Abstract
Skeletal muscles comprise hundreds of individual muscle fibers, with each possessing specialized contractile properties. Skeletal muscles are recognized as being highly plastic, meaning that the physiological properties of single muscle fibers can change with appropriate use. During fiber type transitions, one myosin heavy chain isoform is exchanged for another and over time the fundamental nature of the fiber adapts to become a different fiber type. Within the rat triceps surae complex, the soleus muscle starts out as a muscle comprised of a mixture type IIA and type I fibers. As neonatal rats grow and mature, the soleus undergoes a near complete transition into a muscle with close to 100% type I fibers at maturity. We used immunohistochemistry and single fiber SDS-PAGE to track the transformation of type IIA into type I fibers. We found that transitioning fibers progressively incorporate new myofibrils containing type I myosin into existing type IIA fibers. During this exchange, distinct type I-containing myofibrils are segregated among IIA myofibrils. The individual myofibrils within existing muscle fibers thus appear to represent the functional unit that is exchanged during fiber type transitions that occur as part of normal muscle development:
Keywords: muscle development, muscle plasticity, myofibrillar turnover, postnatal development
Introduction
Skeletal muscles are recognized as being highly plastic tissues, meaning that they are capable of dramatic changes in their physiological properties in response to conditions including exercise, electrical stimulation, development, and disease.1 Although multiple parameters are coordinated in these transformations, the myosin heavy chain (MHC) proteins that form the molecular motors in muscles are of particular relevance. MHCs in mammalian muscles are encoded by a multigene family and are expressed in tissue-specific patterns. Eleven different MHC isoforms are expressed in different mammalian muscles, but of these only six are routinely expressed in the limb muscles.2 The specific MHC isoform expressed is the main determinant of important physiological properties, including muscle shortening velocity, power output, and efficiency. Collectively, the different MHCs provide for a continuum of these properties, because each MHC possesses a different shortening velocity: I < IIA < IIX < IIB.1
During embryonic and postnatal muscle development, an orderly transition in MHC isoform expression proceeds, resulting in the formation of adult muscles with a particular pattern of MHC expression.2 An embryonic MHC (MYH3; MHCemb) isoform is initially expressed, but this MHC is then exchanged for a neonatal MHC (MYH8; MHCneo). In rodents, the embryonic and neonatal MHCs are still present at the time of birth, but are soon replaced by adult MHC isoforms over time.2–5 The MHCemb is completely replaced within the first week of birth, while the MHCneo persists for up to several weeks.2–5 Both the MHCemb and MHCneo may be understood to effectively function as “placeholders” during muscle development, because they are ultimately completely replaced by the adult MHCs in most muscles.2
The number of muscle fibers is fixed soon after birth, meaning that individual muscle fibers are not replaced with new ones during development, but instead existing fibers must be completely remodeled.6 Myofibrillar proteins display relatively slow turnover, and a complete transformation of fiber type requires a period of several weeks. During this transition period, an individual fiber must possess at least two MHC isoforms and these transitional fibers are termed hybrid fibers.7,8 Generally, MHC transitions occur in an orderly “nearest neighbor” process, whereby the new MHC is one isoform faster or slower than the previous one.9 Hybrid fibers are therefore essential components of fiber type transitions, because any fiber undergoing remodeling must become a hybrid for some period of time. Consistent with this premise, hybrid fibers are particularly common in the muscles of young animals as they experience fiber type transitions.3,7,8,10,11
A central question in skeletal muscle development is where nascent MHC isoforms are incorporated into the existing muscle fibers. This is an important process, because each MHC has a specific set of kinetic properties and where in the fiber the new isoforms are incorporated has the capacity to affect muscle function. Individual muscle fibers form the contractile motors of the motor unit and are broadly assumed to contract in unison. A potential problem arises if one region of a fiber contracts more rapidly than its adjacent region. One possible outcome could be that the slower sarcomeres could be stretched apart as their faster partners shorten. Indeed, physiological studies of isolated frog fibers demonstrated that adjacent regions of single fibers can possess different shortening speeds and that these properties are an outcome of different MHC isoforms being expressed along the length of the fibers.12,13 More recently, we have found that many of the hybrid fibers in developing mouse muscles exhibit differences in MHC expression along the length of the fibers.7,8
In the current study, we studied hybrid fibers and fiber type transitions occurring during the normal development of rat skeletal muscles. We chose to focus on muscles of the rat for two reasons. First, a significant proportion of fibers from rat limb muscles are hybrids, even in normal, mature animals.14 Second, many of these fibers undergo dramatic developmental transitions during the early periods of postnatal growth and development.3,5,15 The soleus muscle in particular begins as a muscle of mixed fiber types, but experiences a nearly complete conversion into slow fibers by adulthood.15 Our hypothesis was that the proportion of hybrid fibers would be greatest in newly weaned animals, but would then decline over the subsequent weeks. We were also interested in documenting where within the existing fibers the new MHC isoforms were incorporated. We used complementary approaches of single fiber SDS-PAGE and immunohistochemistry to study the fibers of rat muscles from ages 3 to 12 weeks of age.
Materials and Methods
Animals and Muscle Collection
Three-, six-, nine-, and twelve-week old Sprague Dawley rats were purchased from Taconic Biosciences Inc (Rensselaer, NY, USA). Animals were euthanized upon receipt with an overdose of CO2 as described in a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at SUNY Fredonia. Muscles of the triceps surae (gastrocnemius, plantaris, and soleus) were dissected from the animals immediately after the rats were euthanized. The muscles from one side were placed in a relaxation buffer containing (50% glycerol, 100 mM KCl, 2 mM EGTA, 1 mM MgCl2, 4 mM ATP, and 10 mM imidazol, pH 7.0) and stored in sealed plastic vials at -20C. Muscles from the contralateral side were coated with O.C.T. compound (Tissue-Tek; Sakura Finetek, Torrance, CA, USA) and frozen rapidly in isopentane cooled in liquid N2.
Rat Growth
The growth of rats over the ~12 week time period of the study was assessed using multiple complementary parameters. First, whole animal body mass was recorded at the time of sacrifice. Second, the wet masses of the shank flexor muscles (triceps surae: gastrocnemius, plantaris, and soleus) were measured immediately after they were removed from the animals. Finally, the length of the bones of the shank (tibia and fibula) was measured from isolated bones. Briefly, bones of the shank were removed from animals and immersed in 1 M NaOH overnight to dissolve away muscles and other soft tissues. Bones were then thoroughly rinsed in deionized water and then air dried. Bone dimensions were then determined using electronic calipers to the nearest 0.1 mm. Each of these parameters was plotted as a function of age in weeks. Growth curves were fit using a second order polynomial line of best fit.
SDS-PAGE of Single Fibers
Single muscle fibers from the soleus muscle were dissected from the whole muscle with fine forceps under a stereomicroscope. Single fibers were examined visually to verify that only single fibers were collected. Individual fibers were then placed into 1.5 ml microcentrifuge tubes containing 30 µl of sample buffer containing 8 M urea, 2 M thiourea, 50 mM Tris base, 75 mM dithiothreitol (DTT), 3% sodium dodecyl sulfate (SDS), and 0.004% bromophenol blue, pH 6.8.16 Samples were heated for 15 min at 65C and then 10 µl of the sample were loaded per well.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels were used with a Hoeffer SE 600 to identify MHC isoforms present within single muscle fibers as performed previously in our lab.7,17,18 Resolving gels consisted of 9% acrylamide (200:1 acrylamide: methylene-bis-acrylamide), 12% glycerol, 0.675 M Tris base (pH 8.8), and 0.1% SDS. Stacking gels included 4% acrylamide (20:1 acrylamide:methylene-bis-acrylamide), 0.125 M Tris base (pH 6.8), and 0.1% SDS. The running buffer contained 0.192 M glycine, 25 mM Tris base, 0.1% SDS, and 0.08% 2-mercaptoethanol. Gels were run with constant current of 20 mA for ~41 hr at 8C. After a gel run was completed, the gels were fixed with 50% MeOH containing 0.037% formaldehyde overnight at 4C. Gels were then quickly rinsed in deionized water for ~5 min before being stained with silver.19
ATPase Histochemistry
Muscles removed from the rats were rapidly frozen in isopentane chilled in liquid N2 and stored at -20C until they were processed for sectioning. Sections of the entire shank flexor muscles were cut on a Leica cryostat at thickness of 10 µm and then stored at -20C until the time of labeling. ATPase histochemistry followed the methods of Dubowitz and Sewry20 using a pH 9.4 buffer and this technique has been described in greater detail previously.17,18 Under these conditions, all fast fibers stain dark brown to black and slow fibers are light staining.
Immunohistochemical Analyses
Skeletal muscle fiber types within the shank were identified using a rapid approach that identifies MHC expression through multicolor immunofluorescence.21 Briefly, this approach permits highly specific labeling of different MHC isoforms in a single labeling step by using cocktails of monoclonal antibodies that are of different antibody subtypes. We used a monoclonal antibody specific for type I MHC (MYH7) (4.84; IgM) and for type IIA MHC (MYH2) (4.74; IgG1) obtained from the Developmental Studies Hybridoma Bank (DSHB) at the University of Iowa. Previous studies have demonstrated that 4.84 is specific for type I MHC and that 4.74 reacts primarily with type IIA MHC and to a lesser extent with type IIX MHC (MYH1) in rat skeletal muscles.22–25 The results of this immunofluorescence approach are highly specific labeling of multiple skeletal muscle fiber types, including hybrids expressing multiple MHC isoforms.21
Muscle sections were air dried for 30 min and then covered with blocking buffer (2% bovine serum albumin in TBS) for 1 hr. At this point, blocking solution was removed and replaced with the cocktail containing a mixture of the primary antibodies 4.74 and 4.84 diluted 1:10 in the blocking solution. Sections were incubated with primary antibody cocktail for a minimum of 1 hr, but were generally left to incubate overnight at 4C. Sections were then washed 3X in TTBS for 5 min each wash. The sections were then incubated in a cocktail of secondary antibodies specific for 4.84 (Alexafluor anti-mouse IgM 350) and for 4.74 (Alexafluor anti-mouse IgG1 555). Sections were incubated in the secondary antibody cocktail for 1 hr and then rinsed 3X in TTBS as before. Sections were then mounted in FluormountG (Southern Biotech, Birmingham, AL, USA) and observed with an Olympus BX51 microscope equipped with filter sets appropriate for visualizing blue (excitation: BP 360–370 nm; emission BA 420 nm) and red (excitation: BP 520–550 nm; emission: BA 590 nm) secondary antibodies. Individual images for each secondary antibody were captured with a camera coupled to a computer running Spot software 5.0 (Diagnostics Instruments, Inc). Images from each channel were then overlaid into a single RGB image using Adobe Photoshop CS6 software (version 13.0 x64).
Statistical Analyses
The relative proportions of fiber types comprising the soleus muscle at different stages of postnatal development were determined primarily from single fiber SDS-PAGE of a total of 1212 fibers. Supplemental data were obtained from counts of immunohistochemically stained sections from 3-week-old mice, because it is often difficult to obtain adequate data from the small fibers at this age. A total of 501 fibers were used for fiber type determination from these stained sections from 3 mice. Relative proportions of fiber types from muscles of different aged rats (3, 6, 9, and 12 weeks) were compared by analysis of variance (ANOVA). Significant differences between means from each age group were determined with a Tukey-Kramer post-ANOVA test. All analyses were performed using JMP 10.0.2 software (SAS Institute, Cary, NC, USA).
Results
Growth of Rats
We focused on a period of early postnatal development where overall growth is dramatic. At 3 weeks of age, rats have just recently been weaned and are becoming more physically active. Overall growth as measured from multiple parameters (body mass, muscle mass, long bone length, etc.) is particularly fast during the period of time between 3 and 6 weeks (Fig. 1). Each growth curve was significantly fit with a second-degree polynomial curve of best fit (p<0.001). During this time period, the body mass increased by roughly 4-fold, the mass of the shank flexors by 3-fold, and the length of the tibia and fibula by 2-fold.
Figure 1.
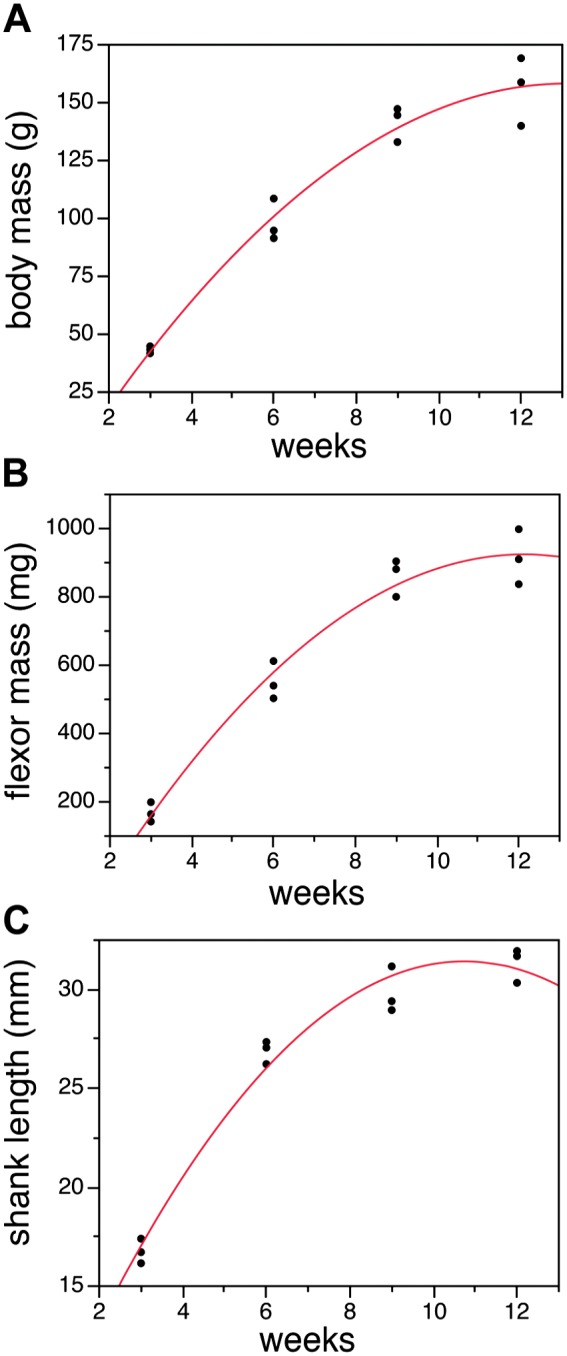
Rat growth during the period of the study. Body mass (A), shank flexors (B), and shank length (C) all increase dramatically at this stage of development. Body mass increased by approximately 3-fold (A), muscle mass increased by 4-fold, and shank length approximately doubled in rats between 3 and 12 weeks of age. Growth is especially rapid between 3 and 9 weeks, but then begins to slow. Lines are second-order polynomial curves fit to the data.
Changes in Fiber Type Proportions
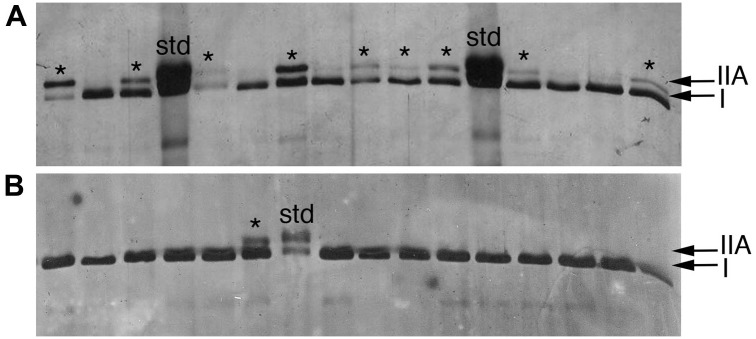
We used single fiber SDS-PAGE to quantitate the relative proportions of fiber types within the soleus muscles of rats at 3, 6, 9, and 12 weeks of age. In addition, these data were supplemented with single fiber counts from immunohistochemically labeled 3-week-old rats, because the single fibers are challenging to dissect and contain small amounts of protein. At 3 weeks of age, the soleus is comprised of roughly 60% type I fibers, with approximately equal proportions (~20%) being made of I/IIA hybrids and pure type IIA fibers (Figs. 2 and 3). As the IIA-containing fibers transition into type I fibers, the relative proportion of slow, type I fibers increased to approximately 90% of the soleus. Histochemical and immunohistochemical results were consistent with this pattern (Figs. 3 and 4). ANOVA demonstrated that the proportion of slow fibers was significantly less at 3 weeks of age, but did not change significantly after 6 weeks of age (DF: 3; F-ratio: 26.78; p<0.0001; Tukey-Kramer post hoc test p<0.05) (Fig. 5). The relative proportions of pure type IIA and hybrid fibers were not significantly different at different time periods, although the type IIA fibers represented fewer than 1% of the fibers at 12 weeks of age.
Figure 2.
Single soleus fibers run on SDS-PAGE gel to identify fiber types. Each lane is the MHC extracted from a single isolated muscle fiber. (A) At 3 weeks of age, many of the soleus fibers are I/IIA hybrids (asterisks). (B) By 12 weeks, the majority of fibers (all but one in this example) are type I. This example shows the transformation of the hybrid fibers into pure type I fibers. Gels of individual, isolated muscle fibers were used for the quantitative analyses of fiber type proportions.
Figure 3.
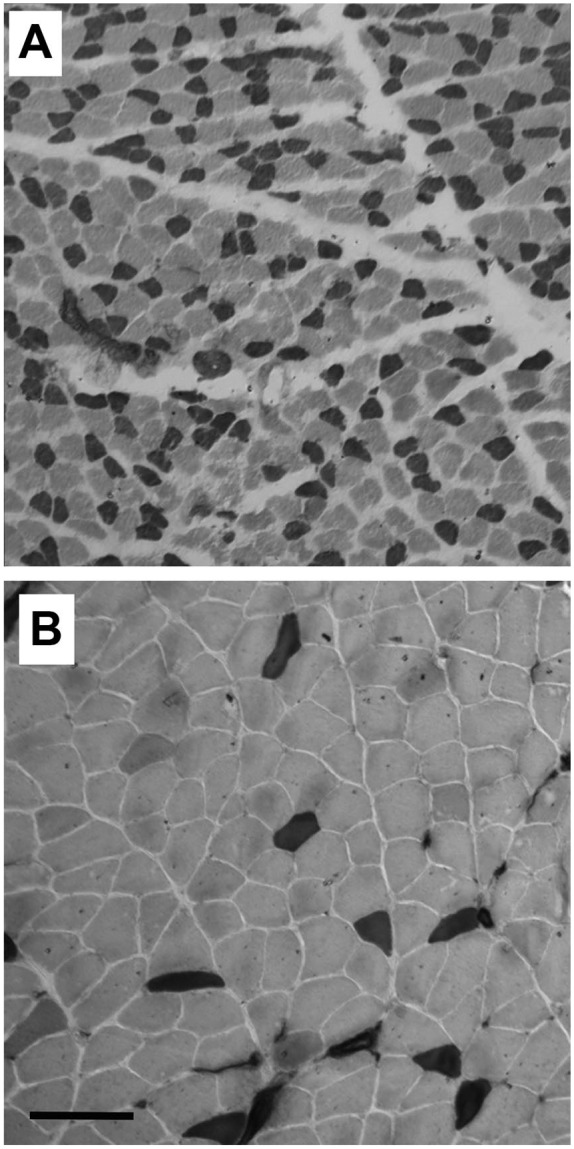
ATPase histochemistry (pH 9.6) demonstrating differences in muscle fiber types and size in 3-week (A) versus 12-week (B) old rats. Dark staining fibers are fast, while light staining fibers are slow. The average fiber diameters in this figure were 17 µm for fast fibers and 22 µm for slow fibers at 3 weeks of age. At 12 weeks of age, these diameters increased to 24 and 41 µm, respectively. In the sample shown, the 3-week-old soleus comprised approximately 50% fast fibers. At 12 weeks, the proportion of fast fibers was fewer than 10%. Scale bar = 100 µm.
Figure 4.
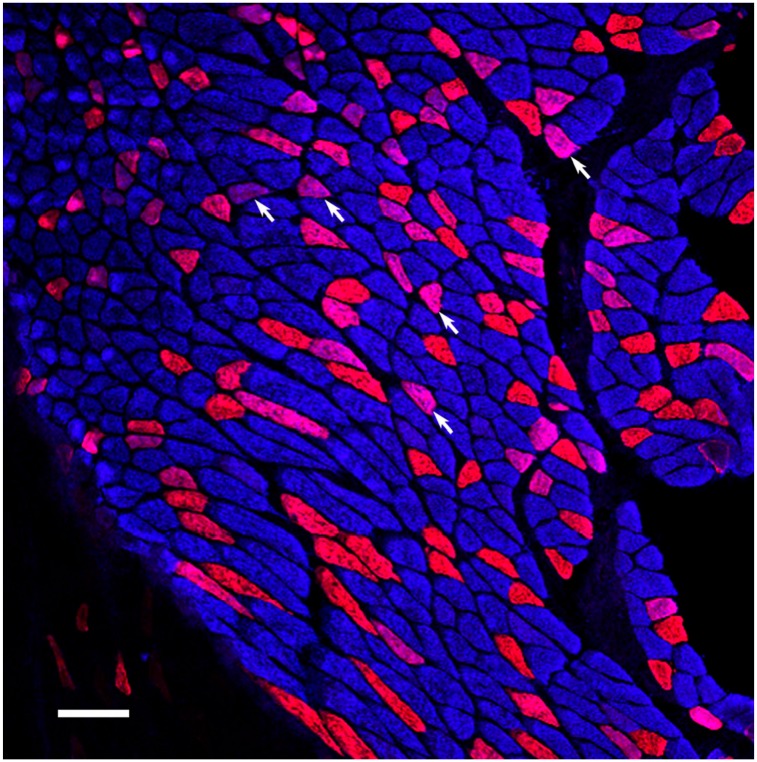
Fiber composition of 6–week-old rat soleus. Type I fibers are blue and represent the majority of fibers. Type IIA are red and hybrid fibers (arrows) are purple. Since the fiber type transition is toward type I fibers, the purple hybrid fibers are understood to be in transition from type IIA (red) to type I (blue) fibers. Scale bar = 100 µm.
Figure 5.
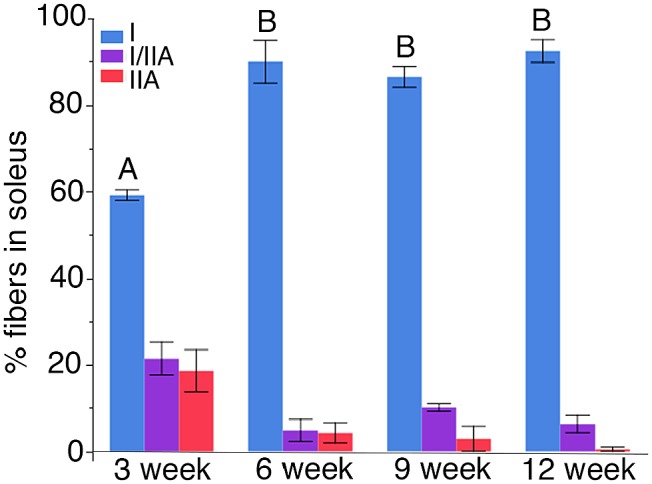
Relative proportion of fiber types in the rat soleus at different developmental ages. At 3 weeks of age, the soleus is comprised of about 60% type I fibers, with the rest being hybrid I/IIA or type IIA. By 6 weeks, approximately 90% of the fibers are type I, with only about 10% being made of hybrids or IIA fibers. The portion of type I fibers was significantly lower at 3 weeks, but the proportions were no different between the other ages. This indicates that the major transformation of fibers is complete by about 6 weeks.
MHC Isoform Distribution Within Single Hybrid Fibers
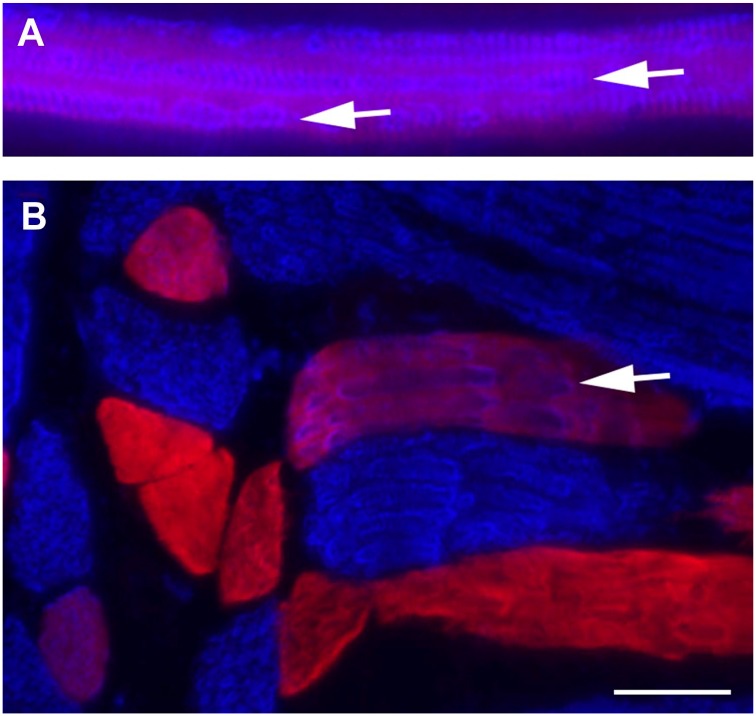
Careful examination of the hybrid fibers identified using monoclonal antibodies specific for type I or type IIA MHC revealed that different MHC isoforms can be segregated among individual myofibrils (Figs. 4 and 6). When hybrid fibers are viewed in cross section, punctate staining for type I MHC (blue) can be seen within a background of type IIA (red) or hybrid (purple) staining. In longitudinal section, individual type I (blue) myofibrils are also apparent (Fig. 6). In other instances, myofibrils within single fibers appear as purple, which we interpret as being mixtures of both isoforms within a myofibril. It is also possible that hybrid thick filaments, containing mixtures of I and IIA MHC were present.
Figure 6.
Longitudinal sections of 6-week-old rat labeled for type I (blue) or type IIA (red) fibers. Hybrid fibers are seen as purple. Closer examination of staining patterns within the hybrids shows that individual myofibrils comprised of type I MHC are distinct within single hybrid fibers (arrows). Sarcomeric banding is clearly seen within myofibrils in A. Figs A and B are different regions of the same muscle. Scale bar = 25 µm.
Discussion
We focused on soleus muscle development in the rat as a model of progressive fiber type transition toward a single muscle fiber type. Previous studies of the adult rat soleus have demonstrated a high proportion of slow type I fiber in the adult, ranging from ~80% to 95% of the total fibers.14,15,26 Early in postnatal development, the soleus comprises a mixture of approximately 40% fast and hybrid fibers, but this proportion is reduced over the first several weeks of postnatal development (Figs. 2–5).15 Functionally, the fast MHC containing fibers exhibit higher shortening velocities and the precise velocity is determined by the relative proportion of fast MHC contained within single hybrid fibers.27 This shift in muscle composition thus represents a tractable model for investigating fiber type transitions, because fibers are changing in a steady progression from fast or mixed fiber types, toward uniformly slow fibers. Interestingly, unloading or clenbuterol treatment of the adult rat soleus drives a fiber type transition in the opposite direction, from slow to fast.28,29 Focusing on the developmental fiber transformations, we can deduce that hybrid fibers co-expressing both IIA and I MHC are in the process of transitioning into pure type I fibers. This pattern indicates that the type I MHC in these fibers has been synthesized in the fiber more recently than the type IIA MHC. Using the highly specific methods for MHC identification,21 we were able to examine the distribution of these two MHC isoforms within the same single fiber with high resolution (Fig. 6).
In many cases, we found that the specific MHC isoforms were segregated among myofibrils within the same fiber. In other words, some of the myofibrils were predominantly or entirely made of type IIA MHC, while others contained type I MHC (Fig. 6). This co-mingling of myofibrils containing different MHC isoforms within single muscle fibers is fundamentally the same pattern identified in developing bird muscles.30 These patterns show that during fiber type transitions myofibrils with “old” MHC are replaced with myofibrils containing “new” MHC in a progressive manner. In this way, myofibrils within an existing muscle fiber may be understood as the unit of exchange during fiber type transitions (Fig. 7). Myofibrillar turnover is a normal part of skeletal muscle plasticity.31 Muscle hypertrophy is the result of an increased number of myofibrils within existing muscle fibers, while atrophy results from a net loss of myofibrils.32 When existing myofibrils are progressively exchanged for new myofibrils containing a different MHC isoform, the fiber type changes over time.
Figure 7.
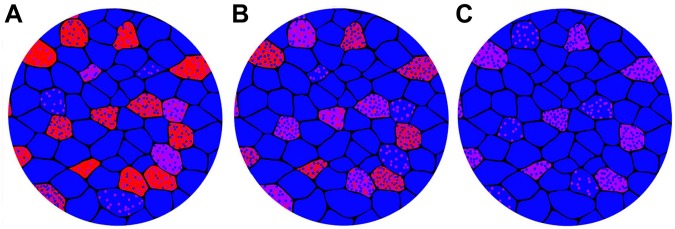
Proposed model of gradual transformation toward slower fiber composition through myofibrillar exchange. Myofibrils containing slow MHC are represented by blue points, fast IIA MHC containing myofibrils by red points, and myofibrils containing a mixture of the two are shown as purple. (A) A substantial proportion of individual fibers contain large amounts of IIA MHC. (B) As slow type I MHC increases in expression and fast containing myofibrils are removed, type I containing myofibrils replace those with type IIA. (C) This progressive exchange of type IIA for type I myofibrils continues until most of the fibers are type I. This is the general pattern of myofibrillar exchange that takes place in the rat soleus during neonatal development.
Skeletal muscles are highly plastic tissues, meaning that their fiber type composition can change in response to a variety of stimuli. Fiber type transitions typically take place in an orderly transition from slowest to fastest isoforms: I ←→ IIA ←→ IIX ←→ IIB.33,34 During these transitions, expression of an existing MHC isoform must be down regulated, while expression of the replacing isoform must be increased. Hybrid fibers are of particular relevance to understanding how these transitions proceed, because any fiber changing from one type to another becomes hybrid for a period of time.7,8,17,35 Fiber number within a muscle is fixed from near the time of birth, so changes in fiber types represent remodeling of existing muscle fibers.5,6 Although hybrids clearly play a central role in fiber type transitions, hybrid fibers can also be phenotypically stable, and represent physiologically intermediate phenotypes.7,17,21,36–39 In the developing rat soleus, hybrid fibers represent transitional fibers switching from the IIA phenotype toward type I fibers. When both types I and IIA MHC isoforms are present in a single fiber, it is straightforward to conclude that the type I MHC represents the “new” isoform replacing the “old” IIA MHC (Fig. 7).
The first several weeks following birth represent a period of intense growth and the rats in the current study were still growing rapidly (Fig. 1). Individual skeletal muscle fibers grow dramatically during postnatal development, and one feature of this growth is increased packing of myofibrils into the growing fibers.5 This period is also a particularly dynamic period of fiber type transitions, marked by dramatic shifts in MHC expression. In addition to the adult MHC isoforms expressed in limb muscles, embryonic, and neonatal MHCs (MHCemb and MHCneo) are also expressed early in development.2–5,10 Other examples of hybrid fibers as transitional fibers during muscle development in mammals have been identified.3,8,10,11 In postnatal mouse muscles, IIX/IIB hybrids principally transition into pure IIB fibers in the tibialis anterior, while those of the brachioradialis predominantly become IIX fibers.8 This example demonstrates that the same hybrid fiber phenotype may transition into distinct pure fiber types in different muscles. In the rat soleus, the transition is relatively simple, with type IIA or I/IIA hybrids gradually becoming pure type I fibers.
An outstanding question about hybrid muscle fibers is how the different MHC isoforms are arranged spatially within the same fiber.40 In the current study, we found that the type I and type IIA MHCs were frequently segregated among the myofibrils within single fibers (Fig. 6). A different spatial arrangement of MHC isoforms within single fibers occurs when isoform expression changes along the length of muscle fibers. A significant number of studies have now reported that unique MHC isoforms are frequently expressed within different segments of the same muscle fiber.7,8,13,41–48 A common interpretation of asymmetric MHC expression is that different myonuclear domains within single fibers may be uncoordinated in their myofibrillar gene expression.7,8,42,46 Individual muscle fibers can be up to several millimeters long, and typically contain hundreds of myonuclei.49,50 These myonuclei are derived from heterogeneous populations of myoblasts during development, and continue to incorporate new nuclei from satellite cells throughout life. Each nucleus controls a limited myonuclear domain within perhaps at most hundreds of micrometers around the nucleus.49,50 If different myonuclear domains contain unique MHC isoforms from one another, then the documented fiber type differences along the length of fibers could result.40 Studies of cultured skeletal muscle fibers have shown that individual nuclei within single fibers exhibit a level of independence in their patterns of regulated nuclear import and patterns of gene expression.51,52 Further research is needed to better understand the unique arrangements of different MHC isoforms within hybrid fibers and what these patterns reveal about basic muscle function.
Acknowledgments
This research was conducted in partial fulfillment of a master’s degree at SUNY Fredonia (Lauren Larson). Lauren Larson, Jordan Johnson, and Jessica Lioy were each supported by Holmberg Summer Research Fellowships at SUNY Fredonia. A preliminary version of this study was presented at the SICB Conference in January, 2015 in West Palm Beach, FL. The monoclonal antibodies used in this study (A4.840 and A4.74) were developed by Helen Blau’s Lab at Stanford University and were obtained from the Developmental Studies Hybridoma Bank (DSHB) at the University of Iowa. Olivia Connor created the images depicting transitioning fibers in Fig. 7.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: LL and JL collected muscles and performed ATPase histochemistry and immunohistochemistry. LL and JJ isolated single fibers and performed SDS-PAGE. LL and SM conducted data analyses and SM designed the study and authored the manuscript. Each of the contributing authors read and approved the manuscript prior to submission.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Summer research funds were provided to students by the Holmberg Foundation.
Contributor Information
Lauren Larson, Biology Department, State University of New York at Fredonia, Fredonia, NY, USA.
Jessica Lioy, Biology Department, State University of New York at Fredonia, Fredonia, NY, USA.
Jordan Johnson, Biology Department, State University of New York at Fredonia, Fredonia, NY, USA.
Scott Medler, Biology Department, State University of New York at Fredonia, Fredonia, NY, USA.
Literature Cited
- 1. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–531. [DOI] [PubMed] [Google Scholar]
- 2. Schiaffino S, Rossi RC, Smerdu V, Leinwand LA, Reggiani C. Developmental myosins: expression patterns and funcional significance. Skelet Muscle. 2015;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Maso NA, Caiozzo VJ, Baldwin KM. Single-fiber myosin heavy chain polymorphism during postnatal development: modulation by hypothyroidism. Am J Physiol Regul Integr Comp Physiol. 2000;278(4):R1099–106. [DOI] [PubMed] [Google Scholar]
- 4. Agbulut O, Noirez P, Beaumont F, Butler-Browne G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell. 2003;95(6):399–406. doi: 10.1016/s0248-4900(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 5. Gokhin DS, Ward SR, Bremner SN, Lieber RL. Quantitative analysis of neonatal skeletal muscle functional improvement in the mouse. J Exp Biol. 2008;211(Pt 6):837–43. doi: 10.1242/jeb.014340. [DOI] [PubMed] [Google Scholar]
- 6. White RB, Bierinx AS, Gnocchi VF, Zammit PS. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev Biol. 2010;10:21. doi: 10.1186/1471-213x-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang MY, Zhang WJ, Medler S. The continuum of hybrid IIX/IIB fibers in normal mouse muscles: MHC isoform proportions and spatial distribution within single fibers. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brummer H, Zhang MY, Piddoubny M, Medler S. Hybrid fibers transform into distinct fiber types in maturing mouse muscles. Cells Tissues Organs. 2013;198:227–36. [DOI] [PubMed] [Google Scholar]
- 9. Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol. 2001;115(5):359–72. [DOI] [PubMed] [Google Scholar]
- 10. Unguez GA, Talmadge RJ, Roy RR, Dalponte D, Edgerton VR. Distinct myosin heavy chain isoform transitions in developing slow and fast cat hindlimb muscles. Cells Tissues Organs. 2000;167(2–3):138–52. doi: 10.1159/000016777. [DOI] [PubMed] [Google Scholar]
- 11. Strbenc M, Smerdu V, Pogacnik A, Fazarinc G. Myosin heavy chain isoform transitions in canine skeletal muscles during postnatal growth. J Anat. 2006;209(2):149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edman KAP, Reggiani CTE, Kronnie GT. Differences in maximum velocity of shortening along single muscle-fibers of the frog. J Physiol. 1985. August;365:147–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edman KAP, Reggiani C, Schiaffino STE, Kronnie G. Maximum velocity of shortening related to myosin isoform composition in frog skeletal muscle fibres. J Physiol. 1988;395:679–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caiozzo VJ, Baker MJ, Huang K, Chou H, Wu Y, Baldwin K. Single-fiber myosin heavy chain polymorphism: how many patterns and what proportions? Am J Physiol Regul Integr Comp Physiol. 2003;285(3):R570–80. [DOI] [PubMed] [Google Scholar]
- 15. Wigston DJ, English AW. Fiber-type proportions in mammalian soleus muscle during postnatal development. J Neurobiol. 1992;23(1):61–70. [DOI] [PubMed] [Google Scholar]
- 16. Blough E, Rennie E, Zhang F, Reiser P. Enhanced electrophoretic separation and resolution of myosin heavy chains in mammalian and avian skeletal muscles. Anal Biochem. 1996;233:31–5. [DOI] [PubMed] [Google Scholar]
- 17. Glaser B, You G, Zhang M, Medler S. Relative proportions of hybrid fibres are unaffected by 6 weeks of running exercise in mouse skeletal muscles. Exp Physiol. 2010;95:211–21. [DOI] [PubMed] [Google Scholar]
- 18. DeNies MS, Johnson J, Maliphol AB, Bruno M, Kim A, Rizvi A, Rustici K, Medler S. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol Rep. 2014;2:e00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wray W, Boulikas T, Wray VP, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118:197–203. [DOI] [PubMed] [Google Scholar]
- 20. Dubowitz V, Sewry CA. Muscle biopsy: a practical approach. Shanghai, China: Elsevier; 2007. [Google Scholar]
- 21. Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscles using multicolor immunofluorescence analysis. PLoS ONE. 2012;7(4):e35273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hughes SM, Blau HM. Muscle fiber pattern is independent of cell lineage in postnatal rodent development. Cell. 1992;68(4):659–71. doi: 10.1016/0092-8674(92)90142-y. [DOI] [PubMed] [Google Scholar]
- 23. Hughes SM, Cho M, Karschmizrachi I, Travis M, Silberstein L, Leinwand LA, Blau HM. Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol. 1993;158(1):183–99. doi: 10.1006/dbio.1993.1178. [DOI] [PubMed] [Google Scholar]
- 24. Jergovic D, Stal P, Lidman D, Lindvall B, Hildebrand C. Changes in a rat facial muscle after facial nerve injury and repair. Muscle Nerve. 2001;24(9):1202–12. doi: 10.1002/mus.1133. [DOI] [PubMed] [Google Scholar]
- 25. Smerdu V, Soukup T. Demonstration of myosin heavy chain isoforms in rat and humans: the specificity of seven available monoclonal antibodies used in immunohistochemical and immunoblotting methods. Eur J Histochem. 2008;52(3):179–89. [DOI] [PubMed] [Google Scholar]
- 26. Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171(3):259–72. [DOI] [PubMed] [Google Scholar]
- 27. Reiser PJ, Kasper CE, Greaser ML, Moss RL. Functional significance of myosin transitions in single fibers of developing soleus muscle. Am J Physiol. 1988;254(5):C605–13. [DOI] [PubMed] [Google Scholar]
- 28. Stevens L, Bastide B, Bozzo C, Mounier Y. Hybrid fibres under slow-to-fast transformations: expression is of myosin heavy and light chains in rat soleus muscle. Pflugers Arch. 2004;448(5):507–14. [DOI] [PubMed] [Google Scholar]
- 29. Desaphy JF, Pierno S, Liantonio A, De Luca A, Didonna MP, Frigeri A, Nicchia GP, Svelto M, Camerino C, Zallone A, Camerino DC. Recovery of the soleus muscle after short-and long-term disuse induced by hindlimb unloading: effects on the electrical properties and myosin heavy chain profile. Neurobiol Dis. 2005;18(2):356–65. doi: 10.1016/j.nbd.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 30. Gauthier GF. Differential distribution of myosin isoforms among the myofibrils of individual developing muscle fibers. J Cell Biol. 1990;110(3):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russell B, Motlagh D, Ashley WW. Form follows function: how muscle shape is regulated by work. J Appl Physiol. 2000;88(3):1127–32. [DOI] [PubMed] [Google Scholar]
- 32. Boonyarom O, Inui K. Atrophy and hypertrophy of skeletal muscles: structural and functional aspects. Acta Physiol. 2006;188(2):77–89. doi: 10.1111/j.1748-1716.2006.01613.x. [DOI] [PubMed] [Google Scholar]
- 33. Staron RS, Pette D. The continuum of pure and hybrid myosin heavy chain-based fiber types in rat skeletal-muscle. Histochemistry. 1993;100(2):149–53. [DOI] [PubMed] [Google Scholar]
- 34. Blaauw B, Schiaffino S, Reggiani C. Mechanisms modulating skeletal muscle phenotype. Compr Physiol. 2013;3(4):1645–87. doi: 10.1002/cphy.c130009. [DOI] [PubMed] [Google Scholar]
- 35. Stephenson GMM. Hybrid skeletal muscle fibres: a rare or common phenomenon? Clin Exp Pharmacol Physiol. 2001;28(8):692–702. [DOI] [PubMed] [Google Scholar]
- 36. Perry MJ, Tait J, Hu J, White SC, Medler S. Skeletal muscle fiber types in the ghost crab, Ocypode quadrata: implications for running performance. J Exp Biol. 2009;212(5):673–83. doi: 10.1242/jeb.023481. [DOI] [PubMed] [Google Scholar]
- 37. Medler S, Lilley T, Mykles DL. Fiber polymorphism in skeletal muscles of the American lobster, Homarus americanus: continuum between slow-twitch (S1) and slow-tonic (S2) fibers. J Exp Biol. 2004;207(16):2755–67. [DOI] [PubMed] [Google Scholar]
- 38. Acevedo LM, Rivero JL. New insights into skeletal muscle fibre types in the dog with particular focus towards hybrid myosin phenotypes. Cell Tissue Res. 2006;323(2):283–303. doi: 10.1007/s00441-005-0057-4. [DOI] [PubMed] [Google Scholar]
- 39. Williamson DL, Gallagher PM, Carroll CC, Raue U, Trappe SW. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol. 2001;91(5):1955–61. [DOI] [PubMed] [Google Scholar]
- 40. Medler S. Mixing it up: the biological significance of hybrid skeletal muscle fibers. J Exp Biol. in press. [DOI] [PubMed] [Google Scholar]
- 41. Korfage JAM, Kwee KE, Everts V, Langenbach GEJ. Myosin heavy chain expression can vary over the length of jaw and leg muscles. Cells Tissues Organs. 2015;201:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang M, Gould M. Segmental distribution of myosin heavy chain isoforms within single muscle fibers. Anat Rec. 2017;300:1636–42. [DOI] [PubMed] [Google Scholar]
- 43. Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13(1):40–7. [DOI] [PubMed] [Google Scholar]
- 44. Schiaffino S, Gorza L, Pitton G, Saggin L, Ausoni S, Sartore S, Lømo T. Embryonic and neonatal myosin heavy-chain in denervated and paralyzed rat skeletal-muscle. Dev Biol. 1988;127(1):1–11. [DOI] [PubMed] [Google Scholar]
- 45. Peuker H, Pette D. Quantitative analyses of myosin heavy-chain mRNA and protein isoforms in single fibers reveal a pronounced fiber heterogeneity in normal rabbit muscles. Eur J Biochem. 1997;247(1):30–6. [DOI] [PubMed] [Google Scholar]
- 46. McLoon LK, Park HN, Kim JH, Pedrosa-Domellof F, Thompson LV. A continuum of myofibers in adult rabbit extraocular muscle: force, shortening velocity, and patterns of myosin heavy chain colocalization. J Appl Physiol. 2011;111(4):1178–89. doi: 10.1152/japplphysiol.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lutz GJ, Sirsi SR, Shapard-Palmer SA, Bremner SN, Lieber RL. Influence of myosin isoforms on contractile properties of intact muscle fibers from Rana pipiens. Am J Physiol Cell Physiol. 2002;282(4):C835–44. [DOI] [PubMed] [Google Scholar]
- 48. Snow LM, Sanchez OA, McLoon LK, Serfass RC, Thompson LV. Effect of endurance exercise on myosin heavy chain isoform expression in diabetic rats with peripheral neuropathy. Am J Phys Med Rehabil. 2005;84(10):770–9. doi: 10.1097/01.phm.0000176350.61935.d6. [DOI] [PubMed] [Google Scholar]
- 49. Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22(10):1350–60. [DOI] [PubMed] [Google Scholar]
- 50. Liu JX, Höglund AS, Karlsson P, Lindblad J, Qaisar R, Aare S, Bengtsson E, Larsson L. Myonuclear domain size and myosin isoform expression in muscle fibres from mammals representing 100 000-fold differences in body size. Exp Physiol. 2009;94:117–29. [DOI] [PubMed] [Google Scholar]
- 51. Newlands S, Levitt LK, Robinson CS, Karpf ABC, Hodgson VRM, Wade RP, Hardeman EC. Transcription occurs in pulses in muscle fibers. Genes Dev. 1998;12(17):2748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cutler AA, Jackson JB, Corbett AH, Pavlath GK. Non-equivalence of nuclear import among nuclei in multinucleated skeletal muscle cells. J Cell Sci. 2018;131(3):jcs207670. doi: 10.1242/jcs.207670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Larson LR, Johnson J, Medler S. Fiber type asymmetries in growing skeletal muscles. Integr Comp Biol. 2015;55:E289. [Google Scholar]