Abstract
Mice deficient in the antioxidant enzyme Cu/Zn-superoxide dismutase (Sod1KO mice) have a significant reduction in lifespan, exhibit many phenotypes of accelerated aging, and have high levels of oxidative stress in various tissues. Age-associated cognitive decline is a hallmark of aging and the increase in oxidative stress/damage with age is one of the mechanisms proposed for cognitive decline with age. Therefore, the goal of this study was to determine if Sod1KO mice exhibit an accelerated loss in cognitive function similar to that observed in aged animals. Cognition was assessed in Sod1KO and wild type (WT) mice using an automated home-cage testing apparatus (Noldus PhenoTyper) that included an initial discrimination and reversal task. Comparison of the total distance moved by the mice during light and dark phases of the study demonstrated that the Sod1KO mice do not show a deficit in movement. Assessment of cognitive function showed no significant difference between Sod1KO and WT mice during the initial discrimination phase of learning. However, during the reversal task, Sod1KO mice showed a significantly greater number of incorrect entries compared to WT mice indicating a decline in cognition similar to that observed in aged animals. Markers of oxidative stress (4-Hydroxynonenal, 4-HNE) and neuroinflammation [proinflammatory cytokines (IL6 and IL-1β) and neuroinflammatory markers (CD68, TLR4, and MCP1)] were significantly elevated in the hippocampus of male and female Sod1KO compared to WT mice. This study provides important evidence that increases in oxidative stress alone are sufficient to induce neuroinflammation and cognitive dysfunction that parallels the memory deficits seen in advanced aging and neurodegenerative diseases.
Keywords: Cu/Zn-superoxide dismutase, Cognition, Accelerating aging, Oxidative stress, Neuroinflammation
Introduction
Cu/Zn-Superoxide dismutase (Cu/ZnSOD) is a major antioxidant enzyme, which converts superoxide radicals into hydrogen peroxide, and is found in the cytosol and the intermembrane space of the mitochondria (Okado-Matsumoto and Fridovich 2001). Mice null for Cu/ZnSOD [Sod1 knockout (Sod1KO) mice] were generated by Reaume et al. (1996), and Elchuri et al. (2005) reported that Sod1KO mice show a ~30% decrease in lifespan (both sexes combined), which was confirmed by Zhang et al. (2013) using female Sod1KO mice. Sod1KO mice exhibit high levels of oxidative stress in various tissues and plasma (Muller et al. 2006; Zhang et al. 2016). Based on these data, it was proposed that the reduced longevity of the Sod1KO mice arose due to accelerated aging as predicted by the oxidative stress theory of aging (Salmon et al. 2010). This concept was supported by the observation that dietary restriction reduced oxidative damage in tissues of the Sod1KO mice and increased the lifespan of Sod1KO mice to that of normal, wild type (WT) mice (Zhang et al. 2013).
Over the past decade, several studies have compared various physiological functions in Sod1KO and WT mice, and these studies support the concept that the Sod1KO mice exhibit accelerated aging. For example, the Sod1KO mice demonstrate hearing loss (Keithley et al. 2005), increased incidence of cataracts (Olofsson et al. 2007), and delayed wound healing (Iuchi et al. 2010). In addition, Sod1KO mice exhibit accelerated sarcopenia, i.e., the loss of muscle mass with age (Muller et al. 2006). The decrease in muscle mass was paralleled by lower force generating capacity of muscle (Larkin et al. 2011) and reduced grip strength (Deepa et al. 2017). A decrease in muscle mass and strength with age is a universal phenotype observed in all vertebrates. In addition, Sod1KO mice showed a ~30% decrease in rotarod performance (Muller et al. 2006) and reduced endurance measured by treadmill running to exhaustion (Jang et al. 2010). More recently, our group showed that the overall severity of pathological lesions of Sod1KO mice was dramatically increased in adult (9-month-old) compared to age-matched WT mice as measured by the geropathology grading score (Snider et al. 2018). Because increased pathology is a hallmark of aging, an increase in pathological lesions globally in the Sod1KO mice is consistent with these mice showing an accelerated aging phenotype.
Because the Sod1KO mice exhibit increased loss of many physical functions, we investigated whether the Sod1KO mice also exhibit an accelerated loss in cognition. Studies in humans demonstrate that there is a strong correlation between age-related changes in physical performance and cognition (Ko et al. 2018; Suire et al. 2017; Rosso et al. 2017; Watson et al. 2010; Mielke et al. 2013). Numerous studies have also shown that cognition declines with advancing age in rats and mice using various measures of cognitive function, e.g., Morris water maze, object recognition, and Y maze (Logan et al. 2018a; VanGuilder Starkey et al. 2013; VanGuilder et al. 2011; Ashpole et al. 2017). In addition, several studies suggest that oxidative stress/damage can negatively impact cognition in laboratory rodents (Fukui et al. 2001, 2002; Morrison et al. 2010; Iguchi et al. 2014). Oxidative stress, assessed as increases in the level of isoprostanes, is increased in the brain of Sod1KO mice (Zhang et al. 2016). Interestingly, deficiency of Sod1 in an amyloid precursor protein-overexpressing Alzheimer’s mouse model is reported to accelerate memory deficits due to increased oxidative damage, compared to control Alzheimer’s mouse model (Murakami et al. 2011). Nevertheless, it is unknown whether a primary increase in oxidative stress alone is sufficient to induce cognitive dysfunction. Therefore, we compared cognitive function in Sod1KO and WT mice in this study using a novel, non-invasive assay that allows one to measure cognition in the animal’s home cage. Logan et al. (2018) recently showed that this assay detects deficits in cognition in old WT mice. In this article, we show for the first time that cognition is dramatically decreased in both male and female Sod1KO mice compared to control mice. These data demonstrate that the Sod1KO mice exhibit an accelerated aging phenotype with respect to cognition, which parallels the decline in a large number of other physical functions making the Sod1KO mouse an excellent model for studying accelerated aging and that a primary increase in oxidative stress results in cognitive impairment.
Materials and methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Oklahoma Health Sciences Center (OUHSC). The Sod1KO mice were generated as described previously (Elchuri et al. 2005; Muller et al. 2006). Experimental cohorts were raised in the OUHSC Rodent facility. Median lifespan of female Sod1KO mice is ~22 months (Zhang et al. 2013). In the study, we used 13 to 15-month-old male Sod1KO mice (n = 10) and WT controls (n = 12). For analysis in females, we used 16 to 19-month-old female Sod1 KO mice (n = 6) and WT (n = 12) controls. The mice were group housed in ventilated cages 20 ± 2 °C, 12-h/12-h dark/light cycle and were fed rodent chow (5053 Pico Lab, Purina Mills, Richmond, IN) ad libitum. Four days prior to the initiation of testing, animals were singly housed and fed dustless precision rodent pellets (F05684, Bio-Serv, Flemington, NJ) for adaptation purposes, and water was available ad libitum.
Assessment of activity and cognition using PhenoTyper
Spontaneous activity, initial discrimination learning, and reversal learning were assessed using an automated home-cage testing apparatus, PhenoTyper (Model 3000, Noldus Information Technology, Netherlands), as described previously (Maroteaux et al. 2012; Loos et al. 2014; Logan et al., 2018) and activity of the mice were recorded using EthoVision software (Noldus). Body weights of mice were measured immediately before the initiation of the study and immediately after completing the behavioral tasks. In the PhenoTyper, behavior was tracked by video and the food dispenser was triggered by specific behaviors of the mouse (Maroteaux et al. 2012), while water was available ad libitum. The cages were transparent plastic (L = 30 × W = 30 × H = 35 cm) and bedding was cellulose-free paper (Pure-o’Cel; The Andersons, Maumee, OH). Mice were individually placed in the PhenoTyper for a 6-h adaptation period and beginning at 1600 h on day 1, the activity of the animals was continuously recorded for 90 h. All movements were recorded with an infrared-sensitive video camera above the arena using the X-Y coordinates of the center of gravity of each animal. These data were sampled at a rate of 15 frames per second (fps) and processed by Lowess smoothing using EthoVision software (EthoVision XT 11.5, Noldus Information Technology, Wageningen, The Netherlands) (Maroteaux et al. 2012; Loos et al. 2014). The experimental protocol for monitoring mice within the PhenoTyper cage was fully automated.
During the cognitive testing phase, mice were continuously monitored during the light and dark cycles. Diurnal spontaneous activity was tracked as previously reported (Logan et al., 2018), and the total distance traveled by each mouse was calculated for the 90 h of the study. During cognitive testing, mice are rewarded with a food pellet after five successful entries into the correct hole (FR5; fixed ratio 5) as previously described (Logan et al., 2018). The percentage of correct entries made in a moving window of the trailing 30 entries was calculated and trials to reach an 80% success rate determined. Thus, when the mouse achieved 80% correct entries within that window, the criteria was met and the number of entries plotted vs the percentage of mice that achieved criteria for the group. These methods have been described in detail in our previous publication (Logan et al., 2018). Results were then summarized for the dark and light phases of the L:D cycle as previously reported. For the acquisition phase of the test, mice were required to pass through the left entrance of the CognitionWall during initial discrimination to obtain a food reward (Dustless Precision Rodent Pellets, F05684, Bio-Serv, Flemington, NJ), which was dispensed using a FR5 schedule. After 45 h of discrimination learning, the task was modified and the correct response was changed to the right entry requiring the animal to extinguish the previous learning and acquire a new response, again rewarded at a FR5 schedule. Success rates for both initial discrimination and reversal learning were calculated post hoc and defined as the percentage of correct entries of the trailing 30 entries through any of the entrances of the CognitionWall. The data were exported from EthoVision as a text file and then processed using Python programming language. The following dependent variables to reach a specific success rate were calculated for both initial discrimination and reversal learning: percent of animals reaching criteria, entries to criteria, errors to criteria, and time (hours) to criteria (Logan et al., 2018). The Cumulative Leaning Index was calculated as correct entries minus the incorrect entries divided by the total number of entries.
Detection of 4-Hydroxynonenal (4-HNE) adducts
Following cognitive testing, animals were euthanized hippocampi were dissected and immediately frozen in liquid nitrogen and stored at −80 °C until further use. For Western blotting, one hippocampus was homogenized in RIPA lysis and extraction buffer (ThermoFisher Scientific, Waltham, MA). For the detection of 4-HNE modified proteins, equal amounts of protein (40 μg/lane) were separated by SDS–PAGE, transferred to polyvinylidene difluoride membranes and treated with 250 mM sodium borohydride in 100 mM (3-(N-morpholino)propanesulfonic acid, MOPS), pH 8.0 for 15 min. The membrane was washed with water, followed by Tris buffered saline with Tween 20 (TBS-T), and blocked with 5% non-fat milk/TBS-T. The membrane was incubated with a 1:2000 dilution of polyclonal antibody against 4-HNE, as described (Uchida et al. 1993; Bhaskaran et al. 2018). The antibody recognizes cysteine, lysine, and histidine 4-HNE protein adducts and is highly specific to 4-HNE derived protein adducts (gift from Dr. Luke Szweda, Oklahoma Medical Research Foundation) (Uchida et al. 1995). This was followed by incubation with anti-rabbit IgG HRP conjugated antibody and the blot was developed using Pierce ECL Western Blotting Substrate (ThermoFisher Scientific, Waltham, MA). Images were taken using a G:BOX imaging system (Syngene, Frederick, MD) and quantified using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Quantitative real-time PCR
Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA, USA) from one frozen hippocampus. First-strand cDNA was synthesized using a high capacity cDNA reverse transcription kit [ThermoFisher Scientific (Applied Biosystems), Waltham, MA] and quantitative real-time PCR was performed with ABI Prism using Power SYBR Green PCR Master Mix [ThermoFisher Scientific (Applied Biosystems), Waltham, MA] with the primers listed in Table 1. Calculations were performed by a comparative method (2−ΔΔCt) using β-microglobulin, actin, and 18S as controls as described previously (Bhaskaran et al. 2018).
Table 1.
Primer sequences for quantitative RT-PCR
| Gene | Primer |
|---|---|
| Tumor Necrosis Factor alpha (TNFα) |
Forward: 5′- CACAGAAAGCATGATCCGCGACGT-3’ Reverse: 5′- CGGCAGAGAGGAGGTTGACTTTCT-3′ |
| Interleukin 6 (IL6) |
Forward: 5’-GCTACCAAACTGGATATAATCAGGA-3’ Reverse: 5’-CCAGGTAGCTATGGTACTCCAGAA-3′, |
| Interleukin 1 beta (IL1β) |
Forward: 5’-CCCTGCAGCTGGAGAGTGTGGA-3’ Reverse: 5’-TGTGCTCTGCTTGTGAGGTGCTG-3’ |
| Cluster of Differentiation 68 (CD68) |
Forward: 5’-CCACAGGCAGCACAGTGGACA-3’ Reverse: 5’-TCCACAGCAGAAGCTTTGGCCC-3’ |
| Toll Like Receptor 4 (TLR4) |
Forward: 5′-ATGGCATGGCTTACACCACC-3′ Reverse: 5′- GAGGCCAATTTTGTCTCCACA-3′ |
| Monocyte Chemoattractant Protein-1 (MCP1) |
Forward: 5’-TTAAAAACCTGGATCGGAACCAA-3’ Reverse: 5’-GCATTAGCTTCAGATTTACGGGT-3’ |
| β2-microglobulin |
Forward: 5′-CACTGACCGGCCTGTATGC-3′ Reverse: 5′-GGGTGGCGTGAGTATACTTGAAT-3′ |
| β-actin |
Forward: 5′-AATCGTGCGTGACATCAAAGAG-3′ Reverse: 5′-GCCATCTCCTGCTCGAAGTC-3′ |
| 18S |
Forward: 5′-CTGAGAAACGGCTACCACATC-3′ Reverse: 5′-CGCTCCCAAGATCCAACTAC-3′. |
Statistical analyses
Data obtained from male and female mice were analyzed separately since the cognitive tests were run sequentially and the ages varied by several months potentially impacting the direct comparison of the learning measures. Data were analyzed using ANOVA (after log 10 transformation). A Learning Index [(Correct entries-Incorrect entries)/Total Entries] was calculated for each animal per hour and analyzed using JMP software (SAS, Cary NC) using a Group x Time repeated measures analysis of variance. Modeling assumptions were evaluated and transformations made, as necessary. Pairwise comparisons were made using the Tukey HSD test as appropriate, p < 0.05 was considered statistically significant. For visualization of performance over the study, a Cumulative Learning Index was calculated, however, the statistical analysis was only performed on the original Learning Index data. To assess the relationship between learning and measures of oxidative stress and inflammation, a multiple regression model was used using maximum learning performance during first 10 h of the reversal phase of the experiment as the predicted variable.
Results
Movement data
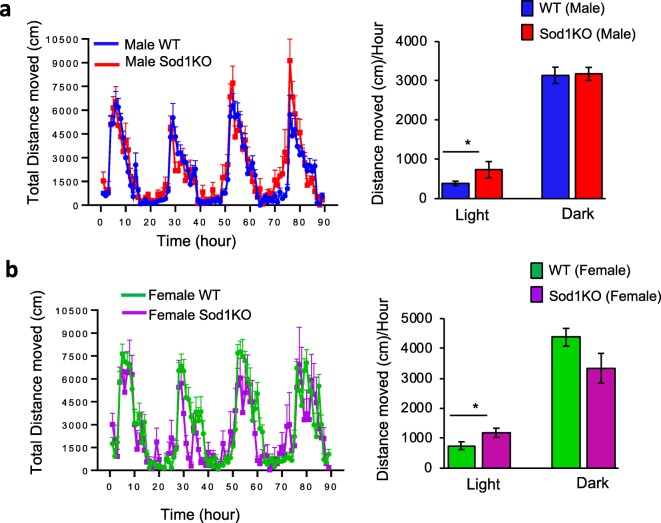
In this study, we measured the activity of 13 to 15-month-old WT and Sod1KO male mice continuously over the light and dark phases of the 90-h study, and data are summarized in Fig. 1a. Figure 1b summarizes the activity of 16 to 19-month-old WT and Sod1KO female mice during the dark and light phases of the study. As expected, both male and female WT and Sod1KO mice have increased activity in the dark phase of the study. No marked differences were found in male or female Sod1KO mice compared to their respective control groups during the dark testing period. Interestingly, during the light phase, the total activity of the male and female Sod1KO mice was significantly higher than their respective control groups, [90% higher for Sod1KO male mice compared WT male mice (p = 0.02) and 57% higher for Sod1KO female mice compared to WT female mice (p = 0.02)]. These data suggest sleep fragmentation may be occurring in Sod1KO mice. Importantly, the data provide strong evidence that the Sod1KO mice do not show a movement deficit that might complicate measuring cognitive function. This is consistent with a previous study showing that there is no difference in spontaneous activity between Sod1KO and WT mice over a 4-day period, assessed using a photocell-based mouse-monitoring system (Muller et al. 2006).
Fig. 1.
Left panel: Total distance moved by (a) male WT (n = 12) and Sod1KO (n = 10) mice, and (b) female WT (n = 12) and Sod1KO (n = 6) mice obtained by tracking animal movements during the 90-h experimental period. Data are averaged over hourly intervals during both the light and dark phases of the light-dark cycle. Dark horizontal lines represent the dark phase of the cycle. Data represent mean + SEM calculated for hourly intervals. Right panel: Summary of the total distance moved over the 90-h period by male (a) and female (b) WT and Sod1KO mice during light and dark cycles. Data represent mean ± SEM for each group. *p < 0.05
Cognitive function
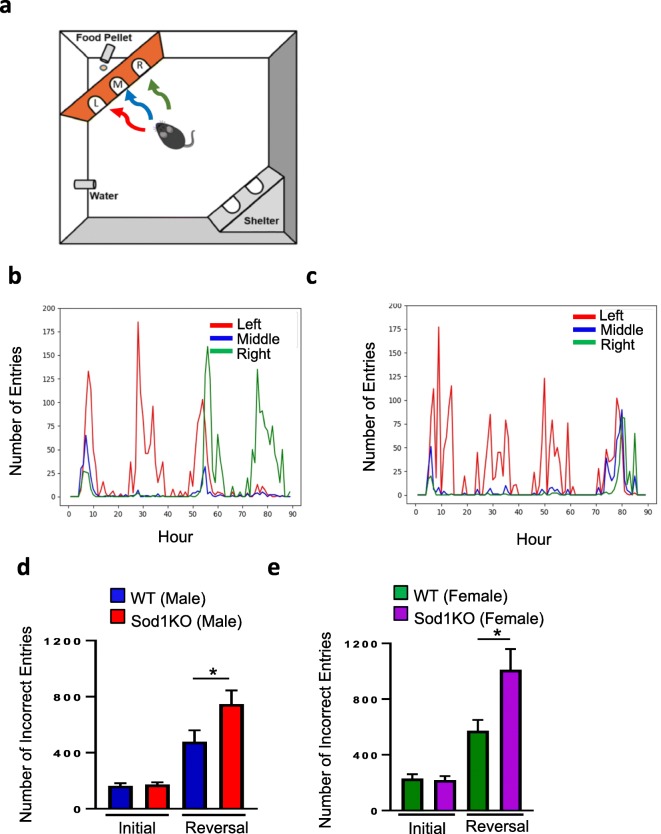
The PhenoTyper/Cognition Wall measures spatial learning and working memory based on a food reward-based task that is divided into two phases: (1) initial discrimination learning during the first 45-h period, and (2) reversal learning during the second 45-h period. Importantly, cognition is measured with minimal stress and without human intervention during both light and dark cycles, the latter cycle during which the mice are most active. Figure 2a diagrammatically shows how the PhenoTyper assesses cognition, with examples of the performance of a selected WT and Sod1KO male mouse (Fig. 2b, c, respectively). Both the WT and Sod1KO mice quickly learned to enter through the left entrance (denoted in red) for food reward during the initial discrimination phase. However, Sod1KO mice perform poorly in the reversal phase (beginning at the 48 h when the correct entry is switched to the right side (denoted in green), which is a challenging behavioral task. The reversal phase is a measure of cognitive flexibility whereby the mice process the extinction of a learned memory while consolidating a new task [learning to enter through the right entrance (green) to receive food]. Figure 2d shows the number of incorrect entries for the male WT and Sod1KO mice over the first 45 h of the learning phase and the second 45 h of reversal phase. There were no significant differences in the number of incorrect entries for WT and Sod1KO mice during the initial 45-h learning phase. However, the number of incorrect entries significantly increased during the reversal phase for both WT and Sod1KO mice and the number of incorrect entries by Sod1KO mice during reversal phase was 56% higher than WT mice (p = 0.05). Next, we compared the number of incorrect entries for the WT and Sod1KO female mice for the learning phase and reversal phase. Female Sod1KO mice also showed increased incorrect entries only during the reversal phase, 76% higher than that of WT female mice (Fig. 2e) (p = 0.03).
Fig. 2.
(a) Illustration of the Ethovision Phenotyper to assess cognitive function in mice. A pellet dispenser and a Cognition Wall were inserted into the PhenoTyper immediately before the initiation of the experiment. The CognitionWall was placed in front of the food dispenser and has three entrances: left (L), middle (M), and right (R). To obtain the food pellet, the animals were required to enter the Cognition Wall through the left entrance (red arrow) during the initial discrimination phase of the study, and through the right entrance (green arrow) during the reversal phase. Representative images showing entry of a WT (b) and Sod1KO (c) male mouse into the three entrances (red left, blue middle, and green right) during the 90-h time period. (d, e) Number of errors (incorrect entries) to reach 80% during the initial discrimination and reversal phases of the test by male mice (d) and female mice (e). Data were analyzed using the raw Learning Index data using a GroupxTime repeated measures ANOVA. Data are mean ± SEM for each group. *p < 0.05
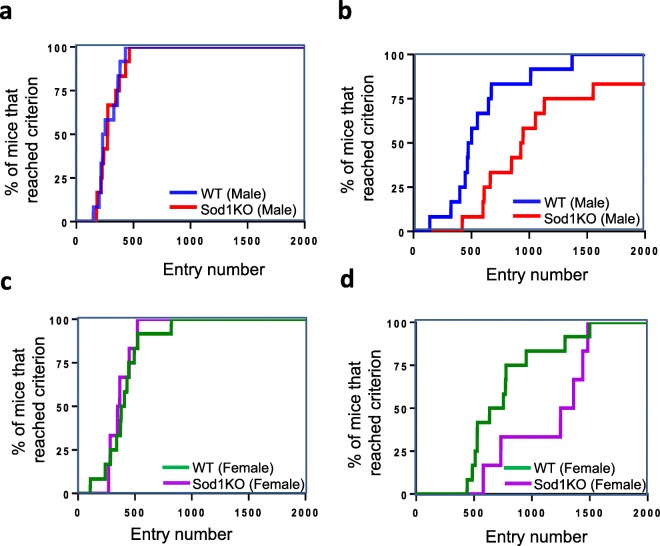
The criteria we have selected for the number of entries required that the animal successfully learn each task based on a success rate of 80% over the trailing 30 entries as described by Logan et al. (2018) and Loos et al. (2014). Fig. 3 shows the number of entries required to reach the 80% success rate for male WT and Sod1KO mice during the initial discrimination (Fig. 3a) and reversal phase of learning (Fig. 3b). Male Sod1KO mice have similar success rates of learning in the initial discrimination task as male WT mice (Fig. 3a). In contrast, during the reversal learning phase, we observed a difference between male Sod1KO and WT mice (Fig. 3b). While all the WT mice were able to complete the task, two of the male Sod1KO male mice were unable to achieve a success rate of 80% after over 2000 entries (Fig. 3b). During the reversal phase of learning, the male Sod1KO mice required a larger number of entries before achieving the 80% success criterion than WT mice. An 80% success rate after 1000 entries was achieved by 90% of WT male mice, whereas only 58% of Sod1KO male mice reached the 80% success rate after 1000 entries (Fig. 3b).
Fig. 3.
(a, b) Entries required to reach 80% success rate for male WT and Sod1KO mice during the initial discrimination phase (a) and reversal learning phase (b) of the study. (c, d) Entries required to reach 80% success rate for female WT and Sod1KO mice during the initial discrimination phase (c) and reversal learning phase (d) of the study. Each ‘step’ represents a mouse that reached the learning criterion at the respective number of entries
The number of entries required to reach the 80% success rate for female WT and Sod1KO mice during the initial discrimination and reversal phase of learning of the study are shown in Fig. 3c and d. All the WT and Sod1KO female mice were able to complete the task. However, during the reversal phase approximately 80% of the WT mice reached criteria by 1000 entries whereas only 33% of Sod1KO female mice reached the 80% success rate at that time (Fig. 3d). In agreement with this observation, the number of food rewards was significantly reduced for Sod1KO mice during the dark phase of reversal learning: 44% reduction for male Sod1KO mice compared to male WT mice (p = 0.004) and 48% reduction for female Sod1KO mice compared to female WT mice (p = 0.04) (data not shown).
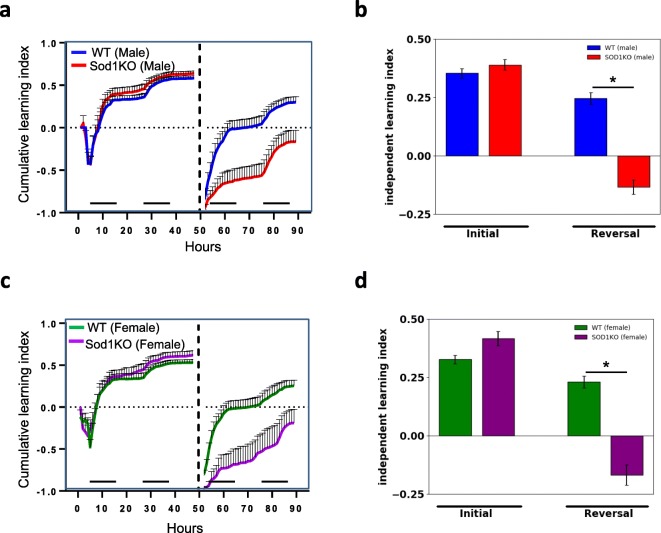
We also evaluated cognitive function by measuring the learning index (Fig. 4). This analysis allows us to measure the accuracy/efficiency of entry choice over time for each mouse normalized to the total number of entries. Furthermore, the cumulative learning index (Figs. 4 a, c) allows us to visually assess the rate of learning (i.e., the slope of the curve during initial discrimination and reversal learning) as well as the maximum learning capacity during the specific testing period. Statistical significance was assessed on the independent learning index values. Sod1KO males showed no differences in the initial discrimination phase but were impaired in their learning performance in the reversal phase (p = 0.0021) compared to controls (Fig. 4b). Similar to male Sod1KO mice, female Sod1KO mice also showed no differences in the initial discrimination phase, but were impaired during the reversal phase compared to female WT mice (p = 0.0035) (Fig. 4d).
Fig. 4.
Assessment of cognitive performance by learning index for male (a, b) and female (c, d) WT and Sod1KO mice during the initial discrimination and reversal phase of learning. Figs. a and c represent the cumulative learning index and Figs b and d represent the independent learning index over the period of the study. Learning behavior during the initial discrimination phase was not statistically significant between WT and Sod1KO in male or female mice. Statistically significant changes were observed in the reversal phase of learning based on analysis of the independent learning index. Horizontal bars in Figs. a and c indicate the dark phase of the L:D cycle. *p < 0.05
Oxidative stress and neuroinflammation
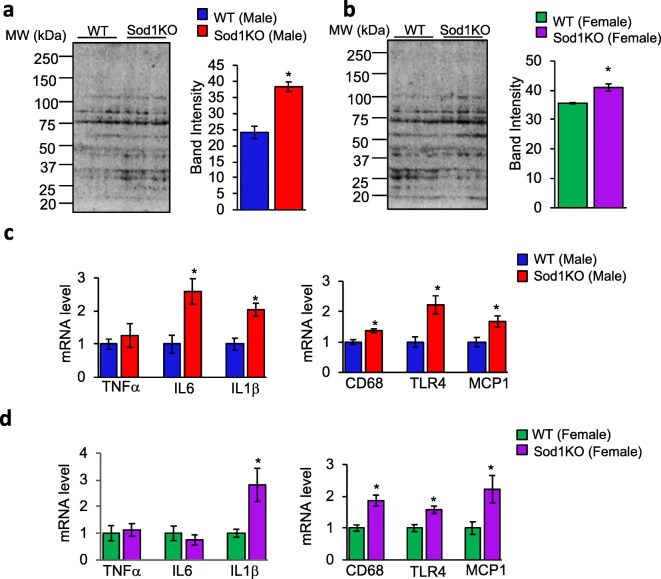
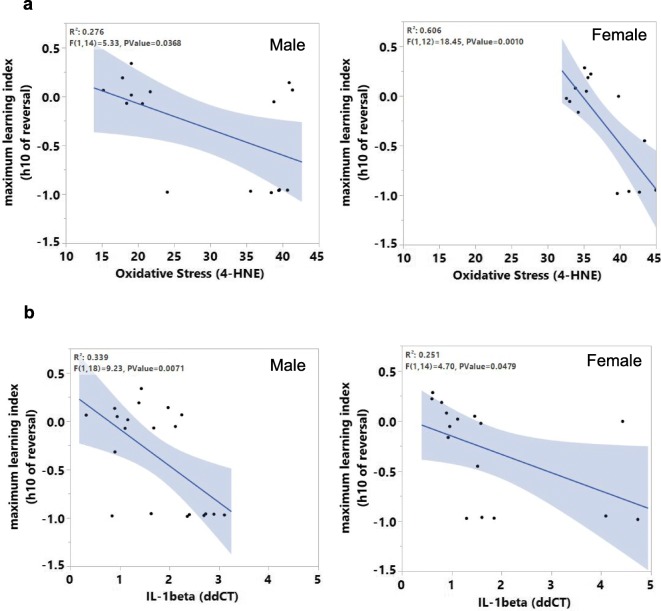
Next, changes in oxidative stress in the hippocampus of male and female WT and Sod1KO mice were assessed. We measured the levels of 4-hydroxy-2-nonenal (4-HNE), which is a product of lipid peroxidation and routinely used as a biomarker of oxidative stress (Dalleau et al. 2013; Breitzig et al. 2016). Western blotting of hippocampal extracts showed a significant increase in 4-HNE levels in male (1.6-fold) and female (1.2-fold) Sod1KO mice, compared to male and female WT mice (Fig. 5a and b). Because an increase in oxidative stress can cause neuroinflammation and is associated with cognitive decline (d'Avila et al. 2018; Hussain et al. 2016), we assessed changes in the expression of proinflammatory cytokines and neuroinflammatory markers in the hippocampus of WT and Sod1KO mice. In the hippocampus of male Sod1KO mice, transcript levels of proinflammatory cytokines IL6 and IL-1β were increased by 2.5- and 2.0-fold, respectively, compared to WT male mice, whereas TNFα levels were similar in WT and Sod1KO male mice (Fig. 5c, left panel). In the hippocampus of female Sod1KO mice, transcript level of the proinflammatory cytokine IL-1β was increased by 2.8-fold, compared to WT female mice, whereas IL6 and TNFα levels were similar in WT and Sod1KO female mice (Fig. 5d, left panel). Similarly, transcript levels of CD68 (1.4-fold), TLR4 (2.2-fold), and MCP1 (1.7-fold), which are markers of activated microglia, are elevated in the hippocampus of Sod1KO male mice compared to WT mice (Fig. 5c, right panel). Transcript levels of CD68 (1.8-fold), TLR4 (1.5-fold), and MCP1 (2.2-fold) were also increased in the hippocampus of Sod1KO female mice compared to WT female mice (Fig. 5d, right panel). Together these findings indicate that increased oxidative stress and neuroinflammation occur in the hippocampus of Sod1KO male and female mice compared to WT male and female mice, respectively. We investigated whether oxidative stress or the levels of cytokines were associated with the decline in cognitive function using a multiple regression approach and the first 10 h of the reversal phase as the measure of learning. Relationships between these measures were found for males (p = 0.0368) and females (p = 0.0010) (Fig. 6a). Similarly, correlation between IL-1β and maximum learning index at hour 10 of reversal phase showed significant effect for males (p value = 0.007) and females (p value = 0.047) (Fig. 6b).
Fig. 5.
Markers of oxidative stress and inflammation are increased in the hippocampus of Sod1KO mice. Western blots of hippocampal extracts from male (a, left panel) and female (b, left panel) WT and Sod1KO mice for 4-HNE (n = 6–8 mice/group). Right panel of a and b: Quantification of band intensity of the entire lane represented graphically. (c, d) Transcript levels of TNFα, IL6, and IL-1β in the hippocampus of WT and Sod1KO male (c, left panel) and female (d, left panel) (n = 6–8 mice/group). Transcript levels of CD68, TLR4, and MCP1 in the hippocampus of WT and Sod1KO male (c, right panel) and female (d, right panel) (n = 6–8 mice/group). Data are mean ± SEM for each group. *p < 0.05
Fig. 6.
(a) Correlation analysis of oxidative stress with maximum learning index for males (left) and females (right). (b) Correlation analysis of IL-1β with maximum learning index for males (left) and females (right)
Discussion
In this study, we assessed cognitive function of Sod1KO and WT mice using an automated home-cage based system where discrimination learning was assessed in the absence of experimenter handling. Middle-aged Sod1KO mice are phenotypically comparable to old wild type mice. Therefore, we used 13 to 15-month old Sod1KO male mice for the current study. Once we identified cognitive deficits in male Sod1KO mice, we were interested in testing whether female Sod1KO mice also exhibit a similar phenotype. For analysis in females, we used 16 to 19-month old female Sod1KO and WT mice. We concluded that it was important to determine if female mice with a deficiency of Sod1 also showed a similar cognition deficit.
The initial discrimination phase assessed working reference memory, where the mice learn to strategically determine the correct response (Dudchenko 2004). Both male and female Sod1KO mice showed no differences during the initial discrimination phase, however, the reversal learning that requires animals to extinguish the previous rewarded paradigm and learn the new reward paradigm was markedly reduced in Sod1KO mice compared to control mice. Reversal learning in mice has been evaluated in a variety of behavioral tasks, including spatial learning with Morris water, T-maze (Bannerman et al. 2003), eight-arm maze (El-Ghundi et al. 2003), etc. Our data demonstrate that Sod1KO mice show a decline in cognitive flexibility, as assessed by the increase in incorrect entries during the reversal phase of learning, compared to age-matched WT mice. Importantly, we conclude that the decline in cognition in Sod1KO mice was not due to differences in activity or aversion to the food reward since the activity and cognitive function during the initial phase of discrimination learning was comparable between the two groups. Furthermore, we found that the decline in cognition exhibited by Sod1KO mice demonstrates marked similarity to the decline in cognition observed with age in 27-month-old male C57BL/6 mice (Logan et al., 2018), assessed using the PhenoTyper suggesting that Sod1KO mice have an accelerated aging phenotype.
The decline in cognition with advanced age is a characteristic feature of aging in humans (Harada et al. 2013; Salthouse 2010). For example, a measurable decline in specific cognitive domains such as attention, memory, executive cognitive function, language, and visuospatial abilities has been reported with increasing age in humans (Murman 2015). Spatial navigation testing, the human analog of the Morris Water Maze, has shown that older humans (71–84 years old males and females) have deficits in allocentric (world-centered, hippocampus-dependent) navigation compared to young, 18 to 26-year-old subjects (Gazova et al. 2013). Similarly, various aspects of free recall are impaired in older humans, as assessed by memory performance on a multi-trial verbal recall task and 1-day delay free recall and recognition trials (Davis et al. 2003). In another study, a virtual Morris water maze (MWM) that is similar to rodent-based MWM was used to study search performance of healthy young and older adults. The study identified that poor-performing older adults exhibit low search accuracy, whereas high-performing older adults exhibited search accuracy comparable to that of the younger adults (Zhong et al. 2017). Similar to humans, laboratory rodents are also reported to show an age-related decline in cognition. A significant deficit in spatial memory was reported in 18-month-old male C57Bl/6 mice (compared to 2-month-old mice), and in 22 to 24-month-old male Long-Evans rats (compared to 6 to 8-month-old rats) as measured by the Barnes Maze and Morris Water maze, respectively (Barreto et al. 2010; Robitsek et al. 2008; Markowska et al. 1998). Analysis of general learning abilities with a battery of seven learning tasks (Lashley Maze, passive avoidance, fear conditioning, odor discrimination, spatial water maze, spatial radial arm maze, and reinforced alternation) in young (3 to 5-month-old) and old (19 to 21-month-old) male and female Balb/C mice showed that old animals have deficits in five of the seven tasks (Matzel et al. 2008). Thus, our finding that middle-aged Sod1KO mice exhibit a decline in cognition similar to 27-month-old male C57BL/6 mice is consistent with accelerated aging. The decline in cognition in the Sod1KO mice is associated with alterations in other age-related parameters, e.g., reduced lifespan (Elchuri et al. 2005; Zhang et al.2013), hearing loss (Keithley et al. 2005), cataracts (Olofsson et al. 2007), skin thinning and delayed wound healing (Iuchi et al. 2010), loss of muscle mass (Muller et al. 2006), and dramatic increases in pathological lesions in mice (Snider et al. 2018).
Even though the age-associated decline in learning and memory is well documented in humans (Davis et al. 2003) and laboratory rodents (Barnes 1988; Forster et al. 1996), the molecular mechanisms underlying the decline in cognition is not clearly understood. Accumulation of oxidative stress with age is one of the proposed mechanisms for age-associated cognitive decline (Dröge and Schipper 2007; Gemma et al. 2007). Structural and functional integrity of the hippocampus is critical for learning and memory, and increased oxidative stress within in the hippocampus has been associated with age-related cognitive decline (Geinisman et al. 1995; Stebbings et al. 2016; Lynch 1998). In our study we found that Sod1KO mice have increased oxidative stress in the hippocampus, as assessed by the levels of 4-HNE and that this was highly correlated with cognitive performance. Previously, it has been shown that oxidative stress, as measured by the level of F2-isoprostanes, is increased by 50% in the brains of 12 to 14-month-old Sod1KO mice, compared to WT mice (Zhang et al. 2016), and Sod1KO mice show a dramatic increase in oxidative damage in response to diquat (Han et al. 2008). Furthermore, loss of Sod1 in a mouse model of Alzheimer’s disease increased oxidative damage in the brain and exacerbated the memory loss, further supporting the role of oxidative stress in cognitive decline (Murakami et al. 2011). Oxidative stress increases in the brain of humans and rodents with age (Calabrese et al. 2004; Cini and Moretti 1995; Hamilton et al. 2001; O’Donnell and Lynch 1998; Siqueira et al. 2005; Sohal et al. 1994) and a recent study in humans suggested that oxidative stress may be an early biomarker of cognitive decline with aging (Hajjar et al. 2018). Chronic or subchronic treatment with antioxidants have been shown to partially reverse the age-related increase in oxidative stress and reverse the decline in learning and memory in old mice further supporting the role of oxidative stress in cognitive decline (Liu et al. 2002;Carney et al. 1991;Fredriksson and Archer1996;Floyd et al. 2002). Finally, oxidative stress in hippocampus is associated with impairments in retention of spatial memory in C57BL/6 J mice and age-associated cognitive decline in rats (Liu et al. 2014; Nicolle et al. 2001). Thus, our observation that the high levels of oxidative stress observed in the hippocampus of Sod1KO mice are associated with a decline in cognition supports the concept that oxidative stress has an important and primary role in the age-related decline in cognition. In our study, Sod1KO mice exhibited a deficit in reversal learning, not initial discrimination, that is indicative of a dysfunction in the coordinated actions of brain regions such as the hippocampus, orbitofrontal cortex, dorsal striatum, and amygdala (Vilà-Balló et al. 2017, Ragozzino 2007; Tait and Brown 2007; Bissonette and Powell, 2012; Floresco and Jentsch 2011). In Sprague-Dawley rats, reversal learning assessed using the Morris water maze is impaired by blocking hippocampal long-term depression (LTD), suggesting hippocampal LTD as an integral component of extinction of a learned task and acquisition of new information (Dong et al. 2013). Similarly, in C57BL6/N mice, hippocampal lesions induced by ibotenic acid disrupted re-learning in water cross maze (Kleinknecht et al., 2012). Future studies examining oxidative stress in various brain regions of Sod1KO mice will be important to understand the role of regional differences in oxidative stress on cognitive function.
Chronic oxidative stress initiates signaling pathways that activate production of proinflammatory cytokines by microglia and astrocytes leading to neuroinflammation and neurodegeneration (Solleiro-Villavicencio and Rivas-Arancibia 2018). Neuroinflammation is a characteristic feature of aging as well as various neurodegenerative diseases, and is strongly associated with cognitive decline in both humans and rodents (Ojala et al. 2009; Griffin 2006; Simen et al. 2011; Bettio et al. 2017; Valcarcel-Ares et al. 2019). We found increased neuroinflammation in the hippocampus of Sod1KO mice, as shown by the increased expression of proinflammatory cytokines IL6 and/or IL-1β, marker of activated microglia CD68 (Taipa et al. 2018), microglial activation marker toll-like receptor 4 (TLR4) (Yao et al. 2013), and marker of microglial recruitment/activation, the monocyte chemoattractant protein-1 (MCP1) (Yang et al. 2011). A previous study has reported an increase in the expression of IL-1β, TNFα, and IL-6 in the hippocampus of senescence-accelerated mice (SAM), which is another mouse model for accelerated aging that shows a decline in cognition (Tha et al. 2000).
IL-1β, IL-6, and TNFα are the three major cytokines associated with age-associated inflammation (Hager et al. 1994; Pedersen et al. 2003; Ferrucci et al. 2005; Roubenoff et al. 1998). Among these cytokines, IL-1β is reported to affect hippocampal-dependent learning: an intracerebroventricular injection of IL-1β in rats resulted in poor performance in the Morris water maze (Oitzl et al. 1993); an increase in hippocampal IL-1β concentration following intraperitoneal or intrahippocampal injection of IL-1β impaired hippocampal dependent tasks in rats (Gibertini et al. 1995; Barrientos et al. 2002); and chronic overexpression of IL-1β in the hippocampus impaired long-term contextual and spatial memory in spatial memory in mice (Moore et al. 2009; Hein et al. 2010). Importantly, IL-1β was the only cytokine increased in both male and female Sod1KO mice suggesting that it has an important role in cognitive dysfunction in response to increased oxidative stress.
As we are using a whole body Sod1KO mice, the effect of vascular oxidative stress on the observed cognitive deficits cannot be ignored. Studies focusing on the cerebral microvasculature have shown that oxidative stress can cause endothelial dysfunction, impaired cerebral blood flow dysregulation and cognitive decline (Toth et al. 2017). In humans, decline in vascular health has been demonstrated to predict cognitive decline during aging (Csipo et al. 2019). Increasing oxidative stress by genetic deletion of Nrf2, the master transcription factor that regulates anti-oxidant genes, has been shown to increase cerebrovascular inflammation, affect neurovascular coupling responses, promote senescence and results in cognitive dysfunction (Tarantini et al. 2018a; Fulop et al. 2018). Furthermore, interventions that attenuate vascular oxidative stress has been shown to restore microvascular function and cognition (Tarantini et al. 2018b, 2019).
In summary, our data combined with previous data on Sod1KO mice demonstrates that this mouse model exhibits accelerated aging in all tissues and physiological functions tested, including cognition (Keithley et al. 2005; Olofsson et al. 2007; Iuchi et al. 2010; Muller et al. 2006; Larkin et al. 2011; Deepa et al. 2017). Our study also supports a role of oxidative stress and inflammation in cognitive decline in the Sod1KO mice, which may also underlie age-associated learning and memory deficits in neurodegenerative diseases. Therefore, antioxidant or anti-inflammatory interventions may improve cognitive performance with age and impede progression of neurodegenerative diseases, such as Alzheimer’s disease.
Acknowledgements
This work was supported by NIH/NIA R01 AG059718, Oklahoma Center for the Advancement of Science and Technology research grant (HR18-053) and Presbyterian Health Foundation (OUHSC) Seed grant to Dr. Sathyaseelan S Deepa; National Institute on Aging K99 AG056662 to Dr. Sreemathi Logan; T32 AG052363 and R01 NS056218 to Dr. William Sonntag; R01 AG057424 to Drs. William Sonntag and Arlan Richardson. The research was also partially supported by grants awarded to Dr. Arlan Richardson from the National Institute on Aging (P01AG020591, R01AG045693).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Seeburg PH, Rawlins JN. GluR-A-deficient mice display normal acquisition of a hippocampus-dependent spatial reference memory task but are impaired during spatial reversal. Behav Neurosci. 2003;117:866–870. doi: 10.1037/0735-7044.117.4.866. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Aging and the physiology of spatial memory. Neurobiol Aging. 1988;9:563–568. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- Barreto G, Huang TT, Giffard RG. Age-related defects in sensorimotor activity, spatial learning, and memory in C57BL/6 mice. J Neurosurg Anesthesiol. 2010;22:214–219. doi: 10.1097/ANA.0b013e3181d56c98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Bettio LEB, Rajendran L, Gil-Mohapel J. The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev. 2017;79:66–86. doi: 10.1016/j.neubiorev.2017.04.030. [DOI] [PubMed] [Google Scholar]
- Bhaskaran S, Pharaoh G, Ranjit R, Murphy A, Matsuzaki S, Nair BC, Forbes B, Gispert S, Auburger G, Humphries KM, Kinter M, Griffin TM, Deepa SS (2018) Loss of mitochondrial protease ClpP protects mice from diet-induced obesity and insulin resistance. EMBO 19. 10.15252/embr.201745009 [DOI] [PMC free article] [PubMed]
- Bissonette GB, Powell EM. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 2012;62:1168–1174. doi: 10.1016/j.neuropharm.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitzig M, Bhimineni C, Lockey R, Kolliputi N. 4-Hydroxy-2-nonenal: a critical target in oxidative stress? Am J Physiol Cell Physiol. 2016;311:C537–C543. doi: 10.1152/ajpcell.00101.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Stella AM, Butterfield DA, Scapagnini G. Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brain stress tolerance. Antioxid Redox Signal. 2004;6:895–913. doi: 10.1089/ars.2004.6.895. [DOI] [PubMed] [Google Scholar]
- Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci U S A. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cini M, Moretti A. Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiol Aging. 1995;16:53–57. doi: 10.1016/0197-4580(95)80007-e. [DOI] [PubMed] [Google Scholar]
- Csipo T, Lipecz A, Fulop GA, Hand RA, Ngo BN, Dzialendzik M, Tarantini S, Balasubramanian P, Kiss T, Yabluchanska V, Silva-Palacios F, Courtney DL, Dasari TW, Sorond F, Sonntag WE, Csiszar A, Ungvari Z, Yabluchanskiy A. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience. 2019;41:125–136. doi: 10.1007/s11357-019-00063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Avila JC, Siqueira LD, Mazeraud A, Azevedo EP, Foguel D, Castro-Faria-Neto HC, Sharshar T, Chrétien F, Bozza FA. Age-related cognitive impairment is associated with long-term neuroinflammation and oxidative stress in a mouse model of episodic systemic inflammation. J Neuroinflammation. 2018;15:28. doi: 10.1186/s12974-018-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalleau S, Baradat M, Guéraud F, Huc L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013;20:1615–1630. doi: 10.1038/cdd.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Small SA, Stern Y, Mayeux R, Feldstein SN, Keller FR. Acquisition, recall, and forgetting of verbal information in long-term memory by young, middle-aged, and elderly individuals. Cortex. 2003;39:1063–1091. doi: 10.1016/s0010-9452(08)70878-5. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A. A new mouse model of frailty: the cu/Zn superoxide dismutase knockout mouse. Geroscience. 2017;39(2):187–198. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Bai Y, Wu X, Li H, Gong B, Howland JG, Huang Y, He W, Li T, Wang YT. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology. 2013;64:65–73. doi: 10.1016/j.neuropharm.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Dröge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, O'Dowd BF, Erclik M, George SR. Attenuation of sucrose reinforcement in dopamine D1 receptor deficient mice. Eur J Neurosci. 2003;17:851–862. doi: 10.1046/j.1460-9568.2003.02496.x. [DOI] [PubMed] [Google Scholar]
- Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Jentsch JD. Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology. 2011;36:227–2250. doi: 10.1038/npp.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA, Hensley K, Forster MJ, Kelleher-Anderson JA, Wood PL. Nitrones as neuroprotectants and antiaging drugs. Ann N Y Acad Sci. 2002;959:321–329. doi: 10.1111/j.1749-6632.2002.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson A, Archer T. Alpha-phenyl-tert-butyl-nitrone (PBN) reverses age-related maze learning performance and motor activity deficits in C57 BL/6 mice. Behav Pharmacol. 1996;7:245–253. [PubMed] [Google Scholar]
- Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S. Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann N Y Acad Sci. 2001;928:168–175. doi: 10.1111/j.1749-6632.2001.tb05646.x. [DOI] [PubMed] [Google Scholar]
- Fukui K, Omoi NO, Hayasaka T, Shinnkai T, Suzuki S, Abe K, Urano S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann N Y Acad Sci. 2002;959:275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazova I, Laczó J, Rubinova E, Mokrisova I, Hyncicova E, Andel R, Vyhnalek M, Sheardova K, Coulson EJ, Hort J. Spatial navigation in young versus older adults. Front Aging Neurosci. 2013;5:94. doi: 10.3389/fnagi.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE. Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog Neurobiol. 1995;45:223–252. doi: 10.1016/0301-0082(94)00047-l. [DOI] [PubMed] [Google Scholar]
- Gemma C, Vila J, Bachstetter A, Bickford PC. Oxidative stress and the aging brain: from theory to prevention. In: Riddle DR, editor. Brain aging: models, methods, and mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–1128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Griffin WS. Inflammation and neurodegenerative diseases. Am J Clin Nutr. 2006;83:470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- Hager K, Machein U, Krieger S, Platt D, Seefried G, Bauer J. Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiol Aging. 1994;15:771–772. doi: 10.1016/0197-4580(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Hajjar I, Hayek SS, Goldstein FC, Martin G, Jones DP, Quyyumi A. Oxidative stress predicts cognitive decline with aging in healthy adults: an observational study. J Neuroinflammation. 2018;15:17. doi: 10.1186/s12974-017-1026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ES, Muller FL, Pérez VI, Qi W, Liang H, Xi L, Fu C, Doyle E, Hickey M, Cornell J, Epstein CJ, Roberts LJ, Van Remmen H, Richardson A. The in vivo gene expression signature of oxidative stress. Physiol Genomics. 2008;34:112–126. doi: 10.1152/physiolgenomics.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29:737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O'Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxidative Med Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi Y, Kosugi S, Nishikawa H, Lin Z, Minabe Y, Toda S. Repeated exposure of adult rats to transient oxidative stress induces various long-lasting alterations in cognitive and behavioral functions. PLoS One. 2014;9:e114024. doi: 10.1371/journal.pone.0114024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi Y, Roy D, Okada F, Kibe N, Tsunoda S, Suzuki S, Takahashi M, Yokoyama H, Yoshitake J, Kondo S, Fujii J. Spontaneous skin damage and delayed wound healing in SOD1-deficient mice. Mol Cell Biochem. 2010;341:181–194. doi: 10.1007/s11010-010-0449-y. [DOI] [PubMed] [Google Scholar]
- Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, Salmon AB, Brooks SV, Larkin L, Hayworth CR, Richardson A, Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res. 2005;209:76–85. doi: 10.1016/j.heares.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinknecht KR, Bedenk BT, Kaltwasser SF, Grünecker B, Yen YC, Czisch M, Wotjak CT. Hippocampus-dependent place learning enables spatial flexibility in C57BL6/N mice. Front Behav Neurosci. 2012;6:87. doi: 10.3389/fnbeh.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SU, Jerome GJ, Simonsick EM, Studenski S, Ferrucci L. Differential gait patterns by history of falls and knee pain status in healthy older adults: results from the Baltimore longitudinal study of aging. J Aging Phys Act. 2018;26:577–582. doi: 10.1123/japa.2017-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin LM, Davis CS, Sims-Robinson C, Kostrominova TY, Van Remmen H, Richardson A, Feldman EL, Brooks SV. Skeletal muscle weakness due to deficiency of CuZn-superoxide dismutase is associated with loss of functional innervation. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1400–R1407. doi: 10.1152/ajpregu.00093.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci. 2002;959:133–166. doi: 10.1111/j.1749-6632.2002.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Liu YZ, Chen JK, Li ZP, Zhao T, Ni M, Li DJ, Jiang CL, Shen FM. High-salt diet enhances hippocampal oxidative stress and cognitive impairment in mice. Neurobiol Learn Mem. 2014;114:10–15. doi: 10.1016/j.nlm.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Logan S, Pharaoh GA, Marlin MC, Masser DR, Matsuzaki S, Wronowski B, Yeganeh A, Parks EE, Premkumar P, Farley JA, Owen DB, Humphries KM, Kinter M, Freeman WM, Szweda LI, Van Remmen H, Sonntag WE. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-β uptake in astrocytes. Mol Metab. 2018;9:141–155. doi: 10.1016/j.molmet.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S, Owen D, Chen S, Chen WJ, Ungvari Z, Farley J, Csiszar A, Sharpe A, Loos M, Koopmans B, Richardson A, Sonntag WE. Simultaneous assessment of cognitive function, circadian rhythm, and spontaneous activity in aging mice. Geroscience. 2018;40:123–137. doi: 10.1007/s11357-018-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M, Koopmans B, Aarts E, Maroteaux G, van der Sluis S, Neuro-BSIK Mouse Phenomics Consortium. Verhage M, Smit AB. Sheltering behavior and locomotor activity in 11 genetically diverse common inbred mouse strains using home-cage monitoring. PLoS One. 2014;9:e108563. doi: 10.1371/journal.pone.0108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Analysis of the mechanisms underlying the age-related impairment in long-term potentiation in the rat. Rev Neurosci. 1998;9:169–201. doi: 10.1515/revneuro.1998.9.3.169. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Maroteaux G, Loos M, van der Sluis S, Koopmans B, Aarts E, van Gassen K, Geurts A, NeuroBSIK Mouse Phenomics Consortium. Largaespada DA, Spruijt BM, Stiedl O, Smit AB, Verhage M. High-throughput phenotyping of avoidance learning in mice discriminates different genotypes and identifies a novel gene. Genes Brain Behav. 2012;11:772–784. doi: 10.1111/j.1601-183X.2012.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Grossman H, Light K, Townsend D, Kolata S. Age-related declines in general cognitive abilities of Balb/C mice are associated with disparities in working memory, body weight, and general activity. Learn Mem. 2008;15:733–746. doi: 10.1101/lm.954808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Roberts RO, Savica R, Cha R, Drubach DI, Christianson T, Pankratz VS, Geda YE, Machulda MM, Ivnik RJ, Knopman DS, Boeve BF, Rocca WA, Petersen RC. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic study of aging. J Gerontol A Biol Sci Med Sci. 2013;68:929–937. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AH, Wu M, Shaftel SS, Graham KA, O'Banion MK. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164:1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, 2nd, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Murakami K, Murata N, Noda Y, Tahara S, Kaneko T, Kinoshita N, Hatsuta H, Murayama S, Barnham KJ, Irie K, Shirasawa T, Shimizu T. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid β protein oligomerization and memory loss in mouse model of Alzheimer disease. J Biol Chem. 2011;286:44557–44568. doi: 10.1074/jbc.M111.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murman DL. The impact of age on cognition. Semin Hear. 2015;36:111–121. doi: 10.1055/s-0035-1555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, van Oers H, Schöbitz B, de Kloet ER. Interleukin-1 beta, but not interleukin-6, impairs spatial navigation learning. Brain Res. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- Ojala J, Alafuzoff I, Herukka SK, van Groen T, Tanila H, Pirttilä T. Expression of interleukin-18 is increased in the brains of Alzheimer's disease patients. Neurobiol Aging. 2009;30:198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- Olofsson EM, Marklund SL, Behndig A. Glucose-induced cataract in CuZn-SOD null lenses: an effect of nitric oxide? Free Radic Biol Med. 2007;42:1098–1105. doi: 10.1016/j.freeradbiomed.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Pedersen M, Bruunsgaard H, Weis N, Hendel HW, Andreassen BU, Eldrup E, Dela F, Pedersen BK. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev. 2003;124:495–502. doi: 10.1016/s0047-6374(03)00027-7. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, Scott RW, Snider WD. Motor neurons in cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci. 2008;28:8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, Verghese J, Metti AL, Boudreau RM, Aizenstein HJ, Kritchevsky S, Harris T, Yaffe K, Satterfield S, Studenski S, Rosano C. Slowing gait and risk for cognitive impairment: the hippocampus as a shared neural substrate. Neurology. 2017;89:336–342. doi: 10.1212/WNL.0000000000004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53:M20–M26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Richardson A, Pérez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16:754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Ther Adv Chronic Dis. 2011;2:175–195. doi: 10.1177/2040622311399145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira IR, Fochesatto C, de Andrade A, Santos M, Hagen M, Bello-Klein A, Netto CA. Total antioxidant capacity is impaired in different structures from aged rat brain. Int J Dev Neurosci. 2005;23:663–671. doi: 10.1016/j.ijdevneu.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Snider TA, Richardson A, Stoner JA, Deepa SS. The Geropathology grading platform demonstrates that mice null for cu/Zn-superoxide dismutase show accelerated biological aging. Geroscience. 2018;40:97–103. doi: 10.1007/s11357-018-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- Solleiro-Villavicencio H, Rivas-Arancibia S. Effect of chronic oxidative stress on Neuroinflammatory response mediated by CD4(+)T cells in neurodegenerative diseases. Front Cell Neurosci. 2018;12:114. doi: 10.3389/fncel.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings KA, Choi HW, Ravindra A, Llano DA. The impact of aging, hearing loss, and body weight on mouse hippocampal redox state, measured in brain slices using fluorescence imaging. Neurobiol Aging. 2016;42:101–109. doi: 10.1016/j.neurobiolaging.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suire CN, Eitan E, Shaffer NC, Tian Q, Studenski S, Mattson MP, Kapogiannis D. Walking speed decline in older adults is associated with elevated pro-BDNF in plasma extracellular vesicles. Exp Gerontol. 2017;98:209–216. doi: 10.1016/j.exger.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipa R, Ferreira V, Brochado P, Robinson A, Reis I, Marques F, Mann DM, Melo-Pires M, Sousa N. Inflammatory pathology markers (activated microglia and reactive astrocytes) in early and late onset Alzheimer disease: a post mortem study. Neuropathol Appl Neurobiol. 2018;44:298–313. doi: 10.1111/nan.12445. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Difficulty overcoming learned non-reward during reversal learning in rats with ibotenic acid lesions of orbital prefrontal cortex. Ann N Y Acad Sci. 2007;1121:407–420. doi: 10.1196/annals.1401.010. [DOI] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott MH, Kinter MT, Deak F, Ungvari Z, Csiszar A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, Neuroinflammation, Amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2018;73:853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Süle Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tha KK, Okuma Y, Miyazaki H, Murayama T, Uehara T, Hatakeyama R, Hayashi Y, Nomura Y. Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res. 2000;885:25–31. doi: 10.1016/s0006-8993(00)02883-3. [DOI] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Szweda LI, Chae HZ, Stadtman ER. Immunochemical detection of 4-hydroxynonenal protein adducts in oxidized hepatocytes. Proc Natl Acad Sci U S A. 1993;90:8742–8746. doi: 10.1073/pnas.90.18.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Itakura K, Kawakishi S, Hiai H, Toyokuni S, Stadtman ER. characterization of epitopes recognized by 4-hydroxy-2-nonenal specific antibodies. Arch Biochem Biophys. 1995;324(2):241–248. doi: 10.1006/abbi.1995.0036. [DOI] [PubMed] [Google Scholar]
- Valcarcel-Ares MN, Tucsek Z, Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Galvan V, Ballabh P, Richardson A, Freeman WM, Wren JD, Deak F, Ungvari Z, Csiszar A. Obesity in aging exacerbates Neuroinflammation, dysregulating synaptic function-related genes and altering eicosanoid synthesis in the mouse Hippocampus: potential role in impaired synaptic plasticity and cognitive decline. J Gerontol A Biol Sci Med Sci. 2019;74:290–298. doi: 10.1093/gerona/gly127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Farley JA, Yan H, Van Kirk CA, Mitschelen M, Sonntag WE, Freeman WM. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011;43:201–212. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder Starkey HD, Bixler GV, Sonntag WE, Freeman WM. Expression of NgR1-antagonizing proteins decreases with aging and cognitive decline in rat hippocampus. Cell Mol Neurobiol. 2013;33:483–488. doi: 10.1007/s10571-013-9929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilà-Balló A, Mas-Herrero E, Ripollés P, Simó M, Miró J, Cucurell D, López-Barroso D, Juncadella M, Marco-Pallarés J, Falip M, Rodríguez-Fornells A. Unraveling the role of the Hippocampus in reversal learning. J Neurosci. 2017;37:6686–6697. doi: 10.1523/JNEUROSCI.3212-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson NL, Rosano C, Boudreau RM, Simonsick EM, Ferrucci L, Sutton-Tyrrell K, Hardy SE, Atkinson HH, Yaffe K, Satterfield S, Harris TB, Newman AB, Health ABC Study Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65:1093–1100. doi: 10.1093/gerona/glq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Meng Y, Li W, Yong Y, Fan Z, Ding H, Wei Y, Luo J, Ke ZJ. Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain Pathol. 2011;21:279–297. doi: 10.1111/j.1750-3639.2010.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur C, Ling EA. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation. 2013;10:23. doi: 10.1186/1742-2094-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ikeno Y, Bokov A, Gelfond J, Jaramillo C, Zhang HM, Liu Y, Qi W, Hubbard G, Richardson A, Van Remmen H. Dietary restriction attenuates the accelerated aging phenotype of Sod1(−/−) mice. Free Radic Biol Med. 2013;60:300–306. doi: 10.1016/j.freeradbiomed.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Walsh M, Bokov A, Ikeno Y, Jang YC, Perez VI, Van Remmen H, Richardson A. Liver specific expression of cu/ZnSOD extends the lifespan of Sod1 null mice. Mech Ageing Dev. 2016;154:1–8. doi: 10.1016/j.mad.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong JY, Magnusson KR, Swarts ME, Clendinen CA, Reynolds NC, Moffat SD. The application of a rodent-based Morris water maze (MWM) protocol to an investigation of age-related differences in human spatial learning. Behav Neurosci. 2017;131:470–482. doi: 10.1037/bne0000219. [DOI] [PMC free article] [PubMed] [Google Scholar]