Abstract
Proteins embedded in the inner mitochondrial membrane (IMM) perform essential cellular functions. Maintaining the folding state of these proteins is therefore of the utmost importance, and this is ensured by IMM chaperones and proteases that refold and degrade unassembled and misfolded proteins. However, the physiological consequences specific to IMM protein misfolding remain obscure because deletion of these chaperones/proteases (the typical experimental strategy) often affects many mitochondrial processes other than protein folding and turnover. Thus, novel experimental systems are needed to evaluate the direct effects of misfolded protein on the membrane. Such a system has been developed in recent years. Studies suggest that numerous pathogenic mutations in isoform 1 of adenine nucleotide translocase (Ant1) cause its misfolding on the IMM. In this review, we first discuss potential mechanisms by which dominant Ant1 mutations may cause disease, highlighting IMM protein misfolding, per se, as a likely pathological factor. Then we discuss the intramitochondrial effects of Ant1 misfolding such as IMM proteostatic stress, respiratory chain dysfunction, and mtDNA instability. Finally, we summarize the mounting evidence that IMM proteostatic stress can perturb mitochondrial protein import to cause the toxic accumulation of mitochondrial proteins in the cytosol: a cell stress mechanism termed mitochondrial Precursor Overaccumulation Stress (mPOS).
Keywords: mitochondria, mitochondrial carrier, Ant1, misfolding, mPOS
Introduction
Mitochondria are essential organelles involved in many different cellular processes such as energy production, calcium signaling, phospholipid metabolism, iron sulfur biosynthesis and redox homeostasis. They were derived from ancient independent-living α-proteobacteria after engulfment by another cell to become an intracellular organelle some 2 billion years ago (Gray, 2012). Since then, most of their genome has been transferred to the nucleus. In humans, only 13 of the ~1500 mitochondrial proteins are encoded by mitochondrial DNA (mtDNA). The rest are synthesized in the cytosol and imported into mitochondria. This necessitates an intimate relationship between cytosolic and mitochondrial protein homeostasis (proteostasis) that has co-evolved throughout eukaryotic history. We are still in the early stages of understanding this proteomic interaction.
All of mitochondria’s functions noted above occur, at least in part, on the inner mitochondrial membrane (IMM). The IMM is densely packed with proteins and is characterized by relatively low phospholipid/protein ratio (Ardail et al., 1990; Simbeni et al., 1991). To provide an adequate micro-environment for efficient assembly of respiratory enzymes and other metabolic complexes on the IMM, eukaryotic cells evolved specific quality control systems to assist protein folding, and to degrade unassembled, misfolded, mutated and perhaps also mistargeted proteins. These protein quality control modules include several metalloproteases such as the m-AAA and i-AAA proteases, as well as the stress-sensing OMA1 protease (Bohovych et al., 2015; Gerdes et al., 2012; Rugarli and Langer, 2012). Under current paradigm, the goal of maintaining IMM proteostasis is to preserve oxidative phosphorylation (OXPHOS). This function may become critical under various pathophysiological conditions that may compromise IMM proteostasis. Many conditions should be considered in this context, such as protein overloading into the membrane, excessive oxidative damage to the IMM, altered phospholipid properties, defects in protein complex assembly, imbalanced nuclear and mitochondrial gene expression, and physiological aging. The importance of IMM protection is underscored by the discovery of various human diseases caused by mutations in the mitochondrial protein quality control proteases (Martinelli and Rugarli, 2010; Rugarli and Langer, 2012).
The mechanism(s) by which deficiency of IMM proteases affects cell fitness and induces diseases is not fully understood. One of the pertinent questions is whether accumulation of misfolded proteins is a primary cause of disease. This question arises following recent studies showing that the protein quality control proteases are multifunctional. For example, in addition to protein turnover, the AAA proteases are involved in respiratory complex assembly, ribosomal maturation, mitochondrial dynamics, organelle transport, phospholipid synthesis and control of IMM permeability barrier (Kondadi et al., 2014; Martinelli and Rugarli, 2010; Nolden et al., 2005; Potting et al., 2013; Shanmughapriya et al., 2015). Thus, it remains unresolved whether deficiency in the protein quality control proteases causes diseases by affecting one of these functions, or whether it also involves other deleterious effects that may result from the accumulation of misfolded proteins on the IMM. Novel experimental systems are clearly needed to directly evaluate the physiological consequences of protein misfolding on the IMM.
Recent studies have shown that several missense mutations in adenine nucleotide translocase 1, an abundant IMM protein primarily involved in ATP/ADP exchange, cause protein misfolding and cell death independent of its translocase activity. In this review, we will focus on the use of this unique experimental system to address some of the important questions related to the physiological effects of protein misfolding on the IMM.
1. Missense mutations in adenine nucleotide translocase 1 (Ant1) cause dominant disease, protein misfolding, and proteostatic stress on the IMM.
Mitochondrial carrier family (MCF) proteins are integral IMM proteins involved in the transport of respiratory substrates, nucleotides, cofactors and a variety of metabolic intermediates across the membrane (Palmieri and Monne, 2016). Loss of mitochondrial carrier function usually leads to recessive metabolic disorders due to substrate transport failure (Palmieri, 2014). Adenine nucleotide translocase (Ant) is the prototypical member of the MCF proteins, and it is primarily involved in ADP/ATP exchange. Under respiring conditions, ATP4− generated by oxidative phosphorylation is exported to the cytosol. ATP4− export is strictly coupled with the import of ADP3− into the mitochondrial matrix to serve as a substrate for ATP4− synthesis. Ant1 is the muscle/heart/brain isoform of Ant. Like in many bioenergetic diseases affecting muscle tissues, complete loss of Ant1 function due to homozygous transport-defective or null mutations cause cardiomyopathy, myopathy and lactic acidosis (Echaniz-Laguna et al., 2012; Korver-Keularts et al., 2015; Palmieri et al., 2005; Strauss et al., 2013; Tosserams et al., 2018; von Renesse et al., 2019). Interestingly, several missense mutations in ANT1 were found to cause dominant pathology. These ANT1 mutations cause the adult- and late-onset disease, autosomal dominant Progressive External Ophthalmoplegia (adPEO), manifested by ptosis, ophthalmoplegia and skeletal muscle weakness with multiple mtDNA deletions. Patients with dominant ANT1 mutations also present with neurological symptoms such as sensorineural hearing loss, clonic seizures, bipolar disorder, cortical atrophy and dementia (Deschauer et al., 2005; Kaukonen et al., 2000; Kaukonen et al., 1999; Komaki et al., 2002; Liu and Chen, 2013; Napoli et al., 2001; Siciliano et al., 2003; Simoncini et al., 2017). Some of these dominant mutations have been shown to affect adenine nucleotide transport activity and substrate preference (Fontanesi et al., 2004; Kawamata et al., 2011). Consistent with this, when similar mutations were introduced into the yeast AAC2 gene, the homolog of human ANT1, the mutant alleles are clearly deficient in supporting respiratory growth of haploid cells lacking the endogenous wild-type AAC2. This suggests that the mutants have lost or reduced ATP/ADP exchange activity on the IMM (Fontanesi et al., 2004; Kaukonen et al., 2000; Lodi et al., 2006). Other investigators generated an Aac2/Ant1 chimera that carries the adPEO A114P mutation (de Marcos-Lousa et al., 2006). The mutant protein also exhibited low activity in supporting the respiratory growth of yeast cells relative to the Aac2/Ant1 chimera lacking the mutation. These data suggest a haploinsufficiency or dominant-negative model of dominant Ant1 disease. However, as discussed in detail below, these hypotheses are inconsistent with many observations. To start, the clinical manifestations of Ant1-induced adPEO do not overlap with those seen in patients with the recessive homozygous null mutations of ANT1, making a haploinsufficiency mechanism unlikely (Strauss et al., 2013). In addition, increasing evidence supports a monomeric form of Ant1, making a dominant-negative mechanism unlikely (Kunji and Crichton, 2010; Pebay-Peyroula et al., 2003; Ruprecht et al., 2019). Thus, an alternative mechanism may be underlying disease. This led to the hypothesis that the dominant mutations in Ant1 may lead to the gain of protein toxicity that causes mitochondrial and cellular damage.
The first indication that dominant mutations in Ant1 may interfere with cell function beyond bioenergetic defects came from studies in the yeast model. When the A106D, M114P and A128P alleles of the yeast AAC2 gene, equivalent to the familial adPEO-type A90D, L98P and A114P mutations in human ANT1 (Fig. 1), are co-expressed with a wild-type copy of AAC2, the mutant alleles do not inhibit respiratory growth at optimal growth temperature (30°C) (Fontanesi et al., 2004; Wang et al., 2008a). This suggests that they do not have an obvious dominant-negative effect on the ADP(cytosol)/ATP(matrix) exchange activity of the endogenous AAC2 gene. Instead, the mutant alleles of aac2 strongly inhibit cell growth on fermentable carbon sources (i.e. glucose) in a dominant manner when cells were grown at 25°C (Wang et al., 2008a). Moderate overexpression of the mutant aac2 alleles, but not the wild-type AAC2, kills cells even at the optimal temperature of 30°C on glucose medium. Because Saccharomyces cerevisiae generates sufficient ATP by fermentation to support cell growth on glucose medium, these findings indicate that the mutant proteins have lethal effects beyond oxidative phosphorylation. Moreover, the R252I, R253I and R254I mutations in Aac2, which inactivate the nucleotide transport activity, do not suppress the cell-killing effect of the A128P allele (Wang et al., 2008b). Cellular damage by the mutant Aac2 is therefore independent of nucleotide transport. It was noted, however, that despite a failure of aac2 mutants to inhibit respiratory growth, cell death on fermentable medium is accompanied by broad structural and functional damage to mitochondria. These include respiratory uncoupling, IMM depolarization, mitochondrial swelling, mtDNA destabilization, and reduced expression and processing of mtDNA-encoded proteins (Chen, 2002; Wang et al., 2008a).
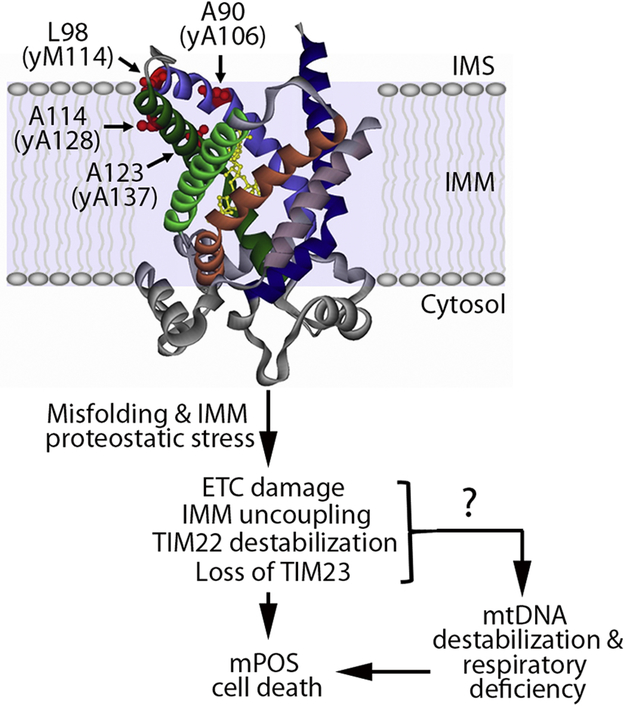
Figure 1. The effects of pathogenic missense mutations in adenine nucleotide translocase 1 (Ant1).
Crystal structure of bovine Ant1 in the cytosol-facing conformation bound by CATR (yellow) (Pebay-Peyroula et al., 2003). Structure contains projected location of the residues where pathogenic Ant1 missense mutations occur: A90, L98, A114 and A123. The yeast-equivalent residues are indicated in parentheses. In yeast, these mutations induce the protein to misfold in the inner mitochondrial membrane (IMM), which has the indicated downstream consequences. TTM22 and TIM23 are the protein import complexes on the IMM. ETC, electron transport chain; mPOS, mitochondrial Precursor Overaccumulation Stress; mtDNA, mitochondrial DNA.
A salient feature associated with the adPEO-type aac2 mutations is that they are synthetically lethal with the disruption of genes involved in IMM proteostasis and membrane biogenesis. These genes encode the Yme1 protease, subunits of the prohibitin complex, the Oxa1 insertase, and the Psd1 protein involved in phosphophatidylethanolamine on the IMM(Liu et al., 2015; Wang et al., 2008a; Wang et al., 2008b). These genetic interactions suggest that mutant Aac2 is misfolded, which induces proteostatic stress on the IMM and, consequently, cell death. Mutant Aac2 most likely interferes with the IMM proteostatic network, which renders cells hypersensitive to further reduction in IMM protein quality control and to altered phospholipid properties. This hypothesis was supported by the finding that expression of the adPEO-type mutations reduces the degradation of an unstable variant of the IMM protein, Ndel, by the i-AAA protease Yme1(Liu et al., 2015). In addition to the loss of IMM proteases and chaperones, the growth of yeast cells co-expressing the wild-type and the adPEO-type aac2 mutations is completely inhibited by antimycin and the elimination of mtDNA (ρ°-lethal) (Liu et al., 2015; Wang et al., 2008a). It is possible that the latter conditions directly or indirectly affect IMM proteostasis, and/or protein import that causes proteostatic stress in the cytosol (see below).
More recent biochemical studies confirmed that the adPEO-type mutations in Aac2 cause misfolding. It was shown by native PAGE that the mutant proteins form high molecular weight species >720 kDa when isolated mitochondria were incubated at 25°C, the same temperature at which mutant aac2 strongly reduces cell viability(Liu et al., 2015). These large molecular species are likely protein aggregates formed by the mutant Aac2, possibly together with other proteins. This striking observation suggests that the mutant Aac2 proteins are misfolded. Consistent with this is the finding that the steady state levels of human Ant1 with several adPEO-type mutations is reduced by >20 fold compared with the wild-type(Liu et al., 2019). Reduced accumulation suggests misfolding and increased attack by quality control proteases. Low accumulation of the adPEO-type Aac2A128P protein has also been reported in yeast (Fontanesi et al., 2004; Liu et al., 2015). The misfolded Aac2 and Ant1 could have increased exposure of hydrophilic patches to the lipid bilayer (Fig. 1), which would be recognized by IMM proteases for degradation. Indeed, overexpression of the Yme1 protease suppresses Aac2A128P-induced cell death(Liu et al., 2015). Consistent with protein misfolding underlying mutant aac2 toxicity, low temperature stabilizes the mutant proteins, and correspondingly enhances cell death(Liu et al., 2015). This suggests dose-dependent toxicity. In sum, it is now clear that aac2A106D, aac2M114P and aac2A128P are misfolded on the IMM.
Several groups have evaluated the physiological effects of the adPEO-type mutations. As noted above, aac2A106D, aac2M114P and aac2A128P alleles do not dominantly inhibit respiratory growth. However, they were found to dominantly reduce cytochrome content and mitochondrial respiration(Fontanesi et al., 2004; Wang et al., 2008a). Consistent with this, biochemical analyses showed that Aac2A106D, Aac2M114P and Aac2A128P cause the loss of respiratory supercomplexes and the ATP synthase when cells are cultured at 25° C(Liu et al., 2015). Moreover, the TIM23 and TIM22 protein import complexes are drastically reduced and destabilized, respectively. These results suggest that misfolded Aac2 has broad effects on the assembly and stability of protein complexes on the IMM. Loss of TIM22 and TIM23 may affect protein import. The mechanism by which the misfolded Aac2 affects protein complex biogenesis and/or stability is yet to be investigated. One possibility may involve the abundance of Ant protein on the IMM(Brand et al., 2005). Perhaps the mutant proteins trap proteases and chaperones, which in turn reduces the assembly and quality control of many other proteins on the IMM. Another possibility may involve the physical interactions Aac2 has with other IMM protein complexes, such as the respiratory supercomplexes (Claypool et al., 2008; Dienhart and Stuart, 2008; Mehnert et al., 2014). The misfolded Aac2 may engage in ectopic interactions with these IMM proteins thereby affecting their maturation, assembly and stability (see Fig. 4, top right panel).
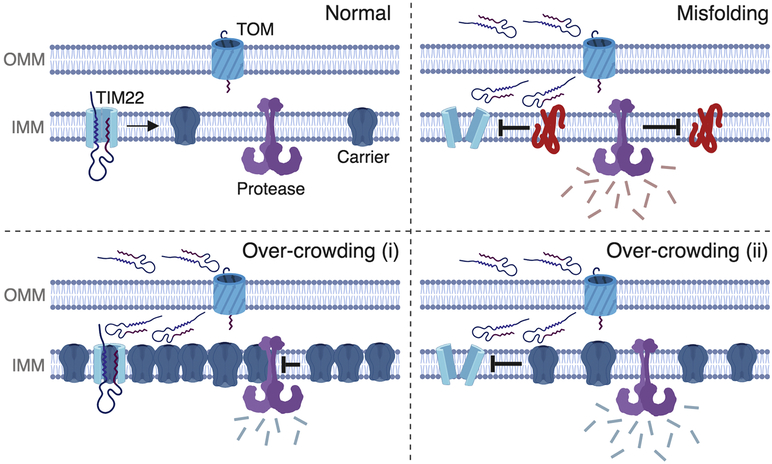
Figure 4. Potential mechanisms by which IMM carrier proteins may reduce protein import.
Four scenarios are depicted on the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM). Under normal circumstances (upper left), mitochondrial carrier proteins are imported by the TOM and the TIM22 complex, and then transferred laterally into the IMM where they co-exist with other proteins such as quality control proteases (depicted in purple). Upon IMM protein misfolding (upper right), the TTM22 translocase is destabilized and protein import is reduced to cause mPOS, as depicted by the unfolded proteins in the intermembrane space and cytosol. In the bottom two panels, we propose two possible mechanisms by which wild-type carrier protein over-crowding may cause reduce import: (i) it could cause a backup of the entire import process due to reduced ability to insert additional proteins into the protein-saturated membrane, and/or (ii) it could cause IMM proteostatic stress through ectopic protein-protein interactions and reduce import by destabilizing protein translocase complexes. (Created with BioRender.)
Other missense mutations in Ant1 are seemingly associated with additional diseases other than adPEO. One such mutation, A123D, may also misfold on the IMM. Palmieri et al. reported a patient homozygous for an A123D mutation in ANT1. This patient presented with juvenile-onset hypertrophic cardiomyopathy and myopathy(Palmieri et al., 2005), a phenotype commonly associated with the loss-of-function ANT1 mutations. Indeed, biochemical analysis revealed that Ant1A123D lacks detectable nucleotide transport activity. Interestingly, multiple mtDNA deletions were detected in diseased muscles as well. This is consistent with observations in Ant1 knockout mice (Esposito et al., 1999). While no PEO phenotype was reported in this 25-year-old patient, PEO does not typically present until middle age or later in life. Interestingly, yeast cells expressing Aac2A137D, equivalent to human Ant1A123D, share many dominant phenotypes with yeast expressing the adPEO-type mutations described above. These include ρ°-lethality, cold-induced mtDNA destabilization, hypersensitivity to moderate overexpression, uncoupled respiration, and synthetic lethality with the disruption of i-AAA protease YME1(Wang et al., 2008a). These data would support a dominant effect of Aac2A137D on IMM proteostasis. However, Aac2A137D does not form visible aggregates on native PAGE like the other mutants, despite a similar reduction in the monomeric form compared with SDS-PAGE (Liu et al., 2015). Additional insights into Aac2A137D-induced cell stress come from the Claypool group. They recently showed that a null allele of AAC2 reduces mitochondrial protein translation and cytochrome c oxidase activity, due to defective nucleotide transport. Interestingly, these defects are greater in cells expressing the transport-inactive Aac2A137D, supporting a possible dominant effect of the mutant protein (Ogunbona et al., 2018). Furthermore, the Claypool group showed that the mutant Aac2 appears to increase the degradation of nascent mtDNA-encoded proteins such as Cox3p. In addition, reduced level of Cox2p has been observed in cells expressing Aac2A137D as well as Aac2A128P (Wang et al., 2008a; Wang et al., 2008b). In context of the dominant effect of Aac2A137D on IMM proteostasis, it is possible that Aac2A137D-induced IMM stress affects an early step in the assembly of the cytochrome c oxidase complex, which leads to increased turnover of specific unassembled subunits.
More recent studies revealed that de novo dominant R80H, R235G and K33N mutations in ANT1 cause severe childhood-onset diseases(King et al., 2018; Thompson et al., 2016). Clinical manifestations include respiratory distress, myopathy, hypertrophic cardiomyopathy, hypotonia, clonic seizure, hydrocephalus and severely reduced mtDNA copy number. Ant1R80H, Ant1R235G and Ant1K33N have severely reduced nucleotide transport activity. Similar mutations in the yeast AAC2 gene have been shown to dominantly reduce respiratory growth, consistent with decreased cytochrome content, respiratory activity and mtDNA stability(Dallabona et al., 2017). Interestingly, in some cases, the steady state levels of the total Ant1 in human patients are reduced to only 10-30% of the healthy controls. More detailed analyses are required to determine whether these proteins are misfolded to induce proteostatic stress on the IMM like the adPEO-type mutations, or whether they cause dominant mitochondrial and cellular damage by a different mechanism.
2. Adenine nucleotide translocase 1 and mtDNA stability.
mtDNA instability is typically associated with mutations in replicative enzymes and those involved in nucleotide biosynthesis (Suomalainen and Isohanni, 2010). Ant1-induced adPEO is also manifested by multiple mtDNA deletions in skeletal muscle (Kaukonen et al., 2000). Given that mutations in the mitochondrial DNA polymerase (Polγ) and Twinkle helicase leads to adPEO, it is reasonable to suggest that the adPEO-type mutations in Ant1 may directly affect mtDNA replication. A long-held view is that altered adenine nucleotide specificity and transport kinetics affect dATP levels and perturb dNTP pool balance in mitochondria. This in turn was proposed to account for the increased mtDNA instability, respiratory deficiency and pathogenesis. Indeed, this may be true for the mtDNA deletions observed in Ant1 knock-out mice, although oxidative stress is believed to be a more likely candidate (Esposito et al., 1999). On the other hand, for the dominant adPEO-type mutations, this model is inconsistent with the data from the Podospora anserine.
In the aerobic filamentous fungus Podospora anserine, mutant alleles equivalent to Ant1L98P and Ant1L114P dominantly cause severe growth defects and reduced lifespan, concomitant with reduced membrane potential and large-scale mtDNA deletions (El-Khoury and Sainsard-Chanet, 2009). Interestingly, lifespan and mtDNA instability, but not membrane potential, were rescued by mutating a cytosolic ribosomal protein. Thus adPEO-type mutations in Ant1 can be rescued independent of nucleotide transport.
Yeast work suggests that Ant1 misfolding and IMM proteostatic stress itself can cause mtDNA instability. Expression of the dominant alleles of AAC2 renders the cells ρ°-lethal in many laboratory strains, meaning that cells cannot grow when mtDNA is depleted. This precludes further study of the potential link between mutant Aac2 and mtDNA instability. However, yeast strains of W303 background expressing the mutant aac2 alleles are generally more tolerant to IMM damage and can survive the loss of mtDNA on glucose medium. Using these strains, mtDNA destabilization was recapitulated when cells co-express the wild-type and the adPEO-type aac2 alleles (Dallabona et al., 2017; Fontanesi et al., 2004; Wang et al., 2008a). Interestingly, the aac2A137D allele, that is completely transport-inactive, also induces mtDNA instability(Wang et al., 2008a). This observation strongly argues that it is the mutant protein, rather than any associated nucleotide transport activity, that is responsible for mtDNA destabilization. The precise mechanism by which mtDNA is destabilized remains unsolved. It is possible that the misfolded Aac2 primarily cause IMM damage, which increases ROS production and mtDNA damage. However, antioxidant responses were not detected in yeast and human cells expressing the mutant Aac2 or Ant1(Liu et al., 2019; Wang and Chen, 2015). It is also possible that proteostasis on the IMM affects the import of proteins and substrates that are critical for mtDNA metabolism. On the other hand, antioxidants have been reported to improve cell survival and mtDNA stability in yeast cells that express mutant aac2(Dallabona et al., 2017; Palmieri et al., 2005). It remains to be determined whether antioxidants directly protect mtDNA or instead improve proteostasis on the IMM, as oxidative damage to IMM proteins could pose a significant proteostatic challenge. Taken together, the data indicate that mtDNA instability is a consequence of IMM proteostatic stress, rather than nucleotide imbalance.
3. Downstream consequences of IMM protein misfolding: mitochondrial Precursor Overaccumulation Stress (mPOS)
What are the downstream consequences of IMM protein misfolding that ultimately kill cells? In addition to the effects on mitochondria discussed above, IMM protein misfolding has lethal effects on global cytosolic proteostasis. Cytosolic proteostasis is disrupted by the accumulation of mitochondrial proteins, which are blocked from import into mitochondria by disrupted IMM proteostasis. This stress, termed mitochondrial Precursor Overaccumulation Stress (mPOS), can kill cells independent of bioenergetics (Fig. 2) (Coyne and Chen, 2018; Guaragnella et al., 2018; Liu et al., 2019; Wang and Chen, 2015). In this section we detail how IMM proteostatic stress causes mPOS in yeast, as well as more recent results suggesting that mPOS occurs in higher eukaryotes. When relevant, we briefly discuss the accumulating evidence that cells have protective mechanisms that respond to mPOS and benefit cell survival.
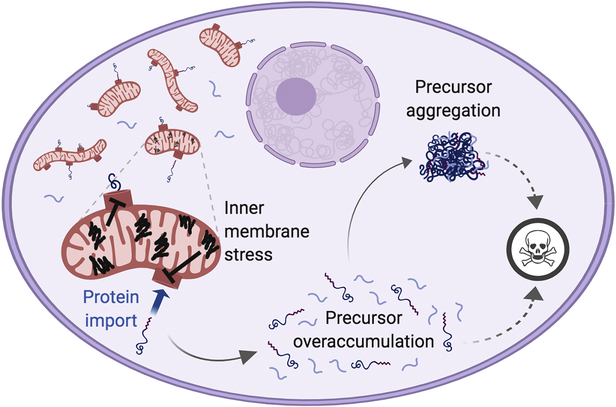
Figure 2. Inner mitochondrial membrane protein misfolding causes mitochondrial Precursor Overaccumulation Stress (mPOS).
Misfolded proteins on the IMM (black lines) disrupts proteostasis in the membrane, thereby reducing protein import into mitochondria. This leads to the unimported mitochondrial precursor proteins accumulating and aggregating in the cytosol, which causes cell stress (specifically, mitochondrial Precursor Overaccumulation Stress, mPOS). This stress kill cell by an unknown mechanism (dotted lines). (Created with BioRender.)
Discoveries made in yeast: mitochondrial Precursor Overaccumulation Stress (mPOS) and anti-mPOS responses
Wang et al. used the misfolded Aac2A128P to determine the mechanism by which IMM stress kills cells (Wang et al., 2008b). The first indication that cytosolic proteostasis may play a deleterious role downstream of IMM proteostatic stress came from the observation that genetic, pharmacological, and environmental interventions that reduced protein synthesis can suppress Aac2A128P-induced cell death. Importantly, the interventions did not reduce the abundance of Aac2A128P, and the effects were independent of bioenergetic crisis, as the yeast were grown in fermentative conditions. Before discussing the effects of Aac2 misfolding in the cytosol, it should be noted that reduced cytosolic protein translation was shown to benefit mitochondrial function. This was demonstrated by its ability to recover mitochondrial membrane potential and improve mitochondrial gene expression in Aac2A128P-expressing cells. More recent studies provided farther support for the idea that reduced global protein synthesis promotes mitochondrial functions in cells with IMM stress. Reduced cytosolic protein synthesis by disruption of genes encoding the cytosolic ribosomal subunit Rpl6b and the ribosome biogenesis factor Rei1 were found to restore OXPHOS and respiratory growth of yeast cells lacking the cardiolipin-modifying enzyme tafazzin (de Taffin de Tilques et al., 2018). Surprisingly, OXPHOS is restored despite a failure to correct cardiolipin remodeling. A similar effect was found in higher eukaryotes as well, where inhibition of cytosolic translation with cycloheximide rescued cell viability and respiratory capacity in human cell models of respiratory complex deficiencies (Peng et al., 2015). Similar to yeast, these models showed restored mitochondrial translation, mass, and membrane potential downstream of cycloheximide treatment. The mechanism by which cytosolic translation inhibition benefits mitochondrial function in these studies remains unclear. As suggested (Wang et al., 2008b), it is possible that reduced protein loading into the membrane may alleviate proteostatic stress on the IMM, which would likely benefit mtDNA stability, mitochondrial biogenesis and cell viability.
It was not until recently that clinically relevant proteostatic stress on the IMM was demonstrated to cause a massive accumulation of mitochondrial proteins in the cytosol. This leads to cell death, which can be suppressed by interventions that improve cytosolic proteostasis, including a reduction of protein translation. Thus, it appears that inhibiting cytosolic translation benefits cells with IMM stress by at least two mechanisms: improving mitochondrial function and preserving cytosolic proteostasis. A global proteostatic challenge induced by IMM protein misfolding was ultimately demonstrated by Wang and Chen’s analysis of the cytosolic proteome of Aac2A128P-expressing yeast (Wang and Chen, 2015). Mass spectrometry revealed that many mitochondrial proteins accumulate in the cytosol of the mutant cells. Most likely, these proteins are relatively resistant to cytosolic proteolysis when their import is delayed or blocked. Perhaps some of these mitochondrial proteins are folded in the cytosol (or some other organelle) to play metabolic and/or signaling functions, though this remains untested. In worms, failed protein import causes the nuclear translocation of the otherwise mitochondrial protein ATFS-1, which has been shown to play a key role in readjusting gene expression to restore mitochondrial homeostasis (Nargund et al., 2015; Nargund et al., 2012). One limitation with Wang & Chen’s proteomic snapshot is that it does not provide information on the potential retention of mitochondrial membrane proteins in the cytosol, as polytopic membrane proteins may escape detection by mass spectrometry. Conceptually, cytosolic retention of mitochondrial proteins, particularly the polytopic IMM proteins, would be highly problematic because they are generally kept unfolded and chaperoned in the cytosol to maintain import competency (Cichocki et al., 2018; Endo and Yamano, 2010; Hansen et al., 2018; Hoseini et al., 2016; Itakura et al., 2016; Jores et al., 2018; Opalinski et al., 2018; Papic et al., 2013; Sahi et al., 2013; Young et al., 2003). One might therefore expect these proteins to impose substantial proteostatic stress in the cytosol. Wang and Chen named this stress as mitochondrial Precursor Overaccumulation Stress (mPOS), to reflect the vulnerability of cytosolic proteostasis to the accumulation of unimported mitochondrial proteins, which results, in this case, from proteostatic stress on the IMM – not from direct perturbation of the import machinery.
Additional lines of evidence support the presence of cytosolic proteostatic stress, or mPOS, downstream of IMM proteostatic stress (Wang and Chen, 2015). Upon Aac2A128P expression, it was found that the mitochondrial matrix protein aconitase has an increased propensity to form aggregates in the cytosol. Furthermore, co-expression of Aac2A128P and the aggregation-prone, polyglutamine-expanded (25Q) Huntingtin protein is synthetically lethal. These observations suggest that the cytosol has a limited capacity in maintaining the solubility of unimported mitochondrial proteins after IMM damage, and that this limits the cell’s ability to handle aggregation-prone proteins. Further support for a role on IMM damage in affecting cytosolic proteostasis came from the observation that disruption of several genes involved in proteasomal biogenesis (Blml0 and Rpn4) is synthetically lethal with the loss of the IMM protease, Yme1.
How cells respond to IMM stress is also indicative of mPOS (Fig. 3). Aac2A128P-expressing cells drastically reduce protein synthesis, apparently to accommodate the accumulation of mitochondrial proteins in the cytosol (Wang and Chen, 2015). A similar effect is observed when the IMM is stressed by different mechanisms, such as deletion of the m-AAA quality control protease subunit afg3 (Delaney et al., 2013), or deletion of the cardiolipin-modifying enzyme tafazzin (de Taffin de Tilques et al., 2018). For Aac2A128P expression and taffazin deletion, this appears to occur at least partly via ribosomal remodeling, as suggested by reduced polysome levels. Specifically in Aac2A128P-expressing cells, it was shown that the ribosome-associated proteins Nog2, Gis2 and Tma7 proteins are upregulated, which may be mechanistically involved in ribosomal remodeling and suppression of other activities related to protein translation (Fig. 3). Indeed, ectopic overexpression of all 3 of these proteins can suppress mPOS-induced cell death. Clearly, reduction of cytosolic protein synthesis is a robust response to IMM proteostatic stress, possibly in an attempt to alleviate mPOS.
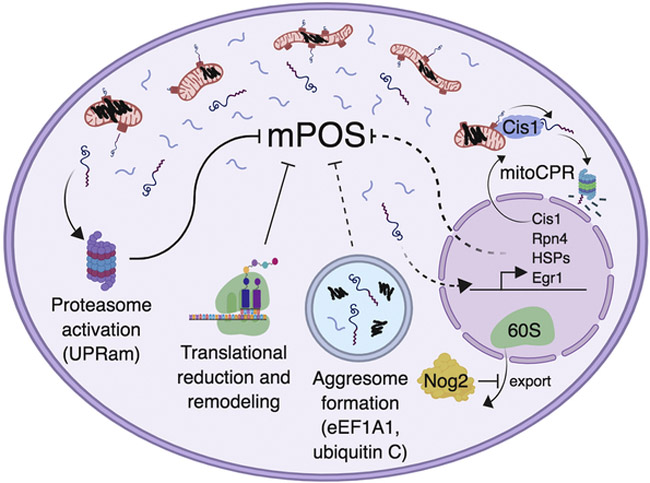
Figure 3. Cellular responses to mPOS.
IMM protein misfolding (black squiggles) causes mPOS (free floating blue lines surrounding mitochondria). mPOS activates proteasomal function (i.e. the unfolded protein response activated by mistargeting of proteins, UPRam), translational remodeling (e.g. reduced protein synthesis), and inhibition of nuclear export of the 60S ribosome by Nog2. When import is impaired, yeast increase transcription of Cis1, which then travels to the mitochondrial surface to facilitate the proteasomal degradation of unimported proteins, a process termed mitochondrial Compromised Protein import Response (mitoCPR). Rpn4 is similarly upregulated in yeast with deficient import, and then transcriptionally activates the proteasome to mitigate mPOS. We speculate (dotted lines) that the observed aggresome formation and increased transcription of Heat Shock Proteins (HSPs), Egr1 and its downstream targets play a role in alleviating mPOS in human cells. Current evidence suggests that eEF1A1 is involved in the formation of mitochondrial carrier-induced cytosolic aggresomes. (Created with BioRender.)
Reduced cytosolic protein synthesis has also been observed when the protein import machinery is directly blocked. The Chacinska group found that direct impairment in the mitochondrial protein import pathway reduces ribosomal biogenesis and protein synthesis (Wrobel et al. 2015). Furthermore, they found that impaired import also activates proteasomal function. These adaptive programs were collectively named the Unfolded Protein Response activated by protein mistargeting (UPRam, Fig. 3) (Wrobel et al., 2015). In support of this model, Boos et al. demonstrated upregulation of genes involved in proteasomal function, in an Rpn4-dependent manner, when the mitochondrial protein import channel is clogged by slow-moving substrates (Boos et al., 2019). They also observed the upregulation of several cytosolic chaperones including those involved in the Hsp70, Hsp90 and TRiC/CCT systems. Overexpression of CCT6, encoding a member of the cytosolic TRiC chaperone complex, has been previously shown to suppress the ρ°-lethality phenotype of tim18Δ cells(Dunn and Jensen, 2003). Collectively, these findings demonstrate that cytosol-directed cell responses are consistent between cells with directly impaired import and cells expressing the misfolded Aac2A128P, further suggesting import is impaired upon IMM proteostatic stress. In addition, these parallels demonstrate that mPOS itself may trigger cellular response mechanisms such as the UPRam (Fig. 3).
The misfolding of Aac2A128P on the IMM also leads to the upregulation of specific proteins (Wang and Chen, 2015). Notably, these include Pdr5, Ypr172W and Ymr102C, which are known to be activated by the multidrug resistance regulatory network. In an elegant study published recently, it was demonstrated that upregulation of this particular regulatory network is critical to induce the expression of Cis1. Cis1 plays an important role in degrading unimported proteins at the mitochondrial translocase when protein import is impaired by precursor overloading (Fig. 3) (Weidberg and Amon, 2018). This pathway was termed mitochondrial Compromised Protein import Response (mitoCPR, Fig. 3).
Using an unbiased screen for multicopy suppressors of Aac2A128P-induced cell death, Wang and Chen identified almost exclusively cytosolic pathways that would be predicted to improve proteostasis (Wang and Chen, 2015). First, the screen confirmed the observations noted above, namely that reduced ribosomal biogenesis, proteasomal activation and increased protein chaperoning can rescue Aac2A128P-induced cell death (Wang and Chen, 2015). Importantly, additional pathways were uncovered as well, such as TOR signaling mRNA silencing/turnover, tRNA methylation and cap-independent translation. Many of these suppressor genes were also found to suppress the ρ°-lethality phenotype of null mutants of YME1, ATP1, TOM70 and MGR2. ρ° cells have low membrane potential, which reduces protein import (Bedwell et al., 1989). As described earlier, YME1 is involved in protein quality control on the IMM. ATP1 is required for the maintenance of membrane potential under ρ° conditions (Chen and Clark-Walker, 1999). TOM70 and MGR2 are directly involved in protein import (Ieva et al., 2014; Wiedemann and Pfanner, 2017). Several suppressor genes of Aac2A128P-induced cell death such as SSB1 and PBP1, involved in the chaperoning of nascent polypeptides and mRNA processing/silencing, also suppress ρ°-lethality of several TIM22 mutants (Dunn and Jensen, 2003). These studies established that a variety of mitochondrial stresses can affect protein import efficiency, which can induce proteostatic stress in the cytosol independent of bioenergetics. mPOS is therefore a common factor that limits cell survival upon various mitochondrial stress conditions that directly or indirectly affect protein import.
IMM proteostatic stress and mPOS in higher eukaryotes
Since the discovery that IMM proteostatic stress can kill yeast cells via mPOS, a number of groups have demonstrated mPOS in higher eukaryotes. Unlike in yeast, however, whether protein misfolding on the IMM is sufficient to cause mPOS in these model systems remains to be determined. Nevertheless, this hypothesis is suggested by a number of observations.
Defective IMM protein quality control affects cytosolic proteostasis. In C. elegans, acute knockdown of the m-AAA protease subunit phi-23 (orthologous to yeast AFG3) causes premature cytosolic aggregation of an ectopically expressed polyglutamine-expanded protein (Nollen et al., 2004). This finding was later reproduced in human cells (Teuling et al., 2011) as well as in vivo in mice, where it was found that loss of the m-AAA protease causes Alzheimer’s disease-related protein aggregation in neurons (Kondadi et al., 2014). Taken together, there is an undeniable connection between loss of m-AAA protease function and cytosolic proteostasis, and mPOS has the potential to rationalize these observations.
Similarly, loss of the IMM protein quality control chaperone prohibitin also has proteostatic effects on the cytosol. In yeast, loss of the prohibitin complex causes growth defects that can be suppressed by reducing cytosolic protein synthesis (Wang et al., 2008b). This occurs in conditions where OXPHOS is dispensable suggesting cytosolic proteostatic stress, not bioenergetics, is limiting cell growth in prohibitin mutant cells. In mice, neuron-specific deletion of prohibitin causes Alzheimer’s-related cytosolic protein aggregation (Merkwirth et al., 2012). These observations implicate mPOS downstream of prohibitin defects.
The above suggestions that mPOS occurs downstream of IMM stress in mammalian systems are problematic due to the multifunctionality of the IMM protein quality control machinery, as discussed in the Introduction. More recently, two strategies were used to induce IMM proteostatic stress and to evaluate cellular consequences in human cells: overloading the IMM with protein, and introducing misfolded Ant1 onto the membrane (Fig. 4) (Liu et al., 2019). Both strategies clearly induced mPOS and cell death, either by directly causing stress on the IMM, or by saturation of an import step prior to the IMM due to overexpression.
Overexpression of wild-type IMM carrier proteins, but not matrix proteins, proved to be a potent strategy for inducing mPOS in human (HEK293T) cells (Liu et al., 2019). It causes widespread protein ubiquitination and the formation of massive (sometimes >5 μm diameter) cytosolic sequestrations of aggregated proteins. As in yeast, carrier-induced mPOS imposes substantial cellular stress that appears independent of bioenergetics. Despite a considerable increase in apoptosis, minimal bioenergetic deficiency is observed.
Importantly, these sequestrations of aggregated proteins, tentatively named Mitochondria-Induced Cytosolic Aggresomes (MICA), not only contain the over-expressed carrier protein, but also appear to contain endogenously expressed mitochondrial proteins. This suggests a global defect in mitochondrial protein import. Precisely how the unimported mitochondrial proteins are recognized and trafficked to form aggresome is still unclear. However, available evidence implicates the ubiquilin family proteins in recognition, and the eukaryotic translation elongation factor 1 α 1 (eEF1α1) in trafficking. In an elegant study, Itakura et al. demonstrated that ubiquilins chaperone hydrophobic mitochondrial membrane proteins in the cytosol to prevent aggregation before import. If import is delayed, ubiquilins recruit an E3 ligase to ubiquitinate their bound mitochondrial protein clients (Itakura et al., 2016). It is possible that these ubiquitinated clients are then trafficked to aggresomes in extreme conditions such as carrier protein overloading in HEK293T cells. The trafficking of misfolded cytosolic proteins to aggresomes has been shown to begin with direct binding by eEF1α1 (Park et al., 2017). Indeed, eEF1α1 is upregulated and preferentially insoluble upon carrier protein overloading, suggesting it is directly involved in aggresome targeting of mitochondrial proteins under mPOS conditions (Liu et al., 2019). Finally, monomeric ubiquitin C was found to be upregulated in this system as well (Fig. 3). This may play a role in the processing of MICA (Hao et al., 2013).
As noted above, it is unclear whether disturbed IMM proteostasis plays a role in mPOS induced by overloading mitochondria with wild-type carrier proteins. It is possible that IMM stress occurs due to space limitations in the two-dimensional membrane, which would set the stage for a protein “over-crowding effect.” The IMM may be especially vulnerable to an overcrowding effect as it is already one of the most protein-dense lipid bilayers in eukaryotic cells (Ardail et al., 1990; Simbeni et al., 1991). Over-crowding could lead to mPOS by at least two mechanisms (Fig. 4): (i) it could cause a backup and clogging of the entire import process because of an inability to insert additional proteins into the membrane, and/or (ii) it could cause IMM proteostatic stress through ectopic protein-protein interactions and cause mPOS by destabilizing protein translocase complexes. The latter may be more likely, as biochemical experiments suggest that the mitochondrial pool of the overexpressed wild-type carrier is on the IMM (Liu et al., 2019). Alternatively, it is possible that an earlier step of protein import is saturated. However, none of these potential mechanisms are mutually exclusive.
It is important to note that MICA formation was not simply a result of accumulation of the hydrophobic carrier protein. This is because expression of unstable, misfolded Ant1 variants similarly induced mPOS and aggresome formation while only accumulating to <5% of the wild-type protein level (Fig. 5) (Liu et al., 2019). Although this observation directly implicates IMM protein misfolding as a cause of mPOS in human cells, it is still possible that the wild-type and misfolded protein are saturating protein import at an earlier step (e.g. TOM translocation or IMS chaperoning), and the mutant is subsequently recognized and degraded on the IMM. In this case, overloading the protein import machinery, as opposed to IMM stress, would be the primary cause of mPOS.
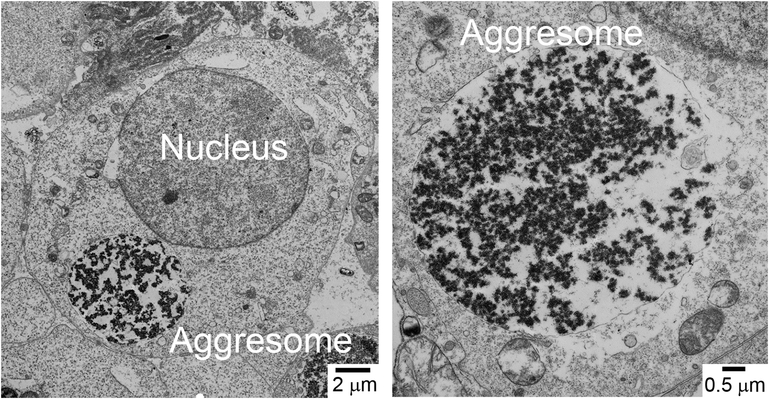
Figure 5. Mitochondria-Induced Cytosolic Aggresomes (MICA).
Left panel, transmission electron micrograph of an HEK293T cell transiently transfected with a plasmid expressing Ant1A90D, L98P, A114P, A123D. Right panel, high magnification of an Ant1A90D, L98P, A114P, A123D-induced aggresome (i.e. a MICA). For details, see (Liu et al., 2019).
Interestingly, while inducing the misfolding of Ant1 did not appear to affect the level of mPOS despite apparently being degraded to <5% of its potential expression level, it did trigger additional cellular responses (Liu et al., 2019). First, unlike wild-type Ant1, a misfolded Ant1 variant containing four missense mutations caused proteasome activation, corroborating the UPRam discovered in yeast (Wrobel et al., 2015). Second, unlike wild-type Ant1, the quadruple mutant Ant led to a reduction in mTOR signaling, which could be an anti-mPOS response to reduce protein synthesis and/or activate autophagy. Third, unlike wild-type Ant1, the quadruple mutant led to the increased transcription of genes encoding the cytosolic Hsp70 and Hsp90 chaperones, reminiscent of yeast cells when protein import is clogged (Fig. 3) (Boos et al., 2019). Overall, it appears that transcription programming was especially sensitive to the folding state of Ant1. Overexpression of Ant1 with three and four missense mutations significantly changed the expression of 206 and 560 genes, respectively. By comparison, wild-type Ant1 changed the expression of just 20 genes. These data suggest an intriguing transcriptional “dose response” to the degree ofmisfolding of Ant1. One commonality between transcriptional responses to wild-type and mutant Ant1 was upregulation of the transcription factor Egr1, as well as its downstream targets(Liu et al., 2019). We speculate this may play a role in MICA formation, though this remains untested.
Finally, we note here that experimental data also emerged in the fly and mouse model systems that are consistent with the occurrence of mPOS, albeit independent of IMM stress and concurrent with severe bioenergetic defects. In flies, knockdown of Tom40 (the channel forming protein of the TOM complex) caused the accumulation of mitochondrial precursors in large autophagosome-like structures, which were similar to aggresomes in size, but distinct in electron density pattern (Liu et al., 2018). In mice, defects in a specific mitochondrial tRNA modification caused cytosolic mitochondrial protein aggregation in culture and in vivo (Fakruddin et al., 2018). Furthermore, reducing the aggregation by the chemical chaperone tauroursodeoxycholate not only improved cell growth, but also benefited mitochondrial cristae organization, akin to the mitochondrial benefits of improved cytosolic proteostasis noted above. With these latest developments, it seems that IMM proteostatic stress may be just one of many physiologically relevant conditions capable of inducing mPOS.
4. Future Perspectives
Proteostatic stress on the IMM is implicated in a multitude of human diseases. Recent advances have indicated that pathogenic mutations in the IMM protein Ant1 induce its misfolding in the membrane, which results in proteostatic stress not only on the IMM but also in the cytosol. These cell-based studies suggest IMM protein misfolding itself may indeed be pathogenic. Ultimately, animal models and studies involving patient samples will determine the pathogenic potential of misfolded proteins on the IMM.
The pathogenic Ant1 alleles provide a unique tool with which to study the basic biology of IMM proteostatic stress. This stress has profound and far-reaching cellular consequences. Specifically, modeling IMM proteostatic stress led to the discovery of mitochondrial Precursor Overaccumulation Stress (mPOS). mPOS occurs when mitochondrial damage (e.g. IMM protein misfolding) blocks the import of proteins into mitochondria, thus causing their aberrant accumulation and aggregation in the cytosol, which poisons cells. To be clear, mPOS is a form of cellular stress, not a stress response mechanism, and this cell stress process is consistent with mounting data generated from yeast, human cells, flies, mouse cells and newborn mice. These studies strongly support the view that the cytosol has a limited capacity to degrade unimported mitochondrial proteins, and that we can dispel the dogma that mitochondrial proteins are universally degraded when import fails. Moving forward, it will be essential to test the pathological relevance of mPOS.
Misfolded variants of adenine nucleotide translocase 1 (Ant1) were recently identified that cause dominant human diseases.
Ant1 misfolding is sufficient to trigger proteostatic stress on the inner mitochondrial membrane (IMM) and to affect protein complex assemblyand mtDNA stability.
IMM stress induces cell death by the mechanism of mitochondrial Precursor Overaccumulation Stress (mPOS) in the cytosol independent of bioenergetics.
Anti-mPOS therapy may offer a potential treatment for diseases caused by mitochondria-induced proteostatic stress.
Acknowledgements:
This work was supported by the National Institutes of Health through grants AG023731 and AG061204 to X.J.C., and a pre-doctoral fellowship AG060702 to L.P.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Ardail D, Privat JP, Egret-Charlier M, Levrat C, Lerme F, Louisot P, 1990. Mitochondrial contact sites. Lipid composition and dynamics. J Biol Chem 265, 18797–18802. [PubMed] [Google Scholar]
- Bedwell DM, Strobel SA, Yun K, Jongeward GD, Emr SD, 1989. Sequence and structural requirements of a mitochondrial protein import signal defined by saturation cassette mutagenesis. Mol Cell Biol 9, 1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohovych I, Chan SS, Khalimonchuk O, 2015. Mitochondrial protein quality control: the mechanisms guarding mitochondrial health. Antioxid Redox Signal 22, 977–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos F, Kramer L, Groh C, Jung F, Haberkant P, Stein F, Wollweber F, Gackstatter A, Zoller E, van der Laan M, Savitski MM, Benes V, Herrmann JM, 2019. Mitochondrial protein-induced stress triggers a global adaptive transcriptional programme. Nat Cell Biol 21, 442–451. [DOI] [PubMed] [Google Scholar]
- Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, Cornwall EJ, 2005. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J 392, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XJ, 2002. Induction of an unregulated channel by mutations in adenine nucleotide translocase suggests an explanation for human ophthalmoplegia. Hum Mol Genet 11, 1835–1843. [DOI] [PubMed] [Google Scholar]
- Chen XJ, Clark-Walker GD, 1999. Alpha and beta subunits of F1-ATPase are required for survival of petite mutants in Saccharomyces cerevisiae. Mol Gen Genet 262, 898–908. [DOI] [PubMed] [Google Scholar]
- Cichocki BA, Krumpe K, Vitali DG, Rapaport D, 2018. Pex19 is involved in importing dually targeted tail-anchored proteins to both mitochondria and peroxisomes. Traffic 19, 770–785. [DOI] [PubMed] [Google Scholar]
- Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM, 2008. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J Cell Biol 182, 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne LP, Chen XJ, 2018. mPOS is a novel mitochondrial trigger of cell death - implications for neurodegeneration. FEBS Lett 592, 759–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallabona C, Baruffini E, Goffrini P, Lodi T, 2017. Dominance of yeast aac2(R96H) and aac2(R252G) mutations, equivalent to pathological mutations in ant1, is due to gain of function. Biochem Biophys Res Commun 493, 909–913. [DOI] [PubMed] [Google Scholar]
- de Marcos-Lousa C, Sideris DP, Tokatlidis K, 2006. Translocation of mitochondrial inner-membrane proteins: conformation matters. Trends Biochem Sci 31, 259–267. [DOI] [PubMed] [Google Scholar]
- de Taffin de Tilques M, Lasserre JP, Godard F, Sardin E, Bouhier M, Le Guedard M, Kucharczyk R, Petit PX, Testet E, di Rago JP, Tribouillard-Tanvier D, 2018. Decreasing cytosolic translation is beneficial to yeast and human Tafazzin-deficient cells. Microb Cell 5, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney JR, Ahmed U, Chou A, Sim S, Carr D, Murakami CJ, Schleit J, Sutphin GL, An EH, Castanza A, Fletcher M, Higgins S, Jelic M, Klum S, Muller B, Peng ZJ, Rai D, Ros V, Singh M, Wende HV, Kennedy BK, Kaeberlein M, 2013. Stress profiling of longevity mutants identifies Afg3 as a mitochondrial determinant of cytoplasmic mRNA translation and aging. Aging Cell 12, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschauer M, Hudson G, Muller T, Taylor RW, Chinnery PF, Zierz S, 2005. A novel ANT1 gene mutation with probable germline mosaicism in autosomal dominant progressive external ophthalmoplegia. Neuromuscul Disord 15, 311–315. [DOI] [PubMed] [Google Scholar]
- Dienhart MK, Stuart RA, 2008. The yeast Aac2 protein exists in physical association with the cytochrome bc1-COX supercomplex and the TIM23 machinery. Mol Biol Cell 19, 3934–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CD, Jensen RE, 2003. Suppression of a defect in mitochondrial protein import identifies cytosolic proteins required for viability of yeast cells lacking mitochondrial DNA. Genetics 165, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echaniz-Laguna A, Chassagne M, Ceresuela J, Rouvet I, Padet S, Acquaviva C, Nataf S, Vinzio S, Bozon D, Mousson de Camaret B, 2012. Complete loss of expression of the ANT1 gene causing cardiomyopathy and myopathy. J Med Genet 49, 146–150. [DOI] [PubMed] [Google Scholar]
- El-Khoury R, Sainsard-Chanet A, 2009. Suppression of mitochondrial DNA instability of autosomal dominant forms of progressive external ophthalmoplegia-associated ANT1 mutations in Podospora anserina. Genetics 183, 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Yamano K, 2010. Transport of proteins across or into the mitochondrial outer membrane. Biochim Biophys Acta 1803, 706–714. [DOI] [PubMed] [Google Scholar]
- Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC, 1999. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A 96, 4820–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakruddin M, Wei FY, Suzuki T, Asano K, Kaieda T, Omori A, Izumi R, Fujimura A, Kaitsuka T, Miyata K, Araki K, Oike Y, Scorrano L, Suzuki T, Tomizawa K, 2018. Defective Mitochondrial tRNA Taurine Modification Activates Global Proteostress and Leads to Mitochondrial Disease. Cell Rep 22, 482–496. [DOI] [PubMed] [Google Scholar]
- Fontanesi F, Palmieri L, Scarcia P, Lodi T, Donnini C, Limongelli A, Tiranti V, Zeviani M, Ferrero I, Viola AM, 2004. Mutations in AAC2, equivalent to human adPEO-associated ANT1 mutations, lead to defective oxidative phosphorylation in Saccharomyces cerevisiae and affect mitochondrial DNA stability. Hum Mol Genet 13, 923–934. [DOI] [PubMed] [Google Scholar]
- Gerdes F, Tatsuta T, Langer T, 2012. Mitochondrial AAA proteases--towards a molecular understanding of membrane-bound proteolytic machines. Biochim Biophys Acta 1823, 49–55. [DOI] [PubMed] [Google Scholar]
- Gray MW, 2012. Mitochondrial evolution. Cold Spring Harb Perspect Biol 4, a011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaragnella N, Coyne LP, Chen XJ, Giannattasio S, 2018. Mitochondria-cytosol-nucleus crosstalk: learning from Saccharomyces cerevisiae. FEMS Yeast Res 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KG, Aviram N, Laborenz J, Bibi C, Meyer M, Spang A, Schuldiner M, Herrmann JM, 2018. An ER surface retrieval pathway safeguards the import of mitochondrial membrane proteins in yeast. Science 361, 1118–1122. [DOI] [PubMed] [Google Scholar]
- Hao R, Nanduri P, Rao Y, Panichelli RS, Ito A, Yoshida M, Yao TP, 2013. Proteasomes activate aggresome disassembly and clearance by producing unanchored ubiquitin chains. Mol Cell 51, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseini H, Pandey S, Jores T, Schmitt A, Franz-Wachtel M, Macek B, Buchner J, Dimmer KS, Rapaport D, 2016. The cytosolic cochaperone Sti1 is relevant for mitochondrial biogenesis and morphology. FEBS J 283, 3338–3352. [DOI] [PubMed] [Google Scholar]
- Ieva R, Schrempp SG, Opalinski L, Wollweber F, Hoss P, Heisswolf AK, Gebert M, Zhang Y, Guiard B, Rospert S, Becker T, Chacinska A, Pfanner N, van der Laan M, 2014. Mgr2 functions as lateral gatekeeper for preprotein sorting in the mitochondrial inner membrane. Mol Cell 56, 641–652. [DOI] [PubMed] [Google Scholar]
- Itakura E, Zavodszky E, Shao S, Wohlever ML, Keenan RJ, Hegde RS, 2016. Ubiquilins Chaperone and Triage Mitochondrial Membrane Proteins for Degradation. Mol Cell 63, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jores T, Lawatscheck J, Beke V, Franz-Wachtel M, Yunoki K, Fitzgerald JC, Macek B, Endo T, Kalbacher H, Buchner J, Rapaport D, 2018. Cytosolic Hsp70 and Hsp40 chaperones enable the biogenesis of mitochondrial beta-barrel proteins. J Cell Biol 217, 3091–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukonen J, Juselius JK, Tiranti V, Kyttala A, Zeviani M, Comi GP, Keranen S, Peltonen L, Suomalainen A, 2000. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science 289, 782–785. [DOI] [PubMed] [Google Scholar]
- Kaukonen J, Zeviani M, Comi GP, Piscaglia MG, Peltonen L, Suomalainen A, 1999. A third locus predisposing to multiple deletions of mtDNA in autosomal dominant progressive external ophthalmoplegia. Am J Hum Genet 65, 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata H, Tiranti V, Magrane J, Chinopoulos C, Manfredi G, 2011. adPEO mutations in ANT1 impair ADP-ATP translocation in muscle mitochondria. Hum Mol Genet 20, 2964–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MS, Thompson K, Hopton S, He L, Kunji ERS, Taylor RW, Ortiz-Gonzalez XR, 2018. Expanding the phenotype ofde novo SLC25A4-linked mitochondrial disease to include mild myopathy. Neurol Genet 4, e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaki H, Fukazawa T, Houzen H, Yoshida K, Nonaka I, Goto Y, 2002. A novel D104G mutation in the adenine nucleotide translocator 1 gene in autosomal dominant progressive external ophthalmoplegia patients with mitochondrial DNA with multiple deletions. Ann Neurol 51, 645–648. [DOI] [PubMed] [Google Scholar]
- Kondadi AK, Wang S, Montagner S, Kladt N, Korwitz A, Martinelli P, Herholz D, Baker MJ, Schauss AC, Langer T, Rugarli EI, 2014. Loss of the m-AAA protease subunit AFG(3)L(2) causes mitochondrial transport defects and tau hyperphosphorylation. EMBO J 33, 1011–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korver-Keularts IM, de Visser M, Bakker HD, Wanders RJ, Vansenne F, Scholte HR, Dorland L, Nicolaes GA, Spaapen LM, Smeets HJ, Hendrickx AT, van den Bosch BJ, 2015. Two Novel Mutations in the SLC25A4 Gene in a Patient with Mitochondrial Myopathy. JIMD Rep 22, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunji ER, Crichton PG, 2010. Mitochondrial carriers function as monomers. Biochim Biophys Acta 1797, 817–831. [DOI] [PubMed] [Google Scholar]
- Liu W, Duan X, Fang X, Shang W, Tong C, 2018. Mitochondrial protein import regulates cytosolic protein homeostasis and neuronal integrity. Autophagy 14, 1293–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen XJ, 2013. Adenine nucleotide translocase, mitochondrial stress, and degenerative cell death. Oxid Med Cell Longev 2013, 146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang X, Chen XJ, 2015. Misfolding of mutant adenine nucleotide translocase in yeast supports a novel mechanism of Ant1-induced muscle diseases. Mol Biol Cell 26, 1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang X, Coyne LP, Yang Y, Qi Y, Middleton FA, Chen XJ, 2019. Mitochondrial carrier protein overloading and misfolding induce aggresomes and proteostatic adaptations in the cytosol. Mol Biol Cell, mbcE19010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi T, Bove C, Fontanesi F, Viola AM, Ferrero I, 2006. Mutation D104G in ANT1 gene: complementation study in Saccharomyces cerevisiae as a model system. Biochem Biophys Res Commun 341, 810–815. [DOI] [PubMed] [Google Scholar]
- Martinelli P, Rugarli EI, 2010. Emerging roles of mitochondrial proteases in neurodegeneration. Biochim Biophys Acta 1797, 1–10. [DOI] [PubMed] [Google Scholar]
- Mehnert CS, Rampelt H, Gebert M, Oeljeklaus S, Schrempp SG, Kochbeck L, Guiard B, Warscheid B, van der Laan M, 2014. The mitochondrial ADP/ATP carrier associates with the inner membrane presequence translocase in a stoichiometric manner. J Biol Chem 289, 27352–27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkwirth C, Martinelli P, Korwitz A, Morbin M, Bronneke HS, Jordan SD, Rugarli EI, Langer T, 2012. Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphosphorylation and neurodegeneration. PLoS Genet 8, e1003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli L, Bordoni A, Zeviani M, Hadjigeorgiou GM, Sciacco M, Tiranti V, Terentiou A, Moggio M, Papadimitriou A, Scarlato G, Comi GP, 2001. A novel missense adenine nucleotide translocator-1 gene mutation in a Greek adPEO family. Neurology 57, 2295–2298. [DOI] [PubMed] [Google Scholar]
- Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM, 2015. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt). Mol Cell 58, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM, 2012. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337, 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolden M, Ehses S, Koppen M, Bernacchia A, Rugarli EI, Langer T, 2005. The m-AAA protease defective in hereditary spastic paraplegia controls ribosome assembly in mitochondria. Cell 123, 277–289. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Garcia SM, van Haaften G, Kim S, Chavez A, Morimoto RI, Plasterk RH, 2004. Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proc Natl Acad Sci U S A 101, 6403–6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunbona OB, Baile MG, Claypool SM, 2018. Cardiomyopathy-associated mutation in the ADP/ATP carrier reveals translation-dependent regulation of cytochrome c oxidase activity. Mol Biol Cell 29, 1449–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opalinski L, Song J, Priesnitz C, Wenz LS, Oeljeklaus S, Warscheid B, Pfanner N, Becker T, 2018. Recruitment of Cytosolic J-Proteins by TOM Receptors Promotes Mitochondrial Protein Biogenesis. Cell Rep 25, 2036–2043 e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F, 2014. Mitochondrial transporters of the SLC25 family and associated diseases: a review. J Inherit Metab Dis 37, 565–575. [DOI] [PubMed] [Google Scholar]
- Palmieri F, Monne M, 2016. Discoveries, metabolic roles and diseases of mitochondrial carriers: A review. Biochim Biophys Acta 1863, 2362–2378. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Alberio S, Pisano I, Lodi T, Meznaric-Petrusa M, Zidar J, Santoro A, Scarcia P, Fontanesi F, Lamantea E, Ferrero I, Zeviani M, 2005. Complete loss-of-function of the heart/muscle-specific adenine nucleotide translocator is associated with mitochondrial myopathy and cardiomyopathy. Hum Mol Genet 14, 3079–3088. [DOI] [PubMed] [Google Scholar]
- Papic D, Elbaz-Alon Y, Koerdt SN, Leopold K, Worm D, Jung M, Schuldiner M, Rapaport D, 2013. The role of Djp1 in import of the mitochondrial protein Mim1 demonstrates specificity between a cochaperone and its substrate protein. Mol Cell Biol 33, 4083–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Park Y, Ryu I, Choi MH, Lee HJ, Oh N, Kim K, Kim KM, Choe J, Lee C, Baik JH, Kim YK, 2017. Misfolded polypeptides are selectively recognized and transported toward aggresomes by a CED complex. Nat Commun 8, 15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G, 2003. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426, 39–44. [DOI] [PubMed] [Google Scholar]
- Peng M, Ostrovsky J, Kwon YJ, Polyak E, Licata J, Tsukikawa M, Marty E, Thomas J, Felix CA, Xiao R, Zhang Z, Gasser DL, Argon Y, Falk MJ, 2015. Inhibiting cytosolic translation and autophagy improves health in mitochondrial disease. Hum Mol Genet 24, 4829–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potting C, Tatsuta T, Konig T, Haag M, Wai T, Aaltonen MJ, Langer T, 2013. TRIAP1/PRELI complexes prevent apoptosis by mediating intramitochondrial transport of phosphatidic acid. Cell Metab 18, 287–295. [DOI] [PubMed] [Google Scholar]
- Rugarli EI, Langer T, 2012. Mitochondrial quality control: a matter of life and death for neurons. EMBO J 31, 1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht JJ, King MS, Zogg T, Aleksandrova AA, Pardon E, Crichton PG, Steyaert J, Kunji ERS, 2019. The Molecular Mechanism of Transport by the Mitochondrial ADP/ATP Carrier. Cell 176, 435–447 e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahi C, Kominek J, Ziegelhoffer T, Yu HY, Baranowski M, Marszalek J, Craig EA, 2013. Sequential duplications of an ancient member of the DnaJ-family expanded the functional chaperone network in the eukaryotic cytosol. Mol Biol Evol 30, 985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmughapriya S, Rajan S, Hoffman NE, Higgins AM, Tomar D, Nemani N, Hines KJ, Smith DJ, Eguchi A, Vallem S, Shaikh F, Cheung M, Leonard NJ, Stolakis RS, Wolfers MP, Ibetti J, Chuprun JK, Jog NR, Houser SR, Koch WJ, Elrod JW, Madesh M, 2015. SPG7 Is an Essential and Conserved Component of the Mitochondrial Permeability Transition Pore. Mol Cell 60, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano G, Tessa A, Petrini S, Mancuso M, Bruno C, Grieco GS, Malandrini A, DeFlorio L, Martini B, Federico A, Nappi G, Santorelli FM, Murri L, 2003. Autosomal dominant external ophthalmoplegia and bipolar affective disorder associated with a mutation in the ANT1 gene. Neuromuscul Disord 13, 162–165. [DOI] [PubMed] [Google Scholar]
- Simbeni R, Pon L, Zinser E, Paltauf F, Daum G, 1991. Mitochondrial membrane contact sites of yeast. Characterization of lipid components and possible involvement in intramitochondrial translocation of phospholipids. J Biol Chem 266, 10047–10049. [PubMed] [Google Scholar]
- Simoncini C, Siciliano G, Tognoni G, Mancuso M, 2017. Mitochondrial ANT-1 related adPEO leading to cognitive impairment: is there a link? Acta Myol 36, 25–27. [PMC free article] [PubMed] [Google Scholar]
- Strauss KA, DuBiner L, Simon M, Zaragoza M, Sengupta PP, Li P, Narula N, Dreike S, Platt J, Procaccio V, Ortiz-Gonzalez XR, Puffenberger EG, Kelley RI, Morton DH, Narula J, Wallace DC, 2013. Severity of cardiomyopathy associated with adenine nucleotide translocator-1 deficiency correlates with mtDNA haplogroup. Proc Natl Acad Sci U S A 110, 3453–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen A, Isohanni P, 2010. Mitochondrial DNA depletion syndromes--many genes, common mechanisms. Neuromuscul Disord 20, 429–437. [DOI] [PubMed] [Google Scholar]
- Teuling E, Bourgonje A, Veenje S, Thijssen K, de Boer J, van der Velde J, Swertz M, Nollen E, 2011. Modifiers of mutant huntingtin aggregation: functional conservation of C. elegans-modifiers of polyglutamine aggregation. PLoS Curr 3, RRN1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K, Majd H, Dallabona C, Reinson K, King MS, Alston CL, He L, Lodi T, Jones SA, Fattal-Valevski A, Fraenkel ND, Saada A, Haham A, Isohanni P, Vara R, Barbosa IA, Simpson MA, Deshpande C, Puusepp S, Bonnen PE, Rodenburg RJ, Suomalainen A, Ounap K, Elpeleg O, Ferrero I, McFarland R, Kunji ERS, Taylor RW, 2016. Recurrent De Novo Dominant Mutations in SLC25A4 Cause Severe Early-Onset Mitochondrial Disease and Loss of Mitochondrial DNA Copy Number. Am J Hum Genet 99, 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosserams A, Papadopoulos C, Jardel C, Lemiere I, Romero NB, De Lonlay P, Wahbi K, Voermans N, Hogrel JY, Laforet P, 2018. Two new cases of mitochondrial myopathy with exercise intolerance, hyperlactatemia and cardiomyopathy, caused by recessive SLC25A4 mutations. Mitochondrion 39, 26–29. [DOI] [PubMed] [Google Scholar]
- von Renesse A, Morales-Gonzalez S, Gill E, Salomons GS, Stenzel W, Schuelke M, 2019. Muscle Weakness, Cardiomyopathy, and L-2-Hydroxyglutaric Aciduria Associated with a Novel Recessive SLC25A4 Mutation. JIMD Rep 43, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen XJ, 2015. A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death. Nature 524, 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Salinas K, Zuo X, Kucejova B, Chen XJ, 2008a. Dominant membrane uncoupling by mutant adenine nucleotide translocase in mitochondrial diseases. Hum Mol Genet 17, 4036–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zuo X, Kucejova B, Chen XJ, 2008b. Reduced cytosolic protein synthesis suppresses mitochondrial degeneration. Nat Cell Biol 10, 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidberg H, Amon A, 2018. MitoCPR-A surveillance pathway that protects mitochondria in response to protein import stress. Science 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann N, Pfanner N, 2017. Mitochondrial Machineries for Protein Import and Assembly. Annu Rev Biochem 86, 685–714. [DOI] [PubMed] [Google Scholar]
- Wrobel L, Topf U, Bragoszewski P, Wiese S, Sztolsztener ME, Oeljeklaus S, Varabyova A, Lirski M, Chroscicki P, Mroczek S, Januszewicz E, Dziembowski A, Koblowska M, Warscheid B, Chacinska A, 2015. Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature 524, 485–488. [DOI] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU, 2003. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112, 41–50. [DOI] [PubMed] [Google Scholar]