Abstract
Background
People with end‐stage kidney disease (ESKD) treated with dialysis are frequently affected by major depression. Dialysis patients have prioritised depression as a critically important clinical outcome in nephrology trials. Psychological and social support are potential treatments for depression, although a Cochrane review in 2005 identified zero eligible studies. This is an update of the Cochrane review first published in 2005.
Objectives
To assess the effect of using psychosocial interventions versus usual care or a second psychosocial intervention for preventing and treating depression in patients with ESKD treated with dialysis.
Search methods
We searched Cochrane Kidney and Transplant's Register of Studies up to 21 June 2019 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs of psychosocial interventions for prevention and treatment of depression among adults treated with long‐term dialysis. We assessed effects of interventions on changes in mental state (depression, anxiety, cognition), suicide, health‐related quality of life (HRQoL), withdrawal from dialysis treatment, withdrawal from intervention, death (any cause), hospitalisation and adverse events.
Data collection and analysis
Two authors independently selected studies for inclusion and extracted study data. We applied the Cochrane 'Risk of Bias' tool and used the GRADE process to assess evidence certainty. We estimated treatment effects using random‐effects meta‐analysis. Results for continuous outcomes were expressed as a mean difference (MD) or as a standardised mean difference (SMD) when investigators used different scales. Dichotomous outcomes were expressed as risk ratios. All estimates were reported together with 95% confidence intervals (CI).
Main results
We included 33 studies enrolling 2056 participants. Twenty‐six new studies were added to this 2019 update. Seven studies originally excluded from the 2005 review were included as they met the updated review eligibility criteria, which have been expanded to include RCTs in which participants did not meet criteria for depression as an inclusion criterion.
Psychosocial interventions included acupressure, cognitive‐behavioural therapy, counselling, education, exercise, meditation, motivational interviewing, relaxation techniques, social activity, spiritual practices, support groups, telephone support, visualisation, and voice‐recording of a psychological intervention.
The duration of study follow‐up ranged between three weeks and one year. Studies included between nine and 235 participants. The mean study age ranged between 36.1 and 73.9 years.
Random sequence generation and allocation concealment were at low risk of bias in eight and one studies respectively. One study reported low risk methods for blinding of participants and investigators, and outcome assessment was blinded in seven studies. Twelve studies were at low risk of attrition bias, eight studies were at low risk of selective reporting bias, and 21 studies were at low risk of other potential sources of bias.
Cognitive behavioural therapy probably improves depressive symptoms measured using the Beck Depression Inventory (4 studies, 230 participants: MD ‐6.10, 95% CI ‐8.63 to ‐3.57), based on moderate certainty evidence. Cognitive behavioural therapy compared to usual care probably improves HRQoL measured either with the Kidney Disease Quality of Life Instrument Short Form or the Quality of Life Scale, with a 0.5 standardised mean difference representing a moderate effect size (4 studies, 230 participants: SMD 0.51, 95% CI 0.19 to 0.83) , based on moderate certainty evidence. Cognitive behavioural therapy may reduce major depression symptoms (one study) and anxiety, and increase self‐efficacy (one study). Cognitive behavioural therapy studies did not report hospitalisation.
We found low‐certainty evidence that counselling may slightly reduce depressive symptoms measured with the Beck Depression Inventory (3 studies, 99 participants: MD ‐3.84, 95% CI ‐6.14 to ‐1.53) compared to usual care. Counselling reported no difference in HRQoL (one study). Counselling studies did not measure risk of major depression, suicide, or hospitalisation.
Exercise may reduce or prevent major depression (3 studies, 108 participants: RR 0.47, 95% CI 0.27 to 0.81), depression of any severity (3 studies, 108 participants: RR 0.69, 95% CI 0.54 to 0.87) and improve HRQoL measured with Quality of Life Index score (2 studies, 64 participants: MD 3.06, 95% CI 2.29 to 3.83) compared to usual care with low certainty. With moderate certainty, exercise probably improves depression symptoms measured with the Beck Depression Inventory (3 studies, 108 participants: MD ‐7.61, 95% CI ‐9.59 to ‐5.63). Exercise may reduce anxiety (one study). No exercise studies measured suicide risk or withdrawal from dialysis.
We found moderate‐certainty evidence that relaxation techniques probably reduce depressive symptoms measured with the Beck Depression Inventory (2 studies, 122 participants: MD ‐5.77, 95% CI ‐8.76 to ‐2.78). Relaxation techniques reported no difference in HRQoL (one study). Relaxation studies did not measure risk of major depression or suicide.
Spiritual practices have uncertain effects on depressive symptoms measured either with the Beck Depression Inventory or the Brief Symptom Inventory (2 studies, 116 participants: SMD ‐1.00, 95% CI ‐3.52 to 1.53; very low certainty evidence). No differences between spiritual practices and usual care were reported on anxiety (one study), and HRQoL (one study). No study of spiritual practices evaluated effects on suicide risk, withdrawal from dialysis or hospitalisation.
There were few or no data on acupressure, telephone support, meditation and adverse events related to psychosocial interventions.
Authors' conclusions
Cognitive behavioural therapy, exercise or relaxation techniques probably reduce depressive symptoms (moderate‐certainty evidence) for adults with ESKD treated with dialysis. Cognitive behavioural therapy probably increases health‐related quality of life. Evidence for spiritual practices, acupressure, telephone support, and meditation is of low certainty . Similarly, evidence for effects of psychosocial interventions on suicide risk, major depression, hospitalisation, withdrawal from dialysis, and adverse events is of low or very low certainty.
Plain language summary
Are psychosocial interventions effective for treating depression among people on dialysis?
What is the issue?
Depression is frequently experienced by people treated with dialysis. Dialysis patients consider treatments that help with depression to be a high priority. Despite that fact that psychosocial interventions have been shown to decrease depression in various chronic diseases, we are very uncertain about whether treatments prevent or treat depression for dialysis patients as studies are rare.
What did we do?
This evidence is current to June 2019. We searched the medical literature and identified 33 studies with 2056 participants treated by dialysis. Studies evaluated a range of possible treatments including acupressure, cognitive‐behavioural therapy (CBT), counselling, education, exercise, meditation, motivational interviewing, relaxation techniques, social activity, spiritual practices, support groups, telephone support, visualisation, and voice control compared to usual care or other psychosocial treatments. We also checked the quality of the information in the studies to learn how certain we could be about the results.
What did we find?
We are moderately certain that CBT, exercise, and relaxation techniques probably decrease symptoms of depression for patients treated with long‐term dialysis. Counselling may slightly decrease depression symptoms, while we are uncertain whether acupressure, telephone support, or meditation make any difference. We found moderate certainty evidence that CBT provides higher quality of life for dialysis patients. Studies did not measure effects of psychosocial treatments on major depression, suicide risk, and whether therapies made any difference to anxiety, hospital admissions, or withdrawal from dialysis treated is uncertain. Adverse events from treatment is very uncertain.
Some study authors did not report the methods for their studies clearly, so we could not be certain whether patients truly had a random chance of being in each treatment group or whether the trial results were assessed by people knowing which treatments that patients actually received. For most outcomes, we identified very few studies, which decreased our confidence in the results.
Conclusions
CBT, exercise, and relaxation techniques probably decrease depressive symptoms for dialysis patients while CBT also improves life quality. Counselling may slightly reduce depression among those receiving dialysis. We are not certain whether interventions prevent or treat major depression, anxiety, suicide risk, or withdrawal from dialysis care before death or whether psychological and social treatments have adverse effects.
Summary of findings
Background
Description of the condition
Depression is the most common psychological problem in patients undergoing dialysis (Finkelstien 2000; Kimmel 1993; Levenson 1991). Approximately one‐quarter of dialysis patients meet diagnostic criteria for major depression (Palmer 2013a; Szeifert 2012). The main factors that contribute to the develop of depressive symptoms are medications, reduction of physical function and dietary restrictions (Farrokhi 2014). The Beck Depression Inventory (BDI), Patient Health Questionnaire and Center for Epidemiologic Studies Depression Scale are validated tools for depression in people undergoing haemodialysis (HD), although the optimal screening tool is still uncertain (King‐Wing Ma 2016).
Depression can adversely affect the well‐being of patients receiving long‐term dialysis in several ways. Health‐related quality of life (HRQoL) of patients on chronic dialysis has been shown to correlate more strongly with depression than with dialysis adequacy measures (Martin 2000; Steele 1996). Depression in dialysis patients is associated with lower adherence to dialysis prescriptions (Kaveh 2001; Kimmel 1995) and recommended fluid restrictions (Everett 1993), which may lead to poorer clinical outcomes (Chilcot 2018). One study (Davies 2003) found a significant association between depression and intolerance to antihypertensive drugs because of nonspecific adverse effects in the general population. This may be of relevance to patients treated with long‐term dialysis as 80% of HD and 50% of peritoneal dialysis (PD) patients are hypertensive (Levey 1998) and hypertension may contribute to the burden of cardiovascular disease in the dialysis population. Depressed patients on PD have been shown to have higher rates of peritonitis (Juergenson 1996). Depression has been associated with increased death for dialysis patients (Hedayati 2010; Palmer 2013b; Weisbord 2014). The risk of hospitalisation is increased in patients with depression (Flythe 2017; Lopes 2002).
Description of the intervention
Depression can be treated by both physical (drugs and electro‐convulsive therapy (ECT)) and psychosocial interventions.
Psychosocial interventions can be defined as those interventions that provide psychological, emotional, or social support without using pharmacological substances. These may include counselling, social group support, cognitive‐behavioural therapy (CBT), relaxation or visualisation techniques, exercise, education, or individual social support including by telephone. Therapies may vary in their mode of delivery, intensity, or methodology, and level of contact with an individual therapist or support worker. Psychosocial interventions may help reduce distressing symptoms, increase coping strategies, increase social connectedness, assist in strategies to address specific disease‐related problems, and decrease anxiety and stress.
How the intervention might work
Several meta‐analysis of psychosocial interventions have found such therapies to be effective treatments for depression in the wider population (Churchill 2001; Dobson 1989; Robinson 1990; Scoggin 1994). In some studies, although participants did not report a specific diagnosis of depression when they were enrolled, psychosocial interventions were effective to prevent depression and impede the progression of the disease (Heshmatifar 2015). Dialysis patients, caregivers, and health professionals have identified depression as a critical outcome for evaluation in nephrology research (Tong 2017); however a previous version of this Cochrane review published in 2005 (Rabindranath 2005) did not identify any randomised controlled trials (RCTs) of psychosocial interventions to treat depression in the dialysis setting. Psychosocial interventions may be especially appropriate for patients on dialysis, since they avoid potential drug interactions and adverse effects of antidepressant medication. Psychosocial interventions are also known to be acceptable to patients and form a core recommendation in guidelines for the treatment of depression in adults (NICE 2018).
Why it is important to do this review
Depression is common for dialysis patients and may increase the substantial burden of symptoms and treatment. Patients, health professional and policy‐makers have identified research on the psychosocial impact of chronic kidney disease (CKD) as a priority (Tong 2015). This is an update of a Cochrane review that was first published in 2005, which identified no relevant studies of psychosocial interventions to treat depression in dialysis patients (Rabindranath 2005). Similarly, a Cochrane review in 2016 of antidepressants for treatment depression in adults with end‐stage kidney disease (ESKD) treated with dialysis included four studies including 170 participants (Palmer 2016). In very low certainty or ungraded evidence, antidepressant therapy had uncertain effects on quality of life (QoL), might reduce depression symptoms and might incur nausea.
Given the priority placed on psychosocial support for dialysis by patients and health professionals, the very low certainty of existing evidence for depression treatment, and the poor outcomes associated with depression in the dialysis setting, our aim was to provide an updated summary of the evidence of the benefits and potential harms of psychosocial interventions among adults with ESKD treated with dialysis.
Objectives
To assess the effect of using psychosocial interventions versus usual care or a second psychosocial intervention for preventing and treating depression in patients with ESKD treated with dialysis.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs (e.g. studies in which the method of assignment is based on alternation, date of birth or medical record number) of psychosocial interventions for prevention and treatment of depression among adults treated with long‐term dialysis.
Types of participants
Inclusion criteria
We included participants aged 18 years or above undergoing dialysis (either HD or PD) for ESKD with or without a diagnosis of depression.
Exclusion criteria
We excluded studies evaluating treatment for other psychiatric disorders including bipolar affective disorder.
Types of interventions
We included studies that compared a psychosocial intervention (such as cognitive and behavioural therapies, exercise training, and counselling) versus usual care or a second psychosocial intervention. We excluded studies comparing psychosocial interventions with drugs or ECT.
Types of outcome measures
We did not exclude studies that did not measure or report review outcomes.
We collected outcome data for depression by any measure and at any time point including incidence of major depression, depression (any severity), and depression score at end of treatment (any measure).
Primary outcomes
Depression (any measure)
HRQoL
Secondary outcomes
Anxiety (any measure)
Cognitive function (any measure)
Hospitalisation
Death from any cause
Suicide or suicide attempts
Adherence to dialysis treatment
Withdrawal from dialysis treatment
Withdrawal from trial intervention
Adverse events
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 21 June 2019.The specialised register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
For the original review, the American College of Physicians Database and PsycINFO were also searched.
Data collection and analysis
Selection of studies
For this 2019 update, two authors independently reviewed study titles and abstracts. Full text articles of studies considered potentially relevant were obtained and reviewed for eligibility by both authors. We consulted a third author to resolve discrepancies if necessary. We reassessed eligibility of studies excluded in the last version of the review (Rabindranath 2005) because of changes to the review criteria.
Data extraction and management
For this update, data extraction and assessment of risk of bias was performed by two authors using standardised data extraction forms. Disagreements not resolved by discussion between authors could be referred to a third author. Studies reported in languages other than English were translated before data extraction. Where more than one report of a study was identified, data were extracted from all reports. Where there were discrepancies between reports, data from the primary source were used. Study authors were contacted for additional information about studies.
We extracted the following information:
Methods: type of study design, setting, country, funding sources, time frame, duration of follow‐up
Participants: number of participants randomised to each group, number of analysed participants, inclusion criteria, exclusion criteria, age, sex, antidepressant medication
Interventions: details of intervention
Outcomes: all outcomes measured by study authors summary statistics of continuous data (mean, standard deviation (SD) and dichotomous data (number who experienced endpoint and number at risk).
Assessment of risk of bias in included studies
Two authors independently assessed methodological reporting using the Cochrane risk of bias assessment tool (Higgins 2011) (see Appendix 2).
We assessed the following:
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Dichotomous data
For dichotomous outcomes (hospitalisation, death, suicide or suicide attempts, withdrawal from trial treatment, withdrawal from dialysis, adverse events), results were expressed as risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
Where continuous scales of measurement were used to assess the effects of treatment (HRQoL, depression score), we used mean differences (MD) where the studies employed the same outcome measure. Where the studies used different scales to assess a given outcome, we used the standardised mean difference (SMD). We considered SMD of 0.2 a small effect size, SMD 0.5 a medium effect size and SMD 0.8 a large effect size (Cohen 1988).
Change scores and missing standard deviations
We included change scores and missing standard deviations (SD) according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Unit of analysis issues
Cross‐over studies
A primary concern with cross‐over trials is the "carry‐over" effect in which the effect of the intervention treatment influences the participant's response to the subsequent intervention in the second phase of the study. As a consequence, participants entering the second phase of the study may differ systematically from their "baseline" state even after a wash‐out phase. To minimise the carry‐over effect, we only extracted data from the first phase of the study, prior to cross‐over.
Dealing with missing data
Any further information required from the original author was requested by written correspondence and any relevant information obtained was to be included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I2 values was as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011).
Assessment of reporting biases
Reporting bias may occur when the direction and/or magnitude of a study's results influence a decision to publish the study. Empirical evidence suggests that studies with statistically significant findings are more likely to be submitted and accepted for publication, and may lead to over‐estimation of the true treatment effect. To assess whether studies in our meta‐analyses may be affected by publication bias, we planned to enter data into a funnel plot when a meta‐analysis included the results of at least 10 studies and in the absence of moderate or substantial heterogeneity (Higgins 2011). In this version of the review, there were insufficient data to generate funnel plots.
Data synthesis
Data were summarised using the random‐effects model and the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Although subgroup analyses have to be treated with caution, as they are hypothesis‐forming rather than hypothesis‐testing, we considered conducting a priori defined analyses in order to explore whether methodological and clinical differences between the trials may have systematically influenced the differences that were observed in the treatment outcomes.
Sensitivity analysis
We considered performing sensitivity analyses to explore the influence of the following factors on effect size, although in this version of the review, there were insufficient data to generate sensitivity analyses.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), or country.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b).
We used the GRADE process to assess the certainty of the body of the evidence associated with the following outcomes.
Major depression
Depression score (any measure)
Anxiety
QoL
Withdrawal from dialysis
Withdrawal from intervention
Death (any cause)
We constructed four 'Summary of Findings' tables for the following comparisons in this review.
CBT versus usual care
Counselling versus usual care
Exercise versus usual care
Relaxation techniques versus usual care
Spiritual practice versus usual care
One author completed the tables in consultation with a second author.
Results
Description of studies
Results of the search
Search results are shown in Figure 1. For this 2019 review update, we screened 72 titles and abstracts. We reclassified seven studies in seven publications from the 2005 review as eligible due to the expanded criteria in this review to include participants without depression at baseline. We removed 21 studies (21 publications) from the 2005 review as they were not RCTs. From the 2019 search update, we identified 26 new studies (33 reports) that met the review eligibility criteria (Characteristics of included studies); 11 studies (29 reports) were and excluded, and there are 7 ongoing studies.
1.
Study flow diagram
Included studies
See Characteristics of included studies.
We included 33 studies in 42 publications. One study (Cukor 2014) was a cross‐over study design in which participants were administered each of the study interventions sequentially without a washout period. One study (Dziubek 2016) was a quasi‐randomised study.
Study design, setting and characteristics
Study duration varied from three weeks to one year. Studies were conducted in fifteen different countries including Brazil (Duarte 2009), Canada (Thomas 2017), Greece (Kouidi 1997; Kouidi 2010; Ouzouni 2009), Indonesia (Sofia 2013), Iran (Babamohamadi 2017; Bahmani 2016; Espahbodi 2015; Heshmatifar 2015; Kargar Jahromi 2016), Jordan (Al Saraireh 2018), Malaysia (Hmwe 2015), Mexico (Lerma 2017), Poland (Bargiel‐Matusiewicz 2011; Bargiel‐Matusiewicz 2011a; Dziubek 2016), Singapore (HED‐SMART 2011), Taiwan (Lii 2007; Tsai 2015), UK (iDiD 2016; Krespi 2009; Vogt 2016), Tunisia (Frih 2017), Turkey (Sertoz 2009), and USA (Beder 1999; Carney 1987; Cukor 2014; Erdley 2014; Frey 1999; Leake 1999; Mathers 1999; Matthews 2001). One study (Thomas 2017) received at least some funding from companies, while 32 studies provided no specific details about funding sources.
Study participants
The 33 studies included 2056 randomised participants treated with HD. The sample size varied from 9 participants (Vogt 2016) to 235 participants (HED‐SMART 2011). One study (Sofia 2013) did not report the number of participants. The mean study age ranged from 36.1 years (Carney 1987) to 73.9 years (Erdley 2014), with a median of 52.4 years.
Interventions
Details of interventions in each study are presented in the Characteristics of included studies and in Table 6.
1. TIDieR framework of interventions descriptions for included studies.
| Study ID | Intervention | Control | Aim | What | How | Who, where, when | Tailoring/modification | How well: planned | How well: actual |
| Al Saraireh 2018 | CBT | Education | To assess the level of depression among patients undergoing HD and to compare the effectiveness of CBT versus psycho education | Traditional CBT sessions protocol was compared with psycho educational therapy | Both groups attended seven sessions of one hour. The control group discussed on education about stress management and relaxation, focusing on optimism, deep breathing and problem‐solving skills | In private rooms within the dialysis units by two expert researchers, for 7 sessions (3 months) | ‐ | All sessions were administered on one‐to‐one basis | 105 completed the study |
| Babamohamadi 2017 | Spiritual practice | Usual care | To examine the effect of the Holy Qur'an recitation (music therapy) on depressive symptoms | Holy Qur'an was recited aloud with the voice of Shateri (a well‐known actor of the Qur'an) | Listened to the Qur'an recitation (adds religious content to the pleasant, adding further to the relaxation, focus on pleasant sounds, and distraction from negative ruminations) using an MP3 player with headphones | Shateri provided the intervention 3 times a week for 20 min each during 1‐month, in the clinic | ‐ | ‐ | 54 completed the study |
| Bahmani 2016 | Counselling | Usual care | To examine a method that considers the needs of patients under special treatment such as dialysis | Combination of treatment including some elements of "existentialism" philosophy and a "cognitive" approach | Discussions on social support, face loneliness, isolation, death, losing the opportunity to job, losing education, emotional difficulties and the pain of treatment | Researcher provided the intervention for 12 sessions of 90 min, for 3 months, in the clinic | ‐ | ‐ | 20 completed the study |
| Bargiel‐Matusiewicz 2011 | Voice recording | Usual care | To assess the influence of a psychological intervention on the cognitive appraisal | Based on the principles of the Ericksonian therapy and used therapeutic metaphors | Listened to a CD (20 min) with a recorded psychological intervention | Twice a day for 3 weeks. Researchers carried out the intervention in a natural environment | ‐ | ‐ | 60 completed the study |
| Bargiel‐Matusiewicz 2011a | Counselling | Usual care | To assess if psychological intervention improve the level of acceptance of illness | Participants attended in meetings | ‐ | Psychologist provided the intervention for 5 weeks | ‐ | ‐ | The number of subject who completed the study was not clear |
| Beder 1999 | Counselling | Usual care | To provide existing dialysis programs with a supported model of service | Basic social support consisted of a psycho‐educational and support components | Providing information on patients' needs and received additional social support component | Renal social workers provided the intervention in the hospital for 3 months | Helping the patients in mobilizing and assessing and accessing additional supports as needed | ‐ | 46 completed the study |
| Carney 1987 | Exercise | Support group | To assess the effect of exercise training on the psychosocial rehabilitation | 5‐min sessions on a stationary bicycle ergometer and fast walking interspersed with 5‐mi rest periods | All patients were provided with bicycle ergometers for home use and jogging 1 to 2 laps | 3 time a week at home for 45 to 60 min for 6 months | ‐ | ‐ | 17 completed the study |
| Cukor 2014 | CBT | Usual care | To test the efficacy of an individual chairside CBT | Standard CBT intervention for depression was adapted for HD patients | At the end of the first period, treatments were inverted | Psychologists provided the intervention in chairside, during dialysis, for 3 months | ‐ | ‐ | 59 completed the study |
| Duarte 2009 | CBT | Usual care | To assess the effectiveness of CBT in ESKD patients with a diagnosis of major depression | Educating patients on several aspects of kidney disease, dialysis, depression | Provided self‐monitoring of mood status; cognitive restructuring; programming pleasant activities; training on social abilities; exercises | Psychologists provided the intervention (1 hour and 30 min) during dialysis, for 3 months | ‐ | Another psychologist checked written records to monitor the intervention | 74 completed the study |
| Dziubek 2016 | Exercise | Exercise | To evaluate the effects of exercise on depression and anxiety, and compared 2 different types of training in dialysis | Endurance and resistance training were performed | Ergonometer group performed short warm‐up, 10 to 15 min of training using a motorized exercise therapy device and 35 to 50 min ride on the cycle ergometer. Resistance group performed strength exercises with weights, balls and Thera Bands and final relaxation | Nephrologist and cardiologist supervised the training performed 3 times a week for 6 months in the clinic | The number of evolutions and load were constant, individually tailored to the patient depending on the tolerance of the exercise | Heart rate, blood pressure and the degree of fatigue were monitored | 28 participants completed the study |
| Erdley 2014 | Counselling | Usual care | To assess the efficacy of problem‐solving therapy in reducing depressive symptoms. | Providing intervention to manage problems and improve individual coping and problem‐solving ability. | Provided orientation to problem solving, evaluating and choosing solutions, and identifying steps to achieve solutions. | Meeting with social workers were provided once weekly in the dialysis unit for 1 hour, for 6 weeks. | ‐ | ‐ | 33 completed the study. |
| Espahbodi 2015 | Education | Usual care | To investigate psychological impacts of psycho education on anxiety and depression | Psycho education sessions | Sessions of anatomy, pathophysiology, variety of treatments, stress management, problem‐solving skills, and muscle relaxation | 3 sessions of one‐hour, in the clinic with a psychiatrist was delivered for 1 month | ‐ | ‐ | 55 completed the study |
| Frey 1999 | Exercise | Usual care | To evaluate the difference in kilocalorie and protein intake in ESKD patients who perform or not perform exercise | Exercise patients cycled on stationary bicycle ergometers | 5 minutes warm‐up and 5 minutes cool down. Cycling periods were followed by gradually increasing tension | Investigator supervised the intervention for 12 weeks | ‐ | ‐ | All participants completed the study |
| Frih 2017 | Spiritual practice | Exercise | To determine whether listening to Holy Qur'an recitation improve the effects of exercise on physiological measures | Resistance training consisted of dynamic exercises. Endurance training consisted of ergo cycle exercise. The intervention group listened to the Holy Qur'an | The Holy Qur'an was recited by the reader Al‐Dosari, who reads with a relaxing and calming voice. The recitation was played through headphones on MP3 | The reader Al‐Dosari recited and participants listened verses 3 times a week during 24 weeks, (20 min), in the clinic | The volume was adjusted according to the patient’s comfort | A Borg score of 5 to 6 for dyspnoea or fatigue was set as a target | All participants completed the study |
| HED‐SMART 2011 | Counselling | Usual care | To determine the efficacy of the intervention on biochemical markers, clinical status, QoL and patient satisfaction | Participants performed the NKF‐NUS self‐management intervention to take control of their condition | 3 main interactive sessions held every two weeks and one booster session Intervention components will include problem solving, overcoming barriers, challenging beliefs, conducting brainstorming sessions, goal setting, and reinforcement. Participants also received an educational booklet | 4 sessions (90 min each) were facilitated by two health care professionals | Used feedback, modelling of problem‐solving strategies through group support and guidance for individual self‐management efforts | Patients were contacted by telephone to assess the progress they were making with their goals | All participants completed the study |
| Heshmatifar 2015 | Relaxation | Usual care | To assess the efficacy of Benson technique to improve depressive symptoms | The training sessions included discussions about relaxation. The participants were asked to perform the relaxation exercises | Participants performed exercises for 20 minutes. The training method, an educational pamphlet and a CD were handed to subjects to perform the exercises | Researcher provided the intervention in the clinic, for 1 month | ‐ | Subjects’ compliance was ensured through text messages | All participants completed the study |
| Hmwe 2015 | Acupressure | Usual care | To evaluate the effects of acupressure on depression, stress, anxiety and general psychological distress | Acupoints for depression, anxiety, and stress was based on the concepts of Chinese medicine | Acupressure is performed by applying consistent fingertip pressure on selected acupoints with rotational movements | Investigator provided acupressure 3 times/ week over 4 weeks, in the clinic | ‐ | A supervisor monitored to ensure that the intervention was performed as the protocol declared | 102 the study. 108 were analysed (intention to treat) |
| iDiD 2016 | Telephone support + CBT | CBT | To explore adherence and examine the efficacy of online CBT sessions and therapist support calls | All patients had access to the iDiD online intervention Patients in the supported arm received three 30 min telephone calls | iDiD sessions were designed to last approximately 60 min. iPads were available at dialysis units. The researcher guided the patient to the most relevant components of sessions | Telephone support was delivered by a trained psychological well‐being researcher | For 6 patients was generate an email address and provide brief Internet education, thus these patients received a higher degree of technical support and face to face contact | Patients received reminders. Support calls were audio recorded for supervision and checks | 23 completed the study |
| Kargar Jahromi 2016 | Telephone support | Usual care | To evaluate the effect of nurse‐led telephone follow‐up on depression, anxiety and stress | The intervention group received telephone follow‐up after dialysis and conventional treatment | Key subjects: communication, cognition/development, breathing/circulation, nutrition, elimination, sleep, tissue, pain, sexuality, activity and psychosocial/spirituality/culture | Researchers provided every session (30 minutes), for a month | ‐ | The content of the call followed a script to ensure consistency | 54 completed the study |
| Kouidi 1997 | Exercise | Usual care | To assess the psychosocial effects of exercise training. | The intervention was the exercise training rehabilitation program. | The telemetric spirometer assessed the maximal oxygen consumption during the performance. | Psychologist and trainer supervised the exercise, for 6 months | The intensity and duration of the exercise sessions was gradually increased. | ‐ | 31 completed the study. |
| Kouidi 2010 | Exercise | Usual care | To investigate the effects of exercise on emotional parameters in HD patients | The intervention was the exercise training rehabilitation program | 5 min warm‐up, 30 to 60 min of cycling, 20 min strengthening time followed by a 5 min cooling off | 3 times a week during the first 2 hours of HD session | ‐ | ‐ | 38 completed the study |
| Krespi 2009 | Relaxation |
Control 1 Voice control Control 2 Usual care |
To investigate the effects of relaxation | The experimental group received specific visual imagery (using metaphors), delivered by audiotapes | Each tape lasted for 25 minutes, relaxation and imagery took 20 minutes for the techniques and 5 minutes for the specific imaging technique | Researchers provided the intervention 3 to 4 times/week for 6 weeks, during HD | ‐ | The procedures support patients and answer questions | 103 completed the study |
| Leake 1999 | Motivational interviewing |
Control 1 Motivational interviewing Control 2 Video recording |
To evaluate the therapeutic benefits of strategic self‐presentation | Patients participated in a videotaped interview where they portrayed their coping strategies | Patients were asked to provide candid testimonies about their own difficulties on problems with chronic illness | The researchers provided the interview for 1 month | ‐ | Patients were given the opportunity to revise the video tape | 41 completed the study |
| Lerma 2017 | CBT | Usual care | To reduce mild and moderate depression and anxiety symptoms in patients | Intervention consisted of positive self‐reinforcement, deep breathing, muscle relaxation, and cognitive restructuring | All components of the programme were adapted to the clinical context of patients with ESRD using images, examples, words, exercises, and everyday scenarios that were relevant to them | The therapist provided the intervention in the clinic during 5 weekly sessions that lasted 2 hours each | Patients who were identified as having severe depression symptoms (BDI > 29 points) were referred for appropriate psychiatric evaluation and care | The therapist recorded comments and received feedback from an expert | 49 completed the study |
| Lii 2007 | CBT | Usual care | To investigate the effects of intervention on depression and QoL | The treatment helped participants to evaluate problem and solve irrational beliefs | Self‐management of depression; restructuring beliefs; stress management; and health education | Nurses provided the intervention in the clinic, once a week, for 2 hours | ‐ | ‐ | 48 completed the study |
| Mathers 1999 | Education | Usual care | To determine if the psychosocial education sessions had an effect on the adaptation level | 7 audiotapes and a companion text module, provided information | Each module consisted in several questions to help participants focus on the topic of the day | Investigators provided 7 sessions, 2 days a week (20 min each), for 4.5 weeks | The investigator was available to answer any questions | ‐ | 6 completed the study |
| Matthews 2001 | Spiritual practice |
Control 1 Visualisation Control 2 Usual care |
To explore the effect of intercessory prayer, positive visualisation, and outcome expectancy | Christian prayer group and transpersonal (nonreligious) positive visualisation group improved illness | In the intervention group there were 6 intercessors who preyed. In the positive visualisation group, 6 psychology interns focused on patients' problems, using audiotapes | The prayers and the visualisation process took 5 to 15 min/5 days, for 6 weeks | ‐ | An individual checked if the intercessory prayer was performed | The number of subject who completed vary (absent during data collection) |
| Ouzouni 2009 | Exercise | Usual care | To assess the effects of intradialytic exercise training on HRQoL | Patients in the exercise group followed an exercise rehabilitation programme | Each exercise session included 30 min of cycling and 30 min of strengthening and flexibility exercises (20 min cycling at desired workload and 5 min cool‐down) | Physiologists provided the exercised 3 times weekly (90 min each), per 10 months (in the centre) | For the cycling exercise specific devices, which were adjusted to each patient’s bed, were used | Their cardiac rhythm and blood pressure were monitored continuously | 33 completed the study |
| Sertoz 2009 | Social activity | Usual care | To investigate the impact of social activity on anxiety, depression, self‐esteem and QoL | Patients were engaged in a play. The play chosen was one by Tuncay Cucenoglu (drama organisation) | “The Painter”, by displaying the social structure and diversity of ideas, maintains the audience’s curiosity. The control group participated as the audience | At the baseline and after 4 months | ‐ | ‐ | The number of subject who completed the study was not clear |
| Sofia 2013 | Relaxation | Control | To determine the effect of Latihan Pasrah Diri (LPD) on QoL in HD patients with depression | The intervention group received LPD | LPD was a method combining relaxation and remembrance with a focus on breathing exercises and words contained in the "zirk" (relaxation and repetitive prayer) | 21 days | ‐ | ‐ | ‐ |
| Thomas 2017 | Meditation | Usual care | To determine whether the intervention reduced depression and anxiety symptoms | Chairside meditative practices were performed 4 meditation techniques drawn from mindfulness‐based. |
Cognitive therapy were practiced in alternating fashion, on the basis of patient preference. In these techniques, the participant is guided to direct their attention toward specific elements of their experience | Interventionists performed 3 times/week, during HD, lasting 10 to 15 min, for 8 weeks | ‐ | Patients were encouraged to practice the techniques at home, but did not have formal logs | 32 completed the study |
| Tsai 2015 | Relaxation | Control | To examine the efficacy of a nurse‐led, in reducing depressive symptoms and improving sleep | The dialysis nurse administered the audio device–guided breathing training in a quiet room | Patients listened to pre‐recorded instructions on breathing technique and then practiced the breathing exercise | A trained nurse provided the intervention in the clinic: 8 sessions, twice weekly for 4 weeks | Each patient received an individual coaching session | The nurse supervised to ensure that participants performed them correctly | 57 completed the study |
| Vogt 2016 | Counselling | Usual care | To examine the feasibility and appropriateness of the intervention | Participants received Acceptance and Commitment Therapy (ACT) | The intervention was based on a self‐help manual with weekly telephone support | 6 weeks. | ‐ | ‐ | ‐ |
CBT ‐ cognitive behavioural therapy; ESKD ‐ end‐stage kidney disease; HD ‐ haemodialysis
Interventions included acupressure (Hmwe 2015) (108 participants), CBT in five studies (Al Saraireh 2018; Cukor 2014; Duarte 2009; Lerma 2017; Lii 2007) (405 participants), counselling in six studies (Bahmani 2016; Bargiel‐Matusiewicz 2011a; Beder 1999; Erdley 2014; HED‐SMART 2011; Vogt 2016) (527 participants), education in two studies (Espahbodi 2015; Mathers 1999) (70 participants), exercise in six studies (Carney 1987; Dziubek 2016; Frey 1999; Kouidi 1997; Kouidi 2010; Ouzouni 2009) (190 participants), meditation in Thomas 2017 (41 participants), motivational interviewing in Leake 1999 (42 participants), relaxation in four studies (Heshmatifar 2015; Krespi 2009; Sofia 2013; Tsai 2015) (287 participants), social activity in Sertoz 2009 (31 participants), spiritual practice in three studies (Babamohamadi 2017; Frih 2017; Matthews 2001) (208 participants), telephone support in Kargar Jahromi 2016 (60 participants), telephone support and CBT in iDiD 2016 (25 participants) and audio‐recording of a psychological intervention in Bargiel‐Matusiewicz 2011 (62 participants).
Three studies reported three treatment groups. In Leake 1999, motivational interviewing was compared with another motivational interviewing or video recording. In Krespi 2009, relaxation was compared with voice control or usual care. Matthews 2001 compared spiritual practice with visualisation or usual care.
The methods for implementation, tailoring, and measurement of adherence of interventions are provided in Table 6 using a TIDIeR [Template for Intervention Description and Replication] checklist (Hoffmann 2014).
Excluded studies
We excluded 14 studies (33 reports) as the intervention or treatment comparison were judged as not eligible, the study did not include the population of interest, or the study was not an RCT. See Characteristics of excluded studies.
Ongoing studies
Our search identified seven studies that have yet to been completed (DOHP 2016; NCT02011139; NCT03162770; NCT03330938; NCT03406845; van der Borg 2016; WICKD 2019). Study comparisons include:
Structured information/workbook, psychosocial and educational supports compared to skills building to usual care (DOHP 2016)
CBT for 12 weeks compared to usual care (NCT02011139)
Meditation for 8 weeks compared to usual care (NCT03162770)
CBT together with resilience training for eight weeks compared to CBT alone (NCT03330938)
Meditation for eight weeks compared to a program of health education, diet, music, exercise, and positive life changes (NCT03406845)
Counselling by a social worker for 16 weeks compared to usual care (van der Borg 2016)
Early treatment with motivational care planning compared to delayed treatment with motivational care planning and usual care (WICKD 2019).
Risk of bias in included studies
The risk of bias for studies overall are summarised in Figure 2 and the risk of bias in each individual study is reported in Figure 3.
2.
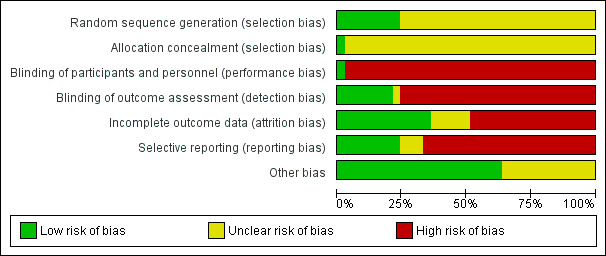
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
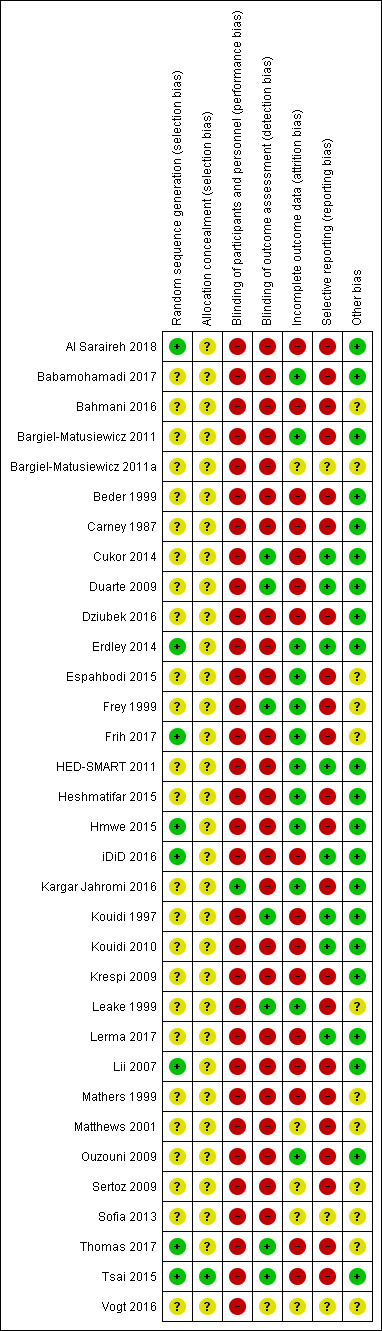
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Methods for generating the random sequence were deemed to be at low risk of bias in eight studies (Al Saraireh 2018; Erdley 2014; Frih 2017; Hmwe 2015; iDiD 2016; Lii 2007; Thomas 2017; Tsai 2015). In the remaining 25 studies, the method for generating the random sequence was unclear.
Allocation concealment was adjudicated as low risk of bias in one study (Tsai 2015). The risk of bias for allocation concealment was unclear in the remaining 32 studies.
Blinding
One study was blinded and considered to be at low risk of bias for performance bias (Kargar Jahromi 2016). The remaining 32 studies were not blinded and were considered at high risk of performance bias.
Blinding of outcome assessment was assessed to be at low risk in seven studies (Cukor 2014; Duarte 2009; Frey 1999; Kouidi 1997; Leake 1999; Thomas 2017; Tsai 2015). The risk of bias for blinding of outcome assessment was unclear in one study (Vogt 2016). The remaining 25 studies were considered at high risk of detection bias.
Incomplete outcome data
Twelve studies met criteria for low risk of attrition bias (Babamohamadi 2017; Bargiel‐Matusiewicz 2011; Erdley 2014; Espahbodi 2015; Frey 1999; Frih 2017; HED‐SMART 2011; Heshmatifar 2015; Hmwe 2015; Kargar Jahromi 2016; Leake 1999; Ouzouni 2009). Sixteen studies were considered at high risk of attrition bias when there was differential loss to follow‐up between treatment groups and high attrition rates (Al Saraireh 2018; Bahmani 2016; Beder 1999; Carney 1987; Cukor 2014; Duarte 2009; Dziubek 2016; iDiD 2016; Kouidi 1997; Kouidi 2010; Krespi 2009; Lerma 2017; Lii 2007; Mathers 1999; Thomas 2017; Tsai 2015). In the remaining five studies, attrition bias was considered unclear. Loss to follow‐up was commonly due to death, hospitalisation, transplantation, withdrawal of consent, or medical problems.
Selective reporting
Eight studies reported expected and clinically‐relevant outcomes and were deemed to be at low risk of bias (Cukor 2014; Duarte 2009; Erdley 2014; iDiD 2016; HED‐SMART 2011; Kouidi 1997; Kouidi 2010; Lerma 2017). The risk of bias for reporting bias was unclear in three studies (Bargiel‐Matusiewicz 2011a; Sofia 2013; Vogt 2016). The remaining 22 studies did not report patient‐centred outcomes of life participation, fatigue, dialysis withdrawal, adverse events, or death.
Other potential sources of bias
Twenty‐one studies appeared to be free from other sources of bias (Al Saraireh 2018; Babamohamadi 2017; Bargiel‐Matusiewicz 2011; Beder 1999; Carney 1987; Cukor 2014; Duarte 2009; Dziubek 2016; Erdley 2014; HED‐SMART 2011; Heshmatifar 2015; Hmwe 2015; iDiD 2016; Kargar Jahromi 2016; Kouidi 1997; Kouidi 2010; Krespi 2009; Lerma 2017; Lii 2007; Ouzouni 2009; Tsai 2015). It was unclear whether the remaining 12 studies had other sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Cognitive‐behavioural therapy versus usual care.
| Cognitive‐behavioural therapy (CBT) versus with usual care for depression in people treated with dialysis | ||||||
|
Patient or population: people with ESKD Settings: dialysis Intervention: CBT Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | CBT | |||||
|
Major depression Mini International Neuropsychiatric Interview (MINI) (median follow‐up: 39.6 weeks) |
Not estimable1 | Not estimable | Not estimable | Insufficient data observations | Not estimable | Studies were not designed to measure effects of Cognitive behavioural therapy on major depression |
|
Depression (any severity, including mild, moderate and severe depression) Investigators measured depression using the Beck Depression Inventory (BDI). A higher score is indicative of more depressive symptoms. (median follow‐up: 17.7 weeks) |
The mean Beck Depression Inventory ranged across control groups from 14.5 to 21.39 | The mean Beck Depression Inventory score in the intervention groups was 6.10 lower (95% CI ‐8.63 to ‐3.57) |
MD ‐6.10 (95% CI ‐8.63 to ‐3.57) |
230 (4) | ⊕⊕⊕⊝ moderate 2 | Cognitive behavioural therapy probably decreases depressive symptoms |
|
Health‐related quality of life Investigators measured health‐related quality of life using different instruments: Quality of Life Scale (QoL) and HRQoL Short Form‐36, Kidney Disease and Quality of Life‐Short Form (KDQOL‐SF‐36) (median follow‐up: 17.7 weeks). A higher score is indicative of higher perceived of QoL. |
The mean quality of life score ranged across control groups from 40.46 to 110.6 | The mean QoL score in the intervention groups was 0.51 standard deviations higher (95% CI 0.19 to 0.83) |
SMD 0.51 (95% CI 0.19 to 0.83) |
230 (4) | ⊕⊕⊕⊝ moderate 2 | As a rule of thumb, 0.2 SMD represents a small effect size, 0.5 SMD a moderate effect size and 0.8 SMD a large effect size. Cognitive behavioural therapy probably moderately improves health‐related quality of life |
|
Anxiety Beck Anxiety Inventory (BAI) (median follow‐up: 9 weeks) |
Not estimable3 | Not estimable | Not estimable | Insufficient data observations | Not estimable. | Studies were not designed to measure effects of cognitive behavioural therapy on anxiety |
| Withdrawal from dialysis | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable. | Studies were not designed to measure effects of cognitive behavioural therapy on withdrawal from dialysis |
| Withdrawal from intervention | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable. | Studies were not designed to measure effects of cognitive behavioural therapy on withdrawal from intervention |
|
Death (any cause) (median follow‐up: 24.3 weeks) |
13.8 per 1000 | ‐1.2 per 1000 (95% CI 4.83 to 47.61) | RR 1.09 (95% CI 0.35 to 3.45) | 145 (2) | ⊕⊕⊝⊝ low 4, 5 | It is uncertain whether CBT makes any difference to death (any cause) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ESKD: end‐stage kidney disease; CI: Confidence interval; MD: mean difference; SMD: standardised mean difference; RR: Risk Ratio; HRQoL: health‐related quality of life | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The estimated risk of major depression was not estimable as a single study reported this outcome.
2 All studies had unclear risks of bias for allocation concealment and high risk of blinding of participants or investigators. Two studies (Cukor 2014; Duarte 2009) reported low risk methods for blinding of outcome assessment.
3 The estimated risk of anxiety was not estimable as a single study reported this outcome.
4 All studies had high or unclear risks of bias for allocation concealment and blinding of participants or investigators. One out of two studies reported low risk methods for blinding of outcome assessment.
5 The certainty in the evidence was downgraded due to imprecision in the treatment estimates, consistent with benefit or harm.
Summary of findings 2. Counselling versus usual care.
| Counselling versus usual care for depressive outcomes in people treated with dialysis | ||||||
|
Patient or population: people with ESKD Settings: dialysis Intervention: counselling1 Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Counselling | |||||
| Major depression | No data observations | Not estimable | No observations. | Insufficient data observations | Not estimable | Studies were not designed to measure effects of counselling on major depression |
|
Depression (any severity, including mild, moderate and severe depression) Investigators measured depression using the Beck Depression Inventory (BDI). A higher score is indicative of more depressive symptoms. (median follow‐up: 13.2 weeks) |
The mean depression score ranged across control groups from ‐2.43 to 18.54 | The mean depression score in the intervention groups was 3.84 lower (95% CI ‐6.14 to ‐1.53) |
MD ‐3.84 (95% CI ‐6.14 to ‐1.53) |
99 (3) | ⊕⊕⊝⊝ low 2,3 | Counselling may decrease depressive symptoms |
|
HRQoL Kidney Disease Quality of Life (KDQOL‐36) (median follow‐up: 6 weeks) |
Not estimable4 | Not estimable | Not estimable | Insufficient data observations | Not estimable | Studies were not designed to measure effects of counselling on health related quality of life |
| Anxiety | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of counselling on anxiety |
| Withdrawal from dialysis | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of counselling on withdrawal from dialysis |
|
Withdrawal from intervention (median follow‐up: 6 weeks) |
Not estimable5 | Not estimable | Not estimable | Insufficient data observations | Not estimable | Studies were not designed to measure effects of counselling on withdrawal from intervention |
|
Death (any cause) (median follow‐up: 22.8 weeks) |
2.5 per 1000 | ‐1.7 per 1000 (95% CI 0.8 to 22.03) | RR 1.69 (95% CI 0.32 to 8.81) | 270 (2) | ⊕⊕⊝⊝ low 2,6 | It is uncertain whether counselling makes any difference to death |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ESKD: end‐stage kidney disease; CI: Confidence interval; MD: mean difference; RR: Risk Ratio; HRQoL: health‐related quality of life | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Counselling included existentialism philosophy and cognitive approach, counselling component, problem‐solving therapy and NKF‐NUS self‐management intervention.
2 All studies had high or unclear risks of bias for allocation concealment, blinding of participants or investigators, and blinding of outcome assessment.
3 The certainty in the evidence was downgraded due to imprecision in the treatment estimates for the limited number of participants, according with Optimal Information Size (OIS).
4 The estimated risk of quality of life was not estimable as a single study reported this outcome.
5 The estimated risk of withdrawal from intervention was not estimable as a single study reported this outcome.
6The certainty in the evidence was downgraded due to imprecision in the treatment estimates, consistent with benefit or harm.
Summary of findings 3. Exercise versus usual care.
| Exercise versus usual care for depression in people treated with dialysis | ||||||
|
Patient or population: people with ESKD Settings: dialysis Intervention: exercise Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Exercise | |||||
|
Major depression (median follow‐up: 44 weeks) |
86.02 per 1000 | 45.59 per 1000 (95% CI 23.23 to 69.68) |
RR 0.47 (95% CI 0.27 to 0.81) |
108 (3) | ⊕⊕⊝⊝ low 1,2 | Exercise may decrease risk of major depression |
|
Depression (any severity, including mild, moderate and severe depression) Investigators measured depression using the Beck Depression Inventory (BDI). A higher score is indicative of more depressive symptoms (median follow‐up: 44 weeks) |
The mean depression score ranged across control groups from 19.4 to22.1 | The mean depression score in the intervention groups was 7.61 lower (95% CI ‐9.59 to ‐5.63) |
MD ‐7.61 (95% CI ‐9.59 to ‐5.63) |
108 (3) | ⊕⊕⊕⊝ moderate 1 | Exercise probably decreases depressive symptoms |
|
HRQoL Investigators measured health related quality of life using the Quality of Life Index (QLI). A higher score is indicative of higher perceived of QoL (median follow‐up: 35.2 weeks) |
The mean QoL score ranged across control groups from 5.6 to 6.3 | The mean QoL score in the intervention groups was 3.06 higher (95% CI 2.29 to 3.83) |
MD 3.06 (95% CI 2.29 to 3.83) |
64 (2) | ⊕⊕⊝⊝ low 1,3 | Exercise may improve HRQoL |
|
Anxiety Hospital Anxiety and Depression Scale (HADS) (median follow‐up: 52.1 weeks) |
Not estimable4 | Not estimable | Not estimable | Insufficient data observations | Not estimable | Studies were not designed to measure effects of exercise on anxiety |
| Withdrawal from dialysis | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of exercise on withdrawal from dialysis |
| Withdrawal from intervention | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of exercise on withdrawal from intervention |
| Death (any cause) | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of exercise on death |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ESKD: end‐stage kidney disease; CI: Confidence interval; RR: Risk Ratio; MD: mean difference; HRQoL: health‐related quality of life | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All studies had high or unclear risks of bias for allocation concealment and blinding of participants or investigators. One study out of four reported low risk methods for blinding of outcome assessment.
2 There was moderate heterogeneity in the findings of available studies.
3 The certainty in the evidence was downgraded due to imprecision in the treatment estimates for the limited number of participants, according with Optimal Information Size (OIS).
4 The estimated risk of anxiety was not estimable as a single study reported this outcome.
Summary of findings 4. Relaxation techniques versus usual care.
| Relaxation techniques versus usual care for depression in people treated with dialysis | ||||||
|
Patient or population: people with ESKD Settings: dialysis Intervention: relaxation techniques1 Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Relaxation techniques | |||||
| Major depression | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of relaxation techniques on major depression |
|
Depression (any severity, including mild, moderate and severe depression) Investigators measured depression using the Beck Depression Inventory (BDI). A higher score is indicative of more depressive symptoms. (median follow‐up: 4.2 weeks) |
The mean depression score ranged across control groups from 9.56 to 30.83 | The mean depression score in the intervention group was 5.77 lower (95% CI ‐8.76 to ‐2.78) |
MD ‐5.77 (95% CI ‐8.76 to ‐2.78) |
122 (2) | ⊕⊕⊕⊝ moderate 2 | Relaxation techniques probably decrease depressive symptoms |
|
HRQoL Investigators measured health‐related quality of life using the Health Status Questionnaire Short Form (SF‐36) (median follow‐up: 6 weeks) |
Not estimable3 | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of relaxation techniques on HRQoL |
| Anxiety | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of relaxation techniques on anxiety |
| Withdrawal from dialysis | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of relaxation techniques on withdrawal from dialysis |
| Withdrawal from intervention | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of relaxation techniques on withdrawal from intervention |
| Death (any cause) | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of relaxation techniques death |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ESKD: end‐stage kidney disease; CI: Confidence interval; MD: mean difference; HRQoL: health‐related quality of life | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Relaxation techniques included Benson relaxation technique and nurse‐led breathing training.
2 Studies had high or unclear risks of bias for allocation concealment, blinding of participants or investigators, and blinding of outcome assessment.
3 Treatment effects on HRQoL was not estimable as a single study reported this outcome.
Summary of findings 5. Spiritual practice versus usual care.
| Spiritual practice versus usual care for depression in people treated with dialysis | ||||||
|
Patient or population: people with ESKD Settings: dialysis Intervention: spiritual practice1 Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Spiritual practice | |||||
| Major depression | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of spiritual practice on major depression |
|
Depression (any severity, including mild, moderate and severe depression) Investigators measured depression using different instruments: Beck Depression Inventory (BDI) and Brief Symptom Inventory (BSI). A higher score is indicative of more depressive symptoms. (median follow‐up: 5.2 weeks) |
The mean depression score ranged across control groups from 31.6 to 53.53 | The mean depression score in the intervention groups was 1.00 standard deviations lower (95% CI ‐3.52 to 1.53) |
SMD ‐1.00 (95% CI ‐3.52 to 1.53) |
116 (2) | ⊕⊝⊝⊝ very low 2,3,4 | As a rule of thumb, 0.2 SMD represents a small effect size, 0.5 SMD a moderate effect size and 0.8 SMD a large effect size. As SMD is ‐1.00, it is very uncertain whether spiritual practice makes any difference to depressive symptoms |
|
Health‐related quality of life Health Status Questionnaire Short Form (SF‐36) (median follow‐up: 6 weeks) |
Not estimable5 | Not estimable | Not estimable | Insufficient data observations | Not estimable | Studies were not designed to measure effects of spiritual practice on quality of life |
|
Anxiety Brief Symptom Inventory (BSI) (median follow‐up: 6 weeks) |
Not estimable6 | Not estimable | Not estimable | Insufficient data observations | Not estimable | Studies were not designed to measure effects of spiritual practice on anxiety |
| Withdrawal from dialysis | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of spiritual practice on withdrawal from dialysis |
| Withdrawal from intervention | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of spiritual practice on withdrawal from intervention |
| Death (any cause) | No data observations | Not estimable | No observations | Insufficient data observations | Not estimable | Studies were not designed to measure effects of spiritual practice on death |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ESKD: end‐stage kidney disease; CI: Confidence interval; SMD: standardised mean difference; HRQoL: health‐related quality of life | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Spiritual practice included Holy Qur'an recitation and Christian prayer.
2 All studies had high or unclear risks of bias for allocation concealment, blinding of participants or investigators, and blinding of outcome assessment.
3 There was substantial heterogeneity in the findings of available studies (two downgrades).
4 The certainty in the evidence was downgraded due to imprecision in the treatment estimates, consistent with benefit or harm.
5 The estimated risk of anxiety was not estimable as a single study reported this outcome.
6 The estimated risk of quality of life was not estimable as a single study reported this outcome.
See: Table 1 CBT versus to usual care; Table 2 Counselling versus usual care; Table 3 Exercise versus usual care; Table 4 Relaxation techniques versus usual care; Table 5 Spiritual practice versus usual care.
Acupressure versus usual care
Hmwe 2015 reported outcome measures for acupressure compared to usual care for four weeks. Depression was assessed as a continuous outcome either as major (or severe) depression and depression (end of treatment), reported as a score. Since the Depression Anxiety Stress Scales (DASS) score showed that all participants reported depressive symptoms at the baseline, the intervention was delivered to treat depression. The study measured major depression and HRQoL using the General Health Questionnaire (GHQ), and depression, anxiety and stress scores using DASS.
Hmwe 2015 (108 participants) reported no differences between acupressure and usual care for major depression (Analysis 1.1: GHQ score MD ‐0.89, 95% CI ‐2.42 to 0.64), depression (Analysis 1.2: DASS score MD ‐0.93, 95% CI ‐3.95 to 2.09), anxiety (Analysis 1.4: DASS score MD 0.23, 95% CI ‐2.21 to 2.67), stress (Analysis 1.5: DASS score MD ‐1.48, 95% CI ‐4.32 to 1.36), withdrawal from treatment (Analysis 1.6: RR 7.00, 95% CI 0.37 to 132.35), and hospitalisation (Analysis 1.7: RR 3.00, 95% CI 0.12 to 72.05). Acupuncture may improve HRQoL (Analysis 1.3: GHQ score MD ‐5.00, 95% CI ‐9.59 to ‐0.41). Adverse events of acupressure were rarely reported (Table 7).
1.1. Analysis.
Comparison 1 Acupressure versus usual care, Outcome 1 Major depression.
1.2. Analysis.
Comparison 1 Acupressure versus usual care, Outcome 2 Depression.
1.4. Analysis.
Comparison 1 Acupressure versus usual care, Outcome 4 Anxiety.
1.5. Analysis.
Comparison 1 Acupressure versus usual care, Outcome 5 Stress.
1.6. Analysis.
Comparison 1 Acupressure versus usual care, Outcome 6 Withdrawal from intervention.
1.7. Analysis.
Comparison 1 Acupressure versus usual care, Outcome 7 Hospitalisation.
1.3. Analysis.
Comparison 1 Acupressure versus usual care, Outcome 3 Health‐related quality of life.
2. Table of studies reporting adverse events.
| Study ID | Intervention | Control | Adverse events in the intervention arm | Adverse events in the control arm | Comments |
| HED‐SMART 2011 | Counselling | Usual care | Adverse events were reported for the overall population | Adverse events were reported for the overall population | Quote: "Four participants died of cardiovascular causes during the course of the study (2 from each study arm). No other adverse events were reported." |
| Hmwe 2015 | Acupressure | Usual care | Adverse events were reported for the overall population | Adverse events were reported for the overall population | Quote: "All patients were closely monitored for the occurrence of adverse effects (if any) during the intervention period. [...] In the current study, intra‐dialytic hypotension occurred in 11 patients, with 2 of them discontinuing the intervention because hypotensive episodes constantly occurred." |
| iDiD 2016 | Telephone support + CBT | CBT | No participants experienced an adverse event related to the intervention | No participants experienced an adverse event related to the intervention | Quote: "No trial adverse events occurred.[...] A total of 10 adverse events were detected. None were deemed related to the study. An additional two events occurred that the study team were unaware of and were self‐reported by patients. Both included a hospital admission related to a routine renal procedure (e.g. fistula plasty)." |
| Kouidi 1997 | Exercise | Usual care | No participants experienced an adverse event | No participants experienced an adverse event | Quote: "There were no adverse effects or other complications associated with the training session." |
| Kouidi 2010 | Exercise | Usual care | No participants experienced an adverse event | No participants experienced an adverse event | Quote: "There were no adverse effects or other complications associated with the training session." |
| Ouzouni 2009 | Exercise | Usual care | No participants experienced musculoskeletal, cardiovascular and other complication related to exercise training. Other adverse events were not reported | No participants experienced musculoskeletal, cardiovascular and other complication related to exercise training. Other adverse events were not reported | Quote: "There was no musculoskeletal, cardiovascular or other complication related to exercise training during the study." |
| Thomas 2017 | Meditation | Usual care | No participants experienced an adverse event | No participants experienced an adverse event | Quote: "No adverse events were observed." |
CBT ‐ cognitive behavioural therapy
Cognitive‐behavioural therapy versus usual care
Four studies reported outcomes for CBT (Cukor 2014; Duarte 2009; Lerma 2017; Lii 2007). Studies involved HD patients in centres in the USA, Brazil, Mexico, and Taiwan. CBT was administered chairside during dialysis in one study and in groups in the remaining three studies. The duration of treatment ranged between five weeks and three months. Cukor 2014 used a cross‐over design and Lerma 2017 administered CBT to the control group after five weeks.
Depression was assessed as a continuous outcome either as major (or severe) depression and depression (end of treatment). Depression (any severity) was reported as a dichotomous outcome. All studies reported depression score using the Beck Depression Inventory (BDI). Since BDI score showed that not all participants reported depressive symptoms at the baseline, or depression was not an inclusion criterion, the intervention was delivered both to prevent and to treat depression. Duarte 2009 measured major depression and suicides using the Mini International Neuropsychiatric Interview (MINI). Lerma 2017 reported anxiety using the Beck Anxiety Inventory (BAI) and distorted thinking using the Distorted Thought Scale (DTS), and Lii 2007 reported self‐efficacy using the Strategies Used by People to Promote Health (SUPPH). Three studies (Cukor 2014; Duarte 2009; Lii 2007) reported HRQoL using the Kidney Disease Quality of Life Instrument Short Form (KDQOL‐SF36), while Lerma 2017 used the Quality of Life (QoL) Scale.
Duarte 2009 (74 participants) reported CBT may improve major depression compared to usual care (Analysis 2.1: MINI score MD ‐1.50, 95% CI ‐2.87 to ‐0.13). Cukor 2014 reported CBT may reduce the number with depression (any severity) during 6 months of follow‐up (Analysis 2.2: RR 0.17, 95% CI 0.04 to 0.69). Four studies reported the depression score at end of treatment (median follow‐up was 17.7 weeks) using BDI. CBT probably improves depressive symptoms to a clinically‐important extent (Analysis 2.3 (4 studies, 230 participants): BDI score MD ‐6.10, 95% CI ‐8.63 to ‐3.57; I2 = 0%; moderate‐certainty evidence). As a rule of thumb, 0.5 SMD represented a moderate effect size (Cohen 1988), and CBT probably improves HRQoL compared to usual care, measured either with KDQOL‐SF36 or QoL scale, during a median follow‐up of 17.7 weeks (Analysis 2.4 (4 studies, 230 participants): KDQOL‐SF36 and QoL scale SMD 0.51, 95% CI 0.19 to 0.83; I2 = 31%; moderate‐certainty evidence).
2.1. Analysis.
Comparison 2 Cognitive‐behavioural therapy versus usual care, Outcome 1 Major depression.
2.2. Analysis.
Comparison 2 Cognitive‐behavioural therapy versus usual care, Outcome 2 Depression (any severity).
2.3. Analysis.
Comparison 2 Cognitive‐behavioural therapy versus usual care, Outcome 3 Depression.
2.4. Analysis.
Comparison 2 Cognitive‐behavioural therapy versus usual care, Outcome 4 Health‐related quality of life.
Lerma 2017 (49 participants) reported CBT may reduce anxiety (Analysis 2.5: BAI score MD ‐8.70, 95% CI ‐15.67 to ‐1.73) and distorted thinking during follow‐up (Analysis 2.8: DTS score MD ‐11.80, 95% CI ‐22.87 to ‐0.73) compared to usual care.
2.5. Analysis.
Comparison 2 Cognitive‐behavioural therapy versus usual care, Outcome 5 Anxiety.
2.8. Analysis.
Comparison 2 Cognitive‐behavioural therapy versus usual care, Outcome 8 Distorted thinking.
Duarte 2009 reported no difference in suicide (Analysis 2.6: MINI score MD 0.00, 95% CI ‐0.75 to 0.75) between CBT and usual care.
2.6. Analysis.
Comparison 2 Cognitive‐behavioural therapy versus usual care, Outcome 6 Suicide.
Lii 2007 (48 participants) reported CBT may improve self‐efficacy compared to usual care (Analysis 2.7: SUPPH score MD 22.30, 95% CI 12.65 to 31.95).
2.7. Analysis.
Comparison 2 Cognitive‐behavioural therapy versus usual care, Outcome 7 Self‐efficacy.
We found that CBT had uncertain effects on death (any cause), during a median follow‐up of 24.3 weeks (Analysis 2.9 (2 studies, 145 participants): RR 1.09, 95% CI 0.35 to 3.45; I2 = 0%; low‐certainty evidence).
2.9. Analysis.
Comparison 2 Cognitive‐behavioural therapy versus usual care, Outcome 9 Death (any cause).
Adverse events were not reported in studies of CBT.
No study measured the outcomes of hospitalisation, withdrawal from dialysis, withdrawal from intervention, or adherence to dialysis treatment.
Cognitive‐behavioural therapy versus education
Al Saraireh 2018 compared counselling (CBT) to psychoeducation for seven sessions during three months. Since the Hamilton inventory (HAM‐D) score showed that all participants reported depressive symptoms at the baseline, the intervention was delivered to treat depression. The study did not measure major depression or depression (any severity) as an outcome.
Al Saraireh 2018 (105 participants) reported psychoeducation may reduce depression (end of treatment) compared to CBT (Analysis 3.1: HAM‐D score MD 3.90, 95% CI 2.27 to 5.53).
3.1. Analysis.
Comparison 3 Cognitive‐behavioural therapy (CBT) versus education, Outcome 1 Depression.
No other review outcomes were measured.
Counselling versus usual care
Four studies evaluated counselling compared to usual care. Bahmani 2016 evaluated cognitive‐existential group therapy twice a week for 12 sessions, Erdley 2014 evaluated problem‐solving therapy for six weekly sessions with an individual counsellor, Beder 1999 evaluated social worker‐based counselling and support during the first three months of dialysis care, and HED‐SMART 2011 evaluated NKF‐NUS self‐management intervention for four sessions during nine months. Three studies(Bahmani 2016; Beder 1999; Erdley 2014) measured depression score using BDI, Since BDI score showed that not all participants reported depressive symptoms at the baseline, or depression was not an inclusion criterion, the intervention was delivered both to prevent and to treat depression. Erdley 2014 reported HRQoL using KDQOL‐SF36 and Beder 1999 reported coping using the Psychosocial Adjustment to Illness (PAIS).
None of the four studies measured the outcomes of major depression, depression (any severity), anxiety, or withdrawal from dialysis.
Counselling may reduce depressive symptoms compared to usual care (median follow‐up was 13.2 weeks) (Analysis 4.1 (3 studies, 99 participants): BDI score MD ‐3.84, 95% CI ‐6.14 to ‐1.53; I2 = 31%; low‐certainty evidence).
4.1. Analysis.
Comparison 4 Counselling versus usual care, Outcome 1 Depression.
Erdley 2014 (33 participants) reported no difference in HRQoL (Analysis 4.2: KDQOL‐SF36 score MD 3.28, 95% CI ‐3.57 to 10.13) between counselling and usual care.
4.2. Analysis.
Comparison 4 Counselling versus usual care, Outcome 2 Health‐related quality of life.
Beder 1999 (46 participants) reported coping may improve (Analysis 4.3: PAIS scale MD ‐13.70, 95% CI ‐16.79 to ‐10.60) with counselling compared to usual care.
4.3. Analysis.
Comparison 4 Counselling versus usual care, Outcome 3 Coping.
Erdley 2014 reported no difference in withdrawal from treatment (Analysis 4.4: RR 5.28, 95% CI 0.27 to 102.58) between counselling and usual care.
4.4. Analysis.
Comparison 4 Counselling versus usual care, Outcome 4 Withdrawal from intervention.
Counselling had uncertain effects on death (any cause), during a median follow‐up of 22.8 weeks (Analysis 4.5 (2 studies, 270 participants): RR 1.69, 95% CI 0.32 to 8.81; I2 = 0%; low‐certainty evidence).
4.5. Analysis.
Comparison 4 Counselling versus usual care, Outcome 5 Death (any cause).
Education versus usual care
Espahbodi 2015 reported outcomes for an education intervention during one month in one hour sessions. The group educational sessions provided information about anatomy, pathophysiology, explanation of the causes of kidney failure and treatment, education about dialysis care, problem‐solving skills, stress management, adaptive responses, and muscle relaxation. The study measured the depression and anxiety score using the Hospital Anxiety and Depression Scale (HADS) at the end of the study. The study did not measure major depression or depression (any severity) as an outcome. Since the HADS score showed that not all participants reported depressive symptoms at the baseline, the intervention was delivered both to prevent and to treat depression.
Espahbodi 2015 (55 participants) reported no differences in depression (Analysis 5.1: HADS scale MD ‐1.78, 95% CI ‐3.66 to 0.10) or anxiety (Analysis 5.2: HADS scale MD ‐1.26, 95% CI ‐2.99 to 0.47) scores between education and usual care.
5.1. Analysis.
Comparison 5 Education versus usual care, Outcome 1 Depression.
5.2. Analysis.
Comparison 5 Education versus usual care, Outcome 2 Anxiety.
Exercise versus usual care
Four studies evaluating exercise compared to usual care. In Ouzouni 2009, participants followed a 10‐month exercise programme during HD treatment 3 times/week for 60 to 90 minutes of cycling and flexibility exercises. In Kouidi 1997, participants did three weekly sessions of exercise training for 6 months. In Kouidi 2010, participants did between 60 and 90 minutes of exercise during the first two hours of dialysis for one year. In Frey 1999, participants cycled on a stationary bicycle ergometers for 3 days/week for 12 weeks.
Depression was assessed as a continuous outcome either as major (or severe) depression and depression (end of treatment). Depression (any severity) was reported as a dichotomous outcome. Since all participants had depressive symptoms, the intervention was delivered to treat depression. Three studies(Kouidi 1997; Kouidi 2010; Ouzouni 2009) measured depression score using BDI, and Kouidi 2010 reported anxiety using HADS.
Exercise may reduce the risk of major depression (Analysis 6.1 (3 studies, 108 participants): RR 0.47, 95% CI 0.27 to 0.81; I2 = 50%; low‐certainty evidence) after a median study follow‐up of 44 weeks. Exercise probably decreases risk of depression of any severity (Analysis 6.2 (3 studies, 108 participants): RR 0.69, 95% CI 0.54 to 0.87; I2 = 38%; moderate‐certainty evidence), and probably decreases depressive symptoms (Analysis 6.3 (3 studies, 108 participants): BDI score MD ‐7.61, 95% CI ‐9.59 to ‐5.63; I2 = 0%; moderate‐certainty evidence) during a median follow‐up of 44 weeks.
6.1. Analysis.
Comparison 6 Exercise versus usual care, Outcome 1 Major depression.
6.2. Analysis.
Comparison 6 Exercise versus usual care, Outcome 2 Depression (any severity).
6.3. Analysis.
Comparison 6 Exercise versus usual care, Outcome 3 Depression.
Two studies (Kouidi 1997; Ouzouni 2009) measured HRQoL using the Quality of Life Index (Spitzer Index) (QLI) translated for a Greek population. Exercise may improve HRQoL (Analysis 6.4 (64 participants): QLI score MD 3.06, 95% CI 2.29 to 3.83; I2 = 0%; low‐certainty evidence) after a median study follow‐up of 35.2 weeks. All three studies reported that no adverse events occurred (Table 7).
6.4. Analysis.
Comparison 6 Exercise versus usual care, Outcome 4 Health‐related quality of life.
Kouidi 2010 (44 participants) reported exercise may reduce anxiety compared to sedentary control group (Analysis 6.5: HADS score MD ‐2.27, 95% CI ‐3.55 to ‐0.99).
6.5. Analysis.
Comparison 6 Exercise versus usual care, Outcome 5 Anxiety.
Frey 1999 (11 participants) reported two hospitalisations in the exercise group (Analysis 6.6: RR 5.83, 95% CI 0.34 to 99.23).
6.6. Analysis.
Comparison 6 Exercise versus usual care, Outcome 6 Hospitalisation.
None of the studies measured withdrawal from dialysis, withdrawal from intervention, or death from any cause.
Exercise versus exercise
Dziubek 2016 reported outcomes for two different types of exercise during six months for three times a week. The intervention group performed the endurance training, while the control group performed the resistance training during the first two hours of HD. Depression was measured using BDI. Since BDI score showed that not all participants reported depressive symptoms at the baseline, the intervention was delivered both to prevent and to treat depression. The study did not measure major depression as an outcome.
Dziubek 2016 (28 participants) reported no differences in the depression score (Analysis 7.1: BDI score MD 0.90, 95% CI ‐5.44 to 7.24) or death (any cause) (Analysis 7.2: RR 0.25, 95% CI 0.03 to 2.22) between the two types of exercise.
7.1. Analysis.
Comparison 7 Exercise versus exercise, Outcome 1 Depression.
7.2. Analysis.
Comparison 7 Exercise versus exercise, Outcome 2 Death (any cause).
No other review outcomes were measured.
Exercise versus support group
Carney 1987 compared exercise (three weekly exercise for 45 to 60 minutes of callisthenics, stationary bicycling, and walking at 50‐60% of V02max) for six months compared to a support group for 60 to 90 minutes twice a week. The study measured treatment effects on major depression, depression score using BDI, and HRQoL according to the Pleasant Events Schedule (PES). Depression was assessed as a continuous outcome either as major (or severe) depression and depression (end of treatment). Since depression was not an inclusion criterion, the intervention was delivered both to prevent and to treat depression.
Carney 1987 (17 participants) reported exercise may reduce major depression (Analysis 8.1: RR 0.14, 95% CI 0.02 to 0.95) and depression score (Analysis 8.2: BDI score MD ‐6.80, 95% CI ‐9.46 to ‐4.14) compared to support group, while there was no difference in HRQoL between the groups (Analysis 8.3: PES score MD ‐20.50, 95% CI ‐65.07 to 24.07).
8.1. Analysis.
Comparison 8 Exercise versus support group, Outcome 1 Major depression.
8.2. Analysis.
Comparison 8 Exercise versus support group, Outcome 2 Depression.
8.3. Analysis.
Comparison 8 Exercise versus support group, Outcome 3 Health‐related quality of life.
No other review outcomes were measured.
Meditation versus usual care
Thomas 2017 compared mindfulness meditative practice three times/week during HD (body scan, guided meditation, silent meditation, and arm movement for 10 to 15 minutes) for eight weeks to usual care. Symptoms of depression and anxiety were measured using the Patient Health Questionnaire (PHQ) and the General Anxiety Disorder (GAD), respectively. The study did not measure major depression or depression (any severity) as an outcome. Since the included population had depressive symptoms in the inclusion criteria, the intervention was delivered to treat depression.
Thomas 2017 (32 participants) reported no differences between the two groups for either depression (Analysis 9.1: PHQ score MD 2.00, 95% CI ‐1.90 to 5.90) or anxiety scores (Analysis 9.2: GAD score MD 1.90, 95% CI ‐1.31 to 5.11).
9.1. Analysis.
Comparison 9 Meditation versus usual care, Outcome 1 Depression.
9.2. Analysis.
Comparison 9 Meditation versus usual care, Outcome 2 Anxiety.
The investigators reported that no adverse events occurred (Table 7).
Motivational interviewing versus motivational interviewing
Leake 1999 compared motivational interviewing with another motivational interviewing technique for one month, with no extractable data for meta‐analysis.
Motivational interviewing versus education
Leake 1999 compared motivational interviewing with education about dialysis delivered by video for one month, with no extractable data for meta‐analysis.
Relaxation techniques versus usual care
Two studies compared relaxation techniques to usual care. In Heshmatifar 2015, participants did relaxation exercises (Benson technique) for 20 minutes during each HD session as well as twice a day (for 20 minutes) at home over one month. In Tsai 2015, participants did eight sessions of breathing training (guided by an audio device) over four weeks. The studies did not measure major depression or depression (any severity) as an outcome. These studies measured depression score using BDI. Since BDI score showed that not all participants reported depressive symptoms at the baseline, or the enrolled participants did not report depressive symptoms at the beginning of the study, the intervention was delivered both to prevent and to treat depression.
Relaxation techniques may reduce depressive symptoms (Analysis 10.1 (2 studies, 122 participants): BDI score MD ‐5.77, 95% CI ‐8.76 to ‐2.78; I2 = 0%; moderate‐certainty evidence), after a median study follow‐up of 4.2 weeks.
10.1. Analysis.
Comparison 10 Relaxation techniques versus usual care, Outcome 1 Depression.
Tsai 2015 measured HRQoL using the Short Form Health Survey (SF‐36). Tsai 2015 (64 participants) reported no differences between relaxation and usual care for either HRQoL (Analysis 10.2: SF‐36 score MD 2.36, 95% CI ‐4.72 to 9.44) or hospitalisation (Analysis 10.3: RR 0.14, 95% CI 0.01 to 2.66).
10.2. Analysis.
Comparison 10 Relaxation techniques versus usual care, Outcome 2 Health‐related quality of life.
10.3. Analysis.
Comparison 10 Relaxation techniques versus usual care, Outcome 3 Hospitalisation.
Other review outcomes including adverse events were not measured.
Relaxation with imagery techniques versus imagery techniques
Krespi 2009 compared relaxation techniques with imagery visualisation delivered using audio recordings with imagery visualisation alone for nine weeks, with no extractable data for meta‐analysis.
Spiritual practice versus usual care
Spiritual practices were evaluated in two studies. In Matthews 2001, prayer (prayers offered by religious personnel over five minutes for five days a week for six weeks and in a group once a week) was compared to usual care or positive visualization in a factorial study design. In Babamohamadi 2017, participants listened to a Qur'an recitation three times a week for 20 minutes for one month. Neither of the two studies measured major depression, withdrawal from dialysis, withdrawal from intervention, or death from any cause. The studies did not measure major depression or depression (any severity) as an outcome. Matthews 2001 measured anxiety and psychological symptoms using the Brief Symptom Inventory (BSI), and HRQoL using SF‐36. Since depression was not an inclusion criterion, the intervention was delivered both to prevent and to treat depression. Depression scores were measured using either the BDI (Babamohamadi 2017) or BSI (Matthews 2001).
As a rule of thumb, at least 0.8 SMD represented a large effect size (Cohen 1988), and spiritual practices had uncertain effects on depressive symptoms (Analysis 11.2 (2 studies, 116 participants): BDI and BSI score SMD ‐1.00, 95% CI ‐3.52 to 1.53; I2 = 97%; very low certainty evidence), after a median study follow‐up of 5.2 weeks.
11.2. Analysis.
Comparison 11 Spiritual practice versus usual care, Outcome 2 Depression.
Matthews 2001 (60 participants) reported no differences between spiritual practice versus usual care for HRQoL (Analysis 11.1: SF‐36 score MD ‐1.02, 95% CI ‐3.31 to 1.27), anxiety (Analysis 11.3: BSI score MD ‐0.18, 95% CI ‐5.48 to 5.12) or psychological symptoms (Analysis 11.4: BSI score MD 0.82, 95% CI ‐1.54 to 3.18).
11.1. Analysis.
Comparison 11 Spiritual practice versus usual care, Outcome 1 Quality of life.
11.3. Analysis.
Comparison 11 Spiritual practice versus usual care, Outcome 3 Anxiety.
11.4. Analysis.
Comparison 11 Spiritual practice versus usual care, Outcome 4 Psychological symptoms.
Spiritual practice versus exercise
Frih 2017 compared the spiritual practice (listening to Holy Qur'an recitation) to recitation practice with endurance resistance physical training, or physical training alone and measured depression score using HADS, HRQoL (SF‐36) and anxiety score (HADS) at 24 weeks. The study did not measure major depression or depression (any severity) as an outcome. Since the HADS score showed that all participants reported depressive symptoms at the baseline, the intervention was delivered to treat depression.
Frih 2017 (53 participants) reported that spiritual practice may reduce depression (Analysis 12.1: HADS score MD ‐1.90, 95% CI ‐2.95 to ‐0.85) and anxiety (Analysis 12.4: HADS score MD ‐3.90, 95% CI ‐4.79 to ‐3.01), and may improve QoL (mental component summary: Analysis 12.2: SF‐36 score MD 15.60, 95% CI 9.84 to 21.36; physical component summary: Analysis 12.3: SF‐36 score MD 5.10, 95% CI ‐0.19 to 10.39).
12.1. Analysis.
Comparison 12 Spiritual practice versus exercise, Outcome 1 Depression.
12.4. Analysis.
Comparison 12 Spiritual practice versus exercise, Outcome 4 Anxiety.
12.2. Analysis.
Comparison 12 Spiritual practice versus exercise, Outcome 2 Quality of life (mental component summary).
12.3. Analysis.
Comparison 12 Spiritual practice versus exercise, Outcome 3 Quality of life (physical component summary).
Other review outcomes including adverse events were not measured.
Spiritual practice versus visualisation
Matthews 2001 evaluated spiritual practice versus positive visualisation for six weeks. The study did not measure major depression or depression (any severity) as an outcome. This study measured depression, anxiety and psychological symptoms using BSI, and HRQoL using SF‐36. Since depression was not an inclusion criterion, the intervention was delivered both to prevent and to treat depression.
Matthews 2001 reported no differences in scores for depression (Analysis 13.1: BSI score MD 2.86, 95% CI ‐2.91 to 8.63), HRQoL (Analysis 13.2: SF‐36 score MD ‐1.03, 95% CI ‐10.80 to 8.74), anxiety (Analysis 13.3: BSI score MD ‐1.20, 95% CI ‐4.76 to 2.36), and psychological symptoms (Analysis 13.4: BSI score MD 1.25, 95% CI ‐1.10 to 3.60).
13.1. Analysis.
Comparison 13 Spiritual practice versus visualisation, Outcome 1 Depression.
13.2. Analysis.
Comparison 13 Spiritual practice versus visualisation, Outcome 2 Health‐related quality of life.
13.3. Analysis.
Comparison 13 Spiritual practice versus visualisation, Outcome 3 Anxiety.
13.4. Analysis.
Comparison 13 Spiritual practice versus visualisation, Outcome 4 Psychological symptoms.
Social activity versus usual care
Sertoz 2009 evaluated social activity (rehearsing and performing in a theatre play for 4 months) versus usual care. The study did not measure major depression or depression (any severity) as an outcome. Since BDI score showed that not all participants reported depressive symptoms at the baseline, the intervention was delivered both to prevent and to treat depression. The study measured depression score using BDI, HRQoL using the Turkish version of the World Health Organization Quality of Life Scale short form (WHOQOL‐Bref), anxiety using BAI, and self‐esteem using the Turkish version of the Rosenberg Self‐Esteem Scale (RSES).
Sertoz 2009 (31 participants) reported no differences in scores for depression (Analysis 14.1: BDI score MD ‐2.60, 95% CI ‐7.03 to 1.83), HRQoL (Analysis 14.2: WHOQOL‐BREF score MD ‐1.70, 95% CI ‐3.55 to 0.15), anxiety (Analysis 14.3: BAI score MD 1.60, 95% CI ‐7.00 to 10.20), and self‐esteem (Analysis 14.4: RSES score MD ‐0.40, 95% CI ‐1.36 to 0.56).
14.1. Analysis.
Comparison 14 Social activity versus usual care, Outcome 1 Depression.
14.2. Analysis.
Comparison 14 Social activity versus usual care, Outcome 2 Health‐related quality of life.
14.3. Analysis.
Comparison 14 Social activity versus usual care, Outcome 3 Anxiety.
14.4. Analysis.
Comparison 14 Social activity versus usual care, Outcome 4 Self‐esteem.
Telephone support versus usual care
Kargar Jahromi 2016 evaluated telephone support (tele‐nursing consisting of a 30 minutes phone call 30 days after a dialysis shift to discuss communication, cognition/development, breathing/circulation, nutrition, elimination, sleep, pain/perception, skin/tissue, sexuality/reproduction, activity, psychosocial/spirituality/culture) versus usual care. The study did not measure major depression or depression (any severity) as an outcome. Since DASS score showed that all participants reported depressive symptoms at the baseline, the intervention was delivered to treat depression. Depression, anxiety, and stress scores were measured using DASS. Outcomes of withdrawal from dialysis and death from any cause were measured.
Kargar Jahromi 2016 reported telephone support may improve depression (Analysis 15.1: DASS score MD ‐7.24, 95% CI ‐7.99 to ‐6.49), anxiety (Analysis 15.2: DASS score MD ‐8.04, 95% CI ‐8.86 to ‐7.22), and stress scores (Analysis 15.3: DASS score MD ‐5.40, 95% CI ‐6.07 to ‐4.73), but made no difference to withdrawal from dialysis (Analysis 15.4: RR 5.00, 95% CI 0.25 to 99.95) or death (Analysis 15.5: RR 0.33, 95% CI 0.01 to 7.87).
15.1. Analysis.
Comparison 15 Telephone support versus usual care, Outcome 1 Depression.
15.2. Analysis.
Comparison 15 Telephone support versus usual care, Outcome 2 Anxiety.
15.3. Analysis.
Comparison 15 Telephone support versus usual care, Outcome 3 Stress.
15.4. Analysis.
Comparison 15 Telephone support versus usual care, Outcome 4 Withdrawal from dialysis.
15.5. Analysis.
Comparison 15 Telephone support versus usual care, Outcome 5 Death (any cause).
Telephone support and cognitive‐behavioural therapy versus cognitive‐behavioural therapy
iDiD 2016 evaluated telephone support and CBT versus CBT for 12 weeks. The study did not measure major depression or depression (any severity) as an outcome. Since all the included population had depressive symptoms in the inclusion criteria, the intervention was delivered to treat depression. This study measured depression score using PHQ, anxiety using GAD and QoL using the EuroQoL scale (EQ‐5D),
iDiD 2016 reported no differences in depression (Analysis 16.1: PHQ score MD ‐0.10, 95% CI ‐4.47 to 4.27), QoL (Analysis 16.2: EQ‐5D score MD 4.90, 95% CI ‐10.42 to 20.22), anxiety (Analysis 16.3: GAD score MD 0.50, 95% CI ‐2.84 to 3.84) and death (Analysis 16.4: RR 1.26, 95% CI 0.06 to 27.82).
16.1. Analysis.
Comparison 16 Telephone support + cognitive behavioural therapy versus cognitive behavioural therapy, Outcome 1 Depression.
16.2. Analysis.
Comparison 16 Telephone support + cognitive behavioural therapy versus cognitive behavioural therapy, Outcome 2 Quality of life.
16.3. Analysis.
Comparison 16 Telephone support + cognitive behavioural therapy versus cognitive behavioural therapy, Outcome 3 Anxiety.
16.4. Analysis.
Comparison 16 Telephone support + cognitive behavioural therapy versus cognitive behavioural therapy, Outcome 4 Death (any cause).
Adverse events of telephone support and CBT were rarely reported (Table 7).
Voice recording versus usual care
Bargiel‐Matusiewicz 2011a evaluated a voice recording of a psychological intervention listened to twice a day for 3 weeks versus usual care. Data could not be extracted.
Discussion
Summary of main results
In this update, we included 33 studies (2056 participants) comparing a psychosocial intervention with a second psychosocial intervention or usual care on depression, HRQoL, anxiety, hospitalisation, withdrawal from treatment, or death (any cause) in adult patients with ESKD treated with dialysis. All studies involved patients treated with HD. In addition, we identified seven ongoing studies.
Interventions included (in alphabetical order) acupressure, CBT, counselling, education, exercise, meditation, relaxation techniques, spiritual practice, social activity, and telephone support. The primary outcome of depression was predominantly measured as a depression score using BDI. HRQoL was measured using a range of instruments. Adverse events were infrequently reported and evidence of adverse events was very uncertain.
The duration of study follow‐up ranged between three weeks and one year. Studies included between nine and 235 participants. The mean study age ranged between 36.1 and 73.9 years.
We noted that random sequence generation and allocation concealment were at low risk of bias in eight and one studies, respectively. One study reported low risk methods for blinding of participants and investigators, and outcome assessment was blinded in seven studies. Twelve studies were at low risk of attrition bias, eight studies were at low risk of selective reporting bias, and twenty‐one studies were at low risk of other potential sources of bias.
Depressive outcomes were assessed in heterogeneous way. Depression was assessed as a continuous outcome either as major (or severe) depression and depression (end of treatment). In addition, the severity of depressive symptoms was assessed as a dichotomous outcome. Since the enrolled population had/did not have depressive symptoms in the inclusion criteria, the intervention was delivered both to treat and to prevent depression.
We found moderate certainty evidence that CBT probably improves depression symptoms (4 studies, 230 participants: BDI score MD ‐6.10, 95% CI ‐8.63 to ‐3.57) and HRQoL (4 studies, 230 participants: KDQOL‐SF36 and QoL scale SMD 0.51, 95% CI 0.19 to 0.83) compared to usual care. CBT makes no difference in suicide risk (one study), but may reduce anxiety (one study) and distorted thinking (one study) compared to usual care. No studies measured hospitalisation.
We found low‐certainty evidence that counselling may reduce depressive symptoms slightly (3 studies, 99 participants: BDI score MD ‐3.84, 95% CI ‐6.14 to ‐1.53) compared to usual care. Counselling makes no difference in HRQoL (one study) compared to usual care. Counselling studies did not measure risk of major depression, suicide, or hospitalisation.
Low‐certainty evidence indicates exercise may reduce or prevent major depression (3 studies, 108 participants: RR 0.47, 95% CI 0.27 to 0.81), depression of any severity (3 studies, 108 participants: RR 0.69, 95% CI 0.54 to 0.87) and HRQoL (2 studies, 64 participants: QLI score MD 3.06, 95% CI 2.29 to 3.83) compared to usual care. In moderate‐certainty evidence, exercise probably improves depression symptoms (3 studies, 108 participants: BDI score MD ‐7.61, 95% CI ‐9.59 to ‐5.63). Exercise may reduce anxiety (one study) compared to sedentary control group. No studies measured suicide risk or withdrawal from dialysis.
We found moderate‐certainty evidence that relaxation techniques probably reduce depressive symptoms (2 studies, 122 participants: BDI score MD ‐5.77, 95% CI ‐8.76 to ‐2.78). Relaxation techniques make no differences in HRQoL (one study) and hospitalisation (one study) compared to usual care. Counselling studies did not measure risk of major depression or suicide.
Spiritual practices have uncertain effects on depressive symptoms, since a rule of thumb, at least 0.8 SMD represented a large effect size (Cohen 1988) (2 studies, 116 participants: BDI and BSI score SMD ‐1.00, 95% CI ‐3.52 to 1.53; very low certainty evidence). Spiritual practices report no difference in anxiety (one study), psychological symptoms (one study) and HRQoL (one study), when compared with usual care. No study measured suicide risk, withdrawal from dialysis, or hospitalisation.
There were few or no data on acupressure, telephone support, meditation and adverse events related to psychosocial interventions.
Overall, there was insufficient evidence to conduct subgroup and sensitivity analyses.
Overall completeness and applicability of evidence
This review found that studies evaluating specific psychosocial interventions to prevent and treat depression for adult dialysis patients are few. Meta‐analyses for the primary outcome of depression included four of fewer studies for all interventions. Due to the small number of studies and heterogeneity of psychosocial interventions, it was not possible to assess whether treatment effects differed according to duration of treatment or patient clinical and demographic characteristics. Studies did not measure effects of treatment in patients treated with peritoneal dialysis. The psychosocial interventions were not standardised, and we could not be certain whether comparisons by type of intervention were always equivalent. In addition, due to the large variability of psychosocial interventions, the assessment and the implementation in clinical practice and the associated resource use might be challenging. The external validity of the review may be limited as most of the studies were not specifically designed to examine interventions in patients with a prespecified diagnosis of depression, were conducted in higher income countries, and were frequently continued for a few weeks.
Standardisation of outcome reporting in future psychosocial intervention trials as prioritised by the Standardised Outcomes in Nephrology (SONG) by patients, caregivers and health professionals may assist to improve the evidence base for nephrology trials. In the HD setting, this would include the compulsory reporting of end points for fatigue, cardiovascular disease, vascular access, and death (SONG‐HD). Based on SONG‐HD, priority outcomes for trials of psychosocial interventions might include the outcomes of depression, the ability to travel, ability to work, dialysis‐free time, impact on family/friends, mobility, pain, cognition, financial impact, food enjoyment, itching, nausea/vomiting, restless legs syndrome, sexual function, and sleep. Consistent measures for these outcomes would improve our confidence in the results of available studies.
Potential adverse events are not well understood based on existing studies.
Quality of the evidence
We used the GRADE process to consider the effect of study limitations on our outcomes. The overall certainty of the evidence for depression outcomes was moderate, meaning additional studies will increase our confidence in the results. We found that many studies did not report adequate methods of randomisation and due to the nature of the interventions, blinding of investigators and participants was not possible. Empirical evidence suggests that treatment effects may be exaggerated when allocation concealment and blinding are not reported within trials, although this is particularly relevant for subjective outcomes including symptoms and adverse events (Wood 2008). As many clinical outcomes such as depression, HRQoL, and anxiety were measured using a self‐rating scale by participants who were aware of treatment assignment, many studies were at high risk of bias for outcome assessment. Minimisation of selection and detection bias in future research studies would increase the certainty of treatment benefits and harms. The limited number of studies prevented exploration of potential sources of heterogeneity in the analyses.
Potential biases in the review process
This review was carried out using standard Cochrane methods. Each step was completed independently by at least two authors including selection of studies, data management, and risk of bias assessment, thus reducing the risks of errors in identification of eligible studies and adjudication of evidence certainty. A highly sensitive search of the Cochrane Kidney Transplant specialised register was completed without language restriction in June 2019. The registry contains hand‐searched literature and conference proceedings, maximising the inclusion of grey literature in this review. Many studies did not report key outcomes in a format available for meta‐analysis. Formal assessment for publication bias through visualisation of asymmetry in funnel plots was precluded for many treatments and outcomes because of few studies.
Agreements and disagreements with other studies or reviews
Few studies have examined the efficacy of psychosocial interventions for people with CKD and the number of meta‐analysis published in this field is limited. The current Cochrane review is consistent with the findings of systematic review and meta‐analysis of published RCTs evaluating psychosocial interventions for depressive and anxiety symptoms in individuals with CKD (Pascoe 2017). In that review that included eight studies, the authors found that psychosocial interventions (empowerment program, QoL therapy, liquid‐intake program, preparing patients for end‐of‐life) reduced depressive symptoms and slightly improved HRQoL for patients and caregivers. Differences between Pascoe 2017 and this review update were related to the inclusion of all patients in CKD (stages 3 to 5) and adults approved for kidney transplantation, languages restrictions in the search strategy, and limited consideration of evidence certainty when drawing conclusions about treatment effects. A second meta‐analysis of RCTs evaluating psychological interventions to prevent or treat depression in HD patients included eight studies. Interventions included CBT, rational‐emotive therapy, adaptation training programme, and visual imagery (Xing 2016). In that analysis, GRADE was not used to evaluate evidence certainty, and the outcome of depression symptoms included different measurement tools. Psychological interventions decreased depressive symptoms but did not improve HRQoL.
In our previous Cochrane review of antidepressant medication (4 studies, 170 participants) for treating depression in adults with ESKD treated with dialysis, medication may reduce depressive symptoms when compared to placebo, but there was low certainty about whether medication made any difference to depression symptoms compared to psychological interventions (Palmer 2016).
Authors' conclusions
Implications for practice.
Our updated review suggests there is now moderate certainty that CBT, exercise and relaxation techniques probably reduce depressive symptoms for patients treated with long‐term dialysis when compared to usual care, although the small number of studies with few enrolled participants lead to considerable uncertainty, and may not provide sufficient evidence to inform clinical practice. The evidence to support improvements in HRQoL with psychosocial strategies is of lower certainty, and current studies are limited to short‐term follow‐up. Since CBT probably decreases depression and improves HRQoL, Internet‐based treatments could reduce waiting‐lists and save therapist time compared with traditional interventions (Cuijpers 2008). In most studies, interventions were very brief (often a few weeks) and variable in structure and delivery. Other interventions such as spiritual practices and meditation had uncertain effects on depression, anxiety, and HRQoL. It was not possible to detect whether treatment effects differed by intensity (one‐on‐one or group, frequency) or for different patient groups. Evidence is largely lacking in the setting of PD or home‐based HD. It is not possible to definitively establish the impact of psychosocial interventions on major depression, anxiety, withdrawal from dialysis, or death from any cause. The potential adverse events of treatment are largely unknown.
Implications for research.
Further research is likely to change the estimated effects of different psychosocial interventions in dialysis patients with or without depressive symptoms, and increase our certainty of the evidence based on limitations in existing studies and a paucity of evidence for specific clinical questions. Given the high symptom burden experienced by dialysis patients, together with the prioritisation of research informing symptom management, new research initiatives for preventing and treating depression would address important clinical uncertainties. Depression was assessed using different tools, outcomes data were measured in heterogeneous ways and the aim of the intervention was delivered either to prevent or to treat dialysis patients with or without depressive symptoms. The findings of this review suggest that CBT, exercise, and relaxation techniques are promising interventions for improving symptoms for dialysis patients that warrant further research. Based on this review, future studies of exercise and CBT would increase our certainty about whether these interventions improve patient well‐being. Systematic assessment of adverse events would inform the design of such interventions for wider use.
Researchers investigating psychosocial treatments should consider standardised interventions, efficient study design to provide adequate statistical power to detect outcome measures, blinding of outcome assessment for subjective outcomes, and inclusion of all participants in the outcome assessments regardless of whether they complete the intervention as designed. Future psychosocial interventions studies should be designed to evaluate patient‐centred core outcomes based on SONG‐HD such as HRQoL, impaired mobility, and inability to participate in life and work that are becoming new priorities to aid in clinical decision‐making.
What's new
| Date | Event | Description |
|---|---|---|
| 16 October 2019 | New citation required and conclusions have changed | New studies added |
| 16 October 2019 | New search has been performed | Expanded inclusion criteria ‐ including trials of participants with and without depression |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 14 October 2008 | Amended | Converted to new review format. |
| 12 May 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The earlier versions of this Cochrane review were funded by the National Kidney Research Fund (UK). The 2019 review update received no specific funding. We wish to thank Fiona Russell, Gail Higgins, and Narelle Willis from Cochrane Kidney and Transplant for support during the preparation of this 2019 update. We thank the reviewers and editors in the review process. We thank all the authors who provided additional details of their studies. We also acknowledge Conal Daly, Janet Butler, Paul Roderick, Sheila Wallace, Alison MacLeod (who were authors on the protocol and the review) and Jasjot Maggo (who contributed to this update).
Appendices
Appendix 1. Electronic search strategies
| DATABASE | Search Terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Acupressure versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Stress | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Withdrawal from intervention | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7 Hospitalisation | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Cognitive‐behavioural therapy versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Depression (any severity) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3 Depression | 4 | 230 | Mean Difference (IV, Random, 95% CI) | ‐6.10 [‐8.63, ‐3.57] |
| 4 Health‐related quality of life | 4 | 230 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.19, 0.83] |
| 5 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Suicide | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Self‐efficacy | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Distorted thinking | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Death (any cause) | 2 | 145 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.35, 3.45] |
Comparison 3. Cognitive‐behavioural therapy (CBT) versus education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Counselling versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 3 | 99 | Mean Difference (IV, Random, 95% CI) | ‐3.84 [‐6.14, ‐1.54] |
| 2 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Coping | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Withdrawal from intervention | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 5 Death (any cause) | 2 | 270 | Risk Ratio (IV, Random, 95% CI) | 1.69 [0.32, 8.81] |
Comparison 5. Education versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Exercise versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major depression | 3 | 108 | Risk Ratio (IV, Random, 95% CI) | 0.47 [0.27, 0.81] |
| 2 Depression (any severity) | 3 | 108 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.54, 0.87] |
| 3 Depression | 3 | 108 | Mean Difference (IV, Random, 95% CI) | ‐7.61 [‐9.59, ‐5.63] |
| 4 Health‐related quality of life | 2 | 64 | Mean Difference (IV, Random, 95% CI) | 3.06 [2.29, 3.83] |
| 5 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Hospitalisation | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 7. Exercise versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Death (any cause) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Exercise versus support group.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major depression | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 9. Meditation versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 10. Relaxation techniques versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐8.76, ‐2.78] |
| 2 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Hospitalisation | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 11. Spiritual practice versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Depression | 2 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [‐3.52, 1.53] |
| 3 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Psychological symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 12. Spiritual practice versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Quality of life (mental component summary) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Quality of life (physical component summary) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 13. Spiritual practice versus visualisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Psychological symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 14. Social activity versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Self‐esteem | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 15. Telephone support versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Stress | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Withdrawal from dialysis | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 5 Death (any cause) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 16. Telephone support + cognitive behavioural therapy versus cognitive behavioural therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Death (any cause) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al Saraireh 2018.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This study was a randomised clinical trial in which patients were randomly assigned to one of two treatment groups using a random number generator." Comment: Random number generator is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. However, the methods of intervention and control treatment were physically different, and therefore masking of intervention was unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Hamilton depression rating scale was completed by the participants in both groups prior to the therapies and after completion." Comment: The Hamilton depression rating scale was completed by participants before and after interventions. Participants were likely to be aware of the intervention they received. Therefore, the outcome assessment for depression was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Only 130 patients agreed to participate in the study, and were randomly assigned to one of the two groups (N = 65 in each). Of the 130 participants, 14 dropped out from the psychoeducation group and 11 from the CBT group, making the number of participants who completed the study 105 (51 and 54)." Comment: 54/65 in the treatment group and 51/65 in the control group completed the study. |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events, death) |
| Other bias | Low risk | No evidence of other sources of bias |
Babamohamadi 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The present study was a clinical trial involving 60 haemodialysis patients randomly assigned to either an experimental or a control group." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. However, the methods of intervention and control treatment were physically different, and therefore masking of intervention was unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The Beck Depression Inventory (BDI‐II) was self‐completed at baseline before the start of dialysis and the first session, and then again 1 month from baseline when the intervention was completed." Comment: The BDI‐II was completed by participants before and after interventions. Participants were likely to be aware of the intervention they received. Therefore, the outcome assessment for depression was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three participants in each group did not complete the follow‐up evaluation at 1 month because of deterioration in their medical or psychiatric condition that prevented further participation or inability to complete the follow‐up evaluation." Comment: 3/30 in the intervention group and 3/30 in the control group were lost to the follow‐up (10% loss to follow‐up; there was not a differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events, death) |
| Other bias | Low risk | There was no evidence of imbalance at baseline. No interim analyses were reported. Funding was not involved into the analysis. There were no other apparent sources of bias |
Bahmani 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned into two groups of experimental and control conditions." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. However, the methods of intervention and control treatment were physically different, and therefore masking of intervention was unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The tool is used for self‐report of signs of depression for individuals above 13 years old and higher." Comment: BDI‐II was completed by participants. Participants were likely to be aware of the intervention they received. Therefore, the outcome assessment for depression was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Two of the participants in the experimental group withdrew their participation due to personal problems." Comment: 2/11 in the intervention group and 0/11 in the control group were lost to the follow‐up for reasons that appeared unrelated to the treatment (> 10% loss to follow‐up; there was a differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events, death) |
| Other bias | Unclear risk | It was not clear if there was evidence of imbalance at baseline. Not reported in sufficient detail to perform an adjudication |
Bargiel‐Matusiewicz 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The dialyzed patients were randomly assigned to the control or experimental group and filled a set of questionnaires during researchers’ first visit." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. However, the methods of intervention and control treatment were physically different, and therefore masking of intervention was unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The instruments were the Cognitive Appraisal Inventory and the State‐Trait Anxiety Inventory (STAI)." Comment: The STAI was completed by participants before and after interventions. Participants were likely to be aware of the intervention they received. Therefore, the outcome assessment for anxiety was not blinded. It was not reported who completed the cognitive assessment measure. Therefore it was unclear whether the completion of this outcome was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants randomised was 62. The number of participants included in analyses was 60. The reasons for withdrawal or non‐inclusion in analyses were not reported |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events, depression) |
| Other bias | Low risk | No evidence of other sources of bias |
Bargiel‐Matusiewicz 2011a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. However, the methods of intervention and control treatment were physically different, and therefore masking of intervention was unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The AIS was a self‐reported measurement. As such, the outcome assessment was conducted by participants who could be aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Other bias | Unclear risk | It was not clear if there was evidence of imbalance at baseline. Not reported in sufficient detail to perform an adjudication |
Beder 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random assignment to each group–the process whereby cases are assigned to experimental and control groups–ensured that each case had the same probability of being assigned to either group." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The issue of confidentiality was assured as each patient participating in the study was assigned a number by the researcher. All records pertaining to the study were kept off‐site in the office of the researcher." Comment: Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were not told whether they were in the experimental or control group." Comment: The methods of intervention and control treatment were physically different, and therefore masking of treatment allocation for participants and investigators was unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Upon gaining consent to participate, patients were administered the Beck Depression Inventory (BDI‐II) and the Psychosocial Adjustment to Illness Scale by the researcher." Comment: BDI‐II and the Psychosocial Adjustment to Illness Scale used a subjective measure which was likely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote. "A total of 55 participants were initially interviewed in the study. Before reaching their three month re interview date, four participants died, one began dialysis at home, and four were hospitalised too long to remain in the study (over one week). The final sample consisted of 46 participants; 23 participants were in the experimental group and 23 formed the control group." Comment: Overall, 9/55 were lost to the follow up for reasons that appeared unrelated to treatment (> 10% loss to follow‐up, it seems that there was not a differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events) |
| Other bias | Low risk | No evidence of other sources of bias |
Carney 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to either and exercise training group or to a support group." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different, and therefore masking of treatment allocation for participants and investigators was unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The patients completed three psychological tests." Comment: The outcome related to the oxygen concentration was considered objective. The Minnesota Multiphasic Personality Inventory (MMPI), BDI, the Pleasant Events Schedule (PES) and the Unpleasant Events Schedule (UES) were self‐reported measurements. As such, the outcome assessment was conducted by participants who could be aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Three patients (1 in the exercise training, 2 in the support group) were unable to remain in treatment due to time pressure related to employment. One patient in the support group refused to complete the baseline psychological assessment. Thus, 10 patients in the exercise training group and 7 patients in the support group completed the study." Comment: 1/11 in the intervention group and 3/10 in the support group were lost to the follow‐up for reasons possibly related to the treatment |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events, death) |
| Other bias | Low risk | No evidence of other sources of bias |
Cukor 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and investigators were not blinded to treatment assignment. As the intervention and comparator were physically different, it was unlikely that participants and investigators were unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All assessments were conducted by an independent assessor who was blind to the participant’s treatment condition and diagnostic history. [...] The sample’s pretreatment mean scores placed participants in the moderately depressed range, as measured by both clinician administered measures (Hamilton Depression Rating Scale [HAM‐D]; mean 15.2 [SD 6.4]) and self‐report mean depression scores (Beck Depression Inventory II [BDI‐II]; mean 23.3 [SD 9.6])." Comment: The assessor who administered all questionnaires was unaware of the treatment allocation group. Haematological and biochemical data were objective measure of the outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | As reported in the flow chart, 6/65 participants dropped out (2 spent too many days in hospital, 1 drop out, 1 transplant, 2 switched dialysis centres), 5 from treatment group and 1 from control group. As there was a differential loss between groups that may have related to the intervention or outcome, this bias domain was adjudicated as high risk |
| Selective reporting (reporting bias) | Low risk | Study reported many outcomes usual for this type of study. Unclear whether outcomes were reported according to pre‐specified protocol |
| Other bias | Low risk | Study reported statistical methods appropriate for the cross‐over study design. Funding was not involved into the analysis. There were no other apparent sources of bias |
Duarte 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | List for patient allocation was prepared by the research coordination centre. Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The envelopes with the code were sealed and kept at the study site and were consecutively opened when a new patient was selected for inclusion." Comment: The methods did not report whether envelopes were opaque and/or consecutively numbered. Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The interventions were physically different. Therefore it was unlikely that participants and investigators were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The questionnaires were administered and rated by a trained psychologist who was blinded to the treatment group allocation." Comment: The risk of bias for outcome assessment was therefore considered to be low risk for the depression and health related quality of life outcomes. All‐cause death was an objective outcome which was considered to be low risk of bias for outcome assessment despite non‐blinding of participants and investigator. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16 patients (18%) loss to follow up and excluded from analysis. 10/46 were lost to follow‐up from the CBT group (3 transplant; 2 withdrawn; 1 excluded; 4 deaths) and 6/44 were lost to follow‐up from the control group (4 deaths; 1 transplant; 1 withdrawn). As there was differential loss between the two groups that may have related to the treatment or outcome measurement, this risk domain was adjudicated as high risk of bias |
| Selective reporting (reporting bias) | Low risk | Study reported many outcomes usual for this type of study. Unclear whether outcomes were reported according to pre‐specified protocol |
| Other bias | Low risk | Randomisation was not performed using random block size. There was no evidence of substantial imbalance at baseline. No interim analyses were reported |
Dziubek 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The interventions were physically different. Therefore it was unlikely that participants and investigators were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Each patient filled in a personal questionnaire once at the start of the training, and the Beck Depression Inventory (BDI) and the State‐Trait Anxiety Inventory (STAI) twice, at the start of the training and after 6 months. [...] The questionnaire was self‐administered, however, an assistant was available to answer any questions or explain how to fill in the form." Comment: BDI and the STAI were self‐reported measurements. As such, the outcome assessment was conducted by participants who could be aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "A total of 28 patients completed the study." Comment: 1/21 participants in endurance training group and 8/16 in resistance training group were lost to the follow‐up for reasons that appeared unrelated to treatment. There was differential loss between the two groups |
| Selective reporting (reporting bias) | High risk | This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events, hospitalisation) |
| Other bias | Low risk | No evidence of other sources of bias |
Erdley 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The actual treatment condition given to an in‐centre dialysis patient was determined by a random scheme produced by computer software that incorporated a standard procedure for generating random numbers with an allocation ratio of 1:1—that is, to either the Problem‐Solving Therapy + usual care group (n=15) or the usual care only control group (n = 18)." Comment: The computer generation is considered a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The generation of the allocation sequence and the assignment of participants were performed by the Haemodialysis Center secretary." Comment: Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This pilot study used an unblinded design, and participants were informed of their allocation sequence upon completing their baseline measures." Comment: The methods of intervention and control treatment were physically different, participants and investigators were aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The Beck Depression Inventory (BDI) was used to measure depressive symptoms. The Beck Depression Inventory (BDI) is a 21‐item self‐administered questionnaire. [...] The Patient‐Health Questionnaire (PHQ‐9) was used to measure depressive symptoms. The Patient‐Health Questionnaire (PHQ‐9) is a self‐administered version. [...] Secondary outcomes of health related quality of life were assessed by means of the Kidney Disease Quality of Life (KDQOL‐36). [...] The Jaloweic Coping Scale (JCS) was used to measure individual coping skills ability. [...] The Social Problem Solving Inventory, Revised Short Form (SPSI‐R) was used to examine subject‐perceived social‐problem ability across 5 dimensions. The Social Problem Solving Inventory, Revised Short Form (SPSI‐R) is a 25‐item self‐report measure." Comment: The BDI, the Patient‐Health Questionnaire (PHQ‐9) and the Social Problem Solving Inventory, Revised Short Form (SPSI‐R) were completed by participants. Participants were aware of the intervention they received. Therefore, the outcome assessment for depression and social problem solving was not blinded. It was not reported who completed the quality of life assessment measure. Therefore it was unclear whether the completion of this outcome was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Figure 3 shows numbers of recruitment, exclusions, refusal, and dropouts throughout the study. Post randomisation, one participant in the intervention group withdrew due to illness and a second participant died shortly after completing pretest measures." Comment: 2/17 in the intervention group and 0/18 in the control group were lost to the follow‐up for reasons that appeared unrelated to treatment |
| Selective reporting (reporting bias) | Low risk | There was no published protocol for this study. This study report many patient‐centred outcomes that might be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Espahbodi 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were divided into two groups by a random allocation after being somewhat matched according to intervening factors such as age, gender, marital status, education level, duration of dialysis and number of dialysis per week." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The interventions were physically different. Therefore it was unlikely that participants and investigators were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Hospital Anxiety Depression Scale (HADS) questionnaire was completed in both groups before the intervention (by patient and oversight of a psychiatrist)." Comment: The HADS was a self‐reported measurement. As such, the outcome assessment was conducted by participants who could be aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients were excluded from the dialysis group with psycho education. This happened due to a change in dialysis schedules. Besides, one patient was excluded from this group because of having a new stressor during the study. Similarly, in control group (the dialysis group without psycho education) two patients were excluded, one due to changes in dialysis schedule and another due to having a new stressor. Therefore, this study was followed by 27 patients in the dialysis group with psycho education and 28 patients in the control group." Comment: 3/30 in the intervention group (2 changed the dialysis shift, 1 new stressor) and 2/30 in the control group (1 changed the dialysis shift, 1 new stressor) were lost to the follow‐up for reasons that appeared unrelated to treatment |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events, death) |
| Other bias | Unclear risk | It was not clear if there was evidence of imbalance at baseline. Not reported in sufficient detail to perform an adjudication |
Frey 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eleven patients were randomly assigned to two groups." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different, participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes were considered objective as they related to laboratory data. Therefore, the trial was at low risk of bias for outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Eleven patients completed the study, with five participants in the exercise group exercising at the suggested heart rate and six participants in the non‐exercise group remaining sedentary." Comment: All participants completed the study |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events, depression) |
| Other bias | Unclear risk | It was not clear if there was evidence of imbalance at baseline. Not reported in sufficient detail to perform an adjudication |
Frih 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were randomised into the intervention group or the control group using a computer randomisation list." Comment: Computer randomisation list is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different, participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The dialysis adequacy was calculated for each patient according to the formula. [...] The surveys were completed independently during dialysis by those patients capable of doing so. Patients unable to complete them independently because of vision or language problems were assisted by the study staff. [...] All data were collected through face‐to‐face interviews by educated nurses in the Nephrology Department." Comment: The outcome related to Kt/V and functional capacity were considered objective. The Hospital Anxiety and Depression Scale (HADS) and the Short‐Form Health Survey (SF‐36) were self‐reported measurements. As such, the outcome assessment was conducted by participants/investigators who could be aware of the treatment assigned. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | As reported in table 1, all participants completed the study |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events, death) |
| Other bias | Unclear risk | It was not clear if there was evidence of imbalance at baseline. Not reported in sufficient detail to perform an adjudication |
HED‐SMART 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "To minimize contamination, the unit of randomisation was the dialysis shift within each of the participating dialysis centres, using computerized randomisation (1:1 allocation ratio)." Comment: It was unclear if it was a computer random number generator. Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation of randomisation was concealed from study participants until baseline assessment was completed." Comment: Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The was a parallel‐group, blinded, cluster randomised controlled trial. [...] Research assessors and all other staff remained blind to allocation at all
assessment points." Comment: Blinding of participants was not reported. The methods of intervention and control treatment were physically different. Participants could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Depression was assessed using the HADS. The HADS was a self‐reported measurement. Participants could be aware of the treatment assigned. We judged the outcome assessment to be at high risk of bias for this outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Primary analyses were based on the intention‐to‐treat population (all randomly assigned participants, including those without post baseline observations). Missing values were imputed using the last‐observation‐carried‐forward method." Comment: As reported in Figure 1, ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | There was a published protocol for this study. This study report many patient‐centred outcomes that might be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Heshmatifar 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This randomised, controlled, clinical trial was performed on 70 haemodialysis patients." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different, participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Depression was assessed using the BDI‐II. The BDI‐II was a self‐reported measurement. As such, the outcome assessment was conducted by participants who could be aware of the treatment assigned. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The study started with 65 subjects including 33 cases in the intervention group and 32 cases in the control group." Comment: all patients were included into the analysis |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. fatigue, dialysis withdrawal, adverse events) |
| Other bias | Low risk | No evidence of other sources of bias |
Hmwe 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A total of 108 patients with haemodialysis were randomly recruited into the acupressure group (n = 54) and the control group (n = 54). Random sequence allocation was performed using a computer random number generator." Comment: Computer random number generator is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Blinding and allocation concealment were not applied in this study" Comment: Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Open‐label randomised controlled trial." Comment: An open‐label trial is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Questionnaires were subsequently administered by the primary investigator and the staff nurses from the respective haemodialysis centres. Patients responded to the questionnaires during haemodialysis treatment." Comment: The DASS‐21 and General Health Questionnaire‐28 were self‐reported measurements. As such, the outcome assessment was conducted by participants/investigators who could be aware of the treatment assigned. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 102 (94.5%) out of 108 patients completed the study, with 6 (5.6%) declining to complete the study. In the acupressure group, three patients discontinued their participation in the final week. The reasons for the discontinuation of the intervention were intra‐dialytic hypotension (n = 2) and hospital admission due to hypoglycaemic coma (n = 1). Three patients who stopped the intervention early responded to the post‐test questionnaires, thus the baseline data and outcome data were retained. However, in the control group, three patients did not respond to the post‐test questionnaires, thus the outcome data were imputed from their baseline data (pretest data) with the assumption that there was no difference in pre‐test and post‐test. The flow diagram for enrolment, randomised allocation, follow‐up and final analysis is shown in Figure 2." Comment: As reported in Figure 2, intention‐to‐treat analysis was performed: in the end, 108 patients were analysed. However, 3/54 in the intervention group (2 intra‐dialytic hypotension, 1 hospital admission) and 3/54 in the control group (reasons were not reported) were lost‐to‐follow‐up (< 10% loss‐to‐follow‐up; there was not a differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. fatigue, dialysis withdrawal, life participation, death) |
| Other bias | Low risk | No evidence of other sources of bias |
iDiD 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group:
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomisation occurred via Lifeguide which is a software used to program online interventions. An automated random number generator with a 1:1 ratio was used to randomise patients to either therapist supported online cognitive‐behavioural therapy (CBT) or online cognitive‐behavioural therapy (CBT) only." Comment: Computer random number generator is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patient was informed of their group allocation via the online cognitive‐behavioural therapy (CBT) program. The patient and trial coordinator also received an automated email. [...] The nature of the trial meant patients were unblinded to allocated treatments." Comment: The methods of intervention and control treatment were physically different, participants and investigators were aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Patients completed self‐report outcomes at baseline and 12 weeks post‐randomisation." Comment: The Patient Health Questionnaire (PHQ‐9), the Generalised Anxiety Disorder (GAD‐7), the EuroQoL scale (EQ‐5D) and the Brief Illness Perception Questionnaire were self‐reported measurements. As such, the outcome assessment was conducted by participants/investigators who were aware of the treatment assigned. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Twenty‐five patients were randomised to the supported (N =18) or unsupported arm (N =7); 92% were retained at follow‐up." Comment: As reported in Figure 2, 2/18 in the intervention group (1 death, 1 did not like web site) and 0/7 in the control group were lost to the follow‐up (> 10% loss to follow‐up; there was a differential loss between groups) |
| Selective reporting (reporting bias) | Low risk | Protocol was published. This study reported many patient‐centred outcomes that might be expected for a study of this type (i.e. death, depression, adverse events, life participation) |
| Other bias | Low risk | There was no evidence of imbalance at baseline. Funding was not involved into the analysis. There were no other apparent sources of bias |
Kargar Jahromi 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects of the study who were selected based on double blind randomised clinical trial consisted of 60 patients with advanced chronic renal disease treated with haemodialysis." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind randomised clinical trial." Comment: A double blind study is considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Depression was assessed using the DASS‐21. The DASS‐21 was a self‐reported measurement. As such, the outcome assessment was conducted by participants who could be aware of the treatment assigned. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In total, 54 patients completed the study. Despite the attempt of researchers to prevent attrition of samples through attending in the field and telephone follow up, but some of the patients did not complete the study. During the research, three patients in the control group and three patients in the intervention group (one patient because of death, two due to major complications, one patient due to inaccessibility by the researcher, and two patients because of declining to do haemodialysis) were excluded from the study." Comment: 3/30 in the intervention group and 3/30 in the control group were lost to the follow‐up for reasons that appeared unrelated to treatment (10% loss to follow‐up; there was not a differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. fatigue, dialysis withdrawal, life participation, adverse events) |
| Other bias | Low risk | No evidence of other sources of bias |
Kouidi 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the initial evaluation, 24 patients (group A) were randomly assigned to participate in a 6‐months exercise renal rehabilitation program (ERRP) at the Sports Medicine Laboratory, whereas the other 12 patients (group B) served as control subjects." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware of the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A formal psychosocial assessment, which included affective Beck Depression Inventory (BDI‐II), Quality of Life Index (QLI), and Eysenck Personality Questionnaire (EPQ) parameters, was performed with validated questionnaires at the beginning and at the end of the exercise renal rehabilitation program. [...] Psychological tests were administered to all participants at the onset and at the end of the study by the same physician, who was not familiar with the patients, nor with the cardiovascular test or the rehabilitation program." Comment: The physician who administered all questionnaires was unaware of the treatment allocation group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Four patients of group A and one of group B withdrew from the study before the 6‐months testing." Comment: 4/24 in the intervention group and 1/12 in the control group were lost to the follow‐up (> 10% loss to follow‐up; there was a differential loss between groups) |
| Selective reporting (reporting bias) | Low risk | There was no published protocol for this study. This study reported many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, quality of life, depression, adverse events) |
| Other bias | Low risk | No evidence of other sources of bias |
Kouidi 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fifty participants met these criteria and were assigned to either an exercise training (group A) or to a sedentary control group (group B) through complete randomisation." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The Beck Depression Inventory (BDI‐II) is a self‐rating questionnaire for the assessment of the severity of depression. [...] The Hospital Anxiety and Depression Scale (HADS) is a self‐administered questionnaire for assessing depression and anxiety of general hospital patients." Comment: The BDI‐II and the HADS were completed by participants. Participants were aware of the intervention they received. Therefore, the outcome assessment for depression and anxiety was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In 24 patients of group A (one dropped out of the exercise training program), a final similar exercise test was carried out after the completion of the last training session. Five patients of group B were lost to follow‐up." Comment: 1/24 in the intervention group and 5/20 in the control group were lost to the follow‐up (> 10% loss to follow‐up; there was a differential loss between groups) |
| Selective reporting (reporting bias) | Low risk | There was no published protocol for this study. This study reported many patient‐centred outcomes that might be expected for a study of this type (i.e. depression; anxiety; adverse events) |
| Other bias | Low risk | No evidence of other sources of bias |
Krespi 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not reported. However, the methods of intervention and control treatment were physically different. The participants and investigators could be aware of the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were considered subjective. The HADS and BDI‐II were self‐reported measurements. As such, the outcome assessment was conducted by participants who were aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Twenty‐three patients were reported to have not completed follow‐up (10 patients died, one patient had a kidney transplant, and one patient changed dialysis treatment modality; it was not clear the reasons why the remaining participants did not complete study follow‐up) 23/153 (15%) participants did not complete the study. It was unclear whether there was differential loss between treatment groups. The proportion lost to follow‐up was > 10%. We judged study attrition to be at high risk of bias |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, dialysis withdrawal, adverse events) |
| Other bias | Low risk | No evidence of other sources of bias |
Leake 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Blocks of patients were first categorised by sex, months on dialysis and whether they had a comorbid diagnosis of diabetes; there were then randomly assigned to a condition." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients completed a questionnaire that served as a baseline measure of adjustment. [...] Four female research assistants, who were unaware of the conditions and hypotheses of the study, conducted the interviews. [...] The first author, who was unaware of the condition to which each patient was assigned, administered the questionnaires at the three time points." Comment: The first author administered the questionnaires and he was unaware on the treatment assigned group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Data for the 1‐month post‐treatment assessment were not available for 1 male patient who ceased treatment before the 1‐month assessment." Comment: Overall, 1/42 participants was lost to the follow‐up (< 10% loss to follow‐up, it was not clear if there was a substantial differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, fatigue, death, dialysis withdrawal, adverse events) |
| Other bias | Unclear risk | It was not clear if there was evidence of imbalance at baseline. Not reported in sufficient detail to perform an adjudication |
Lerma 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants in the study were randomly assigned to either the intervention or the control group. [...] The assignment to one of the groups was providing using random numbers." Comment: It was not clear if randomisation was provided using a computer. Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A single‐blind, randomised controlled design was used to compare patients with ESKD under haemodialysis treatment with and without the cognitive behavioural intervention. [...] The assignment to one of the groups was concealed to the therapist health‐related staff, and administrative personnel. After enrolment, patients became aware of their experimental or control allocation because the waiting list group was informed of the wait period in line with ethical requirements." Comment: A single‐blind study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The BDI‐II, the Beck Anxiety Inventory (BAI), the Quality of Life Scale score, the Distorted Thought Scale (DTS), the Dysfunctional Attitudes Scale (DAS‐A) and the Cognition Check List were self‐reported questionnaires. Participants were aware of the intervention they received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | As reported in Figure 1, 7/38 in the intervention group (2 deaths, 3 transportation troubles, 2 personal reasons) and 4/22 (1 death, 3 personal troubles) in the control group were lost to the follow‐up for reasons that appeared unrelated to treatment (> 10% loss to follow‐up; there was not a differential loss between groups). |
| Selective reporting (reporting bias) | Low risk | There was no published protocol for this study. This study reported many patient‐centred outcomes that might be expected for a study of this type (i.e. life participation, depression, death) |
| Other bias | Low risk | No evidence of other sources of bias |
Lii 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent research assistant (unaware of the baseline data) carried out the concealed randomisation procedure using a random computer‐generated list." Comment: Random computer‐generated list is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "An independent research assistant (unaware of the baseline data) carried out the concealed randomisation procedure using a random computer‐generated list." Comment: Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were considered subjective. The Strategies Used by People to Promote Health (SUPPH), the BDI‐II and the Short Form‐36 (SF‐36) were self‐reported measurements. As such, the outcome assessment was conducted by participants who could be aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Out of 60 original patients randomly assigned into experimental or control groups, 48 completed the study. [...] After intervention for eight weeks, there were 12 patients who dropped out, including 10 in the treatment group and two in the control group. Reasons for dropout included obligations at home, transfers to another haemodialysis unit or time conflicts." Comment: 10/30 in the intervention group and 2/30 in the control group were lost to the follow‐up (> 10% loss to follow‐up; there was a differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. fatigue, death, dialysis withdrawal, adverse events) |
| Other bias | Low risk | No evidence of other sources of bias |
Mathers 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly selected and stratified according to gender, with 2 males and 3 females assigned to either an experimental or control group." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were considered subjective. The Psychosocial Adjustment to Illness Scale, Self‐Report (PAIS‐SR) was self‐reported measurements. As such, the outcome assessment was conducted by participants who could be aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Six out of 10 original patients, 1 male and 2 females in each of the experimental and control group, completed the study." Comment: 2/5 in the intervention group and 2/5 in the control group were lost to the follow‐up (> 10% loss to follow‐up; there was not a differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. depression, life participation, fatigue, death, dialysis withdrawal, adverse events) |
| Other bias | Unclear risk | It was not clear if there was evidence of imbalance at baseline. Not reported in sufficient detail to perform an adjudication |
Matthews 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A volunteer, blinded to the purpose and the hypothesis of the study, randomly assigned each subject to one of the six treatment condition." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "A total of 10 self‐reported dependent measures were analysed." Comment: Laboratory data were considered as objective data. However, all questionnaires were self‐reported measurements. As such, the outcome assessment was conducted by participants who could be aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | As reported in Tables 2 and 3, the number of subjects vary because some subjects were not available at the time of data collection due to their medical condition. The overall number of patients who were lost to the follow‐up and the potential differential loss between groups were unclear |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. fatigue, death, dialysis withdrawal, adverse events) |
| Other bias | Unclear risk | It was not clear if there was evidence of imbalance at baseline. Not reported in sufficient detail to perform an adjudication |
Ouzouni 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomised to either a 10‐months supervised exercise‐training programme during their haemodialysis sessions (group A – 20 patients) or control status (group B – 15 patients)." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All patients were requested to complete the following five different questionnaires." Comment: Laboratory and spiroergometry data were considered as objective data. However, all questionnaires were self‐reported measurements. As such, the outcome assessment was conducted by participants who could be aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "After the randomisation two patients, one of each group, dropped out of the study. One patient in group A stopped training because of medical problems unrelated to exercise, while a patient in group B refused to repeat the functional test at the end of the study." Comment: 1/20 in the intervention group and 1/15 in the control group were lost to the follow‐up for reasons that were unrelated to the treatment (<10% loss to follow‐up; there was not a differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. fatigue, death, dialysis withdrawal, adverse events) |
| Other bias | Low risk | No evidence of other sources of bias |
Sertoz 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "These were then randomly assigned to two groups." Comment: Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Both Beck Depression Inventory (BDI‐II) and Beck Anxiety Inventory (BAI) are self‐report questionnaires consisting of 21 items." Comment: All questionnaires were self‐reported measurements. As such, the outcome assessment was conducted by participants who could be aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Table 3 reported that 15 participants in the intervention group completed the study (0/15 lost to follow‐up). However, the number of participants who completed the study in the control group (group B) was not reported |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. fatigue, death, dialysis withdrawal, adverse events) |
| Other bias | Unclear risk | It was not clear if there was evidence of imbalance at baseline. Not reported in sufficient detail to perform an adjudication |
Sofia 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | KDQOL‐SF was a self‐reported measurement. As such, the outcome assessment was conducted by participants who could be aware of the treatment received. We judged the outcome assessment to be at high risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Other bias | Unclear risk | Not reported in sufficient detail to perform an adjudication |
Thomas 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The interventionists, who were not involved in the recruitment process and patient assessment, randomised the participant codes to the intervention group or the control group, using a simple 1:1 computer‐generated sequence." Comment: Computer‐generated sequence is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not reported. The methods of intervention and control treatment were physically different. Participants could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both the assessor and the statistical associate were blinded to randomisation allocation. [...] Participants completed questionnaires with an independent assessor who then assigned each of them an anonymous code." Comment: The outcome assessment was conducted by the assessor who was unaware of the treatment received. We judged the outcome assessment to be at low risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the participants randomised to the intervention group, 71% completed the study." Comment: As reported in Figure 1, 4/21 in the intervention group and 5/20 in the control group did not complete the post‐questionnaire. Moreover, in the intervention group 6/21 patients did not complete the intervention assigned (> 10% loss to follow‐up; there was a differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. fatigue, death, dialysis withdrawal, life participation) |
| Other bias | Unclear risk | It was not clear if funding was involved into the analysis. There was no evidence of substantial imbalance at baseline. No interim analyses were reported |
Tsai 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random allocation sequence was generated using free online software providing randomly permuted blocks and random block sizes". Comment: Investigators describes a random component in the sequence generation that could be considered as low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Another independent research assistant who did not participate in participant enrolment, data collection, or data analyses generated the allocation sequence. The allocation sequence was concealed in sequentially numbered, opaque, sealed envelopes that were safeguarded by the primary investigator (one of us, P‐ST) until it was time to assign the participants to groups. The dialysis nurse who delivered the intervention ensured that each envelope was still sealed, wrote a participant’s name." Comment: investigators could not foresee assignment and it could be considered as low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This was an outcome assessor–blind, randomisation controlled trial. [...] An independent research assistant (one of us, S‐HT) who was not involved in implementing the intervention and who was blinded to participants’ group allocation performed the outcome assessment." Comment: The outcome assessment was conducted by the assessor who was unaware of the treatment received. We judged the outcome assessment to be at low risk of bias for these outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Sixty‐four participants were randomised equally to either the intervention or the control group. Three participants in the control group subsequently withdrew because of hospitalisation; and four participants in the control group refused to complete post‐test questionnaires at Week 6. Only the 57 participants who completed the posttest questionnaires were included in the data analysis." Comment: As reported in the flow chart, 0/32 in nurse‐led breathing training group and 7/32 in control group were lost to follow up (> 10% loss to follow up, there was a differential loss between groups) |
| Selective reporting (reporting bias) | High risk | There was no published protocol for this study. This study did not report many patient‐centred outcomes that might be expected for a study of this type (i.e. fatigue, death, dialysis withdrawal, adverse events) |
| Other bias | Low risk | No evidence of other sources of bias |
Vogt 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to perform an adjudication |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to perform an adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and/or the investigators was not reported. The methods of intervention and control treatment were physically different. Participants and investigators could be aware on the treatment allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Other bias | Unclear risk | Not reported in sufficient detail to perform an adjudication |
AIS ‐ Acceptance of Illness Scale; BDI ‐ Beck Depression Index; BP ‐ blood pressure; CBT ‐ cognitive‐behavioural therapy; CES‐D ‐ Center for Epidemiological Studies Depression; CKD ‐ chronic kidney disease; DASS ‐ Depression Anxiety Stress Scales; DBP ‐ diastolic blood pressure; DSM ‐ Diagnostic and Statistical Manual of Mental Disorders; ESKD ‐ end‐stage kidney disease; HCT ‐ hematocrit; HD ‐ haemodialysis; HADS ‐ Hospital Anxiety Depression Scale; HAM‐D ‐ Hamilton Depression Rating Scale; HRQoL ‐health‐related quality of life; IDWG ‐ interdialytic weight gain; KDQOL‐SF ‐ Kidney Disease and Quality of Life‐Short Form; Kt/V ‐ dialyser urea clearance adequacy; M/F ‐ male/female; MHS ‐ Miller Hope Scale; MINI ‐ Mini International Neuropsychiatric Interview; NYHA ‐ New York Heart Association; QoL ‐ quality of life; RCT ‐ randomised controlled trial; SBP ‐ systolic blood pressure; SCID ‐ structured clinical interview for DSM; SD ‐ standard deviation; STAI ‐ State‐Trait Anxiety Inventory; SCr ‐ serum creatinine; URR ‐ urea reduction ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allmann 1990 | Wrong intervention/control: this study evaluated the glucose polymer Polycose among people treated with HD (not a psychosocial intervention) |
| ASCEND 2016 | Wrong intervention/control: a psychosocial intervention was compared with a pharmacological intervention |
| Binik 1993 | Wrong population: participants were identified before dialysis was required to preserve their lives |
| Briggs 2004 | Wrong intervention: this study evaluated advanced care planning (not a psychosocial intervention; subject of another Cochrane review) |
| Burkhalter 2015 | Wrong population: kidney transplant recipients more than 1 year post‐transplant |
| dos Rios Santos 2013 | Wrong intervention/control: examined sleep and autonomic sleep function before and after HD (not a psychosocial intervention) |
| Hosseini 2012 | Wrong intervention/control: psychosocial intervention was compared with a pharmacological intervention |
| IRCT2017020311885N8 | Wrong intervention/control: evaluated trans‐cranial stimulation (physical intervention) |
| Kao 2012 | Wrong population: participants with CKD who did not receive dialysis treatment were randomised |
| NCT1225458 | Wrong intervention/control: evaluated additional clinical assessments (not a psychosocial intervention) |
| Sangill 2006 | Wrong intervention/control: evaluated glucose added to the dialysis fluid (not a psychosocial intervention) |
| SMILE 2010 | Wrong intervention/control: evaluated the effects of feedback to renal providers or nurse management of patients to treat symptoms related to ESKD (not psychosocial interventions) |
| Watson 2015 | Wrong population: participants were in pre‐dialysis CKD (stages 3 and 4) |
| Zhao 2017 | Wrong intervention/control: psychosocial intervention was compared with a pharmacological intervention |
CKD ‐ chronic kidney disease; ESKD ‐ end‐stage kidney disease; HD ‐ haemodialysis
Characteristics of ongoing studies [ordered by study ID]
DOHP 2016.
| Trial name or title | Design and protocol for the Dialysis Optimal Health Program (DOHP) randomised controlled trial |
| Methods | The study design is a prospective randomised controlled trial. Ninety‐six adult patients initiating HD or PD will be randomly allocated to either the intervention (DOHP) or usual care group. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | March 2015 |
| Contact information | Chantal F. Ski; Email: Chantal.Ski@acu.edu.au. |
| Notes | Setting: Nephrology unit of St Vincent’s Hospital, Melbourne, Australia. Trial Registration number: ACTRN12615000810516. |
NCT02011139.
| Trial name or title | Cognitive‐behavioural (CBT) in ESRD patients with depression |
| Methods | Patients in HD were divided in CBT group and control group |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | October 2013 |
| Contact information | C. S. Lim; Email: cslimjy@snu.ac.kr |
| Notes | Setting: Seoul National University Boramae Hospital, Seoul, Korea. Trial registration number: NCT02011139. |
NCT03162770.
| Trial name or title | Mindfulness meditation practice during haemodialysis |
| Methods | Fifty patients will be randomly separated in two groups, twenty five each group, half of them in the control group and the other half in the intervention group |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
Then after the evaluations, the control group will receive the intervention of meditation, while the intervention group will not receive any intervention |
| Outcomes |
|
| Starting date | March 2018 |
| Contact information | Erika Bevilaqua Rangel; Email: not reported |
| Notes | Setting: Hospital Israelita Albert Einstein. Brazil. Trial registration number: NCT03162770 |
NCT03330938.
| Trial name or title | Decreasing depression and anxiety and their effect on QoL of ESRD patients (end‐stage renal disease) (ESRD) |
| Methods | Depressed patients with ESKD were randomised to the cognitive‐behavioural intervention and resilience group (intervention group) or cognitive‐behavioural Intervention without resilience |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | December 2017 |
| Contact information | Cristina Jazmín Gonzalez Flores; Email: crisjaz_10@hotmail.com |
| Notes | Setting: Hospital Civil de Guadalajara, Jalisco, Mexico. Trial registration number: NCT03330938 |
NCT03406845.
| Trial name or title | Mindfulness and HEP in dialysis patients with depression and anxiety |
| Methods | HD patients with depression were randomly assigned in chair‐side mindfulness intervention or Health Enhancement Plan (HEP) group |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | May 2018 |
| Contact information | Marouane Nassim; Email: marouane.nassim@mail.mcgill.ca |
| Notes | Setting: McGill University Health Center, Toronto. Trial registration number: NCT03406845. |
van der Borg 2016.
| Trial name or title | Protocol of a mixed method, randomised controlled study to assess the efficacy of a psychosocial intervention to reduce fatigue in patients with End‐stage renal disease (ESRD) |
| Methods | This study follows a mixed‐methods design in which both quantitative and qualitative data will be collected. A multi‐centre, RCT with repeated measures will be conducted to quantitatively assess the efficacy of the psychosocial intervention in reducing fatigue and improving QoL in ESKD patients. Additional secondary outcomes and medical parameters will be assessed. Outcomes will be compared to patients receiving usual care. A sample of 74 severely fatigued dialysis patients will be recruited from 10 dialysis centres. Patients will be randomly assigned to the intervention or control group. Outcomes will be assessed at baseline, post intervention/16 weeks, and at three and six‐month follow‐ups. A qualitative process evaluation will be conducted parallel to/following the effectiveness RCT. Interviews and focus groups will be conducted to gain insight into patients’ and social workers’ perspectives on outcomes and implementation procedures. Implementation fidelity will be assessed by audio‐taped and written registrations. Participatory methods ensure the continuous input of experiential knowledge, improving the quality of study procedures and the applicability of outcomes. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | Not reported |
| Contact information | W. van der Borg; Email: w.vanderborg@vumc.nl |
| Notes | Setting: Department of Medical Humanities, VU University Medical Center/EMGO, Amsterdam, The Netherlands. Trial registration number: NTR5366 |
WICKD 2019.
| Trial name or title | Wellbeing intervention for chronic kidney disease (WICKD): a randomised controlled trial study protocol |
| Methods | This is a 3‐arm, wait list, single‐blind randomised controlled trial testing the efficacy of the Stay Strong App in improving psychological distress, depressive symptoms, QoL and treatment adherence among Indigenous clients undergoing HD in Alice Springs and Darwin with follow‐up over two periods of 3 months (total of 6 months observation). The study compares the efficacy of MCP using the AIMhi Stay Strong App with two control groups (control app intervention and treatment as usual). |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group 1
Control group 2
|
| Outcomes |
|
| Starting date | February 2017 |
| Contact information | Kylie M. Dingwall; kylie.dingwall@menzies.edu.au |
| Notes | Protocol. Setting: Alice Springs and Charles Darwin University, Australia. Trial registration number: ACTRN12617000249358 |
AKI ‐ acute kidney injury; BAI ‐ Beck Anxiety Inventory; BDI‐II ‐ Beck Depression Inventory‐II; CBT ‐ cognitive behaviour therapy; ESKD ‐ end‐stage kidney disease; HADS ‐ Hospital Anxiety and Depression Scale; HD ‐ haemodialysis; HRQoL ‐ health‐related quality of life; KDQOL ‐ Kidney Disease Quality of Life; PD ‐ peritoneal dialysis; PSQI ‐ Pittsburgh Sleep Quality Index; QoL ‐ quality of life
Differences between protocol and review
The inclusion criteria for the 2019 Cochrane review update were expanded on discussion with the editorial group to include participants with or without depression at baseline. Furthermore, seven additional studies included in the 2005 review were identified in the updated search and added to the review (Beder 1999; Carney 1987; Frey 1999; Kouidi 1997; Leake 1999; Mathers 1999; Matthews 2001).
During the process of this review update, 21 studies were removed from the 2005 review as these studies were not relevant for our review.
We have included Summary of Findings tables for the comparisons of: CBT versus usual care; counselling versus usual care; exercise versus usual care; relaxation techniques versus usual care and spiritual practice versus usual care.
Contributions of authors
Writing of protocol and review: PN, SCP, MR, KR, GFMS
Screening of titles and abstracts: SCP, PN, MR
Assessment for inclusion: SCP, PN, MR
Quality assessment: SCP, PN, MR
Data extraction: SCP, PN, MR
Data entry into RevMan: SCP, PN
Data analysis: SCP, PN, VS, KR, GFMS
Disagreement resolution: SCP, GFMS
Sources of support
Internal sources
No sources of support supplied
External sources
National Kidney Research Fund, UK.
Declarations of interest
Giovanni Strippoli has in the past received consulting fees from Diaverum AB.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Al Saraireh 2018 {published data only}
- Al Saraireh FA, Aloush SM, Al Azzam M, Al Bashtawy M. The effectiveness of cognitive behavioral therapy versus psychoeducation in the management of depression among patients undergoing haemodialysis. Issues in Mental Health Nursing 2018;39(6):514‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Babamohamadi 2017 {published data only}
- Babamohamadi H, Sotodehasl N, Koenig HG, Al Zaben F, Jahani C, Ghorbani R. The effect of Holy Qur'an recitation on depressive symptoms in hemodialysis patients: a randomized clinical trial. Journal of Religion & Health 2017;56(1):345‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bahmani 2016 {published data only}
- Bahmani B, Motamed NM, Sayyah M, Shafi‐Abadi A, Haddad KH. The effectiveness of cognitive‐existential group therapy on increasing hope and decreasing depression in women‐treated with haemodialysis. Global Journal of Health Science 2016;8(6):219‐25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bargiel‐Matusiewicz 2011 {published data only}
- Bargiel‐Matusiewicz K, Trzcieniecka‐Green A, Kozlowska A. The influence of psychological intervention on cognitive appraisal and level of anxiety in dialysis patients: a pilot study. Open Nutraceuticals Journal 2011;4(Special Issue):61‐4. [EMBASE: 2011372258] [Google Scholar]
Bargiel‐Matusiewicz 2011a {published data only}
- Bargiel‐Matusiewicz K, Januszek M, Kroemeke A. The influence of psychological intervention on the level of acceptance of illness in dialysis patients and patients with multiple sclerosis [abstract]. Psychology & Health 2011;26(2 Suppl):86. [EMBASE: 2011372258] [Google Scholar]
Beder 1999 {published data only}
- Beder J. Evaluation research on the effectiveness of social work intervention on dialysis patients: the first three months. Social Work in Health Care 1999;30(1):15‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carney 1987 {published data only}
- Carney RM, Templeton B, Hong BA, Harter HR, Hagberg JM, Schechtman KB, et al. Exercise training reduces depression and increases the performance of pleasant activities in hemodialysis patients. Nephron 1987;47(3):194‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cukor 2014 {published data only}
- Cukor D, Ver Halen N, Asher DR, Coplan JD, Weedon J, Wyka KE, et al. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. Journal of the American Society of Nephrology 2014;25(1):196‐206. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duarte 2009 {published data only}
- Duarte PS, Miyazaki MC, Blay SL, Sesso R. Cognitive‐behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney International 2009;76(4):414‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sesso R, Miyasaki MC, Duarte PS. Effectiveness of a cognitive‐behavioral therapy in hemodialysis patients with depression [abstract no: F‐FC232]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):52A. [CENTRAL: CN‐00740531] [Google Scholar]
Dziubek 2016 {published data only}
- Dziubek W, Kowalska J, Kusztal M, Rogowski L, Golebiowski T, Nikifur M, et al. The level of anxiety and depression in dialysis patients undertaking regular physical exercise training‐‐a preliminary study. Kidney & Blood Pressure Research 2016;41(1):86‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Erdley 2014 {published data only}
- Erdley SD, Gellis ZD, Bogner HA, Kass DS, Green JA, Perkins RM. Problem‐solving therapy to improve depression scores among older hemodialysis patients: a pilot randomized trial. Clinical Nephrology 2014;82(1):26‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Espahbodi 2015 {published data only}
- Espahbodi F, Hosseini H, Mirzade MM, Shafaat AB. Effect of psycho education on depression and anxiety symptoms in patients on hemodialysis. Iranian Journal of Psychiatry and Behavioral Sciences 2015;9(1):e227. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frey 1999 {published data only}
- Frey S, Mir AR, Lucas M. Visceral protein status and caloric intake in exercising versus nonexercising individuals with end‐stage renal disease. Journal of Renal Nutrition 1999;9(2):71‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frih 2017 {published data only}
- Frih B, Mkacher W, Bouzguenda A, Jaafar H, ALkandari SA, Ben Salah Z, et al. Effects of listening to Holy Qur'an recitation and physical training on dialysis efficacy, functional capacity, and psychosocial outcomes in elderly patients undergoing haemodialysis. Libyan Journal of Medicine 2017;12(1):1372032. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
HED‐SMART 2011 {published data only}
- Griva K, Lam KF, Nandakumar M, Ng JH, McBain H, Newman SP. The effect of brief self‐management intervention for hemodialysis patients (HED‐SMART) on trajectories of depressive and anxious symptoms. Journal of Psychosomatic Research 2018;113:37‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Griva K, Mooppil N, Krishnan DS, McBain H, Newman SP. Short and long‐term outcomes of the hemodialysis self management intervention randomised trial (HED‐SMART) ‐ a practical low intensity intervention to improve adherence and clinical markers [abstract]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i472. [CENTRAL: CN‐01027019; EMBASE: 71076463] [Google Scholar]
- Griva K, Mooppil N, Pala DS, Hayley J, Newman S, Ng J. A self‐management intervention to improve treatment adherence in hemodialysis patients‐a randomized controlled trial [abstract]. Psychology & Health 2011;26(Suppl 2):293. [CENTRAL: CN‐01658204] [Google Scholar]
- Griva K, Mooppil N, Seet P, Krishnan DS, James H, Newman SP. The NKF‐NUS hemodialysis trial protocol ‐ a randomized controlled trial to determine the effectiveness of a self management intervention for hemodialysis patients. BMC Nephrology 2011;12:4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griva K, Nandakumar M, Ng JH, Lam KF, McBain H, Newman SP. Hemodialysis Self‐management Intervention Randomized Trial (HED‐SMART): a practical low‐Intensity intervention to improve adherence and clinical markers in patients receiving hemodialysis [Erratum appears in Am J Kidney Dis. 2018 Jul;72 (1):157‐158; PMID: 29937025]. American Journal of Kidney Diseases 2018;71(3):371‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lam KF, Mooppil N, Ng HJ, Kang AW, McBain H, Newman S, et al. Longitudinal trajectories of depressive and anxious symptoms following a self‐management intervention for haemodialysis patients [abstract]. Value in Health 2016;19(7):A847. [EMBASE: 613235990] [Google Scholar]
Heshmatifar 2015 {published data only}
- Heshmatifar N, Sadeghi H, Mahdavi A, Shegarf Nakhaie MR, Rakhshani MH. The effect of benson relaxation technique on depression in patients undergoing hemodialysis. Journal of Babol University of Medical Sciences 2015;17(8):34‐40. [EMBASE: 2015254548] [Google Scholar]
Hmwe 2015 {published data only}
- Hmwe NT, Subramanian P, Tan LP, Chong WK. The effects of acupressure on depression, anxiety and stress in patients with hemodialysis: a randomized controlled trial. International Journal of Nursing Studies 2015;52(2):509‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
iDiD 2016 {published data only}
- Hudson JL, Moss‐Morris R, Game D, Carroll A, McCrone P, Hotopf M, et al. Improving distress in dialysis (iDiD): a feasibility two‐arm parallel randomised controlled trial of an online cognitive behavioural therapy intervention with and without therapist‐led telephone support for psychological distress in patients undergoing haemodialysis. BMJ Open 2016;6(4):e011286. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JL, Moss‐Morris R, Norton S, Picariello F, Game D, Carroll A, et al. Tailored online cognitive behavioural therapy with or without therapist support calls to target psychological distress in adults receiving haemodialysis: a feasibility randomised controlled trial. Journal of Psychosomatic Research 2017;102:61‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kargar Jahromi 2016 {published data only}
- Kargar Jahromi M, Javadpour S, Taheri L, Poorgholami F. Effect of nurse‐led telephone follow ups (tele‐nursing) on depression, anxiety and stress in hemodialysis patients. Global Journal of Health Science 2016;8(3):168‐73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kouidi 1997 {published data only}
- Kouidi E, Iacovides A, Iordanides P, Vassiliou S, Deligiannis A, Ierodiakonou Ch, et al. Exercise renal rehabilitation program (ERRP): psychosocial effects [abstract]. Nephrology Dialysis Transplantation 1995;10(6):1015. [CENTRAL: CN‐00261120] [DOI] [PubMed] [Google Scholar]
- Kouidi E, Iacovides A, Iordanidis P, Vassiliou S, Deligiannis A, Ierodiakonou C, et al. Exercise renal rehabilitation program: psychosocial effects. Nephron 1997;77(2):152‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kouidi 2010 {published data only}
- Kouidi E, Karagiannis V, Grekas D, Iakovides A, Kaprinis G, Tourkantonis A, et al. Depression, heart rate variability, and exercise training in dialysis patients. European Journal of Cardiovascular Prevention & Rehabilitation 2010;17(2):160‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krespi 2009 {published data only}
- Krespi MR, Oakley D, Bone M, Ahmad R, Salmon P. The effects of visual imagery on adjustment and quality in life of hemodialysis patients [Gorsel Imgelemenin Hemodiyaliz Hastalarinin Uyum ve Yasam Kalitesine Etkisi]. Turk Psikiyatri Dergisi 2009;20(3):255‐68. [MEDLINE: ] [PubMed] [Google Scholar]
Leake 1999 {published data only}
- Leake R, Friend R, Wadhwa N. Improving adjustment to chronic illness through strategic self‐presentation: an experimental study on a renal dialysis unit. Health Psychology 1999;18(1):54‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lerma 2017 {published data only}
- Lerma A, Perez‐Grovas H, Bermudez L, Peralta‐Pedrero ML, Robles‐Garcia R, Lerma C. Brief cognitive behavioural intervention for depression and anxiety symptoms improves quality of life in chronic haemodialysis patients. Psychology & Psychotherapy: Theory, Research & Practice 2017;90(1):105‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lii 2007 {published data only}
- Lii YC, Tsay SL, Wang TJ. Group intervention to improve quality of life in haemodialysis patients. Journal of Clinical Nursing 2007;16(11C):268‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mathers 1999 {published data only}
- Mathers TR. Effects of psychosocial education on adaptation in elderly hemodialysis patients. ANNA Journal 1999;26(6):587‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Matthews 2001 {published data only}
- Conti JM. The effects of intercessory prayer and transpersonal positive visualization on a hemodialysis population [dissertation]. Dissertation Abstracts International 1999, (2‐B):1‐162. [CENTRAL: CN‐00671878] [Google Scholar]
- Matthews WJ, Conti JM, Sireci SG. The effects of intercessory prayer, positive visualization, and expectancy on the well‐being of kidney dialysis patients. Alternative Therapies in Health & Medicine 2001;7(5):42‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Ouzouni 2009 {published data only}
- Ouzouni S, Kouidi E, Sioulis A, Grekas D, Deligiannis A. Effects of intradialytic exercise training on health‐related quality of life indices in haemodialysis patients. Clinical Rehabilitation 2009;23(1):53‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sertoz 2009 {published data only}
- Sertoz OO, Asci G, Toz F, Duman S, Elbi H, Ok E. Planning a social activity to improve psychological well‐being and quality of life of hemodialysis patients: a pilot study. Therapeutic Apheresis & Dialysis 2009;13(4):366‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sofia 2013 {published data only}
- Sofia NA, Widyaningrum Siswanto A, Djarwoto B, Asdie AH, Shatri H, et al. The influences of relaxation training (Latihan Pasrah Diri) on quality life in haemodialysis patients with depressive symptoms [abstract no: 384]. Psychotherapy & Psychosomatics 2013;82(Suppl 1):106. [EMBASE: 71183866] [Google Scholar]
Thomas 2017 {published data only}
- Thomas Z, Novak M, Platas SG, Gautier M, Holgin AP, Fox R, et al. Brief mindfulness meditation for depression and anxiety symptoms in patients undergoing hemodialysis: a pilot feasibility study. Clinical Journal of the American Society of Nephrology: CJASN 2017;12(12):2008‐15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2015 {published data only}
- Tsai SH, Wang MY, Miao NF, Chian PC, Chen TH, Tsai PS. CE: original research: The efficacy of a nurse‐led breathing training program in reducing depressive symptoms in patients on hemodialysis: a randomized controlled trial. American Journal of Nursing 2015;115(4):24‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vogt 2016 {published data only}
- Vogt W. Acceptance and commitment therapy self‐help intervention for depression in haemodialysis patients: a feasibility randomised controlled trial [abstract no: P227]. UK Kidney Week. Joint Meeting of the British Renal Society & the Renal Association; 2016 Jun 7‐10; Birmingham, UK. 2016.
References to studies excluded from this review
Allmann 1990 {published data only}
- Allman MA, Stewart PM, Tiller DJ, Horvath JS, Duggin GG, Truswell AS. Energy supplementation and the nutritional status of hemodialysis patients. American Journal of Clinical Nutrition 1990;51(4):558‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ASCEND 2016 {published data only}
- Hedayati SS, Daniel DM, Cohen S, Comstock B, Cukor D, Diaz‐Linhart Y, et al. Rationale and design of a trial of sertraline vs. cognitive behavioral therapy for end‐stage renal disease patients with depression (ASCEND). Contemporary Clinical Trials 2016;47:1‐11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Binik 1993 {published data only}
- Binik YM, Devins GM, Barre PE, Guttmann RD, Hollomby DJ, Mandin H, et al. Live and learn: patient education delays the need to initiate renal replacement therapy in end‐stage renal disease. Journal of Nervous & Mental Disease 1993;181(6):371‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Devins GM, Mendelssohn DC, Barre PE, Taub K, Binik YM. Predialysis psychoeducational intervention extends survival in CKD: a 20‐year follow‐up. American Journal of Kidney Diseases 2005;46(6):1088‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Briggs 2004 {published data only}
- Briggs LA, Kirchhoff KT, Hammes BJ, Song MK, Colvin ER. Patient‐centered advance care planning in special patient populations: a pilot study. Journal of Professional Nursing 2004;20(1):47‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burkhalter 2015 {published data only}
- Burkhalter H, Denhaerynck K, Wirz‐Justice A, Cajochen C, Weaver T, Steiger J, et al. A pilot randomized controlled study of light therapy for sleep‐wake disturbances in renal transplant recipients [abstract no: PO46]. Transplant International 2013;26(Suppl 2):194. [EMBASE: 71359678] [Google Scholar]
- Burkhalter H, Wirz‐Justice A, Denhaerynck K, Fehr T, Steiger J, Venzin R, et al. A pilot multi‐center randomized controlled study of bright light therapy for sleep‐wake disturbances in renal transplant recipients [abstract no: D2728]. Transplantation 2014;98(Suppl 1):843. [EMBASE: 71546414] [Google Scholar]
- Burkhalter H, Wirz‐Justice A, Denhaerynck K, Fehr T, Steiger J, Venzin RM, et al. The effect of bright light therapy on sleep and circadian rhythms in renal transplant recipients: a pilot randomized, multicentre wait‐list controlled trial. Transplant International 2015;28(1):59‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
dos Rios Santos 2013 {published data only}
- dos Reis Santos I, Danaga AR, Carvalho Aguiar I, Oliveira EF, Dias IS, Urbano JJ, et al. Cardiovascular risk and mortality in end‐stage renal disease patients undergoing dialysis: sleep study, pulmonary function, respiratory mechanics, upper airway collapsibility, autonomic nervous activity, depression, anxiety, stress and quality of life: a prospective, double blind, randomized controlled clinical trial. BMC Nephrology 2013;14(1):215. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosseini 2012 {published data only}
- Hosseini SH, Espahbodi F, Mirzadeh Goudarzi SM. Citalopram versus psychological training for depression and anxiety symptoms in hemodialysis patients. Iranian Journal of Kidney Diseases 2012;6(6):446‐51. [MEDLINE: ] [PubMed] [Google Scholar]
IRCT2017020311885N8 {published data only}
- Aalishah A. Effects of transcranial direct currency stimulation (tDCS) on the treatment of depressive and anxiety symptoms and improve quality of sleep in patients with chronic renal diseases on dialysis ‐ a randomized double‐blind placebo controlled trial. en.search.irct ir/view/35779 (first received 1 April 2017).
Kao 2012 {published data only}
- Kao YH, Huang Y, Chen P, Wang K. The effects of exercise education intervention on the exercise behaviour, depression, and fatigue status of chronic kidney disease patients. Health Education 2012;112(6):472‐84. [CENTRAL: CN‐00852837] [Google Scholar]
NCT1225458 {published data only}
- NCT01225458. Behaviour and cognitive evaluation for dialysis elderly patients (BCDE). www.clinicaltrials.gov/show/nct01225458 (first received 21 October 2010).
Sangill 2006 {published data only}
- Sangill M, Pedersen EB. The effect of glucose added to the dialysis fluid on blood pressure, blood glucose and quality of life in hemodialysis patients. A placebo controlled cross‐over trial. Lower blood pressure with glucose in dialysis water [abstract no: SP606]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv219. [CENTRAL: CN‐00583924] [DOI] [PubMed] [Google Scholar]
- Sangill M, Pedersen EB. The effect of glucose added to the dialysis fluid on blood pressure, blood glucose, and quality of life in hemodialysis patients: a placebo‐controlled crossover study. American Journal of Kidney Diseases 2006;47(4):636‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SMILE 2010 {published data only}
- Belayev LY, Mor MK, Sevick MA, Shields AM, Rollman BL, Palevsky PM, et al. Longitudinal associations of depressive symptoms and pain with quality of life in patients receiving chronic hemodialysis. Hemodialysis International 2015;19(2):216‐24. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Mor MK, Shields A, Sevick MA, Arnold RM, Palevsky PM, et al. Health literacy and outcomes in hemodialysis patients [abstract no: TH‐PO639]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):260A. [Google Scholar]
- Green JA, Mor MK, Shields AM, Sevick MA, Arnold RM, Palevsky PM, et al. Associations of health literacy with dialysis adherence and health resource utilization in patients receiving maintenance hemodialysis. American Journal of Kidney Diseases 2013;62(1):73‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Green JA, Mor MK, Shields AM, Sevick MA, Palevsky PM, Fine MJ, et al. Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(6):1354‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Mor MK, Shields AM, Sevik MA, Palevsky PM, Fine MJ, et al. Renal provider perceptions and practice patterns regarding the management of pain, sexual dysfunction, and depression in hemodialysis patients. Journal of Palliative Medicine 2012;15(2):163‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mor MK, Sevick MA, Shields AM, Green JA, Palevsky PM, Arnold RM, et al. Sexual function, activity, and satisfaction among women receiving maintenance hemodialysis. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(1):128‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena‐Polanco JE, Mor MK, Tohme FA, Fine MJ, Palevsky PM, Weisbord SD. Acceptance of antidepressant treatment by patients on hemodialysis and their renal providers. Clinical Journal of The American Society of Nephrology: CJASN 2017;12(2):298‐303. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbord SD, Mor MK, Green JA, Sevick MA, Shields AM, Zhao X, et al. Comparison of symptom management strategies for pain, erectile dysfunction, and depression in patients receiving chronic hemodialysis: a cluster randomized effectiveness trial. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(1):90‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbord SD, Mor MK, Sevick MA, Shields AM, Rollman BL, Palevsky PM, et al. Associations of depressive symptoms and pain with dialysis adherence, health resource utilization, and mortality in patients receiving chronic hemodialysis. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(9):1594‐602. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbord SD, Shields AM, Mor MK, Sevick MA, Homer M, Peternel J, et al. Methodology of a randomized clinical trial of symptom management strategies in patients receiving chronic hemodialysis: the SMILE study. Contemporary Clinical Trials 2010;31(5):491‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Watson 2015 {published data only}
- Viana JL, Watson EL, Greening NJ, Barratt J, Smith AC. Skeletal muscle cytokine gene expression after resistance exercise in chronic kidney disease [abstract no: FR‐OR055]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):59A. [Google Scholar]
- Watson E, Greening N, Aulakh J, Young H, Dungey M, Burton J, et al. Benefits of resistance exercise training in CKD: a randomised controlled trial [abstract no: P385]. British Transplantation Society & the Renal Association Joint Congress; 2013 Mar 13‐15; Bournemouth, UK. 2013. [CENTRAL: CN‐01657766]
- Watson E, Wimbury D, Viana JL, Greening N, Barratt J, Smith AC. Resistance exercise does not increase markers of muscle protein degradation in patients with advanced CKD [abstract no: SP453]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii529. [EMBASE: 72207779] [Google Scholar]
- Watson EL, Greening N, Aulakha J, Ruttanaporn N, Young H, Dungey M, et al. Resistance exercise increases muscle mass and preserves intramuscular amino acid concentrations in pre‐dialysis CKD: a randomised controlled trial [abstract no: O28]. British Renal Society Conference; 2013 May 14‐16; Manchester, UK. 2013. [CENTRAL: CN‐01658454]
- Watson EL, Greening NJ, Ruttanaporn N, Barratt J, Smith AC. Effects of resistance exercise on Akt signalling and protein degradation in chronic kidney disease [abstract no: FR‐OR016]. Journal of the American Society of Nephrology 2013;24(Abstracts):39A. [CENTRAL: CN‐01658455] [Google Scholar]
- Watson EL, Greening NJ, Viana JL, Aulakh J, Bodicoat DH, Barratt J, et al. Progressive resistance exercise training in CKD: a feasibility study. American Journal of Kidney Diseases 2015;66(2):249‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Watson EL, Greening NJ, Viana JL, Barratt J, Smith AC. Effects of resistance exercise training on myogenic response to acute exercise in chronic kidney disease [abstract no: O59]. British Renal Society. UK Kidney Week; 2014 Apr 29‐ May 2; Glasgow, UK. 2014. [CENTRAL: CN‐01658453]
Zhao 2017 {published data only}
- Zhao C, Ma H, Yang L, Xiao Y. Long‐term bicycle riding ameliorates the depression of the patients undergoing hemodialysis by affecting the levels of interleukin‐6 and interleukin‐18. Neuropsychiatric Disease & Treatment 2017;13:91‐100. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
DOHP 2016 {published data only}
- Knowles SR, Ski CF, Langham R, O'Flaherty E, Thompson DR, Rossell SL, et al. Design and protocol for the Dialysis Optimal Health Program (DOHP) randomised controlled trial. Trials [Electronic Resource] 2016;17(1):447. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02011139 {published data only}
- Lim CS. Cognitive‐behavioral (CBT) in ESRD patients with depression. www.clinicaltrials.gov/show/nct02011139 (first received 13 December 2013).
NCT03162770 {published data only}
- NCT03162770. Mindfulness meditation practice during hemodialysis. www.clinicaltrials.gov/show/NCT03162770 (first received 22 May 2017).
NCT03330938 {published data only}
- Gonzales Flores CJ. Decreasing depression and anxiety and their effect on QoL of ESRD patients (end‐stage renal disease). www.clinicaltrials.gov/show/nct03330938 (first received 6 November 2017).
NCT03406845 {published data only}
- Nassim M. Mindfulness and HEP in dialysis patients with depression and anxiety. www.clinicaltrials.gov/show/NCT03406845 (first received 23 January 2018).
van der Borg 2016 {published data only}
- Borg WE, Schipper K, Abma TA. Protocol of a mixed method, randomized controlled study to assess the efficacy of a psychosocial intervention to reduce fatigue in patients with end‐stage renal disease (ESRD). BMC Nephrology 2016;17(1):73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WICKD 2019 {published data only}
- Dingwall KM, Nagel T, Hughes JT, Kavanagh DJ, Cass A, Howard K, et al. Wellbeing intervention for chronic kidney disease (WICKD): a randomised controlled trial study protocol. BMC psychology 2019;7(1):2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Chilcot 2018
- Chilcot J, Guirguis A, Friedli K, Almond M, Day C, Silva‐Gane M, et al. Depression symptoms in haemodialysis patients predict all‐cause mortality but not kidney transplantation: a cause‐specific outcome analysis. Annals of Behavioral Medicine 2018;52(1):1‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Churchill 2001
- Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al. A systematic review of controlled trials of the effectiveness and cost‐effectiveness of brief psychological treatments for depression. Health Technology Assessment 2001;5(35):1‐173. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Edition. New York: Routledge, 1988. [ISBN: 9780805802832] [Google Scholar]
Cuijpers 2008
- Cuijpers P, Straten A, Andersson G. Internet‐administered cognitive behavior therapy for health problems: a systematic review. Journal of Behavioral Medicine 2008;31(2):169‐77. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2003
- Davies SJ, Jackson PR, Ramsay LE, Gharamani P. Drug intolerance due to nonspecific adverse effects related to psychiatric morbidity in hypertensive patients. Archives of Internal Medicine 2003;163(5):592‐600. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dobson 1989
- Dobson KS. A meta‐analysis of the efficacy of cognitive therapy for depression. Journal of Consulting & Clinical Psychology 1989;57(3):414‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Everett 1993
- Everett KD, Sletten C, Carmack C, Brantly PJ, Jones GN, McKnight GT. Predicting noncompliance to fluid restrictions in hemodialysis patients. Dialysis & Transplantation 1993;22(10):614‐22. [EMBASE: 23292111] [Google Scholar]
Farrokhi 2014
- Farrokhi F, Abedi N, Beyene J, Kurdyak P, Jassal SV. Association between depression and mortality in patients receiving long‐term dialysis: a systematic review and meta‐analysis. American Journal of Kidney Diseases 2014;63(4):623‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Finkelstien 2000
- Finkelstein FO, Finkelstein SH. Depression in chronic dialysis patients: assessment and treatment. Nephrology Dialysis Transplantation 2000;15(12):1911‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flythe 2017
- Flythe JE, Hilbert J, Kshirsagar AV, Gilet CA. Psychosocial factors and 30‐day hospital readmission among individuals receiving maintenance dialysis: a prospective study. American Journal of Nephrology 2017;45(5):400–8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hedayati 2010
- Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA 2010;303(19):1946‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Juergenson 1996
- Juergensen PH, Juergensen DM, Wuerth DB, Finkelstein SH, Steele TE, Kliger AS, et al. Psychosocial factors and incidence of peritonitis. Advances in Peritoneal Dialysis 1996;12:196‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Kaveh 2001
- Kaveh K, Kimmel PL. Compliance in hemodialysis patients: Multidimensional measures in search of a gold standard. American Journal of Kidney Diseases 2001;37(2):244‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kimmel 1993
- Kimmel PL, Weihs KL, Peterson RA. Survival in hemodialysis patients: the role of depression. Journal of the American Society of Nephrology 1993;41(1):12‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kimmel 1995
- Kimmel PL, Peterson RA, Weihs KL, Simmens SJ, Boyle DH, Verme D, et al. Behavioral compliance with dialysis prescription in hemodialysis patients. Journal of the American Society of Nephrology 1995;5(10):1826‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
King‐Wing Ma 2016
- King‐Wing Ma T, Kam‐Tao Li P. Depression in dialysis patients. Nephrology 2016;21(8):639‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levenson 1991
- Levenson JL, Glocheski S. Psychological factors affecting end stage renal disease: a review. Psychosomatics 1991;32(4):382‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levey 1998
- Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. American Journal of Kidney Diseases 1998;32(5):853‐906. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lopes 2002
- Lopes AA, Bragg J, Young E, Goodkin D, Mapes D, Combe C, et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney International 2002;62(1):199‐207. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martin 2000
- Martin CR, Thompson DR. Prediction of quality of life in patients with end‐stage renal disease. British Journal of Health Psychology 2000;5(1):41‐55. [EMBASE: 30088784] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Depression in adults: recognition and management. www.nice.org.uk/guidance/cg90 (Published date: October 2009; last updated: April 2018). [PubMed]
Palmer 2013a
- Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. Prevalence of depression in chronic kidney disease: systematic review and meta‐analysis of observational studies. Kidney International 2013;84(1):179‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmer 2013b
- Palmer SC, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. Association between depression and death in people with CKD: a meta‐analysis of cohort studies. American Journal of Kidney Diseases 2013;62(3):493‐505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmer 2016
- Palmer SC, Natale P, Ruospo M, Saglimbene VM, Rabindranath KS, Craig JC, et al. Antidepressants for treating depression in adults with end‐stage kidney disease treated with dialysis. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD004541.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pascoe 2017
- Pascoe MC, Thompson DR, Castle DJ, McEvedy SM, Ski CF. Psychosocial interventions for depressive and anxiety symptoms in individuals with chronic kidney disease: systematic review and meta‐analysis. Frontiers in Psychology 2017;8:992. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 1990
- Robinson LA, Berman JS, Neimayer RA. Psychotherapy for the treatment of depression: a comprehensive review of controlled outcome research. Psychological Bulletin 1990;108(1):30‐49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Scoggin 1994
- Scoggin F, McElreath L. Efficacy of psychosocial treatments for geriatric depression: a quantitative review. Journal of Consulting & Clinical Psychology 1994;62(1):69‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Steele 1996
- Steele TE, Baltimore D, Finkelstein SH, Jeurgenson P, Kliger AS, Finkelstein FO. Quality of life in peritoneal dialysis patients. Journal of Nervous & Mental Disease 1996;184(6):368‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Szeifert 2012
- Szeifert L Bragg‐Gresham JL, Thumma J, Gillespie BW, Mucsi I, Robinson BM, et al. Psychosocial variables are associated with being wait‐listed, but not with receiving a kidney transplant in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrology Dialysis Transplantation 2012;27(5):2107–13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tong 2015
- Tong A, Chando S, Crowe S, Manns B, Winkelmayer WC, Hemmelgarn B, et al. Research priority setting in kidney disease: a systematic review. American Journal of Kidney Disease 2015;65(5):674‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tong 2017
- Tong A, Manns B, Hemmelgarn B, Wheeler DC, Evangelidis N, Tugwell P, et al. Establishing core outcome domains in hemodialysis: report of the Standardized Outcomes in Nephrology‐Hemodialysis (SONG‐HD) Consensus Workshop. American Journal of Kidney Diseases 2017;69(1):97‐107. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisbord 2014
- Weisbord SD, Mor MK, Sevick MA, Shields AM, Rollman BL, Palevsky PM, et al. Associations of depressive symptoms and pain with dialysis adherence, health resource utilization, and mortality in patients receiving chronic hemodialysis. Clinical Journal of The American Society of Nephrology: CJASN 2014;9(9):1594–602. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xing 2016
- Xing L, Chen R, Diao Y, Qian J, You C, Jiang X. Do psychological interventions reduce depression in hemodialysis patients?: A meta‐analysis of randomized controlled trials following PRISMA. Medicine 2016;95(34):e4675. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Rabindranath 2003
- Rabindranath KS, MacLeod AM, Daly C, Roderick P, Butler J, Wallace S. Psychosocial interventions for depression in dialysis patients. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004542] [DOI] [PubMed] [Google Scholar]
Rabindranath 2005
- Rabindranath KS, Daly C, Butler JA, Roderick PJ, Wallace S, Macleod AM. Psychosocial interventions for depression in dialysis patients. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004542.pub2] [DOI] [PubMed] [Google Scholar]


