Abstract
Dystonia is a movement disorder characterized by involuntary muscle contractions, twisting movements, and abnormal postures that may affect one or multiple body regions. Dystonia is the third most common movement disorder after Parkinson’s disease and essential tremor. Despite its relative frequency, small molecule therapeutics for dystonia are limited. Development of new therapeutics is further hampered by the heterogeneity of both clinical symptoms and etiologies in dystonia. Recent advances in both animal and cell-based models have helped clarify divergent etiologies in dystonia and have facilitated the identification of new therapeutic targets. Advances in medicinal chemistry have also made available novel compounds for testing in biochemical, physiological, and behavioral models of dystonia. Here, we briefly review motor circuit anatomy and the anatomical and functional abnormalities in dystonia. We then discuss recently identified therapeutic targets in dystonia based on recent preclinical animal studies and clinical trials investigating novel therapeutics.
Keywords: basal ganglia, cerebellum, drug discovery, anatomy, therapy, animal models
Introduction
Dystonia, the third most common movement disorder after tremor and Parkinson’s disease (Defazio, 2010), is characterized by involuntary muscle contractions that cause twisting movements and postures (Albanese et al., 2013). The causes of dystonia are diverse. It may occur as a sporadic (idiopathic) or inherited disorder (Schwarz and Bressman, 2009; Tanabe et al., 2009) and can sometimes occur as a result of brain injury (Frei et al., 2004; Krauss and Jankovic, 2002; Lo et al., 2005). The clinical features of dystonia are also heterogeneous. In some patients, dystonia affects only a small number of muscles, such as those of the hand in writer’s cramp or those of the neck in torticollis. In others, muscles throughout the body are involved. Although dystonia is not lethal, it is debilitating. Indeed, standardized quality of life scores for dystonia fall into the same range as Parkinson’s disease, stroke and multiple sclerosis (Camfield et al., 2002). Despite the prevalence and detrimental impact on quality of life, patients have very limited treatment options and struggle to find off-label alternatives that are rarely efficacious. Therefore, this review examines promising drug targets for dystonia, and presents a summary of recent preclinical, animal studies and clinical trials supporting their efficacy.
Motor Circuit Anatomy
The scarcity of small molecule drugs for the treatment of dystonia is directly attributable to the lack of clearly validated therapeutic targets; dystonia is not typically associated with degeneration or evident neuropathological abnormalities and the underlying cellular mechanisms are largely unknown. However, convergent evidence from clinical investigation and experimental models supports the view that dystonia is a circuit disorder, involving both the basal ganglia-thalamo-cortical and cerebello-thalamo-cortical pathways. Thus, a better understanding of how these networks function both normally and in dystonia is critical for discovering new therapeutics.
The Cortico-Basal Ganglia-Thalamo-Cortical Pathway
The basal ganglia are subcortical nuclei involved in diverse functions, including motor control and motor learning, executive function, and emotion. The basal ganglia include the striatum (caudate-putamen and nucleus accumbens), globus pallidus, subthalamic nucleus (STN), substantia nigra, and pedunculopontine nucleus (PPN). In brief, information flows through the basal ganglia back to the cortex through two pathways with opposing effects on movement execution: the “direct pathway” and the “indirect pathway” (Albin et al., 1989; DeLong, 1990). The original model of basal ganglia organization was based on experimental and clinical evidence of opposite functional effects of the direct and indirect projections on the output structures, which facilitate or inhibit movements, respectively (Gerfen, 2000). This model, which is supported by evidence from genetic, lesion and optogenetic studies, proposes that during normal behavior the direct pathway promotes a specific motor program while the indirect pathway inhibits competing motor programs (Bateup et al., 2010; Durieux et al., 2009; Kravitz et al., 2010). However, this model has undergone significant revisions to reflect more recent evidence demonstrating that direct and indirect pathways are not as segregated as originally proposed (Cui, 2013; Tecuapetla et al., 2016).
The striatum is the input nucleus of the basal ganglia. The striatum receives dopaminergic efferents (DA) from neurons originating in the substantia nigra pars compacta (SNc) and ventral tegmental area. The striatum also receives glutamatergic input from the cerebral cortex and thalamus. Dopaminergic and glutamatergic input to the striatum is integrated by GABAergic spiny projection neurons (SPNs), the major output neurons of the striatum. SPNs compose ≥ 95% of neurons in the striatum (Kawaguchi, 1993; Tepper, 2010). SPNs of the indirect striatopallidal pathway innervate the external segment of the globus pallidus (GPe) and express the dopamine (DA) D2 receptor. SPNs of the direct striatonigral pathway express the D1 DA receptor, and project directly to the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr). The GABAergic GPi and SNr neurons send inhibitory input to the ventral and intralaminar thalamic nuclei. Thalamic glutamatergic neurons then provide excitatory input to the motor cortex, which projects to spinal motor neurons or provides feedback to the striatum. In the indirect pathway, striatopallidal neurons provide inhibitory GABAergic input to GABAergic GPe neurons, which in turn provide inhibitory input to the STN. Glutamatergic neurons in the STN provide excitatory input to the GPi and SNr, which inhibit the thalamus and cortex (Albin et al., 1989; Parent and Hazrati, 1995).
The remaining ~5% of striatal neurons are represented by several different classes of interneurons, including: cholinergic interneurons (ChIs), GABAergic fast-spiking interneurons (FSIs), and other peptidergic interneurons (Kawaguchi, 1997). Some striatal interneurons, such as ChIs and FSIs, respond to dopaminergic signaling and reciprocally modulate both SPNs and other interneuron populations (Gittis, 2010; Gritton, 2019; Tepper et al., 2008).
The Basal Ganglia Connections with the Cerebello-Thalamo-Cortical Pathway
The cerebellum forms complex, large-scale connections with other brain regions and has a well-characterized role in motor learning and control. The cerebellar cortex has a trilaminar cytoarchitecture composed of the granular layer, the molecular layer, and the Purkinje cell layer. In the granular layer, mossy fiber afferents from extracerebellar regions activate granule cells and Golgi cells. Granule cells form ascending axons, called parallel fibers, which extend within the molecular layer. In the molecular layer, parallel fibers innervate inhibitory stellate and basket interneurons as well as Purkinje cells. Purkinje cells are also activated by climbing fibers, which originate in the inferior olive. The Purkinje cells project to the deep cerebellar nuclei, which in turn convey cerebellar output to other brain regions.
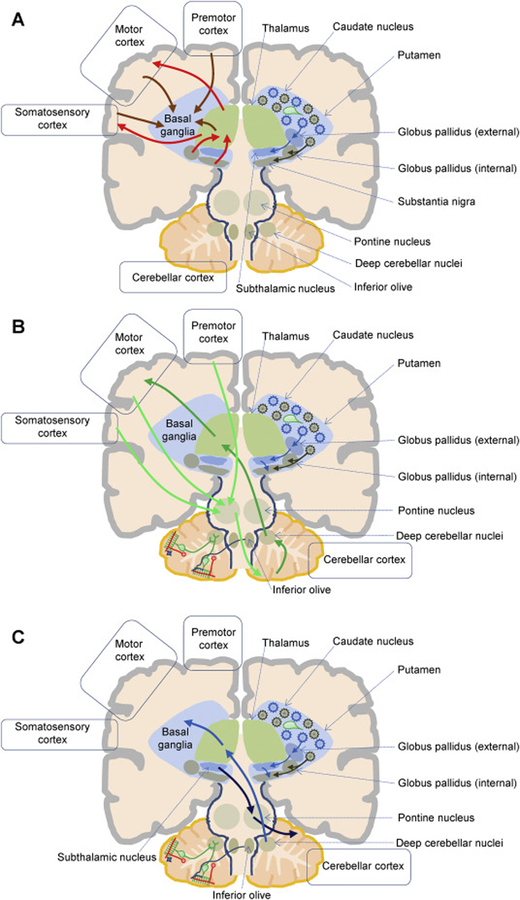
Anatomical and functional data support several bidirectional cerebello-thalamo-cortical circuits connecting the cerebellar cortex to motor, sensory, or associative cerebral cortical areas (Gornati, 2018; Krienen and Buckner, 2009; Schmahmann et al., 2019). Recent work uncovered an anatomical substrate between the basal ganglia and cerebellum, suggesting these structures may form an integrated functional network. Bidirectional communication occurs through a disynaptic projection from the dentate nucleus of the cerebellum to the striatum (Hoshi et al., 2005) and a disynaptic projection from the subthalamic nucleus of the basal ganglia to the cerebellar cortex (Bostan, 2010). This network is topographically organized, whereby the motor, cognitive and affective territories of the basal ganglia, cerebellum and cerebral cortex are interconnected. It is now accepted that the basal ganglia, cerebellum, and cerebral cortex form an integrated network, and this basal ganglia-to-cerebellar pathway plays a role in dystonic movements (Fig. 1) (Bostan, 2018; Neychev et al., 2008; Shakkottai et al., 2017).
Figure 1. Simplified cartoon of basal ganglia-cerebellum anatomical connections.
A. The cartoon depicts a simplified cortico-basal ganglia-thalamo-cortical circuit. Afferents from the cortex and thalamus (left, orange arrows) reach caudate and putamen neuronal populations of cholinergic interneurons (right, green) and projection neurons. Spiny projection neurons give rise to the direct (right, brown) and indirect (right, blue) output pathways, ultimately reaching the basal ganglia output nuclei (globus pallidus externus and substantia nigra pars reticulata), which send the processed information to the thalamus (left, red arrows). The thalamus then project back to the cortex (left, red arrow). B. The cartoon depicts simplified cortico-cerebello-thalamo-cortical connections. Left. The cerebellar cortex has a trilaminar cytoarchitecture composed of the Purkinje cell layer and the molecular layer (green), and of the granular layer (granule cells, red). Parallel fibers (red), originating from granule cells, reach the molecular layer and innervate inhibitory stellate (blue) and basket interneurons, as well as Purkinje cells (green). Purkinje cells are also activated by climbing fibers (blue), which originate in the inferior olive. The pontine nucleus receives cortical afferents and is a source of mossy fibers reaching the contralateral cerebellar cortex (light green arrow). Purkinje cells project to the deep cerebellar nuclei, which in turn convey cerebellar output to the cortex via the thalamus (green arrows). C. Retrograde transneuronal transport of rabies virus in monkeys revealed bidirectional disynaptic connections between the cerebellum and the basal ganglia. Specifically, a pathway originating from the deep cerebellar nuclei and reaching the putamen (blue arrows; Hoshi et al., 2005), and a pathway connecting the subthalamic nucleus to the cerebellar cortex (light blue arrows; Bostan et al., 2010) were identified.
Anatomical Basis of Dystonia
Historically, dystonia has been viewed as a disease of the basal ganglia due to the wealth of evidence implicating this region. In patients with hemidystonia, focal brain lesions were identified in the basal ganglia or associated regions (Bhatia and Marsden, 1994). Further, comorbidity of dystonia with other diseases of the basal ganglia, such as Parkinson’s disease (Tolosa and Compta, 2006) and Huntington’s disease (Louis et al., 1999), suggested involvement of the basal ganglia in the pathology of dystonia.
Human imaging studies have also implicated the basal ganglia in dystonia. In summary, functional magnetic resonance imaging studies of dystonia patients that reveal changes in basal ganglia activity generally show an increase in activation, particularly in the caudate, putamen and globus pallidus (Blood et al., 2004; Haslinger et al., 2010; Schneider et al., 2010). Voxel-based morphometry studies reveal alterations in volume of basal ganglia nuclei. In these studies, regional volume was increased in the caudate and decreased in the putamen of patients with cervical dystonia and blepharospasm (Obermann et al., 2007), though others have observed opposing trends within these regions (Draganski et al., 2009). Within the globus pallidus, regional volume is generally increased (Draganski et al., 2009; Draganski et al., 2003; Egger et al., 2007). Positron emission tomography (PET) imaging studies demonstrate a reduction in D2 DA receptor availability in patients with DYT1 dystonia and writer’s cramp and spasmodic dysphonia (Asanuma et al., 2005b; Berman et al., 2013; Simonyan et al., 2013), and an increase in the availability of D1 DA receptors in individuals with laryngeal dystonia and focal hand dystonia (Simonyan et al., 2017), which may contribute to an imbalance in the direct and indirect basal ganglia pathways. [18F]-fluorodeoxyglucose-PET imaging demonstrate that glucose metabolism is increased within the caudate and putamen in patients with cervical dystonia and DYT1 dystonia (Carbon et al., 2004; Galardi et al., 1996), though others have noted a reduction in activity in the putamen in dopa-responsive dystonia (DRD) patients (Asanuma et al., 2005b). These findings have been reviewed extensively (Asanuma et al., 2005a; Jinnah et al., 2017; Lehericy et al., 2013; Neychev et al., 2011; Simonyan, 2018) and point to the many associations between basal ganglia dysfunction and dystonia. Targeted interventions to basal ganglia nuclei for the treatment of dystonia such as pallidotomy (Lozano et al., 1997) and deep-brain stimulation of the GPi and STN (Ostrem et al., 2017; Ostrem and Starr, 2008) are also compelling evidence.
Although abundant evidence supports the role of the basal ganglia in dystonia, other regions, including the cerebellum, are also involved (Bologna and Berardelli, 2018; Neychev et al., 2011; Shakkottai et al., 2017). For example, cerebellar lesions occur in patients with cervical dystonia (LeDoux and Brady, 2003). Further, post-mortem analysis of brains from patients with cervical dystonia revealed loss of Purkinje cells, increased gliosis, and inclusions of torpedo bodies within Purkinje cells (Prudente et al., 2013). Additionally, there is an increase in metabolic activity in the cerebellum in myoclonus dystonia and cervical dystonia as assessed by PET imaging (Carbon et al., 2013; Eidelberg et al., 1998), and fMRI studies show alterations in cerebellar activation in individuals with dystonia (Filip et al., 2017; Prudente et al., 2016). Moreover, evidence from animal models also implicates the cerebellum in dystonia. Dystonia in both tottering mice (Campbell et al., 1999) and the Dt rat (LeDoux, 2011) arises from cerebellar dysfunction. Additionally, cerebellar knockdown of Tor1a, the gene associated with DYT1 dystonia, results in dystonic movements in wild-type mice, while striatal knockdown does not induce dystonia (Fremont et al., 2017). Dystonia can also be induced in normal mice by pharmacological manipulation of cerebellar signaling using glutamatergic agonists (Fan et al., 2018; Pizoli et al., 2002) or the sodium-potassium pump blocker ouabain (Calderon et al., 2011).
Although the basal ganglia and the cerebellum have been the primary focus of dystonia research, other regions are implicated in dystonia. For example, brainstem lesions are sometimes associated with dystonia, and thalamic stimulation or ablation has been used to treat hand dystonia (Shimizu et al., 2018). Involvement of these other regions has been reviewed elsewhere (Jinnah et al., 2017; Neychev et al., 2011; Schirinzi et al., 2018) and has led to the development of the motor network model of dystonia. Understanding these anatomical substrates and their interactions provides a platform for the development of improved therapies for the treatment of dystonia.
Existing treatments for dystonia
Current treatments for dystonia include oral pharmaceuticals, botulinum toxin, and surgical procedures (Cloud and Jinnah, 2010; Thenganatt and Jankovic, 2014). Here, the focus is on oral pharmaceuticals. Trihexyphenidyl (THP), a nonselective muscarinic acetylcholine receptor (mAChR) antagonist is the only widely used oral pharmaceutical that has been studied in a double-blind controlled trial for dystonia. This clinical trial demonstrated that THP was effective at alleviating dystonia in approximately 70% of patients (Burke et al., 1986). While THP is an effective treatment, it is often poorly tolerated due to significant side effects, including: cognitive impairments, memory loss, nightmares, dry mouth, constipation, blurred vision, and urinary retention (Bymaster et al., 2003; Jabbari et al., 1989; Lumsden et al., 2016). These side effects are often exacerbated by the high doses of THP needed to treat dystonia (Lang, 1986). Other nonselective mAChR antagonists have been used clinically, including atropine (Lang et al., 1982), procyclidine (Paulson, 1960), benztropine (Lang et al., 1982; Stern and Anderson, 1979), and biperiden (Cloud and Jinnah, 2010).
Despite the clear involvement of dopaminergic transmission in the pathophysiology of dystonia (Karimi and Perlmutter, 2015; Thompson et al., 2011), DA agonists, including direct D1/D2 receptor agonists and indirect agonists such as l-DOPA and amphetamine, are generally not effective treatments for dystonia. The exception is the use of l-DOPA to treat patients with DRD, a form of dystonia caused by mutations in genes involved in catecholamine synthesis. However, there are some reports that suggest DA receptor agonists may be effective in subsets of dystonia patients in addition to those with DRD (Fan et al., 2018).
Other pharmaceuticals have been used to treat dystonia, with varying degrees of success. Baclofen, a presynaptic GABAB receptor antagonist, is sometimes used in dystonia. However, a meta-analysis of clinical trials and case reports found that baclofen improved symptoms in only 20% of patients with dystonia (Greene et al., 1988). Other pharmaceuticals used to treat dystonia are: benzodiazepines, muscle relaxants, and anti-epilepsy medications, although there is limited evidence for the efficacy of any of these for the treatment of dystonia (Thenganatt and Jankovic, 2014).
New experimental therapeutic and pharmacological targets
In light of the limited and sometimes ineffective treatments for dystonia, there is significant interest in developing new therapeutics for the treatment of dystonia. There are several broad approaches to identifying new therapeutics: refining existing therapeutics to improve efficacy and limit off-target effects, identifying pharmacological targets that are implicated in multiple forms of dystonia, or investigating pharmacological targets that are implicated in other movement disorders, such as ʟ-DOPA induced dyskinesias (LIDs). Below, we review recent preclinical and clinical work to identify new therapeutics and therapeutic targets. To date, most preclinical studies have focused on therapeutic targets in the basal ganglia. Although the cerebellum is clearly implicated (Tewari et al., 2017), less is known about targets in this region. For a summary of recent preclinical and clinical studies see Table 1.
Table 1.
| Antimuscarinics | Clinical trial (randomized, double-blind, placebo) | 31 adults (Dystonia) | Trihexyphenidyl | Improved dystonia | (Burke et al., 1986) |
| Clinical trial (open label) | 23 (children) 52 (adults) dystonia | Trihexyphenidyl ethopropazine | Improved dystonia | (Fahn, 1983) | |
| Animal study | Dyt1 knockin mice | Trihexyphenidyl | Normalized DA release | (Downs et al., 2019) | |
| Animal study | Dyt1 knockin mice | Trihexyphenidyl VU0255035 (M1 antagonist) | Normalized corticostriatal plasticity | (Maltese et al., 2014) | |
| Animal study | dtsz hamster | Trihexyphenidyl tropicamide | Improved dystonia | (Hamann et al., 2017) | |
| Nicotinic receptors | Case reports | 2 adults (hemidystonia) | Nicotine (lozenges and transdermal patch) | Improved dystonia | (Lees, 1984) (Vaughan et al., 1997) |
| Animal study | Dyt1 knockin mice | AZD1446 (non-desensitizing nChR agonist) | No effect on DA release/overflow | (Zimmerman et al., 2017) | |
| mGluR5 receptors | Animal study | Dyt1 knockin mice | ADX48621 (mGluR5 NAM) | Normalized corticostriatal plasticity | (Sciamanna et al., 2014) |
| A2A adenosine receptors | Animal study | Dyt1 knockin mice | KW6002 (A2A receptor antagonist) | Normalized Corticostriatal plasticity | (Napolitano et al., 2010) |
| Animal study | Dyt11 knockin mice | SCH 58261 (A2A receptor antagonist) | Normalized Corticostriatal plasticity | (Maltese et al., 2017) | |
| Animal study | dtsz hamster | CPA (N(6)-cyclopentyladenosine (A1A receptor agonist) CGS 21680 (A2A receptor agonist) | Improved dystonia | (Richter and Hamann, 2001) | |
| Animal study | dtsz hamster | Non-selective adenosine receptor antagonists A1A receptor antagonists | Worsened dystonia | (Richter and Hamann, 2001) | |
| Endocannabinoid receptors (CB1) | Animal study | dtsz hamster | WIN 55,212–2 (CB1 agonist) | Improved dystonia | (Richter and Loscher, 1994) |
| Animal study | dtsz hamster | WIN 55,212–2 (CB1 agonist) | Improved dystonia | (Richter and Loscher, 2002) | |
| Clinical trial (randomized, double-blind, Placebo controlled) | 15 (primary dystonia) | Nabilone (CB1/CB2) agonist | No improvement | (Fox et al., 2002) | |
| Clinical trial (randomized, placebo controlled) | 9 (cervical dystonia) | Dronabinol (CB1/CB2) agonist | No improvement | (Zadikoff et al., 2011) | |
| Clinical trial (open-label) | 5 (undefined dystonia) | Cannabidiol (CB1/CB2) partial agonist | Improved dystonia | (Consroe et al., 1986) | |
| NMDA receptors | Animal study | dtsz hamster | NVP-AAM077 (NR2A antagonist) | Improved dystonia | (Avchalumov et al., 2014) |
| Animal study | dtsz hamster | Ro 25–6981 (NR2B antagonist) | Worsened dystonia | (Richter, 2003a) | |
| SV2A modulators | Animal study | dtsz hamster | Piracetam Levetiracetam | Improved dystonia | (Loscher and Richter, 2000) |
| Animal study | dtsz hamster | Brivaracetam Seletracetam | Improved dystonia | (Hamann et al., 2008) | |
| Clinical trial | 7 (cranial and oromandibular dystonia) | Levetiracetam | No improvement | (Park et al., 2017) | |
| Clinical trial | 10 (generalized/Segmental dystonia) | Levetiracetam | No improvement | (Hering et al., 2007) | |
| Case report | 1 (generalized dystonia) | Levetiracetam | Significant improvement | (Sullivan et al., 2005) | |
| Case report | 1 (Meige’s syndrome) | Levetiracetam | Significant improvement | (Yardimci et al., 2006) | |
| EIF2α signaling | Animal study | Dyt1 knockin mice | Salubrinal (eIF2α Dephosphorylation inhibitor) | Rescued ER function and abnormal torsinA localization | (Rittiner et al., 2016) |
| BDNF signaling | Animal study | Dyt1 knockin mice | ANA-12 (TrkB inhibitor) | Normalized Corticostriatal plasticity | (Maltese et al., 2018) |
Antimuscarinic receptor antagonists
While nonselective mAChR antagonists are effective in many forms of dystonia, they are often poorly tolerated due to debilitating side effects. Several studies have attempted to improve the therapeutic potential of mAChR antagonists by identifying the specific mAChR subtype(s) that mediate the therapeutic effects of THP and other nonselective mAChR antagonists. These studies have been facilitated by the recent development of truly selective mAChR antagonists and modulators (Bender et al., 2018; Lewis et al., 2008; Marlo et al., 2009; Wood et al., 2017).
In general, mAChR antagonists are thought to improve dystonia by modulating ACh in the striatum. ChIs and cholinergic afferents from the PPN and laterodorsal tegmental nuclei provide ACh to the striatum, although most research has focused on ChIs (Brimblecombe et al., 2018; Dautan et al., 2016). ChIs constitute less than 1% of all neurons in the striatum, but they have broad axonal arborizations that cover most of the striatum (Gonzales and Smith, 2015). ChIs are tonically active and are modulated by dopaminergic and glutamatergic afferents as well as GABAergic neurons in the striatum (Cai and Ford, 2018; Dawson et al., 1990; Kosillo et al., 2016; Tepper et al., 2008); for review see: (Bonsi et al., 2011)). ACh modulates striatal activity via both nicotinic acetylcholine receptors (nAChR) and mAChR. In the striatum, nAChR are expressed on presynaptic terminals of nigral dopaminergic afferents and glutamatergic cortical and thalamic afferents (Exley et al., 2008). mAChRs are divided into two broad classes: M1, M3, and M5 subtypes coupled to Gαq, and M2 and M4 subtypes coupled to Gαi/o. Corticostriatal terminals express M2, M3, and M4 mAChR that modulate glutamate release (Girasole and Nelson, 2015; Pancani et al., 2014). Direct SPNs express M1 and M4 mAChR, while indirect SPNs express only M1 mAChR (Alcantara et al., 2001; Harrison et al., 1996; Hernandez-Flores et al., 2015). Both mAChR subtypes on SPNs modulate corticostriatal plasticity. In contrast, M5 mAChR are restricted to DA neurons and modulate DA release (Foster et al., 2014). ChIs express M2 and M4 mAChR autoreceptors that reduce tonic firing and inhibit ACh release (Bonsi et al., 2008; Dawson et al., 1990; Girasole and Nelson, 2015; Threlfell et al., 2010).
To date, two mechanisms have been proposed to mediate the therapeutic effects of THP in dystonia, although these mechanisms are not mutually exclusive. A recent study found THP normalizes DA release in a mouse knockin model of DYT1 dystonia. This effect is likely mediated by M2 and/or M4 mAChRs on ChIs and depends on nAChR to indirectly mediate the effect of THP on DA release (Downs et al., 2019). However, it is worth noting that this study did not specifically rule out other mAChR subtypes that may mediate the increase in DA release after THP administration. Indeed, previous studies have shown that M1, M4, and M5 mAChRs modulate DA release in striatal sections (Zhang et al., 2002). THP may also produce therapeutic effects by normalizing corticostriatal plasticity in dystonia. Previous studies have identified abnormal corticostriatal long-term depression (LTD) as a common pathology in Dyt1 mouse models (Maltese et al., 2018; Martella et al., 2014; Martella et al., 2009). Abnormal LTD is thought to be mediated by ChI dysfunction, which has been consistently identified in Dyt1 knockin mice (Scarduzio et al., 2017; Sciamanna et al., 2012). THP restores normal patterns of corticostriatal LTD (Dang et al., 2012; Martella et al., 2014) and this effect is mimicked by the M1 mAChR selective antagonist VU0255035 (Maltese et al., 2014). Alternatively, M1 mAChR antagonists may simply block excitatory M1 mAChR inputs to ameliorate dystonia.
However, THP may improve dystonia by other mechanisms, which may involve other mAChR subtypes and other brain regions. A study in a mouse model of DRD found that THP improves dystonic movements, although it is unclear if its therapeutic effects are restricted to the striatum or involves other brain regions (Rose et al., 2015). mAChRs are expressed throughout the basal ganglia and other areas of the brain (Weiner et al., 1990), and, accordingly, the therapeutic actions of THP may not be restricted to the striatum. One study in the dtSZ hamster demonstrated that M1- and M4-preferring mAChR antagonists improved dystonia, although the mechanism of action was less clear given the limited mAChR subtype selectivity of the compounds used (Hamann et al., 2017). Therapeutics targeting specific mAChR subtypes may enhance the treatment of dystonia by minimizing off-target side effects. However, additional studies are needed to further characterize the role of each mAChR subtype in dystonia.
Nicotinic receptor agonists
Nicotinic receptor (nAChR) agonists have been proposed as novel therapeutics for dystonia based on evidence implicating abnormal striatal ACh signaling in animal models of dystonia (Eskow Jaunarajs et al., 2015), and from two case reports that show nicotine lozenges and patches are effective in treating dystonia (Lees, 1984; Vaughan et al., 1997). Nicotine is thought to improve dystonia by activating nAChRs on DA terminals in the striatum or DA cell bodies in the SNc, which increases the firing rate of DA neurons, increases striatal DA release, and changes the amplitude of DA release in response to tonic and phasic firing of DA neurons (Grenhoff et al., 1986; Rice and Cragg, 2004; Threlfell and Cragg, 2011). However, a recent study in a knockin mouse model of DYT1 dystonia showed the non-desensitizing agonist, AZD1446, did not normalize or increase extracellular DA in this model (Zimmerman et al., 2017). In contrast to nAChR agonists, nAChR positive allosteric modulators may warrant further investigation because they might subtly increase DA release without desensitizing nAChRs.
Dopamine receptor agonists
DA modulates the activity of most striatal neurons including SPNs, the sole output neurons of the striatum. The two main classes of DA receptors are defined based on their ability to modulate adenylyl cyclase: D1-class DA receptors (D1, D5) which couple to Gαolf or Gαs, and D2-class DA receptors (D2, D3, D4) which couple to Gαi/o (Andersen et al., 1990; Kebabian and Calne, 1979; Niznik and Van Tol, 1992; Sibley and Monsma, 1992; Tiberi et al., 1991). Within the striatum, D1 receptors are almost exclusively expressed on direct SPNs (Corvol et al., 2001; Zhuang et al., 2000). In contrast, D2 receptors are expressed on indirect SPNs and ChIs, where they reduce neuronal firing, and on presynaptic DA terminals, where they inhibit DA release and regulate DA synthesis (Dawson et al., 1990; De Mei et al., 2009; Giros et al., 1989; Monsma et al., 1989; Usiello et al., 2000). D3, D4, and D5 receptors are also found in the basal ganglia at lower levels (Gainetdinov et al., 1996; Huntley et al., 1992; Missale et al., 1998; Rivera, 2002; Rondou et al., 2010).
Clinical research suggests a common cellular mechanism underlying various forms of dystonia is disrupted DA neurotransmission (Nemeth, 2002; Perlmutter and Mink, 2004; Wichmann, 2008). Reduced DA neurotransmission is associated with dystonia in inherited disorders such as DOPA-responsive dystonia (Furukawa et al., 1996; Ichinose et al., 2000; Ichinose et al., 1995), DA transporter deficiency syndrome (Kurian et al., 2011), amino acid decarboxylase deficiency (Brun et al., 2010), VMAT2 deficiency (Rilstone et al., 2013), and Lesch-Nyhan disease (Lloyd et al., 1981). Reduced DA neurotransmission is also observed in more common idiopathic dystonias such as writer’s cramp (Berman et al., 2013) and spasmodic dysphonia (Simonyan et al., 2013). Further, dystonia can occur in response to therapy with DA receptor antagonists (i.e. tardive dystonia) (Mahmoudi et al., 2014) and is often co-morbid with degenerative disorders that affect DA neurons such as Parkinson’s disease (Lopez-Ariztegui et al., 2009). These observations suggest that restoring striatal DA with direct or indirect DA agonists should improve dystonia (Karimi and Perlmutter, 2015).
Paradoxically, DA agonists are seldom used to treat dystonia, with the exception of DRD. However, a recent meta-analysis suggests there is insufficient evidence in the literature to make definitive conclusions about the efficacy of DA agonists in dystonia (Fan et al., 2018). In fact, previous case reports and small clinical studies demonstrate that amphetamine (Myerson and Loman, 1942), apomorphine (Micheli et al., 1982), bromocriptine (Stahl and Berger, 1981), and lisuride (Micheli and Fernandez Pardal, 1986) are effective in some patients with idiopathic dystonia. Apomorphine is a partial agonist at both D1 and D2 class receptors, whereas bromocriptine and lisuride are D2-like receptor agonists. D2-like receptors agonists are more commonly used to treat dystonia, whereas D1-like receptor-selective agonists are not yet available for use in humans, although they are effective in animal models of dystonia (Fan et al., 2018; Rose et al., 2015). It is thought that D2 and D1/D2 agonists improve dystonia by mimicking normal DA receptor activation of SPNs, which normalizes the activity of the direct and indirect pathways in the basal ganglia (Rose et al., 2015).
Recent studies in animal models of dystonia, especially Dyt1 mice, have suggested that D2 receptors on ChI may be a target for therapeutics. ChIs exhibit abnormal responses to D2 receptor activation in several Dyt1 mouse models. While D2 receptor activation normally reduces the firing rate of ChIs, D2 receptor activation increases ChI firing rate in Dyt1 mice (Napolitano et al., 2010; Sciamanna et al., 2014; Sciamanna et al., 2012). This abnormal D2 receptor signaling is mediated by a switch from Gαi/o protein signaling to β-arrestin signaling (Bonsi et al., 2018; Scarduzio et al., 2017). Furthermore, a recent study found that overexpressing the striatal-specific RGS9–2 regulatory protein functionally inhibits β-arrestin and restores normal D2 receptor function in ChIs of Dyt1 mice (Bonsi et al., 2018). Taken together, these studies suggest that D2 receptor agonists biased towards G-protein mediated signaling may be effective therapeutics in DYT1 dystonia. These studies also identify RGS9–2, a target previously proposed for other disorders (Sjögren, 2017; Traynor et al., 2009), as a novel therapeutic target for dystonia. Future studies should evaluate the therapeutic efficacy of RGS9–2 potentiators in dystonia.
mGluR5 negative allosteric modulators (NAMS)
The effects of glutamate are mediated by two families of receptors, metabotropic and ionotropic, which are categorized by their mode of signal transduction. Metabotropic glutamate receptors are subdivided into three groups: Group I (mGluRs 1 and 5), Group II (mGluRs 2 and 3), and Group III (mGluRs 4, 6, 7, and 8) (Conn and Pin, 1997). In general, Group I mGluRs are located postsynaptically and couple to Gαq/11, whereas Groups II and III are localized presynaptically and couple to Gαi/o (Niswender and Conn, 2010). Group I mGluRs have been most extensively evaluated for therapeutic efficacy in dystonia. Within the striatum, mGluR5 receptors are expressed on SPNs as well as FSIs, ChIs, and low-threshold spike interneurons (Conn et al., 2005; Gubellini et al., 2004). On SPNs, mGluR5 and NMDA receptors are tethered by scaffolding proteins and activation of mGluR5 receptors amplifies NMDA receptor-mediated currents (Conn et al., 2005; Pisani et al., 2001). On indirect SPNs, mGluR5 receptors physically interact with A2A adenosine receptors to synergistically promote MAPK signaling, thereby counteracting the effect of D2 receptor activation (Ferre et al., 2002; Nishi et al., 2003). Further, Group I mGluRs are expressed on cell bodies and terminals of dopaminergic neurons from the SNc (Conn et al., 2005; Hubert et al., 2001). Outside of the striatum, Group I mGluRs are expressed on GABAergic neurons located in the globus pallidus and SNr.
Given the broad distribution of mGluRs in the basal ganglia, mGluR5 negative allosteric modulators (NAMs) have been proposed as a new therapeutic in dystonia. One study in Dyt1 knockin mice showed that the mGluR5 NAM ADX48621 restores normal responses to D2 receptor activation of striatal ChIs (Sciamanna et al., 2014). Neither mGluR5 NAMs nor antagonists have been tested in dystonia patients, but tests in both animals models of LIDS and patients with LIDs have shown promising results (Bezard et al., 2014; Dekundy et al., 2011; Tison et al., 2016). Future studies examining metabotropic glutamate receptors in dystonia will be aided by the recent discovery of a number of subtype-selective mGluR negative and positive allosteric modulators (Bollinger et al., 2017; Nickols et al., 2016; Panarese et al., 2019; Reed et al., 2019).
NMDA receptor antagonists
Ionotropic glutamate receptors are subdivided into three classes named according to their affinity for their selective agonists: α-amino-3hydroxy-5-methyl-4-isoxazole propionate (AMPA), kainic acid or kainate (KA), and N-methyl-D-aspartate (NMDA) (Traynelis et al., 2010). NMDA receptor antagonists have been most extensively studied for treatment of dystonia. NMDA receptor complexes are composed of NR1, NR2A–D and NR3A-B subunits (Kew and Kemp, 2005). The NR1 subunit is expressed ubiquitously throughout the brain, whereas the NR2 subunits have regional patterns of distribution and confer distinct electrophysiological and pharmacological properties to NMDA receptor complexes (Traynelis et al., 2010). In the adult brain, striatal SPNs are enriched for NR2A and NR2B subunits (Dunah and Standaert, 2003; Goebel and Poosch, 1999; Kosinski et al., 1998; Landwehrmeyer et al., 1995; Monyer et al., 1994; Smeal et al., 2008; Standaert et al., 1994). In contrast, the globus pallidus and STN primarily express NR2A and NR2D subunits (Wenzel et al., 1997; Wenzel et al., 1996).
NMDA receptor antagonists, such as amantadine, are sometimes used to treat dystonia (Borison, 1983; Gilbert, 1971). The use of amantadine is limited due to significant side effects including: constipation, cardiovascular dysfunction, hallucinations, delirium, and anxiety (Perez- Lloret and Rascol, 2018). In Dyt1 knockin mice, there is a selective increase in NR2A, but not NR2B, subunits at striatal postsynaptic sites (Maltese et al., 2018). In the dtSZ hamster, an NR2A-preferring antagonist improved dystonia, while NR2B-preferring antagonist worsened dystonia (Avchalumov et al., 2014; Richter, 2003b). Additional studies in the dtSZ hamster found that antagonists with equal affinity for NR2A/NR2B produced moderate improvement (Sander and Richter, 2007). Interestingly, this is in agreement with studies in rodent models of LIDs, in which NR2A-preferring antagonists were effective in reducing abnormal movements (Gardoni et al., 2012), while NR2B-preferring antagonists exacerbated abnormal movements (Nash et al., 2004). These studies suggest that more selective NR2A-preferring antagonists, or even NAMs, might be effective therapeutics for dystonia and could reduce some of the negative side effects that limit the use of nonselective NMDA receptor antagonists. NMDA receptor antagonists have not been tested in dystonia patients. However, one clinical trial found that the NR2B selective NMDA receptor antagonist MK-0657 did not improve dyskinesias or motor symptoms in patients with Parkinson’s disease, which agrees with preclinical studies (Herring et al., 2017).
Adenosine A2A receptor antagonists
Adenosine acts on two different G-protein coupled receptors within the basal ganglia: A1 and A2A. A1 adenosine receptors are located presynaptically on corticostriatal afferents, and postsynaptically on striatonigral and striatopallidal SPNs as well as ChIs (Alexander and Reddington, 1989; Fastbom et al., 1987; Ferre et al., 1996; Preston, 2000). Activation of Gαi/o- coupled A1 adenosine receptors decreases cAMP levels, increases K+ conductance, and decreases transient Ca2+ levels (Ambrosio et al., 1996), thereby decreasing the likelihood of neurotransmitter release from presynaptic terminals (Fredholm, 1995). A2A adenosine receptors are selectively expressed on striatopallidal SPNs and ChIs (Fink et al., 1992; Preston et al., 2000; Schiffmann et al., 1991). Activation of Gαolf-coupled A2A adenosine receptors increases cAMP levels, activating PKA and MAPK signaling (Schulte and Fredholm, 2003). Interestingly, A2A adenosine receptors also form heteromeric complexes with D2 receptors (Canals et al., 2003; Ferre et al., 2008; Fuxe et al., 2005; Hillion et al., 2002) and mGluR5s (Ferre et al., 2002; Morelli et al., 2007).
Studies in animal models suggest A2A adenosine receptor antagonists may be effective therapeutics. A2A adenosine receptor antagonists restore normal patterns of corticostriatal plasticity in Dyt1 (Napolitano et al., 2010) and Dyt11 mice (Maltese et al., 2017). In contrast, studies in the dtSZ hamster showed that A1- and A2A-selective adenosine receptor agonists improved dystonia, while nonselective adenosine receptor antagonists worsened dystonia. A2A- selective adenosine receptor antagonists had no effect on dystonia severity in these hamsters (Richter and Hamann, 2001). Clinical trials using adenosine receptor compounds have not been conducted in dystonia patients. However, A2A adenosine receptor antagonists have been investigated in animal models of Parkinson’s disease and produce limited improvement in dyskinesia symptoms in clinical trials (LeWitt et al., 2008; Nunez et al., 2018; Wang et al., 2017).
Cannabinoid receptor agonists
Most research has focused on type 1 (CB1) and type 2 (CB2) cannabinoid receptors. Both receptors are coupled to Gαi/o and are activated by retrograde signaling of the endocannabinoids arachidonoyl ethanolamide (anandamide or AEA) and 2-arachidonoyl glycerol (2-AG) (Freund et al., 2003; Kreitzer and Malenka, 2005; Mechoulam and Parker, 2013; Tanimura et al., 2010). The CB1 receptor is densely expressed in the striatum (Fusco et al., 2004; Herkenham et al., 1991; Matyas et al., 2006). CB1 receptors are localized to corticostriatal terminals, where they modulate cortical excitation of SPNs (Gerdeman and Lovinger, 2001; Wu et al., 2015). Additionally, CB1 receptors are robustly expressed on SPN axon terminals and have also been observed on striatal parvalbumin-containing GABA interneurons (Hohmann and Herkenham, 2000; Uchigashima et al., 2007). Unlike the CB1 receptor, the CB2 receptor was historically considered a peripheral cannabinoid receptor. However, the CB2 receptor is also expressed in the striatum and globus pallidus, albeit at significantly lower levels of expression than the CB1 receptor (Callen et al., 2012; Gong et al., 2006; Lanciego et al., 2011). A recent study found that CB2 receptors are expressed on striatal DA terminals and decrease striatal DA release through retrograde endocannabinoid signaling (Foster et al., 2016).
There has been significant interest in endocannabinoids in the treatment of movement disorders (Kluger et al., 2015; Koppel, 2015; Lim et al., 2017; Peres et al., 2018; Saft et al., 2018). Two studies in the dtSZ hamster model of dystonia found that the CB1/CB2 receptor agonist WIN 55,212–2 alleviated dystonia, and this effect was blocked by a CB1 receptor-specific antagonist (Richter and Loscher, 1994; Richter and Loscher, 2002). While animal studies have been generally positive, the few clinical trials and case reports in dystonia have been mixed. No improvement was observed in response to the CB1/CB2 receptor agonist nabilone (Fox et al., 2002), and another small placebo-controlled trial found no improvement for cervical dystonia in response to the CB1/CB2 agonist dronabinol (Zadikoff et al., 2011). However, an earlier study found significant improvement with the CB1/CB2 partial agonist cannabidiol in a small open-label trial (Consroe et al., 1986). Given the limited size of past clinical trials and inconsistency in results, further exploration of endocannabinoids for the treatment of dystonia is warranted.
Synaptic vesicle protein 2A (SV2A) modulators
SV2A modulators, including levetiracetam, were originally developed for epilepsy, but studies in rodents and humans have suggested therapeutic potential in dystonia. Levetiracetam is specific to the synaptic vesicle associated protein SV2A, which is found in all neurons in the CNS (Bajjalieh et al., 1993). Levetiracetam is thought to modulate the function of SV2A to reduce neurotransmitter release (Lynch et al., 2004). Several different SV2A modulators, including levetiracetam, improve dystonia in the dtSZ hamster model (Hamann et al., 2008; Loscher and Richter, 2000). However, the exact mechanism of action of levetiracetam in dystonia is unknown. One possibility is that SV2A modulators reduce cortical excitability, because an increase in cortical excitability is observed in multiple forms of dystonia in humans (Calabresi et al., 2016; Erro et al., 2018; Furuya et al., 2018; Hallett, 2006).
Results of clinical trials and case studies with SV2A modulators have been mixed. In two small clinical trials, levetiracetam was ineffective in patients with cranial/oromandibular or segmental/generalized dystonia (Hering et al., 2007; Park et al., 2017). However, in two different case reports, levetiracetam improved dystonia in two patients, one with generalized dystonia (Sullivan et al., 2005) and the other with Meige syndrome (Yardimci et al., 2006). Given the limited size of past clinical trials and inconsistency in results, further exploration of SV2A modulators in dystonia treatment is warranted. The prospect of using SV2A modulators or other compounds that modulate synaptic vesicle release is especially interesting in light of the use of tetrabenazine, a VMAT2 blocker, for the treatment of some dystonias and other movement disorders (Swash et al., 1972). This suggests that modulating synaptic vesicle release, either globally with a SV2A modulator or more specifically with a drug targeted to more restricted vesicular proteins, may represent a future therapeutic approach to dystonia treatment.
eIF2α signaling
Endoplasmic reticulum (ER) dysfunction and abnormal eukaryotic initiation factor α (eIF2α) signaling have been linked to a variety of neurological conditions, including dystonia (Moon et al., 2018; Rittiner et al., 2016). eIF2α is a required component for eukaryotic translation initiation and is tightly regulated by phosphorylation/dephosphorylation to control the rate of protein translation and prevent ER stress (Donnelly et al., 2013). Studies in human-derived iPSCs and mouse models of DYT1 dystonia have demonstrated that while torsinA is normally localized in the endoplasmic reticulum, mutant torsinA (torsinA (ΔE) is primarily localized in the nuclear envelope (Hewett et al., 2003; Oberlin et al., 2004; Vander Heyden et al., 2009). Further, ER protein processing and eIF2α signaling is abnormal in Dyt1 knockin mice and transgenic Dyt1 rats which suggests that neurons carrying the mutation in TorsinA may be highly susceptible to ER stress (Beauvais et al., 2016; Beauvais et al., 2018a; Beauvais et al., 2018b). Genetic studies in patients with sporadic cervical dystonia and DYT16 have revealed mutations in proteins involved in eIF2α signaling (Rittiner et al., 2016). Furthermore, a recent study in DYT6 (THAP1) mice has also demonstrated abnormal eIF2α signaling (Zakirova et al., 2018), which suggests that eiF2α signaling might be a common dysfunction in multiple forms of dystonia. Importantly, pharmacologically modifying eIF2α signaling with the eIF2α dephosphorylation inhibitor salubrinal rescued both abnormal torsinA localization and abnormal ER function (Rittiner et al., 2016). Taken together, these studies support eIf2α as a druggable target in dystonia.
Phosphodiesterase inhibitors (PDE10A)
Phosphodiesterases are responsible for terminating cellular cAMP and cGMP signaling. Accordingly, PDE inhibitors are expected to increase neuronal activity in the brain by enhancing cAMP and cGMP signaling. PDE10A is of specific interest to movement disorders because it is highly expressed throughout the basal ganglia, including direct and indirect pathway SPNs (Svedberg et al., 2019). A recent study in a transgenic mouse model of DYT1 dystonia found inverse changes in PDE10A expression in direct and indirect pathway SPNs (D’Angelo et al.,2017). PDE10A expression was decreased in direct SPNs, but increased in indirect SPNs. These changes are consistent with increased activation of the direct striatonigral pathway and reduced activity in the indirect striatopallidal pathway. There are no clinical trials of PDE10A inhibitors in dystonia. However, PDE10A inhibitors improve motor abnormalities in a mouse model of Huntington’s disease and a non-human primate model of LIDs (Beaumont et al., 2016; Beck et al., 2018). Future studies are needed to determine if PDE10A inhibition modifies dystonia.
Brain derived neurotrophic growth factor (BDNF)
Brain derived neurotrophic growth factor (BDNF) is a widely distributed neurotrophin in the mammalian brain. BDNF binds and activates TrkB to regulate a variety of cellular processes related to neuronal and glial development and synaptic plasticity (Huang and Reichardt, 2001). Because of the importance of BDNF to neuronal plasticity, it is of significant interest in many neurodevelopmental diseases. A previous study has shown that, in early development, there is increased expression of BDNF in Dyt1 mice relative to WT littermates. This parallels the development of abnormal synaptic plasticity in Dyt1 mice and suggests a critical period for targeted intervention. In vivo administration of a TrkB inhibitor during this critical window rescued the abnormal synaptic plasticity, which identifies TrkB/BDNF signaling as a potential therapeutic target for DYT1 dystonia (Maltese et al., 2018). In the dtSZ hamster, changes in BDNF mRNA or protein expression were not detected (Bode, 2017). There is some evidence to suggest a polymorphism in the prodomain region of the BDNF gene may confer risk for developing cervical dystonia and/or blepharospasm (Chen et al., 2013; Siokas, 2018). The SNP rs6265 (G/A) (AF50.1896) results in the substitution of Val in amino acid position 66 with Met (val66met), and healthy carriers of the val66met have been reported to show differences in brain structure and abnormal motor cortex plasticity (Cheeran, 2008; Pezawas, 2004). However, a direct relationship between the val66met variant, abnormal motor cortex plasticity, and dystonia has yet to be uncovered. Taken together, these human genetics and preclinical studies support further investigation of TrkB/BDNF signaling as a potential therapeutic target in dystonia, as proposed for other neurological and psychiatric disorders (Deng, 2016; Longo, 2013; Nagahara, 2011).
Future directions for novel experimental therapeutics in dystonia
Despite advances in our understanding of the genetic and anatomical bases of dystonia, pathogenesis-targeting or disease-modifying therapies remain limited. While the limited oral therapeutics for dystonia are sometimes effective, patients often discontinue treatment due to dose-limiting side-effects, insufficient efficacy, or both. Recent advances in our understanding of the pathophysiological mechanisms of dystonia have revealed new therapeutic targets that deserve careful validation for clinical use. One challenge for drug discovery in dystonia is the heterogeneity of etiologies and disease presentations in the clinical population. The availability of a multitude of animal models for different forms of dystonia with construct and predictive validity will be instrumental for identifying promising common molecular targets. This, in turn, will facilitate clinical trials by identifying the most appropriate patient populations in which to test new, targeted therapies based on shared biological processes and molecular targets. Overall, recent advances in both basic and clinical research provide promising new platforms for the development of novel therapeutics.
Acknowledgments
This work was supported by United States Department of Defense grant W81XWH-15-1-0545 and United States National Institute of Health Grants R01 NS 088528 F31 NS103363 and T32 GM008602 to EJH, and by the Italian Ministry of Health (Ricerca Corrente) to AP and PB. The authors are grateful for the support of the Dystonia Medical Research Foundation.
Footnotes
Declarations of interest: none
References
- Albanese A, et al. , 2013. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 28, 863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, et al. , 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–75. [DOI] [PubMed] [Google Scholar]
- Alcantara AA, et al. , 2001. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 434, 445–60. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Reddington M, 1989. The cellular localization of adenosine receptors in rat neostriatum. Neuroscience. 28, 645–51. [DOI] [PubMed] [Google Scholar]
- Ambrosio AF, et al. , 1996. Modulation of Ca2+ channels by activation of adenosine A1 receptors in rat striatal glutamatergic nerve terminals. Neurosci Lett. 220, 163–6. [DOI] [PubMed] [Google Scholar]
- Andersen PH, et al. , 1990. Dopamine receptor subtypes: beyond the D1/D2 classification. Trends Pharmacol Sci. 11, 231–6. [DOI] [PubMed] [Google Scholar]
- Asanuma K, et al. , 2005a. Neuroimaging in human dystonia. J Med Invest. 52 Suppl, 272–9. [DOI] [PubMed] [Google Scholar]
- Asanuma K, et al. , 2005b. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 64, 347–9. [DOI] [PubMed] [Google Scholar]
- Avchalumov Y, et al. , 2014. Role of striatal NMDA receptor subunits in a model of paroxysmal dystonia. Exp Neurol. 261, 677–84. [DOI] [PubMed] [Google Scholar]
- Bajjalieh SM, et al. , 1993. Brain contains two forms of synaptic vesicle protein 2. Proc Natl Acad Sci USA. 90, 2150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, et al. , 2010. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA. 107, 14845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont V, et al. , 2016. Phosphodiesterase 10A Inhibition Improves Cortico-Basal Ganglia Function in Huntington’s Disease Models. Neuron. 92, 1220–1237. [DOI] [PubMed] [Google Scholar]
- Beauvais G, et al. , 2016. Disruption of Protein Processing in the Endoplasmic Reticulum of DYT1 Knockin Mice Implicates Novel Pathways in Dystonia Pathogenesis. J Neurosci. 36, 10245–10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais G, et al. , 2018a. Exploring the Interaction Between eIF2alpha Dysregulation, Acute Endoplasmic Reticulum Stress and DYT1 Dystonia in the Mammalian Brain. Neuroscience. 371, 455–468. [DOI] [PubMed] [Google Scholar]
- Beauvais G, et al. , 2018b. Efficient RNA interference-based knockdown of mutant torsinA reveals reversibility of PERK-eIF2alpha pathway dysregulation in DYT1 transgenic rats in vivo. Brain Res. [DOI] [PubMed] [Google Scholar]
- Beck G, et al. , 2018. A Selective Phosphodiesterase 10A Inhibitor Reduces L-Dopa-Induced Dyskinesias in Parkinsonian Monkeys. Mov Disord. 33, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AM, et al. , 2018. Discovery and Optimization of Potent and CNS Penetrant M5-Preferring Positive Allosteric Modulators Derived from a Novel, Chiral N-(Indanyl)piperidine Amide Scaffold. ACS Chem Neurosci. 9, 1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman BD, et al. , 2013. Striatal dopaminergic dysfunction at rest and during task performance in writer’s cramp. Brain. 136, 3645–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, et al. , 2014. The mGluR5 negative allosteric modulator dipraglurant reduces dyskinesia in the MPTP macaque model. Mov Disord. 29, 1074–9. [DOI] [PubMed] [Google Scholar]
- Bhatia KP, Marsden CD, 1994. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 117 (Pt 4), 859–76. [DOI] [PubMed] [Google Scholar]
- Blood AJ, et al. , 2004. Basal ganglia activity remains elevated after movement in focal hand dystonia. Ann Neurol. 55, 744–8. [DOI] [PubMed] [Google Scholar]
- Bode C a. R. F a. S. C a. B. T a. B. A a. F. S a. F. J-M a. R. A, 2017. Altered postnatal maturation of striatal GABAergic interneurons in a phenotypic animal model of dystonia. Experimental Neurology. 287, 44–53. [DOI] [PubMed] [Google Scholar]
- Bollinger KA, et al. , 2017. Design and Synthesis of mGlu2 NAMs with Improved Potency and CNS Penetration Based on a Truncated Picolinamide Core. ACS Med Chem Lett. 8, 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologna M, Berardelli A, 2018. The cerebellum and dystonia. Handb Clin Neurol. 155, 259–272. [DOI] [PubMed] [Google Scholar]
- Bonsi P, et al. , 2011. Centrality of striatal cholinergic transmission in basal ganglia function. Frontiers in Neuroanatomy. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, et al. , 2008. Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. Journal of Neuroscience. 28, 6258–6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, et al. , 2018. RGS9–2 rescues dopamine D2 receptor levels and signaling in DYT1 dystonia mouse models. EMBO Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borison RL, 1983. Amantadine in the management of extrapyramidal side effects. Clin Neuropharmacol. 6 Suppl 1, S57–63. [DOI] [PubMed] [Google Scholar]
- Bostan AC a. D. RP a. S. PL, 2010. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA. 107, 8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC a. S. PL, 2018. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci. 19, 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe KR, et al. , 2018. Targeted Activation of Cholinergic Interneurons Accounts for the Modulation of Dopamine by Striatal Nicotinic Receptors. eNeuro. 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun L, et al. , 2010. Clinical and biochemical features of aromatic L-amino acid decarboxylase deficiency. Neurology. 75, 64–71. [DOI] [PubMed] [Google Scholar]
- Burke RE, et al. , 1986. Torsion dystonia: a double-blind, prospective trial of high-dosage trihexyphenidyl. Neurology. 36, 160–4. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, et al. , 2003. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 17, 1403–10. [DOI] [PubMed] [Google Scholar]
- Cai Y, Ford CP, 2018. Dopamine Cells Differentially Regulate Striatal Cholinergic Transmission across Regions through Corelease of Dopamine and Glutamate. Cell Rep. 25, 3148–3157.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, et al. , 2016. Hyperkinetic disorders and loss of synaptic downscaling. Nat Neurosci. 19, 868–75. [DOI] [PubMed] [Google Scholar]
- Calderon DP, et al. , 2011. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat Neurosci. 14, 357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callen L, et al. , 2012. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J Biol Chem. 287, 20851–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camfield L, et al. , 2002. Impact of cervical dystonia on quality of life. Mov Disord. 17, 838–41. [DOI] [PubMed] [Google Scholar]
- Campbell DB, et al. , 1999. Tottering mouse motor dysfunction is abolished on the Purkinje cell degeneration (pcd) mutant background. Exp Neurol. 160, 268–78. [DOI] [PubMed] [Google Scholar]
- Canals M, et al. , 2003. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 278, 46741–9. [DOI] [PubMed] [Google Scholar]
- Carbon M, et al. , 2013. Metabolic changes in DYT11 myoclonus-dystonia. Neurology. 80, 385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, et al. , 2004. Regional metabolism in primary torsion dystonia: effects of penetrance and genotype. Neurology. 62, 1384–90. [DOI] [PubMed] [Google Scholar]
- Cheeran B a. T. P a. M. F a. K. G a. S. A a. E. M a. H. H a. B. K a. G. R a. R. JC, 2008. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. The Journal of Physiology. 586, 5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. , 2013. Association of the Val66Met polymorphism of the BDNF gene with primary cranial- cervical dystonia patients from South-west China. Parkinsonism Relat Disord. 19, 1043–5. [DOI] [PubMed] [Google Scholar]
- Cloud LJ, Jinnah HA, 2010. Treatment strategies for dystonia. Expert Opin Pharmacother. 11, 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, et al. , 2005. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 6, 787–98. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP, 1997. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 37, 205–37. [DOI] [PubMed] [Google Scholar]
- Consroe P, et al. , 1986. Open label evaluation of cannabidiol in dystonic movement disorders. Int J Neurosci. 30, 277–82. [DOI] [PubMed] [Google Scholar]
- Corvol JC, et al. , 2001. Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem. 76, 1585–8. [DOI] [PubMed] [Google Scholar]
- Cui G a. J. SB a. J. X a. P. MD a. V. SS a. L. DM a. C. RM, 2013. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 494, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo V, et al. , 2017. Phosphodiesterase-10A Inverse Changes in Striatopallidal and Striatoentopeduncular Pathways of a Transgenic Mouse Model of DYT1 Dystonia. J Neurosci. 37, 2112–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang MT, et al. , 2012. An anticholinergic reverses motor control and corticostriatal LTD deficits in Dyt1 DeltaGAG knock-in mice. Behav Brain Res. 226, 465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautan D, et al. , 2016. Extrinsic Sources of Cholinergic Innervation of the Striatal Complex: A Whole- Brain Mapping Analysis. Front Neuroanat. 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson VL, et al. , 1990. Muscarinic and dopaminergic receptor subtypes on striatal cholinergic interneurons. Brain Res Bull. 25, 903–12. [DOI] [PubMed] [Google Scholar]
- De Mei C, et al. , 2009. Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol. 9, 53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defazio G, 2010. The epidemiology of primary dystonia: current evidence and perspectives. Eur J Neurol. 17 Suppl 1, 9–14. [DOI] [PubMed] [Google Scholar]
- Dekundy A, et al. , 2011. Pharmacological characterization of MRZ-8676, a novel negative allosteric modulator of subtype 5 metabotropic glutamate receptors (mGluR5): focus on L: -DOPA-induced dyskinesia. J Neural Transm (Vienna). 118, 1703–16. [DOI] [PubMed] [Google Scholar]
- DeLong MR, 1990. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–5. [DOI] [PubMed] [Google Scholar]
- Deng P a. A. JD a. Y. AS a. A. G a. F. KD a. N. JA, 2016. Engineered BDNF producing cells as a potential treatment for neurologic disease. Expert Opinion on Biological Therapy. 16, 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly N, et al. , 2013. The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci. 70, 3493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs AM, et al. , 2019. Trihexyphenidyl rescues the deficit in dopamine neurotransmission in a mouse model of DYT1 dystonia. Neurobiol Dis. 125, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, et al. , 2009. Genotype-phenotype interactions in primary dystonias revealed by differential changes in brain structure. Neuroimage. 47, 1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, et al. , 2003. “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology. 61, 1228–31. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG, 2003. Subcellular segregation of distinct heteromeric NMDA glutamate receptors in the striatum. J Neurochem. 85, 935–43. [DOI] [PubMed] [Google Scholar]
- Durieux PF, et al. , 2009. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 12, 393–5. [DOI] [PubMed] [Google Scholar]
- Egger K, et al. , 2007. Voxel based morphometry reveals specific gray matter changes in primary dystonia. Mov Disord. 22, 1538–42. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, et al. , 1998. Functional brain networks in DYT1 dystonia. Ann Neurol. 44, 303–12. [DOI] [PubMed] [Google Scholar]
- Erro R, et al. , 2018. High frequency somatosensory stimulation in dystonia: Evidence fordefective inhibitory plasticity. Mov Disord. 33, 1902–1909. [DOI] [PubMed] [Google Scholar]
- Eskow Jaunarajs KL, et al. , 2015. Striatal cholinergic dysfunction as a unifying theme in the pathophysiology of dystonia. Prog Neurobiol. 127–128, 91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, et al. , 2008. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 33, 2158–66. [DOI] [PubMed] [Google Scholar]
- Fahn S, 1983. High-dosage anticholinergic therapy in dystonia. Adv Neurol. 37, 177–88. [PubMed] [Google Scholar]
- Fan X, et al. , 2018. Dopamine Receptor Agonist Treatment of Idiopathic Dystonia: A Reappraisal in Humans and Mice. J Pharmacol Exp Ther. 365, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fastbom J, et al. , 1987. Adenosine A1 receptors in the human brain: a quantitative autoradiographic study. Neuroscience. 22, 827–39. [DOI] [PubMed] [Google Scholar]
- Ferre S, et al. , 2002. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci USA. 99, 11940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, et al. , 1996. Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur J Neurosci. 8, 1545–53. [DOI] [PubMed] [Google Scholar]
- Ferre S, et al. , 2008. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des. 14, 1468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip P, et al. , 2017. Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov Disord. 32, 757–768. [DOI] [PubMed] [Google Scholar]
- Fink JS, et al. , 1992. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 14, 186–95. [DOI] [PubMed] [Google Scholar]
- Foster DJ, et al. , 2014. M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location. J Neurosci. 34, 3253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, et al. , 2016. Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release. Neuron. 91, 1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SH, et al. , 2002. Randomised, double-blind, placebo-controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov Disord. 17, 145–9. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, 1995. Purinoceptors in the nervous system. Pharmacol Toxicol. 76, 228–39. [DOI] [PubMed] [Google Scholar]
- Frei KP, et al. , 2004. Natural history of posttraumatic cervical dystonia. Mov Disord. 19, 1492–8. [DOI] [PubMed] [Google Scholar]
- Fremont R, et al. , 2017. A role for cerebellum in the hereditary dystonia DYT1. Elife. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, et al. , 2003. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 83, 1017–66. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, et al. , 1996. GTP-cyclohydrolase I gene mutations in hereditary progressive and dopa-responsive dystonia. Ann Neurol. 39, 609–17. [DOI] [PubMed] [Google Scholar]
- Furuya S, et al. , 2018. Aberrant cortical excitability reflects the loss of hand dexterity in musician’s dystonia. J Physiol. 596, 2397–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco FR, et al. , 2004. Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse. 53, 159–67. [DOI] [PubMed] [Google Scholar]
- Fuxe K, et al. , 2005. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J Mol Neurosci. 26, 209–20. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, et al. , 1996. In vivo evidence for preferential role of dopamine D3 receptor in the presynaptic regulation of dopamine release but not synthesis. Eur J Pharmacol. 308, 261–9. [DOI] [PubMed] [Google Scholar]
- Galardi G, et al. , 1996. Basal ganglia and thalamo-cortical hypermetabolism in patients with spasmodic torticollis. Acta Neurol Scand. 94, 172–6. [DOI] [PubMed] [Google Scholar]
- Gardoni F, et al. , 2012. Targeting NR2A-containing NMDA receptors reduces L-DOPA-induced dyskinesias. Neurobiol Aging. 33, 2138–44. [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM, 2001. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 85, 468–71. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, 2000. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 23, S64–70. [DOI] [PubMed] [Google Scholar]
- Gilbert GJ, 1971. Spasmodic torticollis treated effectively by medical means. N Engl J Med. 284, 896–8. [DOI] [PubMed] [Google Scholar]
- Girasole AE, Nelson AB, 2015. Bridging the Gap: Muscarinic M4 Receptors Promote Striatal Plasticity in Health and Disease. Neuron. 88, 621–3. [DOI] [PubMed] [Google Scholar]
- Giros B, et al. , 1989. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 342, 923–6. [DOI] [PubMed] [Google Scholar]
- Gittis AH a. N. AB a. T. MT a. P. JJ a. K. AC, 2010. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci. 30, 2223–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel DJ, Poosch MS, 1999. NMDA receptor subunit gene expression in the rat brain: a quantitative analysis of endogenous mRNA levels of NR1Com, NR2A, NR2B, NR2C, NR2D and NR3A. Brain Res Mol Brain Res. 69, 164–70. [DOI] [PubMed] [Google Scholar]
- Gong JP, et al. , 2006. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 1071, 10–23. [DOI] [PubMed] [Google Scholar]
- Gonzales KK, Smith Y, 2015. Cholinergic interneurons in the dorsal and ventral striatum: anatomical and functional considerations in normal and diseased conditions. Ann N Y Acad Sci. 1349, 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornati SV a. S. CB a. E. R. OHJ a. N. AL a. D. Z. CI a. H. FE, 2018. Differentiating cerebellar impact on thalamic nuclei. Cell Rep. 23, 2690–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene P, et al. , 1988. Analysis of open-label trials in torsion dystonia using high dosages of anticholinergics and other drugs. Mov Disord. 3, 46–60. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, et al. , 1986. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 128, 351–8. [DOI] [PubMed] [Google Scholar]
- Gritton HJ a. H. WM a. R. MF a., 2019. Unique contributions of parvalbumin and cholinergic interneurons in organizing striatal networks during movement. Nat Neurosci. 22, 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, et al. , 2004. Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog Neurobiol. 74, 271–300. [DOI] [PubMed] [Google Scholar]
- Hallett M, 2006. Pathophysiology of dystonia. J Neural Transm Suppl. 485–8. [DOI] [PubMed] [Google Scholar]
- Hamann M, et al. , 2017. Alterations of M1 and M4 acetylcholine receptors in the genetically dystonic (dt(sz)) hamster and moderate antidystonic efficacy of M1 and M4 anticholinergics. Neuroscience. 357, 84–98. [DOI] [PubMed] [Google Scholar]
- Hamann M, et al. , 2008. Brivaracetam and seletracetam, two new SV2A ligands, improve paroxysmal dystonia in the dt sz mutant hamster. Eur J Pharmacol. 601, 99–102. [DOI] [PubMed] [Google Scholar]
- Harrison MB, et al. , 1996. Expression of m1 and m4 muscarinic receptor mRNA in the striatum following a selective lesion of striatonigral neurons. Brain Res. 734, 323–6. [PubMed] [Google Scholar]
- Haslinger B, et al. , 2010. Sensorimotor overactivity as a pathophysiologic trait of embouchure dystonia. Neurology. 74, 1790–7. [DOI] [PubMed] [Google Scholar]
- Hering S, et al. , 2007. An open trial of levetiracetam for segmental and generalized dystonia. Mov Disord. 22, 1649–51. [DOI] [PubMed] [Google Scholar]
- Herkenham M, et al. , 1991. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 547, 267–74. [DOI] [PubMed] [Google Scholar]
- Hernandez-Flores T, et al. , 2015. Modulation of direct pathway striatal projection neurons by muscarinic M(4)-type receptors. Neuropharmacology. 89, 232–44. [DOI] [PubMed] [Google Scholar]
- Herring WJ, et al. , 2017. A Phase Ib Randomized Controlled Study to Evaluate the Effectiveness of a Single-Dose of the NR2B Selective N-Methyl-D-Aspartate Antagonist MK-0657 on Levodopa-Induced Dyskinesias and Motor Symptoms in Patients With Parkinson Disease. Clin Neuropharmacol. 40, 255–260. [DOI] [PubMed] [Google Scholar]
- Hewett J, et al. , 2003. TorsinA in PC12 cells: localization in the endoplasmic reticulum and response to stress. J Neurosci Res. 72, 158–68. [DOI] [PubMed] [Google Scholar]
- Hillion J, et al. , 2002. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 277, 18091–7. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M, 2000. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 37, 71–80. [DOI] [PubMed] [Google Scholar]
- Hoshi E, et al. , 2005. The cerebellum communicates with the basal ganglia. Nat Neurosci. 8, 1491–3. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF, 2001. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 24, 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, et al. , 2001. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. J Neurosci. 21, 1838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley GW, et al. , 1992. Localization of multiple dopamine receptor subtype mRNAs in human and monkey motor cortex and striatum. Brain Res Mol Brain Res. 15, 181–8. [DOI] [PubMed] [Google Scholar]
- Ichinose H, et al. , 2000. Molecular mechanisms of hereditary progressive dystonia with marked diurnal fluctuation, Segawa’s disease. Brain Dev. 22 Suppl 1, S107–10. [DOI] [PubMed] [Google Scholar]
- Ichinose H, et al. , 1995. GTP cyclohydrolase I gene in hereditary progressive dystonia with marked diurnal fluctuation. Neurosci Lett. 196, 5–8. [DOI] [PubMed] [Google Scholar]
- Jabbari B, et al. , 1989. Treatment of movement disorders with trihexyphenidyl. Mov Disord. 4, 202–12. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, et al. , 2017. The Anatomical Basis for Dystonia: The Motor Network Model. Tremor Other Hyperkinet Mov (N Y). 7, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Perlmutter JS, 2015. The role of dopamine and dopaminergic pathways in dystonia: insights from neuroimaging. Tremor Other Hyperkinet Mov (N Y). 5, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, 1993. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 13, 4908–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, 1997. Neostriatal cell subtypes and their functional roles. Neurosci Res. 27, 1–8. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB, 1979. Multiple receptors for dopamine. Nature. 277, 93–6. [DOI] [PubMed] [Google Scholar]
- Kew JN, Kemp JA, 2005. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl). 179, 4–29. [DOI] [PubMed] [Google Scholar]
- Kluger B, et al. , 2015. The therapeutic potential of cannabinoids for movement disorders. Mov Disord. 30, 313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel BS, 2015. Cannabis in the Treatment of Dystonia, Dyskinesias, and Tics. Neurotherapeutics. 12, 788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosillo P, et al. , 2016. Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski CM, et al. , 1998. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: striatum and globus pallidus. J Comp Neurol. 390, 63–74. [PubMed] [Google Scholar]
- Krauss JK, Jankovic J, 2002. Head injury and posttraumatic movement disorders. Neurosurgery. 50, 927–39; discussion 939–40. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, et al. , 2010. Regulation of parkinsonian motor behaviors by optogenetic control of basal ganglia circuitry. Nature. 466, 622–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC, 2005. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 25, 10537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL, 2009. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 19, 2485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian MA, et al. , 2011. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol. 10, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, et al. , 2011. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol. 25, 97–104. [DOI] [PubMed] [Google Scholar]
- Landwehrmeyer GB, et al. , 1995. NMDA receptor subunit mRNA expression by projection neurons and interneurons in rat striatum. J Neurosci. 15, 5297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, 1986. High dose anticholinergic therapy in adult dystonia. Can J Neurol Sci. 13, 42–6. [DOI] [PubMed] [Google Scholar]
- Lang AE, et al. , 1982. Anticholinergics in adult-onset focal dystonia. Can J Neurol Sci. 9, 313–9. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, 2011. Animal models of dystonia: Lessons from a mutant rat. Neurobiol Dis. 42, 152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux MS, Brady KA, 2003. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 18, 60–9. [DOI] [PubMed] [Google Scholar]
- Lees AJ, 1984. Hemidystonia relieved by nicotine. Lancet. 2, 871. [DOI] [PubMed] [Google Scholar]
- Lehericy S, et al. , 2013. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord. 28, 944–57. [DOI] [PubMed] [Google Scholar]
- Lewis LM, et al. , 2008. Synthesis and SAR of selective muscarinic acetylcholine receptor subtype 1 (M1 mAChR) antagonists. Bioorg Med Chem Lett. 18, 885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWitt PA, et al. , 2008. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol. 63, 295–302. [DOI] [PubMed] [Google Scholar]
- Lim K, et al. , 2017. A Systematic Review of the Effectiveness of Medical Cannabis for Psychiatric, Movement and Neurodegenerative Disorders. Clin Psychopharmacol Neurosci. 15, 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd KG, et al. , 1981. Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N Engl J Med. 305, 1106–11. [DOI] [PubMed] [Google Scholar]
- Lo SE, et al. , 2005. Identification and treatment of cervical and oromandibular dystonia in acutely brain-injured patients. Neurocrit Care. 3, 139–45. [DOI] [PubMed] [Google Scholar]
- Longo FM a. M. SM, 2013. Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nature Reviews. Drug Discovery. 12, 507–525. [DOI] [PubMed] [Google Scholar]
- Lopez-Ariztegui N, et al. , 2009. Central levodopa influx and the clinical motor response to levodopa in patients with Parkinson disease complicated with motor fluctuations and dyskinesias. Clin Neuropharmacol. 32, 321–5. [DOI] [PubMed] [Google Scholar]
- Loscher W, Richter A, 2000. Piracetam and levetiracetam, two pyrrolidone derivatives, exert antidystonic activity in a hamster model of paroxysmal dystonia. Eur J Pharmacol. 391, 251–4. [DOI] [PubMed] [Google Scholar]
- Louis ED, et al. , 1999. Dystonia in Huntington’s disease: prevalence and clinical characteristics. Mov Disord. 14, 95–101. [DOI] [PubMed] [Google Scholar]
- Lozano AM, et al. , 1997. Globus pallidus internus pallidotomy for generalized dystonia. Mov Disord. 12, 865–70. [DOI] [PubMed] [Google Scholar]
- Lumsden DE, et al. , 2016. Medication use in childhood dystonia. Eur J Paediatr Neurol. 20, 625–9. [DOI] [PubMed] [Google Scholar]
- Lynch BA, et al. , 2004. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 101, 9861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi S, et al. , 2014. Upregulation of dopamine D3, not D2, receptors correlates with tardive dyskinesia in a primate model. Mov Disord. 29, 1125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese M, et al. , 2017. Abnormal striatal plasticity in a DYT11/SGCE myoclonus dystonia mouse model is reversed by adenosine A2A receptor inhibition. Neurobiol Dis. 108, 128–139. [DOI] [PubMed] [Google Scholar]
- Maltese M, et al. , 2014. Anticholinergic drugs rescue synaptic plasticity in DYT1 dystonia: role of M1 muscarinic receptors. Mov Disord. 29, 1655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese M, et al. , 2018. Early structural and functional plasticity alterations in a susceptibility period of DYT1 dystonia mouse striatum. Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlo JE, et al. , 2009. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharmacol. 75, 577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella G, et al. , 2014. Regional specificity of synaptic plasticity deficits in a knock-in mouse model of DYT1 dystonia. Neurobiol Dis. 65, 124–32. [DOI] [PubMed] [Google Scholar]
- Martella G, et al. , 2009. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain. 132, 2336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, et al. , 2006. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 137, 337–61. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, 2013. The endocannabinoid system and the brain. Annu Rev Psychol. 64, 21–47. [DOI] [PubMed] [Google Scholar]
- Micheli F, Fernandez Pardal MM, 1986. Responses to lisuride in Meige’s disease and chorea. Neurology. 36, 445–445-a. [DOI] [PubMed] [Google Scholar]
- Micheli F, et al. , 1982. Beneficial effects of lisuride in Meige disease. Neurology. 32, 432–432. [DOI] [PubMed] [Google Scholar]
- Missale C, et al. , 1998. Dopamine receptors: from structure to function. Physiol Rev. 78, 189–225. [DOI] [PubMed] [Google Scholar]
- Monsma FJ Jr., et al. , 1989. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 342, 926–9. [DOI] [PubMed] [Google Scholar]
- Monyer H, et al. , 1994. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 12, 529–40. [DOI] [PubMed] [Google Scholar]
- Moon SL, et al. , 2018. Neuronal Regulation of eIF2alpha Function in Health and Neurological Disorders. Trends Mol Med. 24, 575–589. [DOI] [PubMed] [Google Scholar]
- Morelli M, et al. , 2007. Role of adenosine A2A receptors in parkinsonian motor impairment and l-DOPA-induced motor complications. Prog Neurobiol. 83, 293–309. [DOI] [PubMed] [Google Scholar]
- Myerson A, Loman J, 1942. Amphetamine sulfate in treatment of spasmodic torticollis: Report of two cases. Archives of Neurology & Psychiatry. 48, 823–828. [Google Scholar]
- Nagahara AH a. T. M. H., 2011. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nature Reviews. Drug Discovery. 10, 209–219. [DOI] [PubMed] [Google Scholar]
- Napolitano F, et al. , 2010. Dopamine D2 receptor dysfunction is rescued by adenosine A2A receptor antagonism in a model of DYT1 dystonia. Neurobiol Dis. 38, 434–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JE, et al. , 2004. The NR2B-selective NMDA receptor antagonist CP-101,606 exacerbates L-DOPA- induced dyskinesia and provides mild potentiation of anti-parkinsonian effects of L-DOPA in the MPTP-lesioned marmoset model of Parkinson’s disease. Exp Neurol. 188, 471–9. [DOI] [PubMed] [Google Scholar]
- Nemeth AH, 2002. The genetics of primary dystonias and related disorders. Brain. 125, 695–721. [DOI] [PubMed] [Google Scholar]
- Neychev VK, et al. , 2008. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 131, 2499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neychev VK, et al. , 2011. The functional neuroanatomy of dystonia. Neurobiol Dis. 42, 185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols HH, et al. , 2016. VU0477573: Partial Negative Allosteric Modulator of the Subtype 5 Metabotropic Glutamate Receptor with In Vivo Efficacy. J Pharmacol Exp Ther. 356, 123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, et al. , 2003. Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci USA. 100, 1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ, 2010. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 50, 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niznik HB, Van Tol HH, 1992. Dopamine receptor genes: new tools for molecular psychiatry. J Psychiatry Neurosci. 17, 158–80. [PMC free article] [PubMed] [Google Scholar]
- Nunez F, et al. , 2018. PBF509, an Adenosine A2A Receptor Antagonist With Efficacy in Rodent Models of Movement Disorders. Front Pharmacol. 9, 1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin SR, et al. , 2004. Development and anatomic localization of torsinA. Adv Neurol. 94, 61–5. [PubMed] [Google Scholar]
- Obermann M, et al. , 2007. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord. 22, 1117–23. [DOI] [PubMed] [Google Scholar]
- Ostrem JL, et al. , 2017. Subthalamic nucleus deep brain stimulation in isolated dystonia: A 3-year follow-up study. Neurology. 88, 25–35. [DOI] [PubMed] [Google Scholar]
- Ostrem JL, Starr PA, 2008. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. 5, 320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panarese JD, et al. , 2019. The discovery of VU0652957 (VU2957, Valiglurax): SAR and DMPK challenges en route to an mGlu4 PAM development candidate. Bioorg Med Chem Lett. 29, 342–346. [DOI] [PubMed] [Google Scholar]
- Pancani T, et al. , 2014. M4 mAChR-mediated modulation of glutamatergic transmission at corticostriatal synapses. ACS Chem Neurosci. 5, 318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN, 1995. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 20, 91–127. [DOI] [PubMed] [Google Scholar]
- Park JE, et al. , 2017. Lack of efficacy of levetiracetam in oromandibular and cranial dystonia. Acta Neurol Scand. 136, 103–108. [DOI] [PubMed] [Google Scholar]
- Paulson G, 1960. Procyclidine for dystonia caused by phenothiazine derivatives. Dis Nerv Syst. 21, 447–8. [PubMed] [Google Scholar]
- Peres FF, et al. , 2018. Cannabidiol as a Promising Strategy to Treat and Prevent Movement Disorders? Front Pharmacol. 9, 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Lloret S, Rascol O, 2018. Efficacy and safety of amantadine for the treatment of L-DOPA-induced dyskinesia. J Neural Transm (Vienna). 125, 1237–1250. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW, 2004. Dysfunction of dopaminergic pathways in dystonia. Adv Neurol. 94, 163–70. [PubMed] [Google Scholar]
- Pezawas L a. V. BA a. M. VS a. C. JH a. K. BS a. S. RE a. E. MF a. M.-. 2004. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. The Journal of Neuroscience. 24, 10099–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, et al. , 2001. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 106, 579–587. [DOI] [PubMed] [Google Scholar]
- Pizoli CE, et al. , 2002. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 22, 7825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston Z, et al. , 2000. Adenosine receptor expression and function in rat striatal cholinergic interneurons. Br J Pharmacol. 130, 886–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston Z a. L. K a. W. L a. F. TC a. D. AK a. R. PJ, 2000. Adenosine receptor expression and function in rat striatal cholinergic interneurons. Br J Pharmacol. 130, 886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente CN, et al. , 2013. Neuropathology of cervical dystonia. Exp Neurol. 241, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente CN, et al. , 2016. A Functional Magnetic Resonance Imaging Study of Head Movements in Cervical Dystonia. Front Neurol. 7, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CW, et al. , 2019. Discovery of an orally bioavailable and Central Nervous System (CNS) penetrant mGlu7 Negative Allosteric Modulator (NAM) in vivo tool compound: N-(2-(1H-1,2,4-triazol-1-yl)-5-(trifluoromethoxy)phenyl)-4-(cyclopropylmethoxy)-3 -methoxybenzamide (VU6012962). J Med Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, 2004. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 7, 583–4. [DOI] [PubMed] [Google Scholar]
- Richter A, 2003a. The NMDA receptor NR2B subtype selective antagonist Ro 25–6981 aggravates paroxysmal dyskinesia in the dt(sz) mutant. Eur J Pharmacol. 458, 107–10. [DOI] [PubMed] [Google Scholar]
- Richter A, 2003b. The NMDA receptor NR2B subtype selective antagonist Ro 25–6981 aggravates paroxysmal dyskinesia in the dtsz mutant. European journal of pharmacology. 458, 107–110. [DOI] [PubMed] [Google Scholar]
- Richter A, Hamann M, 2001. Effects of adenosine receptor agonists and antagonists in a genetic animal model of primary paroxysmal dystonia. Br J Pharmacol. 134, 343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, Loscher W, 1994. (+)-WIN 55,212–2, a novel cannabinoid receptor agonist, exerts antidystonic effects in mutant dystonic hamsters. Eur J Pharmacol. 264, 371–7. [DOI] [PubMed] [Google Scholar]
- Richter A, Loscher W, 2002. Effects of pharmacological manipulations of cannabinoid receptors on severity of dystonia in a genetic model of paroxysmal dyskinesia. Eur J Pharmacol. 454, 145–51. [DOI] [PubMed] [Google Scholar]
- Rilstone JJ, et al. , 2013. Brain dopamine-serotonin vesicular transport disease and its treatment. N Engl J Med. 368, 543–50. [DOI] [PubMed] [Google Scholar]
- Rittiner JE, et al. , 2016. Functional Genomic Analyses of Mendelian and Sporadic Disease Identify Impaired eIF2alpha Signaling as a Generalizable Mechanism for Dystonia. Neuron. 92, 1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A a. A. I a. M. AB a. N. JA a. d. l. C. A a. M. R, 2002. Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. The European Journal of Neuroscience. 16, 2049–2058. [DOI] [PubMed] [Google Scholar]
- Rondou P, et al. , 2010. The dopamine D4 receptor: biochemical and signalling properties. Cell Mol Life Sci. 67, 1971–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SJ, et al. , 2015. A new knock-in mouse model of l-DOPA-responsive dystonia. Brain. 138, 2987–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saft C, et al. , 2018. Cannabinoids for Treatment of Dystonia in Huntington’s Disease. J Huntingtons Dis. 7, 167–173. [DOI] [PubMed] [Google Scholar]
- Sander SE, Richter A, 2007. Effects of intrastriatal injections of glutamate receptor antagonists on the severity of paroxysmal dystonia in the dtsz mutant. Eur J Pharmacol. 563, 102–8. [DOI] [PubMed] [Google Scholar]
- Scarduzio M, et al. , 2017. Strength of cholinergic tone dictates the polarity of dopamine D2 receptor modulation of striatal cholinergic interneuron excitability in DYT1 dystonia. Exp Neurol. 295, 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, et al. , 1991. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 57, 1062–7. [DOI] [PubMed] [Google Scholar]
- Schirinzi T, et al. , 2018. Dystonia as a network disorder: a concept in evolution. Curr Opin Neurol. 31, 498–503. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, et al. , 2019. The Theory and Neuroscience of Cerebellar Cognition. Annu Rev Neurosci. [DOI] [PubMed] [Google Scholar]
- Schneider SA, et al. , 2010. Modulatory effects of 5Hz rTMS over the primary somatosensory cortex in focal dystonia--an fMRI-TMS study. Mov Disord. 25, 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte G, Fredholm BB, 2003. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 15, 813–27. [DOI] [PubMed] [Google Scholar]
- Schwarz CS, Bressman SB, 2009. Genetics and treatment of dystonia. Neurol Clin. 27, 697–718, vi. [DOI] [PubMed] [Google Scholar]
- Sciamanna G, et al. , 2014. Negative allosteric modulation of mGlu5 receptor rescues striatal D2 dopamine receptor dysfunction in rodent models of DYT1 dystonia. Neuropharmacology. 85, 440–50. [DOI] [PubMed] [Google Scholar]
- Sciamanna G, et al. , 2012. Cholinergic dysfunction alters synaptic integration between thalamostriatal and corticostriatal inputs in DYT1 dystonia. J Neurosci. 32, 11991–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakkottai VG, et al. , 2017. Current Opinions and Areas of Consensus on the Role of the Cerebellum in Dystonia. Cerebellum. 16, 577–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, et al. , 2018. Stereotactic Lesioning of the Thalamic Vo Nucleus for the Treatment of Writer’s Cramp (Focal Hand Dystonia). Front Neurol. 9, 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ Jr., 1992. Molecular biology of dopamine receptors. Trends Pharmacol Sci. 13, 61–9. [DOI] [PubMed] [Google Scholar]
- Simonyan K, 2018. Neuroimaging Applications in Dystonia. Int Rev Neurobiol. 143, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, et al. , 2013. Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. J Neurosci. 33, 14705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, et al. , 2017. The direct basal ganglia pathway is hyperfunctional in focal dystonia. Brain. 140, 3179–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siokas V a. A. A-M a. T. Z a. M. A a. M. A. FA a. D. E, 2018. Risk Factor Genes in Patients with Dystonia: A Comprehensive Review. Tremor Other Hyperkinet Mov (N Y). 8, 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren B, 2017. The evolution of regulators of G protein signalling proteins as drug targets - 20 years in the making: IUPHAR Review 21. Br J Pharmacol. 174, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal RM, et al. , 2008. Differences in excitatory transmission between thalamic and cortical afferents to single spiny efferent neurons of rat dorsal striatum. Eur J Neurosci. 28, 2041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM, Berger PA, 1981. Bromocriptine in dystonia. Lancet. 2, 745. [DOI] [PubMed] [Google Scholar]
- Standaert DG, et al. , 1994. Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol. 343, 1–16. [DOI] [PubMed] [Google Scholar]
- Stern TA, Anderson WH, 1979. Benztropine prophylaxis of dystonic reactions. Psychopharmacology (Berl). 61, 261–2. [DOI] [PubMed] [Google Scholar]
- Sullivan KL, et al. , 2005. Levetiracetam for the treatment of generalized dystonia. Parkinsonism Relat Disord. 11, 469–71. [DOI] [PubMed] [Google Scholar]
- Svedberg MM, et al. , 2019. In vitro phosphodiesterase 10A (PDE10A) binding in whole hemisphere human brain using the PET radioligand [(18)F]MNI-659. Brain Res. [DOI] [PubMed] [Google Scholar]
- Swash M, et al. , 1972. Treatment of involuntary movement disorders with tetrabenazine. J Neurol Neurosurg Psychiatry. 35, 186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe LM, et al. , 2009. Primary dystonia: molecules and mechanisms. Nat Rev Neurol. 5, 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, et al. , 2010. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 65, 320–7. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, et al. , 2016. Complementary Contributions of Striatal Projection Pathways to Action Initiation and Execution. Cell. 166, 703–715. [DOI] [PubMed] [Google Scholar]
- Tepper JM, et al. , 2008. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res Rev. 58, 272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM a. T. F a. K. T a. I.-S. O, 2010. Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat. 4, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari A, et al. , 2017. It’s not just the basal ganglia: Cerebellum as a target for dystonia therapeutics. Mov Disord. 32, 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thenganatt MA, Jankovic J, 2014. Treatment of dystonia. Neurotherapeutics. 11, 139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson VB, et al. , 2011. Convergent mechanisms in etiologically-diverse dystonias. Expert Opin Ther Targets. 15, 1387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, et al. , 2010. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 30, 3398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Cragg SJ, 2011. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Syst Neurosci. 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiberi M, et al. , 1991. Cloning, molecular characterization, and chromosomal assignment of a gene encoding a second D1 dopamine receptor subtype: differential expression pattern in rat brain compared with the D1A receptor. Proc Natl Acad Sci USA. 88, 7491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tison F, et al. , 2016. A Phase 2A Trial of the Novel mGluR5-Negative Allosteric Modulator Dipraglurant for Levodopa-Induced Dyskinesia in Parkinson’s Disease. Mov Disord. 31, 1373–80. [DOI] [PubMed] [Google Scholar]
- Tolosa E, Compta Y, 2006. Dystonia in Parkinson’s disease. J Neurol. 253 Suppl 7, Vii7–13. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, et al. , 2010. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 62, 405–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor JR, et al. , 2009. RGS9–2: probing an intracellular modulator of behavior as a drug target. Trends Pharmacol Sci. 30, 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, et al. , 2007. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 27, 3663–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, et al. , 2000. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 408, 199–203. [DOI] [PubMed] [Google Scholar]
- Vander Heyden AB, et al. , 2009. LULL1 retargets TorsinA to the nuclear envelope revealing an activity that is impaired by the DYT1 dystonia mutation. Mol Biol Cell. 20, 2661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CJ, et al. , 1997. Treatment of spastic dystonia with transdermal nicotine. Lancet. 350, 565. [DOI] [PubMed] [Google Scholar]
- Wang WW, et al. , 2017. A Meta-Analysis of Adenosine A2A Receptor Antagonists on Levodopa-Induced Dyskinesia In Vivo. Front Neurol. 8, 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, et al. , 1990. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci USA. 87, 7050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, et al. , 1997. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 68, 469–78. [DOI] [PubMed] [Google Scholar]
- Wenzel A, et al. , 1996. Developmental and regional expression of NMDA receptor subtypes containing the NR2D subunit in rat brain. J Neurochem. 66, 1240–8. [DOI] [PubMed] [Google Scholar]
- Wichmann T, 2008. Commentary: Dopaminergic dysfunction in DYT1 dystonia. Exp Neurol. 212, 242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MR, et al. , 2017. Discovery of VU0467485/AZ13713945: An M4 PAM Evaluated as a Preclinical Candidate for the Treatment of Schizophrenia . ACS Med Chem Lett. 8, 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, et al. , 2015. Input- and cell-type-specific endocannabinoid-dependent LTD in the striatum. Cell Rep. 10, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardimci N, et al. , 2006. Levetiracetam in Meige’s syndrome. Acta Neurol Scand. 114, 63–6. [DOI] [PubMed] [Google Scholar]
- Zadikoff C, et al. , 2011. Cannabinoid, CB1 agonists in cervical dystonia: Failure in a phase IIa randomized controlled trial. Basal Ganglia. 1, 91–95. [Google Scholar]
- Zakirova Z, et al. , 2018. Mutations in THAP1/DYT6 reveal that diverse dystonia genes disrupt similar neuronal pathways and functions. PLoS Genet. 14, e1007169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, et al. , 2002. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 22, 6347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, et al. , 2000. G(olf)alpha mediates dopamine D1 receptor signaling. J Neurosci. 20, Rc91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CN, et al. , 2017. Evaluation of AZD1446 as a Therapeutic in DYT1 Dystonia. Front Syst Neurosci. 11, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]