Abstract
In this article, we suggest that motivation serves to anticipate the energy of the body and meet those needs before they arise, called allostasis. We describe motivation as the output of energy computations that include estimates about future energy/metabolic needs and the value of effort required for potential behaviors (i.e., whether the cost of effort is worthwhile). We bring neuroscience evidence to bear to support this hypothesis. We outline a system of brain networks that have been shown to be important for motivation, and focus in on one hub in this network, the anterior mid-cingulate cortex (aMCC), and discuss its importance for establishing motivation in the service of allostasis. We present evidence that the aMCC, positioned at the intersection of multiple brain networks, is wired to integrate signals relating to allostasis with its sensory consequences, termed interoception, as well as with cognitive control processes, sensory and motor functions. This integration guides the nervous system towards the optimal effort required to achieve a desired goal. Across a variety of task domains, we discuss the role of aMCC in motivation, including a) processing of the value of prior and expected rewards, b) assessment of energetic costs in the brain and the body, c) selectively learning and encoding prediction errors (unexpected changes) that are relevant for allostasis, d) computations for monitoring of internal states of the body and e) modulating the internal state of the body to prepare for action. Finally, we discuss the link between individual differences in aMCC processing and variation in two extreme ends of the range of motivational states, tenacity and apathy.
Keywords: allostasis, energy balance, anterior mid-cingulate cortex, motivation, tenacity, apathy
Introduction
Motivation has been defined as the willingness to invest resources to achieve a potentially rewarding goal (Mitchell and Daniels, 2003). A person can be motivated to move, think, pay attention, and learn, which impacts the capacity to survive and reproduce (James, 1890; Sterling and Laughlin, 2015). However, it is not always possible to pursue every goal that might lead to reward because the body has limited energy resources. Correspondingly, classical theories recognized early on the importance of energy balance and efficient energy regulation in motivation. According to one evolutionary theory of motivation, for example, individuals are motivated to adopt strategies that allow them to consume the most energy while expending the least amount of energy (Bernard et al., 2005). The drive-reduction motivational theory also suggests that humans are motivated to achieve a state of biological equilibrium, that is to satisfy physiological needs while correcting disturbances of homeostasis (a self-regulating process by which biological systems tend to maintain stability) (Hull, 1943). The need for achieving optimal energy balance extends beyond biological needs to include psychological states. For instance, the arousal theory of motivation holds that individuals are motivated to engage in behaviors that promote an optimal state of arousal (Berlyne, 1970; Yerkes and Dodson, 1908). Likewise, cognitive approaches to motivation point to the importance of achieving a state of cognitive equilibrium, a state where cognitive dissonance (uncertainty or conflicts between attitudes, beliefs, feelings) is minimized (Cooper, 2007; Festinger, 1957).
Efficient energy regulation requires a full accounting of the costs and benefits of each potential action. Consequently, modern theories of motivation increasingly describe motivational states as the output of a computation (calculation), where the expected value of an action is weighed against anticipated costs. These include energetic costs, as all goal pursuit will require the expenditure of metabolic energy, opportunity costs, as the pursuit of one goal might block progress towards another, hedonic costs, such as the unpleasantness of effort (Botvinick and Braver, 2015; Holroyd and Yeung, 2012; Shenhav et al., 2017). In psychological theory, the availability of physiological resources that can influence motivational computations in multiple ways has received less attention as an important component of these cost-benefit calculations. As the body’s energy supplies are limited, the potential cost of a behavior must be understood in relation to the momentary energy state of the body (and potentially in relation to future states). Similarly, research indicates that the value of a reward is dependent upon the status of physiological needs, and this is true, even for primary rewards like food (Cassidy and Tong, 2017). As a consequence, for example, the computed value of foraging behavior might depend not only on the calories expended seeking food vs. those gained by obtaining it, but must also be weighted considering how much metabolic energy is available for foraging, and how urgently new energy resources are needed.
As both the state of the body and the environment are constantly changing, a crucial component of efficient energy regulation is the ability of the brain to anticipate the future energetic needs of the body, and prepare to satisfy them before they arise. Predictive energy regulation of this sort has been termed allostasis ((Sterling, 2012) see also (Barrett, 2017a, b; Barrett and Simmons, 2015)). Traditional theories of motivation draw on a model of the brain as a fundamentally reactive ‘stimulus-response’ organ and therefore largely fail to take allostasis, as a predictive process, into account. Recent developments in theoretical and empirical neuroscience, however, suggest a new understanding of the brain as a dynamic, predictive machine that uses prior experiences to guide behavior towards goals. According to these accounts the brain is not a passive organ, but instead is constantly drawing on prior experience to construct a model which actively generates predictions about external events and internal energy economy. These predictions are continuously compared to sensory input, and when there is a mismatch, prediction errors are used by the brain to revise its model of the world (Barrett, 2017b, a; Barrett and Simmons, 2015; Friston, 2010, 2013). By constantly updating its predictions to minimize prediction error, the brain can effectively learn and adapt to changing circumstances.
In this paper, we will suggest that motivation is a manifestation of the dynamic regulation of energy resources to predictively meet emerging needs (allostasis). We first consider contemporary predictive theories of motivation. Although consistent with the predictive function of the brain, these theories fail to account for the influence of momentary energy states of the body on motivation. To fill this conceptual gap, we propose a framework for understanding motivation that integrates the brain’s predictive function with its role in promoting and maintaining allostasis. In support of our framework, we present evidence from brain connectomics to briefly describe the neural systems involved in allostasis, focusing on a hub region at the intersection of multiple networks, the anterior mid-cingulate cortex (aMCC). We hypothesize that the aMCC predicts the energy needs and guides behavior toward energy balance by launching the predictions that control the inner systems of the body while integrating ascending interoceptive inputs (e.g., signals related to the glucose levels in the blood) thereby guiding the computation of reward, cognitive control, and motor control. Drawing on evidence from neuroimaging during psychological tasks, we will show how this integration guides the nervous system towards the optimal effort required to achieve a desired goal. Finally, we will consider evidence from neuroimaging and lesion studies pointing to the crucial role of aMCC in two extreme ends of the range of motivational states, apathy and tenacity, i.e., a highly motivated state of defying challenges to achieve goals, to demonstrate how individual variation in motivation can be understood in an allostatic framework.
Motivation as a predictive process
The earliest accounts of motivation described it as a property of an organism or a property of a stimulus that was presumed to provoke a response from the organism. For example, some hypotheses focused on pleasure and pain as the primary drivers of behavior (Bentham, 1789; James, 1890), or on a set of distinct, fundamental psychological needs (Maslow, 1943; Murray, 1938) , each independently driving approach and avoidance of relevant stimuli (Elliot, 2008). Other hypotheses focused on the idea that behavior is extrinsically driven by an external reinforcing stimulus such as monetary reward (incentive theory, Vroom, 1964).
Later theories focused more on prediction as important to motivation. In an early example of such a predictive model, motivation includes not only a desire for a reward (valence), but also the expected probability that some goal will be achieved (expectancy) and that this will lead to that reward (instrumentality) (Lewin et al., 1944). When faced with the option of pursuing some goal, uncertain factors, such as the probability of success, must be estimated in advance of the generation of motivational states. Later theories (Eccles and Wigfield, 2002; Shenhav et al., 2013) added the predicted cost of acting to the equation, suggesting that a motivation to act depends on the expected value of action exceeding expected costs. These accounts focus primarily on predictions of external outcomes. Motivation-related computations may also include other factors, including predictions about the energy state of the body, however, incorporating new insights about the brain as a predictive organ that uses prior occurrences and statistical inference to guide action and construct experience in a fundamentally motivated way.
More recent models of motivated behavior have suggested that multiple components of motivational computations span multiple levels of complexity, with higher levels regulating lower ones (Pezzulo et al., 2018). For example, in the case of an individual deciding whether or not to have dessert, this view proposed that information signaling glucose levels in the blood is represented at the lowest level of motivational computations. The motivation to eat may be tempered, however, by the higher-level general goal of dieting. At the same time, though, episodic (context) details specific to the situation may intervene; if one is celebrating a birthday, the value of dessert may be represented as unusually high. Meanwhile, control processes integrate contextual details, constraining behavior towards actions predicted to successfully achieve desired goals; i.e., once dessert has been decided upon, one might need only to open the refrigerator if at home, but at a restaurant it must be ordered and paid for (Pezzulo et al., 2018). Thus, on this view, motivational computations integrate predictions about physiological needs with longer-term goals maintained by cognitive control and exteroception (sensory information originating outside of the body) about the specifics of the current environment. An implication of this model is that control and motivational processes, while related, are computationally distinct, and may involve different anatomical substrates. The brain has to infer (1) how to achieve goals based on control processes mediated by structures in the prefrontal cortex and the thalamus and (2) which goals are worth pursuing based on motivational processing that occurs in structures in striatum and basal ganglia (Pezzulo et al., 2018).
This more modern model of motivation recognizes both the fundamentally predictive nature of the brain and the importance of current information from the body in generating motivational states. It fails to acknowledge that predictions about the internal environment -- the body’s energetic needs and available energetic resources -- are as important to motivation as predictions of external outcomes. As both the internal and external environments are ever changing, both must be estimated in advance so that energy resources can be appropriately allocated before needs arise. Furthermore, the model fails to take into account predictions that span a longer temporal window, e.g., a goal of dieting requires predicting how eating dessert will impact your weight tomorrow or at some future time (a week, a month). In the next section, we consider this need for maintaining physiological regulation through prediction of internal states in understanding motivation, bridging physical and psychological aspects to propose a computational framework for motivation and related concepts like goals, reward and effort.
Motivation in the service of allostasis
Building on previous computational theories and recent findings in neuroimaging, we hypothesize that motivation results from neural computations whose goal is the maintenance of allostasis, or the predictive regulation of energy resources (Sterling, 2012). Allostasis is related to the widely used concept of homeostasis, but is distinct in that homeostasis focuses on maintaining stability in a reactive way, while allostasis is fundamentally predictive. Conventional theory of homeostasis holds that the body actively seeks physiologically optimal set-points (Cannon, 1932). When a departure from one of these set-points is detected, the body seeks to remedy this either by adjusting physiological processes to compensate (e.g., raising body temperature in cold environments), or motivating behavior that promotes homeostasis (e.g, foraging in conditions of hunger) through the generation of an aversive ‘drive’ state (Hull, 1943). However, there is no single optimal physiological state of the body; energy needs are constantly shifting due to changing environmental conditions and behavioral plans (Sterling, 2012). Thus, in order to maintain energy balance, bodily states must be matched to present energy needs. Thus a reactive approach to energy regulation would lead to a body equipped to meet the needs of the past, not the present. For maximally efficient energy regulation, it is necessary to predict future energy needs, so that the body can begin to move towards energy balance in advance. This predictive energy regulation is termed allostasis (for a model of allostasis see Barrett, 2017a, b; Barrett and Simmons, 2015; Sterling, 2012).
We hypothesize that motivation results from the brain’s attempts to maintain allostasis. The brain continually estimates future energy/metabolic needs (Barrett 2017a,b; Barrett & Simmons, 2015). This estimation includes not only predicted environmental conditions, but also whether or not effort required for potential behaviors are worthwhile. At the same time, predicted energy needs are continually compared to available resources. The output of this energy computation we term motivation. If this computation predicts an energy deficit, and a potential behavior is predicted to promote energy balance, an organism will be motivated to perform that behavior, while the body simultaneously marshals the necessary resources to meet task needs. Alternatively if predicted resources are computed to match predicted needs, motivation to act will be low. Thus motivation, on this view, is simply one part of an ensemble of metabolic changes that prepare the body for future demands, and an incidental consequence of the brain’s endeavor to carry out its most fundamental duty: to support the human body in metabolism and energy regulation. Understanding how the brain achieves allostasis, therefore, is equivalent to understanding how the brain achieves motivation.
Recent neuroscience research points to a large-scale system in the brain supporting allostasis, which connects predictions about energy requirements to systems that monitor and regulate the state of the body (Kleckner et al., 2017). This system includes two of the brain’s major intrinsic networks involved in sensing, thinking, and feeling (known as the salience and default mode network) (see Figure 1; (Kleckner et al., 2017). Each network is comprised of cortical and subcortical regions interconnected by the brain’s hub regions, including but not limited to regions important for motivation such as the anterior subregion of the mid-cingulate cortex (aMCC) (Kleckner et al., 2017). According to the allostasis model (Barrett 2017a,b; Barrett & Simmons, 2015), cortical regions issue physiological and visceromotor predictions to the autonomic nervous system, the immune system and the endocrine systems of the body (see Figure 2; (Barrett, 2017a, b; Barrett and Simmons, 2015). These regions are also hypothesized to send interoceptive predictions (predicted sensory consequences of upcoming changes relevant to allostasis) to the primary interoceptive cortex (mid/posterior insula) (Barrett and Simmons, 2015). Ascending interoceptive input from the body arrives at the primary interoceptive cortex (mid/posterior insula) and is compared with the interoceptive predictions. The difference is computed as prediction error signals (Barrett & Simmons, 2015) (see Figure 2).
Figure 1.
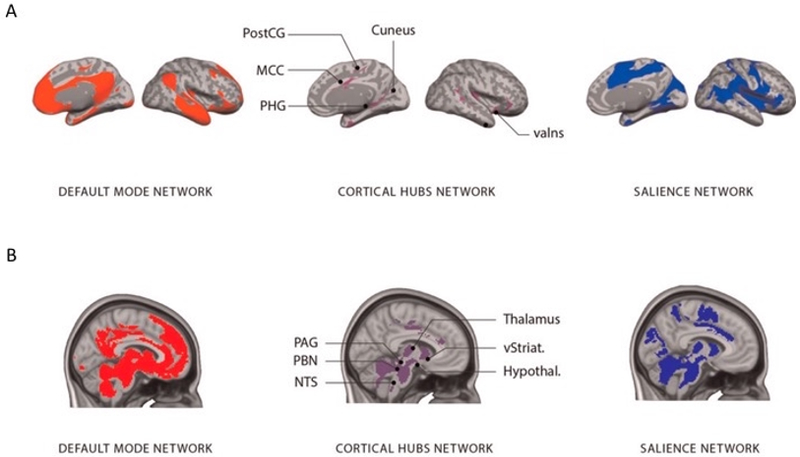
A large-scale system for allostasis and interoception in the human brain. (A) The system implementing allostasis and interoception is composed of two large-scale intrinsic networks (default mode network on the left; salience network on the right) that are interconnected by several hubs (shown in the middle; for coordinates, see Kleckner et al. (2017)). (B) The allostasis/interoception system, including subcortical connections. Note: valns, Ventral anterior insula; MCC, midcingulate cortex; PHG, parahippocampal gyrus; PostCG, postcentral gyrus; PAG, periaqueductal gray; PBN, parabrachial nucleus; NTS, the nucleus of the solitary tract; vStriat., ventral striatum; Hypothal., hypothalamus. Adapted with permission from Kleckner et al. (2017) as adapted in Barrett (Barrett, 2017).
Figure 2.
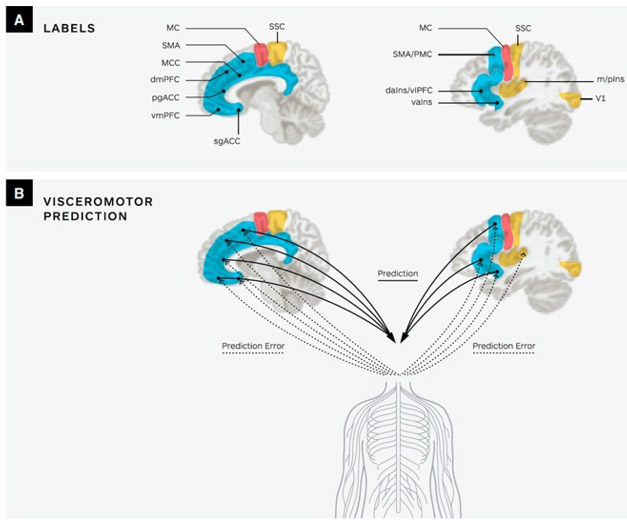
A depiction of visceromotor predictive coding in the human brain. (A) Key limbic and paralimbic cortices (SMA, MCC, dmPFC, pgACC, vmPFC, sgACC) provide cortical control of the body internal milieu. The primary motor cortex is labeled as MC. For simplicity, only primary visual cortex (V1), interoceptive cortex (m/pIns), and somatosensory cortex (SSC) are shown. Subcortical regions are not shown. (B) Limbic cortices initiate visceromotor predictions that descend to the body via the hypothalamus and brainstem nuclei (e.g. PAG, PBN and nucleus of the solitary tract) to regulate the autonomic, neuroendocrine and immune systems (solid lines). The ascending sensory inputs from the internal milieu of the body are carried along the vagus nerve and small diameter C and Aδ fibers to limbic regions (dotted lines). Comparisons between prediction signals and ascending sensory input result in prediction error that is available to update the brain’s internal model. Supplementary motor area-SMA; middle cingulate cortex- MCC; dorsomedial prefrontal cortex- dmPFC; pregenual anterior cingulate cortex - pgACC; ventromedial prefrontal cortex- vmPFC; subgenual anterior cingulate cortex- sgACC; middle and posterior insula- m/pIns. Adapted with permission from Barrett (2017).
An implication of this model of allostasis is that motor plans must be integrated with visceromotor signals to ensure that the body is prepared to meet task needs. The ability to dynamically regulate energy in this way is crucial for maintaining motivation in the face of difficulty. A number of neuroimaging studies (Holroyd and Yeung, 2012; Vassena et al., 2017; Vogt, 2016) suggest that the aMCC is a cortical structural and functional hub that could fulfill such a role of integrating motor and visceromotor signals to motivate behavior. Indeed, the aMCC is connected to regions important for motor (e.g., supplementary motor area) and visceromotor functions (e.g., anterior insula), reward (e.g. basal ganglia), attention (e.g., supramarginal gyrus) and effort (e.g., dorsolateral prefrontal cortex) (Beckmann et al., 2009; Wager et al., 2016).
In the following sections, we will review neuroimaging evidence for an aMCC role in motivation in the service of allostasis. We will first consider neuroanatomical evidence showing that the aMCC is a highly connected network hub, capable of integrating diverse inputs. We will then review neuroimaging evidence of aMCC engagement during tasks involving various computations relevant to motivation: a) processing of the value of prior and expected rewards, b) assessment of energetic costs in the brain and the body, c) selectively learning and encoding prediction errors (unexpected changes) that are relevant for allostasis, d) computations for monitoring of internal states of the body and e) modulating the internal state of the body to prepare for action.
Motivation in the brain: the role of anterior Mid-Cingulate Cortex in allostasis
Anterior Mid-Cingulate Cortex as a structural and functional “Hub”
Situated at the intersection of several of the brain’s major intrinsic networks, the aMCC is one of the most connected regions in the brain (Beckmann et al., 2009; van den Heuvel and Sporns, 2013a)(see Figure 3). Evidence from brain connectivity studies show that the aMCC participates in systems associated with visceromotor functions such as the “salience” (Seeley et al., 2007; Touroutoglou et al., 2012) and “allostatic-interoceptive” networks (Kleckner et al., 2017) as well as networks associated with executive function, attention, and motor control such as “frontoparietal control” (Vincent et al., 2008), “ventral attention” (Fox et al., 2006), and “cingulo-operculum control” networks (Dosenbach et al., 2007; Nelson et al., 2010). Additionally, it has been demonstrated that aMCC is as a key region in a multimodal network that integrates information originating from primary sensory regions (e.g., visual, auditory, and somatosensory) (Sepulcre et al., 2012). Consequently the aMCC has been described as one of an ensemble of “rich club” regions that synchronize information flow across the brain (van den Heuvel and Sporns, 2013a).
Figure 3.
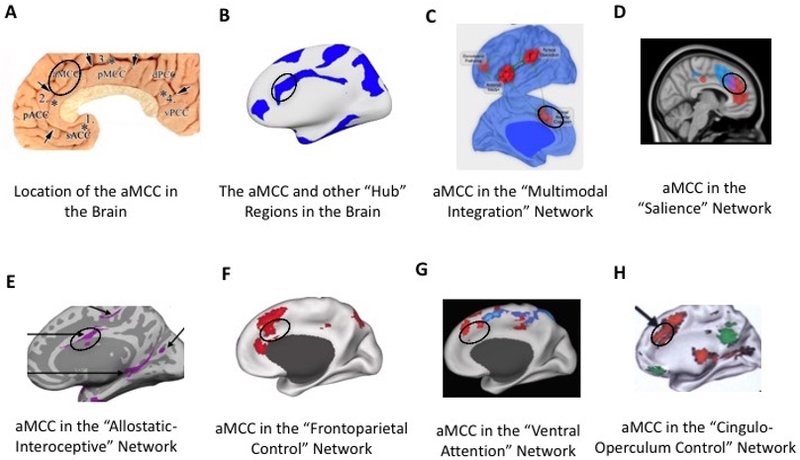
Neuroanatomy and connectivity of the aMCC (black circle). The aMCC region proposed by Vogt (A)(Vogt, 2005); aMCC as a member of the brain’s ‘rich club’ hubs (B)(van den Heuvel and Sporns, 2013b); aMCC (labeled dorsal anterior cingulate cortex) as a key region of the multimodal integration network (C)(Sepulcre et al., 2012); aMCC sits at the nexus (purple) of two salience subsystems; dorsal salience subsystem (blue) associated with attention and the ventral salience subsystem (red) associated with visceroautonomic processing (D)(Touroutoglou et al., 2012); aMCC as a key region of the large-scale allostatic/interoceptive system (E)(Kleckner et al., 2017), frontoparietal control system (F)(Vincent et al., 2008), ventral attention system (G)(Fox et al., 2006), and cinguloopercular network (H)(Dosenbach et al., 2007).
Consistent with its broad connectivity, the aMCC has been implicated in a wide variety of tasks. Indeed, meta-analyses indicate that it is among the most frequently reported areas of activation in functional MRI research (Clark-Polner et al., 2016; Nelson et al., 2010; Yarkoni et al., 2011), with reported activations in processes including pain, emotion, reward, conflict monitoring, error detection, memory, and social cognition (Beckmann et al., 2009; Wager et al., 2016). We will now review evidence of aMCC involvement in these various domains, focusing on its participation in each of the multiple motivational computations that serve allostasis.
Computations of the value of prior and expected rewards
A central component of motivational computation is the processing of the value of prior and expected rewards. Multiple neuroimaging studies indicate the aMCC is involved in reward-based decision-making tasks (Bahlmann et al., 2015). In particular, the aMCC seems to track both the magnitude and the probability of predicted rewards (Kouneiher et al., 2009), increasing its response to a task when reward value is either reduced (Bush et al., 2002), or increased (Rushworth and Behrens, 2008). The aMCC involvement in the processing of both increases and reductions of the expected rewards, suggests a more general role for the region in the computation of hedonic value. Indeed, a recent meta-analysis of 206 imaging studies showed that aMCC activity increases in response to changes in the magnitude of both expected rewarding and aversive outcomes, suggesting an underlying U-shaped function, indicative of signal related to arousal or salience processing (Bartra et al., 2013).
Assessment of energetic costs in the brain and the body
Motivational computations also require the assessment of energetic costs in the brain and the body, in terms of effort expended. Current research indicates that the aMCC encodes not only the value of a reward but also the cost of the effort required to obtain it (Harris and Lim, 2016). Numerous studies indicate that aMCC is engaged by cognitively demanding tasks involving executive function, motor function, memory, attention, language, and mathematics (Cole and Schneider, 2007; Duncan, 2010, 2013; Duncan and Owen, 2000; Power and Petersen, 2013; Hoffstaedter et al., 2014). Such complex tasks are generally experienced as effortful. In one study, Fedorenko and colleagues (2013) presented easy and difficult versions of several distinct tasks involving language, arithmetic, working memory and response inhibition in the same experiment, and found that the more difficult variant evoked significantly greater aMCC activity in every case, irrespective of task differences (Fedorenko et al., 2013). This suggests the possibility that aMCC activity represents a domain-general sense of subjective effort.
Sheth and colleagues (2012) provide evidence suggesting that in addition to monitoring current effort expenditure, the aMCC also serves to predict future effort requirements (Sheth et al., 2012). Their findings show that aMCC activity during a variable difficulty task is modulated by activity during previous trials in a way that accelerates reactions to cues of similar difficulty as previous trials, while slowing reactions when difficulty levels change. In this way, they suggest the aMCC provides a continuously updated prediction of expected cognitive demands. Such a predictive mechanism would allow the body to increase arousal and deploy cognitive control resources in advance of anticipated difficulties to meet task needs.
Selectively learning and encoding of prediction errors relevant for allostasis
Maintaining motivation in the face of unexpected difficulty requires rapid recalibration of allostatic computations when expectations are violated. In these cases, it is crucial that attention be immediately directed towards unexpected demands, such that changing energetic needs can be quickly assessed. Multiple studies indicate that the aMCC is preferentially activated by outcomes that defy expectations, such as when unexpected events occur (Jessup et al., 2010; Nee et al., 2011) errors are detected (Ullsperger and von Cramon, 2001) or available options are in conflict (Barch et al., 2001; Botvinick et al., 2004). Similarly, aMCC response is greatest when a task is novel (and task demands are largely unknown) but declines as a task becomes familiar (and task demands become predictable) (Bush et al., 1998; Raichle et al., 1994).
Indeed, the greater the prediction error (the more events deviate from expectations), the more likely the aMCC is to activate, leading some to suggest a role for aMCC in preparing control systems for future demands by predicting behavioral outcomes and adjusting to prediction errors (Alexander and Brown, 2011, 2017; Barrett and Simmons, 2015). Consistent with this view, feedback signaling a need for larger behavioral adaptations produces a substantially larger aMCC response than feedback signaling a need for smaller or no change (Jocham et al., 2009), and individuals with damage to their aMCC exhibit a marked impairment at feedback-based behavioral adaptation (Sheth et al., 2012). Given that encoding prediction errors is inherently energetically costly, it is necessary to set thresholds of precision for the detection of errors or deviations from prediction that are relevant for allostasis. A very sensitive system could be energetically wasteful while a less sensitive system might miss allostatically relevant information. The aMCC as a region engaged by prediction error may play a role in setting these thresholds to minimize costs such that we only learn information that is helpful to allostasis in the future.
Computations for monitoring of internal states of the body
Monitoring the internal state of the body is also crucial to motivational computations, as maintaining allostasis requires an accurate assessment of available physiological energy resources. Multiple studies have implicated the aMCC in tasks involving interoception. The resting activity of aMCC is significantly correlated with that of ventral anterior insular cortex (Taylor et al., 2009), which is believed to play an important role in predicting the state of the body (Barrett and Simmons, 2015). In task-related studies, when participants are asked to attend closely to their physiological state (by judging the timing of their own heartbeats), the aMCC is robustly engaged (Critchley et al., 2004). Furthermore, the magnitude of aMCC activity during heartbeat detection tasks significantly predicts interoceptive accuracy (Pollatos et al., 2007).
Additionally, substantial research indicates that the aMCC is engaged by various other interoceptive experiences, notably the experience of pain (Derbyshire et al., 2004; Lieberman and Eisenberger, 2015; Lindquist et al., 2012; Shackman et al., 2011; Vogt, 2005; Yarkoni et al., 2011). The aMCC has also been implicated in the processing of various other crucial physiological needs, such as hunger, thirst, and breathlessness (Lieberman and Eisenberger, 2015). Consistent with these findings, Lieberman (2015) has suggested that the aMCC functions as a ‘neural alarm’, which directs attention toward potential conflicts with enduring survival goals.
Modulating the arousal state of the body to prepare for action
Maintaining motivation appropriate to the present circumstances also requires regulation of the state of the body. After potential costs and benefits have been computed, and compared to available resources, it may be necessary to deploy additional energy resources to support task demands, or to limit resources in order to suppress costly and unproductive behavior. A potential mechanism through which this could be achieved is by the regulation of arousal. An increase in arousal can serve to generate additional available metabolic energy in times of stress. Additionally, changes in arousal levels can alter the set-points of various physiological processes (i.e., hunger is suppressed in times of stress), which can further influence motivational computations (Morville et al., In press).
The aMCC is well equipped to modulate states of arousal through its connections to mid-brain nuclei (Bar et al., 2016), and substantial research indicates that the aMCC indeed exerts regulatory control over various autonomic processes. For example, cognitive and social stressors known to evoke autonomic stress responses robustly engage the aMCC (Gianaros and Wager, 2015; Wager et al., 2009;), and the magnitude of aMCC responses have been associated with various stress-induced physiological changes. Increased blood flow in the aMCC is correlated with increases in blood pressure and heart rate variability, evoked by both mental (working memory) and physical (isometric exercise) exertion (Critchley et al., 2000; Critchley et al., 2003). Changes in pupil dilation, another marker of sympathetic activity, have also been associated with aMCC activity (Critchley, 2009). Acute stressors of the sort that evoke stress hormone release also activate the aMCC (Gianaros and Wager, 2015), and the degree of stress-evoked activation predicts the magnitude of the hormonal stress response (Hermans et al., 2011).
Thus, we propose that the aMCC performs computations to predict the value of planned behaviors and other events, and then translates these predictions into changes in physiological function (e.g., alterations in blood pressure, heart rate, hormonal responses, etc.) in order to deal with the situation at hand.
Mid-Cingulate in Motivated Behavior: Tenacity and Apathy
Taken together, the findings above suggest that the aMCC operates as a hub of communication, synchronizing information from the diverse systems that support motivation. We have seen how aMCC contributes to the individual components of motivational computations. In this section, we consider direct behavioral evidence relating aMCC to motivated behavior. To this end, we will focus on the extreme ends of the motivational distributions, considering how the aMCC contributes to persistence in the face of extreme challenge, and how disruption of aMCC function can lead to profound motivational deficits.
Tenacity and the anterior Mid-Cingulate Cortex
Evidence from a number of sources indicates that a healthy and well-connected aMCC is associated with persistent and strong motivation in the face of challenge, here termed tenacity.
Individuals with greater grey matter volume in the aMCC exhibit greater behavioral persistence (Van Schuerbeek et al., 2011). In addition to structural integrity, greater aMCC function is also associated with higher degrees of tenacity. The activity of the aMCC during a difficult task has been found to positively correlate with self-reports of the degree of effort exerted (Mulert et al., 2005). Similarly, greater aMCC activity during cost/benefit comparison is associated with a greater willingness to exert effort (Bonnelle et al., 2016; Chong et al., 2017). Additionally, when individuals are asked to choose between a more difficult task vs. an easier one, the aMCC is preferentially engaged by the choice of the more difficult option (Scholl et al., 2015), and the magnitude of aMCC activity during such a choice predicts measures of behavioral persistence (Kurniawan et al., 2010).
Greater motivation is also predicted by more efficient communication between aMCC and other motivationally relevant regions (Spielberg et al., 2012). Indeed, greater aMCC connectivity is associated with higher scores on survey measures of grit, a scale of persistence in the face of challenge (Myers et al., 2016), as well as with better academic performance (Wang et al., 2017). As excellence in academics requires sustained effort and motivation, this finding indicates that improved mid-cingulate function predicts better outcomes for life challenges outside of the laboratory.
The integrity of the aMCC also seems to play a role in maintaining cognitive function in successful aging. Recent exciting findings in aging show that aMCC structure is associated with superior memory performance in “superagers”, a group of older adults who maintain exceptionally youthful memory abilities (Harrison et al., 2012; Sun et al., 2016). Similar to ‘high-grit’ young people, superagers exhibit a more highly connected aMCC than older adults (Zhang et al., submitted).
Greater aMCC function has also been associated with success in achieving difficult life goals, notably in the area of exercise and weight loss. Maintaining a weight loss regimen requires substantial motivation; indeed, individual differences in grit predict adherence to physical exercise (Reed et al., 2013). Consistent with this observation, studies of brain metabolism during exercise indicate that a larger aMCC response is associated with greater exercise intensity (Kemppainen et al., 2005). Similarly, when individuals are presented with a choice between healthy foods and more calorically dense options, taking the healthy choice is associated with greater aMCC activity (Harding et al., 2017). Furthermore, preliminary evidence further suggests that direct stimulation of aMCC can bolster motivation to adhere to one’s weight loss goals. Leong et al. (2018) demonstrated in obese women that transcranial pink noise stimulation at the aMCC region results in reduced self-reported appetite on a ‘desire to eat’ scale (Leong et al., 2018).
Perhaps the most striking evidence for aMCC role in maintaining motivation can be found in the work of Parvizi and colleagues (2013), who report that direct stimulation of the aMCC produced an increase in “the will to persevere”. Patients described their experience of aMCC stimulation as evoking the feeling of preparing for a difficult challenge. In one patient’s words: “I started getting this feeling like … I was driving into a storm[…] and you’ve got to get across the hill”
Apathy and the anterior Mid-Cingulate Cortex
Just as more robust aMCC function has been associated with higher degrees of motivation, studies of individuals with disruptions of function in this region have reported profound motivational difficulties such as apathy.
Studies of clinical depression, a disorder marked by a profound disruption of motivation (Pizzagalli, 2014), have consistently observed aMCC dysfunction in depressed individuals (Holroyd & Umemoto, 2016; Vogt, 2016). Depressed individuals exhibit reduced activation of aMCC during complex and effortful tasks (Elliott et al., 1997) as well as reduced aMCC gray matter (Goodkind et al., 2015). Furthermore, the degree of reduction in aMCC volume predicts the severity of apathetic symptoms in depression (Lavretsky et al., 2007). Disruption of aMCC function could mediate apathy in multiple ways. One possibility is a disruption of the processing of reward (Holroyd and Umemoto, 2016). Consistent with this view, the depressed show substantially reduced aMCC activity during reward learning tasks (Kumar et al., 2008). Impaired ability to effectively learn from processing prediction error may also play a role. In one study employing a gambling task (Steele et al., 2007), healthy individuals responded to negative feedback with aMCC activation and improved reaction times, while depressed individuals showed neither aMCC engagement or behavioral improvement following errors. This inability to adjust behavior was also correlated with the degree of anhedonia (Steele et al., 2007). Apathy in depression may also result from a disruption of energy regulation. If the aMCC is not receiving accurate information about the energy state of the body, it could fail to properly predict levels of arousal, resulting in a deficit of energy resources relative to current needs. Indeed, recent studies indicate that individuals suffering from depression exhibit significantly decreased interoceptive sensitivity (Avery et al., 2014).
More direct evidence of the connection between aMCC dysfunction and apathy can be observed in clinical populations where the aMCC or its connections have been damaged. Multiple studies have demonstrated that apathy in patients with lesions in aMCC is robustly associated with atrophy in aMCC ((Marin and Wilkosz, 2005) for a review see (Ducharme et al., 2017)). Notably, in a case study, Naccache et al. (2005) report that a patient with extensive aMCC damage was able to complete cognitive control tasks at varying levels of difficulty but reported no difference in the subjective experience of effort between difficulty levels. Thus, damage to the aMCC can result both in reductions of motivation and an impaired ability to assess energetic costs in terms of effort, crucial for motivational computations towards achieving energy balance.
Conclusions
In this article, we present a model of motivation as a process whose primary function is the maintenance of allostasis, the efficient regulation of energy resources via prediction. This view, which places energy regulation and metabolism at the core of the concept of motivation, has a number of implications for theory and research practice.
If, as we suggest, motivation is the output of computations aimed at the promotion of allostasis, then motivational states should be understood as ubiquitous, as the body seeks allostasis at all times. Thus, motivation is not a mechanism engaged in only in circumstances of deprivation or great potential reward, but rather a continuous measure of the match between anticipated energy needs and resources, which can equally lead to tenacious action or lethargy. Additionally, this view has implications for our understanding of reward, suggesting that the rewarding property of any stimulus or behavior derives entirely from the extent to which it promotes allostasis.
Our framework holds that the brain actively predicts future energy requirements, and uses mismatches between predicted and perceived energy levels (prediction errors) to preemptively allocate energy resources. In this way, the brain can prepare the body to act before needs arise (e.g., blood pressure is adjusted prior to standing, to prevent fainting). Effective allostatic energy regulation requires various computations, including a) processing of the value of prior and expected rewards, b) assessment of energetic costs in the brain and the body, c) selectively learning and encoding prediction errors that are relevant for allostasis, d) monitoring of internal states of the body and e) modulating the internal state of the body to prepare for action.
The performance of these computations requires the integration of signal from diverse brain regions within large-scale intrinsic brain networks that serve allostasis. As a ‘hub’ region situated at the intersection of these networks, and receiving diverse inputs from brain regions involved in interoception, reward processing, and cognitive control, the aMCC is well equipped to integrate the various inputs to allostatic computations. By integrating these inputs, the aMCC serves to compute the predicted value and costs of planned behaviors, compare those costs to available physiological resources, and deploy additional resources when costly actions are judged to be worthwhile. In this way, the motivational calculations of the aMCC serve to guide behavior towards efficient (optimal) energy balance. Indeed, converging evidence from studies of brain connectivity, task-related activity, and neuropsychiatric case studies indicate that healthy aMCC function is associated with higher degrees of motivation, leading to tenacity and persistence in the face of challenge, while aMCC dysfunction is associated with profound behavioral apathy.
These findings suggest that future research in motivation should attend more closely to the physiological state of participants, as variation in the internal energy states of participants could exercise a potent influence over motivational computations. Factors such as the time of day, the amount of sleep on the night prior, and the time since last meal could all influence the amount of available metabolic resources of research participants, which could change the amount of effort expended in pursuit of a reward, or whether an outcome is considered rewarding at all. Crucially, variability in the physiological states of participants is not a confound to be controlled, but rather central to understanding the phenomenon of motivation. Moreover, since motivational computations are relevant to all processes requiring metabolic energy, these factors could potentially influence outcomes in all domains of psychology.
References
- Alexander WH, Brown JW (2011) Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci 14:1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, Brown JW (2017) The Role of the Anterior Cingulate Cortex in Prediction Error and Signaling Surprise. Top Cogn Sci. [DOI] [PubMed] [Google Scholar]
- Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK (2014) Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry 76:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahlmann J, Aarts E, D’Esposito M (2015) Influence of motivation on control hierarchy in the human frontal cortex. J Neurosci 35:3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar KJ, de la Cruz F, Schumann A, Koehler S, Sauer H, Critchley H, Wagner G (2016) Functional connectivity and network analysis of midbrain and brainstem nuclei. Neuroimage 134:53–63. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A (2001) Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex 11:837–848. [DOI] [PubMed] [Google Scholar]
- Barrett LF (2017a) The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci 12:1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF (2017b) How emotions are made: The secret life of the brain. . New York: Houghton Mifflin Harcourt. [Google Scholar]
- Barrett LF, Simmons WK (2015) Interoceptive predictions in the brain. Nat Rev Neurosci 16:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW (2013) The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF (2009) Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 29:1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentham J (1789) An Introduction to the Principles of Morals and Legislation. Clarendon Press: Oxford. [Google Scholar]
- Berlyne DE (1970) Novelty, complexity, and hedonic value. . Perception and Psychophysics 8: 279–286. [Google Scholar]
- Bernard LC, Mills M, Swenson L, Walsh RP (2005) An evolutionary theory of human motivation. Genet Soc Gen Psychol Monogr 131:129–184. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Manohar S, Behrens T, Husain M (2016) Individual Differences in Premotor Brain Systems Underlie Behavioral Apathy. Cereb Cortex 26:807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Braver T (2015) Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol 66:83–113. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8:539–546. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998) The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp 6:Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR (2002) Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A 99:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon W (1932) The Wisdom of the Body: W.W. Norton and Company. [Google Scholar]
- Cassidy RM, Tong Q (2017) Hunger and Satiety Gauge Reward Sensitivity. Front Endocrinol (Lausanne) 8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong TT, Apps M, Giehl K, Sillence A, Grima LL, Husain M (2017) Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol 15:e1002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Polner E, Wager TD, Satpute AB, Barrett LF (2016) Neural fingerprinting: Meta-analysis, variation, and the search for brain-based essences in the science of emotion In: Handbook of emotions, 4th Edition (Barrett LF, Lewis M, Haviland-Jones JM, eds). New York: Guilford. [Google Scholar]
- Cole MW, Schneider W (2007) The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage 37:343–360. [DOI] [PubMed] [Google Scholar]
- Cooper J (2007) Cognitive dissonance: Fifty years of a classic theory. . London: Sage. [Google Scholar]
- Critchley HD (2009) Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol 73:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ (2000) Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 523 Pt 1:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rothstein P, Ohman A, Dolan RJ (2004) Neural systems supporting interoceptive awareness. Nature Neuroscience 7:189–195. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ (2003) Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126:2139–2152. [DOI] [PubMed] [Google Scholar]
- Deci EL (1971) Effects of externally mediated rewards on intrinsic motivation. Journal of Personality and Social Psychology 18:105–115. [Google Scholar]
- Derbyshire SW, Whalley MG, Stenger VA, Oakley DA (2004) Cerebral activation during hypnotically induced and imagined pain. Neuroimage 23:392–401. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Price BH, Dickerson BC (2017) Apathy: a neurocircuitry model based on frontotemporal dementia. J Neurol Neurosurg Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J (2010) The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 14:172–179. [DOI] [PubMed] [Google Scholar]
- Duncan J (2013) The structure of cognition: attentional episodes in mind and brain. Neuron 80:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM (2000) Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23:475–483. [DOI] [PubMed] [Google Scholar]
- Eccles JS, Wigfield A (2002) Motivational Beliefs, Values, and Goals. Annual Review of Psychology 53:109–132. [DOI] [PubMed] [Google Scholar]
- Elliot AJ (2008) Handbook of Approach and Avoidance Motivation. New York: Taylor & Francis Group. [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O’Leary DA, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ (1997) Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med 27:931–942. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N (2013) Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A 110:16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger L (1957) A Theory of Cognitive Dissonance. Stanford, CA: Stanford University Press. [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (2010) The free-energy principle: a unified brain theory? Nat Rev Neurosci 11:127–138. [DOI] [PubMed] [Google Scholar]
- Friston K (2013) Active inference and free energy. Behav Brain Sci 36:212–213. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, Wethington E (2010) Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychol Rev 117:134–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD (2015) Brain-Body Pathways Linking Psychological Stress and Physical Health. Curr Dir Psychol Sci 24:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A (2015) Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding IH, Andrews ZB, Mata F, Orlandea S, Martinez-Zalacain I, Soriano-Mas C, Stice E, Verdejo-Garcia A (2017) Brain substrates of unhealthy versus healthy food choices: influence of homeostatic status and body mass index. Int J Obes (Lond). [DOI] [PubMed] [Google Scholar]
- Harris A, Lim SL (2016) Temporal Dynamics of Sensorimotor Networks in Effort-Based Cost-Benefit Valuation: Early Emergence and Late Net Value Integration. J Neurosci 36:7167–7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Weintraub S, Mesulam MM, Rogalski E (2012) Superior memory and higher cortical volumes in unusually successful cognitive aging. J Int Neuropsychol Soc 18:1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernandez G (2011) Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334:1151–1153. [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, Fox PT, Eickhoff SB (2014) The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp 35:2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N (2012) Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci 16:122–128. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Umemoto A (2016) The research domain criteria framework: The case for anterior cingulate cortex. Neurosci Biobehav Rev 71:418–443. [DOI] [PubMed] [Google Scholar]
- Hull CL (1943) Principles of Behavior: An Introduction to Behavior Theory. Oxford, England: D. Appelton-Century Company, Incorporated. [Google Scholar]
- James W (1890) The Principles of Psychology. New York City: Henry Holt and Company. [Google Scholar]
- Jessup RK, Busemeyer JR, Brown JW (2010) Error effects in anterior cingulate cortex reverse when error likelihood is high. J Neurosci 30:3467–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham G, Neumann J, Klein TA, Danielmeier C, Ullsperger M (2009) Adaptive coding of action values in the human rostral cingulate zone. J Neurosci 29:7489–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen J, Aalto S, Fujimoto T, Kalliokoski KK, Langsjo J, Oikonen V, Rinne J, Nuutila P, Knuuti J (2005) High intensity exercise decreases global brain glucose uptake in humans. J Physiol 568:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, Barrett LF (2017) Evidence for a Large-Scale Brain System Supporting Allostasis and Interoception in Humans. Nat Hum Behav 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E (2009) Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 12:939–945. [DOI] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD (2008) Abnormal temporal difference reward-learning signals in major depression. Brain 131:2084–2093. [DOI] [PubMed] [Google Scholar]
- Kurniawan IT, Seymour B, Talmi D, Yoshida W, Chater N, Dolan RJ (2010) Choosing to make an effort: the role of striatum in signaling physical effort of a chosen action. J Neurophysiol 104:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Ballmaier M, Pham D, Toga A, Kumar A (2007) Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. Am J Geriatr Psychiatry 15:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SL, De Ridder D, Vanneste S, Sutherland W, Ross S, Manning P (2018) High definition transcranial pink noise stimulation of anterior cingulate cortex on food craving: An explorative study. Appetite 120:673–678. [DOI] [PubMed] [Google Scholar]
- Lewin K, Dembo T, Festinger L & Sears R (1944). Level of aspiration In McV.Hunt J (Ed.), Personality and the behavioral disorders (pp. 333–278). New York: Ronald Press. [Google Scholar]
- Lieberman MD, Eisenberger NI (2015) The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proc Natl Acad Sci U S A 112:15250–15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF (2012) The brain basis of emotion: a meta-analytic review. Behav Brain Sci 35:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin RS, Wilkosz PA (2005) Disorders of diminished motivation. J Head Trauma Rehabil 20:377–388. [DOI] [PubMed] [Google Scholar]
- Maslow AH (1943) Preface to motivation theory. Psychosomatic Medicine 5:85–92. [Google Scholar]
- McEwen BS, Gianaros PJ (2010) Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 1186:190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TR, Daniels D (2003) Motivation In: Handbook of psychology (Borman W, Ilgen D, Klimoski R, eds), pp 225–254. New York: Wiley. [Google Scholar]
- Morville T, Friston K, Burdakov D, Siebner HR, Hulme OJ (In press) The Homeostatic Logic of Reward. bioRxiv. [Google Scholar]
- Mulert C, Menzinger E, Leicht G, Pogarell O, Hegerl U (2005) Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. Int J Psychophysiol 56:65–80. [DOI] [PubMed] [Google Scholar]
- Murray HA (1938) Explorations in Personality. New York: Oxford University Press. [Google Scholar]
- Naccache L, Dehaene S, Cohen L, Habert MO, Guichart-Gomez E, Galanaud D, Willer JC (2005) Effortless control: executive attention and conscious feeling of mental effort are dissociable. Neuropsychologia 43:1318–1328. [DOI] [PubMed] [Google Scholar]
- Nee DE, Kastner S, Brown JW (2011) Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. Neuroimage 54:528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE (2010) Role of the anterior insula in task-level control and focal attention. Brain Structure and Function 214:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius Michael D (2013) The Will to Persevere Induced by Electrical Stimulation of the Human Cingulate Gyrus. Neuron 80:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD (2006) Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442:1042–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G, Rigoli F, Friston KJ (2018) Hierarchical Active Inference: A Theory of Motivated Control. Trends in Cognitive Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA (2014) Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol 10:393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C (2007) Brain structures mediating cardiovascular arousal and interoceptive awareness. . Brain Research 1141:178–187. [DOI] [PubMed] [Google Scholar]
- Power JD, Petersen SE (2013) Control-related systems in the human brain. Curr Opin Neurobiol 23:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AK, Pardo JV, Fox PT, Petersen SE (1994) Practice-related Changes in Human Brain Functional Anatomy during Nonmotor Learnin. Cereb Cortex 4 1047–1321. [DOI] [PubMed] [Google Scholar]
- Reed J, Pritschet BL, Cutton DM (2013) Grit, conscientiousness, and the transtheoretical model of change for exercise behavior. J Health Psychol 18:612–619. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE (2008) Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci 11:389–397. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Deci EL (2000) Intrinsic and Extrinsic Motivations: Classic Definitions and New Directions. Contemp Educ Psychol 25:54–67. [DOI] [PubMed] [Google Scholar]
- Scholl J, Kolling N, Nelissen N, Wittmann MK, Harmer CJ, Rushworth MF (2015) The Good, the Bad, and the Irrelevant: Neural Mechanisms of Learning Real and Hypothetical Rewards and Effort. J Neurosci 35:11233–11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. Journal of Neuroscience 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Sabuncu MR, Yeo TB, Liu H, Johnson KA (2012) Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J Neurosci 32:10649–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD (2013) The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Musslick S, Lieder F, Kool W, Griffiths TL, Cohen JD, Botvinick MM (2017) Toward a Rational and Mechanistic Account of Mental Effort. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN (2012) Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Miller GA, Warren SL, Engels AS, Crocker LD, Banich MT, Sutton BP, Heller W (2012) A brain network instantiating approach and avoidance motivation. Psychophysiology 49:1200–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JD, Kumar P, Ebmeier KP (2007) Blunted response to feedback information in depressive illness. Brain 130:2367–2374. [DOI] [PubMed] [Google Scholar]
- Sterling P (2012) Allostasis: a model of predictive regulation. Physiol Behav 106:5–15. [DOI] [PubMed] [Google Scholar]
- Sterling P, Laughlin SB (2015) Principles of neural design. Cambridge, MA: : MIT Press. [Google Scholar]
- Sun FW, Stepanovic MR, Andreano J, Barrett LF, Touroutoglou A, Dickerson BC (2016) Youthful Brains in Older Adults: Preserved Neuroanatomy in the Default Mode and Salience Networks Contributes to Youthful Memory in Superaging. J Neurosci 36:9659–9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Seminowicz DA, Davis KD (2009) Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping 30:2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L (2012) Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage 60:1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY (2001) Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage 14:1387–1401. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2013a) Network hubs in the human brain. Trends Cogn Sci 17:683–696. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2013b) An anatomical substrate for integration among functional networks in human cortex. J Neurosci 33:14489–14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schuerbeek P, Baeken C, De Raedt R, De Mey J, Luypaert R (2011) Individual differences in local gray and white matter volumes reflect differences in temperament and character: a voxel-based morphometry study in healthy young females. Brain Res 1371:32–42. [DOI] [PubMed] [Google Scholar]
- Vassena E, Holroyd CB, Alexander WH (2017) Computational Models of Anterior Cingulate Cortex: At the Crossroads between Prediction and Effort. Front Neurosci 11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008) Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. Journal of Neurophysiology 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA (2016) Midcingulate cortex: Structure, connections, homologies, functions and diseases. J Chem Neuroanat 74:28–46. [DOI] [PubMed] [Google Scholar]
- Vroom VH (1964) Work and motivation. Oxford, England: Wiley. [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN (2009) Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage 47:836–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Botvinick MM, Chang LJ, Coghill RC, Davis KD, Iannetti GD, Poldrack RA, Shackman AJ, Yarkoni T (2016) Pain in the ACC? Proc Natl Acad Sci U S A 113:E2474–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhou M, Chen T, Yang X, Chen G, Wang M, Gong Q (2017) Grit and the brain: spontaneous activity of the dorsomedial prefrontal cortex mediates the relationship between the trait grit and academic performance. Soc Cogn Affect Neurosci 12:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011) Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD (1908) The relation of strength of stimulus to rapidity of habit-formation Journal of Comparative Neurology and Psychology 18:459–482. [Google Scholar]
- Zhang J, Andreano J, Dickerson BC, Touroutoglou A, Barrett LF (submitted) Preserved functional connectivity in the default mode and salience networks is associated with youthful memory in superaging. [DOI] [PMC free article] [PubMed] [Google Scholar]


