Abstract
The accumulation of senescent cells in tissues is causally linked to the development of several age-related diseases; the removal of senescent glial cells in animal models prevents Tau accumulation and cognitive decline. Senescent cells can arise through several distinct mechanisms; one such mechanism is dysregulation of alternative splicing. In this study, we characterised the senescent cell phenotype in primary human astrocytes in terms of SA-β-Gal staining and SASP secretion, and then assessed splicing factor expression and candidate gene splicing patterns. Finally, we assessed associations between expression of dysregulated isoforms and premature cognitive decline in 197 samples from the InCHIANTI study of ageing, where expression was present in both blood and brain. We demonstrate here that senescent astrocytes secrete a modified SASP characterised by increased IL8, MMP3, MMP10, and TIMP2 but decreased IL10 levels. We identified significant changes in splicing factor expression for 10/20 splicing factors tested in senescent astrocytes compared with early passage cells, as well as dysregulation of isoform levels for 8/13 brain or senescence genes tested. Finally, associations were identified between peripheral blood GFAPα, TAU3, and CDKN2A (P14ARF) isoform levels and mild or severe cognitive decline over a 3–7-year period. Our data are suggestive that some of the features of cognitive decline may arise from dysregulated splicing of important genes in senescent brain support cells, and that defects in alternative splicing or splicing regulator expression deserve exploration as points of therapeutic intervention in the future.
Keywords: Neurodegenerative disease, Alternative splicing, Senescence, Gene expression
Introduction
Age is a major risk factor for cognitive decline and neurodegenerative disease, as it is for most common, chronic disorders (Niccoli and Partridge, 2012). The accumulation of senescent cells in tissues and organs has emerged as a major driver of the ageing process and age-related disease in mammals; selective removal of such cells in genetically engineered animals has been demonstrated to reverse or delay aspects of ageing (Baker et al., 2016, Baker et al., 2011). Senescent cells are alive and metabolically active, but have altered function compared with their non-senescent counterparts. A major feature of senescence is secretion of the senescence-associated secretory phenotype (SASP) which can act in a paracrine manner on neighbouring cells to drive organismal ageing (Freund et al., 2010). Although terminally differentiated cells such as neurones may not undergo replicative senescence in the accepted sense, other important support cells in the brain such as astrocytes that do have proliferative capacity are subject to this phenomenon. Astrocytes have a pivotal role in maintenance of trophic factors, neurotransmitters, and the support and strengthening of neuronal connections (Sidoryk-Wegrzynowicz et al., 2011). Previous evidence has suggested that the accumulation of senescent astrocytes may drive neurodegenerative disorders (Di Malta et al., 2012) and recent work shows that clearance of senescent glial cells may prevent the accumulation of Tau aggregates and improve cognitive function in mouse models (Bussian et al., 2018, Baker and Petersen, 2018). Pharmacological impairment of astrocytic function recapitulates cognitive deficits that are observed in old age (Tarantini et al., 2017). It is also noteworthy that irradiation-induced accumulation of senescent cells is associated with cerebromicrovascular dysfunction in animal models (Ungvari et al., 2017).
Cells enter a senescent state for a number of reasons, one of which is repeated exposure to internal and external stressors (de Magalhaes and Passos, 2018). Exposure to cellular stress elicits an adaptive and plastic transcriptional response, which is partly orchestrated by alternative splicing (Mastrangelo et al., 2012). Alternative splicing is mediated by the combinatorial binding of a series of splicing activators and inhibitors to splicing enhancer and silencer sequences around the splice sites to determine whether or not each splice site is used (Fu and Ares, 2014, Smith and Valcarcel, 2000). Supporting the notion of a pivotal role for dysregulated alternative splicing in senescence, transcripts associated with chronological age in humans are enriched in gene ontology pathways involved in splice site choice (Harries et al., 2011) and splicing factor expression is also associated with cellular senescence in in vitro models (Holly et al., 2013), with human ageing phenotypes (Lee et al., 2019) and with lifespan in long-lived mice (Lee et al., 2016). Perhaps most persuasively, restoration of splicing factor levels is associated with rescue of cellular senescence in multiple human primary human cell types (Latorre et al., 2017, Latorre et al., 2018a, Latorre et al., 2018c). Changes to the regulation of splicing have previously been reported in Alzheimer’s Disease (Wong, 2013) and global dysregulation of splicing is a characteristic of several neurodegenerative conditions such as Huntington’s disease (Lin et al., 2016), frontotemporal lobar dementia (Gao et al., 2017), and Parkinson’s Disease (Soreq et al., 2012). Specially, alterations to the splicing pattern of the MAPT gene which encodes Tau protein are known to contribute to neurofibrillary tangles (Goedert and Jakes, 1990).
We hypothesised that senescent astrocytes would display differential expression of splicing regulatory factors and altered patterns of alternative splicing in vitro, and that some of these isoform changes may be detectable in peripheral blood and show statistical associations with cognitive phenotypes in human populations. We first characterised the astrocyte SASP in terms of cytokine and MMP production, and then determined the splicing factor repertoire and patterns of alternative splicing for a panel of brain or senescence candidate genes in senescent human primary astrocytes. Where dysregulation of splicing patterns was demonstrated and expression was conserved in blood, we then assessed associations between peripheral blood isoform levels and measures of cognitive dysfunction from 197 individuals from the InCHIANTI study of ageing, a longitudinal and cross-sectional population study of individuals from the Tuscany region of Italy (Ferrucci et al., 2000). We identified that senescent astrocytes display a modified SASP, consisting of elevated IL8, MMP, MMP10, and TIMP2 levels, but decreased IL10. Of the splicing regulatory factors tested, 50% demonstrated dysregulated expression in senescent astrocytes; this was accompanied by altered splicing of 7/13 of candidate genes tested. Furthermore, when we assessed the relationship between peripheral blood expression of isoforms dysregulated in astrocytes and cognitive decline as measured my Mini Mental State Exam (MMSE), GFAPα and TAU3 transcript levels were positively correlated with cognitive decline, whereas GFAPΑ transcript levels were negatively associated with cognitive decline over a 3–7-year period in participants from the InCHIANTI study of ageing. Our data are in agreement with the hypothesis that senescent astrocytes display differential expression of splicing regulatory factors and altered patterns of alternative splicing, and that some of these isoform changes may reflect those in peripheral blood. Such changes may show statistical associations with cognitive phenotypes in human populations.
Experimental protocols
Culture of early and senescent astrocytes
These studies used cultures of early passage and late passage human primary astrocytes (HPA) previously isolated from a block of sub-ventricular deep white matter tissue in a 17-year-old male donor immediately post-mortem with consent from next-of-kin. Ethical approval was granted by the North and East Devon Research Ethics Committee. Astrocytes were isolated from tissue blocks as previously described (Holley et al., 2005). Cells were maintained in humidified incubators with 95% O2/5% CO2 in HPA stock media (Celprogen Inc., Torrance, CA, USA). For the production of senescent cultures, cells were counted and equal numbers of cells seeded (4 × 103 cell/cm2) at each passage in continuous culture until the growth of the culture slowed to less than 0.5 population doublings (PD)/week. Astrocyte cultures underwent continuous culture until the onset of replicative senescence and growth arrest in 3 biological replicates. Early passage astrocytes at PD = 24 and late passage astrocytes at PD = 84 were used.
Quantification of senescent cell load
Cell senescence was assessed in 3 biological replicates by the biochemical senescence marker senescence-associated β-galactosidase (SA-β-Gal) using a commercial kit (Sigma Aldrich, UK) according to the manufacturer’s instructions, with a minimum of 100 cells assessed per replicate. Senescence was also quantified by assessing the expression of the CDKN2A gene (a known molecular marker of cell senescence) and by changes in cell morphology typical of senescence as in our previous work (Latorre et al., 2017, Holly et al., 2013). Total RNA (100 ng) was reverse transcribed in 20 μl reactions using EvoScript reverse transcriptase (Roche Life Sciences, Burgess Hill, UK). Total CDKN2A expression was measured by qRT-PCR relative to 3 empirically determined endogenous control genes (GUSB, PPIA, and GADPH) on the QuantStudio 12K Flex platform (Applied Biosystems, Foster City, USA). PCR reactions contained 2.5 μl TaqMan Universal Mastermix (no AMPerase) (Applied Biosystems, Foster City, USA), 900 nM of each primer, 250 nM probe, and 0.5 μl cDNA in a total volume of 5 μl. Cycling conditions were a single cycle of 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min.
Characterisation of the senescence-associated secretory phenotype in early and late passage cells
Cells were seeded in two biological replicates of 10 × 104 cells in a 25-cm2 flasks and cultured until 80% confluence. Cell supernatants were then harvested and stored at − 80 °C. The senescence-associated secretory phenotype was then quantified in the culture media following 48 h incubation using the ABCAM Human Cytokine Antibody Array (ab133997; Abcam, Cambridge, UK) and the ABCAM Human MMP Antibody Array (ab134004; Abcam, Cambridge, UK). SASP components measured were as follows: IL-1B, IL-2, Il-6, IL-8, IL-10, TNF-α, IFN-γ, GM-CSF, Angiogenin, ENA78, GRO-α, MMP3, MMP10, and TIMP2. Results were normalised using positive and negative controls as per the kit instructions using the LI-COR Odyssey® CLx imaging system (Lincoln, NE, USA). An unpaired two tailed t test was used to assess statistical significance in secreted matrix metalloproteinases and inflammatory cytokine secretion using Minitab 18 software package (Minitab, Centre County, USA).
Quantification of splicing factor expression
Splicing factors have previously been demonstrated to be associated with cellular senescence with evidence suggesting they may be drivers of this process in some tissues (Latorre et al., 2017, Latorre et al., 2018a, Latorre et al., 2018c). We measured the expression levels of an a priori panel of 20 splicing factors previously associated with age, lifespan, and cellular senescence in different tissue types in our previous work (Harries et al., 2011, Holly et al., 2013, Latorre et al., 2018b). This panel included the splicing inhibitors HNRNPA0, HNRNPA1, HNRNPA2B1, HNRNPD, HNRNPH3, HNRNPK, HNRNPM, HNRNPUL2, the splicing activators AKAP17A, PNISR, SRSF1, SRSF2, SRSF3, SRSF6, TRA2B, SRSF7 and the SF3B1, IMP3, LSM14A, and LSM2 components of the core spliceosome. Splicing factor expression was measured in 3 biological and 2 technical replicates by qRT-PCR using custom TaqMan Low Density Arrays (TLDA) on the Quantstudio 12K Flex platform as previously described (Holly et al., 2013). Transcript levels were expressed relative to the geometric mean of the GUSB and PPIA endogenous control genes and normalised to their expression in RNA from early passage cells. We then performed tests for equality of variance and t test using IBM SPSS Statistics 25.
Quantification of candidate gene expression in early passage and senescent astrocytes
A panel of candidate genes were selected for analysis on the basis of biological relevance (known links with brain function, neurodegenerative disease, or senescence) and where available evidence from the literature that alternatively expressed isoforms may have differential function to allow interpretation of changes. The identity of genes tested and the rationale for their inclusion are given in Table 1. TaqMan Assays specific to particular isoforms were designed to unique regions of the transcripts in question (assay sequences are available upon request). Assays were validated by standard curve analysis using 7 serial 1:2 dilutions of cDNA derived from the whole brain lysate. Reverse transcription and qRT-PCR conditions are described above. Experiments were carried out in 3 biological and 3 technical replicates. Transcript levels were expressed relative to GUSB and PPIA endogenous control genes and normalised to their expression in RNA from early passage cells. We then performed tests for equality of variance and t test using IBM SPSS Statistics 25.
Table 1.
Transcript isoforms identified for expression analysis
| Gene | Transcript accession | Isoform/transcript function |
|---|---|---|
| ATM | NM_000051.3 | DNA damage repair |
| AQP4 | ||
| AQP4M1 | NM_001650.6 | Pore-forming integral membrane protein |
| AQP4M23 | NM_004028.4 | Pore-forming integral membrane protein |
| SLC1A2 | ||
| EAAT2A | NM_004171.4 | Excitatory amino acid transporter |
| EAAT2B | NM_001252652.1 | Excitatory amino acid transporter |
| GFAP | ||
| GFAPΑ | NM_002055 | Astrocyte intermediate filament protein |
| GFAP(B) | NM_001131019.1 | Astrocyte intermediate filament protein |
| KL | ||
| KLOTHO mKl | NM_004795 | Membrane bound isoform, coreceptor for FGF23 |
| KLOTHO msKl | NM_004795.3 | Secreted isoform, endocrine factor which improves cognitive performance in ageing |
| CDKN2A | ||
| p14ARF | NM_058195 | p53 pathway to cell cycle cessation |
| p16INK4A | NM_001195132 | RB1 pathway to cell cycle cessation (Takahashi et al., 2007) |
| CDKN1A | ||
| p21a | NM_078467 | Inhibits proliferation |
| p21b | NM_000389 | Promotes proliferation |
| TP53 | Cell cycle regulation | |
| PSEN2 | NM_012486.2 | Processing of β-amyloid |
| MAPT | ||
| TAU3 | Microtubule protein involved in neurofibrillary tangles | |
| TAU4 | Microtubule protein involved in neurofibrillary tangles | |
The table gives the identity of the isoforms selected for analysis, the relevant NM accession numbers, and a brief description of their function
Assessment of candidate gene expression levels with cognitive decline in a longitudinal human population
The InCHIANTI study of ageing is a population study of ageing with detailed assessment of health and lifestyle parameters at baseline, and again at 4 subsequent follow-ups (FU2; 2004–2006, FU3; 2007–2009, and FU4; 2012–2014) (Ferrucci et al., 2000). We selected 197 participants for study. Inclusion criteria were age at FU3 > 64 years with an MMSE score > 18 to avoid those already on a declining cognitive trajectory and availability of an FU3 RNA sample with clinical information available at both FU3 and FU4. Participants were categorised into ‘mild’ or ‘severe’ groups depending their change in the MMSE score between FU3 and FU4; individuals declining between 2 and 8 points were defined as ‘mild’, whereas those declining between 9 and 22 points were categorised as ‘severe’. These thresholds were chosen on the basis of previously defined criteria, where a ‘severe’ decline was categorised as a drop in MMSE score > 3 points per annum (Clark et al., 1999, McCarten et al., 2004, Hensel et al., 2007). Ethical approval was granted by the Instituto Nazionale Riposo e Cura Anziani institutional review board in Italy. Methods were carried out in accordance with the relevant guidelines and regulations. Informed consent was obtained from all participants.
Blood was collected into PAXgene tubes (BD Biosciences) at FU3 and extracted using the PAXgene blood RNA kit (Qiagen, Paisley, UK). One hundred nanograms RNA was then reverse transcribed using the EvoScript Universal cDNA Master kit (Roche Life Sciences, Burgess Hill, UK) according to the manufacturer’s instructions except for a change to the extension phase of the reaction: a step of 30 min at 65 °C was used instead of 15 min at 65 °C. Expression levels for transcripts showing senescence-related dysregulation in levels in astrocytes were quantified in peripheral blood as described above. Samples were assessed in 3 technical replicates. Data were log transformed and multivariate linear regression models were performed using STATA© SE 15 to investigate the association between transcript expression and cognitive decline as measured by change (Δ) in MMSE score. Models were adjusted for age, gender, smoking (lifetime pack-years), study site, education level, and white blood cell subtype counts (% neutrophils, monocytes, basophils, eosinophils).
Results
Characterisation of senescent astrocytes
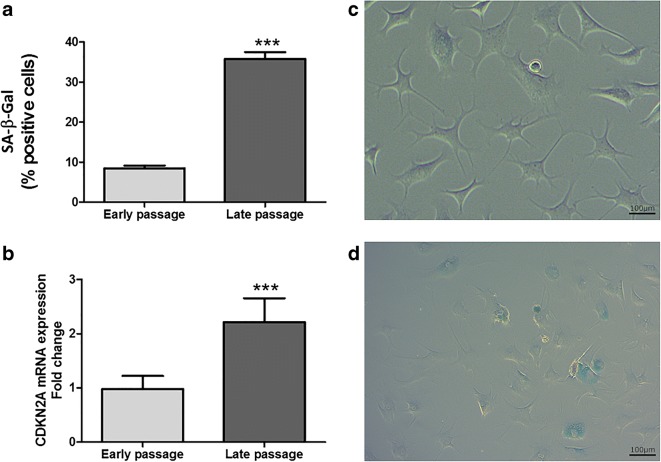
Human primary astrocyte cultures were considered senescent at PD84 at which point they were considered senescent. This was verified by molecular and biochemical characterisation of the growth kinetics of the cultures; senescence-associated β-galactosidase (SA-β-gal) staining demonstrated a significant increase in the number of senescent cells from 8% in early passage cells to 36% in late passage cells (Fig. 1a) which was mirrored by a concurrent increase in the expression of the CDKN2A gene (Fig. 1b). SASP factors in conditioned media derived from senescent cells demonstrated altered levels for several key SASP proteins; we observed elevated IL-8, GM-CSF, Angiogenin, ENA78, GRO-α, MMP-3, MMP-10, and TIMP2 levels, but reduced IL-10 levels (Table 2).
Fig. 1.
Senescent cell quantification in early passage and late passage human primary astrocytes. a This graph gives the change in senescent cell load as determined by senescence-associated β-galactosidase (SA-β-Gal) staining between early passage and late passage human primary astrocytes. The percentage of cells staining positive for SA-β-Gal is given on the Y axis and the identity of the cell culture on the X axis. b Change in senescent cell load as determined by CDKN2A expression between early passage and late passage human primary astrocytes. Levels of CDKN2A transcripts relative to the endogenous control genes (GUSB, PPIA, and GADPH) and normalised to the expression levels in early passage cells are given on the Y axis and the identity of the cell culture on the X axis. Early passage cells are population doubling (PD) 24; late passage cells are at PD = 84. Results are from 3 biological replicates. *p < 0.01, **p < 0.01, ***p < 0.001. Error bars refer to standard deviation of measurement. c Senescence-associated β-galactosidase staining in early passage astrocytes (PD 24). d Senescence-associated β-galactosidase staining in late passage astrocytes (PD 84)
Table 2.
Evaluation of astrocyte SASP. Level of secreted cytokines and matrix metalloproteinase proteins in senescent and non-senescent human primary astrocytes is given below
| Cytokine | Early passage (SE) | Late passage (SE) | p value |
|---|---|---|---|
| IL-1B | 274.233 (24.37) | 286.058 (36.82) | 0.798 |
| IL2 | 74.659 (23.99) | 111.541 (18.97) | 0.273 |
| IL-6 | 78.752 (11.81) | 73.490 (9.49) | 0.740 |
| IL-8 | 409.541 (55.96) | 1097.730 (128.17) | 0.003 |
| IL-10 | 56.560 (9.53) | 18.219 (4.79) | 0.011 |
| TNF-α | 10.214 (6.50) | 16.812 (8.12) | 0.576 |
| IFN-γ | 120.787 (20.05) | 137.933 (23.89) | 0.602 |
| GM-CSF | 27.060 (3.82) | 66.130 (7.63) | 0.004 |
| Angiogenin | 881.4542 (106.96) | 1397.6704 (103.33) | 0.013 |
| ENA78 | 160.618 (28.74) | 270.255 (27.36) | 0.033 |
| GRO-α | 256.152 (27.78) | 461.5086 (65.09) | 0.027 |
| MMP-3 | 75.897 (7.49) | 128.3126 (15.34) | 0.022 |
| MMP-10 | 6.284 (3.15) | 18.566 (2.58) | 0.023 |
| TIMP-2 | 4723.167 (190.92) | 8402.9399 (505.84) | < 0.001 |
SASP, senescence-associated secretory phenotype. Cytokines demonstrating significant differences in expression are given in boldface. Cytokine expression is measured as arbitrary units (AU). N = 2 biological and 2 technical replicates per sample. SE, standard error
Changes in splicing factor expression and patterns of alternative splicing in senescent astrocytes
We have previously demonstrated changes in splicing factor expression in senescent primary human cells of different lineages (Holly et al., 2013, Latorre et al., 2017, Latorre et al., 2018b, Latorre et al., 2018c). Similar changes were also apparent in senescent astrocytes, where 10/20 of the splicing factors tested demonstrated lower expression in late passage cells compared with earlier passage cells (Table 3). Both HNRNP splicing inhibitors and serine-arginine (SR) rich splicing activator transcripts demonstrated dysregulation; 4/8 (50%) transcripts encoding splicing inhibitors, 3/8 splicing activator transcripts, and 3/4 (75%) of core spliceosomal transcripts demonstrated changed expression in senescent astrocytes. Most genes selected for study were expressed in astrocytes; only AQP4M1, EAAT2A, and EAAT2B were not. Eight of the remaining 13 transcripts demonstrated changes to their splicing patterns (Table 4).
Table 3.
Comparison of splicing factor levels in senescent and non-senescent astrocytes
| Splicing factor | Early passage astrocytes | Late passage astrocytes | p value |
|---|---|---|---|
| AKAP17A | 1.035 (0.090) | 1.01 (0.044) | 0.077 |
| HNRNPA0 | 1.024 (0.077) | 0.65 (0.031) | 0.005 |
| HNRNPA1 | 1.236 (0.340) | 1.863 (0.416) | 0.449 |
| HNRNPA2B1 | 1.343 (0.353) | 0.6 (0.108) | 0.257 |
| HNRNPD | 1.094 (0.180) | 0.646 (0.048) | 0.03 |
| HNRNPH3 | 1.071 (0.130) | 0.743 (0.037) | 0.003 |
| HNRNPK | 1.153 (0.176) | 1.37 (0.209) | 0.551 |
| HNRNPM | 1.021 (0.068) | 0.66 (0.067) | 0.012 |
| HNRNPUL2 | 1.074 (0.148) | 0.784 (0.106) | 0.089 |
| IMP3 | 1.088 (0.160) | 0.62 (0.11) | 0.035 |
| LSM14A | 1.042 (0.113) | 0.315 (0.047) | 0.005 |
| LSM2 | 1.377 (0.426) | 0.369 (0.045) | 0.065 |
| PNISR | 1.041 (0.098) | 0.777 (0.030) | 0.005 |
| SF3B1 | 1.018 (0.070) | 0.716 (0.030) | 0.018 |
| SRSF1 | 1.06 (0.140) | 0.885 (0.038) | 0.065 |
| SRSF2 | 1.038 (0.095) | 1.017 (0.045) | 0.121 |
| SRSF3 | 1.077 (0.175) | 0.697 (0.032) | 0.095 |
| SRSF6 | 1.07 (0.126) | 0.762 (0.104) | 0.098 |
| TRA2B | 1.022 (0.081) | 0.757 (0.057) | 0.031 |
| SRSF7 | 1.119 (0.198) | 0.51 (0.063) | 0.015 |
Values given refer to the mean expression of each splicing factor in either early or late passage cells. Values in parentheses are the standard error of the mean. Splicing factors demonstrating significant differences in expression are given in boldface. N = 3 biological and 3 technical replicates per sample
Table 4.
Comparison of alternative isoforms of selected brain or senescence genes in senescent and non-senescent astrocytes
| Early passage astrocytes | Late passage astrocytes | p value | |
|---|---|---|---|
| GFAPΑ | 0.809 (0.208) | 0.062 (0.013) | 0.0021 |
| sKLOTHO | 1.155 (0.209) | 0.498 (0.275) | 0.131 |
| mKLOTHO | 1.059 (0.122) | 0.381 (0.012) | 0.005 |
| AQPM23 | 1.252 (0.459) | 1.067 (0.434) | 0.784 |
| TAU3 | 1.044 (0.114) | 0.603 (0.043) | 0.023 |
| TAU4 | 1.23 (0.674) | 1.542 (0.196) | 0.679 |
| PSEN2 | 1.009 (0.12) | 1.921 (0.158) | 0.01 |
| CDKN2A-p14ARF | 1.043 (0.044) | 0.532 (0.048) | 0.001 |
| CDKN2A-p16Ink4A | 1.061 (0.076) | 2.133 (0.127) | 0.002 |
| p21a | 1.079 (0.085) | 1.666 (0.087) | 0.009 |
| p21b | 1.016 (0.095) | 2.041 (0.077) | 0.001 |
| TP53 | 0.974 (0.052) | 1.038 (0.082) | 0.541 |
| ATM | 0.926 (0.097) | 0.612 (0.069) | 0.057 |
Values given refer to the mean expression of each splicing factor in either early or late passage cells. Values in parentheses are the standard error of the mean. Transcripts demonstrating significant differences in expression are given in boldface. N = 3 biological and 3 technical replicates per sample
Association of senescence-related transcripts with cognitive decline in a longitudinal human population
We next assessed whether any of the transcripts demonstrating senescence-related changes in aged primary human astrocytes were associated with cognitive decline as assessed by change in MMSE score between FU3 and FU4, in peripheral blood mRNA from individuals in the InCHIANTI study of ageing. Of the 8 transcripts demonstrating associations with senescence in late passage astrocytes, TAU3, GFAPα, mKLOTHO CDKN2A(p14ARF), CDKN2A(p16INK4A), CDKN1A(p21a), CDKN1A(p21b), and PSEN2 were also expressed in peripheral blood, and suitable for ongoing analysis. CDKN2A (p14ARF) and TAU3 were positively associated with mild cognitive decline (CDKN2A (p14ARF) beta coefficient 0.122, 95% CI 0.01 to 0.24; p = 0.04, TAU3 beta coefficient 0.170, 95% CI 0.042 to 0.297; p = 0.01), whereas GFAPα was negatively associated with mild cognitive decline (beta coefficient − 0.196 (95% CI − 0.36 to 0.032; p = 0.02; Fig. 2; Table 5). Interestingly, the only association we found with severe cognitive decline was a negative association with TAU3 (beta coefficient − 0.286, 95% CI − 0.56 to 0.04; p = 0.04). GFAPα and TAU3 demonstrated significant differences in level of isoform expression between mild and severe cognitive decline (Fig. 2, Table 5).
Fig. 2.
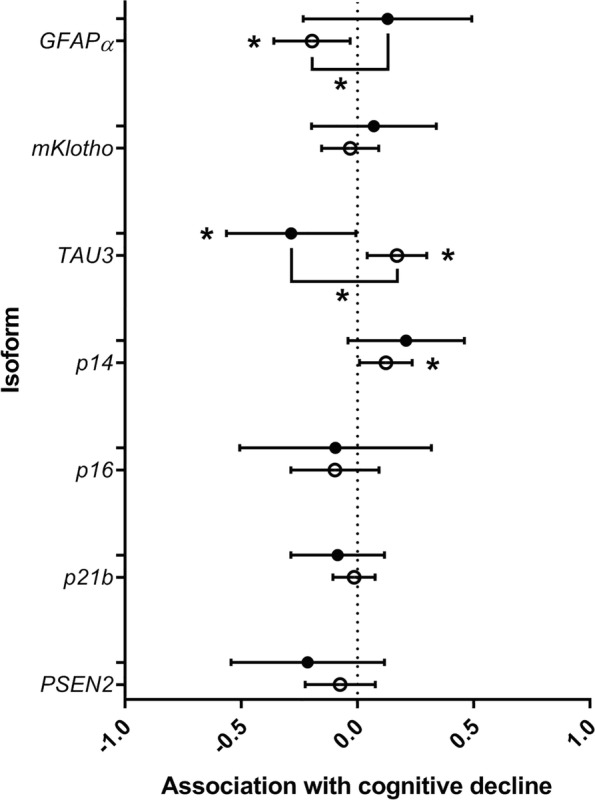
The association between isoform expression and cognitive decline in the InCHIANTI study of ageing. The graph indicates the associations between peripheral blood expression of alternatively expressed transcripts of genes with known links with neurodegenerative disease or cellular senescence and mild or severe cognitive decline is given in this figure. Mild decline is denoted by open circles; severe decline is denoted by closed circles. *p < 0.05. The null association point is given by the dotted line. Beta coefficients of association are given on the X axis and transcript identity is given on the Y axis. Data are from 197 participants classified as either mild cognitive decline (a reduction of 2–8 points in MMSE performance over a 3–7-year period) or severe decline (a reduction of 9–22 points in MMSE performance over the same period). Statistical differences in transcript expression between mild and severe decline are also given
Table 5.
Association between blood-based isoform production, induced by alternative splicing and cognitive decline in participants from the InCHIANTI population study of ageing. The table below gives the beta coefficients, 95% confidence intervals (95% CI), and p values for the association between candidate transcript expression and cognitive decline as assessed by change in MMSE for 197 individuals in a 3–7-year period. Mild cognitive decline is categorised as a decline of between 2 and 8 points in MMSE between FU3 and FU4, whereas severe cognitive decline is characterised as a decline of between 9 and 22 points. Transcripts demonstrating significant differences in expression are given in boldface
| Mild cognitive decline (2–8 point decline in MMSE) | Severe cognitive decline (9–22 point decline in MMSE) | Difference between mild and severe decline in MMSE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform | Beta | 95% CI | p value | Isoform | Beta | 95% CI | p value | Isoform | Beta | 95% CI | p value |
| GFAPα | − 0.196 | − 0.36 to 0.03 | 0.02 | GFAPα | 0.129 | − 0.23 to 0.49 | 0.48 | GFAPα | 0.363 | 0.08 to 0.65 | 0.01 |
| mKLOTHO | − 0.032 | − 0.16 to 0.09 | 0.61 | mKLOTHO | 0.070 | − 0.20 to 0.34 | 0.61 | mKLOTHO | 0.093 | − 0.18 to 0.37 | 0.50 |
| p14 | 0.122 | 0.01 to 0.24 | 0.04 | p14 | 0.209 | − 0.04 to 0.46 | 0.10 | p14 | 0.069 | − 0.18 to 0.32 | 0.59 |
| P16 | − 0.097 | − 0.29 to 0.09 | 0.31 | P16 | − 0.095 | − 0.51 to 0.32 | 0.65 | P16 | − 0.029 | − 0.45 to 0.40 | 0.89 |
| P21b | − 0.015 | − 0.11 to 0.08 | 0.74 | P21b | − 0.085 | − 0.29 to 0.12 | 0.40 | P21b | − 0.030 | − 0.22 to 0.16 | 0.75 |
| PSEN2 | − 0.075 | − 0.23 to 0.08 | 0.33 | PSEN2 | − 0.215 | − 0.55 to 0.12 | 0.20 | PSEN2 | − 0.133 | − 0.47 to 0.20 | 0.43 |
| TAU3 | 0.170 | 0.04 to 0.30 | 0.01 | TAU3 | − 0.286 | − 0.56 to − 0.01 | 0.04 | TAU3 | − 0.460 | − 0.75 to − 0.17 | 0.01 |
Discussion
The accumulation of senescent cells as a result of repeated cell stresses is thought to be a major contributor to ageing and age-related disease. The ablation of senescent cells is able to bring about improvements in multiple age-related phenotypes in animal models (Baker and Petersen, 2018, Baker et al., 2011). Furthermore, correlations exist between circulating levels of senescence markers such as p16INK4a and HMGB2 and functional status in humans (Lawrence et al., 2018). Suppression of senescent cell characteristics such as SASP using the flavonoid apigenin has also been demonstrated to reduce aggressive behaviour in breast cancer cells, providing further evidence reduction of senescent cell load may bring benefits for age-related diseases (Perrott et al., 2017).
Age-related dysregulation of alternative splicing may contribute to the accumulation of senescent cells during ageing. Alternative splicing, a key part of the molecular stress response that is a critical mediator of cellular plasticity and adaptability, has been shown to be disrupted during human ageing; restoration of splicing factor expression is able to reverse cellular senescence and bring about rejuvenation of multiple cell types in culture. We hypothesised that senescent astrocytes would display differential expression of splicing regulatory factors and altered patterns of alternative splicing in vitro, and that some of these isoform changes may be detectable in peripheral blood and show statistical associations with cognitive phenotypes in human populations. We demonstrated that 50% of splicing factor transcripts we measured demonstrated dysregulated expression in senescent astrocytes, and splicing changes were evident for almost half of the alternatively spliced genes in an a priori panel of candidate genes. Eight of the 13 candidate isoforms demonstrated splicing alterations in senescent cells, and were also expressed in human peripheral blood. Of these, 3 (TAU3, GFAPα, and CDKN2A (p14ARF)) were associated with mild cognitive decline in ageing humans. The TAU3 isoform was also associated with severe cognitive decline, but in an opposing direction. Our data are consistent with a model, whereby age-related splicing factor changes may lead to splicing patterns for genes with roles in brain function or senescence, which may influence the development of cognitive decline in the human population. The links between splicing factor changes and isoform changes are impossible to predict from global measures. Each individual splice site in each gene is regulated by a unique and specific combination of activators and inhibitors which determine its usage or not, which makes prediction impossible from levels alone. What our data do indicate is that senescent astrocytes have disrupted expression of many splicing factors, which would be predicted to alter the splicing patterns. We have demonstrated that this holds true for a number of genes important in senescence or in astrocyte function.
Some of the adverse effects of the presence of senescent cells arise because of the paracrine signalling effects of the SASP on neighbouring cells. The presence of senescent cells has been suggested to contribute to shortened overall lifespan (Baker et al., 2016), and clearance of such cells was able to bring about a delay in the appearance of ageing phenotypes in ageing mice (Baker et al., 2011). Other groups have reported increased lifespan, rejuvenation of ageing phenotypes such as thinning fur and improved kidney function in old mice that have undergone targeted removal of senescent cells (Baar et al., 2017). Ablation of senescent glial cells has been demonstrated to lead to a reduction of Tau-dependent pathology and improve cognitive function in mice (Bussian et al., 2018). Accordingly, we observe the generation of a strong SASP in astrocytes that have undergone replicative senescence, which may be contributory to the inflammatory increases evident in the pathophysiology of neurodegeneration. The SASP profile exhibited by the primary human astrocytes does not match that profile seen in other cell lines from our previous work (Latorre et al., 2017, Latorre et al., 2018b, Latorre et al., 2018c); however, cell type specificity in SASP has previously been described (Coppe et al., 2010). The reasons behind these variations are unclear, but may reflect histologically discrete programmes, dysregulation patterns specific to existing transcriptomic profiles, or simply arbitrary patterns of cytokine production which represent the stochastic molecular dysregulation which is occurring. Our observations, together with the marked changes in splicing factor expression, are suggestive that the intersection between disrupted regulation of splicing, cellular senescence, and its associated inflammatory phenotype in astrocytes may contribute to astrocyte dysfunction and have some bearing on eventual cognitive decline. Similar changes may also be occurring in other important proliferative brain cell types such as microglia.
The changes in astrocyte splicing factor expression we have identified here probably do not act directly on splicing regulation in blood, but rather reflect related cognition-associated changes to splicing factor expression in blood as we have recently described (Lee et al., 2019). Similarly, the altered expression of alternatively expressed transcripts in blood may be reflective of similar changes that we demonstrate here also occur in astrocytes. We identified positive associations between CDKN2A (p14ARF) and TAU3 expression in peripheral blood and mild cognitive decline, and a negative correlation between GFAPα expression and mild cognitive decline. The associations between GFAPΑ and CDKN2A (p14ARF) were not apparent in severe cognitive decline, although this may reflect low power imposed by the inherent variability of human biological samples, since p14 expression in severe decline is trending in the same direction. The association of TAU3 expression with severe cognitive decline was still evident, but was negatively, rather than positively correlated. GFAPα expression also demonstrates opposing direction of effect between mild and severe decline. This may represent differences in cell subtype populations, or altered cell characteristics between disease states. GFAPα, which along with other intermediate filaments forms the cytoskeleton, is important for signal transduction and structural properties (Thomsen et al., 2013). GFAPα is highly expressed during key developmental stages during gestation and has also been observed to have elevated expression levels in brain damage and a range of neurological diseases (Thomsen et al., 2013). Some studies have reported no change in whole blood GFAPα protein levels and hypothesised that without rapid astroglial destruction, GFAPα levels may not climb to a detectable level and thus may not be a good indicator of neurological pathology (Mayer et al., 2013). Such studies however may not detect changes at the level of isoforms, if appropriate antibodies capable of specifically identifying individual splice variants are not used.
Increased levels of CDKN2A (p14ARF) may be reflective of an increased load of senescent cells, and is one of two proteins produced by alternate reading frame of the CDKN2A locus (Aram et al., 2016). p14ARF inhibits the activity of MDM2, a protein which sequesters the p53 protein (Wei et al., 2001). Once p53 stabilises and accumulates, it can trigger DNA repair or the apoptosis program of cell death (Fridman and Lowe, 2003). The consequences of increased p14ARF levels are complex and in places conflicted. It has been demonstrated to induce either cellular senescence or apoptosis in a p53-dependant manner (Aram et al., 2016). There have also been some reports that although CDKN2A (p14ARF) transcripts are upregulated in senescent cells, TP53 and MDM2 levels can remain unchanged (Wei et al., 2001). Ectopic expression of p14ARF is capable of inducing senescence but overexpression must be maintained to commit cells to a senescent state (Tokarsky-Amiel et al., 2013). TAU3 transcript expression in the blood was observed to be correlated positively with mild cognitive decline, but interestingly, negatively correlated with severe cognitive decline. Microtubule-associated protein Tau (MAPT) is an alternatively spliced regulator of microtubule dynamics—which is essential for cellular functions from structure to transport (Morris et al., 2011). An ever-growing body of research in human and animal models of tauopathies is demonstrating that the delicately balanced 2:1 3R/4R TAU ratio (Chen et al., 2010), which is achieved through alternative splicing of exon 10, is imperative for maintaining healthy function in cells in the brain, and shifts to disrupt this balance in either direction can be catastrophic (Schoch et al., 2016, Espindola et al., 2018, Avale et al., 2013, Chen et al., 2010). A number of neurodegenerative diseases, including Alzheimer’s and frontotemporal dementia (FTD), have had tau isoform balance (specifically 3R and 4R isoforms) implicated in their pathophysiology (Jakes et al., 1991, Lacovich et al., 2017, Morris et al., 2011). These include those examples with premature cognitive decline (Hu et al., 2007). The precise mechanisms by which TAU isoforms specifically contribute to each neurological pathology are still being investigated. The directionality of association between TAU3 transcript levels and mild and severe decline is consistent with existing literature suggesting imbalances in 3R/4R isoform ratios, rather than absolute isoform levels per se, are identified in cases of dementia and examples of neurodegenerative disease (Chen et al., 2010, Iqbal et al., 2010).
Our finding of dysregulated splicing factor expression in human primary astrocytes is novel and consistent with reports from senescent cells of other tissue types (Holly et al., 2013, Latorre et al., 2017, Latorre et al., 2018b, Latorre et al., 2018c). Our study benefits from a systematic cells-to-populations approach including primary human cell lines and an exquisitely characterised longitudinal population study. Weaknesses of this study are the initial assessment in isolated astrocytes, which may not represent the holistic nature of the cytological and molecular mechanisms involved in cognitive decline and may not capture the extent of cross talk between other cell types in the human brain. It also need to be recognised that the astrocytes used in this study derive from a single donor, and that differences reported here would benefit from confirmation in individuals of different genetic background. Cell lines from a wider variety of donors may return more genes of interest; however, these tissues are rare and difficult to acquire. In addition, our priori list of genes does not include many other isoforms and genes involved in the process. Several of the isoforms selected for study are also not strictly specific for astrocytes. This is particularly true of the senescence genes tested, which reflect players in a more global mechanism. Our scope was narrowed by the need for these genes to be expressed in the blood, for assessment of association with living population. Further work may benefit from the whole transcriptome sequencing and transcriptional profiling in a number of different cell types.
Our data are consistent with a model by which accumulation of senescent astrocytes (and doubtless other important brain cell subtypes), their associated disrupted splicing patterns, and the increased inflammatory microenvironment may contribute to premature cognitive decline. Inflammation and related cellular stresses are capable of activating cell signalling pathways and lead to further dysregulation of splicing factor expression (Latorre et al., 2018a), so it is possible that an auto-regulated feedback loop involving SASP-derived increases in inflammation, dysregulated splicing regulation, and subsequent further increases in senescent cell load may occur as a result of positive feedback. The idea that some of the features of cognitive decline could therefore arise from dysregulated splicing of genes important in the support cells of the brain requires further exploration. This could be explored further by the selective manipulation of specific isoform levels, followed by assessment of effects on astrocyte cell kinetics or astrocyte function in cells and in systems. Our observations suggest that the role of splicing factor expression and dysregulated alternative splicing in cognitive decline may represent an interesting line of future investigation.
Acknowledgements
We are grateful to Dr Paul Weightman Potter for the initial help in establishment of optimised culture conditions for senescent astrocytes.
Authors’ contributions
JL and EL carried out the experiments, analysed the data, and contributed to the manuscript. BPL contributed to data analysis and reviewed the manuscript. SB is the curator of the InCHIANTI study and reviewed the manuscript. NG and JH carried out the initial establishment of the astrocyte cultures from the post-mortem tissue. LF and SB are responsible for the sample cohort and contributed to the manuscript. LWH designed and managed the study, interpreted the data, and reviewed the manuscript.
Funding information
This work was supported by a grant awarded to LWH from the Dunhill Medical Trust (grant number R386/1114) and the Intramural Research Program of the NIH, National institute on Ageing.
Compliance with ethical standards
Declarations of interests
The authors report no competing interests.
Footnotes
Jed J. Lye and Eva Latorre are joint first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aram, K., Su Yeon, H., And Jaewhan, S., LT, SUP, GT, LT, SUP & GT (2016) Dynamics of arf regulation that control senescence and cancer. Bmb Reports 49, 598–606 [DOI] [PMC free article] [PubMed]
- Avale ME, Rodríguez-Martín T, Gallo J-M. Trans-splicing correction of tau isoform imbalance in a mouse model of tau mis-splicing. Hum Mol Genet. 2013;22:2603–2611. doi: 10.1093/hmg/ddt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar, M., Brandt, R., Putavet, D., Klein, J., Derks, K., Bourgeois, B., Stryeck, S., Rijksen, Y., Van Willigenburg, H., Feijtel, D., Van Der Pluijm, I., Essers, J., Van Cappellen, W. A., Van I Jcksen, W., Houtsmuller, A. B., Pothof, J., De Bruin, R. W., Madi, T., Hoeijmakers, J. G., Campisi, J. & De Keizer, P. 2017. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 133 To 147 [DOI] [PMC free article] [PubMed]
- Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128:1208–1216. doi: 10.1172/JCI95145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, Lebrasseur NK, Childs BG, Van De Sluis B, Kirkland JL, Van Deursen JM. Clearance of p16ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, Van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, Van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Townsend K, Goldberg TE, Davies P, Conejero-Goldberg C. MAPT isoforms: differential transcriptional profiles related to 3R and 4R splice variants. J Alzheimer’s Dis: Jad. 2010;22:1313–1329. doi: 10.3233/JAD-2010-101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Sheppard L, Fillenbaum GG, Galasko D, Morris JC, Koss E, Mohs R, Heyman A. Variability in annual mini-mental state examination score in patients with probable Alzheimer disease: a clinical perspective of data from the consortium to establish a registry for Alzheimer’s disease. Arch Neurol. 1999;56:857–862. doi: 10.1001/archneur.56.7.857. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Magalhaes JP, Passos JF. Stress, cell senescence and organismal ageing. Mech Ageing Dev. 2018;170:2–9. doi: 10.1016/j.mad.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Di Malta C, Fryer JD, Settembre C, Ballabio A. Astrocyte dysfunction triggers neurodegeneration in a lysosomal storage disorder. Proc Natl Acad Sci U S A. 2012;109:E2334–E2342. doi: 10.1073/pnas.1209577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espindola SL, Damianich A, Alvarez RJ, Sartor M, Belforte JE, Ferrario JE, Gallo JM, Avale ME. Modulation of tau isoforms imbalance precludes tau pathology and cognitive decline in a mouse model of tauopathy. Cell Rep. 2018;23:709–715. doi: 10.1016/j.celrep.2018.03.079. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez P-Y, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman JS, Lowe SW. Control Of Apoptosis By P53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- Fu XD, Ares M., Jr Context-dependent control of alternative splicing by RNA-binding proteins. Nat Rev Genet. 2014;15:689–701. doi: 10.1038/nrg3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FB, Almeida S, Lopez-Gonzalez R. Dysregulated molecular pathways in amyotrophic lateral sclerosis-frontotemporal dementia spectrum disorder. Embo J. 2017;36:2931–2950. doi: 10.15252/embj.201797568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. Embo J. 1990;9:4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries LW, Hernandez D, Henley W, Wood AR, Holly AC, Bradley-Smith RM, Yaghootkar H, Dutta A, Murray A, Frayling TM, Guralnik JM, Bandinelli S, Singleton A, Ferrucci L, Melzer D. Human aging is characterized by focused changes in gene expression and deregulation of alternative splicing. Aging Cell. 2011;10:868–878. doi: 10.1111/j.1474-9726.2011.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the mini-mental state examination. J Neurol Neurosurg Psychiatry. 2007;78:1298–1303. doi: 10.1136/jnnp.2006.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley JE, Gveric D, Whatmore JL, Gutowski NJ. Tenascin C induces a quiescent phenotype in cultured adult human astrocytes. Glia. 2005;52:53–58. doi: 10.1002/glia.20231. [DOI] [PubMed] [Google Scholar]
- Holly AC, Melzer D, Pilling LC, Fellows AC, Tanaka T, Ferrucci L, Harries LW. Changes in splicing factor expression are associated with advancing age in man. Mech Ageing Dev. 2013;134:356–366. doi: 10.1016/j.mad.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WT, Parisi JE, Knopman DS, Boeve BF, Dickson DW, Ahlskog JE, Petersen RC, Josephs KA. Clinical features and survival of 3R And 4R tauopathies presenting as behavioral variant frontotemporal dementia. Alzheimer Dis Assoc Disord. 2007;21:S39–S43. doi: 10.1097/WAD.0b013e31815bf5e5. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes R, Novak M, Davison M, Wischik CM. Identification of 3- and 4-repeat tau isoforms within the PHF in Alzheimer’s disease. Embo J. 1991;10:2725–2729. doi: 10.1002/j.1460-2075.1991.tb07820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacovich V, Espindola SL, Alloatti M, Pozo Devoto V, Cromberg LE, Carna ME, Forte G, Gallo JM, Bruno L, Stokin GB, Avale ME, Falzone TL. Tau isoforms imbalance impairs the axonal transport of the amyloid precursor protein in human neurons. J Neurosci. 2017;37:58–69. doi: 10.1523/JNEUROSCI.2305-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre E, Birar VC, Sheerin AN, Jeynes JCC, Hooper A, Dawe HR, Melzer D, Cox LS, Faragher RGA, Ostler EL, Harries LW. Small molecule modulation of splicing factor expression is associated with rescue from cellular senescence. Bmc Cell Biol. 2017;18:31. doi: 10.1186/s12860-017-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre E, Ostler EO, Faragher RGA, Harries LW. Foxo1 and Etv6 genes may represent novel regulators of splicing factor expression in cellular senescence. Faseb J. 2018;33:1086–1097. doi: 10.1096/fj.201801154R. [DOI] [PubMed] [Google Scholar]
- Latorre E, Pilling LC, Lee BP, Bandinelli S, Melzer D, Ferrucci L, Harries LW. The VEGFA156b isoform is dysregulated in senescent endothelial cells and may be associated with prevalent and incident coronary heart disease. Clin Sci (Lond) 2018;132:313–325. doi: 10.1042/CS20171556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre E, Torregrossa R, Wood ME, Whiteman M, Harries LW. Mitochondria-targeted hydrogen sulfide attenuates endothelial senescence by selective induction of splicing factors HNRNPD and SRSF2. Aging (Albany Ny) 2018;10:1666–1681. doi: 10.18632/aging.101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence I, Bene M, Nacarelli T, Azar A, Cohen J, Torres C, Johnanes G, Sell C. Correlations between age, functional status, and the senescence-associated proteins HMGB2 and p16INK4a. Geroscience. 2018;40:193–199. doi: 10.1007/s11357-018-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BP, Pilling LC, Emond F, Flurkey K, Harrison DE, Yuan R, Peters LL, Kuchel GA, Ferrucci L, Melzer D, Harries LW. Changes in the expression of splicing factor transcripts and variations in alternative splicing are associated with lifespan in mice and humans. Aging Cell. 2016;15:903–913. doi: 10.1111/acel.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BP, Pilling LC, Bandinelli S, Ferrucci L, Melzer D, Harries LW. The transcript expression levels of HNRNPM, HNRNPA0 and AKAP17A splicing factors may be predictively associated with aging phenotypes in human peripheral blood. Biogerontology. 2019;20:649–663. doi: 10.1007/s10522-019-09819-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Park JW, Ramachandran S, Zhang Y, Tseng YT, Shen S, Waldvogel HJ, Curtis MA, Faull RL, Troncoso JC, Pletnikova O, Ross CA, Davidson BL, Xing Y. Transcriptome sequencing reveals aberrant alternative splicing in Huntington’s disease. Hum Mol Genet. 2016;25:3454–3466. doi: 10.1093/hmg/ddw187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo AM, Marone D, Laido G, De Leonardis AM, De Vita P. Alternative splicing: enhancing ability to cope with stress via transcriptome plasticity. Plant Sci. 2012;185-186:40–49. doi: 10.1016/j.plantsci.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Mayer CA, Brunkhorst R, Niessner M, Pfeilschifter W, Steinmetz H, Foerch C. Blood levels of glial fibrillary acidic protein (Gfap) in patients with neurological diseases. Plos One. 2013;8:E62101–E62101. doi: 10.1371/journal.pone.0062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccarten JR, Rottunda SJ, Kuskowski MA. Change in the mini-mental state exam in Alzheimer’s disease over 2 years: the experience of a dementia clinic. J Alzheimers Dis. 2004;6:11–15. doi: 10.3233/jad-2004-6102. [DOI] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22:R741–R752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Perrott K, Wiley CD, Desprez PY, Campisi J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience. 2017;39:161–173. doi: 10.1007/s11357-017-9970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch KM, Devos SL, Miller RL, Chun SJ, Norrbom M, Wozniak DF, Dawson HN, Bennett CF, Rigo F, Miller TM. Increased 4R-tau induces pathological changes in a human-tau mouse model. Neuron. 2016;90:941–947. doi: 10.1016/j.neuron.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, Bowman AB, Aschner M. Role of astrocytes in brain function and disease. Toxicol Pathol. 2011;39:115–123. doi: 10.1177/0192623310385254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CW, Valcarcel J. Alternative pre-MRNA splicing: the logic of combinatorial control. Trends Biochem Sci. 2000;25:381–388. doi: 10.1016/s0968-0004(00)01604-2. [DOI] [PubMed] [Google Scholar]
- Soreq L, Bergman H, Israel Z, Soreq H. Exon arrays reveal alternative splicing aberrations in Parkinson’s disease leukocytes. Neurodegener Dis. 2012;10:203–206. doi: 10.1159/000332598. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Ohtani N, Hara E. Irreversibility of cellular senescence: dual roles of p16INK4a/Rb-pathway in cell cycle control. Cell Div. 2007;2:10–10. doi: 10.1186/1747-1028-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fulop G, Hertelendy P, Valcarcel-Ares M, Kiss T, Bagwell J, O’connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen R, Daugaard TF, Holm IE, Nielsen AL. Alternative mRNA splicing from the glial fibrillary acidic protein (Gfap) gene generates isoforms with distinct subcellular mRNA localization patterns in astrocytes. Plos One. 2013;8:E72110–E72110. doi: 10.1371/journal.pone.0072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarsky-Amiel R, Azazmeh N, Helman A, Stein Y, Hassan A, Maly A, Ben-Porath I. Dynamics of senescent cell formation and retention revealed by p14Arf induction in the epidermis. Cancer Res. 2013;73:2829–2839. doi: 10.1158/0008-5472.CAN-12-3730. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares M, Fulop G, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanksiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Hemmer RM, Sedivy JM. Role of P14(Arf) in replicative and induced senescence of human fibroblasts. Mol Cell Biol. 2001;21:6748–6757. doi: 10.1128/MCB.21.20.6748-6757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. Altered expression of RNA splicing proteins in Alzheimer’s disease patients: evidence from two microarray studies. Dement Geriatr Cogn Dis Extra. 2013;3:74–85. doi: 10.1159/000348406. [DOI] [PMC free article] [PubMed] [Google Scholar]