Abstract
Background: Self-rated health (SRH) and the surprise question (SQ) capture perceptions of health and are independent risk factors for poor outcomes. Little is known about their association with physiologic and functional decline.
Objective: Determine the association of SRH and SQ with frailty and functional status in older adults with chronic kidney disease (CKD) and their utility as screening tools.
Design: Prospective cohort study.
Setting/Subjects: Two hundred seventy-two adults, age ≥60 years, with advanced CKD seen in nephrology clinic.
Measurements: Patients completed SRH and were evaluated for frailty (Fried criteria and Clinical Frailty Scale [CFS]) and functional status (Katz and Lawton indices of activities of daily living [ADLs] and instrumental ADLs [iADLs]). Providers completed the SQ. Correlations were evaluated using Spearman's rho.
Results: Fifteen percent of patients were frail, 8% had ≥1 ADL deficit, and 29% had ≥1 iADL deficit. SRH and SQ were moderately correlated with frailty and iADLs. A SRH of excellent, very good, or good was predictive of nonfrail status (Fried negative predictive value [NPV]: 0.92; CFS NPV: 0.92) and preserved ADL function (NPV for ≥1 deficit: 0.96). A SQ response of 5, 4, or 3 (i.e., surprised) was predictive of nonfrail status and preserved ADL function (CFS NPV: 0.90; ADL ≥1 deficit NPV: 0.95). A SQ response of 1 or 2 had a positive predictive value of 0.64 for ≥1 iADL deficit.
Conclusions: Subjective health measures may be useful screening tools for frailty and functional status.
Keywords: activities of daily living, chronic, diagnostic self-evaluation, disability evaluation, frailty, prospective studies, renal insufficiency
Introduction
Chronic kidney disease (CKD) is common in older adults,1 is associated with frailty and functional limitations,2,3 and has a heterogenous natural history.4,5 Patients and providers struggle with uncertainty regarding CKD course and quality of life when anticipating health trajectory and preparing for future disability, long-term placement, or treatments like dialysis.6–9 As countries worldwide face aging populations with increasing chronic disease burdens, simple tools to identify patients who would benefit from careful geriatric assessments, rehabilitation, and advance care planning discussions are necessary.
Subjective health assessments are simple efficient measures that capture patients' and providers' perceptions of health10–16 and are independent risk factors for poor outcomes in the general population and in patients with CKD.10–12,14,17–20 However, the relationship between subjective health assessments and common geriatric conditions, including frailty and disability in activities of daily living (ADLs) or instrumental ADLs (iADLs), has not been well studied.
One tool for assessing a patient's subjective health is self-rated health (SRH), a single item question: “In general, would you say your health is: excellent, very good, good, fair, or poor.” One provider-based subjective health measure is the surprise question (SQ): “Would you be surprised if this patient died in the next 12 months?” Both measures have been found to be valid and reliable in the CKD population through association with key outcomes such as mortality and kidney disease progression.15–18
Objective
To investigate the association between subjective health assessments and Fried frailty phenotype, clinical frailty, and measures of functional status (i.e., ADLs, iADLs) in older adults with advanced CKD and to assess their performance as screening tools for frailty and functional status.
Methods
Study design, setting, and participants
As part of a larger prospective cohort study,21 we enrolled adults aged 60 years or older with nondialysis dependent CKD stages 4 to 5 being followed by a provider at an academic nephrology clinic and conducted subjective health assessments, Fried frailty measures, clinical frailty assessments, and ADL/iADL assessments. Exclusion criteria were dialysis dependence, history of kidney transplantation, initial visit with the provider, or recent acute kidney injury. Because the study included a provider-based subjective health measure, we excluded visits where providers evaluated a patient for the first time to allow greater familiarity. Providers included 12 attending physicians and 1 nurse practitioner.
From November 2016 through January 2018, 293 patients were approached and 277 consented (with 16 [5%] declining to participate). Six patients were missing a subjective health assessment, leaving 271 patients in the current analysis. The study was approved by the Vanderbilt University Medical Center Institutional Review Board (No. 161523) and adhered to the principles of the Declaration of Helsinki.
Subjective health measures
Patients completed the SRH assessment (“In general, would you say your health is: excellent, very good, good, fair, or poor”) item during their enrollment visit. SRH has been shown to have excellent reliability and validity in the general population10–12,14,16,19,20 and predicts adverse outcomes in CKD.17
Providers' answered the SQ (“Would you be surprised if this patient died within the next 12 months?”) immediately following their clinic visit. Responses included a binary (i.e., “yes” or “no”) and five-point Likert scale (i.e., very surprised to not at all surprised). The SQ has been shown to predict mortality in CKD and end-stage renal disease22–24 and has shown adequate reliability in advanced CKD.18 For this study, we a priori chose to use providers' Likert scale responses for the SQ since these responses take advantage of providers' demonstrated ability to rank order patients according to prognosis (even when their estimated survival times are generally inaccurate),25 provided enhanced specificity to the SQ in our prior evaluation,18 and allowed additional granularity when evaluating rank order correlations than a binary tool.
Frailty phenotype
We used the Fried frailty phenotype26: grip strength, walk speed, physical exhaustion, involuntary weight loss, and low physical activity. A patient with ≥3 criteria was considered frail and 1–2 criteria was considered prefrail (see Supplementary Data for further details). In determining the correlation with other measures, we scored Fried frailty using a 5-point ordinal scale (i.e., 1 point for each criterion). The Fried frailty phenotype has been associated with death, hospitalizations, and disability.27
Clinical frailty
We used the Clinical Frailty Scale (CFS), a validated measure of clinical frailty, that consists of a single-item ordinal scale ranging from 1 to 9 with scores ≥5 indicating the presence of frailty.28,29 Trained research personnel interviewed the patient and reviewed the electronic health record (EHR) to assess clinical frailty. In determining the correlation with other measures, the full range of scores was used.
Functional status
We measured ADLs and iADLs using the Katz and Lawton indices, respectively.30,31 Scores on the Katz excluded continence with an index range from 0 to 5, while Lawton index scores range from 0 to 8. Higher scores indicate greater independence for both scales.
Covariates
As part of their initial assessment, patients completed a medical history questionnaire. Members of the research team also performed manual review of the EHR using standardized chart abstraction forms to supplement this information. We used these data to define comorbidities and to calculate the Charlson comorbidity index.32
Data analysis
Relationships between trinary SRH and trinary SQ with frailty and functional status categories were assessed using Pearson's chi-squared test. Rank correlations between SRH, SQ (Likert scale), Fried Frailty, CFS, ADLs, and iADLs were determined using Spearman's Rank correlation. Because the variables of interest were ordinal and the estimates of their rank correlations could be effected by the frequency of ties, correlations were also examined by Goodman and Kruskal's Gamma.33–35 When determining correlation coefficients, the SQ and Katz and Lawton indices were reverse coded so that higher scores represented poorer health or function in all variables of interest. Separate proportional odds models were also used to test for interactions between the variables of interest and age, gender, presence of diabetes or cardiovascular disease, and Charlson comorbidity index score. Age was included in the models as a nonlinear term with three knots.
To assess test characteristics of the subjective health assessments, we set an SRH threshold of excellent, very good, or good and a SQ threshold of 5, 4, or 3 (i.e., surprised to ambivalent) to calculate the sensitivity, specificity, and negative and positive predictive values (given the sample prevalence) for Fried frailty, clinical frailty, ≥1 ADL deficiency, and ≥1 iADL deficiency. The binomial exact method was used to calculate the 95% confidence intervals. p-Values less than 0.05 were considered statistically significant, and all analyses were performed using R (version 3.4.4).36
Results
Participants had a median age of 71 years (25th, 75th percentile: 66, 77), 81% were white, 46% were female, 49% had diabetes, and 43% had cardiovascular disease. Participants had a median Charlson comorbidity score of 5 (25th, 75th percentile: 3, 7) (Table 1 and Supplementary Table S1). Patients reported their health as poor, fair, good, very good, and excellent in 23 (8%), 105 (39%), 100 (37%), 40 (15%), and 3 (1%) instances, respectively. Providers responded 1 (not at all surprised), 2, 3, 4, and 5 (very surprised) for 10 (4%), 40 (15%), 50 (18%), 85 (31%), and 86 (32%) patients, respectively (Supplementary Table S2). Patients who rated their health as “poor” or “fair” tended to be more likely to identify as female, have diabetes, and have a modestly higher Charlson comorbidity score, and they were less likely to have graduated college.
Table 1.
Baseline Characteristics by Trinary Self-Rated Health Response
| SRH | ||||
|---|---|---|---|---|
| Total cohort (N = 271) | Excellent or very good (N = 43) | Good (N = 100) | Fair or poor (N = 128) | |
| Age | 71.0 [66.0, 77.0] | 72.0 [68.0, 77.5] | 71.0 [66.0, 76.0] | 70.5 [65.0, 78.0] |
| Female | 126 (46%) | 11 (26%) | 43 (43%) | 72 (56%) |
| Race | ||||
| White/other | 223 (82%) | 35 (86%) | 79 (81%) | 105 (82%) |
| Black | 48 (18%) | 6 (14%) | 19 (19%) | 23 (18%) |
| Marital status | ||||
| Divorced | 33 (12%) | 3 (7%) | 14 (14%) | 16 (12%) |
| Married | 173 (64%) | 30 (70%) | 57 (57%) | 86 (67%) |
| Single/other | 18 (7%) | 2 (5%) | 10 (10%) | 6 (5%) |
| Widow/widower | 47 (17%) | 8 (19%) | 19 (19%) | 20 (16%) |
| Education | ||||
| Less than 12th grade | 29 (11%) | 3 (7%) | 7 (7%) | 19 (15%) |
| 12th grade | 68 (25%) | 7 (16%) | 24 (24%) | 37 (29%) |
| Some college | 60 (22%) | 5 (12%) | 23 (23%) | 32 (25%) |
| College degree or higher | 114 (42%) | 28 (65%) | 46 (46%) | 40 (31%) |
| Incomea | ||||
| Less than $20,000 | 42 (15%) | 3 (7%) | 9 (9%) | 30 (23%) |
| $20,000 to $39,999 | 69 (25%) | 8 (19%) | 28 (28%) | 33 (26%) |
| $40,000 to $59,999 | 51 (19%) | 9 (21%) | 24 (24%) | 18 (14%) |
| $60,000 to $79,999 | 43 (16%) | 11 (26%) | 12 (12%) | 20 (16%) |
| $80,000 to $99,999 | 16 (6%) | 3 (7%) | 8 (8%) | 5 (4%) |
| $100,000 or above | 46 (17%) | 8 (19%) | 17 (17%) | 21 (16%) |
| Insurance | ||||
| Private | 77 (28%) | 14 (33%) | 30 (30%) | 33 (26%) |
| Medicaid | 12 (4%) | 0 (0%) | 3 (3%) | 9 (7%) |
| Medicare | 182 (67%) | 29 (67%) | 67 (67%) | 86 (67%) |
| Hypertension | 265 (98%) | 42 (98%) | 97 (97%) | 126 (98%) |
| Diabetes | 133 (49%) | 13 (30%) | 47 (47%) | 73 (57%) |
| Cardiovascular disease | 117 (43%) | 12 (28%) | 45 (45%) | 60 (47%) |
| Heart failure | 65 (24%) | 4 (9%) | 28 (28%) | 33 (26%) |
| Chronic lung disease | 36 (13%) | 5 (12%) | 9 (9%) | 22 (17%) |
| Malignancy | 68 (25%) | 12 (28%) | 26 (26%) | 30 (23%) |
| CCI | 5 [3, 6] | 4 [2, 5] | 5 [3, 6] | 5 [4, 6] |
| CCI | ||||
| [2–4] | 126 (46%) | 29 (67%) | 48 (48%) | 49 (38%) |
| [5–12] | 145 (54%) | 14 (33%) | 52 (52%) | 79 (62%) |
| BMIb | 30 [26, 35] | 29 [25, 33] | 30 [26, 35] | 31 [26, 37] |
| eGFRc | 23 [17, 28] | 25 [17, 30] | 22 [18, 28] | 22 [16, 28] |
Continuous variables expressed as median (25% percentile, 75% percentile); categorical variables expressed as n (%). Patient characteristics grouped into trinary responses for parsimony and due to limited responses at the extremes.
Four patients declined to answer.
One patient with no BMI measurement.
Calculated using the Modification of Diet in Renal Disease equation.
ADL, activities of daily living; BMI, body mass index; CCI, Charlson comorbidity index; eGFR, estimated glomerular filtration rate; iADL, instrumental ADL.
Associations of SRH and SQ with frailty and functional status
About 15% (n = 40) and 15% (n = 41) of patients were frail by the Fried criteria and CFS score, respectively. Nearly three quarters (73%, n = 197) of patients were prefrail or frail by the Fried criteria. About 8% (n = 21) and 29% (n = 79) of patients had at least 1 ADL or iADL deficit, respectively.
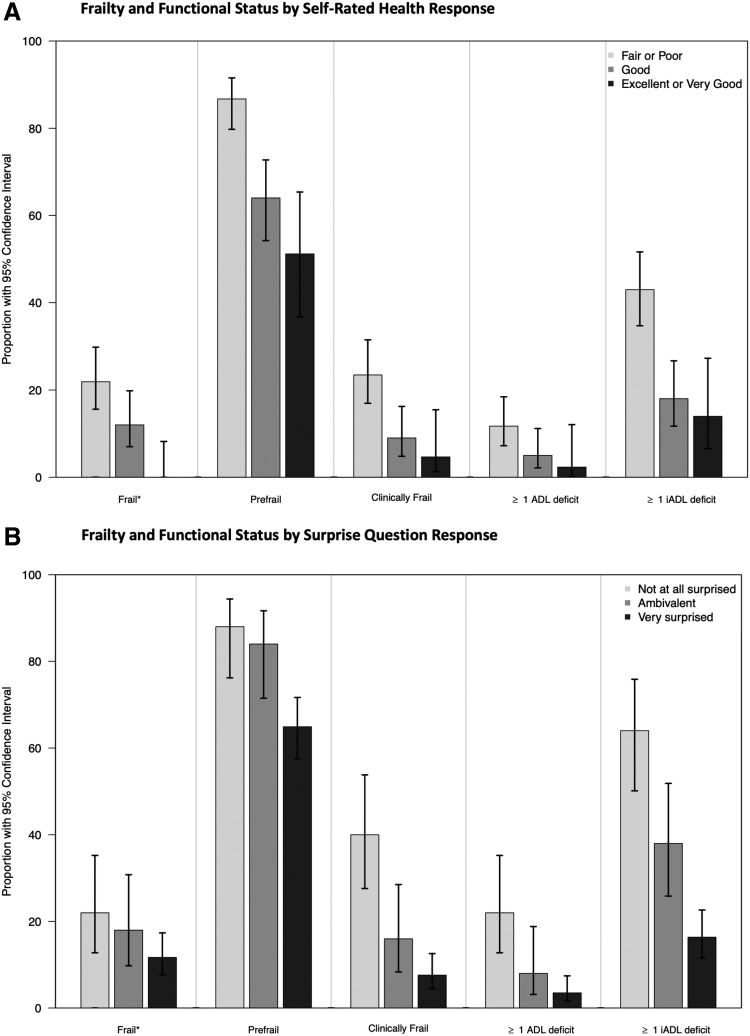
We observed significant associations between SRH and frailty (by Fried criteria), prefrail status (by Fried criteria), clinical frailty (by CFS), ADL scores, and iADL scores (Fig. 1a). Similarly, we observed significant associations between SQ and prefrail status, clinical frailty, ADL scores, and iADL scores (Fig. 1b).
FIG. 1.
(A) Self-rated health response is associated with frailty and functional status, (B) Surprise question response is associated with prefrailty, clinical frailty, and functional status. *Frail or prefrail determined using Fried criteria scores ≥3 or ≥1, respectively. Clinical frailty determined using Clinical Frailty Scale score ≥5. ADL, activity of daily living; iADL, instrumental ADL.
Spearman correlations between SRH and Fried frailty score, CFS score, and iADL score were fair to moderate (ranging from 0.33 to 0.45) (Table 2). Similarly, Spearman correlations between the SQ Likert scale and Fried frailty score, CFS score, and iADL score were fair to moderate (ranging from 0.31 to 0.45) (Table 2). ADLs were weakly correlated with both SRH and the SQ with Spearman Rho values of 0.16 (95% CI 0.04–0.26) and 0.23 (95% CI 0.11–0.36), respectively (Table 2). Correlations assessed using Goodman and Kruskal's Gamma to account for ties in ordinal ranking showed similar or stronger correlations (Supplementary Table S3). When testing for interactions, we found that only the relationship between the SQ and CFS had significant interaction terms (see Results section in Supplementary Data and Supplementary Fig. S1).
Table 2.
Correlation between Subjective Health Measures, Frailty, and Functional Status
| Fried frailty | Clinical Frailty Scale | ADL | iADL | SRH | |
|---|---|---|---|---|---|
| SRH | 0.43 (0.32–0.52) | 0.45 (0.35–0.54) | 0.16 (0.04–0.26) | 0.33 (0.23–0.44) | — |
| SQ | 0.31 (0.20–0.42) | 0.45 (0.40–0.58) | 0.23 (0.11–0.36) | 0.40 (0.29–0.50) | 0.29 (0.18–0.40) |
Correlations are presented as Spearman's rho with 95% confidence intervals.
SQ, surprise question; SRH, self-rated health.
Correlation of patient and provider-based subjective health assessments
SRH and the SQ were correlated with each other (rho 0.29; 95% CI 0.18–0.40), and we did not find evidence of significant interaction terms for this association (see also Results section in Supplementary Data).
Test characteristics
When using a SRH response of “Poor” or “Fair” as a positive test, we found negative predictive values ranging from 0.83 to 0.96 for frailty and impairments in ADLs and iADLs (Table 3). Similarly, a SQ Likert response of not surprised (using the 5-point Likert scale) had negative predictive values ranging from 0.87 to 0.95 for frailty or impairments in ADLs (Table 3). In general, SRH appeared more sensitive (ranging from 0.56 to 0.73) in screening for frailty and functional impairments, while the SQ was more specific (ranging from 0.83 to 0.91). Notably, a SQ score of 1 or 2 had a positive predictive value for ≥1 iADL deficit of 0.64 (95% CI 0.49–0.77) in a sample with a prevalence rate for iADL deficits of 29%.
Table 3.
Test Characteristics for Self-Rated Health Value of “Poor” or “Fair” and Surprise Question Value of “Not Surprised”*
| SRH: poor or fair | Fried frailty | Fried prefrailty | Clinical Frailty Score | ADL impairment | iADL impairment |
|---|---|---|---|---|---|
| Sensitivity | 0.70 (0.53–0.83) | 0.56 (0.49–0.63) | 0.73 (0.57–0.86) | 0.71 (0.48–0.89) | 0.70 (0.58–0.79) |
| Specificity | 0.57 (0.50–0.63) | 0.77 (0.66–0.86) | 0.57 (0.51–0.64) | 0.55 (0.48–0.61) | 0.62 (0.55–0.69) |
| PPV | 0.22 (0.15–0.30) | 0.87 (0.80–0.92) | 0.23 (0.16–0.32) | 0.12 (0.07–0.19) | 0.43 (0.34–0.52) |
| NPV | 0.92 (0.86–0.96) | 0.40 (0.32–0.48) | 0.92 (0.87–0.96) | 0.96 (0.91–0.98) | 0.83 (0.76–0.89) |
| SQ: not surprised | Fried frailty | Fried prefrailty | Clinical Frailty Score | ADL impairment | iADL impairment |
|---|---|---|---|---|---|
| Sensitivity | 0.28 (0.15–0.44) | 0.22 (0.17–0.29) | 0.49 (0.33–0.65) | 0.52 (0.30–0.74) | 0.41 (0.30–0.52) |
| Specificity | 0.83 (0.78–0.88) | 0.92 (0.83–0.97) | 0.87 (0.82–0.91) | 0.84 (0.79–0.89) | 0.91 (0.86–0.94) |
| PPV | 0.22 (0.12–0.36) | 0.88 (0.76–0.95) | 0.40 (0.26–0.55) | 0.22 (0.12–0.36) | 0.64 (0.49–0.77) |
| NPV | 0.87 (0.82–0.91) | 0.31 (0.25–0.37) | 0.90 (0.86–0.94) | 0.95 (0.92–0.98) | 0.79 (0.73–0.84) |
Patients were determined to be frail or prefrail by Fried criteria with scores ≥3 or ≥1, respectively, and clinically frail by Clinical Frailty Scale scores ≥5. Prevalence rates: Fried frailty: 15%, Fried prefrailty or frailty: 73%, clinical frailty: 15%, ≥1 ADL impairment: 8%, ≥1 iADL impairment: 29%
Table values represent proportions.
NPV, negative predictive value; PPV, positive predictive value.
Discussion
Findings
We found that in older adults with advanced CKD, both patient and provider subjective health assessments were moderately correlated with Fried frailty score, CFS score, and measures of functional status. We also found that SRH and the SQ demonstrated a fair correlation with each other. These findings were generally consistent regardless of age, the presence of diabetes or cardiovascular disease, or overall comorbidity burden. In addition, we found that SRH was generally more sensitive, while the SQ was more specific when testing for Fried frailty, clinical frailty, and ADL/iADL disabilities. Our study provides further evidence for the criterion validity of SRH and the SQ as measures of overall health status in patients with advanced CKD. It also suggests that patient and provider subjective health assessments should be further evaluated as a clinical strategy to identify patients with advanced CKD who could benefit from advance care planning.
Potential for clinical integration
Older patients with advanced CKD are the fastest growing dialysis subgroup,37 and for some, the burdens of dialysis outweigh the benefits.38–41 Many older patients who initiate dialysis experience high rates of disability, mortality, and declines in life satisfaction.40–42 Similarly, many advanced CKD patients who are hospitalized undergo aggressive and invasive procedures that are not consistent with their stated health care preferences.43,44 Furthermore, the care delivered to older CKD patients near the end of life frequently conflicts with their stated preferences.44,45 These findings emphasize the need for providers to engage older patients with advanced CKD in longitudinal shared decision making and advance care planning conversations.46–48 Indeed, many older, advanced CKD patients expect their practitioners to engage in advance care planning.47,49 Our findings suggest that patient- and provider-based subjective health assessments can be used to identify patients who are at higher risk for poor outcomes. Providers should target these patients for thoughtful discussions of the trade-offs of maximal conservative management and dialytic therapies. Similarly, these patients are likely to benefit from longitudinal advance care planning conversations, where treatment preferences and goals of care are carefully considered, and a health care proxy is identified.
Anticipating health trajectory
Clinicians and researchers could benefit from pragmatic measures that help capture the risk of disability and death in patients with chronic illnesses. For older patients with CKD, all-cause mortality is 10- to 13-fold more likely end point than end-stage renal disease.8,50 For patients ≥80 years, the relative likelihood of long-term placement compared to developing dialysis dependence reaches 30-fold.8 However, identifying patients at increased risk for disability and nursing home residence has garnered less research attention than understanding risk factors for kidney disease progression.51,52 Pragmatic and efficient methods to identify patients at increased risk for future disability and long-term institutionalization may facilitate efforts to uncover modifiable and nonmodifiable risk factors that contribute to these outcomes. Our study provides evidence that subjective health measures are associated with geriatric syndromes and could serve a useful role in risk stratifying older populations for adverse outcomes beyond mortality. Longitudinal research will need to assess how strongly SRH and the SQ associate with outcomes like disability and institutionalization and how well they track with health trajectories of functional status.
Implications for frailty assessment
Frailty rates are high in the CKD population, and multiple studies have shown frailty to be an independent risk factor for death and other poor outcomes.27,53–55 While frailty measures have been used to monitor health status in CKD,56 widespread implementation of frailty assessments in this setting and other chronic disease settings is limited, likely related to feasibility concerns.57–59 Our findings that patients who reported SRH as excellent, very good, or good were unlikely to be frail or have functional deficits suggest that this can be a simple screening tool for frailty in patients with advanced CKD. This approach may be useful in clinical settings where more thorough evaluations (e.g., comprehensive geriatric assessments) can be targeted, thereby reducing the associated resource burden. Similarly, we found that the SQ (Likert-scale response “not surprised”) had a positive predictive value for ≥1 iADL deficit of 64%. This suggests that the SQ Likert response may be helpful in identifying patients with higher order disabilities. Future studies should examine pairing the SQ with geriatric assessments, exploration of support services available in the patient's current home environment, and advance care planning.
Implications for subjective health measures
While one prior study showed fair correlation between self and provider rated health in the general population,60 we are not aware of studies that have examined the relationship between patient SRH and provider SQ assessments. The fair correlation of these measures is not surprising as they arguably assess related although distinct concepts, general health, and the perceived risk of near-term death.18,45 Notably, in patients whose providers responded “No” to the SQ, the correlation between SRH and the SQ was not changed. Unfortunately, the previously documented low rates of advance care planning conversations in our study setting precluded a meaningful assessment of the impact of advance care planning conversations on the correlation of patient and provider subjective health measures.45 Future research should examine whether patients who have had advance care planning discussions with their providers have a higher correlation between patient and provider subjective health assessments.
Strengths and weaknesses
Our study had several strengths. First, our study measures have previously demonstrated predictive validity in the CKD population. Second, our study focused on older adults with advanced CKD. Patients within this population have substantial variability in health trajectory, and new tools need to be explored to best stratify this population. Third, 95% of the patients we approached to enroll in this study consented, reducing selection bias. Finally, the consistent association of subjective health assessments across multiple measures related to aging and physical function supports our findings.
We also acknowledge several weaknesses. First, while patient age, diabetes, and comorbidity did not modify most of the associations we observed, we were underpowered to detect these effects. Similarly, the correlation of the SQ and SRH may demonstrate significant heterogeneity in other populations and settings with disparate educational attainment and racial backgrounds. Second, our population was recruited from a nephrology clinic at an Academic Medical Center. Our findings may not be generalizable to settings where the educational attainment is lower and the patient case mix or provider characteristics are different. Finally, our study was cross-sectional and did not assess whether subjective health measures were associated with dialysis initiation or institutionalization. However, we previously showed that the SQ associates with mortality in the same environment.18,45
Conclusion
In conclusion, SRH and the SQ are associated with frailty and disability and may be helpful in screening patients for these conditions. Further studies should explore their usefulness as screening tools in clinical research and routine practice, as well as understanding how these measures associate with patients' clinical course.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Stevens LA, Li S, Wang C, et al. : Prevalence of CKD and comorbid illness in elderly patients in the United States: Results From the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2010;55:S23–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fried LF, Jung SL, Shlipak M, et al. : Chronic kidney disease and functional limitation in older people: Health, aging and body composition study. J Am Geriatr Soc 2006;54:750–756 [DOI] [PubMed] [Google Scholar]
- 3. Walker SR, Gill K, Macdonald K, et al. : Association of frailty and physical function in patients with non-dialysis CKD: A systematic review. BMC Nephrol 2013;14:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan D, et al. : Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 5. Hall YN, Himmelfarb J: The CKD classification system in the precision medicine era. Clin J Am Soc Nephrol 2017;12:346–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schell JO, Patel UD, Steinhauser KE, et al. : Discussions of the kidney disease trajectory by elderly patients and nephrologists: A qualitative study. Am J Kidney Dis 2012;59:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdel-Kader K, Unruh ML, Weisbord SD: Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 2009;4:1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tonelli M, Wiebe N, James MT, et al. : A population-based cohort study defines prognoses in severe chronic kidney disease. Kidney Int 2018;93:1217–1226 [DOI] [PubMed] [Google Scholar]
- 9. Turin TC, Tonelli M, Manns BJ, et al. : Lifetime risk of ESRD. J Am Soc Nephrol 2012;23:1569–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mossey JM, Shapiro E: Self-rated health: A predictor of mortality among the elderly. Am J Public Health 1982;72:800–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Idler EI, Benyamini Y: Self-rated health and mortality: A review of twenty-seven community studies. J Health Soc Behav 1997;38:21–37 [PubMed] [Google Scholar]
- 12. Desalvo KB, Bloser N, Reynolds K, et al. : Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med 2006;21:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jylhä M: What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med 2009;69:307–316 [DOI] [PubMed] [Google Scholar]
- 14. Weinberger M, Darnell JC, Tierney WM, et al. : Self-rated health as a predictor of hospital admission and nursing home placement in elderly public housing tenants. Am J Public Health 1986;76:457–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thong MSY, Kaptein AA, Benyamini Y, et al. : Association between a self-rated health question and mortality in young and old dialysis patients: A cohort study. Am J Kidney Dis 2008;52:111–117 [DOI] [PubMed] [Google Scholar]
- 16. Zajacova A, Dowd JB: Reliability of self-rated health in US Adults. Am J Epidemiol 2011;174:977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robinson-Cohen C, Hall YN, Katz R, et al. : Self-rated health and adverse events in CKD. Clin J Am Soc Nephrol 2014;9:2044–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Javier AD, Figueroa R, Salat H, et al. : Reliability and utility of the surprise question in CKD stages 4 to 5. Am J Kidney Dis 2017;70:93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaplan GA, Camacho T: Perceived health and mortality: A nine-year follow-up of the human population laboratory cohort. Am J Epidemiol 1983;117:292–304 [DOI] [PubMed] [Google Scholar]
- 20. Idler EL, Angel RJ: Self-rated health and mortality in the NHANES-I epidemiologic follow-up study. Am J Public Health 1990;80:446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramer SJ, McCall NN, Robinson-Cohen C, et al. : Health outcome priorities of older adults with advanced CKD and concordance with their nephrology providers' perceptions. J Am Soc Nephrol 2018;29:2870–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pang WF, Kwan BCH, Chow KM, et al. : Predicting 12-month mortality for peritoneal dialysis patients using the ‘surprise’ question. Perit Dial Int 2013;33:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moss AH, Lunney JR, Culp S, et al. : Prognostic significance of the “Surprise” question in cancer patients. J Palliat Med 2010;13:837–840 [DOI] [PubMed] [Google Scholar]
- 24. Moss AH, Ganjoo J, Sharma S, et al. : Utility of the ‘Surprise’ question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol 2008;3:1379–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glare P, Virik K, Jones M, et al. : A systematic review of physicians' survival predictions in terminally ill cancer patients. BMJ 2003;327:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fried LP, Tangen CM, Walston J, et al. : Frailty in older adults: Evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci 2001;56:M146–M157 [DOI] [PubMed] [Google Scholar]
- 27. Roshanravan B, Khatri M, Robinson-Cohen C, et al. : A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 2012;60:912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rockwood K, Song X, MacKnight C, et al. : A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alfaadhel TA, Soroka SD, Kiberd BA, et al. : Frailty and mortality in dialysis: Evaluation of a clinical frailty scale. Clin J Am Soc Nephrol 2015;10:832–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katz S: Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983;31:721–727 [DOI] [PubMed] [Google Scholar]
- 31. Lawton MP, Brody EM: Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186 [PubMed] [Google Scholar]
- 32. Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 33. Agresti A: Categorical Data Analysis. 2002. [Epub ahead of print]; DOI: 10.1198/tech.2003.s28 [DOI] [Google Scholar]
- 34. Goodman LA, Kruskal WH: Measures of association for cross classifications. J Am Stat Assoc 1954;49:737–764 [Google Scholar]
- 35. Goodman LA, Kruskal WH: Measures of association for cross classifications III: Approximate sampling theory. J Am Stat Assoc 1963;58:310–364 [Google Scholar]
- 36. R Foundation for Statistical Computing: R: A Language and Environment for Statistical Computing. 2013. www.r-project.org (last accessed March15, 2019)
- 37. United States Renal Data System: 2017. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2017 [Google Scholar]
- 38. Kurella M, Covinsky KE, Collins AJ, et al. : Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007;146:177–183 [DOI] [PubMed] [Google Scholar]
- 39. Murtagh FEM, Marsh JE, Donohoe P, et al. : Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007;22:1955–1962 [DOI] [PubMed] [Google Scholar]
- 40. Jassal SV, Watson D: Dialysis in late life: Benefit or burden. Clin J Am Soc Nephrol 2009;4:2008–2012 [DOI] [PubMed] [Google Scholar]
- 41. Da Silva-Gane M, Wellsted D, Greenshields H, et al. : Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol 2012;7:2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kurella Tamura M, Covinsky KE, Chertow GM, et al. : Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009;361:1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Hare AM, Rodriguez RA, Hailpern SM, et al. : Regional variation in health care intensity and treatment practices for end-stage renal disease in older adults. JAMA - J Am Med Assoc 2010;304:180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong SPY, Kreuter W, O'Hare AM: Treatment intensity at the end of life in older adults receiving long-term dialysis. Arch Intern Med 2012;172:661–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salat H, Javier A, Siew ED, et al. : Nephrology provider prognostic perceptions and care delivered to older adults with advanced kidney disease. Clin J Am Soc Nephrol 2017;12:1762–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Golper TA, Martin J: Schreiber J. Issues in Dialysis—The Course of Therapy: Changing the Paradigm. New York: Nova Science Publishers, Inc., 2013 [Google Scholar]
- 47. Davison SN: End-of-life care preferences and needs: Perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010;5:195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Renal Physicians Association: Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. Renal Physicians Association, Rockville, MD, 2010 [Google Scholar]
- 49. Fine A, Fontaine B, Kraushar MM, et al. : Nephrologists should voluntarily divulge survival data to potential dialysis patients: A questionnaire study. Perit Dial Int 2005;25:269–273 [PubMed] [Google Scholar]
- 50. Dalrymple LS, Katz R, Kestenbaum B, et al. : Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med 2010;26:379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tangri N, Kitsios GD, Inker LA, et al. : Risk prediction models for patients with chronic kidney disease. Ann Intern Med 2013;158:596. [DOI] [PubMed] [Google Scholar]
- 52. Tangri N: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011;305:1553. [DOI] [PubMed] [Google Scholar]
- 53. Shlipak MG, Stehman-Breen C, Fried LF, et al. : The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis 2004;43:861–867 [DOI] [PubMed] [Google Scholar]
- 54. Pugh J, Aggett J, Goodland A, et al. : Frailty and comorbidity are independent predictors of outcome in patients referred for pre-dialysis education. Clin Kidney J 2016;9:324–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chowdhury R, Peel NM, Krosch M, et al. : Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr 2017;68:135–142 [DOI] [PubMed] [Google Scholar]
- 56. McAdams-DeMarco MA, Isaacs K, Darko L, et al. : Changes in frailty after kidney transplantation. J Am Geriatr Soc 2015;63:2152–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Walston J, Buta B, Xue QL: Frailty screening and interventions: Considerations for clinical practice. Clin Geriatr Med 2018;34:25–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Drubbel I, Numans ME, Kranenburg G, et al. : Screening for frailty in primary care: A systematic review of the psychometric properties of the frailty index in community-dwelling older people. BMC Geriatr 2014;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee L, Patel T, Costa A, et al. : Screening for frailty in primary care Accuracy of gait speed and hand-grip strength. Can Fam Physician 2017;63:e51–e57 [PMC free article] [PubMed] [Google Scholar]
- 60. Kivinen P, Halonen P, Eronen M, et al. : Self-rated health, physician-rated health and associated factors among elderly men: The Finnish cohorts of the Seven Countries Study. Age Ageing 1998;27:41–47 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.