Abstract
The gut microbiome as a potential therapeutic target for mental illness is a hot topic in psychiatry. Trillions of bacteria reside in the human gut and have been shown to play a crucial role in gut–brain communication through an influence on neural, immune, and endocrine pathways. Patients with various psychiatric disorders including depression, bipolar disorder, schizophrenia, and autism spectrum disorder have been shown to have significant differences in the composition of their gut microbiome. Enhancing beneficial bacteria in the gut, for example, through the use of probiotics, prebiotics, or dietary change, has the potential to improve mood and reduce anxiety in both healthy people and patient groups. Much attention is being given to this subject in the general media, and patients are becoming increasingly interested in the potential to treat mental illness with microbiome-based therapies. It is imperative that those working with people with mental illness are aware of the rationale and current evidence base for such treatment strategies. In this review, we provide an overview of the gut microbiome, what it is, and what it does in relation to gut–brain communication and psychological function. We describe the fundamental principles and basic techniques used in microbiome–gut–brain axis research in an accessible way for a clinician audience. We summarize the current evidence in relation to microbiome-based strategies for various psychiatric disorders and provide some practical advice that can be given to patients seeking to try a probiotic for mental health benefit.
Keywords: microbiome, microbiota, biological psychiatry, psychobiotics, probiotics, depressive disorders, anxiety, gut–brain axis
Abstract
Le microbiote intestinal à titre de cible thérapeutique potentielle pour la maladie mentale est un sujet d’actualité en psychiatrie. Des milliards de bactéries résident dans l’intestin humain et il a été démontré qu’elles jouent un rôle essentiel dans la communication intestin-cerveau grâce à une influence sur les voies neuronales, immunes et endocriniennes. Les patients souffrant de divers troubles psychiatriques, notamment la dépression, le trouble bipolaire, la schizophrénie et le trouble du spectre de l’autisme se sont révélés avoir des différences significatives dans la composition de leur microbiote intestinal. Accroître les bactéries bénéfiques dans l’intestin, par exemple, en utilisant des probiotiques, des prébiotiques, ou un changement alimentaire, a le potentiel d’améliorer l’humeur et de réduire l’anxiété tant chez les personnes en santé que dans les groupes de patients. Ce sujet a fait l’objet d’une attention soutenue des médias généraux et les patients s’intéressent de plus en plus à la possibilité de traiter la maladie mentale à l’aide de thérapies basées sur le microbiote. Les personnes qui travaillent auprès de personnes souffrant de maladie mentale doivent absolument connaître la raison d’être et les données probantes actuelles de ces stratégies de traitement. Dans cette étude, nous présentons un aperçu du microbiote intestinal, ce qu’il est et ce qu’il fait en relation avec la communication intestin-cerveau et la fonction psychologique. Nous décrivons les principes fondamentaux et les techniques de base utilisés dans la recherche sur l’axe microbiote-intestin-cerveau de façon accessible à un auditoire clinicien. Nous résumons les données probantes actuelles relatives aux stratégies axées sur le microbiote pour divers troubles psychiatriques et prodiguons des conseils pratiques qui peuvent être transmis aux patients cherchant à essayer un probiotique pour un bénéfice de santé mentale.
The Microbiome–Gut–Brain Axis
The human gastrointestinal tract (GIT) harbors an immense collection of microorganisms termed the gut microbiota. This consists predominantly of bacteria but also includes viruses, protozoa, fungi, and archaea. Although more conservative than previously reported, recent estimates place the number of bacteria in the human gut at approximately 3.8 × 1013, slightly in excess of the total number of human cells.1 The collective genome of these bacterial cells, the gut microbiome, vastly exceeds the amount of human DNA present in the body, such that, for every one human gene, we have over 100 bacterial genes.2 Given the enormous genetic potential of the microbiota, it is unsurprising that it appears to play a role in almost all physiological processes in the human body.
The concept of the “gut–brain axis” is not a new one. Gastrointestinal symptoms are often reported in psychiatric illness. Disturbances in appetite and weight change are key features of major depressive disorder (MDD),3 while symptoms of diarrhoea and nausea are frequent complaints in patients with anxiety disorders.4 Gastrointestinal problems commonly coexist with autism spectrum disorder (ASD),5 schizophrenia,6 and Parkinson disease.7 Likewise, gastroenterologists are no strangers to psychopathology. Mood disturbances, anxiety, and stress are well recognized as playing a role in functional gastrointestinal disorders such as irritable bowel syndrome (IBS) along with organic conditions including inflammatory bowel disease8 and peptic ulceration.9
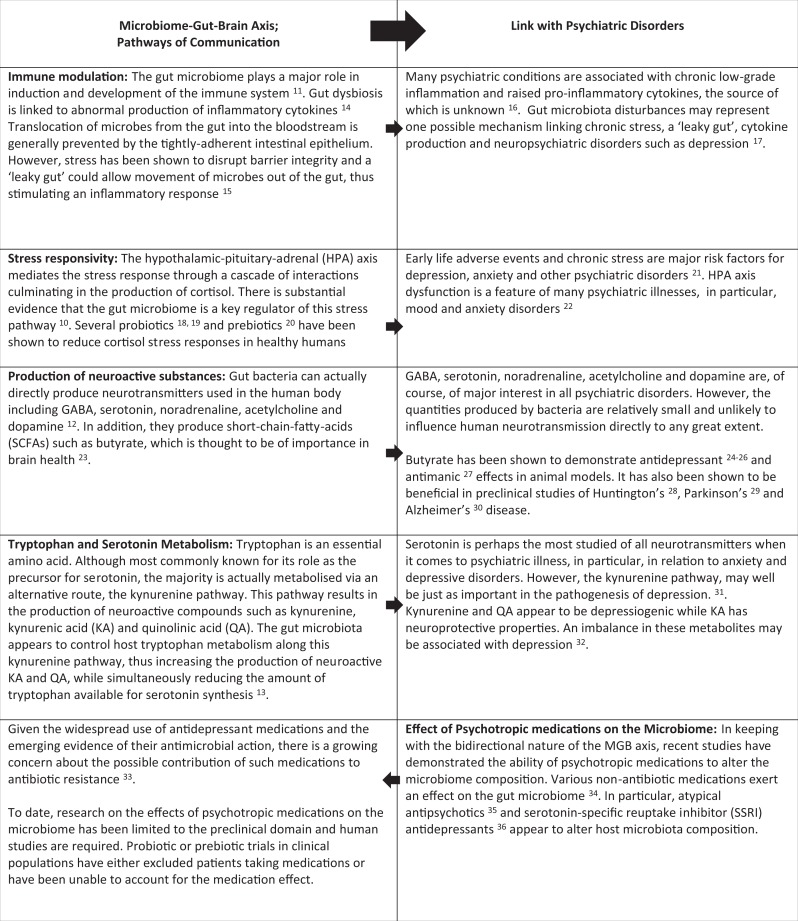
The bidirectional communication between the brain and GIT is a complex and dynamic system, capable of continuously transmitting, interpreting, and responding to information. Within this vast communication matrix lies the gut microbiome, which we now recognize as playing a vital role. The mechanisms by which our gut bacteria communicate with, and influence, the central nervous system are gradually being uncovered and span neural, endocrine, and immune systems. There is a striking overlap between those pathways influenced by the microbiome and those involved in mental illness (see Table 1). The gut microbiome has been shown to play a major role in the development and function of the hypothalamic–pituitary–adrenal (HPA) axis,10 which mediates the stress response and is of interest in a range of psychiatric disorders, in particular depression and anxiety disorders. Our gut bacteria also significantly influence the immune system11 and may represent a link with the immune dysfunction that is characteristic of mental illnesses such as depression and schizophrenia. Interestingly, the gut microbiome also impacts neurotransmission. As well as being capable of directly producing various neurotransmitters such as serotonin, noradrenaline, dopamine, and γ-aminobutyric acid,12 gut bacteria have been shown to modulate tryptophan metabolism and serotonin production.13 These pathways of communication between the microbiome, gut, and brain and their relevance to psychiatric illness are further explored in Table 1.
Table 1.
Microbiome–Gut–Brain Axis Communication Pathways and Their Relevance to the Pathogenesis of Psychiatric Disorders.
|
Development of the Human Gut Microbiome
It is generally accepted that the uterus is a sterile environment and that bacterial colonization begins during birth.37 The neonatal microbiome varies according to mode of delivery, with that of vaginally delivered infants resembling the maternal vaginal microbiome and that of those delivered by cesarean section resembling the maternal skin microbiome.38 Various other factors influence the developing neonatal microbiome including premature birth, mode of feeding,39 and of course, the administration of perinatal antibiotics.40 The simple infant microbiome continues to adapt and diversify, and early disparities resolve quite quickly. Microbiome differences based on delivery mode are no longer evident by the sixth week of life,41 and by 1 year, the infant has a diverse, differentiated adult-like microbiome.42 Throughout adulthood, the major determinant of gut microbiome composition seems to be diet. Rapid and dramatic shifts in microbiome composition occur in response to changes in dietary intake with distinct patterns apparent in plant-based versus animal-based diets.43,44 Nutritional factors continue to be of relevance in the elderly population, and the microbiome appears to be a major determinant of health status and frailty levels as one ages.45 Interestingly, while environmental proximity to another person does not, in itself, increase the similarity of microbiome composition between individuals, the quality of human relationships does seem to have an impact. A recent study found that married couples who described a close relationship had similar microbiome profiles, while no differences in similarity were found between couples who did not report such a close bond.46 Although the same study reported no differences in microbiome similarity between sibling pairs and unrelated pairs, another paper described the similarity indices of monozygotic twins to be significantly higher than those of unrelated individuals, suggesting that host genotype does also play a role in shaping the microbiome.47
Fundamental Principles of Microbiome Research
To understand the current status of microbiome research, it is helpful to become acquainted with some of the basic research methods employed. In this section, we explain the essential laboratory techniques used to identify bacteria within a fecal sample and the various tools applied to investigate the mechanisms of communication between the gut microbiome and brain.
Laboratory Techniques for Microbiome Analysis
Historically, bacteria could only be investigated by culture techniques that involved plating samples on appropriate media and identifying the resultant bacterial growth.48 The problem with this method was that many microorganisms were not suitable for culture and thus were unable to be identified. The advent of “metagenomics,” a culture-independent system, which allows for direct analysis of the genetic material in a sample, has meant that it has become possible to identify all the microorganisms present.49
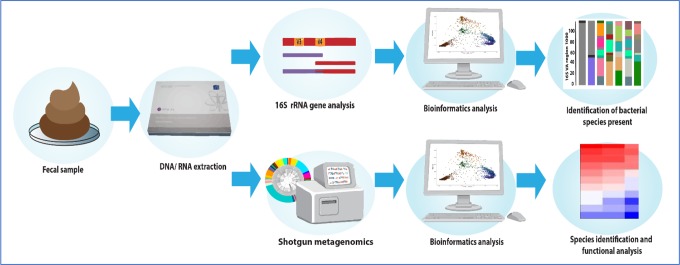
Once a fecal sample has been collected, it undergoes processing to extract the DNA and RNA (see Figure 1). The resulting genetic material can be analyzed in two ways. The first and most commonly employed technique is 16S ribosomal RNA (rRNA) gene analysis. The 16S rRNA gene is a highly conserved gene present in almost all bacteria. The extracted RNA undergoes polymerase chain reaction processing which, using pre-made 16S rRNA primers, identifies and amplifies these genes. The resultant genes are then sequenced allowing identification of the different bacteria present in the sample.50 The second, more expensive, method is “shotgun metagenomics,” also called “whole genome shotgun sequencing.” This is a technique whereby all the extracted DNA in the sample are sequenced, as opposed to only one target gene. It not only identifies which bacteria are present in a sample but also enables an assessment of their function from analysis of all the genes they contain. It is more expensive than 16S rRNA sequencing but very useful for functional, along with compositional, microbiome analysis.51
Figure 1.
Microbiome analysis: Analysis of the gut microbiome from a fecal sample can be done in two ways. The more basic method is using 16S ribosomal RNA analysis, which identifies all the bacterial genera and species present in the sample. Shotgun metagenomics is a more complex and expensive process but provides information on the functional capacity of the microbiome along with bacterial identification.
While traditional DNA sequencing was an extremely slow and expensive process, high-throughput “next generation sequencing” technology has revolutionized the microbiome field by allowing billions of DNA strands to be sequenced in parallel, making genome analysis faster, cheaper, and more accessible.52 Following sequencing, huge data sets are generated and can be analyzed using specialized bioinformatics packages. The DNA sequence reads are clustered with similar reads into “operational taxonomic units,” each of which signifies a specific bacterial genera or species.
Manipulating the Microbiome
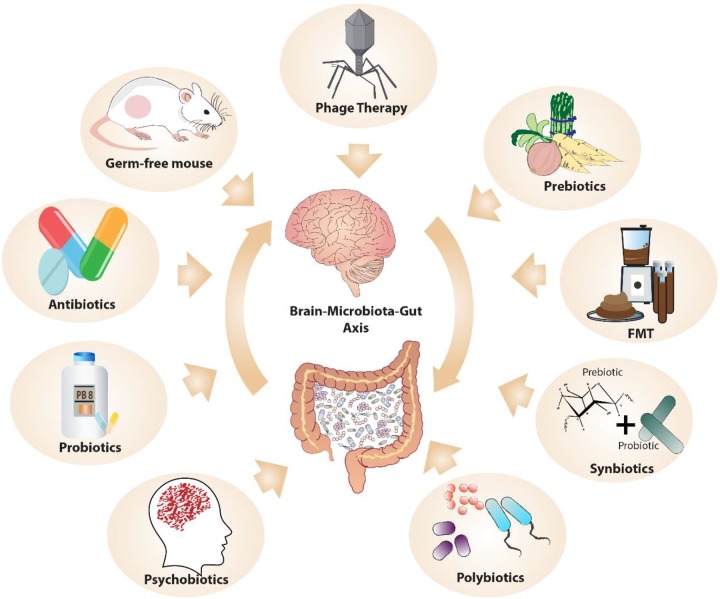
A key method of investigating the pathways of microbiota–gut–brain (MGB) communication is to alter the microbiota in various ways (see Figure 2) and explore the consequences on the brain and behavior. Rodent models are an invaluable resource in this regard. A state of complete absence of the microbiome can be examined by the use of germ-free (GF) animals (animals born and maintained in a sterile environment) and has been extremely useful in proof-of-principle studies, elucidating a role for the microbiome in stress responsivity, anxiety, social behavior, and cognition.53 A less extreme and more clinically relevant model is microbiome depletion, whereby various antibiotics are used to modify the microbiome in predictable and reproducible ways.54
Figure 2.
Manipulating the microbiome: The microbiome can be altered in various ways to investigate the impact on the brain and psychological function.
The microbiome can also be altered by the addition or enhancement of specific bacteria. Probiotics, defined as living bacteria that, when administered in adequate amounts, confer a health benefit on the host,55 are easily administered. They allow investigation of individual species or bacterial combinations, termed polybiotics, on different parameters in both health and disease states. A less specific, but possibly more effective, method of enhancing specific bacteria is through the use of prebiotics, defined as substrates, usually but not necessarily carbohydrates, which selectively enhance the growth of certain bacteria.56 They can be administered with their preferred bacterial targets for greater efficacy, the combination being referred to as a “synbiotic.”57 Another term widely used in the microbiome arena is that of the “psychobiotic” which refers specifically to pro-, pre-, or synbiotics that have been shown to confer a mental health benefit.58
A further means of altering the microbiome is through the use of fecal microbiota transplantation (FMT) that involves the transfer of fecal matter from one individual to another, thereby passing on the donor’s microbiota. It has been used to investigate the ability of the microbiota, from a donor with a specific disorder such as depression, to transfer the disease phenotype to an animal.59 It has also been shown to be effective therapeutically, predominantly in the treatment of the gastrointestinal infection, Clostridium difficile,60 but more recently extending into the psychiatric domain. Two small studies investigating FMT in the treatment of IBS reported improvements in mood symptoms,61,62 and a small open-label trial demonstrated promising results using FMT as a potential therapy for ASD.63
A new and exciting method of altering the microbiome is through the use of phage therapy. Phages, short for bacteriophages, are viruses that infect specific bacteria. Although they have been around for over a century, interest in their use as a method of eliminating pathogenic bacteria largely subsided with the advent of antibiotics. However, renewed curiosity about their therapeutic potential has developed with the emergence of antibiotic resistance.64 The success of FMT in treating resistant gastrointestinal infections such as Clostridium difficile is generally attributed to the transfer and colonization of bacteria. However, it has been shown that the viral component from donor FMT can colonize the recipient gut for up to 12 months and may play a much greater role than is currently appreciated.65 As a modulator of microbiome composition, the use of phage to target the MGB axis is highly plausible, although very much limited to the research domain at present.
“Postbiotics” refer to nonviable bacterial products or bacterial metabolites that have biologic activity in the host. The postbiotics of most interest in relation to the brain are the short-chain fatty acids (SCFAs), namely butyrate, propionate, and acetate, which are produced by colonic bacteria from the fermentation of nondigestible carbohydrates. As such, their production is particularly encouraged by a high-fiber diet, something that has long been associated with better health outcomes. Butyrate, especially, appears to have neuroprotective properties and has been demonstrated to have antidepressant potential in animal models23 although human studies are lacking.
The Microbiome in Psychiatric Disorders: Current Evidence
There is no doubt that the gut microbiome influences brain function, and the vast array of preclinical studies provide us with insights into the mechanisms by which this may be occurring. However, the major question for psychiatrists is whether the science actually translates to the clinic or remains an academic pursuit. The concept of the MGB axis is an exciting one, but does it actually mean anything in the management of mental illness in our patients? Although the human data are certainly lagging behind the laboratory discoveries, application of microbiome-based hypotheses is gradually being tested in clinical populations. In this section, we will review the current evidence base across the spectrum of psychiatric illness, from the characterization of microbiome composition in patients with various disorders to the potential for treatment using microbiome-based interventions.
MDD
The gut microbiome of patients with depression has significant compositional differences when compared with that of healthy controls.59,66–69 Although several case-control studies have confirmed this differential microbiome profile, there does not appear to be an identifiable “depression” signature, and in fact, some findings have been contradictory. This may be partly explained by the fact that microbiome composition shows major interindividual variability, and these MDD studies were small, ranging from only 34 to 60 subjects in patient groups. A Belgian group has attempted to address the issue recently by a large-scale population study that used data from the Flemish Gut Flora Project to investigate the relationships between microbiome composition and quality of life and depression (diagnosed by a general practitioner) in 1,045 people. They found that two bacterial genera, Coprococcus and Dialister, were depleted in patients with depression irrespective of antidepressant treatment and that butyrate-producing Faecalibacterium and Coprococcus bacteria were consistently associated with higher quality of life measures.70 A role for the microbiome in MDD is further supported by the striking observation that when mice are colonized with the microbiome from a depressed patient, through the process of FMT, they begin to exhibit depressive-like symptoms.59,67
Numerous trials have investigated the effect of probiotics on mood, in both healthy population and those diagnosed with depression. Recent meta-analyses of the data, for the most part, confirm the beneficial effects of certain probiotics on mood.71-75 However, several caveats are worth noting. Probiotics appear to be of limited efficacy in those with normal baseline mood, and a beneficial effect is predominantly seen in those exhibiting depressive symptoms.73,75 In addition, the antidepressant effects of probiotics seem to be limited to younger adults and not evident in those over the age of 65 years.74 Another area of concern is the major interstudy discrepancies in relation to probiotic dosing and duration of treatment, which has reduced the comparability of current clinical trials. Likewise, the use of different bacterial species and strains poses a similar challenge. While those probiotics that appear to have antidepressant effects are predominantly of the Bifidobacterium and Lactobacillus genera, there are many different species and strains within these genera, and properties are not generalizable. Prebiotics have also been studied for potential antidepressant properties, but a recent meta-analysis has found no benefit over placebo in relation to mood improvement.75
Bipolar Affective Disorder (BPAD)
Several studies have investigated the microbiome composition in patients with BPAD.76 The first, a relatively large study involving 115 patients, reported decreased levels of Faecalibacterium. This finding was replicated in an Austrian study of 32 patients with bipolar disorder77 and also demonstrated consistency with a study in patients with MDD where similar underrepresentation of the bacterium was reported.66 However, a Danish study that compared the microbiome of 113 patients with newly diagnosed BPAD with unaffected first-degree relatives and healthy individuals found no differences in Faecalibacterium. They reported that Flavonifractor, a bacterial genus that may induce oxidative stress and inflammation, was associated with bipolar disorder.78
Interestingly, two recent clinical trials have demonstrated a beneficial effect of adjunctive probiotics in patients with BPAD. One was an uncontrolled pilot study that reported subtle cognitive improvements in 20 euthymic individuals following 3 months consumption of a probiotic containing nine different strains of Lactobacillus or Bifidobacterium. 79 The second was a randomized controlled trial (RCT) involving 66 patients who had recently been hospitalized for mania.80 After discharge, these patients were randomly assigned to receive 24 weeks of an adjunctive Lactobacillus/Bifidobacterium probiotic or adjunctive placebo. Rehospitalization rates were significantly lower in those individuals who were taking the probiotic. Thus, as seen in MDD, probiotics of the Lactobacillus and Bifidobacterium genera appear to hold therapeutic potential in BPAD.
Anxiety and Related Disorders
There is a wealth of preclinical evidence supporting a role for the gut microbiome in HPA axis development, stress responsivity, and anxiety-related behaviors in animal models.81 While probiotics have consistently demonstrated an ability to reduce anxiety in rodents, evidence for the similar anxiolytic effects in humans is far from established.82 Many probiotic trials in healthy human populations have included a stress or anxiety outcome, and although results have been inconsistent, they generate cautious optimism.71,72 A small cross-sectional study, which would support the potential of microbiome-based treatments for anxiety disorders, found that higher intake of fermented, probiotic-containing foods by healthy students appeared to be protective against developing social anxiety disorder in those who had high baseline levels of neuroticism.83
There has only been a single publication to date reporting on the microbiome composition in those with a specific anxiety disorder. This small study investigated the microbiome composition in post-traumatic stress disorder (PTSD). Authors analyzed the microbiome profile of 18 individuals suffering from PTSD and compared it to that of 12 subjects who, despite exposure to trauma, did not develop PTSD. Although overall diversity measures were similar, the relative abundances of Actinobacteria, Lentisphaerae, and Verrucomicrobia phyla were decreased in PTSD subjects and able to distinguish PTSD from controls with a high degree of accuracy.84 Unfortunately, there have been no other compositional or interventional studies in people with clinically relevant anxiety. In addition, PTSD is quite different from other “primary” anxiety disorders, such as social anxiety disorder (social phobia), panic disorder, agoraphobia, and generalized anxiety disorder, which has been reflected by its recent reclassification in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. This is a gaping hole in the microbiome literature, especially given the promising preclinical results.
Schizophrenia and Psychotic Disorders
Several researchers have proposed a link between the gut microbiome and schizophrenia, hypothesizing about a possible etiological role given the enormous genetic potential of the microbiome85 and its influence on the immune system, a major pathophysiological feature of the illness.86 The microbiome in patients with first-episode psychosis (FEP) and schizophrenia has been shown to be compositionally distinct form healthy controls. In patients with schizophrenia, the oropharyngeal microbiome displays an increased abundance of lactic acid bacteria87 along with increased levels of Lactobacillus phage.88 The fecal microbiome shows increased representation of the phylum, Proteobacteria, accounted for predominantly by increased levels of the genus, Succinivibrio. 89 While a more recent study did not find any major differences at a phylum level, they did report significant separation of several taxa at a family level and demonstrated behavioral and central neurotransmitter changes in mice who received an FMT from schizophrenia patients.90
There have been two studies investigating the microbiome in patients with FEP. A Finnish group compared the microbiome composition in 28 FEP patients with that of 16 healthy matched controls and explored whether there was an association with symptom response up to 12 months after treatment. They found that, although bacterial numbers showed no statistically significant difference between the two groups, numbers of Lactobacillus group bacteria were elevated in FEP patients and significantly correlated with symptom severity. In addition, a subgroup of FEP patients with the strongest microbiota differences showed poor treatment response at 12-month follow-up.91 A larger Chinese study aimed to further explore the microbiome–psychosis link by analyzing the fecal microbiome along with magnetic resonance spectroscopy (MRS) brain imaging of patients at high risk (HR) and ultrahigh risk (UHR) of psychosis. They found that the orders Clostridiales, Lactobacillales, and Bacteroidales and genera Lactobacillus and Prevotella were increased in UHRs compared with HR patients and healthy controls. They also found increased choline levels on imaging, a marker of cell membrane dysfunction. They suggested that the microbiome changes could, through alterations in SCFA production, lead to microglia activation and cell membrane dysfunction,92 a conceivable, but highly speculative, hypothesis.
Neurodegenerative Disorders
Although Parkinson disease (PD) has been the most intensively studied, the microbiome is of interest across a range of neurogenerative disorders including Alzheimer disease (AD), multiple sclerosis, and amyotrophic lateral sclerosis.93 PD may be of particular relevance, given the high prevalence of gastrointestinal disturbances that often precede the more well-recognized motor symptoms. Although findings have been varied, there are some clear trends evident in the microbiome composition of patients with PD. Several studies showed an increase of Lactobacillus, Bifidobacterium, Akkermansia, and Verrucomicrobiaceae in PD, while Faecalibacterium, Coprococcus, Blautia, and Prevotella appear to be underrepresented.94 Conversely, Bifidobacterium appears to be decreased in AD.95 Interestingly, the microbiome composition in PD is strikingly similar to that seen in idiopathic rapid eye movement sleep behavior disorder, a disorder that is considered a prodrome of PD, thus suggesting that the microbiome changes may precede the development of PD symptoms.96
An RCT investigating the use of a probiotic (Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum) in 60 patients with PD reported that probiotic consumption had favorable effects on motor symptoms as well as on various metabolic parameters including C-reactive protein (CRP), glutathione, and insulin metabolism.97 The same Iranian research group also undertook an RCT in 60 patients with AD using a slightly different multispecies probiotic (Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum). They reported an improvement in mini-mental state examination scores following 12 weeks of the intervention.98 Although these trials are encouraging, they need to be replicated. Microbiome manipulation in the treatment of neurodegenerative disorders may hold therapeutic promise, but at present, this research is very much in its infancy.
ASD
The relationship between diet and neurodevelopmental disorders such as ASD and attention-deficit hyperactivity disorder (ADHD) has been the focus of much research. Particular attention has been paid to the role of food additives, refined sugar, food allergies, and fatty acid metabolism, but there is no conclusive evidence in relation to the beneficial effects of any dietary interventions.99 The high prevalence of gastrointestinal symptoms in children with ASD and the potential impact of diet on autism symptoms have led to a keen interest in the role of the microbiome. Differences in the gut microbiome profile of people with autism have been found. While results have been quite variable, replicated findings have included increased abundance of Clostridium species100-103 and elevated Sutterella levels.104,105 In addition, the oral microbiome of autistic children differs from that of neurotypical children in several taxa predominantly related to energy metabolism and lysine degradation pathways.106 In a similar way to the aforementioned depression, a recent study of FMT demonstrated that transplantation of the gut microbiota from human donors with ASD into GF mice was sufficient to induce hallmark autistic behaviors in the recipient animals.107
Although several studies have attempted to investigate the effects of various probiotics on autism symptoms, results are greatly limited by small sample sizes and methodological difficulties, and it is difficult to draw any conclusions.108 A recent small open-label pilot study that involved an FMT from neurotypical donors to ASD children over a period of 8 weeks demonstrated very promising results. Significant improvements in gastrointestinal and behavioral symptoms were seen in patients following up to 8 weeks following microbiome transfer,63 and notably, many of these improvements were maintained at follow-up 2 years later.109
Probiotics, Mood, and Anxiety: Practical Advice for Patients
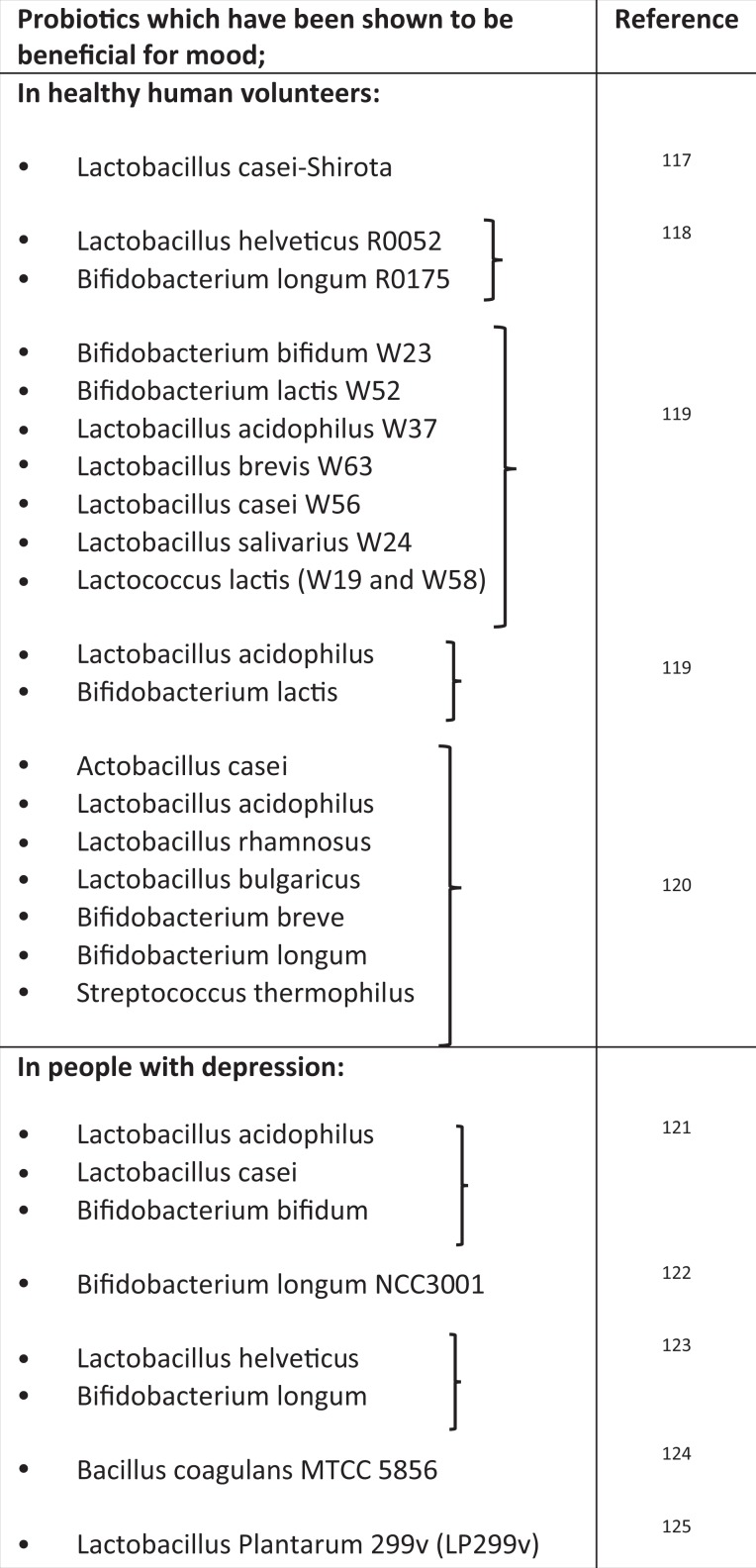
The concept of the MGB axis has gained traction in the mainstream arena in recent years, and it is not uncommon for patients attending the psychiatric clinic to have read about the potential for probiotics to treat depression or anxiety. It is imperative that psychiatrists understand the current status of evidence and can make accurate and informed recommendations to patients about probiotics and microbiome-based interventions (see Table 2). Current trends suggest that the global probiotics market size could reach over US$ 66 billion by 2024,115 and choosing a probiotic from the ever-expanding selection of commercially available products can be daunting for patients. A consumer guide has been developed by the International Scientific Association for Probiotics and Prebiotics (2016) and can be a helpful resource. Most bacteria that have been shown to have psychobiotic effects hail from two genera, Lactobacillus and Bifidobacterium. However, there are many different species and strains within these two genera, with differential psychological effects. For example, Lactobacillus rhamnosus (strain JB-1) failed to impact mood or anxiety levels in healthy males,116 while Lactobacillus casei (strain Shirota) demonstrated an ability to improve mood in healthy volunteers with low baseline mood scores.117 Thus, any claims of efficacy should be species- and strain-specific and have been proven in human trials. In Table 3, we provide a list of probiotics which have been proven to have a positive impact on mood in human subjects.
Table 2.
Advice for Patients in Relation to the Use of Probiotics and Dietary Interventions for Mental Health.
| References | |
|---|---|
General advice about buying probiotics
|
110 |
General advice about diet
|
|
Depression and probiotics
|
See text for references |
Anxiety and probiotics:
|
See text for references |
Table 3.
Bacterial Species and Strains that Have Been Demonstrated to Have, Either Alone or in Combination, a Positive Effect on Mood in Human Studies.
|
Future Directions
The MGB axis has provided psychiatry with a new, and much-needed, paradigm from which to approach mental illness. Even with our comprehensive biopsychosocial approach to the management of psychiatric disease, many patients continue to experience distressing psychological symptoms. A recent large-scale population study confirmed that people with severe mental illnesses, such as schizophrenia, BPAD, and MDD, have higher intakes of obesogenic nutrients and more inflammatory diets than the general population.126 Notably, the poorest dietary patterns were seen in those with schizophrenia, an unsurprising finding given the particularly high prevalence of metabolic disorders and reduced life expectancy in this group. Although much remains to be discovered about the mechanisms by which the gut microbiome influences the brain and mental functioning, the area of nutrition and gut health are beginning to represent an important component in holistic psychiatric care. As society in the developed world becomes increasingly conscious of dietary intake and food choice, targeting mental health through dietary change and other microbiome-based interventions is likely to become an acceptable and widespread practice. However, it is important to recognize that this field is really only in its infancy. The major challenge for microbiome researchers is moving the exciting preclinical discoveries out of the academic domain and into the psychiatric clinic, a step that is far from straightforward. While a new psychotherapeutic may appear hopeful in preclinical phases of development, this does not always ensure efficacy in humans, a narrative well illustrated in recent years by the translational failure of corticotrophin-releasing factor antagonists in the treatment of addiction.127 It would be premature to suggest that probiotics or other microbiome interventions could replace evidence-based pharmacological or psychological treatments. Indeed, if probiotics were subject to the same rigor and scrutiny as antidepressant medications, it is uncertain whether they would pass through all phases of development. There is some debate around how best to regulate the development of probiotics and prebiotics, and if one is to promote these substances for the treatment of clinical conditions such as depression, it is reasonable to suggest that they should be subject to the same process as antidepressant medications. Bearing this in mind, the regulatory structure needs to be flexible enough to allow for research on new probiotic products and not discourage progress in the area by excessively prohibitive regulatory controls.128 It may be that microbiome change can be best achieved through whole diet interventions and by introducing probiotic-rich fermented foods such as kombucha, kefir, or sauerkraut to the diet, although human studies assessing the effect of such interventions on the microbiome are lacking. Despite the challenges, the idea that treatment of psychiatric illness might, in the future, involve a psychobiotic or nutritional prescription alongside a traditional psychotropic medication is certainly plausible. The sentiment that one might consider most appropriate at present, with regard to the field of the gut microbiome and nutritional psychiatry, is a cautious but justifiable optimism.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mary I. Butler  https://orcid.org/0000-0002-7918-533X
https://orcid.org/0000-0002-7918-533X
References
- 1. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. [DOI] [PubMed] [Google Scholar]
- 3. Privitera GJ, Misenheimer ML, Doraiswamy PM. From weight loss to weight gain: appetite changes in major depressive disorder as a mirror into brain-environment interactions. Front Psychol. 2013;4:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mussell M, Kroenke K, Spitzer RL, Williams JB, Herzog W, Lowe B. Gastrointestinal symptoms in primary care: prevalence and association with depression and anxiety. J Psychosom Res. 2008;64(6):605–612. [DOI] [PubMed] [Google Scholar]
- 5. McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133(5):872–883. [DOI] [PubMed] [Google Scholar]
- 6. Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. 2015;17(5):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards L, Quigley EMM, Hofman R, Pfeiffer RF. Gastrointestinal symptoms in Parkinson disease: 18-month follow-up study. Mov Disord. 1993;8(1):83–86. [DOI] [PubMed] [Google Scholar]
- 8. Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15(7):1105–1118. [DOI] [PubMed] [Google Scholar]
- 9. Lim WY, Subramaniam M, Abdin E, Vaingankar J, Chong SA. Peptic ulcer disease and mental illnesses. Gen Hosp Psychiatry. 2014;36(1):63–67. [DOI] [PubMed] [Google Scholar]
- 10. Rea K, Dinan TG, Cryan JF. The microbiome: a key regulator of stress and neuroinflammation. Neurobiol Stress. 2016;4:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roshchina V. Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells Lyte M, Freestone PPE, editors. Microbial endocrinology: Interkingdom signaling in infectious disease and health. New York (NY): Springer; 2010. p. 17–52. [Google Scholar]
- 13. O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- 14. Schirmer M, Smeekens SP, Vlamakis H, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167(4):1125–1136.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci. 2019;1437(1):57–67. [DOI] [PubMed] [Google Scholar]
- 17. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takada M, Nishida K, Kataoka-Kato A, et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol Motil. 2016;28(7):1027–1036. [DOI] [PubMed] [Google Scholar]
- 19. Allen AP, Hutch W, Borre YE, et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016;6(11):e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PW. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl). 2015;232(10):1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agid O, Kohn Y, Lerer B. Environmental stress and psychiatric illness. Biomed Pharmacother. 2000;54(3):135–141. [DOI] [PubMed] [Google Scholar]
- 22. Keller PA, McCluskey A, Morgan J, O’Connor SM. The role of the HPA axis in psychiatric disorders and CRF antagonists as potential treatments. Arch Pharm (Weinheim). 2006;339(7):346–355. [DOI] [PubMed] [Google Scholar]
- 23. Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei Y, Melas PA, Wegener G, Mathe AA, Lavebratt C. Antidepressant-like effect of sodium butyrate is associated with an increase in TET1 and in 5-hydroxymethylation levels in the Bdnf gene. Int J Neuropsychopharmacol. 2014;18(2):pii: pyu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valvassori SS, Resende WR, Budni J, et al. Sodium butyrate, a histone deacetylase inhibitor, reverses behavioral and mitochondrial alterations in animal models of depression induced by early- or late-life stress. Curr Neurovasc Res. 2015;12(4):312–320. [DOI] [PubMed] [Google Scholar]
- 26. Yamawaki Y, Yoshioka N, Nozaki K, et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 2018;1680:13–38. [DOI] [PubMed] [Google Scholar]
- 27. Resende WR, Valvassori SS, Reus GZ, et al. Effects of sodium butyrate in animal models of mania and depression: implications as a new mood stabilizer. Behav Pharmacol. 2013;24(7):569–579. [DOI] [PubMed] [Google Scholar]
- 28. Ferrante RJ, Kubilus JK, Lee J, et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci. 2003;23(28):9418–9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma S, Taliyan R, Singh S. Beneficial effects of sodium butyrate in 6-OHDA induced neurotoxicity and behavioral abnormalities: modulation of histone deacetylase activity. Behav Brain Res. 2015;291:306–314. [DOI] [PubMed] [Google Scholar]
- 30. Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 2011;26(1):187–197. [DOI] [PubMed] [Google Scholar]
- 31. Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98(1-2):143–151. [DOI] [PubMed] [Google Scholar]
- 32. Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new “5-HT” hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):702–721. [DOI] [PubMed] [Google Scholar]
- 33. Macedo D, Filho A, Soares de Sousa CN, et al. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord. 2017;208:22–32. [DOI] [PubMed] [Google Scholar]
- 34. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davey KJ, Cotter PD, O’Sullivan O, et al. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry. 2013;3:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cussotto S, Strain CR, Fouhy F, et al. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology (Berl). 2019;236(5):1671–1685. [DOI] [PubMed] [Google Scholar]
- 37. Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thanabalasuriar A, Kubes P. Neonates, antibiotics and the microbiome. Nat Med. 2014;20:469. [DOI] [PubMed] [Google Scholar]
- 41. Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23(3):314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vaishampayan PA, Kuehl JV, Froula JL, Morgan JL, Ochman H, Francino MP. Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Biol Evol. 2010;2:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812 doi:10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 45. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. [DOI] [PubMed] [Google Scholar]
- 46. Dill-McFarland KA, Tang ZZ, Kemis JH, et al. Close social relationships correlate with human gut microbiota composition. Sci Rep. 2019;9(1):703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Erwin GZ, Akkermans ADL, Vliet WMA, de Visser JAGM, de Vos WM. The host genotype affects the bacterial community in the human gastrointestinal tract. Microb Ecol Health D. 2001;13(3):129–134. [Google Scholar]
- 48. Lagier JC, Edouard S, Pagnier I, Mediannikov O, Drancourt M, Raoult D. Current and past strategies for bacterial culture in clinical microbiology. Clin Microbiol Rev. 2015;28(1):208–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hugenholtz P, Goebel BM, Pace NR. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol. 1998;180(18):4765–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Srinivasan R, Karaoz U, Volegova M, et al. Use of 16S rRNA gene for identification of a broad range of clinically relevant bacterial pathogens. Plos One. 2015;10(2):e0117617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Claesson MJ, Clooney AG, O’Toole PW. A clinician’s guide to microbiome analysis. Nat Rev Gastroenterol Hepatol. 2017;14(10):585–595. [DOI] [PubMed] [Google Scholar]
- 52. Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luczynski P, McVey Neufeld KA, Oriach CS, Clarke G, Dinan TG, Cryan JF. Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol. 2016;19(8):pii: pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kennedy EA, King KY, Baldridge MT. Mouse microbiota models: comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front Physiol. 2018;9:1534 doi:10.3389/fphys.2018.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Behnsen J, Deriu E, Sassone-Corsi M, Raffatellu M. Probiotics: properties, examples, and specific applications. Cold Spring Harb Perspect Med. 2013;3:a010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. [DOI] [PubMed] [Google Scholar]
- 57. Kolida S, Gibson GR. Synbiotics in health and disease. Annu Rev Food Sci Technol. 2011;2:373–393. [DOI] [PubMed] [Google Scholar]
- 58. Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720–726. [DOI] [PubMed] [Google Scholar]
- 59. Kelly JR, Borre Y, O’Brien C, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. [DOI] [PubMed] [Google Scholar]
- 60. Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kurokawa S, Kishimoto T, Mizuno S, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord. 2018;235:506–512. [DOI] [PubMed] [Google Scholar]
- 62. Mazzawi T, Lied GA, Sangnes DA, et al. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS One. 2018;13(11):e0194904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kang DW, Adams JB, Gregory AC, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lin DM, Koskella B, Lin HC. Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. 2017;8(3):162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Draper LA, Ryan FJ, Smith MK, et al. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome. 2018;6(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. [DOI] [PubMed] [Google Scholar]
- 67. Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21(6):786–796. [DOI] [PubMed] [Google Scholar]
- 68. Lin P, Ding B, Feng C, et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J Affect Disord. 2017;207:300–304. [DOI] [PubMed] [Google Scholar]
- 69. Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26(8):1155–1162. [DOI] [PubMed] [Google Scholar]
- 70. Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4(4):623–632. [DOI] [PubMed] [Google Scholar]
- 71. Pirbaglou M, Katz J, de Souza RJ, Stearns JC, Motamed M, Ritvo P. Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr Res. 2016;36(9):889–898. [DOI] [PubMed] [Google Scholar]
- 72. Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. 2017;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ng QX, Peters C, Ho CYX, Lim DY, Yeo WS. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J Affect Disord. 2018;228:13–19. [DOI] [PubMed] [Google Scholar]
- 74. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):pii: E483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Evans SJ, Bassis CM, Hein R, et al. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res. 2017;87:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Painold A, Morkl S, Kashofer K, et al. A step ahead: exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019;21(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Coello K, Hansen TH, Sorensen N, et al. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav Immun. 2019;75:112–118. [DOI] [PubMed] [Google Scholar]
- 79. Reininghaus EZ, Wetzlmair LC, Fellendorf FT, et al. The impact of probiotic supplements on cognitive parameters in euthymic individuals with bipolar disorder: a pilot study. Neuropsychobiology. 2018;51(4):1–8. [DOI] [PubMed] [Google Scholar]
- 80. Dickerson F, Adamos M, Katsafanas E, et al. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: a randomized controlled trial. Bipolar Disord. 2018;20(7):614–621. [DOI] [PubMed] [Google Scholar]
- 81. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. [DOI] [PubMed] [Google Scholar]
- 82. Reis DJ, Ilardi SS, Punt SEW. The anxiolytic effect of probiotics: a systematic review and meta-analysis of the clinical and preclinical literature. PloS One. 2018;13(6):e0199041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hilimire MR, DeVylder JE, Forestell CA. Fermented foods, neuroticism, and social anxiety: an interaction model. Psychiatry Res. 2015;228(2):203–208. [DOI] [PubMed] [Google Scholar]
- 84. Hemmings SMJ, Malan-Muller S, van den Heuvel LL, et al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med. 2017;79(8):936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dinan TG, Borre YE, Cryan JF. Genomics of schizophrenia: time to consider the gut microbiome? Mol Psychiatry. 2014;19(12):1252–1257. [DOI] [PubMed] [Google Scholar]
- 86. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Castro-Nallar E, Bendall ML, Perez-Losada M, et al. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. Peer J. 2015;3:e1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yolken RH, Severance EG, Sabunciyan S, et al. Metagenomic sequencing indicates that the oropharyngeal phageome of individuals with schizophrenia differs from that of controls. Schizophr Bull. 2015;41(5):1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shen Y, Xu J, Li Z, et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: a cross-sectional study. Schizophr Res. 2018;197:470–477. [DOI] [PubMed] [Google Scholar]
- 90. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schwarz E, Maukonen J, Hyytiainen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398–403. [DOI] [PubMed] [Google Scholar]
- 92. He Y, Kosciolek T, Tang J, et al. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. Eur Psychiatry. 2018;53:37–45. [DOI] [PubMed] [Google Scholar]
- 93. Quigley EMM. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17(12):94. [DOI] [PubMed] [Google Scholar]
- 94. Gerhardt S, Mohajeri MH. Changes of colonic bacterial composition in Parkinson’s disease and other neurodegenerative diseases. Nutrients. 2018;10(6):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Heintz-Buschart A, Pandey U, Wicke T, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33(1):88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2018;38(3):1031–1035. [DOI] [PubMed] [Google Scholar]
- 98. Akbari E, Asemi Z, Daneshvar Kakhaki R, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Marti LF. Dietary interventions in children with autism spectrum disorders—an updated review of the research evidence. Curr Clin Pharmacol. 2014;9(4):335–349. [DOI] [PubMed] [Google Scholar]
- 100. De Angelis M, Piccolo M, Vannini L, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8(10):e76993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Song Y, Liu C, Finegold SM. Real-time PCR quantitation of Clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70(11):6459–6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Finegold SM, Molitoris D, Song Y, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35(1 Suppl):S6–S16. [DOI] [PubMed] [Google Scholar]
- 103. Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54(Pt 10):987–991. [DOI] [PubMed] [Google Scholar]
- 104. Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. Mbio. 2012;3(1):e00261–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hicks SD, Uhlig R, Afshari P, et al. Oral microbiome activity in children with autism spectrum disorder. Autism Res. 2018;11(9):1286–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sharon G, Cruz NJ, Kang DW, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600–1618. e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Srinivasjois R, Rao S, Patole S. Probiotic supplementation in children with autism spectrum disorder. Archives of Disease in Childhood 2015;100(5):505–506. [DOI] [PubMed] [Google Scholar]
- 109. Kang D-W, Adams JB, Coleman DM, et al. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Scientific Report. 2019;9:5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. International Scientific Association for Probiotics and Prebiotics. 2016. The P’s and Q’s of probiotics: a consumer guide for making smart choices. [Accessed 2019 Aug 28]. https://4cau4jsaler1zglkq3wnmje1-wpengine.netdna-ssl.com/wp-content/uploads/2016/02/Consumer-Guidelines-probiotic.pdf.
- 111. Kim B, Hong VM, Yang J, et al. A review of fermented foods with beneficial effects on brain and cognitive function. Prev Nutri Food Sci. 2016;21(4):297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lassale C, Batty GD, Baghdadli A, et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. 2019;24(7):965–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jacka FN, O’Neil A, Opie R, et al. A randomised controlled trial of dietary improvement for adults with major depression (the “SMILES” trial). BMC Med. 2017;15(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Reportbuyer.com. 2018. Probiotics market size, forecast and trend analysis, 2014–2024. [Accessed 2019 Aug 28]. https://www.reportbuyer.com/product/5583946/probiotics-market-size-forecast-and-trend-analysis-2014–2024.
- 116. Kelly JR, Allen AP, Temko A, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun. 2017;61:50–59. [DOI] [PubMed] [Google Scholar]
- 117. Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61(3):355–361. [DOI] [PubMed] [Google Scholar]
- 118. Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105(5):755–764. [DOI] [PubMed] [Google Scholar]
- 119. Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–264. [DOI] [PubMed] [Google Scholar]
- 120. Mohammadi AA, Jazayeri S, Khosravi-Darani K, et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci. 2016;19(9):387–395. [DOI] [PubMed] [Google Scholar]
- 121. Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32(3):315–320. [DOI] [PubMed] [Google Scholar]
- 122. Pinto-Sanchez MI, Hall GB, Ghajar K, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–459. e448. [DOI] [PubMed] [Google Scholar]
- 123. Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr. 2019;38(2):522–528. [DOI] [PubMed] [Google Scholar]
- 124. Majeed M, Nagabhushanam K, Arumugam S, Majeed S, Ali F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr Res. 2018;62 doi:10.29219/fnr.v29262.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rudzki L, Ostrowska L, Pawlak D, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–222. [DOI] [PubMed] [Google Scholar]
- 126. Firth J, Stubbs B, Teasdale SB, et al. Diet as a hot topic in psychiatry: a population-scale study of nutritional intake and inflammatory potential in severe mental illness. World Psychiatry. 2018;17(3):365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Spierling SR, Zorrilla EP. Don’t stress about CRF: assessing the translational failures of CRF1 antagonists. Psychopharmacology (Berl). 2017;234(9-10):1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hoffmann DE, Fraser CM, Palumbo F, Ravel J, Rowthorn V, Schwartz J. Probiotics: achieving a better regulatory fit. Food Drug Law J 2014;69(2):237–272. [PMC free article] [PubMed] [Google Scholar]