Abstract
The formation of an effective vascular network can promote peripheral angiogenesis, ensuring an effective supply of blood, oxygen, and nutrients to an engineered bladder, which is important for bladder tissue engineering. Stromal vascular fraction cells (SVFs) promote vascularization and improve the function of injured tissues. In this study, adipose tissue-derived SVFs were introduced as an angiogenic cell source and seeded into the bladder acellular matrix (BAM) to generate a SVF-BAM complex for bladder reconstruction. The morphological regeneration and functional restoration of the engineered bladder were evaluated. In addition, we also explored the role of the Wnt5a/sFlt-1 noncanonical Wnt signaling pathway in regulating the angiogenesis of SVFs, and in maintaining the rational capability of SVFs to differentiate into vasculature in regenerated tissues. Histological assessment indicated that the SVF-BAM complex was more effective in promoting smooth muscle, vascular, and nerve regeneration than BAM alone and subsequently led to the restoration of bladder volume and bladder compliance. Moreover, exogenous Wnt5a was able to enhance angiogenesis by increasing the activity of MMP2, MMP9, and VEGFR2. Simultaneously, the expression of sFlt-1 was also increased, which enhanced the stability of the SVFs angiogenic capability. SVFs may be a potential cell source for tissue-engineered bladders. The Wnt5a/sFlt-1 pathway is involved in the regulation of autologous vascular formation by SVFs. The rational regulation of this pathway can promote neo-microvascularization in tissue-engineered bladders.
Keywords: Bladder augmentation, stromal vascular fraction cells, bladder acellular matrix, Wnt5a
Introduction
Massive bladder defect repair has always been a challenge for urological surgery. Autologous gastrointestinal segment transplantation remains the most commonly used approach for bladder reconstruction. However, this type of operation usually leads to various complications, including metabolic acidosis, bladder stones, urinary tract infection, and tissue contracture. Bladder tissue engineering is a promising technique for promoting bladder regeneration that uses a combination of biological scaffold materials, stem cells, biological factors, and physiological and chemical stimuli.1 Similar to other processes of tissue engineering, bladder regeneration also requires a process to induce neovascularization. Insufficient neovascularization in bladder grafts has been confirmed to suppress the integration of grafts and hosts, thereby leading to graft contracture and ischemic necrosis, among other complications.1,2 Several strategies have been utilized to promote angiogenesis, including the use of mesenchymal stem cells derived from multiple sources, growth factors, other biochemical angiogenic stimuli, and the immune regulation of adaptive immune cells. Nevertheless, the vascularization of massive bladder graft structures remains a challenge.2 Although endothelial cells (ECs) are generally used for coculture with MSCs, the proliferative capability of ECs is limited. Hence, a combination of seed cell selection and scaffolding has received more attention. This approach aims to solve problems stemming from the insufficient preservation and the integration of pro-angiogenic factors into host tissues. It is critical to obtain an adequate number of seed cells with differentiation potential and angiogenic capability in a short period.
Stromal vascular fraction cells (SVFs) comprise a heterogeneous cell population containing adipose tissue with self-renewing capability and differentiation potential. SVFs consist of ECs, smooth muscle cells, blood cells, and mesenchymal cells.3 SVFs have the potential to differentiate into various mesodermal lineages and are able to secrete growth factors, including hepatocyte growth factor, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF), and possess the ideal characteristics of candidate cell populations for cell repair therapy in tissue engineering.4–6 Notably, in animal models of peripheral ischemic diseases and myocardial infarction, SVFs have been verified to promote microvascularization and have the potential to improve organ function. Their unique angiogenic advantage may be the reason for the improved therapeutic effects that have been observed.5,7,8 Therefore, SVFs could provide a relatively accessible source of autologous seed cells. In our previous studies, we established a bladder acellular matrix (BAM) from a pig bladder that was suitable for cell infiltration, angiogenesis and nutrient spread, especially in massive defects. It is considered an ideal model to serve as a delivery system for bioactive factors. BAM, which retains the bladder structure in the absence of cellular components, is a collagen-based heterologous biomaterial with excellent biocompatibility and biodegradability.9
In recent studies, Wnt5a has been demonstrated to be closely associated with the regulation of angiogenesis, indicating the significant function and value of Wnt5a in treating angiogenic diseases. Wnt5a is a component of the noncanonical Wnt pathway. Recent studies have shown that Wnt5a can promote EC differentiation, thereby forming the inner wall of blood vessels through the Wnt/β-Catenin and Protein kinase C signaling pathways in embryonic stem cells. Wnt5a can activate CamKII to subsequently activate the Wnt/Ca2+ signaling pathway to regulate EC proliferation. The noncanonical Wnt5a signaling pathway can control tube formation in the retina in mice, possibly by changing the splicing pattern of Flt-1 to produce sFlt-1, which acts as a negative regulator of angiogenesis to suppress angiogenesis.10
In this study, we evaluated a tissue-engineered bladder comprised of BAM biodegradable constructs loaded with SVFs and attempted to determine whether it significantly improved bladder function. In addition, we also explored the role of the noncanonical Wnt5a/sFlt-1 signaling pathway in the regulation of angiogenesis in SVFs and in maintaining the rational capability of SVFs to differentiate into vasculature in injured tissues.
Materials and methods
Preparation of BAM
BAM was prepared from porcine bladder according to our previously described protocol.2 Briefly, the bladder was trimmed to obtain a crude sample of the lamina propria, which was placed into 0.25% trypsin/0.038% ethylenediaminetetraacetic acid (EDTA) (Gibco, Valencia, CA, USA) for 2 h at room temperature. The bladder was transferred into an ice-cold hypotonic solution overnight. Subsequently, the bladder was transferred to hypertonic solution and incubated for 24 h at room temperature. Then, the bladder was incubated in 10 mM Tris buffer (pH = 7.6) containing 50 U/mL DNase I (Sigma-Aldrich, St. Louis, MO, USA) for 24 h with stirring by an orbital shaker (150 r/min; Gesellschaft Fur Labortechnik; Germany) to remove any cell debris to obtain a scaffold of 0.2–0.4 mm in thickness. The decellularized tissues were finally sterilized by ethylene oxide and stored at −20°C.
Animals
A total of 36 eight-week-old female Sprague-Dawley (SD) rats weighing 250–300 g were used in this study. The rats were fed in a standard room with controlled temperature and humidity with a 12 h light/dark cycle, and provided with food and water ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Affiliated Nanjing First Hospital of Nanjing Medical University. This work was performed in accordance with the institutional and national guidelines for laboratory animals.
The SD rats were divided into three groups: the BAM group (n = 12), the BAM scaffold seeded with SVF group (SVF-BAM group, n = 12), and the cystotomy group (n = 12). The bladders of all animals were harvested at 4 and 12 weeks postoperatively.
SVFs extraction, characterization, tracking, and culture
As described previously,5 SVFs were isolated from epididymic adipose tissue. Rats were anesthetized using an intraperitoneal injection of ketamine (100 mg/kg). The rats were shaved, washed, and disinfected, and then their lower abdomens were cut open along the medioventral line to expose and remove the epididymic adipose tissue. Subsequently, the abdominal wall and skin were sutured, and the rats were fed again. The epididymic adipose tissue was washed with ice-cold sterile phosphate-buffered saline (PBS) three times, cut into small pieces, digested with 0.075% type I collagenase at 37°C, and stirred rapidly for 40 min. The tissue was filtered with 200 μm nylon mesh, and the filtrate was collected and centrifuged at 400 g for 5 min. The deposited cells were treated with red blood cell lysis buffer for 1 min, followed by neutralization with ice-cold PBS and centrifugation.
The cellular markers of uncultured SVFs were detected by flow cytometry analysis with the following antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-CD31 (Bioss Inc., Woburn, MA, USA), FITC-conjugated anti-VEGFR2 (Bioss Inc.), FITC-conjugated anti-CD90 (Bioss Inc.), FITC-conjugated anti-CD45 (BioLegend, San Diego, CA, USA), phycoerythrin (PE)-conjugated anti-CD11b/c (BioLegend), PE-conjugated anti-CD34 (Bioss Inc.), PE-conjugated anti-CD106 (Bioss Inc.), and PE-conjugated anti-CD133 (Novus Biologicals, Centennial, CO, USA). The labeled SVFs were analyzed with a FACSCalibur instrument (BD Biosciences, San Diego, CA, USA). An isotype-matched IgG was used for each procedure. In addition, colony-forming unit assays were performed in 3.5 cm dishes, in which the individual wells were seeded with 1000 passage 1 SVFs, and the cells were incubated at 37°C in 5% CO2 for 12 days, after which the colonies were fixed with 10% methanol and stained with 1% crystal violet.
For cell tracking, the SVFs were collected and incubated with the lipophilic fluorochrome chloromethylbenzamido dialkylcarbo-cyanine (CM-DiI; Molecular Probes, Eugene, OR, USA) in accordance with the manufacturer’s protocol. The SVFs were cultured in DMEM supplemented with 10% fetal bovine serum, 1% penicillin, and 1% streptomycin at a density of 2 × 105/mL. Then, they were co-cultured with BAM in an area of 1 cm × 1 cm at 37°C in a humidified 5% CO2 environment 24 h before bladder implantation.
Tube formation assay
Ten microliters of reduced growth factor Matrigel (BD Biosciences, San Diego, CA, USA) was coated onto each well of a prechilled μ-Slide angiogenesis plate (Ibidi, Gräfelfing, Germany). The plates were then incubated at 37°C for 30 min to form a layer of Matrigel. SVFs were seeded onto solidified Matrigel at a density of 104 cells/well and incubated with serum-free EC growth medium 2 (EGM-2; Lonza, Basel, Switzerland) to facilitate tube formation. The SVFs were incubated at 37°C for 6 h in a 5% CO2 humidified atmosphere to allow tube formation and then examined using phase-contrast microscopy. The lengths of the tubes were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Matrigel plug angiogenesis assay
Matrigel plug angiogenesis assays were performed as previously described.11 Briefly, 2.5 × 105 SVFs were treated with five different concentrations of recombinant human Wnt5a (0, 10, 50, 100, and 200 ng/mL; R&D Systems, Minneapolis, MN, USA) or neutralizing antibodies against sFlt-1 (10 µg/mL; R&D Systems) premixed with Matrigel (1 mg/mL) and EGM-2, and injected subcutaneously into nude mice in both inguinal regions. After 14 days, the plugs were excised and the hemoglobin content of the plugs was determined with Drabkin’s reagent kit (Sigma-Aldrich).
Surgery procedure
The peritoneum of rats was excised via the medioventral line to resect 50% of the bladder (top and upper half), followed by suturing of the BAM and residual bladder using interrupted absorbable sutures of 8-0 polyglycolic acid. Penicillin and buprenorphine were administered for 3 days after the operation.
Urodynamic examination
Rats were anesthetized by the intraperitoneal injection of pentobarbital (30 mg/kg). Afterward, a PE-50 polyethylene catheter was inserted into the bladder dome of each rat, followed by the connection of the catheter to the pressure sensor and the infusion pump through a three-way cock valve. Residual urine was removed from the rat bladder, followed by the injection of preheated sterile normal saline at a rate of 100 µL/min. During the process, the bladder pressure and excretion were recorded, and three urination cycles were recorded for each rat. In addition, the threshold micturition pressure (ΔP), maximal bladder capacity (ΔV), and bladder compliance (ΔV / ΔP) were recorded and evaluated.
Histological and immunohistochemical analyses
Animals were sacrificed at the corresponding time points, and the tissues were excised and fixed in 10% neutral formalin buffer, followed by dehydration and paraffin embedding. A 5-mm-thick section was cut from the paraffin-embedded tissue for and hematoxylin and eosin (H&E) or immunohistochemical staining. Immunohistochemical analysis was performed to detect the urothelium-associated protein AE1/AE3, the contractile smooth muscle marker alpha smooth muscle actin (α-SMA), the endothelial marker CD31, and the neuronal marker S-100 (all obtained from Abcam, Cambridge, MA, USA). Ten independent fields were randomly chosen to analyze the expression of the relevant proteins, the vascular density using ImageJ software (National Institutes of Health).
Western blot analysis
Cultured cells were lysed in 1 × SDS sample buffer. The rat bladder tissues were lysed using RIPA lysis buffer containing protease inhibitor cocktail (Roche, Shanghai, China). The supernatants were collected after centrifugation at 13,000 g at 4°C for 30 min. The protein concentration was quantified by a BCA protein assay kit (Beyotime, Nanjing, China). Equal amounts of protein were subject to 10% or 15% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA), followed by blocking in 5% nonfat milk in distilled water for 2 h at room temperature. The membrane was then incubated overnight with the diluted primary antibody at 4°C, followed by incubation with horseradish-peroxidase-conjugated secondary antibody (Proteintech, Chicago, IL, USA) at room temperature for 2 h. The primary antibodies used were anti-matrix metalloproteinase (MMP) 2, anti-MMP9, anti-VEGF receptor 2, anti-Wnt5a, and anti-sFlt-1 (all obtained from Abcam). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control (Abcam). The membranes with protein bands were analyzed using the ChemiDoc™ XRS system (Bio-Rad, Hercules, CA, USA). Densities of the bands of the Western blot images were evaluated using ImageJ software (National Institutes of Health).12
Statistical analyses
All data are presented as the mean ± standard deviation (SD). The t-test and one-way analysis of variance with a Tukey test were used for the statistical analysis. Statistical analyses were performed with GraphPad 7.0 (GraphPad, La Jolla, CA, USA); p < 0.05 was considered statistically significant.
Results
Characterization of SVFs
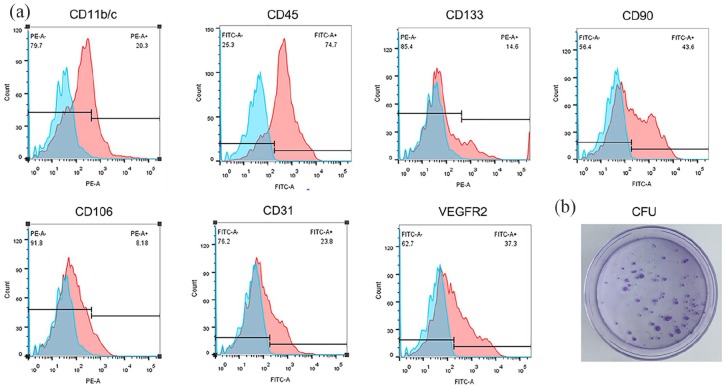
Flow cytometry analysis indicated that the freshly extracted SVFs comprised a heterogeneous population that expressed hematopoietic (CD11b/c (27.64 ± 8.16%), CD45 (68.2 ± 8.41%), and CD133 (16.24 ± 4.62)), mesenchymal (CD90 (40.32 ± 7.40%) and CD106 (12.62 ± 7.61%)), and endothelial markers (CD31 (25.41 ± 4.66%) and VEGFR2 (35.61 ± 7.85%)) (Figure 1(a)). The SVFs were diluted with DMEM and inoculated into a 3.5 cm culture dish at a final concentration of 1000 cells per dish, and the cloning capability of the SVFs was confirmed (Figure 1(b)).
Figure 1.
SVFs extraction and identification: (a) Characterization of freshly isolated SVFs was performed by flow cytometry. The representative flow cytometry histogram of SVFs shows that freshly isolated SVFs could express hematopoietic, mesenchymal, and endothelial cell markers and (b) colony-forming unit (CFU) assay and staining.
Gross analysis
Rats with SVFs seed cell-loaded BAM generally showed better tolerance than those with BAM alone. Two rats died before the scheduled sampling time (two rats in the BAM alone group) due to urinary leakage and stone formation. Other rats survived until euthanasia without obvious infection, urinary leakage, or diverticulum formation. After 4 weeks, gross examination revealed that the surface of the bladder tissue was loosely connected to the adjacent adipose tissue and the bladder graft contracture was clearly more severe in the BAM group than in the SVF-BAM group with irregular morphology. As shown in Table 1, three animals in the BAM group (two animals at 4 weeks and one animal at 12 weeks) and one animal in the SVF-BAM group (12 weeks) suffered from lithogenesis. In addition, three animals in the BAM group (one animal at 4 weeks and two animals at 12 weeks) and one animal in the SVF-BAM group (4 weeks) suffered from graft contracture.
Table 1.
Overview of the animals after surgery.
| 4 weeks |
12 weeks |
||||
|---|---|---|---|---|---|
| BAM | SVF-BAM | BAM | SVF-BAM | cystotomy | |
| Number of lithogenesis | 2 | 0 | 1 | 1 | 0 |
| Graft contracture | 1 | 1 | 2 | 0 | 0 |
BAM: bladder acellular matrix; SVF: stromal vascular fraction cell.
Rat subcutaneous transplantation model
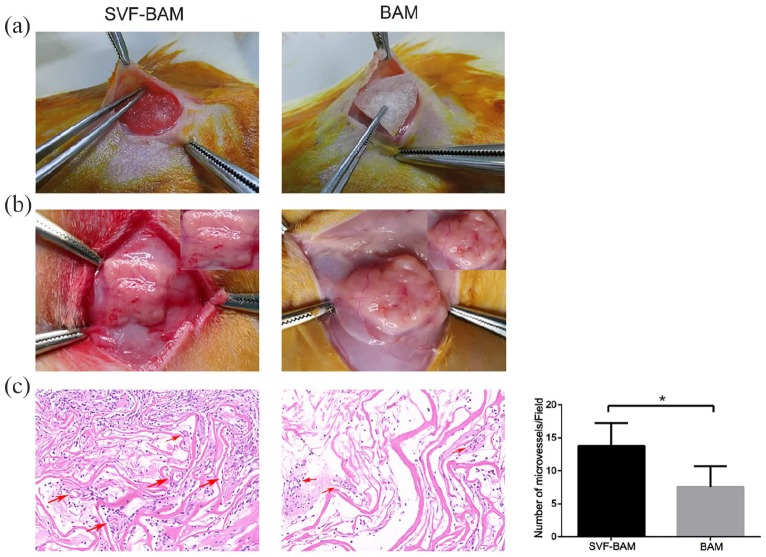
The isolated SVFs were seeded into the previously prepared BAM for subcutaneous autotransplantation in rats, and the autologous subcutaneous transplantation was examined (Figure 2(a)). After 2 weeks, the graft was removed. Gross observation revealed obvious angiogenesis in the BAM inoculated with SVFs, but not in uninoculated BAM (Figure 2(b)). H&E staining revealed significantly greater microvessel density in BAM seeded with SVFs than that in the unseeded BAM (Figure 2(c)), suggesting that the SVFs had a significant effect on angiogenesis.
Figure 2.
The rat subcutaneous transplantation model: (a) BAM seeded with SVFs was placed and fixed in the subcutaneous space in a rat, (b) BAM was placed and fixed in the subcutaneous space in a rat, and (c) photomicrographs of an H&E stained sample 14 days following transplantation.
Representative microvessel structures were marked with arrows. Magnification ×200.
*p < 0.05.
In vivo retention of SVF
Four weeks after the operation, CM-DiI was used to assess the preservation of SVFs in frozen sections. Red staining of markers with CM-DiI was observed in BAM, indicating a biological role (Figure 3).
Figure 3.
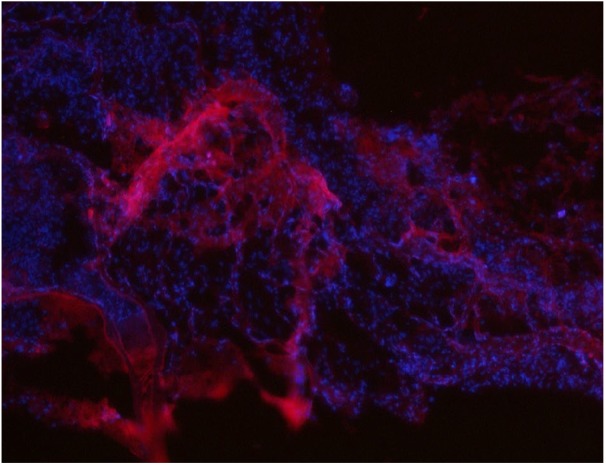
CM-Dil-positive SVFs were clearly observed to be involved in bladder tissue reconstruction at 1 month after surgery, magnification ×100.
Histological assessment
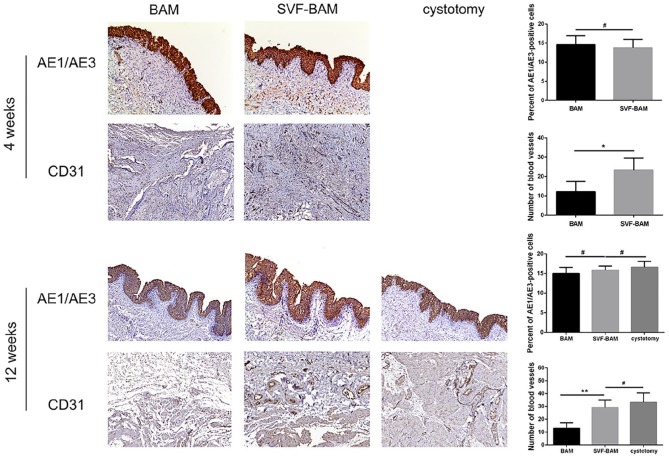
Immunohistochemistry was performed on tissues from both groups to assess the generation of urothelium (AE1/AE3), blood vessels (CD31), smooth muscle bundles (α-SMA), and nerve bundles (S-100) in the grafted area at designated time points. Multilayered structures resembling normal bladders that included urothelium, lamina propria, and basal lamina were observed in all groups 4 weeks after the operation. The thickness of the urothelium was quantified by the uroepithelial index AE1/AE3, which revealed no statistically significant differences. Four weeks and 12 weeks after the operation, the expression of the vascular-specific marker CD31 demonstrated that the vascular area distribution was more extensive in rats in the SVF-BAM group and was similar to that in the cystotomy group (Figure 4).
Figure 4.
Immunohistochemical assessments of regenerated bladders in all groups. Photomicrographs of urothelial markers (AE1/AE3) and a blood vessel endothelial marker (CD31) in all groups, at 1 and 3 months following transplantation. For all panels, magnification ×100.
*p < 0.05; **p < 0.01. #No significant difference when compared with the BAM group.
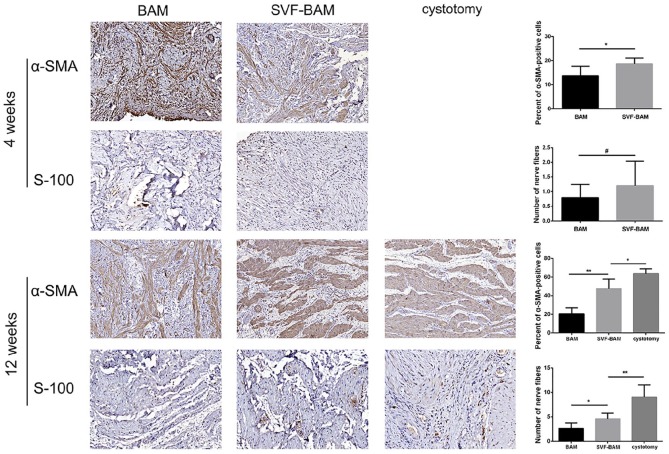
Further analysis at the same time point indicated that 4 weeks after the operation, the smooth muscle was unevenly distributed without bundle distribution in the BAM group without SVFs seeding. Twelve weeks after the operation, the proportion of α-SMA positive cells was significantly higher in the SVF-BAM group compared to that in the BAM group. In addition, the cells were distributed in a more organized way with bundle distribution (Figure 5).
Figure 5.
Immunohistochemical assessments of regenerated bladders in all groups: Photomicrographs of the smooth contractile muscle marker (α-SMA) post transplantation and Photomicrographs of the neuronal marker (S-100) post transplantation.
Magnification ×100 in the α-SMA panels; magnification ×200 in the S-100 panels.
*p < 0.05; **p < 0.01. #No significant difference when compared with the BAM group.
In each group, the expression of the neuronal marker S-100 increased over time. Four weeks after the operation, there was no statistically significant difference between the SVF-BAM group and the BAM group. However, 12 weeks after the operation, the density of S-100 positive cells was significantly higher in the SVF-BAM group than in the BAM group, and it was still significantly different from that in the cystotomy group (Figure 5).
Mean bladder capacity
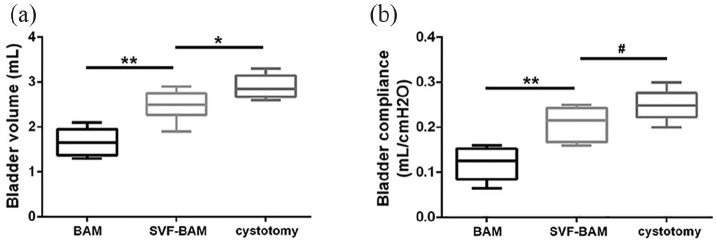
Urodynamic examinations were utilized to evaluate the function of the tissue-engineered neo-bladder. Normal saline was continuously injected into the bladder of all rats, and the intravesical pressure gradually increased. When it reached the critical pressure point, urination occurred. After urination, the intravesical pressure dropped to the baseline level, and the mean bladder capacity (MBC) in each group was recorded. Twelve weeks after the operation, the MBC in the SVF-BAM group was significantly higher than that in the BAM group, but still lower than the cystotomy group. Moreover, the rats in the SVF-BAM group exhibited better bladder compliance that was similar to that observed in the cystotomy group (Figure 6). Overall, these data demonstrated that the inoculation of BAM with SVFs was beneficial in recovering the biological function of the bladder.
Figure 6.
Quantification of the urodynamic parameters at 3 months after bladder reconstruction. The bladder capacity (a) and bladder compliance (b) in the SVF group were significantly greater than those in the BAM group and were similar to those in the cystotomy groups.
*p < 0.05; **p < 0.01. #No significant difference when compared with the cystotomy group.
Wnt5a enhances the angiogenic effects of SVF
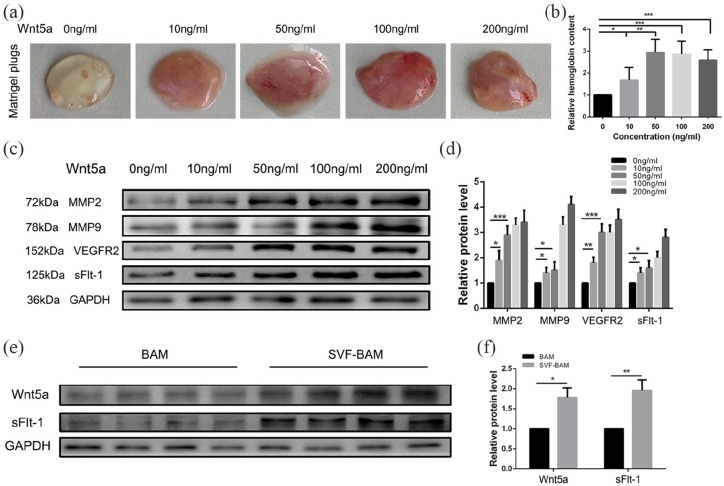
To explore the effect of Wnt5a on the angiogenic capability of SVFs, different doses of recombinant human Wnt5a were used in the formation of the SVFs autologous vascular network. A standard Matrigel plug assay was performed.11 In brief, different concentrations of recombinant human Wnt5a protein were used to stimulate SVFs in Matrigel plugs, which were transplanted subcutaneously into nude mice. The mice were monitored for 2 weeks to allow the formation of vascular structures and were subsequently euthanized. Angiogenesis was evident in the Matrigel plugs, and the angiogenic capability of the SVFs was increased with increasing Wnt5a concentration and tended to be stable when the concentration reached 50 ng/mL. Specifically, 10 and 50 ng/mL Wnt5a increased the hemoglobin content by 1.68 ± 0.26 and 2.94 ± 0.27-fold, respectively. However, when the Wnt5a dosage was increased to 100 and 200 ng/mL, the hemoglobin content no longer increased and remained steady at 2.86 ± 0.26 and 2.60 ± 0.21 times, respectively (Figure 7(a) and (b)).
Figure 7.
Exogenous recombinant Wnt5a mediates SVFs vascular self-assembly. SVFs were treated with increasing concentrations of recombinant Wnt5a: (a and b) The effects of various concentrations of recombinant Wnt5a on neovascularization in Matrigel plugs. After stimulation with different concentrations of recombinant Wnt5a, the plugs were removed on day 14 after Matrigel injection for the visualization and quantification of angiogenesis. Representative photographs of plugs from groups of five animals are shown. Quantification of angiogenesis within the Matrigel plugs is shown for all conditions. (c and d) During stimulation with different concentrations of Wnt5a, the expression of the vascularization-stimulating factors MMP2, MMP9, VEGFR2, and sFlt-1 in SVFs gradually increased. (e and f) At 4 weeks, the expression of Wnt5a and the vascular inhibitor sFlt-1 in the SVF-BAM group simultaneously increased.
*p < 0.05; **p < 0.01; ***p < 0.005.
Given that Wnt5a is associated with angiogenesis, the expression of angiogenesis-associated proteins was assessed in SVFs. The protein expression of Wnt5a receptors was investigated by Western blotting. As shown in Figure 7(c) and (d), three bands appeared at 72, 78, and 152 kDa that corresponded to activated MMP2, MMP9, and VEGFR2, respectively. Along with the increased concentration of Wnt5a, the expression of pro-angiogenic factors, including MMP2, MMP9, and VEGFR2, was upregulated. Intriguingly, the expression of sFlt-1, an inhibitor of angiogenesis, was also gradually increased. Similarly, the expressions of Wnt5a and sFlt-1 were simultaneously increased in tissue-engineered neo-bladders in the SVF-BAM group at 4 weeks (Figure 7(e) and (f)).
sFlt-1 suppresses the angiogenic effects of SVF
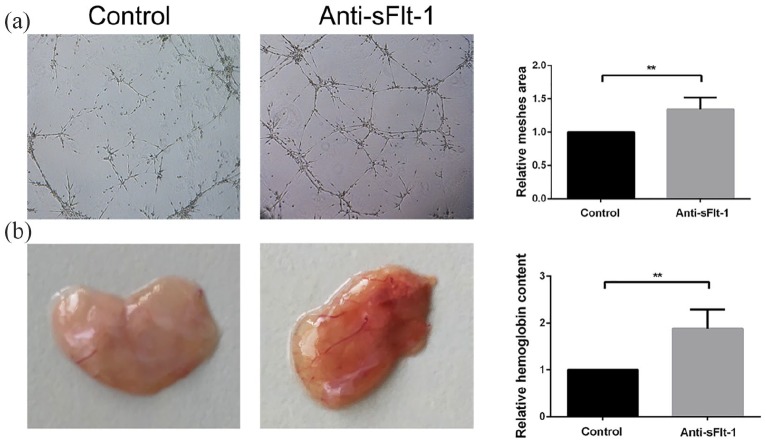
sFlt-1 plays a critical role in the suppression of angiogenesis.10 To investigate the potential role of sFlt-1 in angiogenesis in SVFs, SVFs were exposed to a sFlt-1 neutralizing antibody. Compared to that observed in the negative control group, the administration of anti-sFlt-1 at 10 μg/mL significantly increased the total lengths of blood vessels (Figure 8(a)). Similar results were found in the Matrigel plug assay, and the hemoglobin content was increased in the presence of the sFlt-1 neutralizing antibody (Figure 8(b)).
Figure 8.
sFlt-1 suppresses the angiogenesis of SVFs: (a) Tube formation assays in SVFs treated with sFlt-1 neutralizing antibody, magnification ×40 and (b) Matrigel plug angiogenesis assay in SVFs treated with sFlt-1 neutralizing antibody.
**p < 0.01.
Discussion and conclusion
The vascularization of the bladder is a key factor in the generation of tissue-engineered bladders. Recent clinical trials using tissue-engineered bladder implants have emphasized the essential role of angiogenesis in preventing the contracture and necrosis of the graft. The formation of an effective graft vascular network may ensure, along with the promotion of angiogenesis in the surrounding host tissue, an effective blood supply to the graft, thus avoiding ischemia-related severe complications.1,13 Few studies have assessed the use of stem cells to promote angiogenesis in tissue-engineered bladders. The addition of angiogenic factors, such as VEGF, platelet-derived growth factor-BB, and bFGF, is a common approach used to enhance angiogenesis in tissue-engineered bladders. However, due to the short half-life of these proteins and their rapid diffusion from the target tissues, frequent high doses are necessary to obtain significant efficacy.2,14–16 In addition, more extensive and rapid angiogenesis is required for bladder reconstruction in large animals. Their effects on the promotion of cell metastasis and the proliferation of bioactive factors are limited. Hence, cell implantation is a better method.
SVFs can be easily obtained by minimally invasive methods and have similar characteristics to those of adipose-derived mesenchymal stem cells, and also have intrinsic angiogenic capability.13 In a study of erectile function in a rat model of cavernous nerve injury, SVFs treatment produced statistically significant benefits compared with a single treatment with adipose-derived stem cells, especially for the smooth muscle/collagen ratio and EC content.17 In animal models of peripheral ischemic diseases and myocardial infarction, SVFs have been confirmed to promote microvascularization and have the potential to improve organ function. Moreover, SVFs have been shown to generate a variety of angiogenic growth factors and to function as perivascular cells.6,18–20 Multiple phase I clinical trials using various fat-derived SVFs preparations as therapeutic mesenchymal cells have demonstrated the superior safety of these cells.21,22
Presently, SVFs were implanted into BAM at 37°C for 12 h and reimplanted subcutaneously in rats. The SVFs effectively promoted the vascularization of BAM. CM-Dil staining showed that the SVFs survived and continued to play a role in the BAM 4 weeks later, when a complete multilayer urothelium with AE1/AE3-positive cells was present on the entire luminal surface of the new bladder wall in the SVF-BAM group. In the BAM tissue framework with implanted SVFs, the extent of both regenerated smooth muscle and blood vessels improved over time and reached values similar to those found in the cystotomy group 12 weeks after implantation. These results indicate that bladder reconstruction using the SVF-BAM framework can achieve satisfactory regeneration of both smooth muscle and blood vessels. Although SVFs were insufficient to regenerate nerves when compared to that in the cystotomy group, SVFs did play a positive role in promoting nerve regeneration when compared with that found in the BAM group. Twelve weeks after implantation, the bladder capacity was increased by at least 40%. In contrast, in the BAM group, urodynamic examination showed that bladder capacity and compliance were decreased compared to the SVF-BAM group, which may be due to increased fibrotic production and limited innervation.
There could be two different mechanisms involved in the role of SVFs implantation in bladder regeneration. The first mechanism involves the differentiation of SVFs into urinary tract and smooth muscle cells, which constitute the regenerated bladder wall.23 The other possibility is that SVFs produce nutrients and angiogenic factors to promote tissue regeneration.24 In addition, SVFs have been shown to inhibit inflammatory reactions by exogenous cell transplantation, which may also be beneficial to the regeneration of injured bladder tissues.5 One of the mechanisms by which SVFs regulate angiogenesis in development may involve a noncanonical Wnt5a/sFlt-1 signaling pathway.10,25 We investigated the effect of Wnt5a on SVFs angiogenesis and the potential underlying mechanisms. Previous studies have confirmed that increased Wnt5a can promote angiogenesis through the Wnt5a/β-catenin signaling axis, subsequently promoting the maturation and stability of blood vessels by increasing the expression of tie-2 and activating the VEGF/VEGFR2 signaling pathway to participate in the development of angiogenesis.26 Yao et al. found that Wnt5a is overexpressed in human non-small cell lung cancer tissues and is closely associated with tumor angiogenesis. The authors described that the increased expression of Wnt5a elevates the expression of MMP2 and MMP9 by activating the canonical Wnt signaling pathway, thereby leading to angiogenesis.27 Masckauchan et al.28 found that the Wnt5a-mediated Wnt/Ca2+ signaling pathway can promote the expression of MMP1 and tie-2, thereby inducing the proliferation of ECs and accelerating the formation of lumenal tissue.
The anti-angiogenic factor sFlt-1 is an alternatively spliced variant of VEGFR1 that lacks both the transmembrane and cytoplasmic domains. sFlt-1 binds to VEGF and suppresses the interaction between VEGF and VEGFR1 in the circulation. In addition, sFlt-1 has been reported to suppress angiogenesis.29,30 The circulating level of sFlt-1 is 10 times higher in pregnant women than that in nonpregnant women and is significantly increased at 36 weeks of gestation and throughout late pregnancy.30 From the middle of pregnancy, the circulating level of sFlt-1 in preeclampsia patients is significantly higher than that in normal pregnant women.31 The noncanonical Wnt5a signaling pathway can control vascular formation in the retina in mice, possibly by changing the splicing pattern of VEGFR1 to produce sFlt-1, which acts as a negative regulator of angiogenesis to suppress angiogenesis.10
High levels of pro-angiogenic factors can lead to uncontrollable side effects both locally and systemically, including the high permeability of blood vessels, stimulation of tumor growth, abnormal vascular function, and vascular overgrowth.15 In this study, we observed a dual effect of Wnt5a on SVFs angiogenesis. In vitro experiments showed that increased Wnt5a concentration within a certain range increased the angiogenic capability. However, as the concentration of Wnt5a continued to increase, the expression of the vascular inhibitor sFlt-1 also increased; therefore, the angiogenic capacity of SVFs were stable. The expression of Wnt5a and sFlt-1 were simultaneously increased in vivo. These findings demonstrate that SVFs implantation into BAM could promote angiogenesis, increase the survival rate of grafts, and enhance tissue angiogenesis by upregulating Wnt5a, which is also counterbalanced by sFlt-1 to avoid an increased risk of excessive vascularization.
In summary, we verified for the first time that a combination of SVFs and BAM could enhance the vascularization of tissue-engineered neo-bladders, generation of smooth muscle, and recovery of bladder function. After seeding BAM bladders with SVFs, the activity of Wnt5a in neo-bladders was upregulated, thereby promoting tissue vascularization and accelerating tissue repair. Hence, the manipulation of Wnt5a could enhance vascularization by SVFs in tissue engineering.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (81902602, 31500785, 81570613), Jiangsu Provincial Social Development Project (BE2017615), Jiangsu Provincial Medical Youth Talent (QNRC2016071), Nanjing Medical Science and Technology Development Project (JQX17007, ZDX16006), and Science and Technology Foundation from Nanjing Medical University (NMUB2018316).
ORCID iD: Feng Zhao  https://orcid.org/0000-0001-7111-4812
https://orcid.org/0000-0001-7111-4812
References
- 1. Alberti C. Whyever bladder tissue engineering clinical applications still remain unusual even though many intriguing technological advances have been reached. G Chir 2016; 37(1): 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou L, Yang B, Sun C, et al. Coadministration of platelet-derived growth factor-BB and vascular endothelial growth factor with bladder acellular matrix enhances smooth muscle regeneration and vascularization for bladder augmentation in a rabbit model. Tissue Eng Part A 2013; 19(1–2): 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010; 77(1): 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin G, Garcia M, Ning H, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev 2008; 17(6): 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou L, Xu L, Shen J, et al. Preischemic administration of nonexpanded adipose stromal vascular fraction attenuates acute renal ischemia/reperfusion injury and fibrosis. Stem Cells Transl Med 2016; 5(9): 1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leblanc AJ, Touroo JS, Hoying JB, et al. Adipose stromal vascular fraction cell construct sustains coronary microvascular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol 2012; 302(4): H973–H982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Dijk A, Naaijkens BA, Jurgens WJ, et al. Reduction of infarct size by intravenous injection of uncultured adipose derived stromal cells in a rat model is dependent on the time point of application. Stem Cell Res 2011; 7(3): 219–229. [DOI] [PubMed] [Google Scholar]
- 8. Semon JA, Zhang X, Pandey AC, et al. Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl Med 2013; 2(10): 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hou X, Shi C, Chen W, et al. Transplantation of human adipose-derived mesenchymal stem cells on a bladder acellular matrix for bladder regeneration in a canine model. Biomed Mater 2016; 11(3): 031001. [DOI] [PubMed] [Google Scholar]
- 10. Murdoch CE, Bachschmid MM, Matsui R. Regulation of neovascularization by S-glutathionylation via the Wnt5a/sFlt-1 pathway. Biochem Soc Trans 2014; 42(6): 1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Birbrair A, Sattiraju A, Zhu D, et al. Novel peripherally derived neural-like stem cells as therapeutic carriers for treating glioblastomas. Stem Cells Transl Med 2017; 6(2): 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9(7): 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nunes SS, Maijub JG, Krishnan L, et al. Generation of a functional liver tissue mimic using adipose stromal vascular fraction cell-derived vasculatures. Sci Rep 2013; 3: 2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen BS, Xie H, Zhang SL, et al. Tissue engineering of bladder using vascular endothelial growth factor gene-modified endothelial progenitor cells. Int J Artif Organs 2011; 34(12): 1137–1146. [DOI] [PubMed] [Google Scholar]
- 15. Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res 2002; 60(4): 668–678. [DOI] [PubMed] [Google Scholar]
- 16. Pike DB, Cai S, Pomraning KR, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials 2006; 27(30): 5242–5251. [DOI] [PubMed] [Google Scholar]
- 17. You D, Jang MJ, Kim BH, et al. Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem Cells Transl Med 2015; 4(4): 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cervelli V, Gentile P, De Angelis B, et al. Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res 2011; 6(2): 103–111. [DOI] [PubMed] [Google Scholar]
- 19. Koh YJ, Koh BI, Kim H, et al. Stromal vascular fraction from adipose tissue forms profound vascular network through the dynamic reassembly of blood endothelial cells. Arterioscler Thromb Vasc Biol 2011; 31(5): 1141–1150. [DOI] [PubMed] [Google Scholar]
- 20. Premaratne GU, Ma LP, Fujita M, et al. Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: anti-inflammatory role. J Cardiothorac Surg 2011; 6: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mizuno H, Tobita M, Uysal AC. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012; 30(5): 804–810. [DOI] [PubMed] [Google Scholar]
- 22. Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 2017; 8(1): 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodríguez LV, Alfonso Z, Zhang R, et al. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A 2006; 103(32): 12167–12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004; 109(10): 1292–1298. [DOI] [PubMed] [Google Scholar]
- 25. Murdoch CE, Shuler M, Haeussler DJ, et al. Glutaredoxin-1 up-regulation induces soluble vascular endothelial growth factor receptor 1, attenuating post-ischemia limb revascularization. J Biol Chem 2014; 289(12): 8633–8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu H, He J, Ye L, et al. Mechanisms of angiogenesis in a Curculigoside A-treated rat model of cerebral ischemia and reperfusion injury. Toxicol Appl Pharmacol 2015; 288(3): 313–321. [DOI] [PubMed] [Google Scholar]
- 27. Yao L, Sun B, Zhao X, et al. Overexpression of Wnt5a promotes angiogenesis in NSCLC. Biomed Res Int 2014; 2014: 832562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Masckauchan TN, Agalliu D, Vorontchikhina M, et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell 2006; 17(12): 5163–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levine RJ, Maynard S, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004; 350(7): 672–683. [DOI] [PubMed] [Google Scholar]
- 30. Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem Pharmacol 2015; 95(4): 211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dias-Junior CA, Chen J, Cui N, et al. Angiogenic imbalance and diminished matrix metalloproteinase-2 and -9 underlie regional decreases in uteroplacental vascularization and feto-placental growth in hypertensive pregnancy. Biochem Pharmacol 2017; 146: 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]