Abstract
Mitochondrial transcription factor A (TFAM) plays an important role in mitochondrial DNA (mtDNA) transcription and replication. In some experimental settings, TFAM expression parallels parameters of mitochondrial biogenesis, which led to a widespread acceptance of TFAM as marker of mitochondrial biogenesis. We modulated TFAM expression in several experimental systems and observed that it fails to consistently parallel mtDNA copy number and expression of mtDNA-encoded polypeptides. We suggest that the use of TFAM as a marker of mitochondrial biogenesis should be avoided outside of systems in which its performance has been carefully validated.
1. Introduction
In most mammalian cells, mitochondria generate the bulk of ATP required to sustain diverse cellular processes while also playing important roles in intracellular calcium signaling 1, apoptosis 2, reactive oxygen species (ROS) production 3, and biosynthesis of heme and iron-sulfur clusters4,5. Mitochondria also house part of the total cell genome within mitochondrial DNA (mtDNA). Therefore, the synthesis and assembly of mitochondrial components, known as mitochondrial biogenesis, is of fundamental importance to cellular functions. Because only 13 of the estimated 1500 polypeptides in the mitochondrial proteome are encoded by mtDNA 6, mitochondrial biogenesis involves coordinated expression and assembly of proteins encoded in both nuclear and mitochondrial genomes.
Despite significant progress achieved in recent years, our understanding of mitochondrial biogenesis remains incomplete. It has been established that peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1a and 1β (PGC-1α and PGC1β), as well as a PGC-1-related coactivator (PRC), play critical roles in driving mitochondrial biogenesis and function in mitochondria-rich organs 7–11. Downstream of these coactivators, nuclear transcription factors NRF-1 and NRF-2 were shown to regulate expression of subunits of mitochondrial respiratory complexes 12 as well as mitochondrial transcription factor A (TFAM)13. The latter is required for both mtDNA transcription 14–16 and replication 17. In a high-profile publication, TFAM has been called a “master regulator of mitochondrial biogenesis”18. Indeed, available evidence suggests that, at least in some experimental systems, such constituents of mitochondrial biogenesis as mtDNA copy number, mtDNA transcription and translation of mtDNA-encoded polypeptides as well as some mitochondrial functions may closely parallel TFAM expression 19–23. It has also been reported that in some 20,21,24–26, but not all15,19,27, experimental systems endogenous TFAM levels are sufficient to completely cover mtDNA assuming that in vivo TFAM footprints on mtDNA are of the same size as they are in vitro (23-30 bp,15,28). It is often thought that strictly proportional abundance of the TFAM and mtDNA observed in some studies may be dictated by mutual stabilization of these two components of mitochondrial nucleoids. Therefore, a model emerged that describes TFAM’s involvement in mitochondrial biogenesis 29.
Notwithstanding the evidence in support of the close positive correlation between TFAM expression and some measures of mitochondrial biogenesis, herein we provide evidence of discordance between TFAM expression and mtDNA copy number, mitochondrial transcription and mitochondrial mass. We suggest that the use of TFAM as marker of mitochondrial biogenesis should be approached judiciously.
2. Materials and methods
2.1. Cell growth and treatment
Cells were propagated in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% Fetal Bovine Serum, gentamycin (50 μg/ml), uridine (50 μg/ml) and sodium pyruvate (1 mM) in a humidified atmosphere containing 5% CO2 at 37°C. When indicated, this medium was supplemented with doxycycline (1 μg/ml).
2.2. Recombinant DNA
Plasmids and viral constructs were generated using standard techniques 30. Retroviral constructs rv.2641 and rv.2739 encode the reverse tetracycline-regulated transactivator (rtTA advanced) and are identical, except that rv.2739 lacks EGFP 31. In order to produce inducible shRNA knockdown (KD) constructs, oligonucleotides (Supplementary Table 1) were annealed and cloned into BspMI-digested pMA2867 31, thus generating pMA3643-6. The lentiviral vector pMA4281 is identical to pMA3643-6, except the coding sequence for fluorescent protein mCherry was substituted with the coding sequence for Gaussia luciferase. Lentiviruses lv.3775 and lv.4054 encode human and mouse TFAM cDNAs, respectively, (Supplementary Figure 1).
2.3. Viral transduction
HeLa cells were seeded at 20%-40% confluence in wells of a 6-well plate and allowed to attach for 4-24h. Once cells attached, the medium was replaced with 2 ml of a mixture consisting of fresh DMEM (1 ml) plus retroviral supernatant (1 ml) and supplemented with polybrene (10 μg/ml). After overnight incubation, the medium was replaced with 2 ml of fresh DMEM medium and cells were grown in this medium for another 24 h, trypsinized, serially diluted and plated into 150-mm dishes in DMEM medium supplemented with appropriate antibiotics. After colonies appeared, they were picked into 24-well plates, expanded and analyzed by polymerase chain reaction (PCR).
2.4. Mitochondrial transcription and mtDNA copy number
Quantitation of mitochondrial transcripts was performed by qPCR using primers listed in Table 1. RNA was isolated using the EZNAI total RNA isolation kit (Omega Bio-tek, Atlanta, GA) and treated with the gDNA removal kit (Enzo Life Sciences, Farmingdale, NY) to reduce mtDNA contamination prior to reverse transcription with a SensiFast cDNA synthesis kit (Bioline USA, Taunton, MA). To increase sensitivity, reverse transcription reactions were supplemented with a specific primer for MT-ND6. Transcripts representative of 3 mitochondrial promoters were quantitated: MT-ND6 (for the light strand promoter, LSP), MT-RNR2 (for the heavy strand promoter 1, HSP1), and MT-ND1, MT-ND5, MT-CO1 and/or MT-CO2 transcripts (for HSP2). mtDNA copy number was determined using duplex TaqMan qPCR as described previously 32 using primers listed in Supplementary Table 1 and serial dilutions of a plasmid containing cloned nuclear and mitochondrial targets as the standard.
2.5. Semi-quantitative western blotting
Total cell lysates were prepared by homogenization in a buffer containing Tris (1%), sodium dodecyl sulfate (0.5%) and a mixture of protease inhibitors (Halt, Fisher Scientific, Hampton, NH) at pH 8.0. Western blotting was performed as described previously 33. Antibodies used were: anti-MT-CO1 and anti-MT-CO2 (Abcam, Cambridge, MA), anti-hTFAM (Cell Signaling Technology, Danvers, MA), and anti-HSP60 (BD Biosciences, Franklin Lakes, NJ). Western blots were developed with HRP-conjugated secondary antibodies (Boster Bio, Pleasanton, CA) and imaged with a Bio-Rad ChemiDoc XRS HQ imaging system (Bio-Rad Laboratories, Hercules, CA). Digital images were quantitated with Multi Gauge software (Fujifilm, Edison, NJ) using mitochondrial heat shock protein 60 (HSP60) as an internal control for normalization.
2.6. Determination of mitochondrial mass
Mitochondrial mass was determined with 10-N-nonyl acridine orange (NOA). Cells were plated in 24-well plates (40,000-60,000 cells per well) and incubated overnight to enable attachment. Cells were stained with NOA (100 nM) in a 24-well plate for 30 min at 37°C in a humidified atmosphere of 5% CO2, trypsinized and analyzed with fluorescence-activated cell sorting (BD FACSCanto, BD Biosciences).
2.7. Statistical analysis
All experiments were repeated 2-8 times. Due to variability between some replicas, figures present representative experiments and deviations from a representative experiment are described in the text. Pairwise comparisons were assessed using a 2-tailed t-test assuming unequal variance. Comparisons in groups were made using 2-way analysis of variance (ANOVA) with a post-hoc Tukey test.
3. Results
3.1. Effects of TFAM knockdown in human cervical epithelial cells
The only known effects of TFAM on mitochondrial biogenesis are through regulation of mtDNA replication and transcription 29. Therefore, in this study, we used a highly tractable Tet-On system to either knock down (KD) or overexpress TFAM in different cell lines. First, we established a system for regulated TFAM KD using a previously described Tet-On system 31. HeLa cells were transduced with a retrovirus rv.2641 and a lentivirus lv.3643-6. The retrovirus encoded rtTA advanced, EGFP and blasticidin resistance, and the lentivirus encoded mCherry and hTFAM shRNA (chosen after evaluating a set of 6 shRNAs) under the control of an rtTA-responsive promoter and puromycin resistance.
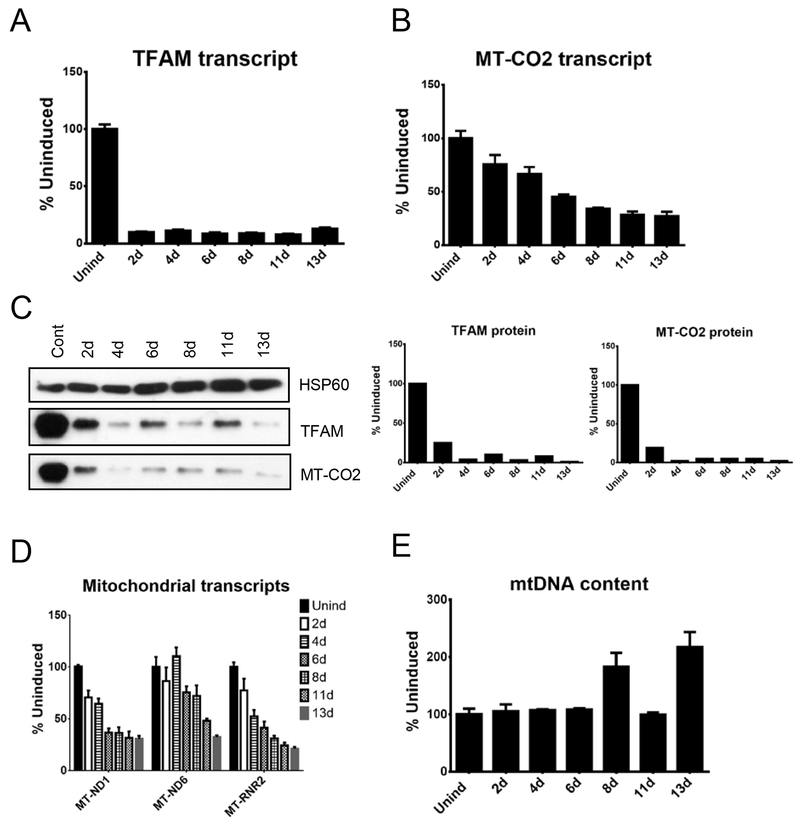
In response to induction, steady-state levels of the TFAM mRNA in these cells decreased to ~7%-12% of the uninduced level. This drop in TFAM mRNA was matched by a (predictably) delayed decrease in the steady-state level of the TFAM protein level to 1% - 10% of an uninduced level (Figure 1A, C). A decrease in the steady-state level of the mtDNA-encoded MT-CO2 mRNA was further delayed in time relative to a decrease in intracellular TFAM protein. Unexpectedly, the decrease in the MT-CO2 transcript was much less pronounced than the decrease in TFAM protein or MT-CO2 protein (Figure 1B, C). To elucidate whether this discrepancy is a spurious one, or represents a trend, we evaluated levels of transcripts generated by three mitochondrial promoters, MT-ND6 (LSP promoter), MT-RNR2 (HSP1 promoter), and MT-ND1 (HSP2 promoter). Indeed, there was a trend in that mitochondrial transcripts were affected to a lesser extent than TFAM protein (Figure 1D). Furthermore, more than a 10-fold reduction in the abundance of TFAM protein was without apparent effect on mtDNA content in HeLa cells (Figure 1E).
Figure 1.
Effects of mitochondrial transcription factor A (TFAM) knockdown in human cervical epithelial cells. HeLa cells were engineered for doxycycline-inducible expression of the hTFAM shRNA, and various mitochondrial parameters were followed during induction. (A) TFAM transcripts. (B) Mitochondrially Encoded Cytochrome C Oxidase II (MT-CO2) transcript. (C) TFAM and MT-CO2 protein expression. (D) Mitochondrial transcription. (E) mtDNA content.
To evaluate the effects of TFAM KD on mitochondrial mass, which is a gross parameter of mitochondrial biogenesis, we engineered HeLa cells with rv.2739 and lv.4281 to avoid the expression of fluorescent proteins that may interefere with FACS measurements. In these doubly transduced cells, TFAM KD resulted in a small, transient, but statistically significant increase in mitochondrial mass at 2 days after induction, which returned to baseline levels at 4 days (results not shown), indicating the lack of positive correlation between TFAM knockdown and gross mitochondrial biogenesis.
3.2. Effects of TFAM KD in human osteosarcoma cells
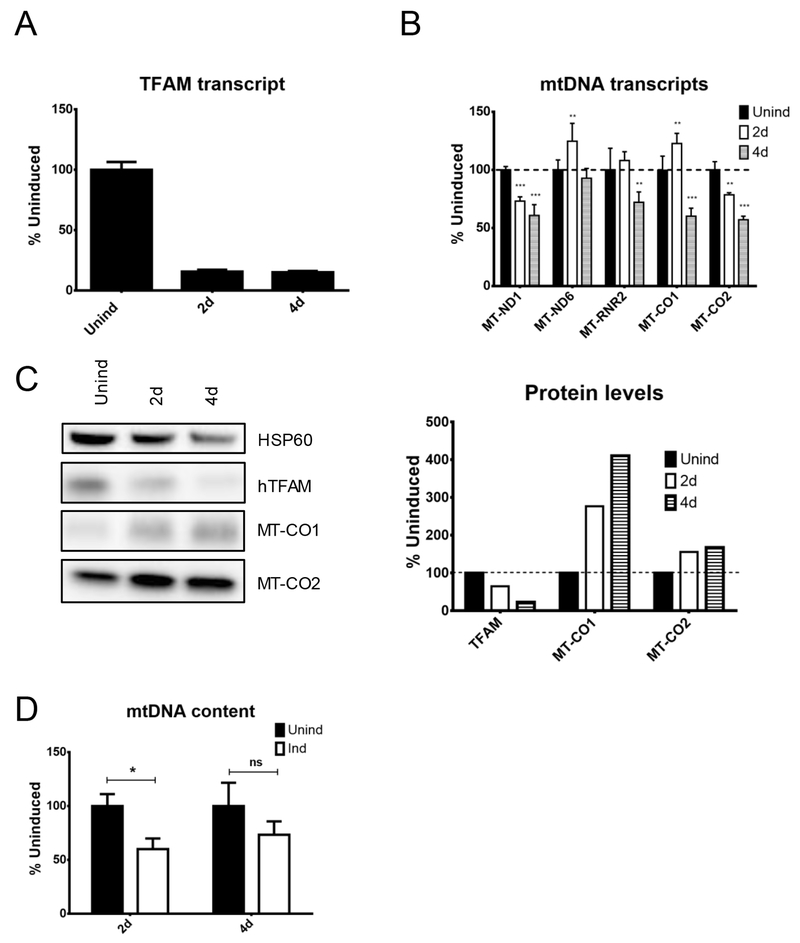
To determine whether the effects of altered TFAM expression very between different cell types, as they do between different tissues in genetically altered animals 17, we engineered the human osteosarcoma cell line 143B, similar to Hela cells, except for lv.4281 in which Gaussia luciferase was substituted for mCherry instead of lv.3643-6, and repeated the measurements (Figure 2). Even though the level of TFAM mRNA KD was similar between 143B cells (~85%) and HeLa cells (~88%), the residual TFAM protein levels were considerably higher in 143B cells (23%) than HeLa cells (4%) at 4 days after induction. Nevertheless, the steady-state levels of mtDNA transcripts were affected to a similar extent in both cell lines. Contrary to expectations based on the notion of TFAM as a marker of mitochondrial biogenesis, expression of the MT-CO2 and another mtDNA-encoded subunit of the oxidative phosphorylation (OxPhos) system Complex IV, MT-CO1 was not negatively affected. If anything, the expression of these two proteins was increased at 4 days after induction (Figure 2C). When the experiment was repeated, expression of MT-CO1, again, increased, while the expression of MT-CO2 remained unaltered (results not shown). Also, in contrast with observations in HeLa cells, the mtDNA content in 143B cells was reduced 2 days after induction (Figure 2D). In a biological repetition, reduction in the mtDNA copy number was significant at both 2 and 4 days after induction (results not shown).
Figure 2.
Effects of mitochondrial transcription factor A (TFAM) knockdown in human osteosarcoma cells (143B cells). The 143B cells were transduced with rv.2641 and lv.4281 for doxycycline-inducible expression of hTFAM shRNA, and mitochondrial parameters were followed during induction. (A) hTFAM transcript. (B) mtDNA transcripts. (C) Protein content (D) mtDNA content. *p < 0.05; **p < 0.01; ***p < 0.001.
3.3. Effects of heterologous hTFAM overexpression in mouse fibroblasts
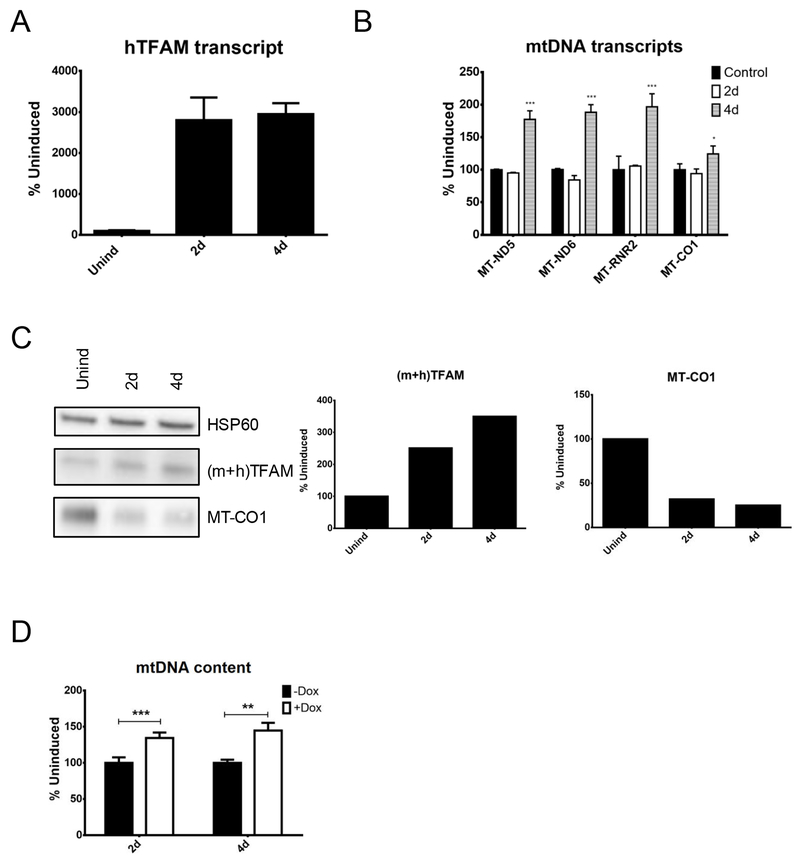
One piece of evidence often cited in support of TFAM as a marker of mitochondrial biogenesis is the observation that in mice transgenic for PAC encoding hTFAM 21 or for hTFAM cDNA 34,35 mtDNA copy number is increased. This increase in mtDNA content is accompanied, in some instances, by increased transcription 21. Therefore, we validated these observations in cultured cells. The 4B6 cell line (Tet-On mouse embryonic fibroblasts 33) was transduced by lv.3775, which expresses hTFAM under the control of the doxycycline-inducible promoter. Induction of the transduced cells resulted in upregulation of the hTFAM transcript and total TFAM protein levels (Figure 3). This increase in TFAM was followed by a substantial increase in steady-state levels of MT-ND5, MT-ND6 and MT-RNR2 transcripts, whereas MT-CO1 transcript increased only marginally, consistent with previous observations 21. However, MT-CO1 protein levels decreased. The increase in heterologous TFAM expression resulted in upregulation of the mtDNA copy number (Figure 3) in mouse fibroblasts, similar to results with transgenic animals 21.
Figure 3.
Effects of heterologous human mitochondrial transcription factor A (hTFAM) overexpression in mouse fibroblasts. 4B6 immortalized mouse embryonic fibroblasts were transduced with lv.3775 for doxycycline-regulated expression of hTFAM cDNA, and mitochondrial parameters were followed during induction. (A) hTFAM transcript. (B) mtDNA transcripts. (C) Protein levels. (D) mtDNA content. *p < 0.05; **p < 0.01; ***p < 0.001.
3.4. Effects of heterologous mTFAM overexpression in HeLa cells
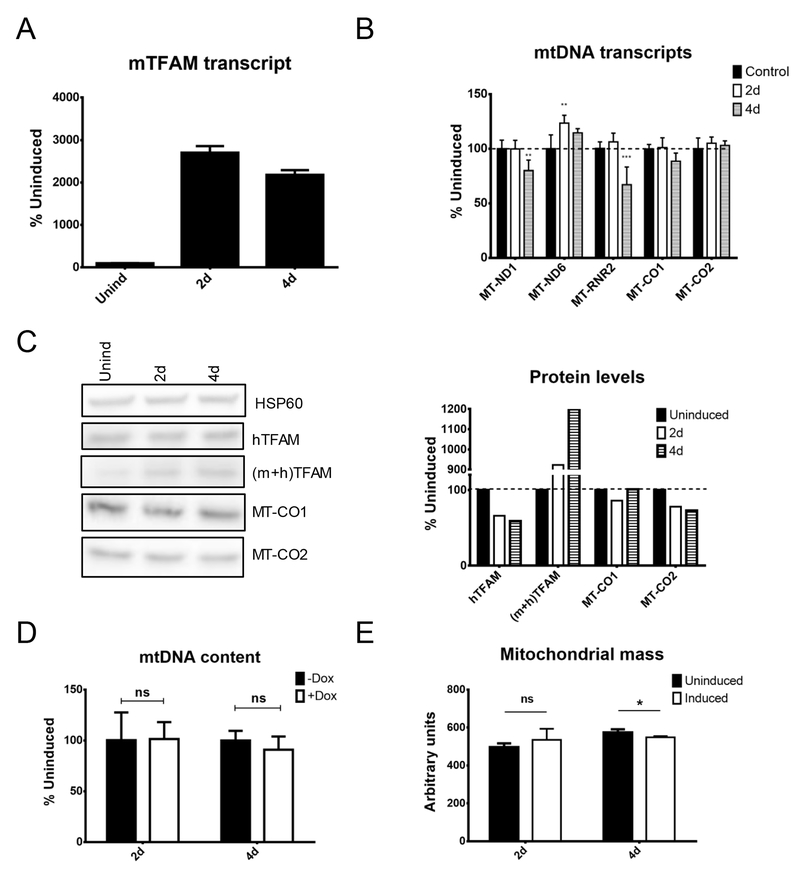
To evaluate the effects of heterologous mTFAM overexpression in human cells, HeLa cells were transduced with rv.2739 and lv.4054. As neither of the constructs encodes a fluorescent protein, these cells enable the evaluation of mitochondrial mass, which is a global measure of mitochondrial biogenesis. This was performed by flow cytometry after staining cells with 10-N-nonyl acridine orange (NOA), which binds cardiolipin in the mitochondrial inner membrane in a membrane potential-independent manner 36.
Induction of mTFAM expression in human cells was associated with accumulation of the mTFAM mRNA and a 10-fold increase in TFAM protein levels, which occurred exclusively because of increased mTFAM expression. This increase in TFAM expression, however, did not stimulate mitochondrial transcription. MT-ND1 and MT-RNR2 transcripts and MT-CO2 protein were downregulated at 4 days after induction. As with TFAM KD, mTFAM overexpression in HeLa cells had no effect on mtDNA content. In addition, mTFAM overexpression in human cells had no effect on the mitochondrial mass at 2 days after induction. At 4 days after induction, there was a slight, but statistically significant decrease in the mitochondrial mass, which, however, was not replicated in a follow-up experiment (Figure 4 and results not shown). In contrast, overexpression of mTFAM in 143B cells led to increased steady-state levels of MT-ND1, MT-ND6 and MT-CO2 transcripts and mtDNA copy number (Supplementary Figure 2).
Figure 4.
Effects of heterologous mouse mitochondrial transcription factor A (mTFAM) overexpression in HeLa cells. HeLa cells were transduced with rv.2739 and lv.4054 for doxycycline-regulated expression of mTFAM cDNA, and mitochondrial parameters were followed during induction. (A) mTFAM transcript. (B) mtDNA transcripts. (C) Protein levels. (D) mtDNA content. (E) Mitochondrial mass. *p < 0.05; **p < 0.01; ***p < 0.001.
3.5. Effects of hTFAM overexpression in human cells
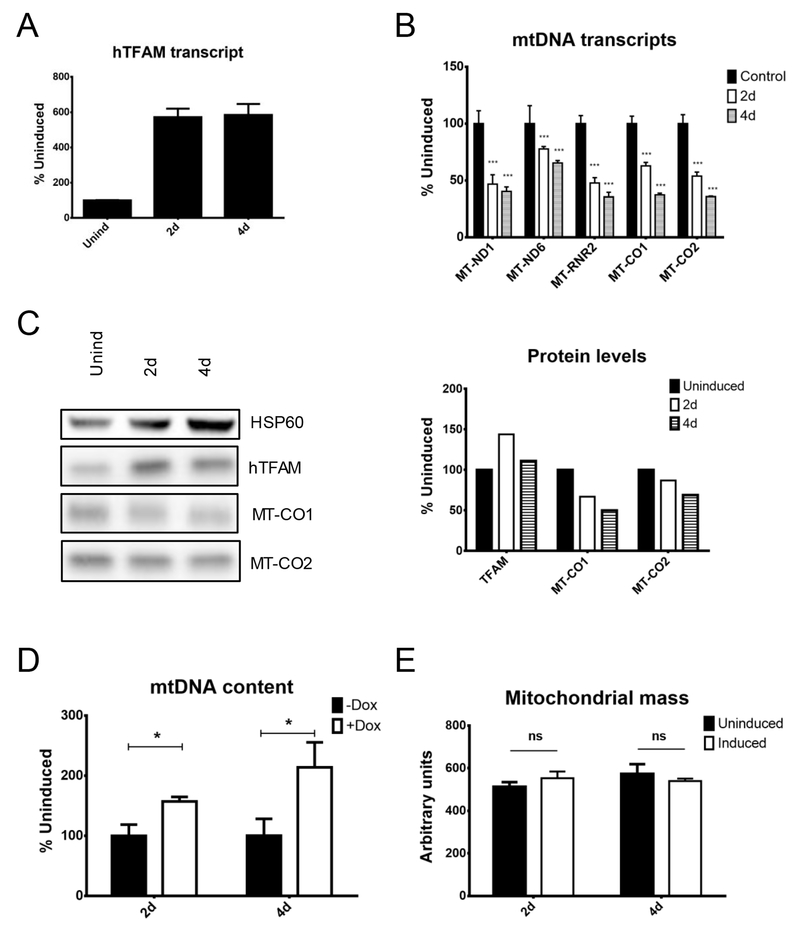
The effects of hTFAM overexpression in human cells were evaluated using HeLa cells transduced with rv.2739 and lv.3775. Upon induction, hTFAM transcript and hTFAM protein levels increased, consistent with the notion that in the absence of a complementary amount of mtDNA, hTFAM is phosphorylated, destabilized and degraded by Lon protease 37. Overexpression of hTFAM resulted in a uniform decrease in all mitochondrial transcripts, consistent with in vitro studies showing that excessive TFAM levels suppress transcription from mitochondrial promoters and with some experiments conducted in cultured cells 38,39. The reduction in MT-CO1 and MT-CO2 transcripts led to a reduction in steady state levels of corresponding proteins (Figure 5). The mtDNA copy number in induced cells increased despite the observed decrease in transcription. Finally, TFAM overexpression had no effect on mitochondrial mass (Figure 5).
Figure 5.
Effects of endogenous human mitochondrial transcription factor A (hTFAM) overexpression in human cells. HeLa cells were engineered with rv.2739 and lv.3775 for doxycycline-regulated expression of hTFAM cDNA, and mitochondrial parameters were followed during induction. (A) hTFAM transcript. (B) mtDNA transcripts. (C) Protein levels. (D) mtDNA content. (E) Mitochondrial mass. *p < 0.05; **p < 0.01; ***p < 0.001.
4. Discussion
While it is generally accepted that TFAM plays the central role in mtDNA metabolism, many aspects of this protein’s function remain poorly understood or even puzzling. In recent years, a substantial body of experimental evidence has accumulated suggesting that while TFAM may be critically involved in mtDNA replication and transcription, this protein’s contribution to mitochondrial biogenesis is likely to be more nuanced than previously thought. Two studies conducted by the same group reported either a complete loss 17 or ~30% residual levels 21 of mtDNA in the embryos of TFAM knockout (KO) animals. To put these observations into perspective, an mtDNA content between 40% and 150% of average in the population is considered clinically normal40. Moreover, while the whole-body TFAM KO is lethal17,21, mice with heart-specific TFAM KO survive to 12 weeks of age 41. The mtDNA copy number in the hearts of these animals, surprisingly, increases between 2 and 4 weeks of age and remains steady up to 8 weeks of age42. Furthermore, TFAM overexpression in flies did not affect mtDNA copy number43. In cultured cells, recovery of TFAM levels after ethidium bromide-induced mtDNA depletion lagged behind the recovery of mtDNA copy numbers, suggesting that in this experimental setting mitochondrial biogenesis (mtDNA replication) can occur without a proportional increase in TFAM expression 44. Conversely, a transient TFAM overexpression in cultured cells did not affect mtDNA copy number 19. Other investigators observed in developing muscle cells a decrease in mtDNA copy number despite a fourfold increase in TFAM expression 45, indicating that at least in some settings increased TFAM expression does not drive increased mitochondrial biogenesis. Similarly, a recent study demonstrated that in a tissue-specific POLRMT KO, normal levels of TFAM are insufficient to override reduced levels of the POLRMT, and mtDNA in these cells is maintained at reduced levels46, thus emphasizing the fact that TFAM expression, by itself, is insufficient to drive mitochondrial biogenesis through mtDNA replication. Finally, in a patient with myoclonic epilepsy with ragged red fibers (MERRF), the tissue with the highest mtDNA copy number had the lowest levels of TFAM 47. Collectively, this evidence indicates that a strong positive relationship between TFAM expression and mtDNA replication observed in some systems is not universal.
Incongruency between TFAM expression and mtDNA copy number deserves attention in the context of the models for mtDNA replication and packaging into nucleoids. Despite the dissenting reports15,19,27, the prevailing view is that TFAM is present in cells in quantities sufficient to completely cover mtDNA20,21,24–26. It has also been reported that some cells lacking mtDNA have reduced TFAM expression compared to parental cells containing mtDNA and that the release of TFAM from complexes with mtDNA is by Lon-mediated degradation 37. This appears to suggest a mandatory stoichiometric relationship between TFAM and mtDNA. However, a recent study indicates that in a tissue-specific POLRMT KO TFAM expression remains unchanged despite severely reduced mtDNA copy number. This TFAM persists free of mtDNA and is not degraded by Lon, which suggests TFAM/mtDNA stoichiometry is not a universal phenomenon 46. These observations also suggest that even though TFAM may be present in quantities sufficient to completely cover mtDNA, mtDNA in vivo may, in fact, be only partially covered, and a significant pool of “free” TFAM may exist in mitochondria. This letter consideration agrees well with a recent “sliding” model of mtDNA transcription28 and with cited above observations in the MERRF patient47.
There is also evidence that the relationship between TFAM expression and mitochondrial transcription is not strong enough to qualify this protein as a marker of mitochondrial biogenesis. Indeed, the very first study that addressed this issue reported that the effects of reduced TFAM expression were tissue-specific, and mitochondrial transcription in the liver and muscle was unaffected (~8% of wt)17. Furthermore, TFAM overexpression in transgenic mice decreased mitochondrial transcription instead of stimulating it34. Similarly, TFAM overexpression in flies had no effect on mitochondrial transcription 43. Conversely, TFAM KD in cultured Drosophila cells reduced TFAM abundance down to 5% of normal levels but did not affect mitochondrial transcription 48. In cultured chicken cells, TFAM overexpression resulted in a 4-fold increase in mtDNA content, but only marginally increased transcription, indicating a decrease in transcription on a per-mtDNA molecule basis 20. Taken together, this evidence points out that effects of TFAM expression on mitochondrial transcription may be cell type-specific and/or modulated by other factors to an extent that severely diminishes TFAM’s utility as a biomarker of mitochondrial biogenesis.
The lack of consensus in the available literature provided a strong rationale for the re-evaluation of TFAM’s role in mitochondrial biogenesis, which we undertook in this study. Here, we evaluated TFAM’s contributions to mitochondrial biogenesis through 1) its role in mtDNA replication (biogenesis of mitochondrial nucleoids) and 2) its role in mtDNA transcription, through which TFAM can contribute to the biogenesis of mitochondrial respiratory complexes. While our findings show that in some experiments changes in TFAM expression may positively correlate with changes in such parameters of mitochondrial biogenesis as mtDNA copy number, mtDNA transcription and/or changes in steady-state levels of mtDNA-encoded polypeptides, they do not in other experiments, sometimes within the same series.
In HeLa cells, TFAM KD correlated with reduced levels of mtDNA-encoded transcripts and MT-CO2 protein, supporting the utility of TFAM as a marker of mitochondrial biogenesis. However, no changes in mtDNA copy number were observed, which suggests the opposite conclusion (Figure 1). In human osteosarcoma cells, TFAM KD also resulted in reduced levels of mitochondrial transcripts (except for MT-ND6) and even reduced mtDNA levels, again supporting TFAM’s utility as a marker of mitochondrial biogenesis, yet levels of the MT-CO1 and MT-CO2 polypeptides increased, which is puzzling (Figure 2). In mouse fibroblasts, heterologous overexpression of hTFAM resulted in increased mitochondrial transcription and mtDNA copy number, yet levels of the MT-CO1 polypeptide dropped (Figure 3). In contrast, in HeLa cells, heterologous overexpression of mTFAM failed to promote unidirectional changes in mitochondrial transcription or to effect increases in mtDNA-encoded polypeptides, mtDNA or mitochondrial mass, which argues against TFAM’s utility as a marker of mitochondrial biogenesis (Figure 4). Finally, overexpression of hTFAM in human cells increased mtDNA content, which aligns well with the notion of TFAM as a marker of mitochondrial biogenesis. However, mitochondrial transcription and levels of mtDNA-encoded polypeptides decreased in this scenario, which argues against this notion (Figure 5). Collectively, none of the experimental systems examined in this study provided uniform support for the utility of TFAM as a marker of mitochondrial biogenesis. Notably, the lack of changes in mitochondrial mass during overexpression of either heterologous (Figure 4) or endogenous (Figure 5) TFAM in human cells also did not support this concept. Therefore, our findings do not provide uniform support for TFAM’s usefulness as a marker of mitochondrial biogenesis.
It is also important to note that TFAM-regulated transcripts supply only ~15% of all components of the OxPhos system with the remaining 85% encoded in the nuclear genome. Therefore, while it is plausible that reduced TFAM expression may downregulate OxPhos biogenesis by reducing the supply of mtDNA-encoded subunits, the converse is unlikely to be true. Indeed, an increase in OxPhos biogenesis in response to an increased supply of mtDNA-encoded subunits would require the existence of an (at this point, hypothetical) mechanism for the simultaneous upregulation of the nuclear-encoded subunits. In contrast, TFAM overexpression in transgenic animals resulted in increased mtDNA content and in an increase in MT-ND6 transcript but effected no increase in respiratory chain activity, suggesting no net gain in respiratory complexes 21. Conversely, in various tissues of animals with reduced TFAM expression, phenotypes ranged from no change in either mitochondrial transcripts or mtDNA-encoded MT-CO1 polypeptide levels, to reduced transcription without a change in MT-CO1, to reduction in transcription, MT-CO1 polypeptide and respiratory complex activity 17.
Limitations of this study.
This discussion would be incomplete without acknowledging certain limitations of the presented experiments. One limitation stems from the Tet-On system used in all experiments. Doxycycline, which was used to induce TFAM overexpression and knockdown is known to inhibit mitochondrial protein synthesis. This may explain the seemingly paradoxical outcome of the experiments presented in Figure 3 where increased levels of MT-CO1 transcripts were accompanied by decreased expression of the corresponding polypeptide. However, in Figure 2 we see that while application of doxycycline effected reduced MT-CO1 and MT-CO2 transcripts, the levels of corresponding proteins were considerably increased. This observation does not agree well with the notion that doxycycline concentrations used in this study inhibit mitochondrial protein synthesis. While not shown in the manuscript, our studies also indicate that at concentrations used in this study, doxycycline does not affect doubling times of the parental cell lines.
Another limitation of this study is the western blotting technique itself, which, when used in conjunction with chemiluminescent detection, is semi-quantitative at best. A better quantitation can be achieved when using fluorescently labeled secondary antibodies. Therefore, quantitative assessments of western blots in this study are tenuous, and the emphasis is on qualitative trends, which are not affected by this particular weakness.
5. Conclusion
Our findings, the available literature as well as theoretical considerations all suggest that the relationship between TFAM expression and mitochondrial biogenesis is more complex than is often appreciated and can be ambiguous, and that the use of this protein as a marker of mitochondrial biogenesis, while justified in limited circumstances, should be avoided in systems in which its performance as a biomarker has not been previously validated. It is suggested that definitive measures of mitochondrial mass/biogenesis be used whenever possible (e.g., VDAC or subunits of mitochondrial respiratory complexes for western blotting, or mtDNA copy number as determined by qPCR or ddPCR).
Supplementary Material
Supplementary Figure 1. Viral constructs used in this study. Abbreviations: lv, lentivirus; rv, retrovirus. Retroviruses rv.2641 and rv.2739 encode the advanced version of the reverse tetracycline-controlled transactivator (rtTA) and blasticidin resistance gene (Blast). rv.2641 also encodes enhanced green fluorescent protein (EGFP), which is fused to the blasticidin resistance gene. The rtTA advanced expression is driven by the long terminal repeat (LTR) promoter and linked to the expression of the blasticidin resistance or EGFP-Blast fusion through encephalomyocarditis virus (EMV) internal ribosome entry site (IRES). In lentiviral constructs, the transgene expression is driven by the rtTA-regulated promoter Ptet. shRNA is embedded into the miR scaffold and is cotranscribed with mCherry (lv.3643-6) or Gaussia princeps luciferase gene (Gluc in lv.4281). Expression of hTFAM and mTFAM in lv.3775 and lv.4054 is driven by Ptet. All lentiviruses encode an SV40 promoter-driven puromycin resistance gene (Puro) for selection. RRE, rev response element; wPRE, Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element.
Supplementary Figure 2. Effects of heterologous mouse mitochondrial transcription factor A (mTFAM) overexpression in HeLa cells. 143B cells were transduced with rv.2641 and lv.4054 for doxycycline-regulated expression of mTFAM cDNA, and mitochondrial parameters were followed during induction. (A) mTFAM transcript. (B) mtDNA transcripts. (C) mtDNA content. *p < 0.05; **p < 0.01; ***p < 0.001.
Supplementary Table 1. Oligonucleotides used in this study.
Highlights:
Changes in mtDNA content are not always proportional to changes in TFAM expression
Changes in mitochondrial transcription are not always proportional to changes in TFAM expression
Changes in TFAM expression have negligible effect on mitochondrial mass
Effects of changes in TFAM expression may be cell-type-specific
Changes in TFAM expression should not be used to make inferences about mitochondrial biogenesis
Acknowledgments
The authors wish to acknowledge Viktoriya Pastukh for expert technical assistance with generation of viral constructs and Elli Trepman for editorial support. MA was supported by NIH grants OD010944 and HL66299 and by the Office of the Assistant Secretary of Defense for Health Affairs under Award No. W81XWH-16-1-0096. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no competing financial interests.
References
- 1.Blackstone NW The impact of mitochondrial endosymbiosis on the evolution of calcium signaling. Cell Calcium 57, 133–139, doi: 10.1016/j.ceca.2014.11.006 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Elkholi R, Renault TT, Serasinghe MN & Chipuk JE Putting the pieces together: How is the mitochondrial pathway of apoptosis regulated in cancer and chemotherapy? Cancer & metabolism 2, 16, doi: 10.1186/2049-3002-2-16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zorov DB, Juhaszova M & Sollott SJ Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev 94, 909–950, doi: 10.1152/physrev.00026.2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah DI et al. Mitochondrial Atpif1 regulates haem synthesis in developing erythroblasts. Nature 491, 608–612, doi: 10.1038/nature11536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lill R, Srinivasan V & Muhlenhoff U The role of mitochondria in cytosolic-nuclear iron-sulfur protein biogenesis and in cellular iron regulation. Curr. Opin. Microbiol 22C, 111–119, doi: 10.1016/j.mib.2014.09.015 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Taylor SW, Fahy E & Ghosh SS Global organellar proteomics. Trends Biotechnol. 21, 82–88 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Puigserver P, Donovan J, Tarr P & Spiegelman BM Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta ), a novel PGC-1-related transcription coactivator associated with host cell factor. J. Biol. Chem 211, 1645–1648, doi: 10.1074/jbc.C100631200 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Andersson U & Scarpulla RC Pgc-1-related coactivator, a novel, serum-inducible coactivator of nuclear respiratory factor 1-dependent transcription in mammalian cells. Mol. Cell. Biol 21, 3738–3749, doi: 10.1128/MCB.21.11.3738-3749.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehman JJ et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Invest 106, 847–856, doi: 10.1172/JCI10268 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arany Z et al. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc. Natl. Acad. Sci. U. S. A 103, 10086–10091, doi: 10.1073/pnas.0603615103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai L et al. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev. 22, 1948–1961, doi: 10.1101/gad.1661708 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh J, Kawana N & Yamamoto Y Pathway Analysis of ChIP-Seq-Based NRF1 Target Genes Suggests a Logical Hypothesis of their Involvement in the Pathogenesis of Neurodegenerative Diseases. Gene Regul. Syst. Bio 7, 139–152, doi: 10.4137/GRSB.S13204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleyzer N, Vercauteren K & Scarpulla RC Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell. Biol 25, 1354–1366, doi: 10.1128/MCB.25.4.1354-1366.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher RP & Clayton DA A transcription factor required for promoter recognition by human mitochondrial RNA polymerase. Accurate initiation at the heavy- and light-strand promoters dissected and reconstituted in vitro. J. Biol. Chem 260, 11330–11338 (1985). [PubMed] [Google Scholar]
- 15.Fisher RP & Clayton DA Purification and characterization of human mitochondrial transcription factor 1. Mol. Cell. Biol 8, 3496–3509 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher RP, Parisi MA & Clayton DA Flexible recognition of rapidly evolving promoter sequences by mitochondrial transcription factor 1. Genes Dev. 3, 2202–2217 (1989). [DOI] [PubMed] [Google Scholar]
- 17.Larsson NG et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet 18, 231–236 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Nisoli E et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317, doi: 10.1126/science.1117728 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Maniura-Weber K, Goffart S, Garstka HL, Montoya J & Wiesner RJ Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells. Nucleic Acids Res. 32, 6015–6027, doi:32/20/6015 [pii] 10.1093/nar/gkh921 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushima Y et al. Functional domains of chicken mitochondrial transcription factor A for the maintenance of mitochondrial DNA copy number in lymphoma cell line DT40. J. Biol. Chem 278, 31149–31158, doi: 10.1074/jbc.M303842200 M303842200 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 21.Ekstrand MI et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet 13, 935–944, doi: 10.1093/hmg/ddh109ddh109 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- 22.Matsushima Y, Garesse R & Kaguni LS Drosophila mitochondrial transcription factor B2 regulates mitochondrial DNA copy number and transcription in schneider cells. J. Biol. Chem 279, 26900–26905, doi: 10.1074/jbc.M401643200M401643200 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- 23.Kanki T et al. Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol. Cell. Biol 24, 9823–9834 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kukat C et al. Super-resolution microscopy reveals that mammalian mitochondrial nucleoids have a uniform size and frequently contain a single copy of mtDNA. Proc. Natl. Acad. Sci. U. S. A 108, 13534–13539, doi: 10.1073/pnas.1109263108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellegrini M et al. MTERF2 is a nucleoid component in mammalian mitochondria. Biochim. Biophys. Acta, doi:S0005–2728(09)00035–8 [pii] 10.1016/j.bbabio.2009.01.018 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Alam TI et al. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 31, 1640–1645 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotney J, Wang Z & Shadel GS Relative abundance of the human mitochondrial transcription system and distinct roles for h-mtTFB1 and h-mtTFB2 in mitochondrial biogenesis and gene expression. Nucleic Acids Res. 35, 4042–4054, doi: 10.1093/nar/gkm424 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farge G et al. Protein sliding and DNA denaturation are essential for DNA organization by human mitochondrial transcription factor A. Nature communications 3, 1013, doi: 10.1038/ncomms2001ncomms2001 [pii] (2012). [DOI] [PubMed] [Google Scholar]
- 29.Picca A & Lezza AM Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions: Useful insights from aging and calorie restriction studies. Mitochondrion 25, 67–75, doi: 10.1016/j.mito.2015.10.001 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J & Russel DW Molecular Cloning. A laboratory manual. (Cold Spring Harbor Laboratory Press, 2001). [Google Scholar]
- 31.Alexeyev MF, Fayzulin R, Shokolenko IN & Pastukh V A retro-lentiviral system for doxycycline-inducible gene expression and gene knockdown in cells with limited proliferative capacity. Mol. Biol. Rep 37, 1987–1991, doi: 10.1007/s11033-009-9647-7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khozhukhar N, Spadafora D, Rodriguez Y & Alexeyev M Elimination of Mitochondrial DNA from Mammalian Cells. Curr. Protoc. Cell Biol 78, 20 11 21–20 11 14, doi: 10.1002/cpcb.39 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shokolenko IN et al. Mitochondrial DNA ligase is dispensable for the viability of cultured cells but essential for mtDNA maintenance. J. Biol. Chem 288, 26594–26605, doi: 10.1074/jbc.M113.472977 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeuchi M et al. Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation 112, 683–690 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Chandrasekaran K et al. Mitochondrial Transcription Factor A Regulation of Mitochondrial Degeneration in Experimental Diabetic Neuropathy. Am. J. Physiol. Endocrinol. Metab, ajpendo 00620 02014, doi: 10.1152/ajpendo.00620.2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maftah A, Petit JM, Ratinaud MH & Julien R 10-N nonyl-acridine orange: a fluorescent probe which stains mitochondria independently of their energetic state. Biochem. Biophys. Res. Commun 164, 185–190 (1989). [DOI] [PubMed] [Google Scholar]
- 37.Lu B et al. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol. Cell 49, 121–132, doi: 10.1016/j.molcel.2012.10.023 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pohjoismaki JL et al. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res. 34, 5815–5828, doi:gkl703 [pii] 10.1093/nar/gkl703 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsushima Y, Goto YI & Kaguni LS Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM). Proc. Natl. Acad. Sci. U. S. A, doi:1008924107 [pii] 10.1073/pnas.1008924107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shokolenko IN & Alexeyev MF Mitochondrial DNA: A disposable genome? Biochim. Biophys. Acta 1852, 1805–1809, doi: 10.1016/j.bbadis.2015.05.016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freyer C et al. Maintenance of respiratory chain function in mouse hearts with severely impaired mtDNA transcription. Nucleic Acids Res, doi:gkq527 [pii] 10.1093/nar/gkq527 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansson A et al. A switch in metabolism precedes increased mitochondrial biogenesis in respiratory chain-deficient mouse hearts. Proc. Natl. Acad. Sci. U. S. A 101, 3136–3141 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuda T, Kanki T, Tanimura T, Kang D & Matsuura ET Effects of overexpression of mitochondrial transcription factor A on lifespan and oxidative stress response in Drosophila melanogaster. Biochem. Biophys. Res. Commun 430, 717–721, doi: 10.1016/j.bbrc.2012.11.084 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Seidel-Rogol BL & Shadel GS Modulation of mitochondrial transcription in response to mtDNA depletion and repletion in HeLa cells. Nucleic Acids Res. 30, 1929–1934 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franko A et al. CREB-1alpha is recruited to and mediates upregulation of the cytochrome c promoter during enhanced mitochondrial biogenesis accompanying skeletal muscle differentiation. Mol. Cell. Biol 28, 2446–2459, doi: 10.1128/MCB.00980-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhl I et al. POLRMT regulates the switch between replication primer formation and gene expression of mammalian mtDNA. Science advances 2, e1600963, doi: 10.1126/sciadv.1600963 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinckmann A et al. Regionalized pathology correlates with augmentation of mtDNA copy numbers in a patient with myoclonic epilepsy with ragged-red fibers (MERRF-syndrome). PLoS One 5, e13513, doi: 10.1371/journal.pone.0013513 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goto A, Matsushima Y, Kadowaki T & Kitagawa Y Drosophila mitochondrial transcription factor A (d-TFAM) is dispensable for the transcription of mitochondrial DNA in Kc167 cells. Biochem. J 354, 243–248 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Viral constructs used in this study. Abbreviations: lv, lentivirus; rv, retrovirus. Retroviruses rv.2641 and rv.2739 encode the advanced version of the reverse tetracycline-controlled transactivator (rtTA) and blasticidin resistance gene (Blast). rv.2641 also encodes enhanced green fluorescent protein (EGFP), which is fused to the blasticidin resistance gene. The rtTA advanced expression is driven by the long terminal repeat (LTR) promoter and linked to the expression of the blasticidin resistance or EGFP-Blast fusion through encephalomyocarditis virus (EMV) internal ribosome entry site (IRES). In lentiviral constructs, the transgene expression is driven by the rtTA-regulated promoter Ptet. shRNA is embedded into the miR scaffold and is cotranscribed with mCherry (lv.3643-6) or Gaussia princeps luciferase gene (Gluc in lv.4281). Expression of hTFAM and mTFAM in lv.3775 and lv.4054 is driven by Ptet. All lentiviruses encode an SV40 promoter-driven puromycin resistance gene (Puro) for selection. RRE, rev response element; wPRE, Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element.
Supplementary Figure 2. Effects of heterologous mouse mitochondrial transcription factor A (mTFAM) overexpression in HeLa cells. 143B cells were transduced with rv.2641 and lv.4054 for doxycycline-regulated expression of mTFAM cDNA, and mitochondrial parameters were followed during induction. (A) mTFAM transcript. (B) mtDNA transcripts. (C) mtDNA content. *p < 0.05; **p < 0.01; ***p < 0.001.
Supplementary Table 1. Oligonucleotides used in this study.