Abstract
We have designed de novo and synthesized ten 26-residue D-conformation amphipathic α-helical cationic antimicrobial peptides (AMPs), seven with “specificity determinants”, which provide specificity for prokaryotic cells over eukaryotic cells. The ten AMPs contain five or six positively charged residues (D-Arg, D-Lys, D-Orn, L-Dab, or L-Dap) on the polar face to understand their role in hemolytic activity against human red blood cells and antimicrobial activity against seven Acinetobacter baumannii strains, resistant to polymyxin B and colistin, and 20 A. baumannii worldwide isolates from 2016 and 2017 with antibiotic resistance to 18 different antibiotics. AMPs with specificity determinants and with L-Dab and L-Dap residues on the polar face have essentially no hemolytic activity at 1000 μg/mL (380 μM), showing for the first time the importance of these unusual amino acid residues in solving longstanding hemolysis issues of AMPs. Specificity determinants maintained excellent antimicrobial activity in the presence of human sera.
Graphical Abstract
1. INTRODUCTION
Antimicrobial peptides (AMPs) represent a ubiquitous response in nature to microbial infections. They are produced by a wide variety of organisms, including bacteria, fungi, plants, insects, amphibians, crustaceans, fish, and mammals, including humans, either constitutively or in response to the presence of a microbe.1 This immune response to bacterial infection has taken on considerable significance in recent years because of the extensive clinical use of classical antibiotics having led to an ever-growing emergence of many medically resistant strains of bacteria. Indeed, there are now “Superbugs” that are resistant to most or all of available antibiotics.2 Clearly, the development of a new class of antibiotics has become critical,3 with AMPs (specifically, cationic AMPs)4 representing such a new class of antibiotics with immense potential.4–17
AMPs are rapidly bactericidal and generally have broad spectrum activity. In addition, it is difficult for bacteria to develop resistance to AMPs that do not have specific targets (unlike traditional antibiotics) as their mode of action generally involves nonspecific interactions with the cytoplasmic membrane of bacteria, whereby peptide accumulation in the membrane causes increased permeability and loss of barrier function.5,6,9,10,12–22 However, despite such advantages over traditional antibiotics, the major barrier to the use of AMPs as antibiotics is their ability to lyse eukaryotic cell membranes as well as bacterial membranes. Indeed, such hemolytic activity and toxicity has always been the main criticism and prevented its widespread usage of such peptide-based drugs. Interestingly, polymyxin B and polymyxin E (also known as colistin) are cationic peptide antibacterials, which saw widespread use in the 1960s. However, their clinical use in the 1970s was scaled back considerably because of serious toxicity issues.23,24 With the advent of prevalent Gram-negative bacteria with multidrug resistance in the 1970s, there was a revival in the use of these two peptides, which have become antibiotics of the last resort. Unfortunately, the emergence of polymyxin-resistant “Superbugs”23,24 now means that it is vital that newly developed antibiotics are effective against both polymyxin B- and colistin-resistant microorganisms.
From numerous structure/activity studies on both natural and synthetic AMPs, factors important for antimicrobial activity were identified, including the presence of both hydrophobic and basic residues, an amphipathic nature, and preformed or inducible secondary structure (α-helix or β-sheet).13 Clearly, to be useful as antibiotics, it is necessary to dissociate anti-eukaryotic activity from antimicrobial activity, that is, increase antimicrobial activity and reduce lysis to normal cells (as determined by the degree of lysis of human red blood cells). We have always believed that a synthetic peptide approach to examining the effect of small incremental changes in hydrophilicity/hydrophobicity, amphipathicity, and helicity of α-helical cationic AMPs would enable rapid progress in the rational design of peptide antibiotics.
In an earlier study,13 we utilized the structural framework of a 26-residue amphipathic α-helical AMP, denoted V681, with excellent antimicrobial activity but with strong hemolytic activity, to study the effects of peptide hydrophilicity/hydrophobicity, amphipathicity, and helicity on biological activities through single D- or L-amino acid substitutions in the center of either the polar or nonpolar faces of the amphipathic helix. Such an amphipathic α-helical conformation permits insertion of a well-defined hydrophobic sector into a lipid bilayer.8 The helix destabilizing properties of D-amino acids in a helix of otherwise all-L-amino acids is well known.25–29 Indeed, we had previously demonstrated how the helix-destabilizing properties of D-amino acids offered a systematic approach to the controlled alteration of hydrophobicity, amphipathicity, and helicity of amphipathic α-helical model peptides.29 Controlled disruption of the α-helical structure of V681 (disruption under aqueous conditions, thus preventing or reducing self-association, and the inducible helical structure in hydrophobic conditions) seemed to be related to strong antimicrobial activity and reduced hemolytic activity. In addition, a higher ability to self-associate in solution was correlated with weaker antimicrobial activity and stronger hemolytic activity of the peptides. Concomitant with such work was our development of a sensitive reversed-phase chromatography (RP-HPLC) approach to monitoring self-association of small amphipathic molecules.13,30–38 Further, and as had been observed previously, the D-enantiomers showed excellent stability against proteolytic digestion,31,39–46 a key property for such AMPs to be useful as injectable antibiotics. Subsequent work in our laboratory involved determining the effect of varying the hydrophobicity of the nonpolar face32 or the number of positively charged residues on the polar face on the biological activity of V13K.33 In summary, the results showed that the number of positively charged residues on the polar face and net charge are both important for both antimicrobial and hemolytic activity,33 while there is threshold hydrophobicity at which optimal antimicrobial activity can be obtained.32 In addition, both of these papers solidified the choice of V13K as a promising lead compound at the time.
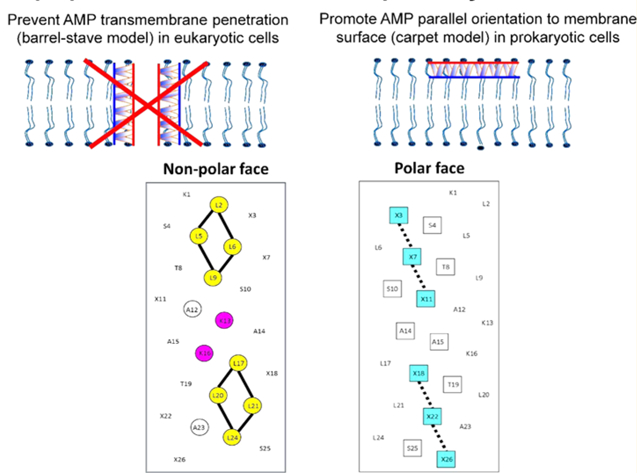
To this point, the purpose had been the development of a class of potentially broad-spectrum antimicrobial agents. However, our discovery of the effectiveness of a single Val to Lys substitution in the middle of the nonpolar face of a highly hemolytic peptide in dramatically reducing hemolytic activity,31 coupled with our assessment of the effects of varying the nonpolar and polar face,32,33 led to a major change in our approach; we could take a broad spectrum 26-residue AMP in the all-D conformation (V13K) and use a rational design approach to enhance further the biological properties if the focus was to develop a better Gram-negative AMP rather than to maintain broad-spectrum activity. An advantage to this approach is that limiting studies to one class of organism, in this case Gram-negative organisms, would hasten development of such antibiotics relative to attempting a “one size fits all” approach. Thus, the disruption of peptide self-association by the positively charged Lys residue on the nonpolar face prevents penetration of the membrane bilayer as a transmembrane helix in eukaryotic cells by a “barrel-stave” mechanism,17,47 thus preventing hemolysis. In contrast, for prokaryotic organisms, the induced α-helical structure of peptide monomers results in the peptides lying at the interface, parallel with the membrane surface, followed by disruption of the membrane via a “carpet” mechanism.17,48,49 What dictates the two different modes of interaction is the difference in lipid composition of prokaryotic and eukaryotic membranes. Thus, the mode of interaction of our AMPs is a “membrane discrimination mechanism”,31 with the Lys residue inserted into the nonpolar face of the helix now being referred to as a “specificity determinant”. Briefly, by utilizing charged, specificity determinants (Lys residues at positions 13 and 16 in the center of the nonpolar face), total hydrophobicity, hydrophobe type, and location as design parameters, we demonstrated not only improvements in antimicrobial activity but also further dramatic reductions in hemolytic activity, concomitant with unprecedented improvements in the therapeutic indices (hemolytic activity/antimicrobial activity, HC50 value/MICGM value).36,38 Of profound importance to the future of peptide-based AMPs as therapeutic agents was our demonstration that modification of native AMPs (Piscidin 1 isolated from fish and dermaseptin S4 isolated from frog) with Lys specificity determinants in the nonpolar faces of these amphipathic α-helical peptides (22 residues and 28 residues, respectively) produced similar results of improved antimicrobial activity and dramatically decreased hemolytic activity.37,50 Thus, specificity determinants are the key to removing hemolytic activity of nature’s AMPs.
The current study now turns to an examination of the role of the type of positively charged residues on the polar face of our model 26-residue AMP template in hemolytic activity against human red blood cells and antimicrobial activity against Acinetobacter baumannii strains resistant to polymyxin B and colistin and 2016/2017 worldwide isolates of the same organism resistant to 18 different antibiotics. Specifically, we examined the effect of replacing Lys residues on the polar face with positively charged residues: Arg or unusual amino acids ornithine (Orn),4,51–56 diaminobutyric acid (Dab),4,51–56 or diaminopropionic acid (Dap)4,51–57 in the absence or presence of two Lys specificity determinants in the nonpolar face.
2. RESULTS AND DISCUSSION
2.1. Peptide Design, Specificity Determinants, and the Type of Positively Charged Residue on the Polar Face
Enantiomeric forms of AMPs with all-D-amino acids have shown equal activities to their all-L-enantiomers.31,39–46 In this study, we designed de novo, synthesized, purified, and characterized ten potentially amphipathic α-helical AMPs. Seven AMPs have specificity determinants, lysine residues on the nonpolar face at positions 13 and 16, and three control AMPs are without these determinants, where the lysine residues at positions 13 and 16 were replaced with alanine residues (Table 1). Five AMPs have six positively charged residues on the polar face which contain Arg, Lys, Orn, Dab, or Dap residues at positions 3, 7, 11, 18, 22, and 26; two AMPs have only five positively charged residues on the polar face at positions 3, 7, 11, 18, and 22, containing either five Lys or five Dab residues (position 26 has been replaced with Ser) (Table 1). All ten peptides have a lysine residue at position 1, and the net charges on these peptides are either +9 or +8 for the AMPs with specificity determinants or +7 for the control AMPs without such determinants (Table 1).
Table 1.
Polar Face Substitutions of Positively Charged Residues in AMPs
| Peptide Namea | Net Charge | Sequenceb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With specificity determinants (Lys13/Lys16) | |||||||||||||
| 1 | 3 | 7 | 11 | 18 | 22 | 26 | |||||||
| KL | X1 | SLL | X2 | TLS | X3 | AKAAKL | X4 | TLL | X5 | ALS | X6 | ||
| D87(Lys1-6 Arg-1) | +9 | Ac-KL(Arg)SLL(Arg)TLS(Arg)AKAAKL(Arg)TLL(Arg)ALS(Arg)-amide | |||||||||||
| D84(Lys1-6 Lys-1) | +9 | Ac-KL(Lys)SLL(Lys)TLS(Lys)AKAAKL(Lys)TLL(Lys)ALS(Lys)-amide | |||||||||||
| D85(Lys1-6 Orn-1) | +9 | Ac-KL(Orn)SLL(Orn)TLS(Orn)AKAAKL(Orn)TLL(Orn)ALS(Orn)-amide | |||||||||||
| D86(Lys1-6 Dab-1) | +9 | Ac-KL(Dab)SLL(Dab)TLS(Dab)AKAAKL(Dab)TLL(Dab)ALS(Dab)-amide | |||||||||||
| D105(Lys1-6 Dap-1) | +9 | Ac-KL(Dap)SLL(Dap)TLS(Dap)AKAAKL(Dap)TLL(Dap)ALS(Dap)-amide | |||||||||||
| D101(Lys1Ser26-5 Lys-1) | +8 | Ac-KL(Lys)SLL(Lys)TLS(Lys)AKAAKL(Lys)TLL(Lys)ALSS-amide | |||||||||||
| D102(Lys1Ser26-5 Dab-1) | +8 | Ac-KL(Dab)SLL(Dab)TLS(Dab)AKAAKL(Dab)TLL(Dab)ALSS-amide | |||||||||||
| Without specificity determinants (Ala13/Ala16) | |||||||||||||
| 1 | 3 | 7 | 11 | 18 | 22 | 26 | |||||||
| KL | X1 | SLL | X2 | TLS | X3 | AAAAAL | X4 | TLL | X5 | ALS | X6 | ||
| D85(K13A/K16A)-(Lys1-6 Orn-1) | +7 | Ac-KL(Orn)SLL(Orn)TLS(Orn)AAAAAL(Orn)TLL(Orn)ALS(Orn)-amide | |||||||||||
| D86(K13A/K16A)-(Lys1-6 Dab-1) | +7 | Ac-KL(Dab)SLL(Dab)TLS(Dab)AAAAAL(Dab)TLL(Dab)ALS(Dab)-amide | |||||||||||
| D105(K13A/K16A)-(Lys1-6 Dap-1) | +7 | Ac-KL(Dap)SLL(Dap)TLS(Dap)AAAAAL(Dap)TLL(Dap)ALS(Dap)-amide | |||||||||||
D denotes that all amino acid residues in each peptide are in the D-conformation except for Dab and Dap which are in the L-conformation. Specificity determinants are positively charged residues in the center of the nonpolar face (Lys13/Lys16). Without specificity determinants means replacement with Ala residues (Ala13/Ala16) (Figure 2).
Peptide sequences are shown using the one-letter code for all amino acid residues except at X1, X2, X3, X4, X5, and (except for D101 and D102) X6, where the three-letter code is used. Ac denotes Nα-acetyl and amide denotes Cα-amide. Positions X1, X2, X3, X4, X5, and (except for D101 and D102) X6 are positively charged residues (Arg, Lys, Orn, Dab, and Dap) on the polar face of the amphipathic α-helix (Figure 1); −1 denotes 6 positively charged residues on the polar face at positions 3, 7, 11, 18, 22, and 26 or 5 positively charged residues on the polar face at positions 3, 7, 11, 18, and 22 (position 26 is substituted by Ser).
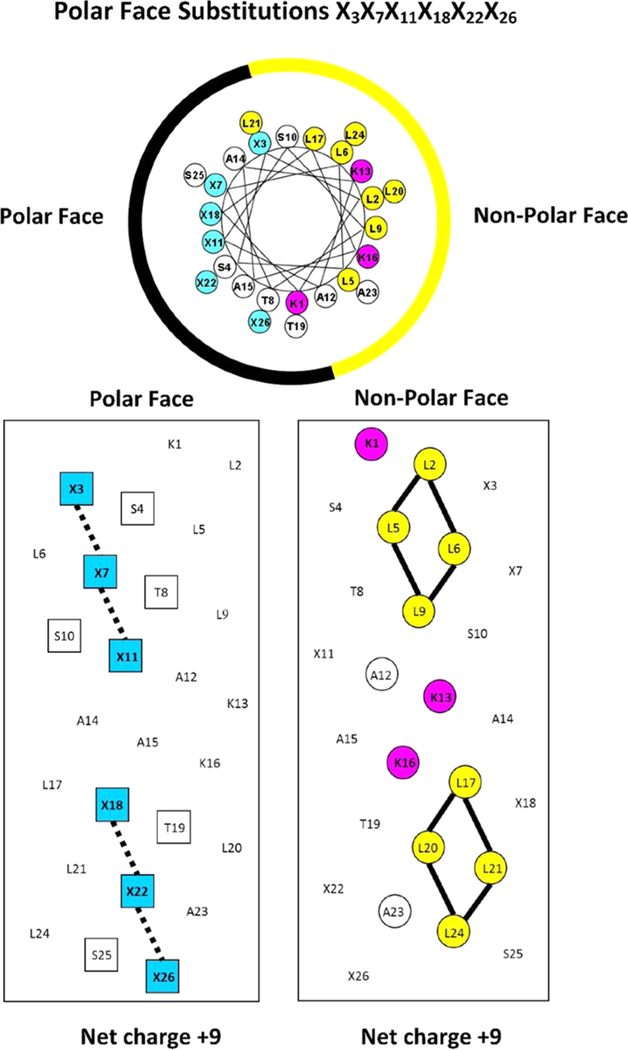
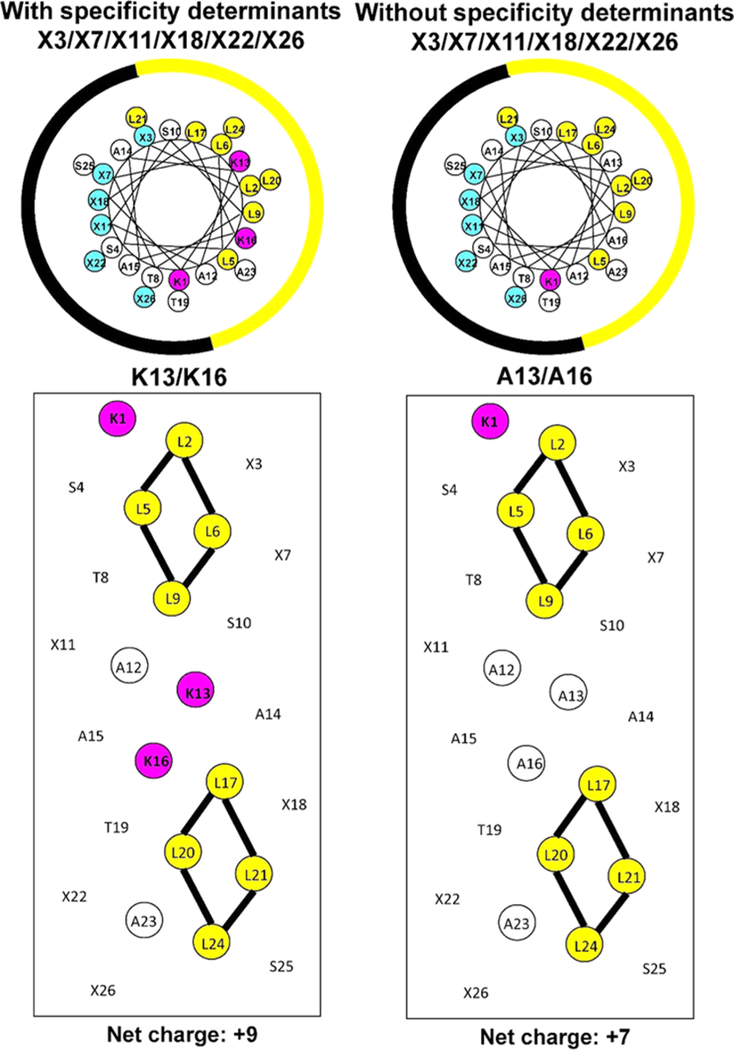
These peptides are unstructured in aqueous medium and structured amphipathic α-helices in the presence of hydrophobic medium. Thus, Figure 1 shows a general amino acid sequence in a helical wheel and helical net representations where X3X7X11X18X22X26 shows the positions on the polar face of the positively charged residues (colored blue). We have displayed two versions of the helical nets where the polar residues are displayed along the center of the helical net (left side) and where the nonpolar residues are displayed along the center of the helical net (right side). The hydrophobic/nonpolar faces of the seven peptides with specificity determinants have eight Leu residues in two clusters of four (colored yellow) separated by the two Lys residues (specificity determinants in the center of the nonpolar face (colored red)). Lys 1 is also on the nonpolar face and colored red. Figure 2 shows the difference between the peptides with these determinants (Lys 13 and Lys 16) in the center of the nonpolar face and those without (Ala 13 and Ala 16). Thus, the positive charge on the nonpolar face decreases from +3 to +1 and the overall net charge on the AMPs decreases from +9 to +7.
Figure 1.
Helical wheel (upper panel) and helical net (lower panels) representations of our amphipathic helical AMPs. In the helical wheel, the nonpolar face is indicated as a yellow arc (Leu residues are colored yellow and the Lys “specificity determinants” at positions 13 and 16 are colored red). Lys 1 is also on the nonpolar face and colored red. The polar face is indicated as a black arc (positively charged residues at positions 3, 7, 11, 18, 22, and 26 are denoted by X and are colored blue). In the helical net (left side), the residues on the polar face are boxed and shown along the center of the net with the positively charged residues at positions 3, 7, 11, 18, 22, and 26 also colored blue. In the helical net (right side), the residues on the nonpolar face are circled (Leu residues are colored yellow) and shown along the center of the net. The Lys “specificity determinants” are at positions 13 and 16 in the center of the nonpolar face between the hydrophobic clusters of Leu residues.
Figure 2.
Helical wheel (upper panels) and helical net (lower panels) representations of an AMP with and without “specificity determinants”. Lys 13 and Lys 16 (colored red) are in the center of the nonpolar face (left side) between the two clusters of hydrophobic Leu residues (colored yellow). The right side shows the helical net in the absence of specificity determinants, where Lys residues are replaced with Ala 13 and Ala 16, thus maintaining a continuous hydrophobic surface along the center of the helix. The positively charged residues on the polar face are indicated in the helical wheels with an X at positions 3, 7, 11, 18, 22, and 26.
2.2. Antibacterial Activity
Tables 2 and S1 show the antibacterial activities against seven different A. baumannii strains resistant to polymyxin B and colistin (antibiotics of last resort). The geometric mean MIC values for the five AMPs, where the positively charged residue was varied from Arg, Lys, Orn, Dab, and Dap, ranged from 0.5 μM (six Lys- and six Orn-containing peptides) to 0.8 μM for 6 Arg, 1.0 μM for 6 Dab, and 1.2 μM for 6 Dap residues on the polar face. Thus, shortening the number of carbon atoms between the side-chain amino group and the α-carbon atom from four (Lys) to one (diaminopropionic acid) had very little effect on antibacterial activity (Tables 2 and S1). We also determined the antibacterial activity of our five AMPs against 20 additional A. baumannii isolates from four continents, 12 different countries, and 17 different cities that were resistant to 18 different antibiotics. As shown in Tables 2 and S2, the geometric mean MIC value was 0.7 μM for the 6 Lys-containing AMP to 1.0 μM for 6 Dab- or 6 Dap-containing AMPs. Thus, it is very clear that changing the type of positively charged residue on the polar face of these AMPs had very little effect on the antibacterial activities. Removing the C-terminal positively charged residue from the peptides D84(Lys26Ser) and D86(Dab26Ser) to give peptides D101 and D102 had a small effect or actually enhanced the geometric mean MIC value (D84 0.5 μM vs D101 0.8 μM) and (D86 1.0 μM vs D102 0.7 μM) (Tables 2 and S1).
Table 2.
Antibacterial Activity against 7 Strains of A. baumannii Resistant to Polymyxin B and Colistin and 20 Worldwide 2016 and 2017 Isolates Resistant to 18 Classical Antibiotics
| peptide namea | peptide mass | MICGM (μM)b | MICGM (μM)c |
|---|---|---|---|
| With Specificity Determinants | |||
| D87(Lys1-6 Arg-1) | 3033.7 | 0.8 | nd |
| D84(Lys1-6 Lys-1) | 2865.6 | 0.5 | 0.7 |
| D85(Lys1-6 Orn-1) | 2781.5 | 0.5 | nd |
| D86(Lys1-6 Dab-1) | 2697.3 | 1.0 | 1.0 |
| D105(Lys1-6 Dap-1) | 2613.1 | 1.2 | 1.0 |
| D101(Lys1Ser26-5 Lys-1) | 2824.5 | 0.8 | 1.0 |
| D102(Lys1Ser26-5 Dab-1) | 2684.3 | 0.7 | 0.6 |
| Without Specificity Determinants | |||
| D85(K13A/K16A)-(Lys1-6 Orn-1) | 2667.3 | 2.0 | 1.9 |
| D86(K13A/K16A)-(Lys1-6 Dab-1) | 2583.1 | 0.9 | 0.9 |
| D105(K13A/K16A)-(Lys1-6 Dap-1) | 2499.0 | 1.3 | 2.0 |
| Colistin | 1155.5 | >28 | nd |
| Polymyxin B | 1301.6 | >25 | nd |
6 Lys-1 denotes 6 Lys residues on the polar face at positions 3,7, 11, 18, 22, and 26; 5 Lys-1 denotes 5 Lys residues on polar face at positions 3, 7, 11, 18, and 22 (position 26 is substituted by Ser).
MIC is minimal inhibitory concentration (μM) that inhibited growth of different strains in MH medium at 37 °C after 24 h, with the MIC based on three sets of replicates; MICGM is the geometric mean of the MIC values from seven different strains of A. baumanii resistant to polymyxin B and colistin, antibiotics of last resort.
MICGM is the geometric mean of the MIC values for 20 worldwide 2016 and 2017 isolates resistant to 18 classical antibiotics.
2.3. Hemolytic Activity and Therapeutic Indices
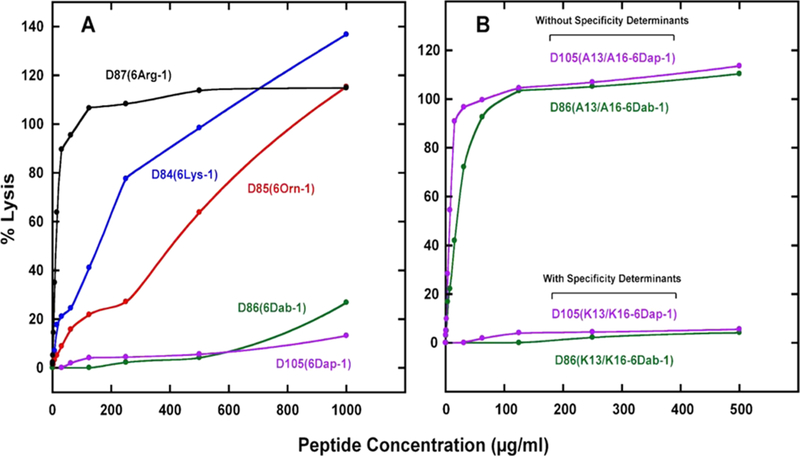
The biological activities of the 10 peptide analogs, with and without specificity determinants are shown in Table 3. The three peptides without specificity determinants are extremely hemolytic, with HC50 values (the peptide concentration required for 50% hemolysis) of 0.9–7.7 μM, which is of comparable magnitude to the antimicrobial activity of 0.9–2.0 μM. Thus, the therapeutic indices vary from 0.5 to 8.6, depending on the positively charged residue used on the polar face (Table 3). The specificity determinants have very little effect on antimicrobial activity where the geometric mean MIC ranges from 0.5 to 1.2 μM for the seven AMPs compared to 0.9–2.0 μM without determinants. The specificity determinants result in dramatic decreases in hemolytic activities from a range of 0.9–7.7 μM for HC50 for AMPs lacking these determinants to 4.0 to >1148 μM, depending on the positively charged residue on the polar face of the AMP. This corresponds to increases in the therapeutic indices from 5.0 to >1012 depending on the AMP. Our best AMP shows an increase in the therapeutic index of >202-fold relative to the Arg-containing peptide (Table 3). Our results show that the improvements in the hemolytic activity and thus the therapeutic indices depend on the type of positively charged residue used on the polar face. The HC50 value for Arg in the six polar face positions (3, 7, 11, 18, 22, and 26) is 4.0 μM compared to Lys (54.3 μM) and Orn (146.1 μM). Thus, the use of Orn residues instead of Arg provides a 37-fold decrease in the hemolytic activity or a 58-fold improvement in the therapeutic index. The dramatic and unexpected decrease in hemolytic activity resulted from the use of the two unusual amino acid residues Dab and Dap on the polar face. There was >186-fold decrease in hemolytic activity relative to Arg-containing AMP when using diaminobutyric acid (4 to >742 μM) and >287-fold decrease in hemolytic activity when using diaminopropionic acid (4 to >1148 μM). Because the antimicrobial activity does not vary significantly between peptides, the changes in therapeutic indices show a similar large increase in fold improvement relative to Arg as the amino acid residues are changed systematically from Arg to Lys, Orn, Dab, and Dap. We are systematically decreasing the number of carbon atoms in the side chain from four in the case of lysine to one in the case of a diaminopropionic acid residue. This results in a change in hemolytic activity from 54.3 μM for Lys to >1148 μM for Dap, a >21-fold change in hemolytic activity, resulting in a therapeutic index of 108.6 for Lys and >957 for Dap (Table 3). The significance of this change from Arg, Lys, Orn to Dab or Dap is observed graphically in a plot of percent lysis of human red blood cells versus peptide concentration up to 1000 μg/mL [Figure 3, panel (A)]. No significant lysis is observed for Dab- or Dap-containing peptides. The importance of using these unusual amino acids in place of Lys or Arg residues in AMPs on the polar face cannot be over emphasized. Removing the C-terminal positively charged residue has no effect on the hemolytic activity or therapeutic index; compare peptide D101(5 Lys-1) to D84(6 Lys-1) or D102(5 Dab-1) to D86(6 Dab-1) (Table 3). The importance of specificity determinants is shown in Figure 3, panel (B) where the 6 Dab- and 6 Dap- containing peptides without determinants (Ala13/Ala16) are extremely hemolytic compared to the same peptides with determinants (Lys13/Lys16) which show no lysis of human red blood cells. This is an unprecedented result.
Table 3.
Polar Face Substitutions of Positively Charged Residues
| peptide namea | net charge | peptide Mass | HC50 (μg/mL)b | HC50 (μM)b | HC50 fold improvementc | MICGM (μM)d | T.I.e | T.I. fold improvementf |
|---|---|---|---|---|---|---|---|---|
| with specificity determinants | ||||||||
| D87(Lys1-6 Arg-1) | +9 | 3033.7 | 12 | 4.0 | 1.0 | 0.8 | 5.0 | 1.0 |
| D84(Lys1-6 Lys-1) | +9 | 2865.6 | 155.5 | 54.3 | 13.6 | 0.5 | 108.6 | 21.7 |
| D85(Lys1-6 Orn-1) | +9 | 2781.5 | 406.5 | 146.1 | 36.5 | 0.5 | 292.2 | 58.4 |
| D86(Lys1-6 Dab-1) | +9 | 2697.3 | >2000 | >742 | >186 | 1.0 | >742 | >148 |
| D105(Lys1-6 Dap-1) | +9 | 2613.1 | >3000 | >1148 | >287 | 1.2 | >957 | >191 |
| D101(Lys1Ser26-5 Lys-1) | +8 | 2824.5 | 279 | 103.9 | 26.0 | 0.8 | 129.9 | 26.0 |
| D102(Lys1Ser26-5 Dab-1) | +8 | 2684.3 | >2000 | >708 | >177 | 0.7 | >1012 | >202 |
| without specificity determinants | fold decreasec | fold decreasef | ||||||
| D85(K13A/K16A)-(Lys1-6 Orn-1) | +7 | 2667.3 | 2.3 | 0.9 | −162.3 | 2.0 | 0.5 | −584.4 |
| D86(K13A/K16A)-(Lys1-6 Dab-1) | +7 | 2583.1 | 20.0 | 7.7 | >−96.4 | 0.9 | 8.6 | >−86.3 |
| D105(K13A/K16A)-(Lys1-6 Dap-1) | +7 | 2499.0 | 7.2 | 2.9 | >−395.9 | 1.3 | 2.2 | >−435.0 |
6 Lys-1 denotes 6 Lys residues on the polar face at positions 3,7,11,18, 22, and 26. 5 Lys-1 denotes 5 Lys residues on polar face at positions 3, 7,11,18, and 22 (position 26 is substituted by Ser).
Hemolytic activity, HC50, is the concentration of peptide that results in 50% hemolysis of human red blood cells after 18 h at 37°C.
Fold improvement in hemolytic activity, HC50, relative to the Arg-containing peptide. Fold decrease compares the same peptide with and without specificity determinants. That without specificity determinants shows a dramatic fold decrease in HC50.
MIC is minimal inhibitory concentration (μM) that inhibited growth of different strains in MH medium at 37°C after 24 h, with the MIC based on three sets of replicates; MICGM is the geometric mean of the MIC values from seven different strains of A. baumanii resistant to polymyxin B and colistin, antibiotics of last resort.
Therapeutic index (T.l.) was calculated from HC50 (μM)/MICGM (μM). Fold decrease compares the same peptide with and without specificity determinants. That without specificity determinants shows a dramatic fold decrease in the therapeutic index.
Fold improvement in the therapeutic index, relative to the Arg containing peptide.
Figure 3.
Percent lysis of human red blood cells vs peptide concentration of AMPs. Panel (A): The sequences of the five peptides (all containing Lys specificity determinants at positions 13 and 16 of the nonpolar face; Figure 1) are shown in Table 1. Note that peptide denotations in this figure have been abbreviated from those shown in Table 1; for example, D87(Lys1-6 Arg-1) has been shortened to D87(6Arg-1), where 6Arg-1 denotes six Arg residues on the polar face at positions 3, 7, 11, 18, 22, and 26. Panel (B): Comparison of percent lysis of the Dap- and Dab-containing peptides in the presence and absence of Lys specificity determinants at positions 13 and 16. The sequences of the peptides are shown in Table 1. HC50 values (concentration of peptide that results in 50% hemolysis of human red blood cells after 18 h at 37 °C) derived from such data are shown in Table 4.
2.4. Comparison of Antibacterial and Hemolytic Activity to Other Studies Utilizing Dab and Dap in AMPs
As noted above, much of the significance of our results lies in the maintenance of excellent antimicrobial activity (Tables 2, S1 and S2) whilst essentially eliminating hemolytic activity (Table 3, Figure 3), resulting in huge increases in the therapeutic index (Table 3). Furthermore, the hemolysis results of the present study are even more impressive when one considers the peptide/blood cell incubation time of 18 h compared to the 0.5–2 h incubation time characteristic of other studies. Three of these studies4,52,56 reflect a similar approach to the present study, whereby substitutions of unusual amino acids are made in the polar face of an amphipathic α-helical AMP. Thus, Uggerhoj et al.,4 when substituting for one Lys in the hydrophilic face of a 10-residue amphipathic α-helix (MIC = 100 μM), reported little effect on antimicrobial activity against Escherichia coli for Dab (MIC = 75 μM) or Dap (100 μM). Note the MIC values for the Dap and Dab analogs of the present study are just 1.0–1.2 μM (Tables 2, S1 and S2). These other researchers reported no change of hemolytic activity within the range of concentrations tested (≤∼500 μM for 1 h incubation at 37 °C). Interestingly, these authors stated that Dap should not be used in the design of AMPs, this statement being qualified in that the data they presented were only valid for the specific group of short (10 residue) cationic α-helical AMPs under consideration. Zelezetsky et al.52 replaced four positively charged residues in the polar face of a 19-residue amphipathic α-helical AMP with Dab or Dap. MIC values against Gram-negative bacteria were generally maintained by Dab or Dap substitutions in the range of 1–4 μM relative to the Lys analog (0.5–1 μM); however, hemolytic activity, although an improvement of that over the Lys analog (90 ± 10% for 100 μM peptide) was still considerable for the Dab (55 ± 15%) and Dap (30 ± 7%) analogs (incubation time not reported). When Kohn et al.56 replaced seven Lys residues in the polar face of an 18-residue amphipathic α-helical AMP with Dap residues, the MIC values (1.875–7.5 μM) increased, decreased, or remained constant, depending on the Gram-negative microorganism being tested. Although reported as the peptides inducing hemolysis only to a very low extent (∼9% for the Lys peptide vs ∼2% for the Dap analog), these results were obtained with just 15 μM of the peptide and a 1 h incubation for hemolysis study. This is in stark contrast to the present study, with 18 h incubation up to 1000 μg/mL (380 μM) of the peptide and essentially no hemolysis at concentrations of the Dab and Dap peptides of 1000 μg/mL (∼380 μM) (Figure 3).
Although it is important to compare the results of the present study with a similar template (amphipathic α-helix) and substitutions (Lys for Dap/Dab in the hydrophilic face of the helix), other studies of interest are represented by refs 53 and 55. Thus, Arias et al.55 substituted Dab or Dap at the N- and C-termini of an α-helix-like AMP (two residues substituted at each end of the peptide sequence). Compared to the Lys analog (MIC = 16 μM), the Dab and Dap analogs exhibited improved MIC values of 4–8 and 4 μM, respectively. However, membrane permeabilization assays (mimicking erythrocyte membranes) showed increasing permeabilization with just 0.2 μM of the peptide producing 50% leakage with the Dap analog compared to 20% for the Lys peptide. Zou et al.53 substituted three Arg residues in 30-residue α-defensives (3-stranded β-sheet with three intramolecular disulfide bridges) with Lys, Orn, Dab or Dap. Briefly, antibacterial activity decreased with decreasing side chain length (Lys < Orn < Dab < Dap) with little difference between the analogs in terms of very high vesicle leakage at just 100 μg/mL peptide.
Clearly, by comparing our present results to other studies, including those employing similar amphipathic α-helical templates, the effects of introducing Dab or Dap residues into the polar face of our 26-residue amphipathic α-helical AMP template are consistently superior when one considers the combination of maintenance of excellent MIC and dramatic lowering/elimination of hemolysis, the latter being evident even at high peptide concentrations and long incubation times.
The critical issue for other researchers is not to use short incubation times 0.5–2 h in hemolytic assays because it is well known that extending incubation time to 18 h dramatically increases hemolysis and hemolysis is dependent on peptide concentration which must be increased to 1000 μg/mL (380 μM) for reliable information to be obtained.
Interestingly, the present results also reflect previous observations concerning the “snorkel effect”, which determines the penetration depth of the peptide.4,52 Thus, the longer the aliphatic chain of the substituted positively charged residue on the polar face (Lys > Orn > Dab > Dap), the deeper the insertion of the helix (parallel to the membrane surface) with the positive charge interacting with the polar head groups on the lipid bilayer. In particular, Zelezetsky et al.52 with a 19-residue amphipathic α-helical AMP template noted a strong correlation of hemolytic activity with the hydrophobic sector depth in a similar manner to the results of the present study, that is, decreasing hemolysis with decreasing aliphatic chain length (Table 3). In addition, the small effect of decreasing the aliphatic chain length on antimicrobial activity in the present study (Table 3) was also apparent with the amphipathic α-helical model of Zelezetsky et al.52 who described a similar small effect of helix insertion depth on antimicrobial activity.
2.5. Antimicrobial Activity of AMPs in the Presence of Human Sera
An important consideration for the routine use of AMPs to treat Gram-negative bacterial infections is whether, and to what extent, the peptides bind to human serum proteins. AMPs bound to serum proteins are unable to interact with the therapeutic target, thus lowering their efficacy. We addressed this issue by determining the MIC values of our AMPs in the presence of Mueller Hinton (MH) medium and MH medium supplemented with 25% (v/v) human sera. This assay allows an estimation of the in vivo bioavailability of our AMPs, where an increase in MIC in the presence of serum is attributed to binding to serum proteins and, hence, lowering of antimicrobial activity. From Table 4, the three AMPs without specificity determinants have reduced antimicrobial activity against A. baumannii blood strain 649 in the presence of 25% human sera except for AMP D105 which shows a 4-fold decrease in MIC. Peptide D85, without specificity determinants, caused precipitation when peptides were added to the assay mixture containing serum (after 18 h incubation, more precipitation was observed), while D86 and D105, without specificity determinants, resulted in a 4-fold and 16-fold loss of antimicrobial activity, respectively (Table 4). These results emphasize the importance of specificity determinants in preventing any significant loss of antimicrobial activity. Thus, specificity determinants have three major roles: maintain or enhance antimicrobial activity, prevent binding to serum proteins, and decrease α-helical structure in aqueous conditions but allow inducible helical structure in the presence of the hydrophobicity of the membrane.
Table 4.
Antimicrobial Activity against A. baumannii Strain 649 in the Presence and Absence of 25% Human Sera
| MIC(μM) |
||
|---|---|---|
| peptide name | no serum | 25% human serum |
| With Specificity Determinants (Lys13Lys16) | ||
| D87(Lys1-6 Arg-1) | 0.7 | 1.3 |
| D84(Lys1-6 Lys-1) | 0.3 | 0.3 |
| D85(Lys1-6 Orn-1) | 0.4 | 0.2 |
| D86(Lys1-6 Dab-1) | 0.7 | 0.2 |
| Dl05(Lys1-6 Dap-1) | 0.8 | 3.0 |
| Dl01(Lys1Ser26-5 Lys-1) | 0.7 | 0.7 |
| D102(Lys1Ser26-5 Dab-1) | 0.4 | 0.7 |
| Without Specificity Determinants (Ala13Ala16) | ||
| D85(K13A/K16A)-(Lys1-6 Orn-1) | 1.5 | precipitate |
| D86(K13A/K16A)-(Lys1-6 Dab-1) | 0.8 | 3.0 |
| D105(K13A/K16A)-(Lys1-6 Dap-1) | 0.8 | 12.5 |
2.6. Peptide Hydrophobicity
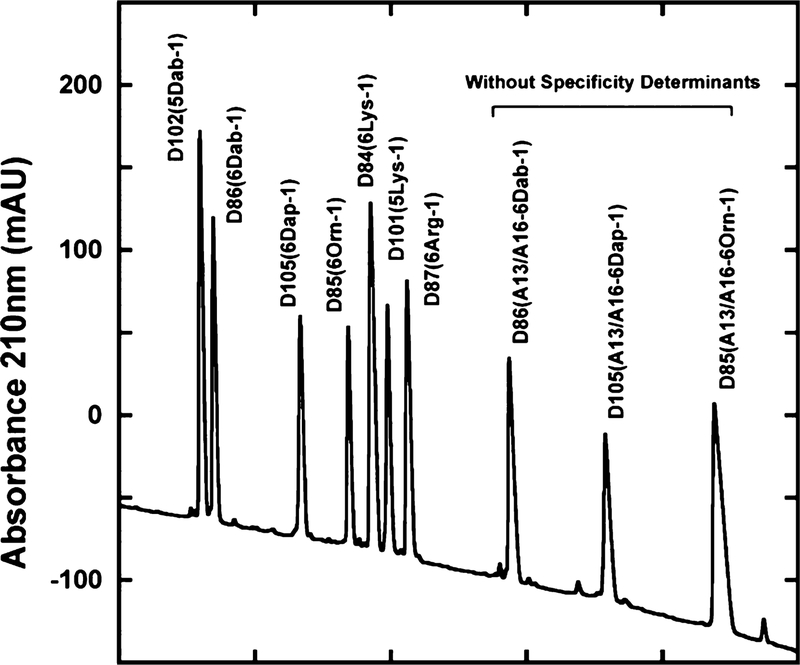
Retention behavior in RP-HPLC is an excellent method to represent overall peptide hydrophobicity. Retention times of amphipathic α-helical peptides are highly sensitive to the conformational status of the peptides upon interaction with the hydrophobic surface of the column matrix.29,58 The nonpolar face of amphipathic α-helical peptides represents the preferred binding domain for interaction with the hydrophobic matrix of the reversed-phase column.29,58 In this study, the observed peptide retention times are relative hydrophobicities because they are dependent on the trifluoroacetic acid (TFA) concentration and the organic solvent in the mobile phase, gradient rate, column temperature, flow-rate, and column used. The three control AMPs without specificity determinants have hydrophobic residues on the nonpolar face of the helix 8 Leu residues in two clusters, colored yellow L2, L4, L6, and L9 in the N-terminal cluster, L17, L20, L21, and L24 in the C-terminal cluster, and 2 Ala residues at positions 13 and 16 between the two clusters of Leu residues (Figure 2). This hydrophobic surface is the preferred binding domain for binding to the hydrophobic surface on the column matrix; however, the overall hydrophobicity is also affected by the composition of residues on the polar face which contains six positively charged residues (Figure 2). The amino acid composition on the polar face has the positively charged residues in the same positions (3, 7, 11, 18, 22, and 26) but varies the type of positively charged residue from either six Arg, Lys, Orn, Dab, or Dap residues. The seven AMPs with specificity determinants have two Lys residues between the two hydrophobic clusters (Figure 2), thus decreasing the overall hydrophobicity. Thus, the overall hydrophobicity varied from 115.8 to 143.2 min considerably less than the peptides without specificity determinants which varied from 158.3 to 188.7 min (Figure 4 and Table 5). All ten peptides used in this study could be readily separated by RP-HPLC with the seven peptides with specificity determinants being much more hydrophilic than the three control peptides without specificity determinants (Figure 4). The type of positively charged residue on the polar face has a dramatic effect on the overall hydrophobicity, with the Dab residue being more hydrophilic (less hydrophobic) than the Dap residue, even though the Dab residues are a carbon atom larger in their side chain compared to the Dap residues (Dab peptide (D86) retention time 115.8 min compared to Dap peptide (D105) retention time of 127.9 min). This can be explained by the Dab residues stabilizing the α-helical structure considerably more than Dap residues. This means that the polar face of Dab residues is interacting more with the hydrophobic matrix than the polar face of Dap residues, which results in large decrease in the retention time (tR for Dap is 127.9 min and tR for Dab is 115.8 min, i.e., a decrease of 12.1 min), even though each of the six Dab residues has one more carbon atom in its side chain than the Dap residue (Table 5).
Figure 4.
Relative hydrophobicity of AMPs as expressed by RP-HPLC elution time. Column: Zorbax 300 SB-C8, 150 × 2.1 mm ID, 5 μm particle size, 300 Å pore size; conditions, linear AB gradient (0.25% acetonitrile/min) at a flow rate of 0.3 mL/min, where eluant A was 20 mM aq TFA and eluant B was 20 mM TFA in acetonitrile and the temperature was 25 °C. The sequences of the ten peptides (with seven peptides containing Lys specificity determinants at positions 13 and 16 of the nonpolar face (Figure 1) are shown in Table 1. Note that peptide denotations in this figure have been abbreviated from those shown in Table 1; for example, D87(Lys1-6 Arg-1) has been shortened to D87(6 Arg-1). For the peptides without specificity determinants, we have added A13/A16 to the peptide denotations.
Table 5.
Biophysical Data
| hydrophobicityb |
aqueous pH 7 |
50% TFE |
Δ[θ]222 |
amphipathicityg |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| peptidenamea | net charge | pH 2tR | [θ]222c | % helixd | [θ]222c | TFE-aqueous | % helixd induced | Tpe (°C) | PAf (min) | pH 2 |
| With Specificity Determinants | ||||||||||
| D87(Lys1-6 Arg-1) | +9 | 143.2 | 10192 | 33 | 31308 | 21116 | 67 | 21.2 | 5.9 | 3.625 |
| D84(Lys1-6 Lys-1) | +9 | 138.1 | 8230 | 24 | 33653 | 25423 | 76 | 21.2 | 5.9 | 3.327 |
| D85(Lys1-6 Orn-1) | +9 | 134.7 | 4808 | 13 | 38423 | 33615 | 87 | 17.3 | 4.1 | 3.420 |
| D86(Lys1-6 Dab-1) | +9 | 115.8 | 1769 | 6 | 27923 | 26154 | 94 | 5.0 | 0 | 3.879 |
| D105(Lys1-6 Dap-1) | +9 | 127.9 | 5961 | 25 | 24192 | 18231 | 75 | 16.9 | 3.7 | 3.570 |
| D101(Lys1Ser26-5 Lys-1) | +8 | 140.3 | 2538 | 7 | 38538 | 36000 | 93 | 21.2 | 6.0 | 3.419 |
| D102(Lys 1Ser26-5 Dab-1) | +8 | 113.9 | 5962 | 25 | 23385 | 17423 | 75 | 5.0 | 0 | 3.842 |
| Without Specificity Determinants | ||||||||||
| D85(K13A/K16A)-(Lys1-6 Orn-1) | +7 | 188.7 | 9269 | 51 | 18038 | 8769 | 49 | 41.0 | 28.0 | 4.631 |
| D86(K13A/K16A)-(Lys1-6 Dab-1) | +7 | 158.3 | 2308 | 12 | 18731 | 16423 | 88 | 31.0 | 12.5 | 5.135 |
| D105(K13A/K16A)-(Lys1-6 Dap-1) | +7 | 172.3 | 9577 | 53 | 18038 | 8461 | 47 | 39.0 | 18.0 | 4.797 |
D denotes all amino acids in each peptide are in the D-conformation.
tR denotes retention time in RP-HPLC at pH 2 at a temperature of 25 °C and is a measure of overall peptide hydrophobicity.
The mean residue molar ellipticities [θ]222 (mdeg cm2/(dmol × res)) at a wavelength of 222 nm were measured at 25 °C in aqueous conditions (100 mM KC1, 50 mM Na2HPO4/NaH2PO4, pH 7.0) or in aqueous buffer containing 50% trifluoroethanol (TFE) by circular dichroism spectroscopy.
The helical content (as a percentage) of a peptide is relative to the molar ellipticity value of the peptide in the presence of 50% TFE. % helix induced is the increase in molar ellipticity (as a percentage) of the peptide in the presence of 50% TFE.
Tp, temperature at which maximum retention time is observed over the temperature range 5–77 °C.
PA denotes the self-association parameter (dimerization/oligomerization) of each peptide during RP-HPLC temperature profiling, which is the maximal retention time difference of (tRt – tR5 for peptide analogs) – (tRt – tR5 for control peptide RC) within the temperature range; tRt – tR5 is the retention time difference of a peptide at a specific temperature (tRt) compared with that at 5 °C (tR5). The sequence of the random coil control peptide (RC) is Ac-ELEKGGLEGEKGGKELEK-amide.
Amphipathicity was determined by calculation of the hydrophobic moment [Eisenberg et.al 1982]59 using hydrophobicity coefficients determined by RP-HPLC at pH 2.
All our AMPs shown in Table 1 have the identical hydrophobic density with eight Leu residues on the nonpolar face. The hydrophobic density of our de novo-designed AMPs is similar to that observed for native AMPs of 22–27 residues (see review by Hodges et al., 2012).16 Hydrophobic density is calculated by the sum of the hydrophobicity values of nonpolar residues (Pro, Val, Ile, Leu, Met, Tyr, Phe, and Trp) in the AMP divided by the number of residues in the peptide.
Systematic decreases or increases of hydrophobicity of the nonpolar face of our model AMP (leading to an overall decrease or increase in peptide hydrophobicity, as expressed by RPC retention behavior) showed that increasing peptide hydrophobicity resulted in increased hemolysis.32 Interestingly, this result was also observed in the present study where, just taking into account the RPC retention behavior of the AMPs with specificity determinants, there is a general trend of increasing hemolysis (Table 3) with an increase in the peptide retention time/hydrophobicity (Figure 4). For instance, D102(5Dab-1), D86(6Dab-1), and D105(6Dap-1), the most hydrophilic peptides, exhibit HC50 values of >2000 μg/mL to >3000 μg/mL compared to the most hydrophobic of these peptides, D85(6Arg-1), with an HC50 value of 12 μg/mL. Further, this relationship also extends to the control peptides without specificity determinants with noninterrupted nonpolar faces and, hence, longer RPC retention time. Thus, retention values of 158.3, 172.3, and 188.7 min for peptides D86(K13A/K16A)6-Dab-1, D105(K13A/K16A)6-Dap-1, and D85(K13A/K16A)6Orn-1, respectively (Figure 4), correlate with HC50 values of 20, 7.2, and 2.3 μg/mL (Table 3).
In contrast to hemolytic activity, there is no correlation between overall peptide hydrophobicity and antimicrobial activity. This is not surprising given the complex relationship seen in the previous study (as described in the Introduction), where changes to the nonpolar face of the peptides were substantial with systematic substitutions of very nonpolar Leu residues for mildly nonpolar Ala residues. This would, in turn, lead to substantial increases in overall peptide hydrophobicity as Leu residues replaced Ala residues. In contrast, in the present study, the changes in overall peptide hydrophobicity are much more subtle for most of the analogs, where substitutions of positively charged residues are made on the polar face of the peptides (i.e., the nonpreferred binding face in RPC) and the nonpolar face remains constant for the seven peptides with specificity determinants and the three peptides without the determinants.
2.7. Peptide Secondary Structure and Amphipathicity
Table 5 shows the circular dichroism (CD) results for the 10 peptides used in this study in conditions of pH 7 (50 mM PO4, 100 mM KCl) and in the presence of 50% trifluoroethanol (TFE), a mimic of the hydrophobicity and the α-helix inducing ability of the hydrophobic membrane. The two Lys specificity determinants substituted in the center of the nonpolar face was to disrupt the continuous hydrophobic surface on the nonpolar face. A continuous hydrophobic surface stabilizes the α-helical structure. Our design concept was to minimize the α-helical structure in aqueous conditions and maximize the inducible α-helical structure in the presence of the hydrophobicity of the membrane. The % helix induced in 50% TFE varied from 67 to 94% for the AMPs with specificity determinants depending on the type of positively charged residue used on the polar face (Table 5). It is interesting that when using six diaminobutyric acid residues on the polar face in aqueous conditions, the peptide had the least α-helical structure (6%) and the highest inducible α-helical structure in the presence of 50% TFE (94%). For peptides with specificity determinants, the amphipathicity ranged from a low value of 3.327–3.879, depending on the positively charged residue used on the polar face. As expected, removing the Lys specificity determinants at positions 13 and 16 and replacing them with Ala residues increased amphipathicity which ranged from 4.631 to 5.135, depending on the positively charged residue used on the polar face. All the +9 peptides shown in Table 1 are identical in sequence except for the six polar face substitutions which were either Arg, Lys, Orn, Dab, or Dap residues. Thus, amphipathicity is affected by the hydrophobicity coefficient used for the calculation of amphipathicity. These values were Arg (0.6), Lys (2.8), Orn (2.1), Dab (−1.2), and Dap (1.0). Thus, the peptide with the highest amphipathicity was the Dab-containing peptide 3.879 (with specificity determinants) and 5.135 without specificity determinants (Table 5). The amphipathicity values of our AMPs are similar to those observed for native AMPs in the length of 22–27 residues (see review by Hodges et al., 2012).16 In summary, both amphipathicity and the inducible α-helical structure play a critical role in providing AMPs with the desired properties.
2.8. Peptide Self-Association
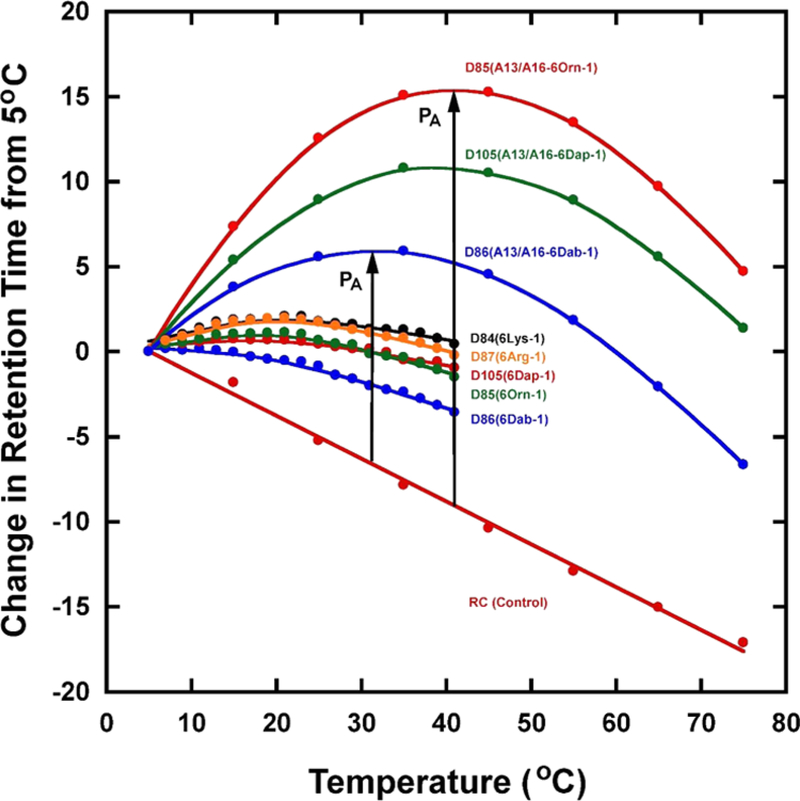
We have proposed that monomeric random-coil AMPs in aqueous solution are best able to pass through a polysaccharide capsule, followed by the outer membrane lipopolysaccharide and the cell wall peptidoglycan layer of Gram-negative bacteria before the cytoplasmic membrane is penetrated. The membrane then induces the α-helical structure and disrupts the membrane structure, leading to cell leakage and death of bacterial cells. In contrast, if self-association of an AMP in aqueous medium is too strong via interaction of their non-polar faces, this decreases the ability of the peptide to dissociate to the monomer, with the dimer/oligomer being too large to pass through the capsule and cell wall efficiently in order to reach the cytoplasmic membrane. The self-association tendency of an AMP was determined by a RP-HPLC technique developed in our laboratory,13,30–38 termed temperature profiling. Figure 5 illustrates the RP-HPLC retention behavior of three control AMPs without specificity determinants (D85(K13A/K16A-6 Orn-1); D105(K13A/K16A-6 Dap-1); and D86(K13A/K16A-6 Dab-1), at the top of the figure, and five AMPs with specificity determinants (D87–6 Arg-1; D84–6 Lys-1; D86–6 Orn-1); D86–6 Dab-1 and D105–6 Dap-1) over the temperature range 5–77 °C. The temperature profiling behavior of these eight AMPs was compared to a control peptide, denoted RC, an 18-residue monomeric random-coil peptide in both aqueous and hydrophobic environments. This control peptide, which exhibits a linear decrease in retention time with increasing temperature, represents peptides which are unable to self-associate during RP-HPLC. Thus, the observed linear decrease in retention time with increasing temperature serves to represent the general effects of increasing temperature, that is, greater solute diffusivity and enhanced mass transfer between the mobile and stationary phases of RP-HPLC. The difference in RP-HPLC retention time between an amphipathic α-helical AMP and the control peptide represents the degree of self-association of the AMP. The association parameter, PA, is larger for AMPs without specificity determinants (Ala 13 and Ala 16), ranging from 12.5 to 28.0 min (Table 5), denoted by a black arrow in Figure 5. Conversely, the association parameter, PA, is smaller for AMPs with specificity determinants (Lys 13 and Lys 16, in the center of the nonpolar face), ranging from 0 to 6 min (Table 5). Thus, the specificity determinants clearly inhibit peptide self-association, a critical property of effective AMPs, as described in the Introduction. We have shown previously the relationship of antimicrobial activity (MIC) and peptide hydrophobicity (expressed as retention times of peptides in RP-HPLC) as a U-shaped relationship.32 Hydrophobicity has two effects on peptide antimicrobial activity: at a relatively lower level of hydrophobicity, an increase in peptide hydrophobicity caused an improvement in antimicrobial activity until an optimum hydrophobicity was reached; in contrast, peptide antimicrobial activity was weakened dramatically with further increase in hydrophobicity beyond the optimum, even resulting in the dramatic loss of antimicrobial activity.32 AMPs that strongly self-associate by their hydrophobic face, that is, too hydrophobic on their nonpolar face, are inactive. Thus, we have a monomer/dimer peptide equilibrium where the stronger the dimerization, the more time that the peptide spends as a dimer, leading to lessening of the antimicrobial activity effectiveness (i.e., higher MIC value).32 Too little or too much hydrophobicity precluded efficient antimicrobial effectiveness. Hence, in the present study, we are working in the region of this optimum peptide nonpolar face hydrophobicity in the presence or absence of specificity determinants, and the substitution of polar residues on the polar face of the peptides is having only very subtle effects on overall peptide hydrophobicity. Thus, the relative effects on antimicrobial activity would be expected to be small in the absence or presence of the specificity determinants which was in fact observed.
Figure 5.
Self-association of AMPs determined by temperature profiling in RP-HPLC. Retention behavior from eight AMPs after normalization to their retention times at 5 °C over the temperature range 5–41 °C in 2 °C—increments or 5–75 °C in 10°C—increments (details shown in Section 2.3). The sequences of the eight peptides are shown in Table 1. D85(A13/A16–6Orn-1), D86(A13/A16–6Dab-1) and D105(A13/A16–6Dap-1) do not contain Lys specificity determinants, whereas the remaining AMPs contain Lys specificity determinants at positions 13 and 16 on the non-polar face (Figure 1). Note that peptide denotations in Figure 5 have been abbreviated from those shown in Table 1; for example, D87(Lys1-6 Arg-1) has been shortened to D87(6 Arg-1). RC is a random coil control peptide (Ac-ELEKGGLEGEKGGKELEK-amide) used for RP-HPLC temperature profiling. The peptide self-association parameter, PA, represents the maximum change in peptide retention time relative to the random coil peptide, RC (PA values shown in Table 5).
3. CONCLUSIONS
The main goal of this study was to eliminate hemolysis of human red blood cells by changing the type of positively charged residues used on the polar face of amphipathic α-helical AMPs. We used the most rigorous test of hemolytic activity (18 h at 37 °C and up to 1000 μg/mL, or >350 μM, of AMP). We have shown that substantially greater hemolysis is observed when the exposure time is increased from less than 2 to 18 h.16 For example, we previously showed a dramatic shift in minimal hemolytic concentration from 250 μg/mL at 12 h13 to 31.3 μg/mL at 18 h.31 Thus, we strongly suggest that hemolysis should be measured on human red blood cells using a time course approach extending to 18 h. Anything less can be misleading.
The template we chose was a de novo designed α-helical AMP of 26 residues extensively studied in our laboratory.13 We maintained the nonpolar face with two Lys specificity determinants at positions 13 and 16 in the center of the nonpolar face and investigated the role of changing the type of positively charged residues on the polar face at positions 3, 7, 11, 18, 22, and 26 (Figures 1 and 2), where we substituted all six positions with either Arg, Lys, Orn, Dab, and Dap residues. As shown in Figure 3, we have eliminated lysis of human red blood cells with the substitution of six Dab or six Dap residues on the polar face using the most stringent test for hemolysis. The present results clearly demonstrate the importance of the peptide template in which substitutions are made and we again verified the importance of the specificity determinants (Figure 3), with the combination of specificity determinants (Lys residues on the nonpolar face at positions 13 and 16) and the use of Dab and Dap residues on the polar face (positions 3, 7, 11, 18, 22, and 26), resulting in the elimination of hemolysis [Figure 3, Panel (B)].
We have also shown here that the specificity determinants have another key role, that is, they eliminate binding to human serum proteins. Thus, for AMPs without such determinants, antimicrobial activity against A. baumannii blood strain 649 in the presence of 25% human serum is greatly reduced. In terms of biological activities, we determined the antimicrobial activity against seven A. baumannii-resistant strains (resistant to antibiotics of last resort, colistin, and polymyxin B). The geometric mean MIC (μM) ranged from 0.5 to 1.2 μM for the seven AMPs (Tables 2 and S1). In addition, we obtained twenty A. baumannii worldwide isolates from 2016/2017 which came from four continents, 12 different countries, and 17 different cities. These isolates were resistant to 18 different antibiotics (amikacin, ampicillin–sulbactam, aztreonam, cefepime, ceftazidime, ceftizidime–avibactam, ciprofloxacin, colistin, doripenem, doxycycline, gentamicin, imipenem, levofloxacin, meropenem, minocycline, piperacillin–tazobactam, tetracycline, and tobramycin). The results ranged from 0.6 to 1.0 μM for the five AMPs tested (Tables 2 and S2). Thus, over the 27 strains of A. baumannii tested, the antimicrobial activity shown was identical regardless of the AMP tested. Thus, the sequence changes had little effect on antimicrobial activity but as shown in the summary Table 3, the positively charged residue used on the polar face had a major effect on hemolytic activity where the fold improvement relative to the Arg containing peptide in the HC50 value was >287-fold resulting in a fold improvement in the therapeutic index as high as >202-fold (Table 3).
One of the most interesting phenomena of this study was the substitution of Dab and Dap in the L-conformation into an all D-AMP. These substitutions were made on the polar face of the amphipathic α-helix because it has always been our objective to have as little α-helical structure as possible in aqueous conditions but maximum inducible α-helical structure in the presence of the hydrophobicity of the membrane. We were able to substitute 6 L-amino acid substitutions of either Dab or Dap in this 26-residue D-peptide and maintain a high inducible helical structure in the presence of 50% TFE, our mimic of the hydrophobicity of the membrane. The significant advantage of this effect was complete removal of hemolytic activity against human red blood cells while maintaining excellent antimicrobial activity and thus achieving exceptional therapeutic indices. This study clearly shows the potential of amphipathic α-helical AMPs with specificity determinants and concomitant Dab and Dap residues on the polar face as potential therapeutics to replace existing antibiotics.
4. EXPERIMENTAL SECTION
4.1. Evaluation of Purity
We have used LC/MS with an analytical reversed-phase HPLC column from Agilent, poroshell 120 EC C18 1.9 μm particle size, 2.1 mm I.D. × 50 mm on an Agilent 1260 Infinity II series HPLC and electrospray mass spectrometer. Run conditions were as follows: linear AB gradient 1% acetonitrile/min, starting from 2% acetonitrile at a flow rate of 200 μL/min, where eluant A is 0.1% aq TFA and eluant B is 0.1% TFA in acetonitrile; temperature, 50 °C. Peptides were dissolved in 1 mL of H2O at a concentration of 1 mg/mL and 2 μL injected. Detection wavelengths were 210, 215, and 280 nm. Correct mass was determined from the +2, +3, +4, +5, and +6 charged species and the correct mass was observed within 0.1–0.4 Da. All peptides had purity ≥95% as shown in the 10 HPLC traces supplied to the Journal.
4.2. Solid-Phase Peptide Synthesis
Standard solid-phase peptide synthesis methodology using 9-fluorenylmethoxycarbonyl (Fmoc) chemistry and Fmoc-rink amide 4-methylbenzhydrylamine resin (P3 BioSystems, Louisville, KY) (substitution 0.65 mmol/g) using a Focus-XC peptide synthesizer (Aapptec, Louisville, KY). The coupling procedure used 5 equivalents of Fmoc-D-amino acid or Fmoc-L-amino acid and HCTU (O-(1H-6-chlorobenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) and 6-Cl-HOBt (1-hydroxy-6-chloro-benztriazole) at 4.5 equiv in DMF (Fisher Scientific, Denver, CO) and N-diisopropylethylamine (DIPEA) 10 equiv (Oakwood Products, Inc., Estill, SC) in N-methyl-2-pyrrolidinone (NMP) (Fisher Scientific, Denver, CO) with the first and second couplings at room temperature for 30 min each. The deprotection procedure (removal of the Fmoc-protecting group) was carried out by treatment of the resin with 0.1 M HOBt (1-hydroxybenzotriazole) in DMF with 20% piperidine for 30 min. After completion of the synthesis, the peptide resin was dried under vacuum, and the peptide was cleaved from the resin with a mixture of 94% TFA, 2.5% water, 2.5% 1,2-ethanedithiol, and 1% triisopropylsilane for 2 h. The resin was removed by filtration, and the peptide was precipitated with ice-cooled ethyl ether on ice for 15 min. Ether was decanted, the peptide was washed twice with ether and redissolved in acetonitrile/water (1:1, with 0.2% TFA), and the solution lyophilized to obtain the crude peptide.
4.3. Analytical and Preparative Purification by RP-HPLC
4.3.1. Analytical RP-HPLC
Column, Luna C18 (2), 250 × 4.6 mm I.D., 5 μm particle size, 100 Å pore size from Phenomenex (Torrance, CA). Run conditions were as follows: linear AB gradient (1% acetonitrile/min, starting from 2% acetonitrile) at a flow-rate of 1 mL/min, where eluant A is 0.2% aq TFA and eluant B is 0.18% TFA in acetonitrile; temperature, 30 °C. Detection wavelengths used were 210, 215, and 280 nm. Preparative RP-HPLC was performed as follows: Peptides were dissolved in 0.2% aq TFA containing 2% acetonitrile to a final concentration of 10 mg/mL. Following filtration through a 0.45 μm Millipore filter and subsequently through a 0.22 μm filter, the peptide solutions were loaded onto the column via multiple 20 mL injections into a 20 mL injection loop at a flow rate of 10 mL/min. Column, Luna C18 (2), 250 × 30 mm I.D., 5 μm particle size, 100 Å pore size from Phenomenex. Run conditions were as follows: 2% acetonitrile/min gradient up to an acetonitrile concentration 10% below that required to elute the peptide during analytical RP-HPLC, then shallow gradient elution (0.05 or 0.1% acetonitrile/min depending on the complexity of impurities near the product of interest on the analytical run of the crude peptide) at a flow rate of 10 mL/min (same eluants as shown above for analytical RP-HPLC); and room temperature. Detection wavelength was 215 nm.
4.4. Temperature Profiling of Peptides on Reversed-Phase HPLC to Measure Dimerization/Oligomerization
Purified peptides were analyzed on an Agilent 1200 series liquid chromatograph for temperature profiling using a Zorbax 300 SB-C8 column (150 mm × 2.1 mm I.D.; 5 μm particle size, 300 Å pore size) from Agilent Technologies. Conditions were as follows: linear AB gradient (0.5% acetonitrile/min) and a flow rate of 0.30 mL/min, where eluant A was 0.20% aq TFA, pH 2 and eluant B was 0.18% TFA in acetonitrile. Temperature profiling was carried out on peptide mixtures run at each temperature in 2 °C increments from 5 to 41 and 10 °C increments from 5 to 75 °C. Twenty minutes was allowed between runs for temperature equilibration.
4.5. Characterization of the Helical Structure
The mean residue molar ellipticities of peptides were determined by CD spectroscopy using a Jasco J-815 spectropolarimeter (Jasco Inc. Easton, MD, USA) at 25 °C under KP buffer (50 mM NaH2PO4/Na2HPO4/100 mM KCl, pH 7.0) as well as in the presence of an α-helix inducing solvent, 2,2,2-TFE (50 mM NaH2PO4/Na2HPO4/100 mM KC1, pH 7.0 buffer/50% TFE). A 10-fold dilution of an approximately 500 μM stock solution of the peptide analogs was loaded into a 0.1 cm quartz cell and its ellipticity scanned from 195 to 250 nm. Peptide concentrations were determined by amino acid analysis.
4.6. Determination of Peptide Amphipathicity
Amphipathicity of peptides at pH 2 was determined by the calculation of hydrophobic moment59 using the software package EMBOSS 6.5.7 and the hmoment application, modified to include hydrophobicity scales determined in our laboratory.60,61 The hydrophobicity scale used in this study is listed as follows: at pH 2, these coefficients were determined in 20 mM TFA, Trp, 32.4; Phe, 29.1; Leu, 23.3; Ile, 21.4; Met, 15.7; Tyr, 14.7; Val, 13.4; Pro, 9.0; Cys, 7.6; Ala, 2.8; Glu, 2.8; Thr, 2.3; Asp, 1.6; Gln, 0.6; Ser, 0.0; Asn, −0.6; Gly, 0.0; Arg. 0.6; His, 0.0; Lys, 2.8; Orn, 2.1; Dab, −1.2; and Dap, 1.0 (polar face), Lys, −18.48 (center of non-polar face). This HPLC-derived scale reflects the relative difference in hydrophilicity/hydrophobicity of the 20 amino acid sidechains more accurately than previously determined scales (see a recent review where this scale was compared to other scales61). The hydrophobicity/hydrophilicity coefficients for Lys residues in the center of the nonpolar face at pH 2.0 were assigned values of −18.48 determined by RP-HPLC of the identical peptides where Ala was substituted by Lys on the nonpolar face at positions 13 and 16. Position X was placed in the sequence where these values are to be used in the hmoment calculations when Lys is in the center of the nonpolar face. In the program, J, B, and Z were used to denote Orn, Dab, and Dap, respectively.
4.7. Amino Acid Analysis for Peptide Quantitation
Amino acid analysis was performed according to the method described by Cohen and Michaud.62 Briefly, 20 μL of each peptide sample was aliquoted into glass tubes and lyophilized. To these tubes, 300 μL of 6 M HCl w/0.1% phenol was added, and the resulting solution was heated to 110 °C for 48 h in order to hydrolyze the peptide bonds in the sample. Each sample tube was allowed to come to room temperature and then vacuum-dried to remove the HCl. Each sample was then resuspended in 10 mM HCl, and 20 μL of the sample was added to 60 μL of 0.2 M sodium borate buffer, pH 8.8. To this mixture, 20 μL of 6-aminoquinoyl-N-hydroxysuccinimidyl carbamate in acetonitrile was added to derivatize the amino acids present in the sample. After this addition, the derivatized sample was heated to 55 °C for 15 min to convert Tyr byproducts to one form. HPLC using an Agilent 1260 series instrument and a Waters AccQ Tag 3.9 × 150 mm column was used to separate and quantify the derivatized amino acids present in each sample. Quantification was accomplished by UV absorbance at 254 nm.
4.8. Gram-Negative Bacteria Strains Used in This Study
The A. baumannii strains used in this study consisted of seven strains obtained from Merck (M89941, M89949, M89951, M89952, M89953, M89955, and M89963). These seven A. baumannii strains were resistant to polymyxin B and colistin. In addition, we obtained 20 A. baumannii strains from JMI Laboratories, North liberty, IA, 2017/2018 worldwide isolates with resistance to antibiotics. These isolates came from four continents [Asia-W. Pacific (collection number 965463, 981650, 1035794, 1018887), Europe (963618, 963659, 964304, 968886, 1017395, 1010245, 1010282, 1035166), Latin America (977751, 1002956), and North America (961997, 9383370, 952654, 1021371, 1007660, 1001611], 12 different countries, and 17 different cities. These isolates were screened against 18 different antibiotics (amikacin (17/20), ampicillin–sulbactam (17/20), aztreonam (20/20), cefepime (18/20), ceftazidime (17/20), ceftazidime–avibactam (17/20), ciprofloxacin (15/20), colistin (10/20), doripenem (17/20), doxycycline (13/17), gentamicin (18/20), imipenem (17/20), levofloxacin (14/20), meropenem (17/20), minocycline (13/17), piperacillin–tazobactam (19/20), tetracycline (15/17), and tobramycin (18/20). The values in brackets show the number of resistant isolates for that antibiotic out of the number of isolates screened. The blood strain 649 of A. baumannii used to determine the antimicrobial activity in the presence of human serum was obtained from the collection of Dr. Anthony A. Campagnari at the University of Buffalo.
4.9. Measurement of Antimicrobial Activity (MIC)
The minimal inhibitory concentration (MIC) is defined as the lowest peptide concentration that inhibited bacterial growth. MICs were measured by a standard microtiter dilution method in MH medium. Briefly, cells were grown overnight at 37 °C in MH broth and were diluted in the same medium. Serial dilutions of the peptides were added to the microtiter plates in a volume of 50 μL, followed by the addition of 50 μL of bacteria to give a final inoculum of 5 × 105 colony-forming units (cfu)/mL. The plates were incubated at 37 °C for 24 h, and the MICs were determined.
4.10. Measurement of Hemolytic Activity
Peptide samples (concentrations determined by amino acid analysis) were added to 1% human erythrocytes in phosphate-buffered saline (100 mM NaCl, 80 mM Na2HPO4, 20 mM NaH2PO4, pH 7.4), and the reaction mixtures were incubated at 37 °C for 18 h in microtiter plates. Two-fold serial dilutions of the peptide samples were carried out. This determination was made by withdrawing aliquots from the hemolysis assays and removing unlysed erythrocytes by centrifugation (800g). Hemoglobin release was determined spectrophotometrically at 570 nm. The control for 100% hemolysis was a sample of erythrocytes treated with water. The control for no release of hemoglobin was a sample of 1% erythrocytes without any peptide added. Because erythrocytes were in an isotonic medium, no detectable release (<1% of that released upon complete hemolysis) of hemoglobin was observed from this control during the course of the assay. The hemolytic activity is generally determined as the peptide concentration that causes 50% hemolysis of erythrocytes after 18 h (HC50). HC50 was determined from a plot of percent lysis versus peptide concentration (μM). Hemolysis data are determined at 12 different concentrations up to 1000 μg per mL for 18 h at 37 °C. The average of 3 replicates is used with an average variance of less than 4%. Fresh human blood was obtained from Vitalant, Denver, CO, USA.
4.11. Calculation of the Therapeutic Index
The therapeutic index is a widely accepted parameter to represent the specificity of AMPs for prokaryotic versus eukaryotic cells. It is calculated by the ratio of hemolytic activity and antimicrobial activity (MIC); thus, larger values of therapeutic index indicate greater specificity for prokaryotic cells. With the peptides used in this study, we used the HC50/MIC ratio value to calculate the therapeutic index (T.I.).
Supplementary Material
ACKNOWLEDGMENTS
We thank S.B., Facility Manager of the Biophysics Core Facility at the University of Colorado, School of Medicine, for performing the CD experiments and carrying out the quantitative amino acid analysis of our AMPs. We thank David Farrell for modifying the hmoment application from the EMBOSS 6.5.7 package with our hydrophobicity/hydrophilicity coefficients at pH 2. We thank JMI Laboratories for providing the 20 strains (2016–2017) of A. baumannii resistant to some 18 different antibiotics. We acknowledge the John Stewart Endowed Chair in Peptide Chemistry to R.S.H. for providing financial support for this project and NIH SBIR grant to AMP Discovery, LLC, R43 AI 131870 (R.S.H., PI). Vitalant, Denver, CO for providing the freshly collected human blood for our hemolytic assays.
ABBREVIATIONS
- AMP
antimicrobial peptide
- Dab
diaminobutyric acid
- Dap
diaminopropionic acid
- HC50
hemolytic activity (peptide concentration that results in 50% hemolysis)
- RPC
reversed-phase chromatography
- RP-HPLC
reversed-phase high-performance liquid chromatography
- MICGM
minimal inhibitory concentration geometric mean
- T.I.
therapeutic index
- TFE
2,2,2-trifluoroethanol
- Orn
ornithine
- MH
Mueller Hinton medium
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.8b01785.
Tables presenting information on antibacterial activity and HPLC traces to show greater than 95 purity (PDF)
REFERENCES
- (1).Jenssen H; Hamill P; Hancock REW Peptide antimicrobial agents. Clin. Microbiol. Rev 2006, 19, 491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Coast J; Smith RD; Millar MR Superbugs: should antimicrobial resistance be included as a cost in economic evaluation? Health Econ. 1996, 5, 217–226. [DOI] [PubMed] [Google Scholar]
- (3).Laxminarayan R; Duse A; Wattal C; Zaidi AKM; Wertheim HFL; Sumpradit N; Vlieghe E; Hara GL; Gould IM; Goossens H; Greko C; So AD; Bigdeli M; Tomson G; Woodhouse W; Ombaka E; Peralta AQ; Qamar FN; Mir F; Kariuki S; Bhutta ZA; Coates A; Bergstrom R; Wright GD; Brown ED; Cars O Antibiotic resistance-the need for global solutions. Lancet Infect. Dis 2013, 13, 1057–1098. [DOI] [PubMed] [Google Scholar]
- (4).Uggerhoj LE; Poulsen TJ; Munk JK; Fredborg M; Sondergaard TE; Frimodt-Moller N; Hansen RP; Wimmer R Rational design of alpha-helical antimicrobial peptides: Do’s and Don’ts. ChemBioChem 2015, 16, 242–253. [DOI] [PubMed] [Google Scholar]
- (5).Wieprecht T; Dathe M; Krause E; Beyermann M; Maloy WL; MacDonald DL; Bienert M Modulation of membrane activity of amphipathic, antibacterial peptides by slight modifications of the hydrophobic moment. FEBS Lett. 1997, 417, 135–140. [DOI] [PubMed] [Google Scholar]
- (6).Dathe M; Wieprecht T; Nikolenko H; Handel L; Maloy WL; MacDonald DL; Beyermann M; Bienert M Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997, 403, 208–212. [DOI] [PubMed] [Google Scholar]
- (7).Hancock REW; Chapple DS Peptide antibiotics. Antimicrob. Agents Chemother 1999, 43, 1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Tossi A; Sandri L; Giangaspero A Amphipathic, α-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [DOI] [PubMed] [Google Scholar]
- (9).Dathe M; Nikolenko H; Meyer J; Beyermann M; Bienert M Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Lett. 2001, 501, 146–150. [DOI] [PubMed] [Google Scholar]
- (10).Giangaspero A; Sandri L; Tossi A Amphipathic alpha helical antimicrobial peptides.. A systematic study of the effects of structural and physical properties on biological activity. Eur. J. Biochem 2001, 268, 5589–5600. [DOI] [PubMed] [Google Scholar]
- (11).Zasloff M Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [DOI] [PubMed] [Google Scholar]
- (12).Brogden KA Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature 2005, 3, 238–250. [DOI] [PubMed] [Google Scholar]
- (13).Chen Y; Mant CT; Farmer SW; Hancock REW; Vasil ML; Hodges RS Rational Design of α-Helical Antimicrobial Peptides with Enhanced Activities and Specificity/Therapeutic Index. J. Biol. Chem 2005, 280, 12316–12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Nizet V Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol 2006, 8, 11–26. [PubMed] [Google Scholar]
- (15).Wang G Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; CABI Publishing: Wallingford, Oxon, GBR, 2010. [Google Scholar]
- (16).Hodges RS; Jiang Z; Whitehurst J; Mant CT Development of antimicrobial peptides as therapeutic agents In Development of Therapeutic Agents Handbook; Wiley and Sons Inc., 2012; pp 285–358. [Google Scholar]
- (17).Kumar P; Kizhakkedathu J; Straus S Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Duclohier H; Molle G; Spach G Antimicrobial peptide magainin I from xenopus skin forms anion-permeable channels in planar lipid bilayers. Biophys. J 1989, 56, 1017–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hancock REW; Lehrer R Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998, 16, 82–88. [DOI] [PubMed] [Google Scholar]
- (20).Oren Z; Shai Y Mode of action of linear amphipathic α-helical antimicrobial peptides. Biopolymers 1998, 47, 451–463. [DOI] [PubMed] [Google Scholar]
- (21).Huang HW Action of Antimicrobial Peptides: Two-State Model. Biochemistry 2000, 39, 8347–8352. [DOI] [PubMed] [Google Scholar]
- (22).Shai Y Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [DOI] [PubMed] [Google Scholar]
- (23).Biswas S; Brunel J-M; Dubus J-C; Reynaud-Gaubert M; Rolain J-M Colistin: an update on the antibiotic of the 21st century. Expert Rev. Anti Infect. Ther 2012, 10, 917–934. [DOI] [PubMed] [Google Scholar]
- (24).Yu Z; Qin W; Lin J; Fang S; Qiu J Antibacterial mechanisms of polymyxin and bacterial resistance. BioMed Res. Int 2015, 2015, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Aguilar MI; Mougos S; Boublik J; Rivier J; Hearn MTW High-performance liquid chromatography of amino acids, peptides and proteins CXXVIII. Effect of d-amino acid substitutions on the reversed-phase high-performance liquid chromatography retention behaviour of neuropeptide Y[18–36] analogues. J. Chromatogr 1993, 646, 53–65. [DOI] [PubMed] [Google Scholar]
- (26).Rothemund S; Beyermann M; Krause E; Krause G; Bienert M; Hodges RS; Sykes BD; Soennichsen FD Structure effects of double D-amino acid replacements: a nuclear magnetic resonance and circular dichroism study using amphipathic model helices. Biochemistry 1995, 34, 12954–12962. [DOI] [PubMed] [Google Scholar]
- (27).Rothemund S; Krause E; Beyermann M; Dathe M; Bienert M; Hodges RS; Sykes BD; Sönnichsen, F. D. Peptide destabilization by two adjacent D-amino acids in single-stranded amphipathic alpha-helices. Pept Res 1996, 9, 79–87. [PubMed] [Google Scholar]
- (28).Krause E; Bienert M; Schmieder P; Wenschuh H The Helix-Destabilizing Propensity Scale of D-Amino Acids: The Influence of Side Chain Steric Effects. J. Am. Chem. Soc 2000, 122, 4865–4870. [Google Scholar]
- (29).Chen Y; Mant CT; Hodges RS Determination of stereochemistry stability coefficients of amino acid side-chains in an amphipathic alpha-helix. J. Pept. Res 2002, 59, 18–33. [DOI] [PubMed] [Google Scholar]
- (30).Lee DL; Mant CT; Hodges RS A Novel Method to Measure Self-association of Small Amphipathic Molecules. J. Biol. Chem 2003, 278, 22918–22927. [DOI] [PubMed] [Google Scholar]
- (31).Chen Y; Vasil AI; Rehaume L; Mant CT; Burns JL; Vasil ML; Hancock REW; Hodges RS Comparison of Biophysical and Biologic Properties of alpha-Helical Enantiomeric Antimicrobial Peptides. Chem. Biol. Drug Des 2006, 67, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Chen Y; Guarnieri MT; Vasil AI; Vasil ML; Mant CT; Hodges RS Role of Peptide Hydrophobicity in the Mechanism of Action of alpha-Helical Antimicrobial Peptides. Antimicrob. Agents Chemother 2007, 51, 1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Jiang Z; Vasil AI; Hale JD; Hancock REW; Vasil ML; Hodges RS Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Biopolymers 2008, 90, 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Jiang Z; Kullberg BJ; van der Lee H; Vasil AI; Hale JD; Mant CT; Hancock REW; Vasil ML; Netea MG; Hodges RS Effects of Hydrophobicity on the Antifungal Activity of α-Helical Antimicrobial Peptides. Chem. Biol. Drug Des 2008, 72, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Jiang Z; Higgins MP; Whitehurst J; Kisich KO; Voskuil MI; Hodges RS Anti-tuberculosis activity of α-helical antimicrobial peptides: de novo designed L- and D-enantiomers versus L- and D-LL37. Protein Pept. Lett 2011, 18, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Jiang Z; Vasil AI; Gera L; Vasil ML; Hodges RS Rational Design of α-Helical Antimicrobial Peptides to Target Gram-negative Pathogens, Acinetobacter baumannii and Pseudomonas aeruginosa: Utilization of Charge, “Specificity Determinants,” Total Hydrophobicity, Hydrophobe Type and Location as Design Parameters to Improve the Therapeutic Ratio. Chem. Biol. Drug Des 2011, 77, 225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Jiang Z; Vasil AI; Vasil ML; Hodges RS “Specificity Determinants” Improve Therapeutic Indices of Two Antimicrobial Peptides Piscidin 1 and Dermaseptin S4 Against the Gram-negative Pathogens Acinetobacter baumannii and Pseudomonas aeruginosa. Pharmaceuticals 2014, 7, 366–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Jiang Z; Mant CT; Vasil M; Hodges RS Role of positively charged residues on the polar and non-polar faces of amphipathic α-helical antimicrobial peptides on specificity and selectivity for Gram-negative pathogens. Chem. Biol. Drug Des 2018, 91, 75–92. [DOI] [PubMed] [Google Scholar]
- (39).Wade D; Boman A; Wahlin B; Drain CM; Andreu D; Boman HG; Merrifield RB All-D amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. U.S.A 1990, 87, 4761–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Cribbs DH; Pike CJ; Weinstein SL; Velazquez P; Cotman CW All-D-Enantiomers of β-Amyloid Exhibit Similar Biological Properties to All-L-β-Amyloids. J. Biol. Chem 1997, 272, 7431–7436. [DOI] [PubMed] [Google Scholar]
- (41).Hong SY; Oh JE; Lee K-H Effect of D-amino acid substitution on the stability, the secondary structure, and the activity of membrane-active peptide. Biochem. Pharmacol 1999, 58, 1775–1780. [DOI] [PubMed] [Google Scholar]
- (42).Wakabayashi H; Matsumoto H; Hashimoto K; Teraguchi S; Takase M; Hayasawa H N-Acylated and d Enantiomer Derivatives of a Nonamer Core Peptide of Lactoferricin B Showing Improved Antimicrobial Activity. Antimicrob. Agents Chemother 1999, 43, 1267–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).De Lucca AJ; Bland JM; Vigo CB; Jacks TJ; Peter J; Walsh TJ D-cecropin B: proteolytic resistance, lethality for pathogenic fungi and binding properties. Med. Mycol 2000, 38, 301–308. [DOI] [PubMed] [Google Scholar]
- (44).Bland JM; de Lucca AJ; Vigo CB; Vigo CB All-Dcecropin B: synthesis, conformation, lipopolysaccharide binding, and antibacterial activity. Mol. Cell. Biochem 2001, 218, 105–111. [DOI] [PubMed] [Google Scholar]
- (45).Hamamoto K; Kida Y; Zhang Y; Shimizu T; Kuwano K Antimicrobial activity and stability to proteolysis of small linear cationic peptides with D-amino acid substitutions. Microbiol. Immunol 2002, 46, 741–749. [DOI] [PubMed] [Google Scholar]
- (46).Elmquist A; Langel U In vitro uptake and stability study of pVEC and its all-D analog. Biol. Chem 2003, 384, 387–393. [DOI] [PubMed] [Google Scholar]
- (47).Ehrenstein G; Lecar H Electrically gated ionic channels in lipid bilayers. Q. Rev. Biophys 1977, 10, 1–34. [DOI] [PubMed] [Google Scholar]
- (48).Pouny Y; Rapaport D; Mor A; Nicolas P; Shai Y Interaction of antimicrobial dermaseptin and its fluorescently labeled analogs with phospholipid membranes. Biochemistry 1992, 31, 12416–12423. [DOI] [PubMed] [Google Scholar]
- (49).Shai Y Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta, Biomembr 1999, 1462, 55–70. [DOI] [PubMed] [Google Scholar]
- (50).Jiang Z; Gera L; Mant CT; Hodges RS Design of new antimicrobial peptides (AMPs) with “Specificity Determinants” that encode selectivity for Gram-negative pathogens and remove both Gram-positive activity and hemolytic activity from broad-spectrum AMPs. Enabling Peptide Research from Basic Research to Drug Discovery, Proceedings of the 24th American Peptide Symposium; American Peptide Society and Propt Scientific Publishing: Orlando, 2015; pp 245–248. [Google Scholar]
- (51).Padmanabhan S; York EJ; Stewart JM; Baldwin RL Helix Propensities of Basic Amino Acids Increase with the Length of the Side-chain. J. Mol. Biol 1996, 257, 726–734. [DOI] [PubMed] [Google Scholar]
- (52).Zelezetsky I; Pacor S; Pag U; Papo N; Shai Y; Sahl H-G; Tossi A Controlled alteration of the shape and conformational stability of α-helical cell-lytic peptides: effect on mode of action and cell specificity. Biochem. J 2005, 390, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Zou G; de Leeuw E; Li C; Pazgier M; Li C; Zeng P; Lu W-Y; Lubkowski J; Lu W Toward Understanding the Cationicity of Defensins. J. Biol. Chem 2007, 282, 19653–19665. [DOI] [PubMed] [Google Scholar]
- (54).Khara JS; Priestman M; Uhía I; Hamilton MS; Krishnan N; Wang Y; Yang YY; Langford PR; Newton SM; Robertson BD; Ee PLR Unnatural amino acid analogues of membrane-active helical peptides with anti-mycobacterial activity and improved stability. J. Antimicrob. Chemother 2016, 71, 2181–2191. [DOI] [PubMed] [Google Scholar]
- (55).Arias M; Piga K; Hyndman M; Vogel H Improving the activity of Trp-rich antimicrobial peptides by Arg/Lys substitutions and changing the length of cationic residues. Biomolecules 2018, 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Kohn E; Shirley D; Arotsky L; Picciano A; Ridgway Z; Urban M; Carone B; Caputo G Role of Cationic Side Chains in the Antimicrobial Activity of C18G. Molecules 2018, 23, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Lan Y; Langlet-Bertin B; Abbate V; Vermeer LS; Kong X; Sullivan KE; Leborgne C; Scherman D; Hider RC; Drake AF; Bansal SS; Kichler A; Mason AJ Incorporation of 2,3-Diaminopropionic Acid into Linear Cationic Amphipathic Peptides Produces pH-Sensitive Vectors. ChemBioChem 2010, 11, 1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Zhou NE; Mant CT; Hodges RS Effect of preferred binding domains on peptide retention behavior in reversed-phase chromatography: amphipathic alpha-helices. Pept Res. 1990, 3, 8–20. [PubMed] [Google Scholar]
- (59).Eisenberg D; Weiss RM; Terwilliger TC The helical hydrophobic moment: a measure of the amphiphilicity of a helix. Nature 1982, 299, 371–374. [DOI] [PubMed] [Google Scholar]
- (60).Kovacs JM; Mant CT; Hodges RS Determination of intrinsic hydrophilicity/hydrophobicity of amino acid side chains in peptides in the absence of nearest-neighbor or conformational effects. Biopolymers 2006, 84, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Mant CT; Kovacs JM; Kim H-M; Pollock DD; Hodges RS Intrinsic amino acid side-chain hydrophilicity/hydrophobicity coefficients determined by reversed-phase high-performance liquid chromatography of model peptides: comparison with other hydrophilicity/hydrophobicity scales. Biopolymers 2009, 92, 573–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Cohen SA; Michaud DP Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem 1993, 211, 279–287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.