Abstract
Background
Many therapies exist for the treatment of low‐back pain including spinal manipulative therapy (SMT), which is a worldwide, extensively practised intervention. This report is an update of the earlier Cochrane review, first published in January 2004 with the last search for studies up to January 2000.
Objectives
To examine the effects of SMT for acute low‐back pain, which is defined as pain of less than six weeks duration.
Search methods
A comprehensive search was conducted on 31 March 2011 in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, PEDro, and the Index to Chiropractic Literature. Other search strategies were employed for completeness. No limitations were placed on language or publication status.
Selection criteria
Randomized controlled trials (RCTs) which examined the effectiveness of spinal manipulation or mobilization in adults with acute low‐back pain were included. In addition, studies were included if the pain was predominantly in the lower back but the study allowed mixed populations, including participants with radiation of pain into the buttocks and legs. Studies which exclusively evaluated sciatica were excluded. No other restrictions were placed on the setting nor the type of pain. The primary outcomes were back pain, back‐pain specific functional status, and perceived recovery. Secondary outcomes were return‐to‐work and quality of life. SMT was defined as any hands‐on therapy directed towards the spine, which includes both manipulation and mobilization, and includes studies from chiropractors, manual therapists, and osteopaths.
Data collection and analysis
Two review authors independently conducted the study selection and risk of bias (RoB) assessment. Data extraction was checked by the second review author. The effects were examined in the following comparisons: SMT versus 1) inert interventions, 2) sham SMT, 3) other interventions, and 4) SMT as an additional therapy. In addition, we examined the effects of different SMT techniques compared to one another. GRADE was used to assess the quality of the evidence. Authors were contacted, where possible, for missing or unclear data. Outcomes were evaluated at the following time intervals: short‐term (one week and one month), intermediate (three to six months), and long‐term (12 months or longer). Clinical relevance was defined as: 1) small, mean difference (MD) < 10% of the scale or standardized mean difference (SMD) < 0.4; 2) medium, MD = 10% to 20% of the scale or SMD = 0.41 to 0.7; and 3) large, MD > 20% of the scale or SMD > 0.7.
Main results
We identified 20 RCTs (total number of participants = 2674), 12 (60%) of which were not included in the previous review. Sample sizes ranged from 36 to 323 (median (IQR) = 108 (61 to 189)). In total, six trials (30% of all included studies) had a low RoB. At most, three RCTs could be identified per comparison, outcome, and time interval; therefore, the amount of data should not be considered robust. In general, for the primary outcomes, there is low to very low quality evidence suggesting no difference in effect for SMT when compared to inert interventions, sham SMT, or when added to another intervention. There was varying quality of evidence (from very low to moderate) suggesting no difference in effect for SMT when compared with other interventions, with the exception of low quality evidence from one trial demonstrating a significant and moderately clinically relevant short‐term effect of SMT on pain relief when compared to inert interventions, as well as low quality evidence demonstrating a significant short‐term and moderately clinically relevant effect of SMT on functional status when added to another intervention. In general, side‐lying and supine thrust SMT techniques demonstrate a short‐term significant difference when compared to non‐thrust SMT techniques for the outcomes of pain, functional status, and recovery.
Authors' conclusions
SMT is no more effective in participants with acute low‐back pain than inert interventions, sham SMT, or when added to another intervention. SMT also appears to be no better than other recommended therapies. Our evaluation is limited by the small number of studies per comparison, outcome, and time interval. Therefore, future research is likely to have an important impact on these estimates. The decision to refer patients for SMT should be based upon costs, preferences of the patients and providers, and relative safety of SMT compared to other treatment options. Future RCTs should examine specific subgroups and include an economic evaluation.
Keywords: Adult; Humans; Low Back Pain; Low Back Pain/therapy; Manipulation, Spinal; Manipulation, Spinal/methods; Randomized Controlled Trials as Topic
Spinal manipulative therapy for acute low‐back pain
Low‐back pain is a common and disabling disorder, representing a great burden both to the individual and society. It often results in reduced quality of life, time lost from work, and substantial medical expense. Spinal manipulative therapy (SMT) is widely practised by a variety of healthcare professionals worldwide and is a common choice for the treatment of low‐back pain. The effectiveness of this form of therapy for the management of acute low‐back pain is, however, not without dispute.
For this review, acute low‐back pain was defined as pain lasting less than six weeks. Only cases of low‐back pain not caused by a known underlying condition, for example, infection, tumour, or fracture, were included. Also included were patients whose pain was predominantly in the lower back but may also have radiated (spread) into the buttocks and legs.
SMT is known as a 'hands‐on' treatment directed towards the spine, which includes both manipulation and mobilization. The therapist applies manual mobilization by passively moving the spinal joints within the patient’s range of motion using slow, passive movements, beginning with a small range and gradually increasing to a larger range of motion. Manipulation is a passive technique whereby the therapist applies a specifically directed manual impulse, or thrust, to a joint at or near the end of the passive (or physiological) range of motion. This is often accompanied by an audible ‘crack’.
In this review, a total of 20 randomized controlled trials (RCTs) (representing 2674 participants) assessing the effects of SMT in patients with acute low‐back pain were identified. Treatment was delivered by a variety of practitioners, including chiropractors, manual therapists, and osteopaths. Approximately one‐third of the trials were considered to be of high methodological quality, meaning these studies provided a high level of confidence in the outcome of SMT.
Overall, we found generally low to very low quality evidence suggesting that SMT is no more effective in the treatment of patients with acute low‐back pain than inert interventions, sham (or fake) SMT, or when added to another treatment such as standard medical care. SMT also appears to be no more effective than other recommended therapies. SMT appears to be safe when compared to other treatment options but other considerations include costs of care.
Summary of findings
Summary of findings for the main comparison.
Spinal manipulative therapy compared to other interventions for acute low‐back pain
| Spinal manipulative therapy compared to other interventions for acute low‐back pain | ||||||
| Patient or population: Patients with acute low‐back pain Settings: Primary or tertiary care Intervention: Spinal manipulative therapy Comparison: Other interventions (e.g. physiotherapy, exercise, back school) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other interventions | Spinal manipulative therapy | |||||
| Pain at one week 0 (no pain) to 10 (worse pain) | The mean pain at one week ranged across control groups from 2.6 to 3.5 points | The mean pain at one week in the intervention groups was 0.1 higher (0.5 lower to 0.7 higher) | 383 (3 studies) | ⊕⊕⊝⊝ low1,2 | Small, not clinically‐relevant effect. | |
| Pain at one month 0 (no pain) to 10 (worse pain) | The mean pain at one month ranged across control groups from 0.5 to 2.3 points | The mean pain at one month in the intervention groups was 0.2 lower (0.5 lower to 0.2 higher) | 606 (3 studies) | ⊕⊕⊕⊝ moderate1 | Small, not clinically‐relevant effect. | |
| Functional status at one week Roland Morris Disability Questionnaire. Scale from: 0 (no dysfunction) to 24 (worse function) | The mean functional status at one week in the control groups was 7.2 points | The mean functional status at one week in the intervention groups was 0.1 standard deviations higher (0.2 lower to 0.3 higher) | 241 (1 study) | ⊕⊕⊝⊝ low2,3 | Small, not clinically‐relevant effect. | |
| Functional status at one month Roland Morris Disability Questionnaire. Scale from: 0 (no dysfunction) to 24 (worse function) | The mean functional status at one month in the control groups was 4.1 points | The mean functional status at one month in the intervention groups was 0.5 points lower (1.2 lower to 0.2 higher) | 681 (3 studies) | ⊕⊕⊕⊝ moderate1 | Small, not clinically‐relevant effect. Based on pooled SMD: ‐0.11 (‐0.26 to 0.05).4 | |
| Recovery at one month | Study population | RR 1.06 (0.94 to 1.21) | 117 (2 studies) | ⊕⊕⊝⊝ low1,5 | Small, not clinically‐relevant effect. | |
| 87 per 100 | 92 per 100 (81 to 100) | |||||
| Serious adverse events | Study population | Not estimable | 2 studies | Total 578 participants. No serious adverse events were observed in the SMT group. | ||
| CI: Confidence interval; RR: Risk ratio;⊕⊕⊝⊝ = these symbols indicate how many of the items were fulfilled (for each ⊕, one item was fulfilled and corresponds to the different levels of evidence). | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High RoB 2 N<400 subjects. 3 Only one study reported the outcome; therefore, data are inconsistent and imprecise. 4 RMDQ based upon Cherkin 1998. 5 N<300 events.
Summary of findings 2.
Spinal manipulative therapy plus another intervention compared to the intervention alone for acute low‐back pain
| Spinal manipulative therapy plus another intervention compared to the intervention alone for acute low‐back pain | ||||||
| Patient or population: Patients with acute low‐back pain Settings: Primary or tertiary care Intervention: Spinal manipulative therapy plus another intervention Comparison: The intervention alone (e.g. usual care, exercise) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| The intervention alone | Spinal manipulative therapy plus another intervention | |||||
| Pain at one week Scale from: 0 (no pain) to 10 (worse pain) | The mean pain at one week in the control groups was 1.9 points | The mean pain at one week in the intervention groups was 0.8 points higher (0.04 lower to 1.7 higher) | 102 (1 study) | ⊕⊕⊝⊝ low1 | Small, not clinically‐relevant effect. | |
| Pain at 3 to 6 months Scale from: 0 (no pain) to 10 (worse pain) | The mean pain at 3 to 6 months in the control groups was 1.5 points | The mean pain at 3 to 6 months in the intervention groups was 0.7 points higher (0.3 lower to 1.6 higher) | 104 (1 study) | ⊕⊕⊝⊝ low1 | Small, not clinically‐relevant effect. | |
| Functional status at one week Oswestry Disability Index. Scale from: 0 (no dysfunction) to 100 (worse function). | The mean functional status at one week in the control groups was 33 points | The mean functional status at one week in the intervention groups was 5.7 points lower (10.1 to 1.4 lower) | 225 (2 studies) | ⊕⊕⊝⊝ low2,3 | Moderately clinically‐relevant effect. Based on pooled SMD: ‐0.41 (‐0.73 to ‐0.10).4 | |
| Functional status at 3 to 6 months Oswestry Disability Index. Scale from: 0 (no dysfunction) to 100 (worse function) | The mean functional status at 3 to 6 months in the control groups was 24.4 points | The mean functional status at 3 to 6 months in the intervention groups was 3.8 points lower (10.6 lower to 2.8 higher) | 225 (2 studies) | ⊕⊕⊝⊝ low2,3 | Small, not clinically‐relevant effect. Based on pooled SMD: ‐0.22 (‐0.61 to 0.16).4 | |
| Recovery at one week | Study population | RR 0.89 (0.32 to 2.47) | 196 (2 studies) | ⊕⊝⊝⊝ very low2,5,6 | Small, not clinically‐relevant effect. Based on pooled RR: 0.88 (0.36 to 2.19). | |
| 16 per 100 | 14 per 100 (5 to 40) | |||||
| Recovery at 3 to 6 months | Study population | RR 0.75 (0.51 to 1.1) | 195 (2 studies) | ⊕⊝⊝⊝ very low2,6 | Small, not clinically‐relevant effect. Based on pooled RR: 0.96 (0.71 to 1.31). I2=57%. | |
| 64 per 100 | 48 per 100 (33 to 70) | |||||
| Serious adverse events | Study population | Not estimable | 2 studies | Total 199 participants. In one of the studies, two serious adverse events were observed in the SMT group; however, they "appeared not to be related to the treatment". An equal number of adverse events were seen in the control group (Juni 2009). | ||
| CI: Confidence interval; RR: Risk ratio;⊕⊕⊝⊝ = these symbols indicate how many of the items were fulfilled (for each ⊕, one item was fulfilled and corresponds to the different levels of evidence). | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Only one study reported the outcome; therefore, the data are inconsistent and imprecise. 2 High RoB. 3 N<400 subjects. 4 ODI based upon Childs 2004. 5 Widely varying estimates of effect. 6N<300 events.
Summary of findings 3.
Spinal manipulative therapy compared to inert interventions for acute low‐back pain
| Spinal manipulative therapy compared to inert interventions for acute low‐back pain | ||||||
| Patient or population: Patients with acute low‐back pain Settings: Primary or tertiary care Intervention: Spinal manipulative therapy Comparison: Inert interventions (e.g. educational booklet, detuned diathermy) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inert interventions | Spinal manipulative therapy | |||||
| Pain at one week Scale from: 0 (no pain) to 10 (worse pain). | The mean pain at one week ranged across control groups from 2 to 4.2 points | The mean pain at one week in the intervention groups was 0.1 points higher (0.7 lower to 1 higher) | 311 (3 studies) | ⊕⊕⊝⊝ low1,2 | Small, not clinically‐relevant effect. | |
| Pain at one month Scale from: 0 (no pain) to 10 (worse pain). | The mean pain at one month in the control groups was 3.1 points | The mean pain at one month in the intervention groups was 1.2 points lower (2 to 0.4 lower) | 178 (1 study) | ⊕⊕⊝⊝ low3 | Moderately clinically‐relevant effect. | |
| Functional status at one week Roland Morris Disability Questionnaire. Scale from: 0 (no dysfunction) to 24 (worse function) | The mean functional status at one week in the control groups was 7.8 points | The mean functional status at one week in the intervention groups was 0.3 points lower (1.5 lower to 0.8 higher) | 205 (2 studies) | ⊕⊕⊕⊝ moderate2 | Small, not clinically‐relevant effect. Based on pooled SMD: ‐0.08 (‐0.37 to 0.21).4 | |
| Functional status at one month Roland Morris Disability Questionnaire. Scale from: 0 (no dysfunction) to 24 (worse function) | The mean functional status at one month in the control groups was 4.9 points | The mean functional status at one month in the intervention groups was 0.3 standard deviations lower (0.6 lower to 0.04 higher) | 178 (1 study) | ⊕⊕⊝⊝ low3 | Small, not clinically‐relevant effect. | |
| Recovery at one week | Study population | RR 0.96 (0.5 to 1.85) | 263 (2 studies) | ⊕⊕⊝⊝ low5,6 | Small, not clinically‐relevant effect. | |
| 33 per 100 | 31 per 100 (16 to 60) | |||||
| Serious adverse events | Study population | Not estimable | 2 studies | Total 427 participants. No serious adverse events were observed in the SMT group. | ||
| CI: Confidence interval; RR: Risk ratio;⊕⊕⊝⊝ = these symbols indicate how many of the items were fulfilled (for each ⊕, one item was fulfilled and corresponds to the different levels of evidence). | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High RoB 2 N<400 subjects 3 Only one study reported the outcome; therefore, the data are inconsistent and imprecise. 4 RMDQ based upon Cherkin 1998. 5 I2=58% 6 N<300 events
Summary of findings 4.
Spinal manipulative therapy (SMT) compared to sham SMT for acute low‐back pain
| Spinal manipulative therapy (SMT) compared to sham SMT for acute low‐back pain | ||||||
| Patient or population: Patients with acute low‐back pain Settings: Primary or tertiary care Intervention: Spinal manipulative therapy (SMT) Comparison: Sham SMT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham SMT | Spinal manipulative therapy (SMT) | |||||
| Pain at one month 0 (no pain) to 10 (worse pain) | The mean pain at one month in the control groups was 2.2 points | The mean pain at one month in the intervention groups was 0.5 lower (1.4 lower to 0.4 higher) | 74 (1 study) | ⊕⊝⊝⊝ very low1,2 | Small, not clinically‐relevant effect. | |
| Functional status at one month Oswestry Disability Index. Scale from: 0 (no dysfunction) to 100 (worse function) | The mean functional status at one month in the control groups was 16.3 points | The mean functional status at one month in the intervention groups was 0.4 standard deviations lower (0.8 lower to 0.1 higher) | 94 (1 study) | ⊕⊝⊝⊝ very low1,2 | Small, not clinically‐relevant effect. | |
| Recovery at one month | Study population | Not estimable | 0 studies | No data were available. | ||
| Serious adverse events | Study population | Not estimable | 0 studies | No data were available. | ||
| CI: Confidence interval; RR: Risk ratio;⊕⊕⊝⊝ = these symbols indicate how many of the items were fulfilled (for each ⊕, one item was fulfilled and corresponds to the different levels of evidence). | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High RoB 2 Only one study reported the outcome; therefore, the data are inconsistent and imprecise.
Background
Low‐back pain is a common and disabling disorder in western society which represents a great societal and financial burden (Dagenais 2008). Therefore, adequate treatment of low‐back pain is an important issue for patients, clinicians, and healthcare policy makers. One widely used intervention for low‐back pain is spinal manipulative therapy (SMT), which has been examined in numerous randomized controlled trials (RCTs). These trials have been summarized in recent systematic reviews (Bronfort 2004a; Cherkin 2003; Brown 2007) that have formed the basis for recommendations in clinical guidelines (Chou 2007; van Tulder 2006). However, these recommendations are largely based on an earlier version of this Cochrane review (Assendelft 2004), which reported that SMT was superior only to sham therapy or therapies judged to be ineffective or even harmful, and concluded that there was no evidence that SMT is superior to other standard treatments for patients with acute low‐back pain. The effect sizes, however, were small and arguably not clinically relevant. Furthermore, these estimates were based mainly on small studies with a high risk of bias.
SMT is delivered by various professional groups, including chiropractors, manual therapists, and osteopaths, and is included in many national guidelines for the management of acute low‐back pain (Koes 2001; van Tulder 2004). These recommendations vary however. In most guidelines, SMT is considered to be a therapeutic option in the acute phase of a low‐back pain episode. The USA, UK, New Zealand, and Danish guidelines consider SMT a useful treatment, whereas the Dutch, Australian, and Israeli guidelines do not recommend SMT for the acute phase (van Tulder 2006).
This report is an update of the previous Cochrane review and follows the most recent guidelines developed by The Cochrane Collaboration in general (Higgins 2011) and by the Cochrane Back Review Group (Furlan 2009) in particular. The current review was split into two parts according to duration of the complaint, namely acute and chronic low‐back pain. The review on chronic low‐back pain has since been published (Rubinstein 2011). The present review focuses on the effectiveness of SMT for acute low‐back pain (Rubinstein 2010) and follows the same methodology as the review for chronic low‐back pain.
Description of the condition
Low‐back pain is defined as pain and discomfort that is localised below the costal margin and above the inferior gluteal folds, with or without referred leg pain. Acute low‐back pain is defined as the duration of an episode persisting for no longer than six weeks. This condition is considered to be typically self‐limiting, with a recovery rate of 90% within six weeks of the initial episode, while 2% to 7% develop chronic low‐back pain (van Tulder 2006). Non‐specific low‐back pain is operationally defined as low‐back pain not attributed to a recognisable, specific pathology (for example infection, tumor, or fracture).
Description of the intervention
In this review, SMT is considered to be any hands‐on treatment that includes manipulation, mobilization, or both, directed towards the spine. Mobilizations use low‐grade velocity, small or large amplitude passive movement techniques within the patient's joint range of motion and control. Manipulation, on the other hand, uses a high velocity impulse or thrust applied to a synovial joint over a short amplitude at or near the end of the passive or physiologic range of motion, which is often accompanied by an audible 'crack' (Sandoz 1969). The cracking sound is caused by cavitation of the joint, which is a term used to describe the formation and activity of bubbles within the fluid (Evans 2002; Unsworth 1971). Various practitioners, including chiropractors, manual therapists (physiotherapists trained in manipulative techniques), orthomanual therapists (medical doctors trained in manipulation), or osteopaths use this intervention. However, the focus of the treatment, education, diagnostic procedures used, treatment objectives, techniques, as well as the philosophy of the various professions differ, often considerably. For example, the focus of orthomanual therapy is on correcting abnormal positions of the skeleton and establishing symmetry in the spine through mobilization. Manual therapy focuses on correcting functional disorders of the musculoskeletal system through predominantly passive mobilization and sometimes using high‐velocity low‐amplitude (HVLA) techniques. Chiropractors, on the other hand, focus on correcting disorders of the neuromusculoskeletal system by using predominantly HVLA manipulative techniques (van de Veen 2005).
How the intervention might work
Many hypotheses exist regarding the mechanism of action for spinal manipulation and mobilization (Bronfort 2008; Khalsa 2006; Pickar 2002), which to some extent is due to the difference in opinions between the various professional groups. Some have postulated that mobilization and manipulation should be assessed as separate entities given their theoretically different mechanisms of action (Evans 2002). The modes of action might be roughly divided into mechanical and neurophysiologic. The mechanistic approach suggests that SMT acts on a manipulable lesion (often called the functional spinal lesion or subluxation) and proposes that forces to reduce internal mechanical stresses result in reduced symptoms (Triano 2001). The neurophysiologic approach suggests that SMT impacts the primary afferent neurons from paraspinal tissues, the motor control system, and pain processing (Pickar 2002). In conclusion, it would appear that the actual mechanism remains debatable (Evans 2002; Khalsa 2006).
Why it is important to do this review
SMT is a worldwide, extensively practised intervention; however, its effectiveness for acute low‐back pain is not without dispute. Although numerous systematic reviews have examined the effectiveness of SMT for low‐back pain (Airaksinen 2006; Chou 2007), very few have conducted a meta‐analysis, especially for acute low‐back pain. The previous Cochrane review (Assendelft 2004) last searched for studies up to January 2000. Numerous RCTs have been identified since then. In addition, the methodology for conducting systematic reviews, including the criteria for evaluating the risk of bias and the GRADE system for evaluating the strength of the evidence, have been substantially revised; therefore, this update is thought to shed a more reliable overview on this issue (Higgins 2011).
Objectives
The objective of this review was to examine the effectiveness of SMT on primary (that is pain, functional status, and recovery) and secondary outcomes (that is return‐to‐work, quality of life) as compared to inert interventions, sham, and all other treatments for adults with acute low‐back pain. The effects were examined for short‐term (closest to one month), intermediate (closest to three to six months), and long‐term follow‐up (closest to 12 months).
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) were included with the exception of those that used inappropriate randomization procedures (for example alternate allocation, birth dates). In addition, studies with follow‐up of less than one day were excluded.
Types of participants
Inclusion criteria
Adult participants (> 18 years of age) with a mean duration of low‐back pain < six weeks
Participants with or without radiating pain
No limits were placed on the setting (that is whether from primary, secondary, or tertiary care).
Exclusion criteria
Participants with:
post‐partum low‐back pain or pelvic pain due to pregnancy,
pain not related to the low‐back, e.g. coccydynia,
post‐operative studies or participants with 'failed‐back syndrome';
or studies which:
examined 'maintenance care' or prevention,
exclusively examined specific pathologies, including sciatica. Of note: Studies of sciatica were excluded because it is a prognostic factor associated with worse pain, disability, or both (Bronfort 2004; Bouter 1998), especially with SMT (Axen 2005; Malmqvist 2008). It is thought to represent a pathology different than non‐specific low‐back pain.
Types of interventions
Experimental intervention
The experimental interventions examined in this review included both spinal manipulation and mobilization of the spine. Unless otherwise indicated, SMT refers to both modes of 'hands‐on' treatment of the spine.
Types of comparisons
Studies were included for consideration if the study design used indicated that the observed differences were due to the unique contribution of SMT. This excludes studies with a multi‐modal treatment as one of the interventions (for example standard physician care + spinal manipulation + exercise therapy) and either a different type of intervention or only one intervention from the multi‐modal therapy as the comparison (for example standard physician care alone) since this would make it impossible to decipher the actual effect of SMT.
Comparison therapies were combined into the following main clusters:
1) SMT versus inert interventions; 2) SMT versus sham SMT; 3) SMT versus all other therapies; 4) SMT plus any intervention versus that same intervention alone (i.e. SMT as an adjunct therapy); 5) SMT versus another SMT technique (e.g. side‐lying thrust SMT versus non‐thrust side‐lying technique, supine thrust SMT versus side‐lying thrust SMT).
Inert interventions include detuned diathermy and detuned ultrasound. Sham SMT was defined as any manipulation or mobilization technique that was ostensibly indistinguishable for the patient from the true technique, meaning the patient did not know if he or she was receiving the real' (or active component) or the placebo or 'fake' therapy. Sham SMT was considered acceptable if this was queried among the participants post‐treatment and the blinding appeared to be successful.
Types of outcome measures
Only patient‐reported outcome measures were evaluated. Physiological measures, such as spinal flexibility or degrees achieved with a straight leg raise test (that is Lasegue’s test), were not considered clinically‐relevant outcomes and were not included in the analyses.
Primary outcomes
Pain, measured by a visual analogue or other pain scale (e.g. visual analogue scale (VAS), numerical rating scale (NRS), McGill pain score)
Back‐pain specific functional status, measured by a back‐pain specific scale (e.g. Roland‐Morris Disability Questionnaire (RMDQ), Oswestry Disability Index (ODI))
Global improvement or perceived recovery, measured by an ordinal or dichotomous scale (defined as the number of patients reported to be recovered or nearly recovered)
Secondary outcomes
Perceived health status or quality of life (e.g. subscale from the SF‐36, the EuroQol thermometer)
Return‐to‐work
Search methods for identification of studies
Electronic searches
RCTs and systematic reviews were identified by electronically searching the following databases (search date: 31 March 2011). The search was limited to studies published since 2000. Studies published prior to this date were included in the previous Cochrane review and were also considered for inclusion in this updated review.
Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1).
MEDLINE (Appendix 2).
EMBASE (Appendix 3).
CINAHL (Appendix 4).
PEDro.
Index to Chiropractic Literature.
The search strategy developed by the Cochrane Back Group was followed using free text words and medical subject headings (MeSH). The search was conducted by a clinical librarian with experience in searching for articles for systematic reviews. The search was updated on July 18, 2012.
Searching other resources
We also screened the reference lists of all included studies and (systematic) reviews pertinent to this topic. We reviewed grey literature that is available electronically from clinical trials registers and the websites recommended by the Chiropractic Library Collaboration. We searched for registered trials in the US Clinical Trials database and the World Health Organization International Clinical Trials Registry Platform (ICTRP). Selected researchers familiar with this literature were also approached in order to confirm whether our selection of studies was complete.
Data collection and analysis
Two review authors (SMR, CBT) independently conducted the selection of studies and performed the risk of bias assessment. Both qualitative and quantitative data were extracted by one review author and checked for accuracy against the original paper by the second review author. All disagreements were resolved through consensus and it was not necessary to consult a third review author (MWvT).
Selection of studies
We screened titles and abstracts from the search results. Potentially relevant studies were obtained in full text and independently assessed for inclusion. Disagreements were resolved through discussion. Only full papers were evaluated. Abstracts and proceedings from congresses or any other 'grey literature' were excluded. No language restrictions were imposed.
Data extraction and management
A standardized form was used to extract the qualitative data. The following were extracted: study characteristics (for example country where the study was conducted, recruitment modality, source of funding, risk of bias), patient characteristics (for example number of participants, age, gender), description of the experimental and control interventions, duration of follow‐up, types of outcomes assessed, and the authors' results and conclusions. Data relating to the primary outcomes were assessed for inclusion in the meta‐analyses. Data were not extracted from those studies thought to have a fatal flaw, which was defined as: 1) a drop‐out rate greater than 50% at the first and subsequent follow‐up measurements; or 2) statistically and clinically‐relevant, important baseline differences for one or more primary outcomes (that is pain, functional status) indicating unsuccessful randomization. Final value scores were used for the meta‐analyses only, meaning data were estimated when change scores were presented. Outcomes were assessed at one week as well as at one, three and 12 months and were categorized according to the time closest to these intervals. In some cases outcome data were not available for the three month interval but were available for six months, in which case these data were extracted and labelled as such (that is three to six months).
Assessment of risk of bias in included studies
The risk of bias assessment for RCTs was conducted using the 12 criteria recommended by the Cochrane Back Review Group. These criteria are standard for evaluating effectiveness of interventions for low‐back pain (Appendix 5) (Higgins 2011) and includes blinding of the patient, treatment provider, and outcomes assessor. For the purpose of this review, any attempt to blind the outcome assessor was considered irrelevant because the patient is viewed to be the outcome assessor when evaluating subjective, self‐reported measures such as pain, functional status, or recovery. Therefore, if the patient was not blinded the outcome assessor was also considered not blinded. The criteria were scored as at 'high' or 'low' risk of bias and were reported in the 'Risk of bias' table. A study with a low risk of bias was defined as fulfilling six or more of the criteria, which is supported by empirical evidence (van Tulder 2009). In all cases and where possible, an attempt was made to contact authors for clarification on methodological issues, if necessary, or for unpublished data. In addition, we attempted to contact all authors from the previous decade with our risk of bias assessment and they were given the opportunity to provide feedback. Where necessary, this was discussed among the research team members. No attempt was made to contact authors for publications earlier than 2000. The review authors were not blinded to the authors of the individual studies, institution, or journal.
Measures of treatment effect
Pain was examined as a mean difference while functional status was examined as a standardized mean difference (SMD) because different instruments were used to assess functional status. For the mean difference, results were assessed on an 0 to 10 point scale and converted when necessary. A negative effect size indicates that SMT is more beneficial than the comparison therapy, meaning participants have less pain and better functional status. For dichotomous outcomes (that is recovery, return‐to‐work) a risk ratio (RR) was calculated and the event defined as the number of participants recovered or returned to work. A RR > 1 indicates that SMT leads to a greater chance of recovery or return‐to‐work. A random‐effects model was used because there was a substantial amount of clinical and unexplained heterogeneity across studies. Funnel plots were constructed using all data from the outcomes pain and functional status in order to evaluate possible publication bias, thus regardless of the type of comparison or follow‐up interval. For each treatment comparison, an effect size and a 95% confidence interval (CI) were calculated. All analyses were conducted in Review Manager 5.1.
Assessment of clinical relevance
Clinical relevance (Cohen 1988; Higgins 2011), as measured by the pooled effect size, was defined as follows.
Small: MD < 10% of the scale (e.g. < 1 mm on a 10 mm VAS); SMD < 0.4; RR < 1.25.
Medium: MD = 10% to 20% of the scale; SMD = 0.41 to 0.7; RR = 1.25 to 2.0.
Large: MD > 20% of the scale; SMD > 0.7; RR > 2.0.
For the interpretation of minimal important change (MIC), from the patient's perspective, the following absolute cut‐offs were considered: 2 points for 0 to 10 on the NRS, 5 points for the Rolland Morris Disability Questionnaire, and 10 points for the Oswestry Disability Index (Ostelo 2008).
Unit of analysis issues
The numbers of participants were accordingly reduced for those studies where multiple comparisons were examined and included in the same comparison in the meta‐analysis. This was conducted in order to prevent overestimating the number of participants for the 'shared' intervention (that is SMT).
Dealing with missing data
When data were reported in a graph only, we estimated the means and standard deviations. We attempted to contact authors when standard deviations were not reported. If the standard deviations for follow‐up measurements was missing, the baseline measure was used for the subsequent follow‐ups. Finally, if no measure of variation was reported anywhere we estimated the standard deviation based upon other studies with a similar population and risk of bias.
Assessment of heterogeneity
Heterogeneity was explored in two manners, by subjective interpretation ('eye‐ball test') and by formally testing using the Q‐test (Chi²) and I² statistic; however, the decision regarding heterogeneity was dependent upon the I² (Higgins 2011) and we used a cut‐off of 40%. Results were described in the text when the results were thought to be too heterogeneous to meaningfully report a pooled value.
Assessment of reporting biases
We searched for protocols of the studies in ClinicalTrials.org and ISRCTN.org, particularly when studies did not reference their protocol and when we were not able to contact the original authors.
Data synthesis
The overall quality of the evidence and strength of the recommendations were evaluated using GRADE (Guyatt 2008) and discussed by three principal members of the group (SMR, CBT, MWvT). Quality of the evidence is defined as follows.
High quality: further research is very unlikely to change the level of evidence. There are sufficient data with narrow confidence intervals. There are no known or suspected reporting biases.
Moderate quality: further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate; one of the domains is not met.
Low quality: further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change it; two of the domains are not met.
Very low quality: great uncertainty about the estimate; three of the domains are not met.
No evidence: no evidence from RCTs.
The quality of the evidence for a specific outcome was based upon five domains and subsequently downgraded from high quality to moderate, low, or very low quality depending upon how many of the domains were fulfilled. For each domain that was not met quality was reduced by one level. The domains are as follows: 1) limitations in design (downgraded if > 25% of the participants were from studies with a high risk of bias); 2) inconsistency of results (downgraded in the presence of significant heterogeneity (I² > 40%) or inconsistent findings (in the presence of widely differing estimates of the treatment effect, that is individual studies favouring the intervention or control group)); 3) indirectness (that is generalizability of the findings; downgraded if > 50% of the participants were outside the target group, for example studies which exclusively examined older participants or included inexperienced treating physicians); 4) imprecision (downgraded if less than 400 subjects for continuous data and less than 300 events for dichotomous data (Mueller 2007)); and 5) other (for example publication bias). Comparisons that included only a single study (N < 400 for continuous outcomes, N < 300 for dichotomous outcomes) were considered inconsistent and imprecise and thought to provide 'low quality evidence', which could be further downgraded to 'very low quality evidence' if limitations in design or indirectness were also present. 'Summary of finding' tables were generated for the primary analyses and for the primary outcome measures only, regardless of statistical heterogeneity.
Subgroup analysis and investigation of heterogeneity
Regardless of possible heterogeneity, stratified analyses were conducted by the control groups as defined in 'Types of interventions' and by the duration of follow‐up.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Table 11.
Table 1.
Specific clinical and treatment characteristics of the individual studies
| Author | Presence/absence of radiating pain | Duration LBP (according to inclusion criteria) | Duration LBP (current episode) | Type of manipulator (n= # of manipulators); experience (if stated) | Type of manipulation | No. txs SMT allowed and duration |
| Bergquist‐Ullman 1977 | No radiation below knee | < 8 wks | >50% less than 4 wks | Physiotherapist (n=?) | Manipulation/MOB according to Cyriax | 4 tx's (mean), 10 (max) |
| Brennan 2006 | Absence of nerve root compression | <3 mo. | Median (IQR): 16d (10, 41) | Physiotherapist (n=?) | Thrust manipulation or low‐amplitude mobilization | ? median range: 6.5 to 7 sessions |
| Cherkin 1998 | No sciatica | Duration was not listed in inclusion criteria | 78% < 6 wks | Chiropractors (n=?); collectively 6 to14 yrs. experience | Short‐lever HVLA SMT | 6.9 tx's (mean) |
| Childs 2004 | Absence of nerve root compression | ? | Median = 27 d | Physiotherapist (n=14) | HVLA SMT | ? |
| Cleland 2009 | Absence of nerve root compression | Duration was not per se an inclusion criteria | Median (IQR) = 45 (27 to 60) | Physiotherapists (n=17); collectively avg. 9 yrs. experience | HVLA SMT or low‐amplitude mobilization | Total 2 sessions |
| Cramer 1993 | Without compressive neuropathy | < 2 wks | ? | Chiropractors (n=?) | Side‐lying (short‐lever?) HVLA? SMT | 3 to 5 times over a 10‐d period |
| Farrell 1982 | Without neurological signs | < 3 wks | ? | Physiotherapists (n=?) | Manipulation/MOB according to Maitland | 3x/wk for 3 weeks. Tx was continued, prn |
| Glover 1974 | Without neurological signs | ? | 52% <7 d | Osteopathic physician? (n=1) | Rotational manipulation | 1 tx (followed by 4d of detuned diathermy) |
| Hadler 1987 | With or without signs of radiculopathy | < 4 wks | ? | Osteopathic physician? (n=1) | Long‐lever high‐velocity SMT | One visit? |
| Hallegraeff 2009 | No symptoms distal to the knee | <16 d. | 69% <3 wks | Manual therapists (n=?) | HVLA SMT | 4 visits over 2 1/2 wks |
| Hancock 2007 | Absence of nerve root compromise | < 6 wks | Mean = 9.13 d | Physiotherapists (n=15) | Most (97%) received low‐velocity mobilization; a small proportion (5%) also received high‐velocity thrusts | 2 to 3x/wk for a max. of 12 txs. over 4 wks |
| Hoehler 1981 | ? | Duration was not listed in the inclusion criteria | 52% of SMT grp & 48% of ctrl. grp < 1 mo | ? | High‐velocity thrust | ? |
| Hoiriis 2004 | No neuropathy | 2 to 6 wks | Total for all subjects = 3.7 wks | Chiropractors (n=?) | HVLA SMT | Most attended 7 chiropractic visits |
| Juni 2008 | Absence of nerve root compression, no radiation below the knee | < 4 wks | 54% of SMT grp & 75% of ctrl. grp <7d with LBP | Medical manipulator (n=2), osteopathy (n=1) | HVLA SMT | Median (IQR): 3 (2, 4) |
| MacDonald 1990 | Absence of nerve root compromise | ? | 55% of both grps<14d LBP | Osteopathy (n=?) | HVLA SMT | 4.7 tx's (mean), 87% were delivered within the first 2.5 wks. |
| Postacchini 1988 | With or without radiation to the knee | grp.A="acute" | Mean duration: 15d & 17d | Chiropractor (n=?) | Manipulation | 12 over 6 wks |
| Rasmussen 1979 | Without signs of nerve root pressure | < 3 wks | ? | Physiotherapist (n=1?) or medical manipulator (n=1?) | Rotational manipulation in the pain‐free direction | 3x/wk for 2 wks |
| Seferlis 1998 | With or without sciatica | < 2 wks | ? | Physiotherapist (n=?) | "Manipulation of the lumbar facet and SI joint" | 10 txs (mean) |
| Skagren 1997 | Absence of nerve root signs | Duration was not listed in the inclusion criteria | 55% of SMT grp. & 48% of control grp <4 wks with LBP | Chiropractors (n=6), collectively 9.9 yrs experience (range 1‐15) | HVLA SMT | 4.9 txs (mean) in 4.1 wks |
| Sutlive 2009 | Absence of nerve root compression | Duration was not a required item | 62% w/ LBP <16d | Physiotherapist (n=1?) | HVLA SMT | 1 tx only |
ctrl.=control group; d=day; HVLA=high‐velocity low‐amplitude; prn=as necessary; SI=sacroiliac joint; SMT=spinal manipulative therapy; tx=treatment; wk(s)=week or weeks; ?=unclear or unspecified. Note: The description of the type of radiating pain allowed in the individual trials is reflective of the language used in those reports.
Results of the search
In total, 20 trials were identified which fulfilled the inclusion criteria: eight (40%) of the trials were published since the previous review (Brennan 2006; Childs 2004; Cleland 2009; Hallegraeff 2009; Hancock 2007; Hoiriis 2004; Juni 2009; Sutlive 2009) (Figure 1). One of the trials (Seferlis 1998) was awaiting assessment at the time of publication of the previous review and, therefore, not included in the previous assessment.
Figure 1.
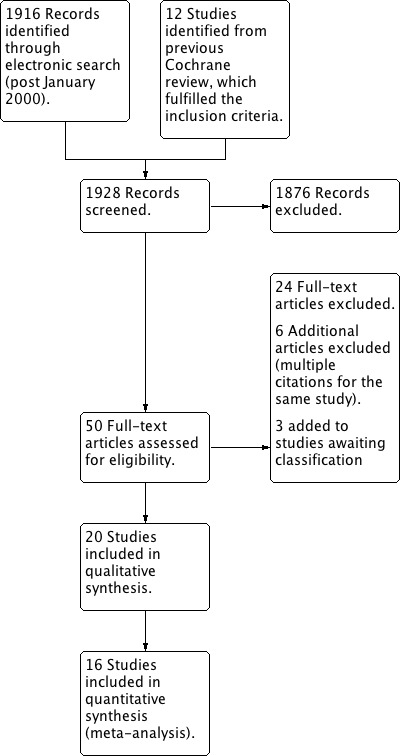
Study flow diagram. Summary of selection process. (Updated July 25, 2012)
A search of ongoing trials in ClinicalTrials.gov and the WHO ICTRP Search Portal revealed three trials examining acute or subacute low‐back pain. A preliminary report of one of the studies revealed that the majority of participants recruited thus far have subacute pain (NCT01211613). Another study was identified which according to the trial registry was completed in 2007; however, a search in PubMed and contact with a colleague of the principal investigator suggests that it has not (yet) been submitted for publication (NCT00497861). A third study was identified as a feasibility study that is in the final stages of manuscript preparation (NCT00632060) and examined participants with acute low‐back pain in a military setting.
The countries in which the studies were conducted varied but were largely limited to North America and Europe: nine were conducted in the USA (Brennan 2006; Cherkin 1998; Childs 2004; Cleland 2009; Cramer 1993; Hadler 1987; Hoehler 1981; Hoiriis 2004; Sutlive 2009); three in Sweden (Bergquist‐Ullman 1977; Seferlis 1998; Skargren 1997); two in Australia (Farrell 1982; Hancock 2007) and the UK (Glover 1974; MacDonald 1990); and one in each of Denmark (Rasmussen 1979), Italy (Postacchini 1988), Netherlands (Hallegraeff 2009), and Switzerland (Juni 2009). All trials were published in English.
Included studies
In total, 2674 participants were examined in the trials. Study sample sizes ranged from 36 to 323 (median (IQR) = 108 (61, 189)). A sample size calculation was performed in eight (40%) of the studies based upon determining a minimally clinically‐relevant difference for one or more of the primary outcome measures (Brennan 2006; Cherkin 1998; Childs 2004; Cleland 2009; Hallegraeff 2009; Hancock 2007; Juni 2009; Sutlive 2009).
Types of studies
Slightly less than half of the studies examined multiple comparisons: three arms (Bergquist‐Ullman 1977; Brennan 2006; Cherkin 1998; Cleland 2009; Hoiriis 2004; Seferlis 1998); fours arms (Hancock 2007); and six arms (Postacchini 1988). The following comparisons were identified.
1) Seven studies compared SMT to inert interventions (i.e. educational booklet (Cherkin 1998), detuned ultrasound and cold packs (Cramer 1993), detuned ultrasound (Hancock 2007), detuned short‐wave diathermy (Glover 1974), anti‐oedema gel spread over the lumbar region (Postacchini 1988), bed rest (Postacchini 1988), and short‐wave diathermy (Bergquist‐Ullman 1977; Rasmussen 1979)). No studies were identified which compared SMT to no intervention or a waiting list control.
2) One study compared SMT to sham SMT (Hoiriis 2004).
3) Eight studies compared SMT to any other intervention (i.e. exercise (Brennan 2006; Seferlis 1998), physical therapy (Bergquist‐Ullman 1977; Cherkin 1998 (according to McKenzie principles); Farrell 1982; Postacchini 1988; Skargren 1997), massage (Hoehler 1981), standard general practitioner (GP) care consisting primarily of prescription (diclofenac or codeine) or non‐prescription medication (paracetamol), or both (Postacchini 1988; Seferlis 1998), back school (Bergquist‐Ullman 1977; Postacchini 1988)).
4) Four studies examined the additional benefit of SMT to another intervention (i.e. consisting of GP visits where advice was given on posture, exercise, and avoidance of occupational distress (MacDonald 1990), medication as necessary (Juni 2009), exercise (Childs 2004), and physiotherapy (Hallegraeff 2009)).
5) Three studies compared different SMT techniques one to another (Cleland 2009; Hadler 1987; Sutlive 2009).
Study population
Most participants were middle‐aged, recruited from primary or secondary care. In one study the vast majority were male (because this was a study conducted in an industrial setting) (Glover 1974) and another study included exclusively male participants (Rasmussen 1979). Two studies were conducted in an occupational setting (Bergquist‐Ullman 1977; Glover 1974). Virtually all studies included patients with or without radiating pain and most were clear that patients with nerve root signs or compressive neuropathy were excluded (Brennan 2006; Cherkin 1998; Childs 2004; Cleland 2009; Cramer 1993; Farrell 1982; Glover 1974; Hallegraeff 2009; Hancock 2007; Hoiriis 2004; Juni 2009; MacDonald 1990; Rasmussen 1979; Skargren 1997; Sutlive 2009). Other studies allowed those with sciatica or radiculopathy (Hadler 1987 ("some had signs of radiculopathy"); in Seferlis 1998 78% had low‐back pain only; and others did not specify if patients with radiating pain were included or not (Hoehler 1981) (Table 11). Virtually all studies included patients with less than four weeks of low‐back pain. Approximately half of the studies included patients with exclusively acute (< six weeks) low‐back pain (Cramer 1993; Farrell 1982; Hadler 1987; Hallegraeff 2009; Hancock 2007; Hoiriis 2004; Juni 2009; Rasmussen 1979; Seferlis 1998), while others included a mixed population (that is acute and subacute (Bergquist‐Ullman 1977; Brennan 2006; Cleland 2009; Sutlive 2009) or acute, subacute, or chronic (Cherkin 1998; Childs 2004; Hoehler 1981; MacDonald 1990; Postacchini 1988; Skargren 1997)). In one study it was unclear what proportion of participants had acute low‐back pain; however, data were stratified by duration (< seven days and > seven days) and therefore we used the data for < seven days only (Glover 1974). Another study also included participants with neck pain but the vast majority (78%, n = 253/323) had low‐back pain (Skargren 1997).
Technique: type, practitioner, number and duration of treatments
The studies were rather diverse with regards to the type of manipulator or practitioner and manipulation and the number and duration of treatments delivered. Most treatments were delivered either by physiotherapists (Bergquist‐Ullman 1977; Brennan 2006; Childs 2004; Cleland 2009; Farrell 1982; Hallegraeff 2009; Hancock 2007; Seferlis 1998; Sutlive 2009) or chiropractors (Cherkin 1998; Cramer 1993; Hoiriis 2004; Postacchini 1988; Skargren 1997), while in other cases either an osteopathic physician (Hadler 1987; Hoehler 1981), combination physiotherapist or medical manipulator (Rasmussen 1979), medical manipulator or osteopath (Glover 1974; Juni 2009) delivered care. In three studies care was delivered by a relatively large number of practitioners (Childs 2004 (n = 14); Cleland 2009 (n = 17); Hancock 2007 (n = 15)) while in other cases care was delivered either by one or a few select practitioners (Glover 1974; Juni 2009; Rasmussen 1979); in all other cases the practitioner was unspecified or unclear. In most cases a high‐velocity thrust was delivered (Cherkin 1998; Childs 2004; Cleland 2009; Cramer 1993; Hadler 1987; Hallegraeff 2009; Hoehler 1981; Hoiriis 2004; Juni 2009; MacDonald 1990; Postacchini 1988; Skargren 1997; Sutlive 2009), while in other cases it was unclear if a high‐velocity thrust was used or not (Glover 1974; Rasmussen 1979; Seferlis 1998) or a combination of manipulation or mobilization or both techniques was used (Bergquist‐Ullman 1977; Brennan 2006; Farrell 1982; Hancock 2007). The mean (or median) number of treatments delivered in the SMT group was reported by slightly more than half of the studies and ranged from one (Glover 1974; Sutlive 2009) to 10 (Seferlis 1998).
Outcome measures: type, timing
Primary outcomes
Pain: all but one study (Hadler 1987) measured pain. In most cases it was measured via a visual analogue (VAS) or numerical rating (NRS) scale; in other cases it was not specified (Rasmussen 1979), was measured using a four or five point ordinal scale (Postacchini 1988; Hoehler 1981) respectively, or was measured by a 0 to 70 (or 75) point scale (Bergquist‐Ullman 1977; MacDonald 1990) making it unclear how this relates to the more common VAS or NRS. In addition, in only a minority of studies was it clear what time‐contingent aspect of pain was being measured, which in all cases where it was stated was current pain or pain in the previous 24 hours (Brennan 2006; Cherkin 1998; Childs 2004; Cleland 2009; Hallegraeff 2009; Hancock 2007; Juni 2009; Seferlis 1998).
Functional status: functional status was measured by most studies using a validated instrument, such as the Oswestry Disability Index (ODI) (Brennan 2006; Childs 2004; Cleland 2009; Cramer 1993; Hallegraeff 2009; Hoiriis 2004; Seferlis 1998; Skargren 1997; Sutlive 2009) or the Roland‐Morris Disability Questionnaire (Cherkin 1998; Hadler 1987; Hancock 2007; Juni 2009), while other older studies assessed this construct by questioned participants about their ability to perform a number of specific back‐related activities, such as the ability to walk across a room or to sit up or get up out of a low chair (Bergquist‐Ullman 1977; Farrell 1982; Hoehler 1981; MacDonald 1990; Postacchini 1988). Two studies did not assess functional status (Glover 1974; Rasmussen 1979).
Recovery: while most assessed this construct, few assessed it via the global improvement or similar (3, 5, or 7 point Likert) scale (Cherkin 1998; Glover 1974; Hadler 1987; Skargren 1997). Other studies used, for example, a composite score consisting of various instruments or measures in order to determine whether their participants were recovered or not (Farrell 1982; Hoiriis 2004; Rasmussen 1979), examined number of days to recovery and plotted a Kaplan‐Meier curve (Hancock 2007; Juni 2009), based recovery on 50% improvement as measured by the ODI (Childs 2004; Cleland 2009), asked participants whether they were recovered or not (Hallegraeff 2009; MacDonald 1990) or whether they thought the treatment was effective (Hoehler 1981). Six studies did not measure recovery (Bergquist‐Ullman 1977; Brennan 2006; Cramer 1993; Postacchini 1988; Seferlis 1998; Sutlive 2009).
Secondary outcomes
Seven studies measured return‐to‐work (Bergquist‐Ullman 1977; Cherkin 1998; Childs 2004; MacDonald 1990; Rasmussen 1979; Seferlis 1998; Skargren 1997) and two studies measured general functional status (Hancock 2007; Skargren 1997).
Other outcomes
Two studies conducted cost‐effectiveness analyses (Seferlis 1998; Skargren 1997). Five studies examined medication usage (Childs 2004; Hoiriis 2004; Juni 2009; Seferlis 1998; Skargren 1997).
Follow‐up
More than half of the studies limited follow‐up to short‐term measurements only (that is < 3 months) (Cramer 1993; Farrell 1982; Glover 1974; Hadler 1987; Hallegraeff 2009; Hancock 2007; Hoehler 1981; Hoiriis 2004; MacDonald 1990; Rasmussen 1979) including, in particular, one study that measured the effect two days post‐treatment only (Sutlive 2009). Five studies measured the long‐term (that is > 12 months) effects of the treatments (Bergquist‐Ullman 1977; Brennan 2006; Cherkin 1998; Seferlis 1998; Skargren 1997).
Safety
Six studies, with a total of 1195 participants, reported on adverse events (Cherkin 1998; Cleland 2009; Hancock 2007; Juni 2009; MacDonald 1990; Skargren 1997). One study reported four serious adverse events, occurring equally in both the experimental and control groups; however, "neither of the events appeared to be related to the allocated treatment strategies" (Juni 2009). In another study 25% of the participants reported at least one side‐effect of treatment; however, there were no differences between the groups and all symptoms resolved within 48 hours of onset (Cleland 2009).
Excluded studies
Many studies were excluded because: the proportion of participants with acute low‐back pain was unclear or unspecified (Beyerman 2006; Bronfort 1989; Doran 1975; Kinalski 1989; Meade 1990; Rupert 1985; Sims‐Williams 1978; Sims‐Williams 1979; Williams 2003; Wreje 1992; Zylbergold 1981); the contribution of SMT to the overall treatment effect could not be determined (Bishop 2010; Blomberg 1994; Delitto 1993; Erhard 1994; Godfrey 1984; Grunnesjo 2004; Waterworth 1985); participants had predominantly subacute or chronic low‐back pain (Hsieh 2002; Hurley 2004; Andersson 1999), or exclusively sciatica (Mathews 1987; Santilli 2006). Other reasons for exclusion were: the study was a pseudo‐RCT (for example alternate inclusion) (Coyer 1955; Nwuga 1982); the authors did not evaluate their participants beyond one day (Gemmell 1995; Sanders 1990); no relevant outcome was measured (Helliwell 1987); or asymptomatic participants were included (Terrett 1984).
Risk of bias in included studies
The results from the risk of bias (RoB) analysis for the individual studies are summarized in Figure 2. In total, approximately one‐third of the studies were considered to have a low RoB (Cherkin 1998; Cleland 2009; Hallegraeff 2009; Hancock 2007; Juni 2009; Sutlive 2009), representing 34% of all participants. Overall RoB scores ranged from zero to nine (median (IQR) 3 (2, 6)). It should be noted that personal contact with Hallegraeff et al resulted in this study being given an overall low RoB although the original evaluation resulted in a high RoB. Only two other trial authors responded to our assessment of the RoB for their study; which did not result in any other modifications.
Figure 2.
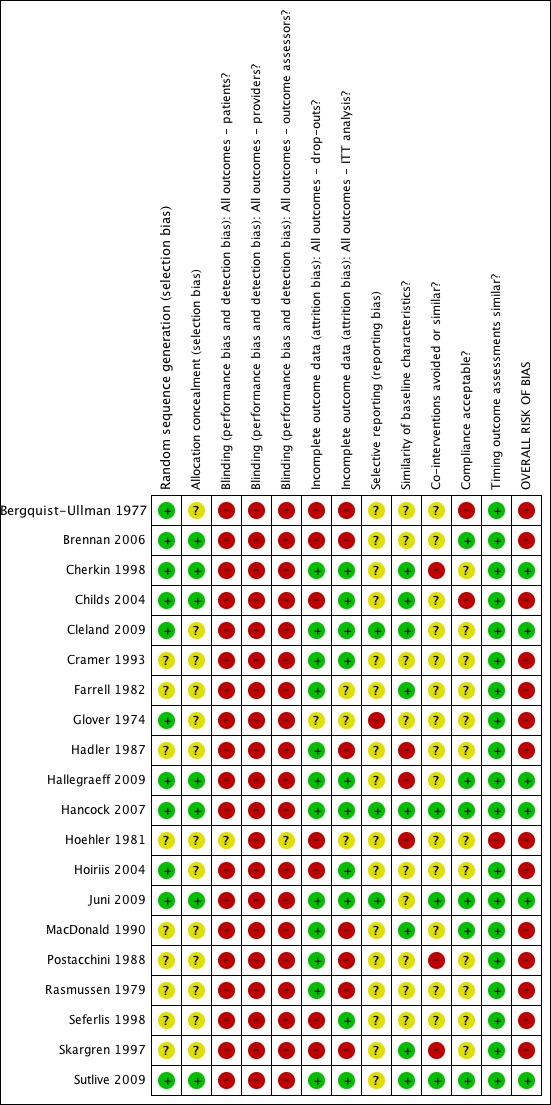
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In seven studies (35%) both the sequence generation and allocation procedure were conducted properly (Brennan 2006; Cherkin 1998; Childs 2004; Hallegraeff 2009; Hancock 2007; Juni 2009; Sutlive 2009). In an additional four studies (20%) the sequence generation was conducted properly but they were questionable regarding the allocation because this was inadequately described (Bergquist‐Ullman 1977; Cleland 2009; Glover 1974; Hoiriis 2004). In the remaining studies it was unclear whether the sequence generation and allocation were properly conducted.
Blinding
One study attempted to blind participants to treatment type (Hoiriis 2004); however, the results suggest that the participants were able to decipher their group allocation.
Incomplete outcome data
In one study loss to follow‐up exceeded 50% of the population at the second follow‐up measurement (three weeks) (Bergquist‐Ullman 1977), representing a fatal flaw. In various other studies the loss to follow‐up exceeded the 30% cut‐off for long‐term data, representing potentially biased results.
Selective reporting
Eight studies (40%) were published in the 21st century. It was, therefore, expected that few studies would fulfil this criterion because it has only been relatively recently (that is since July 2005) that trial protocols are required to be registered (Cleland 2009; Hancock 2007; Juni 2009). It is noteworthy that one older study indicated that recovery had been recorded at one month but did not report this, nor other secondary outcomes (Glover 1974); while in other studies return‐to‐work was measured but not reported (Rasmussen 1979) and similarly for recovery in another study (Hallegraeff 2009).
Other potential sources of bias
Publication bias: no firm conclusions could be drawn from the funnel plots that were suggestive of publication bias (Figure 3; Figure 4).
Figure 3.
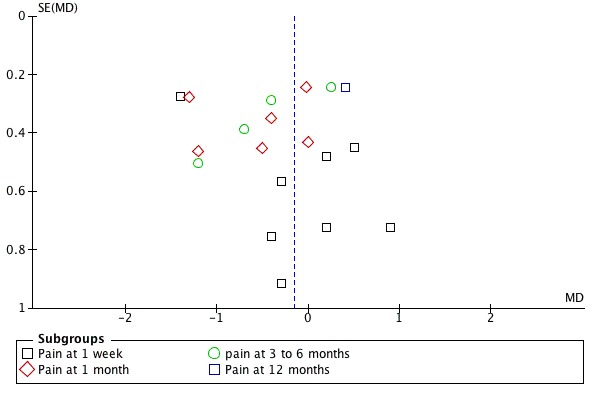
Funnel plot of comparison: 5 SMT versus all comparisons ‐ for the outcome 'Pain'. Note: negative values favour SMT.
Figure 4.
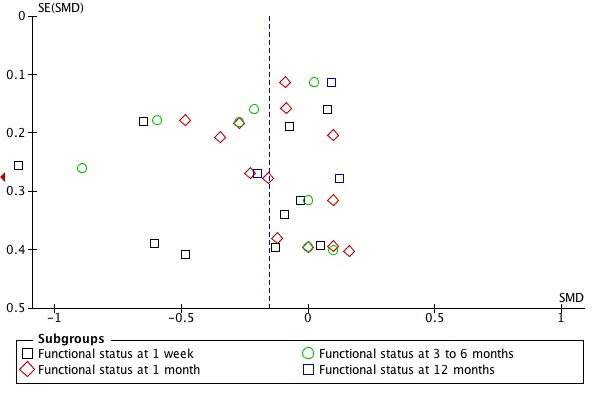
Funnel plot of comparison: 5 SMT versus all comparisons ‐ for the outcome 'Functional status'. Note: negative values favour SMT.
Source of funding: most studies were funded by non‐profit organizations (Brennan 2006; Childs 2004; Cleland 2009; Cramer 1993; Farrell 1982; Glover 1974; Hadler 1987; Hoehler 1981; Hoiriis 2004; Postacchini 1988) or governmental sources (Cherkin 1998; Hancock 2007; Skargren 1997), while in other cases a combination of funding sources were used including industry (Bergquist‐Ullman 1977; Juni 2009; MacDonald 1990). In other cases it was unclear or unspecified (Hallegraeff 2009; Rasmussen 1979; Seferlis 1998; Sutlive 2009).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Data were not extracted from one study beyond the one week follow‐up due to excessive drop‐outs (that is > 50%) (Bergquist‐Ullman 1977); and not extracted from a second study thought to have a fatal flaw as it demonstrated a significant difference between groups for baseline pain (Hallegraeff 2009). In addition, data could not be extracted from three studies (Glover 1974; Postacchini 1988; Seferlis 1998) and these are described below. The quality of the evidence is summarized in the 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4).
Effect of SMT versus inert interventions
Data were available for extraction from two studies with a low RoB (Cherkin 1998; Hancock 2007) and three studies with a high RoB (Bergquist‐Ullman 1977; Cramer 1993; Rasmussen 1979). For the outcome of pain, there was low quality evidence (high RoB, imprecision) from three studies (Bergquist‐Ullman 1977; Cherkin 1998; Cramer 1993) that SMT was not significantly better than inert interventions at one week follow‐up (MD 0.14, 95% CI ‐0.69 to 0.96) and low quality evidence (inconsistency, imprecision) from one study (Cherkin 1998) that SMT was significantly better at one and three month follow‐up (MD ‐1.20, 95% CI ‐2.01 to ‐0.39; MD ‐1.20, 95% CI ‐2.11 to ‐0.29, respectively) (Analysis 1.1). Data from one small study with a high RoB (n = 44) (Glover 1974) could not be extracted but the results suggested a significant immediate effect on pain relief when SMT was compared to detuned diathermy; however there were no significant differences between the groups thereafter, including at one week follow‐up.
For the outcome of functional status, there was moderate quality evidence (imprecision) from two studies (Cherkin 1998; Cramer 1993) that SMT was not significantly better than inert interventions at one week follow‐up (SMD ‐0.08, 95% CI ‐0.37 to 0.21) and low quality evidence (inconsistency, imprecision) from one study (Cherkin 1998) that SMT was not significantly better at one and three months (SMD ‐0.27, 95% CI ‐0.58 to 0.04; SMD ‐0.28, 95% CI ‐0.59 to 0.02, respectively) (Analysis 1.2).
In a separate analysis, one study with a low RoB (Hancock 2007) examined the effect of SMT versus detuned ultrasound in those participants who received either diclofenac or placebo. For the outcomes of pain and functional status, there were no significant differences at 1, 2, 4 or 12 week follow‐up; with the exclusion of the 2 week follow‐up for functional status, which favoured SMT (MD: ‐1.4, 95% CI: ‐2.7 to ‐0.1). These data were not presented in the pooled analyses because they were not available from the publication.
For the outcome of recovery, evidence was available from two studies (Hancock 2007; Rasmussen 1979) at one week follow‐up. They demonstrated non‐significant but conflicting results. One relatively large study (n = 239) with a low RoB (Hancock 2007) suggested benefit in favour of inert interventions (RR 0.74, 95% CI 0.50 to 1.09) while the other relatively small study (n = 24) (Rasmussen 1979) suggested benefit in favour of SMT (RR 3.50, 95% CI 0.91 to 13.53). Further, there was low quality evidence (inconsistency, imprecision) from one study (Hancock 2007) that SMT was not significantly better at one and three months (RR 0.98, 95% CI 0.86 to 1.11; RR 1.00, 95% CI 0.98 to 1.02) (Analysis 1.3).
No data were available for quality of life, return‐to‐work, or cost‐effectiveness.
Effect of SMT versus sham SMT
One study was identified (Hoiriis 2004). For the outcomes of pain and functional status, there was very low quality evidence (high RoB, inconsistency, imprecision) from one study (Hoiriis 2004) that SMT was not significantly better than sham SMT at one month follow‐up (MD ‐0.50, 95% CI ‐1.39 to 0.39; SMD ‐0.35, 95% CI ‐0.76 to 0.06, respectively) (Analysis 2.1 and 2.2). No data were available for recovery, quality of life, return‐to‐work, or cost‐effectiveness.
Effect of SMT versus all other interventions
Data were available for extraction from one study with a low RoB (Cherkin 1998) and six studies with a high RoB (Bergquist‐Ullman 1977; Brennan 2006; Farrell 1982; Hoehler 1981; Rasmussen 1979; Skargren 1997). For the outcome of pain, there was low quality evidence (high RoB, imprecision) from three studies (Bergquist‐Ullman 1977; Cherkin 1998; Farrell 1982) that SMT was not significantly better than other interventions at one week follow‐up (MD 0.06, 95% CI ‐0.53 to 0.65); moderate quality evidence (high RoB) from three studies (Cherkin 1998; Farrell 1982; Skargren 1997) that SMT was not significantly better at one month follow‐up (MD ‐0.15, 95% CI ‐0.49 to 0.18); low quality evidence (high RoB, inconsistency (I2 = 81%)) from two studies (Cherkin 1998; Skargren 1997) that SMT was not significantly better (MD ‐0.20, 95% CI ‐1.13 to 0.73) at three to six month follow‐up; and very low quality evidence (high RoB, inconsistency, imprecision) from one study (Skargren 1997) that SMT was not significantly better (MD 0.40, 95% CI ‐0.08 to 0.88) (Figure 5).
Figure 5.
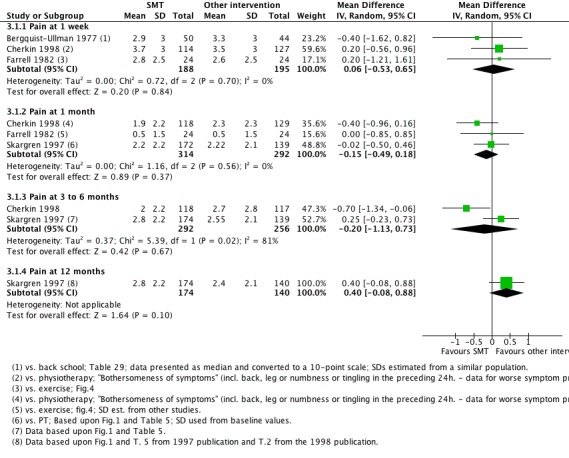
Forest plot of comparison: 3 Spinal manipulative therapy versus all other therapies, outcome 3.1 'Pain'.
For the outcome of functional status, there was low quality evidence (inconsistency, imprecision) from one study (Cherkin 1998) that SMT was not significantly better than other interventions at one week follow‐up (SMD 0.07, 95% CI ‐0.18 to 0.33); moderate quality evidence (high RoB) from three studies (Brennan 2006; Cherkin 1998; Skargren 1997) that SMT was not significantly better at one month follow‐up (SMD ‐0.11, 95% CI ‐0.26 to 0.05); low quality evidence (high RoB, inconsistency (I2 = 51%)) that SMT was not significantly better at three to six month follow‐up (SMD ‐0.09, 95% CI ‐0.33 to 0.15); and low quality evidence (high RoB, imprecision) that SMT was not significantly better at 12 month follow‐up (SMD 0.06, 95% CI ‐0.14 to 0.25) (Figure 6).
Figure 6.
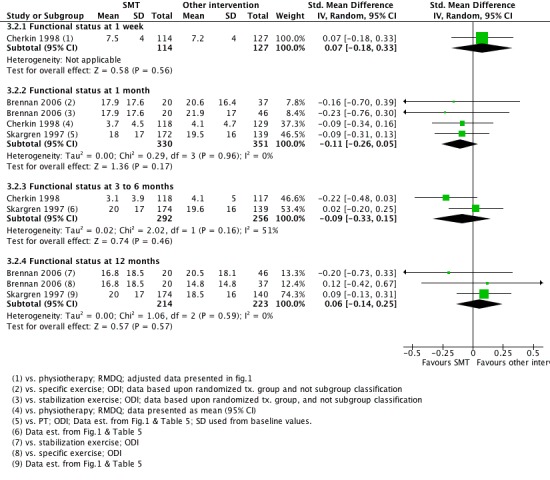
Forest plot of comparison: 3 Spinal manipulative therapy versus all other therapies, outcome 3.2 'Functional status'.
For the outcome of recovery, there was low quality evidence (high RoB, imprecision) from two studies (Farrell 1982; Hoehler 1981) that there was no significant difference at one month (RR 1.06, 95% CI 0.94 to 1.21) and very low quality evidence (high RoB, inconsistency, imprecision) from one study (Hoehler 1981) that SMT did not result in significantly better recovery at three months (RR 1.29, 95% CI: 0.96 to 1.74) (Analysis 3.3).
For return‐to‐work, data was available from one study with a high RoB (Skargren 1997). This study demonstrated similar proportions of participants during the treatment phase and at six months who were no longer on sick leave.
Data not able to be extracted from one study (Seferlis 1998) examined the effects of SMT compared to exercise and standard GP care. At one month follow‐up, there were no significant differences between the interventions for the outcomes of pain, functional status, or socioeconomic disability (including sick‐leave, low‐back pain recurrence; and change of job due to low‐back pain).
Two studies conducted cost‐effectiveness analyses. One study conducted a cost‐minimization analysis (Seferlis 1998), which demonstrated that the differences in costs over one year follow‐up for SMT compared to GP care alone or an exercise program were small; however, no formal statistical comparison was conducted. Furthermore, cost data were not entirely complete (that is only the costs of treatment, the investigations (that is imaging), and operations were collected as direct costs). In addition, cost‐minimization analyses may have limited application of results because it assumes that the outcomes are equivalent; therefore, these results should be viewed with some caution. Another study (Skargren 1997) examined differences in costs at one year between those participants receiving chiropractic care and physiotherapy. The study demonstrated small, non‐significant differences in costs.
No data were available for quality of life.
Effect of SMT plus another intervention versus the intervention alone
Data were available for extraction from one study with a low RoB (Juni 2009) and two studies with a high RoB (Childs 2004; MacDonald 1990). For the outcome of pain, there was low quality evidence (inconsistency, imprecision) from one study (Juni 2009) that SMT plus another intervention was not significantly better than the intervention alone at one week or three to six month follow‐up (MD 0.84, 95% CI ‐0.04 to 1.72; MD 0.65, 95% CI ‐0.32 to 1.62, respectively) (Analysis 4.1).
For the outcome of functional status, there was low quality evidence (high RoB, imprecision) from two studies (Childs 2004; MacDonald 1990) that SMT plus another intervention was significantly better at one week follow‐up (SMD ‐0.41, 95% CI ‐0.73 to ‐0.10); low quality evidence (high RoB, imprecision) from three studies (Childs 2004; Juni 2009; MacDonald 1990) that SMT was not significantly better at one month (SMD ‐0.09, 95% CI ‐0.39 to 0.21) and low quality evidence (high RoB, imprecision) from two studies (Childs 2004; MacDonald 1990) that SMT was not significantly better at three months (SMD ‐0.22, 95% CI ‐0.61 to 0.16) (Analysis 4.2). The study reported in Childs 2004 demonstrated a strong, clinically‐relevant short‐term effect (SMD ‐0.65, 95% CI ‐1.00 to ‐0.30).
For the outcome of recovery, there were conflicting results from one study with a low RoB (Juni 2009) and two studies with a high RoB (Childs 2004; MacDonald 1990). There was low quality evidence (inconsistency, imprecision) from the one study with a low RoB (Juni 2009) which demonstrated no significant effect on recovery at one week or three to six months (RR 0.89, 95% CI 0.32 to 2.47; RR 0.75, 95% CI 0.51 to 1.10, respectively). One relatively large study (n = 131) (Childs 2004) with a high RoB demonstrated a weak, significant effect (RR 1.74, 95% CI 1.19 to 2.55) in favour of SMT at one month. The remaining study (MacDonald 1990), which had a high RoB, examined various subgroups which were defined by duration of the baseline pain. The results were conflicting and by and large they were non‐significant (Analysis 4.3). One of the subgroup comparisons from MacDonald 1990 represented a moderate, significant effect on recovery at three to six months (RR 2.06, 95%CI 1.07 to 3.97).
For the outcome of return‐to‐work there was data from one study with a high RoB (Childs 2004). There was very low quality evidence (high RoB, inconsistency, imprecision) that there was no significant effect on return‐to‐work (RR 1.21, 95% CI 0.99 to 1.47) (Analysis 4.4).
No data were available for quality of life or cost‐effectiveness.
Effect of SMT versus another SMT technique
Data were not pooled for this comparison because it was thought that a pooled estimate would not represent a clinically‐meaningful assessment as various different techniques were being compared to one another; therefore, the individual estimates are described here. In general, side‐lying and supine thrust SMT techniques demonstrated a short‐term statistically significant favourable difference compared to non‐thrust SMT techniques for the outcomes of pain, functional status and recovery, and a significant difference at six months for the outcome of functional status but not pain or recovery (Analysis 5.1 to 5.3) (Cleland 2009). No significant difference was identified between the different thrust techniques for any outcome or time interval.
In a second study, no short‐term effect on functional status was observed for high‐velocity SMT versus mobilization (Analysis 5.2). In a third study, the short‐term effect (48 hours post‐treatment) of two different side‐lying SMT techniques were compared to one another (lumbar pelvic versus neutral‐gap SMT) (Sutlive 2009). No statistically significant difference was observed between the two techniques for pain or functional status (Analysis 5.1 and 5.2).
In a third study, the effects of high‐velocity SMT were compared to mobilization. No significant differences were found for short‐term functional status (Analysis 5.1).
No data were available for quality of life or cost‐effectiveness.
Other clinical variables and sensitivity analyses
Data were insufficient per comparison, outcome, and follow‐up measurement to allow us to assess the effect of SMT for any of the planned sensitivity analyses (for example by risk of bias, success of randomization, specific type of SMT technique used). Nevertheless, only two studies demonstrated a strong clinically‐relevant effect: a small study (n = 24) with a high RoB (Rasmussen 1979) and to a lesser extent the study by Childs 2004 (also with a high RoB).
Discussion
Summary of main results
In general, for the primary outcomes there is low to very low quality evidence of no difference in effect of SMT compared to inert interventions, sham SMT, or when added to another intervention; and varying quality of evidence (from very low to moderate) of no significant difference in effect of SMT compared with other interventions. There are two minor exceptions. There is a statistically significant short‐term but not clinically‐relevant effect of SMT on pain relief compared to inert interventions (one RCT, MD ‐1.20, 95% CI ‐2.01 to ‐0.39) and a moderate short‐term effect of SMT on functional status when added to another intervention (two RCTs, SMD ‐0.41, 95% CI ‐0.73 to ‐0.10). Furthermore, two studies demonstrated a positive, in some cases clinically‐relevant effect of SMT as an adjuvant therapy for functional status (one week change in Oswestry of 9.2, 95% CI 4.4 to 14.1) and recovery (RR 2.06, 95% CI 1.07 to 3.97) (Childs 2004; MacDonald 1990), respectively; although these were isolated effects in studies with a high risk of bias.
To some extent, these results seem inconsistent because one would expect the effect of SMT compared to sham treatment or inert interventions to be greater than compared to other (effective) interventions, such as exercise or physiotherapy. The observation that there is no difference across the various control groups is confusing. In part, these results might be explained by the low quality level of evidence, which is a result of the small numbers of studies identified per comparison, outcome, and time interval, and typically investigated by studies with a high risk of bias. More importantly, the six RCTs with a low risk of bias demonstrated no clinically‐relevant effect of SMT across the various comparisons (Cherkin 1998; Cleland 2009; Hallegraeff 2009; Hancock 2007; Juni 2009; Sutlive 2009). In light of these findings, it is difficult to come to any strong conclusions or make recommendations regarding the use of SMT for acute low‐back pain.
Two important factors might have influenced these results. Firstly, acute low‐back pain is known for its favourable natural history (Dunn 2004); therefore, demonstrating a clinically‐relevant difference represents a unique challenge. Secondly, baseline pain and functional status were, on average, at moderate levels for the study populations in most studies. Therefore, so‐called floor effects (meaning there is too little room for improvement) cannot be discounted.
It is noteworthy that the majority of studies that are registered and currently being conducted are investigating the effect of SMT for subacute and chronic low‐back pain. Consequently, the issue of effectiveness of SMT for acute low‐back pain is not likely to be resolved in the near future. Importantly, there was no evidence of serious adverse events demonstrated in any of the trials, although all RCTs were too small to give any reliable and precise estimate of these types of events; these have been described elsewhere (Assendelft 1996). However, two large cohort studies of SMT failed to identify any serious adverse events following more than 6500 SMT treatments to the neck or low‐back, or both (Leboeuf‐Yde 1997; Senstad 1997).
Overall completeness and applicability of evidence
Virtually all the studies included in this review were conducted in North America or Europe and include a rather broad category of participants (that is most participants were middle‐aged, had little to no radiating pain, and were recruited from primary or tertiary care). Furthermore, care was provided by a variety of practitioners, including chiropractors, osteopaths, and manual therapists; therefore, the results of this review might be generalized to various settings. Nevertheless, there are concerns that applying SMT to such a heterogenous population as aspecific low‐back pain is likely to inevitably lead to small‐to‐moderate effects. In contrast, there is evidence from studies evaluated in this review that (perhaps) clinically‐relevant differences are obtained when clinical prediction rules (CPRs) or forms of subgrouping are applied (Brennan 2006; Childs 2004). Although the trial conducted by Brennan et al was intended to compare the outcomes of those receiving treatments that were matched (or unmatched) to specific subgroups based upon their initial clinical presentation, our analysis did not take this into account; rather, we extracted the data from the unmatched patient assignment. Thus, while SMT might be an effective therapy for specific subgroups, there is too little information at present to draw any strong conclusions. In addition, to our knowledge only two studies have examined CPRs for SMT in patients with acute low‐back pain, namely the CPR of Childs 2004 which failed to be validated in another trial (Hancock 2008).
Other factors that might have influenced these results are the specific features of the treatment, namely frequency and duration. However, that is difficult for us to evaluate because only slightly more than half of the studies reported this feature. Future reviews could benefit from studies that provide more insight into the details of the intervention as well as providing details regarding the practitioner.
Quality of the evidence
Although many questions remain about the effect of SMT, especially given the fact that two‐thirds of the included studies demonstrated a high risk of bias, questions may also be raised regarding the quality of the extracted data. In many cases, particularly for the older studies published before 2000, data were estimated from figures or graphs, which in most cases lacked a measure of variance. Furthermore, we extracted final scores or values rather than change scores or values adjusted for various confounders because the vast majority of studies presented only the former. Therefore, the reader should not place too much emphasis on the precision of the pooled estimates, meaning the pooled point estimates might be compromised. Lastly, relatively few participants were identified for any of the principal outcome measures; therefore, none of the findings should be considered robust.
Potential biases in the review process
The most important and obvious limitation is the large number of studies with a high risk of bias. While there is empirical evidence in the field of low‐back pain that studies with a high risk of bias tend to yield a larger effect (van Tulder 2009), it is unclear to what extent this might have influenced the overall results. An additional limitation is the low numbers of studies and small sample sizes identified per comparison, outcome, and time interval, which prohibited us from conducting any meaningful sensitivity analyses. Other limitations include potential publication bias. Published trials are generally larger and may show an overall greater treatment effect than studies published in the 'grey' literature (Hopewell 2004); therefore, it is important to include these latter studies in systematic reviews (McAuley 2000). Although we only searched online sources for grey literature, funnel plots did not suggest this was an issue.
In addition, the source of funding is an important consideration because of potential financial conflicts and influence from industry‐sponsored research (Bekelman 2003; Okike 2008); however, most of the studies were funded by non‐profit or governmental institutions so this would not appear to be an important concern. Finally, it must be declared that the principal author of this review (SMR) is a chiropractor and uses SMT in his daily practice; however, any potential bias associated with that authorship must be offset by a team of review authors with impeccable academic reputations and who have no financial gain from the conclusions drawn in this review.
Agreements and disagreements with other studies or reviews
In principal, the results and conclusions of this updated review are consistent with the previous edition of the review, namely that SMT is no better than standard interventions for acute low‐back pain. However, one important conclusion from the previous review was that SMT demonstrated a short‐term, clinically‐relevant effect on pain relief compared to sham SMT or other therapies thought to be ineffective or harmful. This is in contrast to the findings of this updated review. Although we found a moderate clinically‐relevant, short‐term effect of SMT compared to inert interventions for pain relief, this was from just one study (Cherkin 1998), albeit a study with a low risk of bias. Importantly, no significant effect was found for functional improvement. Importantly, some of the studies included in the previous review were excluded from this update for the various reasons listed for the excluded studies. Therefore, we believe this update to be a better reflection of the effect of SMT for acute low‐back pain.
This review is not in agreement with a recent systematic review, which was much more positive (Dagenais 2010). Approximately one‐third (n = 5/14) of the studies in that review were not included in this review because they either evaluated patients with sciatica exclusively (n = 2), and therefore were thought to represent a subgroup of patients with low‐back pain not evaluated here, or included studies with subacute (n = 1) or a mix of subacute and chronic pain (n = 1) or included studies in which the contribution of SMT could not be properly determined (n = 1). However, our findings are consistent with other recent systematic reviews (Chou 2007; van Tulder 2006).
Authors' conclusions
No high quality evidence was provided for any comparison, outcome, or time interval; therefore, no strong conclusions or recommendations can be made for the use of SMT for acute low‐back pain. SMT appears to be no better than other existing therapies for pain reduction and improvement of functional status. The decision to refer for SMT should be based upon costs, preferences of the patient and providers, and relative safety of the various treatment options.
It would appear from the continuing 'disappointing' results from the trials included in this review (at least from the perspective of the clinician) that either further research on such heterogenous populations with acute low‐back pain is a waste of funding or that something more fundamental is lacking in our approach. The small to moderate effects seen in clinical trials covering both pharmacological and non‐pharmacological interventions have been a point of contention and discussion by numerous authors (Foster 2010; Lamb 2010) while clinicians wonder why the dramatic effects sometimes observed in their clinical practice are not reflected in these trials. At least one lesson should be drawn from this review, continuing in the same vein seems pointless. After all, there are currently more than 100 RCTs of SMT for low‐back pain (Rubinstein 2012). Despite the disappointing quality of the evidence examined here, a more precise estimate of the effect of SMT for acute low‐back pain, a condition with a rather benign natural history, does not appear to be the way forward. Preventing the onset of chronic low‐back pain, which is disabling and expensive, may be a much more clinically relevant question. Relatively few of the studies included in this review followed patients long enough to identify chronicity or recurrence as an outcome, although any such studies would have to be sufficiently large and powered to adequately address this. Observational designs might sooner be the design of choice to identify who develops chronic complaints and recurrent symptoms.
There remain various avenues yet to be explored. Examples include better identification of subgroups likely to respond to SMT, such as through the use of clinical prediction rules. Other examples include better definitions and reporting in trials of SMT so that interpretation of the results are more transparent. Various initiatives are underway. For example, our research group is currently conducting a large‐scale international survey using a Delphi process designed to reach consensus as to which items should be included in a description of SMT in future trials. This effort is designed to represent an extension of the CONSORT statement.
Other areas to be considered include whether we should abandon the search for a better diagnosis or better identification of the pain generators in favour of a different approach. Apart from identifying those with serious pathology, radicular pain, and psychosocial factors (Rubinstein 2008), it would appear that we have not proceeded beyond the aspecific (or 'uncomplicated') back pain model. Various examination procedures and tests can be conducted, which include advanced imaging, neuromuscular testing, or diagnostic blocks, all of which remove aspecific low‐back pain from the primary care arena; however, it is unclear to what extent this might influence clinician behaviour and, more importantly, whether the patient is likely to benefit (Haldeman 2011). It seems unlikely that the search for a better diagnosis through better identification of pain generators or better identification of pathology will lead in the right direction. Alternative approaches include dropping the aspecific back pain model, which includes a rather heterogenous group of patients, in favour of better classification of patients through identification of pain through movement, such as directional preference or mechanical diagnosis and therapy (that is the McKenzie approach). Other approaches might include use of diagnostic algorithms, such as those that include components of the diagnostic triage and directional preference.
These are but a few examples of the way to proceed and it seems imperative that these problems be resolved before further research is conducted. Finally, it is imperative that any future studies include an economic evaluation.
Acknowledgements
The review authors thank the members of the Editorial Board of the Cochrane Back Review Group for constructive comments on the protocol of this review and Ms Rachel Couban for her assistance with the development of the search strategies. They also thank Dr Sally Morton, Mrs Emily Yu, Ms Marika J Suttorp, and Dr Paul Shekelle for their work on the previous version of this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Back explode all trees
#2 MeSH descriptor Buttocks, this term only
#3 MeSH descriptor Leg, this term only
#4 MeSH descriptor Back Pain explode tree 1
#5 MeSH descriptor Back Injuries explode all trees
#6 MeSH descriptor Low Back Pain, this term only
#7 MeSH descriptor Sciatica, this term only
#8 (low next back next pain)
#9 (lbp)
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Musculoskeletal Manipulations explode all trees
#12 MeSH descriptor Chiropractic explode all trees
#13 manip*
#14 MeSH descriptor Osteopathic Medicine explode all trees
#15 osteopath*
#16 chiropract*
#17 (#11 OR #12 OR #13 OR #14 OR #15 OR #16)
#18 (#17 AND #10
#19 (#18)
Appendix 2. MEDLINE search strategy
Clinical Trial.pt.
randomized.ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐7
Animals/
Humans/
9 not (9 and 10)
8 not 11
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatica/
spondylosis.ti,ab.
lumbago.ti,ab.
exp low back pain/
or/13‐23
exp Manipulation, Chiropractic/
exp Manipulation, Orthopedic/
exp Manipulation, Osteopathic/
exp Manipulation, Spinal/
exp Musculoskeletal Manipulations/
exp Chiropractic/
manipulation.mp.
manipulate.mp.
exp Orthopedics/
exp Osteopathic Medicine/
or/25‐34
12 and 24 and 35
Appendix 3. EMBASE search strategy
Clinical Article/
exp Clinical Study/
Clinical Trial/
Controlled Study/
Randomized Controlled Trial/
Major Clinical Study/
Double Blind Procedure/
Multicenter Study/
Single Blind Procedure/
Phase 3 Clinical Trial/
Phase 4 Clinical Trial/
crossover procedure/
placebo/
or/1‐13
allocat$.mp.
assign$.mp.
blind$.mp.
(clinic$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/15‐29
14 and 30
human/
Nonhuman/
exp ANIMAL/
Animal Experiment/
33 or 34 or 35
32 not 36
31 not 36
37 and 38
38 or 39
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
exp Low back pain/
or/41‐51
exp CHIROPRACTIC/
exp Orthopedic Manipulation/
exp Manipulative Medicine/
exp Osteopathic Medicine/
manipulation.mp.
manipulate.mp.
exp Orthopedics/
osteopathy.mp.
or/53‐60
40 and 52 and 6
Appendix 4. CINAHL search strategy
Randomized Controlled Trials.mp.
clinical trial.pt.
exp Clinical Trials/
(clin$ adj25 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
exp PLACEBOS/
placebo$.tw.
random$.tw.
exp Study Design/
(latin adj square).tw.
exp Comparative Studies/
exp Evaluation Research/
Follow‐Up Studies.mp.
exp Prospective Studies/
(control$ or prospectiv$ or volunteer$).tw.
Animals/
or/1‐15
17 not 16
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
exp SCIATICA/
spondylosis.ti,ab.
lumbago.ti,ab.
exp low back pain/
or/19‐29
exp CHIROPRACTIC/
exp MANIPULATION, CHIROPRACTIC/
exp MANIPULATION, ORTHOPEDIC/
exp MANIPULATION, OSTEOPATHIC/
manipulation.mp.
manipulate.mp.
exp Manual Therapy/
exp ORTHOPEDICS/
exp OSTEOPATHY/
or/31‐39
18 and 30 and 40
Appendix 5. Criteria for risk of bias assessment for RCTs (Higgins 2011)
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomized sequence
There is a low risk of selection bias if the investigators describe a random component in the sequence generation process such as: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random).
There is a high risk of selection bias if the investigators describe a non‐random component in the sequence generation process, such as: sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number; or allocation by judgment of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment
There is a low risk of selection bias if the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomization); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
There is a high risk of bias if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; or other explicitly unconcealed procedures.
Blinding of participants
Performance bias due to knowledge of the allocated interventions by participants during the study
There is a low risk of performance bias if blinding of participants was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of personnel/care providers (performance bias)
Performance bias due to knowledge of the allocated interventions by personnel/care providers during the study
There is a low risk of performance bias if blinding of personnel was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of outcome assessor (detection bias)
Detection bias due to knowledge of the allocated interventions by outcome assessors
There is low risk of detection bias if the blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or:
for patient‐reported outcomes in which the patient was the outcome assessor (e.g. pain, disability): there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding (Boutron 2005);
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g. co‐interventions, length of hospitalization, treatment failure), in which the care provider is the outcome assessor: there is a low risk of bias for outcome assessors if there is a low risk of bias for care providers (Boutron 2005);
for outcome criteria that are assessed from data from medical forms: there is a low risk of bias if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Incomplete outcome data (attrition bias)
Attrition bias due to amount, nature or handling of incomplete outcome data
There is a low risk of attrition bias if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardized difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if drop‐outs are very large, imputation using even 'acceptable' methods may still suggest a high risk of bias) (van Tulder 2003). The percentage of withdrawals and drop‐outs should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but arbitrary, not supported by literature) (van Tulder 2003).
Selective reporting (reporting bias)
Reporting bias due to selective outcome reporting
There is low risk of reporting bias if the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way, or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
There is a high risk of reporting bias if not all of the study's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Group similarity at baseline (selection bias)
Bias due to dissimilarity at baseline for the most important prognostic indicators.
There is low risk of bias if groups are similar at baseline for demographic factors, value of main outcome measure(s), and important prognostic factors (examples in the field of back and neck pain are duration and severity of complaints, vocational status, percentage of patients with neurological symptoms) (van Tulder 2003).
Co‐interventions (performance bias)
Bias because co‐interventions were different across groups
There is low risk of bias if there were no co‐interventions or they were similar between the index and control groups (van Tulder 2003).
Compliance (performance bias)
Bias due to inappropriate compliance with interventions across groups
There is low risk of bias if compliance with the interventions was acceptable, based on the reported intensity/dosage, duration, number and frequency for both the index and control intervention(s). For single‐session interventions (e.g. surgery), this item is irrelevant (van Tulder 2003).
Intention‐to‐treat analysis
There is low risk of bias if all randomized patients were reported/analyzed in the group to which they were allocated by randomization.
Timing of outcome assessments (detection bias)
Bias because important outcomes were not measured at the same time across groups
There is low risk of bias if all important outcome assessments for all intervention groups were measured at the same time (van Tulder 2003).
Other bias
Bias due to problems not covered elsewhere in the table
There is a low risk of bias if the study appears to be free of other sources of bias not addressed elsewhere (e.g. study funding).
Data and analyses
Comparison 1.
Spinal manipulative therapy versus inert interventions
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Pain at 1 week | 3 | 311 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.69, 0.96] |
| 1.2 Pain at 1 month | 1 | 178 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.01, ‐0.39] |
| 1.3 pain at 3 months | 1 | 181 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.11, ‐0.29] |
| 2 Functional status | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Functional status at 1 week | 2 | 205 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.37, 0.21] |
| 2.2 Functional status at 1 month | 1 | 178 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.58, 0.04] |
| 2.3 Functional status at 3 months | 1 | 181 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.59, 0.02] |
| 3 Recovery | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Recovery at 1 week | 2 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.50, 1.85] |
| 3.2 Recovery at 1 month | 1 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.10] |
| 3.3 Recovery at 3 months | 1 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.98, 1.02] |
Analysis 1.1.
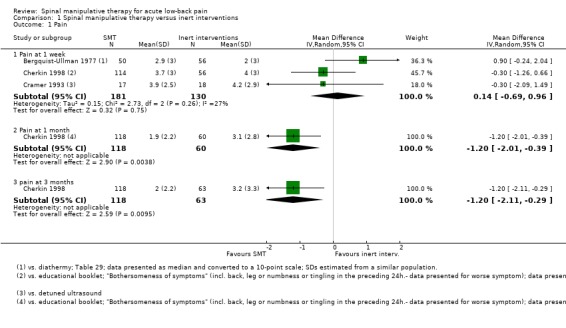
Comparison 1 Spinal manipulative therapy versus inert interventions, Outcome 1 Pain.
Analysis 1.2.
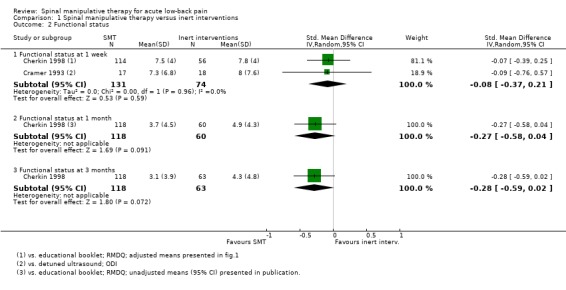
Comparison 1 Spinal manipulative therapy versus inert interventions, Outcome 2 Functional status.
Analysis 1.3.
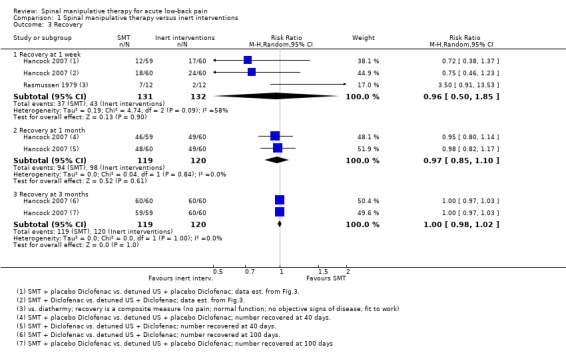
Comparison 1 Spinal manipulative therapy versus inert interventions, Outcome 3 Recovery.
Comparison 2.
Spinal manipulative therapy versus sham SMT
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Pain at 1 month | 1 | 74 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.39, 0.39] |
| 2 Functional status | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Functional status at 1 month | 1 | 94 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.76, 0.06] |
Analysis 2.1.
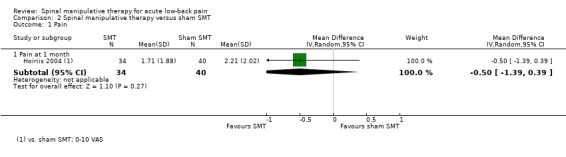
Comparison 2 Spinal manipulative therapy versus sham SMT, Outcome 1 Pain.
Analysis 2.2.
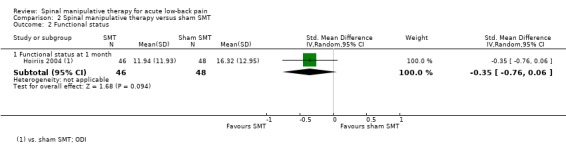
Comparison 2 Spinal manipulative therapy versus sham SMT, Outcome 2 Functional status.
Comparison 3.
Spinal manipulative therapy versus all other therapies
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Pain at 1 week | 3 | 383 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.53, 0.65] |
| 1.2 Pain at 1 month | 3 | 606 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.49, 0.18] |
| 1.3 Pain at 3 to 6 months | 2 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.13, 0.73] |
| 1.4 Pain at 12 months | 1 | 314 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.08, 0.88] |
| 2 Functional status | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Functional status at 1 week | 1 | 241 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.18, 0.33] |
| 2.2 Functional status at 1 month | 3 | 681 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.26, 0.05] |
| 2.3 Functional status at 3 to 6 months | 2 | 548 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.33, 0.15] |
| 2.4 Functional status at 12 months | 2 | 437 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.14, 0.25] |
| 3 Recovery | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Recovery at 1 month | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.21] |
| 3.2 Recovery at 3 months | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.96, 1.74] |
| 4 Return‐to‐work | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 return to work at 1 month | 1 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.12] |
| 4.2 Return‐to‐work at 6 months | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.98, 1.16] |
Analysis 3.1.
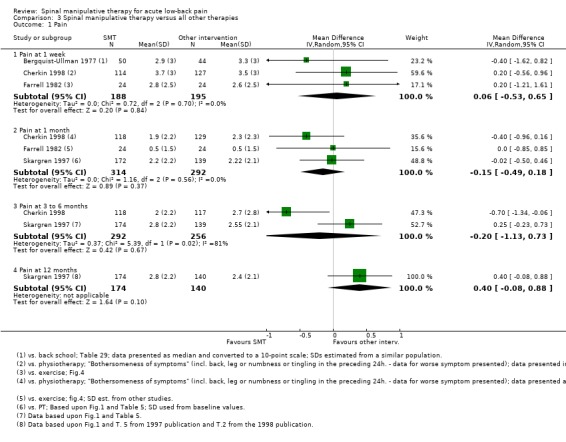
Comparison 3 Spinal manipulative therapy versus all other therapies, Outcome 1 Pain.
Analysis 3.2.
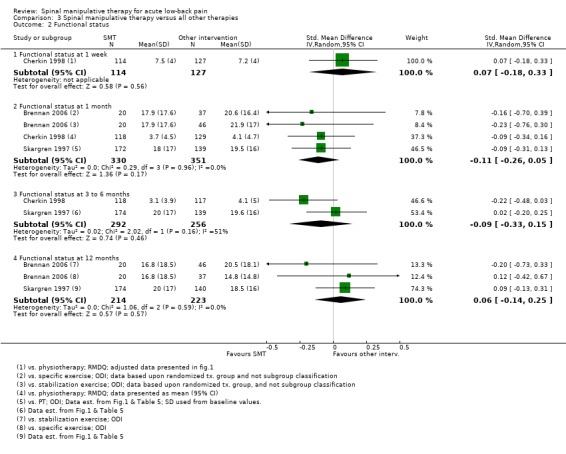
Comparison 3 Spinal manipulative therapy versus all other therapies, Outcome 2 Functional status.
Analysis 3.3.
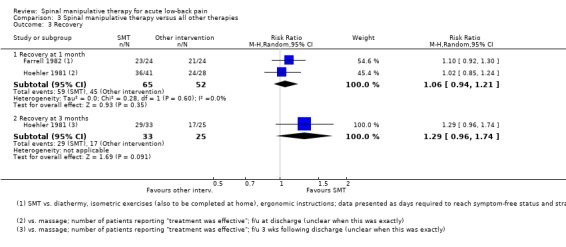
Comparison 3 Spinal manipulative therapy versus all other therapies, Outcome 3 Recovery.
Analysis 3.4.
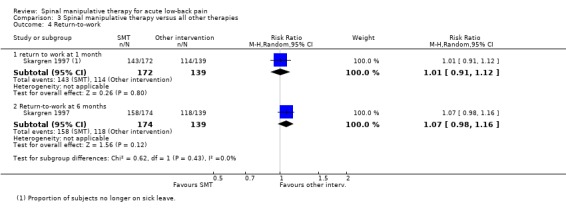
Comparison 3 Spinal manipulative therapy versus all other therapies, Outcome 4 Return‐to‐work.
Comparison 4.
Spinal manipulative therapy plus any intervention versus that same intervention alone
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Pain at 1 week | 1 | 102 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐0.04, 1.72] |
| 1.2 Pain at 3 to 6 months | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.32, 1.62] |
| 2 Functional status | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Functional status at 1 week | 2 | 225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.73, ‐0.10] |
| 2.2 Functional status at 1 month | 3 | 322 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.39, 0.21] |
| 2.3 Functional status at 3 to 6 months | 2 | 225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.61, 0.16] |
| 3 Recovery | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Recovery at 1 week | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.36, 2.19] |
| 3.2 Recovery at 1 month | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.19] |
| 3.3 Recovery at 3 to 6 months | 2 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.71, 1.31] |
| 4 Return‐to‐work | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Return‐to‐work at 6 months | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.99, 1.47] |
Analysis 4.1.
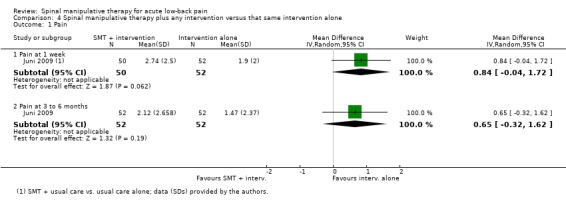
Comparison 4 Spinal manipulative therapy plus any intervention versus that same intervention alone, Outcome 1 Pain.
Analysis 4.2.
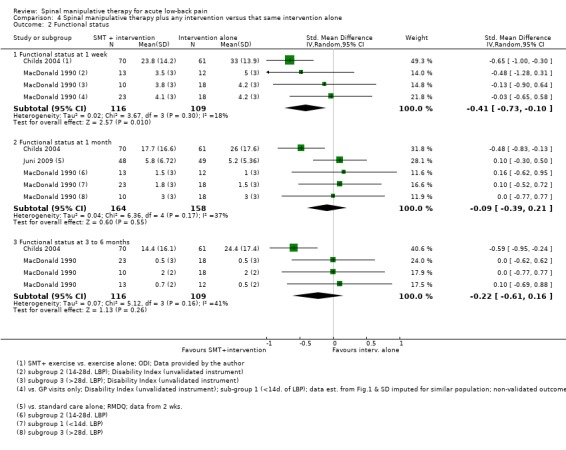
Comparison 4 Spinal manipulative therapy plus any intervention versus that same intervention alone, Outcome 2 Functional status.
Analysis 4.3.
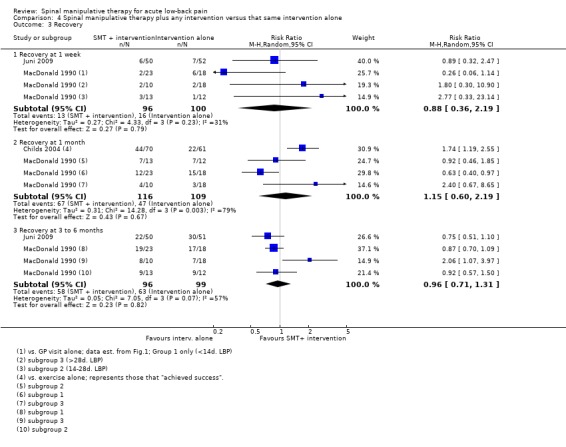
Comparison 4 Spinal manipulative therapy plus any intervention versus that same intervention alone, Outcome 3 Recovery.
Analysis 4.4.
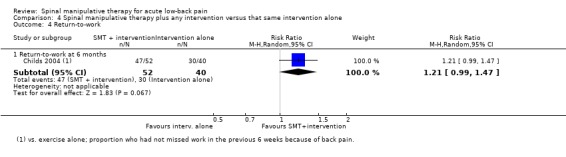
Comparison 4 Spinal manipulative therapy plus any intervention versus that same intervention alone, Outcome 4 Return‐to‐work.
Comparison 5.
Spinal manipulative therapy (SMT) versus another SMT technique
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Pain at 1 week | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain at 1 month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pain at 3 to 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional status | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Functional status at 1 week | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Functional status at 1 month | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Functional status at 3 to 6 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Recovery | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Recovery at 1 week | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Recovery at 1 month | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Recovery at 3 to 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 5.1.
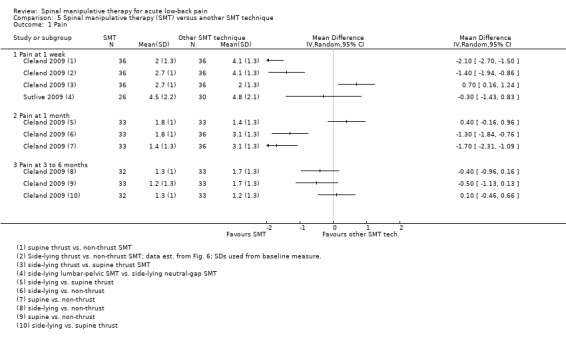
Comparison 5 Spinal manipulative therapy (SMT) versus another SMT technique, Outcome 1 Pain.
Analysis 5.2.
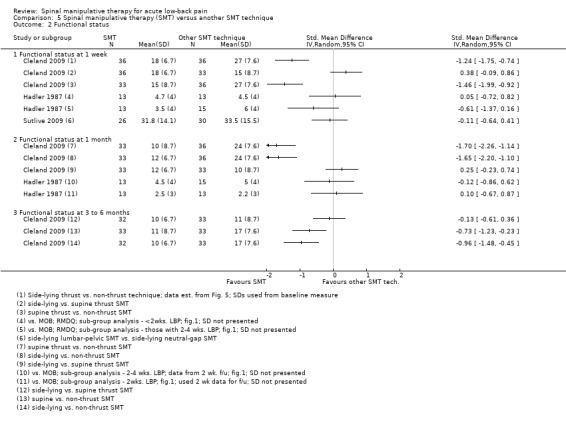
Comparison 5 Spinal manipulative therapy (SMT) versus another SMT technique, Outcome 2 Functional status.
Analysis 5.3.
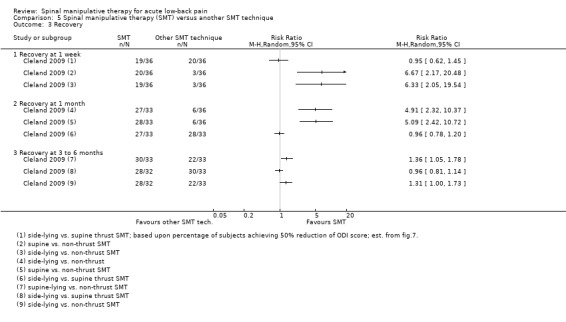
Comparison 5 Spinal manipulative therapy (SMT) versus another SMT technique, Outcome 3 Recovery.
Comparison 6.
SMT versus all comparisons ‐ for construction of funnel plot
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain ‐ For funnel plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Pain at 1 week | 6 | 704 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.82, 0.56] |
| 1.2 Pain at 1 month | 5 | 809 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.07, ‐0.06] |
| 1.3 pain at 3 to 6 months | 3 | 676 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.00, 0.17] |
| 1.4 Pain at 12 months | 1 | 314 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.08, 0.88] |
| 2 Functional status ‐ For funnel plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Functional status at 1 week | 6 | 683 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.59, ‐0.03] |
| 2.2 Functional status at 1 month | 9 | 1280 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.42, ‐0.03] |
| 2.3 Functional status at 3 to 6 months | 5 | 901 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.49, ‐0.02] |
| 2.4 Functional status at 12 months | 2 | 437 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.14, 0.25] |
Analysis 6.1.
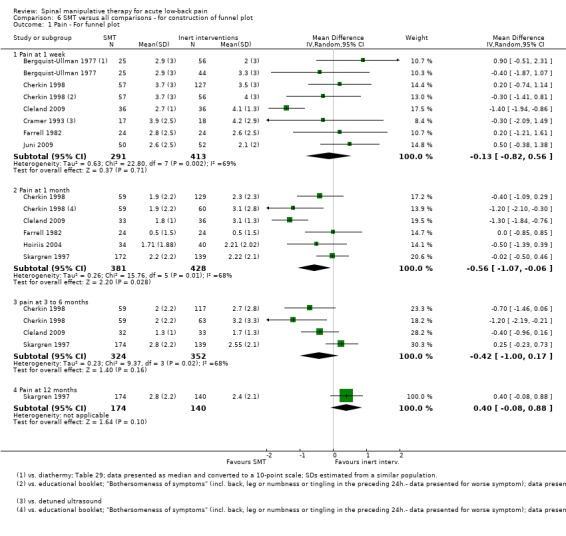
Comparison 6 SMT versus all comparisons ‐ for construction of funnel plot, Outcome 1 Pain ‐ For funnel plot.
Analysis 6.2.
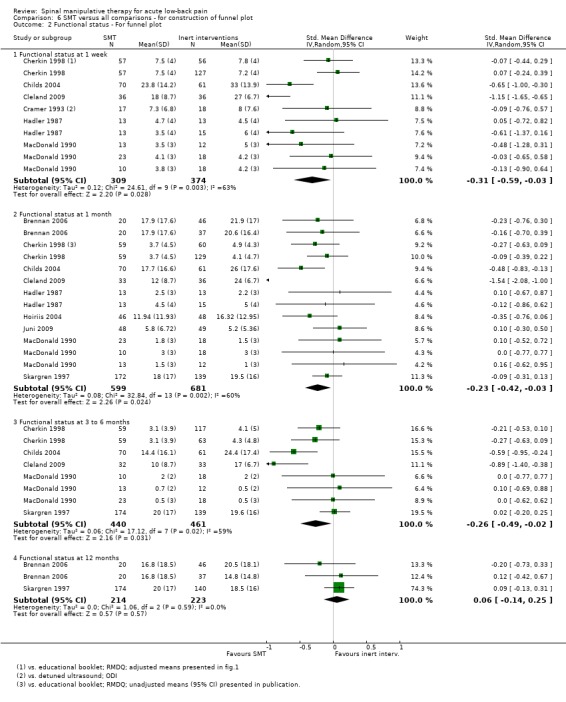
Comparison 6 SMT versus all comparisons ‐ for construction of funnel plot, Outcome 2 Functional status ‐ For funnel plot.
What's new
| Date | Event | Description |
|---|---|---|
| 14 November 2012 | Amended | Updated author affiliations |
Differences between protocol and review
An extra follow‐up measure was added to the data extraction and analyses because the authors realized that short‐term follow‐up (that is at one‐week) was an important interval that would have otherwise been missed. In addition, another comparison group was added, namely SMT versus another SMT technique. It did not seem correct to include these data with any of the other comparison groups.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Method of sequence generation considered adequate. Allocation concealment unclear. Statistical analysis: Contingency tables and Chi2 tests were used when comparing absence from work, number and length of recurrences in one year in the three groups of therapy. Differences in pain index were analysed using analysis of variance. A covariance analysis was used when comparing the duration of symptoms following the first treatment in the three groups (using logarithms of the values). No sample size determination was performed. |
|
| Participants | 217 subjects; study setting: occupational (Automotive Division of AB Volvo, mainly manual workers (light industrial work with a predominance of assembly‐line work) and clerks and executives; country: Sweden Period and mode of recruitment: via the healthcare centre of the company Age: median 34.5 years (range 17‐64) Gender: 13% women Inclusion criteria: Acute or subacute back pain localized to the lumbosacral region with or without radiation to the thigh; duration of pain not longer than three months; a pain‐free year before the onset of the current episode. Exclusion criteria: chronic pain; rhizopathy; pregnancy; spondylolisthesis; infections; tumours; ankylosing spondylitis; senile osteoporosis, structural scoliosis. |
|
| Interventions | I) Manipulation and mobilization according to Cyriax, Kaltenborn, Lewit and Janda (n=72); postural advice and strengthening exercises were also allowed; avg. number of treatments = 4 (max. 10). C1) Back school (including instruction and exercise) (n=70); avg. number of treatments = 4 sessions of 45 minutes given during a 2 week period. C2) Short‐wave diathermy (considered a placebo treatment) (n=75); avg. number of treatments = 5 (max. 10). |
|
| Outcomes | 1. Pain index (range: 0‐70) 2. Back specific functional status: 10 items, 4‐point scale 3. Recovery: not reported 4. Spinal mobility: via Schober's test 5. RTW: patient and insurance data based upon work absenteeism 6. Adverse events: not reported. Note: outcomes were not defined as primary or secondary Period of follow‐up: 10 days; 3, 6 weeks; 12 months |
|
| Notes | Authors' results and conclusions: 70% of the studied group recovered from the initial episode within two months and 86% within three months, regardless of the treatment given. The 95% confidence interval for the difference between the combined physiotherapy group (antilogarithms of the adjusted mean value 15.8 days) and the placebo group (28.7 days) was 0.59 ± 0.37. No difference was detected between the Back School group (14.8 days) and the combined physiotherapy group. There were significantly more patients with a shorter duration of sick‐leave in the Back School group compared to the placebo group (P<0.01). A similar decrease in the pain index was observed in the three groups. No relation was found between the type of treatment and the number of recurrences of pain or total duration of absence from work. "There is enough evidence in this study to conclude that Back School and combined physiotherapy are superior to "placebo" treatment in acute low back pain." Funded by: the Swedish Work Environment Fund (government) and AB Volvo (Industry) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation (based on vocational and psychologic factors). "Separate tables of random numbers were used" (p. 39). |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No mention of an attempt to blind the patients to therapy. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Care provider was not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient was not blinded; therefore, outcomes assessor also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Percentage drop‐out. At 10 days: Back school ‐ 37% (n=26/70); SMT ‐ 31% (n=22/72); Diathermy ‐ 24% (n=18/74) At 3 weeks: Back school ‐ 64% (n=45/70); SMT ‐ 74% (n=53/72); Diathermy ‐ 57% (n=42/74) At 6 weeks: Back school ‐ 80% (n=14/70); SMT ‐ 78% (n=56/72); Diathermy ‐ 80% (n=59/74) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | "22 patients were excluded from the analysis as they refused treatment. Four patients were initially randomly allocated to the physiotherapy group and 18 patients were allocated to the "placebo" group. Since a predominance of patients from the placebo group refused treatment, a separate comparison........ was carried out." |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available |
| Similarity of baseline characteristics? | Unclear risk | Not described |
| Co‐interventions avoided or similar? | Unclear risk | Not described |
| Compliance acceptable? | High risk | Seemingly differences between groups: "All patients attended all four sessions of the back school"; "...four patients (6%) allocated to manual therapy did not attend a single session"; "....16 patients (21%) of those allocated to diathermy did not follow treatment." |
| Timing outcome assessments similar? | Low risk | 1 year follow‐up |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Adequate sequence generation and allocation procedures. Statistical analysis: ANOVA for treatment group, and classification subgroup. ODI was the principal dependent variable. Last variable carried forward was used to impute missing data. Sample size calculation based upon determining a MCID (of 6 points) for the ODI. |
|
| Participants | 123 subjects; study setting: physical therapy clinics; Country: USA Period and mode of recruitment: Primary recruitment occurred at one clinic between January 1, 2000 and July 1, 2003. Additional recruitment occurred at two other clinics between January 1, 2002 and September 1, 2002. Age (all) (mean (SD)): 37.7 (10.7) yrs Gender (all) (% female): 45% Inclusion criteria: Patients between 18 and 65 years with a primary complaint of LBP of less than 90 days, with or without referral into the lower extremity, and an ODI>25% were eligible. Exclusion criteria were a visible lateral shift or acute kyphotic deformity, signs of nerve root compression (positive straight leg raise test and reflex or strength deficits), any red flags indicating a serious pathology such as spinal neoplasm, infection, or fracture, an inability to reproduce any symptoms with lumbar spine active range of motion (AROM) or palpation, current pregnancy, or prior surgery to the lumbar and/or sacral region. |
|
| Interventions | I) Manipulation (n=40): manual therapy techniques that could include thrust manipulation, or low amplitude mobilization procedures directed to the lumbosacral region, along with instruction in a lumbar AROM exercise. The therapist performing the treatment was permitted to reexamine the patient and could choose one of two manual therapy techniques. The choice of which technique to use was left to the therapists’ discretion, but one of the two techniques had to be used. In the first technique, the patient was supine, with the lumbar spine placed into side‐bending and rotation to the opposite direction. The therapist delivered a force through the patient’s pelvis in a posterior and inferior direction. For the second technique, the patient was side‐lying. The lumbar spine was positioned in either flexion or extension followed by rotation in an attempt to isolate forces to a particular spinal level. The therapist delivered the force through the patient’s pelvis and trunk. The choice of technique was left to the discretion of the therapist. The AROM exercise was performed by instructing the patient to alternately flex and extend the lumbar spine while in a quadruped position. C1) Specific exercise (n=37): received instruction in repeated ROM exercises into either lumbar flexion or extension. All patients in this group had to be treated with directional exercises; however, the direction of the exercise was determined by the treating therapist based on a reassessment of the patient’s response to movement testing and symptom response to positions of sitting, standing, or walking. Flexion exercises were used for patients who centralized with or had a preference for flexion movements or positions (i.e., sitting), whereas extension exercises were used for patients who centralized or had a preference for extension (i.e., standing or walking). Either flexion or extension exercises were used, but not both. Flexion exercises were performed with the patient sitting, supine, or quadruped. Extension exercises were performed in prone, using prone on elbows or prone press‐up activities. C2) Stabilization (n=46): treated with a program of trunk strengthening and stabilization exercises. Patients were instructed to perform abdominal bracing exercises in supine and quadruped positions, progressing to more functional positions and activities. Patients were also instructed in alternating arm and leg extension exercises in quadruped to strengthen the lumbar extensor muscles. Strengthening for the oblique abdominals included curl‐up and side support exercises. |
|
| Outcomes | 1. Pain: 11‐pt. NRS, current pain 2. Back specific functional status: modified ODI 3. Recovery: not reported 4. FABQ: including two subscales (work and physical activity) 5. Adverse events: not reported. Note: outcomes were not designated as primary or secondary. Follow‐up: at completion of treatment (˜4 wks. after baseline assessment), 1 year. |
|
| Notes | Authors results and conclusions: Patients receiving matched treatments experienced greater short‐ and long‐term reductions in disability than those receiving unmatched treatments. After 4 weeks, the difference favouring the matched treatment group was 6.6 ODI points (95% CI, 0.70–12.5), and at long‐term follow‐up the difference was 8.3 points (95% CI, 2.5–14.1). Compliers‐only analysis of long‐term outcomes yielded a similar result. Conclusions. Nonspecific low back pain should not be viewed as a homogenous condition. Outcomes can be improved when subgrouping is used to guide treatment decision‐making. Funded by Deseret Foundation (non‐profit). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random number generator was used to generate a randomization list before initiation of the study. The list was maintained by the secretarial staff of the participating clinics." |
| Allocation concealment (selection bias) | Low risk | "Before the first treatment session, the secretarial staff consulted the randomization list and assigned the patient to one of three groups." |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No attempts were made to blind the patient. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | No attempts were conducted to blind the provider. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient was not blinded; therefore, outcome assessor is also not blinded. Physiological measures were examined, including lumbar active ROM, judgement of centralization or peripheralization, aberrant movements occurring during lumbar active ROM (indicating poss. instability), and mobility of each level of the lumbar spine was assessed. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | "66% (n=81) completed the long‐term follow‐up, with no differences in the median number of days between baseline and follow‐up or the proportions of patients with completed follow‐up between patients receiving the matched or unmatched treatments. There were no significant differences between those with complete or incomplete long‐term follow‐up with respect to age, sex, duration of symptoms, baseline pain, OSW, or FABQ scores (P>0.05). The proportion of matched versus unmatched patients did not differ between those with complete or incomplete long‐term follow‐up (P>0.05)." However, patients with complete long‐term follow‐up did have lower 4‐week OSW scores (17.3 vs. 25.0, P=0.02). |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Both ITT and compliers‐only analyses were conducted and are presented in Table 2 and the ITT analysis (for matched and unmatched treatment) is presented in Fig.3. ITT analyses used the last available OSW score carried forward for missing data. A large proportion of patients were lost to the long‐term follow‐up, so a compliers‐only analysis was conducted, including only those patients completing the 1‐year OSW score. This item was scored negatively because data from T.3, which represents the compliers‐only data, were used for data extraction purposes in this review. These data represent randomization without matching. This point is discussed further in the review. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available. Outcomes were presented separately for functional status by type of randomized treatment group and by classification subgroup, but not for pain (scores were presented only for those receiving the matched and unmatched treatments). |
| Similarity of baseline characteristics? | Unclear risk | Data presented only by those receiving matched and unmatched treatments and not by allocation group. Baseline variables presented: age, gender, education level, prior history of LBP, symptoms distal to the knee, duration of current symptoms, missed work or school days related to the current LBP episode, FABQ ‐ work and physical activity sub‐scales, ODI, pain. |
| Co‐interventions avoided or similar? | Unclear risk | Note: This was not stated or not measured. |
| Compliance acceptable? | Low risk | "All patients were scheduled for treatment twice weekly for 4 weeks for a maximum of eight sessions." "Median number of sessions attended by the matched group was 6.5, and 80% attended at least 4 sessions. In the unmatched group median number of sessions was 7, and 77% attended at least 4 sessions". |
| Timing outcome assessments similar? | Low risk | At completion of treatment and 1‐year follow‐up. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Sequence generation and allocation considered adequate. Statistical analysis: For dichotomous outcomes, logistic regression was used with adjustment for baseline values. Sample size calculation was based upon determining a MCID (2.5 points) for the RMDQ and (1.5 points) for the bothersomeness scale. |
|
| Participants | 321 subjects; Study setting: Physical therapy clinics situated within a health maintenance organization (HMO) and SMT was performed in the private practices of chiropractors; Country: USA. Period and mode of recruitment: Patients were recruited from primary care clinics; November 1993 to September 1995. Age (mean (SD) (all): 40.7 (10.7) years (range across groups: 39.7 (9.4) to 41.8 (11.5) Gender (% female) (all): 48% (range across groups: 42% to 53%) Inclusion criteria: 20 to 64 years of age who saw their primary care physician for low back pain and who still had pain seven days later. Exclusion criteria: mild or no pain seven days following the visit to the physician, a history of back surgery, sciatica, systemtic or visceral causes of the pain, osteoporosis, a vertebral fracture or dislocation, severe neurologic signs, spondylolisthesis, coagulation disorders, or a severe concurrent illness. Subjects who had received corticosteroid therapy, were pregnant, were involved in claims for compensation or litigation because of the back injury, had received physical therapy or chiropractic or osteopathic manipulative treatment for their current back pain, or visited practitioners other than their primary care physicians were also excluded. |
|
| Interventions | I) SMT (n=122): The most common method of chiropractic manipulation was used: a short‐lever, high‐velocity thrust directed specifically at a “manipulable lesion.” This procedure is typically performed with the patient lying on his or her side on a segmental table. No other physical treatments were permitted. Chiropractors evaluated patients according to their usual procedures and were allowed to make the same recommendations about exercise and activity restrictions that they usually did. An exercise sheet was used that emphasized stretching and strengthening but excluded extension exercises, an important part of McKenzie therapy. C1) Physical therapy (n=133) following McKenzie principles: Patients were taught to perform exercises that centralize their symptoms and to avoid movements that peripheralize them. This method relies on patient‐generated forces and emphasizes self‐care. McKenzie Institute faculty trained the therapists before the study, and all but one therapist passed an advanced McKenzie credentialing examination. Subjects received McKenzie’s Treat Your Own Back book and a lumbar‐support cushion. Therapists were asked to avoid adjuncts such as heat, ice, transcutaneous electrical nerve stimulation, ultrasonography, and back classes. C2) Educational booklet (n=66): A minimal‐intervention control group received an educational booklet to minimize potential disappointment with not receiving a physical treatment. The booklet discussed causes of back pain, prognosis, appropriate use of imaging studies and specialists, and activities for promoting recovery and preventing recurrences. A previous trial found that the use of this booklet as a supplement to standard care was not associated with improved outcomes. This group was deemed to be similar in some respects to a no‐treatment control group. |
|
| Outcomes | 1. Pain: "bothersomeness" of back pain, leg pain, and numbness or tingeling in the preceding 24 hours; 0‐10 pt. scale (unclear if this was a VAS or NRS) 2. Back specific functional status: RMDQ 3. Recovery: 5‐point scale, ranging from poor to excellent 4. RTW: number of days spent home from work or school 5. Other: number of days spent in bed or with reduced activity (specifically with reference to the back) 6. Adverse events: "No important adverse events of treatment were reported in any of the group" Reviewers note: outcomes were not defined as primary or secondary. Comment reviewers: The score for the most bothersome symptom was used. It is unclear if this was a different measure within the groups at each of the follow‐up measures nor whether it was different between the groups. Follow‐up: 1, 4, 12 weeks; 1 to 2 years. |
|
| Notes | Authors results and conclusions: The McKenzie method of physical therapy and chiropractic manipulation had similar effects (based upon pain and functional status) and costs and patients receiving these treatments had only marginally better outcomes than those receiving the minimal intervention of an educational booklet. Note reviewers: "At both one and four weeks, about 75 percent of the subjects in the physical‐therapy and chiropractic groups rated their care as “very good” to “excellent,” as compared with about 30 percent of the subjects in the booklet group (P<0.001). However, about one quarter of the subjects in the booklet group failed to answer this question, possibly because only 18 percent received care during this period." Funded by: Agency for Health Care Policy and Research (Governmental) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned without stratification .... with the use of sealed, opaque envelopes." |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | There is no mention of attempts to blind the patients to other interventions or their perceptions of potential effectiveness of the different interventions. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | No mention if there were any attempts to blind the care providers to the other groups. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient was not blinded; therefore, this item was scored as "no". |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | "Between 89 and 96 percent of the subjects responded to each of the follow‐up questionnaires." Reviewers note: There was minimal drop‐out and differences between groups, however, no reasons were offered for those who dropped out or were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | "Two subjects were excluded after randomization (one because of a urinary tract infection and one because of pancreatic cancer)." Reviewers note: it was not stated to which group they belonged. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Similarity of baseline characteristics? | Low risk | Variables examined: age, gender, employment, smoker (y/n), general health and mental health perceptions, history of LBP (prior episodes, prior chiropractic and PT care), duration and intensity of current LBP (including pain and functional status), medication usage and expectations of recovery. |
| Co‐interventions avoided or similar? | High risk | Eighteen per cent of the subjects in the booklet group visited a healthcare provider for back pain during the study month, and only 8% of the subjects in the chiropractic group and 9% of those in the physical‐therapy group visited providers other than those assigned. The reported use of exercise was almost identical in the three groups at baseline (about 57 percent) and one month (about 81%). During the month, the percentage of subjects who used back‐pain medication of any type decreased from 82% to 18% in the chiropractic group, from 84% to 27% in the physical‐therapy group, and from 77% to 32% in the booklet group (P<0.05 for the differences among the groups after adjustment for baseline use). Fewer than 2% of the subjects reported using corsets, braces, traction, transcutaneous electrical nerve stimulation, or injections. |
| Compliance acceptable? | Unclear risk | "96% of the chiropractic group and 97% of the physical therapy group visited their assigned provider at least once. The mean number of chiropractic visits exceeded the mean number of physical therapy visits by 50% (6.9 vs. 4.6). According to the subjects' reports, the total amount of time spent with the provider was virtually identical in the two groups (about 145 minutes)." |
| Timing outcome assessments similar? | Low risk | Follow‐up: 1, 4, 12 weeks; 1 to 2 years |
| OVERALL RISK OF BIAS | Low risk | |
| Methods | Adequate sequence generation and allocation concealment. Statistical analysis: Baseline variables between groups were compared using independent t‐tests or Mann‐Whitney U tests for continuous data and Chi2 tests of independence for categorical data. ANOVA was used to examine treatment effect with treatment group, status on the clinical prediction rule as between‐patient variables and time as the within‐patient variable. Potential confounders were controlled for in the modelling. Sensitivity, specificity, positive and negative likelihood ratios were calculated to describe the accuracy of the prediction rule. Number needed to treat (NNT) was also calculated. Sample size calculation was based upon determining a MCID (0.3 effect size) for the ODI. |
|
| Participants | 131 subjects; Study setting: 14 physical therapists in 8 clinics, including 2 academic medical centers and smaller outpatient practices. Country: USA. Period and type of recruitment: March 2002 through March 2003; recruitment was via the clinics Age, y (All, mean (SD)): 33.9 (10.9) Gender (% F): 42% Inclusion criteria: Age 18 to 60 years; a primary symptom of low back pain, with or without referral into the lower extremity; and an ODI>30%. Exclusion criteria: patients who had 'red flags' for a serious spinal condition (for example, tumor, compression fracture, or infection), those who had signs consistent with nerve root compression (that is, positive straight‐leg increase 45 º or diminished reflexes, sensation, or lower‐extremity strength), those who were pregnant, or those who had previous surgery to the lumbar spine or buttock. |
|
| Interventions | I) SMT (n=70): During the first 2 sessions, patients received high‐velocity thrust spinal manipulation and a range‐of‐motion exercise only. First, the physical therapist performed the manipulation by using the same technique used by Flynn and colleagues. Patients were also instructed to perform 10 repetitions of the range‐of‐motion exercise in the clinic and 10 repetitions 3 to 4 times daily on the days they did not attend physiotherapy. Beginning with the third session, patients in the SMT group completed the same exercises as in the comparison (exercise) group. C) Exercise (n=61): We treated patients in the exercise group with a low stress aerobic and lumbar spine strengthening program. The strengthening program was designed to target the trunk musculature identified as important stabilizers of the spine in the biomechanical literature. An aerobic exercise component was also included. Patients began with a goal of 10 minutes of aerobic exercise on a stationary bike or treadmill at a self‐selected pace. The exercise program progressed according to criteria previously described. |
|
| Outcomes | Primary outcome: 1. Back specific functional status: ODI Secondary outcomes: 2. Pain: 11‐point NRS; current pain, best and worst level of pain in the previous 24 hours 3. Recovery: classified as successful if there was at least 50% improvement, all others were classified as non‐successful, based upon % change in the ODI 4. RTW: whether days had been missed at work in the prior 6 weeks due to LBP 5. Medication use: whether medication had been used in the previous week for LBP 6. Adverse events: not reported. Follow‐up: 1, 4 weeks and 6 months. |
|
| Notes | Authors results and conclusions: Outcome from spinal manipulation depends on a patient’s status on the prediction rule. Treatment effects are greatest for the subgroup of patients who were positive on the rule (at least 4 of 5 criteria met); health care utilization among this subgroup was decreased at 6 months. Compared with patients who were negative on the rule and received exercise, the odds of a successful outcome among patients who were positive on the rule and received manipulation were 60.8 (95% CI, 5.2 to 704.7). The odds were 2.4 (CI, 0.83 to 6.9) among patients who were negative on the rule and received manipulation and 1.0 (CI, 0.28 to 3.6) among patients who were positive on the rule and received exercise. A patient who was positive on the rule and received manipulation has a 92% chance of a successful outcome, with an associated number needed to treat for benefit at 4 weeks of 1.9 (CI, 1.4 to 3.5). Conclusions: The spinal manipulation clinical prediction rule can be used to improve decision making for patients with low back pain. Funded by: The Foundation for Physical Therapy, Inc., and the Wilford Hall Medical Center Commander’s Intramural Research Funding Program (non‐profit). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used a random‐number generator to generate a randomization list before the study began. We prepared individual, sequentially numbered index cards with the randomization assignments. We folded the cards and placed them in sealed envelopes. After the baseline examination, the physical therapist who conducted the examination opened the next envelope, indicating the treatment group assignment." |
| Allocation concealment (selection bias) | Low risk | See above. Clarification was requested from the authors regarding the actual allocation and the following was the response: "The individual who performed the randomization process was independent from the study. The PT who performed the baseline examine merely picked the next envelope in the stack, but the randomization itself had already been determined. The treating therapist was a different individual and had nothing to do with the allocation process." |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No mention of blinding the patient to allocation. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Provider not blinded |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patients were not blinded; therefore, outcome assessors were also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Loss to follow‐up. 1 week f/u: SMT: 0% (n=0/70) & none discontinued treatment; Exercise: 0% (n=0/61) & 6 discontinued treatment 4 week f/u: SMT: 0% (n=0/70) & 2 discontinued treatment; Exercise = 2% (n=1/61) & 3 discontinued treatment 6 mo. f/u: SMT: 26% (n=18/70); Exercise: 34% (n=21/61) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | No patients were removed from the analysis due to non‐adherence. No attempt was made to impute missing data. |
| Selective reporting (reporting bias) | Unclear risk | No mention of a published protocol. |
| Similarity of baseline characteristics? | Low risk | Analysed for age, gender, BMI, history of smoking, history of LBP, previous improvement w/ manipulation for LBP, duration of current symptoms, medication use for LBP, missed work for LBP, symptoms distal to the knee, FABQ (physical activity and work sub‐scale), ODI, and pain. |
| Co‐interventions avoided or similar? | Unclear risk | Not stated |
| Compliance acceptable? | High risk | 2 subjects (3%) in the SMT group discontinued treatment at 4 wks; 9 subjects (15%) in the exercise group discontinued treatment at 4 wks. (including the 6 who discontinued earlier at 1 wk). |
| Timing outcome assessments similar? | Low risk | At 1, 4 weeks and 6 months. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Adequate sequence generation and allocation. Statistical analyses: A linear mixed model for repeated measures was used. Time and treatment group were modelled as fixed effects with the ODI as the dependent variable. Missing data was imputed with the last value carried forward method. Separate linear models were also constructed with pain and recovery. Differences between practice settings and proportion of subjects reporting side effects were examined. Sample size calculation was based upon determining a MCID (30% difference from baseline) for the ODI. |
|
| Participants | 112 subjects; Study setting: United States Military Health System and outpatient physiotherapy clinics associated with a hospital, a health care system or the University of Southern California; Country: USA Period and mode of recruitment: subjects were recruited over a 28‐month period (June 2005 to September 2007) via four physiotherapy outpatient clinics. Age (mean, SD), all subjects: 40.3 (11.5) Gender (% F, all subjects): 52% Inclusion criteria: a modified ODI>25%, between 18 and 60 years of age, and to be positive for the SMT clinical prediction rule, which required the presence of at least 4 of 5 findings (i.e. <16 days of LBP; no symptoms distal to the knee; <19 points on the FABQ‐W sub‐scale; >1 hypomobile segment in the lumbar spine; at least one hip with >35° of internal rotation range of motion. Exclusion: Red flags (i.e. tumor, metabolic diseases, RA, osteoporosis, prolonged history of steroid use, etc.); Signs consistent with nerve root compression (e.g. reproduction of low back or leg pain with straight leg raise at less than 45°, muscle weakness involving a major muscle group of the lower extremity, diminished lower extremity muscle reflex, diminished or absent sensation to pinprick in any lower extremity dermatome); Prior surgery to the lumbar spine or buttock; Current pregnancy; Past medical history of osteoporosis or spinal compression fracture; Inability to comply with treatment schedule (weekly sessions for four weeks). |
|
| Interventions | I) Supine thrust SMT (n=37): This treatment group received the manipulation technique that was used in the development and validation of the CPR. The technique is performed with the patient supine. The therapist stands on the side opposite of that to be manipulated. The patient was passively moved into side‐bending towards the side to be manipulated. The patient interlocks the fingers behind his or her head. The therapist passively rotates the patient, then delivers a high velocity, low amplitude thrust to the anterior superior iliac spine in a posterior and inferior direction. A maximum of two attempts per side were permitted in order to achieve joint cavitation. C1) Side‐lying thrust SMT (n=38): The patient was side‐lying with the more painful side up. The therapist flexed the top leg until movement was palpated at the selected segment interspace. The therapist then grasped the patient’s bottom shoulder and arm and introduced side bending and rotation until motion was felt at the selected interspace. Setup was maintained while the patient was rolled toward the therapist. Finally the therapist applied a high‐velocity, low amplitude thrust of the pelvis in an anterior direction. As with the previous technique, a maximum of two attempts per side were permitted. C2) Non‐thrust SMT (n=37): received central lumbar posterior‐anterior nonthrust manipulation procedures directed at L4 and L5. The therapist placed the hypothenar eminence of 1 hand over the spinous process of L4. With the elbows remaining extended, the therapist delivered a low‐velocity, high amplitude oscillatory force (at approximately 2 Hz) directed at L4 for a total 60 seconds. Following a 30‐second rest the therapist performed a similar set of oscillations directed at L5. A second set of oscillations was then performed in a similar manner at L4 and L5. Treatment: Treatment for the 3 groups differed only during the first 2 sessions that were received within the first week after randomization. During these sessions patients received the manual therapy technique to which they were randomized, and a spinal range of motion (ROM) exercise that was common to all groups. Following the first 2 sessions all patients received the same standardized exercise regimen for 3 additional sessions (once weekly for 3 weeks) for a total of 5 treatment sessions over a 4‐week period. |
|
| Outcomes | Primary outcome: 1. Back specific functional status: ODI Secondary outcomes: 2. Pain: current, best and worst levels of pain in the previous 24hours via 11‐point NRS (the avg. of the 3 ratings was used to represent the patients' level of pain) 3. Recovery: defined as 50% improvement on the ODI compared to baseline 4. Other: Fear Avoidance Beliefs Questionnaire (FABQ): the 2 subscales (FABQW and FABQPA) were examined separately 5. Adverse events: "Overall, 28 patients (25%) reported at least one side effect. The percentage did not differ between treatment groups. The most common side effect reported for all groups was aggravation of symptoms, followed by stiffness. All reported side effects began within 4 hours of treatment and were resolved within 48 hours of onset. No serious complications were reported by any patients." Follow‐up: 1, 4 wks.; 6 months |
|
| Notes | Authors results and conclusions: Pair‐wise comparisons revealed no differences between the supine thrust manipulation and side‐lying thrust manipulation at any follow‐up period. Significant differences in the ODI and NRS existed at each follow‐up between the thrust manipulation and the non‐thrust manipulation groups at 1‐week and 4‐weeks. There was also a significant difference in ODI scores at 6‐months in favor of the thrust groups. Conclusion: The results of the study support the generalizability of the CPR to another thrust manipulation technique, but not to the non‐thrust manipulation technique. In general, our results also provided support that the CPR can be generalized to different settings. Funded by: Franklin Pierce University; University of Southern California, Los Angeles, USA (non‐profit). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "....a computer generated randomized table of numbers created for each participating site before the beginning of the study. Individual, sequentially numbered index cards with the random assignment where prepared. The index cards were folded and placed in sealed opaque envelopes." |
| Allocation concealment (selection bias) | Unclear risk | Reviewers note: Unclear who actually was involved in the treatment assignment. |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Unclear what was told to the patient. All patients had similar expectations regarding the effect of manipulation (Table 2). |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Practitioners were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | See blinding patient |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Loss to follow‐up. 1 week f/u: Supine thrust ‐3% (1/37); side‐lying thrust ‐ 5% (2/38); non‐thrust ‐ 3% (1/37) 4 week f/u: Supine thrust ‐ 11% (4/37); side‐lying thrust ‐ 13% (5/38); non‐thrust ‐ 3% (1/37) 6 month f/u: Supine thrust ‐ 11% (4/37); side‐lying thrust ‐ 16% (6/38); non‐thrust ‐ 11% (4/37) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Last value carried forward was used for imputing missing data. |
| Selective reporting (reporting bias) | Low risk | Protocol available: ClinicalTrials.gov (NCT00257998). Reviewers note: None of the secondary outcomes (i.e. pain reduction, recovery) are listed in the trial registry. |
| Similarity of baseline characteristics? | Low risk | Analysed for: age, gender, symptom duration, BMI, outcomes (pain, ODI, FABQ), current medication usage, prior history of LBP, missed work in the prior 6 wks. due to LBP, currently unable to work, current smoker, believe manipulation would improve symptoms. The supine thrust manipulation group had a significantly higher BMI than the side‐lying manipulation group. |
| Co‐interventions avoided or similar? | Unclear risk | Not stated. |
| Compliance acceptable? | Unclear risk | All patients were treated with the same strengthening and stabilization exercises and they were requested to complete the strengthening program daily on they days they did not attend physiotherapy; however, it is unclear if this was assessed post‐treatment. |
| Timing outcome assessments similar? | Low risk | At 1, 4 weeks; 6 months |
| OVERALL RISK OF BIAS | Low risk | |
| Methods | Method of sequence generation and allocation unclear. Statistical analyses: Presented as mean differences (pre‐post testing); t‐Tests used to test for significance. No sample size calculation was performed. |
|
| Participants | 36 subjects; Study setting: outpatient clinic; Country: USA. Period and mode of recruitment: those presenting to the clinic; otherwise not stated. Age (all subjects): 18 to 56 years of age Gender (% female; all subjects): 42% Inclusion criteria: Mechanical low‐back pain less than 2 weeks duration; ODI > 8; VAS > 33 mm; no litigation or workers' compensation; not pregnant. Exclusion criteria: Subjects with clinical evidence of a compressive neuropathy. |
|
| Interventions | I) SMT (n=17): included a side‐lying manipulation to the affected area of the lumbar spine; additional treatment consisted of electrical stimulation and cold‐packs to the lumbar spine (L3‐S1). C) control (n=19): included detuned ultrasound to the low back followed by cold packs (5‐10 min.) and very gentle soft tissue massage (15‐30 sec.). Treatments for both groups were delivered 3 to 5 times over a 10‐day period. |
|
| Outcomes | 1. Pain: 0‐100 (VAS) 2. Back specific functional status: ODI 3. Recovery: not reported 4. Adverse events: not reported 5. Hmax/Mmax ratio were measured. No mention of which were primary or secondary; however, the basis of the report was the Hmax/Mmax ratio. Follow‐up: Pre‐ and post‐testing; Reviewers note: personal communication with the primary author: post‐treatment measurement represents the measurement following the 10‐day period. |
|
| Notes | Authors results and conclusions: The H/M ratio (reviewers: "an objective and clinically useful physiological measure for acute low back pain...") showed greater change in the group which received spinal manipulation, but the change was subtle. The results indicate that the H/M ratio may be of limited value in clinical practice. Funded by: Foundation for Chiropractic Education and Research (non‐profit). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reviewers comment: No mention of the sequence generation procedure nor method of allocation. The abstract indicates that it is a randomized trial and a flow chart is provided suggesting that randomization occurred. |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No mention of any attempts to blind the patient. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | No mention of any attempts to blind the provider. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Outcomes assessor was not blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Unclear, but the tables suggest that all subjects were retained in the study. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Unclear, but presumably all subjects were analysed by allocation treatment and there were no drop‐outs. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Recovery not reported, although selective reporting not likely. |
| Similarity of baseline characteristics? | Unclear risk | Not stated |
| Co‐interventions avoided or similar? | Unclear risk | Not stated |
| Compliance acceptable? | Unclear risk | Not stated |
| Timing outcome assessments similar? | Low risk | Collected post‐treatment over a 10‐day period. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Unclear methods of sequence generation and allocation. Statistical analysis: ANCOVA; number of days required to reach symptom free status (assessed as number of subjects symptom‐free at fixed points (1, 2, 3, and 4 weeks following the first visit). No sample size calculation was performed. |
|
| Participants | 48 subjects; Study setting: private clinics? of physiotherapists; Country: (Western) Australia. Period and mode of recruitment: family‐oriented general practice. Age, y (mean): SMT group = 43.4; comparison group = 41.8 Gender (% F): SMT group = 33%; comparison group = 42% Inclusion criteria: 20 to 65 years of age; pain with lumbar movement or straight leg raising; pain (intermittent or constant) centrally or para vertebrally between T12 and the gluteal folds; symptoms of 3 weeks duration or less; and experienced a pain‐free period of 6 months before the onset of the current episode. Exclusion criteria: other physical treatment for the current episode of LBP; pregnancy; signs of cauda‐equina pressure or altered sensation, reflexes, or muscle weakness in the lower extremity; previous surgery in the lumbar region; past history of fracture in the lower thoracic/lumbar region; evidence of systemic disease, such as rheumatoid arthritis, ankylosing spondylitis or carcinoma. |
|
| Interventions | I) SMT (n=24): passive mobilization and manipulation. The choice of technique included 1) central, posterior‐anterior pressures, 2) unilateral, posteroanterior pressures over the transverse processes, 3) transverse pressures on spinous processes, and 4) mobilization. These techniques have been described by Stoddart and Maitland. C) Physiotherapy (n=24): 1) 15 min. of microwave diathermy, 2) 10 repetitions of isometric abdominal exercises (which the subject was request to perform another 3 to 4 times per day), and 3) ergonomic instructions, including advice on lifting, sitting, standing, carrying objects and rest postures. Each subject was treated 3 times per week for up to 3 weeks. Treatment was discontinued if the subject met the criteria for discharge, which occurred for 8 subjects. Treatment was continued beyond 3 weeks, if necessary. |
|
| Outcomes | 1. Pain: 0 to 10 (unclear if a VAS or NRS) 2. Back specific functional status: according to the questionnaire by Berquist‐Ullman and Larsen (a list of 10‐different functional activities) 3. Recovery: according to the following criteria: 1) subject could perform all functional activities without difficulty, 2) his subjective pain rating was very low (0 or 1 on the 11‐point scale), 3) the objective measures of lumbar movements and straight leg raising were pain‐free, with passive overpressure at the extreme of the patient's active range; Physiological measures: active range of motion and straight leg raising 4. Adverse events: not reported Reviewers note: outcomes were not defined as primary or secondary. Follow‐up: following the first and third treatment, and at 3 weeks. |
|
| Notes | Authors results and conclusions: The duration of LBP symptoms was significantly shorter for subjects receiving mobilization and manipulation. They also achieved symptom‐free status with fewer treatment sessions. Funded by: Spinal Pain Research Foundation of the Western Australian Manipulative Therapy Association (non‐profit). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "....subjects were placed at random into two groups .....". (comment: no other description was provided). |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No mention of any attempt to blind the patients to the procedures. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Provider not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patients were not blinded; therefore, outcomes assessor was also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Drop‐out seems minimal, although reasons for non‐reporting of data are not given: 3 subjects (13%) from the comparison group and 1 subject (4%) from the SMT group were not assessed for symptom‐free status. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | The number of drop‐outs seems minimal and presumably assessed according to allocated assignment, although remains unclear. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Similarity of baseline characteristics? | Low risk | Age, gender, and baseline pain seem roughly similar. No other baseline measures are presented. |
| Co‐interventions avoided or similar? | Unclear risk | Not stated. Treatment was continued beyond 3 weeks if necessary, but not recorded. Unclear if patients sought other therapies. |
| Compliance acceptable? | Unclear risk | The SMT group required 3.5 (1.6) treatments to reach pain‐free status, while the comparison group required 5.8 (2.3) treatments to achieve the same result. |
| Timing outcome assessments similar? | Low risk | Principal outcome measure ‐ days required to reach symptom‐free status. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Adequate sequence generation, but unclear allocation procedure. Statistical analysis: Mann‐Whitney (non‐parametric test). No sample size calculation was performed. |
|
| Participants | 84 subjects (total) (44 subjects w/ LBP <7d.); Study setting: industry ("medium‐sized engineering works") over a period of 15 months; country: UK Age (mean): 34 to 47y. (range for those w/ LBP <7d.) Gender (%F): 11% (of those w/ LBP <7d.) Inclusion criteria: Back pain between the inferior angle of the scapula and the lower end of the sacrum. Exclusion criteria: Bilateral pain and hyperaesthesia; those under treatment by another doctor; abnormal radiological or neurological signs. |
|
| Interventions | I) SMT (n=21 (w/ LBP <7d.)): one lumbar rotational manipulation session of 15 min. or less followed by four daily detuned short‐wave diathermy sessions of 15 min. C) Detuned short‐wave diathermy only (n=23 (w/ LBP <7d.): five 15 min. daily sessions of detuned short‐wave diathermy only. |
|
| Outcomes | 1. Pain: recorded as a percentage of the original pain (ranging from 0% (no relief) to100% (complete relief)) 2. Back specific functional status: not reported 3. Recovery: 3 pt. scale (better, worse, same) ‐ measured but not reported 4. Other: skin hyperaesthesia, deep tenderness, restriction of straight leg raising, forward flexion of the spine. Reviewers note: these other outcomes were to be reported in a subsequent publication, however, no such publication was identified. 5. Adverse events: not reported Reviewers note: outcomes were not defined as primary or secondary. Follow‐up: Days 3 ,7; results from 1 month were not reported. |
|
| Notes | Authors results and conclusions: There was no demonstrable difference between the intervention (SMT) and control groups, except that at the 15‐minute stage, the relief from pain in the manipulated group was always greater than in the controls. Funded by: Nuffield Foundation, UK ("covered the salaries" of the principal researchers (non‐profit)). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated to the manipulation or control series in accordance with a pre‐arranged sequence for that subgroup (reviewers note: four subgroups were made based upon duration of the pain (<7days, >7 days), based on random sampling numbers and contained in a set of sealed envelopes." |
| Allocation concealment (selection bias) | Unclear risk | Note: The person involved in the allocation was also involved in the treatment of the subjects, so it is uncertain to what extent he might have influence the allocation procedure. |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No mention of attempting to blind patients to the intervention. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Provider not blinded |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Subjects were not blinded; therefore, outcomes assessor was also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Data presented for the first week only. Unclear what proportion of subjects dropped out beyond this interval because this data is not presented (although it was reportedly measured). |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Not explicitly stated and difficult to assess for follow‐up beyond one week. |
| Selective reporting (reporting bias) | High risk | Protocol available in which the outcomes are described and time of measurement. "All cases were followed up for one month or more." (Reviewers note: Yet these data are not reported; the author only reports the short‐term (1‐week) follow‐up data. In addition, the authors present only those outcomes related to pain and not the other outcomes, such as those related to hyperaesthesia, tenderness or range of motion). |
| Similarity of baseline characteristics? | Unclear risk | Baseline data reported: age, gender (only). No indication of severity of baseline pain, functional status, etc. |
| Co‐interventions avoided or similar? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported. |
| Timing outcome assessments similar? | Low risk | At 3 and 7 days f/u |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Method of sequence generation and allocation are unclear. Statistical analysis: ANOVA; effects of interaction with time and treatment was examined. No sample size calculation was performed. |
|
| Participants | 54 subjects (excluding one subject who was dropped from the analysis because he/she was inaccessible to follow‐up); Study setting: hospital clinic?; Country: USA. Period and mode of recruitment: via primary physicians from the local community and advertising in the local newspapers; period ‐ not stated. Age (presented as strata, 20 to 29 and 30 to 40 years of age): Manipulation group ‐ 54% (between 20‐29 y/o); Mobilization group ‐ 36% (between 20‐29 y/o) Gender (%F): Manipulation group ‐ 31%; Mobilization group ‐ 54% Inclusion criteria: 18 to 40 years of age; either gender; LBP present for no longer than 1 month; no previous episode of back pain within the prior 6 months; subject was not receiving either workers' compensation or disability insurance at the time and the LBP was not work‐related; no previous experience with spinal manipulation; willing to travel to the Family Practice Center of the North Carolina Memorial Hospital; and be available for telephone interviews over the subsequent 2 weeks. Exclusion criteria: suspicion of inflammatory disease; suggestion of cauda equina syndrome. |
|
| Interventions | I) Manipulation (n=26): Subject was positioned first on the right and then on the left side; positioned in spinal rotation with shoulders and face to the ceiling and pelvis rotated down towards the examining table. A long‐lever high‐velocity thrust was applied to the lower spine. C) MOB (n=28): Subject was positioned first on the right and then on the left side. In each position, the operator stood facing the subject and firmly grasped both knees with one arm while pressing down on the subjects' lower spine with the opposite hand. The subjects legs were then gently, but firmly flexed on the hips twice. No rotational force nor leverage was provided to move facet joints. Treatments were performed by one physician "experienced in the manipulative management of LBP". |
|
| Outcomes | 1. Pain: not reported 2. Back specific functional status: RMDQ 3. Recovery: 7 pts., Likert scale ranging from "much worse" to "much better" ‐ measured immediately following the first treatment only 4. Adverse events: not reported Reviewers note: outcomes were not defined as primary or secondary. Follow‐up: every 3 days for two weeks. |
|
| Notes | Authors results and conclusions: A treatment effect of manipulation was demonstrated only in the strata with more prolonged illness at entry. In the first week following manipulation, these patients improved (significantly) to a greater degree and more rapidly. Funded by: Robert Wood Johnson Foundation (non‐profit). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | ".....assigned by random allocation...". No other text was provided. |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | "The very explicit informed consent form was read, reviewed and discussed. It made clear that there was no placebo arm to the study. Two different interventions were to be compared, both of which are considered by their advocates to be effective." |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | No mention of any attempt to blind the care provider. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient was not blinded; therefore, the outcomes assessor was also blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | One patient proved inaccessible to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | One patient was dropped from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Similarity of baseline characteristics? | High risk | Large differences for age and gender. Duration of symptoms presented in strata: < 2 wks of symptoms, 2 to 4 wks of symptoms (these appear equal). Functional status was essentially equal at baseline. No other baseline characteristics were presented. |
| Co‐interventions avoided or similar? | Unclear risk | Not stated |
| Compliance acceptable? | Unclear risk | Not stated |
| Timing outcome assessments similar? | Low risk | Every 3 days for 2 wks. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Adequate sequence generation, unclear allocation concealment. Statistical analysis: Descriptive statistics were used to analyse the baseline characteristics. A MANOVA was used for analysing the effects on pain, disability and mobility between the treatment groups. Sample size calculation was based upon determining a MCID (50% reduction) for pain. |
|
| Participants | 64 subjects; study setting: physical therapy and manual therapy practices; Country: The Netherlands Period and mode of recruitment: patients presenting to PT clinics in the period 2005‐2006. Age: stratified by age, no mean (SD) is presented; the majority of subjects were between 20 and 50 years of age. Gender (%F): 45% Inclusion criteria: acute (<16d.), non‐specific low‐back pain, between 20 to 55 years of age, with or without previous complaints and no symptoms distal to the knee. Exclusion criteria: specific low back pain (e.g. with neurological signs, rheumatic disease, signs of osteoporotic fractures); inability to fill in the questionnaires. |
|
| Interventions | I) SMT + PT (n=31); C) PT (n=33). ‐SMT consisted of high‐velocity low‐amplitude SMT designed to cause cavitation of the joint and to improve overall joint function, decrease any restrictions in movement at segmental levels in the lumbar spine and sacroiliac joint, and reduce pain. No other technique was applied. The manual therapist chose the techniques on the basis of the location of the dysfunction and in each treatment session, only one manipulation was applied, with an added time investment of approximately four minutes. ‐Physiotherapy consisted of: standard PT care under the principle of increasing physical functioning. Participants participated in a low‐intensity, low‐load endurance exercise programme designed to train specific abdominal muscles, to be completed 5 minutes, two times per day. All participants were also given instructions in staying active and a pamphlet from the Royal Dutch Society for Physical Therapy. |
|
| Outcomes | 1. Pain: 0‐100 VAS (last 24 hours) 2. Back‐specific functional status: ODI 3. Recovery: 2‐point scale, measured at the 4th visit (improved or not improved) 4. Mobility: evaluated using the Sit‐and‐Reach Test (designed to test the mobility of the lower‐body and spinal column) 5. Adverse effects: not reported Note: outcomes not defined as primary or secondary. Follow‐up: at the 4th visit (ca. 2 1/2 weeks post‐baseline). |
|
| Notes | Authors results and conclusions: The addition of SMT to PT care demonstrates a significant effect on improvement in functional status, but not on pain relief or improvement of mobility. This study does not support the efficacy of a clinical prediction rule in the treatment of acute, non‐specific low‐back pain. Funded by: not specified. Note: Primary author provided additional information regarding the RoB assessment, which resulted in a number of items being adjusted from "high" to "low" risk of bias. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent employee, not involved in recruitment of participants, generated a random stratified list where by means of a computerized programme, ...."...patients were assigned to one of the two treatment groups. |
| Allocation concealment (selection bias) | Low risk | Unclear who actually conducted the allocation from the publication; however, we personally contacted the principal author and confirmed that an independent researcher conducted the allocation. |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No attempt was made to blind patients to the intervention. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patients were not blinded; therefore, outcome assessor was also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | According to personal contact with the principal author, only one subject dropped out. No flow chart is provided in the publication. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Stated that an ITT analysis is used; no alternative analyses are presented (e.g. per‐protocol). One patient discontinued care (from the experimental group) due to increasing pain ‐ based upon the numbers it would appear that this subject was removed from the analyses; however, this is not likely to have influenced the overall results. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, although recovery was measured, but not reported. |
| Similarity of baseline characteristics? | High risk | Baseline variables measured: age (stratified by group), gender, duration, onset of LBP (sudden, gradual), presence/absence of relapse, type of physical labour performed, use of medication, outcomes: pain, functional status, mobility. Note: baseline pain for the two groups: SMT + PT = 42.7 (18.4)) vs. PT = 54.0 (17.5). |
| Co‐interventions avoided or similar? | Unclear risk | According to the primary author, "no other technique was applied", but it is unclear if participants sought care elsewhere outside the constructs of the trial. This was not examined. |
| Compliance acceptable? | Low risk | According to the primary author, participants were provided a checklist which was to be completed every day. No differences were found between the groups and it appears that both groups adequately completed their exercises at home. Patients were required to complete 5min. twice per day and this was checked by the checklist. |
| Timing outcome assessments similar? | Low risk | Follow‐up was conducted at the 4th visit (2 1/2 weeks following baseline). |
| OVERALL RISK OF BIAS | Low risk | |
| Methods | Adequate sequence generation and allocation. Statistical analysis: All data were double entered. Primary outcome was examined as Kaplan‐Meier survival curves. Cox regression was performed to estimate the effects of treatment group on recovery and secondary analyses were conducted to examine the effect of potentially important covariates. For the secondary outcomes, effects of the interventions were calculated using linear models and important baseline variables were included in the modelling. Sample size calculation was based upon determining a MCID (20% difference) for recovery. |
|
| Participants | 240 subjects; Study setting: GP practices and private clinics of physiotherapists; Sydney, Australia Period and mode of recruitment: via 40 GP practices Age, y (all (mean (SD)): 40.7 (15.6); range for the groups: 39.5 to 41.9 Gender (%F): 44%; range for the groups: 42% to 46% Inclusion criteria: complaint of pain in the area between the 12th rib and buttock crease causing moderate pain and moderate disability (measured by adaptations of items 7 and 8 of SF‐36). Exclusion criteria: present episode of pain not preceded by a pain‐free period of at least 1 month, in which care was not provided; known or suspected serious spinal pathology; nerve root compromise (with at least two of these signs: myotomal weakness, dermatomal sensory loss, or hyporeflexia of the lower limb reflexes); presently taking NSAIDs or undergoing spinal manipulation; any spinal surgery within the preceding 6 months; and contraindication to paracetamol, diclofenac, or spinal manipulative therapy. |
|
| Interventions | I) SMT + Diclofenac (n=60); C1) Detuned pulsed ultrasound + Diclofenac (n=60); C2) SMT + placebo Diclofenac (n=59); C3) Detuned pulsed ultrasound + placebo Diclofenac (n=60) ‐SMT: consisted of mobilization or high‐velocity thrusts provided by physiotherapists, who had a minimum qualification of a graduate diploma in SMT and who regularly used SMT in their daily practice. The treatment followed an algorithm developed by the researchers on the basis of views of expert clinicians and researchers. The aim of the therapy was due produce motion at the joints of the lumbar and thoracic spine, sacroiliac joint, pelvis and hip. The therapy was allowed to be modified by the clinician in order to suit the patient. ‐Diclofenac: 50 mg., twice daily for a maximum of 4 weeks or until the participant has recovered. ‐Detuned pulsed ultrasound: described as "placebo manipulative therapy". The therapy was designed to match the treatment duration and contact time (30 to 40 minutes at the initial visit and 20 minutes for follow‐up) of SMT. All patients received paracetamol (1g. taken 4 times daily) and advice (consisting of advice to stay active, avoid bed rest and reassurance regarding the prognosis) prior to randomization, which occurred within 2 days of the initial visit with the GP. |
|
| Outcomes | Primary outcomes: 1. Recovery: number of days to recovery as assessed by: 1) first pain‐free day; and 2) first of 7 consecutive days in which the patient had a pain score of 0 or 1 out of 10 Secondary outcomes: 2. Pain: 0‐10 (patients kept a diary that was completed daily) 3. Back specific functional status: RMDQ 4. General function:10‐point Patient Specific Functional Scale 5. Recovery: overall perceived effect 6. Adverse events: no serious adverse events were reported with SMT, 22 (9%) patients reported a possible adverse reaction to medication including gastrointestinal disturbances, dizziness, and heart palpitations Follow‐up: 1, 2, 4, and 12 weeks. |
|
| Notes | Authors results and conclusions: Neither diclofenac nor spinal manipulative therapy appreciably reduced the number of days until recovery compared with placebo drug or placebo manipulative therapy (diclofenac hazard ratio 1·09, 95% CI 0·84–1·42, P=0·516; spinal manipulative therapy hazard ratio 1·01, 95% CI 0·77–1·31, P=0·955). 237 patients (99%) either recovered or were censored 12 weeks after randomisation. 22 patients had possible adverse reactions. Half of these patients were in the active diclofenac group, the other half were taking placebo. Patients with acute low back pain receiving recommended first‐line care do not recover more quickly with the addition of diclofenac or spinal manipulative therapy. Funded by: Australia's National Health and Medical Research Council (governmental). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A statistician not involved in data collection or analysis developed a randomisation schedule and produced 240 consecutively numbered sealed opaque envelopes containing each participant’s allocation. Randomisation was done with randomly permuted blocks of 4, 8, and 12." |
| Allocation concealment (selection bias) | Low risk | "Immediately after collecting baseline data the blinded researcher opened the allocation envelope, which contained a bottle of diclofenac or placebo drug, and gave this bottle to the patient. Active and placebo bottles were identically labelled. Patients were instructed to take their assigned treatment in addition to the paracetamol previously supplied by the GP. The randomisation envelope also contained a second envelope with the participant’s allocation to active or placebo spinal manipulative therapy. This envelope was given to the treating physiotherapist to open in private." |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Subjects were not blinded to SMT, although the authors suggest that they were; however, "placebo manipulative therapy" consisted of detuned pulsed ultrasound. Subjects were, however, blinded to use of diclofenac. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Provider was not blinded to delivery of SMT. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Subjects were not blinded; therefore, outcome assessor was also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | None were lost to follow‐up from the SMT + Diclofenac group nor the detuned ultrasound + placebo Diclofenac group; 3 were lost to follow‐up in the detuned ultrasound + Diclofenac and 1 in the SMT + placebo Diclofenac group. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Stated |
| Selective reporting (reporting bias) | Low risk | Protocol available: ACTRN012605000036617 |
| Similarity of baseline characteristics? | Low risk | Baseline variables presented: Age, gender, duration of current symptoms, number of previous episodes, pain, back specific and general functional status, coping and catastrophising, FABQ ‐ work and activity subscale |
| Co‐interventions avoided or similar? | Low risk | "28 patients took additional co interventions during the study period.The number of patients taking additional interventions was similar between the diclofenac (n=14; 12%) and placebo groups (n=14; 12%) and between the active (n=11; 9%) and placebo manipulative therapy groups (n=17; 14%)." |
| Compliance acceptable? | Low risk | "Compliance with paracetamol across the four groups was not significantly different (p=0·224)....... The mean percentage of full dose taken by the active diclofenac group (69·3% [33·8%]) and placebo group (75·0% [37·7%]) were not significantly different (p=0·225). ...... Median number of sessions per week for the active manipulative therapy group was 2·3 (1·5–3·0) and was 2·3 (1·5–3·0) for the placebo manipulative therapy group." |
| Timing outcome assessments similar? | Low risk | Days to recovery for the primary outcome and at 1, 2, 4, 12 weeks for the secondary outcomes. |
| OVERALL RISK OF BIAS | Low risk | |
| Methods | Unclear sequence generation and allocation. Statistical analysis: Mann‐Whitney U test for non‐parametric data; correlations were evaluated using Spearman rank‐order correlation coefficient. No sample size calculation was performed. |
|
| Participants | 95 subjects; Study setting: medical clinic/department of physical medicine and rehabilitation; Country: USA. Period and mode of recruitment: subjects presenting to University of California, Irvine Medical Center Back clinic between June 1973 to June 1979. Age, y (mean (SD)): SMT group ‐ 30.1 (8.4); control group ‐ 32.1 (9.8) Gender (%F): SMT group ‐ 41%; control group ‐ 41% Inclusion criteria: Presence of palpatory cues indicating hyperalgesia or a restricted or painful range of vertebral motion; absence of any contraindications for vertebral manipulation; absence of any psychosocial problems. Exclusion criteria: Previous experience with manipulation; disability income; pending litigation; previous back surgery; obesity; drug or alcohol abuse; pain not treatable by manipulation of the lumbosacral area. |
|
| Interventions | I) SMT (n=58): rotational manipulation of the lumbar spine. "This was carried out as follows: the patient lies on his or her side on a table facing the manipulator. The inferior leg is extended and the superior leg is flexed, tilting the superior aspect of the pelvis toward the manipulator. The superior shoulder is rotated away from the manipulator and the spine is locked in extension. A short, high‐velocity thrust is then applied to the pelvis. This presumably has the effect of gapping the facet joints and stretching the paravertebral muscles of the lumbosacral area." C) Massage (n=39): soft‐tissue massage to the lumbosacral area only. The number of treatments was variable and left to the discretion of the practitioner. |
|
| Outcomes | 1. Pain: 5‐point ordinal scale, ranging from none to very severe 2. Back specific functional status: listed as specific activities and ability to perform them, such as walking, bending or twisting, sitting down in a chair, sitting up in bed, etc. 3. Recovery: reported as those responding to the question whether the "treatment was effective" (or not) 4. Adverse events: not reported 5. Other: physiological signs: improvement in straight leg raising to pain or to pelvic rotation, and distance of the fingertips to the floor with maximum forward flexion Reviewers note: outcomes were not defined as primary or secondary. Follow‐up: following first treatment; discharge and 3 weeks following discharge. |
|
| Notes | Authors results and conclusions: Patients who received manipulative treatment were much more likely to report immediate relief after the first treatment; and at discharge, there was no significant difference between the 2 groups because both showed substantial improvement. Funded by: Medical Trust, Los Angeles, USA (non‐profit?). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...each patient was randomly assigned to either the experimental or control group." Comment reviewers: no other information was provided. |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Unclear risk | Results from the query post‐treatment (at 3 weeks follow‐up) is as follows (data available for 60% of the baseline sample (note: therefore, possible respondent bias)): 33 received OMT (57% of the original sample (n=33/58)): 21 (65%) perceived it to be OMT and 12 (35%) perceived it to be sham OMT; 25 received sham OMT (64% of the original sample (n=25/39)): 14 (58%) perceived it to be OMT and 11 (42%) perceived it to be sham OMT. The authors claimed the study was successful in comparing manipulation to an appropriate placebo treatment. Reviewers note: This comparison was rated unclear risk because it was unclear what was said to the subjects and whether the subjects were aware that they would receive either soft‐tissue massage or SMT, or whether they were told that they would receive treatment A or treatment B and which was most effective would be evaluated. In other words, if type of treatment was mentioned, the participating subjects could have determined if they thought they received an effective treatment or not, whereas if the type of treatment was not mentioned, the participating subjects would not have had a frame of reference to compare and thus, remain blinded to type of treatment modality. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Unclear risk | See reviewers comment above. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Lost to follow‐up: Discharge: SMT: 29% (n=17/58); control: 28% (n=11/39) 3 wks following discharge: SMT: 43% (n=25/58); control: 36% (n=14/39) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Similarity of baseline characteristics? | High risk | Baseline variables reported: age, gender, proportion with acute and chronic pain, proportion with moderate to very severe pain, proportion with impaired gait and abnormal lumbar curve (as reported by the physician). Difference in percentage of patients with chronic pain: 17 (SMT) versus 29 (control). Difference in percentage of patients with severe to very severe pain: 37 (SMT) versus 16 (control). |
| Co‐interventions avoided or similar? | Unclear risk | Not stated |
| Compliance acceptable? | Unclear risk | Not stated |
| Timing outcome assessments similar? | High risk | Discharge and 3 weeks following discharge, which would have been different for individual subjects. "The number of treatments received was variable, at the discretion of the treating physicians." |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Adequate sequence generation, unclear allocation concealment. Statistical analysis: general linear model ANOVA. No sample size calculation was performed. |
|
| Participants | 192 subjects; Study setting: chiropractic treatments were delivered at a chiropractic institution, but is unclear where the medical treatments were delivered; Country: USA. Period and mode of recruitment: via advertisements (newspaper, radio, television, magazines, Internet posting); period of recruitment was not stated. Age, y (all (mean (SD)): 41.9 (9.9); range for all groups ‐ 40.5 to 43.1 Gender (%F) (all): 43%; range for all groups ‐ 39% to 50% Inclusion criteria: 21 to 59 years of age; "uncomplicated LBP" of 2 to 6 weeks duration. Exclusion criteria: Exclusion criteria included previous spinal surgery, spinal fractures, spinal stenosis, and known or suspected disk herniation; previous LBP within 18 months; neuropathy; spondylitis; vascular disease; malignant disease; cervical complaint; pregnancy; and personal injury litigation. Following informed consent procedures, eligibility was established jointly by doctors of chiropractic and medicine through history taking and a physical examination. |
|
| Interventions | I) SMT + medical placebo (n=50); C1) muscle relaxants + sham adjustments (n=53); C2) medical placebo + sham adjustments (n=53) ‐SMT: Manual spinal adjustments were performed on a drop table with the subject in either a prone or side‐lying position using specific, high‐velocity, low‐amplitude thrusts in the lumbar, pelvic, or sacral spinal region. Supine leg length inequality (LLI) and adjustment vectors were determined according to the Grostic Procedure. The subject was placed in a side‐lying position with the head resting on the mastoid process. Using a handheld instrument with an electro magnetically driven stylus, a high‐velocity, limited excursion thrust was delivered along a lateral‐to‐medial vector with skin surface contact over the level of the atlas (C1 vertebra) transverse process. ‐Drug therapy: The 3 agents used in this study (cyclobenzaprine HCl, 5 mg; carisoprodol, 350 mg; methocarbamol, 750 mg) and their usage instructions were chosen by the medical doctor based on his own clinical experience and were designed to mimic general medical care with a 2‐week duration. Subjects in the medical group were given 3 muscle relaxants. Subjects were instructed to record on a medication log the amount of each drug used and any side effects encountered. The initial dose was 2 capsules at bedtime from bottle A and 2 capsules, 3 times daily from bottle B. Medication from bottles A and B could be doubled or halved as needed. ‐Sham adjustments: Sham procedures were designed to mimic chiropractic adjustments with respect to dialogue, visit length, and physical contact. For lower spine sham procedures, the subject was placed prone on a drop table with the lumbar and pelvic sections activated (lifted but not released) or alternatively, in a side‐lying (semi‐fetal) position on a bench. The chiropractor’s hand was placed over the paravertebral musculature and light pressure was applied. Caution was taken to avoid an actual thrust to the spine. For the cervical sham procedures, the subject was placed in a supine position and the adjusting instrument was positioned over the mastoid. The instrument was disabled so that no thrust was delivered to the spinal articulations. All subjects received acetaminophen as a ‘‘rescue medication’’ to allow assessment of self‐medication. Subjects attended 7 chiropractic visits and self‐administered medication/placebo capsules over 2 weeks. |
|
| Outcomes | 1. Primary outcomes: Pain: 0‐10 VAS 2. Back specific functional status: ODI 3. Recovery: measured as Global Impression of Severity ("....to determine usefulness for assessing temporal aspects of physical examination findings"; "...scores ranged from 0 to 31 and were derived by combining 5 measures determined by a medical doctor performing a blinded evaluation." These 5 measures consisted of the following: limitations in ADLs, tenderness, spasm, Schober's test, and VAS for pain); Depression (Modified Zung Self‐Rating for Depression) 4. Secondary outcomes: Schober's test (to evaluate lumbar flexibility) 5. Acetaminophen usage 6. Adverse events: not reported Follow‐up: 2 and 4 weeks. |
|
| Notes | Authors results and conclusions: When all subjects completing the protocol were combined (N = 146), the data revealed pain, disability, depression, and GIS decreased significantly (P < .0001); lumbar flexibility did not change. Statistical differences across groups were seen for pain, a primary outcome, (chiropractic group improved more than control group) and GIS (chiropractic group improved more than other groups). No significant differences were seen for disability, depression, flexibility, or acetaminophen usage across groups. Conclusion: Chiropractic was more beneficial than placebo in reducing pain and more beneficial than either placebo or muscle relaxants in reducing GIS. Funded by: Research Center of Life University (chiropractic institution) (non‐profit). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were assigned sequential enrolment numbers that provided group assignment based on a computer‐generated randomization chart." |
| Allocation concealment (selection bias) | Unclear risk | No statement on how allocation was conducted nor who was involved. |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | ".....subjects were asked at the end of the study whether they thought they received true chiropractic adjustments and true medications.....This was not the case, and χ2 analysis revealed significant cross‐group differences to both questions (chiropractic adjustments: P < .001; medications: P= .008). Follow‐up pair‐wise comparisons revealed that perception of true chiropractic care was significantly higher (P<.05) in the chiropractic group than either of the other 2 groups...". "....80% of those in the sham SMT group did not believe that they received actual chiropractic treatment, while 88% of those in the chiropractic group did believe they received actual chiropractic treatment" |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | No attempt to blind providers to treatment. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Subjects were not blinded; therefore, outcomes assessors were also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | ".....83% completed the 2‐week care phase and 76% returned 2 weeks thereafter for final data collection. There was no group bias for dropouts.... and most subjects dropped out due to time constraints." Reviewers note: The drop‐out number seems excessive for the 4 wk. assessment. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | "Subjects were analysed in the intervention group to which they were randomized (intent‐to‐treat), but to eliminate erroneous assumptions made for missing data points, data for each outcome measure were restricted to subjects who completed the assessments." Furthermore, ".....data from 3 subjects were discarded because 2 had initiated personal injury litigation (an exclusion criterion) and another inadvertently received both forms of active intervention." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available; however, pain and functional status were measured. |
| Similarity of baseline characteristics? | Unclear risk | Baseline data presented only for those completing the 2‐week care phase. Baseline variables presented: age, gender, pain duration, pain pattern (constant or intermittent), pain onset (gradual or sudden), type of previous back pain treatment, number of previous episodes, previous chiropractic care, pain, functional status (ODI), depression (Zung), Schober's test, and global impression of severity. |
| Co‐interventions avoided or similar? | Unclear risk | Not stated |
| Compliance acceptable? | Unclear risk | "The 2‐week care phase involved a total of 8 visits over a 2‐week period, which was followed by a ninth visit 2 weeks thereafter for a final assessment. The majority of the subject pool that completed the care phase attended all 8 scheduled visits (N= 154, mean = 7.68, SD = 0.72). There was no difference in the number of visits across intervention groups." Reviewers note: Although this represents 80% of those allocated to the various interventions, data were not presented for those not completing the care phase. It is questionable if this is equal between the groups. |
| Timing outcome assessments similar? | Low risk | At 2 and 4 weeks. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Sequence generation and allocation considered adequate. Statistical analysis: Superiority trial with two primary outcomes, pain intensity and analgesic intake. Sample size calculation was based upon determining a MCID (1.1 points) for pain and (40mg.) for diclofenac. |
|
| Participants | 104 subjects; Study setting: Emergency Department of Bern University Hospital and a primary care practice network; Country: Switzerland. Period and mode of recruitment: those presenting between March 2003 and April 2006. Age (all subjects): 20 to 55 years of age Gender (% female; all subjects): 36% Inclusion criteria: men and non‐pregnant women aged 20 to 55 years who presented with acute low back pain (duration of current episode < 4 weeks). Exclusion criteria: signs of nerve root irritation or compression; pain radiating below the knee; cauda equina syndrome; suspected specific cause of low back pain such as fracture, tumor or infection; blood‐coagulation disorder; severe renal or hepatic dysfunction; severe osteoporosis; allergy or intolerance to an administered medication; or epidural corticosteroid injections in the preceding three months. |
|
| Interventions | I) SMT (n=52): "SMT was performed by a specialist in manual medicine, chiropractic and rheumatology, a specialist in physical medicine or an osteopath, all proficient in SMT. SMT was initiated within 24 hours of randomisation, with patients undergoing a maximum of five sessions within 2 weeks; it included a combination of high velocity low amplitude (HVLA) thrusts, spinal mobilizations and muscle energy techniques. Whenever possible, HVLA thrusts were applied, combined with the other techniques as considered necessary in view of the clinical presentation of the patients.......HVLA thrusts were applied in an estimated 80% of all sessions.......". C) Standard care (n=52): "consisted in general advice on rapid return to normal activities and avoidance of bed‐rest in the acute phase and the use of paracetamol, diclofenac or Dihydrocodein according to local guidelines as required. Patients were provided with all three study medications by treating physicians; they were instructed about the maximum daily dosages and advised to use paracetamol as a first line drug. The actual schedule and daily dosage was left at the discretion of patients." Both treatments were given for two weeks. Complications: "Two serious adverse events occurred in the experimental group (4%) and two in the control group (4%). In the experimental group, there was one patient with an acute pancreatitis and one patient with an acute loss of motor and sensory function of the left lumbar segment L5 due to a herniated disk after randomisation, but before any SMT treatment was initiated. In the control group, there was one patient with a symptomatic cholelithiasis and one patient with a femoro‐acetabular impingement syndrome. Neither of these events appeared to be related to the allocated treatment strategies. |
|
| Outcomes | Primary outcomes: 1. Pain: 11‐point NRS 2. Analgesic use based on calculated equivalence doses up to day 14 Secondary outcomes: 3. Back specific functional status: RMDQ at day 14 4. Recovery: proportion of pain‐free patients and the proportion of patients without analgesic intake up to day 14; At 6 months: Pain intensity and the proportion of patients experiencing at least one serious adverse events up to 6 months Follow‐up: daily for the first 14 days and at 6 months. |
|
| Notes | Authors results and conclusions: "We found no evidence for a clinically relevant benefit of SMT in addition to standard care in patients with acute low back pain." "We believe that our trial provide reliable evidence that the majority of patients with acute low back pain can be effectively treated without spinal manipulative therapy." Funded by: Swiss Society for Manual Therapy (Non‐profit), Department of Rheumatology and Clinical Immunology (Non‐profit), Institute of Social and Preventive Medicine and CTU Bern (Non‐profit), and MediX practice network (Industry?). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed on‐site using sealed, opaque, sequentially numbered allocation envelopes, which were produced at the trial coordination centre (ISPM Bern) and only opened by the recruiting physician after a patient had definitely been registered in the trial.The allocation schedule was based on computer‐generated random numbers, blocked and stratified according to trial centre with randomly varied block sizes of 8 and 12. Recruiting physicians were unaware of the block sizes. |
| Allocation concealment (selection bias) | Low risk | Envelopes were monitored by the trial coordination centre to ensure that they were not tampered. |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Patients were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Treating physicians were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient was not blinded; therefore, outcome assessor is also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Seven percent of patients were lost to follow‐up to day 14 and data on pain and analgesic use were missing in about 9% and 25% of observations, respectively. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | "The primary analyses of pain intensity and analgesic intake were based on an intention‐to‐treat approach, with all randomised patients included in the analysis in the group they were originally allocated to." |
| Selective reporting (reporting bias) | Low risk | Outcomes similar as described in the protocol. The trial is registered with clinicaltrials.gov, number NCT00294229. |
| Similarity of baseline characteristics? | Unclear risk | Variables examined: gender, age, type of occupation, pain duration, pain intensity, disability, type of analgesic drug, dose of analgesic drug, diclofenac equivalence dose, fitness for work, health care setting. Table 1: difference in pain duration: %pain <7 days: 54% (SMT+UC) versus 75% (UC alone). Difference in RMDQ: 12.8 (SMT+UC) versus 14.3 (UC alone). |
| Co‐interventions avoided or similar? | Low risk | To avoid performance bias other analgesic drugs or non‐pharmacological treatments (e.g. physiotherapy) were not allowed. "None of the patients allocated to the control group received SMT." |
| Compliance acceptable? | Low risk | Two patients in the experimental group did not receive the allocated treatment. None of the patients allocated to the control group received SMT. The median number of SMT sessions in the experimental group was 3 (inter‐quartile range 2 to 4); High velocity thrusts were applied in an estimated 80% of all sessions, in at least 38 patients allocated to SMT (73%). |
| Timing outcome assessments similar? | Low risk | Daily for the first 14 days and at 6 months |
| OVERALL RISK OF BIAS | Low risk | |
| Methods | Randomization procedure not described. Unclear if patients were aware that they were randomized. Statistical analysis: Results were analysed in three groups according to duration of the present episode: groups 1 up to and including 13 days; group 2: 14‐28 days inclusive; group 3: 29 days and over. t‐test was performed to detect statistically significant differences between groups. No sample size calculation was performed. |
|
| Participants | 95 subjects; Study setting: general practice; Country: UK. Period and mode of recruitment: not stated. Age (all subjects): 16 to 70 years of age Gender (% female): 28% (SMT), 24% (control) Inclusion criteria: 16‐70 y/o; pain partly or wholly between the inferior angles of the scapulas and the buttock folds. Exclusion criteria: Patients suffering from inflammatory joint disease, skeletal metastases or infection, spondylolisthesis, neurological deficit in structures innervated by lumbar or sacral roots that could not be ascribed to a previous resolved episode or other pathology, osteomalacia or osteoporosis, visceral pathology that could refer pain to the low back, pregnancy. Also excluded were those who sought or intended to seek physical treatment outside the practice for their present episode, and transient patients. |
|
| Interventions | I) SMT (n=49): classical range of osteopathic manipulative manoeuvres of the type most likely to be delivered to a patient in the UK from a registered osteopath. The following elements were used in case (if could be applied without pain): direct pressure and stretching to involved musculature, low‐velocity high‐amplitude thrust techniques (HVT) to hypomobile vertebral motion segments, especially those from which the presenting pain was deemed to originate. Treatment continued twice weekly until either patients deemed themselves recovered or the manipulator decided that further treatment was unlikely to produce benefit. C) Control (n=46): controls were seen in the clinical as necessary for examination for incapacity certificates, reinforcement of postural advice, and reassurance. Control patients were told that was no treatment that had been shown to be superior to the program of rest and graded resumption of activities on which they were embarked. Both groups received advice on posture, exercise, and avoidance of occupational distress as appropriate to their situation. |
|
| Outcomes | Primary outcome: 1. Patient‐reported recovery (yes/no) Secondary outcomes: 2. Either patient‐reported recovery or a zero Disability Index (DI) score. Improvement in DI score from presentation to 1, 2 and 3 weeks into the trial were also examined Outcome measures: DI score, asking the subject to mark which of 12 activities were comfortable (scored from 0‐12); VAS pain indicating level of pain between pain‐free (0) and the maximum score of 75, midpoint being the level of pain during the worst 24 hours before presentation; Activity Loss Analog (ALA), a similar VAS ranging from "full normal activity" to "least possible activity"; Index of compliance = ALA divided by VAS pain (activity loss per unit pain); return to partial or full work (or home duties), and reported recovery (yes/no). Follow‐up: 2x/wk for 3 weeks following trial entry and then weekly until the patients deemed themselves recovered or for 3 months, if un recovered. Adverse events: "One control and one manipulated patient developed signs of neurologic deficit soon after trial entry....". No significant difference in development of excess lumbar lordosis or paraesthesias for the 2 treatment groups. |
|
| Notes | Authors results and conclusions: "Even with the small numbers appropriate to a pilot trial, we have confirmed a significant benefit from manipulation to identifiable group of back pain patients. The advantage to manipulated patients was maximal between 1 and 2 weeks after commending treatment, but was not discernable after 4 weeks." Funded by: Osteopathic Trust Ltd (Non‐profit), Rehabilitation Products Ltd (industry ‐ loaned the manipulation tables). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to one of two groups". No further statements on randomization. |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Not stated. It is not clear whether patients were informed about the two treatment arms and the fact that they were randomized. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | No mention of any attempts to blind the provider. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Not stated. It is not clear whether patients were informed about the two treatment arms and the fact that they were randomized. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | "3 control patients were lost to follow‐up less than 2 weeks into the trial and 2 developed complications shortly following trial entry." |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | 5 were excluded from the analyses (2 that developed complications shortly following trial entry and 3 which were lost to follow‐up within the first 2 weeks (n=95). Table 3 shows 94 patients. |
| Selective reporting (reporting bias) | Unclear risk | No protocol. |
| Similarity of baseline characteristics? | Low risk | Variables examined: age, gender, excess lumbar lordosis, straight leg raising, erect pelvic tilt, leg length difference, periods of "pins and needles", periods of "numbness", duration of present episode, previous episodes, disability index, VAS pain, Activity Loss (ALA). The only significant difference was a higher prevalence of complaints of "pins and needles" in the SMT group (P<0.005), and also in the SMT group a higher proportion reported episodes of transient numbness in the leg (P<0.01). |
| Co‐interventions avoided or similar? | Unclear risk | No mention of co‐interventions or avoidance of co‐interventions. |
| Compliance acceptable? | Low risk | 231 manipulative treatments were given: 87% of these in the first 2.5 weeks of the trial. It can be calculated that most patients received 2‐3 treatments. |
| Timing outcome assessments similar? | Low risk | Questionnaires were completed twice weekly for 3 weeks after trial entry and then weekly until the patients deemed themselves recovered, or for 3 months if not recovered. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Sequence generation and allocation procedure unclear Statistical analysis was performed using Wilcoxon signed‐ranks test. No sample size calculation was performed. |
|
| Participants | 159 randomly allocated to 6 treatment groups (of a total 459 who were either lost to follow‐up, changed treatment assignment or had chronic low‐back pain); Study setting: hospital outpatient department (university orthopaedic clinic and a "Static Center" of Rome); Country: Italy. Period and mode of recruitment: January 1985 ‐ October 1986 Age (mean (years)): group1B ‐ 38.4; group2B ‐ 39.5 Gender (%F): group1B ‐ 51% (39/77); group2B ‐ 49% (39/80) Inclusion criteria: low‐back pain, aged 17‐58 years. Pattern of pain radiation: with and without radiation below knee; 2 groups ‐ acute (< 4 weeks) and chronic (> 9 weeks) LBP. Duration of the current LBP (mean): group 1B ‐ 13 months; group 2B ‐ 9 months (all other groups are not relevant for this report). Exclusion criteria: Pregnancy or nursing women, serious general diseases, psychiatric disturbances, medico‐legal litigation. |
|
| Interventions | Two principal grps: group1 ‐ LBP only; group2 ‐ LBP radiating to the buttocks and/or thighs and no neurological changes. Subgroups were defined as: A ‐ LBP <4 wks. duration and no LBP in the preceding 6 months; B ‐ continuous or almost continuous LBP lasting more than 2 months; C ‐ chronic LBP with an episode of acute pain at the time of clinical observation. I) Manipulation by trained chiropractor [at follow‐up: n=87]; no. chronic patients: 12; at a rate of 2 treatment per week C1) Diclofenac "full dose" [at follow‐up: n=81]; duration treatment: 2 weeks C2) Physiotherapy: massage, electrotherapy, infrared, etc. [at follow‐up: n=78]; no. treatment: 15, daily for 3 weeks C3) Bed rest [at follow‐up: n=29]; duration treatment: 6 ‐ 8 days C4) Back school [at follow‐up: n=50]; no. treatment: 4 in 1 week C5) Placebo gel [at follow‐up: n=73]; duration 1 or 2 weeks |
|
| Outcomes | 1. Pain: 4 point scale: ranging from none to most severe pain imaginable 2. Back specific functional status: 4‐point scale: extremely, moderately, slightly or not limited 3. Recovery: not reported. Overall evaluation was based upon a sum score including both subjective and objective measures. 4. Spinal mobility: forward flexion: fingertip to floor distance; Abdominal muscle strength: assessed by the leg‐lowering test, and isometric endurance 5. Adverse events: not reported Note: Outcomes not defined as primary or secondary by the authors. Follow‐up: 3 weeks, 2, 6 months. |
|
| Notes | Authors results and conclusions: In subgroup1B, the best results were obtained with physiotherapy at short‐term and low‐back school at the long‐term. For subgroup2B, physiotherapy gave the best results at both short‐ and long‐term follow‐up. Funded by: Centro Studi di Patologia Vertebrale, Rome (non‐profit). Unequal numbers for the intervention groups because not all interventions applied to the various groups (acute ‐ chronic). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients in each group were randomly assigned to the following treatments. |
| Allocation concealment (selection bias) | Unclear risk | Note: No other information was provided on the sequence generation or allocation. |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | There is no mention of attempts to blind the patients to other interventions or their perceptions of potential effectiveness of the different interventions. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | No mention if there were any attempts to blind the care providers to the other groups. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient was not blinded; therefore, this item was scored as "no". No mention if there were any attempts to blind the outcome assessors to treatment allocation for the subjective or objective outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | 5% (n=23/459) were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | 13% of those randomized were either lost to follow‐up or changed their assigned treatment and subsequently not included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available; recovery not reported. |
| Similarity of baseline characteristics? | Unclear risk | Similar for the 2 groups with chronic LBP (based upon age, gender, and duration of symptoms), but unclear for the baseline scores for functional status. |
| Co‐interventions avoided or similar? | High risk | 8% (38/459) of the subjects had interrupted or changed their assigned treatment. |
| Compliance acceptable? | Unclear risk | Not stated |
| Timing outcome assessments similar? | Low risk | At 3 weeks; 2, 6 months. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Unclear methods of randomization and allocation. Statistical analysis: Mann‐Whitney rank‐sum test. No sample size calculation was performed. |
|
| Participants | 76 subjects; Study setting: Dept. of physical medicine and rheumatology, Alborg Hosptial; Country: Denmark. Period and mode of recruitment: 1975, referred from the general practitioner to the rheumatologist. Age, y (all, mean (SD)): 34.9 (7.3) Gender (%F): 0% Inclusion criteria: males; 20‐50 years of age; LBP without signs of root pressure; duration less than 3 weeks; no treatment except analgesics before entering the trial. Exclusion criteria: contraindication to manipulation; no other exclusion criteria were stated. |
|
| Interventions | I) SMT (n=12): rotational manipulation in the pain‐free direction, delivered by either a physiotherapist or physician using the same technique. C) short‐wave diathermy (n=12): no further description was provided. Therapy was delivered for both groups 3 times per week for 2 weeks. |
|
| Outcomes | 1. Pain: unclear how this was measured 2. Back specific functional status: not reported 3. Recovery: declared restored if subjects fulfilled the following criteria: no pain at all, normal function (according to Schober's test?), no objective signs of disease (unclear how this was determined), and fit to work (not reported) 4. Adverse events: not reported Follow‐up: 1, 2 weeks |
|
| Notes | Authors results and conclusions: There was a significant difference in recovery (92% of patients treated with manipulation were free of symptoms within 2 weeks compared to 25% in the short‐wave diathermy group). All patients in the manipulation group improved function/mobility whereas only half of the patients in the short‐wave group improved (P<0.01). Funded by: Not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... patients were randomized ....". Reviewers note: no other details were provided. |
| Allocation concealment (selection bias) | Unclear risk | See above. |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No mention of an attempt to blind the patients to therapy. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Care provider was not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient was not blinded; therefore, outcomes assessor also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | "92% of the allocated subjects returned for final measurement at 2 weeks." |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Not explicitly stated. In addition, 2 patients (8% of the sample) were dropped from the analysis (one patient dropped out and another did not fulfil the inclusion criteria). |
| Selective reporting (reporting bias) | Unclear risk | No protocol is available. |
| Similarity of baseline characteristics? | Unclear risk | Baseline characteristics for the 2 groups are not presented. |
| Co‐interventions avoided or similar? | Unclear risk | Not stated |
| Compliance acceptable? | Unclear risk | Not stated |
| Timing outcome assessments similar? | Low risk | At 1, 2 weeks. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Unclear sequence generation and allocation procedure. Statistical analysis: ANOVA was used. Differences between the 1 month follow‐up values and the baseline values were calculated for individual patients. Subsequently, the three study programmes were compared with respect to improvement over time. In a second step, a subgroup analysis was conducted for patients with only low‐back pain; patients not improved at 1‐month follow‐up; and patients with only low back pain and not improved at three months follow‐up. No sample size calculation was performed. |
|
| Participants | 180 subjects; Study setting: manual and office workers; Country: Sweden Period and mode of recruitment: Consecutive patients referred from general practitioners, occupational doctors (i.e. physician specialised in occupational related diseases) or from an emergency ward. Age: mean 39 (range 19‐64) Gender (%F): 47% Inclusion criteria: Low‐back pain with or without sciatica requiring sick leave, A sick leave period for LBP less than 2 weeks before entering the study, 18–64 years of age, Employed. Exclusion criteria: Sick listed and/or treated for low‐back pain within 1 month before study entry, Previous spine trauma or surgery, Inflammatory disease, Tumours of the spine, Symptoms from cervical spine, thoracic spine or upper extremities, Clinical symptoms or severe low‐back disease demanding surgery, Severe/major medical disease, Pregnancy, Drug and alcohol addiction, Psychiatric disease/disorder, Unsatisfactory knowledge of the Swedish language. |
|
| Interventions | I) Manual therapy programme (n=60); (C1) Intensive training programme (n=60); (C2) General practitioner care (n=60). ‐Manual therapy programme consisted of: 1. Information, 2. Autotraction, 3. Manipulation of the lumbar facet joint and manipulation of the sacroiliac joint, intended to separate the joint surfaces, 4. General mobilisation of the lumbar spine, 5. Segmental and level‐specific passive mobilisation, 6. Auto‐mobilisation, 7. Muscle Energy Technique (MET), 8. Different types of stretching, 9. Controlled training of co‐ordination and stability in the spine. ‐Intensive training programme consisted of: information, muscle training and general condition training. Muscle training included exercises to decrease muscle fatigue and increase muscle strength and co‐ordination in e.g. abdominal, gluteal, paraspinal, shoulder and lower‐extremity muscles. The training was planned with respect to pain and clinical findings on entry to the study. Most treatments were conducted with patients in small groups. ‐GP care consisted of: rest, sick leave, drug prescription (e.g. analgesics, anti‐inflammatory drugs), advice about posture and information about the self‐curing nature of the disease. Patients in the SMT and training programme groups started treatment 1–3 days after randomisation, while patients in the GP care group started later. Treatment was free for the SMT and training programme, but not for the GPcare patients. The duration of treatment was decided by the therapist and the patients were encouraged to continue with exercises at home after finishing the treatment programme. If a patient had a recurrence during the study year, he or she was referred to the treatment group again for further treatment. |
|
| Outcomes | 1. Pain: Pain questionnaire, developed by Carlsson, with questions on pain intensity, frequency, location and quality, and consumption of analgesics 2. Back specific functional status: ODI 3. Recovery: not reported 4. Adverse events: not reported 5. RTW: presented for 12 month follow‐up; however, data presented as number of days off work for LBP per group, but unclear what proportion of subjects were off work due to their LBP Reviewers note: Data for pain, functional status, socioeconomic disability or impairment are not presented between the groups; however, the authors state that no differences were observed (presumably at any of the f/u intervals). Follow‐up: 1, 3, 12 months. |
|
| Notes | Authors results and conclusions: Patients in all three groups had improved significantly according to outcome variables at the 1‐month follow‐up. "Within the limitations of our study we conclude that manual treatment or intensive training do not give better treatment results than conventional GP care in patients sick listed for acute low‐back pain, although the patients are less satisfied with GP care." Funded by: AMF‐Sjukforsakring, Stockholm, Sweden (non‐profit?). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients fulfilling our inclusion criteria were randomized into one of the three treatment programmes........". Reviewers note: no other details were given regarding the randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No mention of an attempt to blind the patients to therapy. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Care provider was not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient was not blinded; therefore, outcomes assessor also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Loss to follow‐up (Fig.1) 3 months: SMT: 18% (n=11/60); Exercise: 20% (n=12/60); GP: 18% (n=11/60) 12 months: SMT: 33% (n=20/60); Exercise: 30% (n=18/60); GP: 32% (n=19/60) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | "The "intention to treat" principle was mainly followed. Thus, the drop outs remained in the treatment group as far as they still participated in the study". |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available. |
| Similarity of baseline characteristics? | Unclear risk | "Analyses of baseline characteristics between the three treatment groups on entry to the study revealed no differences regarding ergonomic, impairment, pain, sick leave, functional disability, or findings on clinical examination." Reviewers comment: data not shown. |
| Co‐interventions avoided or similar? | Unclear risk | Patients failing to recover in the GP (control group) were often prescribed low‐back school or physiotherapy later. Reviewers comment: unclear what percentage of patients were prescribed these therapies nor whether subjects in the other intervention groups were prohibited from seeking other interventions. |
| Compliance acceptable? | Unclear risk | Not reported |
| Timing outcome assessments similar? | Low risk | 1 month, 2 months, 12 months follow‐up. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Unclear sequence generation and allocation procedure. Statistical analysis: The results of the two treatment groups were compared according to duration of current episode, presence or absence of similar problems during the previous 5 years, and an ODI score at entry of 40% or more. To detect differences between the two treatment groups, χ2 tests were used. The Mann‐Whitney U test was used for pain intensity, general health, and ODI scores. The differences between mean changes in pain intensity, general health, ODI score, and direct and indirect costs were tested by Student's unpaired t‐test. No sample size calculation was performed. |
|
| Participants | 323 subjects; Study setting: primary care centers; Country: Sweden Period and mode of recruitment: general practitioners. Age (mean(SD)): chiropractic group 41.1 (11.6), physiotherapy group 40.5 (11.9) Gender (percentage women): chiropractic group 60%, physiotherapy group 65% Inclusion criteria: 18‐60 yr, no active treatment for low back or neck within the past month, no contraindication for manipulation. Exclusion criteria: evidence of affected nerve root, osteopenia, or suspected infection, having another disease, having been involved in an accident less than 10 days previously, pregnancy, or inability to understand Swedish, or both treatments were considered irrelevant. Mix: acute, subacute and chronic LBP; no radiation below the knee. |
|
| Interventions | I) SMT (n=179): at the discretion of the chiropractor; manipulation (98%) and mobilization (11%); avg. 4.9 treatments in 4.1 weeks; six chiropractors involved, mean practice time 9.9 years (range 1‐15 years). C) Physiotherapy (n=144): at the discretion of the PT, consisting of manipulation (2%)and mobilization (36%), and includes traction, soft‐tissue treatment and McKenzie exercises; avg. 6.4 treatments in 4.7 weeks; 30 PTs involved, mean practice time 10.3 years (range 1‐27 years). |
|
| Outcomes | 1. Pain: 0‐100 VAS, including pain frequency (on a 5‐point scale, ranging from "always, day and night" to "never") and a pain drawing 2. Back specific functional status: ODI 3. Recovery: 7‐point scale for global improvement 4. Generic functional status: 6‐point scale 5. RTW: sick leave 6. Medication usage 7. Adverse events: "No complications attributable to treatment were reported from any therapist or patient during the study period." Note: outcomes were not defined as primary or secondary. Follow‐up: Following the treatment period (4.7 weeks for PT care; 4.1 weeks for chiro. care), 6 and 12 months. |
|
| Notes | Authors results and conclusions: "A highly significant improvement in pain, function, and general health related to the back or neck problems could be measured in both groups according to all variables immediate after the treatment and at the 6‐months follow‐up. No differences in changes could be seen between the two study groups. The effectiveness of chiropractic or physiotherapy as primary treatment were similar to reach the same result after treatment and after 6 months." Funded by: County Council of Ostergötland and the Federation of County Councils Sweden (governmental). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each primary care center received closed envelopes for randomization in proportion to the expected number of patients." Revierwers note: No further details were given regarding the randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No mention of any attempt to blind the patients. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patients were not blinded; therefore, outcomes assessor was also not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | "76 of the randomized patients never contacted the therapist or withdrew from the study before the first treatment session. Another 12 patients refused to participate further after one treatment session". In total 88/411 (21%) drop‐out |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | "The results from 323 patients were analysed according to an intention‐to‐treat approach". "Seventy‐six of the randomized patients never contacted the therapist or withdrew from the study before the first treatment session. Another 12 patients refused to participate further after one treatment session." Reviewers comment: 411 patients were randomized. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available |
| Similarity of baseline characteristics? | Low risk | Baseline variables reported: age, gender, smoking, dissatisfaction with work, similar problems during previous 5 yrs, treated previous 5 yrs for similar problems, expectation of completely restored, pain localization (neck/back), duration of current episode, pain frequency, using pain killers, pain intensity (VAS), ODI score, sick leave, duration of sick leave, general health |
| Co‐interventions avoided or similar? | High risk | "Virtually all the patients treated by chiropractors received mainly manipulation. For 80%, manipulation was the only form of treatment during the treatment period. The treatment forms varied more in the physiotherapy group. Fifty‐two percent of the physiotherapy patients received only one treatment form during the treatment period". Table 4 lists the various co‐interventions: Chiropractic group: 98% manipulation, 11% mobilization, 2% traction, 2% soft‐tissue. PT group: 1% manipulation, 25% mobilization, 15% traction, 25% soft‐tissue treatment, 33% McKenzie, 15% TENS, ultrasound or cryotherapy, 3% acupuncture, 6% relaxation training, 21% individualized training program. |
| Compliance acceptable? | Unclear risk | Not described |
| Timing outcome assessments similar? | Low risk | Following the treatment period (4.7 weeks for PT care; 4.1 weeks for chiro. care), 6 and 12 months. |
| OVERALL RISK OF BIAS | High risk | |
| Methods | Adequate sequence generation and allocation procedure. Statistical analysis: The mean and SDs were calculated for each primary outcome measure with respect to time and treatment group. Descriptive statistics were compared using independent t‐tests or Mann‐Whitney U ‐tests for continuous data and χ2 tests of independence for categorical data. Primary outcome measures were examined using a 2 × 2 repeated‐measures analysis of variance (ANOVA) with group as the between‐subjects variable and time (baseline and 48 hours) as the within‐subjects variable. Last value carried forward was used for imputation of missing data. Sample size calculation based upon detecting a MCID (6 points) for ODI and (2.2 points) for pain. |
|
| Participants | 60 subjects; study setting: physical therapy clinic; Country: USA Period and mode of recruitment: patients recruited from the military health care beneficiary population over an 8‐month period (July 2005 to February 2006). Age (all subjects): 25.5 (9.1) years of age Gender (%F): 48.3% Inclusion criteria: 18–65 years of age, primary complaint of LBP with or without associated lower extremity pain, and have a modified ODI>30%. Additionally, subjects were required to satisfy at least three of the five CPR criteria delineated by Flynn and colleagues and at least one of the criteria had to be present: either a duration of symptoms <16 days or no radiating pain distal to the knee. Exclusion criteria: “red flags” for serious spinal pathology (e.g., tumor, cauda equina symptoms, etc.), any condition for which spinal manipulation was contraindicated (e.g., osteoporosis, rheumatoid arthritis, etc.), pregnancy, a history of surgery to the lumbar spine or buttock, signs consistent with nerve root compression (positive straight‐leg increase <45 degrees or diminished reflexes, sensation, or lower‐extremity strength), those with traumatic injuries to the spine within the last 6 months (motor vehicle or recreational vehicle collision, bicycle accident, and fall of >1 metre), and those with litigation pending for their LBP. |
|
| Interventions | I) Lumbopelvic manipulation + an exercise program (n=30): Subjects were treated in the supine position. The therapist stood next to the subject on the side opposite of that which was to be manipulated and the subject was passively moved into side bending toward the side to be manipulated. This was accomplished by first moving the subject’s legs, then the subject’s upper body into maximum side bending. The subject then interlocked his fingers behind his head. The therapist grasped the subject’s shoulder or threaded his arm through the subject’s arms and passively pulled the subject into rotation toward the therapist. The therapist placed his other hand on the subject’s anterior superior iliac spine (ASIS) and delivered a high‐velocity, low‐amplitude thrust in a posterior and inferior direction. C) Lumbar neutral gap manipulation + an exercise program (n=30): Subjects lay with the painful side up with the therapist standing in front of them. The therapist flexed the top leg until there was movement at the selected segment (e.g., L3–L4) interspace and then placed the subject’s foot in the popliteal fossa of the bottom leg. Next the therapist grasped the subject’s bottom shoulder and arm and introduced left trunk side bending and right rotation until motion was felt at the L3–L4 interspace. The therapist’s right thumb was then placed on the right side of the L3 spinous process and the patient’s arms were positioned around the therapist’s right arm. Setup was maintained while the patient was rolled toward the therapist. Finally the therapist’s left arm was used to apply a high‐velocity, low‐amplitude thrust in an anterior direction. All subjects received a minimum of one manipulation per side and a maximum of two attempts per side. |
|
| Outcomes | Primary outcome: 1. Pain: 0‐10 numerical rating scale Secondary outcomes: 2. Back‐specific functional status: ODI 3. Recovery: not reported 4. Adverse events: not reported Follow‐up: 48 hours post‐treatment. |
|
| Notes | Authors results and conclusions: The two manipulation techniques used in this study were equally effective at reducing pain and disability when compared at 48 hours posttreatment. Clinicians may employ either technique for the treatment of LBP and can expect similar outcomes in those who satisfy the clinical prediction rule (CPR). Funded by: not specified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...an individual not involved with the study used a random‐number generator to create a randomization list and prepared individual, sequentially numbered index cards that indicated the randomization assignment. The cards were then folded and placed in sealed envelopes. After the baseline examination, the examiner opened the envelope, indicating the treatment group assignment." |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Unclear if the patient was aware which treatment they were assigned to receive. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Provider was not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient was not blinded, therefore, outcome assessor was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | 4 were lost to follow‐up in the LP SMT group; none were lost to follow‐up in the NG SMT group. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | ITT analysis is stated; given the one visit treatment and short‐term follow‐up, there is not likely to be any deviations in the analysis nor those included. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available; recovery not reported. |
| Similarity of baseline characteristics? | Low risk | Baseline variables reported: age, gender, pain and functional status (ODI), duration of pain <16 days, FABQ work sub‐scale, presence/absence of the following: lumbar hypomobility, symptoms distal to the knee, >35° hip internal rotation, weight bearing asymmetry. |
| Co‐interventions avoided or similar? | Low risk | Not stated but this is probably not of significance given the very short‐term follow‐up. |
| Compliance acceptable? | Low risk | All subjects were treated just once with SMT. Unclear, however, whether subjects performed the pelvic tilt exercise (but again probably not of significance given the very short‐term follow‐up). |
| Timing outcome assessments similar? | Low risk | Follow‐up at 48 hours post‐treatment. |
| OVERALL RISK OF BIAS | Low risk | |
I = intervention; C = control group
ADLs=activities of daily living; BMI=body mass index; f/u=follow‐up; ODI=Oswestry Disability Index; MCID=minimally clinically important difference; RTW=return‐to‐work. Number of subjects represent the number randomized and not the number analysed.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersson 1999 | Includes a population with primarily subacute and chronic LBP. |
| Beyerman 2006 | Duration not specified. |
| Bishop 2010 | Unique contribution of SMT cannot be determined from the study design. The intervention group (SMT) received "reassurance regarding the natural history of acute mechanical LBP; advice to avoid passive treatment approaches (e.g., bed rest, heat, or the use of back supports/corsets/braces); advice to carry out a progressive walking program (two walks a day, each with an initial duration of between 5 and 15 minutes depending on the patient’s tolerance increasing by 2 minutes each walk per week); acetaminophen, 650mg every 6 to 8 hours as required for 2 to 4 weeks, unless medically contraindicated (e.g., because of allergy, compromised liver function, or acute porphyria); and a maximum 4 weeks of lumbar spinal manipulative therapy using conventional side posture,high‐velocity, low‐amplitude techniques....". The control group "were advised of their diagnosis (i.e., mechanical low back pain) and referred back to their referring family physician with a letter explaining the protocol of the present study." Note: see also Table 3. |
| Blomberg 1994 | Multi‐modal treatments were delivered in the various treatment arms, thus, making the assessment of the effects of SMT impossible. The intervention group consisted of: manipulation (thrust techniques) or "more gentle specific mobilization", muscle stretching (almost all received), auto‐traction (15% of the group received this) followed by "steroid injections, often in combination with needling and local anaesthetics" for those not responding after 1‐2 weeks of treatment (which represents 54% of the intervention group). The conventional care group consisted of: "drugs, low‐back pain school training, active back exercises, corsets, taping, short‐wave ultrasonic waves, transcutaneous nerve stimulation (TNS), transcutaneous electric muscle stimulation (TEMS), heat, cold, postural exercises, and in some cases, plunge‐bath training and massage". |
| Bronfort 1989 | Proportion of subjects with acute low‐back pain is unclear (cut‐off reported in the study was 8 wks). |
| Coyer 1955 | Pseudo‐RCT: alternate inclusion. |
| Delitto 1993 | Unique contribution of SMT cannot be determined from the study design. The intervention group consisted of SMT (mobilization to affect the sacroiliac joint) followed by McKenzie extension‐oriented exercises. The comparison group consisted of flexion‐oriented exercises. "On the return visits, the major focus was to assess how the patient was performing the prescribed exercise regimen." Thus, the value of SMT is unclear and it is uncertain if SMT was delivered only once at the first visit. |
| Doran 1975 | Proportion of subjects with acute low‐back pain is unclear (16% < 1 week; 56% > 1 month (of which 14% > 6 months)). |
| Erhard 1994 | Unique contribution of SMT cannot be determined from the study design. The intervention group consisted of SMT immediately followed by "hand‐heel rocking" (consisting of an exercise designed to induce flexion and extension to the spine). The comparison group included an extension‐oriented treatment regimen as proposed by McKenzie. |
| Gemmell 1995 | No clinical assessment beyond one day. |
| Godfrey 1984 | Unique contribution of SMT cannot be determined from the study design. The experimental group received SMT (according to the technique described by Maigne) followed by soft tissue massage. The comparison group consisted of massage (including light effleurage) administered by a kinesiologist or electrical stimulation to either side of the lumbar spine. |
| Grunnesjo 2004 | Unique contribution of SMT cannot be determined from the study design. Two groups were examined: the reference therapy consisting of the "stay‐active" concept (includes physical training, non‐specific traction, passive physiotherapy modalities) and the experimental group consisting of the "stay active" concept plus SMT and steroid injections (half of the patients) in combination with needling and local anaesthetics, where indicated. In a subsequent publication (Bogefeldt 2008) the groups were isolated into the original 4‐group factorial design so that theoretically the additional effect of SMT could be isolated. However, an examination of Table 1 (Grunnesjo 2004) suggests that many in the experimental group did not also receive passive and active physical training (in contrast to the study design, which examined the additional benefit of stretching, SMT and injections) to the "stay active" concept; therefore, it is our opinion that the effects of SMT cannot be isolated. Furthermore, in Table 2 (Bogefeldt 2008), there are some fundamental differences across the groups and most notably for the SMT group, such as percentage women included, percentage on sick‐leave at the time of inclusion and in the previous two years, and percentage of those with x‐rays due to a previous low‐back pain episode. |
| Helliwell 1987 | No relevant outcome measure. |
| Hsieh 2002 | Overall duration of back pain between 10.7 to 11.8 weeks (SD range from 6.6 to 7.2 weeks). |
| Hurley 2004 | Mean duration of the LBP for the study population was 8 weeks (range 7.6 to 8.3 weeks for the three intervention groups). |
| Kinalski 1989 | Duration not specified. |
| Mathews 1987 | Evaluates subjects with exclusively sciatica. |
| Meade 1990 | Proportion of subjects with acute low‐back pain is unclear (cut‐off that was reported in the study was 1 month: 59% of the chiropractic group and 60% of the hospital group had back pain longer than 1 month). |
| Nwuga 1982 | Pseudo‐RCT ‐ alternate inclusion of subjects. |
| Pope 1994 | Evaluates chronic LBP. |
| Rupert 1985 | Mix of acute, subacute and chronic LBP, and although a subgroup analysis is presented separately for the acute patients, it is unclear what proportion these patients represent as no numbers of subjects are presented for any of the groups separately. |
| Sanders 1990 | Outcome assessment not longer than 1 day. |
| Santilli 2006 | Evaluates subjects with exclusively sciatica. |
| Sims‐Williams 1978 | Duration not specified. |
| Sims‐Williams 1979 | Duration not specified. |
| Terrett 1984 | Asymptomatic subjects |
| Waterworth 1985 | Unique contribution of SMT cannot be determined from the study design. Not all subjects in the SMT group received spinal manipulation: some received exercises (according to McKenzie) and some received SMT alone. The other two comparison groups examined medication (Diflunisal) and standard physiotherapy (heat, ultrasound, flexion and extension spinal exercises). |
| Williams 2003 | Subjects recruited with 2‐12 weeks of spinal pain (of which 61% had low‐back pain only); unclear what percentage of subjects had acute or subacute low‐back pain. In addition, the effect of SMT could not be ascertained: "All patients in the trial continued to receive treatment as usual from their GPs.... The control group did not receive any additional intervention. ...The treatment package consisted mainly of osteopathic spinal manipulation....... Occasionally, if symptoms persisted despite osteopathy, tender ligaments or peripheral joints were injected with corticosteroid and local anaesthetic." |
| Wreje 1992 | Proportion of subjects with acute low‐back pain is unclear (no data were presented regarding duration of the back pain). Exclusion criteria was pain of more than 3 months duration. |
| Zylbergold 1981 | Duration not specified. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | http://ClinicalTrials.gov/show/NCT00497861 Title: Comparison of Mechanical Force, Manually Assisted Activator Manipulation Versus Manual Side Posture Manipulation in Patients With Low Back Pain: a Randomized Pilot Study Purpose: This study compared the treatment effect of Activator Methods Chiropractic Technique (AMCT) and manual Diversified type spinal manipulative therapy in a sample of patients with acute and sub‐acute low back pain. |
| Methods | Allocation: Randomized Endpoint Classification: Safety/Efficacy Study Intervention Model: Parallel Assignment Masking: Open Label Primary Purpose: Treatment |
| Participants | Inclusion Criteria:
Exclusion criteria consisted of the following:
The criteria described above were intended to minimize the likelihood of including subjects with a lumbar disc herniation. |
| Interventions | Manually Assisted Activator Manipulation versus Manual Side Posture Manipulation |
| Outcomes | Primary outcomes measured include pain measurement with a VAS scale, the use of the ODI and the Bournemouth back pain scale questionnaire. |
| Starting date | Study completion date: April 2007 |
| Contact information | Principal Investigator: Mark T. Pfefer, DC, RN; Cleveland Chiropractic College |
| Notes | SMR had contact with a colleague of the PI and stated that as of Sept. 2010, the study had not been submitted for publication; a search in PubMed by SMR in May 2011 did not reveal any listing. |
| Trial name or title | http://clinicaltrials.gov/show/NCT00632060 Title: The Efficacy of Manual and Manipulative Therapy for Low Back Pain in Military Active Duty Personnel: A Feasibility Study The specific aims of this research project are to determine feasibility of, and the comparative treatment effect size for, conducting a larger clinical trial of Manual/Manipulative Therapy (M/MT) in restoring peak performance in military personnel in operational environments and to evaluate the ability of the addition of M/MT to standard care to decrease pain and increase function for patients with low back pain. The following two hypotheses will guide the data collection:
|
| Methods | Allocation: Randomized Endpoint Classification: Efficacy Study Intervention Model: Parallel Assignment Masking: Open Label Primary Purpose: Treatment |
| Participants | Criteria Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | 1. No Intervention Standard Care Control Group ‐ Participants randomized to the standard care group will continue their use of non‐prescription or prescription medication and reduced duty loads, as prescribed by the credentialed medical provider. 2. Experimental Manual / Manipulative Therapy Group: Participants randomized to the M/MT group will receive a course of M/MT along with standard care. The patient will see the chiropractor twice a week for the entire course of the study, regardless of manipulation or not.Intervention: Procedure: Manual / Manipulative Therapy (M/MT). |
| Outcomes | Primary Outcome Measures: Decreased pain [ Time Frame: Baseline, 2 weeks, 4 weeks ] [ Designated as safety issue: No ] Secondary Outcome Measures: Increased function [ Time Frame: Baseline, 2 weeks, 4 weeks ] [ Designated as safety issue: No ] |
| Starting date | February 2008 |
| Contact information | Maria Hondras, DC, MPH (maria.hondras@palmer.edu) |
| Notes | SMR had contact with one of the principal investigators in May 2011: The mean (SD) duration of LBP in the study is 11.5 (8.5) days and the median is 9 days. The investigators are currently in the final stages of manuscript preparation. |
| Trial name or title | http://clinicaltrials.gov/show/NCT01211613 A Comparison of Chiropractic Manipulation Methods and Standard Medical Care for Low Back Pain. Purpose: The investigators will be comparing the effectiveness of two types of chiropractic manipulation and standard medical care for patients with a recent onset of low back pain. The two types of chiropractic treatments being compared will be hands‐on (manual) manipulation and mechanical‐assisted (Activator) manipulation. The standard medical care will consist of a medical examination and prescription for over‐the‐counter anti‐inflammatory medication. |
| Methods | Allocation: Randomized Endpoint Classification: Efficacy Study Intervention Model: Single Group Assignment Masking: Single Blind (Investigator) Primary Purpose: Treatment |
| Participants | Criteria Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Procedure: Manual Manipulation Doctor of chiropractic will apply manual high‐velocity low‐amplitude thrust to lumbar spine of research participants. Device: Mechanically‐assisted manipulation Doctor of chiropractic will use the Activator Instrument to apply a mechanically‐assisted thrust to the lumbar spine of research participants.Other Name: Activator IV Instrument: FDA approval# K003185. Other: Standard Medical Care Patients will receive an examination with a physician who is board certified in physical medicine and rehabilitation. Treatment will consist of medical monitoring of the patient's condition over 4 weeks (baseline and 2 follow up exams) and a prescription for over‐the‐counter anti‐inflammatory medications if indicated. |
| Outcomes | Primary Outcome Measures: ODI [ Time Frame: 4 weeks ] [ Designated as safety issue: No ] Questionnaire of level of self‐reported impairment of ADLs due to low back pain. Secondary Outcome Measures: Numeric Pain Rating Score [ Time Frame: 4 weeks ] [ Designated as safety issue: No ] Likert scale from 0‐10 measuring self‐reported level of low back pain. |
| Starting date | Nov. 2010; Estimated study completion date Nov. 2013 |
| Contact information | Michael J Schneider, PhD, DC Tel. 412.383.6640, mjs5@pitt.edu Christine McFarland Tel. 412.623.6872, mcfarlandce@upmc.edu |
| Notes | http://clinicaltrials.gov/show/NCT01211613 SMR had contact with the PI in May 2011. At that time, 50 subjects had been recruited of which, 13 had acute LBP (<6 weeks), 20 had sub‐acute LBP (6‐12 weeks) and 17 had either acute or sub‐acute LBP (unclear). |
Contributions of authors
Conception and design: SM Rubinstein, MW van Tulder, WJJ Assendelft (previous version of this review) Analysis and interpretation of the data: SM Rubinstein, MR de Boer, MW van Tulder Drafting of the review: all members Critical revision of the article for important intellectual content: all members Final approval of the article: all members Statistical Expertise: MR de Boer Administrative, technical, or logistical support: SM Rubinstein, MR de Boer Collection and assembly of data: SM Rubinstein, CB Terwee, MR de Boer, WJJ Assendelft (studies published before 2000)
Sources of support
Internal sources
Division of Public Health, Department of General Practice, Academic Medical Center, Netherlands.
Dutch College of General Practitioners, Netherlands.
RAND, Santa Monica, USA.
Greater Los Angeles Veterans Affairs Healthcare System, LA, USA.
External sources
No sources of support supplied
Declarations of interest
Maurits van Tulder is Coordinating Editor of the Cochrane Back Review Group. Editors are required to conduct at least one Cochrane review, to ensure that editors are aware of the processes and commitment needed to conduct reviews. This involvement does not seem to be a source of conflict of interest in the Cochrane Back Review Group. Any editor who is a review author is excluded from editorial decisions on the review in which they are contributors.
Sidney Rubinstein is a chiropractor who uses SMT in his clinical practice.
Edited (no change to conclusions)
References
References to studies included in this review
- Bergquist‐Ullman M, Larsson U. Acute low back pain in industry: a controlled prospective study with special reference to therapy and confounding factors. Acta Orthopaedica Scandinavica 1977;170 Suppl:1‐117. [DOI] [PubMed] [Google Scholar]
- Brennan GP, Fritz JM, Hunter SJ, Thackeray A, Delitto A, Erhard RE. Identifying subgroups of patients with acute/subacute "nonspecific" low back pain. Spine 2006;31(6):623‐31. [DOI] [PubMed] [Google Scholar]
- Cherkin DC, Deyo RA, Battie M, Street J, Barlow W. A comparison of physical therapy, chiropractic manipulation, and provision of an educational booklet for the treatment of patients with low back pain. New England Journal of Medicine 1998;339:1021‐9. [DOI] [PubMed] [Google Scholar]
- Childs JD, Flynn TW, Fritz JM. A perspective for considering the risks and benefits of spinal manipulation in patients with low back pain. Manual Therapy 2006;11:316‐20. [DOI] [PubMed] [Google Scholar]; Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, Delitto A. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: A validation study. Annals of Internal Medicine 2004;141:920‐8. [DOI] [PubMed] [Google Scholar]
- Cleland JA, Fritz JM, Kulig K, Davenport TE, Eberhart S, Magel J, Childs JD. Comparison of the effectiveness of three manual physical therapy techniques in a subgroup of patients with low back pain who satisfy a clinical prediction rule. Spine 2009;34(25):2720‐9. [DOI] [PubMed] [Google Scholar]
- Cramer GD, Humphreys CR, Hondras MA, McGregor M, Triano JJ. The Hmax/Mmax ratio as an outcome measure for acute low back pain. Journal of Manipulative and Physiological Therapeutics 1993;16:7‐13. [PubMed] [Google Scholar]
- Farrell JP, Twomey LT. Acute low back pain: comparison of two conservative treatment approaches. The Medical Journal of Australia 1982;1:160‐4. [PubMed] [Google Scholar]
- Glover JR. A clinical trial of rotational manipulation of the spine in back pain cases occurring in a factory. Proceedings of the Royal Society of Medicine 1966;59:847. [PMC free article] [PubMed] [Google Scholar]; Glover JR. Occupational health research and the problem of back pain. The Transactions of the Society of Occupational Medicine 1970;21:2‐12. [DOI] [PubMed] [Google Scholar]; Glover JR, Morris JG, Khosla T. Back pain: A randomized clinical trial of rotational manipulation of the trunk. British Journal of Industrial Medicine 1974;31:59‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadler NM, Curtis P, Gillings DB, Stinnett S. A benefit of spinal manipulation as adjunctive therapy for acute low‐back pain: a stratified controlled trial. Spine 1987;12:703‐5. [PubMed] [Google Scholar]; Hadler NM, Curtis P, Gillings DB, Stinnett S. The use of manipulation in acute low back pain: A group‐controlled study [Der nutzen von manipulationen als zusazliche therapie bei akuten lumbalgie: eine gruppenkontrolierte studie]. Manuelle Medizin 1990;28:2‐6. [Google Scholar]
- Hallegraeff H, Greef M, Winters JC, Lucas C. Manipulative therapy and clinical prediction criteria in treatment of acute nonspecific low back pain. Perceptual and Motor Skills 2009;108(1):196‐208. [DOI] [PubMed] [Google Scholar]
- Hancock MJ, Maher CG, Latimer J, McLachlan AJ, Cooper CW, Day RO, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first‐line treatment for acute low back pain: a randomised controlled trial. Lancet 2007;370:1638‐43. [DOI] [PubMed] [Google Scholar]; Hancock MJ, Maher CG, Latimer J, McLachlan AJ, Cooper CW, O Day R, et al. Manipulative therapy and/or NSAIDs for acute low back pain: design of a randomized controlled trial [ACTRN012605000036617] (study protocol). BMC Musculoskeletal Disorders 2005;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger AA. A controlled trial of rotational manipulation in low back pain. Manuelle Medizin 1980;2:17‐26. [Google Scholar]; Hoehler GK, Tobis JS, Buerger AA. Spinal manipulation for low back pain. JAMA 1981;245:1835‐8. [PubMed] [Google Scholar]; Tobis JS, Hoehler FK. Musculoskeletal manipulation in the treatment of low back pain. Bulletin of the New York Academy of Medicine 1983;7:660‐8. [PMC free article] [PubMed] [Google Scholar]
- Hoiriis KT, Pfleger B, McDuffie FC, Cotsonis G, Elsangak O, Hinson R, Verzosa GT. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. Journal of Manipulative and Physiological Therapeutics 2004;27:388‐98. [DOI] [PubMed] [Google Scholar]
- Juni P, Battaglia M, Nuesch E, Hammerle G, Eser P, Beers R, et al. A randomised controlled trial of spinal manipulative therapy in acute low back pain. Annals of the Rheumatic Diseases 2009;68:1420‐7. [DOI] [PubMed] [Google Scholar]
- MacDonald RS, Bell CMJ. An open controlled assessment of osteopathic manipulation in nonspecific low‐back pain. Spine 1990;15:364‐70. [DOI] [PubMed] [Google Scholar]
- Postacchini F, Facchini M, Palieri P. Efficacy of various forms of conservative treatment in low back pain: a comparative study. Neuro‐Orthopedics 1988;6:28‐35. [Google Scholar]
- Rasmussen GG. Manipulation in treatment of low back pain: a randomized clinical trial. Manuelle Medizin 1979;1:8‐10. [Google Scholar]
- Seferlis T, Lindholm L, Nemeth G. Cost‐minimisation analysis of three conservative treatment programmes in 180 patients sick‐listed for acute low‐back pain. Scandinavian Journal of Primary Health Care 2000;18:53‐7. [DOI] [PubMed] [Google Scholar]; Seferlis T, Nemeth G, Carlsson AM, Gillstrom P. Conservative treatment in patients sick‐listed for acute low‐back pain: a prospective randomised study with 12 months' follow‐up. European Spine Journal 1998;7:461‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skargren EI, Carlsson PG, Oberg BE. One year follow‐up comparison of the cost and effectiveness of chiropractic and physiotherapy as primary management for back pain. Subgroup analysis, recurrence, and additional health care utilization. Spine 1998;23(17):1875‐83. [DOI] [PubMed] [Google Scholar]; Skargren EI, Oberg BE. Predictive factors for 1‐year outcome of low‐back and neck pain in patients treated in primary care: comparison between the treatment strategies chiropractic and physiotherapy. Pain 1998;77(2):201‐7. [DOI] [PubMed] [Google Scholar]; Skargren EI, Oberg BE, Carlsson PG, Gade M. Cost and effectiveness analysis of chiropractic and physiotherapy treatment for low‐back and neck pain. Six month follow‐up. Spine 1997;22(18):2167‐77. [DOI] [PubMed] [Google Scholar]
- Sutlive TG, Mabry LM, Easterling EJ, Durbin JD, Hanson SL, Wainner RS, Childs JD. Comparison of short‐term response to two spinal manipulation techniques for patients with low back pain in a military beneficiary population. Military Medicine 2009;174(7):750‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Andersson GB, Lucente T, Davis AM, Kapler RE, Lipton JA, Leurgans S. A comparison of osteopathic spinal manipulation with spinalcare for patients with low back pain. New England Journal of Medicine 1999;341:1426‐31. [DOI] [PubMed] [Google Scholar]
- Beyerman KL, Palmerino MB, Zohn LE, Kane GM, Foster KA. Efficacy of treating low back pain and dysfunction secondary to osteoarthritis: chiropractic care compared with moist heat alone. Journal of Manipulative and Physiological Therapeutics 2006;29:107‐14. [DOI] [PubMed] [Google Scholar]
- Bishop PB, Quon JA, Fisher CG, Dvorak MFS. The Chiropractic Hospital‐based Interventions Research Outcomes (CHIRO) study: A randomized controlled trial on the effectiveness of clinical practice guidelines in the medical and chiropractic managment of patients with acute mechanical low back pain. The Spine Journal 2010;10:1055‐64. [DOI] [PubMed] [Google Scholar]
- Blomberg S, Hallin G, Grann K, Berg E, Sennerby U. Manual therapy with steroid injections: A new approach to treatment of low back pain. A controlled multicenter trial with an evaluation by orthopedic surgeons. Spine 1994;19:569‐77. [DOI] [PubMed] [Google Scholar]; Blomberg S, Svardsudd K, Mildenberger F. A controlled, multicentre trial of manual therapy in low‐back pain. Initial status, sick‐leave and pain score during follow‐up. Scandinavian Journal of Primary Health Care 1992;10:170‐8. [DOI] [PubMed] [Google Scholar]; Blomberg S, Svardsudd K, Tibblin G. A randomized study of manual therapy with steroid injections in low‐back pain: Telephone interview follow‐up of pain, disability, recovery and drug consumption. European Spine Journal 1994;3:246‐54. [DOI] [PubMed] [Google Scholar]; Blomberg S, Svardsudd K, Tibblin G. Manual therapy with steroid injections in low‐back pain. Improvement of quality of life in a controlled trial with four months' follow‐up. Scandinavian Journal of Primary Health Care 1993;11:83‐90. [DOI] [PubMed] [Google Scholar]; Blomberg S, Tibblin G. A controlled, multicentre trial of manual therapy with steroid injections in low‐back pain: Functional variables, side‐effects and complications during four months follow‐up. Clinical Rehabilitation 1993;7:49‐62. [Google Scholar]
- Bronfort G. Chiropractic versus general medical treatment of low back pain: A small scale controlled clinical trial. American Journal of Chiropractic Medicine 1989;2:145‐50. [Google Scholar]
- Coyer AB, Curwin IH. Low back pain treated by manipulation: A controlled series. British Medical Journal 1955;1(4915):705‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delitto A, Cibulka MT, Erhard RE, Bowling RW, Tenhuis JA. Evidence for use of an extension‐mobilization category in acute low back syndrome: A prescriptive validation pilot study. Physical Therapy 1993;73(4):216‐22. [DOI] [PubMed] [Google Scholar]
- Doran DML, Newell DJ. Manipulation in treatment of low back pain: a multicentre study. British Medical Journal 1975;2:161‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhard RE, Delitto A, Cibulka MT. Relative effectiveness of an extension program and a combined program of manipulation and flexion and extension exercises in patients with acute low back syndrome. Physical Therapy 1994;74:1093‐100. [DOI] [PubMed] [Google Scholar]
- Gemmell HA, Jacobson BH. The immediate effect of activator vs. meric adjustment on acute low‐back pain ‐ a randomized controlled trial. Journal of Manipulative and Physiological Therapeutics 1995;18:453‐6. [PubMed] [Google Scholar]
- Godfrey CM, Morgan PP, Schatzker J. A randomized trial of manipulation for low‐back pain in a medical setting. Spine 1984;9:301‐4. [DOI] [PubMed] [Google Scholar]
- Bogefeldt J, Grunnesjo M, Svardsudd K, Blomberg S. Diagnostic differences between general practitioners and orthopaedic surgeons in a randomised controlled trial in low back pain: The Gotland low back pain study. Upsala Journal of Medical Sciences 2007;112:199‐212. [DOI] [PubMed] [Google Scholar]; Bogefeldt J, Grunnesjo MI, Svardsudd K, Blomberg S. Sick leave reductions from a comprehensive manual therapy programme for low back pain: The Gotland low back pain study. Clinical Rehabilitation 2008;22:529‐41. [DOI] [PubMed] [Google Scholar]; Grunnesjo MI, Bogefeldt JP, Svardsudd KF, Blomberg SIE. A randomized controlled clinical trial of stay‐active care versus manual therapy in addition to stay‐active care: Functional variables and pain. Journal of Manipulative and Physiological Therapeutics 2004;27:431‐41. [DOI] [PubMed] [Google Scholar]
- Helliwell PS, Cunliffe G. Manipulation in low‐back pain. The Physician 1987;April:187‐8. [Google Scholar]
- Hsieh C‐Y, Adams A, Tobis J, Hong C‐Z, Danielson C, Platt K, et al. Effectiveness of four conservative treatments for subacute low back pain. A randomized clinical trial. Spine 2002;27:1142‐8. [DOI] [PubMed] [Google Scholar]
- Hurley DA, McDonough SM, Dempster M, Moore AM, Baxter GD. A randomized clinical trial of manipulative therapy and interferential therapy for acute low back pain. Spine 2004;29(20):2207‐16. [DOI] [PubMed] [Google Scholar]
- Kinalski R, Kuwik W, Pierzak D. The comparison of the results of manual therapy versus physiotherapy methods used in treatment of patients with low back pain syndromes. Manual Medicine 1989;4:44‐6. [Google Scholar]
- Mathews JA, Mills SB, Jenkins VM, Grimes SM, Morkel MJ, Mathews W, et al. Back pain and sciatica: controlled trials of manipulation, traction, sclerosant and epidural injections. British Journal of Rheumatology 1987;26:416‐23. [DOI] [PubMed] [Google Scholar]; Mathews W, Morkel M, Mathews J. Manipulation and traction for lumbago and sciatica: physiotherapeutic techniques used in two controlled trials. Physiotherapy Practice 1988;4:201‐6. [Google Scholar]
- Meade TW, Dyer S, Browne W, Frank AO. Randomised comparison of chiropractic and hospital outpatient management for low‐back pain: results from extended follow‐up. BMJ 1995;311:349‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meade TW, Dyer S, Browne W, Townsend J, Frank AO. Low back pain of mechanical origin: randomised comparison of chiropractic and hospital outpatient treatment. BMJ 1990;300:1431‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwuga VCB. Relative therapeutic efficacy of vertebral manipulation and conventional treatment in back pain management. American Journal of Physical Medicine 1982;61:273‐8. [PubMed] [Google Scholar]
- Pope M, Phillips R, Haugh L, Hsieh C‐Y, MacDonald L, Haldeman S. A prospective randomized three‐week trial of spinal manipulation, transcutaneous muscle stimulation, massage and corset in the treatment of subacute low back pain. Spine 1994;19:2571‐7. [DOI] [PubMed] [Google Scholar]
- Rupert RL, Wagnon R, Thompson P, Ezzeldin MT. Chiropractic adjustments: Results of a controlled clinical trial in Egypt. ICA International Review of Chiropractic 1985;Winter:58‐60. [Google Scholar]
- Sanders GE, Reinert O, Tepe R, Maloney P. Chiropractic adjustive manipulation on subjects with acute low‐back pain: Visual analog pain scores and plasma beta‐endorphin levels. Journal of Manipulative and Physiological Therapeutics 1990;13:390‐5. [PubMed] [Google Scholar]
- Santilli V, Beghi E, Finucci S. Chiropractic manipulation in the treatment of acute back pain and sciatica with disc protrusion: a randomized double‐blind clinical trial of active and simulated spinal manipulations. The Spine Journal 2006;6:131‐7. [DOI] [PubMed] [Google Scholar]
- Sims‐Williams H, Jayson MIV, Young SMS, Baddeley H, Collins E. Controlled trial of mobilisation and manipulation for patients with low back pain in general practice. British Medical Journal 1978;2:1338‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims‐Williams H, Jayson MIV, Young SMS, Baddeley H, Collins E. Controlled trial of mobilisation and manipulation for low back pain: hospital patients. British Medical Journal 1979;2:1318‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrett ACJ, Vernon H. Manipulation and pain tolerance. A controlled study of the effect of spinal manipulation on paraspinal cutaneous pain tolerance levels. American Journal of Physical Medicine 1984;63(5):217‐25. [PubMed] [Google Scholar]
- Waterworth RF, Hunter IA. An open study of diflunisal, conservative and manipulative therapy in the management of acute mechanical low back pain. The New Zealand Medical Journal 1985;98(779):372‐5. [PubMed] [Google Scholar]
- Williams NH, Wilkinson C, Russell I, Edwards RT, Hibbs R, Linck P, Muntz R. Randomized osteopathic manipulation study (ROMANS): pragmatic trial for spinal pain in primary care. Family Practice 2003;20:662‐9. [DOI] [PubMed] [Google Scholar]
- Wreje U, Nordgren B, Aberg H. Treatment of pelvic joint dysfunction in primary care: a controlled study. Scandinavian Journal of Primary Health Care 1992;10:310‐5. [DOI] [PubMed] [Google Scholar]
- Zylbergold RS, Piper MC. Lumbar disc disease: comparative analysis of physical therapy treatments. Archives of Physical Medicine and Rehabilitation 1981;62:176‐9. [PubMed] [Google Scholar]
References to studies awaiting assessment
- Cruser A, Maurer D, Hensel K, Brown SK, White K, Stoll ST. A randomized, controlled trial of osteopathic manipulative treatment for acute low back pain in active duty military personnel. Journal of Manual and Manipulative Therapy 2012;20:5‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali F, Shokri E. The effect of two manipulative therapy techniques and their outcome in patients with sacroiliac joint syndrome. Journal of Bodywork and Movement Therapies 2012;16(1):29‐35. [DOI] [PubMed] [Google Scholar]
- Schenk R, Dionne C, Simon C, Johnson R. Effectiveness of mechanical diagnosis and therapy in patients with back pain who meet a clinical prediction rule for spinal manipulation. Journal of Manual and Manipulative Therapy 2012;20(1):43‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- http://ClinicalTrials.gov/show/NCT00497861Title: Comparison of Mechanical Force, Manually Assisted Activator Manipulation Versus Manual Side Posture Manipulation in Patients With Low Back Pain: a Randomized Pilot StudyPurpose: This study compared the treatment effect of Activator Methods Chiropractic Technique (AMCT) and manual Diversified type spinal manipulative therapy in a sample of patients with acute and sub‐acute low back pain.. Ongoing studyStudy completion date: April 2007.
- http://clinicaltrials.gov/show/NCT00632060Title: The Efficacy of Manual and Manipulative Therapy for Low Back Pain in Military Active Duty Personnel: A Feasibility StudyThe specific aims of this research project are to determine feasibility of, and the comparative treatment effect size for, conducting a larger clinical trial of Manual/Manipulative Therapy (M/MT) in restoring peak performance in military personnel in operational environments and to evaluate the ability of the addition of M/MT to standard care to decrease pain and increase function for patients with low back pain.The following two hypotheses will guide the data collection:The primary hypothesis is that the addition of a course of M/MT to standard care for low back pain will decrease pain at 4 weeks when compared to standard care alone;In addition, the secondary hypothesis will be that the addition of a course of M/MT to standard care for low back pain will decrease pain and increase function over 2 and 4 weeks when compared to standard care alone.. Ongoing studyFebruary 2008.
- http://clinicaltrials.gov/show/NCT01211613A Comparison of Chiropractic Manipulation Methods and Standard Medical Care for Low Back Pain.Purpose: The investigators will be comparing the effectiveness of two types of chiropractic manipulation and standard medical care for patients with a recent onset of low back pain. The two types of chiropractic treatments being compared will be hands‐on (manual) manipulation and mechanical‐assisted (Activator) manipulation. The standard medical care will consist of a medical examination and prescription for over‐the‐counter anti‐inflammatory medication.. Ongoing study Nov. 2010; Estimated study completion date Nov. 2013.
Additional references
- Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber‐Moffett J, Kovacs F, et al. European guidelines for the management of chronic non‐specific low back pain 2004. European Spine Journal 2006;15 Suppl 2:192‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assendelft WJ, Bouter LM, Knipschild PG. Complications of spinal manipulation: A comprehensive review of the literature. Journal of Family Practice 1996;42(5):475‐80. [PubMed] [Google Scholar]
- Axen I, Jones JJ, Rosenbaum A, Lovgren PW, Halasz L, Larsen K, et al. The Nordic Back Pain Subpopulation Program: Validation and improvement of a predictive model for treatment outcome in patients with low back pain receiving chiropractic treatment. Journal of Manipulative and Physiological Therapeutics 2005;28(6):381‐5. [DOI] [PubMed] [Google Scholar]
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: A systematic review. JAMA 2003;289(4):454‐65. [DOI] [PubMed] [Google Scholar]
- Bouter LM, Tulder MW, Koes BW. Methodological issues in low back pain research in primary care. Spine 1998;23:2014‐20. [DOI] [PubMed] [Google Scholar]
- Boutron I, Moher D, Tugwell P, Giraudeau B, Poiraudeau S, Nizard R, et al. A checklist to evaluate a report of a non pharmacological trial (CLEAR NPT) was developed using consensus. Journal of Clinical Epidemiology 2005;58:1233‐40. [DOI] [PubMed] [Google Scholar]
- Bronfort G, Evans RL, Maiers M, Anderson AV. Spinal manipulation, epidural injections, and self‐care for sciatica: A pilot study for a randomized clinical trial. Journal of Manipulative and Physiological Therapeutics 2004;27:503‐8. [DOI] [PubMed] [Google Scholar]
- Bronfort G, Haas M, Evans RL, Bouter LM. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: a systematic review and best evidence synthesis. The Spine Journal 2004;4:335‐56. [DOI] [PubMed] [Google Scholar]
- Bronfort G, Haas M, Evans R, Kawchuk G, Dagenais S. Evidence‐informed management of chronic low back pain with spinal manipulation and mobilization. The Spine Journal 2008;8(1):213‐25. [DOI] [PubMed] [Google Scholar]
- Brown A, Angus D, Chen S, Tang Z, Milne S, Pfaff J, et al. Costs and outcomes of chiropractic treatment for low back pain [technology report no. 56]. Ottawa: Canadian Coordinating Office for Health Technology Assessment2007.
- Cherkin DC, Sherman KJ, Deyo RA, Shekelle PG. A review of the evidence for the effectiveness, safety, and cost of acupuncture, massage therapy, and spinal manipulation for back pain. Annals of Internal Medicine 2003;138:898‐906. [DOI] [PubMed] [Google Scholar]
- Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Annals of Internal Medicine 2007;147(7):492‐504. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioural sciences. Statistical power analysis for the behavioural sciences. New Jersey, USA: Lawrence Earlbaum Associates, Inc., 1988:474pp. [Google Scholar]
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. The Spine Journal 2008;8(1):8‐20. [DOI] [PubMed] [Google Scholar]
- Dagenais S, Gay RE, Tricco AC, Freeman MD, Mayer JM. NASS Contemporary concepts in spine care: Spinal manipulation therapy for acute low back pain. The Spine Journal 2010;10:918‐40. [DOI] [PubMed] [Google Scholar]
- Dunn KM, Croft PR. Epidemiology and natural history of low back pain. Europo Medicophysica 2004;40:9‐13. [PubMed] [Google Scholar]
- Evans DW. Mechanisms and effects of spinal high‐velocity, low‐amplitude thrust manipulation: previous theories. Journal of Manipulative and Physiological Therapeutics 2002;25:251‐62. [DOI] [PubMed] [Google Scholar]
- Foster NE, Hill JC, Hay EM. Subgrouping low‐back pain patients in primary care: Are we getting any better at it?. Manual Therapy 2011;16(1):3‐8. [DOI] [PubMed] [Google Scholar]
- Furlan AD, Pennick V, Bombardier C, Tulder M, Editorial Board Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldeman S. Is it time to discard the term "diagnosis" when examining a person with uncomplicated axial neck pain?. The Spine Journal 2011;11:171‐6. [DOI] [PubMed] [Google Scholar]
- Hancock MJ, Maher CG, Latimer J, Herbert RD, McAuley JH. Independent evaluation of a clinical prediction rule for spinal manipulative therapy: A randomised controlled trial. European Spine Journal 2008;17:936‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta‐analyses of randomized trials of health care interventions. The Cochrane Library 2004;1:Chichester, UK: John Wiley & Sons, Ltd.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa PS, Eberhart A, Cotler A, Nahin R. The 2005 conference on the biology of manual therapies. Journal of Manipulative and Physiological Therapeutics 2006;29:341‐6. [DOI] [PubMed] [Google Scholar]
- Koes BW, Tulder MW, Ostelo R, Burton K, Waddell G. Clinical guidelines for the management of low back pain in primary care: an international comparison. Spine 2001;26:2504‐13. [DOI] [PubMed] [Google Scholar]
- Lamb SE, Hansen Z, Lall R, Castelnuovo E, Withers EJ, Nichols V, Potter R, Underwood MR, Back Skills Training Trial investigators. Group cognitive behavioural treatment for low‐back pain in primary care: A randomised controlled trial and cost‐effectiveness analysis. Lancet 2010;375:916‐23. [DOI] [PubMed] [Google Scholar]
- Leboeuf‐Yde C, Hennius B, Rudberg E, Leufvenmark P, Thunman M. Side‐effects of chiropractic treatment: A prospective study. Journal of Manipulative and Physiological Therapeutics 1997;20:511‐5. [PubMed] [Google Scholar]
- Malmqvist S, Leboeuf‐Yde C, Ahola T, Andersson O, Ekstrom K, Pekkaarinen H, et al. The Nordic back pain subpopulation program: Predicting outcome among chiropractic patients in Finland. Chiropractic & Osteopathy 2008;16:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence the estimates of intervention effectiveness reported in meta‐analyses. Lancet 2000;356:1228‐31. [DOI] [PubMed] [Google Scholar]
- Mueller PS, Montori VM, Bassler D, Koenig BA, Guyatt GH. Ethical issues in stopping randomized trials early because of apparent benefit. Annals of Internal Medicine 2007;146(12):878‐81. [DOI] [PubMed] [Google Scholar]
- Okike K, Kocher MS, Mehlman CT, Bhandari M. Industry‐sponsored research. Injury 2008;39(6):666‐80. [DOI] [PubMed] [Google Scholar]
- Ostelo RWJG, Deyo RA, Stratford P, Waddell G, Croft P, Korff M, Bouter LM, Vet HC. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008;33(1):90‐4. [DOI] [PubMed] [Google Scholar]
- Pickar JG. Neurophysiological effects of spinal manipulation. The Spine Journal 2002;2:357‐71. [DOI] [PubMed] [Google Scholar]
- Rubinstein SM, Tulder M. A best‐evidence review of diagnostic procedures for neck and low‐back pain. Best Practice and Research Clinical Rheumatology 2008;22(3):471‐82. [DOI] [PubMed] [Google Scholar]
- Rubinstein SM, Terwee C, Assendelft WJJ, Boer MR, Tulder MW. Spinal manipulation for acute low‐back pain. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008880] [DOI] [Google Scholar]
- Rubinstein SM, Middelkoop M, Assendelft WJJ, Boer MR, Tulder MW. Spinal manipulative therapy for chronic low‐back pain. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008112.pub2] [DOI] [PubMed] [Google Scholar]
- Rubinstein SM, Terwee CB, Boer MR, Tulder MW. Is the methodological quality of trials on spinal manipulative therapy for low‐back pain improving?. International Journal of Osteopathic Medicine 2012;15:37‐52. [Google Scholar]
- Sandoz R. The significance of the manipulative crack and of other articular noises. Annals of the Swiss Chiropractors' Association 1969;4:47‐68. [Google Scholar]
- Senstad O, Leboeuf‐Yde C, Borchgrevink C. Frequency and characteristics of side‐effects of spinal manipulative therapy. Spine 1997;22:435‐40. [DOI] [PubMed] [Google Scholar]
- Triano JJ. Biomechanics of spinal manipulative therapy. The Spine Journal 2001;1:121‐30. [DOI] [PubMed] [Google Scholar]
- Unsworth A, Dowson D, Wright V. 'Cracking joints'. A bioengineering study of cavitation in the metacarpophalangeal joint. Annals of the Rheumatic Diseases 1971;30:348‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen EA, Vet HC, Pool JJ, Schuller W, Zoete A, Bouter LM. Variance in manual treatment of nonspecific low back pain between orthomanual physicians, manual therapists, and chiropractors. Journal of Manipulative and Physiological Therapeutics 2005;28:108‐16. [DOI] [PubMed] [Google Scholar]
- Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board Cochrane Back Review Group. Updated method guidelines for systemic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
- Tulder MW, Tuut M, Pennick V, Bombardier C, Assendelft WJ. Quality of primary care guidelines for acute low back pain. Spine 2004;29:E357‐62. [DOI] [PubMed] [Google Scholar]
- Tulder MV, Becker A, Bekkering T, Breen A, Real MTG, Hutchinson A, et al. European Guidelines for the management of acute non‐specific low back pain in primary care 2004. European Spine Journal 2006;15 Suppl 2:169‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulder MW, Suttorp M, Morton S, Bouter LM, Shekelle P. Empirical evidence of an association between internal validity and effect size in randomized controlled trials of low‐back pain. Spine 2009;34(16):1685‐92. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Assendelft WJJ, Morton SC, Yu EI, Suttorp MJ, Shekelle PG. Spinal manipulative therapy for low back pain. A meta‐analysis of effectiveness relative to other therapies. Annals of Internal Medicine 2003;138:898‐906. [DOI] [PubMed] [Google Scholar]
- Assendelft WJJ, Morton SC, Yu EI, Suttorp MJ, Shekelle PG. Spinal manipulative therapy for low‐back pain. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000447.pub2] [DOI] [PubMed] [Google Scholar]


