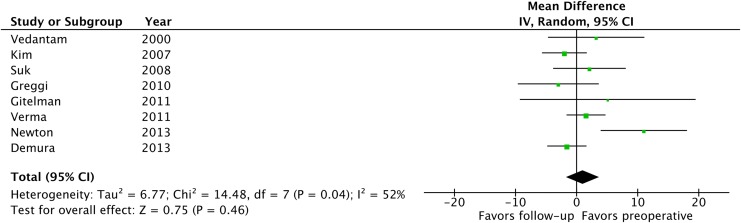Abstract
Study Design:
A systematic review and meta-analysis.
Objectives:
Pulmonary dysfunction is often advocated among the indications for surgical correction of adolescent idiopathic scoliosis (AIS). Previous studies have discussed the effect of scoliosis correction on respiratory function without reaching a definitive conclusion: Some showed that the respiratory function can improve after scoliosis surgery without defining the precise role of anterior, posterior, and combined approaches on this improvement; furthermore, the majority of these studies did not take normal growth into account. As a result, the role of surgery remains to be clarified. The object of the present study was to synthesize the current knowledge regarding changes in respiratory function after posterior corrective surgery for AIS.
Methods:
A comprehensive systematic search was performed to identify all relevant studies in the following electronic databases: MEDLINE, EMBASE, CINAHL (EBSCO). We focused on the studies (1) that discussed posterior fusion surgery for AIS without thoracoplasty, (2) that discussed comparisons of pre- and postoperative percent-predicted values of forced vital capacity (%FVC) or forced expiratory volume (%FEV), and (3) with minimum 2-year follow-up. Forest plots were depicted and Z value was calculated as a test for overall effect.
Results:
Ten studies (6 prospective and 4 retrospective studies) met our inclusion criteria. The overall effect showed that there was no significant difference in %FVC or %FEV between pre- and postoperative measurements (very low evidence).
Conclusions:
Posterior correction surgery for mild to moderate AIS patients showed no significant improvement of postoperative respiratory function measured by relative, percent-predicted values at minimum 2-year follow-up.
Keywords: adolescent idiopathic scoliosis, respiratory function, forced vital capacity, forced expiratory volume, correction, posterior approach, systematic review, meta-analysis
Introduction
Adolescent idiopathic scoliosis (AIS) is a common spinal deformity that derives truncal deformation, and it is known to affect children’s development in many ways, physical and psychological, including self-esteem and mental well-being.1 Although the absolute indications for surgical correction of AIS are yet to be established, the progressive deformity during the growth spurt and the long-term ramifications of untreated curvature have always been of concern. Among them, one of the controversies is whether scoliosis negatively affects respiratory function. Chest wall deformity,2 suboptimal ventilation-perfusion distribution,3 and airway obstruction4 are all known to contribute to pulmonary dysfunction in patients with AIS. Severe scoliosis is known to be associated with respiratory failure in adulthood,5,6 but it is not clear if the majority of AIS cases with only mild to moderate deformity would also eventually suffer from such dysfunction if left untreated, or how much they would respond to surgery.
Based on these controversies, some have argued that a possible preventive effect on future pulmonary dysfunction would justify surgical correction of scoliosis. Indeed, some studies have shown an improvement in respiratory function after scoliosis surgery7,8; their study cohorts, however, were often heterogeneous particularly in terms of surgical approach that included anterior thoracotomies, thoracoscopic approaches, and posterior approaches with or without thoracoplasty. The surgical corridor chosen can itself result in a direct insult on chest cage, affecting lung compliance and thereby affecting negatively the postoperative recovery in respiratory function as well as its long-term improvement.9,10 Moreover, children undergoing scoliosis surgery are often in the midst of their adolescence, and therefore in an age characterized by ongoing respiratory development.11-13 As such, it is challenging to discriminate surgical effect on respiratory function from the normal development, and the absolute values of respiratory function indicators, such as forced vital capacity (FVC) or forced expiratory volume (FEV), cannot be properly discussed without considering the normal development in healthy counterparts. Of note, only a limited number of studies have reported the percent-predicted value of vital capacity (%FVC) in relation to the age-, sex-, and height-matched cohort, where it is generally accepted that %FVC less than 80% indicates restrictive lung disease.14
In designing this systematic review, we focused our attention on the postoperative effect of AIS correction surgery without chest wall violation, that is, posterior approach without thoracoplasty, on long-term respiratory function improvement. The objective of the present study was therefore to provide a critical overview on the current knowledge regarding changes in respiratory function after posterior AIS surgery by means of percent-predicted values of respiratory function.
Methods
Search Strategy
A comprehensive systematic search was performed to identify all relevant studies in the following electronic databases: MEDLINE (Ovid), EMBASE (Ovid), and Cumulative Index to Nursing and Allied Health Literature (CINAHL; EBSCO). We searched all databases from inception to May 2017. Our search strategy included subject headings (eg, Medical Subject Headings [MeSH]) and text words. The 2 main search concepts were scoliosis and respiratory function; in order to maximize recall, the search strategy was composed of controlled vocabulary as well as text words/keywords in accordance to Chapter 6 of the Cochrane Handbook.15 The draft search strategies are listed in full in Appendices A to C. We limited the search to the literatures in English language due to limited time frame, which renders obtaining translations unrealistic.
Study Selection
To highlight postoperative changes in respiratory function after AIS fusion surgery, we defined the following inclusion criteria at time of screening process of the scientific literature: (1) fusion surgery for AIS, (2) up to and including subjects of 24 years of age, (3) comparisons of pre- and postoperative pulmonary function test (PFT) results, and (4) minimum 2-year follow-up. Case reports, literature reviews, commentaries, and technical reports were excluded, and so were abstracts and conference proceedings.
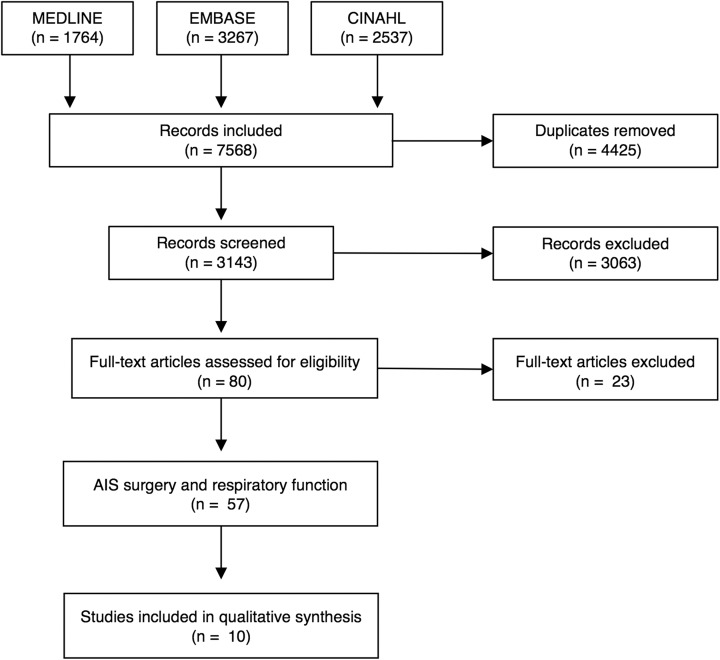
A total of 80 studies, out of the 3143 initially screened by 2 authors (SK and JCM), were assessed for eligibility, and 57 of them were identified to have discussed postoperative respiratory function change after AIS surgery. Finally, we further restricted this initial pool to study cohorts describing posterior surgery and reporting their percent-predicted values of FVC and/or FEV. This yielded to the articles eventually included for meta-analysis and enabled us to comparatively assess the improvement in relation to the normal respiratory development during the study periods. The screening flowchart is shown in Figure 1.
Figure 1.
Screening flowchart of the studies assessed in the present systematic review.
Analysis and Interpretation of Studies
From the selected studies, we extracted the following data: number of the patients, mean and standard deviations of pre- and postoperative percent-predicted FVC (%FVC) and percent-predicted FEV (%FEV). Risk of bias for each study was also assessed. Two authors (SK and MG) assessed the levels of strength of individual papers using the rating advocated by The Journal of Bone & Joint Surgery,16 and the overall level of evidence for each outcome were determined based on the Grades of Recommendation Assessment, Development and Evaluation (GRADE) system.17 A body of evidence that comes from nonrandomized controlled trials was considered “low.” The evidence quality is upgraded based on magnitude of effect or effect of all plausible confounding factors, and downgraded if serious concerns exist in risk of bias, inconsistency, indirectness, imprecision, or publication bias. The statistical analyses were performed by Review Manager (RevMan) Version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Weighted mean differences with 95% confidence intervals (CIs) were summarized and calculated. Heterogeneity among the studies was evaluated by chi-square (χ2) test and I 2statistic. Z value was calculated as a test for overall effect. Finally, the presence of publication bias was assessed by a funnel plot.
Results
Our comprehensive search strategy yielded 10 studies (6 prospective studies9,18-22 and 4 retrospective studies23-26) that met our inclusion criteria, and their results are summarized in the present systematic review. The other studies were excluded due to heterogeneity of the cohort that could not be extracted by stratification, percent-predicted values being not reported, or their standard deviations being not reported.
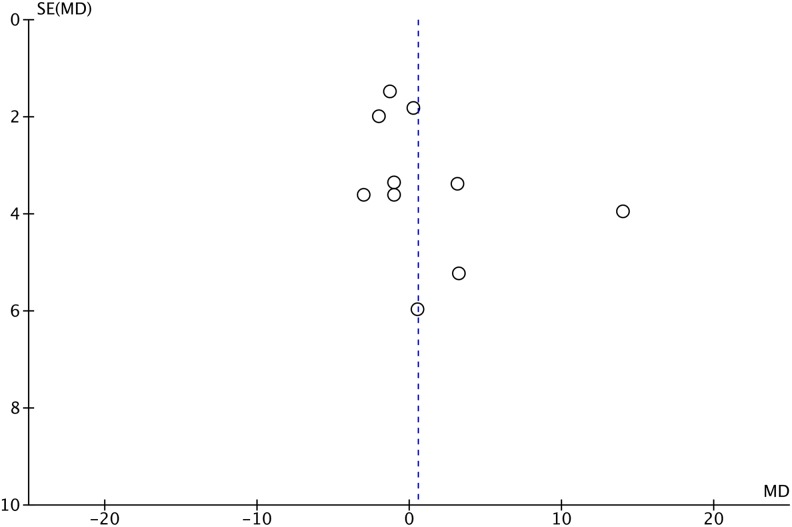
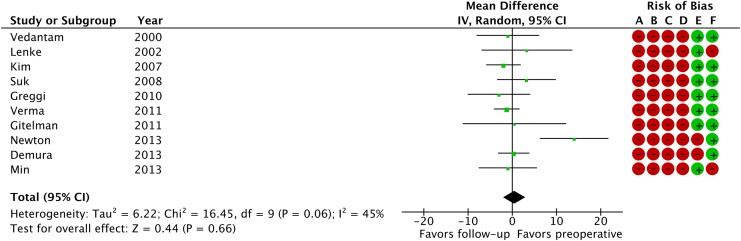
Table 1 summarizes details and patient demographic information for the included studies. Levels of strength are summarized in the same table. From the 10 studies identified, only the groups with posterior correction surgery without thoracoplasty were extracted for meta-analysis. Of note, the average preoperative Cobb angle of the major curve ranged from 49° to 63°, with the maximum curve reported among these studies being 96°. Seven studies focused on thoracic scoliosis,19-24,26 whereas 3 studies included all curve types or did not mention the curve type.9,18,25 Preoperative %FVC averaged from 74% to 87.7% depending on the study, with 2 studies reporting slight restrictive pattern of their cohorts.19,22 A funnel plot showed a slight asymmetry and the tests of heterogeneity revealed that χ2 was 16.45 (P = .06) and I 2 was 45%, indicating there was a moderate heterogeneity (Figure 2). Consequently, a random-effect model was employed. A forest plot depicting the statistics of the 10 studies is shown in Figure 3. Risk of bias assessment is also summarized in Figure 3. Z value was 0.44 (P = .66) and the overall effect showed that there was no significant difference in %FVC between pre- and postoperative measurements. We concluded that the overall strength of evidence was “very low,” given that all of the studies included were non-randomized controlled trials (“Low”) and we downgraded the strength based on the inconsistency of the results. Among the 10 studies identified, only 8 reported %FEV.9,19-21,23-26 Similarly, a forest plot of the 8 studies (Figure 4) showed that postoperative %FEV was not significantly different from preoperative measurements (Z = 0.75, P = .46), and the strength of evidence was “very low” for the same reasons as %FVC.
Table 1.
Summary of the Included Studies.
| Authors | Published Year | Study Design | n | Operative Procedure as Described | Age (Years), Mean (SD) | Gender (M/F) | FU Period (Years) | Cobb Angle (deg) | Curve Type | %FVC, Mean (SD) | %FEV1, Mean (SD) | Level of Evidence | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | FU | Preoperative | FU | |||||||||||
| Vedantam et al9 | 2000 | Prospective | 47 | Posterior spinal arthrodesis with autogenous iliac bone graft | 14.2 (2.2) | NA | 2 | 57 (13) | All | 87.7 (17.8) | 88.7 (17.2) | 85.5 (17.9) | 82.3 (21) | II |
| Lenke, et al18 | 2002 | Prospective | 20 | Posterior fusion | 13.8 (1.4) | 4, 16 | 2 | 60.9 (8.4) | NA | 85.9 (15.0) | 82.6 (18.0) | NA | NA | II |
| Kim et al19 | 2007 | Prospective | 139 | Posterior segmental spinal fusion and instrumentation with iliac crest bone graft | 14.6 (2.19) | 25 114 | 2 | 60 (11.7) | Lenke 1-4 | 79 (16.5) | 81 (16.7) | 75 (16.1) | 77 (15.5) | IVa |
| Suk et al23 | 2008 | Retrospective | 37 | Pedicle screw instrumentation and rod derotation (no thoracoplasty) | 14.7 | 5, 32 | 6.0 | 53.2 (9.1) | Lenke 1, 2 | 81.7 (13.5) | 78.5 (15.6) | 74.8 (13.1) | 72.7 (13.0) | III |
| Greggi et al24 | 2010 | Retrospective | 40 | Posterior segmental fusion without thoracoplasty | 15.2 (1.9) | 5, 35 | 8.3 | 63 (13) | Lenke 1-3 | 84 (14) | 87 (18) | 80 (13) | 83 (17) | III |
| Gitelman et al25 | 2011 | Retrospective | 11 | Posterior spinal fusion/instrumentation with iliac crest bone graft and no chest cage disruption | 15.4 (3.3) | 3, 8 | 10.8 | 53 | All | 85.4 (15.8) | 84.9 (11.9) | 78.3 (15.1) | 73.2 (19.1) | IVb |
| Verma et al26 | 2011 | Retrospective | 70 | Posterior spinal fusion | 15.6 (2.3) | 23, 47 | 2 | 50 (7.5) | Lenke 1-3 | 81.4 (12.4) | 82.66 (0.72) | 80.3 (13.6) | 78.76 (0.60) | III |
| Demura et al20 | 2013 | Prospective | 154 | Posterior spinal fusion without thoracoplasty | 14.6 (2.2) | 42 112 | 2 | 54.8 (11.5) | Lenke 1-4 | 80.9 (14.6) | 80.6 (17.2) | 76.9 (14.8) | 78.5 (14.0) | II |
| Newton et al21 | 2013 | Prospective | 64 | Posterior spinal fusion | NA | NA | 3 | 49 (7) | Lenke 1 | 85 (18) | 71 (26) | 80 (16) | 69 (24) | II |
| Min et al22 | 2013 | Prospective | 48 | Posterior correction with pedicle screw alone instrumentation | 15.3 | 6, 42 | 10 | 58 (12) | Lenke 1, 2 | 74 (21) | 75 (10) | NA | NA | IVa |
Abbreviations: SD, standard deviation; M, male; F, female; FU, follow-up; NA, not available.
aDowngraded due to lack of control groups (case series)
bDowngraded based on a small effect size
Figure 2.
A funnel plot testing the heterogeneity of the studies included in the meta-analysis. SE, standard error; MD, mean difference.
Figure 3.
A forest plot depicting percent-predicted values of forced vital capacity in the 10 studies included. Risk of bias legend: (A) random sequence generation (selection bias), (B) allocation concealment (selection bias), (C) blinding of participants and personnel (performance bias), (D) blinding of outcome assessment (detection bias), (E) incomplete outcome data (attrition bias), (F) selective reporting (reporting bias). Green circle indicates high risk and red circle indicates low risk. IV, inverse variance; CI, confidence interval.
Figure 4.
A forest plot depicting percent-predicted values of forced expiratory volume in the 8 studies included. IV, inverse variance; CI, confidence interval.
Discussion
The present systematic review and meta-analysis revealed that, following posterior correction surgery of mild to moderate AIS, postoperative respiratory function showed no statistically significant difference compared with the preoperative values. This statement is valid in terms of both %FVC and %FEV, and refers to the minimum 2 years of follow-up. Unfortunately, several studies had to be excluded because they only reported the raw values of respiratory function measurements; nonetheless, given the incremental nature of pulmonary function in adolescents,11-13,27 we believe that it is imperative to focus on relative values to healthy counterparts at each developmental stage, pre- and postoperatively.
Respiratory function is thought to be affected by AIS in various aspects. The critical lung development phase in terms of numbers of alveoli is almost complete by the age of 8 years in most children,28 and the majority of AIS patients would have normal function at the onset of their progressive deformity. However, it has been demonstrated that chest wall deformity can, over time, lead to decreased thoracic volume,29 and more importantly, alter the dynamic mechanical property of inhalation and exhalation, therefore disabling energy-efficient respiration.2 Alteration of chest compliance would lead to a restrictive pattern of lung dysfunction. Suboptimal ventilation-perfusion distribution3 and airway obstruction in severe cases4 could also potentially negatively affect respiratory function.
Surgical intervention is thought to reverse these negative effects, thereby maintain or ideally improve the post-operative respiratory function. For example, Wood et al29 reported a significant increase in chest volume measured by computed tomography scan after scoliosis correction obtained with ISOLA instrumentation and sublaminar wiring.29 On the other hand, surgery does not come without its own side effects: Any insult to the chest wall occurring during anterior approaches to the spine, either through thoracotomy or thoracoscopy and even thoracoplasty with rib resections, can increase the frailty of the chest cage and cause pleural adhesions, and thus be detrimental to the chest compliance.9,10 Hence, posterior surgery has been generally believed to be more beneficial for postoperative respiratory function. Indeed, Lee et al,30 in their recently published systematic review and meta-analysis on the effects of surgical approach on pulmonary function in patients with AIS, have argued that anterior fusion did not result in significant change in respiratory function whereas posterior fusion increased respiratory function. However, it is worth considering that posterior scoliosis correction alone also entails the immobilization of a significant portion of chest cage, and this would stunt the remaining axial growth at the adolescence stage as well. As a result, the incremental effect on respiratory function can be theoretical and practically negligible in many cases.
Despite the moderate heterogeneity identified in the studies selected for inclusion in the present meta-analysis, we can conclude that there was no significant difference in respiratory function after posterior surgical correction of AIS. Demura et al20 attempted to elucidate, in their series of 154 cases, whether fusions including high thoracic spine (T1-3) resulted in lower postoperative pulmonary function than fusions stopping at lower levels. Although their focus was not to identify the postoperative change in each group, they concluded that none of percent predicted values showed significant difference in all groups. They also pointed out that absolute values such as FVC and FEV1 showed significant increase, highlighting the importance of reporting the patient demographics, and the normative values, when discussing surgical outcomes and effects on respiratory function. Other relevant studies conducted on large cohorts of patients include the one from Kim et al,19 which showed significant increase in both absolute and percent-predicted values of FVC and FEV at 2-year post-operatively in a total of 139 AIS patients. Noteworthy, they also demonstrated that thoracic pedicle screw instrumentation was associated with significant clinical improvement. On the other hand, Newton et al21 showed that %FVC and %FEV1 were decreased postoperatively regardless of surgical approach, including posterior spinal fusion, although absolute values slightly increased. This inconsistency of the results may be derived from the heterogeneity of surgical techniques and detailed characteristics of scoliosis curvature such as Lenke types. We determined that the overall strength of evidence as “very low” due to this inconsistency found in the current meta-analysis, which warrants future high-quality evidence with well-controlled design.
There are several limitations in the present systematic review. First, the present study only focused on pre- and postoperative respiratory function in AIS patients, thus we can only speculate that surgical correction will not allow AIS patients to catch up with the normative development. Little is known about the natural history of respiratory function of untreated AIS patients,6 but that impaired function may result in accelerated decline in the long-term.31 This said, the protective effects of surgical correction on early respiratory deterioration should not be underestimated. Second, in order to minimize the heterogeneity of the population included, we decided to focus on studies that discussed posterior scoliosis correction only. However, there are several other factors that were not controlled. For instance, the instrumentation strategy ranged from Harrington segmental spinal instrumentation in the oldest case,9 to all pedicle-screw based spinal fusion in more recent cases.22 The present data did not allow us to investigate the postoperative changes in PFT based on Lenke’s classification discriminating cases by the existence of thoracic curves. The studies used in the meta-analysis mainly included mild to moderate AIS with very mild restrictive ventilatory pattern. Indeed, only a portion of AIS patients had preoperative pulmonary symptoms or evidence of poor functional status on respiratory tests. Although our intention was to prove the minimal change in respiratory function in these cases, it is possible that severe scoliosis patients have experienced the improvement in respiratory function, which was diluted out due to the limited number of the patients included in these studies. Thus, it would be wrong to conclude that all AIS patients will not benefit by corrective surgery. As reported by others, the deterioration of pulmonary function mainly depends on the age of onset, Cobb angle, and location of the apex vertebra.32 A stratification of patients would therefore be warranted to clarify how much improvement patients with severe scoliosis could aim for after surgical correction. Preoperative physical exercise and post-operative physiotherapy, which would be relevant for a comprehensive understanding of this topic, were not described in many surgical series. Third, the endpoints for postoperative follow-up and assessment of respiratory function were considerably diverse. We included only studies with minimum 2-year follow-up, but a few studies presented very long-term results, up to 10 years of postoperative follow-up.22-25 It is therefore difficult to identify progressive changes in pulmonary function over time, and this confirms the need for additional studies investigating this research question. Fourth, we have to mention that conventional PFT parameters such as FVC and FEV may not sufficiently reflect the actual patient respiratory function: For example, even with normal or nearly normal PFT results, it has been shown that AIS patients sometimes suffer from decreased exercise tolerance, which is not readily assessed by conventional PFT.18,33 Finally, we focused on percent-predicted values of FVC and FEV, instead of their raw measurements, in the present study. This enabled us to discuss postoperative change in respiratory function against normal development observed in adolescents. One limitation associated with these predicted values is that they were based on patients’ height as well as age and gender.34 Patients with AIS are likely to have slightly shortened axial height in proportion to their truncal growth, given the fact that these patients obtain immediate gain in height after corrective surgery.35 Therefore, %FVC and %FEV in patients with AIS can be overestimated. Unfortunately, none of the studies we investigated have discussed the necessity of this adjustment, with only 1 study having managed to mention the average height in the cohort.18
Conclusion
In conclusion, the present systematic review and meta-analysis revealed that there was no statistically significant improvement in either %FVC or %FEV1 postoperatively after posterior surgical correction of mild to moderate AIS. Given the heterogeneity of the results currently available in the literature, our systematic review suggests that future well designed studies, investigating large, homogeneous cohorts of patients, treated with similar surgical techniques, and followed up for 5 to 10 years with standardized outcome measurement methodology, are warranted to elucidate the long-term respiratory function outcomes after surgical correction of AIS.
Acknowledgments
The authors would like to thank Heather Cunningham, MSc, MLIS, Information Specialist, Biological & Health Sciences, Gerstein Science Information Centre, University of Toronto, for assistance with literature screening process.
Appendix A
MEDLINE (Ovid) Search Strategy
Scoliosis/
scoliosis.mp.
1 or 2
Respiration/
Respiratory Insufficiency/
Lung/
exp Respiratory Function Tests/
respiratory.mp.
lung.mp.
pulmonary.mp.
4 or 5 or 6 or 7 or 8 or 9 or 10
3 and 11
limit 12 to english language
remove duplicates from 13
Appendix B
EMBASE (Ovid) Search Strategy
exp scoliosis/
scoliosis.mp.
1 or 2
exp respiratory function/
exp lung function/
exp lung function test/
total lung capacity/
lung/
respiratory system/
pulmonary.mp.
lung.mp.
respiratory.mp.
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
3 and 13
limit 14 to (human and english language)
remove duplicates from 15
Appendix C
CINAHL (EBSCO) Search Strategy
S10 S4 OR S5 OR S6 OR S7 OR S8 OR S9
S9 respiratory
S8 lung
S7 pulmonary
S6 (MH “Lung”)
S5 (MH “Respiration+”)
S4 (MH “Respiratory Function Tests+”)
S3 S1 OR S2
S2 scoliosis
S1 (MH “Scoliosis+”)
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Agabegi SS, Kazemi N, Sturm PF, Mehlman CT. Natural history of adolescent idiopathic scoliosis in skeletally mature patients: a critical review. J Am Acad Orthop Surg. 2015;23:714–723. [DOI] [PubMed] [Google Scholar]
- 2. Cooper DM, Rojas JV, Mellins RB, Keim HA, Mansell AL. Respiratory mechanics in adolescents with idiopathic scoliosis. Am Rev Respir Dis. 1984;130:16–22. [DOI] [PubMed] [Google Scholar]
- 3. Fujii T, Watanabe K, Toyama Y, Matsumoto M. Pulmonary function recovery demonstrated by ventilation-perfusion scan after posterior vertebral column resection for severe adolescent idiopathic scoliosis: a case report. Spine (Phila Pa 1976). 2014;39:E1190–E1194. [DOI] [PubMed] [Google Scholar]
- 4. Borowitz D, Armstrong D, Cerny F. Relief of central airways obstruction following spinal release in a patient with idiopathic scoliosis. Pediatr Pulmonol. 2001;31:86–88. [DOI] [PubMed] [Google Scholar]
- 5. Weinstein SL, Zavala DC, Ponseti IV. Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Joint Surg Am. 1981;63:702–712. [PubMed] [Google Scholar]
- 6. Pehrsson K, Bake B, Larsson S, Nachemson A. Lung function in adult idiopathic scoliosis: a 20 year follow up. Thorax. 1991;46:474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gagnon S, Jodoin A, Martin R. Pulmonary function test study and after spinal fusion in young idiopathic scoliosis. Spine (Phila Pa 1976). 1989;14:486–490. [DOI] [PubMed] [Google Scholar]
- 8. Kinnear WJ, Johnston ID. Does Harrington instrumentation improve pulmonary function in adolescents with idiopathic scoliosis? A meta-analysis. Spine (Phila Pa 1976). 1993;18:1556–1559. [PubMed] [Google Scholar]
- 9. Vedantam R, Lenke LG, Bridwell KH, Haas J, Linville DA. A prospective evaluation of pulmonary function in patients with adolescent idiopathic scoliosis relative to the surgical approach used for spinal arthrodesis. Spine (Phila Pa 1976). 2000;25:82–90. [DOI] [PubMed] [Google Scholar]
- 10. Kim YJ, Lenke LG, Bridwell KH, Cheh G, Sides B, Whorton J. Prospective pulmonary function comparison of anterior spinal fusion in adolescent idiopathic scoliosis: thoracotomy versus thoracoabdominal approach. Spine (Phila Pa 1976). 2008;33:1055–1060. [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Dockery DW, Wypij D, et al. Pulmonary function growth velocity in children 6 to 18 years of age. Am Rev Respir Dis. 1993;148(6 pt 1):1502–1508. [DOI] [PubMed] [Google Scholar]
- 12. Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koopman M, Zanen P, Kruitwagen CL, van der Ent CK, Arets HG. Reference values for paediatric pulmonary function testing: the Utrecht dataset. Respir Med. 2011;105:15–23. [DOI] [PubMed] [Google Scholar]
- 14. Griner PF, Mayewski RJ, Mushlin AI, Greenland P. Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann Intern Med. 1981;94(4 pt 2):557–592. [PubMed] [Google Scholar]
- 15. Lefebvre C, Manheimer E, Glanville J; Cochrane Information Retrieval Methods Group. Searching for studies In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, England: The Cochrane Collaboration; 2011. [Google Scholar]
- 16. Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. J Bone Joint Surg Am. 2015;97:1–2. [DOI] [PubMed] [Google Scholar]
- 17. Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lenke LG, White DK, Kemp JS, Bridwell KH, Blanke KM, Engsberg JR. Evaluation of ventilatory efficiency during exercise in patients with idiopathic scoliosis undergoing spinal fusion. Spine (Phila Pa 1976). 2002;27:2041–2045. [DOI] [PubMed] [Google Scholar]
- 19. Kim YJ, Lenke LG, Bridwell KH, Cheh G, Whorton J, Sides B. Prospective pulmonary function comparison following posterior segmental spinal instrumentation and fusion of adolescent idiopathic scoliosis: is there a relationship between major thoracic curve correction and pulmonary function test improvement? Spine (Phila Pa 1976). 2007;32:2685–2693. [DOI] [PubMed] [Google Scholar]
- 20. Demura S, Bastrom TP, Schlechter J, Yaszay B, Newton PO. Should postoperative pulmonary function be a criterion that affects upper instrumented vertebra selection in adolescent idiopathic scoliosis surgery? Spine (Phila Pa 1976). 2013;38:1920–1926. [DOI] [PubMed] [Google Scholar]
- 21. Newton PO, Marks MC, Bastrom TP, et al. Harms Study Group. Surgical treatment of lenke 1 main thoracic idiopathic scoliosis: results of a prospective, multicenter study. Spine (Phila Pa 1976). 2013;38:328–338. [DOI] [PubMed] [Google Scholar]
- 22. Min K, Sdzuy C, Farshad M. Posterior correction of thoracic adolescent idiopathic scoliosis with pedicle screw instrumentation: results of 48 patients with minimal 10-year follow-up. Eur Spine J. 2013;22:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suk SI, Kim JH, Kim SS, Lee JJ, Han YT. Thoracoplasty in thoracic adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2008;33:1061–1067. [DOI] [PubMed] [Google Scholar]
- 24. Greggi T, Bakaloudis G, Fusaro I, et al. Pulmonary function after thoracoplasty in the surgical treatment of adolescent idiopathic scoliosis. J Spinal Disord Tech. 2010;23:e63–e69. [DOI] [PubMed] [Google Scholar]
- 25. Gitelman Y, Lenke LG, Bridwell KH, Auerbach JD, Sides BA. Pulmonary function in adolescent idiopathic scoliosis relative to the surgical procedure: a 10-year follow-up analysis. Spine (Phila Pa 1976). 2011;36:1665–1672. [DOI] [PubMed] [Google Scholar]
- 26. Verma K, Lonner BS, Kean KE, Dean LE, Valdevit A. Maximal pulmonary recovery after spinal fusion for adolescent idiopathic scoliosis: how do anterior approaches compare? Spine (Phila Pa 1976). 2011;36:1086–1095. [DOI] [PubMed] [Google Scholar]
- 27. Piccioni P, Tassinari R, Carosso A, Carena C, Bugiani M, Bono R. Lung function changes from childhood to adolescence: a seven-year follow-up study. BMC Pulm Med. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gillingham BL, Fan RA, Akbarnia BA. Early onset idiopathic scoliosis. J Am Acad Orthop Surg. 2006;14:101–112. [DOI] [PubMed] [Google Scholar]
- 29. Wood KB, Schendel MJ, Dekutoski MB, Boachie-Adjei O, Heithoff KH. Thoracic volume changes in scoliosis surgery. Spine (Phila Pa 1976). 1996;21:718–723. [DOI] [PubMed] [Google Scholar]
- 30. Lee ACH, Feger MA, Singla A, Abel MF. Effect of surgical approach on pulmonary function in adolescent idiopathic scoliosis patients a systemic review and meta-analysis. Spine (Phila Pa 1976). 2016;41:E1343–E1355. [DOI] [PubMed] [Google Scholar]
- 31. Gibson AM, Doyle LW. Respiratory outcomes for the tiniest or most immature infants. Semin Fetal Neonatal Med. 2014;19:105–111. [DOI] [PubMed] [Google Scholar]
- 32. Johnston CE, Richards BS, Sucato DJ, et al. Correlation of preoperative deformity magnitude and pulmonary function tests in adolescent idiopathic scoliosis. Spine. 2011;36:1096–1102. [DOI] [PubMed] [Google Scholar]
- 33. Barrios C, Perez-Encinas C, Maruenda JI, Laguia M. Significant ventilatory functional restriction in adolescents with mild or moderate scoliosis during maximal exercise tolerance test. Spine (Phila Pa 1976). 2005;30:1610–1615. [DOI] [PubMed] [Google Scholar]
- 34. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 35. van Popta D, Stephenson J, Verma R. Change in spinal height following correction of adolescent idiopathic scoliosis. Spine J. 2016;16:199–203. [DOI] [PubMed] [Google Scholar]