Abstract
The purpose of this study was to understand the incidence, clinical characteristics and related factors of bronchiectasis in chronic obstructive pulmonary disease (COPD) patients.
From January 2015 to January 2017, 133 patients with moderate to severe COPD admitted to our hospital were enrolled in the study. Bronchiectasis analysis was performed by high resolution CT of the chest, the clinical data of all patients were collected including increasing state of COPD, peripheral blood samples, pulmonary function, blood gas. And sputum samples were collected for detection of microorganisms.
the patients were aged 70.18 ± 8.31 years, and 62.4% of the patients were male. FEV1 accounted for an estimated value of 37.91 ± 10.68%, and 104 (78.2%) were severe COPD, and 43 (32.3%) had bronchiectasis. Bronchiectasis is mainly bilateral, multiple and columnar bronchiectasis. The most easily involved sites are the left lower lobe, left lingual lobe and right middle lobe. Bronchiectasis is associated with history of disease (P = .027), at least one hospitalization exacerbated by COPD in the past year (P = .025), and the separation of potential pathogenic microorganisms from sputum (P = .022). The most commonly isolated pathogen was Pseudomonas aeruginosa (P < .001).
Bronchiectasis should be noted in patients with COPD who often suffer from exacerbation or repeated respiratory infections, especially in those who isolate P aeruginosa from respiratory specimens.
Keywords: bronchiectasis, obstructive, pulmonary disease
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease in developing countries,[1] its morbidity and mortality are increasing year by year,[2,3] seriously affecting the quality of life of patients and placing a huge burden on families and society.[4–6]
Bronchiectasis is a long-term respiratory disease that affects quality of life due to persistent symptoms and repeated infection deterioration.[7] With the wide application of high resolution CT (HRCT), the diagnostic rate of bronchiectasis is greatly improved. Bronchiectasis is more common in moderate to severe COPD.[8–10]
COPD is characterized by persistent airflow restriction, which is associated with chronic abnormal inflammation of respiratory particles and gases.[11,12] Bronchiectasis is not an independent disease. Many diseases that directly or indirectly affect the defensive function of the bronchial wall cause bronchiectasis. Studies have shown that bronchiectasis and chronic obstructive pulmonary disease often occur in men with a long history of smoking. This finding is related to tobacco exposure as one of the most important causes of chronic bronchiolitis, which is considered to be the basic pathogenesis of bronchiectasis.[13–15] The characteristic pathophysiological changes of chronic obstructive pulmonary disease are similar to bronchiectasis. Chronic obstructive pulmonary disease is reported to be associated with bronchiectasis.[8–10,16]
Environmental risk factors and genetic susceptibility are associated with the occurrence of COPD.[17] Paroxysmal cough,[18,19] excessive airway mucus secretion[20,21] are independent risk factors for COPD. Acute exacerbation is an important part of COPD, it accelerates the decline in lung function and increases the risk of death, and is an independent predictor of prognosis for COPD patients.[22,23] bronchiectasis in COPD patients is associated with Pseudomonas aeruginosa,[24] high levels of sputum interleukin-6 (IL-6) and IL-8.[25]
The purpose of this study was to understand the incidence, clinical characteristics and related factors of bronchiectasis in COPD patients, to identify the subgroups of brochiectasis early, and to give appropriate treatment interventions to improve prognosis.
2. Material and methods
2.1. Selected objects
From January 2015 to January 2017, 133 patients with moderate to severe COPD admitted to our hospital were enrolled in the study. COPD is defined as post bronchodilator FEV1/FVC < 70%, and reversibility FEV1 of β2-agonists < 12% and 200 ml; post bronchodilator FEV1 50% ≤ FEV1 <80% is considered to be moderate COPD, FEV1 <50% is considered to be severe COPD. In this study, patients who were diagnosed with bronchiectasis prior to diagnosis of COPD or clinical evidence of bronchiectasis were excluded. Patients diagnosed as bronchiectasis by chest CT before diagnosis of COPD in this study were excluded. This study has been approved by the Ethics Committee of our hospital. All patients or their relatives signed the informed consent.
2.2. Diagnosis of bronchiectasis
All patients underwent high resolution CT chest scan (Siemens 64 slice spiral Definition ASCT SOMA, SIEMENS, Germany) to diagnose the presence of bronchiectasis. HRCT was performed at the end of inspiration (1 mm thick, 10 mm interval) and scanned from apex to bottom of lung. HRCT manifestations of patients with bronchiectasis at admission were as follows:
-
1)
there was no obvious thinning trend in the central bronchial area;
-
2)
bronchial diameter was larger than pulmonary artery diameter;
-
3)
bronchial shadow could be seen at 1 cm of peripheral pleura or mediastinal pleura.[26]
HRCT was independently interpreted by 2 experienced radiologists, and they did not communicate with each other. Small bronchiectasis seen only in one lung segment was ruled out, as it can be seen in many healthy individuals.
2.3. Clinical data, pulmonary function, color Doppler echocardiography, and blood samples
The clinical data collected included general information (age and sex), age at diagnosis of COPD, history of illness, and smoking history. The number of peripheral blood leukocytes, fibrinogen levels, C reactive protein and serum albumin were measured as indicators of systemic inflammation and nutritional status. Pulmonary function tests (MS Diffusion Pulmonary Function Instrument (Yegger, Germany)) including FEV1, FVC, and diastolic tests, and arterial blood gas analysis were performed in those patients. Pulmonary arterial systolic pressure was measured by color Doppler echocardiography (Voluson 730 Expert Color Doppler Ultrasound Diagnostic Instrument (GE Company)). Pulmonary arterial systolic pressure> = 40mmHg is considered as pulmonary hypertension.
2.4. Acute exacerbation
Acute exacerbation of chronic obstructive pulmonary disease (COPD) is defined as 3 clinical symptoms (dyspnea, sputum volume and purulent sputum), at least 2 of which are severe, either requiring urgent treatment or hospitalization, or increasing the use of antibiotics or corticosteroids due to COPD-related respiratory symptoms.
2.5. Sputum samples
Early morning sputum was collected from each patient for microbial analysis. Teach patients how to collect sputum specimens. The sputum specimens were detected within 2 hours after collection. Sputum specimens with low magnification field < 10 squamous epithelial cells and high magnification field > 25 white blood cells were qualified specimens. The sputum smears were cultured after being qualified. Phlegm culture (Zhuhai Dier system) is expressed as colony forming units (CFUs) per milliliter. Sputum more than 103 CFUs is defined as meaningful result.
2.6. Statistical processing
SPSS 13 software was used for statistical analysis. The normal distribution of measurement data was expressed by mean ± standard deviation, and the independent sample t test was used to compare between the two groups. The data of non normal distribution were represented by M (Q), and the rank sum test was used to compare between the 2 groups. The Chi-square test was used to compare the measurement data. The difference of P < .05 was statistically significant.
3. Results
Of the 133 patients with moderate and severe COPD (mean age 70.18 years), 104 (78.2%) patients were severe COPD, and 29 (21.8%) patients were moderate COPD. Bronchiectasis occurred in 43 patients (32.3%). Patients were divided into COPD group and COPD with bronchiectasis group according to the presence of bronchiectasis.
3.1. General and clinical characteristics of the 2 groups
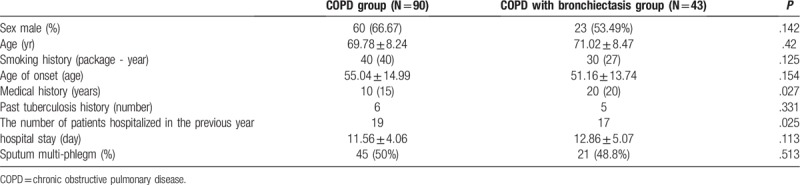
COPD with bronchiectasis patients had a longer medical history (P = .027) and had higher rates of hospitalization at least once a year due to exacerbations of COPD (P = .025). There was no significant difference in the hospitalization time (P = .113) and sputum status (P = .513) between COPD group and COPD with bronchiectasis group (Table 1).
Table 1.
General data and clinical features of COPD group and COPD with bronchiectasis group.
3.2. Comparison of the functional characteristics between COPD group and COPD with bronchiectasis group
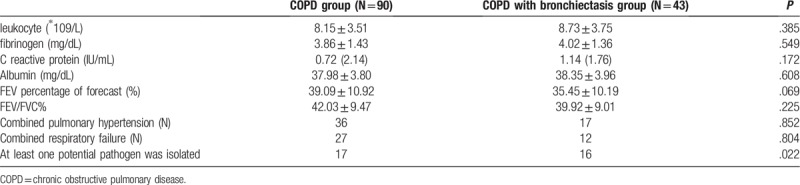
There were no significant differences in the levels of systemic inflammation (peripheral blood leukocytes, fibrinogen and C reactive protein), nutritional status (serum albumin), and pulmonary function, respiratory failure, pulmonary hypertension between the two groups (Table 2) (P > .05).
Table 2.
Microbial and functional characteristics of COPD group and COPD with bronchiectasis group.
3.3. Isolation of pathogenic microorganisms in COPD group and COPD group with bronchiectasis
The positive rate of potential pathogenic microorganisms in sputum of COPD patients with bronchiectasis was higher (P = .022). Potential pathogenic microorganisms were isolated from at least one sputum sample in 33 (24.18%) patients during the study period. The most common pathogenic microorganisms were Klebsiella pneumoniae, P aeruginosa and Acinetobacter baumannii. P aeruginosa was the most common pathogen isolated from COPD patients with bronchiectasis (P < .001) (Table 3).
Table 3.
Isolation of pathogenic microorganisms in COPD group and COPD group with bronchiectasis.

3.4. Characteristics of bronchiectasis
Bilateral bronchiectasis was found in 25 (58.1%) cases, left bronchiectasis in 11 (25.6%) cases and right bronchiectasis in 7 (16.3%) cases. Bronchiectasis involved more than one lobe in 30 (69.8%) patients and only one lobe in 13 (30.2%) patients. There were 33 cases of columnar bronchiectasis (76.7%) and 10 cases of cystic bronchiectasis (23.3%). The most common involved lobes were left lower lobe, right middle lobe and lingual lobe.
4. Discussion
COPD and bronchiectasis are 2 different diseases that occur alone but can coexist. In 2014, the GOLD guidelines noted that COPD can be combined with bronchiectasis.[16] With the increasing application of CT to evaluate COPD patients, it is not uncommon to see bronchiectasis in COPD patients.[8,10,16,27,28] Martínez-García et al reported clinical features in 91 patients with moderate to severe COPD, HRCT scanning results showed that 57.6% patients have bronchiectasis.[29] Jin et al showed that 45.8% of patients with moderate and severe COPD had bronchiectasis.[24] Previous studies have shown that the incidence of bronchiectasis in COPD patients ranged from 25.6% to 69%,[26,33,24,29] suggesting that bronchiectasis in COPD patients is not uncommon. It is not clear whether this group has unique characteristics, represents a particular phenotymic group, and requires different treatments.
The bronchial expansion is mainly bilateral, multiple and column-like bronchial expansion. The most vulnerable areas are the lower left, left tongue and lower right. Broncoching dilating has a long history of being hospitalized at least once in the past year due to COPD exacerbation and potentially pathogenic microorganisms isolated from the sputum. The most common pathogenic microorganism in this paper is Pseudomonas aeruginosa, which is consistent with previous studies. In this study, 32.3% of patients with moderate to severe COPD had bronchiectasis. The main types of bronchiectasis were bilateral, multiple and columnar bronchiectasis. The most vulnerable sites were the left lower lobe, left lingual lobe and right middle lobe. Bronchiectasis is associated with these factors, including a long history of disease, at least one hospitalization for aggravated COPD in the past year, and potential pathogenic microorganisms have been isolated from sputum.
Patel et al observed that bronchiectasis was associated with the increase of potential pathogenic microorganisms in 54 patients with moderate to severe COPD.[9] Martínez-García et al found that bronchiectasis is associated with microbial pathogens, especially P aeruginosa, which may be a predictor of exacerbation.[29] Gatheral et al suggested that bronchiectasis in COPD patients was associated with respiratory tract infection and increased hospital stay, but no coexistence of emphysema and bronchial wall thickening.[10] In a study of 93 COPD patients undergoing mechanical ventilation, it was found that bronchiectasis associated with COPD increased the length of stay in ICU, increased the chance of ventilator-associated pneumonia, but did not increase mortality.[30] These findings suggested that COPD with bronchiectasis is associated with the colonization and infection of pathogenic microorganisms. Potential pathogenic microorganisms and their hydrolysates are the triggering mechanism of bronchiectasis. The colonization of bacteria in respiratory tract and subsequent inflammation play an important role in the formation of bronchiectasis, and bronchiectasis aggravates the colonization of bacteria, thus forming a vicious circle. Therefore, early identification of this subgroup and the use of appropriate antibiotics may break this vicious circle. This study showed that factors associated with bronchiectasis included at least one culture of positive pathogens in sputum samples. The most common pathogenic microorganism in this study was P aeruginosa, which was consistent with previous studies.[9,31–33]
This study found that associated factors of bronchiectasis included long medical history and hospitalization at least once a year due to acute exacerbation of COPD. COPD patients are prone to repeated aggravation and progression, and each acute exacerbation may lead to irreversible destruction of lung structure, leading to further decline of lung function. In addition, bronchiectasis can lead to the loss of airway purification function, microbial colonization and secretion retention, resulting in repeated aggravation of COPD. Wedzicha et al assessed the interaction between structure and function in exacerbation of COPD, suggesting that bronchiectasis can affect the frequency and severity of exacerbation.[34] Patel et al observed that even though the frequency of acute exacerbation of COPD was not related to bronchiectasis, the acute exacerbation of bronchiectasis was more severe.[9] Another study showed that bronchiectasis alone predict mortality in patients with moderate or severe COPD, independent of lung function and other risk factors,[28] suggesting that COPD bronchiectasis may represent a special phenotype, and early detection of this subgroup has important guiding significance for the prognosis and treatment of the disease.[35]
Some studies showed that the incidence of bronchiectasis is negatively correlated with FEV1%, suggesting that airway obstruction is associated with the formation of bronchiectasis.[24,29] In this study, pulmonary function of patients with bronchiectasis was poor, but there was no statistical difference. This may be due to poor lung function and narrow FEV1 range in patients with bronchiectasis.
The predisposing site of bronchiectasis is related to the anatomical structure of bronchus and previous medical history (e.g., tuberculosis). The lower left branch bronchial tube is slender, affected by the heart and blood vessels, and the main bronchial is large in angle. Poor drainage can easily cause bronchial expansion. The tongue-side bronchial opening is close to the lower left lobe and is susceptible to infection in the lower lobe. Therefore, bronchiectasis of the left lower lobe and the lingual bronchus often coexist. Due to the anatomical structures and compression of the surrounding lymph nodes, the middle lobe of the right lung is prone to stenosis and poor drainage, causing repeated infection and secondary bronchiectasis. Bronchiectasis in COPD patients may be related to the location and extent of bronchitis and emphysema in the COPD itself.
In addition, this study showed that there was no significant difference in systemic inflammation and nutritional status between COPD patients with bronchiectasis and COPD patients, suggesting that bronchiectasis mainly affects the local airway of COPD patients, but has little effect on the whole body.
The limitation of this study is that the number of cases included in this study is still relatively small, and the conclusions may be partial and one-sided, and this comment applies to other studies on the same subject.[36] Because the total number of cases is relatively small, and the number of cases detected by pathogens is also small, we may not be able to further study the potential changes in sputum microbiology, we may have insufficient motivation to detect some differences, especially between deteriorating phenotypes.
5. Conclusion
In conclusion, the incidence of bronchiectasis in patients with moderate to severe COPD is higher, and bronchiectasis in the left lower lobe, left lingual lobe and right middle lobe is the most common. Factors associated with bronchiectasis include a long history, hospitalization at least once a year for aggravation, and the isolation of potential pathogenic microorganisms from sputum specimens. Bronchiectasis should be noted in patients with COPD who often suffer from exacerbation or repeated respiratory infections, especially in those who isolate P aeruginosa from respiratory specimens.
Author contributions
Conceptualization: Qihong Yu, Hongyu Qian, Hong Zhang.
Data curation: Qihong Yu, Hong Zhang.
Formal analysis: Qihong Yu, Haiying Peng.
Investigation: Haiying Peng, Hongyu Qian.
Methodology: Haiying Peng.
Resources: Bo Li.
Software: Bo Li, Hongyu Qian.
Supervision: Bo Li.
Writing – original draft: Qihong Yu.
Writing – review & editing: Hong Zhang.
Footnotes
How to cite this article: Yu Q, Peng H, Li B, Qian H, Zhang H. Characteristics and related factors of bronchiectasis in chronic obstructive pulmonary disease. Medicine. 2019;98:47(e17893).
Abbreviations: CFUs = colony forming units, COPD = chronic obstructive pulmonary disease, HRCT = high-resolution CT (SIEMENS 64 slice spiral Definition ASCT SOMA) scan.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Sehgal IS, Dhooria S, Agarwal R. Chronic obstructive pulmonary disease and malnutrition in developing countries. Curr Opin Pulm Med 2017;23:139–48. [DOI] [PubMed] [Google Scholar]
- [2].Landis SH, Muellerova H, Mannino DM, et al. Continuing to Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012–2013. Int J Chron Obstruct Pulmon Dis 2014;9:597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wali SO, Idrees MM, Alamoudi OS, et al. Prevalence of chronic obstructive pulmonary disease in Saudi Arabia. Saudi Med J 2014;35:684–90. [PubMed] [Google Scholar]
- [4].Cai L, Cui W, He J, et al. The economic burden of smoking and secondhand smoke exposure in rural South-West China. J Asthma 2014;51:515–21. [DOI] [PubMed] [Google Scholar]
- [5].Kim C, Yoo KH, Rhee CK, et al. Health care use and economic burden of patients with diagnosed chronic obstructive pulmonary disease in Korea. Int J Tuberc Lung Dis 2014;18:737–43. [DOI] [PubMed] [Google Scholar]
- [6].Itoh M, Tsuji T, Nemoto K, et al. Undernutrition in Patients with COPD and Its Treatment. Nutrients 2013;5:1316–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kelly C, Grundy S, Lynes D, et al. Self-management for bronchiectasis. Cochrane Database Syst Rev 2018;2:CD012528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Martínez-García MA, de la Rosa Carrillo D, Soler-Cataluña JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:823–31. [DOI] [PubMed] [Google Scholar]
- [9].Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:400–7. [DOI] [PubMed] [Google Scholar]
- [10].Gatheral T, Kumar N, Sansom B, et al. COPD-related bronchiectasis; independent impact on disease course and outcomes. COPD 2014;11:605–14. [DOI] [PubMed] [Google Scholar]
- [11].COPD group of Chinese Thoracic Society Guideline for diagnosis and treatment of chronic obstructive pulmonary disease (updated 2013)Zhonghua Jie He He Hu Xi Za Zhi. 2013;36:255–265. [Google Scholar]
- [12].Shen Y, Huang S, Kang J, et al. Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition). Int J Chron Obstruct Pulmon Dis 2018;13:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu Y, Pleasants RA, Croft JB, et al. Smoking duration, respiratory symptoms, and COPD in adults aged ≥45 years with a smoking history. Int J Chron Obstruct Pulmon Dis 2015;10:1409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van Eerd EA, van Rossem CR, Spigt MG, et al. Do we need tailored smoking cessation interventions for smokers with COPD? A comparative study of smokers with and without COPD regarding factors associated with tobacco smoking. Respiration 2015;90:211–9. [DOI] [PubMed] [Google Scholar]
- [15].Schauer GL, Wheaton AG, Malarcher AM, et al. Health-care Provider Screening and Advice for Smoking Cessation Among Smokers With and Without COPD: 2009–2010 National Adult Tobacco Survey. Chest 2016;149:676–84. [DOI] [PubMed] [Google Scholar]
- [16].Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65. [DOI] [PubMed] [Google Scholar]
- [17].Sundar IK, Mullapudi N, Yao H, et al. Lung cancer and its association with chronic obstructive pulmonary disease: update on nexus of epigenetics. Curr Opin Pulm Med 2011;17:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].de Marco R, Accordini S, Cerveri I, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med 2007;175:32–9. [DOI] [PubMed] [Google Scholar]
- [19].Yamane T, Hattori N, Kitahara Y, et al. Productive cough is an independent risk factor for the development of COPD in former smokers. Respirology 2010;15:313–8. [DOI] [PubMed] [Google Scholar]
- [20].Miravitlles M, Guerrero T, Mayordomo C, et al. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration 2000;67:495–501. [DOI] [PubMed] [Google Scholar]
- [21].Hogg JC, Chu FS, Tan WC, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 2007;176:454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011;184:662–71. [DOI] [PubMed] [Google Scholar]
- [23].Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med 2014;35:157–63. [DOI] [PubMed] [Google Scholar]
- [24].Ni Y, Shi G, Yu Y, et al. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2015;10:1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang X, Xu Y, Jin J, et al. Chronic rhinosinusitis is associated with higher prevalence and severity of bronchiectasis in patients with COPD. Int J Chron Obstruct Pulmon Dis 2017;12:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Martínez-García MÁ, Soler-Cataluña JJ, Donat Sanz Y, et al. Factors associated with bronchiectasis in patients with COPD. Chest 2011;140:1130–7. [DOI] [PubMed] [Google Scholar]
- [27].Benfante A, Bellia M, Scichilone N, et al. Airway distensibility by HRCT in asthmatics and COPD with comparable airway obstruction. COPD 2013;10:560–6. [DOI] [PubMed] [Google Scholar]
- [28].Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J Chron Obstruct Pulmon Dis 2017;12:1401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jin J, Yu W, Li S, et al. Factors associated with bronchiectasis in patients with moderate-severe chronic obstructive pulmonary disease. Medicine (Baltimore) 2016;95:e4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gursel G. Does coexistence with bronchiectasis influence intensive care unit outcome in patients with chronic obstructive pulmonary disease? Heart Lung 2006;35:58–65. [DOI] [PubMed] [Google Scholar]
- [31].Kim WJ, Hoffman E, Reilly J, et al. Association of COPD candidate genes with computed tomography emphysema and airway phenotypes in severe COPD. Eur Respir J 2011;37:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mair G, Maclay J, Miller JJ, et al. Airway dimensions in COPD: relationships with clinical variables. Respir Med 2010;104:1683–90. [DOI] [PubMed] [Google Scholar]
- [33].Engler K, Mühlemann K, Garzoni C, et al. Colonisation with Pseudomonas aeruginosa and antibiotic resistance patterns in COPD patients. Swiss Med Wkly 2012;142:w13509. [DOI] [PubMed] [Google Scholar]
- [34].Wedzicha JA, Hurst JR. Structural and functional co-conspirators in chronic obstructive pulmonary disease exacerbations. Proc Am Thorac Soc 2007;4:602–5. [DOI] [PubMed] [Google Scholar]
- [35].Novosad SA, Barker AF. Chronic obstructive pulmonary disease and bronchiectasis. Curr Opin Pulm Med 2013;19:133–9. [DOI] [PubMed] [Google Scholar]
- [36].Martinez FJ, Erb-Downward JR, Huffnagle GB. Significance of the microbiome in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013;10: Suppl: S170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


