Abstract
Small intestinal bacterial overgrowth (SIBO) is a common, yet underrecognized, problem. Its prevalence is unknown because SIBO requires diagnostic testing. Although abdominal bloating, gas, distension, and diarrhea are common symptoms, they do not predict positive diagnosis. Predisposing factors include proton-pump inhibitors, opioids, gastric bypass, colectomy, and dysmotility. Small bowel aspirate/culture with growth of 103–105 cfu/mL is generally accepted as the “best diagnostic method,” but it is invasive. Glucose or lactulose breath testing is noninvasive but an indirect method that requires further standardization and validation for SIBO. Treatment, usually with antibiotics, aims to provide symptom relief through eradication of bacteria in the small intestine. Limited numbers of controlled studies have shown systemic antibiotics (norfloxacin and metronidazole) to be efficacious. However, 15 studies have shown rifaximin, a nonsystemic antibiotic, to be effective against SIBO and well tolerated. Through improved awareness and scientific rigor, the SIBO landscape is poised for transformation.
INTRODUCTION
The adult gastrointestinal (GI) tract has the largest microbial population in the human body (1); the predominant site is the colon, containing 38 trillion bacteria (2). Culture-independent methods, such as next-generation sequencing, show low concentration of distinct bacterial populations in the duodenum of healthy individuals, in contrast with bacterial populations that inhabit the mouth (3). Bacterial concentrations increase progressively along the small intestine (4,5).
Small intestinal bacterial overgrowth (SIBO) is characterized by the presence of an abnormal amount of bacteria in the small intestine together with a constellation of GI symptoms. The purpose of this article is to provide an up-to-date review of SIBO, including symptom patterns, predisposing risk factors, prevalence, specialized diagnostic testing, and potential therapeutic interventions, and to describe gaps in our knowledge and unmet needs.
METHODS
A PubMed search was performed on June 8, 2018, to identify English-language publications of clinical trials pertaining to SIBO in adults since 1985 using the search terms “small bowel bacterial overgrowth,” “small intestinal bacterial overgrowth,” “SIBO,” “epidemiology,” “diagnosis,” “treatment,” “antibiotic (e.g., ciprofloxacin, cotrimoxazole, and metronidazole),” “rifaximin,” or “probiotic.” Clinical studies of rifaximin (n = 15), systemic antibiotics (n = 6), and probiotics (n = 3) in SIBO were included, whereas studies of combination therapies, for example, rifaximin with another antibiotic and/or other combination of systemic antibiotics or probiotics, were excluded from this review. A total of 23 references on predisposing factors and 4 on diagnostic testing for SIBO were included. Although we recognize that SIBO occurs in a wide spectrum of diseases discussed below, most literature on this topic has focused on patients presenting with either unexplained symptoms or symptoms of irritable bowel syndrome (IBS). Our review primarily focuses on these patients, as they are most commonly encountered in gastroenterology clinics, but other conditions are appropriately referenced wherever necessary.
CLINICAL FEATURES, PREVALENCE, AND PATHOETIOLOGY
Symptoms of SIBO are nonspecific and include abdominal pain, belching, bloating, diarrhea, distension, flatulence, and indigestion that overlap and vary in frequency, duration, and severity. Typically, over two-thirds of patients report the aforementioned symptoms (6,7). Diagnosis of SIBO is challenging, as illustrated by 1 study in which mean total symptom scores were similar regardless of whether patients tested positive or negative with duodenal aspirate and breath testing (P = 0.9) (6). Because a SIBO diagnosis requires specialized testing (e.g., microbial culture and breath testing), and owing to variability in patient populations and methods used to establish a diagnosis across studies (8), prevalence has been difficult to estimate. However, SIBO appears to be more prevalent in women and in older individuals (9).
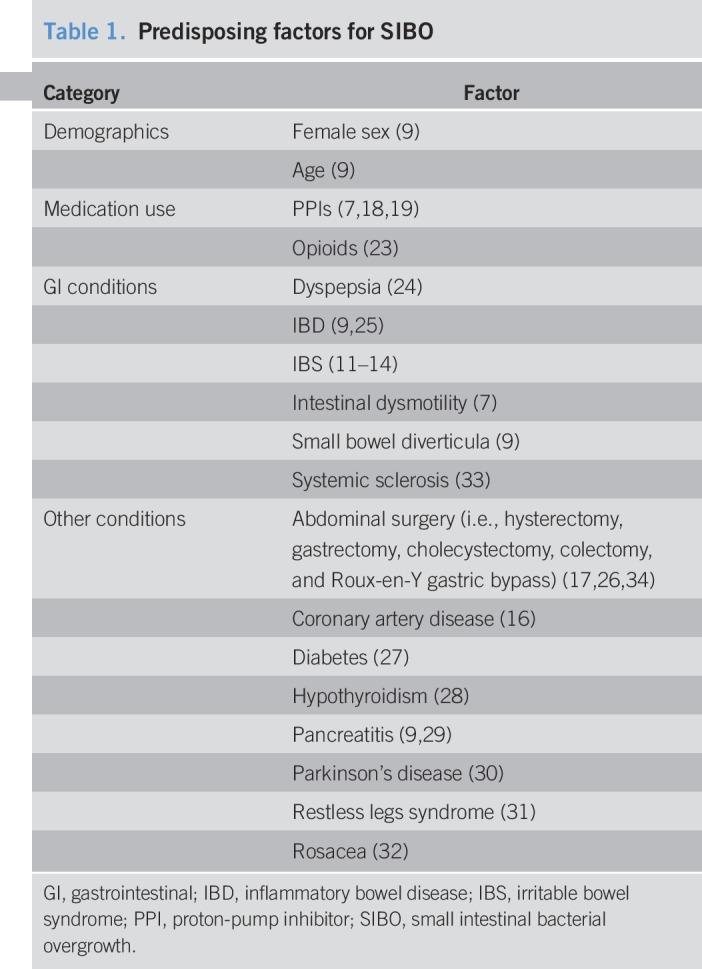
Several factors are associated with or predispose patients to SIBO, including small intestinal dysmotility (10). A study using duodenal aspirate/culture demonstrated that patients with small intestinal dysmotility were at increased risk of SIBO (>103 colony-forming units [cfu]/mL threshold, odds ratio [OR], 3.6; P = 0.0003; >105 cfu/mL threshold, OR, 2.7; P = 0.005) (7). Indeed, a significantly greater percentage of patients with IBS and SIBO were considered to have dysmotility vs patients with IBS without SIBO (86% vs 39%, respectively; P = 0.02) (11). Besides IBS, conditions that have been associated with SIBO include inflammatory bowel disease, dyspepsia, rosacea, restless legs syndrome, small bowel diverticula, pancreatitis, hypothyroidism, Parkinson's disease, diabetes, coronary artery disease, and abdominal surgery (e.g., hysterectomy, gastrectomy, cholecystectomy, and colectomy). However, the prevalence of SIBO in patients with these associated conditions is highly variable (range, 4%–79%) (11–16). In a 2018 case-control study, a significantly greater percentage of patients who underwent colectomy had SIBO compared with patients with long-standing GI complaints without colectomy (62% vs 32%, respectively; P = 0.0005) (17).
Some studies have suggested an association between SIBO and use of proton-pump inhibitors (PPIs) (7,18,19); however, others have not (9,20). PPIs may predispose patients to bacterial overgrowth by decreasing gastric acid (21). An initial study reported that 56% of 25 patients with peptic ulcers who received omeprazole had SIBO compared with none of 15 controls referred for diagnostic endoscopy (P = 0.0003). Subsequent studies have confirmed the association of SIBO with PPIs (18,19), including a retrospective study (n = 1,263 duodenal aspirates), showing that PPI use was significantly greater in patients with positive duodenal culture results compared with negative culture results (52.6% vs 30.2%, respectively; P < 0.0001) (18). Results of a 2017 meta-analysis of 19 studies (N = 7,055) confirmed a higher risk of SIBO with PPI use (OR, 1.7; 95% confidence interval [CI], 1.2–2.4) (22). A recent study demonstrated that probiotic bacteria may colonize the small bowel and predispose patients to SIBO and brain fogginess (23). Thus, numerous factors predispose an individual to the development of SIBO (Table 1) (7,9,11–19,23–34). Interestingly, up to 13% of healthy individuals have also tested positive for SIBO, based on the results of breath testing or small bowel aspirate and culture (26,35–41).
Table 1.
Predisposing factors for SIBO
DIAGNOSIS OF SIBO
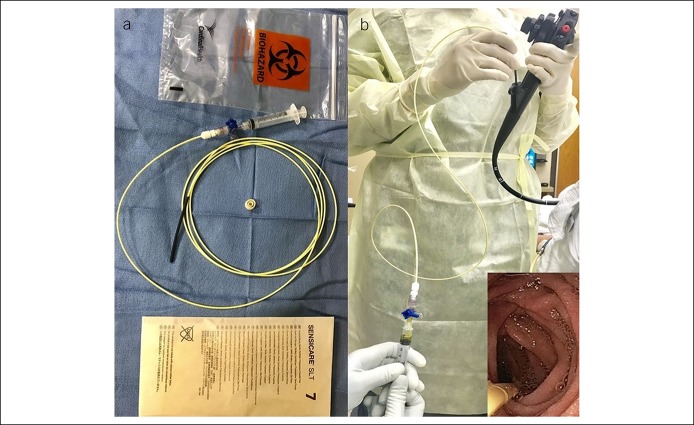
Small bowel culture is widely accepted as the “best diagnostic method” for establishing a diagnosis of SIBO (9); a threshold of ≥103 cfu/mL is recommended as a positive test result for SIBO, especially when performing duodenal aspirate and culture, because of very low bacterial counts in this more acidic environment (6–8). However, some investigators have suggested a higher threshold of ≥105 cfu/mL based on traditional microbiological standards for bacterial infection and for jejunal culture (11–13,42,43). In culture-based diagnostic testing, aseptic technique is critical to minimize cross-contamination from outside the duodenum (6), and standardized methods are needed (8). To this end, aspiration of duodenal juice by endoscopic suction and collection in a container may be fraught with contamination (18). One study described the use of a double-lumen catheter for collection of small bowel aspirate to prevent oropharyngeal cross-contamination of the sample (12), but this device is not commercially available. For more than 2 decades, we have successfully used a 6F Liguory catheter (COOK Medical, Bloomington, IN) with multiple side holes at its tip (Figure 1) for collection of small bowel aspirate. The catheter assembly and aspiration kit is first prepared by wearing sterile gloves before the procedure (Figure 1). Next, a sterilized upper endoscope, kept in a sterile wrap and flushed with sterile water before intubation, is passed into the second/third portion of the duodenum using minimal air insufflation. Thereafter, the staff change to another set of sterile gloves to prevent contamination during specimen collection. The endoscopist then passes the Liguory catheter through the biopsy channel of the scope, using the short overtube to prevent biopsy valve contamination. The technician is usually seated for gravity-assisted suction and flow and gently aspirates fluid by repeated suction using a 5-mL sterile syringe connected to a 3-way stopcock. If the lumen is dry, the liver may be gently massaged to facilitate the flow of bile into the duodenum. Typically, within approximately 2–5 minutes, 3 mL of bile-stained duodenal juice is successfully aspirated (Figure 1) (17). The syringe is capped with a sterile cap, and the specimen is placed in a biohazard bag and immediately sent to the microbiology laboratory for aerobic and anaerobic cultures (6,7,44).
Figure 1.
Description of the procedure for duodenal aspiration, specimen collection, and handling: The technician flushes the scope with sterile water and prepares a sterile field. A Liguory catheter with a stopcock is assembled (a). The scope is passed into the second/third portion of the duodenum with minimal air insufflation and suctioning. The endoscopist and the technician wear sterile gloves and advance the Liguory catheter through the biopsy channel. The technician performs gravity-assisted aspiration by holding the syringe at a height lower than the patient to aid fluid flow. Using gentle suction, ∼3 mL of duodenal fluid is collected and immediately transferred to the microbiology laboratory (b).
Care is taken not to aspirate oral secretions or stomach juices before securing the scope in the duodenum and passing the catheter. In the microbiology laboratory, after vortexing the sample, the following agar plates are inoculated using a 0.001 calibrated loop: blood, chocolate, MacConkey, Columbia nalidixic acid with blood, anaerobic blood, phenyl ethyl alcohol, Remel Anaerobic LPV Blood (Thermo Fisher Scientific, Waltham, MA), which contains paromomycin and vancomycin, inhibitory mold, and mycobiotic. Agar plates are then struck for the colony count. The blood and chocolate agars are held at 37 °C in carbon dioxide for 5 days. MacConkey and Columbia nalidixic acid plates are held in oxygen for 48 hours before being discarded. Anaerobic media are incubated under anaerobic conditions for 5 days. Any bacterial growth ≥1,000 cfu/mL is identified and reported out using colony count numeration. The organisms (i.e., Neisseria sp, Gram-positive bacilli resembling diphtheroids/coryneforms, Lactobacillus species, Streptococcus viridans group, Staphylococcus coagulase negative, and Rothia sp.) are identified based on gram stain, colonial morphology, or spot tests. Wherever appropriate, antibiotic susceptibility panels are performed and reported (17).
The limitations of small bowel culture include its invasive nature, cost, potential inability to detect bacterial strains that are difficult to grow under standard culture conditions, detection of proximal SIBO only, and potential for sample contamination (6,8).
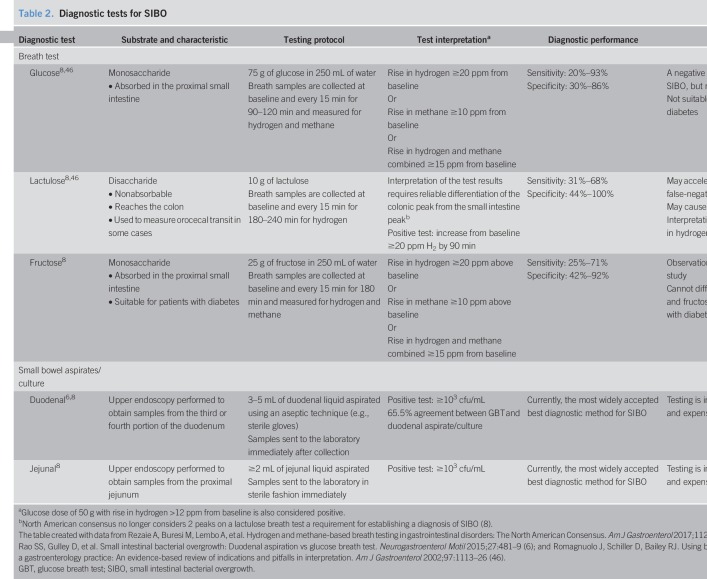
Breath testing is a safe and noninvasive diagnostic method for SIBO. However, there is currently no standard methodology for breath testing (8). During a breath test, patients ingest a carbohydrate substrate that is metabolized when exposed to GI microbes, leading to the production of hydrogen and methane. Some of these gases are absorbed from the GI tract into the blood stream and finally exhaled through the lungs, and therefore, analysis of breath samples after carbohydrate ingestion provides an indirect measure of detecting SIBO (45). Glucose and lactulose are commonly used as breath test substrates for detecting SIBO (Table 2) (6,8,46).
Table 2.
Diagnostic tests for SIBO
In 2017, the North American consensus regarding breath testing (8) provided updates to previously published statements by expert groups from Germany and Italy in 2005 and 2009, respectively (47,48). The consensus recommends that patients avoid treatment with antibiotics for 4 weeks and promotility agents and laxatives for at least 1 week before breath testing (8). Furthermore, a strict bland diet, including avoidance of fermentable foods (e.g., complex carbohydrates), is recommended for the day before administration of the breath test. Patients should also fast 8–12 hours before the breath test, avoid smoking the day of the breath test, and minimize physical exertion during the breath test (8).
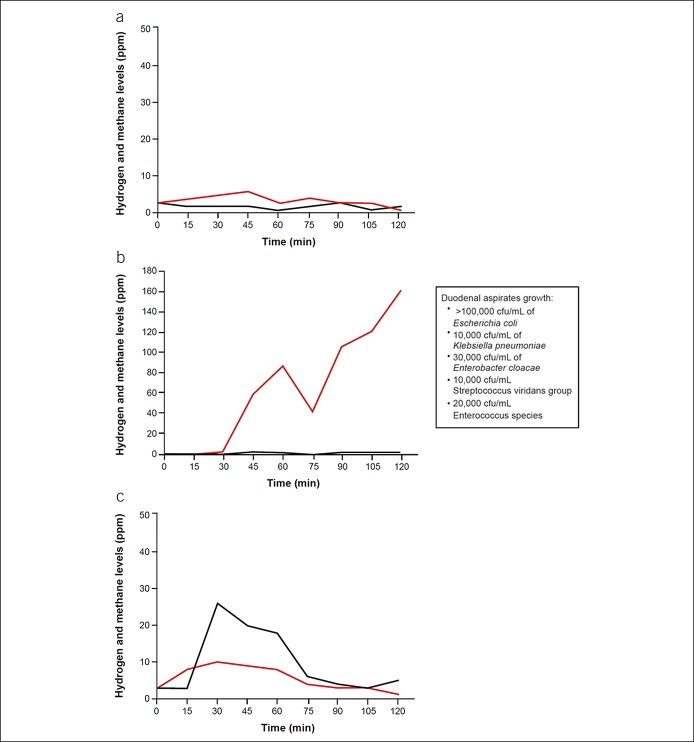
The North American consensus for breath testing recommends administering 75 g of glucose or 10 g of lactulose, taken with or followed by 1 cup of water; breath testing should measure hydrogen, methane, and carbon dioxide (see Table 2 for test characteristics) (8). An increase in hydrogen concentrations of ≥20 ppm from baseline within 90 minutes (8) (Figure 2a,b) (49) and an increase from baseline in methane concentrations of ≥10 ppm within 2 hours are considered diagnostic of SIBO (Figure 2a,c) (8,49). When using lactulose as a substrate, an initial duodenal peak from bacterial overgrowth in the small intestine followed by a second hydrogen peak from colonic bacterial fermentation has been observed (50), although both hydrogen peaks are not required for the diagnosis of SIBO (8). In addition, the first peak must occur within 90 minutes of substrate administration for the test to be considered positive (Table 2) (8). Although lactulose breath testing has higher sensitivity than glucose, scintigraphic studies have shown that the rise in breath hydrogen coincides with the arrival of lactulose in the cecum, raising concerns for a false-positive result; by contrast, the glucose breath test (GBT) has good specificity but low sensitivity, as it detects only proximal SIBO because glucose is completely absorbed in the proximal jejunum (Table 2) (8,46). A study from Lin and Massey (51) demonstrated that rapid transit of glucose into the cecum may provide a false-positive breath test within 90 minutes, possibly because of colonic fermentation, based on GBT combined with nuclear scintigraphy. Although possible, the likelihood of glucose reaching the cecum is low, as glucose is usually completely absorbed in the proximal small bowel. On the other hand, lactulose invariably will reach the cecum, as it is a nonabsorbable disaccharide (52). Arguably, scintigraphy is an imperfect test to localize the cecum. Small bowel loops or diverticula overlying the cecal region could be erroneously reported as a tracer filling this area. Also, the head of the meal may have reached the cecum, but the bulk of the meal could remain in the small intestine undergoing fermentation from SIBO, and both sources of fermentation could produce a rise in breath hydrogen or methane. Consequently, better substrates, better localization methods, and further validation are needed to settle this dilemma.
Figure 2.
Typical example of breath test results: Shown are a negative breath test result (a), a positive hydrogen breath test showing hydrogen concentration rising >20 ppm from baseline (b), and a positive breath test showing methane concentration rising >10 ppm from baseline (c). Red lines show hydrogen concentrations, and black lines show methane concentrations. Duodenal aspirates and culture results are shown in the text inset of (b).
Microbial culture and breath testing used to diagnose SIBO do not always produce similar findings (6,12). In 1 study, duodenal aspirate culture (i.e., ≥103 cfu/mL) and GBT (i.e., increase from baseline of both hydrogen and methane ≥20 ppm, of hydrogen ≥20 ppm, or of methane ≥15 ppm) agreed on the diagnosis of SIBO in 65.5% of 139 patients with GI-related symptoms (i.e., abdominal discomfort, gas, bloating, and diarrhea) considered related to SIBO (6). Another study found that although 39% of 18 patients were positive for SIBO with GBT, these results correlated poorly with jejunal culture results, suggesting that further validation is required. Therefore, 1 testing method may not definitively diagnose SIBO, and additional testing may be necessary when SIBO is suspected (6).
The North American consensus recommended a cutoff of ≥103 cfu/mL when using culture methods for the diagnosis of SIBO (8). Although additional validation studies are warranted to standardize breath testing including optimal cutoff thresholds (8), new approaches of breath testing have been investigated. A 2017 study reported the results of administering glucose through endoscope rather than oral ingestion in patients with a negative oral GBT (53). The results showed an increased yield for the diagnosis of SIBO, implying that endoscopic administration of glucose may facilitate detection of distal SIBO. This requires further validation with combined scintigraphy to reassure that the rise is not from colonic fermentation. A novel, orally ingested capsule technology that can measure in vivo hydrogen and carbon dioxide after ingestion of a carbohydrate meal is currently under development and may provide a better alternative to current breath hydrogen measurement techniques (54). Also, another novel oral diagnostic capsule, a smart capsule bacterial detection system, has been developed and tested ex vivo (55). This system can detect bacteria and, in a noninvasive manner, provides bacterial concentration; however, further clinical trials and validation are needed to assess the use of the smart capsule bacterial detection system for SIBO diagnosis (55).
TREATMENT OF SIBO
The goal of treatment for patients with SIBO is symptom relief by eradicating overgrowth of bacteria. This is typically achieved by treatment with antibiotics. However, some patients may remain symptomatic despite treatment, suggesting that other underlying conditions (e.g., dysmotility and PPI use) may potentially be the cause of symptoms and/or the bacteria may be antibiotic resistant (56). Hence, effective treatment includes eradication of bacteria, treatment of predisposing conditions, and prevention of SIBO. To date, no drugs have received regulatory approval in the United States or Europe specifically for the treatment of SIBO. However, the following treatments have been studied in patients with SIBO.
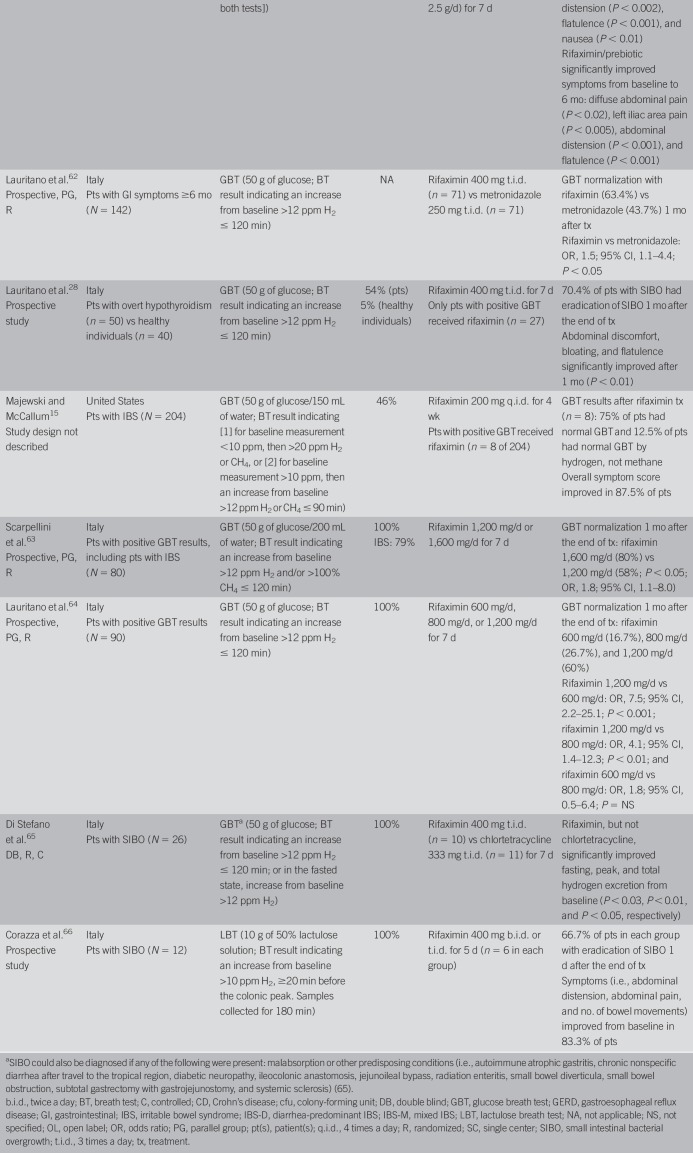
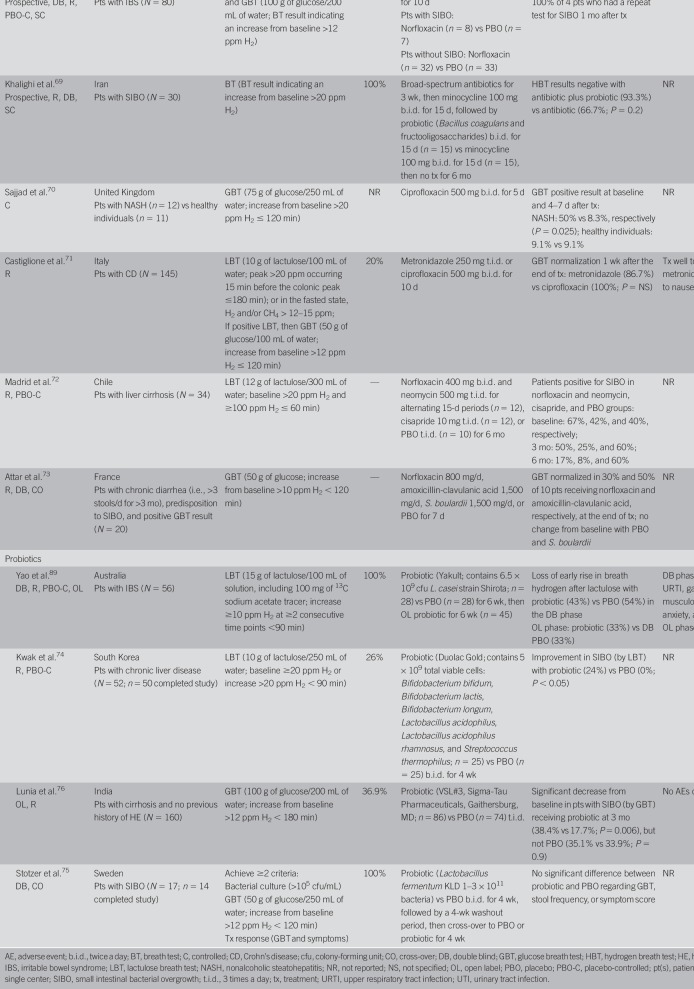
Rifaximin, a nonsystemic antibiotic, is currently the most studied agent for patients with SIBO, with numerous studies demonstrating its efficacy (e.g., eradication of SIBO), although the dose and duration of treatment, SIBO diagnostic methods and definitions, and patient populations vary among studies (Table 3) (15,18,25,28,30,57–66). A systematic review and meta-analysis of rifaximin (dose range: 600–1,600 mg/d; duration of treatment: 5–28 days) reported that SIBO was eradicated (determined by glucose or lactulose breath testing) in 70.8% of patients (26 studies; 95% CI, 61.4–78.2) (67). Adverse events (AEs) were uncommon and occurred in 4.6% of 815 patients from 17 studies reporting safety (67). In the meta-analysis (67), patients discontinued rifaximin treatment because of an AE (5% of 120 patients) in only 1 study (68). Clostridium difficile infection was reported in 1 patient receiving rifaximin 1,200 mg/d for 4 weeks; however, detailed information was lacking (59,67).
Table 3.
Summary of clinical studies of rifaximin
Systemic antibiotics
Studies of systemic antibiotics (e.g., ciprofloxacin, norfloxacin, and metronidazole) also reported eradication of SIBO as determined by either the breath test or bacterial culture (Table 4) (43,69–73). However, the sample sizes are small, and the methodologies used and populations evaluated differ across studies. A meta-analysis of 10 prospective clinical studies of nonsystemic antibiotics in patients with SIBO reported higher rates for breath test normalization with an antibiotic vs placebo (51.1% vs 9.8%, respectively; effectiveness ratio, 2.6; 95% CI, 1.3–5.0; P = 0.03) (56). Normalization of the breath test occurred in 49.5% of the pooled population (n = 325) treated with a nonsystemic antibiotic, rifaximin. The response rate varied widely (21.7%–85.0%), possibly because of rifaximin dosing and the timing of the breath test. Normalization of the breath test with metronidazole, a systemic agent (n = 86), was observed in 51.2% of patients (56). Finally, 70% of patients with SIBO and brain fogginess who received different antibiotics reported significant improvement of SIBO symptoms (P = 0.005), and 85% achieved complete resolution of brain fogginess (P = 0.05) (23).
Table 4.
Summary of clinical studies of systemic antibiotics or probiotics
Probiotics
Probiotics are believed to have beneficial effects on the gut microbiota. However, few clinical studies have examined this option (Table 4) (74–76); furthermore, these studies lack consistency not only in the formulations used but also in the duration of treatment, populations assessed, and methods of diagnosing SIBO (74,75). In a safety study, probiotics were not associated with AEs (76). More recently, a 2017 meta-analysis of 18 studies reported that probiotics were associated with significantly increased clearance of SIBO compared with nonprobiotic therapy (6 studies; relative risk, 1.6; 95% CI, 1.2–2.2), although probiotics were not found to be efficacious for the prevention of SIBO (77). Furthermore, probiotics may inadvertently colonize the small bowel, causing both SIBO and d-lactic acidosis, as well as brain fogginess (23). Some experts consider these findings to be controversial (78).
Nonpharmacologic and dietary therapies
Several nonpharmacologic treatments have been proposed because of the cost and potential adverse effects of antibiotics and probiotics. One such approach is an elemental diet, which contains predigested micronutrients that are mostly absorbed within the proximal small bowel, thus limiting the delivery of nutrients to bacteria in the distal portion of the small intestine (79). In a retrospective review, 124 patients with SIBO received an elemental diet for 14 days; patients without normalization of breath testing continued the diet for an additional 7 days (80). The cumulative symptomatic response rate for an elemental diet was 85% (79/93 patients) (80). A total of 14 patients (12%) were unable to tolerate the diet (80). At 1-month follow-up (n = 63 patients), 28/36 patients with improved IBS symptoms had a normalized breath test result (80). However, these diets are generally not palatable and difficult to adhere to and require a motivated patient (79).
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols has been shown to be beneficial in IBS (81). This may be due to decreasing the exposure of small intestinal bacteria exposure to carbohydrate and its fermentation products, thereby stifling bacterial growth or altering luminal fluid transport and/or colonic gas production. However, there is a lack of sound data to suggest that a low fermentable oligosaccharides, disaccharides, monosaccharides, and polyol diet is beneficial for patients with SIBO.
Recurrent SIBO
Approximately 44% of patients with SIBO may experience a relapse of symptoms within 9 months of initial treatment (82). For these patients, the most effective way to achieve eradication is by first identifying the appropriate organism(s) and providing targeted antibiotic therapy (i.e., “the right drug for the right bug” approach). This is best achieved by small bowel aspiration, culture, and sensitivity. Another strategy is to identify and correct any underlying condition(s), such as avoiding medications that delay gut transit, reduce PPI and opioid use, and improve glycemic control, and adhesiolysis or correction of blind loops (7,83). Prokinetic agents improve motility and could enhance antegrade clearance of bacteria (72). Hence, a trial of prokinetic agents such as cisapride, tegaserod, erythromycin, and prucalopride may be considered, but there are no data to support their use, and some of these agents are not universally available or have risks (72,84,85). Some factors are not reversible, such as radiation enteritis, systemic sclerosis, postgastric resection, and surgical resection of the ileocecal valve (83,86). In such patients, some experts recommend cyclical monthly low-dose antibiotic therapy using 2 or 3 antibiotics (83,86). However, these approaches merit controlled trials.
Treatment failures
Approximately 30%–40% of patients may not have resolution of SIBO symptoms with antibiotic trials as shown in Table 4. In such cases, other overlapping or alternate diagnosis should be considered, such as disaccharide deficiency or food intolerances (87,88). For example, a patient with SIBO and lactose intolerance could present with symptoms of gas, bloating, and diarrhea; antibiotics will only confer partial resolution of symptoms. In addition, the patient will require a lactose-free diet. Therefore, a comprehensive assessment of symptoms with appropriate diagnostic tests and careful exclusion of other conditions is important in a patient with risk factors or in those with suboptimal response to therapy. Rigorous controlled studies are needed to guide clinical management. Moreover, other overlapping conditions such as pancreatic exocrine insufficiency, bile acid malabsorption, hormonal oversecretion, medications, functional bloating, hypersensitivity, and factitious symptoms should all be considered as possible causes.
CONCLUSIONS
SIBO causes nonspecific GI symptoms and is associated with other GI and non-GI conditions. Because of the broad range of symptoms experienced by these patients, symptoms alone cannot be used to establish a SIBO diagnosis. Consequently, diagnostic testing is required. Although inconsistencies exist, bacterial culture of small bowel aspirates is generally accepted as the best diagnostic method for the diagnosis of SIBO, but aseptic precautions and proper technique is key. Although a perfect breath test for SIBO is currently lacking, the 2017 North American consensus document offers clinical direction regarding choice of substrate, testing methodology, and interpretation of results, which requires further refining as new evidence emerges (8). Breath testing is considered a safe and noninvasive diagnostic tool for SIBO, although gaps in knowledge regarding the optimal method(s) of performing and interpreting the breath test remain.
Therapies shown to be efficacious and well tolerated for patients with SIBO include the nonsystemic antibiotic rifaximin and systemic antibiotics. However, given the differences across study populations, diagnostic tests and interpretation, and dosing and duration of antibiotic therapy, large well-designed randomized clinical trials with appropriate patient selection and well-defined symptoms and objective criteria are warranted to guide effective management of SIBO.
CONFLICTS OF INTEREST
Guarantor of the article: Satish S. C. Rao, MD, PhD, is the guarantor of the narrative review and assumes responsibility for the decision to publish.
Specific author contributions: S.S.C.R. and J.B. were involved in interpreting data included in this review, drafting the manuscript, and approving the final draft of the review.
Financial support: This work was supported by Salix Pharmaceuticals, Bridgewater, NJ. S.S.C.R.: Previously received research funding from Salix Pharmaceuticals.
Potential competing interests: None.
ACKNOWLEDGEMENTS
Technical editorial assistance was provided, under the direction of the author, by Mary Beth Moncrief, PhD, and Sophie Bolick, PhD, Synchrony Medical Communications, LLC, West Chester, PA.
REFERENCES
- 1.D'Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta 2015;451:97–102. [DOI] [PubMed] [Google Scholar]
- 2.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stearns JC, Lynch MD, Senadheera DB, et al. Bacterial biogeography of the human digestive tract. Sci Rep 2011;1:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drasar BS, Shiner M, McLeod GM. Studies on the intestinal flora. I. The bacterial flora of the gastrointestinal tract in healthy and achlorhydric persons. Gastroenterology 1969;56:71–9. [PubMed] [Google Scholar]
- 5.Gorbach SL. Population control in the small bowel. Gut 1967;8:530–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erdogan A, Rao SS, Gulley D, et al. Small intestinal bacterial overgrowth: Duodenal aspiration vs glucose breath test. Neurogastroenterol Motil 2015;27:481–9. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs C, Coss Adame E, Attaluri A, et al. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment Pharmacol Ther 2013;37:1103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American Consensus. Am J Gastroenterol 2017;112:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choung RS, Ruff KC, Malhotra A, et al. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment Pharmacol Ther 2011;33:1059–67. [DOI] [PubMed] [Google Scholar]
- 10.Hoog CM, Lindberg G, Sjoqvist U. Findings in patients with chronic intestinal dysmotility investigated by capsule endoscopy. BMC Gastroenterol 2007;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posserud I, Stotzer PO, Björnsson ES, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007;56:802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghoshal UC, Srivastava D, Ghoshal U, et al. Breath tests in the diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome in comparison with quantitative upper gut aspirate culture. Eur J Gastroenterol Hepatol 2014;26:753–60. [DOI] [PubMed] [Google Scholar]
- 13.Pyleris E, Giamarellos-Bourboulis EJ, Tzivras D, et al. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: Relationship with irritable bowel syndrome. Dig Dis Sci 2012;57:1321–9. [DOI] [PubMed] [Google Scholar]
- 14.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000;95:3503–6. [DOI] [PubMed] [Google Scholar]
- 15.Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: Clinical profiles and effects of antibiotic trial. Adv Med Sci 2007;52:139–42. [PubMed] [Google Scholar]
- 16.Fialho A, Fialho A, Kochhar G, et al. Association between small intestinal bacterial overgrowth by glucose breath test and coronary artery disease. Dig Dis Sci 2017;63:412–21. [DOI] [PubMed] [Google Scholar]
- 17.Rao SSC, Tan G, Abdulla H, et al. Does colectomy predispose to small intestinal bacterial (SIBO) and fungal overgrowth (SIFO)? Clin Transl Gastroenterol 2018;9:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franco DL, Disbrow MB, Kahn A, et al. Duodenal aspirates for small intestine bacterial overgrowth: Yield, PPIs, and outcomes after treatment at a tertiary academic medical center. Gastroenterol Res Pract 2015;2015:971582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombardo L, Foti M, Ruggia O, et al. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010;8:504–8. [DOI] [PubMed] [Google Scholar]
- 20.Ratuapli SK, Ellington TG, O'Neill MT, et al. Proton pump inhibitor therapy use does not predispose to small intestinal bacterial overgrowth. Am J Gastroenterol 2012;107:730–5. [DOI] [PubMed] [Google Scholar]
- 21.Tsuda A, Suda W, Morita H, et al. Influence of proton-pump inhibitors on the luminal microbiota in the gastrointestinal tract. Clin Transl Gastroenterol 2015;6:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su T, Lai S, Lee A, et al. Meta-analysis: Proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol 2018;53:27–36. [DOI] [PubMed] [Google Scholar]
- 23.Rao SSC, Rehman A, Yu S, et al. Brain fogginess, gas and bloating: A link between SIBO, probiotics and metabolic acidosis. Clin Transl Gastroenterol 2018;9:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa MB, Azeredo IL, Jr, Marciano RD, et al. Evaluation of small intestine bacterial overgrowth in patients with functional dyspepsia through H2 breath test. Arq Gastroenterol 2012;49:279–83. Portuguese. [DOI] [PubMed] [Google Scholar]
- 25.Greco A, Caviglia GP, Brignolo P, et al. Glucose breath test and Crohn's disease: Diagnosis of small intestinal bacterial overgrowth and evaluation of therapeutic response. Scand J Gastroenterol 2015;50:1376–81. [DOI] [PubMed] [Google Scholar]
- 26.Kim DB, Paik CN, Kim YJ, et al. Positive glucose breath tests in patients with hysterectomy, gastrectomy, and cholecystectomy. Gut Liver 2017;11:237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virally-Monod M, Tielmans D, Kevorkian JP, et al. Chronic diarrhoea and diabetes mellitus: Prevalence of small intestinal bacterial overgrowth. Diabetes Metab 1998;24:530–6. [PubMed] [Google Scholar]
- 28.Lauritano EC, Bilotta AL, Gabrielli M, et al. Association between hypothyroidism and small intestinal bacterial overgrowth. J Clin Endocrinol Metab 2007;92:4180–4. [DOI] [PubMed] [Google Scholar]
- 29.Therrien A, Bouchard S, Sidani S, et al. Prevalence of small intestinal bacterial overgrowth among chronic pancreatitis patients: A case-control study. Can J Gastroenterol Hepatol 2016;2016:7424831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fasano A, Bove F, Gabrielli M, et al. The role of small intestinal bacterial overgrowth in Parkinson's disease. Mov Disord 2013;28:1241–9. [DOI] [PubMed] [Google Scholar]
- 31.Weinstock LB, Walters AS. Restless legs syndrome is associated with irritable bowel syndrome and small intestinal bacterial overgrowth. Sleep Med 2011;12:610–3. [DOI] [PubMed] [Google Scholar]
- 32.Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: A population-based cohort study. Br J Dermatol 2017;176:100–6. [DOI] [PubMed] [Google Scholar]
- 33.Parodi A, Sessarego M, Greco A, et al. Small intestinal bacterial overgrowth in patients suffering from scleroderma: Clinical effectiveness of its eradication. Am J Gastroenterol 2008;103:1257–62. [DOI] [PubMed] [Google Scholar]
- 34.Sabaté JM, Jouët P, Harnois F, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: A contributor to severe hepatic steatosis. Obes Surg 2008;18:371–7. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Liu G, Duan Y, et al. Prevalence of small intestinal bacterial overgrowth in multiple sclerosis: A case-control study from China. J Neuroimmunol 2016;301:83–7. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Yu YM, Zhang YQ, et al. Hydrogen breath test to detect small intestinal bacterial overgrowth: A prevalence case-control study in autism. Eur Child Adolesc Psychiatry 2018;27:233–40. [DOI] [PubMed] [Google Scholar]
- 37.Lasa JS, Zubiaurre I, Fanjul I, et al. Small intestinal bacterial overgrowth prevalence in celiac disease patients is similar in healthy subjects and lower in irritable bowel syndrome patients. Rev Gastroenterol Mex 2015;80:171–4. [DOI] [PubMed] [Google Scholar]
- 38.Rana SV, Sinha SK, Lal S, et al. Small intestinal bacterial overgrowth in North Indian patients with celiac disease. Trop Gastroenterol 2007;28:159–61. [PubMed] [Google Scholar]
- 39.Rubio-Tapia A, Barton SH, Rosenblatt JE, et al. Prevalence of small intestine bacterial overgrowth diagnosed by quantitative culture of intestinal aspirate in celiac disease. J Clin Gastroenterol 2009;43:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niu XL, Liu L, Song ZX, et al. Prevalence of small intestinal bacterial overgrowth in Chinese patients with Parkinson's disease. J Neural Transm (Vienna) 2016;123:1381–6. [DOI] [PubMed] [Google Scholar]
- 41.Gabrielli M, Bonazzi P, Scarpellini E, et al. Prevalence of small intestinal bacterial overgrowth in Parkinson's disease. Mov Disord 2011;26:889–92. [DOI] [PubMed] [Google Scholar]
- 42.Ghoshal UC, Ghoshal U. Small intestinal bacterial overgrowth and other intestinal disorders. Gastroenterol Clin North Am 2017;46:103–20. [DOI] [PubMed] [Google Scholar]
- 43.Ghoshal UC, Srivastava D, Misra A, et al. A proof-of-concept study showing antibiotics to be more effective in irritable bowel syndrome with than without small-intestinal bacterial overgrowth: A randomized, double-blind, placebo-controlled trial. Eur J Gastroenterol Hepatol 2016;28:281–9. [DOI] [PubMed] [Google Scholar]
- 44.Erdogan A, Rao SS. Small intestinal fungal overgrowth. Curr Gastroenterol Rep 2015;17:16. [DOI] [PubMed] [Google Scholar]
- 45.Levitt MD, Bond JH., Jr Volume, composition, and source of intestinal gas. Gastroenterology 1970;59:921–9. [PubMed] [Google Scholar]
- 46.Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: An evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol 2002;97:1113–26. [DOI] [PubMed] [Google Scholar]
- 47.Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment Pharmacol Ther 2009;29(Suppl 1):1–49. [DOI] [PubMed] [Google Scholar]
- 48.Keller J, Franke A, Storr M, et al. Clinically relevant breath tests in gastroenterological diagnostics: Recommendations of the German Society for Neurogastroenterology and Motility as well as the German Society for Digestive and Metabolic Diseases. Z Gastroenterol 2005;43:1071–90. German. [DOI] [PubMed] [Google Scholar]
- 49.Erdogan A, Lee YY, Badger C, et al. What is the optimal threshold for an increase in hydrogen and methane levels with glucose breath test (GBT) for detection of small intestinal bacterial overgrowth (SIBO)? Gastroenterology 2014;146:S–532. [Google Scholar]
- 50.Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil 2011;17:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin EC, Massey BT. Scintigraphy demonstrates high rate of false-positive results from glucose breath tests for small bowel bacterial overgrowth. Clin Gastroenterol Hepatol 2016;14:203–8. [DOI] [PubMed] [Google Scholar]
- 52.Saad RJ, Chey WD. Breath testing for small intestinal bacterial overgrowth: Maximizing test accuracy. Clin Gastroenterol Hepatol 2014;12:1964–72, quiz e1119–1920. [DOI] [PubMed] [Google Scholar]
- 53.Viswanathan L, Larion S, Shaffer N, et al. Endoscopically assisted glucose breath test (EAGBT): A novel diagnostic approach for distal sibo. Gastroenterol 2017;152:S413. [Google Scholar]
- 54.Kalantar-Zadeh K, Berean KJ, Ha N, et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat Electronics 2018;1:79–87. [Google Scholar]
- 55.Singh S, Allan N, Wahl C, et al. Development of a swallowable diagnostic capsule to monitor gastrointestinal health. Paper presented at: AGA 2019. Gastroenterology 2019;156(Suppl 1):s-376. [Google Scholar]
- 56.Shah SC, Day LW, Somsouk M, et al. Meta-analysis: Antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2013;38:925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bae S, Lee KJ, Kim YS, et al. Determination of rifaximin treatment period according to lactulose breath test values in nonconstipated irritable bowel syndrome subjects. J Korean Med Sci 2015;30:757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boltin D, Perets TT, Shporn E, et al. Rifaximin for small intestinal bacterial overgrowth in patients without irritable bowel syndrome. Ann Clin Microbiol Antimicrob 2014;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chedid V, Dhalla S, Clarke JO, et al. Herbal therapy is equivalent to rifaximin for the treatment of small intestinal bacterial overgrowth. Glob Adv Health Med 2014;3:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moraru IG, Portincasa P, Moraru AG, et al. Small intestinal bacterial overgrowth produces symptoms in irritable bowel syndrome which are improved by rifaximin: A pilot study. Rom J Intern Med 2013;51:143–7. [PubMed] [Google Scholar]
- 61.Rosania R, Giorgio F, Principi M, et al. Effect of probiotic or prebiotic supplementation on antibiotic therapy in the small intestinal bacterial overgrowth: A comparative evaluation. Curr Clin Pharmacol 2013;8:169–72. [DOI] [PubMed] [Google Scholar]
- 62.Lauritano EC, Gabrielli M, Scarpellini E, et al. Antibiotic therapy in small intestinal bacterial overgrowth: Rifaximin versus metronidazole. Eur Rev Med Pharmacol Sci 2009;13:111–6. [PubMed] [Google Scholar]
- 63.Scarpellini E, Gabrielli M, Lauritano CE, et al. High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2007;25:781–6. [DOI] [PubMed] [Google Scholar]
- 64.Lauritano EC, Gabrielli M, Lupascu A, et al. Rifaximin dose-finding study for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2005;22:31–5. [DOI] [PubMed] [Google Scholar]
- 65.Di Stefano M, Malservisi S, Veneto G, et al. Rifaximin versus chlortetracycline in the short-term treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2000;14:551–6. [DOI] [PubMed] [Google Scholar]
- 66.Corazza GR, Ventrucci M, Strocchi A, et al. Treatment of small intestine bacterial overgrowth with rifaximin, a non-absorbable rifamycin. J Int Med Res 1988;16:312–6. [DOI] [PubMed] [Google Scholar]
- 67.Gatta L, Scarpignato C. Systematic review with meta-analysis: Rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther 2017;45:604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyrat P, Safroneeva E, Schoepfer AM. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment Pharmacol Ther 2012;36:1084–93. [DOI] [PubMed] [Google Scholar]
- 69.Khalighi AR, Khalighi MR, Behdani R, et al. Evaluating the efficacy of probiotic on treatment in patients with small intestinal bacterial overgrowth (SIBO)–A pilot study. Indian J Med Res 2014;140:604–8. [PMC free article] [PubMed] [Google Scholar]
- 70.Sajjad A, Mottershead M, Syn WK, et al. Ciprofloxacin suppresses bacterial overgrowth, increases fasting insulin but does not correct low acylated ghrelin concentration in non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2005;22:291–9. [DOI] [PubMed] [Google Scholar]
- 71.Castiglione F, Rispo A, Di Girolamo E, et al. Antibiotic treatment of small bowel bacterial overgrowth in patients with Crohn's disease. Aliment Pharmacol Ther 2003;18:1107–12. [DOI] [PubMed] [Google Scholar]
- 72.Madrid AM, Hurtado C, Venegas M, et al. Long-term treatment with cisapride and antibiotics in liver cirrhosis: Effect on small intestinal motility, bacterial overgrowth, and liver function. Am J Gastroenterol 2001;96:1251–5. [DOI] [PubMed] [Google Scholar]
- 73.Attar A, Flourié B, Rambaud JC, et al. Antibiotic efficacy in small intestinal bacterial overgrowth-related chronic diarrhea: A crossover, randomized trial. Gastroenterology 1999;117:794–7. [DOI] [PubMed] [Google Scholar]
- 74.Kwak DS, Jun DW, Seo JG, et al. Short-term probiotic therapy alleviates small intestinal bacterial overgrowth, but does not improve intestinal permeability in chronic liver disease. Eur J Gastroenterol Hepatol 2014;26:1353–9. [DOI] [PubMed] [Google Scholar]
- 75.Stotzer PO, Blomberg L, Conway PL, et al. Probiotic treatment of small intestinal bacterial overgrowth by Lactobacillus fermentum KLD. Scand J Infect Dis 1996;28:615–9. [DOI] [PubMed] [Google Scholar]
- 76.Lunia MK, Sharma BC, Sharma P, et al. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: A randomized controlled trial. Clin Gastroenterol Hepatol 2014;12:1003–8.e1001. [DOI] [PubMed] [Google Scholar]
- 77.Zhong C, Qu C, Wang B, et al. Probiotics for preventing and treating small intestinal bacterial overgrowth: A meta-analysis and systematic review of current evidence. J Clin Gastroenterol 2017;51:300–11. [DOI] [PubMed] [Google Scholar]
- 78.Quigley EMM, Pot B, Sanders ME. “Brain fogginess” and D-lactic acidosis: Probiotics are not the cause. Clin Transl Gastroenterol 2018;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winitz M, Adams RF, Seedman DA, et al. Studies in metabolic nutrition employing chemically defined diets. II. Effects on gut microflora populations. Am J Clin Nutr 1970;23:546–59. [DOI] [PubMed] [Google Scholar]
- 80.Pimentel M, Constantino T, Kong Y, et al. A 14-day elemental diet is highly effective in normalizing the lactulose breath test. Dig Dis Sci 2004;49:73–7. [DOI] [PubMed] [Google Scholar]
- 81.Dionne J, Ford AC, Yuan Y, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. Am J Gastroenterol 2018;113:1290–300. [DOI] [PubMed] [Google Scholar]
- 82.Lauritano EC, Gabrielli M, Scarpellini E, et al. Small intestinal bacterial overgrowth recurrence after antibiotic therapy. Am J Gastroenterol 2008;103:2031–5. [DOI] [PubMed] [Google Scholar]
- 83.Sachdev AH, Pimentel M. Gastrointestinal bacterial overgrowth: Pathogenesis and clinical significance. Ther Adv Chronic Dis 2013;4:223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hennessy S, Leonard CE, Newcomb C, et al. Cisapride and ventricular arrhythmia. Br J Clin Pharmacol 2008;66:375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pimentel M, Morales W, Lezcano S, et al. Low-dose nocturnal tegaserod or erythromycin delays symptom recurrence after treatment of irritable bowel syndrome based on presumed bacterial overgrowth. Gastroenterol Hepatol (N Y) 2009;5:435–42. [PMC free article] [PubMed] [Google Scholar]
- 86.Pittman N, Rawn SM, Wang M, et al. Treatment of small intestinal bacterial overgrowth in systemic sclerosis: A systematic review. Rheumatology (Oxford) 2018;57:1802–11. [DOI] [PubMed] [Google Scholar]
- 87.Viswanathan L, Larion S, Shaffer N, et al. How useful is histological testing of disaccharidase deficiency in the evaluation of unexplained GI symptoms in adults? AGA Abstracts 2017;152:S7–S8. [Google Scholar]
- 88.Philpott H, Yu S, Rao S. Letter to the editor: It's all in the mix: Diagnosis and management of food intolerance. Clin Gastro Hepatol 2016;14:1221–4. [DOI] [PubMed] [Google Scholar]
- 89.Yao CK, Barrett JS, Philpott H, et al. Poor predictive value of breath hydrogen response for probiotic effects in IBS. J Gastroenterol Hepatol 2015;30:1731–9. [DOI] [PubMed] [Google Scholar]