideration the opposite phase relationship between the SCN and peripheral clocks in nocturnal species compared to diurnal species.
Circadian pharmacokinetics
Not only will the experimental manipulations performed during rodent sleep-time affect their translation to humans, administration of drugs at different times of the day can also lead to various outcomes due to variations in pharmacokinetics. Drug pharmacokinetics are governed by its physicochemical characteristics and Absorption, Distribution, Metabolism, and Excretion (ADME) properties. The potential circadian modulation of pharmacokinetics, chronopharmacokinetics, was reviewed as early as the 1980s (see (Reinberg and Smolensky, 1982), and it examined the chronobiological regulation of factors involved in different aspects of ADME. Such interactions range from the macroscopic impact of activity and feeding cycles on factors such as gastric emptying and blood-flow rate on drug absorption and distribution, to circadian regulation of expression levels of enzymes and transporters involved in metabolism and excretion of xenobiotics such as drugs (Erkekoglu and Baydar, 2012).
The ADME properties of all drugs can be subject to large circadian variations, as shown for hundreds of compounds in laboratory rodents and humans (Levi and Schibler, 2007). To some degree, they are affected by circadian rest-activity patterns such as meal timing, the general sleep-wake cycle, and physical activity, all of which modulate blood pressure and flow. The degree of impact depends not only on the routes of administration and excretion, but also on circadian modulation of gastric pH and gastrointestinal motility, both of which influence drug absorption. Blood flow and capillary perfusion, by contrast, impact drug absorption from the gastrointestinal tract, distribution to tissues and target organs, and even excretion through glomerular filtration rate in the kidneys (Ballesta et al., 2017; Dallmann et al., 2014; Dallmann et al., 2016). Recent research demonstrated that xenobiotic efflux by the blood-brain barrier is also under circadian regulation, which could influence the response to drug treatments that target the brain (Zhang et al., 2018).
There are other drug-specific effects that depend on circadian regulation of xenobiotic detoxification pathways (Zmrzljak and Rozman, 2012). The expression of phase I and II drug-metabolizing enzyme families, such as CYP450, SULT, UGT, NQI, EPH, GSTH, and NAT, are regulated by CLOCK/BMAL1, REV-ERB/ROR, or circadian clock-regulated PARbZip transcription factors (Gachon et al., 2006; Kang et al., 2007; Tanimura et al., 2011). CYP450 activity also depends on heme availability, which is under circadian control because the rate-limiting enzyme in heme biosynthesis (ALAS1) is driven by the CLOCK paralogue NPAS2 (Froy, 2009). Finally, phase III detoxification through hepatobiliary or renal excretion and reabsorption of parent drug or metabolites is not only affected by perfusion and activity levels, as described above, but these processes can also be modulated through circadian regulation of transporter protein expression levels, including the ABC/MDR, OAT, OCT, and MRP families (Gachon and Firsov, 2011). Since these same proteins are involved in the transport into and out of target tissues and cells in the organism (Scherrmann, 2009), such circadian regulation can equally affect the absorption and distribution aspects of the drug pharmacokinetic profile.
In summary, the impact of circadian modulation on the ADME properties of (and thus relevance of chronopharmacokinetics for) the therapeutic effect of a given drug will depend strongly on the degree to which the compound interacts with transporters during the processes of absorption, distribution, and excretion, and the amount and role of metabolism it undergoes. Consequently, the pharmacokinetics of a given drug may differ depending on the time of the day it is delivered, an aspect that has not yet been systematically examined in clinical research, drug development, registration by regulatory agencies, or in medical and pharmacy practices. Specific attention should be paid to drugs with a narrow therapeutic window, or when a strong correlation is found between the pharmacodynamic effect and the plasma or tissue levels of a drug that acts on a therapeutic target which is itself under circadian regulation (Bruguerolle and Lemmer, 1993). All of these parameters can profoundly differ in model organisms.
Treatment outcome depends on the time of administration
Drug efficacy may not only depend on drug pharmacokinetics, but also on the internal status of the clock and clock-regulated genes, which will in turn determine the sensitivity of target cells and pathways to the available drug at specific times of the day. Chronopharmacological approaches to treating diseases revealed that efficacy is improved, and side effects reduced, when the administration is appropriately timed. For instance, levels of antibodies in response to influenza vaccination are higher in the morning compared to the afternoon (Long et al., 2016), a phenomenon that could be due to baseline differences in antibody titers, which vary during the day (Kurupati et al., 2017). Patients who received a sustained release formulation of indomethacin for hip or knee osteoarthritis presented a 33% incidence of adverse events after morning dosing, compared to 7% after evening dosing. Antalgic and anti-inflammatory efficacies were most effective following evening dosing in subjects with predominantly nocturnal or morning pain (Levi et al., 1985). In asthmatic children, a sustained-release preparation of theophylline (Theo24R), was recommended to be taken in the evening, based on improved efficacy of standard theophylline preparations dosed at this time of day (Smolensky et al., 1987). However, in a randomized, double-blind, and placebo-controlled study, the serum levels of Theo24R exceeded the toxic threshold of 20 mg/L in the majority of children that took the medication daily for 6 days at 21:00, as compared to none when taken at 06:00 (Smolensky et al., 1987). While Theo24R effectively improved airway functions, there were no statistically significant differences in drug efficacy according to dosing time in this study, in contrast to large differences in steady state pharmacokinetics (Smolensky et al., 1987).
Theo24R later proved to be too toxic (toxicity symptoms ranging from abdominal pain to cardiac arrhythmias to seizures) at the recommended evening intake and was withdrawn from the market (Cooling, 1993; Journey and Bentley, 2018). In oncology, the chronomodulated administration of combination chemotherapy with oxaliplatin, 5-fluorouracil, and leucovorin for the treatment of metastatic colorectal cancer produced severe mucosal toxicities in only 14% of patients as compared to 76% of those who received “standard” infusions at a constant rate for 5 days. This protocol significantly increased the rate of patients with an objective treatment response from 29% to 51% (Levi et al., 1997). A meta-analysis of three international randomized trials that involved 842 patients with metastatic colorectal cancer further demonstrated that the three-drug chronotherapy schedule significantly improved overall survival in men, but not women, independently of all known prognostic factors (Giacchetti et al., 2012). These results triggered further pre-clinical and clinical studies aimed at providing a reliable and real-time metric of the circadian timing system for personalizing chronotherapeutic strategy and delivery. Such circadian biomarker assessments are clearly necessary for customizing chronotherapeutics according to the internal phase of the patient. Moreover, in a pooled analysis of 1077 cancer patients, cases of circadian disruption were associated with significantly worse survival and quality of life, as compared to robustness, a finding that supports the need to develop clock-targeted therapies for these patients (Ballesta et al., 2017).
Several large studies highlight the relevance of circadian timing of treatments for clinical tolerability and/or efficacy in allergic, rheumatologic, sleep, cardiovascular, and malignant diseases (Table 1). It is interesting to note that a majority of drugs (e.g., angiotensin converting-enzyme (ACE) and angiotensin receptor blockers, calcium channel blockers, and diuretics) show greater efficacy upon evening administration. In contrast, glucocorticoids show the opposite effect, with greater efficacy in the morning. For instance, standard prednisone treatment for rheumatoid arthritis was compared with evening intake of a chrono-prednisone tablet, which automatically released the drug during the early morning (04:00 when taken at 20:00); the chrono-release tablet ameliorated median joint stiffness by 22%, as compared to 0.4% for standard prednisone therapy (Buttgereit et al., 2008). This efficacy was replicated in another large, randomized, double-blind, and placebo-controlled study that showed the efficacy of modified-release prednisone treatment for rheumatoid arthritis (Buttgereit et al., 2013). Thus, systems chronopharmacology and circadian biomarker studies are now shaping chronotherapeutics for chronic diseases in the era of precision and personalized medicine (Ballesta et al., 2017). It is interesting to note that the majority of top-selling drugs target genes whose expression is circadian and have half-lives shorter than 6 hours. Thus, the timing of administration is critical for drugs with such short half-lives (Zhang et al., 2014). An example of a short-lived compound is aspirin, which targets circadian clock-regulated genes (e.g. PTGS1 or cyclooxygenase-1, alias COX1), and modulates blood pressure when delivered in low doses at nighttime (Hermida et al., 2005).
Table 1. Efficacy of a morning versus evening schedule of drug administration.
The table is modified from De Georgi et al. (2013), with permission from Elsevier, and includes studies reported in PubMed until September 2018 using the term “chronotherapy”. Studies with n > 100 are reported with the drug used and its classification. The number of subjects and the study design are shown, and the time at which the best efficacy in treatment outcome was observed is reported.
| Drug | Type | Subjects | Type of study | Most Efficacious Delivery Time | Reference |
|---|---|---|---|---|---|
| Ramipril | ACE inhibitors & angiotensin receptor blocker | 115 | Randomized | Evening | (Hermida and Ayala, 2009) |
| Olmesartan | ACE inhibitors & angiotensin receptor blocker | 123 | Randomized | Evening | (Hermida et al., 2009) |
| Telmisartan | ACE inhibitors & angiotensin receptor blocker | 215 | Randomized | Evening | (Hermida et al., 2007) |
| Nifedipine | Calcium channel blocker | 180 | Randomized | Evening | (Hermida et al., 2008b) |
| Torasemide | Diuretics | 113 | Randomized | Evening | (Hermida et al., 2008a) |
| Doxazosin | Diuretics | 111 | Randomized | Evening | (Pickering et al., 1994) |
| Amiodipine/valsartan | Combinations | 203 | Single/Combined | Evening | (Hermida et al., 2010) |
| Hydrochlorothyazide/valsartan | Combinations | 204 | Open-label, blinded end-point | Evening | (Hermida et al., 2011) |
| Simvastatin | Statins | 172 | Double-blind, placebo-controlled | Evening | (Saito et al., 1991) |
| Simvastatin | Statins | 132 | Randomized, double-blind, placebo-controlled | No difference | (Kim et al., 2013) |
| Fluvastatin ER | Statins | 197 | Double-blind, multicenter | No difference | (Scharnagl et al., 2006) |
| Ezetimibe-simvastatin | Statins | 171 | Randomized, Cross-over | Morning | (Yoon et al., 2011) |
| Ketprofen | Analgesics | 117 | Randomized, Double-blind | No difference | (Perpoint et al., 1994) |
| Aspirin | COX inhibitor | 290 | Randomized crossover | No difference | (Bonten et al., 2015) |
| Aspirin | COX inhibitor | 350 | Randomized, double-blind, placebo-controlled | Evening | (Ayala et al., 2013) |
| Prednisone (for arthritic pain) | Corticosteroids | 288 | Randomized, Double-blind | Morning | (Buttgereit et al., 2008) |
| Mometasone (for asthma) | Corticosteroids | 268 | Placebo-controlled | No difference | (Karpel et al., 2005) |
| Tiotropium | Beta(2)-adrenergic agonists | 121 | Randomized, Double-blind, Placebo-controlled | No difference | (Calverley et al., 2003) |
| Montelukast | Leukotriene receptor antagonist | 343 | Randomized, Placebo-controlled | Evening | (Altman et al., 1998) |
Potential solutions to improve clinical outcomes in drug trials
Considerations of time in pre-clinical research
As noted above, a key difficulty in translating bench findings to the clinic results from pre-clinical research performed on rodents. The mismatch between rodent and human phase could easily be corrected by inverting the light-dark cycle of the rodent, thereby eliminating the necessity for the scientist to perform experiments during the night. Is the solution to incorporate diurnal rodents in pre-clinical drug testing? Not necessarily–this design would not avoid the need for testing drugs at different times of the day to reveal their respective times of optimal efficacy and diminished toxicity, since these results may not only depend on the rhythmicity of drug metabolism and target but also on the read-outs. We strongly advocate that the disease model—not its diurnality—should be the strongest argument for testing drugs. Additionally, the number of existing diurnal rodents is small [e.g. the Mongolian gerbil (Meriones unguiculatus), the degu (Octodon degus), the African (Nile) grass rat (Arvicanthis niloticus), and the antelope ground squirrel (Ammospermophilus leucurus)], and their value as experimental models for drug development deserves to be explored (Refinetti and Kenagy, 2018). An easier and more natural approach would be to reverse or alter light-dark cycles in the normal rodent (mouse, rat) housing environment, in order to perform the experimental work during the day without phase related artifacts. For instance, turning off lights from 10:00 to 20:00 would allow animal caretakers to perform their husbandry tasks in light in the morning, while the animal experimentation could take place in darkness (Hawkins and Golledge, 2018). A number of tests demonstrated greater behavioral and cognitive performance at nighttime (Roedel et al., 2006). For instance, tonic pain sensitivity in rodents is greatest during daytime (Perissin et al., 2000), a finding that could have implications for drug efficacy being misinterpreted or completely missed solely due to the rhythmicity of the read-out. One could also use commercially available isolated housing cabinets with independent light-dark cycles, or generalize autonomic animal chronobiologic facilities where light-dark schedules are programmable-in-time (Tampellini et al., 1998). There are obvious animal-facility management implications to such approaches, in order to avoid accidental light contamination (e.g., from corridors) or noise that could entrain the animal’s rhythm. Lamps with light spectra to which rodents have reduced sensitivity (e.g., red or red-orange light, narrow-wave-length sodium vapor lamps) can entrain rodents (Peirson et al., 2018). Night-vision goggles or cameras could provide the means of performing experiments in darkness. Another solution would be to use automated systems to collect data at nighttime in the absence of human interventions.
Improving therapeutics by targeting the clock system
While controlling the animal’s diurnal cycle and considering the time of the day in pre-clinical drug treatments, another way of improving therapeutic outcome is to target the clock system. A recent analysis by Zhang, Lahens, and colleagues showed that disease genes are highly enriched for circadian regulation (Zhang et al., 2014). Many, but not all, of these clock-regulated genes are mutated in diseases such as Alzheimer’s disease, schizophrenia, and Down’s syndrome and are involved in neurodegeneration. Other circadian-related diseases include obesity, type II diabetes, and cancer. Aging causes circadian transcriptome reprogramming, which is differentially regulated in organs (Sato et al., 2017). Given that circadian clocks regulate most human physiology (Skarke et al., 2017), where half of all human protein-coding genes are clock regulated (including drug transporters, metabolizing enzymes, and targets; (Ruben et al., 2018), the clock system has received renewed interest as a pharmacological target. Multiple pilot small-molecule screens identified circadian clock-modifying compounds (e.g., (He et al., 2016; Hirota et al., 2012; Solt et al., 2012) that are under investigation for wide-ranging indications including cancer, neurodegeneration, endocrinology, and asthma. Moreover, an ever-increasing number of studies postulate distinct and elegant mechanisms by which the molecular clockwork can modulate drug metabolizing enzymes, transporters, and targets (Anafi et al., 2014; Pizarro et al., 2013; Zhang et al., 2014). For short-acting molecules, these mechanisms could be exploited to improve the therapeutic index and balance between toxicity and efficacy. However, the pathways to translational application of this knowledge remain unclear. We envision multiple possibilities beyond conventional chronopharmacology per se:
Targeted clock suppression or enhancement. Given that the basic circadian mechanism is a feedback loop of transcriptional/translational activation and repression, any suppression of circadian clock function essentially blocks the mechanism toward one extreme or the other. For example, in wound healing, Bmal1 deletion (the “positive limb” in the molecular clock) results in hyperkeratosis and insufficient cell proliferation, while Per2 deletion (the “negative limb”) results in hyperproliferation of fibroblasts and keratinocytes but insufficient keratin and collagen secretion (Kowalska et al., 2013). Thus, it is possible that transient clock suppression by targeting one or the other limb could be useful to push a normal circadian process toward a therapeutically useful extreme. On the other hand, enhancing the circadian rhythm may offer an elegant ‘do no harm’ mechanism for therapies including cancer (Iurisci et al., 2006; Kiessling et al., 2017). For example, in one study, disrupted circadian clock function led to a progression from non-alcoholic fatty liver disease (NAFLD) to fibrosis and cancer (Kettner et al., 2016). This change occurred on its own, and was even enhanced by genetic disruption of clock genes. Recent literature points to clock system disruption as causal in neurodegeneration (Hastings and Goedert, 2013; Musiek et al., 2018). It stands to reason that improving clock function, e.g., through enhancing BMAL1/CLOCK or inhibiting REV-ERBα activity, could improve outcomes. In support of this hypothesis, a recent study from Montaigne et al. reported that the incidence of major adverse cardiac events was lower in patients who undergo cardiac surgery in the afternoon compared to the morning. Consistent with a greater expression of REV-ERBα in the human myocardium in the morning, deletion of REV-ERBα function or its blockade using a selective antagonist (SR8278) at the sleep-to-wake transition protects mice from myocardial injury (Montaigne et al., 2018).
Leveraging circadian regulation. As pre-clinical researchers discover new mechanisms by which the circadian clock modulates physiology, each of these mechanisms provides a potential intervention point for therapy. For example, circadian clock proteins interact in many different ways to control drug metabolism and transport. Anafi et al. showed that a mechanistic understanding of drug transport enables design and hypothesis testing to improve the therapeutic index (Anafi et al., 2017). GLUT2, a solute carrier and drug transporter, is clock-regulated in mice and humans with high amplitude. Timing the administration of streptozocin, a chemotherapeutic agent and GLUT2 substrate, early in the wake phase dramatically decreases its toxicity, as evidenced by reduced weight loss in comparison to administration early, in the inactive phase. Recently, researchers reported a robust clock machinery in the peripheral auditory system that is associated with greater vulnerability of mice to a noise trauma delivered during the active compared to the inactive phase (Meltser et al., 2014). The improved recovery from the noise exposure during the inactive phase was associated with the ability of the cochlea to increase Bdnf expression in response to noise, a phenomenon that did not occur during the active phase. Interestingly, BDNF is circadian in the brain, where it peaks during the active phase (Marosi and Mattson, 2014), as well as in the cochlea (Basinou et al., 2016). Treatment with an agonist of the BDNF receptor TrkB, namely di-hydroxyflavone (DHF), effectively protects hearing in mice from noise trauma when delivered at night but not during the day (Meltser et al., 2014). If the compound had not been tested during nighttime, the efficacy of this molecule would have been missed, and the fundamental relevance of circadian mechanisms and TrkB signaling in the treatment of hearing disorders would have been ignored. Winter et al. found that myeloid cell recruitment to atherosclerotic lesions in mice oscillates with a peak at the onset of daytime, a process regulated by the rhythmic release of myeloid cell-derived CCL2 (Winter et al., 2018). However, myeloid cell adhesion to microvascular beds peaks at the onset of nighttime. Treatment at nighttime with RS102895, a CCR2 antagonist, reduces atherosclerotic lesion formation, whereas daytime treatment is not effective (Winter et al., 2018). Hundreds of other drug-metabolizing enzymes, transporters, and drug targets are also clock-regulated, many of these with high amplitude. This knowledge could be leveraged to improve the action of existing drugs, but also incorporated into trials of new drug candidates.
Clock independent modulation of gene expression: Finally, another possibility is to develop therapeutic drugs that target clock proteins, irrespective from their clock function. Indeed, comprehensive ChIP-seq studies suggest that individual clock proteins like CRY1 and CRY2 might associate with non-circadian promoters independently of other clock proteins (Koike et al., 2012), and drugs affecting these clock proteins might therefore exert entirely clock-independent effects upon transcription of these genes. Thus, clock protein-targeting drugs could also exert disease-pertinent clock-independent transcriptional effects.
The importance of understanding circadian alignment
Conditional mutagenesis has elucidated the contribution of individual tissue clocks to homeostasis at different times across the sleep/wake cycle. When controlled for time of day, nutrient status (e.g., fasting or feeding), and developmental age (using inducible alleles), such studies reveal that local clocks of distinct organs partition anabolic and catabolic processes to different times of day (Peek et al., 2013; Perelis et al., 2015). An area that remains in its infancy is our understanding of how specialized pacemaker clock neurons and astrocytes might be linked within circuits involved in energy balance, sleep, mood, learning, and memory. As clocks are robust and interconnected, a challenge has been that perturbation of individual factors in an animal may lead to compensatory up-regulation in a second limb. Genetic strategies must control for such compensatory gene regulatory loops. For instance, in experimental design, investigators may consider monitoring behavioral and physiological endpoints under free-running conditions, or using cell-based models including three-dimensional organoids from different tissues (Yamajuku et al., 2012) that are devoid of the complex nutritional factors that modulate rhythmicity in an intact animal. Work in eubacteria and plants (Dodd et al., 2005; Ouyang et al., 1998) demonstrated growth and reproductive advantage when intrinsic clock time is aligned with the external light-dark cycle. Advancing the concept of circadian alignment and its effect in mammalian organisms remains a critical goal in mapping the role of biological clocks in human health.
Conclusions
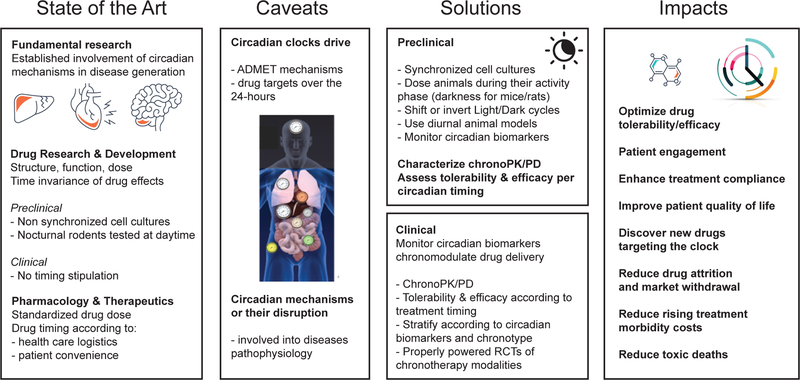
There is clear evidence for the involvement of circadian mechanisms in diseases, yet there is only sparse recognition of its importance in pre-clinical and clinical research. This article has highlighted the hurdles when performing experiments in nocturnal rodents during their sleep-time, and how this experimental design is likely to affect translation to humans. We advocate a paradigm shift in pre-clinical research using circadian knowledge in order to develop therapeutic interventions appropriately timed by considering the drug kinetics and the circadian status of the target and the disease (Figure 1). Chronotherapy, which considers the time of day, may significantly improve clinical trial success and ultimately patient care. Since sleep-wake cycles are driven by the coupling of the circadian clock (oscillator) and the sleep homeostat (hourglass timer) (Guo et al., 2018), sleep-wake cycles (e.g. fragmented sleep, sleep deprivation) will probably have to be considered when designing chronotherapeutic regimens. Additionally, a novel approach would be to develop and exploit drugs that target the clock system. For such knowledge to emerge, there is a need for greater incentives by funding agencies for the inclusion of circadian aspects in grant calls. For instance, circadian-omics may help in creating a phase translation map that would reveal potential translational hurdles and benefits. In parallel, pharmaceutical and biotechnology companies should apply existing circadian knowledge to their drug development pipeline. Finally, circadian biology is often not incorporated in the curriculum of medical schools and hence, circadian education and dissemination among medical practitioners will be important for bridging the gap. It is time to take time seriously.
Figure 1. Towards the use of chronopharmacology for precision and personalized medicine.
The current state of the art indicates that although the impact of circadian rhythms on biological outcomes is acknowledged (e.g. academia and industry are more cautious in performing experiments at similar times of the day), minimal attention is given on the powerful impact that circadian mechanisms may have on drug development. Animal and clinical work show a strong correlation between clock malfunction and disease. However, there are inherent translational challenges in drug R&D such as the use of rodents (nocturnal animals) tested at daytime, which has a large impact on physiology and thus complicates the translation to humans. Clinically, there is no emphasis put on the timing of drug administration, rather the focus is on health care logistics and patient convenience. Important caveats include the fact that drug ADME properties (Absorption, Distribution, Metabolism, and Excretion) are controlled by circadian mechanisms leading to altered bioavailability at different times of the day, and that the circadian status of the pathway targeted by the drug will also impact outcome. Thus, integrating circadian knowledge on ADME properties and the activity of the targeted pathway will lead to increased efficacy and diminished side-effects. Since the majority of FDA approved drugs have circadian targets, timing drug delivery could have a large impact on the effectiveness of target activation or inhibition. What solutions are available to achieve such medical improvements? While the use of diurnal rodents would require decades to develop optimal disease models, using the acquired knowledge in nocturnal animals would be more powerful by including shifts in the light cycle or performing experiments during the animal’s active time – depending on the read-out and the physiology tested. Implementing circadian aspects in pre-clinical research will lead to new discoveries that, once applied in clinical trials (e.g. using chronotype or circadian biomarkers), may improve the impact on human health by optimizing drug efficacy and reducing side-effects. The case of Oxaliplatin is a pioneering example that led to chronomodulated infusion, with maximized treatment efficacy and minimized adverse effects.
Acknowledgments
Research in the Canlon lab is funded by Communication Disorders of the National Institutes of Health R21DC013172 and 1R56DC016415-01, the Swedish Medical Research Council K2014-99X-22478-01-3, the Knut and Alice Wallenberg Foundation (B.C. #KAW2008), the Karolinska Institutet, Tysta Skolan, Hörselforskningsfonden, Magnus Bergvalls, and the EU (H2020-MSCA-ITN, ESIT, C.R.C -project # 722046). B.C. and C.R.C. also received funding from the Office of the Assistant Secretary of Defense for Health Affairs, through the Neurosensory and Rehabilitation program, under Award No. W81XWH-16-1-0032. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. Research in the Helfrich-Förster lab is funded by the German Research Foundation (DFG; FO207/15-1) and the EU (H2020-MSCA-ITN-2017, CINCHRON, project # 765937). J.S.T. is an Investigator in the Howard Hughes Medical Institute. M.H.H. was funded by the UK Medical Research Council, U.K. (MC_U105170643). Research in the UA is funded by the Swiss National Science Foundation (SNF 310030_184667/1) and the Velux Foundation (No. 995). FL was funded by CRUK (C53561/A 19933), MRC (MR/M013170), Philips Respironics (USA), the Mc Grath Family, and Ramsay-Generale-de-Sante (Fr), Dr. Bass’ work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants R01DK090625, R01DK100814, R01DK11301, and R01DK050203; the National Institute on Aging grant P01AG011412; the Chicago Biomedical Consortium S-007; and the University of Chicago Diabetes Research and Training Center grant P60DK020595, FG received support from the Institute for Molecular Bioscience, The University of Queensland; SB is supported by the Swiss National Science Foundation, the Velux Foundation, and the Human Frontiers Science Program, MY is supported by grants from the National Institutes of Health (GM054339).
Footnotes
Jonas Dyhrfjeld-Johnsen is an employee and shareholder of Sensorion. All other authors declare that they have no competing financial interests.
References
- Albrecht U (2007). Per2 has time on its side. Nat Chem Biol 3, 139–140. [DOI] [PubMed] [Google Scholar]
- Allada R, Emery P, Takahashi JS, and Rosbash M (2001). Stopping time: the genetics of fly and mouse circadian clocks. Annual review of neuroscience 24, 1091–1119. [DOI] [PubMed] [Google Scholar]
- Altman LC, Munk Z, Seltzer J, Noonan N, Shingo S, Zhang J, and Reiss TF (1998). A placebo-controlled, dose-ranging study of montelukast, a cysteinyl leukotriene-receptor antagonist. Montelukast Asthma Study Group. J Allergy Clin Immunol 102, 50–56. [DOI] [PubMed] [Google Scholar]
- Anafi RC, Francey LJ, Hogenesch JB, and Kim J (2017). CYCLOPS reveals human transcriptional rhythms in health and disease. Proceedings of the National Academy of Sciences of the United States of America 114, 5312–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anafi RC, Lee Y, Sato TK, Venkataraman A, Ramanathan C, Kavakli IH, Hughes ME, Baggs JE, Growe J, Liu AC, et al. (2014). Machine learning helps identify CHRONO as a circadian clock component. PLoS biology 12, e1001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala DE, Ucieda R, and Hermida RC (2013). Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiology international 30, 260–279. [DOI] [PubMed] [Google Scholar]
- Ballesta A, Innominato PF, Dallmann R, Rand DA, and Levi FA (2017). Systems Chronotherapeutics. Pharmacol Rev 69, 161–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basinou V, Park JS, Cederroth CR, and Canlon B (2016). Circadian regulation of auditory function. Hearing research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, and Lazar MA (2016). Circadian time signatures of fitness and disease. Science 354, 994–999. [DOI] [PubMed] [Google Scholar]
- Bonten TN, Snoep JD, Assendelft WJ, Zwaginga JJ, Eikenboom J, Huisman MV, Rosendaal FR, and van der Bom JG (2015). Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: a randomized cross-over trial. Hypertension 65, 743–750. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Edwards MD, Patton AP, Smyllie NJ, Chesham JE, Maywood ES, and Hastings MH (2019). Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 363, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH (1987). Susceptibility of the fat reserves of mice to natural challenges. J Comp Physiol B 157, 551–554. [DOI] [PubMed] [Google Scholar]
- Bruguerolle B, and Lemmer B (1993). Recent advances in chronopharmacokinetics: methodological problems. Life Sci 52, 1809–1824. [DOI] [PubMed] [Google Scholar]
- Buhr ED, Yoo SH, and Takahashi JS (2010). Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burioka N, Fukuoka Y, Koyanagi S, Miyata M, Takata M, Chikumi H, Takane H, Watanabe M, Endo M, Sako T, et al. (2010). Asthma: Chronopharmacotherapy and the molecular clock. Advanced drug delivery reviews 62, 946–955. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Doering G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, Jeka S, Krueger K, Szechinski J, and Alten R (2008). Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet 371, 205–214. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Mehta D, Kirwan J, Szechinski J, Boers M, Alten RE, Supronik J, Szombati I, Romer U, Witte S, et al. (2013). Low-dose prednisone chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2). Annals of the rheumatic diseases 72, 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, and Shea SA (2012). Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4, 129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PM, Lee A, Towse L, van Noord J, Witek TJ, and Kelsen S (2003). Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax 58, 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PJ, Bolli GB, Cryer PE, and Gerich JE (1985). Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. The New England journal of medicine 312, 1473–1479. [DOI] [PubMed] [Google Scholar]
- Caussanel JP, Levi F, Brienza S, Misset JL, Itzhaki M, Adam R, Milano G, Hecquet B, and Mathe G (1990). Phase I trial of 5-day continuous venous infusion of oxaliplatin at circadian rhythm-modulated rate compared with constant rate. Journal of the National Cancer Institute 82, 1046–1050. [DOI] [PubMed] [Google Scholar]
- Chang AM, Aeschbach D, Duffy JF, and Czeisler CA (2015). Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proceedings of the National Academy of Sciences of the United States of America 112, 1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, and Colwell CS (2002). Circadian modulation of learning and memory in fear-conditioned mice. Behavioural brain research 133, 95–108. [DOI] [PubMed] [Google Scholar]
- Chellappa SL, Morris CJ, and Scheer F (2018). Daily circadian misalignment impairs human cognitive performance task-dependently. Scientific reports 8, 3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, and Zhou QY (2002). Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417, 405–410. [DOI] [PubMed] [Google Scholar]
- Chinoy ED, Duffy JF, and Czeisler CA (2018). Unrestricted evening use of light-emitting tablet computers delays self-selected bedtime and disrupts circadian timing and alertness. Physiological reports 6, e13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JD, Kyriacou CP, and Reppert SM (2001). Keeping time with the human genome. Nature 409, 829–831. [DOI] [PubMed] [Google Scholar]
- Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, and Pangalos MN (2014). Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nature reviews Drug discovery 13, 419–431. [DOI] [PubMed] [Google Scholar]
- Cooling DS (1993). Theophylline toxicity. J Emerg Med 11, 415–425. [DOI] [PubMed] [Google Scholar]
- Crosby P, Hamnett R, Putker M, Hoyle NP, Reed M, Karam CJ, Maywood ES, Stangherlin A, Chesham JE, Hayter EA, et al. (2019). Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell 177, 896–909 e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin KD, Huang ZJ, and Rosbash M (1995). Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron 14, 365–372. [DOI] [PubMed] [Google Scholar]
- Dallmann R, Brown SA, and Gachon F (2014). Chronopharmacology: new insights and therapeutic implications. Annu Rev Pharmacol Toxicol 54, 339–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmann R, Okyar A, and Levi F (2016). Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends Mol Med 22, 430–445. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, and Schibler U (2000). Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes & development 14, 2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, and Albrecht U (2010). The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annual review of physiology 72, 517–549. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, and Czeisler CA (1994). Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neuroscience letters 166, 63–68. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, and Webb AA (2005). Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633. [DOI] [PubMed] [Google Scholar]
- Dohm GL, Tapscott EB, Barakat HA, and Kasperek GJ (1983). Influence of fasting on glycogen depletion in rats during exercise. J Appl Physiol Respir Environ Exerc Physiol 55, 830–833. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, and Young ME (2010). Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circulation research 106, 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkekoglu P, and Baydar T (2012). Chronopharmacokinetics of drugs in toxicological aspects: A short review for pharmacy practitioners. J Res Pharm Pract 1, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extra JM, Espie M, Calvo F, Ferme C, Mignot L, and Marty M (1990). Phase I study of oxaliplatin in patients with advanced cancer. Cancer Chemother Pharmacol 25, 299–303. [DOI] [PubMed] [Google Scholar]
- Falchi F, Cinzano P, Duriscoe D, Kyba CC, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, and Furgoni R (2016). The new world atlas of artificial night sky brightness. Sci Adv 2, e1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froy O (2009). Cytochrome P450 and the biological clock in mammals. Curr Drug Metab 10, 104–115. [DOI] [PubMed] [Google Scholar]
- Gachon F, and Firsov D (2011). The role of circadian timing system on drug metabolism and detoxification. Expert Opin Drug Metab Toxicol 7, 147–158. [DOI] [PubMed] [Google Scholar]
- Gachon F, Olela FF, Schaad O, Descombes P, and Schibler U (2006). The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell metabolism 4, 25–36. [DOI] [PubMed] [Google Scholar]
- Giacchetti S, Dugue PA, Innominato PF, Bjarnason GA, Focan C, Garufi C, Tumolo S, Coudert B, Iacobelli S, Smaaland R, et al. (2012). Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: a meta-analysis. Ann Oncol 23, 3110–3116. [DOI] [PubMed] [Google Scholar]
- Granda TG, D’Attino RM, Filipski E, Vrignaud P, Garufi C, Terzoli E, Bissery MC, and Levi F (2002). Circadian optimisation of irinotecan and oxaliplatin efficacy in mice with Glasgow osteosarcoma. Br J Cancer 86, 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham SC, Agnew HW Jr., and Williams RL (1965). The sleep of depressed patients. An EEG and eye movement study. Archives of general psychiatry 13, 503–507. [DOI] [PubMed] [Google Scholar]
- Guo F, Holla M, Diaz MM, and Rosbash M (2018). A Circadian Output Circuit Controls Sleep-Wake Arousal in Drosophila. Neuron 100, 624–635 e624. [DOI] [PubMed] [Google Scholar]
- Hannibal J, and Fahrenkrug J (2002). Melanopsin: a novel photopigment involved in the photoentrainment of the brain’s biological clock? Annals of medicine 34, 401–407. [DOI] [PubMed] [Google Scholar]
- Hastings MH, and Goedert M (2013). Circadian clocks and neurodegenerative diseases: time to aggregate? Current opinion in neurobiology 23, 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, and Brancaccio M (2018). Generation of circadian rhythms in the suprachiasmatic nucleus. Nature reviews Neuroscience 19, 453–469. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, and Yau KW (2002). Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus E (2007). Chronobiology of hemostasis and inferences for the chronotherapy of coagulation disorders and thrombosis prevention. Advanced drug delivery reviews 59, 966–984. [DOI] [PubMed] [Google Scholar]
- Hawkins P, and Golledge HDR (2018). The 9 to 5 Rodent - Time for Change? Scientific and animal welfare implications of circadian and light effects on laboratory mice and rats. Journal of neuroscience methods 300, 20–25. [DOI] [PubMed] [Google Scholar]
- He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, et al. (2016). The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell metabolism 23, 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Forster C (2004). The circadian clock in the brain: a structural and functional comparison between mammals and insects. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190, 601–613. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C (2009). Does the morning and evening oscillator model fit better for flies or mice? Journal of biological rhythms 24, 259–270. [DOI] [PubMed] [Google Scholar]
- Hermida RC, and Ayala DE (2009). Chronotherapy with the angiotensin-converting enzyme inhibitor ramipril in essential hypertension: improved blood pressure control with bedtime dosing. Hypertension 54, 40–46. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Calvo C, Lopez JE, Mojon A, Rodriguez M, and Fernandez JR (2005). Differing administration time-dependent effects of aspirin on blood pressure in dipper and non-dipper hypertensives. Hypertension 46, 1060–1068. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Chayan L, Mojon A, and Fernandez JR (2009). Administration-time-dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiology international 26, 61–79. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Fernandez JR, and Calvo C (2007). Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension 50, 715–722. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Fontao MJ, Mojon A, and Fernandez JR (2010). Chronotherapy with valsartan/amlodipine fixed combination: improved blood pressure control of essential hypertension with bedtime dosing. Chronobiology international 27, 1287–1303. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Mojon A, Chayan L, Dominguez MJ, Fontao MJ, Soler R, Alonso I, and Fernandez JR (2008a). Comparison of the effects on ambulatory blood pressure of awakening versus bedtime administration of torasemide in essential hypertension. Chronobiology international 25, 950–970. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Mojon A, and Fernandez JR (2008b). Chronotherapy with nifedipine GITS in hypertensive patients: improved efficacy and safety with bedtime dosing. Am J Hypertens 21, 948–954. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Mojon A, Fontao MJ, and Fernandez JR (2011). Chronotherapy with valsartan/hydrochlorothiazide combination in essential hypertension: improved sleep-time blood pressure control with bedtime dosing. Chronobiology international 28, 601–610. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, and Block GD (1998). Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nature neuroscience 1, 708–713. [DOI] [PubMed] [Google Scholar]
- Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA, et al. (2012). Identification of small molecule activators of cryptochrome. Science 337, 1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, and Okamura H (2005). Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell metabolism 2, 297–307. [DOI] [PubMed] [Google Scholar]
- Iurisci I, Filipski E, Reinhardt J, Bach S, Gianella-Borradori A, Iacobelli S, Meijer L, and Levi F (2006). Improved tumor control through circadian clock induction by Seliciclib, a cyclin-dependent kinase inhibitor. Cancer research 66, 10720–10728. [DOI] [PubMed] [Google Scholar]
- Journey JD, and Bentley TP (2018). Theophylline Toxicity In StatPearls (Treasure Island; (FL: )). [PubMed] [Google Scholar]
- Kalsbeek A, La Fleur S, Van Heijningen C, and Buijs RM (2004). Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 7604–7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HS, Angers M, Beak JY, Wu X, Gimble JM, Wada T, Xie W, Collins JB, Grissom SF, and Jetten AM (2007). Gene expression profiling reveals a regulatory role for ROR alpha and ROR gamma in phase I and phase II metabolism. Physiol Genomics 31, 281–294. [DOI] [PubMed] [Google Scholar]
- Karpel JP, Busse WW, Noonan MJ, Monahan ME, Lutsky B, and Staudinger H (2005). Effects of mometasone furoate given once daily in the evening on lung function and symptom control in persistent asthma. Ann Pharmacother 39, 1977–1983. [DOI] [PubMed] [Google Scholar]
- Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, Katchy CA, Lee C, Moore DD, and Fu L (2016). Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell 30, 909–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling S, Beaulieu-Laroche L, Blum ID, Landgraf D, Welsh DK, Storch KF, Labrecque N, and Cermakian N (2017). Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biol 15, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim MK, Seo HS, Hyun MS, Han KR, Cho SW, Kim YK, and Hoon Park S (2013). Efficacy and safety of morning versus evening dose of controlled-release simvastatin tablets in patients with hyperlipidemia: a randomized, double-blind, multicenter phase III trial. Clin Ther 35, 1350–1360 e1351. [DOI] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, and Takahashi JS (2012). Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338, 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska E, Ripperger JA, Hoegger DC, Bruegger P, Buch T, Birchler T, Mueller A, Albrecht U, Contaldo C, and Brown SA (2013). NONO couples the circadian clock to the cell cycle. Proceedings of the National Academy of Sciences of the United States of America 110, 1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Jha P, Challet E, and Kalsbeek A (2015). Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Molecular and cellular endocrinology 418 Pt 1, 74–88. [DOI] [PubMed] [Google Scholar]
- Kurupati RK, Kossenkoff A, Kannan S, Haut LH, Doyle S, Yin X, Schmader KE, Liu Q, Showe L, and Ertl HCJ (2017). The effect of timing of influenza vaccination and sample collection on antibody titers and responses in the aged. Vaccine 35, 3700–3708. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Alaghband Y, Kramar EA, Lopez AJ, Vogel Ciernia A, White AO, Shu G, Rhee D, Michael CM, Montellier E, et al. (2018). Epigenetic regulation of the circadian gene Per1 contributes to age-related changes in hippocampal memory. Nature communications 9, 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, and Buijs RM (2001). A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes 50, 1237–1243. [DOI] [PubMed] [Google Scholar]
- Levi F (2001). Circadian chronotherapy for human cancers. Lancet Oncol 2, 307–315. [DOI] [PubMed] [Google Scholar]
- Levi F, Le Louarn C, and Reinberg A (1985). Timing optimizes sustained-release indomethacin treatment of osteoarthritis. Clin Pharmacol Ther 37, 77–84. [DOI] [PubMed] [Google Scholar]
- Levi F, Misset JL, Brienza S, Adam R, Metzger G, Itzakhi M, Caussanel JP, Kunstlinger F, Lecouturier S, Descorps-Declere A, et al. (1992). A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer 69, 893–900. [DOI] [PubMed] [Google Scholar]
- Levi F, Perpoint B, Garufi C, Focan C, Chollet P, Depres-Brummer P, Zidani R, Brienza S, Itzhaki M, Iacobelli S, et al. (1993). Oxaliplatin activity against metastatic colorectal cancer. A phase II study of 5-day continuous venous infusion at circadian rhythm modulated rate. Eur J Cancer 29A, 1280–1284. [DOI] [PubMed] [Google Scholar]
- Levi F, and Schibler U (2007). Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol 47, 593–628. [DOI] [PubMed] [Google Scholar]
- Levi F, Zidani R, and Misset JL (1997). Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350, 681–686. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, and Bauer VK (2006). The circadian basis of winter depression. Proceedings of the National Academy of Sciences of the United States of America 103, 7414–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh DH, Dragich JM, Kudo T, Schroeder AM, Nakamura TJ, Waschek JA, Block GD, and Colwell CS (2011). Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs. Journal of biological rhythms 26, 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, and Phillips AC (2016). Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 34, 2679–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney M, Bult A, and Smale L (2001). Phase response curve and light-induced fos expression in the suprachiasmatic nucleus and adjacent hypothalamus of Arvicanthis niloticus. Journal of biological rhythms 16, 149–162. [DOI] [PubMed] [Google Scholar]
- Maor R, Dayan T, Ferguson-Gow H, and Jones KE (2017). Temporal niche expansion in mammals from a nocturnal ancestor after dinosaur extinction. Nat Ecol Evol 1, 1889–1895. [DOI] [PubMed] [Google Scholar]
- Marosi K, and Mattson MP (2014). BDNF mediates adaptive brain and body responses to energetic challenges. Trends in endocrinology and metabolism: TEM 25, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, O’Brien JA, and Hastings MH (2011). A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proceedings of the National Academy of Sciences of the United States of America 108, 14306–14311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA (2013). How might circadian rhythms control mood? Let me count the ways. Biological psychiatry 74, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JH, Daan S, Overkamp GJ, and Hermann PM (1990). The two-oscillator circadian system of tree shrews (Tupaia belangeri) and its response to light and dark pulses. Journal of biological rhythms 5, 1–16. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Rusak B, and Harrington ME (1989). Photically responsive neurons in the hypothalamus of a diurnal ground squirrel. Brain research 501, 315–323. [DOI] [PubMed] [Google Scholar]
- Meltser I, Cederroth CR, Basinou V, Savelyev S, Lundkvist GS, and Canlon B (2014). TrkB-mediated protection against circadian sensitivity to noise trauma in the murine cochlea. Current biology : CB 24, 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, and Takahashi JS (2012). Central and peripheral circadian clocks in mammals. Annual review of neuroscience 35, 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, et al. (2018). Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbalpha antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet 391, 59–69. [DOI] [PubMed] [Google Scholar]
- Morgan P, Brown DG, Lennard S, Anderton MJ, Barrett JC, Eriksson U, Fidock M, Hamren B, Johnson A, March RE, et al. (2018). Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nature reviews Drug discovery. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Edelstein K, Hastings MH, and Maywood ES (2001). Cycle of period gene expression in a diurnal mammal (Spermophilus tridecemlineatus): implications for nonphotic phase shifting. Journal of biological rhythms 16, 471–478. [DOI] [PubMed] [Google Scholar]
- Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, et al. (2018). Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, and Ju YS (2018). Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, and Holtzman DM (2016). Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Andersson CR, Kondo T, Golden SS, and Johnson CH (1998). Resonating circadian clocks enhance fitness in cyanobacteria. Proceedings of the National Academy of Sciences of the United States of America 95, 8660–8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Green CB, and Takahashi JS (2014). Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, et al. (2013). Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342, 1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Brown LA, Pothecary CA, Benson LA, and Fisk AS (2018). Light and the laboratory mouse. Journal of neuroscience methods 300, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, et al. (2015). Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350, aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissin L, Facchin P, and Porro CA (2000). Diurnal variations in tonic pain reactions in mice. Life Sci 67, 1477–1488. [DOI] [PubMed] [Google Scholar]
- Perpoint B, Mismetti P, Simitsidis S, Hocquart J, Rambaud C, Buchmuller A, Queneau P, and Decousus H (1994). Dosing time optimizes sustained-release ketoprofen treatment of osteoarthritis. Chronobiology international 11, 119–125. [DOI] [PubMed] [Google Scholar]
- Pevet P (2003). Melatonin in animal models. Dialogues in clinical neuroscience 5, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering TG, Levenstein M, and Walmsley P (1994). Nighttime dosing of doxazosin has peak effect on morning ambulatory blood pressure. Results of the HALT Study. Hypertension and Lipid Trial Study Group. Am J Hypertens 7, 844–847. [DOI] [PubMed] [Google Scholar]
- Pizarro A, Hayer K, Lahens NF, and Hogenesch JB (2013). CircaDB: a database of mammalian circadian gene expression profiles. Nucleic acids research 41, D1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaluppi F, and Hermida RC (2007). Circadian rhythms in cardiac arrhythmias and opportunities for their chronotherapy. Advanced drug delivery reviews 59, 940–951. [DOI] [PubMed] [Google Scholar]
- Refinetti R, and Kenagy GJ (2018). Diurnally active rodents for laboratory research. Lab Anim, 23677218771720. [DOI] [PubMed] [Google Scholar]
- Reinberg A, and Smolensky MH (1982). Circadian changes of drug disposition in man. Clin Pharmacokinet 7, 401–420. [DOI] [PubMed] [Google Scholar]
- Roedel A, Storch C, Holsboer F, and Ohl F (2006). Effects of light or dark phase testing on behavioural and cognitive performance in DBA mice. Lab Anim 40, 371–381. [DOI] [PubMed] [Google Scholar]
- Rosbash M (2009). The implications of multiple circadian clock origins. PLoS biology 7, e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben MD, Wu G, Smith DF, Schmidt RE, Francey LJ, Lee YY, Anafi RC, and Hogenesch JB (2018). A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruger M, and Scheer FA (2009). Effects of circadian disruption on the cardiometabolic system. Rev Endocr Metab Disord 10, 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Yoshida S, Nakaya N, Hata Y, and Goto Y (1991). Comparison between morning and evening doses of simvastatin in hyperlipidemic subjects. A double-blind comparative study. Arterioscler Thromb 11, 816–826. [DOI] [PubMed] [Google Scholar]
- Sandrelli F, Costa R, Kyriacou CP, and Rosato E (2008). Comparative analysis of circadian clock genes in insects. Insect Mol Biol 17, 447–463. [DOI] [PubMed] [Google Scholar]
- Sato S, Solanas G, Peixoto FO, Bee L, Symeonidi A, Schmidt MS, Brenner C, Masri S, Benitah SA, and Sassone-Corsi P (2017). Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell 170, 664–677 e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharnagl H, Vogel M, Abletshauser C, Freisinger F, Stojakovic T, and Marz W (2006). Efficacy and safety of fluvastatin-extended release in hypercholesterolemic patients: morning administration is equivalent to evening administration. Cardiology 106, 241–248. [DOI] [PubMed] [Google Scholar]
- Scherrmann JM (2009). Transporters in absorption, distribution, and elimination. Chem Biodivers 6, 1933–1942. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Reppert SM, Eagan SM, and Moore-Ede MC (1983). In vivo metabolic activity of the suprachiasmatic nuclei: a comparative study. Brain research 274, 184–187. [DOI] [PubMed] [Google Scholar]
- Shafer OT, Rosbash M, and Truman JW (2002). Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 5946–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simasko SM, and Mukherjee S (2009). Novel analysis of sleep patterns in rats separates periods of vigilance cycling from long-duration wake events. Behavioural brain research 196, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarke C, Lahens NF, Rhoades SD, Campbell A, Bittinger K, Bailey A, Hoffmann C, Olson RS, Chen L, Yang G, et al. (2017). A Pilot Characterization of the Human Chronobiome. Scientific reports 7, 17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky MH, Scott PH, Harrist RB, Hiatt PH, Wong TK, Baenziger JC, Klank BJ, Marbella A, and Meltzer A (1987). Administration-time-dependency of the pharmacokinetic behavior and therapeutic effect of a once-a-day theophylline in asthmatic children. Chronobiology international 4, 435–447. [DOI] [PubMed] [Google Scholar]
- Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, et al. (2012). Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, and Menaker M (2001). Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493. [DOI] [PubMed] [Google Scholar]
- Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V, and Group W.H.O.I.A.F.R.o.C.M.W. (2007). Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol 8, 1065–1066. [DOI] [PubMed] [Google Scholar]
- Takahashi JS (2017). Transcriptional architecture of the mammalian circadian clock. Nature reviews Genetics 18, 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini M, Filipski E, Liu XH, Lemaigre G, Li XM, Vrignaud P, Francois E, Bissery MC, and Levi F (1998). Docetaxel chronopharmacology in mice. Cancer research 58, 3896–3904. [PubMed] [Google Scholar]
- Tanimura N, Kusunose N, Matsunaga N, Koyanagi S, and Ohdo S (2011). Aryl hydrocarbon receptor-mediated Cyp1a1 expression is modulated in a CLOCK-dependent circadian manner. Toxicology 290, 203–207. [DOI] [PubMed] [Google Scholar]
- Thosar SS, Butler MP, and Shea SA (2018). Role of the circadian system in cardiovascular disease. The Journal of clinical investigation 128, 2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier BB, Dardente H, Vuillez P, Pevet P, and Challet E (2007). Expression of Tgfalpha in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Neuroscience 145, 1138–1143. [DOI] [PubMed] [Google Scholar]
- van Diepen HC, Ramkisoensing A, Peirson SN, Foster RG, and Meijer JH (2013). Irradiance encoding in the suprachiasmatic nuclei by rod and cone photoreceptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 27, 4204–4212. [DOI] [PubMed] [Google Scholar]
- VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD, and Meijer JH (2007). Seasonal encoding by the circadian pacemaker of the SCN. Current biology : CB 17, 468–473. [DOI] [PubMed] [Google Scholar]
- Vosko AM, Hagenauer MH, Hummer DL, and Lee TM (2009). Period gene expression in the diurnal degu (Octodon degus) differs from the nocturnal laboratory rat (Rattus norvegicus). American journal of physiology Regulatory, integrative and comparative physiology 296, R353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Armstrong ME, Cairns BJ, Key TJ, and Travis RC (2011). Shift work and chronic disease: the epidemiological evidence. Occupational medicine 61, 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, Pairaudeau G, Pennie WD, Pickett SD, Wang J, et al. (2015). An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nature reviews Drug discovery 14, 475–486. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, and Reppert SM (1995). Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697–706. [DOI] [PubMed] [Google Scholar]
- Winter C, Silvestre-Roig C, Ortega-Gomez A, Lemnitzer P, Poelman H, Schumski A, Winter J, Drechsler M, de Jong R, Immler R, et al. (2018). Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell metabolism 28, 175–182 e175. [DOI] [PubMed] [Google Scholar]
- Yamajuku D, Inagaki T, Haruma T, Okubo S, Kataoka Y, Kobayashi S, Ikegami K, Laurent T, Kojima T, Noutomi K, et al. (2012). Real-time monitoring in three-dimensional hepatocytes reveals that insulin acts as a synchronizer for liver clock. Scientific reports 2, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Kim SH, Kim JK, Ko SH, Ko JE, Park SJ, Park MG, Lee JH, and Hyon MS (2011). Comparison of effects of morning versus evening administration of ezetimibe/simvastatin on serum cholesterol in patients with primary hypercholesterolemia. Ann Pharmacother 45, 841–849. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Wulbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, and Helfrich-Forster C (2009). The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila’s clock. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 2597–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MW, and Kay SA (2001). Time zones: a comparative genetics of circadian clocks. Nature reviews Genetics 2, 702–715. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, and Hogenesch JB (2014). A circadian gene expression atlas in mammals: implications for biology and medicine. Proceedings of the National Academy of Sciences of the United States of America 111, 16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yue Z, Arnold DM, Artiushin G, and Sehgal A (2018). A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell 173, 130–139 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Roederer MW, Chen WQ, Fan L, and Zhou HH (2012). Pharmacogenetics of drugs withdrawn from the market. Pharmacogenomics 13, 223–231. [DOI] [PubMed] [Google Scholar]
- Zmrzljak UP, and Rozman D (2012). Circadian regulation of the hepatic endobiotic and xenobitoic detoxification pathways: the time matters. Chem Res Toxicol 25, 811–824. [DOI] [PubMed] [Google Scholar]
- Zung WW, Wilson WP, and Dodson WE (1964). Effect of Depressive Disorders on Sleep Eeg Responses. Archives of general psychiatry 10, 439–445. [DOI] [PubMed] [Google Scholar]