Abstract
Cognitive impairment is one of the most common co-occurring chronic conditions among elderly heart failure patients (incidence: up to ~ 80%); however, the underlying mechanisms are not completely understood. It is hypothesized that in addition to decreased cardiac output, increases in central—and consequentially, cerebral—venous pressure (backward failure) also contribute significantly to the genesis of cognitive impairment. To test this hypothesis and elucidate the specific pathogenic role of venous congestion in the brain, we have established a novel model of increased cerebral venous pressure: mice with jugular vein ligation (JVL). To test the hypothesis that increased venous pressure in the brain contributes to the development of cognitive deficits by causing blood-brain barrier disruption, dysregulation of blood flow, and/or promoting neuroinflammation, in C57BL/6 mice, the internal and external jugular veins were ligated. Cognitive function (radial arm water maze), gait function (CatWalk), and motor coordination (rotarod) were tested post-JVL. Neurovascular coupling responses were assessed by measuring changes in cerebral blood flow in the whisker barrel cortex in response to contralateral whisker stimulation by laser speckle contrast imaging through a closed cranial window. Blood-brain barrier integrity (IgG extravasation) and microglia activation (Iba1 staining) were assessed in brain slices by immunohistochemistry. Neuroinflammation-related gene expression profile was assessed by a targeted qPCR array. After jugular vein ligation, mice exhibited impaired spatial learning and memory, altered motor coordination, and impaired gait function, mimicking important aspects of altered brain function observed in human heart failure patients. JVL did not alter neurovascular coupling responses. In the brains of mice with JVL, significant extravasation of IgG was detected, indicating blood-brain barrier disruption, which was associated with histological markers of neuroinflammation (increased presence of activated microglia) and a pro-inflammatory shift in gene expression profile. Thus, cerebral venous congestion per se can cause blood-brain barrier disruption and neuroinflammation, which likely contribute to the genesis of cognitive impairment. These findings have relevance to the pathogenesis of cognitive decline associated with heart failure as well as increased cerebal venous pressure due to increased jugular venous reflux in elderly human patients.
Keywords: Vascular contributions to cognitive impairment and dementia (VCID), VCI, Vascular cognitive impairment, Vein, Cerebral circulation
Introduction
Chronic heart failure is a significant health problem in the aging population of the Western world that affects over 10% of the elderly (Leto and Feola 2014). Despite important advances in pharmacological therapies and prevention of heart failure, mortality and morbidity are still high and the quality of life of affected patients is poor. Cognitive impairment is an important complication of heart failure among older adults that contributes to decreased quality of life (Woo et al. 2009; Beer et al. 2009; Hooghiemstra et al. 2017) (incidence: from 25 to 80%) (Leto and Feola 2014; Cannon et al. 2017). Heart failure likely exerts multifaceted effects on the brain (Jefferson et al. 2010). It may impair cerebral blood flow (CBF) by decreasing cardiac output (termed “forward failure”) (Roy et al. 2017; Loncar et al. 2011; Gruhn et al. 2001; Cornwell III and Levine 2015; Choi et al. 2006), lowering perfusion pressure, which are recognized as important factors contributing to cognitive decline (Fulop et al. 2019). Importantly, heart failure also causes systemic venous congestion (termed “backward failure”), which raises jugular venous pressure that is transmitted to the cerebral circulation (Watson et al. 2000). It is hypothesized that cerebral venous congestion plays a significant role in the development of cognitive decline (Fulop et al. 2019). Yet, there are no experimental studies extant that attempt to selectively investigate the mechanistic effects of cerebral venous congestions on cognitive function.
The present study was designed to experimentally test the hypothesis that cerebral venous congestion per se can elicit cognitive impairment by promoting blood-brain barrier disruption, neuroinflammation, and/or by impairing regulation of CBF. To achieve this goal and to elucidate the specific pathogenic role of venous congestion in the brain, we have established a novel model of increased cerebral venous pressure: mice with jugular vein ligation. To test the hypothesis that increased venous pressure in the brain contributes to the development of cognitive deficits by causing blood-brain barrier disruption, dysregulation of blood flow, and/or promoting neuroinflammation, in C57BL/6 mice, the internal and texternal jugular veins were ligated. On the second week after the JVL, cognitive function (radial arm water maze), gait function (CatWalk), and motor coordination (rotarod) were tested. Neurovascular coupling responses were assessed by measuring changes in CBF in the whisker barrel cortex in response to contralateral whisker stimulation by laser speckle contrast imaging. Blood-brain barrier integrity (IgG extravasation) and microglia activation (CD68/Iba1 staining) were assessed in brain slices by immunohistochemistry. Neuroinflammation-related gene expression profile was assessed by a targeted qPCR array. In addition, the additive effects of venous congestion and arterial hypertension were studied by treating control and experimental mice with angiotension II.
Methods
Animals, jugular vein ligation
Adult (10 months old, n = 40) male C57BL/6 mice (purchased from the Jackson Laboratories) were used. Animals were housed under specific pathogen-free barrier conditions in the Rodent Barrier Facility at the University of Oklahoma Health Sciences Center under a controlled photoperiod (12-h light; 12-h dark) with unlimited access to water and were fed a standard AIN-93G diet (ad libitum). All procedures were approved by the Institutional Animal Use and Care Committees of the University of Oklahoma Health Sciences Center.
To test the effect of venous congestion on the brain, the mice were divided into two groups: (1) bilateral external and internal jugular vein ligation (JVL), (2) sham-operated control. One group underwent a venous occlusion surgery, according to the published protocol of Auletta and coworkers (Auletta et al. 2017). The other group underwent sham operation. In brief, anesthesia of mice was induced with 4% of isoflurane and was kept between 1.5 and 2% during the surgical procedure. Bilateral occlusion of the external jugular veins and the internal jugular veins was performed according to the protocol of Auletta and coworkers (Auletta et al. 2017). The interruption of blood flow was obtained via surgical ligation. Sham operation consisted of bilateral surgical exposure of both the external jugular veins and the internal jugular veins without surgical ligation, with the same length of anesthesia. Each mouse was placed in a dorsally recumbent position on a heating pad and the hair was removed. A cutaneous midline incision was made on the neck. Blunt dissection of the loose fascia was performed, and the salivary glands were separated and reflected dorsolaterally to expose the external jugular veins. The common trunk of the external jugular veins, which is situated close to the thoracic inlet, was exposed for surgical ligation. The internal jugular veins, located over the carotid arteries next to the vagus nerve, were also exposed for surgical ligation. The surgical ligations were performed using a 6-0 polyfilament surgical suture. Two surgeon’s knots were placed around the veins so that the vessels could be cut between the knots. Once the surgerical ligations were completed, the wounds were closed using a 6-0 nylon monofilament surgical suture with a simple continuous pattern. Antibiotic ointment was applied over the closed wound and the mice were allowed to recover. The medication was applied every day for five consecutive days. The mice were checked daily for suture failure, infection, and bleeding. The overall success rate of the surgical ligation was over 90%. After a recovery period of 7 days, animals underwent behavioral evaluation and terminal experimentation to measure neurovascular coupling responses
Induction of hypertension
The additive effect of venous congestion and arterial hypertension on BBB integrity and neuroinflammation was studied in a separate set of animals with bilateral ligation of external jugular veins and the internal jugular veins or sham operation. Arterial hypertension was induced by a combination treatment with ω-nitro-l-arginine-methyl ether (l-NAME, 100 mg/kg/day, in drinking water) and administration of angiotensin II (Ang II; s.c. via osmotic mini-pumps [Alzet Model 2006, 0.15 μl/h, Durect Co, Cupertino, CA]). Pumps were filled with either saline or solutions of angiotensin II (Sigma Chemical Co., St. Louis, MO, USA) that delivered (subcutaneously) 1 μg/min/kg of angiotensin II, thus generating four experimental groups: (1) JVL + Ang II, (2) JVL + vehicle, (3) sham control + Ang II, and (4) sham control + vehicle. Pumps were placed into the subcutaneous space of ketamine/xylazine-anesthetized mice through a small incision in the interscapular area that was closed with surgical sutures using aseptic techniques. All incision sites healed rapidly without the need for additional medication. Blood pressure of the animals was measured using a tail-cuff blood pressure apparatus (CODA Non-Invasive Blood Pressure System, Kent Scientific Co., Torrington, CT), as described (Toth et al. 2015a). Animals were sacrificed on day 10 post-induction of hypertension and/or JVL for tissue collection.
Standardized neurological examination of mice
Neurological examination was performed as reported (Toth et al. 2015a; Tarantini et al. 2017a), by assessing each animal’s spontaneous activity, symmetry in the movement of the four limbs, forelimb outstretching, climbing ability, body proprioception, response to vibrissae touch, and gait coordination. Each examined animal was provided with a score calculated by the summation of all individual test scores.
Behavioral studies
Behavioral tasks were performed on the second week post-operationally to characterize the effect of jugular vein ligation on learning and memory, sensory-motor function, gait, and locomotion.
Radial arm water maze testing
Spatial memory and long-term memory were tested using the radial arm water maze task, as described (Tarantini et al. 2019; Tarantini et al. 2018a; Ungvari et al. 2017a; Shukitt-Hale et al. 2004). The maze consisted of eight arms 9 cm wide that radiated out from an open central area, with a submerged escape platform located at the end of one of the arms. Paint was added into the water to make it opaque. The maze was surrounded by privacy blinds with extramaze visual cues. Intramaze visual cues were placed at the end of the arms. The mice were monitored by a video tracking system directly above the maze as they waded and parameters were measured using Ethovision software (Noldus Information Technology Inc., Leesburg, VA, USA). Experimenters were unaware of the experimental conditions of the mice at the time of testing. During the learning period each day, mice were given the opportunity to learn the location of the submerged platform during two sessions each consisting of four consecutive acquisition trials. On each trial, the mouse was started in one arm not containing the platform and allowed to wade for up to 1 min to find the escape platform. All mice spent 30 s on the platform following each trial before beginning the next trial. The platform was located in the same arm on each trial. Over the 3 days of training, mice in the young control group gradually improved performance as they learned the procedural aspects of the task. Upon entering an incorrect arm (all four paws within the distal half of the arm) or failing to select an arm after 15 s, the mouse was charged an error. Learning capability was assessed by comparing performance on days 2 and 3 of the learning period. When eventually both groups learned the procedural aspects of the task, the mice were placed in their home cage for 7 days. Then, the mice were administered the retention trial on day 10.
Rotarod, motor skill learning
Motor coordination was assessed in JVL and sham-operated control mice by using an automated four-lane rotarod (Columbus Instruments, Columbus, OH) as described (Tarantini et al. 2015). Analysis of day-to-day changes in performance on the accelerating rotarod test was used to evaluate the motor skill learning. In brief, mice were pre-trained by placing them on the moving rotarod at 10 rpm until they performed at this speed for 120 s. On the days of testing, mice were habituated in their home cages and acclimate to the testing room for at least 15 min. The test phase consisted of 3 trials (separated by 15-min inter-trial intervals) per day for 4 days. The testing apparatus was set to accelerate from 4 to 40 rpm in 300 s. One mouse was then placed on each lane and the rotarod was started with an initial rotation of 4 rpm. The rotational velocity was set to increase every 10 s and the latency to fall was recorded. Latency to fall was recorded in seconds by an infra-red beam across the fall path along with the max rpm sustained by each mouse (MacLaren et al. 2014).
Grip strength test
A grip strength test was used to measure the maximal muscle strength of forelimbs of the mice. Forelimb grip strength was assessed using a grip strength meter (Chatillon Ametek Force Measurement, Brooklyn, New York). The strength measurements of each group of mice were measured three times by the same investigator. The maximum grip strength values were used for subsequent analysis.
Measurement of neurovascular coupling responses
Neurovascular coupling (functional hyperemia) is a critical homeostatic mechanism that ensures adequate adjustment of cerebral blood flow to increases in the oxygen and nutrient demands of activated neurons (Ungvari et al. 2017a; Csiszar et al. 2017; Tarantini et al. 2017b; Tarantini et al. 2017c). Several cardiovascular risk factors promote cognitive decline by impairing neurovascular coupling. In the present study, we tested the hypoythesis that venous congestion also may negatively impact neurovascular coupling responses. To achieve that goal, after behavioral testing, mice in each group were anesthetized with isoflurane (4% induction and 1% maintenance), endotracheally intubated and ventilated (MousVent G500; Kent Scientific Co, Torrington, CT). A thermostatic heating pad (Kent Scientific Co, Torrington, CT) was used to maintain rectal temperature at 37 °C [26]. End-tidal CO2 was controlled between 3.2 and 3.7% to keep blood gas values within the physiological range, as described (Tarantini et al. 2015; Toth et al. 2015b). The right femoral artery was cannulated for arterial blood pressure measurement (Living Systems Instrumentations, Burlington, VT) (Toth et al. 2014). The blood pressure was within the physiological range throughout the experiments (90–110 mmHg). Mice were immobilized and placed on a stereotaxic frame (Leica Microsystems, Buffalo Grove, IL), the scalp and periosteum were pulled aside, and the skull was gently thinned using a dental drill while cooled with dripping buffer. A laser speckle contrast imager (Perimed, Järfälla, Sweden) was placed 10 cm above the thinned skull, and to achieve the highest CBF response, the right whiskers were stimulated for 30 s at 10 Hz from side to side. Differential perfusion maps of the brain surface were captured. Changes in CBF were assessed above the left barrel cortex in six trials in each group, separated by 5–10-min intervals. At the end of the experiments, the animals were transcardially perfused and decapitated. The brains were immediately removed and pieces of the somatosensory and motor cortex were isolated and frozen for subsequent analysis.
Immunofluorescent labeling and confocal microscopy: assessment of blood-brain barrier disruption and microglia activation
Mice were transcardially perfused with PBS; then, brains were removed and hemisected. The left hemispheres were fixed overnight in 4% paraformaldehyde, then were cryoprotected in a series of graded sucrose solutions (10%, 20%, and 30% overnight) and frozen in Cryo-Gel (Electron Microscopy Sciences, Hatfield, PA). Coronal sections of 70 μm were cut through the hippocampus and stored free-floating in cryopreservative solution (25% glycerol, 25% ethylene glycol, 25% 0.2 M phosphate buffer, 25% distilled water) at – 20 °C. Selected sections were ~ 1.6 mm caudal to bregma, representing the more rostral hippocampus. After washing (3× for 5 min with TBS then 3× for 5 min with 1× TBS + 0.25% Triton X-100), sections were treated with boiling sodium citrate buffer (pH 6) for 20 min. After a second washing step (3× for 5 min with distilled water plus 3× for 5 min with 1× TBS) and blocking in 5% BSA/TBS (with 0.5% Triton X-100, 0.3 M glycine, and 1% fish gelatin; for 3 h), sections were immunostained using primary antibodies for 2 nights at 4 °C. The following primary antibodies were used: goat anti-mouse IgG (1:100, FITC conjugated; Cat No.: 005-090-003, Jackson Immuno Research, West Grove, PA) to label extravasated IgG and rabbit anti-mouse Iba1 (1:50, unconjugated; Cat No.: 019-19741, Wako, Richmond, VA) to label microglia. Alexa Fluor 647–labeled donkey anti-rabbit IgG was used as the secondary antibody to label Iba1+ microglia (Abcam, Cambridge, MA). Sections were washed for 3× for 5 min with TBS then 3× for 5 min with 1× TBS + 0.25% Triton X-100. For nuclear counterstaining, DAPI (Life Technologies, Grand Island, NY) was used. Then, the sections were transferred to slides and cover-slipped with prolong antifade mounting medium. Confocal images were captured using a Leica SP8 MP confocal laser scanning microscope.
Immunofluorescent labeling for Iba1 was used to identify activated microglia in the brain, respectively. The relative numbers of Iba1-positive microglia per region of interest in the hippocampus were calculated. In each animal, 4 randomly selected fields from the hippocampus were analyzed in 6 nonadjacent sections. Six animals per group were analyzed.
Assessment of pro-inflammatory gene expression in the hippocampus
In addition to quantifying microglia activation, we analyzed relative abundance of several neuroinflammatory cytokines/chemokines through quantitative real-time PCR with RNA isolated from snap-frozen hippocampal samples using validated TaqMan Gene Expression Assays (Applied Biosystems) and a Strategen MX3000 platform as described (Tucsek et al. 2014a). Total RNA was isolated with RNeasy Mini Kit (QIAGEN) using a fully automated QIAcube-based workflow and was reverse transcribed using the High Capacity RNA-to-cDNA synthesis kit (Applied Biosystems) as described previously (Bailey-Downs et al. 2012). QRT-PCR quantification was performed using the ΔΔCq method. The relative quantities of the reference genes Hprt, Ywhaz, B2m, Actb, and S18 were determined and a normalization factor was calculated based on the geometric mean for internal normalization.
Results
Jugular vein ligation results in impaired cognitive function in mice
To determine how JVL impacts cognitive function in mice, animals were tested in the radial arm water maze (Fig. 1). We compared the learning performance of mice in each experimental group by analyzing the day-to-day changes in the combined error rate. During acquisition, mice from both groups showed a decrease in the combined error rate (Fig. 1b) across days, indicating learning of the task. After the first day of learning, sham-operated mice tended to have lower combined error rate than mice with JVL (Fig. 1b).
Fig. 1.

Cerebral venous conhestion induced by jugular vein ligation (JVL) associates with impaired radial arm water maze (RAWM) performance. a Heatmap representing the percentage of time spent in different locations in the maze for a randomly selected animal from each group during experimental day 3. b Animals with JVL tend to have higher combined error rates throughout the learning phase and probe day 10 as compared with sham-operated controls. c JVL results in higher working memory error rates as compared with sham-operated controls. n = 10–15 for each data point. All data are shown as mean ± SEM. Statistical significance was calculated using one-way ANOVA with Tukey’s post hoc test to determine differences among groups. *P < 0.05
To analyze working memory function (short-term memory that is involved in immediate conscious perception), we examined re-entries into incorrect arms (without hidden platform) that were previously attempted for escape. We found that working memory function was also impaired in mice with JVL as compared with sham-operated control mice (Fig. 1c). The analyses of noncognitive parameters revealed no difference in swimming speed and non-exploratory behavior (the cumulative time the mice spent not actively looking for the platform, e.g., floating) (data not shown). Taken together, the aforementioned results suggest that cerbral venous congestion associated with JVL in mice impairs performance in the radial arm water maze, which results from a decline in hippocampal-dependent spatial learning and memory and not from changes in motor or motivational processes.
Jugular vein ligation does not affect neurovascular coupling responses
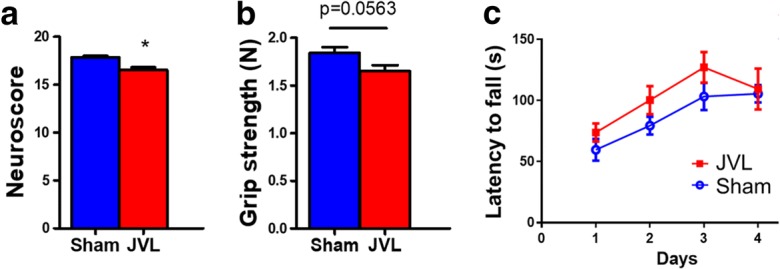
There is strong evidence that impairment of neurovascular coupling responses associates with cognitive decline (Tarantini et al. 2015; Tarantini et al. 2017c). Neurovascular coupling responses are sensitive to a wide range of cardiovascular risk factors (Tarantini et al. 2019; Tarantini et al. 2018a; Toth et al. 2014, 2015b; Tarantini et al. 2017d, 2018b; Toth et al. 2017; Tucsek et al. 2014b), including changes in the hemodynamic environment (De Silva and Faraci 2012; Dunn and Nelson 2014; Faraco et al. 2016; Kazama et al. 2004). Thus, we hypothesized that venous congestion may also promote neurovascular dysfunction. Contrary to our hypothesis, we found that CBF responses in the whisker barrel cortex elicited by contralateral whisker stimulation were unaffected by JVL (Fig. 2).
Fig. 2.
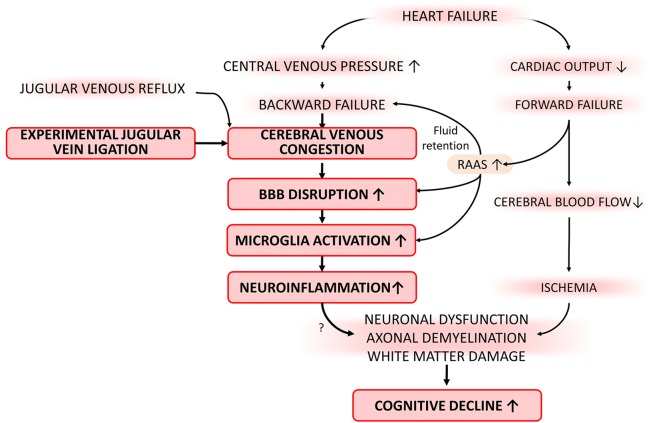
Effects on jugular vein ligation (JVL) on neurological parameters, grip strength, and motor skill learning. a JVL in mice resulted in a significant decline in the composite neuroscore. b Grip strength measured in mice with JVL tended to be weaker than the force values produced by sham-operated controls. c Motor skill learning on the accelerating rotarod. Daily changes in mean latencies to fall for mice with JVL and sham-operated controls are shown. Data are mean ± S.E.M. (n = 10–20 for each data point). *P < 0.05 vs. sham control
Effects of jugular vein ligation on neurological parameters, grip strengths, and motor learning function
We found that JVL resulted in a significant decline in the neurological score (Fig. 3a). To investigate the effects of JVL on the motor performance of mice, we measured performance of the accelerating rotarod and grip strength which evaluate muscle strength, balance, and endurance. We found a discernible trend for decline in grip strength in mice with JVL (Fig. 3b). Motor skill learning was assessed using a modified rotarod test. During a 4-day-long experimental period, mice with JVL displayed a skill learning curve that was similar to sham-operated controls (Fig. 3c).
Fig. 3.
Effects on jugular vein ligation (JVL) on neurovascular coupling responses. a Representative pseudocolour laser speckle flowmetry maps of baseline CBF (upper images) and CBF changes in the whisker barrel field relative to baseline during contralateral whisker stimulation (lower images, right oval, 30 s, 5 Hz) in mice with JVL and sham-operated control mice. Color bar represents CBF as percent change from baseline. b Time course of CBF changes after the start of contralateral whisker stimulation (horizontal bars). Summary data are shown in c. Data are mean ± S.E.M. (n = 6–8 in each group). n.s., not significant
Jugular vein ligation exacerbates BBB disruption in the hippocampus
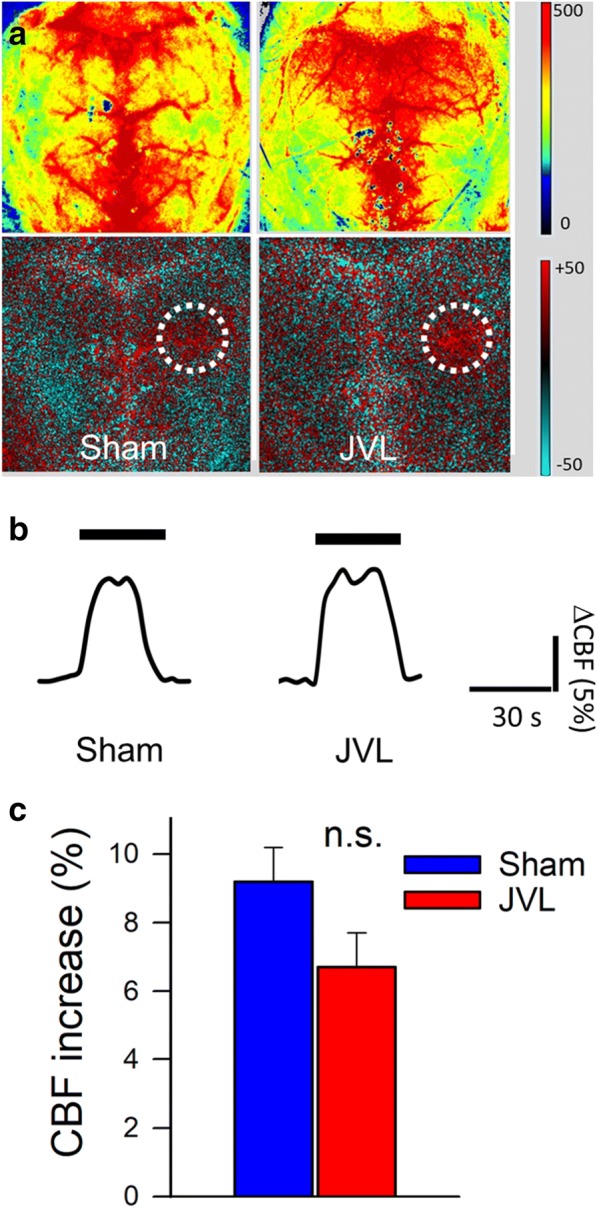
Using extravasated plasma-derived IgG as a marker for increased hippocampal cerebrovascular permeability, we tested the hypothesis that in mice cerebral venous congestion per se is associated with an exacerbated BBB disruption. Immunostaining for plasma-derived IgG revealed significant IgG deposits in the hippocampus of mice with JVL, which was further exacerbated by induction of hypertension (Fig. 4). IgG leakage in the hippocampus of hypertensive sham-operated mice was significantly lower, and there was no detectable IgG leakage in normotensive control mice (Fig. 4).
Fig. 4.
Cerebral venous congestion induced by jugular vein ligation (JVL) exacerbates disruption of the blood-brain barrier. a Confocal microscopy analysis of plasma-derived IgG (green) in the hippocampus of mice with JVL and sham-operated controls with or without angiotensin II (Ang II)–induced hypertension. Bar graphs are summary data (b). Note the increased presence of extravascular IgG deposits in the hippocampus of mice with JVL. Data are mean ± S.E.M. n= 4–6 animals per group. *P < 0.05 vs. sham control, #P < 0.05 vs. no Ang II
Jugular vein ligation exacerbates neuroinflammation in the hippocampus
Previous studies suggest that leakage of plasma-derived factors through the damaged BBB has the potential to induce neuroinflammation by activating microglia (Tucsek et al. 2014a; Zlokovic 2008; Valcarcel-Ares et al. 2018). We found that in the hippocampi of sham-operated control mice, the number of activated Iba1+ microglia was low (Fig. 5a, b). In the hippocampi of mice with JVL, the number of activated Iba1+ microglia was increased (Fig. 5a, b). We found that JVL-induced microglia activation was exacerbated in the hippocampi of hypertensive mice (Fig. 5a, b). Sustained JVL-induced activation of microglia was associated with an increased expression of several pro-inflammatory cytokines and chemokines in the hippocampi and this effect was exacerbated by hypertension (Fig. 5c).
Fig. 5.
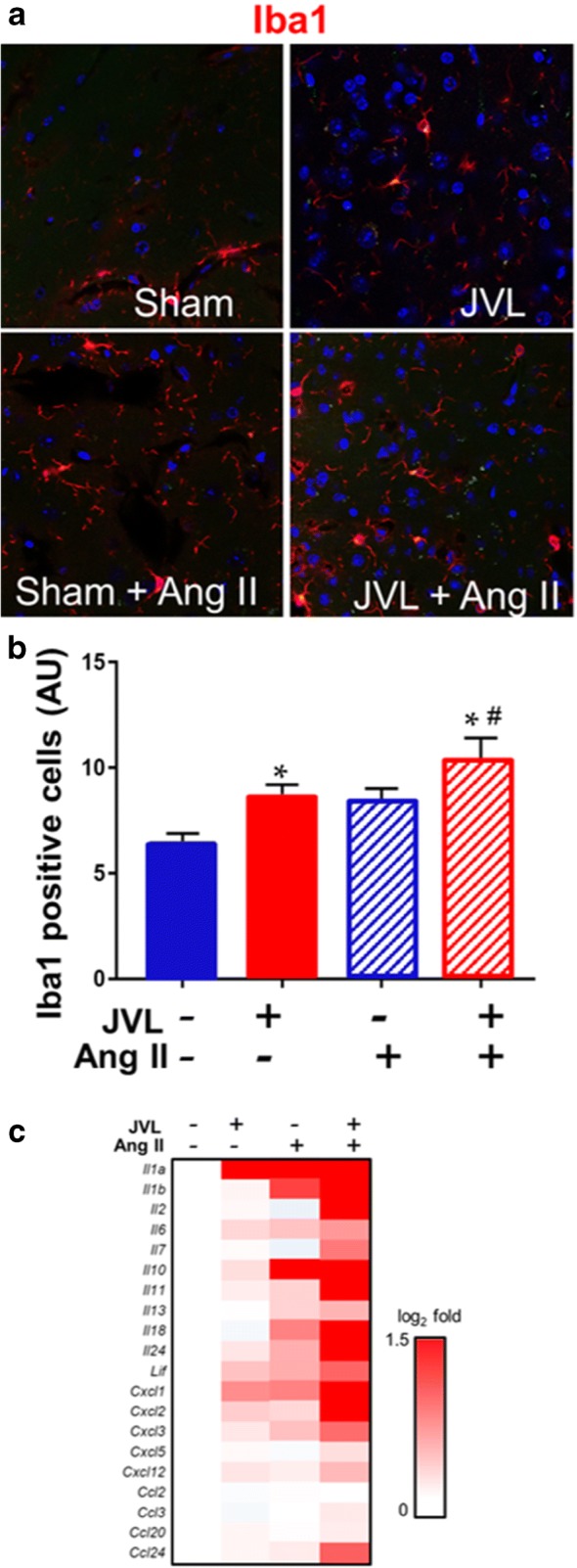
Cerebral venous congestion induced by jugular vein ligation (JVL) exacerbates neuroinflammation. a–d Confocal images showing Iba1-positive (red fluorescence) activated microglia in the hippocampus from mice with JVL and sham-operated control mice with or without angiotensin II (Ang II)–induced hypertension. Blue fluorescence: nuclei. b Summary data of relative changes in the number of IBA1-positive activated microglia in the hippocampus. Data are mean ± S.E.M. n = 4–6 animals per group. *P < 0.05 vs. sham control, #P < 0.05 vs. no Ang II. c Cerebral venous congestion induced by JVL is associated with a pro-inflammatory shift in cytokine expression profiles in the mouse hippocampus. The heat map is a graphic representation of normalized mRNA expression of cytokines and chemokines depicted by color intensity, from highest (bright red) to lowest (white) changes in expression (n = 6 in each group). Mice with JVL-induced venous congestion superimposed upon systemic hypertension have the highest expression of inflammatory markers`
Discussion
The key finding of this study is that cerebral venous congestion per se promotes blood-brain barrier disruption and neuroinflammation, which associate with cognitive impairment in a validated mouse model of increased cerebral venous pressure (Auletta et al. 2017).
This is the first study, to our knowledge, to demonstrate that isolated cerebral venous congestion induced by bilateral jugular vein occlusion associates with cognitive decline in mice. The critical role for increased venous pressure per se in the genesis of neuronal dysfunction in humans is supported by the findings that in addition to patients suffering from backward heart failure, patients with cerebral venous hypertension induced by severe heart valve insufficiency (Sung et al. 2019) or an intracranial dural arteriovenous fistula (Randall et al. 2015; Morparia et al. 2012; Dinc et al. 2019; Geraldes et al. 2012; Hurst et al. 1998; Labeyrie et al. 2014; Racine et al. 2008) often exhibit (potentially reversible) cognitive impairment. In that regard, it is interesting that in a transgenic mouse model slowly developing isolated heart failure at the stage of early left ventricle diastolic dysfunction (with preserved ejection fraction) already associates with cognitive impairment (Adamski et al. 2018). Rodent models of cerebral venous congestion caused by arteriovenous anastomosis also exhibit cognitive impairment, extending the human observations (Hai et al. 2009; Zhang et al. 2019). In addition, elderly patients often exhibit jugular venous reflux, which leads to stagnation or reversal of the internal jugular vein flow, promoting transmission of increased central venous pressure to the cerebral venous circulation. The internal jugular vein valve, which is critical for the prevention of jugular venous reflux (Lepori et al. 1999; Dhanger et al. 2016), is frequently incompetent in the elderly (Uchino et al. 2007; Inano et al. 2010; Kudo et al. 2004; Jang et al. 2013; Kang et al. 2015; Kim et al. 2014) promoting cerebral venous hypertension (e.g., during sustained Valsalva maneuver (Ungvari et al. 2018)) (Zivadinov and Chung 2013). Importantly, incidence of jugular valve incompetence can reach ~ 30 to 90% in the general population (Valecchi et al. 2010; Akkawi et al. 2002).
The mechanisms by which cerebral venous congestion/increased cerebral venous pressure promotes cognitive impairment are likely multifaceted (Fig. 6). Our studies provide direct evidence that cerebral venous congestion promotes BBB disruption, which associates with significant neuroinflammation (microglia activation and upregulation of pro-inflammatory cytokines and chemokines). These results extend previous findings obtained in Sprague-Dawley rats showing that superior venae cavae occlusion, which increases pial venous pressure from ~ 7 to ~ 30 mmHg, also results in significant BBB disruption (Mayhan and Heistad 1986). Signs of venous congestion (enlarged atria) were also reported to associate with compromised BBB permeability in a transgenic mouse model of heart failure (Adamski et al. 2018). The mechanisms of BBB disruption associated with venous congestion likely involve mechanical damage to thin-walled venules and capillaries, including impairment of tight junctions. Our findings demonstrate that through the damaged BBB evident in mice with JVL, plasma constituents, including IgG, enter the brain. Plasma-derived factors can affect neuronal function by multiple mechanisms (Zlokovic 2008; Fernandez-Vizarra et al. 2012), including the induction of neuroinflammation (Tucsek et al. 2014a; Bruce-Keller et al. 2010; Pistell et al. 2010; White et al. 2009). Plasma-derived IgG is a particularly potent stimulus for microglia activation, via activating IgG Fcγ receptors (Tucsek et al. 2014a). Other plasma constituents that can contribute to microglia activation upon BBB disruption include thrombin, fibrinogen, and inflammatory cytokines (Davalos et al. 2012; Carreno-Muller et al. 2003). Here we provide evidence that in mice with cerebral venous congestion, BBB disruption and increased extravasation of IgG and likely other plasma constituents are associated with an exacerbated neuroinflammatory response (Adamski et al. 2018) as shown by the increased number of activated microglia and upregulation of inflammatory mediators in the hippocampi. Pro-inflammatory cytokines, chemokines, proteases, and reactive oxygen species derived from activated microglia have been shown to promote neuronal dysfunction (Gao et al. 2003; Kaneko et al. 2012; Block et al. 2007). Importantly, we found that the deleterious effects of cerebral venous congestion on blood-brain barrier integrity and inflammatory processes are exacerbated by comorbid hypertension. This observation has important clinical relevance. A large percentage of heart failure patients have a history of hypertension and these patients perform worse in cognitive tests than those without such history (Alosco et al. 2012). Future studies should determine that the synergistic negative effects of heart failure and hypertension on cognitive function involve exacerbated BBB disruption and neuroinflammation. Additional mechanisms by which increased cerebral venous pressure may promote cognitive decline include decreases of cerebral blood flow, microhemorrhages (Ungvari et al. 2018, 2017b), and altered cerebrospinal fluid absorption (Fulop et al. 2019). Heart failure patients are also frequently diagnosed with white matter hyperintensities (WMHs, a radiological finding showing damage in the white matter regions of the brain near the lateral ventricles on T2/FLAIR MRI sequences) (Alosco et al. 2013), which predict poorer attention and executive function (Alosco et al. 2015). In addition to tissue ischemia (Makedonov et al. 2013), increased cerebral venous pressure has been causally linked to the pathogenesis of WMHs (Fulop et al. 2019; Moody et al. 1995; Chung and Hu 2010). Further studies are warranted to test the mechanistic roles of BBB disruption and neuroinflammation associated with cerebral venous congestion in the neuropathological changes contributing to WMHs (Jorgensen et al. 2018).
Fig. 6.
Potential contribution of elevated venous pressure to cognitive decline in elderly heart failure patients. In older adults, heart failure was shown to associate with cognitive decline. The scheme depicts putative synergistic effects of heart failure–induced chronic hypoperfusion of the brain and increased venous pressure, which exacerbates blood-brain barrier disruption, neuroinflammation, white matter damage, and neuronal dysfunction, promoting cognitive impairment in elderly patients with heart failure. RAAS, renin-angiotensin-aldosterone system
In conclusion, our preclinical findings provide evidence supporting a potentially important role of cerebral venous congestion in the pathogenesis of cognitive impairment associated with heart failure and other pathological conditions (Fig. 6). New investigations using transgenic animal models and rodent and primate animal models of aging (Fulop et al. 2018; Reglodi et al. 2018; Deepa et al. 2017; Fang et al. 2017; Ashpole et al. 2017; Justice et al. 2017; Bernier et al. 2016; Mattison et al. 2012; Mattison et al. 2014) are needed to better understand the relationships among cerebral venous congestion, neuroinflammation and neuronal dysfunction and/or the pathogenesis of specific age-related diseases (e.g., Alzheimer’s disease). Both rodent (Adamski et al. 2018) and canine models of age-related heart failure could be quite relevant in that regard (Urfer et al. 2017; Labinskyy et al. 2007; Lei et al. 2004; Lionetti et al. 2005). Studies investigating the interaction of cerebral venous congestion–induced neuroinflammation and activation of inflammatory mechanisms by other comorbid conditions (Csiszar et al. 2017; Tucsek et al. 2014a, b; Leng et al. 2017; Tucsek et al. 2017; Toth et al. 2013; Van Skike et al. 2018) are also warranted. Prospective clinical studies on heart failure patients that include in the study design endpoints that reflect jugular venous reflux, cerebral venous pressure, microvascular damage, and neuroinflammation as well as cognitive performance (Carlson et al. 2018) are also needed.
Acknowledgments
The authors acknowledge the support from the NIA-funded Geroscience Training Program in Oklahoma (T32AG052363).
Funding information
This work was supported by grants from the American Heart Association (ST), the Oklahoma Center for the Advancement of Science and Technology (to AC, AY, PB, ZU), the American Federation for Aging Research (to PB), the National Institute on Aging (R01-AG055395, R01-AG047879, R01-AG038747), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS100782, R01-NS056218), and the Department of Veterans Affairs (Merit Number 1I01CX000340); a Pilot Grant from the Stephenson Cancer Center funded by the National Cancer Institute Cancer Center Support Grant P30CA225520 awarded to the University of Oklahoma Stephenson Cancer Center, the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (U54GM104938, to AY), and the Presbyterian Health Foundation (to ZU, AC, AY); the European Union–funded grants EFOP-3.6.1-16-2016-00008, 20765-3/2018/FEKUTSTRAT, EFOP-3.6.2.-16-2017-00008, GINOP-2.3.2-15-2016-00048, and GINOP-2.3.3-15-2016-00032; a grant from the National Research, Development and Innovation Office (NKFI-FK123798); a grant from the Hungarian Academy of Sciences (Bolyai Research Scholarship BO/00634/15); and a grant from the ÚNKP-18-4-PTE-6 New National Excellence Program of the Ministry of Human Capacities (to PT).
Compliance with ethical standards
All procedures were approved by the Institutional Animal Use and Care Committees of the University of Oklahoma Health Sciences Center.
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gabor A. Fulop, Chetan Ahire, Tamas Csipo, Stefano Tarantini and Tamas Kiss contributed equally to this work.
References
- Adamski MG, Sternak M, Mohaissen T, Kaczor D, Wieronska JM, Malinowska M, Czaban I, Byk K, Lyngso KS, Przyborowski K, Hansen PBL, Wilczynski G, Chlopicki S (2018) Vascular cognitive impairment linked to brain endothelium inflammation in early stages of heart failure in mice. J Am Heart Assoc 7 [DOI] [PMC free article] [PubMed]
- Akkawi NM, Agosti C, Borroni B, Rozzini L, Magoni M, Vignolo LA, Padovani A. Jugular valve incompetence: a study using air contrast ultrasonography on a general population. J Ultrasound Med. 2002;21:747–751. doi: 10.7863/jum.2002.21.7.747. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Brickman AM, Spitznagel MB, van Dulmen M, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Hughes J, Rosneck J, Gunstad J. The independent association of hypertension with cognitive function among older adults with heart failure. J Neurol Sci. 2012;323:216–220. doi: 10.1016/j.jns.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Brickman AM, Spitznagel MB, Griffith EY, Narkhede A, Raz N, Cohen R, Sweet LH, Hughes J, Rosneck J, Gunstad J. Independent and interactive effects of blood pressure and cardiac function on brain volume and white matter hyperintensities in heart failure. J Am Soc Hypertens. 2013;7:336–343. doi: 10.1016/j.jash.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Penn MS, Brickman AM, Spitznagel MB, Cleveland MJ, Griffith EY, Narkhede A, Gunstad J. Preliminary observations on MRI correlates of driving independence and performance in persons with heart failure. Int J Neurosci. 2015;125:424–432. doi: 10.3109/00207454.2014.945643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auletta L, Greco A, Albanese S, Meomartino L, Salvatore M, Mancini M. Feasibility and safety of two surgical techniques for the development of an animal model of jugular vein occlusion. Exp Biol Med (Maywood). 2017;242:22–28. doi: 10.1177/1535370216657446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer C, Ebenezer E, Fenner S, Lautenschlager NT, Arnolda L, Flicker L, Almeida OP. Contributors to cognitive impairment in congestive heart failure: a pilot case-control study. Intern Med J. 2009;39:600–605. doi: 10.1111/j.1445-5994.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison JA, Tarantini S, Tucsek Z, Ungvari Z, Csiszar A, Pearson KJ, de Cabo R. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 2016;8:899–916. doi: 10.18632/aging.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, White CL, Gupta S, Knight AG, Pistell PJ, Ingram DK, Morrison CD, Keller JN. NOX activity in brain aging: exacerbation by high fat diet. Free Radic Biol Med. 2010;49:22–30. doi: 10.1016/j.freeradbiomed.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JA, Moffitt P, Perez-Moreno AC, Walters MR, Broomfield NM, McMurray JJV, Quinn TJ. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail. 2017;23:464–475. doi: 10.1016/j.cardfail.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Carlson BW, Craft MA, Carlson JR, Razaq W, Deardeuff KK, Benbrook DM. Accelerated vascular aging and persistent cognitive impairment in older female breast cancer survivors. Geroscience. 2018;40:325–336. doi: 10.1007/s11357-018-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno-Muller E, Herrera AJ, de Pablos RM, Tomas-Camardiel M, Venero JL, Cano J, Machado A. Thrombin induces in vivo degeneration of nigral dopaminergic neurones along with the activation of microglia. J Neurochem. 2003;84:1201–1214. doi: 10.1046/j.1471-4159.2003.01634.x. [DOI] [PubMed] [Google Scholar]
- Choi BR, Kim JS, Yang YJ, Park KM, Lee CW, Kim YH, Hong MK, Song JK, Park SW, Park SJ, Kim JJ. Factors associated with decreased cerebral blood flow in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 2006;97:1365–1369. doi: 10.1016/j.amjcard.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Chung CP, Hu HH. Pathogenesis of leukoaraiosis: role of jugular venous reflux. Med Hypotheses. 2010;75:85–90. doi: 10.1016/j.mehy.2010.01.042. [DOI] [PubMed] [Google Scholar]
- Cornwell WK, III, Levine BD. Patients with heart failure with reduced ejection fraction have exaggerated reductions in cerebral blood flow during upright posture. JACC Heart Fail. 2015;3:176–179. doi: 10.1016/j.jchf.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Tarantini S, Fulop GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 2017;39:359–372. doi: 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, Gonias Murray S, Ling JB, Lassmann H, Degen JL, Ellisman MH, Akassoglou K. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva TM, Faraci FM. Effects of angiotensin II on the cerebral circulation: role of oxidative stress. Front Physiol. 2012;3:484. doi: 10.3389/fphys.2012.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A. A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. Geroscience. 2017;39:187–198. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanger S, Vaidiyanathan B, Tripathy DK. Internal jugular venous valve: well known but mostly neglected. Indian J Anaesth. 2016;60:602–603. doi: 10.4103/0019-5049.187813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinc N, Won SY, Eibach M, Quick-Weller J, Keil F, Berkefeld J, Konczalla J, Marquardt G, Seifert V. Thrombosis of the straight sinus and microbleedings due to deep seated arteriovenous fistula - hemodynamic changes, cognitive impairment and improvement after microsurgery. A technical report. J Clin Neurosci. 2019;68:317–321. doi: 10.1016/j.jocn.2019.07.012. [DOI] [PubMed] [Google Scholar]
- Dunn Kathryn M., Nelson Mark T. Neurovascular signaling in the brain and the pathological consequences of hypertension. American Journal of Physiology-Heart and Circulatory Physiology. 2014;306(1):H1–H14. doi: 10.1152/ajpheart.00364.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, McFadden S, Darcy J, Hill CM, Huber JA, Verhulst S, Kopchick JJ, Miller RA, Sun LY, Bartke A. Differential effects of early-life nutrient restriction in long-lived GHR-KO and normal mice. Geroscience. 2017;39:347–356. doi: 10.1007/s11357-017-9978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, Iadecola C. Hypertension enhances Abeta-induced neurovascular dysfunction, promotes beta-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab. 2016;36:241–252. doi: 10.1038/jcbfm.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Vizarra P, Lopez-Franco O, Mallavia B, Higuera-Matas A, Lopez-Parra V, Ortiz-Munoz G, Ambrosio E, Egido J, Almeida OF, Gomez-Guerrero C. Immunoglobulin G Fc receptor deficiency prevents Alzheimer-like pathology and cognitive impairment in mice. Brain. 2012;135:2826–2837. doi: 10.1093/brain/aws195. [DOI] [PubMed] [Google Scholar]
- Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop GA, Tarantini S, Yabluchanskiy A, Molnar A, Prodan CI, Kiss T, Csipo T, Lipecz A, Balasubramanian P, Farkas E, Toth P, Sorond F, Csiszar A, Ungvari Z. Role of age-related alterations of the cerebral venous circulation in the pathogenesis of vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2019;316:H1124–H1140. doi: 10.1152/ajpheart.00776.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. Faseb J. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Geraldes R, Albuquerque L, Ferro JM, Sousa R, Sequeira P, Campos J. Rapidly progressive cognitive impairment, ataxia, and myoclonus: an unusual presentation of a dural arteriovenous fistula. J Stroke Cerebrovasc Dis. 2012;21:619 e3–619 e5. doi: 10.1016/j.jstrokecerebrovasdis.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- Hai J, Wan JF, Lin Q, Wang F, Zhang L, Li H, Zhang L, Chen YY, Lu Y. Cognitive dysfunction induced by chronic cerebral hypoperfusion in a rat model associated with arteriovenous malformations. Brain Res. 2009;1301:80–88. doi: 10.1016/j.brainres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Hooghiemstra AM, Bertens AS, Leeuwis AE, Bron EE, Bots ML, Brunner-La Rocca HP, de Craen AJM, van der Geest RJ, Greving JP, Kappelle LJ, Niessen WJ, van Oostenbrugge RJ, van Osch MJP, de Roos A, van Rossum AC, Biessels GJ, van Buchem MA, Daemen M, van der Flier WM, Heart-Brain Connection C The missing link in the pathophysiology of vascular cognitive impairment: design of the heart-brain study. Cerebrovasc Dis Extra. 2017;7:140–152. doi: 10.1159/000480738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RW, Bagley LJ, Galetta S, Glosser G, Lieberman AP, Trojanowski J, Sinson G, Stecker M, Zager E, Raps EC, Flamm ES. Dementia resulting from dural arteriovenous fistulas: the pathologic findings of venous hypertensive encephalopathy. AJNR Am J Neuroradiol. 1998;19:1267–1273. [PMC free article] [PubMed] [Google Scholar]
- Inano S, Itoh D, Takao H, Hayashi N, Mori H, Kunimatsu A, Abe O, Aoki S, Ohtomo K. High signal intensity in the dural sinuses on 3D-TOF MR angiography at 3.0 T. Clin Imaging. 2010;34:332–336. doi: 10.1016/j.clinimag.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Jang J, Kim BS, Kim BY, Choi HS, Jung SL, Ahn KJ, Byun JY. Reflux venous flow in dural sinus and internal jugular vein on 3D time-of-flight MR angiography. Neuroradiology. 2013;55:1205–1211. doi: 10.1007/s00234-013-1239-5. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O’Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010;122:690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen DR, Shaaban CE, Wiley CA, Gianaros PJ, Mettenburg J, Rosano C. A population neuroscience approach to the study of cerebral small vessel disease in midlife and late life: an invited review. Am J Physiol Heart Circ Physiol. 2018;314:H1117–H1136. doi: 10.1152/ajpheart.00535.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Silverstein-Metzler MG, Uberseder B, Appt SE, Clarkson TB, Register TC, Kritchevsky SB, Shively CA. Relationships of depressive behavior and sertraline treatment with walking speed and activity in older female nonhuman primates. Geroscience. 2017;39:585–600. doi: 10.1007/s11357-017-9999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko YS, Nakashima A, Mori K, Nagatsu T, Nagatsu I, Ota A. Microglial activation in neuroinflammation: implications for the etiology of neurodegeneration. Neurodegener Dis. 2012;10:100–103. doi: 10.1159/000332936. [DOI] [PubMed] [Google Scholar]
- Kang Y, Kim E, Kim JH, Choi BS, Jung C, Bae YJ, Lee KM, Lee DH. Time of flight MR angiography assessment casts doubt on the association between transient global amnesia and intracranial jugular venous reflux. Eur Radiol. 2015;25:703–709. doi: 10.1007/s00330-014-3448-7. [DOI] [PubMed] [Google Scholar]
- Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- Kim E, Kim JH, Choi BS, Jung C, Lee DH. MRI and MR angiography findings to differentiate jugular venous reflux from cavernous dural arteriovenous fistula. AJR Am J Roentgenol. 2014;202:839–846. doi: 10.2214/AJR.13.11048. [DOI] [PubMed] [Google Scholar]
- Kudo K, Terae S, Ishii A, Omatsu T, Asano T, Tha KK, Miyasaka K. Physiologic change in flow velocity and direction of dural venous sinuses with respiration: MR venography and flow analysis. AJNR Am J Neuroradiol. 2004;25:551–557. [PMC free article] [PubMed] [Google Scholar]
- Labeyrie MA, Lenck S, Saint-Maurice JP, Bresson D, Houdart E. Dural arteriovenous fistulas presenting with reversible dementia are associated with a specific venous drainage. Eur J Neurol. 2014;21:545–547. doi: 10.1111/ene.12300. [DOI] [PubMed] [Google Scholar]
- Labinskyy V, Bellomo M, Chandler MP, Young ME, Lionetti V, Qanud K, Bigazzi F, Sampietro T, Stanley WC, Recchia FA. Chronic activation of peroxisome proliferator-activated receptor-alpha with fenofibrate prevents alterations in cardiac metabolic phenotype without changing the onset of decompensation in pacing-induced heart failure. J Pharmacol Exp Ther. 2007;321:165–171. doi: 10.1124/jpet.106.116871. [DOI] [PubMed] [Google Scholar]
- Lei B, Lionetti V, Young ME, Chandler MP, d’Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, Recchia FA. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol. 2004;36:567–576. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Leng SX, Kamil J, Purdy JG, Lemmermann NA, Reddehase MJ, Goodrum FD. Recent advances in CMV tropism, latency, and diagnosis during aging. Geroscience. 2017;39:251–259. doi: 10.1007/s11357-017-9985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepori D, Capasso P, Fournier D, Genton CY, Schnyder P. High-resolution ultrasound evaluation of internal jugular venous valves. Eur Radiol. 1999;9:1222–1226. doi: 10.1007/s003300050822. [DOI] [PubMed] [Google Scholar]
- Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol. 2014;11:316–328. doi: 10.11909/j.issn.1671-5411.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Linke A, Chandler MP, Young ME, Penn MS, Gupte S, d’Agostino C, Hintze TH, Stanley WC, Recchia FA. Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc Res. 2005;66:454–461. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Loncar G, Bozic B, Lepic T, Dimkovic S, Prodanovic N, Radojicic Z, Cvorovic V, Markovic N, Brajovic M, Despotovic N, Putnikovic B, Popovic-Brkic V. Relationship of reduced cerebral blood flow and heart failure severity in elderly males. Aging Male. 2011;14:59–65. doi: 10.3109/13685538.2010.511326. [DOI] [PubMed] [Google Scholar]
- MacLaren DA, Santini JA, Russell AL, Markovic T, Clark SD. Deficits in motor performance after pedunculopontine lesions in rats - impairment depends on demands of task. Eur J Neurosci. 2014;40:3224–3236. doi: 10.1111/ejn.12666. [DOI] [PubMed] [Google Scholar]
- Makedonov I, Black SE, MacIntosh BJ. Cerebral small vessel disease in aging and Alzheimer’s disease: a comparative study using MRI and SPECT. Eur J Neurol. 2013;20:243–250. doi: 10.1111/j.1468-1331.2012.03785.x. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG, Heistad DD. Role of veins and cerebral venous pressure in disruption of the blood-brain barrier. Circ Res. 1986;59:216–220. doi: 10.1161/01.res.59.2.216. [DOI] [PubMed] [Google Scholar]
- Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: association with leukoaraiosis. Radiology. 1995;194:469–476. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- Morparia N, Miller G, Rabinstein A, Lanzino G, Kumar N. Cognitive decline and hypersomnolence: thalamic manifestations of a tentorial dural arteriovenous fistula (dAVF) Neurocrit Care. 2012;17:429–433. doi: 10.1007/s12028-012-9746-5. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine CA, Lawton MT, Hetts SW, Josephson SA. Neuropyschological profile of reversible cognitive impairment in a patient with a dural arteriovenous fistula. Neurocase. 2008;14:231–238. doi: 10.1080/13554790802232677. [DOI] [PubMed] [Google Scholar]
- Randall A, Ellis R, Hywel B, Davies RR, Alusi SH, Larner AJ. Rapid cognitive decline: not always Creutzfeldt-Jakob disease. J R Coll Physicians Edinb. 2015;45:209–212. doi: 10.4997/JRCPE.2015.307. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Atlasz T, Szabo E, Jungling A, Tamas A, Juhasz T, Fulop BD, Bardosi A. PACAP deficiency as a model of aging. Geroscience. 2018;40:437–452. doi: 10.1007/s11357-018-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Woo MA, Wang DJJ, Fonarow GC, Harper RM, Kumar R. Reduced regional cerebral blood flow in patients with heart failure. Eur J Heart Fail. 2017;19:1294–1302. doi: 10.1002/ejhf.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, McEwen JJ, Szprengiel A, Joseph JA. Effect of age on the radial arm water maze-a test of spatial learning and memory. Neurobiol Aging. 2004;25:223–229. doi: 10.1016/s0197-4580(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Sung SH, Lee CW, Wang PN, Lee HY, Chen CH, Chung CP. Cognitive functions and jugular venous reflux in severe mitral regurgitation: a pilot study. PLoS One. 2019;14:e0207832. doi: 10.1371/journal.pone.0207832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges E, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35(11):1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Fulop GA, Kiss T, Farkas E, Zolei-Szenasi D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini Stefano, Valcarcel-Ares Noa M., Yabluchanskiy Andriy, Fulop Gabor A., Hertelendy Peter, Gautam Tripti, Farkas Eszter, Perz Aleksandra, Rabinovitch Peter S., Sonntag William E., Csiszar Anna, Ungvari Zoltan. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17(2):e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini Stefano, Valcarcel-Ares M Noa, Yabluchanskiy Andriy, Tucsek Zsuzsanna, Hertelendy Peter, Kiss Tamas, Gautam Tripti, Zhang Xin A, Sonntag William E, de Cabo Rafael, Farkas Eszter, Elliott Michael H, Kinter Michael T, Deak Ferenc, Ungvari Zoltan, Csiszar Anna. Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood–Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype. The Journals of Gerontology: Series A. 2017;73(7):853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, Yabluchanskiy A, Fulop G, Gautam T, Orock A, Csiszar A, Deak F, Ungvari Z. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience. 2017;39:385–406. doi: 10.1007/s11357-017-9981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino A, Nomiyama K, Takase Y, Nakazono T, Tominaga Y, Imaizumi T, Kudo S. Retrograde flow in the dural sinuses detected by three-dimensional time-of-flight MR angiography. Neuroradiology. 2007;49:211–215. doi: 10.1007/s00234-006-0186-9. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–H1143. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Yabluchanskiy A, Tarantini S, Toth P, Kirkpatrick AC, Csiszar A, Prodan CI. Repeated Valsalva maneuvers promote symptomatic manifestations of cerebral microhemorrhages: implications for the pathogenesis of vascular cognitive impairment in older adults. Geroscience. 2018;40:485–496. doi: 10.1007/s11357-018-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DE, Kaeberlein M. Asymptomatic heart valve dysfunction in healthy middle-aged companion dogs and its implications for cardiac aging. Geroscience. 2017;39:43–50. doi: 10.1007/s11357-016-9956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcarcel-Ares Marta Noa, Tucsek Zsuzsanna, Kiss Tamas, Giles Cory B, Tarantini Stefano, Yabluchanskiy Andriy, Balasubramanian Priya, Gautam Tripti, Galvan Veronica, Ballabh Praveen, Richardson Arlan, Freeman Willard M, Wren Jonathan D, Deak Ferenc, Ungvari Zoltan, Csiszar Anna. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-Related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. The Journals of Gerontology: Series A. 2018;74(3):290–298. doi: 10.1093/gerona/gly127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valecchi D, Bacci D, Gulisano M, Sgambati E, Sibilio M, Lipomas M, Macchi C. Internal jugular vein valves: an assessment of prevalence, morphology and competence by color Doppler echography in 240 healthy subjects. Ital J Anat Embryol. 2010;115:185–189. [PubMed] [Google Scholar]
- Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, Lechleiter JD, Galvan V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2018;314:H693–H703. doi: 10.1152/ajpheart.00570.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RD, Gibbs CR, Lip GY. ABC of heart failure. Clinical features and complications. BMJ. 2000;320:236–239. doi: 10.1136/bmj.320.7229.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MA, Kumar R, Macey PM, Fonarow GC, Harper RM. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2009;15:214–223. doi: 10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang NN, Zhao KT, Zhao ZA, Chen WL, Xu HB, Chen HS. A novel rat model of cerebral artery occlusion complicated with prior venous stagnation. J Neurosci Methods. 2019;318:100–103. doi: 10.1016/j.jneumeth.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Zivadinov R, Chung CP. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med. 2013;11:260. doi: 10.1186/1741-7015-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]