Abstract
Objective
Malaria infection could result in severe disease with high mortality. Prognostic models and scores predicting severity of infection, complications and mortality could help clinicians prioritise patients. We conducted a systematic review to assess the various models that have been produced to predict disease severity and mortality in patients infected with malaria.
Design
A systematic review.
Data sources
Medline, Global health and CINAHL were searched up to 4 September 2019.
Eligibility criteria for selecting studies
Published articles on models which used at least two points (or variables) of patient data to predict disease severity; potential development of complications (including coma or cerebral malaria; shock; acidosis; severe anaemia; acute kidney injury; hypoglycaemia; respiratory failure and sepsis) and mortality in patients with malaria infection.
Data extraction and synthesis
Two independent reviewers extracted the data and assessed risk of bias using the Prediction model Risk Of Bias Assessment Tool.
Results
A total of 564 articles were screened and 24 articles were retained which described 27 models/scores of interests. Two of the articles described models predicting complications of malaria (severe anaemia in children and development of sepsis); 15 articles described original models predicting mortality in severe malaria; 3 articles described models predicting mortality in different contexts but adapted and validated to predict mortality in malaria; and 4 articles described models predicting severity of the disease. For the models predicting mortality, all the models had neurological dysfunction as a predictor; in children, half of the models contained hypoglycaemia and respiratory failure as a predictor meanwhile, six out of the nine models in adults had respiratory failure as a clinical predictor. Acidosis, renal failure and shock were also common predictors of mortality. Eighteen of the articles described models that could be applicable in real-life settings and all the articles had a high risk of bias due to lack of use of consistent and up-to-date methods of internal validation.
Conclusion
Evidence is lacking on the generalisability of most of these models due lack of external validation. Emphasis should be placed on external validation of existing models and publication of the findings of their use in clinical settings to guide clinicians on management options depending on the priorities of their patients.
PROSPERO registration number
CRD42019130673.
Keywords: malaria, prognostic model, prognostic score, mortality
Strengths and limitations of this study.
This review is the first to comprehensively summarise the various prognostic models that have been produced to identify complications, severity and risk of mortality in patients with severe malaria.
The review covers prognostic models produced worldwide and for all the various malaria species.
The review reduced the risk of bias by using an independent review process for the screening of potential articles and the extraction of data.
Considering the wide variety of statistical methods used to generate and validate these models, there is the risk of heterogeneity in interpretation of the results.
The search was carried out in only one language which could potentially exclude some relevant studies published in different languages.
Introduction
Malaria is a disease caused by infection with a protozoan parasite of the genus Plasmodium. The most relevant of these species is Plasmodium falciparum as it causes most deaths from the disease.1 Another species of relevance is Plasmodium vivax which is predominantly found in Asia and has a wider distribution.2 Malaria infection can result in severe disease and is associated with a high mortality. In about 108 countries where the transmission of the disease still occurs, an estimated 435 000 people died in 2017.3 4
The incidence of malaria cases has decreased by 41% worldwide in the last 10 years, with about 17 countries in Latin America and the Middle East reporting no new cases of malaria over this period.3 5 There are, however, concerns that the fight against malaria might be slowed down by an overemphasis on prevention over treatment.6
Treatment and clinical management of malaria is made difficult due to potential evolution of simple infections into life-threatening severe disease; the multiorgan affection of severe disease; the dilemma of when to admit to intensive care units (ICUs) considering limited resources and the occurrence of concomitant sepsis infection with malaria.7 8 Some of these issues can be addressed with the help of guidelines; scores or models that could help clinicians predict the occurrence of severe disease and complications in order to act appropriately.
We therefore conducted this review to systematically assess the various predictive models or scores available to guide clinicians in the management of severe malaria, whether these models have been validated and if there is any evidence that they are being successfully used in the clinical setting.
Methods
Institutional review board approval and informed consent were not required for this systematic review. We reported our findings according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (online supplementary appendix 1).
bmjopen-2019-030793supp001.pdf (85.2KB, pdf)
Search strategy and selection criteria
We searched MEDLINE, CINAHL and Global Health databases using a tailored search strategy (online supplementary appendix 2) to identify all the relevant titles and abstracts of studies (randomised control trials, cohort, cross-sectional and case–control studies) published in English from inception of the database up to the 4 September 2019, that reported predictive/prognostic scores or models that could be used in the management of malaria. These included:
Scores/models that predicted the severity of disease as this could guide clinicians’ decisions to admit for intensive care management or the use of parenteral treatment.
Scores/models that predicted the potential development of complications (including coma or cerebral malaria; shock; acidosis; severe anaemia; acute kidney injury; hypoglycaemia; respiratory failure and sepsis).
Scores/models that predicted mortality in patients with malaria infection.
The main keywords in the search strategy included: ‘prognostic model/score’, ‘predictive model/score’ and ‘predictive value of tests’ coupled with ‘malaria’, ‘plasmodium’, ‘anti-malarials’, ‘malaria falciparum’, ‘malaria vivax’ and ‘clinical malaria’. We further canvassed the references of eligible papers to identify similar papers for review.
We excluded any duplicate studies, editorials, systematic reviews, case studies, conference abstracts, unpublished studies and expert commentaries. For studies with more than one publication of findings, we selected the most recent publication.
We also excluded studies which contained models or scores that were aimed at the diagnosis of malaria as we intend to limit the scope of the review to only models that could be used to predict severity, mortality or risk of complications—that could guide clinicians in their management options. Studies that used animal models to predict disease severity were also excluded.
Two independent reviewers (TN and BST) screened the titles and abstracts for compliance to the aforementioned inclusion and exclusion criteria and any conflicts were settled by mutual agreement. Articles considered to have data relevant to the topic were assessed in detail and the references cited in these publications were searched to identify further publications.
Data extraction
Data extraction sheets which were prepared prior to screening were used by the two independent reviewers to obtain the following details for inclusion into the final review: last name of first author; date of publication; period of patient recruitment and/or follow-up; country of study; sample size; age group; type of predictive model; name of model; method of internal validation (calibration and discrimination); diagnostic properties of model and evidence of external validation or use in clinical settings.
Definitions
By prognostic/predictive model, we mean a statistical tool which uses at least two points (or variables) of patient data to predict a specific clinical outcome.9 Prognostic models applied in clinical settings are usually used at the discretion of physicians for accurate future predictions based on characteristics gathered in the present.9 10 The information found in prognostic models is usually specific to the patients’ characteristics rather than the disease or treatment and includes: prediction of chance or the duration of survival; classification of patients into risk groups; and prediction of clinical events related to the treatment the patient is receiving.11
For models that used the area under the curve (AUC) or c-statistic to assess discrimination, the following classification was used: 0.90–1—excellent; 0.80–0.90—good; 0.70–0.80—fair; 0.60–0.70—poor and 0.50–0.60—very poor discriminative properties.12
Data synthesis and analysis
We assessed and discussed the selected studies qualitatively to describe the diagnostic properties of the models proposed in the study, their intended purpose and evidence of use of the model in other clinical settings.
We further divided the models into various categories: models used to predict a potential complication of severe malaria; models used to predict mortality as an outcome and models used to predict severity of malaria infection.
Assessment of risk of bias and applicability
The risk of bias and applicability of the models in the various studies were assessed by the two independent reviewers using the Prediction model Risk Of Bias Assessment Tool (PROBAST)13 14 (online supplementary appendix 3). Any disagreements were handled by mutual agreement.
Patient and public involvement
Patients and the public were not involved in the design and conduction of this review.
Results
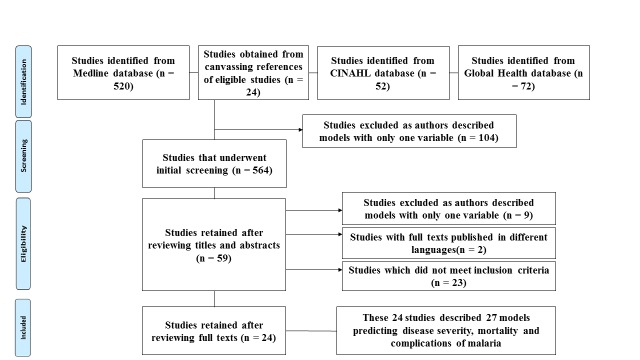
A total of 564 articles were identified by the electronic search of the databases. The titles and abstracts of these articles were screened to retain 59 articles for full text review. These were then evaluated according to the inclusion criteria and 24 articles were identified describing 27 models/scores of interests; after eliminating 23 irrelevant articles, 9 articles which used only one variable to predict an outcome and two articles describing models in other languages (figure 1).
Figure 1.
Flowchart showing reasons for exclusion of various studies from the review.
Two of the articles described models predicting complications of malaria8 15; 15 described original models predicting mortality in severe malaria16–30; 3 described models predicting mortality in different contexts but adapted and validated to predict mortality in malaria31–33; and 4 articles described models predicting severity of the disease.34–37 One of the articles described three models to predict mortality paediatric severe malaria,31 while another described two models to predict mortality in adult severe malaria.24 The rest of the articles described one model each.
Using the PROBAST to assess risk of bias and applicability, none of the studies had a low risk of bias while six studies were not found to be applicable in real-life settings15 16 22 34–36 (online supplementary appendix 3).
The general characteristics of the studies included in the review are summarised in tables 1–4.
Table 1.
Summary of articles with models predicting complications in severe malaria
| N | Study | Year | Period of participant recruitment | Country | Type of study | Sample size | Statistics used | Name of model | Method internal of validation |
Age profiles | Sex profiles |
Outcome predicted | Variables used | Diagnostic properties | External validation | Use in clinical settings |
| Complications of malaria severe anaemia | ||||||||||||||||
| 1 | Weber et al15 | 1997 | July to December 1994 | Gambia | Cohort | 368 | Logistic regression | None | None | Median age: 28 months (IQR: 14–48 months) | Females—49% | Paediatric development of severe anaemia in malaria (packed cell volume <15%) | Pallor of conjunctiva and pallor of palms | Sensitivity of 80% and a specificity of 85%. | None | NE |
| Development of sepsis | ||||||||||||||||
| 2 | Njim et al8 | 2018 | June 2003 to May 2005 | Bangladesh, India, Indonesia and Myanmart | Randomised control trial | 1187 | Logistic regression | None | Bootsrapping | 17–87 years | Female—24.3% | Development of clinical sepsis in adults with severe falciparum malaria | Sex, blood urea nitrogen levels, plasma anion gap, respiratory distress, shock on admission, parasitaemia, coma and jaundice | AUC: 0.789. Sensitivity—70.0%; specificity—69.4% |
None | NE |
AUC, area under the curve; NE, no evidence.
Table 2.
Summary of articles with models predicting mortality in paediatric severe malaria
| N | Study | Year | Period of participant recruitment | Country | Type of study | Sample size | Statistics used | Name of model |
Method internal of validation | Age profiles | Sex profiles |
Outcome predicted | Variables used | Diagnostic properties | External validation | Use in clinical settings |
| Mortality | ||||||||||||||||
| 1 | Jaffar et al21 | 1997 | 1992–1994 | Gambia | Retrospective analysis of data from a randomised control trial | 624 | Logistic regression | None | None | 1–9.5 years | Females—49% | Mortality in paediatric cerebral malaria | Cold periphery, deep coma and hypoglycaemia | Not done | None | NE |
| 2 | Molyneux et al27 | 1989 | January 1987 to June 1988 | Malawi | Cohort | 131 | Univariable analysis | Bedside prognostic index | None | 7 months to 10 years | Females—55.7% | Mortality in paediatric cerebral malaria | Blood glucose, parasitaemia, WBC count, age, coma score, absent corneal reflexes, decerebration, convulsions | Positive predictive value—83%, sensitivity—66% | None | NE |
| 3 | Conroy et al16 | 2012 | 1997–2009 | Malawi | Cohort | 155 | Logistic regression | None | Hosmer-Lemeshow goodness-of-fit test | 8 months to 14 years | Females—54.4% | Mortality in patients with cerebral malaria | Age, Blantyre coma score, respiratory distress, severe anaemia, angiopoietin-1, angiopoietin-2 and sTie-2 levels |
C-index of 0.79 (95% CI 0.72 to 0.84) | None | NE |
| 4 | Krishna et al22 | 1994 | 1988–1989 | Gambia | Cohort study | 115 | Logistic regression | None | Wald statistic and ROC analysis | 18 months to 12 years | NC | Mortality in paediatric severe malaria | Coma score, whole blood lactate/glucose ratio, TNF level | Wald statistic: coma score (4.5), lactate/glucose ratio (8.36), TNF level (6.5) | None | NE |
| 5 | Marsh et al23 | 1995 | May 1989 to November 1991 | Kenya | Cohort | 1844 | Logistic regression | None | None | Mean: 26 months | NC | Mortality in children with severe malaria | Impaired consciousness, respiratory distress, hypoglycemia, and jaundice | Predicted 92.2% of deaths | None | NE |
| 6 | Newton et al28 | 2005 | January 2001 to December 2003 | Malawi, Kenya and Ghana | Cohort | 14 605 | Linear regression | None | AUROC | Mean age: 32–36 months | Females—53%–55% | Mortality in paediatric severe falciparum malaria | Deep breathing, Blantyre Coma Score, inability to sit, weight-for-age Z score, hypoglycaemia, base excess and lactate concentration | c-statistic 0.83–0.88 in the three sites: Blantyre (0.88), Kilifi (0.87) and Kumasi (0.83) | None | NE |
| 7 | Gérardin et al32 | 2006 | 1 October 1997 to 31 March 1999 | Senegal | Cohort | 311 | Logistic regression | PRISM (paediatric risk of mortality) AUC: 0.9240 |
Hosmer-Lemeshow χ2 test | Median: 8 years (IQR: 5–11 years) | Females—40.5% | Mortality in children with falciparum malaria | Systolic blood pressure, temperature, mental status, heart rate, dilatation of pupils, pH, total CO2, PCO2, arterial PaO2, serum glucose, potassium, urea, creatinine, white blood cells, prothrombin time, platelet count | AUROC for acute malaria: 0.89 (95% CI 0.85 to 0.92) and 0.86 (95% CI 0.81 to 0.90) for severe malaria | Yes | NE |
| 8 | Helbok et al20 | 2009 | December 2000 to May 2005 | Gambia, Malawi, Kenya, Ghana, and Gabon | Cohort | 23 890 | Logistic regression | LODS (Lambaréné Organ Dysfunction Score) | Internal validation using Bonferroni correction | Mean: 30–38 months | Females—41%–47% | Mortality in children with severe falciparum malaria | Coma, prostration and deep breathing | AUROC: 0.80 (0.79 to 0.82) | Yes | NE |
| 9 | von Seidlein et al30 | 2012 | 2005–2010 | Gambia, Mozambique, Nigeria, Rwanda, Kenya, DRC, Tanzania, Ghana, Uganda | Retrospective analysis | 5426 | Logistic regression | None | ROC analysis | Median: 2.8 years (1.7, 4.3) | NC | Mortality in paediatric severe falciparum malaria | Base deficit, coma, convulsions, BUN and chronic illness | AUROC: 0.85 (95% CI 0.83 to 0.87) | None | NE |
| 10 | Conroy et al31 | 2015 | NC | Uganda | Cohort | 1589 | Logistic regression | SICK (Signs of Inflammation in Children that Kill) 38—AUC*: 0.887 (sensitivity 84.1% specificity 82.2%) | Hosmer-Lemeshow goodness-of-fit | NC | Females—54.3% | Mortality in malaria | Altered consciousness, temperature, heart rate, respiratory rate, systolic blood pressure, capillary refill time and age | AUROC: 0.846 | Yes | NE |
| LODS57 | Hosmer-Lemeshow goodness-of- fit | NC | Females—54.3% | Mortality in malaria | Prostration, coma (BCS) and deep breathing | AUROC: 0.898 | Yes | NE | ||||||||
| PEDIA39—AUC*: 0.93 (95% CI 0.92 to 0.94) | Hosmer-Lemeshow goodness-of- fit | NC | Females—54.3% | Mortality in malaria | Kwashiokor†, jaundice, subcostal indrawing, prostration (±seizures) and wasting | AUROC: 0.896 | Yes | NE | ||||||||
*Diagnostic properties of original model.
†Not used in present model.
AUROC, area under the receiver operating curve; BCS, Blantyre coma score; BUN, blood urea nitrogen; DRC, Democratic Republic of the Congo; NC, not clear; NE, no evidence; TNF, tissue necrotic factor; WBC, white blood cells.
Table 3.
Summary of articles with models predicting mortality in adult severe malaria
| N | Study | Year | Period of participant recruitment | Country | Type of study | Sample size | Statistics used | Name of model | Method internal of validation | Age profiles | Sex profiles |
Outcome predicted | Variables used | Diagnostic properties | External validation | Use in clinical settings |
| Mortality | ||||||||||||||||
| 1 | Wilairatana and Looareesuwan41 | 1995 | July 1991 to May 1993 |
Thailand | Cohort | 72 | Validation of APACHE II model (Original APACHE II score use clinical judgement and physiological relationships to assign weightings) | APACHE II score58 | ROC analysis | Mean age: 29.9 | Females—33.3% | Mortality in adult patients with cerebral falciparum malaria | MAP, temperature, heart rate, respiratory rate, arterial pH, PaO2, haematocrit, WBC count, creatinine, sodium, potassium and Glasgow coma score | Predicted mortality with 95.8% accuracy | None | NE |
| 2 | Dondorp et al17 | 2004 | NC | Vietnam | Cohort | 268 | Logistic regression | None | Hosmer-Lemeshow goodness-of-fit test | 15–79 years | Females—19% | Mortality in adults with severe falciparum malaria | Plasma lactate, plasma strong anion gap and plasma creatinine | AUROC: 0.81 | None | NE |
| 3 | Mishra et al24 | 2007 | NC | India | Cohort | 212 | Linear regression | MSA (malaria score for adults) | Not done | NC | NC | Mortality in adults with severe malaria | severe anaemia, acute renal failure, respiratory distress, cerebral malaria | Sensitivity: 89.9%, specificity: 70.6%, positive predictive value: 94.1% with cut-off of 5/10 | Yes43 | NE |
| MPS (malaria prediction score) | Not done | NC | NC | Mortality in severe malaria | Age, serum creatinine level, haemoglobin level, cerebral malaria, presence of a pregnancy, use of a ventilator | NE | Yes43 | NE | ||||||||
| 4 | Hanson et al18 | 2010 | June 2003 to May 2005 | Bangladesh, India, Indonesia and Myanmar | Retrospective analysis of a randomised control trial | 789 | Logistic regression | CAM (coma acidosis malaria) score | Hosmer-Lemeshow goodness-of-fit | NC | NC | Mortality in adults with severe malaria | Coma and acidosis (base deficit | AUROC: 0.81 (95% CI 0.77 to 0.84) | Yes59 | NE |
| 5 | Mohapatra and Das26 | 2009 | January 200 to December 2004 | India | Cohort study | 2089 | Logistic regression | MSS (Malaria severity score) | Hosmer-Lemeshow goodness-of-fit (internal validation by splitting data—2089 vs 509) | 18–71 years | Female—34.6% | Mortality in adult patients with severe falciparum malaria | Neurological, renal, haematological, hepatic, respiratory, cardiovascular, and metabolic organ systems | AUROC: 0.9 | None | NE |
| 6 | Newton et al29 | 2013 | 1986–2002 | Thailand | Retrospective analysis | 988 | Logistic regression | MPI (malaria prognostic index) | ROC curve analysis and internal validation by data splitting | 15–74 years | Females—43% | Mortality in adult severe falciparum malaria | Glasgow coma scale, parasitaemia, plasma lactate, serum bilirubin, pigmented parasites and treatment with ACT | AUROC: 0.97 | None | NE |
| 7 | Mohapatra et al25 | 2014 | NC | India | Cohort | 112 | NC | GCBRS (GCS, creatinine, respiratory rate, bilirubin and systolic BP) score | NC | Mean: 35.8±15.1 years | Females—16.1 | Mortality in severe falciparum malaria | Cerebral malaria, renal failure, respiratory distress, jaundice and shock | Sensitivity: 85.3%. Specificity: 95.6% | None | NE |
| 8 | Hanson et al19 | 2014 | 1996–2013 | Bangladesh, India, Indonesia, Vietnam and Myanmar | Randomised control trials and cohort studies | 1801 | Logistic regression | None | Hosmer-Lemeshow goodness-of-fit | 21–45 | Females—24.4 | 48 hours survival and survival to discharge in patients with severe malaria | Shock, oligo-anuria, dysglycaemia, respiratory rate, Glasgow Coma Score and absence of fever | PPV for 48 hour-survival: 99.4% (95% CI 97.8 to 99.9). PPV for survival to discharge: 96.9% (95% CI 94.3 to 98.5) | None | NE |
ACT, artemisinin combined therapy;AUROC, area under the receiver operating curve; NC, not clear; NE, no evidence; PPV, positive predictive value; WBC, white blood cells.
Table 4.
Summary of articles with models predicting severity of malaria infection
| N | Study | Year | Period of participant recruitment | Country | Type of study | Sample size | Statistics used | Name of model | Method internal of validation | Age profiles | Sex profiles |
Outcome predicted | Variables used | Diagnostic properties | External validation | Use in clinical settings |
| Severity of disease | ||||||||||||||||
| 1 | Helbok et al35 | 2003 | 1 October 2001 to 30 January 2002 |
Thailand | Cohort | 22 | NC | MODS (multiorgan dysfunction score)44 | None | 16–41 years | Female—41.8% | Severity of disease in adult patients with uncomplicated falciparum malaria | Ten organ systems: (heart, blood vessel, blood, respiratory system, metabolism, gastrointestinal system, liver, kidney and urinary tract, immune system, and central nervous system) | None | None | NE |
| 2 | Helbok et al34 | 2005 | 1 October 2001 to 30 July 2002 | Thailand | Cohort | 29 | Survival analysis | MODS44 | None | Mean age: 27.1 (±10.6) | Female—27.6% | Severity of disease in adult patients with severe falciparum malaria | Ten organ systems: (heart, blood vessel, blood, respiratory system, metabolism, gastrointestinal system, liver, kidney and urinary tract, immune system and central nervous system) | None | None | NE |
| 3 | Helbok et al36 | 2006 | August 2003 to May 2005 | Gabon | Cohort | 485 | Survival analysis | Simplified MODS35 | ROC analysis | 4–169 months | Females—49% | Severity of disease and disability in children with severe falciparum malaria infection | Ten organ systems: (heart, blood vessel, blood, respiratory system, metabolism, gastrointestinal system, liver, kidney and urinary tract, immune system, and central nervous system) | AUC to predict prolonged disease (>48 hours unable to walk): 0.92 (95% CI 0.89 to 0.95) | None | NE |
| 4 | Grigg et al37 | 2018 | October 2012 to April 2016 | Malaysia | Cohort | 481 patients with Plasmodium knowlesi | Logistic regression | None | None | 33 years (IQR: 21–49) | Female—43.2% | Severity of Plasmodium knowlesi infection using WHO 2014 research criteria45 | Age >45, abdominal pain, shortness of breath, increased parasite count, schizont proportion >10%, bicarbonate<18 mmol | None | None | NE |
AUC, area under the curve; NC, not clear; NE, no evidence; TNF, tissue necrotic factor.
Models predicting the risk of complications in malaria infection
Webber et al15 in 1997 conducted a study to predict the risk of severe anaemia (packed cell volume <15%) in children with severe malaria in the Gambia using logistic regression analysis. This model was not internally validated, and the two predictors identified were pallor of the conjunctiva and pallor of the palms. There is no evidence from this review that the model has been externally validated and is being used in clinical settings.
In 2018, Njim et al8 described a prognostic model for clinical use to predict the risk of sepsis development among adult patients (>16 years old) admitted for severe falciparum malaria in Southeast Asia. They used data from South East Asian Quinine Artesunate Malaria Trial (SEQUAMAT)—a large randomised control trial (RCT) conducted to determine the benefits of intravenous artesunate over quinine treatment for severe malaria. They used a multivariable logistic regression approach with internal validation using bootstrapping to generate a prognostic model with modest discriminative abilities (AUC: 0.789) containing the following predictive variables: female sex, high blood urea nitrogen, high plasma anion gap, respiratory distress, shock on admission, high parasitaemia, coma and jaundice. The model has not been externally validated and there is no evidence of use in clinical settings.
Models predicting mortality in severe malaria
Models predicting mortality in paediatric severe malaria
Ten articles described models that predicted mortality in paediatric severe malaria.16 20–23 27 28 30–32 Three articles described models which predicted mortality in paediatric patients with cerebral malaria16 21 27; two articles described models generated to assess mortality in different conditions that were validated for use in the present studies31 32; and five articles described original models predicting the risk of mortality in children with severe malaria.20 22 23 28 30
Models predicting mortality in paediatric cerebral malaria
Molyneux et al27 in 1989 conducted a study among 131 comatose Malawian children with severe cerebral malaria to determine the prognostic factors for death in these patients. The authors derived a ‘bedside prognostic index’ with: blood glucose ≤2.2 mmol/L; parasitaemia >106 ring forms/μL; white blood cell count >15×10/L; age ≤3 years; coma score (modification of the Glasgow coma score)=0; absent corneal reflexes; signs of decerebration and convulsions; as predictors of mortality with each predictor assigned a score of 1. Individuals with a score ≥4 were more likely to die. This score was calculated only using univariable analysis and internal and external validation were not done.
In 1997 in Gambia, Jaffar et al21 performed a retrospective analysis on data obtained from a randomised control trial during which artemether was compared with quinine and a monoclonal antibody against tumour necrosis factor (TNF) compared with a placebo in patients with cerebral malaria. They used these data to identify predictors of mortality in cerebral malaria using a multivariable logistic regression model. A cold periphery, a coma score of either 0 or 1 (assessed using the Blantyre coma scale measured on a scale of 0–5), and hypoglycaemia were found to be present at admission in 90% of the children who died. This model was not internally validated.
Conroy et al16 in 2012 conducted a study among 155 children aged 8 months—14 years in Malawi to determine predictors of mortality in cerebral malaria. They used a multivariable logistic regression model containing clinical parameters and biomarkers with a modest discriminative ability (C-index of 0.79) after internal validation; which contained the following variables: age, Blantyre coma score, respiratory distress, severe anaemia, angiopoietin-1, angiopoietin-2 and sTie-2 levels. The model was not externally validated.
Original models predicting mortality in paediatric severe malaria
Krishna et al22 in 1994 conducted a study in the Gambia to predict mortality in children aged 8 months to 14 years. They used a multivariable logistic regression model internally validated using the Wald statistic to determine that the coma score (using the Blantyre coma scale), whole blood lactate/glucose ratio and TNF level were the best predictors of death.
In 1995, Marsh et al23 studied 1844 children in Kenya to determine predictors of life-threatening malaria (risk of death) using a multivariable logistic regression model. They determined that impaired consciousness (assessed using the Blantyre coma scale), hypoglycaemia, respiratory distress and jaundice could correctly predict 84.4% of deaths in the sample population. The model was not validated internally or externally.
In 2005, Newton et al28 conducted a study to assess the prognostic value of measures of acid/base balance in paediatric falciparum malaria. They examined 14 605 children in Malawi (Blantyre), Kenya (Kilifi) and Ghana (Kumasi); where they determined that deep breathing, Blantyre Coma Score, inability to sit, and weight-for-age Z score were independent predictors of mortality in all the three sites. Discrimination of the model was performed by calculating the area under the receiver operating curve (AUROC). After addition of laboratory data to these models—hypoglycaemia, base excess and lactate concentrations; the c-statistics obtained were 0.88 (Blantyre), 0.87 (Kilifi) and 0.83 (Kumasi) denoting good discriminative properties of the models.
Helbok et al20 in 2009 produced the the Lambarene Organ Dysfunction Score (LODS) which combined three variables: coma, prostration and deep breathing to generate a model using multivariable logistic regression which predicted death in African children—Banjul (Gambia), Blantyre (Malawi), Kilifi (Kenya), Kumasi (Ghana), and Lambarene and Libreville (Gabon); who were admitted for severe falciparum malaria. Each component of the model was assigned a score of 1 and a LODS of 3 at admission had a 98% specificity and 25% sensitivity in predicting death. Meanwhile a LODS ≥1 had a sensitivity of 85% and a specificity of 63%. The model had good discriminative properties with an AUC of 0.80 (95% CI 0.79 to 0.82). In 2015, Conroy et al31 externally validated this model among 1589 Ugandan children. The model showed good discriminative properties with an AUC of 0.898.
Similarly, in 2012, von Seidlein et al30 conducted an analysis of data from an RCT carried out in several African countries (Gambia, Mozambique, Nigeria, Rwanda, Kenya, DRC, Tanzania, Ghana and Uganda) to generate a model for predicting mortality from severe falciparum malaria using multivariable logistic regression analysis and internally validated by AUROC analysis. After analysis of data from 5426 children, base deficit, impaired consciousness (assessed using the Blantyre Coma Score), convulsions, elevated blood urea and underlying chronic illness were identified in the model to predict mortality with a good discriminative ability—AUROC: 0.85 (95% CI 0.83 to 0.87).
Existing models validated for use in the prediction of mortality in severe malaria in children
As described above, Conroy et al31 externally validated the LODS model among 1589 Ugandan children. The authors further externally validated two other scores: the Signs of Inflammation in Children that Kill (SICK) score which was developed in India as a practical triage tool using variables related to the systemic inflammatory response syndrome, with data collected from 1099 children in 2003 admitted for any paediatric illness38; and the Paediatric Early Death Index for Africa (PEDIA) score which was developed to predict early death among 8091 children in Kenya in 2003 admitted for paediatric illnesses.39 The original SICK score containing the following variables: altered consciousness, temperature, heart rate, respiratory rate, systolic blood pressure, capillary refill time and age; had good discriminative properties with an AUC of 0.887.38 Externally validated against this cohort of 1589 children, the score maintained its good discriminative properties with an AUC of 0.846. Similarly, the PEDIA score which originally had excellent discriminative properties with an AUC of 0.9339 had good discriminative properties (AUC: 0.896) when externally validated on the cohort of 1589 Ugandan children.31 The original PEDIA score contained Kwashiorkor, jaundice, subcostal indrawing, prostration (±seizures) and wasting as variables in the model. However, kwashiorkor was not included in the validation model as it was not measured among the Ugandan children.
In 2006, Gerardin et al32 externally validated the Paediatric Risk of Mortality (PRISM) model which was originally developed in 1988 by Pollack et al40 to reduce the number of physiological variables required for paediatric ICU death risk assessment. The model was developed from data of 1227 patients with 105 deaths and contained 14 variables: systolic blood pressure, temperature, mental status, heart rate, dilatation of pupils, pH, total CO2, PCO2, arterial PaO2, serum glucose, potassium, urea, creatinine, white blood cells, prothrombin time and platelet count. The original score had excellent discriminative properties with an AUC of 0.92.40 Gerardin et al used a cohort of 311 Senegalese children admitted with severe malaria to externally validate this model. The model showed good discriminative properties in predicting death in children with severe malaria—AUC: 0.86 (95% CI 0.81 to 0.90).32
Models predicting mortality in adult severe malaria
There were eight articles assessing models that predicted mortality in adult severe malaria.17–19 24–26 28 41
In 1995, Wilairatana et al41 used the APACHE II score (the acute physiology and chronic health evaluation system score commonly used in ICUs) based on 12 physiological variables—mean arterial pressure (MAP), temperature, heart rate, respiratory rate, arterial pH, PaO2, haematocrit, white blood cells (WBC) count, creatinine, sodium, potassium and Glasgow coma score to predict the risk of mortality in adult patients with cerebral malaria in Thailand. The score was able to predict mortality with a 95.8% accuracy. The original APACHE II model was produced in 1985 by Knaus et al,42 and clinical judgement and physiological relationships were used to assign weightings for the various factors in the model.
Dondorp et al17 in 2004 created a model using logistic regression with laboratory data form 268 patients in Vietnam to determine the risk of mortality in adult patients with severe malaria. This model had a good discriminative value with an AUROC of 0.81. The laboratory variables asscoicated with mortality in this cohort were: plasma lactate, plasma creatinine and a strong anion gap. On the other hand, in 2007, Mishra et al24 created the malaria score for adults (MSA) and the malaria prediction score (MPS) from a cohort of 212 patients in India to predict mortality in severe malaria. The MSA was an upgrade of the malaria prognostic index (MPI) which required laboratory data and included a small proportion of children. The clinical variables included in the MSA were: severe anaemia, acute renal failure, respiratory distress and cerebral malaria and had a sensitivity of 89.9% and a specificity of 70.6%. This model was externally validated by Santos et al43 among 59 patients with imported severe malaria in Portugal and was shown to have good discriminative properties—AUROC: 0.84; 95% CI 0.70 to 0.98.
Similarly, Hanson et al18 produced the coma acidosis malaria (CAM) score after using a logistic regression analysis on data previously collected from the SEQUAMAT. The authors proposed the use of the presence of a coma and base deficit to calculate a five-point score to predict mortality. The score had good discriminative properties with an AUROC of 0.81 (95% CI 0.77 to 0.84). The same author used data from several cohort studies and RCTs carried out in Bangladesh, India, Indonesia, Vietnam and Myanmar to predict 48 hours survival and survival to discharge in patients with severe malaria.19 The model containing the variables: shock, oligoanuria, dysglycaemia, respiratory rate, Glasgow coma score and fever could correctly predict 48-hour survival in 99.4% of the patients and survival to discharge in 96.9% of patients.
Mohapatra et al26 in 2009 carried out a cohort study of 2089 patients in 2009, where they produced the malaria severity score (MSS) to predict mortality in adult patients with severe falciparum malaria in India. They assessed seven organ systems: neurological, renal, haematological, hepatic, respiratory, cardiovascular and metabolic organ systems; assigning a maximum score of 0–3 for each organ system. The model had excellent discriminative propertiens with an AUROC of 0.9. The authors also developed the GCRBS (Glasgow coma scale, creatinine, respiratory rate, bilirubin and systolic BP) score in 2014 as an alternative to other scores like the APACHE II score which was considered cumbersome.25 The score had a sensitivity of 85.3% and a specificity of 95.6% in predicting a fatal outcome in severe malaria.
In 2013 in Thailand, Newton et al29 conducted a retrospective analysis of 988 records with severe falciparum malaria to produce the MPI validated using ROC curve analysis and internal validation by data splitting. The MPI contained the following variables: Glasgow coma scale, parasitaemia, plasma lactate, serum bilirubin, pigmented parasites and treatment with ACT and had excellent discriminative properties with an AUROC of 0.97.
Models predicting the severity of malaria
The multiorgan dysfunction score (MODS) which is an index used in severely ill patients admitted in ICUs to determine the severity of their disease irrespective of the diagnosis.34 44 The score evaluates ten organ systems: heart, blood vessel, blood, respiratory system, metabolism, gastrointestinal system, liver, kidney and urinary tract, immune system and central nervous system—giving a score of 1–5 for each system depending on the level of dysfunction of the system, with a minimum score of 10 and a maximum score of 50.35 Helbok et al assessed the use of this score to predict severity in a small cohort (n=22) of adult patients with uncomplicated falciparum malaria35 and in adults with severe malaria (n=29)34 in Thailand. The score was not internally validated in both studies but the authors showed that higher scores were correlated with symptom severity and duration of hospitalisation. In 2006, the authors used a simplified version of the score—simplified MODS (sMODS); in a cohort of 485 children in Gabon to predict the level of severity of the disease with respect to the amout of disability the children suffered into categories: ability to walk unaided and ability to sit unaided.36 The authors obtained an AUC of 0.92 (95% CI 0.89 to 0.95) in predicting inability to walk ≥48 hours for children with sMODS ≥16 and an AUC of 0.90 (95% CI 0.87 to 0.93) in predicting inability to sit unaided (table 4).
Grigg et al in 2018, used a multivariable logistic regression model to predict the severity of Plasmodium knowlesi malaria infection in a cohort of 481 participants in Malaysia. The authors showed that independent predictors of disease severity using the WHO 2014 research criteria45 were: increasing age, abdominal pain, shortness of breath, increasing parasite count, schizont proportion >10% and serum bicarbonate levels <18 mmol. The model was not internally or externally validated (table 4).
Discussion
In this review, we report on the various prognostic models and scores produced to predict complications, mortality and severity of malaria infection. We showed that there were 2 models produced to predict the risk of developing complications from malaria infection, 12 models that predict mortality from severe malaria in children, 9 models that predict mortality from severe malaria in adults and 4 models that predict disease severity in malaria. Seventeen of these models were internally validated while only seven have been externally validated. There is no published evidence that any of these models are routinely used in clinical settings.
The models identified in this review that were used to predict mortality in children with severe malaria have similar clinical predictors. All the models had neurological dysfunction based on either the Glasgow coma score, impaired consciousness, altered mental status, convulsions, decerabration or coma as a predictor. Similarly, in adults, all the models predicting mortality also had neurological dysfuction as a predictor. Microvascular obstruction in capillaries of the brain due to direct sequestration of red blood cells infected with the malaria parasite could lead to tissue hypoxia.46 The effects of this sequestration and its sequelae in the brain can be directly visualised in both adults and children as retinopathy.16 46–48 This leads to varied results with increased intracranial pressure more pronounced in children than in adults.46 With the increased oxygen demand associated with brain hypoxia and raised intracranial pressure, coma and brain dysfunction could therefore become an important predictor of mortality.
In children, half of the models predicting mortality had hypoglycaemia as a predictor.21–23 27 28 32 Hypoglycaemia is usually implicated as a complication of severe malaria infection. This association has been said to be multifactorial.49 Proposed mechanisms for this association include: increased glucose use by the malaria parasites in the red blood cells, inhibition of gluconeogenesis by the cascade of cytokines released due to infection and prolonged starvation and fasting especially in severely ill children further compounds the problem.49 50 Considering that glucose is the primary source for organs like the brain which is likely suffering from the above highlighted effects of microvascular obstruction and sequestration; depleted glucose sources could lead to neurological dysfuction including seizures, deepening comas and hence death. As above, any factor that significantly affects neurological dysfuction could be highly predictive of mortality or disease severity in patients.
Half of the models in children predicting mortality had respiratory distress (including deep breathing and subcostal indrawing) as a predictor.16 20 23 28 31 Meanwhile, six out of the nine models in adults had respiratory failure as a clinical predictor of mortality.19 24 26 41 The incidence of respiratory distress in severe malaria is quite common as it occurs in about 40% of children with severe falciparum malaria and in 25% of adults.51 It results from acute respiratory distress syndrome (ARDS); metabolic acidosis; fluid overload possibly resulting from increased inflammatory related capillary permeability and endothelial damage8 51; and aspiration pneumonia which could lead to sepsis8—a common association with severe malaria. The high mortality rates (up to 87% in some cases) associated with respiratory failure like in ARDS52 could explain the predictive significance of respiratory distress in predicting mortality in malaria infection. Respiratory failure usually leads to hypoxia and a high probability of acute mortality in patients.
Acidosis was also a prominent predictor of mortality in most of the models predicting mortality. It was present in three of the models predicting mortality in children28 30 32 and five models predicting mortality in adults.17 18 26 29 41 Acidosis usually results from underlying pathologies like respiratory distress, renal failure and shock. These three variables were also common variables in the models predicting mortality in both children and adults identified in this review. Renal failure expressed in these models either as acute renal failure, oligoanuria or estimates of the kidney function using serum urea and creatinine17 19 24–26 30 32 41; is due to acute tubular necrosis that occurs in severe malaria infection as a direct result of microvascular obstruction of capillaries by infected red blood cells leading to the release of inflammatory cytokines like TNF.53 Similarly, shock expressed either as a function of the systolic blood pressure or cold peripheries in three models in children21 31 32 and likewise in two models in adults19 41 could result from peripheral vasodilation which may usually occur concomitantly with sepsis and is a marker of a poor prognosis.8 54 55
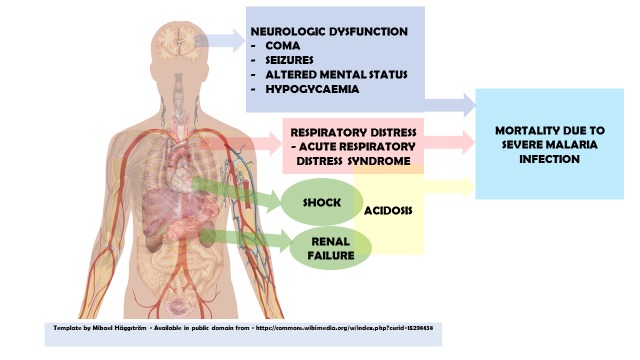
From the above, factors that were predictive of disease severity and mortality seemed to be consistent among these studies. The factors that should therefore be considered by physicians when faced with a patient with malaria infection should include: neurological dysfunction (coma and seizures), acidosis, hypoglycaemia and respiratory distress (figure 2). These factors seem to be highly predictive of mortality and disease severity in most of the articles that were included in the review and should therefore be included in any future studies attempting to predict these outcomes in malaria (table 5).
Figure 2.
Predictive factors of disease severity and mortality in malaria infection.
Table 5.
Findings of review, research gaps and potential for future research
| Findings of review | Research gaps | Potential for future research | Other possible avenues |
| Several models available to predict various outcomes in severe malaria. | Incorporation of produced models into artificial intelligence to help in the fast prediction of risks of adverse outcomes and suggestions of treatment and management modalities. | ||
| Variables consistent in predicting disease severity, mortality and complications include: neurological dysfunction, respiratory distress and acidosis. | Models that take into consideration these major variables. | Studies with robust designs. | |
| Most models have high risk of bias due to lack of use of up-to-date methods of internal validation. | Models without risk of bias that use adequate statistical methods of internal validation. | Internal validation and wide external validation to help integrate models into daily clinical practice. |
We found evidence of external validation in only seven of the models identified in this study.18 20 24 31 32 External validation is an important component as it determines the generalisability of the model and its potential use in different geographical regions.56 As outlined above, most of the models have similar variables highlighting the fact that the predictors of complications, severity and mortality in malaria might be consistent across different settings. Emphasis could therefore be better placed in the validation of existing models and initiating their use in clinical settings to guide clinicians on prioritising patients and anticipating outcomes. Publication of the findings on the use of these models in clincal settings should also be encouraged to guide clinicians on which models work better in various settings.
After assessment of the risk of bias of the various models, 18 of the studies contained models that used variables that could be readily available and hence were applicable in real-life settings. However, all the models had a high risk of bias. This was primarily due to the lack of internal validation in several of the studies or the lack of use of up-to-date methods of validation. Caution should therefore be used when interpreting and using the results from the articles.
This review has some limitations. The search included only articles that were published in English. This could potentially lead to the exclusion of studies and models that could otherwise have been included in the review.
Conclusion
Models predicting severity and mortality of malaria infection identified in this review have similar predictors. Evidence is, however, lacking on the generalisability of most of these models due lack of external validation. Emphasis should therefore be placed on external validation of existing models and publication of the findings of their use in clinical settings to guide clinicians on management options depending on the priorities of their patients.
Supplementary Material
Footnotes
Contributors: Conception: TN. Independent reviews of papers: TN and BST. Writing of initial draft: TN. Manuscript revisions: TN and BST.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. White NJ, Pukrittayakamee S, Hien TT, et al. Malaria. Lancet 2014;383:723–35. 10.1016/S0140-6736(13)60024-0 [DOI] [PubMed] [Google Scholar]
- 2. Tanner M, Greenwood B, Whitty CJM, et al. Malaria eradication and elimination: views on how to translate a vision into reality. BMC Med 2015;13:167 10.1186/s12916-015-0384-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organisation Achieving the malaria mdg target: reversing the incidence of malaria 2000-2015. Geneva, 2015. [Google Scholar]
- 4. World Health Organisation Malaria, 2019. Available: https://www.who.int/news-room/fact-sheets/detail/malaria [Accessed 4 Sep 2019].
- 5. World Health Organisation World malaria report. Geneva: World Health Organisation, 2016. [Google Scholar]
- 6. A single agenda needed for malaria. Lancet Infect Dis 2003;3:317 10.1016/S1473-3099(03)00635-2 [DOI] [PubMed] [Google Scholar]
- 7. Day N, Dondorp AM. The management of patients with severe malaria. Am J Trop Med Hyg 2007;77:29–35. [PubMed] [Google Scholar]
- 8. Njim T, Dondorp A, Mukaka M, et al. Identifying risk factors for the development of sepsis during adult severe malaria. Malar J 2018;17:278–78. 10.1186/s12936-018-2430-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perel P, Edwards P, Wentz R, et al. Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak 2006;6:38 10.1186/1472-6947-6-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vogenberg FR. Predictive and prognostic models: implications for healthcare decision-making in a modern recession. Am Health Drug Benefits 2009;2:218–22. [PMC free article] [PubMed] [Google Scholar]
- 11. Cook NR. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem 2008;54:17–23. 10.1373/clinchem.2007.096529 [DOI] [PubMed] [Google Scholar]
- 12. Mehdi T, Bashardoost N, Ahmadi M. Kernel smoothing for ROC curve and estimation for thyroid stimulating hormone. Int J Public Health Res 2011:239–42. [Google Scholar]
- 13. Moons KGM, Wolff RF, Riley RD, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019;170:W1–33. 10.7326/M18-1377 [DOI] [PubMed] [Google Scholar]
- 14. Wolff RF, Moons KGM, Riley RD, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51–8. 10.7326/M18-1376 [DOI] [PubMed] [Google Scholar]
- 15. Weber MW, Kellingray SD, Palmer A, et al. Pallor as a clinical sign of severe anaemia in children: an investigation in the Gambia. Bull World Health Organ 1997;75 Suppl 1:113–8. [PMC free article] [PubMed] [Google Scholar]
- 16. Conroy AL, Glover SJ, Hawkes M, et al. Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: a retrospective case-control study*. Crit Care Med 2012;40:952–9. 10.1097/CCM.0b013e3182373157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dondorp AM, Chau TTH, Phu NH, et al. Unidentified acids of strong prognostic significance in severe malaria. Crit Care Med 2004;32:1683–8. 10.1097/01.CCM.0000132901.86681.CA [DOI] [PubMed] [Google Scholar]
- 18. Hanson J, Lee SJ, Mohanty S, et al. A simple score to predict the outcome of severe malaria in adults. Clin Infect Dis 2010;50:679–85. 10.1086/649928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanson J, Lee SJ, Mohanty S, et al. Rapid clinical assessment to facilitate the triage of adults with falciparum malaria, a retrospective analysis. PLoS One 2014;9:e87020 10.1371/journal.pone.0087020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Helbok R, Kendjo E, Issifou S, et al. The Lambaréné organ dysfunction score (LODS) is a simple clinical predictor of fatal malaria in African children. J Infect Dis 2009;200:1834–41. 10.1086/648409 [DOI] [PubMed] [Google Scholar]
- 21. Jaffar S, Van Hensbroek MB, Palmer A, et al. Predictors of a fatal outcome following childhood cerebral malaria. Am J Trop Med Hyg 1997;57:20–4. 10.4269/ajtmh.1997.57.20 [DOI] [PubMed] [Google Scholar]
- 22. Krishna S, Waller DW, ter Kuile F, et al. Lactic acidosis and hypoglycaemia in children with severe malaria: pathophysiological and prognostic significance. Trans R Soc Trop Med Hyg 1994;88:67–73. 10.1016/0035-9203(94)90504-5 [DOI] [PubMed] [Google Scholar]
- 23. Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med 1995;332:1399–404. 10.1056/NEJM199505253322102 [DOI] [PubMed] [Google Scholar]
- 24. Mishra SK, Panigrahi P, Mishra R, et al. Prediction of outcome in adults with severe falciparum malaria: a new scoring system. Malar J 2007;6:24 10.1186/1475-2875-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohapatra BN, Jangid SK, Mohanty R. GCRBS score: a new scoring system for predicting outcome in severe falciparum malaria. J Assoc Physicians India 2014;62:14-7. [PubMed] [Google Scholar]
- 26. Mohapatra MK, Das SP. The malaria severity score: a method for severity assessment and risk prediction of hospital mortality for falciparum malaria in adults. J Assoc Physicians India 2009;57:119–26. [PubMed] [Google Scholar]
- 27. Molyneux ME, Taylor TE, Wirima JJ, et al. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med 1989;71:441–59. [PubMed] [Google Scholar]
- 28. Newton CRJC, Valim C, Krishna S, et al. The prognostic value of measures of acid/base balance in pediatric falciparum malaria, compared with other clinical and laboratory parameters. Clin Infect Dis 2005;41:948–57. 10.1086/432941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newton PN, Stepniewska K, Dondorp A, et al. Prognostic indicators in adults hospitalized with falciparum malaria in Western Thailand. Malar J 2013;12:229 10.1186/1475-2875-12-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Seidlein L, Olaosebikan R, Hendriksen ICE, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis 2012;54:1080–90. 10.1093/cid/cis034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conroy AL, Hawkes M, Hayford K, et al. Prospective validation of pediatric disease severity scores to predict mortality in Ugandan children presenting with malaria and non-malaria febrile illness. Crit Care 2015;19:47 10.1186/s13054-015-0773-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gérardin P, Rogier C, Leteurtre S, et al. Evaluation of pediatric risk of mortality (PriSM) scoring in African children with falciparum malaria. Pediatr Crit Care Med 2006;7:45–7. 10.1097/01.PCC.0000192321.66637.E6 [DOI] [PubMed] [Google Scholar]
- 33. Khoo KL, Tan WL, Eng P, et al. Malaria requiring intensive care. Ann Acad Med Singapore 1998;27:353–7. [PubMed] [Google Scholar]
- 34. Helbok R, Dent W, Nacher M, et al. The use of the multi-organ-dysfunction score to discriminate different levels of severity in severe and complicated Plasmodium falciparum malaria. Am J Trop Med Hyg 2005;72:150–4. 10.4269/ajtmh.2005.72.150 [DOI] [PubMed] [Google Scholar]
- 35. Helbok R, Dent W, Nacher M, et al. Use of the multi-organ dysfunction score as a tool to discriminate different levels of severity in uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg 2003;68:372–5. 10.4269/ajtmh.2003.68.372 [DOI] [PubMed] [Google Scholar]
- 36. Helbok R, Issifou S, Matsiegui PB, et al. Simplified multi-organ dysfunction score predicts disability in African children with Plasmodium falciparum malaria. Am J Trop Med Hyg 2006;75:443–7. 10.4269/ajtmh.2006.75.443 [DOI] [PubMed] [Google Scholar]
- 37. Grigg MJ, William T, Barber BE, et al. Age-Related clinical spectrum of Plasmodium knowlesi malaria and predictors of severity. Clin Infect Dis 2018;67:350–9. 10.1093/cid/ciy065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar N, Thomas N, Singhal D, et al. Triage score for severity of illness. Indian Pediatr 2003;40:204–10. [PubMed] [Google Scholar]
- 39. Berkley JA, Ross A, Mwangi I, et al. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ 2003;326:361 10.1136/bmj.326.7385.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med 1988;16:1110–6. 10.1097/00003246-198811000-00006 [DOI] [PubMed] [Google Scholar]
- 41. Wilairatana P, Looareesuwan S. Apache II scoring for predicting outcome in cerebral malaria. J Trop Med Hyg 1995;98:256–60. [PubMed] [Google Scholar]
- 42. Knaus WA, Draper EA, Wagner DP, et al. Apache II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- 43. Santos LC, Abreu CF, Xerinda SM, et al. Severe imported malaria in an intensive care unit: a review of 59 cases. Malar J 2012;11:96 10.1186/1475-2875-11-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weiler T, Baldering HJ, Heinrichs W, et al. [Quality assurance in intensive care medicine. Results of a multicenter study in Germany]. Anasthesiol Intensivmed Notfallmed Schmerzther 1997;32:372–5. 10.1055/s-2007-995073 [DOI] [PubMed] [Google Scholar]
- 45. World Health Organisation Severe malaria. Trop Med Int Health 2014;19 Suppl 1:7–131. 10.1111/tmi.12313_2 [DOI] [PubMed] [Google Scholar]
- 46. Plewes K, Turner GDH, Dondorp AM. Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr Opin Infect Dis 2018;31:69–77. 10.1097/QCO.0000000000000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. White VA, Lewallen S, Beare N, et al. Correlation of retinal haemorrhages with brain haemorrhages in children dying of cerebral malaria in Malawi. Trans R Soc Trop Med Hyg 2001;95:618–21. 10.1016/S0035-9203(01)90097-5 [DOI] [PubMed] [Google Scholar]
- 48. Lewallen S, Bronzan RN, Beare NA, et al. Using malarial retinopathy to improve the classification of children with cerebral malaria. Trans R Soc Trop Med Hyg 2008;102:1089–94. 10.1016/j.trstmh.2008.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ogetii GN, Akech S, Jemutai J, et al. Hypoglycaemia in severe malaria, clinical associations and relationship to quinine dosage. BMC Infect Dis 2010;10:334 10.1186/1471-2334-10-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thien HV, Kager PA, Sauerwein HP. Hypoglycemia in falciparum malaria: is fasting an unrecognized and insufficiently emphasized risk factor? Trends Parasitol 2006;22:410–5. 10.1016/j.pt.2006.06.014 [DOI] [PubMed] [Google Scholar]
- 51. Taylor WRJ, Hanson J, Turner GDH, et al. Respiratory manifestations of malaria. Chest 2012;142:492–505. 10.1378/chest.11-2655 [DOI] [PubMed] [Google Scholar]
- 52. Máca J, Jor O, Holub M, et al. Past and present ARDS mortality rates: a systematic review. Respir Care 2017;62:113–22. 10.4187/respcare.04716 [DOI] [PubMed] [Google Scholar]
- 53. Duvic C, Rabar D, Didelot F, et al. [Acute renal failure during severe malaria: physiopathology and therapeutic management. Apropos of 2 cases]. Med Trop 2000;60:267–70. [PubMed] [Google Scholar]
- 54. Bruneel F, Gachot B, Timsit JF, et al. Shock complicating severe falciparum malaria in European adults. Intensive Care Med 1997;23:698–701. 10.1007/s001340050396 [DOI] [PubMed] [Google Scholar]
- 55. Kuethe F, Pfeifer R, Rummler S, et al. Treatment of a patient with shock complicating severe falciparum malaria: a case report. Cases J 2009;2:6644 10.1186/1757-1626-2-6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Altman DG, Vergouwe Y, Royston P, et al. Prognosis and prognostic research: validating a prognostic model. BMJ 2009;338:b605 10.1136/bmj.b605 [DOI] [PubMed] [Google Scholar]
- 57. Taylor T, Olola C, Valim C, et al. Standardized data collection for multi-center clinical studies of severe malaria in African children: establishing the SMAC network. Trans R Soc Trop Med Hyg 2006;100:615–22. 10.1016/j.trstmh.2005.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Seneff M, Knaus WA. Predicting patient outcome from intensive care: a guide to APACHE, MPM, saps, prism, and other prognostic scoring systems. J Intensive Care Med 1990;5:33–52. 10.1177/088506669000500107 [DOI] [Google Scholar]
- 59. Aggarwal HK, Jain D, Rao A, et al. Role of coma acidosis malaria score in patients with severe malaria among Indian population: a tertiary care center experience. Eurasian J Med 2017;49:30–5. 10.5152/eurasianjmed.2017.16069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-030793supp001.pdf (85.2KB, pdf)