Significance
All plant-based biomass production, and thus our food supply, depends on the slow and inaccurate catalyst Rubisco to transform gaseous carbon dioxide into organic molecules. Rubisco requires a metabolic repair enzyme, Rubisco activase, which acts to remove inhibitors from the active site, keeping it unblocked and functional. The detailed mechanism of activase function remains elusive. Using proteins from rice, we characterize a suite of activase mutants with varying capacities to interact with Rubisco. The activase helper is a machine consisting of 6 identical parts that constantly exchange in solution, which permits an analysis of how mutants poison the wild-type system. Understanding activase function will empower scientists that aim to engineer the rate-limiting CO2-fixing reactions of photosynthesis.
Keywords: Rubisco activase, photosynthesis, AAA+ proteins, Oryza sativa
Abstract
During photosynthesis the AAA+ protein and essential molecular chaperone Rubisco activase (Rca) constantly remodels inhibited active sites of the CO2-fixing enzyme Rubisco (ribulose 1,5-bisphosphate carboxylase/oxygenase) to release tightly bound sugar phosphates. Higher plant Rca is a crop improvement target, but its mechanism remains poorly understood. Here we used structure-guided mutagenesis to probe the Rubisco-interacting surface of rice Rca. Mutations in Ser-23, Lys-148, and Arg-321 uncoupled adenosine triphosphatase and Rca activity, implicating them in the Rubisco interaction. Mutant doping experiments were used to evaluate a suite of known Rubisco-interacting residues for relative importance in the context of the functional hexamer. Hexamers containing some subunits that lack the Rubisco-interacting N-terminal domain displayed a ∼2-fold increase in Rca function. Overall Rubisco-interacting residues located toward the rim of the hexamer were found to be less critical to Rca function than those positioned toward the axial pore. Rca is a key regulator of the rate-limiting CO2-fixing reactions of photosynthesis. A detailed functional understanding will assist the ongoing endeavors to enhance crop CO2 assimilation rate, growth, and yield.
The key photosynthetic enzyme ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) catalyzes the CO2-fixing reaction ultimately responsible for the majority of planetary biomass (1, 2). In contrast to its pivotal role it is slow, catalyzes a metabolite-damaging side reaction with oxygen, and needs metabolic repair by Rubisco activase (Rca), a group of molecular chaperones that release tightly bound sugar phosphates from the active site (3, 4). These Rcas have evolved the same function repeatedly from at least 3 different ancestral chaperones in multiple autotrophic lineages (5). All 3 known classes of Rca belong to the AAA+ (adenosine triphosphatases [ATPases] associated with various cellular activities) family of motor proteins (6). They share the family’s common theme of ATP-driven conformational rearrangements of a macromolecular substrate, but their detailed mechanisms of action are distinct and only partially understood. The most detailed picture to date relates to the red-type Rca found in proteobacteria and red-lineage phytoplankton (7, 8). Detailed biochemical and structural data converge on a model where the disk-shaped hexameric Rca transiently threads the C-tail of the large subunit RbcL through its axial pore (9). This leads to disruption of the closed active site conformation. In contrast the CbbQO class of Rca found in chemoautotrophic bacteria is absolutely dependent on an adaptor protein, which needs to bind to the Rubisco large subunit at an acidic residue located at the βC–βD loop (10).
Green-type Rca is present in all higher plants and green algae (11–13) and is thus arguably the one most critical to human affairs. It is a crop improvement target because it limits CO2 assimilation at elevated temperatures (14) and fluctuating light conditions (15, 16). Its mechanism of action is poorly defined but believed to involve central pore-loop–mediated rearrangements of structural elements other than the RbcL C terminus (17, 18). Our current understanding is governed by a knowledge of Rca residues and elements that if mutated lead to a loss in Rca activity, but not ATPase function. These are the N-terminal domain (especially W15) (19, 20), multiple axial pore loops (17), a surface loop in the nucleotide binding domain (α4–β4) (21), and the specificity helix H9 (22). Only for the last element has the corresponding interaction site on Rubisco been determined; it is the βC–βD loop of the large subunit (23, 24). In combination with structural data (17, 25, 26) this contributes to a model where the hexameric disk binds the inhibited holoenzyme via multiple contact points on its top surface, followed by an ATP-driven conformational rearrangement of an unknown target element on Rubisco using the axial pore loops (18, 27).
Most plants, including rice, possess 2 major Rca isoforms (long Rcaα and short Rcaβ) often produced by alternative splicing (28, 29). Rcaα possesses a ∼30-amino-acid residue extension that confers light-mediated thioredoxin-dependent regulation via reversible oxidation of a conserved pair of cysteines (30, 31).
Toward enriching the density of functional information on plant Rca-mediated Rubisco remodeling, here we combined structurally guided mutagenesis with mutant subunit mixing experiments. While initially assessing the propensity of rice Rca to exchange subunits we found that low-activity Rcaα subunits rapidly heterooligomerize with high-activity Rcaβ subunits, which would cause an overall down-regulation of Rca activity at elevated temperatures without affecting the overall thermostability of the complex. We then used structural information to identify a suite of residues involved in Rca function. Subunit mixing experiments indicate that residues located toward the rim of the disk are less critical for function than those positioned near the axial pore loop, which mediates the conformational rearrangement of Rubisco.
Results
Rice Rca Isoforms Rapidly Exchange and Modulate Heterooligomer Activity.
Mutant subunit doping experiments are commonly used to study AAA+ motor function in cases where subunits are known to exchange rapidly (32–34) (Fig. 1A). The outcome of such experiments can be interpreted by modeling the probability of the distribution of the mutant subunits in the functional hexamers (Fig. 1B). Measured activity can then be compared to predictions made by the model, where the simplest variation assumes that each wild-type (WT) subunit contributes one-sixth of the activity of the hexamer. Cooperativity is then introduced by imposing that hexamers containing more than a certain number of mutant subunits are nonfunctional (Fig. 1C). Here we adopt this approach (35) to provide a framework to analyze the outcome of Rca subunit mixing experiments. We produced and purified a suite of rice activase variants to homogeneity (SI Appendix, Fig. S1), all of which displayed the typical concentration-dependent polydispersity when assayed by analytical size-exclusion chromatography (SI Appendix, Fig. S2).
Fig. 1.
Heterooligomerization of rice Rca isoforms is rapid and modulates biochemical activity. (A) Scheme visualizing the subunit mixing strategy utilized in this work. (B) Theoretical distribution of Rca hexamer populations as a function of increasing the proportion of mutant subunits. (C) Biochemical activity expected from the distribution shown in B when a particular number of mutant subunits is sufficient to eliminate hexamer activity. (D) Rcaβ ATPase activity is poisoned identically irrespective of the mutant isoform identity. Rcaβ was mixed with an increasing proportion of D173A Rcaα or Rcaβ mutants and ATPase activity was measured (5 µM Rca protomer). (E) The loss in activase activity mirrors that of ATPase activity. Rca assays used 0.5 µM RuBP-inhibited Rubisco active sites (ER) and 5 µM Rca protomer. (F) Subunit mixing kinetics is fast and independent of Rca isoform. NADH oxidation-coupled ATPase assay time course is shown for WT and poisoned Rcaβ (solid lines). The kinetics of poisoning by mutant addition is identical for the mutant α and β isoforms (dashed lines). (G) Increasing the proportion of low-activity Rcaα subunits in Rcaβ/α heterooligomers results in a linear decrease in ATPase and activase activity. Protein concentrations as in D and E. (H) Mixtures (1:1) of Rcaα and Rcaβ were heated at the indicated temperature for 10 min in the presence and absence of 1mM ATPγS/5 mM MgCl2. ATPase assays (5 µM Rca protomer) were subsequently performed at 25 °C. Error bars indicate the SD of at least 3 independent experiments.
The polydispersity of plant Rca proteins is due to their occupation of multiple oligomeric states (36–39), and biochemical data implicate the typical AAA+ hexamer to be the functional unit in Rubisco activation (17). In contrast, smaller oligomers are capable of hydrolyzing ATP (17, 40). Rca oligomers exchange subunits rapidly, but this property is highly variable among species. For instance, whereas spinach Rcaβ forms essentially locked hexameric assemblies in the presence of Mg2+ and ATPγS (41), tobacco Rca exchanges subunits at a rate of 0.1 to 0.3 s−1 under comparable conditions (42). To assess these properties in the context of rice Rca, we performed a number of biochemical subunit exchange experiments. First we measured the ATPase activity of Rcaβ mixed at various proportions with the ATPase-inactive Walker B mutant D173A (43, 44) of either Rcaβ or the longer Rcaα isoform (Fig. 1D) (residue numbering throughout refers to rice Rcaβ). In both cases we observed a severe nonlinear decrease in ATPase activity with increasing mutant subunit composition, indicating strong cooperativity between subunits, similar to the result seen for Walker mutants of ClpB earlier (35). Adding either mutant isoform resulted in an identical profile, suggesting that Rcaα and Rcaβ have similar affinities for each other and this property is not modulated by Rcaα’s 33-amino-acid C-terminal extension (Fig. 1D). The same mixing experiment was performed using Rcaβ and its D173A mutant, followed by measurement of relative Rca activity. As observed for the ATPase activity, Rca function decreased in the same manner with increasing mutant subunit populations (Fig. 1E). For Rca function the result suggests that insertion of only ∼2 mutant subunits resulted in nonfunctional hexameric ensembles.
We then decided to estimate the rate of subunit exchange in actively ATP-hydrolyzing Rcaβ complexes, by measuring the time required to transition between fully functional and poisoned ensembles upon addition of the mutant subunits to Rcaβ using the method applied to ClpB previously (35). Following addition of an equimolar amount of mutant subunits to WT Rcaβ, the new steady-state ATP hydrolysis rate was achieved within 100 s, consistent with complete subunit exchange on the minute timescale (Fig. 1F). The kinetics of Rcaβ–Rcaβ and Rcaβ–Rcaα subunit interchange were indistinguishable by this method.
At 5 µM protomer, rice Rcaα presents with a far lower ATPase (16%) and Rubisco activation rate (24%) compared to the Rcaβ isoform (SI Appendix, Fig. S3A) (21, 45). In our assay systems we did not observe the previously reported concentration-dependent sigmoidal effect (45) and both ATPase and Rca activity remained similar at Rca concentrations ranging from 5 to 20 µM protomer (SI Appendix, Fig. S3A). The regulation of Rcaα activity by reduction with thioredoxin-f (Trx-f) has not yet been characterized in rice (31, 46). To clarify this point we produced and characterized the C396S variant of Rcaα, which cannot form the disulfide bridge. RcaαC396S had a 2.8-fold faster ATPase activity than Rcaα and Rubisco activation activity was increased 1.8-fold (SI Appendix, Fig. S3B). We also incubated Rcaα with dithiothreitol and recombinant Trx-f, which when applied together, but not alone, resulted in 35% and 71% increases in ATPase and Rca activity, respectively (SI Appendix, Fig. S3B). We thus conclude that the slow Rcaα purified using our protocol is mostly oxidized, resembling the state expected during the night in vivo.
Given the observed rapid and complete subunit exchange shown in Fig. 1F, the implication is that the slow, oxidized Rcaα will decelerate ATP hydrolysis in Rcaβ, similar to mutant subunit poisoning. We therefore measured ATP hydrolysis and Rubisco activation rates of different Rcaβ/Rcaα mixes. Under our assay conditions the observed reduction in activity for both activase and ATPase was linear with respect to the fraction of Rcaα included in the assay (Fig. 1G), indicating that the slow Rcaα subunits were not significantly impeding Rcaβ subunit function.
The thermolability of Rca correlates with the loss of photosynthetic function at moderately elevated temperatures (47–49). In addition it has repeatedly been observed that different rice varieties increase the ratio of Rcaα to Rcaβ when exposed to heat stress (50, 51). However, rice Rcaα is as thermolabile as and possesses much lower activity than Rcaβ (21, 45). It is conceivable that heterooligomerization of the 2 isoforms will lead to a thermostable complex as reported for the spinach Rca heterooligomer (52, 53). A 1:1 mixture of Rcaα and Rcaβ was subjected to increasing temperatures in the presence and absence of stabilizing Mg-ATPγS. However, the heterooligomer presented with thermostability essentially identical to that of the subunits in isolation (21), losing all activity at ∼42 °C (Fig. 1H).
In summary, our in vitro results suggest that the observed up-regulation of Rcaα in thermally stressed rice plants would result in a net reduction of Rca function due to heterooligomerization of the 2 types of isoforms. This contrasts with hypotheses that predict high-temperature–associated Rca isoforms to be thermostable (50). Instead, down-regulation of Rca function may be adaptive to reduce Calvin cycle flux due to reasons elaborated on previously (54, 55).
Structurally Guided Identification of Rubisco-Interacting Residues.
Combining the availability of experimentally determined Rca structural models (17, 26) with initial mechanistic predictions (18) now provides a framework to identify highly conserved residues in conspicuous locations followed by experimental validation. As the surface of the Rca hexamer is implicated with Rubisco binding (Fig. 2A) we generated amino acid substitutions of residues that could contribute to a Rubisco binding surface. Of particular interest was the highly conserved helix H3 (residues 145 to 161; SI Appendix, Fig. S4), found adjacent to the functionally critical pore loop 1 (17). H3 substantially contributes to the surface of the hexameric disk surrounding the central pore (Fig. 2B). Mutational analysis revealed that a K148A substitution on this helix uncoupled ATPase and activase function consistent with a critical role in Rubisco interaction (Fig. 2C and SI Appendix, Fig. S5A). In contrast, D159A exhibited a higher Rca function than WT Rca with a 50% reduction in ATPase activity (Fig. 2C and SI Appendix, Fig. S5A). This phenotype could be related to a higher affinity for the Rubisco substrate, similar to that observed for the E217Q/A variants characterized earlier (21). Since both ATPase and Rca function was reduced in variants harboring Q152A and E156A mutations (Fig. 2C and SI Appendix, Fig. S5A) no clear evidence for their involvement in the Rubisco–Rca protein–protein interaction could be deduced.
Fig. 2.
Structure-based identification of residues involved in Rca function. (A) Surface representation of the (Rubisco-interacting) top side of the tobacco Rca hexameric model. (Protein Data Bank [PDB] ID code 3ZW6). (B) Close-up view of one subunit. Residues corresponding to those chosen for mutagenesis are shown in stick format and helices are shown as cylinders. Colors used in A and B indicate that alanine substitution either leads to a reduction in both ATPase and Rca activity (magenta), uncouples ATPase and Rca function (green), or enhances Rca function (yellow) as shown in C. The Rubisco-interacting specificity helix H9 is shown in red. See SI Appendix, Fig. S4 for a structure-based sequence alignment. (C) ATPase and Rca activity of the indicated variants was measured using the same conditions as described for Fig. 1. Error bars indicate the SD of at least 3 independent experiments.
Based on its position on the hexameric surface we also decided to probe R321, a residue located adjacent to the specificity helix H9 (residues 314 to 319; SI Appendix, Fig. S4). Although not highly conserved (SI Appendix, Fig. S4), mutagenesis of this residue to alanine also uncoupled ATPase and Rca function, indicating an important role in the interaction between the Rubisco and Rca.
Finally, we recapitulated the phenotype of pore loop 1 residue A143V, which as reported for tobacco Rca (17) resulted in complete uncoupling of ATPase and Rca function confirming the role of the pore loop in the Rubisco–Rca interaction (Fig. 2C). Additionally probing the 2 downstream residues by assaying the G144A and E145A mutant proteins revealed these substitutions severely compromised both ATPase and Rca function (Fig. 2C and SI Appendix, Fig. S5A).
Insights into the Role of the Unstructured N-Terminal Domain.
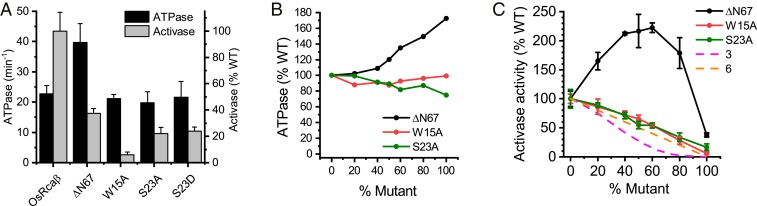
The intrinsically disordered N-terminal domain is known to be critical for Rca function (19, 20), prompting us to generate 4 rice Rcaβ variants. In contrast to the result observed for tobacco Rca (17), removing the N-terminal 67 residues (ΔN67) substantially reduced, but did not completely eliminate, Rca function. In addition, ATPase activity of this protein was increased by 75% (Fig. 3A). We also confirmed the critical role of the Rubisco-interacting W15 (20), where substitution to alanine did not affect ATPase but resulted in a 90% reduction of activase function. Finally, we substituted S23, the residue shown to be phosphorylated in rice Rca (56), with alanine and a phosphomimetic aspartate. This residue is adjacent to and probably functionally equivalent to the threonine 78 (numbering includes transit peptide) phosphorylated in the dark in Arabidopsis (57–59). The 2 variants presented with similar phenotypes, hydrolyzing ATP at the same rate as the WT but having lost ∼80% of activase function. This result suggests that the hydroxyl group of S23A is also involved in the Rca–Rubisco interaction, and that phosphorylation of this residue will interfere with Rubisco binding.
Fig. 3.
The role of the intrinsically disordered N-terminal domain. (A) N-terminal domain variants display severe reductions in Rca but not ATPase function. ATPase and Rca activity was measured using the same conditions as described for Fig. 1. (B) Subunit mixing does not impair ATPase function of the heterooligomers. (C) Increasing the proportion of N-terminal domain variants in WT–mutant mixes indicates the point mutants function noncooperatively, whereas ΔN67 enhances the Rca activity of subunit mixtures. Error bars indicate the SD of at least 3 independent experiments.
The Relative Functional Contribution of Rubisco-Interacting Residues.
The uncoupling of ATPase and Rca function is a strong indicator that a mutated residue is involved in protein–protein interactions with Rubisco, but this phenotype provides no information regarding the element’s relative role in the context of the hexamer. Hence, we applied the previously outlined subunit mixing strategy (Fig. 1 A–C) to assess how the presence of such mutant subunits affected Rca function.
The 2 N-terminal domain mutants W15A and S23A did not modify ATPase activity when mixed with WT subunits (Fig. 3B), and Rca function declined linearly with an increasing concentration of the mutant (Fig. 3C). The result suggests that the function of WT subunits is not negatively affected by the presence of mutant subunits in the same assemblies. In stark contrast, mixtures of the WT subunits with subunits lacking the N-terminal domain (ΔN67) presented with higher ATPase and far higher activase activity than the WT enzyme (Fig. 3 B and C). This enhanced Rca activity was even maintained when assayed at 35 °C (SI Appendix, Fig. S5B). Similar activity-potentiating outcomes are frequently observed when mixing mutant subunits of other AAA+ proteins (33, 34, 60) and are generally explained by functional derepression of adjacent subunits. In our case we propose that although the N-terminal domain is critical for Rubisco engagement, only 1 or 2 domains per hexamer may be required for this to occur. In addition, a hexamer containing only some N-terminal domains exhibits increased Rca function. This could be a direct consequence of the higher ATPase activity of the ΔN67 subunits, or related to reduced steric hindrance of chaperone/Rubisco interactions during the protein remodeling steps that lead to inhibitor release.
Rubisco-Interacting Residues in the Vicinity of the Pore Loop Are More Critical to Rca Function than Those Located toward the Periphery of the Disk.
We performed systematic subunit mixing experiments using those Rca mutants that had uncoupled ATPase and Rca functions. To assess whether these variants maintained WT behavior with regard to subunit interchange we performed mixing experiments with the Walker B mutant RcaβD173A equivalent to those shown in Fig. 1D. All variants but A143V displayed the severe decrease in ATPase activity observed for the WT, indicating equivalent mixing and a highly cooperative ATPase function (SI Appendix, Fig. S6A). Surprisingly, the ATPase activity of the pore loop 1 mutant A143V was reduced in an almost linear fashion in this experiment. This result is consistent with the Walker B mutant’s not influencing the ATPase kinetics of the A143V subunits. This could come about due to a lack of mixing, or alternatively a disruption of the intersubunit communication responsible for the observed cooperativity. We favor the latter interpretation because increasing the proportion of A143V, when mixed with WT Rca, resulted in a severe loss of Rca activity consistent with hexamers containing only 2 mutant subunits’ being completely nonfunctional (Fig. 4A). This result is consistent with an actively remodeling pore, where multiple functional loops need to productively engage and rearrange the Rubisco substrate (61).
Fig. 4.
Assessing the contribution of interacting residues to Rca function by subunit mixing. (A) Subunits mutated in key interacting residues flanking the Rca pore result in drastic loss of Rca function when mixed with WT subunits. (B) Subunits mutated at interacting residues located in the α4–β4 loop (K216) or toward the rim of the hexamer (R321) are well-tolerated, displaying a linear loss of function. (C) Poisoning by using a charge switch H9 mutant is more severe than the alanine substitution. (D) Divergent effect of mixing 2 Rca-activity potentiating subunits. Error bars indicate the SD of at least 3 independent experiments. The dashed lines represent the modeled loss of activity as shown in Fig. 1C and using the same colors.
Other activity-uncoupling alanine substitutions affected Rca function less severely. The pore-flanking K148A and K216A (α4–β4 loop) substitutions displayed some cooperativity, indicating that 3 or 4 mutant subunits completely inactivated the hexamers (Fig. 4 A and B). K315A (specificity helix) and R321A (rim of the ring) had the weakest effect consistent with noncooperative behavior, since increasing mutant subunit concentration resulted in a linear loss of activity (Fig. 4 B and C). The residues not associated with the pore may thus play a more passive role, such as Rubisco docking, which can still be successfully achieved when only 1 or 2 WT subunits remain in a hexamer. However, we also observed that utilizing a more drastic amino acid substitution (e.g., charge switch) can lead to highly cooperative behavior for the same residue in the case of the K315D mutant. The result suggested that the inclusion of multiple (∼3) mutant subunits in the assembly led to nonfunctional hexamers (Fig. 4C), which could be a consequence of electrostatic repulsion between the Rca disk and Rubisco. We attribute the reductions in function seen in the mixing experiments described here to protein–protein interactions alone, since ATPase activity of the subunit mixes was not impaired (SI Appendix, Fig. S6B).
Finally, we evaluated the contribution of the amino acid substitutions that increase Rca activity that we have identified here (Fig. 2C) and previously (21). Only a small fraction of D159A subunits was required to achieve complete potentiation to ∼140% of WT Rca activity (Fig. 4D). In contrast, mixing increasing proportions of E217A with the WT Rca led to a linear increase toward the extraordinary 5-fold increase in Rca activity displayed by this variant. E217 substitutions are predicted to alter the conformation of the α4–β4 loop to lead to an increased affinity for Rubisco. The subunit mixing experiment suggests this effect is additive where each additional mutant subunit enhances Rca activity equally. For both potentiating amino acid substitutions increasing the proportion of the mutant subunit led to a linear decrease in ATPase activity (SI Appendix, Fig. S6B). Similar to potentiation by the ΔN67 subunit, the E217A subunit mixture maintained its high Rca function at an elevated assay temperature of 35 °C (SI Appendix, Fig. S5B). This high functionality was not due to enhanced thermostability as the potentiating variants and mixtures displayed an ATPase temperature activity profile similar to WT (SI Appendix, Fig. S5C).
Discussion
In this work we describe a toolbox of Rca variants that we have generated toward deepening our appreciation of green-type Rca function. During characterization of the tendency of rice Rca subunits to heterooligomerize we come to the conclusion that the reported up-regulation of Rcaα in heat-stressed rice plants likely serves to down-regulate Rca function, rather than generating thermostable Rca heterocomplexes (Fig. 1). In contrast, wheat has just been reported to possess an additional, genuinely thermostable Rca isoform, suggesting this observation may not be universal (62). Comparative genomics indicates the homolog of the thermostable isoform has been lost in rice (63).
By inspecting the Rubisco-interacting surface of the available Rca structural models we successfully implicate multiple previously unstudied residues in the Rubisco–Rca protein–protein interaction (Fig. 2). Analysis of a phosphomimetic S23D substitution in the N-terminal domain indicates that the reported regulatory phosphorylation of this moiety serves to reduce Rca function by interfering with Rubisco engagement (Fig. 3A). This finding is consistent with a recent report showing that Arabidopsis plants expressing a hyperphosphorylated Rca mutant displayed reduced photosynthetic performance (59).
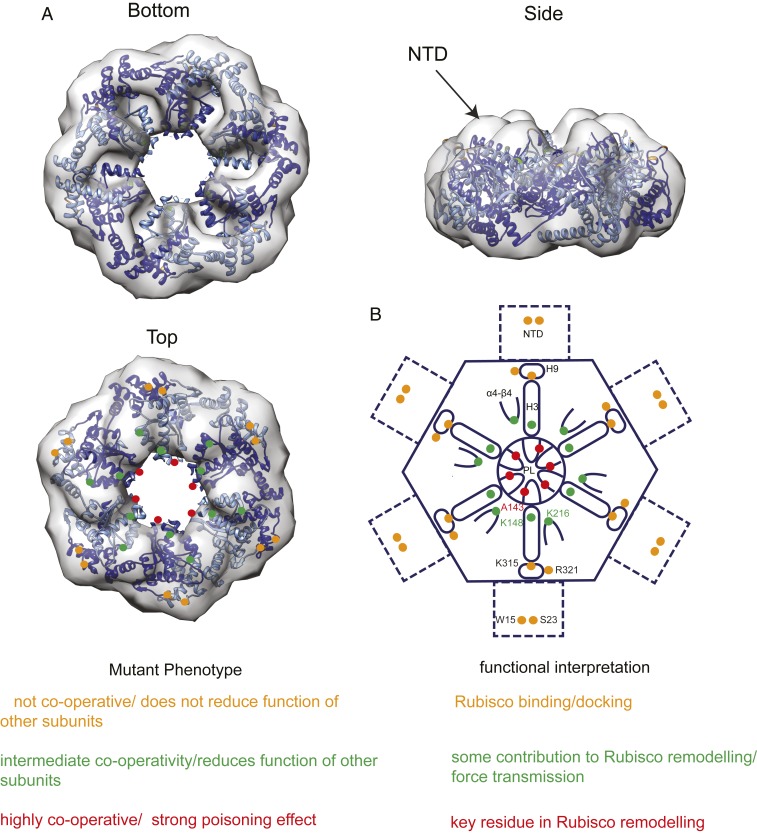
Subunit mixing experiments provide additional information regarding the relative contribution of interacting residues and elements. Whereas most mutated Rca proteins poisoned WT Rca function, unexpectedly mixtures of WT and subunits lacking the N-terminal domain possessed enhanced activase function (Fig. 3C). Subunits mutated at key residues located at or close to the pore loop associated with Rubisco remodeling poison Rca activity cooperatively, whereas mutations positioned toward the periphery of the Rca disk do not appear to negatively affect the activity of the WT subunits (Fig. 4). Mapping these results on the surface of the Rca hexamer suggests that interacting residues positioned toward the periphery of the disk are less critical for function than those located closer to the central pore (Fig. 5). We hypothesize that the residues toward the outer rim of the hexamer and in the as-yet structurally undefined N-terminal domain are involved in passive docking interactions. An increase in importance will reflect involvement in the active force transmission required for Rubisco remodelling, which is most pronounced for the pore loop.
Fig. 5.
The distribution and functional contribution of Rubisco-interacting residues on Rca’s top surface. The Rca hexamer (A) engages Rubisco via multiple interacting residues located on its top surface (scheme in B). Mutant subunit mixing experiments show that the pore loop1 substitution (red dots) has the most critical role, whereas interacting residues on H3 and the α4–β4 loop are of intermediate importance (green dots). Mutations in interacting residues found toward the periphery or on the N-terminal domain (NTD) of the hexamer are well-tolerated (orange dots). The hexameric model (PDB ID code 3ZW6) is superposed on the electron microscopy map (Electron Microscopy Data Bank entry EMD-1940).
Our data support comparable mechanistic functioning between green- and red-type Rca (9), which resembles the central pore-loop threading mechanism of AAA+ proteases (64). However, it is important to note that the Rca mechanism does not involve global protein unfolding but rather a comparatively gentle rearrangement of Rubisco conformation. Such conformational editing functions by AAA+ proteins are an emergent theme and include the remodeling that favors cofactor insertion described for mitochondrial ClpX into ALAS (65, 66) or by NirQ/NorD into nitric oxide reductase (67).
The utility of the toolbox of characterized variants described in this work will be strengthened significantly by the recently achieved breakthrough concerning the successful production of higher plant Rubisco in Escherichia coli (68). We anticipate that combining our collection of Rca mutants with a carefully designed library of complementary higher-plant Rubisco variants will permit elucidation of key questions in Rca-mediated plant Rubisco remodeling, in particular those concerning the identity of additional elements that are recognized and remodeled on Rubisco. Such insights will then be critical to enhance the CO2-fixing reactions of photosynthesis under elevated temperature and during dark-to-light transitions in a crop canopy (13, 69).
Materials and Methods
Molecular Biology and Protein Production.
The QuikChange protocol was used to introduce the desired mutations into pHueOsRcaβ (21). Primers used are listed in SI Appendix, Table S1 and all protein-encoding sequences were verified by DNA sequencing. The gene encoding OsTrx-f (UniProt: Q8S091, residues 73 to 186) was synthesized by Genscript and cloned into the SacII-HindIII site of pHue (70) to give pHueOstrxf.
Rca proteins and OsTrx-f were produced as His6–ubiquitin fusions (70) in E. coli BL21(DE3) cells harboring the required version of pHueOsRcaβ, pHueOsRcaα, or pHueOstrxf (21). Proteins were then purified by a sequence of affinity, anion exchange, and gel filtration chromatography following the protocol described for His6–Ub-tagged proteins previously (10). Rice leaves were a gift from Prakash Kumar, National University of Singapore, Singapore, and rice Rubisco was purified as described (21) using a modification of (71).
Biochemical Assays.
ATPase and Rca activity was measured and quantified exactly as described in ref. 21 using adaptations of the coupled spectrophotometric assays for ATPase (17) and Rubisco activation (72, 73). Rca assay substrate concentrations were 20 mM NaHCO3 and 1 mM RuBP, unless stated otherwise. RuBP was synthesized enzymatically from ribose-5-phosphate (74) and purified by anion-exchange chromatography (75). Activase mixtures were preincubated at 240 to 280 µM Rca protomer for 10 min at 25 °C in storage buffer (20 mM Tris⋅HCl, pH 8, 50 mM NaCl, and 5% vol/vol glycerol) prior to assaying using 5 µM Rca protomer.
To obtain relative activase activities, every sampling day Enzyme–CO2–Mg2+ (ECM) and Enzyme–RuBP (ER) + Rcaβ was assayed as internal controls and Rca activity was quantified (8). Activities of the mutants and mutant/WT mixtures were then determined and quantified as percent WT compared to the same day’s control. The reported values are mean and SD of these percentages.
Model Used to Interpret Subunit Mixture Activities.
The mathematical model used to interpret the activities of subunit mixtures is as developed by ref. 35. The probability P that a hexamer contains x mutant subunits is given by the binomial distribution:
where p is the probability that a mutant subunit is incorporated and is assumed here to be similar to WT, which thus simplifies to the ratio of mutant and WT protein:
The calculated distribution of hexamers then permits a calculation of biochemical activity versus p where the presence of each WT subunit is assigned one-sixth of WT hexamer activity. Cooperativity is then introduced by assigning no activity to hexamers containing more than a specified number of mutant subunits.
Supplementary Material
Acknowledgments
We thank Na Yi Ting and Lynette Liew for technical assistance. This work was funded by a Nanyang Technological University startup grant and Ministry of Education (MOE) of Singapore Tier 2 grant to O.M.-C. (MOE2016-T2-2-088).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data generated and analyzed in this study are available at https://researchdata.ntu.edu.sg/dataverse/cajar.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914245116/-/DCSupplemental.
References
- 1.Parry M. A. J., Andralojc P. J., Mitchell R. A. C., Madgwick P. J., Keys A. J., Manipulation of Rubisco: The amount, activity, function and regulation. J. Exp. Bot. 54, 1321–1333 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Whitney S. M., Houtz R. L., Alonso H., Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiol. 155, 27–35 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat J. Y., Thieulin-Pardo G., Hartl F. U., Hayer-Hartl M., Rubisco activases: AAA+ chaperones adapted to enzyme repair. Front. Mol. Biosci. 4, 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bracher A., Whitney S. M., Hartl F. U., Hayer-Hartl M., Biogenesis and metabolic maintenance of Rubisco. Annu. Rev. Plant Biol. 68, 29–60 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Mueller-Cajar O., The diverse AAA+ machines that Repair inhibited Rubisco active sites. Front. Mol. Biosci. 4, 31 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanson P. I., Whiteheart S. W., AAA+ proteins: Have engine, will work. Nat. Rev. Mol. Cell Biol. 6, 519–529 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Mueller-Cajar O., et al. , Structure and function of the AAA+ protein CbbX, a red-type Rubisco activase. Nature 479, 194–199 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Loganathan N., Tsai Y.-C. C., Mueller-Cajar O., Characterization of the heterooligomeric red-type rubisco activase from red algae. Proc. Natl. Acad. Sci. U.S.A. 113, 14019–14024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhat J. Y., et al. , Mechanism of enzyme repair by the AAA+ chaperone Rubisco activase. Mol. Cell 67, 744–756.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Tsai Y. C., Lapina M. C., Bhushan S., Mueller-Cajar O., Identification and characterization of multiple rubisco activases in chemoautotrophic bacteria. Nat. Commun. 6, 8883 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvucci M. E., Portis A. R. Jr, Ogren W. L., A soluble chloroplast protein catalyzes ribulosebisphosphate carboxylase/oxygenase activation in vivo. Photosynth. Res. 7, 193–201 (1985). [DOI] [PubMed] [Google Scholar]
- 12.Portis A. R., Jr, Rubisco activase–Rubisco’s catalytic chaperone. Photosynth. Res. 75, 11–27 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Carmo-Silva E., Scales J. C., Madgwick P. J., Parry M. A., Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ. 38, 1817–1832 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Kumar A., Li C., Portis A. R. Jr, Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynth. Res. 100, 143–153 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Carmo-Silva A. E., Salvucci M. E., The regulatory properties of Rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiol. 161, 1645–1655 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamori W., Masumoto C., Fukayama H., Makino A., Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 71, 871–880 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Stotz M., et al. , Structure of green-type Rubisco activase from tobacco. Nat. Struct. Mol. Biol. 18, 1366–1370 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Wachter R. M., et al. , Activation of interspecies-hybrid Rubisco enzymes to assess different models for the Rubisco-Rubisco activase interaction. Photosynth. Res. 117, 557–566 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Esau B. D., Snyder G. W., Portis A. R. Jr, Differential effects of N- and C-terminal deletions on the two activities of rubisco activase. Arch. Biochem. Biophys. 326, 100–105 (1996). [DOI] [PubMed] [Google Scholar]
- 20.van de Loo F. J., Salvucci M. E., Activation of ribulose-1,5-biphosphate carboxylase/oxygenase (Rubisco) involves Rubisco activase Trp16. Biochemistry 35, 8143–8148 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Shivhare D., Mueller-Cajar O., In vitro characterization of thermostable CAM Rubisco activase reveals a Rubisco interacting surface loop. Plant Physiol. 174, 1505–1516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C., Salvucci M. E., Portis A. R. Jr, Two residues of rubisco activase involved in recognition of the Rubisco substrate. J. Biol. Chem. 280, 24864–24869 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Ott C. M., Smith B. D., Portis A. R. Jr, Spreitzer R. J., Activase region on chloroplast ribulose-1,5-bisphosphate carboxylase/oxygenase nonconservative substitution in the large subunit alters species specificity of protein interaction. J. Biol. Chem. 275, 26241–26244 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Larson E. M., O’Brien C. M., Zhu G., Spreitzer R. J., Portis A. R. Jr, Specificity for activase is changed by a Pro-89 to Arg substitution in the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. J. Biol. Chem. 272, 17033–17037 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Henderson J. N., Kuriata A. M., Fromme R., Salvucci M. E., Wachter R. M., Atomic resolution x-ray structure of the substrate recognition domain of higher plant ribulose-bisphosphate carboxylase/oxygenase (Rubisco) activase. J. Biol. Chem. 286, 35683–35688 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasse D., Larsson A. M., Andersson I., Structure of Arabidopsis thaliana Rubisco activase. Acta Crystallogr. D Biol. Crystallogr. 71, 800–808 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Hauser T., Popilka L., Hartl F. U., Hayer-Hartl M., Role of auxiliary proteins in Rubisco biogenesis and function. Nat. Plants 1, 15065 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Werneke J. M., Chatfield J. M., Ogren W. L., Alternative mRNA splicing generates the two ribulosebisphosphate carboxylase/oxygenase activase polypeptides in spinach and Arabidopsis. Plant Cell 1, 815–825 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.To K. Y., Suen D. F., Chen S. C. G., Molecular characterization of ribulose-1,5-bisphosphate carboxylase/oxygenase activase in rice leaves. Planta 209, 66–76 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Portis A. R. Jr, Li C., Wang D., Salvucci M. E., Regulation of Rubisco activase and its interaction with Rubisco. J. Exp. Bot. 59, 1597–1604 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Zhang N., Schürmann P., Portis A. R. Jr, Characterization of the regulatory function of the 46-kDa isoform of Rubisco activase from Arabidopsis. Photosynth. Res. 68, 29–37 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Moreau M. J., McGeoch A. T., Lowe A. R., Itzhaki L. S., Bell S. D., ATPase site architecture and helicase mechanism of an archaeal MCM. Mol. Cell 28, 304–314 (2007). [DOI] [PubMed] [Google Scholar]
- 33.DeSantis M. E., et al. , Operational plasticity enables hsp104 to disaggregate diverse amyloid and nonamyloid clients. Cell 151, 778–793 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeny E. A., et al. , The Hsp104 N-terminal domain enables disaggregase plasticity and potentiation. Mol. Cell 57, 836–849 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werbeck N. D., Schlee S., Reinstein J., Coupling and dynamics of subunits in the hexameric AAA+ chaperone ClpB. J. Mol. Biol. 378, 178–190 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Barta C., Dunkle A. M., Wachter R. M., Salvucci M. E., Structural changes associated with the acute thermal instability of Rubisco activase. Arch. Biochem. Biophys. 499, 17–25 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Chakraborty M., et al. , Protein oligomerization monitored by fluorescence fluctuation spectroscopy: Self-assembly of rubisco activase. Biophys. J. 103, 949–958 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keown J. R., Griffin M. D., Mertens H. D., Pearce F. G., Small oligomers of ribulose-bisphosphate carboxylase/oxygenase (Rubisco) activase are required for biological activity. J. Biol. Chem. 288, 20607–20615 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blayney M. J., Whitney S. M., Beck J. L., NanoESI mass spectrometry of Rubisco and Rubisco activase structures and their interactions with nucleotides and sugar phosphates. J. Am. Soc. Mass Spectrom. 22, 1588–1601 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Serban A. J., Breen I. L., Bui H. Q., Levitus M., Wachter R. M., Assembly-disassembly is coupled to the ATPase cycle of tobacco Rubisco activase. J. Biol. Chem. 293, 19451–19465 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson-Forbrook D. S., et al. , Nucleotide dependence of subunit rearrangements in short-form Rubisco activase from spinach. Biochemistry 56, 4906–4921 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Wang Q., Serban A. J., Wachter R. M., Moerner W. E., Single-molecule diffusometry reveals the nucleotide-dependent oligomerization pathways of Nicotiana tabacum Rubisco activase. J. Chem. Phys. 148, 123319 (2018). [DOI] [PubMed] [Google Scholar]
- 43.van de Loo F. J., Salvucci M. E., Involvement of two aspartate residues of Rubisco activase in coordination of the ATP gamma-phosphate and subunit cooperativity. Biochemistry 37, 4621–4625 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Kuriata A. M., et al. , ATP and magnesium promote cotton short-form ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase hexamer formation at low micromolar concentrations. Biochemistry 53, 7232–7246 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Scafaro A. P., et al. , Heat tolerance in a wild Oryza species is attributed to maintenance of Rubisco activation by a thermally stable Rubisco activase ortholog. New Phytol. 211, 899–911 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Zhang N., Portis A. R. Jr, Mechanism of light regulation of Rubisco: A specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-f. Proc. Natl. Acad. Sci. U.S.A. 96, 9438–9443 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurek I., et al. , Enhanced Thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 19, 3230–3241 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crafts-Brandner S. J., Salvucci M. E., Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc. Natl. Acad. Sci. U.S.A. 97, 13430–13435 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salvucci M. E., Crafts-Brandner S. J., Mechanism for deactivation of Rubisco under moderate heat stress. Physiol. Plant. 122, 513–519 (2004). [Google Scholar]
- 50.Wang D., et al. , Two Rubisco activase isoforms may play different roles in photosynthetic heat acclimation in the rice plant. Physiol. Plant. 139, 55–67 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Scafaro A. P., Haynes P. A., Atwell B. J., Physiological and molecular changes in Oryza meridionalis Ng., a heat-tolerant species of wild rice. J. Exp. Bot. 61, 191–202 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keown J. R., Pearce F. G., Characterization of spinach ribulose-1,5-bisphosphate carboxylase/oxygenase activase isoforms reveals hexameric assemblies with increased thermal stability. Biochem. J. 464, 413–423 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Crafts-Brandner S. J., Van De Loo F. J., Salvucci M. E., The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiol. 114, 439–444 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharkey T. D., Badger M. R., von Caemmerer S., Andrews T. J., Increased heat sensitivity of photosynthesis in tobacco plants with reduced Rubisco activase. Photosynth. Res. 67, 147–156 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Sharkey T. D., Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 28, 269–277 (2005). [Google Scholar]
- 56.Baginsky S., Protein phosphorylation in chloroplasts–A survey of phosphorylation targets. J. Exp. Bot. 67, 3873–3882 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Kim S. Y., et al. , The plastid casein kinase 2 phosphorylates Rubisco activase at the Thr-78 site but is not essential for regulation of Rubisco activation state. Front. Plant Sci. 7, 404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boex-Fontvieille E., et al. , Phosphorylation pattern of Rubisco activase in Arabidopsis leaves. Plant Biol (Stuttg) 16, 550–557 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Kim S. Y., et al. , In vivo evidence for a regulatory role of phosphorylation of Arabidopsis Rubisco activase at the Thr78 site. Proc. Natl. Acad. Sci. U.S.A. 116, 18723–18731 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kummer E., et al. , Bacterial and yeast AAA+ disaggregases ClpB and Hsp104 operate through conserved mechanism involving cooperation with Hsp70. J. Mol. Biol. 428, 4378–4391 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Iosefson O., Nager A. R., Baker T. A., Sauer R. T., Coordinated gripping of substrate by subunits of a AAA+ proteolytic machine. Nat. Chem. Biol. 11, 201–206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scafaro A. P., Bautsoens N., den Boer B., Van Rie J., Gallé A., A conserved sequence from heat-adapted species improves Rubisco activase thermostability in wheat. Plant Physiol. 181, 43–54 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagarajan R., Gill K. S., Evolution of Rubisco activase gene in plants. Plant Mol. Biol. 96, 69–87 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Weibezahn J., et al. , Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell 119, 653–665 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Kardon J. R., et al. , Mitochondrial ClpX activates a key enzyme for heme biosynthesis and erythropoiesis. Cell 161, 858–867 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olivares A. O., Baker T. A., Sauer R. T., Mechanistic insights into bacterial AAA+ proteases and protein-remodelling machines. Nat. Rev. Microbiol. 14, 33–44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kahle M., Ter Beek J., Hosler J. P., Ädelroth P., The insertion of the non-heme FeB cofactor into nitric oxide reductase from P. denitrificans depends on NorQ and NorD accessory proteins. Biochim. Biophys. Acta Bioenerg., S0005-2728(18)30138-5 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Aigner H., et al. , Plant RuBisCo assembly in E. coli with five chloroplast chaperones including BSD2. Science 358, 1272–1278 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Ort D. R., et al. , Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. U.S.A. 112, 8529–8536 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker R. T., et al. , Using deubiquitylating enzymes as research tools. Methods Enzymol. 398, 540–554 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Makino A., Mae T., Ohira K., Purification and storage of ribulose 1,5-bisphosphate carboxylase from rice leaves. Plant Cell Physiol. 24, 1169–1173 (1983). [Google Scholar]
- 72.Lan Y., Mott K. A., Determination of apparent K(m) values for ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase using the spectrophotometric assay of Rubisco activity. Plant Physiol. 95, 604–609 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kubien D. S., Brown C. M., Kane H. J., Quantifying the amount and activity of Rubisco in leaves. Methods Mol. Biol. 684, 349–362 (2011). [DOI] [PubMed] [Google Scholar]
- 74.Horecker B. L., Hurwitz J., Weissbach A., Ribulose diphosphate. Biochem. Prep. 6, 83–90 (1958). [Google Scholar]
- 75.Kane H. J., Wilkin J. M., Portis A. R., John Andrews T., Potent inhibition of ribulose-bisphosphate carboxylase by an oxidized impurity in ribulose-1,5-bisphosphate. Plant Physiol. 117, 1059–1069 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.